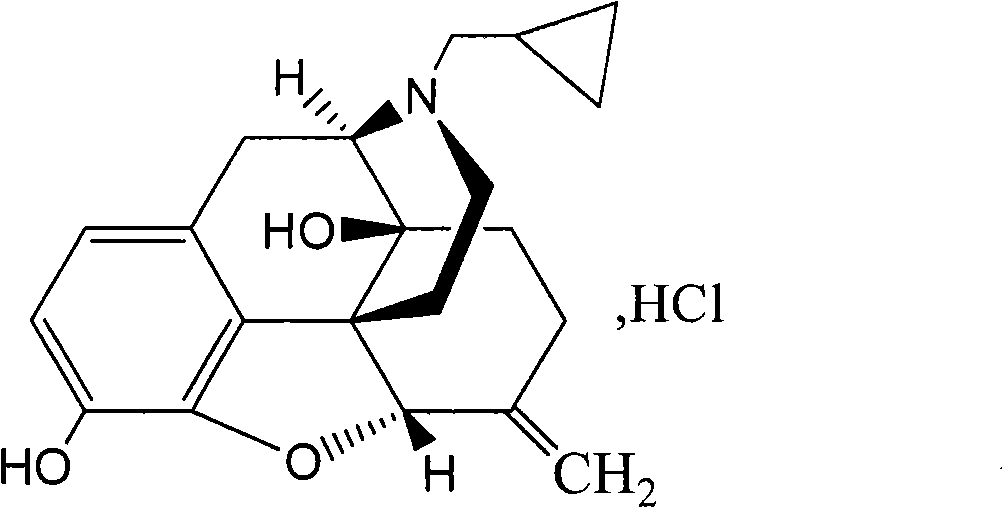
Patents
Literature
54 results about "NALMEFENE HYDROCHLORIDE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Administration of nalmefene at the recommended doses did not prevent analgesia with subsequently administered opioids {01} {19}. Parenteral Dosage Forms Note: The dosing and strengths of the dosage form available are expressed in terms of nalmefene base (not the hydrochloride salt) {01}. NALMEFENE HYDROCHLORIDE INJECTION Usual adult dose
Stabilized nalmefene hydrochloride injection and its preparation
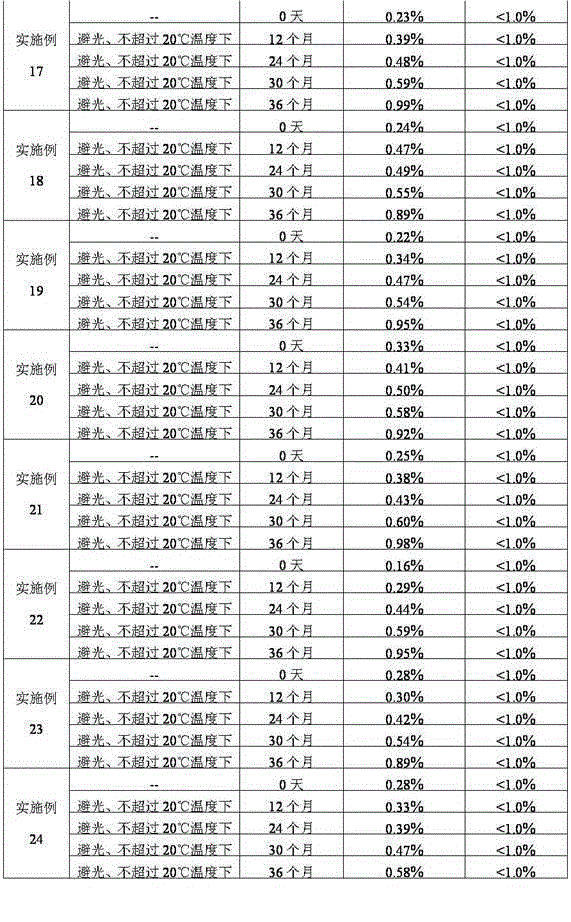
ActiveCN1895251AReasonable compositionSimple processOrganic active ingredientsNervous disorderGlucose polymersD-Glucose
A high-stability nalmefene hydrochloride injection is prepared from nalmefene hydrochloride (0.005-0.2 W / v %) and the medicinal carrier chosen from sodium chloride, glucose, beta-cyclodextrin, dextran, pectose, sorbitol, etc. Its preparing process is also disclosed.
Owner:西藏易明西雅医药科技股份有限公司
Nalmefene injection and preparation method thereof
ActiveCN101406474AReasonable compositionSimple processOrganic active ingredientsNervous disorderActive componentHigh heat
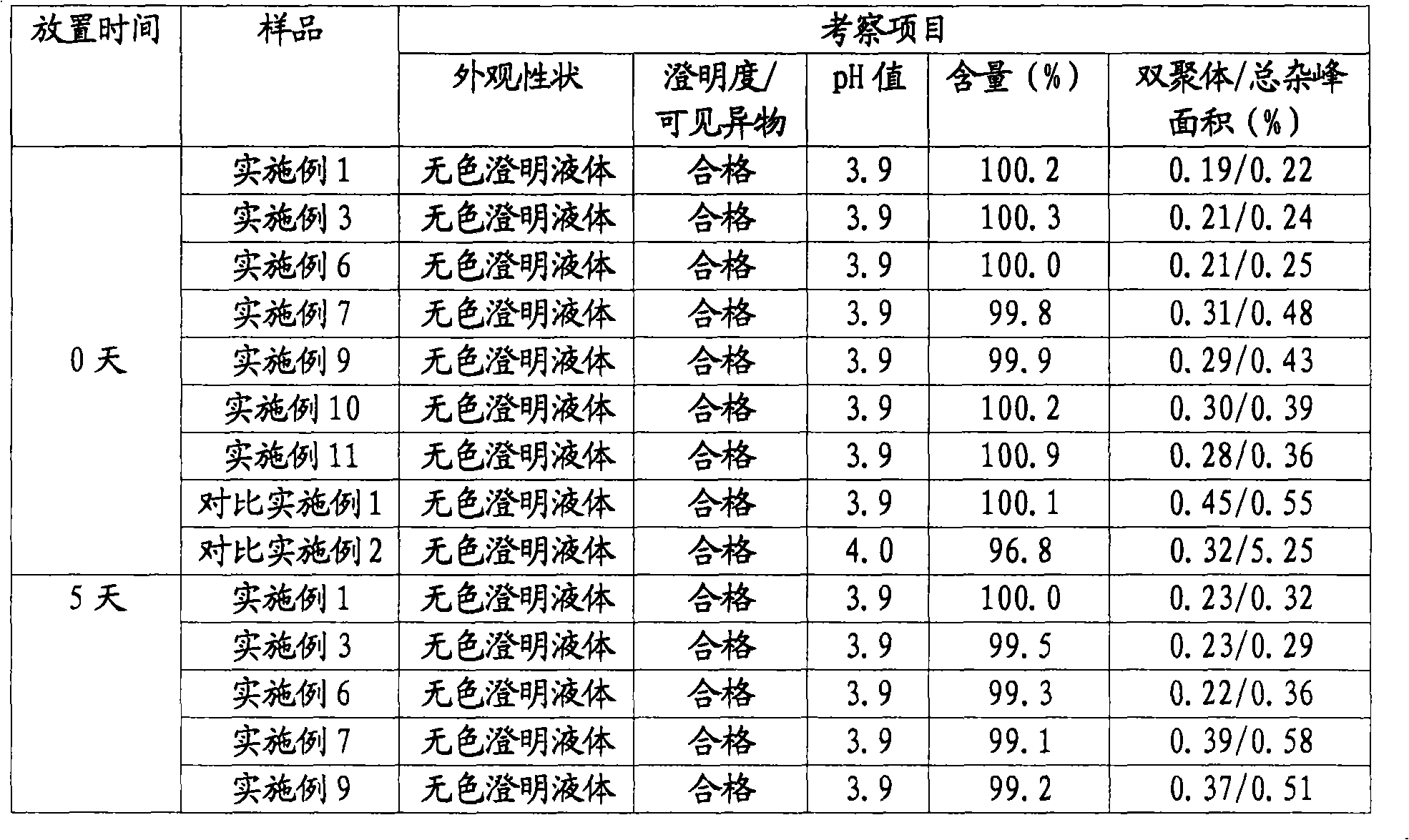
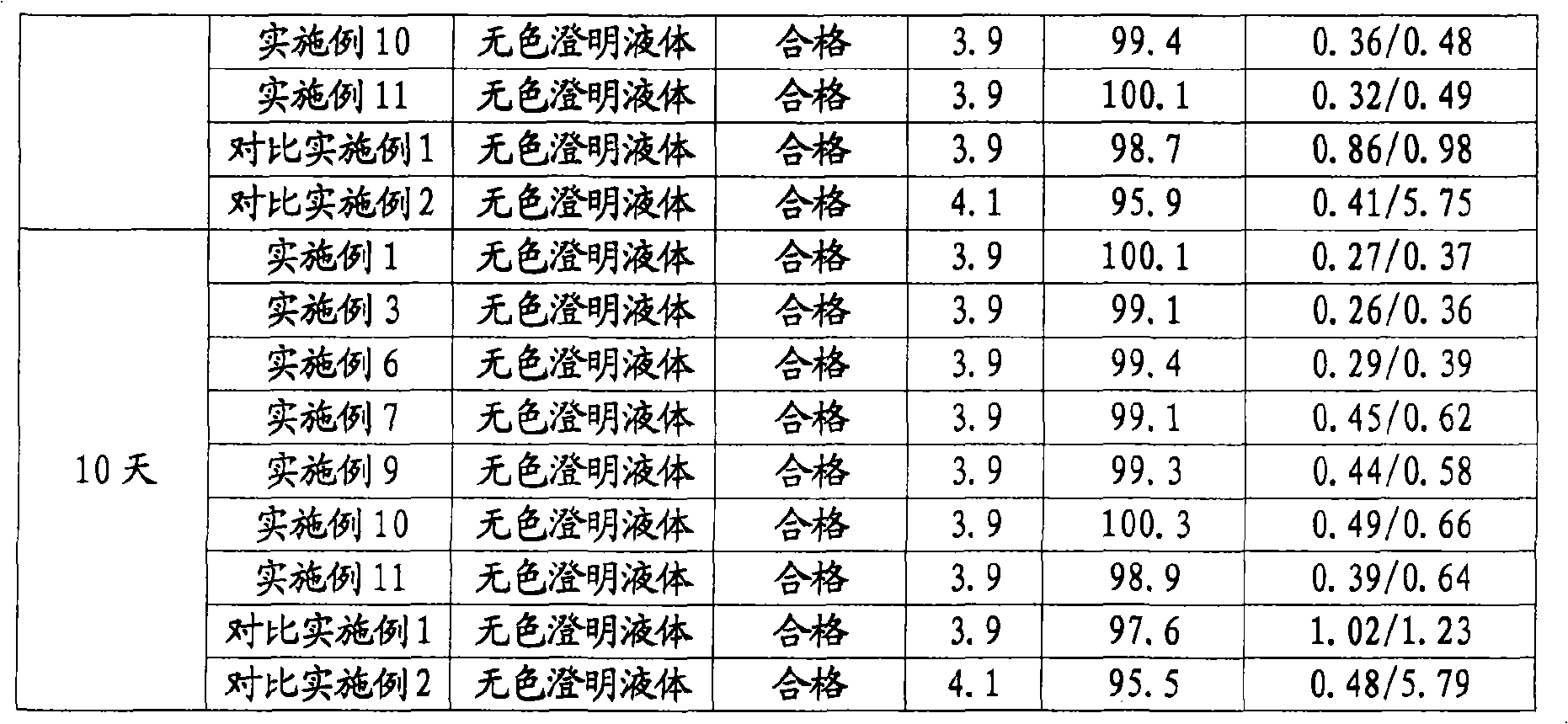
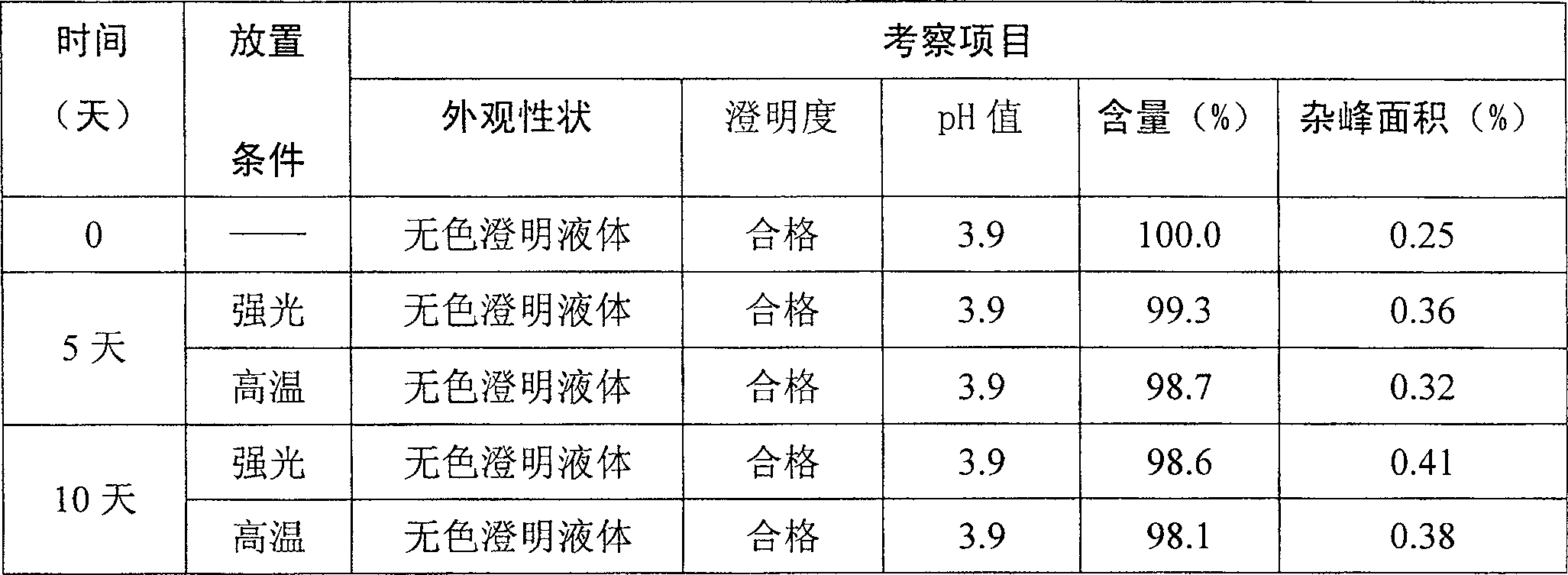
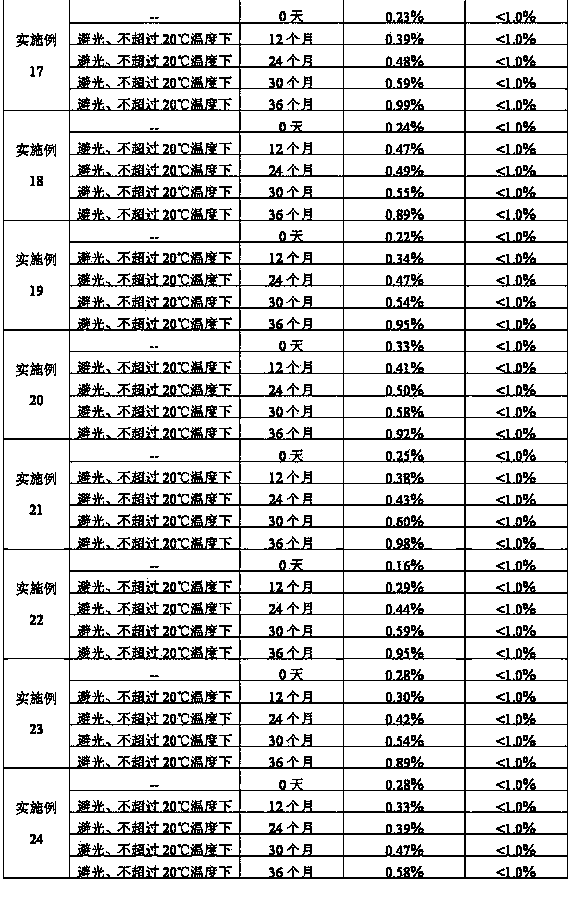
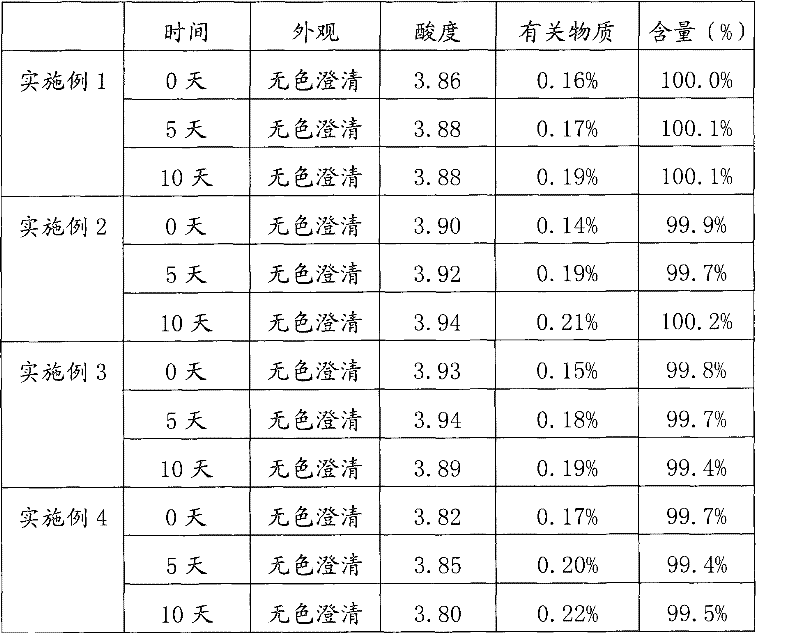
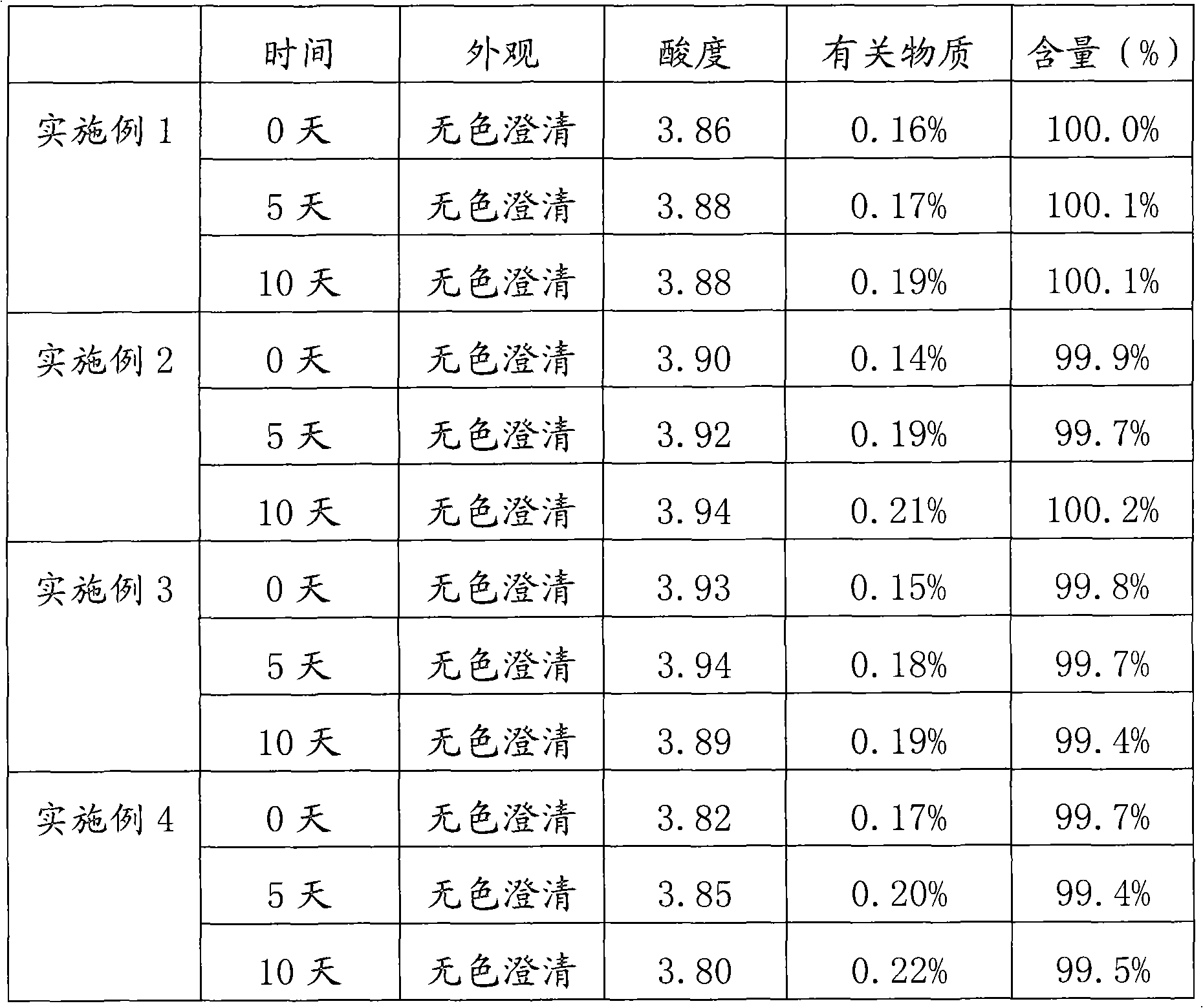
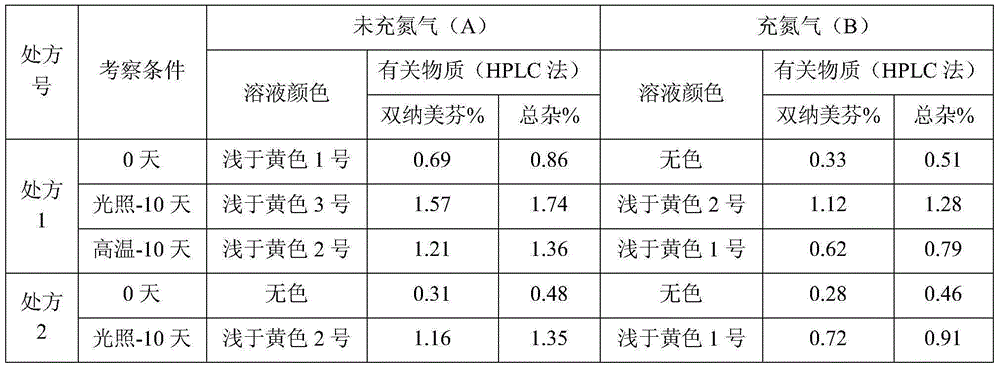
