Patents
Literature
2519 results about "Injection solution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
TRAUMEEL Injection Solution is an anti-inflammatory, anti-edematous, anti-exudative combination formulation of 12 botanical substances and 1 mineral substance. TRAUMEEL Injection Solution is officially classified as a homeopathic combination remedy (1).
Delivery of agents to tissue
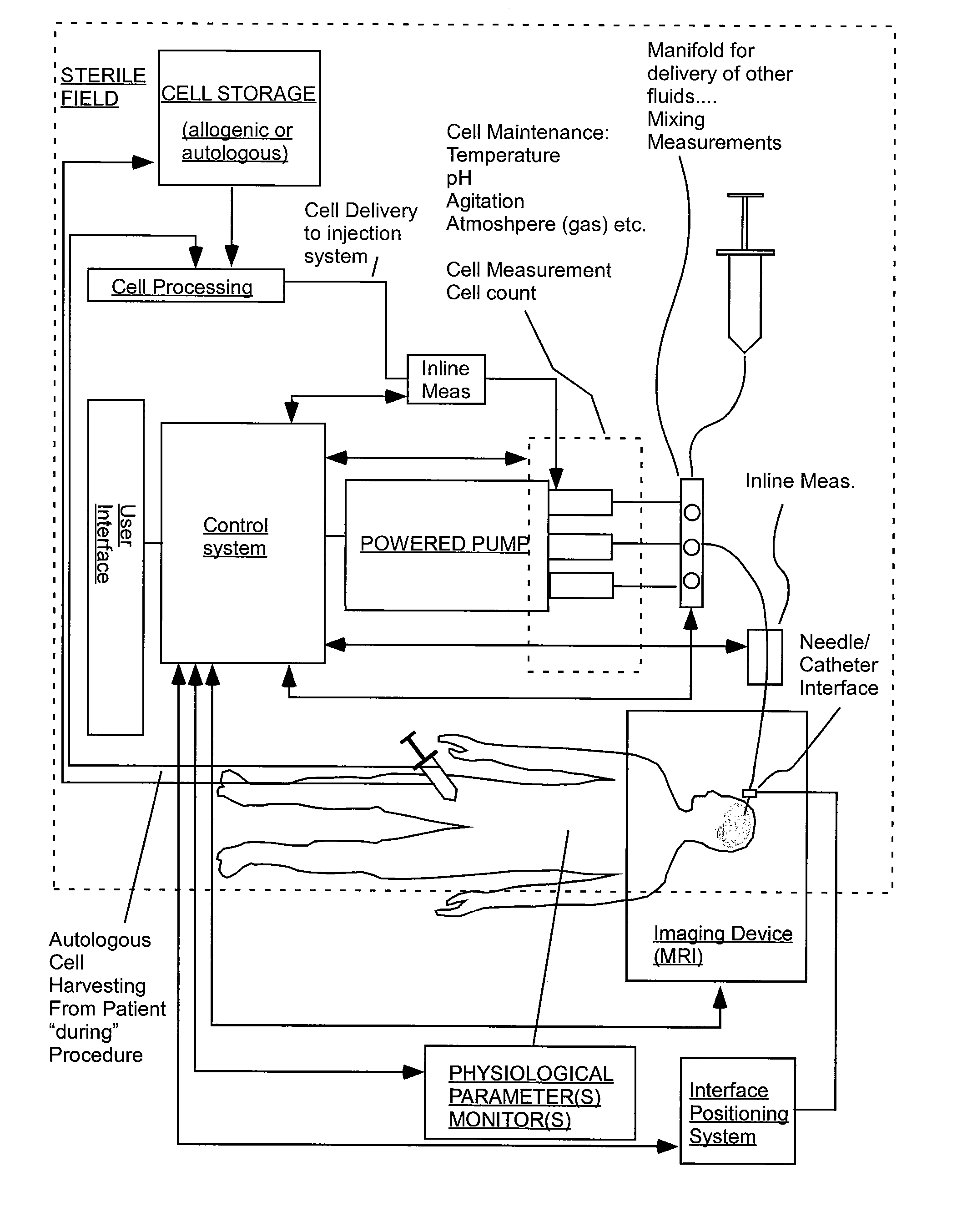
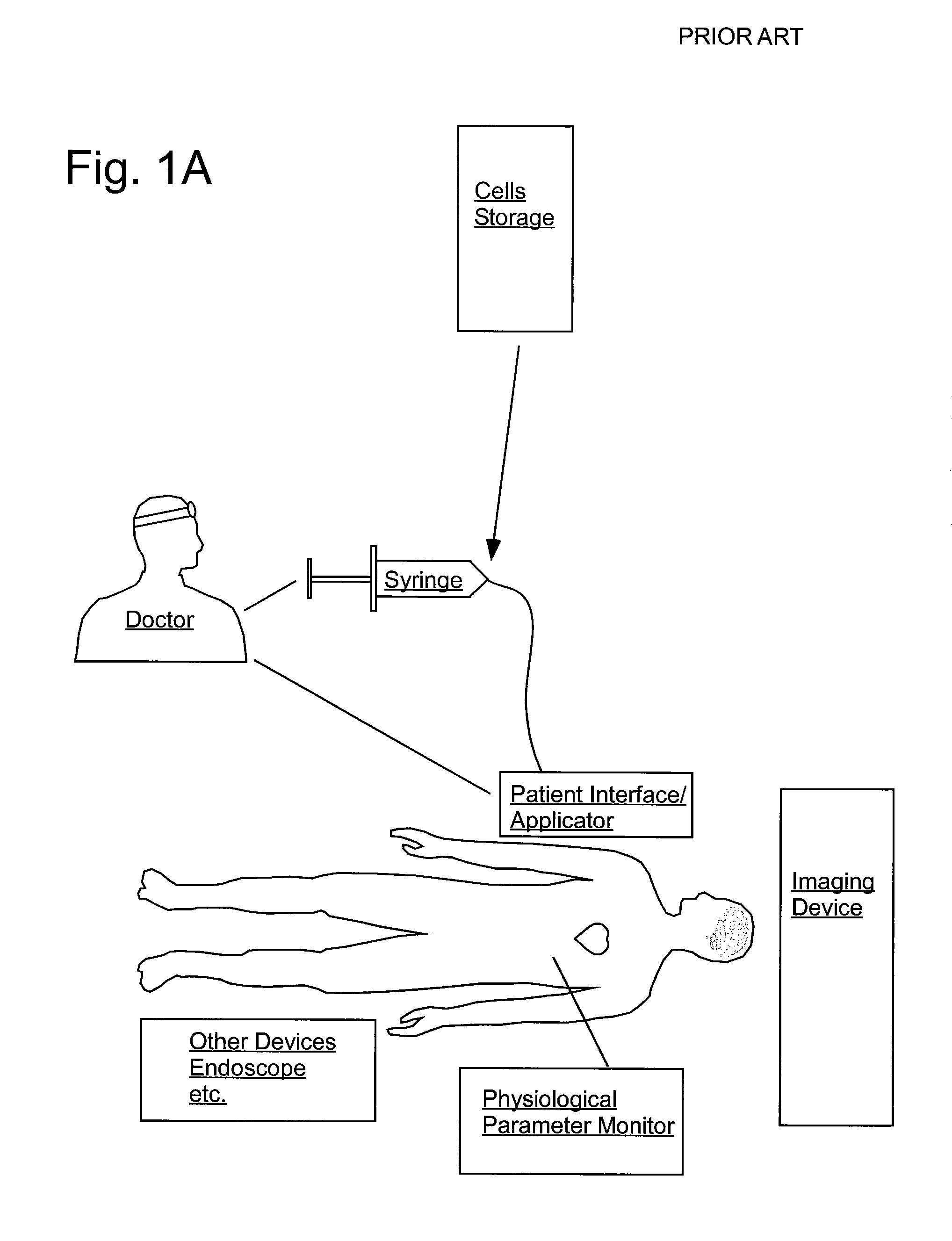
InactiveUS20070106208A1Limit localizationKeep sterileBioreactor/fermenter combinationsBiological substance pretreatmentsStereotactic localizationBiomedical engineering
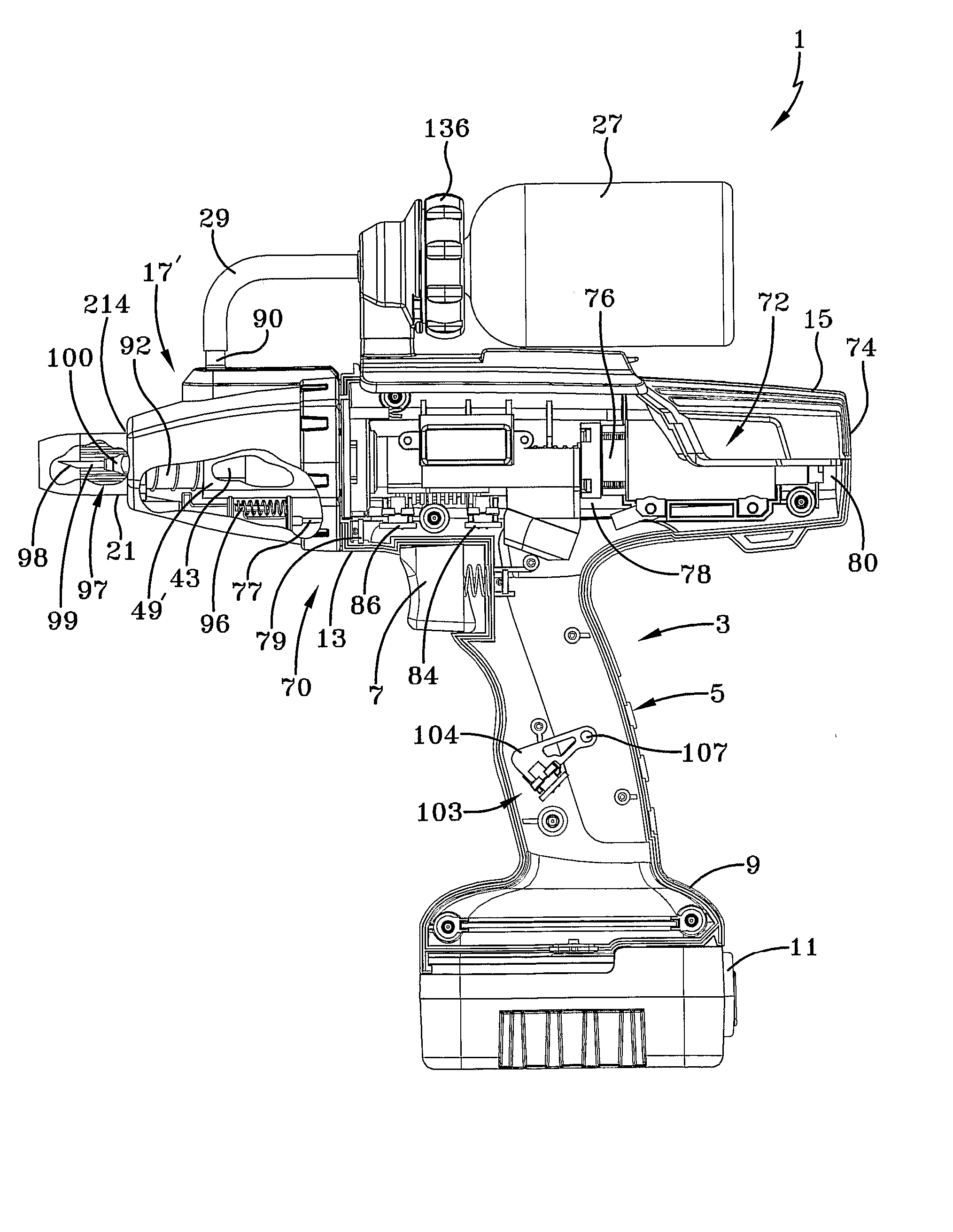
A system for injecting an injectate into patient includes a first pressurizable container for holding the injectate; a patient interface in fluid connection with the first pressurizable container, the patient interface being adapted to pass the injectate into tissue of the patient; a powered injector in operative connection with the first pressurizable container to pressurize the injectate; a controller system in operative connection with powered injector; and a stereotactic localization frame adapted to be placed in operative connection with the patient interface to assist in controlling localization of the patient interface. A system for processing cells (and / or other injectate components) includes a container and a plunger adapted to be slidably positioned within the container. The system includes at least one inlet port through which a fluid can enter the system and at least one effluent port through which an effluent can exit the system. The plunger section forms a sealing engagement with the inner wall of the container such that rearward motion of the plunger is adapted to draw fluid into the system via the inlet and forward motion of the plunger is adapted to force effluent out of the system via the effluent port.
Owner:BAYER HEALTHCARE LLC +1
Novel drug delivery technology
The invention relates to a novel drug delivery technology. More particularly the invention relates to a method of delivering at least one therapeutic compound or a formulation comprising the at least one therapeutic compound to a patient; to a throwaway or reusable device for delivering at least one therapeutic compound or a formulation comprising the at least one therapeutic compound to a patient in a manner as set out by