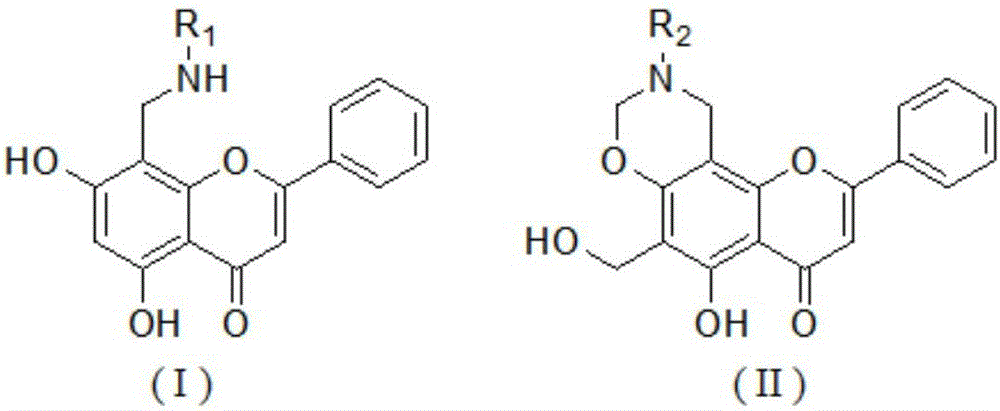
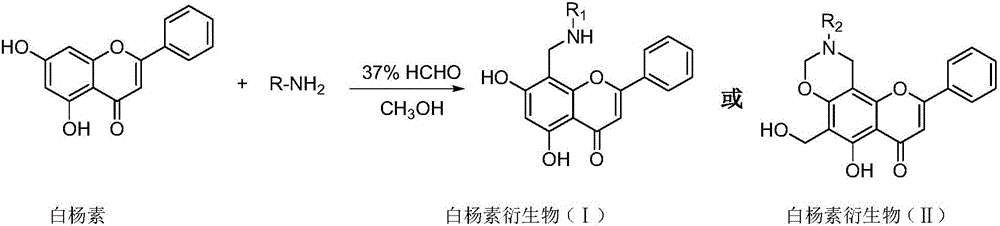
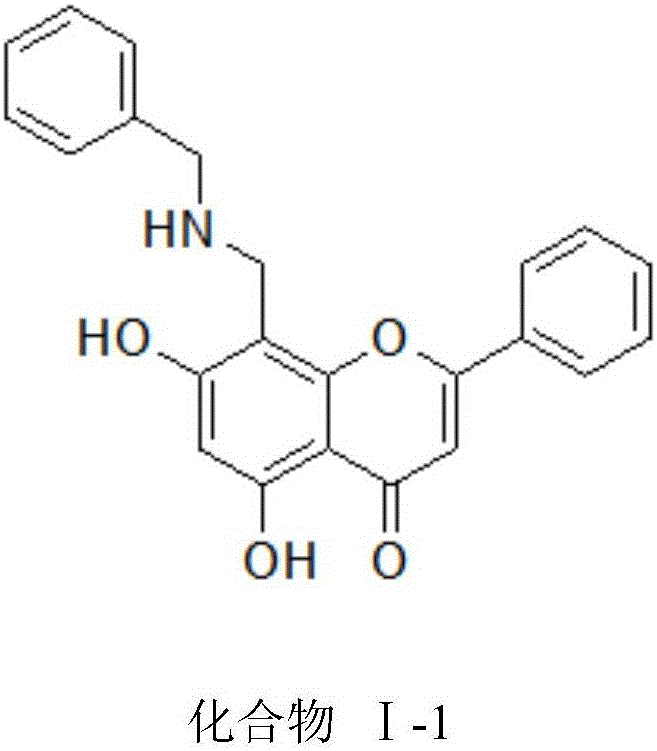
Chrysin derivative and preparation thereof and application thereof in treating hyperuricemia
A technology for hyperuricemia and chrysin, which is applied in the directions of drug combinations, medical preparations containing active ingredients, organic active ingredients, etc., can solve problems such as no reports of chrysin derivatives, and achieve convenient clinical medication and good response. Yield, good operability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation of embodiment 1 compound I-1
[0024] Take 0.254g of chrysin (1mmol) in a reaction flask, add dimethylformamide DMF to dissolve it, 10ml, then add 480ul (5.9mmol) of 37% formaldehyde solution, stir vigorously at 65°C for 30min, then add 160ul of benzylamine (1.5mmol), the reaction solution was stirred at this temperature for 3h and the reaction was completed. The reaction solution was evaporated to dryness under reduced pressure, the residue was dissolved in water, and 5% sodium hydroxide solution was added to adjust the pH value to 9, extracted with ethyl acetate, and combined Ethyl acetate solution was dehydrated with anhydrous MgSO4, filtered, evaporated to dryness under reduced pressure, and subjected to silica gel column chromatography to obtain compound Ⅰ-1.
[0025] Compound Ⅰ-1 5,7-dihydroxy-2-phenyl-8-((benzylamino)methyl)-4-H-benzopyran-4-one
[0026] Light yellow crystals, yield: 58.81%; 1 H-NMR (CDCl 3 ,300MHz)3.95(s,2H,Ar-CH 2 -NH),4.21(...
Embodiment 2
[0028] The preparation of embodiment 2 compound Ⅰ-2
[0029] Take 0.254g chrysin (1mmol) in a reaction flask, add dimethylformamide DMF to dissolve it, 10ml, then add 480ul (5.9mmol) of 37% formaldehyde solution, stir vigorously at 65°C for 30min, then add 2-fluoro Aniline 166ul (1.5mmol), the reaction solution was stirred at this temperature for 2.5h after the reaction was completed, the reaction solution was evaporated to dryness under reduced pressure, the residue was dissolved in water, and 5% sodium hydroxide solution was added to adjust the pH value to 9, ethyl acetate Extract, combine ethyl acetate solution, dehydrate with anhydrous MgSO4, filter, evaporate to dryness under reduced pressure, and go through silica gel column chromatography to obtain compound Ⅰ-2.
[0030] Compound Ⅰ-2 8-((2-fluoroanilino)methyl)-5,7-dihydroxy-2-phenyl-4H-1-benzopyran-4-one
[0031] Light yellow crystals, yield: 47.22%; 1 H-NMR (CDCl 3 ,300MHz)δ:4.59(s,2H,Ar-CH 2 -NH),6.47(s,1H,=C(OH)...
Embodiment 3
[0033] The preparation of embodiment 3 compound Ⅰ-3
[0034]Take 0.254g chrysin (1mmol) in a reaction flask, add dimethylformamide DMF to dissolve it, 10ml, then add 480ul (5.9mmol) of 37% formaldehyde solution, stir vigorously at 65°C for 30min, then add 3-fluoro Aniline 166 (1.5 mmol), the reaction solution was stirred at this temperature for 1.5 h and the reaction was complete. The reaction solution was evaporated to dryness under reduced pressure, the residue was dissolved in water, the pH value was adjusted to 9 by adding 5% sodium hydroxide solution, extracted with ethyl acetate, the ethyl acetate solution was combined, dehydrated with anhydrous MgSO4, filtered, evaporated to dryness under reduced pressure, and purified by silica gel Column chromatography yielded compound Ⅰ-3.
[0035] Compound Ⅰ-3 8-((3-fluoroanilino)methyl)-5,7-dihydroxy-2-phenyl-4H-1-benzopyran-4-one
[0036] Light yellow crystals, yield: 49.78%; 1 H-NMR (CDCl 3 ,300MHz)δ:4.64(s,2H,Ar-CH 2 -NH),6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com