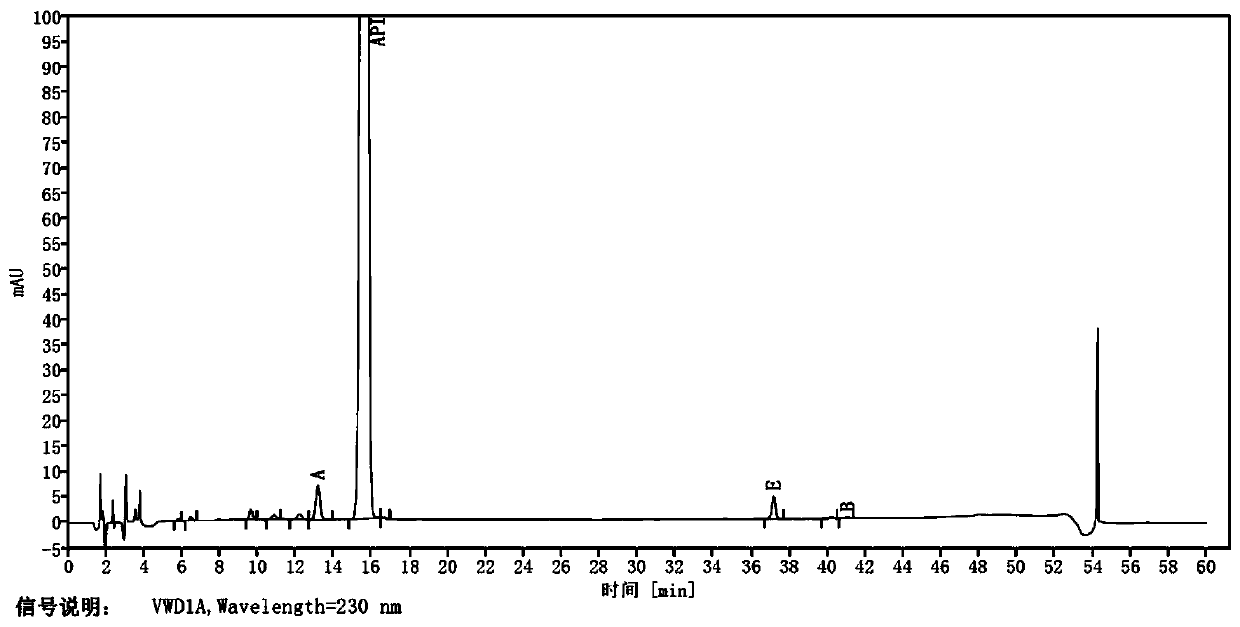
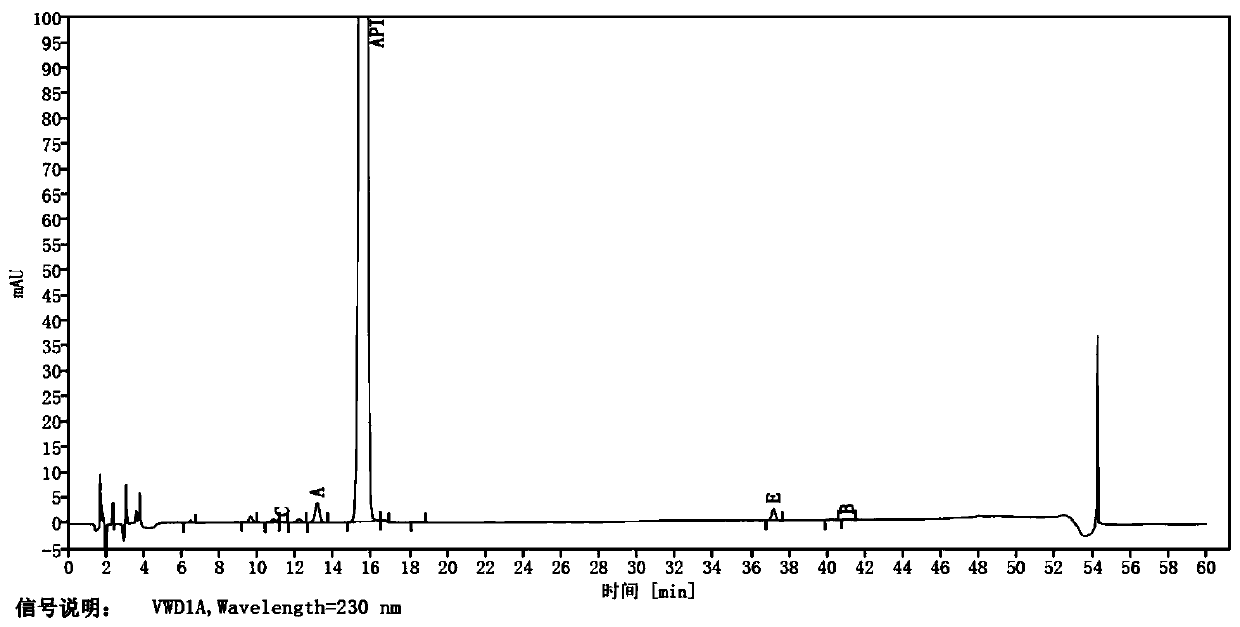
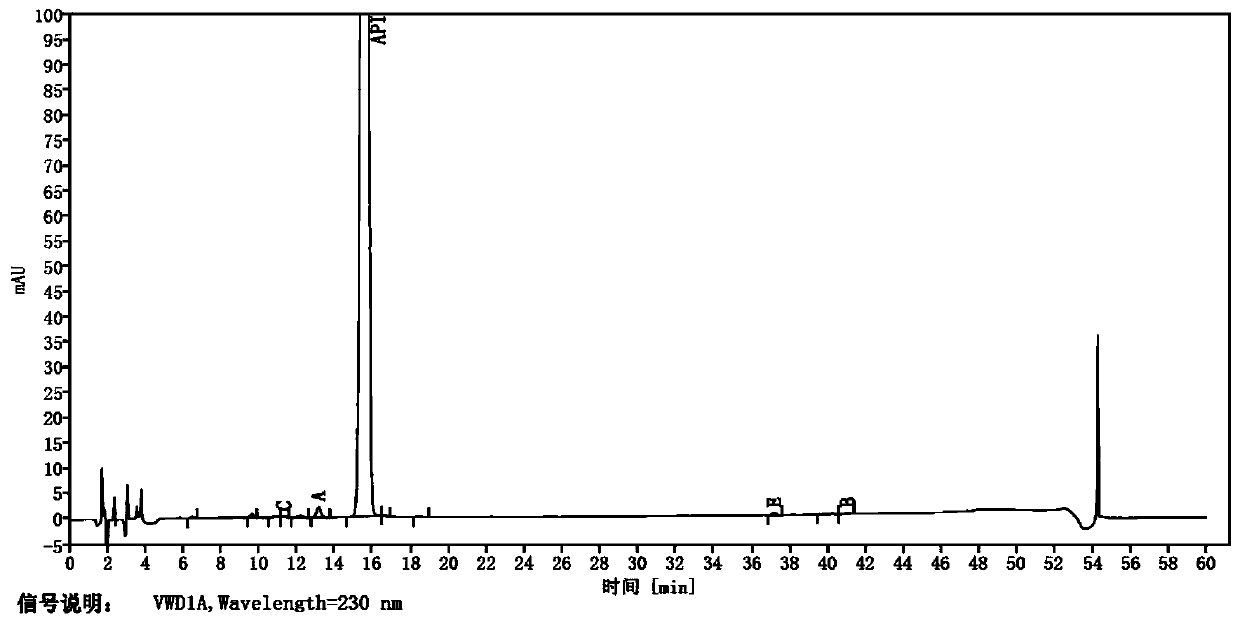
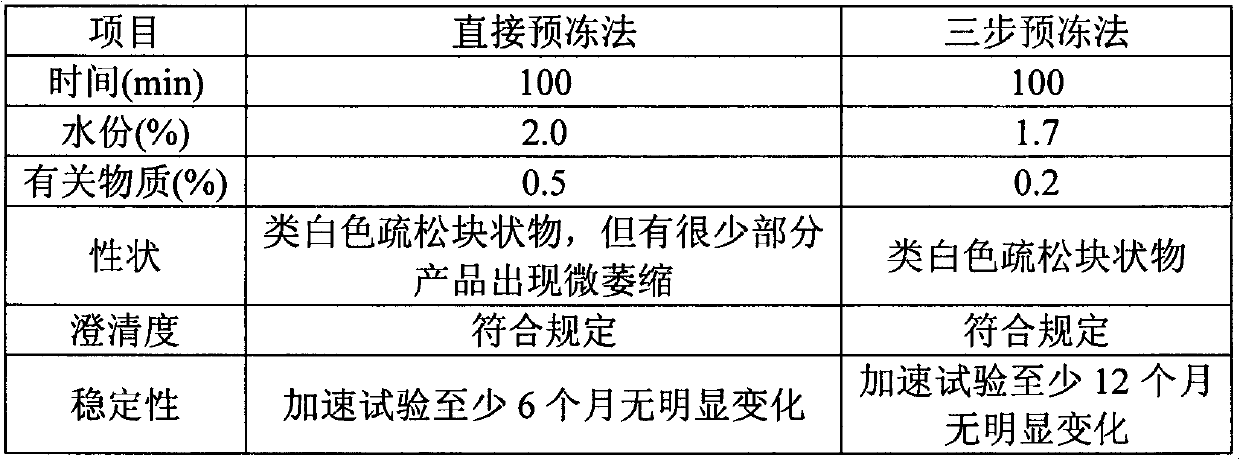
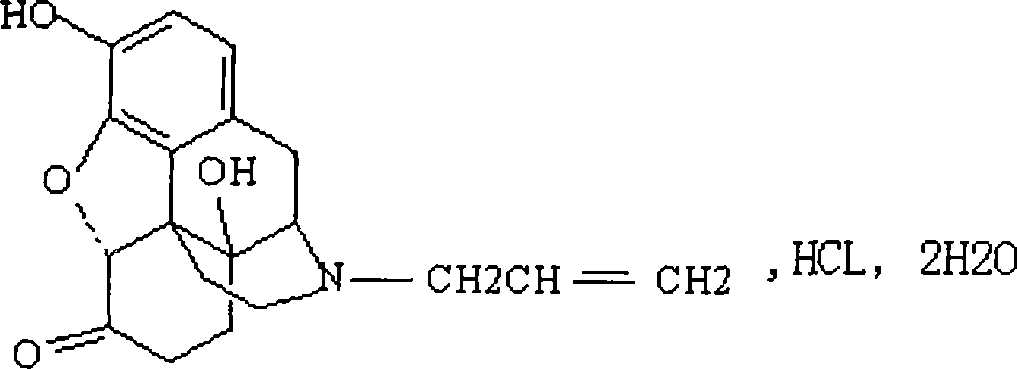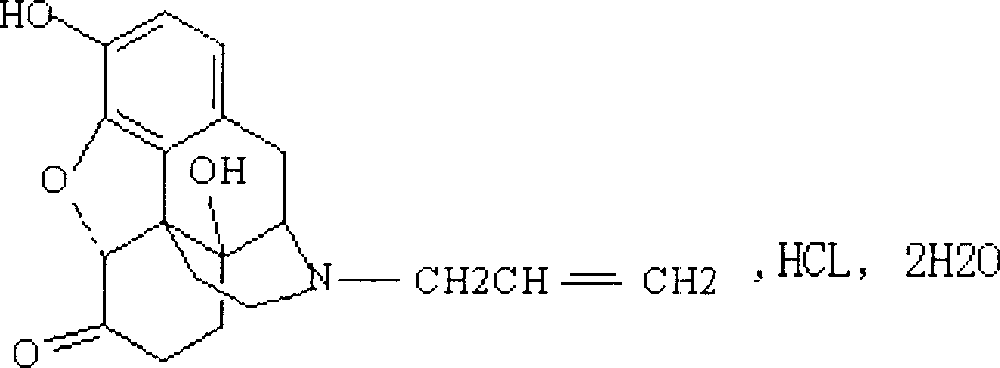Patents
Literature
51 results about "Naloxone Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
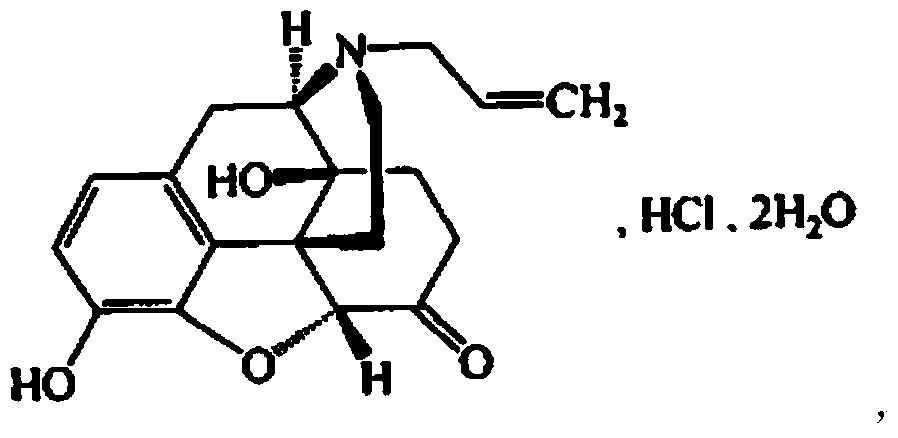
The hydrochloride salt of naloxone, a thebaine derivate with opioid antagonist activity. Naloxone binds to opioid receptors in the CNS in a competitive manner, reversing or inhibiting characteristic opioid effects, including analgesia, euphoria, sedation, respiratory depression, miosis, bradycardia, and physical dependence. This agent binds to mu-opioid receptors with a high affinity, and a lesser degree to kappa- and gamma-opioid receptors.
