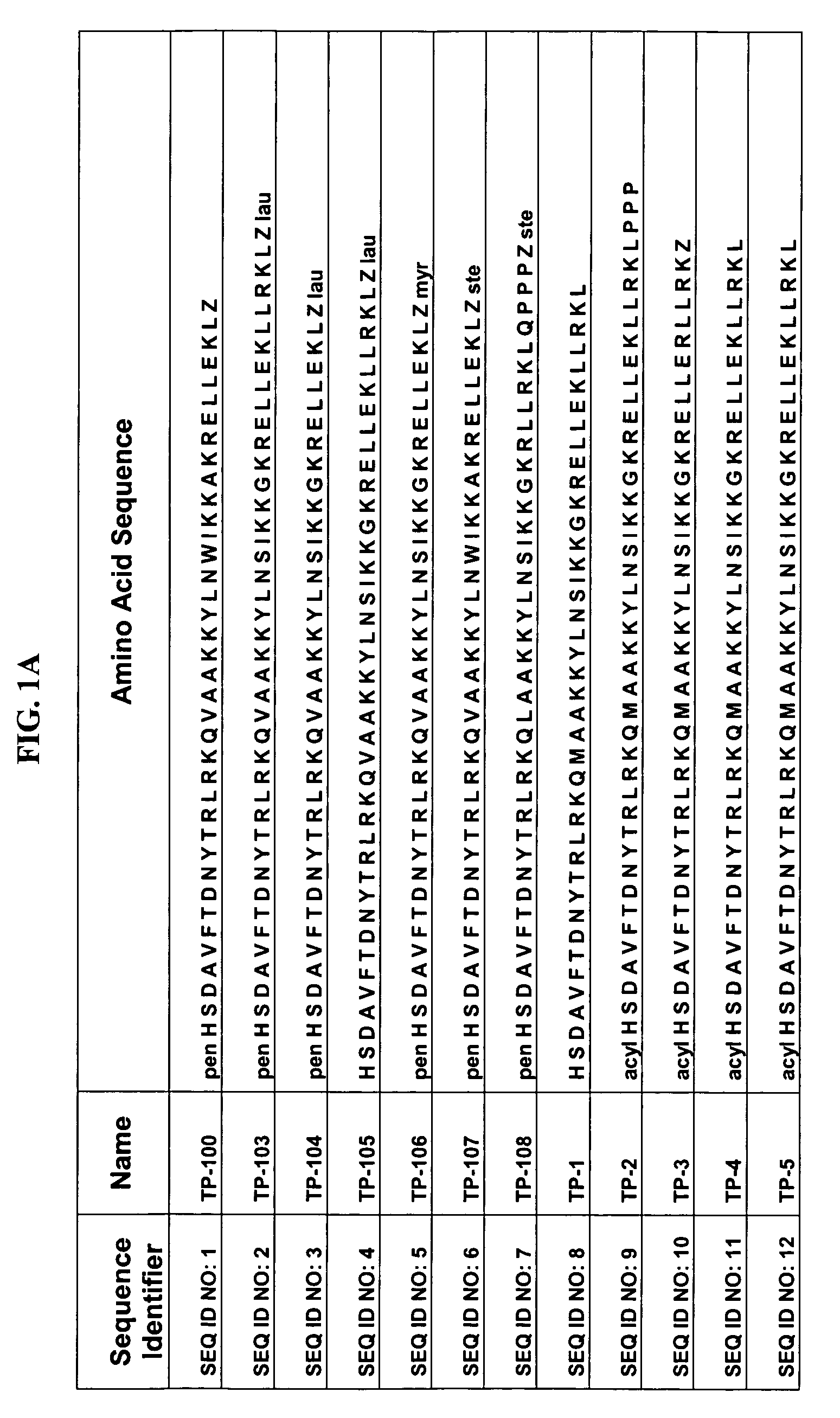
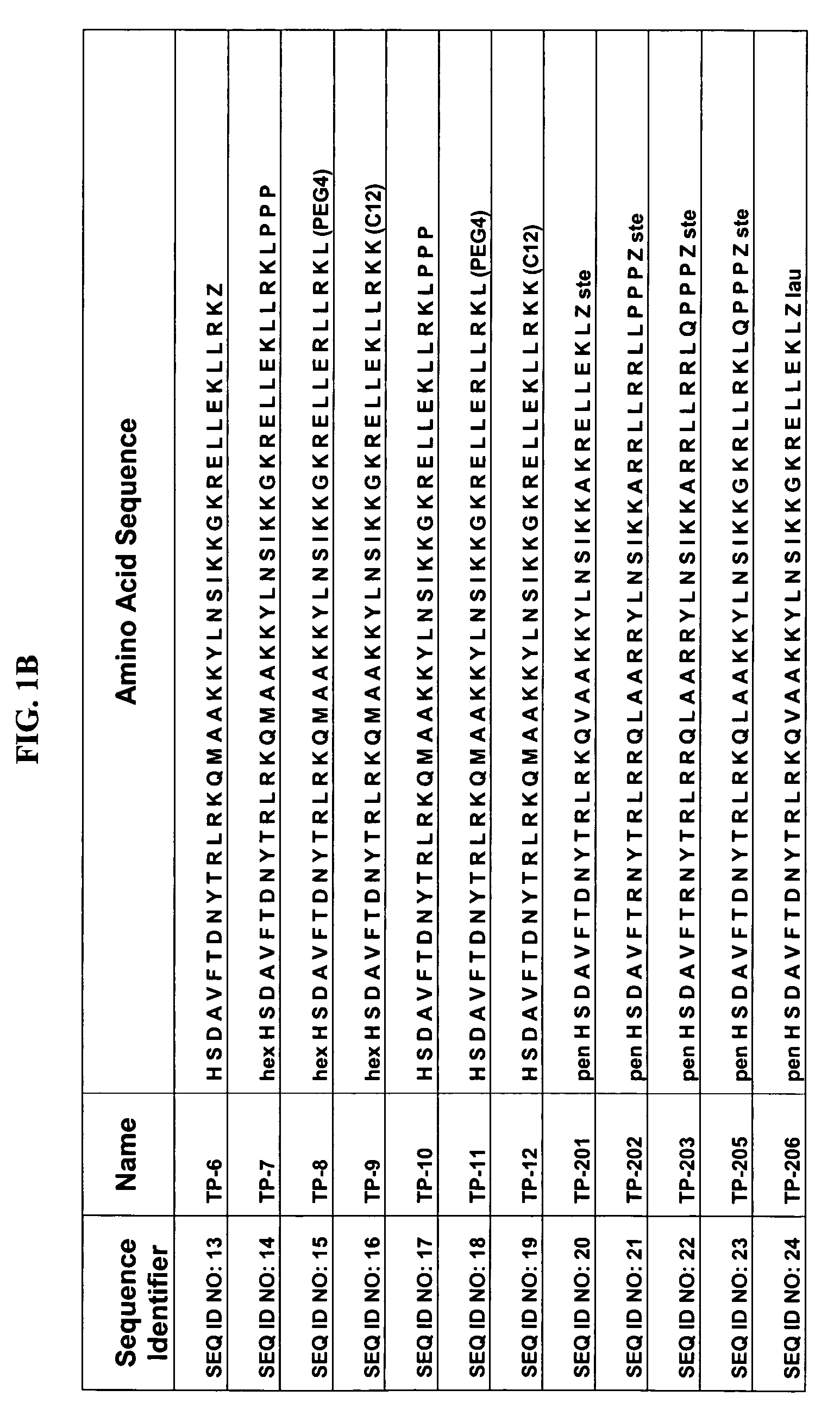
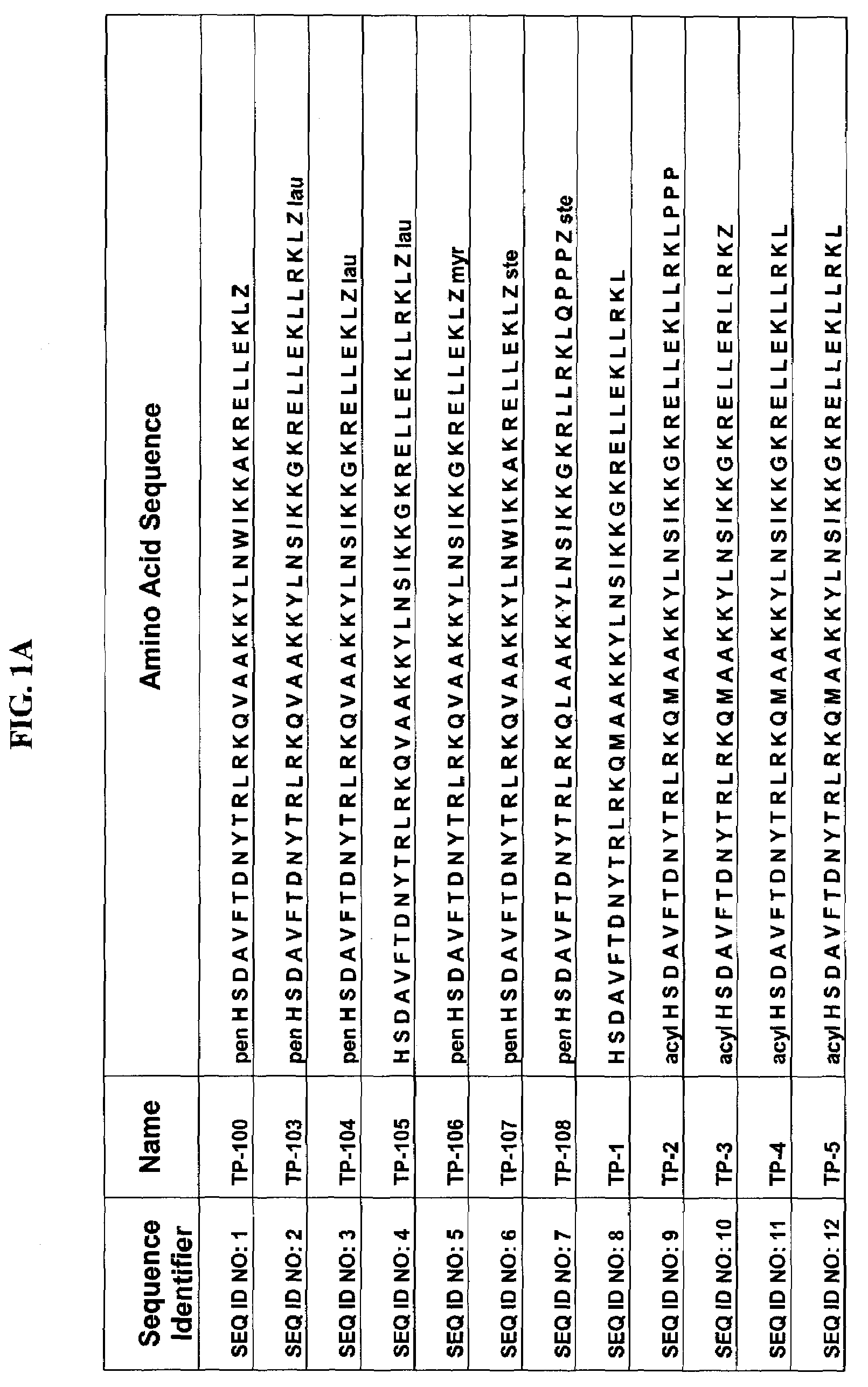
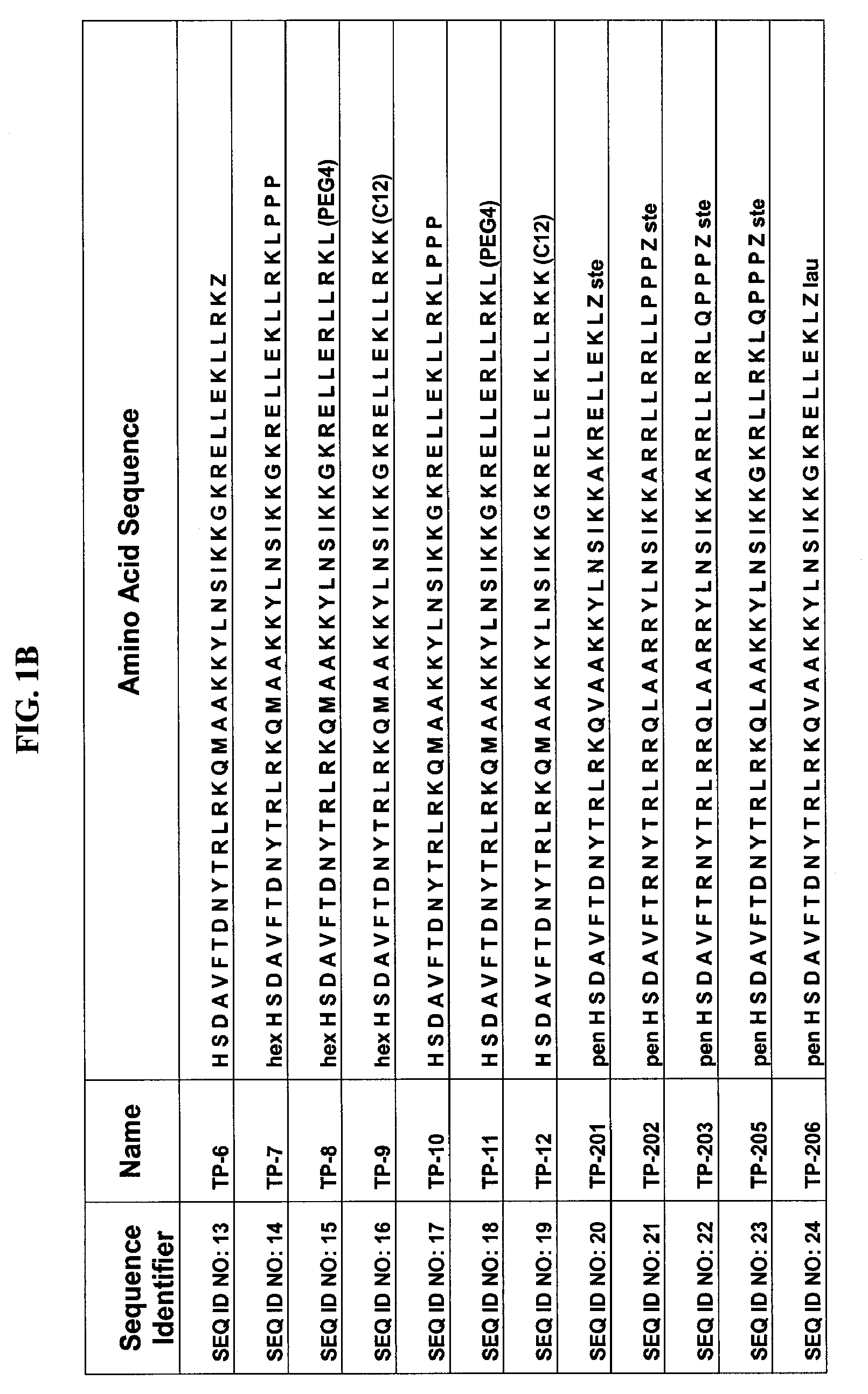
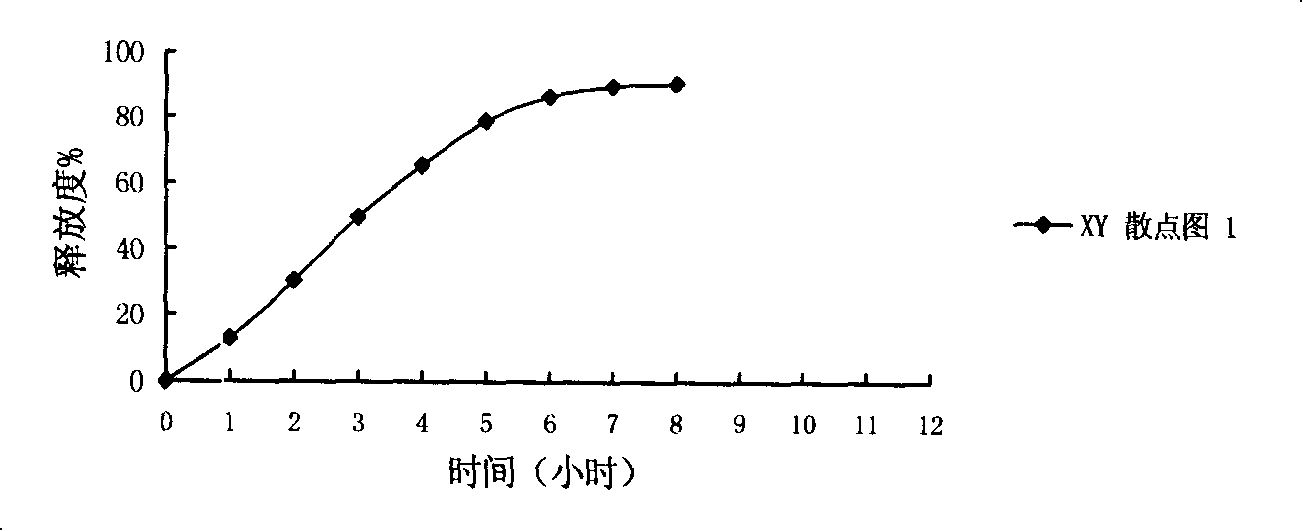
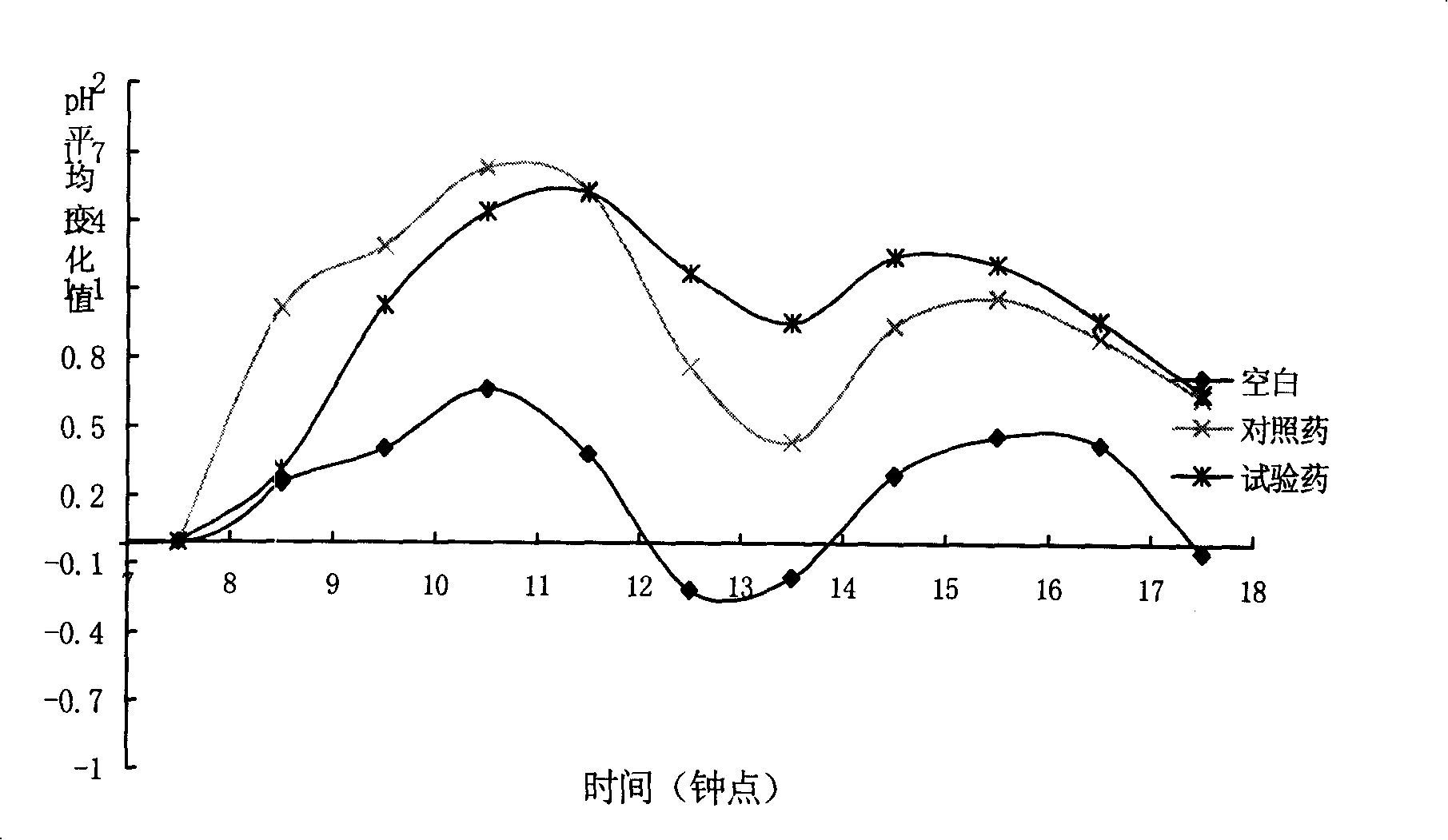
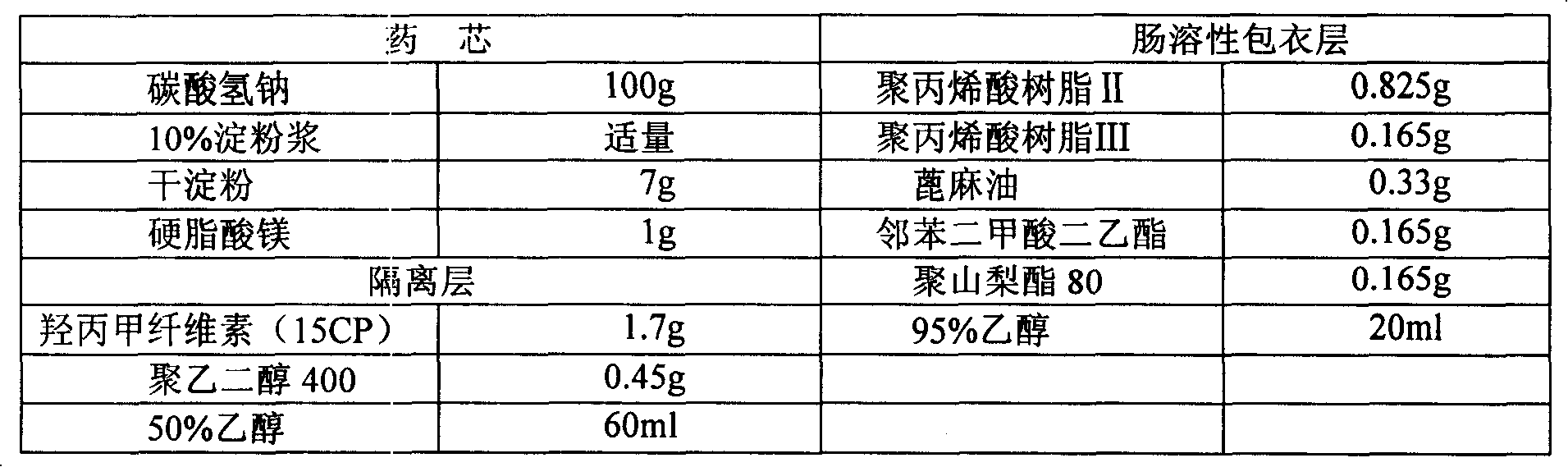
Patents
Literature
71 results about "Metabolic acidosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A condition in which there is excess acid in the body fluids.
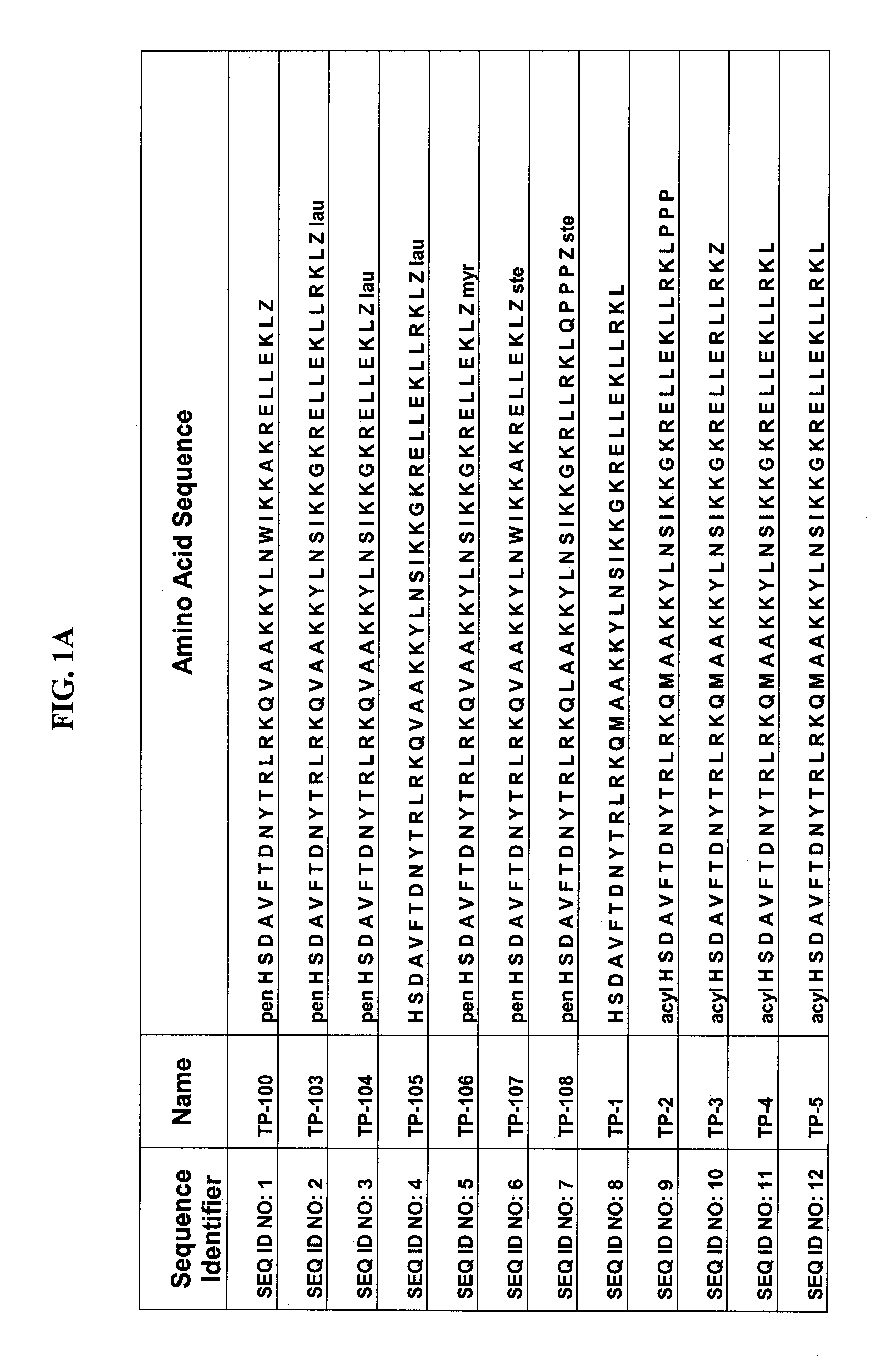
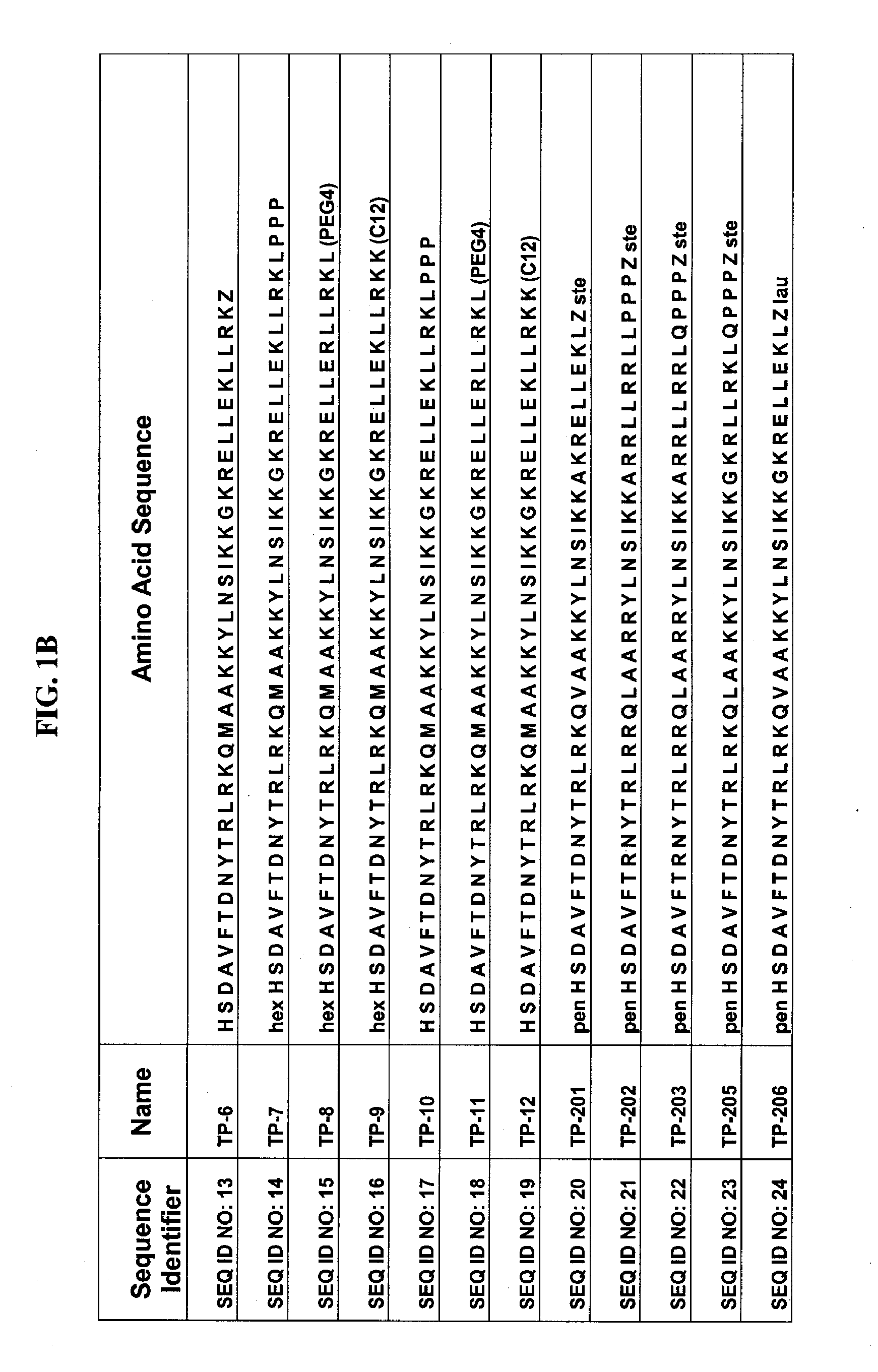
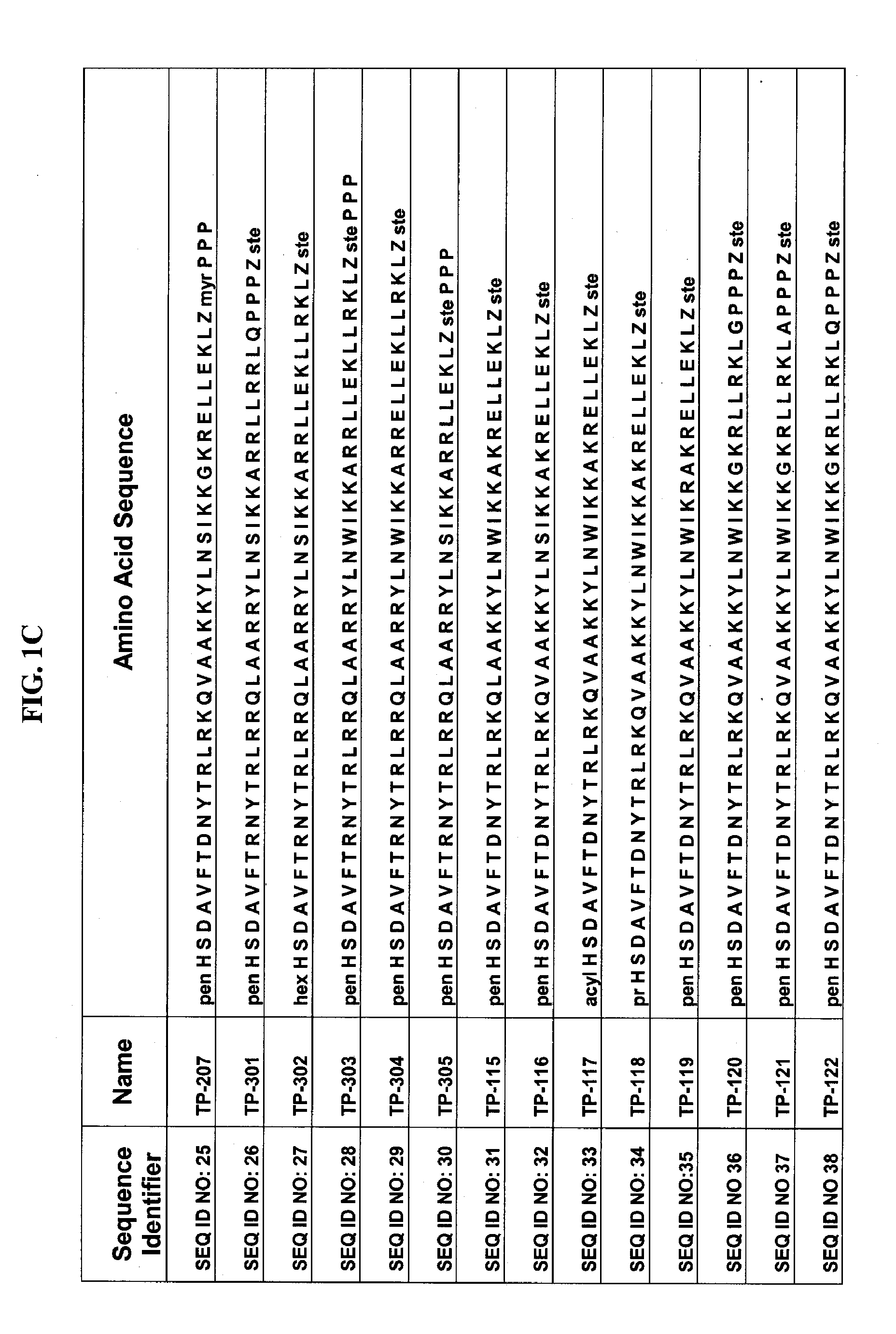
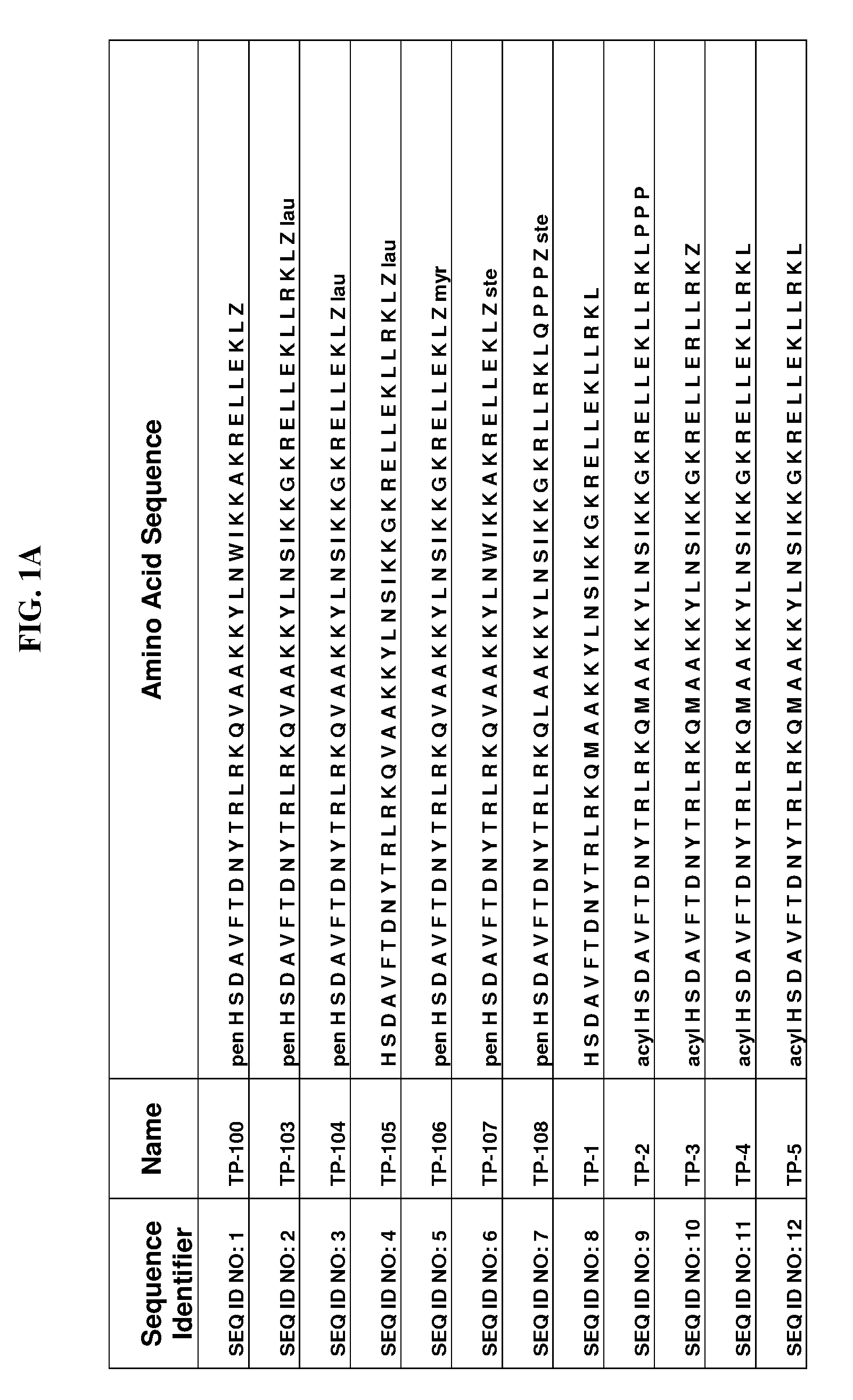
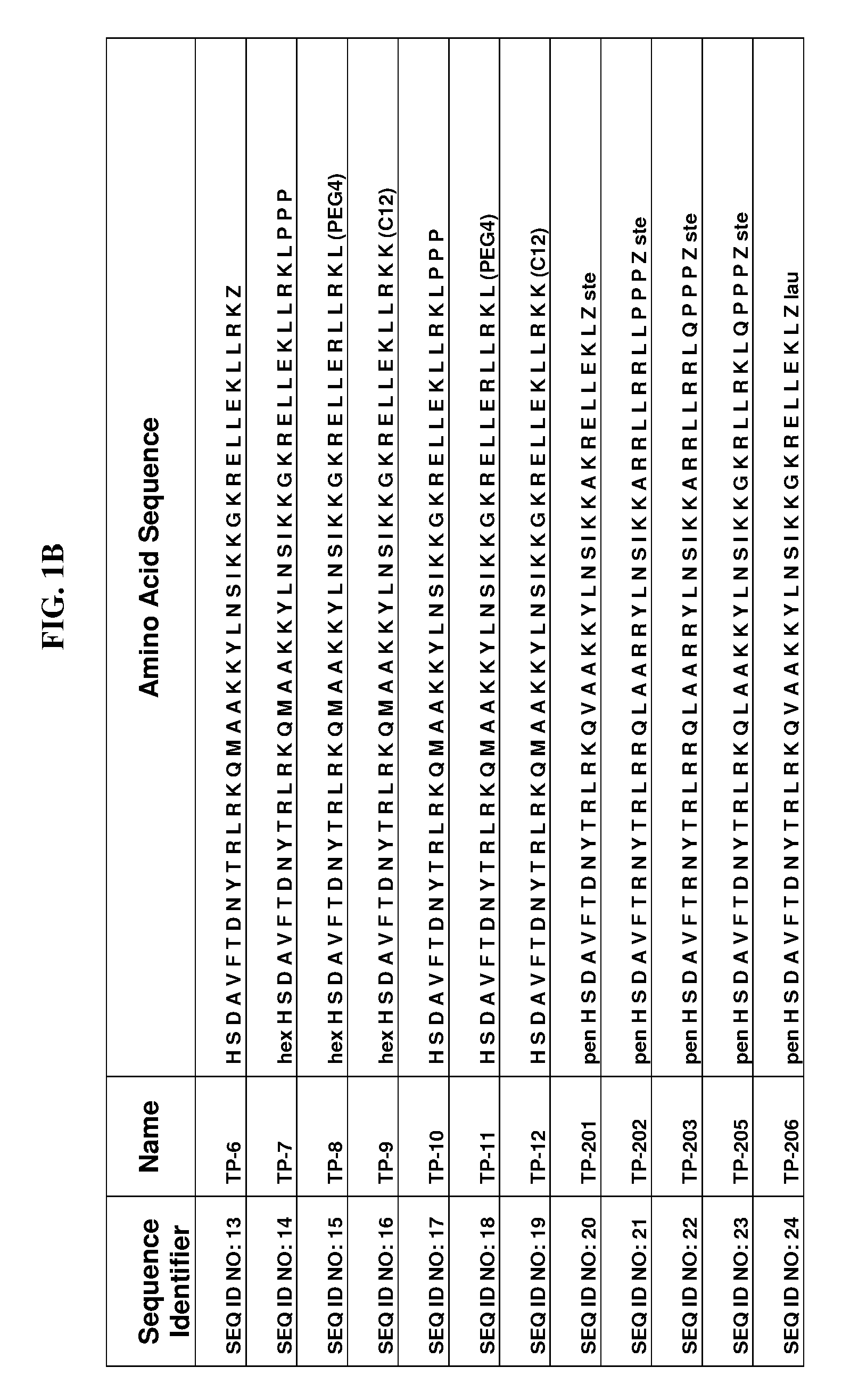
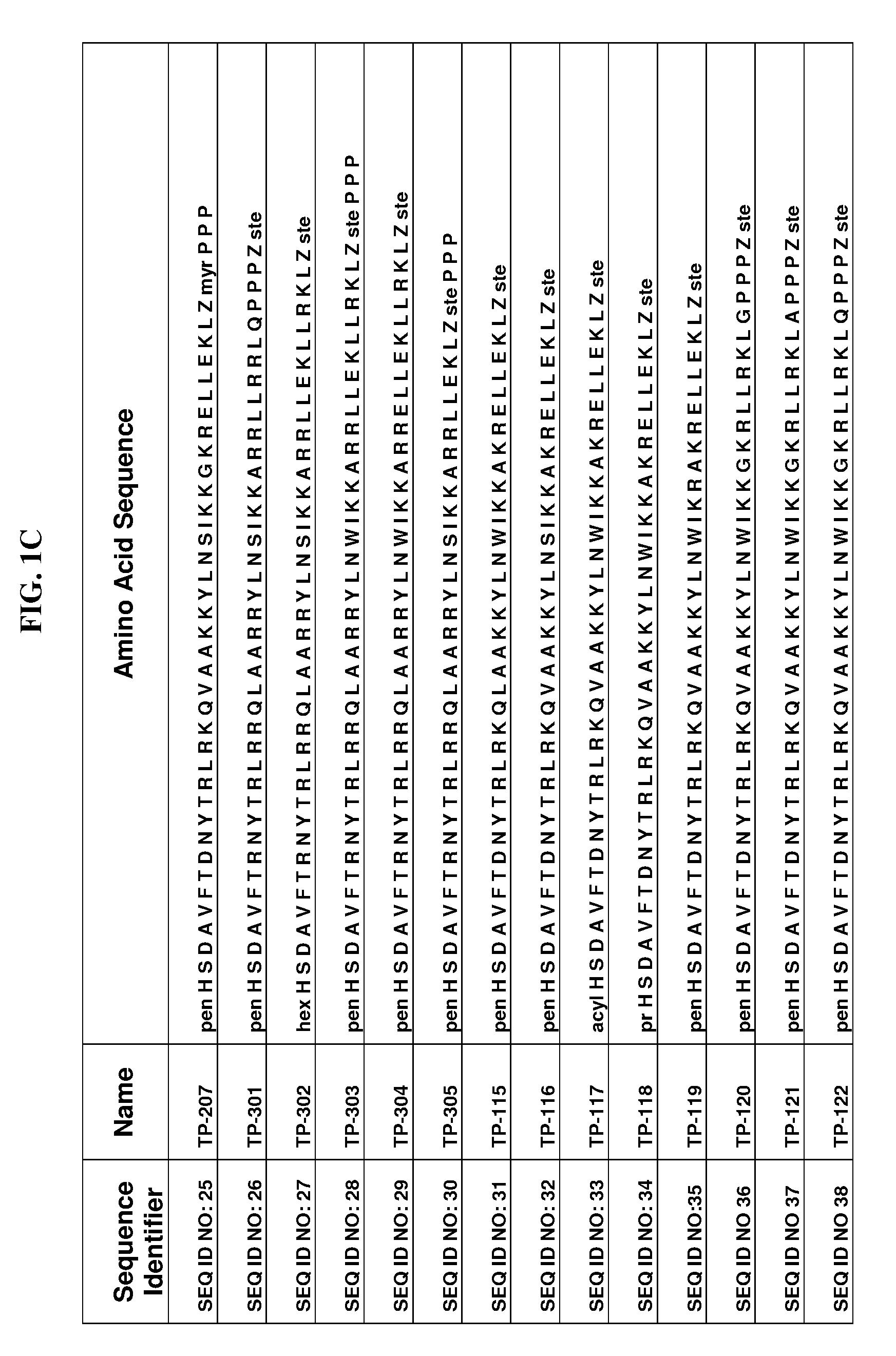
Fluorinated lysine derivatives as dipeptidyl peptidase IV inhibitors
InactiveUS20050043292A1Ease of preparation and detectabilityGood metabolic stabilityBiocideOrganic chemistryDiabetic retinopathyArthritis
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV (“DPP-IV”), pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes, metabolic syndrome (syndrome X or insulin resistance syndrome), hyperglycemia, impaired glucose tolerance, glucosuria, metabolic acidosis, arthritis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
Fluorinated cyclic amides as dipeptidyl peptidase IV inhibitors
InactiveUS6710040B1Easy to prepareEase of detectabilityBiocideOrganic chemistryAcute coronary syndromeDisease progression
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV, pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, hyperglycemia, impaired glucose tolerance, metabolic syndrome (Syndrome X or insulin resistance syndrome), glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
Dipeptidyl peptidase IV inhibiting fluorinated cyclic amides
InactiveUS20040110817A1Ease of preparation and detectabilityGood metabolic stabilityBiocideSenses disorderDiabetic retinopathyDisease progression
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV, pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, hyperglycemia, impaired glucose tolerance, metabolic syndrome (Syndrome X or insulin resistance syndrome), glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
