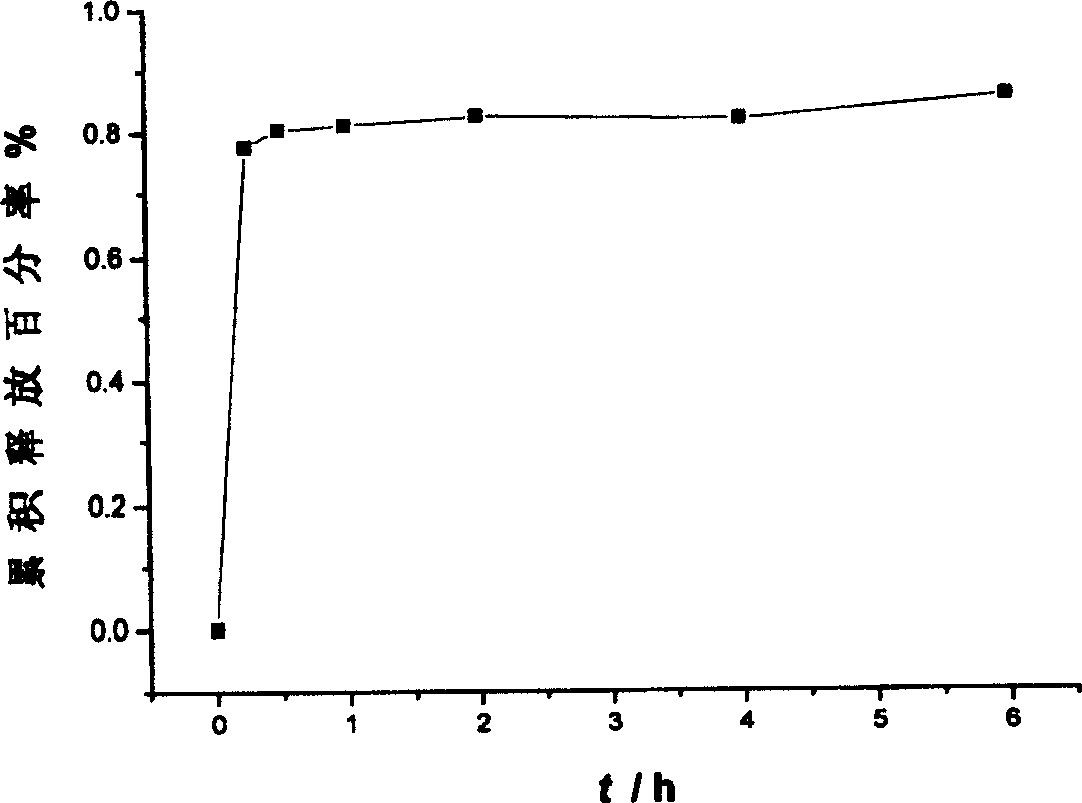
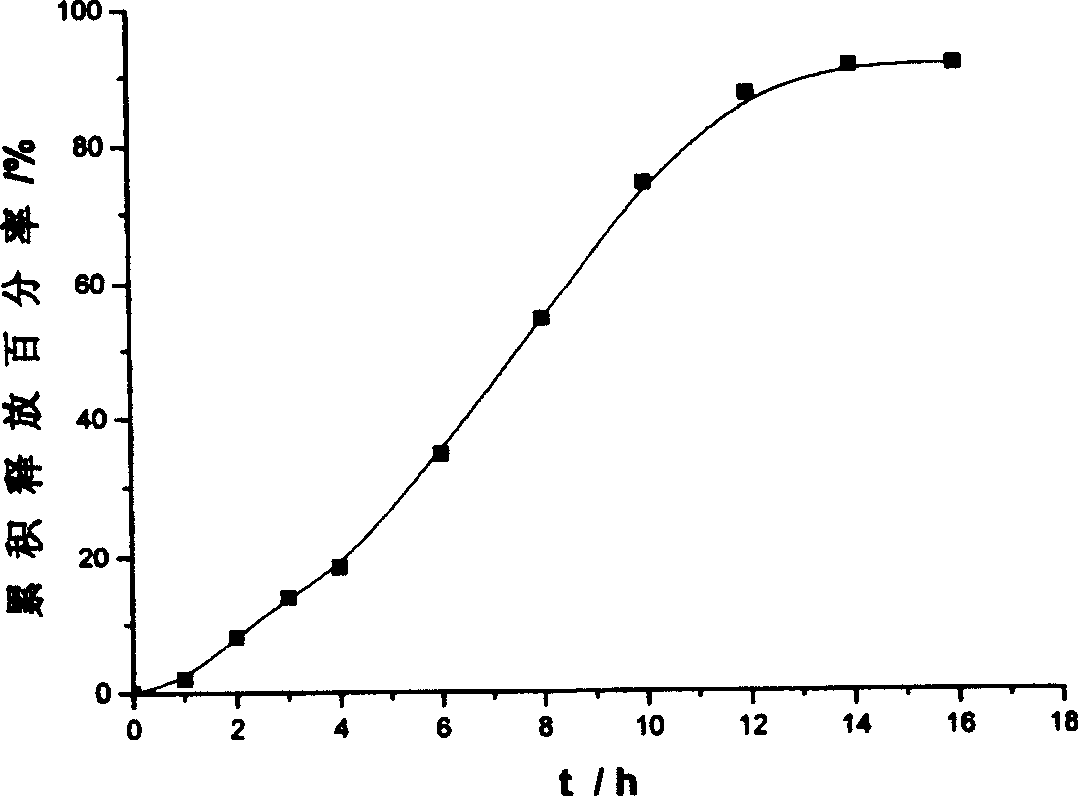
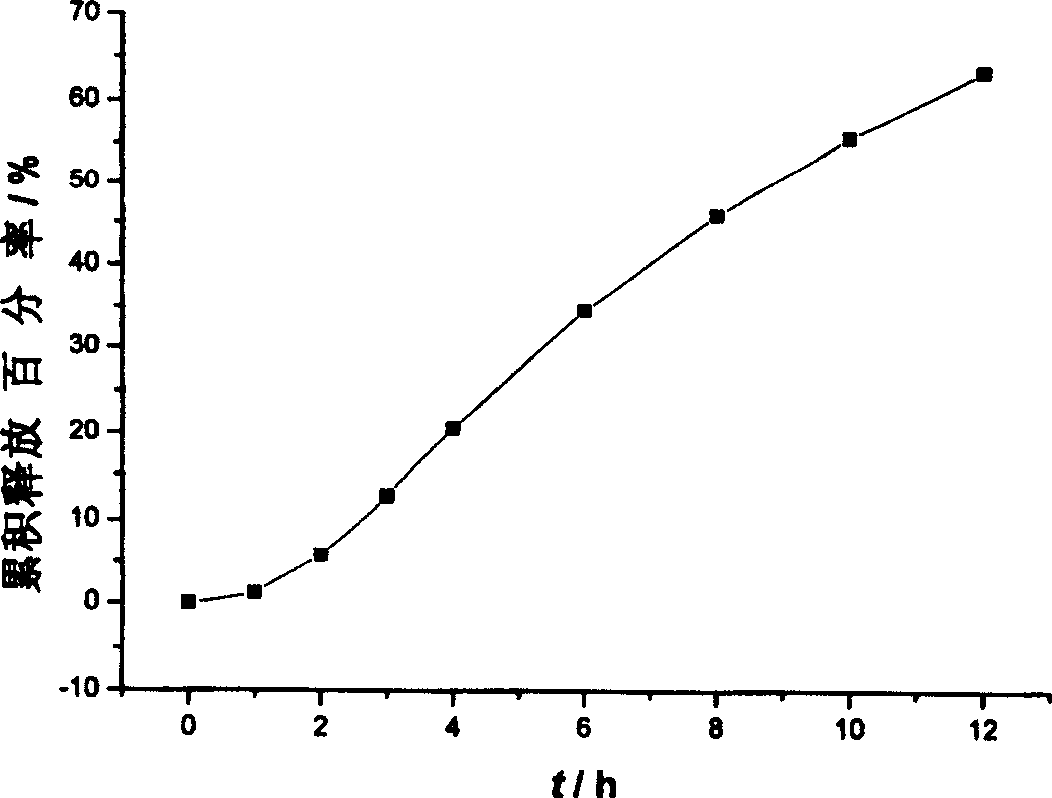
An osmotic pump controlled release tablet of solid dispersion of hydroxy camptothecin and a preparation method thereof
A technology of hydroxycamptothecin and solid dispersion, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc. Release and other issues, to achieve the effect of sustained drug release, improved bioavailability, and stable drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] Among them, the hydroxycamptothecin solid dispersion is prepared by mixing hydroxycamptothecin with a mass ratio of 1:2-1:30 with a polymer carrier, and the preparation method includes: a melting method, a solvent-melting preparation co-precipitation method and Spray drying method, etc.
[0035] The core of the single-layer osmotic pump tablet is prepared according to the following process: the solid dispersion of hydroxycamptothecin, the excipients for controlled release and other excipients are crushed and sieved, and the binders and lubricants in the other excipients After the materials are uniformly mixed, a soft material is made with a binder, sieved and granulated, dried at 50°C, sieved again to be granulated, and lubricant is added to form a tablet core.
