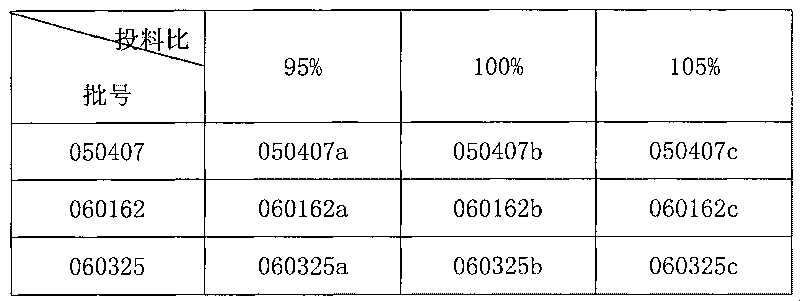
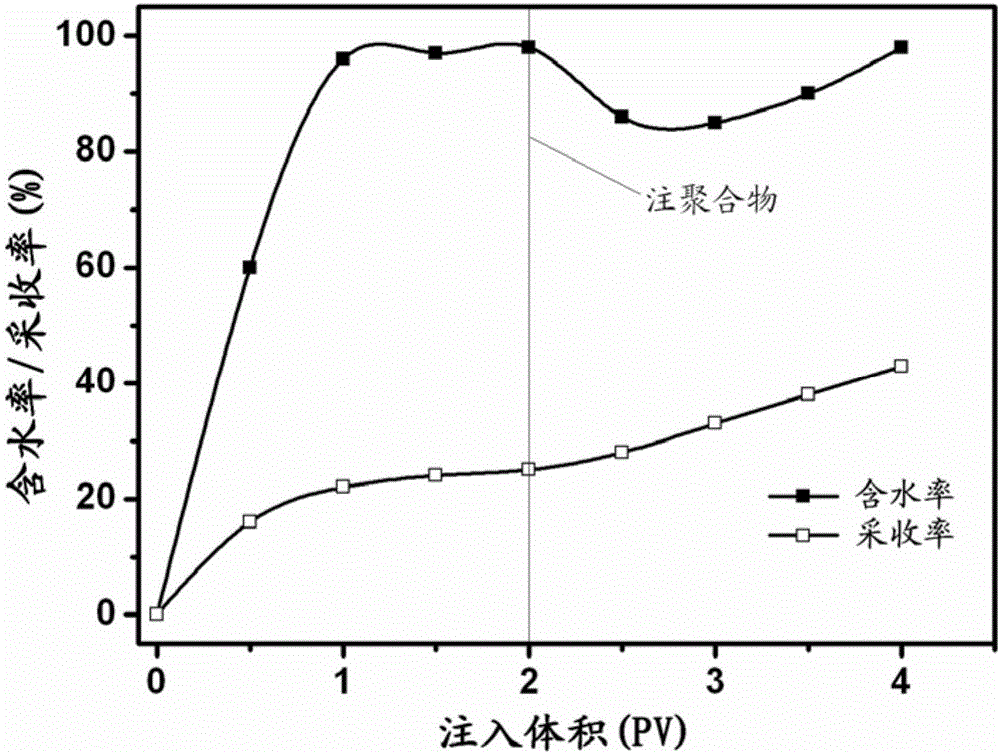
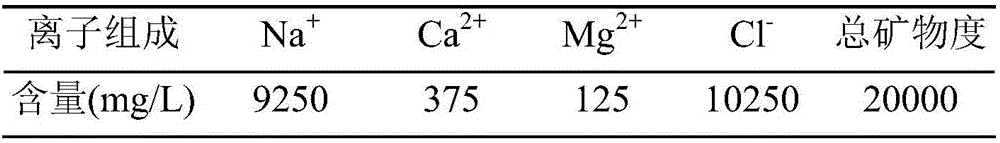
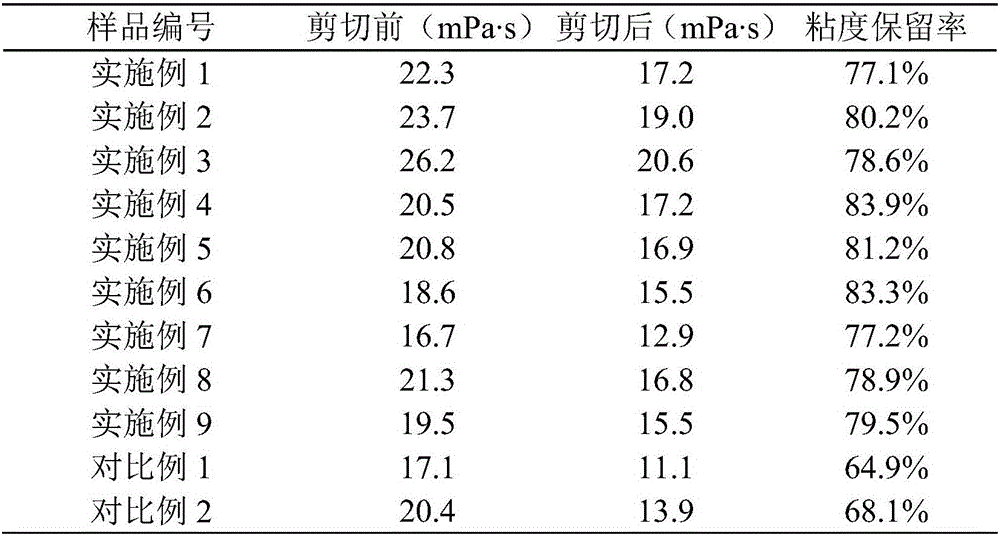
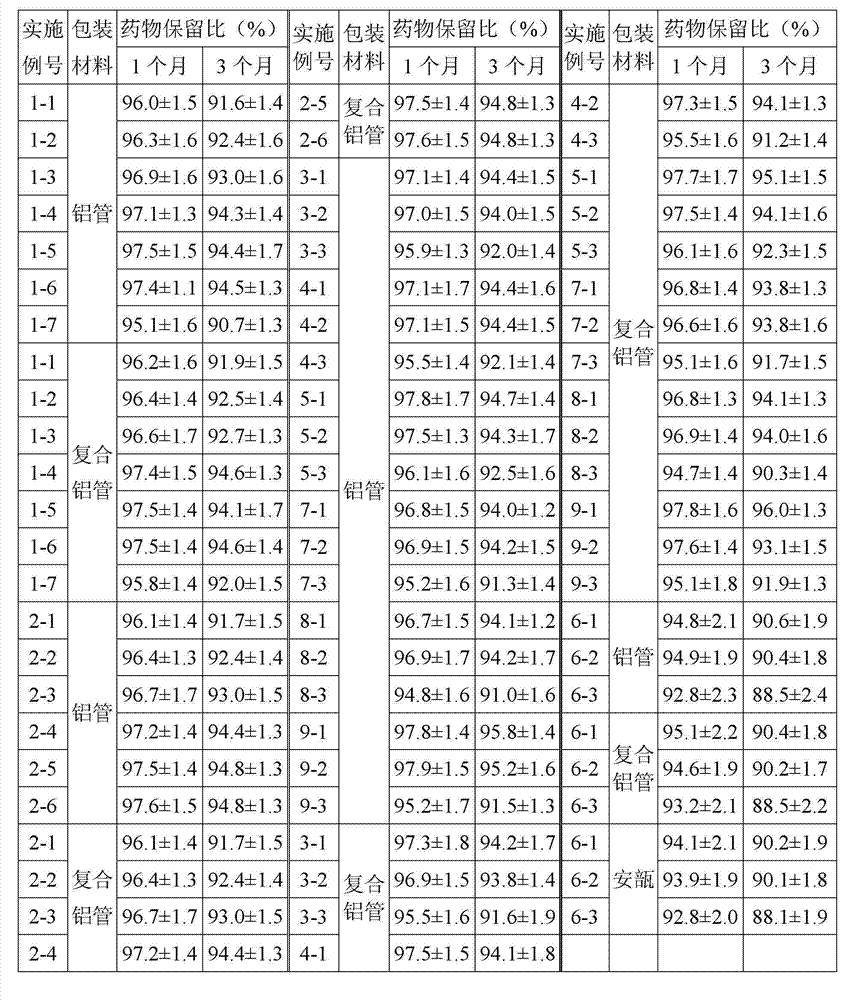
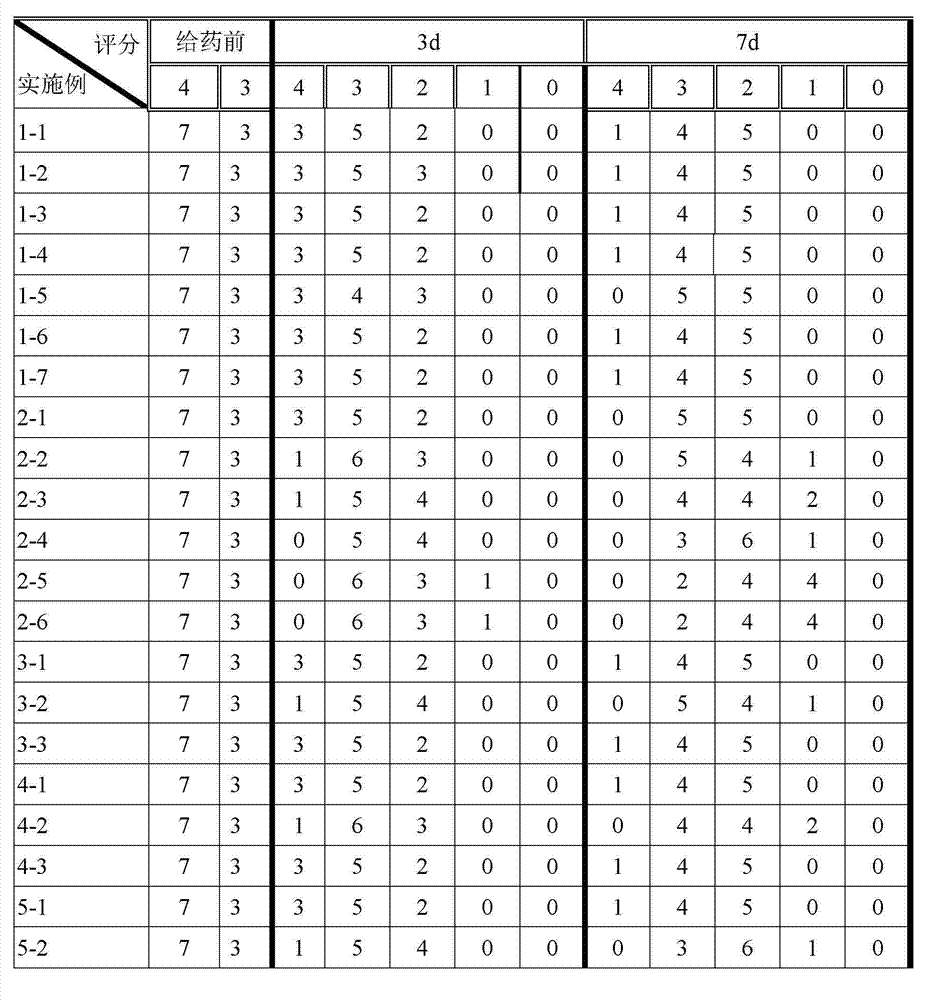
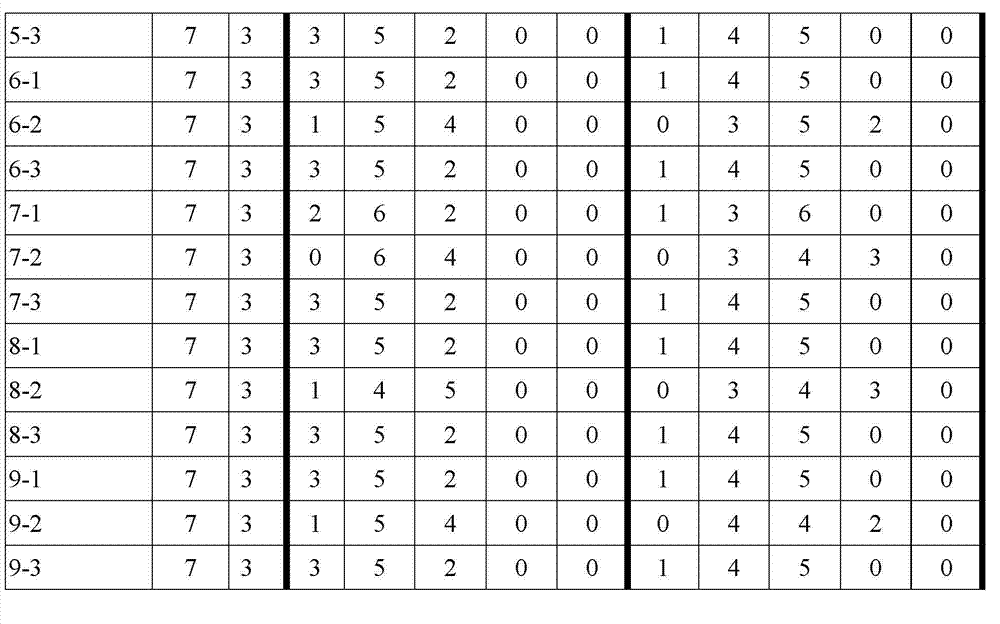
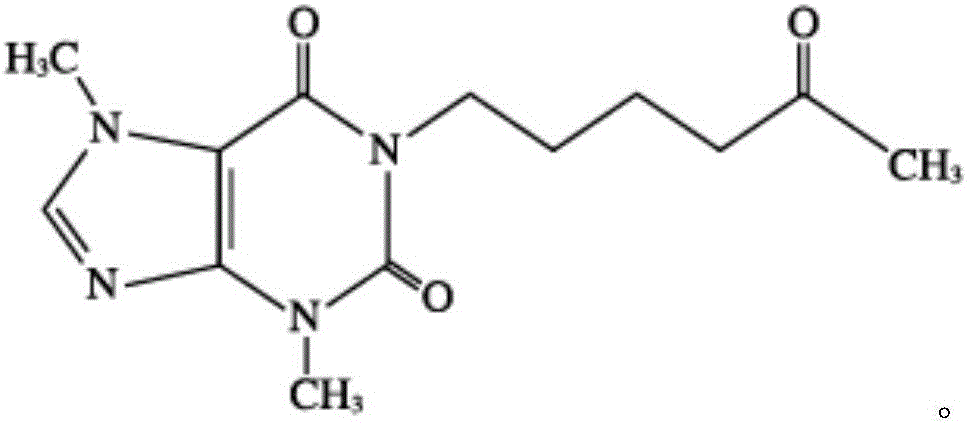
Patents
Literature
492 results about "Disodium Edetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Acne-removing scar-lightening mask solution, manufacturing method and acne-removing scar-lightening mask
InactiveCN104814921APromote transdermal absorptionAnti-agingCosmetic preparationsToilet preparationsDisodium EdetateCuticle
The invention discloses an acne-removing scar-lightening mask solution which is prepared from the following components in percentage by weight: 0.01-1% of high-molecular humectant, 0.01-0.2% of disodium edetate, 0.01-0.5% of thickener, 5-25% of witch hazel extract, 0.01-2.0% of conditioner, 0.01-5% of desensitizer, 1-10% of low-molecular humectant, 1-8% of plant acne-removing agent, 0.1-0.5% of preservative, 0.01-0.5% of mung bean extract, 0.01-5% of epidermal growth factor and the balance of deionized water. The manufacturing method of the acne-removing scar-lightening mask solution is simple. Under the synergistic actions of the plant acne-removing agent and mung bean extract in combination with the epidermal growth factor and witch hazel extract, the mask solution has the quick effects of acne removal, itching relief and sterilization, and can restore the damaged skin and lighten the acne marks and scars while removing the acnes, thereby improving the skin from inside to outside and restoring the smooth, delicate and healthy skin.
Owner:朱敏
Freeze-dried powder injection of pantoprazole sodium and its preparation
ActiveCN1679563ALittle side effectsImprove stabilityOrganic active ingredientsPowder deliveryDisodium EdetateFreeze-drying
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST +1
Pantoprazole sodium freeze-drying medicinal composition for injection and preparation method thereof
ActiveCN101810588AImprove stabilityEliminate side effectsPowder deliveryOrganic active ingredientsFreeze-dryingSulfite salt