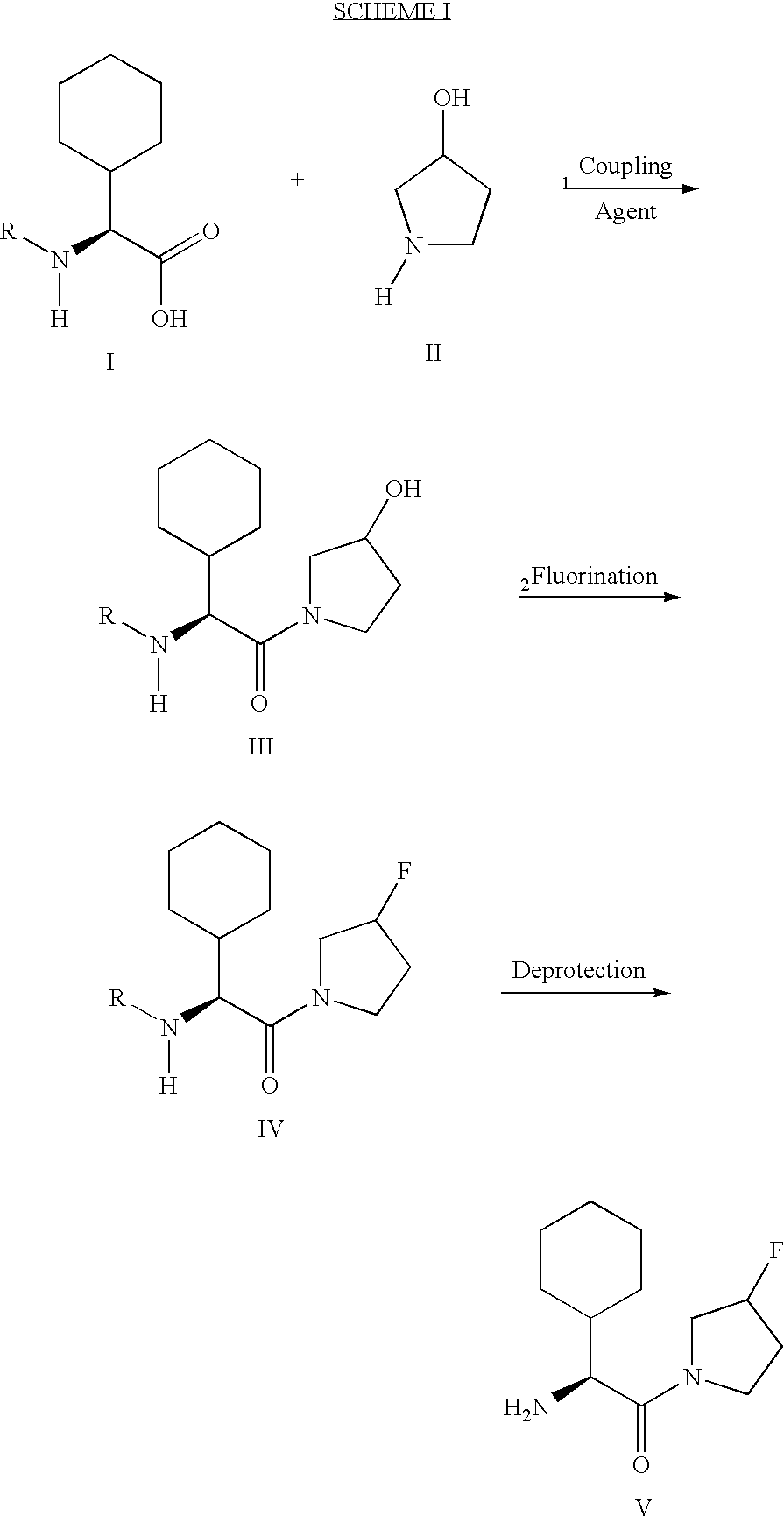
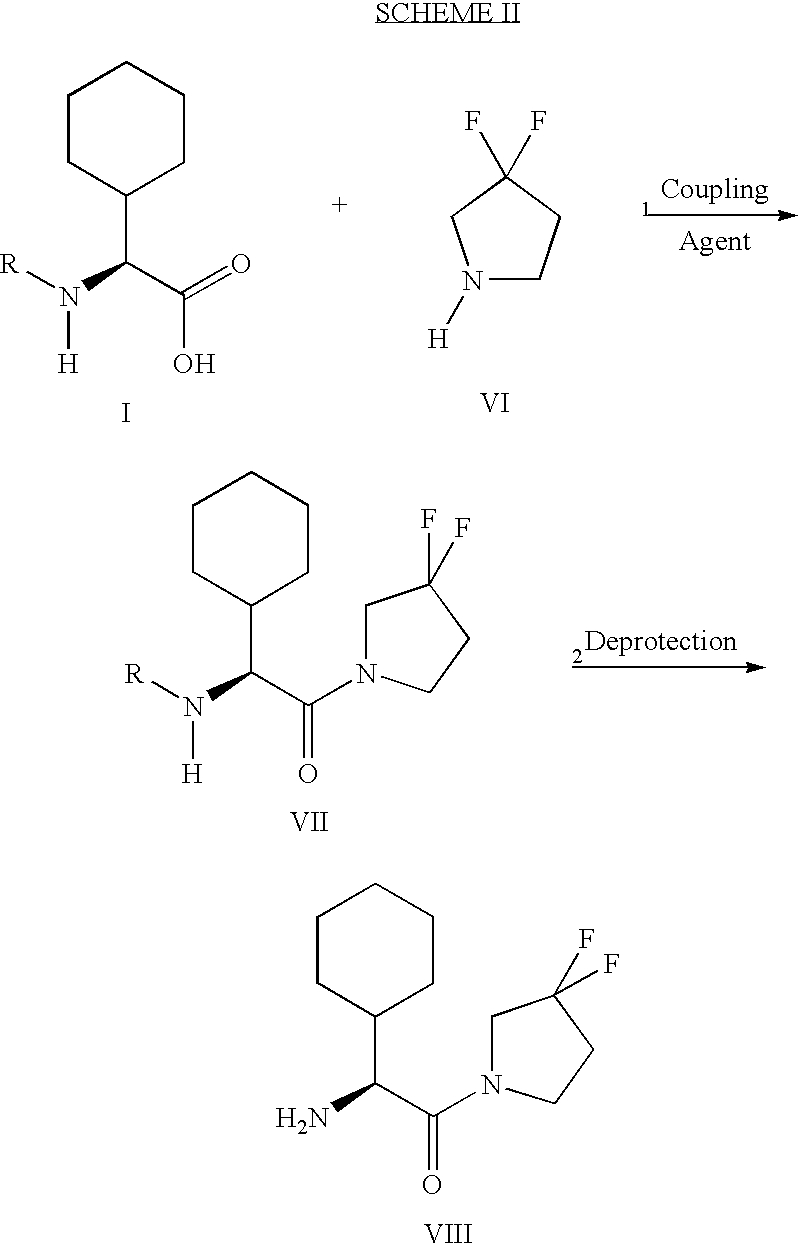
Synthesis of 3,3,4,4-tetrafluoropyrrolidine and novel dipeptidyl peptidase-IV inhibitor compounds
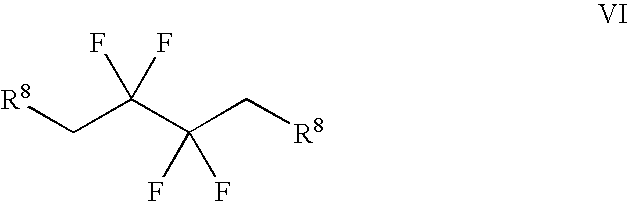
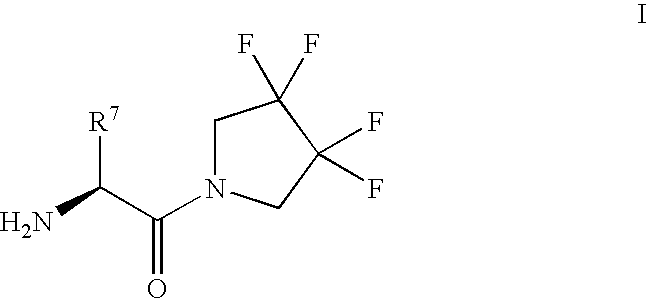
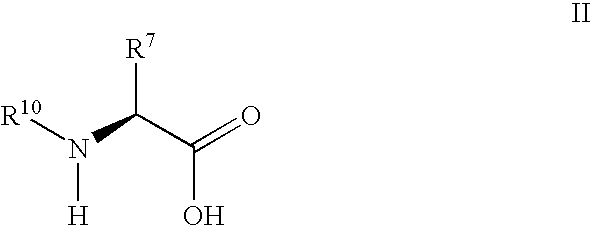
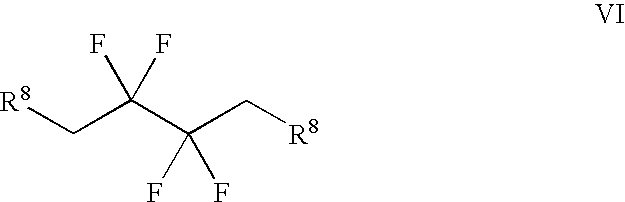
The present invention relates to a method of making novel dipeptidyl peptidase-IV ("DPP-IV') inhibitor compounds useful for treating, inter alia, diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, metabolic syndrome (Syndrome X or insulin resistance syndrome), hyperglycemia, impaired glucose tolerance, glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of making 3,3,4,4-tetrafluoropyrrolidine, a starting material utilized in the afore-mentioned method for preparing DPP-IV compounds.
Owner:PFIZER INC
Synthesis of 3,3,4,4-tetrafluoropyrrolidine and novel dipeptidyl peptidase-IV inhibitor compounds
InactiveUS6812350B2Metabolism disorderPhosphorus organic compoundsDisease progressionDiabetic nephropathy
Owner:PFIZER INC
Substituted amino ketone compounds
InactiveUS20050014946A1Organic active ingredientsOrganic compound preparationIGT - Impaired glucose toleranceNephrosis
The present invention relates to compounds of the general formula I B—(CH—R1)n—C(═X2)-D (I) and pharmaceutically acceptable salts thereof including stereoisomers, to the use of the compounds for the treatment of impaired glucose tolerance, glucosuria, hyperlipidaemia, metabolic acidosis, diabetes mellitus, diabetic neuropathy and nephropathy and of sequelae caused by diabetes mellitus in mammals.
Owner:PROSIDION LIMITED
Synthesis of 3,3,4,4-tetrafluoropyrrolidine and novel dipeptidyl peptidase-IV inhibitor compounds
InactiveUS20040002609A1Easy to cutMetabolism disorderPhosphorus organic compoundsDisease progressionDisease cause
