Patents
Literature
327 results about "Head injury sequelae" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sequelae of traumatic brain injury include headache and dizziness, anxiety, apathy, depression, aggression, cognitive impairments, personality changes, mania, psychosis. Some conditions may be diagnosed retrospectively from their sequelae. An example is pleurisy.
Topical Composition for Treating Pain
ActiveUS20080311167A1Ameliorate and eliminate painFree from painBiocideHydrocarbon active ingredientsSequelaPreventing pain
Topical compositions having as the active ingredient a lipid, fatty acid ester, natural wax, sterol, or combinations thereof referred to herein as “lipophilic vehicle” or “LV” and methods of use, have been developed for the amelioration or prevention of pain or the sequelae of pain. The composition may be in the form of an ointment, cream, gel, lotion, spray, foam, paste, patch, suspension or dispersion. In the preferred embodiment, the formulation is a gel. The LV may contain a penetration enhancer, most preferably one with membrane disruptive properties. The formulation may be applied to or impregnated into a gauze, wrap, bandage, cotton-tipped stick, adhesive bandage strip, or other support wrap or medical bandage or wound cover. For example, the compositions may be are incorporated onto or into disposables such as hemorrhoid wipes, sponge, mouth guards, dental trays; needles or catheters; adult diapers; gloves, socks or wrist bands, for ease of application. The composition is applied topically to a site at or adjacent to a painful region. The composition is reapplied as necessary. Pain relief is typically obtained within minutes and lasts for periods of variable duration ranging from minutes to several hours and even, in some cases, days. The composition is variably effective to treat visceral, somatic and neuropathic pain both acute and chronic as well as muscle pain and stiffness and joint pain and stiffness.
Owner:EPICENTRX
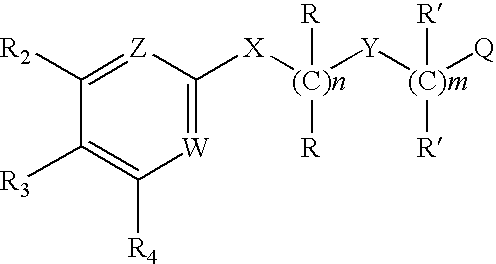
Substituted aryl amides
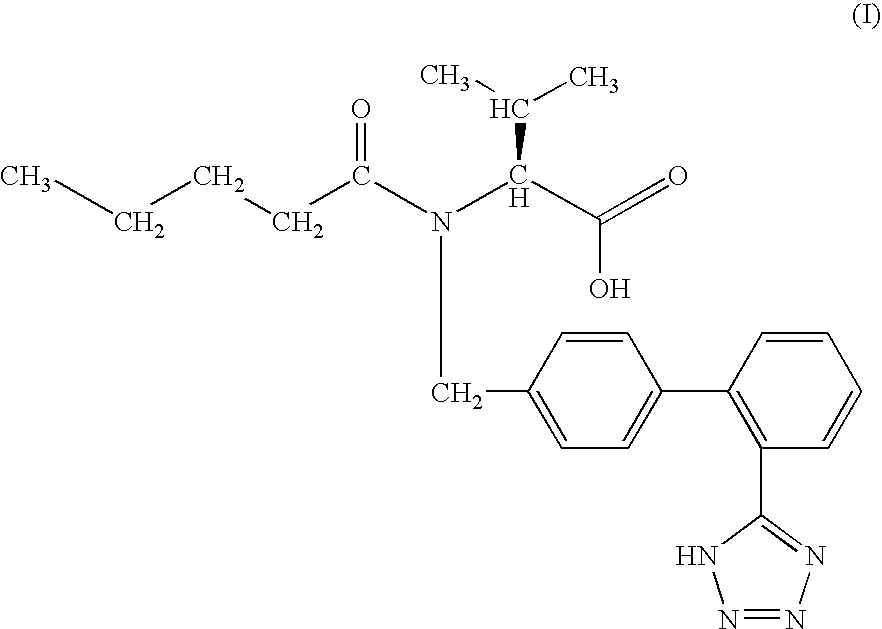
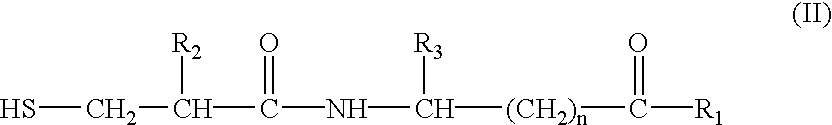
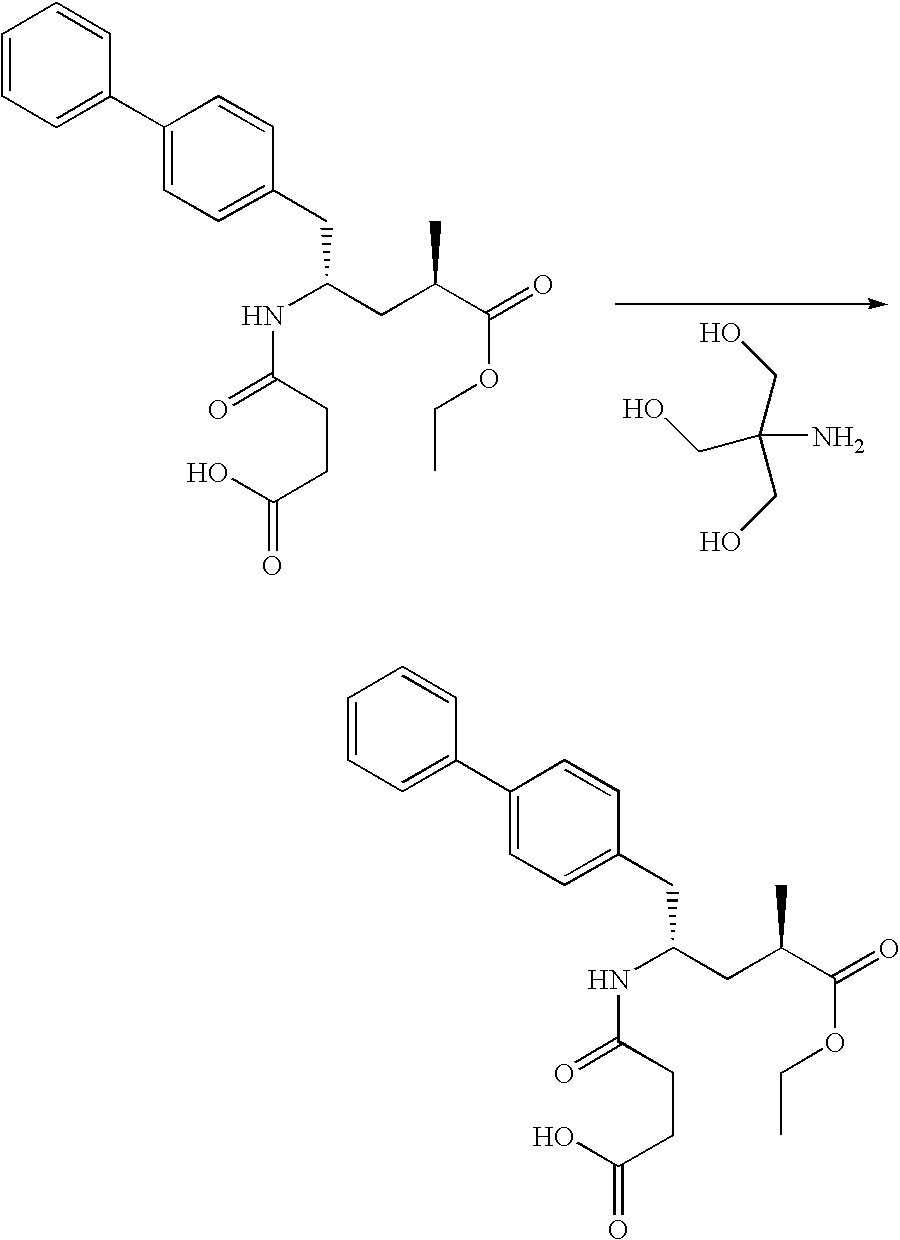
Novel compounds of structural formula (I) are antagonists and / or inverse agonists of the Cannabinoid-1 (CB1) receptor and are useful in the treatment, prevention and suppression of diseases mediated by the CB1 receptor. The compounds of the present invention are useful as psychotropic drugs in the treatment of psychosis, memory deficits, cognitive disorders, migraine, neuropathy, neuro-inflammatory disorders including multiple sclerosis and Guillain-Barre syndrome and the inflammatory sequelae of viral encephalitis, cerebral vascular accidents, and head trauma, anxiety disorders, stress, epilepsy, Parkinson's disease, movement disorders, and schizophrenia. The compounds are also useful for the treatment of substance abuse disorders, the treatment of obesity or eating disorders, as well as, the treatment of asthma, constipation, chronic intestinal pseudo-obstruction, and cirrhosis of the liver.
Owner:MERCK & CO INC
Substituted amides
Novel compounds of the structural formula (I) are antagonists and / or inverse agonists of the Cannabinoid-1 (CB1) receptor and are useful in the treatment, prevention and suppression of diseases mediated by the CB1 receptor. The compounds of the present invention are useful as centrally acting drugs in the treatment of psychosis, memory deficits, cognitive disorders, migraine, neuropathy, neuro-inflammatory disorders including multiple sclerosis and Guillain-Barre syndrome and the inflammatory sequelae of viral encephalitis, cerebral vascular accidents, and head trauma, anxiety disorders, stress, epilepsy, Parkinson's disease, movement disorders, and schizophrenia. The compounds are also useful for the treatment of substance abuse disorders, the treatment of obesity or eating disorders, as well as the treatment of asthma, constipation, chronic intestinal pseudo-obstruction, and cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
Substituted amino ketone compounds
InactiveUS20050014946A1Organic active ingredientsOrganic compound preparationIGT - Impaired glucose toleranceNephrosis
The present invention relates to compounds of the general formula I B—(CH—R1)n—C(═X2)-D (I) and pharmaceutically acceptable salts thereof including stereoisomers, to the use of the compounds for the treatment of impaired glucose tolerance, glucosuria, hyperlipidaemia, metabolic acidosis, diabetes mellitus, diabetic neuropathy and nephropathy and of sequelae caused by diabetes mellitus in mammals.
Owner:PROSIDION LIMITED
Method for increasing therapeutic gain in radiotherapy and chemotherapy
InactiveUS20050272644A1Inhibit tumor growthReduced radiation-induced normal tissue fibrosisBiocideAntipyreticAbnormal tissue growthTumor therapy
The present invention provides compositions and methods for increasing therapeutic gain in radiotherapy and chemotherapy for proliferating malignant or nonmalignant disease to produce high probability of tumor control with low frequency of sequelae of therapy by administering a therapeutically effective amount of a histone deacetylase inhibitor. The compounds are capable of simultaneously stimulating the epithelium regrowth, inhibiting the fibroblast proliferation, decreasing the collagen deposit, suppressing the fibrogenic growth factor, subsiding the proinflammatory cytokine and modulating the expression of cell cycle genes, tumor suppressors and oncogenes, and are useful to increase the therapeutic gain in radiotherapy and chemotherapy, which results in decrease of skin swelling and inflammation, promotion of epithelium healing in mucosa and dermis, decrease of xerostomia, prevention / reduction of severity of plantar-palmar syndrome, prevention of tissue fibrosis, ulceration, necrosis and tumorigenesis, and increase of tumor growth inhibition and tumor therapy effectiveness.