Naloxone hydrochloride nasal spray
The present invention is nasal cavity spray of Naloxine hydrochloride and its preparation process. The preparation of the present invention may be applied in single dosage form or multiple dosage form. The spray of the present invention includes Naloxine hydrochloride, osmotic pressure regulator, preservative, osmotic promoter and water.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Powder and injection preparation of hydrochloric naloxone and preparation method
InactiveCN1626083AQuality improvementEasy to storePowder deliveryOrganic active ingredientsWater solublePowder injection
A powder injection of naloxone hydrochloride is prepared from the naloxone hydrochloride and water-soluble medical auxiliary for injection. Its advantages aer high stability and high quality.
Owner:FUDAN UNIV +1
Naloxone hydrochloride sublingual spraying drug delivery system or composition and its preparation method
InactiveCN101057830APromote absorptionImprove bioavailabilityOrganic active ingredientsNervous disorderMouth mucosaPreservative
The invention discloses a chlorhydric acid naloxone hypoglossal spraying drug delivery system or compound and the preparing method, belonging to pharmacology field. It is characterized in that: it is hypoglossal spraying agent, and the comprised components and their weight proportion are as follows: (1) chlorhydric acid naloxone or naloxone free alkali or naloxone medical salt: 0. 1-30%, (2) absorption promoter: 0. 5-10%; (3) osmotic pressure regulator: 0. 1 -5%; (4) bodying agent: 0. 5-30%; (5) corrigent: 0. 01-20%; (6) conservative 0. 01-0. 5%, (7) water: 45-95%. The invention is characterized by good adjustment, especially suitable for patient in comatose state; high biological utilization rate, convenient utilization. The product is characterized by stable performance, controllable quality and no stimulation to mouth mucosa.
Owner:BEIJING HUMANWELL JUNWEI PHARM TECH CO LTD
Naloxone hydrchloride freeze-dried powder preparation for injection
InactiveCN1615867AImprove solubilitySimple manufacturing processPowder deliveryOrganic active ingredientsMANNITOL/SORBITOLAlcohol
The freeze dried naloxone hydrochloride powder for injection consists of naloxone hydrochloride in effective medicine amount and proper amount of medicinal carrier. The naloxone hydrochloride content is 0.08-70.59 wt% or 0.1-12 mg; and the medicinal carrier may be one or several of mannitol, glucol, sodium chloride, beta-cyclodextrin, dextran, fructose, sorbic alcohol, etc., is preferably mannitol and glucol and has the ratio in the preparation of 29.41-99.92 wt%.
Owner:YANGGUANG RUNHE SCI & TECHNOLGY BEIJING
Process for preparing naloxone hydrochloride injection
InactiveCN101590014APrevent oxidative deteriorationReduce oxygen contentOrganic active ingredientsNervous disorderMedicineOxygen content
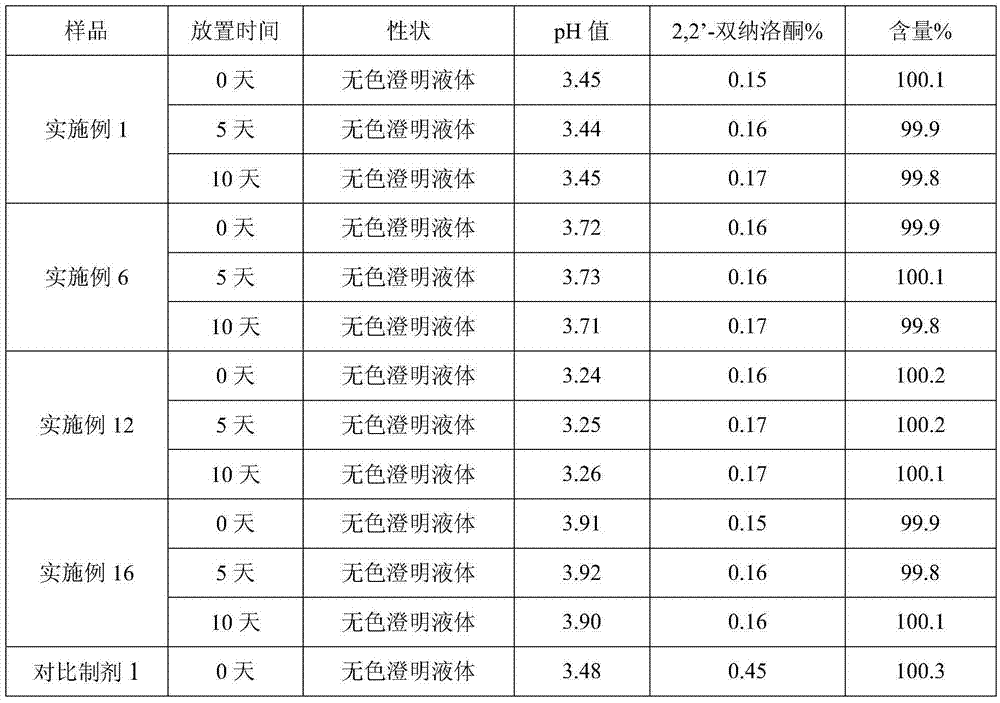
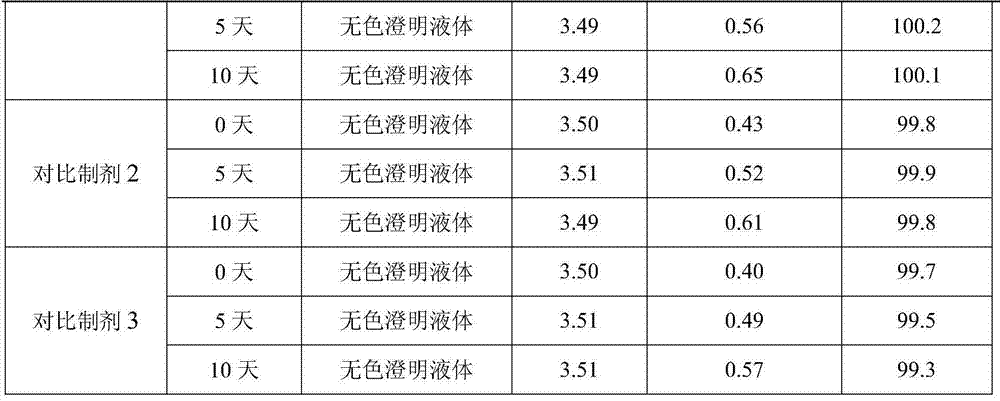
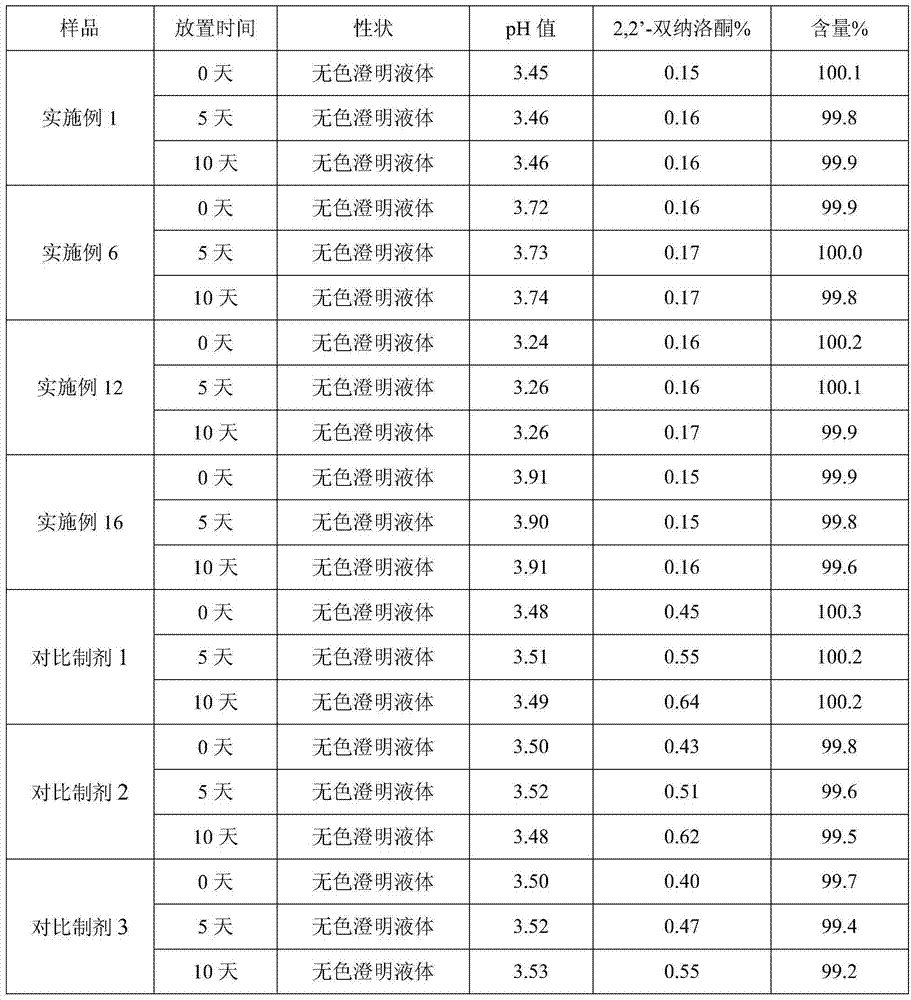
The invention provides a process for preparing naloxone hydrochloride injection. The pH of the naloxone hydrochloride injection can be unchangeable during placing by strictly controlling the pH range in production; and simultaneously, a method for inert gas protection adopted in the production reduces the oxygen content in liquid medicine and an ampoule to the minimum so as to prevent oxidative deterioration of the naloxone hydrochloride injection to reduce related substances. The technology greatly ensures the product quality.
