Pharmaceutical composition containing nalmefene hydrochloride and preparation method of same
A technology for nalmefene hydrochloride and nalmefene, which is applied in the field of medicine, can solve problems such as affecting the medicinal effect of nalmefene, lack of stability tests and data, etc., and achieves simple ingredients, less product impurities and good safety. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
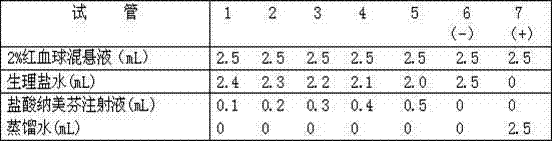
Image
Examples
Embodiment 1
[0021] Material name Quantity
[0022] Nalmefene hydrochloride (calculated as free base) 0.1g
[0024] Disodium edetate 0.3g
[0025] 0.1mol / L hydrochloric acid to pH 3.8
[0026] Appropriate amount of water for injection
[0027]
[0028] Full volume 1000ml
[0029] Nalmefene hydrochloride injection (1ml:0.1mg): its preparation method includes the following steps:
[0030] 1) Weigh the main drug, sodium chloride and edetate disodium according to the prescription amount, add 20% of the total amount of water for injection, stir to dissolve evenly, and then add water for injection to the full amount;
[0031] 2) Use 0.1mol / L hydrochloric acid to adjust the pH value of the solution to 3.8, and filter it through a 0.02um microporous membrane until it becomes clear.
[0032] 3) After passing the inspection (pH, content) of the semi-finished products, they are respectively filled and sealed in ampoules;
[0033] 4) Melt seal; 5) Sterilize in ...
Embodiment 2
[0035] Material name Quantity
[0036] Nalmefene hydrochloride (calculated as free base) 2.0g
[0037] Sodium chloride 18.0g
[0038] Disodium edetate 0.6g
[0039] 0.1mol / L hydrochloric acid to pH 3.80
[0040] Appropriate amount of water for injection
[0041]
[0042]Full volume 2000ml
[0043] Nalmefene Hydrochloride Injection (2ml:2mg): its preparation method is the same as in Example 1:
Embodiment 3
[0045] The prescription of Nalmefene Hydrochloride Injection (1ml:0.1mg) is the same as in Example 1, and its preparation method:
[0046] 1) Weigh disodium edetate according to the prescription amount, add 20% of the total amount of water for injection to dissolve it completely, heat treatment at 75°C for 15 minutes to obtain disodium edetate solution;
[0047] 2) Add the main drug and sodium chloride of the prescribed amount into the disodium edetate solution, add water for injection with 20% of the total amount of water for injection and stir until uniform, then add water for injection to the full amount;
[0048] 3) Use 0.1mol / L hydrochloric acid to adjust the pH value of the solution to 3.8, and filter it through a 0.02um microporous membrane until it becomes clear;
[0049] 4) After passing the inspection (pH, content) of the semi-finished products, they are respectively filled and sealed in ampoules;
[0050] 5) Melt seal; 6) Sterilize in a 121°C water bath for 15 minu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com