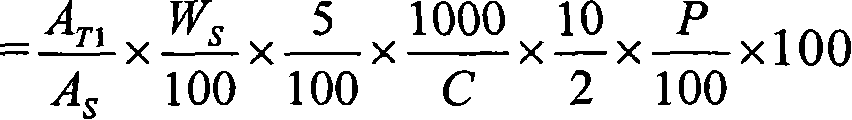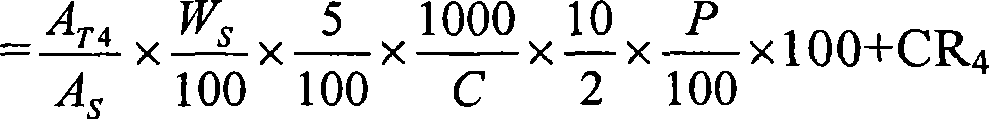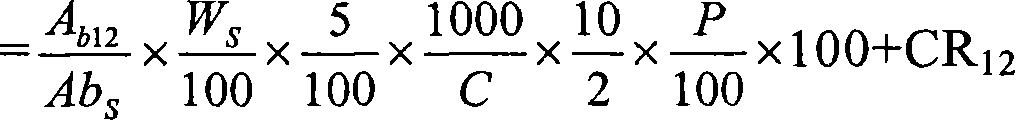Novel pharmaceutical modified release dosage form composition comprising cyclooxygenase enzyme inhibitor
A cyclooxygenase and inhibitor technology, applied in the field of pharmaceutically acceptable carriers, can solve the problems of drug treatment ineffectiveness, toxicity, lack of patient compliance, etc., and achieve simple and cost-effective results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment -1
[0157] Embodiment-1: Nimesulide controlled release tablet
[0158] A) immediate release layer
[0159] No. Ingredient name Quantity / tablet (mg)
[0160] 1. Micronized Nimesulide 50.00
[0161] 2. Lactose 87.03
[0162] 3. Sodium croscarmellose 3.75
[0163] 4. Colloidal silica 3.00
[0164] 5. Cornstarch 19.55
[0165] 6. Povidone (K-30) 3.00
[0166] 7. Docusate Sodium 3.40
[0167] 8. Iron oxide (red) 0.47
[0168] 9. Appropriate amount of purified water
[0169] 10. Magnesium stearate 0.80
[0170] 11. Sodium croscarmellose 7.25
[0171] 12. Colloidal silicon dioxide 2.50
[0172] 13. Povidone (K-30) 1.25
[0173] step
[0174] i) Mix ingredients 1-5 together and sieve through a No. 30 sieve. Ingredient 8 was dissolved in the above mixture.
[0175] ii) Dissolve ingredients 6 and 7 in ingredient 9 to obtain a homogeneous solution.
[0176] iii) The material from step (i) was granulated together with the material from step (ii), then dried and sieved through ...
Embodiment -2
[0201] Example-2: Nimesulide Controlled Release Capsules
[0202] A) Quick release part
[0203] No. Ingredient name Quantity / tablet (mg)
[0204] 1. Nimesulide 50.0
[0205] 2. Mannitol 80.0
[0206] 3. Sodium starch glycolate 5.0
[0207] 4. Colloidal Silica 3.0
[0208] 5. Corn starch 10.0
[0209] 6. Povidone (K-30) 3.0
[0210] 7. Polysorbate 80 1.0
[0211] 8. Purified water is lost during processing
[0212] 9. Magnesium stearate 1.0
[0213] 10. Croscarmellose Sodium 8.0
[0214] step
[0215] i) Mix ingredients 1-5 and sieve through a No. 30 sieve.
[0216] ii) Dissolve ingredients 6 and 7 in ingredient 8 to obtain a homogeneous solution.
[0217] iii) The material from step (i) was granulated together with the material from step (ii), then dried and sieved through a No. 16 sieve.
[0218] iv) Sift ingredients 9 and 10 through a No. 40 sieve.
[0219] v) mixing the material of step (iv) with the material of step (iii).
[0220] B) Sustained release part...
Embodiment -3
[0238] Example-3: Nimesulide Controlled Release Small Tablets Enclosed in Capsules
[0239]A) Quick release part
[0240] No. Ingredient name Quantity / tablet (mg)
[0241] 1. Nimesulide 50.0
[0242] 2. Mannitol 6.5
[0243] 3. Sodium starch glycolate 6.0
[0244] 4. Cornstarch 5.0
[0245] 5. Polysorbate 80 1.0
[0246] 6. Magnesium stearate 1.5
[0247] step
[0248] i) Mix ingredients 1-5 together and sieve through a No. 30 sieve.
[0249] ii) Sieve ingredient 6 through a No. 40 sieve.
[0250] iii) The material from step (i) is mixed with the material from step (ii) and compressed into pellets.
[0251] B) Sustained release part
[0252] No. Ingredient name Quantity / tablet (mg)
[0253] 1. Nimesulide 50.0
[0254] 2. Lactose monohydrate 6.5
[0255] 3. Docusate Sodium 2.0
[0256] 4. Povidone (K-30) 3.0
[0257] 5. Colloidal Silica 3.0
[0258] 6. Magnesium stearate 3.0
[0259] 7. Methacrylate polymers 5.5
[0260] 8. Triethyl citrate 1.5
[0261] 9. Is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com