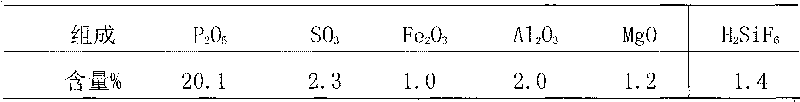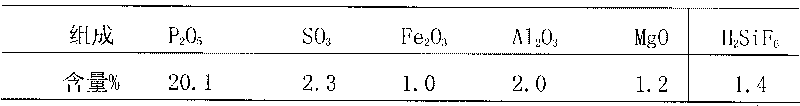Patents
Literature
341 results about "Hexafluorosilicic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hexafluorosilicic acid is an inorganic compound with the chemical formula (H₃O)₂SiF₆ (also written as (H₃O)₂[SiF₆]). It is a colorless liquid rarely encountered undiluted. Hexafluorosilicic acid has a distinctive sour taste and pungent smell. It is produced naturally on a large scale in volcanoes. It is manufactured as a coproduct in the production of phosphate fertilizers. The resulting hexafluorosilicic acid is almost exclusively consumed as a precursor to aluminum trifluoride and synthetic cryolite, which are used in aluminium processing. Salts derived from hexafluorosilicic acid are called hexafluorosilicates.
Method for producing fluorine series compounds and white carbon black
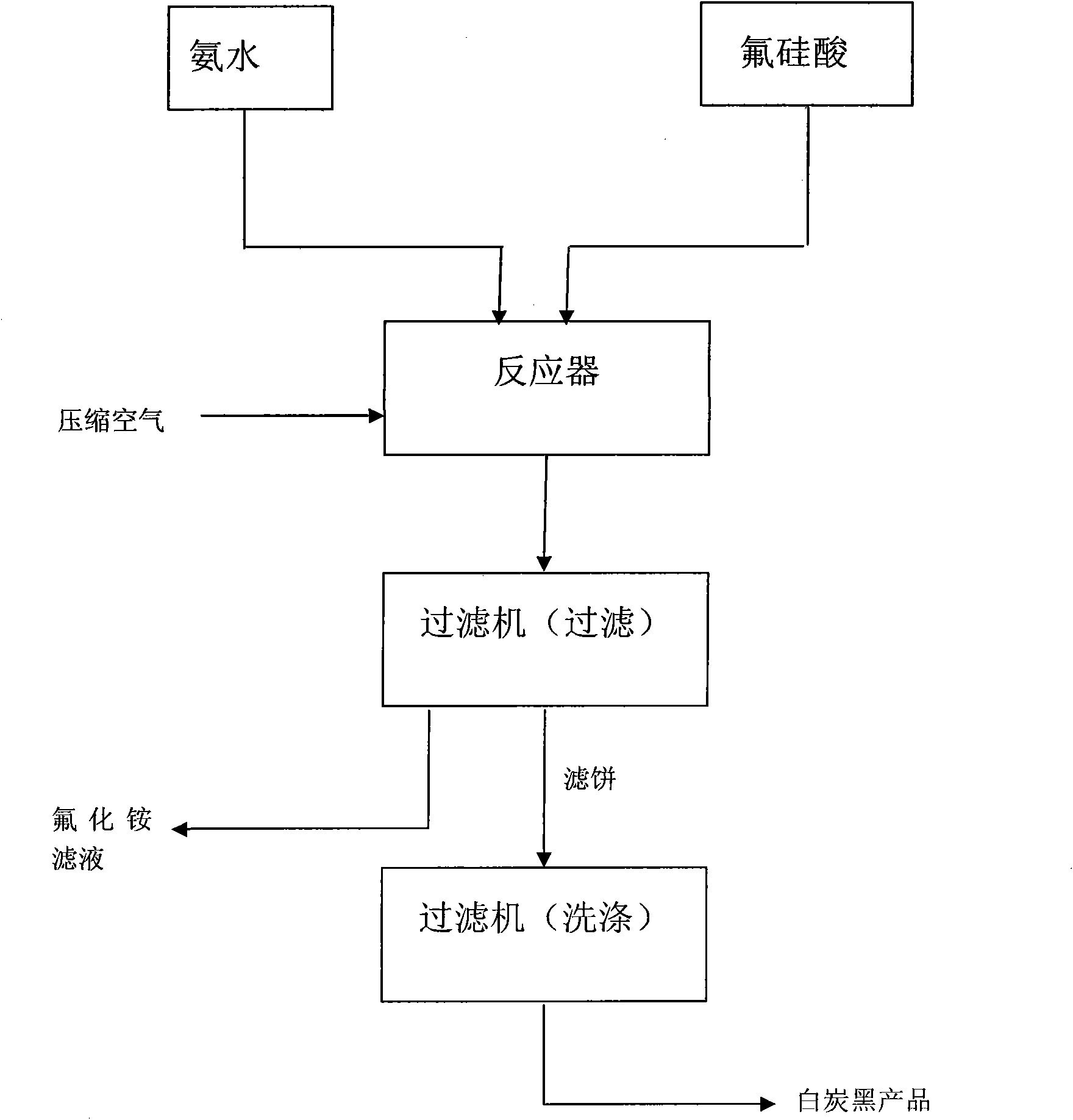
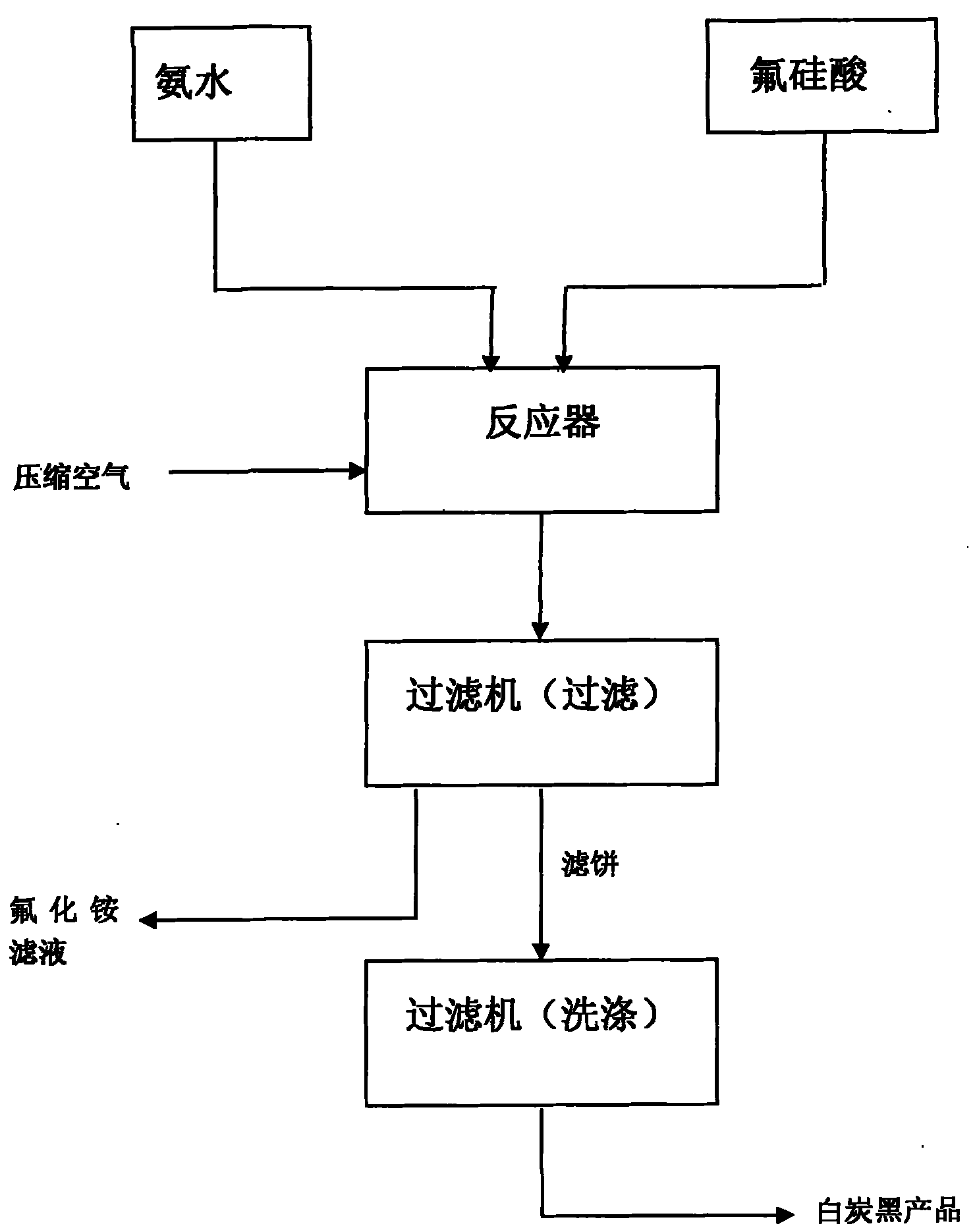
The invention relates to a method utilizing waste gas containing fluoride in fertilizer production or fluorine-containing gas or sodium fluorosilicate in fluorine chemical production as raw materials to product series compounds of fluorine and white carbon black. The ammonium fluoride and / or ammonia are / is introduced into an absorption system to get a (NH4) 2SiF6 solution with the absorbing solution concentration of 25 to 37 percent or the ammonium fluoride and the sodium fluorosilicate react to get a reaction product of sodium fluoride crystallization, fluosilicic acid solution and septenary-fluorine compound NH4F.(NH4)2SiF6 crystallization. The absorption liquid or the reaction product is aminated to get precipitated silica (white carbon black) with the specific surface of 100-180m<2> / g, and the latter can get a sodium fluoride product and an NH4F solution with the concentration of 30 to 45 percent. The solution used as a starting point can nearly prepare all inorganic fluoride chemical products, and ammonium fluoride, sodium fluoride, potassium fluoride, ammonium bifluoride, cryolite, aluminum fluoride, sodium hydrogen diffluoride and hydrofluoric acid production, etc. are main. The method has simplicity, effectiveness and higher economic benefit, and simultaneously, fluorine and silicon in the waste gas containing fluoride of phosphate fertilizer for the pollution of environment can be basically eliminated, and basically no waste water, waste gas and waste residue are discharged.
Owner:夏克立
Processes for preparing color stable manganese-doped phosphors
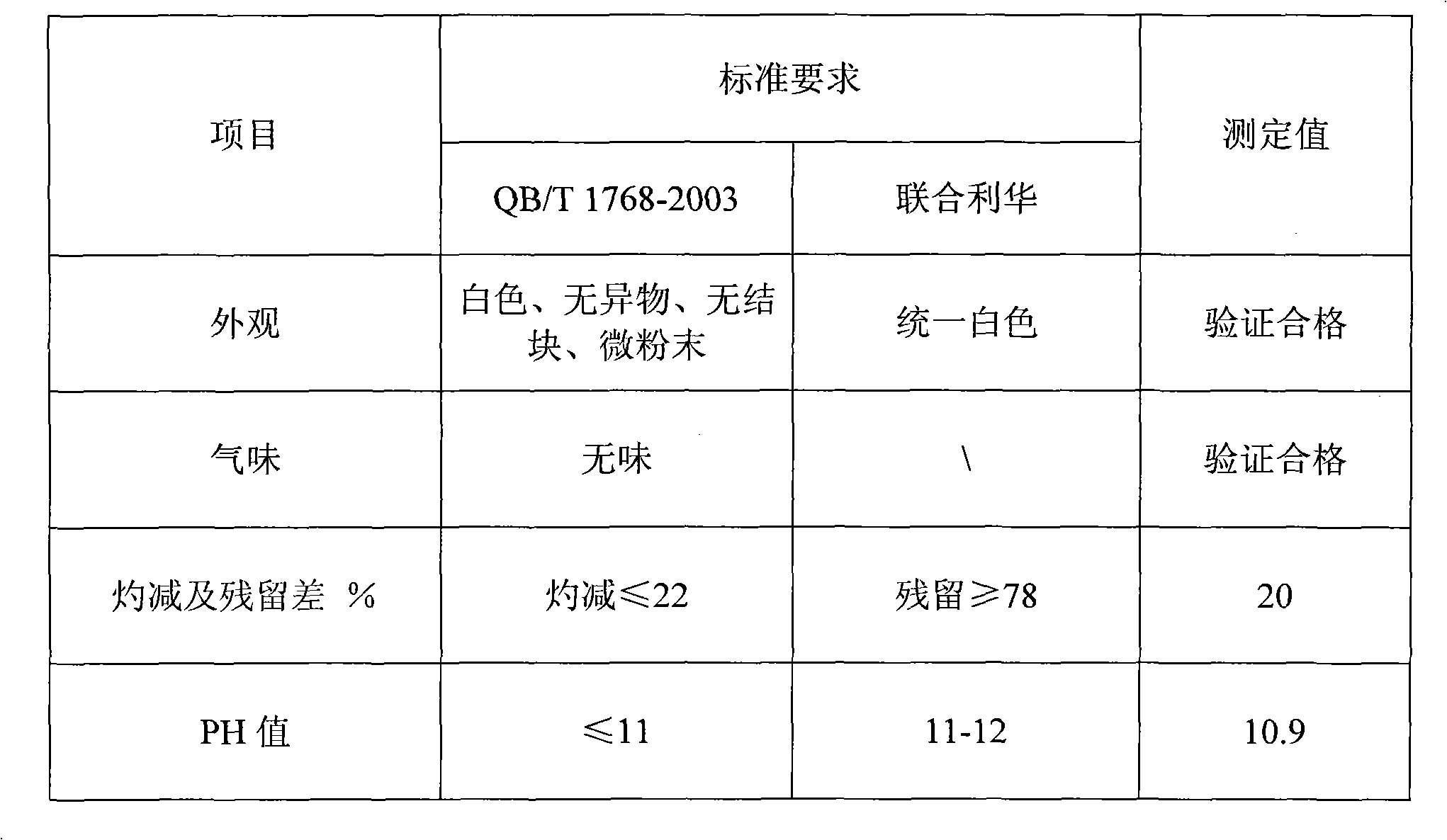
ActiveUS20150054400A1Improved color stabilityGood colorDischarge tube luminescnet screensLamp detailsPhosphorHexafluorosilicic acid
Low-HF or HF-free processes for improving color stability of a Mn+4 doped phosphor of formula I include contacting the phosphor of formula I with a solution that contains hexafluorosilicic acid, and isolating a treated phosphor of formula I having improved color stability relative to an untreated phosphor of formula IAx[MFy]:Mn+4 (I)whereinA is Li, Na, K, Rb, Cs, R4 or a combination thereof;M is Si, Ge, Sn, Ti, Zr, Al, Ga, In, Sc, Y, La, Nb, Ta, Bi, Gd, or a combination thereof;R is H, lower alkyl, or a combination thereof;x is the absolute value of the charge of the [MFy] ion; andy is 5, 6 or 7.
