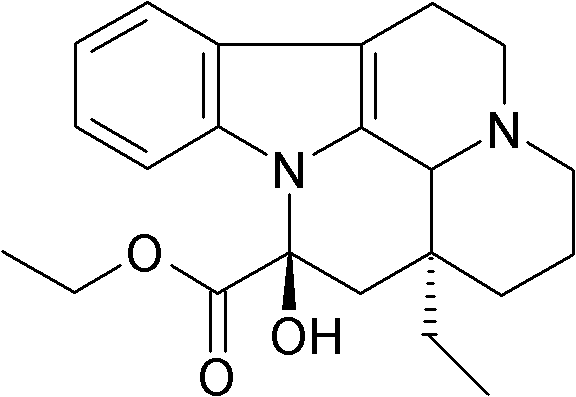
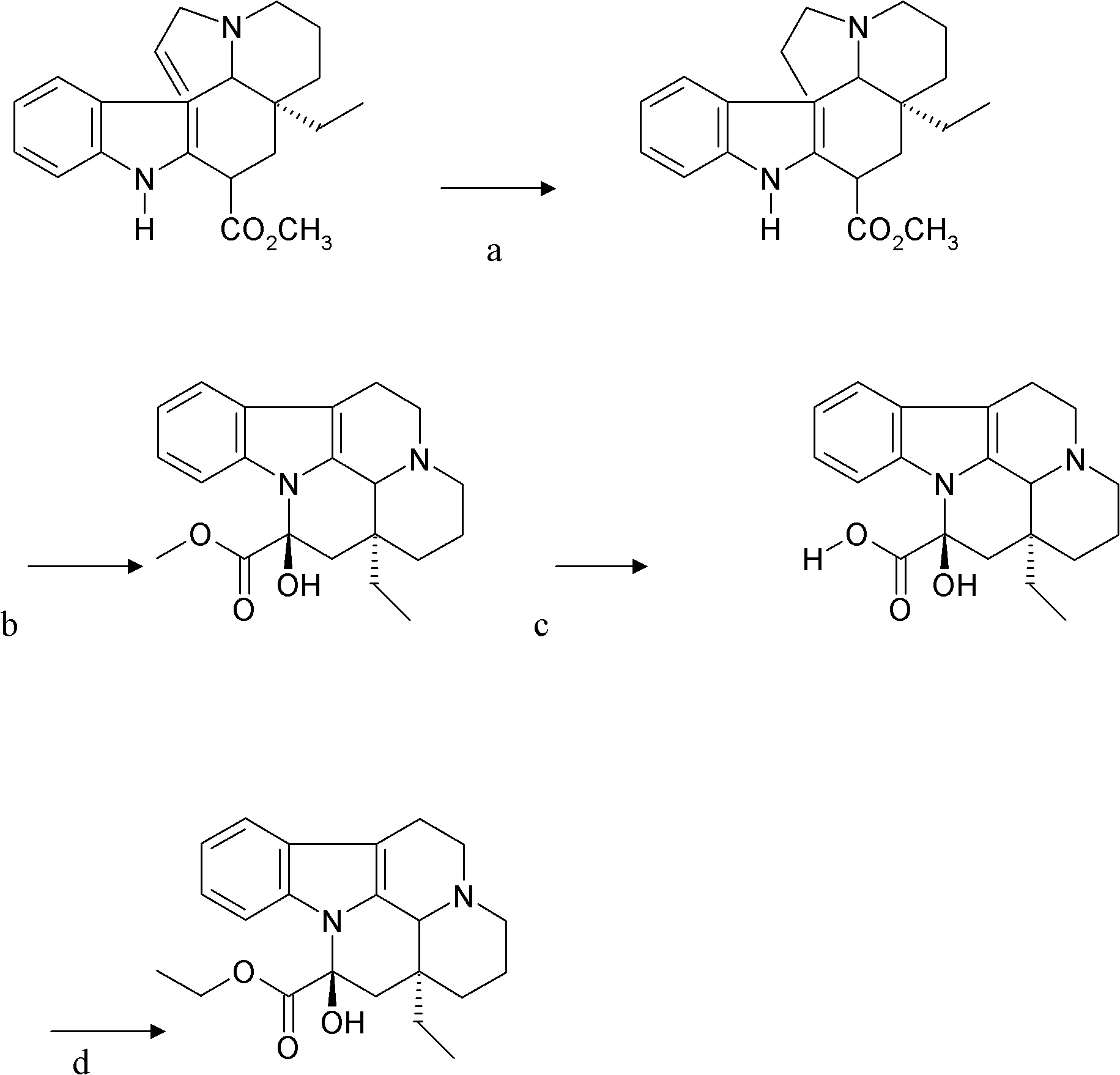
Synthetic method of vinpocetine
A technology of vinpocetine and synthetic method, which is applied in the field of semi-synthesis of active molecules of cerebral vasodilators, can solve the problems of long synthesis steps, low yield, and inability to realize large-scale production, and achieve low cost, high yield, better effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The invention provides a semi-synthetic method for industrialized production of vinpocetine under the premise of ensuring product purity and yield. The invention adopts the African-grown plant tobersonina to extract the raw material tobersonina required for synthesis. Synthesis of vinpocetine step by step.
[0030] The preparation of vinca vinformin includes the hydrogenation of bonin and bonin hydrochloride, the catalyst involves palladium carbon catalyst, and the solvent involves anhydrous methanol, ethanol and other solvents that can dissolve bonin and hydrochloride ;The reaction temperature is 50-60°C, and the hydrogenation pressure is 3-5MP;
[0031] Specifically, first dissolve Tabonin or Tabonin hydrochloride with an extraction content of more than 98% in a solvent of 8 to 12 times the volume, preferably 10 times the volume, and the solvent is generally anhydrous methanol or absolute ethanol , the catalyst is palladium carbon catalyst or platinum oxide catalyst,...
example 1
[0043] 1] The raw material is powdered Taborin, dissolved in 10 times methanol, adding 0.1 times the amount of palladium carbon (10%), hydrogenation at 50-60 °C, pressure 3MP, speed 800rpm, 3-4h, no raw materials by TLC point, remove the catalyst from the reaction solution, dry the methanol solution