The present invention relates to a method of making novel dipeptidyl peptidase-IV ("DPP-IV') inhibitor compounds useful for treating, inter alia, diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, metabolic syndrome (Syndrome X or insulin resistance syndrome), hyperglycemia, impaired glucose tolerance, glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of making 3,3,4,4-tetrafluoropyrrolidine, a starting material utilized in the afore-mentioned method for preparing DPP-IV compounds.
Owner:PFIZER INC
Fluorinated cyclic amides as dipeptidyl peptidase IV inhibitors
InactiveUS20040132713A1Ease of preparation and detectabilityGood metabolic stabilityBiocideOrganic chemistryDisease progressionDiabetic nephropathy
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV, pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, hyperglycemia, impaired glucose tolerance, metabolic syndrome (Syndrome X or insulin resistance syndrome), glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
Dipeptidyl peptidase-IV inhibitors
InactiveUS20050234065A1High activityImproved gastrointestinal permeabilityBiocideNervous disorderDiabetic retinopathyArthritis
The invention provides compounds of Formula (I) or prodrugs thereof, or pharmaceutically acceptable salts of said compounds or prodrugs, or solvates of said compounds, prodrugs or salts, wherein A, N, X and R1 are as defined herein; pharmaceutical compositions thereof; and methods of using the pharmaceutical compositions for the treatment of diseases, including Type 2 diabetes, Type 1 diabetes, impaired glucose tolerance, hyperglycemia, metabolic syndrome (syndrome X and / or insulin resistance syndrome), glucosuria, metabolic acidosis, arthritis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, short stature due to growth hormone deficiency, infertility due to polycystic ovary syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome; short bowel syndrome; and the prevention of disease progression in Type 2 diabetes.
Owner:PFIZER INC
Biochemically balanced peritoneal dialysis solutions