Owner:GE LIGHTING SOLUTIONS LLC
Method for absorbing and extracting phosphor and fluorin from phosphoric acid produced in kiln method
InactiveCN101049920AImprove separation rateHigh puritySilicon halogen compoundsPhosphoric acidHigh concentrationAbsorption column
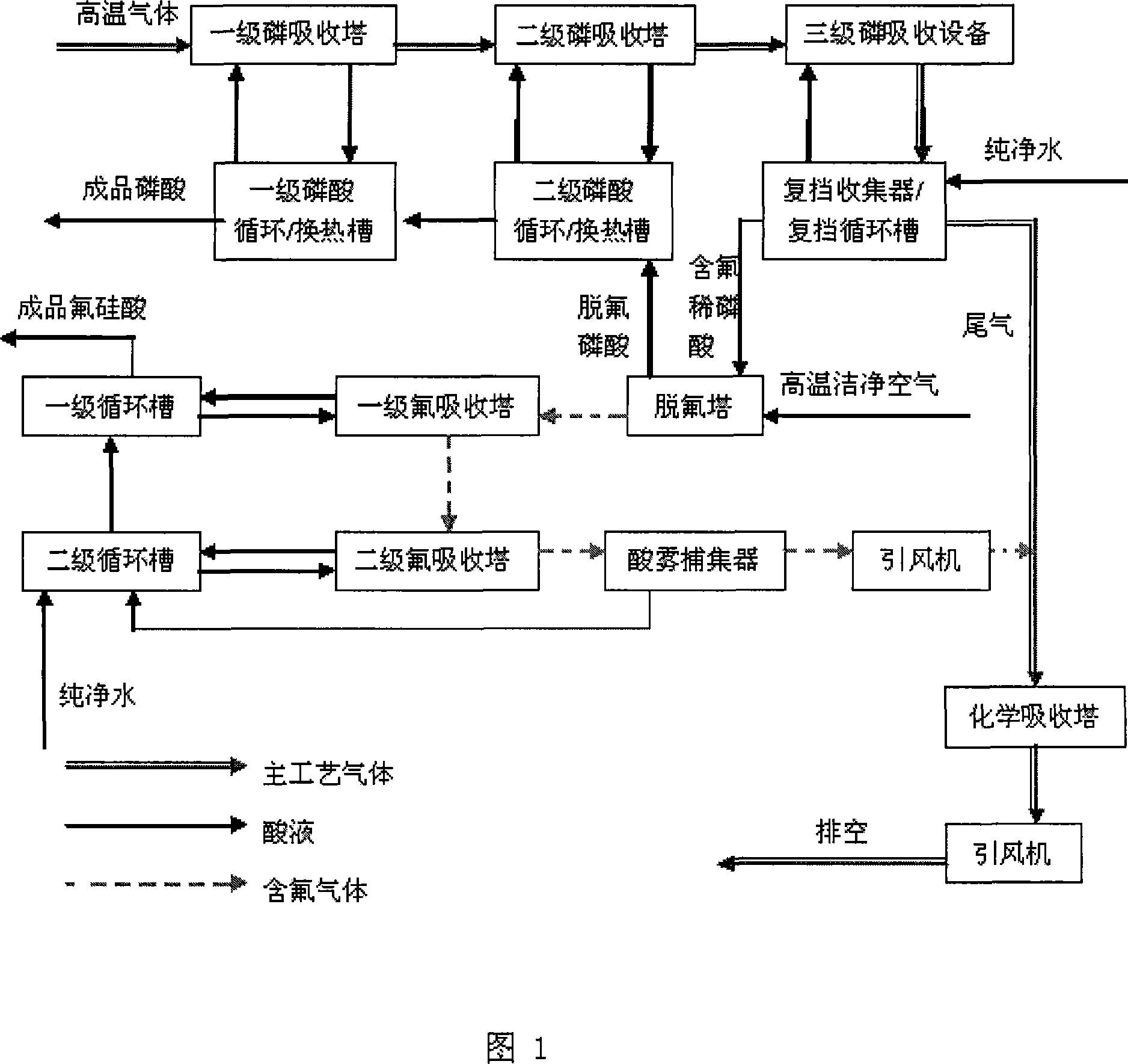
This invention discloses a method for separating phosphorus and fluorine of kiln process phosphoric acid by absorption. The method comprises: sending relatively high temperature phosphoric acid after hydration in primary and secondary hydration columns to a circulation tank for cooling, pumping into the hydration columns for circulation, sending gases after primary and secondary absorption to a Venturi absorber for re-absorption, collecting the residual acid mist by a collector, sending the absorbed gas and tail gas after fluorine absorption together to a chemical absorption column, and exhausting the tail gas. Besides, qualified fluosilicic acid can be obtained from the fluorine-containing diluted H3PO4 in the collector. The method utilizes the air with residual heat in the kiln, and has such advantages as no extra energy consumption, high phosphorus and fluorine separation efficiency, high concentration and low fluorine content in obtained H3PO4, and low P2O5 content in obtained fluosilicic acid. The obtained fluosilicic acid has a wide concentration range and a high purity, and is easy to utilize.
Owner:HUBEI SANXIN PHOSPHORIC ACID
Addition product, production and use thereof as corrosion inhibitor
InactiveUS20040168748A1Rapid and lasting inhibitionOrganic chemistrySolid state diffusion coatingHexafluorotitanic acidHexafluorosilicic acid
The invention relates to an addition product that can be produced from hexafluorosilicic acid, hexafluorotitanic acid, and / or hexafluorozirconic acid by an acid-base reaction with one or several organic bases and a method for production and use thereof. The addition products according to the invention guarantee a rapid and lasting inhibition of corrosion processes; they are in particular suitable for inhibiting the corrosion of light metals.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
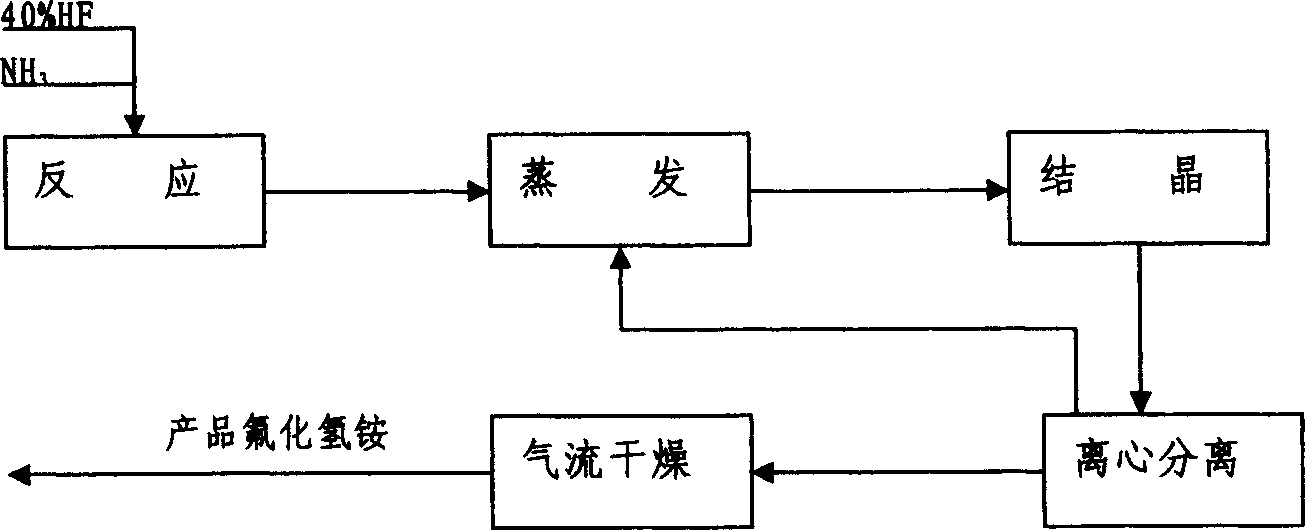
Anhydrous hydrogen fluoride production method
ActiveCN103350985AImprove conversion rateLow costFluorine/hydrogen-fluorideHydrogen fluoridePhysical chemistry
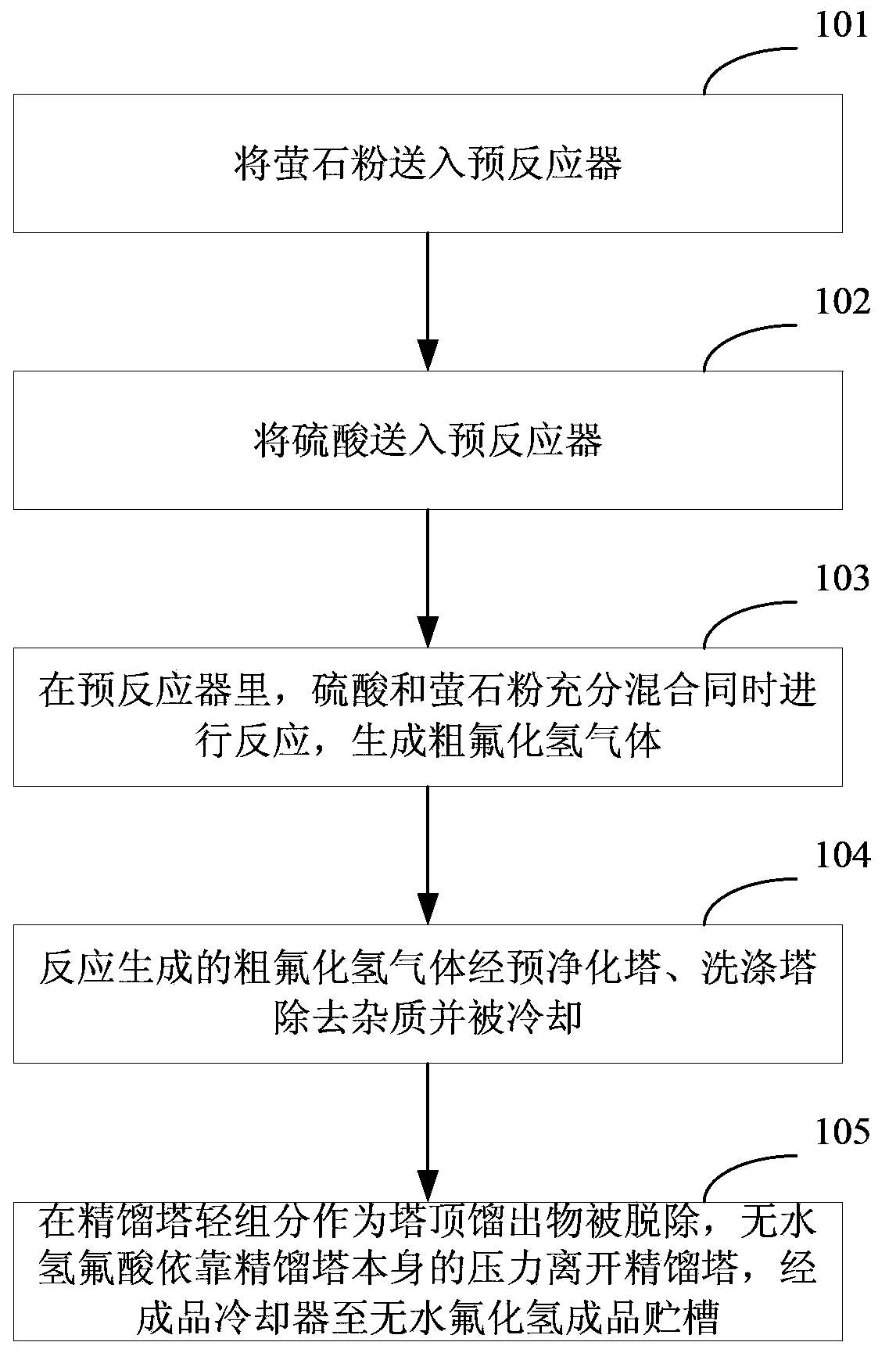
The invention discloses an anhydrous hydrogen fluoride production method which comprises the following steps: conveying fluorite powder and sulfuric acid to a pre-reactor, fully mixing the fluorite powder and the sulfuric acid and enabling the fluorite powder and sulfuric acid to react to generate coarse hydrogen fluoride gas; removing impurities from the coarse hydrogen fluoride gas through a pre-purifying tower and a washing tower, cooling the coarse hydrogen fluoride gas, and conveying the coarse hydrogen fluoride gas to a rectifying tower for refining; enabling the anhydrous hydrofluoric acid to get out of the rectifying tower with the help of the pressure of the tower, and leading the anhydrous hydrofluoric acid to an anhydrous hydrofluoric acid finished product storage tank through a finished product cooler. According to the invention, the hydrogen fluoride discharged from the gas can be recycled, the conversion rate of the product is increased, the cost is lowered, and useful by-products such as gypsum and fluosilicic acid are produced.
