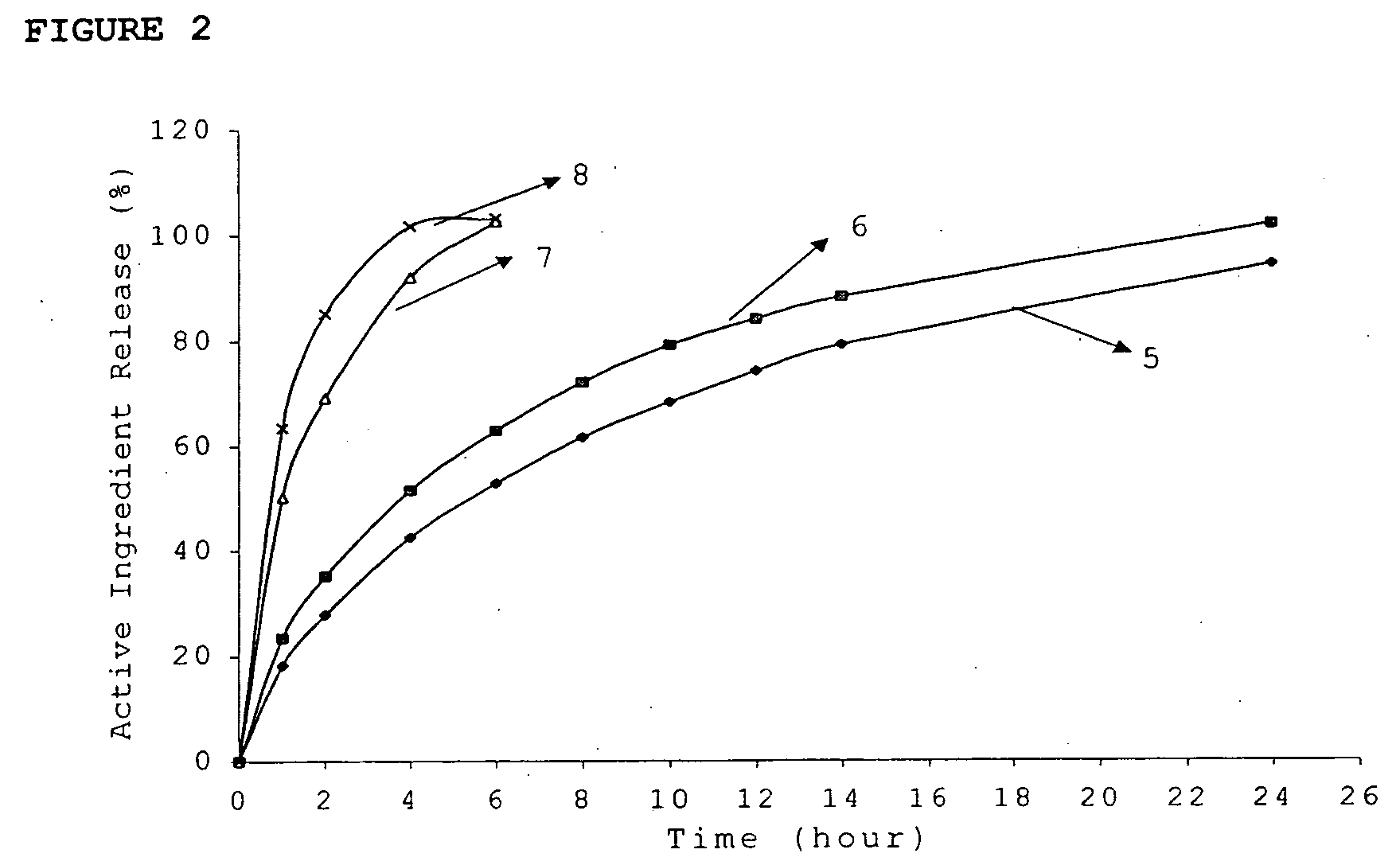
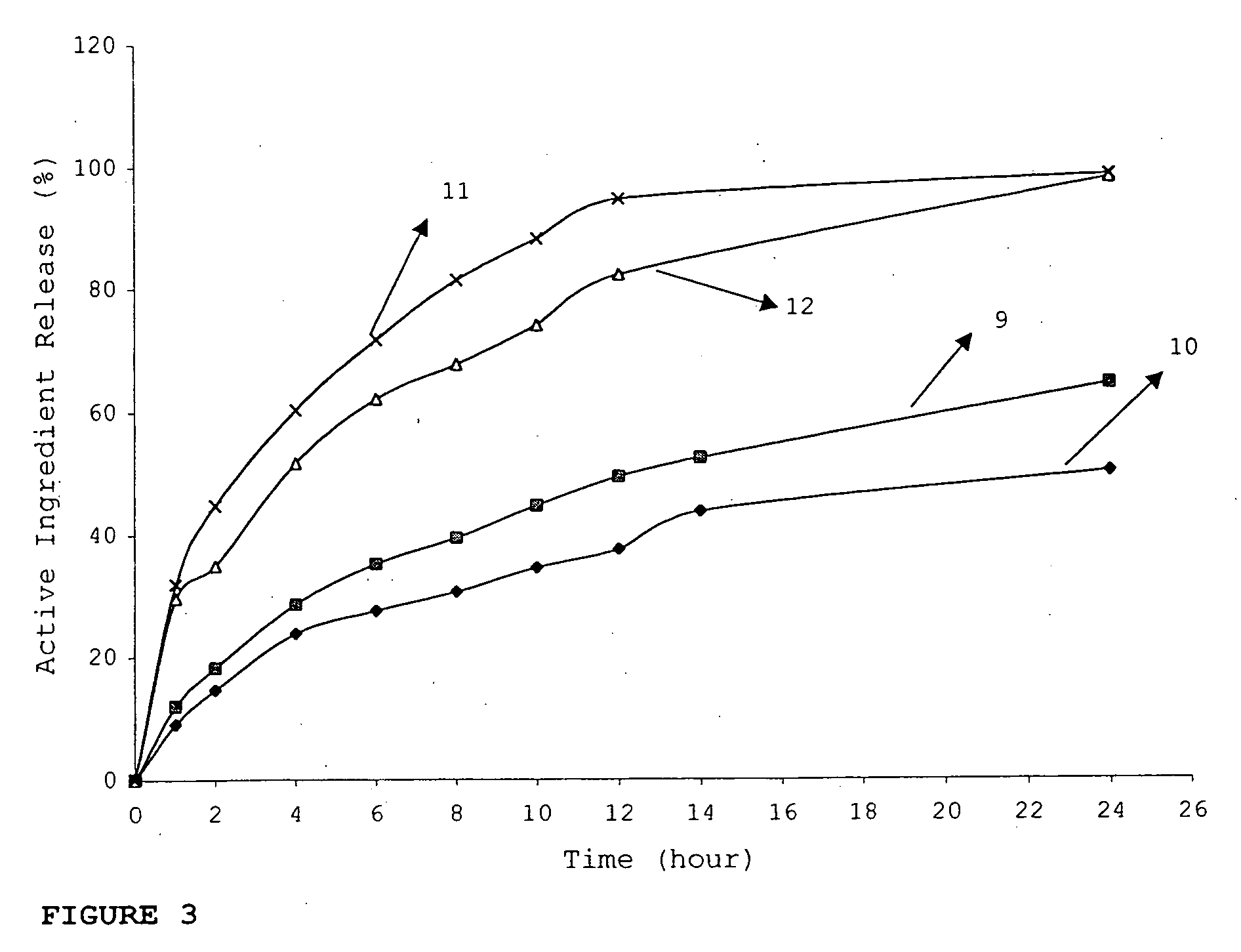
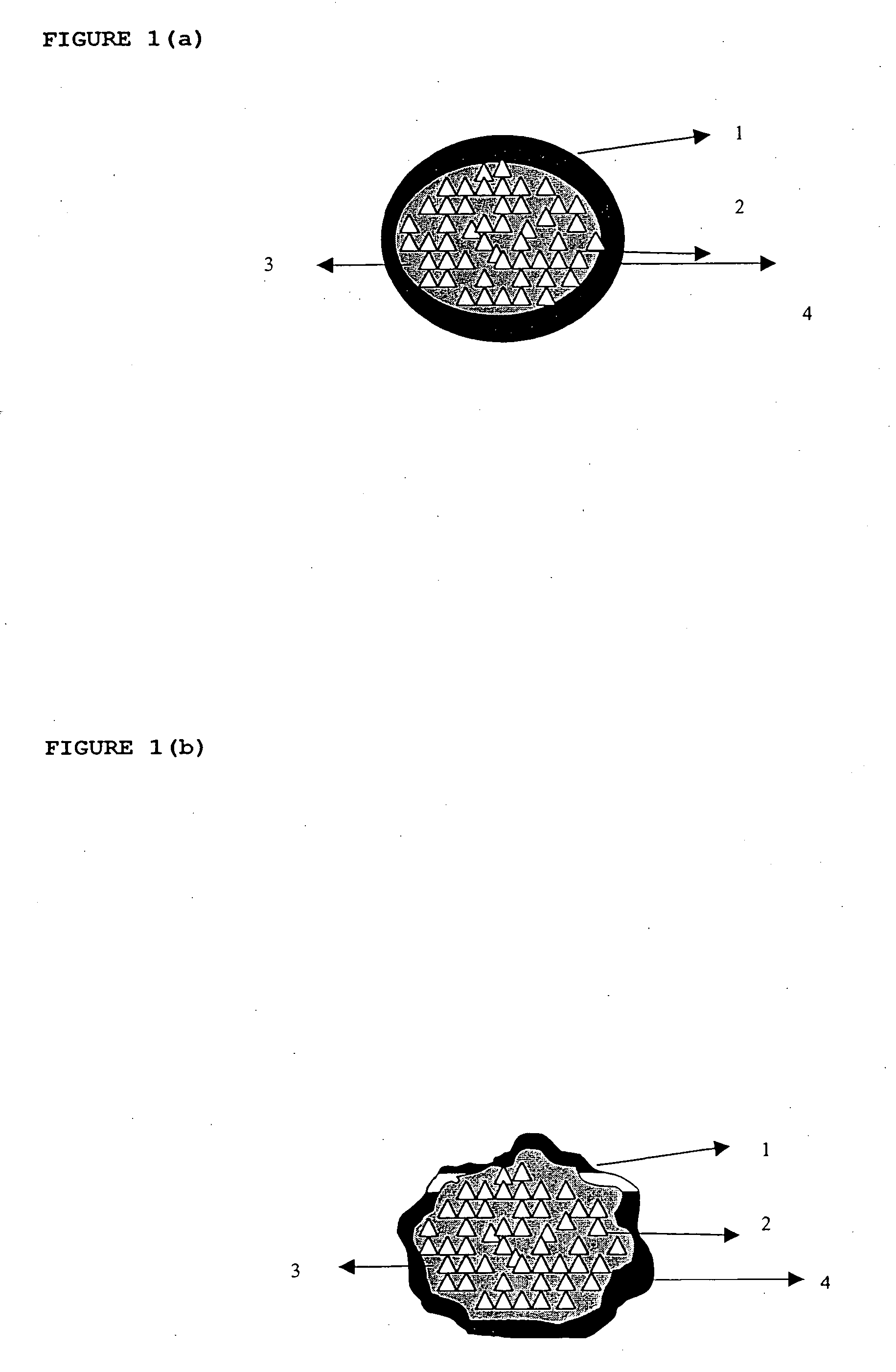
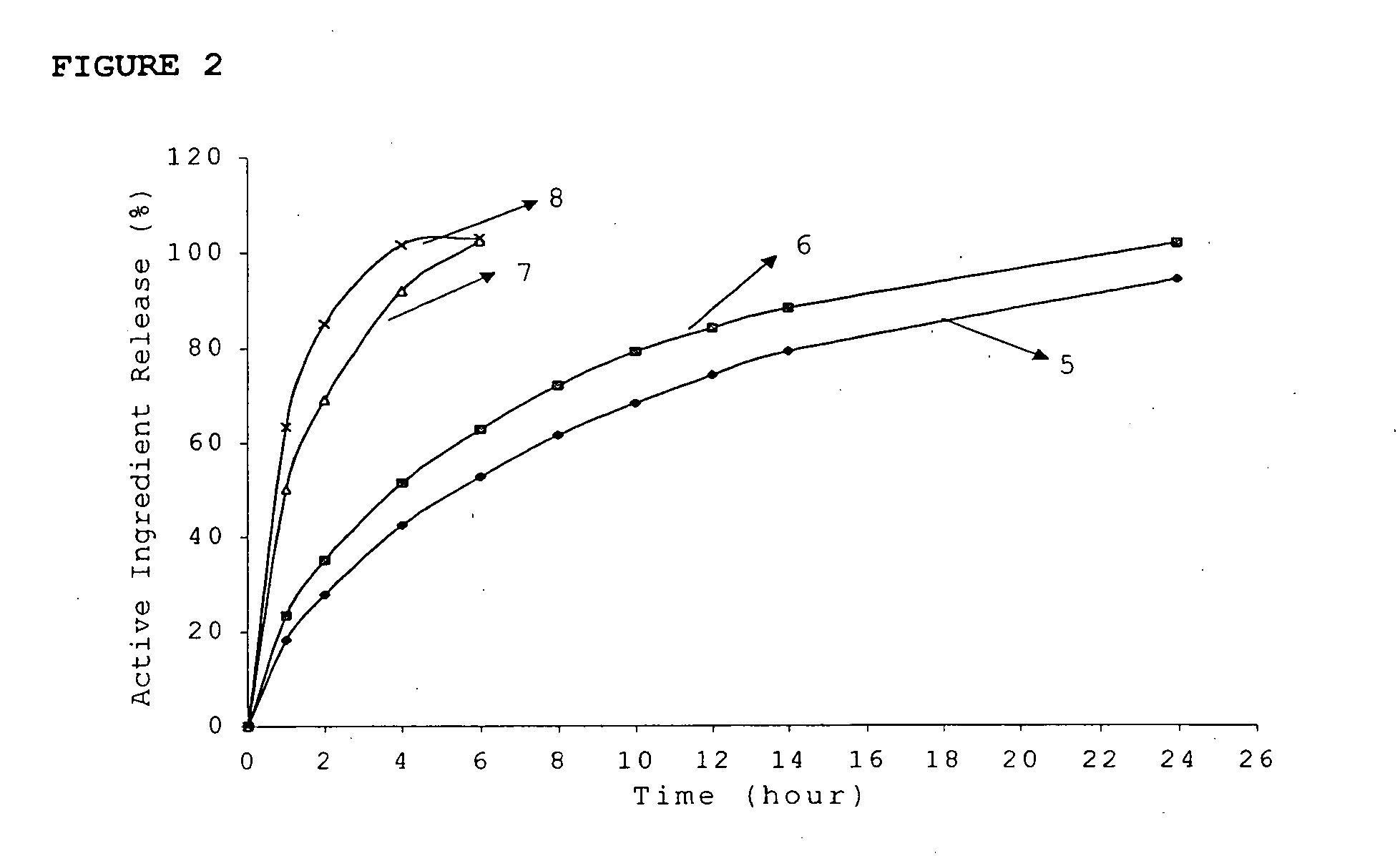
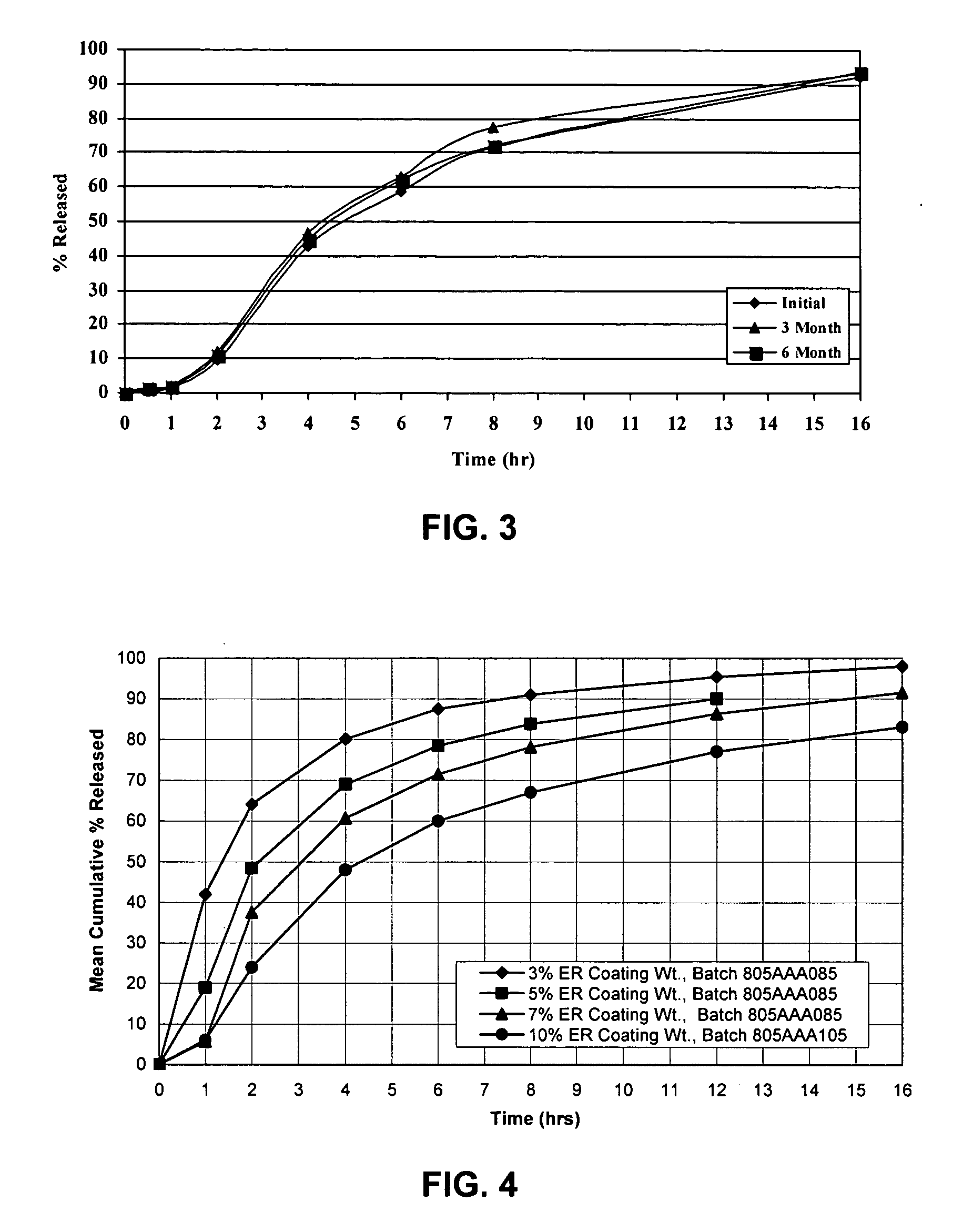
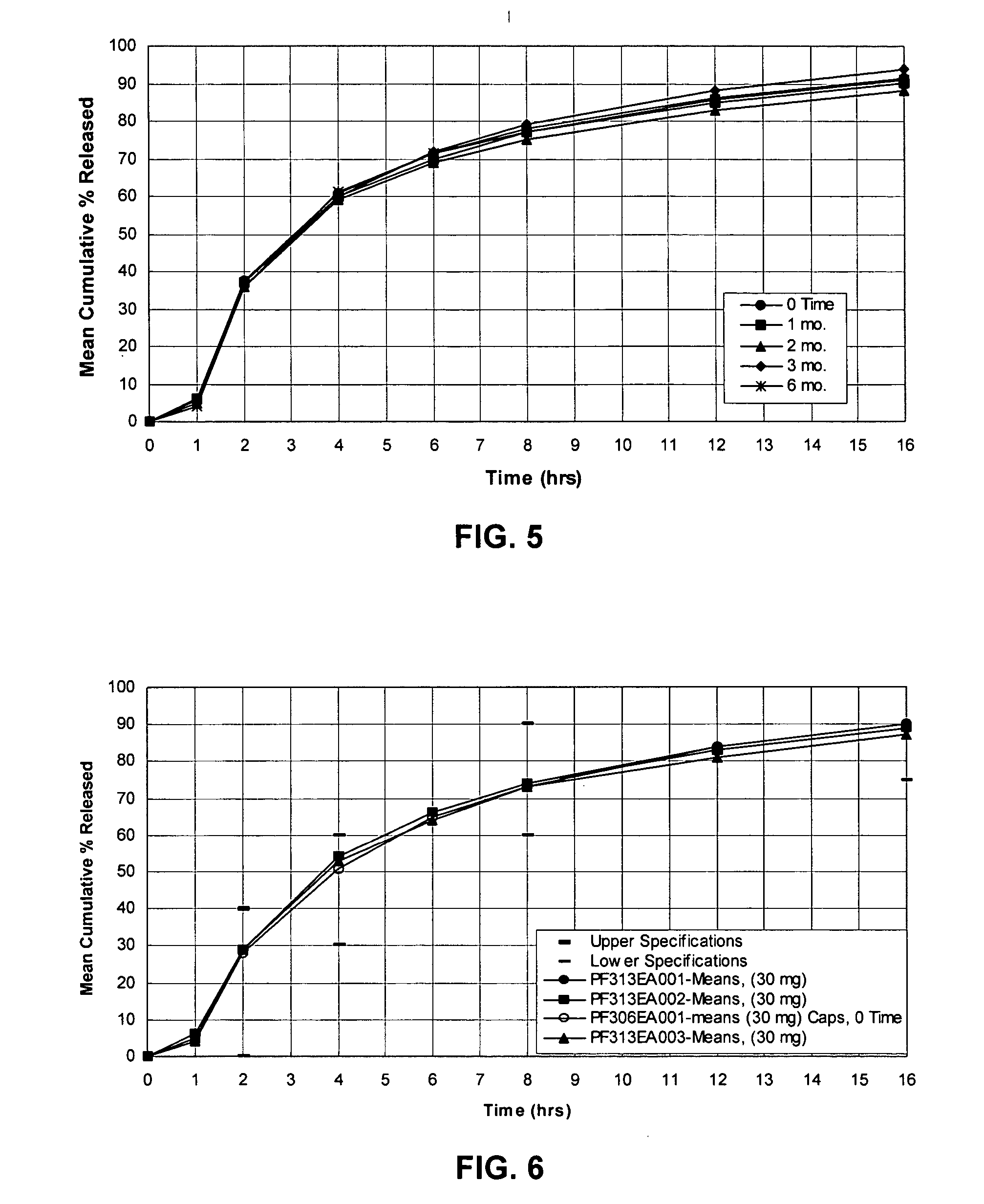
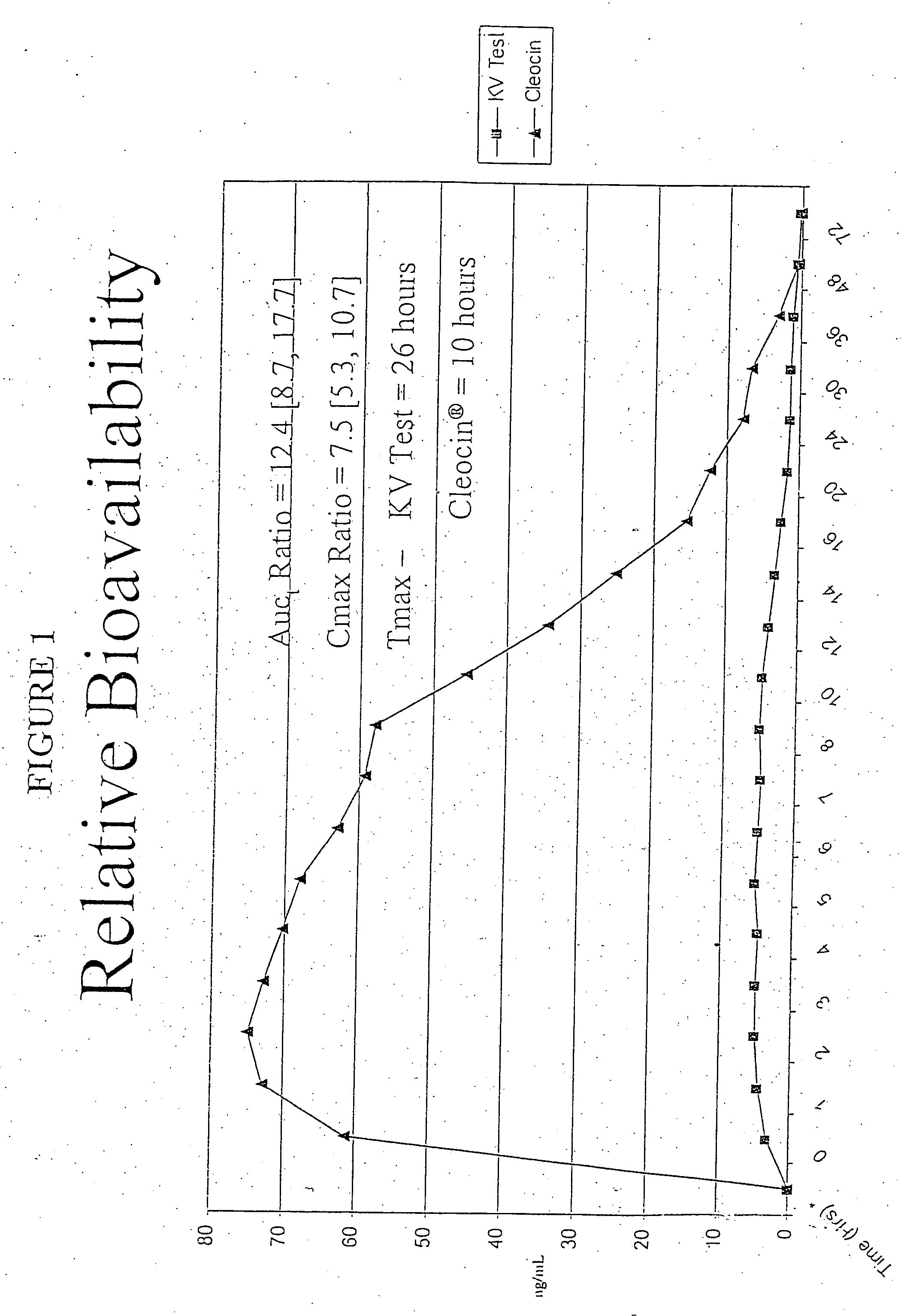
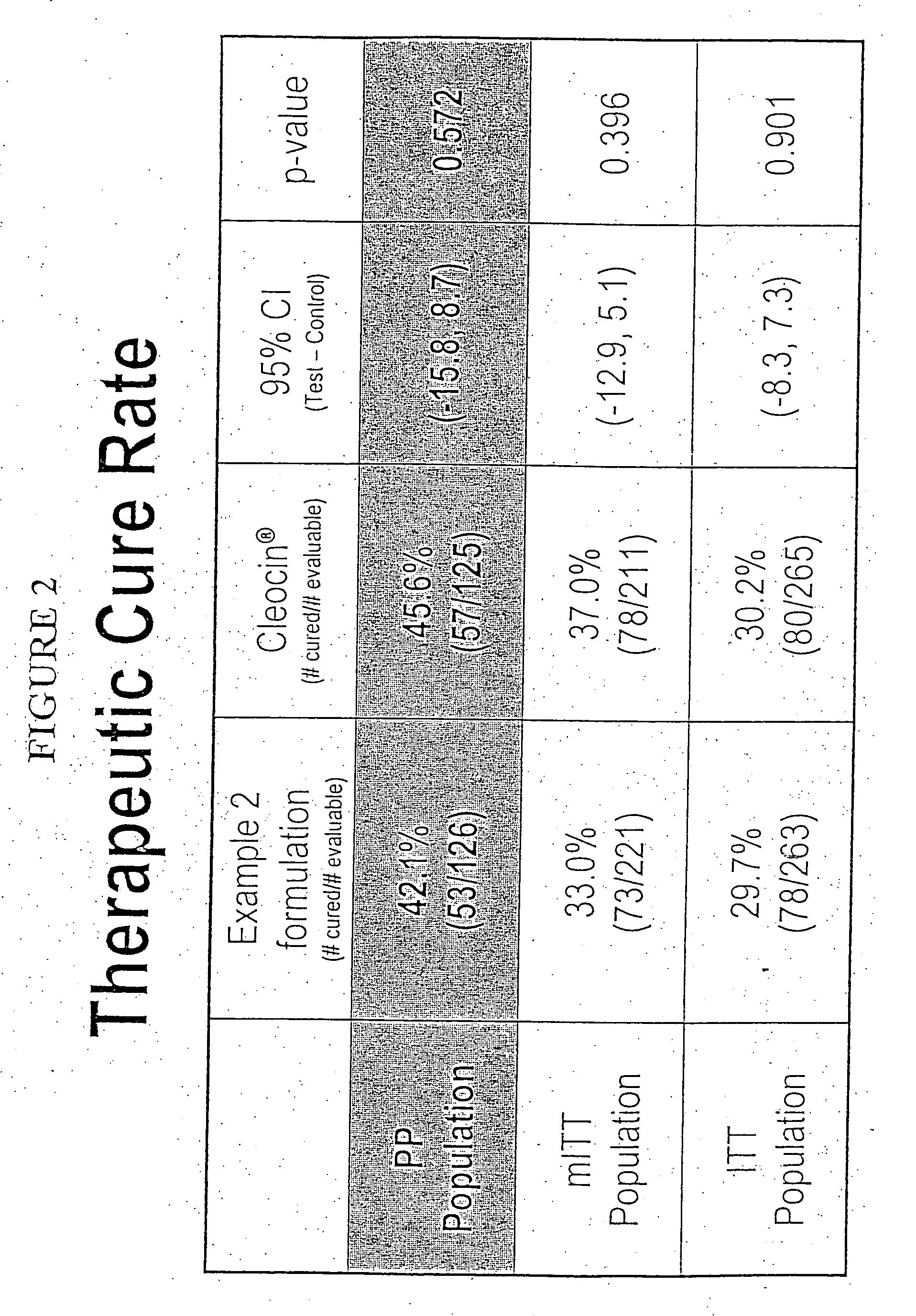
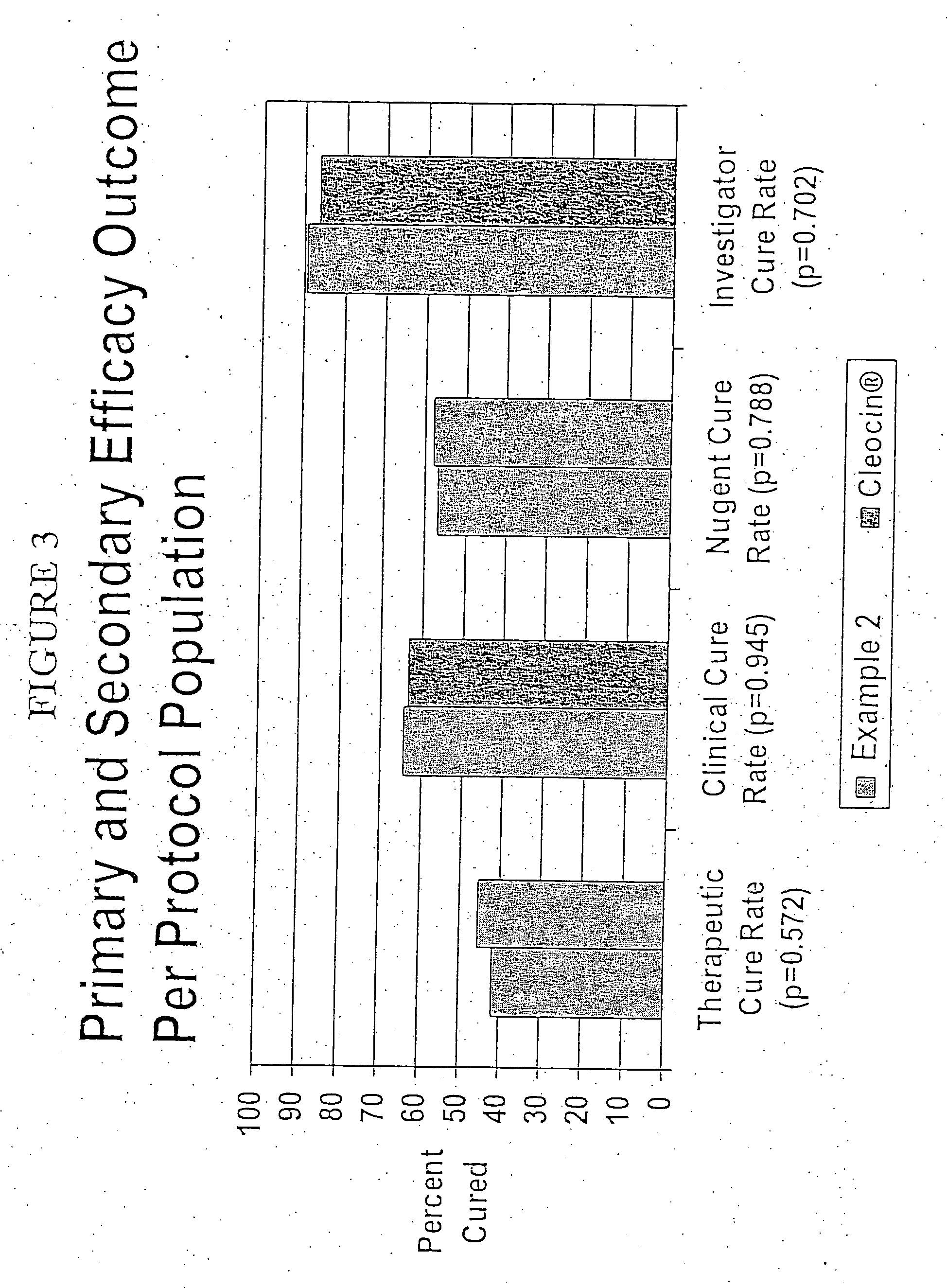
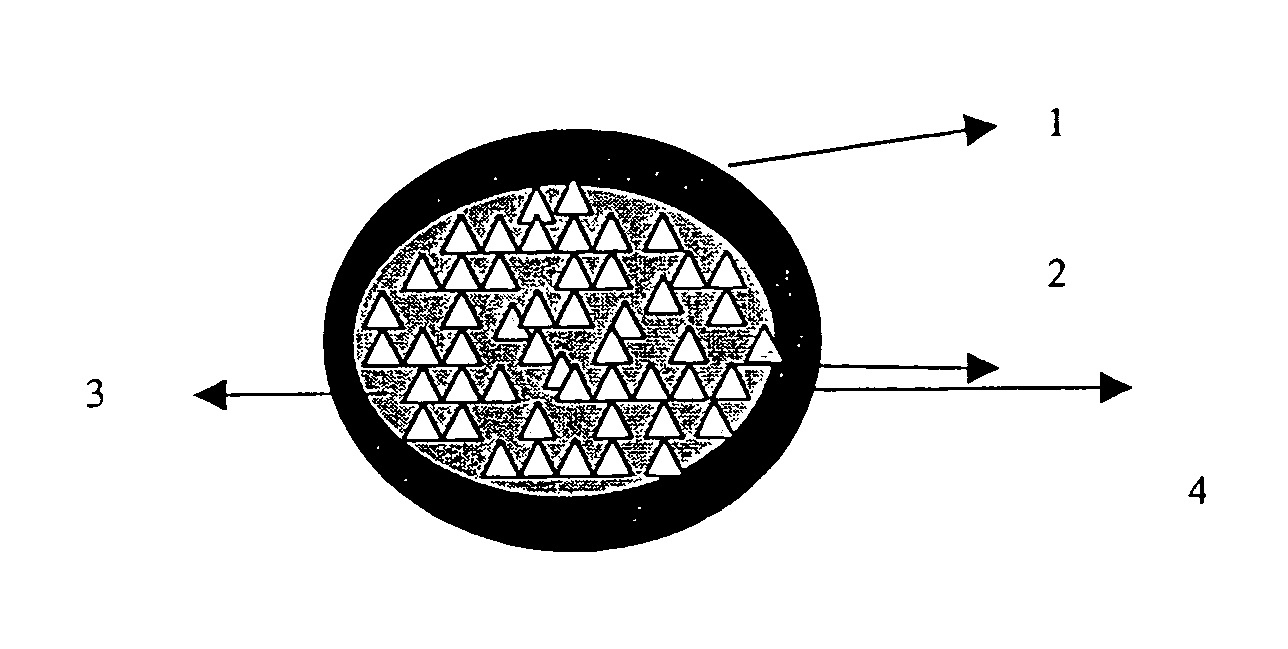
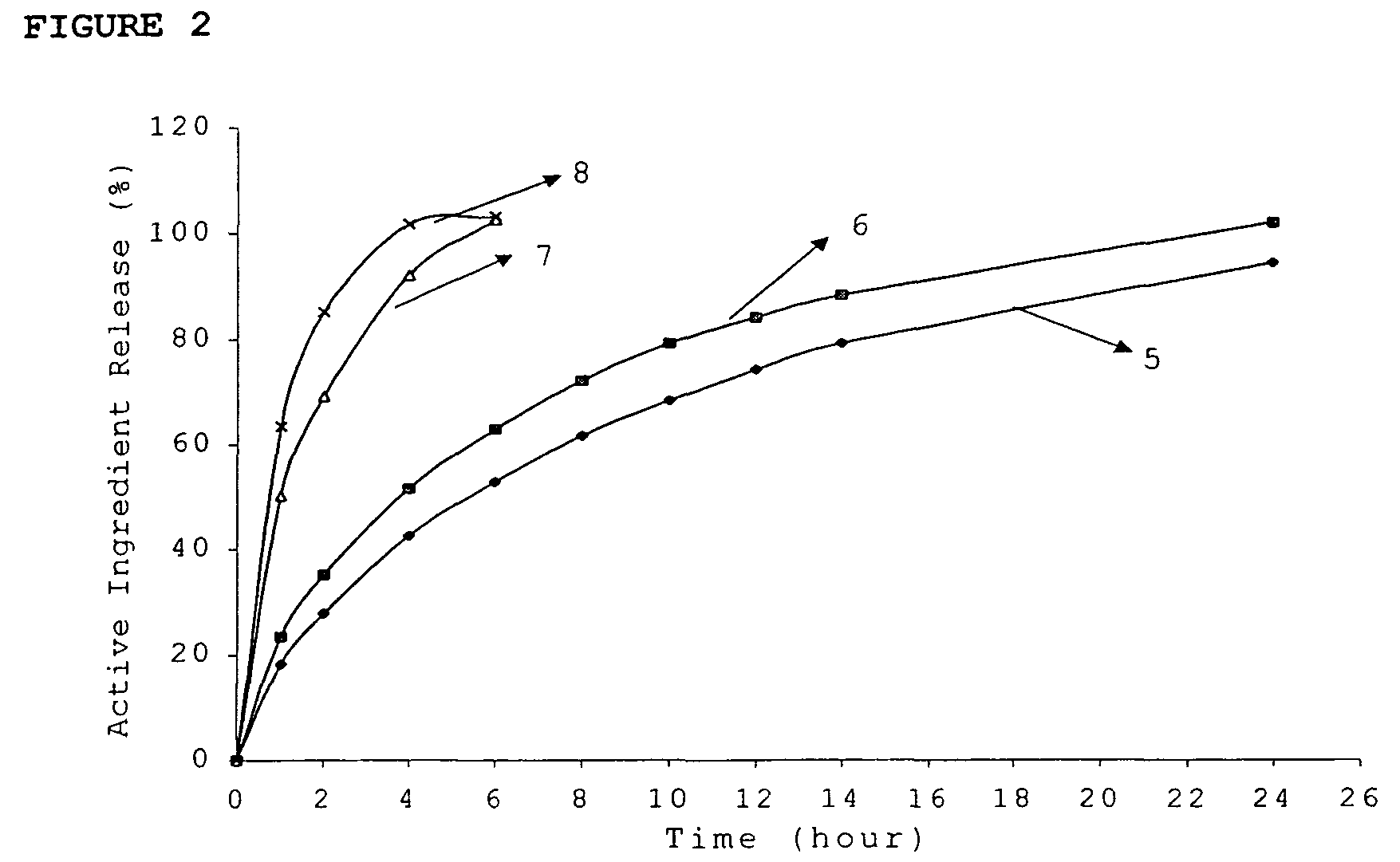
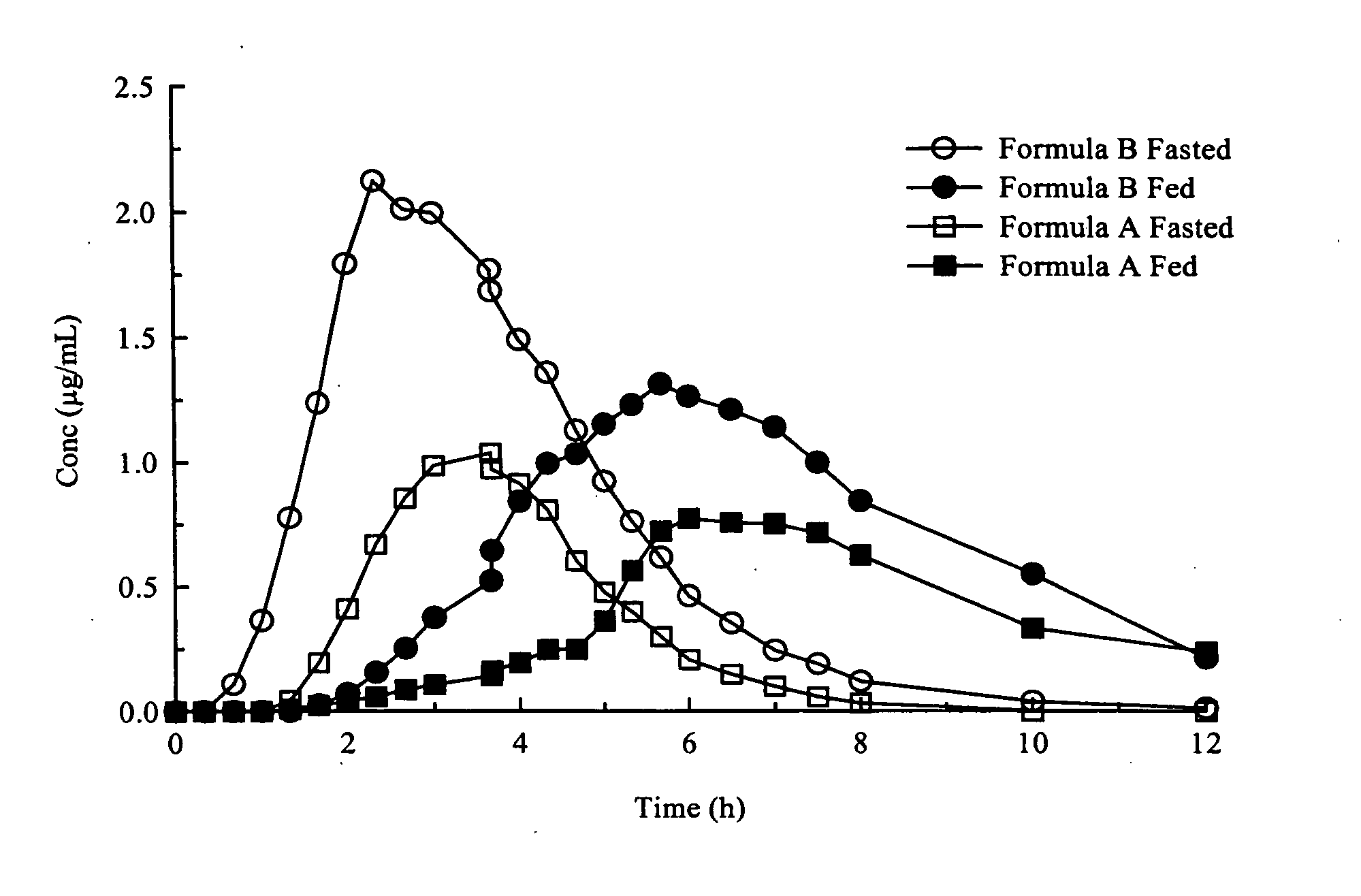
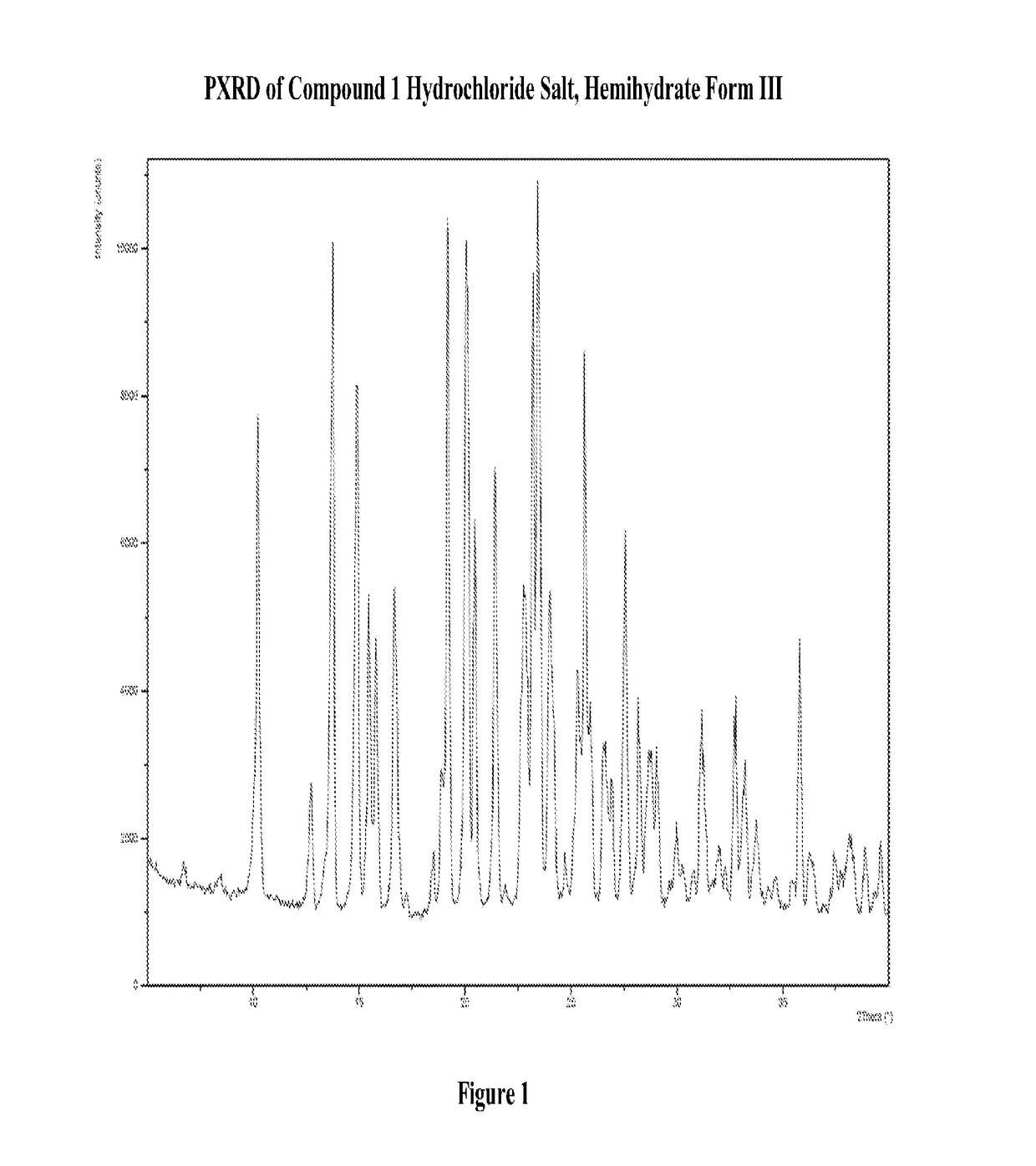
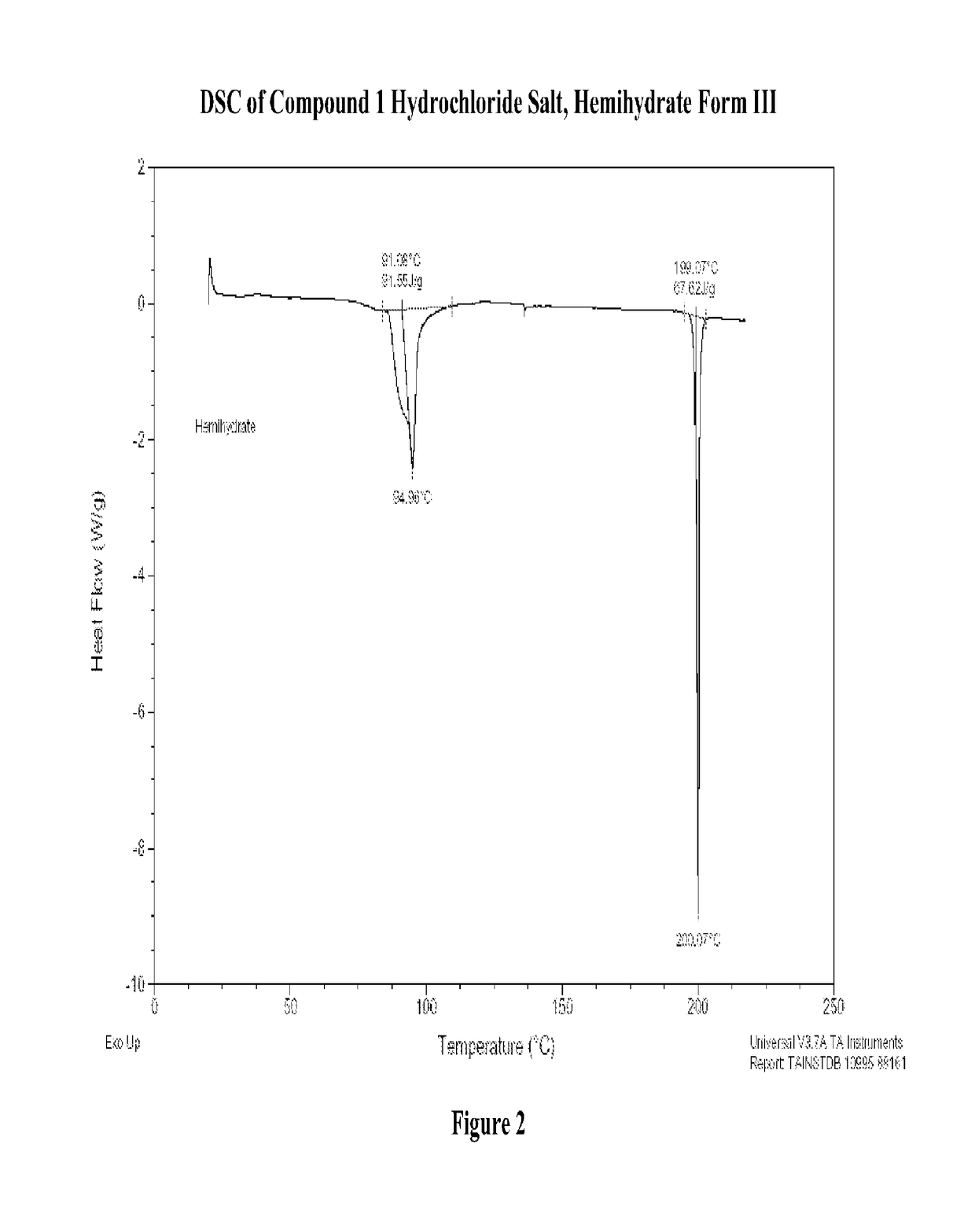
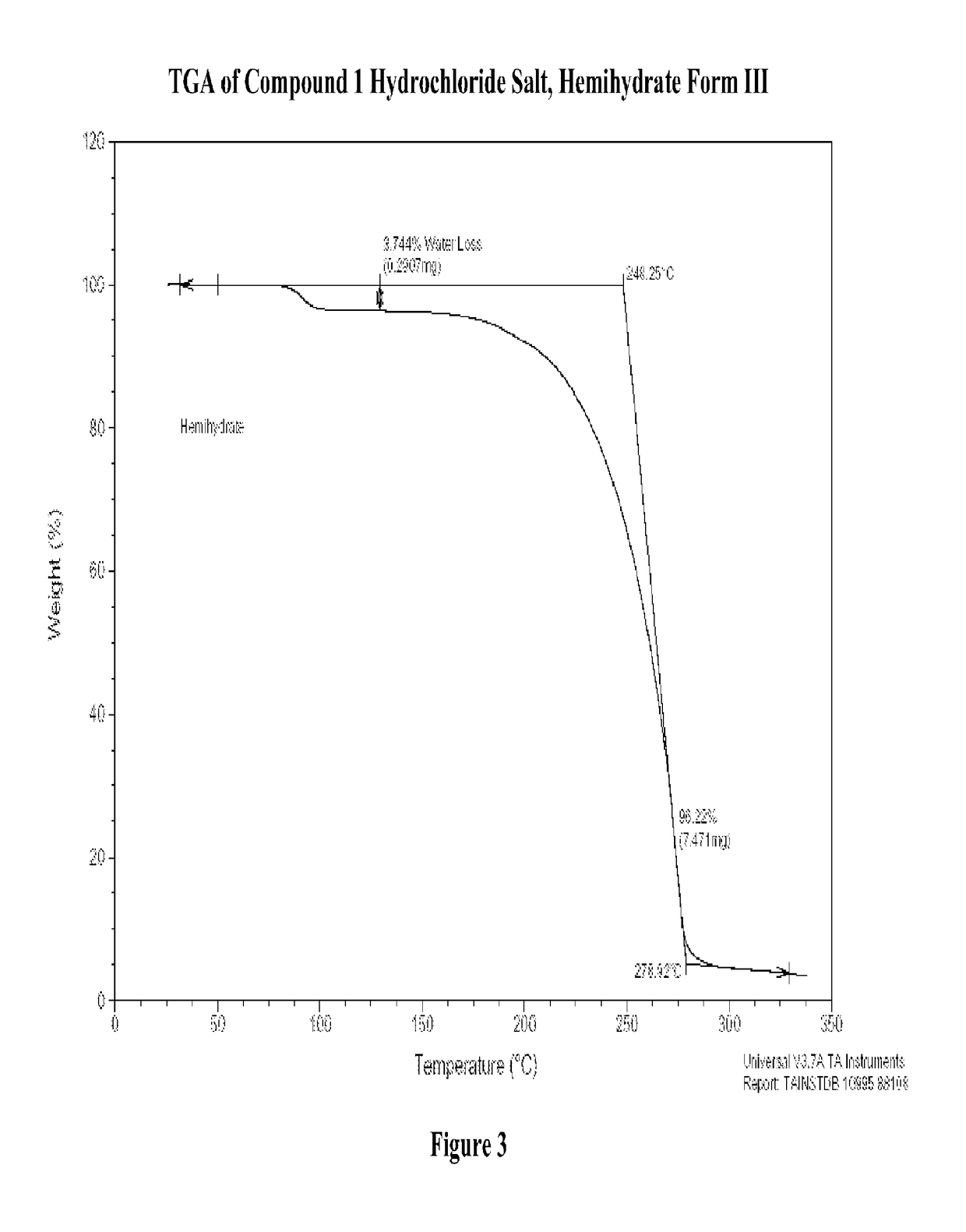
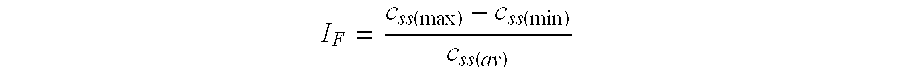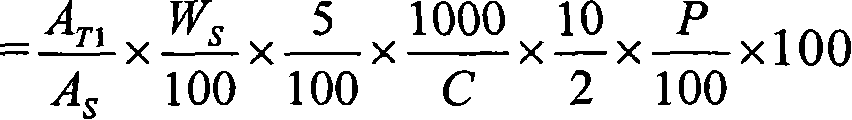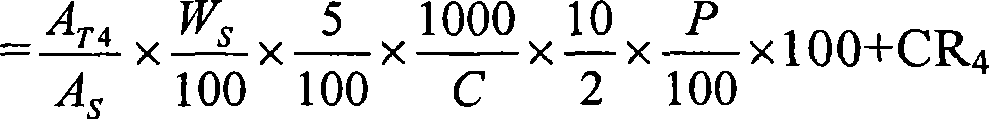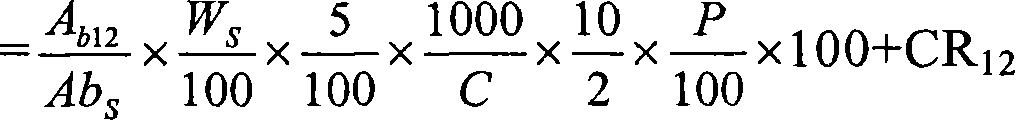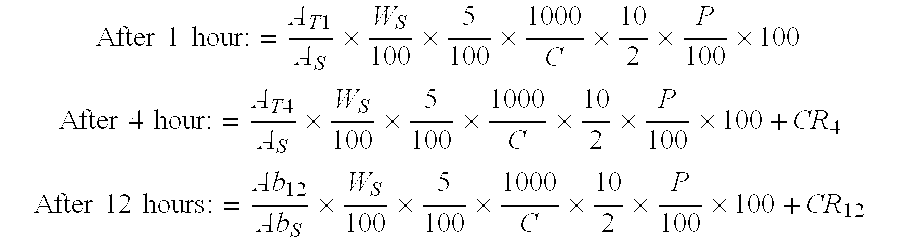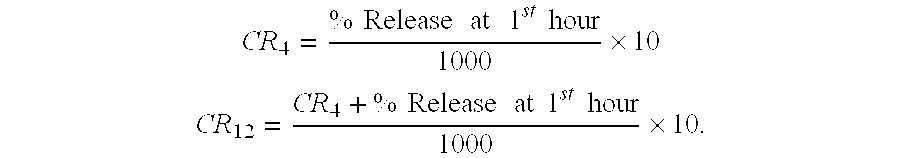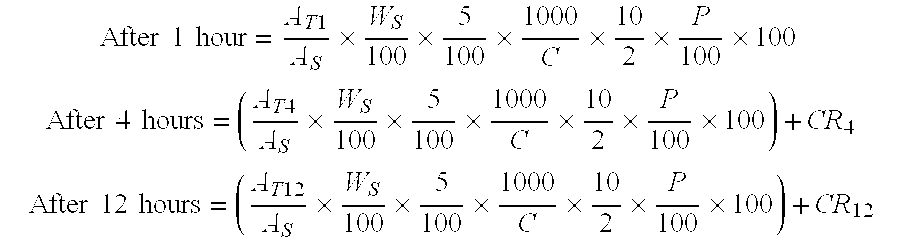Patents
Literature
35 results about "Modified Release Dosage Form" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A solid, semi-solid, solution or suspension exhibiting an altered inherent rate of release of active and/or inert ingredient(s).
Novel drug delivery system
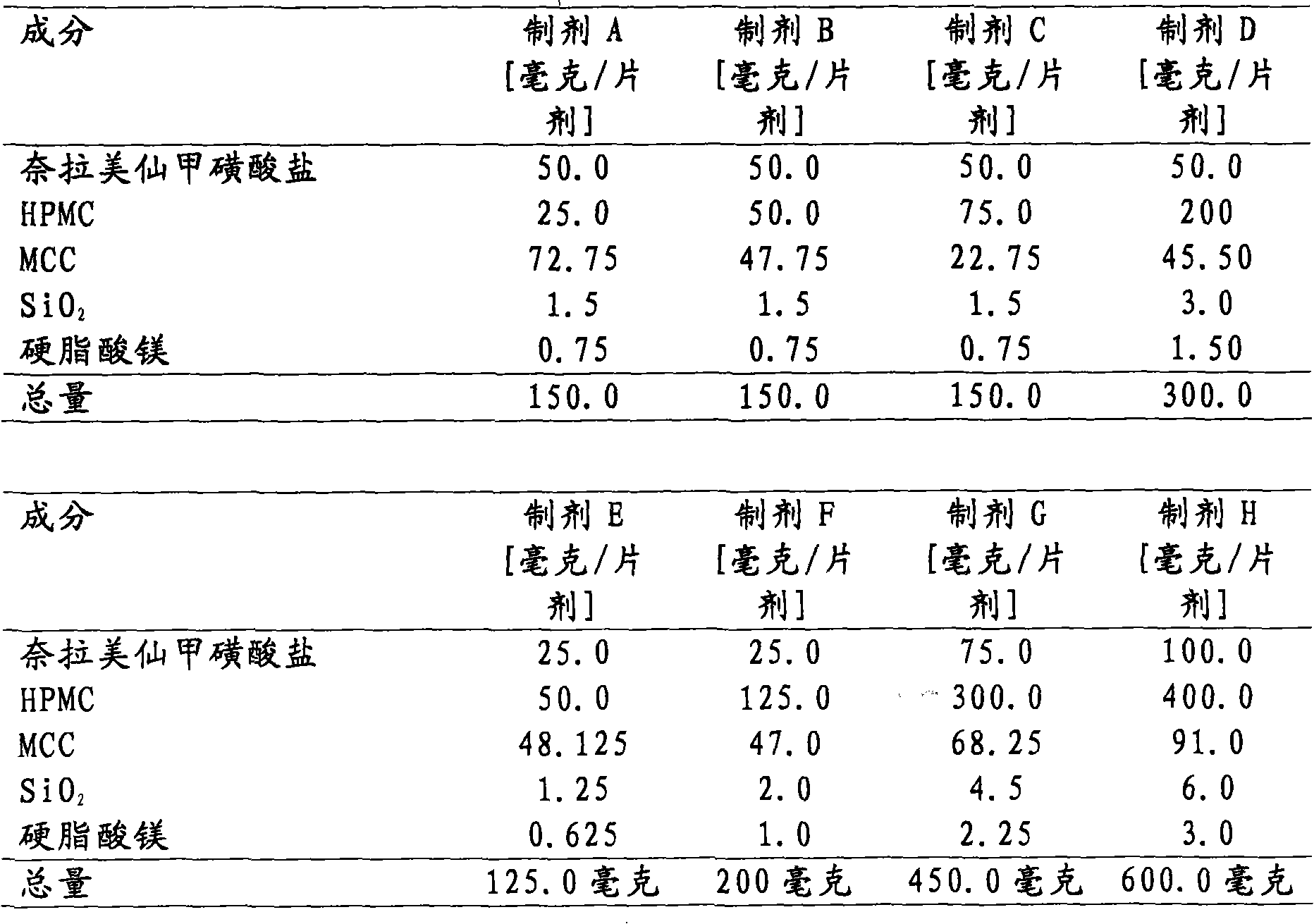
InactiveUS20060018933A1Effectively control release rateSmall sizePill deliveryAnhydride/acid/halide active ingredientsSolubilityModified Release Dosage Form
