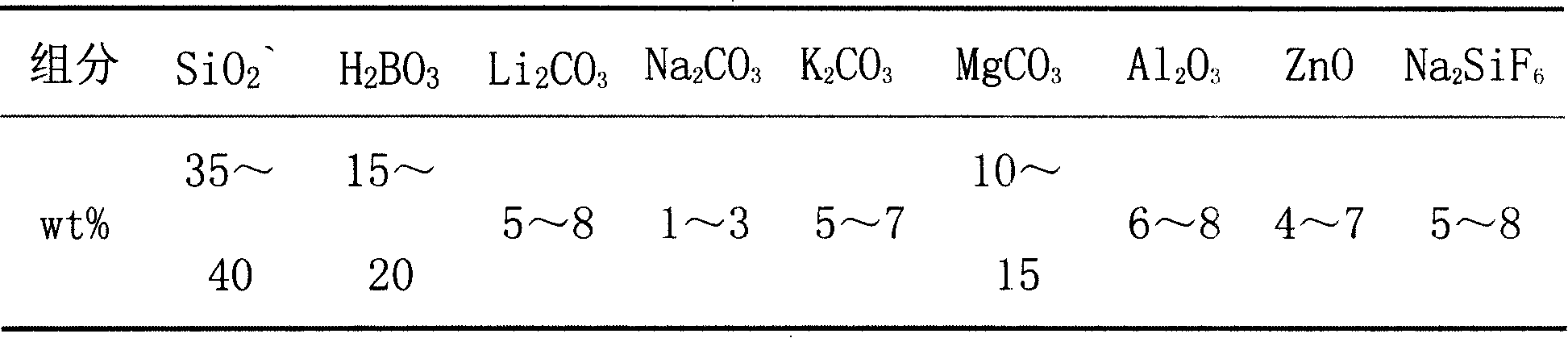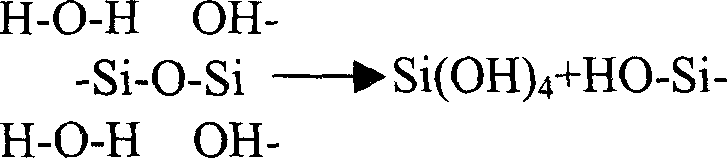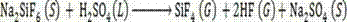Patents
Literature
463 results about "Sodium fluorosilicate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sodium fluorosilicate is a compound with the chemical formula Na₂[SiF₆].
Fiber reinforcement fire-proof thermal insulation plate and preparation process thereof
InactiveUS20150345132A1Low production costResistance to crackingSolid waste managementClimate change adaptationThermal insulationCalcium formate
The present invention discloses a fiber reinforcement fire-proof thermal insulation plate, which is prepared by raw materials having the following weight portions: 80-100 portions of fly ash, 30-50 portions of expanded perlite, 10-20 portions of haydite, 10-20 portions of vegetable fiber, 5-10 portions of winnowing beads, 5-8 portions of sepiolite, 3-5 portions of sodium fluorosilicate, 3-5 portions of calcium formate, 3-5 portions of flame-retardant, 0.1-0.5 portion of triisopropanolamine loeate and 0.1-0.3 portion of dimethoxy-ethane. According to the present invention, industrial production wastes are adopted as main raw materials, so that the production cost is reduced; and defects in the existing building thermal insulation plates can be effectively overcome through the cooperation effect produced by organic combination of the fly ash, light aggregates and additives.
Owner:GUANGDONG NO 1 CONSTRUCTION ENGINEERING CO LTD
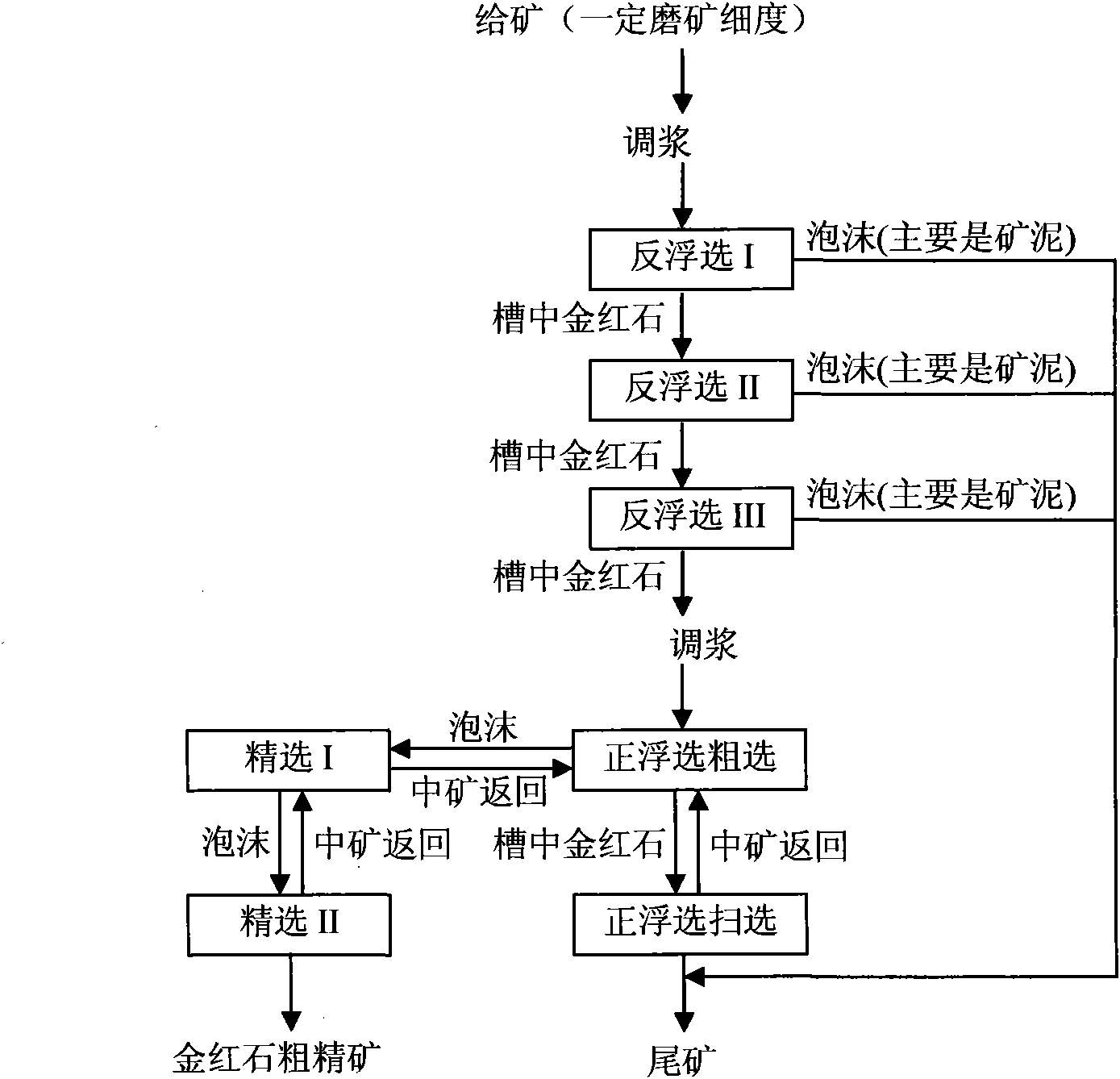
Method for roughing and tailings-discarding of fine rutile ore by multi-stage floatation
The invention provides a method for roughing and tailings-discarding of fine rutile ore by multi-stage floatation, relating to the technique for roughing and tailings-discarding of fine rutile ore by multi-stage floatation and belonging to the technical field of mineral processing engineering. The method of the invention comprises the following steps: firstly, conducting the reverse floatation of rutile by using sodium oleate as a collector according to the characteristics that aluminum sulfate can inhibit the rutile and activate silicate minerals in varying degrees, so as to effectively deslime in the process of reverse floatation; and then, conducting the forward floatation of rutile by using lead nitrate (or lead acetate) as an activator of rutile and using sodium alkyl hydroxamate and benzyl arsonic acid (or styryl phosphonic acid) as a combined collector according to the characteristic that gangue minerals can be inhibited on a combined basis under the synergistic action of sodium fluorosilicate, carboxymethylcellulose and the residual aluminum sulfate in the ore pulp, briefly, the method of the invention can realize the roughing and tailings-discarding of fine rutile by the multi-stage floatation comprising the following steps: firstly, conducting the reverse floatation by inhibiting the rutile; and then, conducting the forward floatation by activating the rutile. The method of the invention has the advantages that the enrichment ratio and recovery rate of rutile are high, the tailings of rutile ore can be discarded thoroughly and the mineral processing cost of rutile can be greatly reduced.
