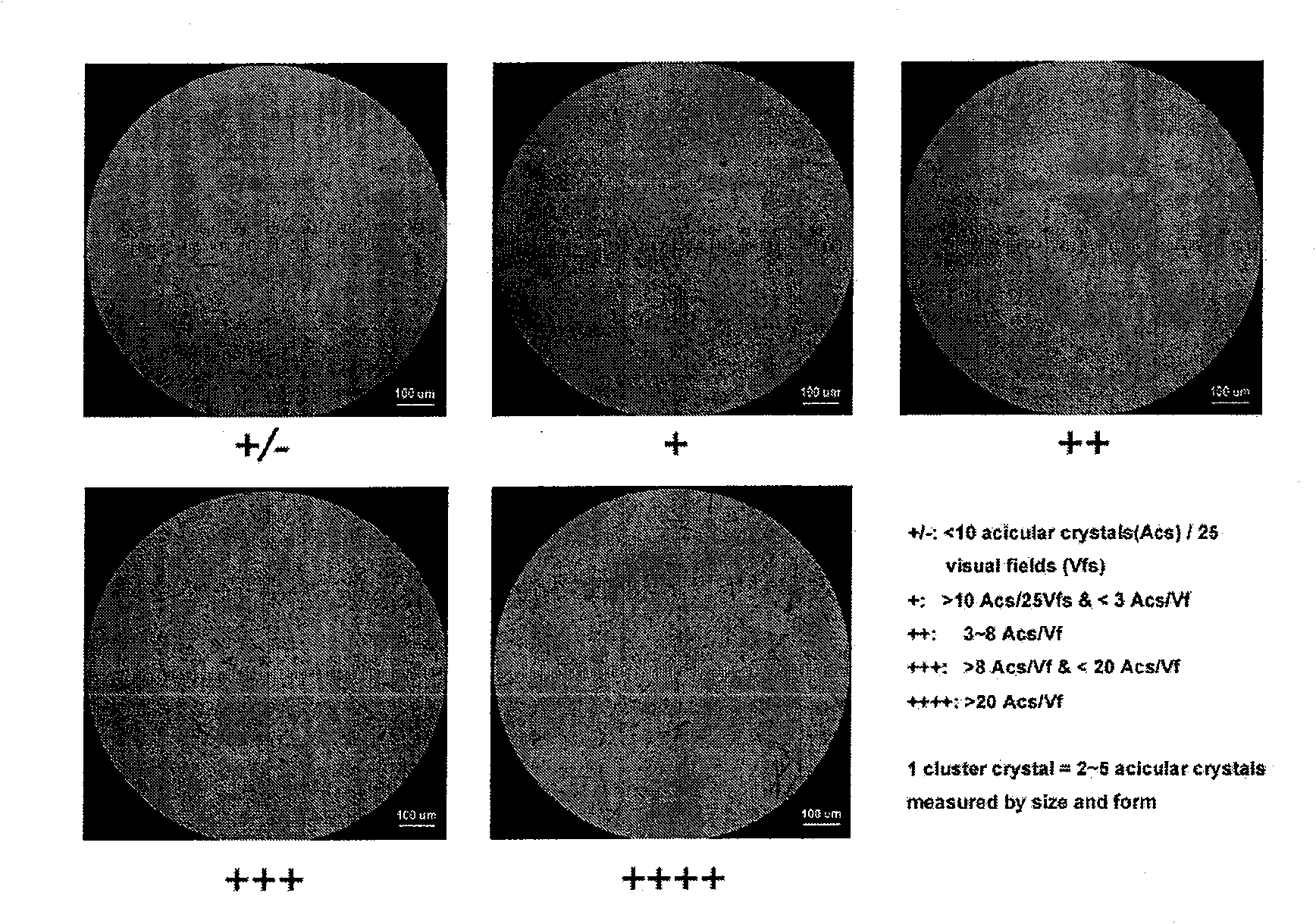
Patents
Literature
327 results about "Glucose Injection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Glucose Injection 5%, 10%, 25%, 50% and 70% Infusion solution. DESCRIPTION. The active ingredient is Glucose (monohydrate) in water for injection. The chemical name of glucose is D-(+) glucopyranose. Molecular formula of glucose is C6H12O6.
Tanshinon IIA sulfonic acid sodium glucose injection liquid and its preparing method
InactiveCN1640391ASolve discolorationFix instabilityOrganic active ingredientsPharmaceutical delivery mechanismAntioxidantTherapeutic effect
The persent invention relates to a sodium tanshinon IIA sulfonate glucose injection and its preparation method. Said injection contains (by wt%) 0.002-0.016% of sodium tanshinon IIA sulfonate, 5-25% of glucose, 0.05-0.2% of antioxidant and the rest is injection water. Said injection can obtain good therapeutic effect, and its preparation method is simple.
Owner:昆明希捷医药研发有限公司
Taxanes medicine preparation for intravenous injection and preparation method thereof
ActiveCN101288642AGood biocompatibilityHigh tolerance in vivoOrganic active ingredientsSolution deliveryDrugs solutionDocetaxel
The invention relates to the technical field of medicine, which is a preparation of a taxane drug for intravenous drug delivery, consisting of two parts of drug solution and an emulsion. The drug solution is composed of paclitaxel or docetaxel, a pH regulator and a solvent for injection, wherein, the solvent for injection is an organic solvent; the emulsion comprises a fat emulsion and is composed of oil for injection, an emulsifier, an antioxidant, an isotonic regulator, a stabilizer, a pH regulator and water for injection. When in use, the drug solution can be added and evenly mixed in the emulsion for direct intravenous drip according to the clinical drug dosage and can also be firstly added in the emulsion with the volume that is not less than 5 times of the volume of the drug solution according to the clinical drug dosage and then added with a certain amount of physiological saline or glucose injection for intravenous drip. The preparation of the invention does not contain solubilizer and has the advantages of little toxicity, safety, effectivity, stability and economy. The fat emulsion can also be taken as a nutrition replenisher for a patient, thus achieving better treatment effects. The physiological saline or the glucose injection can also replace a certain amount of the emulsion, so the storage and the transportation are convenient, and the preparation is more economical.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
Injection composition of fat milk, amino acid and glucose packed with three-cavity bag and preparing method thereof
ActiveCN101015501AAvoid contamination riskEasy to useOrganic active ingredientsMetabolism disorderMicroorganismParticulate pollution
The invention discloses an injection composition packaged in a three-cavity bag. The three cavities separated by diaphragm are respectively filled with fattiness milk injection, compound amino acid injection, and glucose injection. The using method is crimping each cavity in turns, or divulging the isolating bar in each cavity to mix the injections for venoclysis so as to avoid the venture of microorganism and particulates pollution in traditional mix processing. The mix processing can be carried out under any environmental condition which qualifies the small hospital without aseptic preparation condition to use the nutrition, highly reduce the untoward reaction such as transfusion heater response, sanguis infection, and blood poisoning due to traditional mix processing.