A novel modified release dosage form comprising of a high solubility active ingredient, which utilizes dual retard technique to effectively reduce the quantity of release controlling agents. Present invention can optionally comprise additionally another active ingredient as an immediate release form or modified release form. Present invention also relates to a process for preparing the said formulation.
Owner:TORRENT PHARMA LTD
Novel drug delivery system
InactiveUS20060018934A1Effectively control release rateSmall sizePill deliveryMicrocapsulesSolubilityModified Release Dosage Form
A novel modified release dosage form comprising of a high solubility active ingredient, which utilizes dual retard technique to effectively reduce the quantity of release controlling agents. Present invention can optionally comprise additionally another active ingredient as an immediate release form or modified release form. Present invention also relates to a process for preparing the said formulation.
Owner:TORRENT PHARMA LTD
Modified release composition for highly soluble drugs
InactiveUS8268352B2Effectively control release rateSmall sizePill deliveryMicrocapsulesSolubilityModified Release Dosage Form
A novel modified release dosage form comprising of a high solubility active ingredient, which utilizes dual retard technique to effectively reduce the quantity of release controlling agents. Present invention can optionally comprise additionally another active ingredient as an immediate release form or modified release form. Present invention also relates to a process for preparing the said formulation.
Owner:TORRENT PHARMA LTD
Neramexane MR matrix tablet
InactiveUS20070141148A1Low peak concentrationReduce absorptionBiocideSenses disorderRegimenModified Release Dosage Form
The invention provides novel oral modified release dosage forms of neramexane which are useful for the continuous therapy of patients suffering from diseases and conditions such as Alzheimer's dementia and neuropathic pain. The compositions have drug release profiles that are suitable for achieving steady state plasma concentrations of neramexane which have relatively small fluctuation when administered on a twice-daily or even once-daily regimen. The dosage forms may be designed as modified release matrix tablets, which are optionally coated for taste masking. The invention further provides therapeutic methods of treating conditions such as Alzheimer's dementia and neuropathic pain which involve the administration of such dosage forms.