the method; to a pioneer projectile for use in said method; to formulations for use in said method and to an injectate comprising a pioneer projectile and formulation. It also relates to a disposable component containing either a pioneer projectile or an injectate. The invention also relates to a throwaway or reusable device for delivering at least one therapeutic compound, or a formulation comprising the at least one therapeutic compound (hereafter drug) to a patient, and a method for administering a drug to a patient using said device. It also relates to a packaged drug for use with said device.
Owner:ENESI PHARM LTD
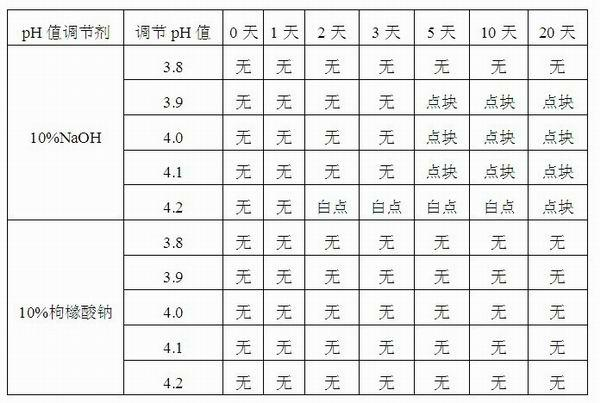
Injection-purpose medicine composition for improving stability of ligustrazine medicine injection formulation and preparation method of injection-purpose medicine composition
ActiveCN102008727AStable pHDecreased substancesOrganic active ingredientsPharmaceutical non-active ingredientsMedication injectionUse medication
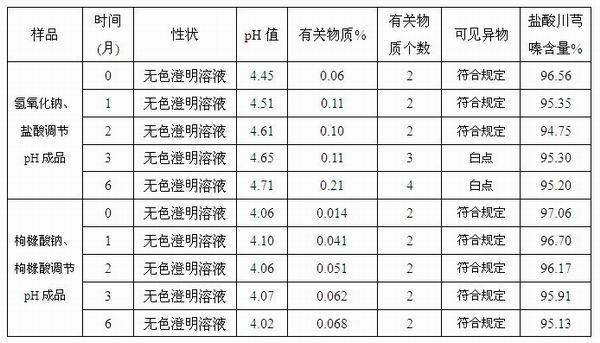
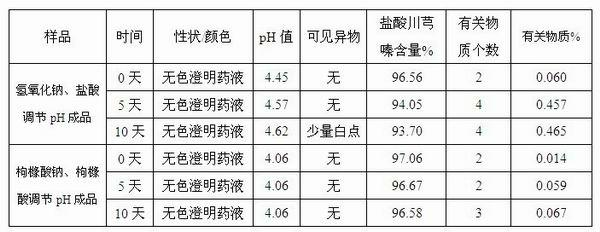
The invention discloses an injection-purpose medicine composition for improving stability of ligustrazine medicine injection formulation and a preparation method of the injection-purpose medicine composition. The injection-purpose medicine composition is prepared by the method comprising following steps: dissolving ligustrazine salt in water for injection, adjusting the pH value of the liquid medicine by adding citric acid and / or sodium citrate used as a pH regulator, wherein the dosage of the citric acid and / or sodium citrate ranges from (0.1mg to 200.0mg) / 100ml. In the invention, pH value of the injection liquid can be more stable, the content of ligustrazine degradation substance is greatly reduced compared with that of the prior art, the clarity of the ligustrazine injection liquid is improved under the condition of not using other cosolvents to increase the clinical application risk, particularly, the problems of small white spots, white blocks and solution turbidity are solved under the condition that the ligustrazine injection liquid is stored for a long time by adopting the prior art, and the medicine composition ensures that the inspection of visible foreign substances of the product is in accordance to the formulation of medicine quality standard, and is convenient for clinical medication and popularization.
