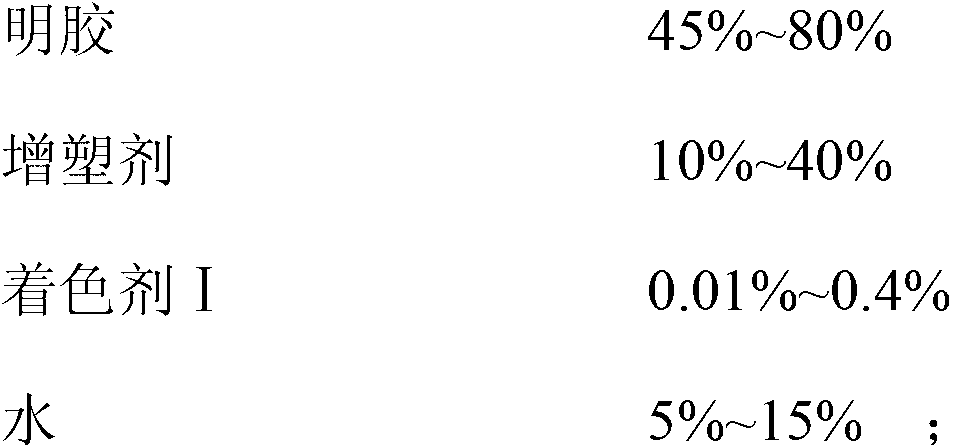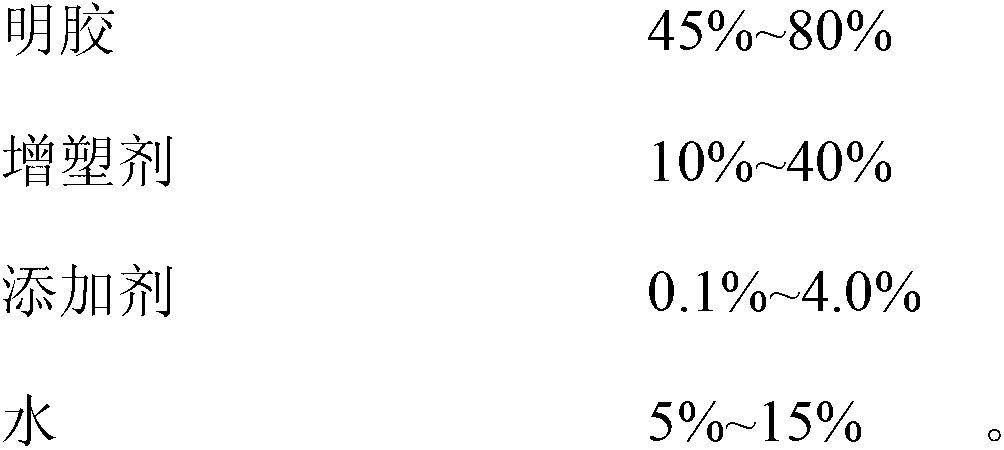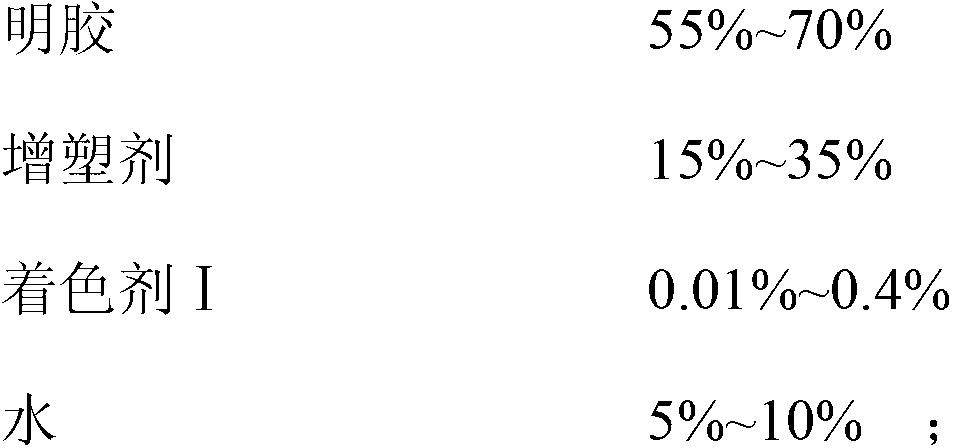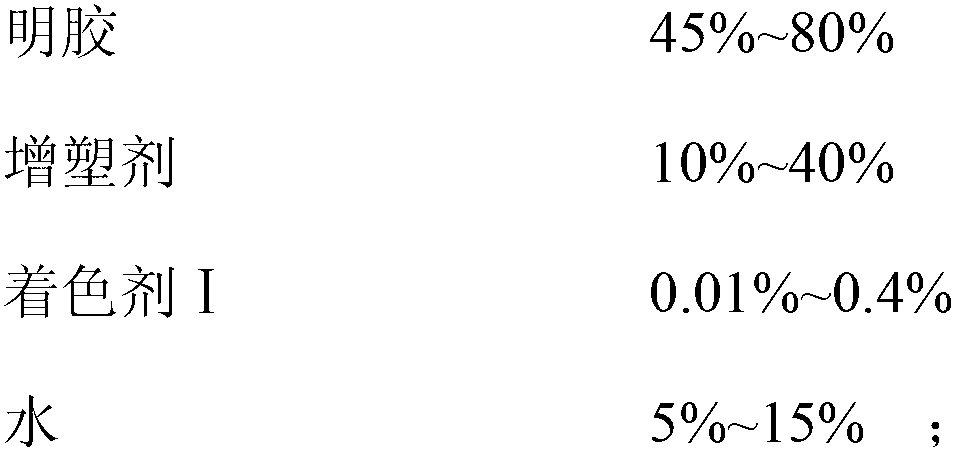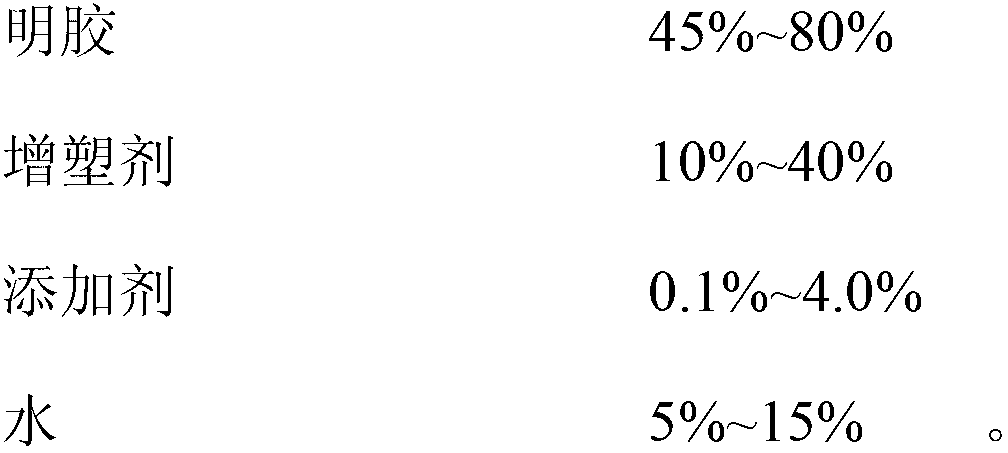Patents
Literature
102 results about "Docusate Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The sodium salt of docusate, a dioctyl salt and an emollient laxative with stool-softening activity. Docusate decreases surface tension and emulsification of fecal matter and allows water to penetrate and mix with stool. As a result, it softens the stool.
Whole sulphate type trivalent chromium plating solution and electroplating method using the same
The invention relates to full sulfate type trivalent chromium electroplating solution. The electroplating solution includes chromic sulfate, potassium sulfate and / or sodium sulfate, boracic acid, complexing agent and water, wherein, the electroplating solution also includes additive A and additive B, the additive A is selected from one or more of OP emulsifier, water soluble borofluoride, water soluble silico fluoride, vinyl diamine acetic acid, cystine, 1,4-butynediol, thiourea and glycol; the additive B is selected from one or more of O-Sulfonylbenaylsuamine, glycerine, water soluble sulphosuccinate, Docusate sodium and water soluble alkyl ether sulfate. The electroplating solution provided by the invention ensures the current density scopes of the coating and the luminous area to be both improved to a great extent.
