Patents
Literature
408 results about "Medication injection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
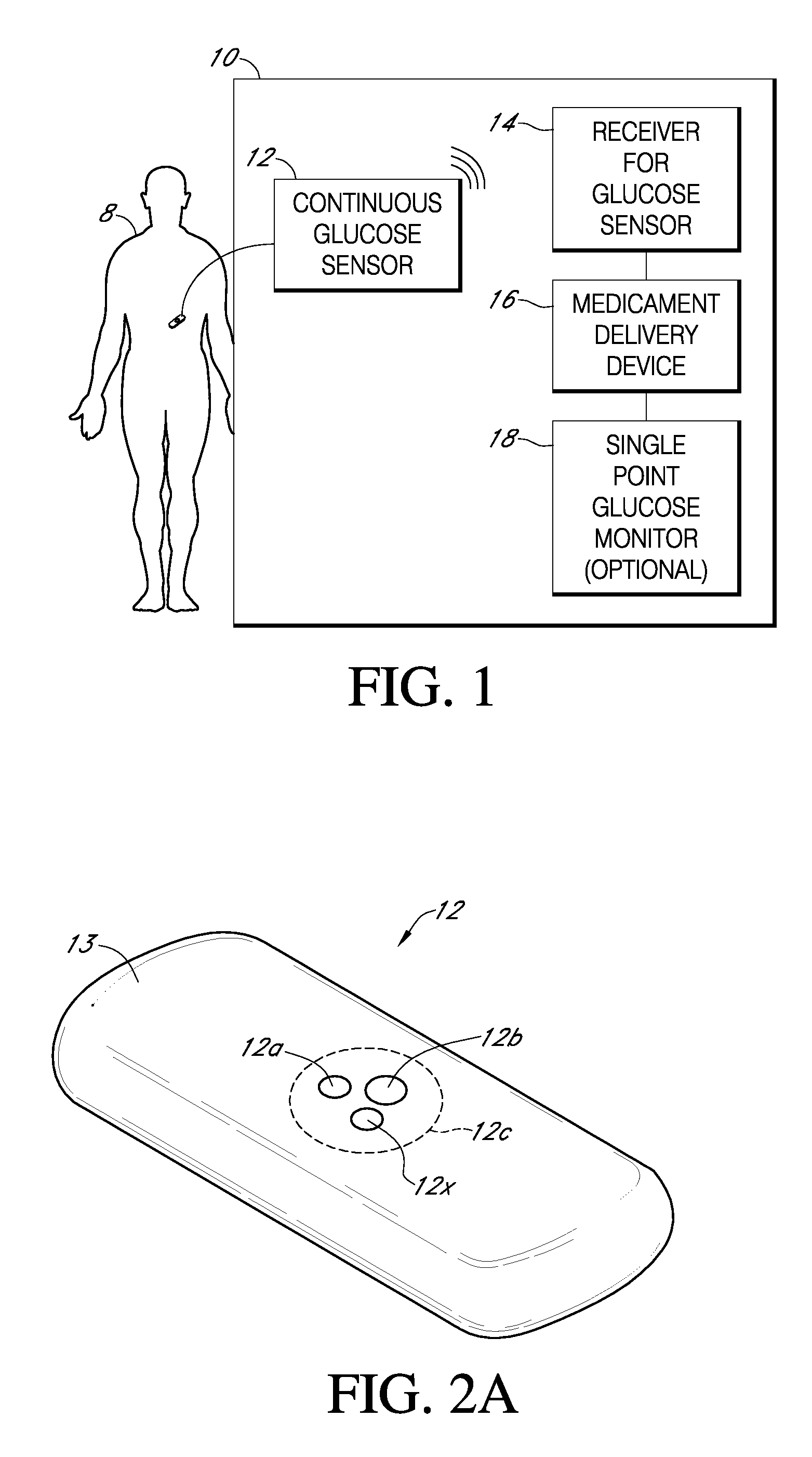
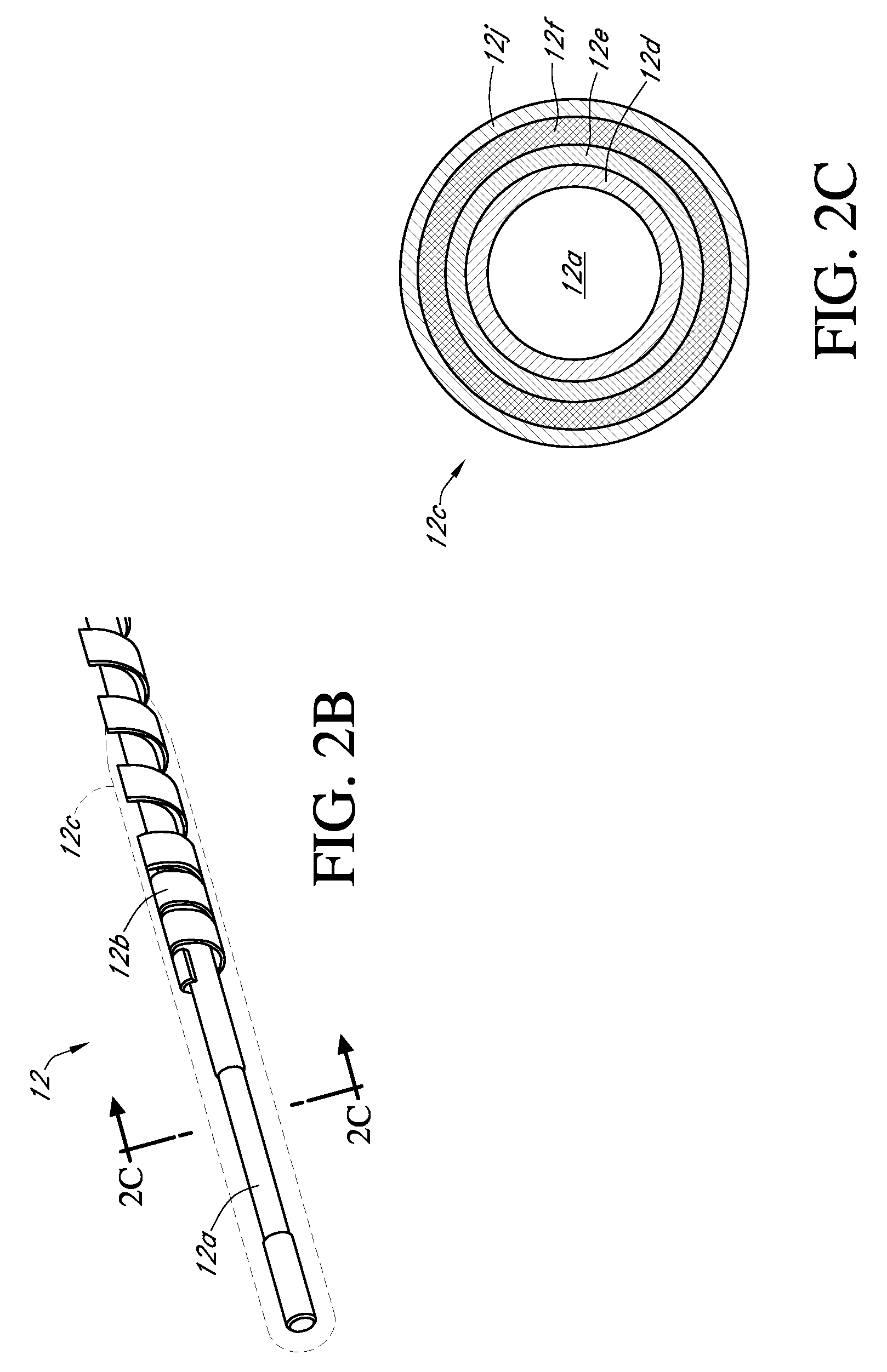
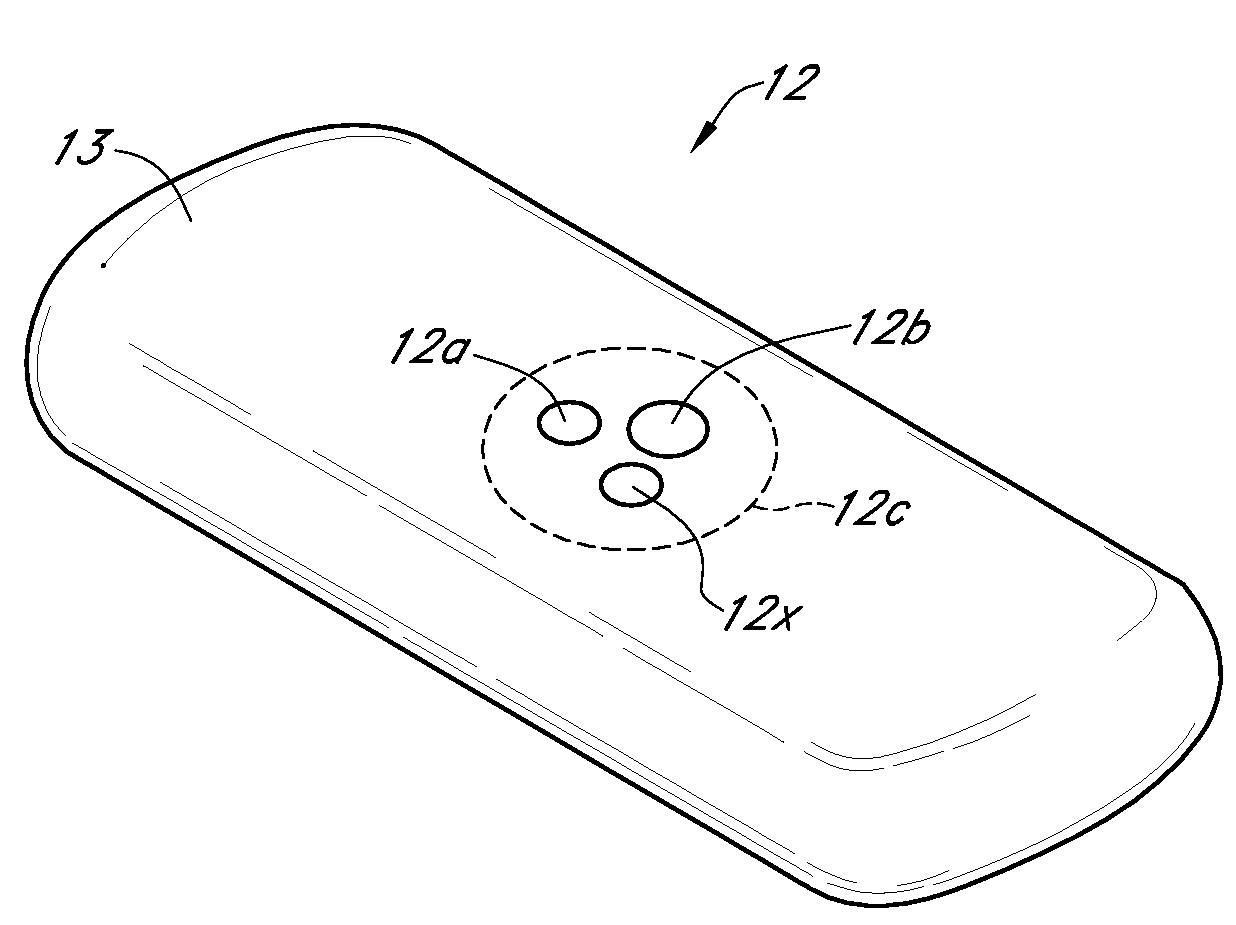
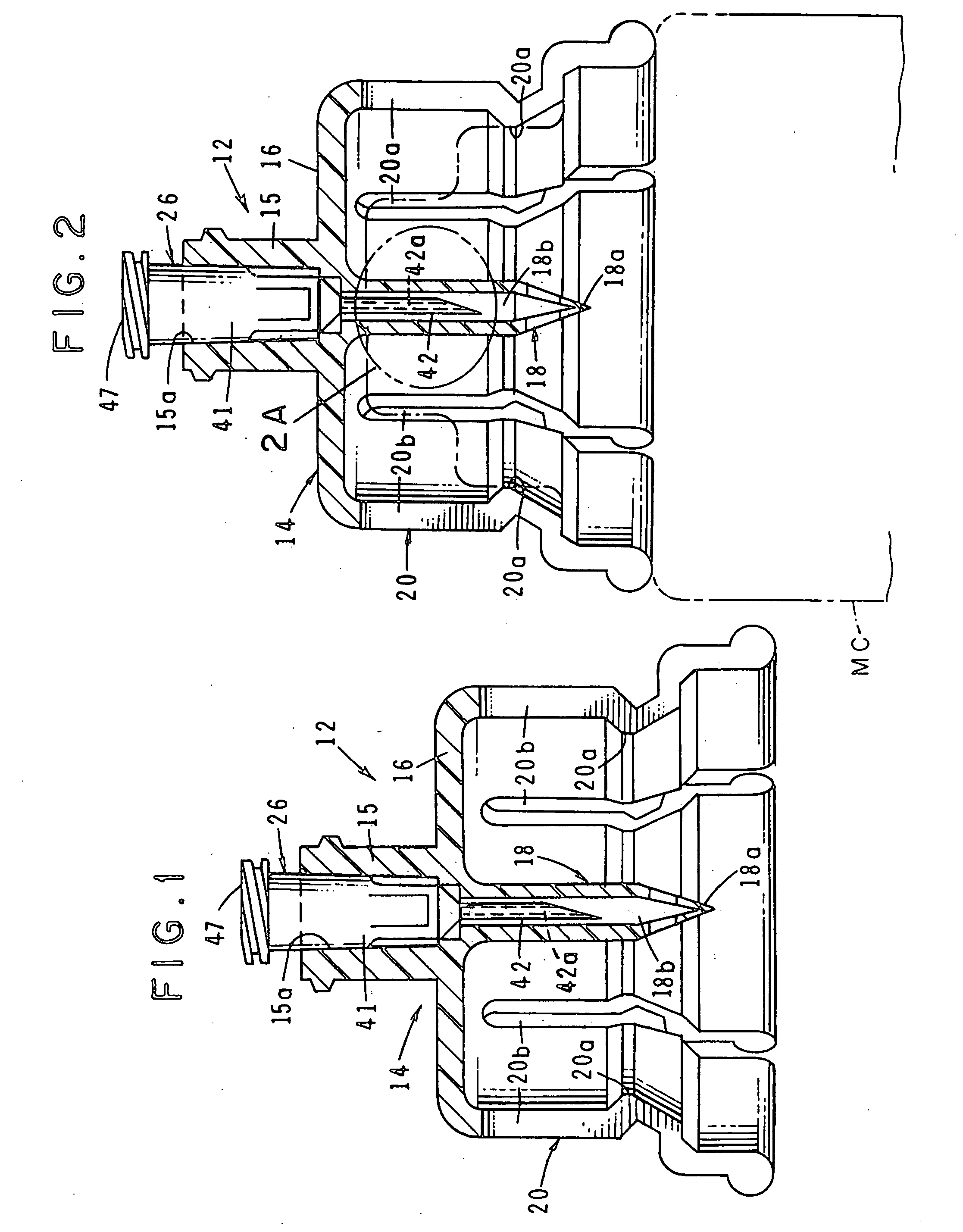
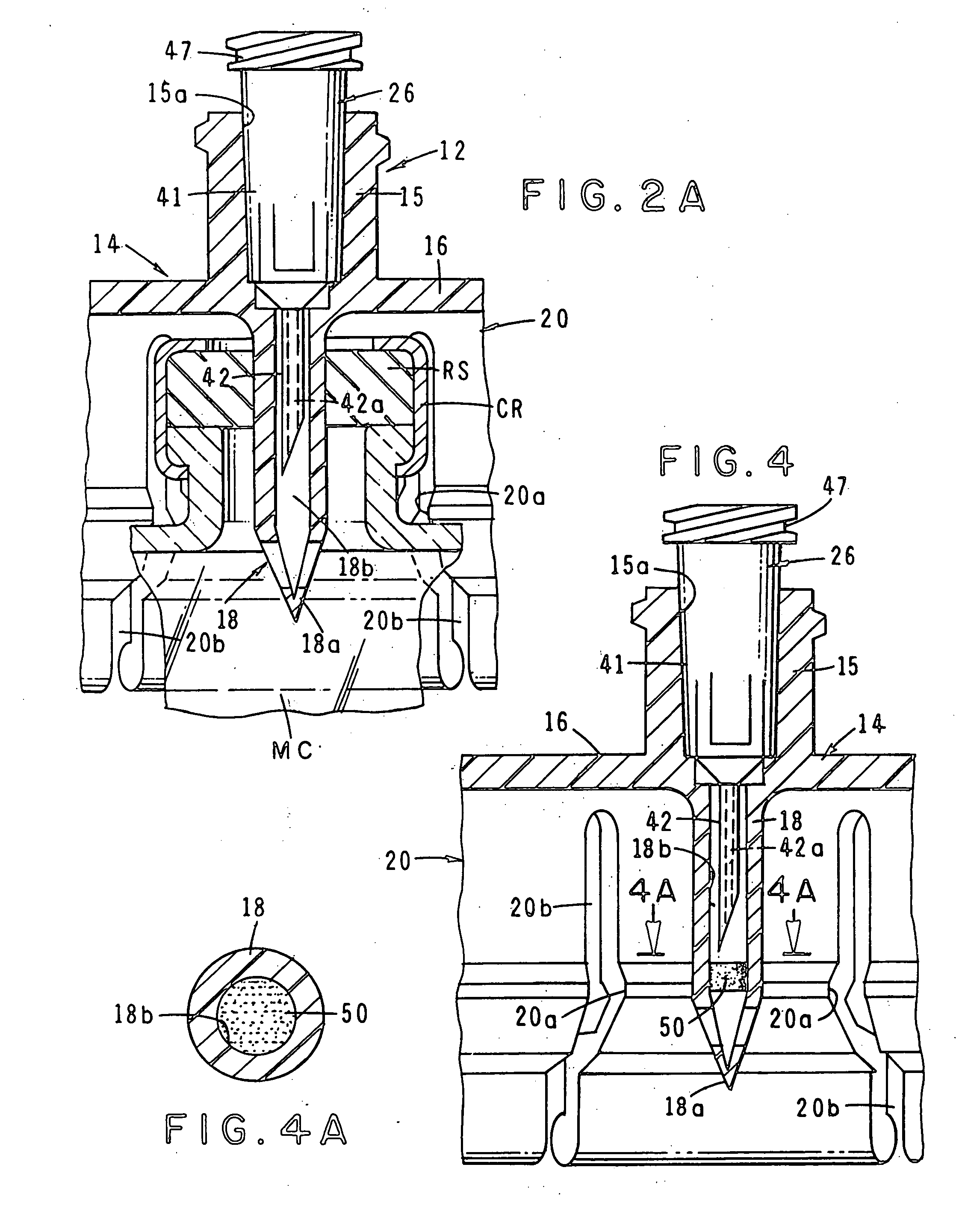
Medications given using a subcutaneous injection. Medications administered by subcutaneous injection include drugs that can be given in small volumes (usually less than 1 mL but up to 2 mL is safe). Insulin and some hormones are commonly administered as subcutaneous injections.
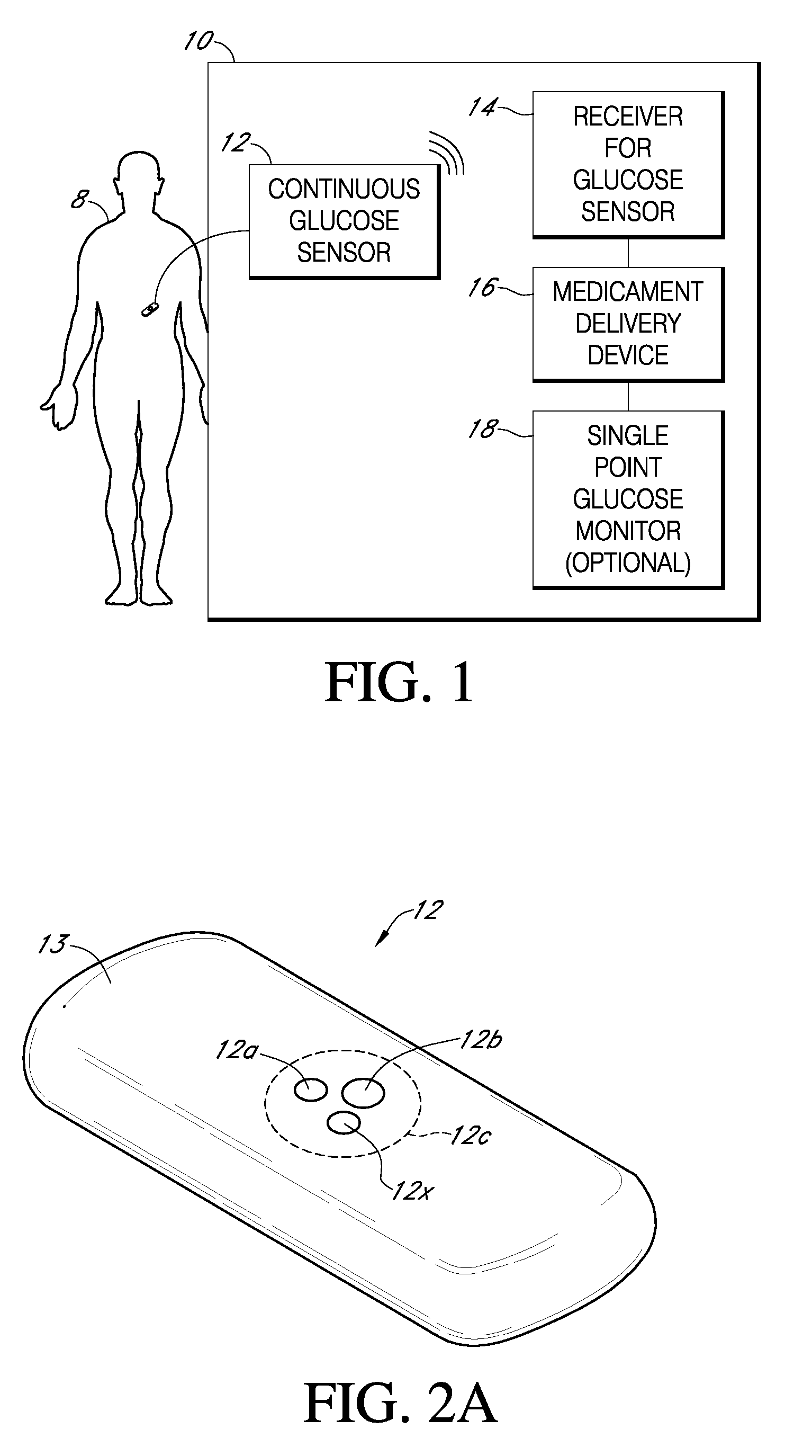
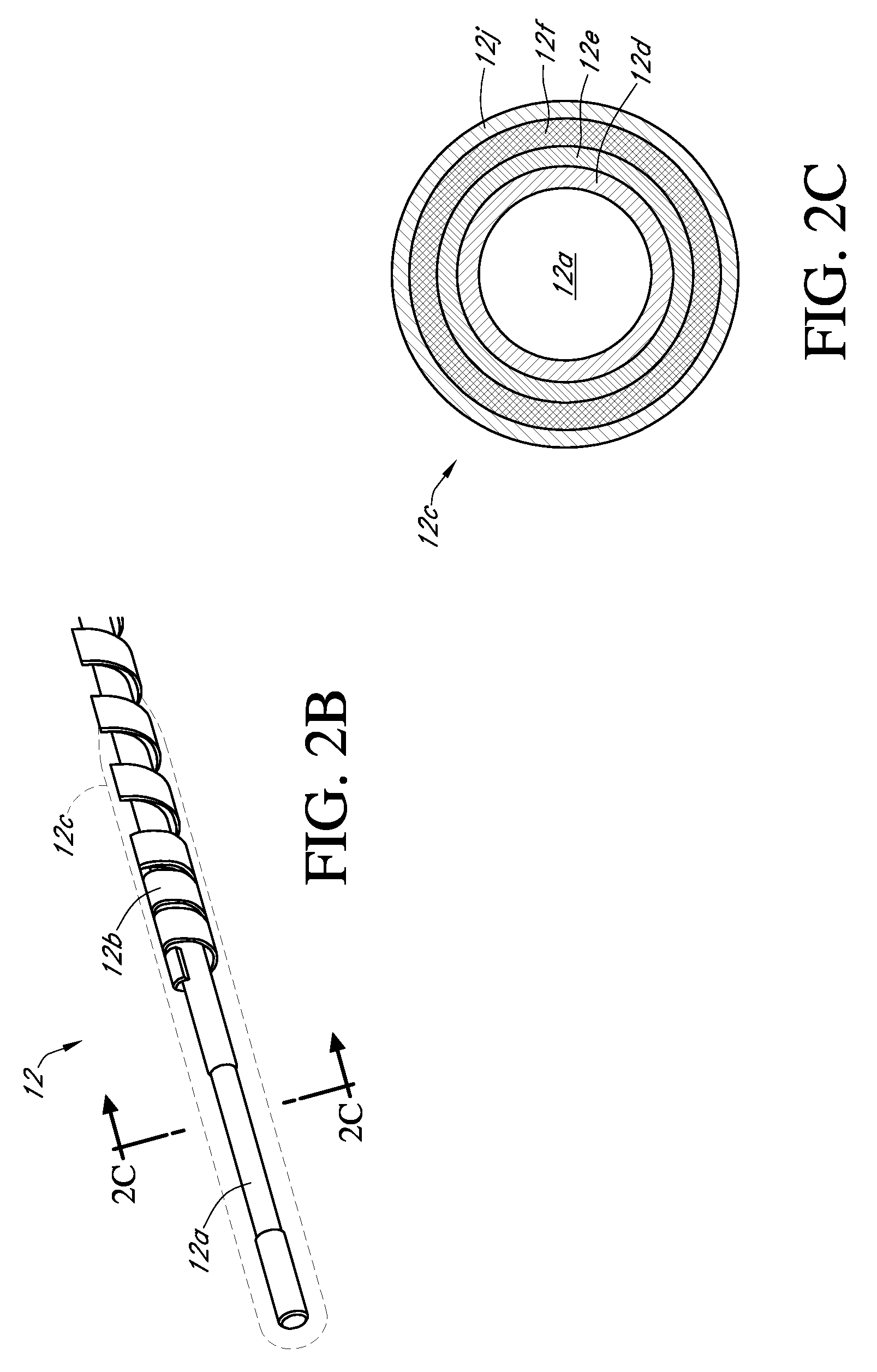
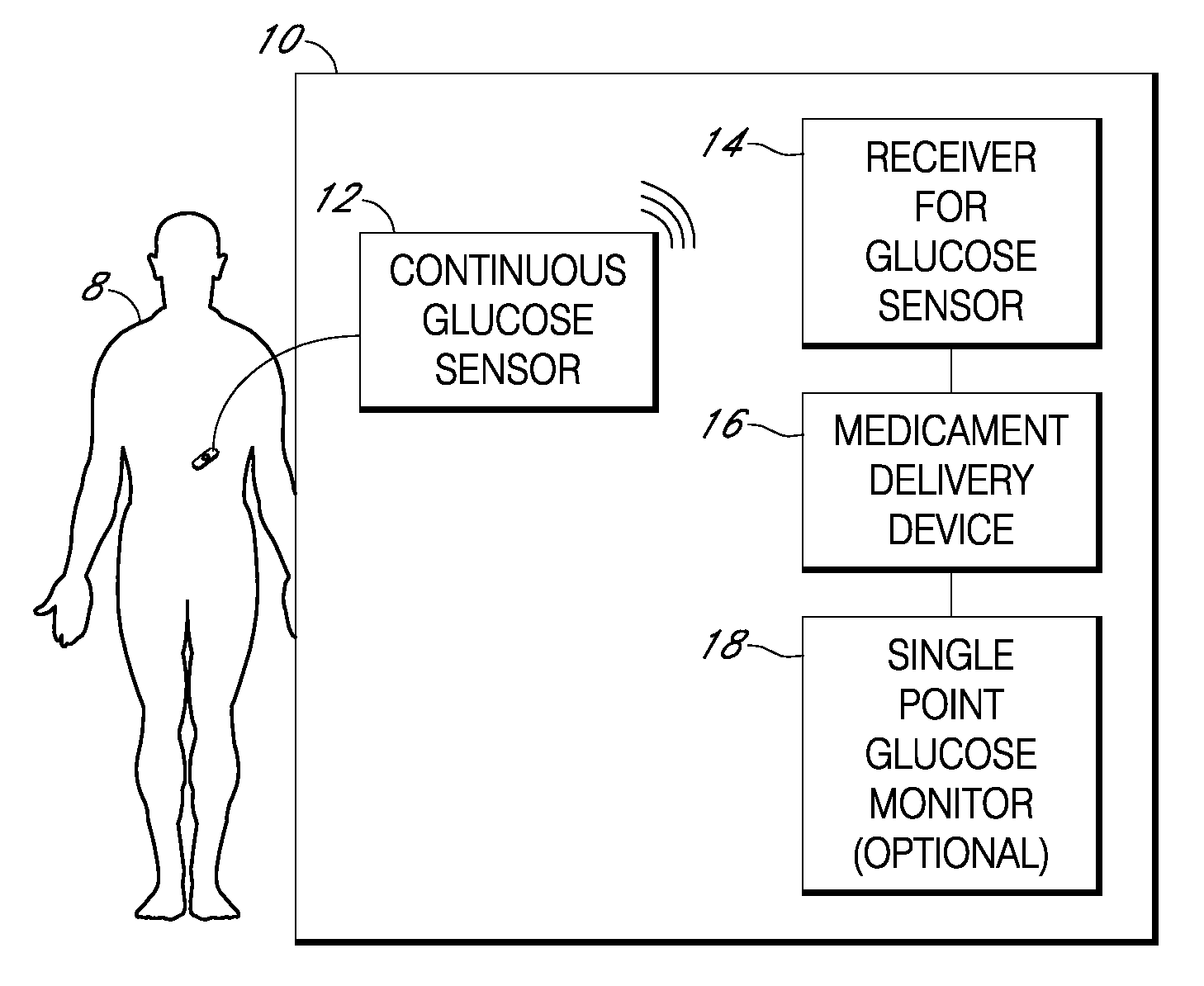
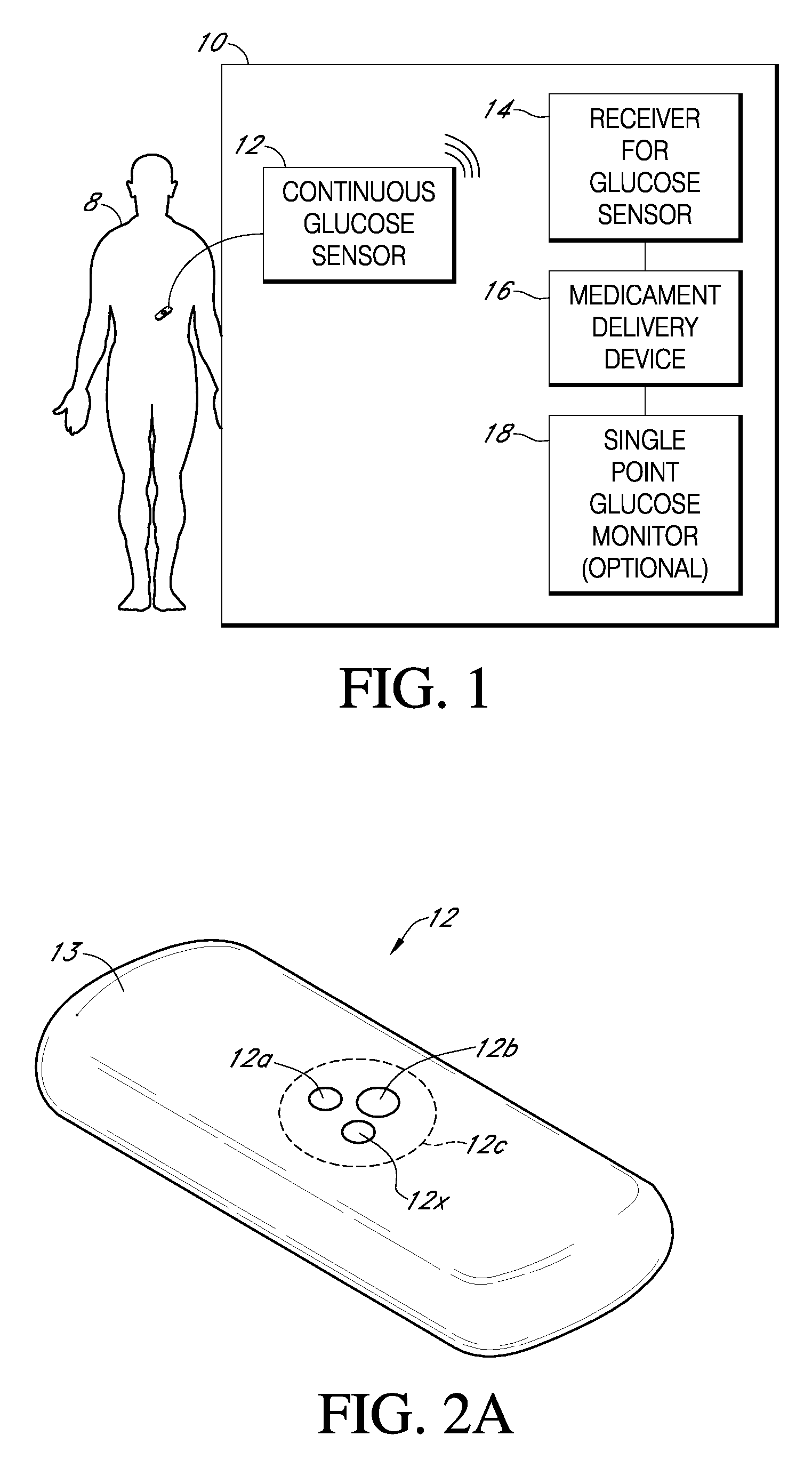
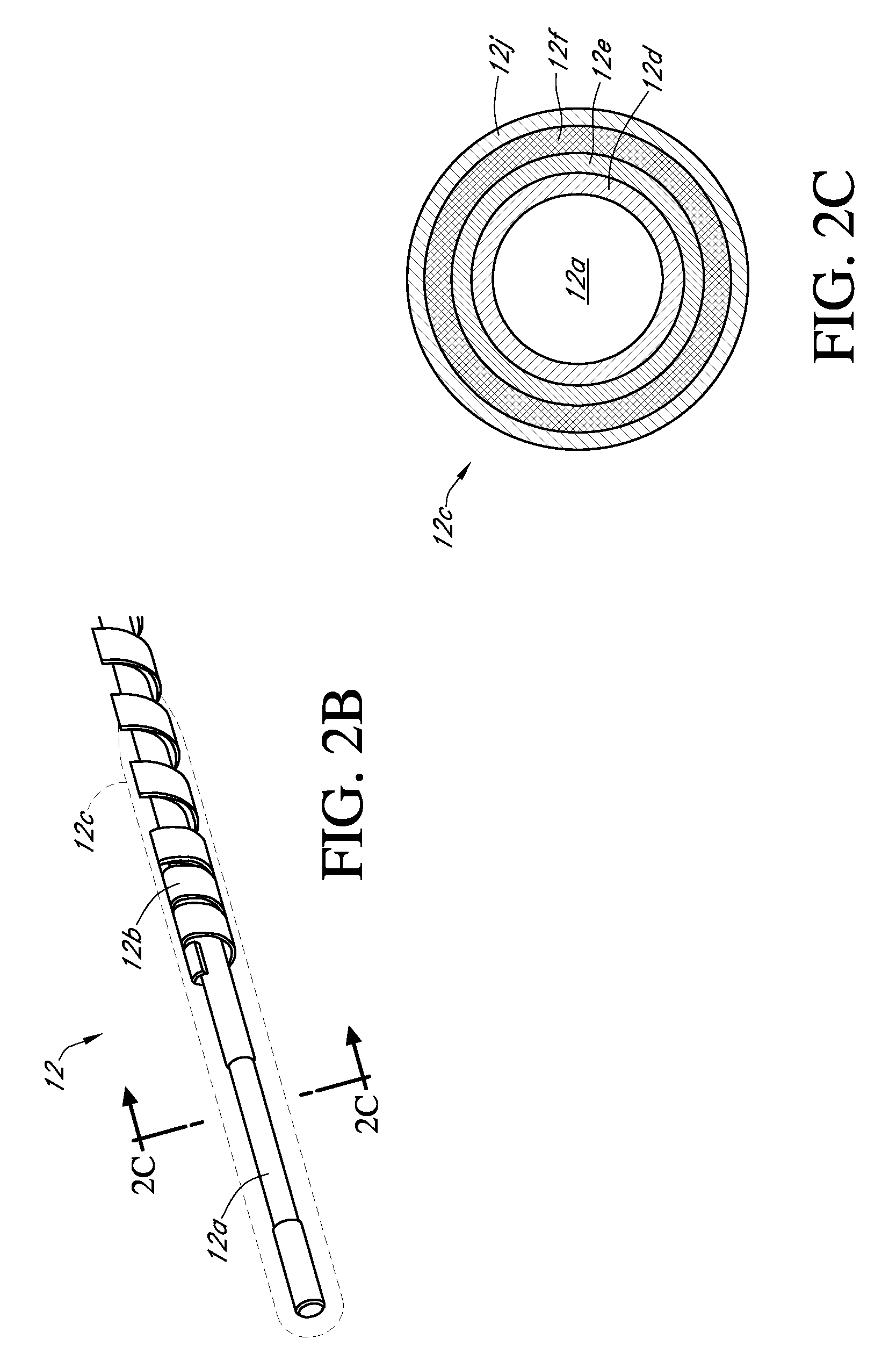
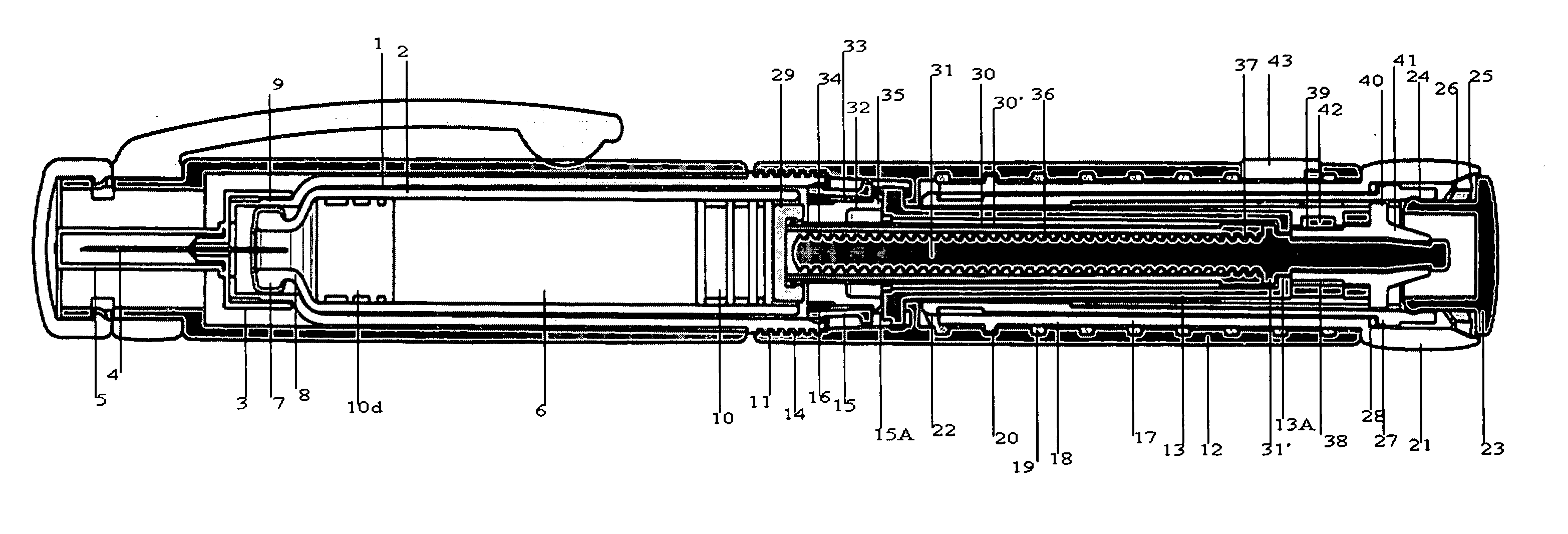
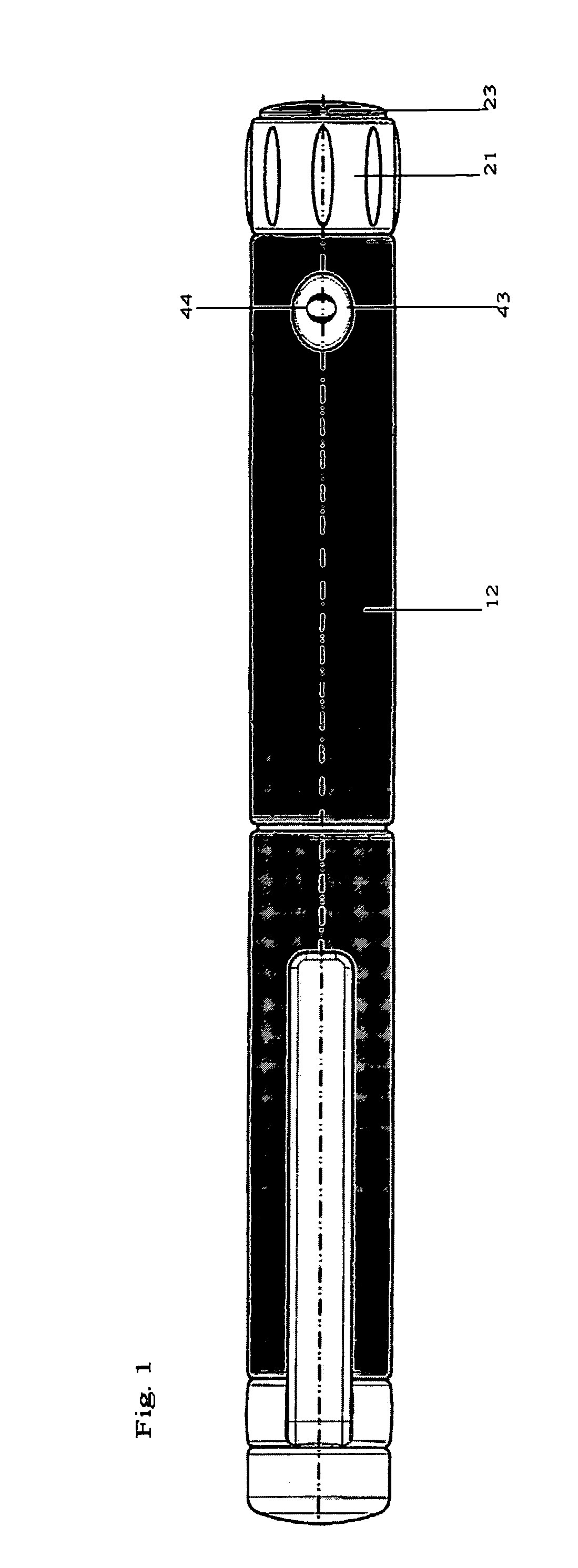
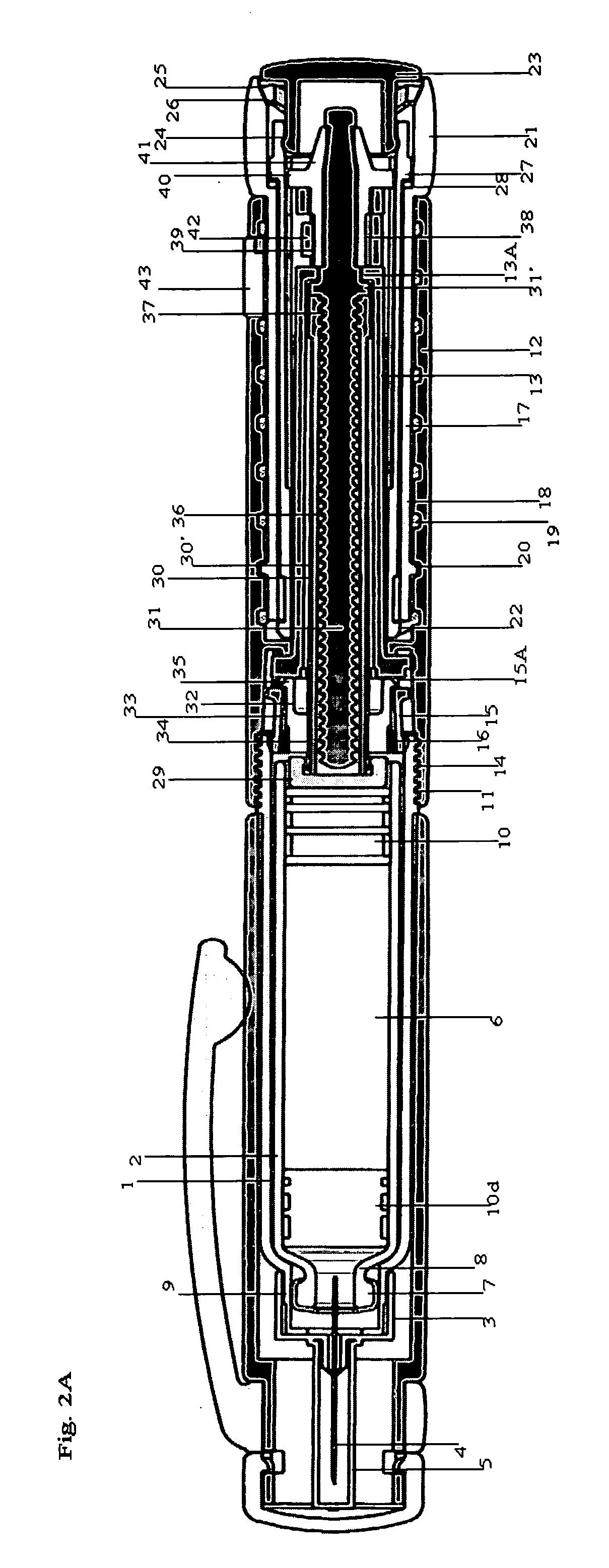
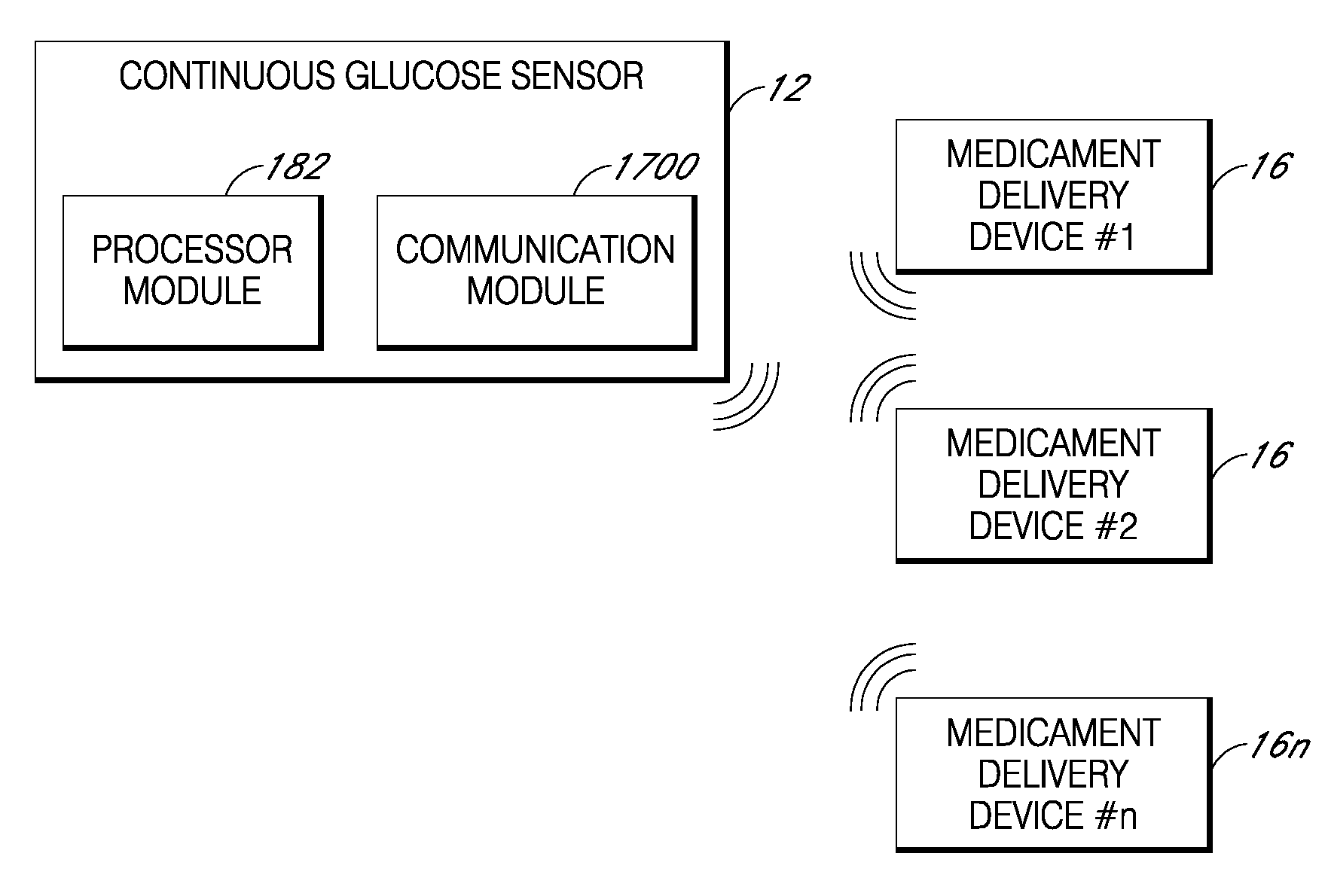
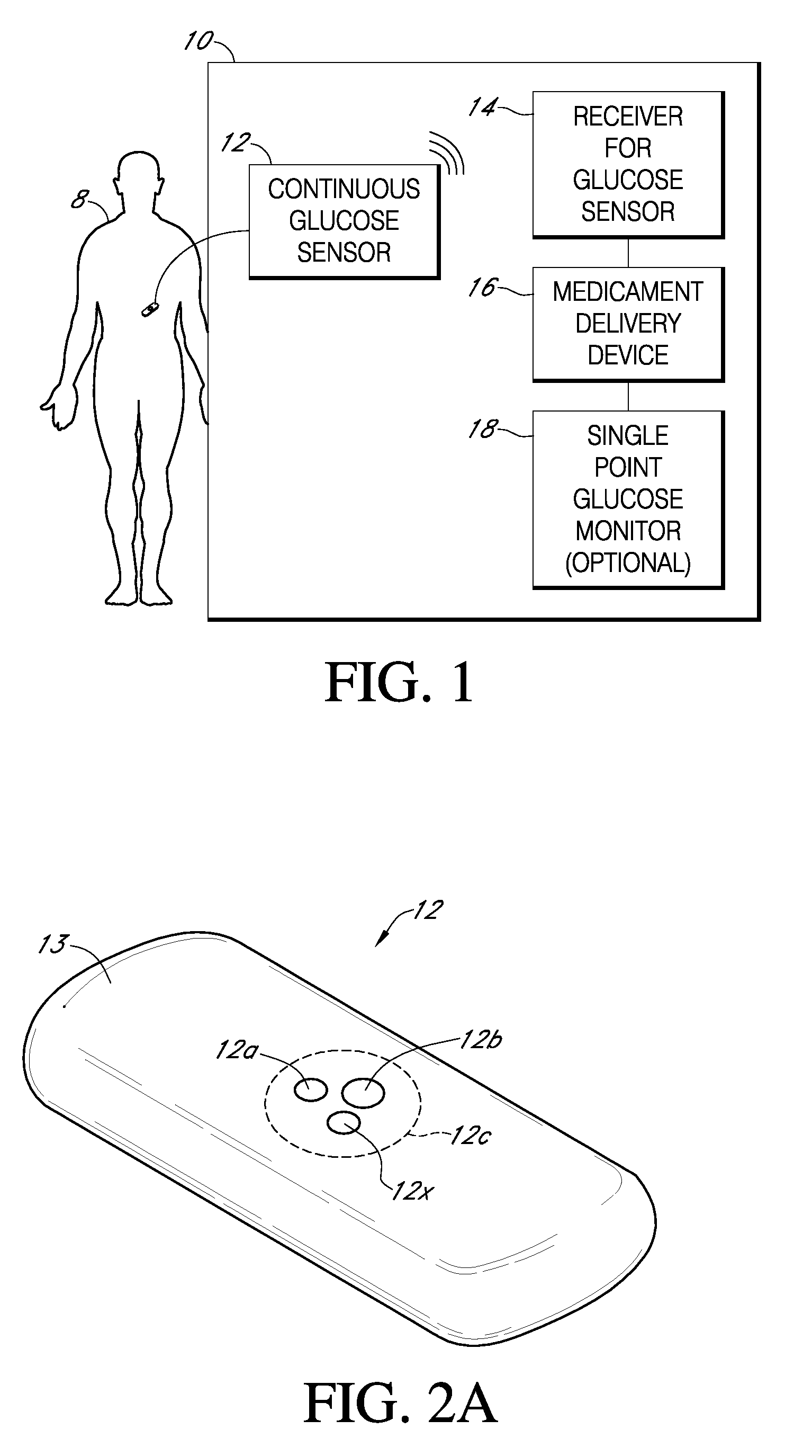
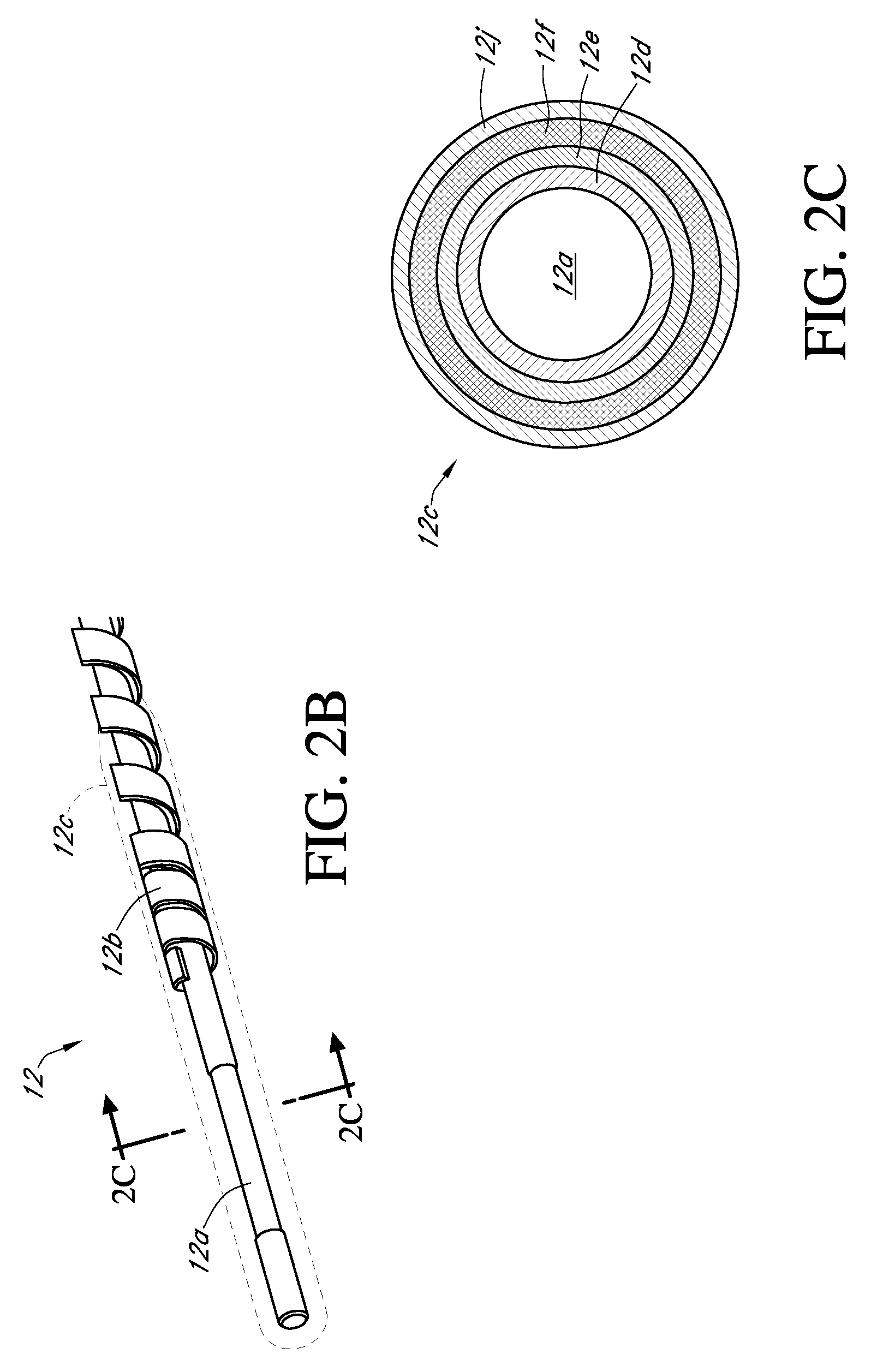
Integrated medicament delivery device for use with continuous analyte sensor
InactiveUS20080306434A1Reduce the burden onSimple processAmpoule syringes2D-image generationDiabetes mellitusMedication injection
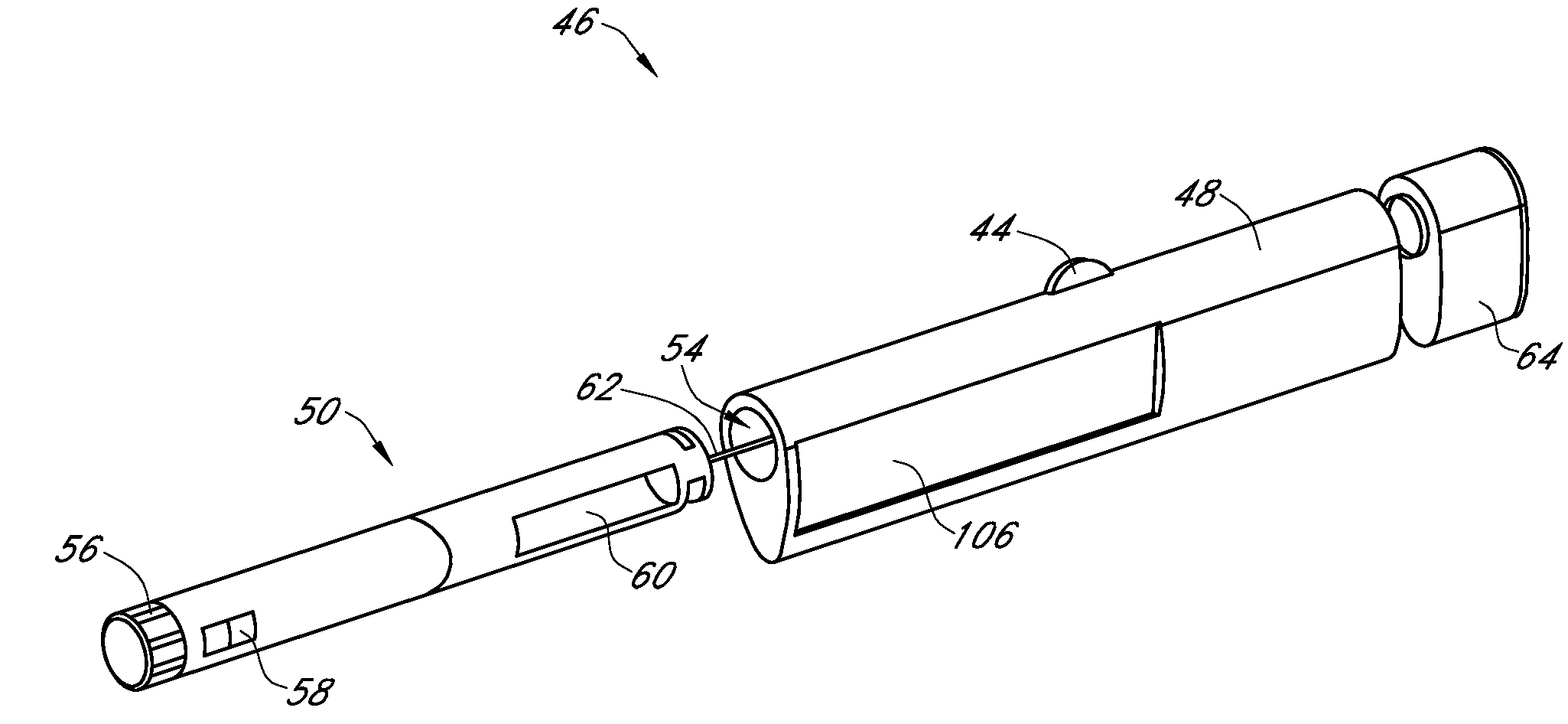
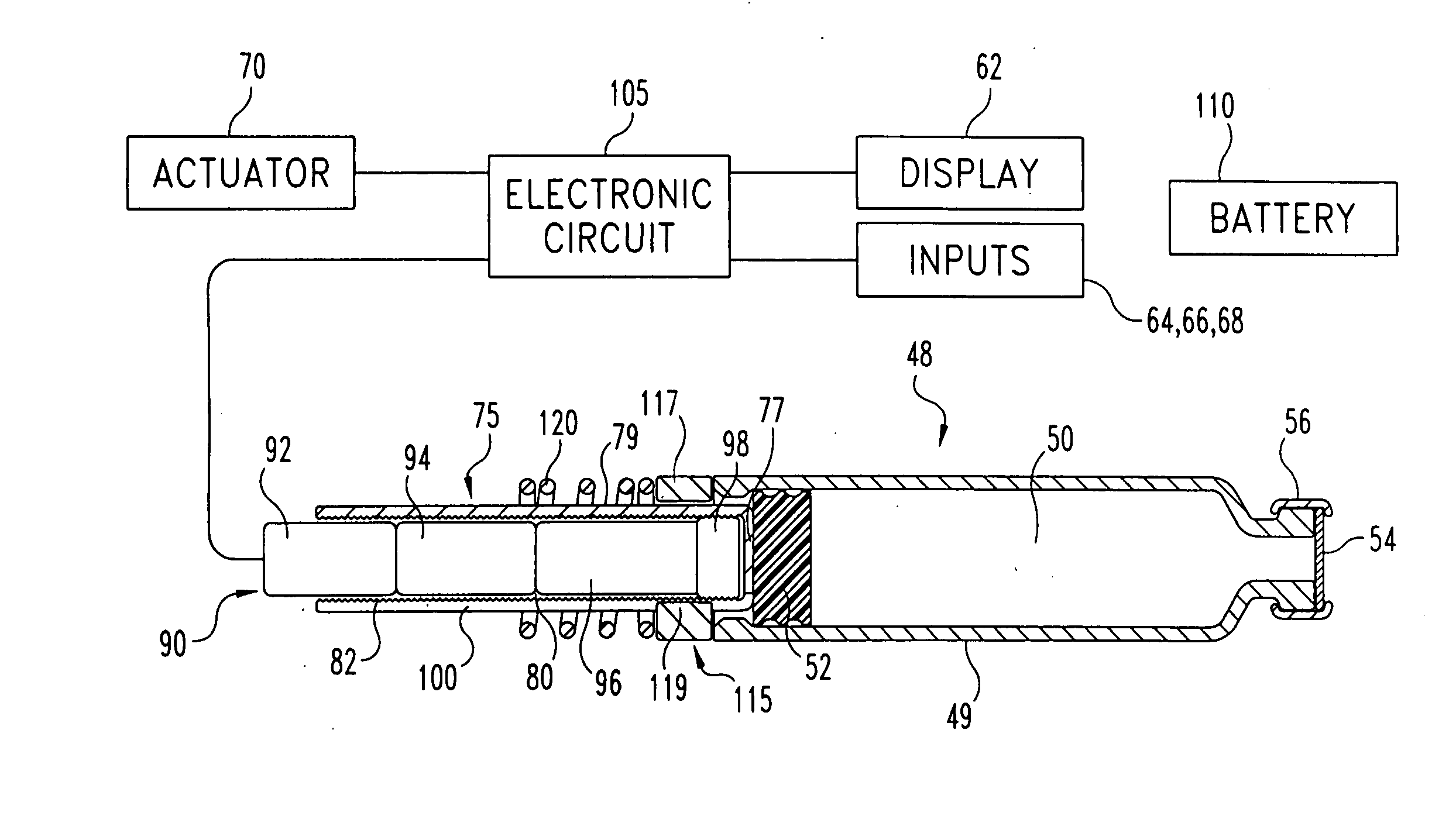
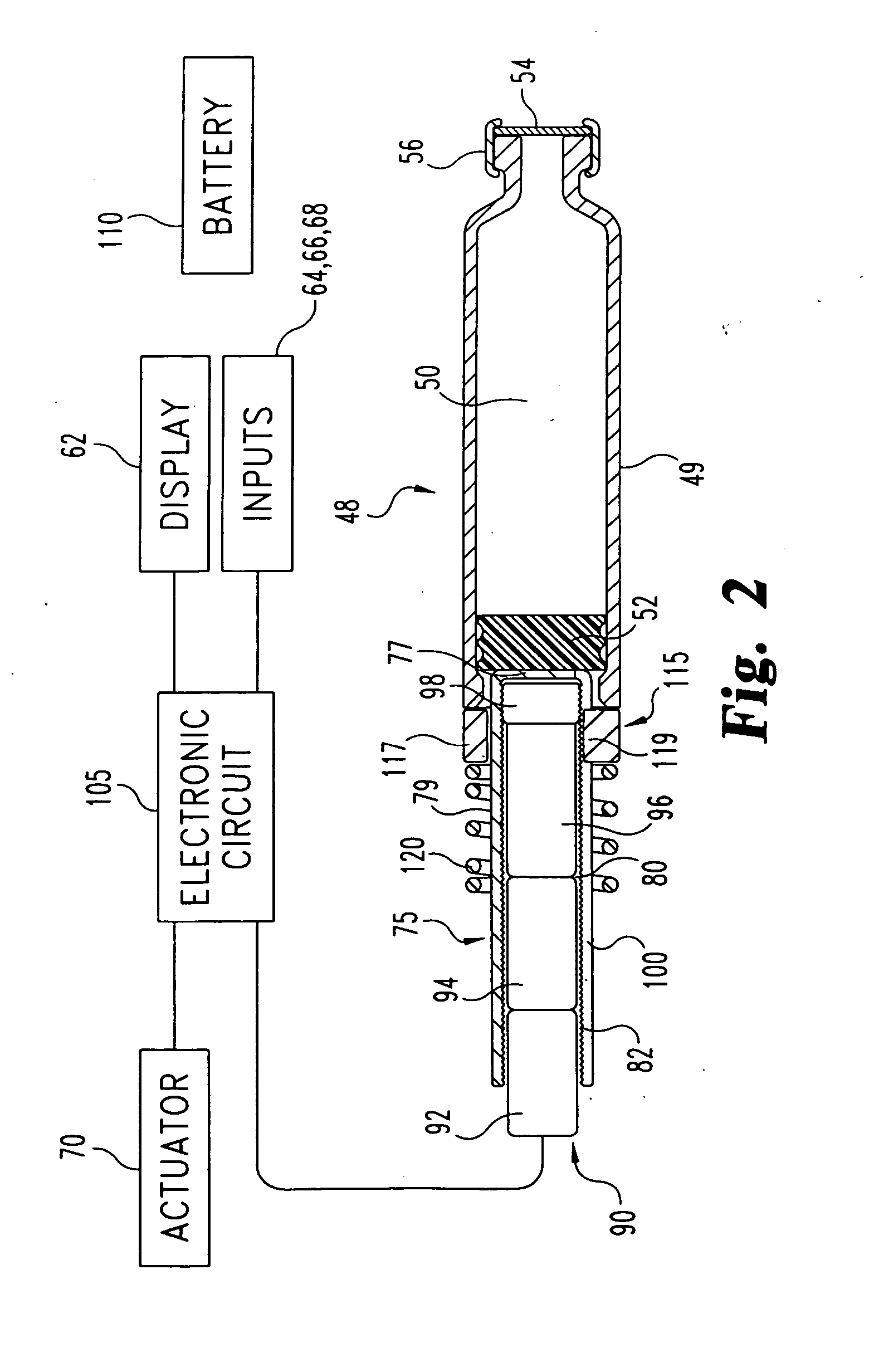
An integrated system for the monitoring and treating diabetes is provided, including an integrated receiver / hand-held medicament injection pen, including electronics, for use with a continuous glucose sensor. In some embodiments, the receiver is configured to receive continuous glucose sensor data, to calculate a medicament therapy (e.g., via the integrated system electronics) and to automatically set a bolus dose of the integrated hand-held medicament injection pen, whereby the user can manually inject the bolus dose of medicament into the host. In some embodiments, the integrated receiver and hand-held medicament injection pen are integrally formed, while in other embodiments they are detachably connected and communicated via mutually engaging electrical contacts and / or via wireless communication.
Owner:DEXCOM
Integrated medicament delivery device for use with continuous analyte sensor
ActiveUS20080306435A1Reduce the burden onSimple processAmpoule syringes2D-image generationMedication injectionDiabetes mellitus
An integrated system for the monitoring and treating diabetes is provided, including an integrated receiver / hand-held medicament injection pen, including electronics, for use with a continuous glucose sensor. In some embodiments, the receiver is configured to receive continuous glucose sensor data, to calculate a medicament therapy (e.g., via the integrated system electronics) and to automatically set a bolus dose of the integrated hand-held medicament injection pen, whereby the user can manually inject the bolus dose of medicament into the host. In some embodiments, the integrated receiver and hand-held medicament injection pen are integrally formed, while in other embodiments they are detachably connected and communicated via mutually engaging electrical contacts and / or via wireless communication.
Owner:DEXCOM
Method and device for painless injection of medication
InactiveUS7637891B2Simple and inexpensive to performSimple and inexpensive to and operateAmpoule syringesAutomatic syringesMedication injectionMedical treatment
A device for painlessly injecting medications, and a method for providing a substantially painless injection of medication into a patient that does not require the use of an anesthetic, that does not require the medical personnel to spend a substantial amount of time performing the injection procedure, that is relatively simple and inexpensive to perform and operate, and that provides a relatively high degree of safety for both the medical personnel and for the patient. The injection needle can have an outside diameter greater than 0.20 mm and less than about 0.38 mm. The medicament can be injected painlessly through the needle and into the patient at a substantially constant volumetric flow rate of about 0.05 μL / s to about 50 μL / s.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Medicine injection devices and methods
A reloadable medicine injector and methods are described in which a barrel with a receiving cavity is adapted to slidably receive a syringe subassembly for axial movement therein. Upon removal of a safety and release of a syringe driver, the syringe driver moves forward and injects the syringe needle. A plurality of penetration controls are shown for controlling injection needle penetration depth. The penetration controls have an abutment and various lengths to provide different needle penetration depth positions. In one form of penetration control a sleeve is used against which the syringe or related parts contact. In another form the front return spring is used as a penetration control. A cushioning ring may be used to reduce syringe breakage. A load distribution and guide ring may be used to distribute loading applied to the syringe and help guide the moving syringe.