Owner:ANHUI JINYANG FLUORINE CHEM
Process method for producing fluorine compounds and silicon compounds by cleanly utilizing fluosilicic acid
ActiveCN101913637AHigh recovery rateTake advantage ofSilicaAlkali metal silicatesChemical industrySlag
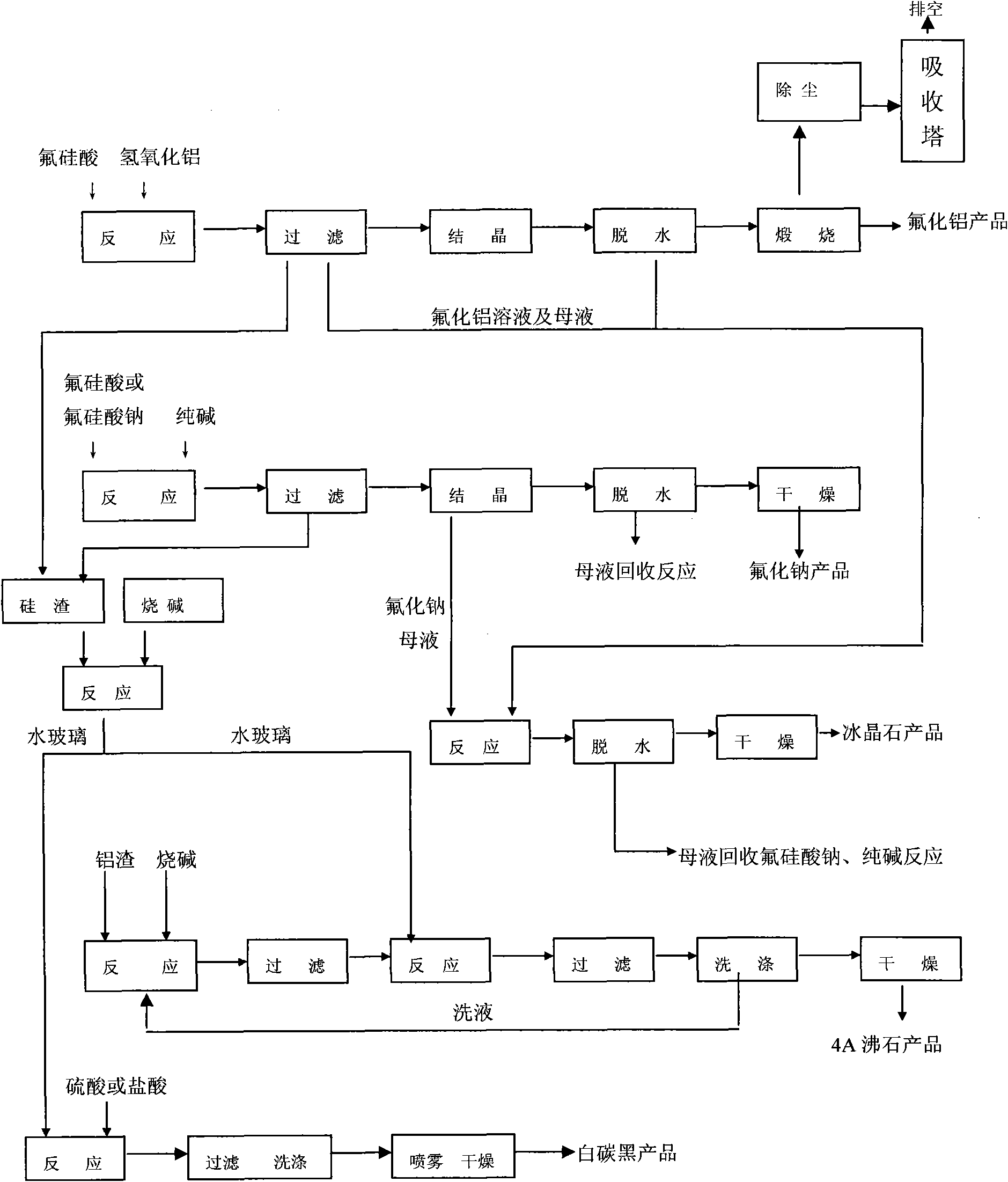
The invention discloses a process method for producing fluorine compounds and silicon compounds by cleanly utilizing fluosilicic acid. The process method comprises the following steps of: preparing fluosilicic acid as a phosphorus chemical by-product into an anhydrous aluminum fluoride product, a sodium fluoride product and a cryolite product, and combining with other industrial waste silicon slags and aluminum slags to prepare a 4A zeolite product. The process method comprehensively utilizes the fluosilicic acid and has high resource recovery rate, wherein the recovery rate of the fluorine element reaches higher than 90 percent. Waste silicon dioxide slags generated in the process can be recycled to produce white carbon black and the 4A zeolite, and a mother liquor, a cleaning solution and waste gas which are generated in the production process are all recycled, thereby the environmental pollution is reduced, and the purposes of zero emission and no pollution are truly achieved, thus the method completely meets the requirement for clean production. The invention has the advantages of advanced production process, good product quality and high value, wherein the fluorine content of the aluminum fluoride is high and between 63-65 percent; the quality index of the obtained white carbon black meets the requirement on the standard of the chemical industry; and the quality of the 4A zeolite product meets the requirements on the national standard and the standard of European and American developed countries.
Owner:四川励志环保科技有限公司
Technique for preparing waterless hydrogen fluoride on high purity
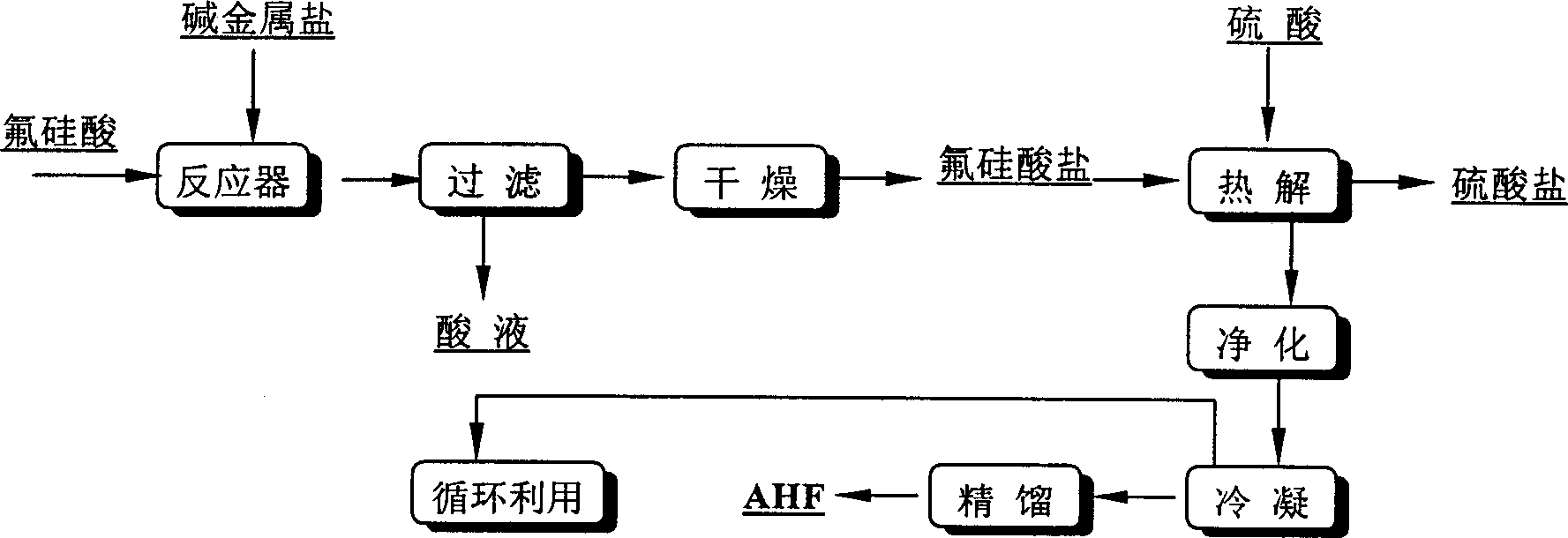
A process for preparing high-purity anhydrous hydrogen fluoride from the fluorosilicic acid as industrial by-product and concentrated sulfuric acid features that the fluorosilicid acid is deposited out in salt mode and the traditional fluorespar approach is then used. The generated sulfate as by-product can be used as precipitant of fluorosilicic acid system.
Owner:SICHUAN UNIV
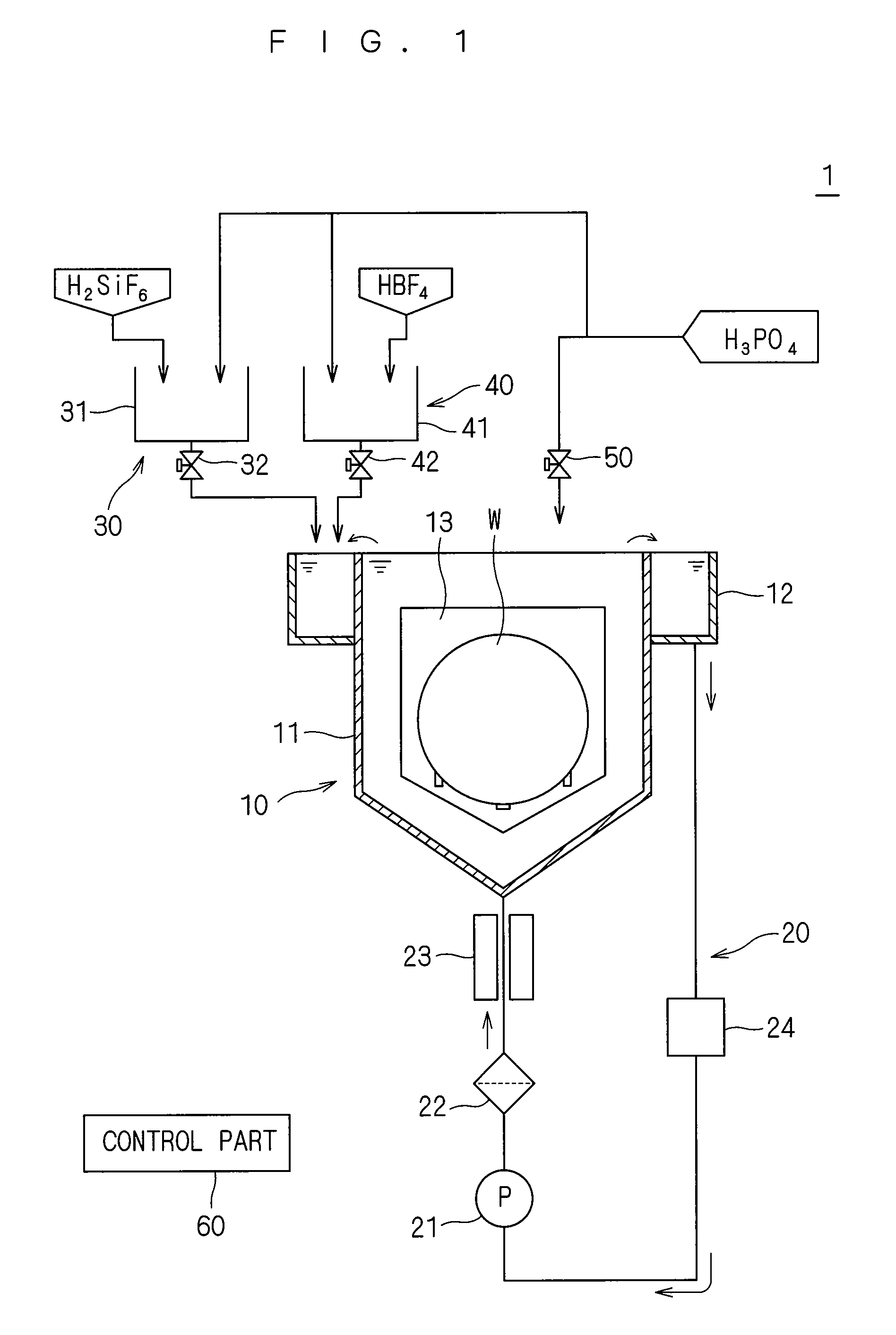
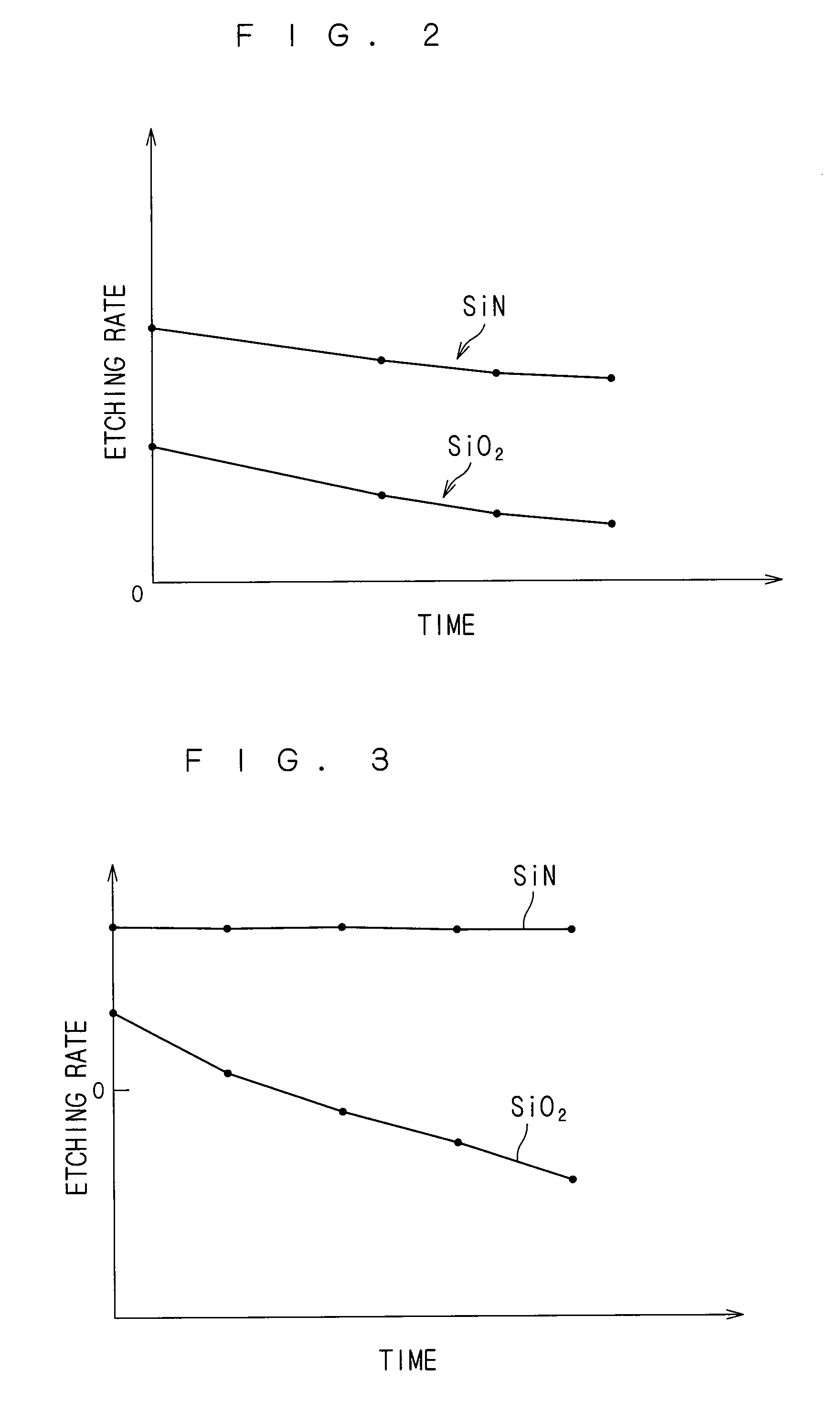
Substrate processing apparatus and substrate processing method for performing etching process with phosphoric acid solution
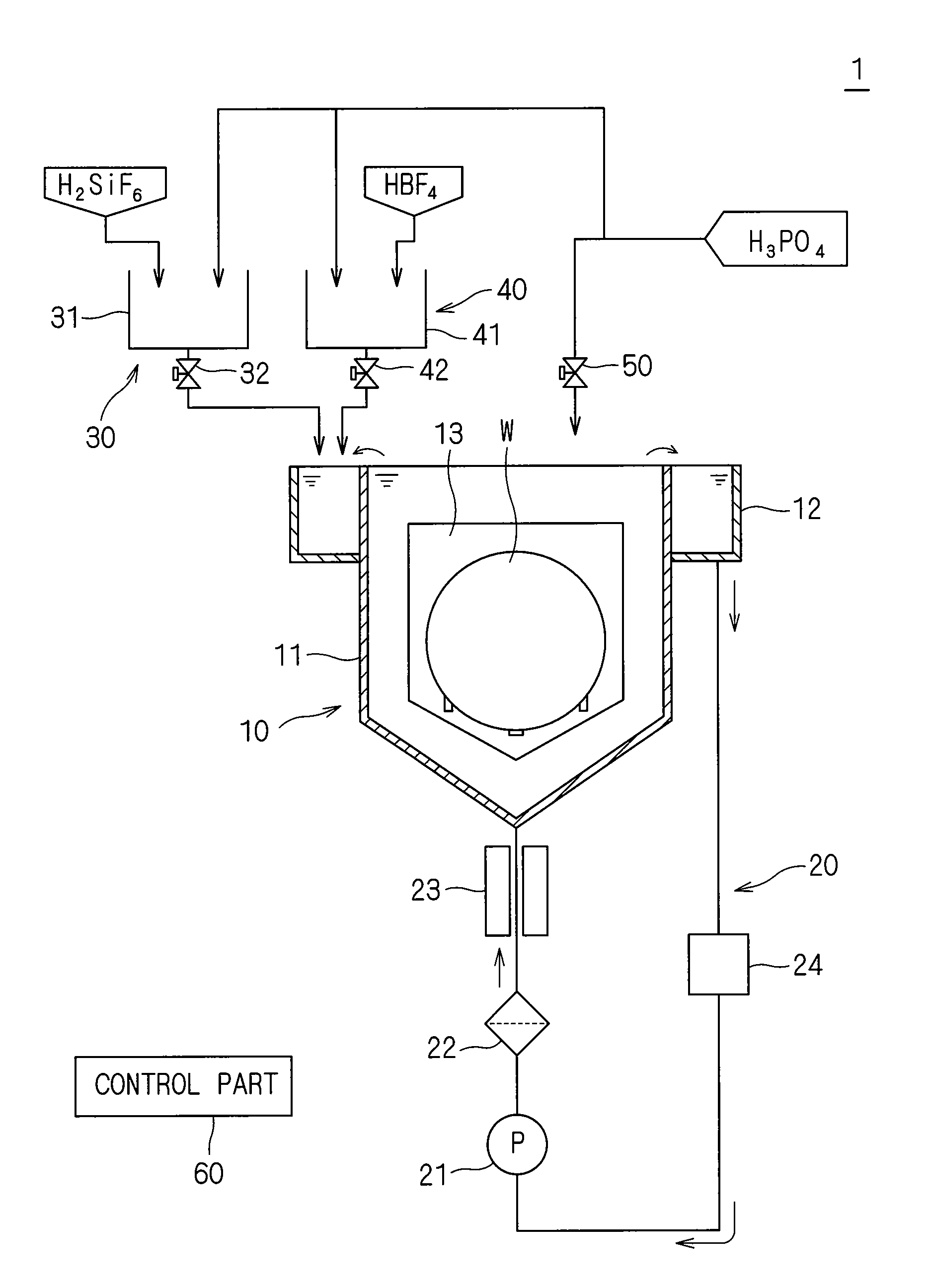
ActiveUS20090081881A1Constant characteristicConstant solutionDecorative surface effectsSemiconductor/solid-state device manufacturingDecompositionPhosphoric acid