Owner:BYD CO LTD
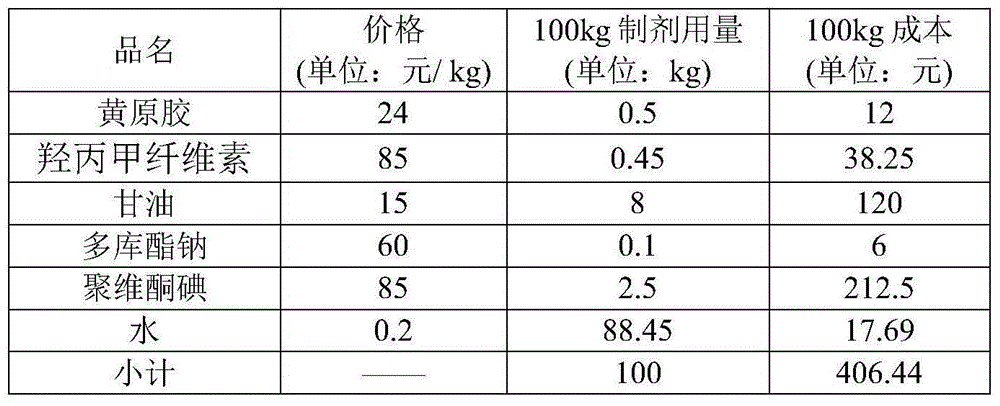
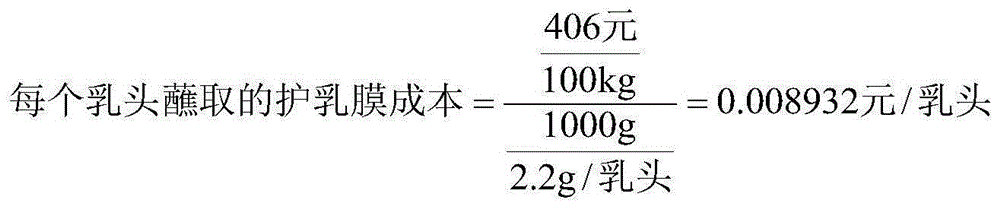
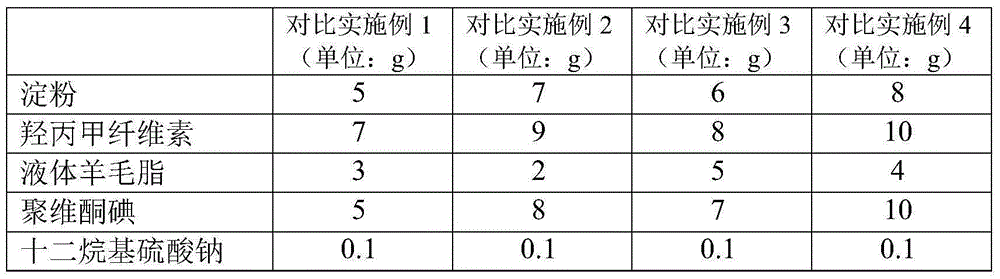
Breast protection membrane for preventing dairy cow mastitis and preparing process of breast protection membrane
ActiveCN105168239AReduce absorptionResidue reductionCosmetic preparationsToilet preparationsIrritationGlycerol
The invention provides a breast protection membrane for preventing dairy cow mastitis and a preparing process of the breast protection membrane. The breast protection membrane is prepared from membrane forming agents, thickening agents, povidone iodine, a wetting agent, skin moisturizing agents and purified water according to a composition proportion being (0.3 to 1):(0.3 to 1):(0.5 to 5):(0.05 to 0.15):(6 to 15):(92.85 to 77.85), wherein the membrane forming agents select hydroxyethyl cellulose, hypromellose, methylcellulose, polyvidone and sodium carboxymethyl cellulose; the thickening agents select xanthan gum, guar gum and carrageenin; the wetting agent selects docusate sodium; and the skin moisturizing agents select glycerol and propylene glycol. The breast protection membrane has the characteristics that the formula has small irritation on the breasts of dairy cows; the action is mild; the bacteriostatic effect is high; the dripping phenomenon on the nipples of the dairy cows seldom occurs; and the cost is low, and the like. The process operation is simple; raw materials can be easily acquired; and the breast protection membrane is suitable for large-scale production.
Owner:济南深蓝动物保健品有限公司
Environment-friendly water-series extinguishing agent
InactiveCN106492395AReduce surface tensionStrong foaming powerFire extinguisherPolyethylene glycolSolvent
The invention discloses an environment-friendly water-series extinguishing agent. Water serves as non-ignitable liquid and is a most adequate natural extinguishing agent, while the water is liable to flow, only a small part of the water plays an extinguishing effect in the actual extinguishing process, the waste rate is high, the reburning preventing efficiency is also poor, and some substances with special effects are added into the water so that the performance of the water can be improved; and various function requirements are met at the same time, and namely, the water-series extinguishing agent is obtained. The environment-friendly water-series extinguishing agent is prepared by the following main raw materials proportionally including, by weight, foaming agents: 4-8 parts of alkyl glycoside, interfacial agents: 3-6.5 parts of sodium dodecyl benzene sulfonate, spreading anti-burning agents: 1-3 parts of polyethylene glycol, tackifiers: 0.5-1.5 parts of hydroxypropyl methyl cellulose, antifreeze agents: 8-16 parts of dipropylene glycol dimethyl ether, cosolvents: 3-6.5 parts of urea, and wetting agents: 0.5-1.5 parts of docusate sodium and 43-87 parts of water.
Owner:于晶
Heavy-oil delivery pipeline cleaning agent and preparation method thereof
InactiveCN104531378ANo corrosionNo damageSurface-active detergent compositionsDetergent compounding agentsPolyoxyethylene castor oilHolboellia latifolia
The invention discloses a heavy-oil delivery pipeline cleaning agent and a preparation method thereof and belongs to the technical field of oil pipeline cleaning. The heavy-oil delivery pipeline cleaning agent is prepared from the following components in parts by weight: 2-4 parts of corn stigma, 3-5 parts of argy wormwood leaves, 1-2 parts of holboellia latifolia wall, 8-12 parts of polyoxyethylene lauryl ether, 7-10 parts of polyoxyethylene castor oil, 6-8 parts of docusate sodium, 2-5 parts of gellan gum, 1-2 parts of sodium citrate, 0.1-0.5 part of polyquaternium, 0.5-1 part of dimercapto ethylglycine, 1-2 parts of polymaleic anhydride which serves as an inhibitor, 20-30 parts of ethanol and 50-60 parts of water. The heavy-oil delivery pipeline cleaning agent disclosed by the invention can be used for removing 99% of oil and wax of the heavy-oil delivery pipeline thoroughly and rapidly and has a good scale inhibiting effect in the subsequent use process so that the cleaning cycle can be prolonged greatly. In addition, the heavy-oil delivery pipeline cleaning agent does not contain strongly acidic or alkaline substances, can not corrode crude oil equipment, is harmless to the human body and is suitable for large-scale production.
Owner:JIANGSU JIANSHEN BIOLOGY AGROCHEM
Docusate sodium soft capsule
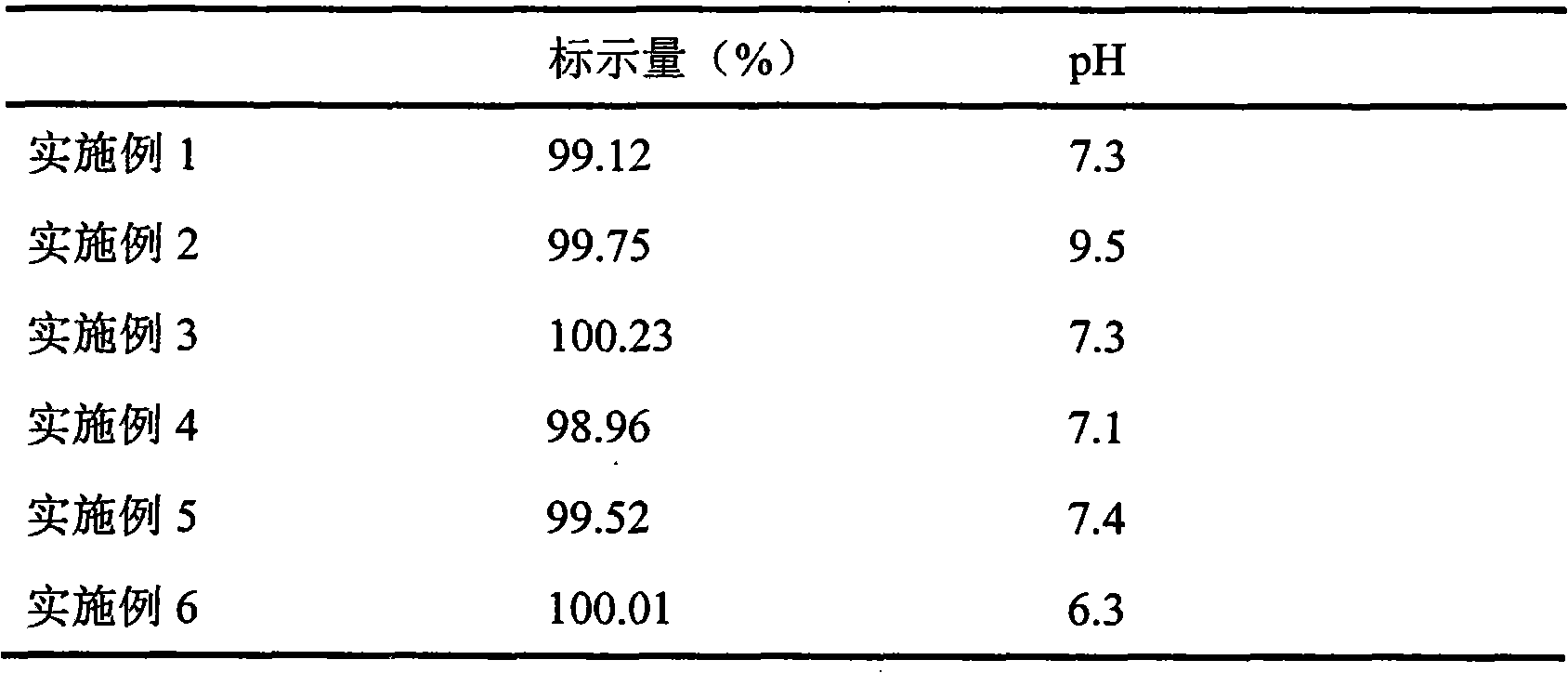
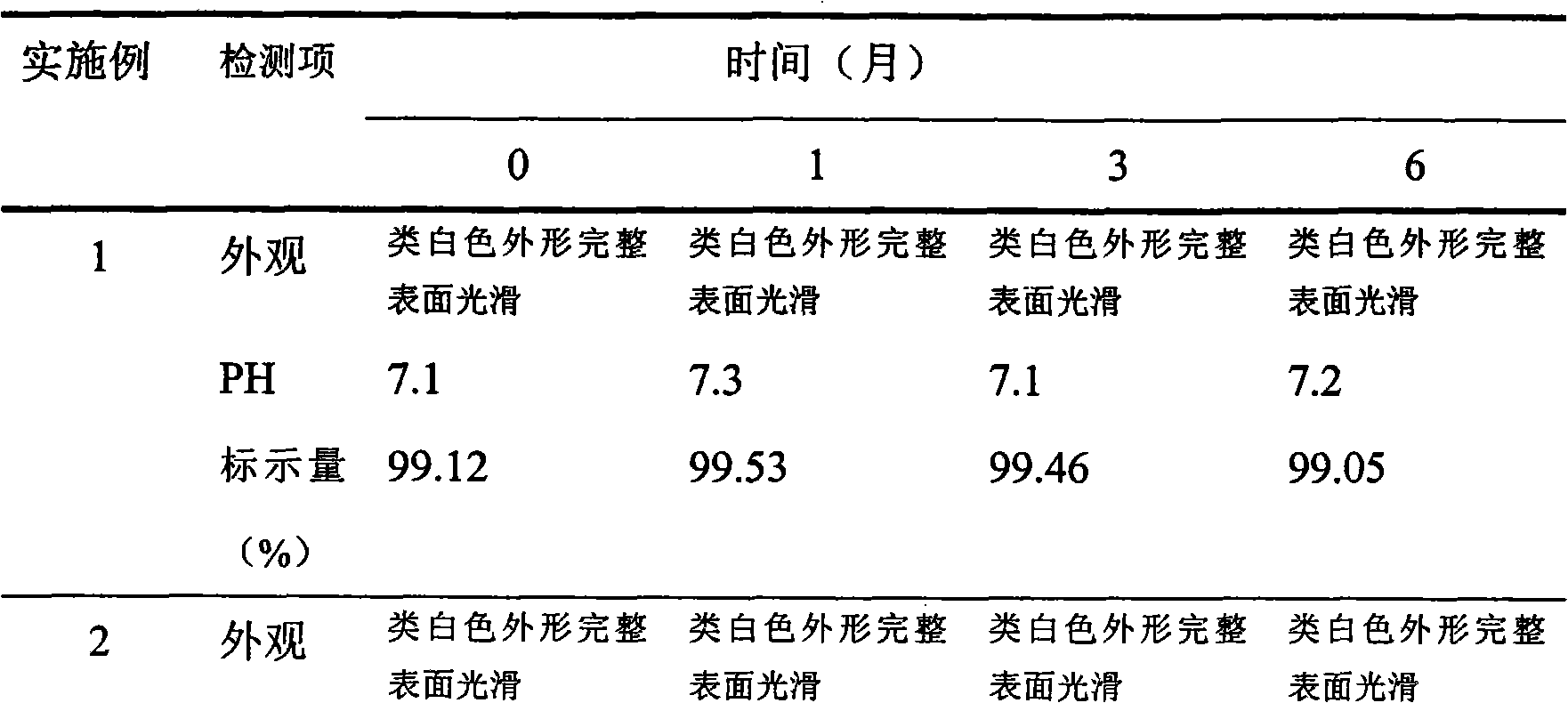
The invention discloses a docusate sodium soft capsule which comprises a soft capsule rubber skin and docusate sodium solution packaged in the soft capsule shell. The docusate sodium soft capsule is characterized in that the docusate sodium solution consists of the following components in percentage by weight: 10% to 50% of docusate sodium, 35% to 90% of polyethylene glycol and 0% to 15% of polyhydric alcohol; the soft capsule shell comprises a red shell and a white shell; the red rubber skin consists of the following components in percentage by weight: 45% to 80% of gelatin, 10% to 40% of plasticizer, 0.01% to 0.4% of colorant I and 5% to 15% of water; and the white shell consists of the following components in percentage by weight: 45% to 80% of gelatin, 10% to 40% of plasticizer, 0.1% to 4.0% of additive and 5% to 15% of water. The invention aims to overcome the defects in the prior art and provide the docusate sodium soft capsule which comprises simple components and has strong adaptability and high response speed.
