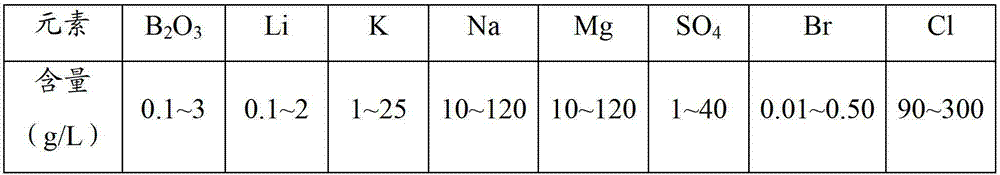
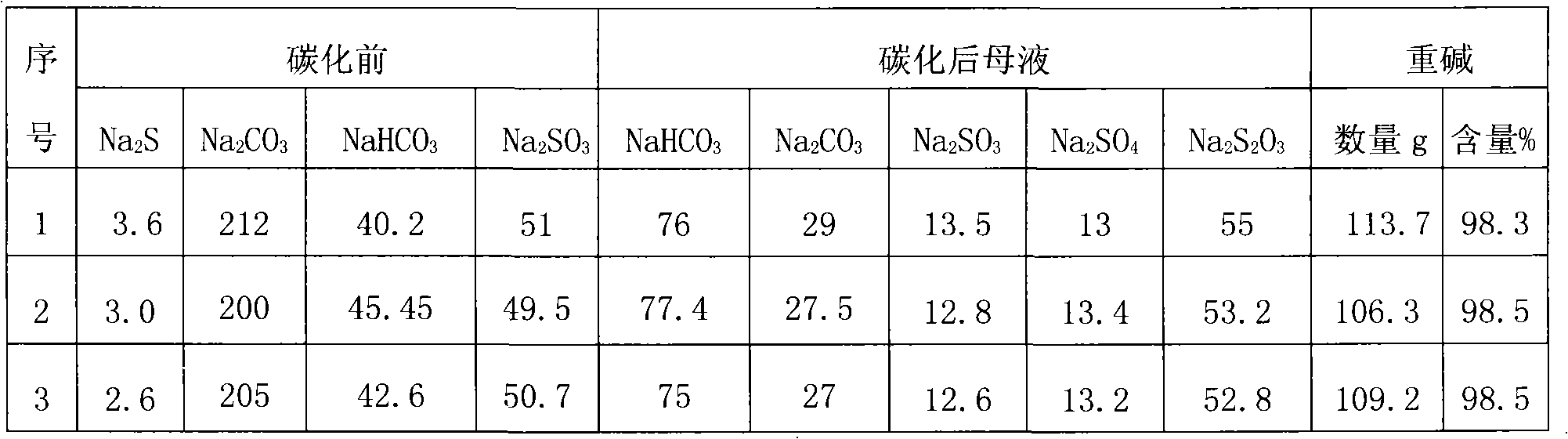
Patents
Literature
45 results about "Thenardite" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Thenardite is an anhydrous sodium sulfate mineral, Na₂SO₄ which occurs in arid evaporite environments. It also occurs in dry caves and old mine workings as an efflorescence and as a crusty sublimate deposit around fumaroles. It occurs in volcanic caves on Mt. Etna, Italy. It was first described in 1825 for an occurrence in the Espartinas Saltworks, Ciempozuelos, Madrid, Spain and was named for the French chemist, Louis Jacques Thénard (1777–1826).
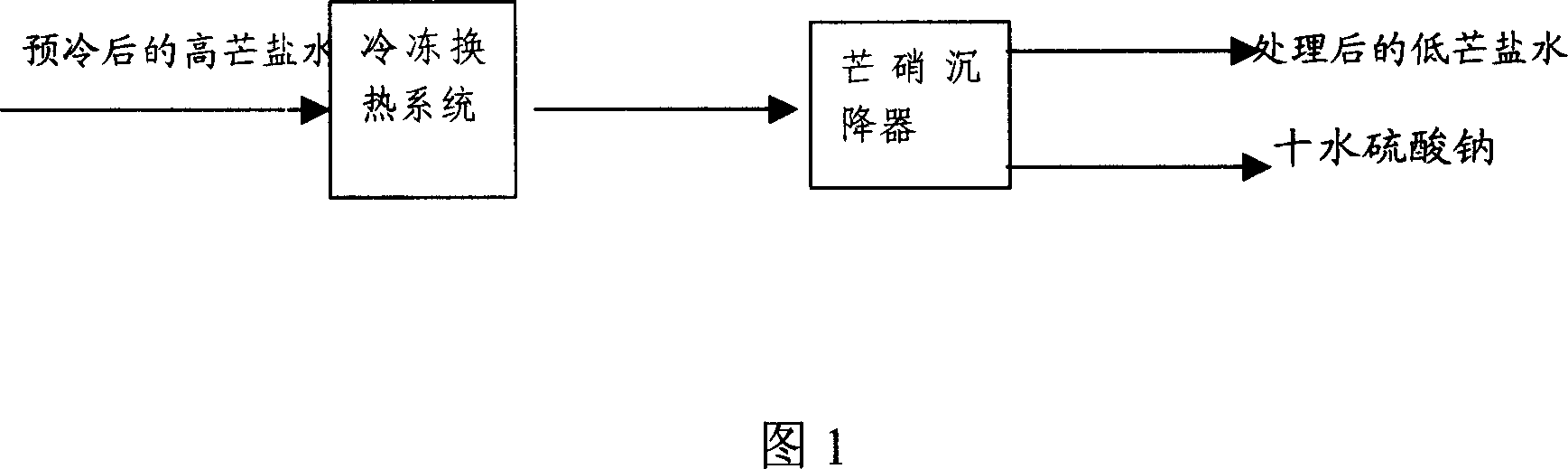
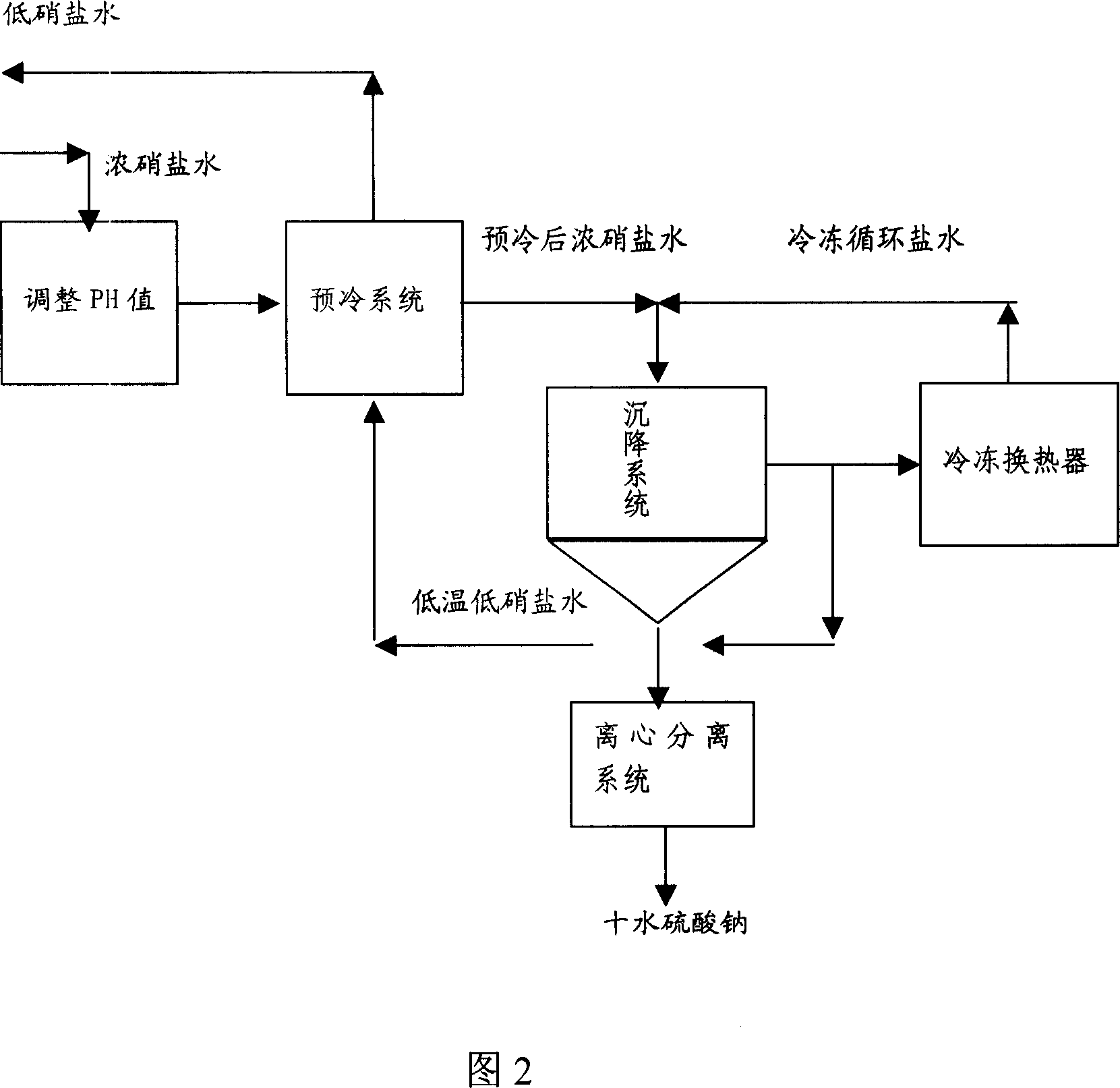
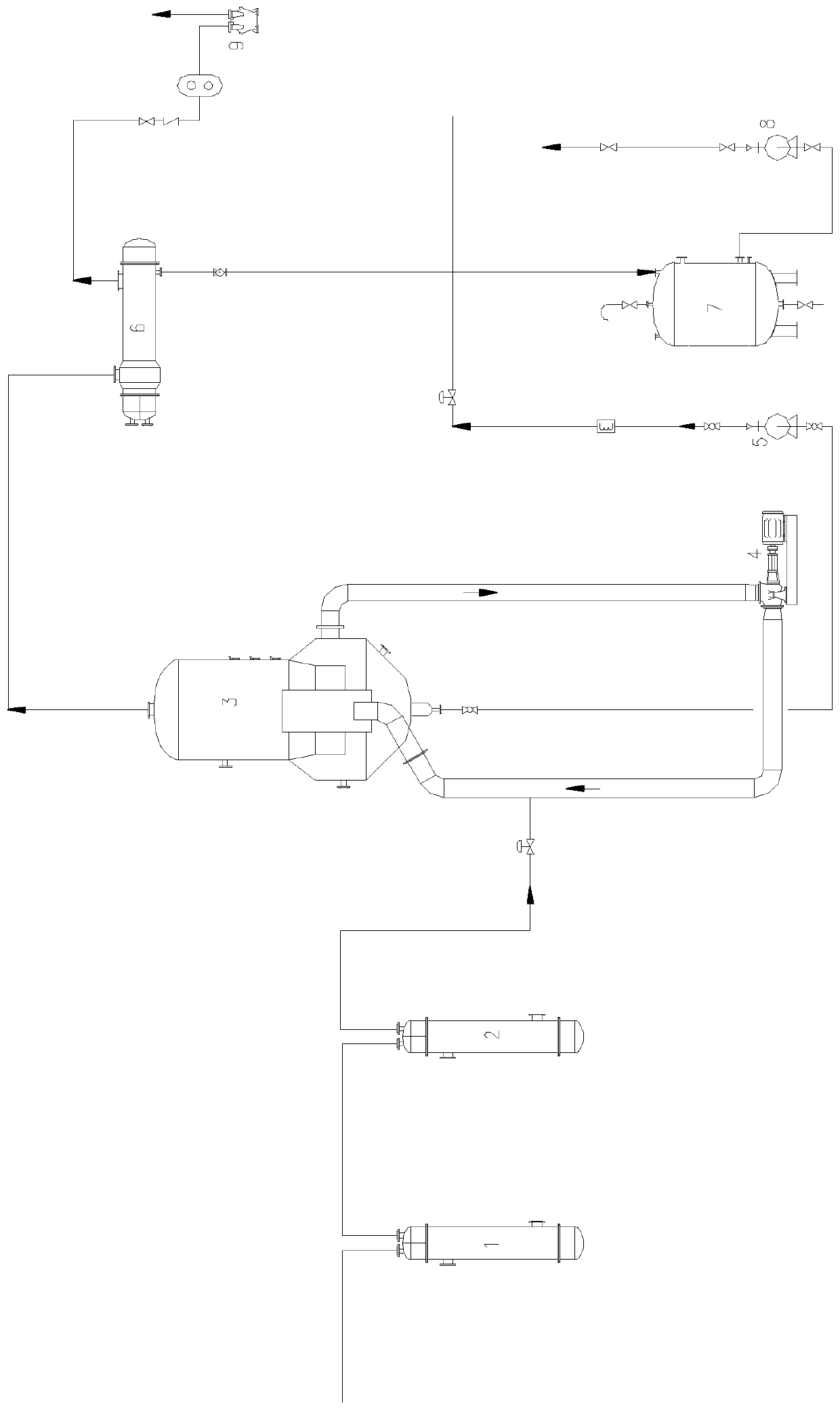
Method freezing separating mirabilite by brine solution
The invention discloses a method of cold separating mirabilite in salt water solution, which comprises the following steps: (1) adjusting pH value of armed salt cake solution as alkalify; (2) proceeding pre-cool in the precooling system; (3) mixing with low-temperature low-nitrate water of incorporating salt cake with lower temperature through demirabilite treatment; controlling temperature at -10-0 deg.c; proceeding solid-liquid separation in salt cake settler; getting ten water sodium sulfate crystal and low nitrate water; (4) lowering temperature of part low nitrate water through freezing heat exchanger; recycling as low temperature nitrate water of step (3); discharging the other part of low nitrate water as byproduct.
