Patents
Literature
240results about How to "Good for clinical use" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gene detecting methods without using PCR
ActiveUS8704165B2Enhanced signalHigh sensitivityMicrobiological testing/measurementParticle spectrometer methodsMicroparticleBiology
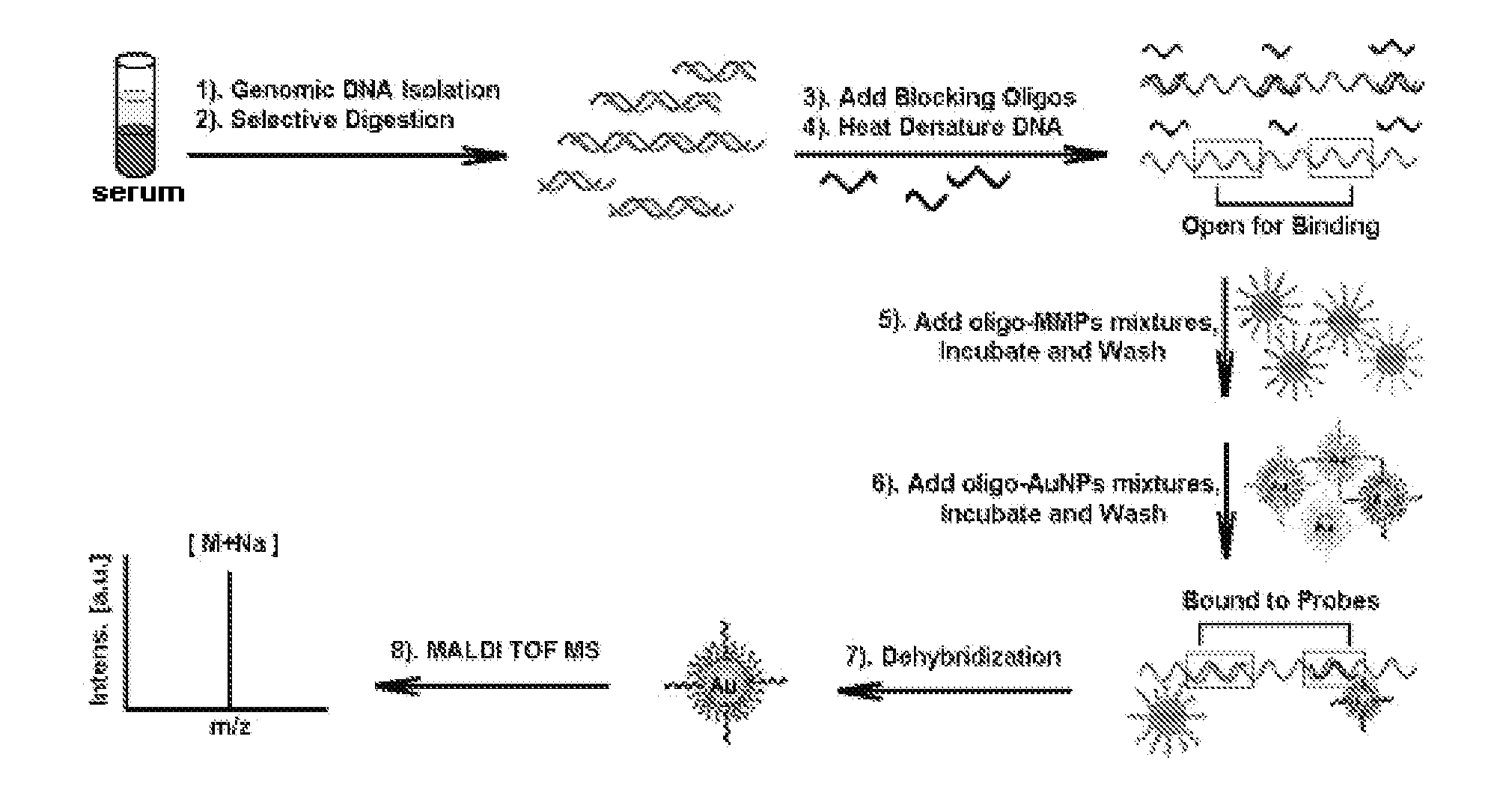
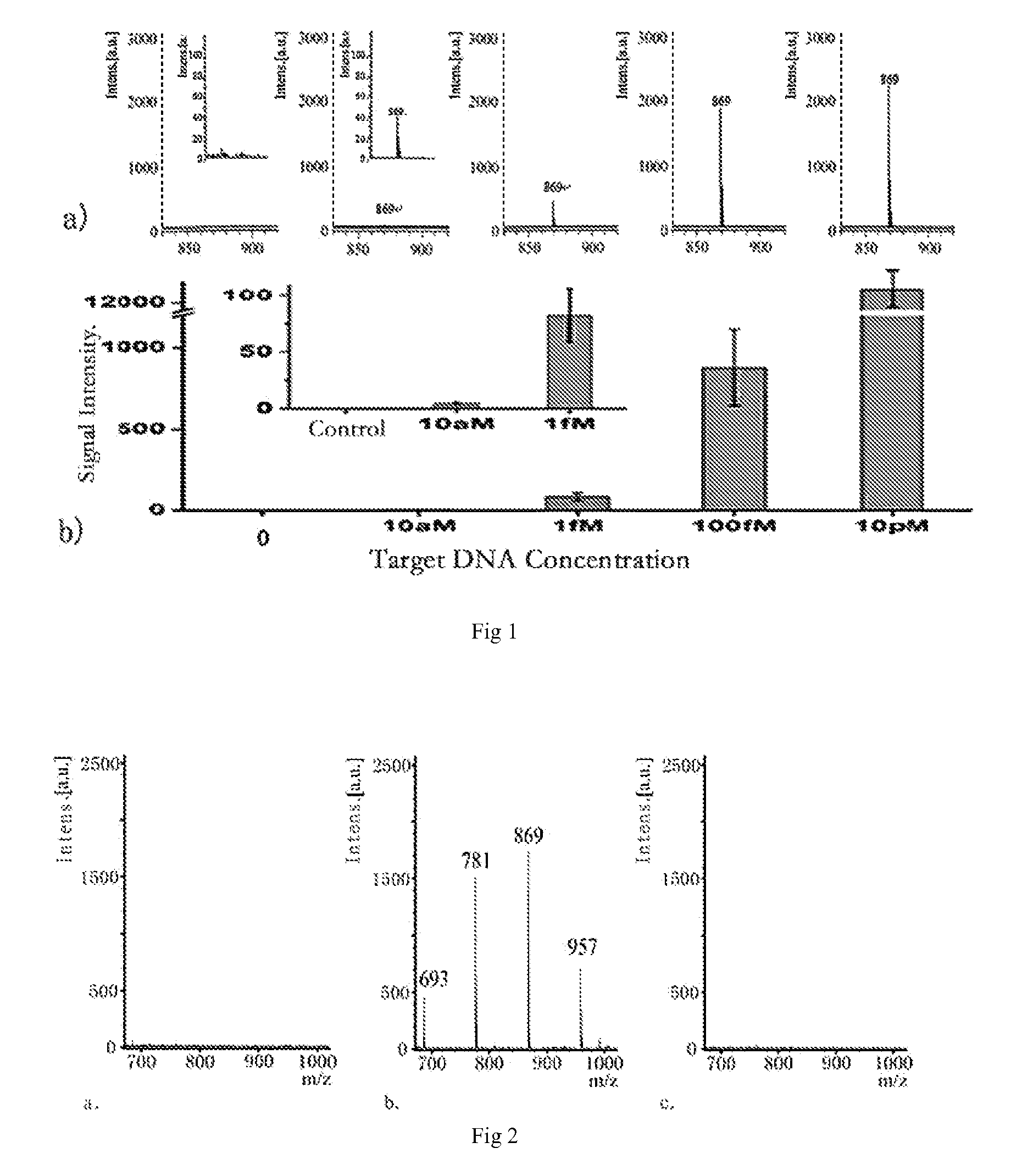
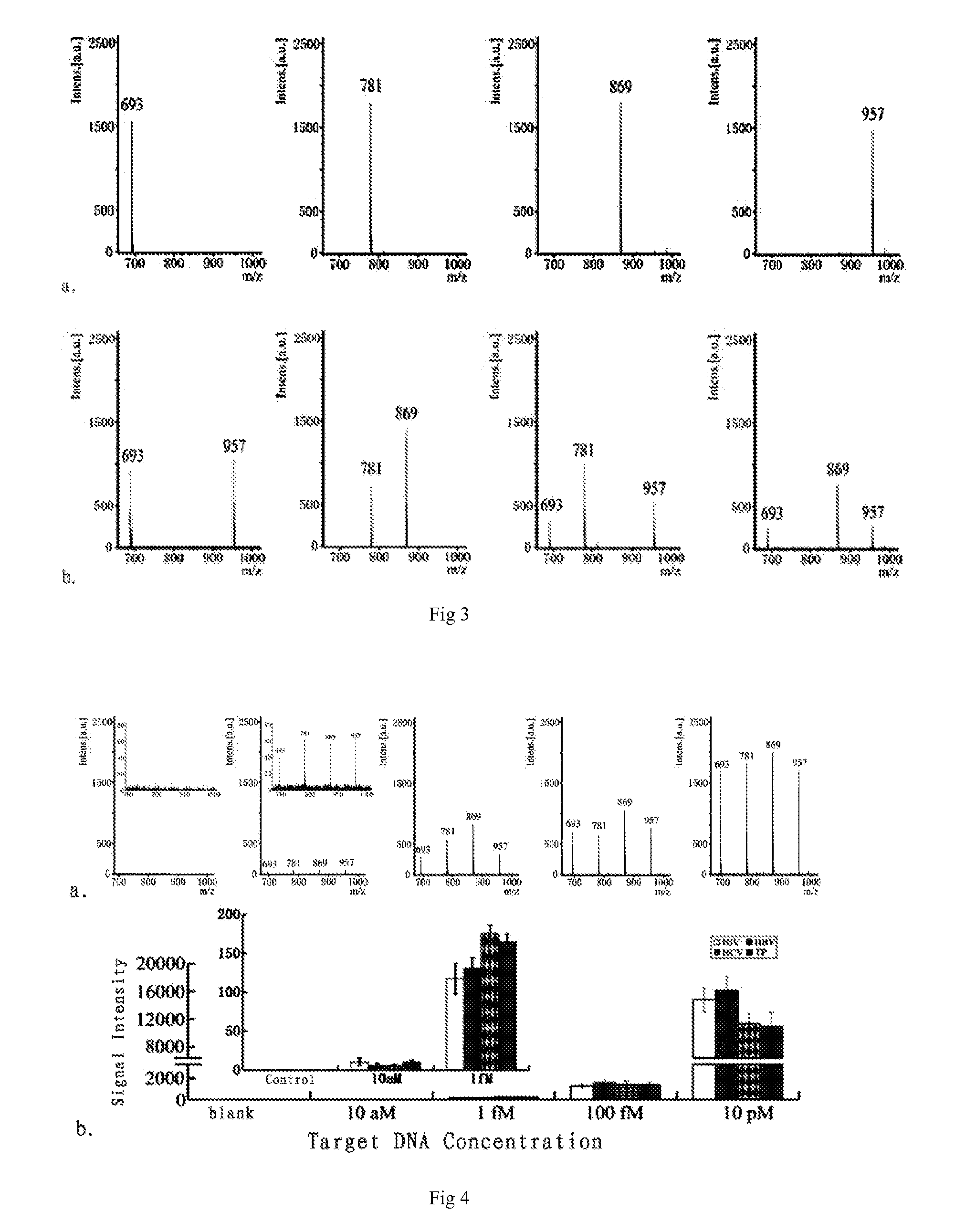
Gene detecting methods without using PCR are disclosed. The methods comprise forming sandwich complexes by target genes with nano-probes and capture probes, wherein nano-probes are modified with recognition molecules and magnetic microparticles modified with capture molecules; then separating the sandwich complexes; releasing the nano-probes; and detecting molecular ion peaks of encoding molecules on the surface of nano-probes by mass spectrometric detection directly, characterized in that the proportions of recognition molecules and encoding molecules on the nano-probes are 300-2000:1.