Owner:费森尤斯卡比华瑞制药有限公司
Medicinal compound of fat-soluble vitamin injection (II) and water-soluble vitamin for injection and preparation method thereof
ActiveCN102429864AImprove securityConvenient sourcePowder deliveryHydroxy compound active ingredientsTreatment effectWater-Soluble Vitamin
The invention relates to a medicinal compound of fat-soluble vitamin injection (II) and a water-soluble vitamin for injection, in particular to a combined application package. The medicinal compound comprises the fat-soluble vitamin injection (II) and the water-soluble vitamin for the injection. When in use, the medicinal compound is dissolved or thinned by using water for the injection or glucose injection liquid first, and then is respectively instilled in a venous way. By using the combined application package provided by the invention, the vitamins needed by a human body are comprehensively replenished; the defects caused by the usage of a single vitamin are avoided; and the treatment effect is improved greatly.
Owner:HAINAN LINGKANG PHARMA CO LTD
Bicyclo-ethanol submicron emulsion and preparation method thereof
ActiveCN101524329AImprove solubilityGood chemical stabilityDigestive systemEmulsion deliveryOrganosolvOil phase
The invention discloses a bicyclo-ethanol submicron emulsion and a preparation method thereof. the preparation method comprises the steps of dissolving bicyclo-ethanol and emulsifying agent in an oil phase, adding an assistant for emulsifying agent, a stabilizing agent, other additive and a water phase, adopting a cutting dispersing and high-pressure homogeneous emulsification process to prepare an oil-in-water(O / W) submicron emulsion with the average grain diameter below 500 nm and drug loading dosage between 0.01mg / ml and 5mg / ml. The bicyclo-ethanol submicron emulsion is injected through vein and used for treating medium and serious hepatitis. The prepared submicron emulsion does not contain solubilizer such as Tween-80 or organic solvent, can be mixed with glucose injection, physiological saline or distilled water according to random proportion, and can not easily generate insoluble particulates when stored, used or matched with other components, thereby having high security and good stability. The invention also relates to various preparations of the bicyclo-ethanol submicron emulsion.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI +1
Self-emulsifying preparation of taxane compound and preparation method thereof
ActiveCN101829052ASimple production processEase of industrial productionOrganic active ingredientsEmulsion deliveryPhospholipidSurface-active agents
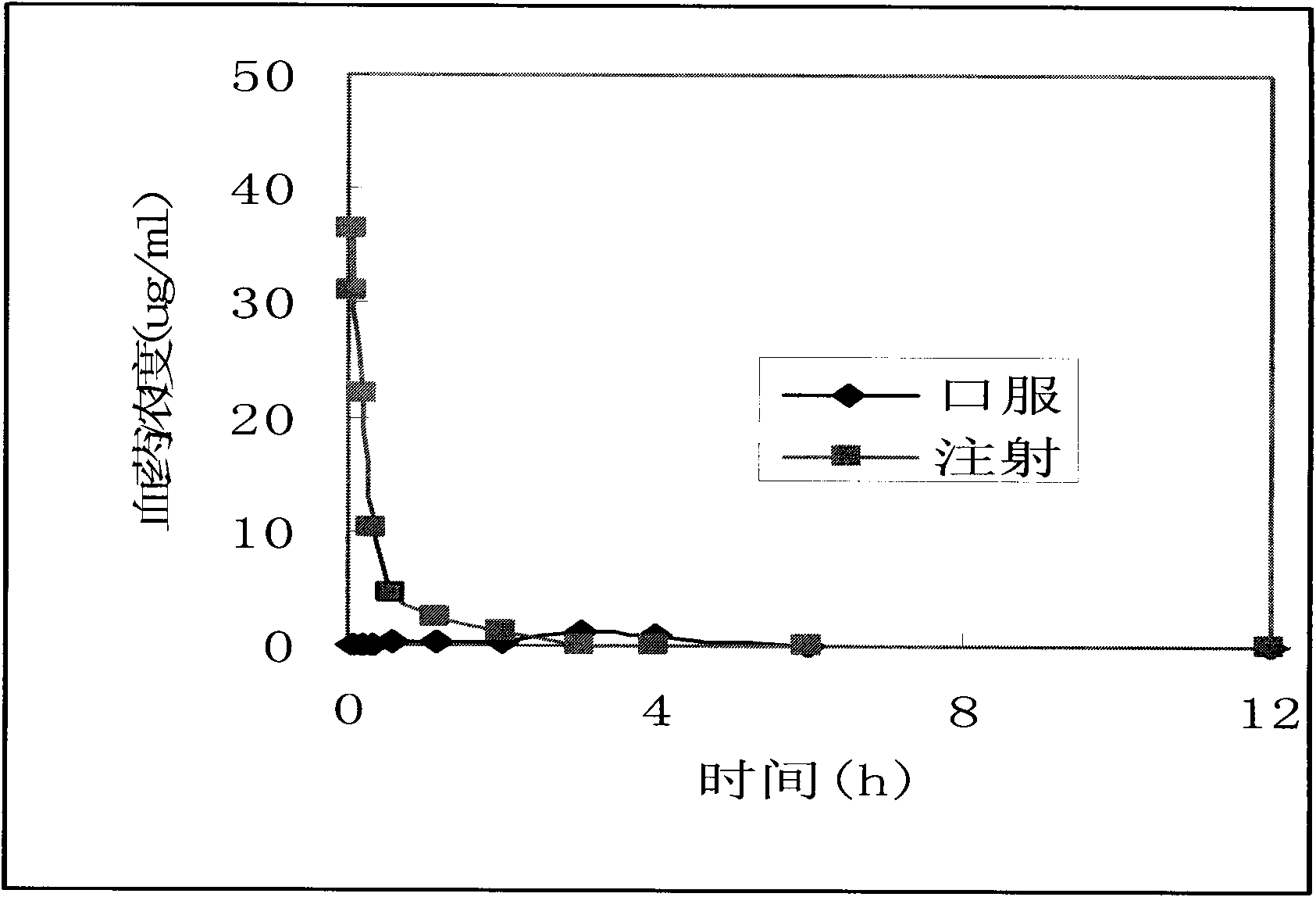
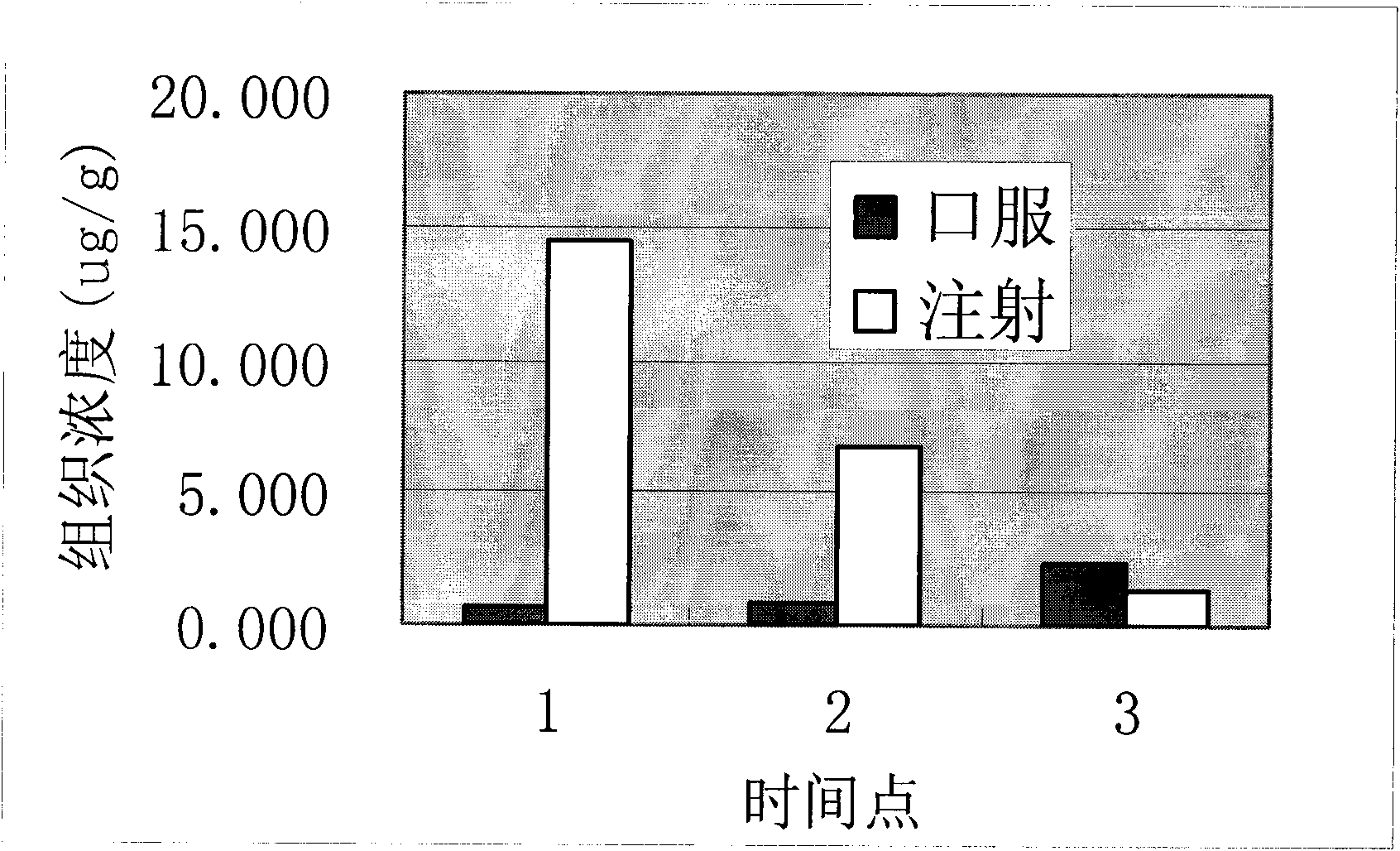
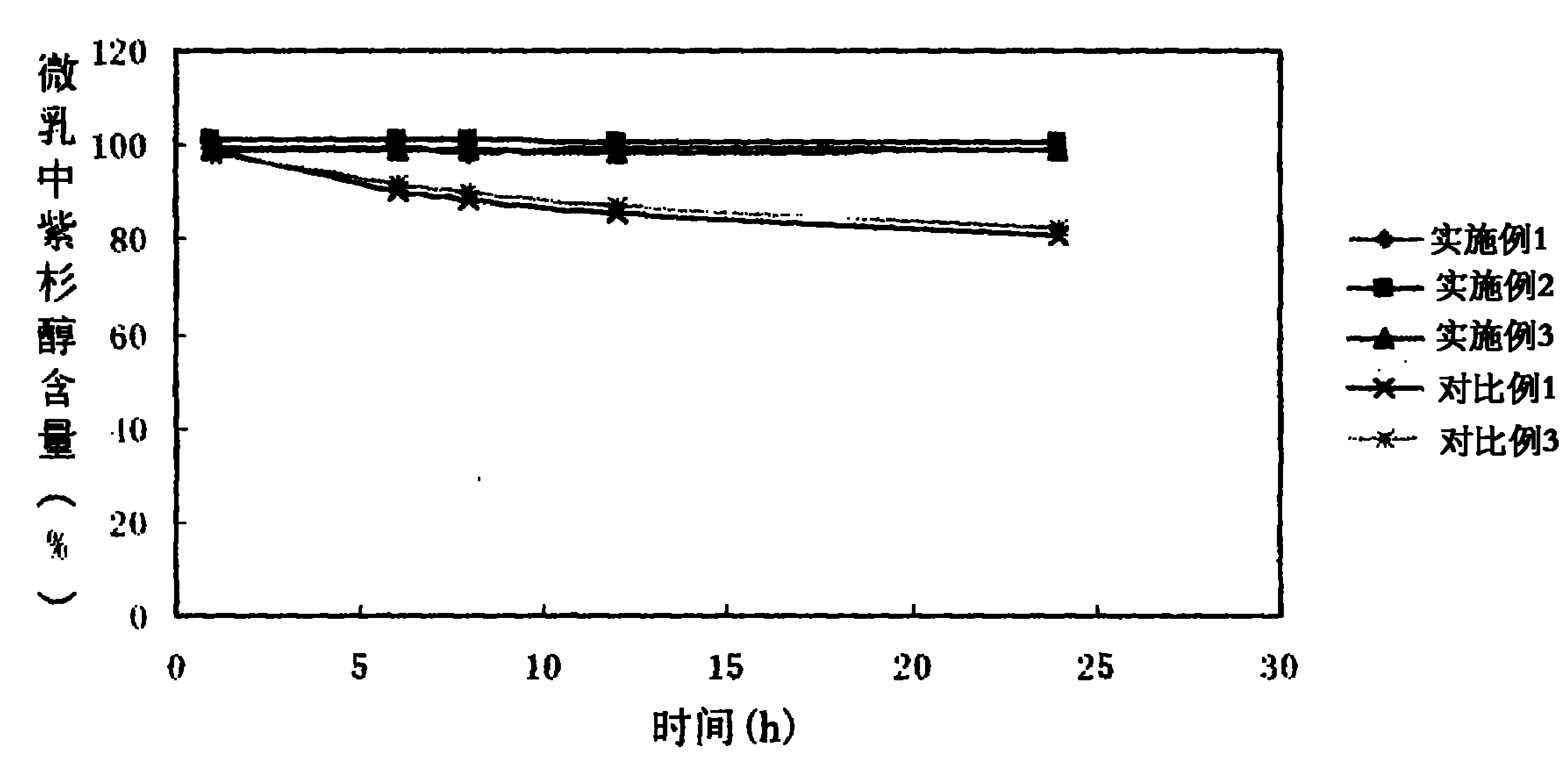
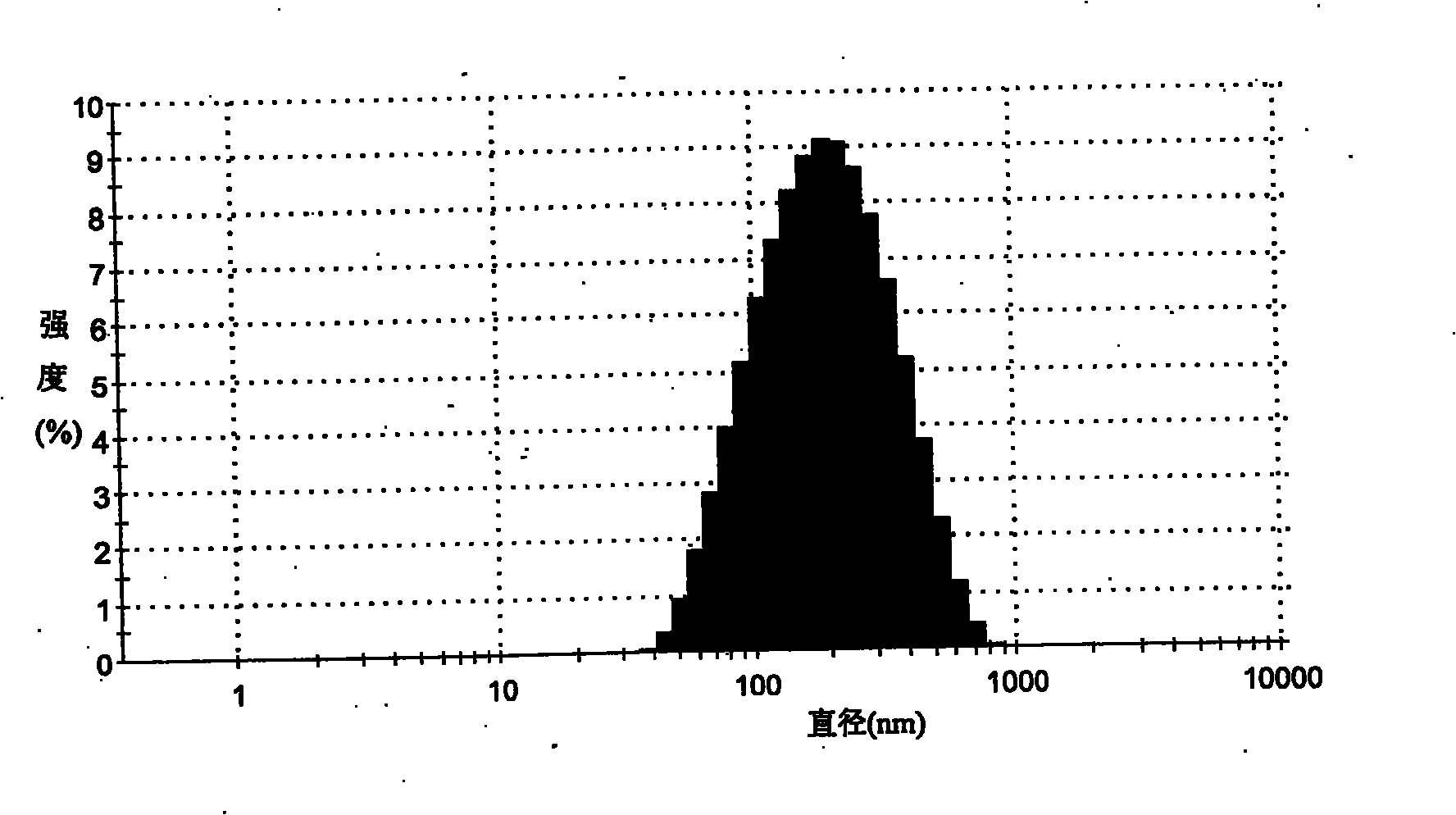
The invention provides a self-emulsifying medical composition for delivering taxane medicaments and a preparation method thereof. The self-emulsifying medical composition is transparent liquid, comprises an effective dose of taxane compound, an oil component, a surface active agent and alcohol and preferentially comprises lactic acid, wherein the surface active agent consists of phospholipid and polyoxyethylenated castor oil. After the self-emulsifying composition is added to a glucose injection, the composition can self-emulsify into micro emulsion the mean grain size of which is 50-300nm, and the micro emulsion can stabilize for more than 12 hours at room temperature. The preferential taxane compound in the invention is taxol.