Owner:WASHINGTON BIOTECH CORP +1
Medication injecting apparatus with fluid container piston-engaging drive member having internal hollow for accommodating drive member shifting mechanism
A medication injecting apparatus having a motor-driven drive member that when advanced inserts into a fluid container for forcing fluid therefrom. The drive member includes an internal hollow in which fits at least a portion of the motorized driver assembly when the drive member is retracted to allow a compact apparatus to be provided.
Owner:ELI LILLY & CO
Injection device for administering a vaccine
InactiveUS7670314B2Simple and inexpensive to prepareSimple and inexpensive to and operateAmpoule syringesAutomatic syringesMedication injectionInjection device
A manually-powered injection device that self-administers a painless injection. The injection device provides a method for substantially painless injections of vaccine and other medication into a patient that does not require the use of an anesthetic, that does not require the medical personnel to spend a substantial amount of time performing the injection procedure, that is relatively simple and inexpensive to perform and operate, and that provides a relatively high degree of safety for both the medical personnel and for the patient. The injection needle can have an outside diameter greater than 0.10 mm and less than about 0.38 mm. The vaccine or other medicament can be injected painlessly through the needle and into the patient at a substantially constant volumetric flow rate of about 0.05 μL / s to about 50 μL / s, typically over a 3- to 5-minute period of time. The injection device is configured for easy handling, and is manually powered by the use of the hand or fingers of the medical technician, patient or other person.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Medicament injection kit and medicament injection method
ActiveUS7297475B2Safely and efficiently in vivoSafely and efficiency in vivoBiocidePeptide/protein ingredientsMedication injectionVein
A medicament injection kit, for use in occluding a renal artery and a renal vein in a kidney and injecting a therapeutic medicament into the kidney so as to pressurize the kidney, includes: an artery catheter which includes a first balloon capable of occluding the renal artery; a vein catheter which includes a second balloon capable of occluding the renal vein; a syringe for injecting the therapeutic medicament, the syringe being capable of being connected to at least one of the artery catheter and the vein catheter; and a syringe for pressurizing the inside of the kidney by injecting a liquid, the syringe being capable of being connected to at least one of the artery catheter and the vein catheter.
Owner:TERUMO KK
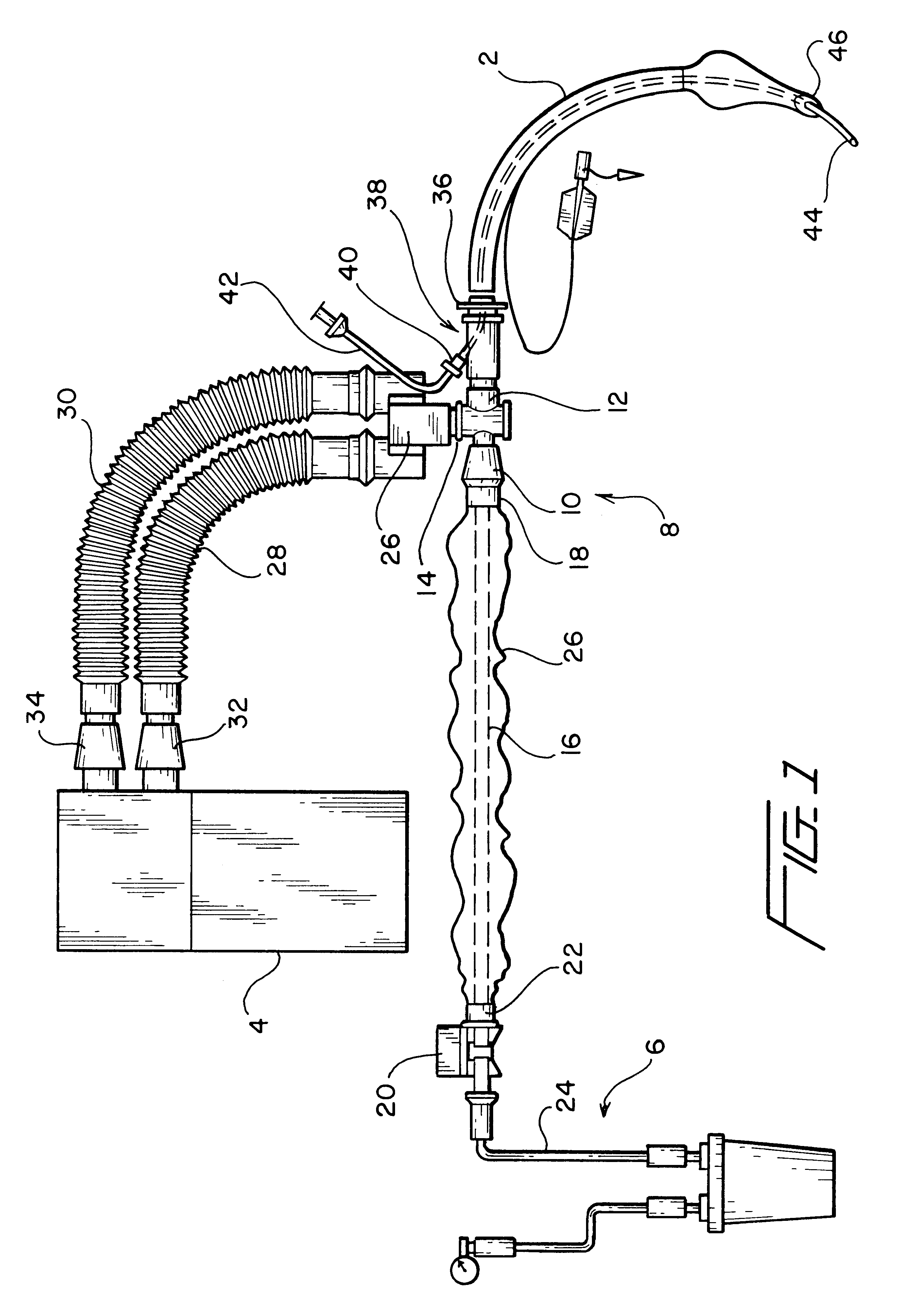
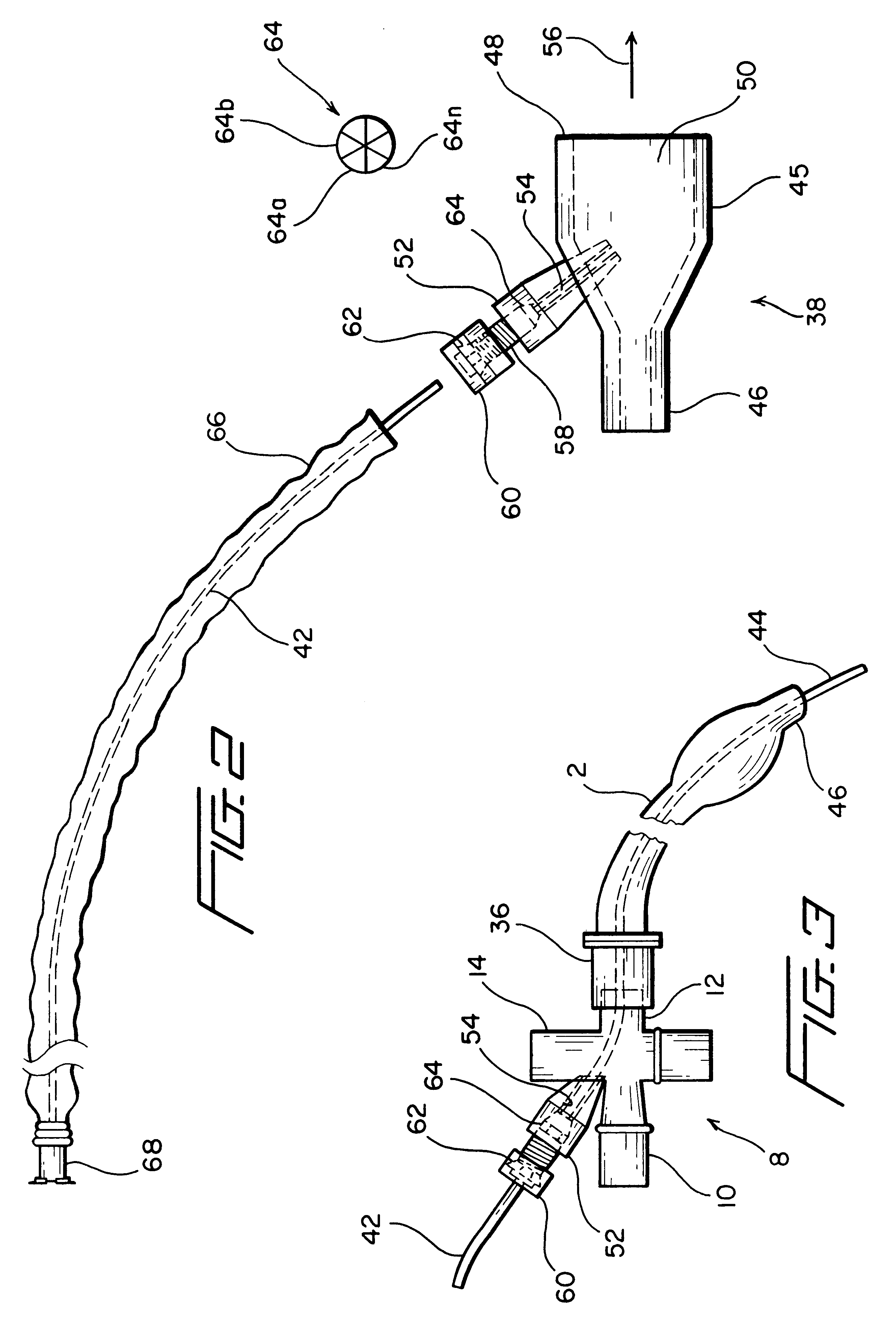
Adapter for localized treatment through a tracheal tube and method for use thereof
InactiveUS6575944B1Prevent backflowPrecise positioningGuide needlesTracheal tubesTracheal tubeBronchial tube
By interposing an adapter between the endotracheal or tracheal tube inserted to a patient and the ventilation and suction systems that are connected to the endotracheal tube, a catheter could be inserted via an input port built into the adapter so as to enable a medical personnel to provide localized treatments in the lungs of a patient without having to disconnect either one of the systems connected to the endotracheal tube. The adapter is configured to have a securing mechanism that allows the medical personnel to secure the medication catheter in place. A one way valve fitted to the apertured arm that forms the input port of the adapter prevents any back flow of fluid from the input port. The catheter is manufactured with calibration markings, most likely equally spaced, and a radiopaque line along its length to enhance the maneuvering and the positioning thereof in the patient so that the distal tip of the catheter could be accurately positioned to the desired location of the patient's tracheal / bronchial tree. As a result, the localized treatment such as the injection of a medicament is accurately provided to the appropriate location where the need is the greatest.
Owner:SMITHS MEDICAL ASD INC
Self-contained medication injection system and method
InactiveUS20080312604A1Eliminate needLow powerAutomatic syringesMedical devicesMedication injectionDrug delivery
