Mixed glue bundle pharmaceutical preparations produced in combination use of multiple surfactant and processes for their preparation
A surfactant, mixed micelle technology, applied in the field of medicine, can solve the problems of unsafe and inconvenient clinical use, hemolysis and allergic reactions, large release of histamine, etc., to achieve convenient clinical medication, strong dilution stability, Toxicity reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Solubilization of docetaxel by HS15-EPC mixed micelles
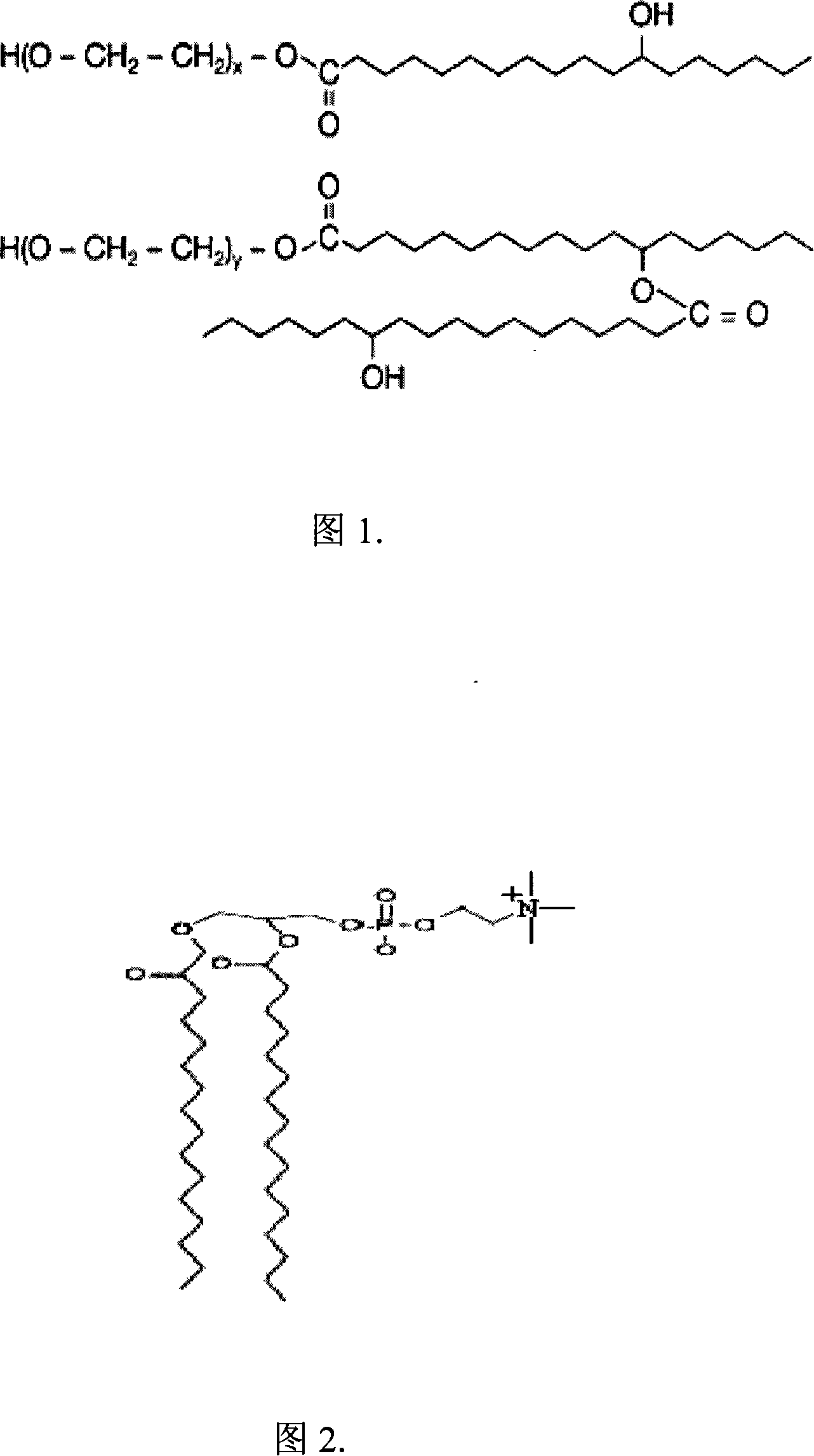
[0043] HS15 (see Figure 1 for structure) and phospholipids form mixed micelles. The main component of lecithin is phosphatidylcholine (PC, the structure is shown in Figure 2). The two acyl chains of PC increase the hydrophobicity inside the micelles, which can increase the affinity for fat-soluble drugs, and its flexibility is good for accommodating drug molecules and forming a stable combination that favors van der Waals forces. In addition, the intervention of PC makes the structure of mixed micelles slightly swell, and the volume is larger than simple micelles, which can hold more fat-soluble drug molecules [Chen Jing, Tu Xide, Huang Feiyun, etc. New adjuvant for injections- --Bile salt / lecithin mixed micellar system. Progress in Pharmacy, 2001, 25(4): 227-230].
[0044] Prescription: 200ml
[0045] HS15 30g
[0046] EPC 7g
[0047] Doc 1g
[0048] Cit a 0.5g
[0049] Preparation: Precisely weigh the pres...
Embodiment 2
[0058] Example 2 Solubilization of docetaxel by HS15-S100 mixed micelles
[0059] Prescription: 200ml
[0060] HS15 30g
[0061] S100 7g
[0062] Doc 1g
[0063] Cit a 0.25g
[0064] Preparation: Replace EPC with S100, and the rest is the same as in Example 1.
[0065] Stability: The stability of the prescription and its dilution (the drug concentration is 1mg / ml) is shown in Table 3-4.
[0066] Table 3 Preparation stability test results (room temperature crystallization situation)
[0067] 0 o'clock
[0068] Table 4 Dilution stability test results (crystallizing at room temperature)
[0069] 2 hours
[0070] Conclusion: The mixed micelle preparation has good stability, and no crystals are observed within 3 months; the difference between the dilution with normal saline and glucose injection is large, and the glucose injection dilution group has partial analysis of crystals after 16 hours, while the normal saline In the dilution group, no crystals were seen...
Embodiment 3
[0071] Example 3 Solubilization of docetaxel by HS15-SPC97 mixed micelles
[0072] Prescription: 200ml
[0073] HS15 30g
[0074] SPC97 10g
[0075] Doc 1g
[0076] Cit a 0.05g
[0077] Preparation: Replace EPC with SPC97, adjust the dosage appropriately according to the prescription ratio, and the rest is the same as in Example 1.
[0078] Stability: See Table 5-6 for the results of the prescription and its stability after dilution.
[0079] Table 5 Preparation stability test results (room temperature crystallization situation)
[0080] 1 hour
[0081] Table.6 Crystallization time after dilution of the formula (room temperature crystallization situation)
[0082] 2 hours
[0083] Conclusion: The mixed micelle preparation has good stability, and no crystals are observed within one month; no crystals are seen 58 hours after dilution, which is beneficial for clinical administration. Its particle size is less than 100nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com