Owner:JIANGSU SINOBIOPHARMA
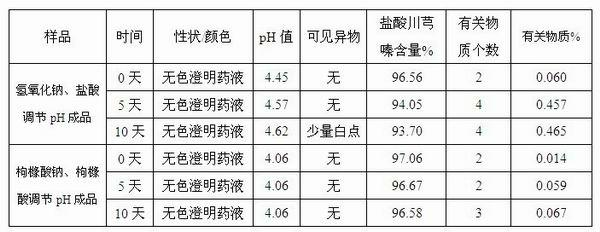
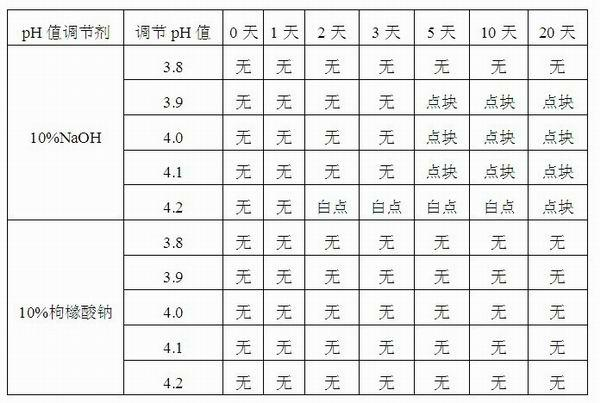
Injection-purpose medicine composition for improving stability of ligustrazine medicine injection formulation and preparation method of injection-purpose medicine composition
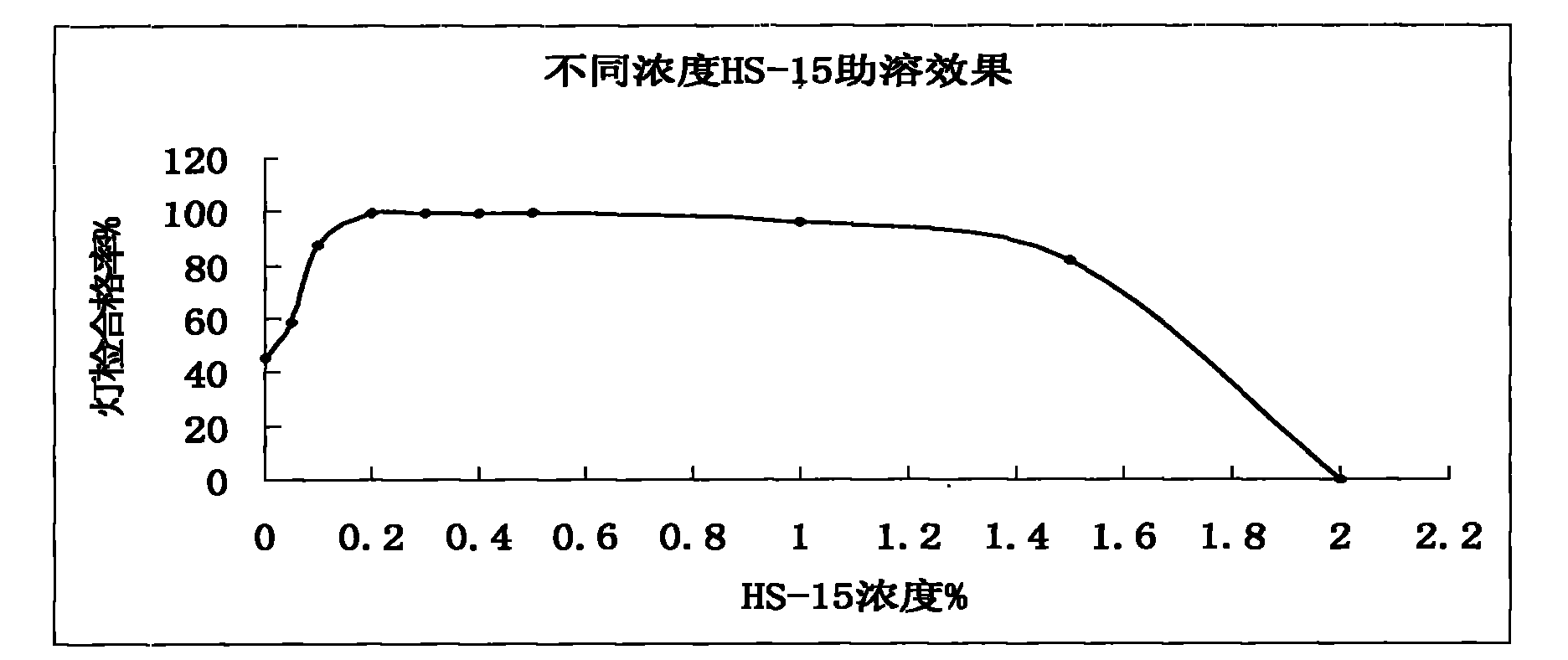
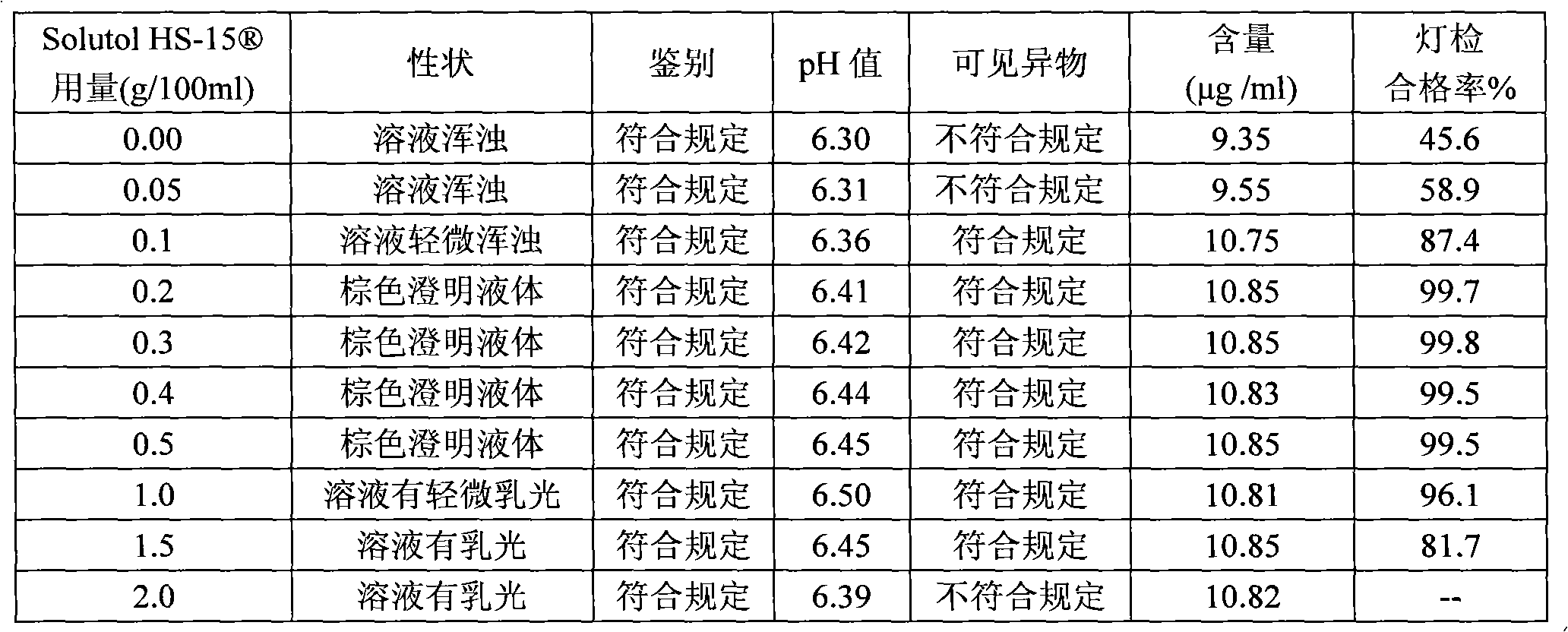
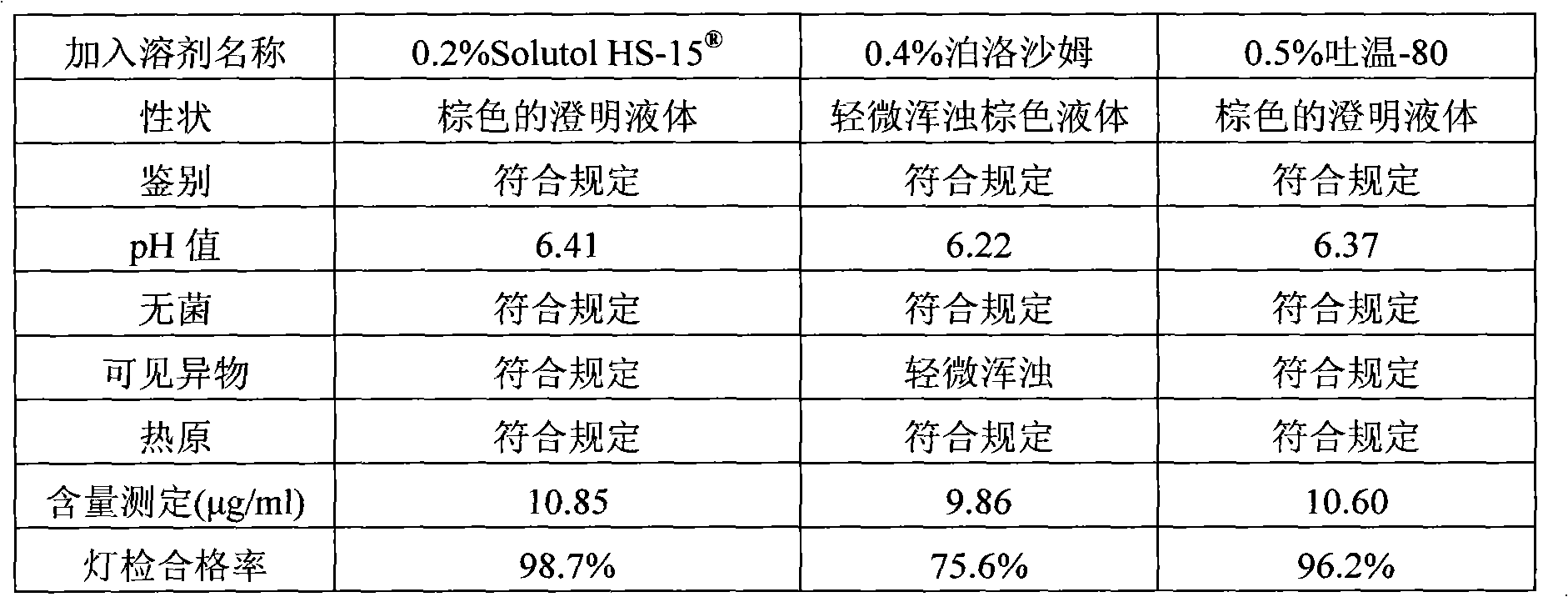
ActiveCN102008727AStable pHDecreased substancesOrganic active ingredientsPharmaceutical non-active ingredientsMedication injectionUse medication
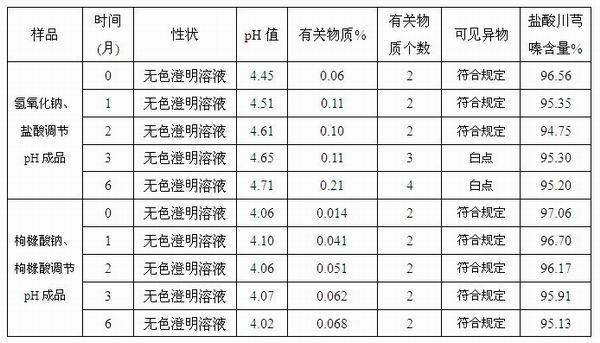
The invention discloses an injection-purpose medicine composition for improving stability of ligustrazine medicine injection formulation and a preparation method of the injection-purpose medicine composition. The injection-purpose medicine composition is prepared by the method comprising following steps: dissolving ligustrazine salt in water for injection, adjusting the pH value of the liquid medicine by adding citric acid and / or sodium citrate used as a pH regulator, wherein the dosage of the citric acid and / or sodium citrate ranges from (0.1mg to 200.0mg) / 100ml. In the invention, pH value of the injection liquid can be more stable, the content of ligustrazine degradation substance is greatly reduced compared with that of the prior art, the clarity of the ligustrazine injection liquid is improved under the condition of not using other cosolvents to increase the clinical application risk, particularly, the problems of small white spots, white blocks and solution turbidity are solved under the condition that the ligustrazine injection liquid is stored for a long time by adopting the prior art, and the medicine composition ensures that the inspection of visible foreign substances of the product is in accordance to the formulation of medicine quality standard, and is convenient for clinical medication and popularization.
Owner:SICHUAN SUNNYHOPE PHARM CO LTD
Mixed glue bundle pharmaceutical preparations produced in combination use of multiple surfactant and processes for their preparation
InactiveCN101138550AStrong dilution stabilityReduce viscosityPharmaceutical non-active ingredientsLiposomal deliverySolubilityMixed micelle
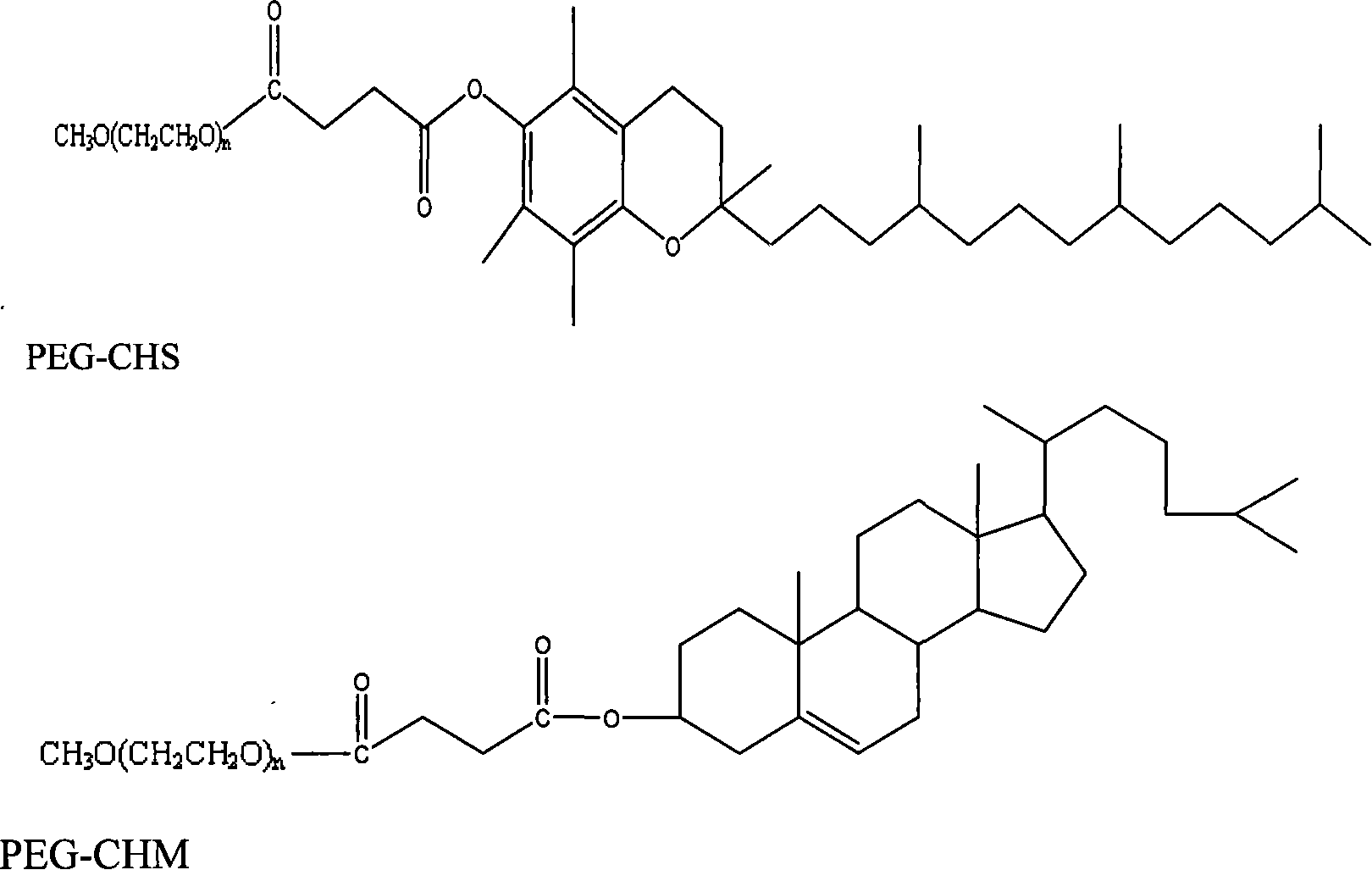
The present invention relates to a mixed micelle medicine preparation and a preparation method, which is prepared by the combination of various kinds of surface acting agents. The mixed micelle consists of the polyethylene glycol-12-hydroxy stearate and the other surface acting agents of one kind or various kinds. The other surface acting agents comprise phospholipid, VE Macrogol succinate, Macrogol-VE-carbonate and Macrogol-VE-succinate. In addition, the mixed micelle also comprises drugs, solvent, a stabilizer with or without other components and a PH conditioner. The amount of the polyethylene glycol-12-hydroxy stearate in the prescription is 4 percentage to 40 percentage, W / V: the amount of the phospholipid is 0 percentage to 30 percentage, W / V: the amount of the activator is 0 percentage to 30 percentage, W / V: the amount of the drug is 0.001 percentage to 10 percentage, W / V: the amount of the solvent is 0 percentage to 90 percentage, W / V. The medicine comprises the hydrophobicity drug and the lip solubility drug, but the medicine is not restricted by the both kinds of drugs. The present invention has the following advantages. Firstly, the preparation has good dilution stability, which can improve the defect in the present preparation and can meet the demanding for clinical drug administration. Secondly, the toxicity is low and the chemical stability is excellent.
Owner:SHENYANG PHARMA UNIVERSITY
Polymer micelle lyophilized agent encapsulating insoluble antitumor drug
ActiveCN102218027ASmall toxicityGood biocompatibilityOrganic active ingredientsPharmaceutical delivery mechanismPolyesterSide effect
The invention belongs to the field of pharmaceutical agents, relates to a polymer micelle lyophilized agent encapsulating an insoluble antitumor drug as well as a preparation method and an application thereof. The polymer micelle lyophilized agent is prepared by carrying out molecular self-assembly on a methoxy poly(ethylene glycol) 2000-polyester block copolymer to form micelles, and then encapsulating the insoluble antitumor drug in a hydrophobic core formed by the polyester. The lyophilized agent has high encapsulation rate, high drug loading and small particle size, can significantly improve the water solubility of the insoluble drug and result in passive targeting of more antitumor drugs to concentrate in the tumor tissues, thus improving an anti-tumor treatment effect and reducing the toxic and side effects of drugs, and can be used to prepare the drugs used for the treatment of lung cancer, intestinal cancer, mammary cancer, ovarian cancer, etc. The lyophilized agent can also be quickly dissolved and dispersed to form a transparent micellar solution after water for injection, normal saline solution and the like are added, and is used for the preparation of the drugs for treating primary intestinal cell carcinoma.