Owner:山东布莱恩化工技术有限公司
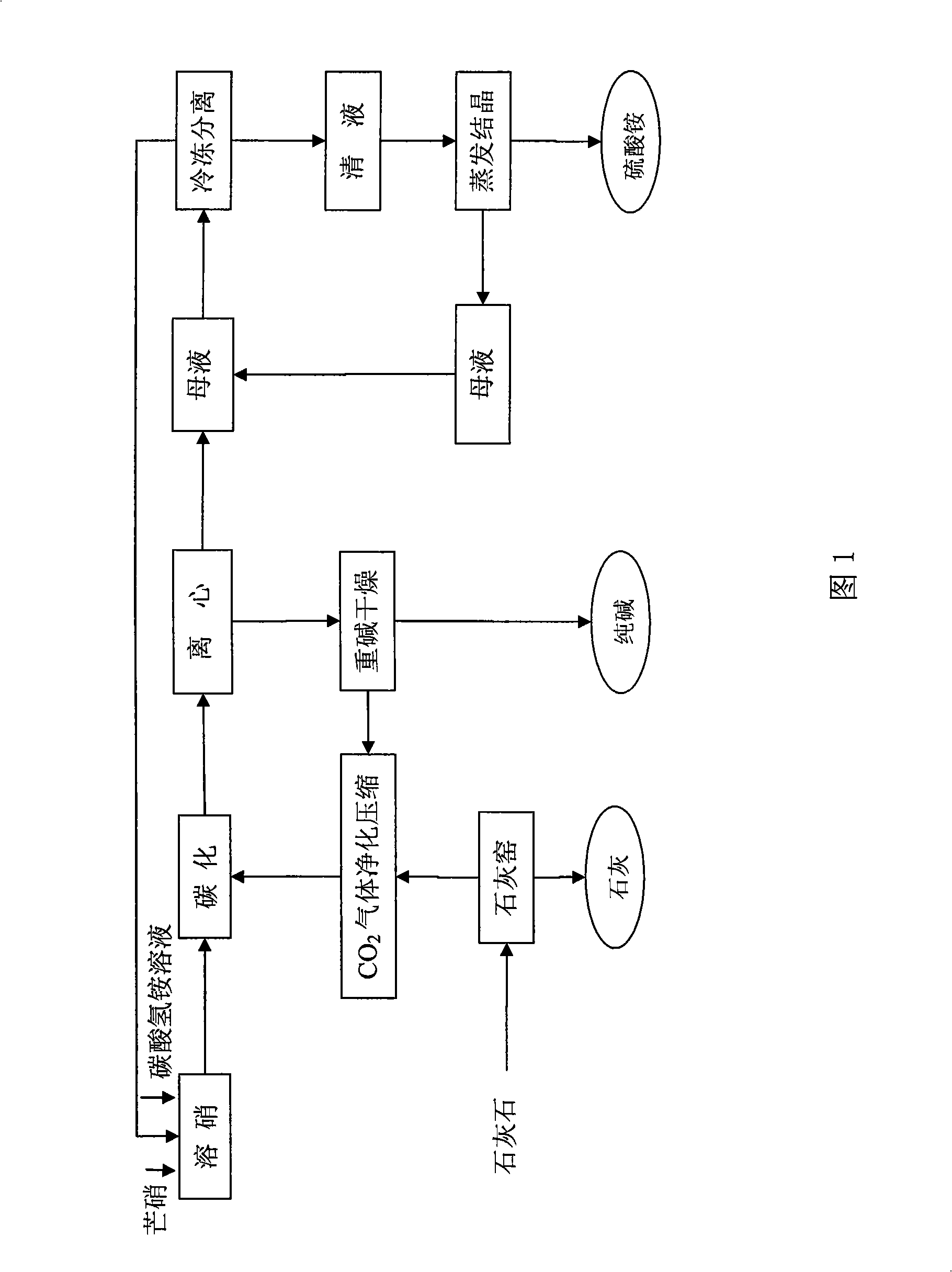
Method for preparing agricultural ammonium sulphate and calcined soda for industry with salt cake ammonia soda process
InactiveCN101318668ARich reservesReserves are cheapCarbonate preparationAmmonia compoundsSodium bicarbonateEvaporation
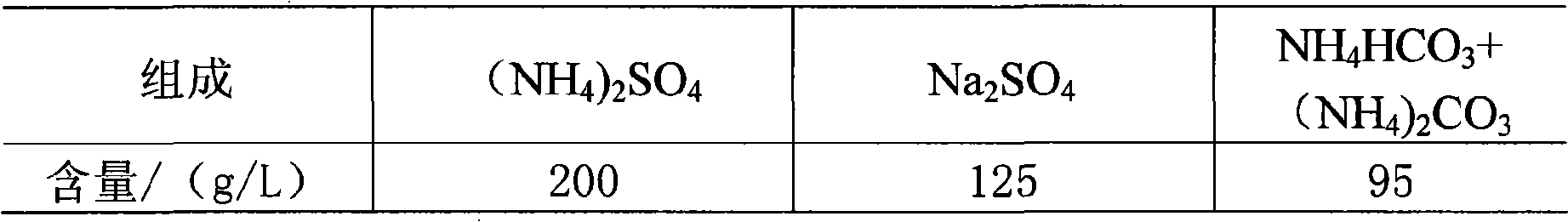
The invention discloses a method for producing an agricultural ammonia sulfate and a commercially pure soda by a glauber salt ammonia-soda process realizing CO2 emission reduction and no waste water emission. The method comprises the following steps: A) carbonization procedure of ammonia water, B) dissolution procedure of glauber salt, C) carbonization procedure, D) soda drying procedure, E) freezing and purification procedure, and F) evaporation and crystallization procedure of ammonia sulfate; a finished product is obtained by dehydration and drying, the filtrate obtained from the evaporation and crystallization is evaporated to a co-saturation point, the co-saturated filtrate is mixed with a mother liquor obtained by separation of sodium bicarbonate in step C and the mixture is frozen, and then the procedures are recycled. The method is characterized in that glauber salt is dissolved in a carbonized ammonia solution or ammonium bicarbonate solution and carbonated by CO2 gas to form and separate out the dense soda NaHCO3 crystal, the crystal is filtered and dried to obtain the standard soda; the mother liquor is mixed with the co-saturated solution obtained from the evaporation of the ammonium sulfate, the mixture is frozen to separate sodium sulfate from the ammonium bicarbonate precipitate, and the ammonium sulfate is obtained by evaporation, crystallization and drying. The method has the advantages of simple process, environmental protection, no pollution, the complete recycle of the mother liquor and no discharge of waste water.
Owner:GANSU JINSHI CHEM
Method for preparing lithium salt ore from plateau sulfate type salt lake brine
ActiveCN103204523ANo pollution in the processReduce manufacturing costChemical industryLithium sulfates/sulfitesHigh magnesiumThenardite
The invention discloses a method for preparing lithium salt ore from plateau sulfate type salt lake brine. The method comprises the following steps of: evaporating sulfate type salt lake brine to be in the sodium chloride saturated state, freezing in winter to separate out mirabilite, and carrying out solid-liquid separation when the content of the sulfate ion in the brine is controlled to be 1g / L to 7g / L; evaporating the brine from which the mirabilite is separated out, so as to separate sodium chloride; evaporating the brine from which the sodium chloride is separated out, so as to separate out sylvine, carnallite and epsomite, carrying out solid-liquid separation when the lithium ion concentration in the brine is controlled to be greater than or equal to 6g / L, wherein the brine subjected to solid-liquid separation is the brine with high magnesium chloride content; mixing the brine with high magnesium chloride content with the mirabilite to react so as to separate out sodium salt and magnesium salt, and carrying out solid-liquid separation when the magnesium-lithium ratio in the solution is controlled to be less than or equal to 8:1, so as to obtain boron-lithium-rich brine; and reacting the boron-lithium-rich brine with water to separate out boron rock, carrying out solid-liquid separation so as to obtain lithium-rich brine; and introducing the lithium-rich brine into a lithium salt pool, and evaporating so as to separate out the lithium salt ore.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI +1
Process for preparing anhydrous mirabilite by freezing method
ActiveCN1837050AEasy to operateReasonable heat transfer temperature differenceChemical industryAlkali metal sulfite/sulfate dehydrationMirabiliteSodium sulfate
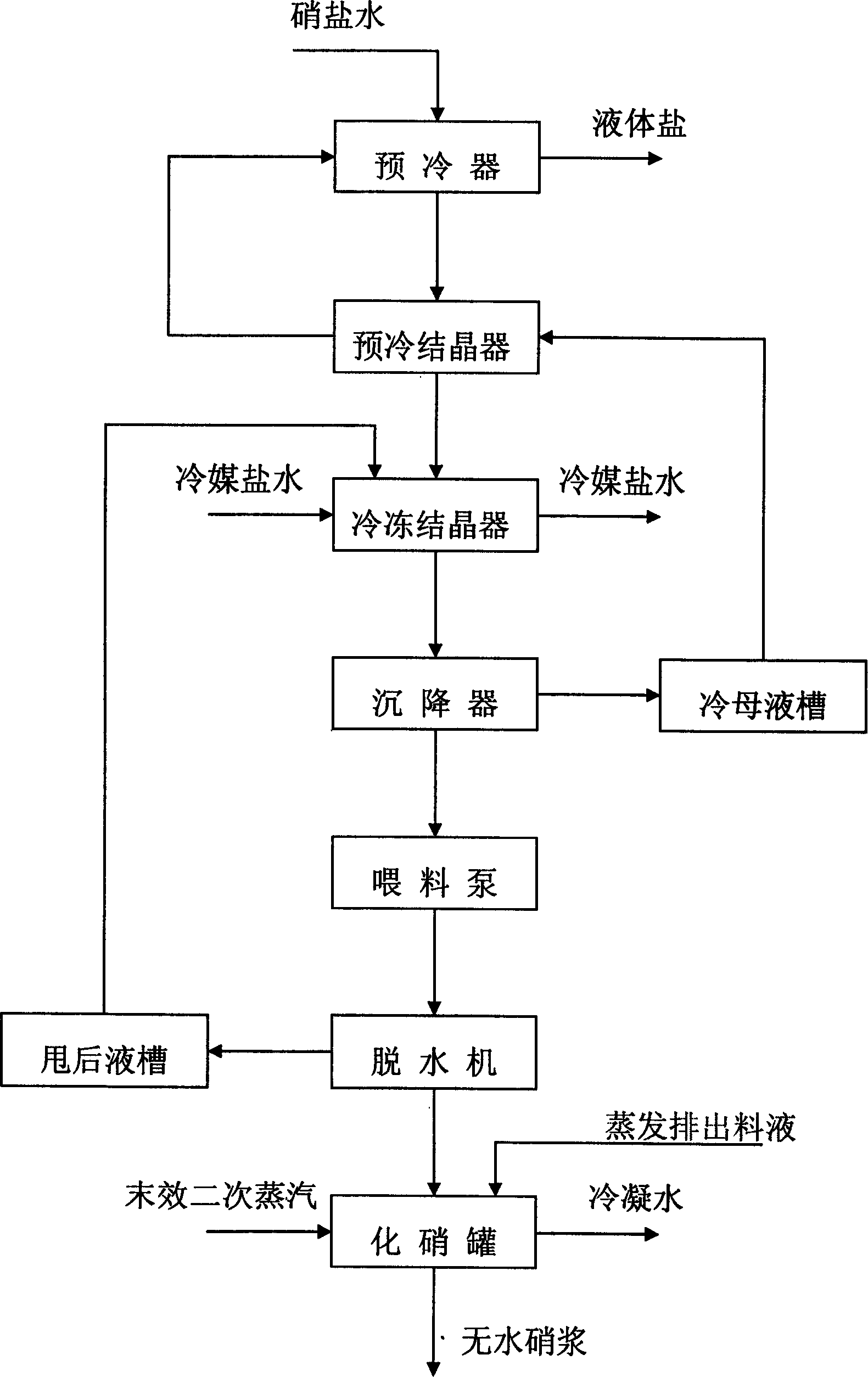
The invention discloses a new technics about the production of freezing method absolute nitrate, containing steps as follows: getting the solution contains saltpeter across precooler to doing coolant elementary cooling by cold mother liquor; switching the feed liquid from precooler to precooler crystallizer; adding the feed liquid from precooler crystallizer in cooler crystallizer to freeze the feed liquid to -5-7Deg C for the saltpeter separating of second phase refrigeration; Adding the feed liquid from cooler crystallizer in depressor for solid-liquid separation; passing the discharged sodium sulfate from precooler through screw pump to feed the dewatering machine; putting the dewatered sodium sulfate in saltpeter melting tank, mixing with the swinged liquid of evaporate discharged feed liquid or absolute nitrate plasm, making sodium sulfate autolyze by end effect quadratic steam heating. In the saltpeter melting procedure of this method, adopting hot material and sodium sulfate mixing melt saltpeter, at the same time, using low-grade second steam heat energy heat up the feed liquid, compared to the former pervaporation, this method is more reasonable, energy saving and ensure the quality of product.
