Pharmaceutical composition containing active component, namely nalmefene hydrochloride
A technology of nalmefene hydrochloride and active ingredients, which is applied in the field of medicine and can solve problems such as dissatisfaction, drug safety, effectiveness risk, failure to achieve the stable effect of the instructions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
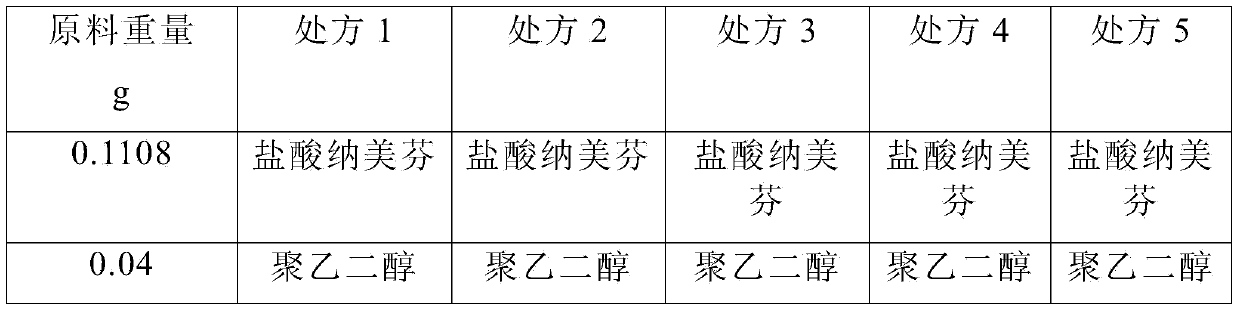
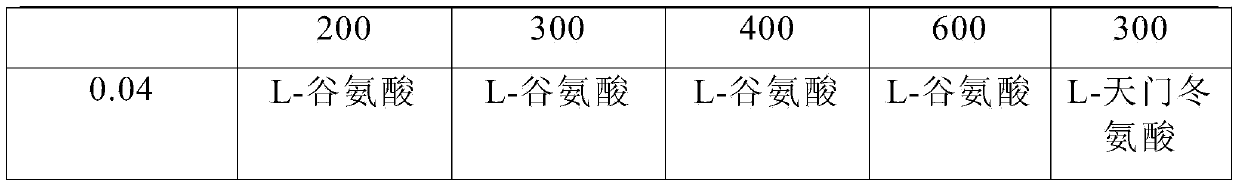
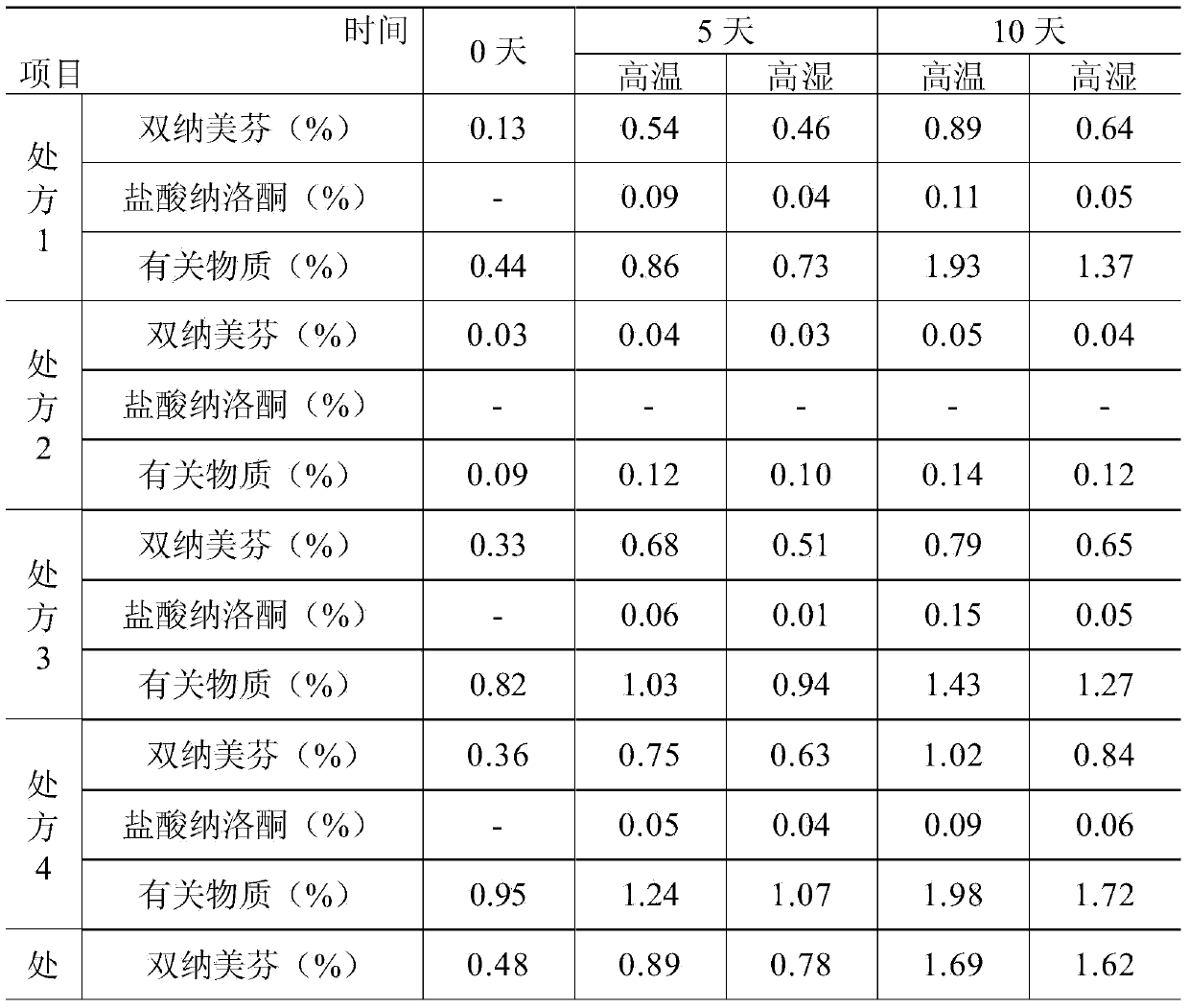
[0054] Under clean conditions, take 7000ml of water for injection at a temperature of 30-40℃, add 0.4g polyethylene glycol 300 to the water for injection, stir to dissolve, add 0.3g L-glutamic acid, stir evenly, add 0.05% (g / ml) activated carbon, stirred and adsorbed for 15 minutes, filtered to remove the carbon, added 1.108g of nalmefene hydrochloride, added 9g of sodium chloride, added water for injection to 10000ml, filtered through a 0.22μm microporous membrane, filled; heated at 121℃ Autoclave for 20 minutes; light inspection; put in storage; get nalmefene hydrochloride injection
Embodiment 2
[0056] Under clean conditions, take 7000ml of water for injection at a temperature of 30-40℃, add 0.5g of polyethylene glycol 300 to the water for injection, stir to dissolve, add 0.35g of L-glutamic acid, stir evenly, add 0.05% (g / ml) activated carbon, stirred and adsorbed for 15 minutes, filtered to remove the carbon, added 1.108g of nalmefene hydrochloride, added 9g of sodium chloride, added water for injection to 10000ml, filtered through a 0.22μm microporous membrane, filled; heated at 121℃ Autoclave for 20 minutes; light inspection; put in storage; then nalmefene hydrochloride injection is obtained.
Embodiment 3
[0058] Under clean conditions, take 7000ml of water for injection at a temperature of 30-40℃, add 0.55g polyethylene glycol 300 to the water for injection, stir to dissolve, add 0.6g L-glutamic acid, stir evenly, add 0.05% (g / ml) activated carbon, stirred and adsorbed for 15 minutes, filtered to remove the carbon, added 1.108g of nalmefene hydrochloride, added 9g of sodium chloride, added water for injection to 10000ml, filtered through a 0.22μm microporous membrane, filled; heated at 121℃ Autoclave for 20 minutes; light inspection; put in storage; then nalmefene hydrochloride injection is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com