Owner:KUNMING UNIV OF SCI & TECH
Glass-ceramics used as ultrahard material grinding wheel bond and preparation method thereof
The invention relates to a microcrystal glass and preparation method used as super-hard grinding wheel bond, which adopts the method that: quartz, boracic acid, alumina, zinc oxide, lithium carbonate, sodium carbonate, potassium carbonate and magnesium carbonate are used as basic glass materials and sodium fluorosilicate is used as nucleation agent, the materials are smelted to form the glass that is quenched and then smashed to glass powder; the glass powder is pressed, molded, and sintered, and crystallized under certain temperature so as to obtain the microcrystal glass; the main crystal phase of the microcrystal glass is fluorophlogopite, the microcrystal is a hexagonal flake structure, the size of the microcrystal is 1um-2um. The microcrystal glass can be used as super-hard grinding wheel bond in place of ceramic so as to improve the strength of super-hard grinding wheel greatly, thereby improving speed and service life of grinding wheel; the invention also has integrated with excellent performances of glass and porcelain enamel, such as wear-resistant, anti-corrosion, good anti-oxidation, excellent electrical property, adjustable expansion coefficient, and good thermal stability. The invention can widely be applied as structural materials, optical materials, and architecture materials.
Owner:HUNAN UNIV
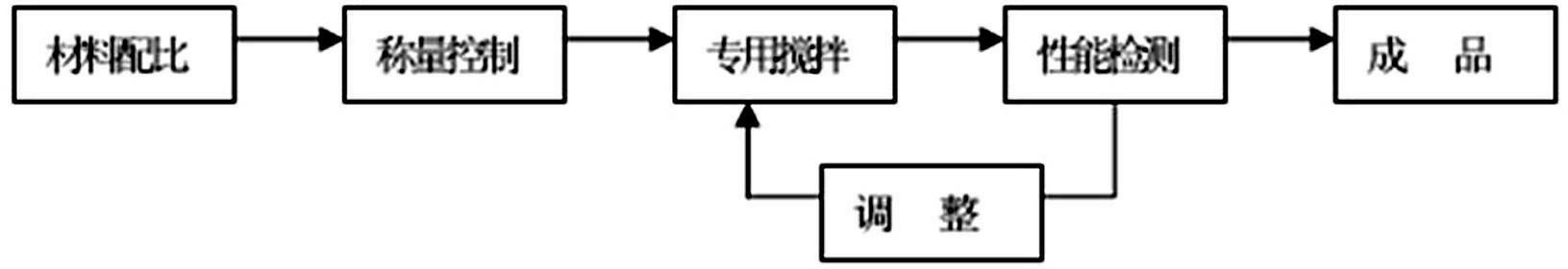
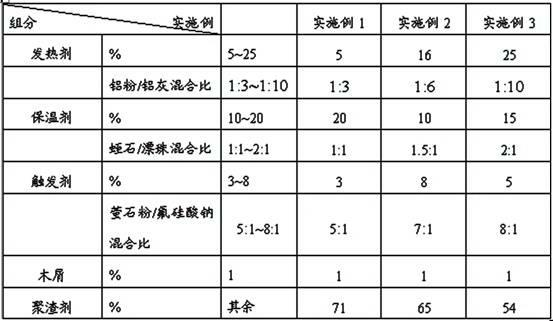
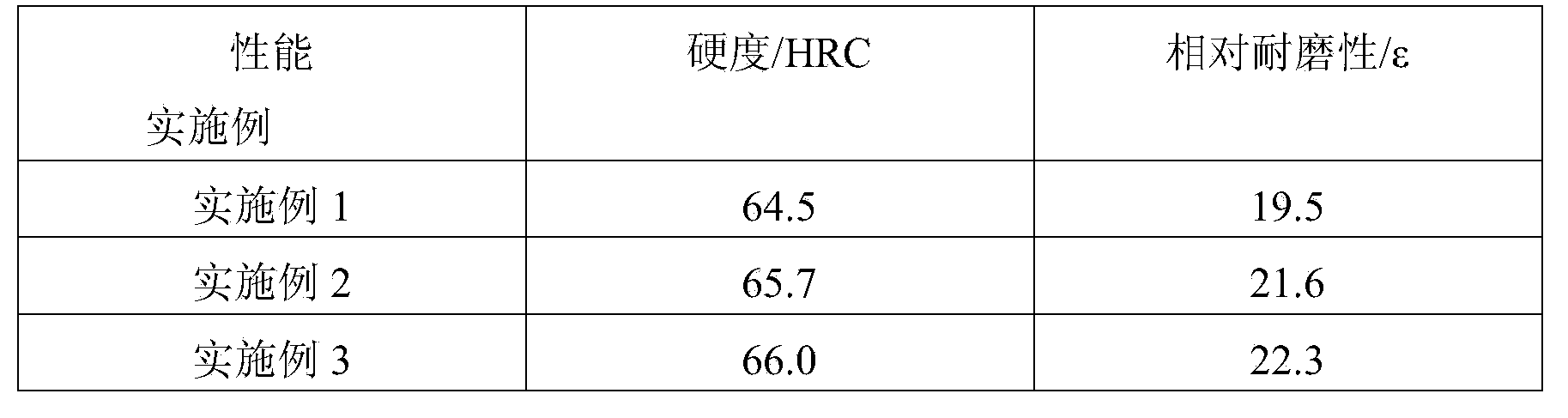
Heating covering agent for casting steel piece
The invention discloses heating covering agent for casting a steel piece. The heating covering agent comprises components by weight percent: 5-25% of heating agent, 10-20% of heat preservation agent, 3-8% of triggering agent, 1% of sawdust, and the balance of slag accretion agent, wherein the heating agent is aluminium powder or aluminium ash, the heat preservation agent is a mixture of vermiculite and floating beads, fluorite powder and sodium fluorosilicate are adopted as the triggering agent, and the slag accretion agent is pearlite. By the study on new materials, the heating covering agent is further improved in the heating and heat preservation performance, and simultaneously realizes an effect of cleaning molten steel. By the application of carbon-free slag accretion materials, the heating covering agent obtains good heat preservation performance while solving problems with recarburization and slag accretion. The carbon-free heating heat preservation covering agent with a slag accretion effect is skillfully designed.
Owner:鑫工艺(上海)材料科技有限公司
Method for preparing fatty hydroximic acid collecting agent and application
The invention discloses a method for preparing a fatty hydroximic acid collecting agent, which is characterized by comprising the following steps of: stirring alkyl hydroximic acid having more than or equal to 5 to 9 carbon atoms, fatty acid having 5 to 22 carbon atoms and mixed solvent oil having 4 to 30 carbon atoms for reaction at room temperature and then separating an oil phase from a water phase, wherein the oil phase is the fatty hydroximic acid collecting agent. The collecting agent of the invention has good selectivity, high collectivity, proper foaming characteristic and easy dissolution in water. Rare earth metal concentrate is obtained by the following steps of: grinding crude rare earth metal oxide ore; adding sodium hydroxide, water glass, sodium fluorosilicate and sodium sulphide into the grinded ore; adding the fatty hydroximic acid collecting agent of the invention into the mixture; then adding modified starch into the mixture; and finally performing flotation. The collecting agent of the invention is a flotation collecting agent for the rare earth metal oxide which is suitable for industrial production and has simple process and high separation efficiency.
