Patents
Literature
163 results about "Vinpocetine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vinpocetine (ethyl apovincaminate) is a synthetic derivative of the vinca alkaloid vincamine. Vincamine is extracted from either the seeds of Voacanga africana or the leaves of Vinca minor (lesser periwinkle).
Compositions and methods for treating cellular proliferation disorders
Compositions and methods for treating patients suffering from a proliferation disorder characterized by an increased voltage gated ion-channel uptake are described. Included are compositions comprised of a compound selected from the group consisting of matrine, oxymatrine, artemisinin, agmatine, and vinpocetine.
Owner:NUTRICOLOGY INC
Compositions and methods for timed release of water-soluble nutritional supplements, green coffee extract
InactiveUS20050181044A1High energyAppetite suppressantPill deliveryGranular deliveryAmylase inhibitorsNiacinamide
Owner:ROMERO JAIME
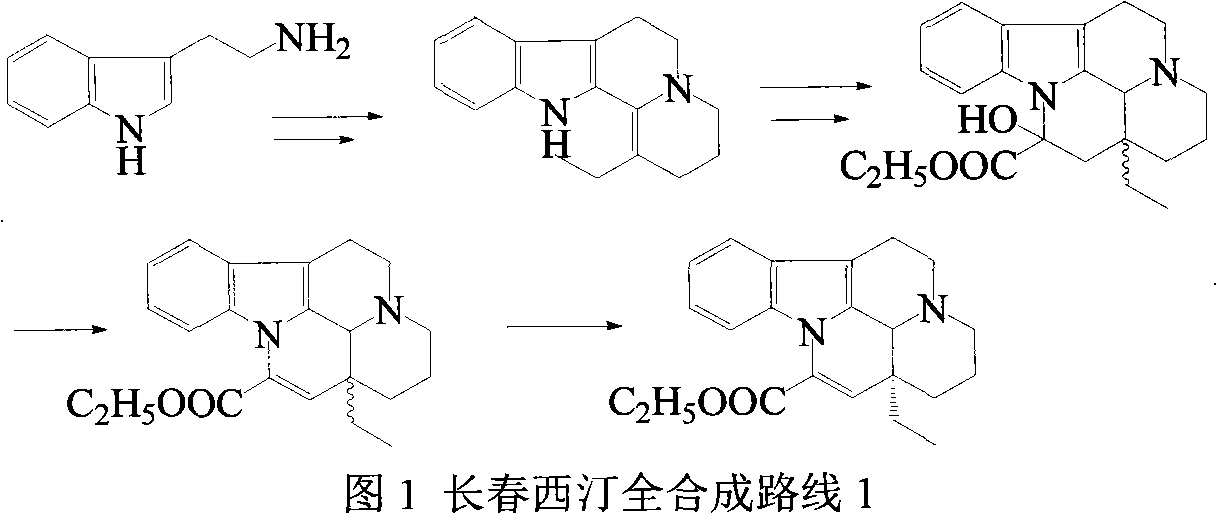
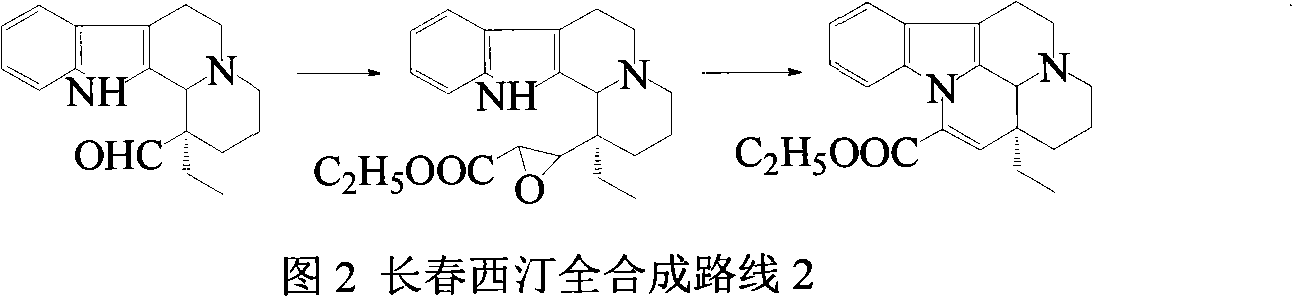
Synthetic method of vinpocetine
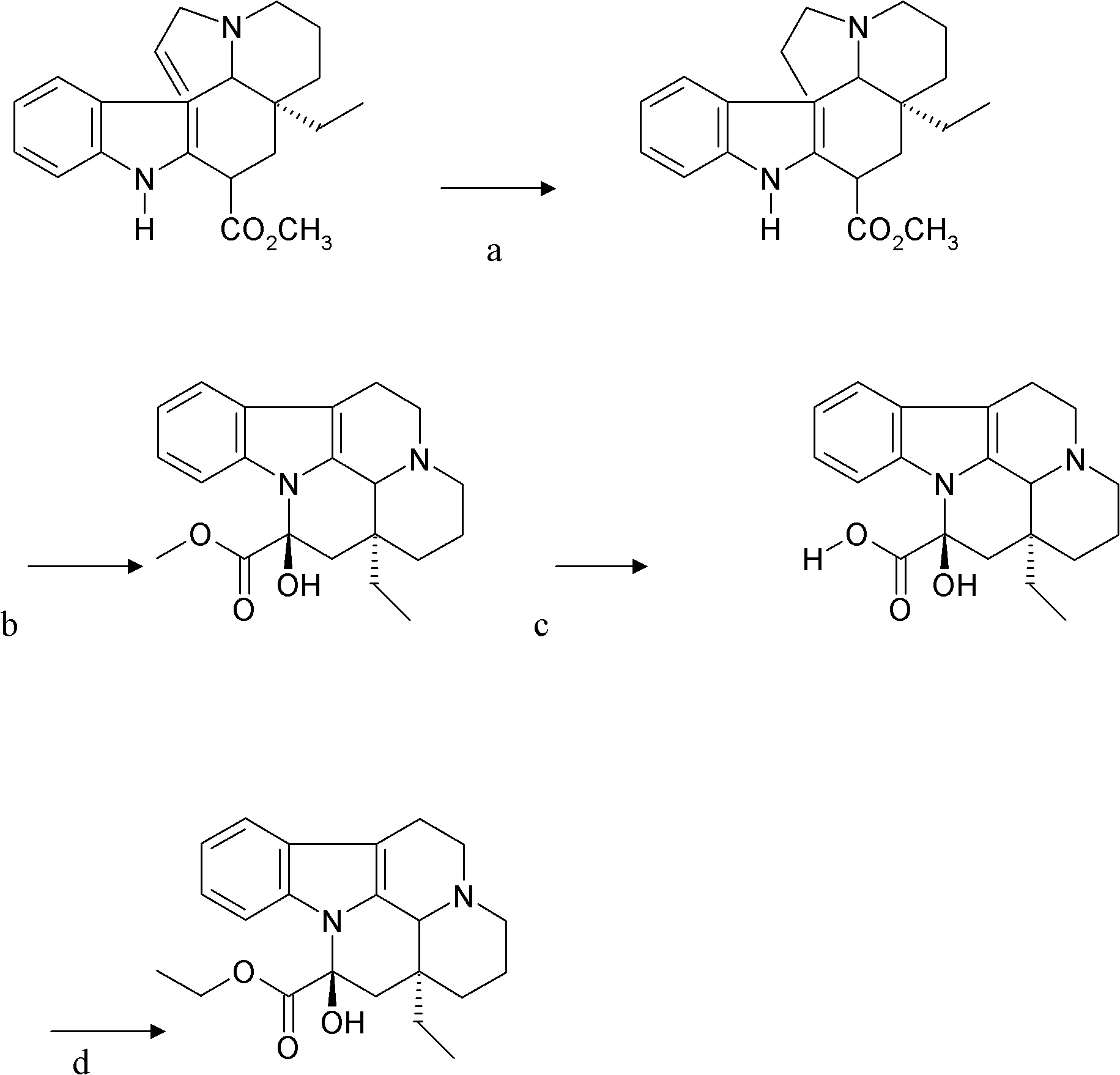
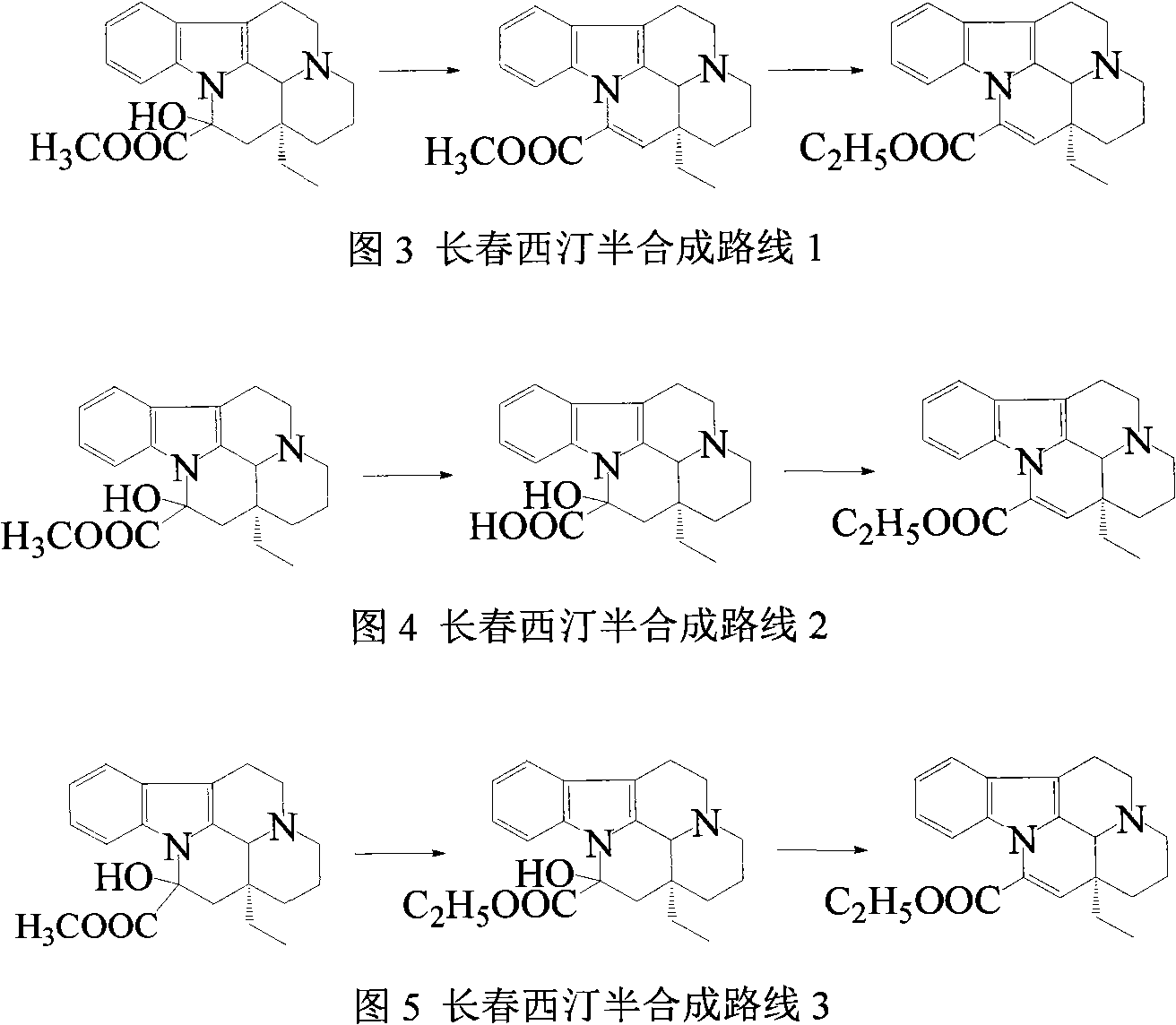
The invention provides a half synthetic method for industrially producing vinpocetine on the premise of ensuring product purity and yield. Voacanga seed grown in Africa is used for extracting raw material-tabersonine required by synthesis. The tabersonine is synthesized into vinpocetine by four steps: 1) preparing vincadifformine; 2) preparing vincamine; 3) preparing vincamine acid; and 4) synthesizing the vinpocetine the content of which is 99%. The invention builds an integral process from tabersonine to vinpocetine, has the advatages of simple process, high yield and lower cost, and can realize the industrial production of vinpocetine on the premise of ensuring the product purity and yield.