Owner:YINGU PHARMA
Ibuprofen injection and preparation method thereof
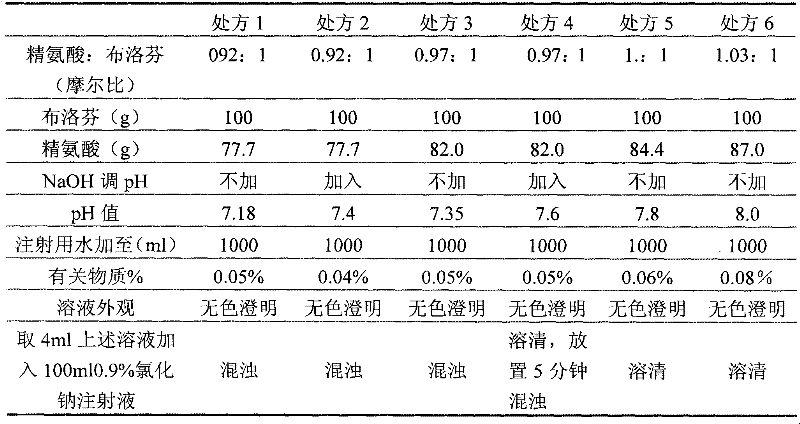
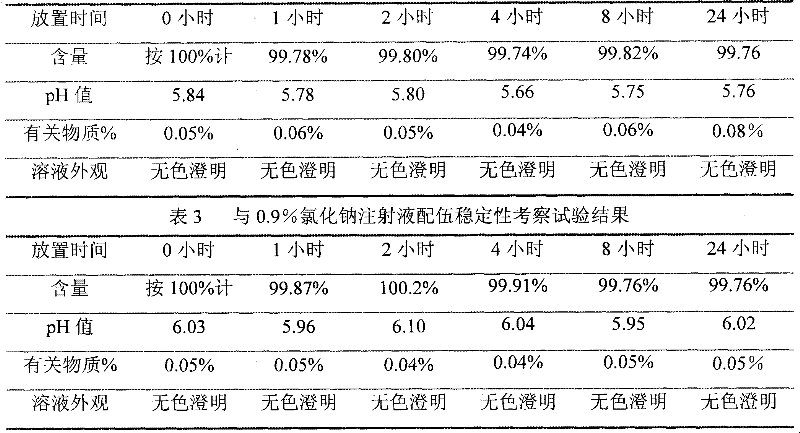
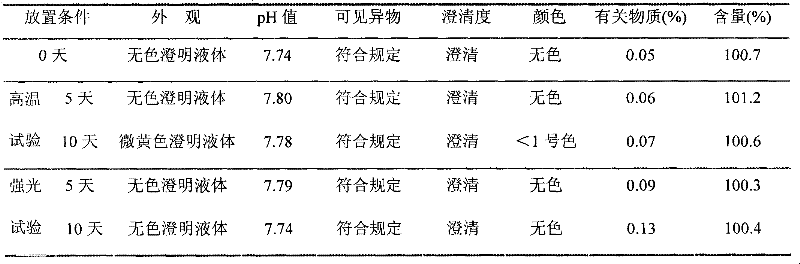
The invention relates to an ibuprofen injection and a preparation method thereof. The ibuprofen injection is characterized in that the concentration of ibuprofen in the injection is 100mg / ml, arginine is used as a cosolvent, and the molar ratio of the arginine to the ibuprofen is greater than or equal to 1:1. The invention also provides a method for preparing the injection. The prepared injection which has good dissolvability is added into a 0.9% sodium chloride injection or a 5% glucose injection, the concentration of the ibuprofen is 4mg / ml or less than 4mg / ml, and the solution is clear and is stabilized for a long time. The ibuprofen injection is suitable for intravenous drip for patients for whom the oral administration is not suitable.
Owner:付正香
Vinpocetine containing high-capacity sodium chloride injection and preparation method thereof
InactiveCN101991530AImprove bioavailabilityQuality improvementOrganic active ingredientsNervous disorderVinpocetineSodium Chloride Injection
The invention provides a vinpocetin containing high-capacity sodium chloride injection and a preparation method thereof, aiming at solving the problems that vinpocetin is mainly applied to middle aged and elderly patients, high-capacity glucose injection contains massive glucose and is not beneficial to treating patients suffering from diabetes at the same time. The invention has the key point that the injection is mainly composed of vinpocetin, osmotic pressure regulator, cosolvent and pH value regulator. The vinpocetin high-capacity sodium chloride injection of the invention has high bioavailability and stable quality, is convenient to use and is applicable to treatment of patients suffering from neurologic disease caused by cerebral circulation restriction, especially the patients suffering from diabetes at the same time.
Owner:SHENYANG ZHIYING PHARMA FACTORY
Separately packed fatty milk, amino acid and glucose injection composition and the prepn process
InactiveCN101019822AEasy to synthesizeScientific and reasonable clinical useMetabolism disorderPharmaceutical delivery mechanismAmino Acid InjectionMedical prescription
The present invention is one kind of three-cavity packed injection composition, and has medium / long chain fatty milk injection, compound amino acid injection and glucose injection packed separately in three diaphragm separated cavities. Before intravenous infusion, the medium / long chain fatty milk injection, the compound amino acid injection and the glucose injection are squeezed out and mixed in common operation condition. The injection composition can be used to patient for meeting the requirement in protein and saccharide.
Owner:费森尤斯卡比华瑞制药有限公司
Separately packed structural fatty milk, aminoacid and glucose injection composition and the prepn process
ActiveCN101019823AMeet metabolic needsAvoid Metabolic AcidosisMetabolism disorderPharmaceutical delivery mechanismAmino Acid InjectionMedical prescription
The present invention is one kind of three-cavity packed injection composition, and has structural fatty milk injection, compound amino acid injection and glucose injection packed separately in three diaphragm separated cavities. Before intravenous infusion, the structural fatty milk injection, the compound amino acid injection and the glucose injection are squeezed out and mixed in common operation condition. The injection composition can be used to patient for meeting the requirement in protein and saccharide.
Owner:费森尤斯卡比华瑞制药有限公司
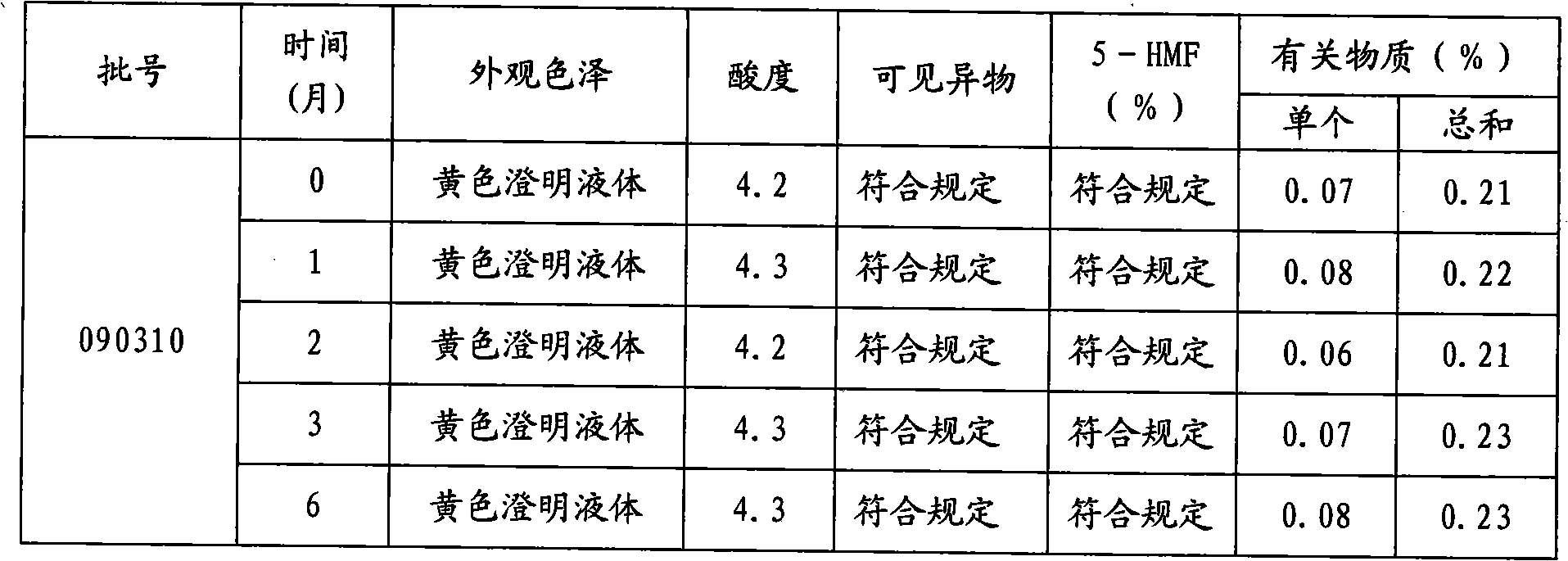
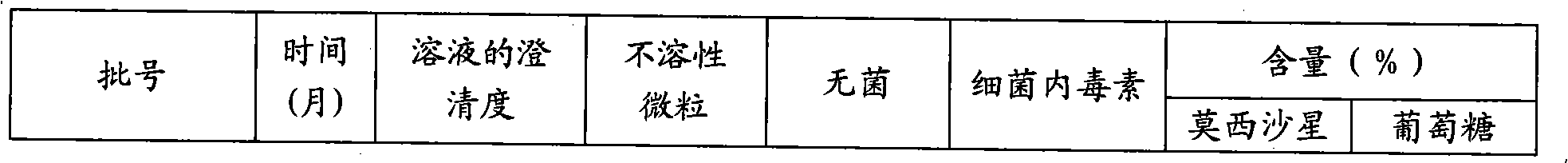
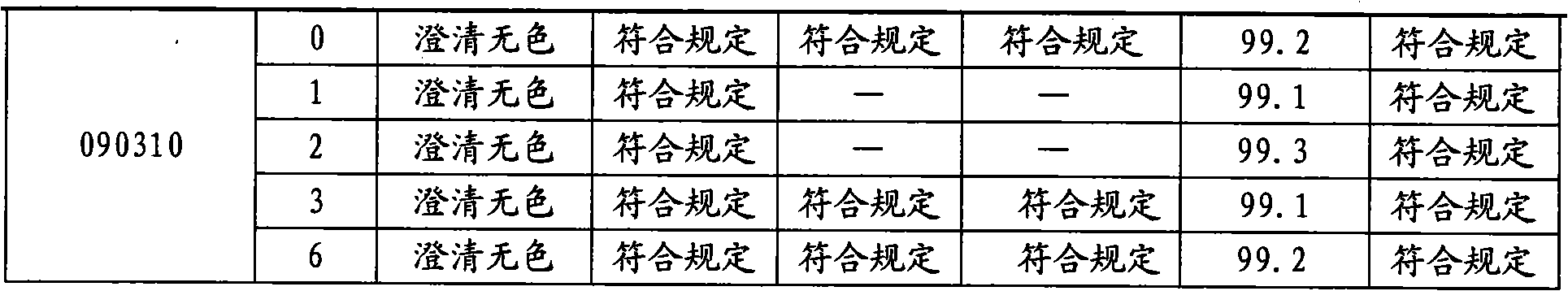
Moxifloxacin hydrochloride glucose injection and preparation method and use thereof
InactiveCN101836950APrecipitation does not occurReduce solubilityAntibacterial agentsOrganic active ingredientsAdditive ingredientMoxifloxacin hydrochloride
The invention provides moxifloxacin hydrochloride glucose injection and a preparation method and use thereof. The method for preparing the injection comprises the following steps of: adding water for injection accounting for 20 to 98 percent of the batch volume into an ingredient tank, and adding glucose, a metal complexing agent and the moxifloxacin hydrochloride in a ratio; after stirring to fully dissolve the components, regulating the pH value to between 4.0 and 4.5 by using 1mol / L hydrochloric acid solution or 1mol / L sodium hydroxide, adding medicinal carbon accounting for 0.05 percent (W / V) of the total volume, uniformly stirring, maintaining the temperature of between 70 and 80 DEG C for 20 minutes, and performing circular filtering for over 20 minutes; replenishing the water for injection to the batch scale, stirring for 5 to 10 minutes, and detecting the pH value of the prepared solution (controlling to between 4.0 and 4.5); after determining that no residual water is present in an elevated tank and a pipeline, opening a valve of the elevated tank, and sampling liquid medicament at a self-circulation pipeline sampling port after the liquid medicament circulates for 20 minutes through a filter element and the elevated tank; detecting according to the intermediate quality standard, requiring that the content of the moxifloxacin hydrochloride is between 1.52 and 1.68 mg / ml, the glucose content is between 47.5 and 52.5 mg / ml, and the pH value is between 4.0 and 4.5; after the intermediate is detected to be qualified, beginning to fill; and conveying the filled semi-finished products into a sterilizing cabinet for sterilization, wherein the sterilization condition is to sterilize for 8 to 30 minutes at 121 DEG C through thermal pressure steam.