Owner:广东省资源综合利用研究所
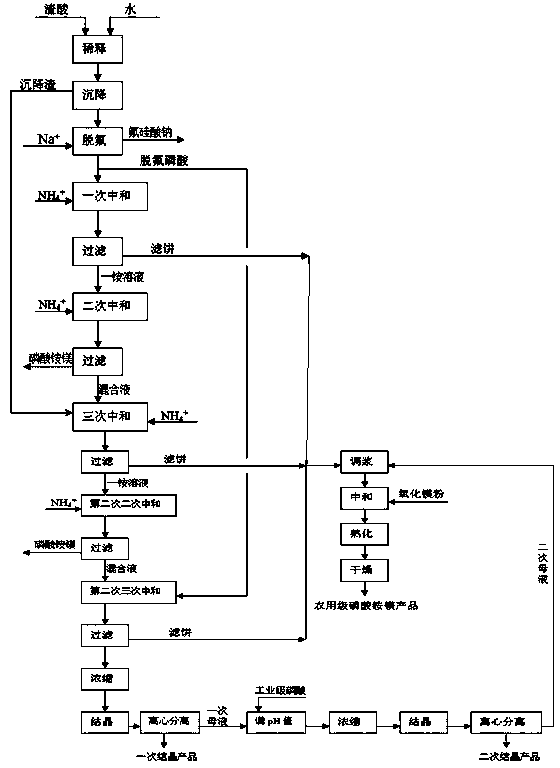
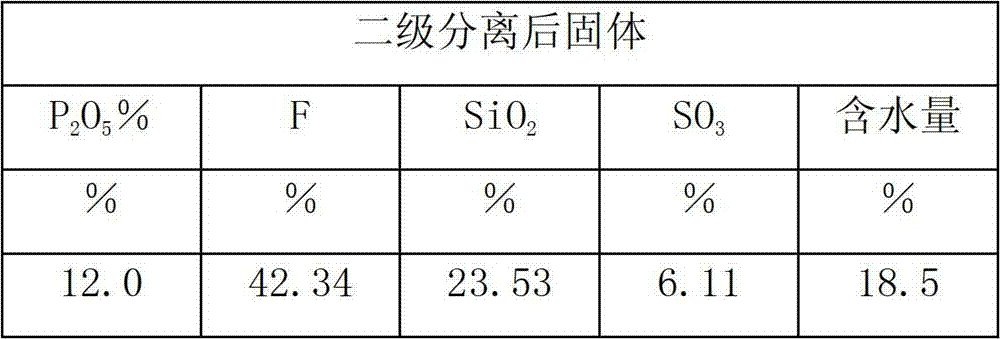
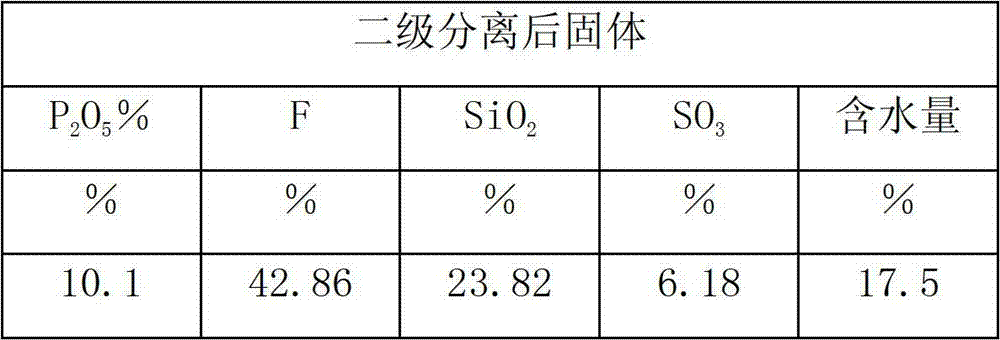
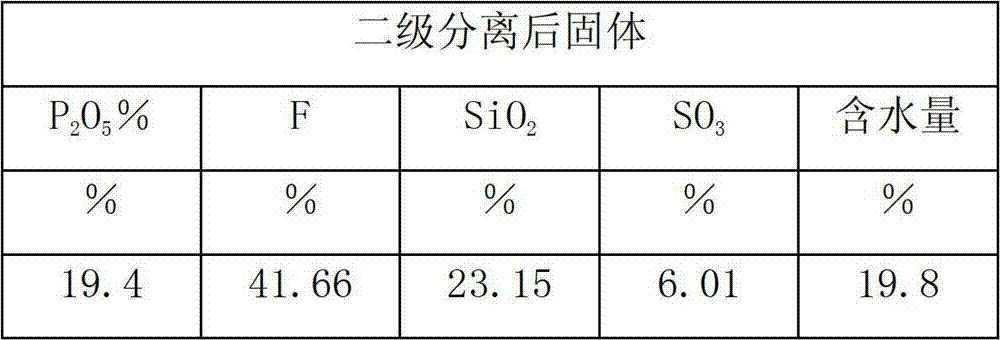
Method for producing monoammonium phosphate and magnesium ammonium phosphate by using wet concentrated phosphoric acid residues
ActiveCN104058378AReduce solid particlesEffective liquid-solid separationPhosphatesMagnesium phosphatePhosphoric acid
The invention provides a method for producing monoammonium phosphate and magnesium ammonium phosphate by using wet concentrated phosphoric acid residues. According to the method, concentrated phosphoric acid residues are adopted as raw materials and sodium fluorosilicate, industrial monoammonium phosphate and magnesium ammonium phosphate are respectively obtained through the processes of dilution and sedimentation, defluorination, fractional multistep neutralization, concentration and crystallization, primary mother liquor pH value adjustment, size mixing of filter cake and secondary mother liquor, magnesia powder neutralization and the like. The method is little in equipment investment, low in production cost and good in product quality and has the advantages that the problem that acid residues of ammonium phosphate plants can be only used for producing low value-added products is solved and the low-cost industrial monoammonium phosphate slow release fertilizer magnesium ammonium phosphate with high quality and inexpensive can be obtained.
Owner:昆明隆祥化工有限公司
Low-cost high-hardness self-protection build up welding flux-cored wire
ActiveCN103753052ASolve the problem of self-protectionHigh hardnessWelding/cutting media/materialsWelding/soldering/cutting articlesSlagFilling rate
The invention provides a low-cost high-hardness self-protection build up welding flux-cored wire which comprises a mild-carbon steel strip and a flux core. The flux core is filled into the steel strip. The flux core comprises, by weight, 85-92% of high carbon ferro-chrome, 2-5% of manganese powder, 1-2% of aluminum magnesium alloy, 0.5-1.5% of silicon iron, 1-3% of graphite, 0.5-2% of ferro-boron and 0.5-1.5% of sodium fluorosilicate. The filling rate of the flux core in a cored solder wire is 58-65%. The low-cost high-hardness self-protection build up welding flux-cored wire is low in cost, high in hardness, good in abrasion resistance, needless of slag removal in a multi-layer welding mode, and extremely small in air hole sensibility.
Owner:北京力佳利焊接材料有限公司
Early strength asater resistant dispersion and anti-dissolving shielding tunnel back lining filling material and its preparation method
ActiveCN1868956AHigh viscosityImprove the coagulation effectSolid waste managementSlagFilling materials
An early-strengthening grouting material for the back liner of tunnel to resist against dispersing in water and denudation is proportionally prepared from powdered coal ash, slag, steel dregs, meta-kaolin, sodium fluorosilicate, stellite, hydroxyethyl methylcellulose, sand, water, and water glass solution. Its preparing process is also disclosed.
Owner:武汉市城市建设投资开发集团有限公司 +3
High-efficient multifunctional metal detergent
This invention discloses a metal cleaning agent with high effect and multi-functions, which uses phosphoric acid and zinc oxide as A material, water, tripoly phosphate, thiourea, and uses tartaric acid, C6H12N4, butanone, thiocyanic acid natrium, triethanolamine, fluorin sodium metasilicate, hydrofluoric acid, lemon acid, nitrous acid natrium, op-10, JFC and alcohol as B material, the main character is that after A material is mixed by a certain weight ratio, it combines B material with a certain weight ratio. This invention combines oil removing, dirty removing, rust preventing, deactivation, inhibiting, phosphate 8 in 1 functions together, which has well cleaning effect, and the work surface quality is high, the dealing technology is simple, the cost is lower.
Owner:凌惠刚
Fiber reinforcement aerated thermal insulation plate and preparation process thereof
InactiveUS20150343666A1Simple stepsGood heat insulationWood working apparatusBuilding constructionsCalcium formateThermal insulation
The present invention discloses a fiber reinforcement aerated thermal insulation plate, which is prepared by raw materials having the following weight portions: 80-100 portions of fly ash, 30-50 portions of expanded perlite, 10-20 portions of haydite, 10-20 portions of vegetable fiber, 5-10 portions of winnowing beads, 5-8 portions of sepiolite, 3-5 portions of sodium fluorosilicate, 3-5 portions of calcium formate, 2-4 portions of foaming agent, 0.1-0.5 portion of triisopropanolamine loeate and 0.1-0.3 portion of dimethoxy-ethane. According to the present invention, industrial production wastes are adopted as main raw materials, so that the production cost is reduced; and defects in the existing building thermal insulation plates can be effectively overcome through the cooperation effect produced by organic combination of the fly ash, light aggregates and additives.
Owner:GUANGDONG NO 1 CONSTRUCTION ENGINEERING CO LTD
Method for producing high-purity nano-zinc oxide by ammonia method using electrolytic zinc acid-leaching residues
ActiveCN102863007AGrowth inhibitionSmall particle sizeZinc oxides/hydroxidesNanotechnologyElectrolysisZno nanoparticles
The invention discloses a method for producing high-purity nano-zinc oxide by an ammonia method using electrolytic zinc acid-leaching residues. The method comprises the following steps of: adding slaked lime being 1-5% of the mass of electrolytic zinc acid-leaching residues before a leaching step to perform activation, then leaching with ammonia-ammonium bicarbonate solution, adding 0.3-0.5kg of sodium fluorosilicate to per cubic meter of ammonia-ammonium bicarbonate solution, and refining after performing purification and impurity removal. According to the method for producing high-purity nano-zinc oxide by ammonia method using electrolytic zinc acid-leaching residues, the electrolytic zinc acid-leaching residues can be leached efficiently, the high-purity nano-zinc oxide with the purity up to above 99.7% can be obtained, and the high-purity nano-zinc oxide has high practical value and economic value; all the valuable and harmful heavy metals in the electrolytic zinc acid-leaching residues are leached and utilized, so that the obtained final leaching residues are converted from electrolytic zinc acid-leaching residues as high hazard wastes into ordinary solid wastes, the environment is protected, and the resources are rationally utilized.