Owner:SHANDONG XINHUA PHARMA CO LTD
Naloxone hydrochloride nano granule powder injection formulation and preparation thereof
InactiveCN101322691AImprove stabilityGuarantee product qualityPowder deliveryOrganic active ingredientsFreeze-dryingSodium bisulfite
The invention discloses a naloxone hydrochloride nano-particle powder injection, containing the raw materials with the following parts by weight: 0.4-4 parts of naloxone hydrochloride, 0.15-2 parts of dextran, 0.5-8 parts of sodium bisulfite, 0.2-4 parts of sodium sulfate, 0.6-10 parts of polyalkylcyano-acrylate, 10-1,200 parts of freeze-drying support agent. The invention further discloses a preparation method of the preparation. The preparation has fine stability and compatibility.
Owner:HAINAN LINGKANG PHARMA CO LTD
Newly packed naloxone hydrochloride injection and its producing method
InactiveCN1748702AAvoid pollutionStable and reliable productionOrganic active ingredientsAntipyreticBottleNaloxone Hydrochloride
The process of producing naloxone hydrochloride injection in new package includes the following steps: preparing naloxone hydrochloride injection intermediate; bacteria-free filtering the intermediate to obtain filtrate; bacteria-free packing the filtrate in Schering bottle; and sterilizing with flowing steam. While bacteria-free filtering, the insoluble particles in the medicine liquid are also eliminated, and this can greatly reduce the phlebitis attacking probability. The product is safe and reliable.
Owner:BEIJING KAWIN TECH SHARE HLDG
Pharmaceutical naloxone hydrochloride composition for injection and preparation method of pharmaceutical naloxone hydrochloride composition
ActiveCN103877578AAvoid degradationPH controlOrganic active ingredientsPharmaceutical non-active ingredientsExcipientFumaric acid
The invention provides a pharmaceutical naloxone hydrochloride composition for injection and a preparation method of the pharmaceutical naloxone hydrochloride composition. In the prescription, trehalose is used as an excipient, and a certain quantity of fumaric acid is added, so that the pH value of a product can be controlled within a reasonable range, meanwhile, the degradation of naloxone hydrochloride in a storage process can also be avoided, and the stability of the product is greatly improved; in addition, the process is easy to operate, and the prepared product is full in appearance, stable in quality and relatively good in dissolvability and compatible stability.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Medicine composition of naloxone hydrochloride and polyethylene glycol and preparation method thereof
ActiveCN101732313AStable in natureEasy to degradeOrganic active ingredientsNervous disorderPolyethylene glycolNaloxone Hydrochloride
The invention belongs to the technical field of medicine, which discloses a medicine composition of naloxone hydrochloride and polyethylene glycol and a preparation method thereof. The weight percent of the naloxone hydrochloride to the polyethylene glycol of the composition is 1:0.1-10. The invention solves the problem that naloxone hydrochloride injection in the prior art is unstable to light, is convenient to master the application quantity and the effective dose and enables the clinical application to be safer and more effective.
Owner:BEIJING SIHUAN PHARMA +2
Naloxone hydrochloride spraying agent for mouth and nose
InactiveCN101036651AEasy to useQuick effectOrganic active ingredientsAerosol deliveryNasal cavitySide effect
The invention discloses a naloxone hydrochloride spraying agent for oral or nasal cavity, which is prepared by naloxone hydrochloride, naloxone free alkali or the other naloxone acceptable in medicine having a ratio by weight of 1:2.5~10 with pharmaceutic edjuvant based on general spraying agent preparation process. Active ingredient of the naloxone hydrochloride spraying agent for oral or nasal cavity in the invention can directly participate intracorporeal circulation after absorbed by capillary tube under oral or nasal mucosa of a patient, thus has advantages of fast absorption and high bioavailability, without stimulating to the oral or nasal mucosa. In particular, the drugs of the invention can be taken with no requirement of use situation, so that it is convenient for using which dosage is controllable and has little side effect.
Owner:薛京 +1
Composition of naloxone hydrochloride and polyvinylpyrrolidone and preparation method thereof
InactiveCN101732314APrevent oxidative breakdownImprove securityPowder deliveryOrganic active ingredientsPolyvinylpyrrolidoneNaloxone Hydrochloride
The invention belongs to the technical field of medicines, in particular a composition of naloxone hydrochloride and polyvinylpyrrolidone and a preparation method thereof. The weight ratio of the naloxone hydrochloride to the polyvinylpyrrolidone in the composition is 1:1-1:10, and the composition can contain auxiliary materials. The composition of the invention solves the problem of instability of the naloxone hydrochloride under the condition of high temperature and illumination intensity, so that the naloxone hydrochloride becomes more stable with easy storage.
Owner:BEIJING SIHUAN PHARMA +1
Method and Composition for the Treatment of Opioid Induced Constipation
InactiveUS20170087150A1Specific and beneficial pharmacokineticsOrganic active ingredientsMicrocapsulesOpioid antagonistHalf-life
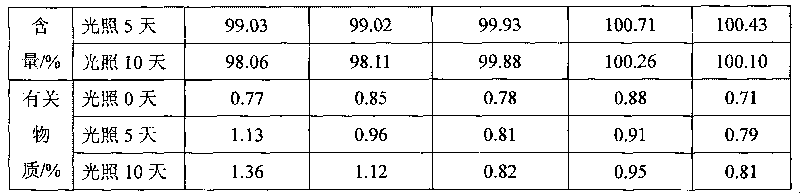
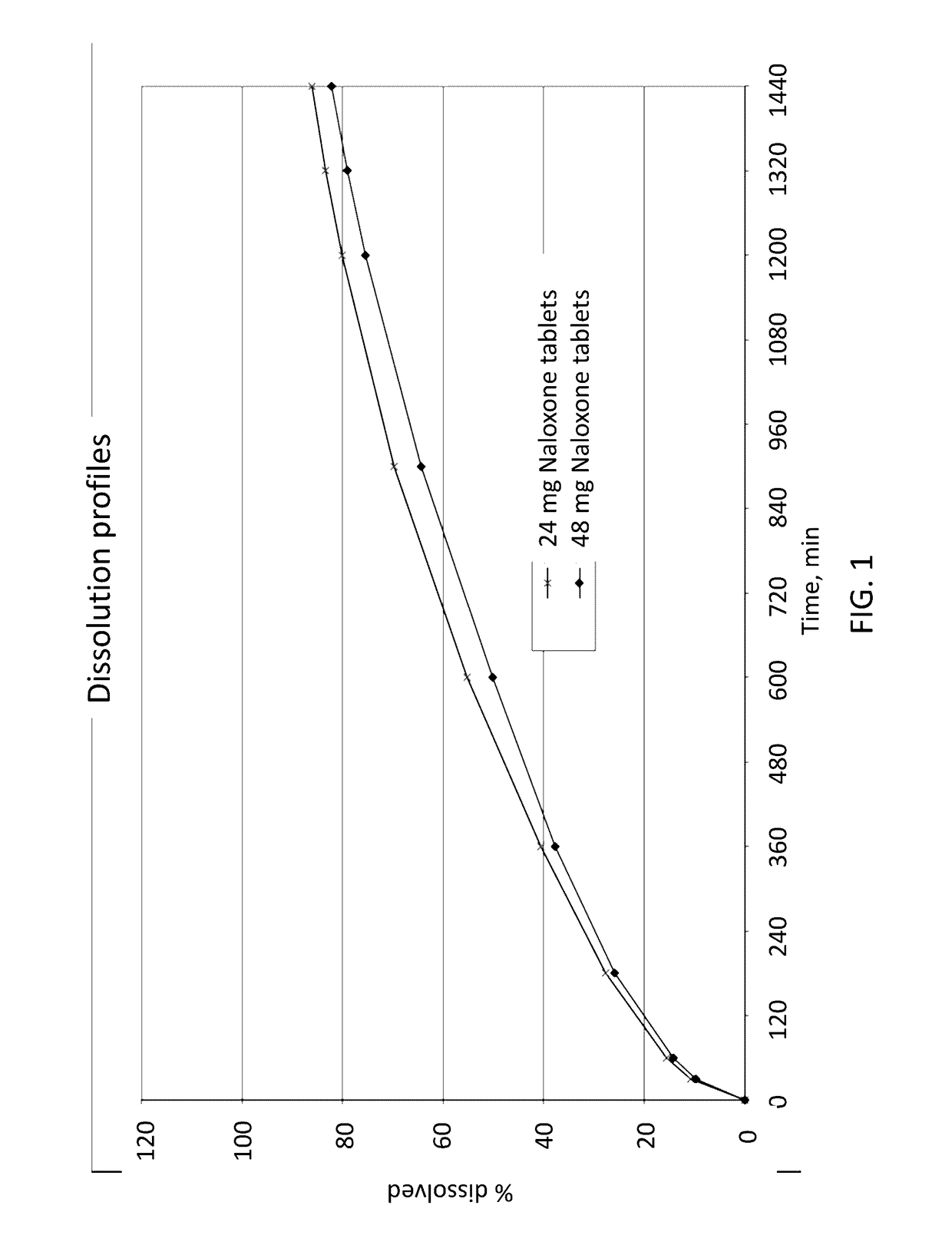
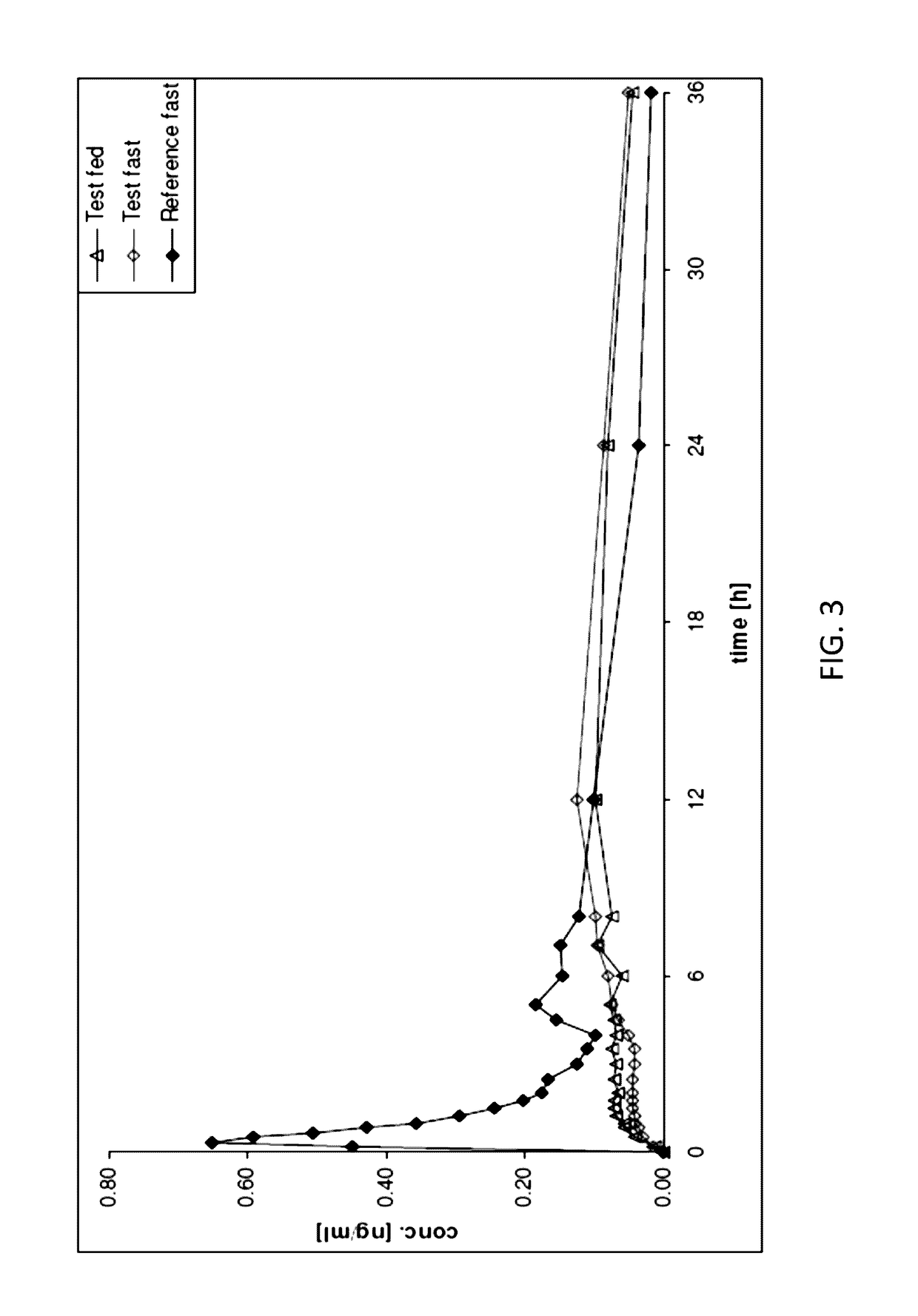
The invention relates to a method of treatment using a solid oral pharmaceutical dosage form comprising an opioid receptor antagonist for use in the treatment of opioid induced constipation comprising the opioid antagonist equivalent to 24 mg of naloxone hydrochloride as twice daily formulation or equivalent to 48 mg of naloxone hydrochloride as once daily formulation, characterized in that the opioid antagonist has a rapid first pass metabolism with a plasma half-life of 2 hours or less in humans within the first 3 hours of oral administration of a solution, wherein the steady state pharmacokinetics result in a constant level of naloxone in the bloodstream, wherein the pharmacokinetics are independent of whether the dosage is administered once or twice daily, wherein the oral dosage form releases the opioid antagonist in a prolonged manner, wherein the in vitro release rate of the opioid antagonist measured using the paddle stirrer method according to Ph. Eur. at 75 rpm in 1000 ml 0.1 N hydrochloric acid at 37° C., is 15%±5% in 1 h, is 26%±5% in 3 h, is 40%±5% in 6 h, is 55%±7% in 10 h, is 67%±8% in 15 h, and is 78%±10% in 20 h.
