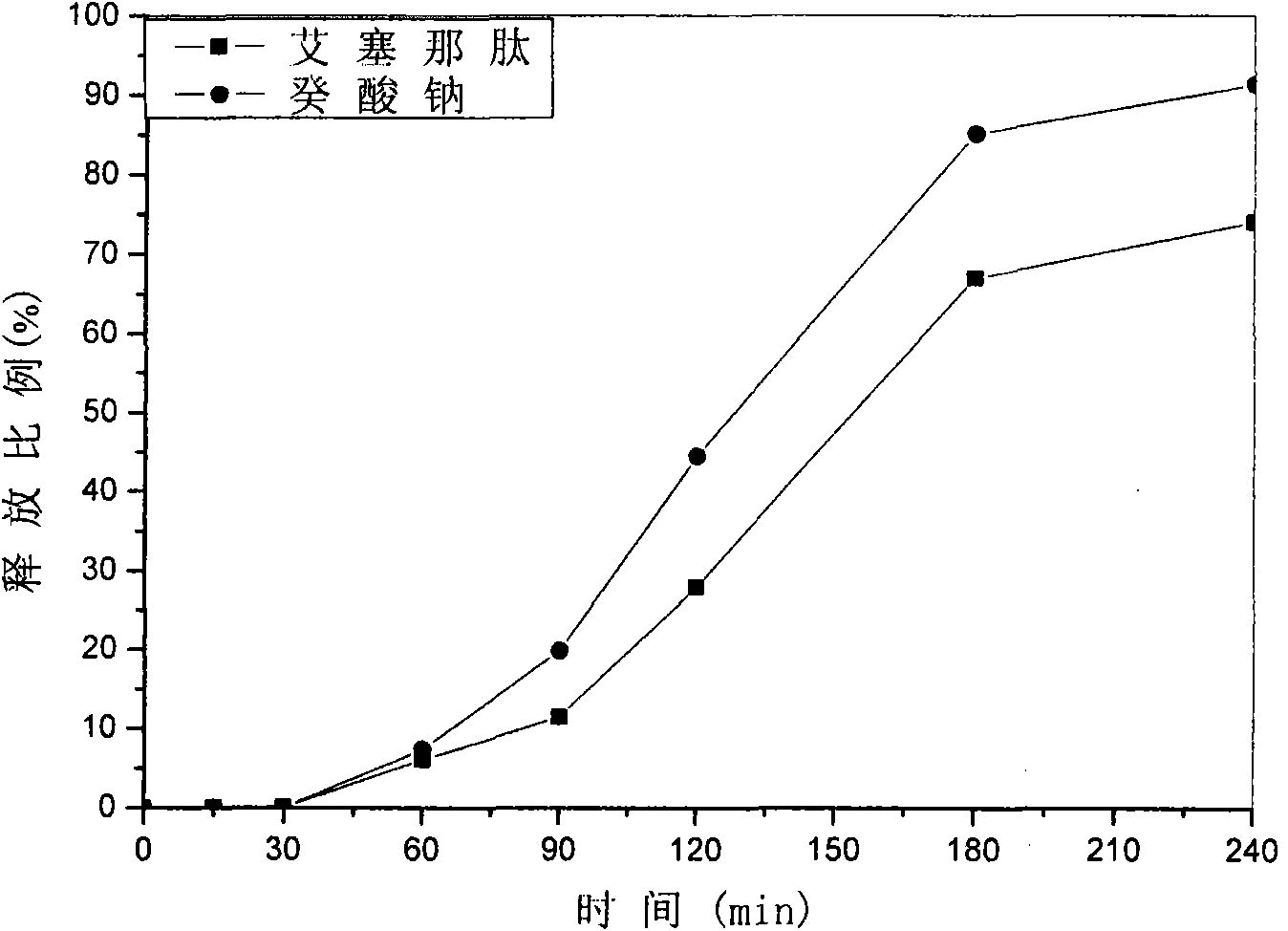
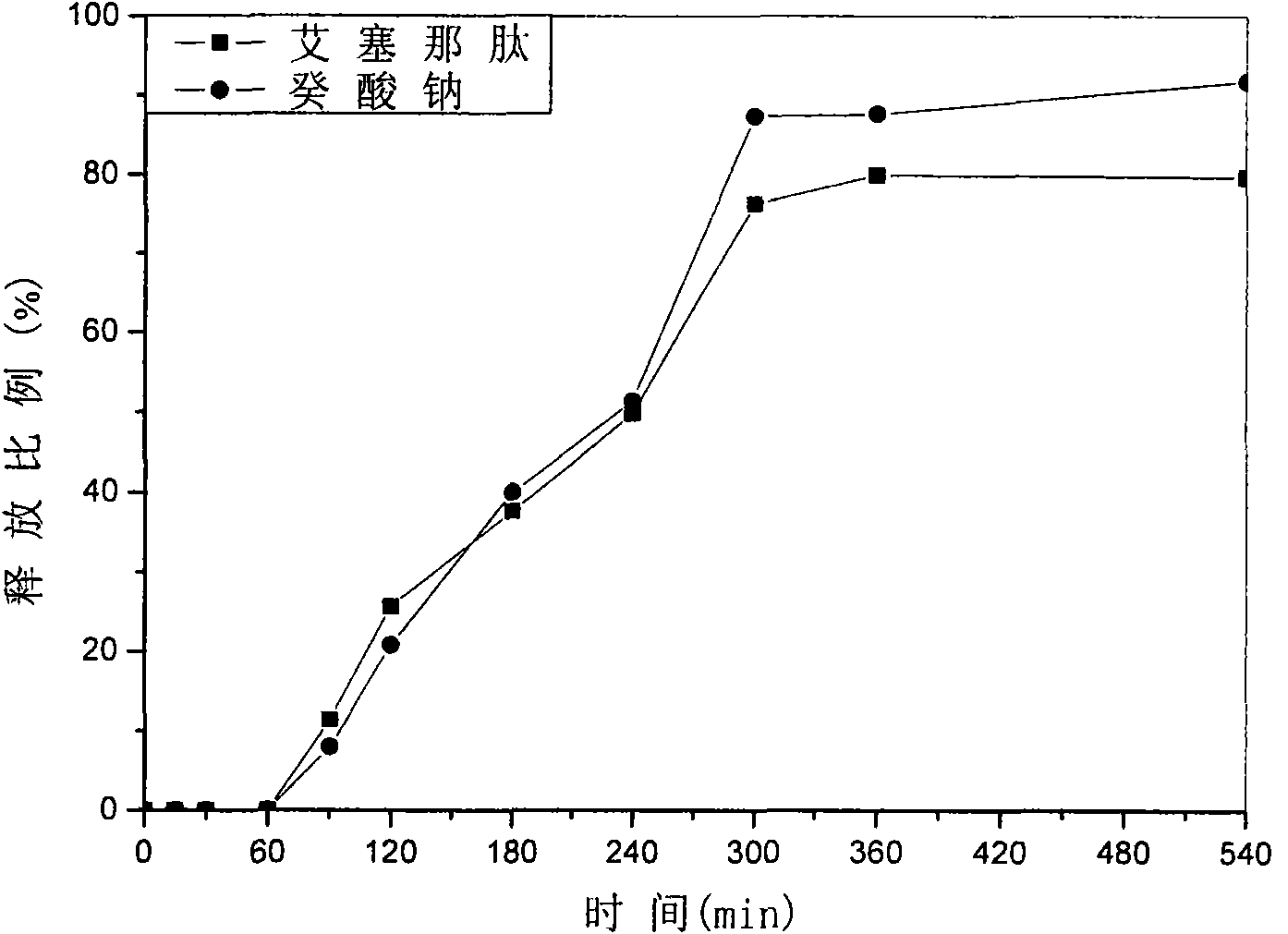
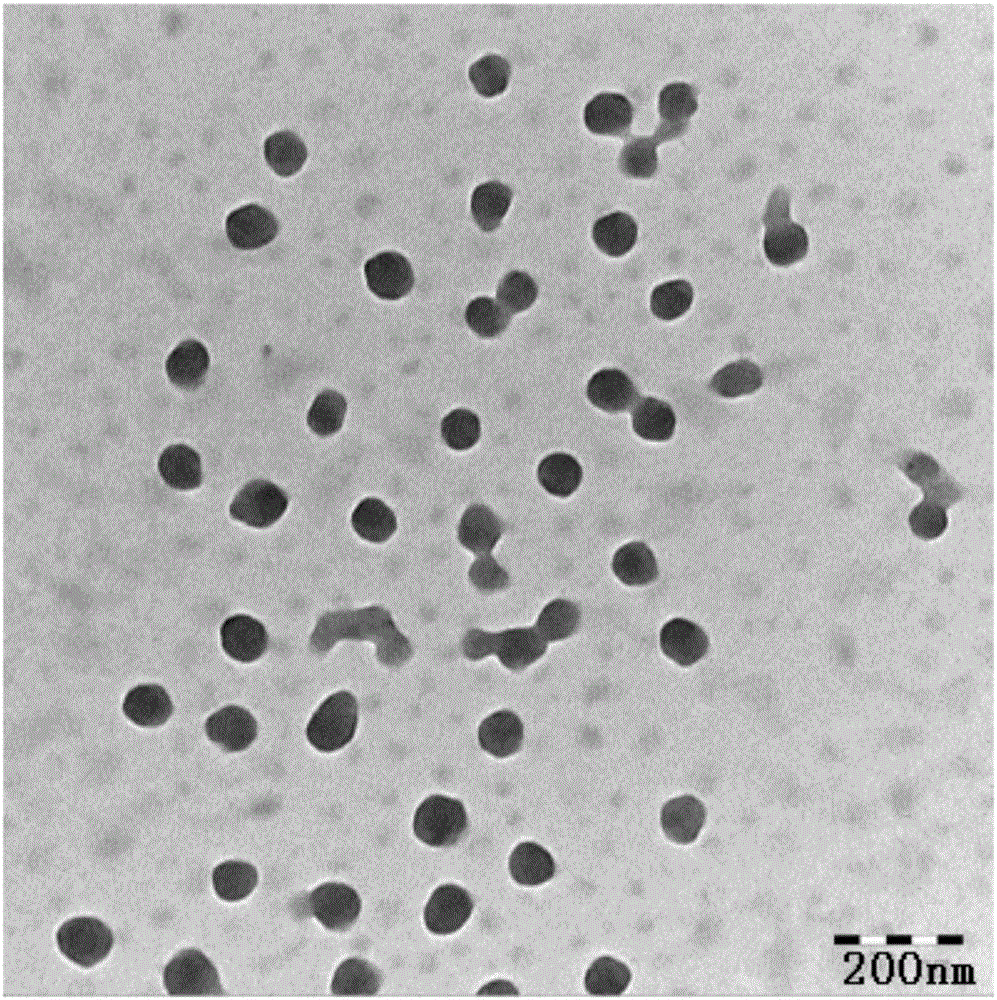
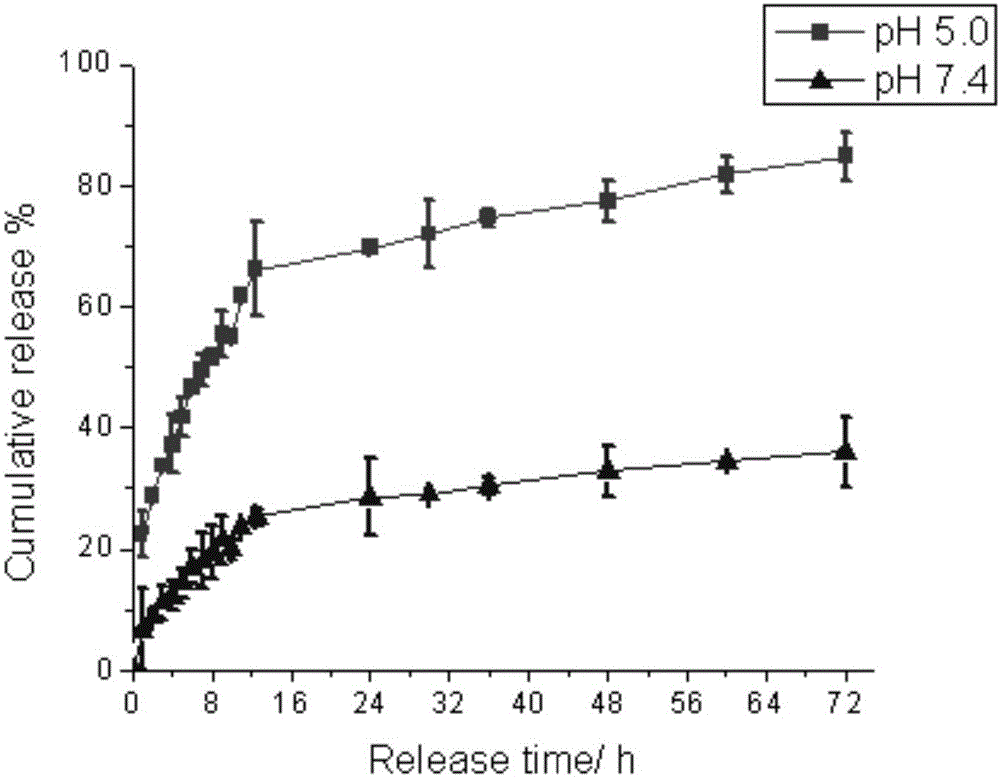
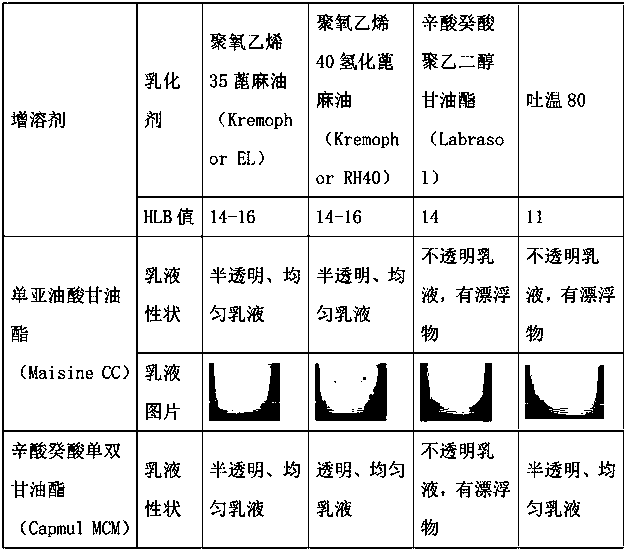
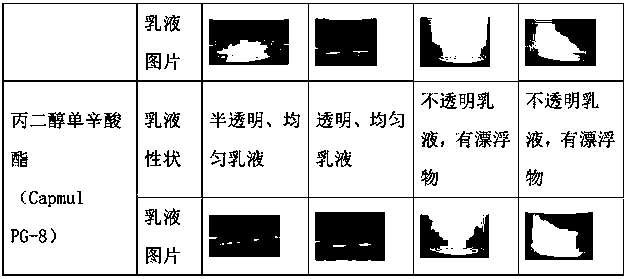
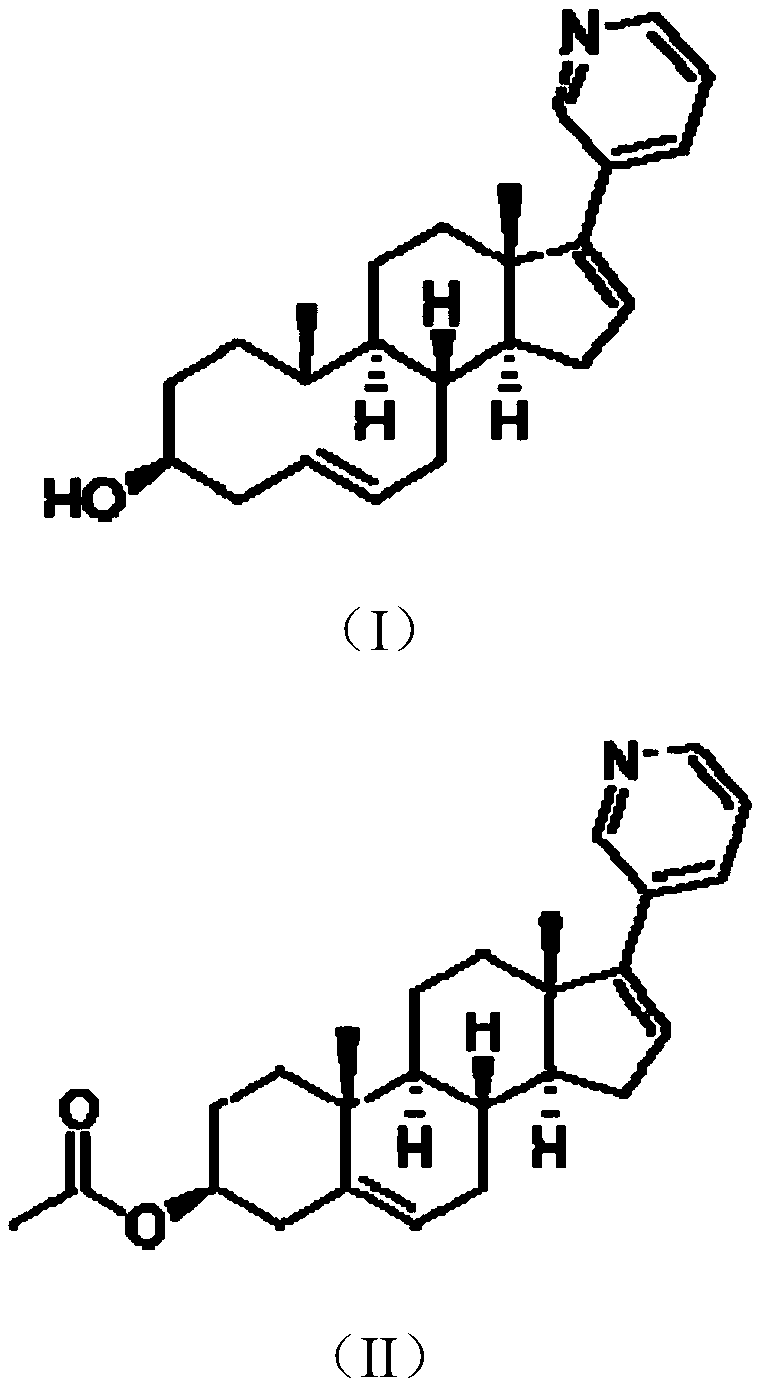
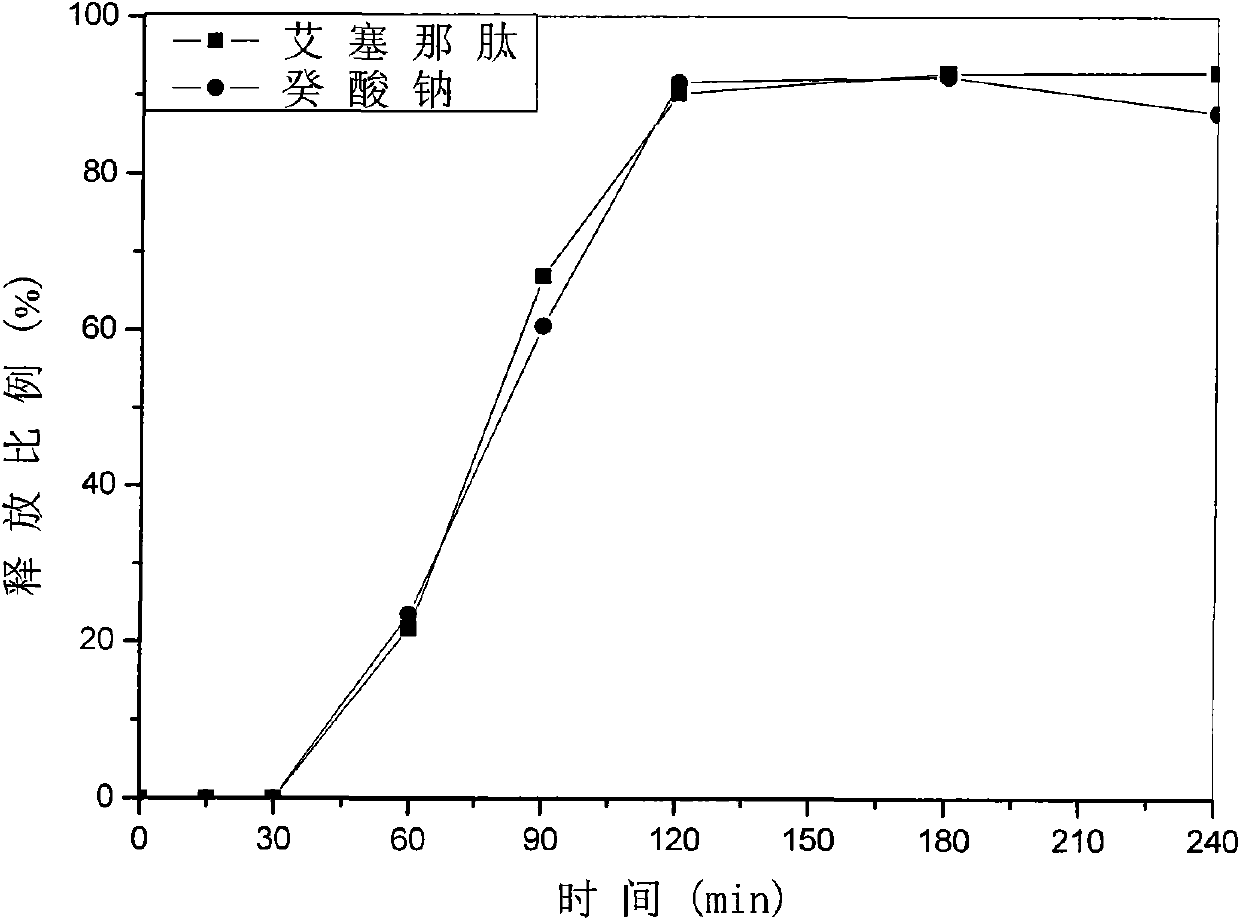
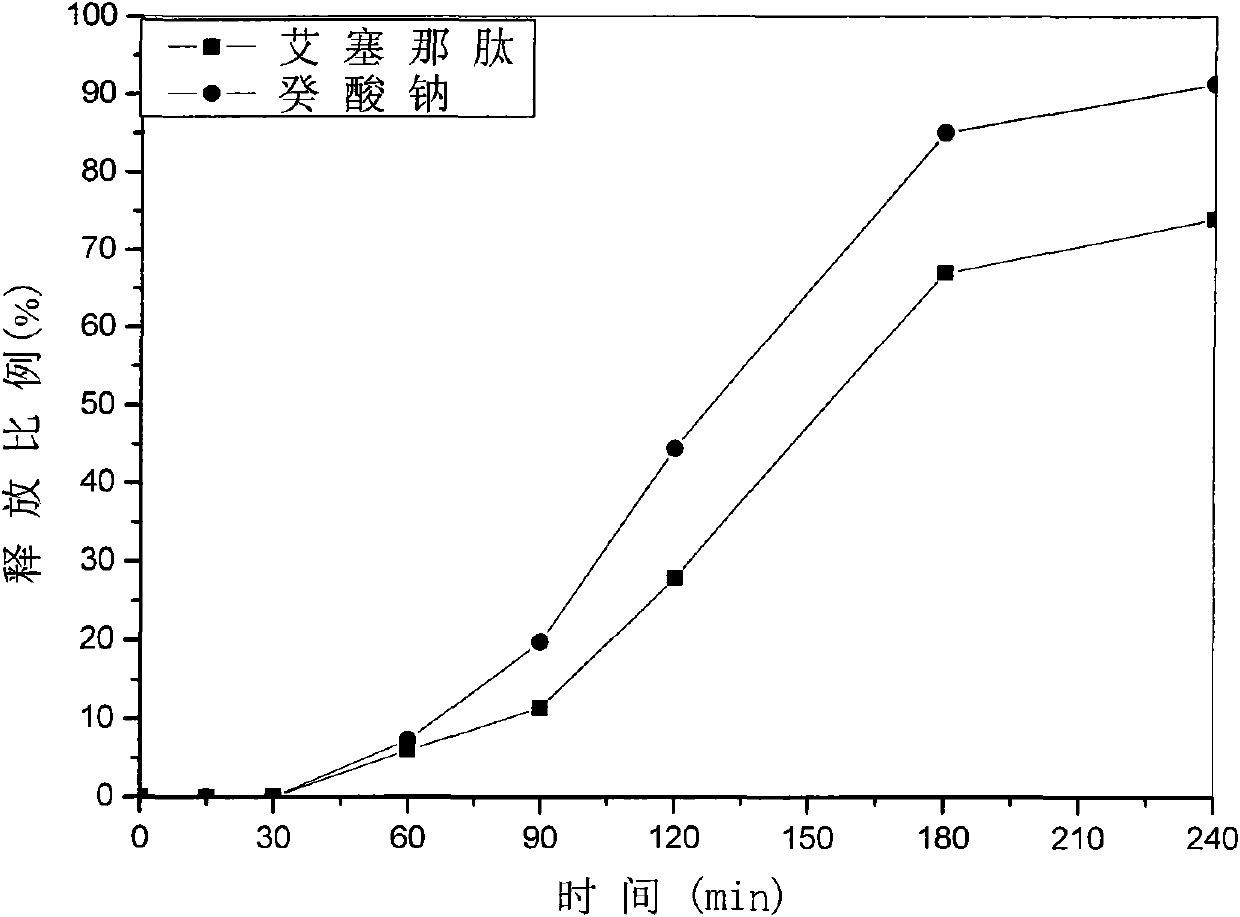
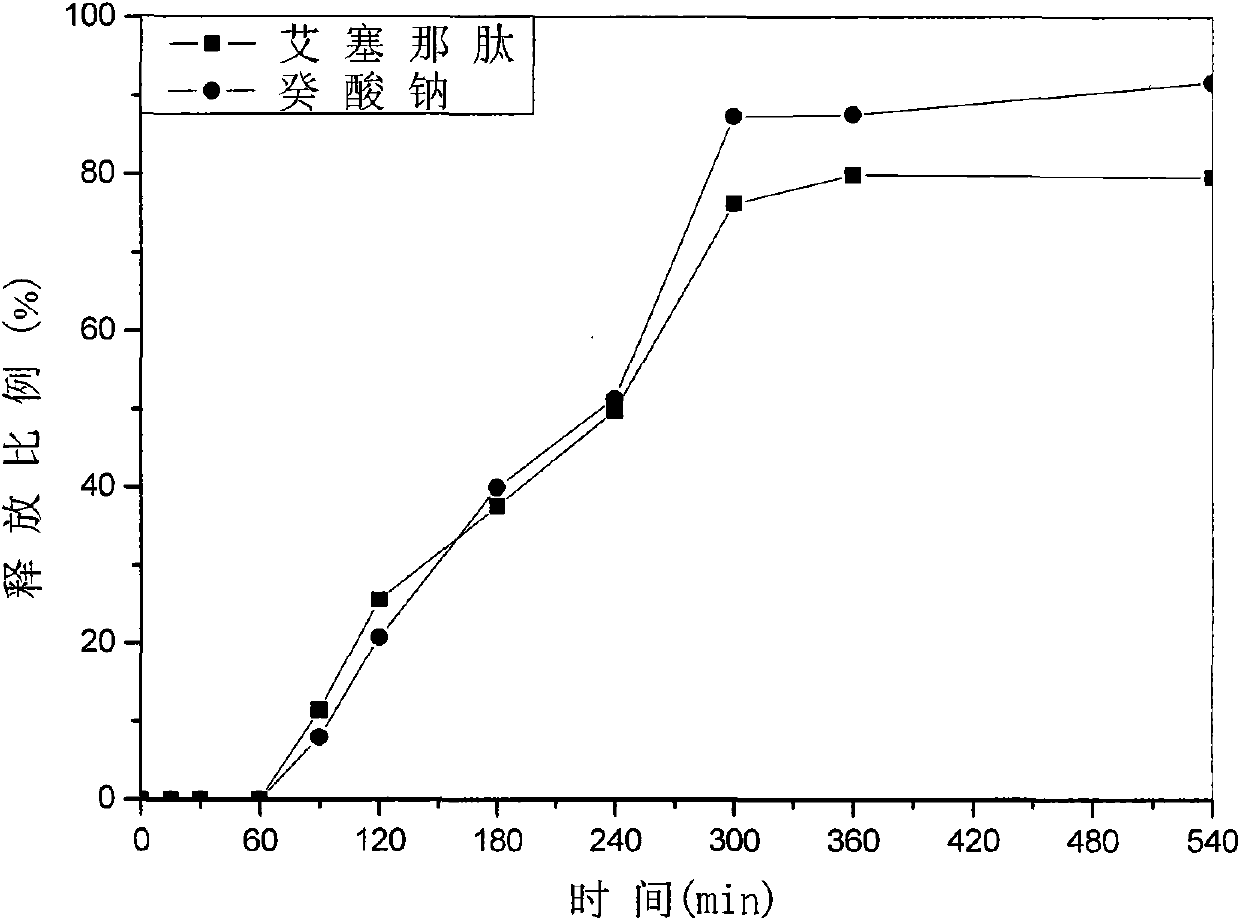
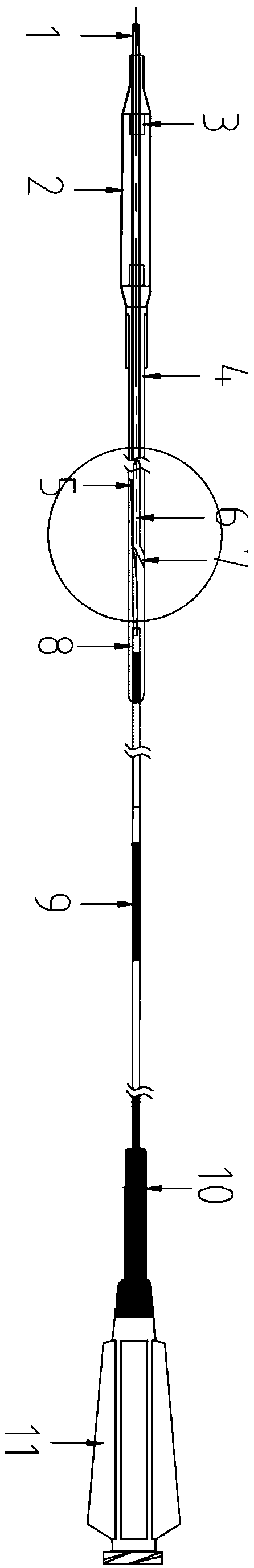
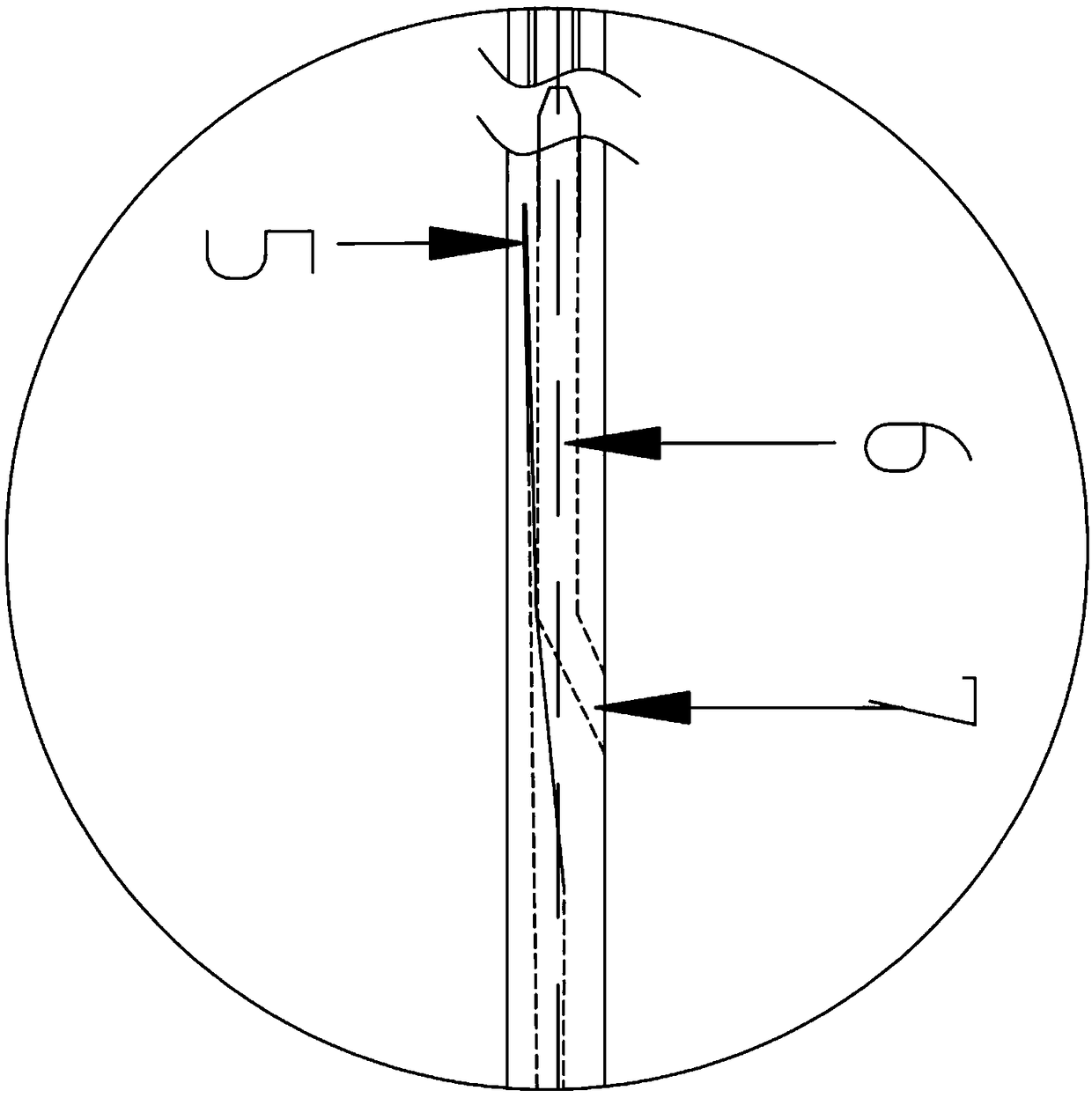
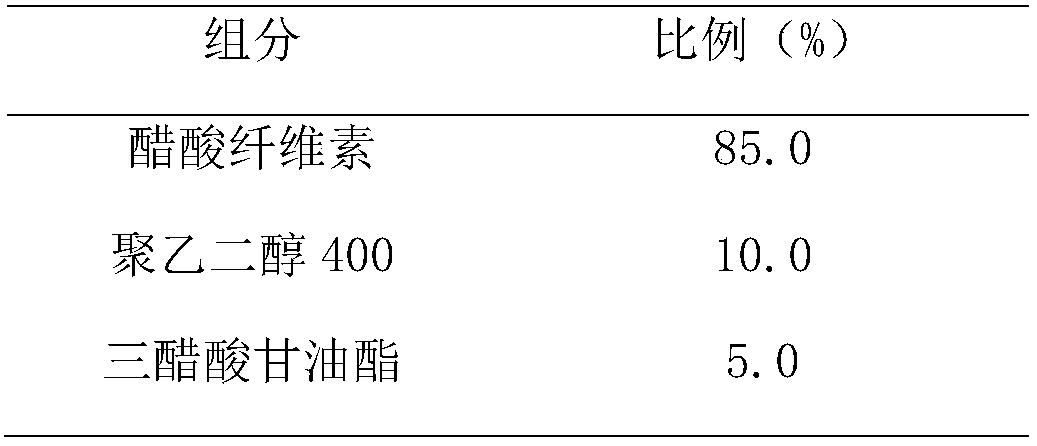
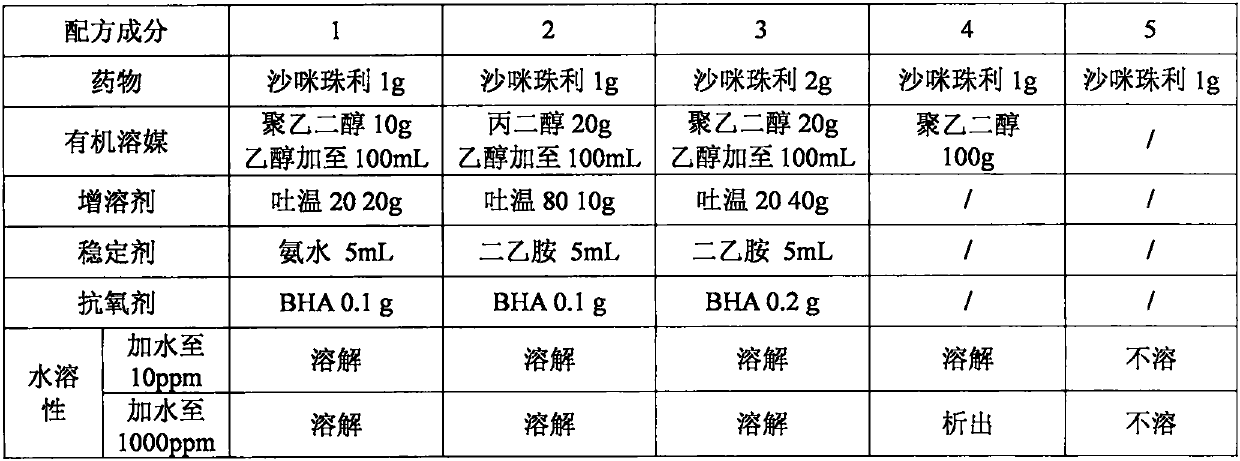
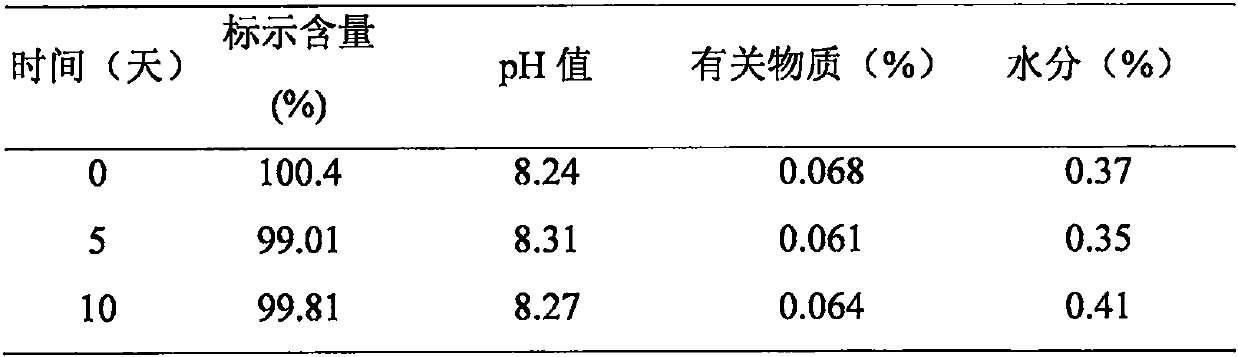
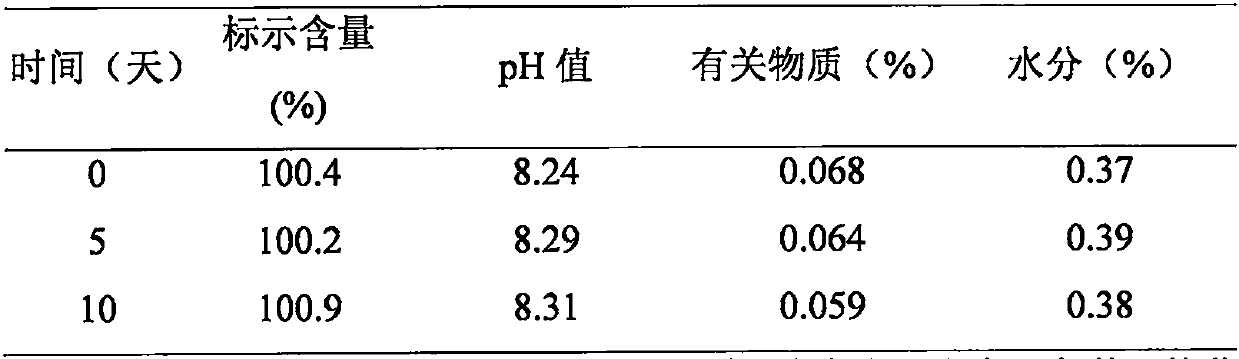
Patents
Literature
126 results about "Drug uptake" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cellular uptake and membrane permeability are important to determine if a drug is working and getting to the parts of the body it needs to. Researchers have developed a new method to better evaluate drug uptake in the body by using biosensors and fluorescent protein detectors.
Controlled regional oral delivery
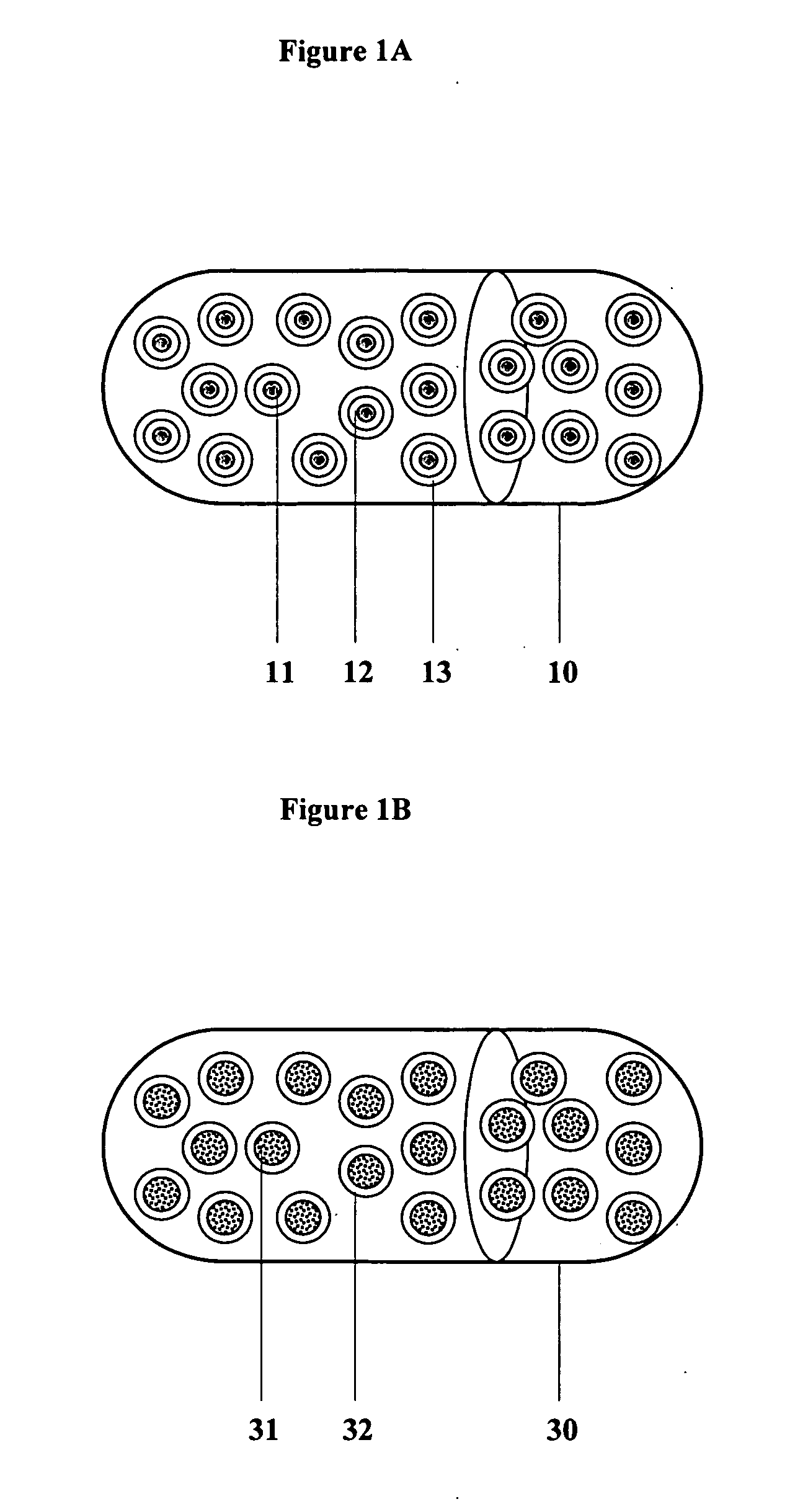
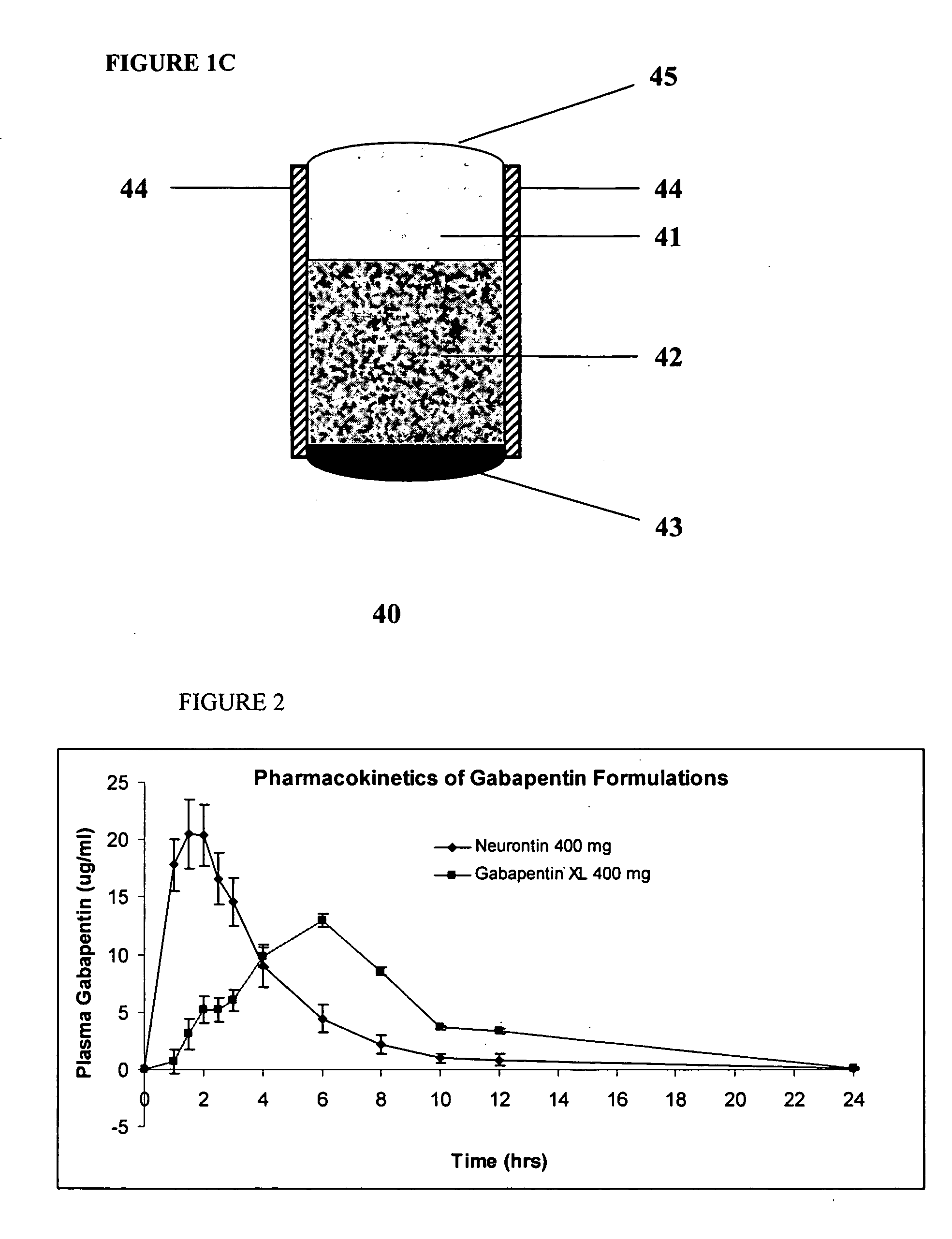
InactiveUS20060045865A1Significant variabilityLow variabilityPill deliveryGranular deliverySolubilityGabapentin
A composite formulation has been developed for selective, high efficacy delivery to specific regions of the mouth and gastrointestinal tract. The formulation is typically in the form of a tablet or capsule, which may include microparticles or beads. The formulation uses bioadhesive and controlled release elements to direct release to specific regions, where the drug is absorbed in enhanced amounts relative to the formulation in the absence of the bioadhesive and / or controlled release elements. This is demonstrated by an example showing delivery of gabapentin with a greater area under the curve (“AUC”) relative to the FDA reference immediate release drug, i.e., the AUC of the composite bioadhesive formulation is greater than 100% of the AUC of the immediate release drug. In the preferred embodiments, the formulation includes drug to be delivered, controlled release elements, and one or more bioadhesive elements. The bioadhesive polymer may be either dispersed in the matrix of the tablet or applied as a direct compressed coating to the solid oral dosage form. The controlled release elements are selected to determine the site of release. The bioadhesive components are selected to provide retention of the formulation at the desired site of uptake and administration. By selecting for both release and retention at a specific site, typically based on time of transit through the gastrointestinal tract, one obtains enhanced efficacy of uptake of the drug. This is particularly useful for drugs with narrow windows of absorption, and drugs with poor solubility such as the BCE class III and class IV drugs.