Owner:ENG TECH INST CO LTD OF CNSIC
Method for producing sodium bicarbonate and sulfur from mirabilite by wet process
InactiveCN101264904AAchieving zero emissionsAchieve recyclingBicarbonate preparationSulfur preparation/purificationSodium bicarbonateCarbonization
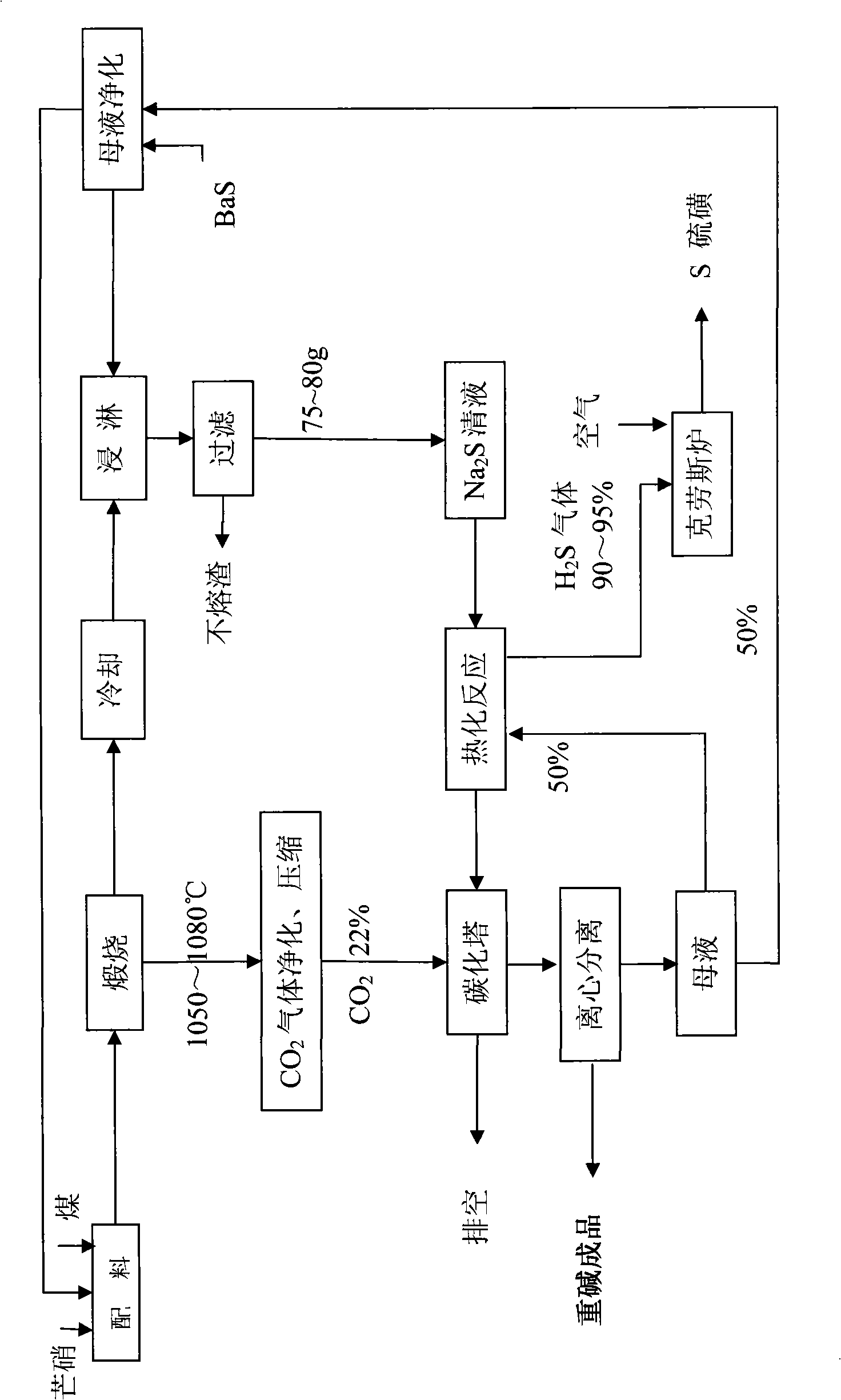
The invention discloses a method for clean method for producing sodium bicarbonate and sulphur by means of Glauber salt wet method for reducing the emission of CO2 and avoiding the discharge of wastewater, which comprises the following steps: A, sodium sulphide preparing process, B, hot carbonatation process, C, sulphur preparing process, D, cold carbonization process, and E, mother liquor purifying. Glauber salt coal is reduced and calcined into sodium sulphide, and the sodium sulphide solution is heated and carbonized into a mixed solution with the soda as the main component by use of sodium bicarbonate, then the mixed solution is cold-carbonated by CO2 to separate out NaHCO3 crystal, which is dried to finally obtain the soda in conformity with relevant standard. The production method has the advantages of simple process, environmental friendliness, no pollution, full circulation of the mother liquor, and no wastewater discharged.
Owner:GANSU JINSHI CHEM
Method for producing lithium hydroxide by dissolving spodumene out of pipeline reactor
InactiveCN104003428ASimple stepsReduce material consumptionLithium oxides/hydroxidesThenarditeLithium hydroxide
The invention provides a method for producing lithium hydroxide by dissolving spodumene out of a pipeline reactor. The method comprises the following steps: carrying out size mixing on water and beta spodumene powder, sodium hydroxide and lime which are obtained by transforming a calcined crystal, delivering into the pipeline reactor by using a pump, mixing by utilizing the flow of slurry in a pipeline, and reacting at a certain temperature and pressure; after the reacted slurry flows out of the pipeline reactor, carrying out solid-liquid separation; and carrying out concentration, impurity removal, crystallization and mother solution circulation on filter liquor to obtain a lithium hydroxide product. The method provided by the invention is simple in lithium hydroxide production process, small in investment, low in production energy consumption and high in lithium extraction rate without sodium sulphate frozen precipitation or sulfuric acid transformation.
Owner:FUZHOU UNIV
Method for refining sodium sulfate powder from chromium-containing mirabilite
ActiveCN103539164AReduced chromium contentHigh yieldAlkali metal sulfites/sulfatesThenarditeSulfite salt
The invention relates to a method for refining sodium sulfate powder from chromium-containing mirabilite. The method comprises the following steps: a) grinding the chromium-containing mirabilite in the presence of water to prepare water-containing slurry; b) regulating the pH of the water-containing slurry obtained in the step a) with sulfuric acid to 2-3, and then adding sodium sulfite to reduce hexavalent chromium in the water-containing slurry to trivalent chromium, thus obtaining a saturated or over-saturated sodium sulfate water solution; c) stirring and crystallizing the saturated or over-saturated water solution which is obtained after reduction in the step b); d) filtering out crystals obtained in the step c); e) washing the crystals obtained in the step d) with the saturated sodium sulfate water solution; f) re-crystallizing the washed crystals obtained in the step e) with a saturated neutral sodium sulfate water solution to obtain the sodium sulfate powder. According to the method provided by the invention, additional heating is not required, the energy consumption is low, the obtained sodium sulfate powder has high yield and high purity, the content of sodium sulfate can achieve 99.00-99.90wt%, and the chromium content does not exceed 5ppm.
Owner:SICHUAN YINHE CHEM
Method for preparing potassium sulfate from sodium sulfate
InactiveCN107857282AReduce solubilityEasy to separateAlkali metal sulfite/sulfate purificationAlkali metal chloridesFiltrationSolvent
The invention discloses a method for preparing potassium sulfate from sodium sulfate. The method is characterized by comprising the following steps: (1) performing a reaction by taking the salt lake sodium sulfate and potassium chloride as raw materials and a water-alcohol system as a solvent, performing a reaction under a room temperature for a period of time, performing filtration to obtain aphthitalite and a mother liquid A, performing concentration on the mother liquid A, performing crystallization, and performing vacuum filtration to obtain sodium chloride and a filtrate A; and (2) weighing the aphthitalite and potassium chloride, performing a reaction by taking a water-alcohol system as a solvent to obtain the potassium sulfate and a mother liquid B, performing concentration on the mother liquid B, performing crystallization, and performing vacuum filtration to obtain sodium chloride and a filtrate B. Compared with the prior art, the method provided by the invention reduces the production energy efficiency, shortens the production time, and improves the yield of the potassium sulfate.
Owner:SHIHEZI UNIVERSITY
Method of separating sodium sulfate crystal from mirabilite mineral
InactiveCN1608996AHigh in Sodium SulfateLow impurity contentAlkali metal sulfite/sulfate purificationThenarditeResource utilization
The method of separating sodium sulfate crystal from mirabilite mineral includes fully mixing mirabilite ore and water in the ratio of 1 to 0.1-5, and separating the mixed slurry with mirabilite ore grains of size larger than 3 mm in a separator to obtain sodium sulfate crystal grains. The direct separation of sodium sulfate crystal from mirabilite mineral has sodium sulfate product with high purity, and the product may be used in preparing weathered saltpeter, anhydrous saltpeter and anhydrous sodium sulfate. The said process has high ore utilization, low cost and high product quality.
Owner:温润宪 +1
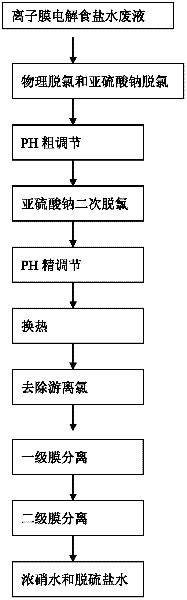
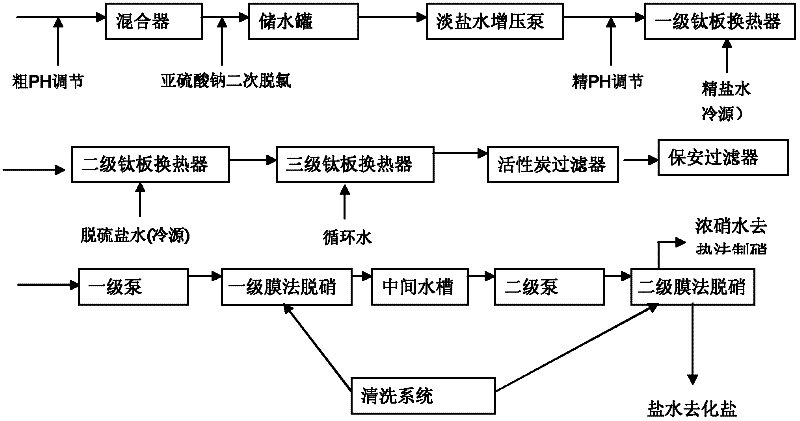
Comprehensive utilization method of ionic membrane electrolytic saline solution waste liquid
ActiveCN102502986AEfficient processing and utilizationEfficient use ofWater/sewage treatment bu osmosis/dialysisMultistage water/sewage treatmentComing outLiquid waste
The invention relates to a comprehensive utilization method of an ionic membrane electrolytic saline solution waste liquid, which comprises the step of sending dechlorinated dilute brine coming out of an electrolytic tank to a membrane process denitration device for denitration. The comprehensive utilization method is characterized by comprising the following steps of: pre-adjusting the pH value of a system to 3-8 by using a saline solution pH value automatic control device, exchanging the heat till the temperature of the system is 25-50 DEG C, removing free chlorine till the content of chlorine is less than or equal to 3 ppm, sending into a primary membrane separation system for primary membrane separation and controlling the primary membrane separation pressure to be not higher than 3.0MPa; and then sending into a secondary membrane separation system for secondary membrane separation, controlling the secondary membrane separation pressure to be not higher than 3.5 MPa, separating to obtain desulfurized brine and concentrated nitrate water, sending the dissolved desulfurized brine to an ionic membrane electrolytic saline solution device for reutilization and sending the concentrated nitrate water to a thermal method denitration device to prepare thenardite. The invention has the beneficial effects that as the two stages of membrane separation systems are adopted, the pressure is more stable, the membrane service life is long, and the separation effect is good; the content of sodium sulfate in the separated concentrated nitrate water can reach above 90 g / l, and the content of sodium sulfate in the desulfurized brine can reach below 3 g / l.
Owner:CHINA PETROLEUM & CHEM CORP +1
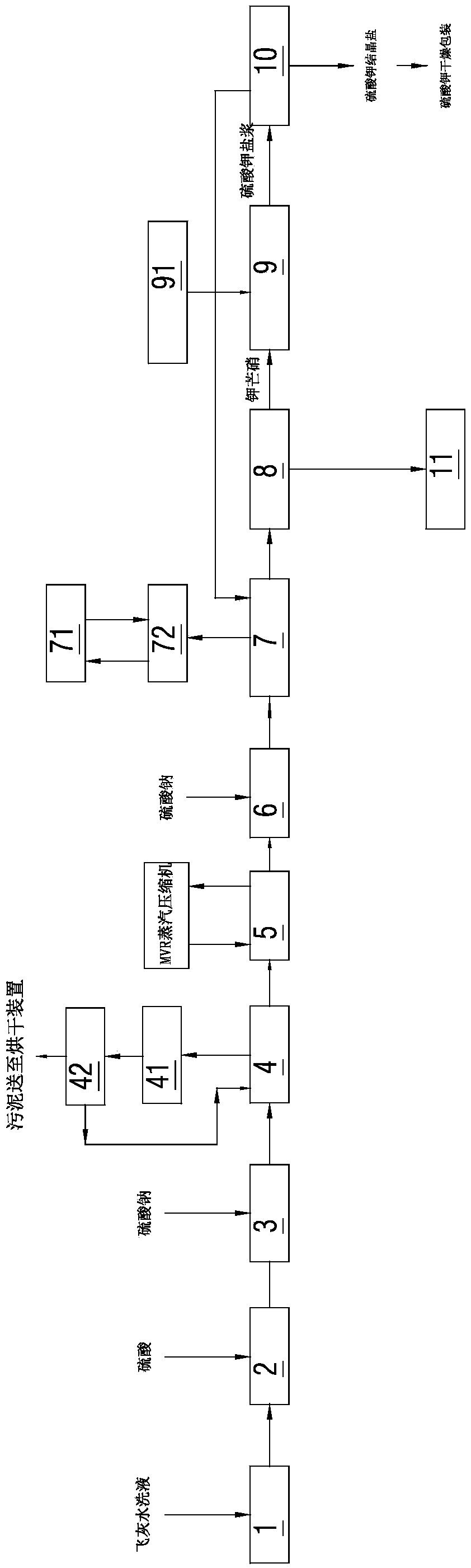
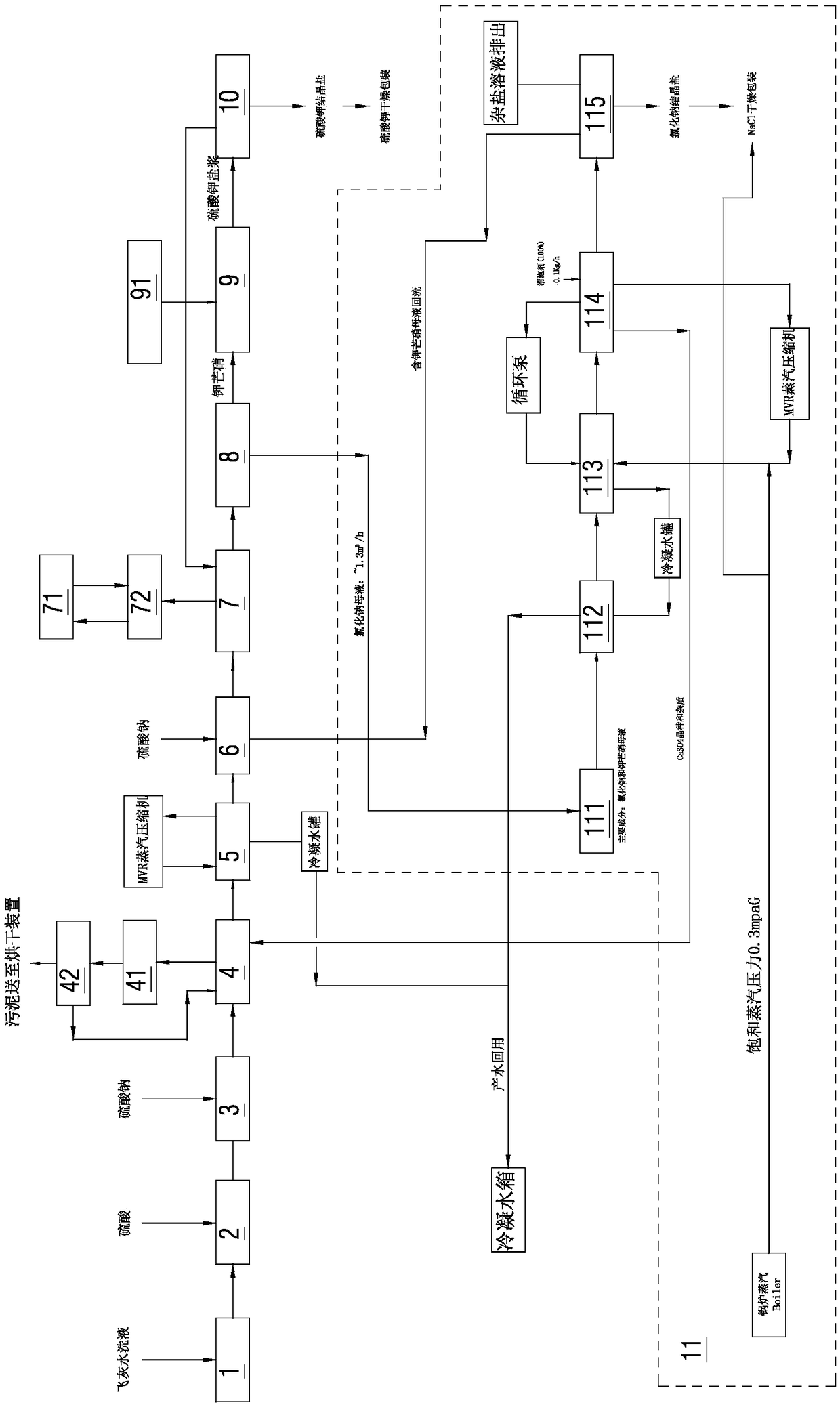
Method for extracting potassium from fly ash hydrolysate
A method for extracting potassium from fly ash hydrolysate comprises the following steps: step A, carrying out front-section filtration on the fly ash hydrolysate and removing suspended matters, thenadding sulfuric acid to regulate pH value, then feeding sodium sulfate, and carrying out clarification and sludge filtration to obtain clarified water; step B, concentrating the clarified water obtained from step A, adding the sodium sulfate in a concentrated solution, then carrying out freezing crystallization, and separating out glaserite solid; and step C, adding the glaserite solid obtained instep B in a potassium chloride regulating solution to obtain potassium sulfate salt mixture slurry, and separating the potassium sulfate salt mixture slurry to obtain potassium sulfate crystallized salt. Compared with an existing process, the method for extracting the potassium from the fly ash hydrolysate has the advantages that the pretreatment process is greatly simplified, and the running cost is greatly reduced; and co-products with high value can be obtained, and therefore, the cost-effectiveness of a treatment process is greatly increased.