Owner:HC SYNTHETIC PHARMA CO LTD
Cryopreserving solution for clinical-grade CAR-T cryopreservation and capable of being directly reinfused through intravenous infusion
InactiveCN108552159AAvoid lostReduce workloadInorganic non-active ingredientsPharmaceutical delivery mechanismClinical gradeHydroxyethyl starch
The invention discloses a cryopreserving solution for clinical-grade CAR-T cryopreservation and capable of being directly reinfused through intravenous infusion. The cryopreserving solution comprisesthe following raw materials: dimethyl sulfoxide, a glycerol fructose sodium chloride injection, an invert sugar electrolyte injection, a dextran 40 glucose injection, a hydroxyethyl starch 130 / 0.4 electrolyte injection, a vitamin C injection, a human serum albumin injection and a 0.9 % sodium chloride injection; the cryopreserving solution has the pH value of 6.8-7.0. When a formula for the clinical-grade CAR-T cryopreservation and capable of being directly reinfused through the intravenous infusion is used for cryopreserving CAR-T cells, the thawed cells and the thawed cryopreserving solutiondo not need centrifugation, resuspension, fluid exchange and other processes and can be directly reinfused through the intravenous infusion, so that the loss of cell varieties due to pollution in-vitro repeated proliferation of the CAR-T cells, or changes of cell morphologies and cell functions are effectively avoided, medical equipment can be also significantly simplified and the workload of medical workers can be reduced.
Owner:WUHAN BIO RAID BIOTECH CO LTD
Clinical mesenchymal stem cell cryopreservation solution and preparation method thereof
InactiveCN109329271AImprove the living environmentImprove protectionDead animal preservationAmino Acid InjectionTrehalose
The invention discloses clinical mesenchymal stem cell cryopreservation solution and a preparation method thereof. The preservation solution comprises the following components in percentage by weight:1-5% of autologous plasma lysate mixed liquor, 1-2% of human serum albumin, 0-1% of VC injection, 5-10% of glucose injection, 20-30% of electrolyte-like solution, 20-30% of glucosamine injection, 20-30% of compound amino acid injection, 2-5% of dimethyl sulfoxide and 2.5-5% of trehalose. The prepared cryopreservation solution can be used for deep low-temperature and long-term storage of cells, and can realize long-distance transportation, so that the distribution range of cells is expanded, the long-distance treatment of stem cells is realized, and the cells can be directly returned after recovery so as to reduce the potential safety hazards caused by complicated operations.
Owner:CHENGDU QINGKE BIOTECH
Fast propagation method of macadimia nut seedlings
InactiveCN107667751APromote hydrationSufficient oxygenGraftingExcrement fertilisersEngineeringWater circulation
The invention discloses a fast propagation method of macadimia nut seedlings. The method comprises the steps of stock cultivation, wherein a macadimia nut germination accelerating device is used, themacadimia nut germination accelerating device comprises a cabinet and germination accelerating boxes, air outlets are formed in the top of the cabinet, multiple atomizing nozzles are arranged on the top of the cabinet, an air inlet is formed in the bottom of the cabinet, and multiple water discharging and ventilation parts for water circulation and ventilation are arranged on the bottom of each germination accelerating box; stock seedbed production, germination accelerating and sowing, stock raising, scion treatment, grafting and field planting, wherein wounds of scions and stocks need to be washed with a mixture of a snail extract, honey, a glucose injection and a normal saline solution before grafting. By means of the method, the growth speed and the grafting survival rate of the seedlings can be improved, so that fast propagation of the macadimia nut seedlings is achieved.
Owner:马山县荷松种植专业合作社
Process for preparing glucose injection and glucose sodium chloride injection
InactiveCN1799551AOvercoming technical biasImprove productivityOrganic active ingredientsMetabolism disorderSodium Chloride InjectionD-Glucose
Owner:樊武良
Method for culturing cordyceps militaris with slant solid strains effectively instead of liquid strains
InactiveCN105733957AReduce energy lossChange color quicklyFungiMicroorganism based processesBiotechnologyFood culture
The invention discloses a method for culturing cordyceps militaris with slant solid strains effectively instead of liquid strains. The method comprises the following steps: culturing carrot slant cordyceps militaris strains, wherein a culture medium is prepared from the following components in parts by weight: 200 parts of juice prepared from carrots, 20 parts of glucose, 0.5 part of magnesium sulfate, 1 part of monopotassium phosphate and 20 parts of agar; performing subpackaging, sterilization, cooling, inoculation, culturing and selection on the culture medium; aseptically transferring the slant strains into a one-off injection syringe, sucking glucose injection, extruding a solution obtained by mixing the slant stains and the glucose injection from a syringe needle, pouring the extruded solution back into the one-off injection syringe, repeating the operation until the carrot slant cordyceps militaris strains are completely and uniformly mixed with the glucose injection, and inoculating prepared solidified cordyceps militaris liquid strains into a food culture medium or silkworm pupae; culturing the food culture medium or the silkworm pupae in a constant temperature incubator. The method is easy to operate, short in culture period, high in fruiting body yield and free of pollution and waste, and factory production can be implemented.
Owner:LUDONG UNIVERSITY
Preparation method of high-purity breviscapinun material used by breviscapinun injection
ActiveCN102188440AHigh purityImprove securityPowder deliveryOrganic active ingredientsAlcoholSodium Chloride Injection
The invention provides a preparation method of high-purity breviscapinun material used by a breviscapinun injection. The method comprises the preparation steps of: carrying out dissolving, alcohol precipitation, acid precipitation, ultrafitration and the like on the breviscapinun to obtain the high-purity breviscapinun material. The purity of the high-purity breviscapinun material can achieve more than 98 percent by detection through high performance liquid chromatography, and the high-purity breviscapinun material can be used for preparing large-capacity injection such as breviscapinun sodium chloride injection or breviscapinun glucose Injection.
Owner:吉林四长制药有限公司
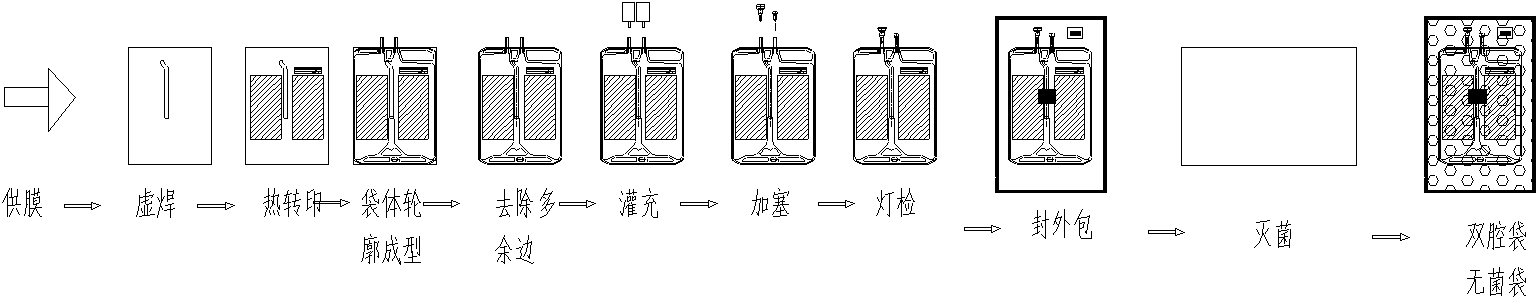
Amino acid and glucose injection packaged by dual-chambered bag, and preparation method of the injection
ActiveCN102600187AAvoid the Maillard reactionImprove stabilityOrganic active ingredientsPeptide/protein ingredientsMaillard reactionSide effect
The invention discloses a preparation method of amino acid and glucose injection packaged by a dual-chambered bag. The method comprises the steps of using glucose and injection water to prepare a solution; using amino acid and injection water to prepare a solution and charging nitrogen into the solution; feeding the glucose solution and the amino acid solution to two chambers of the dual-chambered bag simultaneously; vacuumizing and then filling nitrogen; loading the filled dual-chambered bag into an outer bag with high barrier; vacuumizing, filling nitrogen for several minutes, and packaging; and putting the packaged dual-chambered bag to a sterilizing device to sterilize at 115-125 DEG C. The amino acid and glucose injection provided by the invention is packaged by the dual-chambered bag. Because the amino acid solution and the glucose solution are fed in two chambers separated by cold solder joint, Maillard reaction or other side effects between amino acid and glucose due to long-term contact during the sterilization and storage are prevented, and then the usage safety and efficiency of the product can be ensured.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Stem cell repairing material as well as preparation method and application thereof
InactiveCN102205146ATraumaLittle painMammal material medical ingredientsDermatological disorderMesenchymal stem cellAutologous Fat Graft
The invention relates to a stem cell repairing material as well as a preparation method and application thereof. The stem cell repairing material comprises a stem cell and a cell carrier, wherein the stem cell is an autologous adipose-derived mesenchymal stem cell (ADSC) with a concentration of 105-108 / ml, and the cell carrier is autoserum, normal saline (0.9%) or a glucose injection (5%). In the invention, adopted seed cells are prepared through separating and purifying autologous adipose tissues of patients, thereby avoiding the ethical disputes and the immunological rejection. The material drawing of the adipose tissues can be performed by using an instrument suction method, which is simple in operation and can bring less traumas and pains to the patients. The cell carrier used in the invention is the autoserum, normal saline (0.9%) or glucose injection (5%), therefore, the cell carrier mixed with mesenchymal stem cells and other ingredients can be injected into the patents by virtue of syringes. By using the stem cell repairing material provided by the invention, the operation process is simple, no scar is left, and the wounds and pains for the patients are avoided, therefore, the stem cell repairing material can be easily accepted by the patients.
