Patents
Literature
258 results about "Solid oral dosage form" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This inspection guide provides information regarding the inspection and evaluation of the manufacturing and control processes used to manufacture solid oral dosage form pharmaceutical products.
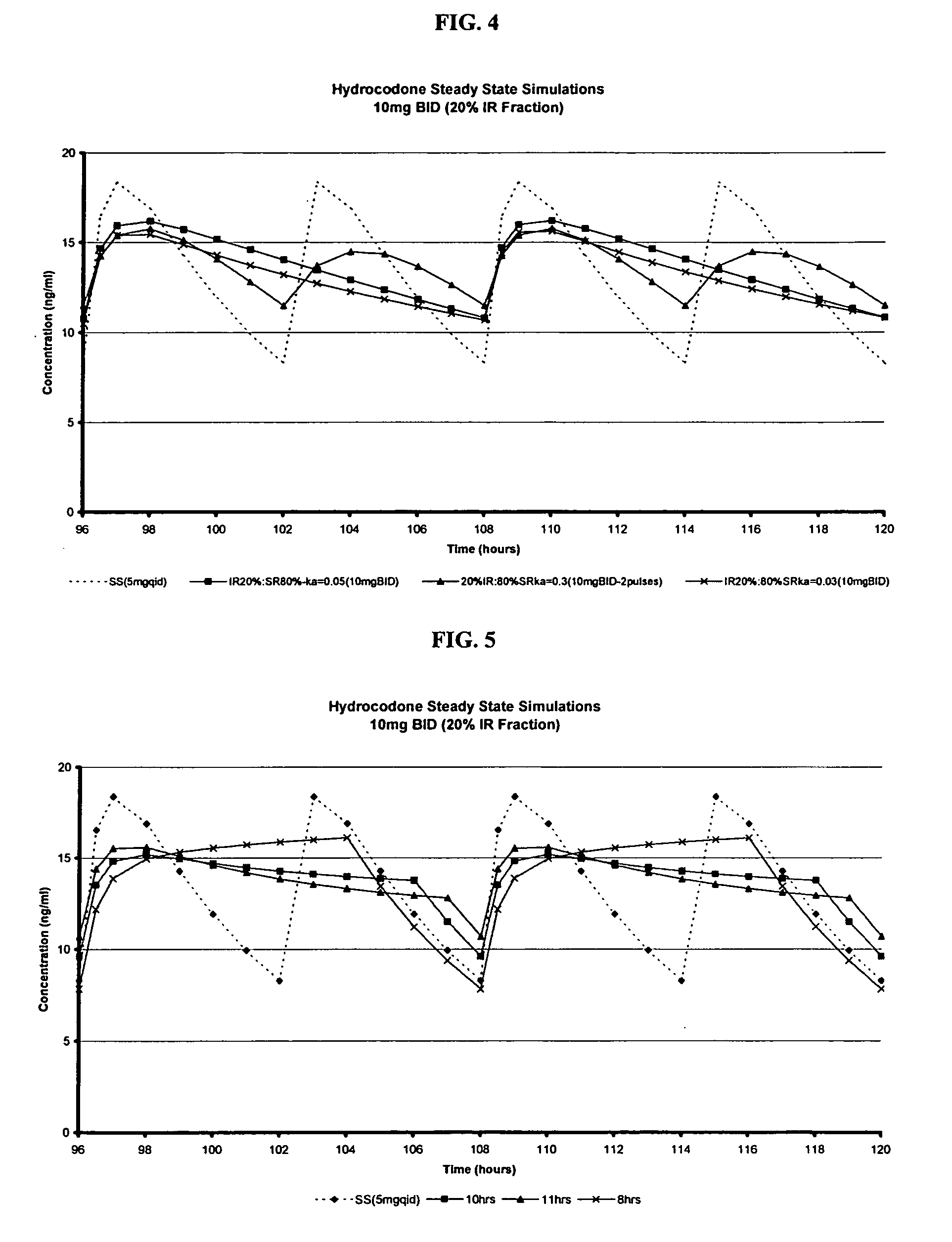
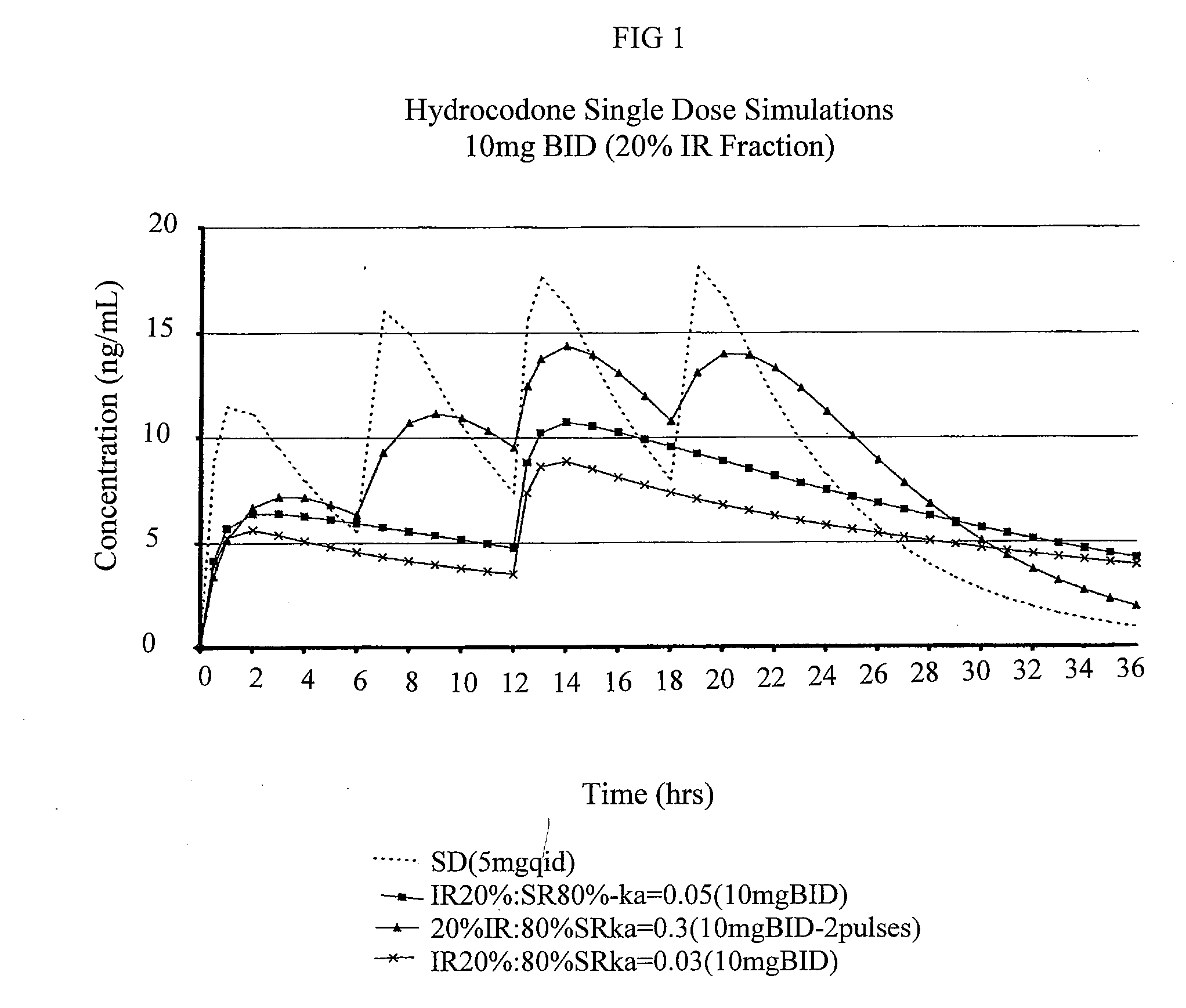
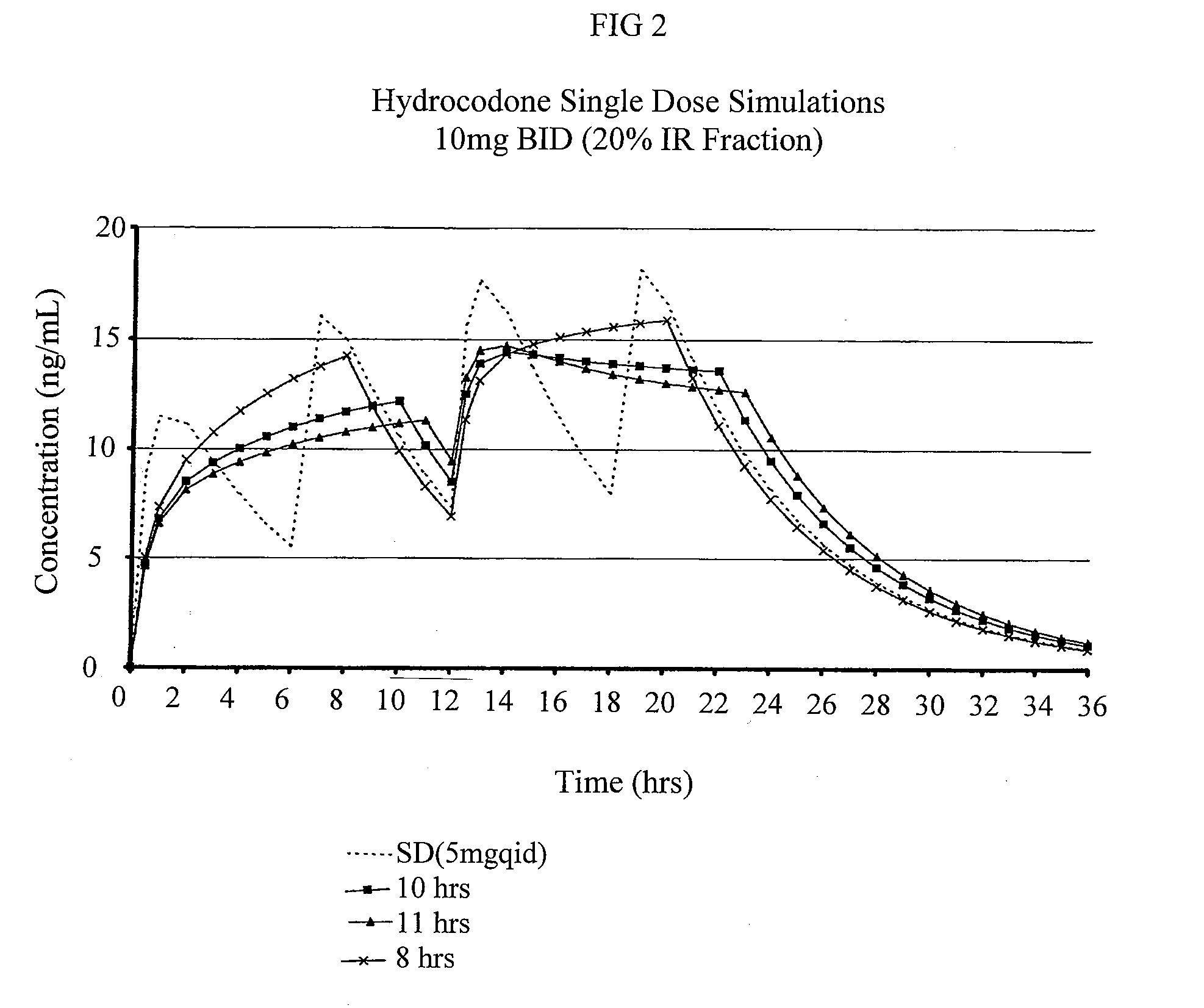
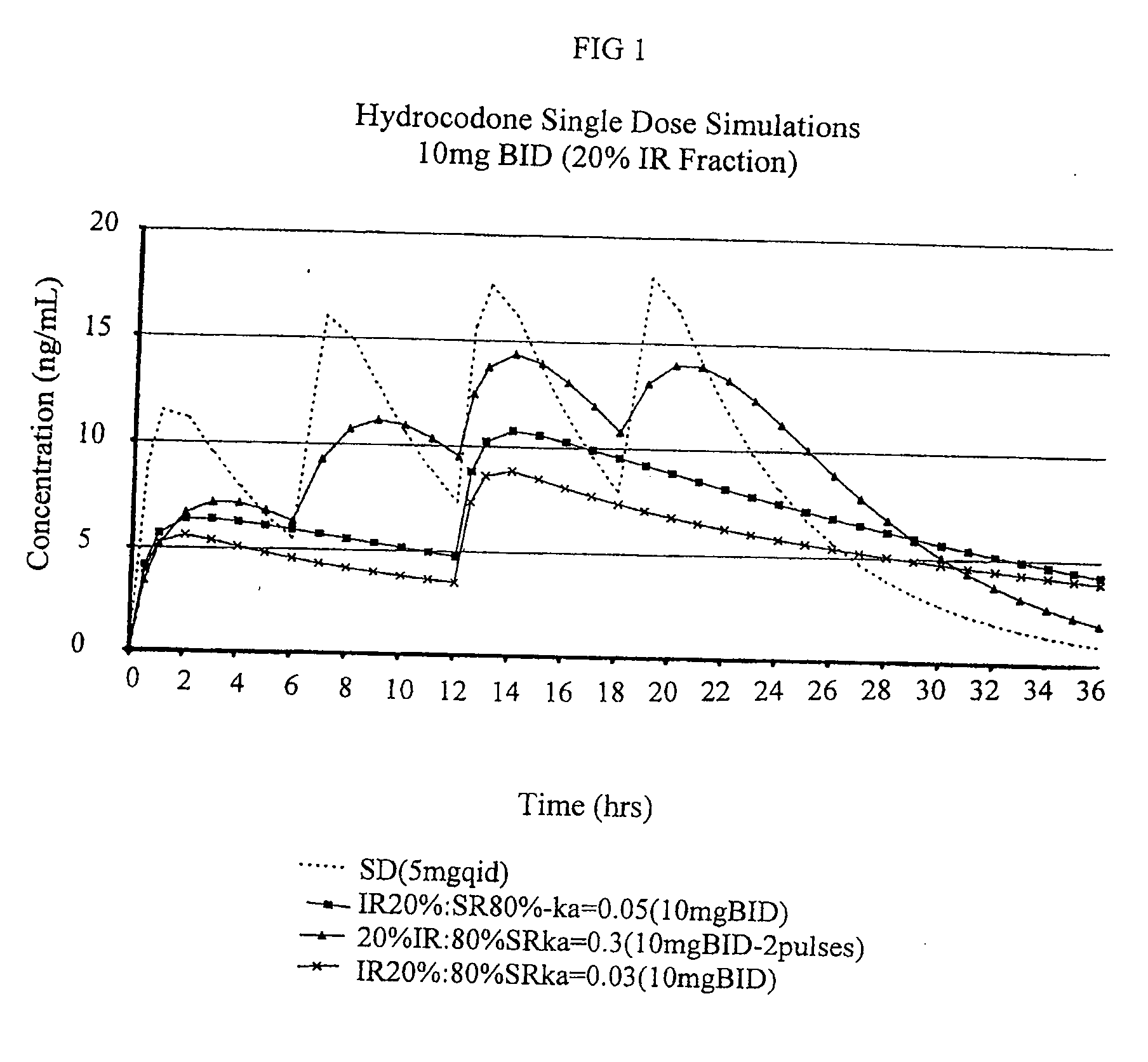
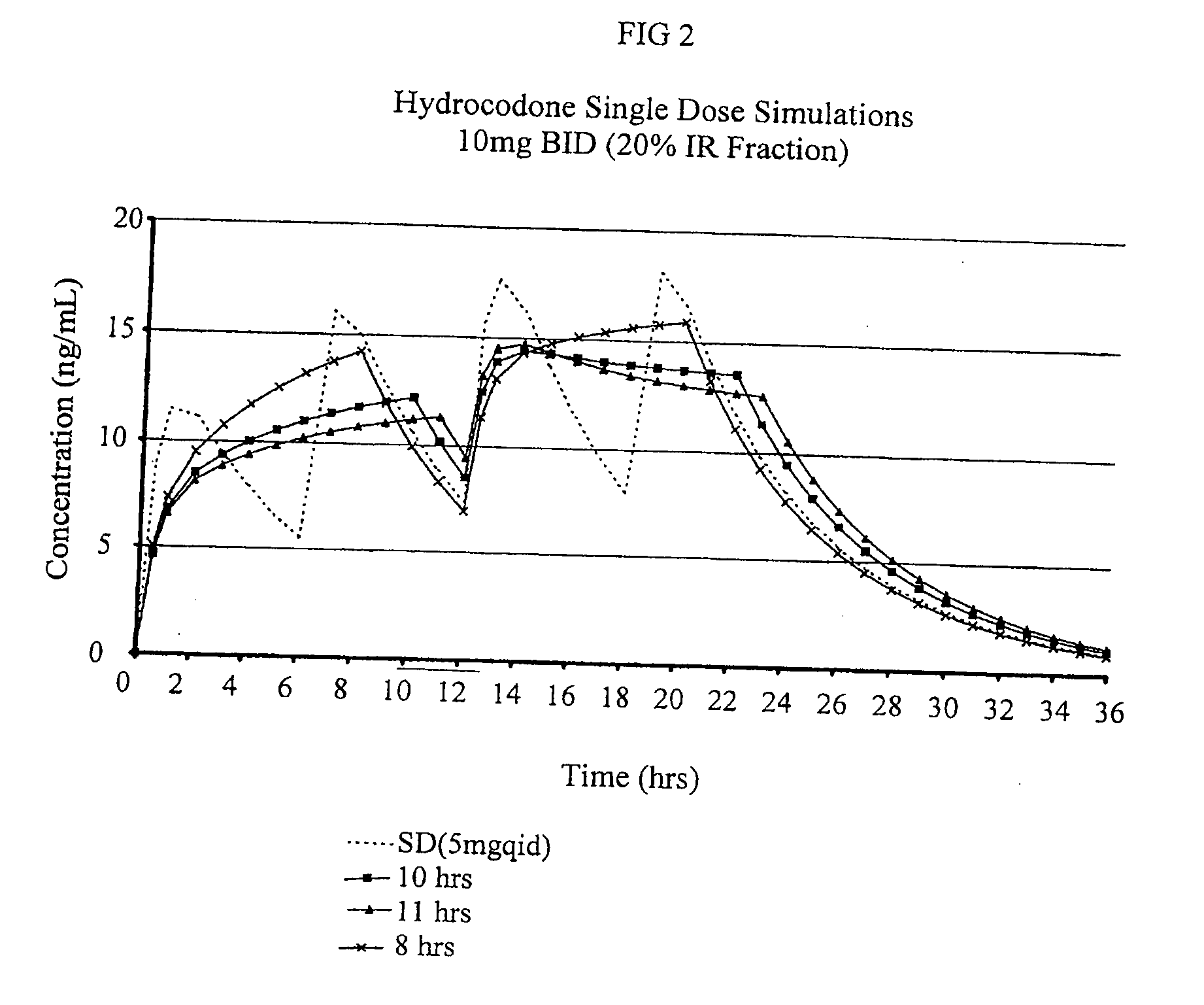
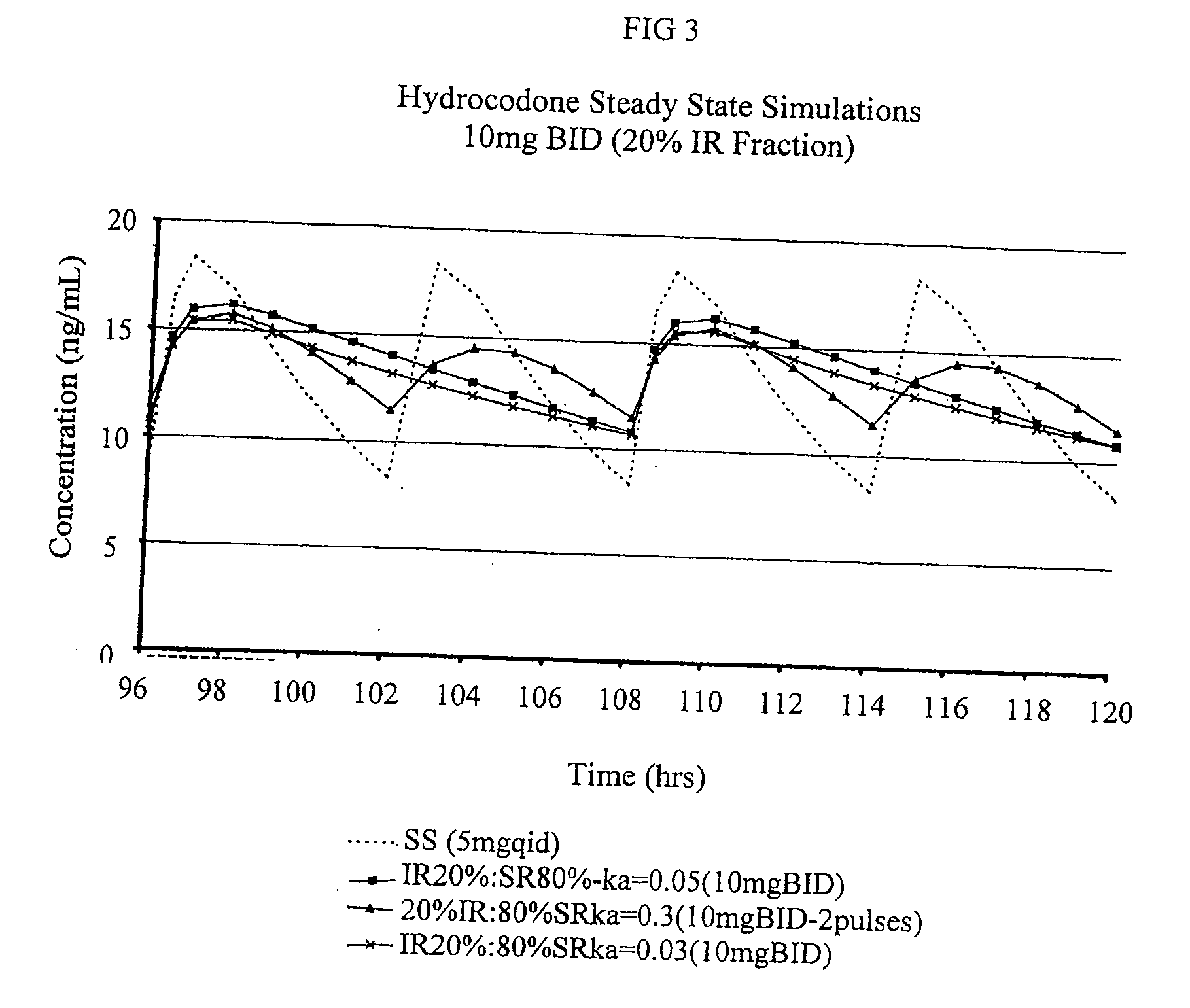
Multiparticulate modified release composition
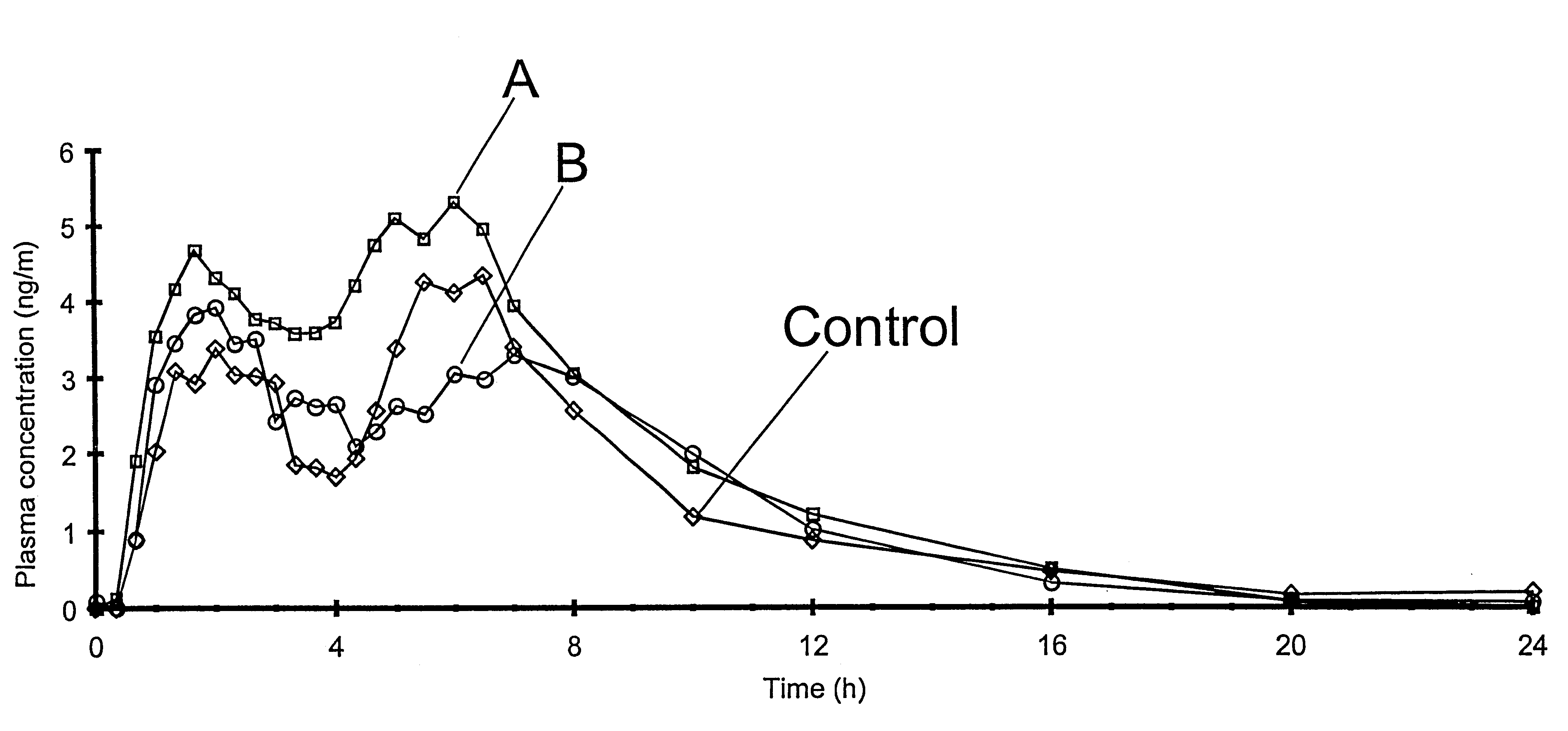
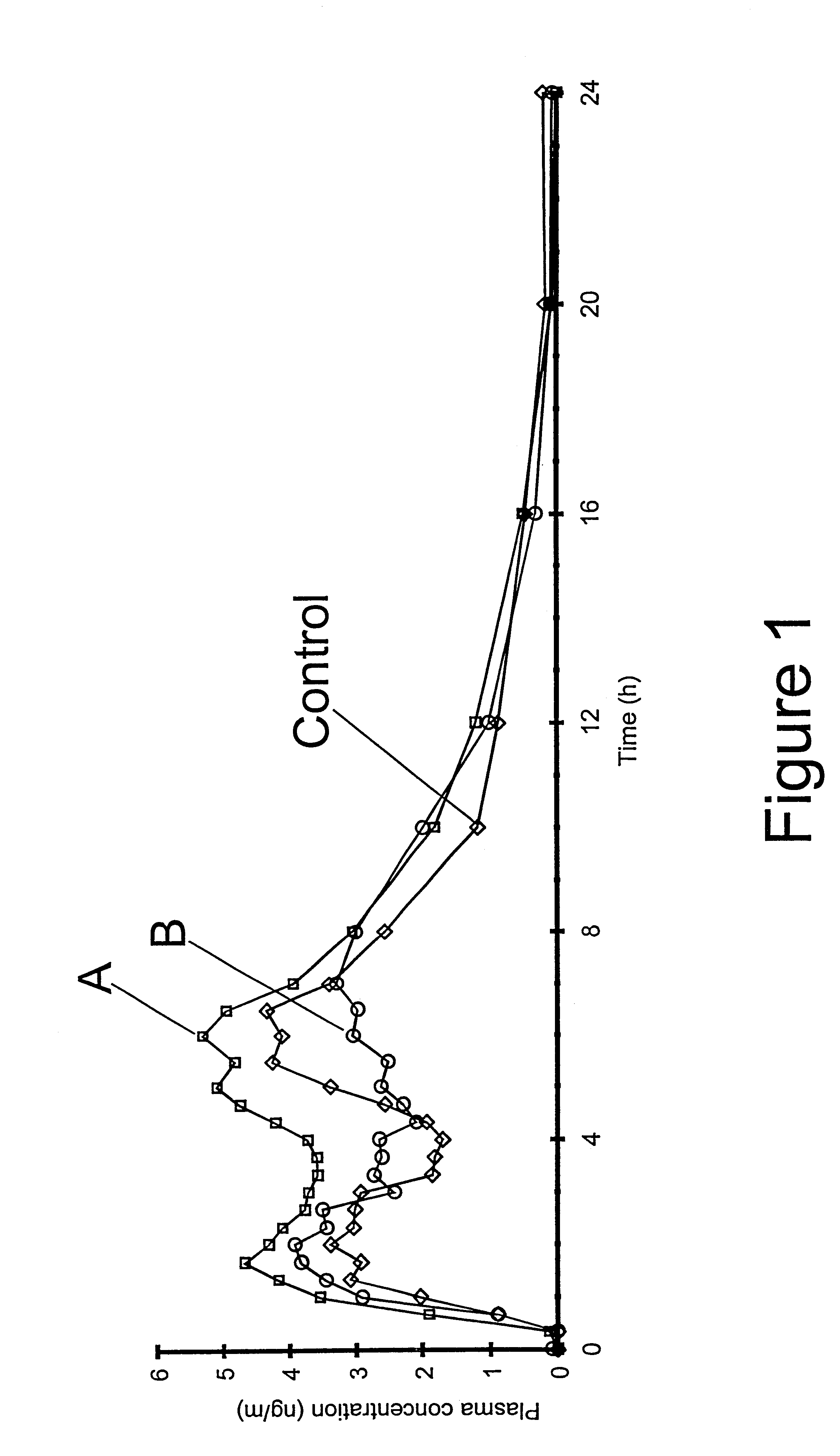
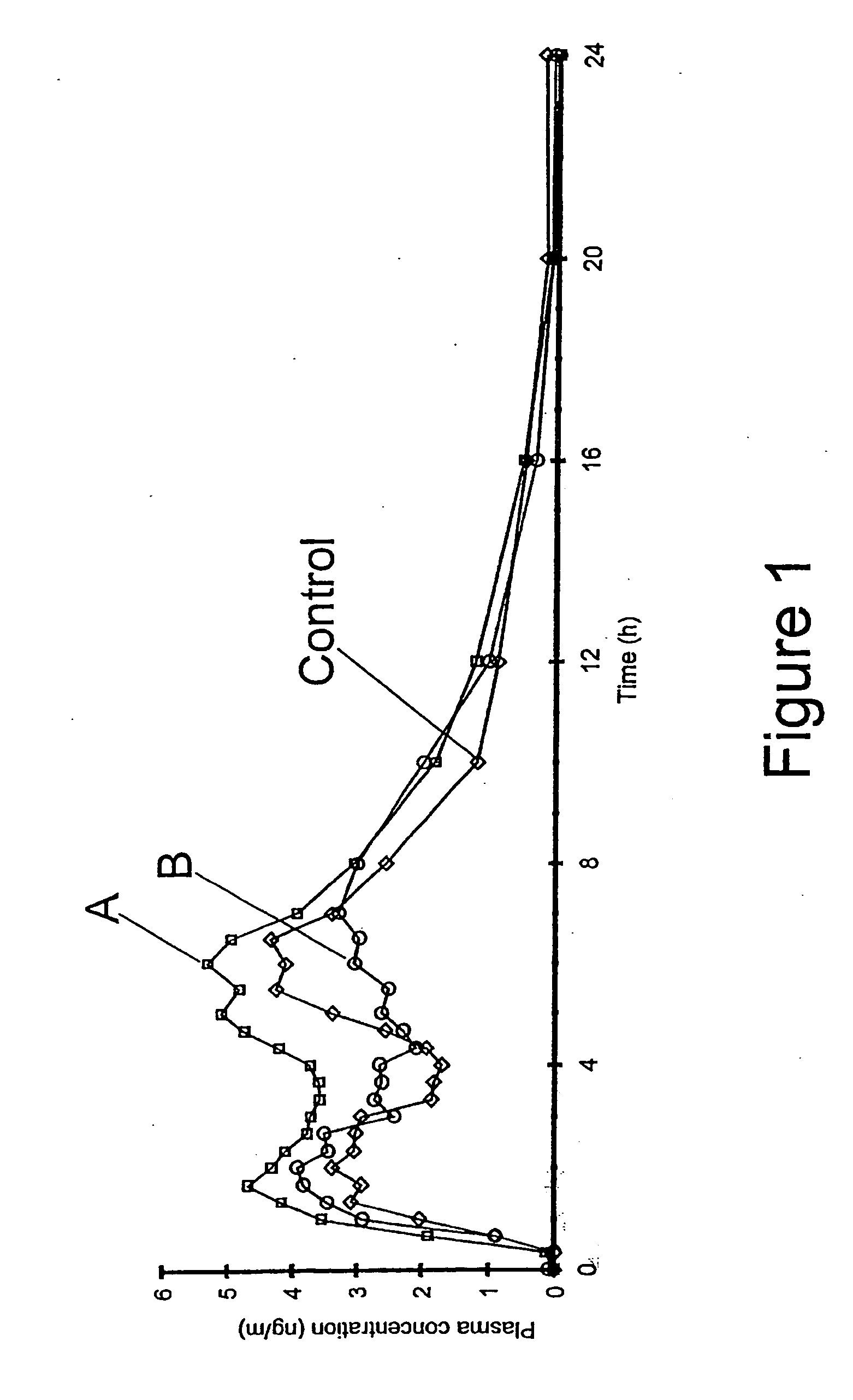
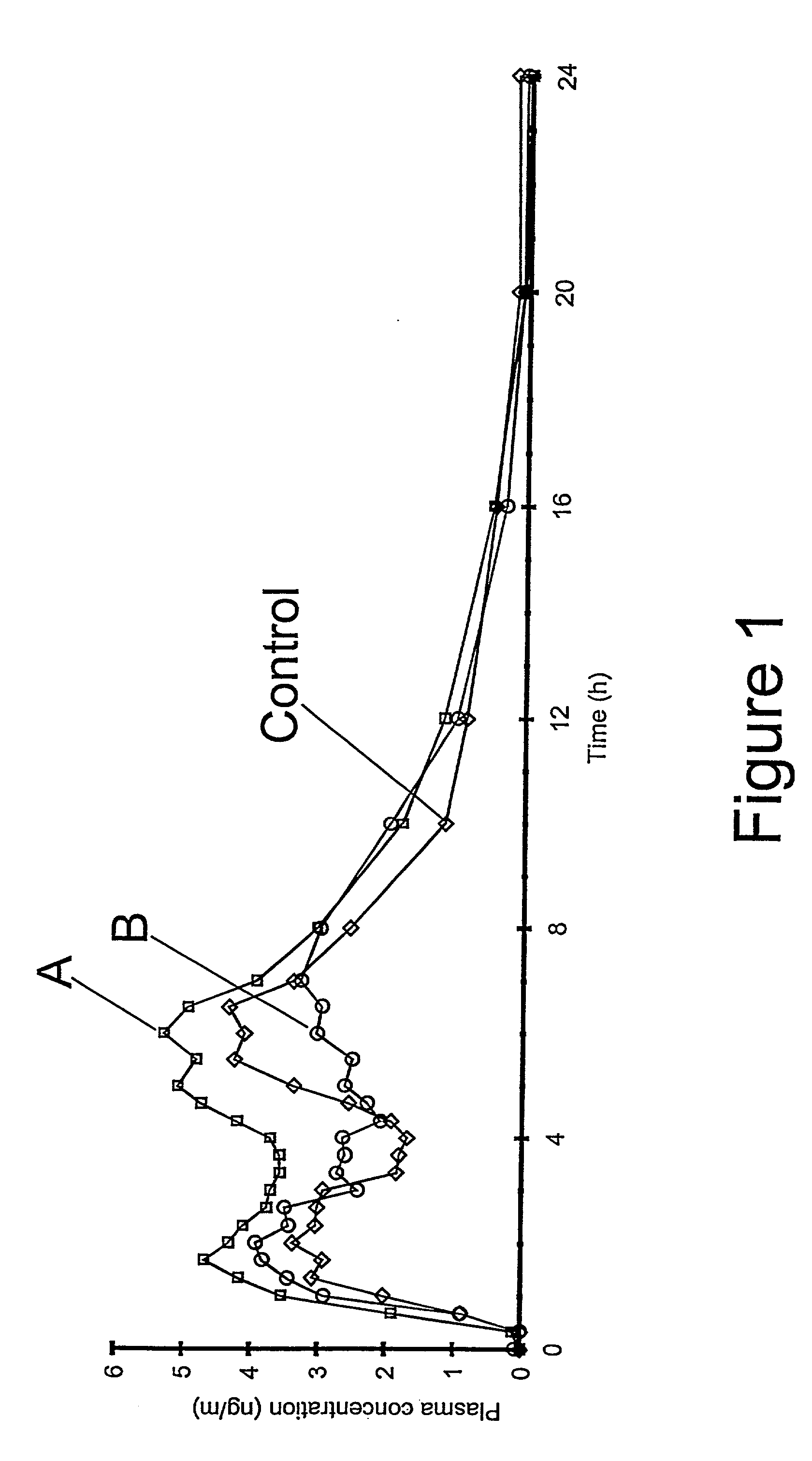
The invention relates to a multiparticulate modified release composition that in operation delivers an active ingredient in a pulsed or bimodal manner. The multiparticulate modified release composition comprises an immediate release component and a modified release component; the immediate release component comprising a first population of active ingredient containing particles and the modified release component compnsimg a second population of active ingredient containing particles coated with a controlled release coating; wherein the combination of the immediate release and modified release components in operation deliver the active ingredient in a pulsed or a bimodal manner. The invention also relates to a solid oral dosage form containing such a multiparticulate modified release composition. The plasma profile achieved by the multiparticulate modified release composition is advantageous in reducing patient tolerance to the active ingredient and in increasing patient compliance by reducing dosage frequency.