Owner:上海谊众药业股份有限公司
Debriding composition from bromelain and methods of production thereof
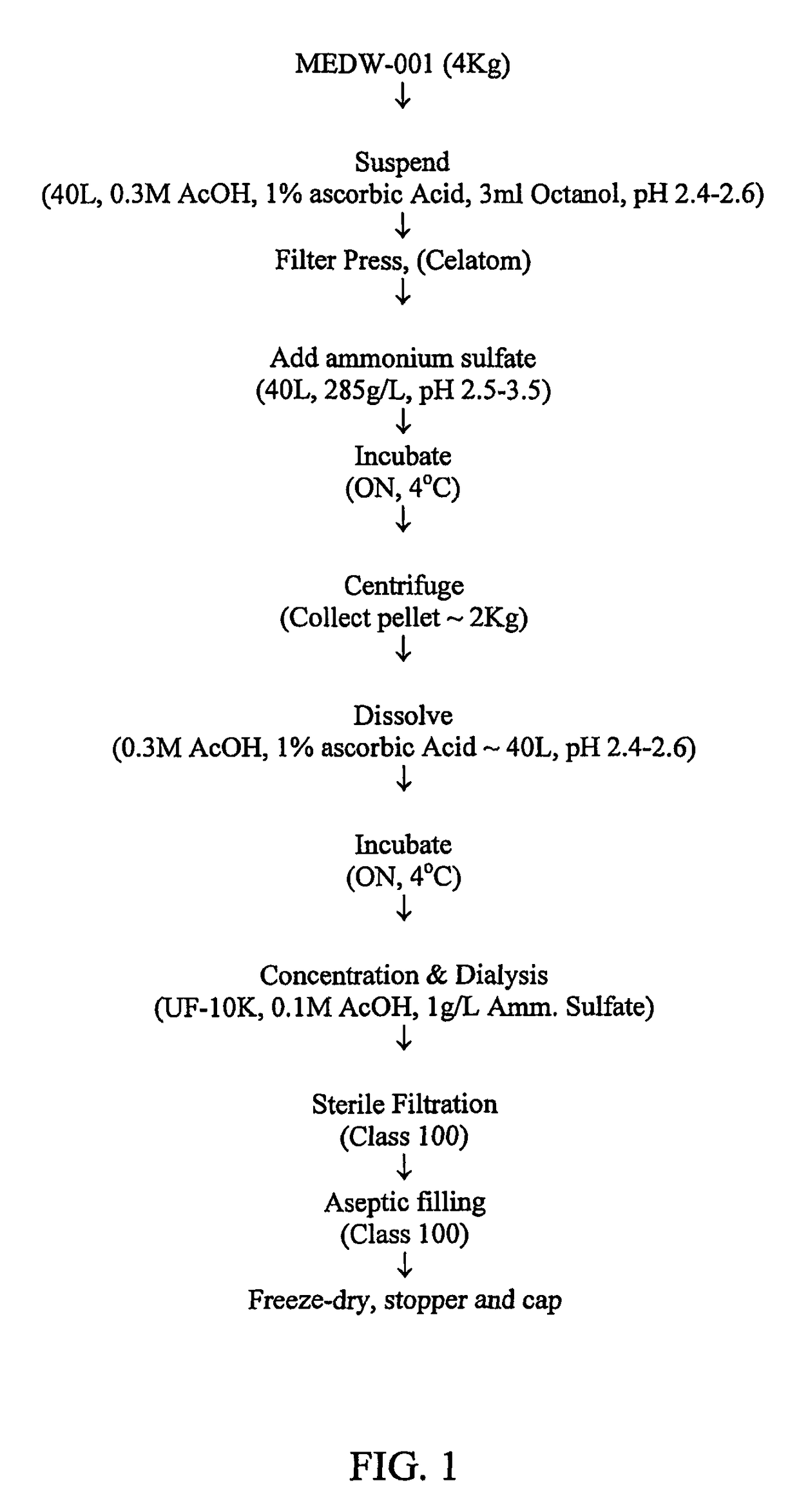
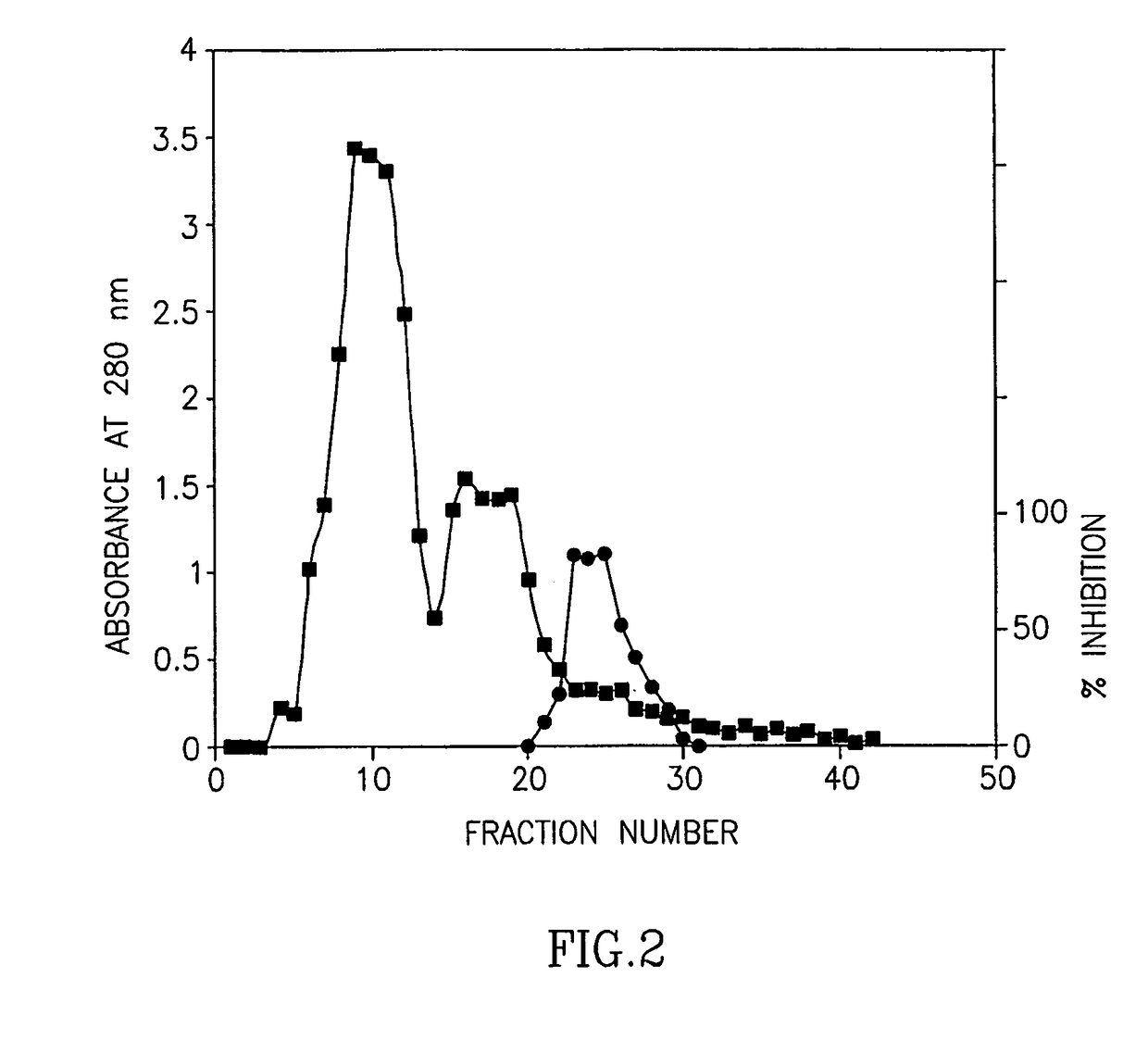
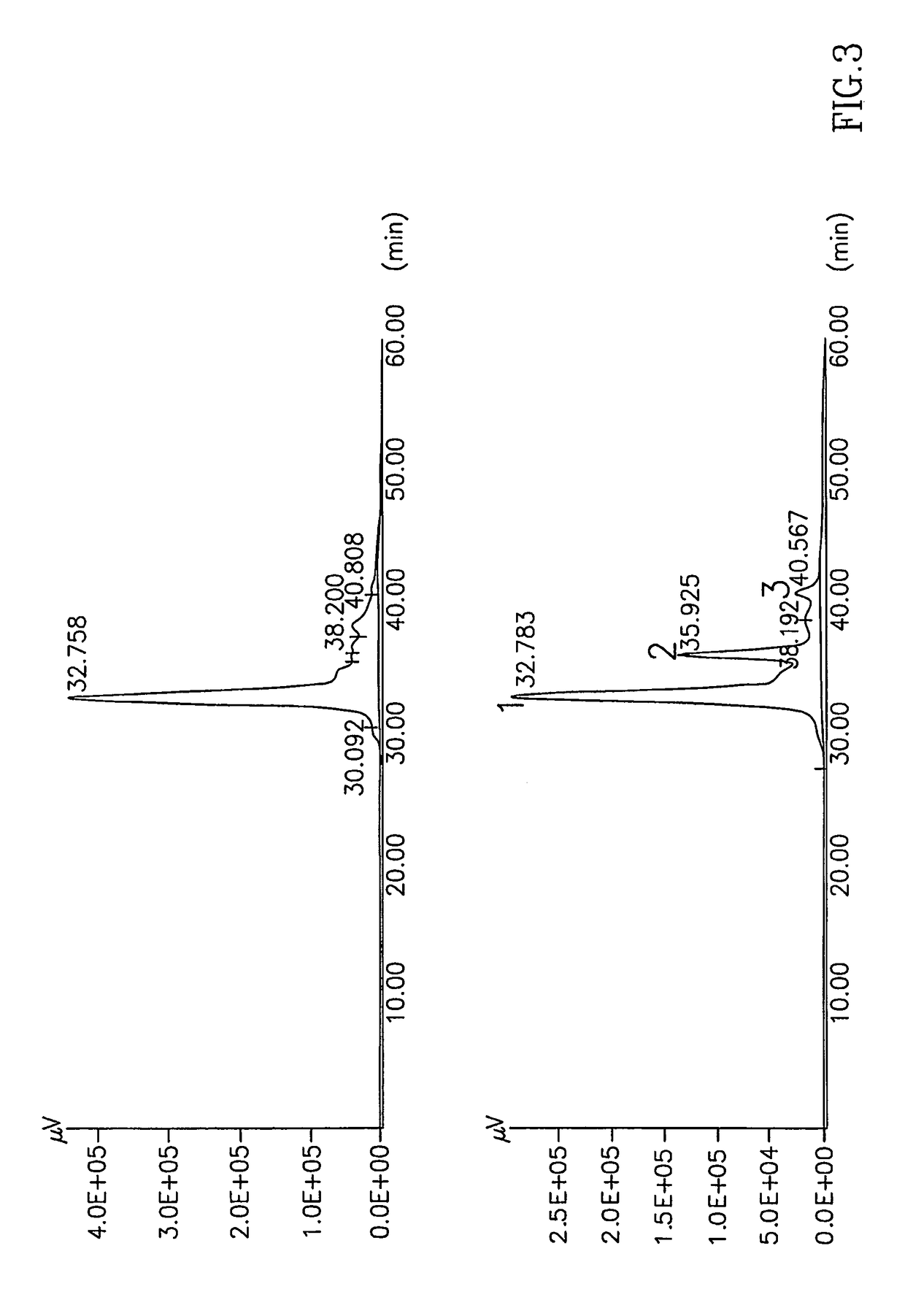
ActiveUS8119124B2Superior debridement activityHigh in proteinOrganic active ingredientsHydrolasesWound healingMedicine
Owner:MEDIWOUND
Process for extracting ginkgolide, ginkgolide injection and process for preparing same
ActiveCN1594319AEasy to operateReduce pollutionOrganic active ingredientsOrganic chemistrySodium acetateGinkgolide
The invention discloses a process for extracting ginkgolide, ginkgolide injection and process for preparing same, wherein the extracting process consists of disintegrating the ginkgo leaves, leaching by diluted acetone solution, recovering acetone, removing impurities, extracting and purifying with acetic ether, removing impurities with sodium acetate again, reclaiming acetic ether, recrystallizing in ethanol or methanol, filtering, low temperature drying, solubilizing the bilobalide with hydroxypropyl-beta-cyclodextrin to obtain the bilobalide injection.
Owner:HEILONGJIANG ZBD PHARMA
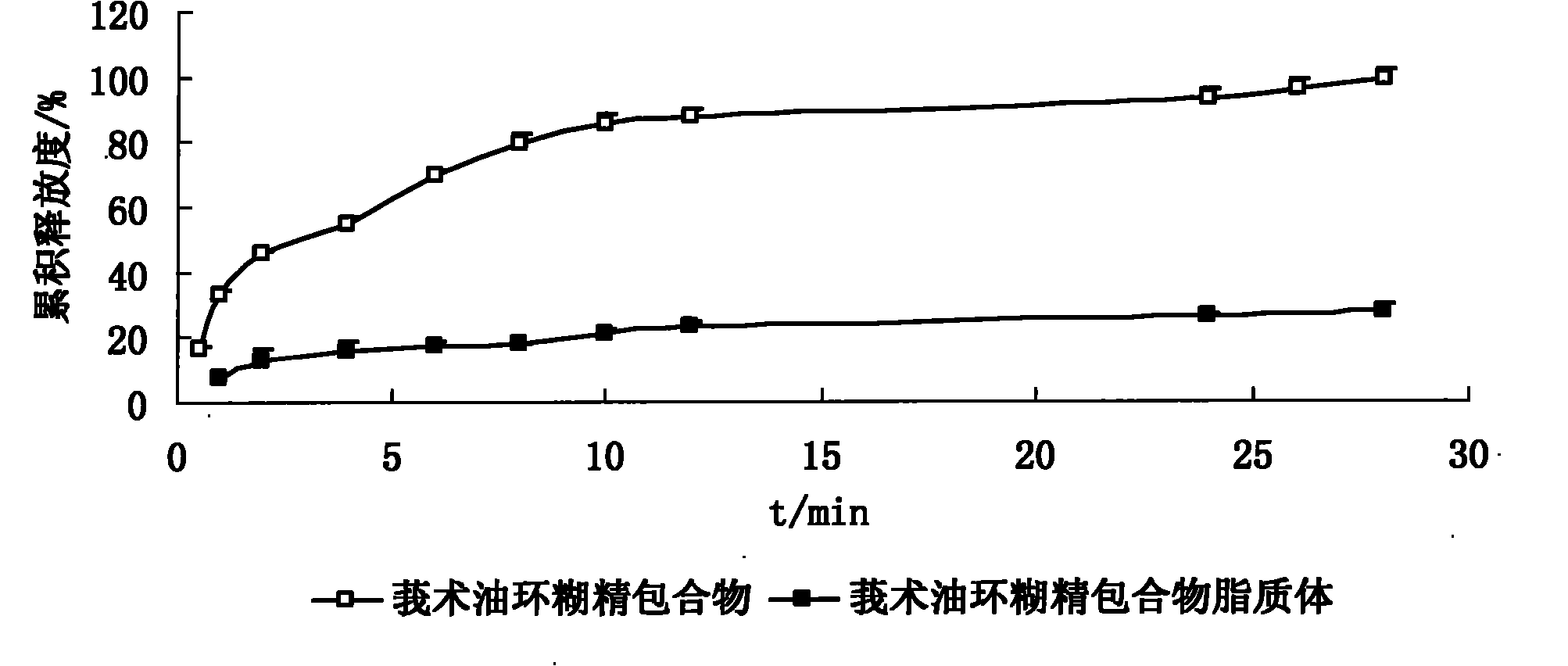
Hydroxypropyl-beta-cyclodextrin inclusion liposome of zedoary turmeric oil and preparation method thereof
InactiveCN101926962ANo hemolytic toxicityObvious slow-release and long-actingPharmaceutical non-active ingredientsAntineoplastic agentsHemolysisEthanol Injection
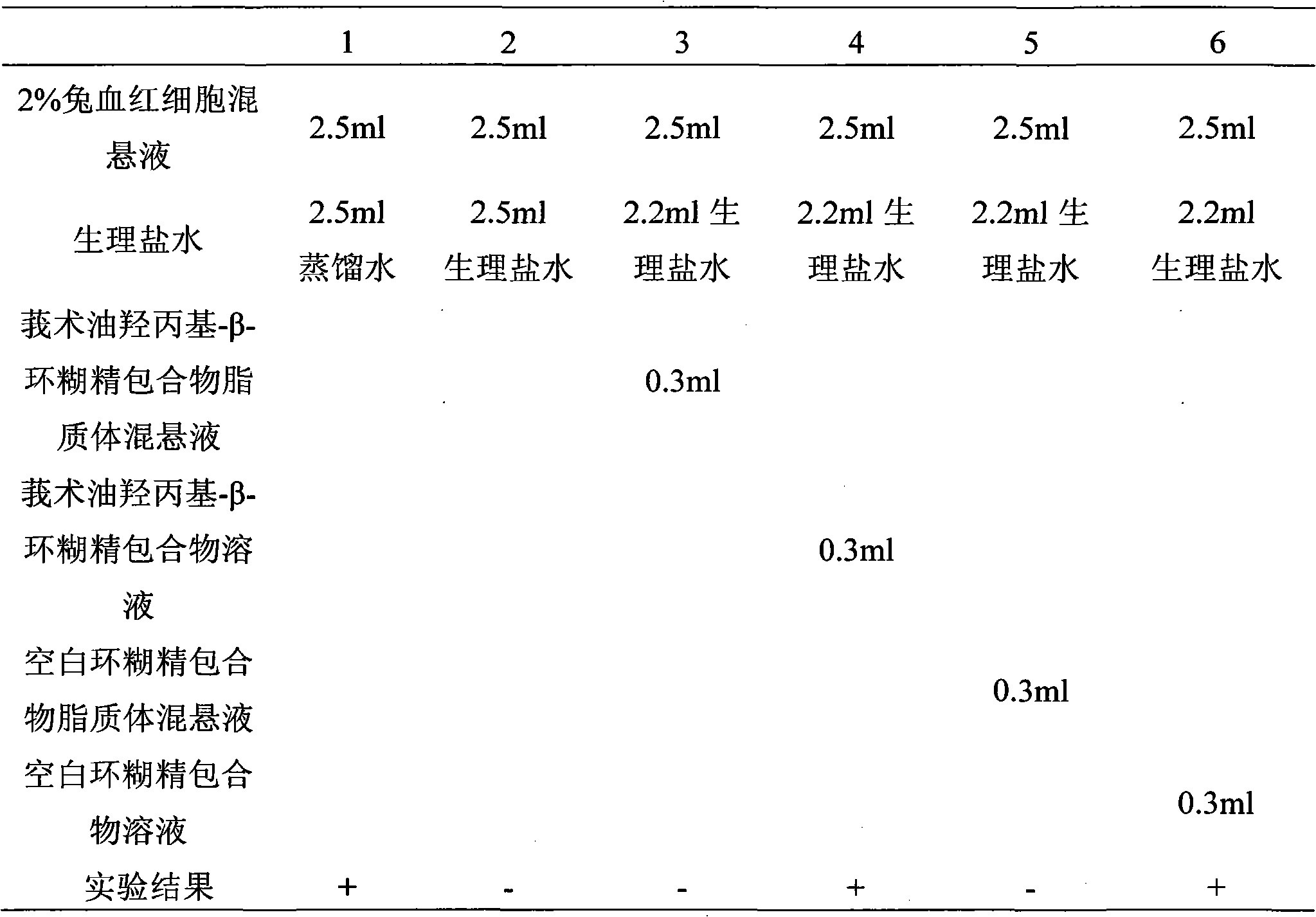
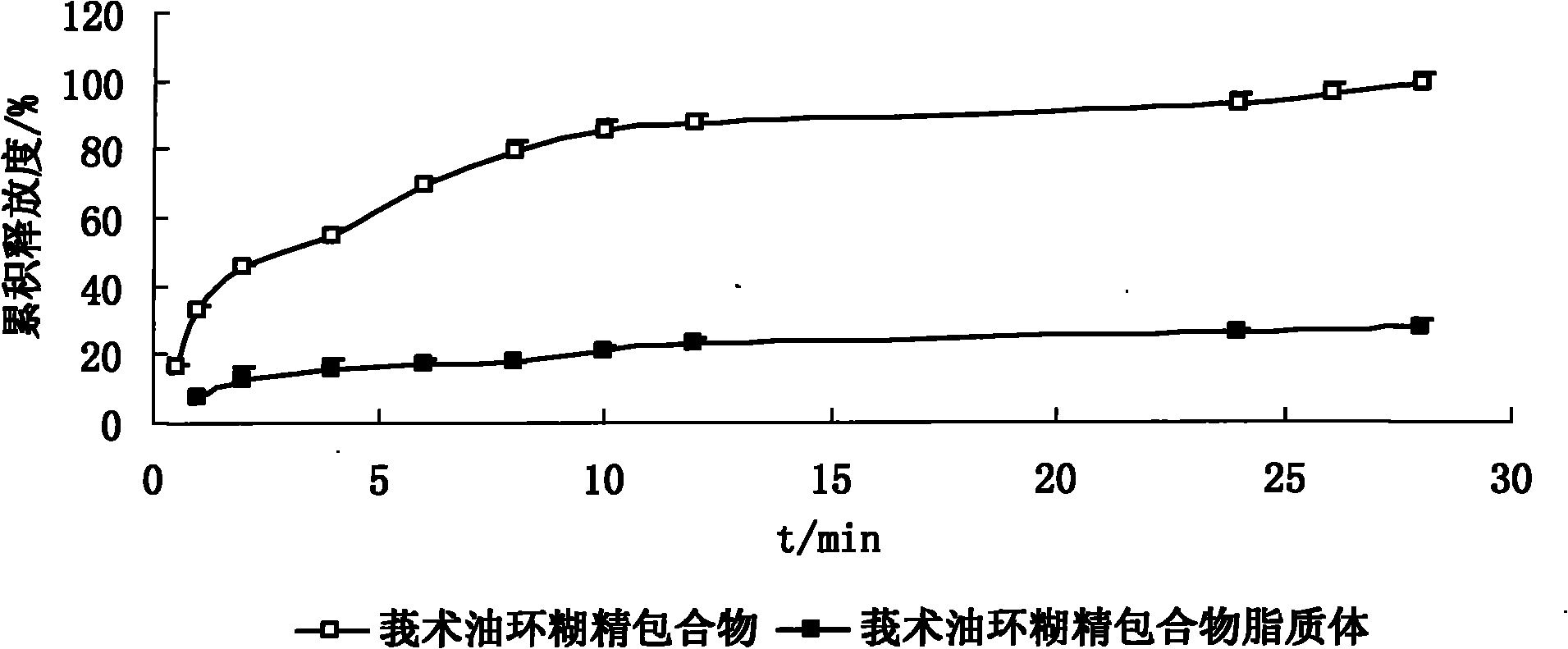
