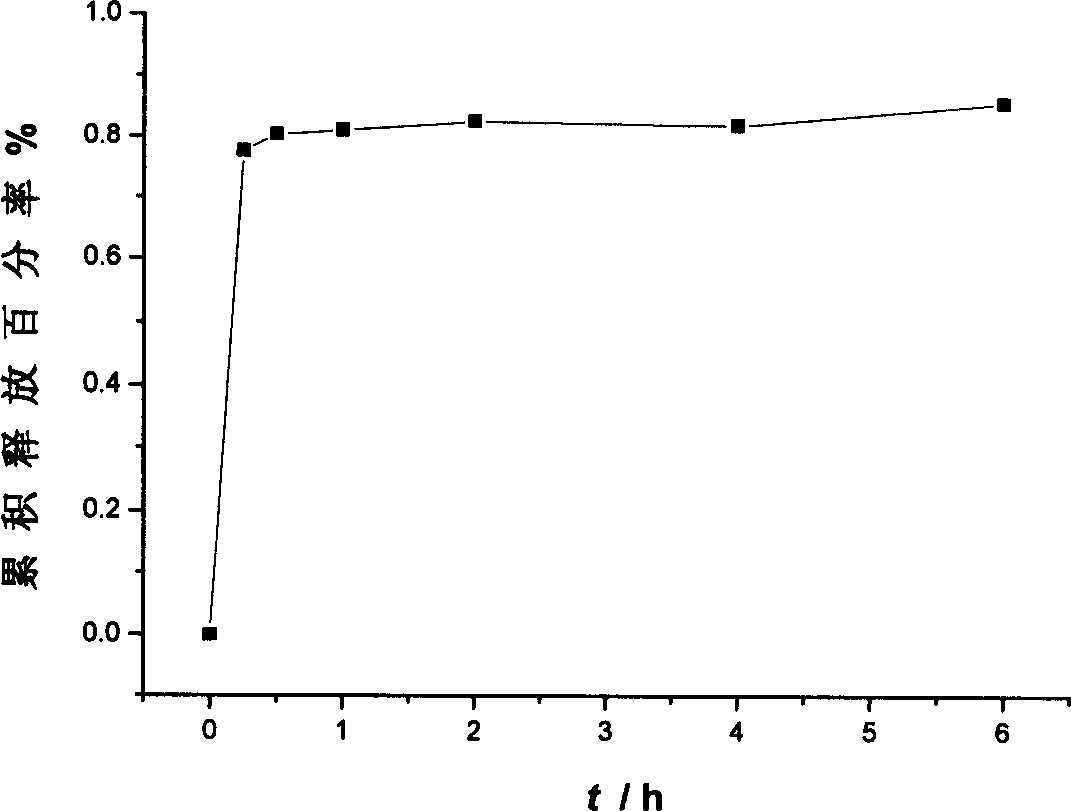
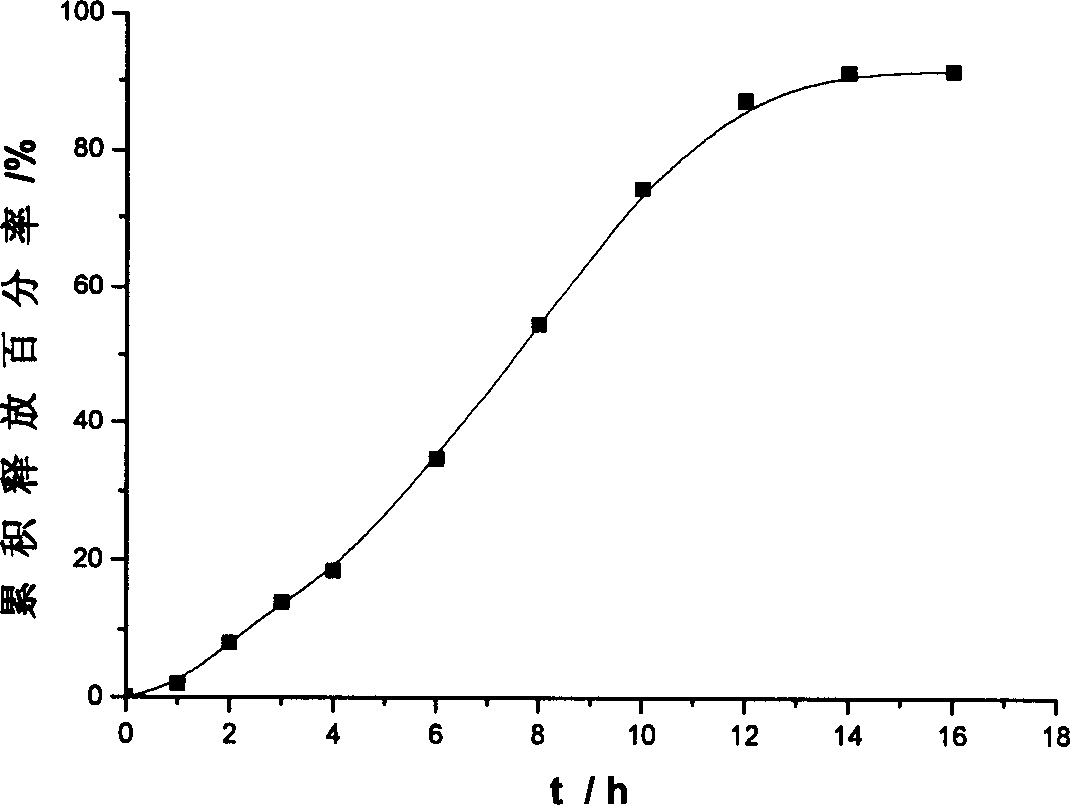
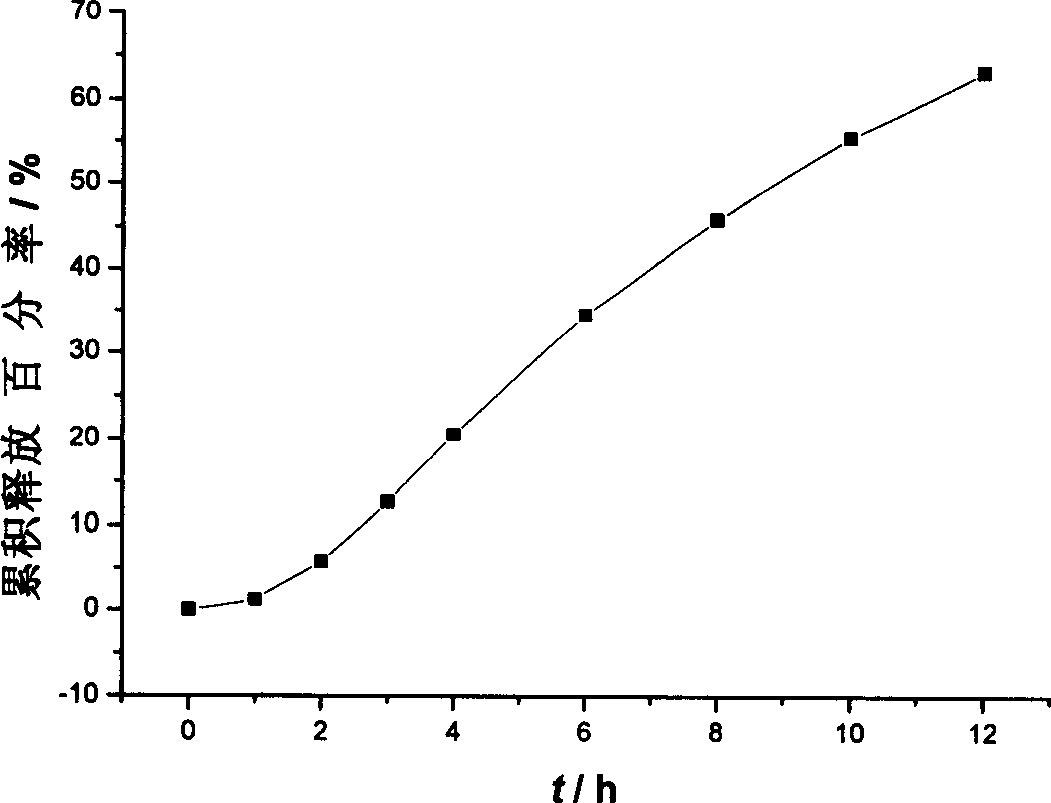
Osmotic pump controlled release tablet of solid dispersion of hydroxy camptothecin and prepn. method
A technology of hydroxycamptothecin and solid dispersions, which is applied in the direction of medical preparations containing non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. Low-level problems, to achieve sustained drug release, improve bioavailability, and constant-rate release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] Wherein, the hydroxycamptothecin solid dispersion is prepared by mixing hydroxycamptothecin with a mass ratio of 1:2-1:30 and a polymer carrier, and the preparation method includes: melting method, solvent-melting preparation coprecipitation method and spray drying, etc.
[0035] The core of the single-layer osmotic pump tablet is prepared according to the following process: the solid dispersion of hydroxycamptothecin, the excipients with controlled release effect and other excipients are pulverized and sieved respectively, mixed evenly, and the soft material is made with a binder, and the sieve is made. After granulation, it is dried at 50°C, sieved again for granulation, and pressed into tablet cores by adding lubricant.