Owner:VAUNNEX
Absorption Enhancers for Drug Administration
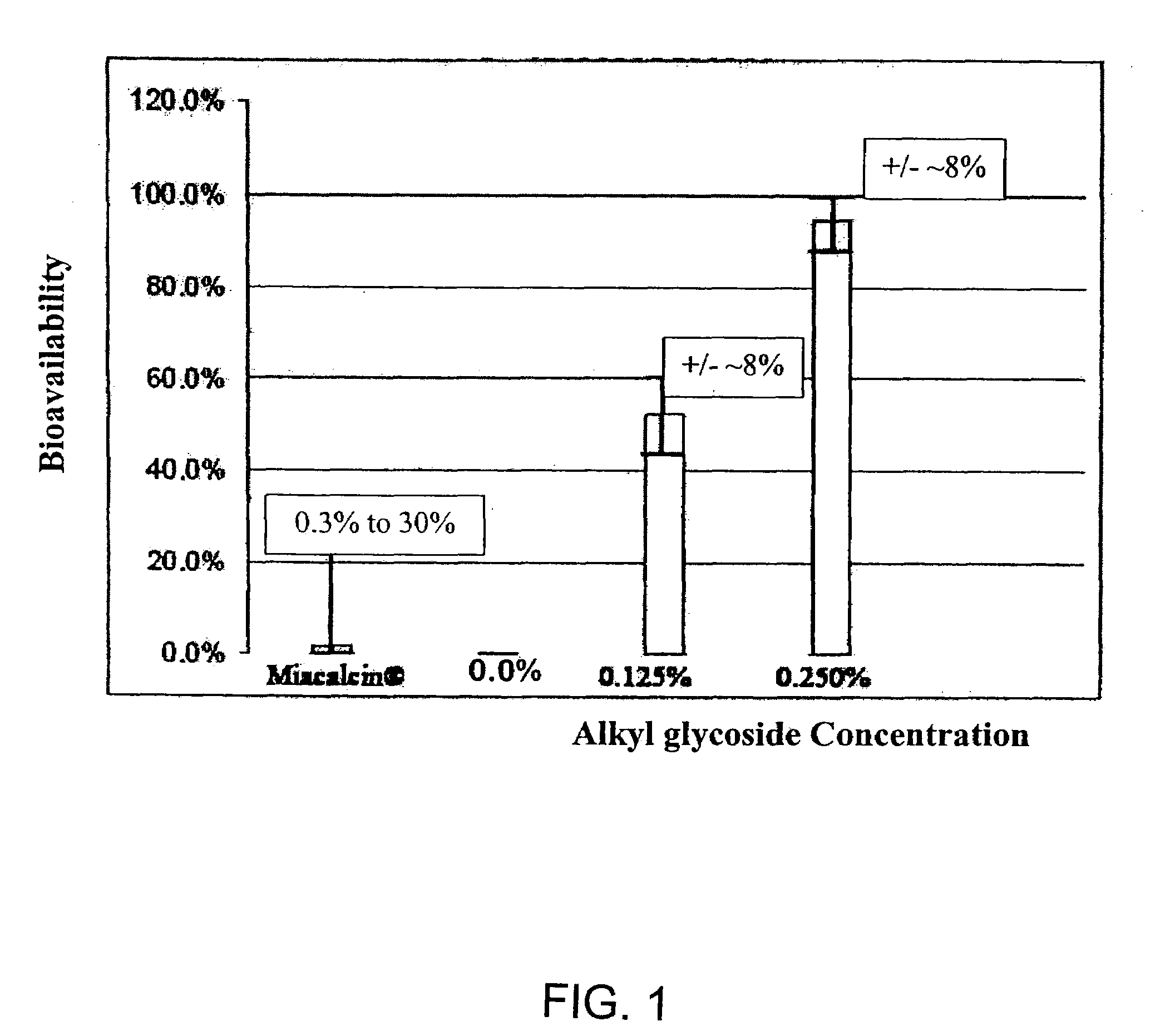
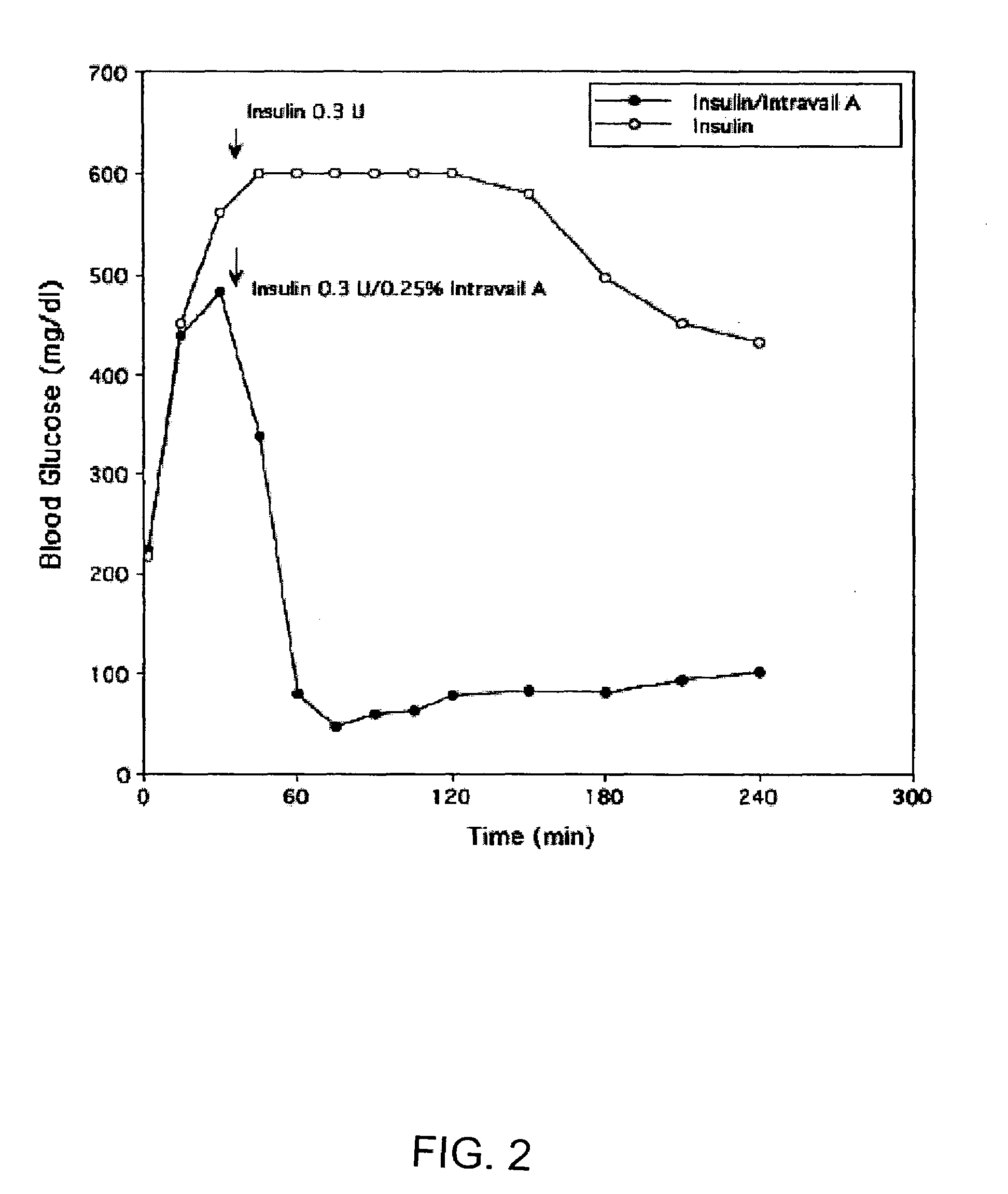
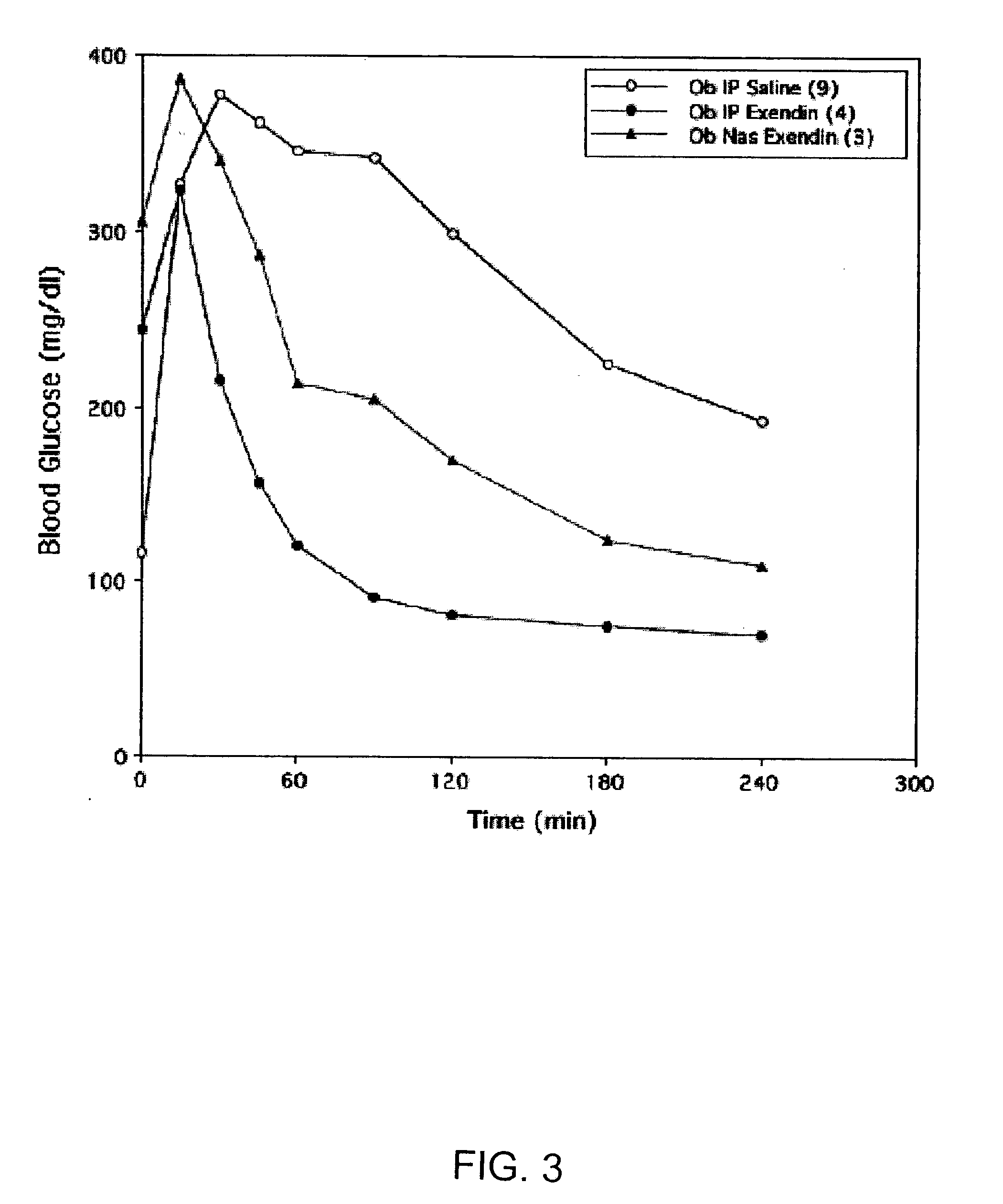
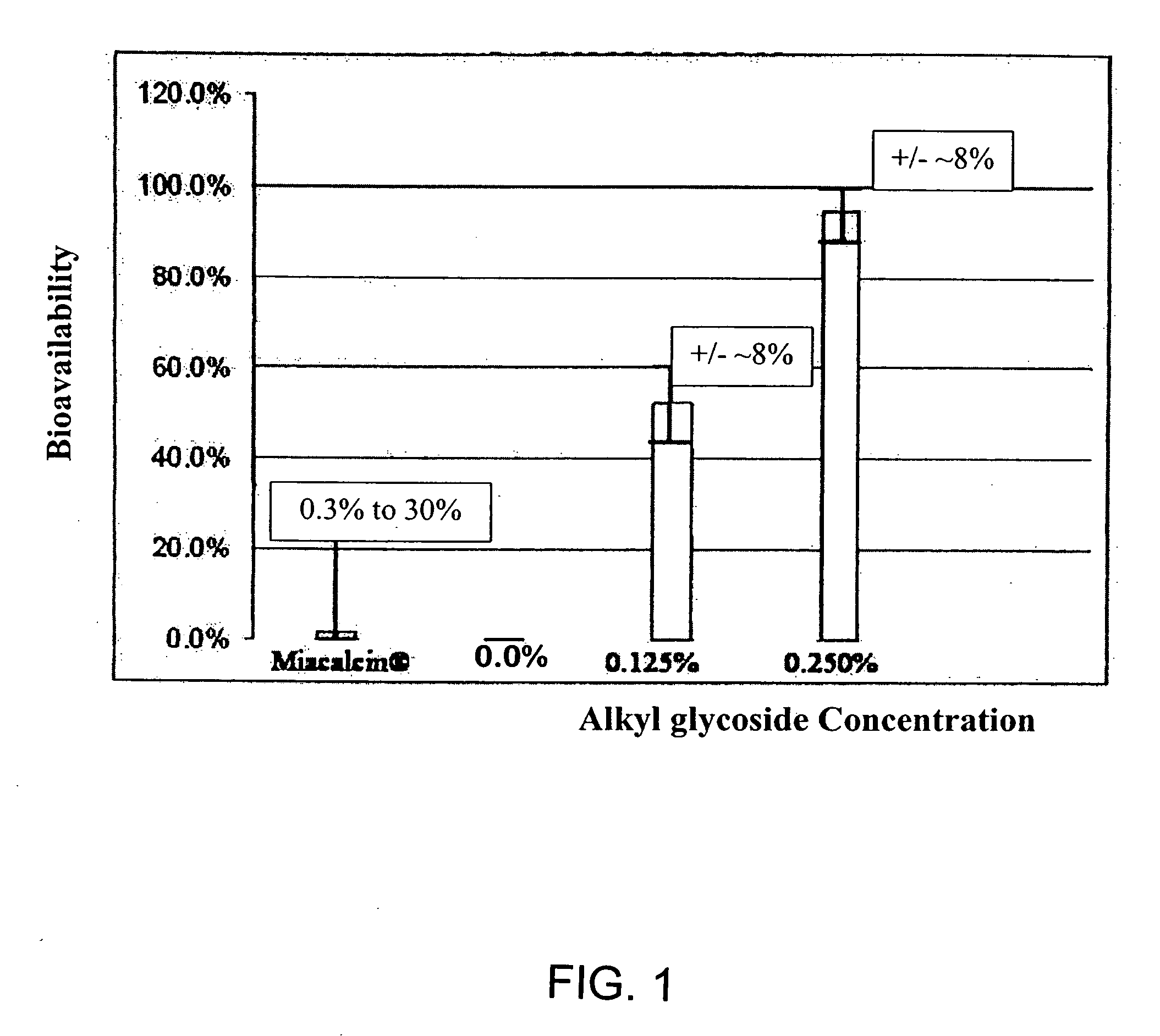
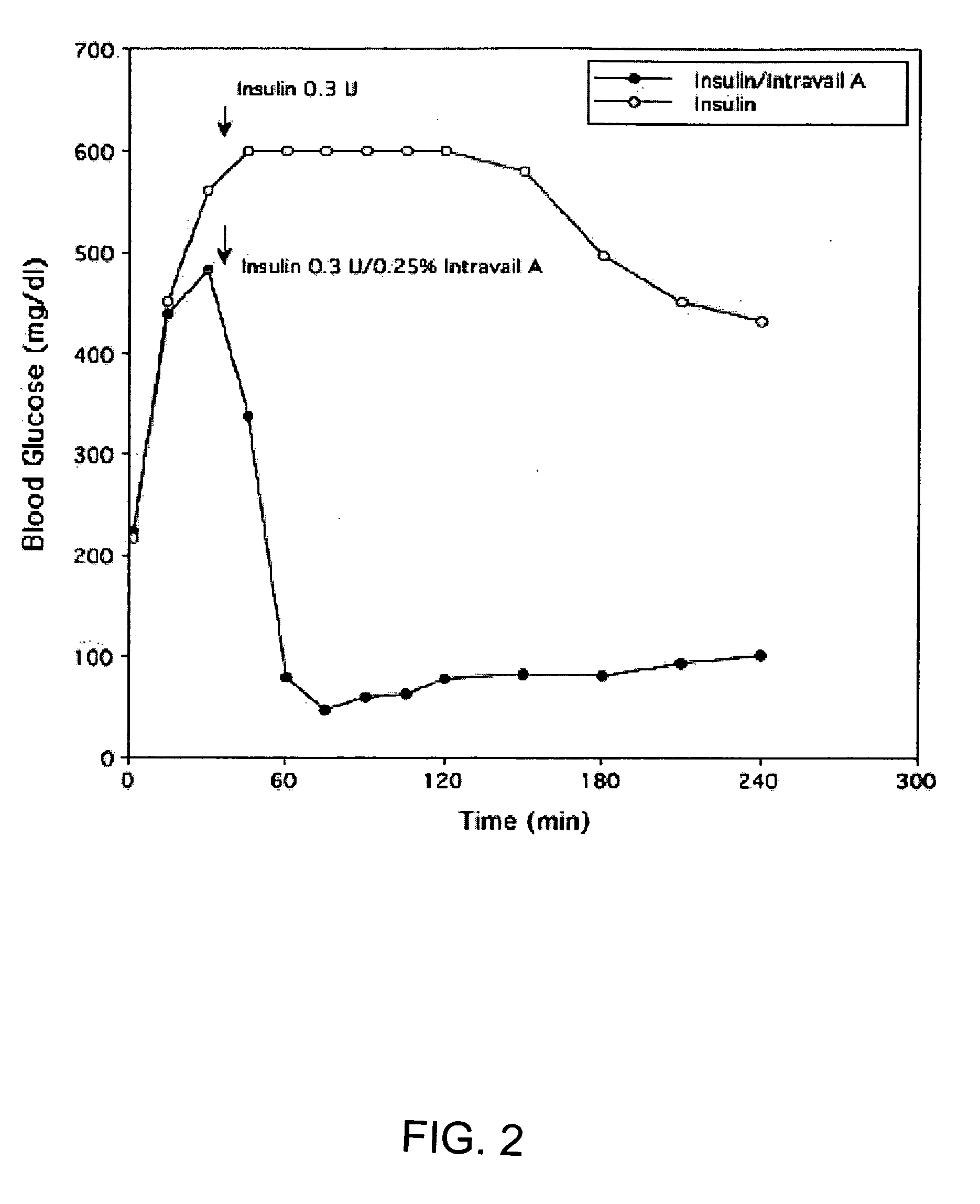
ActiveUS20080299079A1Improve absorption and bioavailabilityToxic effectsBiocideNervous disorderActive agentPancreatic hormone
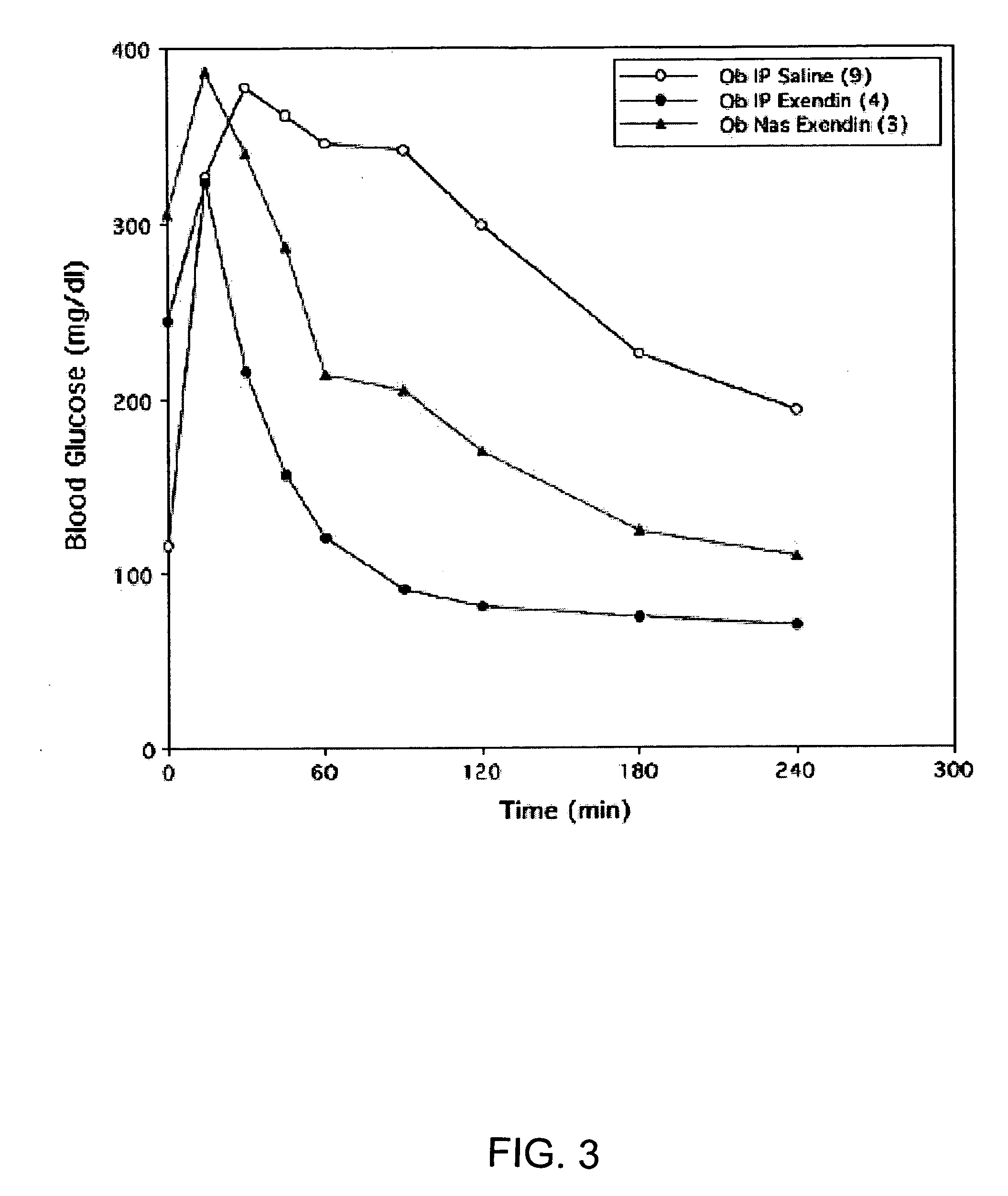
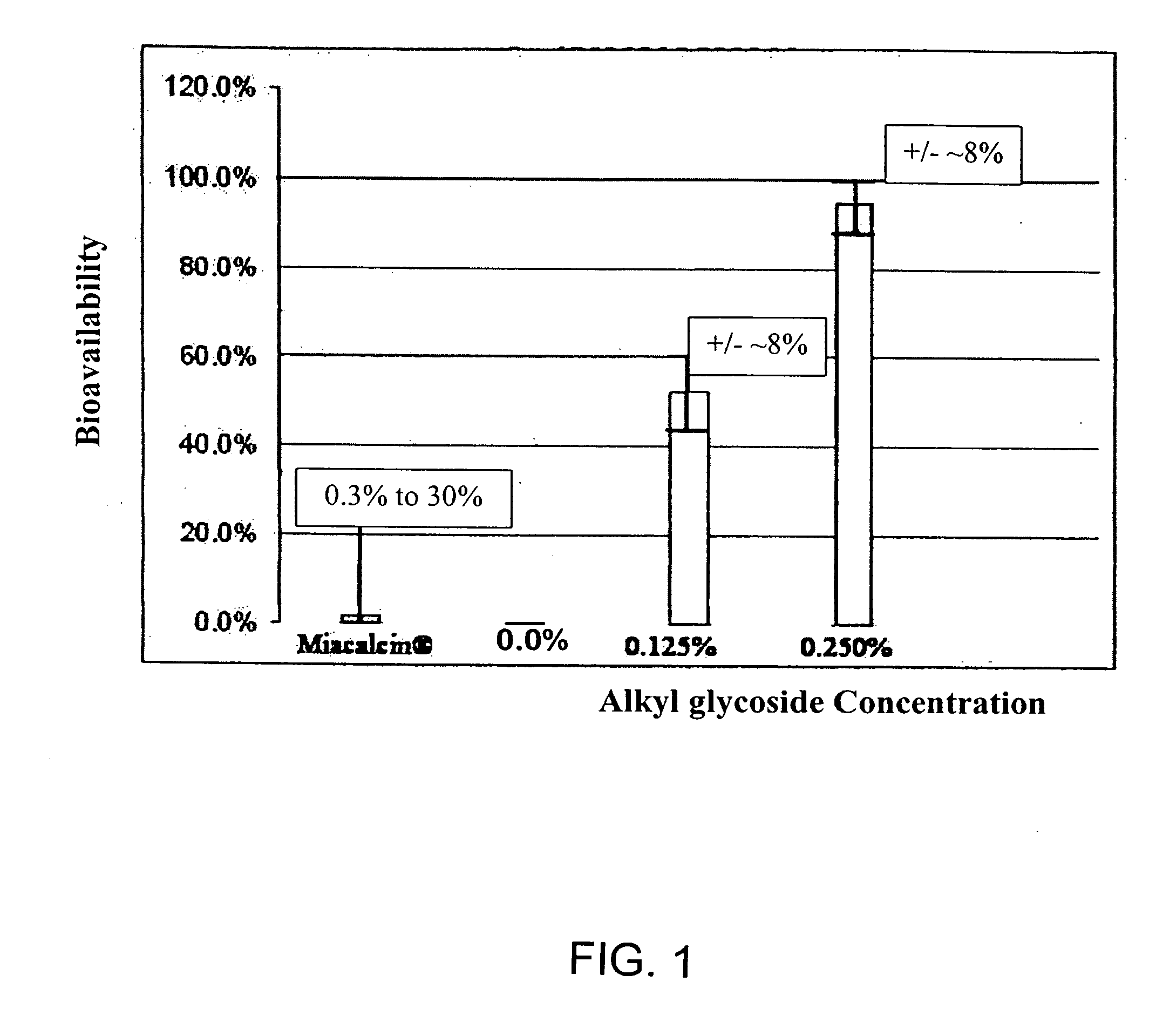
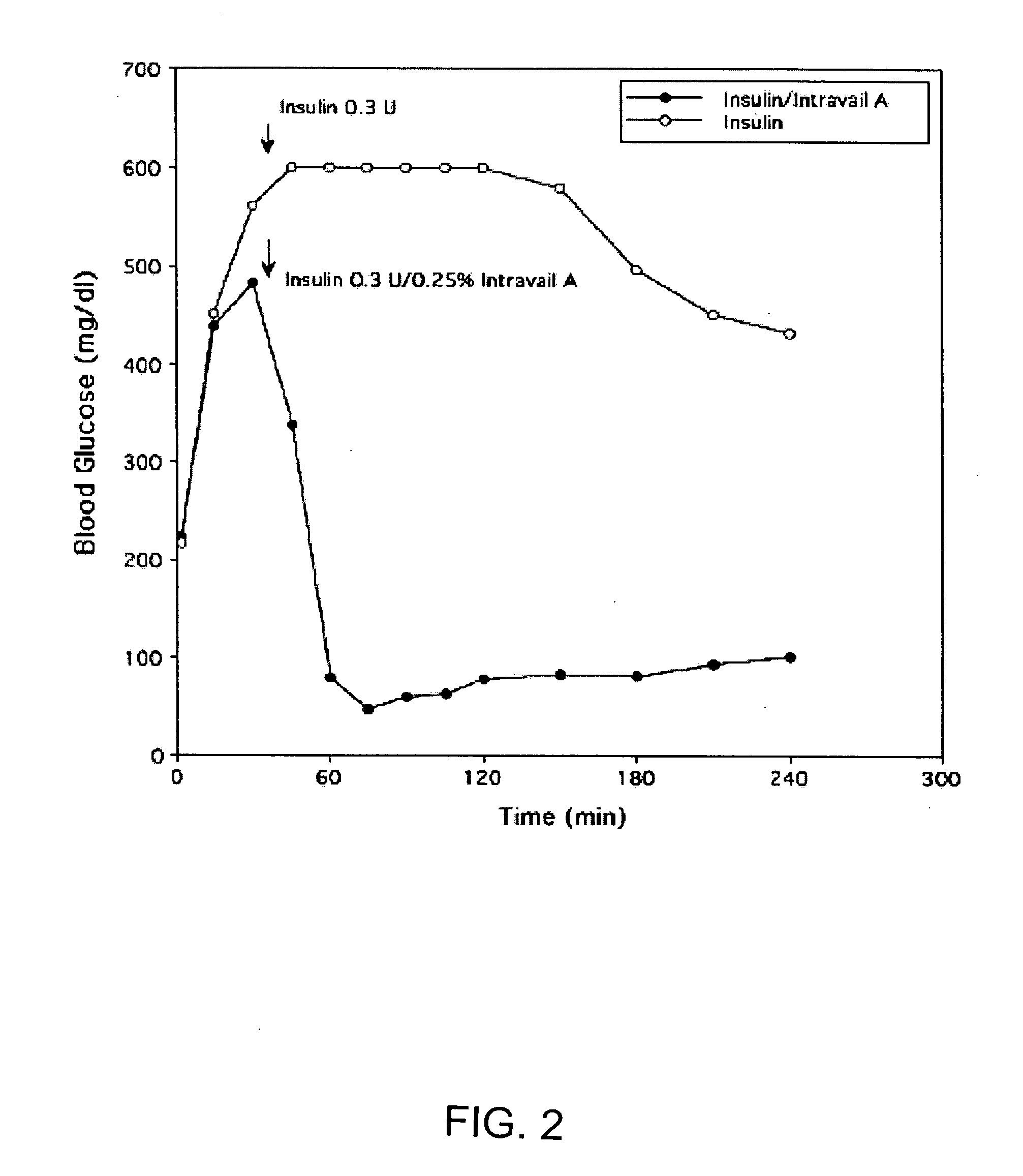
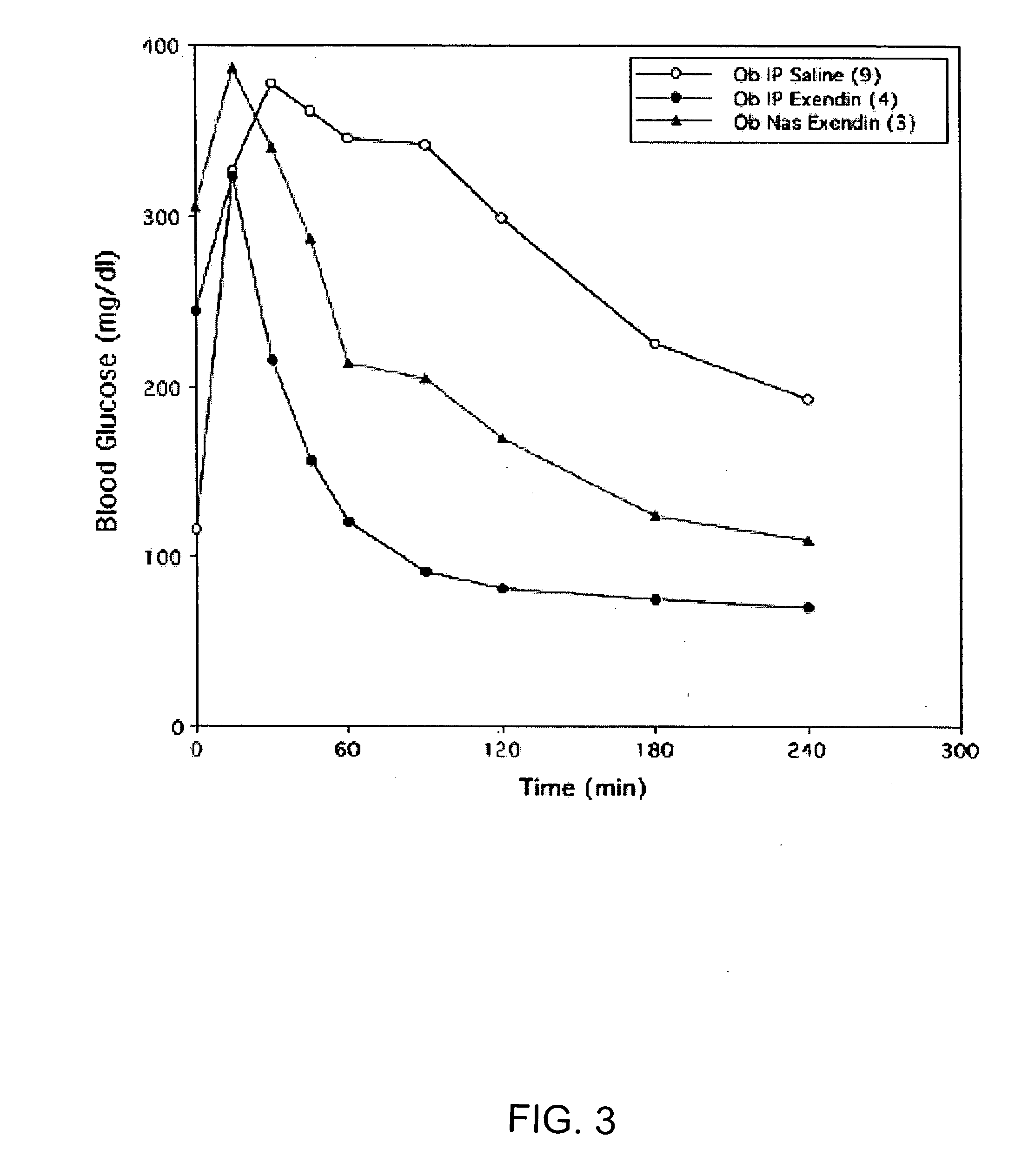
A composition including a surfactant and at least one alkyl glycoside and / or saccharide alkyl ester and a drug. The surfactant composition(s) when admixed with a drug is non-toxic and non-irritating, while stabilizing and increasing the bioavailability of the drug. The invention also provides compositions that enhance absorption of drugs via the oral, ocular, nasal, nasolacrimal, inhalation or pulmonary, oral cavity (sublingual or Buccal cell) or CSF delivery route of a patient, including but not limited to insulin, glucagon and exendin-4.