Owner:MERZ PHARMA GMBH & CO KGAA
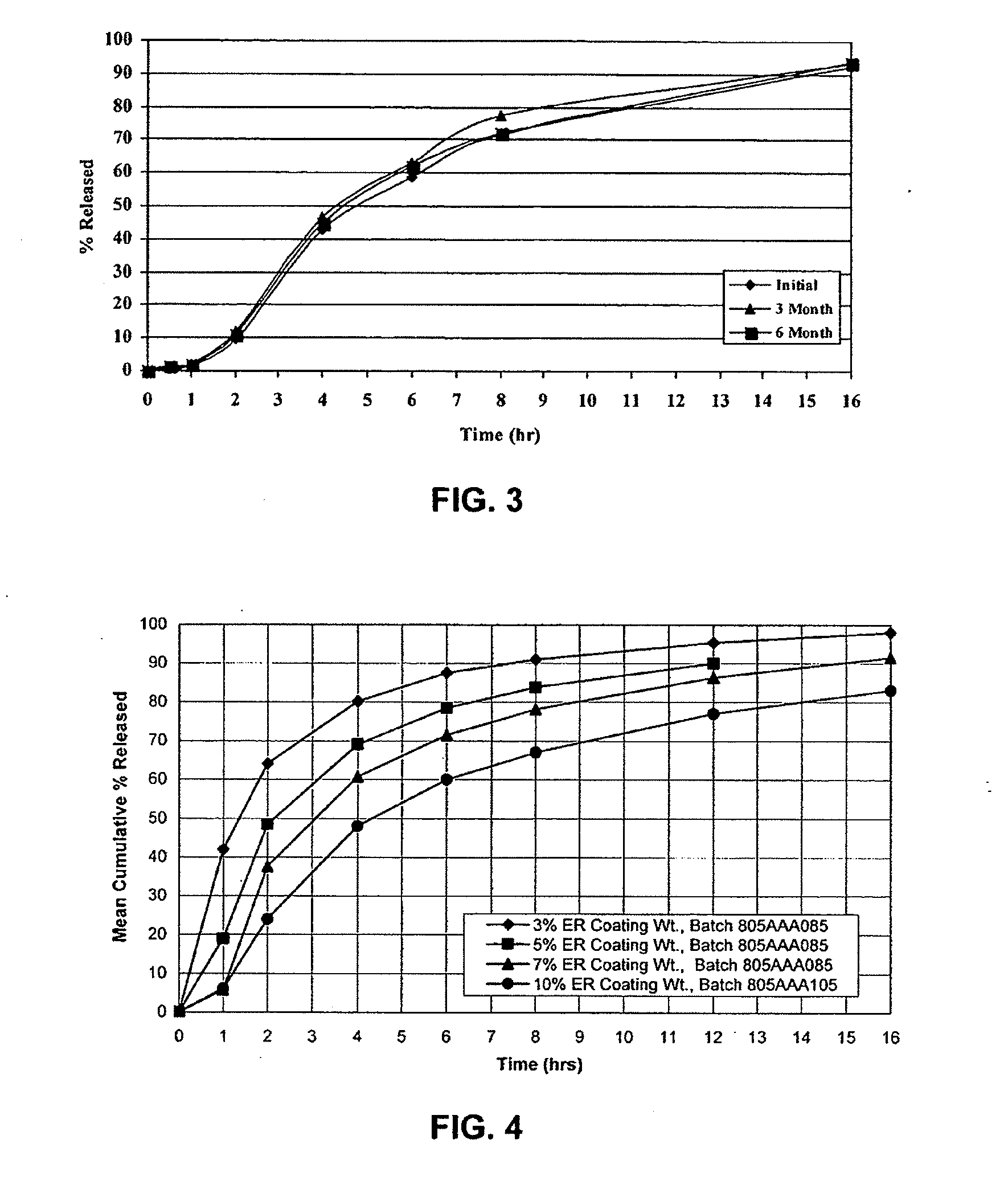
Modified release dosage forms
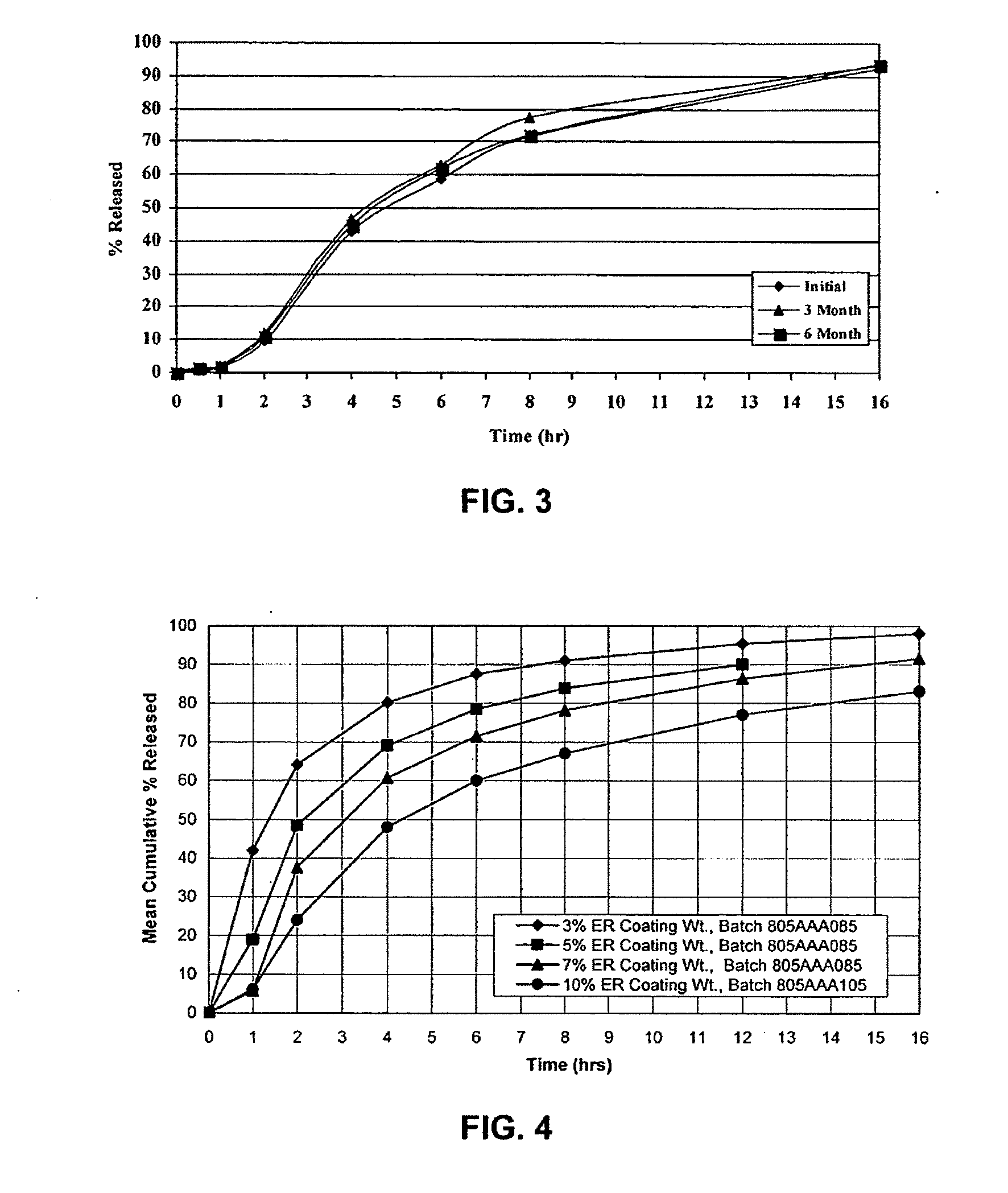
In one embodiment a dosage form comprises at least one active ingredient and a molded matrix which comprises 10-100% of a material having a melting point of less than about 100 degrees C. selected from the stamp consisting of thermoplastic polyalkylene oxides, low melting hydrophobic materials, thermoplastic polymers, thermoplastic starches and combinations thereof, and the matrix is capable of providing modified release of the active ingredient upon contacting of the dosage form with a liquid medium. The dosage form may additionally comprise uncoated particles which may contain at least one active ingredient. In another embodiment, a dosage form comprises at least one active ingredient, a plurality of particles and a molded matrix, wherein at least a portion of the particles are coated. The coated particles, the matrix or both may comprise at least one active ingredient, and the coated particles or the matrix or a combination thereof is capable of providing modified release of the active ingredient upon contacting of the dosage form with a liquid medium.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Modified release dosage forms of skeletal muscle relaxants
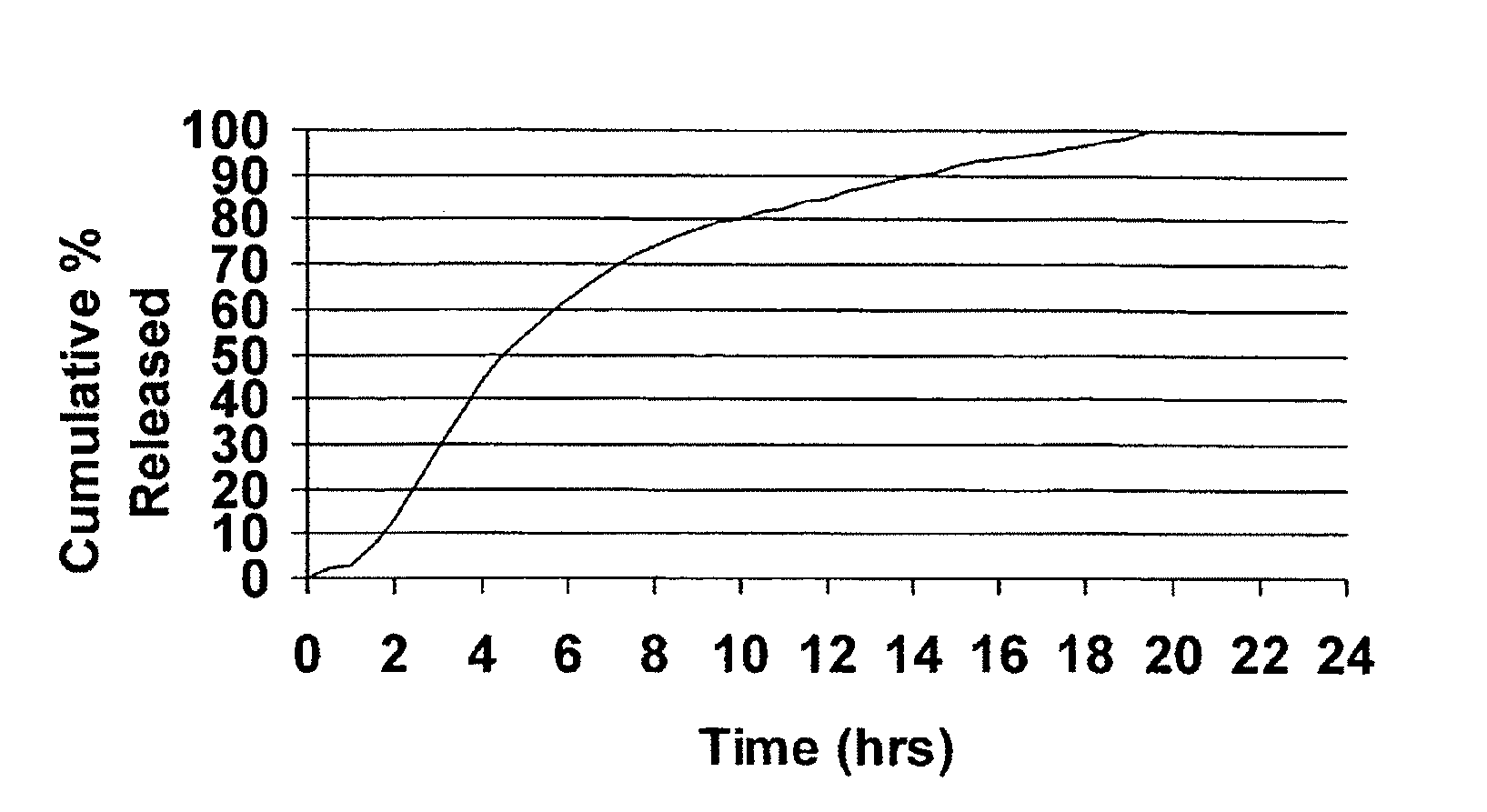
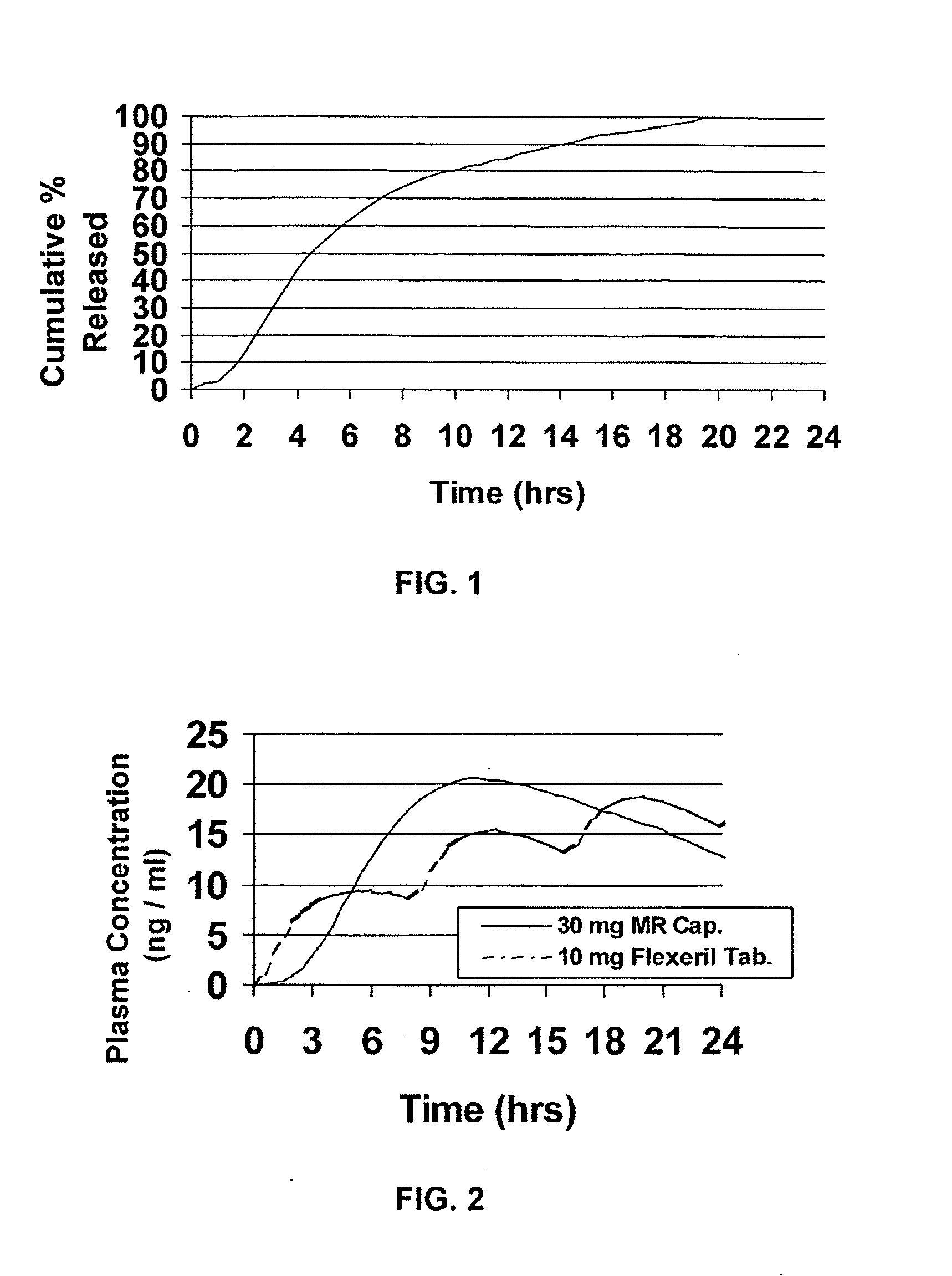
InactiveUS20050106247A1Patient compliance is goodEfficient ConcentrationBiocidePowder deliveryDiseaseModified Release Dosage Form
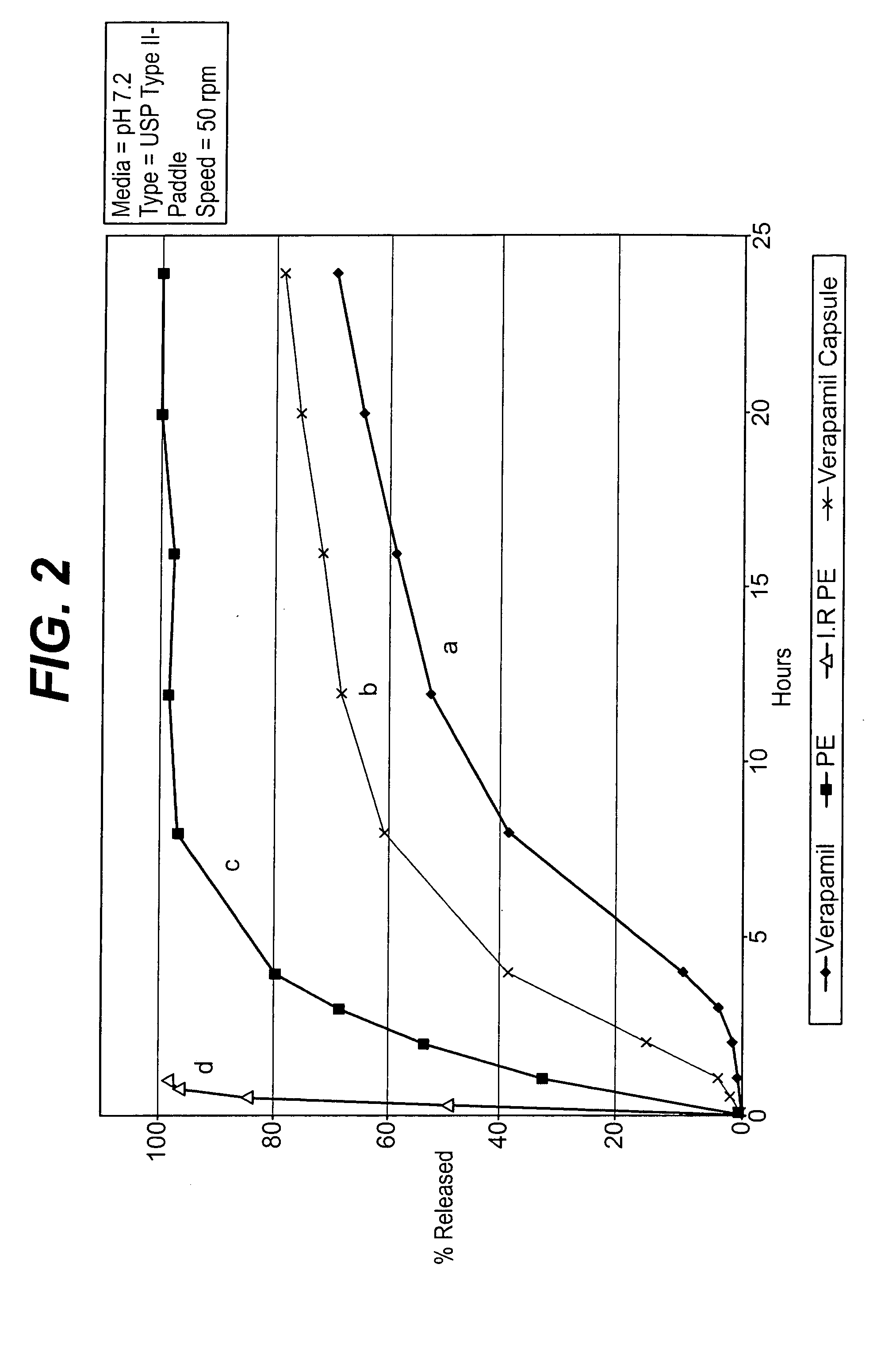
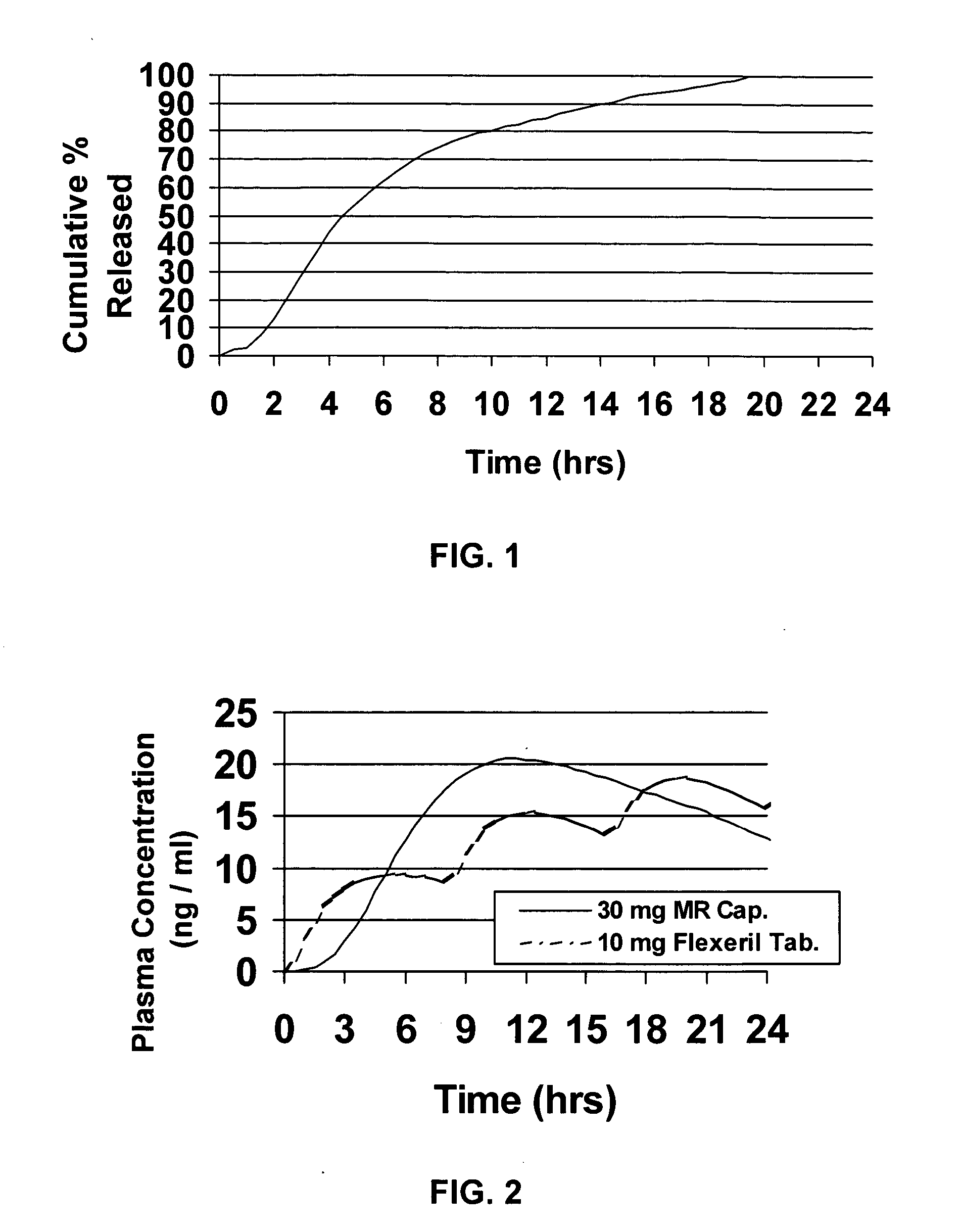