[0036] The core of the push-pull osmotic pump tablet is prepared according to the following process: the solid dispersion of hydroxycamptothecin, the excipients with controlled release effect and other excipients are pulverized and sieved respective...
Embodiment 1
[0039] Example 1: Preparation of hydroxycamptothecin bilayer osmotic pump tablet
[0040] Using film coating technology, wet granulation and tableting, laser drilling is performed on the coating film of the drug-containing layer as drug release holes. Wherein the preparation of hydroxycamptothecin solid dispersion adopts solvent-melting method.
[0041] The composition of the hydroxycamptothecin solid dispersion is (mass percent):
[0042] Hydroxycamptothecin 17%
[0043] Macrogol 6000 83%
[0044] The content composition of each 100 osmotic pump tablets is as follows:
[0045] The composition of the drug-containing layer of the tablet core is:
[0046] Hydroxycamptothecin solid dispersion 9g
[0047] Polyoxyethylene 50 4g
[0049] Lactose 3g
[0050] Magnesium Stearate 0.05g
[0051] The push layer consists of:
[0052] Polyoxyethylene 550 4g
[0053] Hydroxypropyl methylcellulose 1g
[0055] Lactose 3g
[...
Embodiment 2
[0063] Example 2: Preparation of hydroxycamptothecin bilayer osmotic pump tablet
[0064] As in Example 1, hydroxycamptothecin was prepared into a double-layer osmotic pump tablet. In this example, the preparation of the hydroxycamptothecin solid dispersion adopts the coprecipitation method.
[0065] The composition of the hydroxycamptothecin solid dispersion is (mass percent):
[0066] Hydroxycamptothecin 14%
[0067] Polyvinylpyrrolidone (K30) 86%
[0068] The content composition of each 100 osmotic pump tablets is as follows:
[0069] The composition of the drug-containing layer of the tablet core is:
[0070] Hydroxycamptothecin solid dispersion 9g
[0071] Polyoxyethylene 50 4g
[0073] Lactose 3g
[0074] Magnesium Stearate 0.05g
[0075] The push layer consists of:
[0076] Polyoxyethylene 550 4g
[0077] Hydroxypropyl methylcellulose 1g
[0078] Sodium Chloride 2g
[0079] Lactose 3g
[0080] Magnesium Stearate 0.05g
[0081] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com