Owner:SICHUAN JUHONG TECH
Latex and fiber composite foaming material and preparation method thereof
The invention discloses a latex and fiber composite foaming material and a preparation method thereof. The latex and fiber composite foaming material is prepared by mixing the following components in parts by weight: 10-30 parts of fibers, 100 parts of natural latex, 0.3-0.8 part of sodium dodecyl benzene sulfonate, 1.6-3.5 parts of sulfur, 0.6-3.5 parts of zinc dimethyldithiocarbamate, 0.8-2.3 parts of anti-aging agent RD, 15-25 parts of aluminum hydroxide, 1.5-3 pars of zinc oxide, 2.0-2.5 parts of sodium fluorosilicate, 1-8 parts of 3 mass percent formaldehyde and 5-10 parts of latex foaming agent; and the method comprises the steps of preparing the latex, molding the fibers, foaming, putting into a mold, vulcanizing, demolding, washing, drying and the like. The latex and fiber composite foaming material has good elasticity, stable foam structure and good comfort, and overcomes the defects that an un-foamed fiber elastic material is relatively hard and a sponge material is soft in supporting and poor in breathability.
Owner:GUIZHOU DAZIRAN TECH
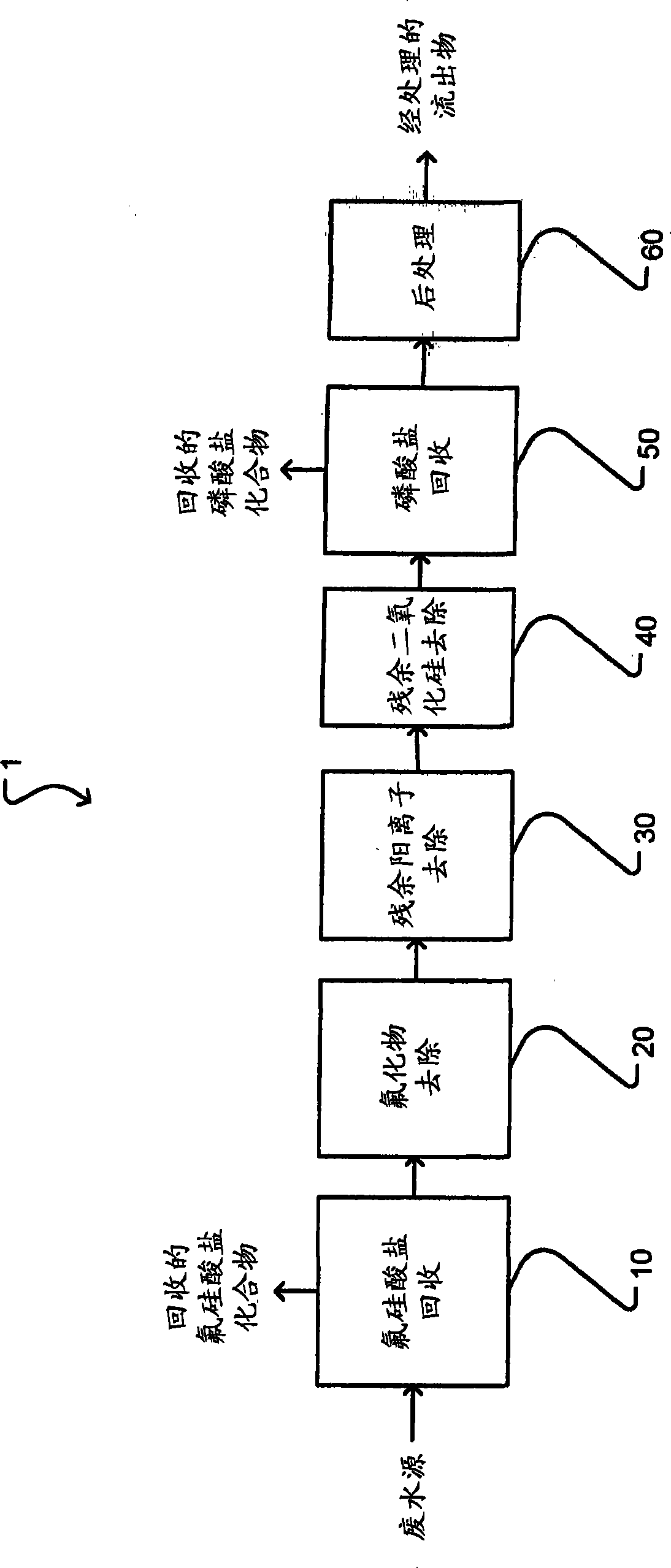
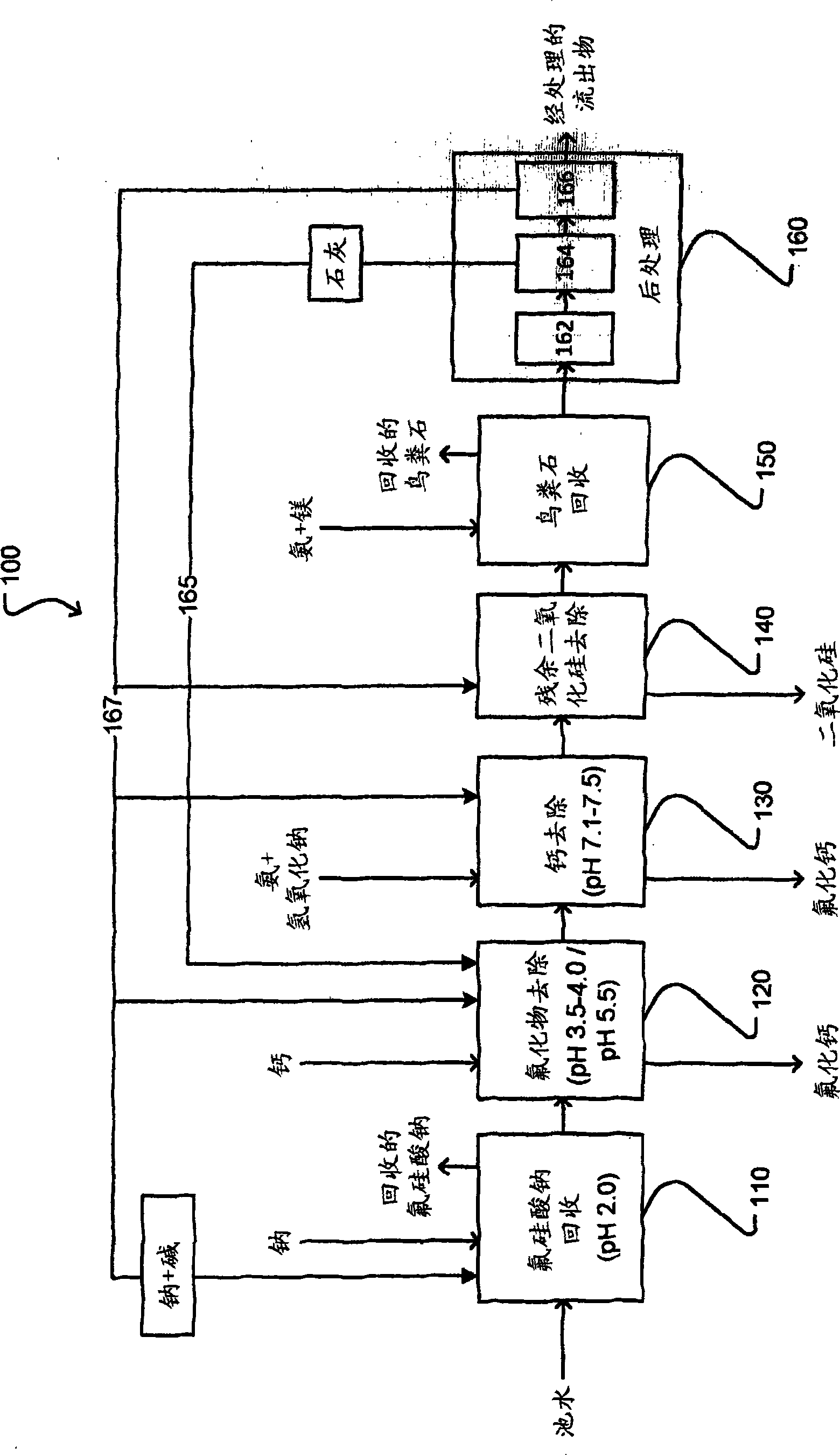
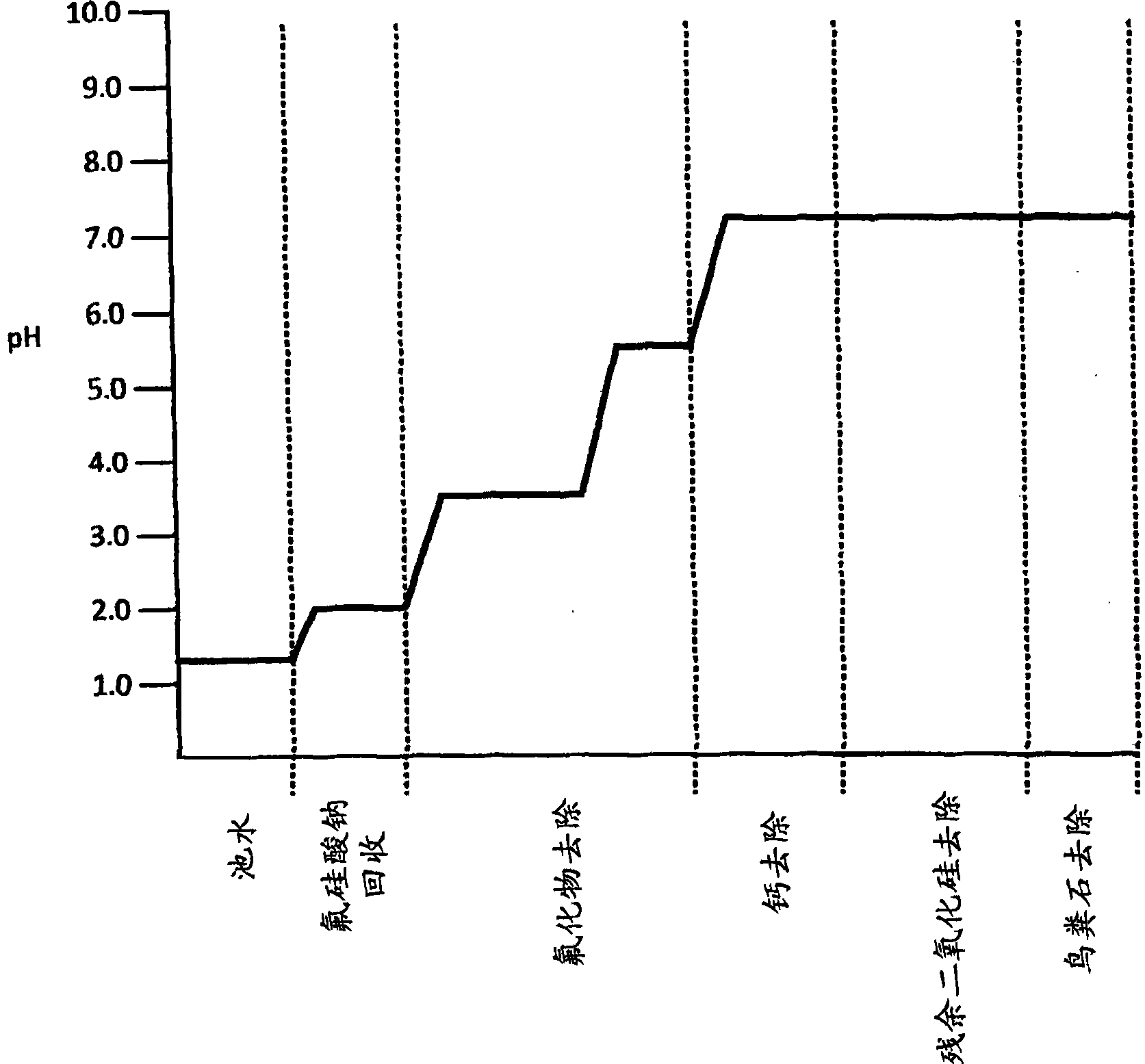
Treatment of phosphate-containing wastewater with fluorosilicate and phosphate recovery
ActiveCN103813987ARaise the pHWater contaminantsWater/sewage treatment bu osmosis/dialysisPhosphateWastewater
A method for treating phosphate-containing wastewater, such as phosphogypsum pond water. The method includes the steps of: (a) adding a first cation to the wastewater to precipitate fluorosilicate from the wastewater; (b) adding a second cation to the wastewater to precipitate fluoride from the wastewater; (c) raising the pH of the wastewater to precipitate the second cation from the wastewater; (d) removing residual silica from the wastewater; and (e) precipitating phosphate from the wastewater. The precipitated fluorosilicate may be sodium fluorosilicate. The precipitated phosphate may be struvite.
Owner:OSTARA NUTRIENT RECOVERY TECH INC
Melt purifying method for oxygen-free copper rod
The invention discloses a melt purifying method for an oxygen-free copper rod. The melt purifying method for the oxygen-free copper rod includes the following steps that S1, a copper material is subjected to alkaline washing and acid pickling, after being dried, the copper material is added into a smelting furnace to be molten, and then refining, standing and slag skimming are performed; S2, a purifying fluxing agent is added into melt and is composed of, by weight percentage, 60%-65% of sodium borate, 10%-15% of sodium fluorosilicate, 10%-15% of cryolite, 5%-10% of sodium chloride and 5%-10% of sodium carbonate, and high-purity nitrogen is continuously