Owner:SICHUAN SUNNYHOPE PHARM CO LTD
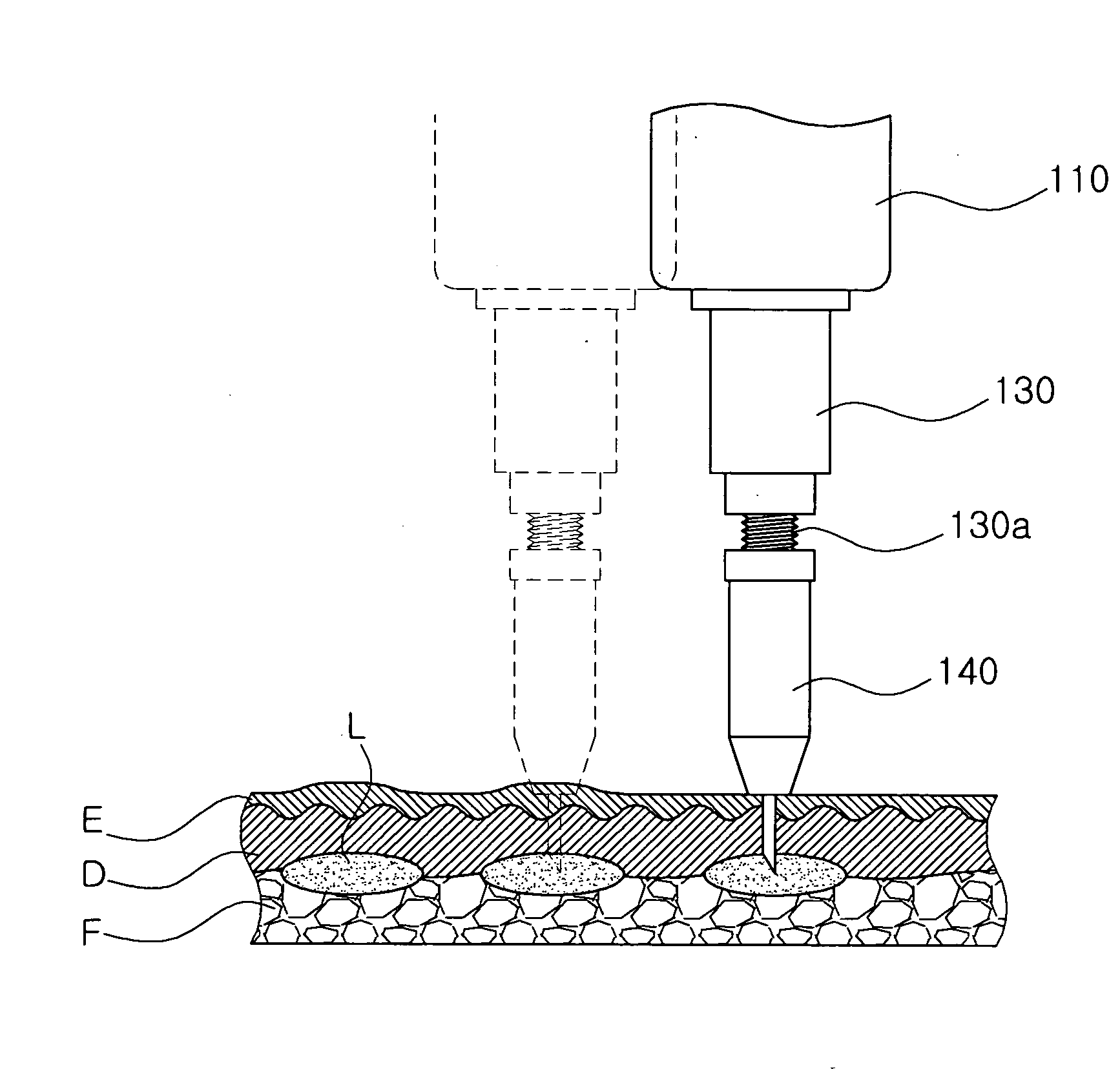
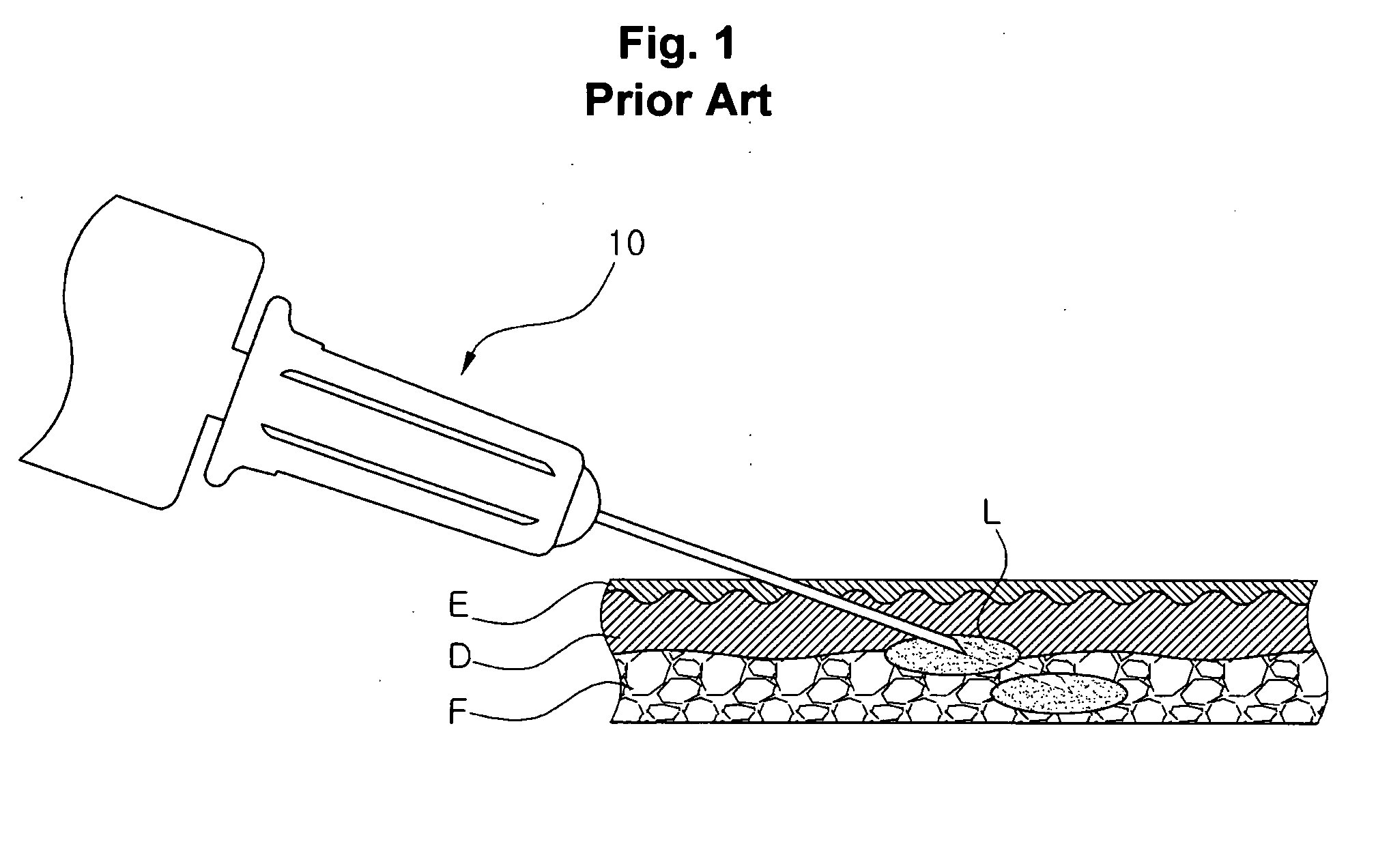
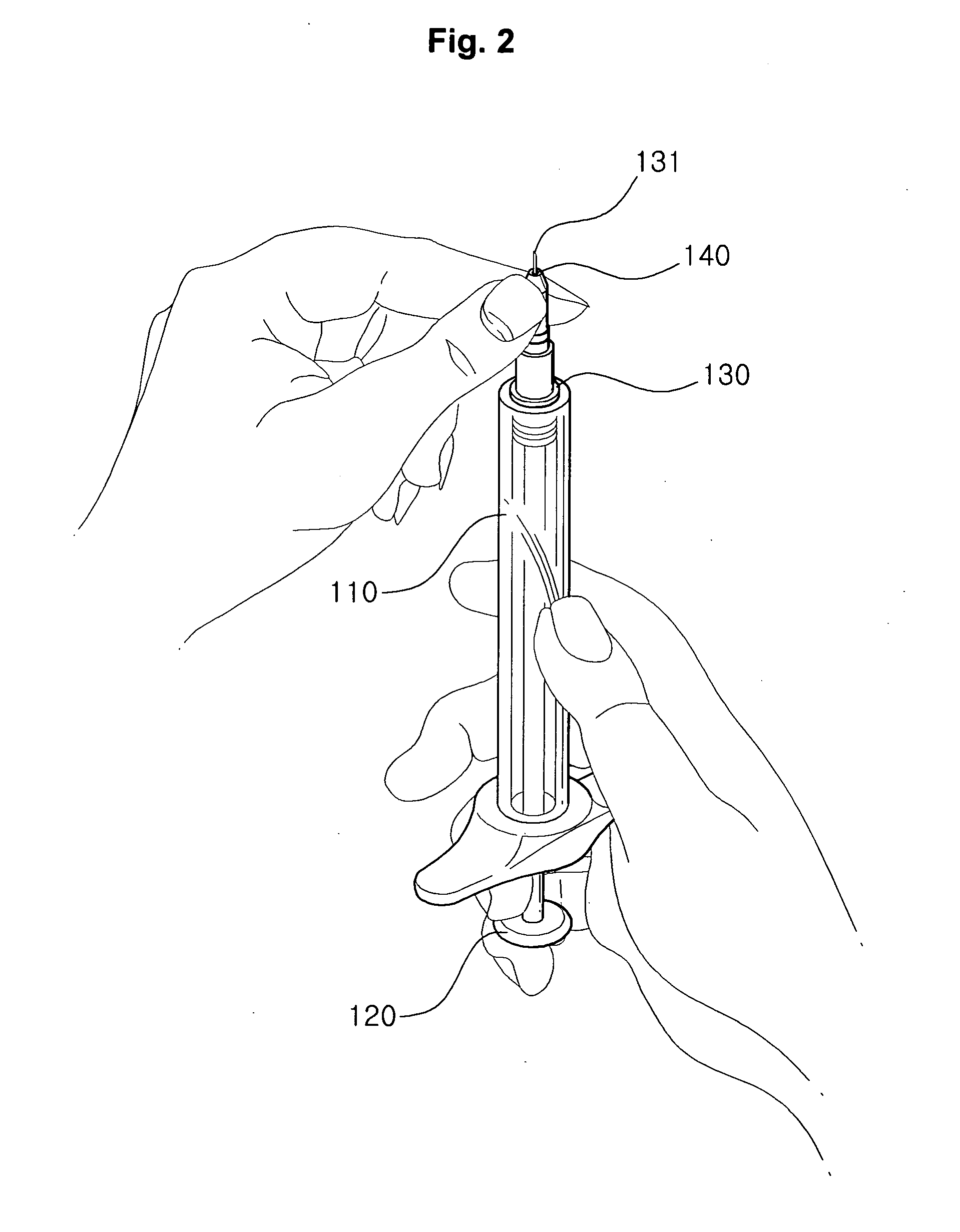
Injection method using injector with length-adjustable needle and injection apparatus using the same
InactiveUS20090259180A1Relieve painEfficient injectionAutomatic syringesMedical devicesThree vesselsInjection device
An injection method using a length-adjustable needle is disclosed, which comprises an adjusting step in which an exposed needle length of a length-adjustable needle of an injector corresponding to a skin tissue of a patient is adjusted; an insertion step in which an injector is positioned on a skin tissue in a vertical direction with respect to the skin tissue, and a needle is inserted; a suction step in which it is checked whether the needle is inserted into a blood vessel; and an injection step in which an injection liquid is injected from the injector inserted into the skin tissue by the depth corresponding to the exposed length of the needle.