Owner:SHAANXI JIAHE PHYTOCHEM
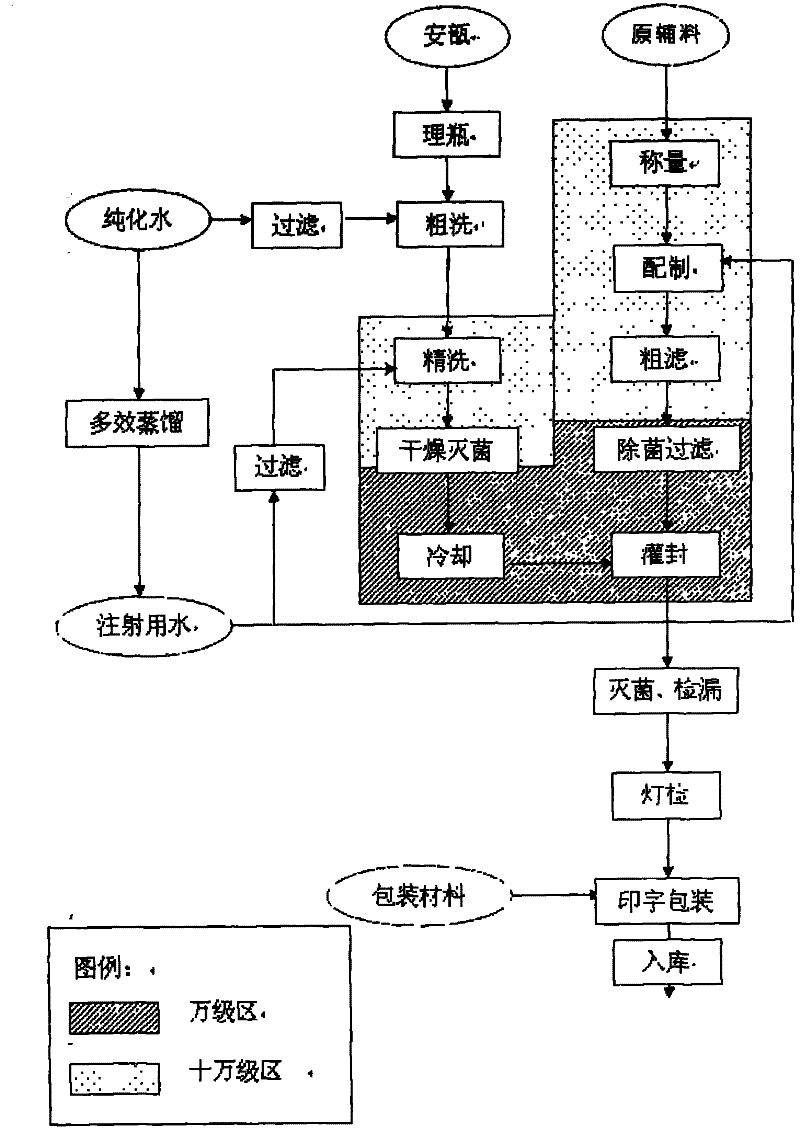
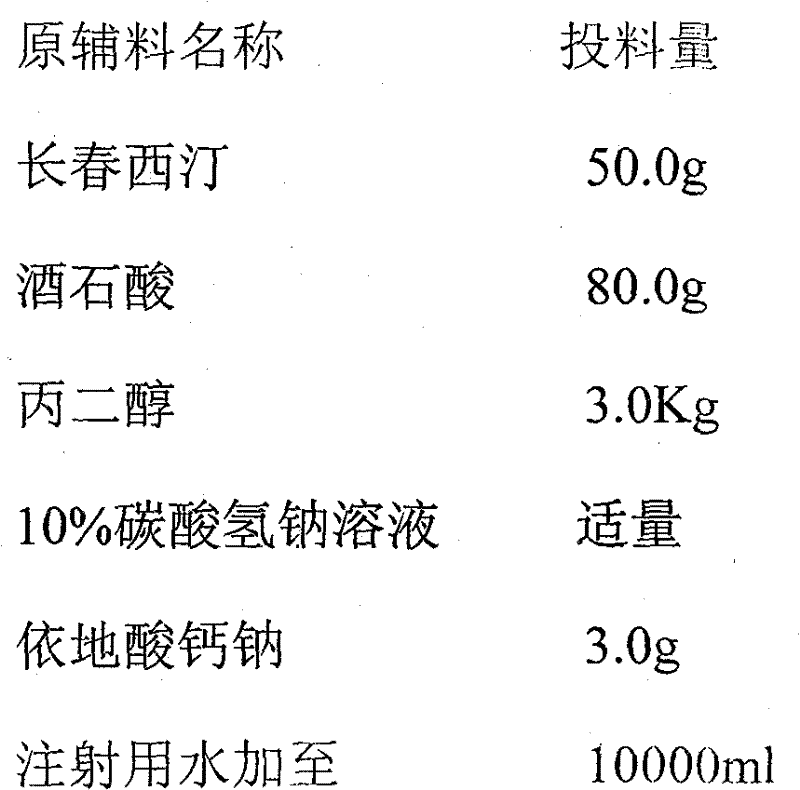
Vinpocetine injection and preparation method thereof
InactiveCN102091030AImprove stabilityShort dissolution timeOrganic active ingredientsPharmaceutical delivery mechanismVinpocetineMedicine
The invention relates to a vinpocetine injection. Each 1000ml of the vinpocetine injection provided by the invention contains 3-15g of vinpocetine, 5-25g of cosolvent, 0.2-2g of antioxidant, 20-100g of physical stabilizer and 10-25g of vessel stimulation regulator. The invention also relates to a preparation method of the vinpocetine injection.
Owner:罗军
Diet supplement for causing rapid weight loss, controlling appetite, managing stress, supporting relaxation, combating fatigue and supporting mental well-being
InactiveUS20060286183A1Rapid weight lossAppetite suppressantBiocidePeptide/protein ingredientsDietary supplementMental health
Compositions and methods for administering to the diet of humans a composition for inducing rapid weight loss, controlling appetite, managing stress and supporting mental well-being, supporting relaxation, and combating fatigue. A diet supplement comprising Calcium and Potassium double salt of Garcinia Cambogia Extract supplying 60% Hydroxycitric Acid, Gymnema Sylvestre Leaf Extract, Rhodiola Rosea Root Extract, Theanine, Astaxanthin Algae Extract, Chromium Polynicotinate, Hoodia Gordonii, N-olyl-phosphatidyl ethanolamine (NOPE) / EGCG blend, Vinpocetine, Russian Tarragon Extract, N-acetyl tyrosine, and Withania Somnifera Root Extract is provided. Said diet supplement is comprised of at least Hoodia Gordonii wherein the extract does not contain any extract from the root of the plant.
Owner:SMARTBURN FORMULATIONS LTD
Weight control compositions and methods
InactiveUS20050025844A1Decreasing body fatIncreasing and maintaining lean body massBiocideUnknown materialsN-MethyltyramineReceptor antagonist
The present invention provides compositions and methods that assist in providing weight control. Compositions comprise Caffeine, an adrenergic amine (e.g. synephrine, hordenine, octopamine, tyramine and N-methyltyramine,) forskolin, Guggulsterones, an alpha-2 receptor antagonist (e.g. Yohimbine) and a vinca alkaloid (e.g. vinpocetine.) Black pepper extract may be added as well in various alternative embodiments. Methods utilizing administration of nutrient compositions are disclosed as well.
Owner:SELLO AZUL
Pharmaceutical composition containing vinpocetine, preparation method and application thereof
The invention provides a pharmaceutical composition containing vinpocetine, a preparation method and an application of the pharmaceutical composition. The composition comprises the following components in parts by weight: 1 part of vinpocetine, 0.5-3 parts of cosolvent, 0.02-0.8 part of antioxidant, 2-20 parts of stabilizer, 1-5 parts of vascular regulator and a proper amount of Ph regulator. The composition is mainly used for treating cardiovascular and cerebrovascular diseases.