[0036] The core of the push-pull osmotic pump tablet is prepared according to the following process: the solid dispersion of hydroxycamptothecin, the excipients for controlling release and other excipients are pulv...
Embodiment 1
[0039] Example 1: Preparation of hydroxycamptothecin double-layer osmotic pump tablets
[0040] Using film coating technology, wet granulation and tableting, laser drilling is performed on the coating film of the drug-containing layer as a drug release hole. Among them, the preparation of the hydroxycamptothecin solid dispersion adopts the solvent-melting method.
[0041] The composition of the solid dispersion of hydroxycamptothecin is (mass percentage):
[0042] Hydroxycamptothecin 17%
[0043] Polyethylene glycol 6000 83%
[0044] (The mass ratio of hydroxycamptothecin to polyethylene glycol 6000 is 17:83).
[0045] The content of every 100 osmotic pump tablets is as follows:
[0046] The composition of the drug-containing layer of the tablet core is:
[0047] Hydroxycamptothecin solid dispersion 9g
[0048] Polyoxyethylene 50 4g
[0050] Lactose 3g
[0051] Magnesium stearate 0.05g
[0052] The composition of the push layer is:
[0053] Polyoxyethyl...
Embodiment 2
[0064] Example 2: Preparation of hydroxycamptothecin double-layer osmotic pump tablets
[0065] As in Example 1, hydroxycamptothecin was prepared as a double-layer osmotic pump tablet. In this example, the preparation of the hydroxycamptothecin solid dispersion adopts the co-precipitation method.
[0066] The composition of the solid dispersion of hydroxycamptothecin is (mass percentage):
[0067] Hydroxycamptothecin 14%
[0068] Polyvinylpyrrolidone (K30) 86%
[0069] (The mass ratio of hydroxycamptothecin to polyvinylpyrrolidone is 7:43)
[0070] The content of every 100 osmotic pump tablets is as follows:
[0071] The composition of the drug-containing layer of the tablet core is:
[0072] Hydroxycamptothecin solid dispersion 9g
[0073] Polyoxyethylene 50 4g
[0075] Lactose 3g
[0076] Magnesium stearate 0.05g
[0077] The composition of the push layer is:
[0078] Polyoxyethylene 550 4g
[0079] Hydroxypropyl methylcellulose 1g
[0080] Sodium chl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com