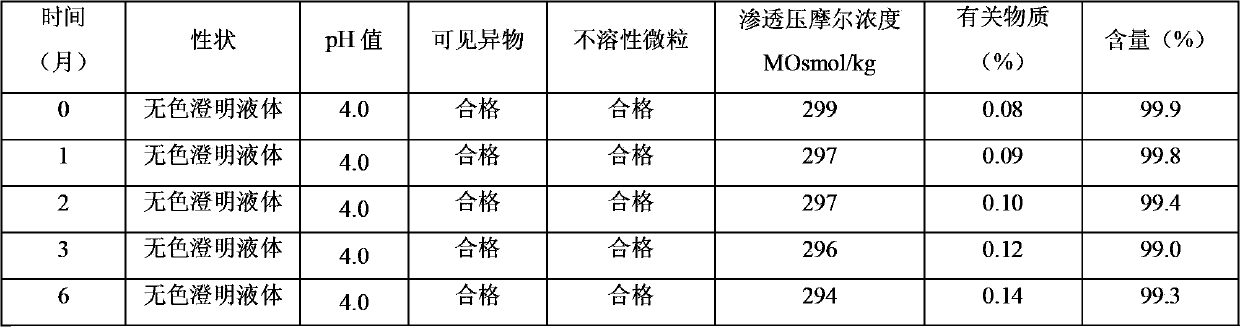
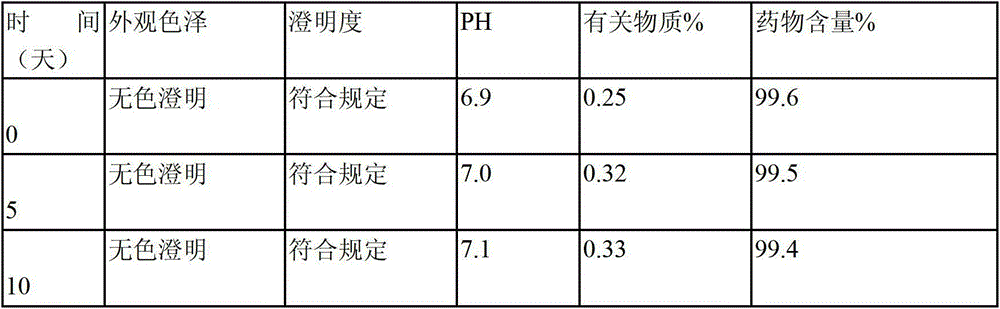
The invention relates to a stable nalmefene injection and a method for preparing the same. The nalmefene hydrochloride injection has the advantages of reasonable formulation, simple process, and good stability. After a test of keeping stand the injection under high temperature condition for 10 days and a test of accelerating at the temperature of 40 DEG C for 6 months, the appearance property, the pH value, the content of active components, the related substances and the like of a sample are not changed obviously.
Owner:云南龙海天然植物药业有限公司
Nalmefene hydro chloride lyophilized powder formulation for injection
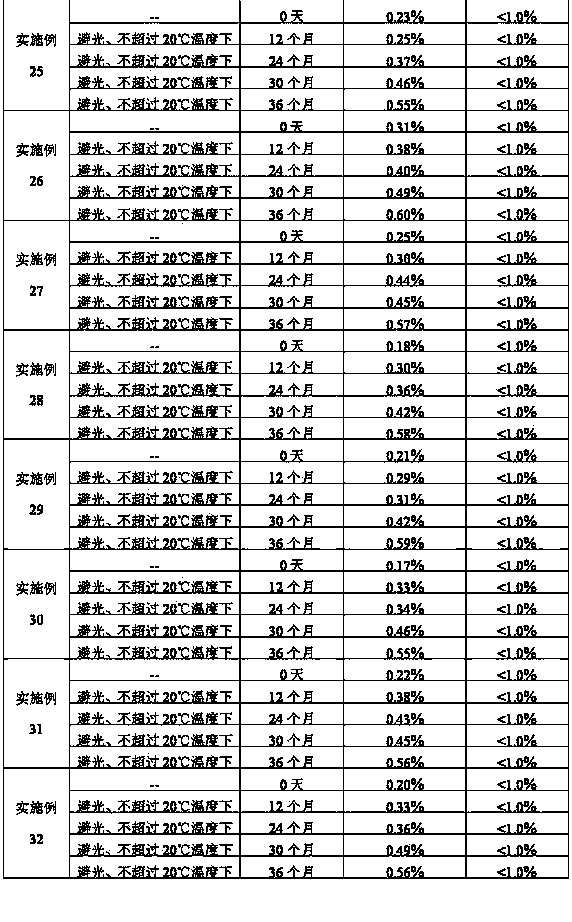
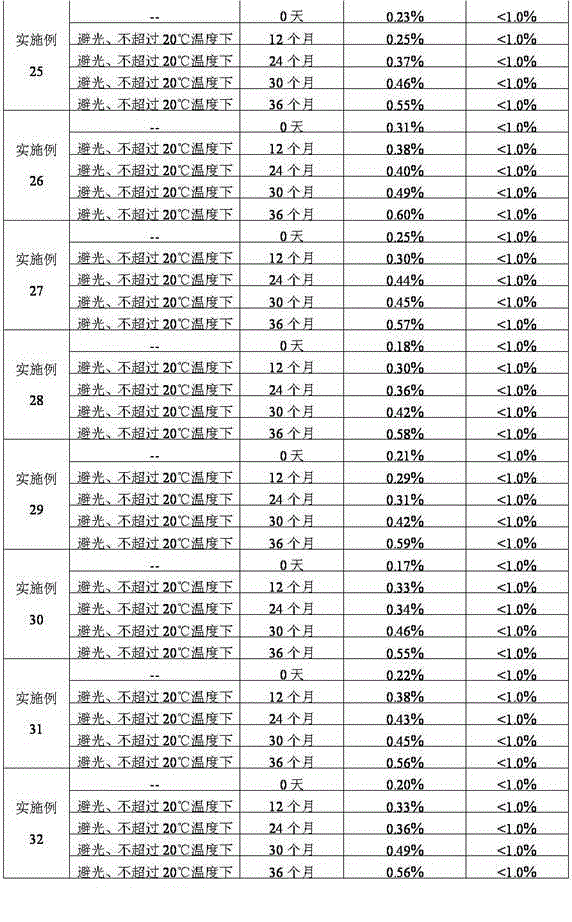
InactiveCN1813739AImprove solubilitySimple manufacturing processOrganic active ingredientsPowder deliveryFreeze-dryingDextran
The present invention relates to a nalmefene hydrochloride freeze-dried powder injection preparation for injection. Said preparation is formed from effective component malmefene hydrochloride and proper medicinal carrier, in which the content range of nalmefene hydrochloride in the preparation is 0.1-4.5 mg generally, the medicinal carrier can be one or several kinds selected from mannitol, glucose, sodium chloride, beta-cyclodextrin, glucosan, fructose and sorbitol. The content range of said medicinal carrier can be 10-100 mg. the pH value of said preparation is 5.0-7.0.
Owner:王颖
Method for preparing nalmefene hydrochloride injection and prepared nalmefene hydrochloride injection
InactiveCN103202806AExcellent pHImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismSample appearanceAdditive ingredient
The invention discloses a method for preparing nalmefene hydrochloride injection. The method comprises the steps of adding a certain amount of citric acid or citrate auxiliary material to the preparation when the nalmefene hydrochloride injection is prepared according to a common method, wherein the adding amount is 5-15g of citric acid or citrate added to each liter of injection; firstly, taking the prescribed auxiliary material in preparation, adding injection water to dissolve, adding active carbon to agitate and adsorb, filtering and removing impurities; adding the prescribed nalmefene hydrochloride under the protection of nitrogen, fully dissolving and sizing to an appointed constant volume by water, adjusting the pH value to 3.5-4.5; and finally conducting split charging, charging nitrogen, sealing and sterilizing after filtering by a filter. By adopting the method disclosed by the invention, the validity period of the nalmefene hydrochloride injection can be obviously prolonged; a specific citric acid pH value stabilizer is adopted; and the nalmefene hydrochloride injection has good stability, and stable sample appearance, pH value, effective ingredient content and related substances, and the like. Therefore, the safety of medication is ensured.