The invention discloses a hydroxypropyl-beta-cyclodextrin inclusion liposome of zedoary turmeric oil and a preparation method thereof. The liposome is prepared by the following steps of: preparing a hydroxypropyl-beta-cyclodextrin inclusion of the zedoary turmeric oil from the zedoary turmeric oil and hydroxypropyl-beta-cyclodextrin through an inclusion process; and preparing the hydroxypropyl-beta-cyclodextrin inclusion liposome of the zedoary turmeric oil from the hydroxypropyl-beta-cyclodextrin inclusion, phospholipid and cholesterol through an ethanol injection method. Experimental results show that: the hydroxypropyl-beta-cyclodextrin inclusion liposome of the zedoary turmeric oil has the advantages of good sustained release and long action, high loading rate, particularly no untoward effect such as hemolysis, and higher safety.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Ultrasonic medicine paste
InactiveUS20100262070A1Increase profitSimple structureSonopheresisUltrasound therapyUltrasonic sensorElectric signal
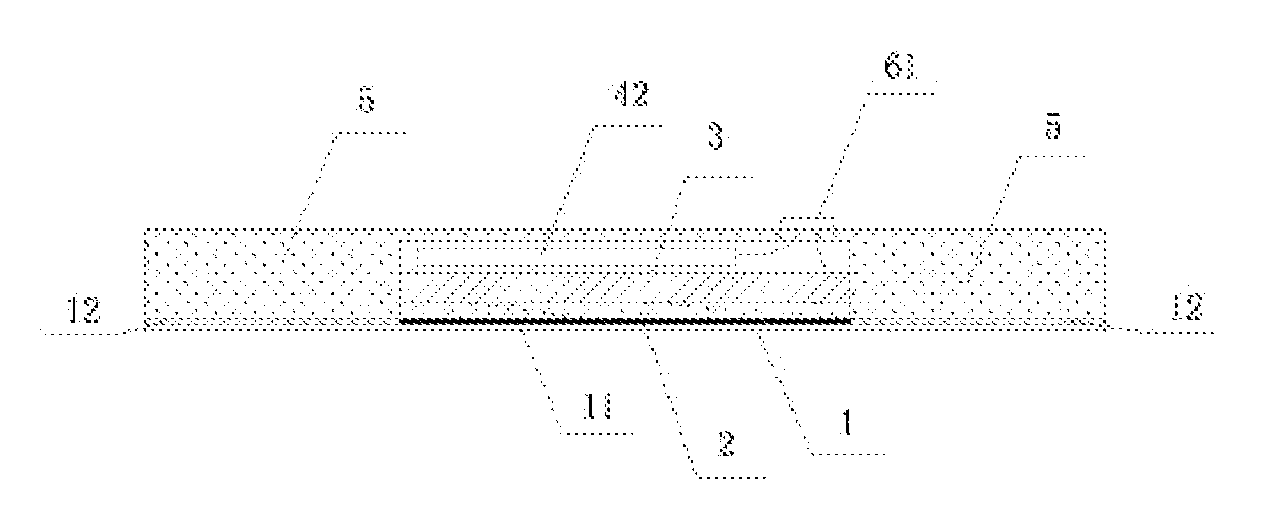
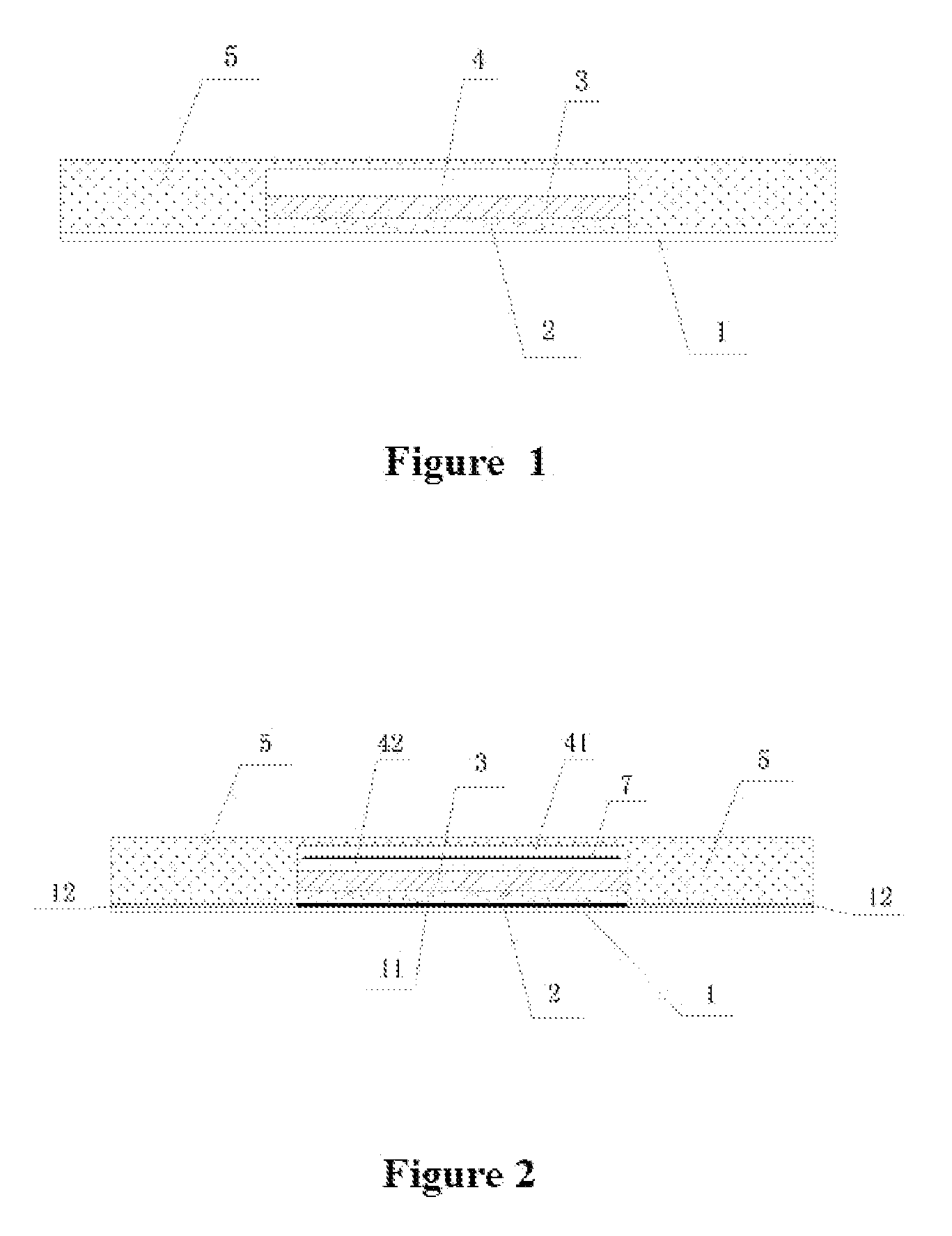
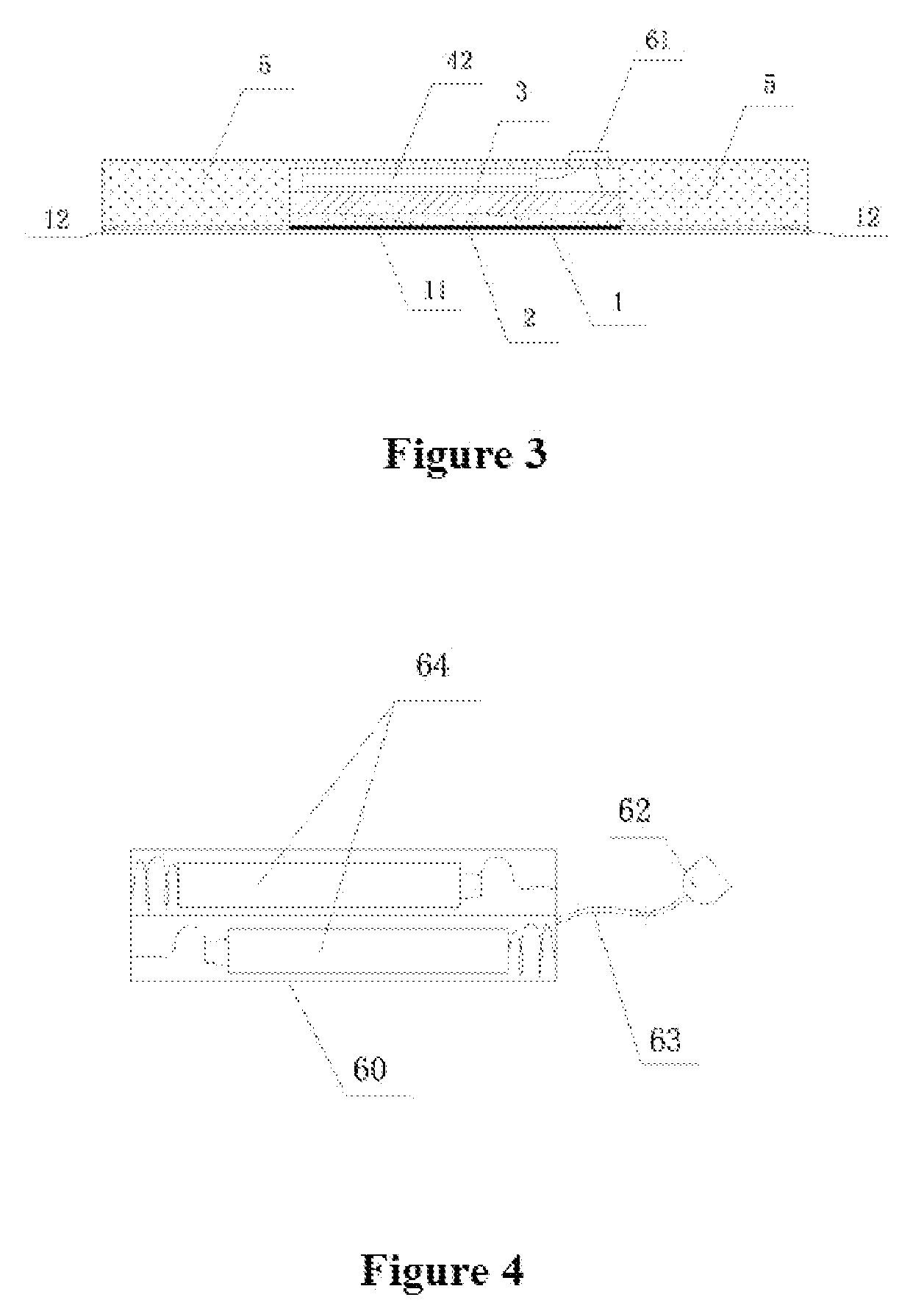
An ultrasonic medicine paste for transdermally permeating a medicine to a body and the manufacture method thereof. The medicine paste includes an adhesive layer (1) as the base layer, a medicine layer (2) adjacent to the adhesive layer (1), and ultrasonic transducer (3) for generating ultrasonic signal, a driving unit (4) that supplies an electric signal and drives the ultrasonic transducer (3) to generate ultrasonic signal, and a coating layer (5) that covers the exterior surface thereof.