A self-contained system for medication injection is activated by the user, who is able to distribute the medication through the self-contained needle system for intradermal or intramuscular application of the medication. The self-contained system may be a medication delivery pen having a pen body assembly comprising a distal end, a proximal end and a needle array containing a plurality of needles disposed within the pen body assembly.
Owner:BOESEN PETER V
Safe intravenous infusion port injectors
We describe connectors for safely and conveniently injecting measured doses of sterile liquid medications into a patient via one or more infusion ports in intravenous access assemblies. Each connector comprises a tubular injector divided by a rigid septum which holds a hollow needle sharp on each end safely recessed in a leading and in a trailing chamber. The leading chamber snugly holds the trailing limb and penetrable cap of an inserted standard infusion port. The trailing recess snugly holds the leading end of a cartridge with a leading penetrable diaphragm, a bore containing liquid medication, a cartridge piston and trailing bore suitable for insertion of a separate cartridge plunger having markers for measuring doses delivered; or, alternatively, the pentrable cap and trailing limb of second similar infusion port attached by trailing tubing to a large measured volume infusion source. When assembled such that the trailing cap of infusion port is penetrated by the needle in the leading chamber and the leading diaphragm of the cartridge or, alternatively, the leading penetrable cap on a second infusion port is penetrated by the needle in the trailing chamber, such that flow can proceed through the needle, precisely measured doses of sterile liquid medication can be injected into the venous access assembly without possibilities for the user to touch, get stuck or finger-contaminate the needle or its contents. Unique features added to increase the efficiency of the system are a biased leading end on the tubular injector to conveniently and securely accommodate a Y-infusion port; an eccentric needle in the connector, such that rotation prevents the leading sharp end of the needle from passing through the infusion port cap via the same track; and an easily removed biased cap for keeping the cartridge diaphragm sterile.
Owner:WALKER JACK M +1
Medicine injection devices and methods
A reloadable medicine injector and methods are described in which a barrel with a receiving cavity is adapted to slidably receive a syringe subassembly for axial movement therein. Upon removal of a safety and release of a syringe driver, the syringe driver moves forward and injects the syringe needle. A plurality of penetration controls are shown for controlling injection needle penetration depth. The penetration controls have an abutment and various lengths to provide different needle penetration depth positions. In one form of penetration control a sleeve is used against which the syringe or related parts contact. In another form the front return spring is used as a penetration control. A cushioning ring may be used to reduce syringe breakage. A load distribution and guide ring may be used to distribute loading applied to the syringe and help guide the moving syringe.
Owner:WASHINGTON BIOTECH CORP +1
Medicatioin injector
InactiveUS20060247579A1Easy to carryLess intimidatingAutomatic syringesInfusion needlesMedication injectionTwo step
A card-shaped disposable auto-injector includes a two-step safety mechanism to prevent inadvertent use or activation of the injection mechanism. In one aspect, the auto-injector is sized and shaped so as to be easy to carry in a wallet-shaped case. In one aspect, it is rectangular in shape so as to facilitate carrying and storage, and to provide a relatively large amount of surface space for instructional text, diagrams, identifying information, warnings, and the like.
Owner:FRIEDMAN STEVEN A
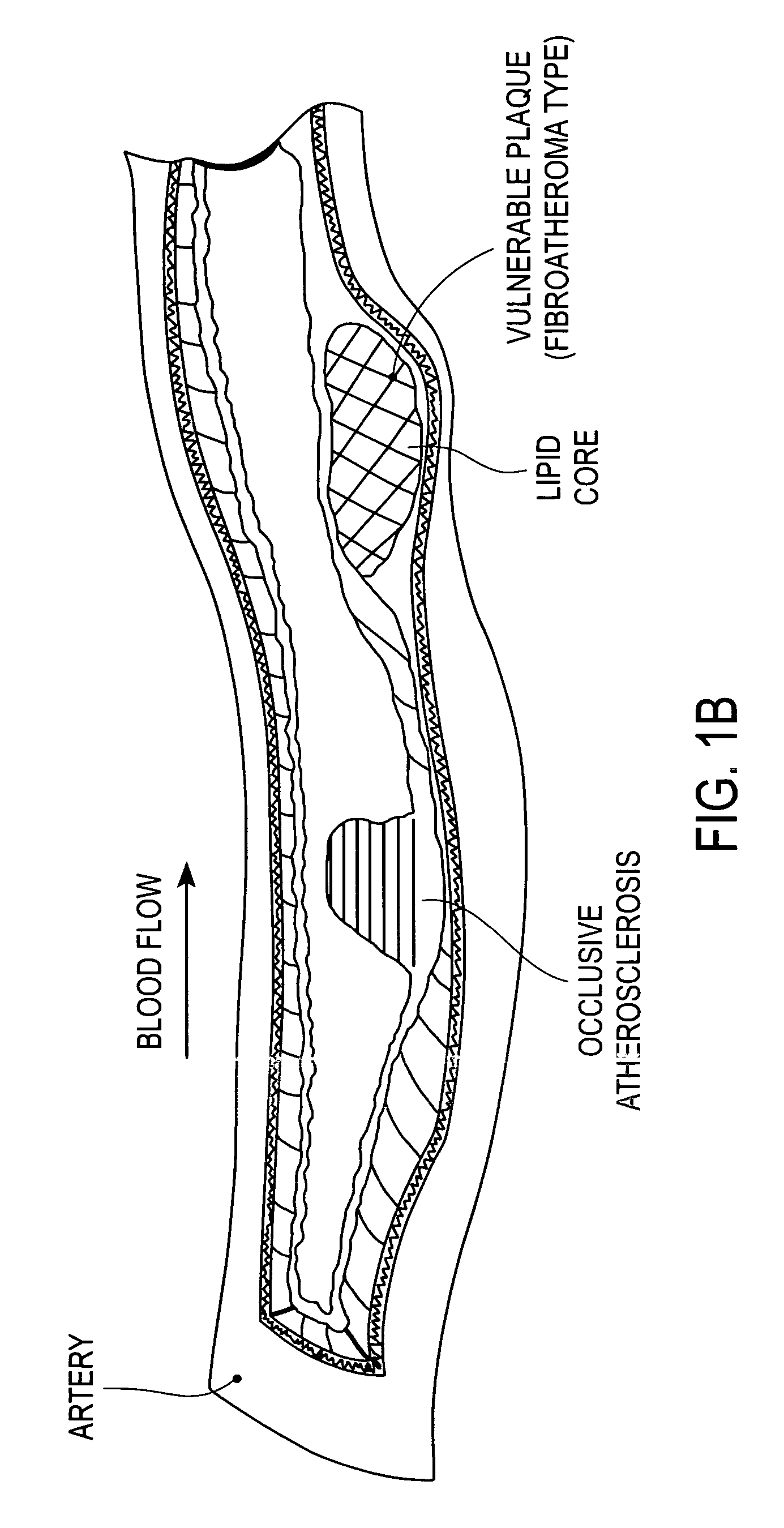
Method and apparatus for treating vulnerable plaque
Various apparatuses and methods are described to treat vulnerable plaque with the use of combination drug therapy. In one embodiment, the apparatus has an elongated catheter body adapted for insertion in a body lumen, with a drug delivery device attached near a distal portion of the elongated body. The drug delivery device is configured to deliver a therapeutic or biologically active agent to stabilize a vulnerable plaque. In another embodiment, a drug eluting stent is delivered and deployed in conjunction with a second apparatus that delivers drug in pressurized retrograde perfusion. Still another embodiment uses a uniquely shaped balloon to deploy a stent while utilizing a drug infusion needle to inject drug into the vessel wall through a hollow guide wire.