Owner:ANHUI HEALSTAR PHARM CO LTD
Pharmaceutical composition containing nalmefene hydrochloride and preparation method of same
ActiveCN102415993ASimple ingredientsLess impuritiesOrganic active ingredientsNervous disorderEthylene diamine tetra aceticEthylene diamine
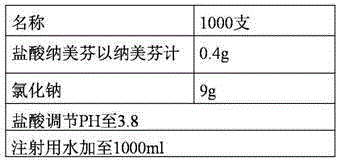
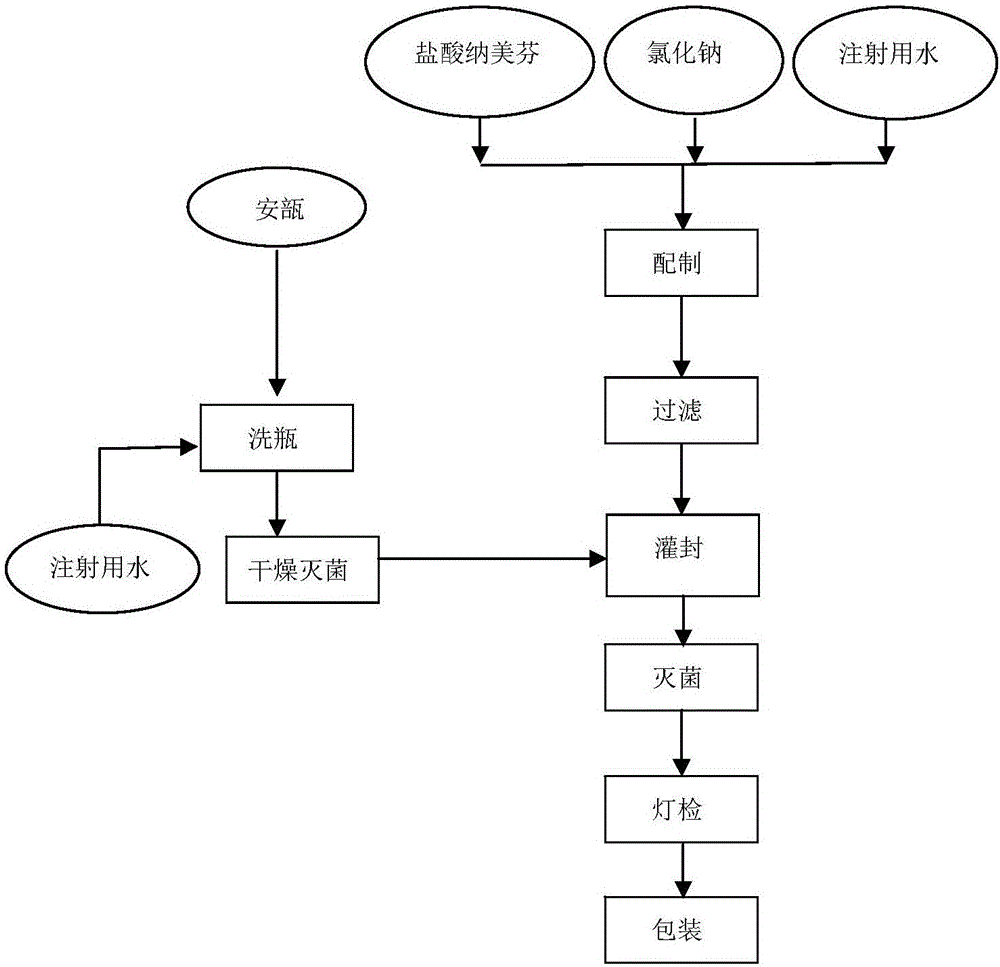
The invention relates to a pharmaceutical composition containing nalmefene hydrochloride. Nalmefene hydrochloride injection is a product of the pharmaceutical composition. 1ml injection consists of 0.1mg or 1mg nalmefene hydrochloride, 9.0 mg sodium chloride, 0.3mg ethylene diamine tetraacetic acid, hydrochloric acid and the remaining of water for injection, wherein the hydrochloric acid is used for regulating pH of the injection to 3.75-3.85. A preparation method of the pharmaceutical composition comprises the following steps of: mixing the nalmefene hydrochloride, the sodium chloride and the ethylene diamine tetraacetic acid, dissolving the mixture in the water for injection, uniformly mixing the mixture, and adding the water for injection to full; adding 0.1mol / L hydrochloric acid in to the mixture to regulate the pH value of medicine liquid to 3.75-3.85; filtering the medicine liquid by using a microporous filtering film with the thickness of 0.22 microns; and sterilizing the medicine liquid in a water bath with the temperature of 121 DEG C for 15 minutes to obtain the nalmefene hydrochloride injection. The pharmaceutical composition containing the nalmefene hydrochloride is simple components; and the product has the advantages of less foreign matters as well as high stability and safety based on tests on influencing factors, long-term stability and safety.
Owner:ZHUHAI TONGYUAN PHARMA CO LTD
Nalmefene injection and preparation method thereof
ActiveCN101406474BImprove stabilityOrganic active ingredientsNervous disorderActive componentHigh-temperature corrosion
Owner:云南龙海天然植物药业有限公司
Stabilized nalmefene hydrochloride injection and its preparation
ActiveCN100536848CReasonable compositionSimple processOrganic active ingredientsNervous disorderAcetic acidD-Glucose
The invention relates to a nalmefene hydrochloride injection for injection. The preparation is composed of an effective amount of nalmefene hydrochloride and an appropriate amount of pharmaceutical carrier, wherein the concentration of nalmefene hydrochloride in the preparation can be 0.005%-0.2%. (w / v), its content range in each unit preparation is generally 0.1-4mg; the pharmaceutical carrier can be one of sodium chloride, glucose, β-cyclodextrin, dextran, fructose, sorbitol, etc. Or several, preferably sodium chloride and glucose, its content range in each unit preparation can be 4.5mg~90mg, or glucose can be 25mg~400mg; Sodium bisulfite can be 0.005mg~0.5mg; Disodium amine tetraacetate can be 0.001mg ~ 0.5mg; appropriate amount of hydrochloric acid or acetic acid, adjust the pH value of the solution before dispensing this product to 3.5 ~ 5.5. The preparation process of nalmefene hydrochloride injection adopts a nitrogen filling process, and the sterilization conditions can be sterilized at 105°C for 45 minutes or autoclaved at 115°C for 20 minutes or autoclaved at 121°C for 15 minutes. It is preferably autoclaved at 115°C for 20 minutes. The appearance, clarity, pH value, content and related substances of nalmefene hydrochloride injection had no obvious changes after 10 days of strong light irradiation and high temperature storage and 6 months after accelerated test.
Owner:西藏易明西雅医药科技股份有限公司
Method for preparing high-purity nalmefene hydrochloride
ActiveCN103012416AReduce typesReduce contentOrganic active ingredientsNervous disorderOrganic solventImpurity
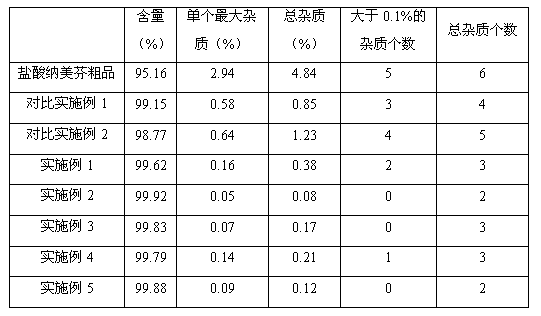
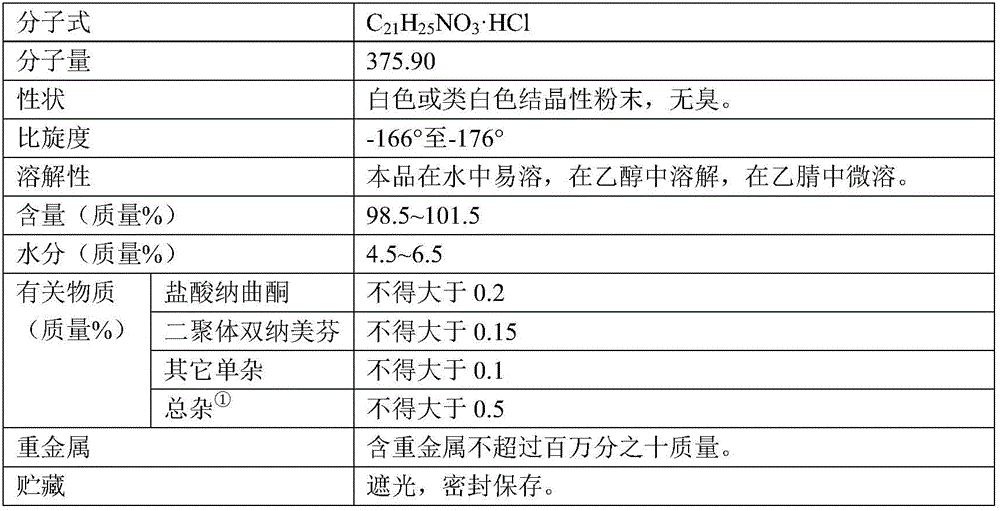
The invention relates to a method for preparing high-purity nalmefene hydrochloride, and in particular relates to a method for refining the nalmefene hydrochloride. The method comprises the steps of dissolving a crude product of the nalmefene hydrochloride in right amount of hot water, cooling and separating out the high-purity nalmefene hydrochloride. The method has the following advantages: (1) through refining, the content of the nalmefene hydrochloride is up to more than 99.5%, the content of the maximum single impurity is less than 0.2%, the content of total impurities is less than 0.5% and the prepared nalmefene hydrochloride is excellent in storage stability and suitable for preparing the preparations of parenteral administration; (2) the method is simple in process, simple and convenient for operation and low in cost and suitable for industrial production; and (3) as no organic solvent is used, the damage to operating personnel and environment is avoided, the finished product has no residual organic solvent problem, the quality inspection step is simplified and the medication safety is guaranteed.
Owner:LIAONING HAISCO PHARMACEUTICAL CO LTD
Medicinal use of nalmefene hydrochloride
ActiveCN102048733AReduce aggregation rateImproved prognosisOrganic active ingredientsDigestive systemFatty liverLiver function
The invention relates to medicinal use of nalmefene hydrochloride. The nalmefene hydrochloride can improve lipid metabolism and improve prognosis of a fatty liver patient by reducing the platelet aggregation rate, improving the liver function state and resisting oxidation. The nalmefene hydrochloride can serve as a medicine for resisting human / animal fatty liver.
