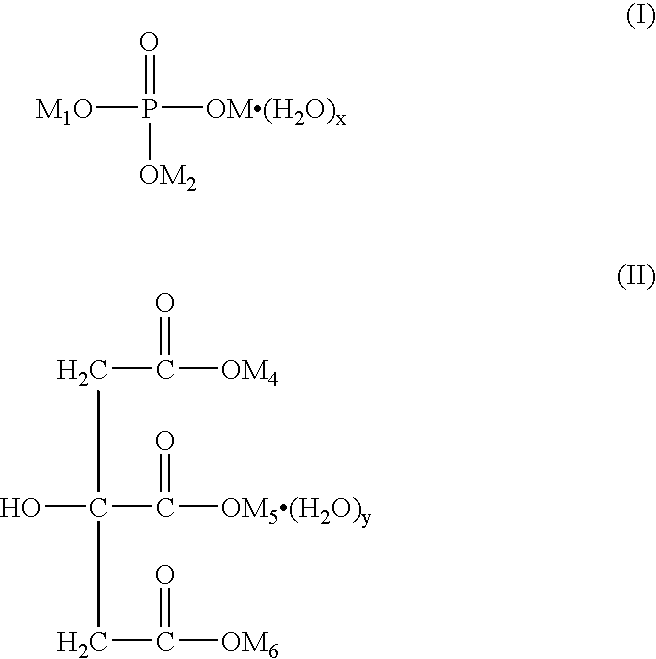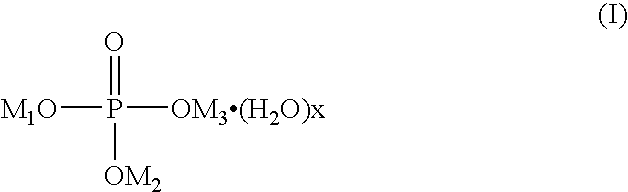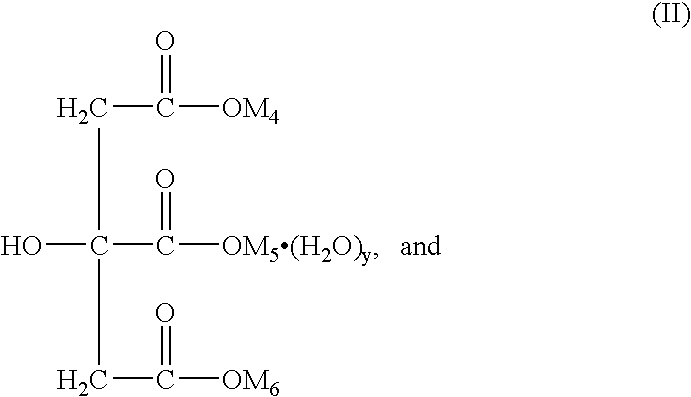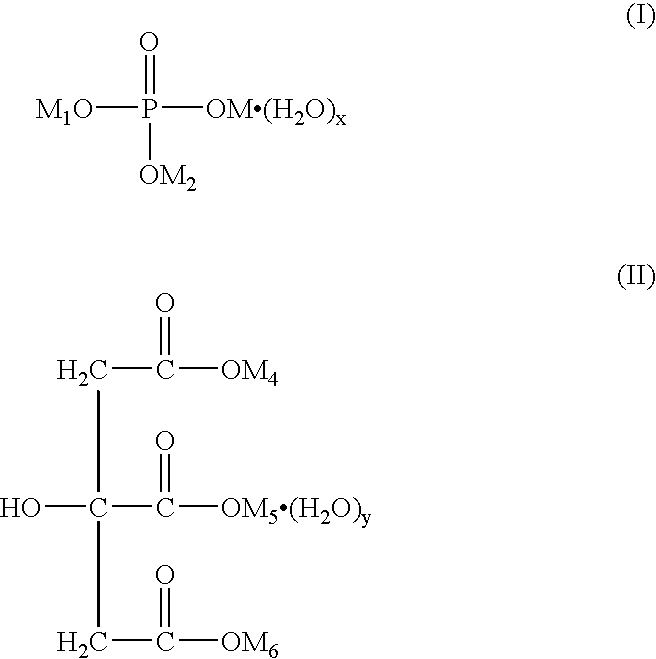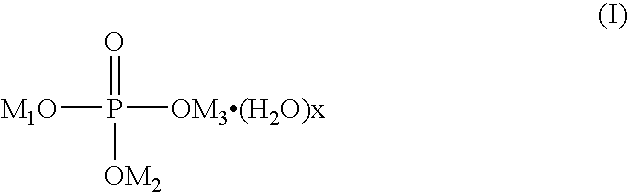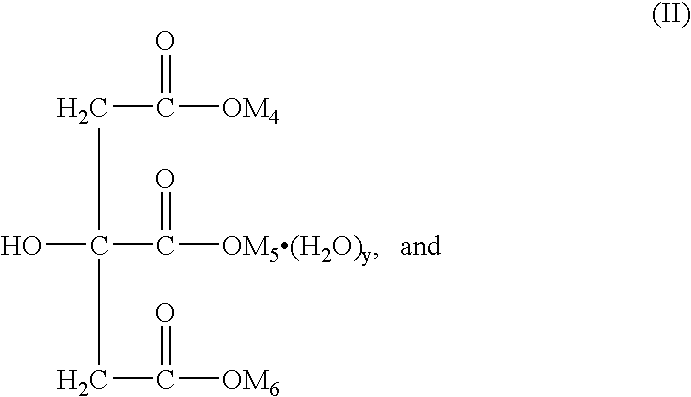Patents
Literature
62results about How to "Decreased substances" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
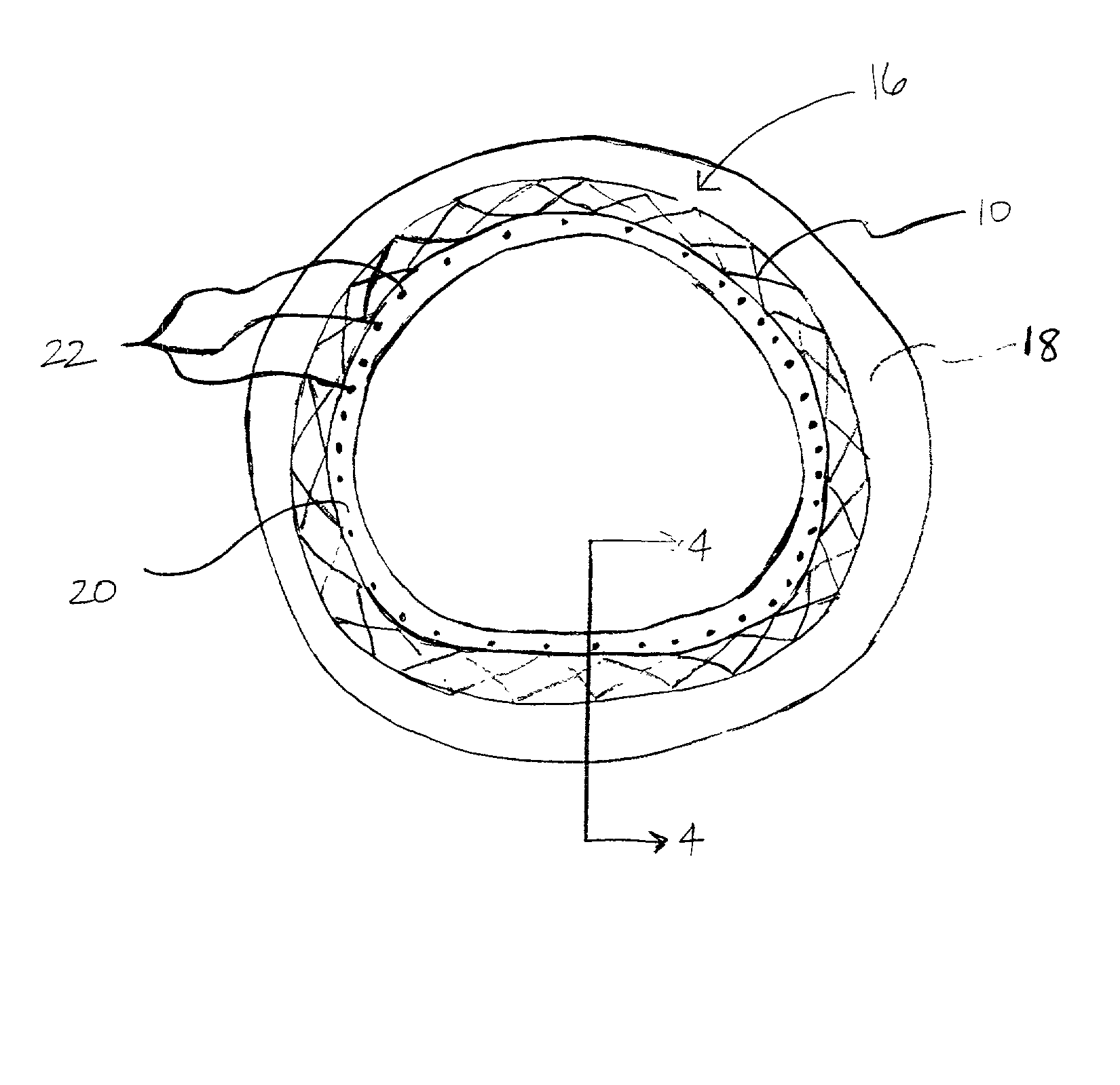
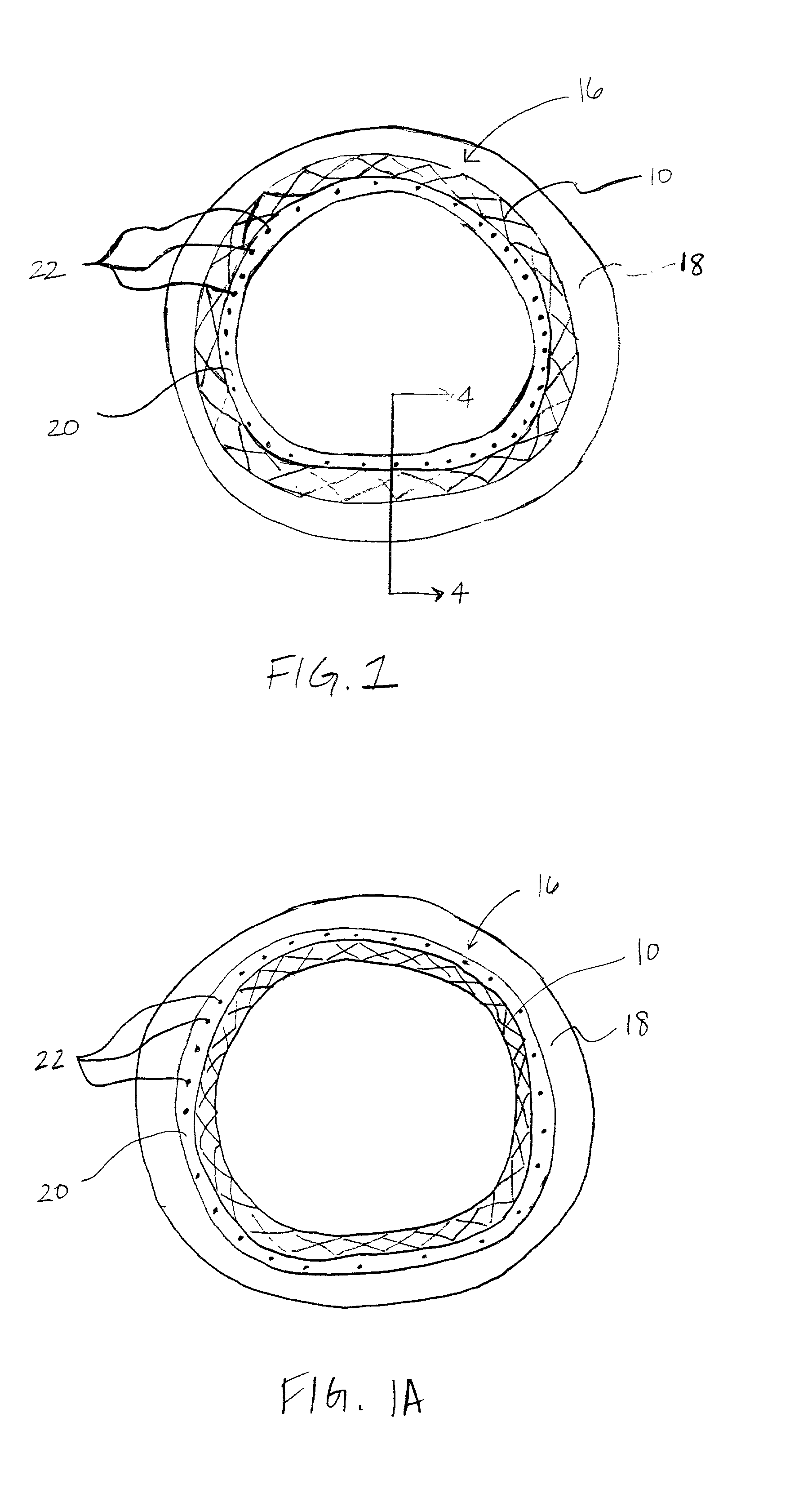
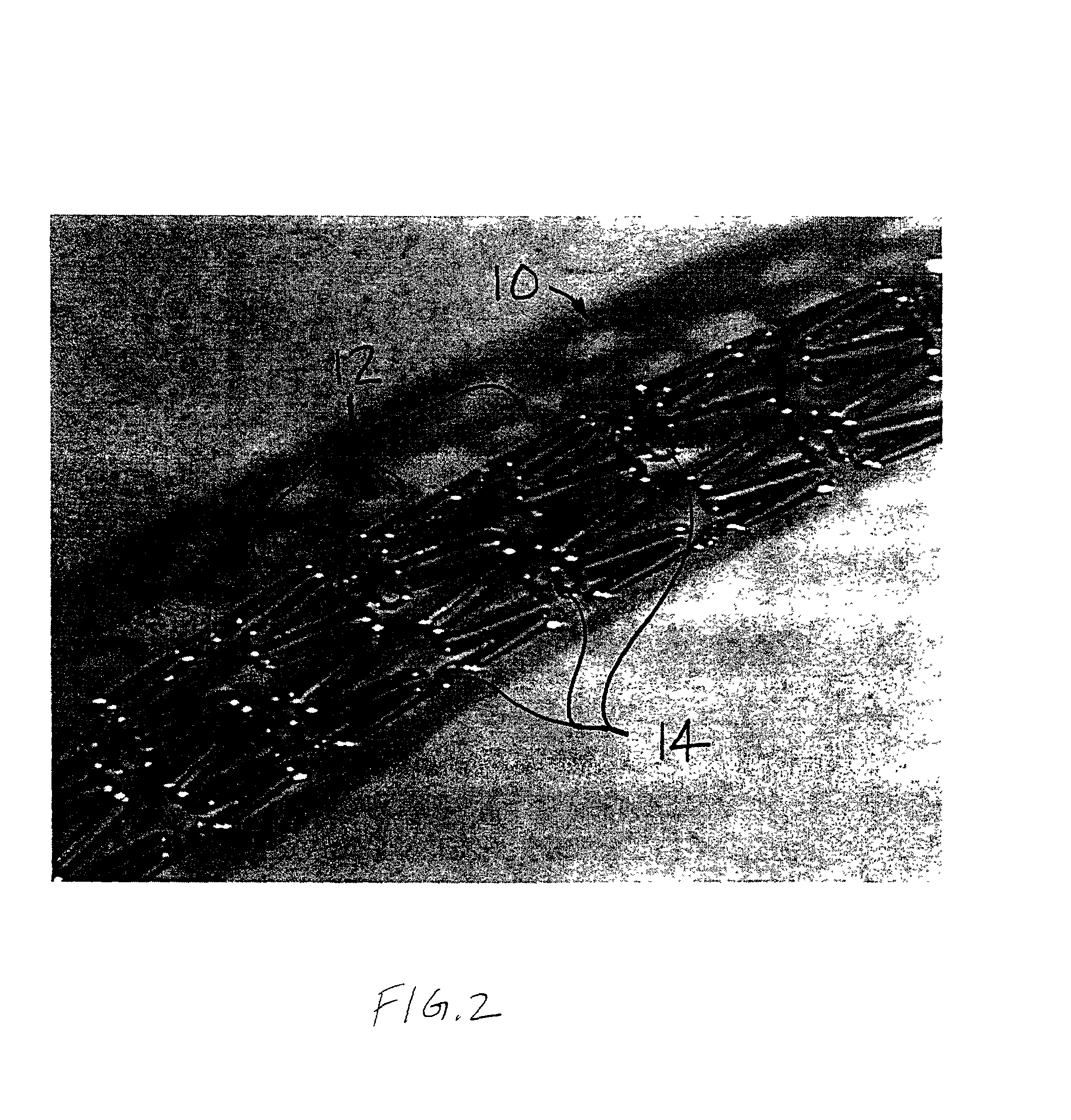
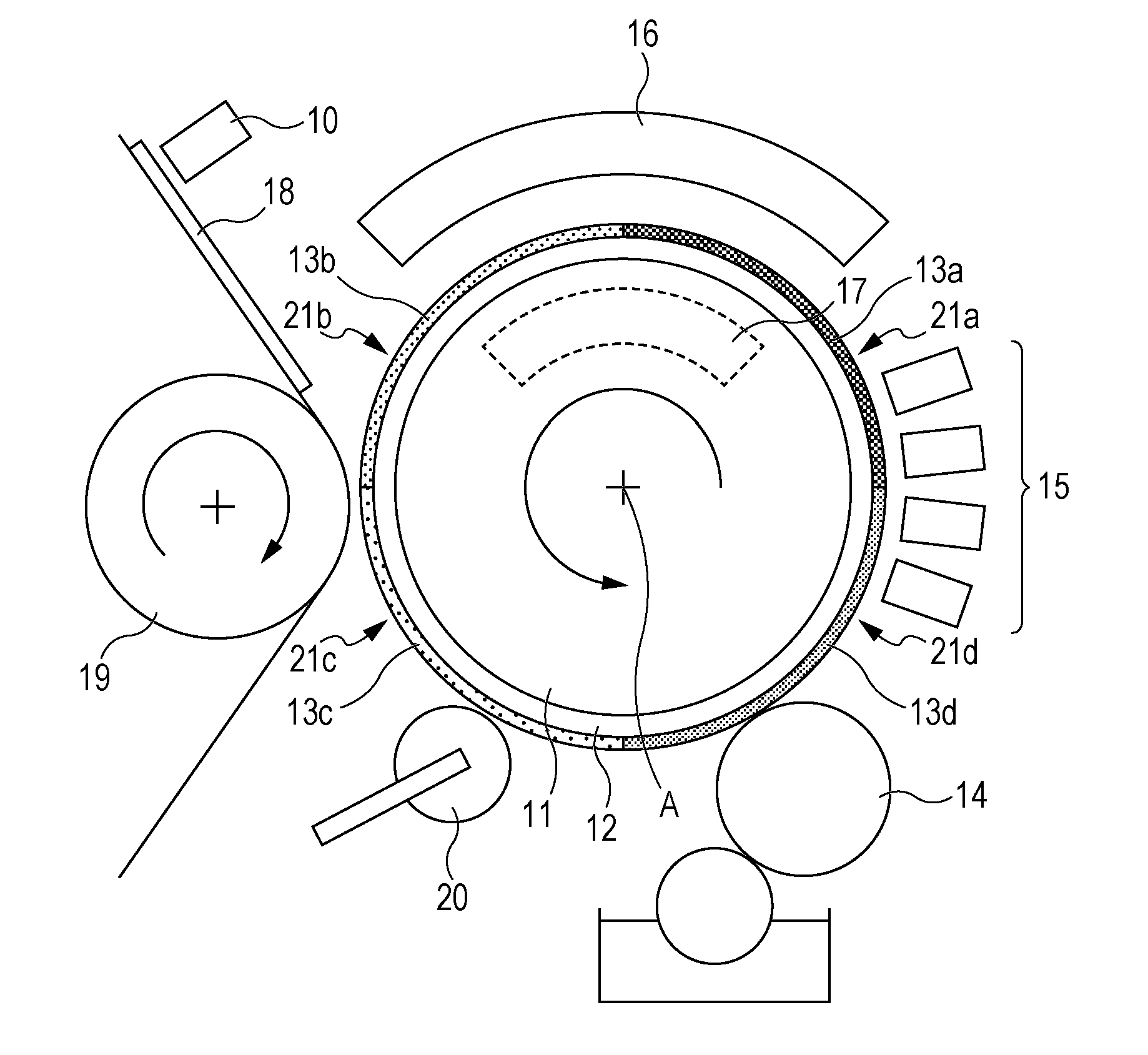
Apparatus and methods for controlled substance delivery from implanted prostheses
InactiveUS20020082685A1Improve drug delivery efficiencyReduce lossesStentsSurgeryPercent Diameter StenosisControl substances
The present invention provides improved devices and methods for inhibiting restenosis and hyperplasia after intravascular intervention. In particular, the present invention provides luminal prostheses which allow for programmed and controlled substance delivery with increased efficacy to selected locations within a patient's vasculature to inhibit restenosis. The luminal delivery prosthesis comprises a scaffold which is implantable within a body lumen and means on the scaffold for releasing a substance from the scaffold. The substance is released over a predetermined time pattern comprising an initial phase wherein the substance delivery rate is below a threshold level and a subsequent phase wherein the substance delivery rate is above a threshold level.
Owner:ALTAI MEDICAL TECH
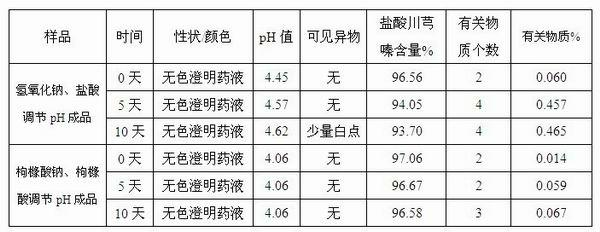
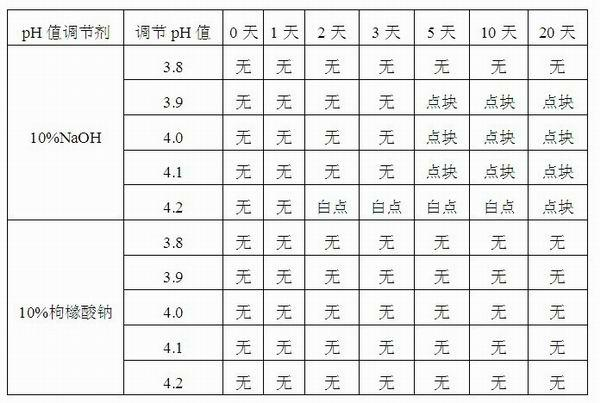
Injection-purpose medicine composition for improving stability of ligustrazine medicine injection formulation and preparation method of injection-purpose medicine composition
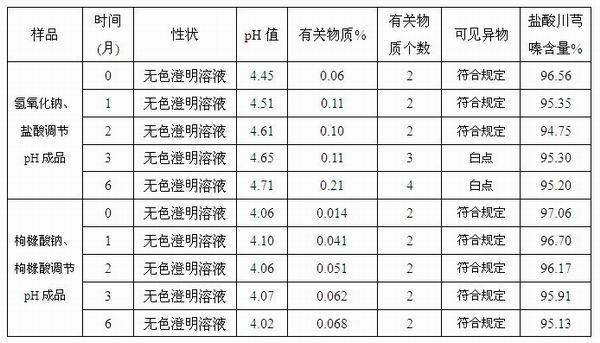
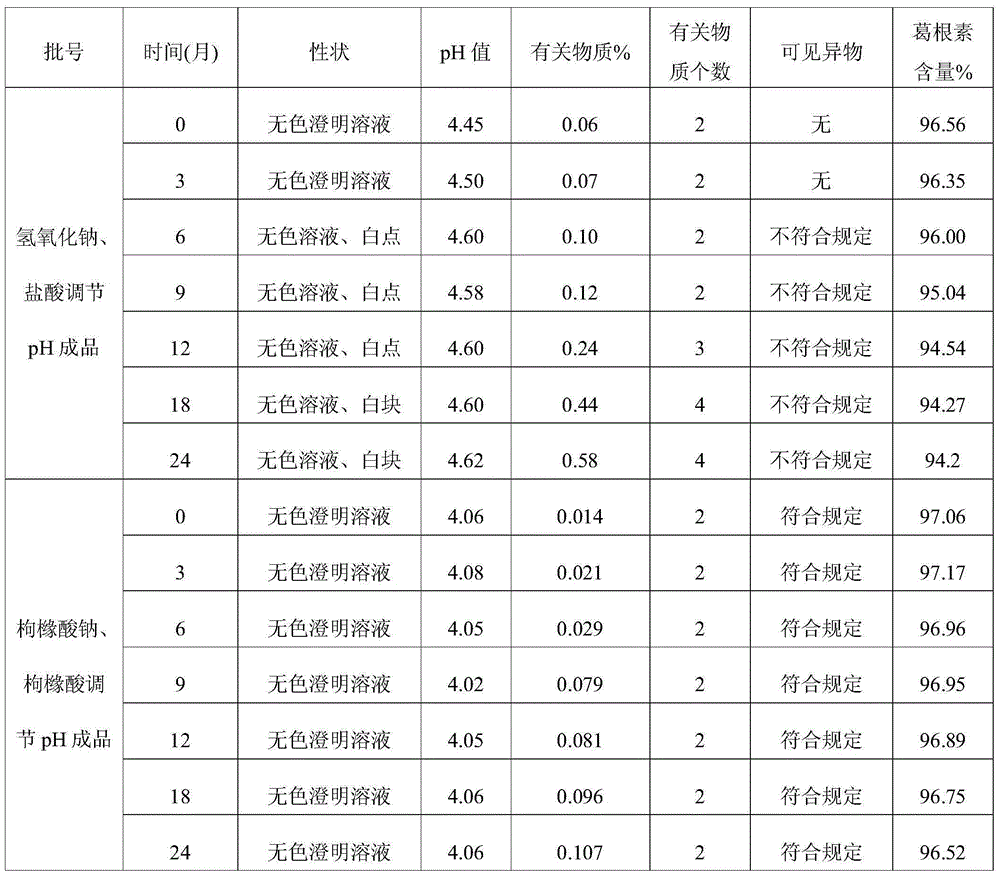
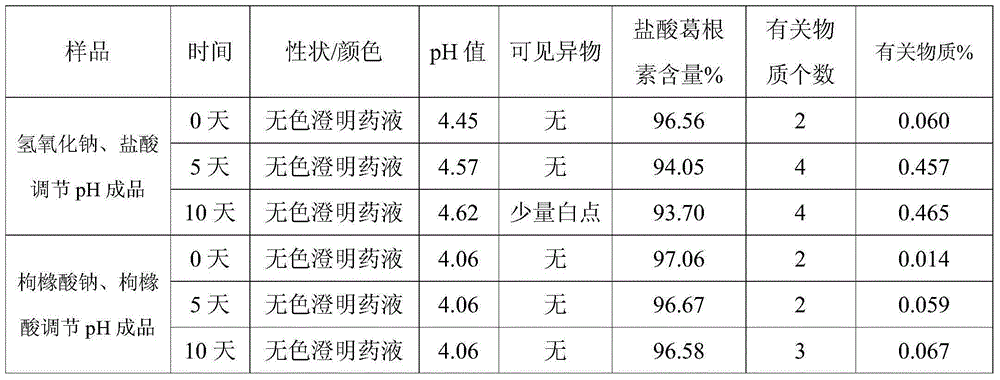
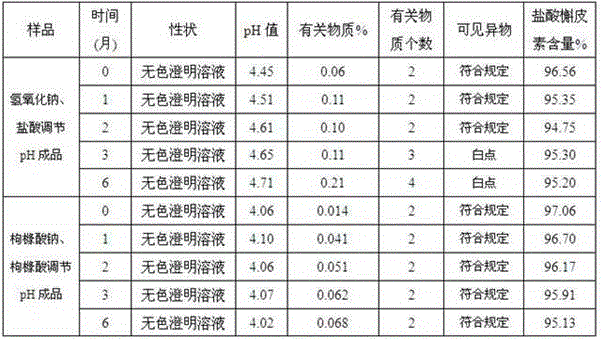