Owner:北京爱力佳医药科技有限公司
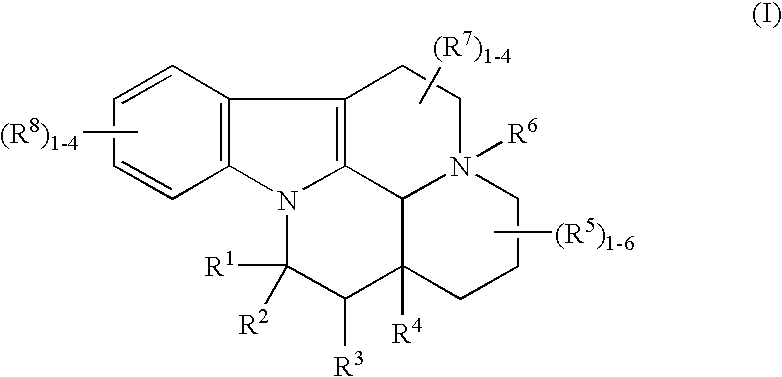
Vinpocetine and eburn amonine derivatives for promoting bone growth
The present invention provides a method of promoting bone growth in a subject in need thereof, by administering to the subject a therapeutically effective amount of a compound of Formula I. The compounds include the salts, hydrates and isomers thereof. The present invention also provides methods for the treatment of renal disease and cancer.
Owner:OSSIFI
Synthesis and micellization application of amphiphilic beta-cyclodextrin star polymer
ActiveCN103396521AIncrease the hydrophobic spaceImprove drug loading capacityOrganic active ingredientsMacromolecular non-active ingredientsPolymer scienceCyclodextrin
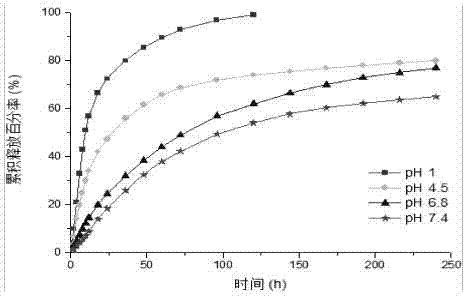
The invention belongs to the technical field of high polymer chemistry and pharmaceutical preparations, relates to a synthesis and a micellization application of an amphiphilic beta-cyclodextrin (beta-CD) star polymer, and provides an amphiphilic beta-cyclodextrin (beta-CD) star polymer taking beta-cyclodextrin (beta-CD) as a core, acrylic acid and a methyl methacrylate copolymer as a lipotropy block and poly (N-vinyl pyrrolidone) as a hydrophilic block, and a synthesizing method of the amphiphilic beta-cyclodextrin (beta-CD) star polymer. The amphiphilic beta-cyclodextrin (beta-CD) star polymer is formed by connecting amphiphilic block polymers to 3-6 hydroxide radicals of the beta-cyclodextrin (beta-CD). The molar mass ratio of the acrylic acid and the methyl methacrylate in the lipotropy block and the poly (N-vinyl pyrrolidone) in the hydrophilic block in the amphiphilic polymer is 4:1:(1-15):1:16. The invention further relates to a vinpocetine micelle taking the amphiphilic beta-cyclodextrin (beta-CD) star polymer as a carrier material and a preparation method of the vinpocetine micelle. The medicine carrying micelle of the amphiphilic beta-cyclodextrin (beta-CD) star polymer provided by the invention has the characteristics of slow-release effect, pH sensitivity and lung targeting.
Owner:GUANGDONG PHARMA UNIV
Vinpocetine medicine composition and preparation method thereof
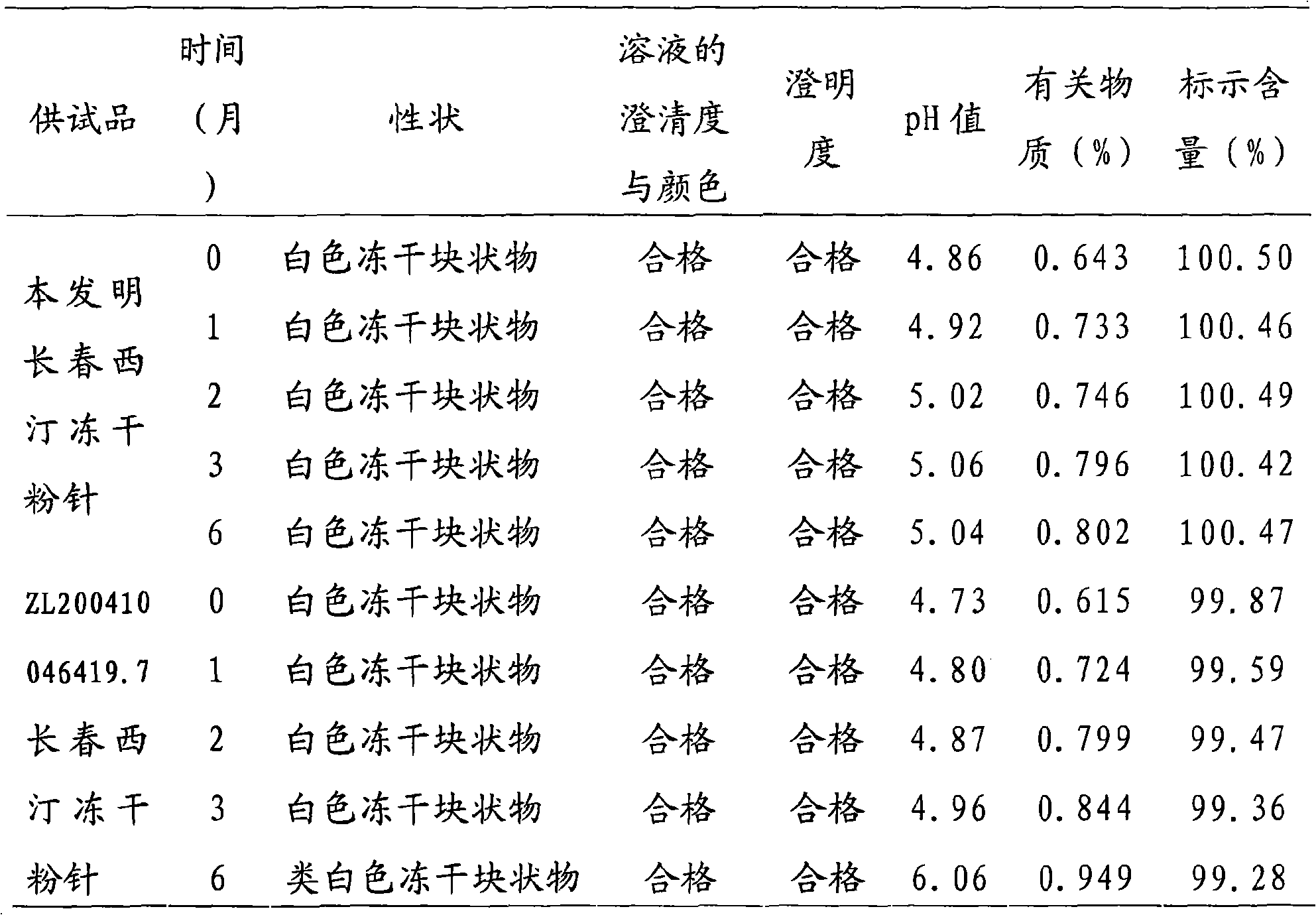
ActiveCN101791310AImprove stabilityReduce contentPowder deliveryOrganic active ingredientsVinpocetineFreeze-drying
The invention relates to a stable vinpocetine medicine composition belonging to the technical field of medicine preparations. The composition is a freeze-dried powder injection and is characterized in that the freeze-dried powder injection is prepared from the following components: 10-30g of vinpocetine, 70g of mannite, 20g of sorbitol, 10-20g of 50-100 ml of phosphoric acid and the balance of water for injection up to 2000ml. The freeze-dried powder injection prepared by the invention has better stability.