Owner:王影
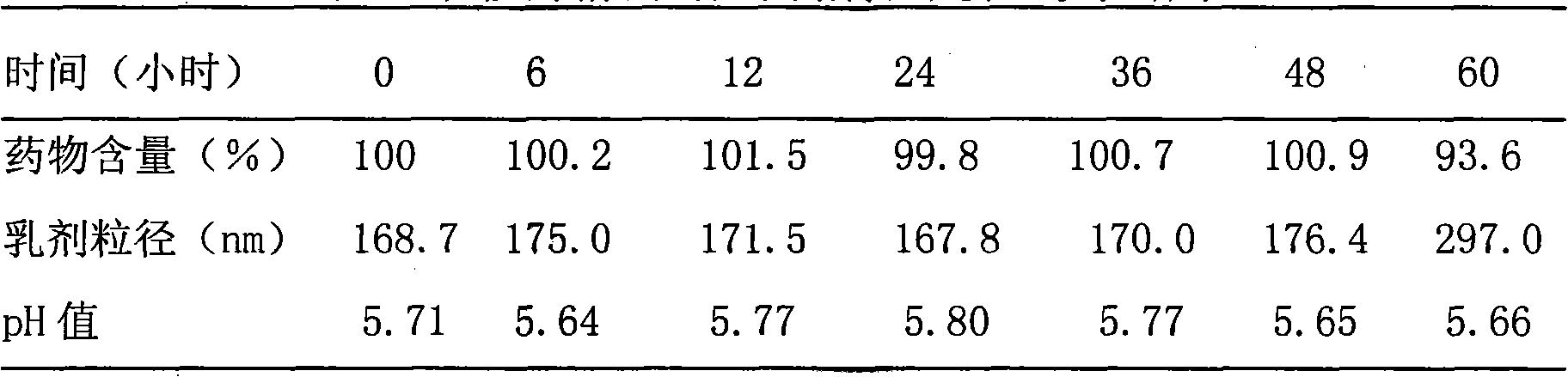
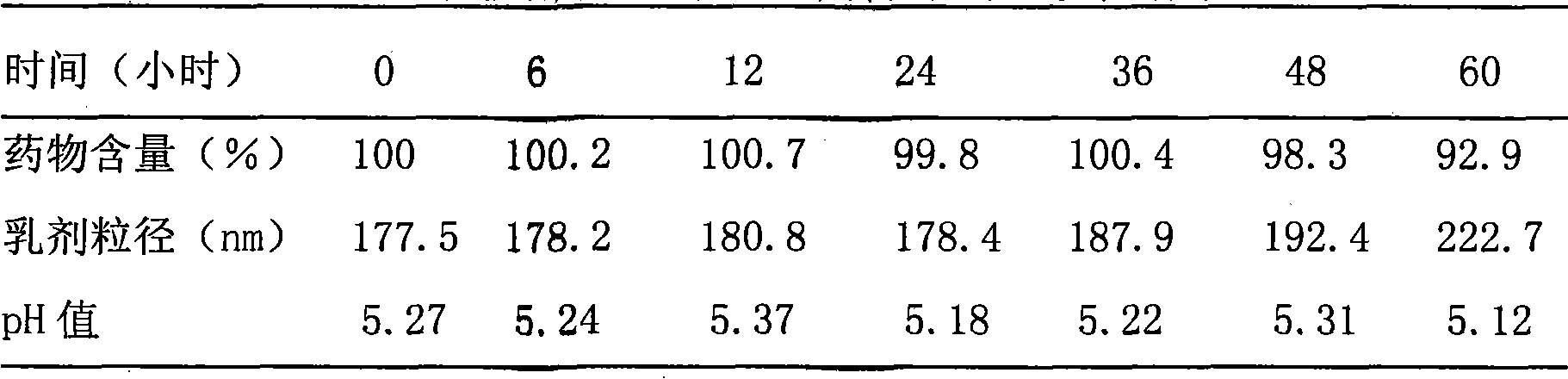
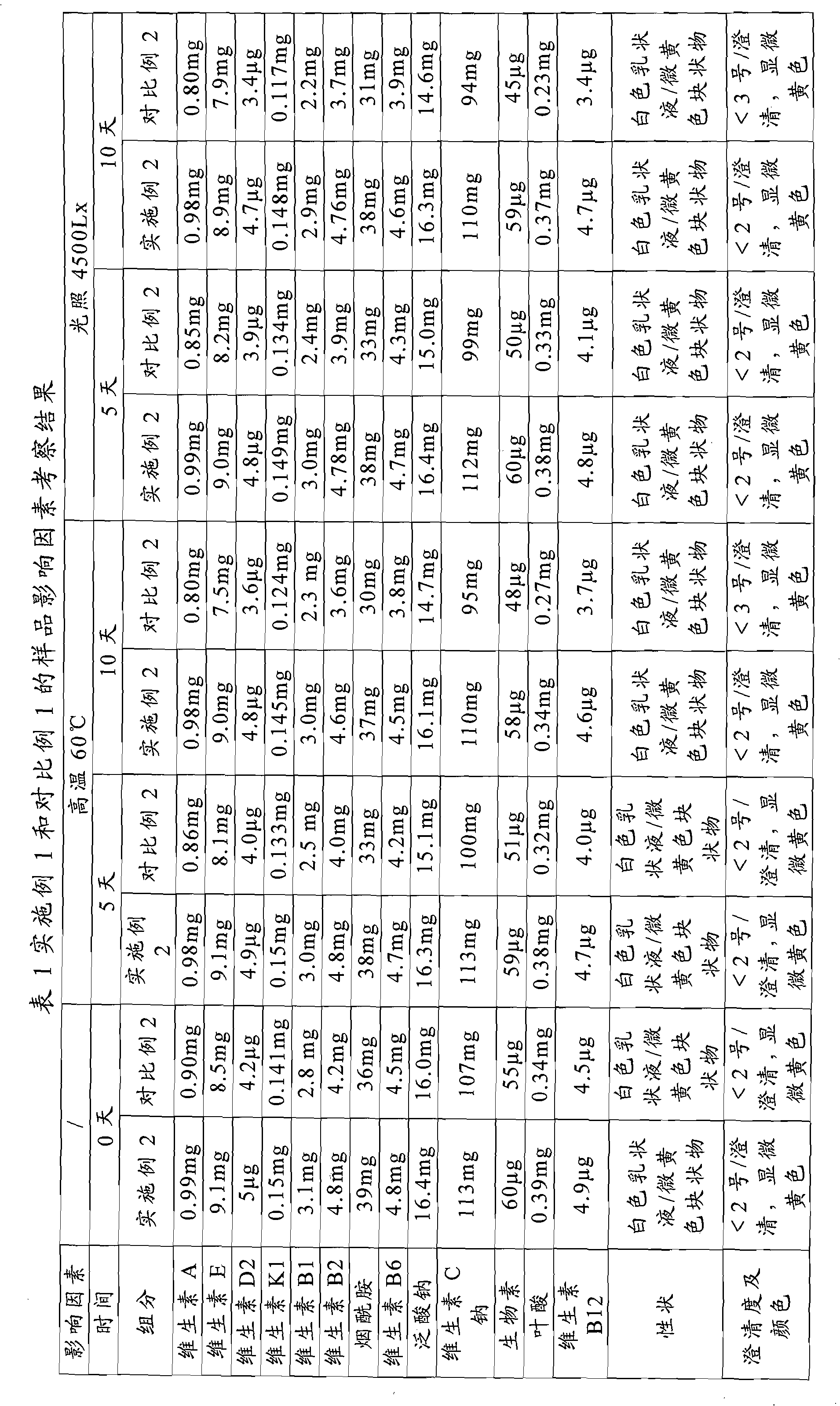
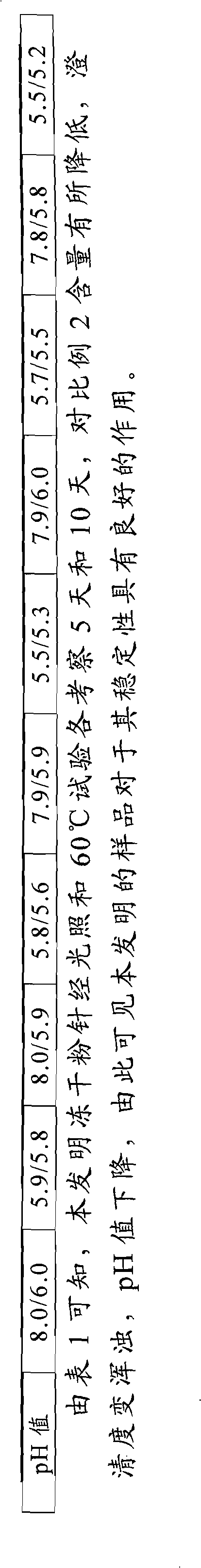
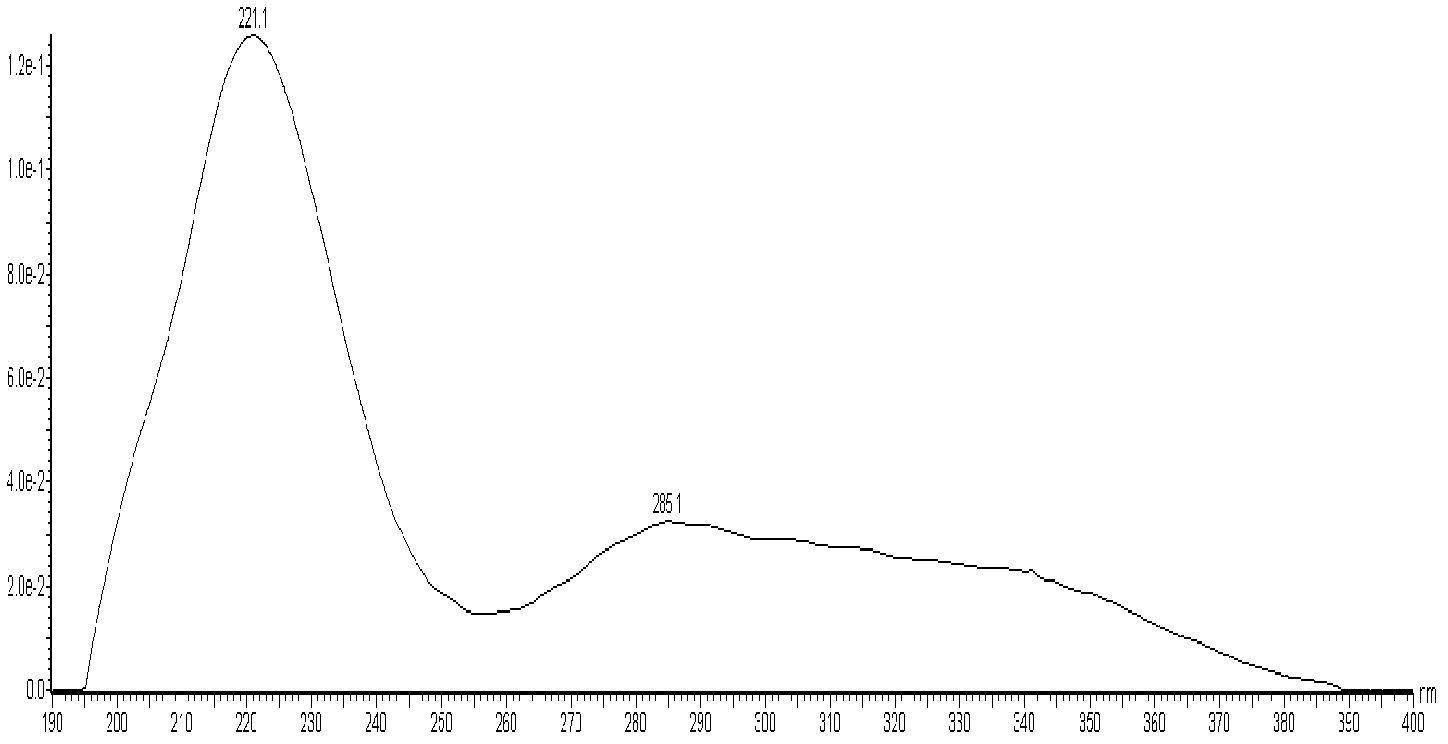
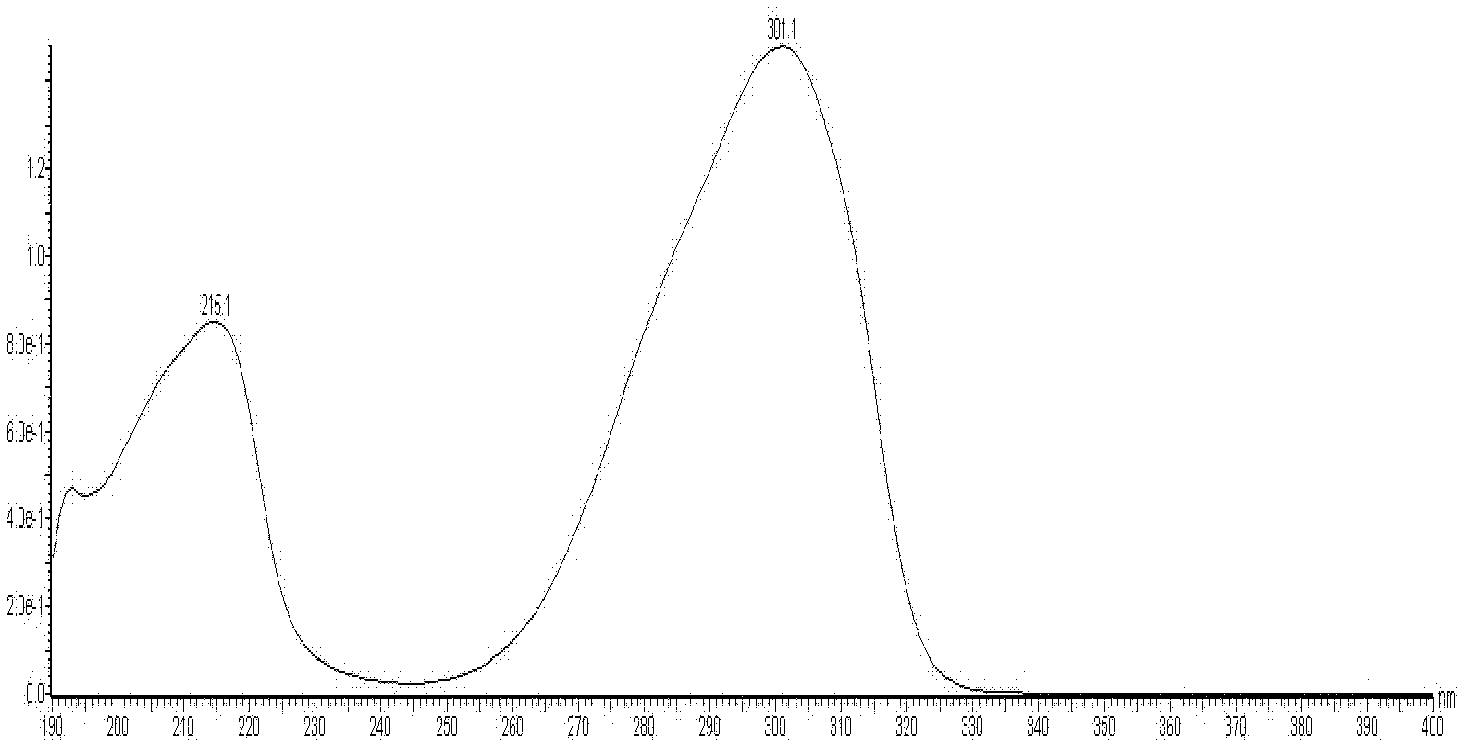
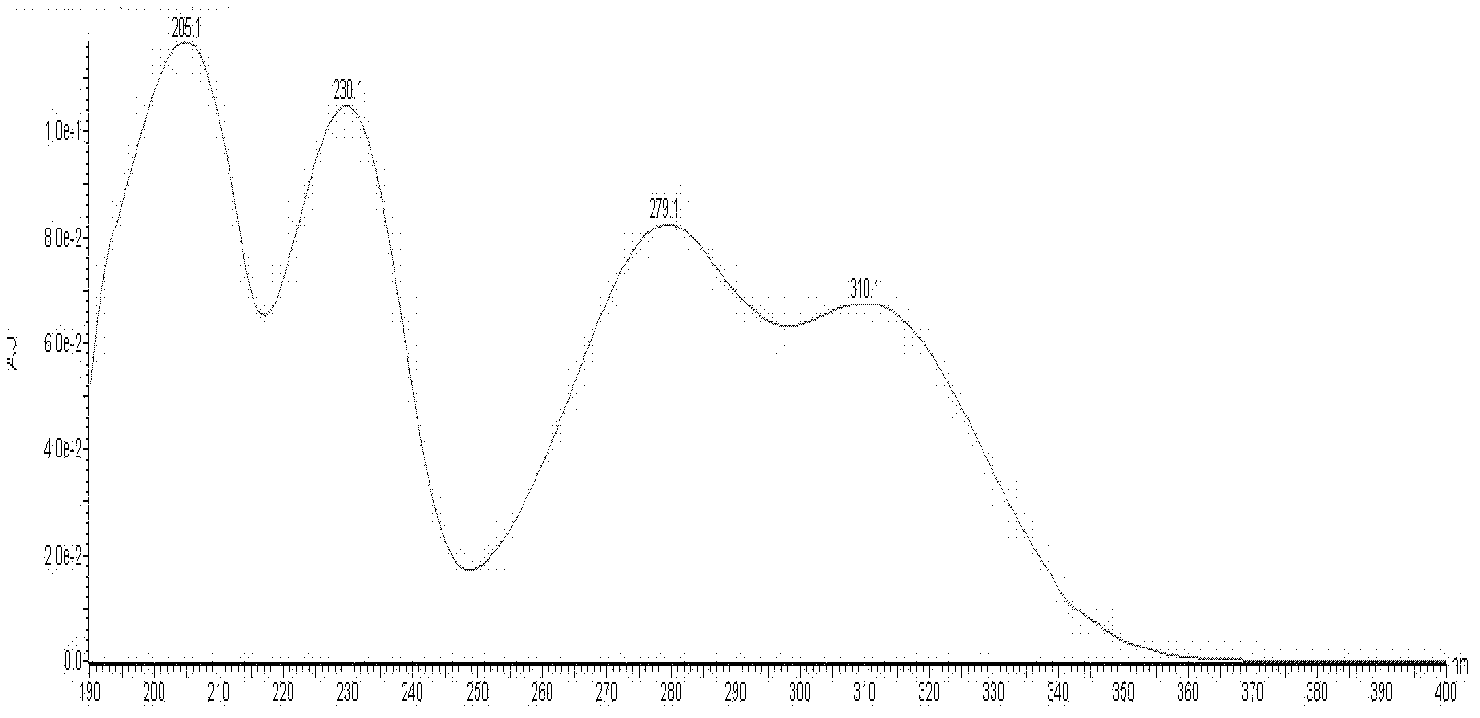
Method for measuring contents of multiple components in Shenxiong glucose injection
The invention belongs to the technical field of quality control of Chinese materia medica preparations, and provides a method for measuring contents of multiple components in Shenxiong glucose injection. According to the invention, the HPLC (high performance liquid chromatography) is adopted to measure contents of six compounds, namely tanshinol, ligustrazine hydrochloride, protocatechuic acid, rosmarinic acid, danshinolic acid B and danshinolic acid A, in the Shenxiong glucose injection under the same chromatographic condition. Compared with the present simple content measurement method for a single component, the method provided by the invention is better in integrity, characteristics and stability, can comprehensively reflect the interior quality of the preparations, and provides test base for further improving the quality control level of the Shenxiong glucose injection and ensuring the quality stability of the products.
Owner:GUIZHOU JINGFENG INJECTION
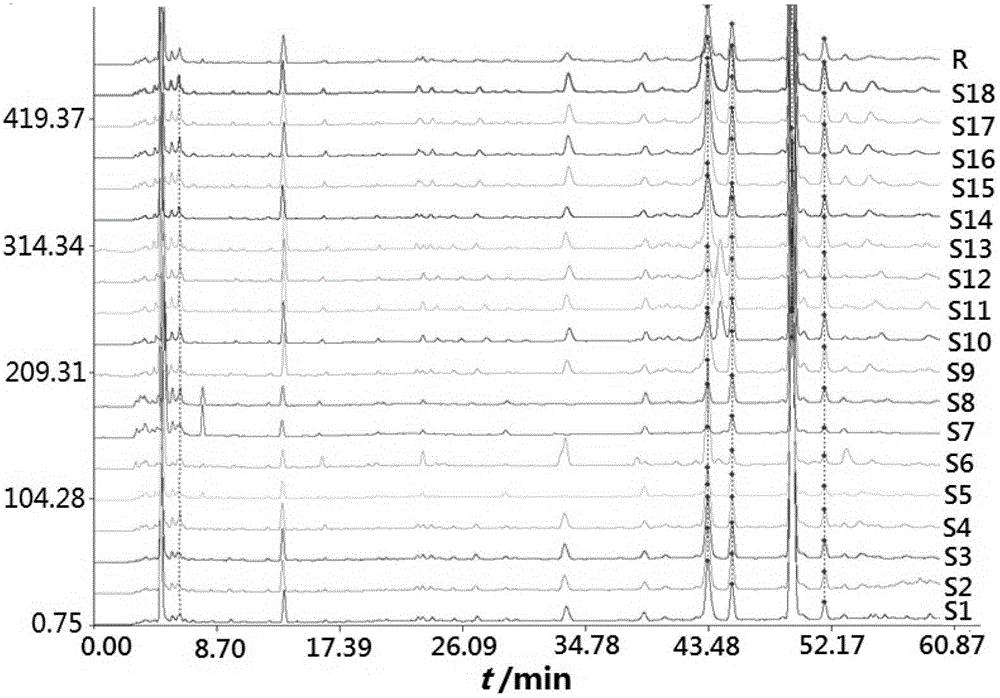
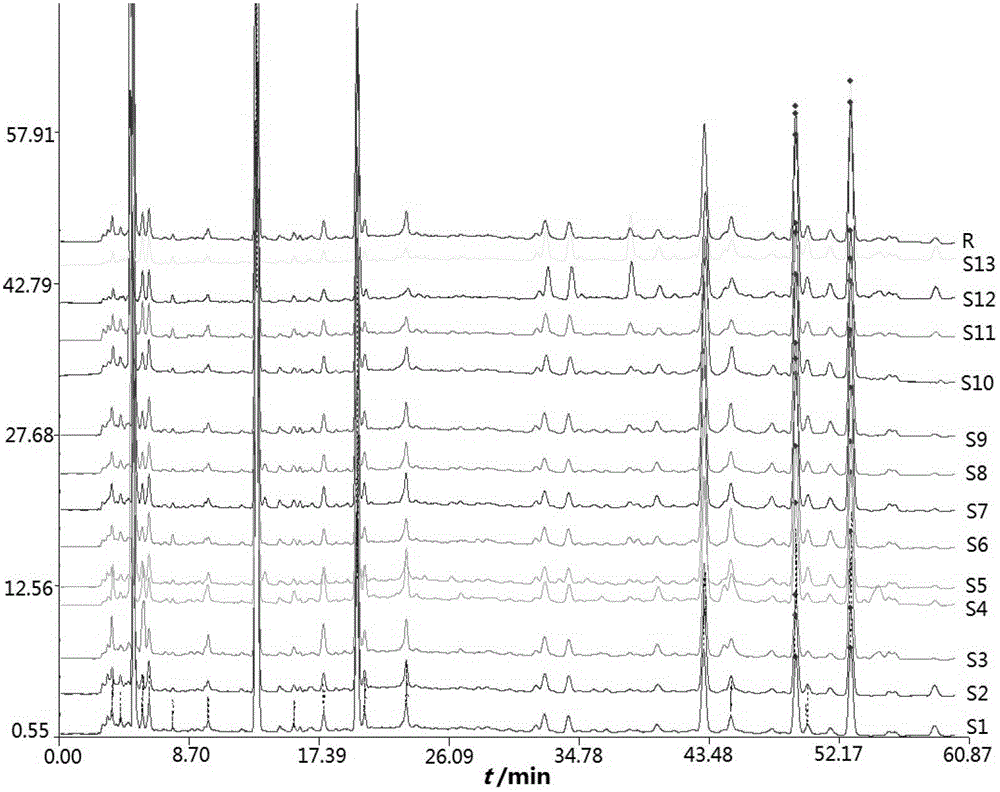
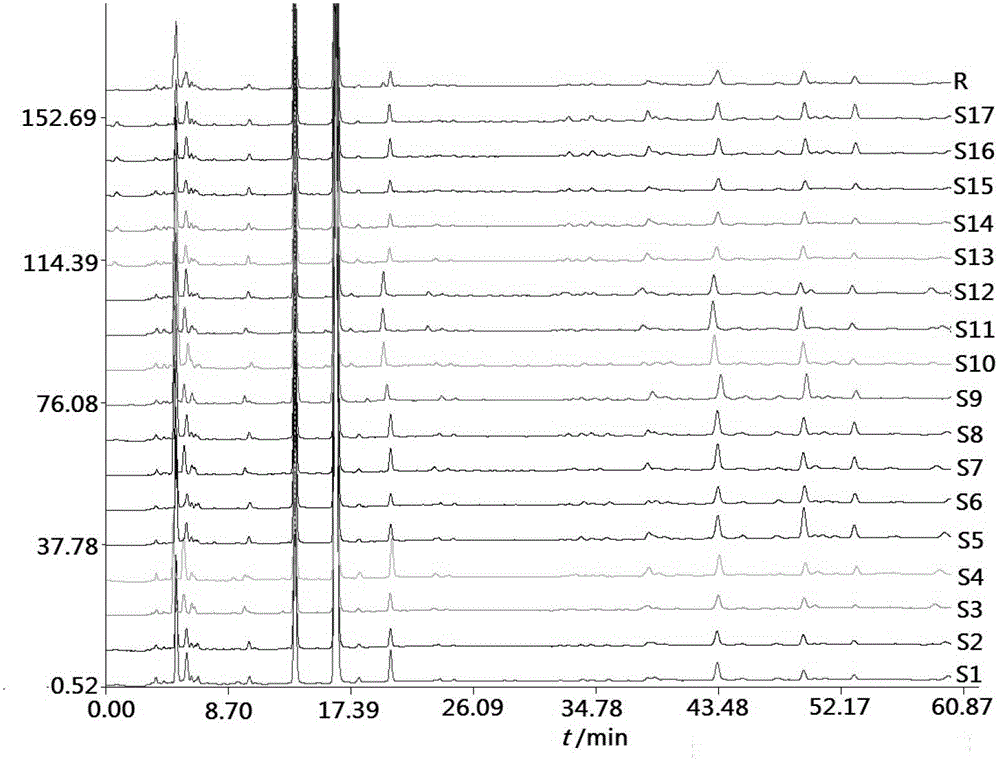
Fingerprint building method and quality control method of Shenxiong glucose injection raw material, Shenxiong glucose injection midbody and Shenxiong glucose injection preparation