ActiveCN102008727AStable pHDecreased substancesOrganic active ingredientsPharmaceutical non-active ingredientsMedication injectionUse medication
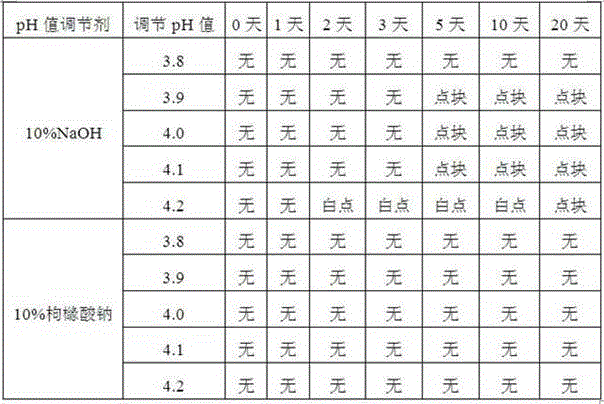
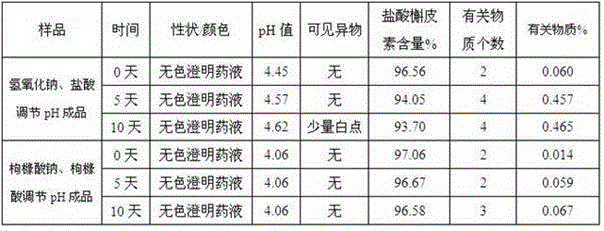
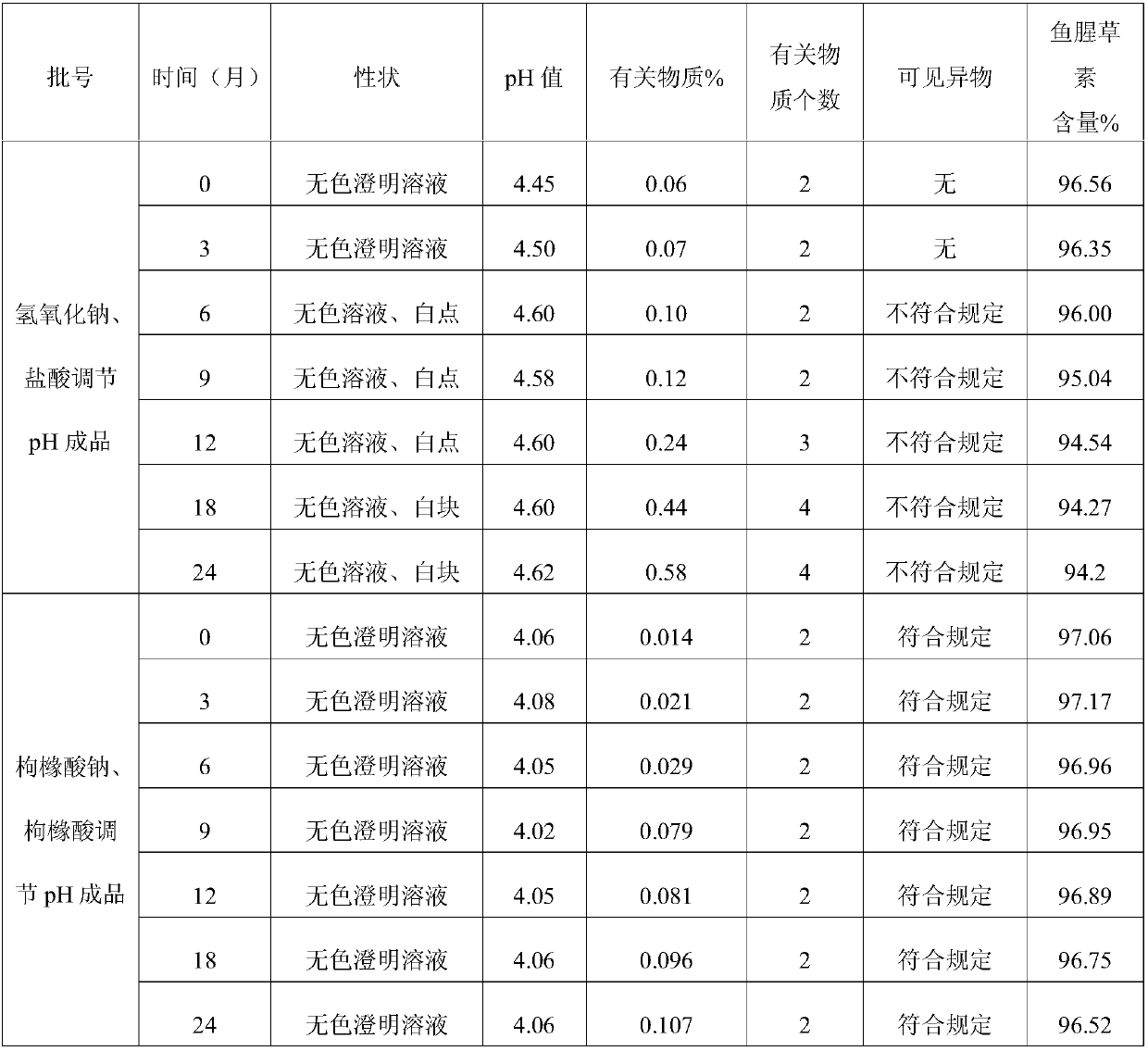
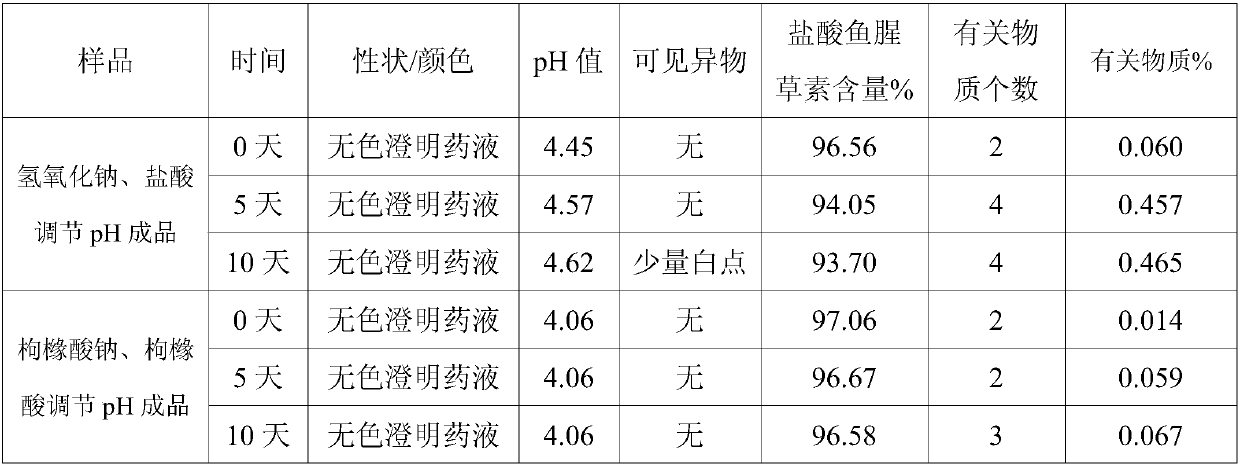
The invention discloses an injection-purpose medicine composition for improving stability of ligustrazine medicine injection formulation and a preparation method of the injection-purpose medicine composition. The injection-purpose medicine composition is prepared by the method comprising following steps: dissolving ligustrazine salt in water for injection, adjusting the pH value of the liquid medicine by adding citric acid and / or sodium citrate used as a pH regulator, wherein the dosage of the citric acid and / or sodium citrate ranges from (0.1mg to 200.0mg) / 100ml. In the invention, pH value of the injection liquid can be more stable, the content of ligustrazine degradation substance is greatly reduced compared with that of the prior art, the clarity of the ligustrazine injection liquid is improved under the condition of not using other cosolvents to increase the clinical application risk, particularly, the problems of small white spots, white blocks and solution turbidity are solved under the condition that the ligustrazine injection liquid is stored for a long time by adopting the prior art, and the medicine composition ensures that the inspection of visible foreign substances of the product is in accordance to the formulation of medicine quality standard, and is convenient for clinical medication and popularization.
Owner:SICHUAN SUNNYHOPE PHARM CO LTD
Transfer type inkjet recording method and transfer type inkjet recording device
InactiveUS20120113203A1Reduce workloadDecreased substancesPrintingIntermediate imageSurface roughness