An additive containing a hexafluorosilicic acid solution (H2SiF6+H2O) is sequentially inputted into a phosphoric acid solution pooled in an immersion bath from an additive input mechanism. Further, a trap agent containing a fluoroboric acid solution (HBF4+H2O) is inputted into the phosphoric acid solution from a trap agent input mechanism. F− which accelerates etching of a silicon nitride film is added as appropriate by sequentially inputting the additive and siloxane which increases by the sequential input is etched with hydrofluoric acid generated by decomposition of the fluoroboric acid, to thereby suppress a significant increase in the concentration of siloxane. This makes it possible to maintain respective initial etching rates of the silicon nitride film and a silicon oxide film.
Owner:DAINIPPON SCREEN MTG CO LTD
Process for removing chromide coatings from metal substrates, and related compositions
InactiveUS6953533B2Decorative surface effectsBlade accessoriesO-Phosphoric AcidHexafluorosilicic acid
A method for removing a chromide coating from the surface of a substrate is described. The coating is treated with a composition which includes an acid having the formula HxAF6, where “A” can be Si, Ge, Ti, Zr, Al, or Ga; and x is 1–6. An exemplary acid is hexafluorosilicic acid. The composition may also include a second acid, such as phosphoric acid or nitric acid. In some instances, a third acid is employed, such as hydrochloric acid. A related repair method for replacing a worn or damaged chromide coating is described. The coating is often applied to portions of turbine engine components made from superalloy materials.
Owner:GENERAL ELECTRIC CO
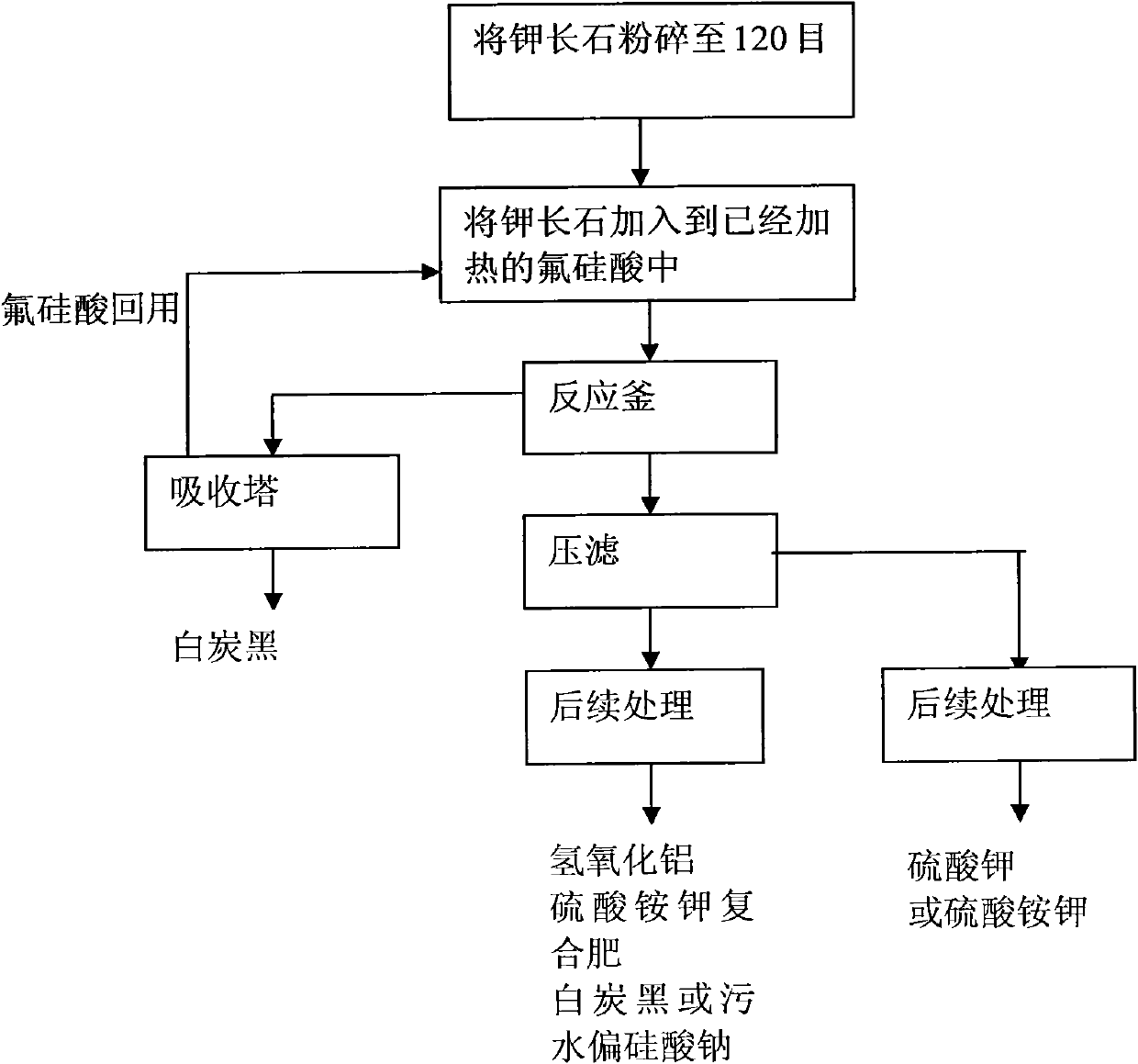
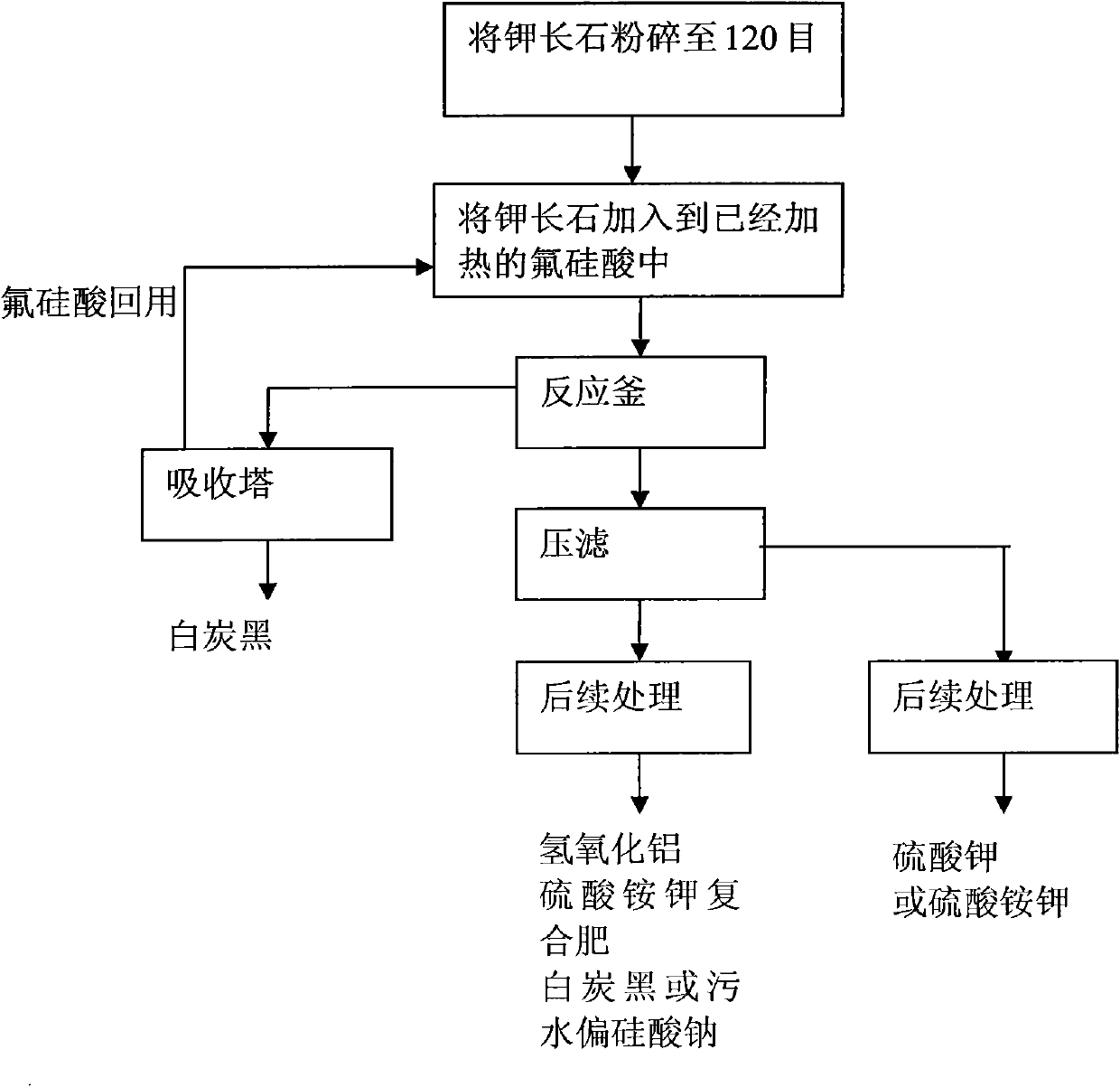
Method for processing potassium-containing rocks
InactiveCN101948115AShort reaction timeReduce energy consumptionSilicaAluminium silicatesChemical treatmentPotassium
The invention discloses a method for processing potassium-containing rocks, which mainly comprises: coarsely and finely crushing the potassium-containing rocks to 100 to 200 meshes; and adding the potassium-containing rocks to fluosilicic acid according to a ratio to react the potassium-containing rocks with fluosilicic acid fully to obtain a mixed material containing potassium fluosilicate, aluminium fluosilicate and silica gel. In the invention, the fluosilicic acid is used alone to decompose the potassium-containing rocks, the fluosilicic acid and ore powder are fed into a reaction kettle in turn under a stirring condition, the reaction kettle is heated to 110 to 170 to allow the reactants to react fully, the reaction time is 1 to 3 hours, the fluosilicic acid suspension is reserved and subjected to subsequent chemical processing, acidification is performed, and the fluosilicic acid is reclaimed. Compared with the conventional mixed acid method, the method can process 1 ton of potassium-containing rock raw material by using 0.2 ton of coal containing 5,000 large calories of heat; and compared with the method of evaporating the mixed acid to dryness, the method can save 60 to 80percent of energy consumption and reduce processing time by 4 to 6 folds.