Owner:SUNNY PHARMTECH
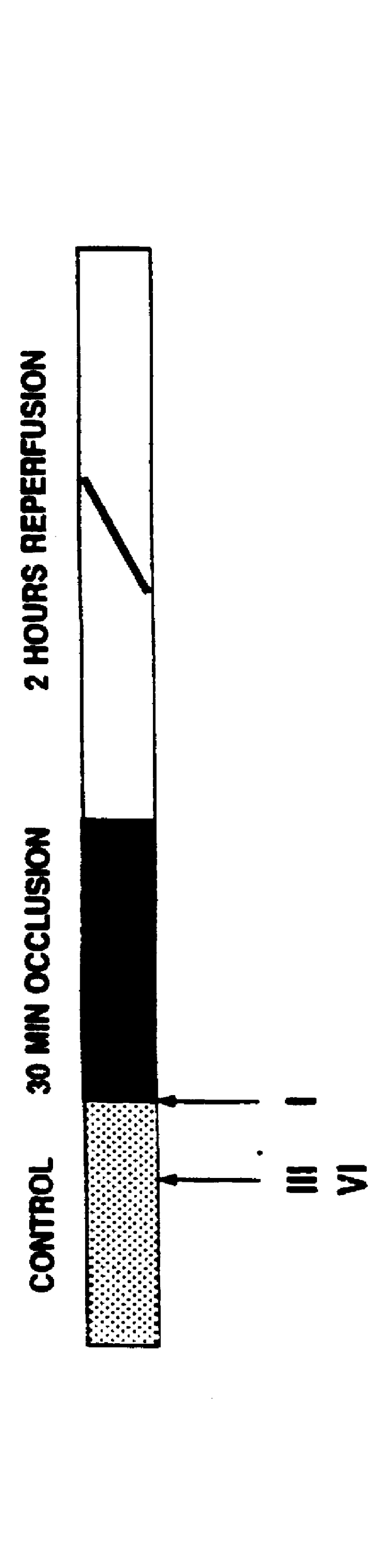
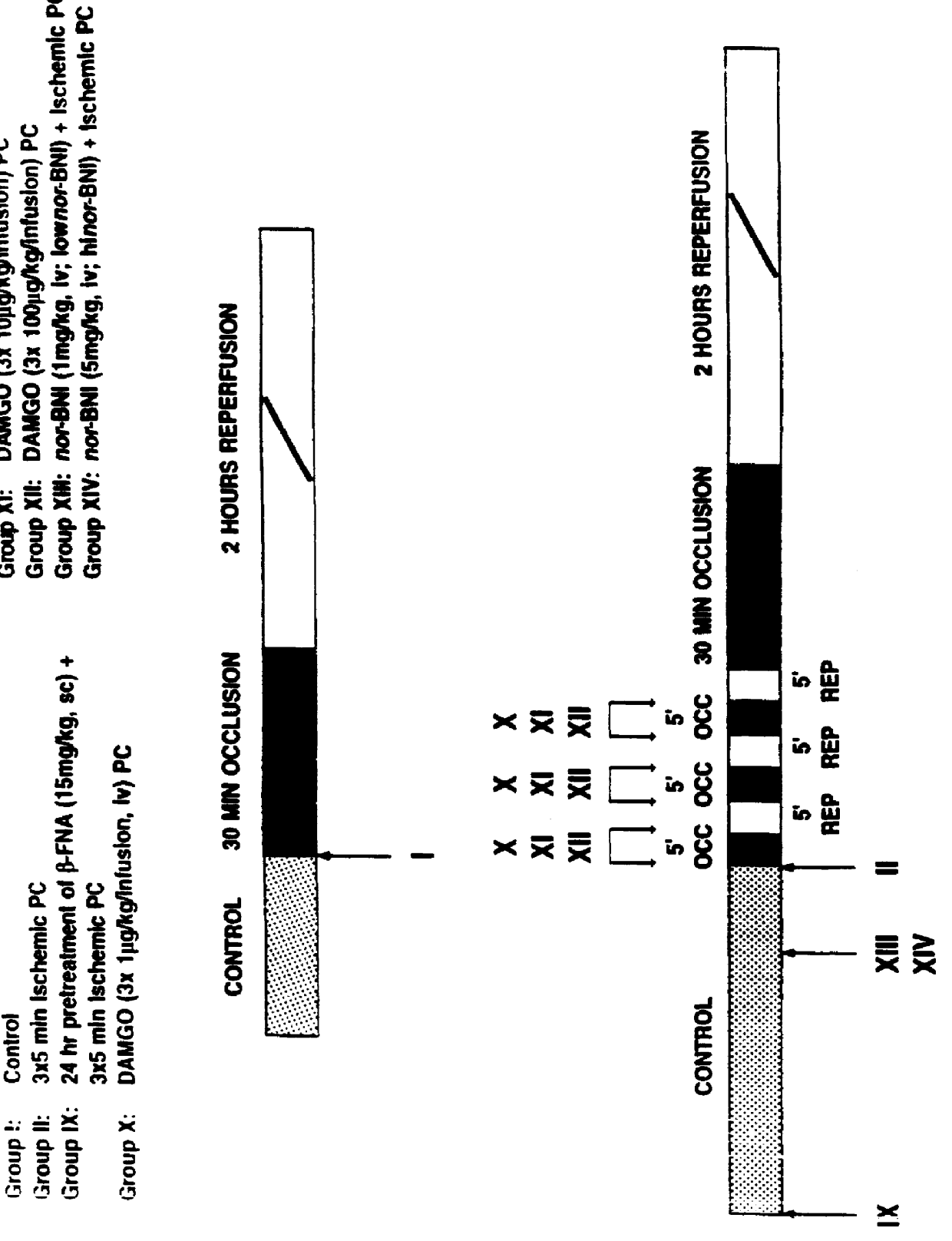
Ischemic preconditioning
InactiveUS6103722AInduce effectAbolished hypotensionBiocideOrganic chemistryCardioprotectionHead injury sequelae
Methods and pharmaceutical compositions of matter are disclosed and claimed relating to cardioprotective effect mediated by delta ( delta ) opioid receptor agonists or more specifically delta-1 ( delta 1)-opioid receptor agonists. Further, methods drawn to reducing ischemic damage to organs and tissues having delta ( delta ) opioid receptor agonists or more specifically delta-1 ( delta 1)-opioid receptors are disclosed and claimed. Specifically, methods and pharmaceutical compositions of matter are taught as a means of providing cardioprotective treatment through the administration of delta ( delta ) opioid receptor agonists or more specifically delta-1 ( delta 1)-opioid receptor agonists, such as TAN67(-). Said methods and pharmaceutical compositions are envisioned as a means of reducing myocardial infarction arising from the onset and sequelae of myocardial ischemia.
Owner:THE MEDICAL COLLEGE OF WISCONSIN INC
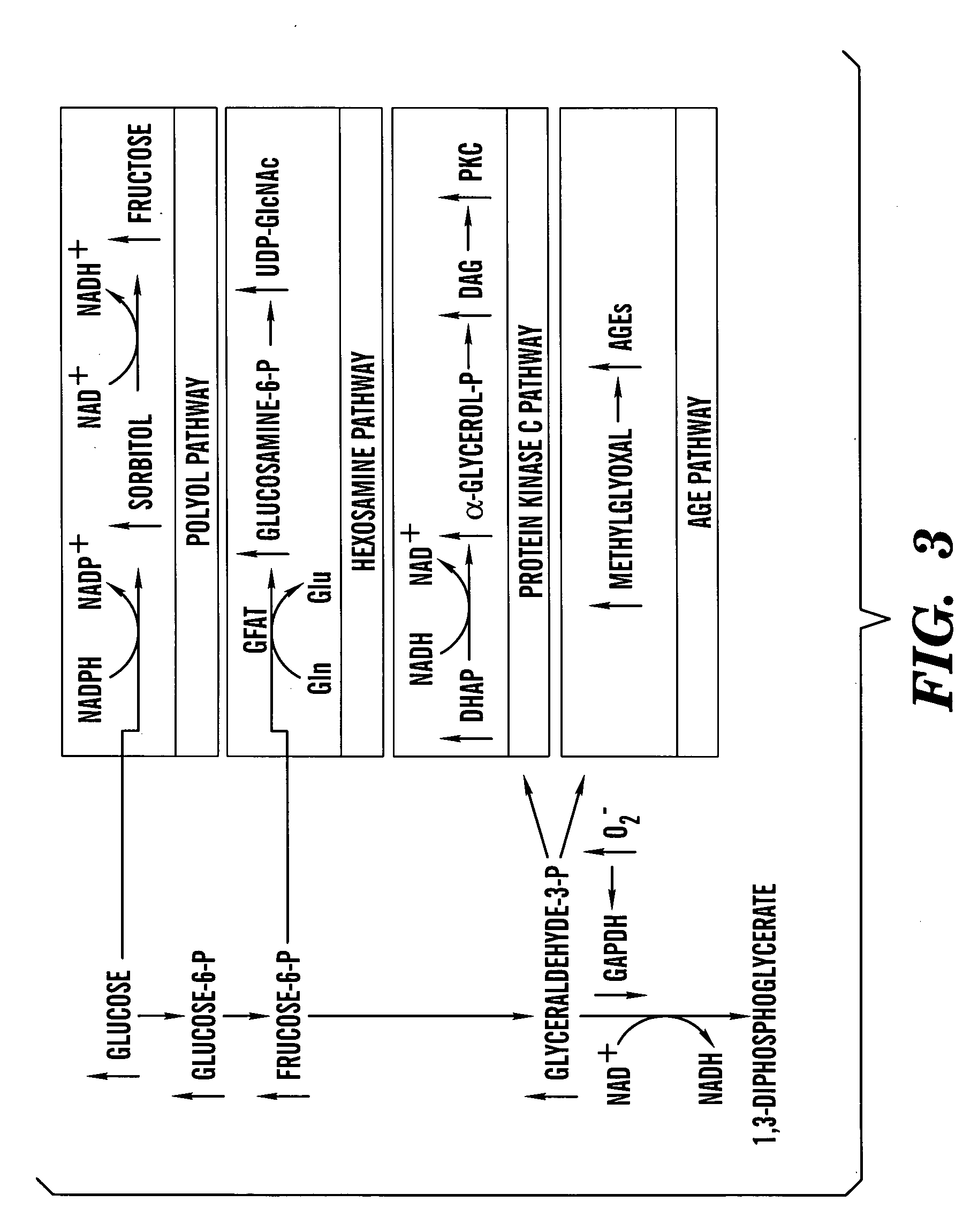
Method of treating or preventing pathologic effects of acute increases in hyperglycemia and/or acute increases of free fatty acid flux
ActiveUS20060100189A1Restoring deficient angiogenesisReduce degradation rateBiocideOrganic active ingredientsAcute hyperglycaemiaAcute hyperglycemia
One aspect of the present invention relates to a method of treating or preventing pathologic sequelae of acute hyperglycemia and / or increased fatty acid flux in a subject. This method involves administering an ROS inhibitor to the subject. In addition, methods of promoting neovascularization, inhibiting oxidation or excessive release of free fatty acids, and identifying compounds suitable for treatment or prevention of ROS-mediated injury are also disclosed.
Owner:GURTNER GEOFFREY C +1
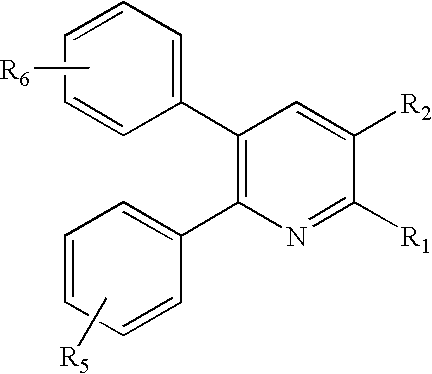
Substituted 2,3-diphenyl pyridines
Novel compounds of the structural formula (I) are antagonists and / or inverse agonists of the Cannabinoid-1 (CB1) receptor and are useful in the treatment, prevention and suppression of diseases mediated by the CB1 receptor. The compounds of the present invention are useful as centrally acting drugs in the treatment of psychosis, memory deficits, cognitive disorders, migraine, neuropathy, neuro-inflammatory disorders including multiple sclerosis and Guillain-Barre syndrome and the inflammatory sequelae of viral encephalitis, cerebral vascular accidents, and head trauma, anxiety disorders, stress, epilepsy, Parkinson s disease, movement disorders, and schizophrenia. The compounds are also useful for the treatment of substance abuse disorders, the treatment of obesity or eating disorders, as well as the treatment of asthma, constipation, chronic intestinal pseudo-obstruction, and cirrhosis of the liver.