Owner:ABBOTT CARDIOVASCULAR
Integrated medicament delivery device for use with continuous analyte sensor
ActiveUS8808228B2Reduce the burden onSimple processAmpoule syringesDrug and medicationsMedication injectionDiabetes mellitus
An integrated system for the monitoring and treating diabetes is provided, including an integrated receiver / hand-held medicament injection pen, including electronics, for use with a continuous glucose sensor. In some embodiments, the receiver is configured to receive continuous glucose sensor data, to calculate a medicament therapy (e.g., via the integrated system electronics) and to automatically set a bolus dose of the integrated hand-held medicament injection pen, whereby the user can manually inject the bolus dose of medicament into the host. In some embodiments, the integrated receiver and hand-held medicament injection pen are integrally formed, while in other embodiments they are detachably connected and communicated via mutually engaging electrical contacts and / or via wireless communication.
Owner:DEXCOM
Two-stage reconstituting injector
ActiveUS8251947B2Easy and effective deliveryAmpoule syringesJet injection syringesMedication injectionCatheter
An injector for injecting a medicament into a patient. The injector includes a container defining a first chamber, which contains a fluid therein, and a second chamber. The injector also includes an injection conduit configured for directing the fluid fired from the container into the patient. A transfer mechanism is operable by a user to transfer the fluid from the first chamber to the second chamber in a first stage of operation, and a firing mechanism is operable by the user for firing the fluid from the second chamber through the injection conduit in a second stage of operation. An energy source is in powering association with the firing mechanism to drive firing mechanism in the first and second stages.
Owner:ANTARES PHARMA INC
Needle assisted jet injector
InactiveUS20050080377A1Prevent re-exposureAmpoule syringesJet injection syringesMedication injectionJet injection
Owner:ANTARES PHARMA INC
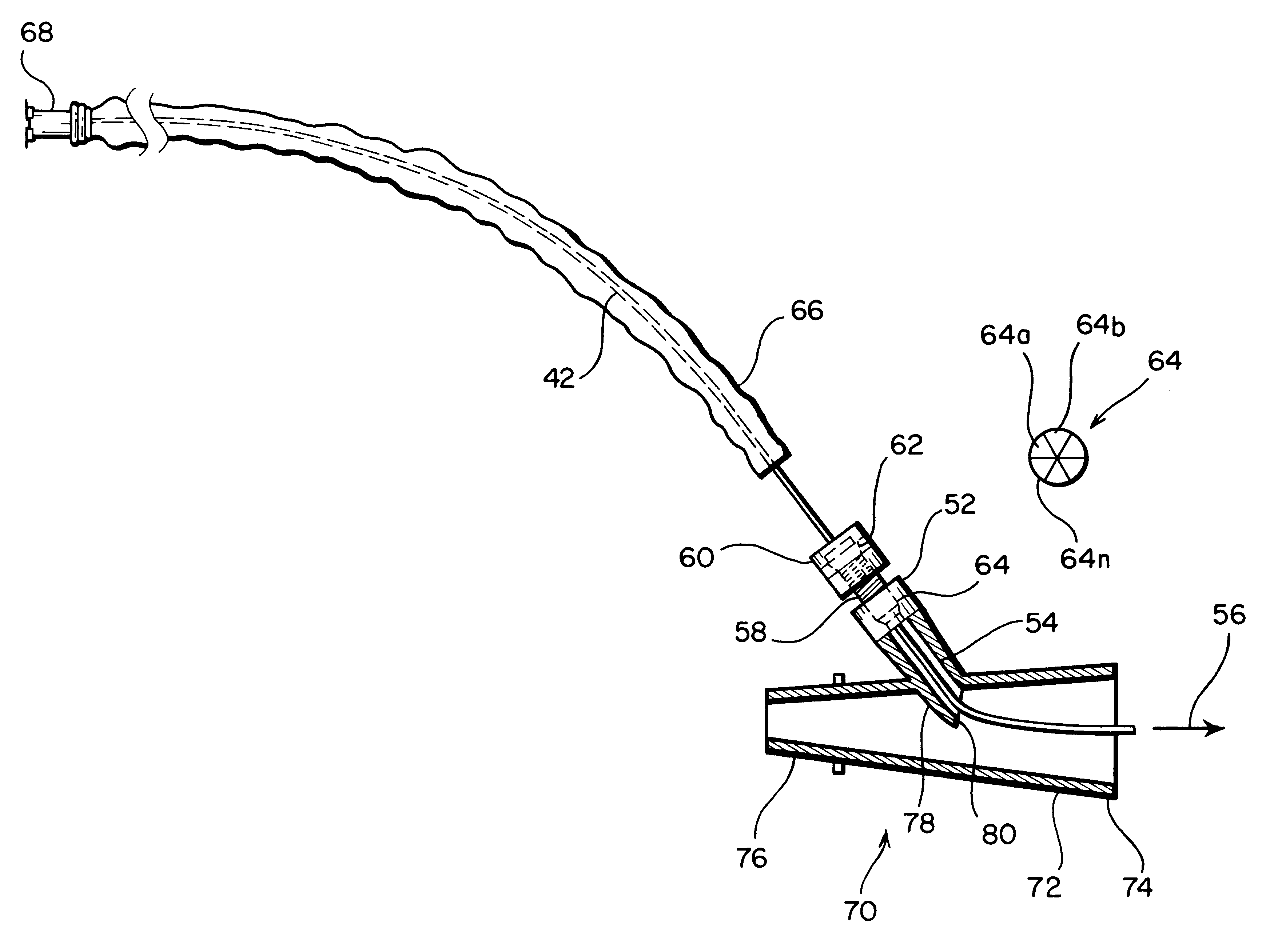
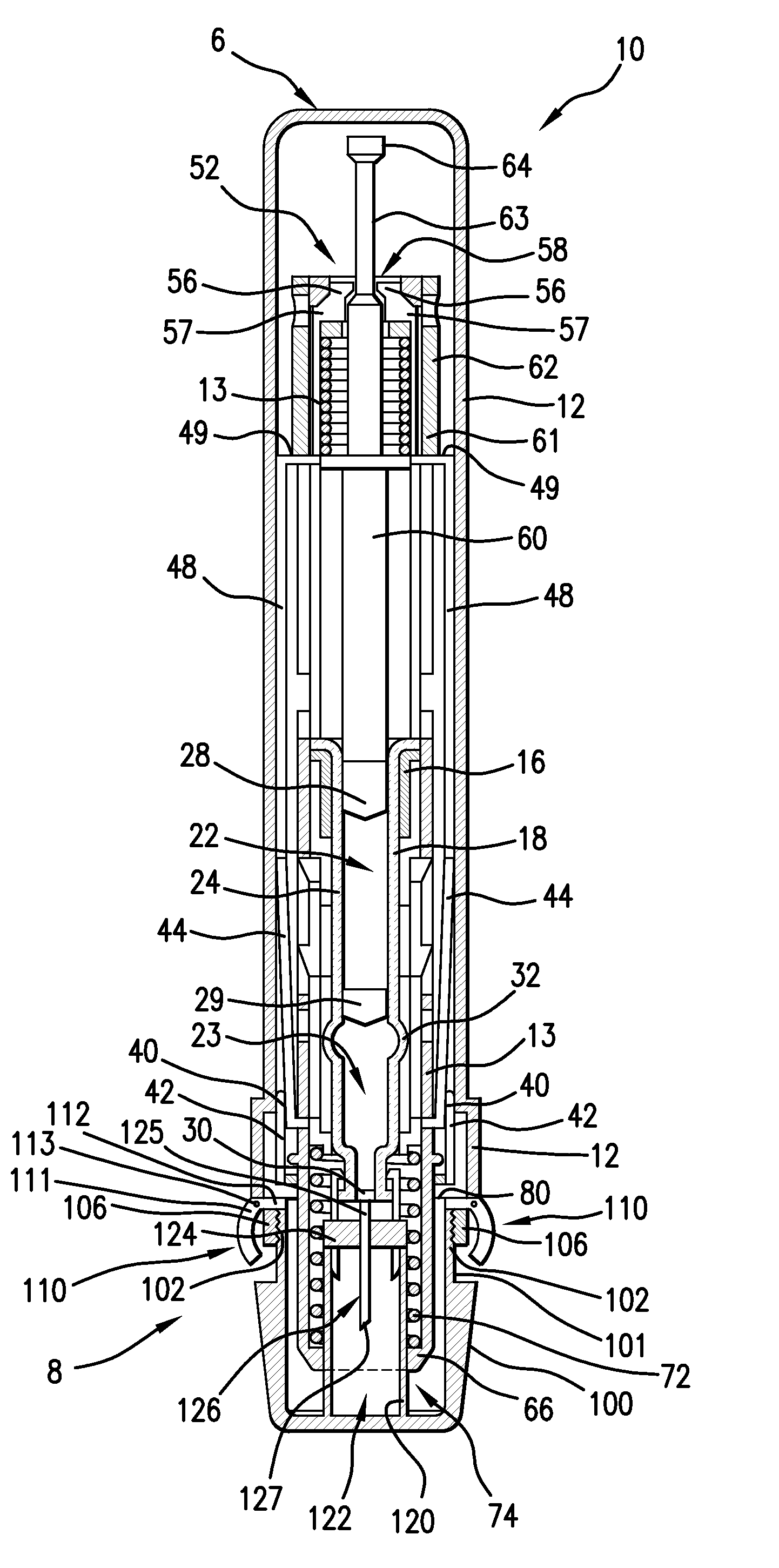
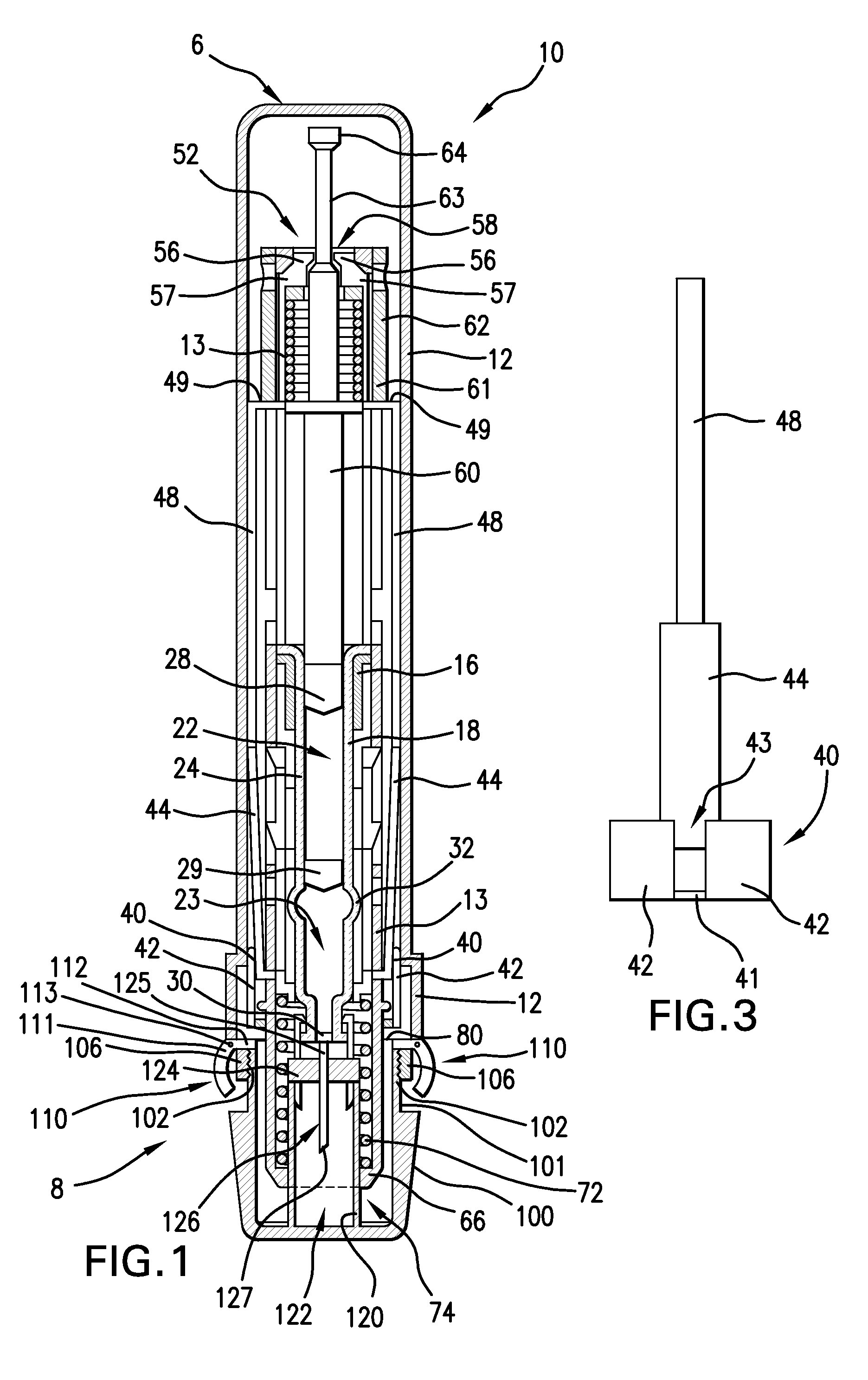
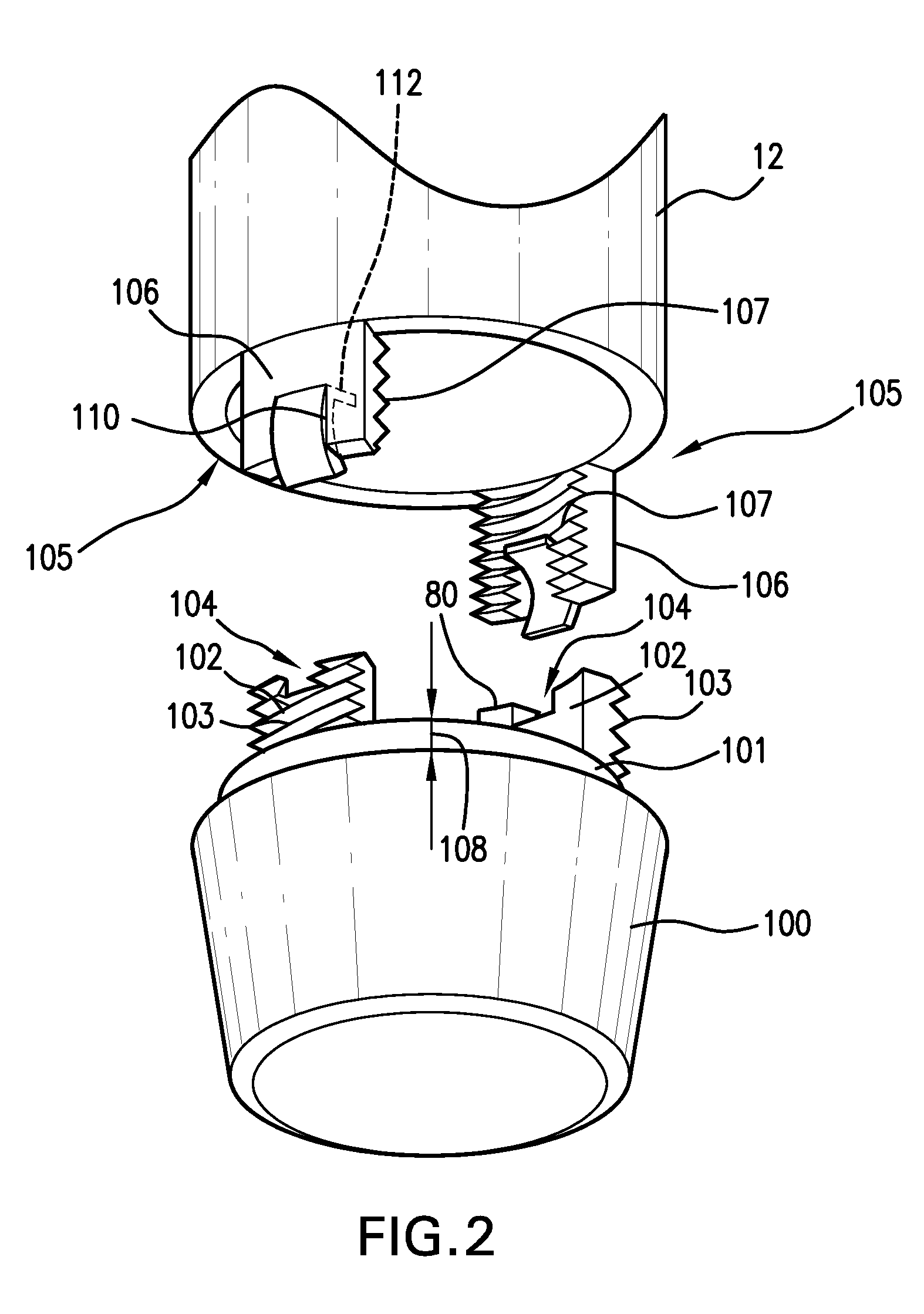
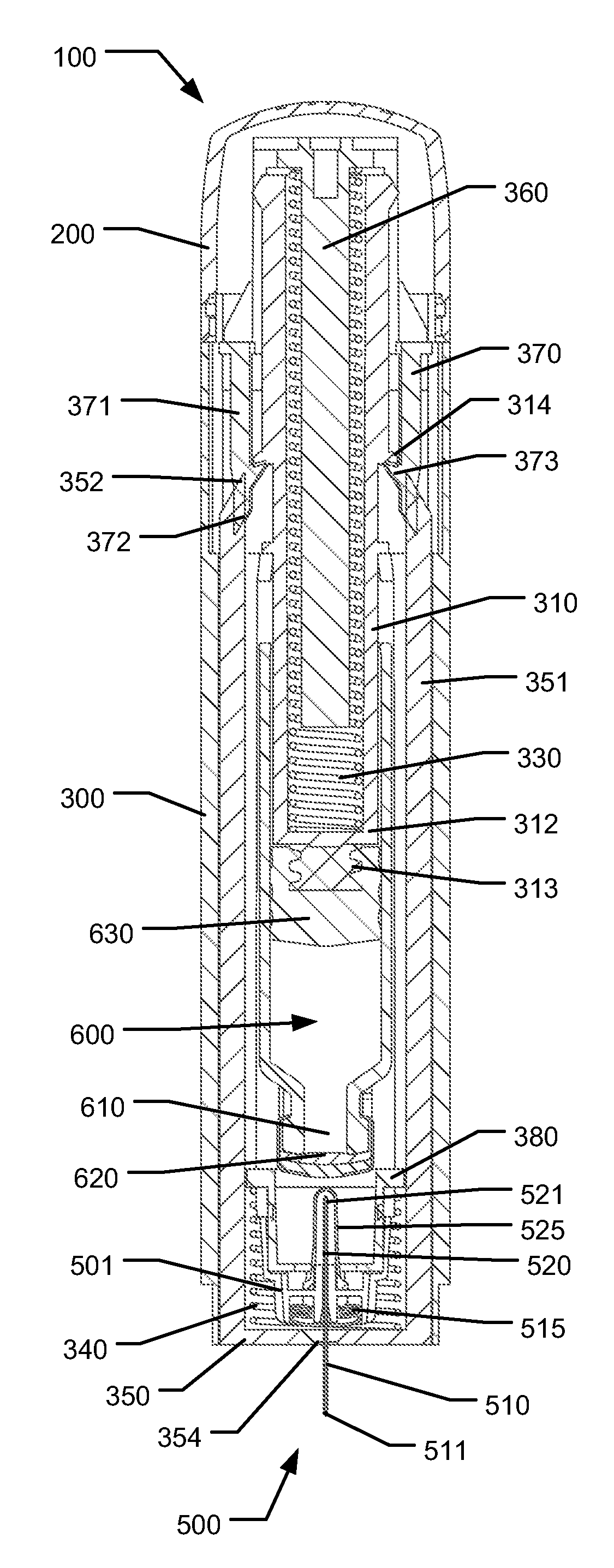
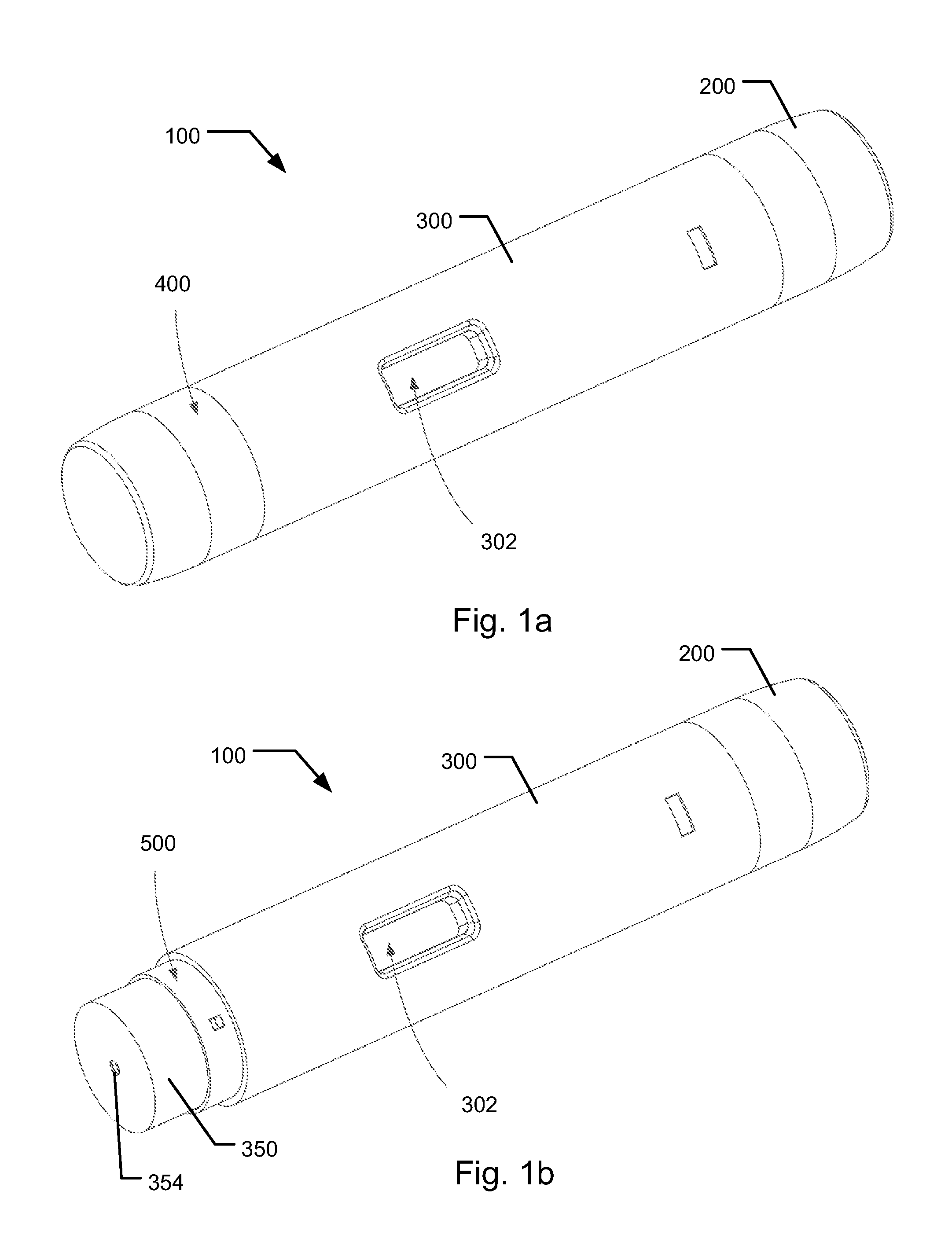
Medical Injection Device
ActiveUS20130211330A1Easy to controlPotential uneasiness for the user can be alleviatedAmpoule syringesAutomatic syringesMedication injectionInjection device
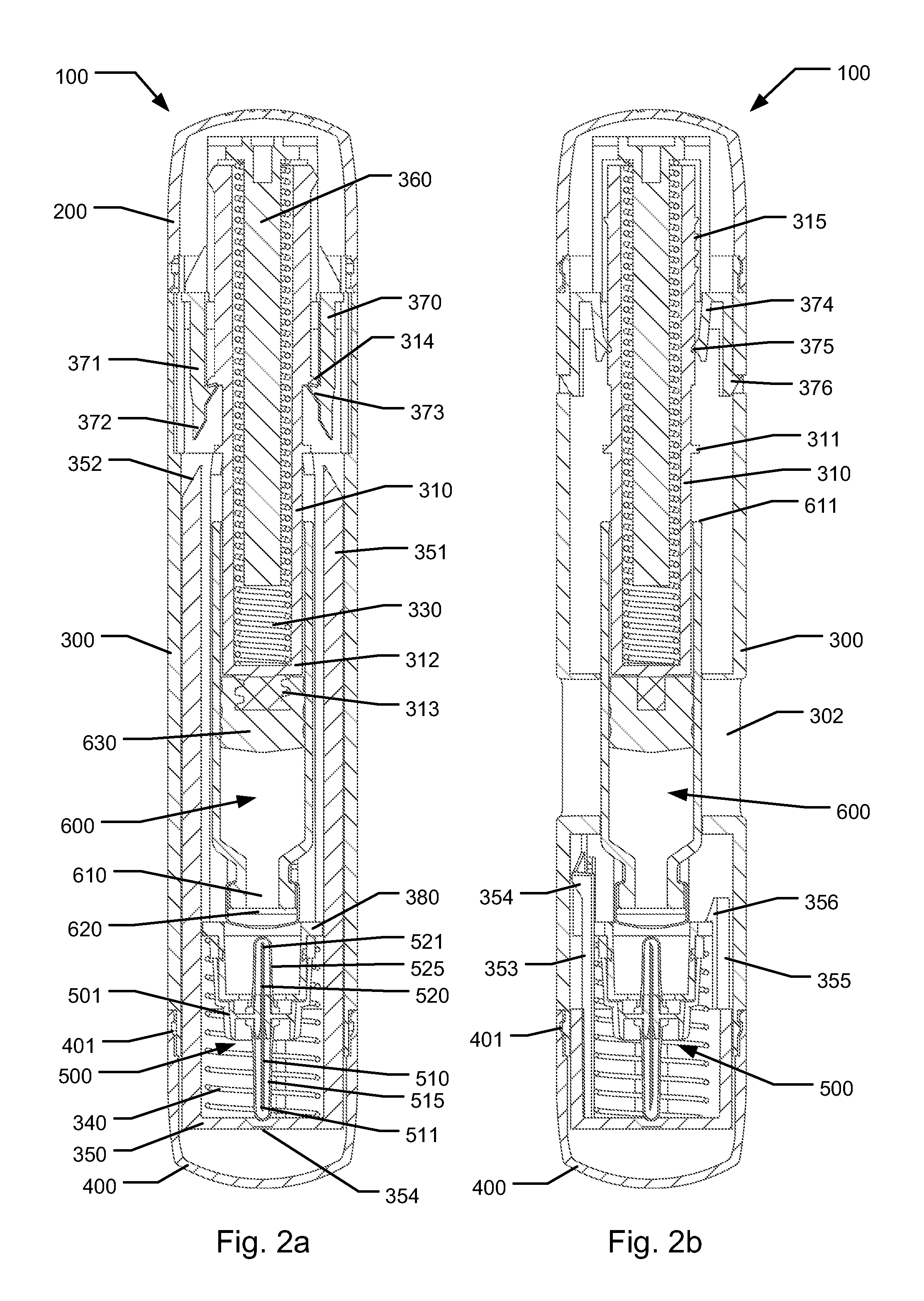
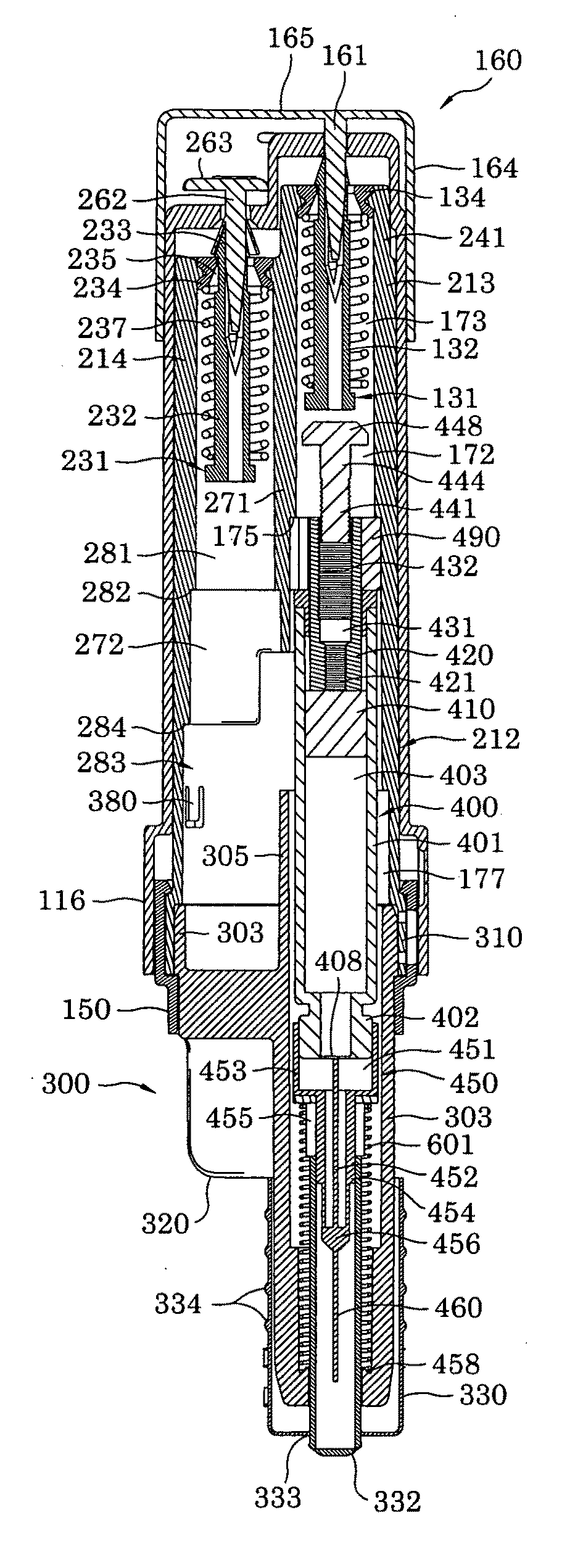
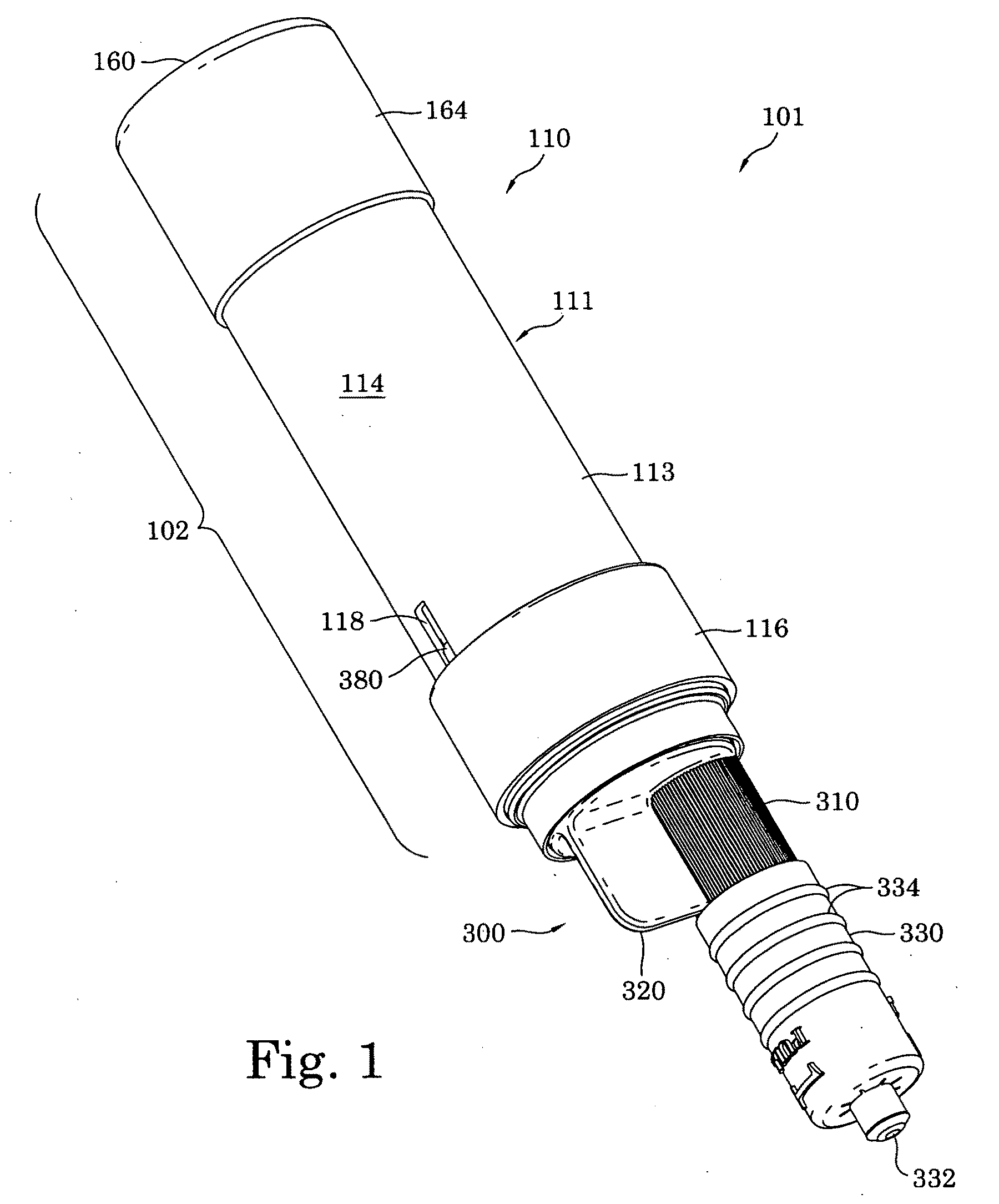
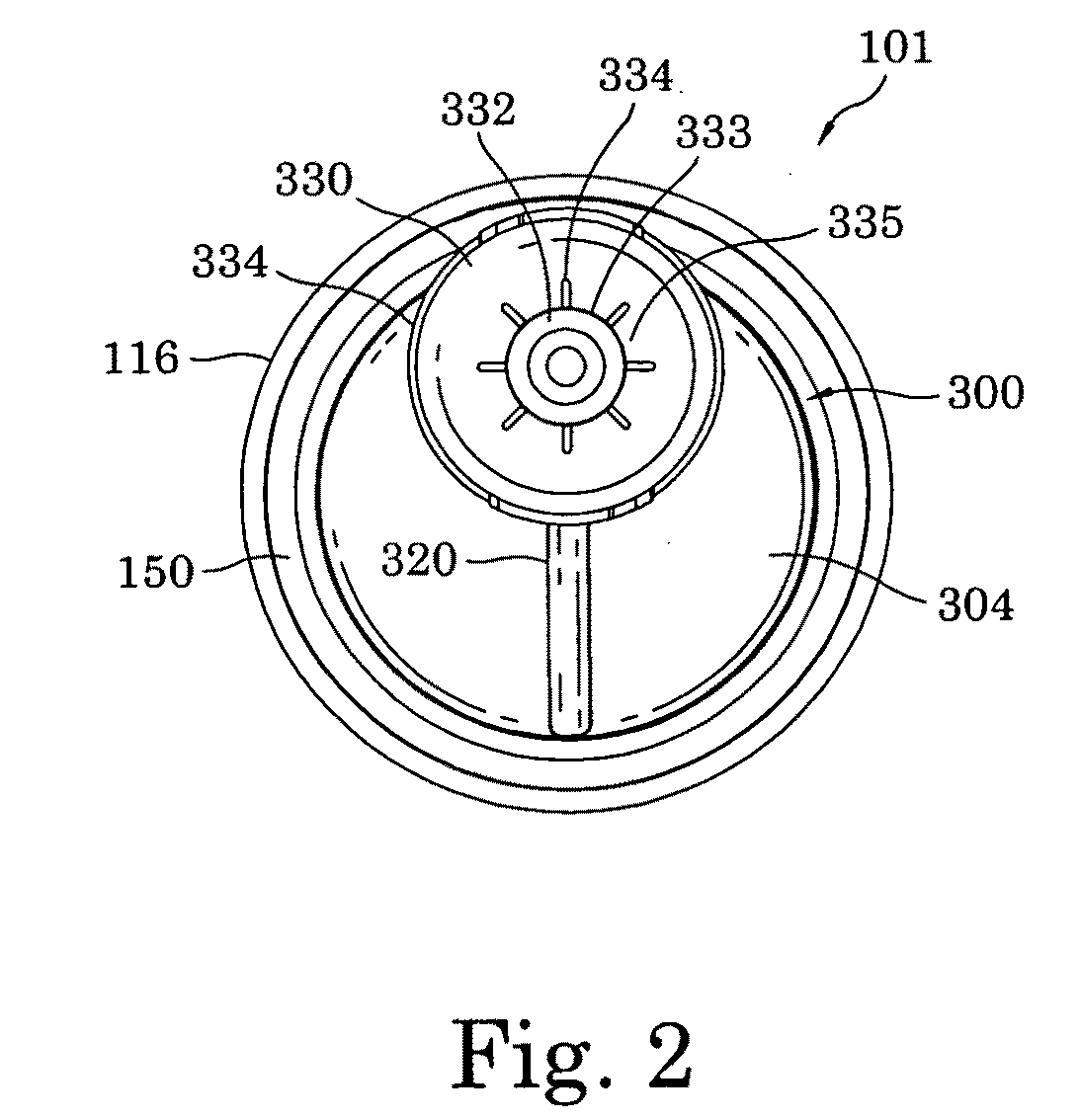
The present invention relates to medicament injection devices (100). A cartridge (600) with a septum and a needle unit (500) having front and rear needles (510, 520) are configured for relative movement from a state where the septum is sealed to a state where the septum is pierced by the rear needle (520). The injection device (100) may include a needle shield (350) and be configured for piercing the septum by the rear needle (520) when the front needle (510) is operated relative to the needle shield (350). The injection device (100) may also include a damping mechanism configured for limiting the speed of movement of the cartridge relative to the needle unit. The injection device (100) may also include an indicator generating a signal when a piston driver has travelled the complete stroke length, wherein the indicator has a deflection element that is deflected prior to or during movement of the cartridge relative to the housing.