The invention relates to a pantoprazole sodium freeze-drying medicinal composition for injection and a preparation method thereof. The pantoprazole sodium freeze-drying medicinal composition for injection comprises the following components in part by weight: 1 part of pantoprazole sodium, 0.01 to 0.1 part of mannitol, 0.02 to 0.03 part of natrium adetate, 0.07 to 0.10 part of sodium sulfite and 0 to 0.1 part of sodium citrate. In the method, the stability of the solution of the pantoprazole sodium is improved, related matters of the solution of the pantoprazole sodium in the process of preparation, packaging or freeze-drying during preparation are not increased obviously, the content of the related matters is not reduced obviously; the prepared pantoprazole sodium freeze-drying powder injection is good in stability in the process of transportation and storage; solution mixed with the injection during clinical use can be placed for a long time, so that the clinical use is more convenient; and simultaneously, hidden troubles of the medication safety of patients due to the increase of impurities (related matters) and the problem of the curative effect on the patients due to content reduction are reduced greatly.
Owner:福建康成医药有限公司
Environment-friendly air conditioner detergent and preparation method thereof
InactiveCN104450263AHigh decontamination efficiencyQuick clearNon-ionic surface-active compoundsDetergent perfumesSodium metasilicateDisodium Edetate
The invention belongs to the field of chemical detergents, and particularly relates to an environment-friendly air conditioner detergent and a preparation method thereof. Raw materials of the environment-friendly air conditioner detergent include sodium metasilicate, sodium lauryl sulfate, disodium edetate, sulphamic acid, sodium chloride, formic acid, ethanol, dibromo-3-nitrilo-propionyl, ethylene glycol polyoxyethylene ether, carboxymethyl cellulose, 2-bromo-5-chlorothiophene and purified water. According to the air conditioner detergent, a weak-acidity solution formed by sulphamic acid and formic acid is matched with substances such as carboxymethyl cellulose and sodium lauryl sulfate, so the air conditioner detergent is improved in decontamination efficiency and free of pungent smells; the chemical substances such as disodium edetate can form a chelate layer on the surface of a metal member so as to play an anti-corrosive protective effect; besides, the compounds such as dibromo-3-nitrilo-propionyl have a sterilization function, so the air conditioner detergent also has a sterilization effect and is applicable to washing and sterilization of automobiles or domestic air conditioners, simple to operate and capable of being used for cleaning the air conditioners by one step.