ActiveCN103149310AGood repeatabilityImprove quality controlComponent separationPhosphoric acidGradient elution
The invention discloses a fingerprint building method and a quality control method of Shenxiong glucose injection raw material, a Shenxiong glucose injection midbody and Shenxiong glucose injection preparation. Fingerprint detection is conducted on the raw material, the midbody and the preparation of the raw material and the midbody under the condition of the same chromatograph. Octadecylsilane chemically bonded silica is used as chromatographic column filling agent, acetonitrile-phosphate aqueous solution is used as mobile phase, gradient elution is conducted, a test sample fingerprint and a contrasted fingerprint have good relevance, similarity is larger than 0.90, a fingerprint of the Shenxiong glucose injection raw material, a fingerprint of the Shenxiong glucose injection midbody and a fingerprint of the Shenxiong glucose injection preparation have good relevance, and each characteristic peak in the preparation fingerprint can be tracked in the raw material and the midbody. The fingerprint building method and the quality control method of the Shenxiong glucose injection raw material, the Shenxiong glucose injection midbody and the Shenxiong glucose injection preparation can be used for controlling conditions and quality of the preparation technology of Shenxiong glucose injection products and ensure stability of the preparation technology and the quality of the products. A fingerprint detecting method has the advantages of being simple and convenient to operate, accurate, reliable, and suitable for controlling the quality of Shenxiong glucose injection.