with anhydrous sodium sulfate, concentrate to 1 / 2 and place it for about 8 hours until the crystallization is complete, filter, and dry at room temperature to obtain the crude product of white vincamine, which is recrystallized with 10 times the volume of petroleum ether , concentrated to 1 / 3 volume, placed to crystallize, suction filtered, and dried to obtain crude product isomorphic vincamine (vinca fermin), which is used for the next step of synthesis;
[0044] 2] Dissolve 50 g of isomorphic vincamine in 2000 ml of anhydrous benzene, then add 80.5 grams of 98% m-chloroperoxybenzoic acid, stir and react at room temperature (26° C.) for 7 hours, until the thin layer detection raw m...
example 2
[0048] 1] Raw material powder 50 grams of Borningin hydrochloride, dissolved in 500ml of ethanol, add 5 grams of palladium carbon (10%), heat up to 50 ~ 60 ° C, pressure 3MP, speed 800rpm, 3 ~ 4h, thin layer Detect no raw material point, remove the catalyst from the reaction solution, dry the methanol solution with anhydrous sodium sulfate, concentrate to 1 / 2 and let it stand for about 8 hours until the crystallization is complete, filter, and dry at room temperature to obtain the crude product of white vincamine, which is mixed with 10 times the volume of petroleum The ether was recrystallized, concentrated to 1 / 3 volume, left to crystallize, suction filtered, and dried to obtain crude vincamine, which was used in the next step of synthesis;
[0049] 2] Dissolve 50 g of isomorphic vincamine in 2000 ml of toluene, add 80.5 g of 98% m-chloroperoxybenzoic acid, stir and react at room temperature (26° C.) for 7 hours, until the thin layer detection raw material point disappears, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com