Owner:安士制药(中山)有限公司
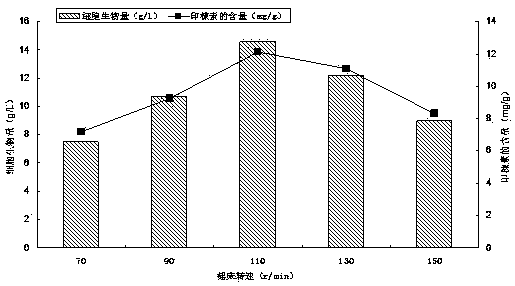
Method for preparing high-purity docusate sodium
InactiveCN104829503AOrganic-compounds/hydrides/coordination-complexes catalystsSulfonic acids salts preparationIsooctyl alcoholHydrogen
The invention provides a method for preparing high-purity docusate sodium. The method includes esterification of maleic anhydride and isooctanol and sulfonation of obtained diesters and sodium hydrogen sulfite in aqueous solution; wherein the esterification and sulfonation are completed under nitrogen protection, and the esterification is divided into 80-95 DEG C pre-esterification and 115-125 DEG C decompression reaction. By the method simple in process, little in toxic solvent, free of pollution and high in yield, the prepared docusate sodium is high in purity.
Owner:BEIJING BANGNIKANGDA MEDICAL TECH CO LTD
Composition for improving treating or preventing constipation comprising Cassia fermented by lactic acid bacteria as an active ingredient and preparation method thereof
InactiveCN105495150AImprove securityConstipation Treatment EnhancementBacteriaDigestive systemSide effectFeces
The invention relates to a composition for improving treating or preventing constipation comprising Cassia fermented by lactic acid bacteria as an active ingredient and a preparation method thereof. The Cassia fermented by lactic acid bacteria can used for a long time, is high in safety, and is low in side effect when be used as a crude drug. The Cassia fermented by lactic acid bacteria has an effect that more than one of the excrement amount, the moisture content and the excrement weight is increased, the effect is the same as that of a commercial Dulcolax-S with sennoside or bisacodyl and docusate sodium as the active ingredient, the commercial Dulcolax-S is an irritant constipation therapeutic agent and is forbidden to be used for health care foods due to the side effect. Thus, the Cassia fermented by lactic acid bacteria can be applied to pharmaceutical compositions for constipation treatment or prevention, or health care foods for constipation improvement or prevention. Furthermore, the Lactobacilluskefiri MJ90 bacterial strain is beneficial to the preparation of Cassia fermented by lactic acid bacteria for improving treating or preventing constipation.
Owner:HEANAM NATURAL FARMING ASSOC COOPERATION
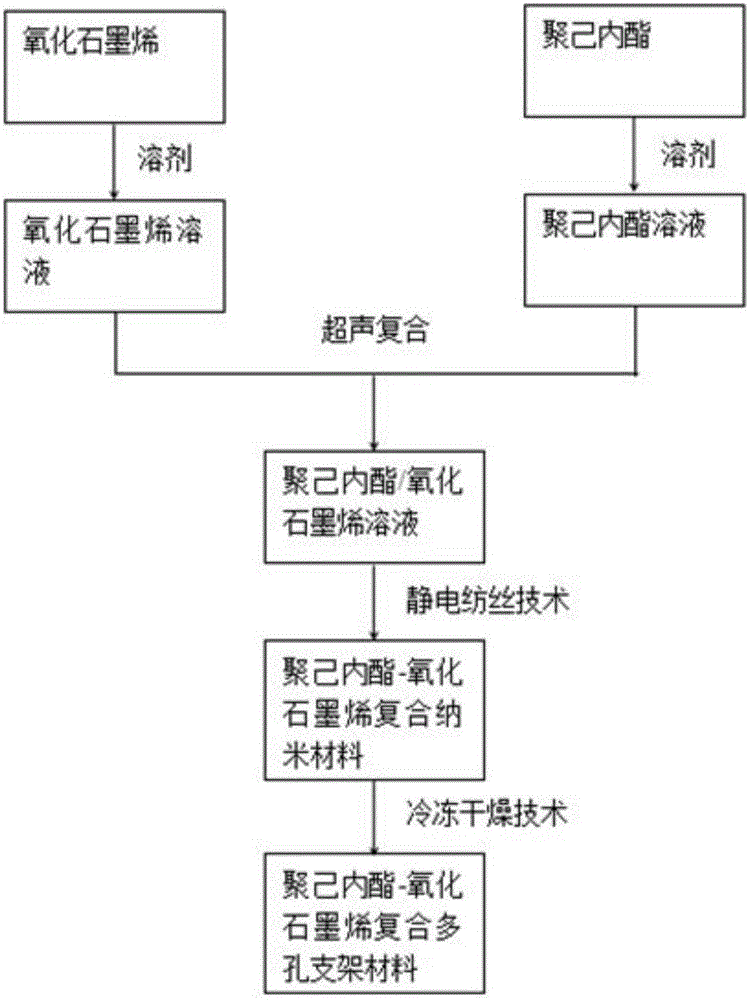
Polycaprolactone-graphene oxide composite porous scaffold material preparation method
PendingCN106581777AHigh porosityGood biocompatibilityTissue regenerationProsthesisFiberFreeze-drying
The invention relates to a polycaprolactone-graphene oxide composite porous scaffold material preparation method, which comprises: (1) simultaneously dissolving graphene oxide and docusate sodium into dimethylformamide to obtain a graphene oxide / docusate sodium dispersion liquid; (2) dissolving polycaprolactone into the mixed liquid of dichloromethane and docusate sodium to obtain a polycaprolactone / (dichloromethane and docusate sodium) solution; (3) mixing the graphene oxide solution and the polycaprolactone solution to obtain a polycaprolactone-graphene oxide solution; and (4) carrying out ultrasonic treatment on the obtained mixed liquid, loading into the syringe of an electrospinning machine, carrying out electrospinning, and carrying out freeze drying on the composite nanometer fiber obtained from the electrospinning to obtain the polycaprolactone-graphene oxide composite porous scaffold material. According to the present invention, the polycaprolactone-graphene oxide composite porous scaffold material prepared through the method has advantages of high porosity, pore communication, good biocompatibility, osteogenic inducing activity, and the like; and with the preparation method, the porous scaffold has the high porosity while the good mechanical and biological properties are provided.
Owner:袁峰 +1
Cold chain coolant and preparation method thereof
InactiveCN110484215AEasy to operateSuitable for large-scale productionOrganic compound preparationHeat-exchange elementsCold chainPolyethylene glycol
The invention discloses a cold chain coolant that is characterized by being prepared from, by mass, 5%-10% of metal chloride, 1%-4% of ammonium chloride, 1%-3% of alcohol, 0.1%-0.3% of active silicon,2%-5% of borax, 0.3%-1% of docusate sodium, 5%-10% of amino-terminated polyethylene glycol amidated pectic acid, 2%-4% of aleuritic acid group organic salt and the balance water, wherein the aleuritic acid group organic salt is prepared by carrying out ion exchange reaction on aleuritic acid and choline. The invention further discloses a preparation method of the cold chain coolant. The cold chain coolant disclosed by the invention has the advantages of large latent heat, small supercooling degree, good heat transfer, good cold chain effect, small toxicity and pollution, high cold chain efficiency and long cold storage time.