Owner:ALKERMES PHARMA IRELAND LTD +1
Multiparticulate modified release composition
InactiveUS20060240105A1Reduce dosing frequencyReduce frequencyBiocideAnimal repellantsBULK ACTIVE INGREDIENTActive ingredient
The invention relates to a multiparticulate modified release composition that, upon administration to a patient, delivers at least one active ingredient in a bimodal or multimodal manner. The multiparticulate modified release composition comprises a first component and at least one subsequent component; the first component comprising a first population of active ingredient containing particles and the at least one subsequent component comprising a second population of active ingredient containing particles wherein the combination of the components exhibit a bimodal or multimodal release profile. The invention also relates to a solid oral dosage form containing such a multiparticulate modified release composition.
Owner:ALKERMES PHARMA IRELAND LTD
Controlled regional oral delivery
InactiveUS20060045865A1Significant variabilityLow variabilityPill deliveryGranular deliverySolubilityGabapentin
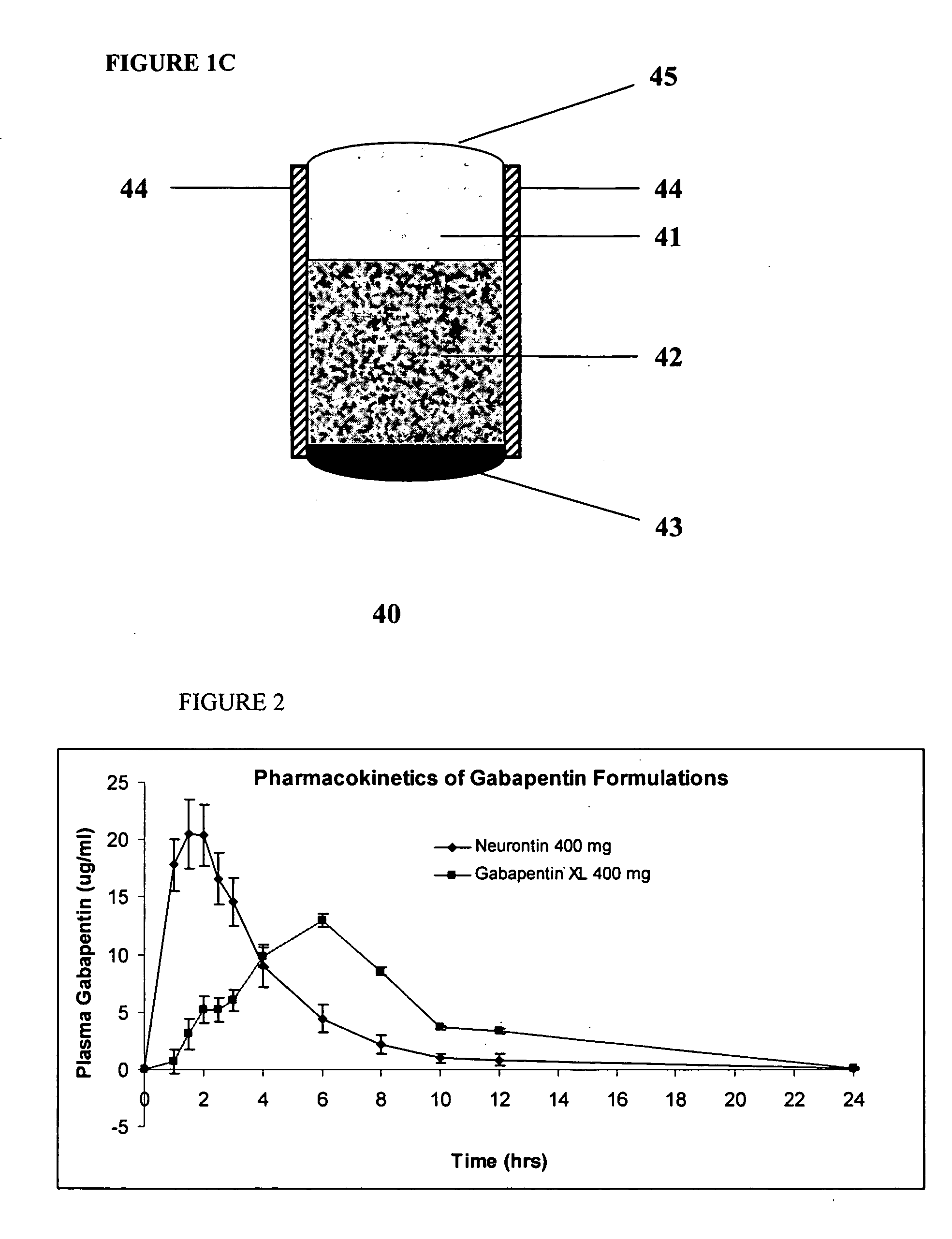
A composite formulation has been developed for selective, high efficacy delivery to specific regions of the mouth and gastrointestinal tract. The formulation is typically in the form of a tablet or capsule, which may include microparticles or beads. The formulation uses bioadhesive and controlled release elements to direct release to specific regions, where the drug is absorbed in enhanced amounts relative to the formulation in the absence of the bioadhesive and / or controlled release elements. This is demonstrated by an example showing delivery of gabapentin with a greater area under the curve (“AUC”) relative to the FDA reference immediate release drug, i.e., the AUC of the composite bioadhesive formulation is greater than 100% of the AUC of the immediate release drug. In the preferred embodiments, the formulation includes drug to be delivered, controlled release elements, and one or more bioadhesive elements. The bioadhesive polymer may be either dispersed in the matrix of the tablet or applied as a direct compressed coating to the solid oral dosage form. The controlled release elements are selected to determine the site of release. The bioadhesive components are selected to provide retention of the formulation at the desired site of uptake and administration. By selecting for both release and retention at a specific site, typically based on time of transit through the gastrointestinal tract, one obtains enhanced efficacy of uptake of the drug. This is particularly useful for drugs with narrow windows of absorption, and drugs with poor solubility such as the BCE class III and class IV drugs.
Owner:VAUNNEX
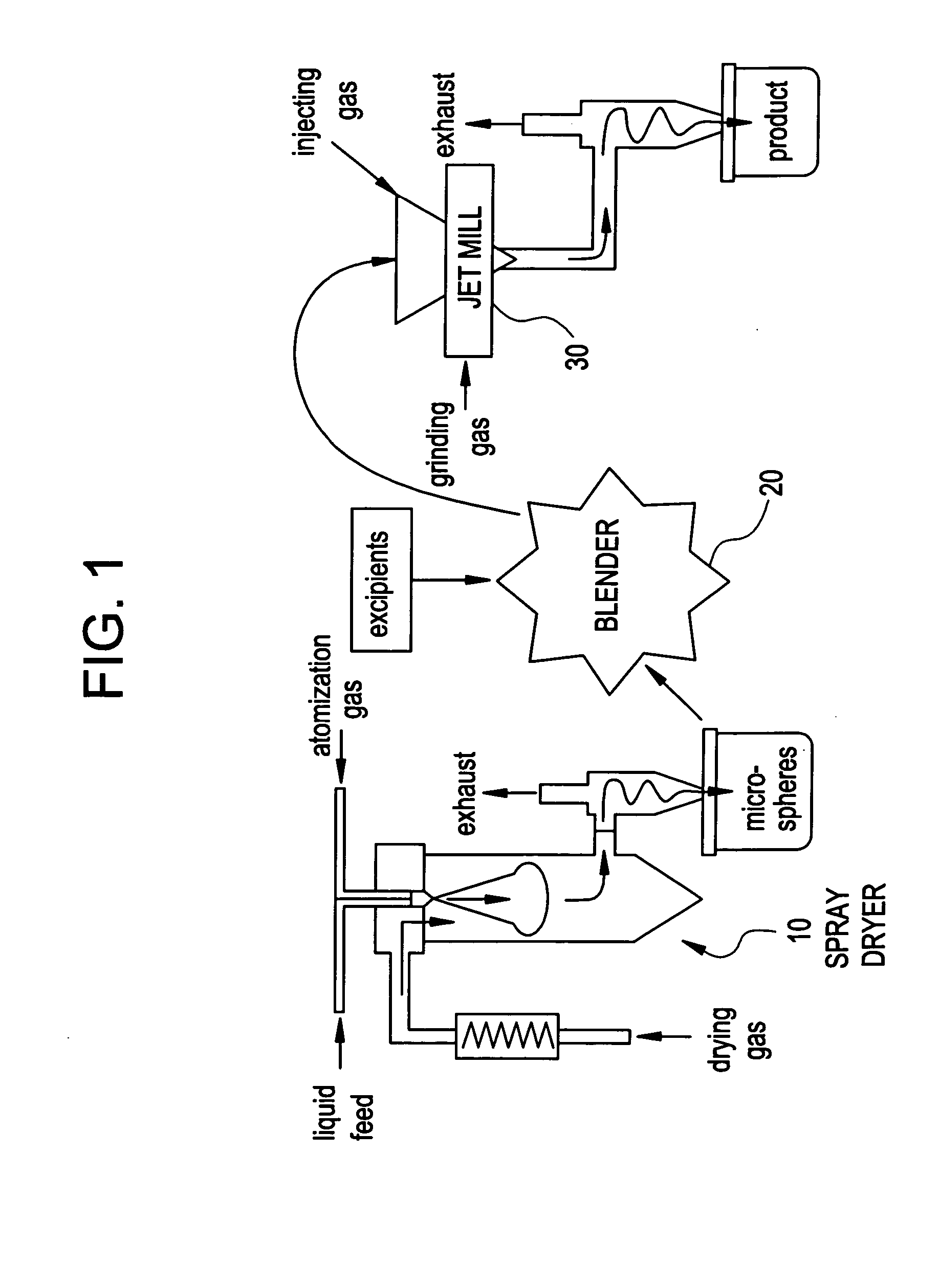
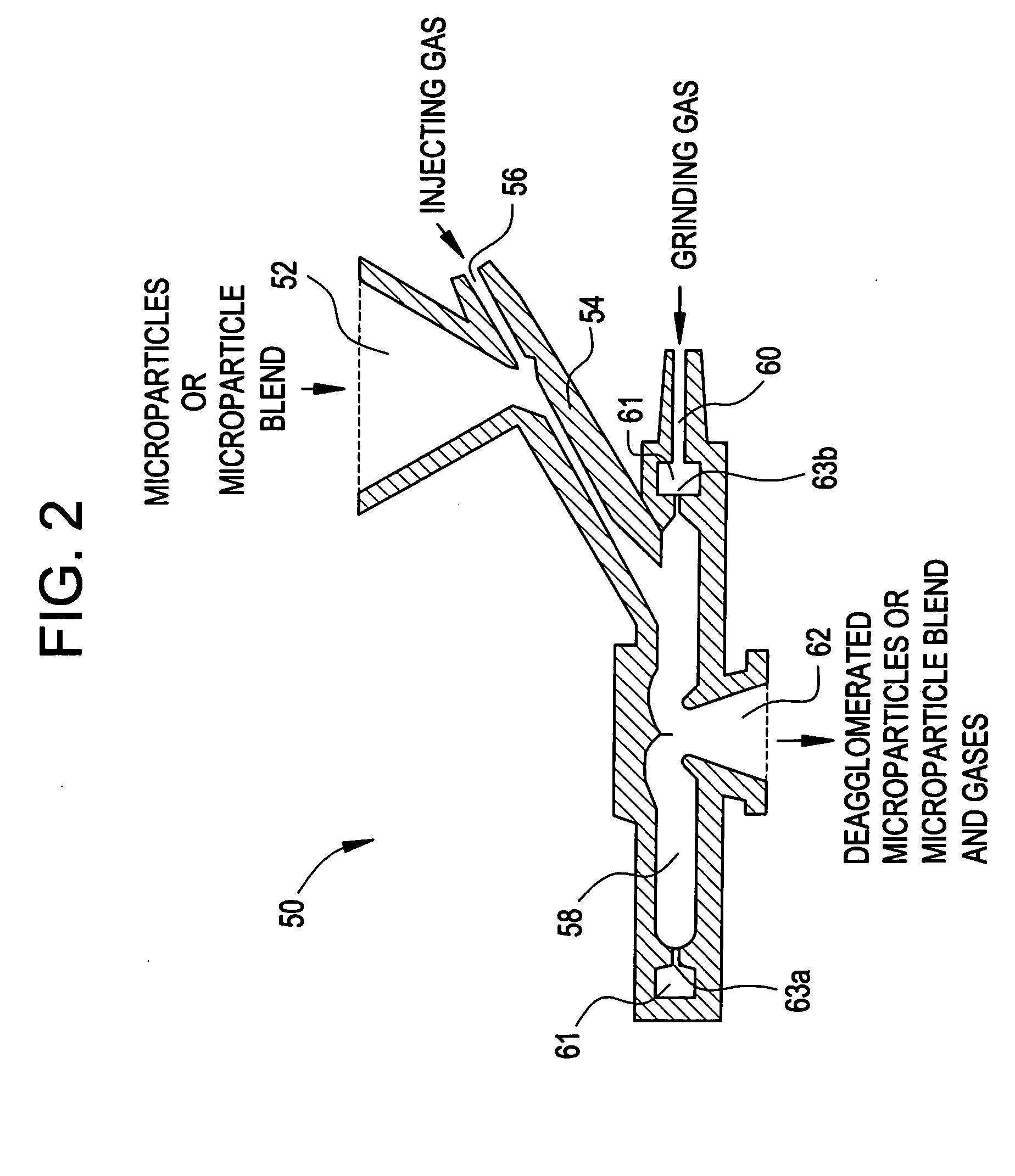
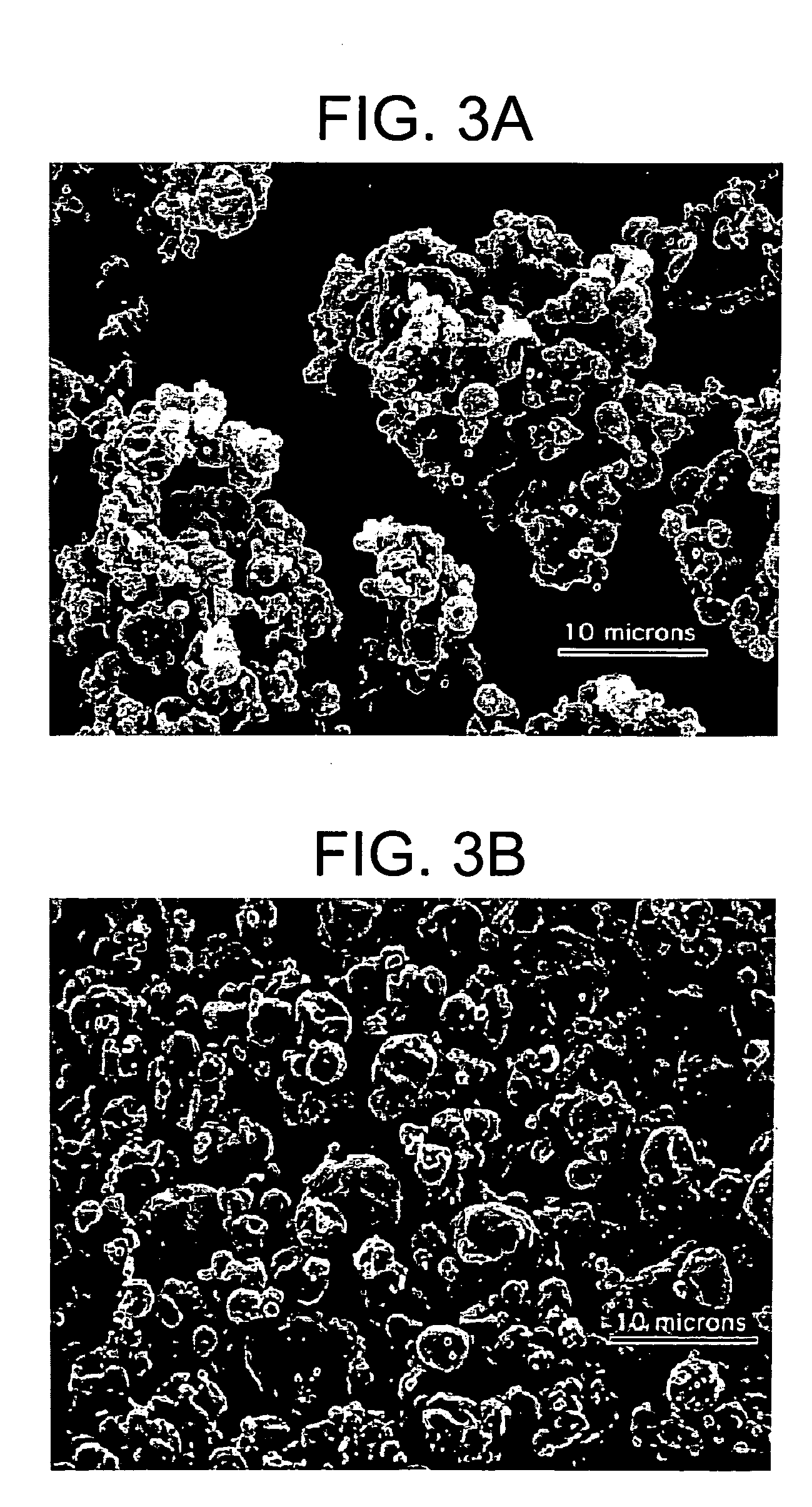
Methods for making pharmaceutical formulations comprising microparticles with improved dispersibility, suspendability or wettability
InactiveUS20050079138A1Good dispersibilityImproved suspendabilityPowder deliveryGranulation by liquid drop formationPowder mixtureMicroparticle
Methods are provided for making a dry powder blend pharmaceutical formulation, comprising the steps of: (a) providing microparticles which comprise a pharmaceutical agent; (b) blending the microparticles with at least one excipient in the form of particles to form a powder blend; and (c) jet milling the powder blend to form a dry powder blend pharmaceutical formulation having improved dispersibility, suspendability, or wettability as compared to the microparticles of step (a) or the powder blend of step (b). The method can further include dispersing the dry powder blend pharmaceutical formulation in a liquid pharmaceutically acceptable vehicle to make an formulation suitable for injection. Alternatively, the method can further include processing the dry powder blend pharmaceutical formulation into a solid oral dosage form. In one embodiment, the microparticles of step (a) are formed by a solvent precipitation or crystallization process.