Owner:周莉莉
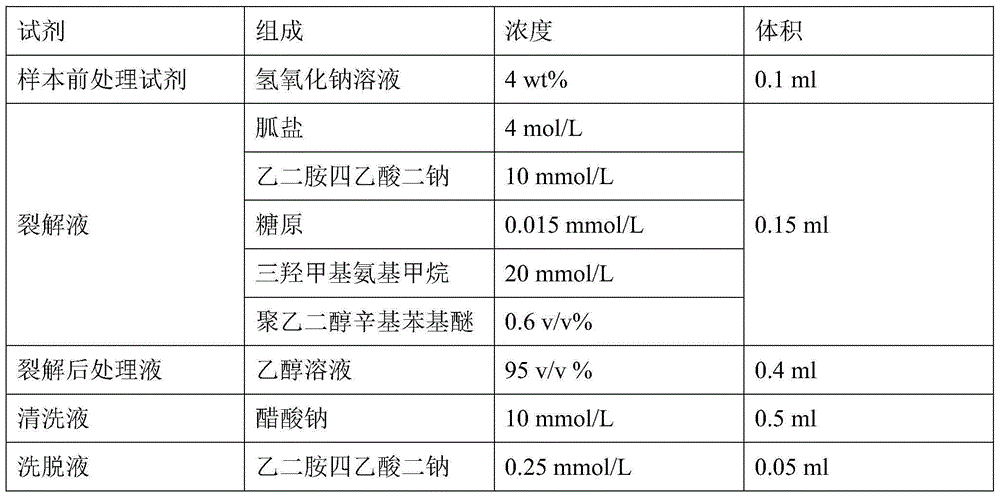
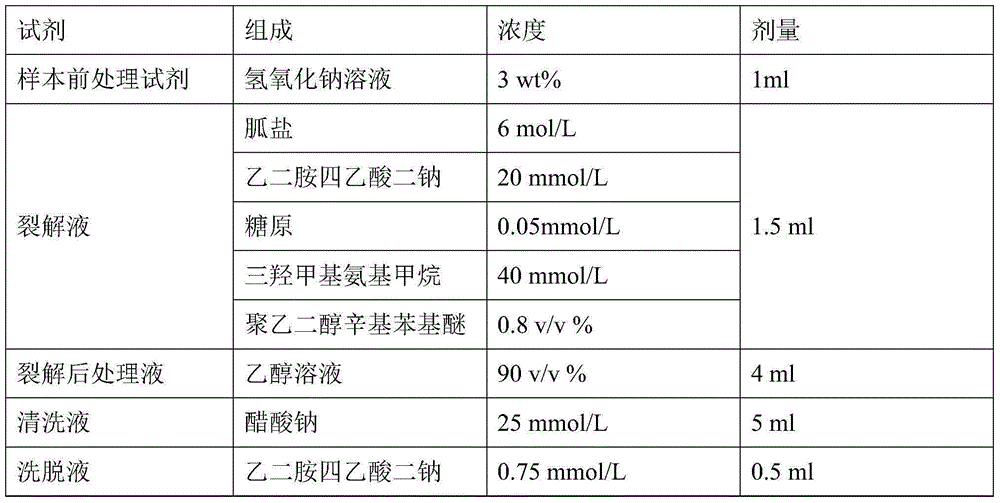
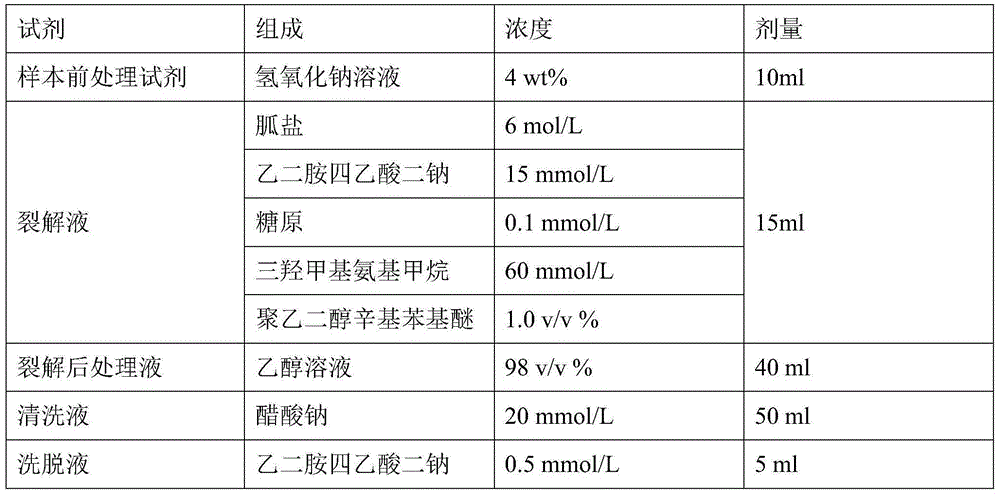
Nucleic acid extraction method and nucleic acid extraction reagent for biological samples
The present invention relates to the field of nucleic acid extraction, particularly to a nucleic acid extraction method and a nucleic acid extraction reagent for biological samples. The specific method comprises: adding a sodium hydroxide solution to a biological sample, adding a lysate, and carrying out lysis to obtain a lysis reaction liquid, wherein the lysate contains a guanidine salt, disodium edetate, a surfactant and a buffer agent; and adding an ethanol solution to the lysis reaction liquid, and achieving nucleic acid adsorption, cleaning and elution by using an instrument-free nucleic acid extraction apparatus having a silica gel membrane or a centrifugal column. According to the present invention, the sodium hydroxide is added to pre-treat and then the lysate is added to carry out lysis, such that the protein in the sample can be well dissolved, and the binding of the nucleic acid and the adsorption film is prompted; and the lysate component is optimized, such that the binding capacity of the nucleic acid molecule and the silica gel membrane under the high salt and high pH value conditions is significantly enhanced, and the good nucleic acid extraction efficiency is ensured.
Owner:USTAR BIOTECHNOLOGIES (HANGZHOU) CO LTD
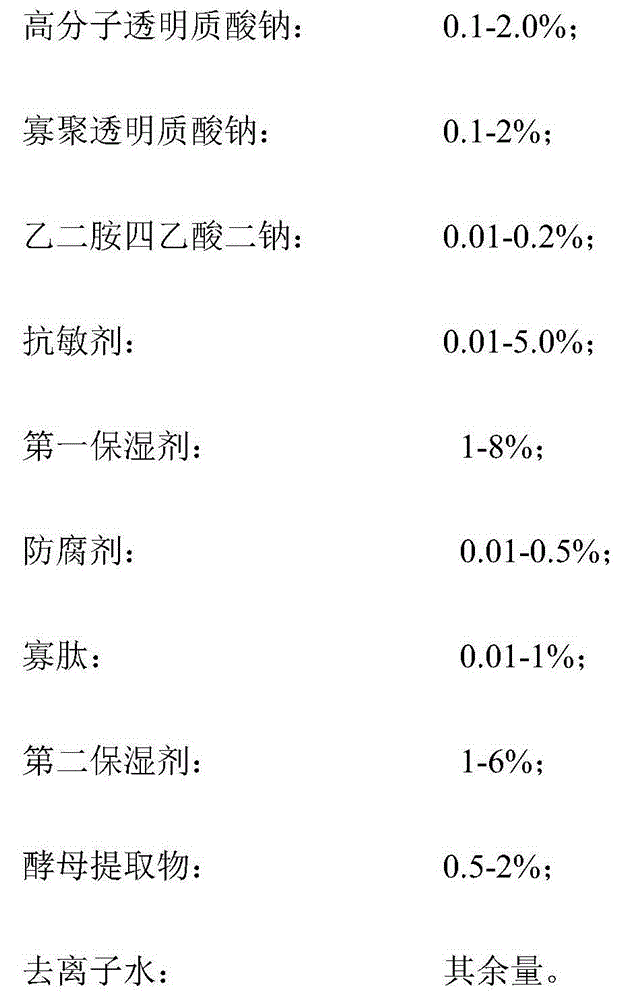
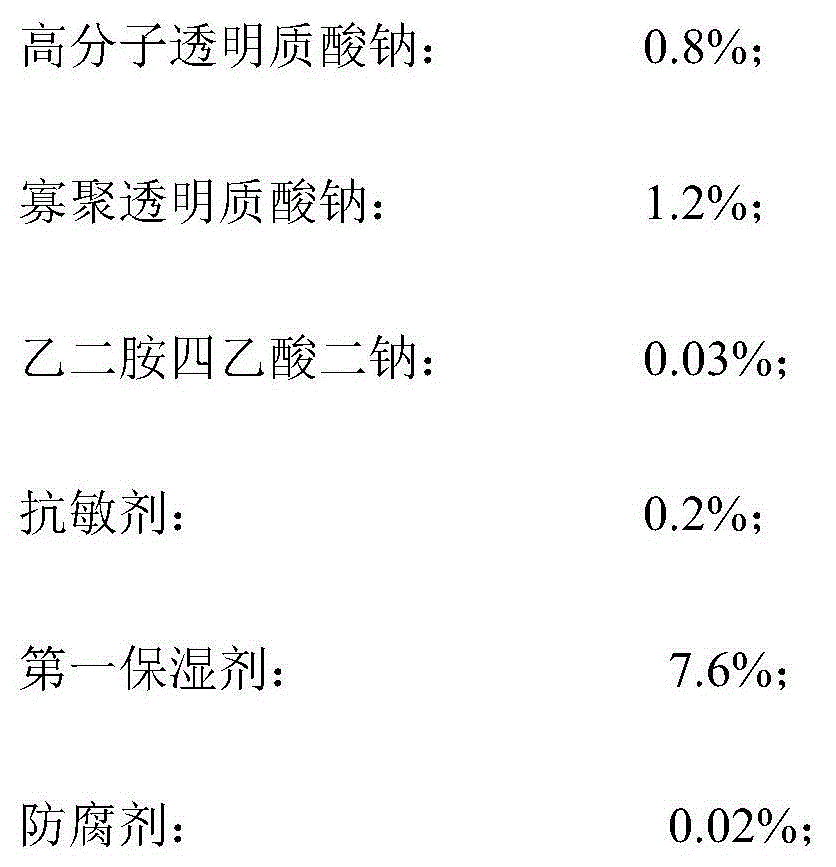
Moisturizing anti-wrinkle mask solution, manufacturing method and moisturizing anti-wrinkle mask
InactiveCN104814919AEasy to prepareSpeed up the newborn processCosmetic preparationsToilet preparationsDisodium EdetateCuticle
The invention discloses a moisturizing anti-wrinkle mask solution and a manufacturing method thereof. The anti-wrinkle mask solution is prepared from the following components in percentage by weight: 0.1-2.0% of high-polymer sodium hyaluronate, 0.1-2% of oligomeric sodium hyaluronate, 0.01-0.2% of disodium edetate, 0.01-5.0% of desensitizer, 1-8% of first moisturizer, 0.01-0.5% of preservative, 0.01-1% of oligopeptide, 1-6% of second moisturizer, 0.5-2% of yeast extract and the balance of deionized water. The preparation method of the mask solution is simple. By using the synergistic actions of the epidermal growth factor oligopeptide and the double moisturizing factors high polymer sodium hyaluronate and oligomeric sodium hyaluronate in combination with the ferment carrier-yeast extract, the mask solution has the functions of restoring the damaged part of the skin, accelerating the skin regeneration progress, promoting the metabolism of the fibrocytes and epidermis cells, improving the skin circulation, enhancing the compactness and fullness of the skin and relieving the skin texture symptom, thereby finally achieving the efficient moisturizing and anti-wrinkle effects.
