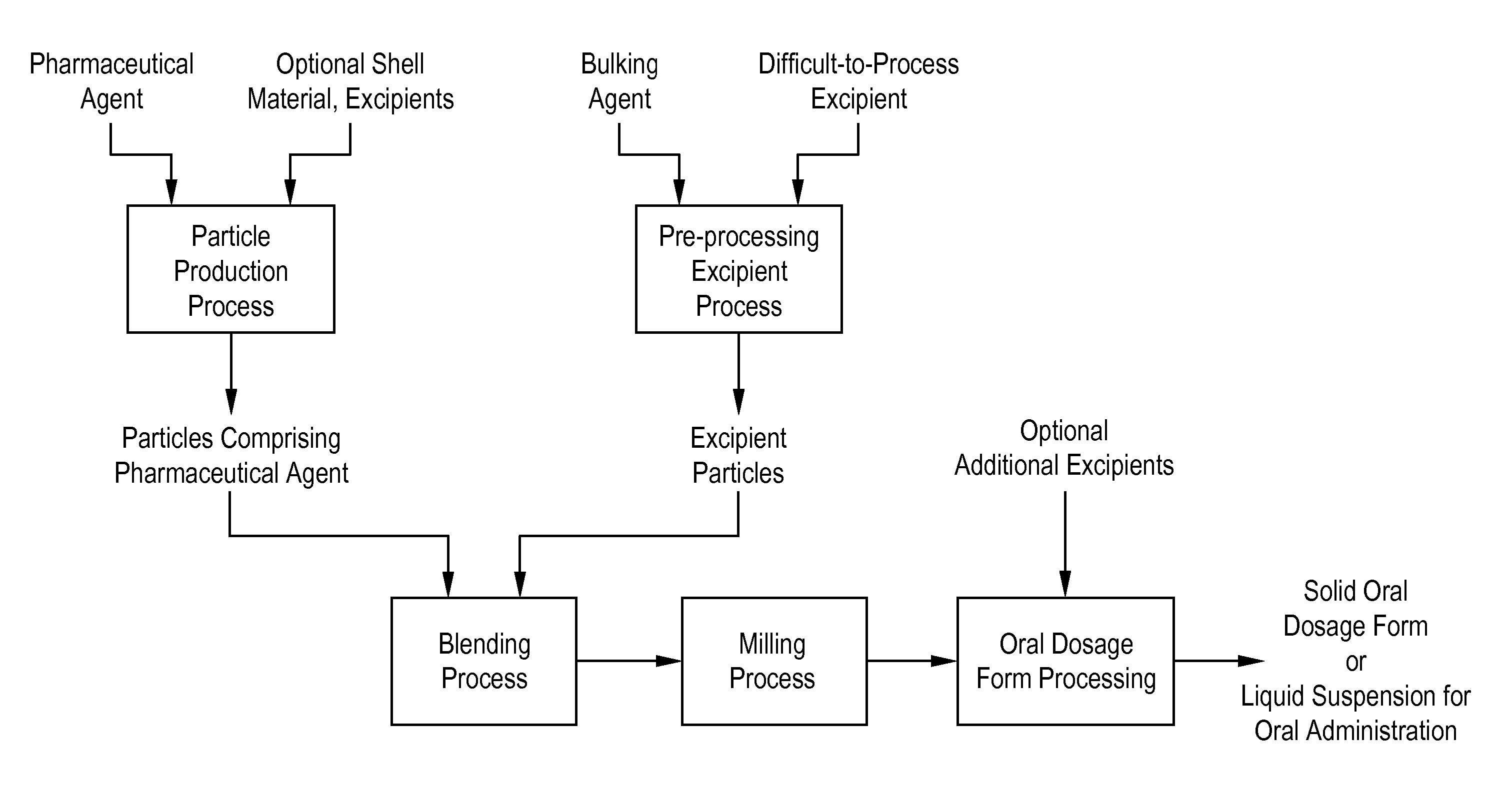
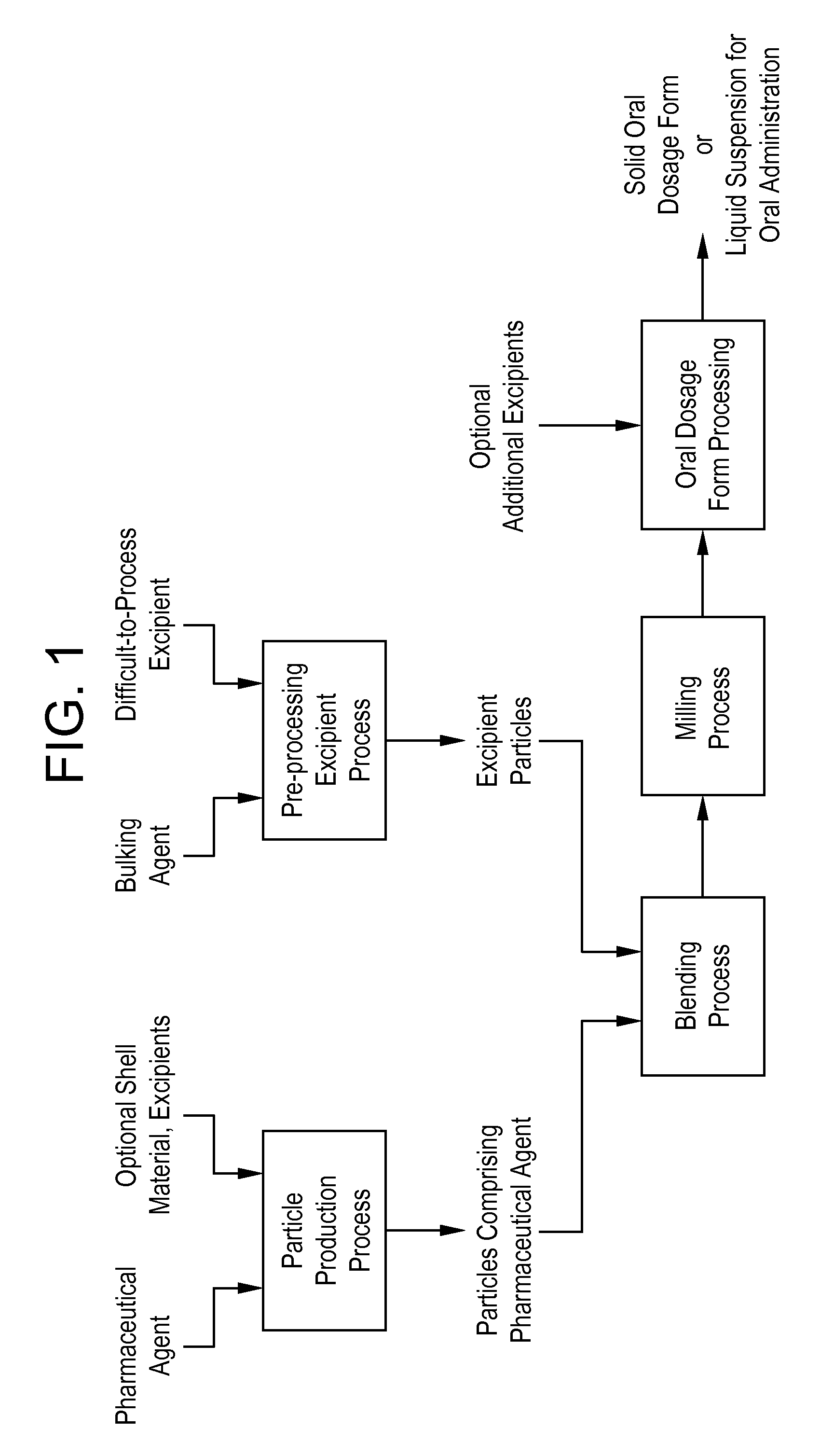
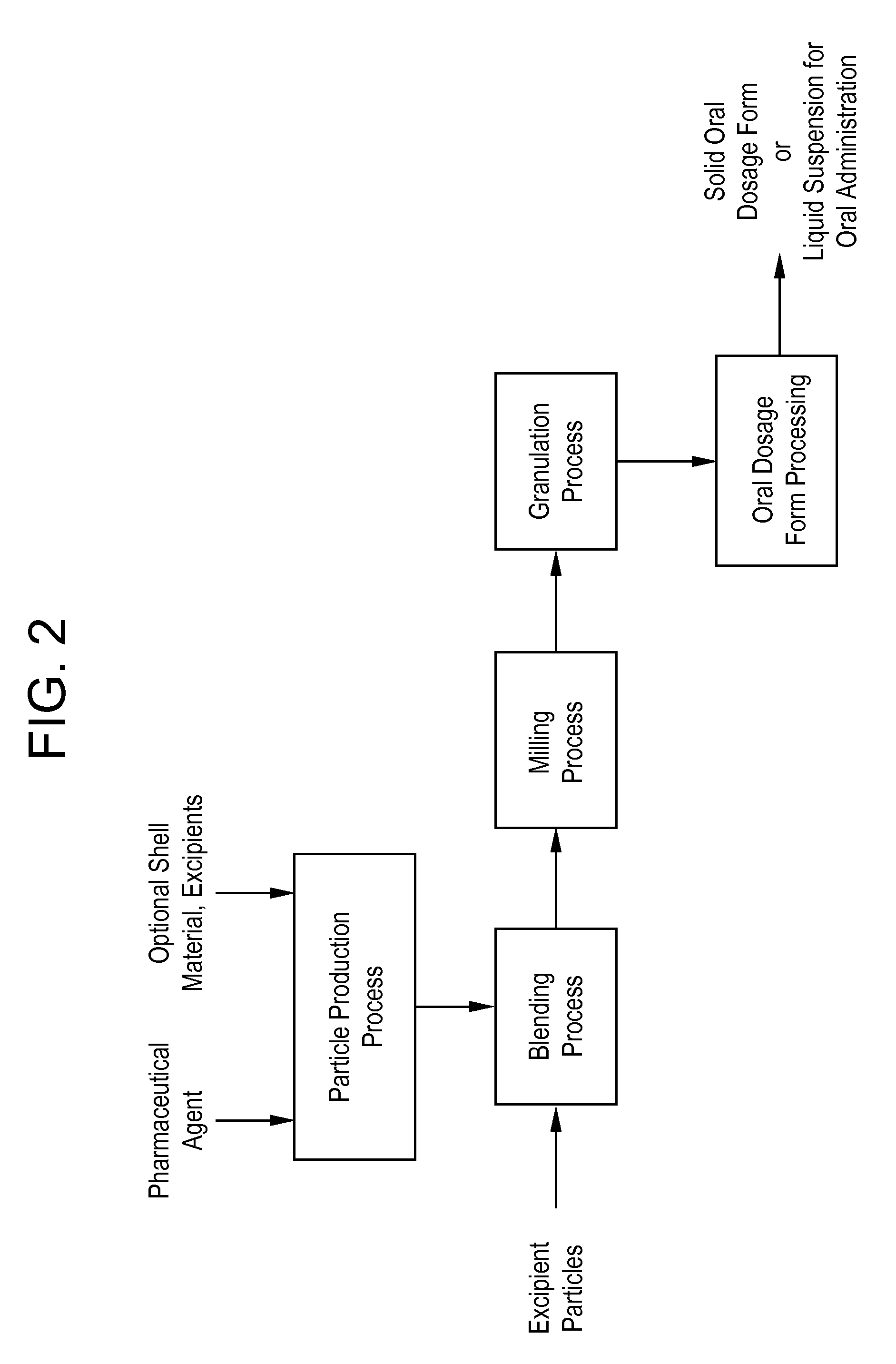
Processes for making particle-based pharmaceutical formulations for oral administration
a technology of pharmaceutical formulations and powders, which is applied in the directions of medicine preparations, powder delivery, capsule delivery, etc., can solve the problems of inability to meet the needs of patients, etc., to achieve the effect of improving the performance and reproducibility of the microparticle formulation, and reducing the cost of production and scaling up
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Jet Milling a Blend of PLGA Microparticles with Pre-Processed Excipient Particles Comprising Tween80 and Mannitol
[0115] Blending was conducted in two steps: a first step in which an excipient was pre-processed into a dry powder form and a second step in which the particles (representing particles of a pharmaceutical agent) were combined with the particles of pre-processed excipient. In the first step, mannitol and Tween80 were blended in liquid form, wherein 500 mL of Tween80 / mannitol vehicle was prepared from Tween80, mannitol, and water. The vehicle was frozen and then subjected to