Owner:CHONGQING RONGHAI ENG RES CENT OF ULTRASONIC MEDICINE
HIV vaccine formulation
ActiveUS20170362280A1Improve stabilityGood for clinical useHydroxy compound active ingredientsViral antigen ingredientsAdjuvantEngineering
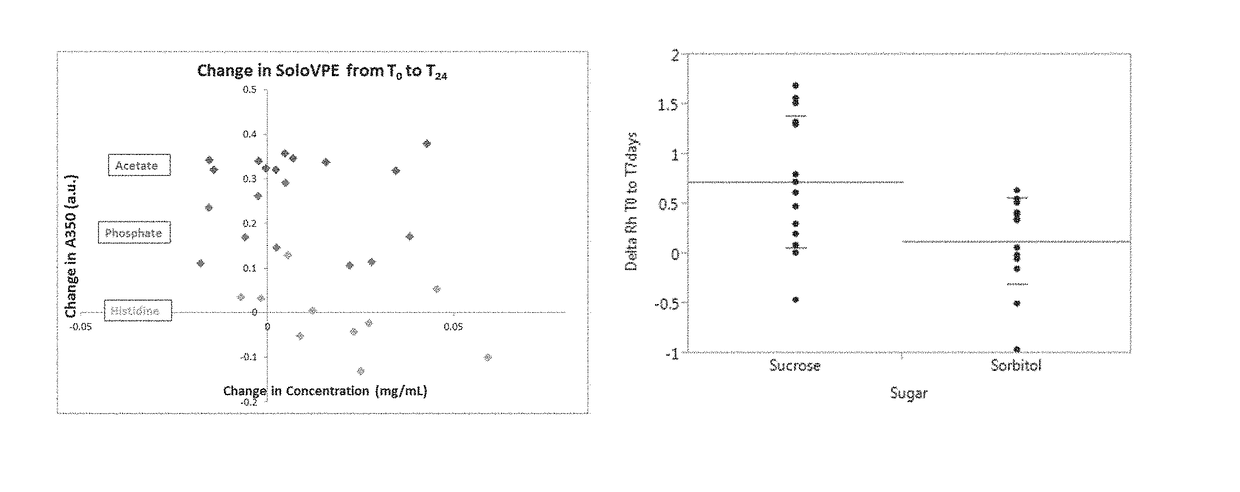
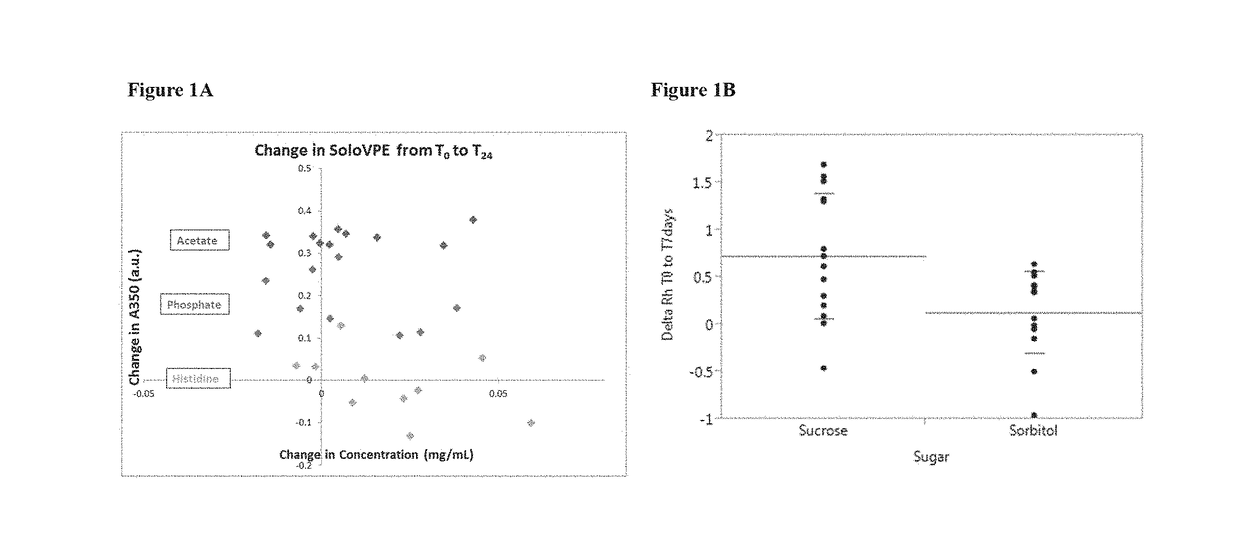
Immunogenic compositions containing a human immunodeficiency virus (HIV) gp140 protein, sorbitol, polysorbate 20, and histidine buffer are described. The described immunogenic compositions are advantageous in that they are stable at refrigerated temperature for extended periods of time, and are compatible with an adjuvant. Also described are methods of using the immunogenic compositions to induce an immune response against an HIV in a subject. The immunogenic compositions can be administered alone, or in combination with one or more additional HIV antigens, or one or more adenovirus vectors encoding the one or more additional HIV antigens.
Owner:JANSSEN VACCINES & PREVENTION BV
Hemostatic anti-adhesive membrane and preparation method thereof
InactiveCN106880867AImprove mechanical propertiesWon't breakSurgical adhesivesFilament/thread formingFiberAcetic acid
The invention relates to a hemostatic anti-adhesive membrane and a preparation method of the hemostatic anti-adhesive membrane. The preparation method of the hemostatic anti-adhesive membrane comprises the following steps: dissolving chitosan in acetic acid aqueous solution, carrying out electrostatic spinning, thus obtaining a chitosan fibrous membrane, removing residual acid in the chitosan fibrous membrane, immersing the chitosan fibrous membrane in acid polysaccharide aqueous solution, thus obtaining a compound fibrous membrane, and finally, carrying out aftertreatment on the compound fibrous membrane, thus obtaining the hemostatic anti-adhesive membrane. The finally obtained hemostatic anti-adhesive membrane is mainly composed of the chitosan fibrous membrane and acid polysaccharose, wherein the content of the acid polysaccharose is 5-80 wt%, the acid polysaccharose is polysaccharose containing carboxylate radical ions, the acid polysaccharose is bonded to chitosan through amido bonds, and thus the chitosan fibers realize covalent cross-linking. The finally prepared hemostatic anti-adhesive membrane is high in tenacity, good in fitting performance, and high in biocompatibility, can completely degrade in the body, and belongs to the ideal hemostatic anti-adhesive material.
Owner:吴欣
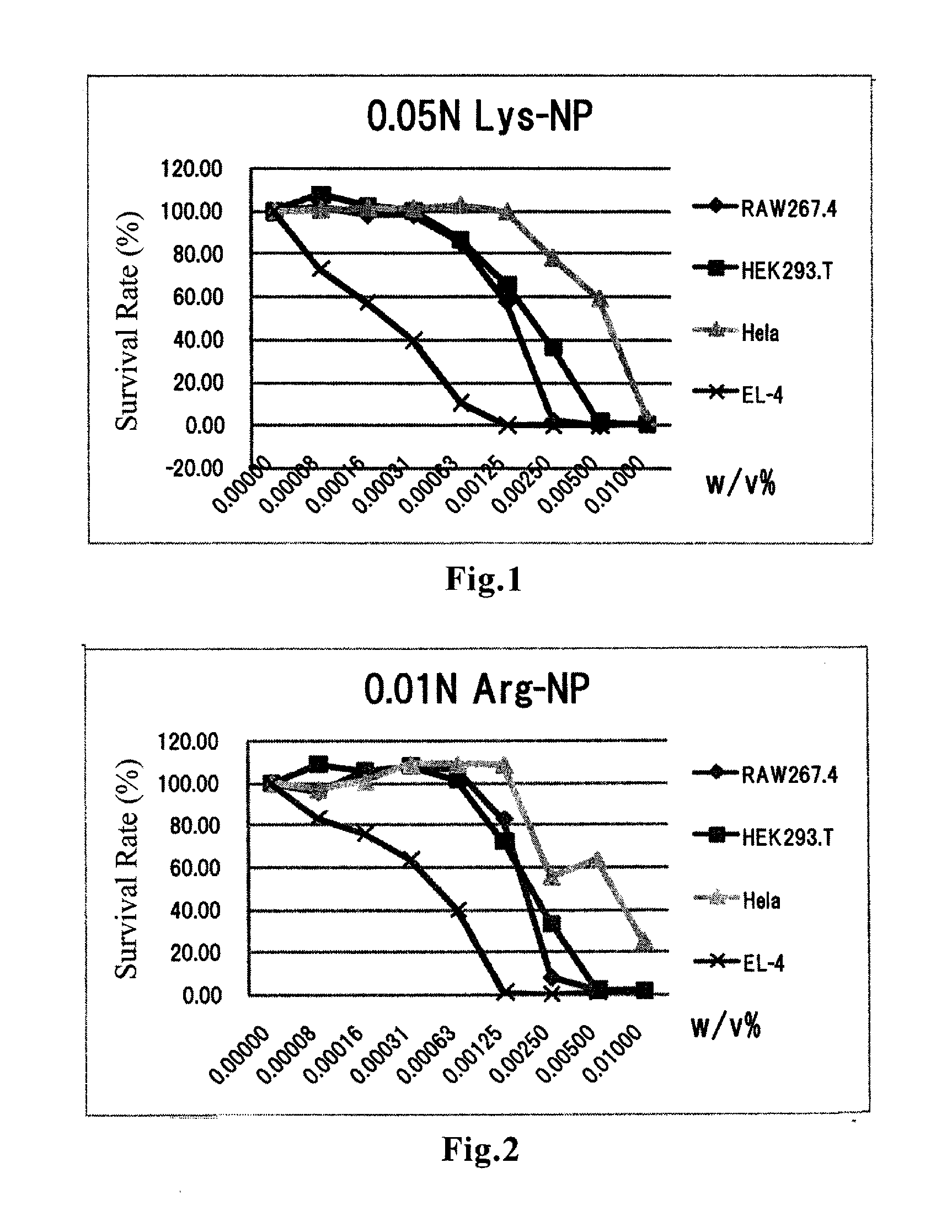
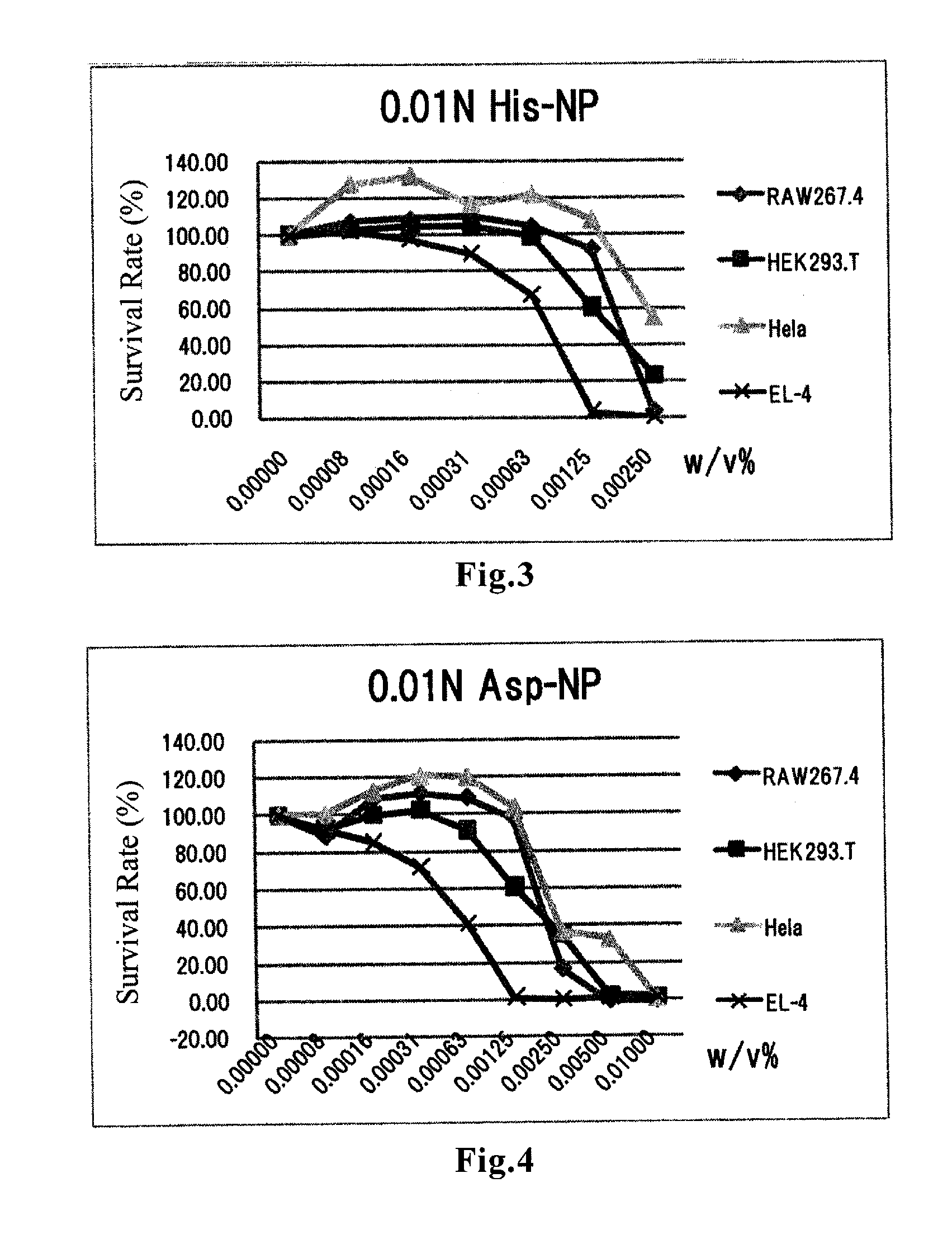
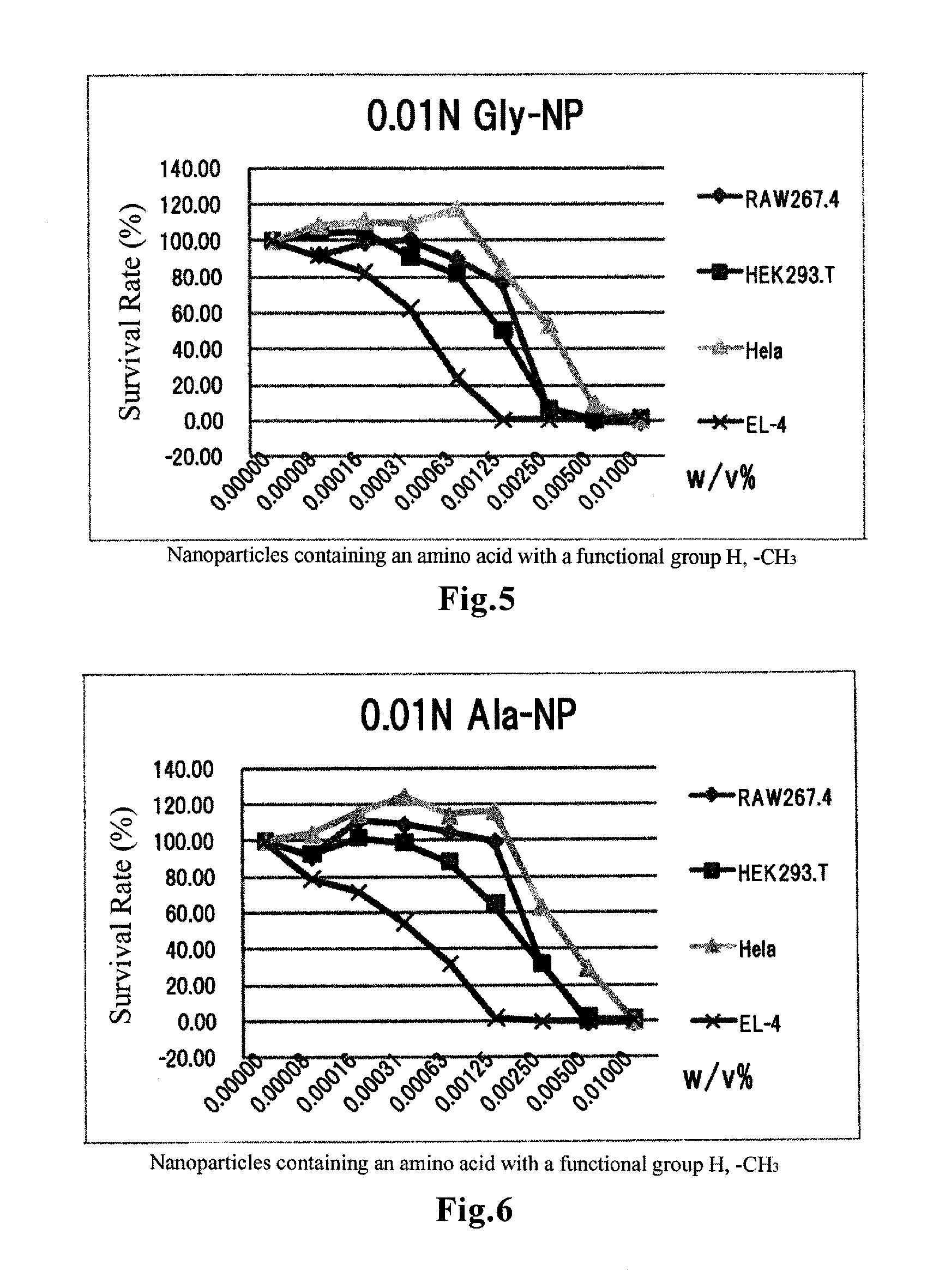
Amino acid-conjugated cyanoacrylate polymer particles
InactiveUS20120027821A1Prevent proliferationGood for clinical usePowder deliveryOrganic active ingredientsCyanoacrylateCancer cell
Disclosed are cyanoacrylate polymer particles which comprise an amino acid(s) and have an average particle diameter of less than 1000 nm. The amino acid-containing particles according to the present invention can kill cancer cells by inducing apoptosis-like cell death. The particles have an especially high affinity for cell lines derived from lymphomas such as T-cell lymphoma and B-cell lymphoma. The particles can also exhibit an antiproliferative effect against some kinds of pancreatic cancer-derived cell lines. Therefore, the particles according to the present invention are useful for prevention and / or treatment of cancers.
