Patents
Literature
1172 results about "Mirabilite" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mirabilite, also known as Glauber's salt, is a hydrous sodium sulfate mineral with the chemical formula Na₂SO₄·10H₂O. It is a vitreous, colorless to white monoclinic mineral that forms as an evaporite from sodium sulfate-bearing brines. It is found around saline springs and along saline playa lakes. Associated minerals include gypsum, halite, thenardite, trona, glauberite, and epsomite.
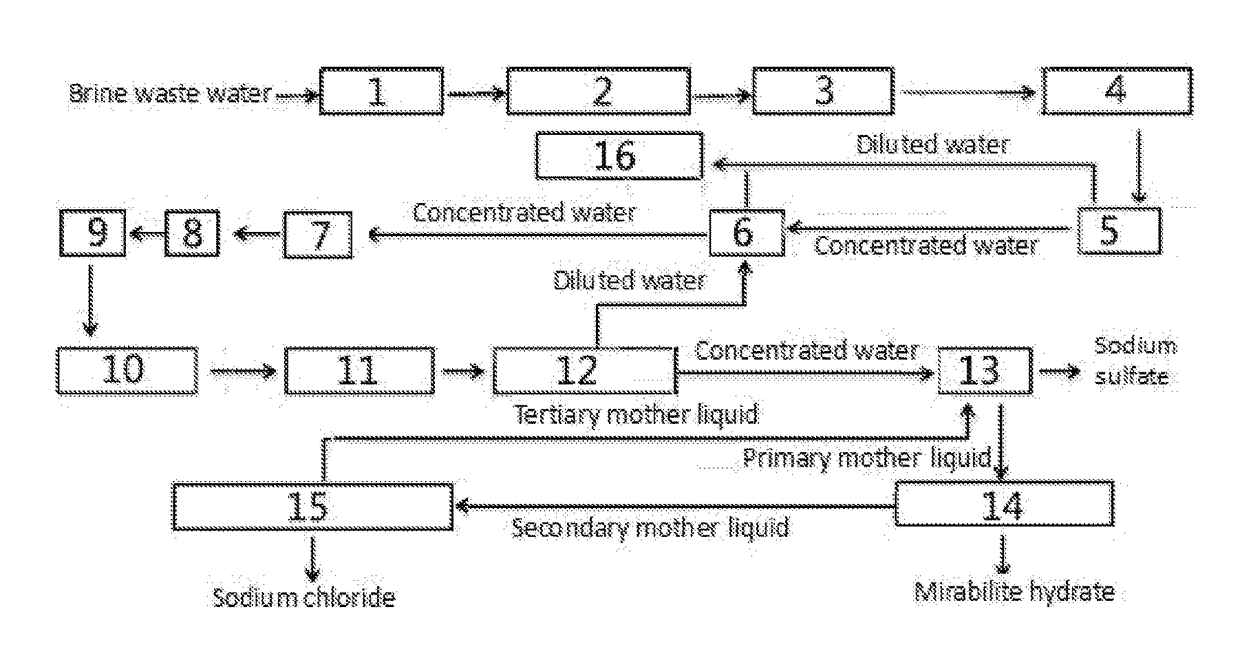
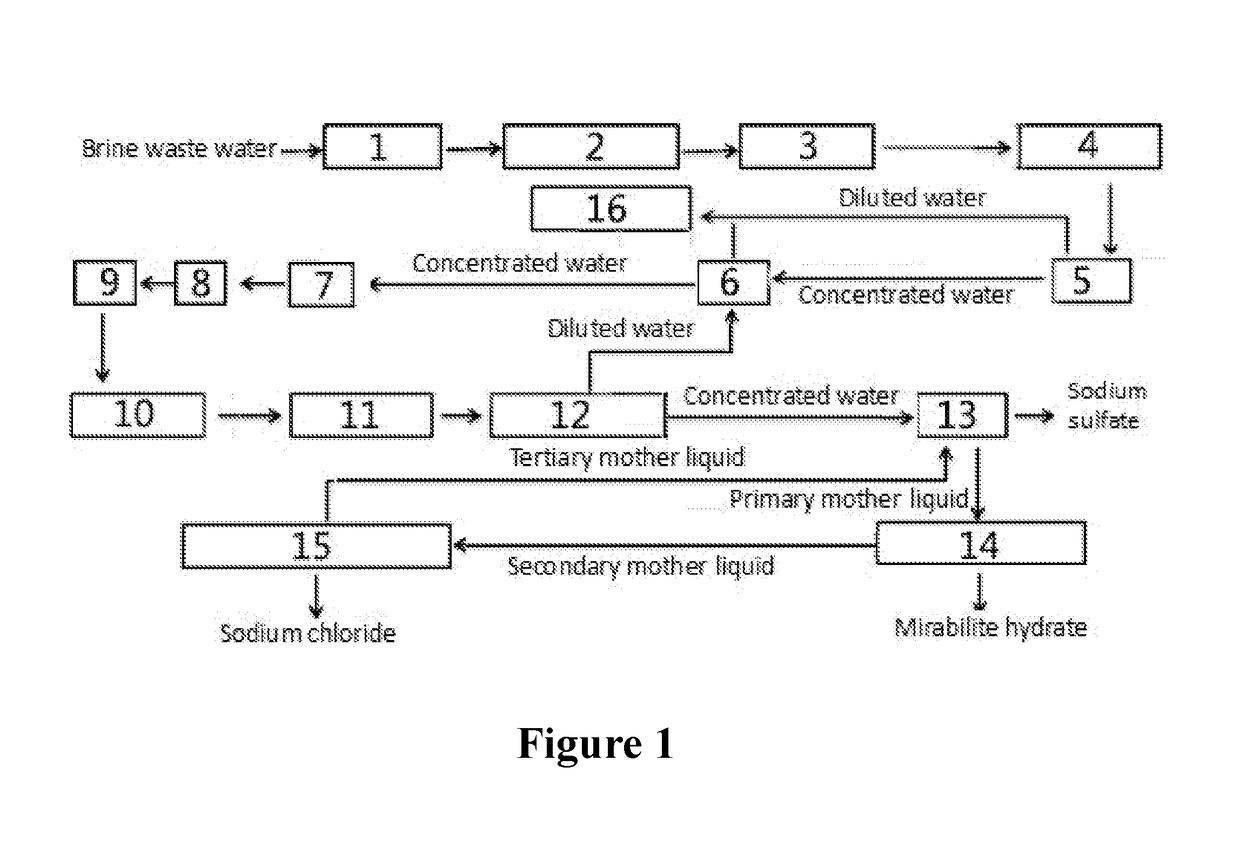
Method and System for Treating Brine Waste Water
ActiveUS20180148350A1Reduce pollutionExtended service lifeScale removal and water softeningSustainable biological treatmentSaline waterWastewater
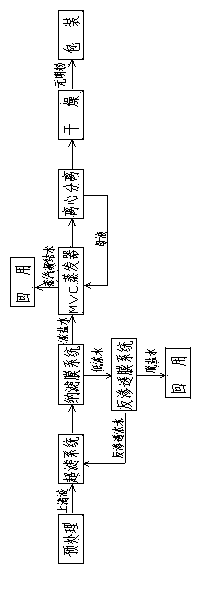
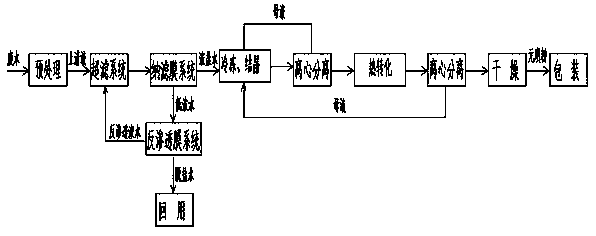
The present invention relates to a method for zero-release treatment of brine waste water, comprising: (1) pretreatment; (2) reverse osmosis treatment; (3) advanced oxidation treatment; (4) biochemical treatment; (5) electrodialysis concentration; (6) circulating crystallization. Compared with the prior art, the method for zero-release treatment of brine waste water provided in the present invention realizes zero release or near zero release of waste water, improves salt recovery efficiency, can recover high-quality sodium sulfate, mirabilite and sodium chloride, and turns crystalline salts into a resource; the membrane treatment unit can operates stably in the process for a long operation period at a low cost, and the entire process has high economic efficiency.
Owner:CHINA PETROCHEMICAL CORP +1
Mineral glass beads for road markings
InactiveCN101857368AHigh reflective brightnessStrong retro-reflective effectSmithsoniteRefractive index
The invention relates to mineral glass beads for road markings, which relates to optical glass beads and solves the problems of low refractive index and poor reflective brightness of the current glass beads for the road markings. The mineral glass beads for the road markings are the optical glass beads, wherein the glass beads are prepared from the following components in part by weight: 40 parts of sandstone, 28 to 32 parts of barite, 1835 to 22.5 parts of diamond, 2.5 parts of datolite, 2 parts of feldspar, 4 parts of mirabilite and 2 parts of smithsonite. The mineral glass beads can be widely used in road markings of airfield runways and high-level roads.
Owner:大庆路通科技有限公司
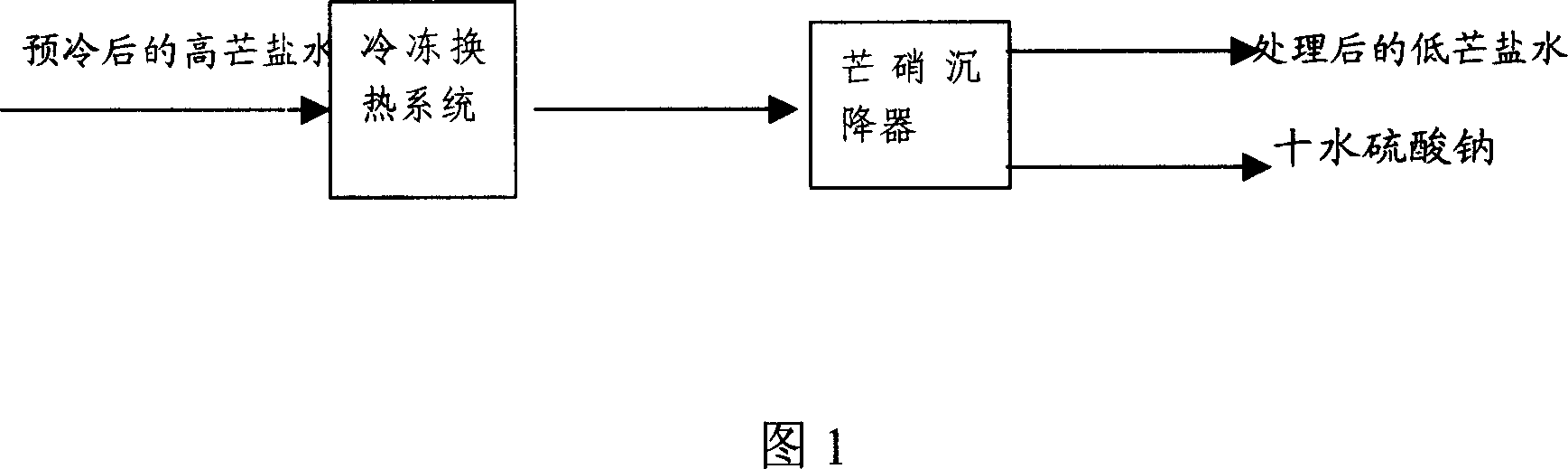
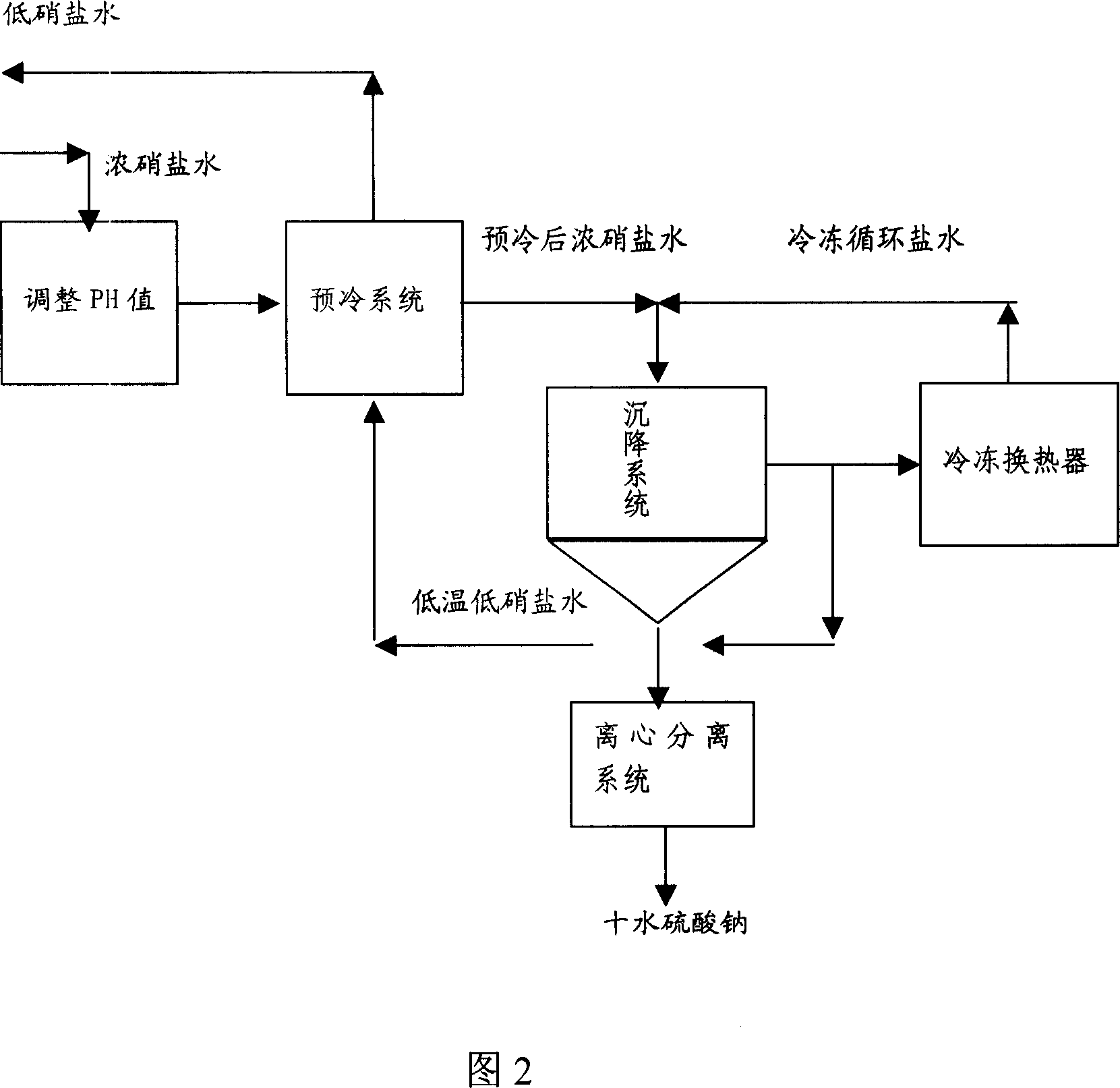
Method freezing separating mirabilite by brine solution
The invention discloses a method of cold separating mirabilite in salt water solution, which comprises the following steps: (1) adjusting pH value of armed salt cake solution as alkalify; (2) proceeding pre-cool in the precooling system; (3) mixing with low-temperature low-nitrate water of incorporating salt cake with lower temperature through demirabilite treatment; controlling temperature at -10-0 deg.c; proceeding solid-liquid separation in salt cake settler; getting ten water sodium sulfate crystal and low nitrate water; (4) lowering temperature of part low nitrate water through freezing heat exchanger; recycling as low temperature nitrate water of step (3); discharging the other part of low nitrate water as byproduct.