Owner:HUNAN QIWEI TECH CO LTD
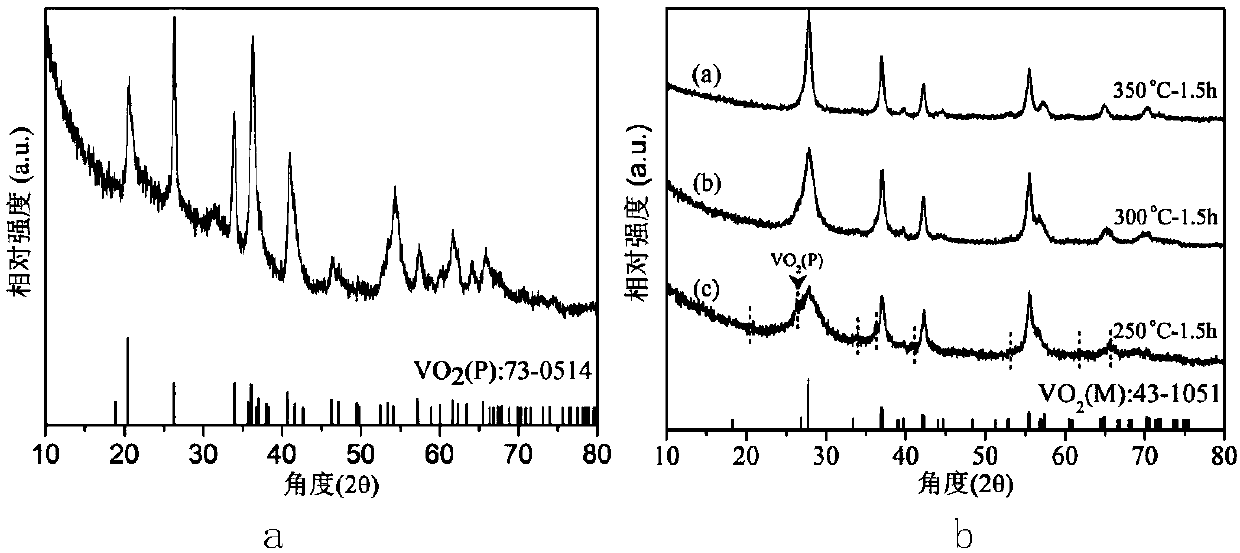
Preparation method of P-phase VO2 nano-powder
ActiveCN106698514ASmall sizeGood dispersionNanotechnologyVanadium oxidesVanadium dioxideDocusate Sodium
The invention discloses a preparation method of P-phase VO2 nano-powder. The method adopts a hydrothermal method and specifically comprises the following steps: firstly, ammonium metavanadate and docusate sodium are added to water and stirred, a mixed solution is obtained, a formic acid solution is added dropwise to the mixed solution under stirring, and a reaction precursor solution is obtained; then the reaction precursor solution is placed at 180-250 DEG C for closed reaction, a reaction liquid is obtained and subjected to solid-liquid separation, washing and drying, and a granular target product with particle size being 50-90 nm, namely, the P-phase VO2 nano-powder, is obtained. The preparation method has the characteristics that environmental protection is realized and nitrogen protection is not needed for follow-up annealing treatment of the target product, conversion of VO2(P) to VO2(M) can be realized easily through low temperature thermal treatment, and accordingly, the final product VO2(M) is applied to the fields of energy-saving windows, sensors, storage devices and the like commercially and widely quite easily.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
Flunarizine-hydrochloride pharmaceutical composition
ActiveCN105998024ALong-term storage stabilitySolve the splinter problemOrganic active ingredientsNervous disorderFlunarizine HydrochlorideMagnesium stearate
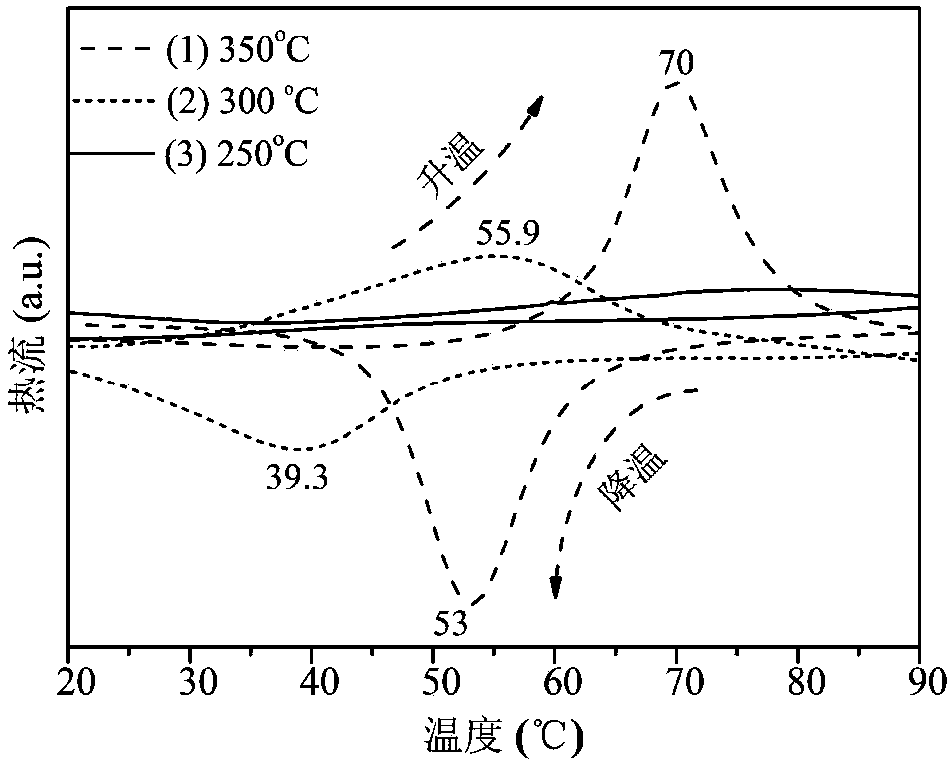
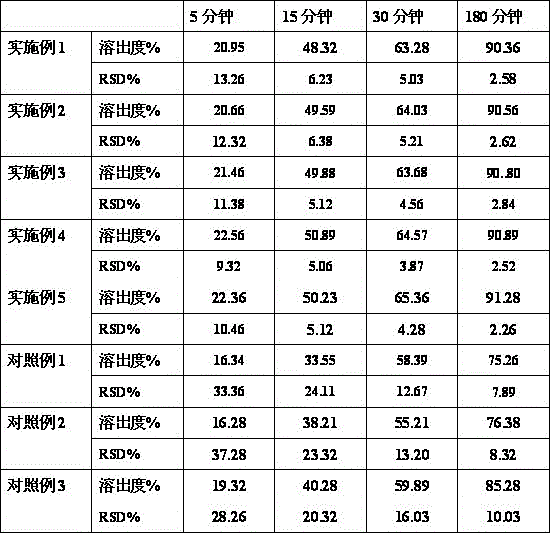
The invention relates to a flunarizine-hydrochloride pharmaceutical composition and a preparing method thereof, and belongs to the technical field of medicine making. The flunarizine-hydrochloride pharmaceutical composition in the technical scheme is characterized in that every one thousand compositions comprise 5.9 g of flunarizine hydrochloride, 62.5 g-82.5 g of lactose, 70 g-90 g of microcrystalline cellulose, 3.2-12.8 g of sodium carboxymethylcellulose, 0.8 g of silicon dioxide, 0.8 g of magnesium stearate and 1-1.6 g of docusate sodium. According to the flunarizine-hydrochloride pharmaceutical composition and the preparing method thereof, a tablet with the small resolving degree RSD is provided through reasonable compatibility, the prepared tablet is stable in long-term storage, and high-quality medicine is provided for a clinic.
Owner:DISHA PHARMA GRP
Modifying agent for preventing calcium halide scaling
ActiveCN105419761AStrong anti-scaling performanceReduce dosageDrilling compositionDocusate SodiumDioctyl Sulfosuccinic Acid
The invention discloses a modifying agent for preventing calcium halide scaling. The modifying agent is prepared from, by mass, 15-25 parts of amino trimethylene phosphonic acid, 4-8 parts of docusate sodium, 2-3 parts of cetyltrimethylammonium chloride, 0.1-0.3 part of tributyl phosphate and the balance water. The modifying agent has the advantages of being high in scaling resistance, small in use amount, easy to construct, not capable of increasing operation workloads, high in flow-back capacity, capable of saving cost, and the like.
Owner:PETROCHINA CO LTD
Biodegradable mulching film with multi-layer structure
InactiveCN108055954AMeet the growth cycle requirementsImprove insulation effectPlant protective coveringsThermal insulationPolyvinyl alcohol
The invention discloses a biodegradable mulching film with a multi-layer structure. The film comprises an anti-ageing layer, a water-resisting layer, an insulating layer and a water retaining layer; the anti-ageing layer is prepared from 100 parts of dimethyl terephthalate copolymer, 10-30 parts of polylactic acid, 30-45 parts of nano calcium carbonate, 0.2-0.5 part of erucic acid amide and 0.5-2pars of ultraviolet absorbent; the water-resisting layer is prepared from 100 parts of chemical modified starch, 30-100 parts of polylactic acid and 2-6 parts of plasticizer; the insulating layer is prepared from 100 parts of polyvinyl alcohol, 10-30 parts of polylactic acid, 20-50 parts of polyhydroxyalkanoate, 3-10 parts of ultrafine talcum powder, 0.2-0.5 part of erucic acid amide and 0.5-1 part of citric acid; the water retaining layer is prepared from 100 parts of polyacrylic acid resin, 20-40 parts of polylactic acid, 5-10 parts of docusate sodium and 2-6 parts of polyethylene glycol. The biodegradable mulching film with the multi-layer structure is biodegradable, the thermal insulation and moisturizing effects are good, the uvioresistant and anti-ageing properties are excellent, andthe service life can meet the requirements of the whole growth cycle of crops.