Owner:南京南环水务科技有限公司
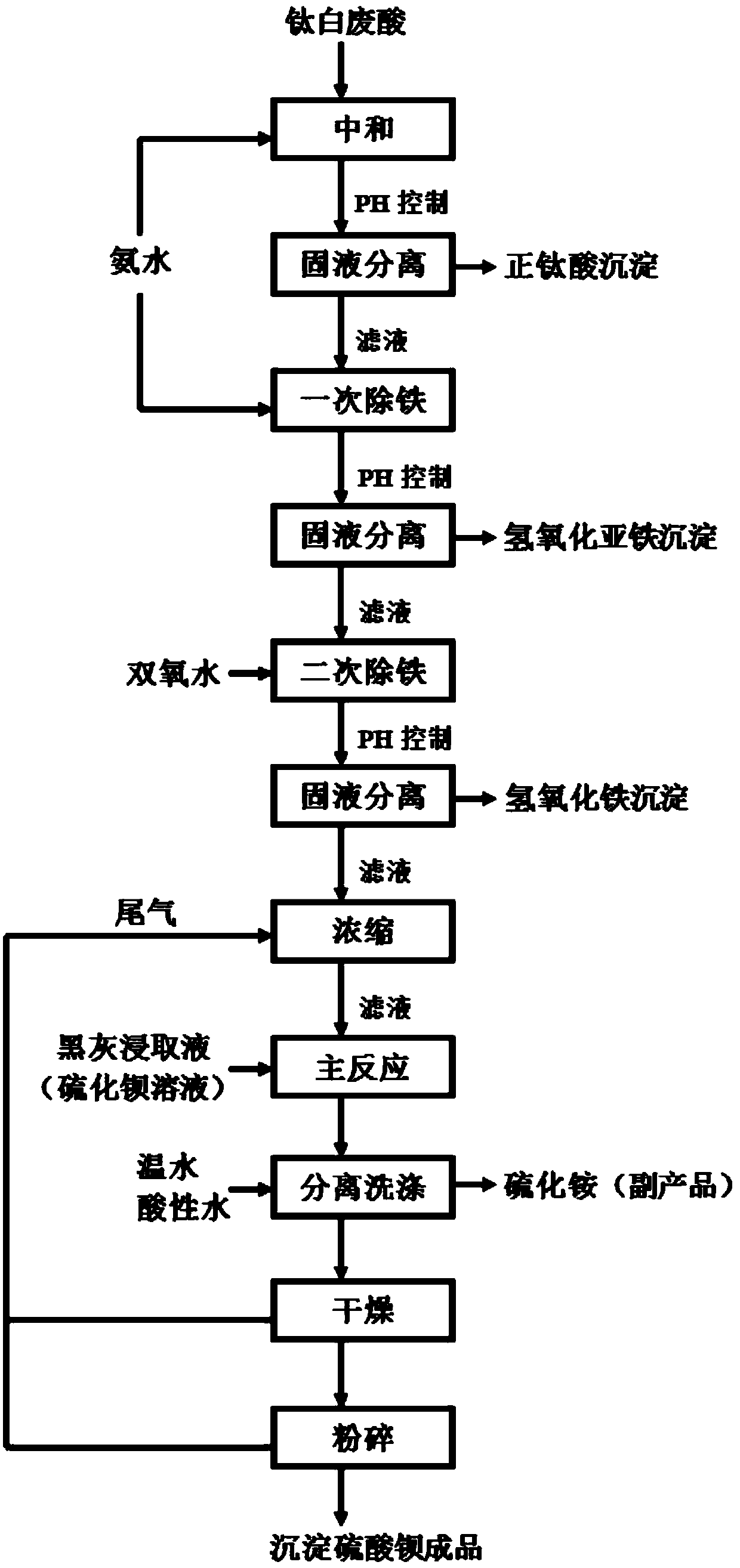
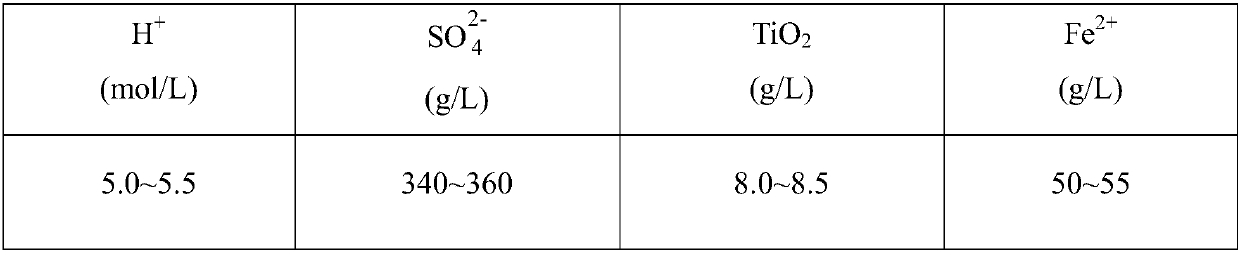
Method for preparing precipitated barium sulfate by utilizing titanium white waste acid
ActiveCN107720801AReduce cost pressureReduce pressure on environmental protectionAmmonium sulfides/polysulfidesCalcium/strontium/barium sulfatesFerric hydroxideSodium sulfate
The invention discloses a method for preparing precipitated barium sulfate by utilizing titanium white waste acid, belonging to the technical field of comprehensive utilization of titanium white wasteacid and preparation of barium sulfate. The method comprises the following steps of adding ammonium hydroxide to neutralize with titanium white waste acid firstly, then generating ammonium sulfate, and controlling a systemic pH to separate out photodegredation sediment; then adding the ammonium hydroxide continuously, and controlling the pH to separate out ferrous hydroxide sediment; then addinghydrogen peroxide in proportion, oxidating residual Fe2<+> into Fe3<+>, and adjusting the pH to separate out ferric hydroxide sediment; condensing an ammonium sulfate solution, then replacing a mirabilite solution in a traditional precipitated barium sulfate production process, enabling the ammonium sulfate solution to generate replacement reaction with a barium sulfide solution, and washing, drying and smashing to prepare a precipitated barium sulfate product. According to the method, after neutralizing the titanium white waste acid with the ammonium hydroxide and performing purification andimpurity removal, a traditional sodium sulfate solution is replaced for producing the precipitated barium sulfate, meanwhile, recycled photodegredation sediment can be further used for titanium dioxide production, not only are resources saved, but also waste can be recycled, and the waste acid treatment costs of titanium dioxide plants and environment protection pressure are reduced.
Owner:ANHUI JXTB GRP
Method for sanitary production of sodium fluosilicate
InactiveCN102923713AReduce pollutionReduce processing costsSilicon halogen compoundsPhosphoric acidHexafluorosilicic acid
The invention discloses a method for sanitary production of sodium fluosilicate. Mirabilite is adopted by the method as raw materials to react with fluosilicic acid to form the sodium fluosilicate and dilute sulphuric acid. The dilute sulphuric acid is used as wet process phosphoric acid to filter dilute acid to digest. In the process of combination, firstly, water and the dilute sulphuric acid are added to the mirabilite, and then the mirabilite is put into a salt melting groove to dissolve, and after dissolving, the mirabilite contains sodium sulfate solutions ranging from 5% to 40%, and the mirabilite is put in a salt water groove to keep warm through steam to be used. Fluosilicic acid is added to a crystallization groove, and then sodium sulfate salt solutions are added to the crystallization groove, and so that sodium fluosilicate and the dilute sulphuric acid are prepared. After being filtered, the sodium fluosilicate and the dilute sulphuric acid are dried to prepare a sodium fluosilicate end product. The dilute sulphuric acid returns to a wet process phosphoric acid device to be used as washing water. The method for sanitary production of the sodium fluosilicate uses mother liquor which is generated in the production of the sodium fluosilicate as wet process phosphoric acid washing water, so that in the production of the sodium fluosilicate, pollution and sewage processing cost is not only reduced, but also in the process of extraction of the wet process phosphoric acid, consumption of sulfuric acid is simultaneously reduced. Therefore, production cost of the sodium fluosilicate and the wet process phosphoric acid is reduced.
Owner:WENGFU (GRP) CO LTD
Process of producing gypsum with mirabilite slag
The process of producing gypsum with mirabilite slag includes the following steps: crushing mirabilite slag in a mill to 8-80 mesh; mixing mirabilite slag powder with water via mechanical stirring; depositing the mixture repeatedly in deposition tanks until the deposit has required low sodium sulfate content; and drying the final deposit to obtain the gypsum. The said process is simple, practical and low in cost, and can eliminate pollution caused by mirabilite slag while producing useful material.
Owner:何宝成
Preparation process of sodium sulfide with low content of iron
The invention discloses a preparation process of sodium sulfide with low content of iron, and involves the field of production of industrial sodium sulfide. The preparation process includes the steps: preparing thenardite from mirabilite as a raw material; mixing thenardite with pulverized coal, and preheating the obtained mixture in a preheater; adding the preheated materials into a converter from the tail part, adding fuel coal into the converter from the head, and reacting the thenardite with the pulverized coal to generate sodium sulfide; conducting hot melting on the sodium sulfide in a hot melting tank, and sending a supernatant obtained after precipitation to a storage tank; sending concentrated alkali water in the storage tank into a clarification tank; sending a clear alkali liquid into a reaction tank, adding an iron removal agent, performing a sufficient reaction of the iron removal agent and impurities in the clear alkali liquid, allowing the obtained material to stand, discharging precipitated insoluble matters into a mud groove and sending alkali liquor after standing into an evaporating pot; concentrating the alkali liquor; sending concentrated alkali liquor into a tableting machine to form flaky sodium sulfide, packaging sodium sulfide and preparing the packaged sodium sulfide for sell. The preparation process can effectively reduce the content of iron in sodium sulfide.
Owner:重庆市庆岩建材有限公司
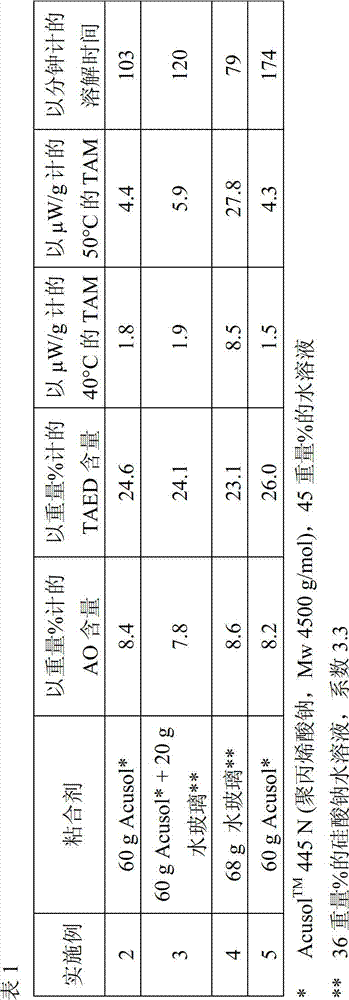
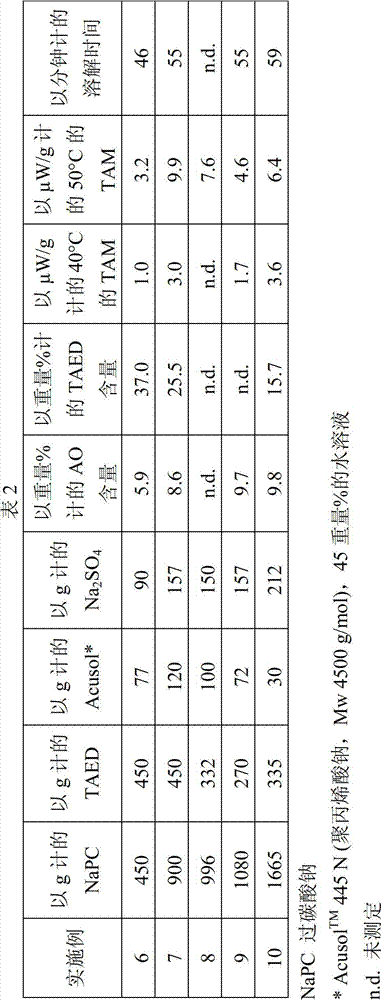
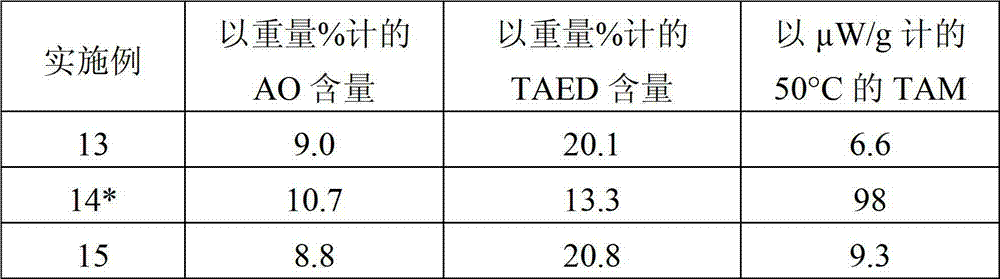
Bleaching agent particles comprising sodium percarbonate and a bleach activator
InactiveCN102858936AInorganic/elemental detergent compounding agentsPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesThenarditeWater soluble
The invention relates to bleaching agent particles, comprising a core made of sodium percarbonate, an inner enveloping layer containing at least 50 wt % of sodium sulfate in the form of thenardite or burkeite and an outer enveloping layer, containing a water-soluble binder and a perhydrolyzable N-acyl compound or O-acyl compound as the bleach activator, wherein said particles are storage-stable, suitable for silo storage and can be safely transported and handled even in a hot and humid climate.