Owner:MERCK SHARP & DOHME CORP
Chinese medicinal composition for diseases of nervus, varscular headache, cerebral blood supply lack and sequel of apolexy
A Chinese medicine for treating neuralgia, vascular headache, cerebral ischemia and cerebral apoplexy sequelae is prepared from 41 Chinese-medicinal materials including ginseng, astragalus root, Chinese angelica root, white peony root, etc.
Owner:董兆元
Chinese patient medicament for treating cardiovascular and cerebrovascular diseases
ActiveCN101879298ASolve the disadvantages of drug resistance and recurrent attacks that cannot be controlledSmooth blood flowAnthropod material medical ingredientsInanimate material medical ingredientsDiseaseSequela
The invention relates to a Chinese patient medicament for treating cardiovascular and cerebrovascular diseases and a preparation method thereof. The Chinese patient medicament is prepared from the following raw materials by weight: 0.3 gram of cornu saigae tatariace powder, 12 grams of tabasheer, 15 grams of grassleaf sweelflag rhizome, 15 grams of sea-ear shell, 10 grams of nacre, 15 grams of raw dragon bone and oyster shell, 12 grams of thinleaf milkwort root-bark, 12 grams of bamboo shavings, 20 grams of salivia chinensis, 15 grams of tall gastrodia tuber, 20 grams of gambir plant, 20 grams of common selfheal fruit-spike, 10 grams of radix geutianae, 20 grams of dendrobium, 30 grams of liquor white paeony root, 30 grams of vinegar white paeony root, 30 grams of raw white paeony root, 12 grams of radix curcumae, 15 grams of vinegar Chinese thorowax root, 15 grams of liquor rhubarb, 0.1 gram of musk, 12 grams of turtle shell, 15 grams of stiff silkworm, 20 grams of sanguisuga, 30 grams of earthworm, 25 grams of kudzuvine root, 10 grams of scorpion, 15 grams of desertliving cistanche, 6 grams of liquorice root, 100 grams of astragalus, 15 grams of szechwon tangshen root, 20 grams of heterophylly falsestarwort root, 15 grams of liquor Chinese angelica, 12 grams of szechuan lovage rhizome, 12 grams of safflower and the like. The Chinese patient medicament has the advantages of preventing the evolution period and sequelae of the cardiovascular and cerebrovascular diseases from generating and overcoming the defect that other treatment methods make patients generate medicinal tolerance to various medicaments.
Owner:王巧玲
Macromolecular conjugates of cystic fibrosis transmembrane conductance regulator protein inhibitors and uses therefor
ActiveUS20080171793A1Increased intestinal fluid secretionAvoid transportBiocideNervous disorderDiseaseSecretory diarrhea
Provided herein are bioactive agents comprising a compound that inhibits the ion transport activity of a cystic fibrosis transmembrane conductance regulator (CFTR) and that is linked to a macromolecule that interacts with a cell that expresses CFTR. The bioactive agents described herein are useful for treating diseases, disorders, and sequelae of diseases, disorders, and conditions that are associated with aberrantly increased CFTR activity, for example, secretory diarrhea.
Owner:RGT UNIV OF CALIFORNIA
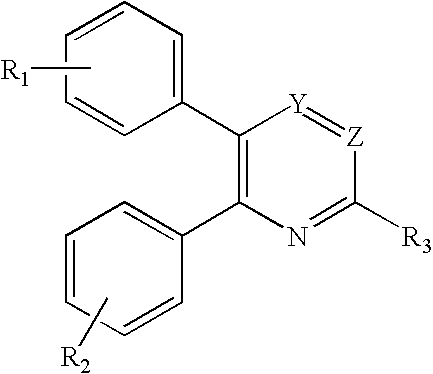
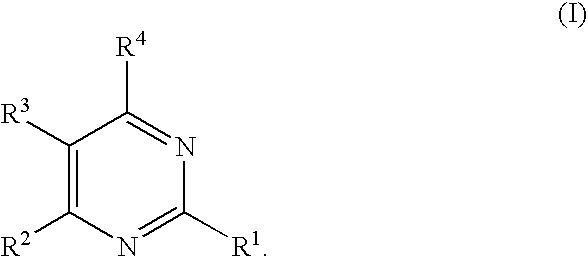
Substituted pyrimidines
Novel compounds of the structural formula (I) are antagonists and / or inverse agonists of the Cannabinoid-1 (CB1) receptor and are useful in the treatment, prevention and suppression of diseases mediated by the CB1 receptor. The compounds of the present invention are useful as centrally acting drugs in the treatment of psychosis, memory deficits, cognitive disorders, migraine, neuropathy, neuro-inflammatory disorders including multiple sclerosis and Guillain-Barre syndrome and the inflammatory sequelae of viral encephalitis, cerebral vascular accidents, and head trauma, anxiety disorders, stress, epilepsy, Parkinson's disease, movement disorders, and schizophrenia. The compounds are also useful for the treatment of substance abuse disorders, the treatment of obesity or eating disorders, as well as the treatment of asthma, constipation, chronic intestinal pseudo-obstruction, and cirrhosis of the liver.
Owner:MERCK SHARP & DOHME CORP
Bicyclic amides
Novel compounds of the structural formula (I) are antagonists and / or inverse agonists of the Cannabinoid-1 (CB1) receptor and are useful in the treatment, prevention and suppression of diseases mediated by the CB1 receptor. The compounds of the present invention are useful as centrally acting drugs in the treatment of psychosis, memory deficits, cognitive disorders, migraine, neuropathy, neuro-inflammatory disorders including multiple sclerosis and Guillain-Barre syndrome and the inflammatory sequelae of viral encephalitis, cerebral vascular accidents, and head trauma, anxiety disorders, stress, epilepsy, Parkinson's disease, movement disorders, and schizophrenia. The compounds are also useful for the treatment of substance abuse disorders, the treatment of obesity or eating disorders, as well as the treatment of asthma, constipation, chronic intestinal pseudo-obstruction, and cirrhosis of the liver.
Owner:MERCK SHARP & DOHME CORP
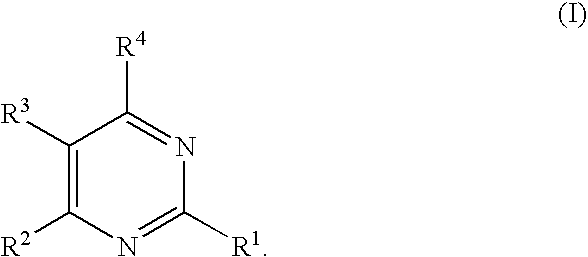
Substituted pyrimidines
Owner:MERCK SHARP & DOHME CORP
Diphenyl cyclopentyl amides as cannabinoid-1 receptor inverse agonists
Novel compounds of structural formula (I) are antagonists and / or inverse agonists of the Cannabinoid-1 (CB1) receptor and are useful in the treatment, prevention and suppression of diseases mediated by the CB1 receptor. The compounds of the present invention are useful as psychotropic drugs in the treatment of psychosis, memory deficits, cognitive disorders, migraine, neuropathy, neuro-inflammatory disorders including multiple sclerosis and Guillain-Barre syndrome and the inflammatory sequelae of viral encephalitis, cerebral vascular accidents, and head trauma, anxiety disorders, stress, epilepsy, Parkinsons disease, movement disorders, and schizophrenia. The compounds are also useful for the treatment of substance abuse disorders, the treatment of obesity or eating disorders, as well as, the treatment of asthma, constipation, chronic intestinal pseudo-obstruction, and cirrhosis of the liver.
Owner:MERCK SHARP & DOHME CORP
Medicament for treating cerebrovascular and cardiovascular disease and apoplexy sequelae
InactiveCN1966004AGood curative effectStroke preventionNervous disorderAnthropod material medical ingredientsSequelaGastrodia
The invention discloses a medicament for treating cardiovascular and cerebrovascular diseases and apoplexy after-effect, which is prepared from Chinese angelica root, Ligusticum wallichii, Sichuan aconite root, poria cocos, dahurian angelica root, agkistrodon acutus, atractylodes rhizome, licorice root, ledebouriella root, Schizonepeta tenuifolia, shiny pricklyash, Chinese ephedra, Dendrobium nobile, scorpion, gastrodia tuber, root of red rooted saliva, fleece-flower root, astragalus root, prepared rehmannia root, radix paeoniae rubrathe, safflower, centipede, hairy deerhorn, earthworm, dried body of ground beetle, eucommia bark, leeches, cherokee rose, and cyathula root.
Owner:李文杰
Pure traditional Chinese medicine preparation for treating cardio-cerebrovascular disease
InactiveCN101396543AConcentrated liquid medicine method is highWay highOrganic active ingredientsAnthropod material medical ingredientsHeracleum hemsleyanumPinellia
A pure traditional Chinese medicine preparation for treating cardiovascular diseases is characterized in that the pure traditional Chinese medicine preparation is prepared by traditional Chinese medicine raw materials with the following parts by weight: 5-10 parts of safflower, 10-20 parts of motherwort herb, 4-8 parts of danshen root, 4-8 parts of Szechuan lovage rhizome, 2-4 parts of medicinal cyathula root, 3-6 parts of peach seed, 2-4 parts of trogopterus dung, 2-4 parts of common burreed rhizome, 2-4 parts of zedoary, 2-4 parts of turmeric, 1-2 parts of dragon blood, 3-6 parts of clematis root, 2-4 parts of gentiana macrophylla, 2-4 parts of heracleum hemsleyanum michaux, 2-4 parts of radix stephaniae tetrandrael, 2-4 parts of sea-ear shell, 2-4 parts of oyster shell, 2-4 parts of puncturevine caltrop fruit, 3-6 parts of uncaria, 1-2 parts of stiff silkworm, 0.2-0.4 part of scorpion, 4-8 parts of rhubarb, 2-4 parts of ephedra herb, 2-4 parts of pepperweed seed, 2-4 parts of bitter apricot seed, 2-4 parts of sessile stemona root, 4-8 parts of unibract fritillary bulb, 2-4 parts of snakegourd fruit, 2-4 parts of arisaema cum bile, 2-4 parts of pinellia tuber, 2-4 parts of nutgrass galingale rhizome, 2-4 parts of rhizoma corydalis, 2-4 parts of Chinese eaglewood, 2-4 parts of cassia seed, 2-4 parts of common selfheal fruit-spike, 1-2 parts of cicada slough, 3-6 parts of honeysuckle flower, 2-4 parts of chrysanthemum, 3-6 parts of baical skullcap root, 2-4 parts of golden thread, 2-4 parts of tree peony bark, 4-8 parts of red paeony root, 4-8 parts of tuber fleeceflower root and 4-8 parts of membranous milk vetch root. The pure traditional Chinese medicine preparation is used for treating various cardiovascular diseases, the sequelae and the complications thereof.
Owner:宁甲保
Prepn process of brain protein hydrolysate injection
InactiveCN101019889ARefractive index qualifiedPrevent oxidationNervous disorderHydrolysed protein ingredientsDiseaseEnzymatic hydrolysis
The brain protein hydrolysate injection is prepared with health swine brain and contains 16 kinds of free amino acids and small amount of peptides. It is used in treating sequelae of craniocerebral trauma and cerebrovascular disease, improving cerebral function, etc. It is prepared through washing, homogenizing, thermal changing, enzyme hydrolysis, freezing, ultrafiltering, sterilizing and other steps.
