Resource comprehensive utilization method for recovering fluorine from wet-process phosphoric acid
A wet-process phosphoric acid and resource technology, applied in the direction of halogenated silicon compounds, etc., can solve the problems of increased amount of waste liquid, single variety, large environmental pollution, etc., and achieve the effect of increasing the economic benefits of enterprises, reducing or eliminating harm, and reducing production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
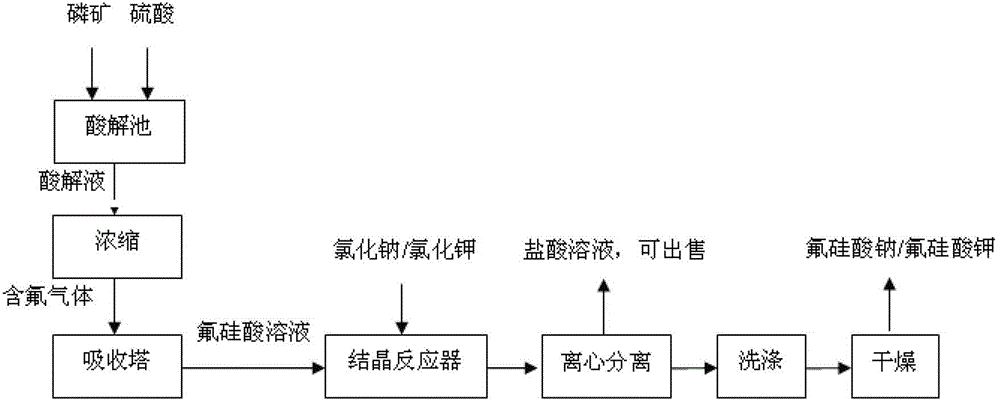
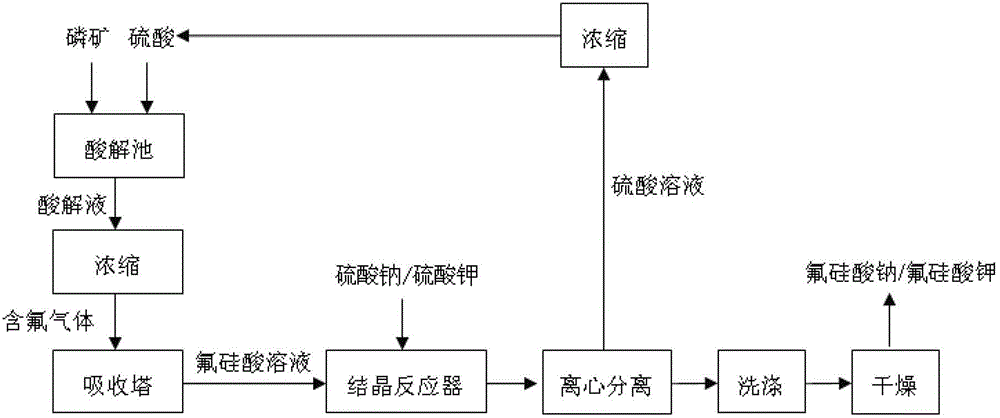
[0037] Absorption of SiF in wet-process phosphoric acid with water 4 and HF gas to make a fluosilicic acid solution with a mass percentage concentration of 15%, take 2000 kg of a mass percentage concentration of 15% fluosilicic acid solution into the reaction crystallizer, and follow the molar ratio of fluosilicic acid: sodium sulfate 1.0 : The proportion of 0.8 was slowly added sodium sulfate solid while stirring, and after 1.5 hours of stirring reaction at 50°C, a large amount of sodium fluorosilicate crystallized out; After washing with water, dry at 100°C for 3 hours to obtain 313 kg of sodium fluorosilicate; the filtrate is a sulfuric acid solution with a mass fraction of 23%, and the sulfuric acid solution is concentrated to a mass fraction of 90% and directly used as a raw material for preparing wet-process phosphoric acid.
Embodiment 2
[0039] As described in Example 1, the difference is that according to fluosilicic acid: the ratio of the molar ratio of potassium sulfate 1.0: 0.9, 2000 kilograms of mass percent concentration is added in the reaction crystallizer to be 15% fluosilicic acid solution and potassium sulfate solid, After stirring and reacting at 60°C for 1 hour, a large amount of potassium fluorosilicate crystallized out; after the reaction, stop stirring, settle for half an hour, and centrifuge to obtain solid and filtrate; wash the solid with water and dry at 120°C for 1 hour to obtain fluorosilicic acid Potassium 412 kilograms, filtrate is the sulfuric acid solution of mass fraction 31%, and sulfuric acid solution is directly used as the raw material of preparing wet process phosphoric acid after being concentrated to mass fraction is 98%.
Embodiment 3
[0041] Absorption of SiF in wet-process phosphoric acid with water 4 and HF gas to make a fluorosilicic acid solution with a mass percent concentration of 20%, add 2400 kg of a fluorosilicic acid solution with a mass percent concentration of 20% in the reaction crystallizer, and follow the molar ratio of fluorosilicic acid: sodium chloride Slowly add solid sodium chloride while stirring at a ratio of 1.0:1.8. After stirring and reacting at 70°C for 0.5 hours, a large amount of sodium fluorosilicate crystallizes out; after the reaction, centrifuge to obtain solid and filtrate; Dry for 2 hours to obtain 565 kg of sodium fluorosilicate, the filtrate is a hydrochloric acid solution with a mass fraction of 28%, and the hydrochloric acid solution is directly sold as a commodity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com