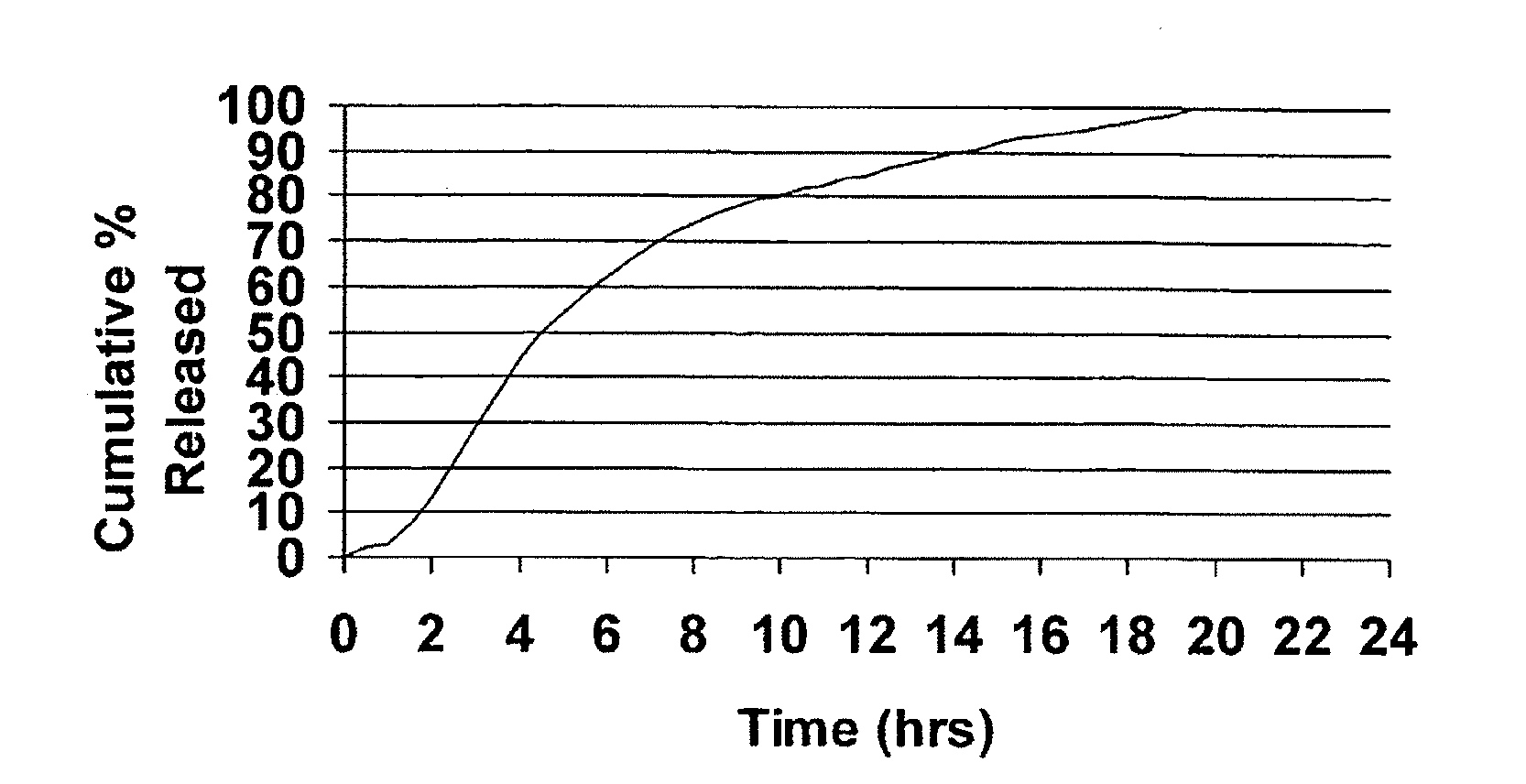
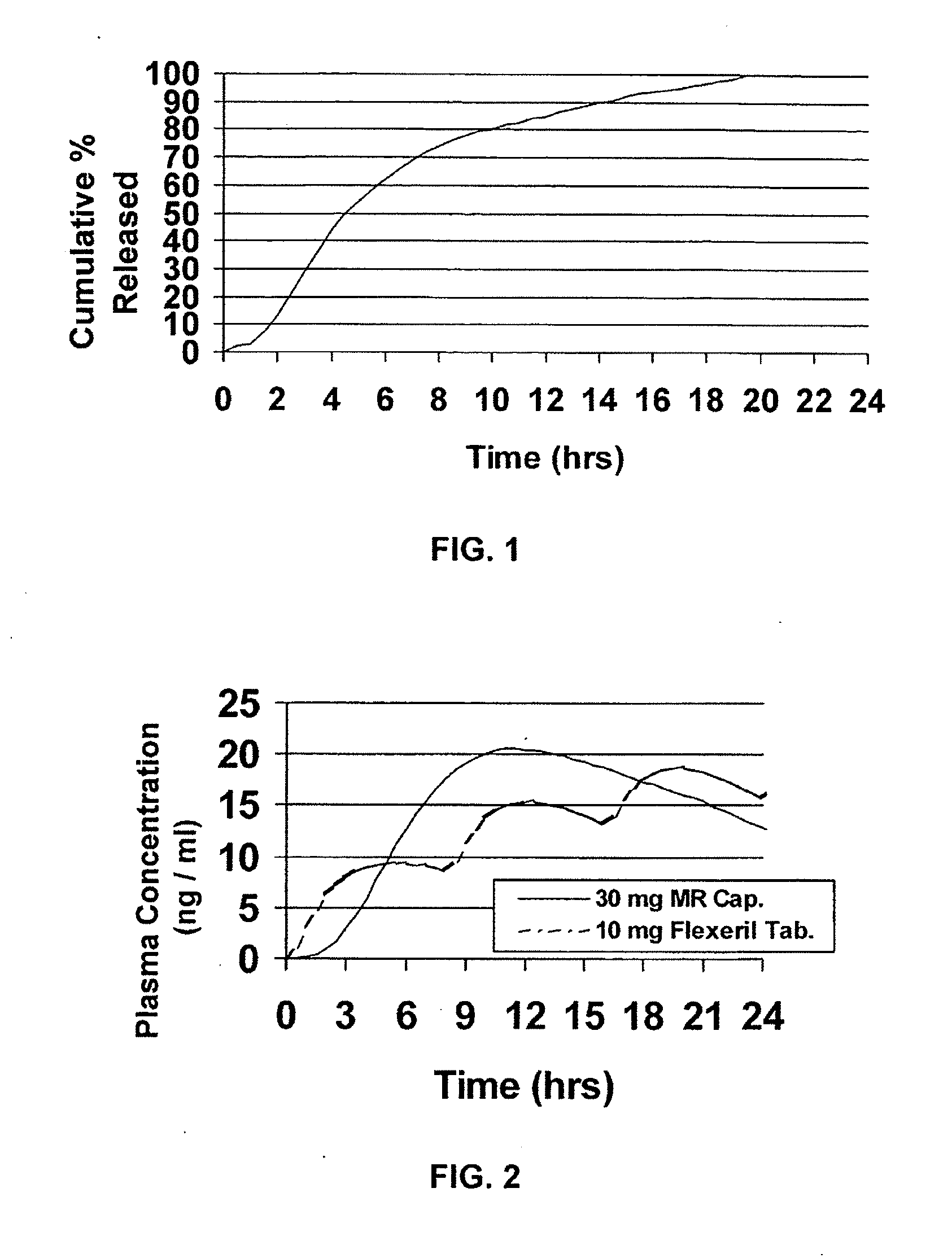
A unit dosage form, such as a capsule or the like, for delivering a skeletal muscle relaxant, such as cyclobenzaprine hydrochloride, into the body in an extended or sustained release fashion comprising one or more populations of drug-containing particles (beads, pellets, granules, etc.) is disclosed. At least one bead population exhibits a pre-designed sustained release profile. Such a drug delivery system is designed for once-daily oral administration to maintain an adequate plasma concentration—time profile, thereby providing relief of muscle spasm associated with painful musculoskeletal conditions over a 24 hour period.
Owner:ADARE PHARM INC
Pharmaceutical delivery system
InactiveUS20050095245A1Reduce adverse effectsEffectively impact the life cycleAntibacterial agentsBiocideDiseaseGynecology
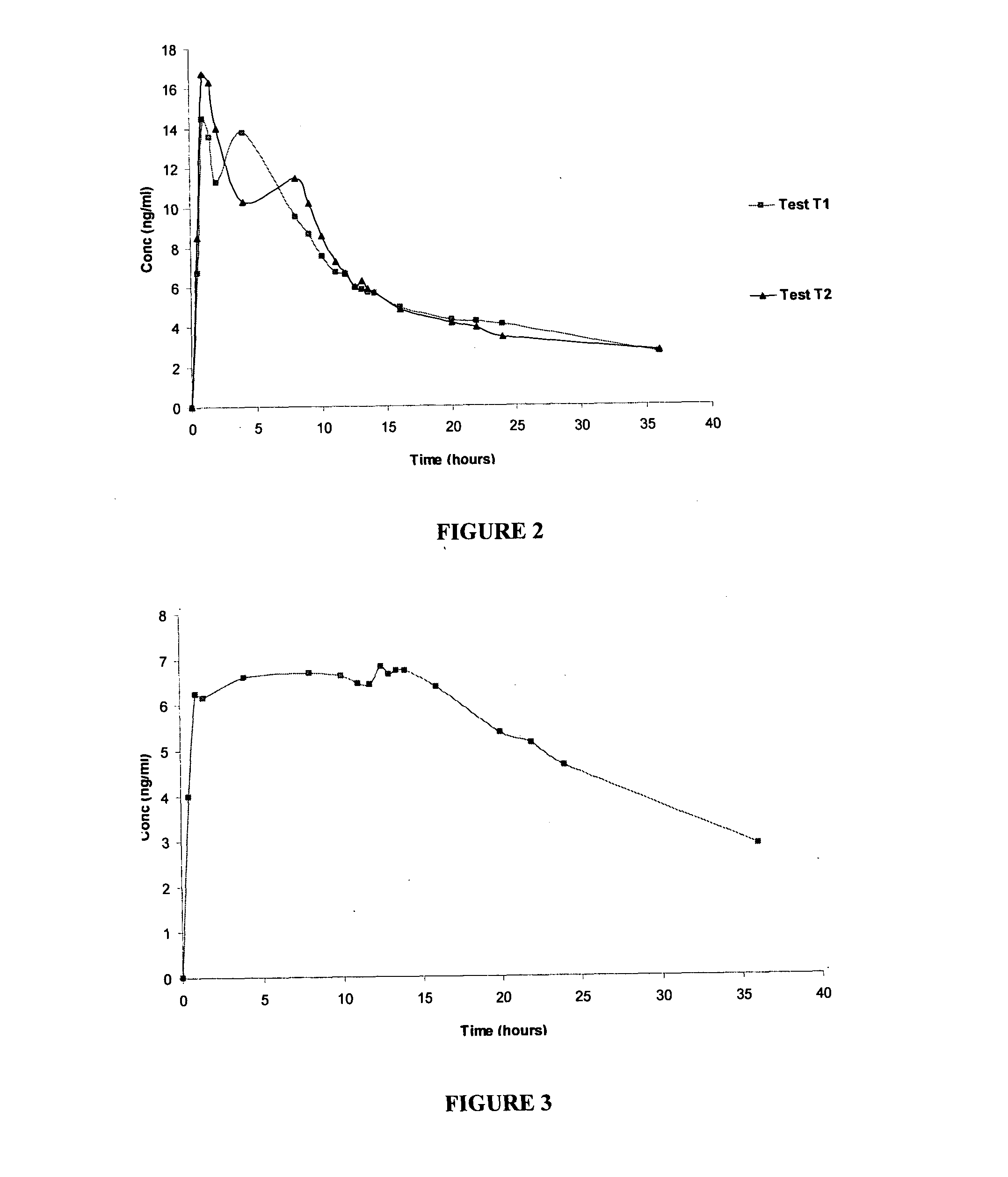
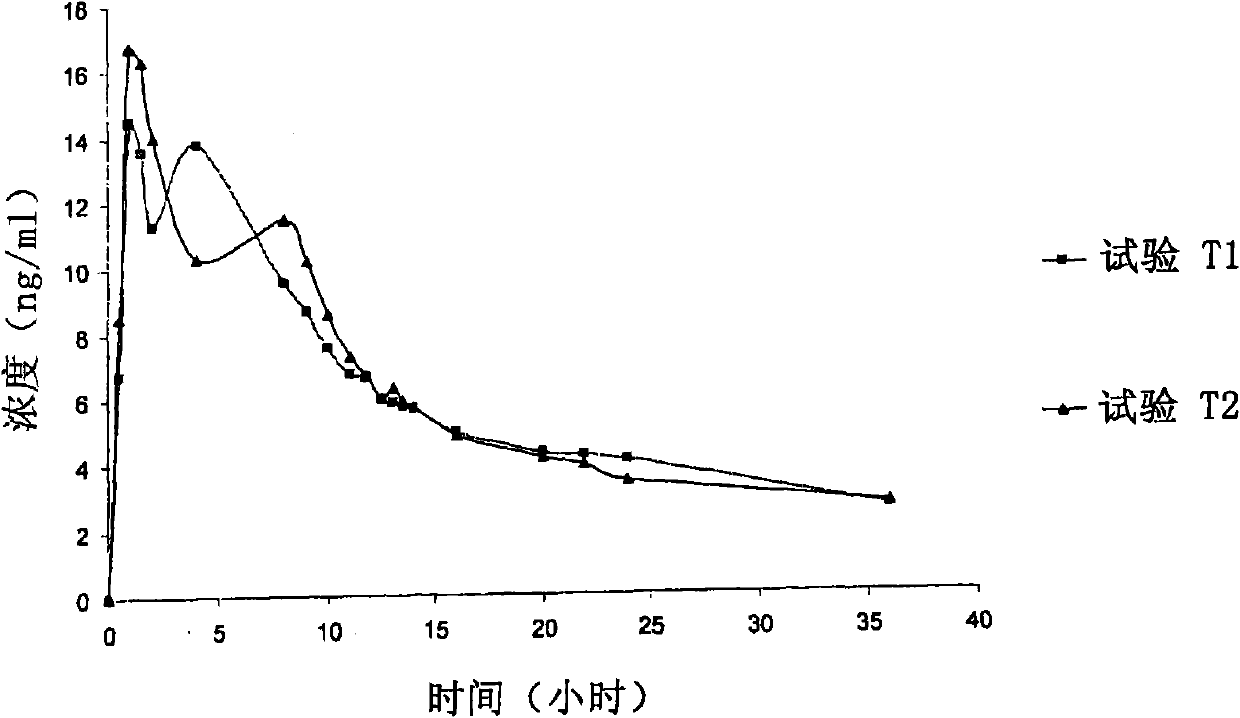
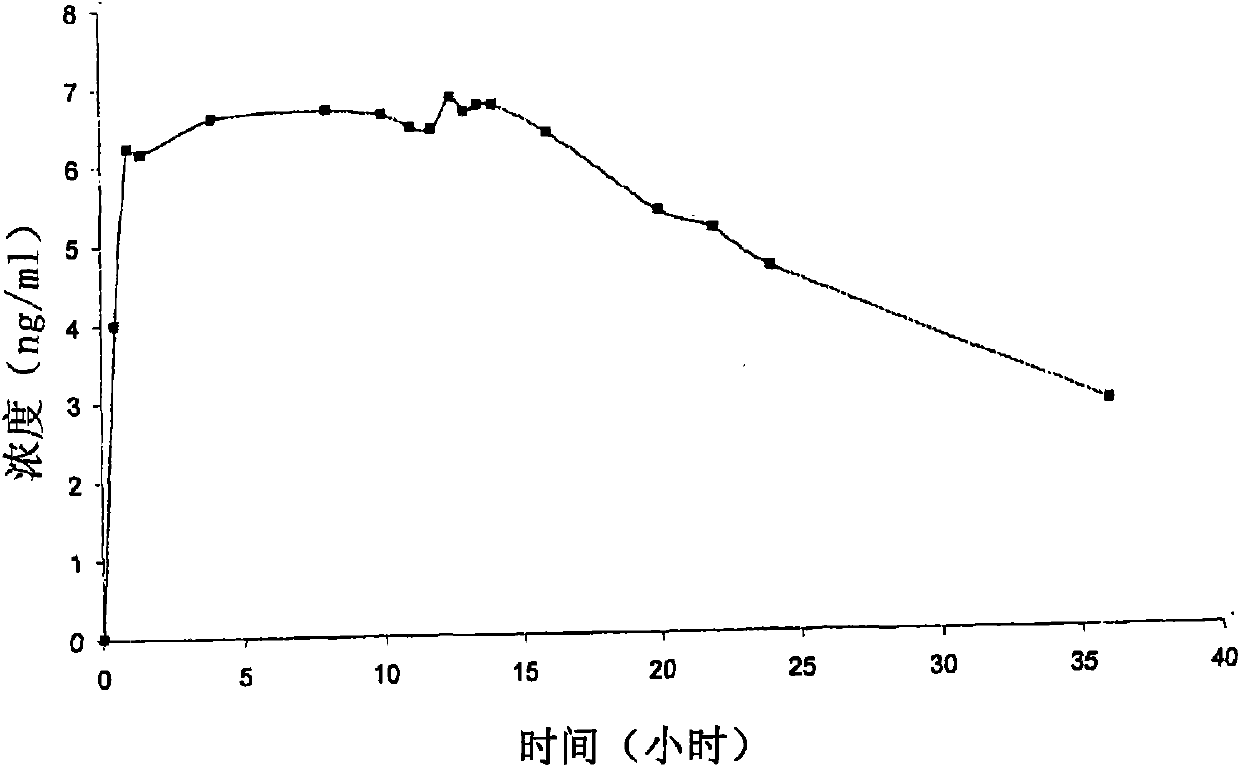
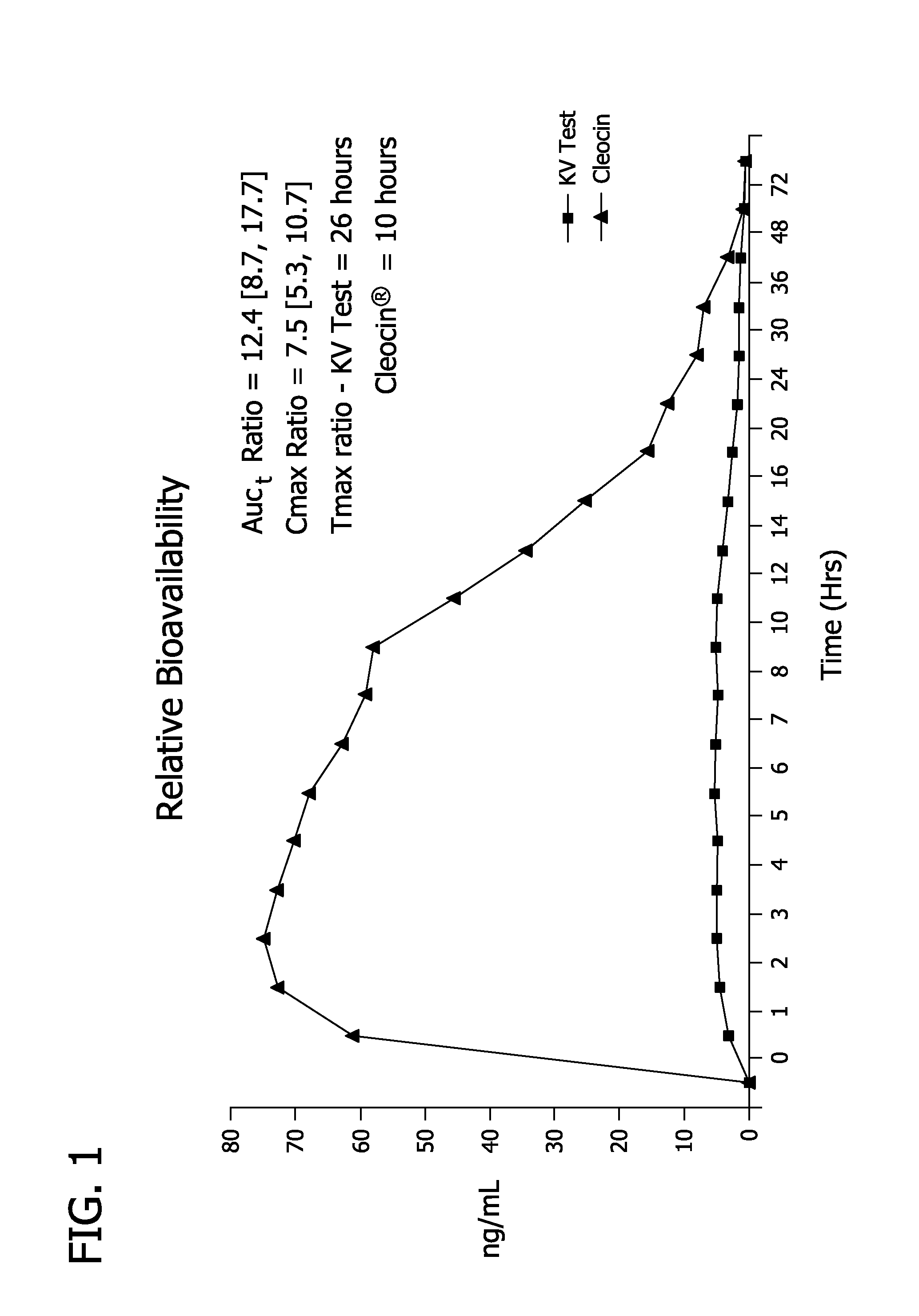
A pharmaceutical formulation to treat vaginal conditions in a human patient comprises: at least one active agent; a modified release dosage form which provides extended release of the anti-infective agent upon vaginal administration to the patient; and wherein the formulation, when containing a total dose of the anti-infective agent of about 25 μg to about 500 mg based on the active agent will produce a plasma concentration versus time curve (ng / mL versus hours) having an area under the curve (AUC) of less than about 600 ng / mL.hr.