InactiveUS7011855B2Improved peritoneal dialysis solutionAvoid lostBiocideSolvent extractionMedicinePeritoneal dialysis solutions
A peritoneal dialysis solution that is biochemically balanced to correct metabolic acidosis associated with chronic renal failure in a more physiological manner. The peritoneal dialysis solution has a physiological pH, e.g., pH of 7.0 to 7.4, and contains bicarbonate at a concentration that is found in normal blood. Additionally, the solution contains carbon dioxide at a partial pressure that is similar to partial pressure of carbon dioxide found in normal blood. The peritoneal dialysis solution also contains a weak acid with a pKa of less than 5.0.
Owner:BAXTER INT INC
Vasoactive Intestinal Polypeptide Compositions
InactiveUS20070293429A1Facilitate duration of actionProlong the action timeAntibacterial agentsPeptide/protein ingredientsObesityBlood vessel
Pharmaceutical compositions relating to vasoactive intestinal polypeptides and methods for the treatment of metabolic disorders, including diabetes, insulin resistance, metabolic acidosis and obesity are presented. Methods of using the vasoactive intestinal polypeptide compositions are also disclosed.
Owner:TRANSITION THERAPEUTICS INC
Vasoactive intestinal polypeptide compositions
InactiveUS20080214440A1Facilitate increased duration of actionProlong the action timePeptide/protein ingredientsAntipyreticObesityInsulin resistance
Pharmaceutical compositions relating to vasoactive intestinal polypeptides and methods for the treatment of metabolic disorders, including diabetes, insulin resistance, metabolic acidosis and obesity are presented. Methods of using the vasoactive intestinal polypeptide compositions are also disclosed.
Owner:TRANSITION THERAPEUTICS INC
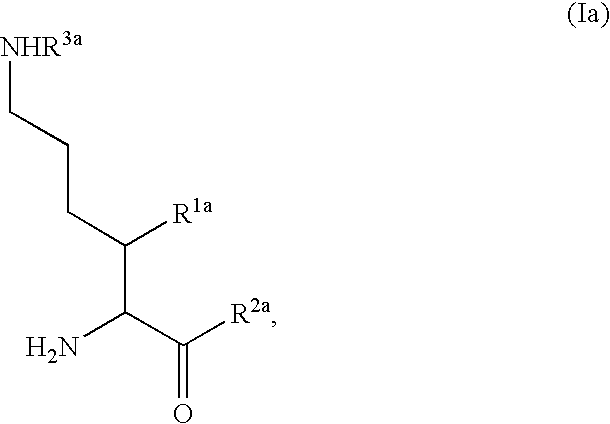
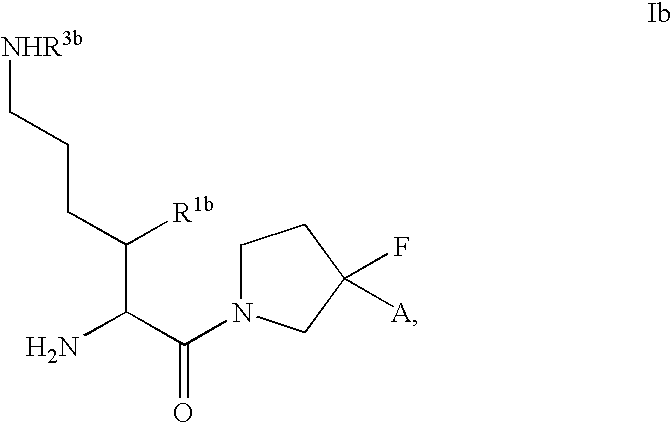
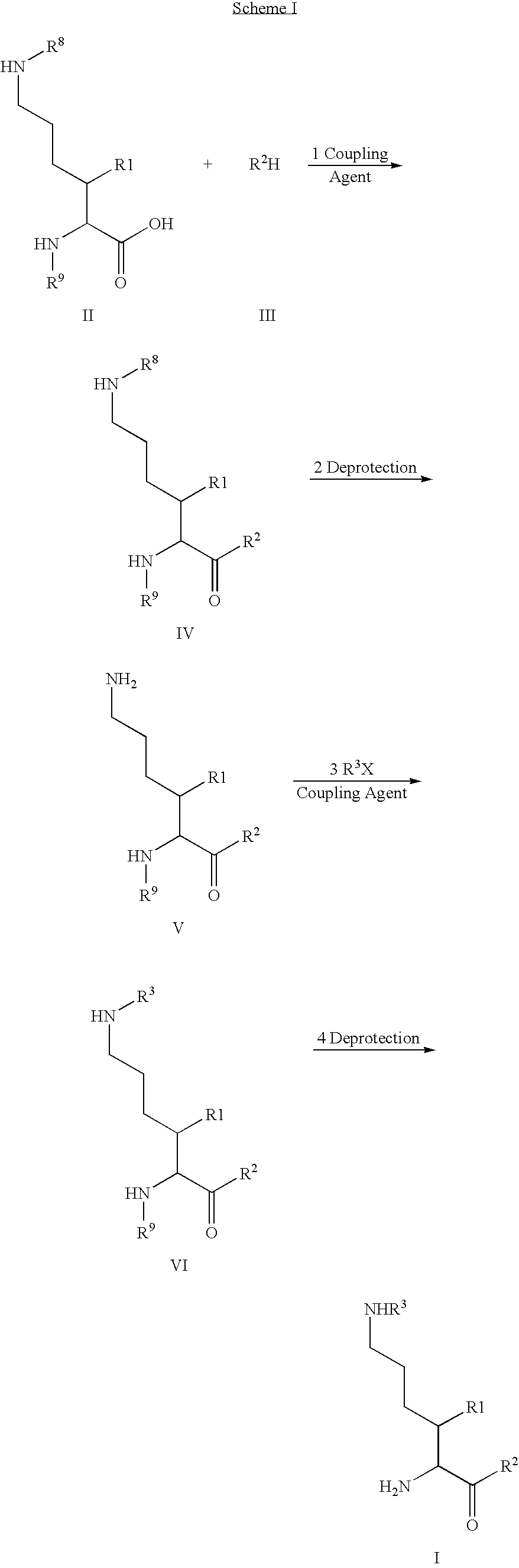
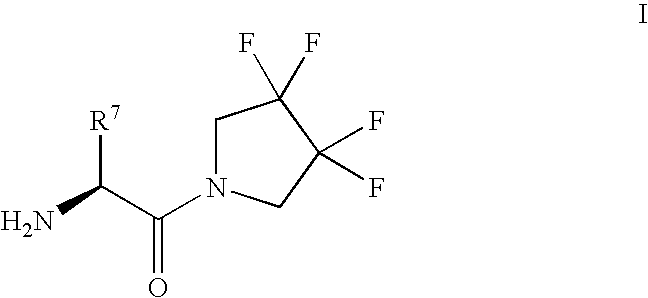
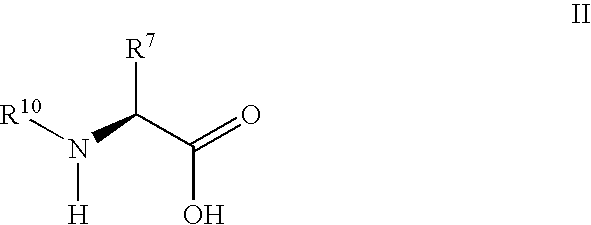
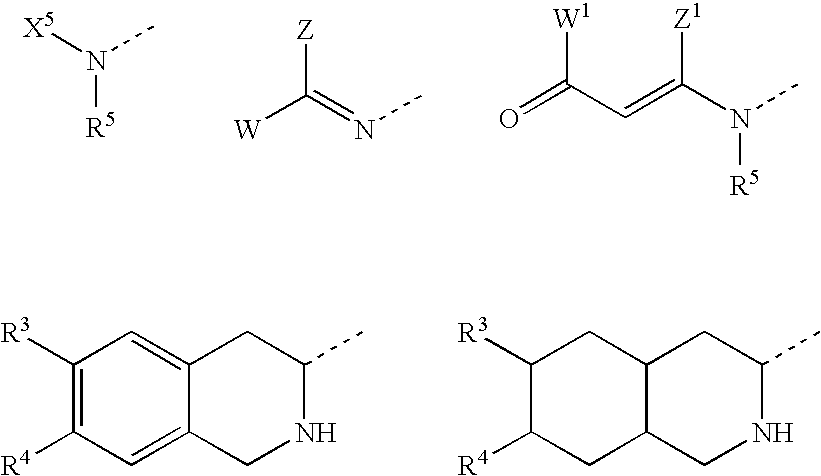
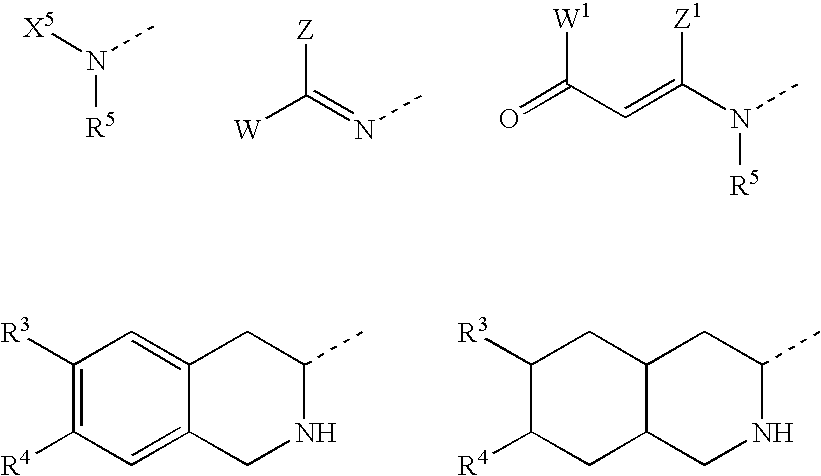