The transfer type inkjet recording method has the intermediate image formation process and the transfer process defined in the specification. The transfer type inkjet recording method includes, in the intermediate image formation process, selecting an intermediate transfer body having a center line surface roughness closest to the center line surface roughness of a recording medium from a plurality of intermediate transfer bodies different from each other in the center line surface roughness, and then an intermediate image is formed on the surface of the selected intermediate transfer body. Moreover, a transfer type inkjet recording which is used for the method is provided.
Owner:CANON KK
LED illumination module
InactiveUS20150049486A1Decreased substancesLoss of optical efficiencyPoint-like light sourceElectric circuit arrangementsFluorescenceEngineering
Provided is a light emitting diode (LED) illumination module. The LED illumination module includes a fluorescent substance plate mounted to be capable of being attached to and detached from an opening formed in a top surface of a heat sink. Also, the LED illumination module includes a lens that covers the opening of the heat sink and is mounted to be capable of being attached to and detached from the heat sink.
Owner:SEOUL SEMICONDUCTOR
Chemically modified hyaluronic acid or salts thereof, and a process for producing thereof
InactiveUS6673919B2Reduce weightHigh viscositySugar derivativesSugar derivatives preparationChlorideChloroform
This invention relates to a chemically modified hyaluronic acid and salts thereof, which are obtained by O-acylating, alkoxylating or crosslinking a complex consisting of hyaluronic acid or a salt thereof and a cationic compound in a nonaqueous solvent, and a process for the production thereof. The nonaqueous solvent used in the invention is preferably one or more solvents selected from the group consisting of chloroform, toluene, methylene chloride and heptane.