Owner:NOVO NORDISK AS
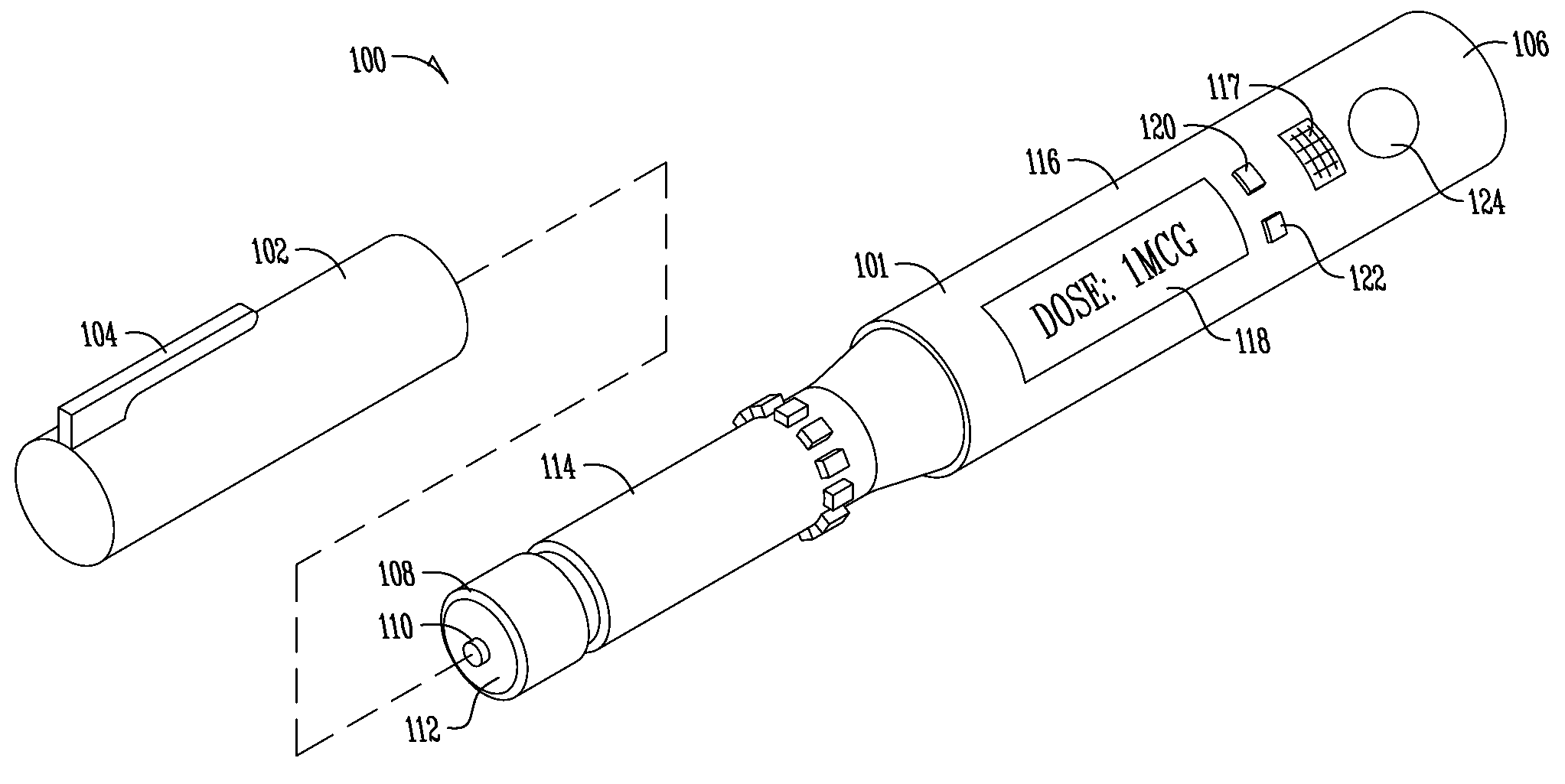
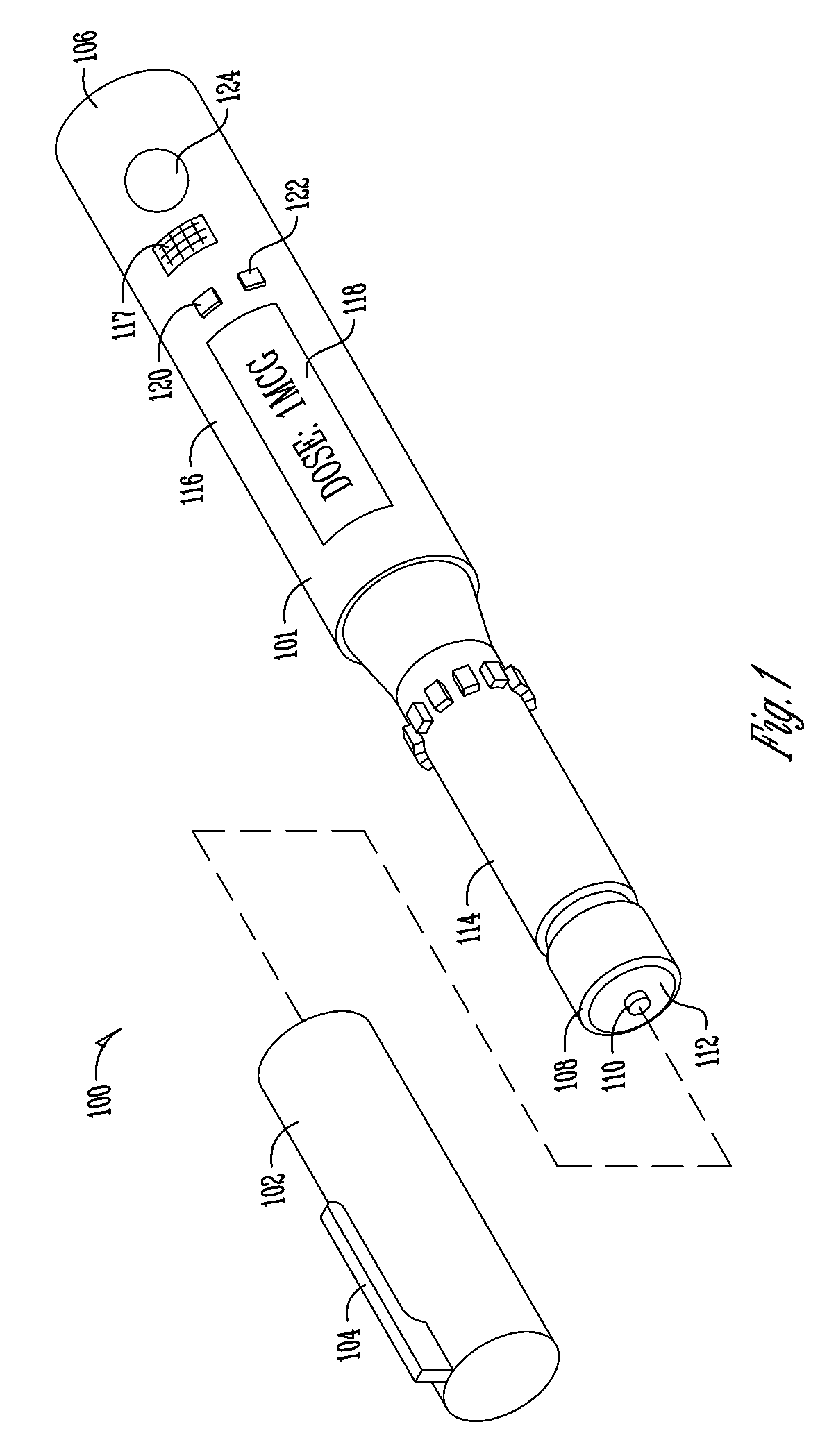
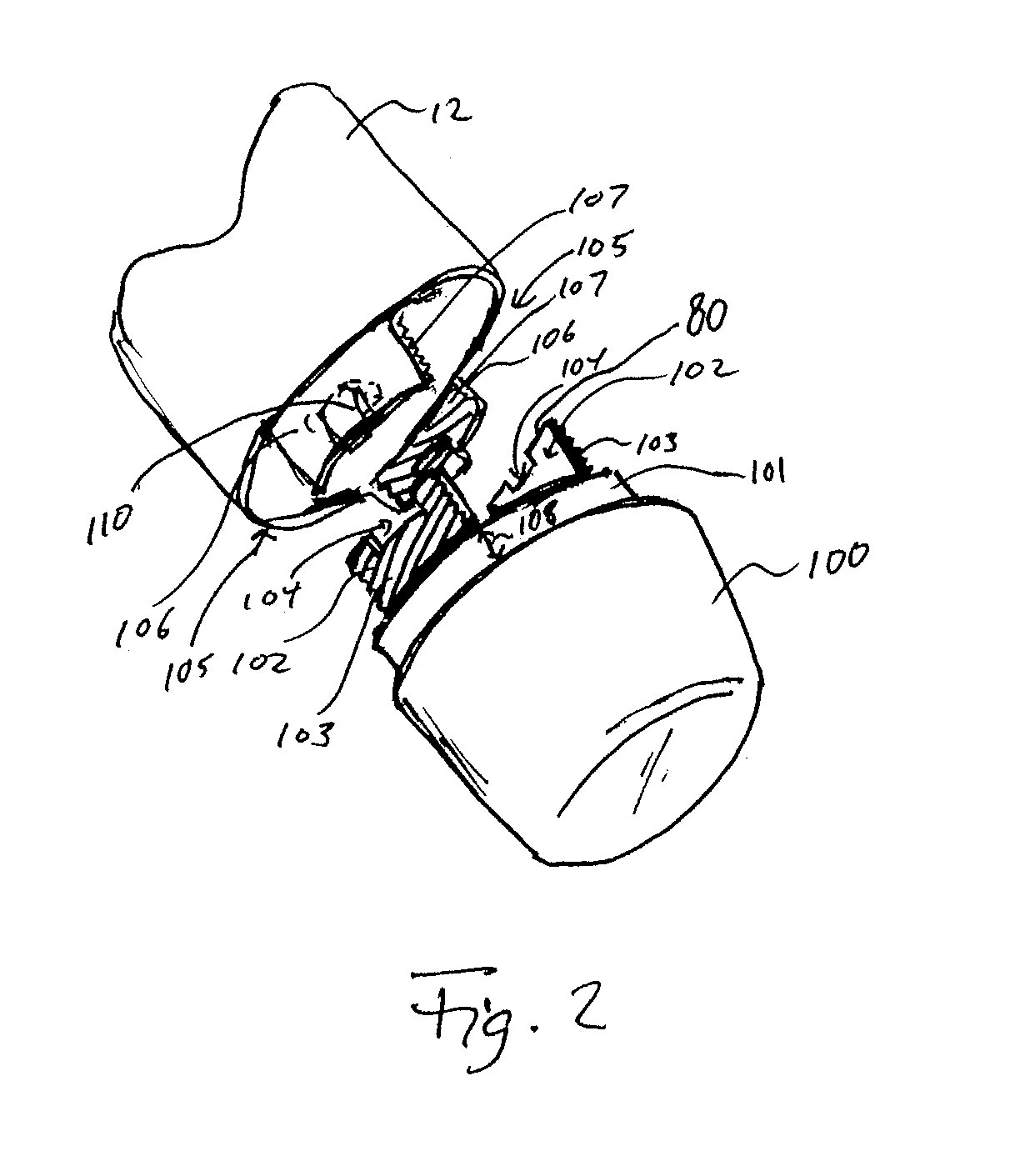
Medication and identification information transfer apparatus
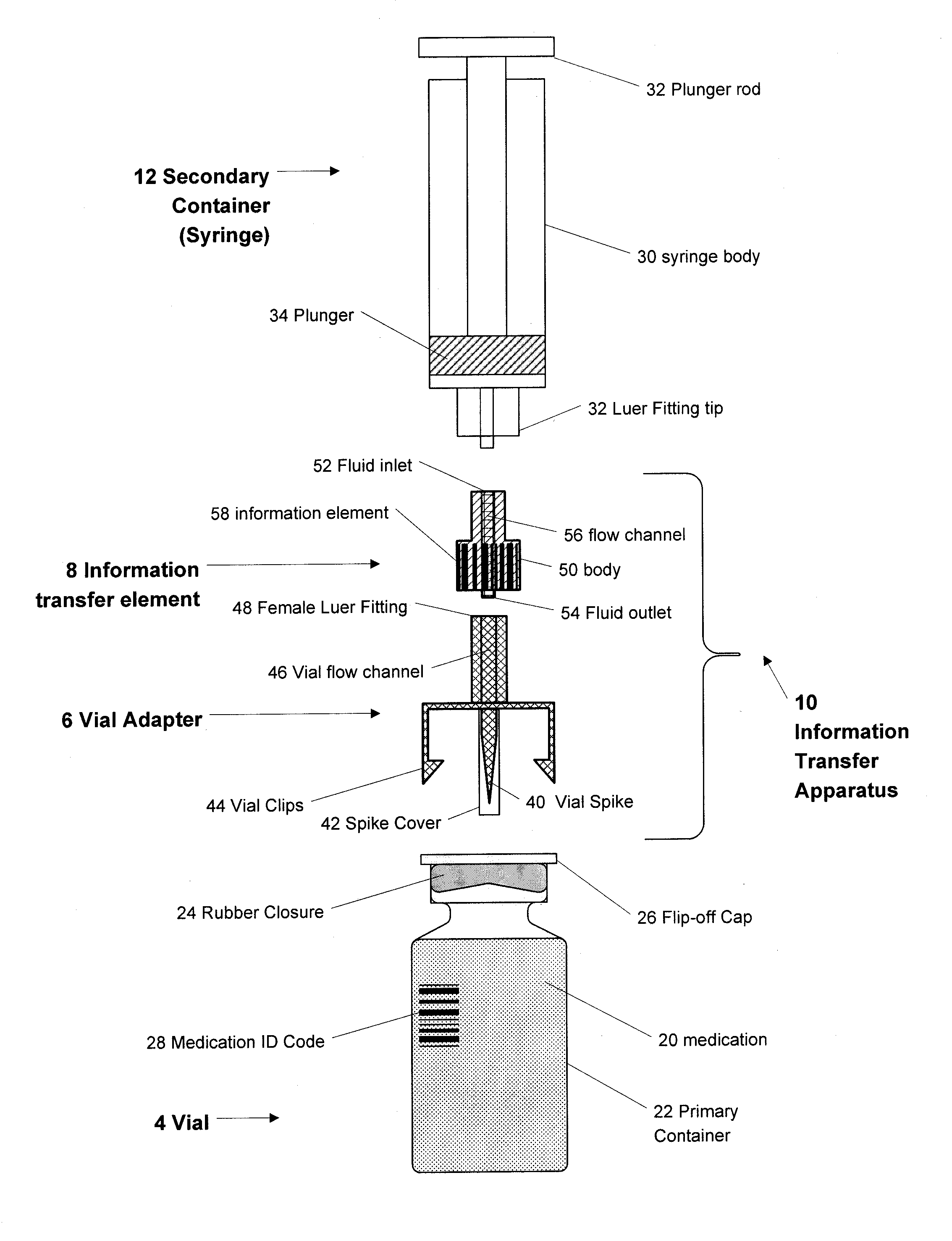
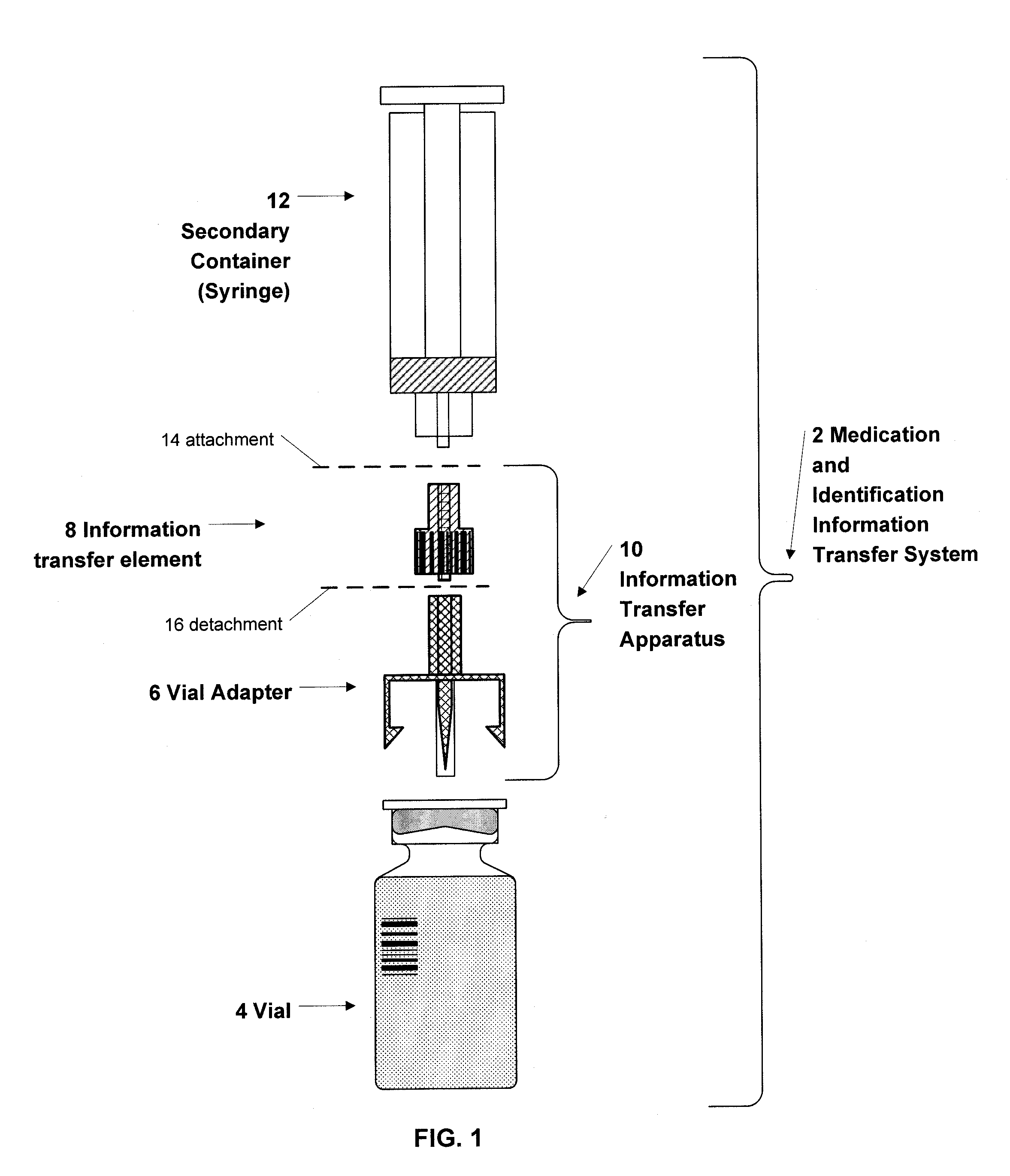
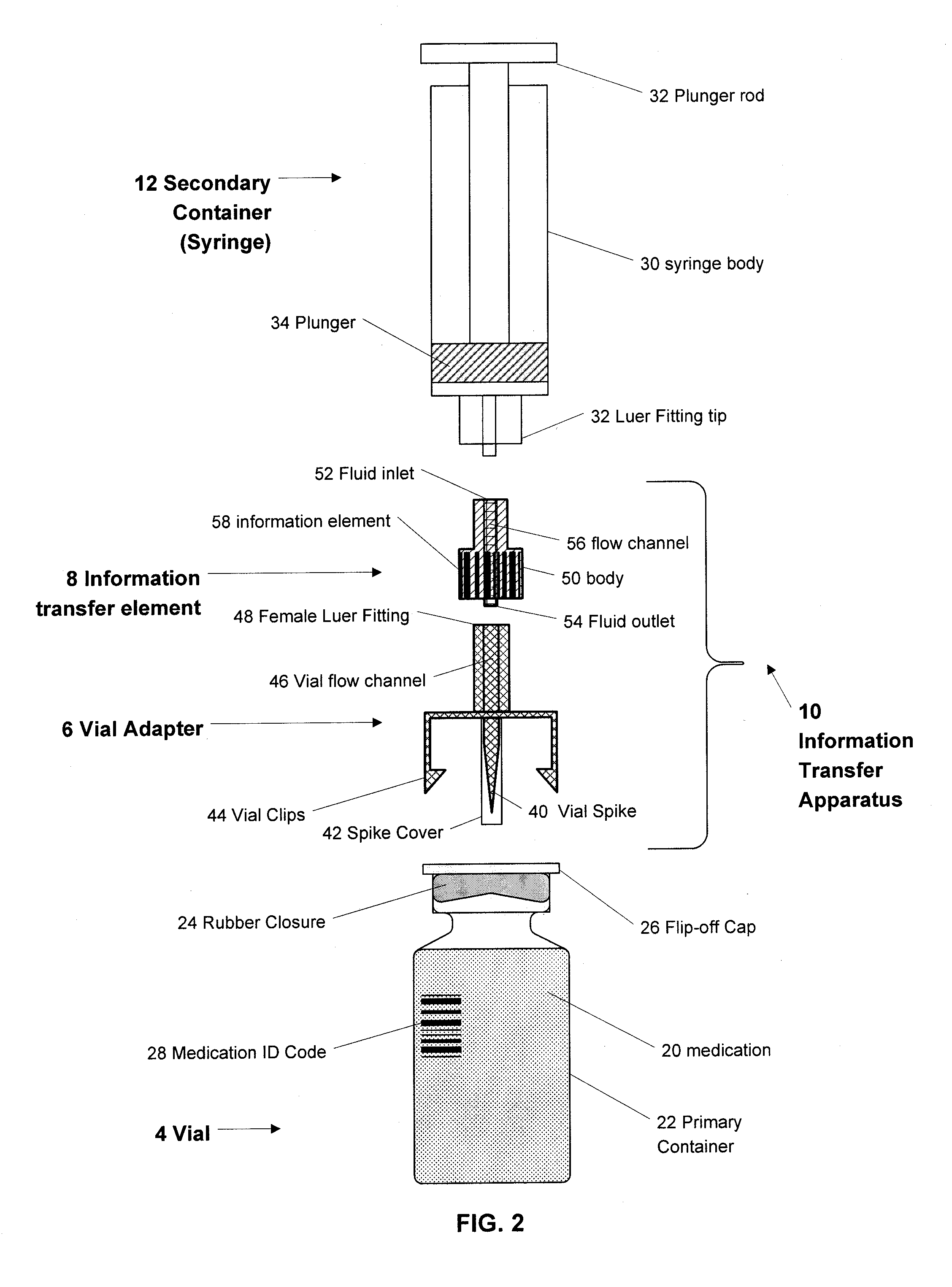
A medication and identification information transfer system is provided that includes a medication vial, a secondary medication container (syringe) and a medication information transfer apparatus. The medication information transfer apparatus, when coupled to a vial, can transfer information indicative of the contents of the vial to an intelligent injection site. The medication information transfer apparatus has a shape and size enabling it to be connected to a vial adapter for removal of medication from the vial transfer it to a syringe for delivery to an injection site while simultaneously transferring information about the medication in the vial to the injection site. In some implementations, the medication injection site can be placed on a fluid delivery line for infusion into a patient. Related apparatus, systems, and kits are also disclosed.