Owner:苏州星火丰盈环保包装有限公司
Method for preparing docusate sodium soft capsules
ActiveCN103070847ANice appearanceStrong complianceDigestive systemCapsule deliveryMelting tankAlcohol
The invention discloses a method for preparing docusate sodium soft capsules, which comprises the following steps: A, preparing red gelatin solution, i.e. dissolving a plasticizer into water, adding the obtained product into a gelatin melting tank, heating to a temperature of 40 to 80 DEG C, adding gelatin, stirring to dissolve the gelatin to obtain gelatin solution, adding a colorant I into the water, uniformly stirring, adding the mixture into the gelatin solution, stirring, vacuumizing and discharging air bubbles to obtain the red gelatin solution for later use; B, preparing white gelatin solution, i.e. adding an additive into the sufficient quantity of water, uniformly dispersing by a colloid mill to obtain solution I, dissolving the plasticizer into the water, adding the solution I and the plasticizer solution into the gelatin melting tank to heat to a temperature of 40 to 80 DEG C, adding gelatin, stirring and dissolving the gelatin, and after totally dissolving the gelatin, vacuumizing and discharging air bubbles to obtain the white gelatin solution for later use; C, preparing contents, i.e. adding polyhydric alcohol into polyethylene glycol solution of docusate sodium and uniformly mixing at a temperature of 30 to 50 DEG C to obtain the contents for later use; and D, preparing the soft capsules, i.e. adding the content liquor obtained in the step C into a feed hopper of a pelleting press, regulating packing volumes and thickness of shells, pelleting, drying, smearing pills and packaging.
Owner:安士制药(中山)有限公司
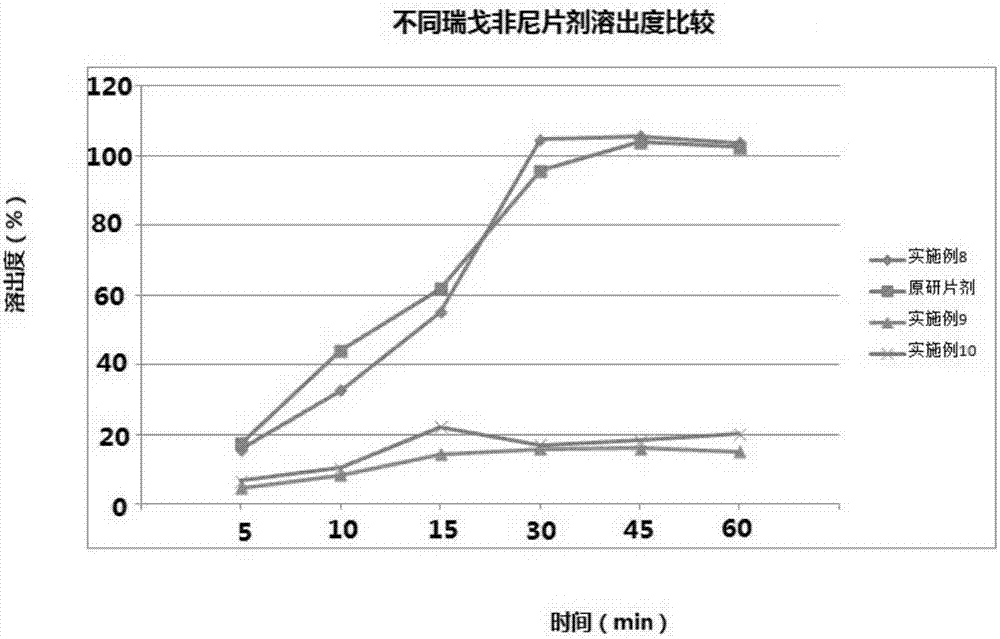
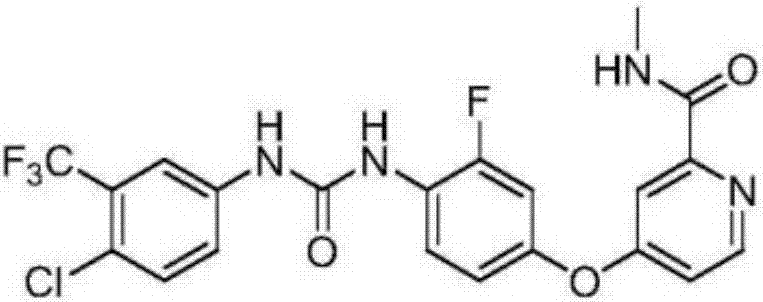
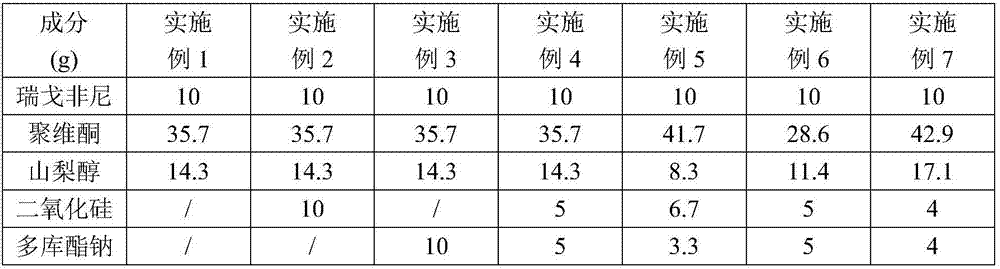
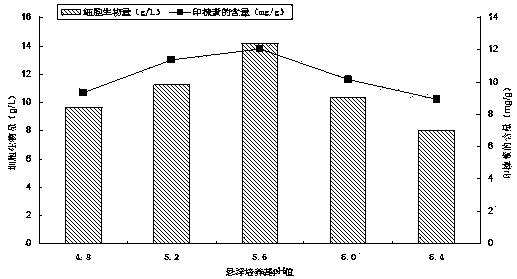
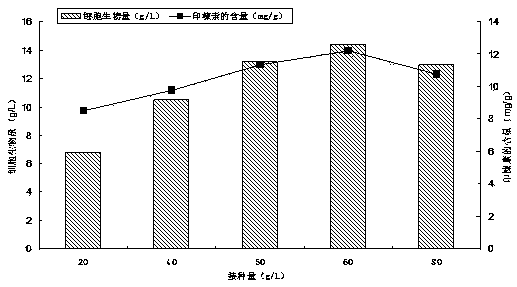
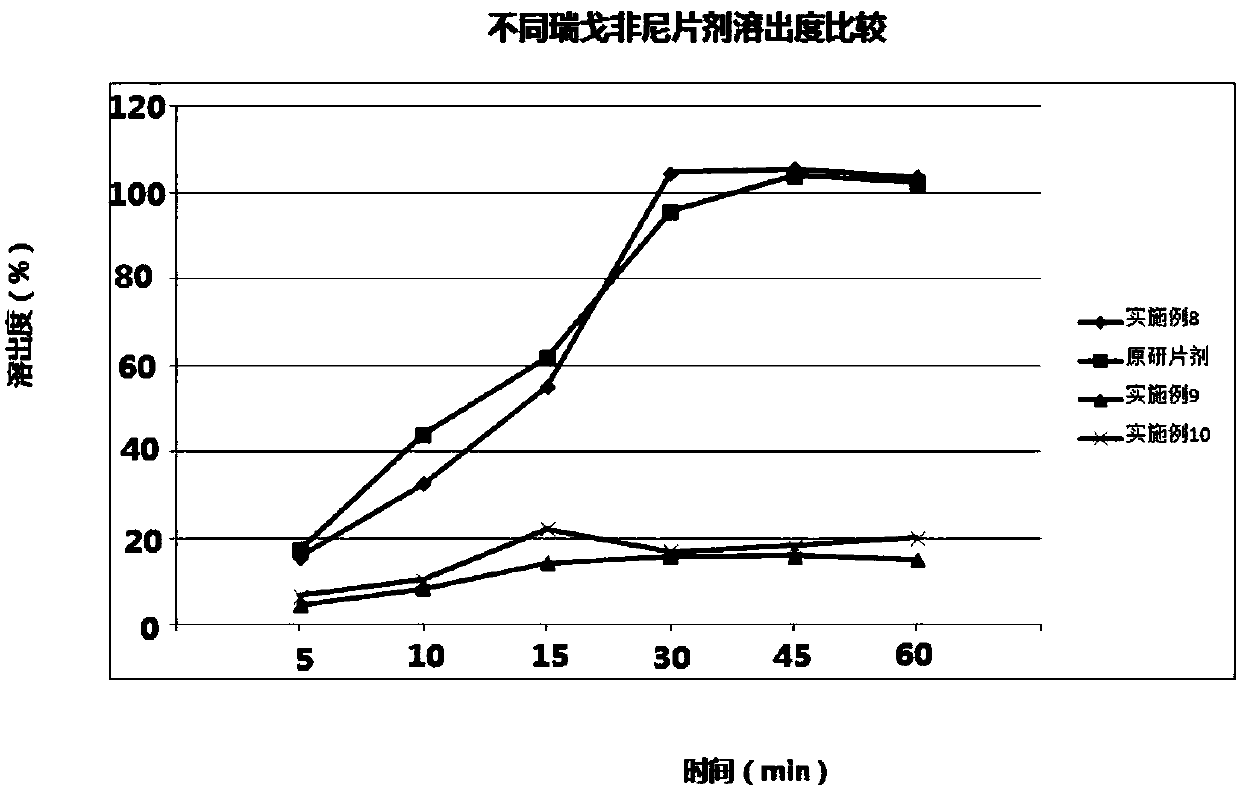
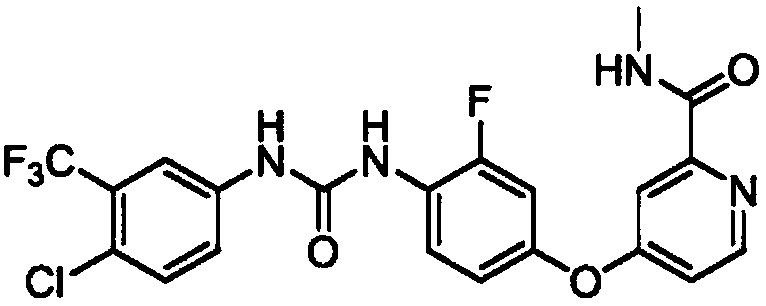
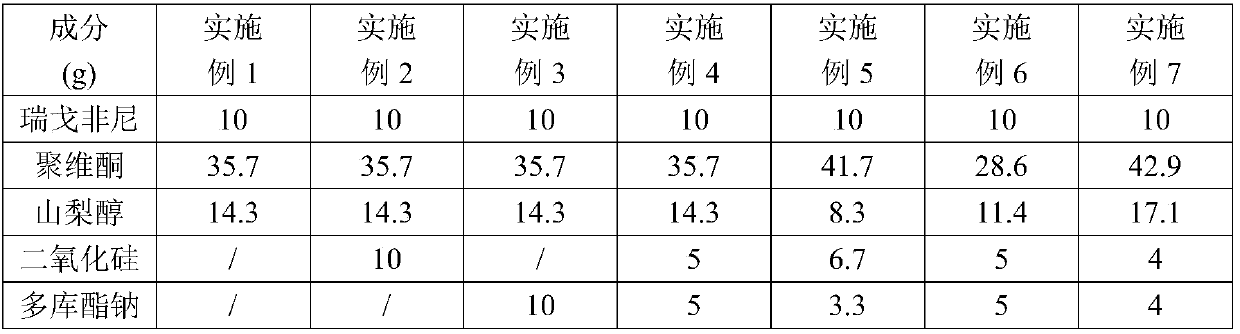
Regorafenib solid dispersion and preparation thereof
ActiveCN107213127AGood water solubilityImprove solubilityPill deliveryPharmaceutical non-active ingredientsSolubilityDocusate Sodium
The invention discloses a regorafenib solid dispersion, which comprises regorafenib, a carrier material and a synergistic agent in the mass ratio of 1: (2-20): (0.2-5), the carrier material comprises povidone and sorbitol in the mass ratio of 1: (0.1-0.5), and the synergistic agent comprises silica and Docusate sodium in the mass ratio of 1: (0.5-1). Through a certain process, the regorafenib is prepared into the sorbitol-regorafenib-povidone sandwich structure solid dispersion, dissolution of the regorafenib from inside to outside is promoted, the solubility of the regorafenib is greatly improved, safe, non-toxic and volatile ethanol is used as a solvent in the preparation process, and no security risk is caused by residual solvent. At the same time, the aging phenomenon in the long time storage process of the regorafenib solid dispersion can be avoided, and stability is improved.