Owner:CHOI JONG SOO
Preparation method of stable fleabane extract injection
InactiveCN1476840AFix stability issuesImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismArginineMedicine
The present invention provides a preparation method of stable breviscapine injection. In the course of preparation the L-arginine can be added, its concentration is 40-80 mg / 100ml, and its pH value is regulated to 4.5-6.5. Said prepared breviscapine injection is stable in quality. It can be stored for above three years.
Owner:上海博泰医药科技有限公司
Ambroxol hydrochloride injection
The invention relates to a medicinal preparation, in particular to an ambroxol hydrochloride injection which is a stable medial composition taking ambroxol hydrochloride as an active ingredient to be combined with carriers acceptable in pharmacy. The carriers comprise a water-soluble filling agent, a pH regulating agent, a stabilizing agent, water used for injection or an osmotic pressure regulating agent and the like. As the carriers are adopted to carry out scientific preparation, the drug quality and medication safety of freeze-dried powder injections, small-needle injections and small infusion solutions are ensured, and the stability of the preparations during storage is improved.
Owner:天津康哲维盛医药科技发展有限公司
Preparation of indissoluble medicament nano granule
InactiveCN101322682AIncrease Saturation SolubilityPassive targetingPowder deliveryPharmaceutical non-active ingredientsSide effectNanocrystal
The invention relates to the technical field of medicine, in particular to a preparation method of nano-particles of insoluble drugs. The method includes the following steps: (1) dissolving the drugs in a first solvent (good solvent) to form solution, (2) blending the solution with a second solvent (poor solvent) to form premixed suspension, (3) applying energy on the premixed suspension to form the nano-particles, the average effective grain diameter of which is less than 2Mum. By adopting the technology combining micro-deposition and homogenization, the invention suspends the drugs in poor solvent (usually water) in a form of pure nano-crystal, thus solving the problem that solution is difficult to be prepared since the drugs are difficult to be dissolved in water and oil; compared with the corresponding injection for intravenous infusion and the oral preparations such as tablets, capsules, and the like, the preparation method of the invention can lower adverse reaction, reduce toxic and side effect, improve bioavailability, has sustained-release effect and is convenient to be used by patients.
Owner:KANGYA OF NINGXIA PHARMA +1
Sulfonated derivative of andrographolide and combination of medication
ActiveCN1687049AClear structureGood antibacterialAntibacterial agentsPowder deliveryDiseaseTonsillitis
The present invention discloses II kinds of andrographolide sulfonated derivatives with the actions of resisting bacteria, relieving inflammation and reducing fever and medicine composition containing them. They can be used for preparing freeze-dried powder, injection or oral preparation, and can be used for curing the diseases of pneumonia, bronchitis, tonsillitis and bacillary dysentery, etc.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Method for in vitro production of three-dimensional vital cartilage tissue and use thereof as transplant material
The invention relates to a remarkably simple method for the in vitro production of three-dimensional, vital and mechanically stable cartilage or bone tissue and to the use thereof as a transplantation material for treating cartilage or bone defects and degenerative diseases such as rheumatism or arthrosis, and to the use thereof in testing active substances and physical factors. The invention is also directed to the cartilage or bone tissue and therapeutical formulations produced thereby, e.g. injection solutions comprising such tissue.
Owner:CO DON AG
Genetic vaccination device and process for forming an injection therefor
InactiveUS6951613B2Ampoule syringesGenetic material ingredientsGenetic MaterialsMembrane configuration
There is disclosed a genetic vaccination device and a process for forming an injection solution therefor, the device comprising a syringe and canula coupled to a membrane adsorber having genetic material adsorbed thereon, and the process comprising eluting the genetic material from the membrane adsorber so as to form an injection solution containing the genetic material.
Owner:SARTORIUS STEDIM BIOTECH GMBH
Injection device
InactiveUS20030236500A1Easy to operateReliably trappedFiltering accessoriesTube connectorsSkin surfaceSterile water
An injection device for injecting an injection solution prepared from a powdered medication in sterile water immediately before use which is administered by a syringe. A winged cannula has a hollow needle and a needle holder with wings. A female Luer Lock connector is joined to the winged cannula by a flexible connecting tube and has an insertion region for the connecting tube and a connection region for placing the syringe, filled with the injection solution, against a skin surface, and also has a continuous bore that is continuous from the insertion region to the connection region of the Luer Lock connector. A porous filter element permeable to the injection solution is provided in the continuous bore of the Luer Lock connector.
Owner:SCHEU ROLF RAINER
Edaravone injection for treating acute cerebral thrombus and its prepn
InactiveCN1440749AAdjust detection sensitivityOrganic active ingredientsSolution deliveryEdaravone InjectionEmulsion
The Edaravone injection of the present invention is a kind of free radical eliminating medicine to treat acute cerebral infarction. It is injection prepared with Edaravone, additive and solvent and in the form of sterilized solution, emulsion or suspension. It may be small capacity sterilized injection, great capacity sterilized injection, bacteria-free powder preparation for injection, freeze dried powder preparation for injection, sterilized suspension injection or sterilized emulsion injection.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Preparation method of polyene-containing taxol nanoparticle mixed micelle preparation and freeze-drying agent