Owner:CRISI MEDICAL SYST
Pen shaped medication injection devices
The present invention provides a delivery device comprising a fluid cartridge holder, housing and a piston drive mechanism that comprises a hollow piston rod and a drive-shaft. The hollow piston rod has an internal thread that mates with the external thread of the drive-shaft, forming a thread connection and being axially restrained in the proximal direction relative to the housing. The drive mechanism is in turn connected to the housing via a one way ratchet such that the piston rod is prevented from moving in the proximal direction.
Owner:WOCKHARDT AMERICAS
Integrated medicament delivery device for use with continuous analyte sensor
ActiveUS8562558B2Reduce the burden onSimple processAmpoule syringes2D-image generationMedication injectionDiabetes mellitus
An integrated system for the monitoring and treating diabetes is provided, including an integrated receiver / hand-held medicament injection pen, including electronics, for use with a continuous glucose sensor. In some embodiments, the receiver is configured to receive continuous glucose sensor data, to calculate a medicament therapy (e.g., via the integrated system electronics) and to automatically set a bolus dose of the integrated hand-held medicament injection pen, whereby the user can manually inject the bolus dose of medicament into the host. In some embodiments, the integrated receiver and hand-held medicament injection pen are integrally formed, while in other embodiments they are detachably connected and communicated via mutually engaging electrical contacts and / or via wireless communication.
Owner:DEXCOM INC
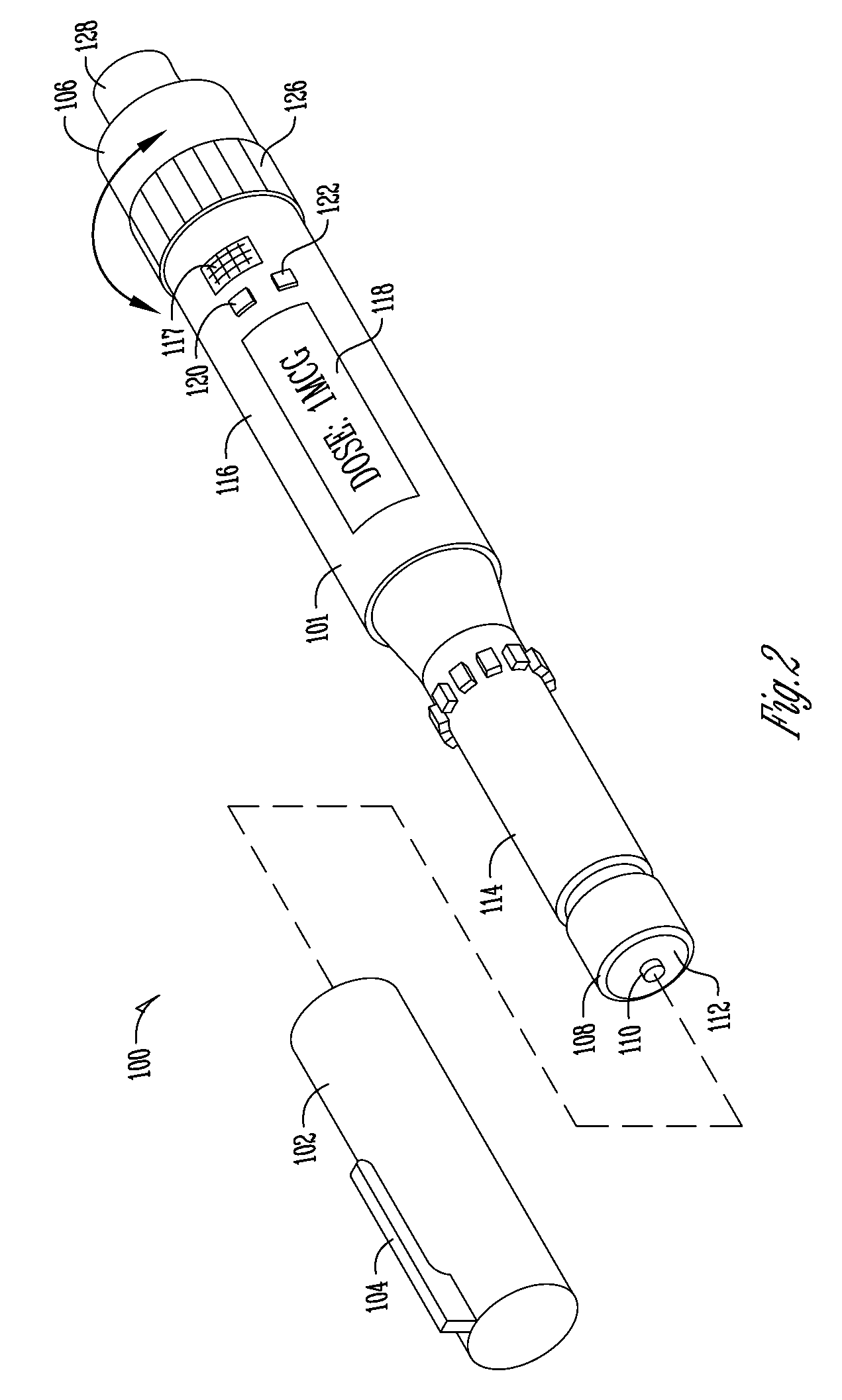
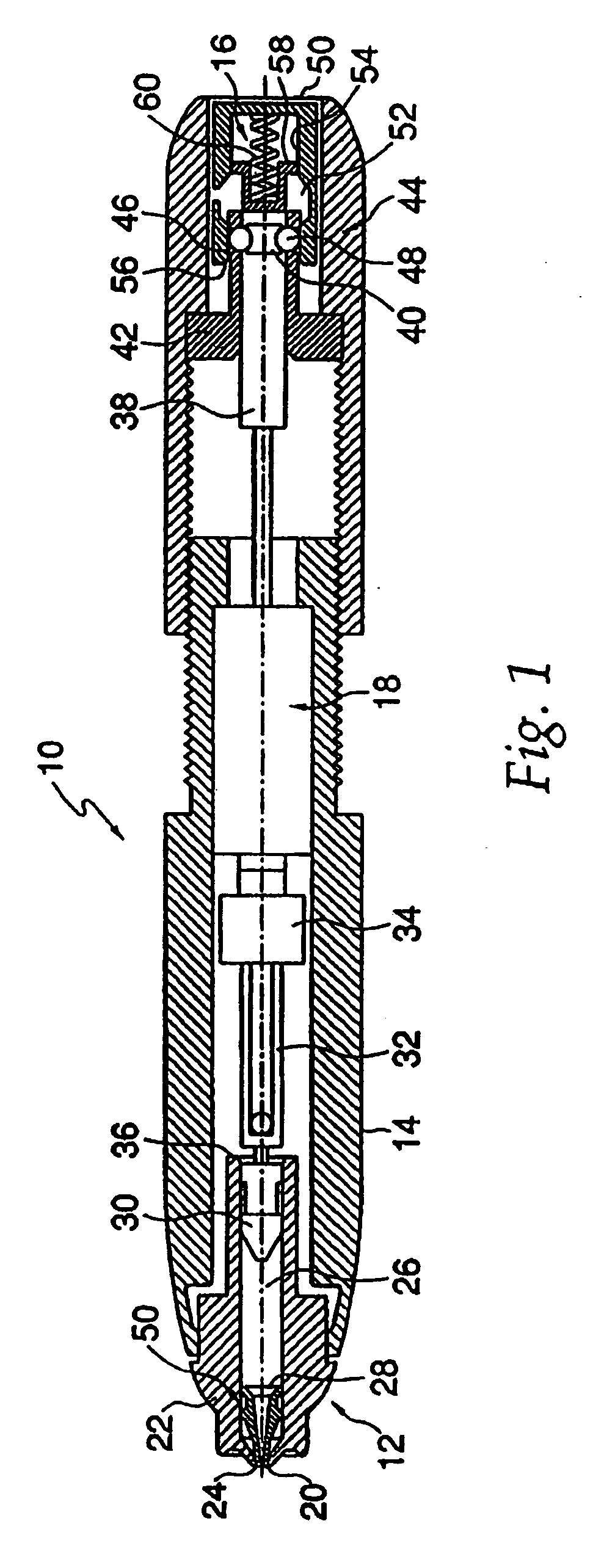
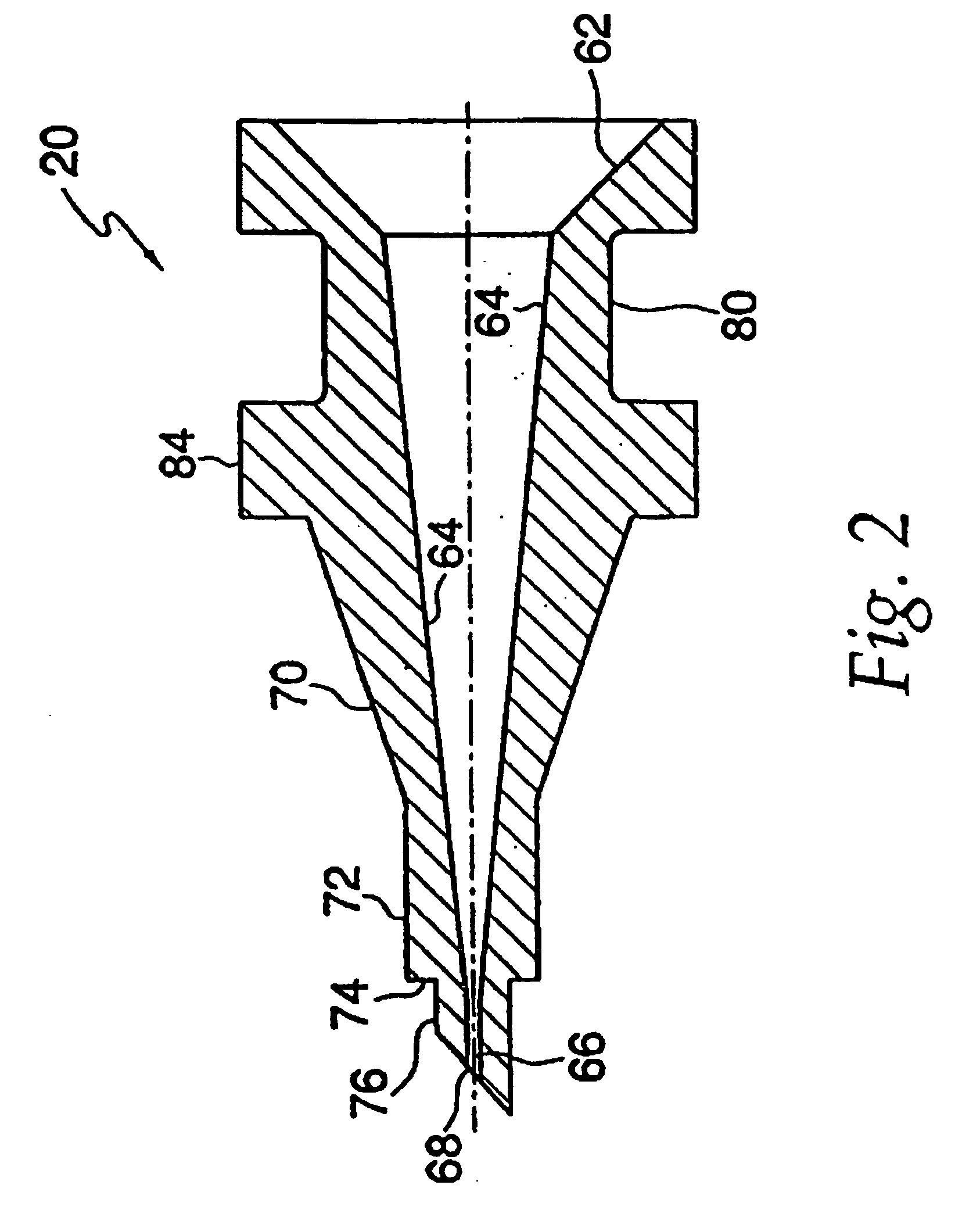
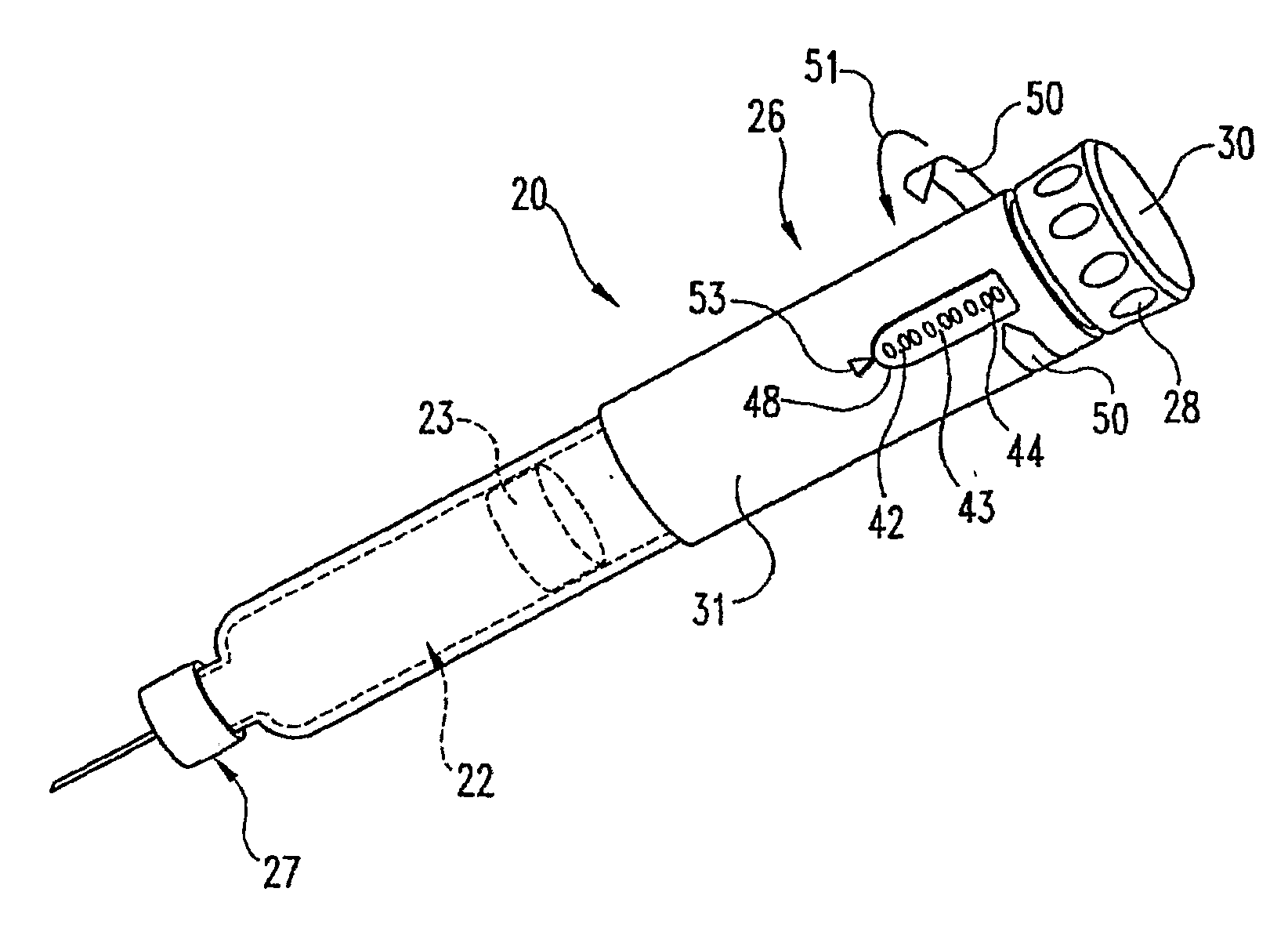
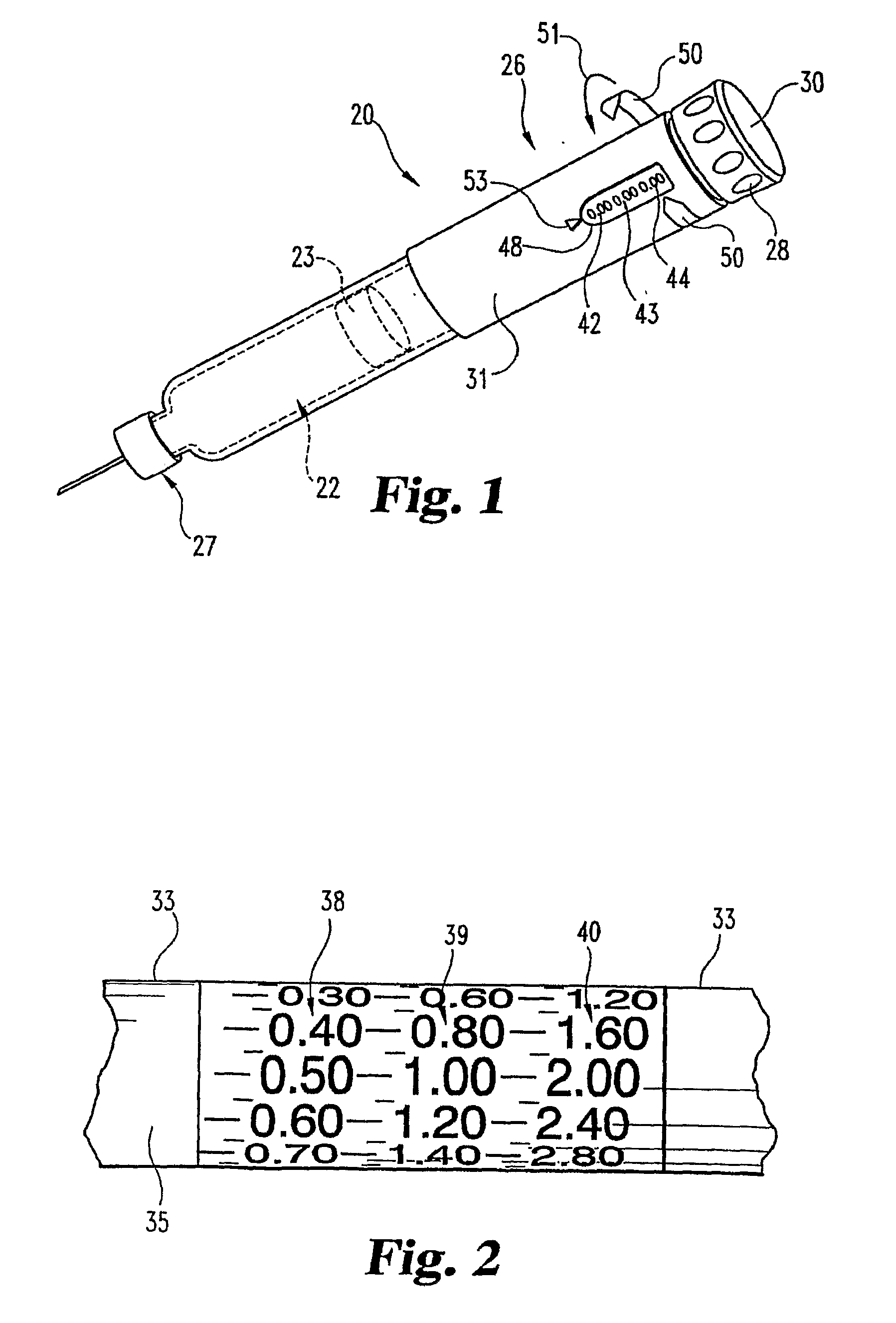
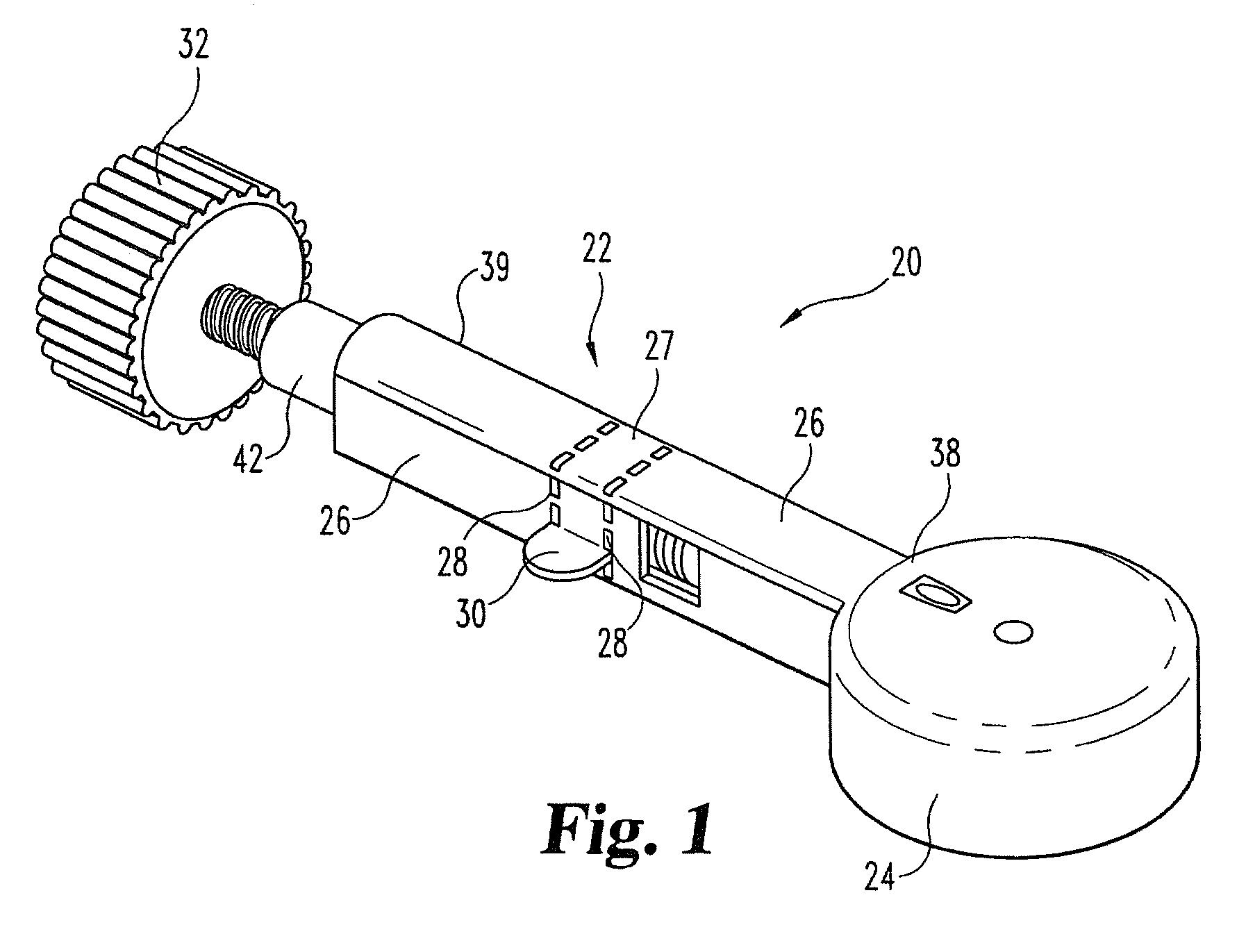
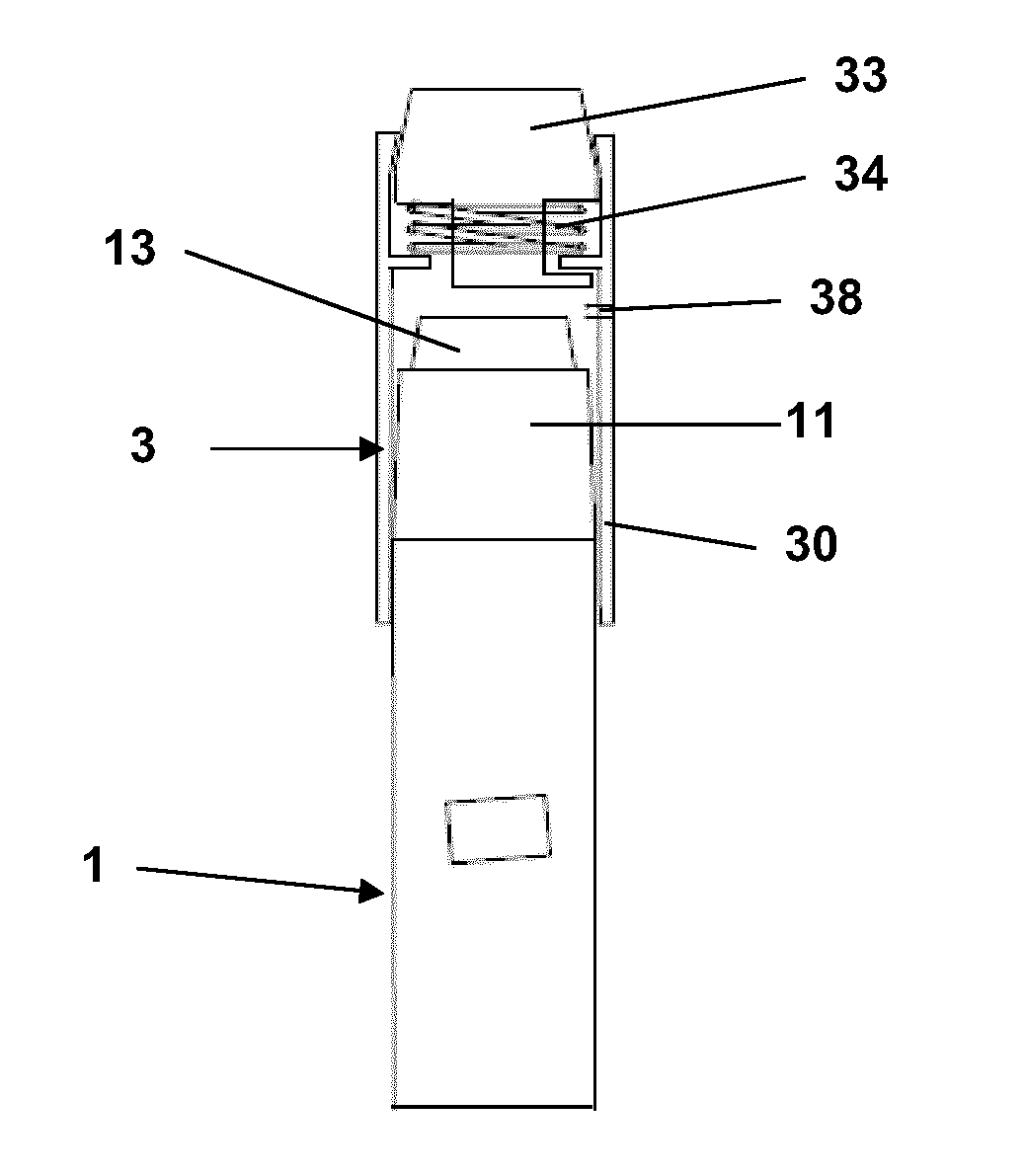
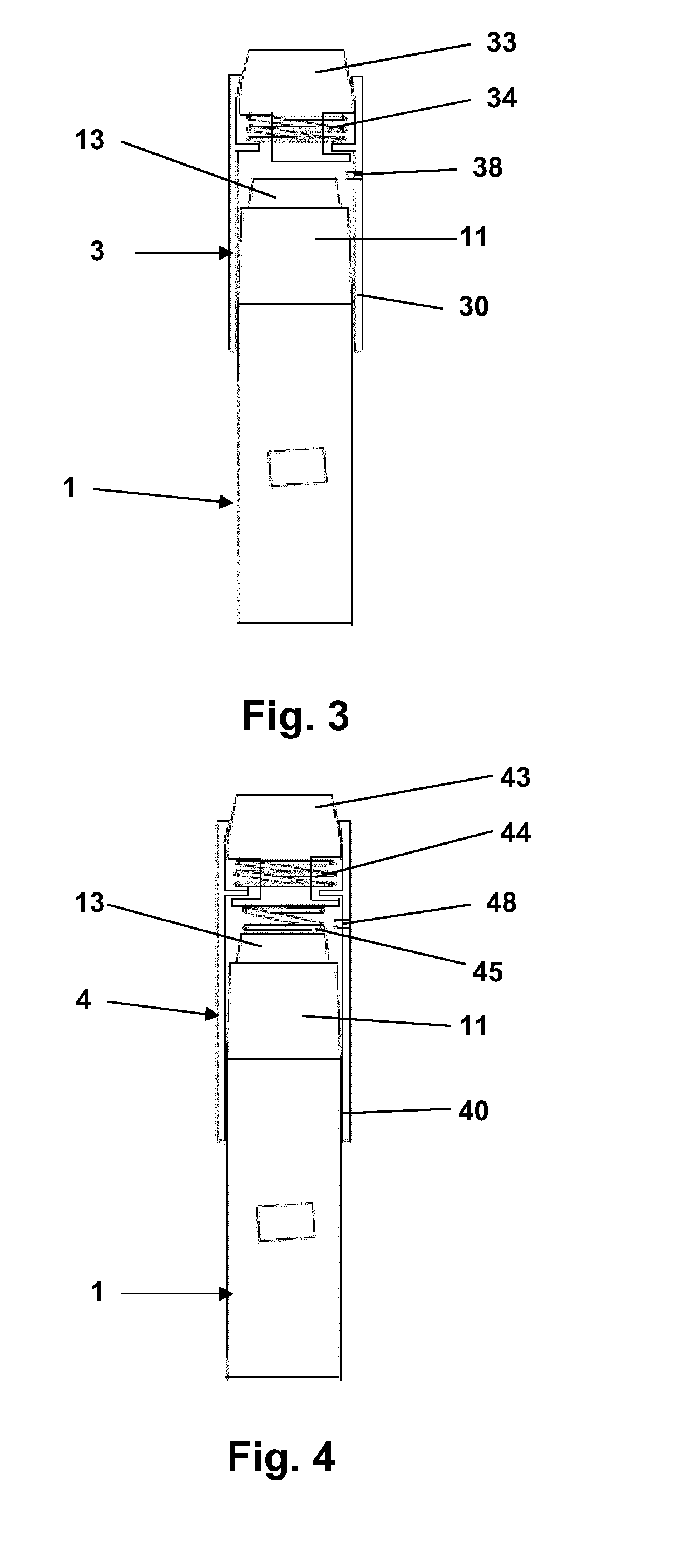
Dose Indicating Assembly of a Pharmaceutical Injection Device
InactiveUS20080269688A1Easy to useConverted easily and quicklyInfusion syringesIntravenous devicesMedication injectionInjection device
A dose indicating assembly in a pharmaceutical injection device (20). The dose indicating assembly includes an external housing barrel (31) extending in an axial direction, and a dial at least partially disposed within the housing barrel. The dial (33) is screwably movable in the axial direction relative to the barrel during dose setting. The dial includes an outer radial periphery with a plurality of parallel arrays (38, 39, 40) of dose indicia provided thereon, each of the plurality of arrays of dose indicia provided in a helical pattern on the periphery. The dose indicating assembly also includes means (50) for viewing the dose indicia of a selectively chosen one of the plurality of arrays.