Owner:石海
Substituted 2,3-diphenyl pyridines
Novel compounds of the structural formula (I) are antagonists and / or inverse agonists of the Cannabinoid-1 (CB1) receptor and are useful in the treatment, prevention and suppression of diseases mediated by the CB1 receptor. The compounds of the present invention are useful as centrally acting drugs in the treatment of psychosis, memory deficits, cognitive disorders, migraine, neuropathy, neuro-inflammatory disorders including multiple sclerosis and Guillain-Barre syndrome and the inflammatory sequelae of viral encephalitis, cerebral vascular accidents, and head trauma, anxiety disorders, stress, epilepsy, Parkinson s disease, movement disorders, and schizophrenia. The compounds are also useful for the treatment of substance abuse disorders, the treatment of obesity or eating disorders, as well as the treatment of asthma, constipation, chronic intestinal pseudo-obstruction, and cirrhosis of the liver.
Owner:MERCK SHARP & DOHME CORP
Chinese medicament for treating neck, shoulder, waist and leg pains
InactiveCN101890104AQuick resultsShort course of treatmentNervous disorderAntipyreticMedicinal herbsDisease
The invention relates to a Chinese medicament, in particular to a Chinese medicament which is prepared from Chinese medicinal herbs and is used for treating neck, shoulder, waist and leg pains. The Chinese medicament is prepared from 16 types of the Chinese medicinal herbs such as root of rehmannia, Chinese taxillus twig, clematis root, dipsacus root, szechuan lovage rhizome, frankincense, myrrh and the like. The Chinese medicament has unique curative effect of treating refractory rheumatoid arthritis, proliferative arthritis, lumbago and scelalgia, frozen shoulder, limb numbness, sciatica, hyperosteogeny, lumbar disc herniation, cervical spondylosis, ankylosing spondylitis, hyperplasia of mammary glands, various intractable diseases and fracture sequelae. The Chinese medicament has scientific compatibility, quick treatment, and the effects of nourishing liver and kidney, promoting blood circulation by removing blood stasis, strengthening sinews and bones, promoting circulation of qi and to relieve pains and balancing yin and yang. The Chinese medicament has the advantages of quick response, short period of treatment, high curative ratio and low expense.
Owner:杨中发
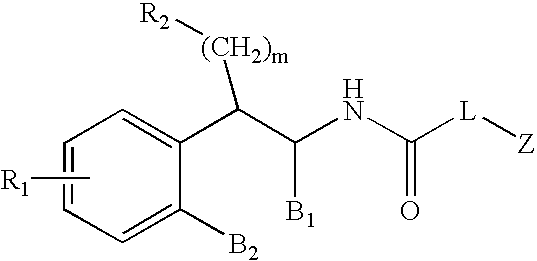
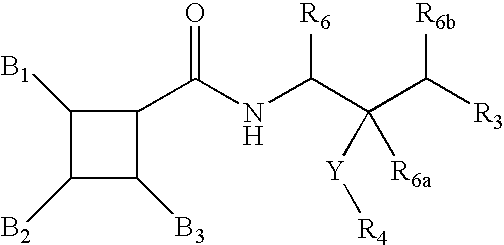
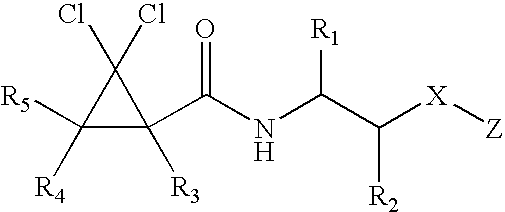
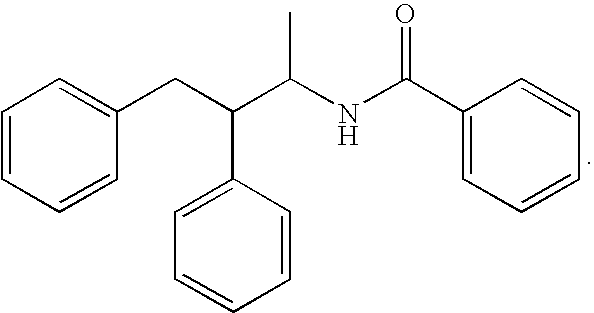
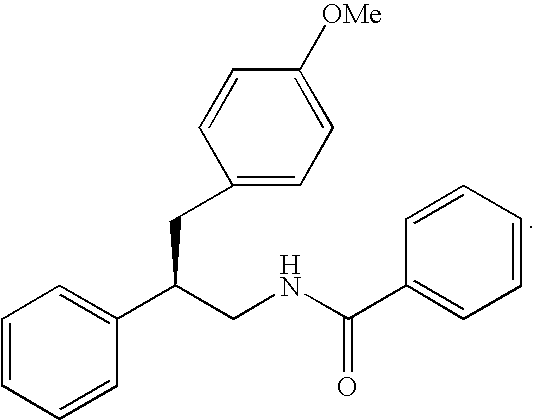
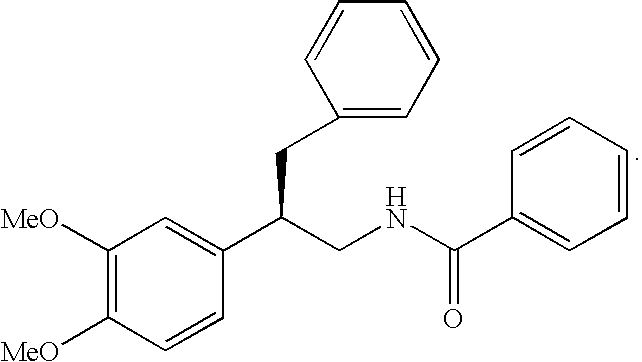
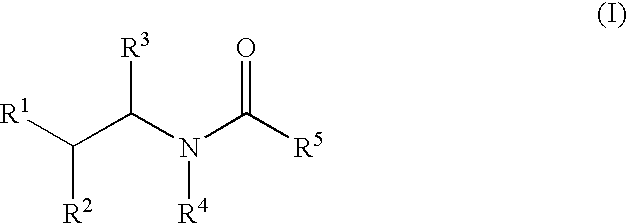
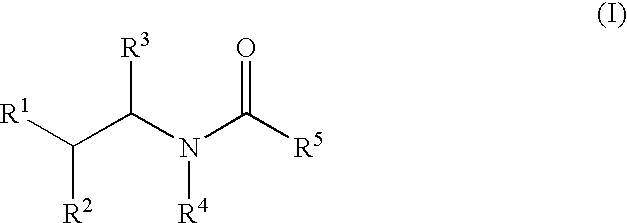
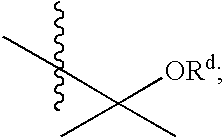
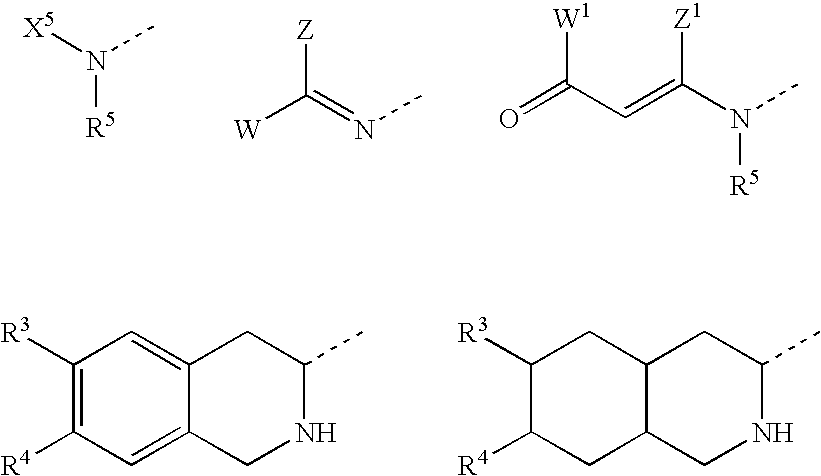
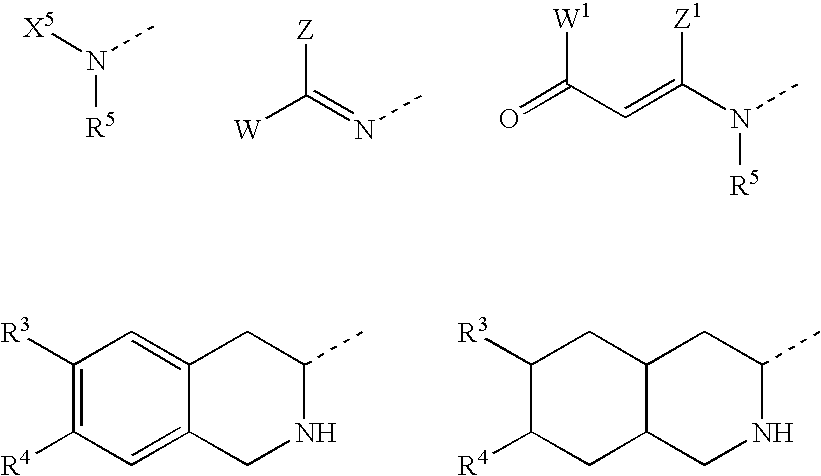
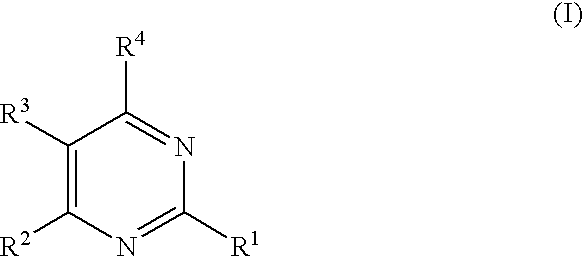
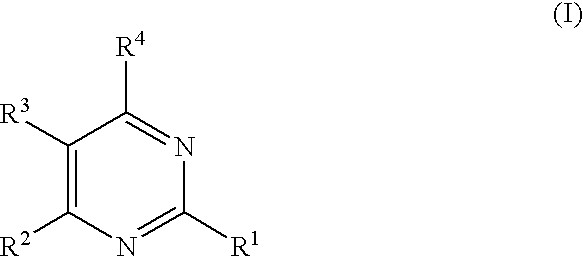
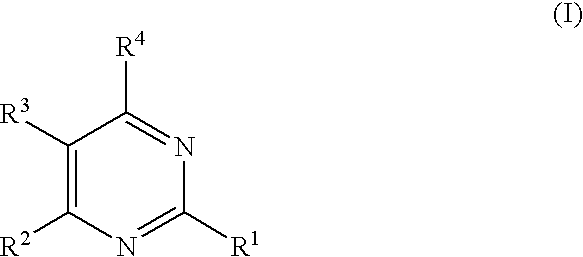
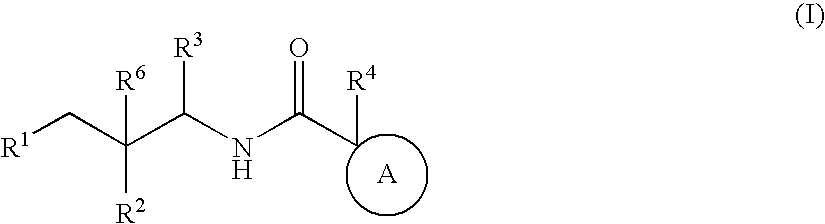
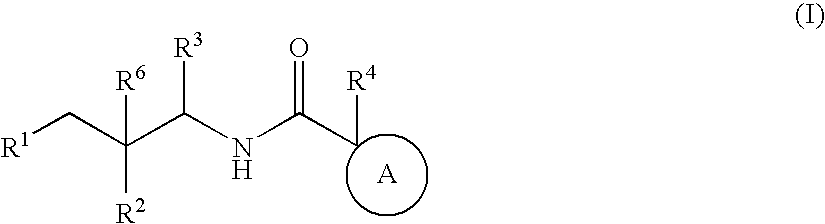
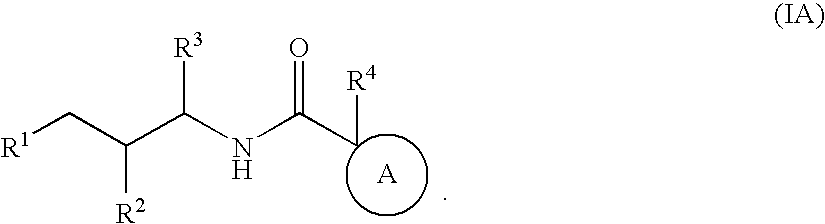
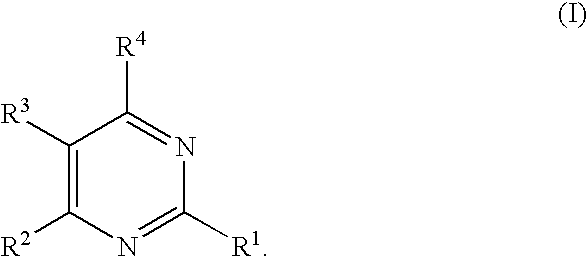
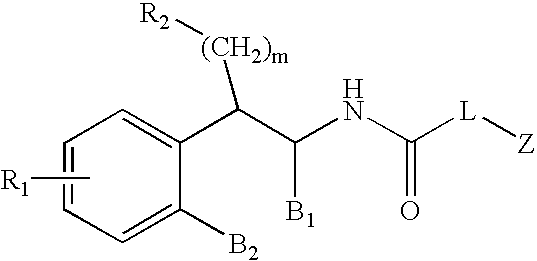
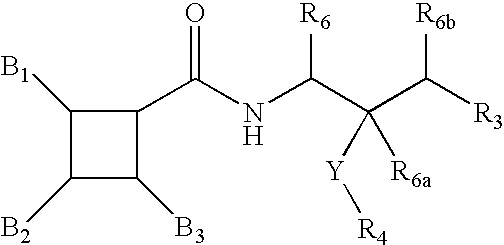
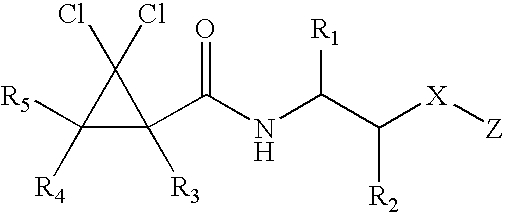
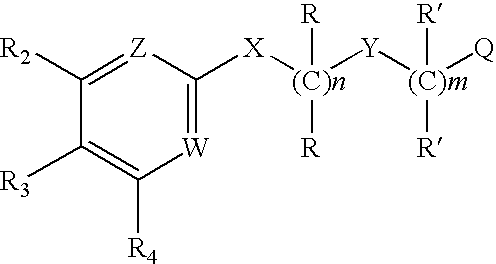
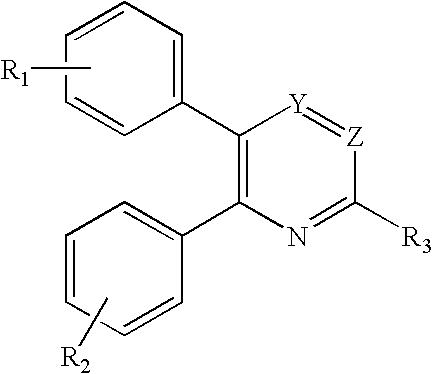
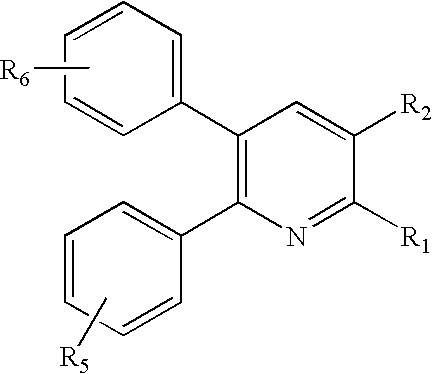
Substituted furo[2,3-b]pyridine derivatives
Novel compounds of the structural formula (I) are antagonists and / or inverse agonists of the Cannabinoid-1 (CB1) receptor and are useful in the treatment, prevention and suppression of diseases mediated by the CB1 receptor. The compounds of the present invention are useful as centrally acting drugs in the treatment of psychosis, memory deficits, cognitive disorders, migraine, neuropathy, neuro-inflammatory disorders including multiple sclerosis and Guillain-Barre syndrome and the inflammatory sequelae of viral encephalitis, cerebral vascular accidents, and head trauma, anxiety disorders, stress, epilepsy, Parkinson's disease, movement disorders, and schizophrenia. The compounds are also useful for the treatment of substance abuse disorders, the treatment of obesity or eating disorders, as well as the treatment of asthma, constipation, chronic intestinal pseudo-obstruction, and cirrhosis of the liver.