Owner:ACUSPHERE INC
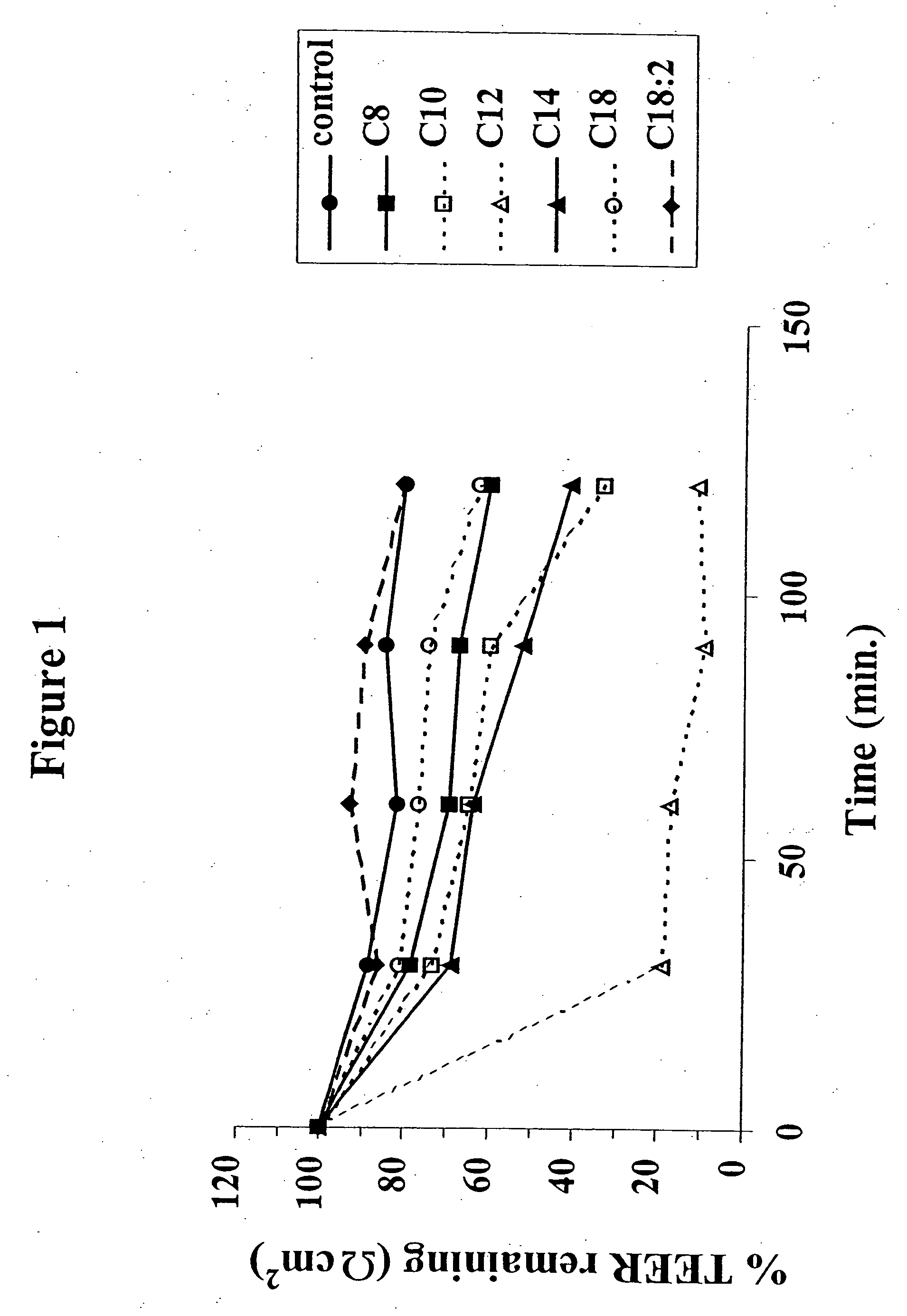
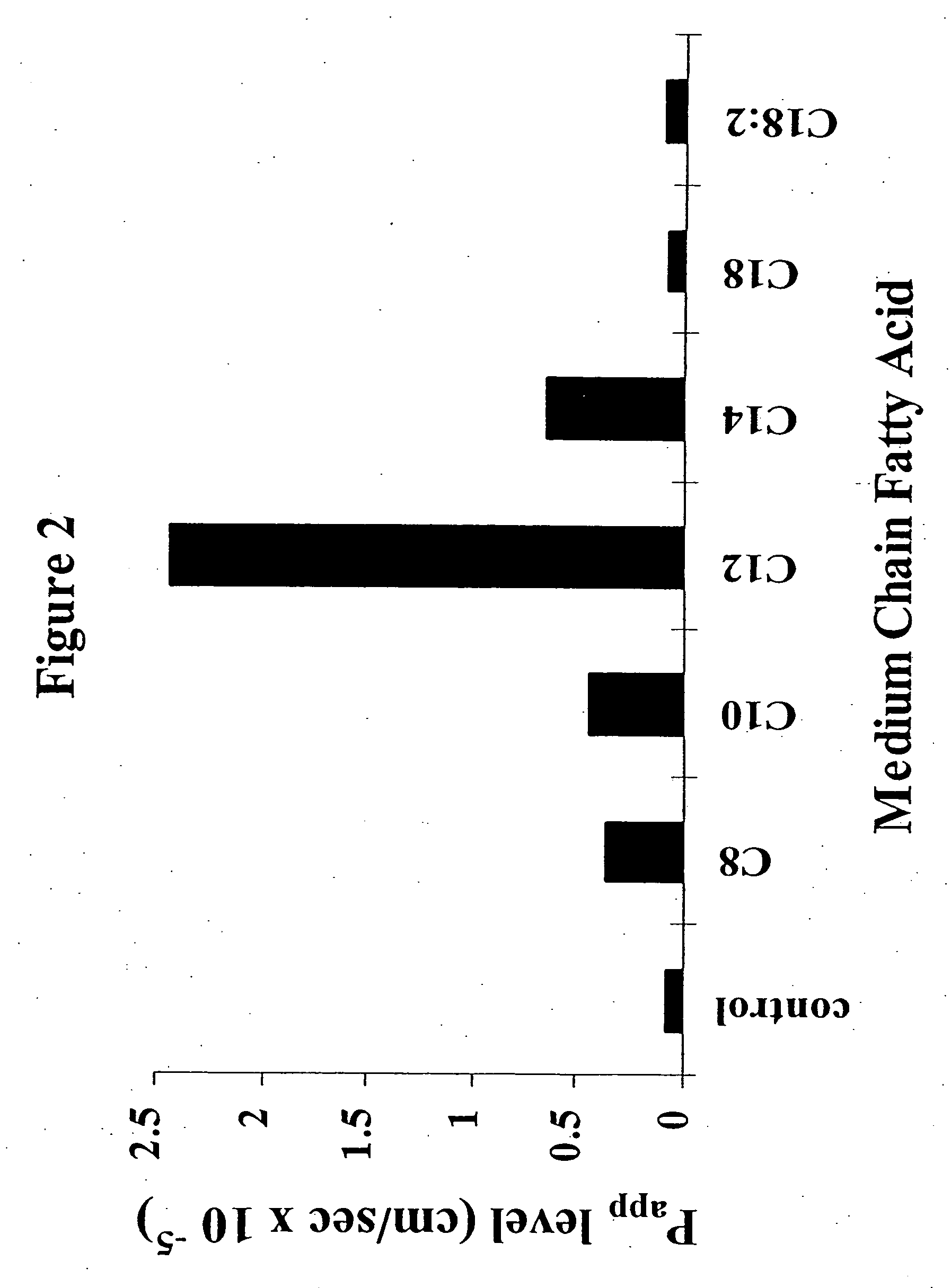
Solid oral dosage form containing an enhancer
InactiveUS8119159B2Minimizes risk of local irritationImprove oral bioavailabilityBiocidePhosphorous compound active ingredientsDelayed Release Dosage FormCarbon chain
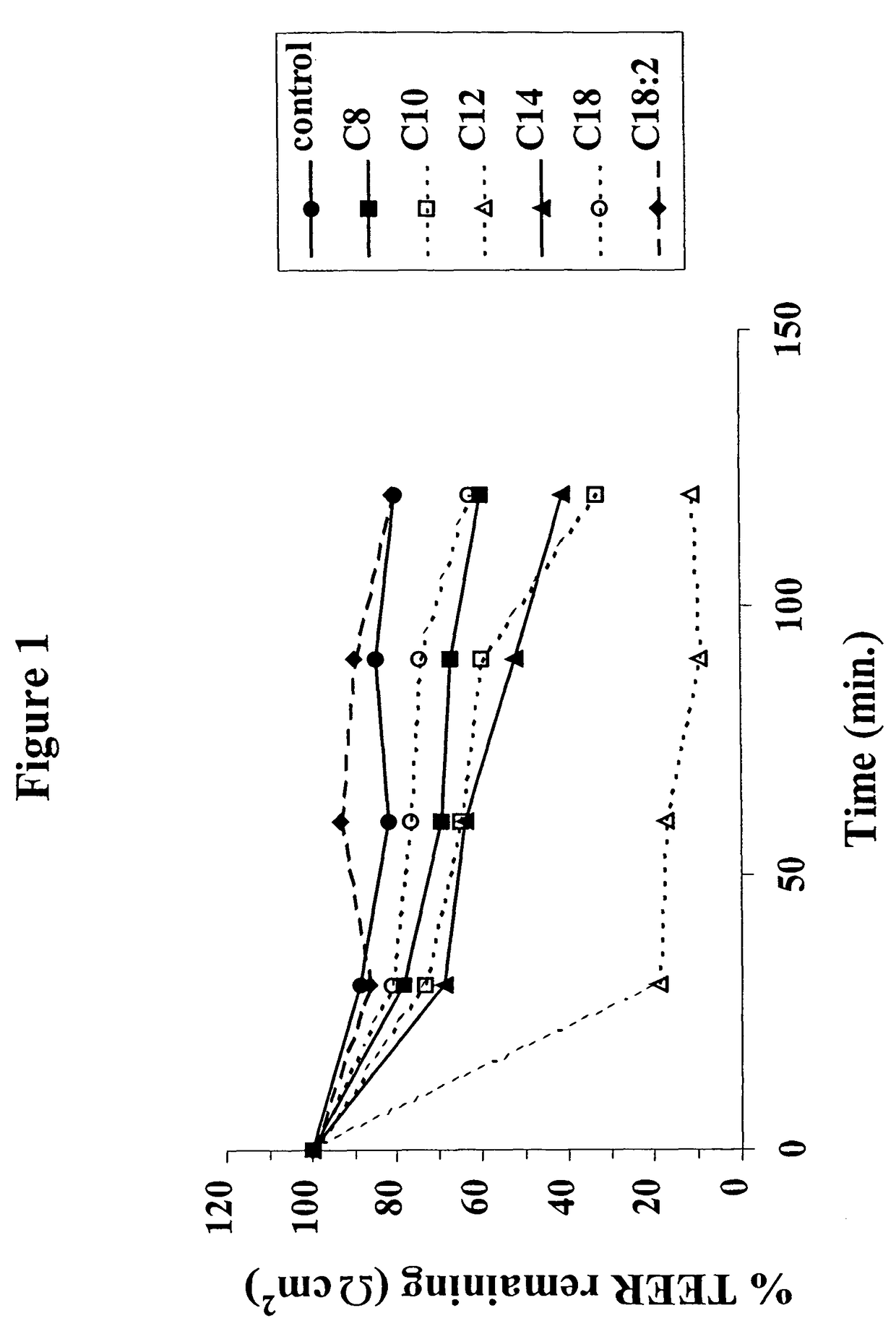
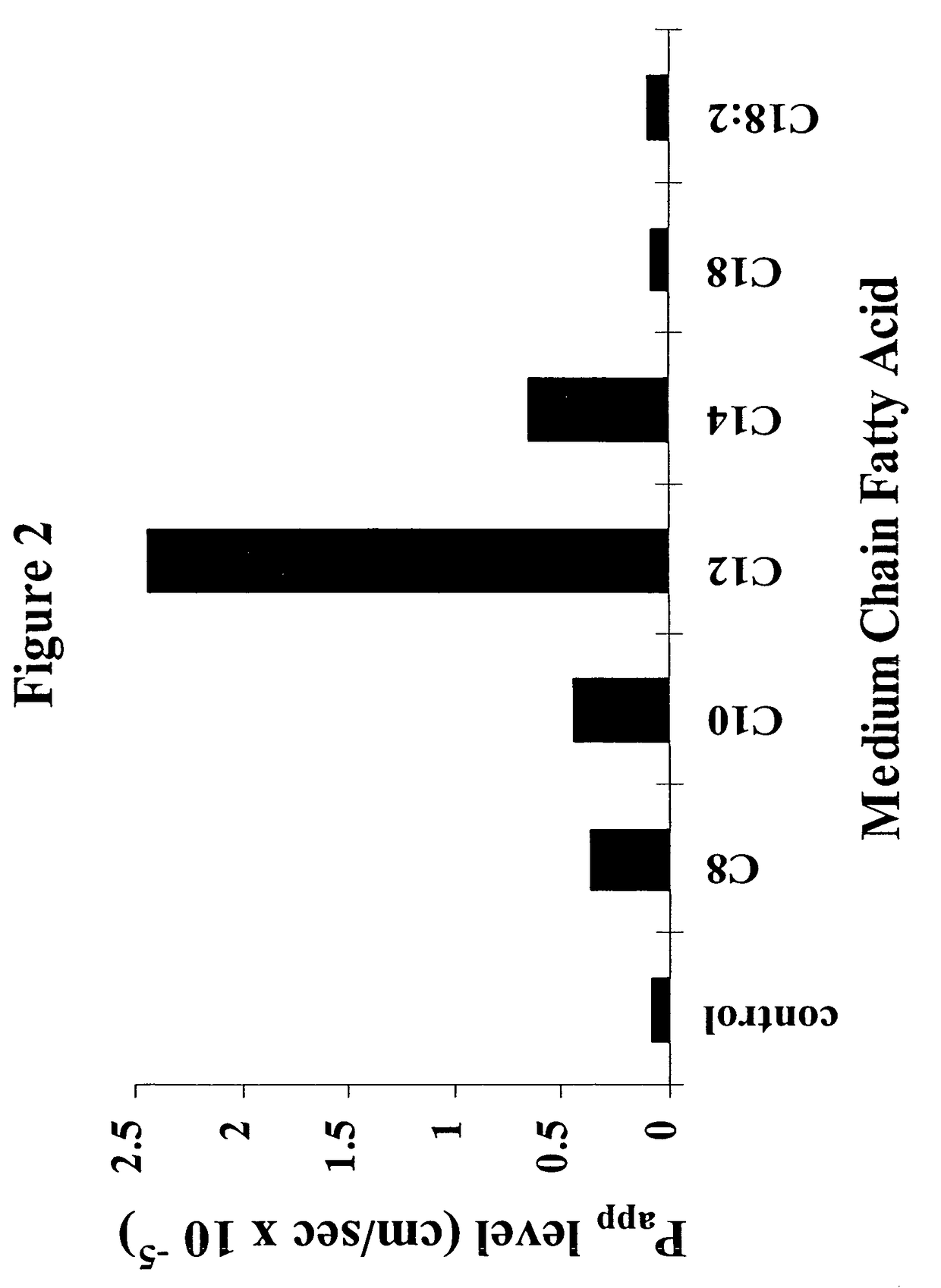
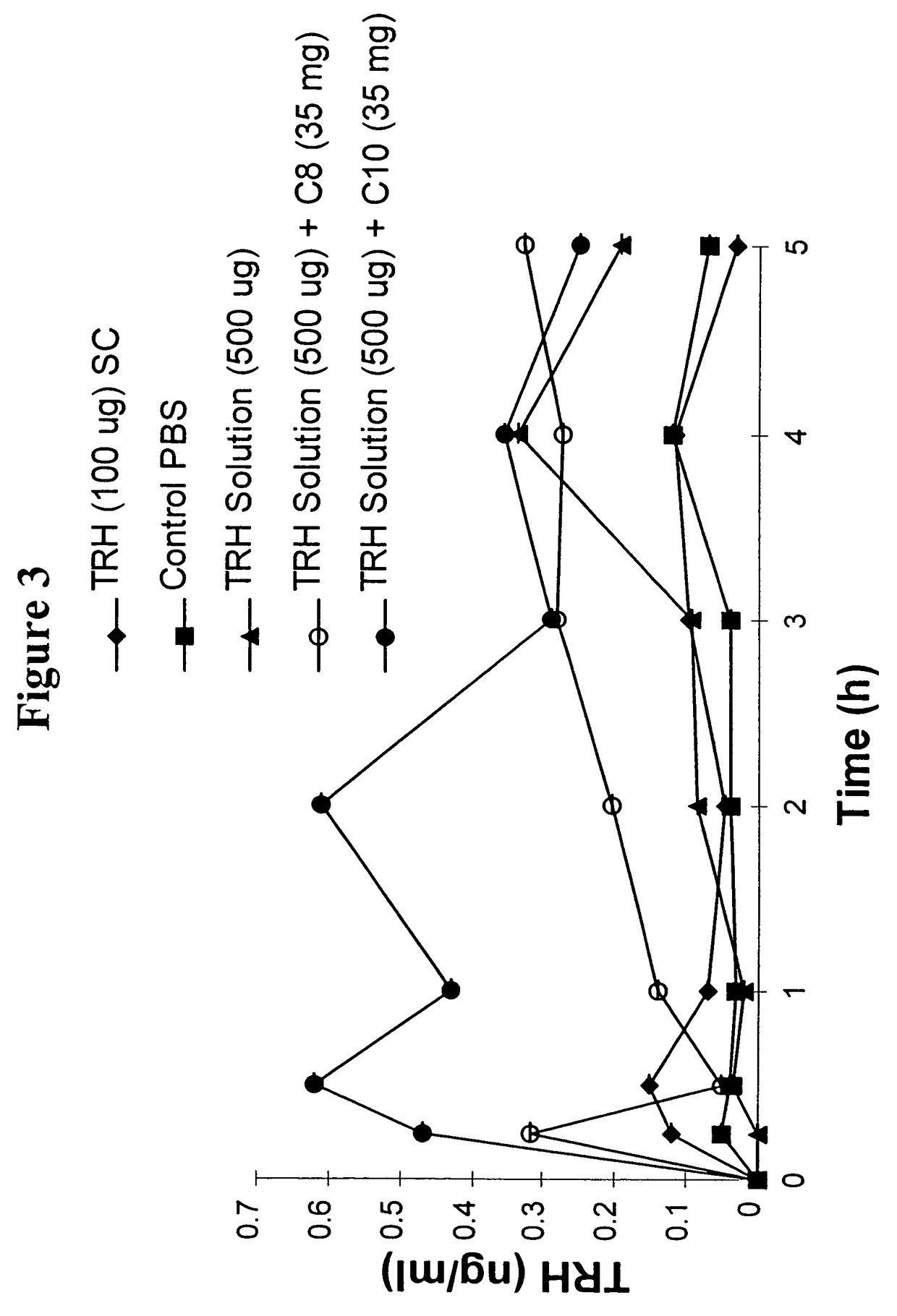
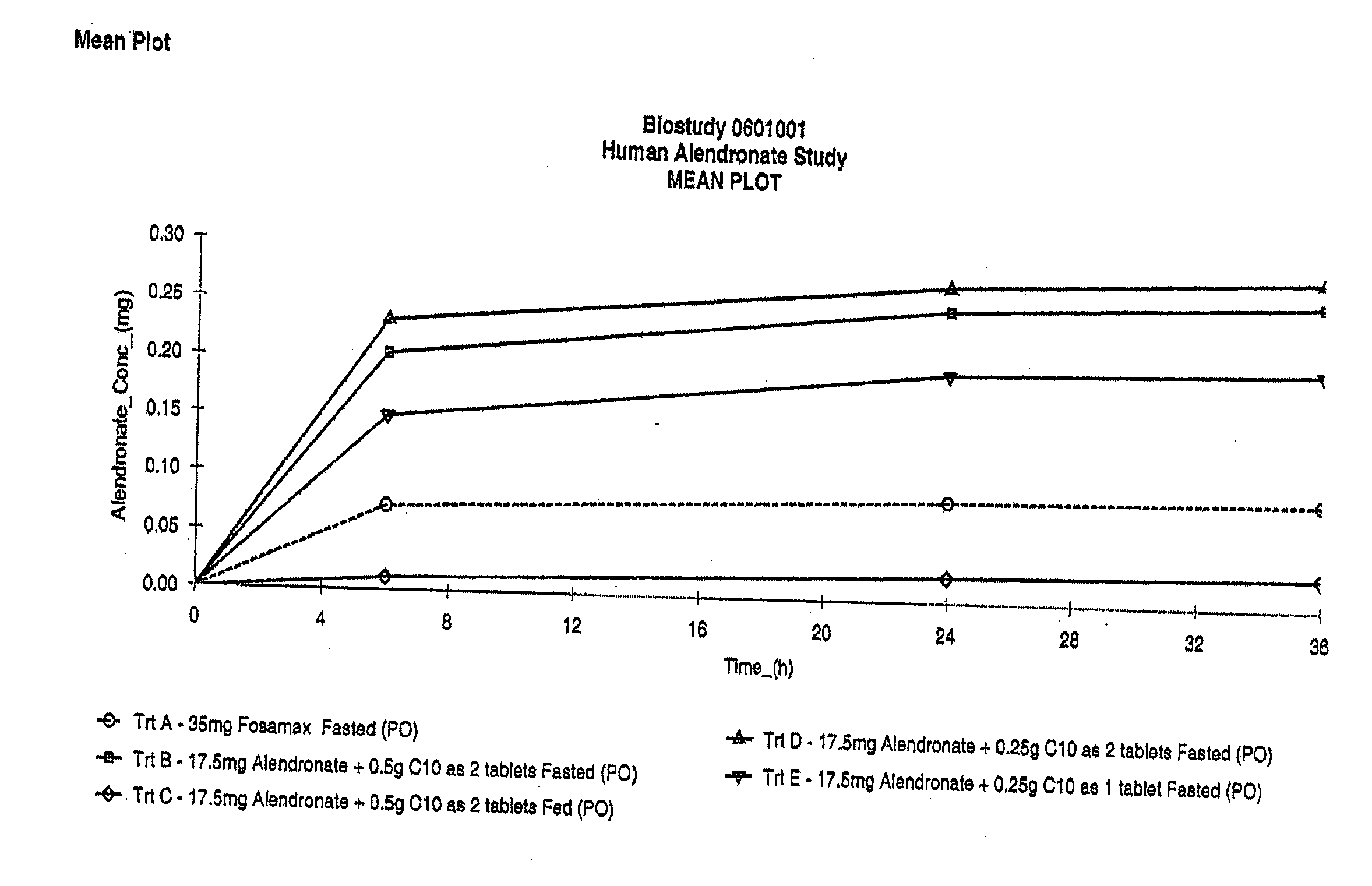
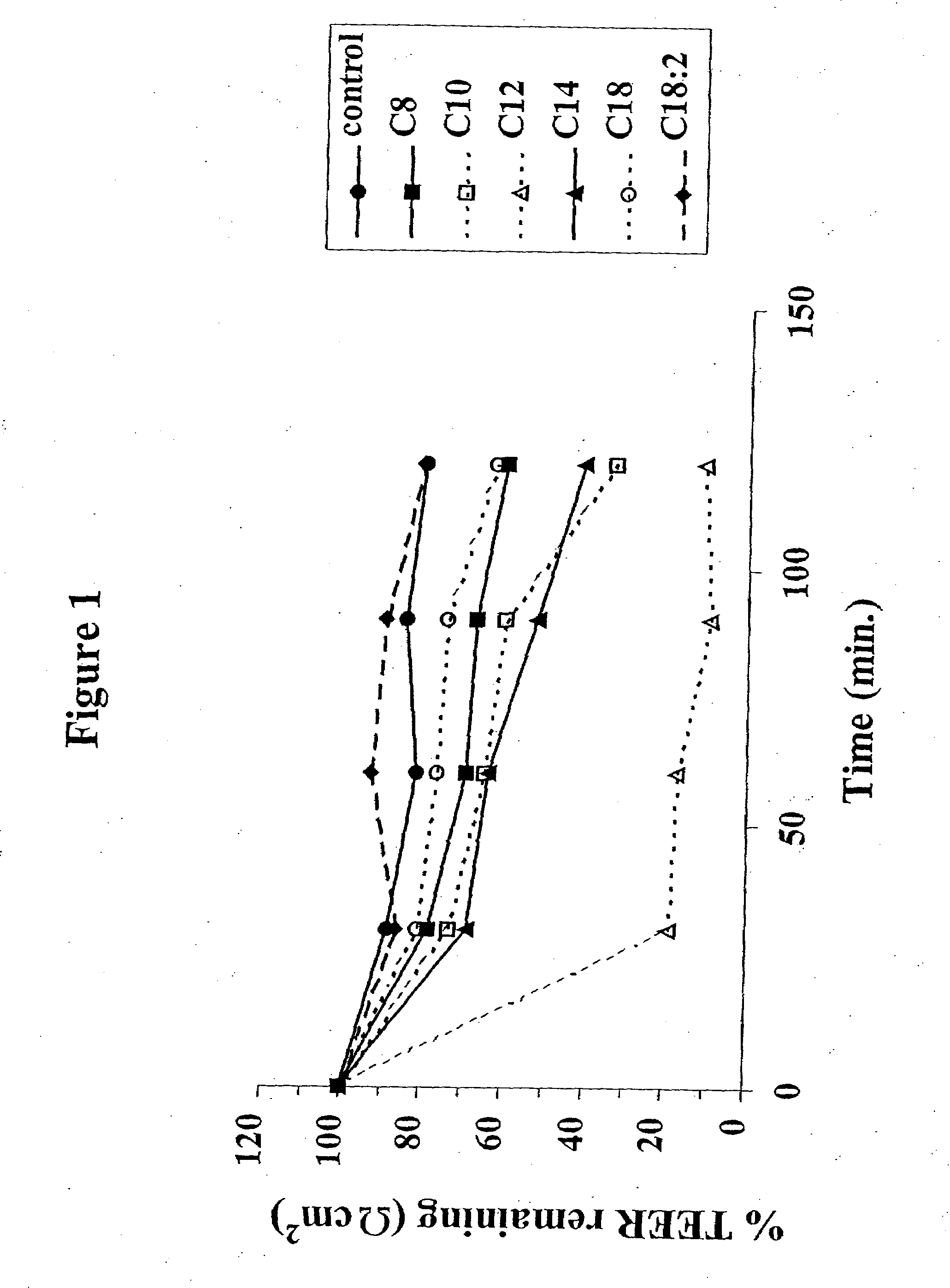
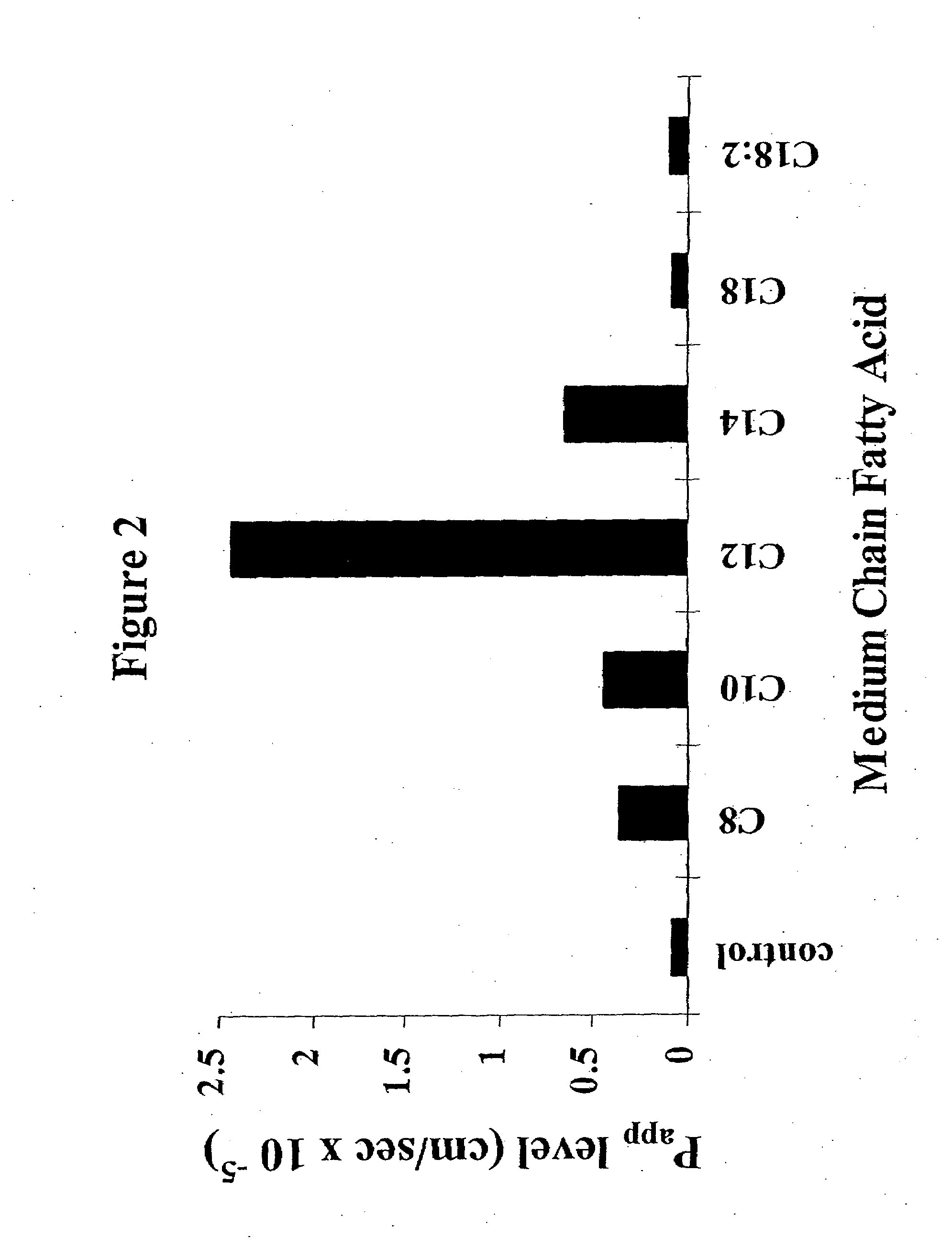
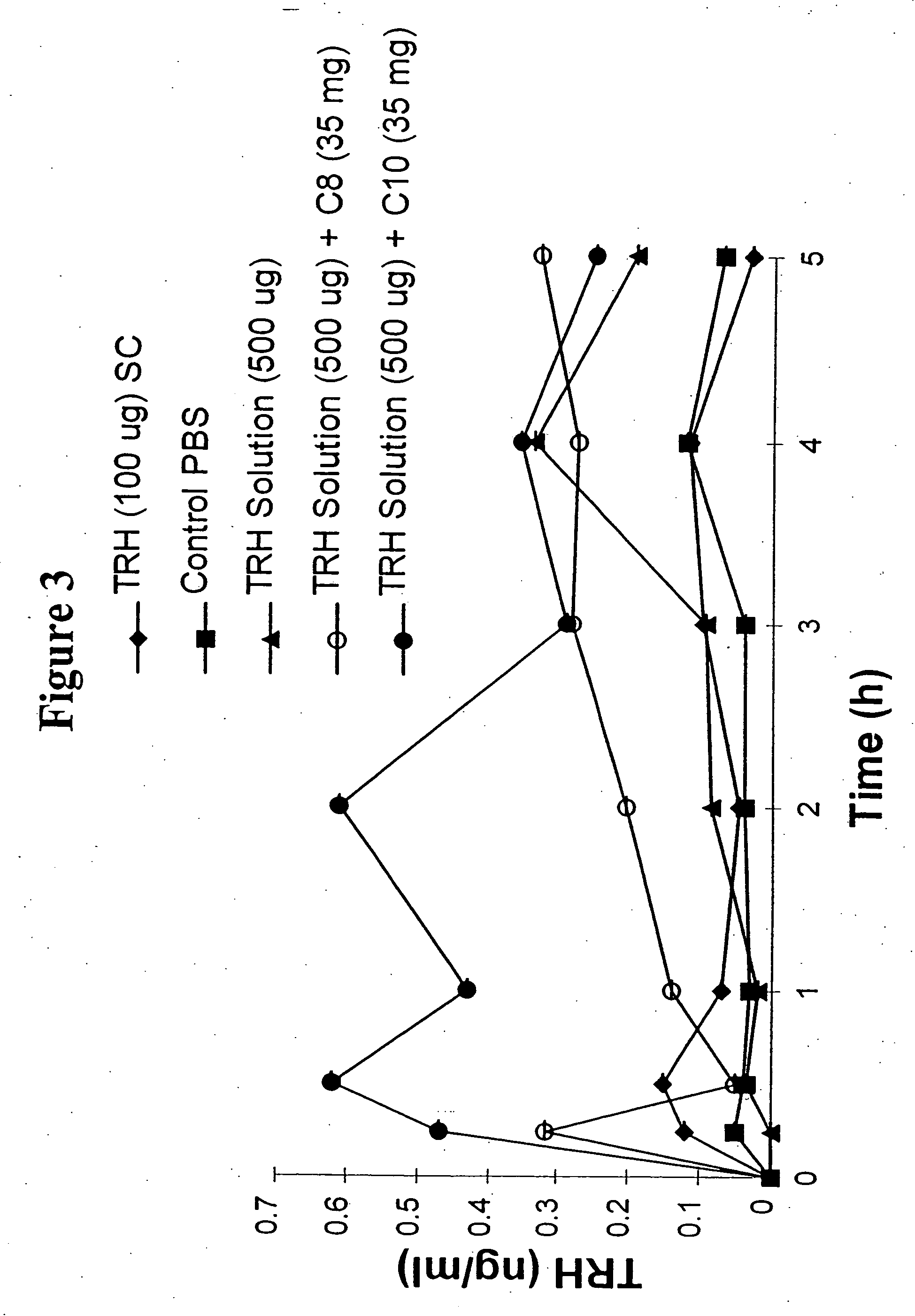
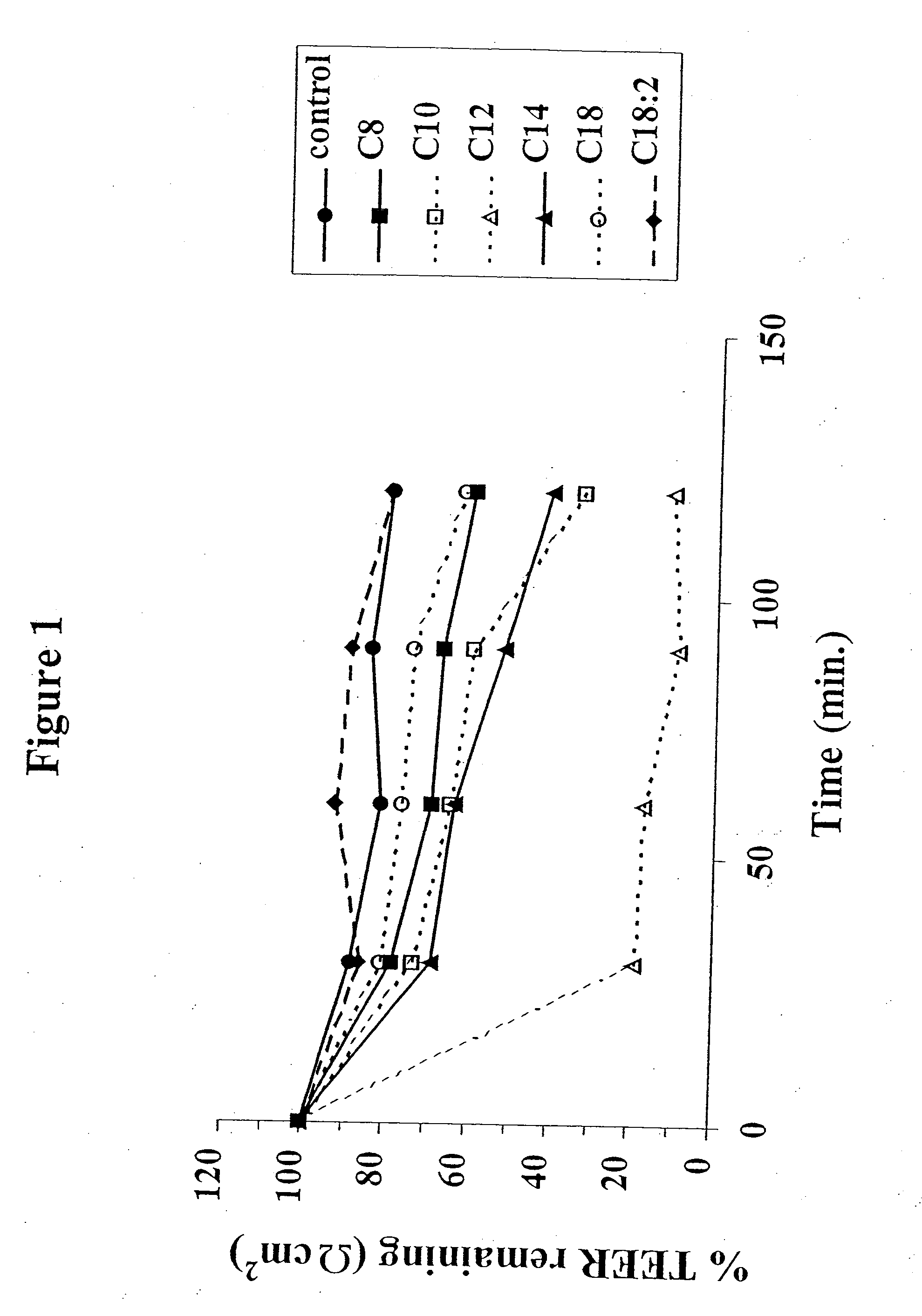
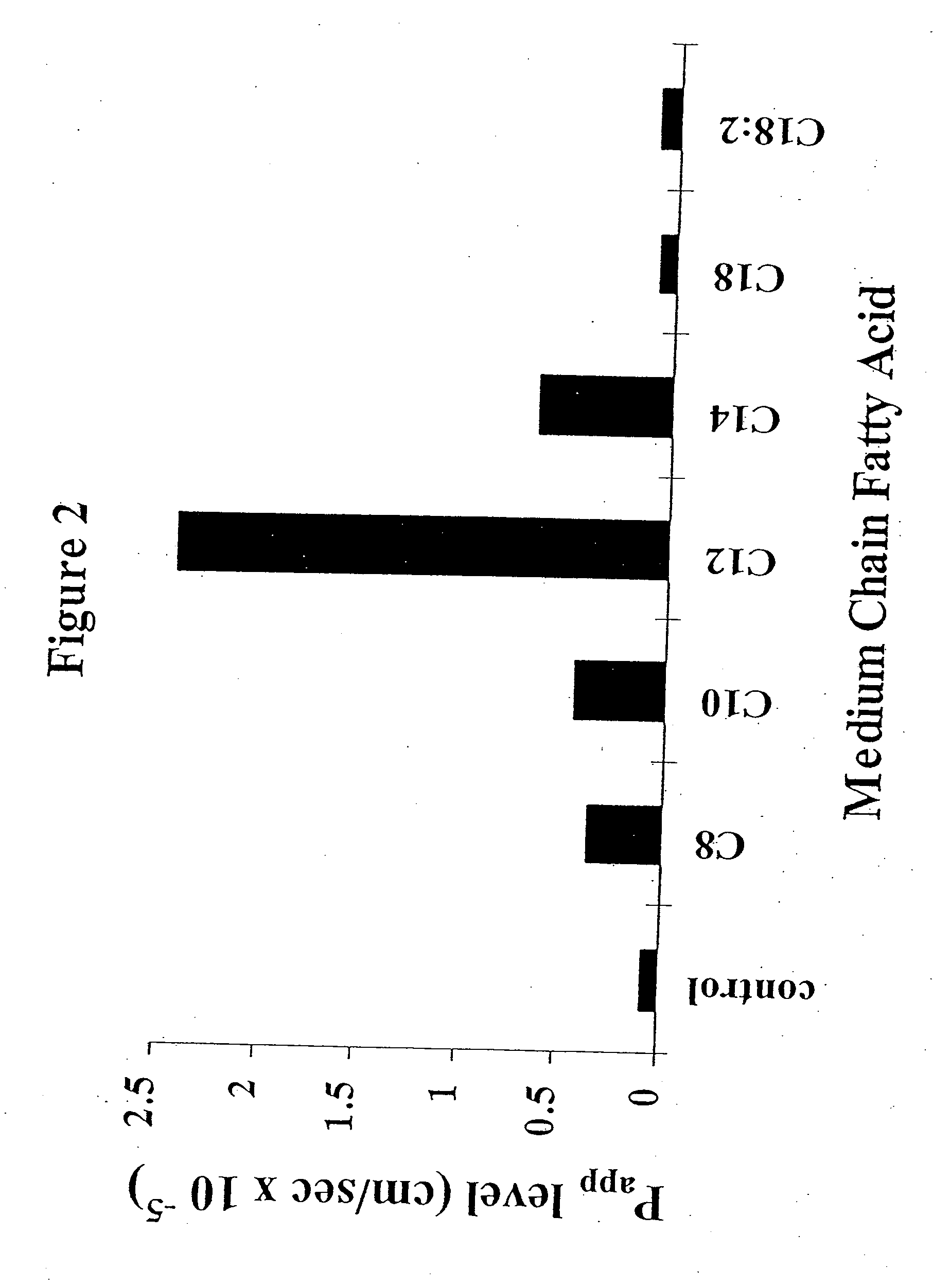
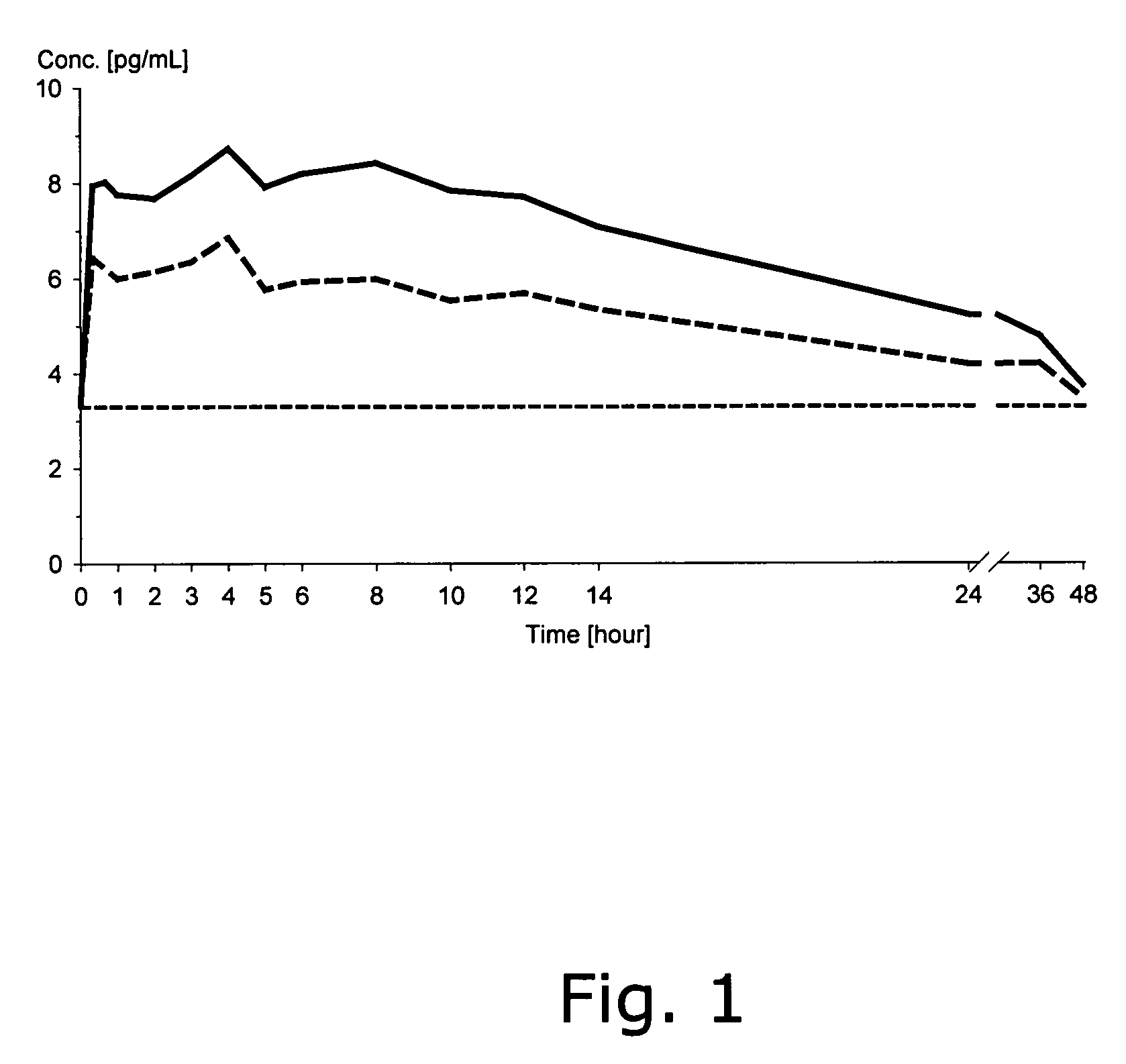
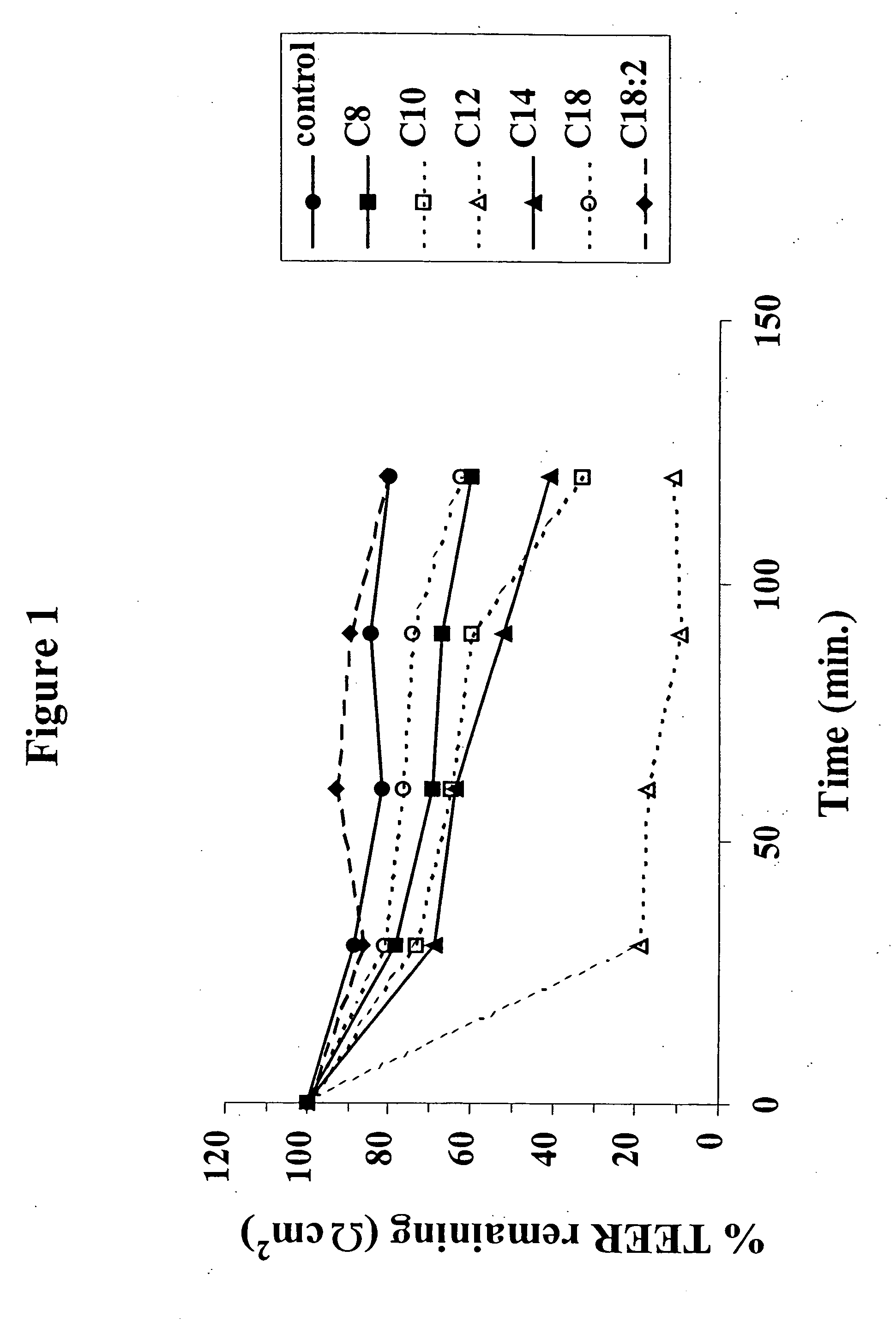
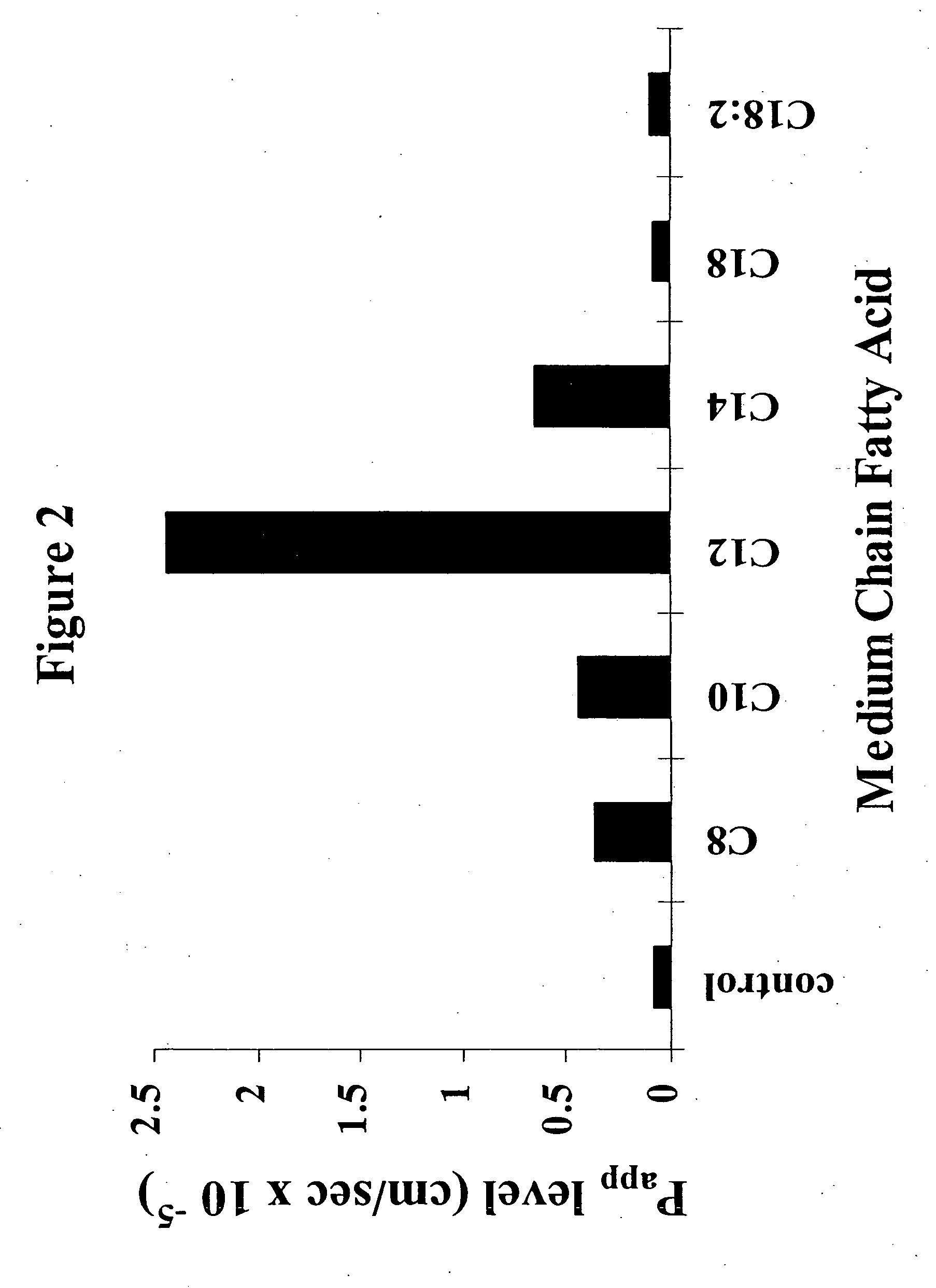
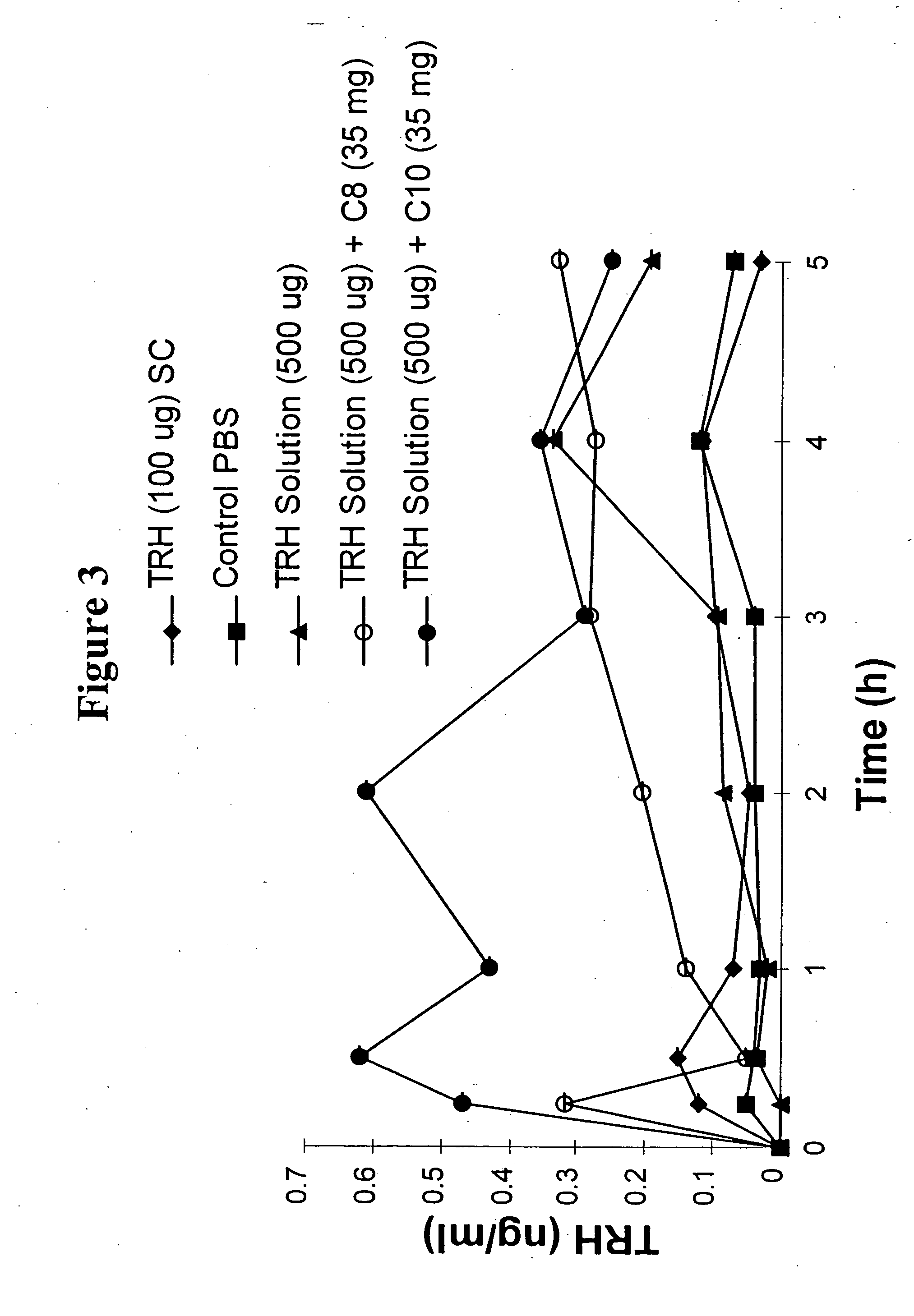
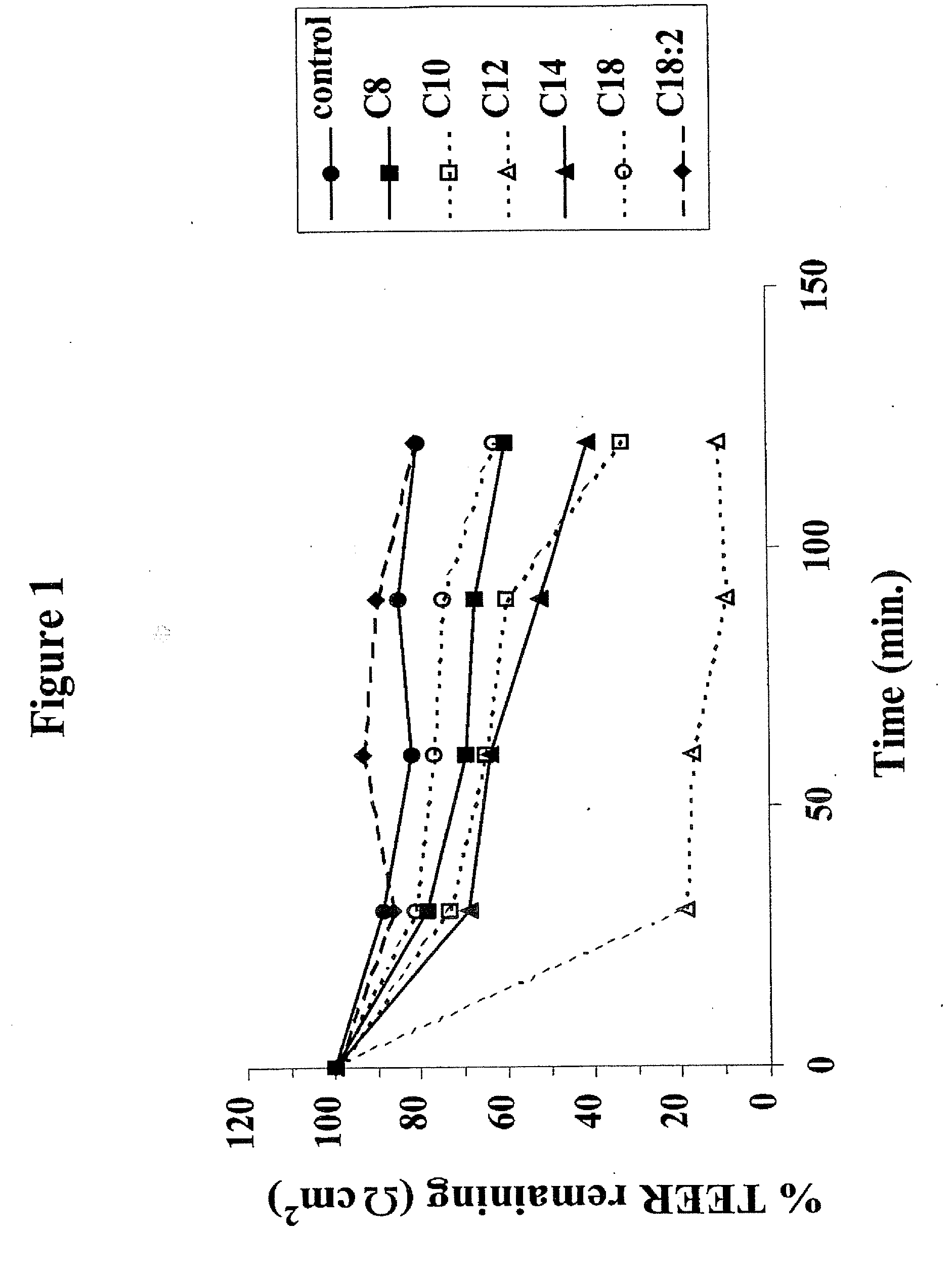
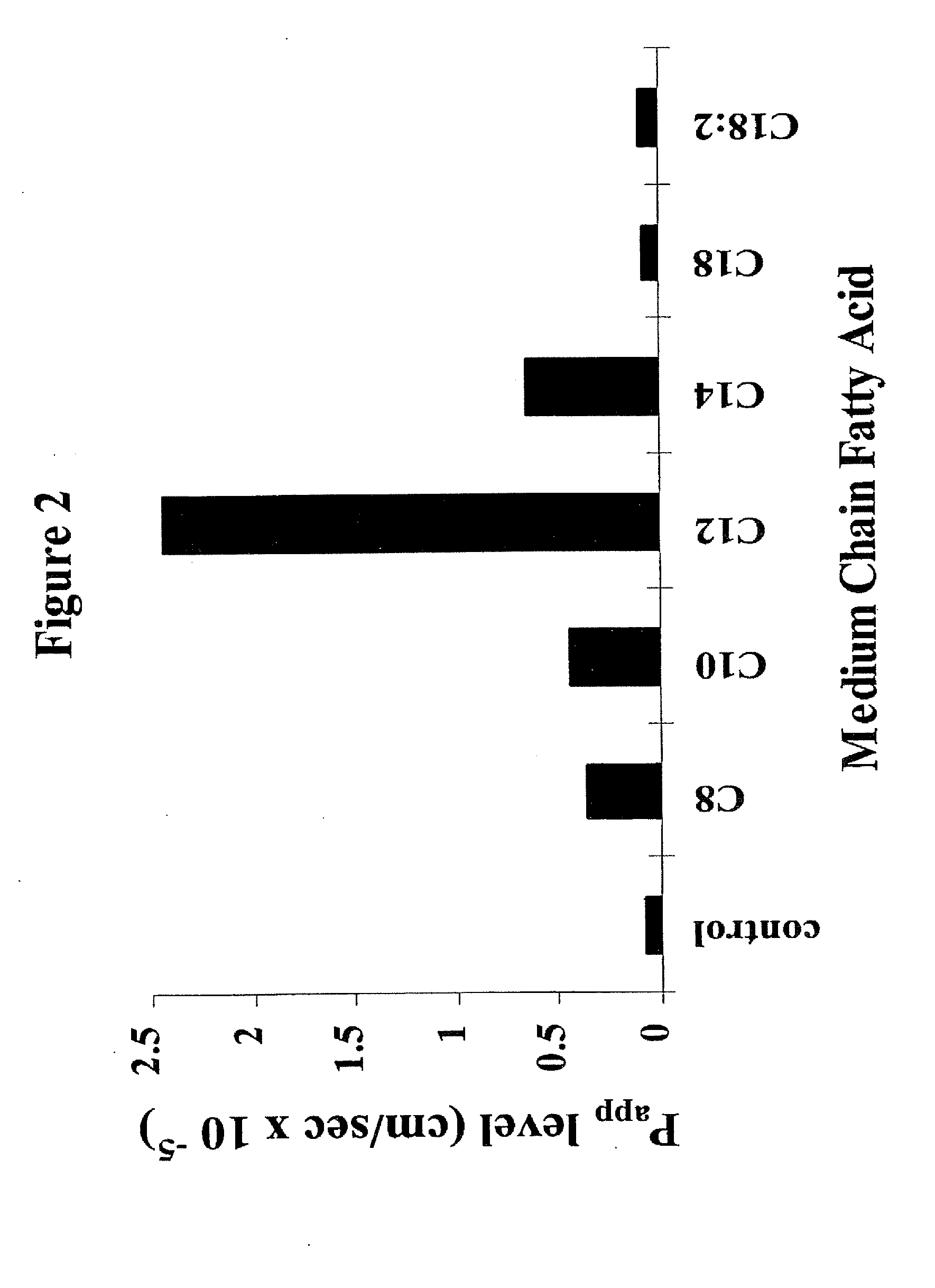
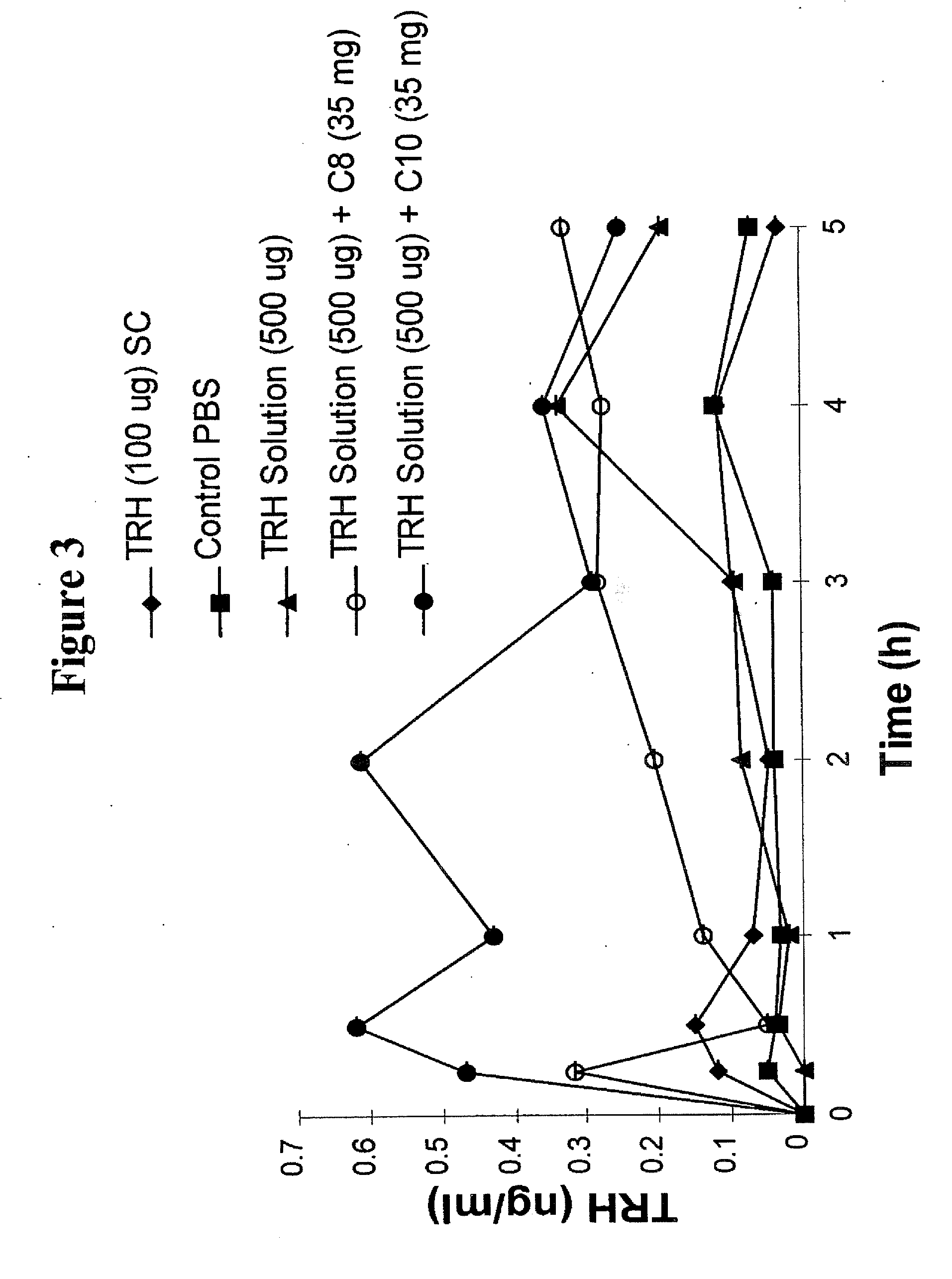
The invention relates to a pharmaceutical composition and oral dosage forms comprising a bisphosphonate in combination with an enhancer to promote absorption of the bisphosphonate at the GIT cell lining. The enhancer is a medium chain fatty acid or a medium chain fatty acid derivative having a carbon chain length of from 6 to 20 carbon atoms. Preferably, the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:NOVO NORDISK AS
Solid Oral Dosage Form Containing an Enhancer
InactiveUS20070238707A1Improve oral bioavailabilityMinimizes risk of local irritationBiocideAntipyreticDelayed Release Dosage FormDiphosphonates