Owner:广东安诺药业股份有限公司
Nutritional supplements for women desiring to become pregnant, and pregnant and nursing women
The present invention relates to nutritional supplements to be administered to, or to be taken by, women desiring to become pregnant, and pregnant and nursing women. The nutritional supplements of this invention have a unique blend of vitamins, minerals, lycopene, co-enzyme Q10, DHA, docusate (such as docusate sodium), folic acid, and a nutritionally acceptable carrier therefor. The invention includes specific nutritional supplements for the uses set forth above.
Owner:ARGENT DEV GROUP
Azadirachta indica cell suspension culture medium and cell suspension culture method
The invention provides an azadirachta indica cell suspension culture medium and a cell suspension culture method. According to the azadirachta indica cell suspension culture medium, based on a basic culture medium, component matching is optimized, and a certain amount of sodium vanadate, L-cysteine, tyrosine, nicotinamide, dithiothreitol, beta-D-galactose, cyclohexanol phosphate, diethylaminoethylhexanoate, 6-BA, ecdysone, sodium nitroprusside, docusate sodium, flaccid knotweed herb juice and henry munronia herb juice is added; the cell suspension culture method comprises the following stepsof 1) obtaining of sterile test-tube plantlets; 2) induction and subculture for calluses; 3) cell suspension culture. By adopting the culture medium and the culture method, not only can the stable azadirachta indica cell suspension culture system be obtained, but also the biomass of suspension cells and the content of azadirachtin in the suspension cells can be significantly improved, the technical support is provided for azadirachtin production through large-scale azadirachta indica cell culture, and important application value is achieved.
Owner:无锡吉成生物科技有限公司
Method for preparing high-purity docusate sodium
The invention relates to a method for preparing docusate sodium. The method comprises the step of esterification between 2-ethyl hexanol and maleic anhydride and the step of sulfonation of the obtained ester and sodium hydrogensulfite in a water solution. The method is characterized in that the method further comprises the following steps of purification of rough products: crystallizing the sulfonated rough products in an alcohol and water system to obtain a water-containing crystal, dissolving the water-containing crystal in alcohol dehydratum, performing reduced pressure distillation to remove a solvent so as to obtain anhydrous docusate sodium, wherein alcohol in the alcohol and water system is selected from a group comprising methyl alcohol, ethanol, normal propyl alcohol and isopropyl alcohol, and the alcohol dehydratum is selected from ethanol, normal propyl alcohol and isopropyl alcohol. By the method provided by the invention, the high-purity docusate sodium can be prepared.
Owner:HUNAN WARRANT PHARMA
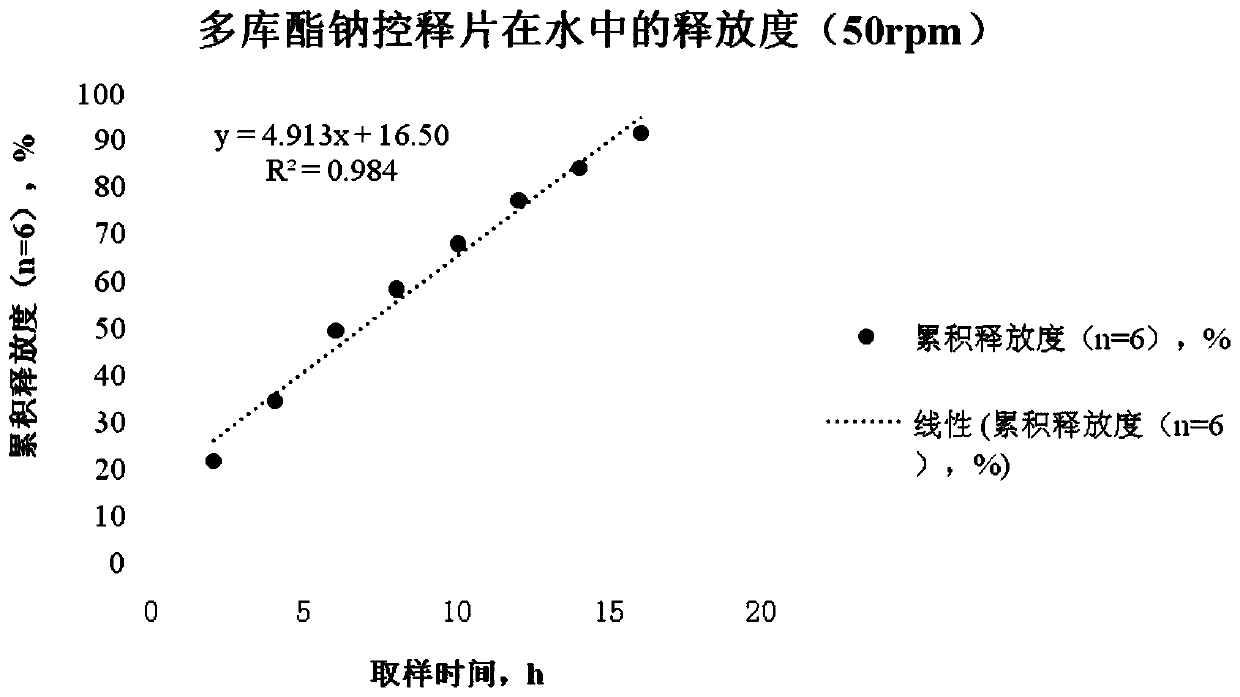
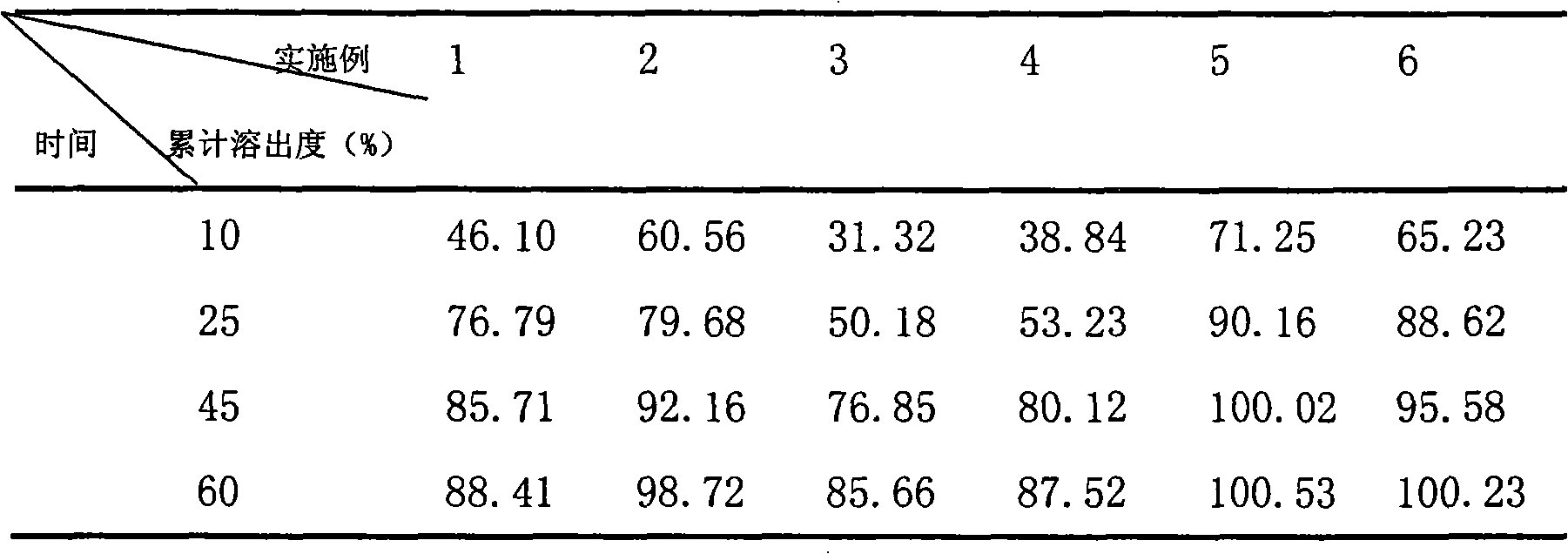
Preparation method of docusate sodium controlled-release tablet
InactiveCN111568870AWide variety of sourcesLow costDigestive systemInorganic non-active ingredientsPhysical chemistryDrugs preparations
The invention relates to a preparation method of a docusate sodium controlled-release tablet, and belongs to the technical field of pharmaceutical preparations. The method comprises the following steps of (1) dissolving docusate sodium in ethanol; (2) adding microcrystalline cellulose and hydroxypropyl methylcellulose into a wet type granulator, setting the rotating speed of a mixing knife and therotating speed of a granulating knife, starting the wet type granulator, and performing mixing; (3) setting the rotating speed of the mixing knife and the rotating speed of the granulating knife, starting the wet type granulator, spraying a mixture obtained in the step (1) by using a spray gun, and then performing stirring; (4) granulating an obtained soft material by using a screen; (5) drying wet granules by using a boiling granulating dryer, and performing straightening granulation by using a screen of a swing straightening granulator; and (6) mixing and tabletting the obtained dry granules, silicon dioxide and talcum powder to obtain the docusate sodium controlled-release tablet. The method is scientific and reasonable in design, is simple and feasible, solves the problems of difficulty in raw material grinding, non-uniform mixing and easiness in raw material caking, and is suitable for large-scale production.