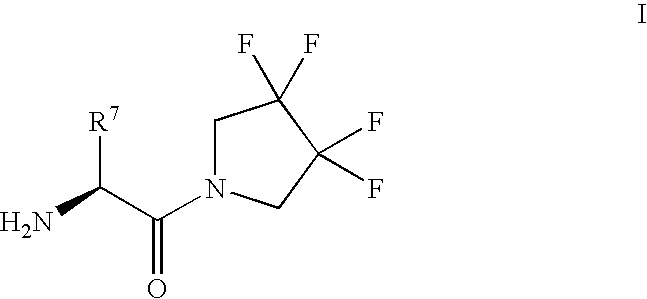
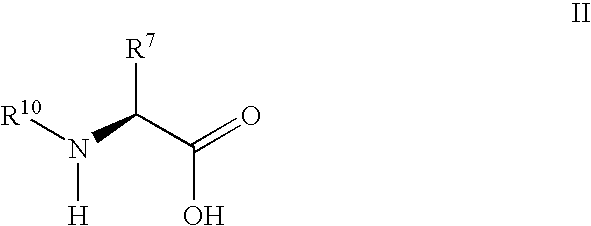
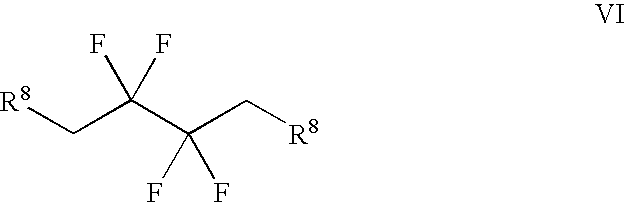
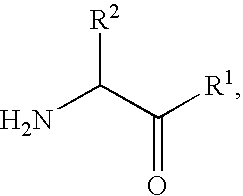
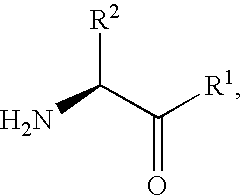
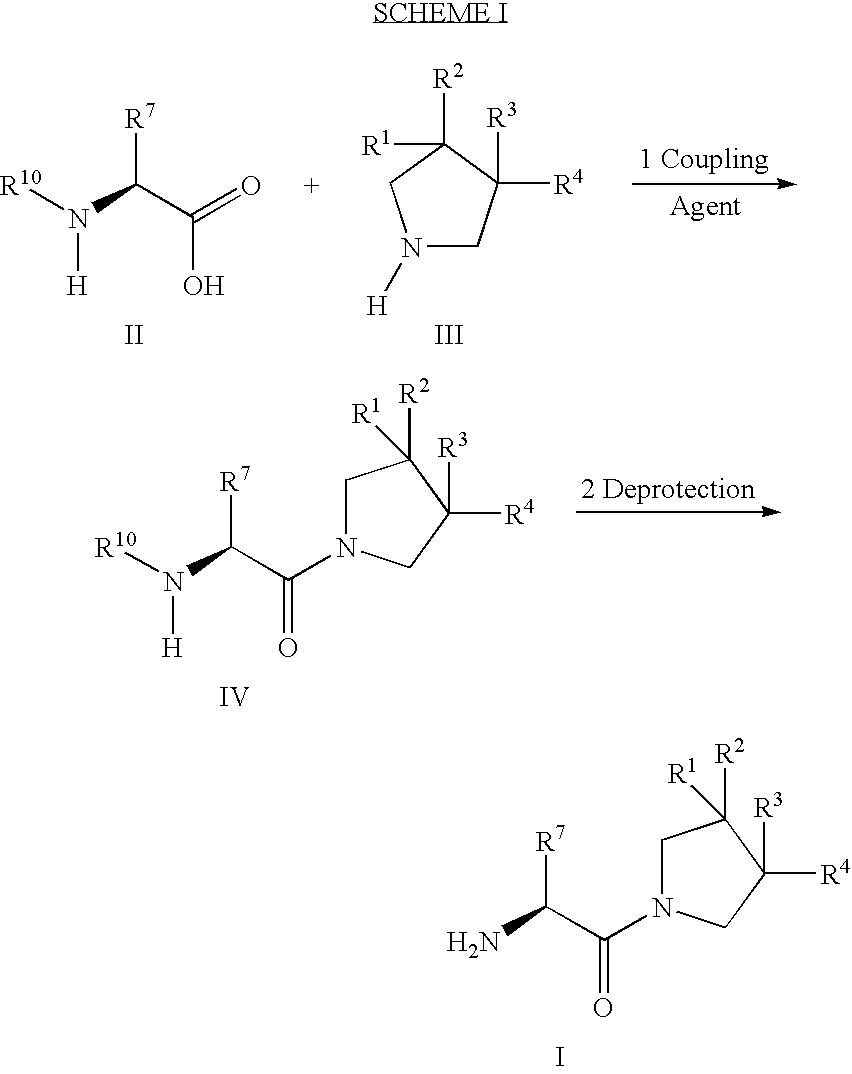
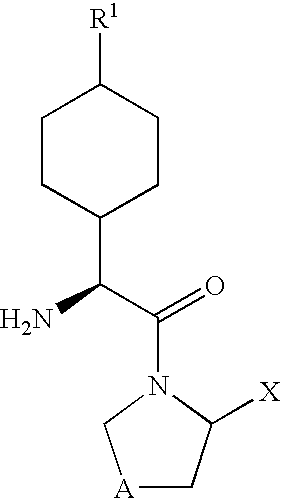
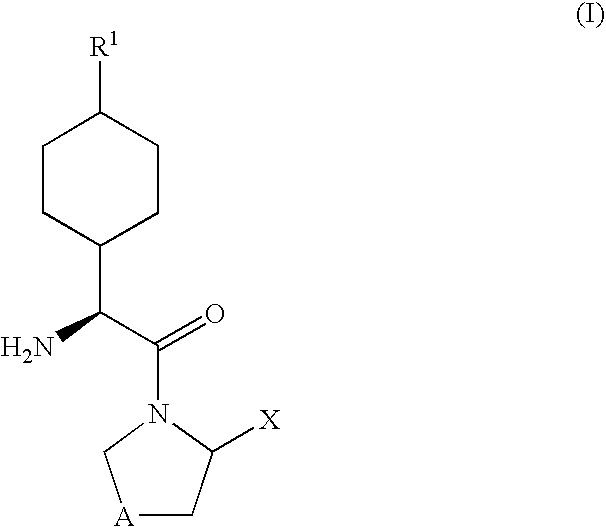
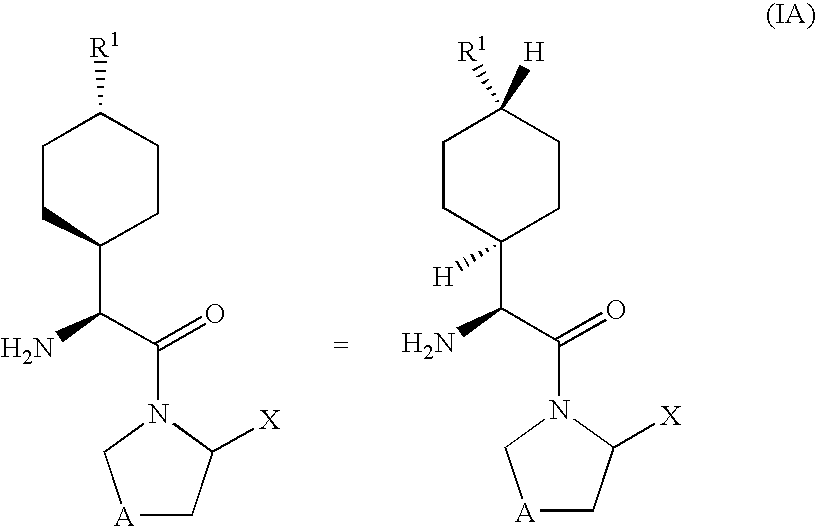
Derivatives of 3-Azabicyclo[3.1.0] Hexane as Dipeptidyl Peptidase-IV Inhibitors
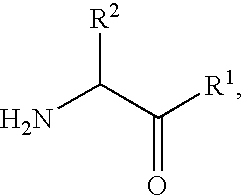
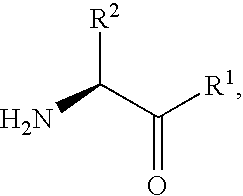
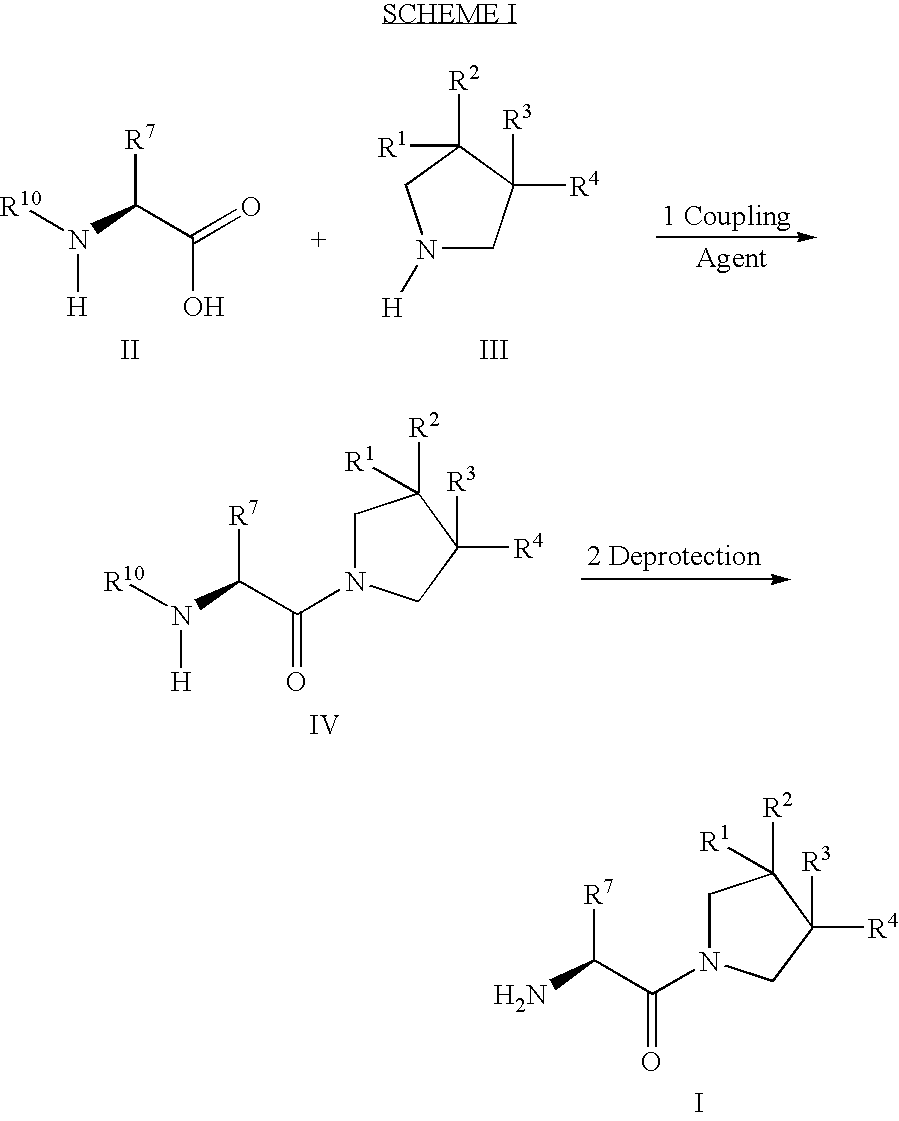
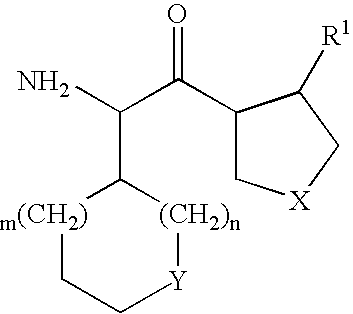
InactiveUS20080300251A1Avoid chemical reactionsBiocideNervous disorderDipeptidyl peptidasePrediabetes
The present invention relates to novel 3-azabicyclo[3.1.0]hexane derivatives as dipeptidyl peptidase-IV inhibitors and the processes for the synthesis of the said compounds. This invention also relates to pharmacological compositions containing the compounds of the present invention, and methods of treating diabetes, especially type 2 diabetes, as well as prediabetes, diabetic dyslipidemia, metabolic acidosis, ketosis, satiety disorders, and obesity. These inhibitors can also be used to treat conditions manifested by a variety of metabolic, neurological, anti-inflammatory, and autoimmune disorders like inflammatory disease, multiple sclerosis, rheumatoid arthritis; viral, cancer and gastrointestinal disorders. The compounds of this invention can also be used for treatment of infertility arising due to polycystic ovary syndrome.
Owner:RANBAXY LAB LTD
Vasoactive intestinal polypeptide pharmaceuticals
InactiveUS7566691B2Facilitate duration of actionExtended durationMetabolism disorderDepsipeptidesVasoactive intestinal peptideObesity
Pharmaceutical compositions relating to vasoactive intestinal polypeptides and methods for the treatment of metabolic disorders, including diabetes, insulin resistance, metabolic acidosis and obesity are presented. Methods of using the vasoactive intestinal polypeptide compositions are also disclosed.