Owner:JNC CORP +1
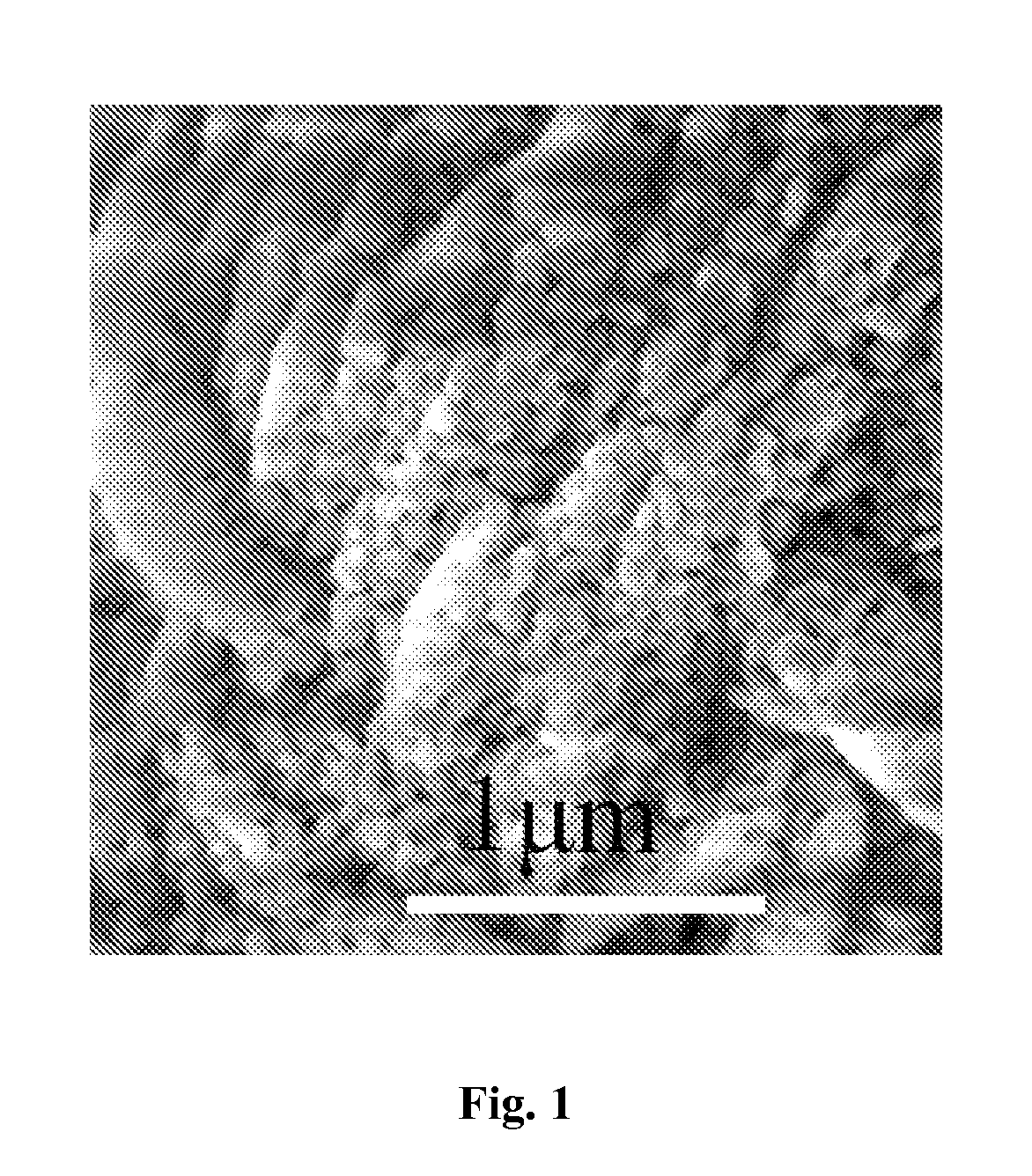
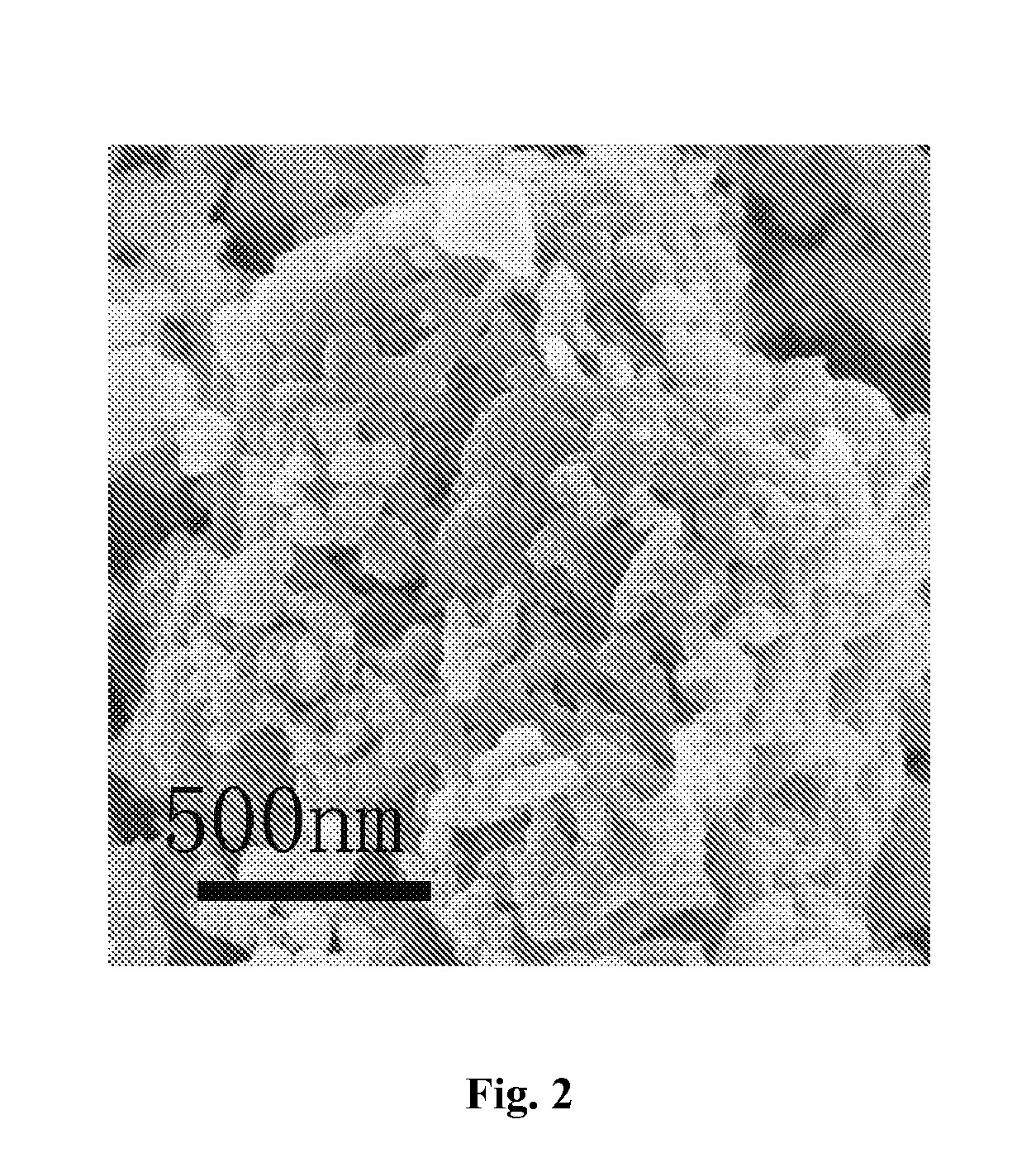
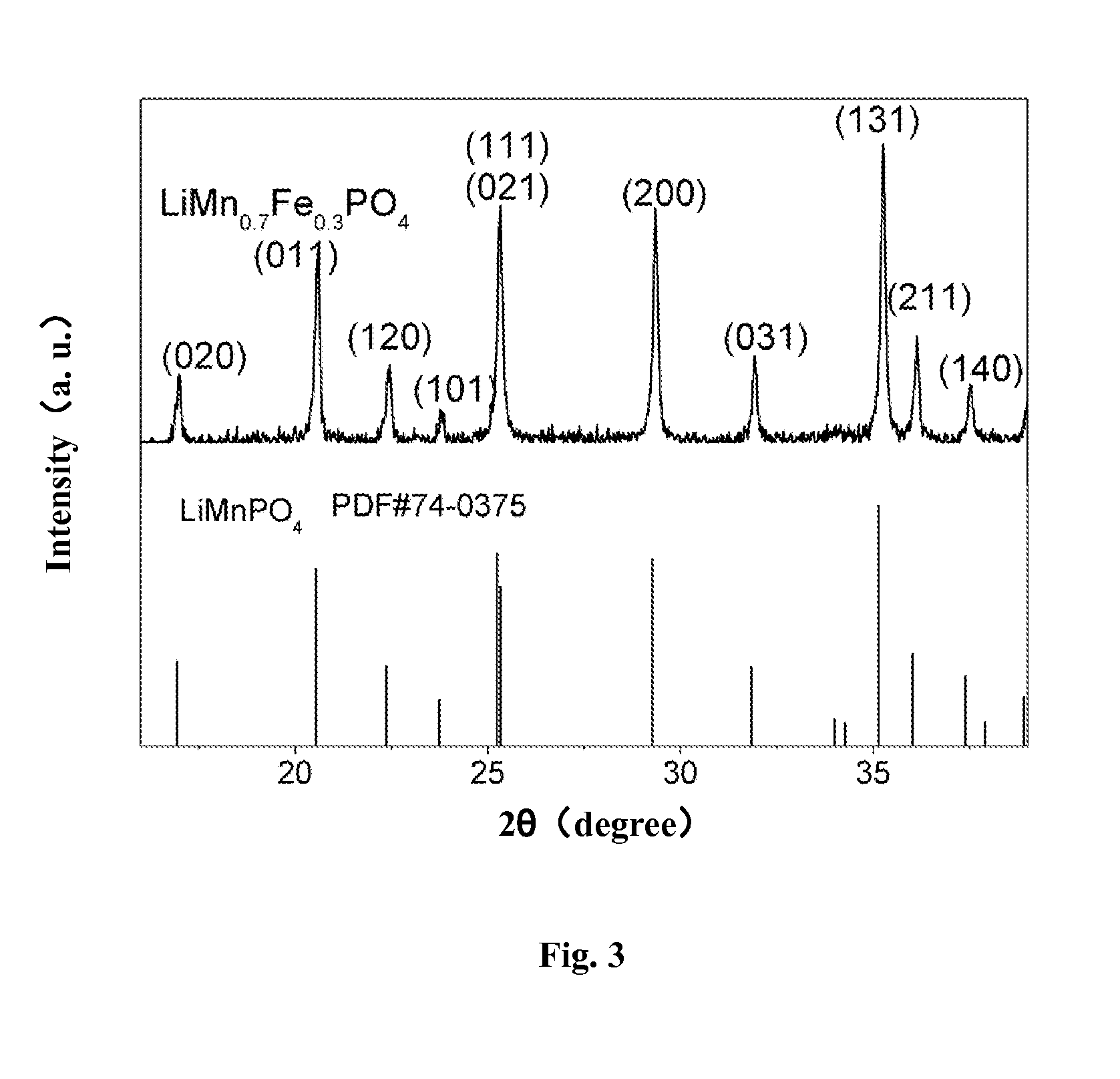
Porous Lithium Mangaense Phosphate-Carbon Composite Material, Preparation Method and Application Thereof
InactiveUS20160013474A1Improve Capacitive PerformanceExcellent rate performanceMaterial nanotechnologyNon-metal conductorsCarbon compositesPhosphate
A porous lithium manganese phosphate-carbon composite material, and a preparation and application thereof. Multiple nano-pores are distributed in the composite material, and the composite material includes a lithium manganese phosphate material and carbon. The method for preparing the porous lithium manganese phosphate-carbon composite material includes the steps of: mixing a porous pyrophosphate material with a doped metal source, a lithium source, phosphate and a carbon source and then drying them to obtain a reaction precursor, and calcining the reaction precursor at a constant temperature under a protective atmosphere to obtain the composite material. The lithium manganese phosphate material contains compounds in a general formula of LiMnxM1−xPO4, and the porous pyrophosphate material contains compounds in a general formula of (MnxM1−x)2P2O7 and 0 wt % to 50 wt % of carbon, where M comprises a transition metal, and 0.6≦x≦1.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
Fluorine-containing compound, resist composition for immersion exposure, and method of forming resist pattern
InactiveUS20090142699A1Affect propertyImprove hydrophobicityOrganic chemistryPhotosensitive materialsResistOrganic group
A fluorine-containing compound represented by a general formula (c-1) shown below:RX-AN-(OR2)a [Chemical Formula 1](c-1)[wherein, RX represents an organic group, AN represents a naphthalene ring that may have a substituent, R2 represents a base dissociable group, and a represents 1 or 2, provided that at least one among AN and said a R2 groups contains a fluorine atom].