Owner:广州致美化妆品有限公司
Compound analgesic agent
InactiveCN1679520ARelieve symptoms of acute and chronic painAntiviralNervous disorderAntipyreticCalcium hydroxideCarboxymethyl cellulose
A compound analgesic for bone fracture, injury, ecchymoma, nerval pain, etc is composed of antisticking layer, hydrophilic gel layer and substrate layer. Said hydrophilic gel layer consists of the medicine prepared from lidocaine hydrochloride and superfine notoginseng powder or its extract and the matrix prepared from gelatin, carboxymethyl cellulose sodium, polyvinyl pyrrolidone, etc.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Nourishing youth-keeping facial cleanser and preparation method thereof
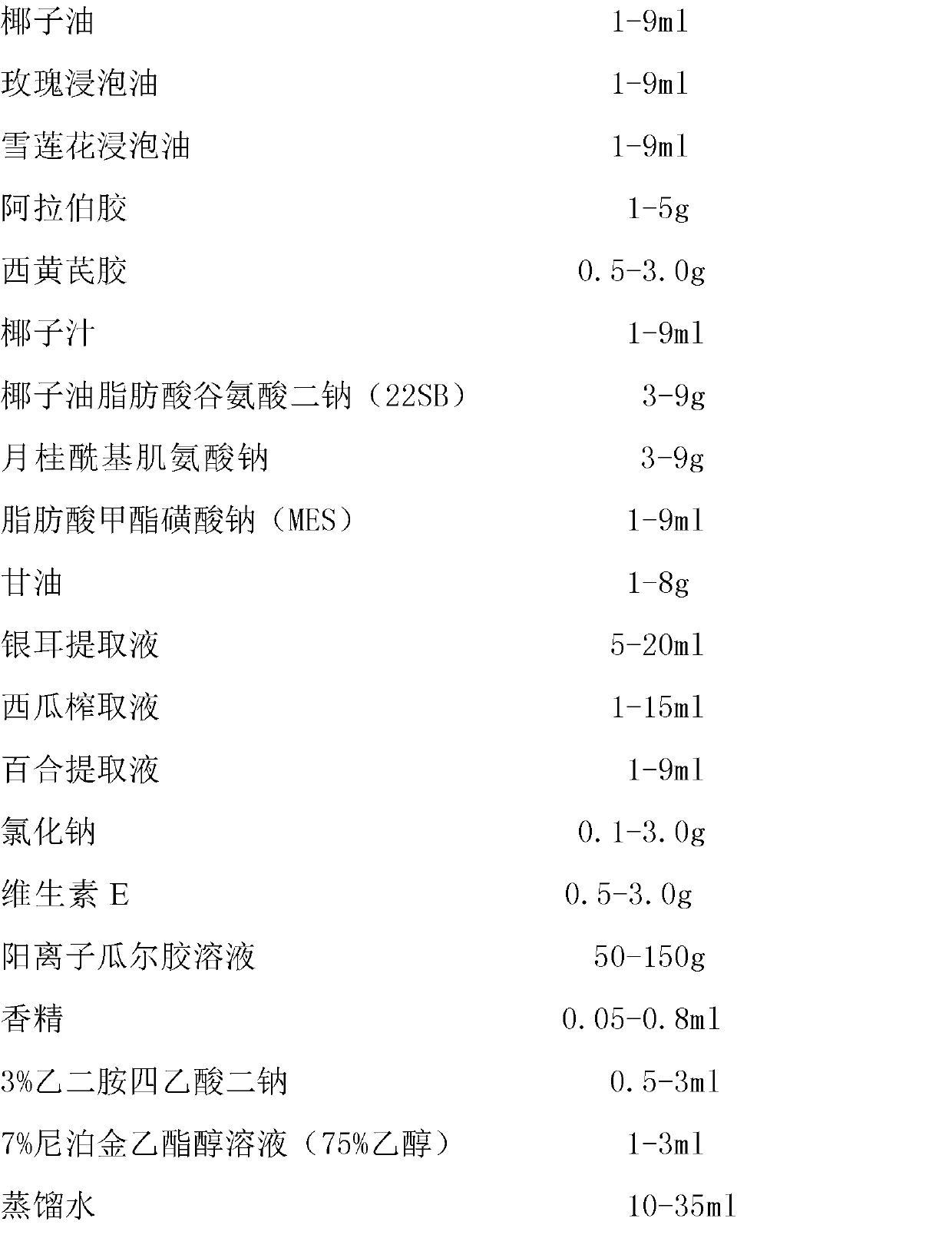
InactiveCN102973486AIncrease moistureReduce sensitivityCosmetic preparationsToilet preparationsDisodium EdetateGuar gum
The invention discloses a nourishing youth-keeping facial cleanser and a preparation method thereof. The facial cleanser comprises the substances of: coconut oil, rose infused oil, snow Lotus herb infused oil, gum Arabic, west tragacanth gum, coconut juice, coconut oil fatty acid disodium glutamate, sodium sarcosinate, fatty acid methyl ester sulfonate, glycerine, distilled water, white fungus extract liquid, watermelon extract liquid, lily extract liquid, sodium chloride, vitamin E, a cationic guar gum solution, an essence, ethyl paraben, and disodium edetate. The nourishing youth-keeping facial cleanser provided by the invention has a milky-white color, and is transparent and crystal. The facial cleaner has a proper viscosity, a proper consistency, good stability, rich foam, and good fragrance. The facial cleanser can provide nutrients and deep nourishing effects for facial skins. With the facial cleanser, the skins can turn moistened and elastic.
Owner:珠海市日新化妆品有限公司
Cataplasm matrix and preparation method thereof
InactiveCN101690818ALong storage timeHigh peel strengthPharmaceutical non-active ingredientsSheet deliveryPhase mixingAcetic acid
The invention discloses a cataplasm matrix and a preparation method thereof. The matrix comprises part of neutralized sodium polyacrylate, dihydroxyaluminium, glycerol, disodium edetate dehydrate, polyvinylpyrrolidone, crospolyvinylpyrrolidone, dihydroxybutanedioic acid, DMDMH, polysorbate 80 and purified water. The preparation method comprises three steps, namely oil phase preparation, aqueous phase preparation and oil-aqueous phase mixing. The matrix has excellent peel strength and sticky consistency, features long water preservation, is difficult to fall off when being stuck on the skin and is free of residue after being uncovered.
Owner:SUZHOU HENGXING MEDICAL MATERIAL
Yellow-removing whitening mask solution, manufacturing method and yellow-removing whitening mask
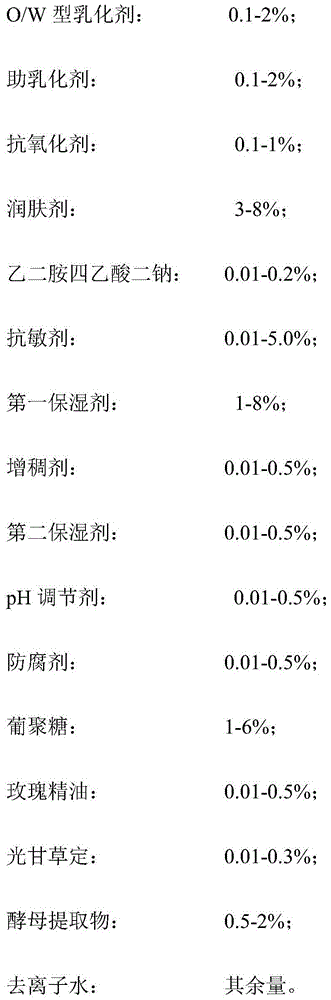
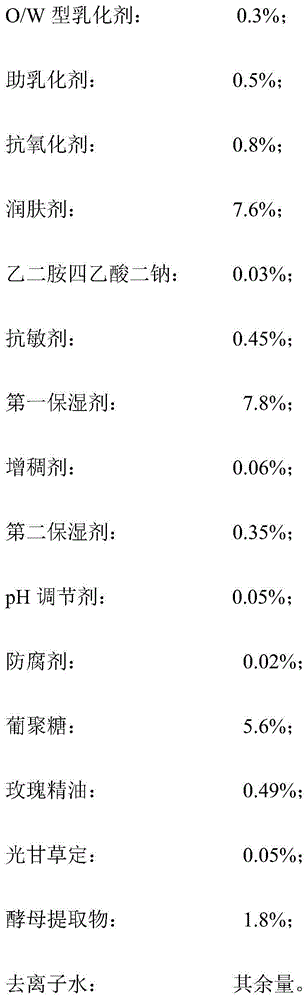
InactiveCN104814920AEasy to preparePromote absorptionCosmetic preparationsToilet preparationsDisodium EdetateAntioxidant
The invention discloses a yellow-removing whitening mask solution and a manufacturing method thereof. The yellow-removing whitening mask solution is prepared from the following components in percentage by weight: 0.1-2% of O / W (oil-in-water) emulsifier, 0.1-2% of co-emulsifier, 0.1-1% of antioxidant, 3-8% of emollient, 0.01-0.2% of disodium edetate, 0.01-5.0% of desensitizer, 1-8% of first humectant, 0.01-0.5% of thickener, 0.01-0.5% of second humectant, 0.01-0.5% of pH regulator, 0.01-0.5% of preservative, 1-6% of glucan, 0.01-0.5% of rose essential oil, 0.01-0.3% of glabridin, 0.5-2% of yeast extract and the balance of deionized water. The manufacturing method is simple. By using the oil-in-water emulsion system, the effective substances in the mask solution can be absorbed more easily, and can penetrate into the skin and keep high activity; and thus, the mask solution has the functions of whitening and high-efficiency oxidation resistance. The mask solution can effectively inhibit activities of multiple enzymes in the melanin generation process, thereby achieving the yellow-removing and whitening effects.