Owner:TRANSITION THERAPEUTICS INC
Vasoactive intestinal polypeptide pharmaceuticals
InactiveUS7595294B2Facilitate duration of actionExtended durationMetabolism disorderDepsipeptidesMedicineVasoactive intestinal peptide
Owner:TRANSITION THERAPEUTICS INC
Vasoactive intestinal polypeptide pharmaceuticals
InactiveUS20060079456A1Facilitate duration of actionProlong the action timeMetabolism disorderDepsipeptidesObesityInsulin resistance
Pharmaceutical compositions relating to vasoactive intestinal polypeptides and methods for the treatment of metabolic disorders, including diabetes, insulin resistance, metabolic acidosis and obesity are presented. Methods of using the vasoactive intestinal polypeptide compositions are also disclosed.
Owner:TRANSITION THERAPEUTICS INC
Use of a layer consisting of hydrophobic linear, or two-dimensional polycyclic aromatics as a barrier layer or an encapsulation and electric components constructed with a layer of this type and comprising organic polymers
The invention relates to cyclic bioisosteres of derivatives of a purine system having a general structural formula R1=—H, —NH2, —Br, —Cl, —OH, —COOH, B=—N═, —CH═, Z=—CH═, —N═, A=—N═ at B=—N═, Z=—CH—, A=—CH═ at B=—N═, Z=—CH—, A=—CH═ at B=—N═, Z=—N═, A=—CH═ at B=—CH═, Z=—CH═, A=—CH═ at B=—CH═, Z=—N═, and their pharmacologically acceptable salts having a normalizing effect on endocellular processes, in particular, it is capable eliminating endocellular metabolic acidosis and capable of binding excessively formed free radicals, in particular, free-radical forms of oxygen, capable of normalizing the nitrergic mechanisms of the cells, and also capable of interreacting with adenosine-sensitive receptors on the membrane of non-nuclear cells and in nuclei-containing cells to decrease the aggregation of thrombocytes. The compounds according to the invention have hepatoprotective effect and can be used for producing pharmaceutical compositions on their base.
Owner:TECH UNIV BRAUNSCHWEIG
Separately packed structural fatty milk, aminoacid and glucose injection composition and the prepn process
ActiveCN101019823AMeet metabolic needsAvoid Metabolic AcidosisMetabolism disorderPharmaceutical delivery mechanismAmino Acid InjectionMedical prescription
The present invention is one kind of three-cavity packed injection composition, and has structural fatty milk injection, compound amino acid injection and glucose injection packed separately in three diaphragm separated cavities. Before intravenous infusion, the structural fatty milk injection, the compound amino acid injection and the glucose injection are squeezed out and mixed in common operation condition. The injection composition can be used to patient for meeting the requirement in protein and saccharide.
Owner:费森尤斯卡比华瑞制药有限公司
Medicinal composition
InactiveCN1813795AImprove scalabilityImprove securityOrganic active ingredientsMetabolism disorderSodium lactateHydroxyethyl starch
The present invention relates to a medicine composition. It is composed of hydroxyethyl starch 130 / 0.4 and electrolyte balancing solution, in which the molecular weight of hydroxyethyl starch is 100000-150000, its content in every 100 ml of said composition is 5.4-6.6 g, and the electrolyte balancing solution is sodium lactate Ringer's injection or compound electrolyte injection. Its production process is simple and its product quality is stable.
Owner:SHANDONG CHENGCHUANG PHARMA R&D
Process for controlling water and electrolyte balance and acid-base equilibrium in human body
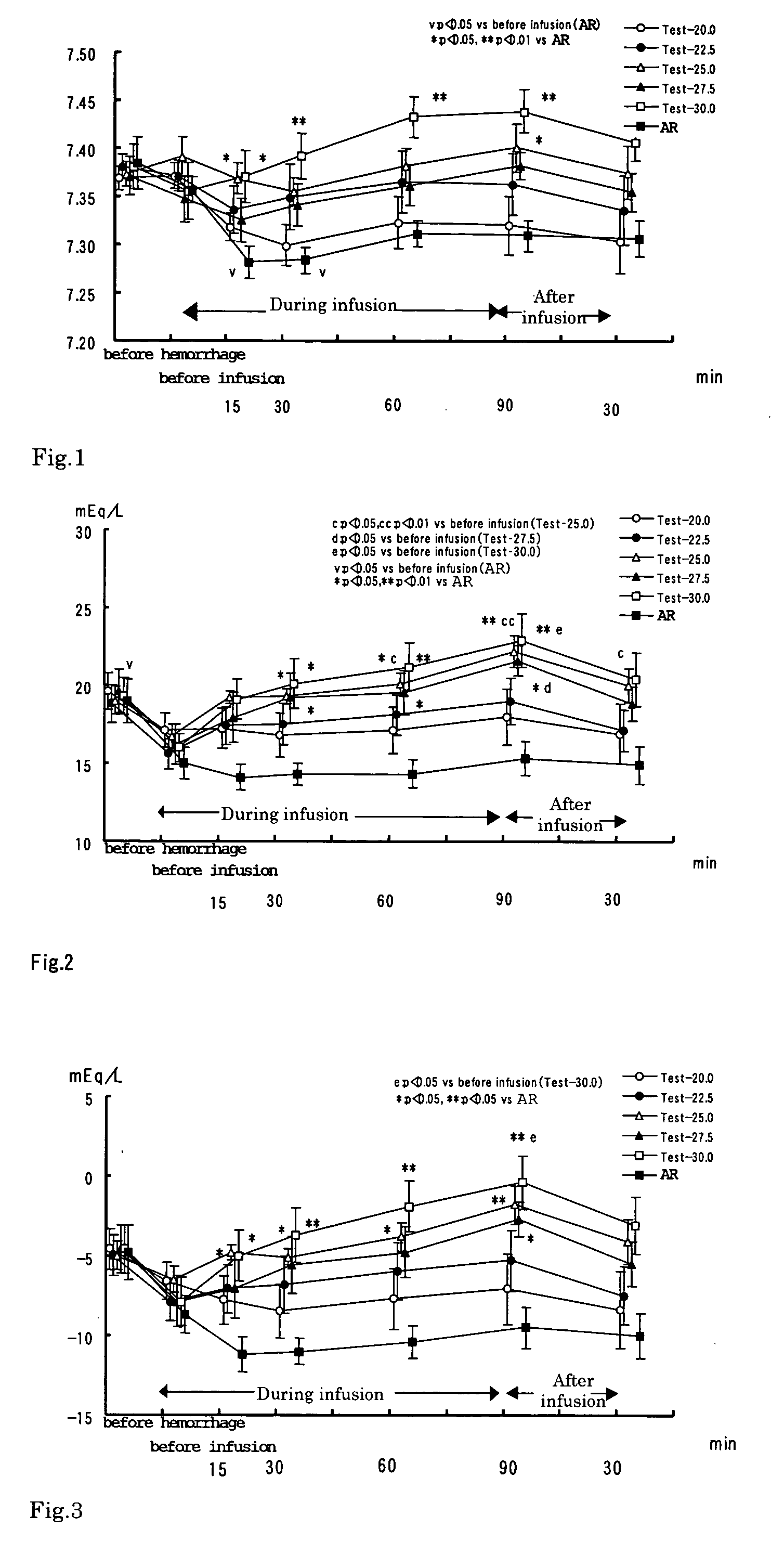
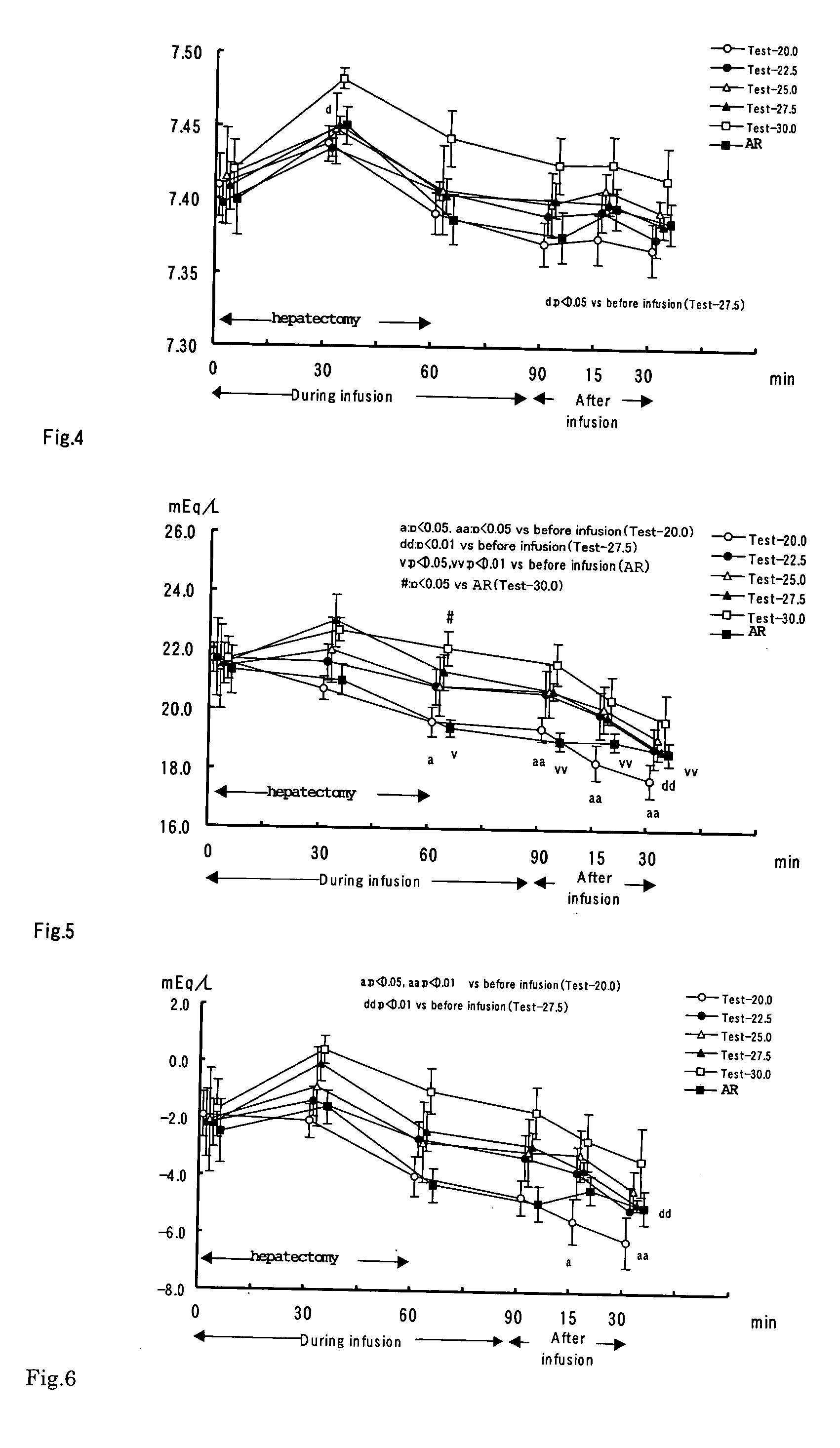
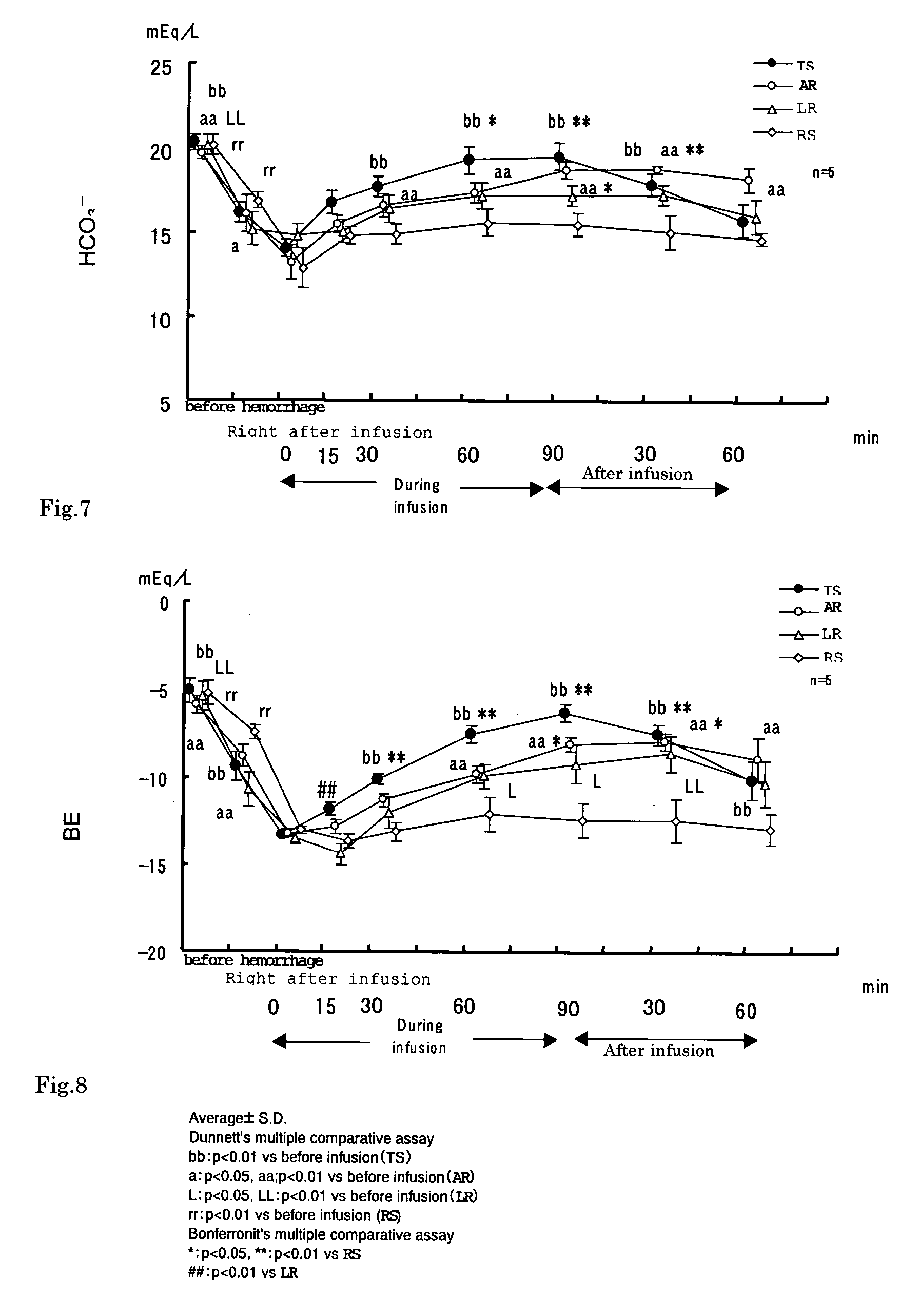
InactiveUS20050100615A1Induce metabolic alkalosisDisappear quicklyBiocidePharmaceutical delivery mechanismSodium bicarbonateHypernatremia
The present invention relates to process for controlling water and electrolyte balance and acid-base equilibrium, and particularly to process for controlling water and electrolyte balance and acid-base equilibrium supervening metabolic acidosis due to burn injury, hemorrhagic shock, multiple organ failure, systemic inflammatory response syndrome (SIRS), and so on. By administering the preparation containing sodium bicarbonate as an alkalizing agent of the present invention, the acidosis correction effect is exhibited immediately after the start of the infusion and disappeared quickly by demedication. And therefore, the preparation of the present invention can be administered safely without inducing metabolic alkalosis during infusion and alkalosis after the infusion. The preparation of the present invention also has no problem with hypernatremia. The controlling water and electrolyte balance and acid-base equilibrium, and particularly to process for controlling water and electrolyte balance can be done by administering the preparation containing bicarbonate at a rate of 2 to 60 mL / kg / hour.
Owner:AJINOMOTO CO INC
Chinese medicinal composition for treating kidney failure diseases and preparation method thereof
InactiveCN101810792AImprove self-coordinationFunction increaseUrinary disorderAluminium/calcium/magnesium active ingredientsNervous systemAdemetionine
The invention discloses a novel Chinese medicinal composition for treating kidney failure diseases and a preparation method thereof. The Chinese medicinal composition mainly comprises the following raw material medicaments: medlar, American ginseng, campanumaea pilosula, raw astragalus, pantotrichum, common yam rhizome, sea horse, medicinal indianmulberry root, cordyceps sinensis, lucid ganoderma, south dodder seed, gordon euryale seed, eucommia bark, radix rehmanniae preparata, Chinese angelica, white paeony root, cochinchnese asparagus root, glossy privet fruit, amur corktree bark, Indian buead, gardenia, plantain seed, longhairy antenoron herb, talc and oriental waterplantain rhizome. The Chinese medicinal composition can be prepared into any common oral preparation by the conventional Chinese medicinal preparation method. The Chinese medicinal composition can obviously improve symptoms such as edemaofface, edema of limbs, inability to get food down, bradypsychia, uroschesis, creatinine, albuminuria, dreaminess, insomnia and the like, and can improve various complications which seriously influence health and life and even cause depth such as infection, diseases of cardiovascular systems, nervous systems and digestive systems, electrolyte disturbance, metabolic acidosis and the like caused by renal function hydrolysis. The Chinese medicinal composition has the advantages of exact clinical effects, obvious curative effect, quick response, low cost, basically no toxic or side effects and the like because the Chinese medicinal composition basically combines medicaments having homology of medicament and food specified in national formulary.