InactiveCN101804021AImprove solubilityHigh metabolic stabilityOrganic active ingredientsPowder deliveryMixed micelleFreeze-drying
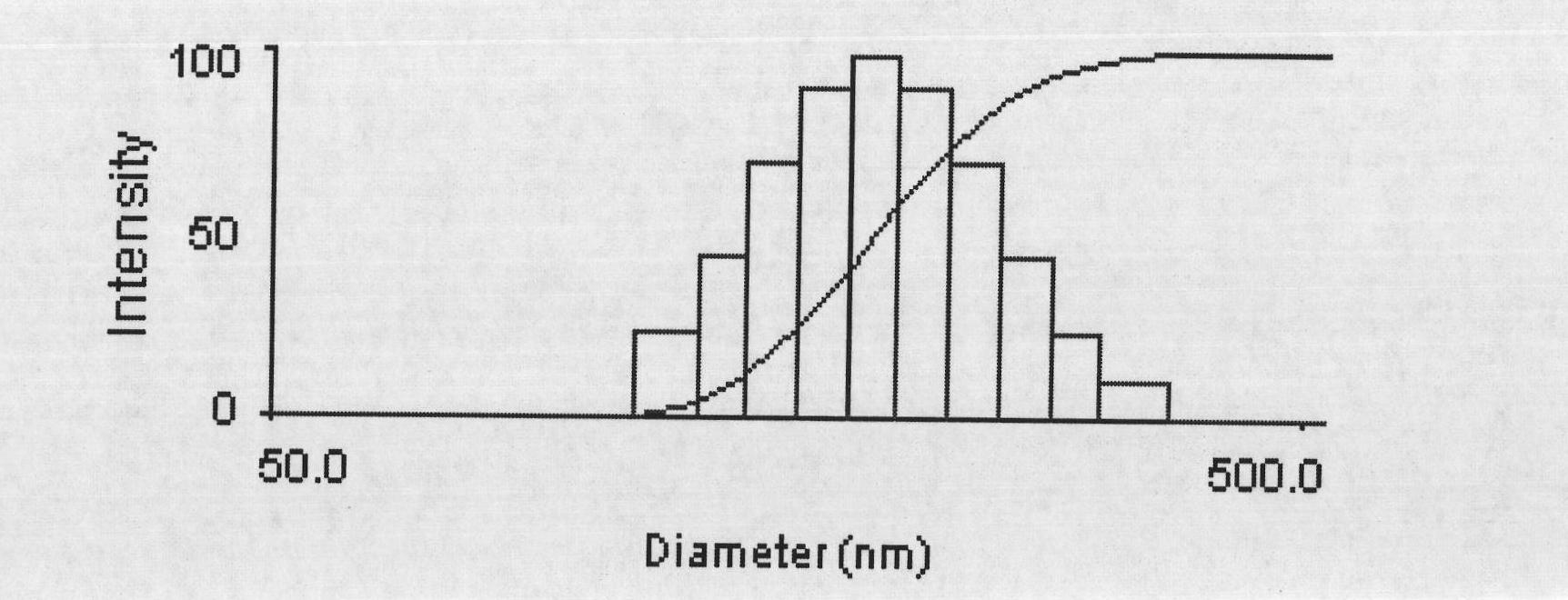
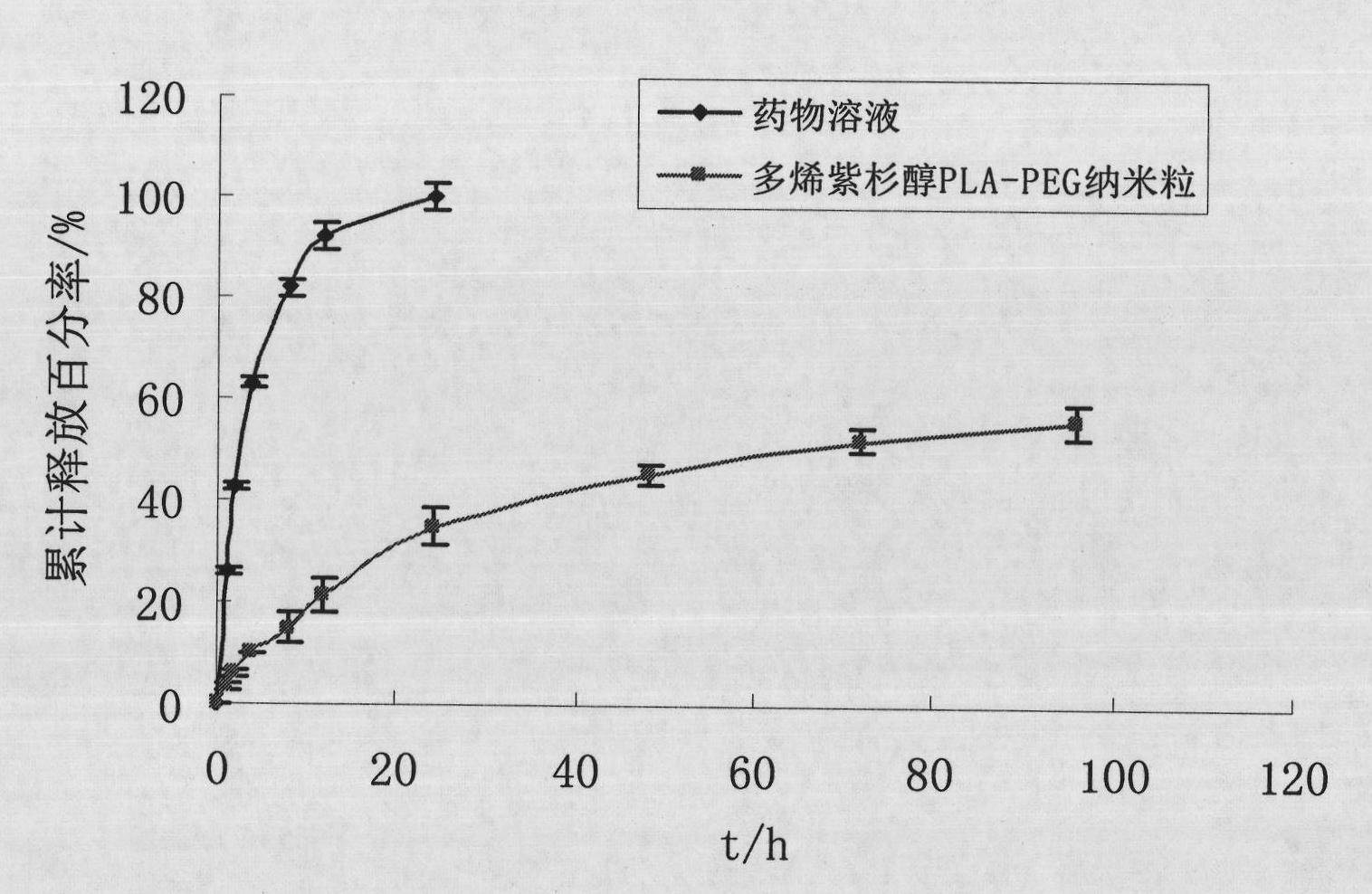
The invention discloses a preparation method of a polyene-containing taxol nanoparticle mixed micelle preparation and a freeze-drying agent, which prepares docetaxel PLA-PEG nanoparticles or micelle or nanoparticle mixed micelle through a modified solvent evaporation method, takes PLA-PEG copolymer as a carrier, and wraps docetaxel in a PLA hydrophobic core. When in use, the docetaxel PLA-PEG containing long cycle freeze-dried preparation only needs to be added with water and is dissolved, and uniform nanoparticle suspension, micellar solution or mixed micellar nanoparticle suspension can be prepared. The preparation method does not need tween-80 and ethanol solubilization, only takes the biodegradable PLA-PEG as the carrier, and does not contain any surfactant; and compared with the docetaxel injection on sale, the preparation can reduce the toxicity and the adverse reactions of the medicine, and improve the clinical application safety of the medicine.
Owner:SHANDONG UNIV
Herbal pharmaceutical composition for treatment of HIV/AIDS patients
Owner:WU TZU SHENG
Stabilized nalmefene hydrochloride injection and its preparation
ActiveCN1895251AReasonable compositionSimple processOrganic active ingredientsNervous disorderGlucose polymersD-Glucose
A high-stability nalmefene hydrochloride injection is prepared from nalmefene hydrochloride (0.005-0.2 W / v %) and the medicinal carrier chosen from sodium chloride, glucose, beta-cyclodextrin, dextran, pectose, sorbitol, etc. Its preparing process is also disclosed.
Owner:西藏易明西雅医药科技股份有限公司
Product having sobering up and liver protecting functions and its preparation method and usage
InactiveCN1698879APromote alcohol metabolismAvoid gatheringNervous disorderPeptide/protein ingredientsGreen Tea PolyphenolsVitamin C
The invention provides a product having sobering up and liver protecting functions and its preparation method and use, which comprises the following raw material (by weight ratio), soybean peptides and soybean extract 2.5-12:0.5, panaxoside or ginkgo leaf extract, green tea polyphenols, glutacid, arginine or lycine, vitamin C, vitamin B6, vitamin B1, vitamin B2 or vitamin B12. The medicament can be made into various dose forms including capsule, oral liquid, tablet, effervescent tablet or injection.
Owner:蔺益民
Nimodipine emulsion injection liquid and method for preparing the same
InactiveCN1732936AImprove solubilityEasy to store and useOrganic active ingredientsNervous disorderSolventEmulsion
The invention provides a high concentration nimodipine emulsion injection for intravenous injection, which is prepared from active component of nimodipine and auxiliary materials including oil for injection, emulsifying agent, auxiliary solvent, isotonic conditioning agent and water for injection.
Owner:SHANGHAI INST OF PHARMA IND +1
Hypodermic Injection System
ActiveUS20080071218A1Easy to controlIncrease chanceJet injection syringesAutomatic syringesEngineeringDrive motor
The invention relates to a hypodermic injection system having a direct-drive motor for moving a ram towards an injectate chamber for discharging injectate therein through a discharge orifice. The system can have an injection head attached to a housing having an injectate chamber for holding injectate to be injected, a remote discharge device with a control apparatus or the structure for holding a cartridge containing injectate. The injectate supply could be a bottle, a remote reservoir or a cartridge. An injection head can have a nose actuator for enabling an injection only if the nose actuator has engaged the body to be injected. A clamping device clamps the body to be injected. Control is effected through a microprocessor to which the electrically-operated parts of the invention are attached. Control and output signals are readable through an electronic display.
Owner:MARK ANDERSON & ASSOCS
Preparing method of oxiracetam injection and products thereof
InactiveCN1424034AReduce workloadThe preparation process is feasibleOrganic active ingredientsDrug compositionsActivated carbonMedical prescription
An oxiracetam injection is prepared from oxiracetam, gluclose (or sodium chloride) for injection and water for injection through proportioning, dissolving the glucose or sodium chloride for injection in the water for injection, adding activated carbon, heating, holding the temp. filtering, adding oxiracetam to the filtrate, stirring for dissolving, adding the water for injection, regulating pH value, fine filtering, bottling and sterilizing at 105-126.5 deg.C.
Owner:诸葛华明 +1
Oxiracetam injection
ActiveCN101396358AGood storage stabilityQuality improvementOrganic active ingredientsNervous disorderInjection solutionIntermediate product
The invention relates to an oxiracetam injection solution which comprises 1 weight portion of oxiracetam, 1.5 to 5.5 weight portions of glucose and 50 to 100 weight portions of injection water, wherein, the pH value of the injection solution is 3.8 to 4.5. The invention also provides a preparation method of the oxiracetam injection solution which comprises the steps as follows: a) the bulk drug oxiracetam and the glucose or sodium chloride are solved in the injection water under the temperature of 40 DEG C to 70 DEG C to obtain dissolved solution; b) the dissolved solution is cooled to the room temperature, active carbon is added for decolorizing, the active carbon is removed by filtering, proper quantity of water is complemented, and citric acid and sodium citrate are added to adjust the pH value to 3.8 to 4.5 to obtain the dissolved solution with adjusted pH value; c) an intermediate product after encapsulation is sterilized; and d) the product after the sterilization is packed. The oxiracetam injection solution manufactured by the prescription and the preparation method of the invention has good storage stability.