Owner:山东布莱恩化工技术有限公司
Resource technology and system for separating salt from high-salinity wastewater
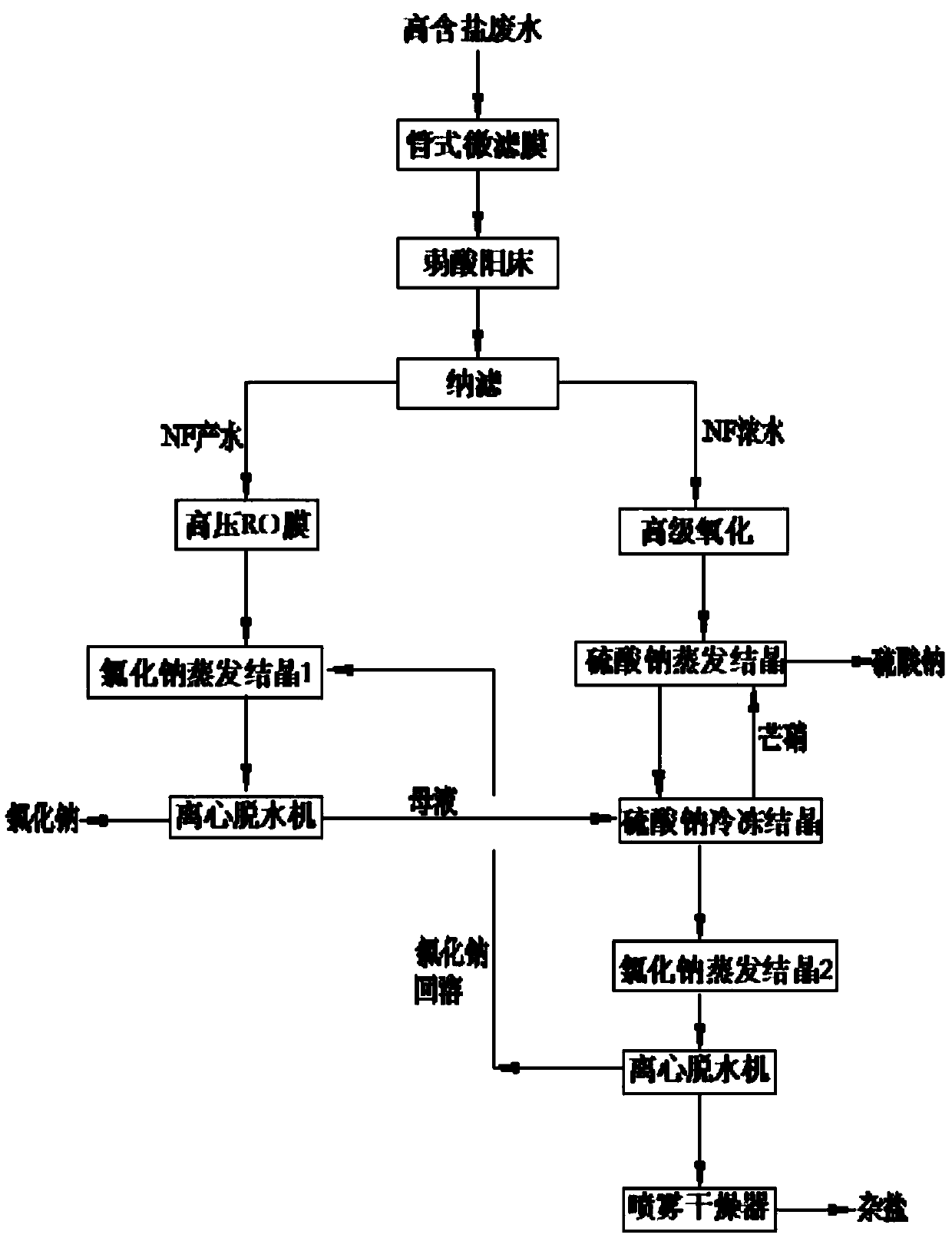
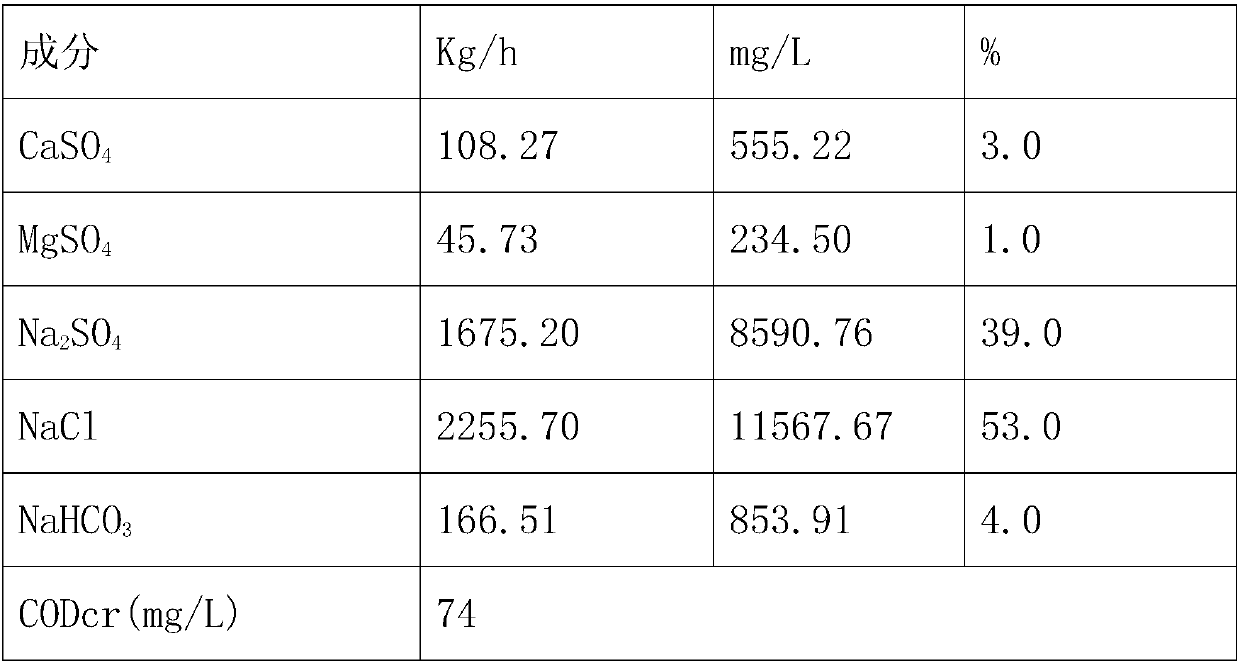
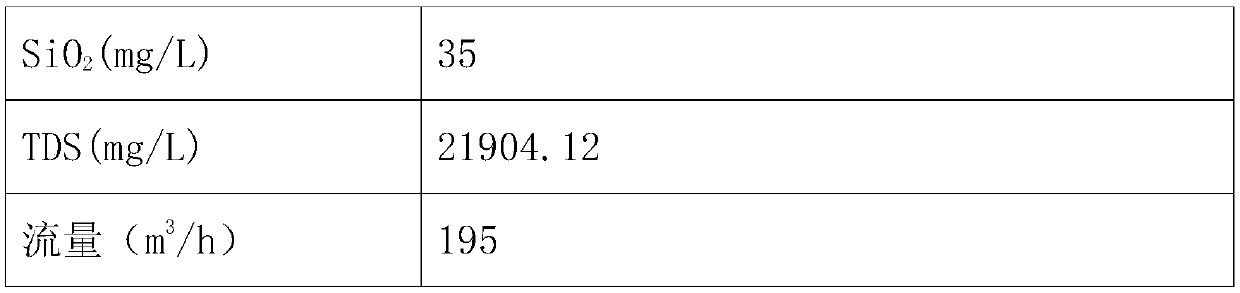
InactiveCN107619144ASolve the problem of pipe blockageReduce energy consumptionMultistage water/sewage treatmentAlkali metal chloridesMirabiliteProduced water
The invention discloses a zero-drainage technology for recycling crystallizing salt from high-salinity wastewater and a treatment system thereof. The treatment system comprises a tubular microfiltration system, a weak acid resin hardness removal system, a nanofiltration membrane salt separating system, a nanofiltration concentrated water oxidizing system, a nanofiltration concentrated water sodiumsulfate evaporating and crystallizing system, a sodium sulfate freezing and crystallizing system and the like. The zero-drainage technology has the advantages that the pretreated wastewater is subject to nanofiltration primary salt separating, the salt component in the produced water is mainly sodium chloride, the sodium chloride with purity no lower than 98.5% is obtained by membrane concentration, evaporating and crystallizing, and the sodium sulfate with purity 99.1% or more is produced by MVR (mechanical vapor recompression) crystallizing after concentrated water oxidizing; a mother liquid after evaporating and crystallizing of sodium sulfate and a mother liquid after nanofiltration evaporating and crystallizing are mixed and frozen, so as to obtain mirabilite, and the mirabilite is converted into anhydrous sodium sulfate after sodium sulfate evaporating and crystallizing; at the premises of ensuring quality, the whole recycling rate of salt reaches 90% or above; finally, a smallamount of mother liquid is sprayed, dried and cured, and the zero-drainage effect of wastewater is realized.