Owner:薛彦辉
Method for continuously preparing white carbon black and ammonium fluoride by carrying out fluosilicic acid ammoniation
ActiveCN101863482AContinuous processProcess stabilitySilicaAmmonium halidesSal ammoniacHexafluorosilicic acid
The invention discloses a method for continuously preparing white carbon black and ammonium fluoride by carrying out fluosilicic acid ammoniation, which relates to a chemical production method, in particular to a method for preparing the white carbon black and the ammonium fluoride. The method comprises the following steps: (a) at a temperature between minus 5 and 30 DEG C, starting a stirrer and an air compressor, blowing compressed air into an outlet pipe of a reactor by the air compressor and adding fluosilicic acid and ammonia into the reactor simultaneously; (b) when the fluosilicic acid and the ammonia are added to the position of an overflow pipe in the reactor, opening an emptying valve to continuously feed and discharge materials and controlling a PH value of the reaction, wherein the period of the continuous reaction is 24 hours; (c) in the reaction process, filtering the materials discharged by the step (b) to obtain a white carbon black filter cake and filtrate of ammonium fluoride, and washing and drying the white carbon black filter cake to obtain a white carbon black finished product, wherein the filtrate is ammonium fluoride solution; and (d) after performing a reaction for 24 hours in the reactor, emptying the materials, cleaning the reactor to be used next time and producing the white carbon black finished product by using the materials according to the step (c), wherein the ammoniation yield of the process can reach 99.99 percent. The method of the invention effectively overcomes the defects of the existing intermittent technology and has the advantages of continuous, stable and simple process and long operation period.
Owner:YUNNAN YUNTIANHUA +1
Laminated film
InactiveCN101130870AIncrease coverageEasy to controlSemiconductor/solid-state device manufacturingHexafluorosilicic acidAlloy
The present invention relates to a compound etching liquor which can be used for etching metal laminated film formed on the semiconductor substrate. Said compound etching liquor contains fluoride and oxidant. The described fluoride is at least one kind selected from metal salt or ammonium salt of hydrofluoric acid, hexafluorosilicic acid, metal salt or ammonium salt of hexafluorosilicic acid, tetrafluoroboric acid and metal salt or ammonium salt of tetrafluoroboric acid.
Owner:KANTO CHEM CO INC
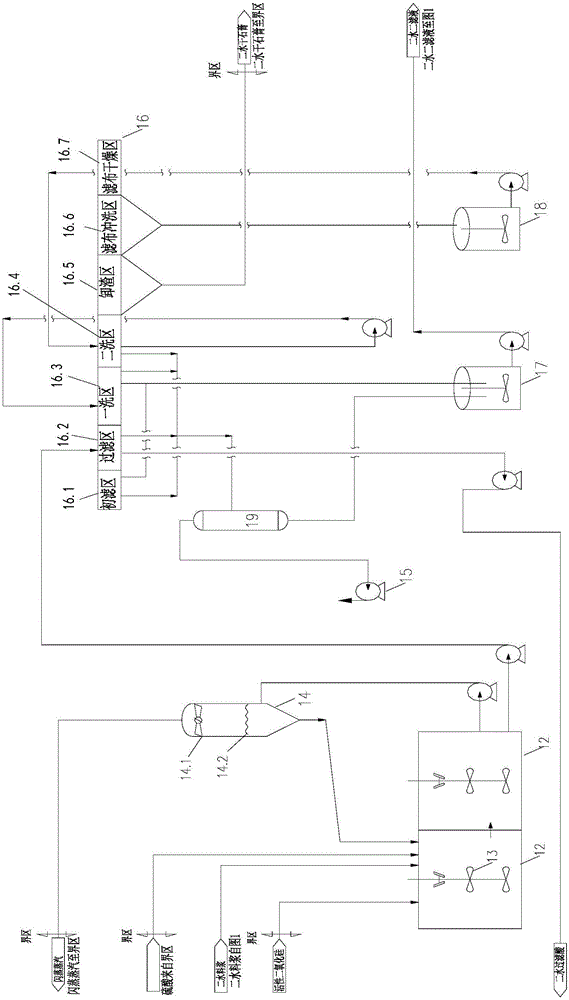
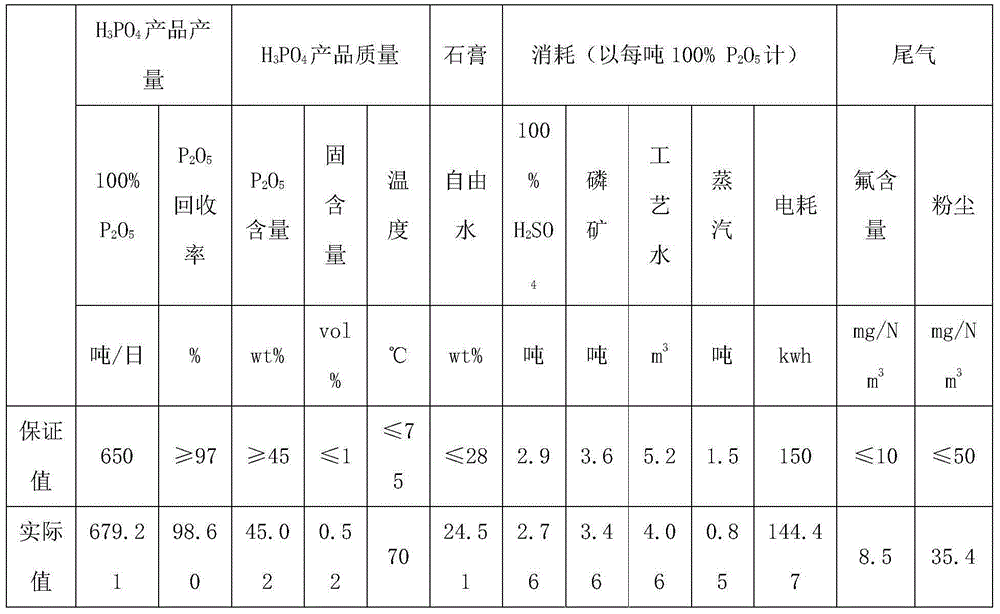
Semi-hydrated-two-hydrated wet process phosphoric acid production process and system thereof
The invention relates to a semi-hydrated-two-hydrated method wet process phosphoric acid production process and a system thereof, which solve the problems of high investment, small device scale, high energy consumption, low device operation rate and low raw material yield of the existing processing process. The technical solution comprises: performing semi-hydrated reaction and filtering by taking phosphorite as a raw material to obtain a product phosphoric acid and semi-hydrated gypsum, and then performing two-hydrated reaction and filtering on the semi-hydrated gypsum to obtain water-hydrated dry gypsum. The system comprises a semi-hydrated reaction and filtering system and a two-hydrated reaction and filtering system. The process is reliable, product phosphoric acid concentration is high, quality of side products fluosilicic acid and dry gypsum is good, energy consumption is low, equipment investment and operation cost are low, and environment friendliness and continuous stable large-scale production (more than or equal to 0.3 million tons / year) are realized.
Owner:WUHUAN ENG
Method for producing defluorinated sulfenyl nitrogen-phosphorus-potassium compound fertilizer
InactiveCN101717290AIncrease profitSave resourcesFertilizer mixturesChemical industryPhosphoric acid
The invention relates to a method for producing defluorinated sulfenyl nitrogen-phosphorus-potassium compound fertilizer, which belongs to the technical field of production in the chemical industry. The method comprises the following steps of: firstly, adding soluble sylvite (or sodium salt) into fluosilicic acid in wet phosphoric acid to generate potassium fluosilicate (or sodium fluosilicate) precipitate, wherein after separating the potassium fluosilicate (or sodium fluosilicate) precipitate, the potassium fluosilicate (or sodium fluosilicate) precipitate is used as byproduct; then mixing the defluorinated wet phosphoric acid with converted potassium bisulfate at low temperature, carrying out neutralization reaction on mixed acid and gas ammonia; and granulating, drying, sieving, cooling and packing the neutralized slurry to prepare the defluorinated sulfenyl nitrogen-phosphorus-potassium compound fertilizer. The invention is mainly used for producing chemical fertilizers.
Owner:SHANDONG HONGRI ACRON CHEM
Method for producing ammonium bifluoride by recovering fluorine resource from fluorine-containing silicon slag
ActiveCN102491370AReduce dosageIncrease concentrationSolid waste disposalAmmonium halidesHydrogen fluoridePhysical chemistry
The invention discloses a method for producing ammonium bifluoride by recovering a fluorine resource from fluorine-containing silicon slag. The method comprises the following steps of: dissolving fluorine-containing silicon slag and an ammonium fluoride solution, cooling an obtained ammonium fluosilicate solution, introducing gas ammonia, and precipitating white carbon black out; filtering and washing to obtain a white carbon black solid and an ammonium fluoride solution; introducing anhydrous hydrogen fluoride into the ammonium fluoride solution, uniformly mixing the anhydrous hydrogen fluoride with the ammonium fluoride solution, and fully reacting till the pH of the solution is 2-4 to obtain an ammonium hydrogen fluoride of which the mass concentration is 21-37 percent; mixing with a crystallized ammonium hydrogen fluoride mother solution, and performing triple-effect evaporation and concentration till the mass concentration of the ammonium hydrogen fluoride is 60-80 percent; feeding into a crystallizer, and cooling and crystallizing for 3-7 hours to obtain an ammonium hydrogen fluoride solid-liquid mixture; and separating to obtain an ammonium bifluoride product and an ammonium bifluoride mother solution. The method has the advantages of low using amount of fluorine hydride, high concentration of ammonium bifluoride, low energy consumption in a concentrating process, low production cost, easiness and convenience for operating, and easiness for realizing industrialization.