Owner:TOKYO OHKA KOGYO CO LTD
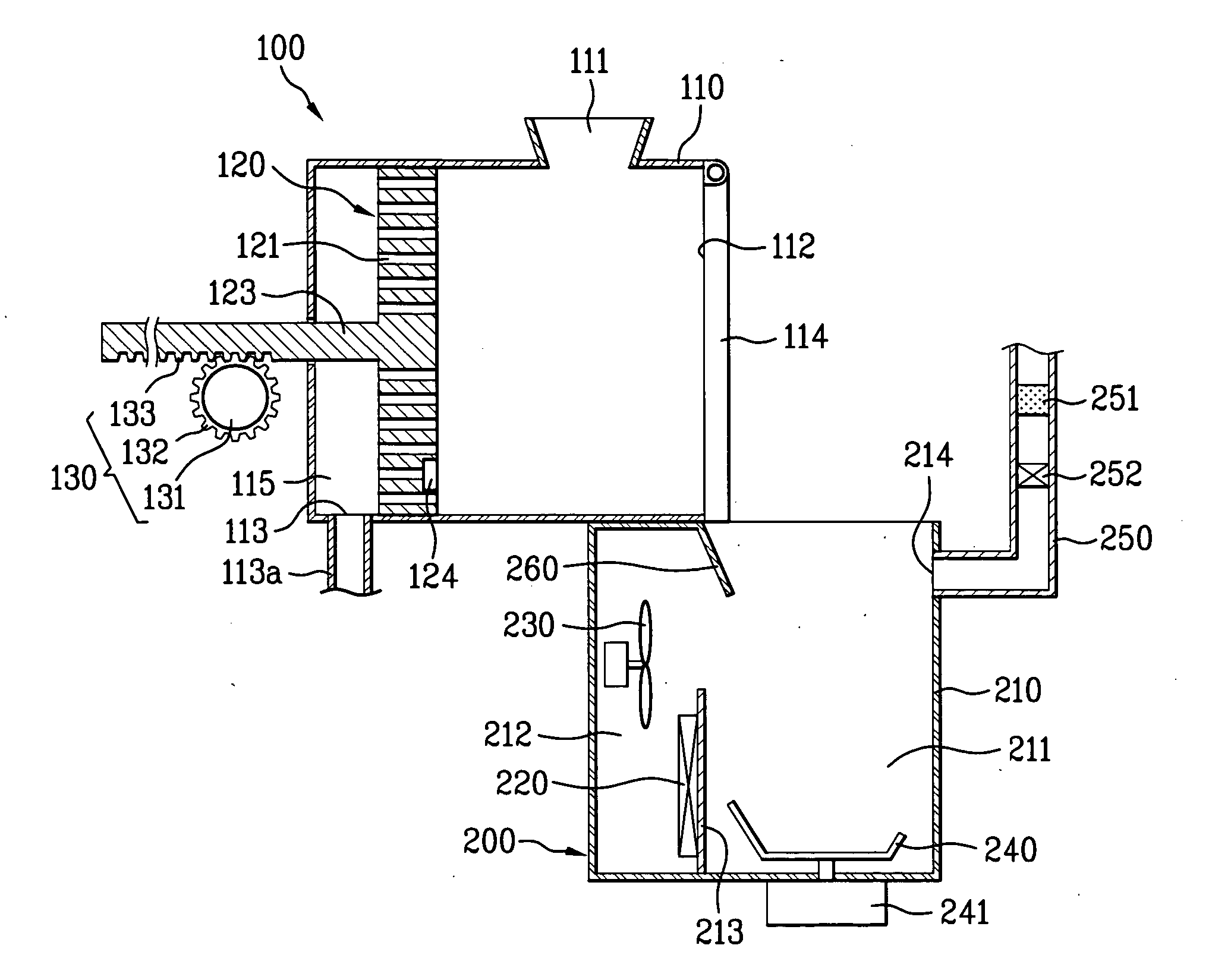
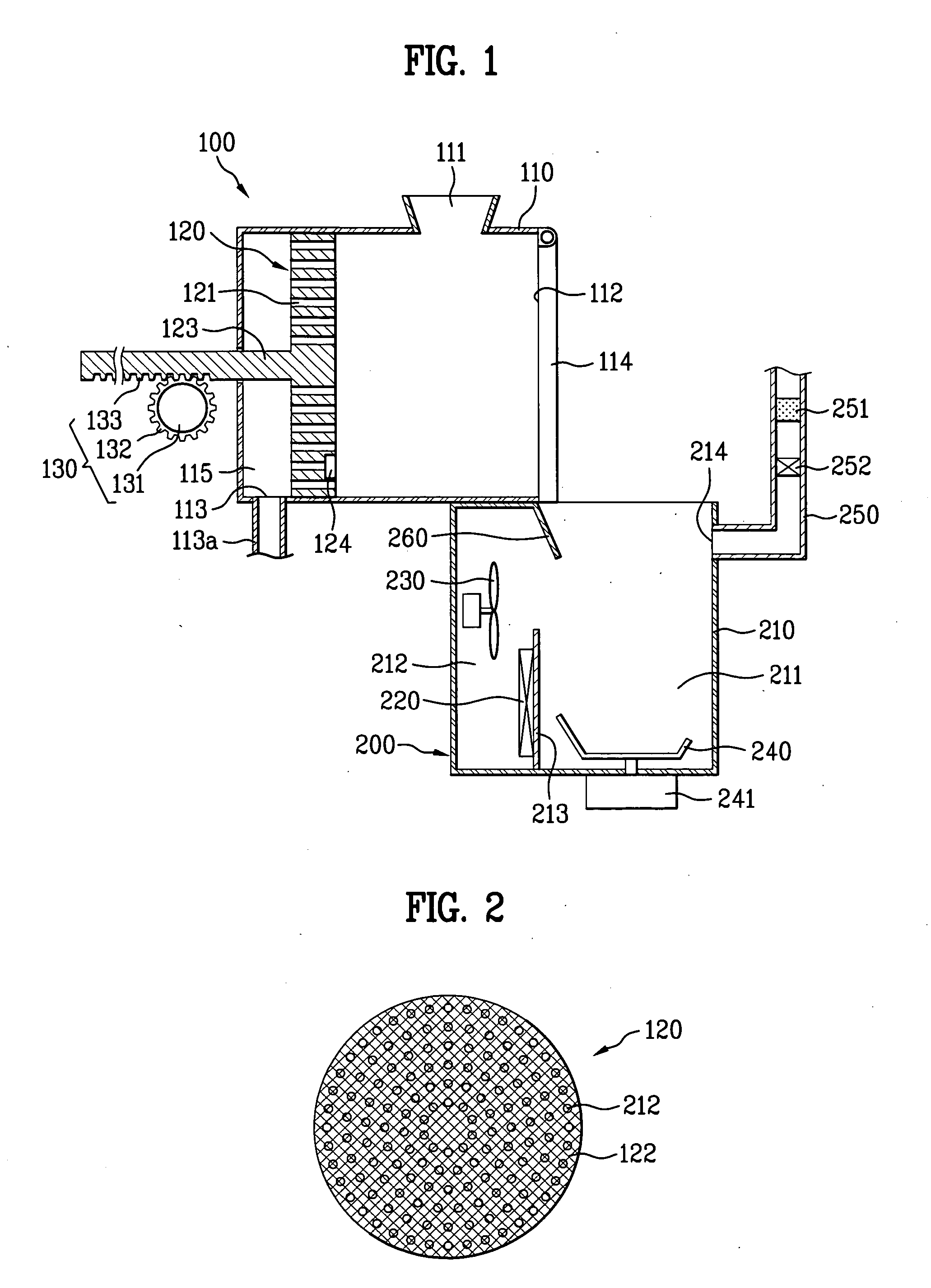
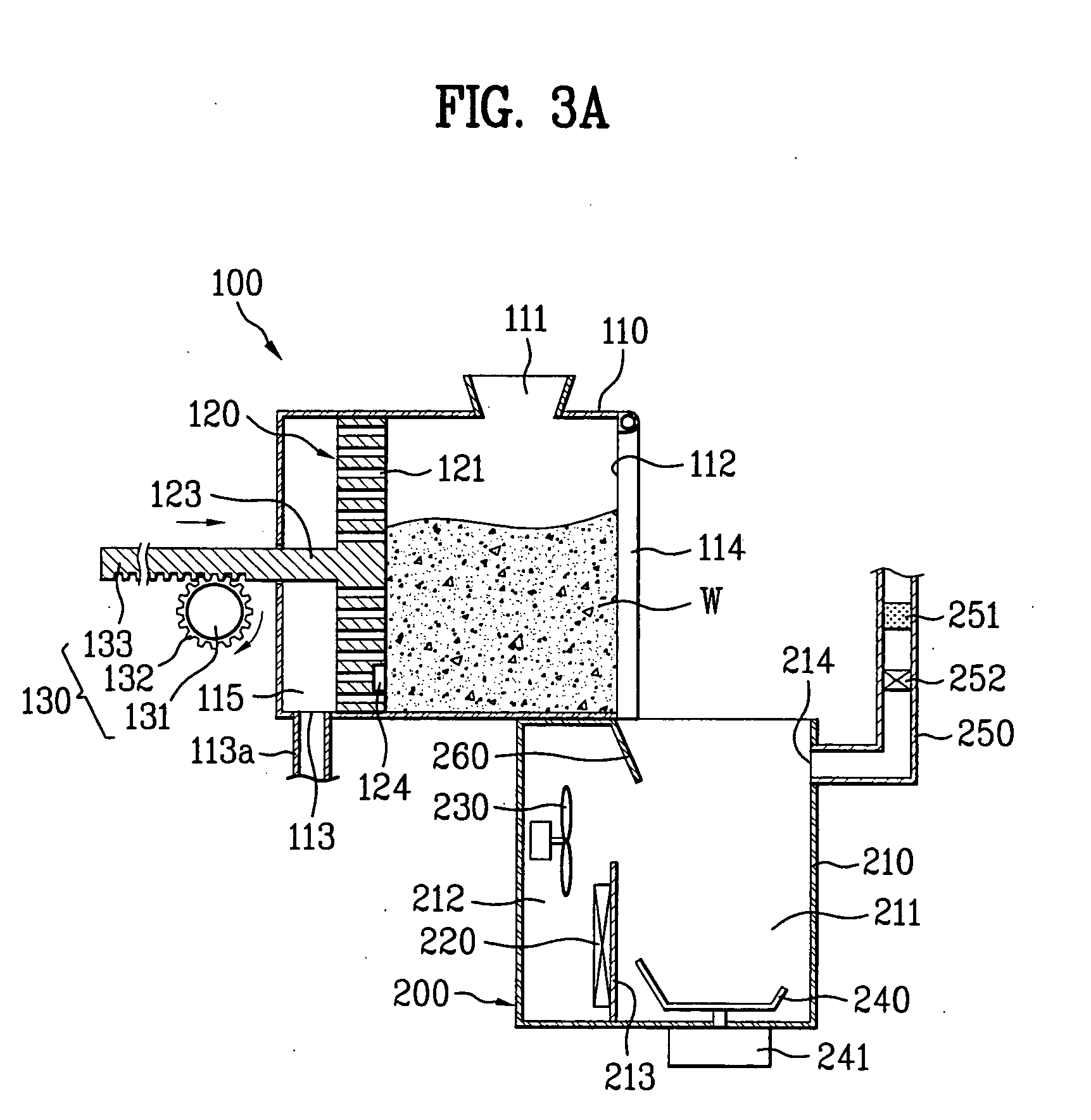
Apparatus for processing organic substances
The present invention provides an apparatus for processing organic substances, by which performance of processing the organic substances is enhanced. The present invention includes a grinding device for grinding inputted organic substances and a drying device for drying the organic substances grinded by the grinding device.
Owner:LG ELECTRONICS INC
Boiler
InactiveUS20090025655A1Less generation amountSubstance reductionFluid heatersFuel supply regulationCombustionConcentration ratio
First, an emission amount of nitrogen oxide can be decreased to zero as much as possible and, an emission amount of carbon monoxide is decreased to a permissible range. Second, energy saving by combustion at a low air ratio close to 1.0 is realized. Third, air ratio control is performed stably in a combustion region at a low air ratio. Provided is a boiler, including: a premixed burner; a water pipe group for, by heat exchanging with a gas produced by the premixed burner, suppressing a temperature of the gas to thereby suppress a concentration of nitrogen oxide to a predetermined value or less; an oxidation catalyst for oxidizing carbon monoxide contained in the gas after the passage through the water pipe group by oxygen and for reducing nitrogen oxide contained therein by carbon monoxide; and an air-ratio adjusting device for adjusting an air ratio of the premixed burner, in which: the premixed burner and the water pipe group have such characteristics that when the air ratio is a set air ratio, a concentration ratio of oxygen, nitrogen oxides, and carbon monoxide in the gas on a primary side of the oxidation catalyst becomes a predetermined concentration ratio; the oxidation catalyst has such characteristics that when the concentration ratio is a predetermined concentration ratio, a concentration of nitrogen oxides on a secondary side of the oxidation catalyst is substantially zero while a concentration of carbon monoxide is decreased to substantially zero or a predetermined value or less; and the predetermined concentration ratio is kept to be constant by being controlled to the set air ratio by the air-ratio adjusting device.
Owner:MIURA COMPANY LIMITED
Antibacterial composition and methods thereof
InactiveUS20060100124A1Easy to aggregateEasy to removeInorganic/elemental detergent compounding agentsBiocideCITRATE ESTERPhosphate
Provided is an antibacterial aqueous solution comprising a phosphate, a citrate, and a silicate; a method of controlling bacterial contamination and / or growth in food substance; a method of prohibiting the formation of, and / or facilitating the removing of, silicate aggregation on metal article; and a method of reducing phosphate usage in industrial antibacterial process.
Owner:AS DE DANSKE SUKKERFABRIKKER
Antibacterial composition and methods thereof comprising a ternary builder mixture
InactiveUS7354888B2Easy to aggregateEasy to removeInorganic/elemental detergent compounding agentsBiocideCITRATE ESTERPhosphate
Provided is an antibacterial aqueous solution comprising a phosphate, a citrate, and a silicate; a method of controlling bacterial contamination and / or growth in food substance; a method of prohibiting the formation of, and / or facilitating the removing of, silicate aggregation on metal article; and a method of reducing phosphate usage in industrial antibacterial process.
Owner:AS DE DANSKE SUKKERFABRIKKER
Liquid crystal display and method thereof
InactiveUS20100007816A1Increase in costReduce light emissionVessels or leading-in conductors manufactureOptical light guidesLiquid-crystal displayEngineering
A liquid crystal display includes a light guide plate guiding light, light sources disposed adjacent to at least one side of the light guide plate, and a lower receptacle. The lower receptacle includes a bottom plate on which the light guide plate and the light sources are disposed, a lower receptacle side wall extending in a direction substantially perpendicular to the bottom plate and from an edge of the bottom plate, and an upper plate extended from the lower receptacle side wall and substantially parallel to the bottom plate. The upper plate does not overlap the light guide plate in a plan view of the liquid crystal display.