Owner:侯新春 +1
Clean production process of plateau sulfate type boron-lithium salt lake brine
InactiveCN102910652AHigh purityReduce the ratio of magnesium to lithiumChemical industryAlkali metal halide purificationHydration reactionSylvinite
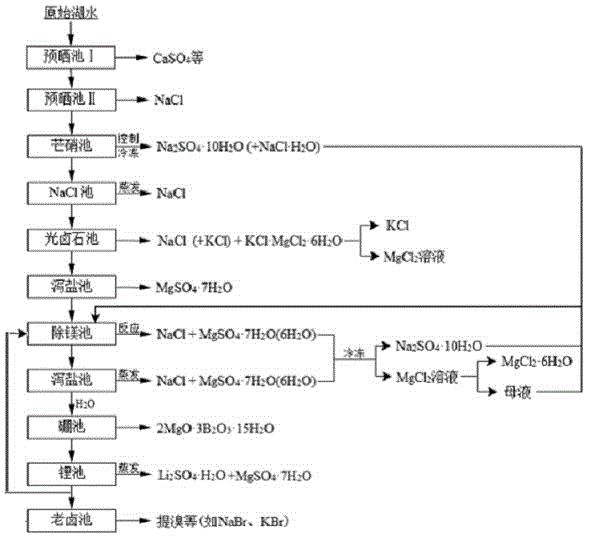
The invention relates to a clean production process of plateau sulfate type boron-lithium salt lake brine. The process comprises the following steps of: (1) arranging a pre-airing pond, a mirabilite pond, a NaCl pond, a carnallite pond, an epsom salt pond I, a magnesium removing pond, an epsom salt pond II, a boron pond, a lithium pond and an old brine pond; (2) controlling the sodium ion concentration in plateau sulfate type boron-lithium salt lake brine, precipitating mirabilite out in winter to obtain brine A, naturally evaporating the brine A, and salting out to obtain brine B; (3) naturally evaporating the brine B, and precipitating sylvine and carnallite out in sequence to obtain brine C; (4) naturally evaporating the brine C, precipitating an epsom salt out, and performing solid-liquid separation to obtain brine D and a solid A; (5) blending the brine D with mirabilite, removing magnesium to obtain brine E, and naturally evaporating brine E to obtain brine F and a solid B; (6) performing a hydration reaction on brine F, naturally evaporating, and precipitating reservoir water / inderite and brine G out; and (7) evaporating brine G or refrigerating for precipitating lithium sulfate, and processing the lithium sulfate into a corresponding product. The process has the advantages of comprehensive utilization of natural energy, saving in energy and environment friendliness.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI +1
Phase-change constant-temperature material and pad body
The invention relates to a phase-change material, in particular relating to a phase-change constant-temperature material. The invention also relates to a pad body, in particular relating to a pad body containing the phase-change constant-temperature material. The phase-change material can ensure constant temperature within a certain period of time, is large in latent heat and stable in property, and can be repeatedly used. The phase-change material is fine and smooth like sand as compared with the original phase-change material. The phase-change material is prepared through the following steps: evenly mixing 16wt%-20wt% of water, 1wt% of thickener, 3wt%-4wt% of nucleating agent and 1wt% of dispersing agent, so that the mixture is of a uniform thickness shape; adding 75wt%-78wt% of mirabilite; and evenly mixing so that the components are evenly dispersed. The phase-change material can be added in the pad body to serve as a cold-hot bag, and insulation is durable. The pad body containing the phase-change material consists of small grids which are of strip shapes, the grids are tightly distributed, and a pouring material port is reserved on each grid and used for pouring the phase-change material.
Owner:JIANGSU YISHUN MEDICAL DEVICES CO LTD
Chinese medicinal herb feed additive for meat rabbits and application thereof
InactiveCN101595947APrevent predisposition to diseaseImprove securityAnimal feeding stuffBulbulHouttuynia
The invention discloses a Chinese medicinal herb feed additive for meat rabbits and application thereof. The additive comprises the following components: ordate houttuynia, magnolia officinalis, bitter orange, phellodendron, Chinese bulbul, liquorice, rhizoma atractylodis, medicated leaven, Chinese yam rhizome, hawthorn, muze, pilose asiabell root and refined mirabilite. The additive fully considers Chinese medicament compatibility principles of eighteen incompatible medicaments and nineteen medicaments of mutual antagonism according to physiological characteristics of the meat rabbits, ensures that pointed components are compatible with each other, then draw the strong points of others to offset own weakness and cooperate with each other, has good disease-preventing, disease-resistant, growth-promoting and fattening effects, and strengthens the disease resistance. The additive can prevent common diseases that the meat rabbits are susceptible to such as mastitis, constipation, abdominal distension and the like, improve the economic benefit of rabbit breeding, has no side effect, and cannot remain harmful substances; the additive can play a larger role by about 2 percent of consumption, and cannot cause the damage to the physiological functions of the meat rabbits; and the Chinese medicinal herb additive has the advantages of broad sources of raw materials, low cost, high safety and remarkable benefit, so the additive can be developed toward a higher level in meat rabbit breeding industry.
Owner:邹振可
White carbon black production wastewater treatment technology
ActiveCN103539281AAchieve enrichmentReduce energy consumptionWater/sewage treatment bu osmosis/dialysisMultistage water/sewage treatmentWater sourceFiltration
The invention belongs to the technical field of chemical wastewater treatment, and in particular relates to a white carbon black production wastewater treatment technology. The technology comprises the steps of pretreating white carbon black production wastewater so that the water quality of the treated wastewater can reach the water quality requirement of a membrane treatment system; carrying out membrane filtration so that enriching sodium sulfate in the wastewater is enriched at one side of a membrane until the content of sodium sulfate is not less than 10% and the produced water at the other side reaches a recycle water index and is recycled to a production system for reuse; carrying out freezing crystallization on the thick water to separate out mirabilite, separating out the crystallization mother liquor so as to obtain the mirabilite and returning the crystallization mother liquor back to a membrane concentration system, and thermally dehydrating the mirabilite so as to obtain anhydrous sodium sulphate which is not less than 98% and used for selling; or evaporating and crystallizing the thick water with the sodium sulfate being not less than 10% through directly utilizing an evaporator so as to separate out the anhydrous sodium sulphate. The water source recycling and chemical raw material recovery are realized through low energy consumption. Through cooperatively using two technologies, the energy consumption for treating each tonnage of wastewater can be greatly reduced, and the water recovery rate of a whole treatment system is not less than 92%; the sodium sulfate content of an anhydrous sodium sulphate product is not less than 98%.
Owner:JINNENG SCI & TECH
Technology for producing sodium bicarbonate from mirabilite
InactiveCN105712382AIncrease profitEmission reductionCalcium/strontium/barium sulfatesCarbonate preparationSodium bicarbonateDistillation
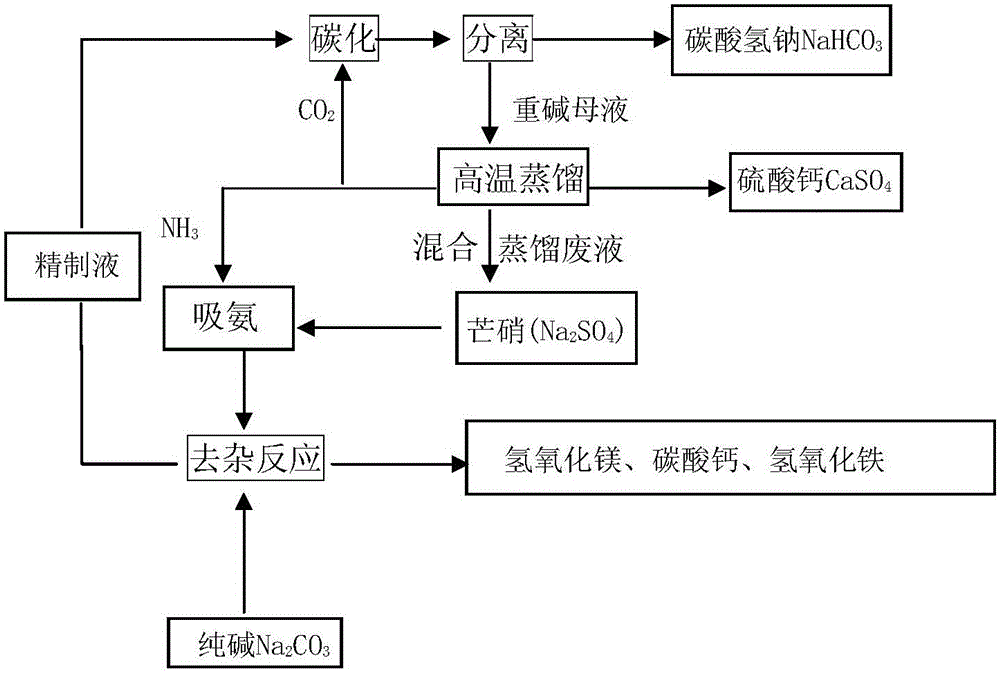
A technology for producing sodium bicarbonate from mirabilite comprises steps as follows: mirabilite and distillation waste liquor mixed liquor is used as a raw material, sodium carbonate is added to the solution after absorption of ammonia, magnesium, iron and calcium ions in the solution are removed after the solution is subjected to a precipitation reaction at 15-50 DEG C, and a refined solution is obtained; carbon dioxide is added to the refined solution for carbonatation, and sodium bicarbonate solids and heavy alkali mother liquor are obtained through separation after the refined solution is subjected to a replacement reaction at 15-50 DEG C; lime is added to the heavy alkali mother liquor, and ammonia gas, carbon dioxide, calcium sulfate and distillation waste liquor are obtained after distillation reaction at 15-120 DEG C; ammonia gas and carbon dioxide are recovered to ammonia absorption and carbonatation procedures respectively; the distillation waste liquor and mirabilite in Step A are mixed to be recycled. The technology has the characteristics that mirabilite is directly utilized, ammonia is recycled, the comprehensive utilization rate of distillation waste liquor waste is high, the production cost is low, the waste residue discharge is low, the technology is environment-friendly, and the like.
Owner:CHINA LIGHT IND INT ENG CO LTD
Traditional Chinese medicine gastrointestinal auxiliary for type-B ultrasonic and preparation method thereof
InactiveCN102949734AEasy to useNo painDigestive systemEchographic/ultrasound-imaging preparationsMedicinal herbsDisease
The invention provides a traditional Chinese medicine gastrointestinal auxiliary for type-B ultrasonic and a preparation method thereof, and the traditional Chinese medicine comprises the following raw medicinal materials: cumin, galangal, rhizome cyperi, three-nerved spicebush roots, cuttlebone, green tangerine peels, medicated leaven, malts, immature bitter orange, rheum officinale, mirabilite, cortex magnoliae officinalis, radix aucklandiae, amomum, agastache rugosa, finger citron, hawthorns, radish seeds, calcined concha arcae, evodia, pinellia ternate, semen arecae, atractylodes macrocephala koidz, rhizoma nardostachyos and fritillary bulb. The traditional Chinese medicine gastrointestinal auxiliary for type-B ultrasonic has the beneficial effects of being safe to use, painless, noninvasive, non-toxic, free from anaphylactic reaction, short in examination time, high in development rate, convenient to use and low in cost, providing a new technology and a new method for corrective early-stage diagnosis of diseases, and powerful basis for clinical diagnosis, and being a desirable novel auxiliary. Furthermore, the preparation process is simple and practical.
