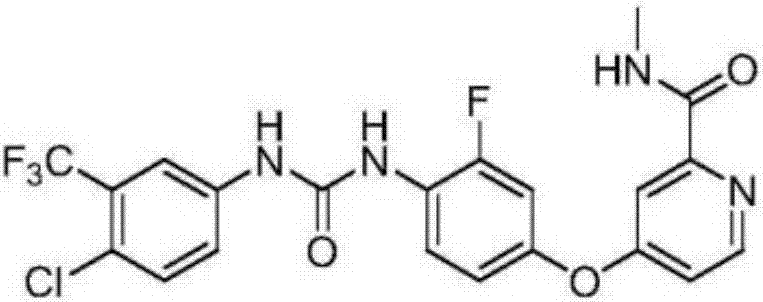
Regorafenib solid dispersion and preparation thereof
A technology of regorafenib solid and solid dispersion, applied in the field of regorafenib solid dispersion and its preparation, and pharmaceutical preparations containing the solid dispersion, can solve the problems of incomplete dissolution and slow dissolution speed, and achieve Improved solubility, low cost, and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-7 and comparative example 1-4
[0033] Preparation of Example 1-7 and Comparative Example 1-4 Regorafenib Solid Dispersion
Embodiment 1-7
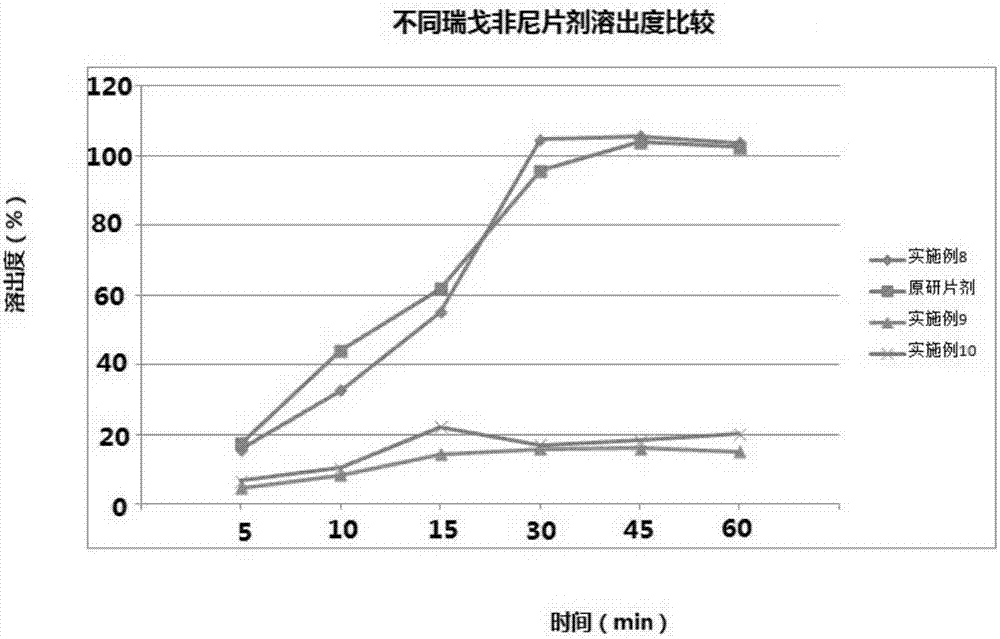
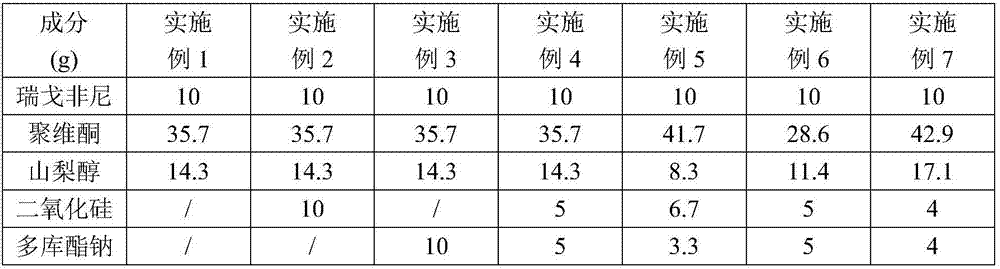
[0034] Example 1-7 The composition of regorafenib solid dispersion is shown in the table below:
[0035]
Embodiment 1
[0038] Example 1 Preparation of regorafenib solid dispersion:
[0039](1) Dissolving regorafenib in absolute ethanol to make a solution with a concentration of 20% (m / v) for later use;
[0040] (2) get sorbitol to be dissolved in water, make the solution that concentration is 35% (m / v), for subsequent use;
[0041] (3) Under stirring conditions, add the sorbitol aqueous solution to the regorafenib ethanol solution, stir for 10 min, then add the pre-dissolved povidone ethanol solution with a concentration of 15% (m / v), and continue stirring for 3 min , Concentrate the solution to dryness under reduced pressure with a rotary evaporator, transfer the product to a vacuum drying oven, and continue drying at room temperature for 24 hours to obtain the product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com