Owner:ZHUHAI TONGYUAN PHARMA CO LTD
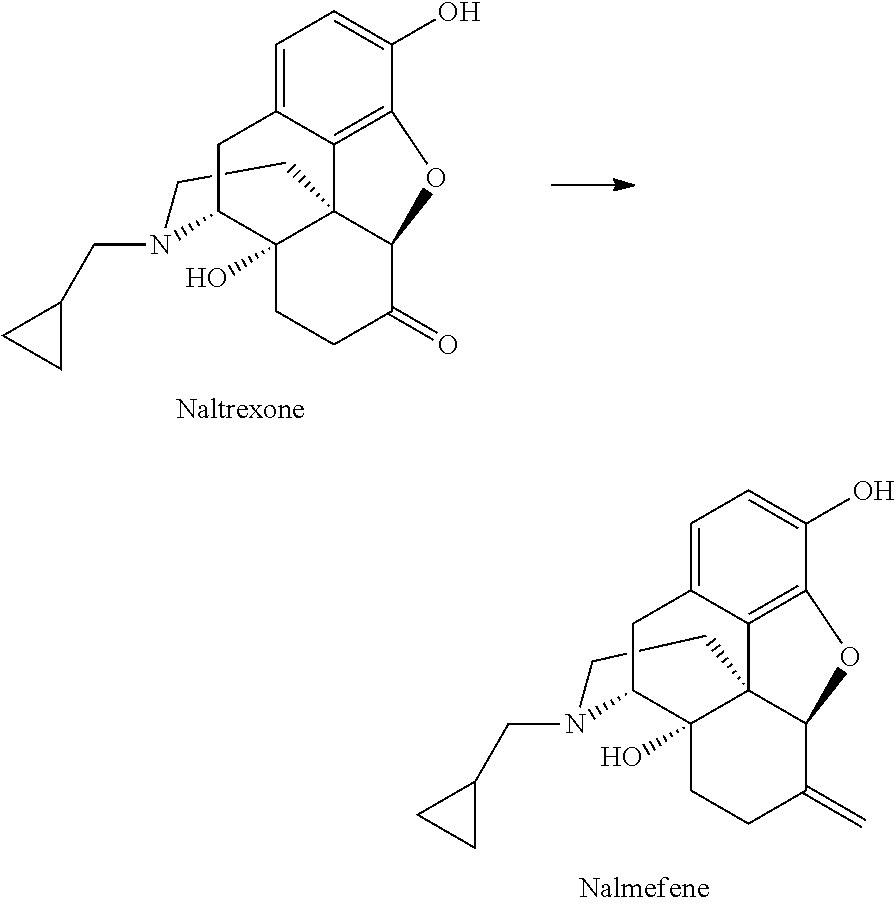
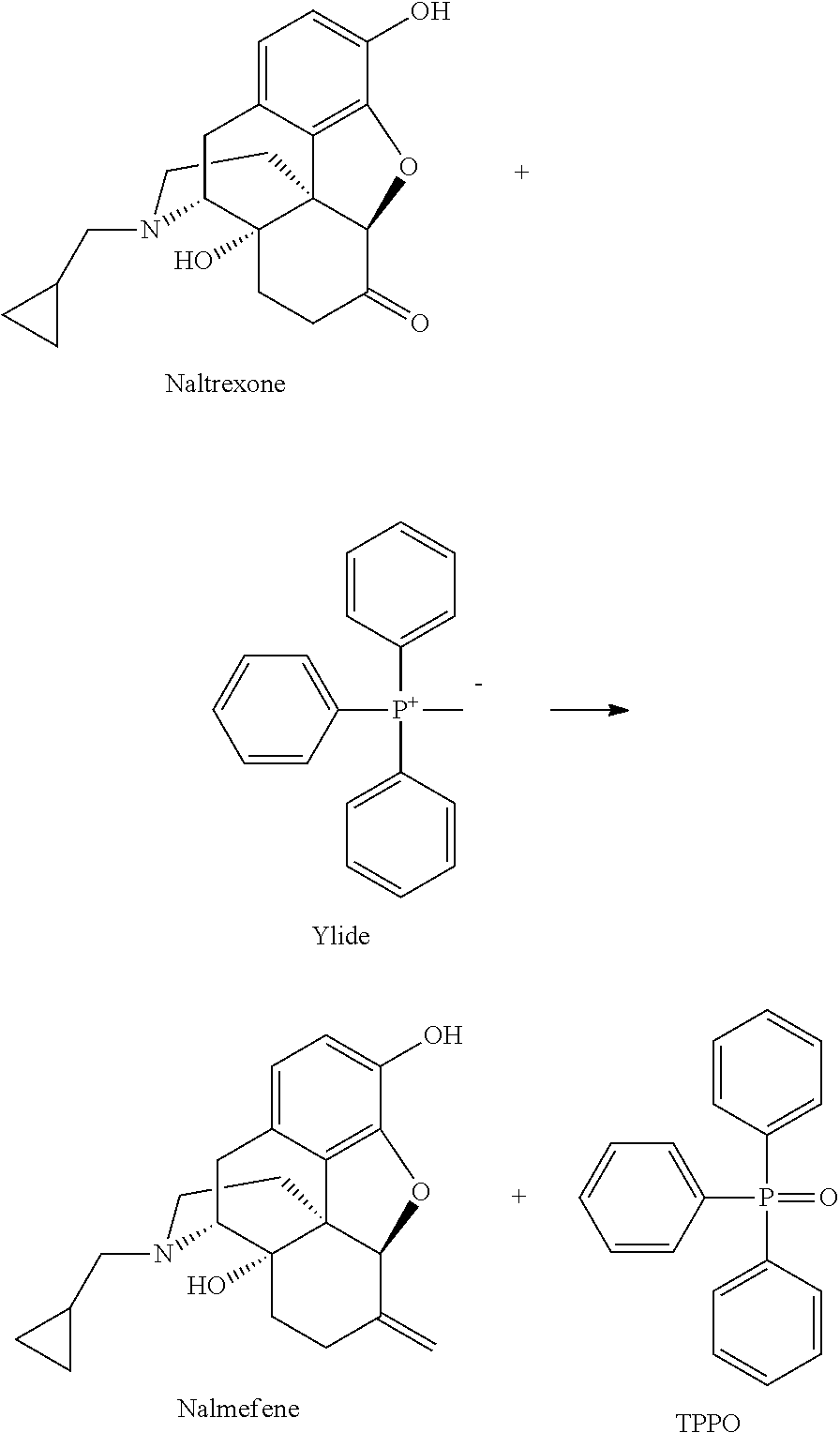
Preparation of nalmefene hydrochloride from naltrexone
Method for producing nalmefene hydrochloride from naltrexone, which method is particular well adapted for large-scale industrial application, and has been found to be efficient, to give a high yield and to afford highly pure nalmefene hydrochloride salt.
Owner:H LUNDBECK AS
Pharmaceutical composition containing nalmefene hydrochloride for injection
ActiveCN103705448AExcellent long-term storage stabilityMaintain binalmefene contentOrganic active ingredientsNervous disorderActivated carbonNalmefene
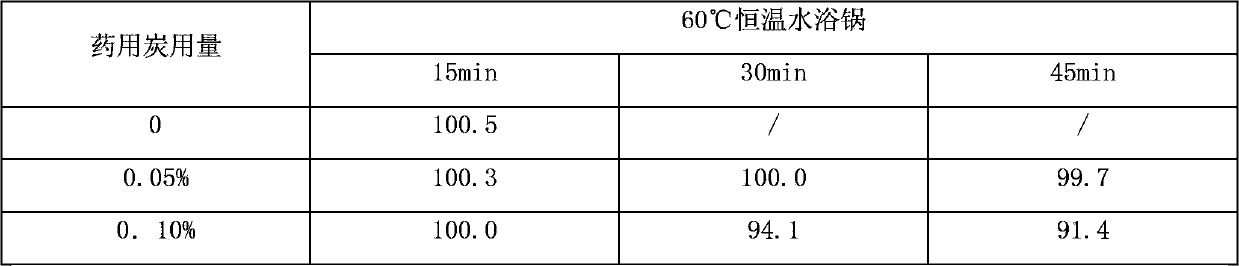
The invention discloses a formula of a pharmaceutical composition containing nalmefene hydrochloride for injection. The pharmaceutical composition is characterized in that an injection does not contain a chelating agent, and comprises effective dose of nalmefene hydrochloride and a defined amount of pharmaceutical carrier, wherein the concentration of the nalmefene hydrochloride in the injection can be 0.01-0.2 percent (w / v), the content of the nalmefene hydrochloride in each unit of injection is 0.1-2.0mg; an osmotic pressure regulator can be sodium chloride or glucose, preferably, sodium chloride, the content of the osmotic pressure regulator in each unit of injection is 4.5-22.5mg; the pH value of the injection is regulated to be 3.2-4.5 by using hydrochloric acid. The invention further discloses a preparation method of the formula of the injection. The preparation method comprises the steps of in a blending process, regulating the pH value of a solution to be 6.0-9.0 by using alkaline in first time, and after adsorbing by using active carbon, regulating the pH value of the solution to be 3.2-4.5 by using acid in second time. The formula of the injection containing the nalmefene hydrochloride is simple, and is stable in quality; the preparation method is simple in process, simple and convenient to operate, and more suitable for industrialized production.
Owner:CHENGDU GUOHONG PHARMA
Nalmefene hydrochloride injection and preparation method thereof
ActiveCN101658489BImprove stabilityEnsure safetyOrganic active ingredientsNervous disorderTert butylPhenol
Owner:BEIJING SIHUAN PHARMA +1
Pharmaceutical composition containing nalmefene hydrochloride for injection
ActiveCN103705448BExcellent long-term storage stabilityMaintain binalmefene contentOrganic active ingredientsNervous disorderActivated carbonNalmefene
Owner:CHENGDU GUOHONG PHARMA
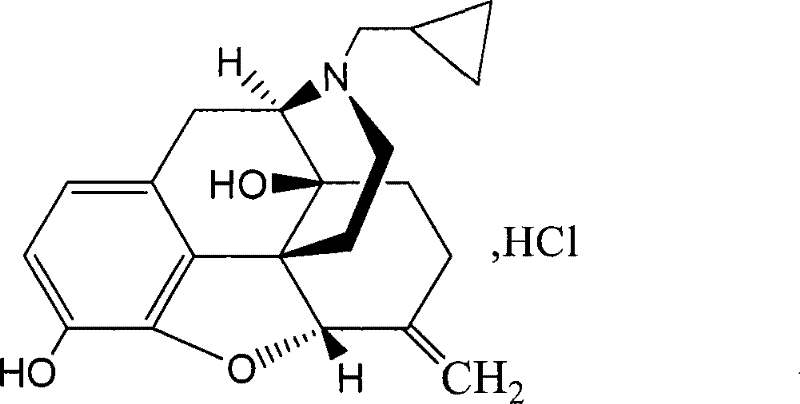
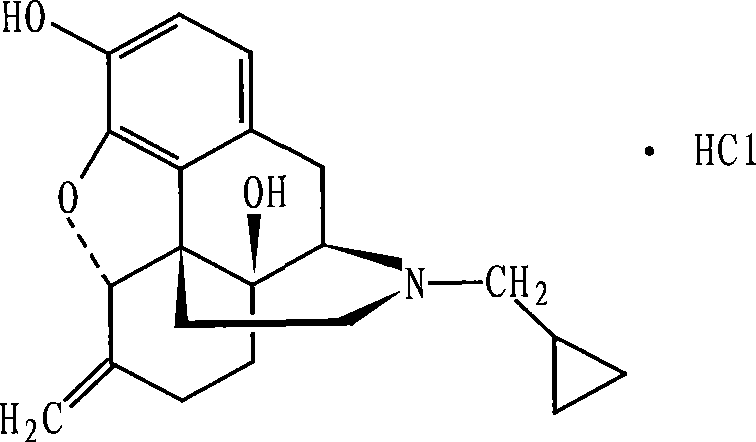
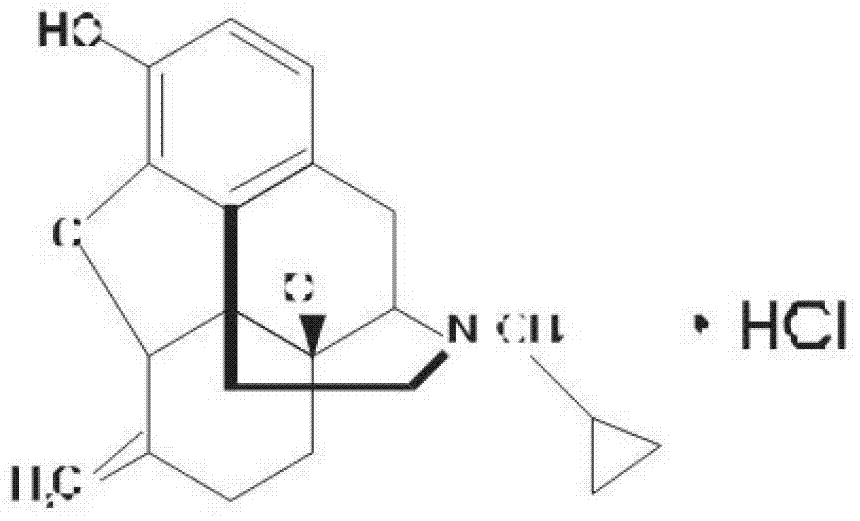
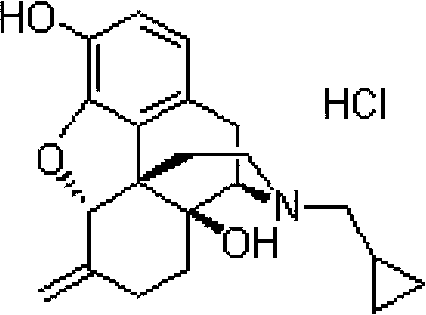
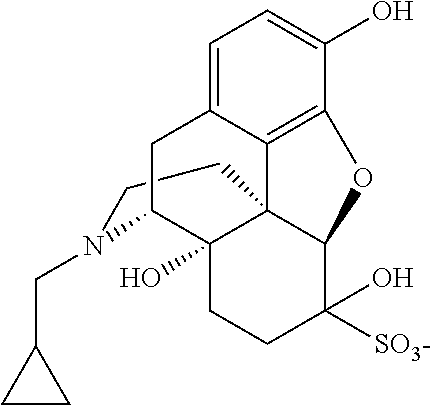