Owner:王玉云
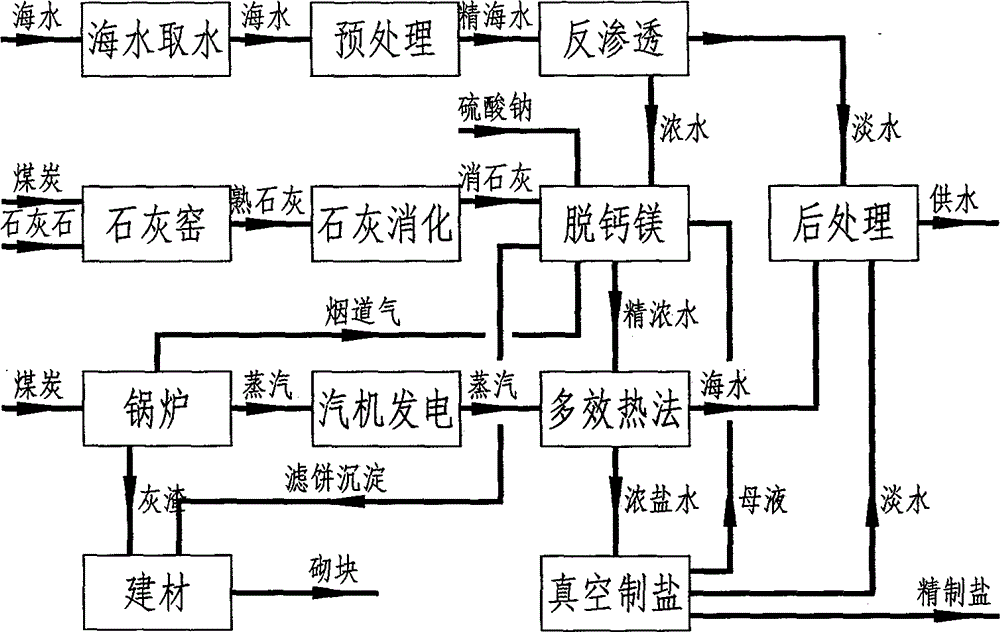
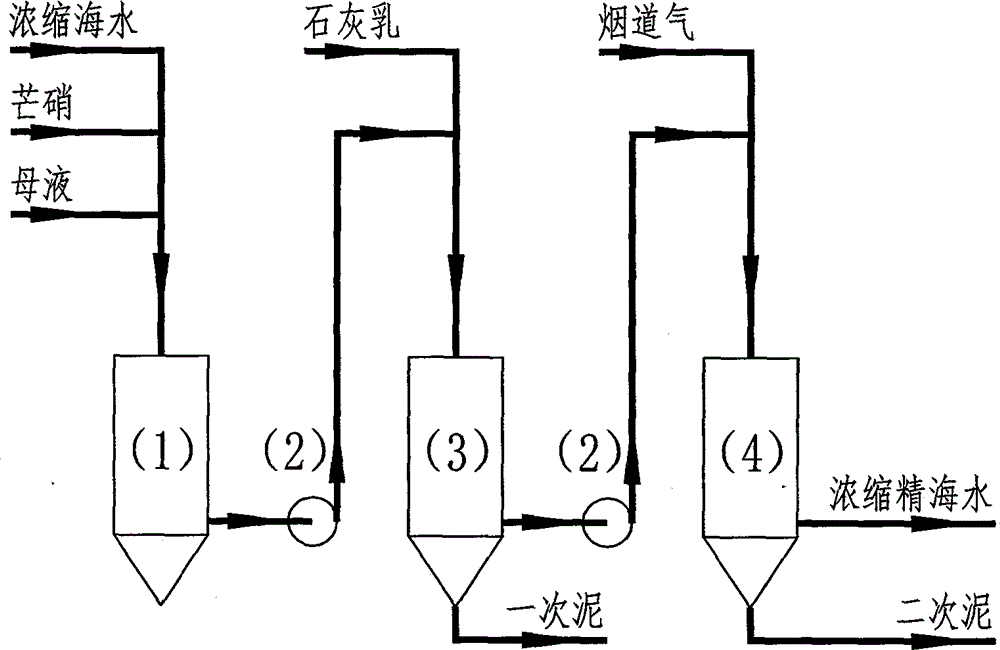
Methods for removing calcium and magnesium and co-producing water and salt by low-cost process during sea water desalination
InactiveCN102795719AScaling limitGeneral water supply conservationSeawater treatmentWater desalinationMirabilite
The invention discloses methods for removing calcium and magnesium and co-producing water and salt by a low-cost process during sea water desalination. By the methods, on the basis of removing the calcium and magnesium in the sea water desalination industry, water and salt producing processes can be integrated, so that fresh water can be produced when the crude salt is produced; sea water is treated by a low-cost method for removing calcium and magnesium ions, so that the sea water cannot be scaled, and the sea water desalination and vacuum salt production are linked; the sea water is refined by a lime-mirabilite-carbon dioxide method, and the used raw materials are lime, mirabilite and carbon dioxide which are cheap and readily available; the process comprises the following steps of removing magnesium, namely adding a proper amount of mirabilite into the sea water, adding lime milk, and reacting the magnesium ions to form magnesium hydroxide precipitations so as to remove the magnesium; causticizing, namely causticizing superfluous calcium hydroxide and sodium sulfate to form calcium hydroxide and sodium sulfate precipitations, and separating the produced sodium sulfate precipitation; removing calcium, namely introducing flue gas into concentrated sea water subjected to magnesium removal and causticizing, reacting the calcium hydroxide and the carbon dioxide to form sodium carbonate, reacting the sodium carbonate and the calcium ions to form calcium carbonate so as to remove calcium.
Owner:王凯勋
Production process of large grain anhydrous sodium sulfate with natural salt lake mirabilite
InactiveCN1986406AChange granularityControl the number of generatedSulfate/bisulfate preparationSalt lakeWarm water
The production process of large grain anhydrous sodium sulfate with natural salt lake mirabilite includes the steps of washing mirabilite, decomposing mirabilite, refining, settlement, neutralizing with sulfuric acid to obtain clear solution, evaporating, centrifuging to dewater, and drying with hot blast to obtain product. It features the addition of surfactant solution comprising sodium dodecyl benzene sulfonate, sodium ethoxy alkyl sulfate and warm water at about 40deg.c into the clear solution before evaporating. The production process can produce anhydrous sodium sulfate of granularity of 200-1000 microns and with high economic value.
Owner:乌鲁木齐市华高商贸有限公司
Method for manufacturing beer bottles through broken glass
A method for manufacturing beer bottles through broken glass includes the steps of adding powder materials in 1000 kilograms of broken glass. For manufacturing white beer bottles, the composition and weight of the powder materials are that: 55-57 kilograms of quartz sand, 15-19 kilograms of sodium carbonate, 15-19 kilograms of calcite, 3-6 kilograms of dolomite, 6-8 kilograms of feldspar, 3-5 kilograms of mirabilite, 5-7 kilograms of fluorite and 1-2 kilograms of sodium nitrate or 0.5-1.5 kilograms of cerium dioxide. For manufacturing green beer bottles, the composition and weight of the powder materials are that: 53-57 kilograms of quartz sand, 17-21 kilograms of sodium carbonate, 7-10 kilograms of calcite, 3-5 kilograms of dolomite, 4-6 kilograms of feldspar, 4-6 kilograms of mirabilite, 5-7 kilograms of fluorite and 0.5-1.5 kilograms of chromium mineral powder. For manufacturing brown beer bottles, the composition and weight of the powder materials are that: 52-56 kilograms of quartz sand, 17-20 kilograms of sodium carbonate, 12-15 kilograms of calcite, 3-5 kilograms of dolomite, 4-6 kilograms of feldspar, 5-7 kilograms of mirabilite, 5-7 kilograms of fluorite and 0.3-1.0 kilogram of carbon powder. The method for manufacturing beer bottles through broken glass can improve utilization rate of broken glass, ensures quality of glass and is suitable for manufacturing of beer bottles.
Owner:WUHAN HUAXIA GLASSWORK
Method for reclaiming lignin and alkali from boiling black liquor
InactiveCN101157459AIncreased recycling profitMake full use ofAlkali metal sulfides/polysulfidesLignin derivativesLiquid wasteCombustion
The invention relates to a method for reclaiming lignin and alkali from steamed black liquor in chemical pulping. The invention adopts addition of H2SO4 solution into the black liquor to precipitate lignin thereof, and after separation, eylogen with high added value is obtained; then NaOH solution is added into the black liquor to adjust the pH value followed by procedures such as evaporation, combustion, causticization, etc. to reclaim alkali and heat energy. The added H2SO4 reacts with NaOH to produce Na2SO4, which can partially or completely substitute replenished mirabilite in the process of alkali reclamation and then transform into NaOH and Na2S for reuse as steaming drugs in production. The invention solves the problems that the combustion method for reclamation of alkali and heat energy from the black liquor does not rationally utilize the lignin resource thereof, and the alkali and the heat energy in the black liquor are not rationally reclaimed in the traditional acid extracting lignin process, as well as waster liquor is difficult to dispose further. The invention has prominent social, environmental and economic benefits; additionally, the method of the invention does not produce harmful substances and has no secondary pollution.
Owner:CHANGSHA UNIVERSITY OF SCIENCE AND TECHNOLOGY
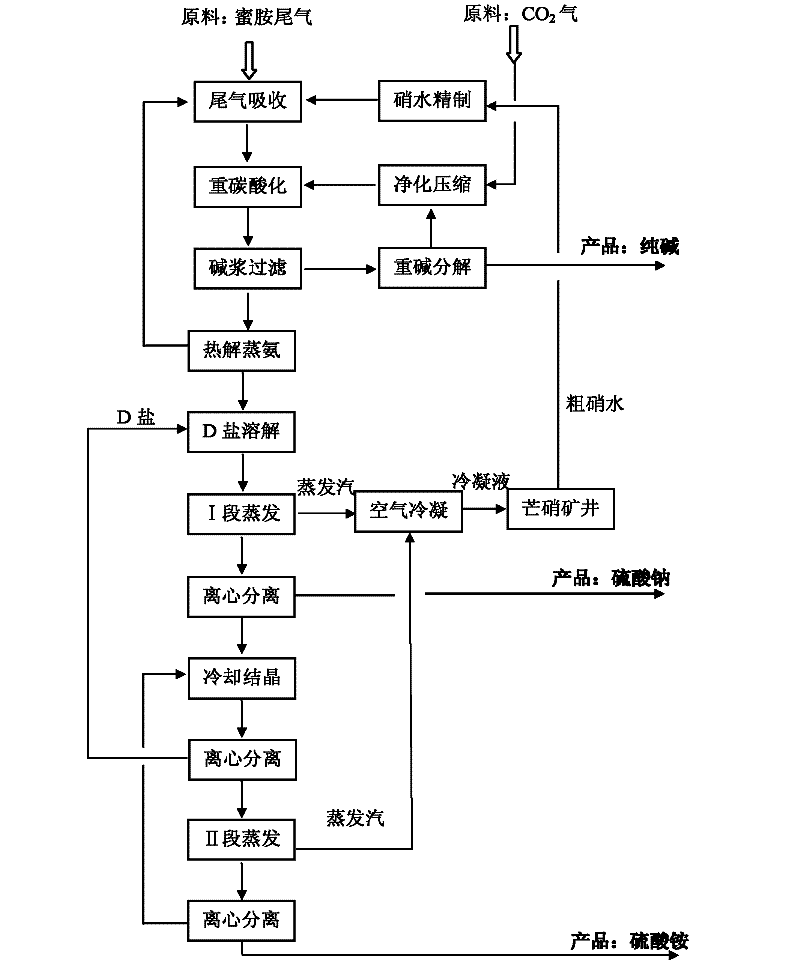
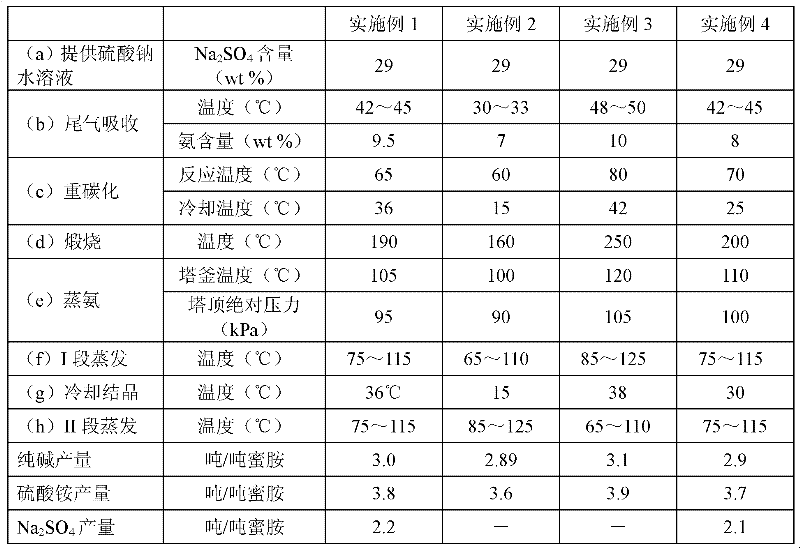
Methods for coproducing sodium carbonate and ammonium sulfate from melamine tail gas and mirabilite
InactiveCN102198953AIncrease profitReduce consumptionAmmonium sulfatesCarbonate preparationSodium bicarbonateSlurry
The invention relates to methods for coproducing sodium carbonate and ammonium sulfate from melamine tail gas and mirabilite. The method for producing the sodium carbonate comprises the following steps of: a, providing a sodium sulfate water solution; b, introducing melamine tail gas into the sodium sulfate water solution to form an ammonium carbonate-sodium sulfate water solution; c, introducing carbon dioxide into the ammonium carbonate-sodium sulfate water solution to obtain a suspension serous fluid of sodium bicarbonate crystals; and d, carrying out solid and liquid separation on the suspension serous fluid to obtain sodium bicarbonate and a mother solution I, calcining sodium bicarbonate solid to obtain the sodium carbonate. The method further comprises the following steps of: e, adding the mother solution I into an ammonia stilling tower, heating, forming tower top mixed gas containing ammonia and carbon dioxide, generating a deamination solution in the tower; f, evaporating the deamination solution for dewatering to obtain sodium sulfate crystals and a mother solution II; g, cooling the mother solution II for crystallizing to obtain Na2SO4.(NH4)2SO4.4H2O salt crystals and a mother solution III; and h, evaporating the mother solution III for dewatering, and separating out ammonium sulfate crystals. The methods have the advantages of high utilization rate of raw materials and low energy consumption.