The invention relates to a pharmaceutical composition and oral dosage forms comprising a bisphosphonate in combination with an enhancer to enhance intestinal delivery of the bisphosphonate to the underlying circulation. Preferably, the enhancer is a medium chain fatty acid or a medium chain fatty acid derivative having a carbon chain length of from 6 to 20 carbon atoms, and the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:NOVO NORDISK AS
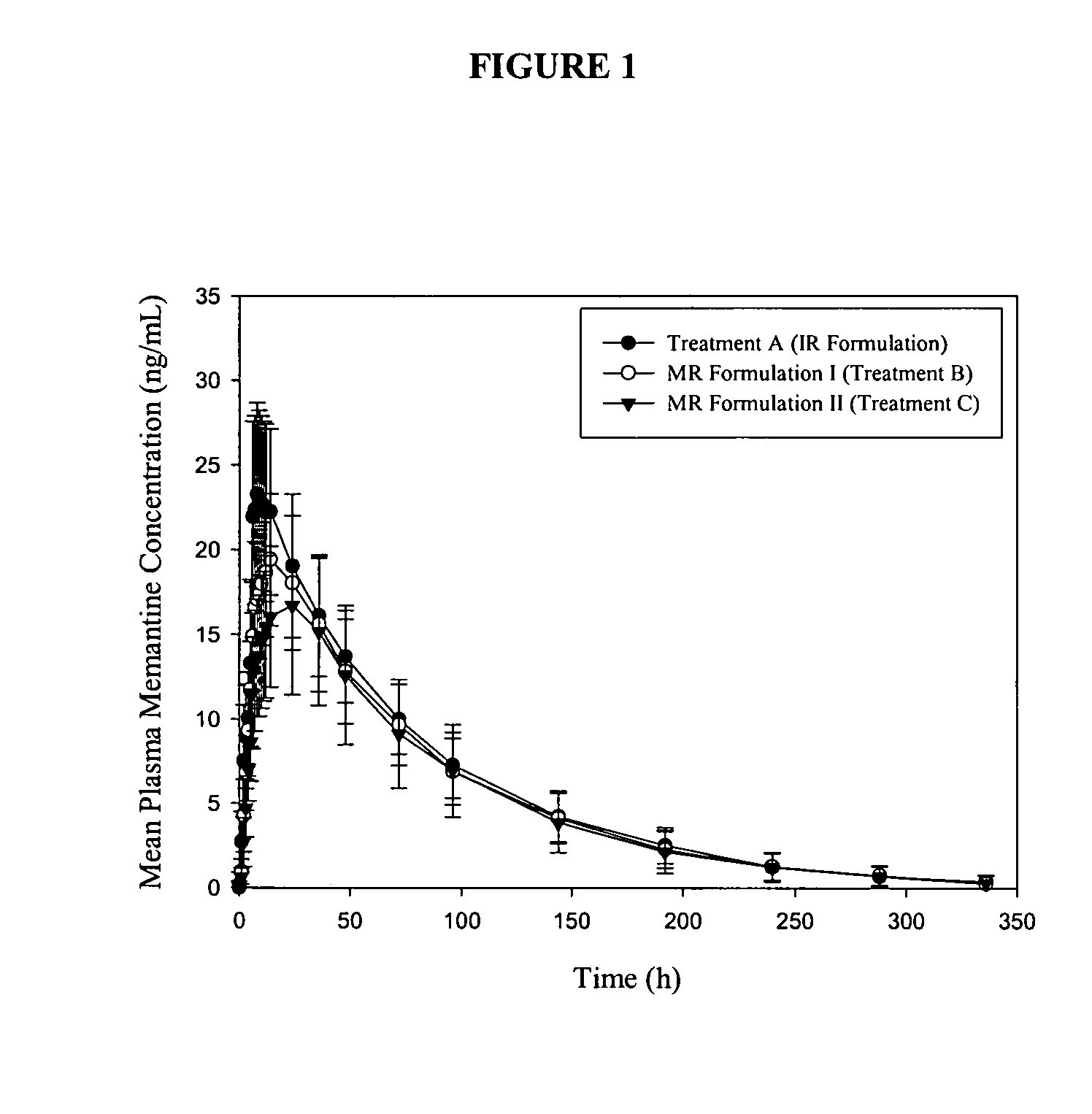
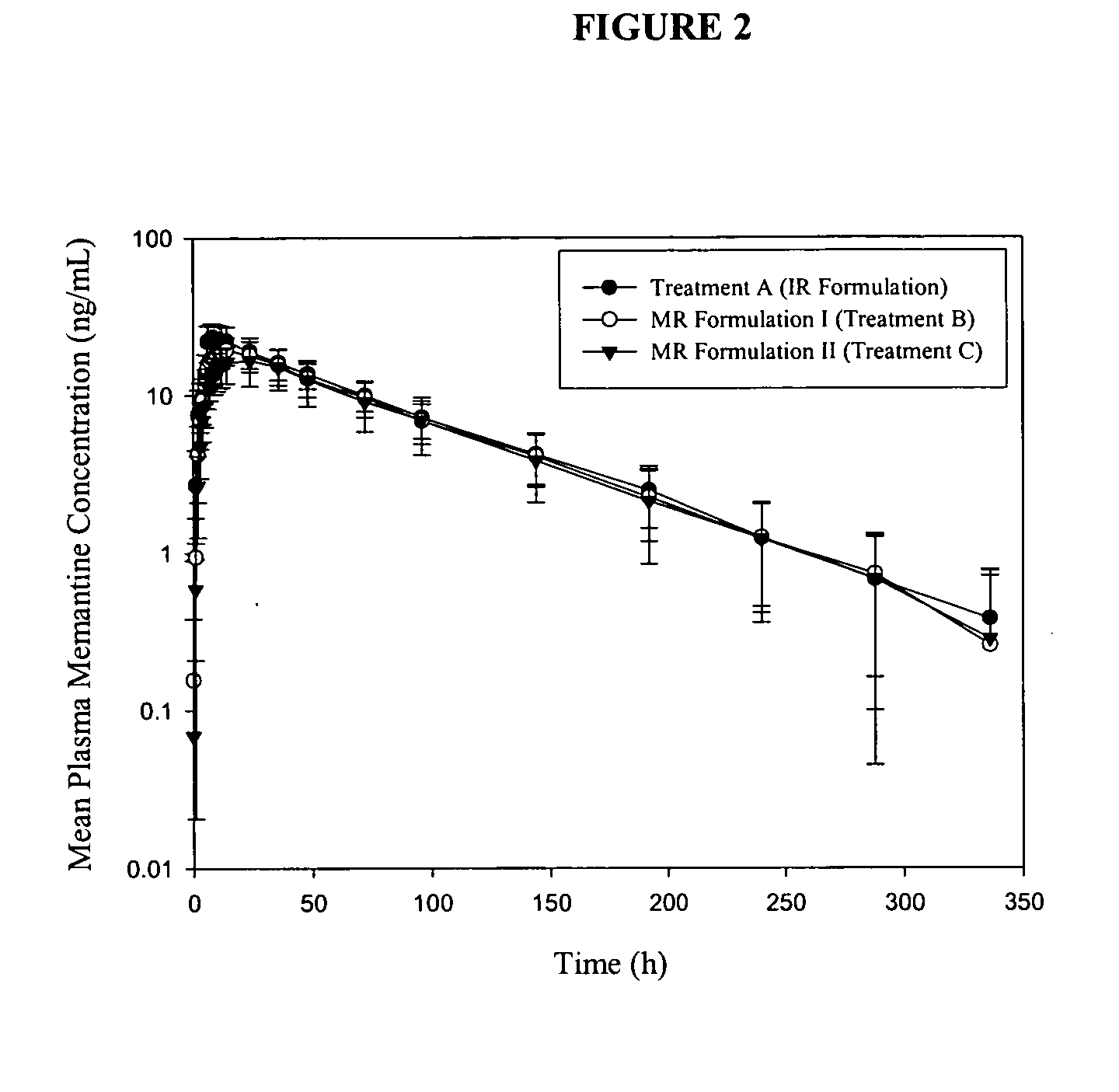
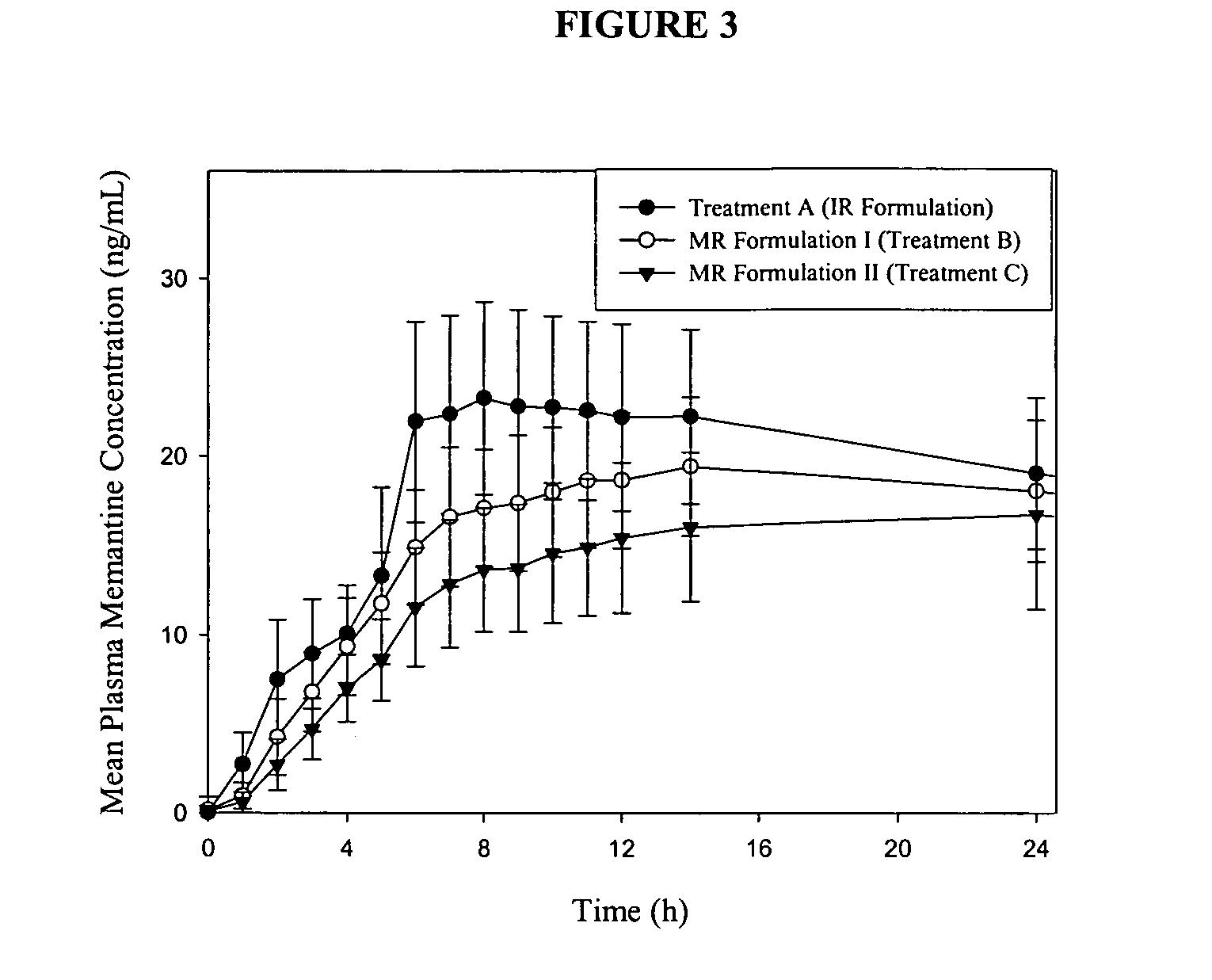
Immediate release formulations of 1-aminocyclohexane compounds, memantine and neramexane
InactiveUS20060002999A1Improve bioavailabilityAdvantageous stability profileBiocideSenses disorderImmediate releasePharmaceutical medicine
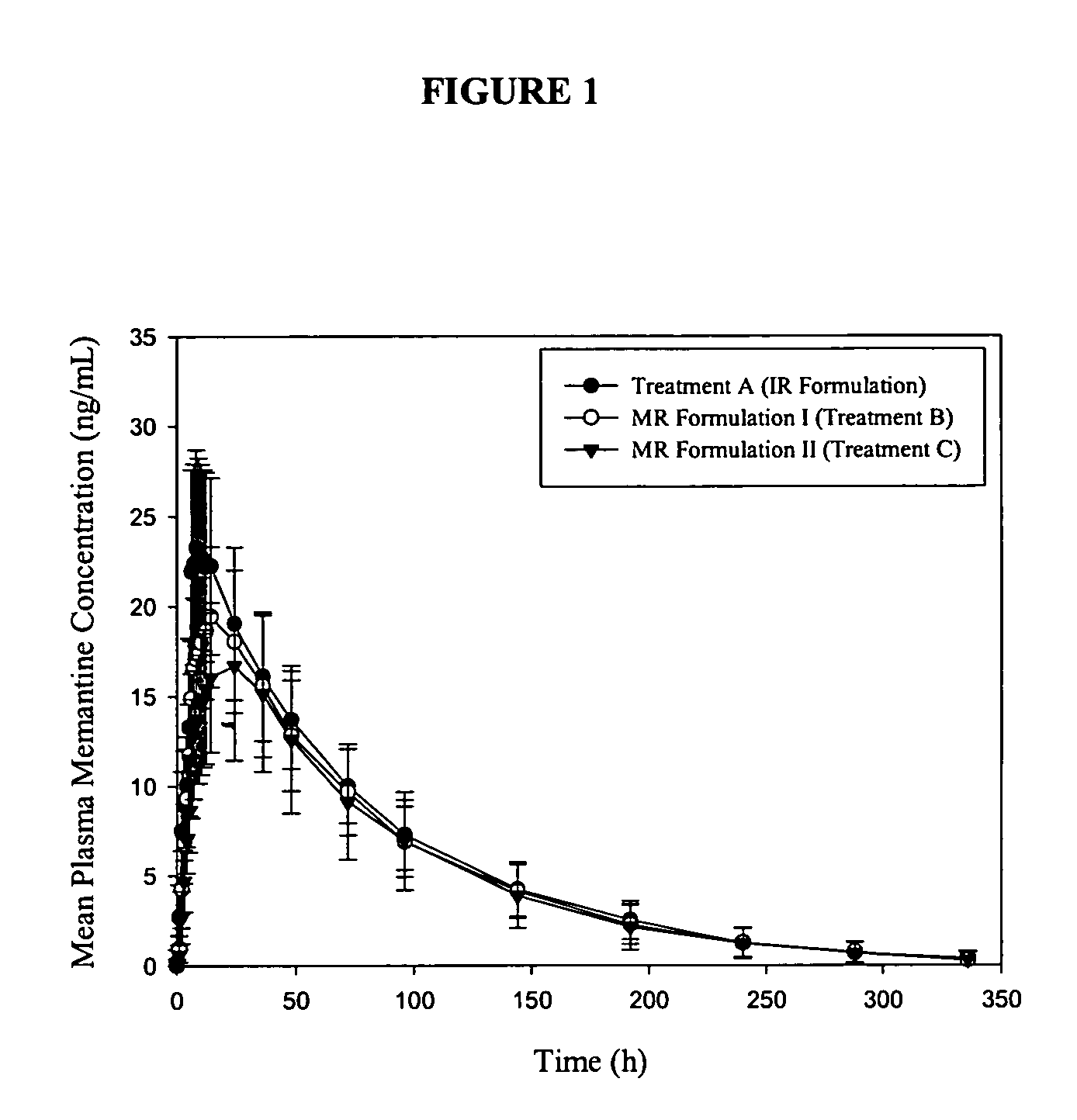
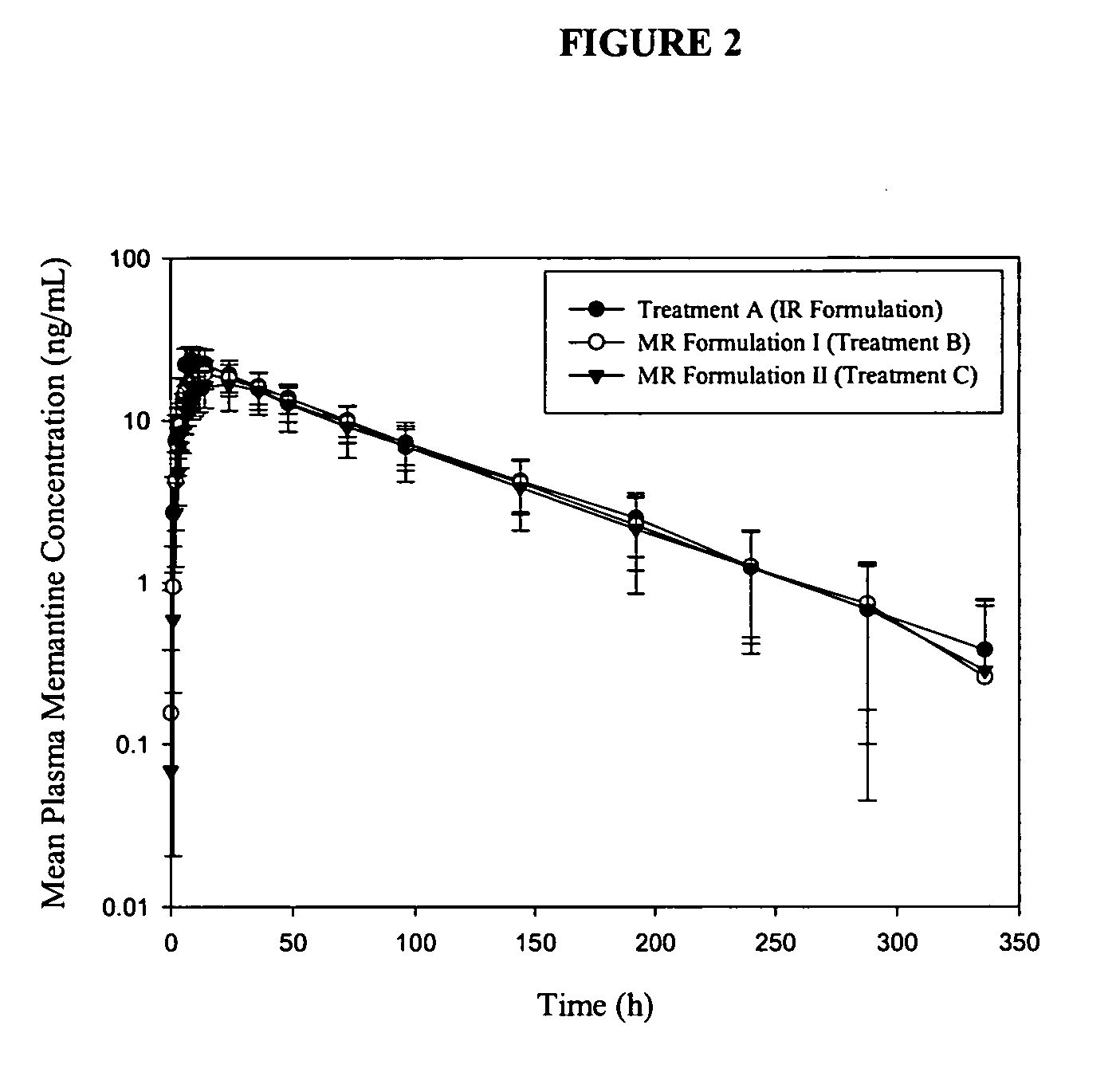
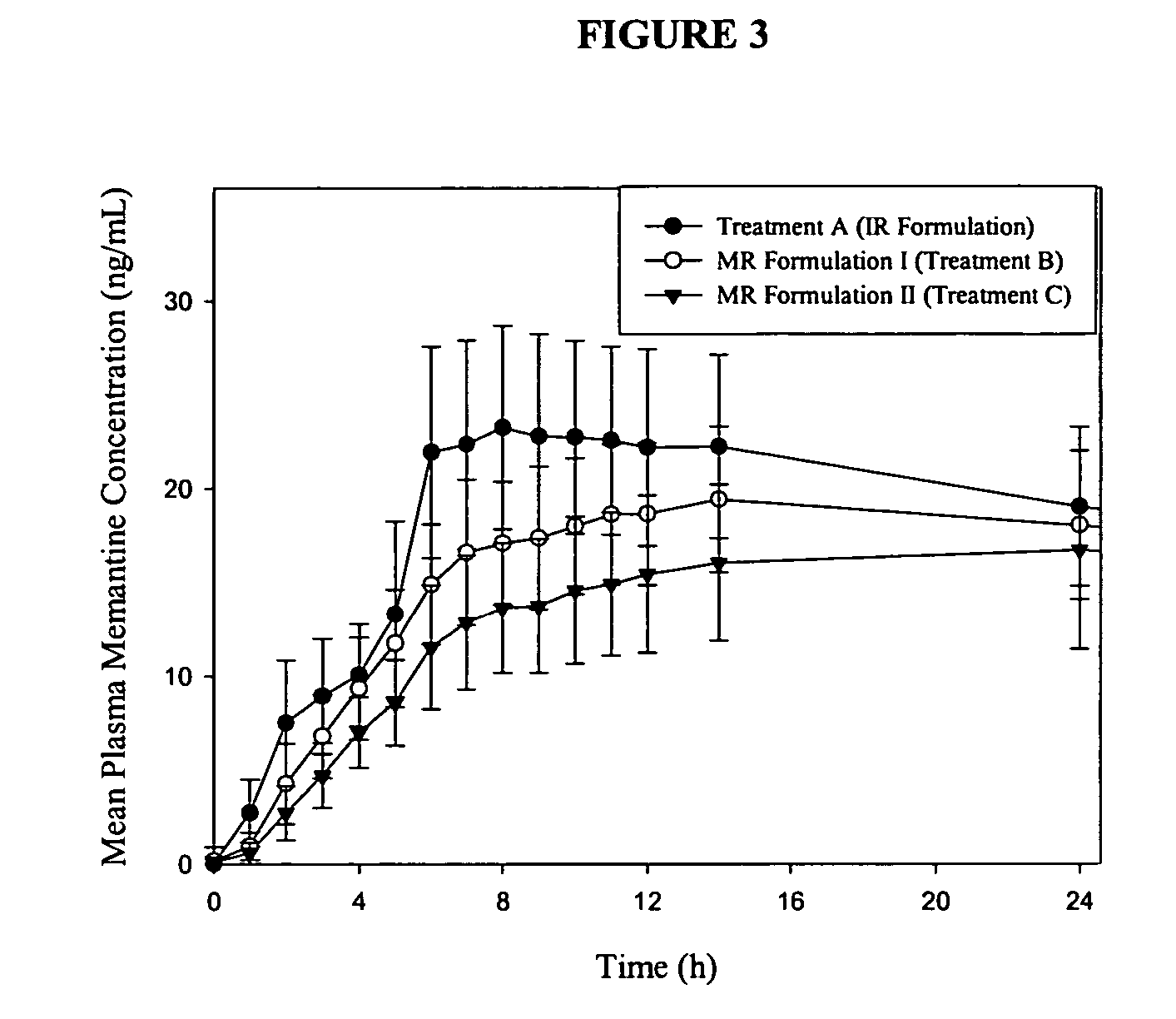
The present invention relates to an immediate release solid oral dosage form containing 1-aminocyclohexanes, preferably memantine or neramexane, and optionally a pharmaceutically acceptable coating, wherein the active ingredient exhibits dose proportionality and is released at a dissolution rate of more than about 80% within about the first 60 minutes following entry of said form into a use environment. The dosage form is direct compressed and has a hardness within the range of between about 3 and about 40 Kp, exhibits an average Tmax within the range of about 2 to