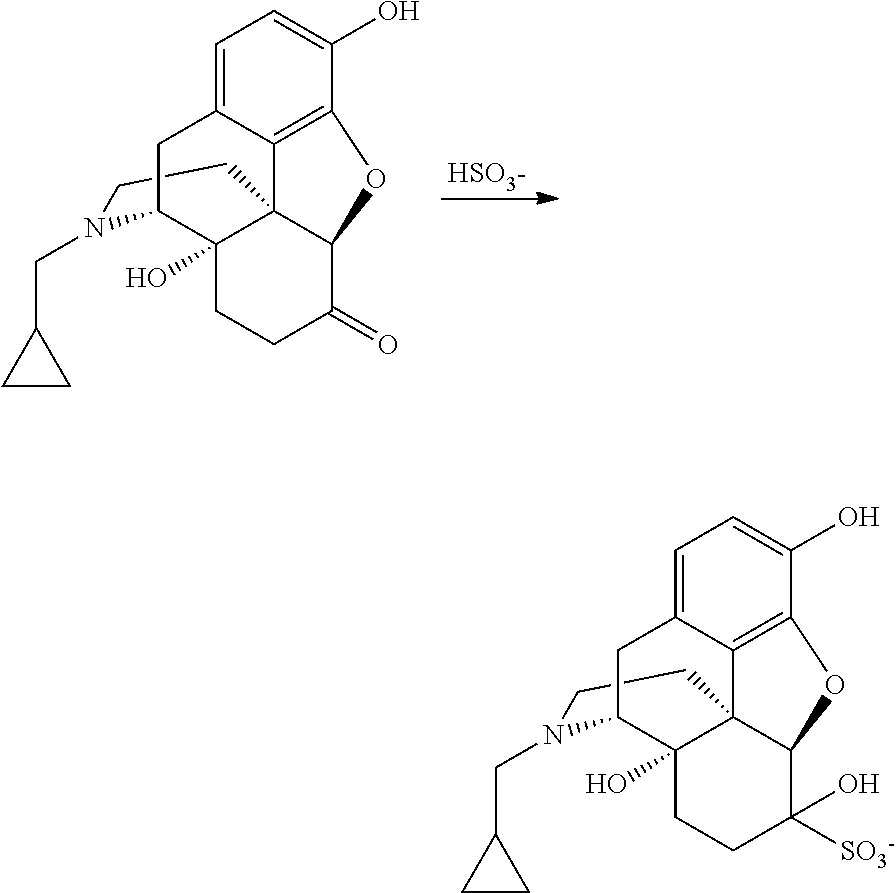
Nalmefene hydrochloride compound and preparation method thereof
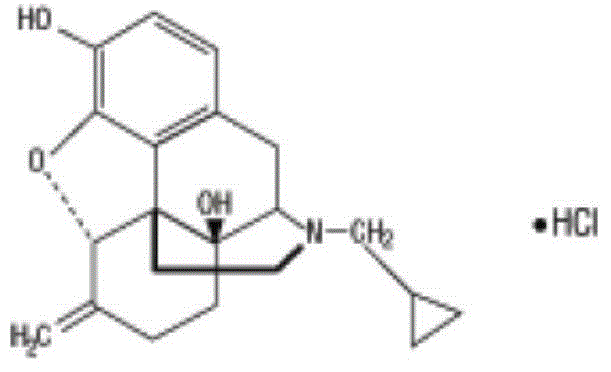
ActiveCN103204859ANot harmful to healthNo pollution in the processOrganic chemistryChemical structureAlcoholisms
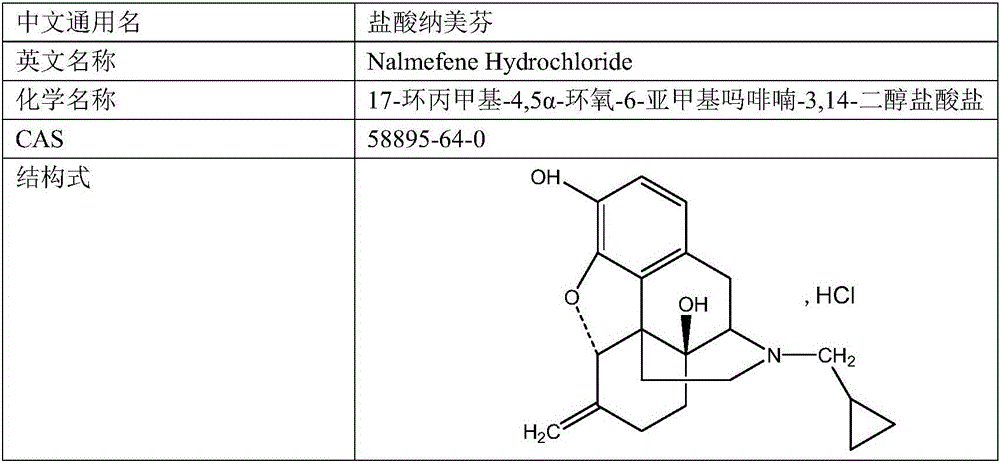
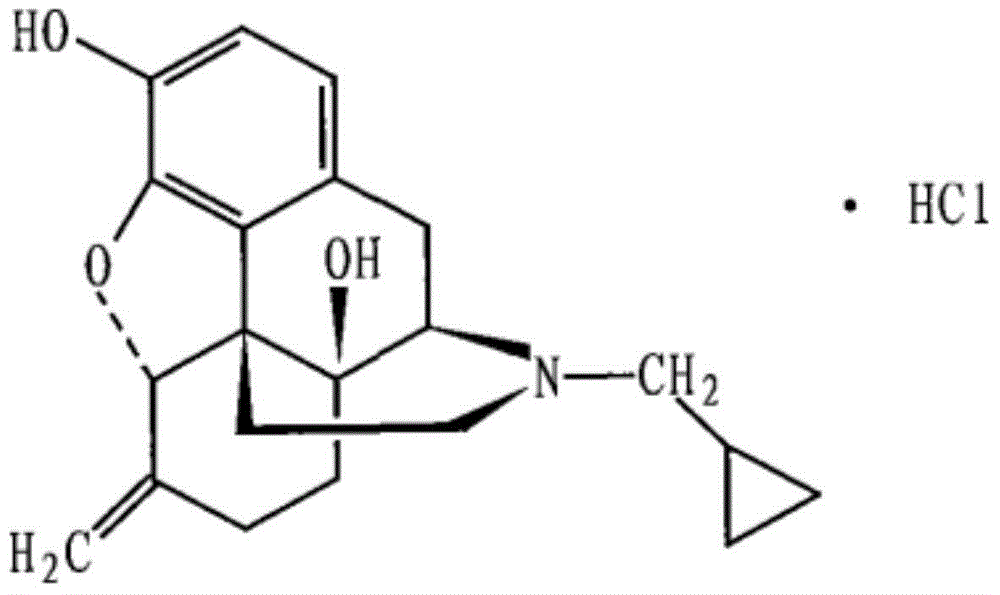
The invention belongs to a technical field of medicines. Specifically, the invention relates to a nalmefene hydrochloride compound and a preparation method thereof. Nalmefene hydrochloride is a derivative of water-soluble naltrexone, and due to the chemical structure of 6-bit methylene of the nalmefene hydrochloride, the nalmefene hydrochloride has the characteristics of long action time, more medication ways, high bioavailability, less side effects, stronger physiological activity, easier biofilm penetration and the like, has different degrees of effects on keeping the normal functions of breathing, circulation, digestion, endocrine and nervous systems, and is applied to the antagonism of respiratory depression of narcotic analgesics, the treatment of heart failure and shock, the treatment of alcoholism and addiction, weight loss and the like. According to the invention, the drug stability is improved, and the medication safety is ensured.
Owner:海思科制药(眉山)有限公司
Nalmefene hydrochloride injection and preparation method thereof
ActiveCN101658489AImprove stabilityEnsure safetyOrganic active ingredientsNervous disorderAntioxidantNalmefene
The invention discloses a nalmefene hydrochloride injection, which is solution prepared from nalmefene hydrochloride and usable pharmaceutical excipients dissolved in injection water. The pharmaceutical excipients comprise an antioxidant and an osmotic pressure modifier. The nalmefene hydrochloride injection is characterized in that: the weight ratio of nalmefene to the antioxidant in the nalmefene hydrochloride is 1:0.1-1.0; and the antioxidant is mixed by one or more of tertiary butyl hydroxy anisole, 2,6-di-tert-butyl-4-methyl-phenol and rosemary. The injection is stable in property and ensures medication safety.
Owner:BEIJING SIHUAN PHARMA +1
Nose cavity administering formulation of nalmefene
The invention provides a nalmefene preparation to be administered through nasal cavity, which comprises nalmefene, namefene free alkali or other pharmaceutically acceptable medicinal salt of nalmefene and absorption promoting agent, a large number of tests have shown that, the nasal cavity administered medicinal preparation by the invention can make the medicament absorbed through the path of nasal mucosa, and enter into blood circulation for actions. The advantages of the preparation include stabilized performance, controlled quality and non-stimulation to nasal cavity.
