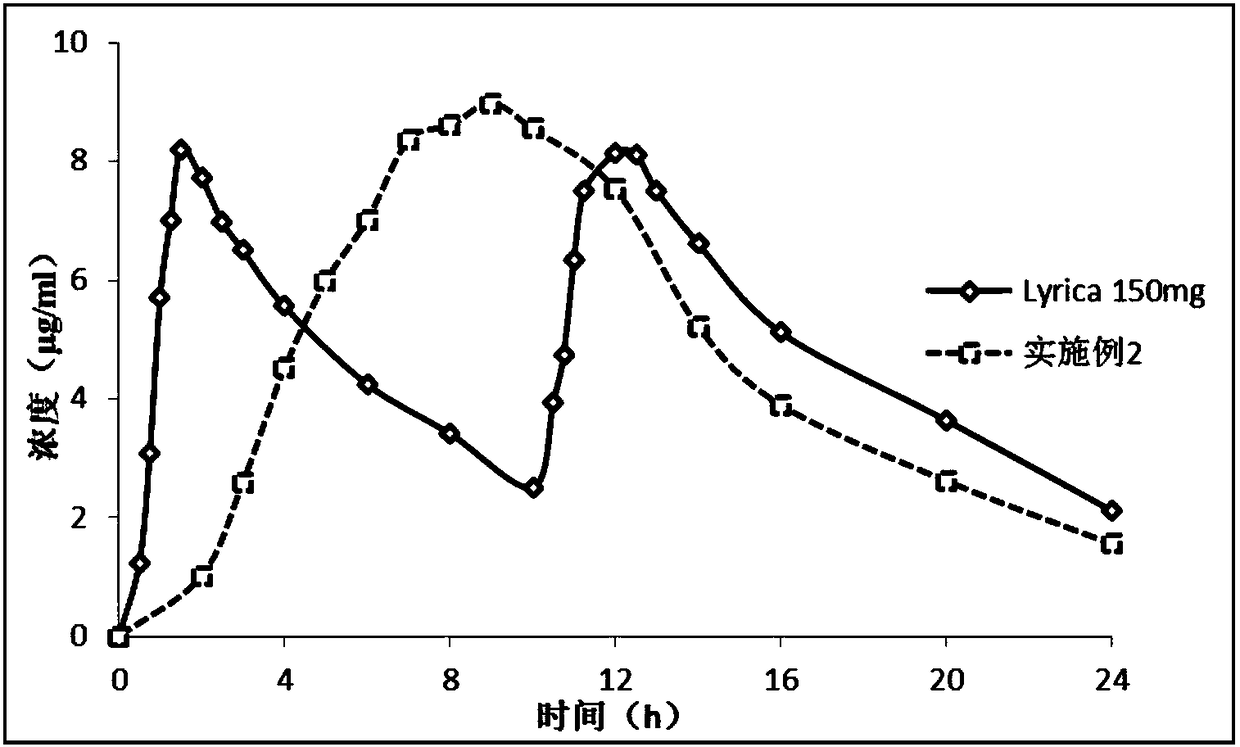
Single-chamber osmotic pump controlled release tablet containing pregabalin
A single-chamber osmotic pump and pregabalin technology, applied in the field of pharmaceutical preparations, can solve the problems of ionic group compatibility risk, process scale-up risk, performance impact, etc., to reduce the number of medications, improve compliance and effective The effect of reducing the peak and valley effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] prescription:
[0027] I chip
[0028]
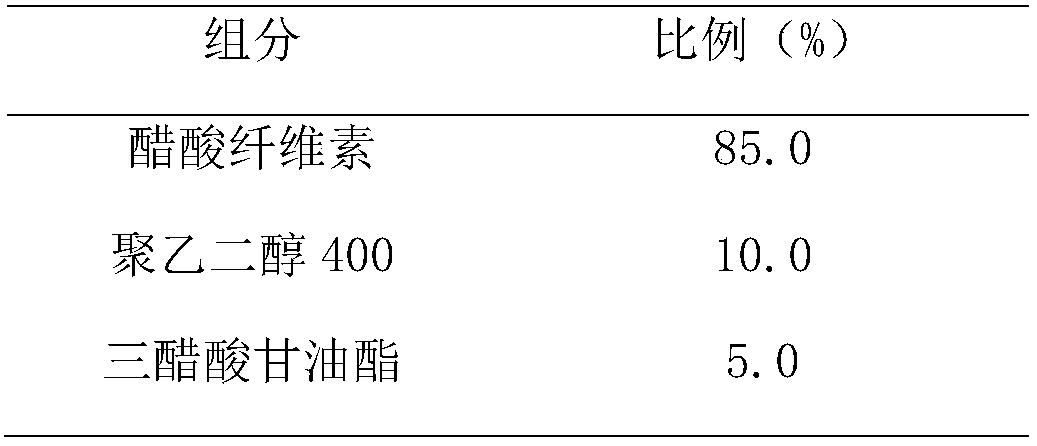
[0029] II controlled release film coat
[0030]
[0031] Preparation Process:
[0032] Mixing: pass pregabalin, mannitol and hydroxypropyl cellulose through a 30-mesh sieve to remove lumps, and mix them in a material mixer for 20 minutes after treatment; pass magnesium stearate through a 30-mesh sieve and then mix with pregabalin powder for 3 The final blend was obtained after 1 min.
[0033] Tablet compression: the blended pregabalin powder is directly compressed with a circular shallow concave punch and a rotary tablet press to control the tablet hardness to 10-20kg.
[0034] Seal coating (optional): A layer of water-soluble seal coating can be coated between the tablet core and the controlled-release film coat, and the coating powder 03A19322-CN is transparent and soluble in pure water. Use a high-efficiency coating pan to coat the tablet core with a sealing layer. The atomization pressure is 0.1-0.2MPa, the tablet ...
Embodiment 2
[0038] prescription:
[0039] I chip
[0040]
[0041] II controlled release film coat
[0042]
[0043] Preparation Process:
[0044] Mixing: pass pregabalin, mannitol and povidone through a 30-mesh sieve to remove lumps, and mix in a material mixer for 20 minutes after treatment; pass magnesium stearate through a 30-mesh sieve and then mix with pregabalin powder for 3 minutes to obtain the final blend.
[0045] Tablet compression: the blended pregabalin powder is directly compressed with a circular shallow concave punch and a rotary tablet press to control the tablet hardness to 10-20kg.
[0046] Seal coating (optional): A layer of water-soluble seal coating can be coated between the tablet core and the controlled-release film coat, and the coating powder 03A19322-CN is transparent and soluble in pure water. Use a high-efficiency coating pan to coat the tablet core with a sealing layer. The atomization pressure is 0.1-0.2MPa, the tablet bed temperature is 30-40℃, ...
Embodiment 3
[0050] prescription
[0051] I chip
[0052]
[0053] II controlled release film coat
[0054]
[0055] Preparation Process:
[0056] Mixing: pass pregabalin, mannitol and hydroxypropyl cellulose through a 30-mesh sieve to remove lumps, and mix them in a material mixer for 20 minutes after treatment; pass magnesium stearate through a 30-mesh sieve and then mix with pregabalin powder for 3 The final blend was obtained after 1 min.
[0057] Tablet compression: the blended pregabalin powder is directly compressed with a circular shallow concave punch and a rotary tablet press to control the tablet hardness to 10-20kg.
[0058] Seal coating (optional): A layer of water-soluble seal coating can be coated between the tablet core and the controlled-release film coat, and the coating powder 03A19322-CN is transparent and soluble in pure water. Use a high-efficiency coating pan to coat the tablet core with a sealing layer. The atomization pressure is 0.1-0.2MPa, the tablet b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com