about 8 hours with an active ingredient load within the range of about 2.5 to about 150 mg. The formulation allows for dose-proportional compositions for once daily or b.i.d. dosing, while maintaining a steady average range of Tmax.
Owner:FOREST LAB HLDG LTD
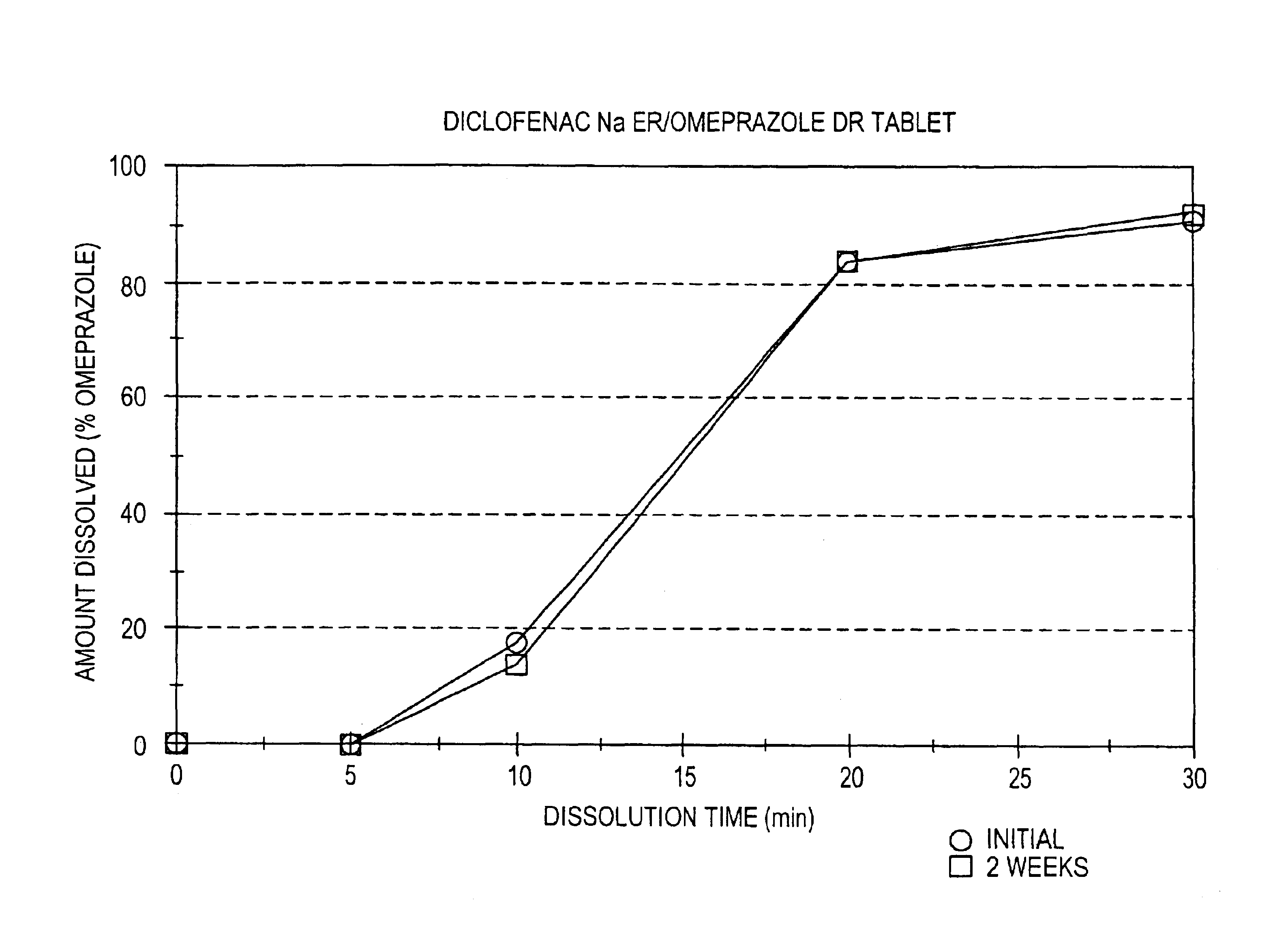
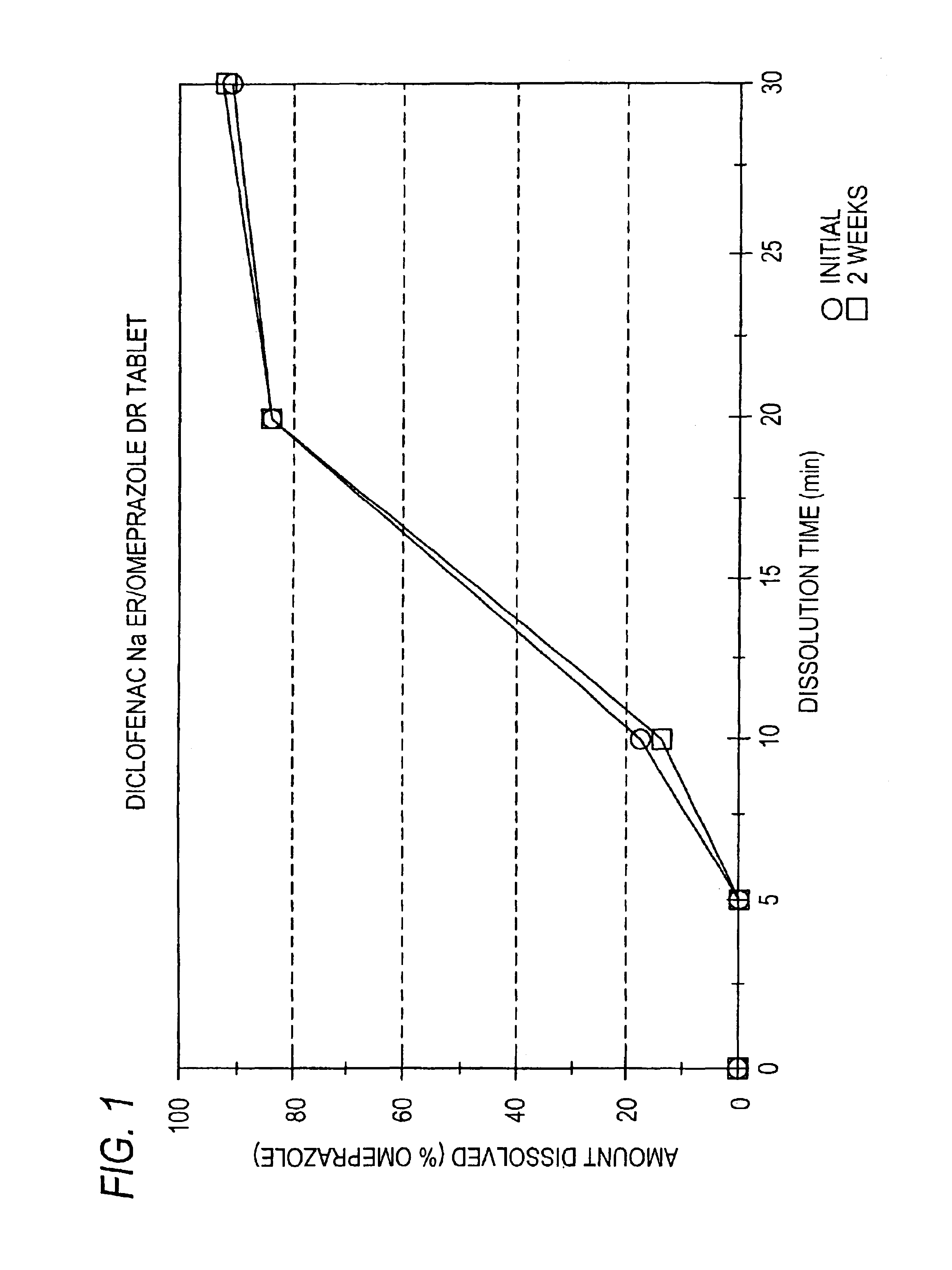
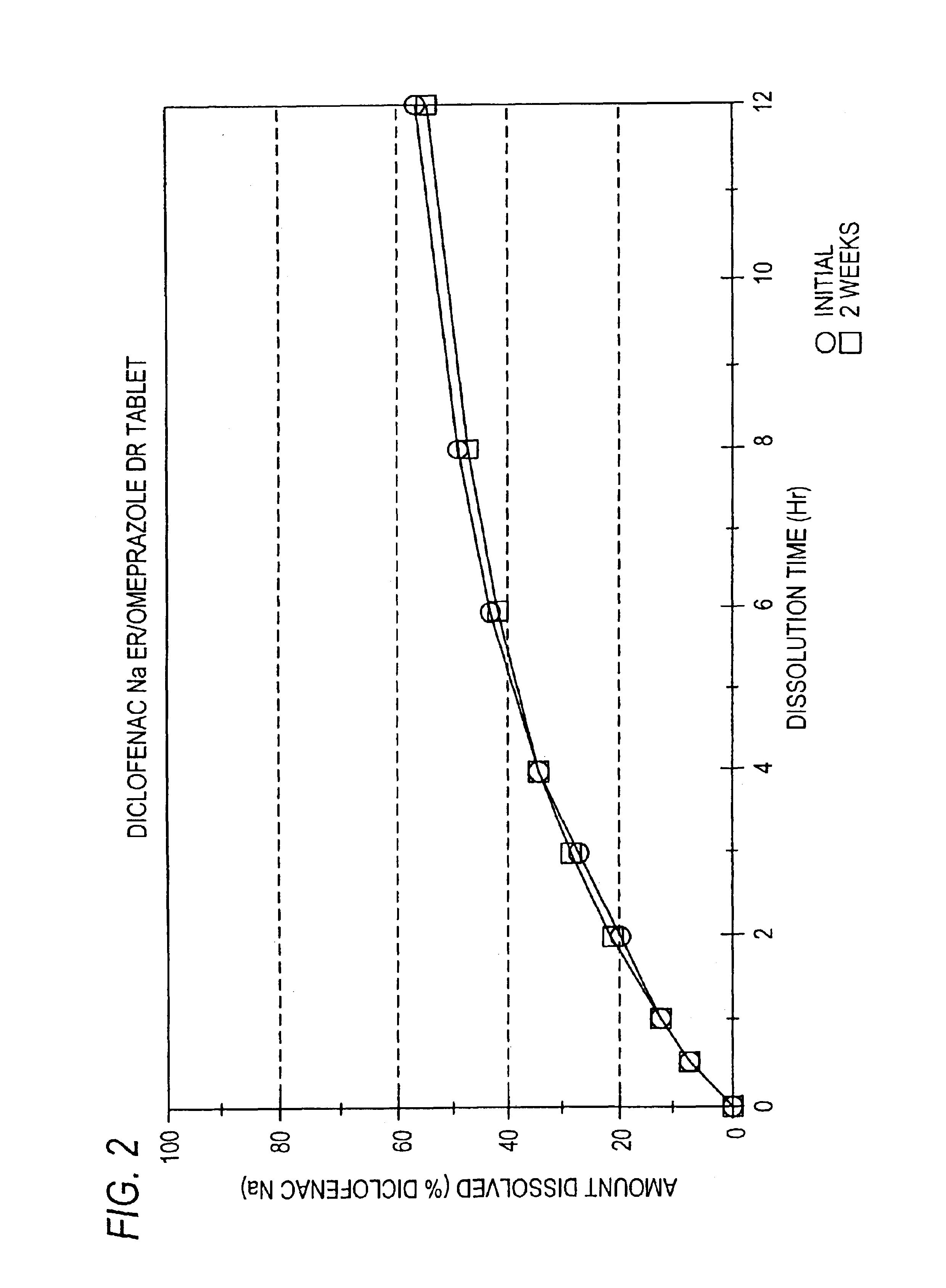
Pharmaceutical formulations containing a non-steroidal antiinflammatory drug and a proton pump inhibitor
InactiveUS6869615B2Decrease risk of development and exacerbationGood curative effectPowder deliveryAntipyreticSide effectDepressant
An oral solid dosage form includes a therapeutically effective amount of an NSAID and a proton pump inhibitor in an amount effective to inhibit or prevent gastrointestinal side effects normally associated with the NSAID. Also disclosed is a method of treating a human patient in need of antiinflammatory, analgesic and / or antipyretic therapy, comprising orally administering to the patient an oral pharmaceutical dosage form which includes a therapeutically effective amount of an NSAID and an amount of a proton pump inhibitor effective to substantially inhibit gastrointestinal side effects of the NSAID. The invention is further related to a method of prophylactically treating a human patient who is on a therapy known to have significant gastrointestinal side effects or is about to begin such a therapy, via concurrent administration of an NSAID and a proton pump inhibitor in a combination (single) oral dosage form.