Owner:MERCK SHARP & DOHME CORP
Methods of treatment and pharmaceutical composition
The invention relates a pharmaceutical composition comprising a combination of:(i) the AT 1-antagonist valsartan or a pharmaceutically acceptable salt thereof; and(ii) a NEP inhibitor or a pharmaceutically acceptable salt thereof and optionally a pharmaceutically acceptable carrier and to a method for the treatment or prevention of a condition or diseaseselected from the group consisting of hypertension, heart failure, such as (acute and chronic) congestive heart failure, left ventricular dysfunction and hypertrophic cardiomyopathy, diabetic cardiac myopathy, supraventricular and ventricular arrhythmias, atrial fibrillation, atrial flutter, detrimental vascular remodeling, myocardial infarction and its sequelae, atherosclerosis, angina (whether unstable or stable), renal insufficiency (diabetic and non-diabetic), heart failure, angina pectoris, diabetes, secondary aldosteronism, primary and secondary pulmonary hypertension, renal failure conditions, such as diabetic nephropathy, glomerulonephritis, scleroderma, glomerular sclerosis, proteinuria of primary renal disease, and also renal vascular hypertension, diabetic retinopathy, the management of other vascular disorders, such as migraine, peripheral vascular disease, Raynaud's disease, luminal hyperplasia, cognitive dysfunction, such as Alzheimer's, glaucoma and stroke, comprising administering a therapeutically effective amount of the pharmaceutical composition to a mammal in need thereof.
Owner:NOVARTIS PHARM CORP
Antimicrobial dental materials, restorations, and prostheses
The present invention relates to compositions and methods for antimicrobial dental materials in the restoration of the sequelae of oral infections including replacement of oral tissues lost to disease and prevention of additional infections.
Owner:JERNBERG GARY R +1
Use of onion extracts to prevent and treat acute and chronic cardiac and vascular complications and their sequelae, as well as to resolve hematomas
Onions (Allium Cepa and its close relatives, as opposed to Allium Sativum) contain potent coagulation modulators, as well as platelet inhibitors. Clinical, in vivo and in vitro testing confirms that the efficacy of commonly consumed quantities of onions in terms of the efficacy of its impact on coagulation and platelet function rivals and in many respects exceeds the potency of any other modalities available today. Additionally, onion compounds have unique effects as described later in this application. The use of onion extracts is thus proposed to prevent and treat acute and chronic cardiac and vascular complications and their sequelae, to prevent and treat thrombosis, to prevent and treat embolization in the setting of thrombosis, various arrhythmias and cardiac abnormalities, as well as to resolve hematomas
Owner:MADY ATTILA +1
Medicinal liquor for treating bone disease and its preparation method
InactiveCN102552693ANo side effectsEasy to useAnthropod material medical ingredientsAntipyreticMyrrhCervical spondylosis
The invention relates to medicinal liquor for treating bone disease, which comprises the following substances by weight: 50 parts of Panax japonicum, 50 parts of tuber santsigu, 50 parts of ground beetle, 50 parts of safflower, 15 parts of Paris polyphylla, 15 parts of rhizoma drynariae, 18 parts of pseudo-ginseng, 30 parts of Sanguis Draconis, 50 parts of radix clematidis, 20 parts of myrrh, 50 parts of gardenia jasminoides, 12 parts of dried orange peel, 30 parts of cortex eucommiae, 30 parts of chaff flower root, 30 parts of pawpaw, 30 parts of buck grass, 30 parts of cassia twig, 50 partsof fiveleaf akebia, 24 parts of coralbean bark and 24 parts of common dodder. The present invention also relates to a method for preparing the medicinal liquor. The medicinal liquor provided by the invention can be used for treating rheumatic numbness of limbs, rheumatic arthritis, cervical spondylosis, periarthritis humeroscapularis, ischialgia caused by lumbar muscle strain, hyperosteogeny, hernia of intervertebral disc, spondylosis and ischemic necrosis of femoral head, traumatic injury, bone fracture, soft tissue contusion sequelae and the like.