Owner:DRAGTEK CORP
Modified release composition of highly soluble drugs
InactiveUS7976871B2Effectively control release rateSmall sizePill deliveryAnhydride/acid/halide active ingredientsSolubilityModified Release Dosage Form
A novel modified release dosage form comprising of a high solubility active ingredient, which utilizes dual retard technique to effectively reduce the quantity of release controlling agents. Present invention can optionally comprise additionally another active ingredient as an immediate release form or modified release form. Present invention also relates to a process for preparing the said formulation.
Owner:TORRENT PHARMA LTD
Enhanced absorption of modified release dosage forms
InactiveUS20050142187A1Effective for bacterial infectionEffective treatmentPill deliveryGranular deliveryIntestinal structureModified Release Dosage Form
Disclosed are products and methods for improving the plasma profile in a patient being treated with a pharmaceutical active agent that is subject to a limited window of absorption, which products and methods comprise orally administering the active agent in multiparticulate form, such that at least a portion thereof is delivered to the intestine while the patient is in the fed condition.
Owner:SHIONOGI INK
Modified release dosage forms of skeletal muscle relaxants
InactiveUS20080124398A1Patient compliance is goodEfficient ConcentrationPowder deliveryOrganic active ingredientsDiseaseModified Release Dosage Form
A unit dosage form, such as a capsule or the like, for delivering a skeletal muscle relaxant, such as cyclobenzaprine hydrochloride, into the body in an extended or sustained release fashion comprising one or more populations of drug-containing particles (beads, pellets, granules, etc.) is disclosed. At least one bead population exhibits a pre-designed sustained release profile. Such a drug delivery system is designed for once-daily oral administration to maintain an adequate plasma concentration—time profile, thereby providing relief of muscle spasm associated with painful musculoskeletal conditions over a 24 hour period.
Owner:ADARE PHARM INC
Novel modified release dosage forms of xanthine oxidoreductase inhibitor or xanthine oxidase inhibitors
InactiveUS20110311620A1Facilitated releaseBiocidePowder deliveryXanthine oxidase inhibitorModified Release Dosage Form
Owner:TAKEDA PHARMA U S A
Modified release dosage form
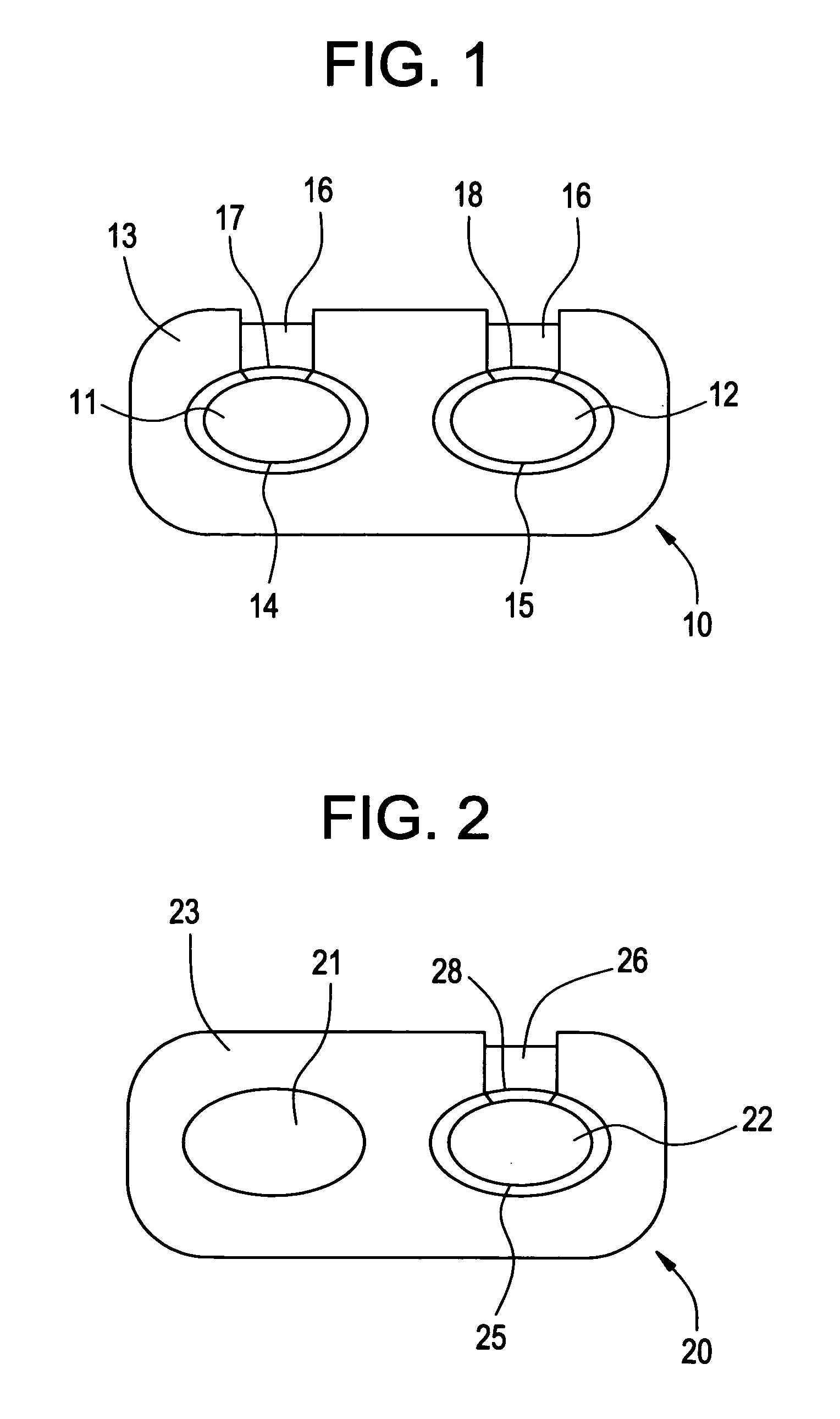
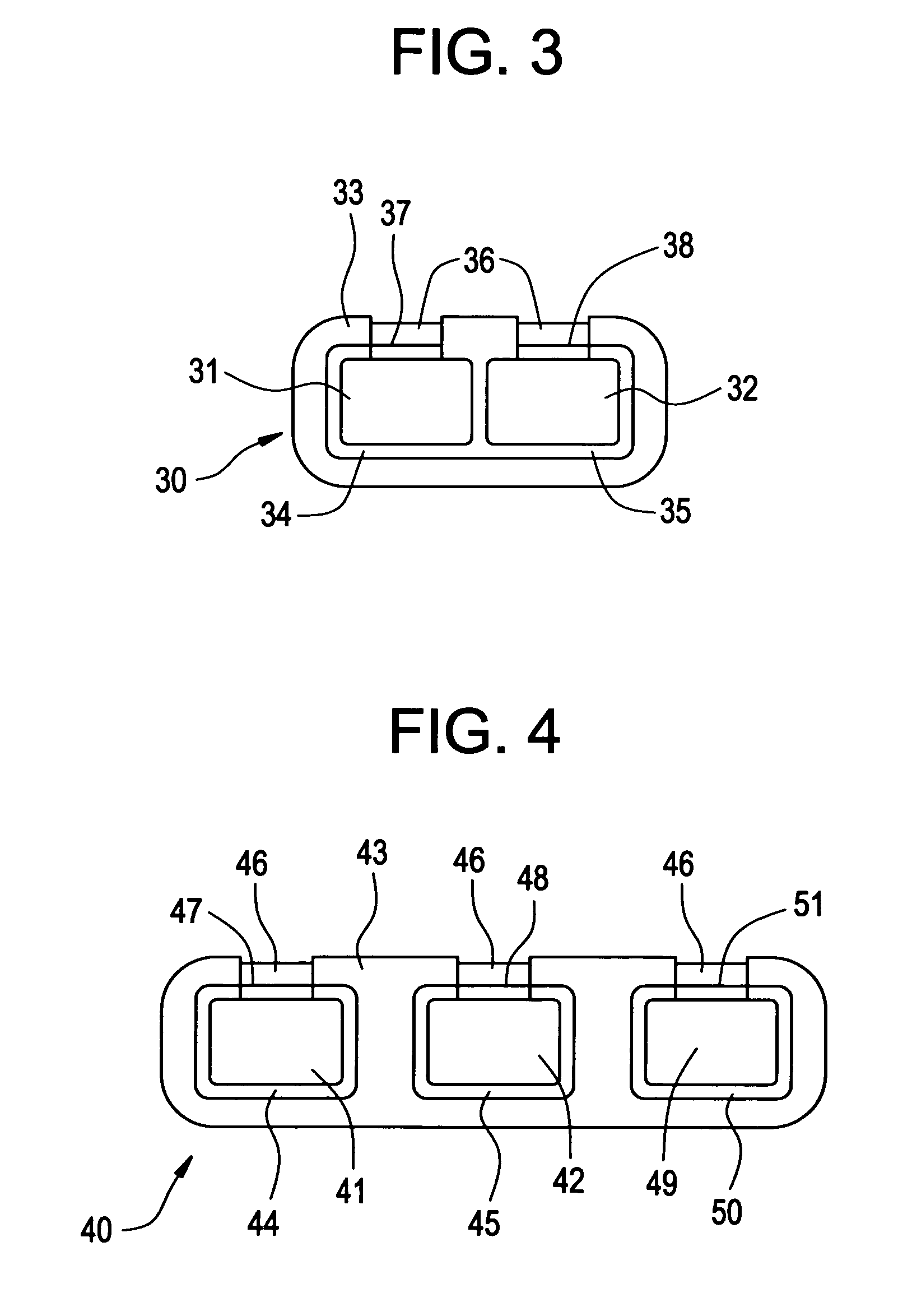
The present invention relates to a medicinal dosage form having a first core, a second core, and a shell that surrounds a first portion of each core and a fill material that covers a second portion of at least one core, wherein the fill material that is provided over at least one core is not in contact with any portion of the other core. e. The inventive dosage forms provide modified release of one or more active ingredients contained therein. The present invention also relates to methods for manufacturing such medicinal dosage forms.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Compositions of (-)-17-(cyclobutylmethyl)morphinan-3,14-diol
ActiveUS9364430B2Increase rangeSlow onsetOrganic active ingredientsGranular deliveryModified Release Dosage FormMorphinan
The present invention is directed to oral, therapeutically effective modified release pharmaceutical compositions of (−)-17-(cyclobutylmethyl)morphinan-3,14-diol and it pharmaceutically acceptable salts and the use thereof, including delayed onset and extended release dosage forms. The present invention is also directed at modified release dosage forms of oral (−)-17-(cyclobutylmethyl)morphinan-3,14-diol which provide robust efficacy and reduced potential for abuse and misuse.
Owner:RELMADA THERAPEUTICS
Modified dosage forms of tacrolimus
InactiveUS20100086592A1Eliminates releaseLower metabolismPowder deliveryBiocideModified Release Dosage FormOral medication
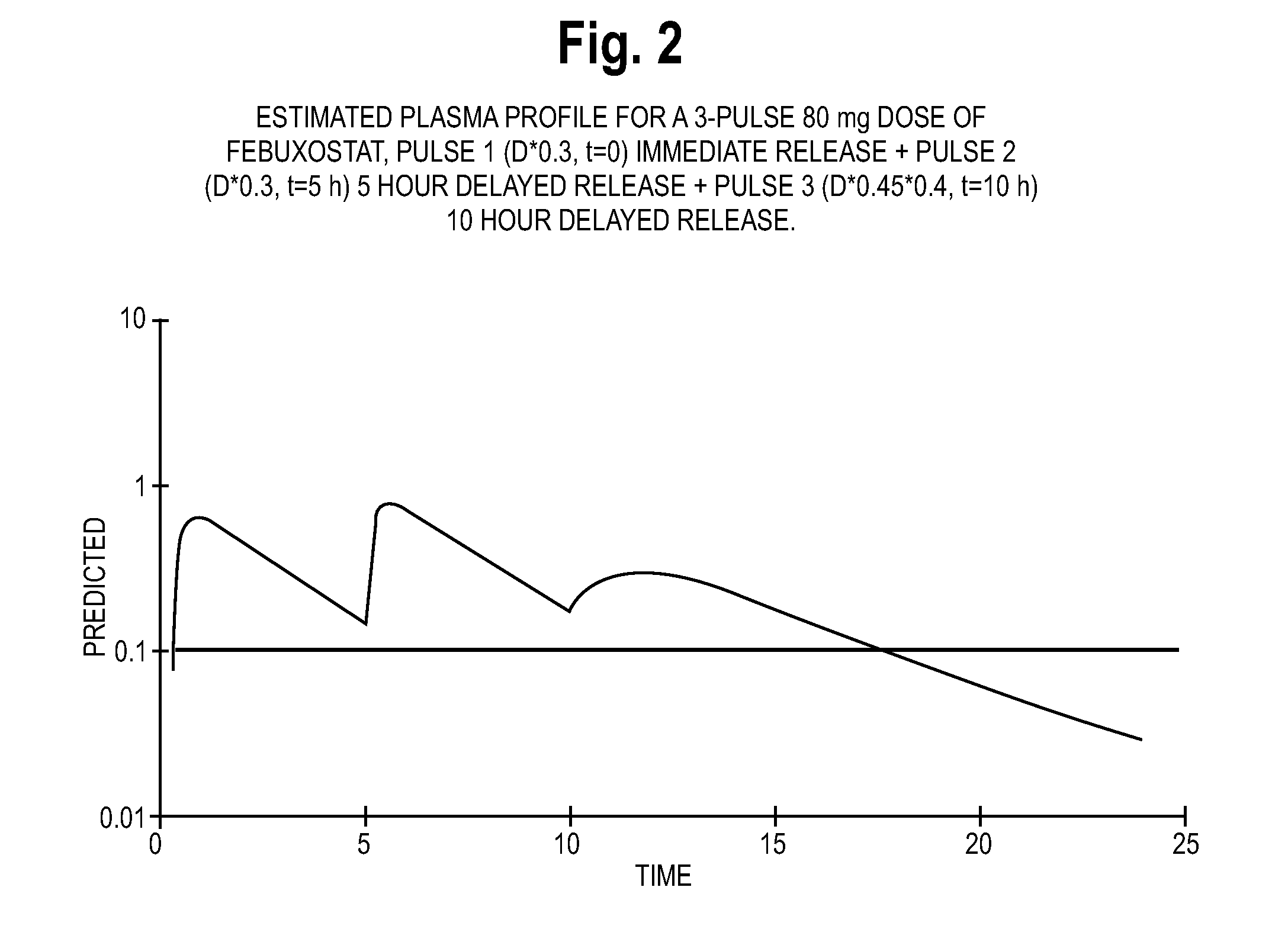
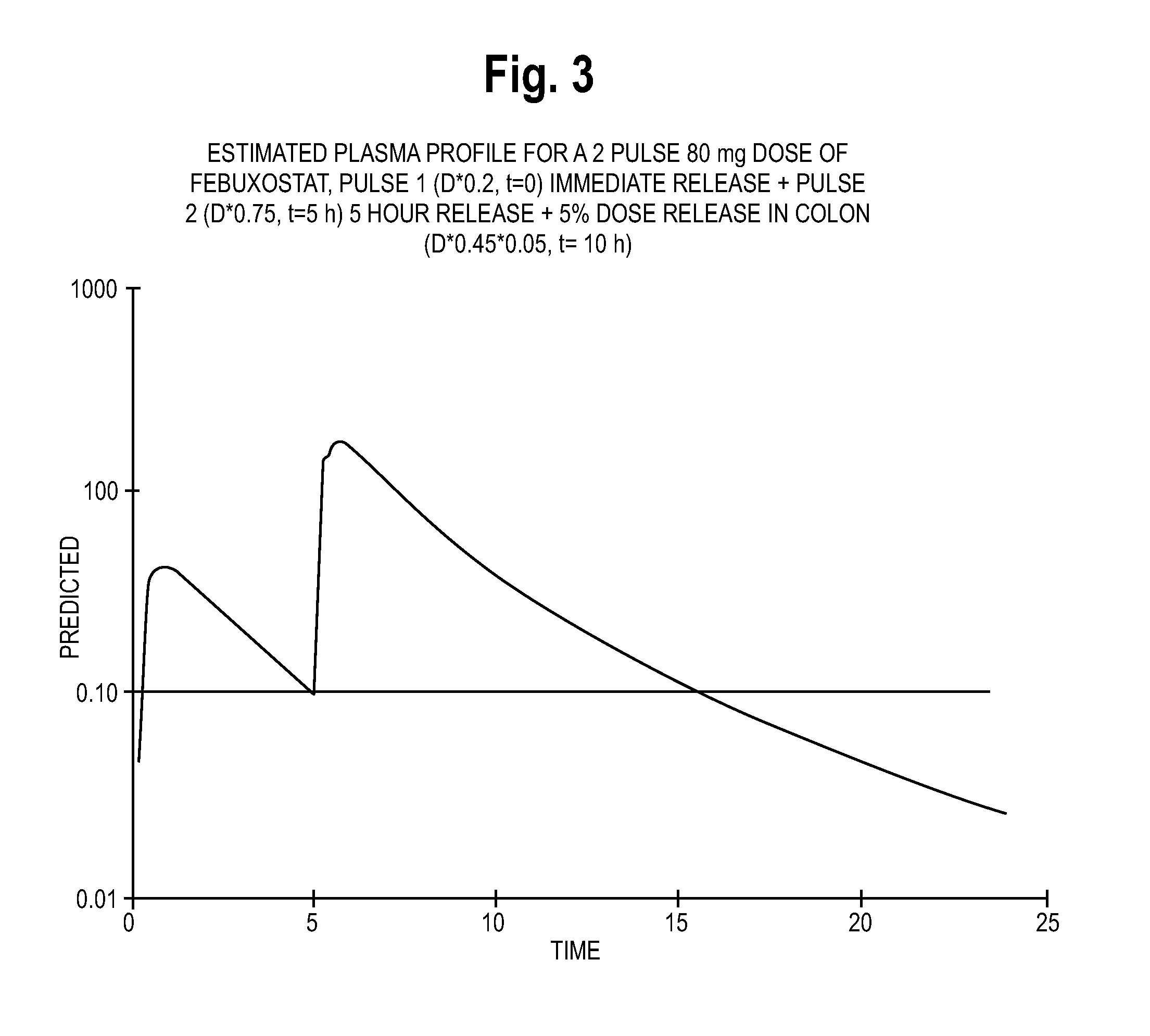
The present invention provides a modified release dosage form of tacrolimus that releases two or more amount of tacrolimus upon oral administration, the first amount of tacrolimus releases from the immediate release dosage unit substantially immediately within 0-2 hours followed by a time interval ranging from about 1-10 hours during which substantially no amount of tacrolimus is released from the dosage form, after which a second amount of tacrolimus is released wherein said second amount is released from the delayed release dosage unit either immediately e.g. within 0-2 hours or over a period of time ranging from about 2-12 hours from its initial release from the delayed release dosage unit. The dosage form may further comprise additional amount of tacrolimus to provide additional pulse of tacrolimus. The dosage forms of tacrolimus exhibit improved bioavailability and reduced flux or fluctuation over existing composition of tacrolimus. A method of preparing the dosage forms is also described.
Owner:PANACEA BIOTEC
Compositions of (-)-17-(Cyclobutylmethyl) Morphinan-3,14-Diol
ActiveUS20140200237A1Increase rangeSlow onsetBiocideGranular deliveryModified Release Dosage FormDiol
The present invention is directed to oral, therapeutically effective modified release pharmaceutical compositions of (−)-17-(cyclobutylmethyl)morphinan-3,14-diol and it pharmaceutically acceptable salts and the use thereof, including delayed onset and extended release dosage forms. The present invention is also directed at modified release dosage forms of oral (−)-17-(cyclobutylmethyl)morphinan-3,14-diol which provide robust efficacy and reduced potential for abuse and misuse.
Owner:RELMADA THERAPEUTICS
Modified release dosage forms of skeletal muscle relaxants
InactiveUS20080124399A1Patient compliance is goodEfficient ConcentrationPowder deliveryCosmetic preparationsDiseaseModified Release Dosage Form
A unit dosage form, such as a capsule or the like, for delivering a skeletal muscle relaxant, such as cyclobenzaprine hydrochloride, into the body in an extended or sustained release fashion comprising one or more populations of drug-containing particles (beads, pellets, granules, etc.) is disclosed. At least one bead population exhibits a pre-designed sustained release profile. Such a drug delivery system is designed for once-daily oral administration to maintain an adequate plasma concentration-time profile, thereby providing relief of muscle spasm associated with painful musculoskeletal conditions over a 24 hour period.
Owner:ADARE PHARM INC
Modified release dosage form
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Modified dosage forms of tacrolimus
InactiveCN101652141AImprove complianceLower metabolismPowder deliveryPill deliveryTime rangeOral medication
The present invention provides a modified release dosage form of tacrolimus that releases two or more amount of tacrolimus upon oral administration, the first amount of tacrolimus releases from the immediate release dosage unit substantially immediately within 0-2 hours followed by a time interval ranging from about 1-10 hours during which substantially no amount of tacrolimus is released from thedosage form, after which a second amount of tacrolimus is released wherein said second amount is released from the delayed release dosage unit either immediately e.g. within 0-2 hours or over a period of time ranging from about 2-12 hours from its initial release from the delayed release dosage unit. The dosage form may further comprise additional amount of tacrolimus to provide additional pulseof tacrolimus. The dosage forms of tacrolimus exhibit improved bioavailability and reduced flux or fluctuation over existing composition of tacrolimus. A method of preparing the dosage forms is also described.
Owner:PANACEA BIOTEC
Modified-release dosage forms of 5-ht2c agonists useful for weight management
InactiveUS20130315994A1Reduce food consumptionIncreasing meal-related satietyBiocideOrganic chemistryBenzeneModified Release Dosage Form
The present invention relates to methods for weight management that utilize modified-release dosage forms comprising (R)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine salts and crystalline forms thereof. The present invention further relates to (R)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine salts, crystalline forms thereof and modified-release dosage forms comprising them.