Owner:朱敏
Tree nutrient solution
InactiveCN106008030AImprove survival rateImprove developmentMagnesium fertilisersAlkali orthophosphate fertiliserDisodium EdetatePotassium hydroxide
The invention discloses a tree nutrient solution and relates to the technical field of tree cultivation. The tree nutrient solution is prepared from, by weight, 1-2 parts of potassium nitrate, 0.1-0.5 part of ammonium phosphate, 0.1-0.5 part of disodium edetate dehydrate, 0.1-0.2 part of ferrous sulfate, 0.1-0.2 part of vitamin B, 0.1-0.5 part of sodium nitrophenolate, 1-2 parts of fulvic acid, 1-2 parts of indolebutyric acid, 1-5 parts of gibberellin, 2-5 parts of chitosan, 4-8 parts of potassium hydroxide, 2-5 parts of ferric chloride, 2-6 parts of fulvic acid, 4-7 parts of glucose, 3-5 parts of calcium nitrate, 6-9 parts of citric acid, 7-9 parts of magnesium sulfate and 40-60 parts of water. The tree nutrient solution has the advantages that the survival rate of large transplanted trees can be greatly increased, the tree nutrient solution has a good rejuvenation effect on trees which are not transplanted and are weak in vigor, and the tree nutrient solution is nutrient, safe, capable of being quickly absorbed by trunks, supplementing nutrition and promoting development of root systems and good in use effect.
Owner:安徽富牧通生物科技有限公司
Preparation of injecting soluble vitamines
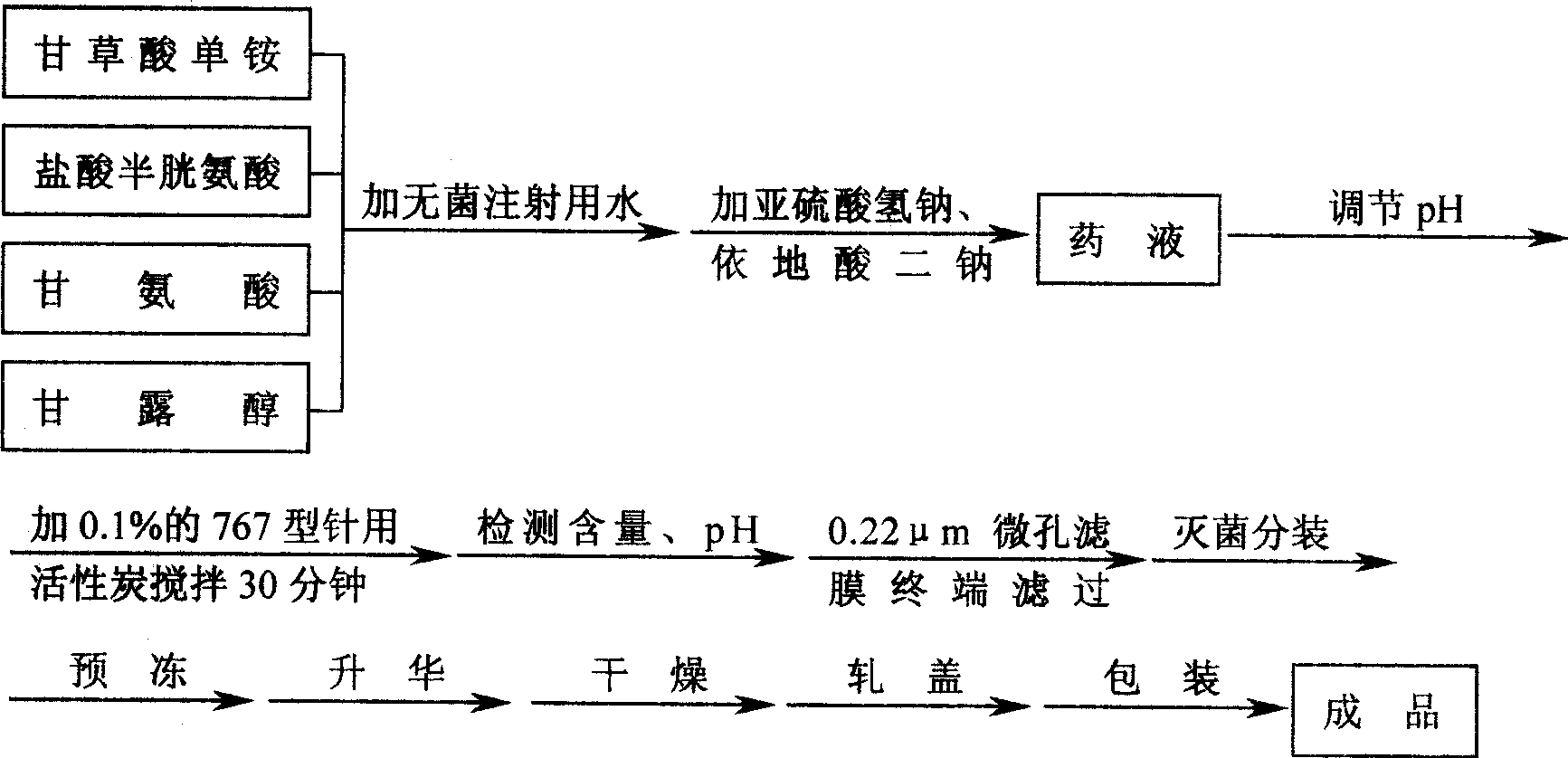
InactiveCN1939333ARaise storage temperatureImprove antioxidant capacityOrganic active ingredientsMetabolism disorderGlycineVitamin C
A process for preparing the injection of water-soluble vitamin includes such steps as thermally dissolving glycine in the water for injection while filling inertial gas, adding 12 compounds including cysteine hydrochloride, endrate disodium, vitamin C sodium, folic acid, etc, stirring, regulating pH=5.6-6.1, adding the water for injection, stirring, aseptic filtering, pouring in containers, pre-freezing, vacuumizing, filling inertial gas, pre-freezing, heating to 30-35 deg.C, holding the temp for 6-10 hr, filling inertial gas, and sealing.
Owner:YAOPHARMA CO LTD
Lipid emulsion with low anisidine value and preparation method thereof
InactiveCN102805727AAchieve antioxidantReduce the value of methoxyanilineEmulsion deliveryOil/fats/waxes non-active ingredientsEdetic AcidDisodium Edetate
The invention relates to a lipid emulsion with a low anisidine value, which contains oily constituents, water, an emulsifying agent and an osmotic pressure regulator, wherein the lipid emulsion contains a metal ion chelant but not an antioxidant. The content of the metal ion chelant is below 100mg / ml. The metal ion chelant is one or more of sodium calcium edentate, edetate disodium, edetate sodium and edetic acid. The invention relates to a method for preparing the lipid emulsion with the low anisidine value as well. According to the invention, in the process of preparing the lipid emulsion, other antioxidants are not needed to add, such as Vitamin E (VE), the antioxidation can be implemented only by using the metal ion chelant, and the low anisidine value can be greatly reduced. The low anisidine value of the lipid emulsion cannot be reduced by adding other antioxidants.
Owner:JIANGSU JIUXU PHARMA +1
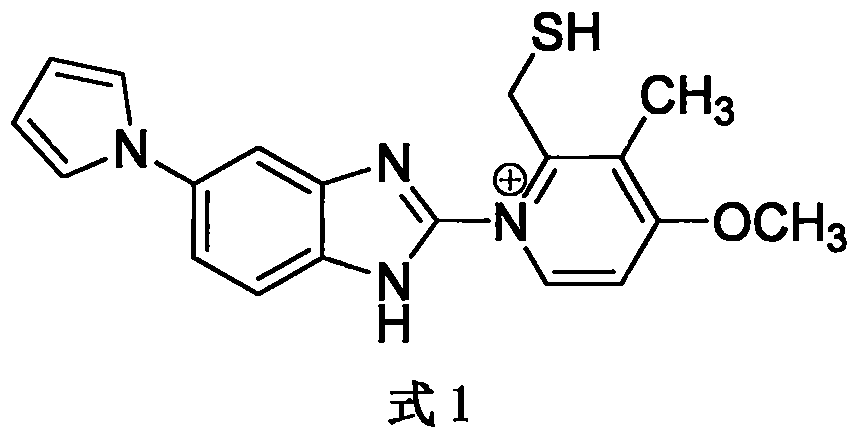
Sodium ilaprazole powder injection and preparation method thereof
The invention provides a sodium ilaprazole powder injection and a preparation method thereof. The powder injection is prepared from, by weight volume, 0.8-1.2% of sodium ilaprazole, 2.4-4.6% of mannitol and 0.05-0.15% of disodium edotato.