Owner:DEVELCO PHARMA SCHWEIZ AG
Abuse potential low compound buprenorphin hydrochloride naloxone hydrochloride sublingual tablet
InactiveCN1943575AReduce Abuse PotentialAvoid abuseOrganic active ingredientsNervous disorderBuprenorphine HydrochlorideAlcohol
A buprenorphine hydrochloride / naloxone hydrochloride sublingual tablets with low misuse potential , said tablets contain medicinal contents of buprenorphine hydrochloride and naloxone hydrochloride, part by weight thereof is 2:1-6:1, 1) using alcohol to dissolve regulator corrigent and bond of PH of prescription amount for use; 2) grinding main medicine buprenorphine hydrochloride / naloxone hydrochloride, filler and lubricant and sifting out thereof; 3) mixing evenly the prescription amount of buprenorphine hydrochloride / naloxone hydrochloride, filler, bond and disintegrating agent, thereafter adding contents in step 1 to prepare into soft stuff, again making into pellet, drying, sorting out, adding lubricant again, mixing evenly and pressing into tablet.
Owner:岳振江
Naloxone Hydrochloride nose powder preparation
The invention relates to hydrochloric naloxone nose powder preparation and its preparation method, the invention also relates to the hydrochloric naloxone single dosage and multiple dosage administration nose powder preparation, which comprises hydrochloric naloxone and one or more selected from mannitol, glucose, and soluble starch, sodium chloride, microcrystalline cellulose and sodium carboxymethylcellulose as medicinal findings.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Pharmaceutical composition of naloxone hydrochloride injection and preparation method thereof
ActiveCN103877016ASimple prescriptionReduce degradationOrganic active ingredientsNervous disorderMalic acidNaloxone Hydrochloride
The invention provides a novel pharmaceutical composition of a naloxone hydrochloride injection and a preparation method thereof. The prescription is simple, the stability of a naloxone hydrochloride water solution is obviously increased after a certain amount of malic acid is added to the prescription, and malic acid is free of poison or thrill, so that the pharmaceutical composition is better in safety; meanwhile, the preparation process disclosed by the invention is easy to operate, and the prepared product is controllable in quality.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Preparator of naloxone hydrochloride injection and preparation method thereof
ActiveCN107485563AEasy to solveGuaranteed efficacyOrganic active ingredientsNervous disorderForeign matterFiltration
The invention discloses a preparator of a naloxone hydrochloride injection and a preparation method thereof. The preparation method comprises the steps that a certain amount of water for injection, sodium chloride and naloxone hydrochloride are added into an upper tank body of the preparator, nitrogen is introduced, mixing and stirring are performed to obtain a mixed solution, then edetate disodium is added to regulate a pH value, and the mixed solution is filtered by a first filter membrane in the middle of the preparator and delivered to a lower tank body; a certain amount of water for injection is added into the lower tank body, stirring is performed under the situation that nitrogen is introduced, and a hydrochloric acid solution is used for regulating the pH value; finally, visible foreign matter checking is performed after filtration of a second filter membrane at the lower portion of the lower tank body, nitrogen charging is performed for filling encapsulation after qualification, and sterilization is performed to obtain the sterilization. No auxiliary material is added, and the purity and use safety of the prepared injection are ensured. The preparation method is simple and easy to operate, and the preparation cost is effectively reduced. In addition, the invention provides the preparator of the naloxone hydrochloride injection. Device replacement in the preparation process of the naloxone hydrochloride injection is avoided, the drug quality is improved, and drug safety is ensured.
Owner:北京市永康药业有限公司
Nasal cavity taken drug system and combination of naloxone hydrochloride and preparation method
A nasal application system or composition of naloxone hydrochloride contains the naloxone hydrochloride or its free alkali or its other medicinal salts, absorption promoter, osmotic pressure regulator, antiseptic, and solvent or other medicinal auxiliaries. Its preparing process s also disclosed.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
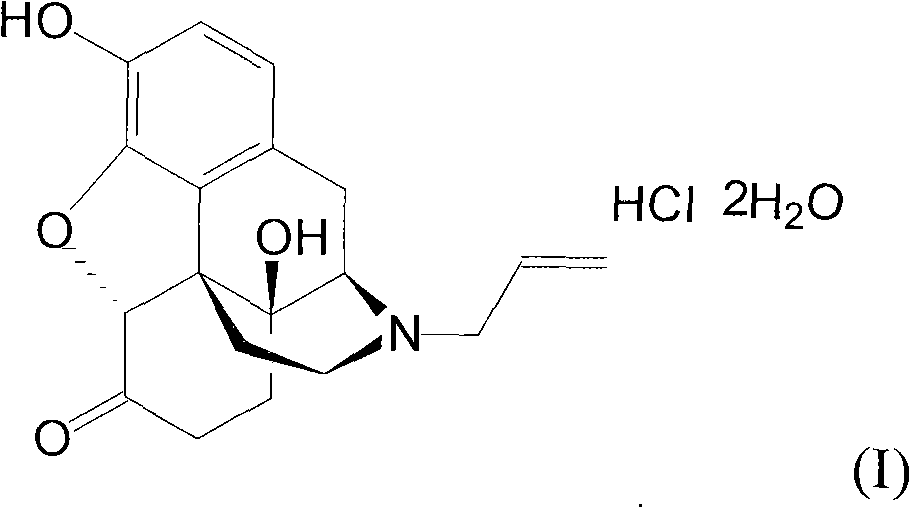
Process for salifying naloxone hydrochloride
The invention belongs to the pharmaceutical and chemical field and particularly relates to a process for salifying naloxone hydrochloride, which comprises the following steps: carrying out allylation reaction on intermediates of 14-hydroxy normorphine ketone and allyl bromide in DMF (dimethyl rormamide) which is used as a solvent, processing allylation reaction solution to obtain naloxone base, refining the naloxone base, and carrying out salifying reaction. Because the naloxone base is refined, the mass content of the naloxone base is increased, less impurities are generated, the accurate amount of HCl (hydrogen chloride) used for salifying is ensured, the colloidal agglomeration during salifying is avoided, the product quality and yield are improved, the column chromatography process is omitted, the production efficiency is improved, and the cost is reduced.
Owner:SHANDONG XINHUA PHARMA CO LTD
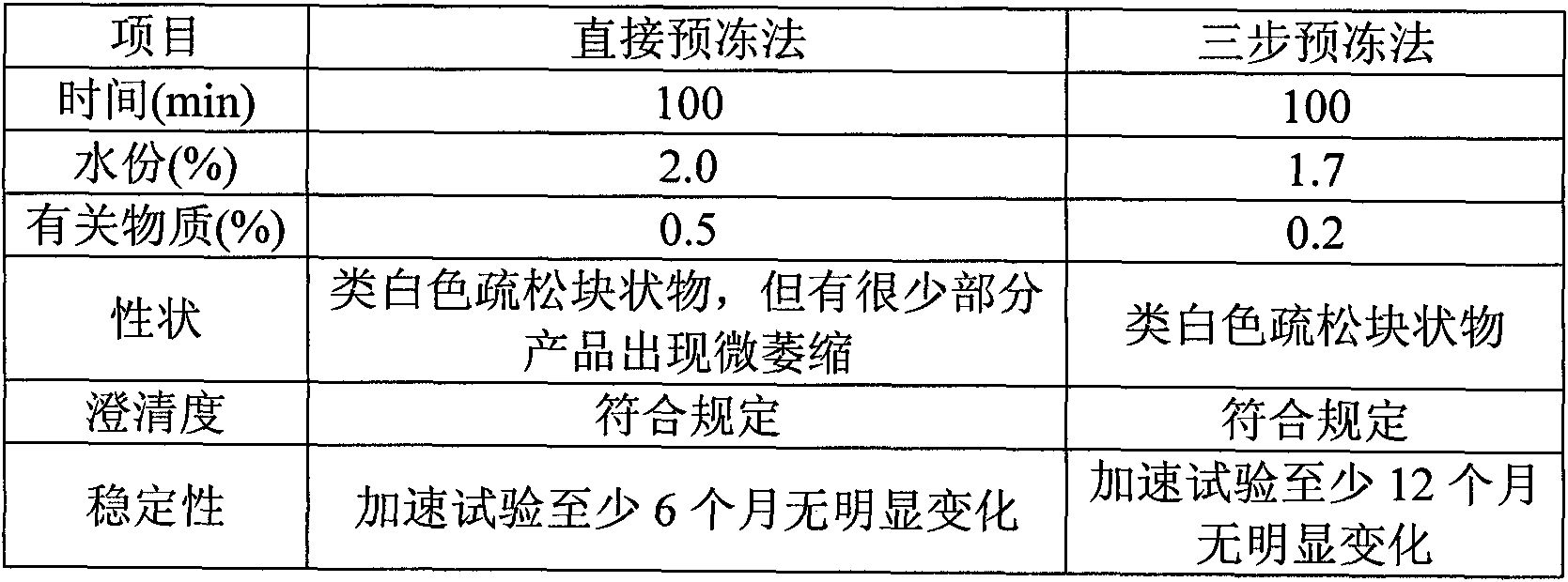
Stable naloxone hydrochloride lyophilized preparation and preparation method thereof
InactiveCN102727449AGood resolubilityHigh clarityPowder deliveryOrganic active ingredientsSolubilityMANNITOL/SORBITOL
A stable naloxone hydrochloride lyophilized preparation comprises naloxone hydrochloride and mannitol in a weight ratio of 1 : 30 - 200. The lyophilized preparation has a simple formula and fewer side effects, and employs an advanced freeze-drying process. The prepared products have full appearance, good solubility, and excellent quality.