Owner:广东世信药业有限公司 +1
Salt of leonurine and its preparation
InactiveCN1415603ALess irritatingGuaranteed stabilityOrganic active ingredientsOrganic chemistryLeonurineOrganic acid
Owner:李晓祥
Taxanes medicine preparation for intravenous injection and preparation method thereof
ActiveCN101288642AGood biocompatibilityHigh tolerance in vivoOrganic active ingredientsSolution deliveryDrugs solutionDocetaxel
The invention relates to the technical field of medicine, which is a preparation of a taxane drug for intravenous drug delivery, consisting of two parts of drug solution and an emulsion. The drug solution is composed of paclitaxel or docetaxel, a pH regulator and a solvent for injection, wherein, the solvent for injection is an organic solvent; the emulsion comprises a fat emulsion and is composed of oil for injection, an emulsifier, an antioxidant, an isotonic regulator, a stabilizer, a pH regulator and water for injection. When in use, the drug solution can be added and evenly mixed in the emulsion for direct intravenous drip according to the clinical drug dosage and can also be firstly added in the emulsion with the volume that is not less than 5 times of the volume of the drug solution according to the clinical drug dosage and then added with a certain amount of physiological saline or glucose injection for intravenous drip. The preparation of the invention does not contain solubilizer and has the advantages of little toxicity, safety, effectivity, stability and economy. The fat emulsion can also be taken as a nutrition replenisher for a patient, thus achieving better treatment effects. The physiological saline or the glucose injection can also replace a certain amount of the emulsion, so the storage and the transportation are convenient, and the preparation is more economical.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
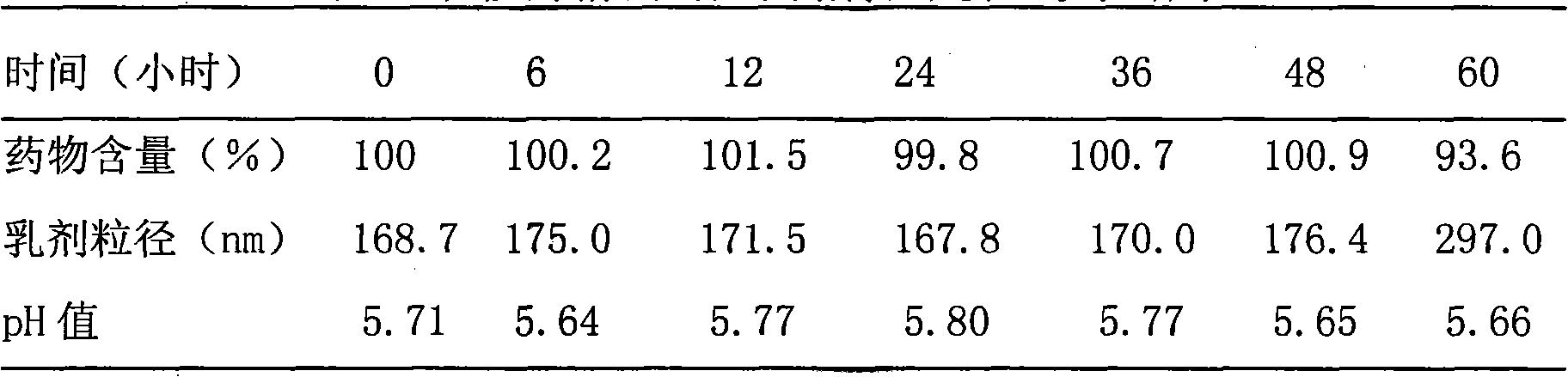
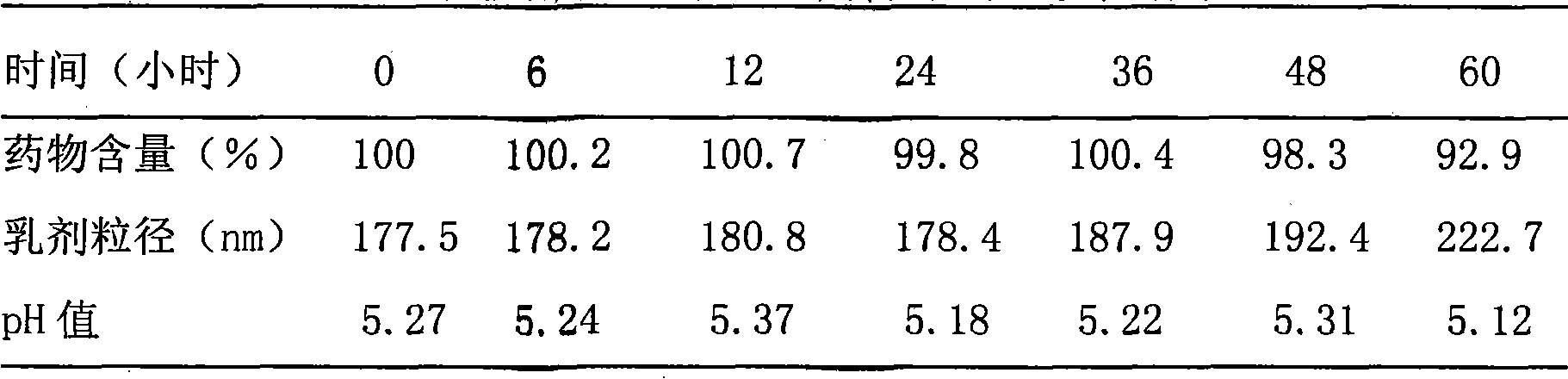
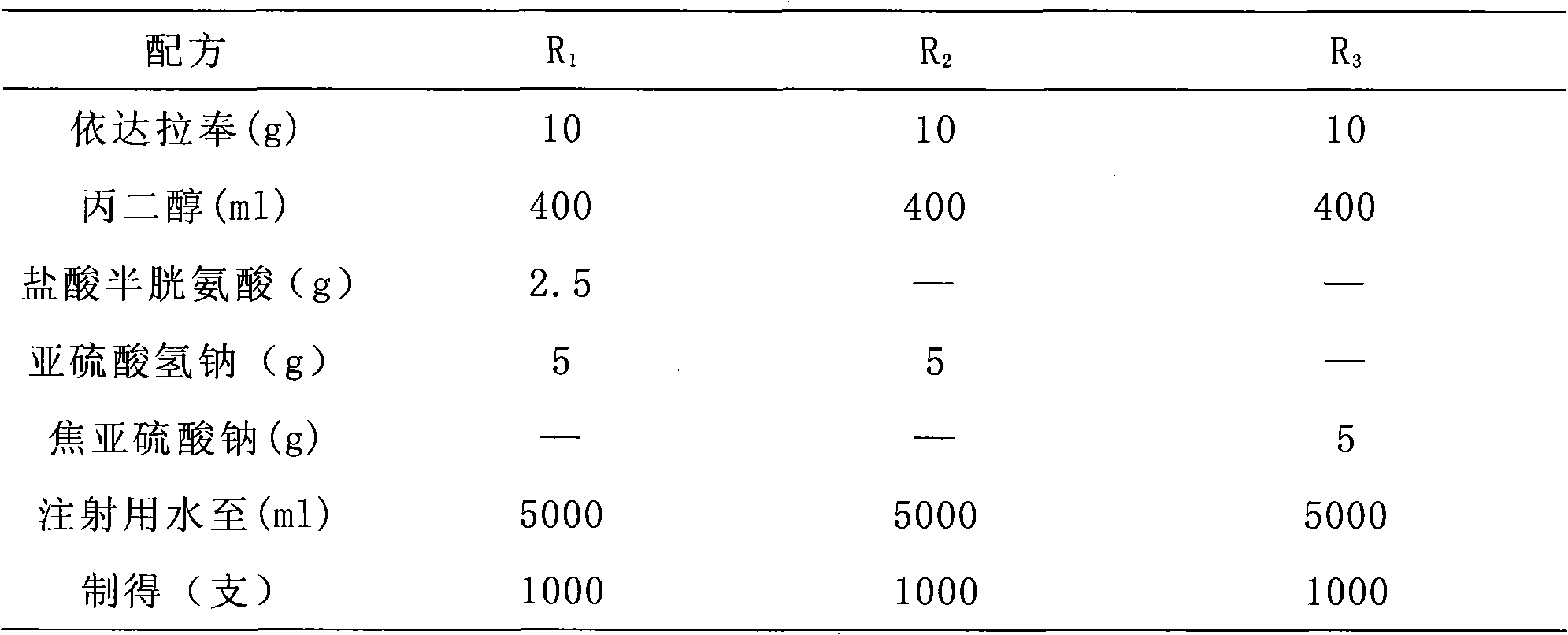
Edaravone injection and preparation method thereof
InactiveCN101933899AImprove stabilityLong validity periodOrganic active ingredientsNervous disorderEdaravone InjectionNitrogen
The invention discloses edaravone injection and a preparation method thereof. The injection contains edaravone serving as an active material, sodium pyrosulfite serving as antioxygen and propylene glycol serving as cosolvent. In the invention, in an edaravone injection production process, the sodium pyrosulfite antioxygen is added and high-purity nitrogen is charged into an ampoule bottle before and after liquid medicine canning and sealing to ensure the residual oxygen in the air above the liquid medicine in the ampoule bottle is less than 3 percent, so the edaravone is kept in a low-oxygen environment all the time. In the invention, the stability of the edaravone injection is improved, the period of validity of the edaravone injection is prolonged, and the safety and effectiveness of the edaravone injection in clinical use are ensured.