Owner:EVONIK TREIBACHER
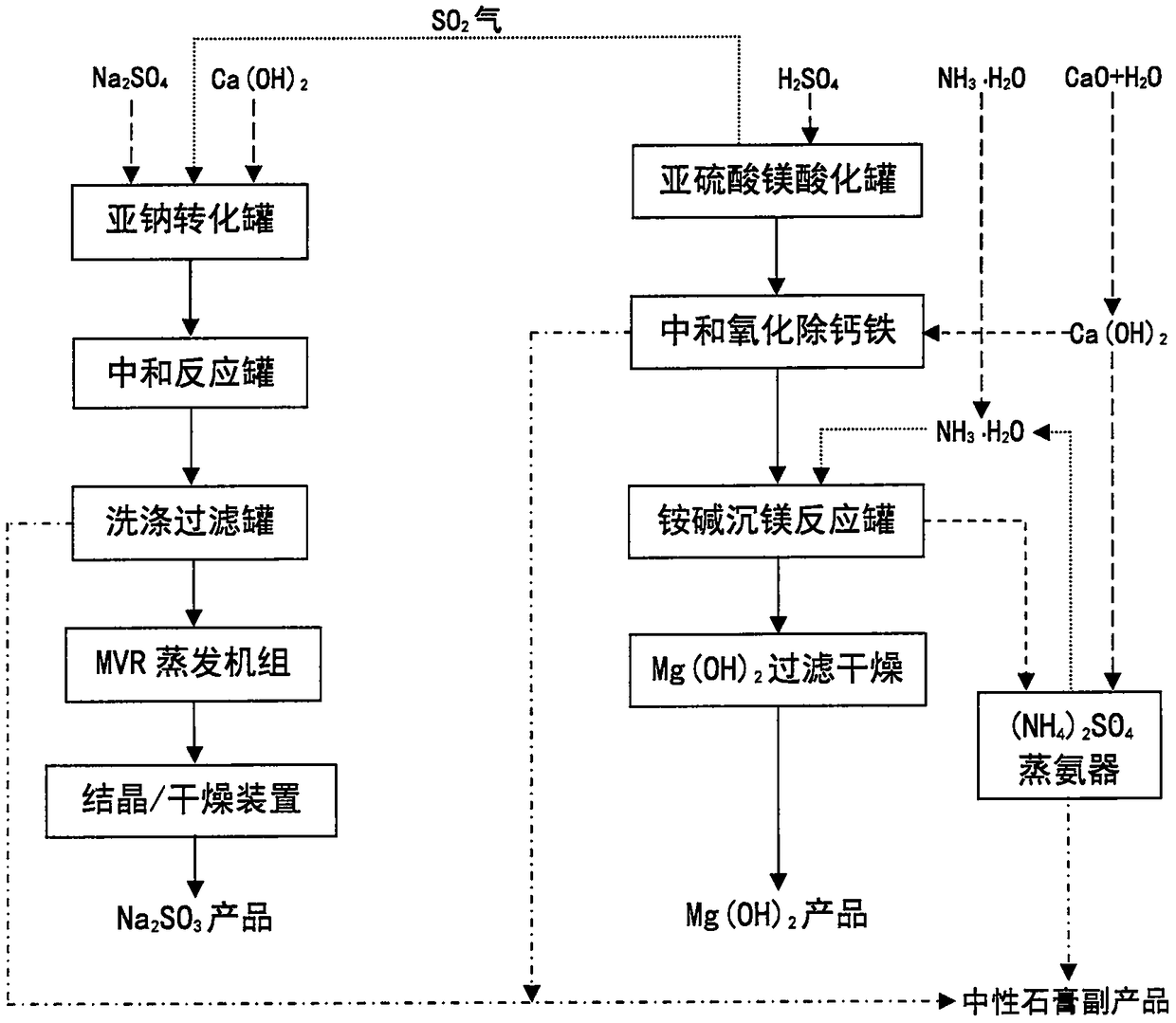
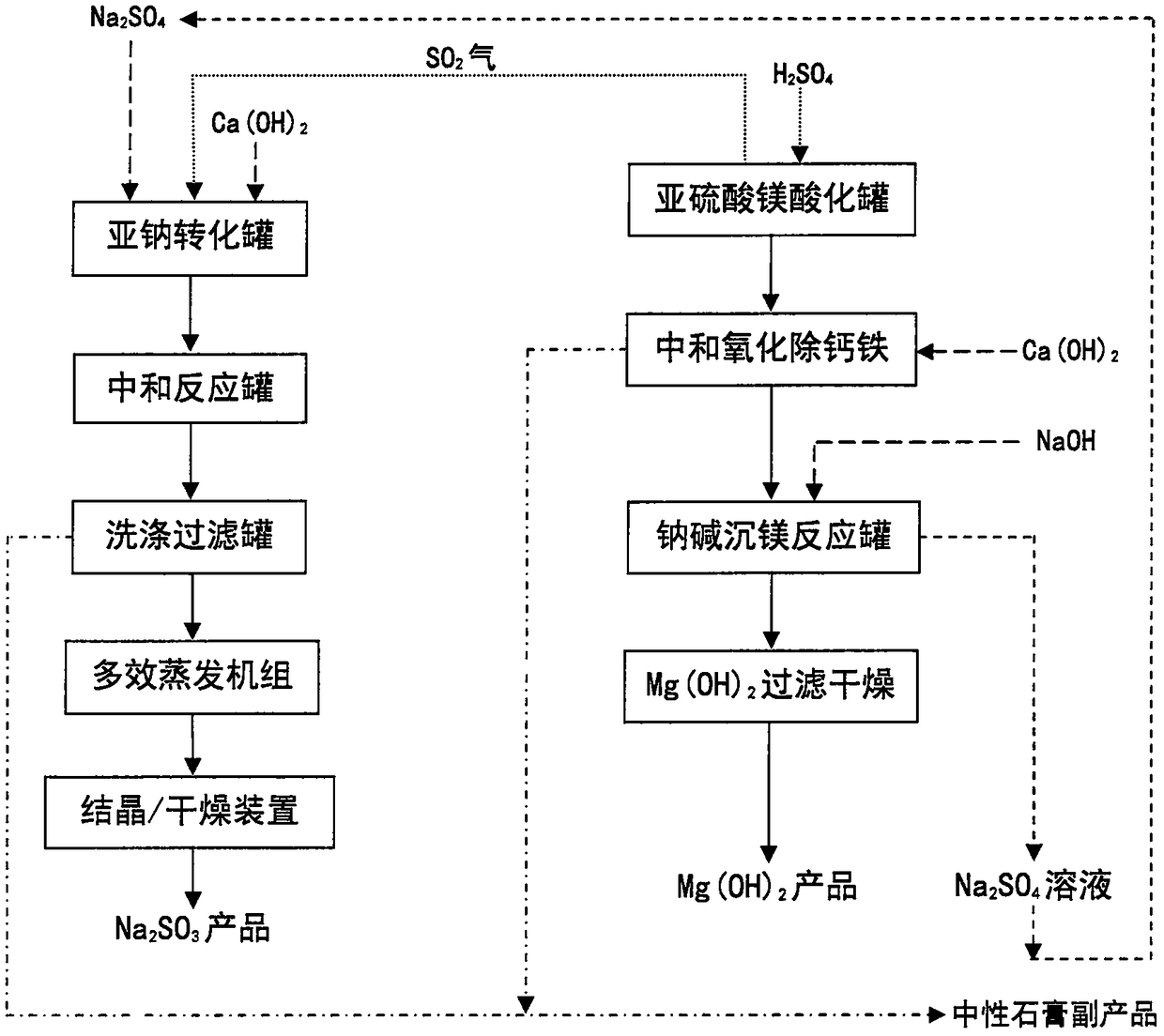
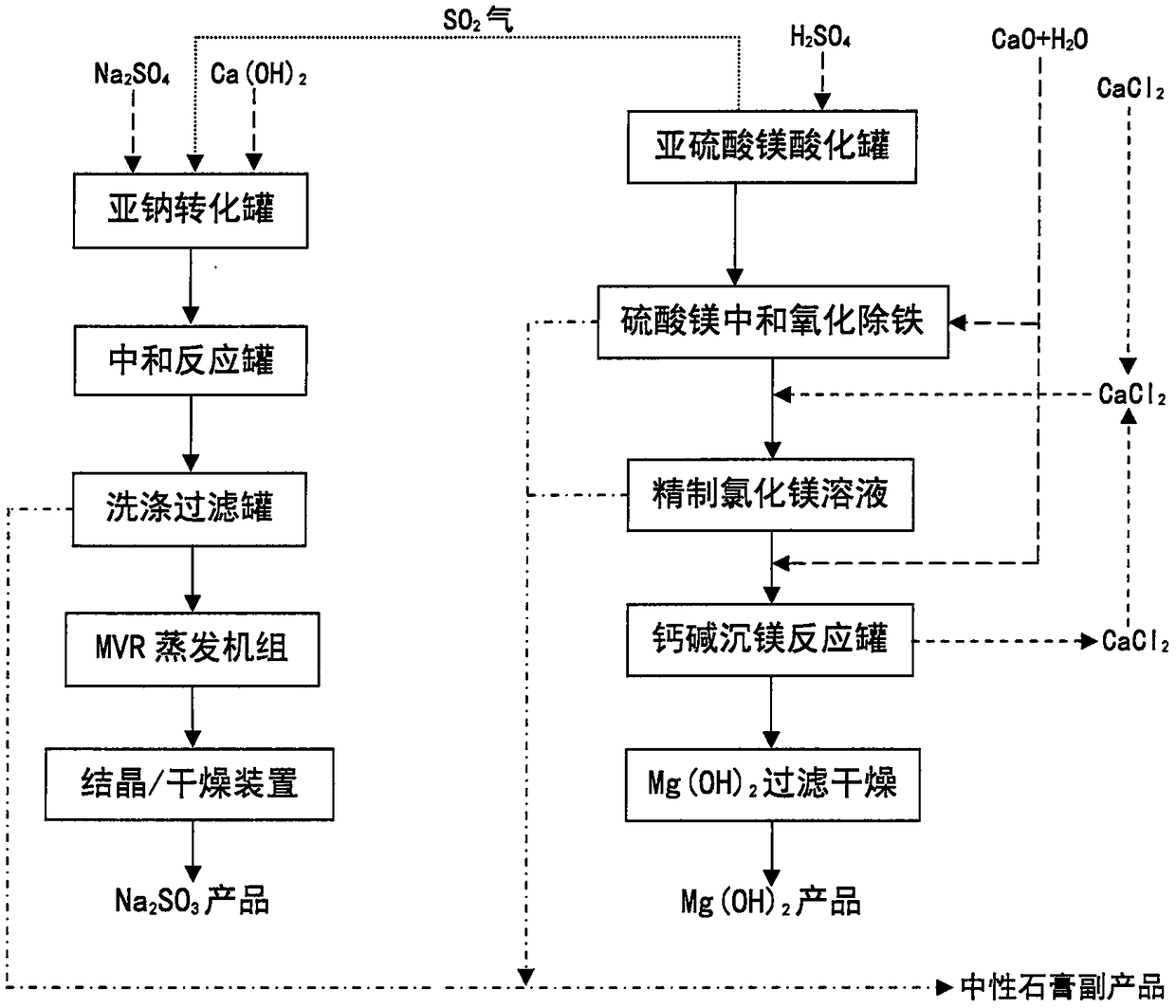
Process for preparing magnesium hydroxide and sodium sulfite by using magnesium sulfite
InactiveCN108394920AWon't affect conversion rateDoes not affect recoveryCalcium/strontium/barium sulfatesAlkali metal sulfite preparationProcess systemsSolubility
The invention discloses a process for preparing a magnesium hydroxide product and a sodium sulfite product by using magnesium sulfite, mirabilite and calcium carbide slag as raw materials. The specific process comprises: acidifying magnesium sulfite to dissolve and release sulfur dioxide gas, neutralizing and oxidizing the acidifying liquid to remove insoluble residue, respectively adding a converting agent, ammonia water, sodium hydroxide and other magnesium precipitation agents to the magnesium salt solution to generate magnesium hydroxide precipitate, and carrying out solid-liquid separation to obtain the magnesium hydroxide product, wherein the filtrate is soluble ammonium sulfate, sodium sulfate, ammonium chloride or calcium chloride and returns to the process system so as to be recycled, or lime milk is continuously added to the ammonium sulfate and ammonium chloride solution to generate ammonia and gypsum so as to regenerate and recycle the ammonia; and introducing the sulfur dioxide gas into a suspension formed from mirabilite and lime milk, carrying out a reaction to convert into sodium sulfite and gypsum precipitate, and carrying out solid-liquid separation to obtain thesodium sulfite product, wherein the filter cake is washed to obtain the by-product gypsum. According to the present invention, the magnesium hydroxide product and the sodium sulfite product are prepared by using the solid waste, such that the production cost is low, and the environmental and economic benefits are significant.