Owner:YAOPHARMA CO LTD
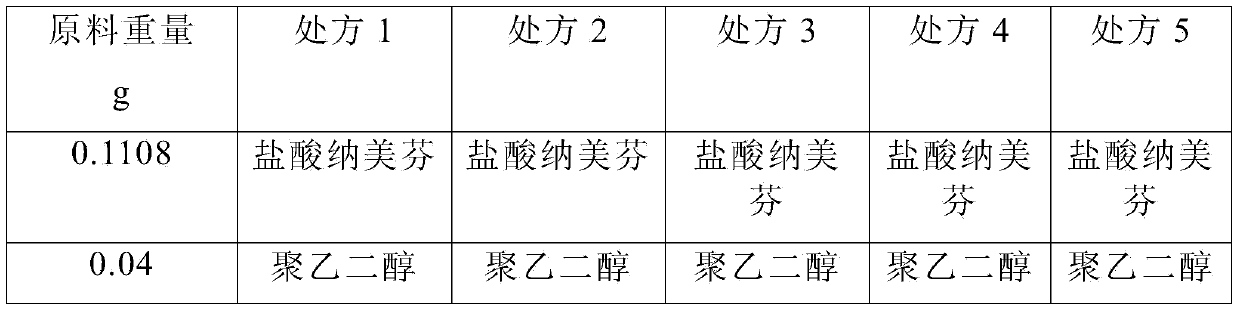
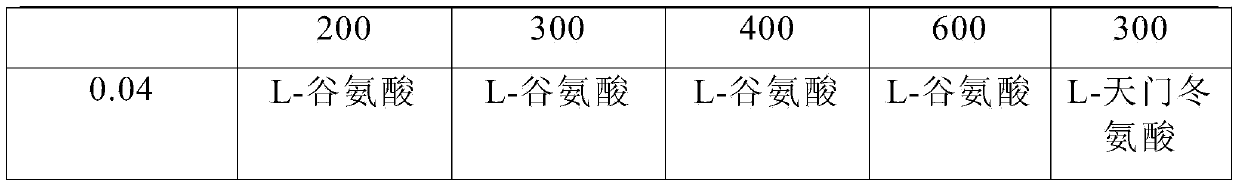
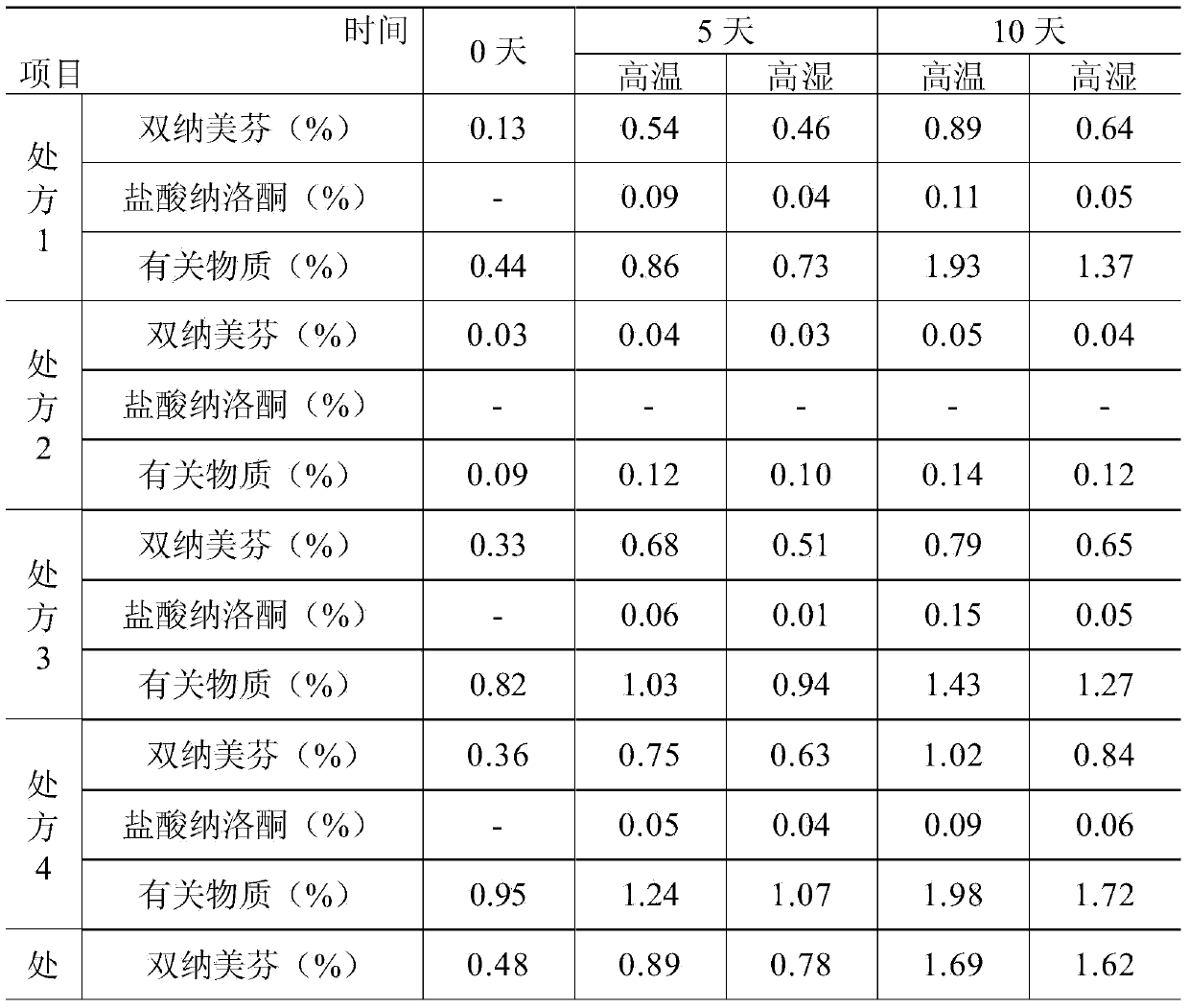
Pharmaceutical composition containing active component, namely nalmefene hydrochloride
ActiveCN104000827AGuaranteed contentOrganic active ingredientsNervous disorderActive componentPolyethylene glycol
The invention belongs to the technical field of medicines, and in particular relates to a pharmaceutical composition containing an active component nalmefene hydrochloride. The pharmaceutical composition disclosed by the invention contains nalmefene hydrochloride, polyethylene glycol 300 and L-glutamic acid. Injection prepared from the pharmaceutical composition disclosed by the invention is good in stability; after placing for 24 months, the content of bis-nalmefene is less than 0.1%; furthermore, the content of naloxone hydrochloride is less than 0.01%; the injection prepared from the pharmaceutical composition disclosed by the invention is convenient to use and beneficial to storing and transporting; the pharmaceutical composition disclosed by the invention is simple in preparation method, easy for industrialization production and low in production cost.
Owner:西藏易明西雅医药科技股份有限公司
Naloxone hydrochloride compound with high purity
The invention relates to a naloxone hydrochloride compound, in particular to a naloxone hydrochloride compound with high purity obtained by a method, belonging to the technical field of medicine. By acid-base reaction, polyamide resin elution and activated carbon absorption, the purity of the naloxone hydrochloride is greatly improved, the product quality of preparation is optimized, and clinic pharmacy safety is ensured; and the method has the advantages of simple process, low cost and high yield, and is suitable for industrialized production.
Owner:HAINAN LINGKANG PHARMA CO LTD
Medicine composition of naloxone hydrochloride and medicine purpose thereof
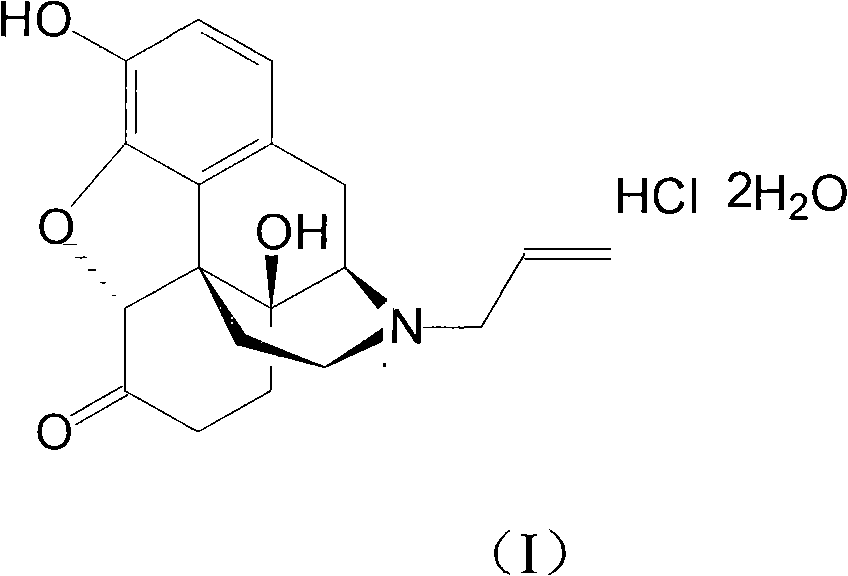
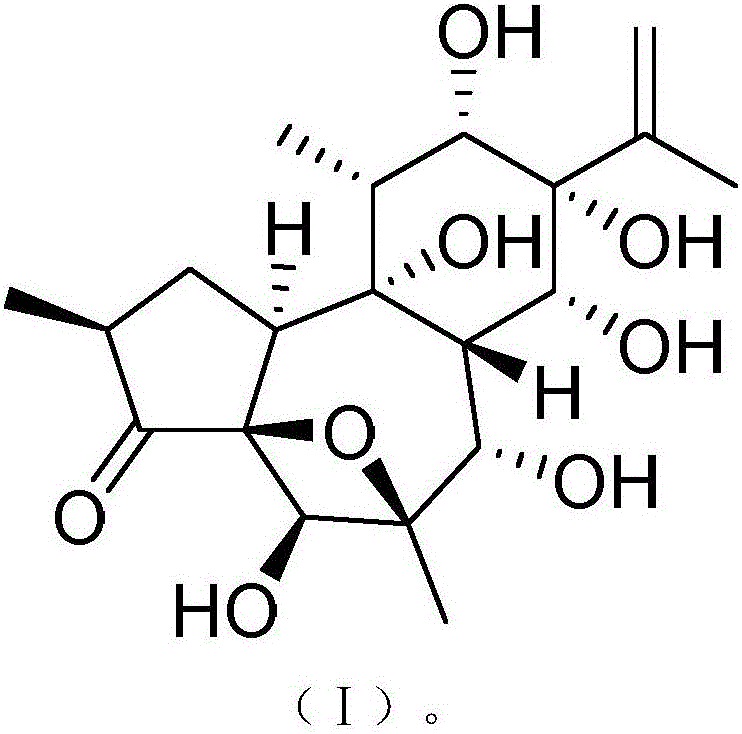
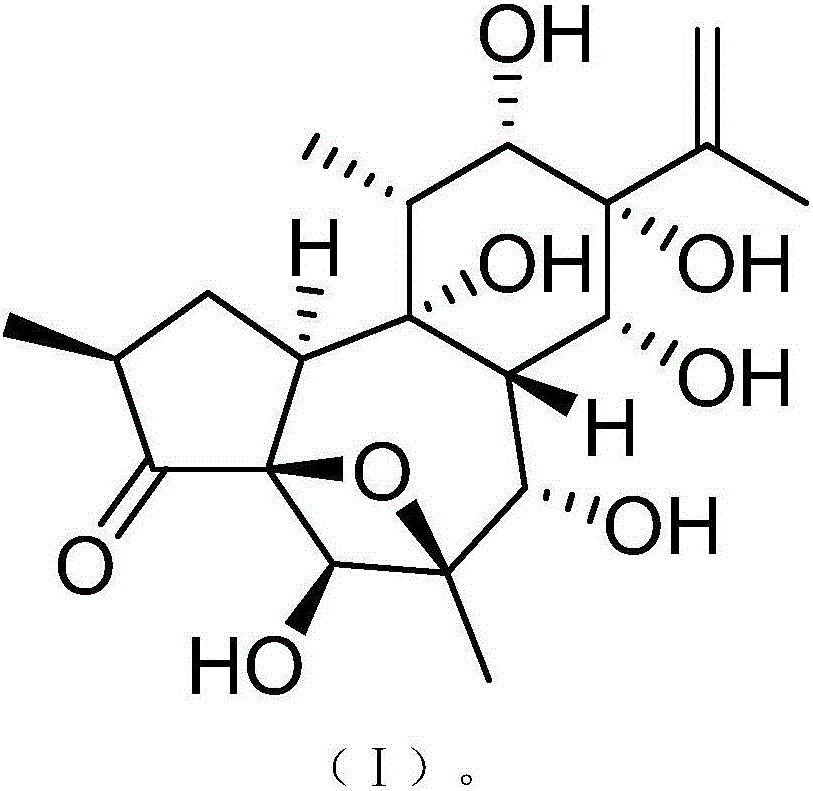
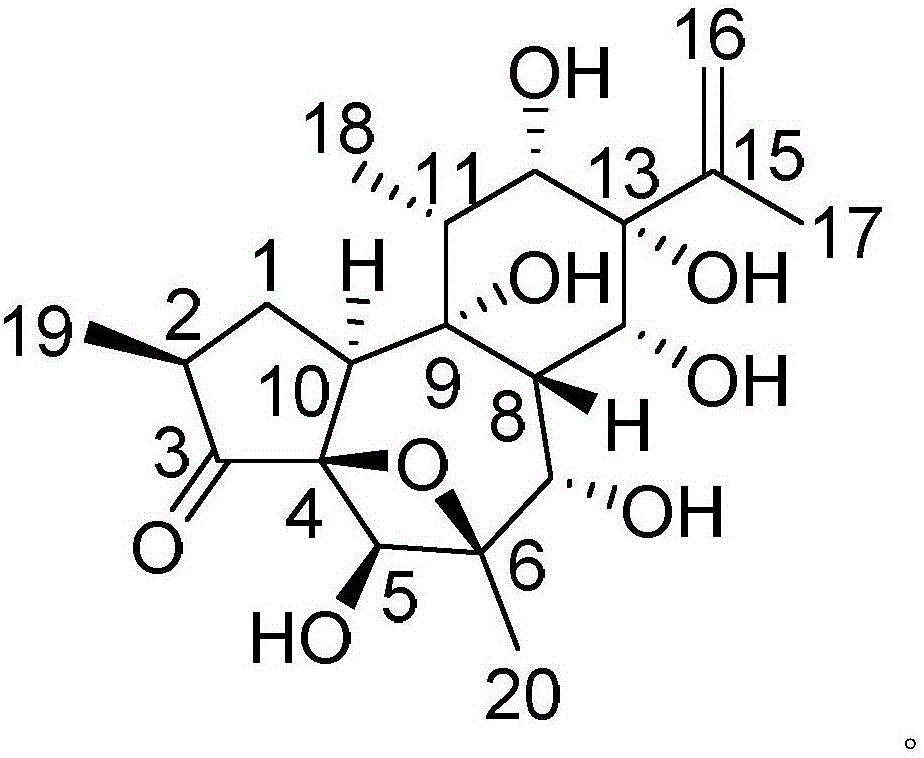
InactiveCN106188087AHas therapeutic effectGood treatment effectOrganic active ingredientsOrganic chemistryNatural productTherapeutic effect
The invention discloses a medicine composition of naloxone hydrochloride and a medicine purpose thereof. The medicine composition of naloxone hydrochloride comprises naloxone hydrochloride and a natural product compound (I) with a novel structure; when the naloxone hydrochloride and the compound (I) singly act, the function of treating cervical cancer is realized; when the naloxone hydrochloride and the compound (I) jointly act, the treatment effect is further improved, and a medicine for treating cervical cancer can be developed. Compared with prior art, the medicine composition has outstanding material characteristic and obvious process.