Owner:ANDRX LABS
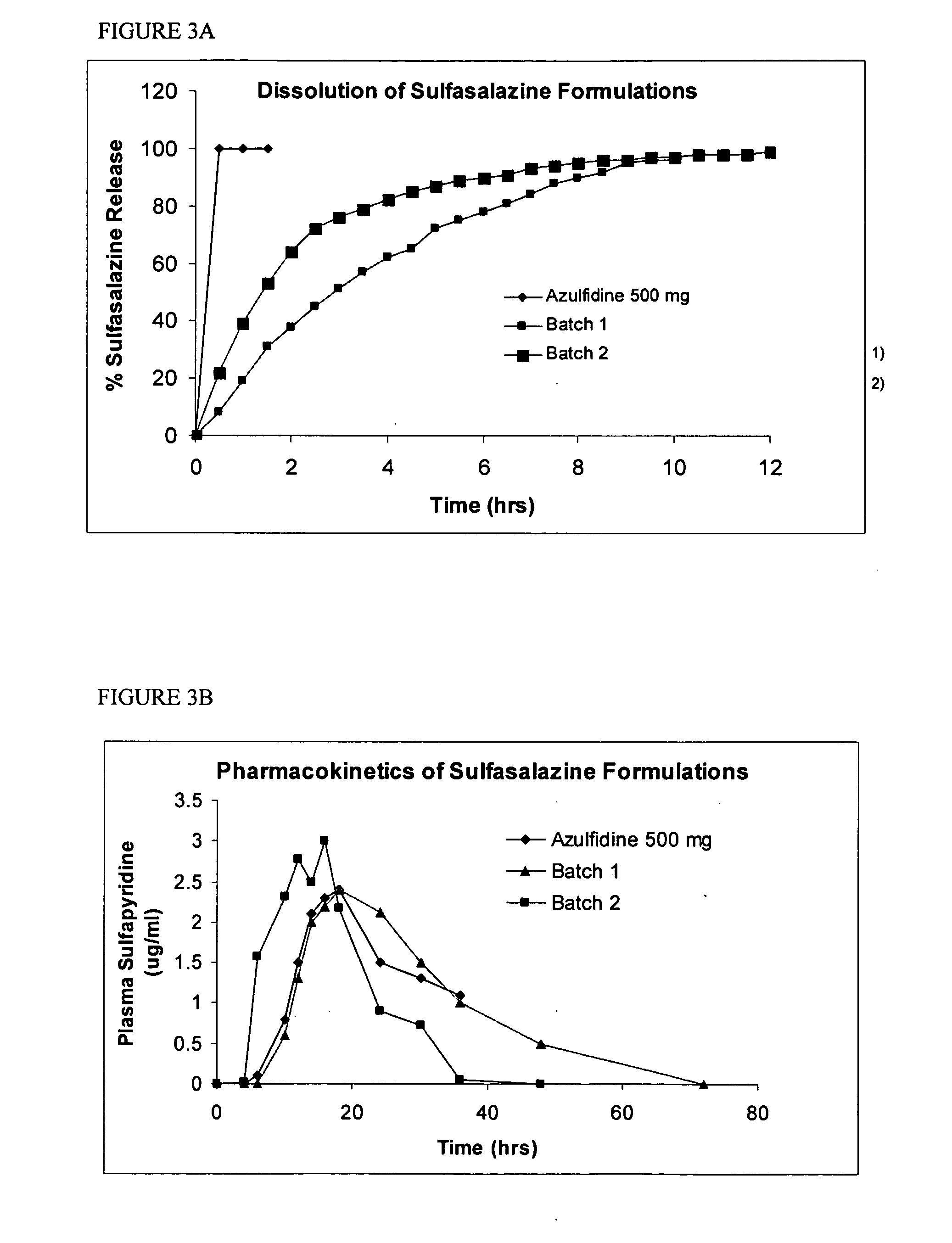
Process for the preparation of a granulate suitable to the preparation of rapidly disintegrable mouth-soluble tablets and compositions obtained thereby
InactiveUS6149938AGood water solubilityPill deliveryPharmaceutical non-active ingredientsParticle compositionSoluble Tablet
A process for making a granulate composition suitable to the preparation of an oral solid form that can disintegrate rapidly inside the buccal cavity is provided as well as the granulate compositions and obtained.
Owner:ALPEX PHARMA SA
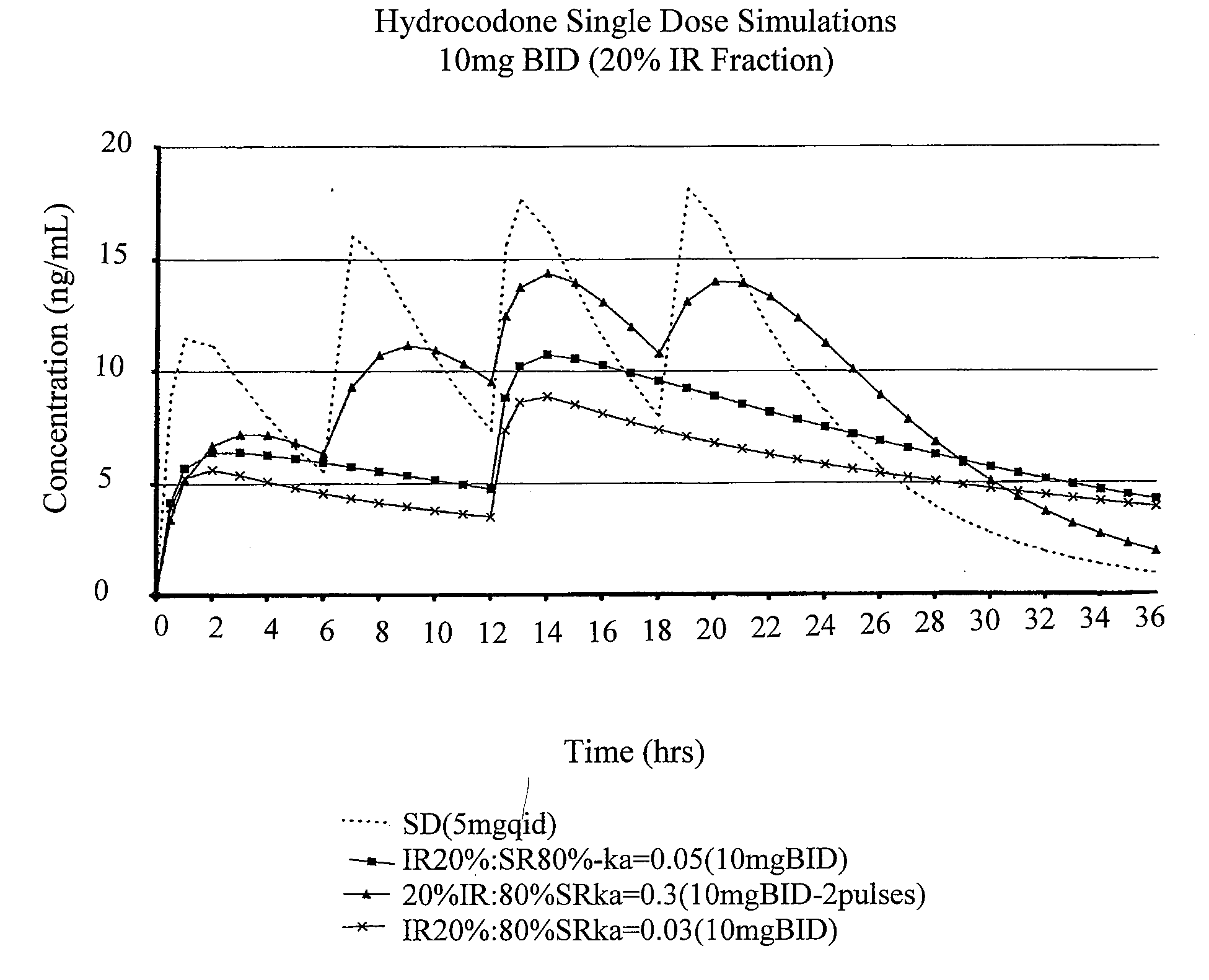
Compositions comprising nanoparticulate meloxicam and controlled release hydrocodone
InactiveUS20080102121A1Increasing patient convenienceImprove complianceBiocidePowder deliveryMeloxicamControl release
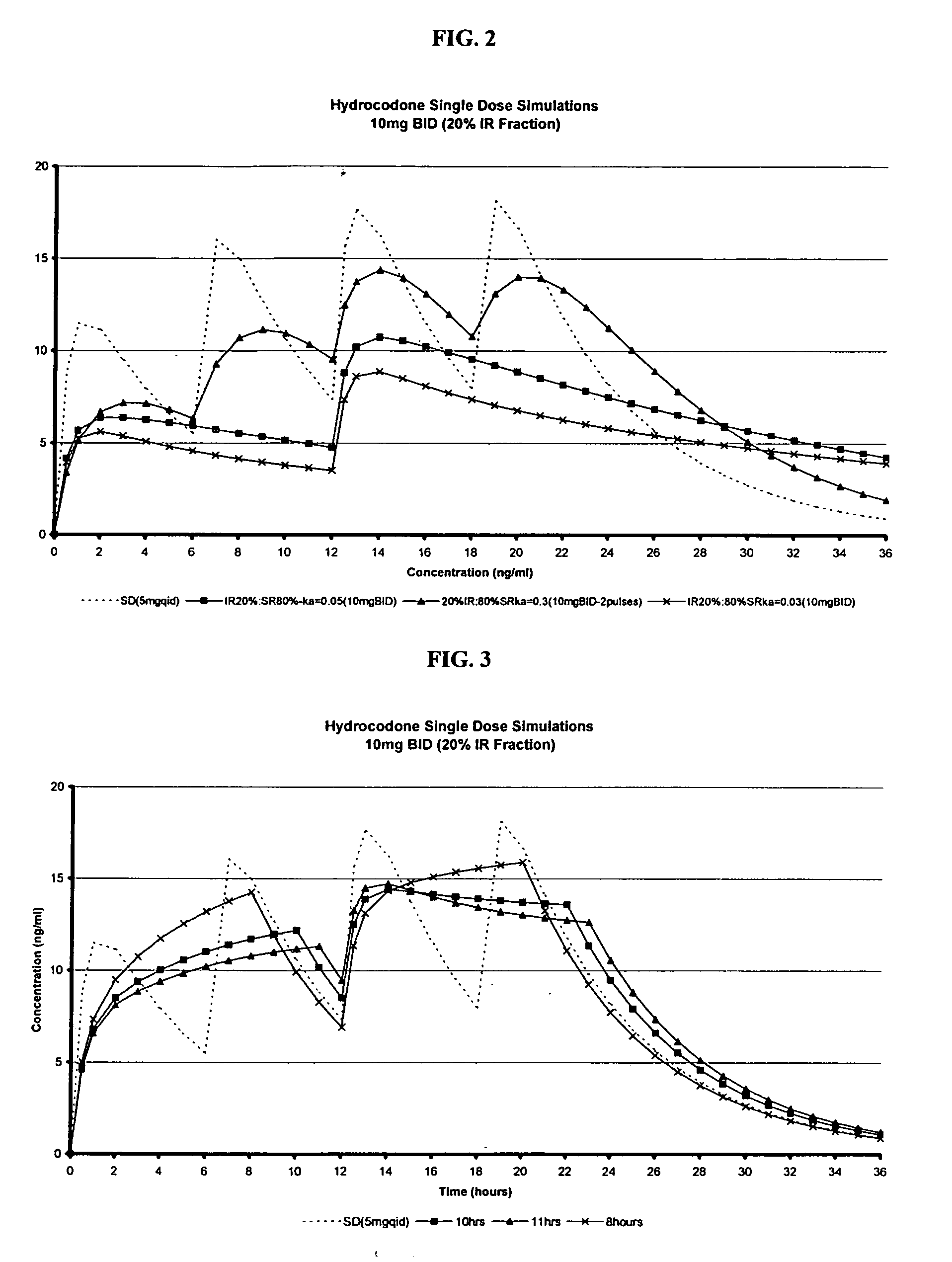
The invention relates to a compositions comprising a nanoparticulate meloxicam composition in combination with a multiparticulate modified release hydrocodone composition that, upon administration to a patient, delivers a hydrocodone in a bimodal or multimodal manner. The multiparticulate modified release composition comprises a first component and at least one subsequent component; the first component comprising a first population of hydrocodone-comprising particles and the at least one subsequent component comprising a second population of hydrocodone-comprising particles, wherein the combination of the components exhibit a bimodal or multimodal release profile. The invention also relates to a solid oral dosage form comprising such a combination composition.
Owner:ELAN PHRMA INT LTD
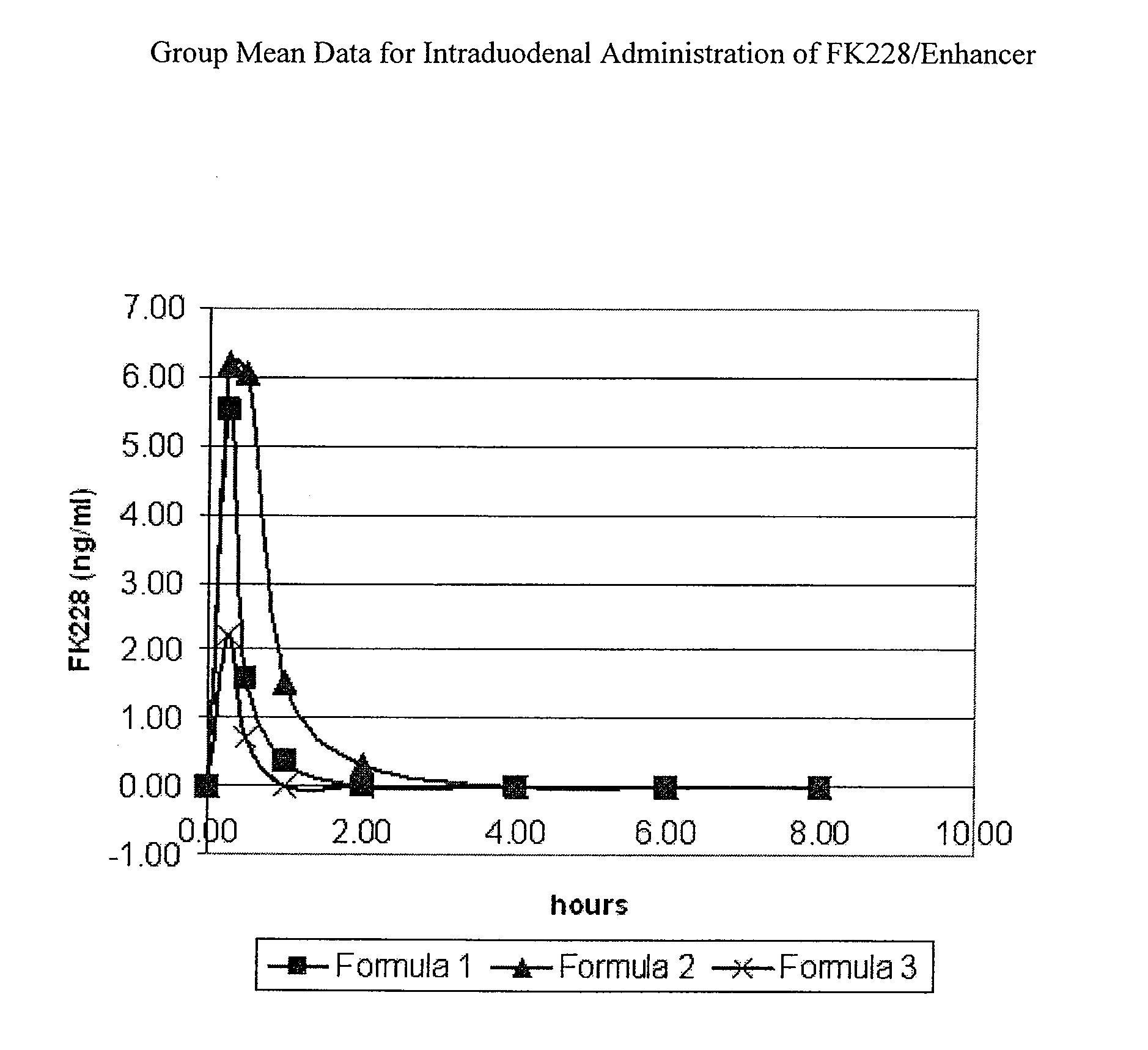
Solid oral dosage form containing an enhancer
InactiveUS20070148228A1BiocideCyclic peptide ingredientsDelayed Release Dosage FormPharmaceutical drug
The invention relates to a pharmaceutical composition and oral dosage forms comprising an HDAC inhibitor in combination with an enhancer to promote absorption of the HDAC inhibitor at the GIT cell lining. The enhancer is a medium chain fatty acid or a medium chain fatty acid derivative having a carbon chain length of from 6 to 20 carbon atoms. Preferably, the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:MERRION RES I
Simethicone solid oral dosage form
The present invention provides a composition for forming a compressed solid dosage form that is a free-flowing compressible admixture of simethicone, an adsorbant, and an optional active agent, wherein the weight ratio of simethicone to adsorbent is at least 1:2.22. Also included are solid dosage forms made from a free-flowing compressible admixture of simethicone, an adsorbant, and an optional active agent, wherein the weight ratio of simethicone to adsorbent is at least 1:2.22.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
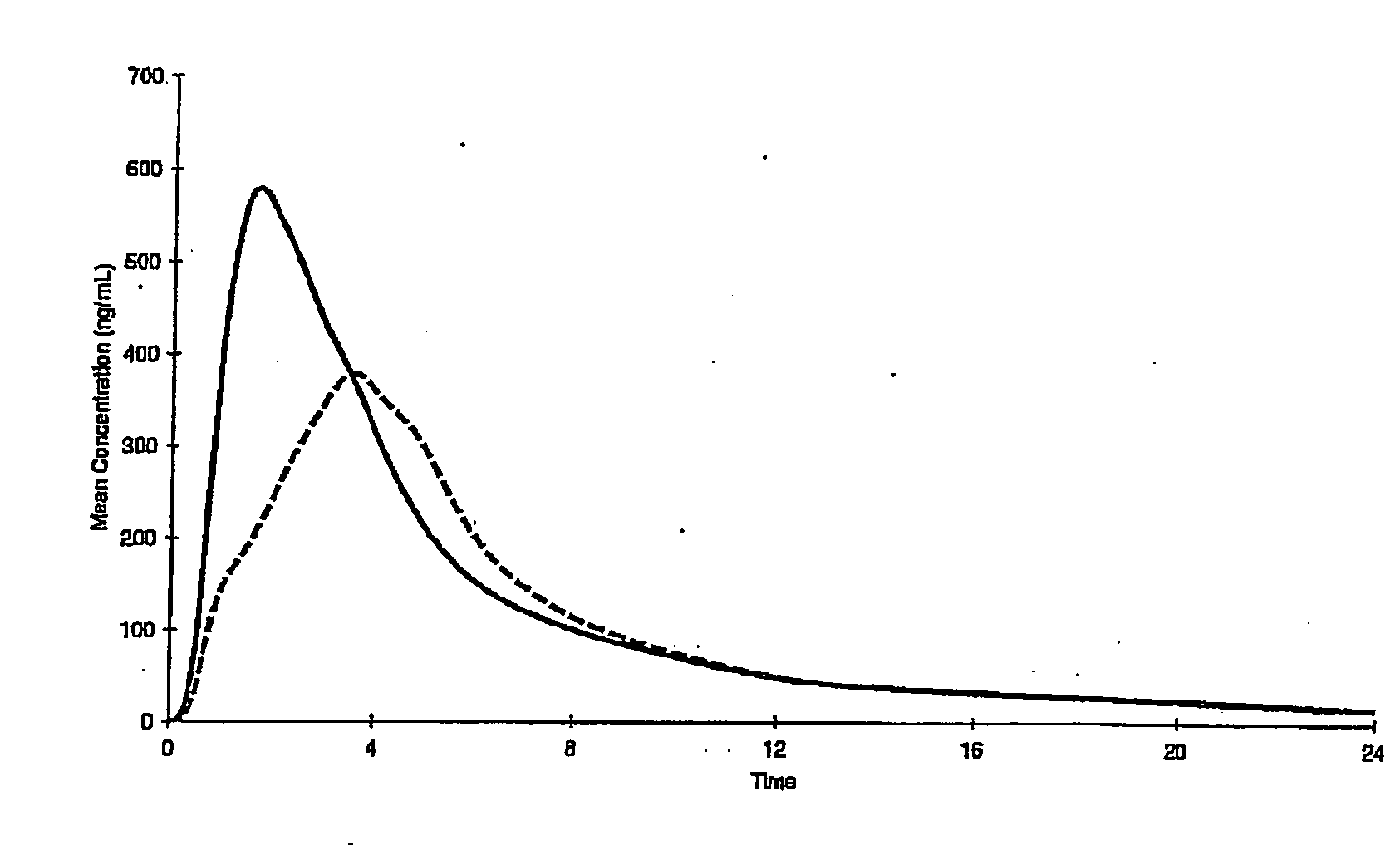
Controlled release metformin compositions
InactiveUS6866866B1Effective controlImprove bioavailabilityOrganic active ingredientsCoatingsCo administrationBlood plasma
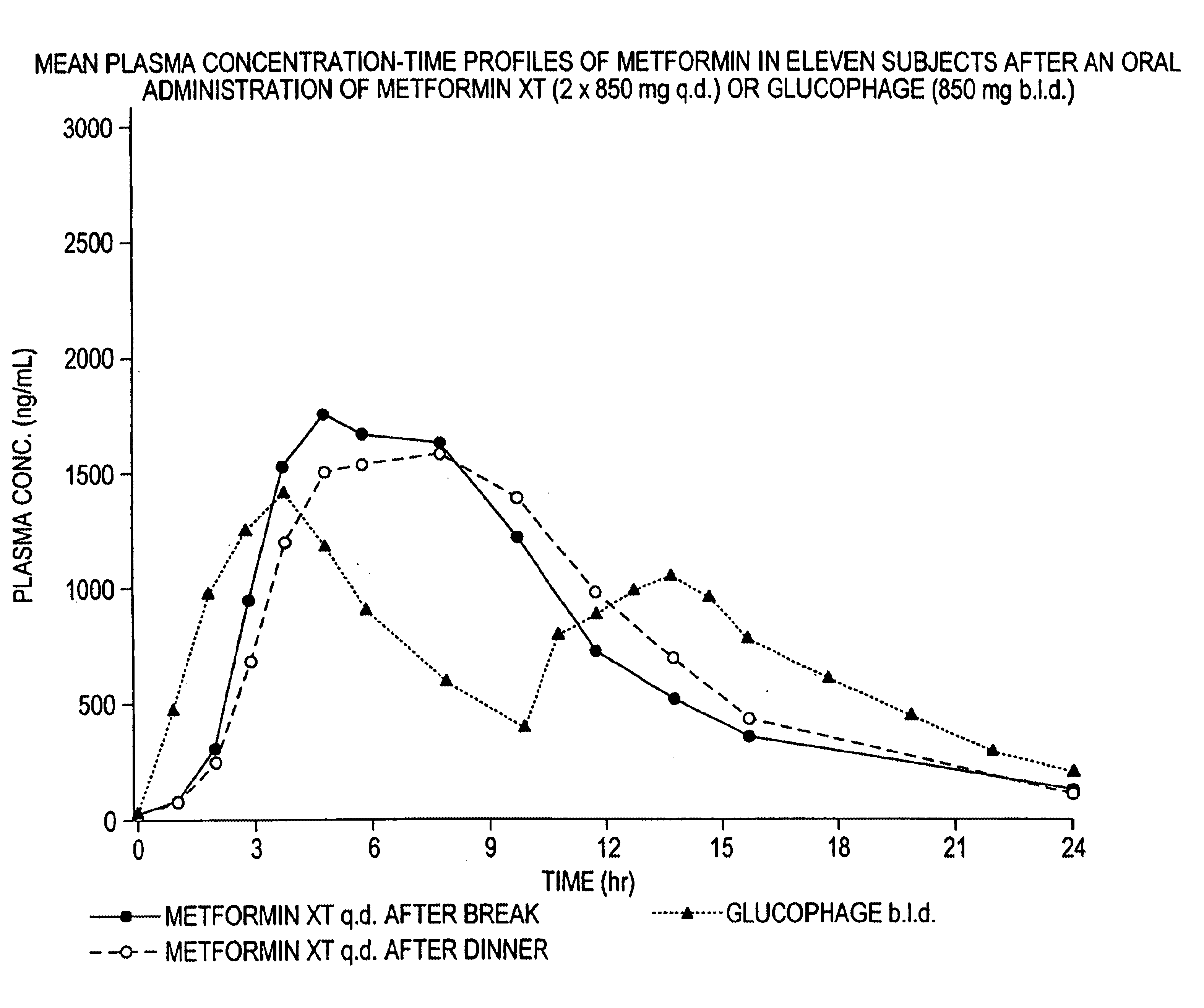
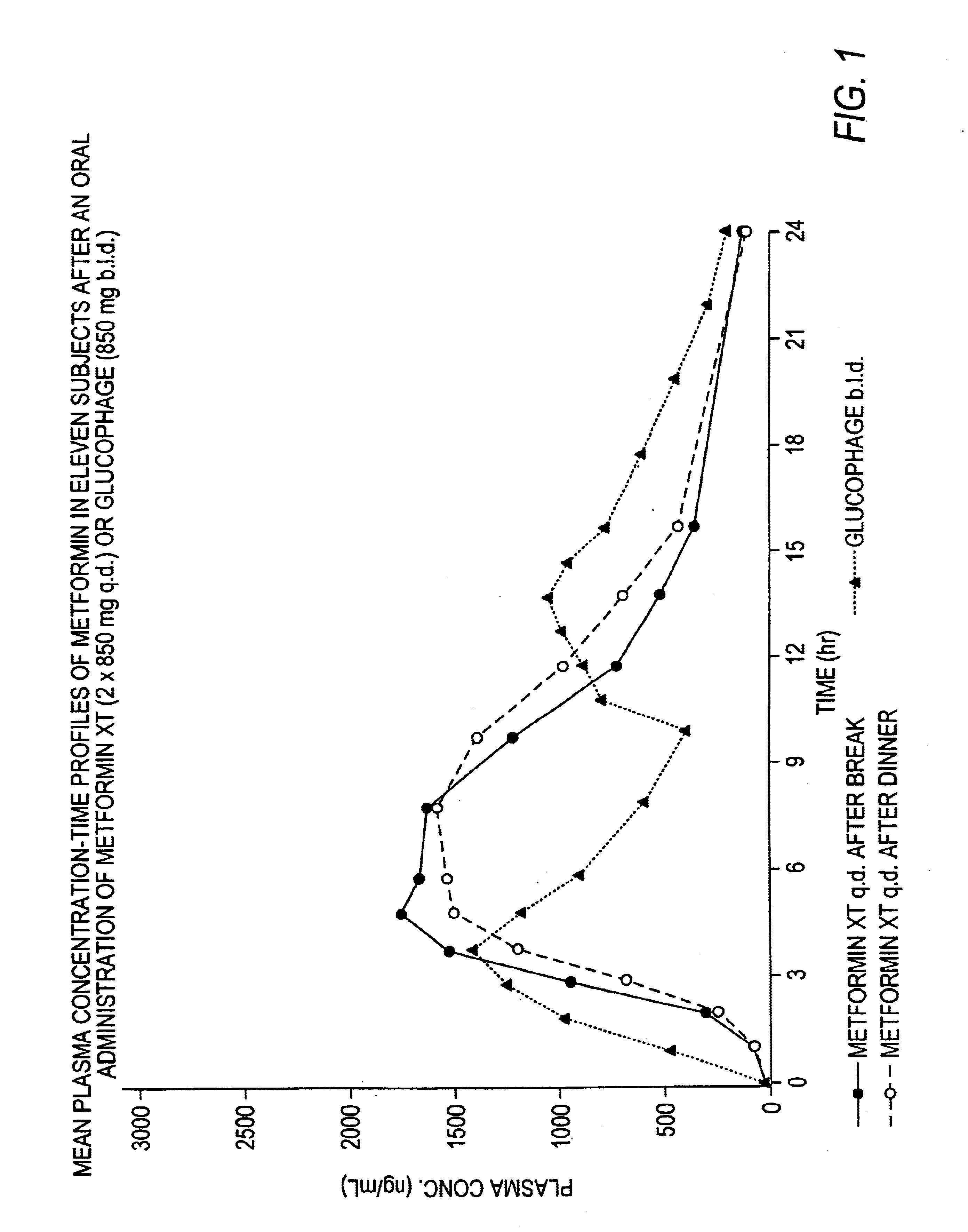
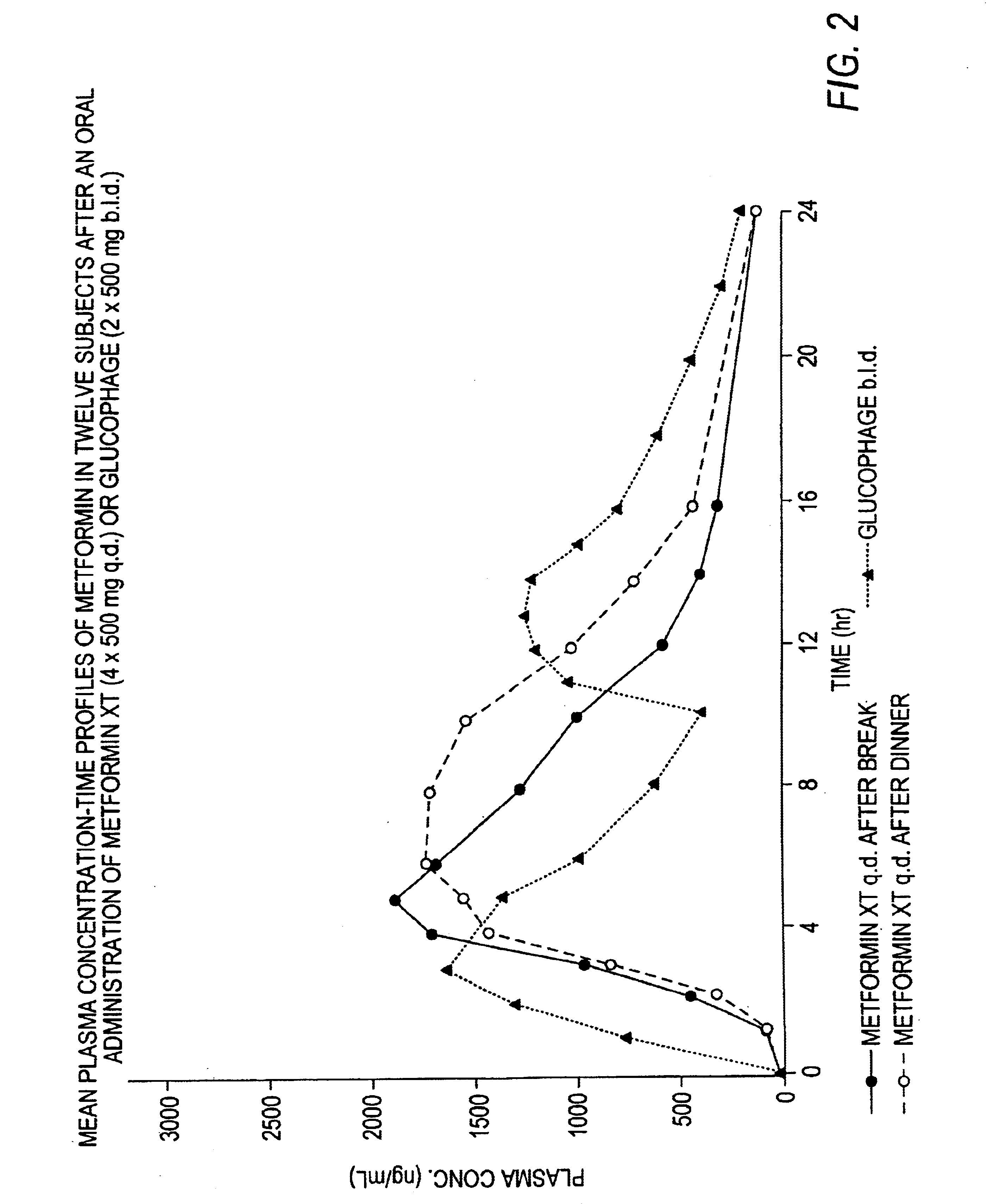
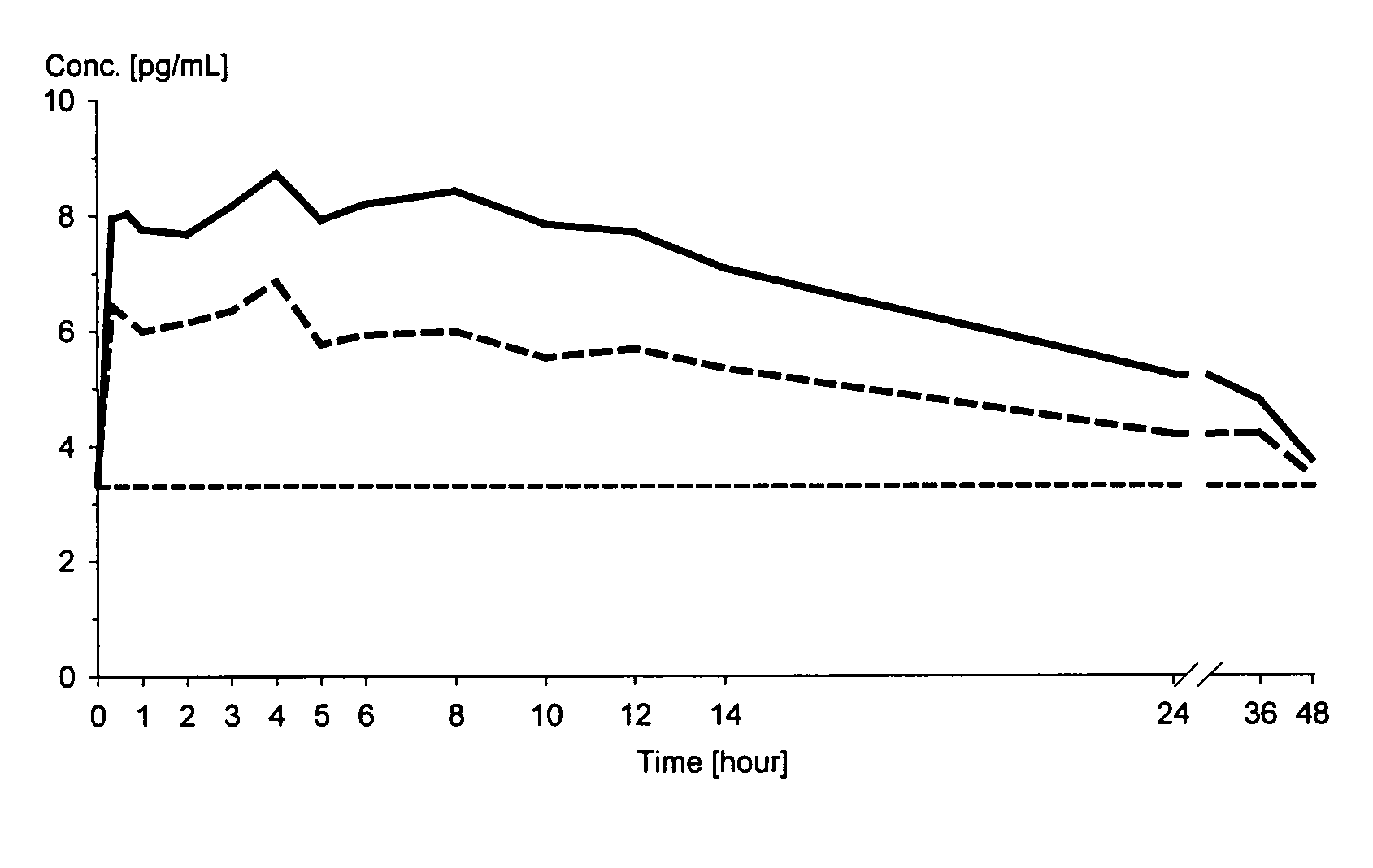
A composition for treating patients having non-insulin-dependent diabetes mellitus (NIDDM) by administering a controlled release oral solid dosage form containing preferably a biguanide drug such as metformin, on a once-a-day basis. The dosage form provides a mean time to maximum plasma concentration (Tmax) of the drug which occurs at 5.5 to 7.5 hours after oral administration on a once-a-day basis to human patients. Preferably, the dose of drug is administered at dinnertime to a patient in the fed state.
Owner:ANDRX LABS
Compositions comprising nanoparticulate naproxen and controlled release hydrocodone
InactiveUS20080113025A1Reduces and eliminates developmentImprove complianceBiocidePowder deliveryControl releaseHydrocodone
The invention relates to a compositions comprising a nanoparticulate naproxen composition in combination with a multiparticulate modified release hydrocodone composition that, upon administration to a patient, delivers a hydrocodone in a bimodal or multimodal manner. The multiparticulate modified release composition comprises a first component and at least one subsequent component; the first component comprising a first population of hydrocodone-comprising particles and the at least one subsequent component comprising a second population of hydrocodone-comprising particles, wherein the combination of the components exhibit a bimodal or multimodal release profile. The invention also relates to a solid oral dosage form comprising such a combination composition.