Owner:PUBLIC UNIV CORP YOKOHAMA CITY UNIV
Aromatic acid pro-drug with nitrogen monoxide donor, and preparation method and application thereof
InactiveCN101885684AImprove solubilityReduce adverse reactionsOrganic active ingredientsBlood disorderDiseaseLipid formation
The invention relates to the field of research into pharmaceutical chemistry, and particularly discloses a novel aromatic acid pro-drug with nitrogen monoxide donor, and a preparation method and application thereof in preventing and treating thrombotic diseases. In the invention, natural aromatic acid compounds with strong antithrombotic actions are structurally modified, and the carboxylic acid groups are incorporated with nitrogen monoxide donor to obtain the nitrogen monoxide donor type aromatic acid pro-drug with favorable liposolubility. The aromatic acid pro-drug with nitrogen monoxide donor has favorable solubility, can effectively permeate into the biomembrane lipid bimolecular layer, and can enhance the bioavailability after the human body takes the pro-drug. The pro-drug can be resolved into aromatic acid compounds and release nitrogen monoxide, and the aromatic acid compounds and nitrogen monoxide can cooperate to perform the pharmacological activities of resisting thrombi, resisting platelet aggregation and the like. The aromatic acid pro-drug with nitrogen monoxide donor can be used for preventing and treating thrombotic diseases and cerebral ischemia diseases.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Enrofloxacin suspension and its prepn process
InactiveCN1706386ASlow absorptionGood curative effectAntibacterial agentsOrganic active ingredientsBlood drug concentrationPropanediol
The present invention is enroloxacin suspension and its preparation process and belongs to veterinary medicine technology. The enroloxacin suspension includes enroloxacin 8-12 wt%, butyl oleate 3-8 wt%, polysorbate 3-8 wt% and propylene glycol 30-50 wt% except water. The enroloxacin suspension is prepared through D phase emulsifying process, and is white and ropy. The enroloxacin suspension has slow release of the effective medicine component, high bioavailability, enhanced medicinal effect, long effective blood medicine concentration maintaining period up to 48 hr, low cost and other advantages.
Owner:史同瑞
A kind of traditional Chinese medicine compound with anti-sickness effect and its preparation method and application
InactiveCN102274441AGood anti-sickness effectThe ratio is scientific and reasonableHeavy metal active ingredientsHydroxy compound active ingredientsMotion sicknessPinellia
The invention discloses a Chinese herbal compound having motion sickness resisting effect and a preparation method and application thereof. The Chinese herbal compound is prepared from galangal, fennel, dahurian angelica root, sweet basil, tsaoko amomum fruit, dried orange peel, mint, wrinkled gianthyssop herb, fortune eupatorium herb, borneol, largehead atractylodes rhizome, Indian buead, pinellia and ruddle. In the Chinese herbal compound provided by the invention, a raw material blending composition is selected according to the traditional Chinese medicine and the pathologic mechanism of motion sickness, and the mixture ratio of each component is scientific and reasonable. As proved by pharmacological experiment and clinical experiment results, the Chinese herbal compound provided by the invention has good effects of clearing orifices, restoring consciousness, strengthening spleen, drying dampness and calming the adverse-rising energy to stop hiccup, has quick response and long duration after being taken by a patient suffering from motion sickness, and has a good motion sickness resisting effect. The preparation method of the Chinese herbal compound provided by the invention has a reasonable process design and high operability, and mass industrial production can be realized.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Scutellarin aglycone methylate product based on in-vivo metabolic mechanism as well as preparation method and application of scutellarin aglycone methylate product
InactiveCN102702155AImprove solubilityImprove bioavailabilityOrganic active ingredientsOrganic chemistrySolubilityProduct base
The invention relates to the field of pharmaceutical chemistry study, in particular to a novel scutellarin aglycone methylate product based on in-vivo metabolic mechanism as well as a preparation method of the novel scutellarin aglycone methylate product and an application of the novel scutellarin aglycone methylate product to thrombus prevention and treatment medicine. Pharmacological experiment results show that the scutellarin aglycone methylate product provided by the invention has pharmacological effects of better solubility, antioxidation, cell damage inhabitation and the like, and can be developed into novel medicine for preventing and treating thrombotic diseases.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
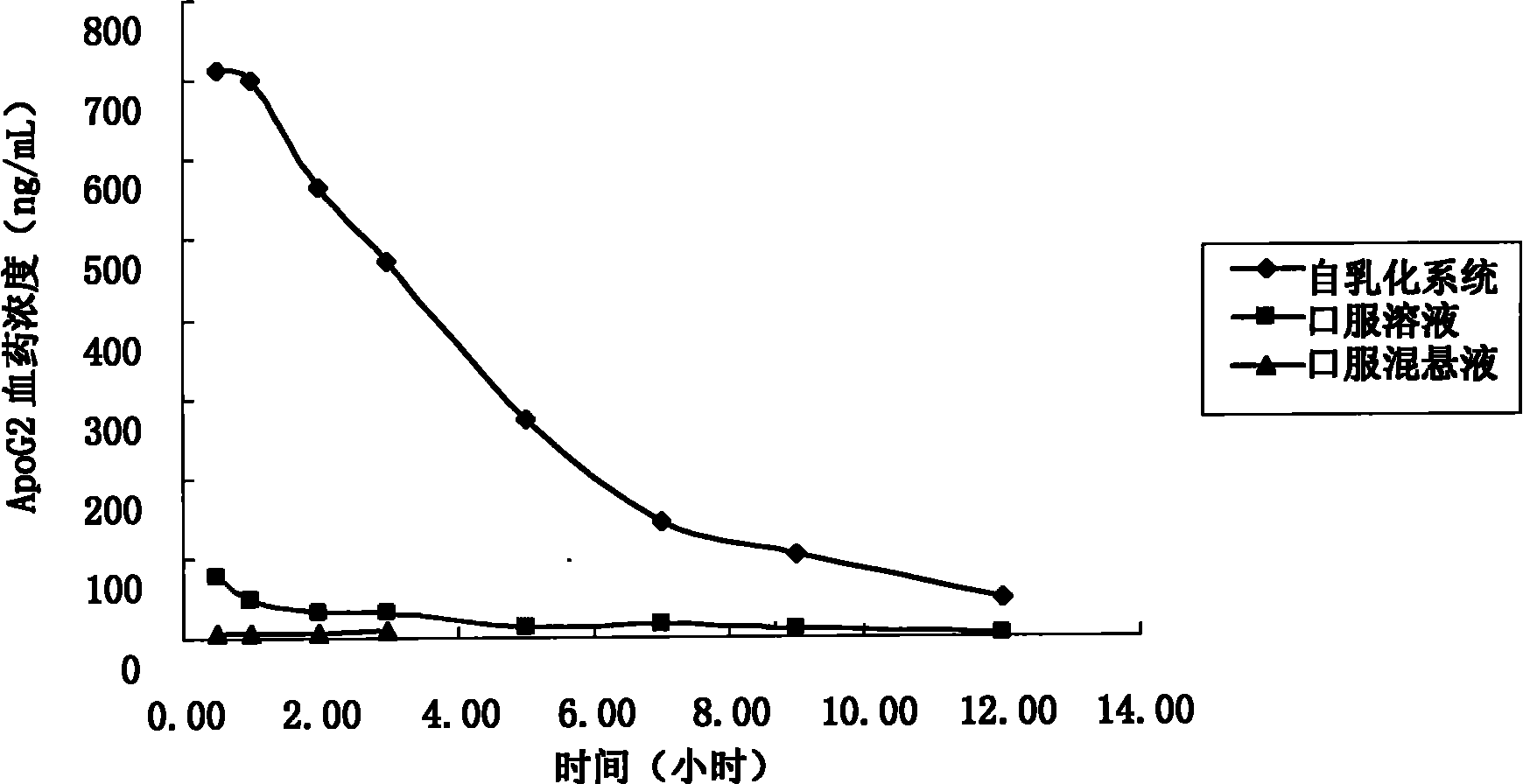
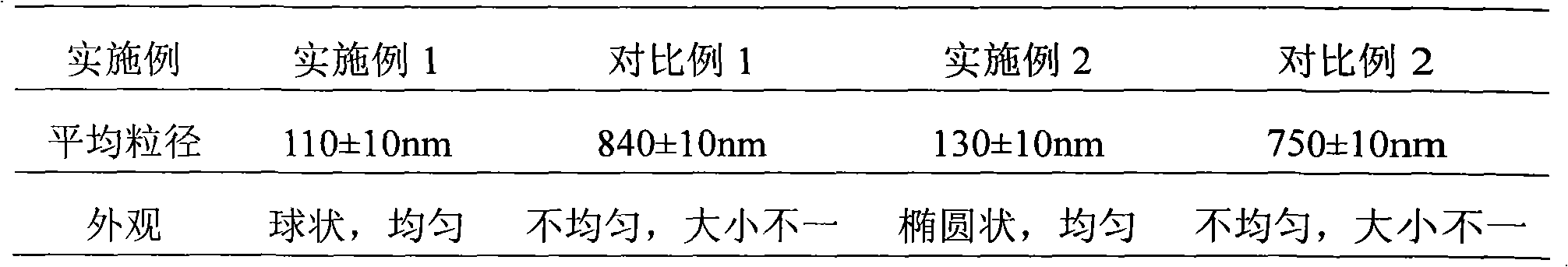
Apogossypolone self-emulsifying drug delivery system and preparation method thereof
InactiveCN102247321AIncrease biofilm permeabilityImprove bioavailabilityPowder deliveryOrganic active ingredientsOrganic acidSolubility
The invention provides an apogossypolone self-emulsifying drug delivery system, which comprises the following components in percentage by mass: 0.2 to 20 percent of apogossypolone, 10 to 89.8 percent of oil phase, 5 to 50 percent of emulsifier, 5 to 50 percent of auxiliary emulsifier and 0 to 20 percent of organic acid, wherein the components form a uniform semisolid or liquid or solid formulation. The invention also provides a method for preparing the apogossypolone self-emulsifying drug delivery system. Compared with the ordinary apogossypolone oral preparation, the apogossypolone self-emulsifying drug delivery system can increase the biomembrane permeability of apogossypolone, improve bioavailability obviously, increase the solubility of the apogossypolone and improve a drug-loading rate. The preparation method is simple and high in operability and industrial degree; and the apogossypolone self-emulsifying drug delivery system is conveniently and stably taken in clinic.
Owner:SHANGHAI YASHENG MEDICAL TECH
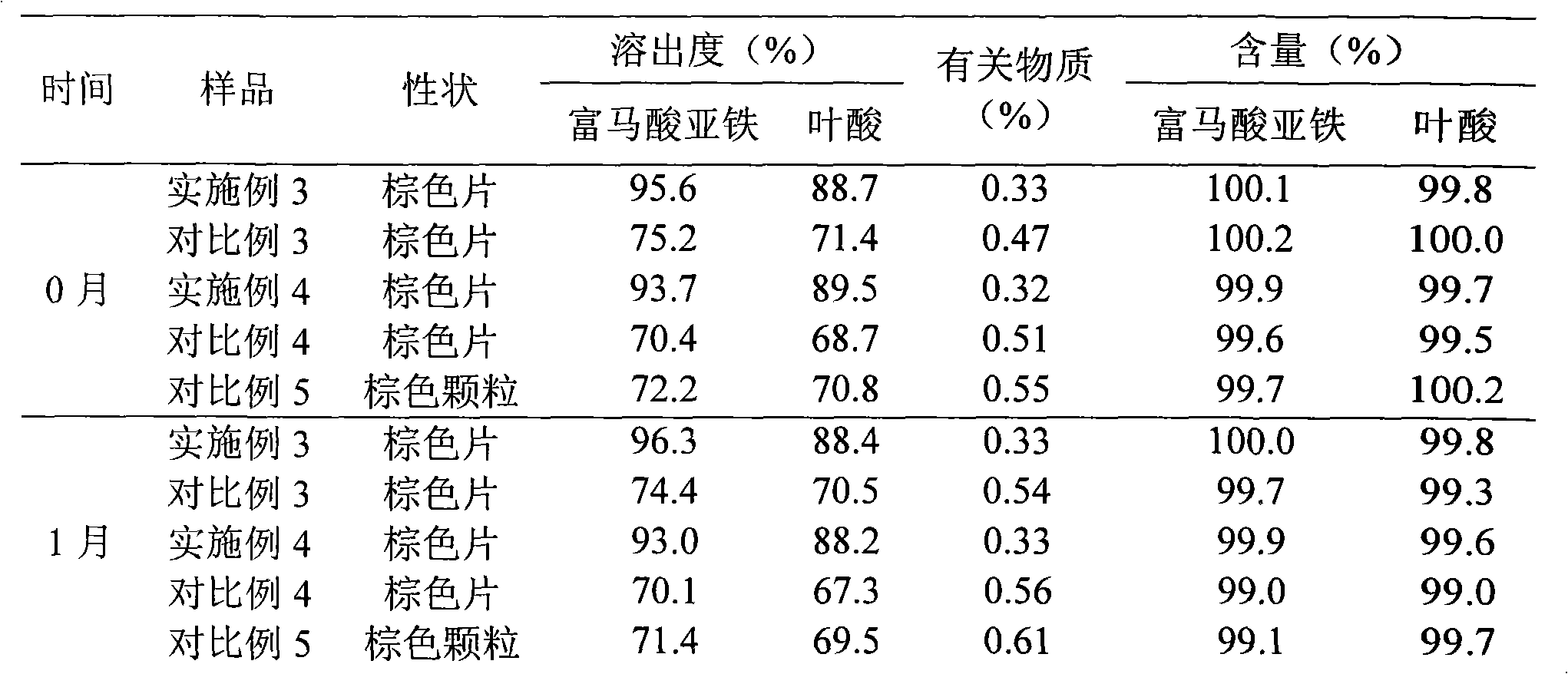
Ferrous fumarate and folic acid pharmaceutical composition and liposome solid preparation thereof
InactiveCN101632675AIron Prevention During PregnancyPrevent Folate DeficiencyOrganic active ingredientsPharmaceutical product form changeAdhesiveLiposome
The invention relates to a ferrous fumarate and folic acid pharmaceutical composition, a liposome solid preparation and a preparation method thereof. The solid preparation contains a liposome of the ferrous fumarate and folic acid pharmaceutical composition, and a pharmaceutically acceptable excipient, and specifically contains 1 part of the liposome of the ferrous fumarate and folic acid pharmaceutical composition, 0.2-1.5 parts of a filling agent, 0.01-0.5 parts of a disintegrant, 0.01-0.2 parts of an adhesive and 0.01-0.3 parts of a lubricant.
Owner:HAINAN YONGTIAN PHARMA INST
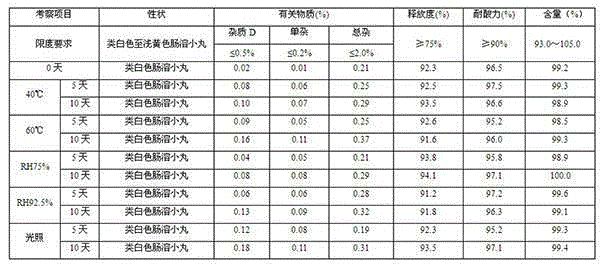
Esomeprazole magnesium enteric capsules and preparation method thereof
ActiveCN105106168AAvoid damageIntegrity guaranteedOrganic active ingredientsDigestive systemActive agentPharmaceutical drug
The invention discloses esomeprazole magnesium enteric capsules and a preparation method thereof. Esomeprazole magnesium is prepared into enteric coated pellets, and the enteric coated pellets are filled into gastric coated capsules. Each enteric coated pellet consists of an empty pellet core, a medicine carrying layer, two isolating layers, an enteric coated layer and a film coating layer. In order to guarantee stability of medicines, alkaline materials are added in the empty pellet cores; alkali stabilizers and antioxidant are added in the medicine carrying layer; and a high-alkalinity modifier and a low-alkalinity modifier are respectively added in the two isolating layers of each enteric coated pellet. In order to guarantee a dissolution effect, surfactant is added in the enteric coated layers; and stability of products is improved owing to the film coating layers wrapping the outer layers of the enteric coated pellets. Owing to optimized formulation and technology of the pellets, smoothness of a technology is improved, work efficiency is also improved, coating time is shortened, consumption of labor and materials is reduced, and production cost is reduced.