Owner:SICHUAN GOLDEN ELEPHANT SINCERITY CHEM CO LTD +1
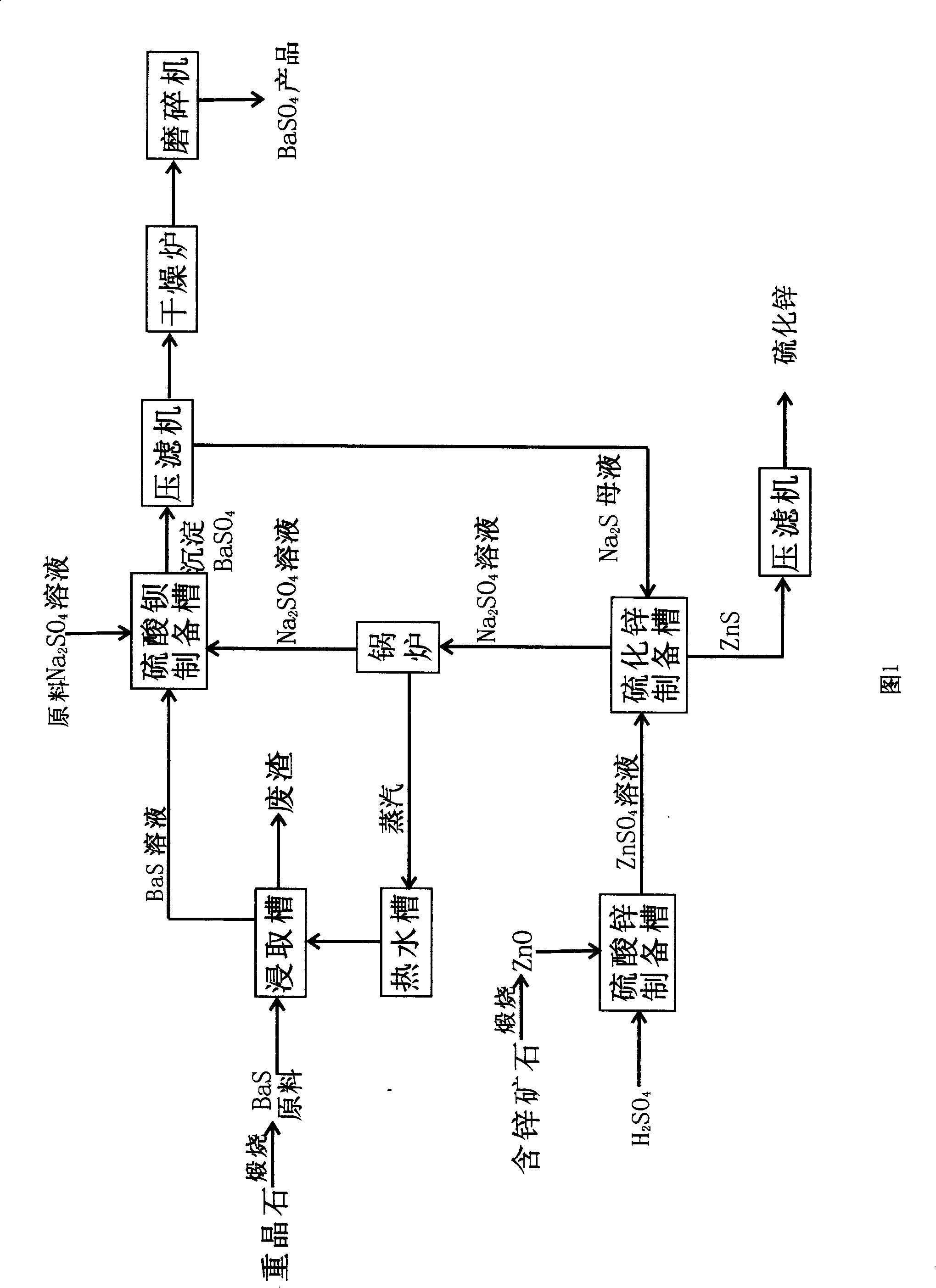
Method for preparing barium sulfate and zinc sulfide
InactiveCN101205077AIncrease profitImprove protectionCalcium/strontium/barium sulfatesZinc sulfidesSulfateMirabilite
The invention discloses a method for preparing barium sulfate and zinc sulphide, which takes barites and zinc containing ore as principal material and includes the following steps: firstly, black ash raw material which contains more than 50% of barium sulphide prepared by the mixed calcinations of barites and coal is leached to get pellucid barium sulphide solution; secondly, the barium sulphide solution reacts with mirabilite to get barium sulfate products after filter pressing separation, drying and grinding; thirdly, zinc oxide obtained by the calcinations of the zinc containing ore reacts with sulphuric acid, and zinc sulfate solution is prepared by the purification of reacting solution; fourthly, depurative zinc sulfate solution reacts with vulcanized alkali containing mother liquor which is prepared after the separation of the barium sulfate from the reacting resultant obtained from step two to get zinc sulphide after filter pressing separation; the sodium sulfate containing mother liquor which is separated from zinc sulphide is put into a boiler for concentration; the steam formed heats the leach barium sulfide water; the concentrated sodium sulfate containing solution reacts with barium sulfide to prepare precipitated barium sulfate.Due to the adoption of closed cycle, the invention has the advantages of simple operation, high yield, energy conservation and environmental protection.
Owner:LUOYANG HONGYUAN BARIUM SALT CHEM IND INST
Universal detoxifying composition
Described is a universal detoxifying composition comprising: from about 10 to about 20% by weight Radix Rehmannica, from about 8 to about 18% by weight Scutellariae Racis, about 2% by weight Rhei Rhizoma, about 1% by weight Mirabilite Pawde, from about 3 to about 13% by weight Herba Taraxaci, from about 3 to about 13% by weight Herba Violae, from about 5 to about 15% by weight Puemariae Radix, from about 1 to about 11% by weight Fructus Viticis, from about 2 to about 12% by weight Fructus Forsythiae, from about 2 to about 12% by weight Gardeniae Fructus, about 3% by weight Sophara Flavescens Aiton, about 1% by weight Gummi Olibanum, about 1% by weight Myrrha, from about 6 to about 14% by weight Cordyceps, and from about 4 to about 12% by weight Young Ji. Also disclosed is a method of treating cancer which comprises administering a therapeutically efficient amount of the composition to a patient.
Owner:YUEN LIU
Medicine composition for clearing heat and purging-fire
InactiveCN1899505AImprove securityObvious toxic reactionOrganic active ingredientsDigestive systemSophora RootForsythia
The heat-clearing and fire-purging medicine composition is prepared with following five groups of material in clinical reasonable amount: group A of at least one in nine kinds of Chinese medicinal materials, including hemsleya root, forsythia, etc; group B of at least one in seven kinds of Chinese medicinal materials, including flavescent sophora root, gentian root, etc; group C of at least one in rhubarb, aloe and mirabilite; group D of at least one in six kinds of Chinese medicinal materials, including figwort, ophiopogon root, etc; and group E of licorice. The heat-clearing and fire-purging medicine composition has excellent functions of clearing heat, purging fire and detoxificating, less adverse reaction and high safety.
Owner:CHENGDU KANGHONG PHARMA GRP
Energy-saving and environmental-protection method for tanning raccoon fur
InactiveCN101921882AReduced hair removal rateReduced rehumidification timeTanning treatmentPre-tanning chemical treatmentMirabiliteSodium sulfate
The invention discloses an energy-saving and environmental-protection method for tanning a raccoon fur, which is characterized by adopting the following processes: (1) shunting and grouping; (2) soaking: preparing a soaking solution which contains a wetting fungicide HAC, a soaking enzyme E38, a degreasing agent S-40 and salts; (3) removing meats by water scalping; (4) re-soaking: preparing a re-soaking solution which contains the wetting fungicide HAC, Supralan80, degreasing agent S-50 and salts; stirring while throwing furs; and taking out of the furs and drying; (5) shaving and deep scalping: cutting off the pachydermia part of the fur to cause each part of the fur to have even thickness; (6) softening and pickling: preparing a softening pickling liquor which contains softening enzyme E40, sulfuric acid, Pelgrassol LP, anhydrous sodium sulfate, JFC, salts, formic acid, Eskatan GLS and fatliquoring agent FSE; throwing the furs into the softening pickling liquior for stirring; and then taking out of the furs and leaching; (7) tanning: preparing a tanning solution which contains tschermigite, JFC, salts, mirabilite, auxiliary tanning agent P-3B and fatliquoring agent FSE; stirring while throwing the furs; successively adding NovaltanPF and aluminium tanning agent AL, adding baking soda, stirring, adding the fatliquoring agent FSE and taking out of the furs; and (8) arranging for later stage. The invention has simple processes, reduces manpower consumption, improves use efficiency and saves usage amount of chemical materials and water.
Owner:HEBEI SUANG FUR & LEATHER
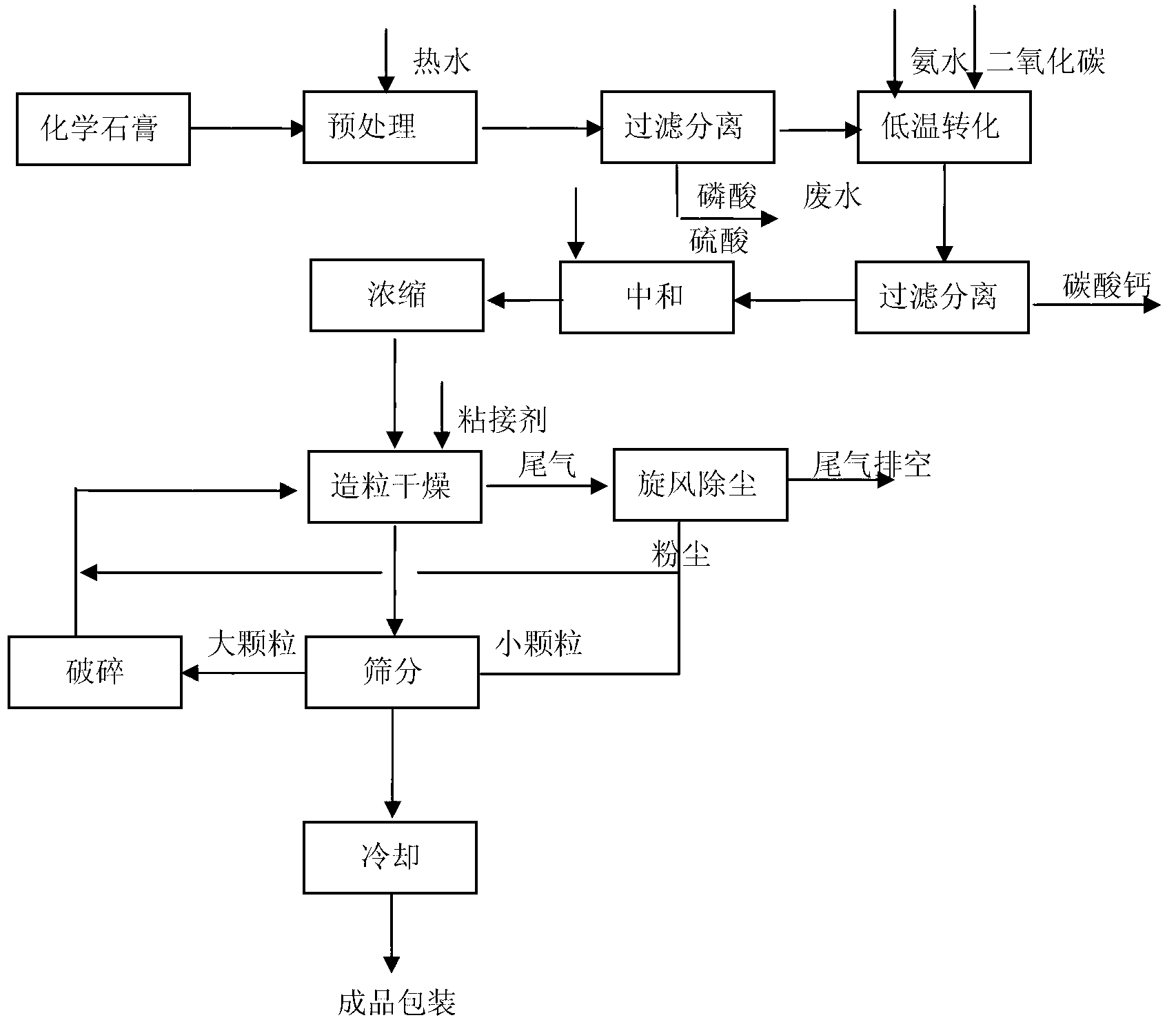
Method for preparing granular ammonium sulfate and calcium carbonate by chemical gypsums via low-temperature transformation
ActiveCN102701256AAvoid secondary pollutionReduce processCalcium/strontium/barium carbonatesAmmonium sulfatesResource utilizationPhosphogypsum
The invention discloses a process for preparing granular ammonium sulfate and calcium carbonate by chemical gypsum via low-temperature transformation, and relates to the field of resource utilization of industrial byproduct gypsums and environmental protection and control. The process is characterized by taking chemical gypsums (such as phosphogypsum, flue gas desulfurization (FGD) gypsum, fluorgypsum, citric gypsum, boron gypsum, titanium gypsum, and mirabilite gypsum) as raw materials and comprising the steps of pretreatment of raw materials, low-temperature transformation of the chemical gypsums, solid-liquid separation and washing, concentration of ammonium sulfate and granulation of ammonium sulfate. The process has the advantages of being wide in raw material sources, solving the land occupation and environment pollution problems of waste residues of the chemical gypsums, and realizing the cyclic utilization of sulfur resources; and according to the process, the large-scale production is easy to realize, the technique is advanced, the energy consumption is low, the benefit is high and no waste residue is discharged.