Owner:ARENA PHARMA
Novel pharmaceutical modified release dosage form composition comprising cyclooxygenase enzyme inhibitor
InactiveCN101227893AExtension of timeNo toxicityNervous disorderAntipyreticModified Release Dosage FormEster prodrug
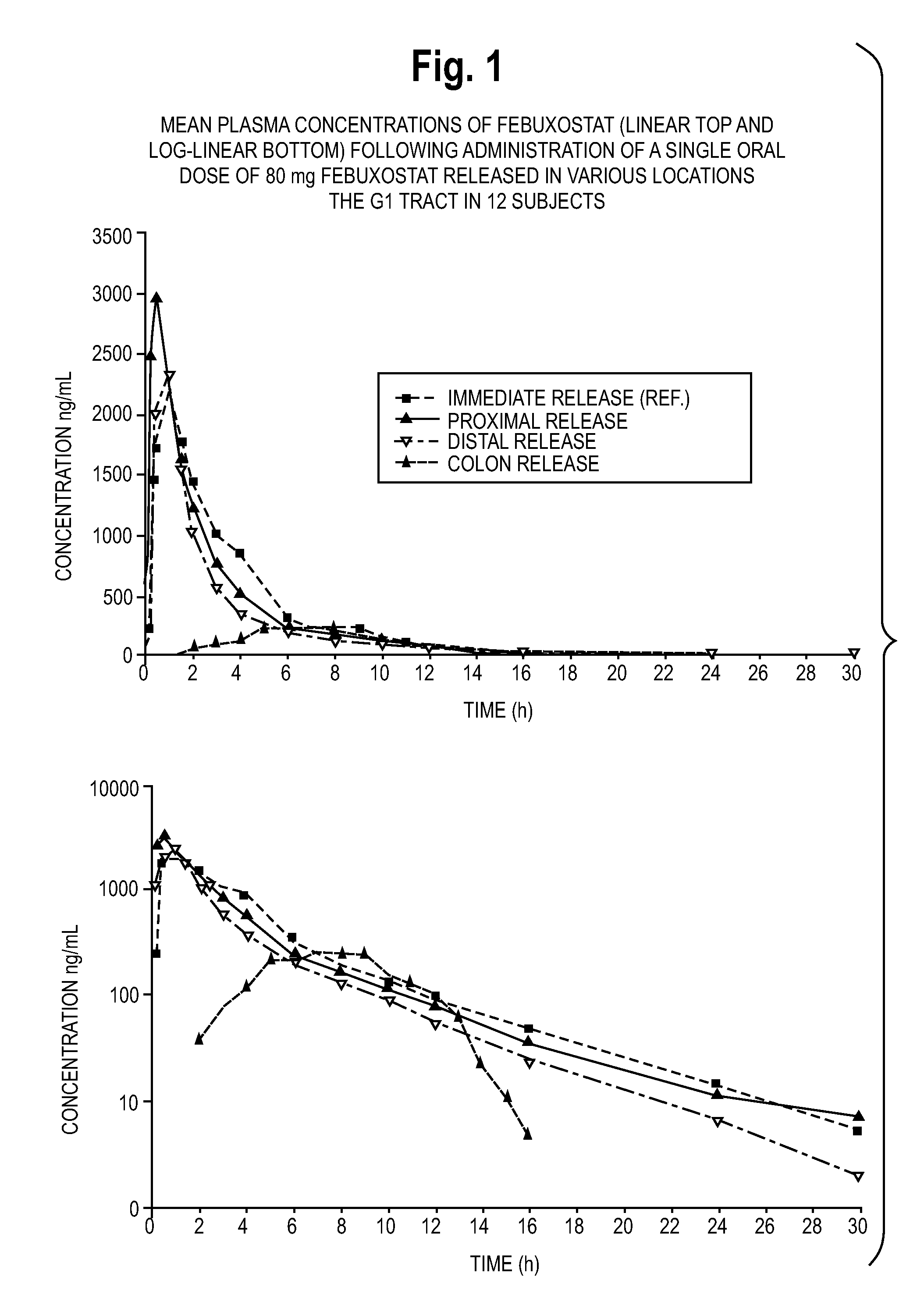
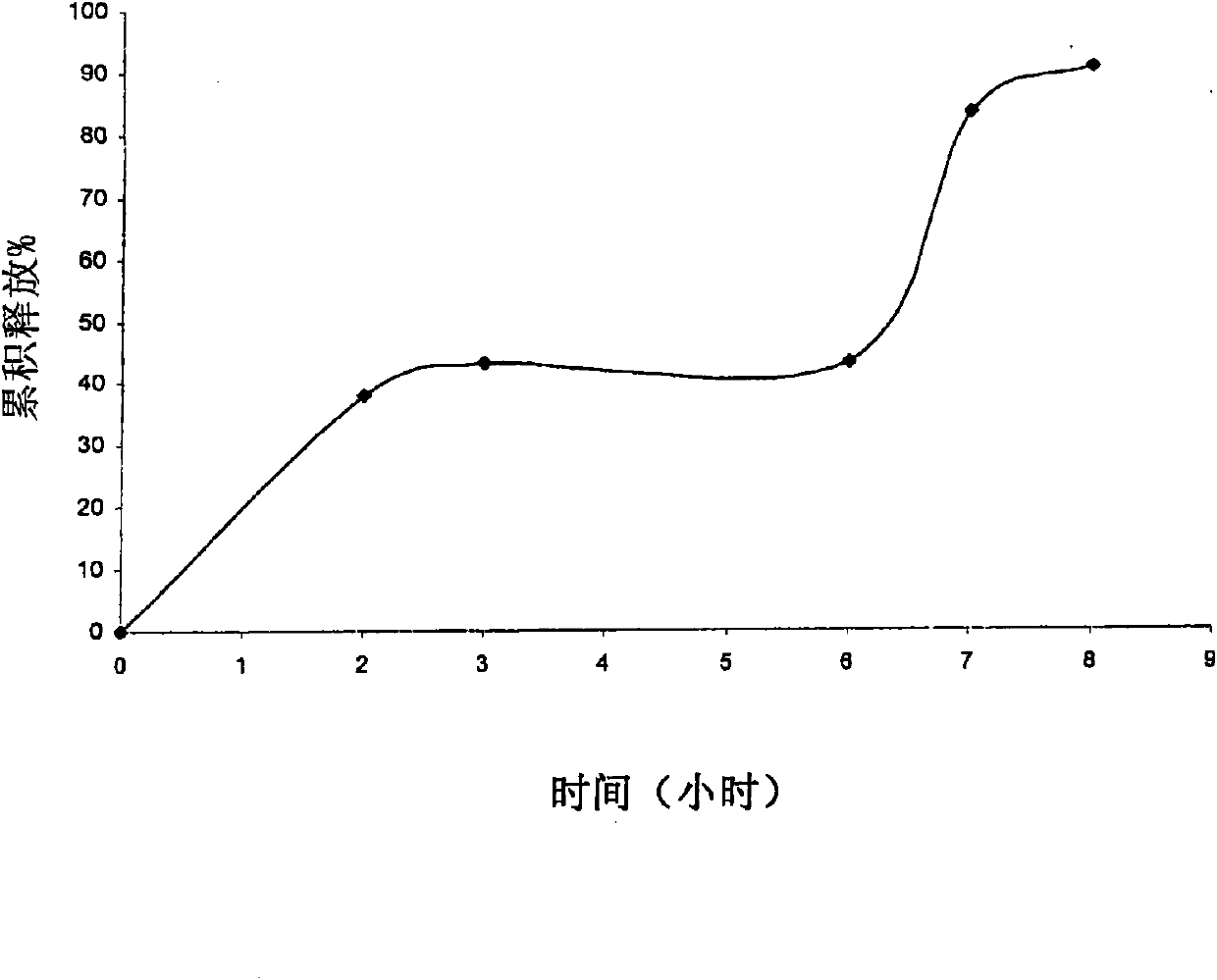
Pharmaceutical modified release dosage form comprising at least one cyclooxygenase enzyme inhibitor or its pharmaceutically acceptable salts, esters, prodrugs, solvates, hydrates, or derivatives thereof as active agent, with a pharmaceutically acceptable carrier for controlling the release of the cyclooxygenase enzyme inhibitor is provided. The dosage form preferably provides a release of not more than about 60 % of the cyclooxygenase enzyme inhibitor in 1 hour and not less than about 75 % of the cyclooxygenase enzyme inhibitor after 12 hours when tested in accordance with the dissolution method (I) described herein employing Distilled water with 2.0 % Sodium lauryl sulphate as the dissolution medium or in accordance with the dissolution method (II) described herein employing pH 7.0 Phosphate buffer with 2.0% Sodium lauryl sulphate as the dissolution medium or in accordance with the dissolution method (III) described herein employing 0.001 N Hydrochloric acid with 1.0 % Sodium lauryl sulphate as dissolution medium. Further, the pharmaceutical composition of the present invention when tested in a group of healthy humans preferably achieves a mean peak plasma concentration (Cmax) after at least about 1 hour of administration of the dosage form,. The present invention also provides process of preparing such dosage form compositions and prophylactic and / or therapeutic methods of using such dosage form.
Owner:PANACEA BIOTEC
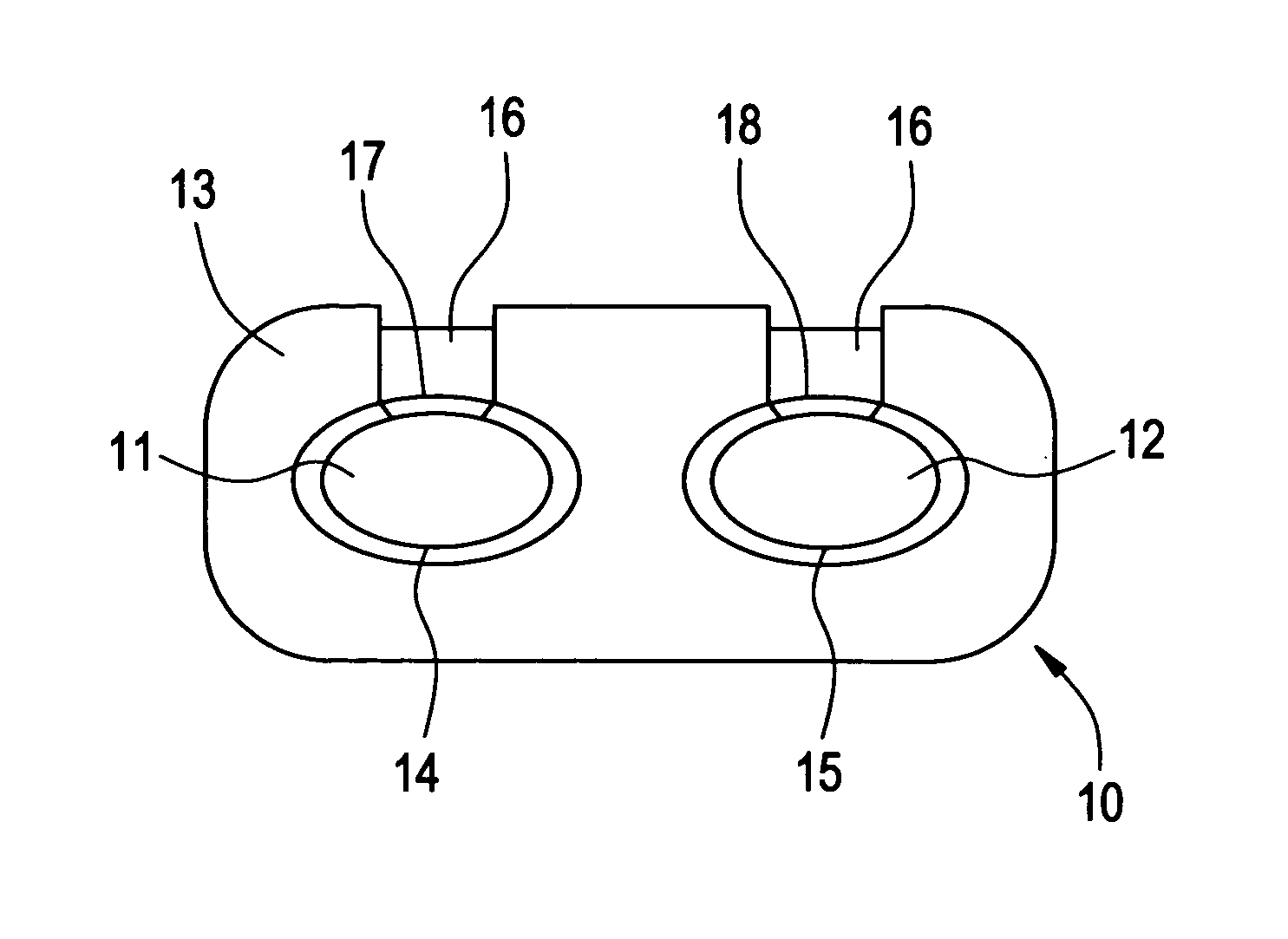
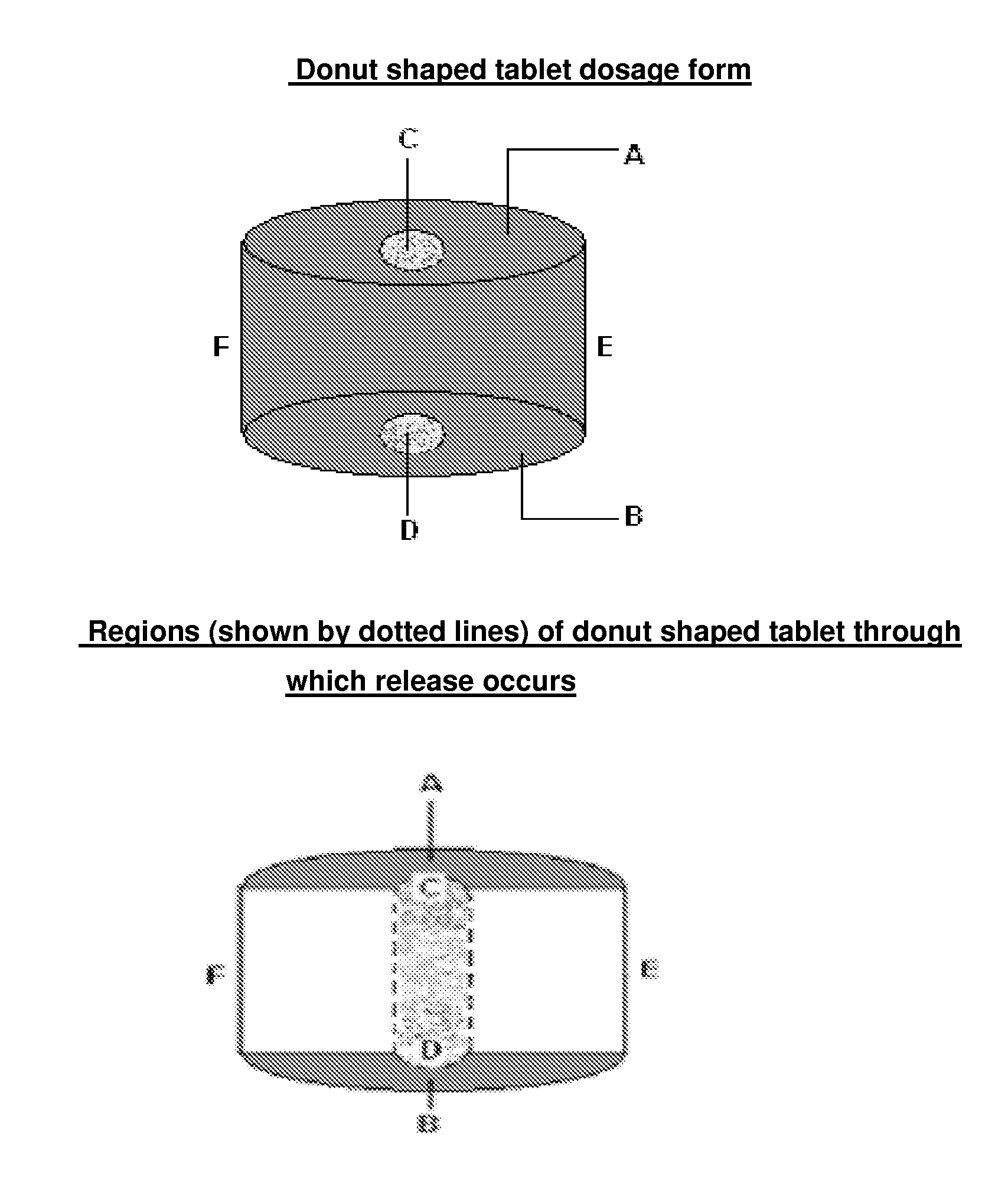
Modified release dosage form
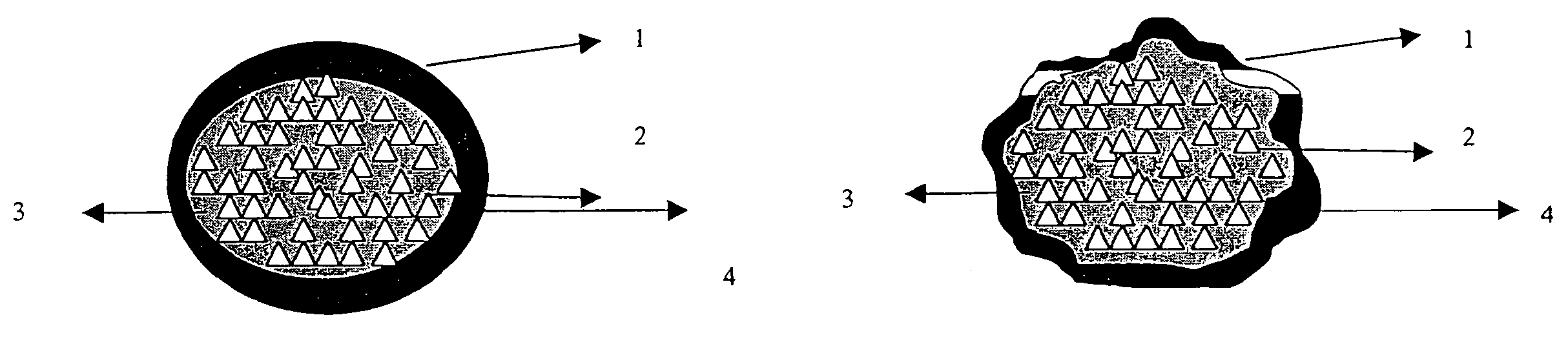
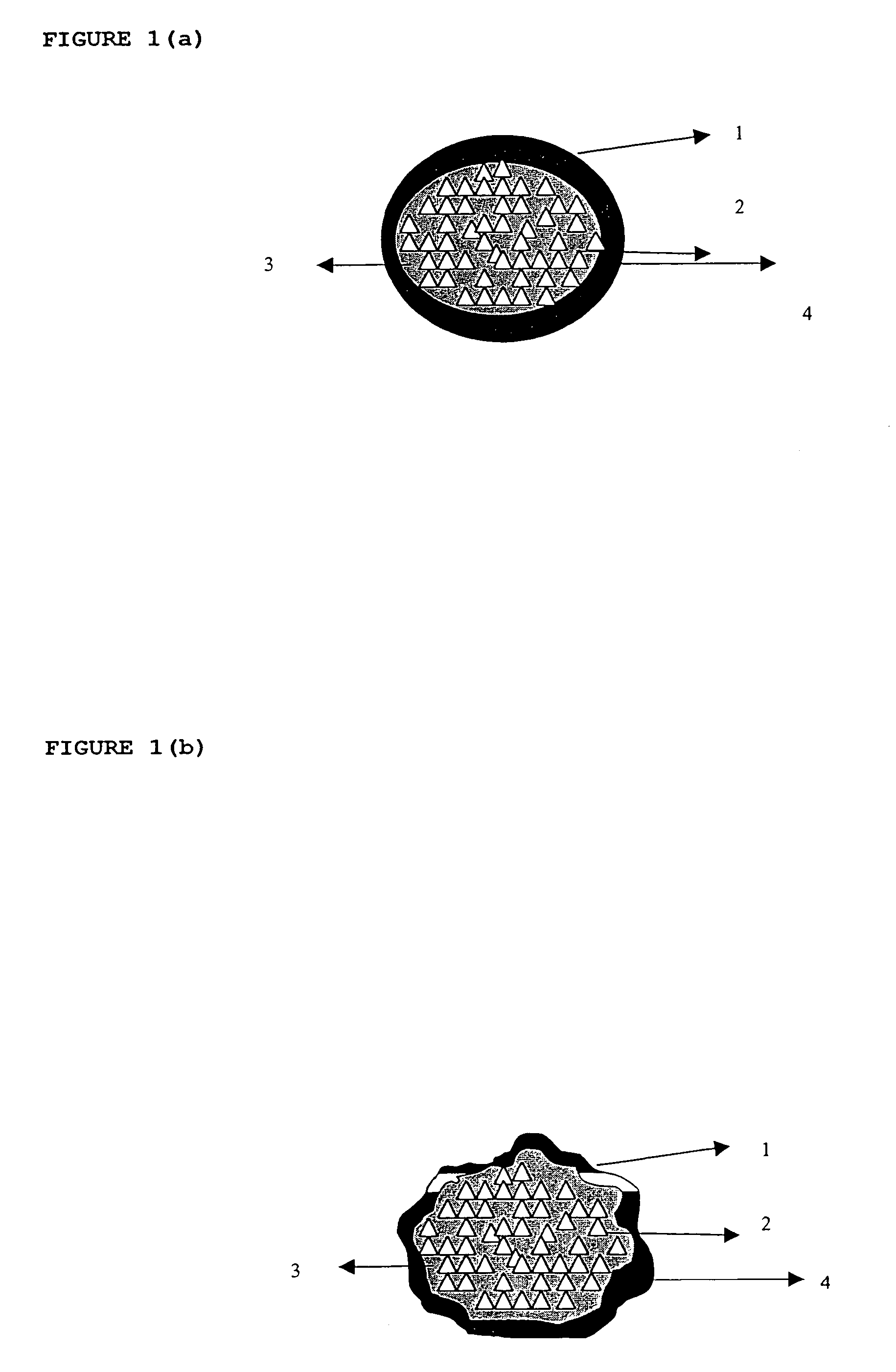
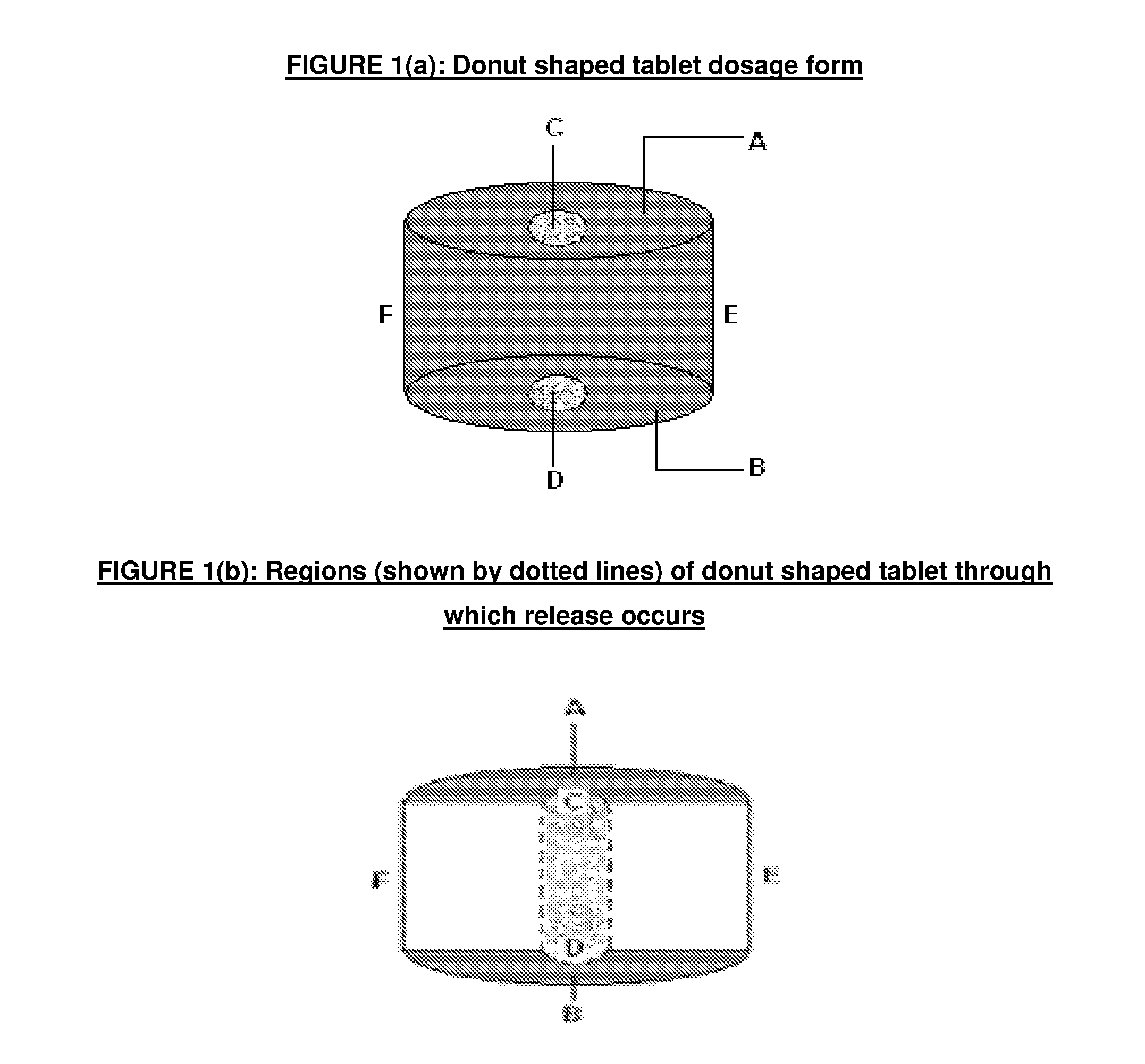
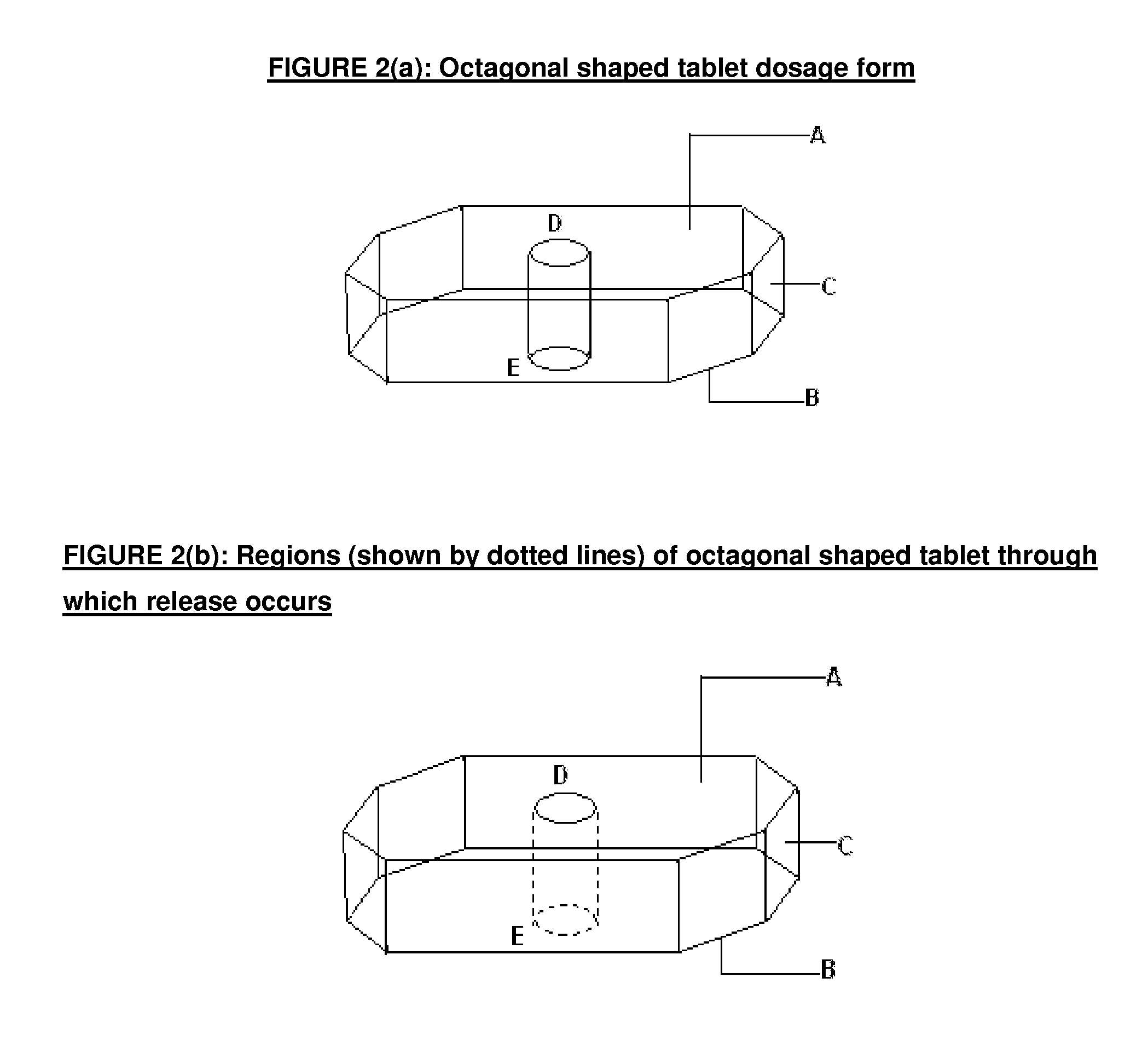
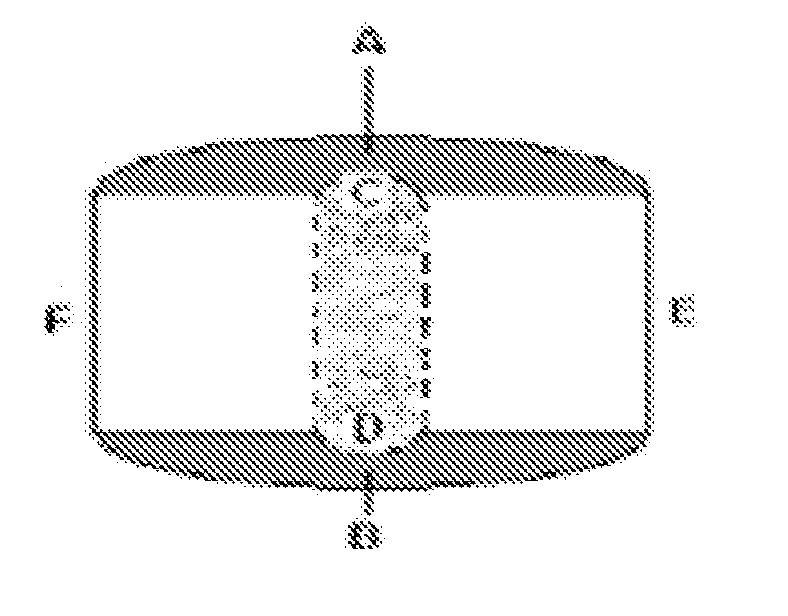
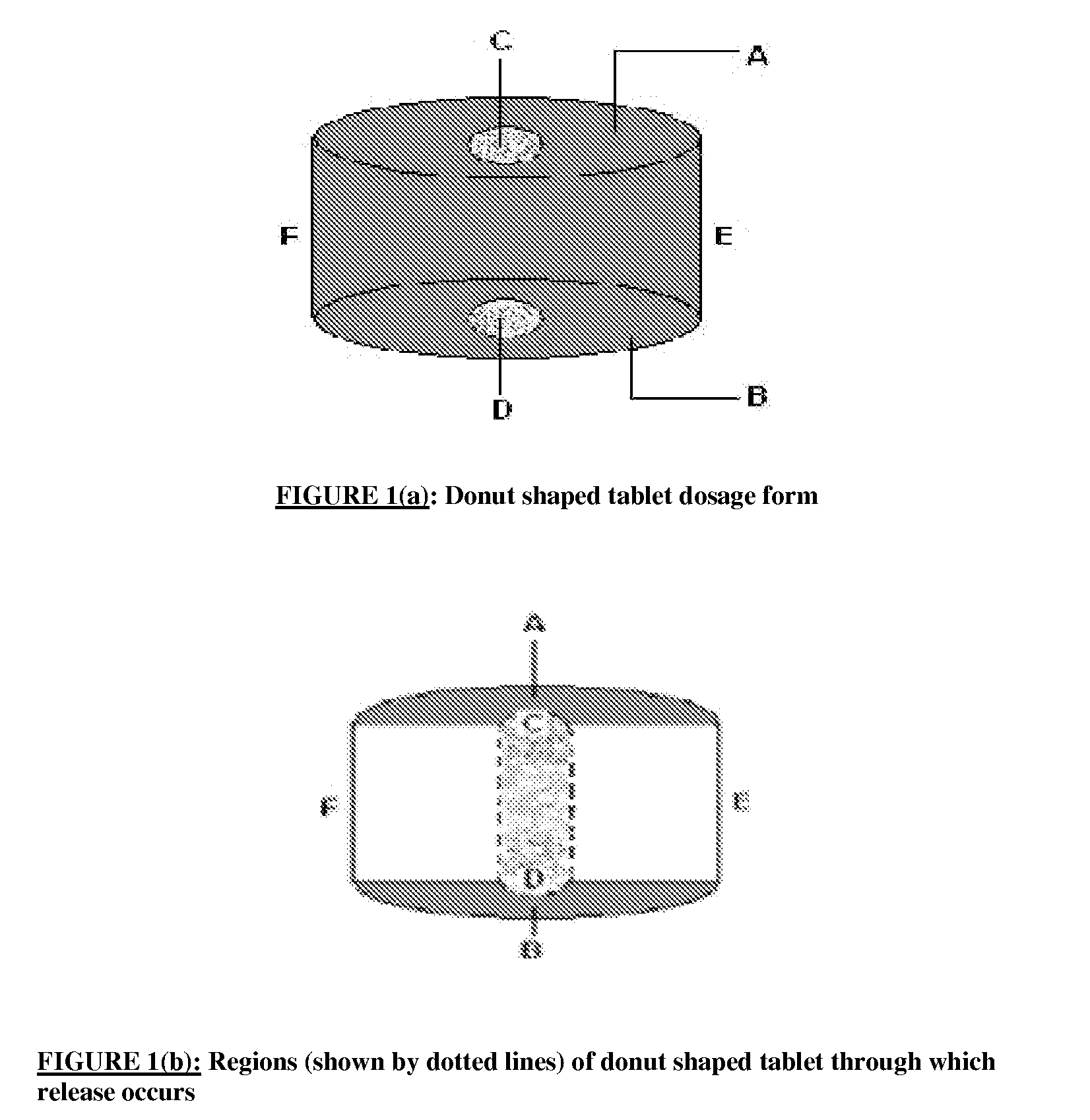
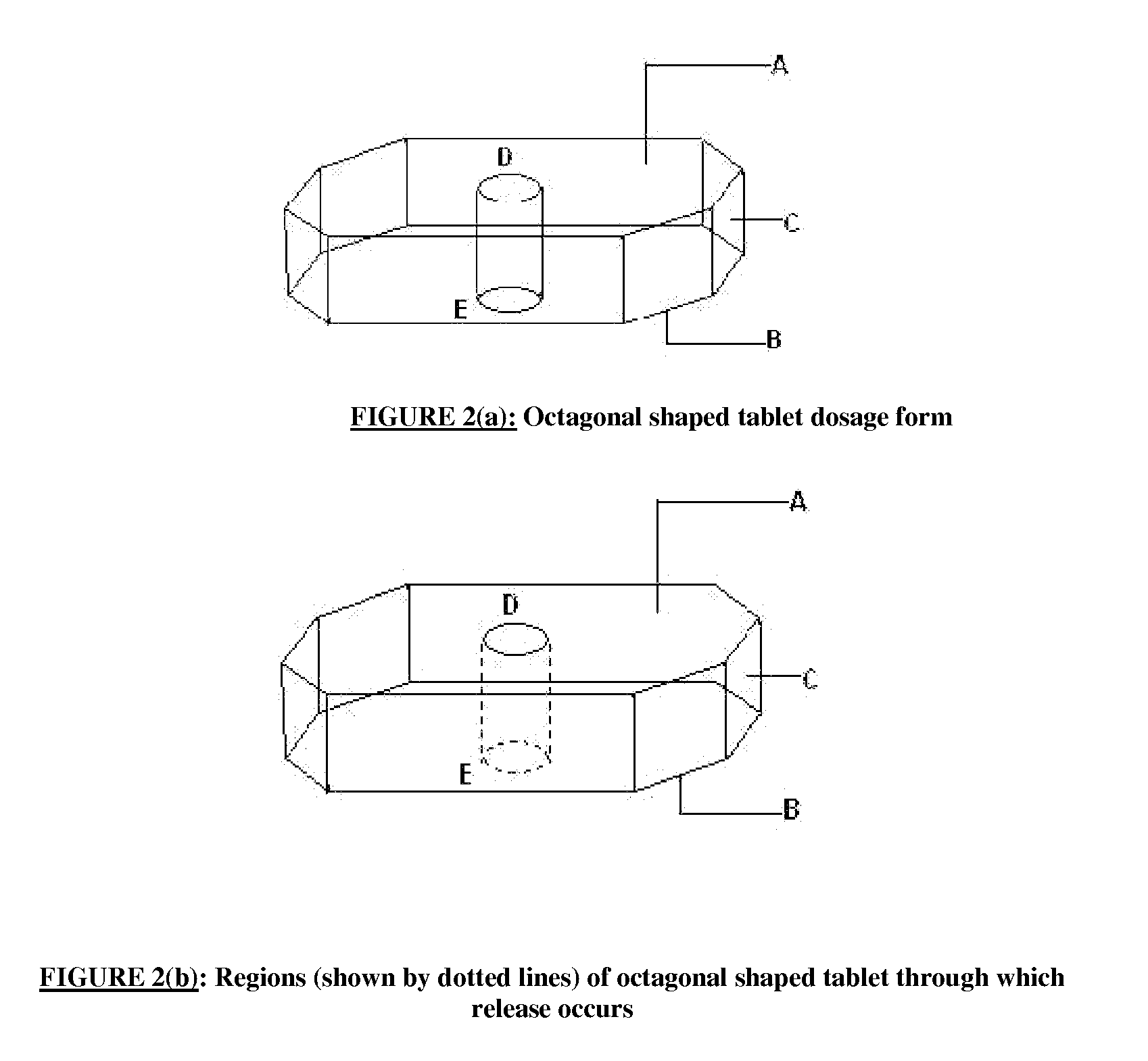
There is provided a modified release dosage form comprising a core coated with a polymeric coat, said polymeric coat comprising one or more rate controlling polymers, said dosage form having a hole extending through the dosage form resulting in an inner radial surface and an outer radial surface, said core comprising at least one therapeutically active ingredient, characterized in that the inner radial surface is partially coated with said polymeric coat.
Owner:GUNDO RAMAKANT KASHINATH +2
Novel Pharmaceutical Modified Release Dosage Form Cyclooxygenase Enzyme Inhibitor
InactiveUS20100204333A1Easy and cost-effectiveBiocideNervous disorderModified Release Dosage FormPhosphate
Pharmaceutical modified release dosage form comprising at least one cyclooxygenase enzyme inhibitor or its pharmaceutically acceptable salts, esters, prodrugs, solvates, hydrates, or derivatives thereof as active agent, with a pharmaceutically acceptable carrier for controlling the release of the cyclooxygenase enzyme inhibitor is provided. The dosage form preferably provides a release of not more than about 60% of the cyclooxygenase enzyme inhibitor in 1 hour and not less than about 75% of the cyclooxygenase enzyme inhibitor after 12 hours when tested in accordance with the dissolution method (I) described herein employing Distilled water with 2.0% Sodium lauryl sulphate as the dissolution medium or in accordance with the dissolution method (II) described herein employing pH 7.0 Phosphate buffer with 2.0% Sodium lauryl sulphate as the dissolution medium or in accordance with the dissolution method (III) described herein employing 0.001 N Hydrochloric acid with 1.0% Sodium lauryl sulphate as dissolution medium. Further, the pharmaceutical composition of the present invention when tested in a group of healthy humans preferably achieves a mean peak plasma concentration (Cmax) after at least about 1 hour of administration of the dosage form. The present invention also provides process of preparing such dosage form compositions and prophylactic and / or therapeutic methods of using such dosage form.
Owner:PANACEA BIOTEC
Osmotic dosage form
InactiveUS20060233882A1Small sizePill deliveryOsmotic deliveryModified Release Dosage FormBULK ACTIVE INGREDIENT