Owner:AEGIS THERAPEUTICS LLC
Absorption enhancers for drug administration
InactiveUS20060045869A1Avoid effectIncrease absorption and bioavailability of drugBiocideNervous disorderDrugDrug administration
A composition including a surfactant and at least one alkyl glycoside and / or saccharide alkyl ester and a drug. The surfactant composition(s) when admixed with a drug is non-toxic and non-irritating, while stabilizing and increasing the bioavailability of the drug. The invention also provides compositions that enhance absorption of drugs via the oral, ocular, nasal, nasolacrimal, inhalation or pulmonary, oral cavity (sublingual or Buccal cell) or CSF delivery route of a patient, including but not limited to insulin, glucagon and exendin-4.
Owner:AEGIS THERAPEUTICS LLC +1
Absorption enhancers for drug administration
InactiveUS20060045868A1Improve absorption and bioavailabilityToxic effectsPowder deliveryBiocideInhalationDrug administration
A composition including a surfactant and at least one alkyl glycoside and / or saccharide alkyl ester and a drug. The surfactant composition(s) when admixed with a drug is non-toxic and non-irritating, while stabilizing and increasing the bioavailability of the drug. The invention also provides compositions that enhance absorption of drugs via the oral, ocular, nasal, nasolacrimal, inhalation or pulmonary, oral cavity (sublingual or Buccal cell) or CSF delivery route of a patient, including but not limited to insulin, glucagon and exendin-4.
Owner:AEGIS THERAPEUTICS LLC +1
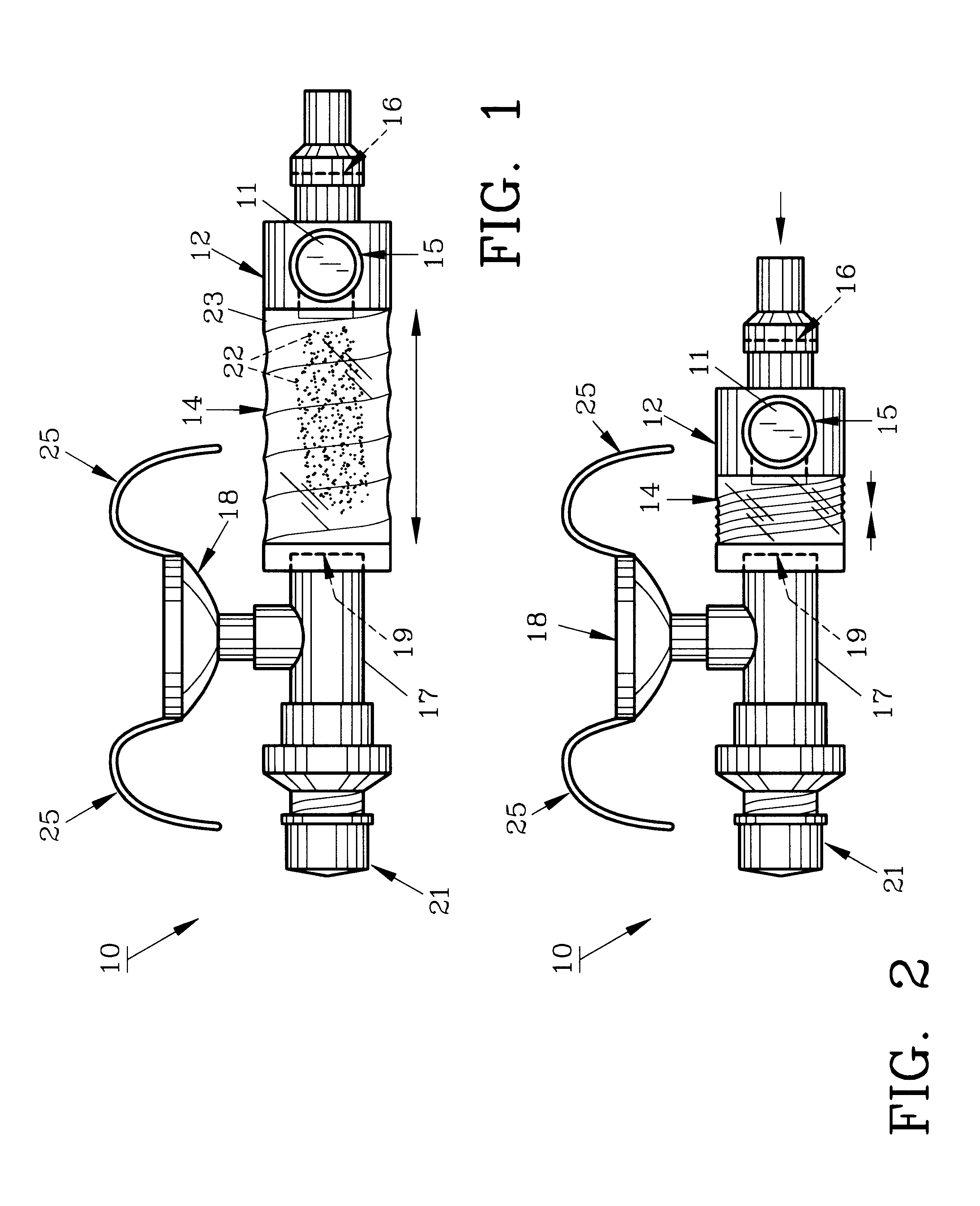
Inhalation therapy assembly and method
InactiveUS20020069870A1Conveniently and easily allowChemical protectionLighting and heating apparatusDose deliveryInhalation
The inhalation therapy assembly and method of use described herein increases the efficiency of metered dose inhalers by allowing delivery of the doses to a collapsible reservoir which can be manually pumped, ensuring that medicants contained therein are properly and completely delivered to the patient. Terminal and proximal valves of the one-way diaphragm type allow flow of the aerosol medicants while preventing improper expulsion. An exhalation valve is adjustable to ensure the patient exhales suitably to permit proper medicant absorption. A conventional metered dosage inhaler having an approved FDA canister provides proper dosage to the patient and is joined to the collapsible reservoir by a connector having a plurality of apertures for receiving the MDI and an accessory T-fitting.
Owner:FARMER MEDICAL
Inhalation therapy assembly and method
InactiveUS6494202B2Conveniently and easily allowBreathing masksRespiratory masksInhalationDose delivery
The inhalation therapy assembly and method of use described herein increases the efficiency of metered dose inhalers by allowing delivery of the doses to a collapsible reservoir which can be manually pumped, ensuring that medicants contained therein are properly and completely delivered to the patient. Terminal and proximal valves of the one-way diaphragm type allow flow of the aerosol medicants while preventing improper expulsion. An exhalation valve is adjustable to ensure the patient expires suitably to permit proper medicant absorption.
Owner:FARMER MEDICAL
Methods of enhancing drug delivery and effectiveness of therapeutic agents
ActiveUS20120076862A1Promote absorptionConvenient treatmentPowder deliveryHeavy metal active ingredientsDiseaseCo administration
The present invention in one aspect provides methods of enhancing uptake of a therapeutic agent in a target tissue as well as methods of treating a disease (such as cancer) or enhancing effectiveness of treatment with a therapeutic agent in an individual by co-administering a composition comprising nanoparticles comprising albumin and a poorly water soluble drug such as a taxane with the therapeutic agent. The present invention in another aspect provides a method of treatment or a method of selecting patients for treatment of a disease (such as cancer) with the combination of a therapeutic agent and a composition comprising nanoparticles comprising albumin and a poorly water soluble drug such as a taxane based on one or more characteristics of the target tissue that correlates or indicates the capability of getting enhanced therapeutic agent uptake as a result of the co-administration of the taxane nanoparticle composition in the target tissue (referred to as “the drug uptake capability”). Also provided are pharmaceutical compositions, article of manufacture, and kits useful for methods described herein.
Owner:ABRAXIS BIOSCI LLC
Controlled release oral drug delivery system
