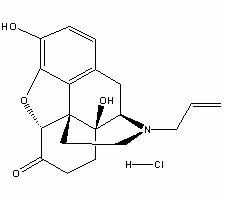
Naloxone hydrochloride freeze-dried powder injection and preparation method thereof
A technology of naloxone hydrochloride and freeze-dried powder injection, which is applied in the field of naloxone hydrochloride freeze-dried powder for injection and its preparation, can solve problems such as poor stability of water injection, and achieve the effect of improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] 1. Raw materials:
[0059] Naloxone hydrochloride 0.4 g
[0060] Mannitol 48 g
[0061] Edetate disodium 0.1 g.
[0062] Two, the preparation process is as follows:
[0063] 1.1 Weighing: Weighing strictly according to the prescription and ingredient instruction list (conventional ingredients 22,000 pieces / box), using a double-check system, weighing prescription quantities of naloxone hydrochloride, mannitol, edetate disodium, activated carbon, , placed in an appropriate container, weighed a small amount of water for injection, dissolved edetate disodium and naloxone hydrochloride respectively for later use, weighed activated carbon according to 0.1% of the total amount prepared, and adjusted the humidity with water for later use;
[0064] 1.2 Preparation:
[0065] 1.2.1 The dilute dispensing tank and feed liquid pipe are sterilized by pure steam and circulated for 30 minutes;
[0066] 1.2.2 Add an appropriate amount of water for injection that has been cooled to r...
Embodiment 2
[0088] 1. Raw materials:
[0089] Naloxone hydrochloride 1 g
[0090] Mannitol 48 g
[0091] Edetate disodium 0.1 g.
[0092] Two, the preparation process is as follows: prepare following 5 kinds of solutions by the method shown in embodiment 1:
[0093] Solution 1: pH value 5.0;
[0094] Solution 2: PH value 4.5;
[0095] Solution three: pH value 4.0;
[0096] Solution 4: pH value 3.5;
[0097] Solution five: pH value 3.0;
[0098] Solution six: pH value 2.5.
Embodiment 3
[0100] 1. Raw materials:
[0101] Naloxone hydrochloride 2 g
[0102] Mannitol 48 g
[0103] Edetate disodium 0.1 g.
[0104] Two, the preparation process is as follows: prepare following 5 kinds of solutions by the method shown in embodiment 1:
[0105] Solution 1: pH value 5.0;
[0106] Solution 2: PH value 4.5;
[0107] Solution three: pH value 4.0;
[0108] Solution 4: pH value 3.5;
[0109] Solution five: pH value 3.0;
[0110] Solution six: pH value 2.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com