Owner:IDEO +1
Method and device for painless injection of medication
InactiveUS20100076400A1Simple and inexpensive to performSimple and inexpensive to and operateAmpoule syringesAutomatic syringesMedication injectionAnesthetic
A method for providing a substantially painless injection of medication into a patient, including inserting an injection needle having an outside diameter greater than 0.20 mm and less than about 0.38 mm, and injecting a medicament through the needle and into the patient at a substantially constant volumetric flow rate of about 0.05 μL / s to about 50 μL / s that provides a painless injection. The method does not require the use of an anesthetic or that the medical personnel to spend a substantial amount of time performing the injection procedure, is relatively simple and inexpensive to perform and operate, and provides a relatively high degree of safety for both the medical personnel and for the patient.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
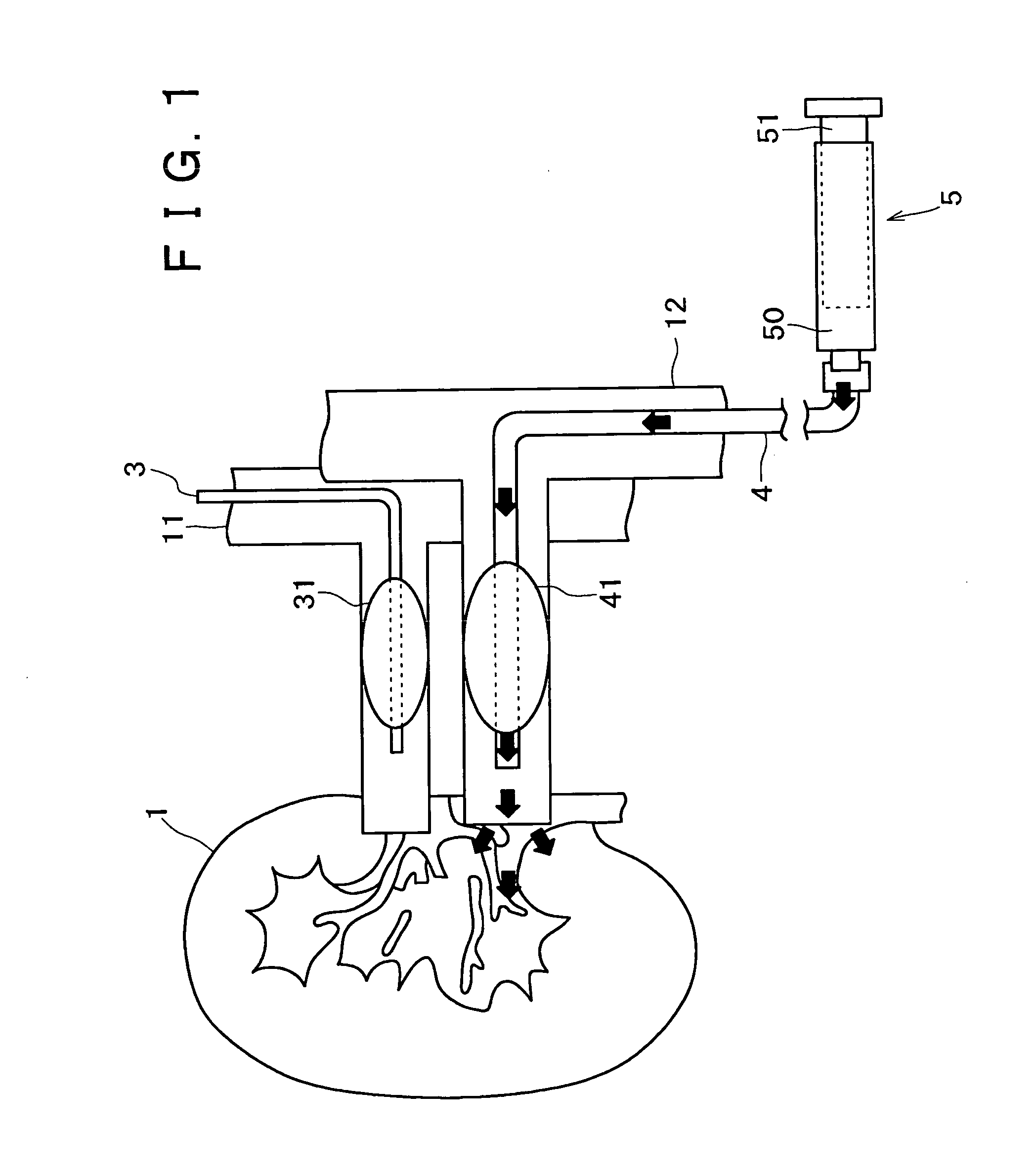
Compact non-electric medicament infuser
ActiveUS20100305507A1Easily attached to patientStable force transmissionIntravenous devicesSuction devicesMedication injectionDriver/operator
An assembly is provided which includes an infusion device coupled to a standard medication syringe. The medication syringe may be coupled to a stopcock valve having multiple ports and to which syringes, vial adapters, infusion tubing, and multiple other items may be coupled. The infusion device includes a source of power based on a resistance force such as vacuum, spring or gas power. The infusion device converts the resistance based force to usable work in the form of a force applicator. The force applicator includes a driver section on one section of a reciprocating arm and an attachment to the power source on another section of the arm. The driver is pulled outward (excursion) to increase the size of the chamber, creating a force that tends to return the driver back inward, causing incursion. The driver can be attached removably to the syringe plunger to induce the infusion process.
Owner:MONUMEDICAL
Medicament administration apparatus
InactiveUS20080287914A1Cost-effectiveSimple designPharmaceutical containersMedical devicesMedication injectionHypodermoclysis
An apparatus for removal of premixed drugs or reconstitution of lyophilized drugs and for the injection of the reconstituted drug into the patient. The apparatus includes a syringe assembly and an adapter assembly that can be removably connected to a medicament container containing a premixed drug or lyophilized medicament. The syringe assembly of the apparatus includes a body portion to form a liquid chamber between the forward end of the body portion and the piston and a syringe cannula assembly. The syringe cannula assembly, which can be removably interconnected with the body portion, comprises a cannula support and a hypodermic needle sealably connected to the cannula support. The adapter assembly comprises an adapter preferably molded from a moldable plastic that includes a top wall, an adapter cannula connected to and extending from the top wall and a variety of connectors connected to the top wall for removably interconnecting the adapter with the medicament container. The adapter assembly further includes syringe connector member connected to the top wall for removably interconnecting the syringe with the adapter in a manner to uniquely position the syringe cannula within the lumen of the adapter cannula wherein it is completely shielded from external contamination to prevent print damage and injury to the user.
Owner:MEDICAL ASSOCS NETWORK
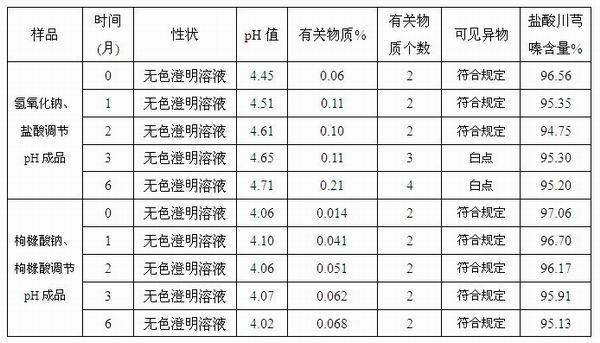
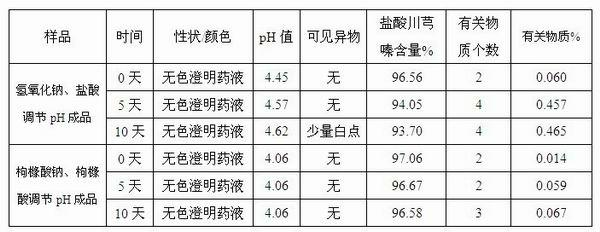
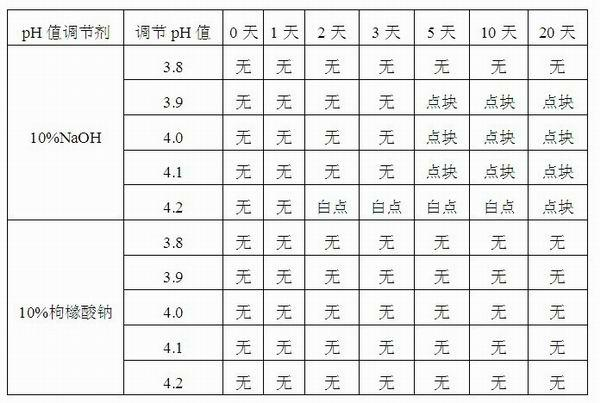
Injection-purpose medicine composition for improving stability of ligustrazine medicine injection formulation and preparation method of injection-purpose medicine composition
ActiveCN102008727AStable pHDecreased substancesOrganic active ingredientsPharmaceutical non-active ingredientsMedication injectionUse medication
The invention discloses an injection-purpose medicine composition for improving stability of ligustrazine medicine injection formulation and a preparation method of the injection-purpose medicine composition. The injection-purpose medicine composition is prepared by the method comprising following steps: dissolving ligustrazine salt in water for injection, adjusting the pH value of the liquid medicine by adding citric acid and / or sodium citrate used as a pH regulator, wherein the dosage of the citric acid and / or sodium citrate ranges from (0.1mg to 200.0mg) / 100ml. In the invention, pH value of the injection liquid can be more stable, the content of ligustrazine degradation substance is greatly reduced compared with that of the prior art, the clarity of the ligustrazine injection liquid is improved under the condition of not using other cosolvents to increase the clinical application risk, particularly, the problems of small white spots, white blocks and solution turbidity are solved under the condition that the ligustrazine injection liquid is stored for a long time by adopting the prior art, and the medicine composition ensures that the inspection of visible foreign substances of the product is in accordance to the formulation of medicine quality standard, and is convenient for clinical medication and popularization.
Owner:SICHUAN SUNNYHOPE PHARM CO LTD
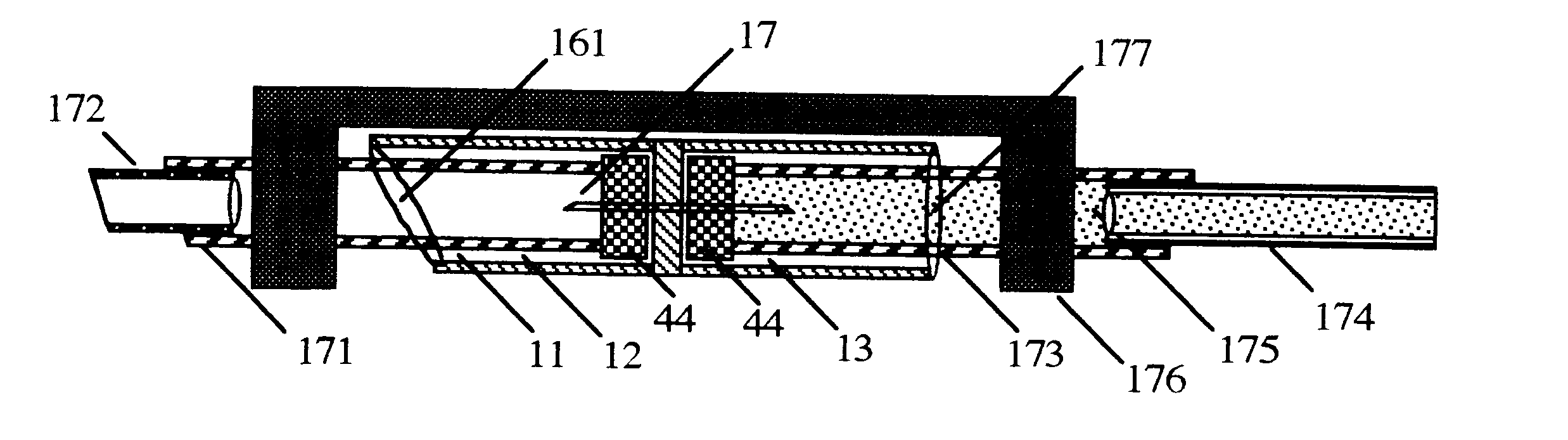
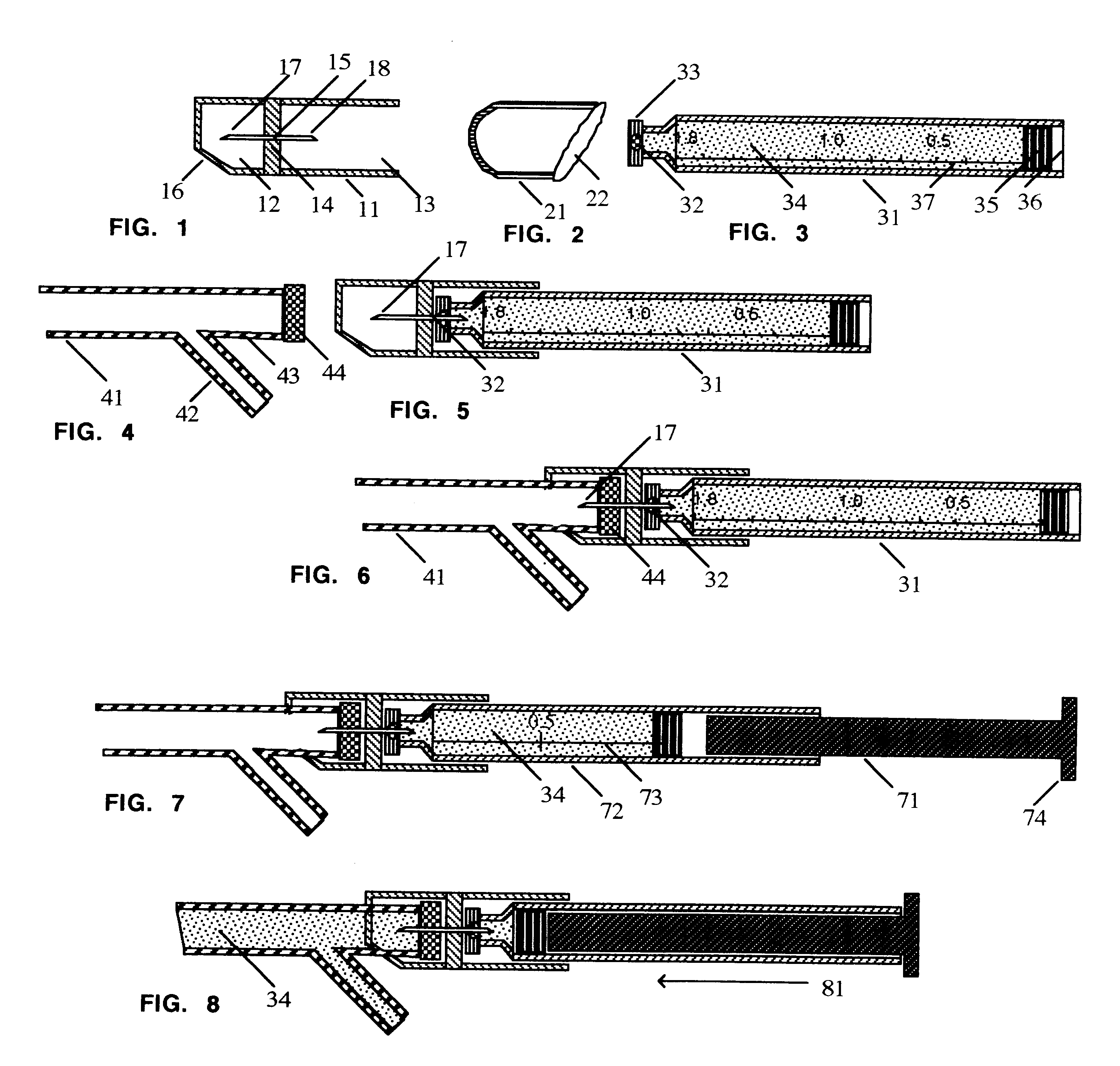
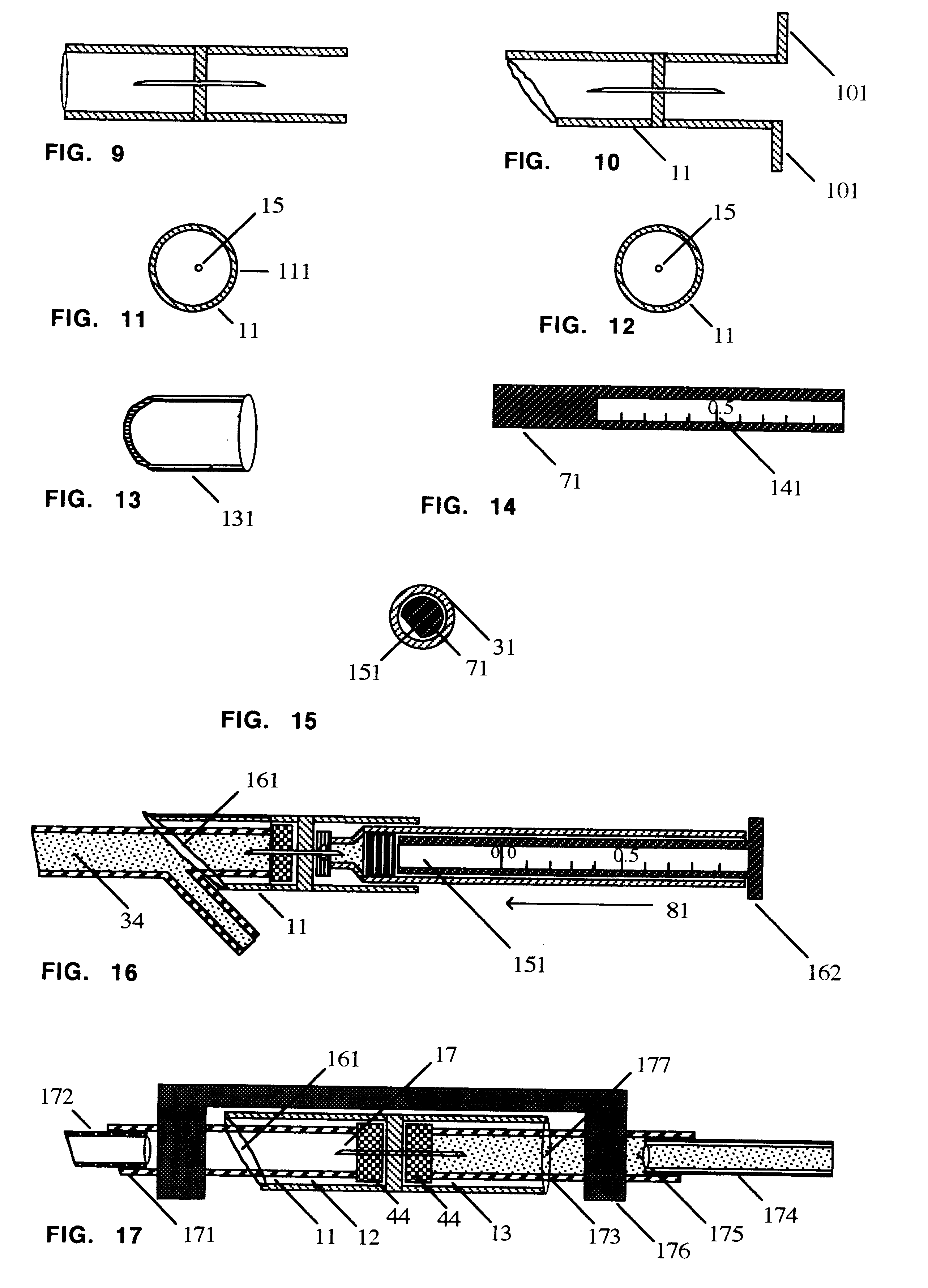
Medicine injection apparatuses
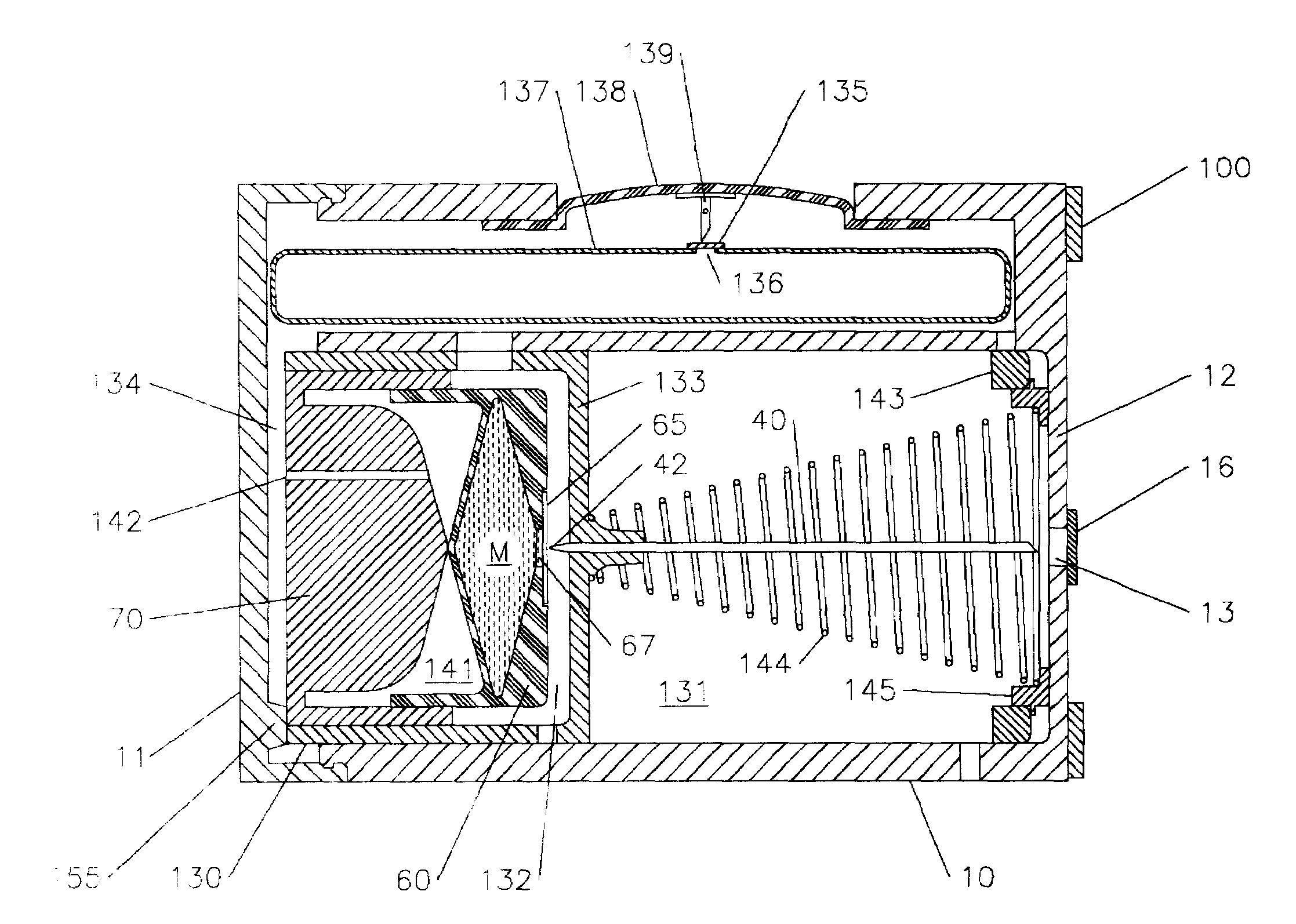
Medicine injection apparatuses having multiple chambers. The chambers may be in lateral relationship. A torsional part is adapted to turn and be repositioned angularly relative to a main body to allow different chambers to be used to receive an injection assembly. The medicine injector can be constructed to administer multiple doses. The apparatuses may also have a storage chamber for storing the injection assembly after use. Plural drivers may be used to administer multiple doses, such as at the different angular positions of the torsional part. The apparatuses may allow multiple automatic injections from different angular positions and storage of an injector after use. Needle fright is reduced by minimizing exposure of an injection needle prior to injection.
Owner:WASHINGTON BIOTECH CORP
Two-stage reconstituting injector
ActiveUS20090292240A1Easy and effective deliveryAmpoule syringesJet injection syringesMedication injectionCatheter
An injector for injecting a medicament into a patient. The injector includes a container defining a first chamber, which contains a fluid therein, and a second chamber. The injector also includes an injection conduit configured for directing the fluid fired from the container into the patient. A transfer mechanism is operable by a user to transfer the fluid from the first chamber to the second chamber in a first stage of operation, and a firing mechanism is operable by the user for firing the fluid from the second chamber through the injection conduit in a second stage of operation. An energy source is in powering association with the firing mechanism to drive firing mechanism in the first and second stages.
Owner:ANTARES PHARMA
Method for providing medicament to tissue
InactiveUS7776025B2Promote angiogenesisImprove angiogenesisElectrotherapyJet injection syringesMedication injectionInjection device
A system for delivering medicaments to tissue including a delivery member and an optical fiber formed together into a unitary structure. The optical fiber has an inlet attached to a laser energy source and an outlet for emitting laser energy. The delivery member has an inlet attached to a medicament source and an outlet for injecting medicament. A handpiece is adapted to receive the ablating and injecting device in a controlled and movable relationship and may include at least one tissue stabilizing member thereon. In use, the distal end of the handpiece is placed against tissue to be ablated. The optical fiber is advanced into the tissue while emitting laser energy thereby ablating the tissue and forming a channel therein. During retraction, medicament may be injected into the channel or into the tissue surrounding the channel.