Owner:GUIZHOU JINGFENG INJECTION
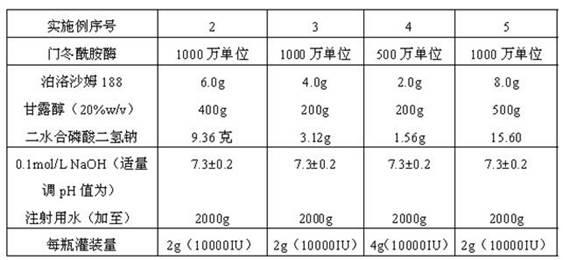
Asparaginase freeze-dried powder injection and preparation method thereof, as well as asparaginase solution
ActiveCN102138909AEasy to useRapid reconstitutionPowder deliveryPeptide/protein ingredientsFreeze-dryingSolvent
The invention discloses an asparaginase freeze-dried powder injection. The injection comprises asparaginase, a buffering agent, a surfactant, medical auxiliary materials and 5% of injection water which is used as a solvent in a preparing process and less than the addition finally, wherein the surfactant is a nonionic surfactant; and the buffering agent and the nonionic surfactant are added into the formula, so that the situation that tiny visible particles exceed standard is prevented when the asparaginase is dissolved and transferred into an injection. The invention also discloses a dedicated solution for dissolving the asparaginase, wherein the solution solvent is the injection water, and the solute comprises the buffering agent and the surfactant. On the premise of not changing the conventional formula of the freeze-dried powder injection, when the freeze-dried powder is dissolved in the solution, and is transferred into sodium chloride or a glucose injection, the situation that tiny visible particles exceed standard is prevented, so that the stimulation to human bodies is reduced in use, and the clinical administration safety is improved.
Owner:CHANGZHOU QIANHONG BIOPHARMA
Pharmaceutical compositions of fat-soluble vitamins for injection and water-soluble vitamins for injection and preparation method thereof
ActiveCN102499939AImprove securityConvenient sourcePowder deliveryHydroxy compound active ingredientsTreatment effectWater soluble
The invention relates to pharmaceutical compositions of fat-soluble vitamins for injection and water-soluble vitamins for injection, in particular to a combined application package including two types, namely the fat-soluble vitamins for injection and the water-soluble vitamins for injection. Water for injection or glucose injection is firstly used for dissolution and intravenous drip is further respectively performed during the use. The combined application package disclosed by the invention can comprehensively supplement the vitamins required for a human body, avoid the defects caused by the use of the single vitamin and greatly improve the treatment effect.
Owner:HAINAN YONGTIAN PHARMA INST
Medicinal composition of liposoluble vitamin injection (I) and water-soluble vitamin for injection and preparation method thereof
ActiveCN102512437AImprove securityConvenient sourcePowder deliveryHydroxy compound active ingredientsVitamin injectionTreatment effect
The invention relates to a medicinal composition of a liposoluble vitamin injection (I) and a water-soluble vitamin for injection, in particular to a combined application package. The medicinal composition contains the liposoluble vitamin injection (I) and the water-soluble vitamin for injection. When in use, the medicinal composition is dissolved or diluted by using water for injection or glucose injection firstly, and then is subjected to intravenous drip respectively. In the combined application package, vitamins required by a human body are comprehensively implemented, the defect caused by using a single vitamin is overcome, and the treatment effect is greatly improved.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Ibuprofen medicinal composition
ActiveCN101978945AGood water solubilityImprove stabilityOrganic active ingredientsAntipyreticSodium lactateArginine
The invention discloses an ibuprofen medicinal composition for injection. By adding tromethamine into solution of ibuprofen and arginine, the stability of the ibuprofen medicinal composition in clinical application is improved. Experiments prove that: after being diluted by the sodium chloride injection or glucose injection or sodium lactate Ringers injection, the composition solution can be stably stored for at least 12 hours.
Owner:茂裕环保科技南通有限公司
Method for preparing injection preparation of freeze drying powder of Xuesaitong
ActiveCN101049332AImprove solubilityAvoid bringing inPowder deliveryLyophilised deliveryRefluxAlcohol
Owner:HEILONGJIANG ZBD PHARMA +1
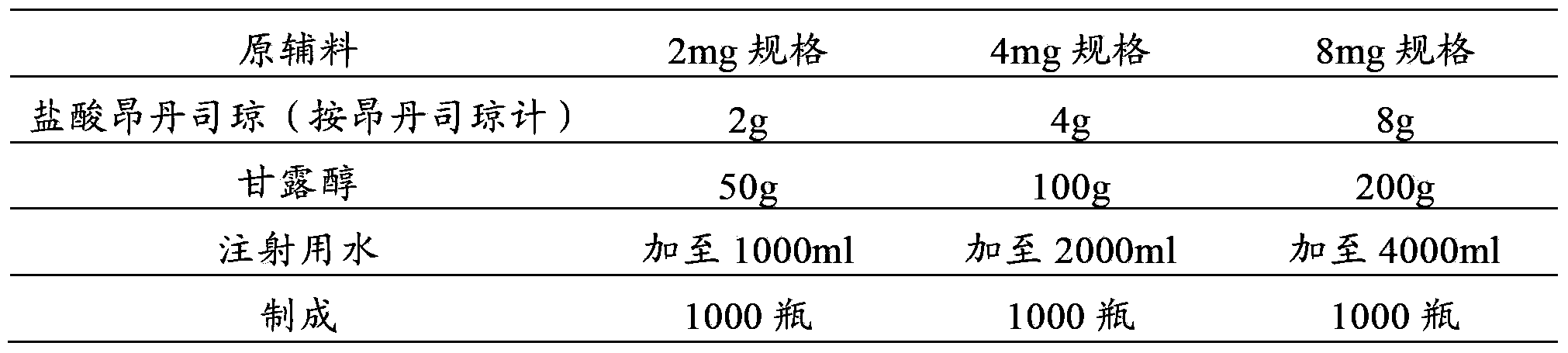
Azasetron hydrochloride glucose injection formulation, and its preparing method and quality control technology
The present invention discloses an antiemetic ayasetron hydrochloride glucose injection preparation, its preparation method and quality control technique. Its composition includes ayasetron hydrochloride 0.1g, glucose 40-50g and sodium hydrogen sulfite 0-20mg. All the above-mentioned components are added into injection water, and dissolved to 1000ml so as to obtain the invented product. Said preparation is a fluid infusion type preparation, and can be used for clinical application.
Owner:余世春
Pharmaceutical composition of ondansetron hydrochloride and dexamethasone
The invention relates to a pharmaceutical composition of ondansetron hydrochloride and dexamethasone, and particularly relates to a combined application package comprising an ondansetron hydrochloride injection and an injection containing dexamethasone. When the combined application package is in use, the ondansetron hydrochloride injection and the injection containing dexamethasone are co-dissolved in normal saline, a Ringer's solution, 5% glucose injection and the like for intravenous infusion, so that a curative effect of stopping vomit can be increased.