Owner:范信
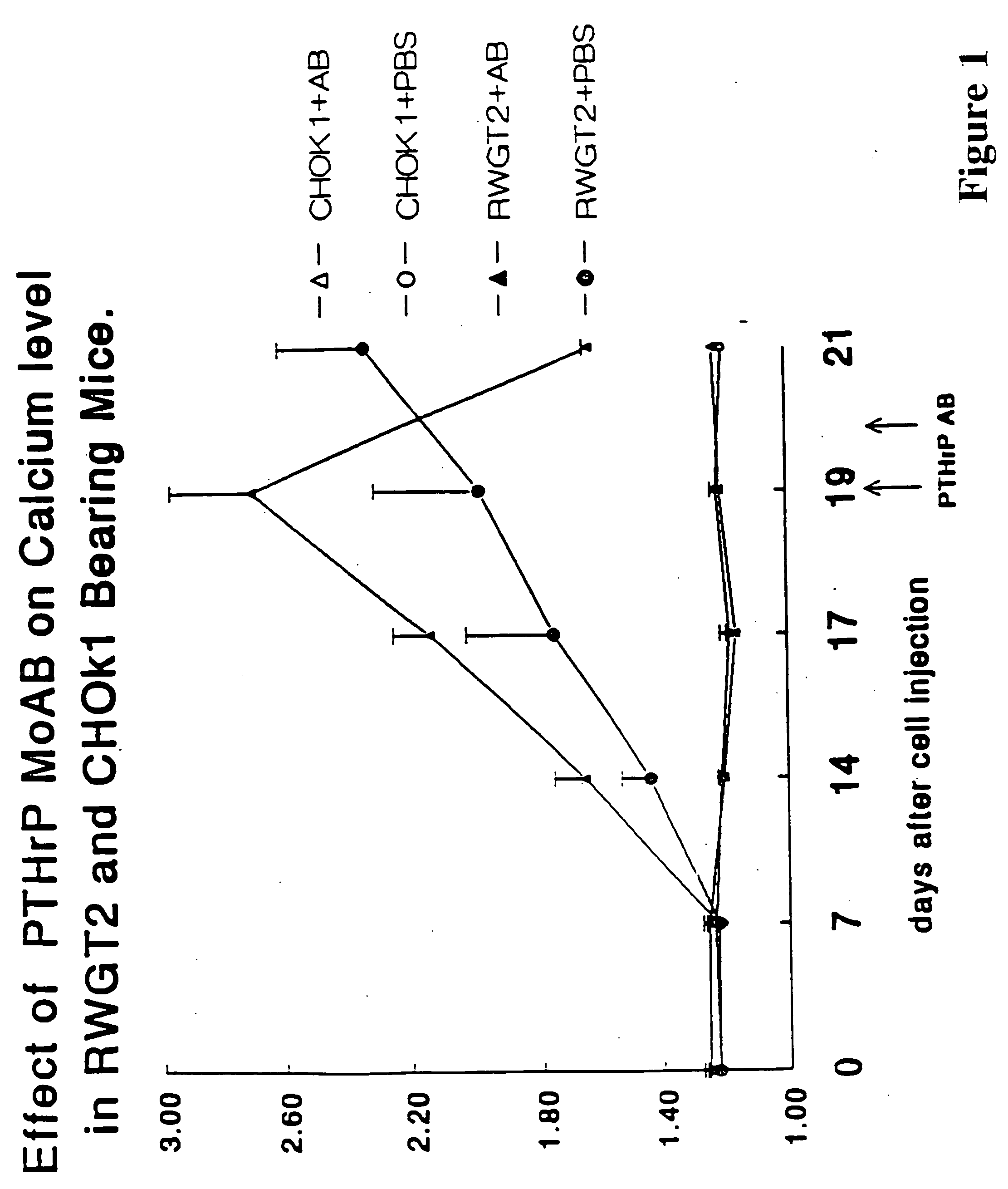
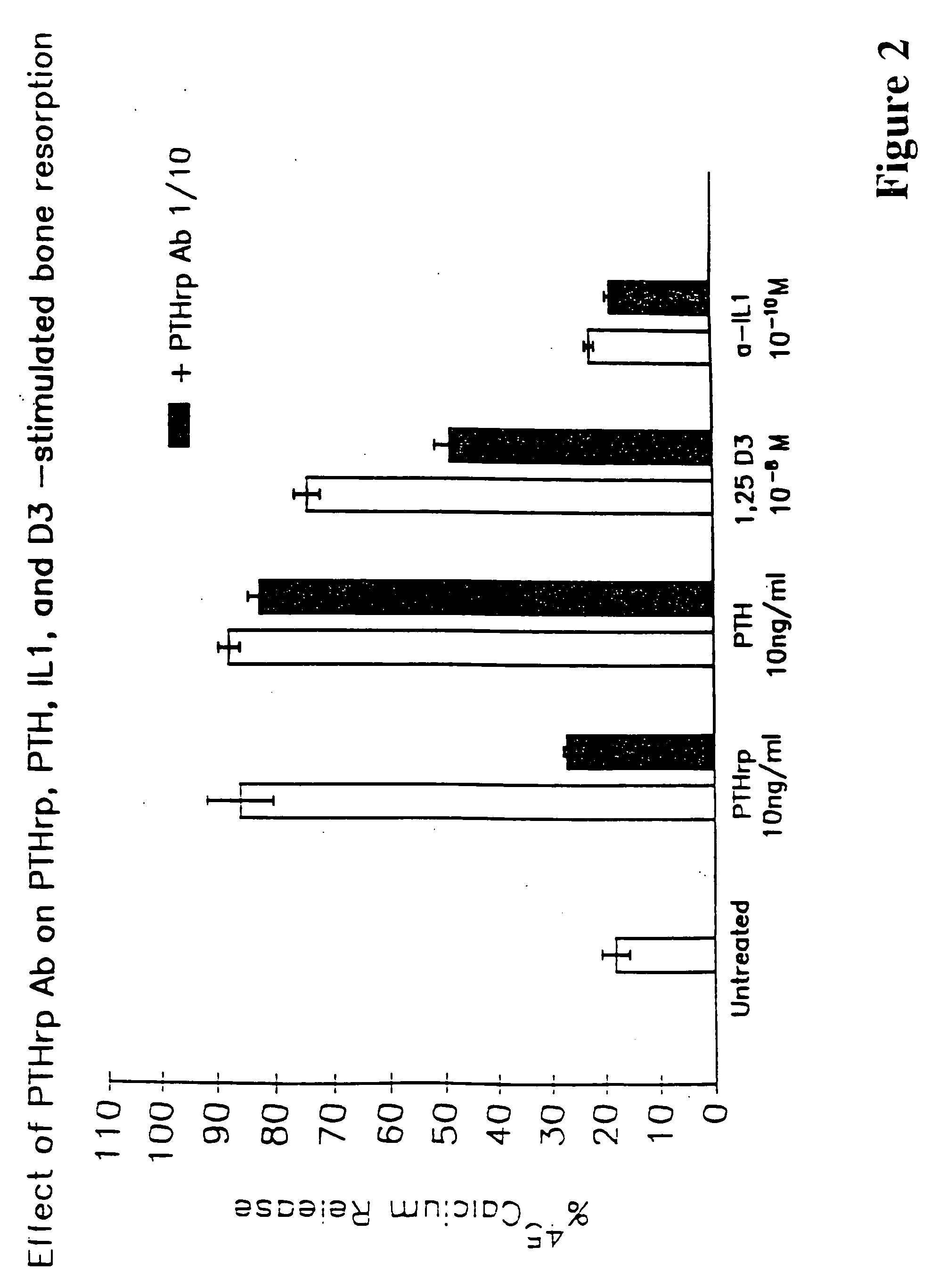
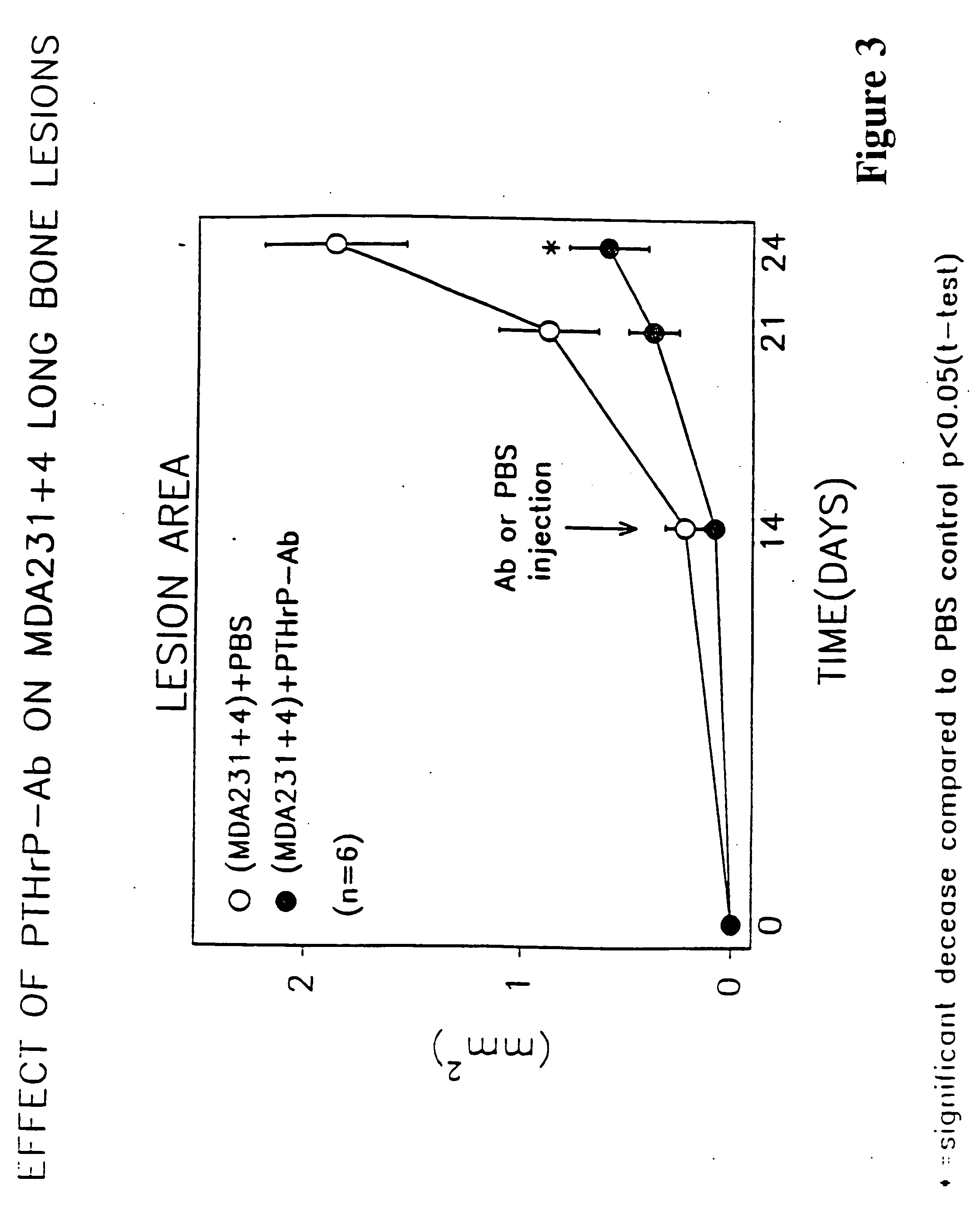
Method to ameliorate osteolysis and metastasis
A therapeutically effective amount of an antibody for a compound selected from the group consisting of PTHrp, TGFα, IL-1α, IL-1β, IL-6, Lymphotoxin, TNF, PGE; 1,25 dihydroxy vitamin D3 and an antigenic fragment thereof used in the treatment of cancer metastasis to bone and cancer cell growth in bone as well as osteolysis and symptomatic sequelae thereof. An antibody immunoreactive with parathyroid hormone-related protein (PTHrp) is particularly preferred. Antibodies with human characteristics are included in the invention for application of the invention method to human subjects. Also, the antibody can be administered in an injectable is formulation in combination with a therapeutically effective amount of a bisphosphonate or pyrophosphate having the general structure formula wherein X is a linking moiety allowing for the interconnection of the phosphonate groups, and pharmaceutically acceptable salts, hydrates and partial hydrates thereof. The antibody and bisphosphonate act synergistically in the treatment of cancer metastases to bone and symptomatic sequelae thereof and particularly as regards bone resorption.
Owner:XENOTECH CALIFORNIA
Topical medicinal liquor for treating rheumatoid bone disease
InactiveCN103110769AQuick resultsShort course of treatmentNervous disorderAnthropod material medical ingredientsMonkshoodsDisease
The invention belongs to the technical field of traditional Chinese medicines, and particularly relates to topical medicinal liquor for treating a rheumatoid bone disease. The topical medicinal liquor is prepared from prepared monkshood, prepared radix aconiti agrestis, eucommia ulmoides, flowers carthami, cinnamon, radix cyathulae, scorpio, long-noded pit viper, radices sileris, angelica sinensis, homalomena occulta, schizonepeta, radix clematidis, as arum, dipsacus root, pseudo-ginseng and white spirit. The prescription disclosed by the invention is appropriate and rigorous in compatibility of medicines, and unique in preparation method, can rapidly cure hyperostosis by the topical medicinal liquor disclosed by the invention, and is rapid to become effective, short in treatment course, high in recovery rate and low in expense. The drug has unique curative effects for rheumatism, rheumatoid arthritis, pain in waist and lower extremities, scapulohumeral periarthritis, limbs anesthesia, ischioneuralgia, hyperostosis, lumbar disc herniation, cervical spondylosis, ankylosing spondylitis, hyperplasia of mammary glands, various pain killer diseases, fracture sequelae and femoral head necrosis.