Owner:SINOFERT HOLDINGS
Production process for glass fiber yarn
ActiveCN105948493AImprove product qualityEfficient use ofGlass making apparatusYarnYarnCooking & baking
The invention relates to a production process for glass fiber yarn. The production process comprises the steps: selecting the following raw material ingredients in percentage by weight: 1% to 30% of waste / used beaded glass, 1% to 40% of cullet, 1% to 30% of quartz sand, 22% to 35% of pyrophyllite, 3% to 15% of feldspar, 5% to 13% of borax, 1% to 4% of calcite, 3% to 7% of dolomite, 0.5% to 2% of fluorite, 2% to 5% of mirabilite and 0.2% to 0.5% of composite clarifier; and carrying out processes such as material preparing, melting, drawing, baking and yarn making, thereby producing the high-quality glass fiber yarn. According to the production process for the glass fiber yarn, disclosed by the invention, the waste / used beaded glass and the cullet serve as main raw materials, so that waste resources can be effectively utilized, and the pollution to environments is reduced; and the produced glass fiber yarn is stable in product quality, and the strength of the produced glass fiber yarn can meet requirements.
Owner:旌德县青川玻纤有限公司
Method for preparing lithium salt ore from plateau sulfate type salt lake brine
ActiveCN103204523ANo pollution in the processReduce manufacturing costChemical industryLithium sulfates/sulfitesHigh magnesiumThenardite
The invention discloses a method for preparing lithium salt ore from plateau sulfate type salt lake brine. The method comprises the following steps of: evaporating sulfate type salt lake brine to be in the sodium chloride saturated state, freezing in winter to separate out mirabilite, and carrying out solid-liquid separation when the content of the sulfate ion in the brine is controlled to be 1g / L to 7g / L; evaporating the brine from which the mirabilite is separated out, so as to separate sodium chloride; evaporating the brine from which the sodium chloride is separated out, so as to separate out sylvine, carnallite and epsomite, carrying out solid-liquid separation when the lithium ion concentration in the brine is controlled to be greater than or equal to 6g / L, wherein the brine subjected to solid-liquid separation is the brine with high magnesium chloride content; mixing the brine with high magnesium chloride content with the mirabilite to react so as to separate out sodium salt and magnesium salt, and carrying out solid-liquid separation when the magnesium-lithium ratio in the solution is controlled to be less than or equal to 8:1, so as to obtain boron-lithium-rich brine; and reacting the boron-lithium-rich brine with water to separate out boron rock, carrying out solid-liquid separation so as to obtain lithium-rich brine; and introducing the lithium-rich brine into a lithium salt pool, and evaporating so as to separate out the lithium salt ore.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI +1
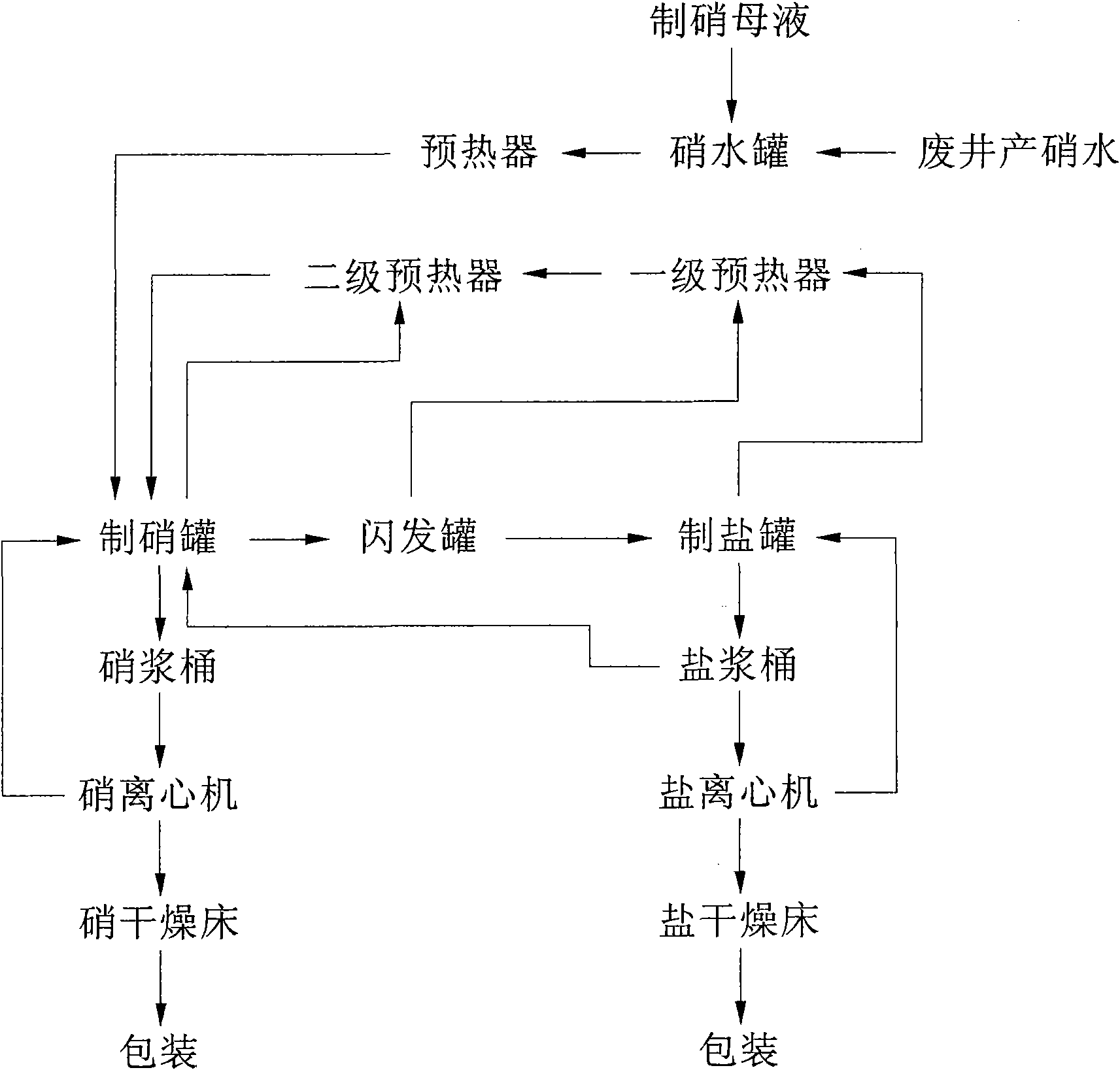
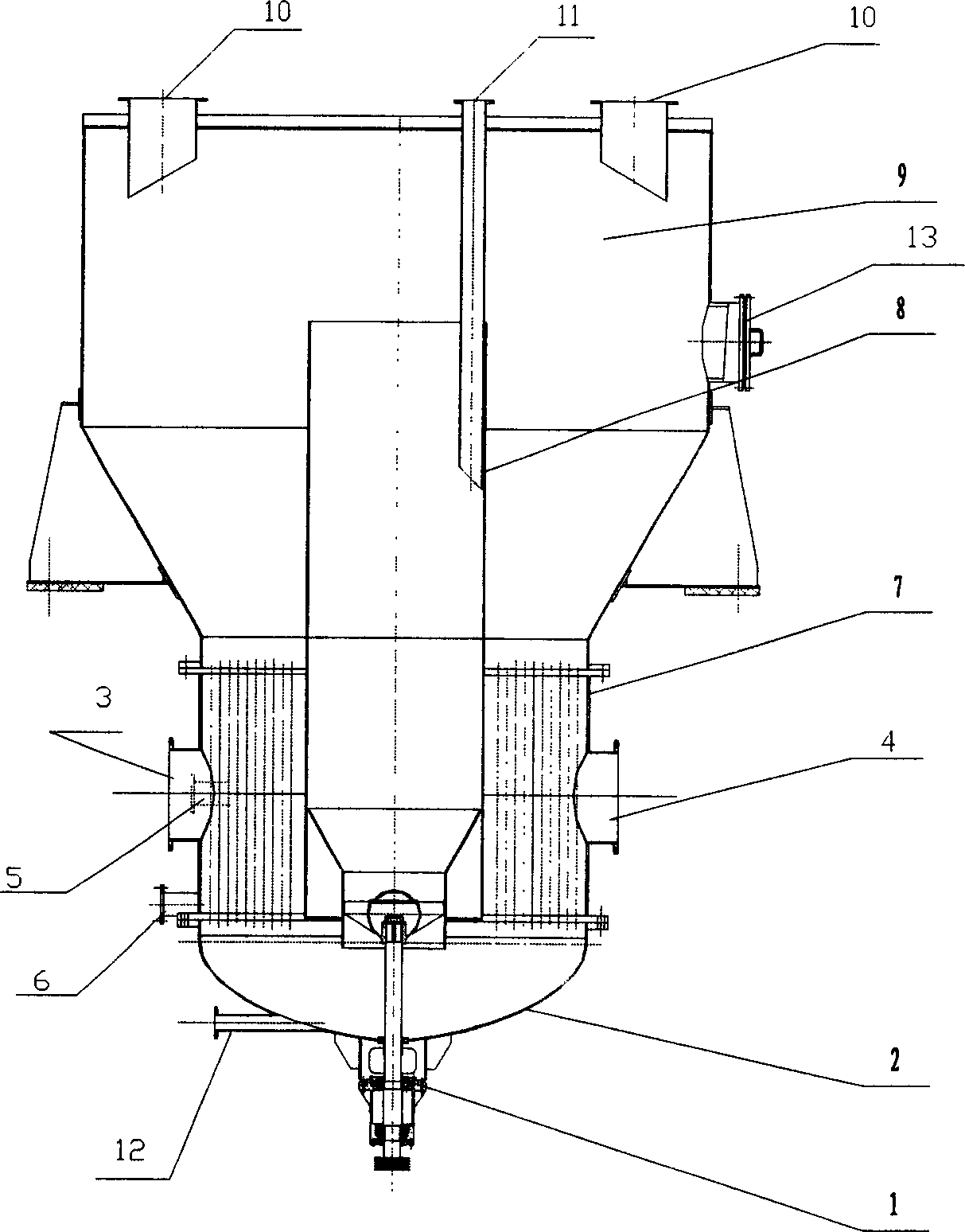
Production method for co-production of mirabilite and salt and production device thereof
ActiveCN101624194AIncrease profitImprove energy savingAlkali metal sulfites/sulfatesMineral SourcesMirabilite
The invention belongs to the technical field of preparing mirabilite, and in particular relates to a production method for the co-production of mirabilite and salt and a production device thereof. The production method comprises the following steps: heating mirabilite preparing solution and separating the mirabilite out of the mirabilite preparing solution; adding salt, namely sodium chloride into high-temperature mirabilite preparing solution to separate the mirabilite out of the solution; and simultaneously, changing the proportions of the mirabilite and the salt in the solution, then using a process principle of 'nitrate co-production' to separate out the salt at a low temperature so as to achieve the separation and the co-production of the mirabilite and the salt, and reclaiming the mother solution containing the salt to the mirabilite preparing solution for recycling. The method and the device can effectively solve the problems of environmental pollution and resource waste caused by the discharge of the mirabilite preparing mother solution by manufacturing enterprises of anhydrous sodium sulfate, improve the utilization rate of mineral resources, save the energy resources, reduce the emissions, protect the environment, and ensure the sustainable development of the anhydrous sodium sulfate industry.