The invention relates to synchronous drug delivery composition comprising a polymeric matrix which comprises hydrogel blended with a hydrophobic polymer, so as to form an erodible matrix, a drug, and, optionally, an agent which enhances intestinal drug absorption and / or an agent which inhibits intestinal drug degradation,wherein erosion of the erodible matrix, permits synchronous release of the drug, the hydrogel and the intestinal drug absorption agent and / or the agent which inhibits intestinal drug degradation.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Methods of enhancing drug delivery and effectiveness of therapeutic agents
InactiveUS20140017323A1Uptake of the therapeutic agent to a target tissuePromote absorptionBiocideOrganic active ingredientsDiseaseNanoparticle
The present invention in one aspect provides methods of enhancing uptake of a therapeutic agent in a target tissue as well as methods of treating a disease (such as cancer) or enhancing effectiveness of treatment with a therapeutic agent in an individual by co-administering a composition comprising nanoparticles comprising albumin and a poorly water soluble drug such as a taxane with the therapeutic agent. The present invention in another aspect provides a method of treatment or a method of selecting patients for treatment of a disease (such as cancer) with the combination of a therapeutic agent and a composition comprising nanoparticles comprising albumin and a poorly water soluble drug such as a taxane based on one or more characteristics of the target tissue that correlates or indicates the capability of getting enhanced therapeutic agent uptake as a result of the co-administration of the taxane nanoparticle composition in the target tissue (referred to as “the drug uptake capability”). Also provided are pharmaceutical compositions, article of manufacture, and kits useful for methods described herein.
Owner:ABRAXIS BIOSCI LLC
Powder Composition for nasal administration
There is provided a powder composition for nasal administration comprising a drug having a particle size of less than 10 mum or a lyophilized drug, a water-absorbing and water-slightly soluble base material, and a water-absorbing and gel-forming base materials. The composition has excellent drug absorbability.
Owner:TEIJIN LTD
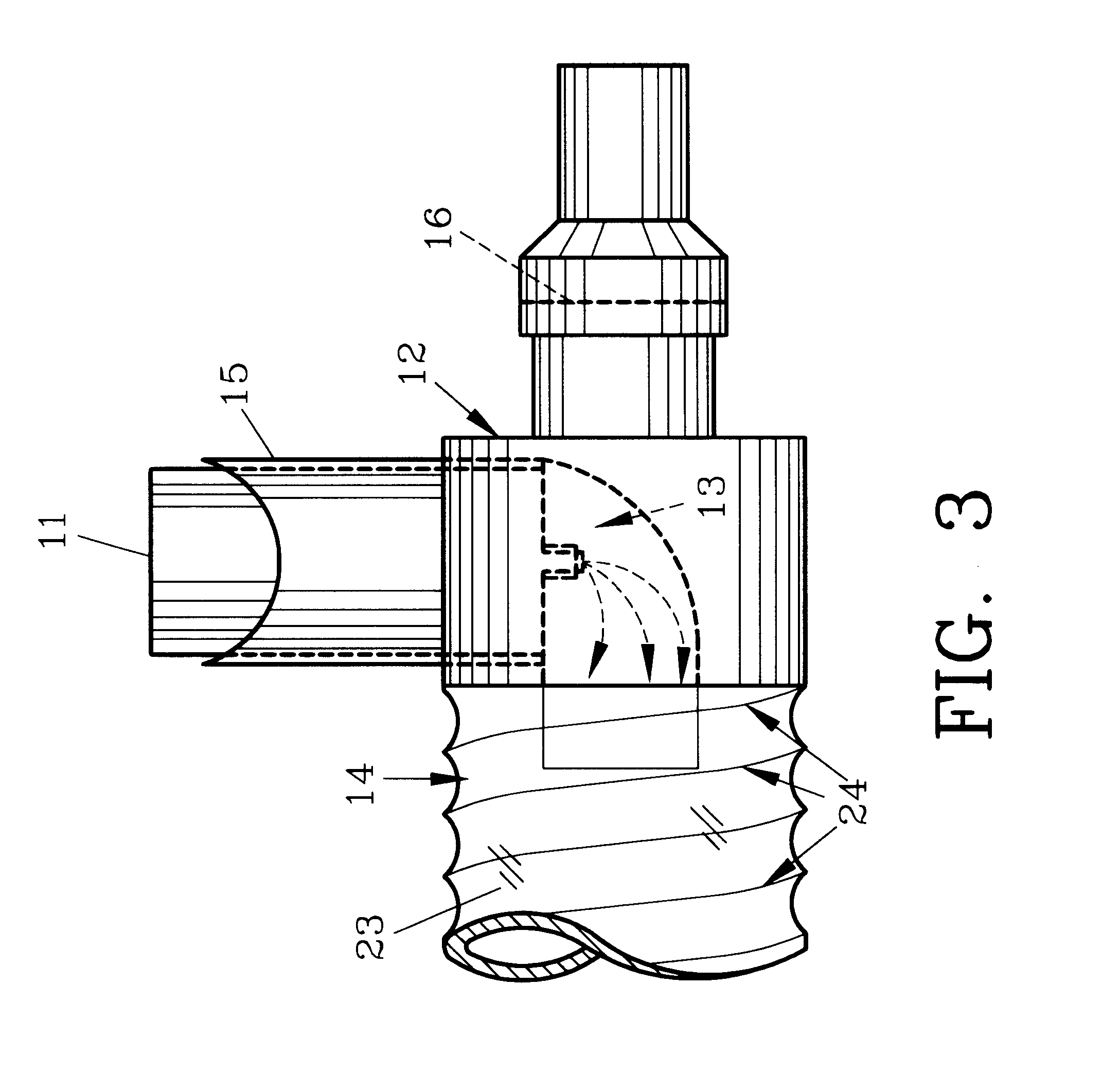
Bifurcated catheter for agent delivery and method of agent delivery
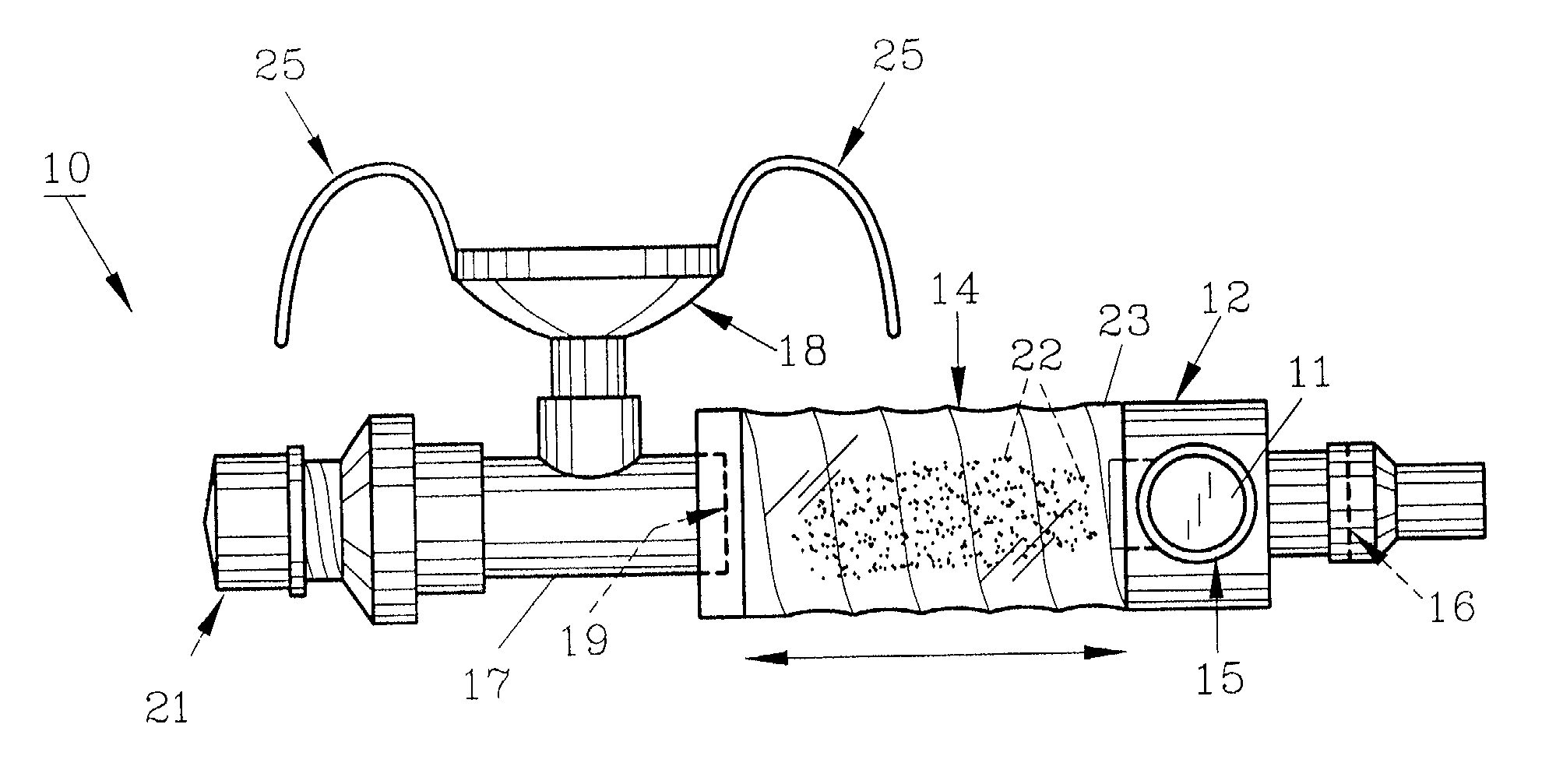
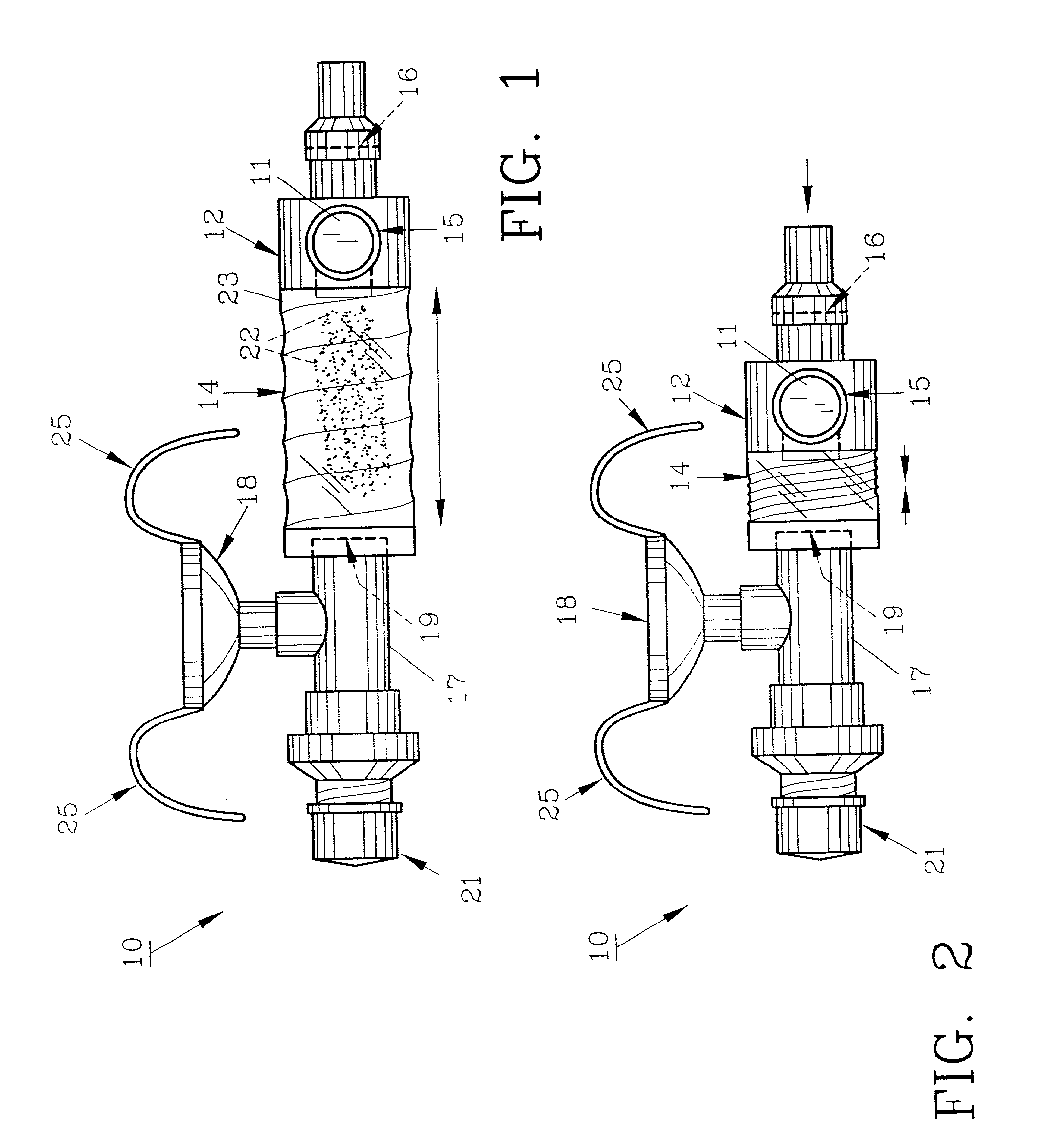
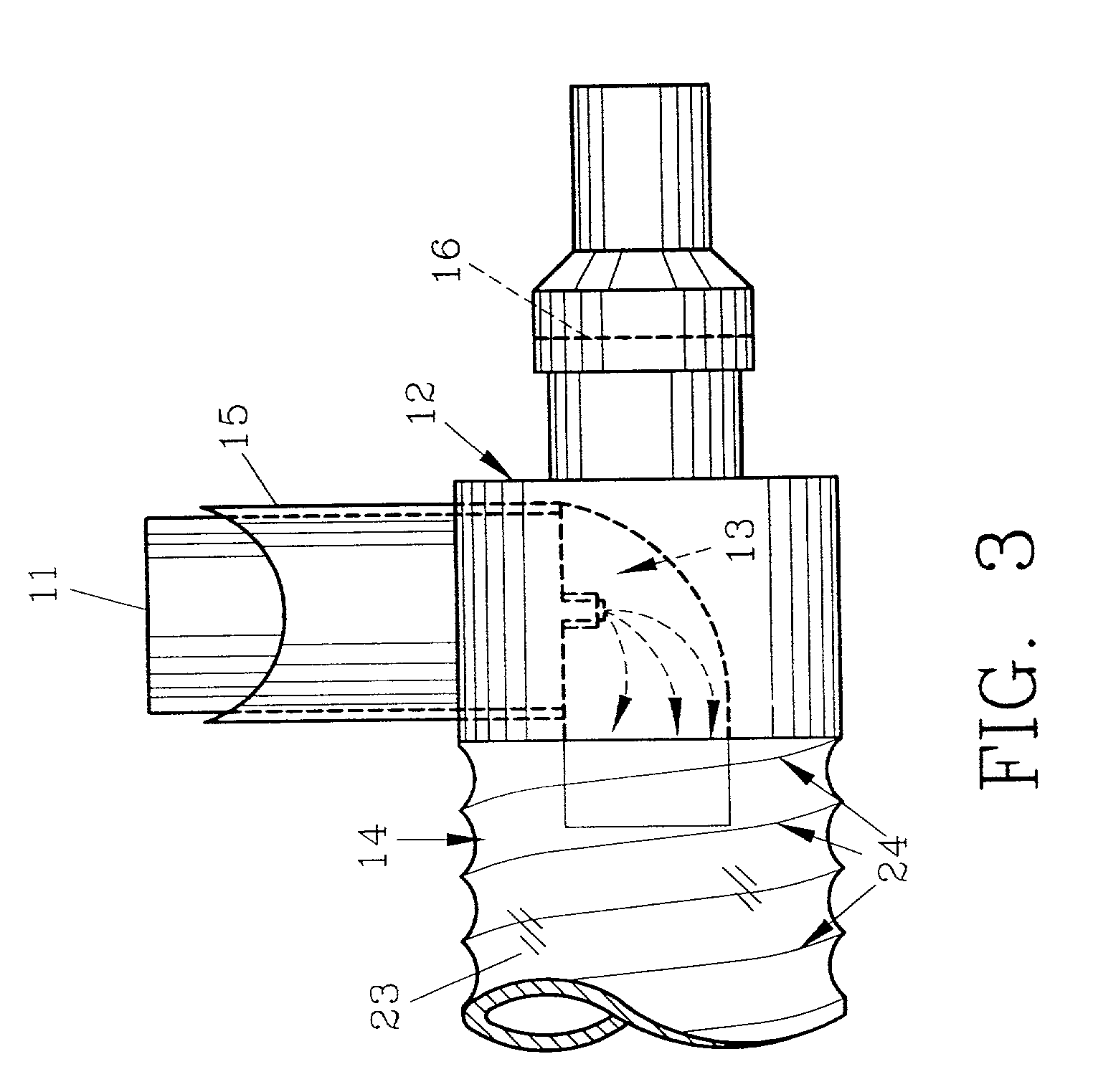
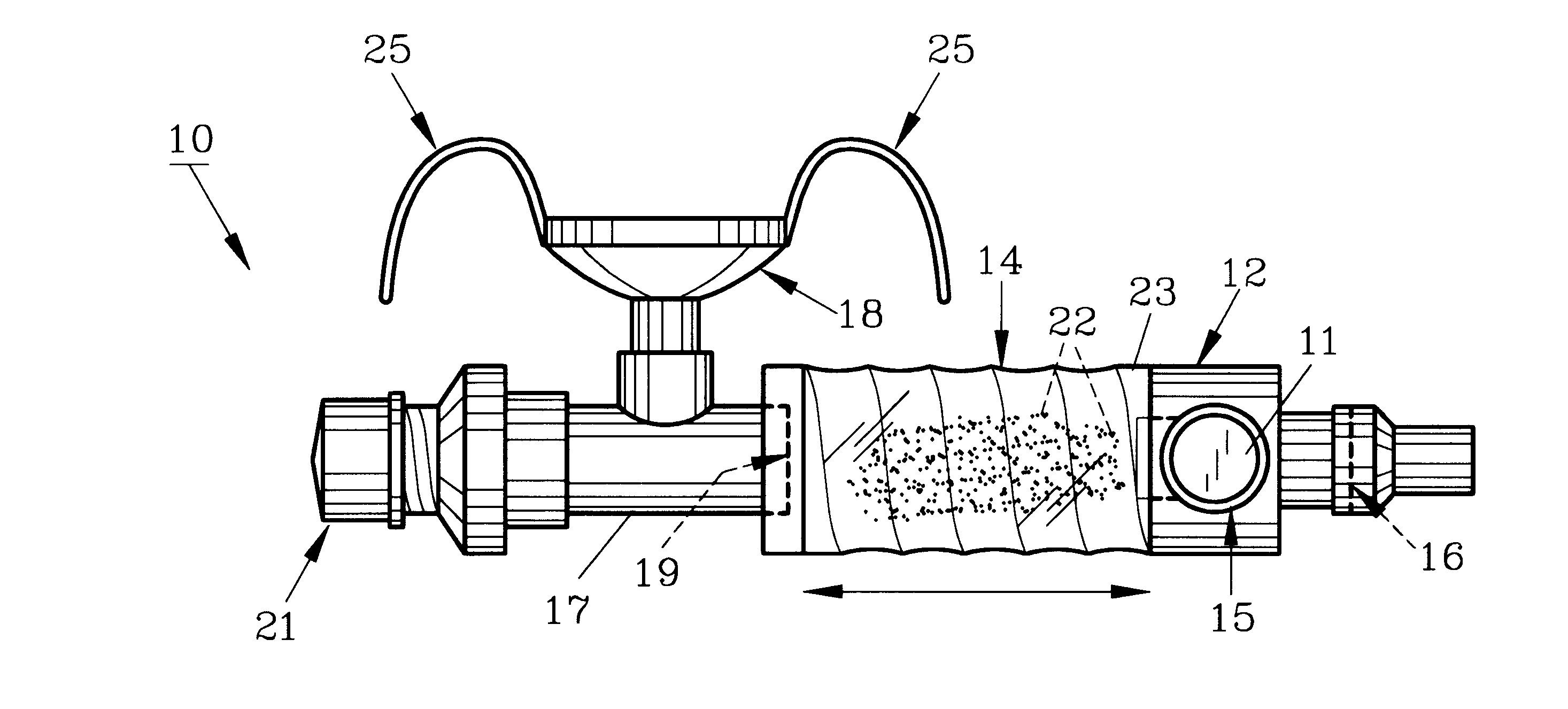
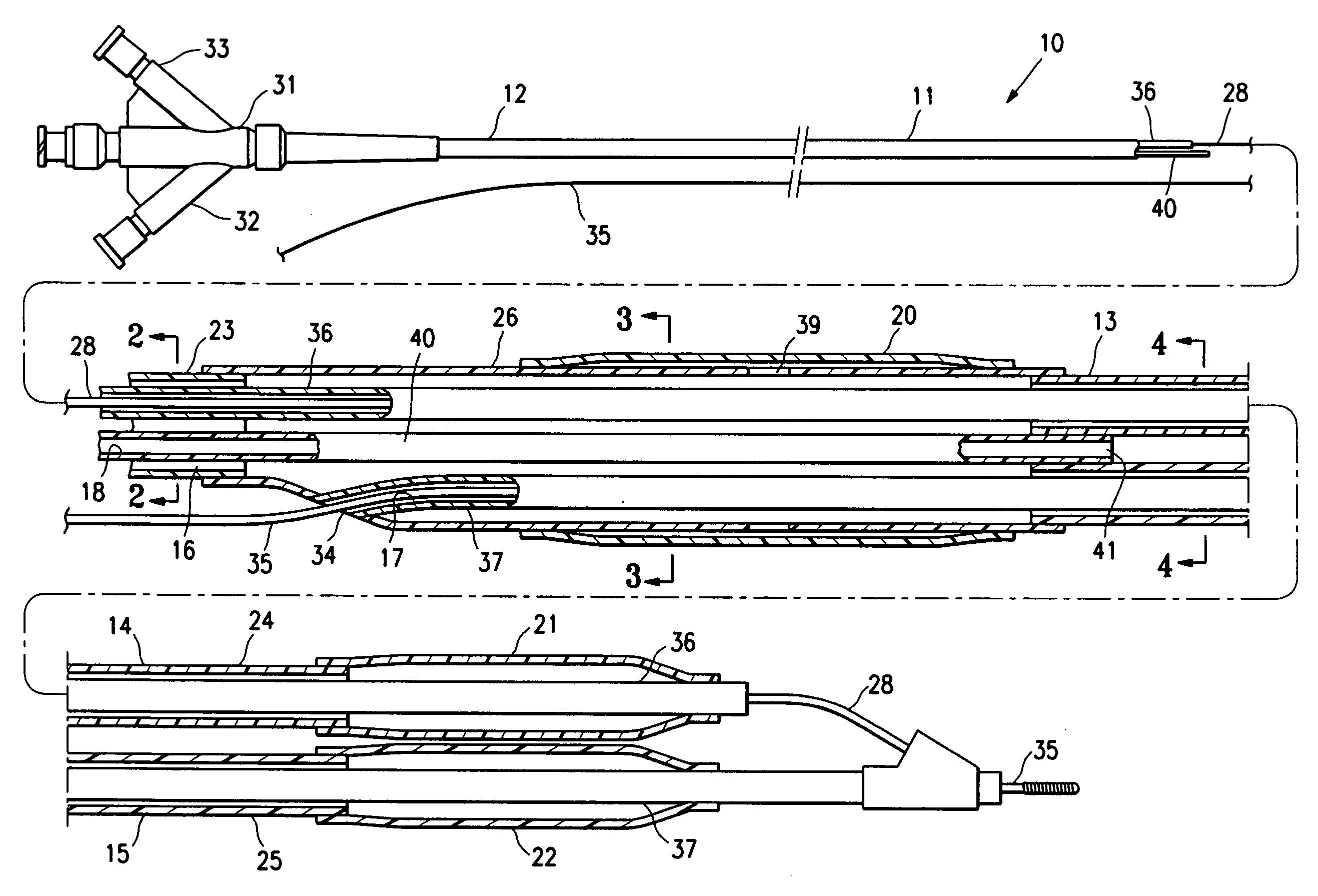
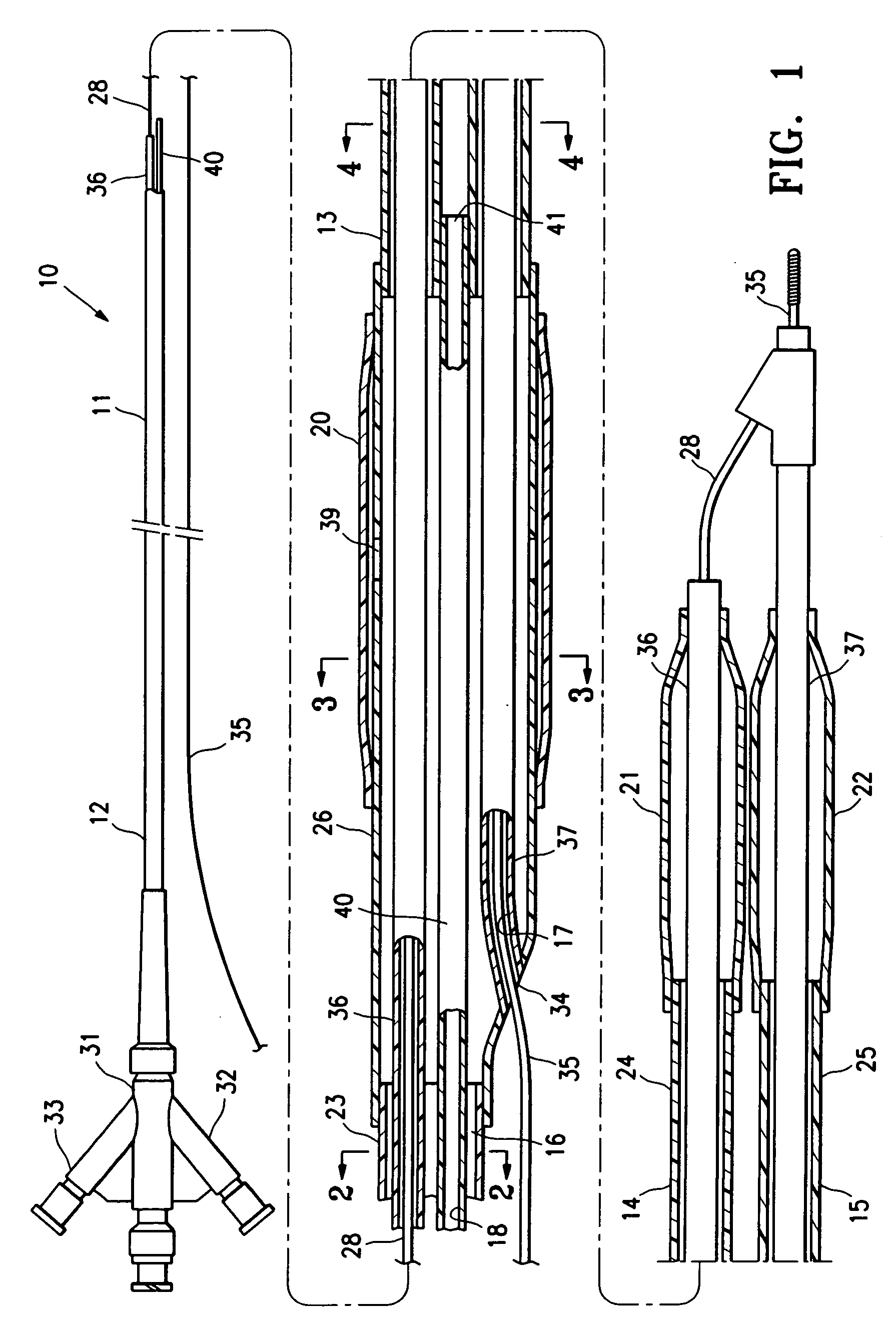
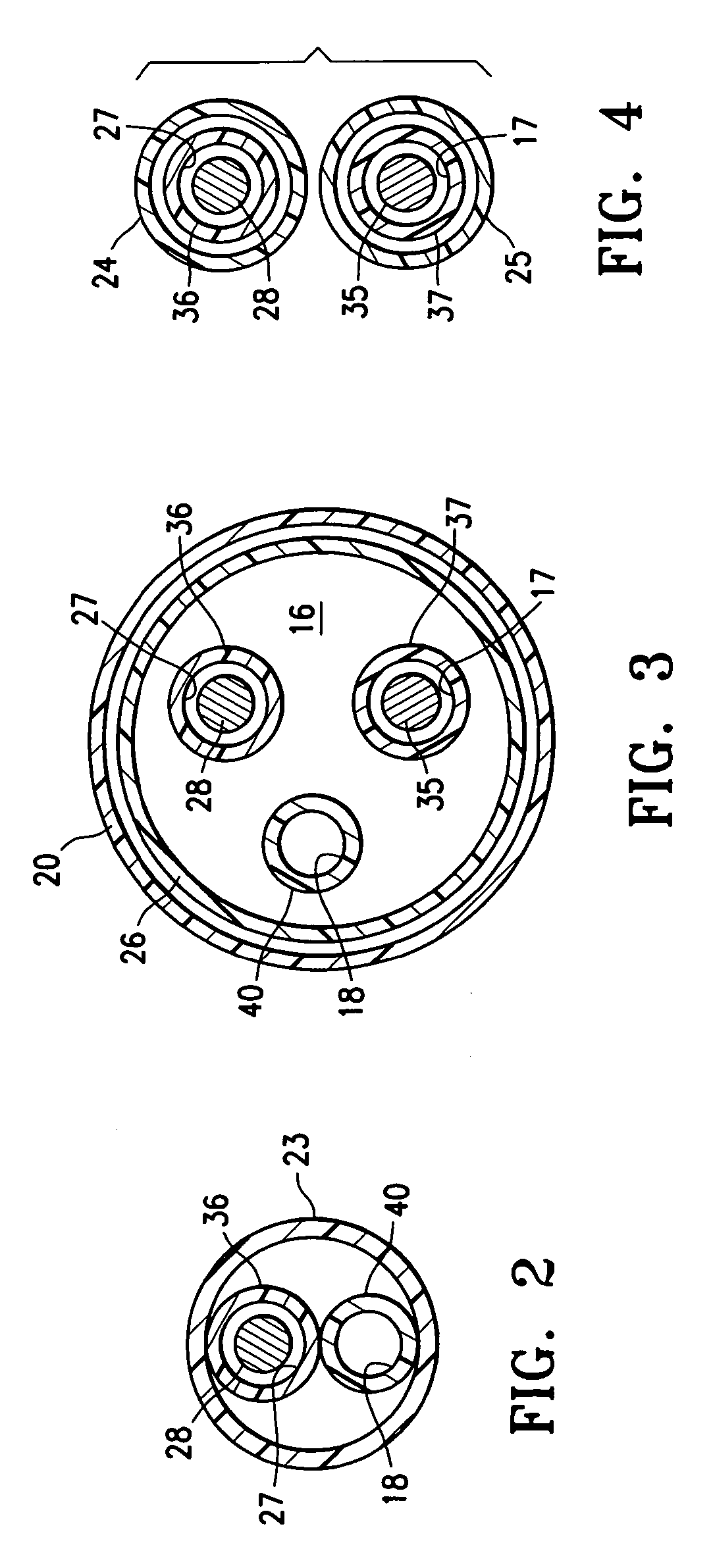
InactiveUS20070142819A1Maximizing efficiency of drugMaximize efficiencyStentsBalloon catheterCatheter deviceDrug uptake
A bifurcated catheter having a branched distal shaft section and one or more porous or nonporous balloons, and combinations thereof, which is configured for delivery of an agent to a patient's bifurcated body lumen. Another aspect of the invention is directed to a method of delivering an agent to a patient's body lumen which facilitates maximizing the efficiency of drug uptake into the tissue at the desired site within the patient's body lumen.
Owner:ABBOTT CARDIOVASCULAR
Sublingual buccal effervescent
InactiveUS20050064030A1Organic active ingredientsWood working apparatusOral medicationAbsorption drugs
A pharmaceutical dosage form adapted to supply a medicament to the oral cavity for buccal, sublingual or gingival absorption of the medicament which contains an orally administrable medicament in combination with an effervescent for use in promoting absorption of the medicament in the oral cavity. The use of an additional pH adjusting substance in combination with the effervescent for promoting the absorption drugs is also disclosed.
Owner:CIMA LABS
Materials and methods for drug delivery and uptake
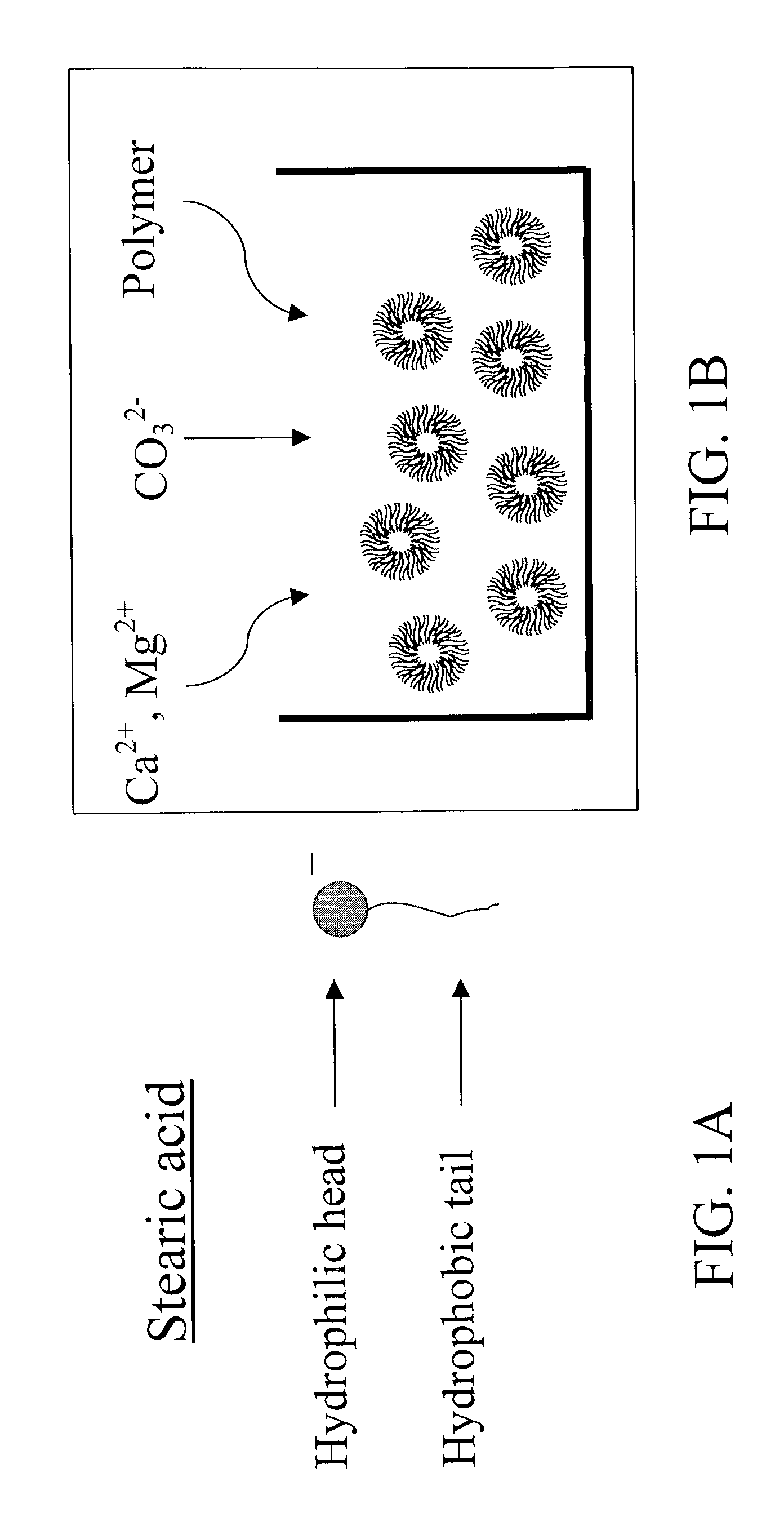
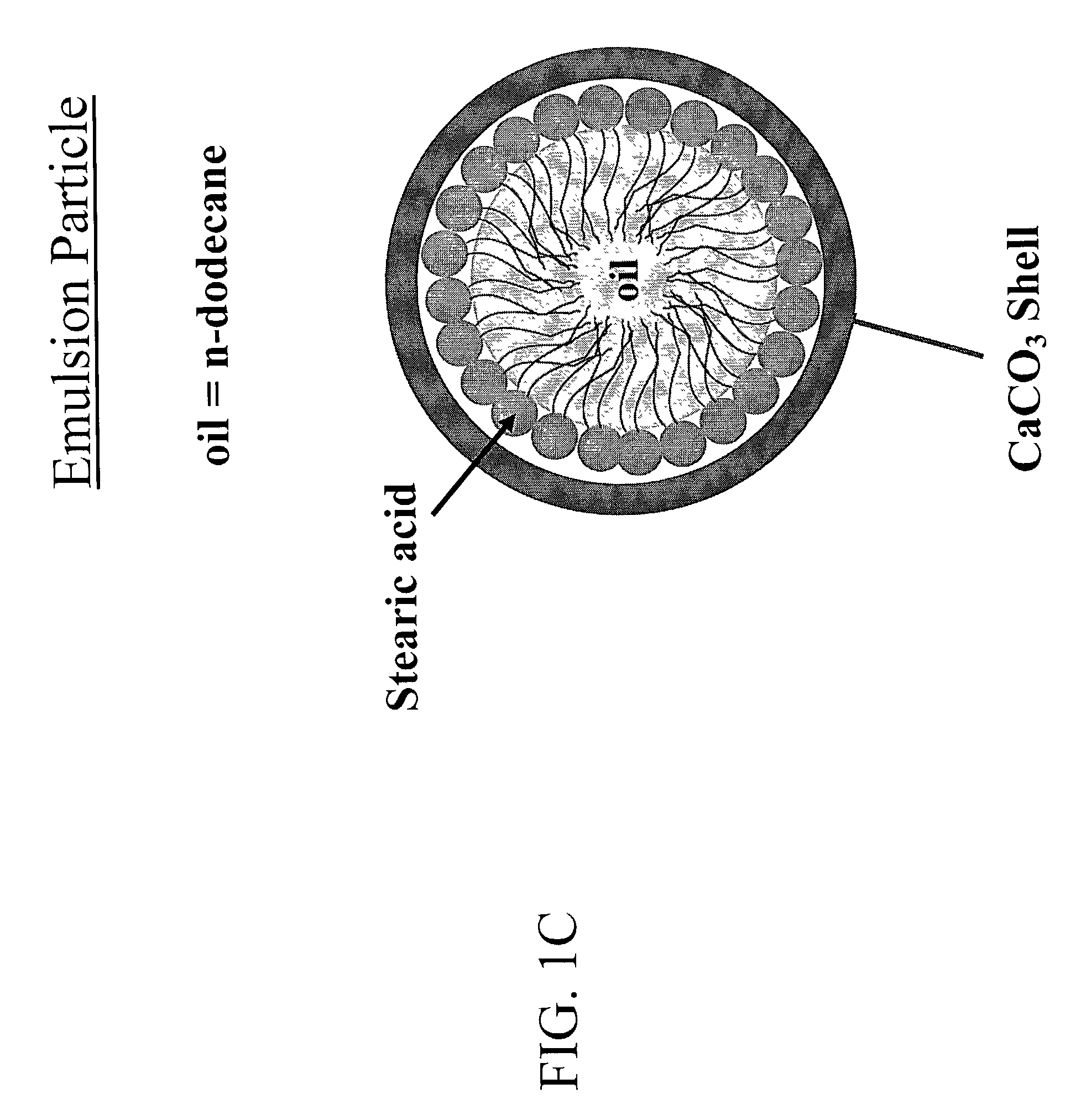
The subject invention pertains to novel materials and methods for use in delivering and sequestering substances, such as pharmacological agents, within a patient. One aspect of the invention is directed towards core-shell particles having a core encapsulated within a calcium carbonate shell, with an intermediate layer composed of an amphiphilic compound surrounding the core. When the particles of the subject invention are administered to a patient, they are capable of removing lipophilic drugs by absorption of the drug through their mineral shell and into their core. The particles of the subject invention can also be administered to a patient as controlled release, drug delivery vehicles. Thus, in another aspect, the subject invention concerns a method of delivering pharmacological agents by administering the core-shell particles of the subject invention to a patient in need of such administration.