Owner:广东同德药业有限公司
Pharmaceutical composition containing active component, namely nalmefene hydrochloride
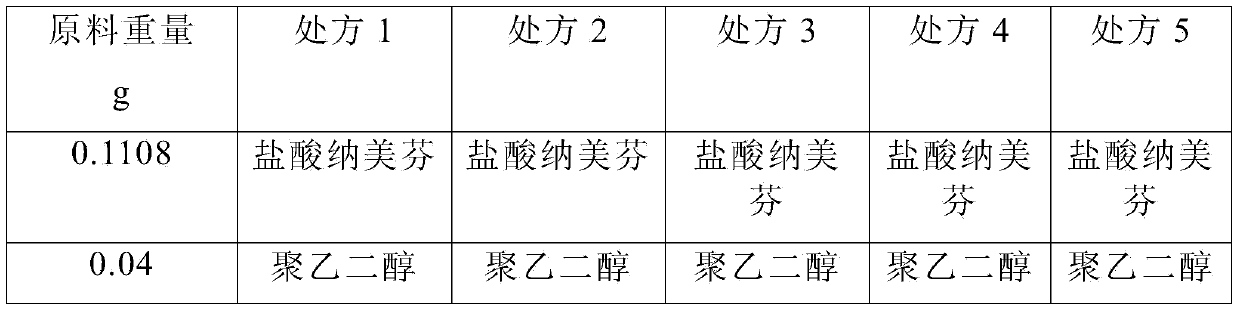
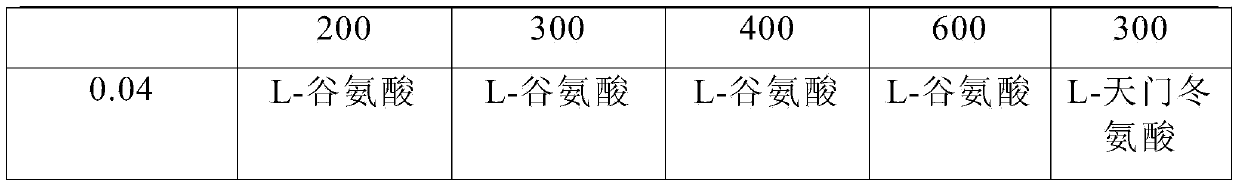
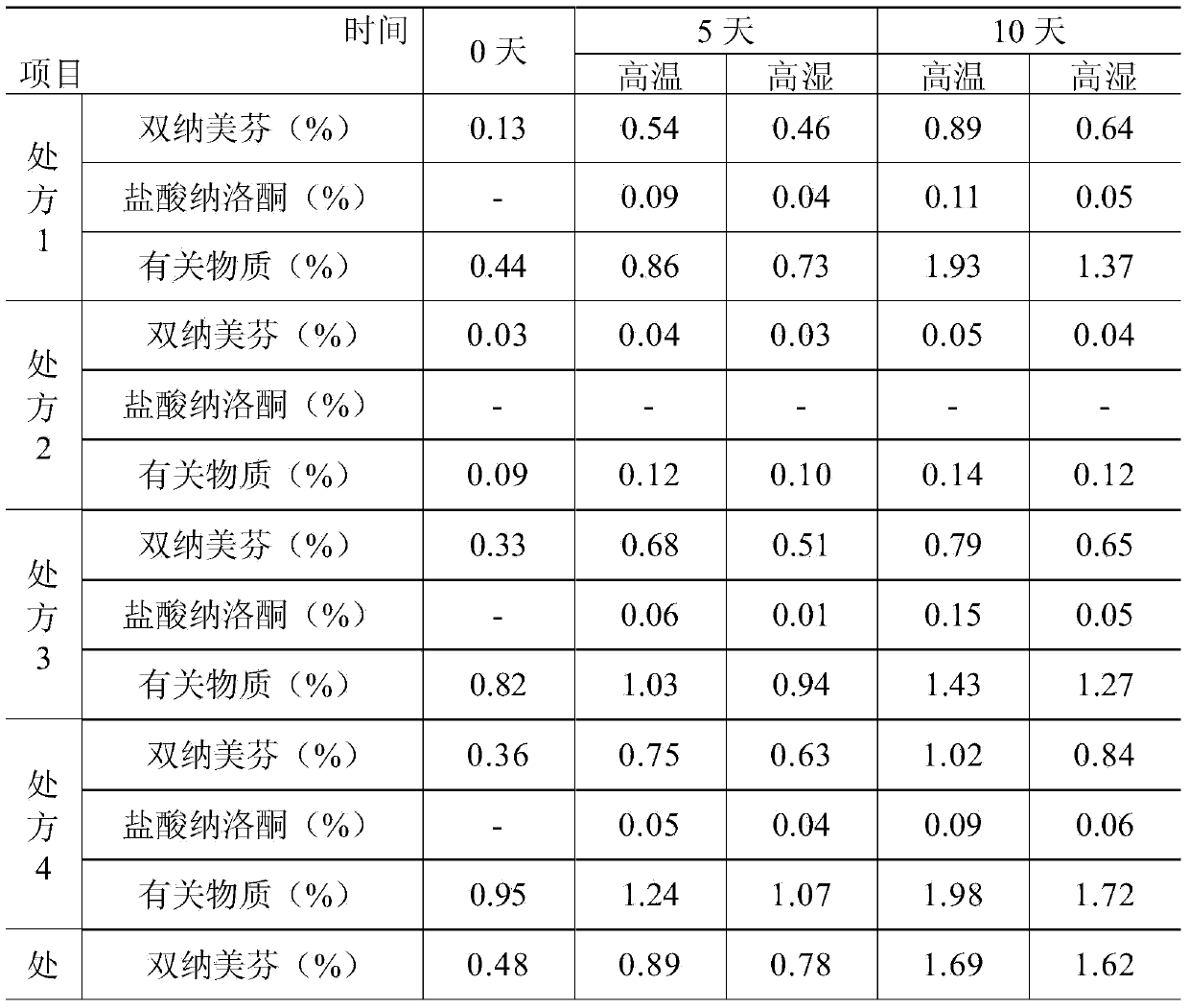
ActiveCN104000827AGuaranteed contentOrganic active ingredientsNervous disorderActive componentPolyethylene glycol
The invention belongs to the technical field of medicines, and in particular relates to a pharmaceutical composition containing an active component nalmefene hydrochloride. The pharmaceutical composition disclosed by the invention contains nalmefene hydrochloride, polyethylene glycol 300 and L-glutamic acid. Injection prepared from the pharmaceutical composition disclosed by the invention is good in stability; after placing for 24 months, the content of bis-nalmefene is less than 0.1%; furthermore, the content of naloxone hydrochloride is less than 0.01%; the injection prepared from the pharmaceutical composition disclosed by the invention is convenient to use and beneficial to storing and transporting; the pharmaceutical composition disclosed by the invention is simple in preparation method, easy for industrialization production and low in production cost.
Owner:西藏易明西雅医药科技股份有限公司
Nalmefene hydrochloride preparation method
InactiveCN104513251AReduce usageReduce three waste discharge pollutionOrganic chemistryOrganic solventPollution
The invention relates to a nalmefene hydrochloride preparation method, nalmefene hydrochloride preparation process is improved, the new process is easier to operate and easier to control, the production cycle is shortened, and the environment pollution is reduced, so that the new technology is more safe and reliable to meet the requirement of large scale production.
Owner:ANHUI YOUCARE KAIYUE PHARMA
Nalmefene hydrochloride injection medicinal composition and preparation method thereof
ActiveCN104997728AAvoid degradationSimple prescriptionOrganic active ingredientsNervous disorderNALMEFENE HYDROCHLORIDEDrug
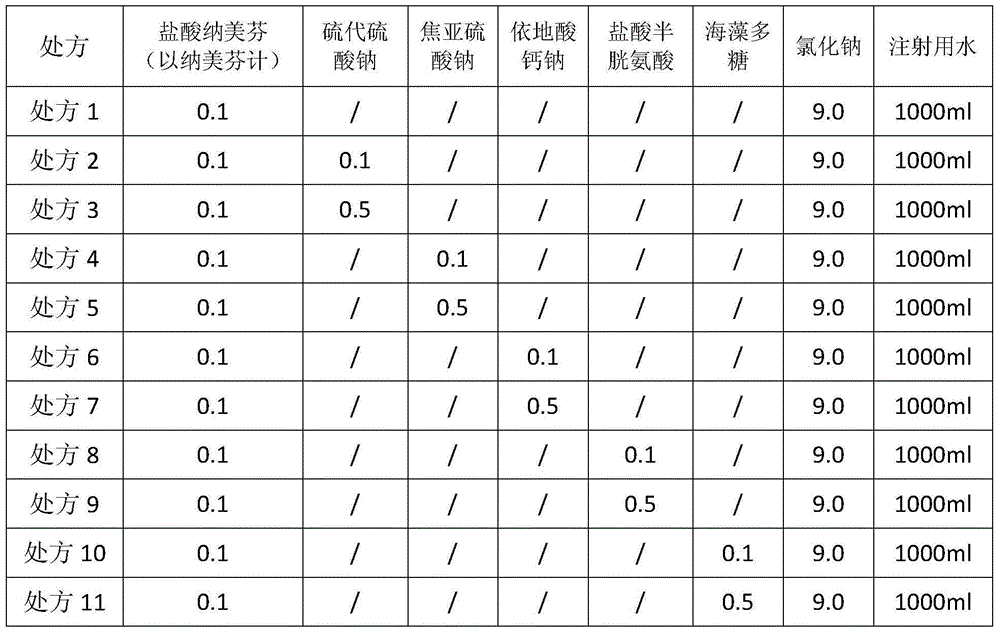
The invention relates to a nalmefene hydrochloride injection medicinal composition and a preparation method thereof. The nalmefene hydrochloride injection medicinal composition is composed of nalmefene hydrochloride, sodium chloride, alga polysaccharides and injection water. The above prescription is simple, and an antioxidant used in the prior art is not used in the method, so untoward effects induced by the antioxidant are avoided, and the safety is guaranteed; a small amount of the alga polysaccharides is added to the prescription, so degradation of nalmefene hydrochloride injection in the storage process is effectively prevented, and the stability of products is obviously enhanced; and a nitrogen introduction loading technology is not needed, so the production steps are simplified, and the cost is reduced.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Injection containing nalmefene hydrochloride
InactiveCN106474054AImprove stabilityPlay a stabilizing roleOrganic active ingredientsNervous disorderImpurityDosage form
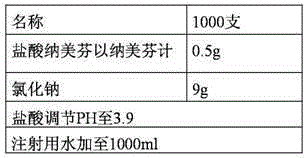
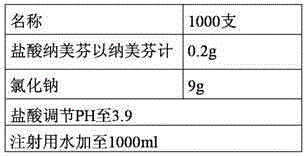
The invention relates to an injection containing nalmefene hydrochloride and a preparation method thereof. The nalmefene hydrochloride injection is a unit dosage form. Each unit dosage form of the injection comprises 0.35 to 0.75 mg of nalmefene hydrochloride (calculated by nalmefene), preferably 0.5 mg of nalmefene hydrochloride (calculated by nalmefene). The injection can maximally reduce the degradation reactions during the storage process. After long term storage, the content of bis-nalmefene impurity is extremely low, the product stability is better, the shelf life is longer, and the safety is higher.