Owner:CHINA CEC ENG +1
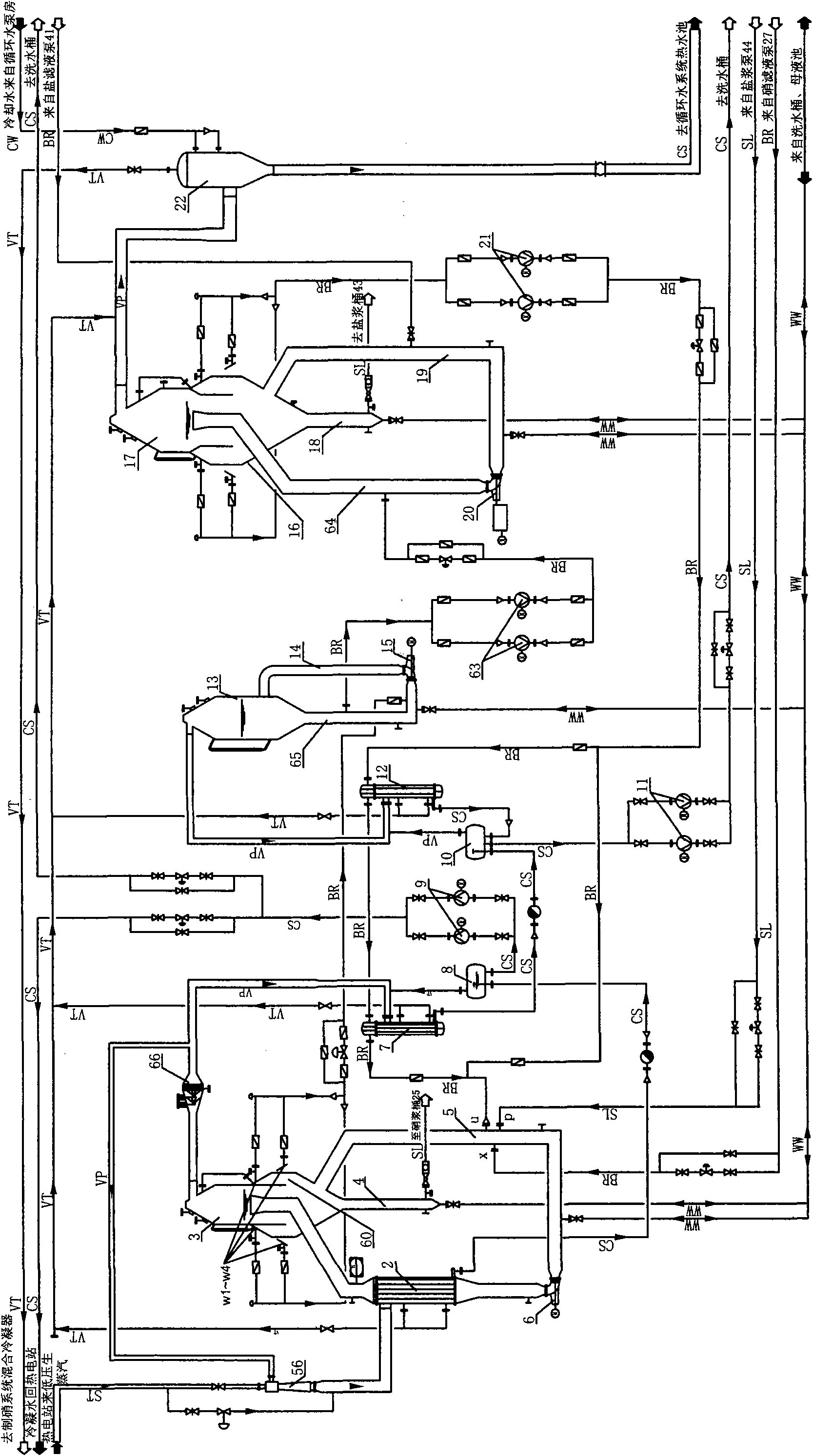
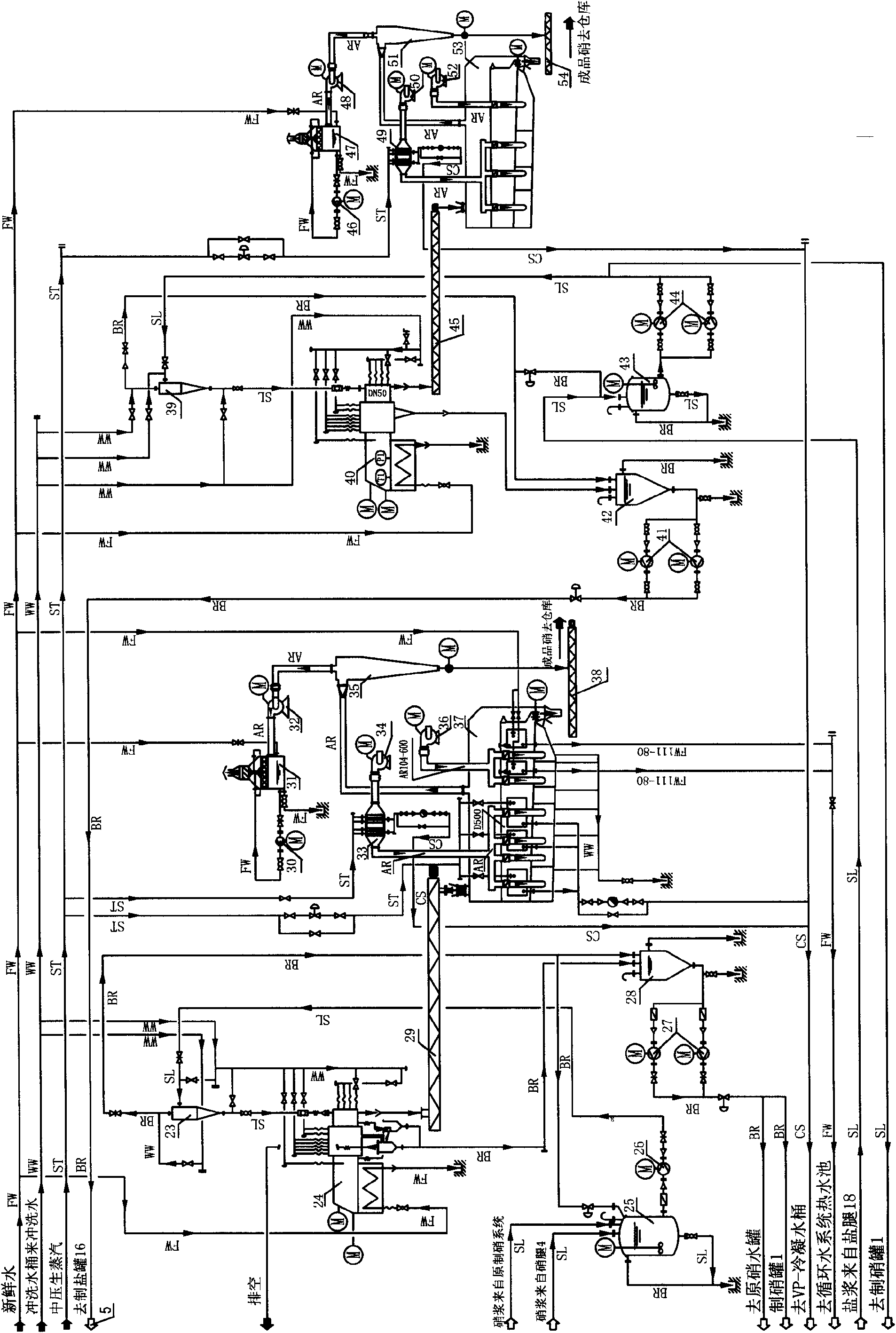
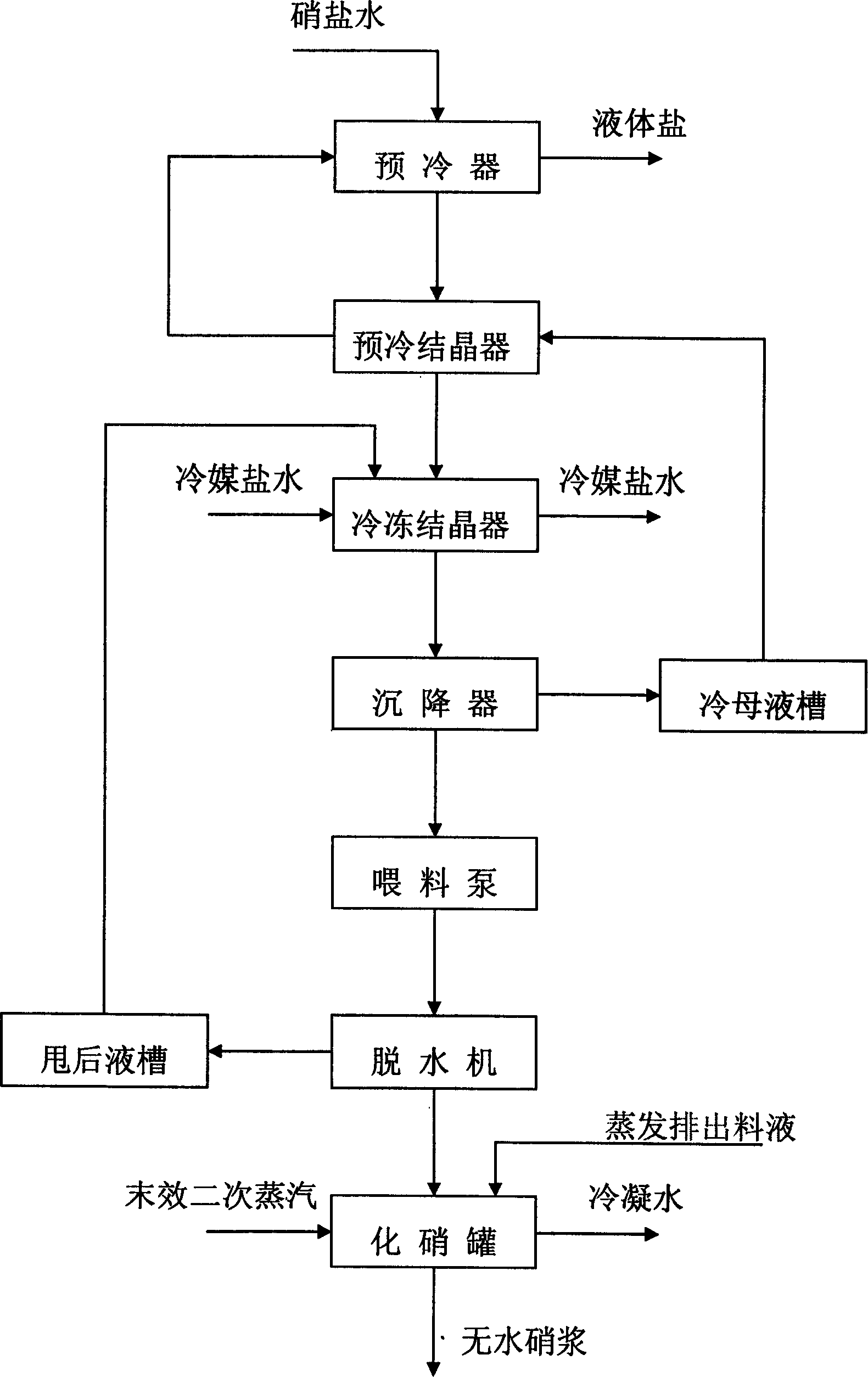
Process for preparing anhydrous mirabilite by freezing method
ActiveCN1837050AEasy to operateReasonable heat transfer temperature differenceChemical industryAlkali metal sulfite/sulfate dehydrationMirabiliteSodium sulfate
The invention discloses a new technics about the production of freezing method absolute nitrate, containing steps as follows: getting the solution contains saltpeter across precooler to doing coolant elementary cooling by cold mother liquor; switching the feed liquid from precooler to precooler crystallizer; adding the feed liquid from precooler crystallizer in cooler crystallizer to freeze the feed liquid to -5-7Deg C for the saltpeter separating of second phase refrigeration; Adding the feed liquid from cooler crystallizer in depressor for solid-liquid separation; passing the discharged sodium sulfate from precooler through screw pump to feed the dewatering machine; putting the dewatered sodium sulfate in saltpeter melting tank, mixing with the swinged liquid of evaporate discharged feed liquid or absolute nitrate plasm, making sodium sulfate autolyze by end effect quadratic steam heating. In the saltpeter melting procedure of this method, adopting hot material and sodium sulfate mixing melt saltpeter, at the same time, using low-grade second steam heat energy heat up the feed liquid, compared to the former pervaporation, this method is more reasonable, energy saving and ensure the quality of product.