Owner:UNIV OF FLORIDA RES FOUNDATION INC +1
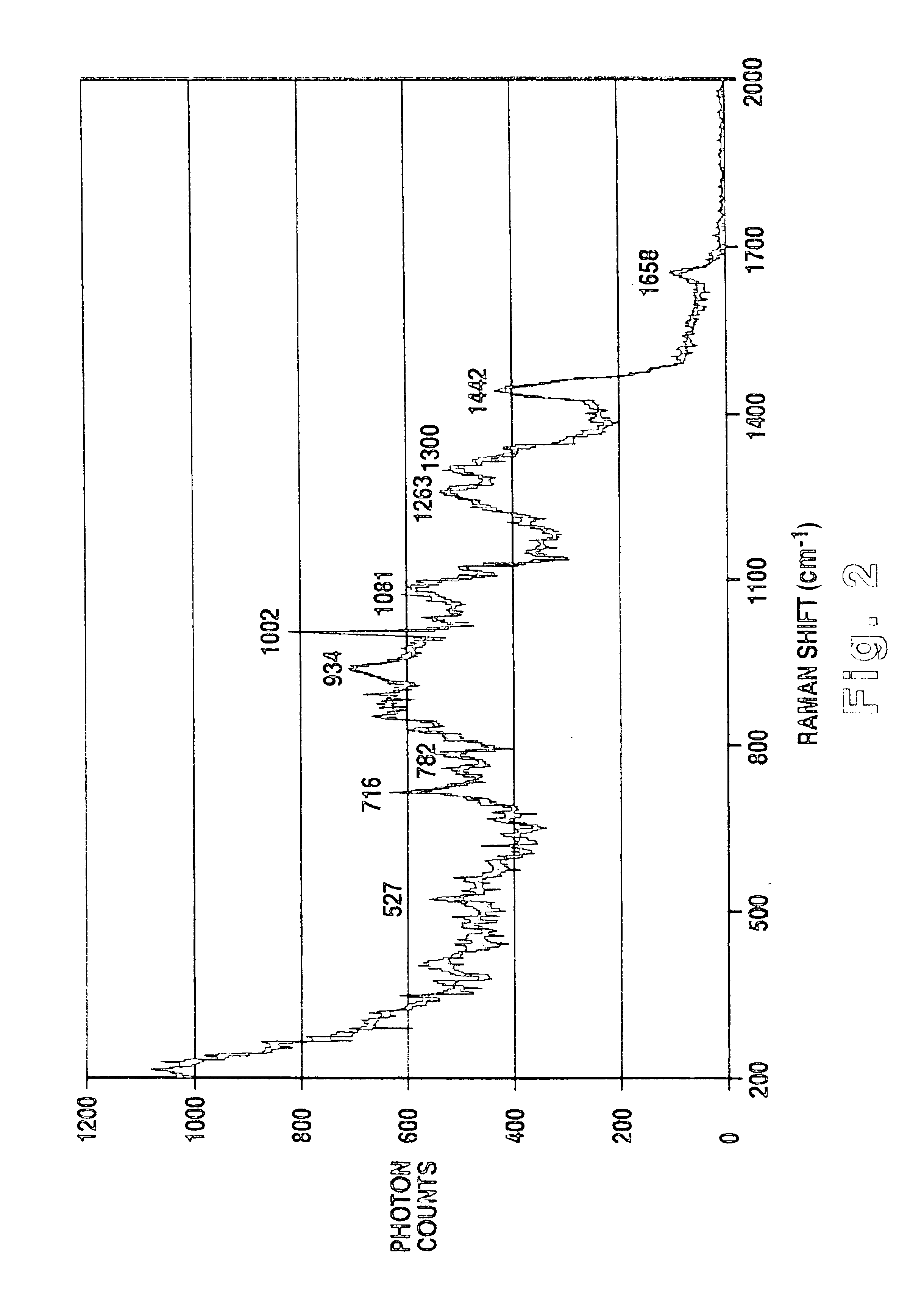
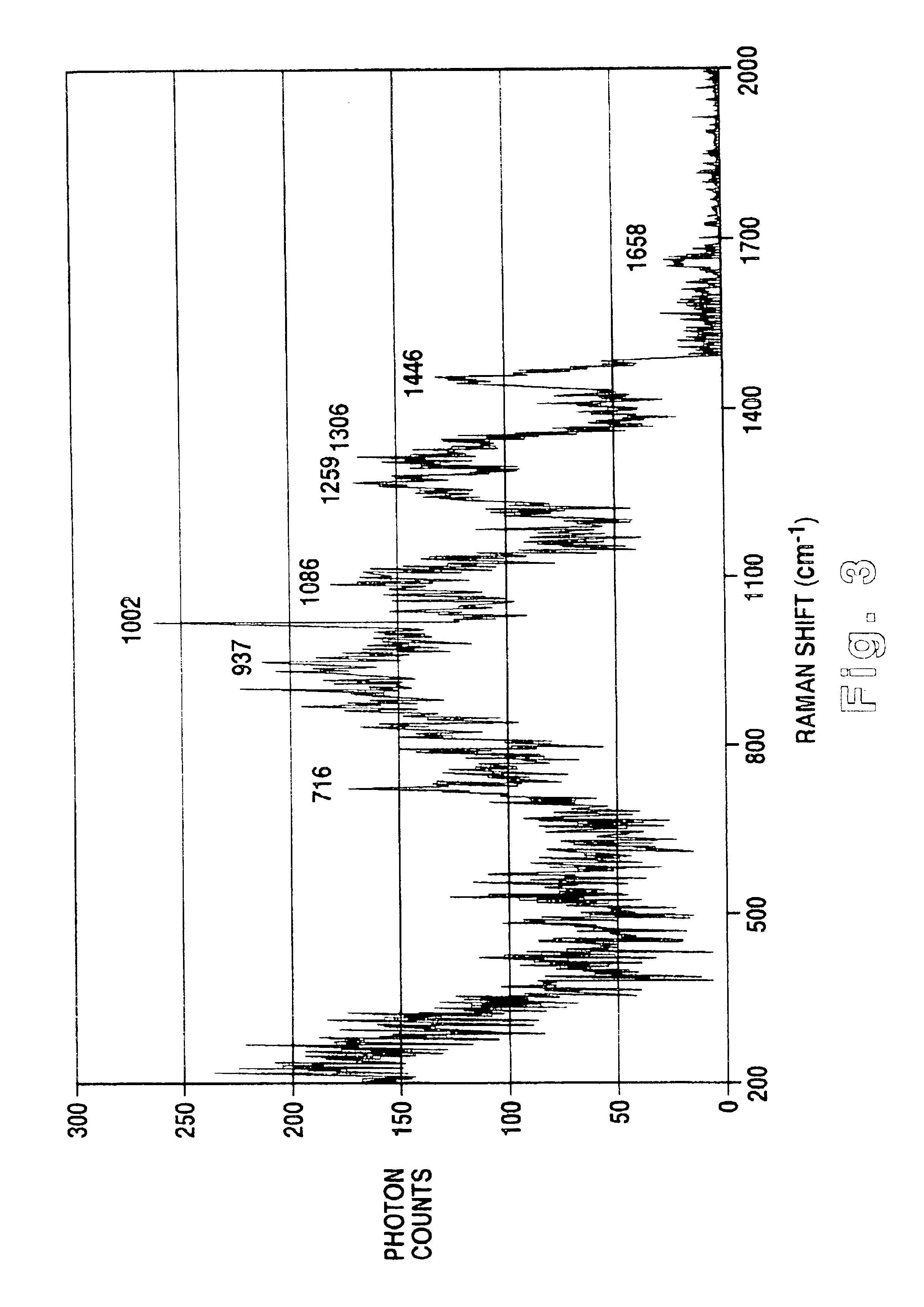
Methodology of using raman imaging microscopy for evaluating drug action within living cells
InactiveUS6939686B2Convenient and cost-effective methodRadiation pyrometryMicrobiological testing/measurementStudy drugRaman imaging
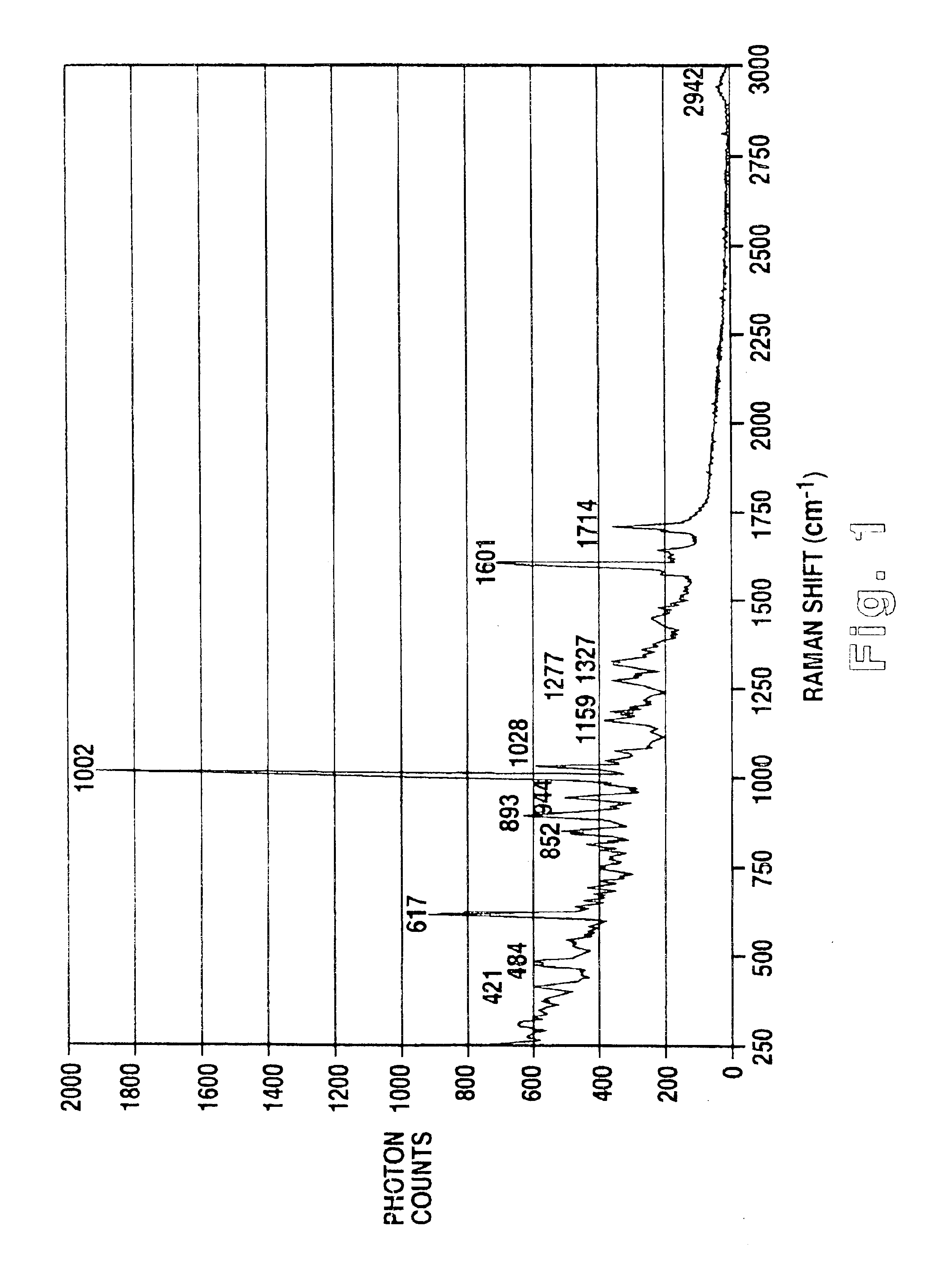
A method of using Raman imaging microscopy to evaluate drug actions in living cells is disclosed. Specifically the invention describes the methods of using Raman imaging microscopy to detect drug uptake, distribution, binding, and metabolism in a single cell, and to study drug pharmacokinetics at the cellular level. The method involves measuring the Raman image of both the drug and the cell. Control images and post-treatment images of the cell were studied. Ratio images were calculated and the requisite information was obtained from a study of the intensity of the bright areas in the ratio images.
Owner:SOUTHWEST RES INST
A kind of azithromycin gel eye drop and its preparation process
ActiveCN102283799AStrong practical significanceStrong application valueAntibacterial agentsOrganic active ingredientsBacterial ConjunctivitisAntioxidant
The invention discloses azithromycin gel eye drops and a preparation process thereof. The eye drops are prepared from a main medicine, namely azithromycin and excipients such as an adhesive, a gel matrix, an isotonic regulator, a preservative, an antioxidant, a buffering agent and the like. The adhesive, namely polycarbophil in the eye drops can increase the biological adhesion of the eye drops, so that the detention time of medicines in eyes is further prolonged. The invention provides a practical, convenient and reliable ophthalmic preparation for treating bacterial conjunctivitis, and solves the problems that the detention time of medicines in an eye drop formulation in the eyes is short, the medicines are not easy to absorb, the bioavailability is low and the like.
Owner:北京乐维生物技术有限公司
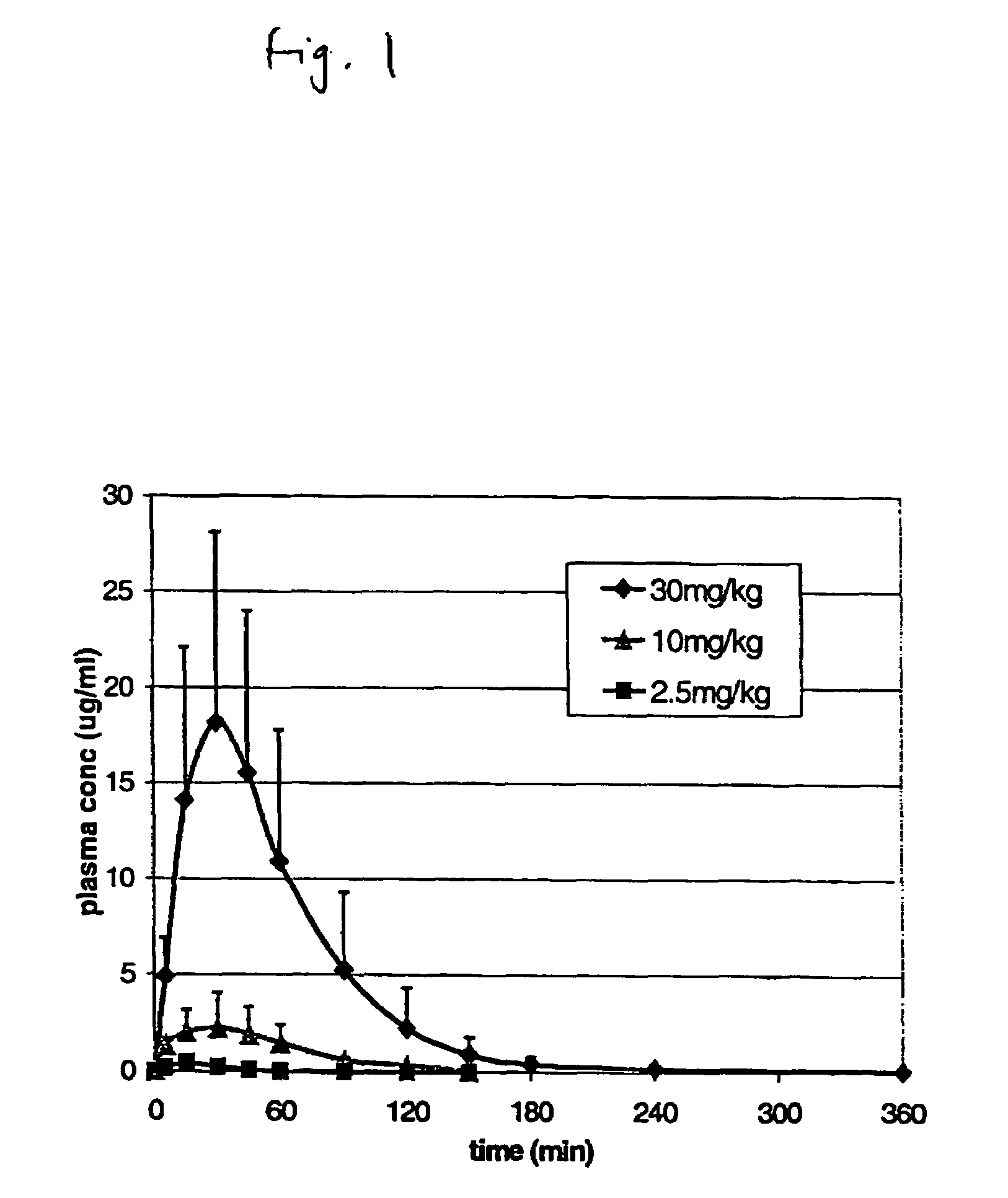
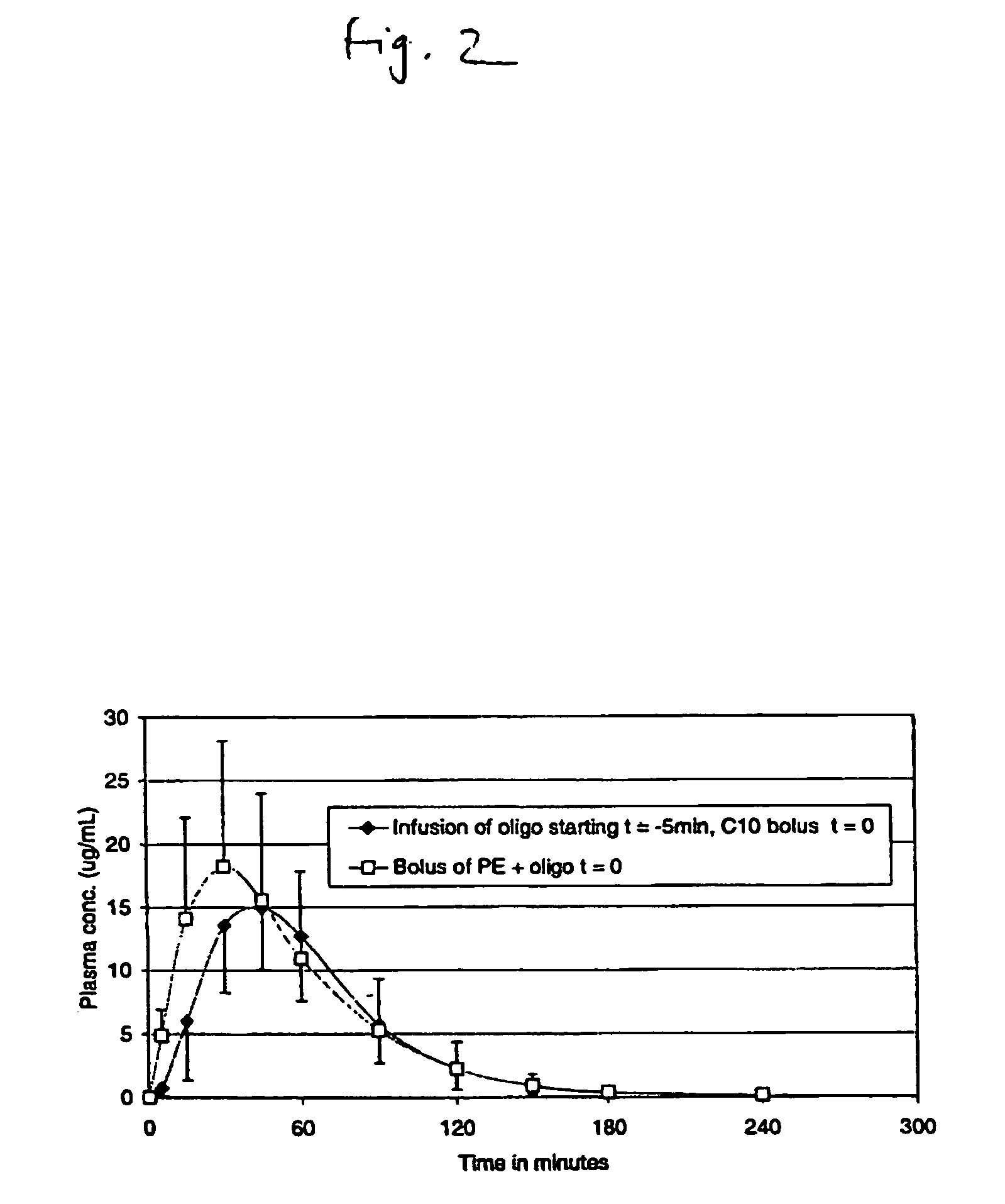
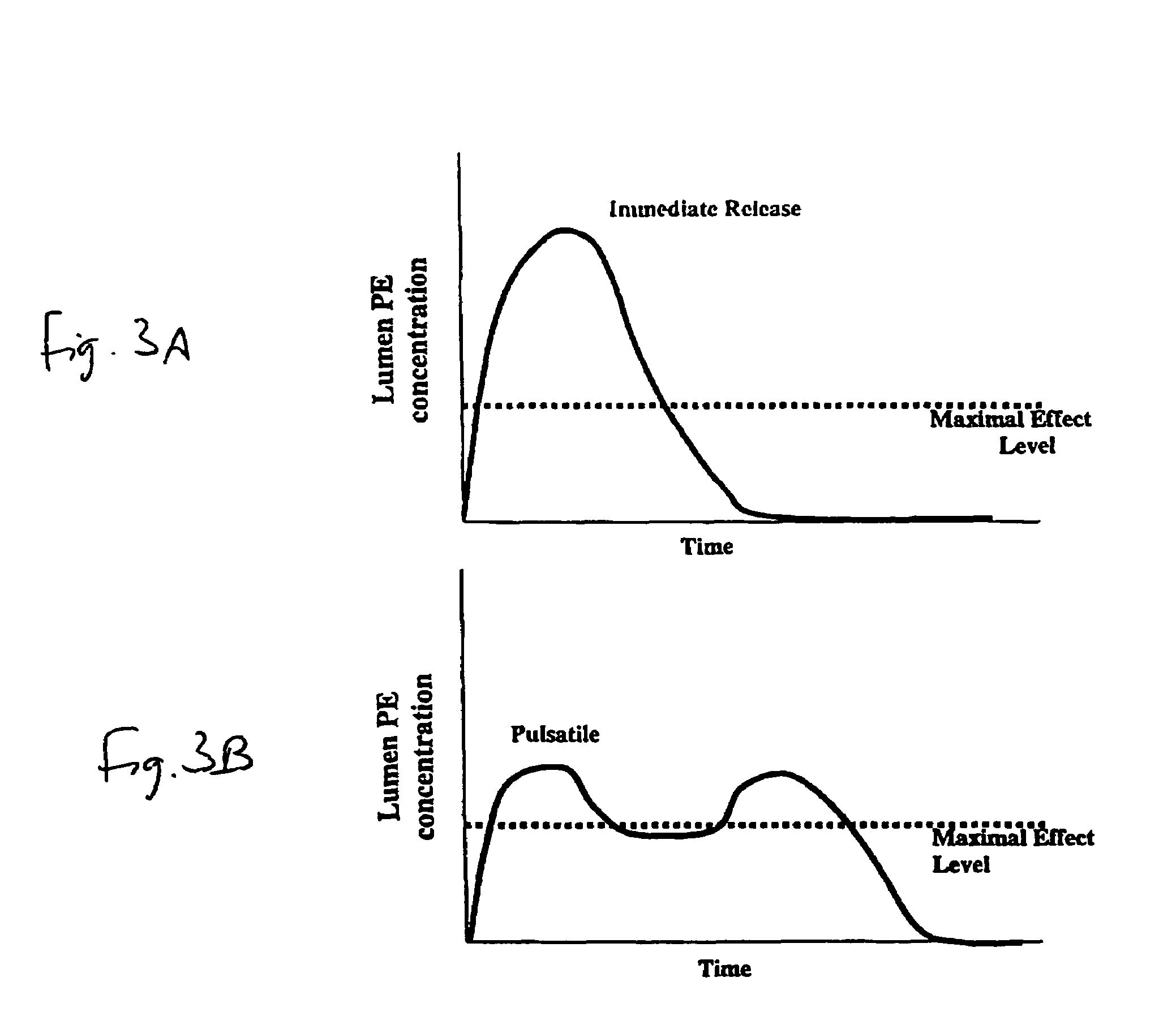
Pulsatile release compositions and methods for enhanced intestinal oligonucleotide drug absorption
Delayed release oral pharmaceutical formulations and methods for enhanced intestinal drug absorption. The formulation comprises a first population of carrier particles comprising a drug and a penetration enhancer which are released at a first location in the intestine, and a second population of carrier particles comprising a penetration enhancer and a delayed release coating or matrix. This penetration enhancer is released at a second location in the intestine downstream from the first location and enhances absorption of the drug when it reaches the second location.