Owner:ELAN PHRMA INT LTD
Solid Oral Dosage Form Containing an Enhancer
The invention relates to a pharmaceutical composition, particularly oral dosage forms, comprising a DAC inhibitor in combination with an enhancer to promote absorption of the DAC inhibitor at the GIT cell lining. The enhancer is a medium chain fatty acid or derivative thereof having a carbon chain length of from 6 to 20 carbon atoms. In certain embodiments, the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:MERRION RES I
Multiparticulate modified release composition
InactiveUS20020054907A1Organic active ingredientsNervous disorderControlled releasePatient compliance
The invention relates to a multiparticulate modified release composition that in operation delivers an active ingredient in a pulsed or bimodal manner. The multiparticulate modified release composition comprises an immediate release component and a modified release component; the immediate release component comprising a first population of active ingredient containing particles and the modified release component comprising a second population of active ingredient containing particles coated with a controlled release coating; wherein the combination of the immediate release and modified release components in operation deliver the active ingredient in a pulsed or a bimodal manner. The invention also relates to a solid oral dosage form containing such a multiparticulate modified release composition. The plasma profile achieved by the multiparticulate modified release composition is advantageous in reducing patient tolerance to the active ingredient and in increasing patient compliance by reducing dosage frequency.
Owner:ALKERMES PHARMA IRELAND LTD
Immediate release formulations of 1-aminocyclohexane compounds, memantine and neramexane
InactiveUS20060198884A1Improve stabilityBiocideSenses disorderImmediate releasePharmaceutical medicine
The present invention relates to an immediate release solid oral dosage form containing 1-aminocyclohexanes, preferably memantine or neramexane, and optionally a pharmaceutically acceptable coating, wherein the active ingredient exhibits dose proportionality and is released at a dissolution rate of more than about 80% within about the first 60 minutes following entry of said form into a use environment. The dosage form is direct compressed and has a hardness within the range of between about 3 and about 40 Kp, exhibits an average Tmax within the range of about 2 to about 8 hours with an active ingredient load within the range of about 2.5 to about 150 mg. The formulation allows for dose-proportional compositions for once daily or b.i.d. dosing, while maintaining a steady average range of Tmax.
Owner:FOREST LAB HLDG LTD
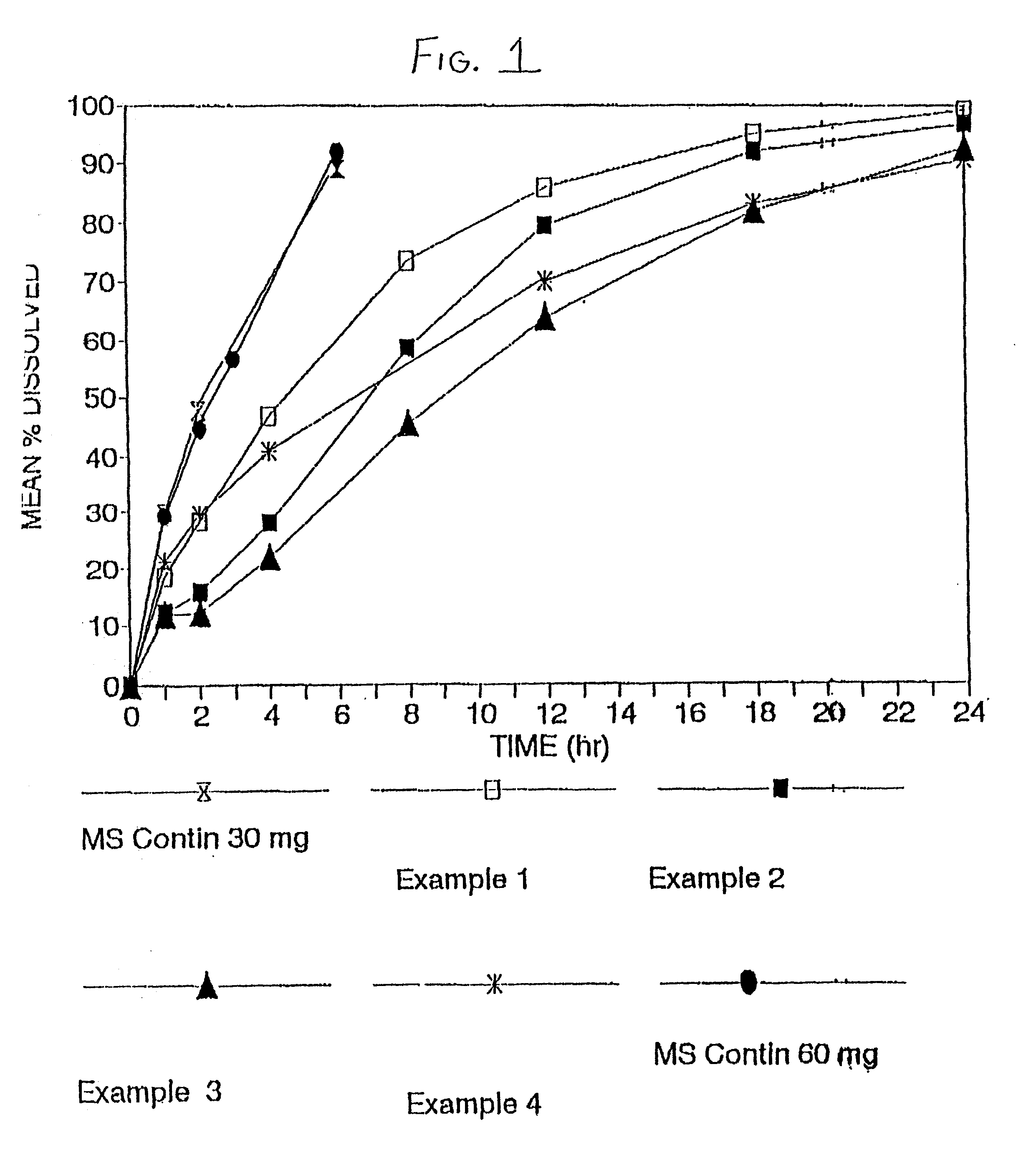
Orally administrable opioid formulations having extended duration of effect
InactiveUS20020081333A1Effective steady-state blood levelPowder deliveryBiocideBlood levelOral medication
Owner:PURDUE PHARMA LP
Tablets of linezolid form iii and processes for their preparation
InactiveUS20070104785A1Reduce gelling tendencyTrend downOrganic active ingredientsPill deliveryLinezolidDissolution
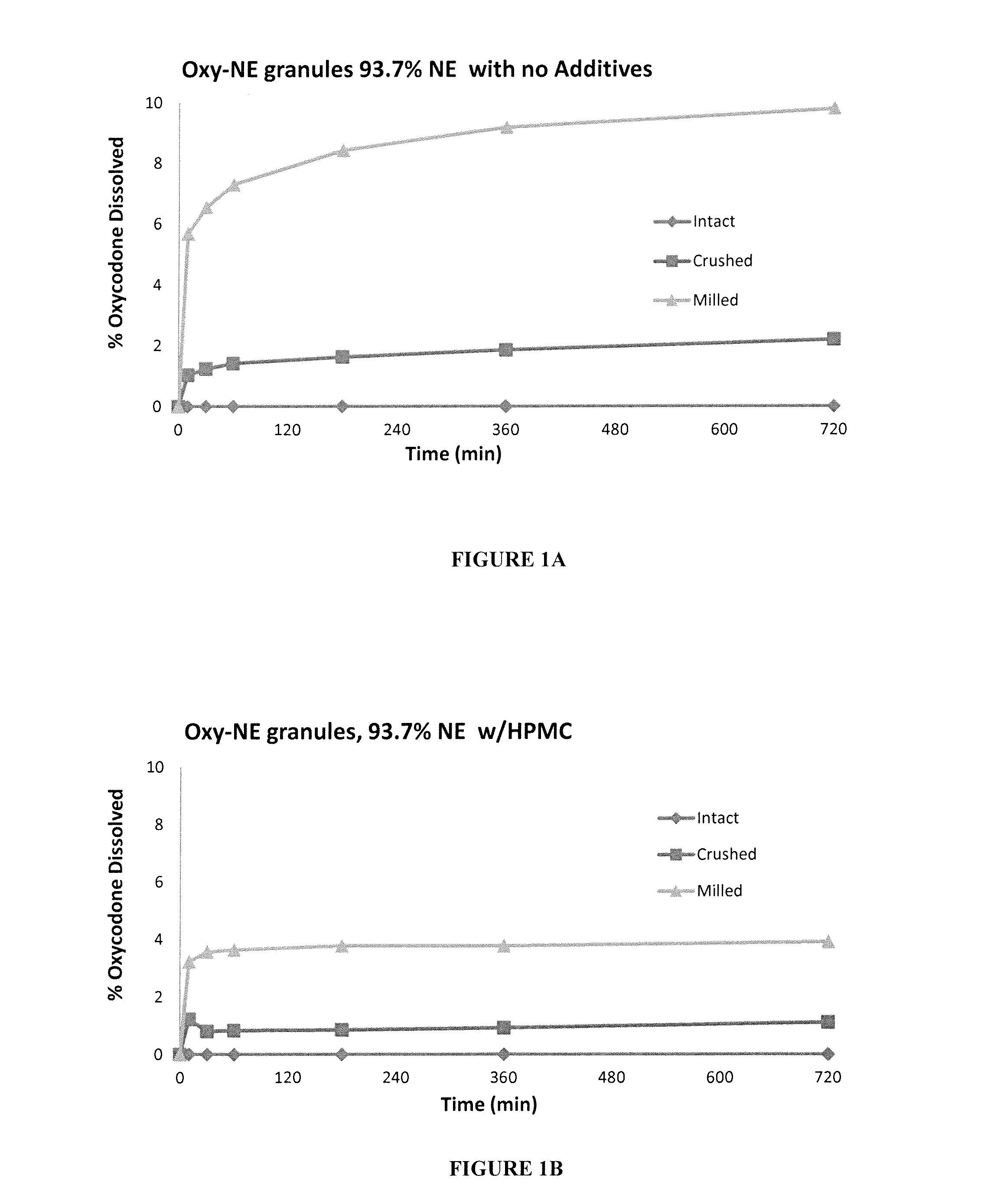
The present invention relates to solid oral dosage forms of linezolid polymorphic Form III with reproducible dissolution profile and processes for their preparation. The solid dosage form includes linezolid Form III, one or more of means to reduce the gelling tendency of linezolid form III, and one or more of pharmaceutically acceptable excipients.
Owner:NAVALE SURYAKANT VAMANRAO +3
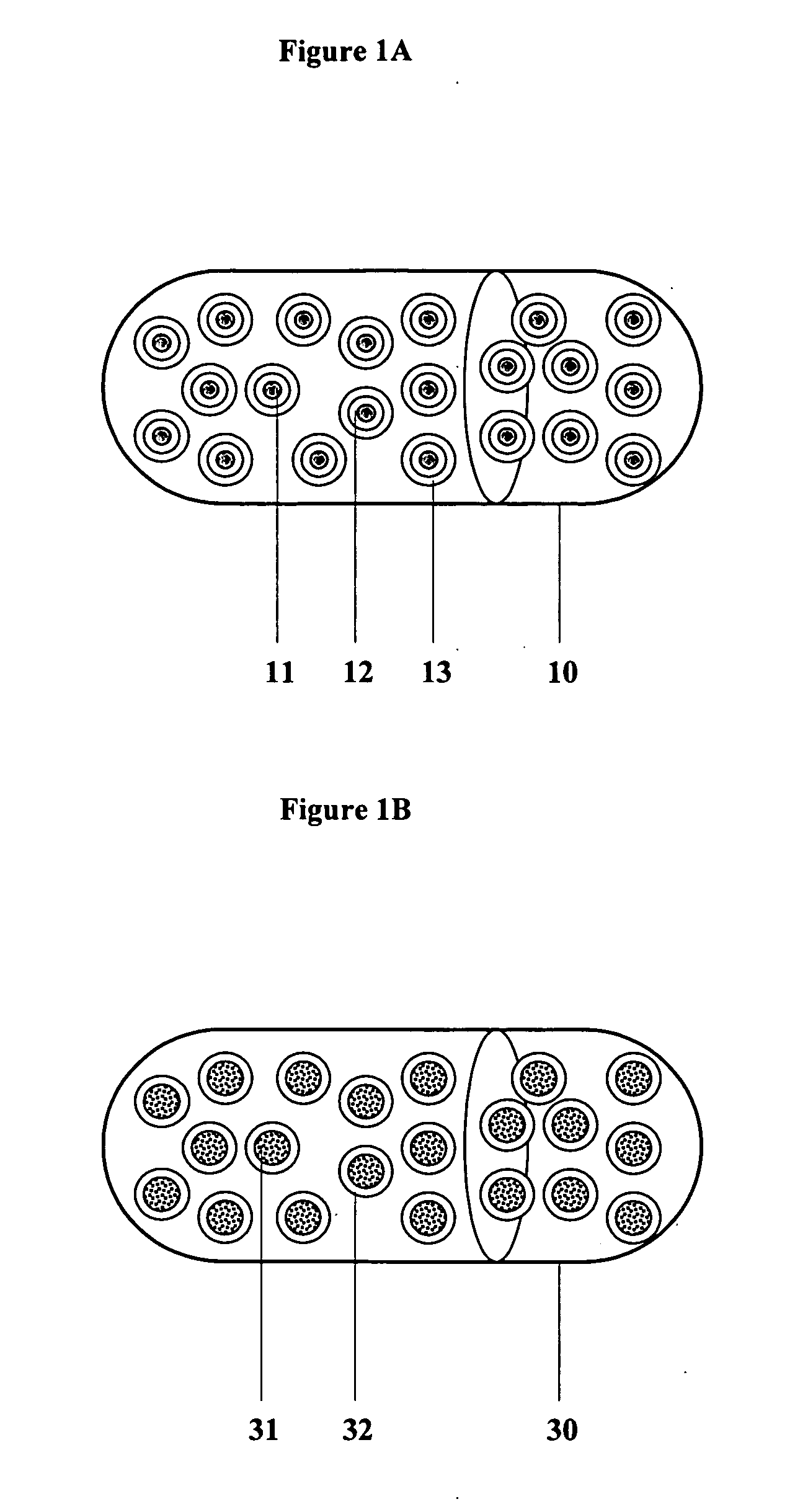
Solid oral dosage form containing seamless microcapsules
InactiveUS20060018965A1Reduce nucleic acid degradationReduce proteolytic degradationDigestive systemImmunological disordersLong chain fatty acidOrganic solvent
This invention relates to a solid oral dosage form containing one or more pharmaceutically active ingredients solubilised or suspended in a pharmaceutically acceptable solvent or liquid phase and encapsulated in seamless controlled release microcapsules. Accordingly the pharmaceutically acceptable solvent or liquid phase may range from aqueous phase, organic solvent(s), glycols, oils and derivatives of including mono-,di, and triglycerides of short, medium and long chain fatty acids. The microcapsules have a diameter of <1 mm to 8 mm and a drug loading of up to 90%. Additionally the microcapsules may be coated to release the pharmaceutically active ingredient at specific sites and for predetermined rates.