Owner:SAMSUNG DISPLAY CO LTD
Positive resist composition and method of forming resist pattern
ActiveUS20110111343A1Use optimizationAffect propertyPhotosensitive materialsSemiconductor/solid-state device manufacturingSolubilityResist
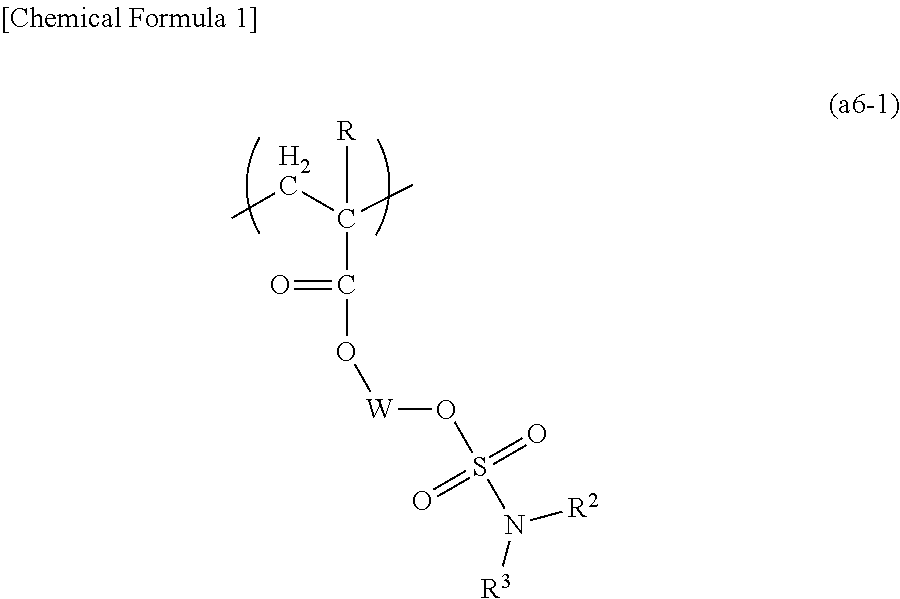
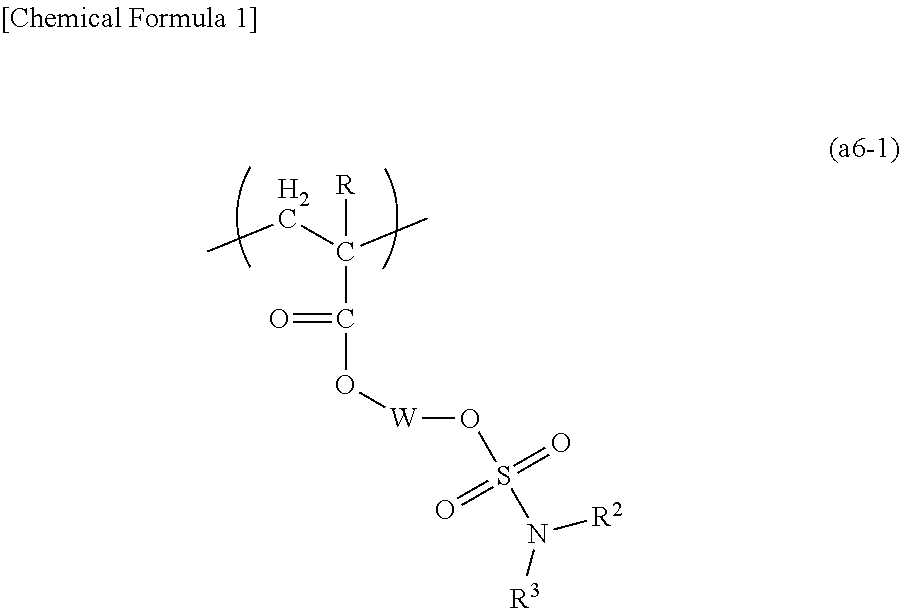
A positive resist composition including a resin component (A) which exhibits increased solubility in an alkali developing solution under the action of acid and an acid-generator component (B), the resin component (A) including a polymeric compound (A1) having a structural unit (a1) containing an acid dissociable, dissolution inhibiting group, a structural unit (a5) containing a base dissociable group an a structural unit (a6) represented by general formula (a6-1) (R represents a hydrogen atom, an alkyl group of 1 to 5 carbon atoms or a halogenated alkyl group of 1 to 5 carbon atoms; each of R2 and R3 independently represents a hydrogen atom or an alkyl group that may contain an oxygen atom at an arbitrary position, or R2 and R3 are bonded together to form an alkylene group; and W represents a cyclic alkylene group that may include an oxygen atom at an arbitrary position).
Owner:TOKYO OHKA KOGYO CO LTD
Composition for oral administration
InactiveUS20130309394A1Reduce bitternessNot impair out appearanceFood preparationPlant ingredientsGum arabicOral medication
A composition for oral administration includes: a water-insolubilized material including a bitter or astringent substance, such as an iso-α acid, and a polyvalent metal salt such as ferric chloride; and a dispersant such as a water-soluble macromolecule such as gelatin or gum arabic.
Owner:FUJIFILM CORP
Container and one-way valve assembly for strong and dispensing substances, and related method
InactiveUS20070095857A1Limit degradationLimit wasteClosuresLiquid flow controllersEngineeringVALVE PORT
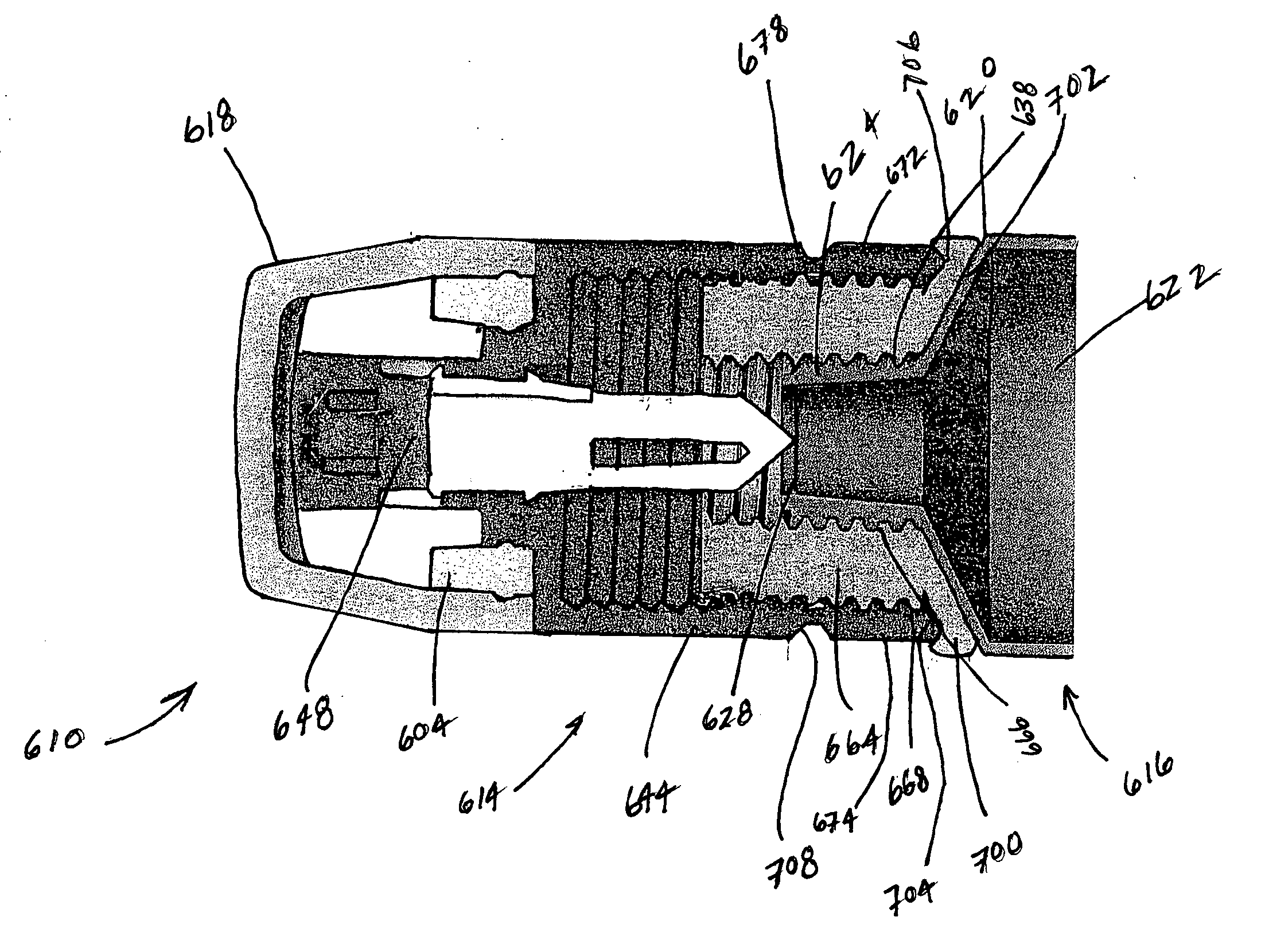
A device for storing and dispensing a substance includes a container having a body defining therein a storage chamber for receiving and storing the substance. The container includes a first passageway that is in fluid communication with the storage chamber of the body and defines a flow path therebetween. The container also includes a pierceable wall located on an opposite side of the first passageway relative to the storage chamber, and a first threaded connecting portion located at one end of the body for connecting another component thereto. The device also includes a one-way valve assembly that includes a valve body including a body base defining a second passageway and a piercing portion mounted within the valve body and engageable with the pierceable wall of the container. At least one of the piercing portion and the pierceable wall is movable relative to the other between a first position wherein the pierceable portion is not piercing the pierceable wall, and a second position wherein the pierceable portion is piercing the pierceable wall and the first passageway of the container is in fluid communication with the second passageway of the valve body for allowing the flow of substance from the storage chamber therethrough. The valve assembly includes a second threaded connecting portion that is threadedly connectable to the first threaded connecting portion of the container for fixedly securing the valve assembly to the container when the valve assembly and container are located in the second position. A manually-engageable and removable member is disposed intermediate the valve body and the container that prevents movement of at least one of the piercing portion and pierceable wall to the second position until the removable member is removed. The valve assembly includes a valve seat and at least one flow aperture extending through the valve body adjacent to the valve seat and in fluid communication with the second passageway for receiving the substance from the storage chamber there through.