introduced into the melt; S3, a deoxidizing agent composed of a Cu-Re alloy and a Cu-B alloy is then added into the melt, and stirring is performed till uniform distribution is achieved; and S4, the melt is led to flow into a heat-preserving furnace, and the surface of the melt is covered with a layer of crystalline flake graphite used for isolating hydrogen and oxygen in air. The melt purifying method for the oxygen-free copper rod has both the impurity removing effect and the gas removing effect, and a guarantee is provided for producing the high-purity and high-conductivity oxygen-free copper rod.
Owner:安徽晋源铜业有限公司
Method for producing aluminun fluoride
The present invention relates to process of producing aluminum fluoride with fluorosilicic acid and aluminum hydroxide as material. The process includes the following steps: 1. reacting fluorosilicic acid solution and sodium sulfate for 10-60 min, filtering to obtain solid sodium fluorosilicate and waste sulfuric acid solution to be treated and drained; 2. decomposing aluminum fluorosilicate at 300-800 deg.c for 1-5 hr to produce solid sodium fluoride and silicon tetrafluoride gas; 3. absorbing silicon tetrafluoride gas with water and hydrolyzing, filtering to obtain fluorosilicic acid solution to be returned to the step 1 and solid silica, and washing and drying silica to obtain carbon white; 4. reacting solid sodium fluoride and sulfuric acid, condensing, rectifying to obtain anhydrous hydrofluoric acid and solid sodium sulfate returned to the step 1; and 5. reacting anhydrous hydrofluoric acid and aluminum hydroxide to produce aluminum fluoride product. The process is environment friendly.
Owner:DO FLUORIDE CHEM CO LTD
Preparation method of pyrite activator
ActiveCN105457760AImprove the activation effectEliminate calcium ionsFlotationDissolutionBiological activation
The invention provides a preparation method of a pyrite activator. The preparation method comprises the following steps: (1) dissolving copper sulfate in water at 95 DEG C and stirring properly until the copper sulfate is dissolved completely; (2) adding copper sulfate pentahydrate and ammonium sulfate in a weight ratio of 1: 1.6 to the completely dissolved copper sulfate solution for complete dissolution and reaction for 5-10 minutes; (3) after the reaction is complete, carrying out cooling precipitation till about 40 DEG C, pouring a supernatant liquid, namely an end solution, and then drying the precipitation solid to obtain light-blue crystals; and (4) carrying out a solid phase blending reaction to the obtained light-blue crystals and sodium fluorosilicate in a weight ratio of 35: 65 for 30 minutes, thereby obtaining a light-blue powdery product, namely the pyrite activator. The activator is capable of realizing activation of pyrites under a high alkalinity condition caused by lime.