Owner:九瑞健康股份有限公司
Vinpocetine freeze-dried powder for injection and preparation thereof
InactiveCN101264064AImprove solubilityGood stabilityOrganic active ingredientsPowder deliverySolubilityMANNITOL/SORBITOL
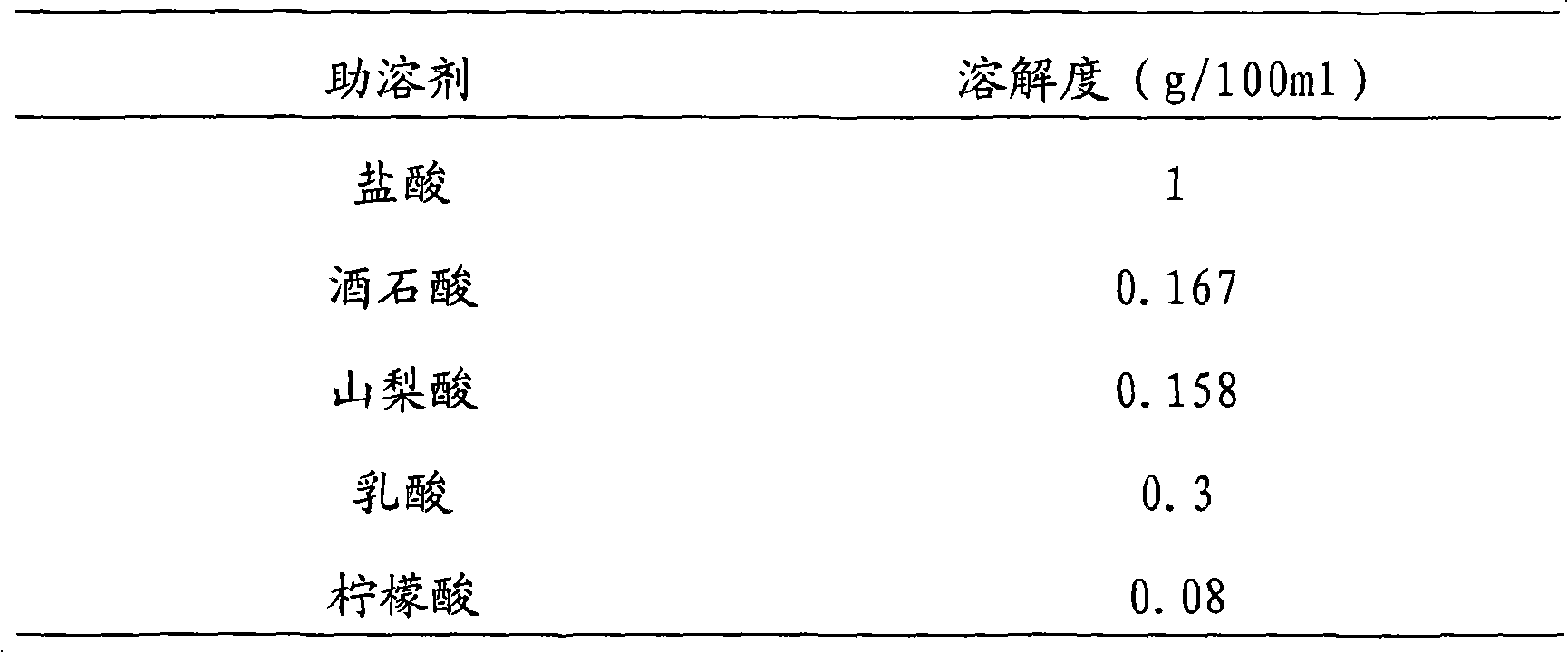
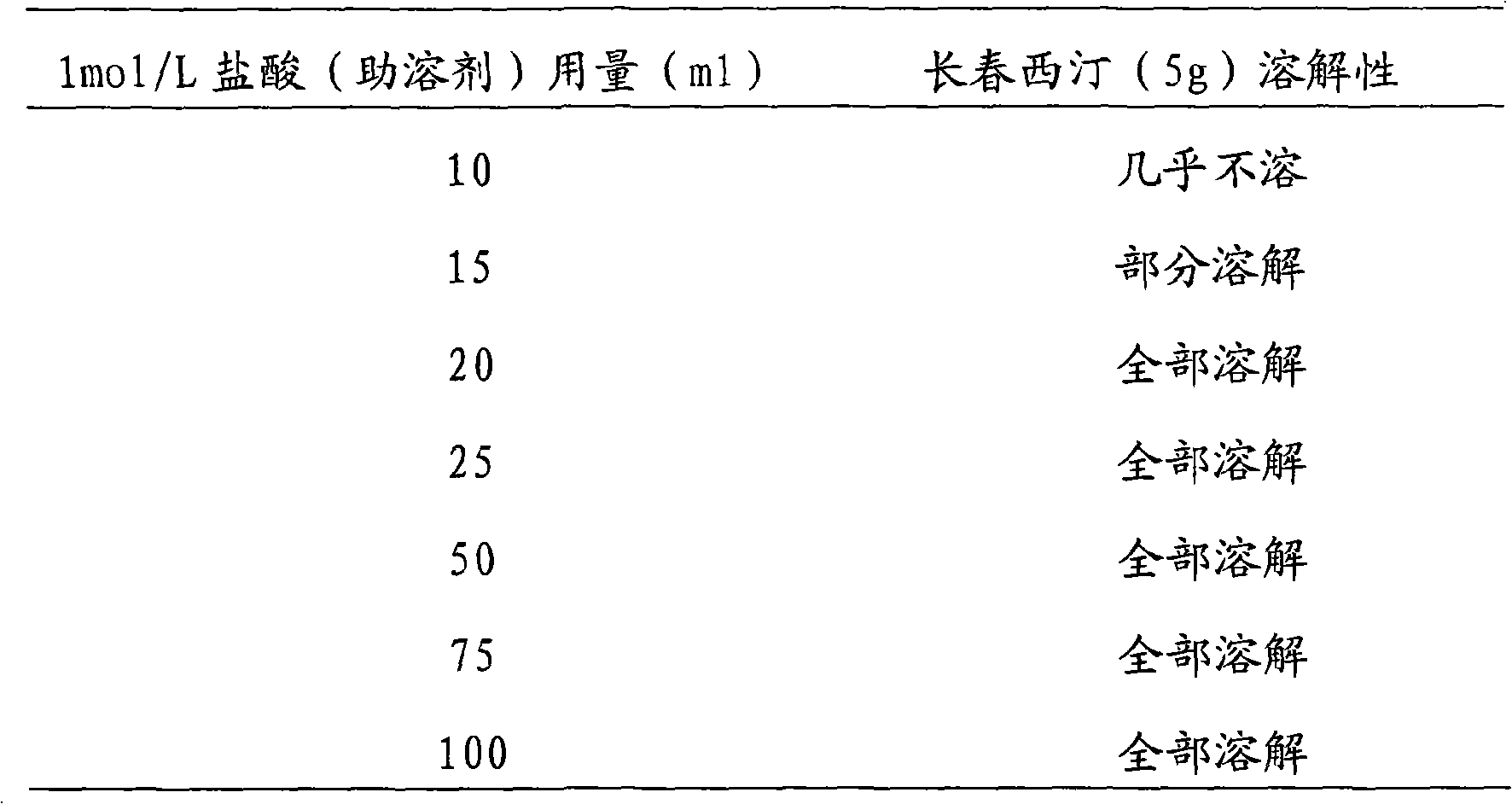
The invention discloses a new vinpocetine freeze-dried injection and the preparation method, which comprises vinpocetine, frozen-dried supporting agent mannitol and cosolvent hydrochloride. The invention is characterized in that: The salt is formed by the hydrochloride with stable and safe physicochemical properties used as cosolvent and the vinpocetine, thereby successfully producing the vinpocetine freeze-dried injection. The vinpocetine freeze-dried injection has the advantages of good solubility, stability and safety compared with prior vinpocetine freeze-dried injection.
Owner:开封康诺药业有限公司
Vinpocetine containing high-capacity sodium chloride injection and preparation method thereof
InactiveCN101991530AImprove bioavailabilityQuality improvementOrganic active ingredientsNervous disorderVinpocetineSodium Chloride Injection
The invention provides a vinpocetin containing high-capacity sodium chloride injection and a preparation method thereof, aiming at solving the problems that vinpocetin is mainly applied to middle aged and elderly patients, high-capacity glucose injection contains massive glucose and is not beneficial to treating patients suffering from diabetes at the same time. The invention has the key point that the injection is mainly composed of vinpocetin, osmotic pressure regulator, cosolvent and pH value regulator. The vinpocetin high-capacity sodium chloride injection of the invention has high bioavailability and stable quality, is convenient to use and is applicable to treatment of patients suffering from neurologic disease caused by cerebral circulation restriction, especially the patients suffering from diabetes at the same time.
Owner:SHENYANG ZHIYING PHARMA FACTORY
Medicament for treating cerebral blood-vessel dilate and preparation method thereof
InactiveCN102113994AImprove securityGood curative effectOrganic active ingredientsPharmaceutical delivery mechanismVitamin CFreeze-drying
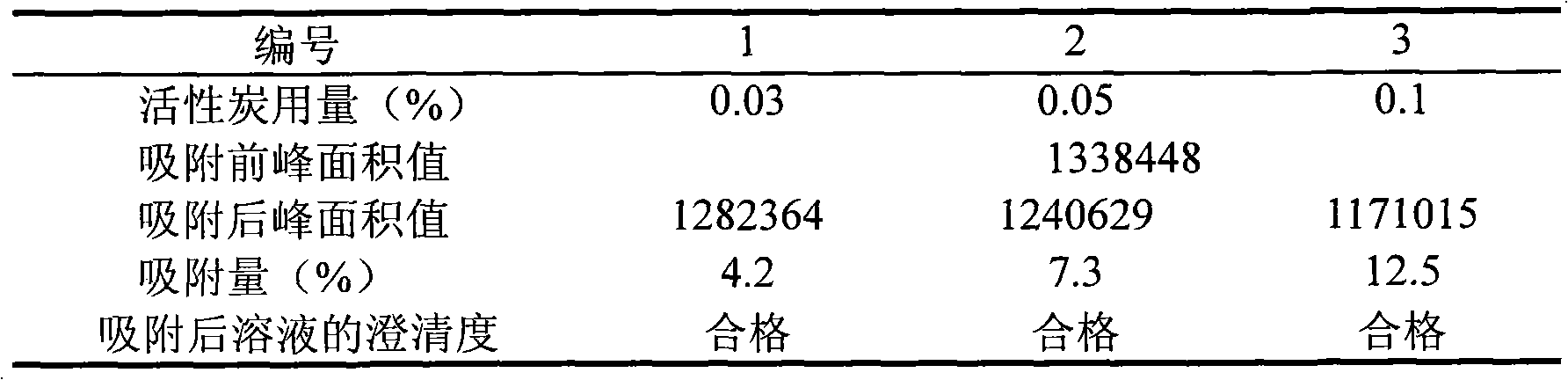
The invention relates to a medicament for treating cerebral blood-vessel dilate and a preparation method thereof, in particular relating to a vinpocetine freeze-dried preparation for treating cerebral blood-vessel dilate. The vinpocetine freeze-dried preparation is prepared from the following raw and auxiliary materials in parts by weight: 5 to 15 parts of the vinpocetine, 0.5 to 5 parts of vitamin C, 1 to 5 parts of sodium pyrosulfite, 100 to 300 parts of mannitol, 50 to 150 parts of sorbitol, and 1 to 10 parts of tartaric acid. The raw material medicaments and auxiliary materials are added according to the conventional method in the field to prepare a preparation for intravenous drip. The preparation has the characteristics of stable quality, safety and obvious curative effect.
Owner:长春海悦药业股份有限公司
Lifeforce liquid supplement
InactiveUS20110189319A1Maximum protectionBiocideOrganic active ingredientsDiseaseAdditive ingredient
Two very important factors in human life—which play vital roles in promoting well being—includes returning the body to homeostasis and giving boost to body called lifeforce to get overall health free from diseases, i.e., its optimal balanced functioning ability. When the body is at homeostasis, there is no place for disease. It is only when the body is out of balance (something is deficient, in excess or stagnating) that pain, illness or disease can occur. The application described is based on returning the body to homeostasis (removing the problem and balancing the body) by providing lifeforce and not on dealing with the symptom (e.g., pain, vitamin deficiency). Imbalance results in various diseases and adverse health conditions (starting from aging to diabetes and cancers). This wonderful composition will interact with the body in a way that allows it to boost lifeforce and reach homeostasis no matter which direction it was. In this combination, in the form of liquid, all the 14 ingredients (Lepidium meyenii (Maca), Croton planstigma (Dragon's blood) tree sap, Uncaria tomentosa (Cat's Claw), Morinda citrifolia (Noni fruit) 4:1 PE, Lutein, Lycopene 5%, Flaxseed Oil (Omega-3-Fatty Acids), Vinpocetine, Phosphatidyl Serine 50%, Korean Ginseng 80%, Bacopa monnieri (Bacopin), CDP Choline (Cognizing), Guaranine (Guarana Seed PE 12%), Yerba Mate Ext. 8%) work as mixture for returning the body to homeostasis and give it lifeforce.