Owner:林翔
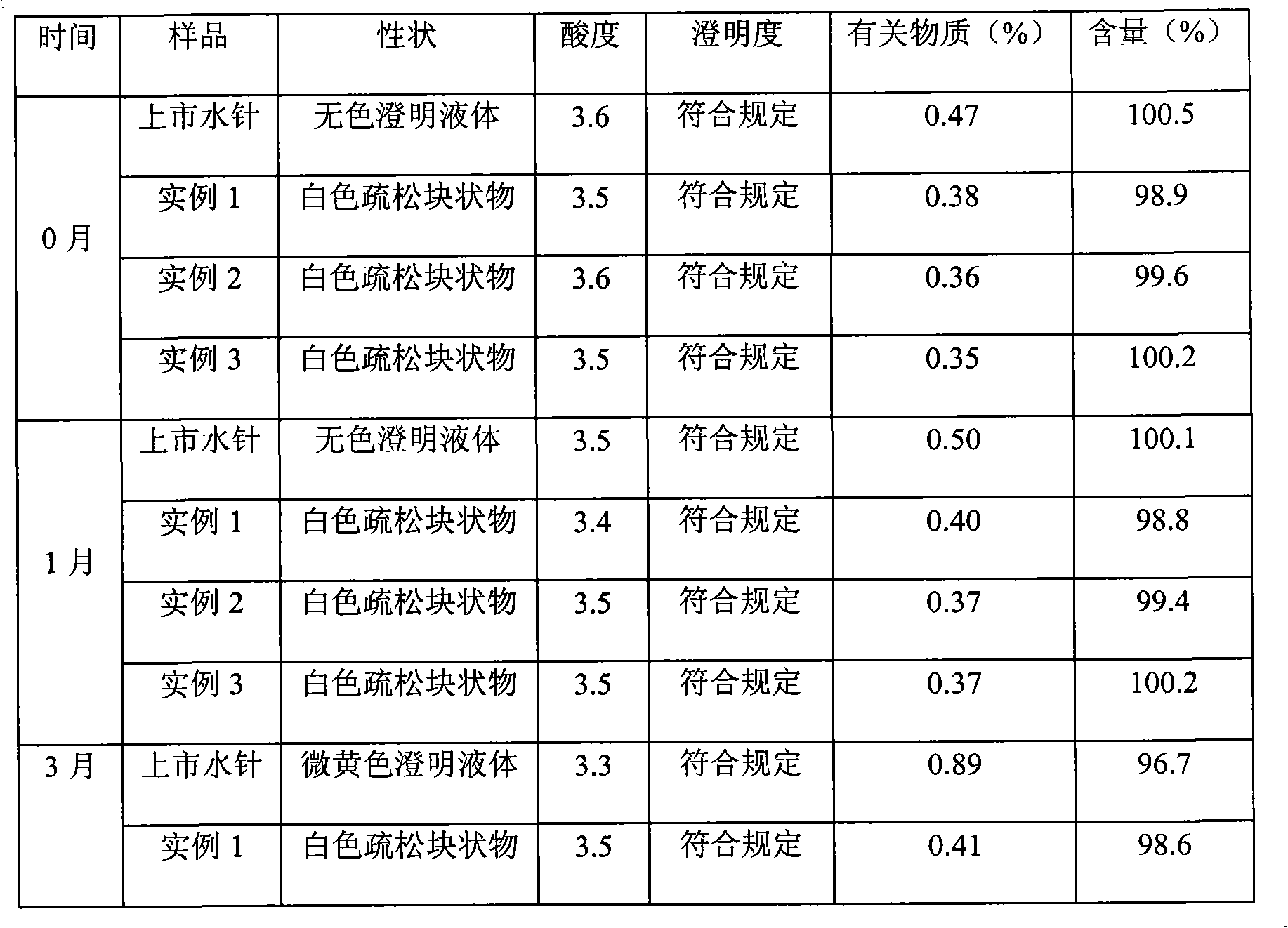
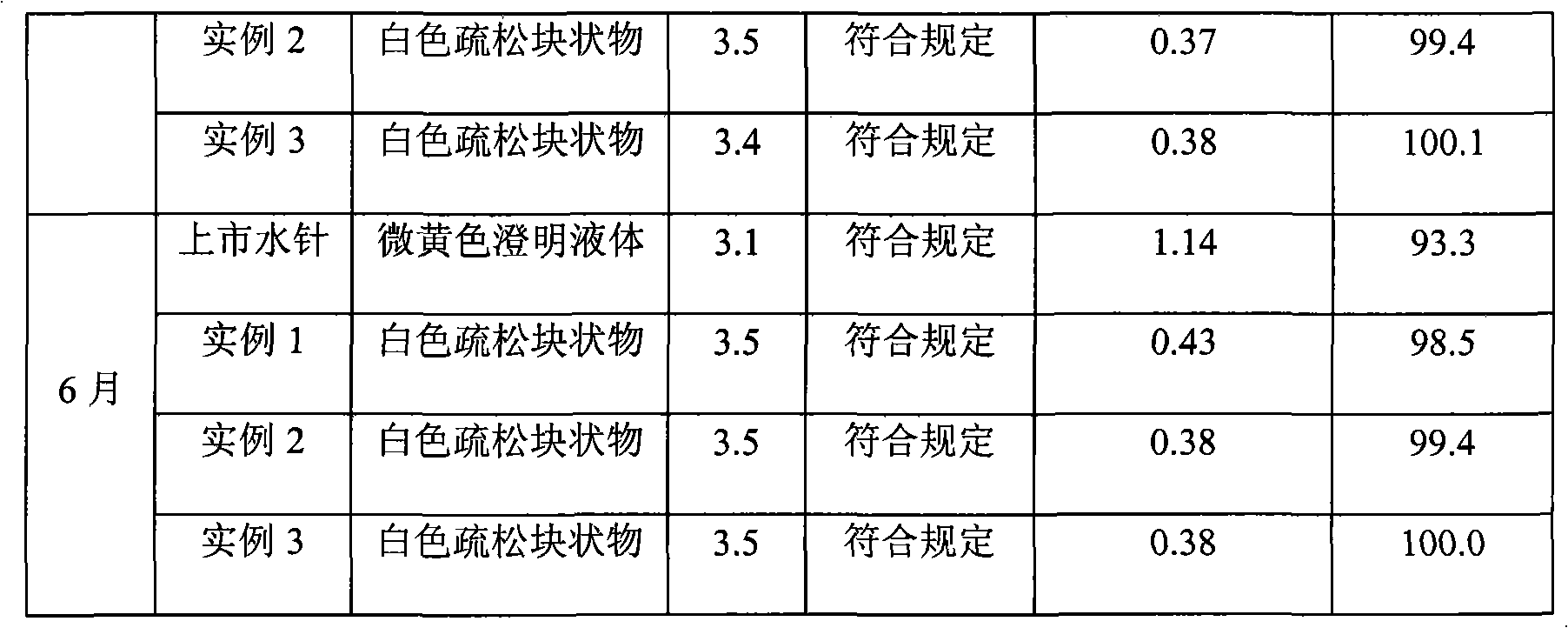
Naloxone hydrochloride injection solution and preparation method thereof
ActiveCN110269837AWide pH rangeLess prescription ingredientsOrganic active ingredientsNervous disorderMedicineInjection solution
The invention belongs to the technical field of medicine and provides a naloxone hydrochloride injection solution. Under the conditions of high-temperature sterilization and long-term storage, the quantity of related substances keeps an extremely low level, and accordingly the high quality of a liquid preparation is maintained. In addition, the invention further provides a preparation method of the naloxone hydrochloride injection solution.
Owner:GUANGDONG QIFANG MEDICINES CO LTD +1
Stable naloxone hydrochloride freeze-dry preparation and preparation method thereof
InactiveCN102166198ASimple recipeLittle side effectsOrganic active ingredientsPowder deliverySolubilityMANNITOL/SORBITOL
The invention provides a stable naloxone hydrochloride freeze-dry preparation, comprising naloxone hydrochloride and mannitol, wherein the weight ratio of the naloxone hydrochloride to the mannitol is 1:(30-200). The freeze-dry preparation has the advantages of simple formula and fewer side effects; and moreover, by using an advanced freeze-drying process, the prepared product has the advantages of full appearance, good quick solubility and excellent quality.