Owner:GUANGXI ZHONGJIN LINGNAN MINING CO LTD
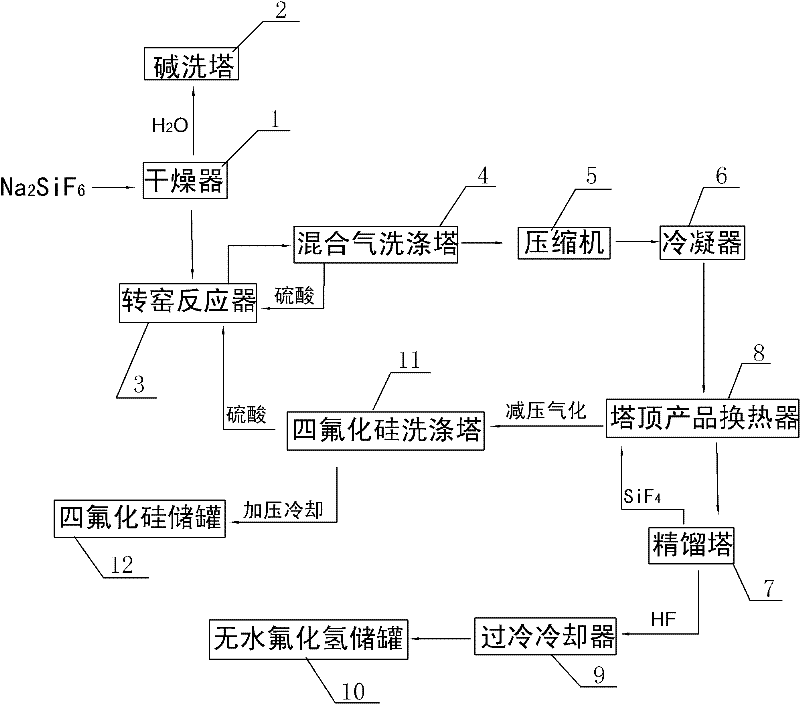
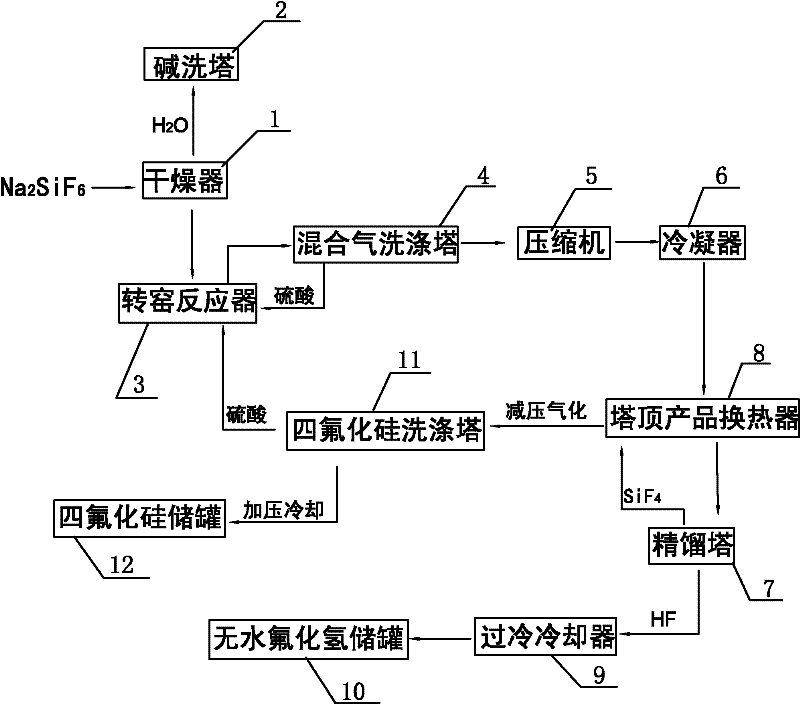
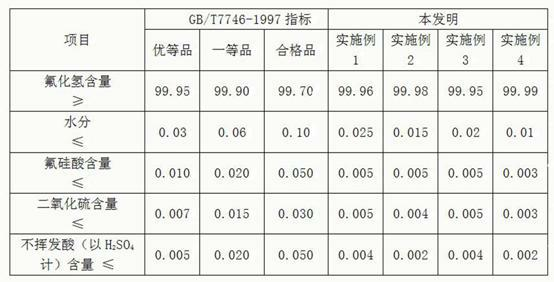
Method for preparing silicon tetrafluoride and anhydrous hydrogen fluoride by taking sodium fluorosilicate as raw material
InactiveCN102557043AWell mixedAdequate responseFluorine/hydrogen-fluorideHalogenated silanesSilicon tetrafluorideSodium sulfate
The invention discloses a method for preparing silicon tetrafluoride and anhydrous hydrogen fluoride by taking sodium fluorosilicate as a raw material. The method is characterized by comprising the following steps of: (1) drying sodium fluorosilicate for removing water; (2) adding the dried sodium fluorosilicate and sulfuric acid into a rotary kiln reactor in the molar ratio of 1:1, and reacting at the temperature of 160-220 DEG C for 30-180 minutes to generate a sodium sulfate solid and a mixed gas of silicon tetrafluoride and hydrogen fluoride; (3) dedusting, pressurizing and precooling to between 20 DEG C below zero and 30 DEG C below zero; (4) feeding the precooled mixed gas into a rectifying tower for separating, controlling the pressure of the rectifying tower at 0.6-0.75 MPaG and the temperature of tower top condenser between 70 DEG C below zero and 80 DEG C below zero, and discharging anhydrous hydrogen fluoride from the tower bottom; and (5) discharging silicon tetrafluoride from the tower top in the form of a liquid phase, decompressing, gasifying, dedusting and feeding into a silicon tetrafluoride washing tower to obtain silicon tetrafluoride gas. The method has the advantages that: corrosion to equipment can be reduced greatly, the obtained silicon tetrafluoride and hydrogen fluoride products have high purity, and the production process has low energy consumption and is environmentally-friendly.
Owner:SEDIN NINGBO ENG
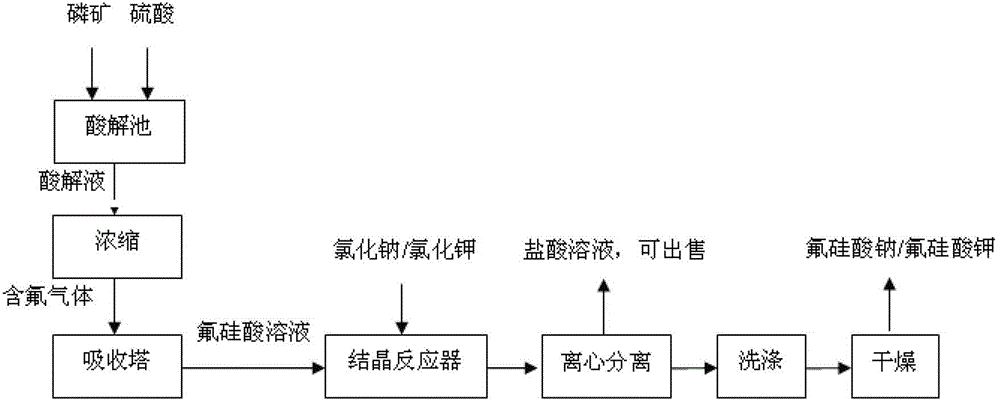
Resource comprehensive utilization method for recovering fluorine from wet-process phosphoric acid
InactiveCN103145131AQuality improvementReduce or eliminate hazardsSilicon halogen compoundsPotassium silicatePhosphoric acid
The invention relates to a resource comprehensive utilization method for recovering fluorine from wet-process phosphoric acid. The method comprises the following steps of: absorbing SiF4 and HF gases generated in the wet-process phosphoric acid by using water, and preparing the gases into a hydrofluosilicic acid solution of which the mass percent concentration is 10-35%; mixing one of four solids including solid sodium sulfate, solid potassium sulfate, solid sodium chloride and solid potassium chloride with the hydrofluosilicic acid solution, and reacting for 0.1-2 hours at 30-80 DEG C under stirring to obtain a mixture; centrifugally separating the obtained mixture to obtain a solid and a filtrate; and washing the solid with water, and drying for 1-3 hours at 100-120 DEG C to obtain a sodium fluorosilicate or potassium fluorosilicate crystal. According to the method, a byproduct hydrofluosilicic acid in the wet-process phosphoric acid and sodium (potassium) sulfates or chlorides which are abundant and easily-available are used as raw materials, so that the cost is low, two products including sodium fluorosilicate and potassium fluorosilicate can be produced at the same time by using one set of equipment, and the maximum utilization ratio of the hydrofluosilicic acid can reach over 99%.
Owner:KINGENTA NORSTERRA CHEM CO LTD
Preparation method of hydrofluoric acid
InactiveCN101134561ASilicon oxidesFluorine/hydrogen-fluorideSilicon tetrafluorideHexafluorosilicic acid
The process of producing hydrofluoric acid with fluorosilicic acid and magnesia as material includes the following steps: 1. reacting fluorosilicic acid solution and solid sodium sulfate for 10-60 min, filtering to obtain solid sodium fluosilicate, and treating waste sulfuric acid before draining; 2. decomposing sodium fluorosilicate at 300-800 deg.c for 1-5 hr to produce solid sodium fluoride and gaseous silicon tetrafluoride; 3. absorbing gaseous silicon tetrafluoride with water and hydrolyzing, and filtering to obtain fluorosilicic acid solution returned to the step 1 and silica, washing and drying to obtain carbon white; and 4. reacting the solid sodium fluoride and 98 % over sulfuric acid, condensing the produced gas condensing and rectifying to obtain hydrofluoric acid, and returning solid sodium sulfate to the step 1. The present invention utilizes fluorosilicic acid as side product from phosphate fertilizer production as material, and has low cost and environment friendship.