Owner:REYOUNG PHARMA
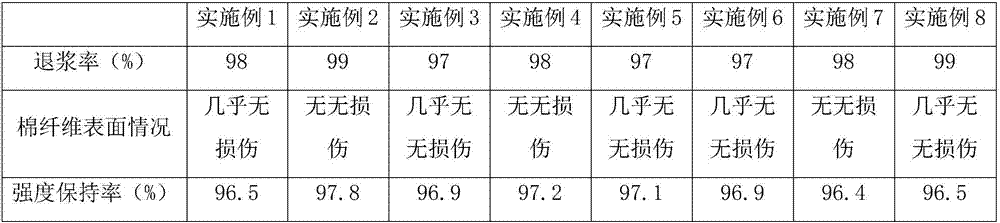
Cotton desizing method based on supercritical carbon dioxide and alkaline biological enzymes
InactiveCN106996034AGood alkali resistanceHigh activityDry-cleaning apparatus for textilesSodium bicarbonateAlpha-amylase
The invention provides a preparation method of a cotton desizing method based on supercritical carbon dioxide and alkaline biological enzymes. The cotton desizing method comprises the following steps: dissolving lignin and sodium bicarbonate weakly-alkaline materials in water, adding alcohol, then adding docusate sodium and chitosan surfactants, and uniformly stirring under room temperature to form a substrate solution; adding alkaline proteases, alpha-amylases and PVA degeneration enzymes into the substrate solution, and uniformly mixing to form a desizing agent; dipping a cotton fabric in the desizing agent, putting the desizing agent and the cotton fabric in a constant-temperature ultrasonic generator, heating and carrying out ultrasonic pretreatment, taking the desizing agent and the cotton fabric out, transferring the desizing agent and the pretreated cotton fabric to a supercritical carbon dioxide fluid device, carrying out desizing treatment, separating and recycling the desizing agent and the supercritical carbon dioxide fluid, taking the fabric out, washing and drying to obtain the desized cotton fabric. The cotton desizing method is capable of efficiently removing various size agents on the surface of the cotton fabric with combination of a biological enzyme technique, an ultrasonic technique and a supercritical carbon dioxide technique, and is green and safe.
Owner:苏州凯邦生物技术有限公司
Preparation method of wool-silk shrink resistant machine washing liquor
InactiveCN106854555AAvoid loweringNatural lusterNon-ionic surface-active compoundsOrganic detergent compounding agentsFiberDishwashing liquid
The invention relates to a preparation method of wool-silk shrink resistant machine washing liquor. When wool-silk garments are washed at ordinary times, washing powder or dishwashing liquid serves as a main washing product, then a wool-silk shrink resistant softener is used for caring, and the effect is not ideal after washing. The wool-silk shrink resistant machine washing liquor is formed by mixing paeonol, raspberry ketone glucoside, nicotinamide, azelaic acid, gleditsia sinensis, an arnica montana extracting solution, hydroxypropyl methyl cellulose, ilex chinensis sims oil, AES, probiotics microflora, an emulgator, a platycladus orientalis extracting solution, citric acid, glucoside powder, sodium cocoyl glutamate, docusate sodium, enzymes, acetic acid, incense and water. The wool-silk shrink resistant machine washing liquor ensures that fabric can effectively prevent shrinkage and static electricity, improves the brightness, rapidly permeates into the deep part of fiber, removes stains inside the fiber, and ensures that washed fabric can effectively prevent shrinkage, and is soft and fluffy and natural in gloss.
Owner:CHANGSHA XIEHAOJI BIOENG CO LTD
Medical preparation for treating astriction and preparation method thereof
The invention relates to a medical preparation for treating astriction and a preparation method thereof. The invention is characterized in that: a suppository for rectal administration is prepared by adding a base material and a proper dispersant into docusate sodium; the quality of the suppository is controllable; the product is stable; the natural of the product is mild; the product can be used for preventing and treating various kinds of astriction, such as astriction caused by hemorrhoids, age and weakness and cerebrovascular diseases and astriction occurring after anal and gynecological operations and other operations, in people of different ages; and the product has a particular clinic application value for patients who cannot bear taking medicaments orally or special patients.
Owner:田普森
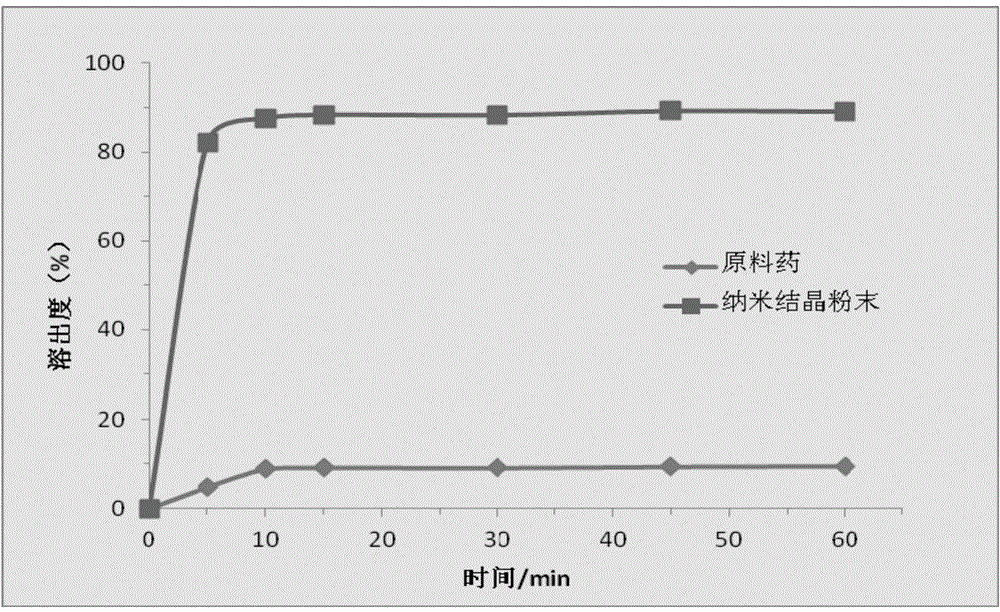
Simvastatin nano suspension and preparation method thereof
The invention relates to a simvastatin nano suspension and a preparation method thereof. The simvastatin nano suspension comprises the following components: 1 weight part of simvastatin and 0.5-20 weight parts of stabilizing agent; wherein the stabilizing agent is selected from lecithin, Poloxamer 188, sodium dodecyl sulfate, hydroxy propyl cellulose, hydroxypropyl methylcellulose, polyvinylpyrrolidone, docusate sodium and its combination.
Owner:SHANGHAI SINE PHARMA LAB
Degreasing and cleaning agent for chemical equipment
The invention provides a degreasing and cleaning agent for chemical equipment. The degreasing and cleaning agent comprises the following components in parts by weight: 3.0 to 5.8 parts of aluminum-magnesium silicate, 3.5 to 8.0 parts of arginine, 5.0 to 8.5 parts of docusate sodium, 5.5 to 7.2 parts of tetrasodium EDTA, 6.5 to 9.3 parts of metal ion chelating agent, 1.6 to 2.8 parts of bentonite, and 2.2 to 5.6 parts of sucrose fatty acid ester. The provided degreasing agent is especially suitable for cleaning and degreasing important chemical equipment, such as stainless steel, pipelines, containers, and the like; is noncorrosive to the surface of metal equipment, is barely affected by acid, alkali, soft water, hard water, and seawater; has a strong degreasing performance and good cleaning effect; is nontoxic and flame retardant, and thus has a wide application prospect. After cleaning and degreasing, the surface is lustrous and does not become black, the degreasing and cleaning agent is user-friendly, and the pollution and cleaning cost are reduced.
Owner:YANCHENG OUHUA CHEM IND
Method for preparing high-purity docusate sodium
The invention relates to a method for preparing docusate sodium. The method comprises the step of esterification between 2-ethyl hexanol and maleic anhydride and the step of sulfonation of the obtained ester and sodium hydrogensulfite in a water solution. The method is characterized in that the method further comprises the following steps of purification of rough products: crystallizing the sulfonated rough products in an alcohol and water system to obtain a water-containing crystal, dissolving the water-containing crystal in alcohol dehydratum, performing reduced pressure distillation to remove a solvent so as to obtain anhydrous docusate sodium, wherein alcohol in the alcohol and water system is selected from a group comprising methyl alcohol, ethanol, normal propyl alcohol and isopropyl alcohol, and the alcohol dehydratum is selected from ethanol, normal propyl alcohol and isopropyl alcohol. By the method provided by the invention, the high-purity docusate sodium can be prepared.