Owner:重庆健能医药开发有限公司
Naloxone hydrochloride dropping pills
InactiveCN101036650AQuick effectImprove bioavailabilityOrganic active ingredientsAntinoxious agentsSide effectIntramuscular injection
A naloxone hydrochloride pill is disclosed in the invention, which is formed by naloxone hydrochloride, naloxone free alkali or the other naloxone acceptable in medicine having a ratio by weight of 1:5~10000 with pharmaceutic edjuvant based on general pill preparation process. Active ingredient of the naloxone hydrochloride pill in the invention can directly participate intracorporeal circulation after absorbed by capillary tube under oral mucosa of a patient, thus has advantages of fast absorption and high bioavailability, additionally, suffering of intramuscular injection of the patient is prevented. In particular, the drugs of the invention can be taken with no requirement of use situation, so that it is convenient for taking the medicine which dosage is controllable and has little side effect.
Owner:薛京 +1
Pharmaceutical composition of naloxone hydrochloride injection and preparation method of pharmaceutical composition
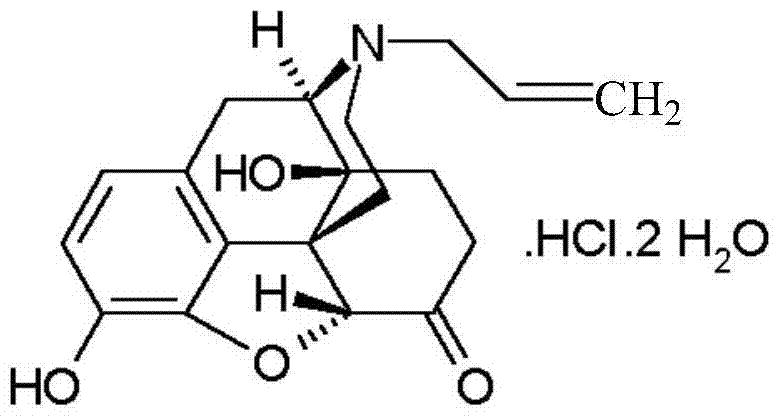
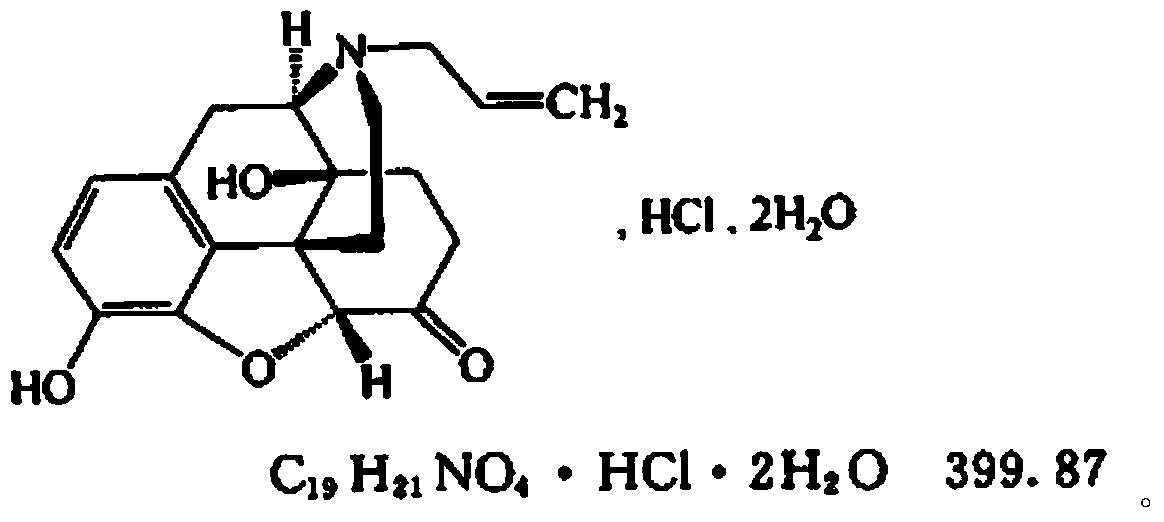
ActiveCN104127380AWake upRescue from acute alcohol poisoningOrganic active ingredientsNervous disorderEpoxyMethadone hydrochloride
The invention belongs to the technical field of medicines, relates to a pharmaceutical composition of a naloxone hydrochloride injection and a preparation method of pharmaceutical composition, and particularly relates to a pharmaceutical composition of a naloxone hydrochloride injection. The pharmaceutical composition comprises naloxone hydrochloride, and particularly comprises naloxone hydrochloride, saccharides, organic acid and injection water. The naloxone hydrochloride in the pharmaceutical composition is added to the composition in a form of 17-allyl-4,5alpha-epoxy-3,14-dihydroxy-pyran-6-methadone hydrochloride dehydrate; the saccharides are selected from one or more of the following components: mannitol, sorbitol, lactose, maltose, glycine, mycose, glucose and the like. The pharmaceutical composition disclosed by the invention can be used after opioid compound anaesthesia to resist respiratory depression caused by the medicine and prompt patients to revive, is used for respiratory depression caused by excessive opioids and complete or partial reversion of the opioids, is used for saving acute alcohol poisoning, and is used for diagnosing excessive acute opioids.
Owner:CHENGDU TIANTAISHAN PHARMA
Sublingual pellicles containing naloxone hydrochloride and preparing method thereof
InactiveCN1813740ADissolution rate is fastFast absorbing effectOrganic active ingredientsNervous disorderPlasticizerWater soluble
The present invention relates to a sublingual membrane preparation containing naloxone hydrochloride. Said preparation composition includes medicinal effective component naloxone hydrochloride, ethylene homopolymer or water-soluble cellulose derivative membrane material and water-soluble plasticizer. Besides, said invention also provides its preparation method and concrete stops.
Owner:岳振江
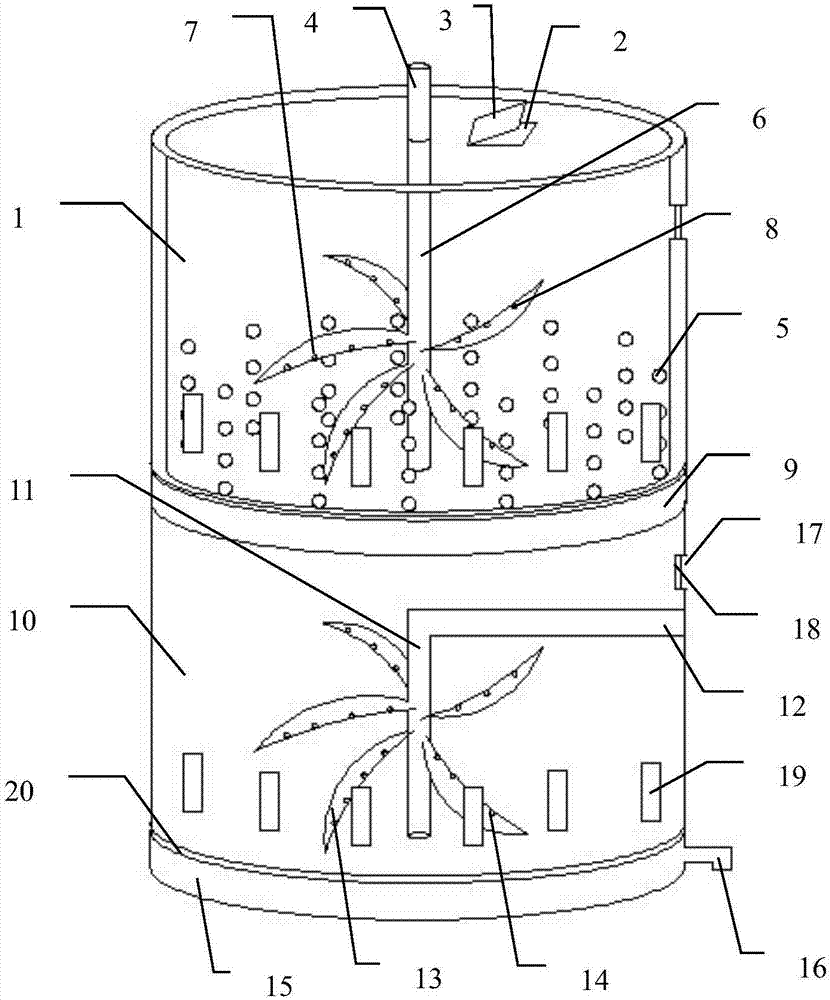
Method for eliminating static electricity in micro-pill room