Owner:LIVZON GROUP LIVZON PHARMA FACTORY
Famotidine injection and preparation method thereof
InactiveCN101716136ADecrease in the marked amount of feedingHigh yieldOrganic active ingredientsDigestive systemDisodium EdetateUltraviolet lights
The invention discloses a preparation method of a famotidine injection. 1000ml of injection contains the following raw materials: 20.0g of famotidine, 3.5g of aspartic acid, 0.2g of natrium adetate, 0.5g of medicinal carbon and the balance of injection water. The preparation method comprises the following steps: (1) adding fresh injection water which accounts for 80% of the preparation liquid to aspartic acid and natrium adetate and stirring to be thoroughly dissolved; (2) adding a formula amount of medicinal carbon and allowing to stand to absorb for 20 minutes; (3) filtering, feeding 102% of famotidine and stirring to be dissolved; (4) adjusting a pH value to 5.2-5.4 with 10% aspartic acid solution, adding injection water to a full amount and uniformly stirring; (5) sequentially filtering by a filter element of 0.45 microns and a filter element of 0.22 microns until clarity is qualified, obtaining a half finished product of famotidine injection and adopting an ultraviolet light-visible light photometry to control the quality of the half finished product; (6) finally, filtering the half finished product by a filter membrane of 0.22 microns after the half finished product is detected to be qualified, filling 2ml of nitrogen and encapsulating; and (7) sterilizing in flowing steam at 100 DEG C for 30 minutes, detecting leakage and lamps, airing, printing characters and packaging to obtain the famotidine injection.
Owner:HENAN FUREN HUAIQINGTANG PHARMA
Temperature-resistant and salt-resistant oil displacing polymer containing nano particles
ActiveCN106832113ARaw materials are easy to getLow priceDrilling compositionPolymer scienceDisodium Edetate
The invention discloses a temperature-resistant and salt-resistant oil displacing polymer containing nano particles. A preparation method of the polymer comprises the following steps that 1, the surface of nanosilicon dioxide is inoculated with amidogen, and modified nanosilicon dioxide is obtained; 2, modified nanosilicon dioxide, water-soluble monomers and water are mixed, the pH of the mixed system is adjusted to range from 4 to 8, nanosilicon dioxide is completely dispersed in the water, disodium edetate dehydrate, ferrous sulfate and tetramethylethylenediamine are added into the mixed system obtained after the pH is adjusted, then azobis(isobutylamidine hydrochloride), ammonium persulfate and hydrogen peroxide are added, the reaction I is carried out in the nitrogen atmosphere, the reaction II is carried out after temperature rise, the pH of the reaction product of the reaction II is adjusted to range from 8 to 9, constant-temperature ageing is carried out, and the polymer can be obtained after drying and smashing are carried out. The dispersing performance and high temperature stability of modified nanosilicon dioxide are utilized, through interaction between nanosilicon dioxide and the polymer, the rigidity of polymer molecules is improved, a network structure among the polymer molecules is formed, and excellent temperature resisting, salt resisting and ageing resisting performance and the like are generated.
Owner:CHINA NATIONAL OFFSHORE OIL (CHINA) CO LTD +1
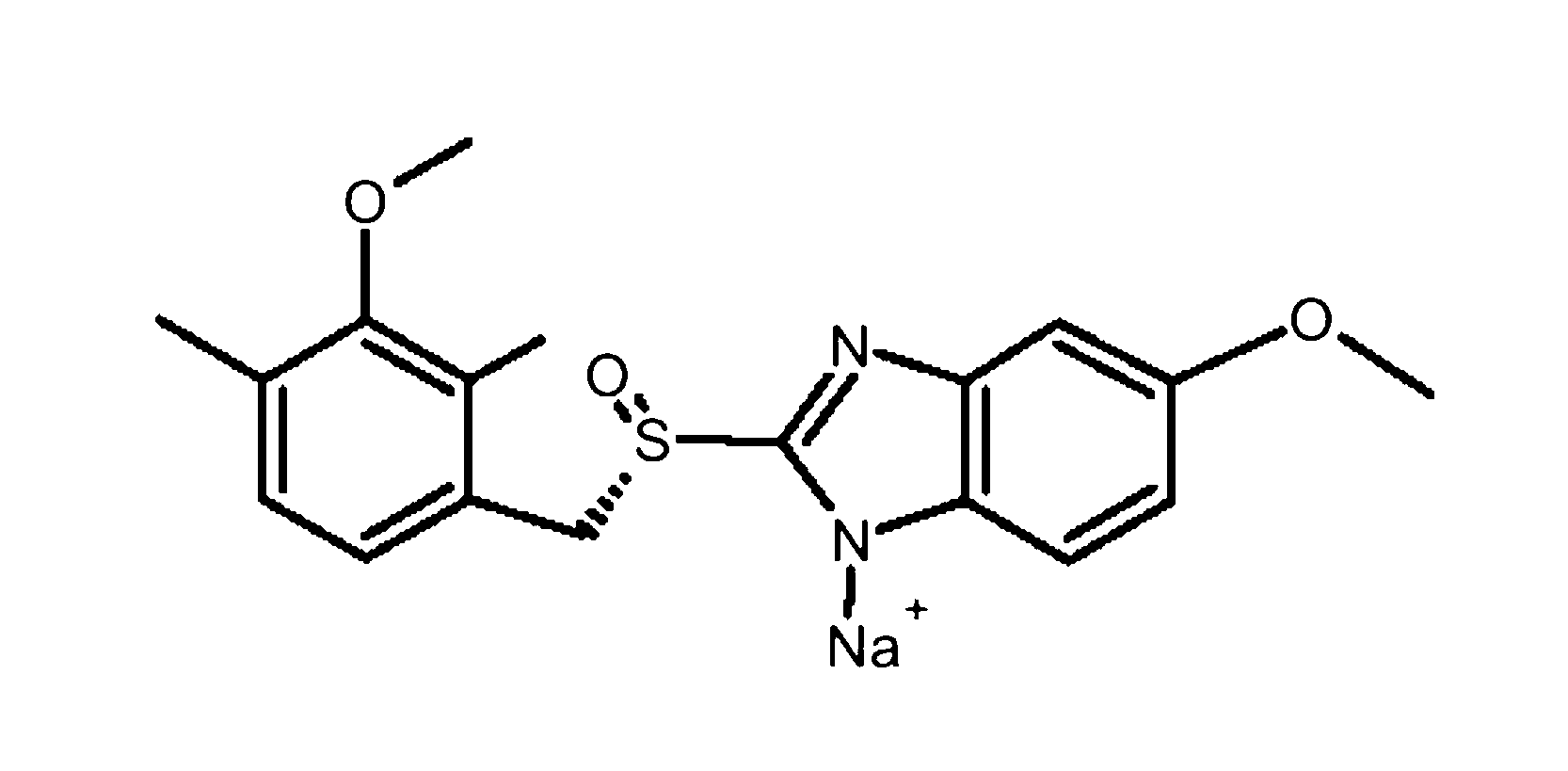
Preparation of injection esomeprazole sodium
ActiveCN103006585AIncrease profitReduce manufacturing costPowder deliveryOrganic active ingredientsEsomeprazole SodiumActivated carbon
The invention discloses preparation of injection esomeprazole sodium. The preparation comprises the following steps of: (a) adding partial injection water into a basic liquid, adding edetate disodium, dissolving and cooling to a temperature of between 10 and 20 DEG C, introducing nitrogen and adding esomeprazole sodium, stirring until the mixture is completely dissolved, adding activated carbon, stirring, decarbonizing and filtering; (b) adding injection at a temperature of between 10 and 20 DEG C to account for 80 to 95 percent of the preparation amount, introducing nitrogen, regulating pH value to be between 11.0 and 11.8 with alkali, adding injection water at a temperature of between 10 and 20 DEG C to the total preparation amount, filtering by using a filter with filter element of 0.22mu m, subpackaging in penicillin bottles, partly covering rubber plugs, and lyophilizing. The finished product prepared by the preparation is more stable in quality, and the production cost is remarkably reduced, so that the preparation is advantageous to mass production, and is good in market prospect, and worthy of popularization.
Owner:哈药集团股份有限公司 +1
Sodium ilaprazole freeze-dried powder injection
ActiveCN105769777AStable in natureAdapt to clinical needsPowder deliveryOrganic active ingredientsDisodium EdetateMannitol
The invention aims at providing a sodium ilaprazole freeze-dried powder injection which has few adverse reactions and is stable in character and suitable for clinic requirements. The sodium ilaprazole freeze-dried powder injection is prepared from, by weight volume, 0.8-1.2% of sodium ilaprazole, mannitol and 0.05-0.15% of disodium edotato.