Owner:SIGMOID PHARM LIMITED
Tamper Resistant Immediate Release Formulations
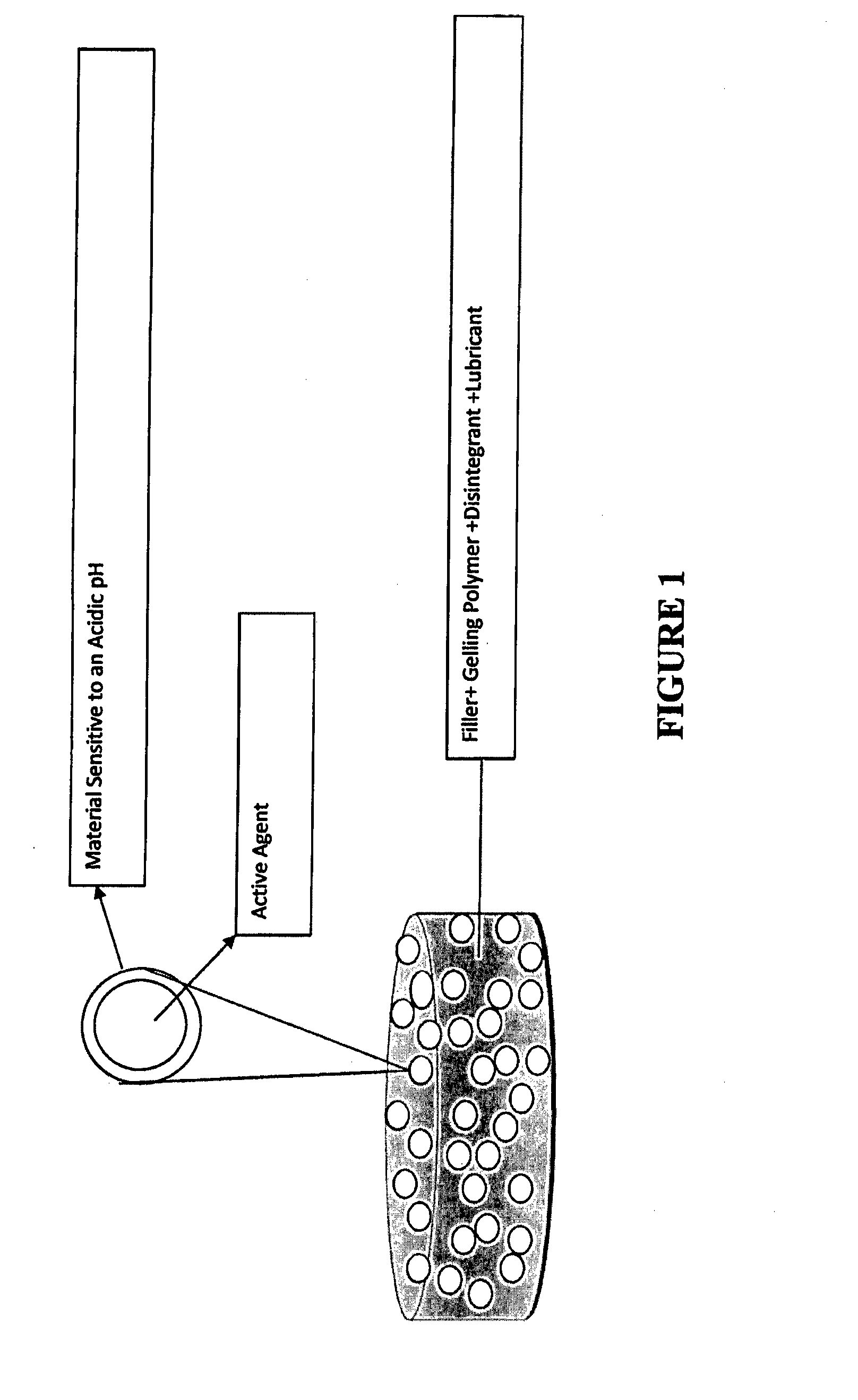
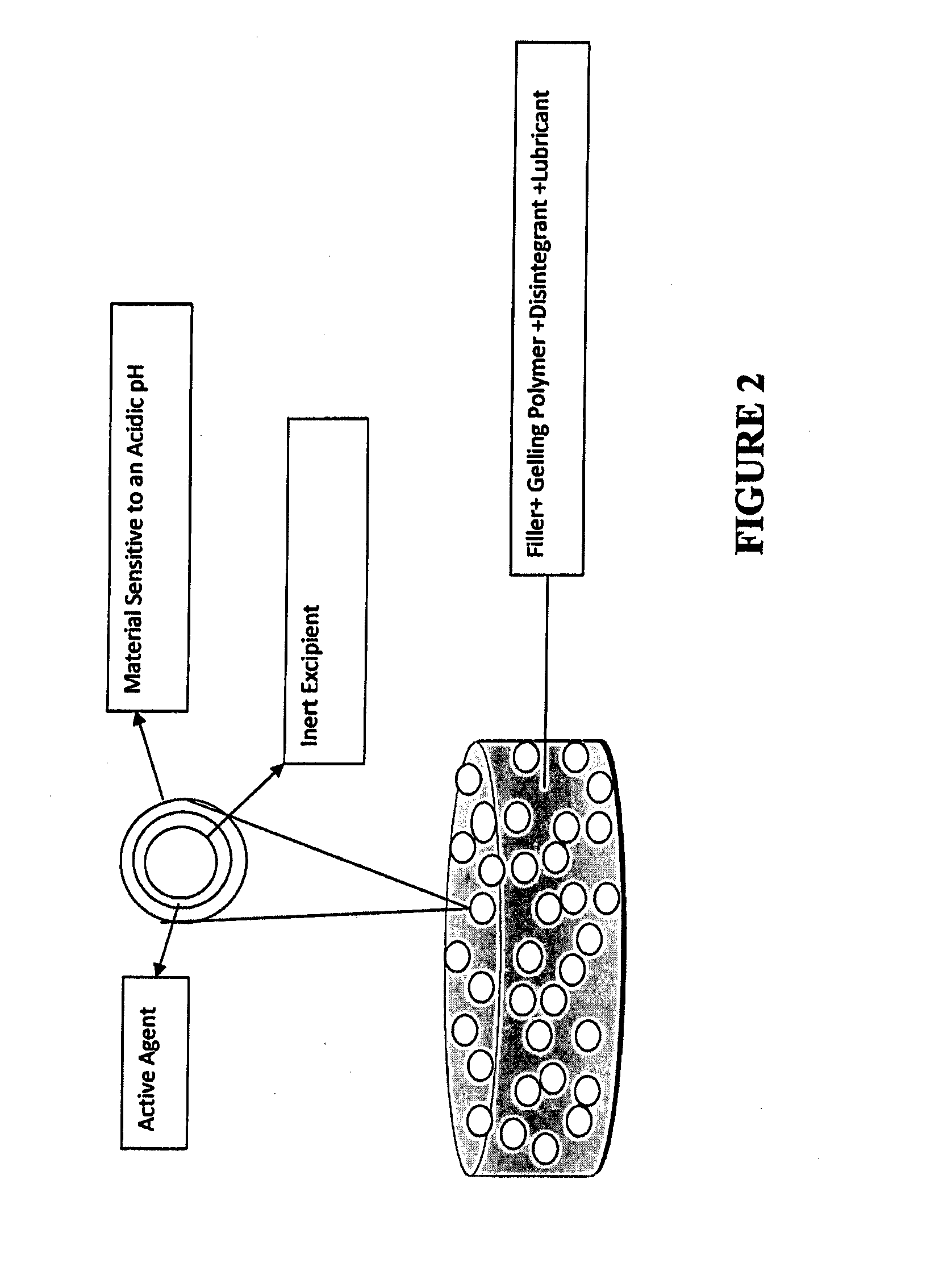
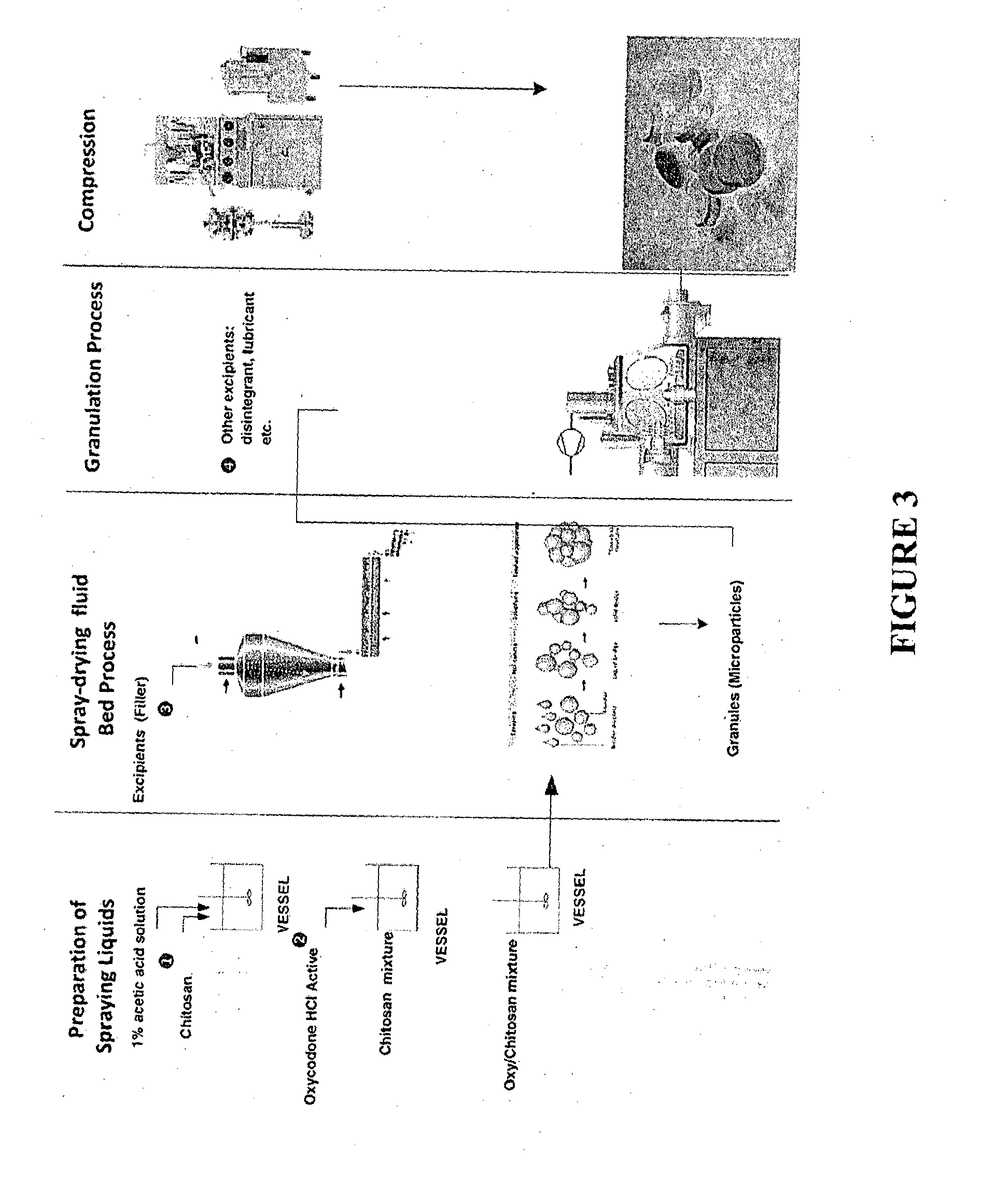
ActiveUS20150030677A1Reducing abuse potential of dosage formPrevent and reduce abilityPowder deliveryBiocideActive agentImmediate release
Disclosed is an immediate release solid oral dosage form comprising (i) an active agent; and (ii) a material that is sensitive to acidic pH;
Owner:RHODES PHARMA LP
Oral solid dosage forms containing a low dose of estradiol
The present invention relates to oral solid dosage forms containing a very low dose of estradiol. The dosage forms are formulated in a manner so as to avoid degradation of the estradiol and to minimise the content of polyvinylpyrrolidone, while still achieving similar fast dissolution of the estradiol. The dosage forms are useful in preventing or treating a physical condition in a woman caused by insufficient endogenous levels of estradiol.
Owner:BAYER SCHERING PHARMA AG
Solid oral dosage form containing an enhancer
InactiveUS20070196464A1Improve oral bioavailabilityMinimizes risk of local irritationBiocidePhosphorous compound active ingredientsDelayed Release Dosage FormCarbon chain
The invention relates to a pharmaceutical composition and oral dosage forms comprising a bisphosphonate in combination with an enhancer to promote absorption of the bisphosphonate at the GIT cell lining. The enhancer is a medium chain fatty acid or a medium chain fatty acid derivative having a carbon chain length of from 6 to 20 carbon atoms. Preferably, the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:NOVO NORDISK AS
Solid oral dosage form containing an enhancer
InactiveUS20080275001A1BiocideOrganic active ingredientsDelayed Release Dosage FormAdditive ingredient
The invention relates to a solid oral dosage form comprising a pharmaceutically active ingredient in combination with an enhancer which enhances the bioavailability and / or the absorption of the active ingredient. Accordingly, a solid oral dosage form comprises a drug and an enhancer wherein the enhancer is a medium chain fatty acid ester, ether or salt or a derivative of a medium chain fatty acid, which is, preferably, solid at room temperature and which has a carbon chain length of from 6 to 20 carbon atoms. Preferably, the solid oral dosage form is controlled release dosage form such as a delayed release dosage form.
Owner:NOVO NORDISK AS
Tamper resistant pharmaceutical formulations
ActiveUS20140271896A1Reducing abuse potential of dosage formMaintenance characteristicBiocideNervous disorderControl releaseAlcohol
Disclosed in certain embodiments is a solid oral dosage form comprising a plurality of particles, each particle comprising (i) a core comprising an active agent susceptible to abuse and an internal adhesion promoter, wherein the cores are (i) dispersed in a matrix comprising a controlled release material or (ii) coated with a controlled release material. The dosage form can also include an alcohol resistant material.
Owner:PURDUE PHARMA LP
Testosterone oral dosage formulations and associated methods
InactiveUS20050100608A1Reduce adverse outcomesPowder deliveryOrganic active ingredientsPolyethylene glycolEthylene glycol
Solid oral dosage forms of testosterone and methods for the preparation thereof are disclosed and described. The solid oral dosage form may include a therapeutically effective amount of testosterone in a substantially solid polyethylene glycol carrier. Such a form has been found to alleviate many of the undesirable consequences of undergoing testosterone therapy, such as the pain of injections and problems with patient noncompliance.
Owner:WATSON PHARMA INC
Processes for making particle-based pharmaceutical formulations for oral administration
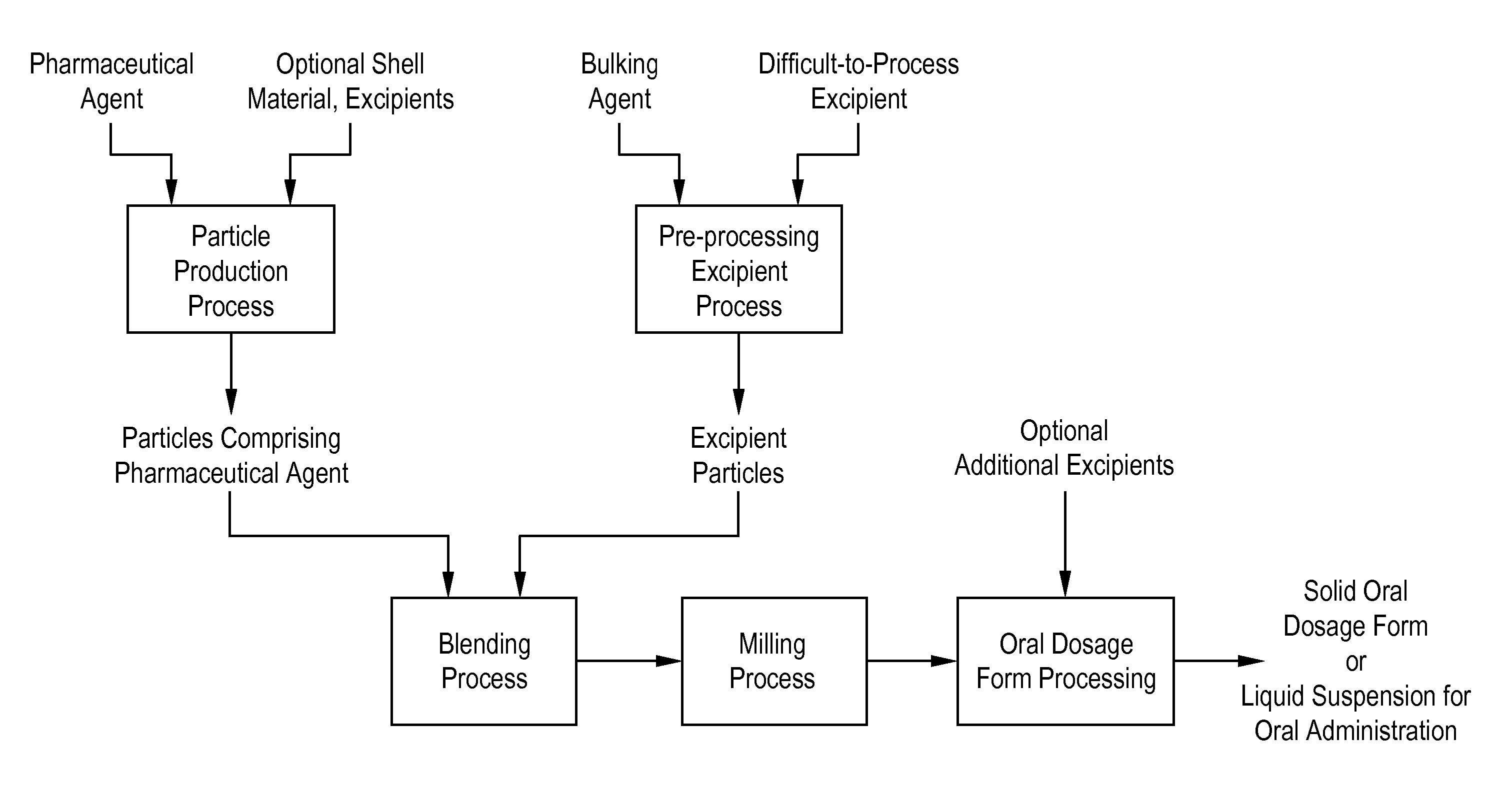
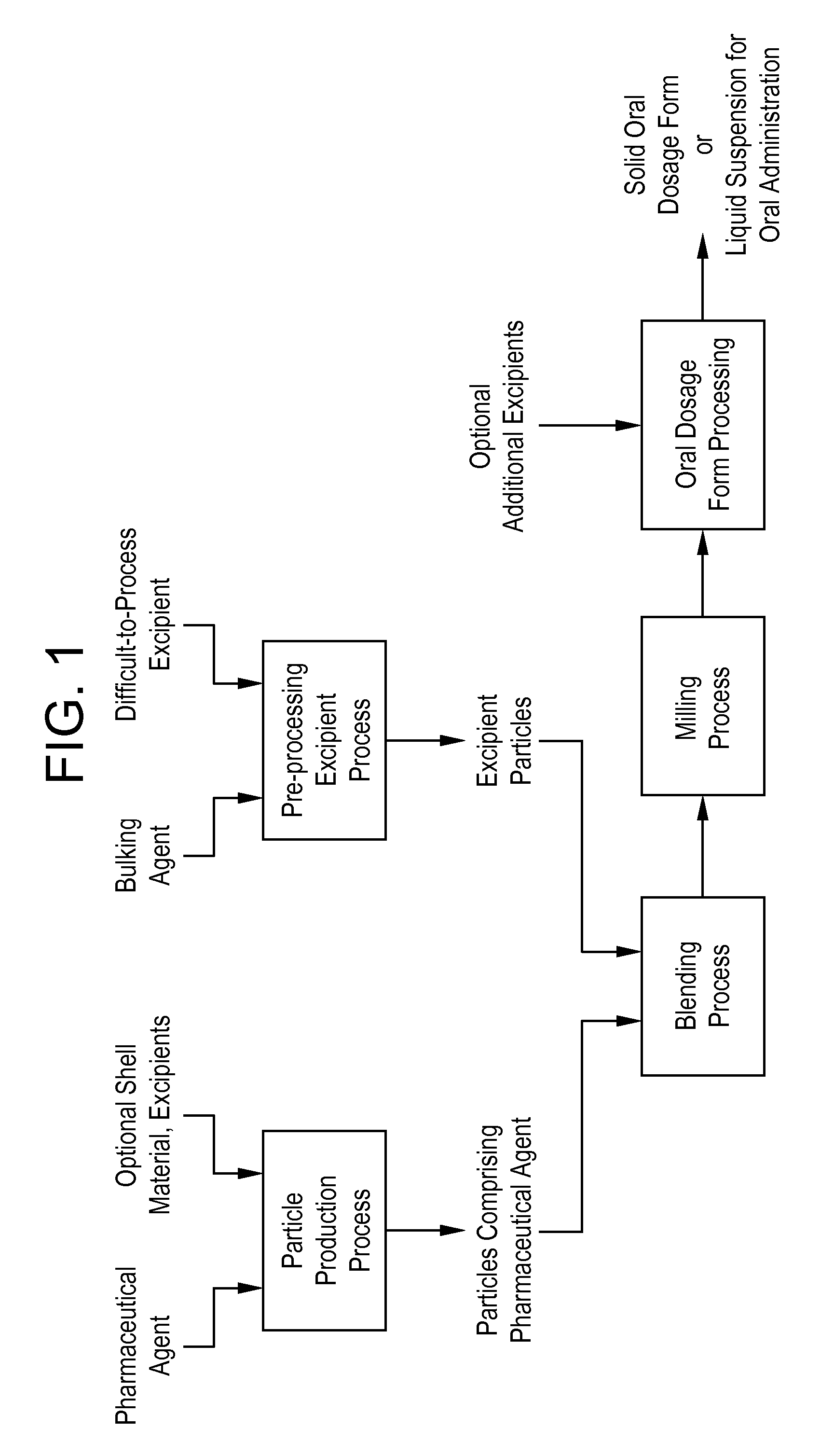
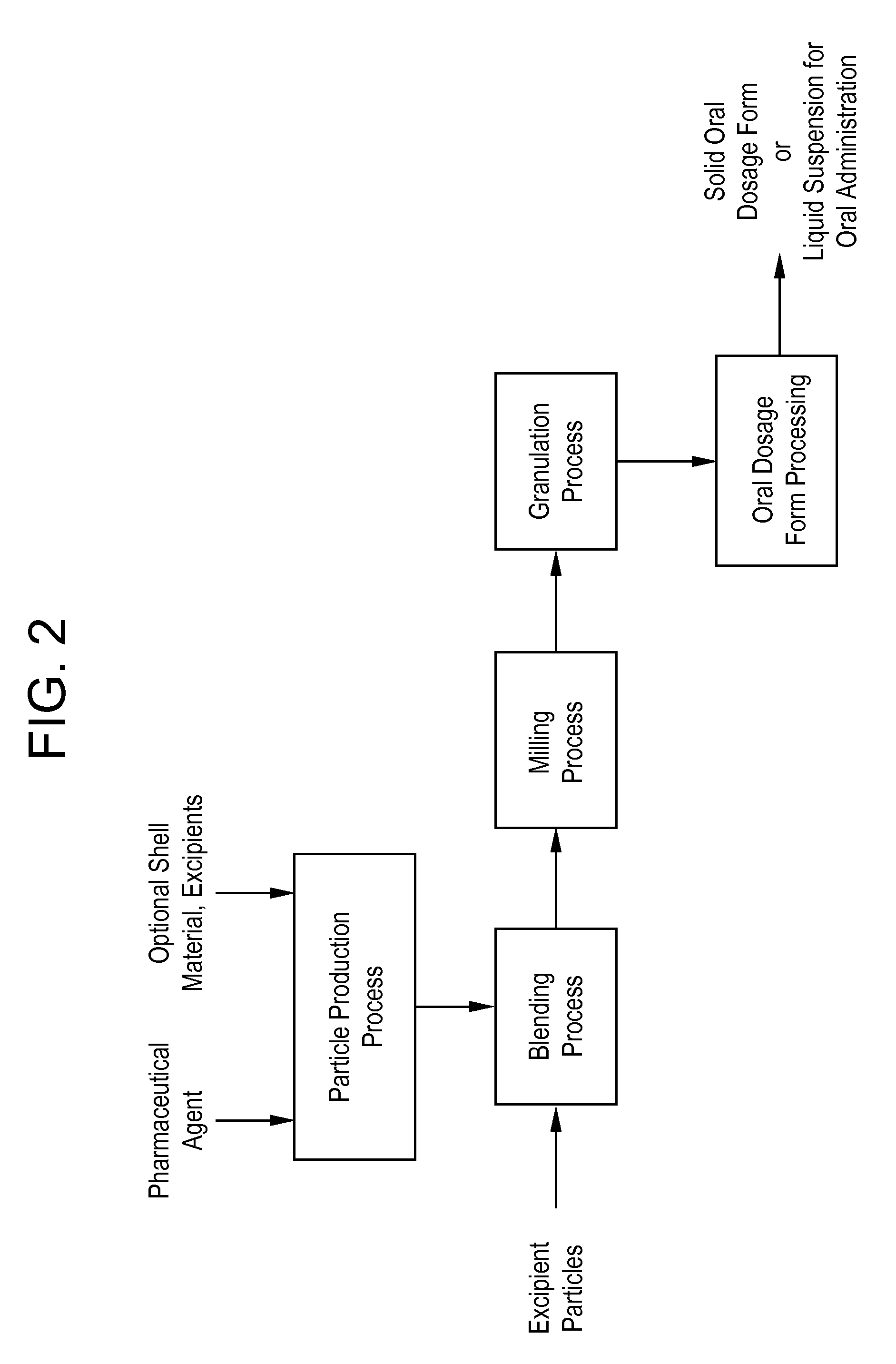
A method is provided for making an oral dosage form of a pharmaceutical agent which includes the steps of (a) providing particles which include a pharmaceutical agent; (b) blending the particles with particles of a pre-processed excipient to form a primary blend, wherein the pre-processed excipient is prepared by (i) dissolving a bulking agent (e.g., a sugar) and at least one non-friable excipient (e.g., a waxy or liquid surfactant) in a solvent to form an excipient solution, and (ii) removing the solvent from the excipient solution to form the pre-processed excipient in dry powder form; (c) milling the primary blend to form a milled pharmaceutical formulation blend that includes microparticles or nanoparticles of the pharmaceutical agent; and (d) processing the milled pharmaceutical formulation blend into a solid oral dosage form or liquid suspension for oral administration. The process yields formulations having improved wettability or dispersibility.
Owner:ACUSPHERE INC
Tamper Resistant Solid Oral Dosage Forms
ActiveUS20140010874A1Reducing the abuse potential of an active agentOrganic active ingredientsNervous disorderActive agentCoating
Disclosed in certain embodiments is a solid oral dosage form comprising: (a) an inert tamper resistant core; and (b) a coating surrounding the core, the coating comprising an active agent.
Owner:PURDUE PHARMA LP
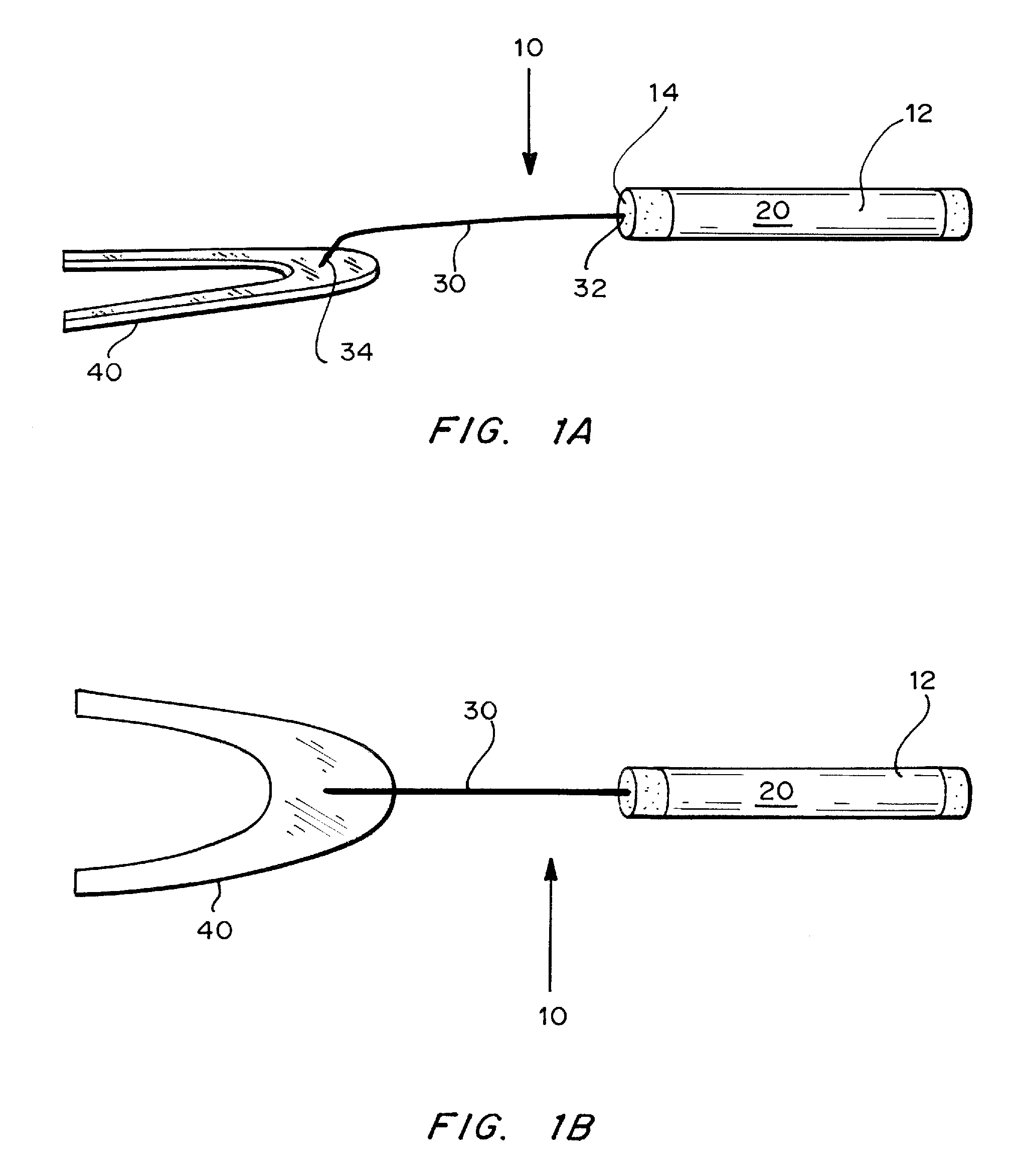
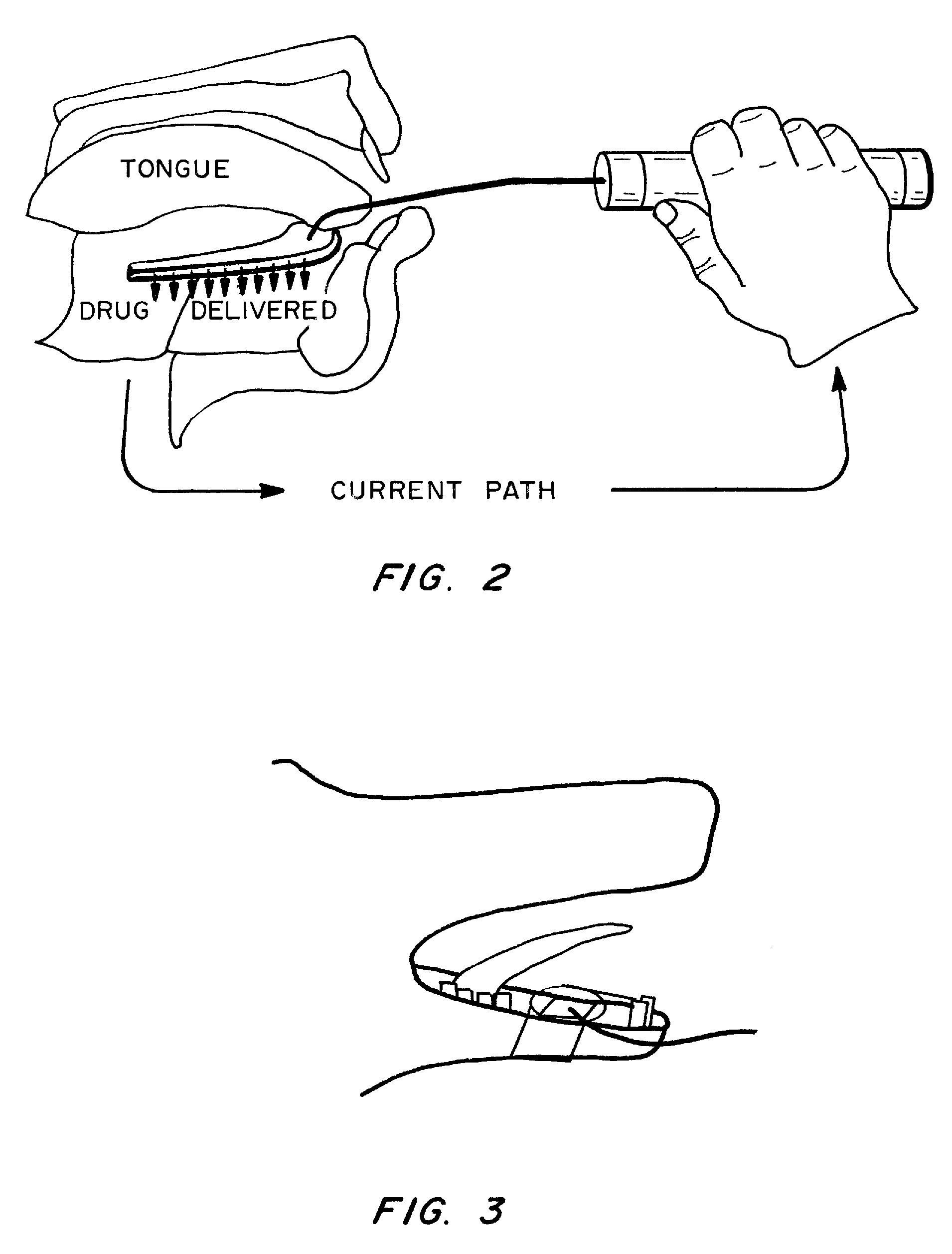
Method and Device for Sublingual Drug Delivery Using Iontophoresis
Methods, devices and kits for sublingual drug delivery using iontophoresis are described herein. An active agent can be administered sublingually by placing a solid oral dosage form containing the active agent in the sublingual region of a patient and applying iontophoresis for a suitable period of time. Preferably up to 4 mA of current are applied to the sublingual region. Different time ranges can be used to administer iontophoresis; preferably iontophoresis is administered for up to 2 minutes at a time. Any suitable device for administering iontophoresis to the sublingual region may be used. The preferred device is a hand-held device that contains a handle, two electrodes, one of which is located on the handle and the other of which is attached to the end of the handle, and a connection to a power source. Optionally, the device contains a timer, which can be used turn off the current at a preset time. The device can be used to administer an active agent by iontophoresis to the sublingual region of a patient, by attaching the second electrode of the device to a solid oral dosage form containing the active agent to be administered. A kit contains the device for administering iontophoresis and one or more solid oral dosage forms, preferably in the form of one or more tabs or wafers. The tabs or wafers may be completely dissolvable or edible, or may contain a non-edible and non-dissolvable component. In a preferred embodiment, the solid oral dosage form contains insulin or an analog thereof and one or more excipients, preferably EDTA and citric acid.
Owner:BIODEL INC
Solid oral dosage forms comprising tadalafil
InactiveUS20110263606A1Improved solubilization and stabilizationPromote absorptionBiocidePharmaceutical delivery mechanismTadalafilBioavailability
Owner:INTELGENX CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com