The present invention is directed to a modified release dosage form for delivering at least one pharmaceutically active ingredient. The dosage form has a first immediate release core for an active ingredient and an osmotic core or osmotic chamber containing at least one pharmaceutically active ingredient that can be the same or a different active ingredient contained in the first immediate release core. A shell having one or more portions surrounds the first immediate release core and osmotic core / chamber. The osmotic chamber includes a barrier layer that is substantially impermeable to the pharmaceutically active ingredient contained therein.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Modified Release Tranexamic Acid Formulation
InactiveUS20120135079A1Providing therapyPowder deliveryBiocideModified Release Dosage FormOral medication
Owner:ACTAVIS HOLDCO US INC
Pharmaceutical delivery system
ActiveUS20130102548A1Reduce adverse effectsEffectively impact the life cycleAntibacterial agentsBiocideGynecologyModified Release Dosage Form
A pharmaceutical formulation to treat vaginal conditions in a human patient comprises:at least one active agent;a modified release dosage form which provides extended release of the anti-infective agent upon vaginal administration to the patient; andwherein the formulation, when containing a total dose of the anti-infective agent of about 25 μg to about 500 mg based on the active agent will produce a plasma concentration versus time curve (ng / mL versus hours) having an area under the curve (AUC) of less than about 600 ng / mL·hr.
Owner:PADAGIS US LLC
Modified release tranexamic acid formulation
InactiveUS8597683B2Providing therapyBiocideOrganic active ingredientsModified Release Dosage FormOral medication
Owner:ACTAVIS HOLDCO US INC
Modified release dosage form comprising desvenlafaxine or salts thereof
InactiveUS20130059917A1Facilitated releaseBiocideOrganic active ingredientsModified Release Dosage FormActive agent
The present invention refers to a modified release pharmaceutical composition comprising desvenlafaxine or salts thereof, a release rate modifying system that controls the release of active agent(s) in both acidic and basic environments. A process of making and method of using the above-described composition is also disclosed.
Owner:WOCKHARDT LTD
Neramexane MR matrix tablet
The invention provides novel oral modified release dosage forms of neramexane which are useful for the continuous therapy of patients suffering from diseases and conditions such as Alzheimer's dementia and neuropathic pain. The compositions have drug release profiles that are suitable for achieving steady state plasma concentrations of neramexane which have relatively small fluctuation when administered on a twice-daily or even once-daily regimen. The dosage forms may be designed as modified release matrix tablets, which are optionally coated for taste masking. The invention further provides therapeutic methods of treating conditions such as Alzheimer's dementia and neuropathic pain which involve the administration of such dosage forms.
Owner:MERZ PHARMA GMBH & CO KGAA
Device For The Manufacture Of A Dosage Form With A Hole And Method Of Manufacture
This invention is related to a device for the manufacture of a dosage form with a hole and method of manufacture. The dosage form may be a modified release dosage form comprising a core coated with a polymeric coat comprising one or more rate controlling polymers, said dosage form having a hole extending through the dosage form resulting in an inner radial surface and an outer radial surface, said core comprising at least one therapeutically active ingredient, characterized in that the inner radial surface is partially coated with said polymeric coat.
Owner:GUNDU RAMAKANT KASHINATH +2
Modified-release dosage forms of 5-ht2c agonists useful for weight management
ActiveUS20170246179A1Reduce food consumptionIncreasing meal-related satietyOrganic chemistryMetabolism disorderBenzeneModified Release Dosage Form
The present invention relates to methods for weight management that utilize modified-release dosage forms comprising (R)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine salts and crystalline forms thereof. The present invention further relates to (R)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine salts, crystalline forms thereof and modified-release dosage forms comprising them.
Owner:ARENA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com