Owner:LEITMAN LORN +1
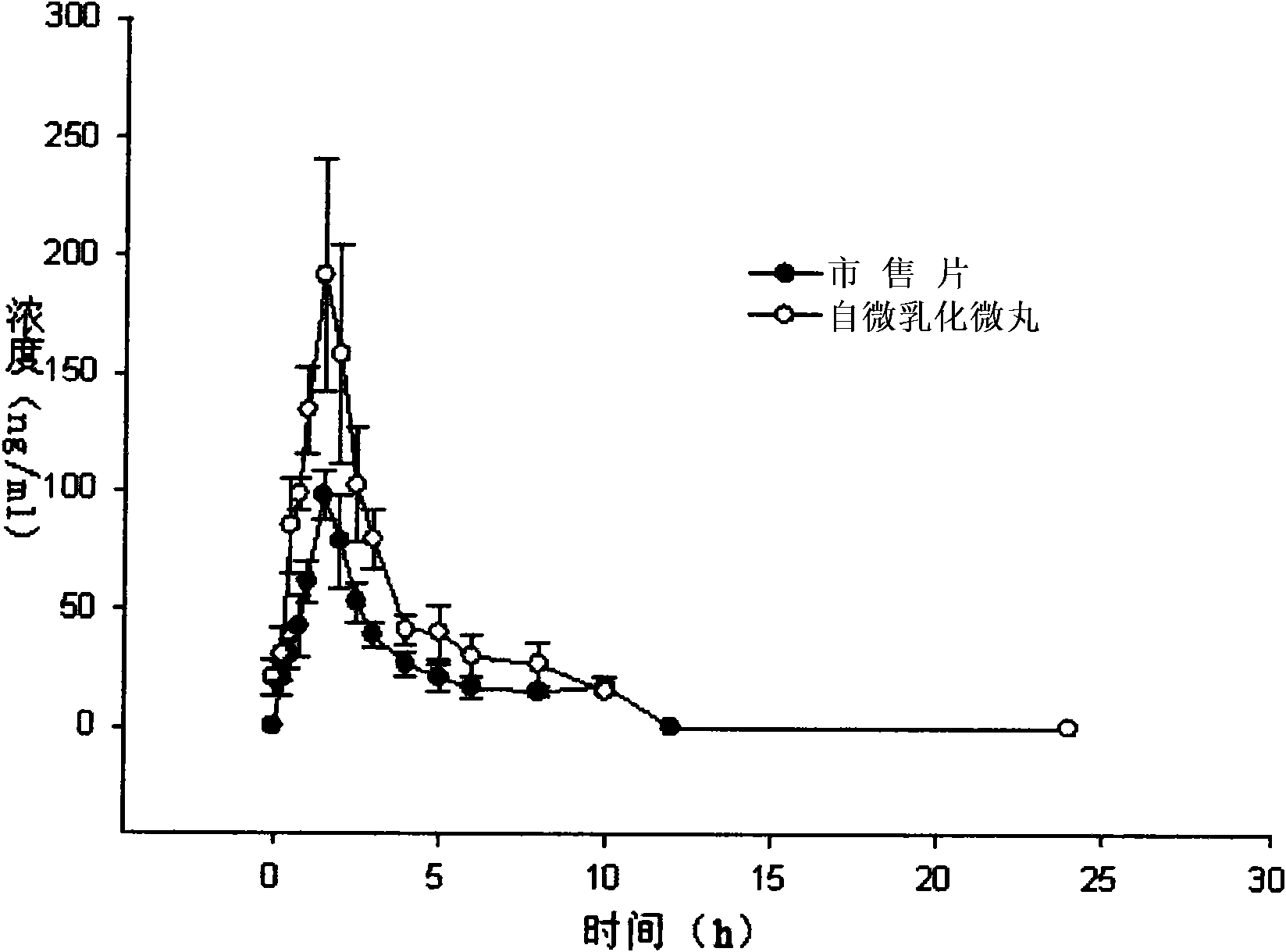
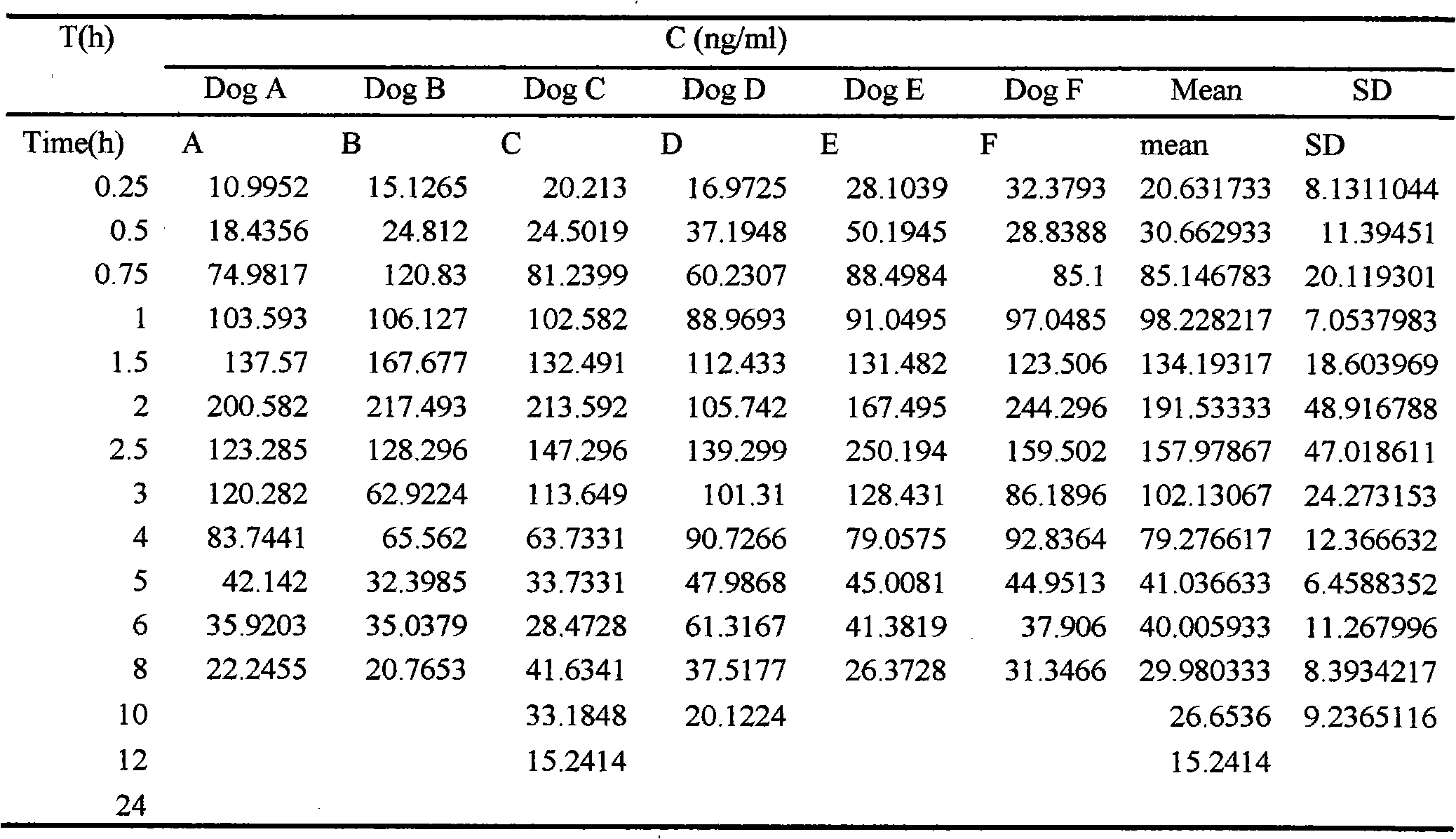
Vinpocetine oral self-micro-emulsification medicine-releasing system and preparation method thereof
InactiveCN101103962AImprove bioavailabilityImprove solubilityOrganic active ingredientsNervous disorderSolubilityEmulsion
The invention relates to a vinpocetine oral self-micro-emulsifying drug delivery system and the preparation method. The vinpocetine oral self-micro-emulsifying drug delivery system is composed of vinpocetine, fat phase, emulsifier and coemulsifier. The mass ratio percentage preferential scope of the ingredients is that the vinpocetine occupies 1-10 per cent, the fat phase occupies 20-60 per cent, the emulsifier occupies 25-70 per cent and the coemulsifier occupies 0-25 per cent. The vinpocetine oral self-micro-emulsifying drug delivery system can be spontaneously emulsified into micro-emulsion with 10-100 mm granule diameter after taken orally, promoting drug to be quickly absorbed. The drug delivery system has the advantages of improving the solubility of the vinpocetine in water, improving the availability of the vinpocetine and eliminating the influence of food to the absorption and bioavailability of the vinpocetine.
Owner:华中科技大学同济医学院
Diet supplement for causing rapid weight loss, controlling appetite, managing stress, supporting relaxation, combating fatigue and supporting mental well-being.
InactiveUS20070237786A1Fast weighingAppetite suppressantBiocideMetabolism disorderDietary supplementMental health
Owner:SMARTBURN FORMULATIONS LTD
Vinthitine liposome
InactiveCN1723896AImprove poor oral absorption and low bioavailabilityGood curative effectOrganic active ingredientsLiposomal deliveryNeurotransmitter systemsCholesterol
A vinpocetine lipid with high absorptivity, biologic utilization rate and stability for treating cardiovascular and cerebropathy caused by senility is prepared proportionally from vinpocetin, phosphatide, cholesterol and the supporting agent chosen from sorbitol, mannitol, cane sugar, sodium chloride, water-soluble starch etc.