Owner:孟宪昴 +1
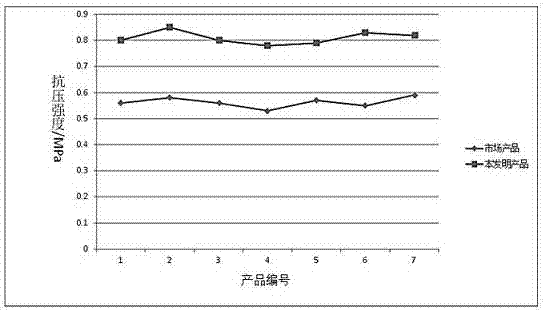
Preparation method for foam glass with high compressive strength
InactiveCN107500552AHigh compressive strengthAvoid breakingGlass shaping apparatusFoaming agentThenardite
The invention relates to a preparation method for foam glass with high compressive strength, belonging to the technical field of building materials. The foam glass comprises the following raw materials by weight: 90-94% of waste glass abrasive powder, 1.00-1.50% of a raw material additive, 0.5-2.0% of sodium hexametaphosphate, 1.0-3.0% of borax, 0.4-0.8% of thenardite, 1.5-4% of calcium carbonate, 1.8-1.9% of a foaming agent, 0.6-0.8% of a foam stabilizer, 0.2-0.4% of a flux and 0.1-0.2% of a reinforcing agent. The preparation method comprises the following steps: sufficient grinding in a conical ball mill; and melting, foaming and cooling in an electromagnetic induction heater. The foam glass prepared by using the method has high compressive strength, uniform stomatal structure, small density and low thermal conductivity.
Owner:LUDONG UNIVERSITY
A kind of method utilizing chromium-containing Glauber's salt to prepare anhydrous sodium sulfate
ActiveCN107934993BAvoid the problem of excessive hexavalent chromiumReduce the content of hexavalent chromiumChromium compoundsAlkali metal sulfites/sulfatesChemical industryChromium trioxide
The invention relates to the field of a chemical industry and particularly relates to a method for preparing anhydrous sodium sulfate from chromium-containing mirabilite as a by-product in the production process of sodium dichromate. The method comprises dissolving a certain concentration of chrome-containing mirabilite in chromium-containing waste water, adding quantitative sodium thiosulfate into the waste water to promote reduction of hexavalent chromium into trivalent chromium under 0.1-1 Mpa at 80-190 DEG C, wherein the trivalent chromium in a form of chromium trioxide hydrate is precipitated, adding a small amount of sulfuric acid to neutralize, filtering to acquire the chromium trioxide hydrate, removing impurities, carrying out decoloration, evaporating to precipitate anhydrous sodium sulfate crystals, and carrying out continuous centrifugal separation and drying. The method is simple and easy, utilizes cheap raw materials, has low energy consumption and a high automation degree and is suitable for large-scale production. The quality of the industrial anhydrous sodium sulfate product obtained by the method meets the requirements of class I products in the GB / T 6009-2014 industrial anhydrous sodium sulfate standard.
Owner:陕西省商南县东正化工有限责任公司
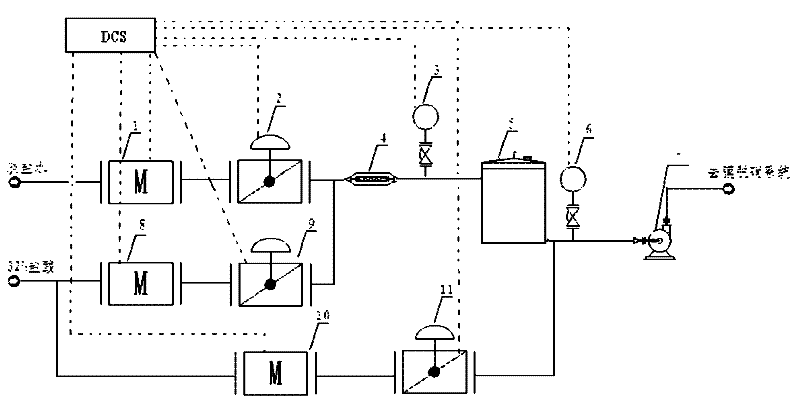
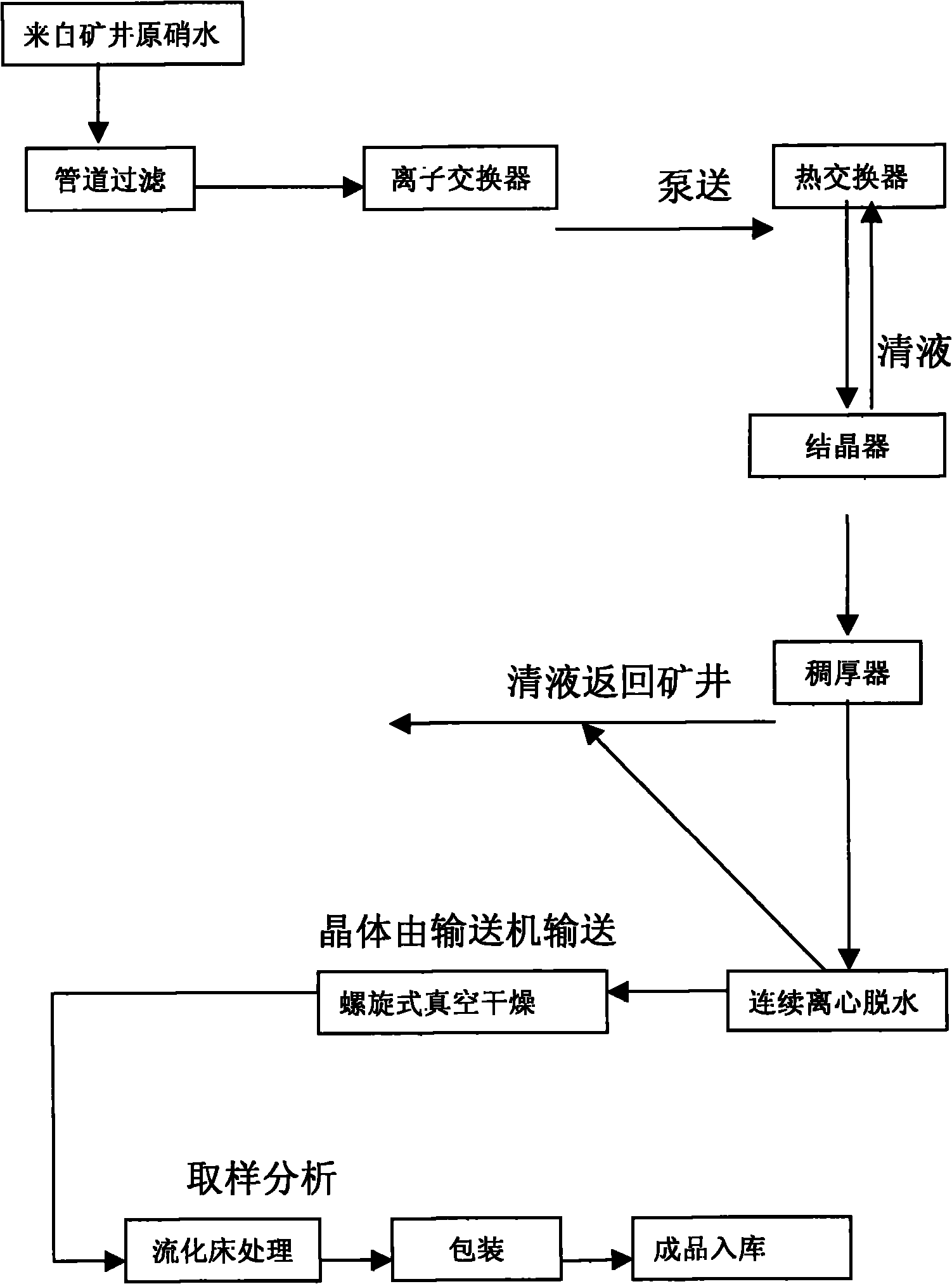
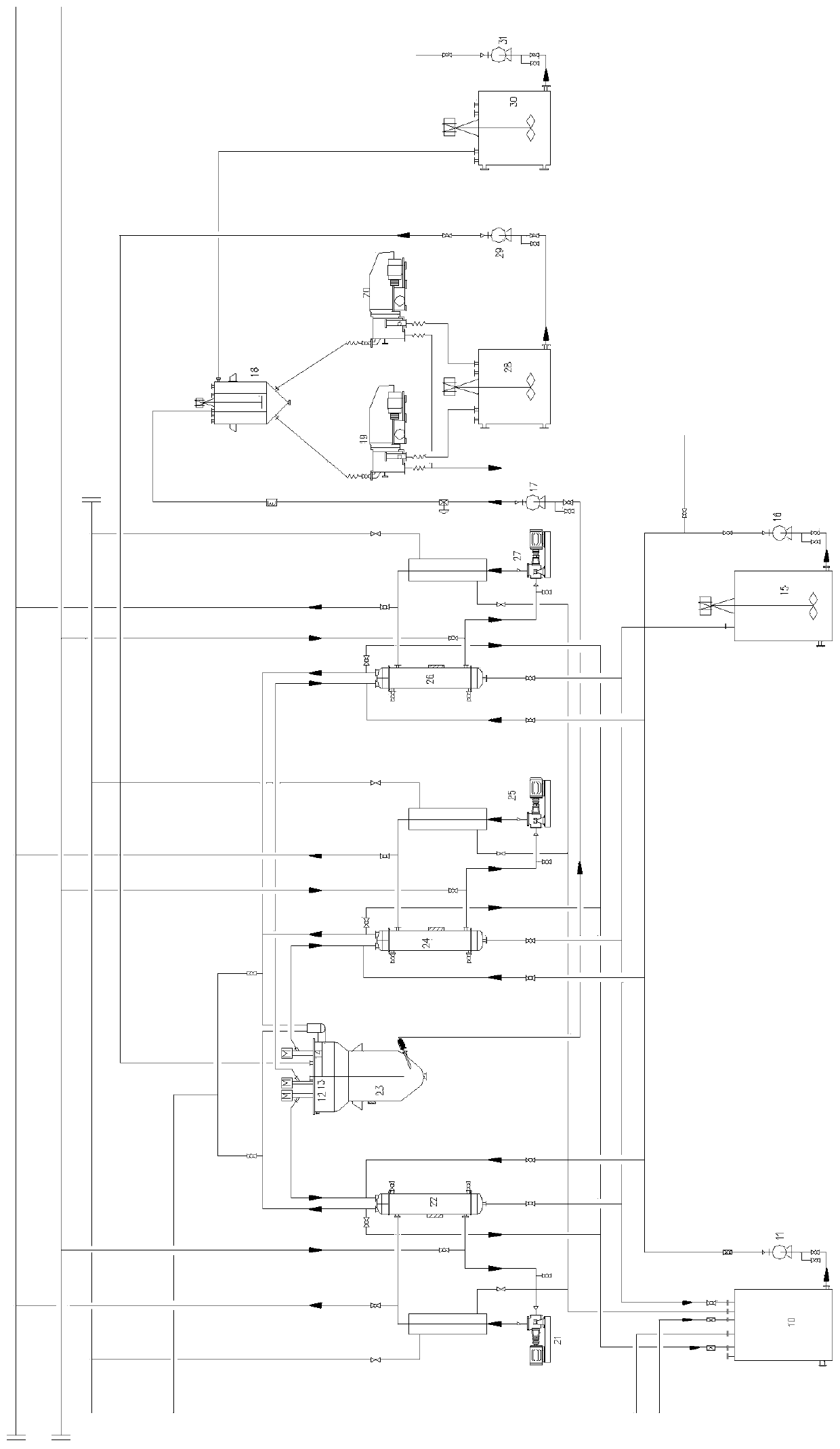
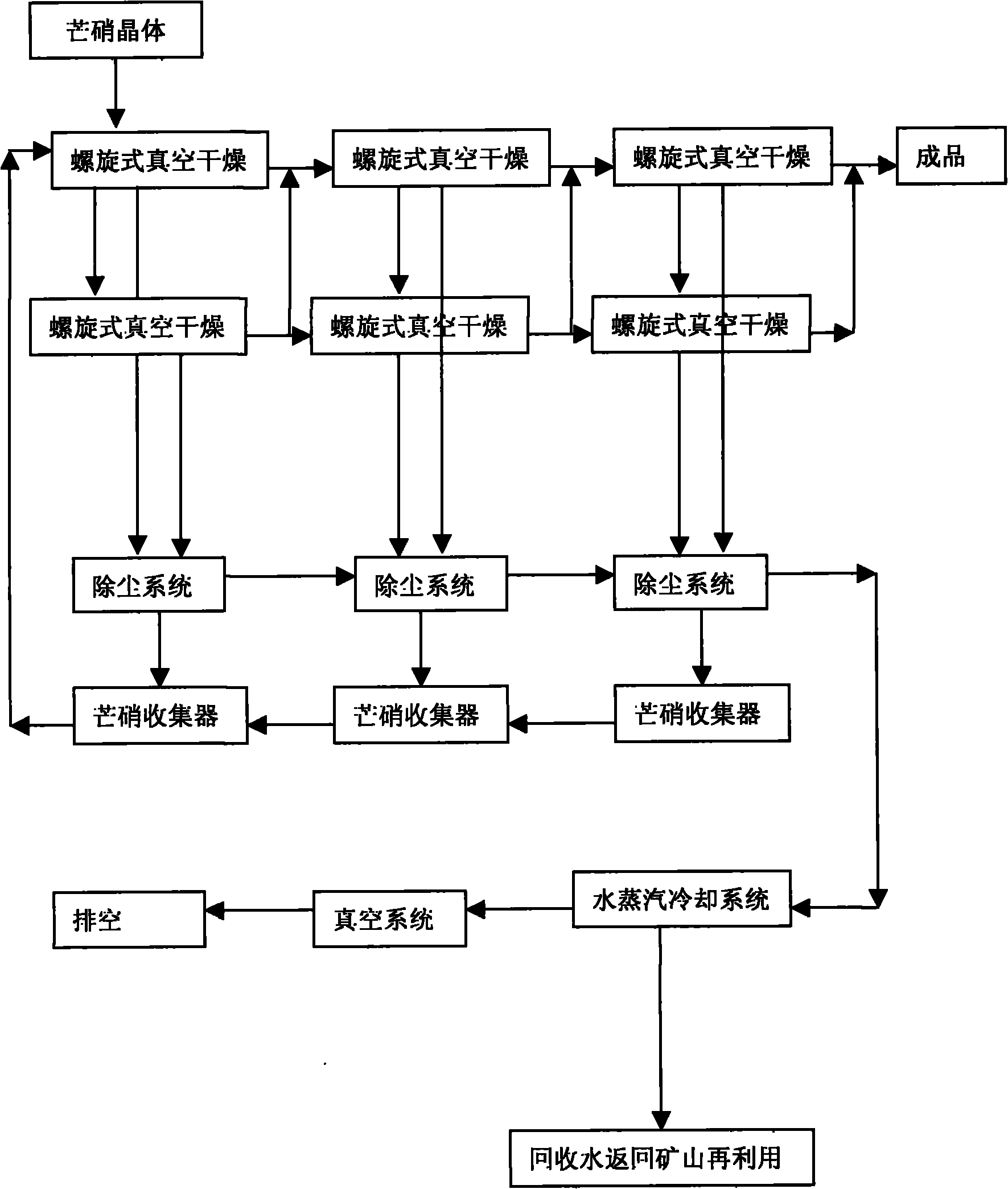
Preparation process of ultrafine particle thenardite
InactiveCN101830486ASave cleanup timeIncrease productivityAlkali metal sulfites/sulfatesProcess systemsThenardite
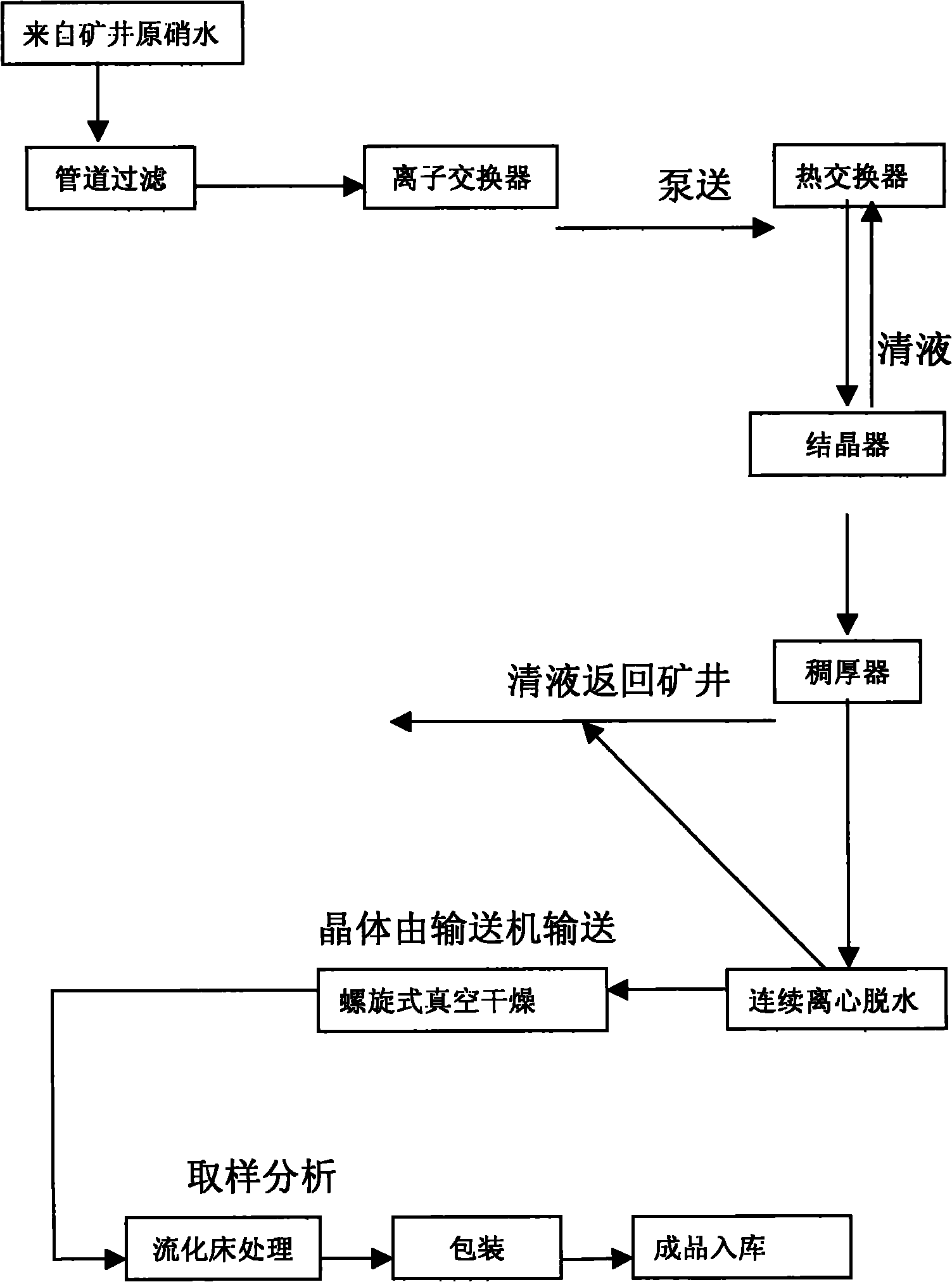
The invention discloses a preparation process of ultrafine particle thenardite. The preparation process comprises the following steps of: under the monitoring of a DCS (Distributed Control System) and the control of an AK liquid balance flowmeter, filtering raw nitrate water which as certain concentration and is conveyed underground by a first-level pipeline filter to remove mechanical impurities, refining and purifying by a four-level ion exchanger to obtain refined nitrate water with ultralow impurity content, inputting the refined nitrate water into a continuous crystallization process system, separating solid and liquid by a first-level centrifuge, then inputting the separated solution to a multi-level spiral vacuum drier to dry and remove water, and inputting the product to a fluidized bed to process to obtain ultrafine particle thenardite.
Owner:四川川眉特种芒硝有限公司
A method for extracting Glauber's salt and industrial salt from high-salt wastewater
InactiveCN105197965BLow yieldLow softening efficiencyAlkali metal chloridesAlkali metal sulfites/sulfatesThenarditeSalt-wasting