Owner:DO FLUORIDE CHEM CO LTD
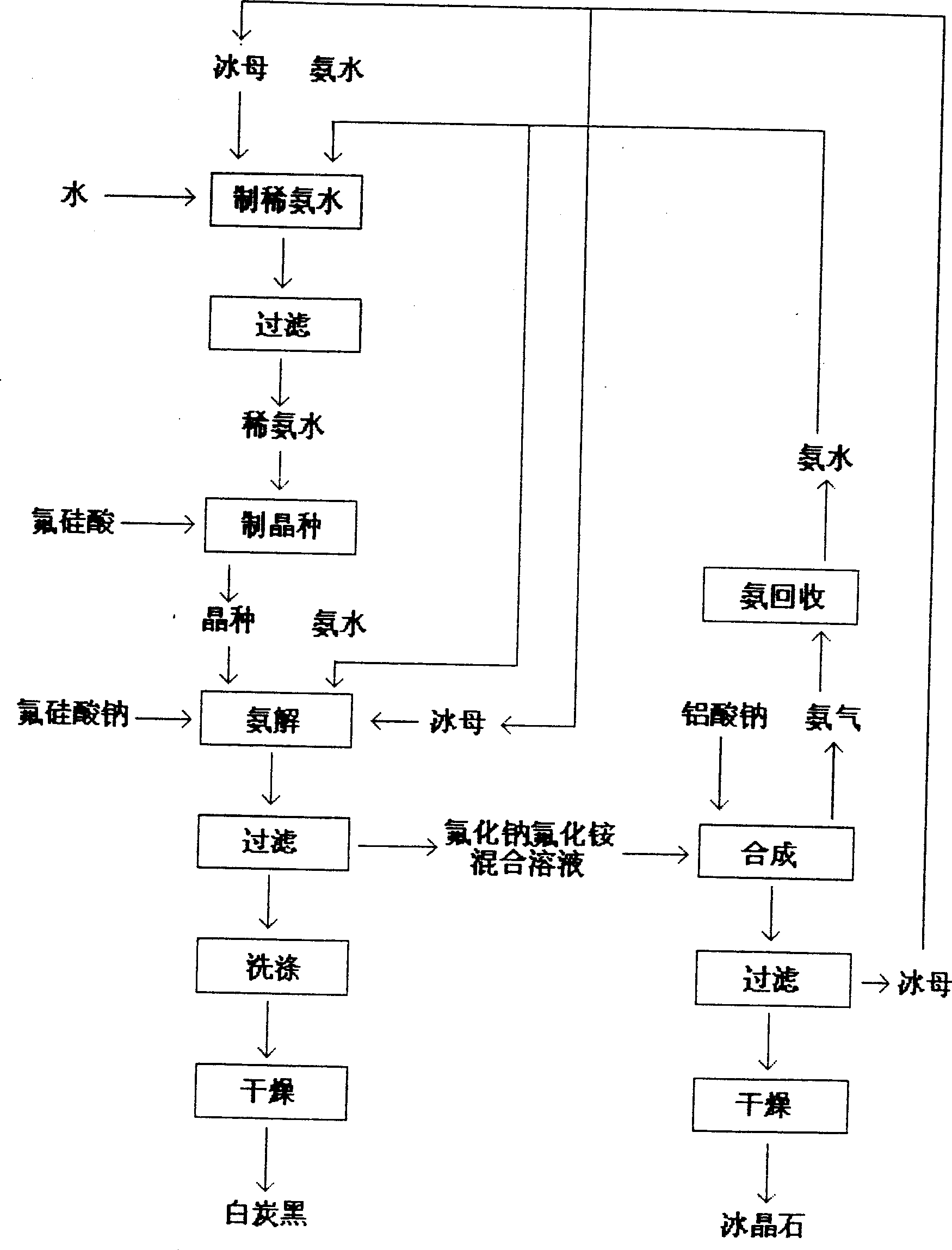
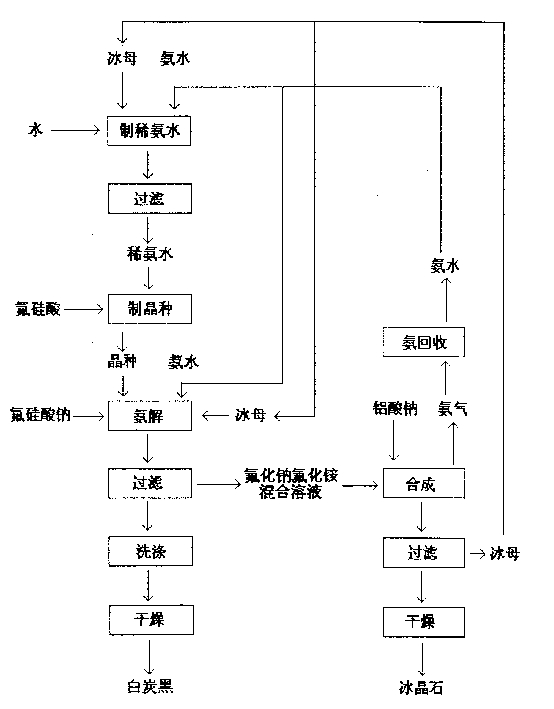
Method for simultaneously producing sodium aluminofluoride and silica white
InactiveCN1515493ALower molecular ratioHigh puritySilicon oxidesAluminium fluoridesReaction temperatureSodium aluminate
The method for producing ice spar and coproducing white carbon black includes the following steps: (1) adding ice mother and ammonia water in water to prepare dilute ammonia water, making fluorosilicic acid solution react with dilute ammonia water to obtain white carbon black crystal seed, controlling pH value of white carbon black in 6-8 and reaction temp. at 30-40 deg.C; (2). adding a certain quantity of white carbon black crystal seed into ammonolysis cell, in which the added quantity of crystal seed can be regulated according to the requirements, adding proper quantity of ice mother, in the presence of crystal seed making sodium fluorosilicate and ammonia water implement ammonolysis reaction at about 60 deg.C to obtain white carbon black slurry, filtering and washing to obtain white carbon black ointment, drying to obtain its finished product; (3). providing the steps for obtain ice spar product.
Owner:DO FLUORIDE CHEM CO LTD
Iron removal agent and iron removal method for recovering waste aluminum
Owner:ZHEJIANG JINFEI MACHINERY GROUP
Thermal insulating material and preparation method thereof
The invention discloses a thermal insulating material. The thermal insulating material is prepared from the following raw materials in parts by weight: 40-55 parts of pitchstone glazed bead, 4-5 parts of light-weight magnesium oxide, 0.5-1.2 parts of sodium fluorosilicate, 0.4-1 part of aluminum dihydrogen phosphate, 0.2-0.5 part of polyvinyl alcohol, 0.6-0.8 part of kaolin, 2-2.5 parts of fly ash, 2-3 parts of attapulgite, 2.5-3.5 parts of sepiolite fibre, 1-1.5 parts of disodium sulfosuccinate and 2-3 parts of cement. As for the thermal insulating material prepared from the raw materials in proportions, disclosed by the invention, the dry density can be less than 400kg / m<3>, the thermal conductivity can be less than 0.085W / (m.k), and the fireproof performance can reach the national A2-level requirements. In addition, the thermal insulating material has excellent characteristics of high strength, aging resistance and sound absorption, and is long in service life.
Owner:广西青龙化学建材有限公司
Production method of high-purity nanometer zinc oxide from low-grade zinc oxide ore by ammonia decarbonization
ActiveCN102849783AControl growthRaise the pHMaterial nanotechnologyZinc oxides/hydroxidesBrickPhase composition
The invention discloses a production method of high-purity nanometer zinc oxide from low-grade zinc oxide ore by ammonia decarbonization. The method comprises the steps of taking ammonia water-ammonium carbonate solution as a leaching agent; adding 0.3-0.5 kg sodium fluorosilicate into per cubic meter leaching agent; leaching low-grade zinc oxide ore with the leaching agent; and adding 50-60 kg slaked lime into per cubic meter leachate to perform decarbonization treatment. The obtained zinc oxide nanopowder has purity not less than 99.7%, uniform particle size distribution (average particle size of 10-28 nm), specific surface area of not less than 107 m<2> / g, good fluidity and dispersibility. The inventive treatment method is low in energy consumption and high in efficiency, and the leaching agent can be recycled. The leaching residue, without destruction of original mineral component phase composition, can still be used for brick making, so as to realize dual purposes of economy and environment protection, and achieve high economic and social benefits.
Owner:SICHUAN JUHONG TECH
Additive for saltresistance series concrete
An additive for preparing the saline-resistant concrete able also to resist against freezing, osmosis and corrosion is proportionally prepared from sodium beta-nephthalenesulfonate-formaldehyde polycondensate, sodium sulfate, rosin thermopolymer, magnesium powder, alkylphenyl sulfonate, sodium fluorosilicate, triethanolamine, sodium nitrite plus sodium sulfate, and hydroxycarboxylic acid.