Owner:LIVZON GROUP LIVZON PHARMA FACTORY
Fosaprepitant dimeglumine injection composition and preparation method thereof
InactiveCN103156813ALow costNo side effectsPowder deliveryOrganic active ingredientsFormularyDisodium Edetate
The invention relates to the field of medicament preparation, and particularly relates to a fosaprepitant dimeglumine injection composition and a preparation method thereof. The pharmaceutical active ingredients of the fosaprepitant dimeglumine injection composition include the following components in parts by weight: 180-250 parts of fosaprepitant dimeglumine, 12.0-20.0 parts of disodium edetate, 50.0-80.0 parts of polysorbate-80, 250-400 parts of lactose and 2000-3000 parts of water for injection. The preparation method comprises the following steps: dissolving, preparing, adsorbing, pre-freezing, performing sublimation drying and mounting an injection piston. The fosaprepitant dimeglumine injection composition provided by the invention is prepared from the low low-cost auxiliary materials according to a self-developed formula, has the same medicament effect in comparison with the existing fosaprepitant medicament available in America, is low in medicament cost and has no side effect basically; and the lactose used as excipient resists infection and is easy to absorb, so that favorable pharmaceutical effect can be achieved, and crushing of the medicament during storage can be avoided.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Ofloxacin injection and preparation process thereof
ActiveCN101693008AImprove solubilityPromote crystallizationAntibacterial agentsOrganic active ingredientsDrugs solutionAcetic acid
The invention discloses an ofloxacin injection which is prepared by ofloxacin, acetic acid, disodium tetracemate, propylene glycol and water for injection. The invention solves the problem that an ofloxacin injection hydro-acupuncture is easy to crystallize through adopting the acetic acid as cosolvent and improving the dissolvability of ofloxacin by adding the propylene glycol to regulate the polarity of solution. The injection provided by the invention has excellent quality stability, solves the problem of crystallization commonly existed in products of ofloxacin injection hydro-acupuncture, solves the weaknesses of instability and short retention period of injection, reduces the number of insoluble particles in medicine solution, and provides the effective guarantee for the safe use in clinics.
Owner:ANHUI FENGYUAN PHARM CO LTD
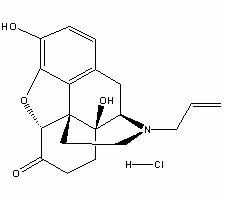
Naloxone hydrochloride freeze-dried powder injection and preparation method thereof
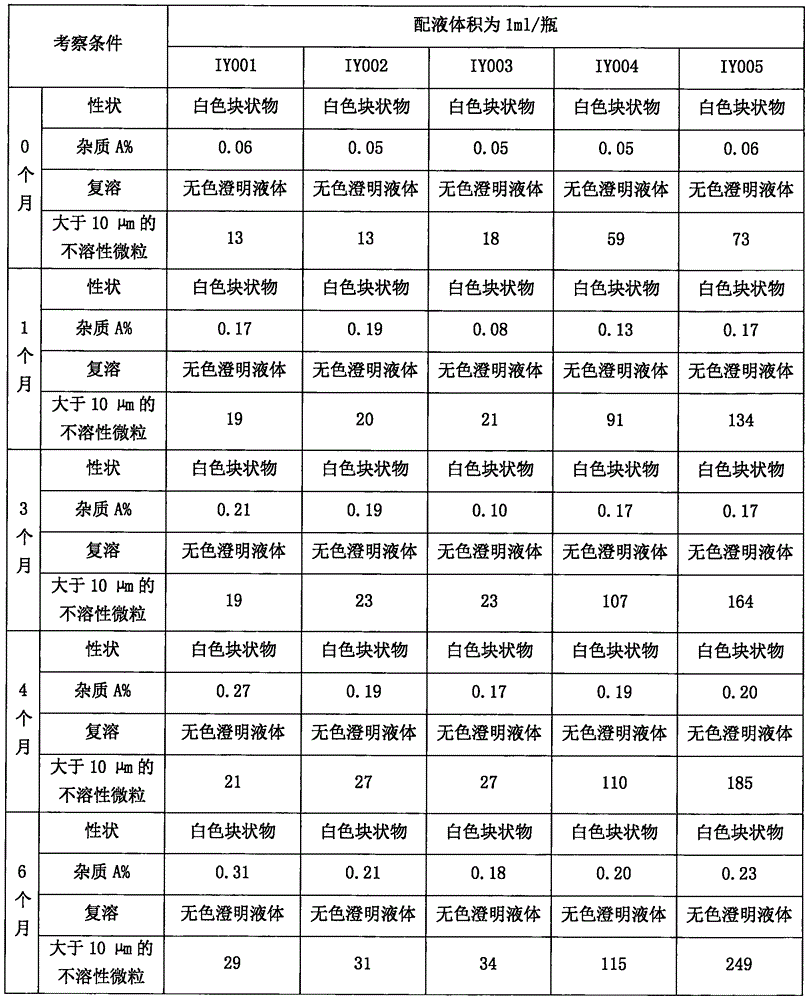
ActiveCN102274196AQuality improvementImprove stabilityOrganic active ingredientsPowder deliveryFreeze-dryingDisodium Edetate
The invention relates to a naloxone hydrochloride freeze-dried powder injection and a preparation method thereof. The naloxone hydrochloride freeze-dried powder injection in every 1000 bottles is obtained by adding water for injection to dissolve the following ingredients and then freeze-drying: 0.1-5g of naloxone hydrochloride, 30-60g of mannitol and 0.01-0.5g of edetate disodium; before the freeze-drying, the pH value of the solution is 2.0 to 5.0. According to the invention, through controlling different pH value regulation ranges during the preparation process of different specifications of the naloxone hydrochloride for injection, the finished products can meet the prescribed standards after the freeze-drying, so that the quality and the stability of the product are improved.
Owner:ZHEJIANG JINHUA CONBA BIO PHARM CO LTD
Special controlled-release fertilizer for oil peony and preparation method thereof
InactiveCN104529662ALong-acting fertilizerCalcareous fertilisersBio-organic fraction processingDisodium EdetatePotassium
The invention discloses a special controlled-release fertilizer for oil peony, which comprises a nutrient medium and an envelope, wherein the envelope comprises the following raw materials in parts by weight: 40-75 parts of lignite wax resin, 5-8 parts of humate, 5-10 parts of resin solvent, 21-25 parts of lignite essence and 70-95 parts of high polymeric chitosan; and the nutrient medium comprises the following raw materials in parts by weight: 600-820 parts of straw, 300-400 parts of animal manure, 10-12 parts of urea, 10-12 parts of calcium chloride, 3-4 parts of ferrous sulfate, 2-3 parts of manganese sulfate, 10-15 parts of soil conditioner, 5-10 parts of pesticide, 4-10 parts of borax, 5-10 parts of bentonite, 10-15 parts of shell powder, 10-15 parts of ammonium phosphate, 1-5 parts of slow-release agent, 20-30 parts of ground phosphorite, 20-30 parts of wheat middling, 1-6 parts of disodium edetate, 30 parts of potassium fulvate and 50 parts of mixed culture powder.
Owner:CHONGQING UNIV OF ARTS & SCI
Exterior medicine composition of fluocinolone acetonide and ester of fluocinolone acetonide
The invention provides an exterior medicine composition of fluocinolone acetonide and ester of fluocinolone acetonide. The exterior medicine composition comprises the fluocinolone acetonide or the ester of the fluocinolone acetonide serving as the active component, and one or more auxiliary materials applied to skin; and the exterior medicine composition is characterized in that the auxiliary materials for skin include sodium calcium edetate or edetate disodium.
Owner:天津金耀药业有限公司
Gel preparation of Ciclopirox Olamine
InactiveCN101049286AImprove adhesionUniform coatingAntimycoticsPharmaceutical delivery mechanismAlcoholDisodium Edetate
A gel medicine containing ciclopirox olamine for skin and vagina is proportionally prepared from ciclopirox olamine, carbomer, alcohol, propanediol, triethanolamine, bisodium versenate, polysorbide, and water. Its preparing process is also disclosed.
Owner:山东省医疗器械研究所
Adrenaline hydrochloride injection and preparation process thereof
InactiveCN102335125AOrganic active ingredientsPharmaceutical delivery mechanismSodium metabisulfiteAdrenaline hydrochloride
The invention discloses an adrenaline hydrochloride injection and a preparation process thereof. The preparation process comprises the following steps: (1) adding water for injection in a container and inserting CO2 for 15 min, wherein, the volume of the water is 90% of the total volume, and the temperature of the water is less than 30 DEG C; (2) dissolving sodium chloride in proper amount of water for injection having a temperature of below 30 DEG C; (3) dissolving disodium edetate with boiled water for injection; (4) dissolving sodium chloride solution, disodium edetate solution, sodium pyrosulfite and epinephrine respectively in water for injection; (5) measuring the pH value of the solution, regulating the pH value to 3.6-4.0 with 1 mol / L hydrochloric acid, adding water for injection to reach the total amount, and uniformly stirring; (6) filtering; and (7) carrying out aseptic filling and sealing, and inserting N2 in ampoules when filling and sealing; or carrying out filling and sealing according to operating procedures of filling and sealing, inserting N2 in the ampoules when filling and sealing , and carrying out disinfection for 30 min at the temperature of 100 DEG C. The process realizes aseptic production, and products produced by the preparation process satisfy the national medicine quality standards.