Owner:TAIYI HEPU BEIJING RES INST OF TCM
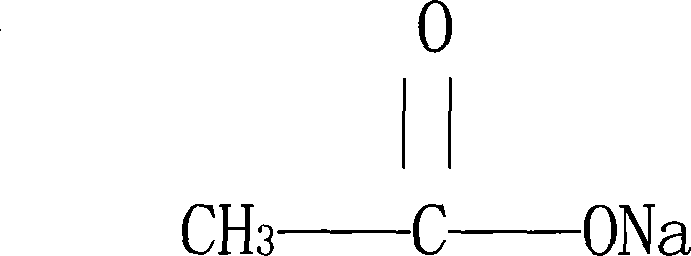
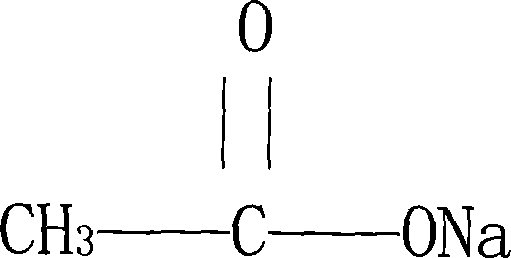
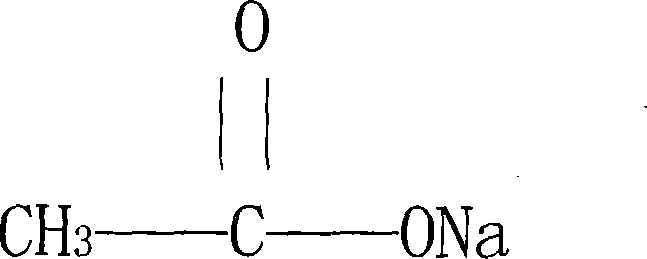
Sodium acetate anhydrous and preparation method and usage thereof
ActiveCN101139280ANo pollution in the processGood chemical stabilityMetabolism disorderCarboxylic acid salt preparationChemical structureSodium acetate
The molecular formula of an anhydrous sodium acetate is C2H3NaO2; the molecular weight is 82.03; and the anhydrous sodium acetate has the following chemical structure. The preparation method is: the sodium acetate trihydrate is got and heated to be between 120 and 130 DEG C; then the sodium acetate trihydrate is decompressed and concentrated, cooled and crystallized, and dried to get the anhydrous sodium acetate. The anhydrous sodium acetate is clinically applied in the circulating blood volume, the supplement of the outer liquid of the cell when the issue liquid is reduced, and the metabolic acidosis. The advantages are: the anhydrous sodium acetate has the good solubility in water; the chemical stability is good, suitable for the human medication requirements; the anhydrous sodium acetate is easy to be stored. Because the anhydrous sodium acetate can be dissolved in water; the anhydrous sodium acetate is more suitable for the pharmaceutical preparation, in particular for the preparation of the injection. The preparation method is characterized in the high collection rate, the good product quality, the low production cost, no environmental pollution, and so on. The anhydrous sodium acetate is clinically applied in the circulating blood volume, the supplement of the outer liquid of the cell when the issue liquid is reduced, and the metabolic acidosis.
Owner:HUBEI DUORUI PHARMA
Pharmaceutical composition of compound amino acid injection 18AA and application thereof
InactiveCN104055766AReduce hyperchloremiaReduce the likelihood of developing hyperchloremiaOrganic active ingredientsMetabolism disorderProtein intakeNutritional status
The invention provides a pharmaceutical composition of compound amino acid injection 18AA. The pharmaceutical composition disclosed by the invention can be used for preparing drug for treating patients with body metabolism needs on amino acid being not unsatisfied due to protein intake deficiency, absorbing barrier, and the like, drug for improving the nutrition status of a postoperative patient, and drug for preventing or treating metabolic acidosis and the like, so that the prepared product has widened application people or better targeting performance and better clinical safety during clinical application.
Owner:刘力
Hydroxyethyl starch injection liquid and preparation method thereof
InactiveCN1994316AReduce demandAvoid damageOrganic active ingredientsInorganic active ingredientsHydroxyethyl starchPlasma Substitutes
The invention provides a hydroxyethyl starch injection used as plasma substitute, process for preparation and method of application, compared with the existing hydroxyethyl starch injections, higher safety and effectiveness can be achieved in clinical application, the injury to kidney function is lowered, thereby causing no high chlorine metabolic acidosis.
Owner:李晓祥
Vasoactive intestinal polypeptide compositions
InactiveUS20090170775A1Facilitate increased duration of actionProlong the action timePeptide/protein ingredientsMetabolism disorderVasoactive intestinal peptideObesity
Pharmaceutical compositions relating to vasoactive intestinal polypeptides and methods for the treatment of metabolic disorders, including diabetes, insulin resistance, metabolic acidosis and obesity are presented. Methods of using the vasoactive intestinal polypeptide compositions are also disclosed.
Owner:TRANSITION THERAPEUTICS INC
Alkaline drug enteric preparation and preparation method thereof
InactiveCN101190185ADoes not affect normal digestion and absorption functionAvoid discomfortDigestive systemPharmaceutical non-active ingredientsDiseaseMetabolic acidosis
Owner:李大鹏
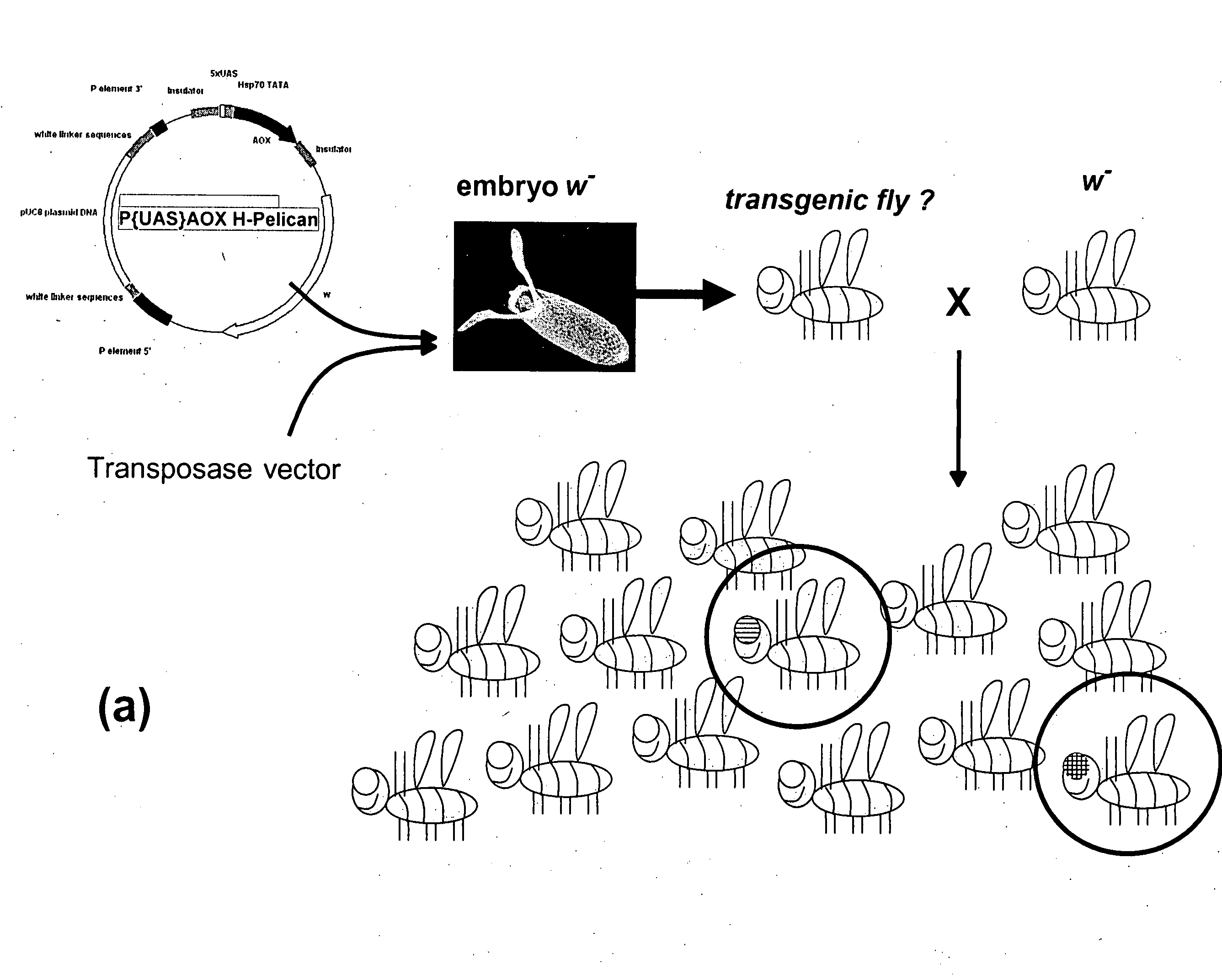
Alternative oxidase and uses thereof
InactiveUS20080103088A1Anti agingExtend your lifeNervous disorderVirusesWhole OrganismPhosphorylation
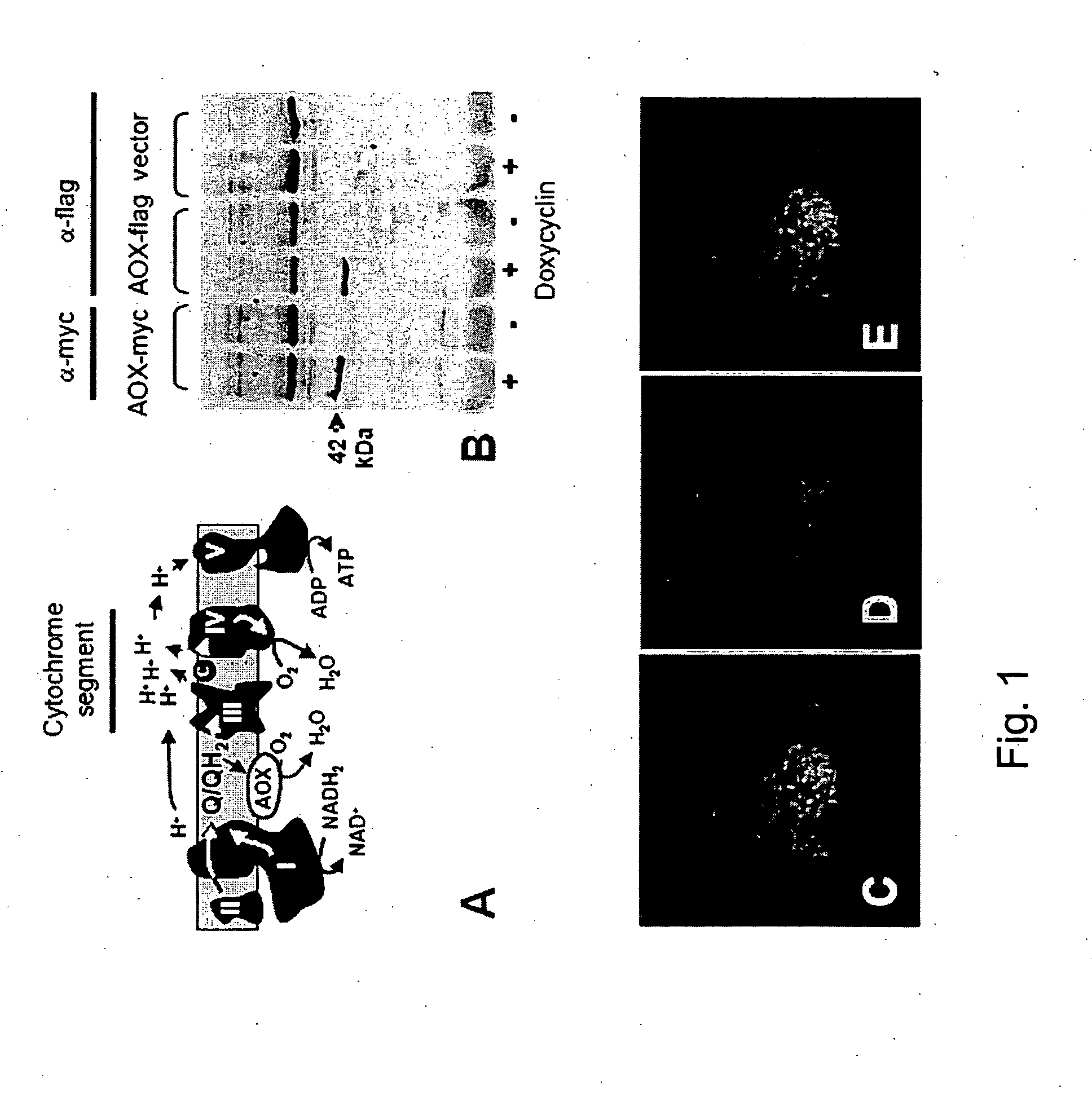
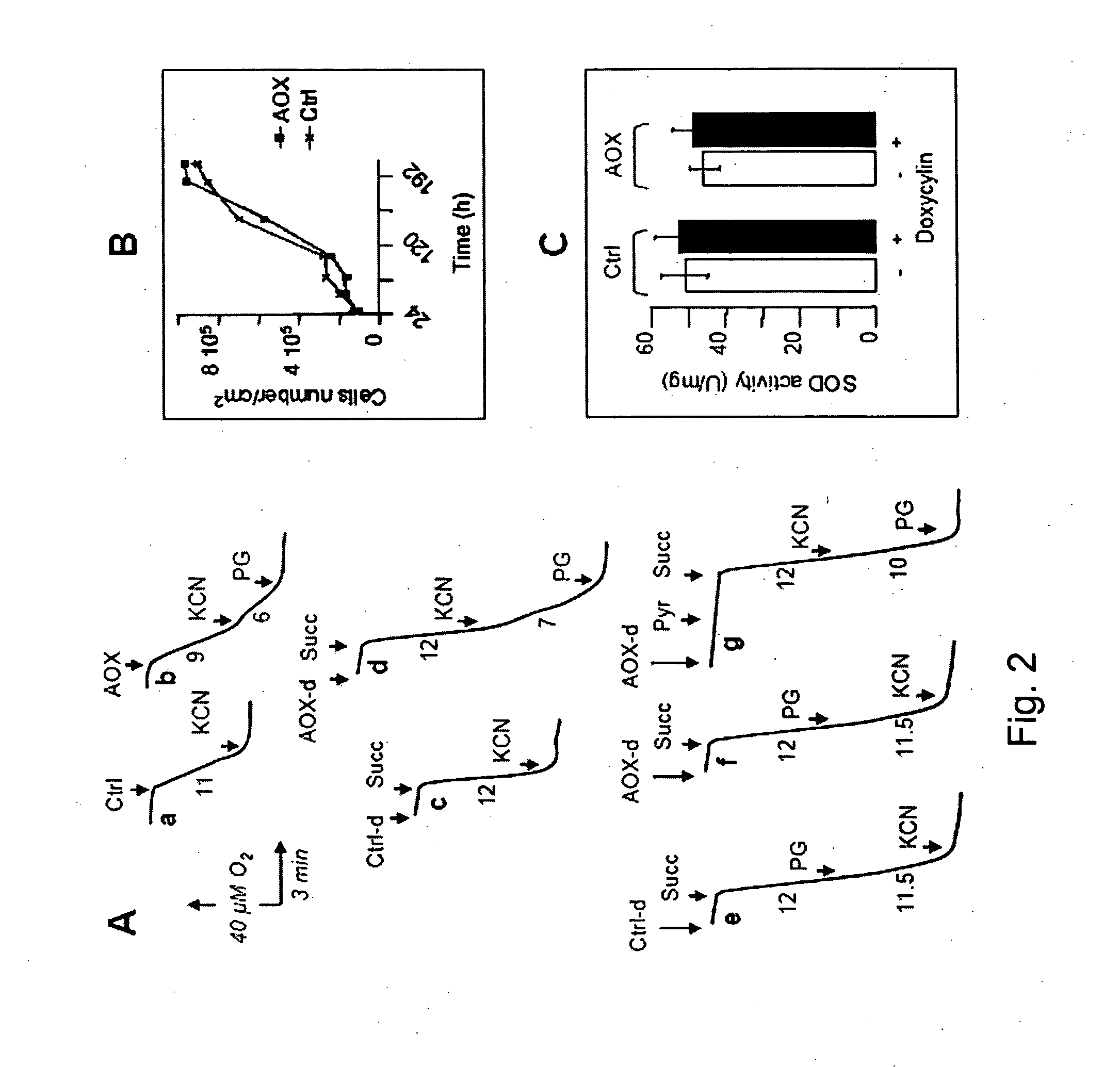
The invention relates to a method for combating disorders affecting the mitochondrial oxidative phosphorylation system by allotopic expression of the cyanide-insensitive alternative oxidase (AOX) in human cells. The successful expression of AOX in human cells and in Drosophila has been shown to confer spectacular cyanide-resistance to mitochondrial substrate oxidation, alleviate oxidative stress, apoptosis susceptibility and metabolic acidosis. AOX is well tolerated when expressed ubiquitously in the whole organism. AOX expression is a valuable tool to limit the deleterious consequences of respiratory chain deficiency.