Owner:WENGFU (GRP) CO LTD +1
Method for producing sodium fluosilicate by utilizing waste water containing sodium sulfate
ActiveCN102020281AQuality impactLow costSilicon halogen compoundsIndustrial waste waterWater resources
The invention discloses a method for producing sodium fluosilicate by utilizing waste water containing sodium sulfate. The method is as follows: generating the sodium fluosilicate by adopting the waste water containing sodium sulfate and fluosilicic acid as a side product for producing hydrofluoric acid as a raw material; and obtaining a sodium fluosilicate product by curing, filtering, cleaning and pneumatic drying. The dilute sulphuric acid as a side product is used for a production line of sodium dichromate. The method provided by the invention has the advantages that the industrial sodium sulfate waste water is utilized to produce the sodium fluosilicate, and the materials are all industrial waste water. The industrial waste water of the sodium sulfate is from waste water obtained by washing chromium sesquioxide produced by sodium dichromate or waste water generated for producing chromium salt, the fluosilicic acid is from waste water in the process for producing the hydrofluoric acid, the produced sodium fluosilicate is used as a commodity for sales, the dilute sulphuric acid as a side product is used for the production line of the sodium dichromate. By utilizing the method, the cost for treating the waste water treatment of the industrial sodium sulfate in the chromium salt industry is reduced, and the reutilization of water resources is realized.
Owner:GANSU JINSHI CHEM
Gas phase hydrolysis and fluoride-silicon separation method of silicon tetrafluoride
InactiveCN103601195ASimple purification methodLow hydrolysis temperatureSilicaFluorine/hydrogen-fluorideChemical industrySilicon tetrafluoride
A two-step method is adopted to carry out low temperature gas phase hydrolysis and fluoride-silicon separation operations of silicon tetrafluoride. In a first step, a silicon tetrafluoride gas is hydrolyzed into a fluosilicic acid gas and silicon dioxide particles at a low temperature; in a second step, a hydrogen fluoride gas is dissolved by a washing operation of high concentrated sulfuric acid so as to promote complete decomposition of the fluosilicic acid into the hydrogen fluoride gas and a silicon tetrafluoride gas; decomposed and separated hydrogen fluoride gas is completely dissolved in high concentrated sulfuric acid; decomposed and separated silicon tetrafluoride can be continuously carried out hydrolysis and fluoride-silicon separation operations; and finally complete separation of the fluoride and silicon elements are realized. The method has the beneficial effects that a gas raw material containing relatively low silicon tetrafluoride content can be used; a method for purifying the raw material is simple; total conversion rate of silicon tetrafluoride introduced into a production apparatus system can reach over 98%; and hydrolysis temperature is relatively low, so that the method can realize industrial production relatively easily. The method is suitable for recovery and utilization of fluoride- and silicon-containing materials from industries such as phosphorus chemical industry, fluorine chemical industry, electron industry, glass processing industry and aluminium alloy processing industry.
Owner:班仁义
Process for treating tail gas of rare earth mineral powder and concentrated sulphuric acid roasting process
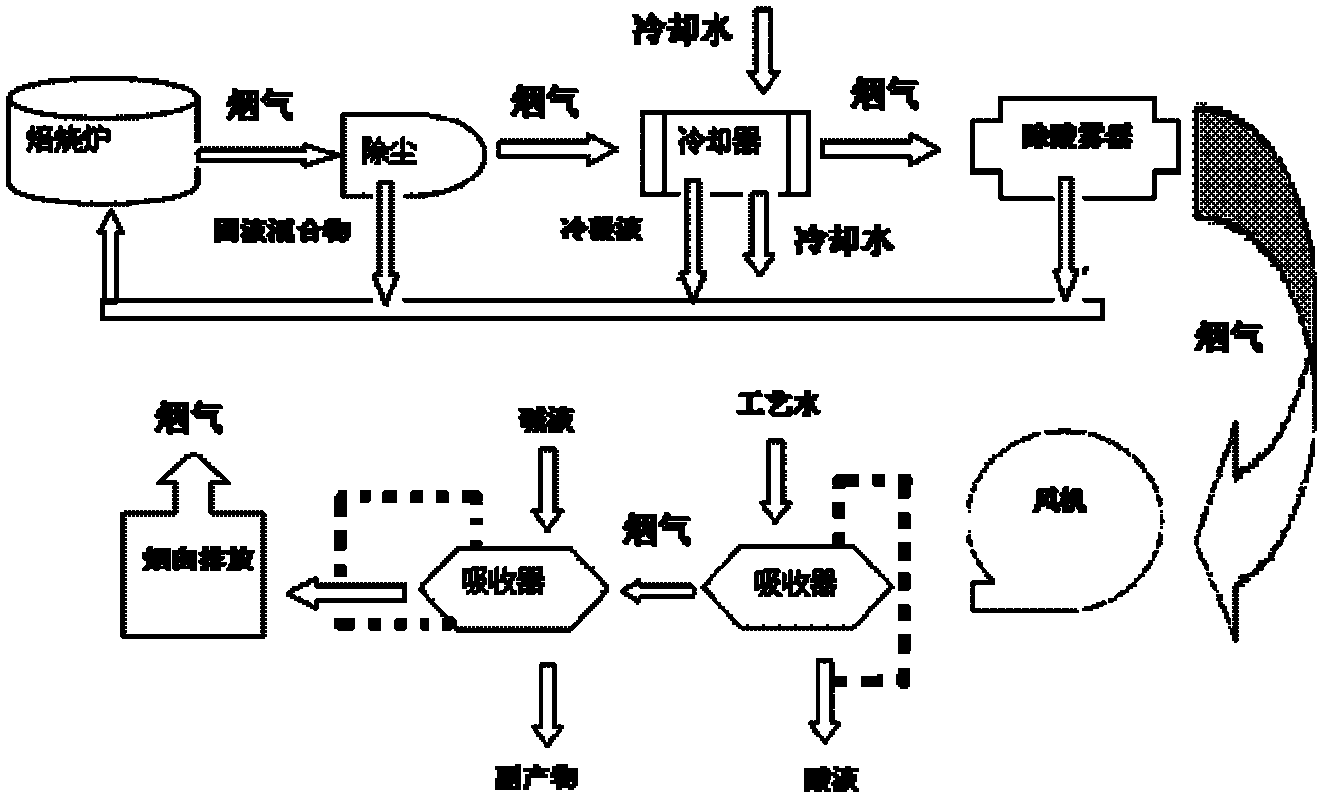
ActiveCN102247708AAvoid energy consumptionAvoid investmentDispersed particle separationVapor condensationHydrogen SulfateHexafluorosilicic acid
The invention relates to a process for treating tail gas of a rare earth mineral powder and concentrated sulphuric acid roasting process. The process comprises the following steps of: introducing the tail gas of 300 DEG C of the process into a dust remover for dust removal with a machine; then introducing the tail gas into a condenser for cooling, condensing massive sulphuric acid fog and water vapour in the smoke and recycling acid liquor; introducing the cooled smoke into an acid fog collector, collecting acid fog and water fog in the smoke for recycling; boosting the treated smoke by a fan, introducing the treated smoke into a first absorber, washing by diluted acid liquor or process water to remove residual sulphuric acid fog, hydrofluoric acid and fluosilicic acid from the smoke; andthen introducing the smoke into a second absorber, washing the smoke by an alkali washing tower to remove residual sulphuric acid fog, hydrofluoric acid and SO2 gas from the smoke, and discharging the smoke out after defogging. The process provided by the invention can save massive smoke cooling water, produce less waste water, reduce waste water treatment cost, and recycle the side product fluoric acid produced by solid mineral substances, sulphuric acid, hydrofluoric acid and the like in the tail gas as a raw material for recycling fluorine.
Owner:北京鸿源龙嘉环保科技有限公司
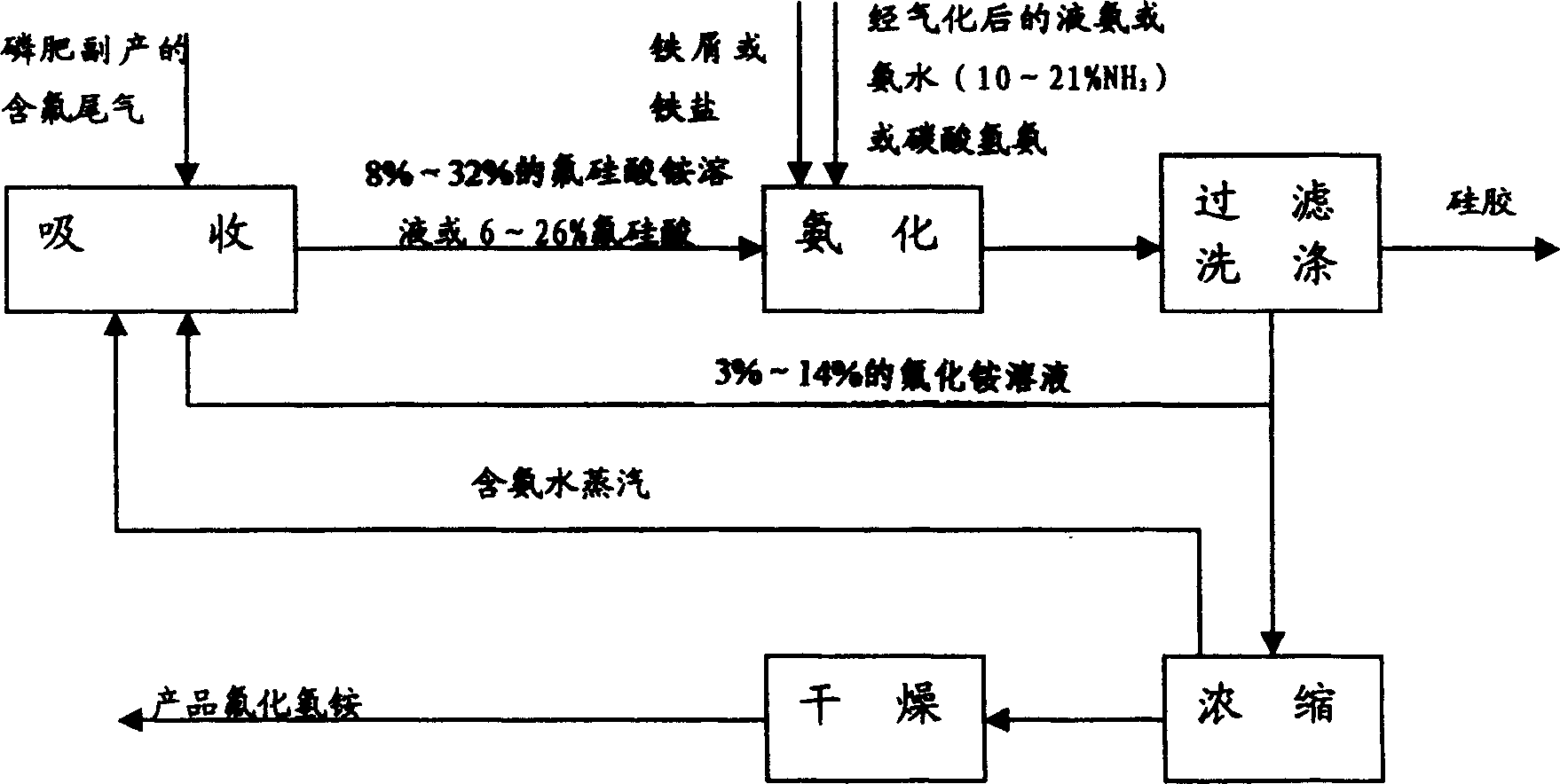
Process for preparing ammonium hydrogen fluoride
InactiveCN1554586AReasonable designReduce material requirementsFluoride preparationSolid waste disposalIron saltsHexafluorosilicic acid
The present invention discloses production process of ammonium hydrogen fluoride. Ammonium fluoride solution in 3-14% concentration is used to absorb fluorine containing tail gas as side product of phosphate fertilizer production to obtain ammonium fluorosilicate solution of 8-12% ammonium fluorosilicate content. Iron scrap or iron salt is added into ammonium fluorosilicate solution or one other side product from phosphate fertilizer production to eliminate phosphorus; and through adding gasified liquid ammonia or ammonia water or ammonium bicarbonate, filtering, washing to eliminate SiO2 precipitate, returning ammonium fluoride solution to phosphate fertilizer absorbing system, concentration and drying, the ammonium hydrogen fluoride product is obtained.