Owner:HUNAN WARRANT PHARMA
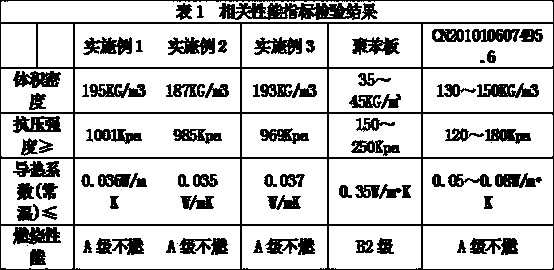
Energy-saving thermal insulation concrete block for building and preparation method thereof
The invention discloses an energy-saving thermal insulation concrete block for a building. The energy-saving thermal insulation concrete block is prepared from the following raw materials in parts by weight: 60-80 parts of ordinary Portland cement, 30-40 parts of waste paper, 9-12 parts of polyimide fiber, 6-8 parts of lithium phosphate, 65-85 parts of water, 9-12 parts of palygorskite, 4-5 parts of pyrophyllite, 7-9 parts of barite, 3-4 parts of a polycarboxylate high-performance water-reducing agent, 2-3 parts of sodium bicarbonate, 4-5 parts of docusate sodium and 1-2 parts of diisopropyl azodicarboxylate. The absolute dry apparent density of a high-strength light-weight thermal insulation concrete block is 180-200kg / m<3>, the strength is 0.95-1.01MPa, the interior of the high-strength light-weight thermal insulation concrete block adopts a closed-hole honeycomb structure, the heat conductivity coefficient is 0.035-0.037W / m.k, and the energy-saving thermal insulation concrete block for the building has the characteristics of high yield, short maintenance time, low raw material cost, simple process, energy conservation and emission reduction.
Owner:汉江城建集团有限公司
Preparation method of benzoyl peroxide gel
PendingCN110433135AObvious gritGood application comfortAerosol deliveryOintment deliveryColloidal silicaBenzoyl peroxide
The invention relates to the technical field of a medicinal preparation, and relates to a preparation method of a benzoyl peroxide gel. The prescription ingredient ratio of the benzoyl peroxide gel ischaracterized in that a main drug comprises 5% of benzoyl peroxide (based on pure basis), a gel matrix is 0.60% to 1.0% of carbomer 980, a wetting agent is 3% to 5% of propylene glycol, a surfactantis 0.1% to 0.3% of poloxamer 188, a chelating agent is 0.1% of disodium edetate, a surfactant is 0.05% of docusate sodium, a suspending agent is 0.02% to 0.05% of colloidal silica, an adsorbent is 1.5% to 2.5% of an acrylate copolymer (polytrap 6603), a humectants is 3 % to 5% of glycerin, a pH regulator is 0.14% of sodium hydroxide and a solvent, and purified water is supplied to 100%. The methodis characterized in that a carcinogenic solvent (benzene) is used in the production process of Carbomer 940, and a part of benzene remains in the commercial Carbomer 940, in the technical scheme, thecarbomer 980 is used instead of carbomer 940, and carbomer 980 does not contain benzene, so the product safety is improved.
Owner:四川明欣药业有限责任公司
A kind of regorafenib solid dispersion and preparation thereof
ActiveCN107213127BImprove solubilityThere is no security riskPill deliveryPharmaceutical non-active ingredientsSolubilityMass ratio
The invention discloses a regorafenib solid dispersion, which comprises regorafenib, a carrier material and a synergistic agent in the mass ratio of 1: (2-20): (0.2-5), the carrier material comprises povidone and sorbitol in the mass ratio of 1: (0.1-0.5), and the synergistic agent comprises silica and Docusate sodium in the mass ratio of 1: (0.5-1). Through a certain process, the regorafenib is prepared into the sorbitol-regorafenib-povidone sandwich structure solid dispersion, dissolution of the regorafenib from inside to outside is promoted, the solubility of the regorafenib is greatly improved, safe, non-toxic and volatile ethanol is used as a solvent in the preparation process, and no security risk is caused by residual solvent. At the same time, the aging phenomenon in the long time storage process of the regorafenib solid dispersion can be avoided, and stability is improved.
Owner:GUANGDONG ANNOL PHARM CO LTD
Phosphorus polluted wastewater treatment adsorbent as well as preparation method and application thereof
InactiveCN108212099ANot easy to cause secondary pollutionWith phosphorus removalOther chemical processesWater contaminantsSorbentCalcium formate
The invention discloses a phosphorus polluted wastewater treatment adsorbent as well as a preparation method and application thereof. The phosphorus polluted wastewater treatment adsorbent is preparedfrom the following raw materials in parts by weight: 20 to 30 parts of maca powder, 15 to 20 parts of docusate sodium, 10 to 20 parts of fly ash, 6 to 15 parts of calcium formate, 6 to 11 parts of ethylene glycol salicylate, 18 to 28 parts of mannitol, 5 to 11 parts of malic acid and 40 to 65 parts of water. The phosphorus polluted wastewater treatment adsorbent disclosed by the invention can beused for treating phosphorus polluted wastewater and treating heavy metal wastewater relatively well; the raw materials do not easily cause secondary pollution and the phosphorus polluted wastewater treatment adsorbent has phosphorus removal and heavy metal adsorption functions; energy-saving and environment-protection requirements can be met relatively well and the phosphorus polluted wastewatertreatment adsorbent has relatively high popularization value.
Owner:SUZHOU RUISHUO ENVIRONMENT TECH CO LTD
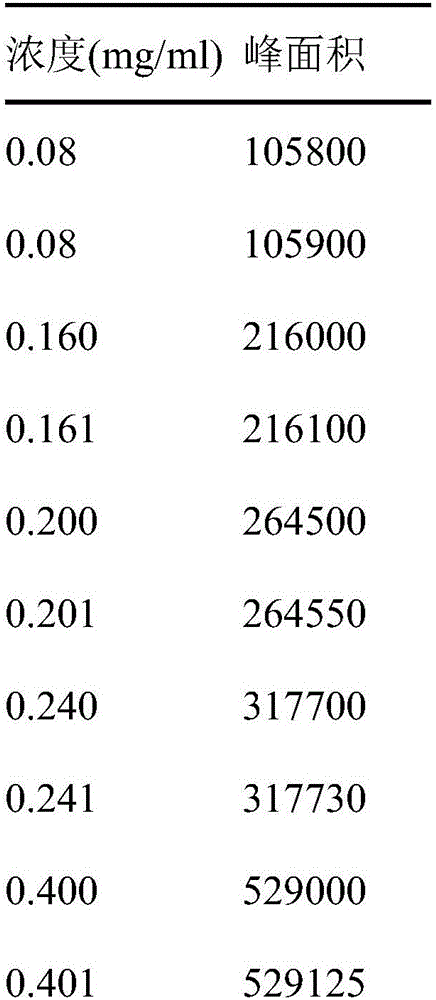
Novel detecting method for docusate sodium content and relevant substance
ActiveCN105891352AAccurate measurementReduce testing costsComponent separationTest articleDocusate Sodium
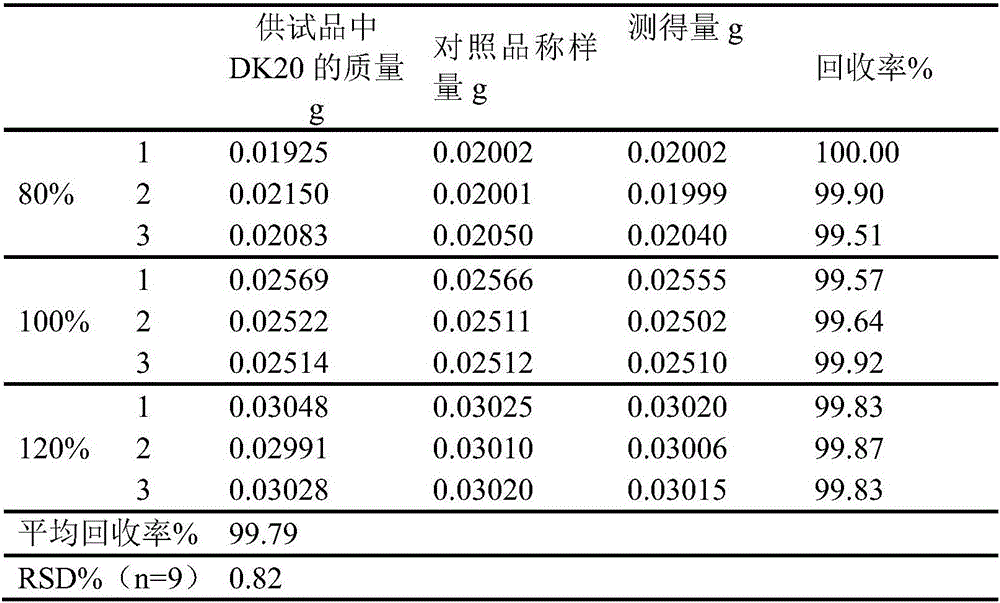
The invention discloses a novel detecting method for the docusate sodium content and a relevant substance. The relevant substance is 2-ethylhexyl ester. The method includes the following steps of preparing a reference substance solution A and a test article solution B, injecting 20 microliters of a sample of the reference solution A and 20 microliters of a sample of the test article solution B, recording the peak area of docusate sodium in a chromatogram, calculating the HPLC content through anhydrous docusate sodium according to an external standard method, preparing an impurity reference substance solution C and a test article solution D, injecting 20 microliters of a sample of the impurity reference solution C and 20 microliters of a sample of the test article solution D, recording the peak areas of docusate sodium and 2-ethylhexyl ester in the chromatogram, and calculating the content of docusate sodium and the limit of the relevant substance, namely 2-ethylhexyl ester, and other total impurities in the docusate sodium sample according to the external standard method. By means of the method, under isocratic liquid phase spectrum conditions, docusate sodium and the relevant substance like DK10 can be effectively separated, and the content of docusate sodium and the limit of the relevant substance like DK10 can be accurately and quantitatively detected, detection cost is low, and detection efficiency is high.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com