Owner:胡才忠
Vinpocetine injection and preparation method thereof
InactiveCN102160853AImprove securityStrong irritantOrganic active ingredientsInorganic non-active ingredientsForeign matterMedicine
The invention relates to a vinpocetine injection and a preparation method thereof. The vinpocetine injection is mainly prepared from vinpocetine, a cosolvent and a physical stabilizer and contains a pH regulator and a metal ion chelator. The pH range is close to the neutrality, the irritation of the vinpocetine injection is small, the stability of the vinpocetine injection is good, and antioxidants and vascular irritation regulators do not need to be added. The invention has the beneficial effects that the chemical stability of the vinpocetine injection is better, the vinpocetine injection can be stored at room temperature, the foreign matter inspection result meets the rules, and the safety of the vinpocetine injection is high.
Owner:方宝林 +2
Semi-synthesis of vinpocetine through one kettle way and preparation of water-soluble vinpocetine salt
InactiveCN102485723AConducive to large-scale industrial productionAtom economy is highOrganic chemistryFood additiveVinpocetine
The invention relates to a semi-synthesis of vinpocetine and a preparation of a vinpocetine salt, wherein vincamine is used as a raw material. The one kettle way for the synthesis of vinpocetine allows the production efficiency to be improved and the total yield to be above 85%; and a method of the preparation of the vinpocetine salt is simple, and the yield is above 85%. Compared with original production methods, the method of the invention, which has the advantages of high atom economy, and concise and controllable process flow, is in favor of the production of GMP (good manufacturing practice) grade bulk medicines and food additives.
Owner:江苏斯威森生物医药工程研究中心有限公司
Concentration and mental performance amplifying formulation
InactiveUS20120177732A1Improve focus performanceIncrease mental performancePowder deliveryBiocideRHODIOLA ROSEA ROOTVinpocetine
A concentration and mental performance amplifying formulation comprising rhodiola rosea and geranium oil, together with at least one of vinpocetine, phosphatidylcholine, caffeine and Salix alba (White Willow Bark). In another aspect, there is disclosed a concentration and mental performance amplifying formulation comprising: rhodiola rosea, geranium oil, and at least one of anhydrous caffeine and nicotine. In yet another aspect, there is disclosed a concentration and mental performance amplifying formulation as last described which further comprises: Vinpocetine, or Vincamine together with Huperzine-A, or Vinca minor (Periwinkle) together with Huperzine-A; PhosphatidylCholine or Bacopa monnieri (Brahmi) or Mucuna pruriens; Nicotinamide Adenine Dinucleotide Hydrate; and Salix alba (White Willow Bark). Also described is a method of amplifying concentration and mental performance in a human subject by administering such formulations to such a subject.
Owner:BUCKLEY MICHAEL SCOTT
Dietary supplement and a method to enhance sleep and lucid dreaming
InactiveUS20080107754A1Enhance lucid dreamingPromote lucid dreamingBiocideNervous disorderAdditive ingredientDietary supplement
A nutritional supplement for enhancing sleep and lucid dreaming in humans. It contains a combination of ingredients in proportions calculated to enhance lucid dreaming. The primary ingredients are Calea zacatechichi, L-5-Hydroxytryptophan (L-5-HTP), and Vinpocetine. In addition, the nutritional supplement may include the secondary ingredient Melatonin and the tertiary ingredients Wild Lettuce Extract, Mugwort Extract, Dimethylaminoethanol Powder (DMAE), Passionflower Extract and Green Tea Extract. Further, various Vitamins may be added such as certain B vitamins, D and C, as well as Zinc, Magnesium and Calcium. The selection and amounts of the ingredients of the nutritional supplement promotes sleep and lucid dreaming in people who have taken the nutritional supplement prior to going to sleep.
Owner:LUCIANO JEFF
Neuro-degenerative inhibitor, neuro-endocrine modulator, and neuro-cerebral metabolism enhancer
InactiveUS20100189819A1Minor side effectsMinimal toxicityBiocideSenses disorderEndocrine functionsNiacinamide
Neuro-metabolic and endocrine-function regulating / modulating compositions, are disclosed. The compositions of the present invention comprise Selegiline Hydrochloride, Procaine Hydrochloride, Vinpocetine, trimethylglycine, and an ingredient selected from a group consisting of N-nicotinoyl-gamma-aminobutyric acid (N-GABA), niacin, niacinamide, gamma-aminobutyric acid (GABA), and combinations thereof. Methods of using the compositions, compositions, and compositions of the present invention are also disclosed.
Owner:INTRATHERAPIES
Preparation technology of high-purity vinpocetine