Owner:DEZHOU DEYAO PHARMA
Enhanced anti-angiogenic activity of permanently charged derivatives of steroid hormones
InactiveUS6083990AImprove anti-tumor activityPotent anti-angiogenic activityBiocideOrganic compound preparationSerotonin AgonistBULK ACTIVE INGREDIENT
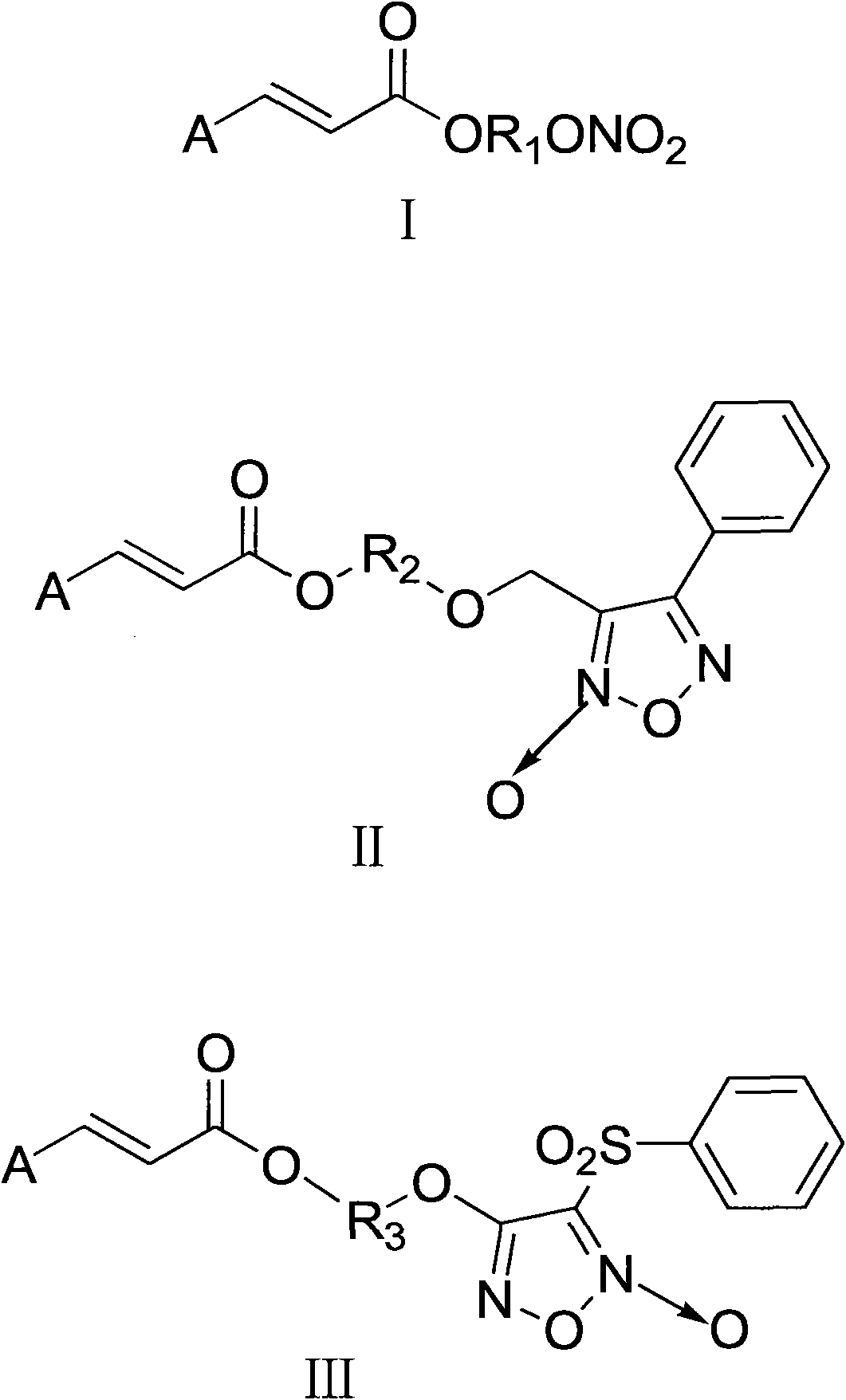
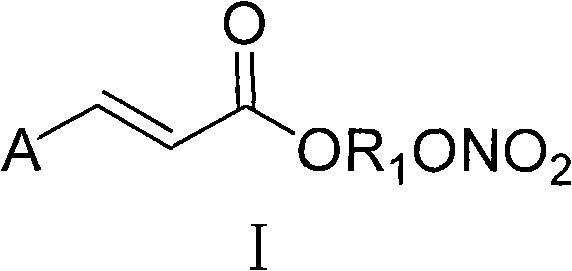
The present invention discloses the use of permanently charged steroid agonists or antagonists as potent anti-angiogenic compositions comprising as an active ingredient a compound of the general formulae I, II or III: wherein DRUG is any steroid agonist or antagonist, a mixed agonist-antagonist, or a partial agonist and the substituents are as defined in the specification.
Owner:PHARMOS
Injectable parenteral medicinal preparation of temozolomide and preparation method thereof
ActiveCN102342931AImprove stabilityEasy to acceptOrganic active ingredientsPowder deliveryVitamin CPharmaceutical formulation
The invention relates to an injectable parenteral medicinal preparation of temozolomide and a preparation method thereof. The medicinal preparation comprises (1) temozolomide or pharmaceutically acceptable salt thereof, (2) at least one stabilizer, and (3) at least one aqueous diluent, wherein the stabilizer is selected from L-alanine, L-glycine, L-cysteine, L-cysteine hydrochloride anhydride, L-cysteine hydrochloride monohydrate, acetyl cysteine, S-carboxymethyl-L-cysteine, L-ethyl cysteine hydrochloride, L-methyl cysteine hydrochloride, vitamin C or a mixture thereof. The invention further relates to lyophilized power containing the medicinal preparation and products thereof.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Chinese herbal compound effective composite for treating dysmenorrhea and preparation method and application thereof
InactiveCN101822762AActive ingredient clearThe ratio is scientific and reasonableOrganic active ingredientsSexual disorderAdditive ingredientGradient elution
The invention discloses a Chinese herbal compound effective composite and a preparation method and application thereof. The effective composite is prepared by carrying out precipitation on Chinese herbal compound water decoction consisting of Chinese angelica, szechuan lovage rhizome, rehmannia glutinosa, white paeony root, nutgrass galingale rhizome, costustoot and rhizoma corydalis by ethanol to remove impurities, then using a macroporous absorption resin column, using ethanol to carry out gradient elution and purification and recovering the solvent of eluent. The invention selects the raw materials to compose prescription compatibly according to the theory of traditional Chinese medicine and the pathogenesis of dysmenorrhea. Pharmacological tests show that the Chinese herbal compound effective composite provided by the invention has good anticoagulation, has effect of preventing formation of blood stasis, has clearer ingredients and lower untoward effect, and is expected to be developed into a novel generation of medicine for treating dysmenorrhea.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Itraconazole oral solution and preparation method thereof
InactiveCN102670490AGood for clinical useImprove Medication AdherenceOrganic active ingredientsAntimycoticsItraconazole Oral SolutionBiological organism
The invention relates to an oral solution of an antifungal medicine, and a preparation method for the oral solution. An itraconazole oral solution consists of slightly soluble bulk pharmaceutical itraconazole, hydroxypropyl-beta-cyclodextrin, preservative and additive. 150 milliliters of the itraconazole oral solution contains 1.0 to 2.0g of itraconazole, 30.0 to 60.0g of hydroxypropyl-beta-cyclodextrin, 30 to 60ml of 1,2-propylene glycol, preservative sorbierite, saccharin sodium and essence additive. The problem of low dissolubility of the itraconazole is solved; a product has the advantages of simple process, low cost, small side effect, convenience in taking, high bioavailability and the like and has an obvious effect on fungus infection; and an oral solution preparation has an obvious curative effect on monilial infection in oral cavity / esophagus.
Owner:NANJING TEFENG PHARMA +2
Compound angelica medicament injection preparation containing polyethylene glycol 12-hydroxystearate and preparation method thereof
InactiveCN101884658AHigh clarityGood for clinical usePowder deliveryAntipyreticPolyethylene glycolHydroxystearic Acid
The invention discloses a compound angelica medicament injection preparation containing polyethylene glycol 12-hydroxystearate and a preparation method thereof. The compound angelica medicament injection preparation is the injection medicament mainly prepared by dissolving an angelica extract, a rhizoma chuanxiong extract, a safflower extract and polyethylene glycol 12-hydroxystearate for improving the clarity of the injection in injection water, wherein the using amount of the polyethylene glycol 12-hydroxystearate is 0.1g-1.0g / 100ml. The compound angelica medicament injection preparation of the invention can improve the clarity of compound angelica injection, stably ensure that the detection for visible foreign matters of the injection complies with the medicament quality standard especially under the condition that the compound angelica injection is preserved for a longer time (over 24 months), solve the problems of small white spots, white blocks and turbidity of the compound angelica medicament injection which adopts the conventional cosolvent (Tween-80) and is preserved for a longer time, and ensure that the detection of the visible foreign matters of the injection complies with the medicament quality standard so as to facilitate clinic administration and popularization.
Owner:SICHUAN SUNNYHOPE PHARM CO LTD
Granular formulation containing cefixime liposomes and preparation method thereof
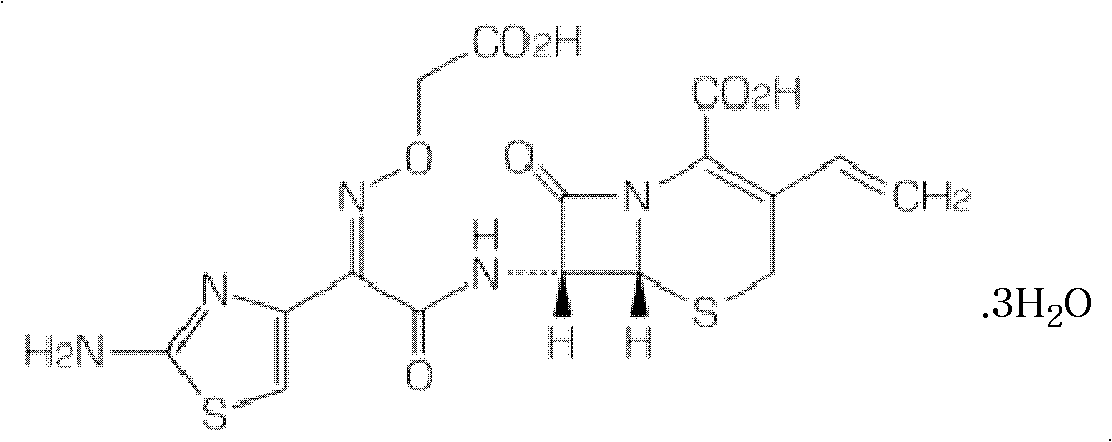
InactiveCN101966154AHigh encapsulation efficiencyReduce leak rateOrganic active ingredientsAntiinfectivesYolkCholesterol
The invention relates to a cefixime liposome, a preparation method and a granular formulation containing the cefixime liposome. The granular formulation comprises the cefixime liposome and a pharmaceutically acceptable vector, wherein the cefixime liposome is prepared from the following components in parts by weight: 1 part of cefixime, 1.25-5 parts of hydrogenated soybean lecithin, 1.25-5 parts of hydrogenated egg yolk lecithin, 2.5-10 parts of cholesterol and 0.1-4.5 parts of polysorbate 80. The granular formulation not only accords with the requirement on Chinese pharmacopoeia, but also has the advantages of stabler storage and rapider drug effect exertion and remarkably improved bioavailability compared with the common pharmaceutical cefixime composition at a room temperature.
Owner:王丽燕
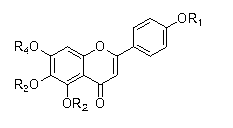
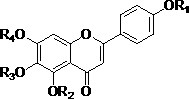
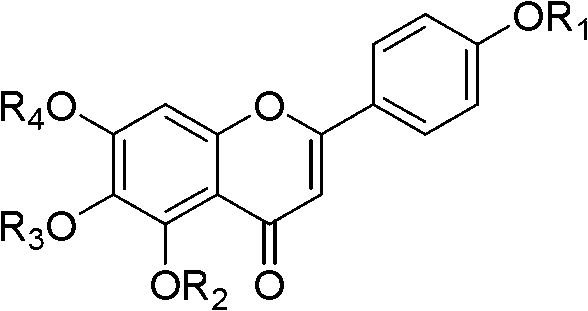
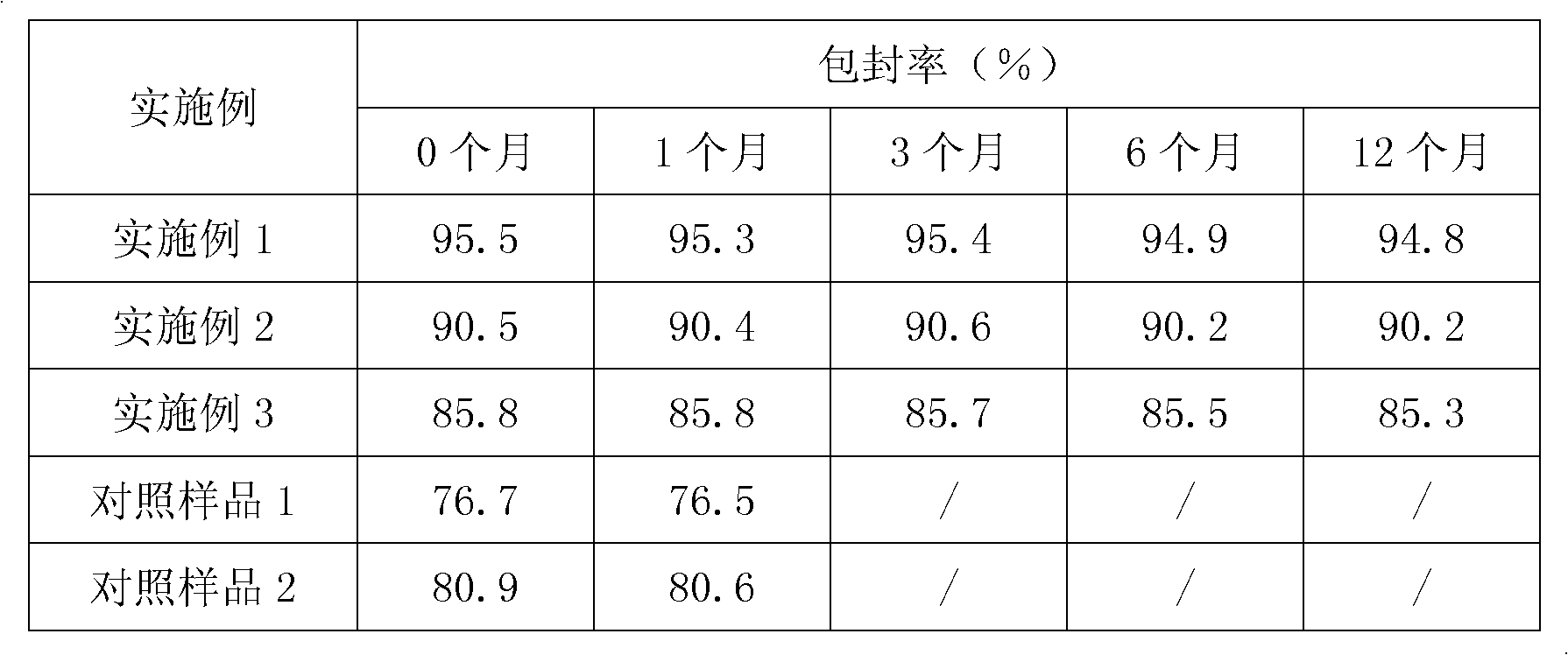
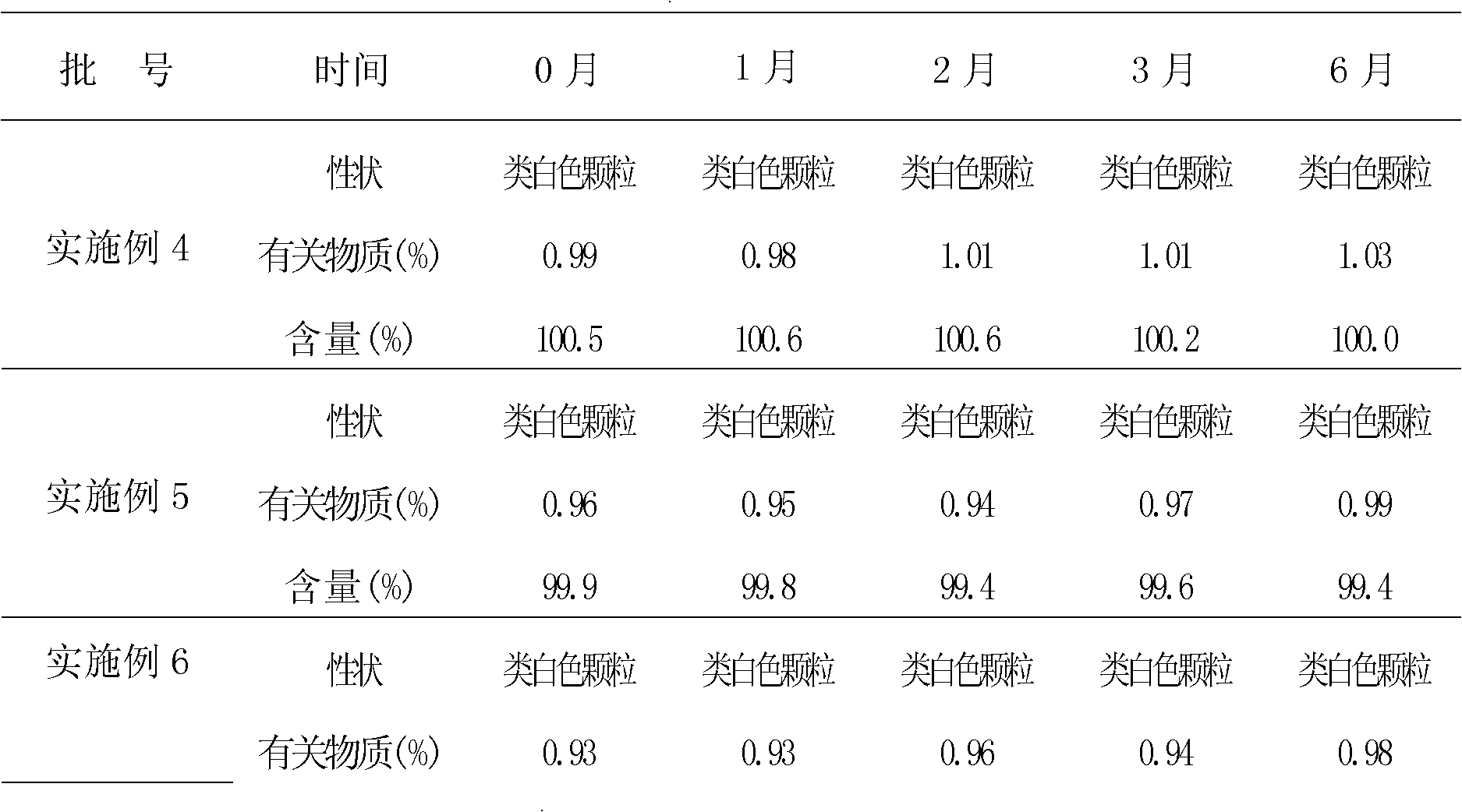
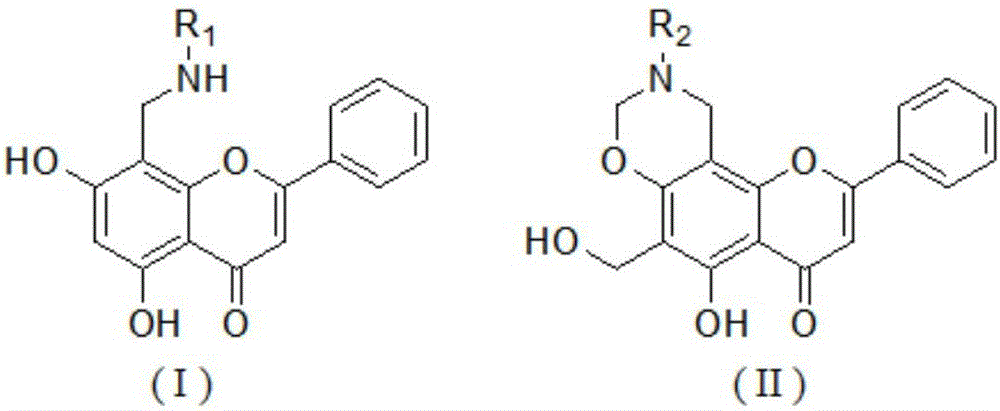
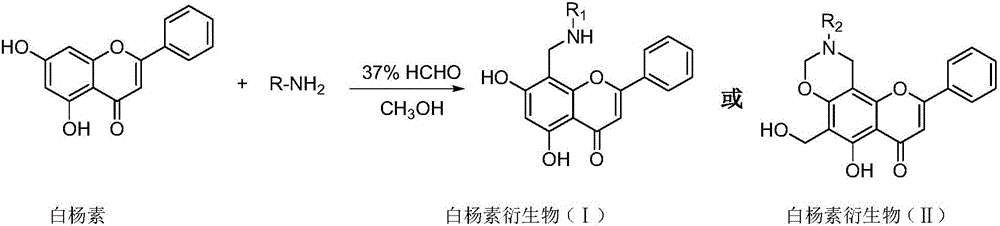