Owner:QINGHAI UNIVERSITY +1
Refractory castable in use for homogeneous stopper plug of floating plug, and preparation method
InactiveCN1760159ASignificant fire resistanceSignificant craftsmanshipManufacturing convertersSlagGranularity
A refractory casting material for the uniform slag plug is prepared from the weighting aggregate (25-40 Wt%) and refractory mixture (60-75) through proportionally mixing metallic filings, super-grade high-Al clinker, brown corundum, silicon micropowder, alpha-Al2O3 powder, water glass, sodium fluorosilicate and melamine, mashing to become blocks, baking, pulverizing, sieving, and proportionally mixing it with refractory mixture.
Owner:武钢集团有限公司
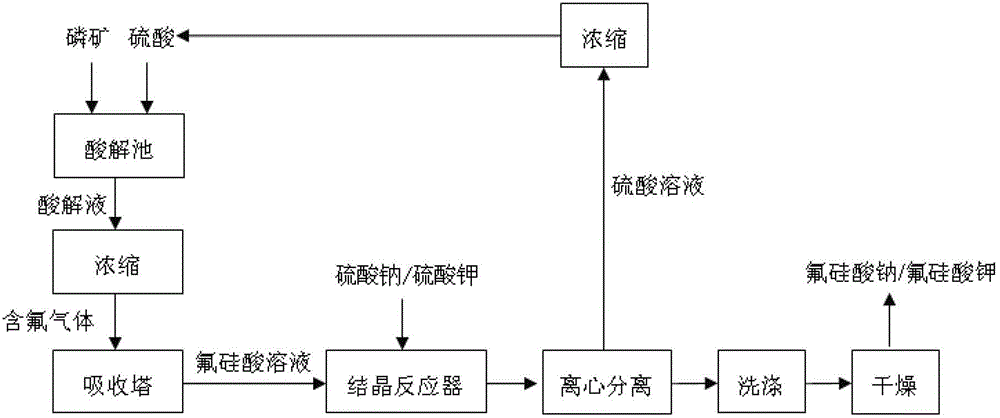
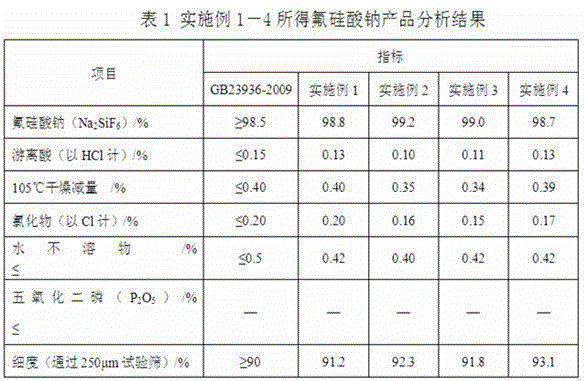
Technology for preparing sodium fluorosilicate through fluoride-containing wastewater
InactiveCN105174270AAchieve recyclingRealize comprehensive utilizationSilicon halogen compoundsSlagEconomic benefits
The invention discloses a technology for preparing sodium fluorosilicate through fluoride-containing wastewater. The technology comprises the following steps that 1, silicon slag is added in the fluoride-containing wastewater at 10 DEG C-35 DEG C, the stirring speed is set at 350-450 r / min, the reaction time is set at 15-35 min, and then filtering is performed to obtain a fluosilicic acid solution; 2, a sodium chloride solution is added in the fluosilicic acid solution, the reaction temperature is controlled at 10 DEG C-35 DEG C, the reaction time is controlled to be 20 min 40 min, filtering is performed, and the sodium fluorosilicate product is obtained when a filter cake is dried. According to the technology for preparing the sodium fluorosilicate through the fluoride-containing wastewater, after the industrial fluoride-containing wastewater is treated, the fluorine content in the wastewater is reduced to 0.1 g / L-0.5 g / L, the content of aluminum and boron is not basically changed, the treated wastewater can be recycled through deep fluoride removal, therefore, recycling of fluorine and silicon resources can be achieved, the production cost is lowered, and the better economic benefit and environmental protection benefit are achieved.
Owner:湖南有色氟化学科技发展有限公司
Method for defluorination, purification and separation of wet-process phosphoric acid
ActiveCN102730657AGuaranteed separation effectFull accessSilicon halogen compoundsPhosphorus compoundsFiltrationBelt filter
The present invention discloses a method for improving phosphorus yield by defluorination, purification and separation of wet-process phosphoric acid, and belongs to the field of wet-process phosphoric acid production. The method comprises the following steps: a, adding a reaction amount of a defluorination agent to wet-process phosphoric acid to carry out a compete reaction; b, adding the material slurry from the step a to a scroll discharge sedimentation centrifuge to carry out solid-liquid separation, and adding the separated liquid to a defluorinated phosphoric acid storage tank; c, adding hot water to the solid-liquid separated solid from the step b to carry out slurry conditioning, wherein density of the material slurry is 1.35-1.50 g / ml; and d, conveying the conditioned material slurry from the step c to a belt filter to carry out filtration and countercurrent washing, wherein the liquid after filtration and countercurrent washing enters the defluorinated phosphoric acid storage tank, and the solid after filtration and countercurrent washing is the by-product sodium fluorosilicate. With the method of the present invention, phosphorus yield in the defluorination purification process can be increased to more than 99% from 95%, and pure sodium fluorosilicate by-product can be obtained, such that comprehensive utilization of resources is achieved.
Owner:GUIZHOU CHANHEN CHEM CO LTD
A method for producing anhydrous hydrogen fluoride and silicon tetrafluoride by utilizing fluosilicic acid
InactiveCN102275877AImprove conversion rateLow costFluorine/hydrogen-fluorideHalogenated silanesSlagHexafluorosilicic acid
The invention discloses a method for producing anhydrous hydrogen fluoride and silicon tetrafluoride by utilizing fluosilicic acid. The method of the invention is specifically to firstly react fluosilicic acid solution with sodium sulfate at normal temperature to obtain sodium fluosilicate and dilute sulfuric acid. Filtration, dilute sulfuric acid is concentrated to make concentrated sulfuric acid, then mixed with filter cake sodium fluorosilicate ointment, added to the pre-reactor, reacted at 50-200 ° C to generate silicon tetrafluoride gas, and the silicon tetrafluoride gas passes through the degassing tower Silicon tetrafluoride products are obtained after treatment; the remaining materials in the pre-reactor are transported to the rotary furnace, and then hydrogen fluoride gas is released in the rotary furnace at 150-350 °C, and the hydrogen fluoride gas is purified to produce anhydrous hydrogen fluoride products. The remaining slag in the rotary kiln is discharged through the furnace tail. The fluosilicic acid solution used in the present invention is derived from the by-products of phosphate fertilizer or anhydrous hydrogen fluoride, the cost of raw materials is low, a new source of fluorine is opened up, and the obtained anhydrous hydrogen fluoride and silicon tetrafluoride products have wide application fields and outstanding economic benefits , high value-added products.
Owner:DO FLUORIDE CHEM CO LTD
Method for producing high-purity nano-zinc oxide by ammonia decarburization of electrolytic zinc acid-leaching residues
ActiveCN102838158AExcellent liquidityExcellent dispersionMaterial nanotechnologyZinc oxides/hydroxidesSocial benefitsElectrolysis
The invention discloses a method for producing high-purity nano-zinc oxide by ammonia decarburization of electrolytic zinc acid-leaching residues, wherein when leaching, 0.3-0.5kg of sodium fluorosilicate is added to per cubic meter of ammonia-ammonium bicarbonate solution, and then 30-60kg of slaked lime is added to per cubic meter of a leaching agent for performing decarburization and refining treatment; the method can obtain nano-zinc oxide powder with the purity greater than and equal to 99.7%, uniform particle size distribution (wherein the average particle size is 10-30nm), the specific surface area greater than and equal to 105m<2> / g, and excellent fluidity and dispersibility; the treatment method provided by the invention has the characteristics of low energy consumption, high efficiency, and higher economic and social values; all the valuable and harmful heavy metals in the electrolytic zinc acid-leaching residues are leached to be rationally used and cleaned with water; and finally, the obtained final leaching residues are turned from the electrolytic zinc acid-leaching residues as high-risk wastes into general solid wastes, thereby creating good economic and social benefits.
Owner:SICHUAN JUHONG TECH
Silicon Production Process
An improved process for producing high purity silicon results from the reaction of sodium with pure silicon tetrafluoride gas, which produces sodium fluoride as a by-product. The silicon tetrafluoride gas is formed by decomposing sodium fluorosilicate. The sodium fluorosilicate is produced by precipitation when fluorosilicic acid (FSA) is reacted with the by-product sodium fluoride in closed loop process. Likewise, the fluorosilicic acid is preferably formed at high purity using a source material that consists essentially of silica by reacting the by-product sodium fluoride with an acid to create reactive fluoride ions.
Owner:CIRCULON HUNGARY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com