Owner:HAINAN LINGKANG PHARMA CO LTD
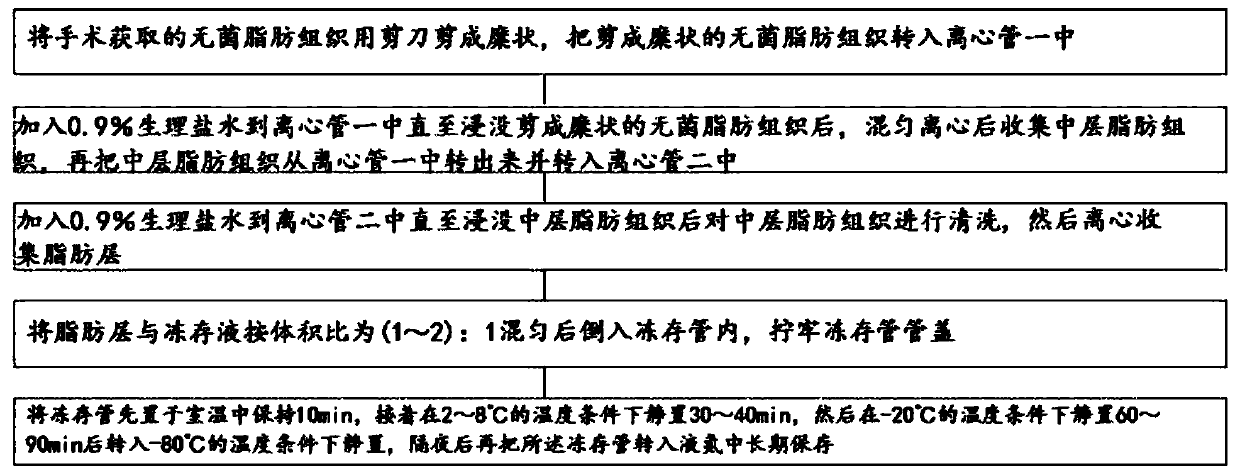
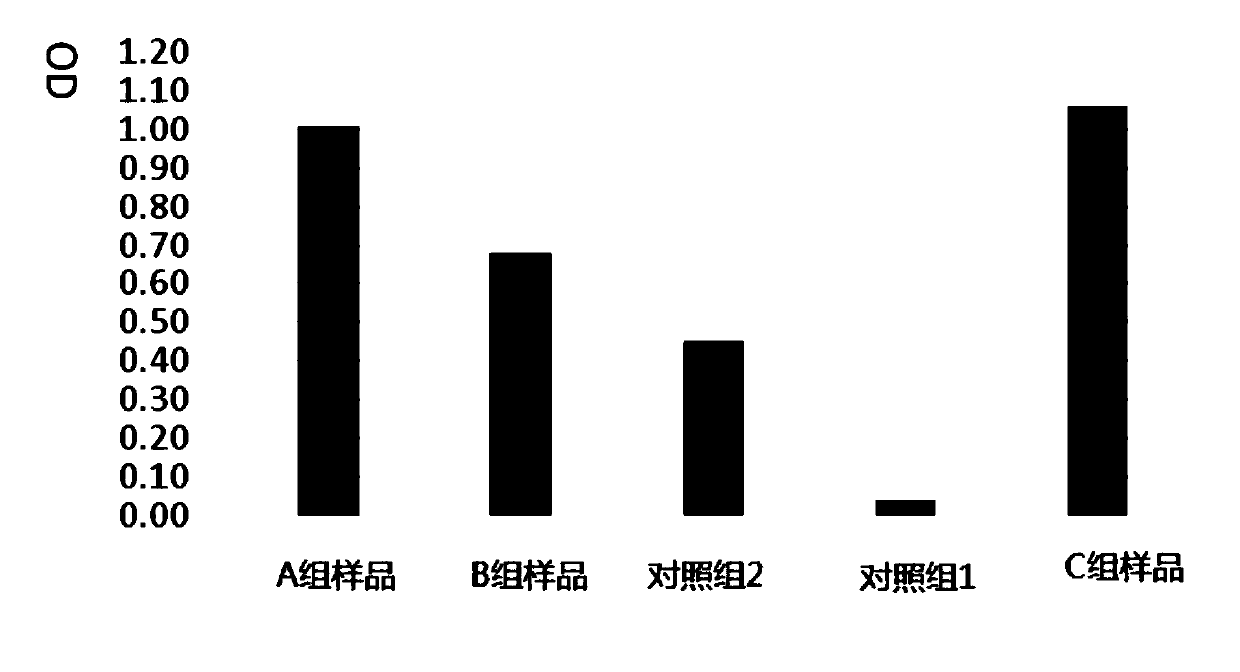
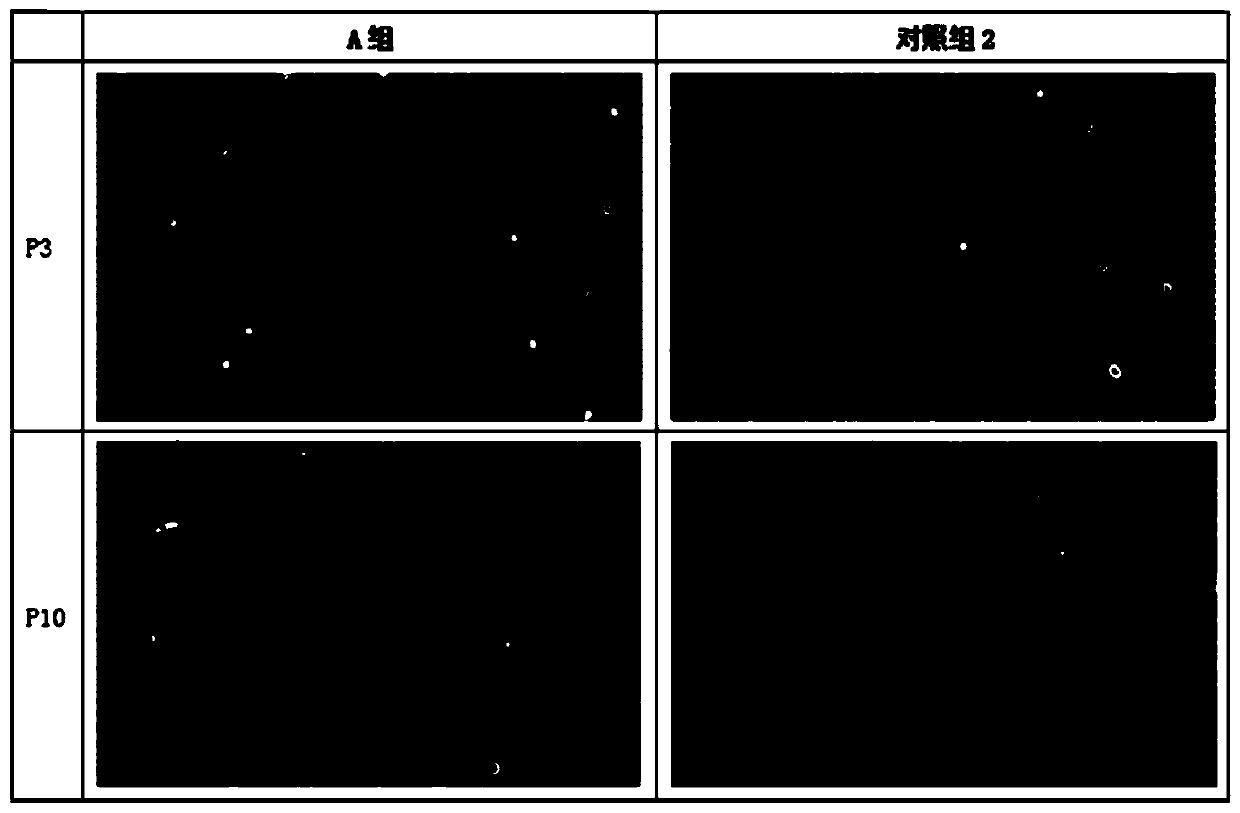
Adipose tissue cryopreservation liquid at clinical application level and cryopreservation method
Adipose tissue cryopreservation liquid at a clinical application level and a cryopreservation method. The adipose tissue cryopreservation liquid at the clinical application level comprises 15-50-mmol / L saccharose, 15-50-mmol / L mannitol injection, 1-5-mmol / L glucose injection, 1-3-mol / L adenosine injection, 0.5-5-mmol / L reduced glutathione, 1-4.5-mol / L trehalose and 0.1-1-mmol / L EDTA-2Na; and the components are mixed to form mixed liquid, and the mixed liquid is the adipose tissue cryopreservation liquid at the clinical application level. By means of the adipose tissue cryopreservation liquid,in combination with the cryopreservation method thereof, at the clinical application level, the defect of adverse reaction due to 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES), phenol red, DMSO, animal serum, blood platelet extracts and the like which are used in the prior art is effectively overcome.
Owner:陕西医赛尔生物科技有限公司
Injection containing sodium, potassium, magnesium, calcium and glucose injection and preparation method of injection
InactiveCN104224829AImprove securityReduce contentMetabolism disorderPharmaceutical delivery mechanismAntioxidantPotassium
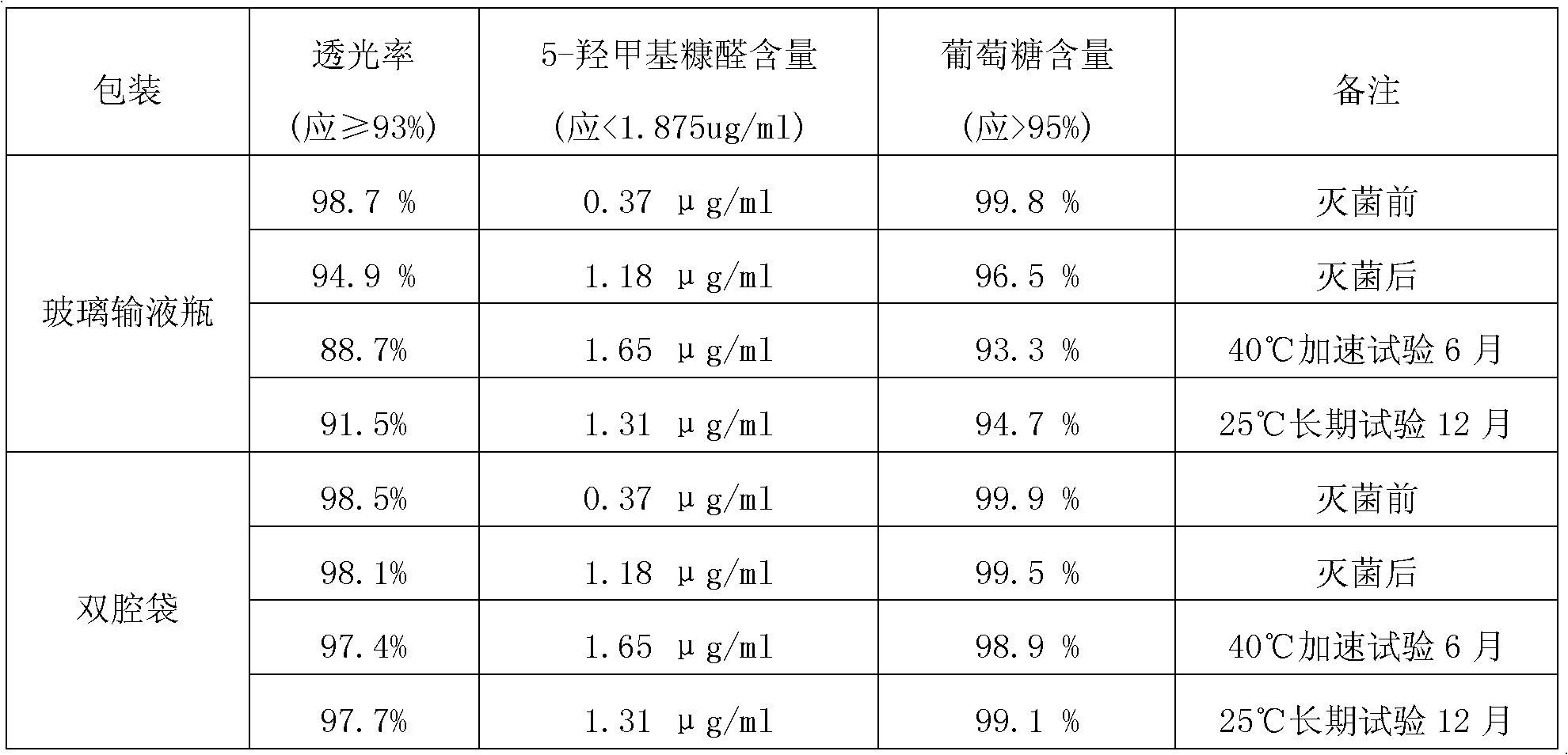
The invention discloses an injection containing sodium, potassium, magnesium, calcium and glucose and a preparation method of the injection. Every 1000 ml of injection contains 5-10 g of sodium chloride, 0.1-0.5 g of potassium chloride, 0.05-0.5 g of magnesium chloride hexahydrate, 1-5 g of sodium acetate trihydrate, 0.1-1 g of sodium citrate dehydrate, 0.1-2 g of calcium gluconate monohydrate, 5-20 g of anhydrous glucose, 0.5-2 g of antioxidant and appropriate water for injection, and a pH regulator solution is used for regulating the pH to be 4.5-6.0. The injection containing sodium, potassium, magnesium, calcium and glucose has the advantages that as appropriate antioxidant is added, the content of 5-hydroxymethyl furfural in the sterilized sodium, potassium, magnesium, calcium and glucose injection is effectively reduced; the sterilization time and injection preparation temperature are optimized, so that glucose being converted into 5-hydroxymethyl furfural at excess temperature is avoided, and the content of 5-hydroxymethyl furfural in the injection containing potassium, calcium and glucose is also effectively reduced; the preparation method is simple and reliable and is suitable for industrial production.
Owner:SICHUAN KELUN PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com