Owner:云南云天化国际化工有限公司 +1
Production method of potassium dihydrogen phosphate
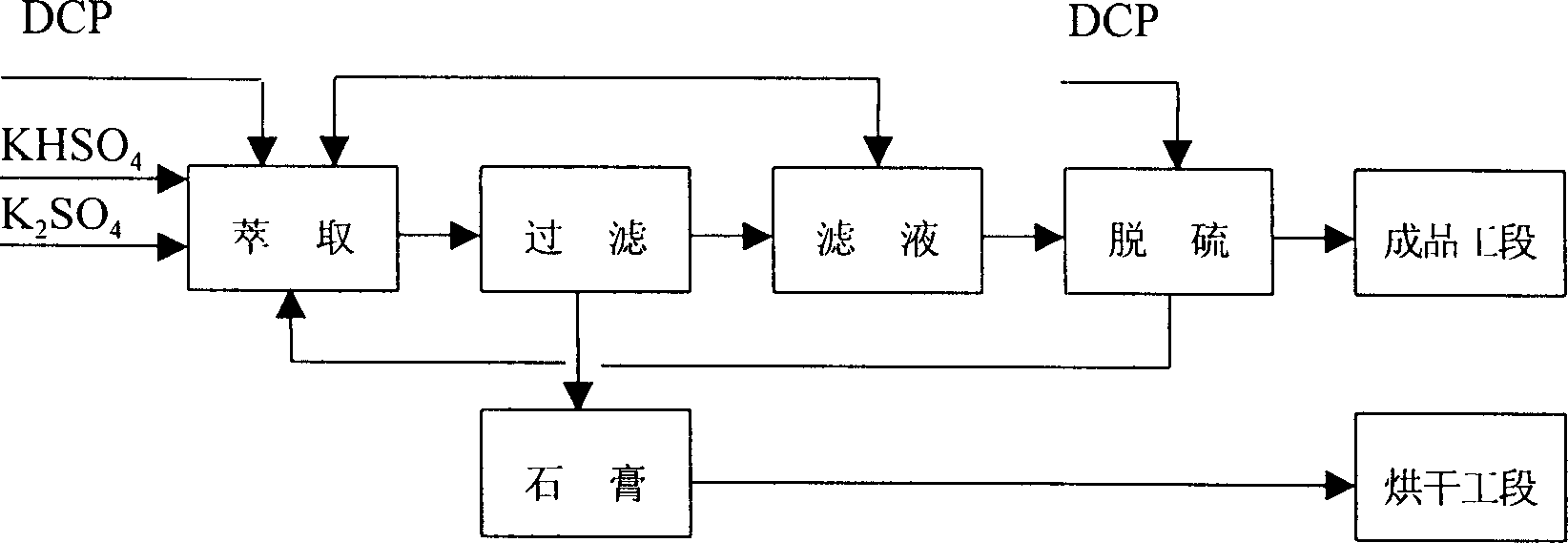
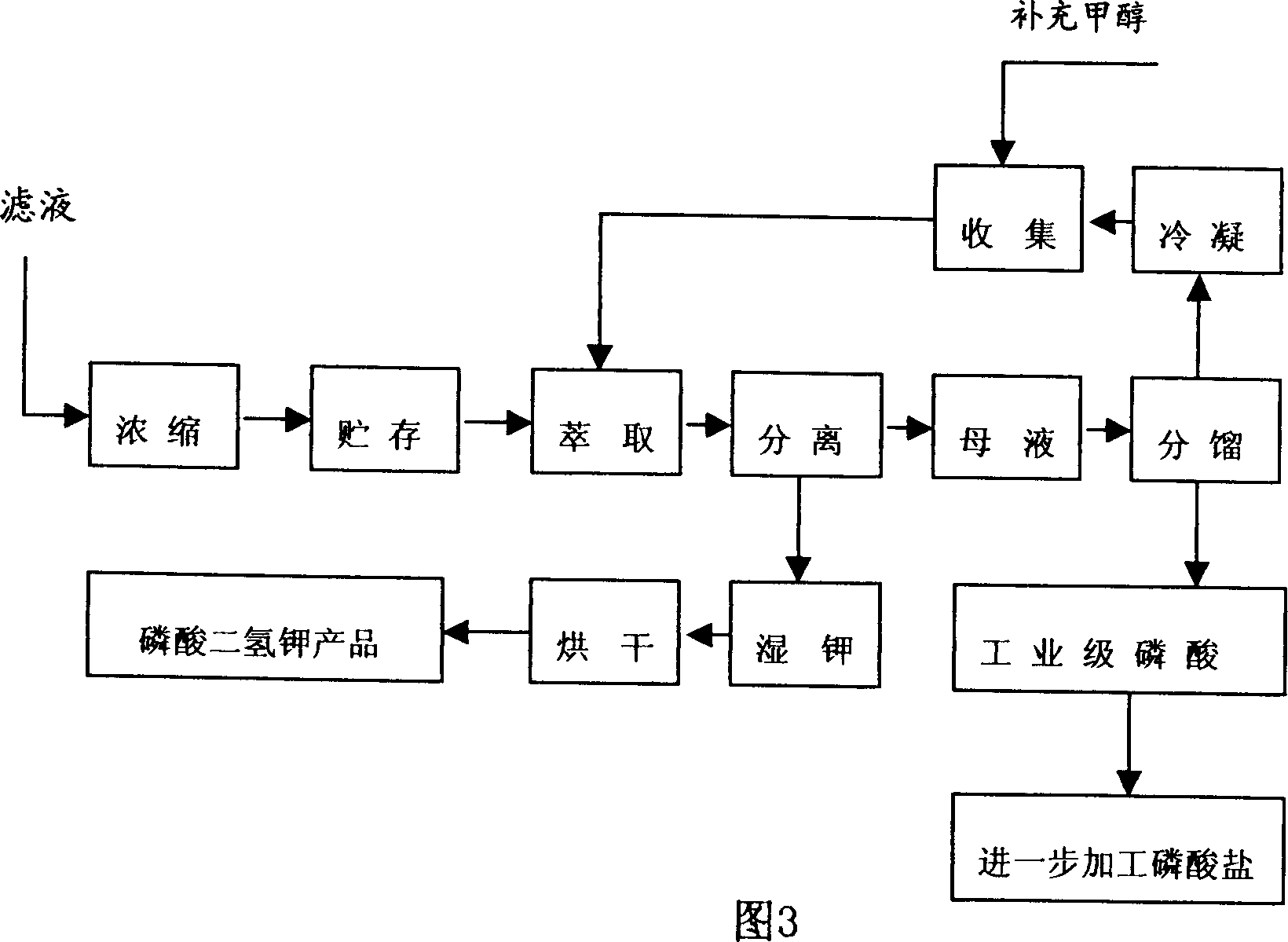
InactiveCN1412107AImprove qualityReliable technologySulfate/bisulfate preparationLiquid solutions solvent extractionHydrogen SulfateFiltration
The production method of potassium dihydrogen phosphate uses the calcium hydrogen phosphate whose impurities of iron, aluminium, fluorine and sulfur are low as raw material, and is implemented by adopting technological processes of potassium hydrogen sulfate preparation, solution extraction, potassium dihydrogen phosphate crystallization and separation ,and its operation includes the following steps: fulfonation, hydrolysis, extraction, filtration, filtrate concentration, crystallization and separation, drying and fractionation. Its reaction condition is moderate, its potassium yield is high,at the same time the quality of potassium dihydrogen phosphate and phosphoric acid can be ensured.
Owner:SICHUAN HONGDA (GRP) CO LTD
Method for preparing hydrogen fluoride from fluosilicic acid
The invention provides a method for preparing hydrogen fluoride from fluosilicic acid, comprising the following steps: (1) enabling the fluosilicic acid as a raw material to react with liquid ammonia, thus obtaining an ammonium fluoride solution and a silicon dioxide solid; (2) enabling the ammonium fluoride solution obtained in the step (1) to react with calcium oxide, thus obtaining calcium fluoride and weak aqua ammonia; (3) rectifying to purify the weak aqua ammonia obtained in the step (1), thus obtaining liquid ammonia, and entering the step (1) for recycling; (4) sequentially drying and calcining the calcium fluoride obtained in the step (2), then leading into a fluidization reaction bed with superheated steam, thus obtaining the hydrogen fluoride and calcium oxide, and returning the step (2) to reuse the calcium oxide for recycling, wherein the calcium oxide reacting with the ammonium fluoride solution for the first time in the step (2) is from purchased calcium oxide, and the calcium oxide in the second and subsequent reactions is the calcium oxide in the step (4). The preparation method realizes recycling of the calcium oxide generated in the hydrogen fluoride preparation process, and the purity of the hydrogen fluoride reaches 99.9999% or more.
Owner:衢州市鼎盛化工科技有限公司
Resource comprehensive utilization method for recovering fluorine from wet-process phosphoric acid
InactiveCN103145131AQuality improvementReduce or eliminate hazardsSilicon halogen compoundsPotassium silicatePhosphoric acid
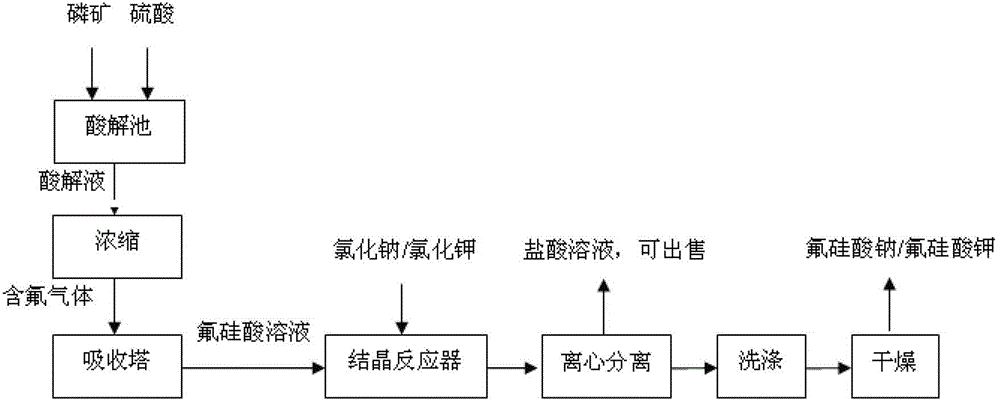
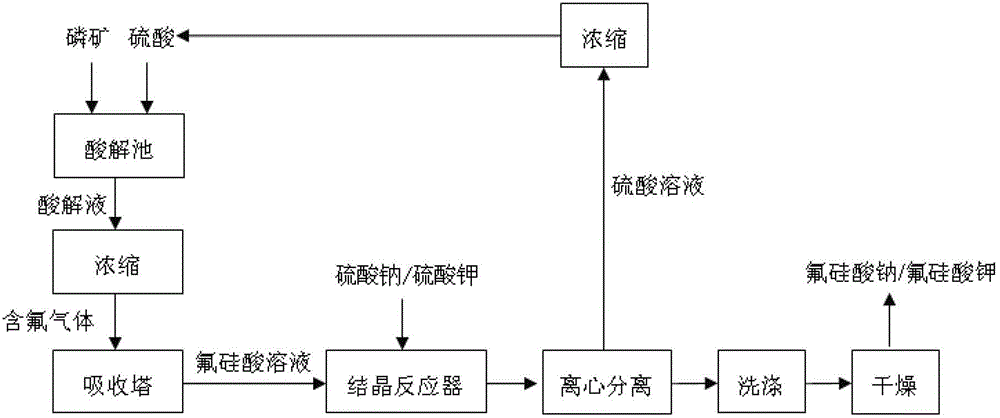
The invention relates to a resource comprehensive utilization method for recovering fluorine from wet-process phosphoric acid. The method comprises the following steps of: absorbing SiF4 and HF gases generated in the wet-process phosphoric acid by using water, and preparing the gases into a hydrofluosilicic acid solution of which the mass percent concentration is 10-35%; mixing one of four solids including solid sodium sulfate, solid potassium sulfate, solid sodium chloride and solid potassium chloride with the hydrofluosilicic acid solution, and reacting for 0.1-2 hours at 30-80 DEG C under stirring to obtain a mixture; centrifugally separating the obtained mixture to obtain a solid and a filtrate; and washing the solid with water, and drying for 1-3 hours at 100-120 DEG C to obtain a sodium fluorosilicate or potassium fluorosilicate crystal. According to the method, a byproduct hydrofluosilicic acid in the wet-process phosphoric acid and sodium (potassium) sulfates or chlorides which are abundant and easily-available are used as raw materials, so that the cost is low, two products including sodium fluorosilicate and potassium fluorosilicate can be produced at the same time by using one set of equipment, and the maximum utilization ratio of the hydrofluosilicic acid can reach over 99%.
Owner:KINGENTA NORSTERRA CHEM CO LTD
Preparation method of hydrofluoric acid
InactiveCN101134561ASilicon oxidesFluorine/hydrogen-fluorideSilicon tetrafluorideHexafluorosilicic acid
The process of producing hydrofluoric acid with fluorosilicic acid and magnesia as material includes the following steps: 1. reacting fluorosilicic acid solution and solid sodium sulfate for 10-60 min, filtering to obtain solid sodium fluosilicate, and treating waste sulfuric acid before draining; 2. decomposing sodium fluorosilicate at 300-800 deg.c for 1-5 hr to produce solid sodium fluoride and gaseous silicon tetrafluoride; 3. absorbing gaseous silicon tetrafluoride with water and hydrolyzing, and filtering to obtain fluorosilicic acid solution returned to the step 1 and silica, washing and drying to obtain carbon white; and 4. reacting the solid sodium fluoride and 98 % over sulfuric acid, condensing the produced gas condensing and rectifying to obtain hydrofluoric acid, and returning solid sodium sulfate to the step 1. The present invention utilizes fluorosilicic acid as side product from phosphate fertilizer production as material, and has low cost and environment friendship.