Owner:EDWARDS LIFESCIENCES CORP
Module for a medication injection device
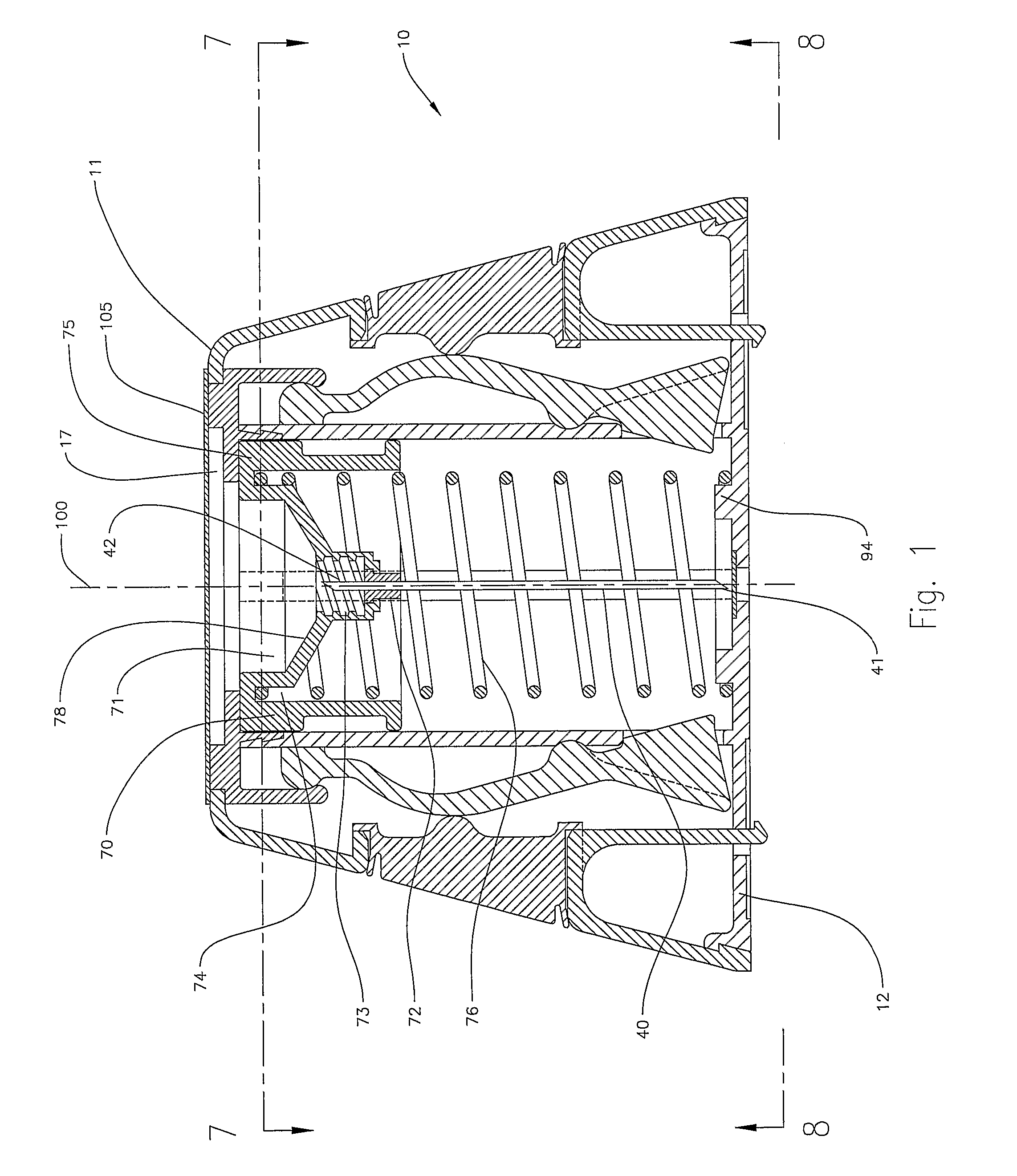
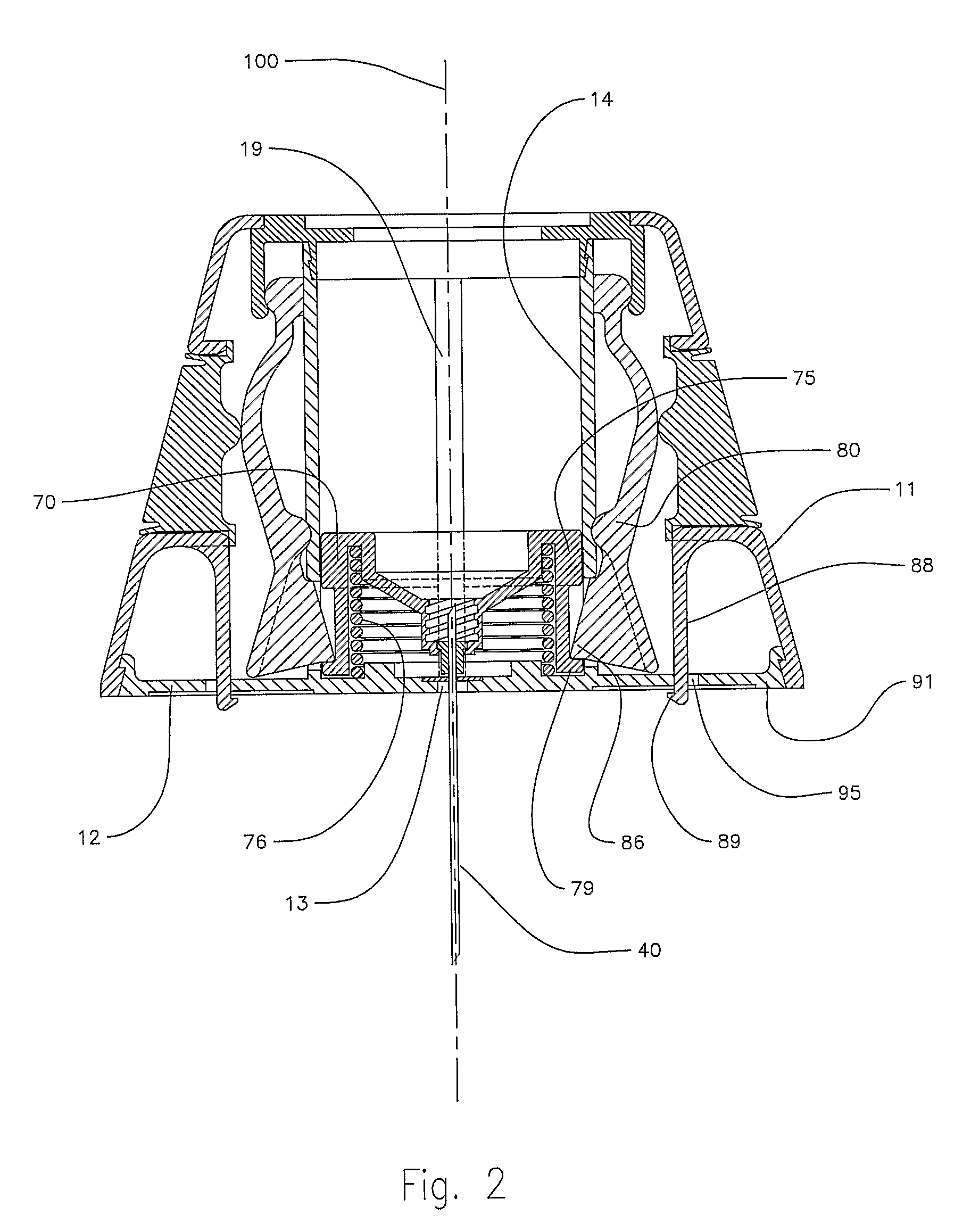
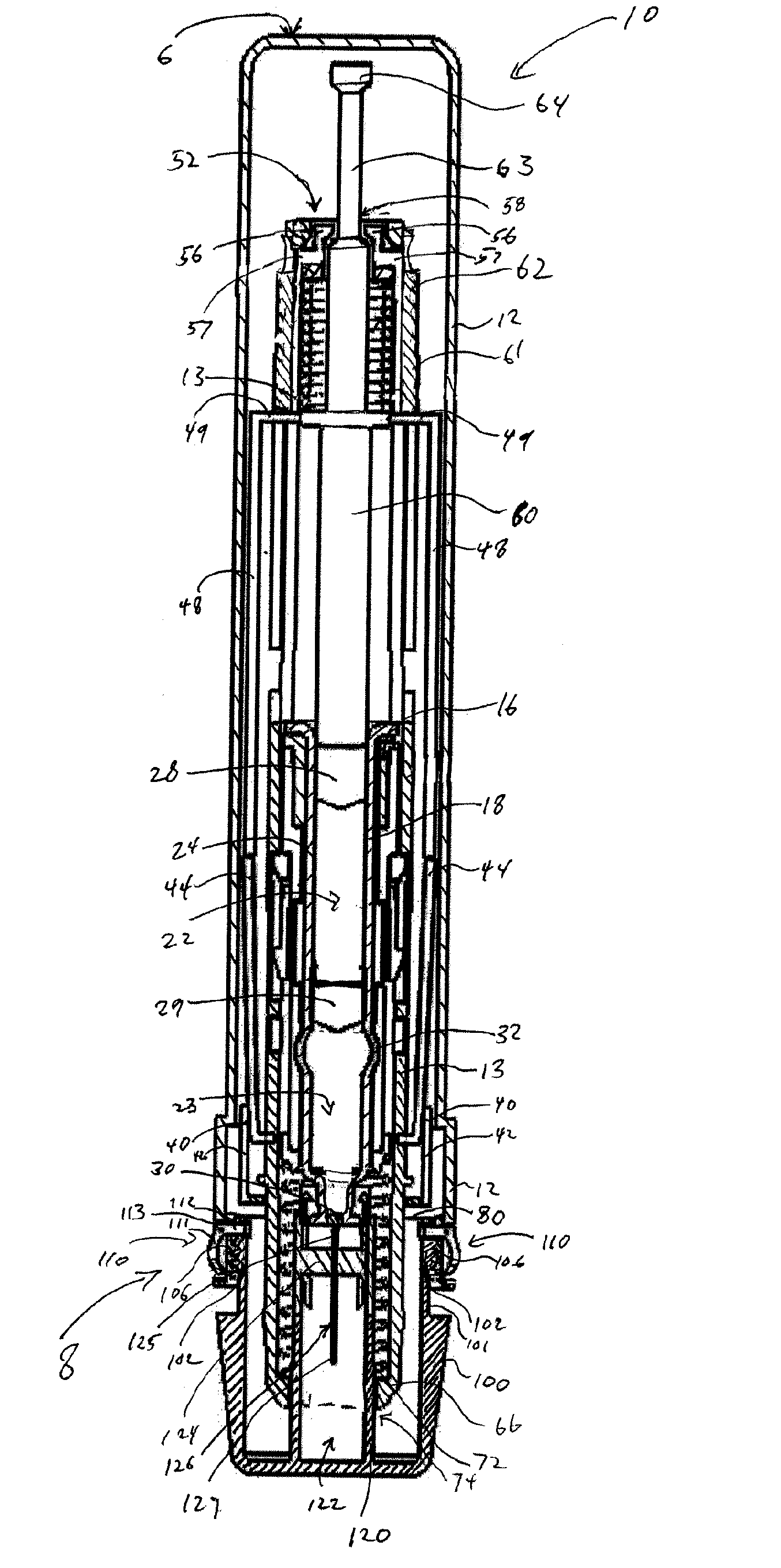
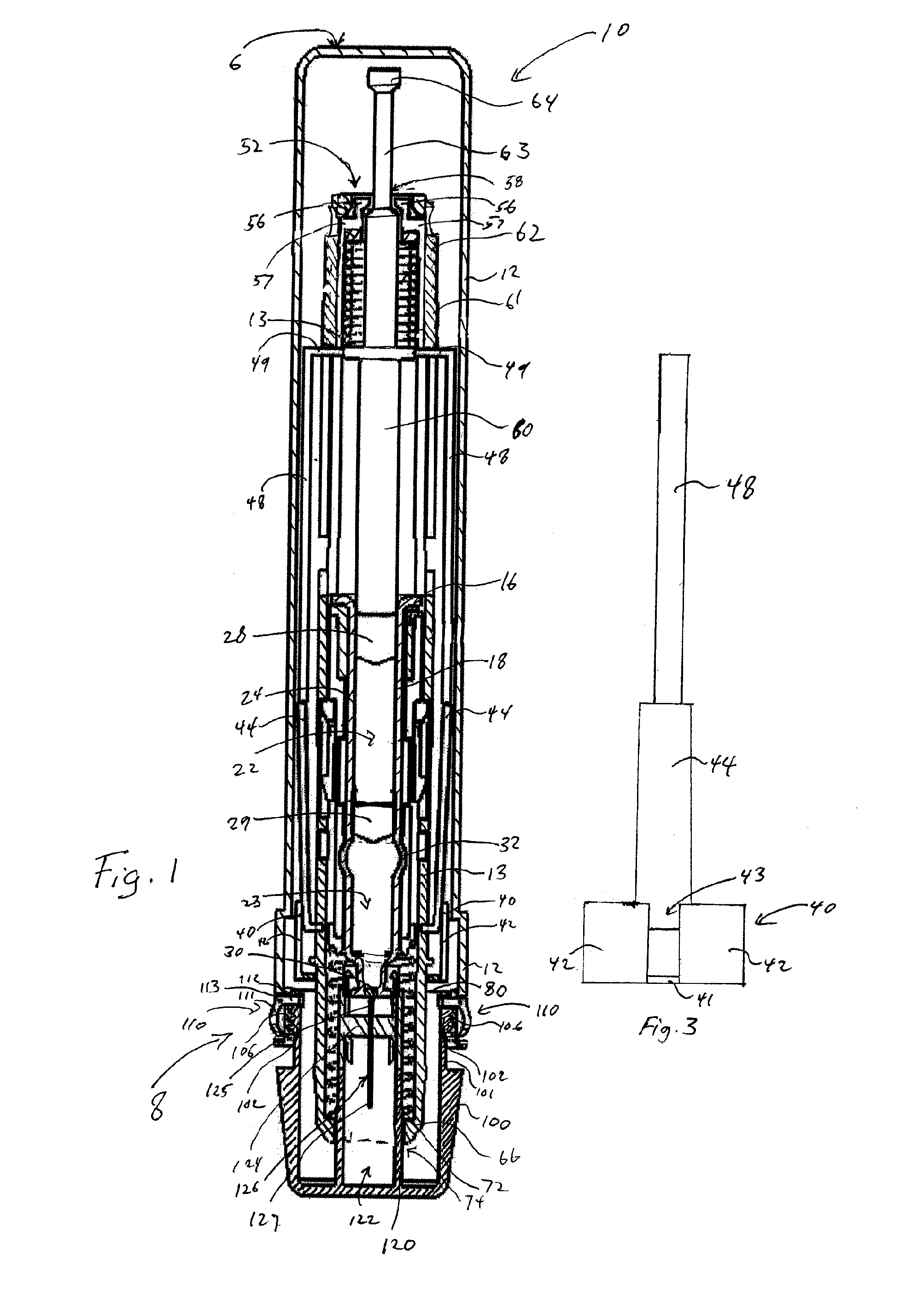
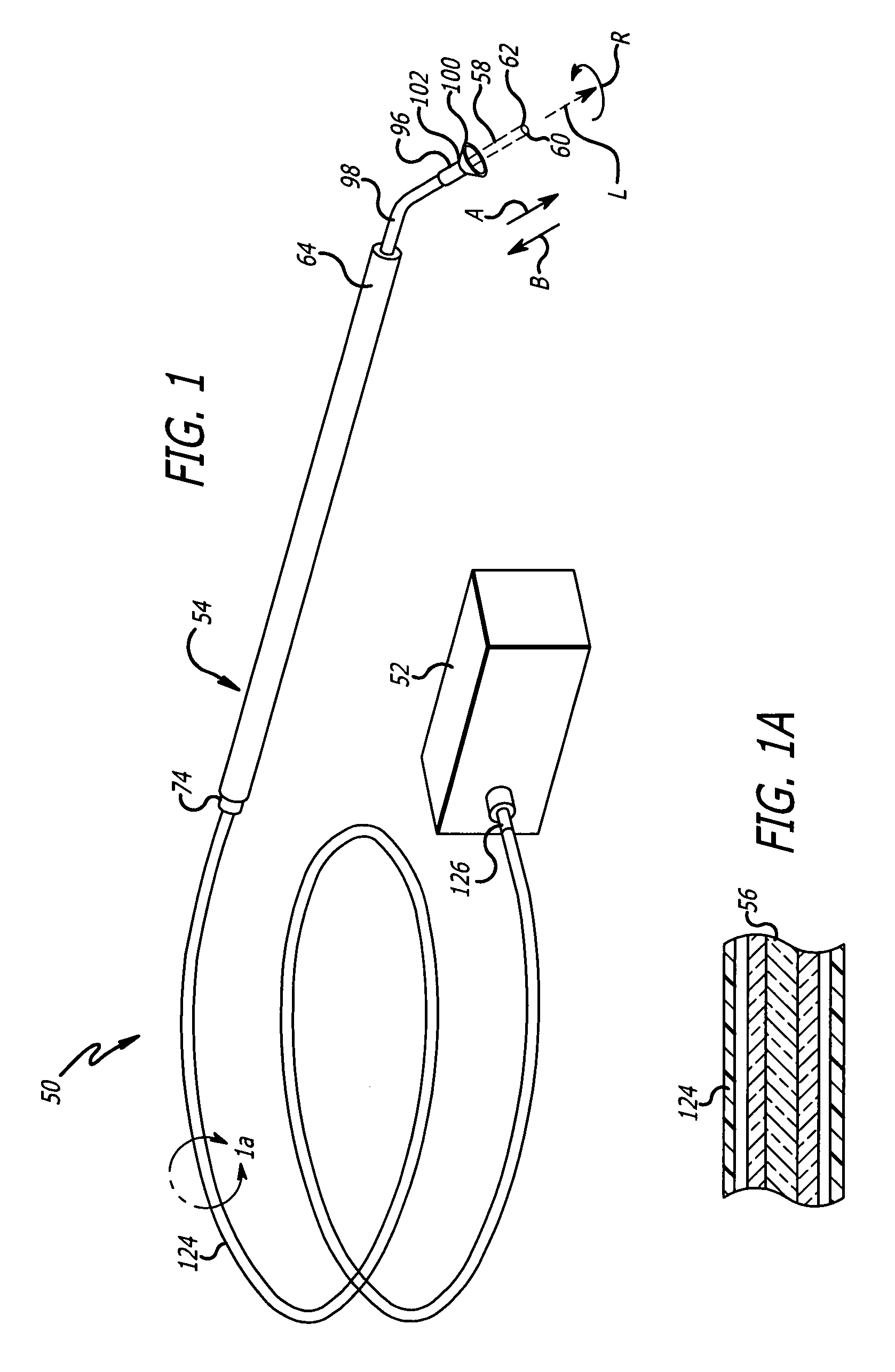
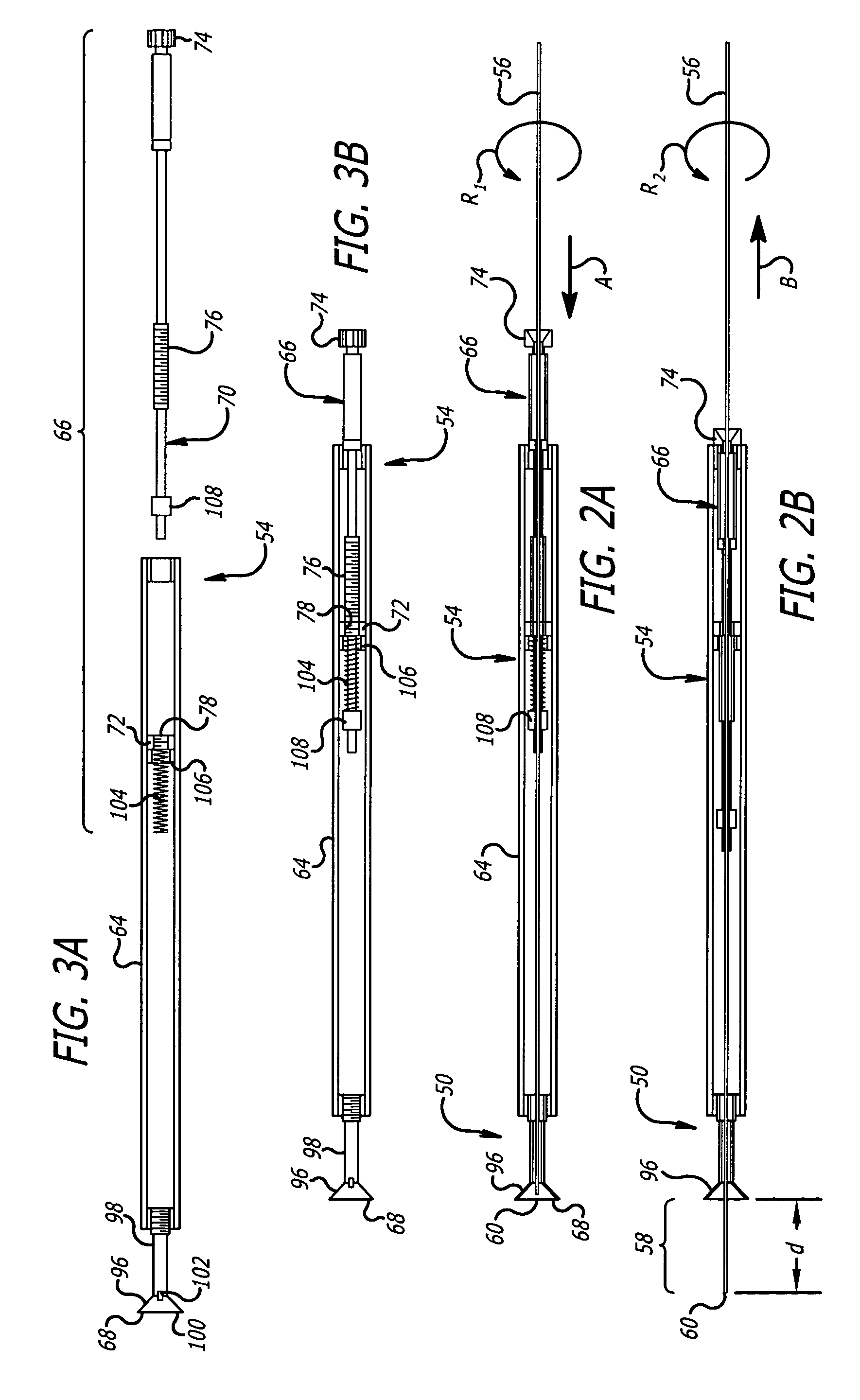
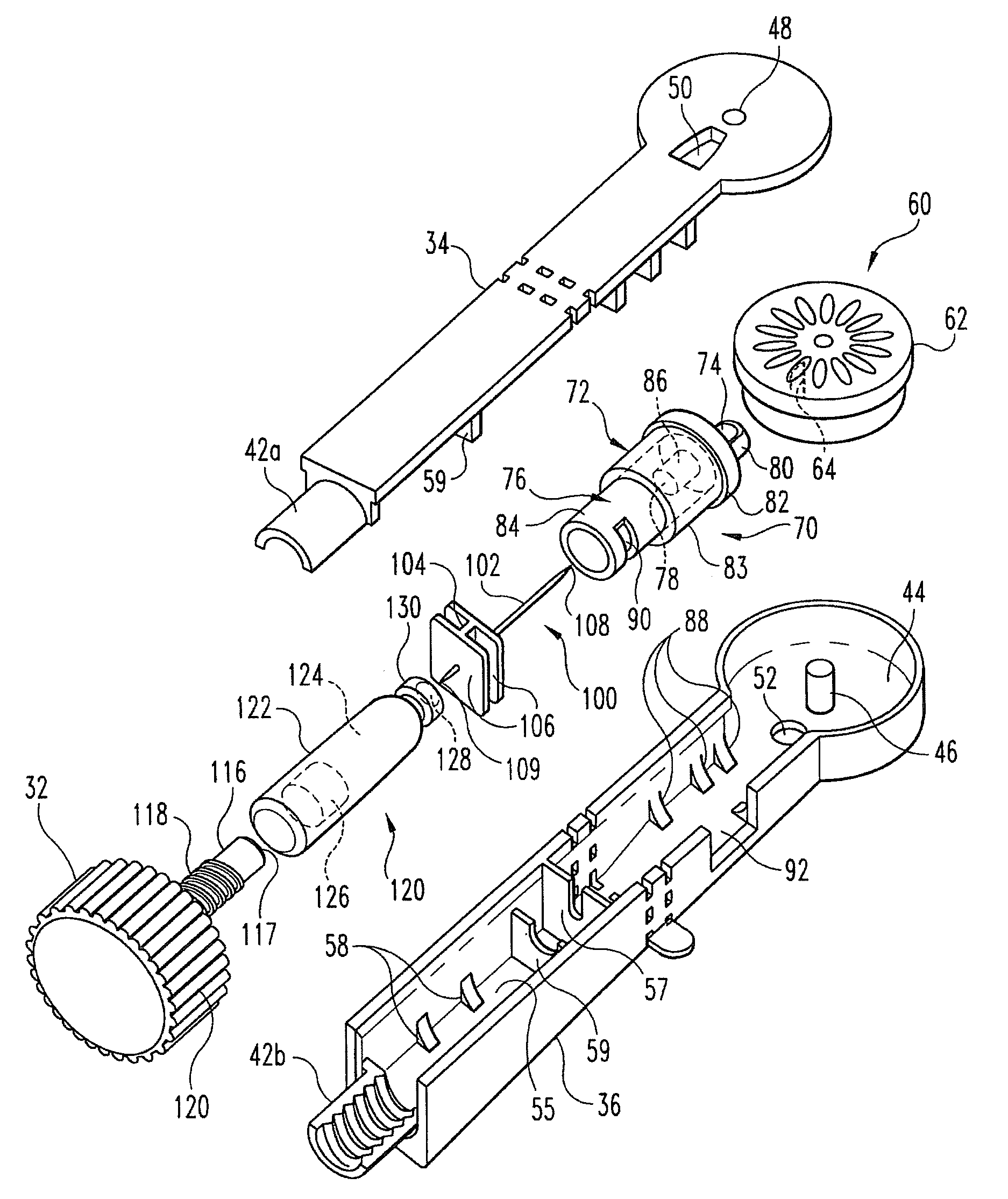
InactiveUS8216192B2Easy to disassembleAmpoule syringesInfusion needlesMedication injectionEngineering
A medication and needle module (20) for an injection device. The module (20) includes a housing (22) including a first portion (39) and a second portion (38) detachably connected together, the second housing portion having a periphery complementarily shaped with a cavity (154) in the injection device (150) to be loadable therein, a primary container (120) within the first housing portion and including a medication filled reservoir (124), a secondary container (70) within the second housing portion and including a medication tillable reservoir, a needle cassette (60) rotatably mounted within the second housing portion and including a plurality of delivery needles (64), and a transfer needle assembly (100) within the housing. The detachability of the second housing portion from the first housing portion permits the second housing portion with the secondary container and the needle cassette to be loaded as a unit independently of the first housing portion into the injection device cavity for use.
Owner:ELI LILLY & CO
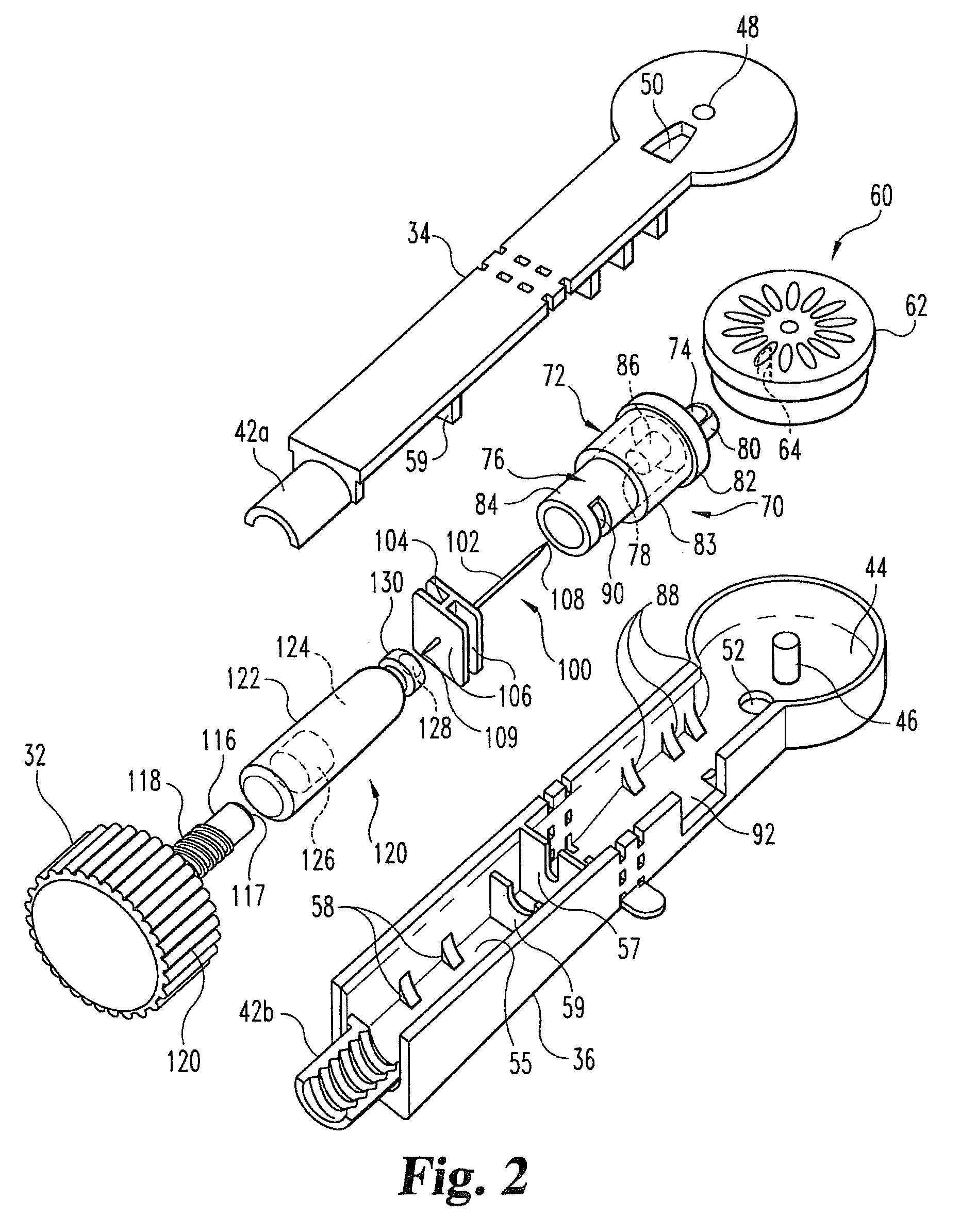
Drug delivery injection pen with add-on dose capturing and display module
A drug injector (1) comprising expelling means for expelling a dose of drug from a reservoir, a release member (13) for releasing the drug expelling means, and an actuation member (33) adapted to be moved by a user between an initial, intermediate and actuated position in which the release member is moved to release the drug expelling means. The injector further comprises an electronic capturing system for capturing data representing a dose of drug to be expelled, and a switch (38) for starting initialization of the data capture system, the switch being actuated when the actuation member is positioned in its intermediate position. A spring (34) provides a biasing force against movement of the actuation member between its initial and intermediate position. Thereby the electronic data capturing system is allowed to initialize during the actuation member's movement between the intermediate and the actuated position.
Owner:NOVO NORDISK AS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com