Owner:UNIVERSITY OF TAMPERE
Novel effectors of dipeptidyl peptidase IV
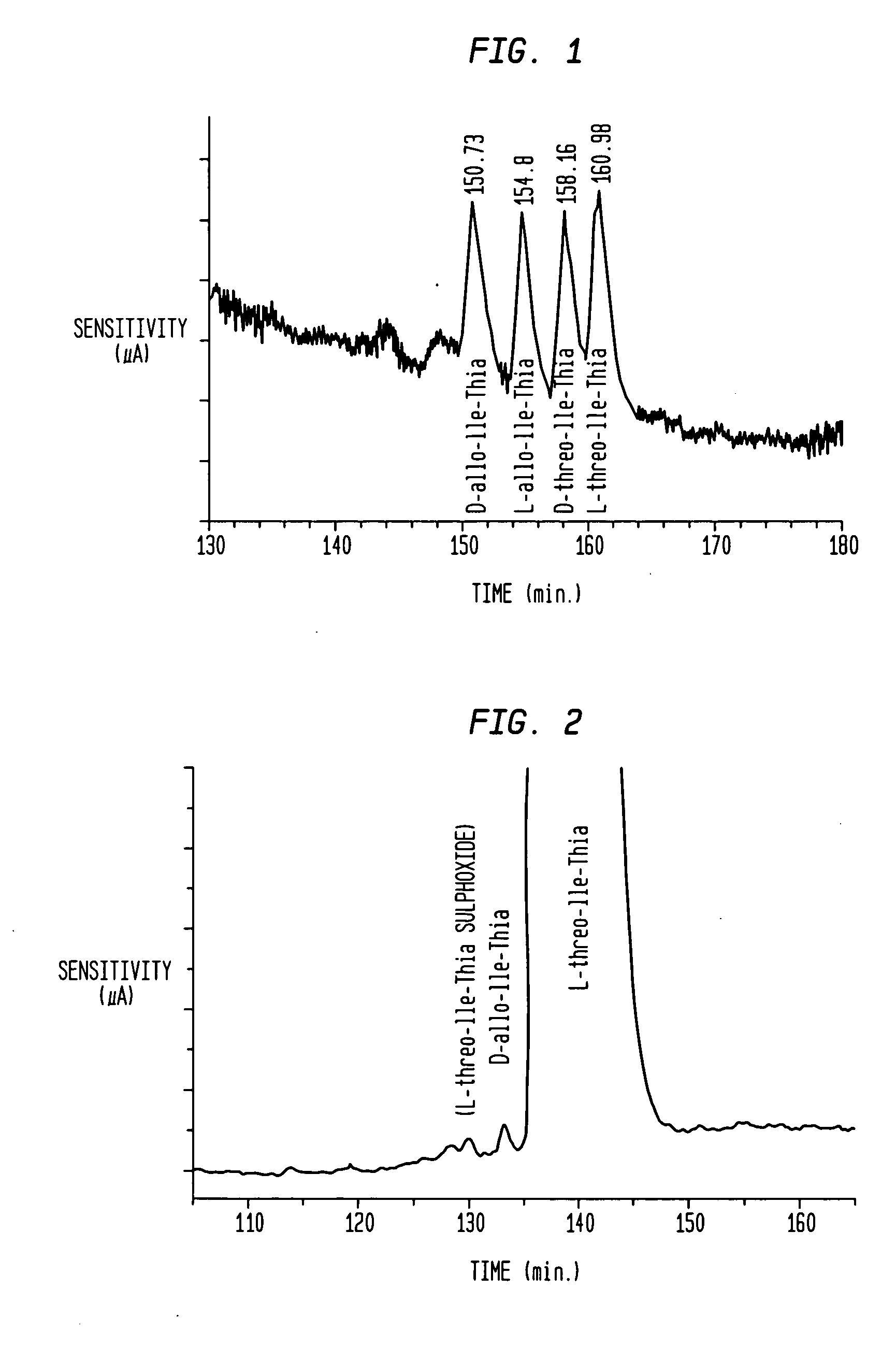
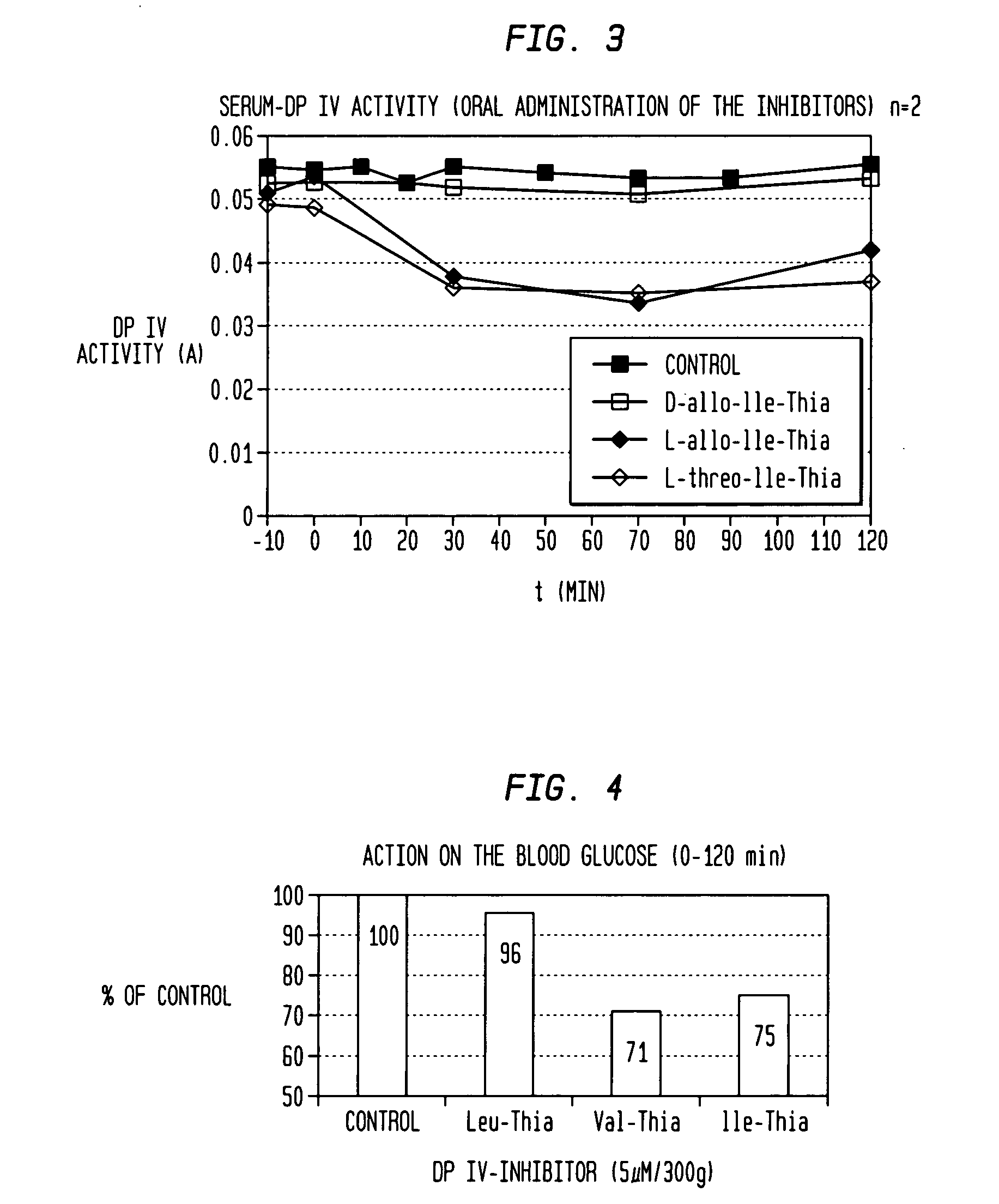
Dipeptide compounds and compounds analogous to dipeptide compounds that are formed from an amino acid and a thiazolidine or pyrrolidine group, and salts thereof used in the treatment of impaired glucose tolerance, glycosuria, hyperlipidaemia, metabolic acidoses, diabetes mellitus, diabetic neuropathy and nephropathy and also of sequelae of diabetes mellitus in mammals.
Owner:PROSIDION LIMITED
Medicament for preventing and controlling atony of proventriculus of ruminant
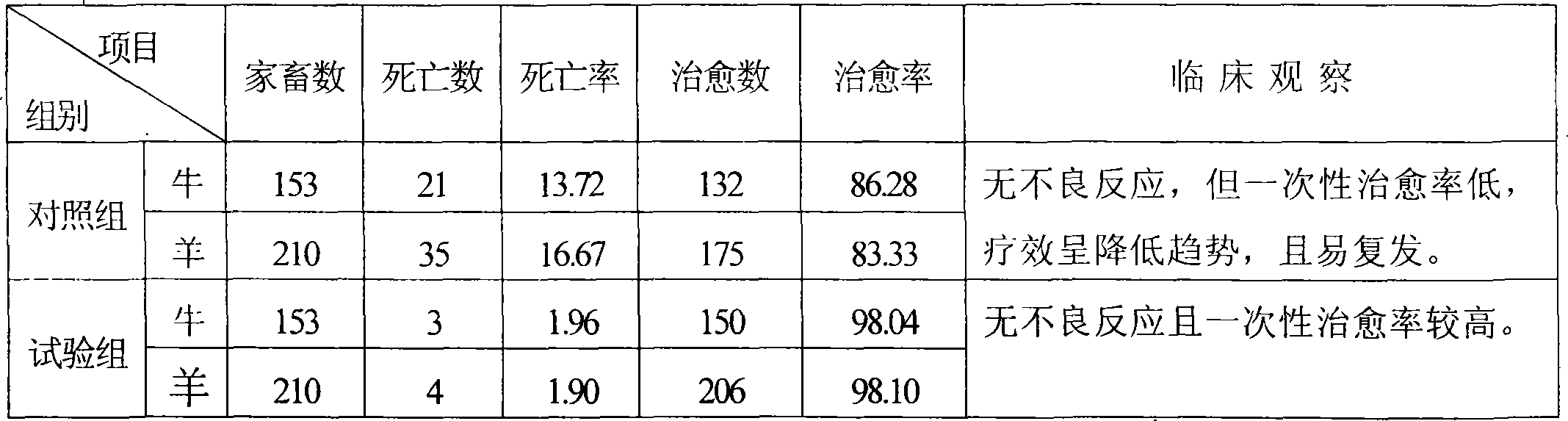
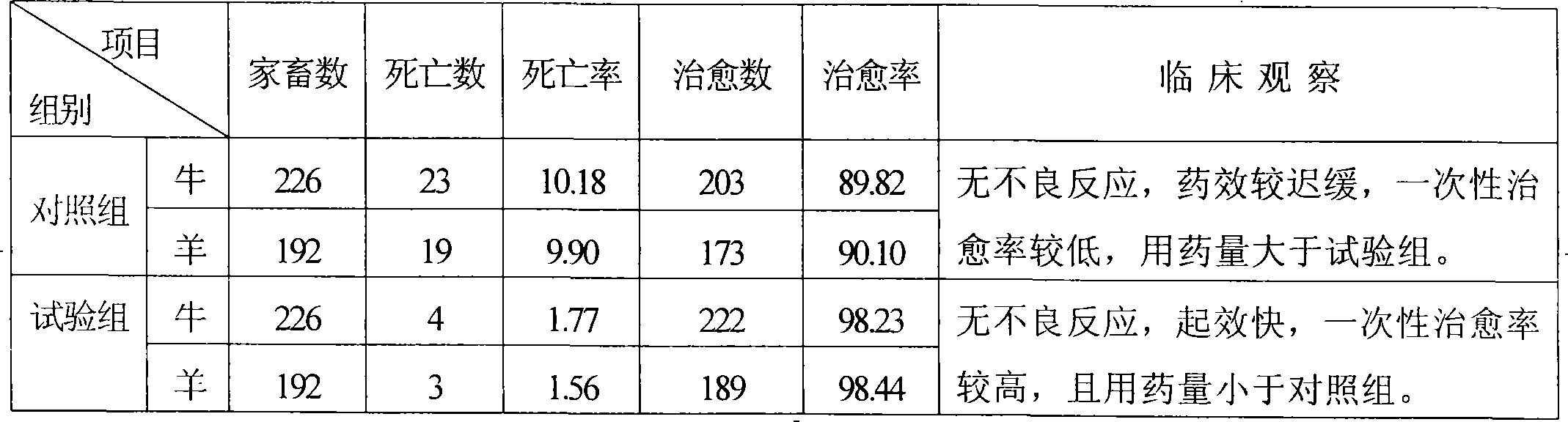
The invention discloses a medicine for preventing and curing forestomach atony of ruminants, being prepared with the medicines according to a certain proportion: (fried) nuxvomica, areca seed, pharbitidis seed, euphorbia root, medicated leaven, hawthorn fruit, malt, Chinese rhubarb, mirabilite, stertite, antimony potassium tartrate and sodium bicarbonate, which (except mirabilite and stertite) are sun-dried, purified, crushed, and then grinded with two western medicines, sieved through an 80 to 100-mesh screen and finally mixed evenly. In clinical practice, the medicines are socked in boiled water for 30-60 minutes, of which the effective ingredients are dissolved and precipitated for improving the absorption rate of the medicine which is one-time drenched when cool. The medicine of the invention has quicker efficacy and smaller dosage compared with the single use of traditional Chinese medicine, longer maintenance time of drug effect and more difficult recurring compared with the single use of western medicine, and is difficult to develop the immunity to drugs, effectively releases or eliminates in vivo metabolic acidosis, greatly reduces death rate without a untoward reaction and with higher clinic cure rate. With simple preparation method and convenient use, the invention can be widely used in the breeding industry.
Owner:HEBEI AGRICULTURAL UNIV.
Vasoactive intestinal polypeptide pharmaceuticals
InactiveUS20060234933A1Facilitate duration of actionExtended durationMetabolism disorderDepsipeptidesObesityBlood vessel
Pharmaceutical compositions relating to vasoactive intestinal polypeptides and methods for the treatment of metabolic disorders, including diabetes, insulin resistance, metabolic acidosis and obesity are presented. Methods of using the vasoactive intestinal polypeptide compositions are also disclosed.
Owner:TRANSITION THERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
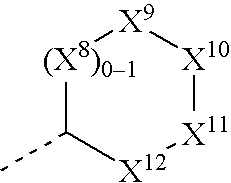
![Derivatives of 3-Azabicyclo[3.1.0] Hexane as Dipeptidyl Peptidase-IV Inhibitors Derivatives of 3-Azabicyclo[3.1.0] Hexane as Dipeptidyl Peptidase-IV Inhibitors](https://images-eureka.patsnap.com/patent_img/b07ea871-988e-445e-b196-080eb11a636d/US20080300251A1-20081204-C00001.png)
![Derivatives of 3-Azabicyclo[3.1.0] Hexane as Dipeptidyl Peptidase-IV Inhibitors Derivatives of 3-Azabicyclo[3.1.0] Hexane as Dipeptidyl Peptidase-IV Inhibitors](https://images-eureka.patsnap.com/patent_img/b07ea871-988e-445e-b196-080eb11a636d/US20080300251A1-20081204-C00002.png)
![Derivatives of 3-Azabicyclo[3.1.0] Hexane as Dipeptidyl Peptidase-IV Inhibitors Derivatives of 3-Azabicyclo[3.1.0] Hexane as Dipeptidyl Peptidase-IV Inhibitors](https://images-eureka.patsnap.com/patent_img/b07ea871-988e-445e-b196-080eb11a636d/US20080300251A1-20081204-C00003.png)