Owner:DO FLUORIDE CHEM CO LTD
Method for producing anhydrous hydrogen fluoride and coproducing silica white from low-grade fluorine resources
ActiveCN102795601ALow impurity contentIncrease profitSilicaFluorine/hydrogen-fluorideSlurryHexafluorosilicic acid
The invention discloses a method for producing anhydrous hydrogen fluoride and coproducing silica white from low-grade fluorine resources, which comprises the following steps: carrying out ammonolysis on a phosphate fertilizer byproduct fluosilicic acid solution to obtain an ammonium fluoride solution and silicon dioxide, washing the filter cake, and drying to obtain the silica white product, wherein the filtrate ammonium fluoride solution is used for the next production step; concentrating the ammonium fluoride solution, and carrying out pyrolysis to obtain an ammonium bifluoride solution and ammonia gas, wherein the ammonium bifluoride solution is used for the next production step, and the ammonia gas is used for ammonolysis of the fluosilicic acid solution; reacting the ammonium bifluoride solution and sodium fluoride to generate a sodium bifluoride slurry, and drying the filter cake to obtain sodium bifluoride, wherein the filtrate ammonium fluoride solution can be recycled; and carrying out pyrolysis on the sodium bifluoride to obtain crude anhydrous hydrogen fluoride and sodium fluoride, and purifying the anhydrous hydrogen fluoride to obtain the anhydrous hydrogen fluoride product, wherein the sodium fluoride can be recycled. The method disclosed by the invention has the advantages of accessible raw materials, low price, simple production technique and lower cost for the anhydrous hydrogen fluoride product, solves the bottleneck of environmental protection in the development of low-grade fluorine resource industry, and does not generate secondary pollution in the production process.
Owner:DO FLUORIDE CHEM CO LTD
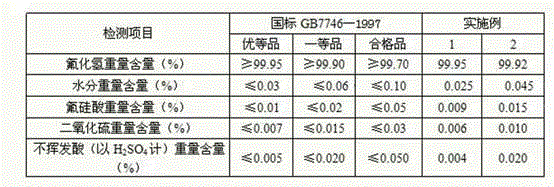
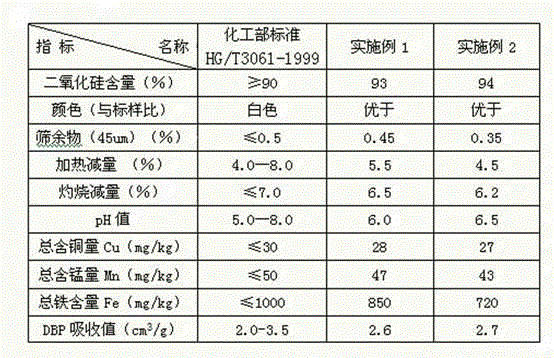
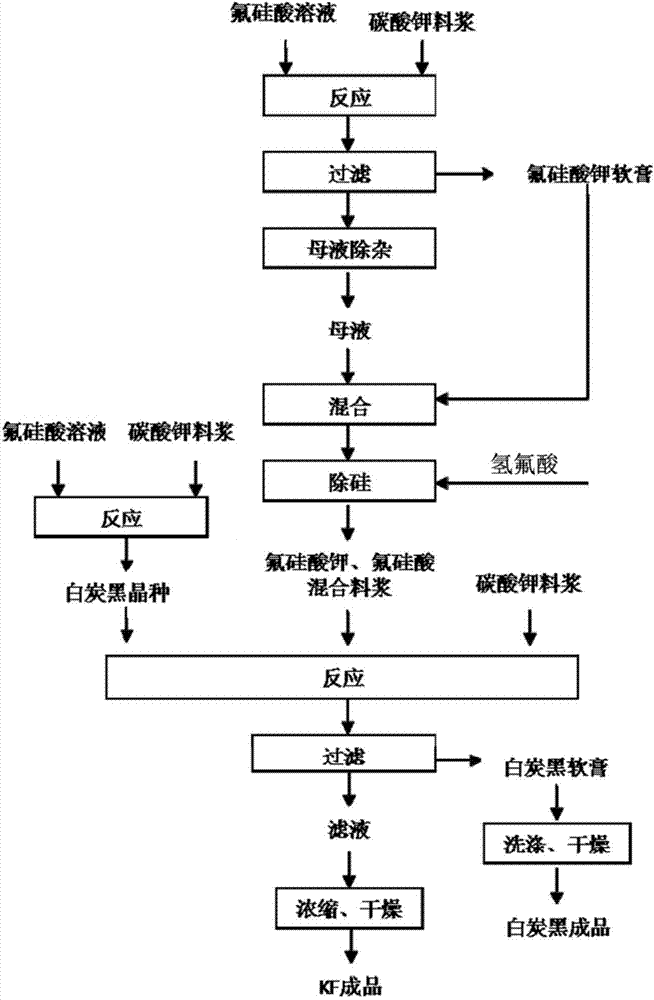
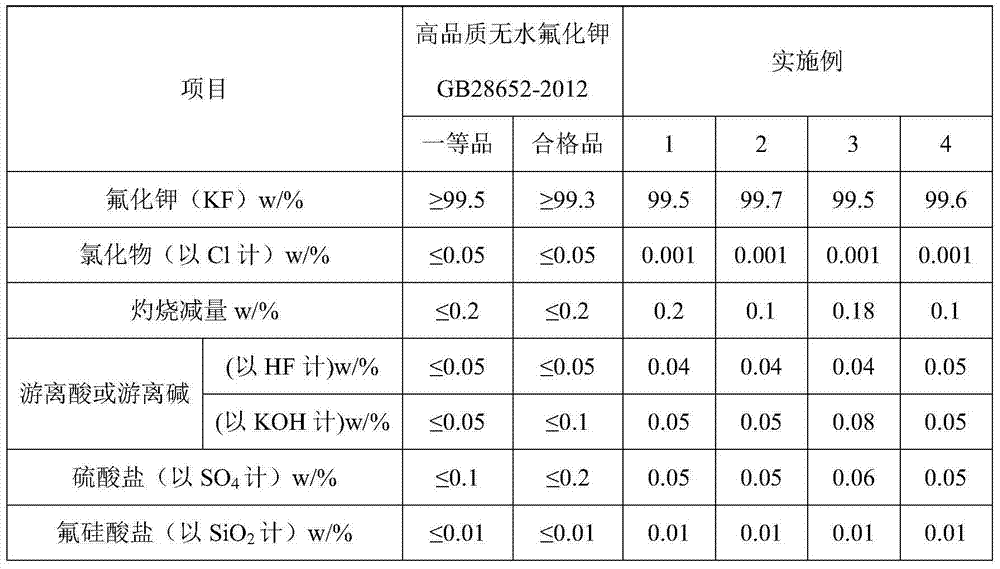
Method for preparing potassium fluoride by employing fluosilicic acid with co-production of white carbon black
ActiveCN104326475ARealize industrial productionHigh activitySilicaAlkali metal fluoridesPotassium fluorideFiltration
The invention discloses a method for preparing potassium fluoride by employing fluosilicic acid with co-production of white carbon black. The method includes following steps: (1) adding potassium carbonate in a fluosilicic acid solution and performing filtration after the reaction to obtain a potassium fluosilicate paste and a mother liquid A; (2) adding calcium oxide or calcium hydroxide to the mother liquid A, performing filtration after the reaction for removing precipitation, and adding potassium carbonate with filtration to obtain a mother liquid B; (3) mixing the potassium fluosilicate paste and the mother liquid B, adding hydrofluoric acid and SiO2 for reaction to obtain a potassium fluosilicate slurry material B; and (4) adding a white carbon black seed crystal to the potassium fluosilicate slurry material B with addition of potassium carbonate for performing an alkaline hydrolysis reaction, performing filtration to obtain a potassium fluoride solution and white carbon black paste and drying the potassium fluoride solution and the white carbon black paste. In the method, by means of the technologies of step-by-step alkaline hydrolysis and impurity removing in the mother liquids, a problem of accumulation of PO4<3-> and SO4<2-> in the raw material fluosilicic acid solution in a product. The potassium fluoride and the white carbon black are high in qualities and are low in production cost. High-quality and cleaning production of fluorine and silicon resources in phosphatic fertilizer industry and hydrofluoric acid industry is achieved.
Owner:DUOFUDUO KUNMING TECH DEV CO LTD
Method for extracting iodine from iodine-containing fluosilicic acid
The invention discloses a method for extracting iodine from fluosilicic acid containing the iodine; the method comprises the following steps of: extracting the iodine from the fluosilicic acid solution produced in the production process of the phosphoric acid, wherein, the fluosilicic acid contains 50 to 110 mg / L of the iodine and the total acidity of the fluosilicic acid is 15 percent to 25 percent; adding a compound oxidizing agent into the fluosilicic acid and oxidizing the ion iodine in the solution into molecular iodine; using air to blow out the molecular iodine; absorbing the moleculariodine by using the acid solution containing SO2 with the PH value of the absorbing liquid ranging from 1 to 6; adding hydrogen peroxide and leading the iodine to be precipitated; carrying out filtering and separation and obtaining the finished iodine. The raw material for extracting the iodine by the invention is fluosilicic acid, in which the content of the iodine is usually below 100mg / L. The method and the compound oxidizing agent obtained by the applicant by experiments can carry out extraction to the iodine with low content. The invention expands the selecting range of extracting the material of the iodine.
Owner:WENGFU (GRP) CO LTD +1
Method for leaching vanadium from vanadous stone coal ore with fluosilicic acid and sulphuric acid
InactiveCN101624649AReduce dosageImprove leaching rateProcess efficiency improvementIndustrial waste waterHexafluorosilicic acid
The invention discloses a method for leaching vanadium from vanadous stone coal ore with fluosilicic acid and sulphuric acid, comprising the following steps: smashing and wet grinding the vanadous stone coal ore so that granularity is less than 0.15mm; adding fluosilicic acid of which dosage is 5-15% of the vanadous stone coal ore by weight percent and is calculated with 100% of fluosilicic acid by weight percent and sulphuric acid of which dosage is 10-25% of the ore by weight percent to leach vanadium at 80-95 DEG C for 2-20 hours; obtaining vanadium-containing leaching liquor by liquid-solid separation after leaching; reducing ferric ion with iron powder; adjusting the pH value to 2.8-3.0 with lime and ammonia; extracting with solvent; oxidizing; adding ammonia to obtain ammonium polyvanadate precipitate; and calcining ammonium polyvanadate to prepare powdered vanadium pentoxide product; wherein, the ratio of the volume of the water in the pulp obtained by wet grinding to the mass of the ore is 1-3:1. The invention has the following beneficial effects: 1. compared with a technology of leaching vanadium from the vanadous stone coal ore solely with the sulphuric acid, the method can save 5%-25% of sulphuric acid; 2. the method creates a condition for leaching vanadium with sulphuric acid, because hydrofluoric acid generated by the hydrolysis of fluosilicic acid can effectively destroy the crystal structure of siliceous-aluminous minerals which contains roscoelite, kaolin, and the like; 3. the fluosilicic acid can be the industrial byproduct fluosilicic acid and the fluosilicic acid-containing industrial waste water; 4. the vanadium leaching rate of the vanadous stone coal ore is high.
Owner:长沙达华矿业技术开发有限公司
Technology for producing sodium fluosilicate
InactiveCN102849744ALight in massSilicon halogen compoundsSulfur-trioxide/sulfuric-acidChemical equationSewage
The invention discloses a technology for producing sodium fluosilicate. The technology comprises the following steps of 1, preparing a sodium sulfate solution having the mass content of 26 to 32%, 2, adding fluosilicic acid having the mass content of 8 to 14% into the sodium sulfate solution with stirring, 3, controlling a use amount of the sodium sulfate solution, adjusting a use amount of fluosilicic acid so that compared with a theoretical use amount calculated by a chemical equation, the use amount of fluosilicic acid is increased by 3 to 6%, 4, carrying out crystallization after a reaction of sodium sulfate and fluosilicic acid, 5, washing, separating sodium fluosilicate crystals, and carrying out centrifugal drying, and 6, recovering sulfuric acid and fluosilicic acid. The technology reduces unit consumption of sodium sulfate, reduces sodion content of sewage and realizes recovery of sulfuric acid in the sewage. Compared with the prior art, the technology has high efficiency, is environmentally friendly, simple and convenient, and has low salt consumption and a low cost. Sewage produced by the technology for producing sodium fluosilicate can be directly recovered and used.
Owner:贵州开磷氟硅化工有限责任公司
New method for producing fluoride and white carbon black by adopting fluosilicic acid or fluosilicate
InactiveCN103979548AHigh content of active ingredientsLow reaction temperatureSilicon halogen compoundsSilicaReaction temperatureWastewater
The invention discloses a new process for producing fluoride and a white carbon black coproduct by adopting fluosilicic acid or fluosilicate. A method for adding SiO2 particles in a process of hydrolyzing fluosilicate for promoting generation of SiO2 crystal nucleus reaction product is adopted, reaction temperature is reduced, reaction efficiency is improved, and a hydrolysis process is more complete. The new method for producing fluoride and white carbon black by adopting fluosilicic acid or fluosilicate provides an effective way for recycling of fluoride-containing waste water.
Owner:SUZHOU HENGRUI BIO PHARMA TECH
Method for producing defluorinated ammonium phosphate
InactiveCN101708832ATake advantage ofAvoid lostPhosphatesSilicon halogen compoundsPhosphoric acidPotassium
The invention relates to a method for producing defluorinated ammonium phosphate and belongs to the technical field of chemical production. In the method, a fluosilicic acid in a wet-process phosphoric acid is made to generate potassium (or sodium) fluosilicate precipitate in a mode of adding soluble potassium (or sodium) salt, potassium (or sodium) fluosilicate is a by-product after the potassium (or sodium) fluosilicate precipitate is separated, the defluorinated wet-process phosphoric acid is subjected to neutralization reaction with gas ammonia, and a neutralized slurry is prepared into the defluorinated ammonium phosphate by the steps of concentrating, drying, packaging and the like. The method for producing the defluorinated ammonium phosphate is mainly used in the field of fertilizer production.
Owner:SHANDONG HONGRI ACRON CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com