Owner:ENG TECH INST CO LTD OF CNSIC
Method for preparing colored foam glass ceramic material from lithium tailings
InactiveCN108191230ASimple preparation processWide firing rangeGlass shaping apparatusPore distributionSlag
The invention provides a method for preparing a colored foam glass ceramic material from lithium tailings. According to the method, the material is prepared from a main material, an auxiliary material, fluxing agents, pore forming agents and colorants by blending and one-time high-temperature sintering, wherein the lithium tailings serve as the main material; cullet serves as the auxiliary material; the fluxing agents comprise sodium fluoride, potassium carbonate, sodium silicate, mirabilite and borax; the pore forming agents comprise calcium carbonate and silicon carbide; the colorants comprise copper oxide, iron oxide, cobalt oxide, chromium oxide, manganese oxide and zinc oxide. The problems of industrial slag accumulation and secondary pollution can be solved effectively with the method, and recycling of resources is realized; the preparation process is simple, the cost is low, and prepared foam glass ceramic is small in bulk density, uniform in pore distribution, adjustable in color, high in compressive strength and high in added value and has good industrial prospect.
Owner:WUHAN UNIV OF TECH
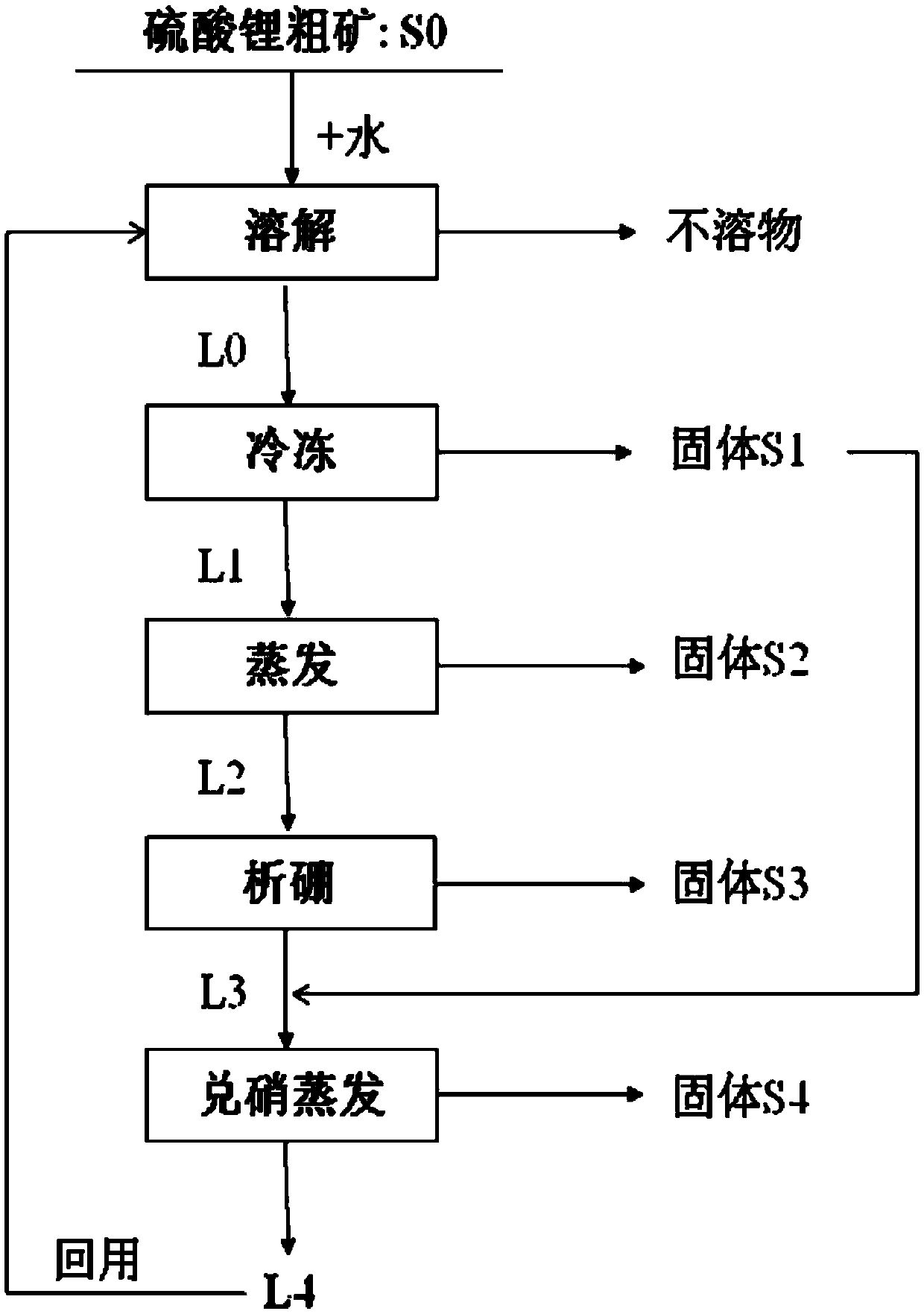
Method for purifying lithium sulfate crude ore
ActiveCN105502440AHigh yieldSimple process routeMagnesium chloridesAlkali metal chloridesLithium sulphateMirabilite
The invention provides a method for purifying lithium sulfate crude ore. The method comprises the following steps: step I, mixing the lithium sulfate crude ore S0 with excessive water to ensure that soluble ingredients in the lithium sulfate crude ore are just dissolved completely, and carrying out solid-liquid separation to obtain a solution L0; step II, freezing the solution L0 to separate out mirabilite, and carrying out solid-liquid separation to obtain a solution L1 and a solid S1; step III, evaporating the solution L1 to separate out a solid phase, and carrying out solid-liquid separation to obtain a solution L2 and a solid S2; step IV, allowing the solution L2 to stand still in a sealed condition for 7 to 50 days at 0 to 40 DEG C to separate out borate, and carrying out solid-liquid separation to obtain a solution L3; step V, mixing the solid S1 obtained in the freezing process in the step II with the solution L3, and evaporating the mixture at 0 to 40 DEG C to separate lithium sulfate concentrate.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
NaCl-Na2SO4-H2O system and process for producing NaHCO3, NH4Cl and NaCl from NH4HCO3
InactiveCN101837995AAdaptableThe main product is of high qualityAmmonium halidesAlkali metal chloridesSodium bicarbonateMirabilite
The invention provides a NaCl-Na2SO4-H2O system and a process for producing NaHCO3, NH4Cl and NaCl from NH4HCO3, comprising the following steps of: (1) carrying out double decomposition reaction on materials of mirabilite bittern and ammonium hydrogen carbonate and then separating to obtain an ammonium hydrogen carbonate product and an alkali-making mother solution containing ammonium chloride, sodium sulfate and sodium chloride, wherein the mirabilite bittern contains sodium chloride and sodium sulfate; (2) evaporating the alkali-making mother solution at high temperature and separating to obtain a mixed salt of sodium chloride and sodium sulfate and a mother solution of making sodium chloride and sodium sulfate; (3) distilling the mother solution of making sodium chloride and sodium sulfate at low temperature and separating to obtain ammonium chloride and an ammonium-making mother solution; (4) mixing the ammonium-making mother solution and the alkali-making mother solution, circulating, evaporating and separating the mixed salt of sodium chloride and sodium sulfate; (5) dissolving the mixed salt of sodium chloride and sodium sulfate in the mirabilite bittern containing sodium chloride and sodium sulfate, separating to obtain a sodium chloride mother solution and a washing mother solution and then introducing the washing mother solution to an integrated utilization system. The invention has the advantages of high quality of major product, strong material applicability, low cost, low energy consumption, no discharge of three wastes, and the like.
Owner:CHINA LIGHT IND INT ENG CO LTD
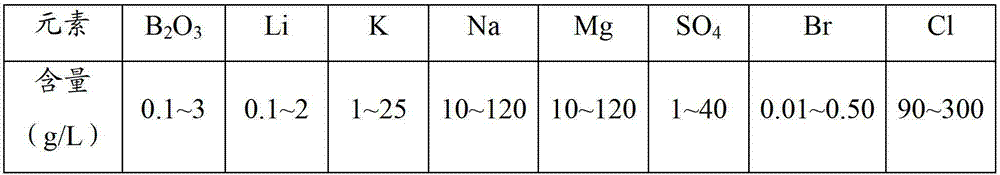
Method for enriching boron and lithium elements in sulfate type salt lake brine
ActiveCN103194622AReduce manufacturing costReduce the ratio of magnesium to lithiumBoron/boridesChemical industrySalt lakePotassium
The invention relates to a method for enriching boron and lithium elements in sulfate type salt lake brine. The method comprises the following steps of: (1) introducing the sulfate type salt lake brine into a pre-sunning pool, and regulating the concentration of sodium ions to a saturation state of sodium chloride; (2) introducing the brine with the sodium chloride in the saturation state into a mirabilite pool, and freezing in winter to precipitate mirabilite; (3) evaporating the brine after the precipitation of the mirabilite in spring and summer to precipitate the sodium chloride; (4) performing potassium removal treatment on the brine after the precipitation of the sodium chloride; (5) introducing the brine after potassium removal into an epsomite pool, and precipitating epsomite to obtain the brine with high content of magnesium chloride; and (6) mixing the brine with high content of magnesium chloride with the mirabilite for reaction, and performing solid-liquid separation to obtain a solution which is rich in boron and lithium elements.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI +1
Flyash excitant
The flyash excitant has two different compositions. That for no-steel bar concrete consists of mirabilite 10-30 wt%, calcium chloride 10-30 wt%, sodium chloride 10-20 wt%, efficient water reducing agent 10-25 wt%, calcium lignosulfonate 1.5-7.0 wt% and ardealite 5-15 wt% other than carrier material. That for reinforced concrete consists of mirabilite 10-30 wt%, calcium chloride 10-15 wt%, sodium chloride 10-20 wt%, calcium nitrite 20-30 wt% efficient water reducing agent 6-10 wt%, calcium lignosulfonate 2-5 wt% and ardealite 5-15 wt% other than carrier material. The flyash excitant can excite the latent activity of flyash effectively so as to increase the added amount of flyash in concrete and lower the cost of concrete product.
Owner:葫芦岛市辽西混凝土外加剂有限公司
Process and equipment for separating salt and nitrate without industrial wastewater discharge
InactiveCN107364877AAchieve separationAchieve recyclingEnergy inputWater/sewage treatment by heatingDischarge - substanceSodium sulfate
The invention discloses a process for separating salt and nitrate without industrial wastewater discharge. The process includes evaporating and concentrating sodium sulfate in high-temperature states until the sodium sulfate is in near saturation states; crystallizing out mirabilite under low-temperature freezing conditions; evaporating and crystallizing out sodium chloride under high-temperature conditions; carrying out hot melting on the mirabilite and crystallizing and dehydrating the mirabilite to dissolve out the sodium sulfate. The invention further discloses equipment for implementing the process. The process and the equipment have the advantages that the sodium sulfate and the sodium chloride in industrial wastewater with the salt and the nitrate can be separated and recycled, accordingly, the wastewater is free of impurity salt, the impurity contents can be reduced, the process and the equipment are free of discharge substances which can cause pollution of water systems, and technical effects of recycling resources without pollution liquid discharge can be realized; the process is low in energy consumption, the treated wastewater can be recycled, and the process and the equipment are suitable for industrially treating the wastewater; industrial-grade sodium sulfate and sodium chloride products further can be obtained, and the solid waste or hazardous waste production probability can be lowered; the equipment is high in heat efficiency and automation degree and low in power consumption and operation cost, and sustainable development requirements can be met by the equipment.
Owner:SHENZHEN SUNEVAP TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com