vacuum drying, yielding a powder comprised of Tween80 homogeneously dispersed with the mannitol. In the second step, poly(lactide-co-glycolide) (50:50) (“PLGA”) microparticles (which represented the pharmaceutical agent particles) were combined with the mannitol / Tween80 blend and mixed in a tumbler mixer to yield a dry blended powder. The PLGA microparticles had an Xn=2.83 micron and Xv 8.07 micron. Th...
example 2
Jet Milling of Celecoxib / Excipient Blend for Improved Microparticle Dispersibility
[0116] Mannitol (89.3 g, Pearlitol 100SD from Roquette America Inc., Keokuk, Iowa), sodium lauryl sulfate (3.46 g), celecoxib (149.0 g), and hypromellose-606 (9.35 g) were added to a stainless steel jar. The jar was then set in a TURBULA™ mixer for 90 minutes at 96 min−1, yielding a dry blended powder. The dry blended powder then was fed manually into a Fluid Energy Aljet jet mill (injector gas pressure 8.0 bar, grinding gas pressure 4.0 bar) to produce well dispersing microparticles.
[0117] The unprocessed celecoxib, the blended celecoxib, and the jet milled blended celecoxib were analyzed using visual inspection and by light microscopy (performed on a hemacytometer slide) following reconstitution in 0.01N HCl. FIGS. 5A, 5B, and 5C show the particles of the bulk celecoxib, the blended powder, and the jet-milled blended powder, respectively. The quality of the suspensions are described in Table 2.
TA...
example 3
Granulation and Tabletting of a Milled Blend Comprising Fenofibrate and a Pre-processed Excipient
[0119] To create a pre-processed excipient, a solution of mannitol (267.7 g, Pearlitol 100SD) and DSS (32.16 g) in 2264 g of water was prepared. The solution was frozen and lyophilized, and the resulting powder was screened through an 850 μm sieve prior to blending with the fenofibrate particles.
[0120] A dry powder blend formulation was prepared by one of three different processes. The blend included fenofibrate, mannitol, DSS, and Plasdone S630 in a 10:10:1.2:2.0 ratio, where the mannitol and DSS were in the form of the pre-processed excipient described above. The total blend amount was 150 g. The three processes were (1: API Blend) blending the fenofibrate and excipient particles without milling, (2: Blend of JM API) separately milling the fenofibrate particles and then blending the milled particles with excipient particles, or (3: JM API Blend) blending the fenofibrate and excipient...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com