ActiveCN102697660AReduce frictionPrevent or reduce morbidityPharmaceutical product form changeGranular deliveryElectricityCurative effect
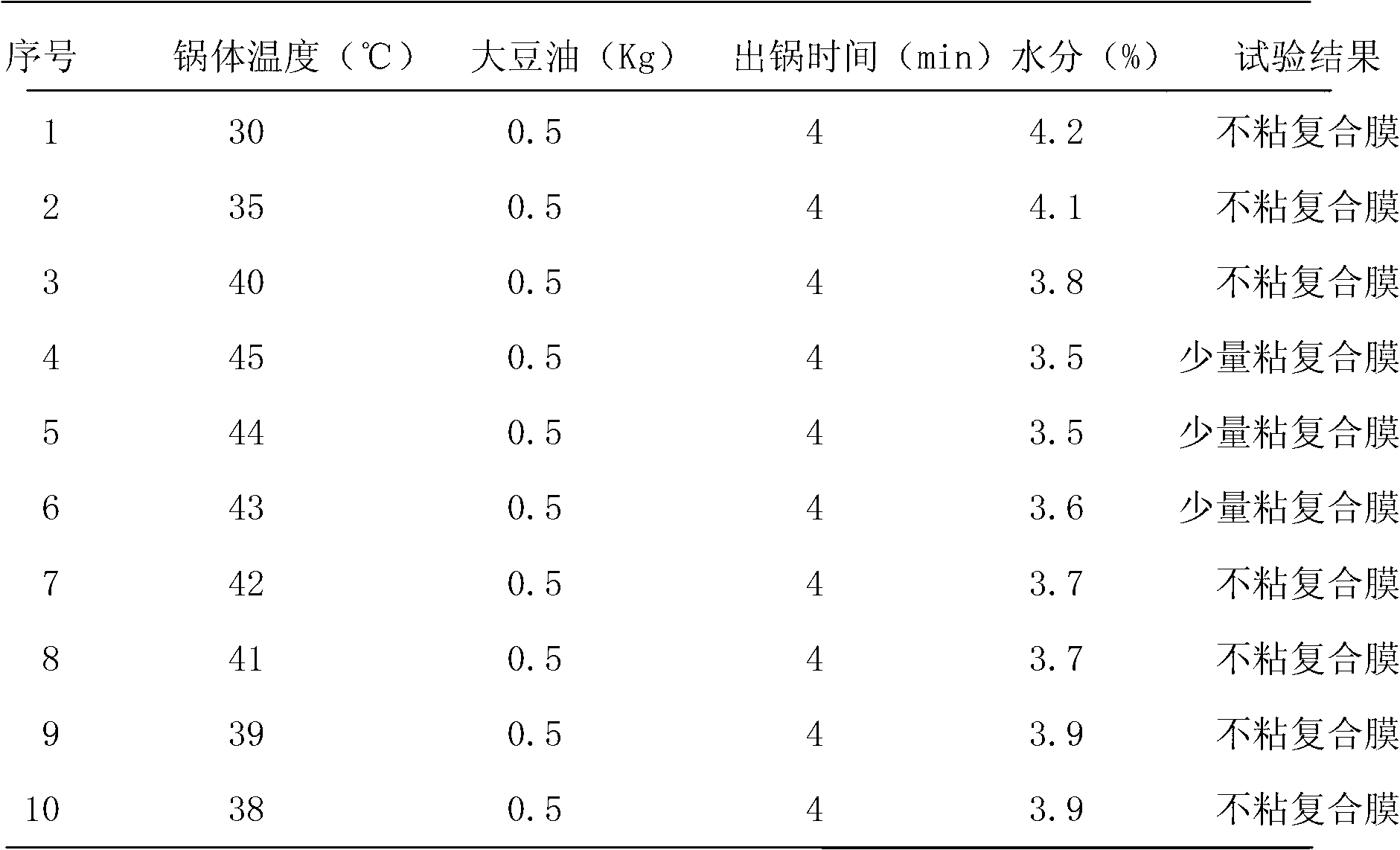
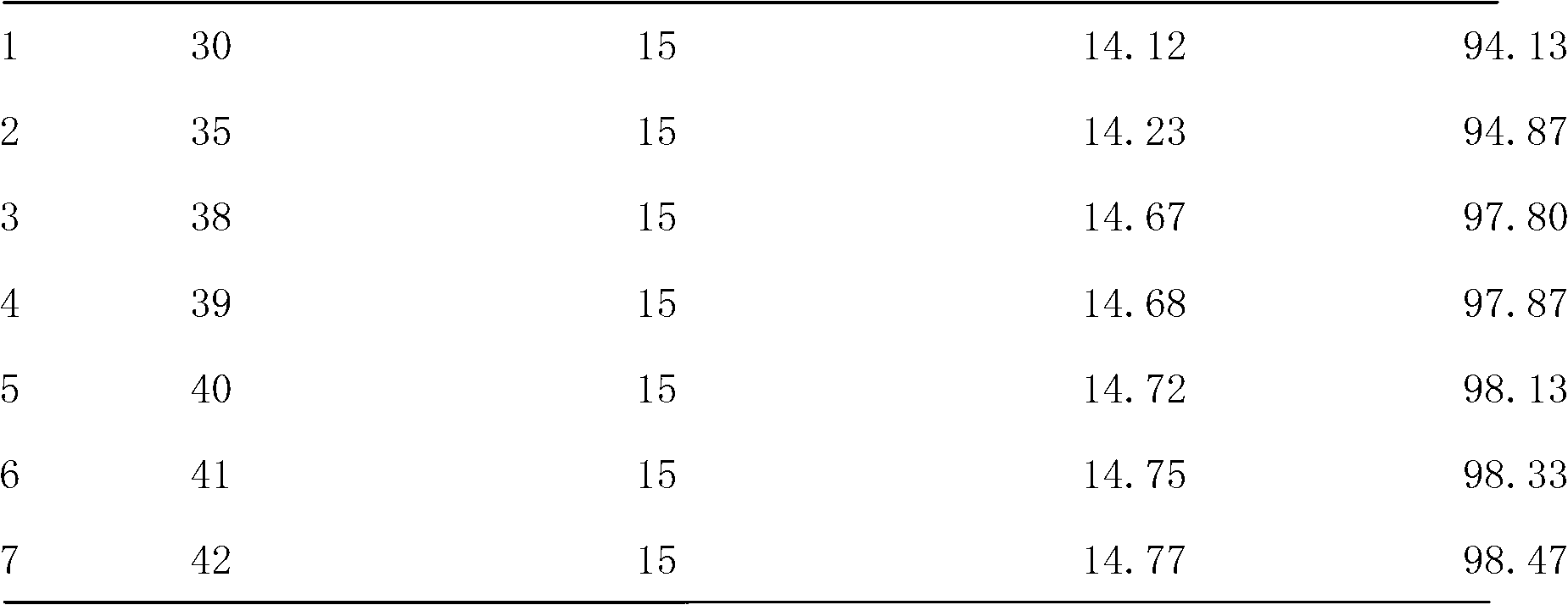
The invention discloses a method for eliminating static electricity in a micro-pill room. The method is characterized by comprising the following steps of: coating micro-pills, and spraying medicinal soybean oil which is 0.03-0.05 percent by weight the mass of the micro-pills into a coating granulator, wherein during spraying of the medicinal soybean oil, the rotating speed of a host machine of the coating granulator is controlled at 50-70 revolutions per minute, the blowing pressure is controlled at 0.2-0.4Pa, and the temperature of a boiler body of the coating granulator is controlled to be less than or equal to 43 DEG C; and after the surfaces of the micro-pills are coated with a layer of medicinal soybean oil, taking out and transferring into a boiling drying furnace for drying. The medicinal soybean oil is added, so that the problem of electric electricity in a pill room is solved, the quality of naloxone hydrochloride pills for injection is ensured, and linoleic acid in soybean oil can play a certain role in enhancing the medicinal effect of a medicament.
Owner:GUIZHOU JINGFENG INJECTION
Naloxone hydrochloride crystal form compound
ActiveCN105503888AOrganic active ingredientsNervous disorderCombinatorial chemistryMedicinal chemistry
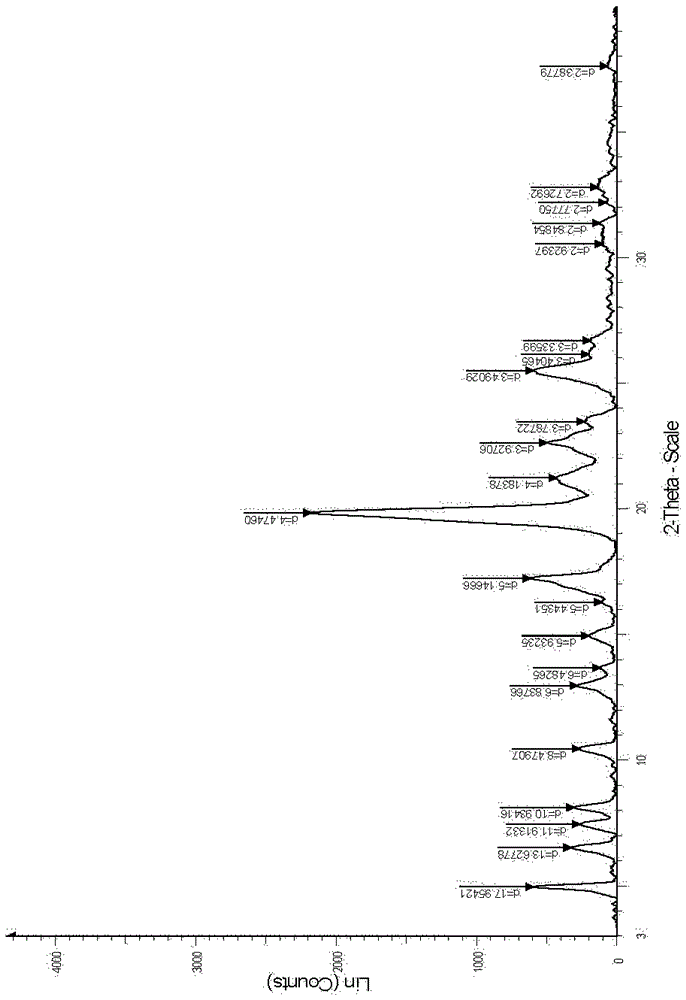
The invention belongs to the technical field of medicines, and particularly relates to a naloxone hydrochloride crystal form compound shown as formula (I). The X-ray powder diffraction pattern obtained by measuring the compound with Cu-Ka rays is shown in figure 1. The naloxone hydrochloride crystal form compound provided by the invention has improved stability; compared with the preparation in the prior art, the preparation prepared by adopting the naloxone hydrochloride crystal form compound disclosed by the invention has a better medicinal effect.
Owner:ZHENGZHOU SIHUAN MEDICINE ARTICLE CO LTD
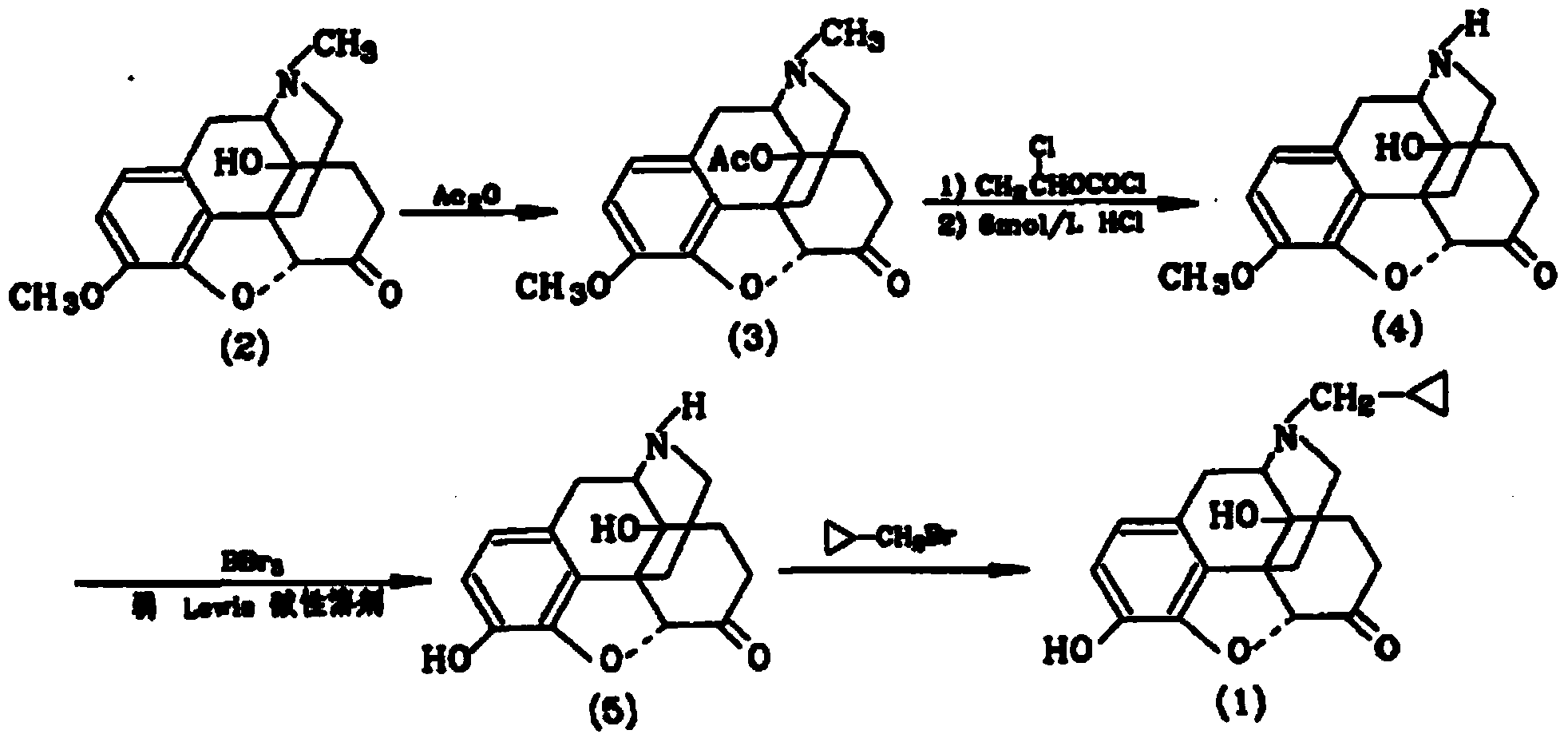
Synthetic method of naloxone hydrochloride
The invention belongs to the technical field of medicament synthesis, and relates to a synthetic method of naloxone hydrochloride. The method comprises the following steps: by adopting thebaine as a starting material, oxidizing and then reducing thebaine to obtain a compound 2, reacting the compound 2 with acetic anhydride to obtain an ester compound 3, then removing methyl and performing hydrolysis to obtain a compound 4, reacting the compound 4 with an alkylating reagent (such as the reagent selected from chloropropene, bromopropene, iodopropylene or the combination thereof), performing allylation on the Nth bit of a mother nucleus structure to obtain a compound 5, then removing methyl to obtain naloxone free alkali, then forming acid addition salts with hydrochloric acid, and then refining to obtain naloxone hydrochloride which can be used as a medicinal raw material medicament. By adopting the method disclosed by the invention, a high-quality medicinal raw material medicament can be prepared.
Owner:CHENGDU TIANTAISHAN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com