Owner:MAEJ LLC ODONNELL & TESSITORE
Control device for analysis device
InactiveUS20170115261A1Shorten analysis timeEfficient analysisComponent separationProgramming languageCorrelation analysis
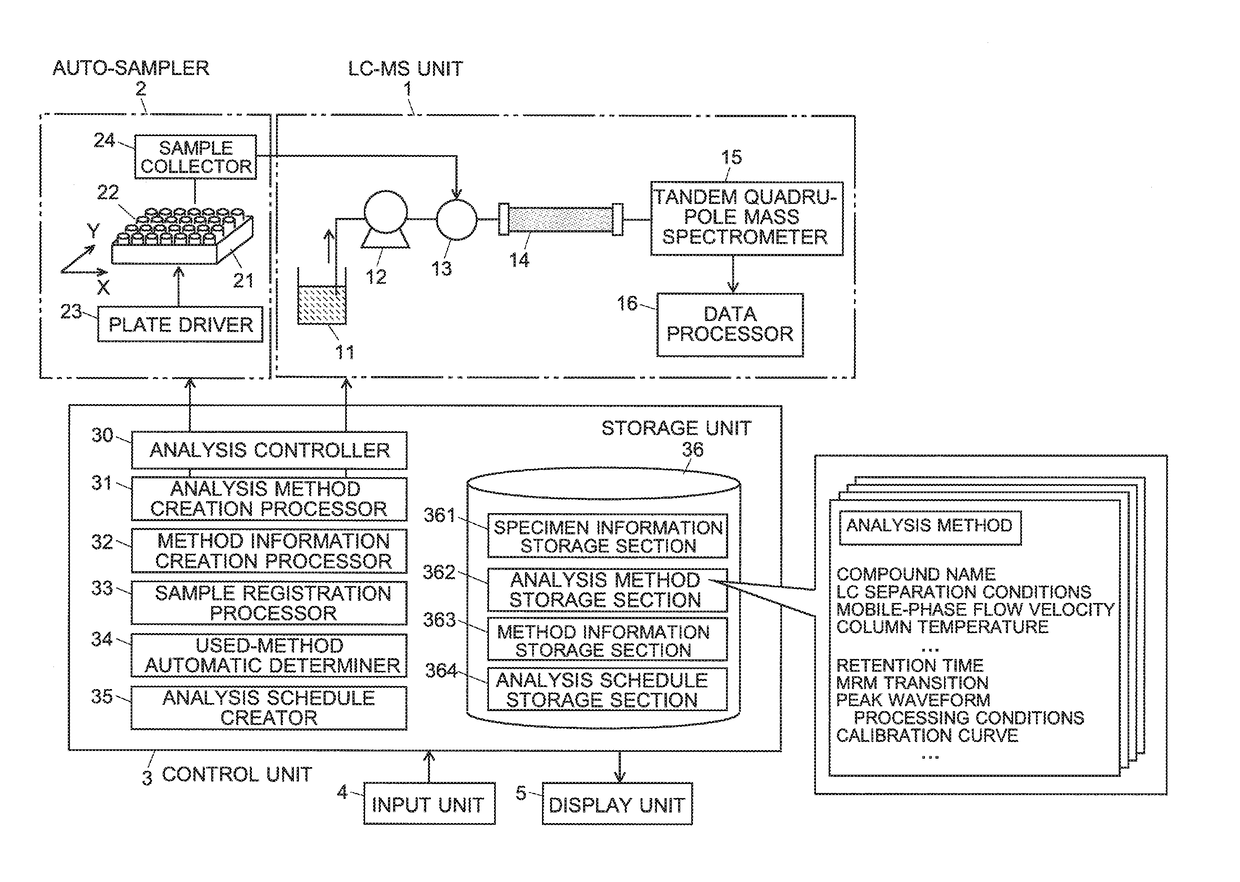
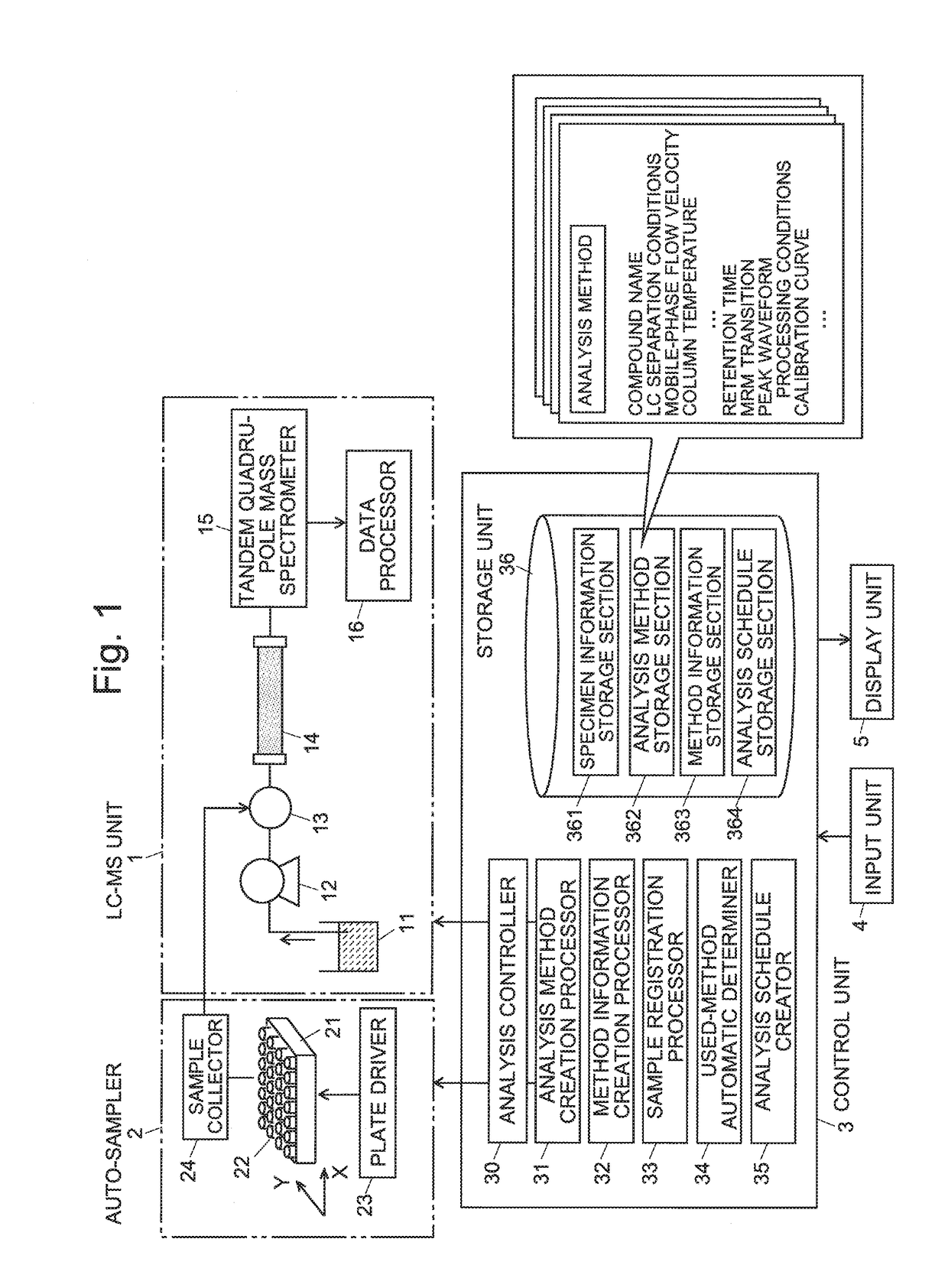
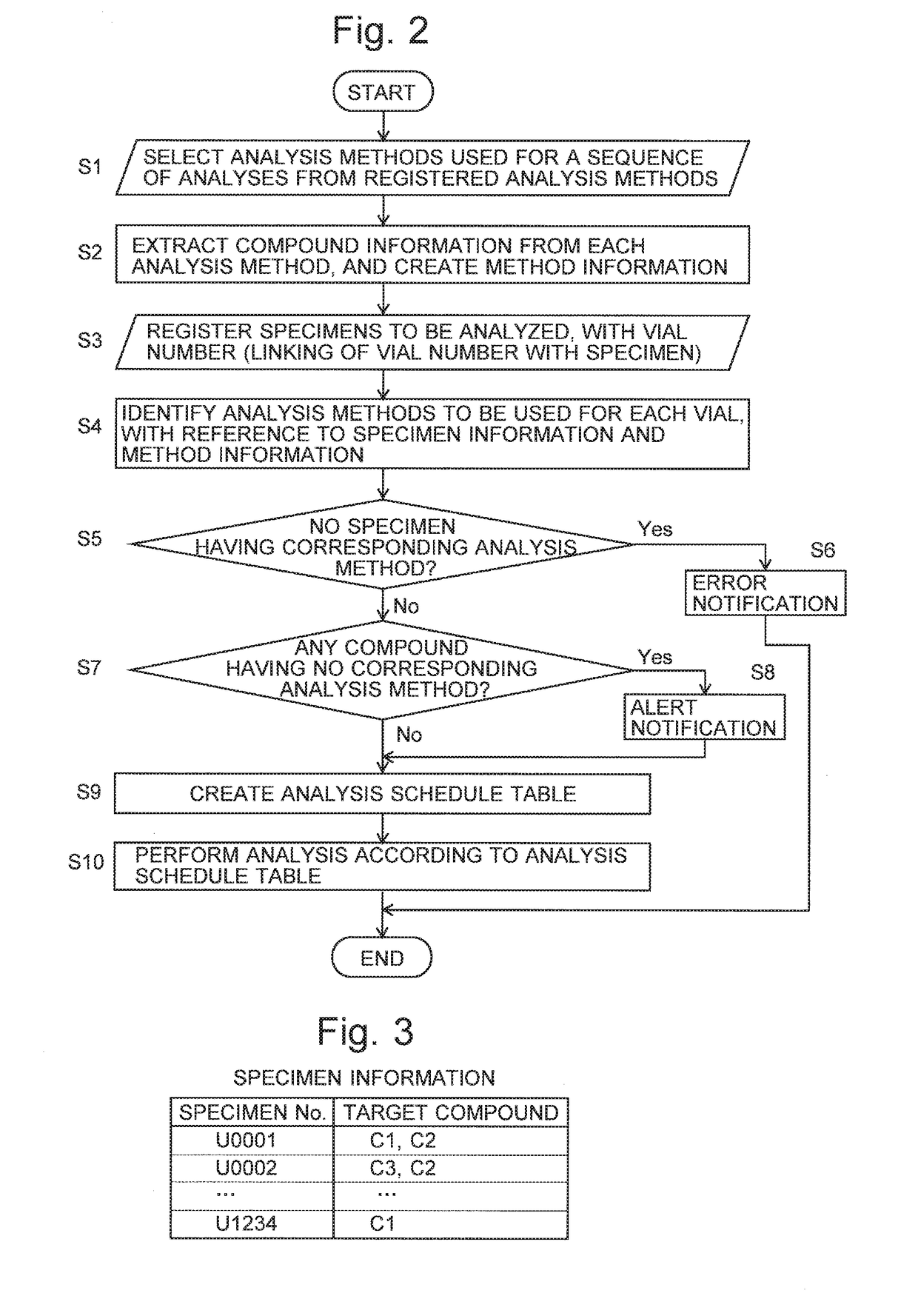
A specimen information storage section (361) holds specimen information showing the relationship between a number of specimens to be analyzed and compounds whose quantities need to be determined. An analysis method storage section (362) holds the files of analysis methods created by an administrator. When an operator selects and indicates analysis methods to be used in an analysis, a method information creation processor (32) extracts compound information from the selected analysis methods and creates method information showing the correspondence between the analysis methods and the compounds to be analyzed. When the operator registers the specimen numbers of the analysis targets, a used-method automatic determiner (34) refers to the specimen information and method information to identify a suitable analysis method for each compound in the registered specimens. An analysis schedule creator (35) creates a schedule table in which the specimens and analysis methods are described in order of the analysis. This schedule includes no useless analysis performed using an analysis method which is unsuitable for the analysis concerned. Consequently, the efficiency of the analysis is improved.
Owner:SHIMADZU CORP
Preparation method of injecting drug improving stability of puerarin drug injection preparation
InactiveCN105125485ADecreased substancesStable pHOrganic active ingredientsNervous disorderDrug injectionMedicine
The invention discloses a preparation method of injecting drug improving the stability of a puerarin drug injection preparation. An injecting drug composition is mainly prepared by dissolving puerarin salt in injecting water, adding citric acid and / or sodium citrate as a pH adjusting agent to adjust the pH value of the medicine liquid, and the dosage of the citric acid and / or sodium citrate is 0.1 to 200 mg / 100ml. By adopting the preparation method, the pH value of the injecting liquid is more stable, puerarin degraded substances are greatly reduced compared with the prior art, under the situation of not utilizing other co-solvent increasing the clinical application risk, the clarity of the puerarin injecting liquid is improved, the problems that the puerarin injecting liquid adopting a product of the prior art has small white points, white blocks and turbidity under the situation that the storage time is relatively long can be solved, the inspection on visible foreign matters of the product is enabled to meet the stipulation of the drug quality standard, and the clinical drug application and popularization are facilitated.
Owner:CHENGDU AIBIKE BIOTECH
Process for obtaining dinitrogen monoxide (N20)
InactiveUS20130171711A1Improve energy balanceHighly climate-damagingWater treatment parameter controlTreatment by combined electrochemical biological processesMicroorganismCell Fraction
In a method for obtaining dinitrogen monoxide by microbiological or enzymatic processes from nitrogen-containing substances, the microorganisms, bacteria, archaea, eukaryotes, fungi, parasites, phages, cells, cell fractions or membrane fractions, and / or enzymes, and / or a combination thereof to be used in this context are selected, or manipulated or partly or entirely reversibly and / or irreversibly inhibited by suitable actions, or the corresponding microbiological or enzymatic processes are controlled, for example, by way of suitable process conditions, so that, in part or entirely, dinitrogen monoxide (N2O) is formed from the nitrogen-containing compounds of the nitrogen-containing substances.
Owner:ROBERT BOSCH GMBH
Apparatus for processing oranic substance
InactiveUS20060086262A1Easy to processImprove performanceDrying using combination processesSolid waste disposalOrganic matterMoisture
The present invention provides an apparatus for processing organic substances such as food and the like, by which time and costs for processing the organic substances are reduced to enhance performance of processing the organic substances. The present invention includes a dewatering means for separating moisture from inputted inorganic substances and a drying means provided to one side of the dewatering means for drying the inorganic substances discharged from the dewatering means.
Owner:LG ELECTRONICS INC
Preparation method of injection medicine for improving stability of quercetin medicine injection preparation
InactiveCN105963247ADecreased substancesStable pHOrganic active ingredientsMetabolism disorderMedicineTurbidity
The invention discloses an injection medicine composition for improving the stability of a quercetin medicine injection preparation, and a preparation method thereof. The injection medicine composition is an injection medicine composition mainly prepared by dissolving salt of quercetin into injection water and adding citric acid and / or sodium citrate as pH regulators to regulate the pH value of medicine liquid. The consumption of the citric acid and / or sodium citrate is 0.1mg to 200.0mg / 100ml. The injection medicine composition has the advantages that the pH value of the injection liquid is more stable; the quercetin degradation substances are greatly reduced through being compared with that in the prior art; under the condition of not using other solutizers increasing the clinic application risks, the clarity of the quercetin injection liquid is improved; the problems of small white points, white blocks and solution turbidity of a product of the quercetin injection liquid in the prior art under the condition of long storage time are particularly solved; the visible foreign matter inspection of the product is enabled to conform to the specification of the medicine quality standard; the clinic medication and popularization are convenient.
Owner:CHENGDU YICHUANGSI BIOLOGICAL SCI & TECH
Method for producing methane gas
InactiveUS7736510B2Efficient use ofDecreased substancesWater treatment parameter controlBiological substance pretreatmentsMethane fermentationProduct gas
A practical technique that enables effective utilization of organic wastes is provided. In order to achieve the above-mentioned object, a method for producing methane gas from organic wastes according to the present invention includes: treating organic wastes with at least one of supercritical water and sub-critical water to convert the organic wastes into low molecular weight substances; and subjecting the low molecular weight substances to methane fermentation. According to the method, initially, the organic wastes are treated with at least one of the supercritical water and the sub-critical water so as to be converted into low molecular weight substances that are easily subjected to methane fermentation. Then, the treated substances are subjected to methane fermentation. Consequently, methane gas can be produced from the organic wastes at a high speed with high digestion efficiency.