Owner:刘梅生
Co-Administration of An Agent Linked to an Internalization Peptide With an Anti-Inflammatory
ActiveUS20120252731A1Reduce capacityInhibit the inflammatory responseNervous disorderPeptide/protein ingredientsCo administrationBiotin
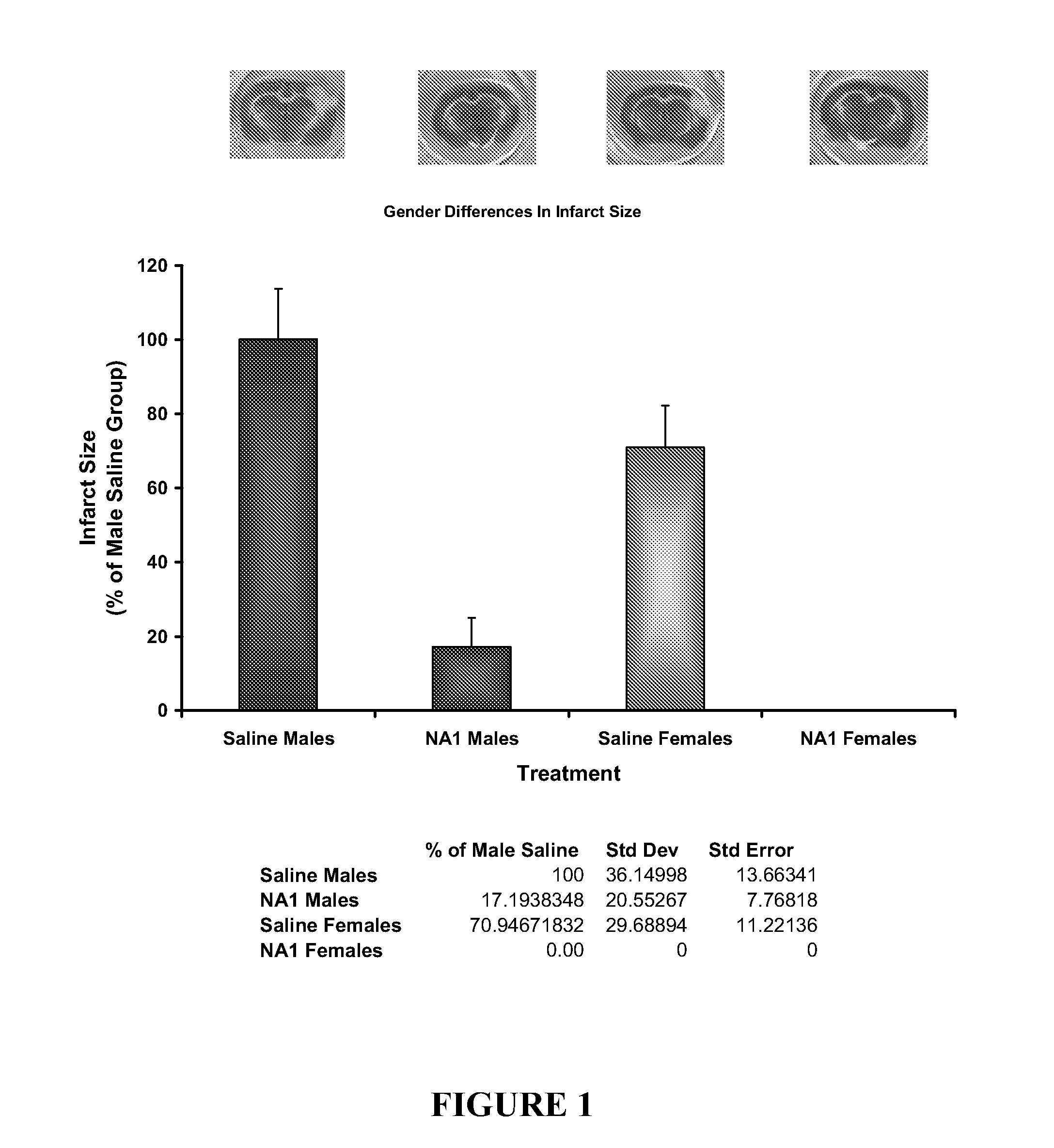
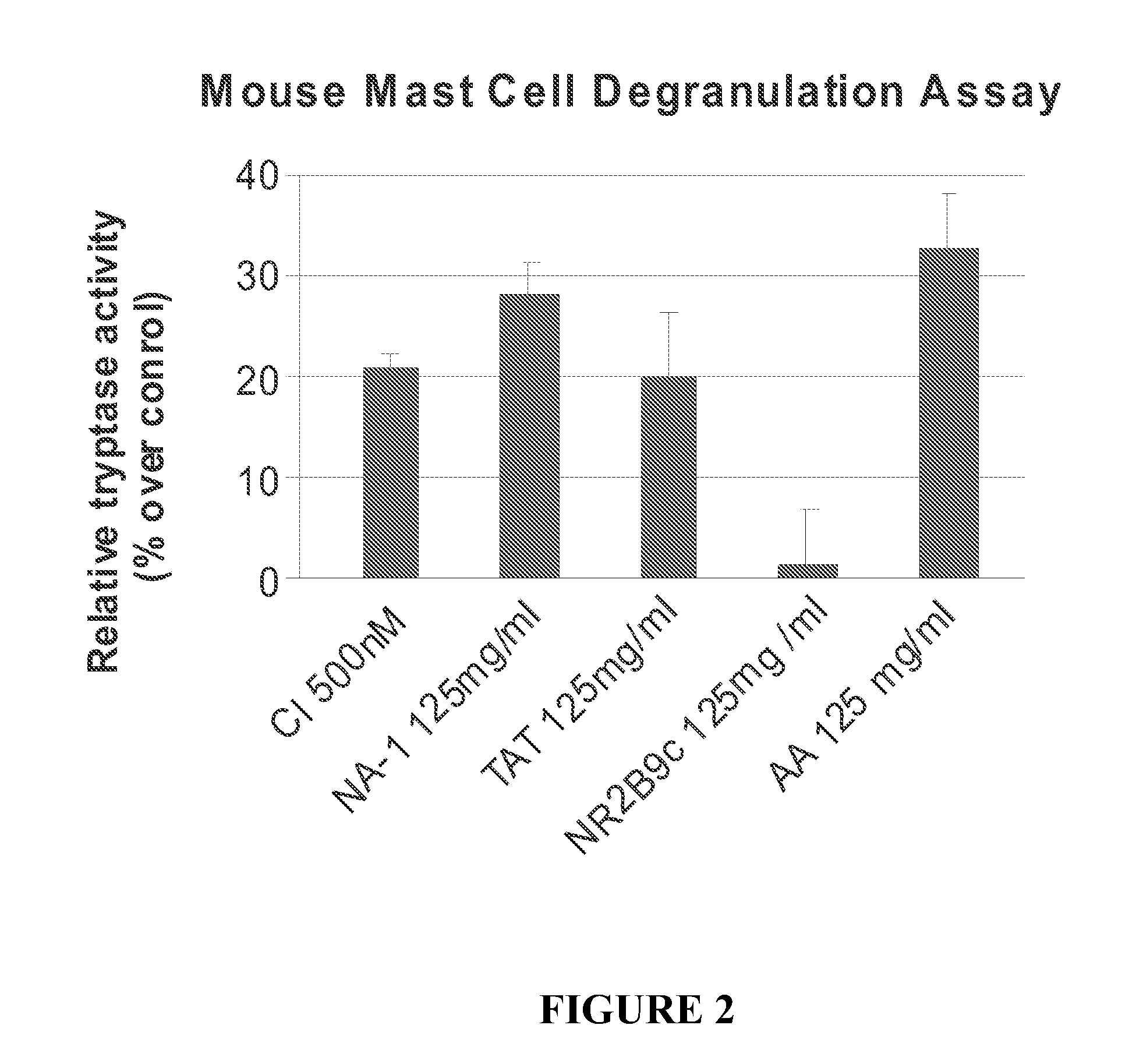
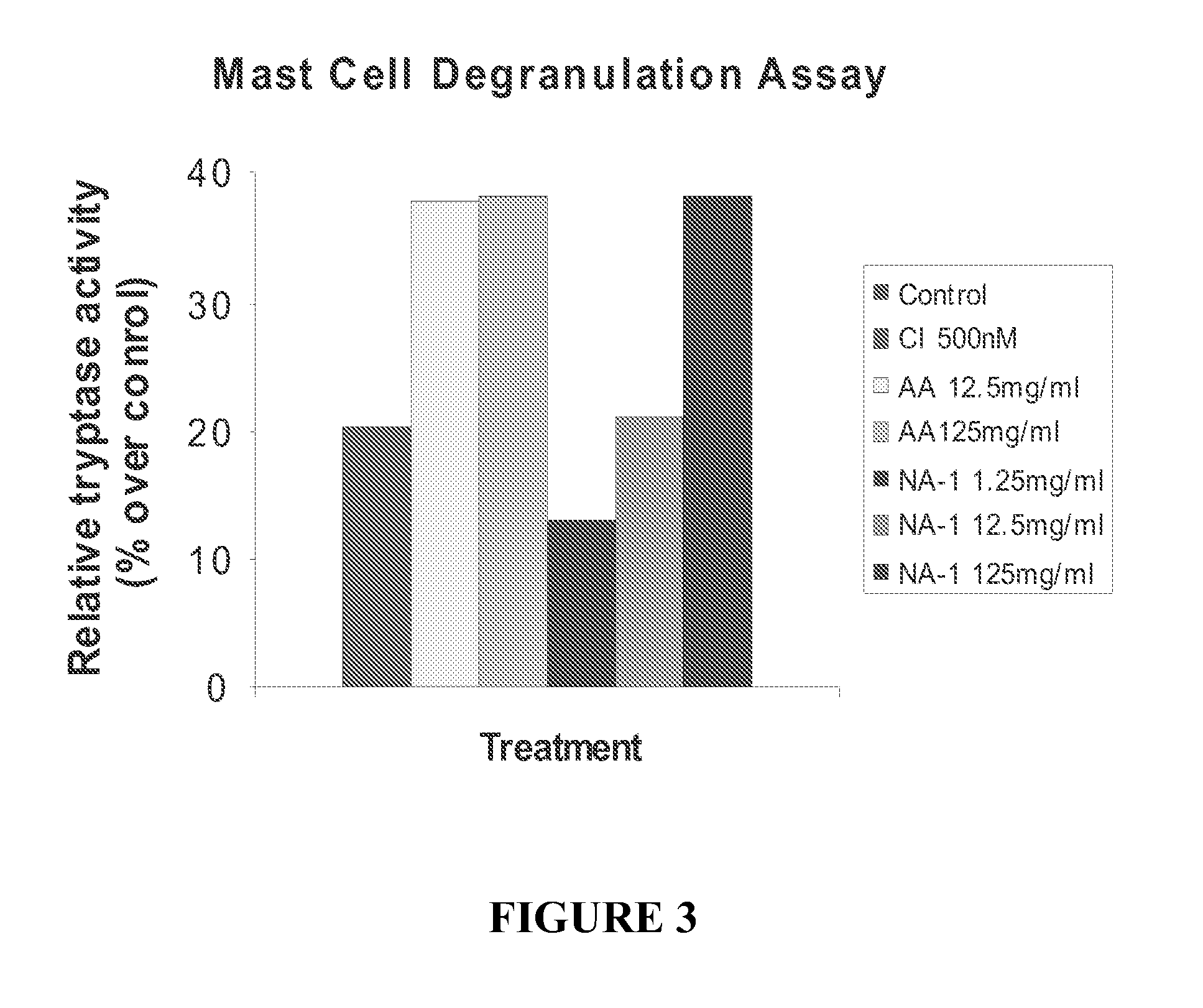
The invention provides methods of delivering pharmacologic agents linked to an internalization peptide, in which an inflammatory response inducible by the internalization peptide is inhibited by co-administration of an anti-inflammatory or by linking the internalization peptide to biotin or similar molecule. Such methods are premised in part on the results described in the examples whereby administration of a pharmacological agent linked to tat at high dosages is closely followed by an inflammatory response, which includes mast cell degranulation, histamine release and the typical sequelae of histamine release, such as redness, heat, swelling, and hypotension.
Owner:NONO INC
Collateral dredging and inflammation diminishing analgesic ointment
InactiveCN104056018ADetoxifying and analgesicAnti-inflammatory and insecticidalAnthropod material medical ingredientsHydroxy compound active ingredientsMyrrhChinese licorice
The invention provides a collateral dredging and inflammation diminishing analgesic ointment, and relates to medicines for treating herpes zoster, scapulohumeral periarthritis and traumatic injuries. The ointment comprises the following medicinal materials: Chinese angelica, red peony root, raw rehmmania root, radix scrophulariae, scutellaria baicalensis, coptidis rhizoma, cortex phellodendri, rheum officinale, lonicera japonica, earthworm, ligusticum wallichii, gecko, ground beetle, leech, safflower, camphor, angelica root, pangolin, frankincense, myrrh, dragon's blood, catechu, fructus forsythiae, nidus vespae, liquorice and borneol. The ointment is prepared by the following steps: soaking raw materials except borneol in 2.0-2.4kg of sesame oil for 5-7 days; decocting until the mixture is black with slow fire; removing dregs, adding 1.0-1.2kg of yellow lead, and keeping on decocting until the dropping mixture forms a bead shape; removing from the fire and cooling; adding borneol into the mixture, uniformly stirring, and spreading into patches. The collateral dredging and inflammation diminishing analgesic ointment has the advantages that the treatment effect is good, the cure rate is high, one patch of the ointment is used for 3 days and can be used for relieving pains in 8-12 hours, pains can be cured in 3 days generally, and certain individual syndromes need two patches and can be cured in 6 days; sequelae are not left; clinical testing on 229 cases indicates that the effective rate of the ointment is 100 percent; the ointment is prepared by using a pure traditional Chinese medicinal formula, and has small toxic and side effects.