Owner:CHENGDU GUOHONG PHARMA
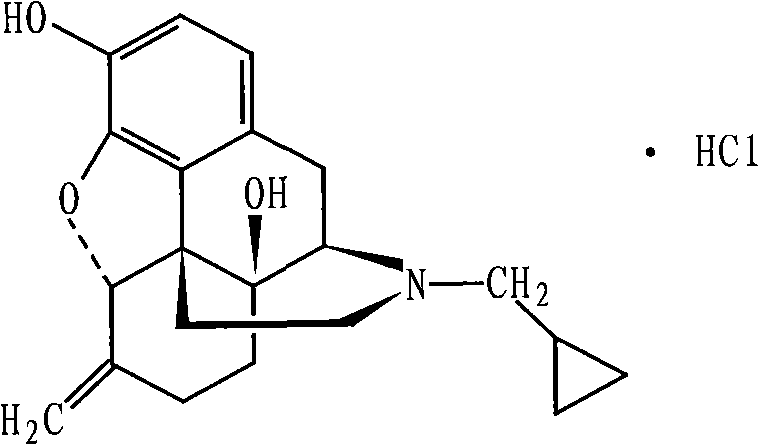
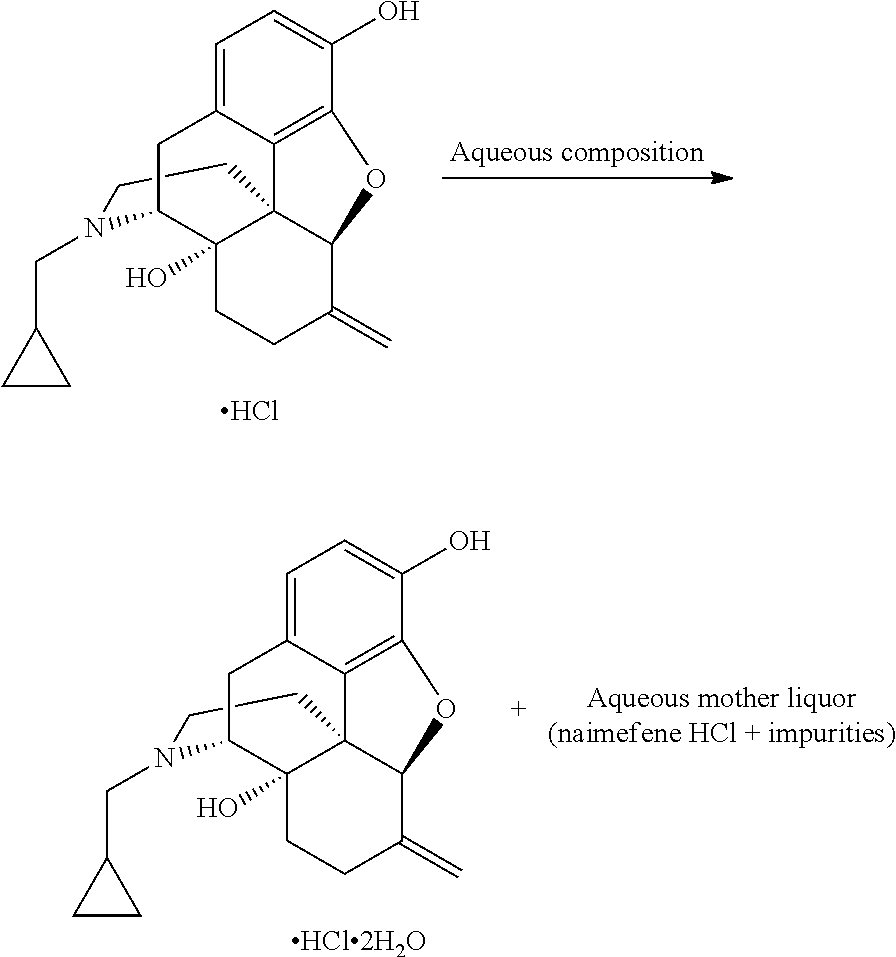
Preparation method of nalmefene hydrochloride monohydrate
The invention discloses a preparation method of a nalmefene hydrochloride monohydrate. According to the method, nalmefene hydrochloride is dispersed in a specific organic solvent, water is added, the dosage is controlled, and crystal transformation is performed. The prepared nalmefene hydrochloride monohydrate has the advantages of high yield, high purity, high stability and good nonabsorbent property.
Owner:南京卓康医药科技有限公司
Nalmefene hydrochloride purification method
ActiveCN106167492AThe production method is economical and practicalProduction method safetyOrganic chemistryAcetic acidPurification methods
The invention belongs to the technical field of medicines, and particularly relates to a nalmefene hydrochloride purification method. Nalmefene is used as a raw material, and nalmefene hydrochloride with purity of 99.95% or more is prepared under the action of solvents such as ethyl acetate, acetone and water.
Owner:西藏易明西雅医药科技股份有限公司
Nose cavity administering formulation of nalmefene
The invention provides a nalmefene preparation to be administered through nasal cavity, which comprises nalmefene, namefene free alkali or other pharmaceutically acceptable medicinal salt of nalmefene and absorption promoting agent, a large number of tests have shown that, the nasal cavity administered medicinal preparation by the invention can make the medicament absorbed through the path of nasal mucosa, and enter into blood circulation for actions. The advantages of the preparation include stabilized performance, controlled quality and non-stimulation to nasal cavity.
Owner:广东同德药业有限公司
Nalmefene hydrochloride injection and preparation method thereof
InactiveCN106727293AGood compatibilityHigh content of active ingredientsOrganic active ingredientsNervous disorderActivated carbonHydrogen
The invention relates to a nalmefene hydrochloride injection and a preparation method thereof. The preparation method of the nalmefene hydrochloride injection comprises the following steps: (1) adding 0.095 to 0.105 g of nalmefene hydrochloride metered according to C21H25NO3 into injection water, thus preparing liquid medicine A of which the concentration is 2.0 to 2.5 mg / ml; adding 8.5 to 9.5 g of sodium chloride in the liquid medicine A, and completely dissolving, thus obtaining liquid medicine B; (2) adding activated carbon in the liquid medicine B, and decarbonizing after stirring and adsorbing, thus obtaining liquid medicine C, wherein the usage of the activated carbon is 0.05 to 0.10 mg / 100 ml of the liquid medicine A; (3) adding the injection water in the liquid medicine C until the volume is 940 to 960 ml, regulating the system pH (Potential of Hydrogen) to be 3.95 to 4.50 by using hydrochloric acid, and replenishing the injection water until the volume is 1000 ml, thus obtaining the nalmefene hydrochloride injection. The nalmefene hydrochloride injection disclosed by the invention is high in effective component content and low in impurity content; the requirements of quality standard are met after the nalmefene hydrochloride injection is placed for a long term, and good long-term stability is obtained.
Owner:HENAN RUNHONG PHARMA
Nalmefene hydrochloride nasal medicine administration preparation
InactiveCN106361700AImprove performanceQuality is easy to controlOrganic active ingredientsNervous disorderDiseaseNasal cavity
The invention provides a nalmefene nasal medicine administration preparation, which is prepared from nalmefene hydrochloride, nalmefene free alkali or pharmaceutically acceptable other nalmefene pharmaceutical salts and sorbefacients. A great number of experiments show that the nasal medicine administration preparation provided by the invention realizes the medicine absorption through a tunica mucosa nasi path, and the medicine enters blood circulation to achieve the effect; the advantages of stable performance, quality controllability and no stimulation on the tunica mucosa nasi can be realized. The nasal medicine administration preparation provided by the invention belongs to the intranasal medicine administration preparation capable of being used for shock resistance, overdose anesthesia rescue in an operation, morphine medicine poisoning and drug dependence person diagnosis, and prevention or treatment on stress diseases of acute alcoholism, cerebral infarction, neonatal asphyxia, drug poisoning and the like.
Owner:威海恒基伟业信息科技发展有限公司
Nalmefene hydrochloride pharmaceutical composition for injection
InactiveCN103655464AFix stability issuesHigh yieldOrganic active ingredientsAntinoxious agentsSynthetic opioidDrug product
The invention relates to a medicine in the field of medicine, and particularly relates to a nalmefene hydrochloride pharmaceutical composition for injection with good stability and a preparation method thereof. As a new generation of opioid receptor antagonist, the composition is used for completely or partially reversing the effects of opioids, including respiratory depression caused by natural or synthetic opioids; moreover, the composition is applied to the treatment of known or suspected overdose of opioids.
Owner:TIANJIN SONGRUI MEDICAL TECH
Process for recovery of nalmefene hydrochloride
Owner:H LUNDBECK AS
Pharmaceutical composition containing nalmefene hydrochloride compound
ActiveCN103040733BImprove stabilityImprove toleranceOrganic active ingredientsNervous disorderEthylene diamineVitamin C
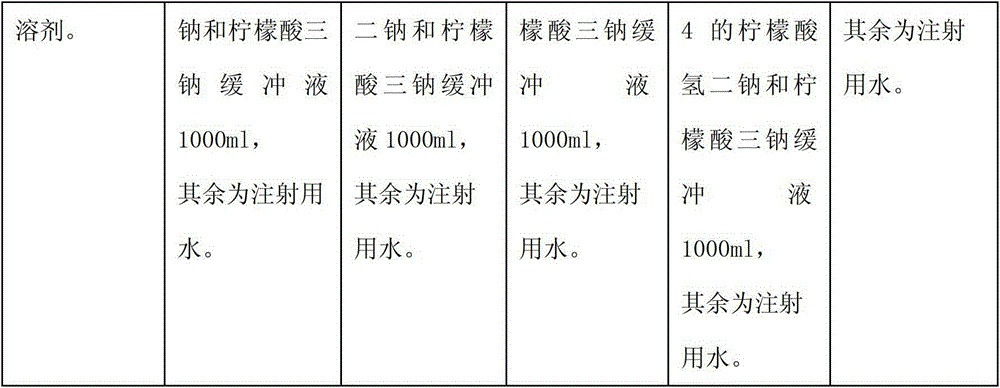
The invention relates to a pharmaceutical composition containing a nalmefene hydrochloride compound. For every 1000 injections, the pharmaceutical composition comprises the following components: 100mg of nalmefene hydrochloride, 1-2g of Tween 80, 1-3g of EDTA (Ethylene Diamine Tetraacetic Acid) calcium, 1-2g of vitamin C, 1000ml of sodium hydrogencitrate and trisodium citrate buffer liquor with molar ratio of 1:4, and the balance of water for injection.
Owner:罗诚
Preparation of sublingual administered nalmefene hydrochloride
InactiveCN101007005AEasy to useQuick effectOrganic active ingredientsNervous disorderSide effectMedicine
The invention disclosed a kind of hydrochloric acid nalmefene sublingually consumed preparation which includes active ingredients and medical accessories at a ratio of 1:1-10000 (by weight), the active ingredients include hydrochloric acid nalmefene, nalmefene liberated alkali or other medically acceptable nalmefene salts. The active ingredients in the invention can enter the vivo circulation through the capillary under patients' mucous membrane of mouth so as to fasten the effects and increase the bioavailability. The invention can be used in any occasions, so it's convenient to use with low taking dosage and less side effects.
Owner:薛京 +1
Nalmefene hydrochloride injection medicine composition and preparation method
The invention belongs to the technical field of medicine, and relates to a nalmefene hydrochloride injection medicine composition and a preparation method, and particularly relates to a nalmefene hydrochloride injection medicine composition. The medicine composition comprises nalmefene hydrochloride. Particularly the medicine composition comprises the nalmefene hydrochloride, inorganic salt and injection water. The nalmefene hydrochloride in the medicine composition is added into the composition in a form of 17-(cyclopropyl)-4, 5alpha-epoxyl-6-methylene morphinan-3, 14-diol hydrochloride. Sugar is selected from one or more of mannitol, glucitol, lactose, maltose, maltitol, glycine, trehalose, glucose, dextran and the like. The medicine composition is an opiates receptor antagonist and is mainly clinically used for completely or partially inverting the effects of opiates drugs, including the respiratory depression caused by natural or synthetic opiates drugs. The injection is good in pharmaceutical properties such as excellent chemical stability and physical stability.
Owner:成都天台山制药股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com