Owner:NANJING SIMCERE DONGYUAN PHARM CO LTD
Method for refining injection-level ambroxol hydrochloride, product and injection thereof
ActiveCN102153482AQuality improvementImprove stabilityOrganic active ingredientsOrganic compound preparationSolventAqueous solution
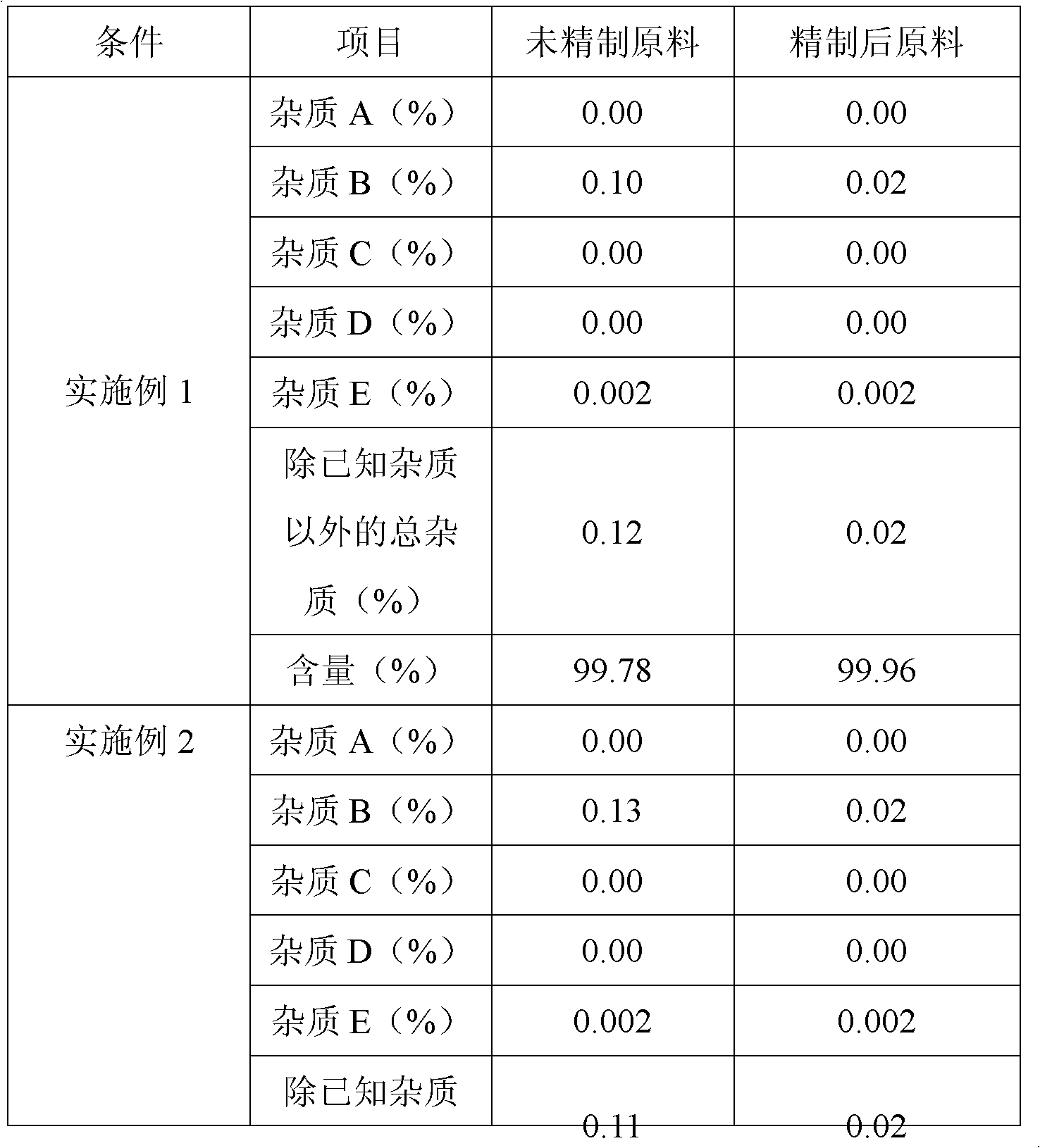
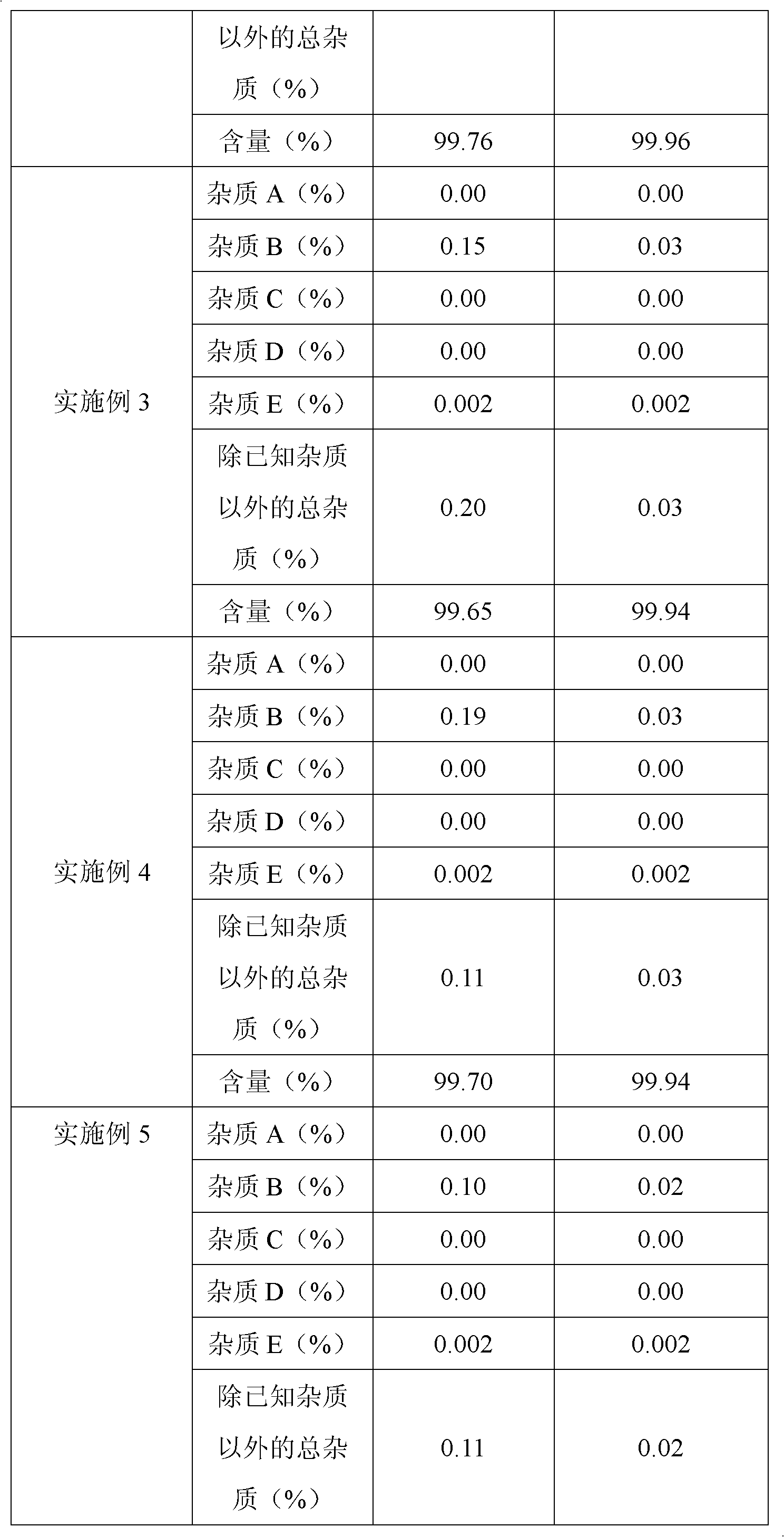
The invention belongs to fields of a method for refining and purifying compounds and products thereof, and particularly relates to a method for refining and purifying an ambroxol hydrochloride Chinese herbal medicine, a product thereof and the field of application. The method for refining the injection-level ambroxol hydrochloride is characterized by comprising the following steps of: adding 70.2to 88 volume percent of aqueous solution of ethanol (g / ml) into an ambroxol hydrochloride raw material (g) of which the purity is over 99.0 percent in a ratio of 1:(5.5-9.2); heating, and distilling until the ambroxol hydrochloride raw material is dissolved fully; stopping heating, cooling and crystallizing to separate ambroxol hydrochloride out; and filtering the solvent to obtain crystals, and drying to obtain the injection-level ambroxol hydrochloride. In the injection-level ambroxol hydrochloride raw material, an impurity B is less than or equal to 0.03 percent, an impurity E less than orequal to 0.002 percent, and total impurities except for the impurities B and E are less than or equal to 0.03 percent; and the purity is over 99.9 percent.