The invention relates to an optimized preparation technology of high-purity vinpocetine. According to the invention, vincamine is used as a raw material, a high-purity dehydrated methyl ester intermediate is obtained through a dehydration reaction, and then the vinpocetine is directly generated by an ester exchange reaction; or the dehydrated methyl ester intermediate is subjected to basic hydrolysis to generate corresponding acid, then thionyl chloride is dropwise added into the absolute ethyl alcohol solution of the acid, and the vinpocetine is generated by an esterification reaction. Through the invention, the production cost is obviously reduced, little environmental pollution is caused, the experimental operation is simple, the product is easy to separate and purify, and the product purity exceeds 99.8%.
Owner:孙新鹏
Vinpocetine polymer micelle preparation and preparation method thereof
ActiveCN102327208AImprove solubilityImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismPolymer scienceOral medication
The invention discloses a vinpocetine polymer micelle preparation and a preparation method thereof, and belongs to the technical field of medicinal preparation. The vinpocetine polymer micelle preparation is formed by embedding vinpocetine into polyethylene glycol polylactic acid, wherein a mass ratio of the vinpocetine to the polyethylene glycol polylactic acid is (1-12):20; and the weight-average molecular weight of the polyethylene glycol polylactic acid is between 7,000 and 45,000, the molecular weight of polyethylene glycol is between 2,000 and 15,000, and the molecular weight of polylactic acid is between 5,000 and 30,000. In the preparation method, the vinpocetine polymer micelle preparation is prepared by a membrane hydration method which is subjected to condition screening, and hydration temperature, hydration time, administration dose and the like are limited optimally, so in the obtained vinpocetine polymer micelle preparation product, the defects of poor oral administration absorption and low bioavailability of the vinpocetine are overcome.
Owner:GUANGDONG PHARMA UNIV
Preparation method for vinpocetine
The invention provides a preparation method for vinpocetine. The method comprises the following steps: with vincamine as a raw material and triethyl formate as an esterification reagent, subjecting vincamine and triethyl formate to a one-step reaction under the action of Lewis acid so as to obtain a crude vinpocetine product; and then carrying out washing with alkali lye, decoloring in alcohol and recrystallization so as to prepare a refined vinpocetine product. Compared with the prior art, the preparation method provided by the invention has the advantages of short process flow, simple operation and suitability for industrial production, and the prepared refined vinpocetine product has yield of more than 89%, a melting point of 148 to 151 DEG C, HPLC of greater than 99%, a whitish color, good quality and low preparation cost.
Owner:HUNAN KEYUAN BIO PRODS
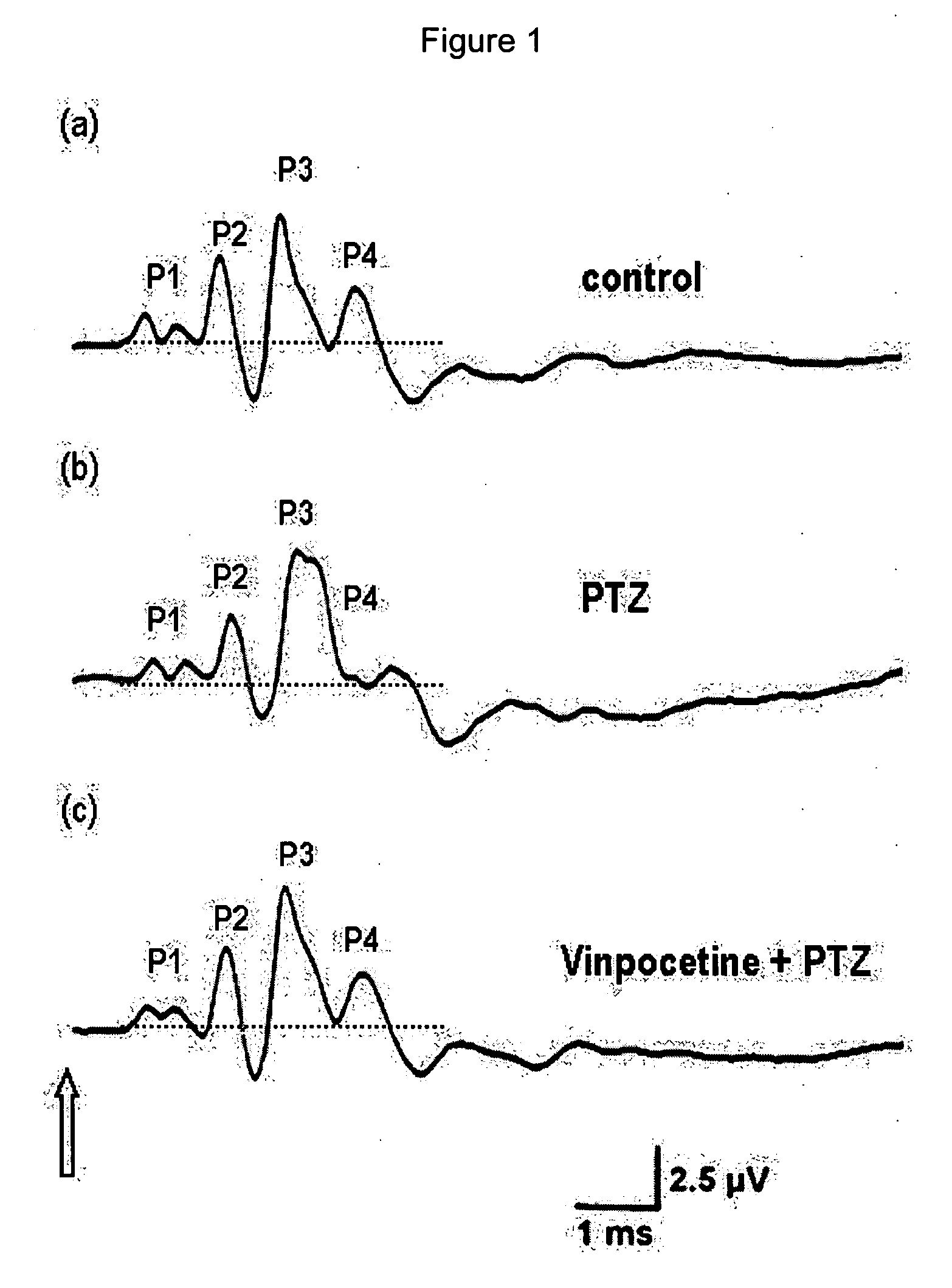
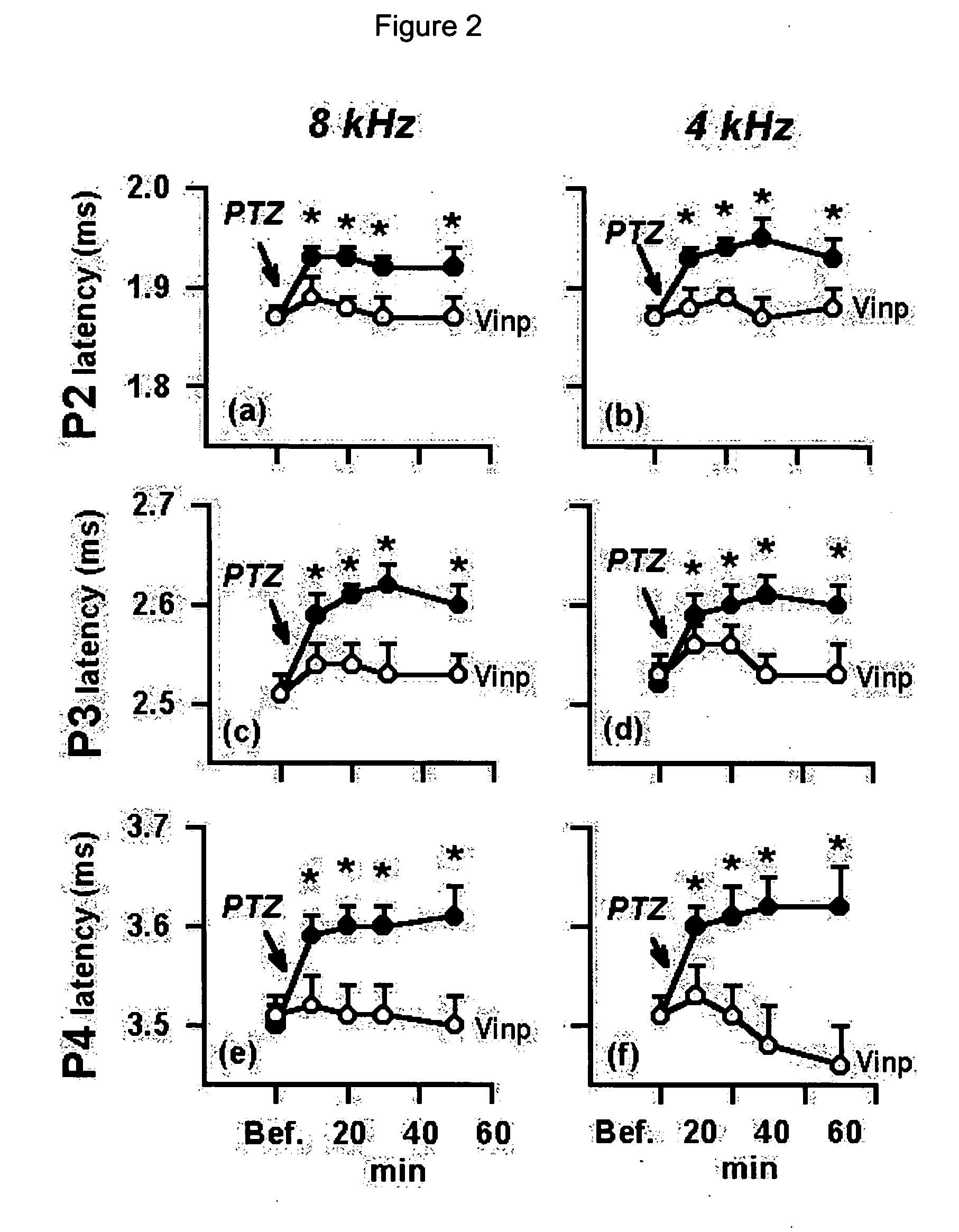
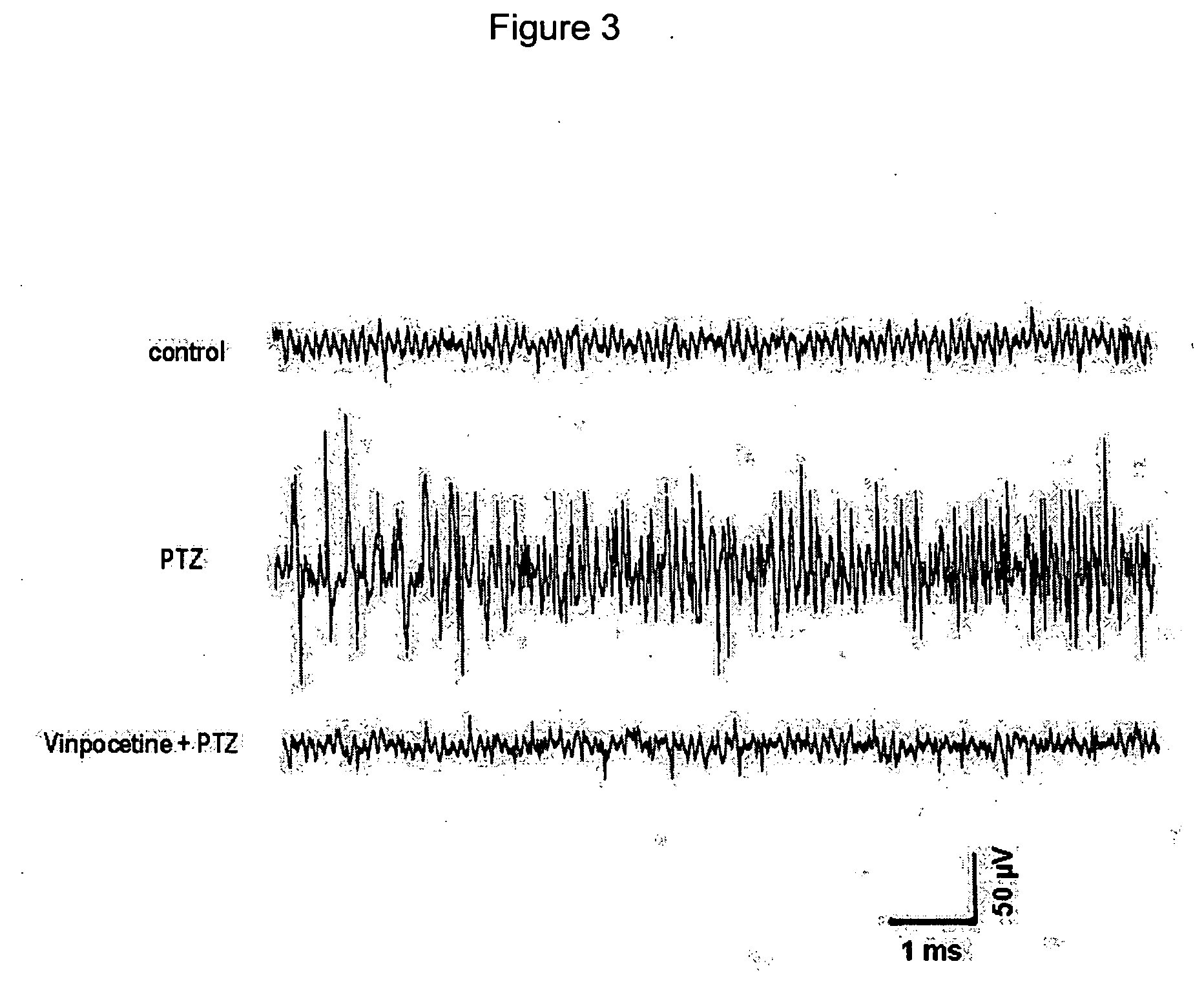
Utilization of vinpocetine to avoid complications in particular those associated to hearing which occur with epilepsy, and treatment thereof
The present invention is related with the use of vinpocetine and the derivates developed from its formula that maintains the same effects for the treatment of epilepsy and its complications. Our results show that vinpocetine prevents all the abnormalities of the ABR waves that accompany the epileptic cortical activity observed for the ictal and post-ictal periods in two experimental models of epilepsy in vivo, that vinpocetine also inhibits the marked hearing loss and the characteristic EEG changes induced by two convulsing agents that differ in their mechanisms of action. These findings also indicate that the capacity of vinpocetine as an antiepileptic drug is not accompanied by adverse secondary effects.
Owner:UNIV NAT AUTONOMA DE MEXICO
Revitalizing hair follicles with total parenteral nutrition or hyperalimentation for maximum hair growth
InactiveUS20070081967A1Promotes hair growthCosmetic preparationsBiocideParenteral nutritionPantothenic acid
The present invention relates to composition and method for revitalizing hair growth, rebuilding and restoring the natural integrity of the hair follicles wherein a first treatment formulation containing active ingredients comprising of vinpocetine, niacin, proanthocyanidins, azelaic acid, isopropyl alcohol, propylene glycol, and water is applied to the scalp as a liquid suspension, lotion, gel or cream, following, a second treatment formulation containing active ingredients comprising of total parenteral nutrition or hyperalimentation, folic acid, propylene glycol, and water is applied to the scalp as a liquid suspension, lotion, gel, or cream, and lastly, a third treatment formulation for men containing sal palmetto, niacin, vinpocetine, folic acid, pantothenic acid, Vitamin A, Vitamin B-6, Zinc, PABA, grape seed extract, thiamine, and biotin, and a third treatment formulation for women comprising of niacin, vitamin A, thiamine, vitamin B-6, pantothenic acid, biotin, PABA, zinc, vitamin e, citrus bioflavonoids, calcium, riboflavin, and inositol.