Owner:IONIS PHARMA INC
An acid-responsive nanometer micelle for drug loading, a preparation method and an application thereof
ActiveCN109172521AProlong blood circulation timeQuick releaseOrganic active ingredientsDispersion deliveryCancers diagnosisPolyethylene glycol
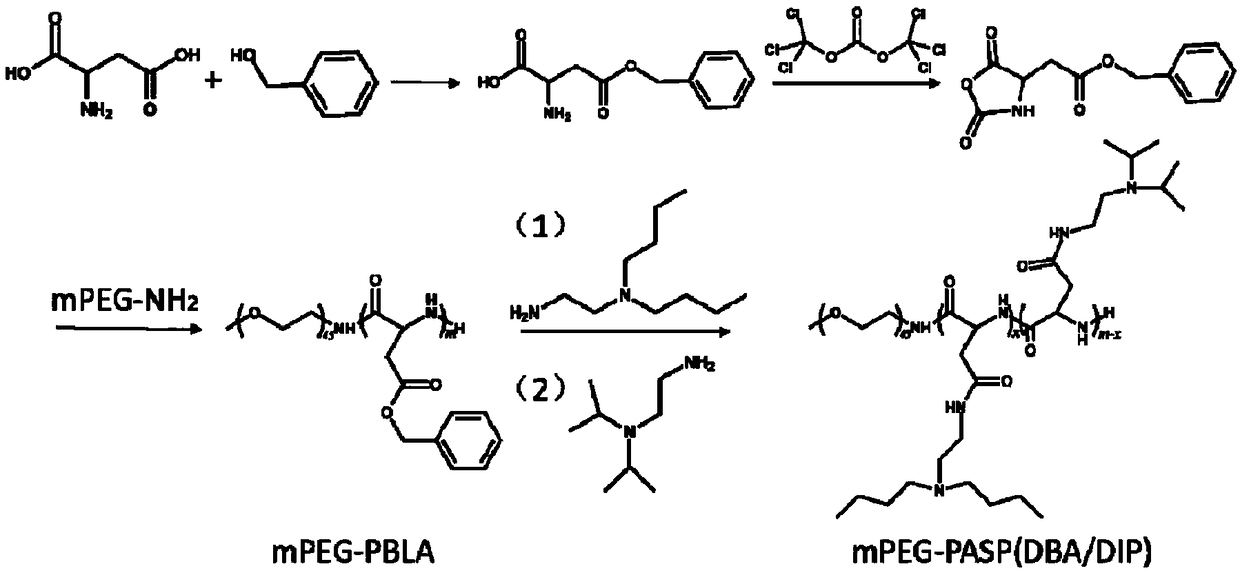
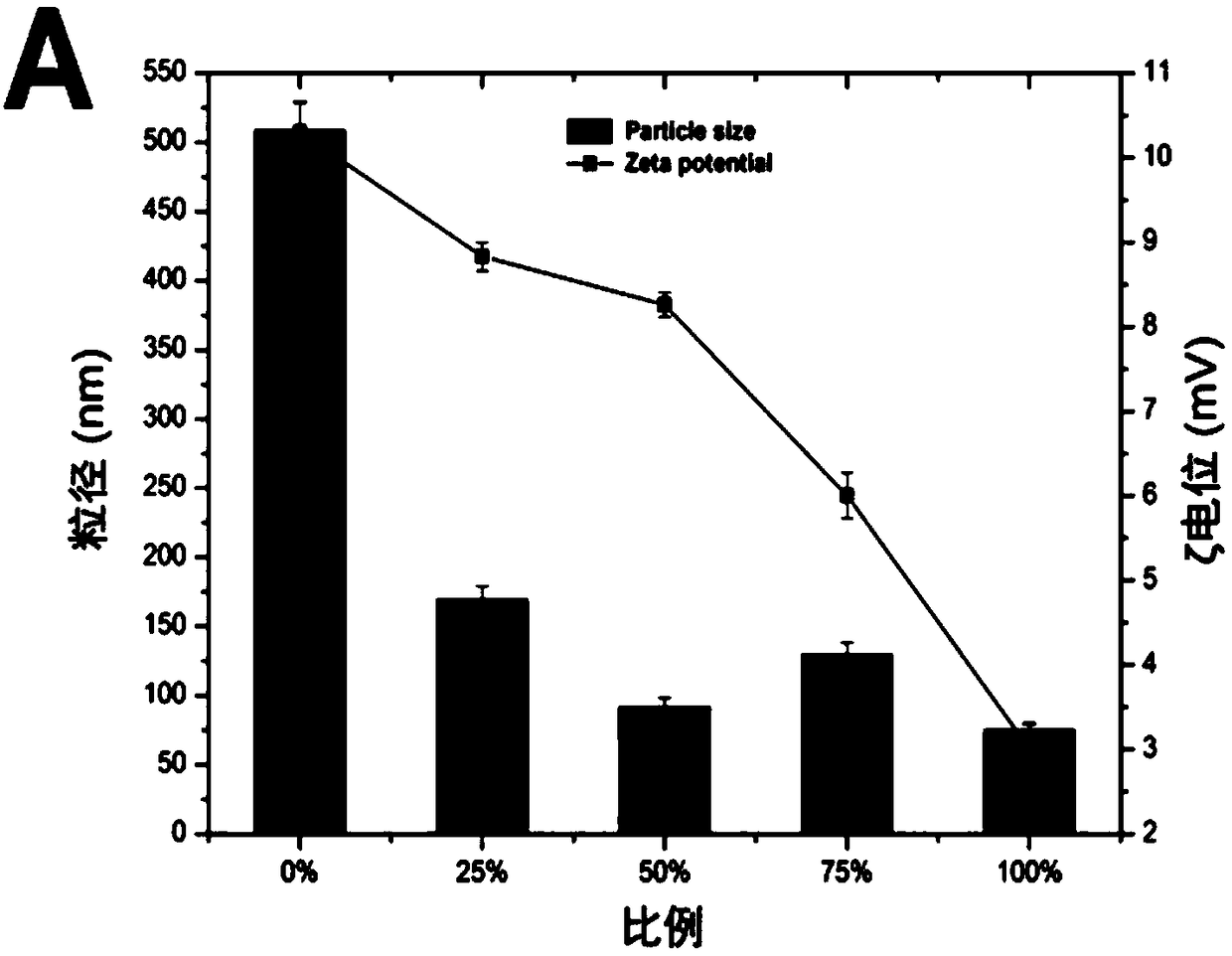
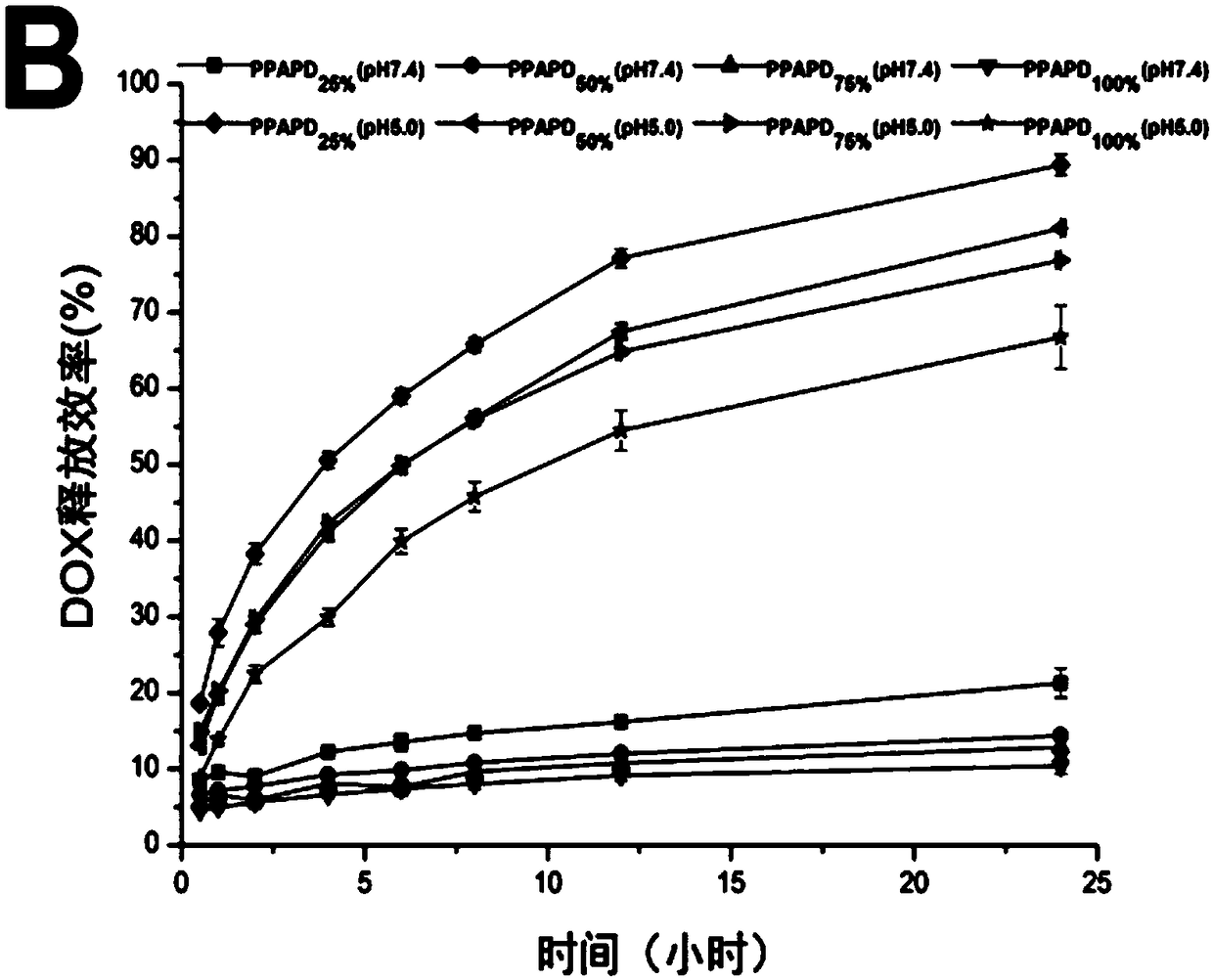
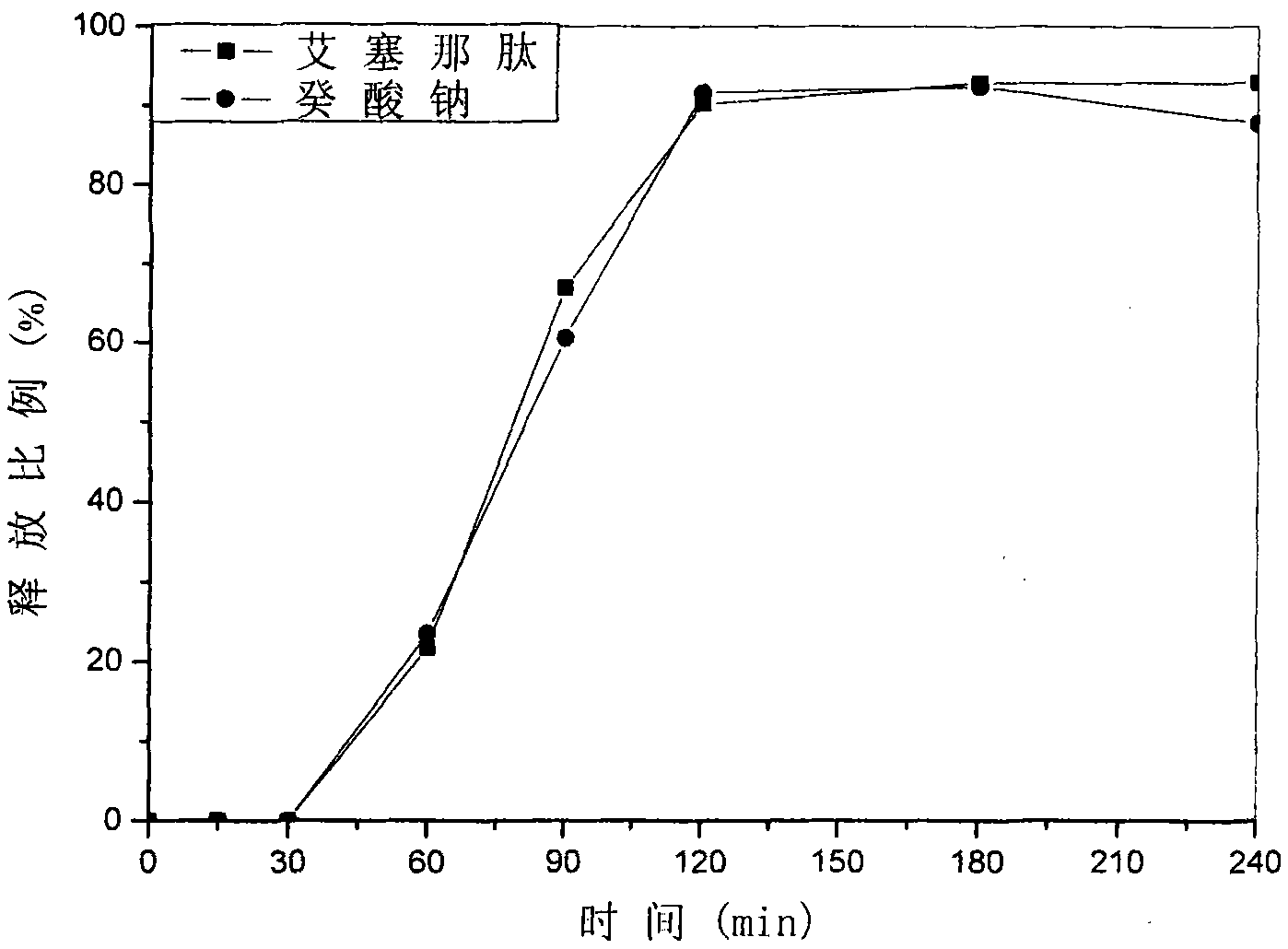
The invention discloses an acid-responsive nanometer micelle for drug loading, a preparation method and an application thereof. The nano-micelles were self-assembled from hydrophilic segment polyethylene glycol (PEG) and hydrophobic segment pH-sensitive polyaspartyl diisopropylethylenediamine / di-n-butylethylenediamine (PAsp (DIP / DBA)). The nano-micelles can prolong the drug circulation time, aggregate in the tumor site, increase the local drug concentration, and respond to the micro-acid environment of the tumor tissue. As a drug carrier of stimulation response, the nano-micelles can rapidly release the loaded chemotherapeutic drug adriamycin in the tumor site, and play a significant anti-tumor effect. At the same time, the nano-micelles loaded with magnetic resonance contrast agent superparamagnetic iron oxide can be used for tumor magnetic resonance imaging and monitoring drug uptake and aggregation. This method utilizes nano-drug aggregation at tumor site and acid responsiveness ofcarrier to realize rapid drug release to improve tumor therapeutic effect, and endows nano-micellar MRI visualization function, which provides a promising innovative strategy for cancer diagnosis andtreatment, and has broad application prospects.
Owner:THE SECOND AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV
Nano-suspension for silybin-phospholipid complex and preparation method thereof
InactiveCN102228430AImprove long-term stabilityFor long-term storageOrganic active ingredientsPowder deliverySolubilityDecreased energy
The invention discloses a nano-suspension for a silybin-phospholipid complex and a preparation method thereof, wherein the nano-suspension for a silybin-phospholipid complex is prepared by combining a technology of promoting medicine absorption by a phospholipid complex with a solubilizing-targeting technology of nano-suspension; the ingredients of the nano-suspension for silybin-phospholipid complex comprise a silybin-phospholipid complex, a stabilizer and water; the nano-suspension exists in the form of suspension or freeze-dried powder and prepared by combining a microprecipitation method with a high-pressure homogenization method; the method of the invention prepares a nano-suspension hard to dissolve in a medicine, without a need to pre-micronize raw material medicines, which greatly decreases energy consumption; the grain diameter distribution range of the prepared nano-grains is narrow, and the medicine highly disperses in a granular state, which increases the wettability, solubility and solution velocity of the medicine, thereby improving the oral bioavailability thereof; the technique process of the preparation method is simple and easy to realize industrial production.
Owner:INST OF CHINESE MATERIA MEDICA CHINA ACAD OF CHINESE MEDICAL SCI
Administration composition and preparation method and using method thereof
ActiveCN102100912APromote absorptionImprove absorption efficiencyPharmaceutical non-active ingredientsPharmaceutical active ingredientsPharmaceutical drugCombinatorial chemistry
The invention provides an administration composition for transferring medicaments and a preparation method and a using method thereof. The administration composition comprises a solid preparation carrying the effective dose of treatment components, a sorbefacient, a pharmaceutic adjuvant and a biologic adhesion layer containing biologic adhesion polymer, and also comprises a selectable anti-permeable or semipermeable coating layer, so that the treatment components and the sorbefacient in the preparation have unidirectional releasing capacity. The administration composition can promote the absorption of medicaments which are difficult to absorb, improves bioavailability, regulate pharmacokinetic characteristics and improve the absorption efficiency of the medicaments, has low cost and is easy to produce relatively.
Owner:SHANGHAI BIOLAXY MEDICAL SCI & TECH
Albumin nanoparticles realizing co-delivery of antitumor drug and MRI (magnetic resonance imaging) contrast medium and preparation method of albumin nanoparticles
ActiveCN105879045AAchieve releaseRealize the combination of diagnosis and treatmentOrganic active ingredientsPowder deliveryMRI contrast agentT1 weighted
The invention discloses albumin nanoparticles realizing co-delivery of an antitumor drug and an MRI (magnetic resonance imaging) contrast medium and a preparation method of the albumin nanoparticles and further provides an albumin nanoparticle vector containing the MRI contrast medium, and drug loading is realized through electrostatic adsorption and coordination between the drug and the vector as well as crosslinking of amino acid residues of the vector. The invention further discloses an optimal preparation technology of the albumin nanoparticles realizing co-delivery of the antitumor drug and the MRI contrast medium and a function of the albumin nanoparticles in diagnosis and treatment combination of tumors. According to the albumin nanoparticles realizing co-delivery of the antitumor drug and the MRI contrast medium, the drug uptake ratio of cells can be increased, and drug efflux caused by drug-resistant cells is reduced, so that drug resistance is reversed, and efficient delivery of the drug is realized; the albumin nanoparticles realizing co-delivery of the antitumor drug and the MRI contrast medium have excellent T1 weighted imaging capability and an effective means is provided for the diagnosis and treatment combination of the tumors.
Owner:CHINA PHARM UNIV
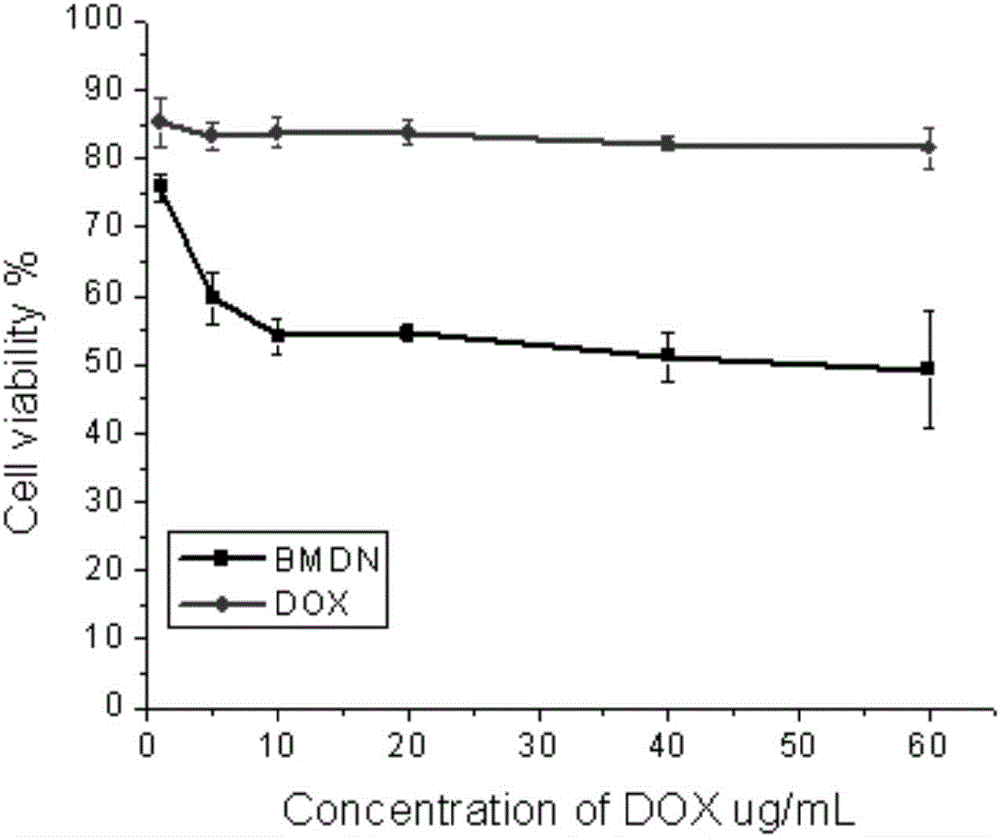
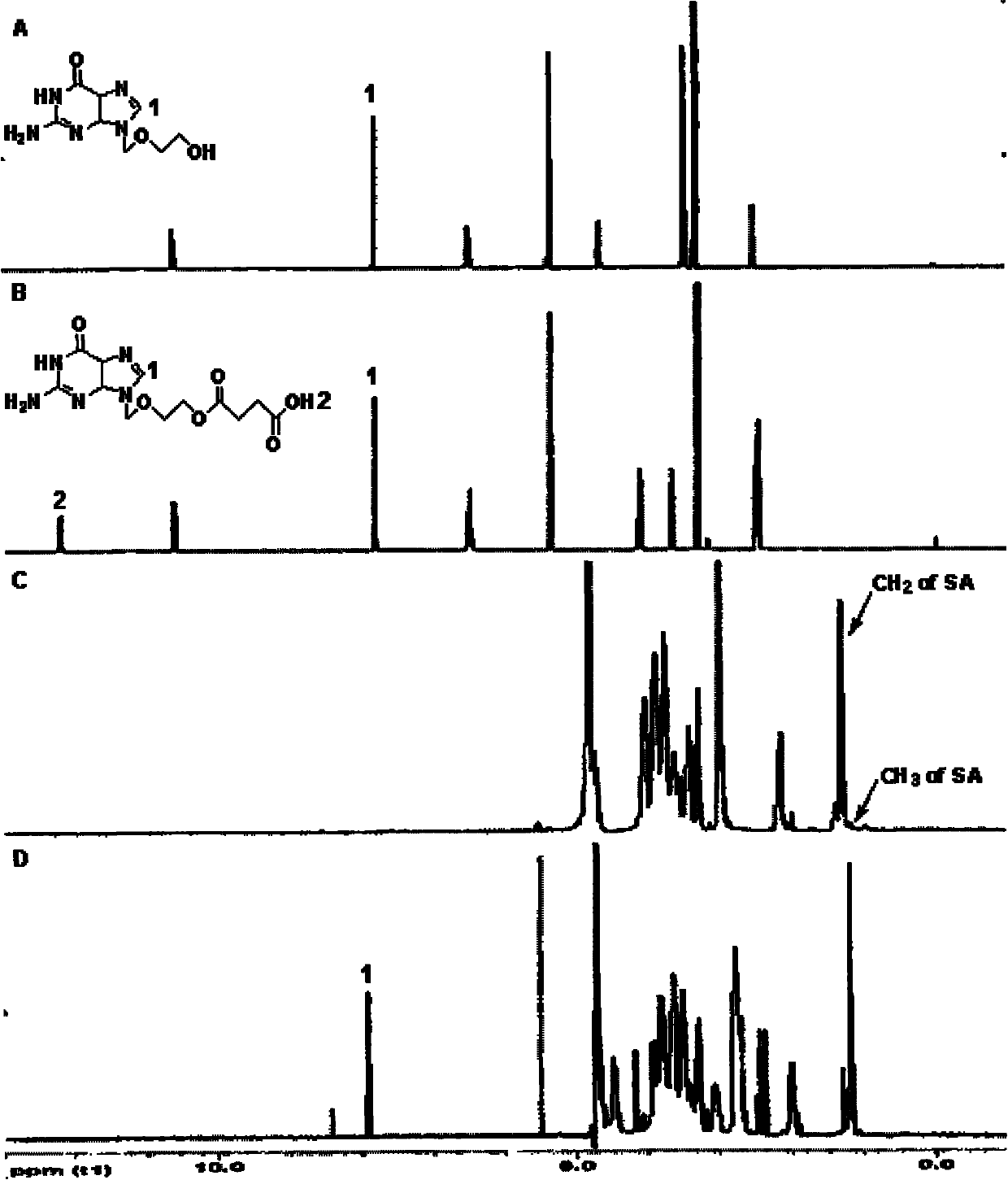
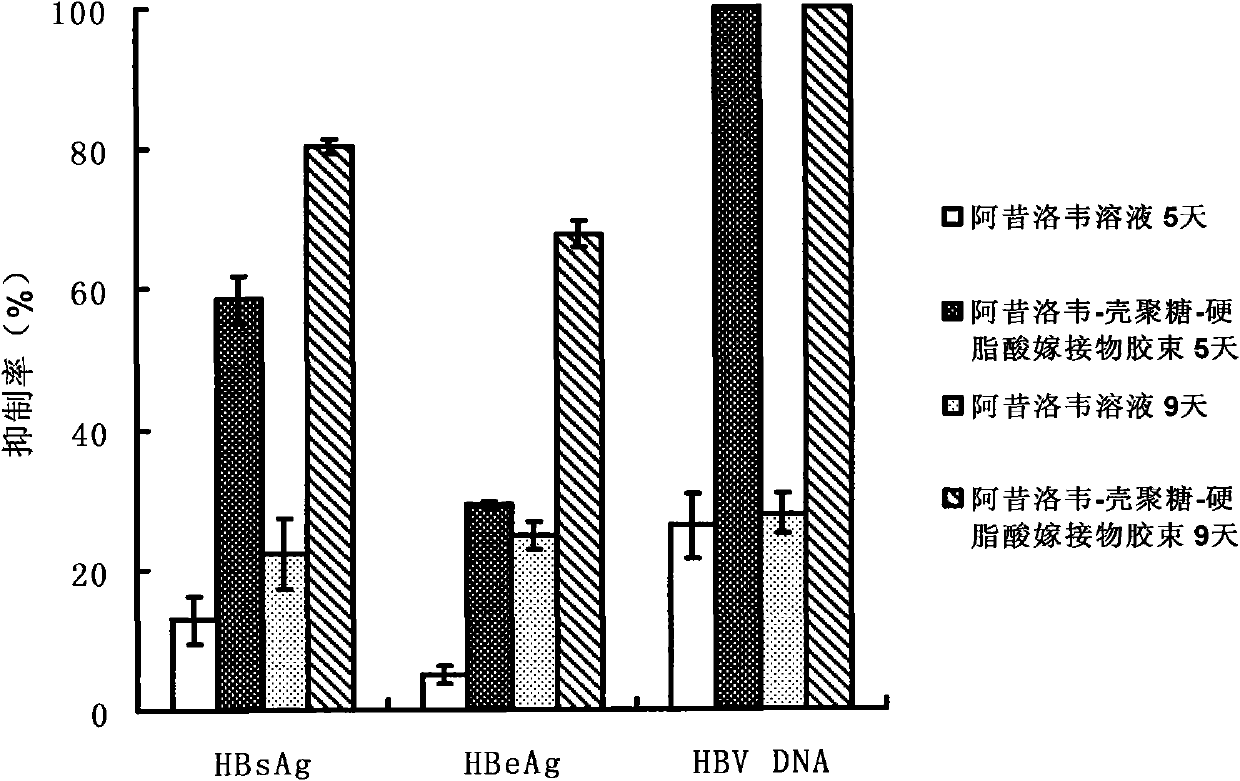
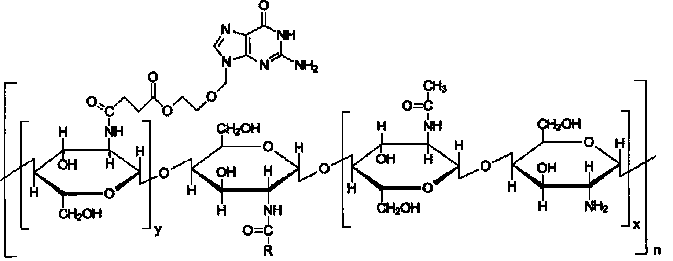
Acyclovir-chitosan-stearic acid grafting, synthetic method and application thereof
InactiveCN101791412AConducive to loadHeavy loadDigestive systemAntiviralsSide effectButanedioic acid
The invention provides a hydrophilic antiviral drug, namely acyclovir-chitosan-stearic acid grafting, which is beneficial to the load of the drug by a target carrier material through chemical grafting by the synthesis of an acyclovir-succinic acid midbody. On the basis, by adopting the load of the antiviral drug in cells of a molecular target mark for a chitosan-stearic acid grafting micelle with high-efficiency cellular uptake and low toxicity, the drug uptake of virus cells is greatly increased, and the drug concentration of the drug molecular target mar position is increased. The acyclovir-chitosan-stearic acid grafting increases the drug uptake of virus cells, is beneficial to reducing the distribution of the drug in normal tissues or cells and reduces the toxic side effect of the drug; and the drug concentration increase of the drug molecular target mark position is beneficial to increasing the healing effect of the antiviral drug. The acyclovir-chitosan-stearic acid grafting has the chemical general formula (disclosed in the specification).