Owner:PUBLIC UNIVERSITY CORPORATION OSAKA CITY UNIVERSITY
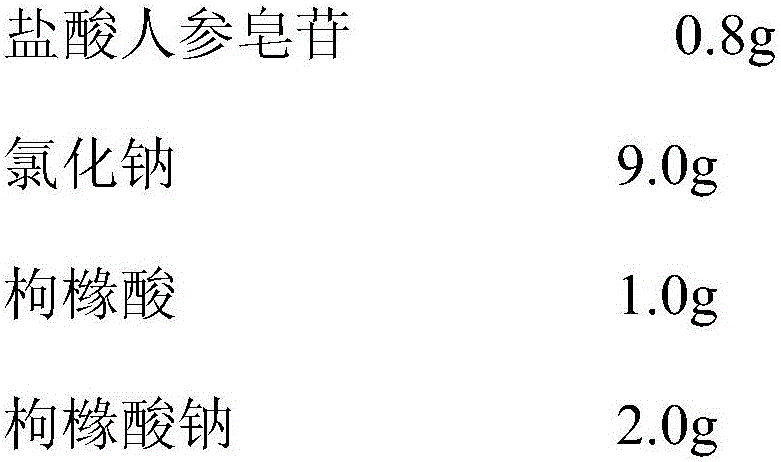
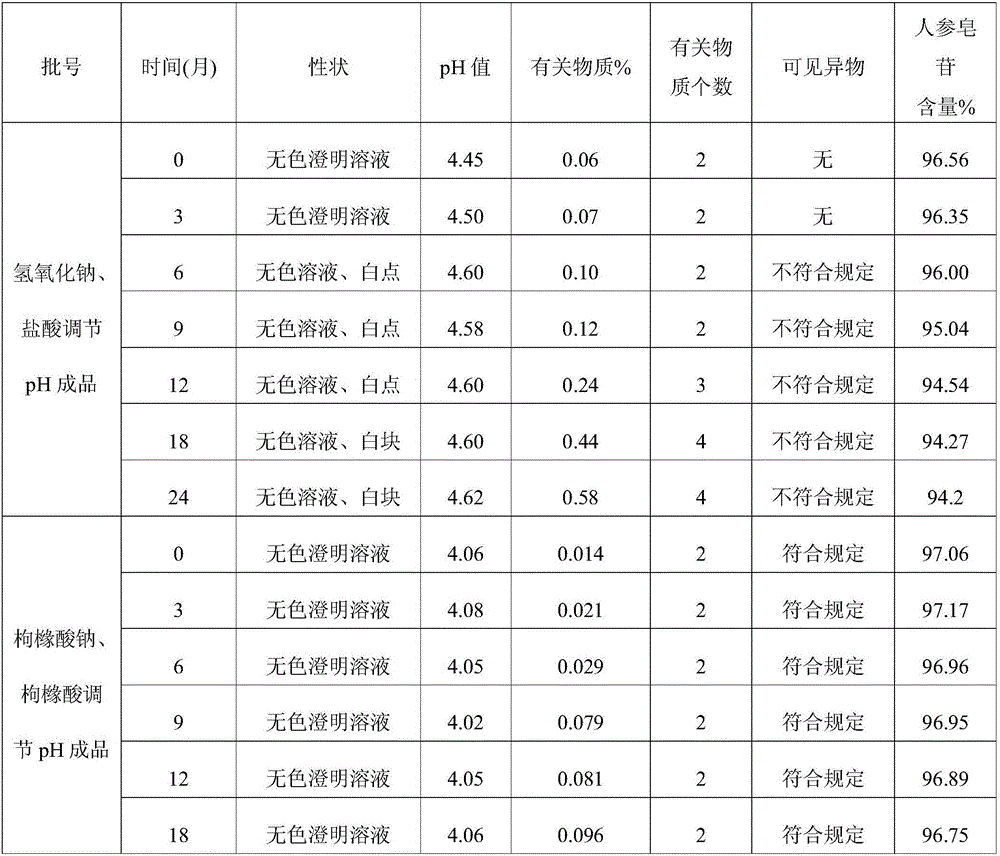
Injecting medicine composition for improving stability of ginsenoside medicine injection
InactiveCN105999278ADecreased substancesStable pHPharmaceutical delivery mechanismPharmaceutical non-active ingredientsMedicineCLARITY
The invention discloses an injecting medicine composition for improving the stability of a ginsenoside medicine injection and a preparation method thereof. The preparation method mainly comprises the following steps: dissolving salt of ginsenoside in injecting water; adding citric acid and / or sodium citrate to serve as a pH regulator, and regulating the pH value of the medicinal liquid, wherein the dosage of the citric acid and / or sodium citrate is 0.1-200.0mg / 100ml. By adopting the preparation method, the pH value of the injection can be stable, the ginsenoside degraded substances are greatly reduced in comparison with that in the prior art, the clarity of ginsenoside injection is improved under a condition that other cosolvent capable of increasing clinical application risks is prevented from being used, and the problems that the ginsenoside injection has small spots, white blocks and turbid solution by adopting the prior art under a condition of long storage period can be especially solved, obviously foreign matter inspection of the injection accords with the specification of medicine quality standard, and clinical medication and popularization can be benefited.
Owner:成都市斯贝佳科技有限公司
Liquid crystal display and method thereof
InactiveUS8582046B2Reduce lossesLow costVessels or leading-in conductors manufactureOptical light guidesLiquid-crystal displayLight guide
Owner:SAMSUNG DISPLAY CO LTD
Container and one-way valve assembly for storing and dispensing substances, and related method
InactiveUS7775398B2Decreased substancesPreventing and limiting wasteClosuresClosure using stoppersHolding roomEngineering
A device for storing and dispensing a substance includes a storage chamber for the substance. The container includes a passageway in fluid communication with the storage chamber. The container includes a pierceable wall located on an opposite side of the passageway relative to the storage chamber. The device includes a one-way valve assembly including a piercing portion. At least one of the piercing portion and the pierceable wall is movable relative to the other between a first position wherein the pierceable portion is not piercing the pierceable wall, and a second position wherein the pierceable portion is piercing the pierceable wall allowing the flow of substance from the storage chamber. A removable member is disposed intermediate the valve and the container that prevents movement of at least one of the piercing portion and pierceable wall to the second position until the removable member is removed.
Owner:MAEJ LLC ODONNELL & TESSITORE
Fluorine-containing ethylene-vinyl alcohol copolymer resin composition as well as mixture and blend thereof
ActiveUS20210198470A1Reduce adhesionGood lookingSynthetic resin layered productsDomestic containersPolymer scienceFluoride
The instant disclosure relates to a fluorine-containing ethylene-vinyl alcohol copolymer (EVOH) resin composition as well as mixture and blend thereof. The fluorine-containing EVOH resin composition comprises EVOH and fluorine-containing particles, wherein the fluorine-containing EVOH resin composition has a total fluoride ion content ranging from 45 to 41000 ppm. The invention can reduce the adhesion of EVOH to the inside of the extruder, and effectively reduce the appearance of gel or gelled substance in subsequent finished products.
Owner:CHANG CHUN PETROCHEMICAL CO LTD
Method for producing methane gas
InactiveUS20060183951A1High-speed methane productionImprove digestion efficiencyWater treatment parameter controlBiological substance pretreatmentsMethane fermentationMethane gas
A practical technique that enables effective utilization of organic wastes is provided. In order to achieve the above-mentioned object, a method for producing methane gas from organic wastes according to the present invention includes: treating organic wastes with at least one of supercritical water and sub-critical water to convert the organic wastes into low molecular weight substances; and subjecting the low molecular weight substances to methane fermentation. According to the method, initially, the organic wastes are treated with at least one of the supercritical water and the sub-critical water so as to be converted into low molecular weight substances that are easily subjected to methane fermentation. Then, the treated substances are subjected to methane fermentation. Consequently, methane gas can be produced from the organic wastes at a high speed with high digestion efficiency.
Owner:OSAKA PREFECTURE UNIV PUBLIC CORP
Pharmaceutical composition for injection for improving stability of illicium henryi medication injection preparation
The invention discloses a pharmaceutical composition for injection for improving stability of an illicium henryi medication injection preparation. The pharmaceutical composition for injection is mainly prepared by dissolving extracting solution of illicium henryi in water for injection, adding caffeic acid and / or sodium caffeate to serve as a pH regulator for regulating the pH value of the liquid medicine, wherein the amount of the caffeic acid and / or sodium caffeate is 1-5g / 100ml. According to the invention, the pH value of the injection can be stable, illicium henryi degradable substances are greatly reduced compared with that in the prior art, the clarity of the illicium henryi injection is improved under the condition that other cosolvents capable of increasing clinical application risk are not used, particularly the problems that the illicium henryi injection has small white spots and white blocks and turbid in solution when stored for a long time by adopting the products in the prior art are solved, inspection of visible foreign matters in the product can accord with regulations of drug standards, and the pharmaceutical composition is convenient for clinical medication and popularization.
Owner:成都佳迪璐莎生物科技有限公司
Resist composition for immersion exposure, method of forming resist pattern, and fluorine-containing resin
ActiveUS20120116038A1Affect propertyImprove hydrophobicityPhotomechanical exposure apparatusResistSolubility
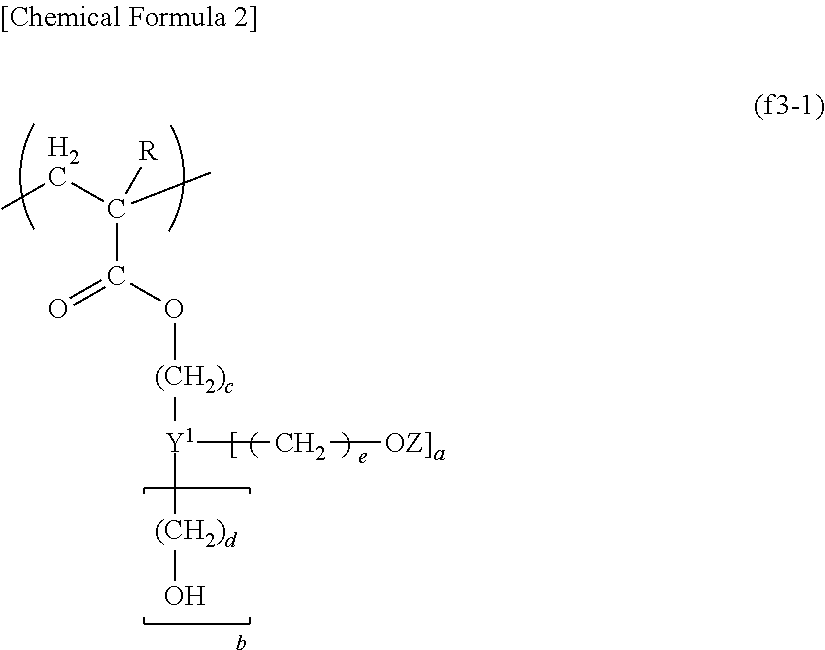
A resist composition for immersion exposure including: a base component (A) which exhibits changed solubility in an alkali developing solution under the action of acid; an acid-generator component (B) which generates acid upon exposure; and a fluorine-containing resin component (F); dissolved in an organic solvent (S), the fluorine-containing resin component (F) including a structural unit (f1) containing a fluorine atom, a structural unit (f2) containing a hydrophilic group-containing aliphatic hydrocarbon group, and a structural unit (f3) derived from an acrylate ester containing a tertiary alkyl group-containing group or an alkoxyalkyl group.
Owner:TOKYO OHKA KOGYO CO LTD
Injection medicinal composition of houttuyfonate
InactiveCN107737101AThe pH of the liquid is stableHouttuyfotin degradation substances decreasedAntibacterial agentsPharmaceutical delivery mechanismCitrate sodiumDrug standards
The invention discloses an injection medicinal composition of houttuyfonate. The injection medicinal composition is mainly prepared by the steps of dissolving salt of houttuyfonate in injection water,and adding citric acid and / or sodium citrate to serve as a pH regulator to adjust the pH value of medicinal liquid, wherein the dose of the citric acid and / or sodium citrate is 0.1-200.0mg / 100ml. According to the injection medicinal composition, the pH value of injection can be more stable, the houttuyfonate recalcitrant substance is greatly reduced in comparison with that in the prior art, the clarity of a houttuyfonate injection can be improved under the condition that other co-solvent increasing clinical application risk is prevented from being used, the problems of small white dots, whiteblocks and solution turbidity occurring in the houttuyfonate injection can be solved when the product is stored for a relatively long time by adopting the prior art, the condition that the visible foreign inspection of a product can accord with the specification of drug standard can be guaranteed, and clinical administration and popularization can be facilitated.
Owner:CHENGDU SHENGSHI GUANGHUA BIOTECH CO LTD
Injection pharmaceutical composition for improving stability of rhizoma dioscoreae nipponicae drug injection preparation
The invention discloses an injection pharmaceutical composition for improving stability of a rhizoma dioscoreae nipponicae drug injection preparation. The injection pharmaceutical composition is mainly prepared by dissolving an extracting solution of rhizoma dioscoreae nipponicae in water, and regulating pH value of medicine liquid with the addition of formic acid and / or sodium formate which serves as a pH regulator, wherein the dosage of the formic acid and / or the sodium formate is 1-3g / 100ml. According to the injection pharmaceutical composition disclosed by the invention, the stability of the pH value of the injection (the injection pharmaceutical composition) is enhanced, the content of rhizoma dioscoreae nipponicae degradable substances is greatly reduced in comparison with the prior art, and the clarity of the rhizoma dioscoreae nipponicae injection is improved under the circumstance of avoiding the use of other cosolvents which can increase the risk of clinical application; especially, the problems of the prior art that small white spots, white plaques and solution turbidity easily occur in a finished rhizoma dioscoreae nipponicae injection product can be solved; the obvious foreign matter inspection of the product (the injection pharmaceutical composition) can conform to the specifications of drug quality standard; and the injection pharmaceutical composition is convenient for clinical medication and popularization.
Owner:成都佳迪璐莎生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com