Owner:CERULLO JOHN +1
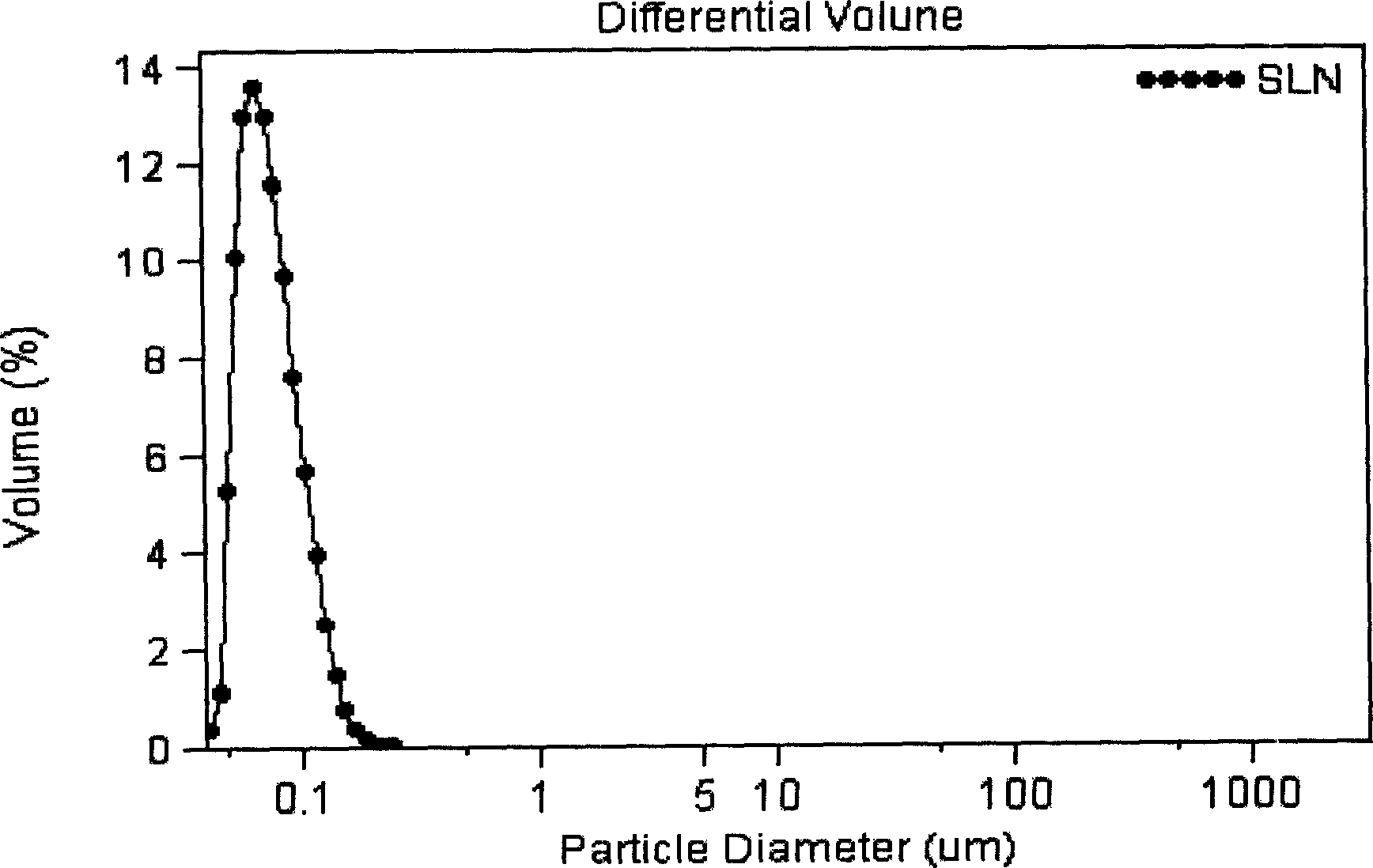
Vinpocetine solid liposome nano-particle and its preparation technology
InactiveCN1823776ASmall particle sizeHigh encapsulation efficiencyOrganic active ingredientsPowder deliveryVinpocetineHead injury sequelae
A solid lipid nanoparticle of vinpocetin used for preparing orally taken medicine or injection for treating the circulatory disturbance type cerebrovascular diseases including cerebral infarction, sequelae of encephalorrhagia, cerebral arteriosclerosis, etc, improving cerebral function and delaying cerebral senility is prepared from xinpocetin, phosphatide, emulsifier and additive.
Owner:SHENYANG PHARMA UNIVERSITY
Method of detecting apovincamine acid and vincamine acid in vinpocetine simultaneously
InactiveCN110455944AEasy detectionEasy to separateComponent separationChromatographic separationPhosphate
The invention provides a method of detecting apovincamine acid and vincamine acid in vinpocetine simultaneously. The method comprises steps of (1) preparing a test solution by using a vinpocetine rawmaterial or vinpocetine injection; (2) diluting the test solution by 1000 times to be used as a control solution; (3) performing chromatographic separation on the test solution and the control solution by using high-performance liquid chromatography, and recording a chromatograph, wherein chromatographic conditions are as follows: detection wavelength is 262nm-266nm, column temperature is 25DEG C-35DEG C, a mobile phase A is a phosphate buffer solution, a mobile phase B is methanol, and eluting flow rate is 0.8-1.5ml / min; and (4) calculating contents of apovincamine acid and vincamine acid inthe test solution according to a self-control method of a principle component with a calibration factor. Peaks of the vincamine acid and apovincamine acid and the peak of vinpocetine can be well separated. The method can highly adapt to a system, and has low detection limit and high precision.
Owner:武汉华龙生物制药有限公司
Vinpocetine oral administration self-microemulsifying pellet as well as preparation method and application thereof
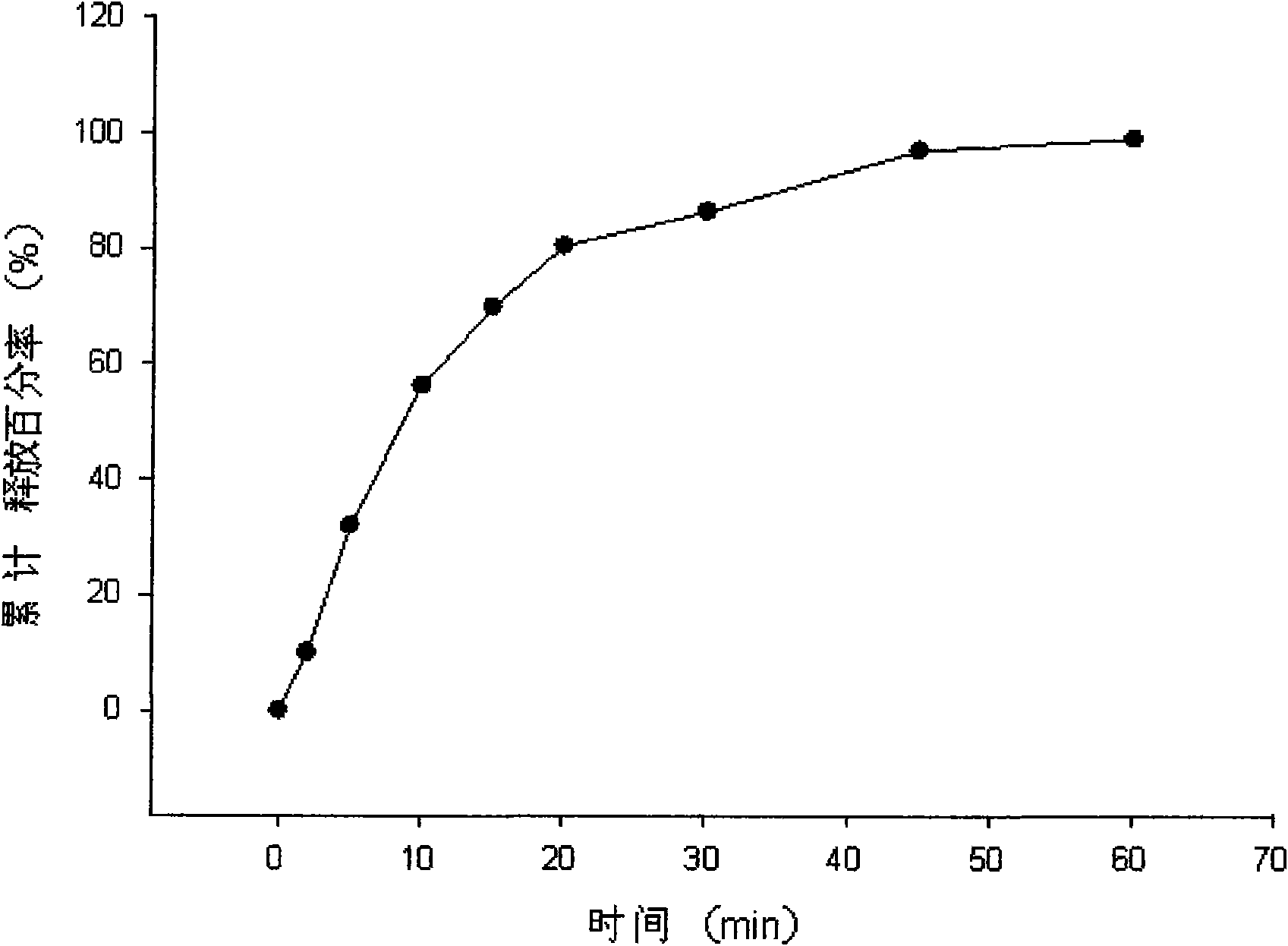
InactiveCN101647782APromote dissolutionImprove clinical efficacyOrganic active ingredientsNervous disorderControlled releaseOral medication
The invention relates to a vinpocetine oral administration self-microemulsifying pellet preparation. After being uniformly mixed, vinpocetine self-microemulsifying concentrated liquor and a common auxiliary material are made into a soft material and further made into a pellet, and an insoluble medicine in the prepared solid preparation can be rapidly dissolved till completion, wherein the vinpocetine self-microemulsifying concentrated liquor comprises the following components in percentage by volume in the preferable range: 1-10 percent of vinpocetine, 10-60 percent of oil phase, 10-70 percentof emulsifying agent and 0-50 percent of assistant emulsifying agent. The pellet prepared in the way can be used for further preparing a medicine release system with properties of slow release and controlled release. The prepared insoluble medicine pellet is rapidly dissolved and can lead about 100 percent of medicines to be released in an hour, and a method is safe and reproducible. The pellet can carry out self-microemulsifying in vivo to form micro-emulsion with the particle diameter of 10-100nm after oral administration, and the dissolution and the absorption of the vinpocetine in water are improved; and the bioavailability of the vinpocetine is improved.
Owner:SHENYANG PHARMA UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com