The present invention relates to the technical field of waste water treatment, extraction and recovery, and specifically relates to a method for extracting mirabilite and industrial salt from high-salt waste water. Finally, the series of steps of preparing industrial salt can extract the mirabilite and industrial salt from the high-salt wastewater for industrial reuse, and the purification rate is high, and the final wastewater can be directly discharged without polluting the environment.
Owner:东莞市英硫净水服务有限公司
Device and process for extracting mirabilite from lithium deposition mother liquor for producing lithium salt from lithium mica
InactiveCN109809436AReduce the temperatureReduce energy consumptionAlkali metal sulfite/sulfate purificationSolution crystallizationLithiumThenardite
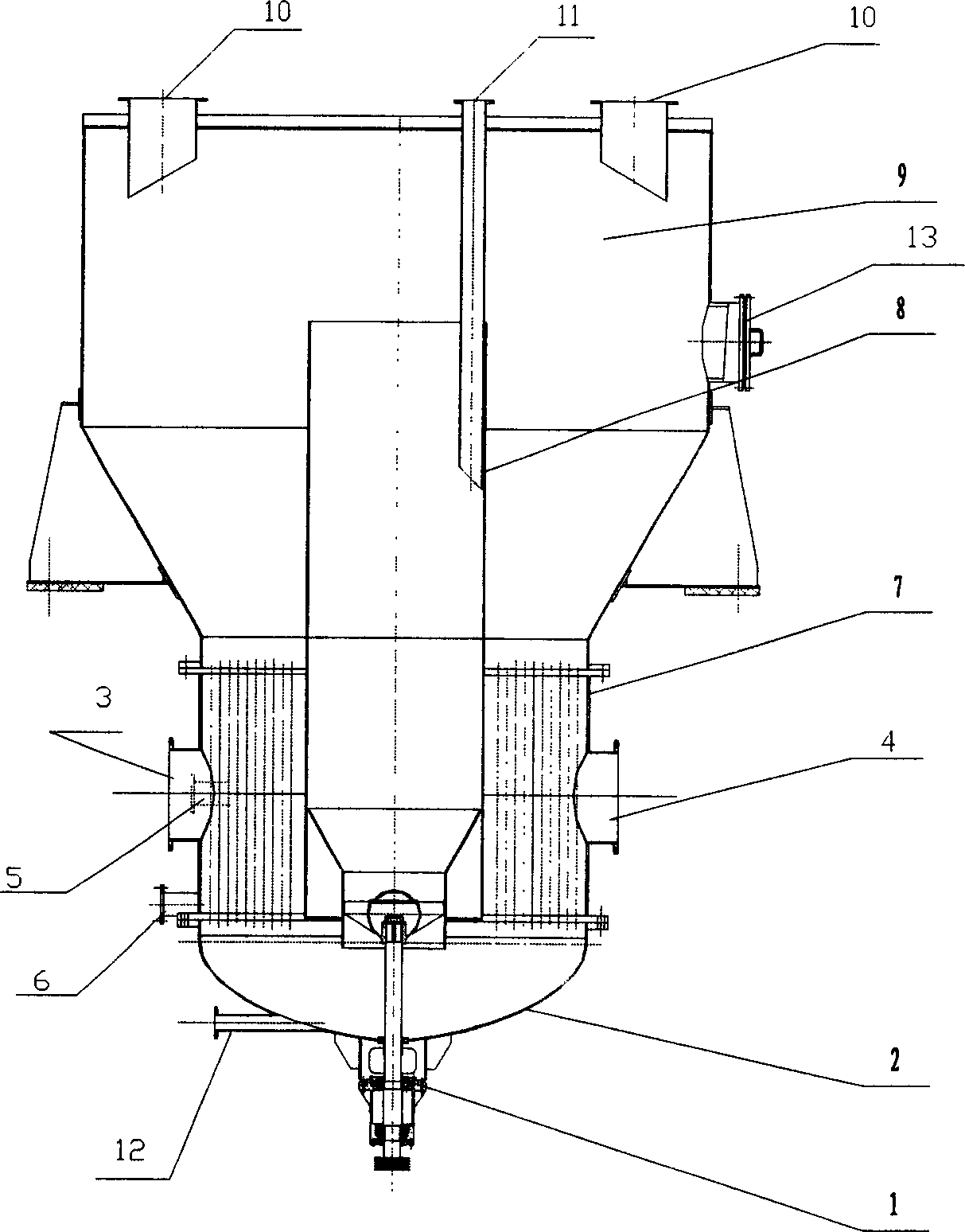
The invention relates to a device for extracting mirabilite from lithium deposition mother liquor for producing lithium salt from lithium mica, comprising a flash crystallization device and a freezingcrystallization device, wherein the flash crystallization device comprises a precooler, a flash crystallizer and a condenser, and the freezing crystallization device comprises a freeze crystallizer,an outer cooler, a thickener and a centrifuge. The invention also provides a process for extracting mirabilite from the lithium deposition mother liquor for producing lithium salt from lithium mica. Firstly the lithium deposition mother liquor is subjected to two-stage pre-cooling treatment, then mirabilite is obtained by recovering in the ways of flash crystallization and freeze crystallization,and a crystallization control scheme, anti-blocking measures and a cleaning system are arranged in the freezing crystallization system. Compared with a conventional process, on one hand, the process greatly reduces the system energy consumption, and on the other hand the risk of equipment clogging during crystallization is reduced.
Owner:河北云瑞化工设备有限公司
Drying process for thenardite
InactiveCN101844778ASimplify the water removal processKeep dryAlkali metal sulfite/sulfate dehydrationProcess systemsThenardite
The invention relates to a drying process for thenardite. The process comprises the following steps of: removing mechanical impurities from original mirabilite liquid with a certain concentration conveyed underground flow by using a pipeline filter and refining and purifying the original mirabilite liquid by using an ion exchanger under the monitoring of a DCS automatic monitoring system and the control of an AK liquid balancing flow meter to obtain refined mirabilite liquid with extremely low impurity content; inputting the refined mirabilite liquid into a continuous crystallizing process system; continuously performing solid-liquid separation by using a first-stage centrifugal machine to obtain mirabilite crystal; and inputting the mirabilite crystal into a multi-stage spiral vacuum drying machine for drying and removing water moisture so as to obtain the thenardite which contains 0.01 to 0.03 percent of free water.
Owner:四川川眉特种芒硝有限公司
Manufacturing process of coarse grained thenardite
InactiveCN101844779ASave cleanup timeIncrease productivityAlkali metal sulfites/sulfatesProcess systemsForeign matter
The invention discloses a manufacturing process of coarse grained thenardite, which is characterized in that under the monitoring of a DCS automatic monitoring system and the control of AK liquid balancing flow-rate meter, raw glass gall with a given concentration which is conveyed in the well is mechanically removed foreign matters by a primary pipeline filter and is precisely purified by a four-stage ion exchanger to obtain refined glass gall with low content of foreign matter, after pH value of the refined glass gall is adjusted, the refined glass gall is inputted to a continuous crystallization process system, after continuous solid-liquid separation in a primary centrifugal, the refined glass gall is re-inputted to a multilevel screw-type vacuum dryer to be dried and dehydrated and then to be conveyed to a fluidized bed to be treated, and after mirabilite with coarse grain is separated, the coarse grained thenardite is obtained.