Owner:姬光勤
Spirocyclic amides as cannabinoid receptor modulators
Novel compounds of structural formula (I) are antagonists and / or inverse agonists of the Cannabinoid-1 (CB1) receptor and are useful in the treatment, prevention and suppression of diseases mediated by the CB1 receptor. The compounds of the present invention are useful as psychotropic drugs in the treatment of psychosis, memory deficits, cognitive disorders, migraine, neuropathy, neuro-inflammatory disorders including multiple sclerosis and Guillain-Barre syndrome and the inflammatory sequelae of viral encephalitis, cerebral vascular accidents, and head trauma, anxiety disorders, stress, epilepsy, Parkinsons disease, movement disorders, and schizophrenia. The compounds are also useful for the treatment of substance abuse disorders, the treatment of obesity or eating disorders, as well as, the treatment of asthma, constipation, chronic intestinal pseudo-obstruction, and cirrhosis of the liver.
Owner:MERCK SHARP & DOHME CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
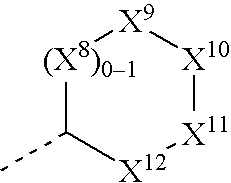
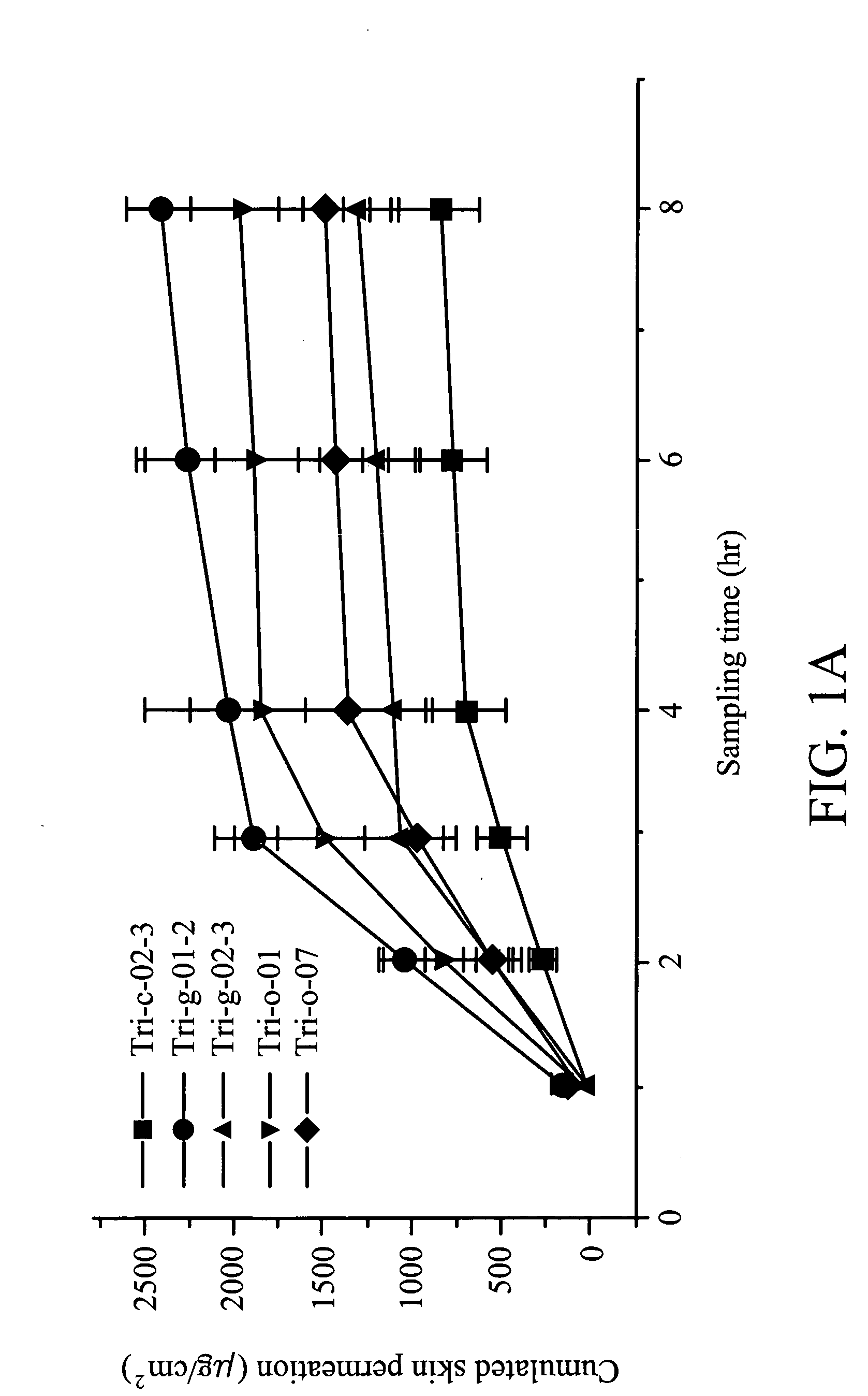
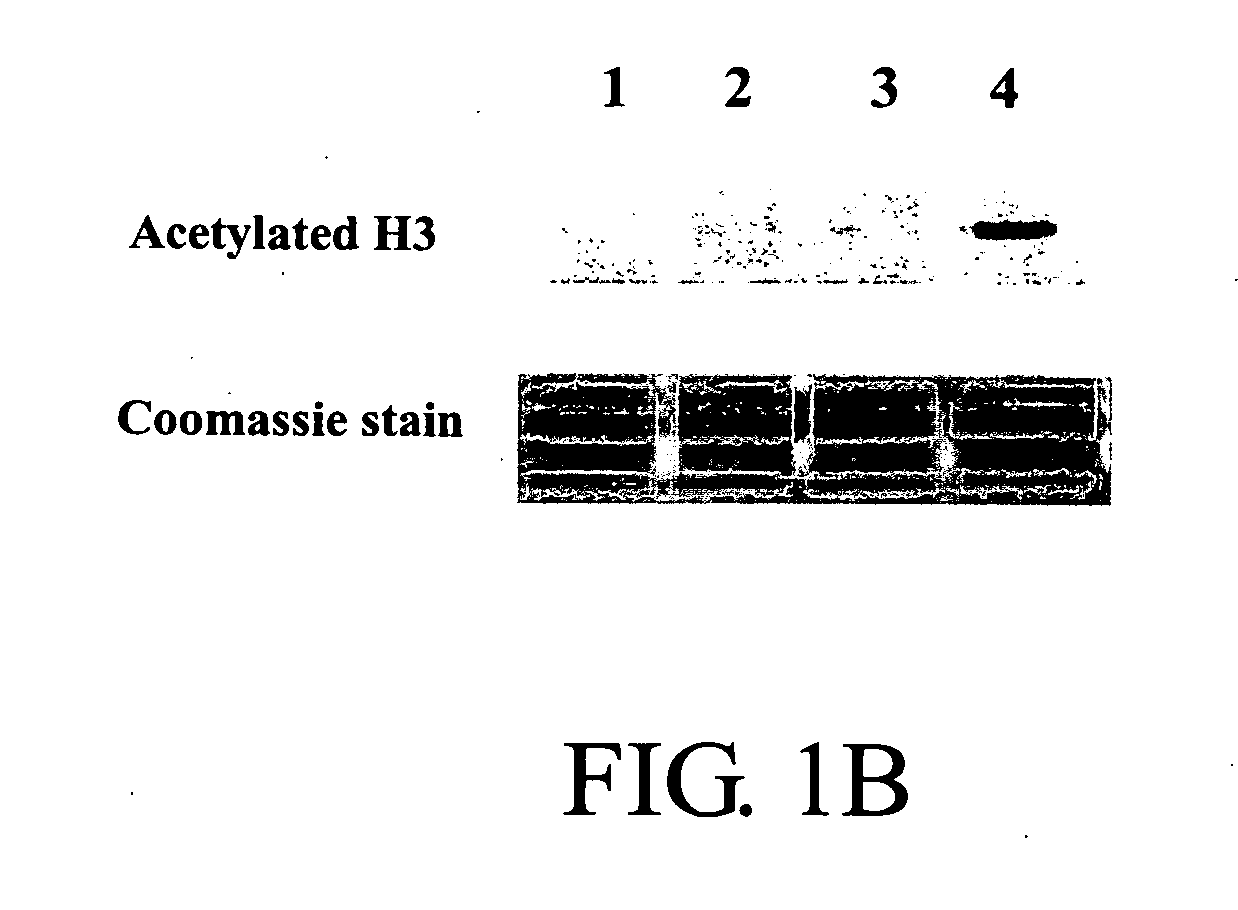
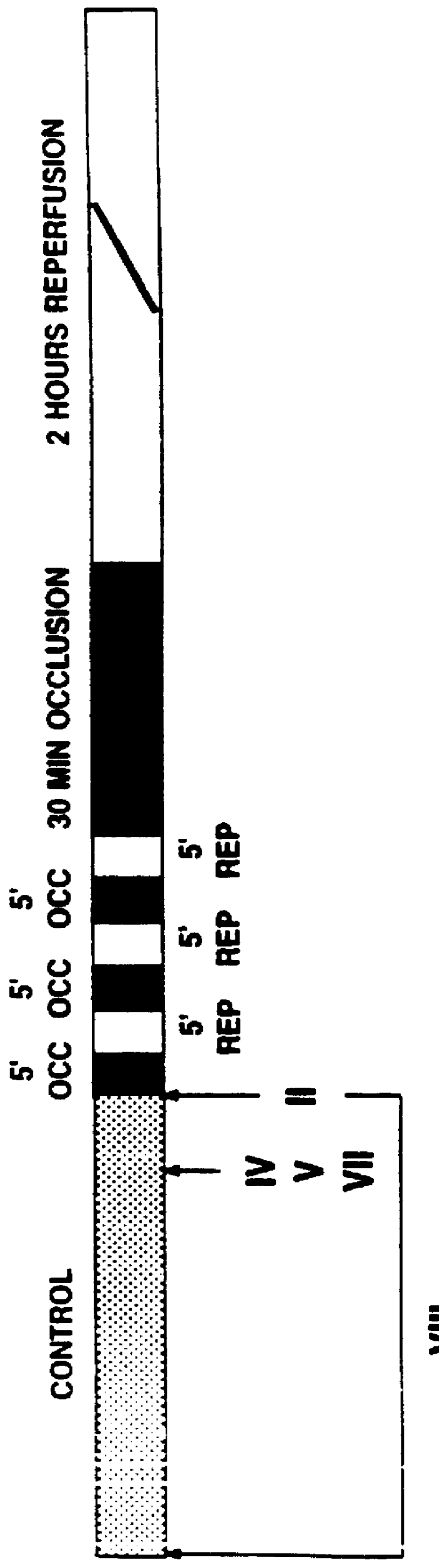
![Substituted furo[2,3-b]pyridine derivatives Substituted furo[2,3-b]pyridine derivatives](https://images-eureka.patsnap.com/patent_img/c9b9cc4e-0710-4977-a22c-471a25d75136/US07091216-20060815-C00001.png)
![Substituted furo[2,3-b]pyridine derivatives Substituted furo[2,3-b]pyridine derivatives](https://images-eureka.patsnap.com/patent_img/c9b9cc4e-0710-4977-a22c-471a25d75136/US07091216-20060815-C00002.png)
![Substituted furo[2,3-b]pyridine derivatives Substituted furo[2,3-b]pyridine derivatives](https://images-eureka.patsnap.com/patent_img/c9b9cc4e-0710-4977-a22c-471a25d75136/US07091216-20060815-C00003.png)