Owner:上海禾丰制药有限公司
Process for preparing pantoprazole sodium for injection
ActiveCN101961309AIncrease profitReduce manufacturing costOrganic active ingredientsDigestive systemMANNITOL/SORBITOLHigh volume manufacturing
The invention relates to a preparation process (preparation method) for pantoprazole sodium for injection, overcomes the defect of the traditional preparation process for the pantoprazole sodium for injection, and provides a preparation process with low cost, high yield and more stable finished product quality. The process comprises the following steps of: (1) feeding materials according to 100 percent of formula quantity, adding mannitol into injection water in an amount which is about 15 percent of preparation quantity, dissolving the materials with stirring, adding medicinal carbon in an amount which is 0.2 percent (weight / volume) of preparation quantity into the solution, boiling the solution for 30 minutes, removing carbon and filtering the solution, cooling the filtered solution to between 10 and 20 DEG C, and introducing nitrogen (the pressure of the nitrogen is 0.3 to 0.5MPa) into the solution for later use; (2) introducing the nitrogen (the pressure of the nitrogen is 0.3 to 0.5MPa) into the 10-20 DEG C injection water in an amount which is about 60 percent of preparation quantity, adding the medicinal carbon in an amount which is 0.1 percent (weight / volume) of preparation quantity into the solution, stirring the solution uniformly, standing the solution for 20 minutes, removing carbon and filtering the solution; and (3) mixing the filtered solution, introducing the nitrogen (the pressure of the nitrogen is 0.3 to 0.5MPa) into the solution for protecting, adjusting the pH value of the solution to between 10.0 and 10.8 by using 10 percent sodium hydroxide solution, adding 10-20 DEG C injection water to the preparation quantity, stirring the solution uniformly, retesting the pH value which should be between 10.0 and 10.8, filtering the solution by using filters with a 0.45mum filter element and a 0.22mum filter element respectively, and filling the product after the submitted semi-finished products are qualified.
Owner:HENAN FUREN HUAIQINGTANG PHARMA
Compound monoammonium glycyrrhizunate S prepn for injection and its prepn process and quality control technology
InactiveCN1706394AImprove stabilityAvoid intermediate linksOrganic active ingredientsDisodium EdetateViral hepatitis
The compound monoammonium glycyrrhizunate S preparation is injection or freeze dried powder preparation compounded with monoammonium glycyrrhizunate S, cysteine hydrochloride, glycine, sodium bisulfite, disodium versenate and mannitol in certain proportion. The present invention also discloses the preparation process and quality control technology. The compound monoammonium glycyrrhizunate S injection preparation is used in treating skin diseases and viral hepatitis mainly, and may be also used in treating acute and chronic unresolved hepatitis, poisoning hepatitis, etc. to result in certain curative effect. Recently, it is also in treating epidemic hemorrhagic fever, epidemic encephalitis, pulmonary tuberculosis, etc and thus has expanded clinical application range.
Owner:余世春
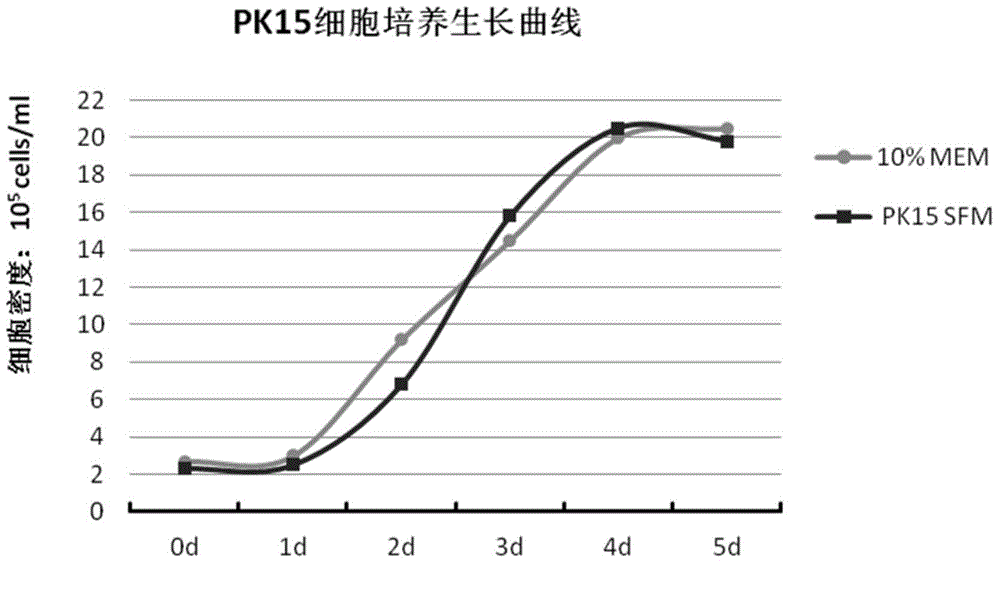
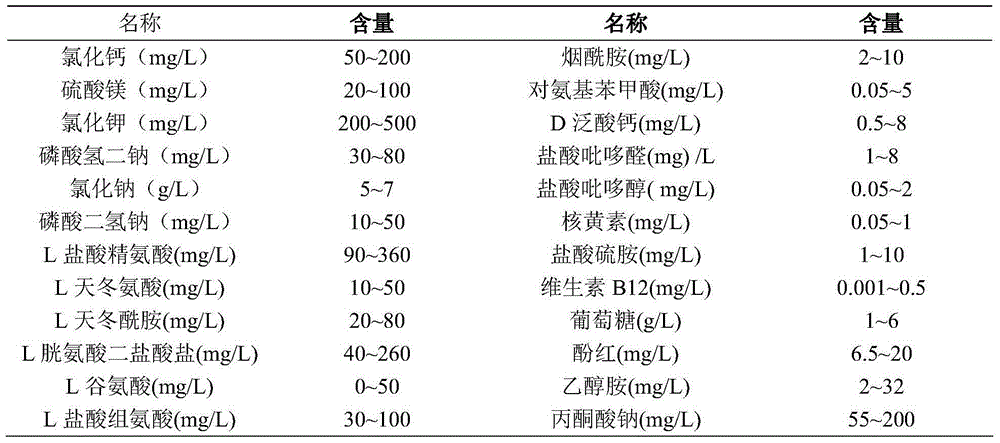
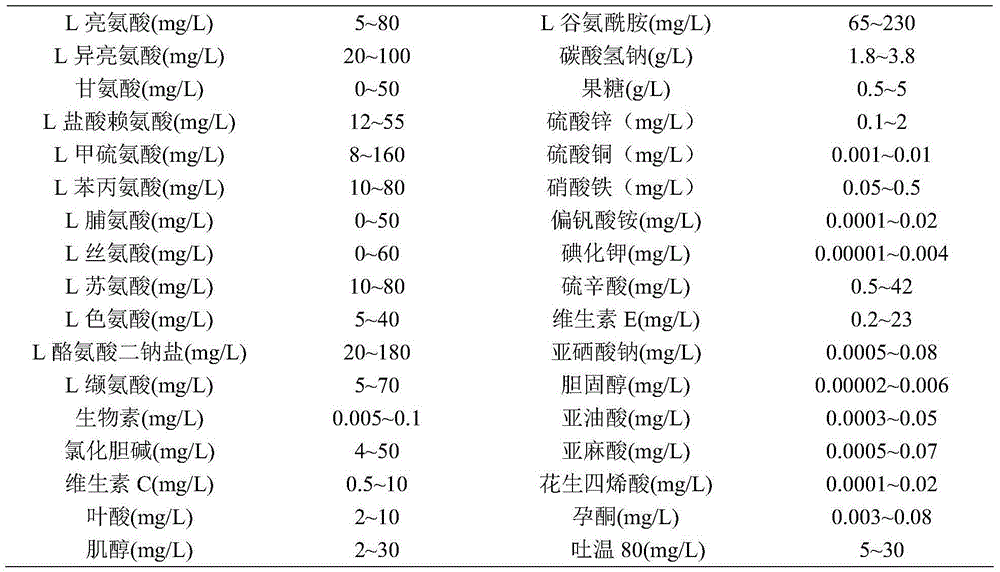
Protein-free, hydrolysate-free and serum-free culture medium and preparation method thereof
ActiveCN104911143AReduce manufacturing costSimplified purification stepsArtificial cell constructsVertebrate cellsDisodium EdetateHydrolysate
The invention relates to a protein-free, hydrolysate-free and serum-free culture medium and a preparation method thereof. The culture medium contains the following substances: manganese gluconate, calcium citrate malate, disodium edetate, progesterone, alanyl-glutamine,glycerophosphate, fructose, vitamin C, vitamin E, sodium selenite, hydroxypropyl-beta-cyclodextrin, cholesterol, malic acid, oxaloacetic acid, vitamin B12, lipoic acid, ferrous sulfate, ferric citrate, uric acid, taurine, reduced glutathione, zinc sulfate, copper sulfate and ferric nitrate. The protein-free, hydrolysate-free and serum-free culture medium has the advantages that the cost is low, the difference among different batches of products is small, the purification step of down-stream products is simplified, and potential safety hazards are reduced.
Owner:SICHUAN BAINUOJI TECH CO LTD
Pharmaceutical composition for external use adopting glucocorticoid as active component
A pharmaceutical composition for external use which adopts glucocorticoid as an active component is disclosed. The pharmaceutical composition comprises the glucocorticoid adopted as the active component, and one or more pharmaceutical excipient. The pharmaceutical excipient comprises edetate disodium or edetate calcium disodium.
Owner:TIANJIN JINYAO GRP
Pentoxifylline injection composition and preparation method thereof
ActiveCN106309362AGood quality and stabilityImprove stabilityNervous disorderAntipyreticMedicineTheobromine
The invention relates to a pentoxifylline injection composition and a preparation method thereof, belonging to the technical field of medical preparations. The injection composition is prepared from pentoxifylline, sodium dihydrogen phosphate, taurate, vitamin B6, disodium edetate and the like. The pentoxifylline injection composition is simple and feasible in technique. After the pentoxifylline injection composition is placed under accelerated test conditions for 6 months, the solution is still colorless, the pH value is basically unchanged, the increase of related substances is not obvious, the pentoxifylline content decrease is not obvious, and the product has obviously higher quality and stability than the control product.
Owner:CSPC OUYI PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com