Owner:四川川眉特种芒硝有限公司
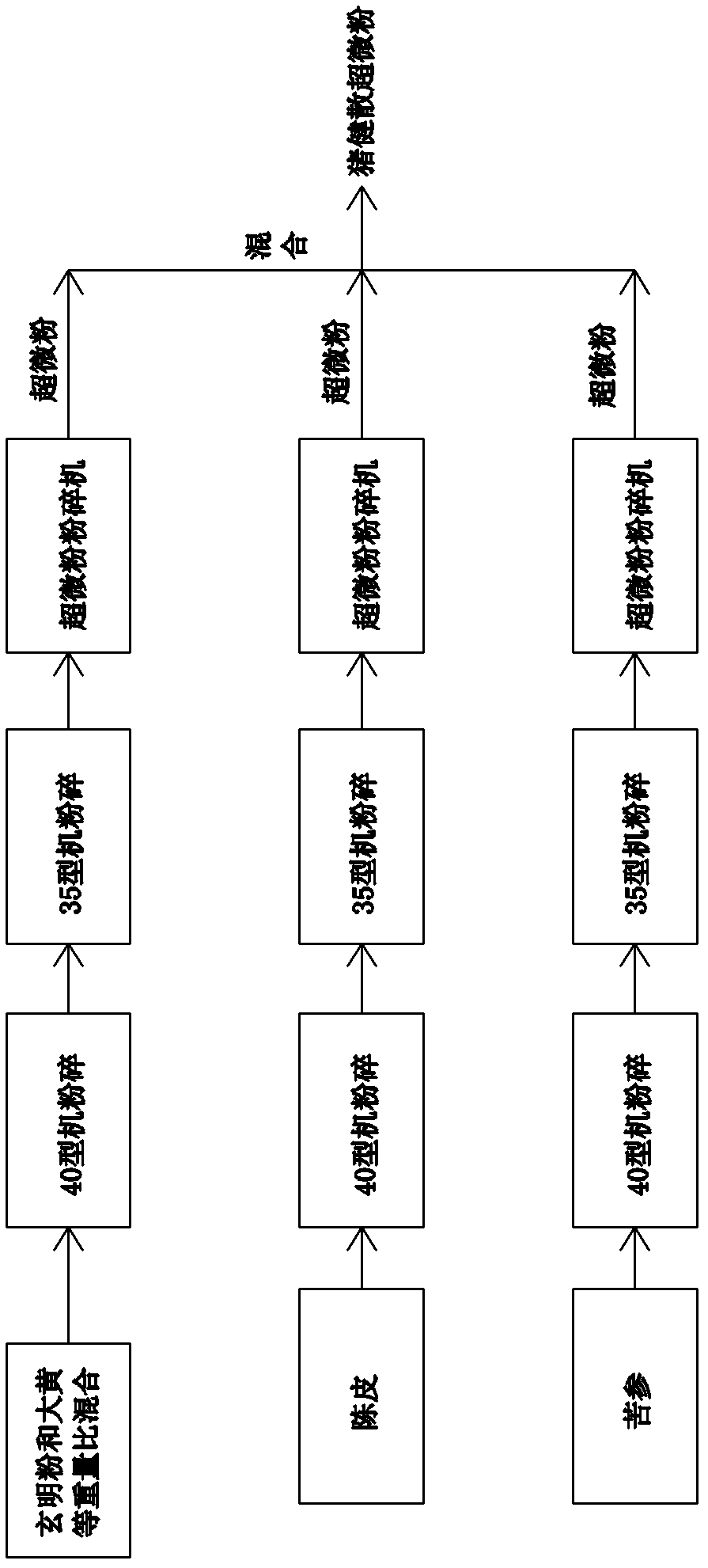
Preparation method for ultramicro powder of pig-strengthening powder
InactiveCN103316122ASmall granularityFine granularityPowder deliveryDigestive systemCelluloseThenardite
A preparation method for an ultramicro powder of a pig-strengthening powder is characterized by comprising the following steps: (1) mixing rheum officinale and thenardite in an equal weight ratio, successively passing the mixture through a 40-type pulverizer for first stage grinding, a 35-type pulverizer for second stage grinding and an ultramicro pulverizer for third stage grinding, and forming a mixed ultramicro powder of rheum officinale and thenardite; (2) successively passing dried orange peel through through the 40-type pulverizer for first stage grinding, the 35-type pulverizer for second stage grinding and the ultramicro pulverizer for third stage grinding, and forming a dried orange peel ultramicro powder; (3) successively passing sophora flavescens through the 40-type pulverizer for first stage grinding, the 35-type pulverizer for second stage grinding and the ultramicro pulverizer for third stage grinding, and forming a sophora flavescens ultramicro powder; and (4) mixing the mixed ultramicro powder of rheum officinale and thenardite, the dried orange peel ultramicro powder and the sophora flavescens ultramicro powder in ratio to form the ultramicro powder of the pig-strengthening powder. The preparation method effectively solves the problems that rheum officinale containing cellulose is hard to grind and the yield of the ultramicro powder of the pig-strengthening powder is low.
Owner:SICHUAN RONGZHOU ANIMAL PHARMA
External application anti-wind-cold medicinal liquor
InactiveCN109288897AEasy to useAnthropod material medical ingredientsRespiratory disorderPeppermintsAngelica dahurica
The invention belongs to the field of health medicinal liquor, and particularly relates to an external application anti-wind-cold medicinal liquor, which comprises, by weight, 8 g of Rhizoma Corydalis, 12 g of White muscardine silkworm, 18 g of thenardite, 5 g of Tree Peony Bark, 7 g of Herba Asari Heterotropoidedis, 11 g of Agastache rugosa leaf, 10 g of Rhizoma et Radix Notopterygii, 6 g of Angelica dahurica, 1 kg of Baijiu, 20 g of ginger juice, 15 g of Ligusticum chuanxiong hort, and 25 g of peppermint. According to the present invention, the external application anti-wind-cold medicinal liquor with effects of wind evil expelling, blood circulation activation, body resistance strengthening, evil eliminating, common cold treating and wind cold treating is provided.
Owner:谢瀚
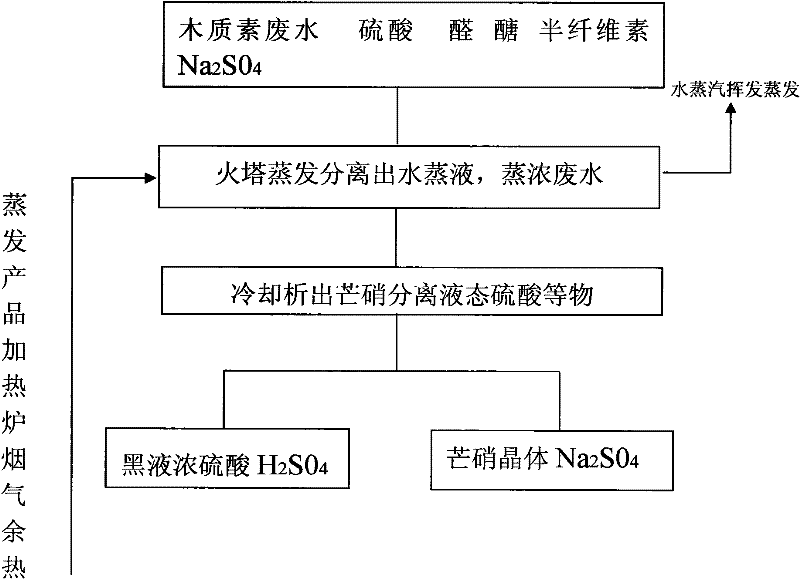
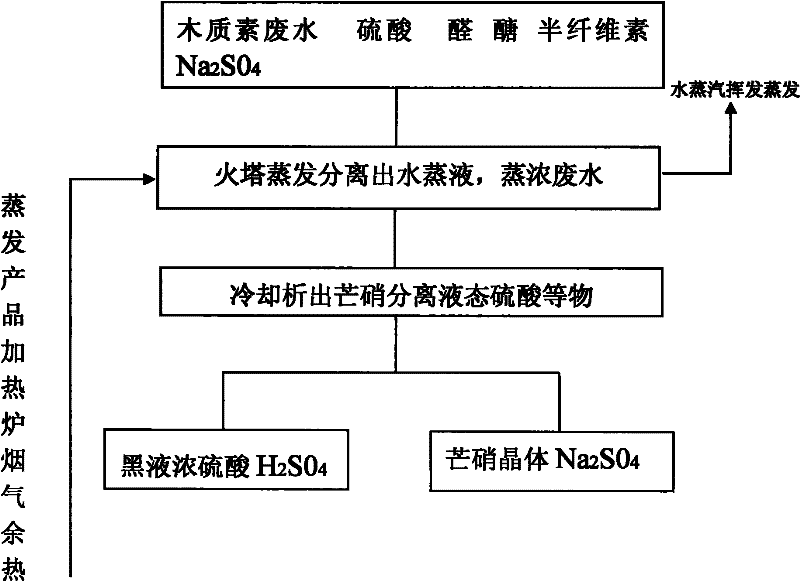
Separation method of sulfuric acid and sodium sulfate in organic sulfonate production wastewater
InactiveCN102295315AWide range of industrial applicationsHigh yieldEnergy inputSulfur-trioxide/sulfuric-acidEvaporationSodium sulfate
The invention relates to a method for separating sulfuric acid and sodium sulfate in organic sulfonate production wastewater, in particular to a method for separating sulfuric acid and sodium sulfate in lignin wastewater. The waste water is heated to evaporate the water; after the evaporated waste liquid enters the circulation pool, it is circulated and evaporated by the circulation pump, and when the concentration of the evaporated waste liquid reaches 24-25 Baume When the temperature is high, it enters the cold circulation pool to separate the thenardite and the black liquor containing sulfuric acid. Heating-evaporation is carried out by using the residual heat of the flue gas, and the temperature of the residual heat of the flue gas is 170°C-270°C. After cooling for many times, Na2SO4·10H2O Na2SO4 · 10H2O mirabilite needle-like white crystals are fully separated. After Glauber's salt is taken out in the circulating pool, the rest is mainly sulfuric acid solution.
Owner:HUBEI SHENGHUA RENEWABLE ENERGY DEV
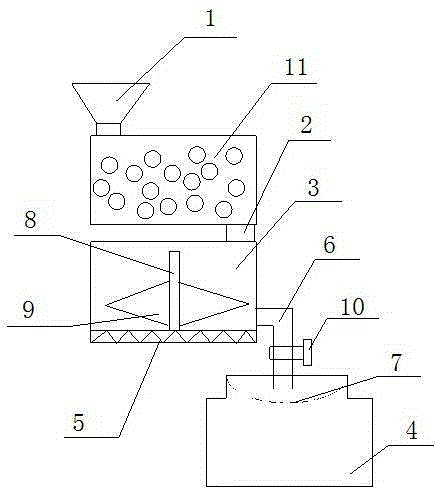
Equipment and method for producing high-purity sodium sulfate from mirabilite ore
InactiveCN106745088AHigh puritySimple structureAlkali metal sulfite/sulfate purificationThenarditeFiltration
The invention discloses equipment for producing high-purity sodium sulfate from mirabilite ore. The equipment comprises a grinding device, an impurity removing device and an air drying device, wherein the top portion of the grinding device is provided with a material feeding port and the bottom portion is provided with a material outlet, the material outlet is connected to the impurity removing device, the impurity removing device comprises a dissolving box, the bottom portion of the dissolving box is provided with a heater, a stirrer is arranged inside the dissolving box, the dissolving box is further provided with a liquid discharging pipe, the liquid discharging pipe is communicated to the air drying device, and a filtration device is arranged between the liquid discharging pipe and the air drying device. The invention further provides a method for producing high-purity sodium sulfate from mirabilite ore, wherein the method comprises grinding, dissolving, precipitation filtration, air drying, and other steps. According to the present invention, the structure and the method are simple, and the produced sodium sulfate has the high purity.
Owner:HUNAN HENGYANG XINLI CHEM
Recycling processing technique for industrial wastewater from anhydrous sodium sulphate production
InactiveCN106219575AAchieving zero emissionsReduce consumptionEnergy inputAlkali metal sulfites/sulfatesThenarditeFlue gas
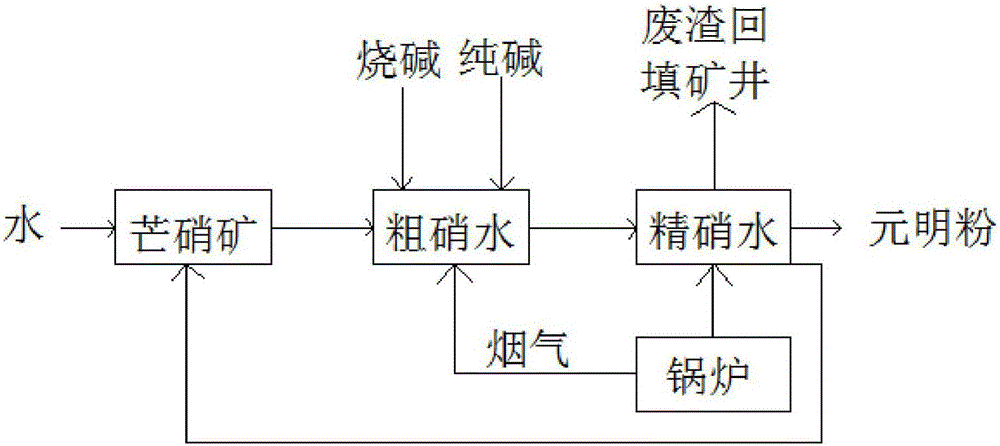
The invention discloses a recycling processing technique for industrial wastewater from anhydrous sodium sulphate production. The technique comprises the following steps: (1) firstly soaking glauber salt ore in water, thereby acquiring rough liquid glauber salt; (2) adding caustic soda and sodium carbonate into the rough liquid glauber salt, thereby acquiring fine liquid glauber salt; (3) heating the fine liquid glauber salt, crystallizing for acquiring the anhydrous sodium sulphate powder product, and separating solid from liquid, thereby acquiring the waste residue and waste water; (4) backfilling the waste residue into a mining well and returning the waste water to the step of soaking sodium sulfafe decahydrate in water. According to the invention, the waste water and steam condensate after the fine liquid glauber salt is crystallized and subjected to solid and liquid separation are used for soaking the sodium sulfafe decahydrate, so that the zero emission of the waste water is realized. Besides, the waste heat of the flue gas of the boiler is utilized to heat the rough liquid glauber salt, so that the energy consumption is saved.
Owner:四川省洪雅青衣江元明粉有限公司
Environment-friendly refining method of sodium sulfide
ActiveCN109455676AIncrease contact areaIncrease productionAlkali metal sulfides/polysulfidesThenarditeEvaporation
The invention discloses an environment-friendly refining method of sodium sulfide. The method is high in production efficiency and less in environmental pollution, and can be used for obtaining the high-purity sodium sulfide product. The method comprises the following steps: (1) evenly mixing pulverized coal and anhydrous thenardite according to a mass ratio of 1 to (3-4), and then calcining at 70-1000 DEG C to generate a rough blank of the sodium sulfide; (2) filling with a thermalized container with billet water at the room temperature, and dissolving the hot rough blank of the sodium sulfide in the billet water at the normal temperature to form heated billet water; when every liter of the heated billet water in the thermalized container contains 370g of the sodium sulfide or more, introducing new low-concentration cleaning alkali sulfide water or clear water from the bottom of the thermalized container, and enabling the heated billet water at the upper part of the thermalized container to be replaced into a sedimentation tank of the next process; (3) settling the sodium sulfide liquid subjected to blank formation in the sedimentation tank, and screening out the primary refined sodium sulfide; (4) enabling the primary refined sodium sulfide to be subjected to evaporation extraction to obtain the sodium sulfide crystals.
Owner:范冲天
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com