Chrysin derivative and preparation thereof and application thereof in treating hyperuricemia
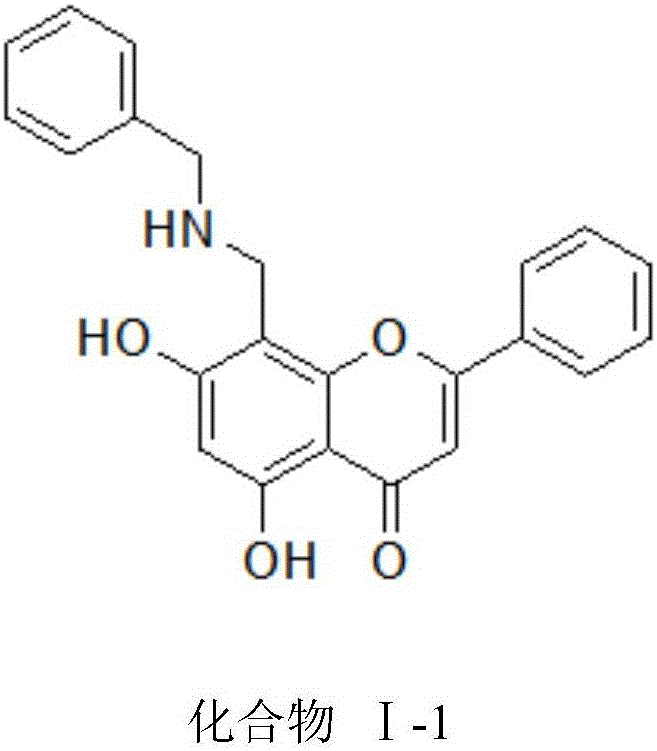
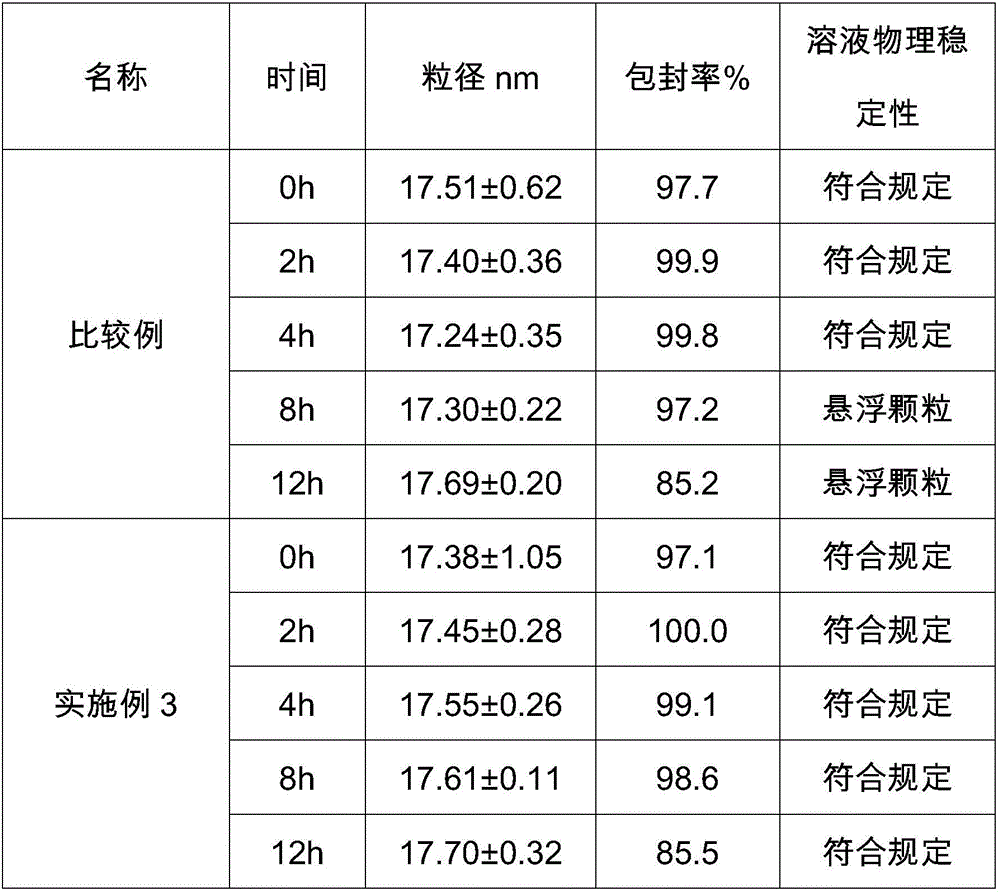
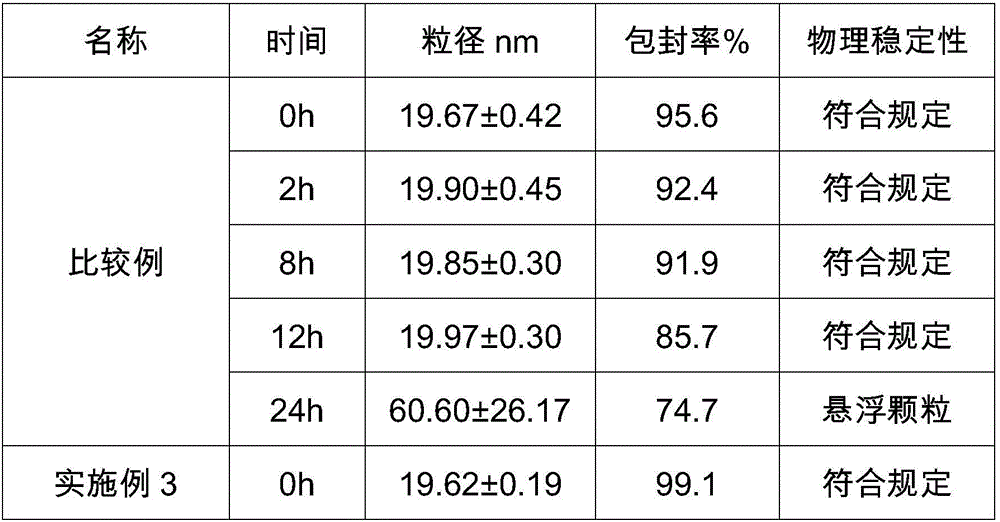
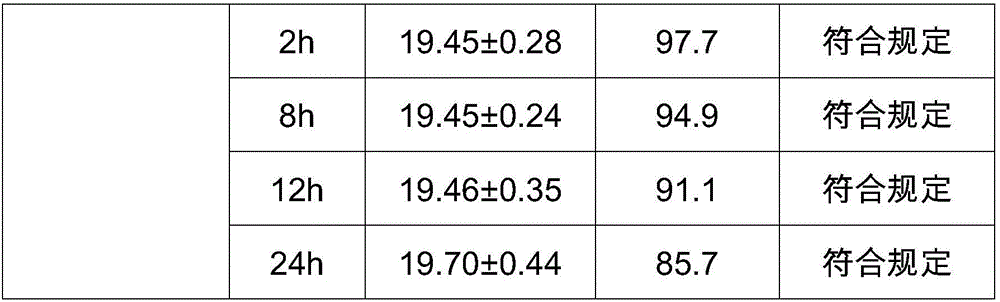
ActiveCN105884735AImprove solubilityGood for clinical useOrganic active ingredientsOrganic chemistryNatural productDrugs synthesis
The invention relates to a chrysin derivative and preparation thereof and application thereof in treating hyperuricemia, and belongs to the field of pharmaceutical synthesis. By using easily-obtained natural product chrysin as a starting material and selectively introducing active functional groups containing fluorine atoms, chrysin derivatives (I) and (II) are synthesized; a synthetic method is simple and is well operable and high in reaction yield, the obtained chrysin derivatives has significantly enhanced anti-hyperuricemia activity when compared to lead compound chrysin and is useful in treating hyperuricemia.
Owner:JILIN ACAD OF TRADITIONAL CHINESE MEDICINE +1
Polymeric micelle freeze-dried preparation of taxane anti-tumor drugs as well as preparation method and application of polymeric micelle freeze-dried preparation
ActiveCN106389355AGood molecular weight uniformityMeet the requirements for intravenous administrationPowder deliveryOrganic active ingredientsPolyesterFreeze-drying
The invention discloses a polymeric micelle freeze-dried preparation of taxane anti-tumor drugs as well as a preparation method and an application of the polymeric micelle freeze-dried preparation. The polymeric micelle freeze-dried preparation consists of a polyether / polyester segmented copolymer and taxane drugs, wherein the weight ratio of the polyether / polyester segmented copolymer to the taxane drugs is at (1-99) to 1; and in the polyether / polyester segmented copolymer, the molecular weight ratio of polyether to polyester is at 1 to (0.5-2). According to the taxane anti-tumor drug polymeric micelle freeze-dried preparation prepared by the invention, the physical stability of a redissolved solution is significantly increased; and meanwhile, the occurrence rate of a guinea pig allergic reaction in the taxane anti-tumor drug polymeric micelle freeze-dried preparation prepared from the polyether / polyester segmented copolymer which is subjected to ultrafiltration and freeze-drying treatment is obviously reduced, so that the safety of the taxane anti-tumor drug polymeric micelle freeze-dried preparation is further enhanced.
Owner:GUANGDONG ZHONGSHENG PHARMA
Ambroxol hydrochloride containing hydroxypropyl beta-cyclodextrin and its preparation
InactiveCN1424026ASolve the problem of water solubilityGood for clinical useAmine active ingredientsRespiratory disorderFreeze-dryingWater soluble
An ambroxol hydrochloride injection containing hydroxypropyl beta-dextrin in the form of "liquid injection", "freeze dried powder injection", "aseptic powder injection", etc is composed of the ambroxol hydrochloride and 2-hydroxypropyl beta-dextrin in Wt ratio of 1:(3-50). Its advantage is high solubility in water, especially in high-pH water.
Owner:SHENYANG PHARMA UNIVERSITY
Composition and preparation for treating novel coronavirus pneumonia and application of composition
ActiveCN111467451AAchieve the purpose of removingSignificant clinical effectAntiviralsRespiratory disorderBiotechnologyRhizome
The present invention relates to a composition for treating novel coronavirus pneumonia. The composition is prepared from the following traditional Chinese medicines in parts by weight: 18-25 parts ofradix bupleuri, 8-15 parts of radix scutellariae, 8-15 parts of rhizoma pinellinae praeparata, 8-15 parts of fructus trichosanthis, 15-20 parts of radix codonopsis pilosulae, 8-15 parts of betel nuts, 15-20 parts of fructus tsaoko, 15-20 parts of officinal magnolia bark, 8-15 parts of rhizoma anemarrhenae, 8-15 parts of radix paeoniae rubra, 8-15 parts of liquorice, 8-15 parts of dried orange peels and 8-15 parts of giant knotweed rhizome. The composition furthest reserves accumulated medicine using experience of traditional Chinese medicine, and is also suitable for modern society, convenient to take and good in taste, widely applied and verified clinically, definite in curative effect and good in treatment effect.
Owner:劲牌持正堂药业有限公司
Chinese medicine compound effective part with effect of treating arthritis
ActiveCN103405487AEffective parts and active ingredients are clearGood anti-inflammatory effectAntipyreticAnalgesicsDiseaseMyrrh
The invention discloses a Chinese medicine compound effective part with an effect of treating arthritis. The effective part is prepared via the steps as follows: 10-50 parts of frankincense and 10-50 parts of myrrh are abstracted via water or alcohol, impurities are removed via alcohol deposition, and the frankincense and the myrrh are placed on a macropore adsorption resin column, and eluted and purified with alcohol gradient with optimized concentrate. The effective part is refined and prepared via modern analytical apparatuses and pharmacological experiments section and according to Chinese medicine theories, the pathogenesis of rheumatic and rheumatoid arthritis. Shown by the experiment result, the Chinese medicine compound effective part provided by the invention has the good effects of antiphlogosis, pain easing, immunization regulation and the like, can be used for treating rheumatic and rheumatoid arthritis, proliferative arthritis, and inflammation pain diseases, is clear in ingredient, low in clinical dosage and lower in adverse reaction, and possibly can be developed as a new generation of medicine for treating the arthritis.
Owner:南京百草和康医药科技有限公司
Sauchinone derivative and preparing method and application thereof
InactiveCN103073560AIncrease biosolubilityImprove bioavailabilityOrganic chemistryDigestive systemDrugChemistry
The invention relates to the field of chemical research of drugs, and particularly discloses a sauchinone derivative and a preparing method and an application thereof. A ketonic group is selectively reduced into a free hydroxyl group, or the hydroxyl group is esterified, so the biological solubility is improved, the biological utilization degree after the sauchinone derivative is taken by people is improved, the pharmacological activity is improved, the adverse reaction is low, the safety in taking of the sauchinone derivative is realized, the sauchinone derivative can be prepared into drug preparations of multiple types, and the convenience in clinical medication is realized. The preparing method provided by the invention has the advantages that the operability is high, the production efficiency is high, the cost is low, and the purity is high. After being proved by experiment results, the sauchinone derivative provided by the invention has the advantages that the solubility is good, the biological utilization degree is higher, the hepatic injury-resistance efficiency is better, and the sauchinone derivative can be developed into new drugs for preventing and controlling hepatic diseases.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com