Owner:ZHEJIANG UNIV
Abiraterone oral emulsion and preparation method thereof
PendingCN111012745AHigh drug loadingImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsAbirateroneDigestion
The invention discloses an abiraterone oral emulsion and a preparation method thereof. The preparation comprises an active component, a solubilizer, an emulsifier and an antioxidant; the active component is abiraterone acetate or abiraterone; and the preparation comprises, by weight, 2%-10% of abiraterone acetate or abiraterone, 20%-70% of the solubilizer, 20%-40% of the emulsifier and 0.01%-1% ofthe solubilizer. According to the invention, the drug loading capacity and the stability of the abiraterone oral emulsion are high; and the abiraterone oral emulsion can be spontaneously emulsified to form an emulsion when meeting water. And the formed emulsion is stable; the digestion influence is low; the dissolution of medicines in gastrointestinal tracts is improved; the absorption of the medicines is promoted; and the bioavailability is improved.
Owner:SHENZHEN NEPTUNUS PHARMA RES INST CO LTD
Administration combination and preparation and use methods thereof
The invention provides an administration combination and a preparation method and a use method thereof. The administration combination comprises treatment components carrying effective doses, absorbefacient, medical auxiliary materials, and solid preparation of a bioadhesive layer containing bioadhesive polymer, and further comprises a selectable impervious or semi-permeable coating layer which ensures that the treatment components and the absorbefacient have unidirectional release capability. A unidirectional release channel with unidirectional release capability is formed through manual or laser ablation technology, and the releasing rates of the treatment components and the absorbefacient in the solid preparation are nearly the same. The invention simultaneously provides the preparation and the use methods of the administration combination. Low-dose solid preparation prepared through a non-solvent granulation process can maintain the uniformity and the stability of the treatment components. The administration combination can promote the absorption of drug difficult to be absorbed, improves the bioavailability, adjusts the pharmacodynamic feature and improves the absorption efficiency of drug, has a low price, and is relatively easy to produce.
Owner:SHANGHAI BIOLAXY MEDICAL SCI & TECH
Dragon's blood external use preparation and its preparing method
InactiveCN1748757AEasy to useStrong permeabilityAntipyreticAerosol deliverySuppositoryBiomedical engineering
The present invention discloses the preparation process of several kinds of externally applied dragoní»s blood preparation, including suppository, ointment and cataplasm. The externally applied dragoní»s blood preparations are prepared through treating dragoní»s blood into fine powder and conventional preparation process with the fine dragoní»s blood powder as active component and pharmaceutically acceptable carrier. The present invention has high medicine quality, high curative effect, convenient clinical application, high medicine absorption and saving in the dragoní»s blood resource.
Owner:FUKANGREN BIO PHARMA
Drug coated balloon dilating catheter and process thereof
InactiveCN108744233AProcess parameter controlShort absorption timeBalloon catheterCoatingsBalloon dilatation catheterPolyethylene oxide
The invention discloses a drug coated balloon dilating catheter and a process thereof. The drug coated balloon dilating catheter comprises a drug coated balloon, an outer cavity tube, an inner cavitytube and a connector. The surface of the drug coated balloon is coated with a drug coating, a release drug in the drug coating is a sirolimus derivative BA9, and a carrier of the release drug is polyethylene oxide. A manufacturing process of the drug coated balloon dilating catheter includes steps of catheter assembly and drug coating. The drug coated balloon has advantages of short tissue absorption time, high drug absorption efficiency and high drug coated balloon release rate, and high tissue safety is realized. Compared with an existing taxol drug coated balloon, the drug coated balloon has advantages of promising application prospect.
Owner:JW MEDICAL SYSTEMS LTD
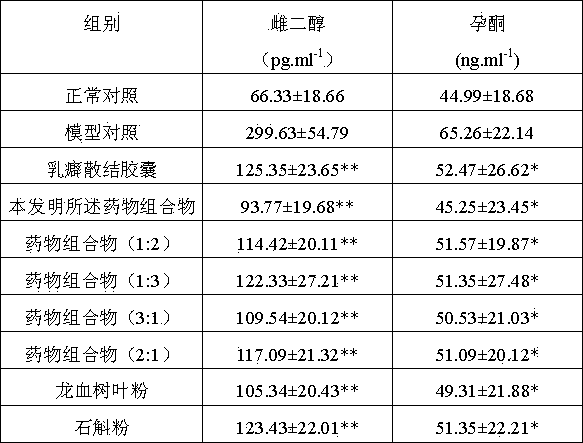
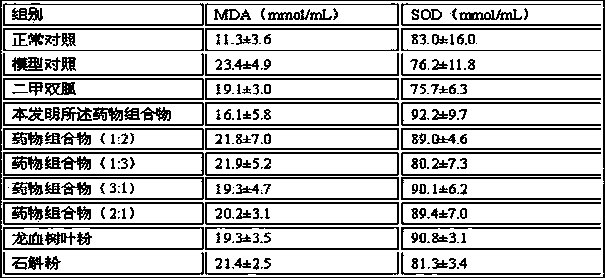
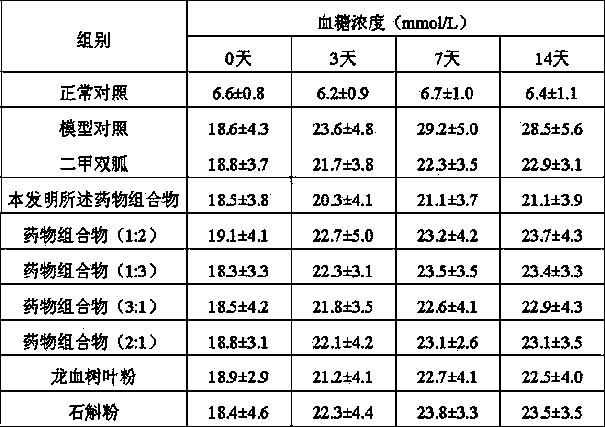
Dragon tree leaf/dendrobe pharmaceutical composition, and preparation method, preparation and application thereof
ActiveCN103800690AReduce lossesReduce active ingredientsMetabolism disorderSexual disorderBiotechnologyDiabetes mellitus
The invention discloses a dragon tree leaf / dendrobe pharmaceutical composition, and a preparation method, preparation and application thereof, belonging to the technical field of preparation of medicines. The pharmaceutical composition comprises 45-55 parts by weight of dragon tree leaf ultrafine powder and 45-55 parts by weight of dendrobe ultrafine powder. The preparation method comprises the following steps: removing impurities from dragon tree leaf and dendrobe, selecting, cleaning, airing, and drying until the water content is at most 3%; respectively pulverizing the dragon tree leaf and dendrobe with a coarse pulverizer, and passing through a 100-140-mesh screen; pulverizing the dragon tree leaf coarse powder by a jet mill to obtain the ultrafine powder with the particle size of 10-15 mu m, and pulverizing the dendrobe coarse powder by a vibromill to obtain the ultrafine powder with the particle size of 6-12 mu m; and evenly mixing the 45-55 parts by weight of dragon tree leaf ultrafine powder and the 45-55 parts by weight of dendrobe ultrafine powder. The preparation is prepared by adding pharmaceutically acceptable auxiliary materials into the pharmaceutical composition. The pharmaceutical composition can be used for preparing medicines for treating hyperplasia of mammary glands and diabetes, and has the characteristics of high bioavailability, high active component leaching speed and favorable drug absorption effect.
Owner:普洱淞茂滇草六味制药股份有限公司
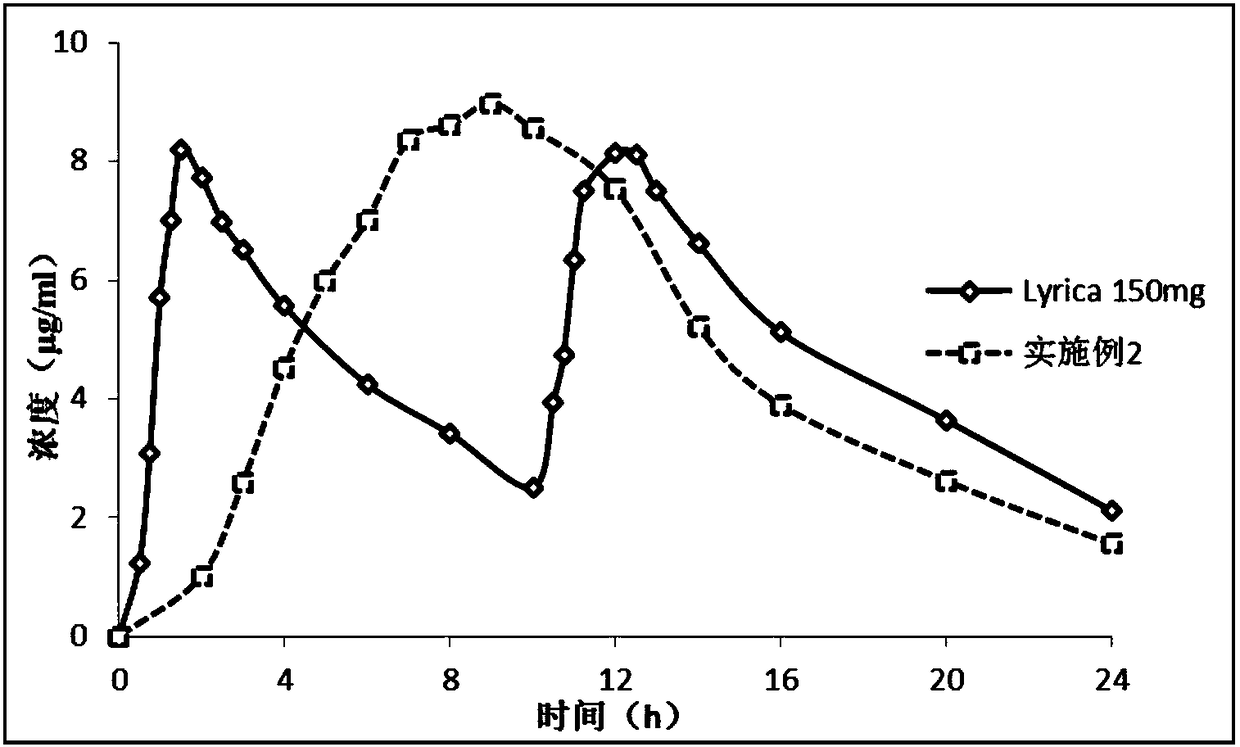
Single-chamber osmotic pump controlled release tablet containing pregabalin
InactiveCN108478537AGood correlation between in vitro and in vivoReduce peak and valley effectOrganic active ingredientsNervous disorderTime rangeWater soluble
The invention discloses a single-chamber osmotic pump controlled release tablet containing pregabalin. The tablet comprises a single-layer piece core, a controlled release film coating layer and at least one laser hole in a controlled release film. The basic composition of the piece core includes pregabalin or salt or hydrate thereof, diluent, adhesive and lubricant, and does not include an expansible polymer. The adopted accessories are simple and economical, the tablet can be prepared through a simple direct tabletting process, and the controlled release film coating layer includes a film forming material, a pore-foaming agent and plasticizer. According to the drug release mechanism, water and a physiological medium dissolve water-soluble active components and the diluent in the piece core through the controlled release film to form trans-film osmotic pressure, the dissolved active components are released out of the piece core through the small holes in the controlled release film, the controlled release tablet can release a therapeutic drug within a certain time range at a constant drug release rate, and the influence of the stomach and intestine difference between different individuals on drug absorption can be minimized.
Owner:南京易亨制药有限公司
Oral drug solution formula with function of resisting coccidium and preparation
InactiveCN110292561AImprove bioavailabilityConvenient for clinical operationPharmaceutical delivery mechanismPharmaceutical non-active ingredientsDrugs solutionSolubility
The invention relates to an oral drug solution formula with the function of resisting coccidium and preparation, in particular to a compound formula for improving the water solubility of a bioactivitylipophilic drug. According to the specific compound formula, the drug is mixed with one or more of organic solvents, and then mixed with various stabilizers. According to the composition formula, thedrug dosage form is made according to a making method of common drug dosage forms. After the drug dosage form made according to the method is clinically used, after the drug is diluted with water inproportion, the drug is administrated to poultry while the poultry drink water, the solubility and stability of the drug in water are improved, drug absorption is improved, and the bioavailability isimproved.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Quadrivalent platinum prodrug bendazac platinum, preparation thereof, and preparation methods and applications of prodrug and preparation
ActiveCN112961188ASolve efficiency problemsGood water solubilityOrganic active ingredientsPlatinum organic compoundsEfficacyBovine serum albumin
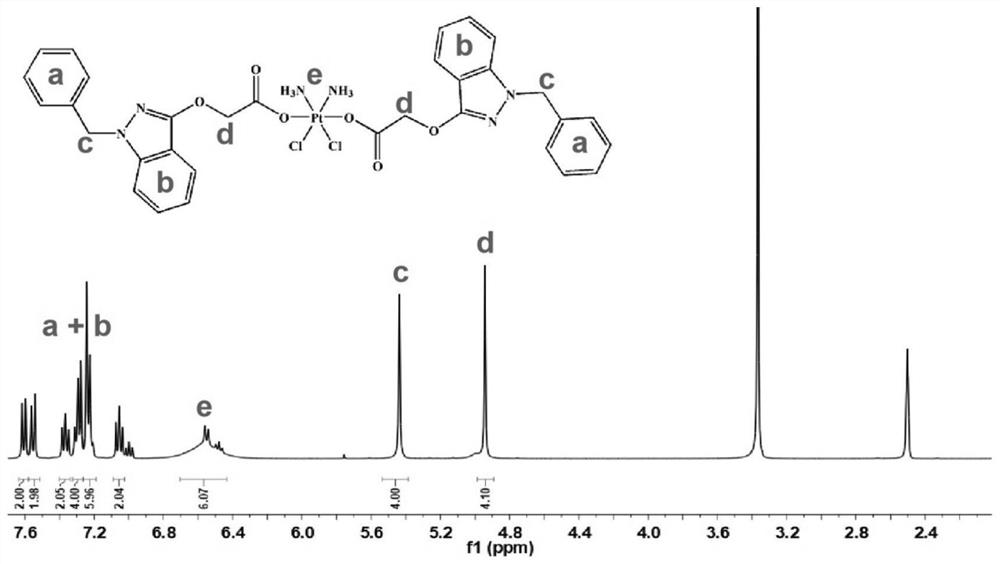
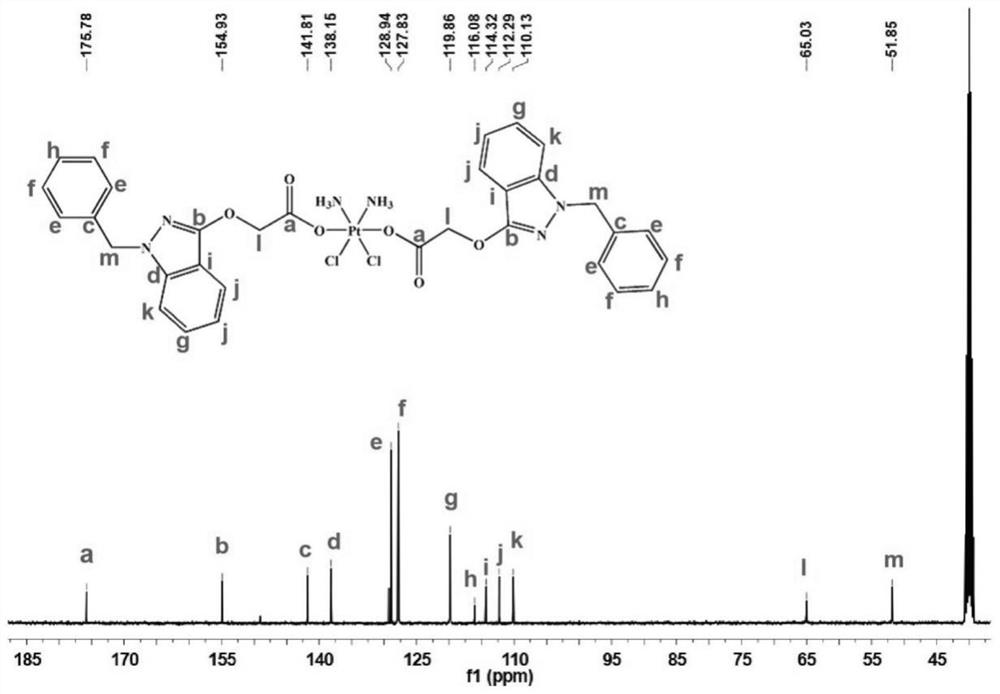
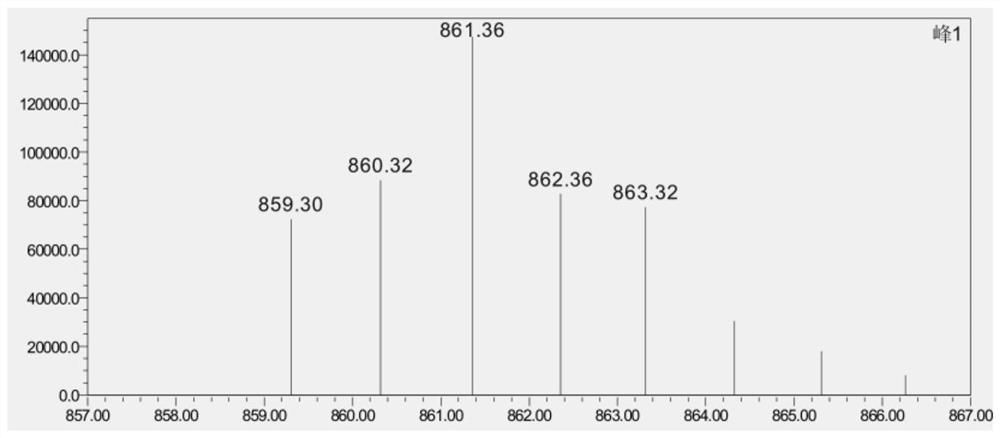
The invention belongs to the technical field of medicines, and particularly relates to a tetravalent platinum prodrug bendazac platinum, a preparation thereof, and preparation methods and applications of the prodrug and the preparation. The structural formula of the prodrug is shown in the specification. The preparation method of the prodrug comprises the following steps: (1) synthesizing hydroxyl platinum; (2) synthesizing bendazac platinum; and (3) purifying the bendazac platinum. The preparation method of the nano preparation comprises the following steps: (1) preparing a bendazac platinum solution; and (2) preparing the nano preparation by taking bovine serum albumin as a carrier. The tetravalent platinum prodrug bendazac platinum and the nano preparation thereof provided by the invention are good in water solubility, small in toxic and side effects and high in anti-tumor efficacy, can be passively targeted to a tumor site, can increase the drug uptake ability of tumor cells, and show a good anti-tumor effect, and the inhibition rates of the tetravalent platinum prodrug bendazac platinum to various tumors can be higher than 90%.
Owner:山东省第二人民医院(山东省耳鼻喉医院、山东省耳鼻喉研究所)
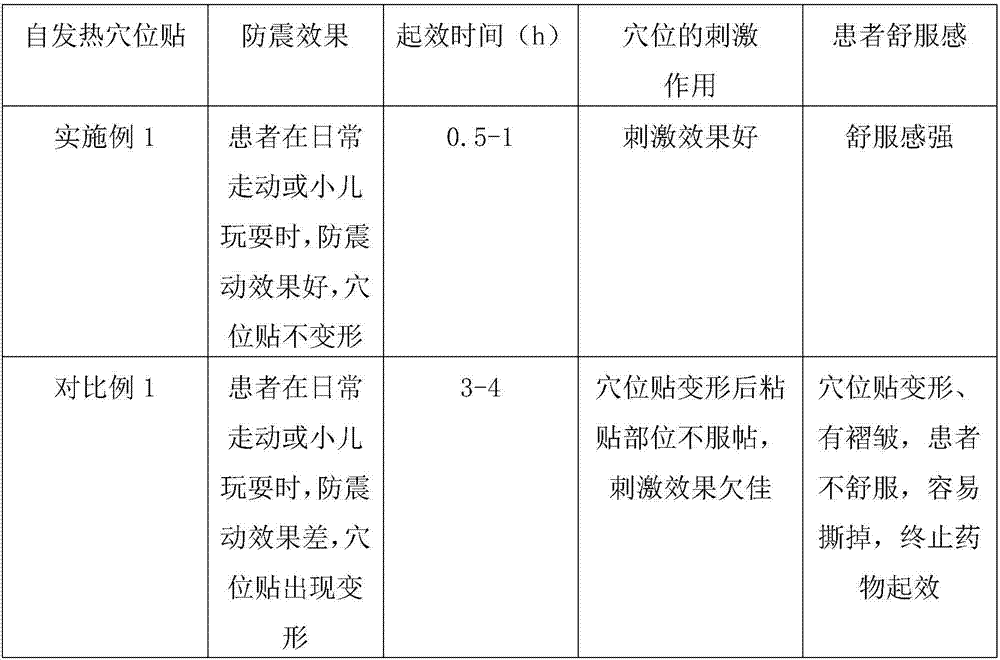
Self-heating acupuncture point patch as well a processing method and application thereof
InactiveCN106924032AFewer allergic side effectsWill not defaceDevices for heating/cooling reflex pointsMedical devicesMedicineBioavailability
The invention relates to the technical field of medical apparatuses, and in particular discloses a multifunctional self-heating acupuncture point patch. The invention also discloses a processing method and an application of the self-heating acupuncture point patch. The self-heating acupuncture point patch comprises a heating device and a medicine layer, wherein a sticky layer is arranged on the upper surface of the heating device; the medicine layer is arranged on the lower surface of the heating device; the medicine layer sleeves inside a locator; a breathable layer covers the medicine layer and the locator; a piece of release paper is arranged on the lower side of the breathable layer; and the sticky layer is stuck with the periphery of the release paper. The problems of an existing self-heating patch that a medicine layer, at high temperature, is poor in anti-shock effect, easy for deformation, slow to take efficacy of medicines and long in treatment time can be solved. With the application of the self-heating acupuncture point patch provided by the invention, a time that medicines take effects is shortened, and meanwhile, bioavailability of the medicines is improved and absorption efficiency of the medicines is greatly improved, so that an effect of stimulating acupuncture points is enhanced; and moreover, the self-heating acupuncture point patch is broad in application scope and conducive to market popularization.
Owner:杭州久遇网络科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com