Owner:天津市铭泰医药科技有限公司
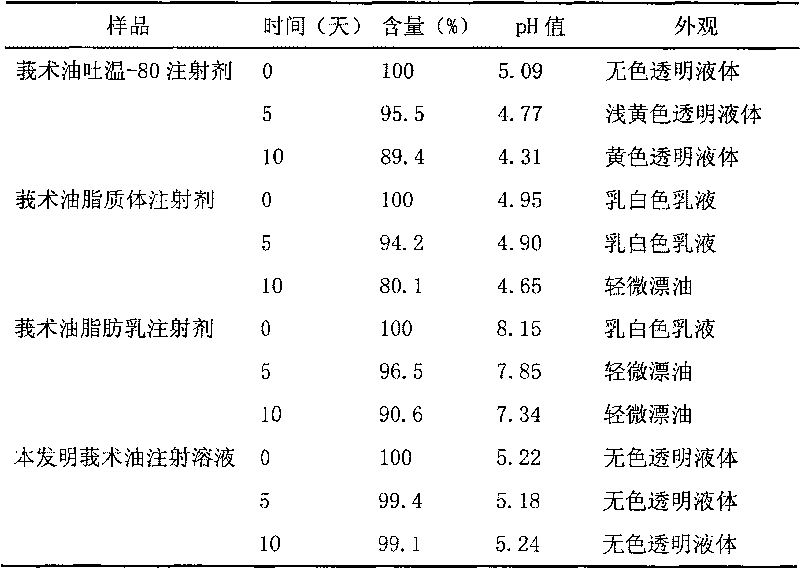
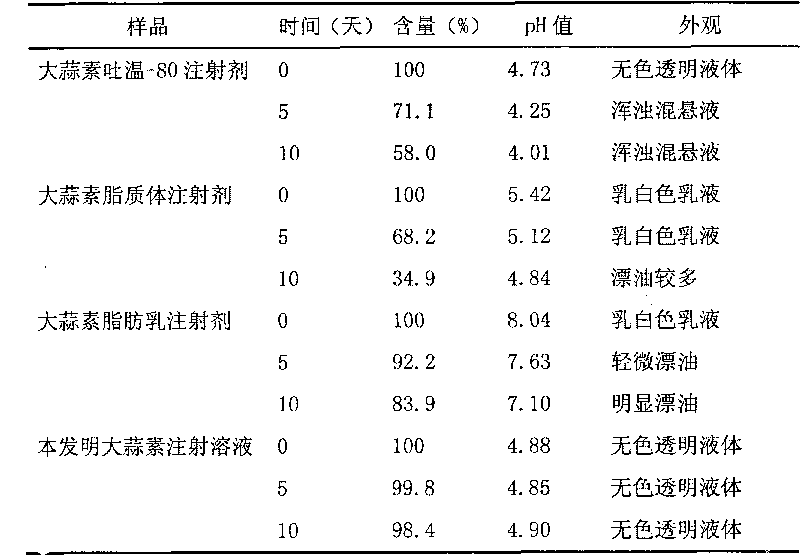
Chinese medicinal essential oil injection solution, injection and preparation method thereof
InactiveCN101708314AFix stability issuesSimple preparation processAntibacterial agentsSulfur/selenium/tellurium active ingredientsEmulsionSolvent
The invention relates to the technical field of medicaments, and discloses a Chinese medicinal essential oil injection solution, an injection thereof and a preparation method thereof. The invention provides a stable and safe Chinese medicinal essential oil injection without Tween-80, which consists of the Chinese medicinal essential oil injection solution and a dispersion medium, wherein the Chinese medicinal essential oil injection solution consists of a Chinese medicinal essential oil, a stabilizing agent, a pH value regulator and a solvent for injection; and the dispersion medium is an emulsion for injection. When in clinical medication, the Chinese medicinal essential oil injection solution is dispersed in the emulsion for the injection and is prepared into the Chinese medicinal essential oil injection, and then the intravenously administrable can be performed. In the invention, the Chinese medicinal essential oil injection solution and the emulsion for the injection are packaged and stored respectively so that the stability of a medicament stored for a long time can be improved. The invention provides a harmfulless, safe and conveniently-stored intravenous injection for the Chinese medicinal essential oil.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Delivery of molecules and complexes to mammalian cells in vivo
InactiveUS7589059B2Improved in vivo deliveryEasy to moveVirusesPeptide/protein ingredientsMedicineIn vivo
Disclosed is a system for providing in vivo delivery of molecules or complexes to extravascular mammalian cells using an intravascular administration route. The molecules or complexes are inserted in an injection solution into a mammalian vasculature. Insertion of the injection solution at an appropriate rate transiently increases the volume of extravascular fluid in the tissue thereby facilitating delivery of the molecule to the cell.
Owner:ROCHE MADISON
Drop pills for treating asthma cough and preparation method thereof
InactiveCN101011494AEasy to keepReduce dosagePill deliveryPharmaceutical non-active ingredientsDrugAsthma
The invention relates to a drop agent used to treat asthma and relative preparation. Via tests, the invention uses the Chinese ephedra and datura flower as main drugs, at 300-600 and 100-300 mass ratios. And the invention can add 100-400 deals of drug X and 100-500 deals of findings. The invention has the functions as resolving phlegm and relieving cough, or the like.
Owner:GUANGDONG PHARMA UNIV
A glycerin and fructose injection and preparation method thereof
InactiveCN1864693AGood isotonic effectExtended shelf lifeHydroxy compound active ingredientsPharmaceutical delivery mechanismFructoseGlycerol
The present invention relates to one kind of glycerine-fructose injection and its preparation process. The injection in 250 liters consists of glycerine fructose in 25 kg, fructose in 12.5 kg, sodium chloride in 2.25 kg and water for injection for the rest, and may be compounded into 1000 bottles of transfused solution of 250 ml each or 500 bottles of transfused solution of 500 ml each. The present invention also provides the preparation process of the injection.
Owner:曾列丹
Oxiracetam injection and preparation method thereof
The invention provides an oxiracetam injection, characterized in that 1000 ml of the injection contains 100-250 g of oxiracetam, and the pH value is adjusted to 4.0-5.5 by using 0.01-0.1 mol / L of a NaOH solution, and the balance of the injection is injection water. The invention also provides a preparation method of the oxiracetam injection. The oxiracetam injection has the advantages of low content of impurities and good stability, and can be stored at room temperature.
Owner:YAOPHARMA CO LTD
MSC (mesenchymal stem cell) injection as well as preparation and application thereof
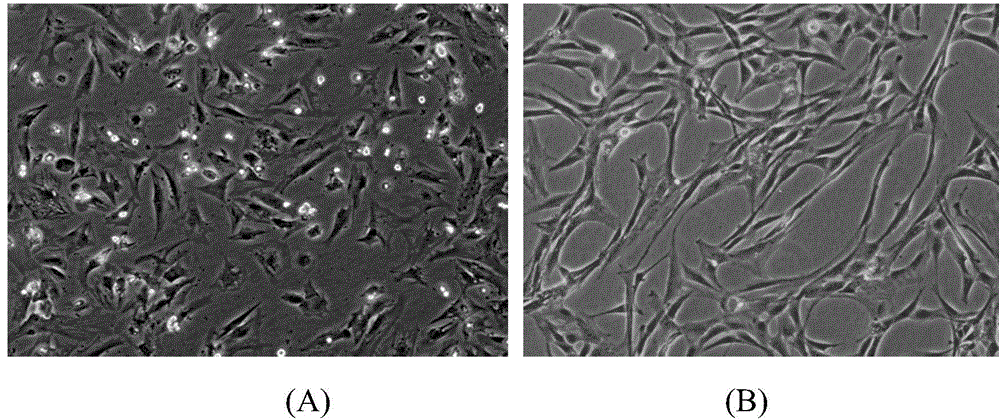
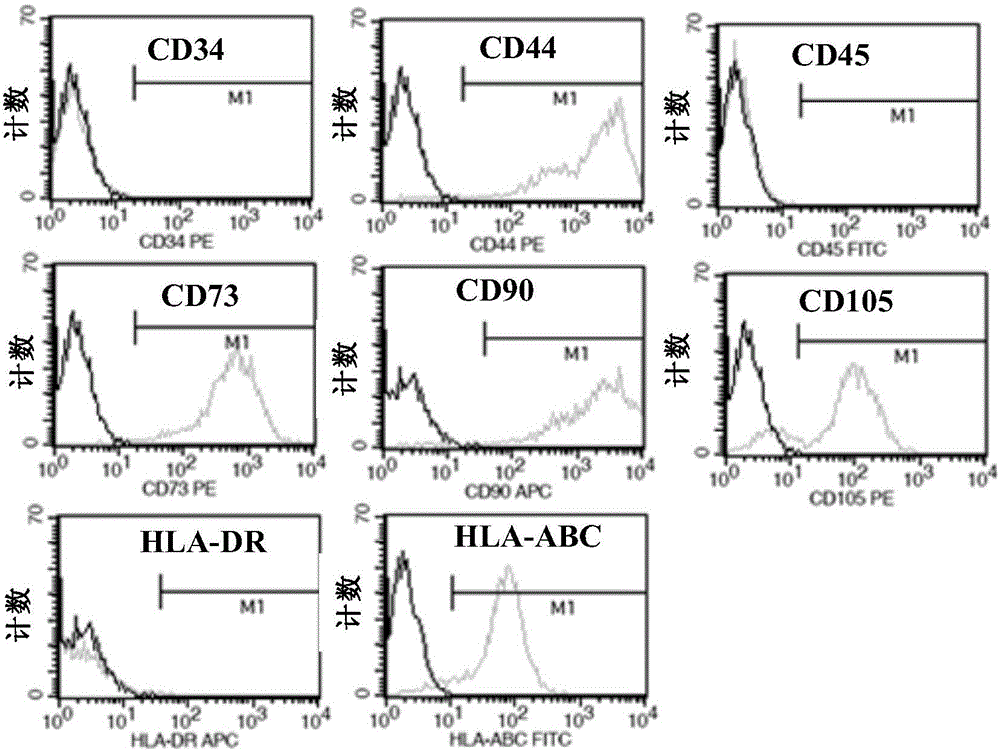
InactiveCN104857022AImprove survival rateAvoid gatheringPharmaceutical delivery mechanismUnknown materialsClinical gradeHydroxyethyl starch
The invention relates to the field of biology, in particular to MSC (mesenchymal stem cell) injection as well as a preparation and an application thereof. The injection comprises MSCs and a cell cryopreservation solution, wherein the cryopreservation solution comprises components in percentage by volume as follows: 25%-70% of an electrolyte balance solution, 5%-20% of clinical-grade DMSO (dimethyl sulfoxide), 1%-50% of 20% human serum albumin, 1%-10% of hydroxyethyl starch 130 / 0.4 and 5%-20% of triphosadenine-disodium magnesium chloride freeze-drying powder. The injection is free of animal serum, has clear ingredients and good cell cryopreservation effect and is safe and controllable, long-term storage and long-distance transport are facilitated, the survival rate of cells after recovery is higher than 95%, the vitality is high, and the injection can effectively relieve injury and inflammation symptoms of lesion tissue of lungs and promote tissue regeneration of the lungs, so that acute lung injury can be fundamentally and comprehensively treated.
Owner:北京青藤谷禧干细胞科技研究院有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com