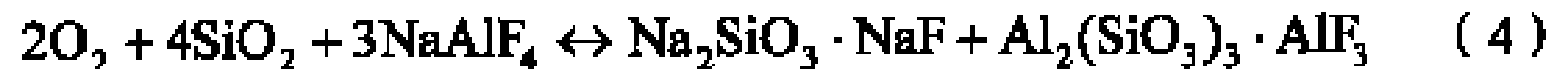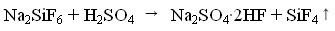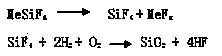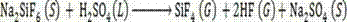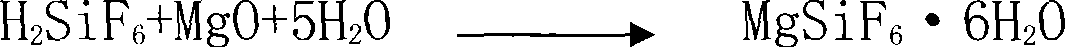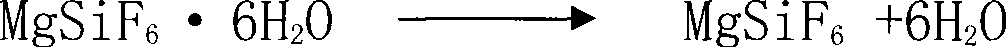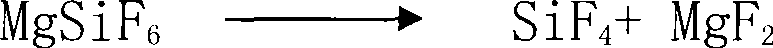Patents
Literature
300 results about "Silicon tetrafluoride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Silicon tetrafluoride or tetrafluorosilane is the chemical compound with the formula SiF₄. This colorless compound is notable for having a narrow liquid range: its boiling point is only 4 °C above its melting point. It was first synthesized by John Davy in 1812. It is a tetrahedral molecule.
Ethylene/tetrafluoroethylene copolymer molded product and method for its production
ActiveUS20090214801A1Highly hydrophilic surfaceGood light transmissionLiquid surface applicatorsChemical vapor deposition coatingSilicon tetrafluorideSilicon oxide
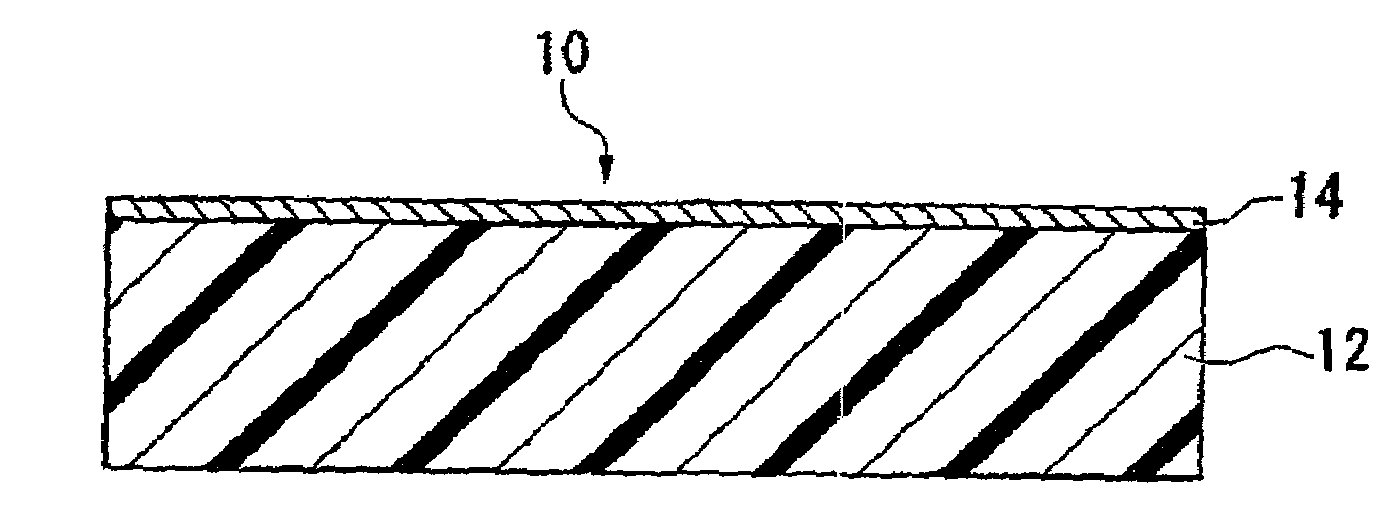
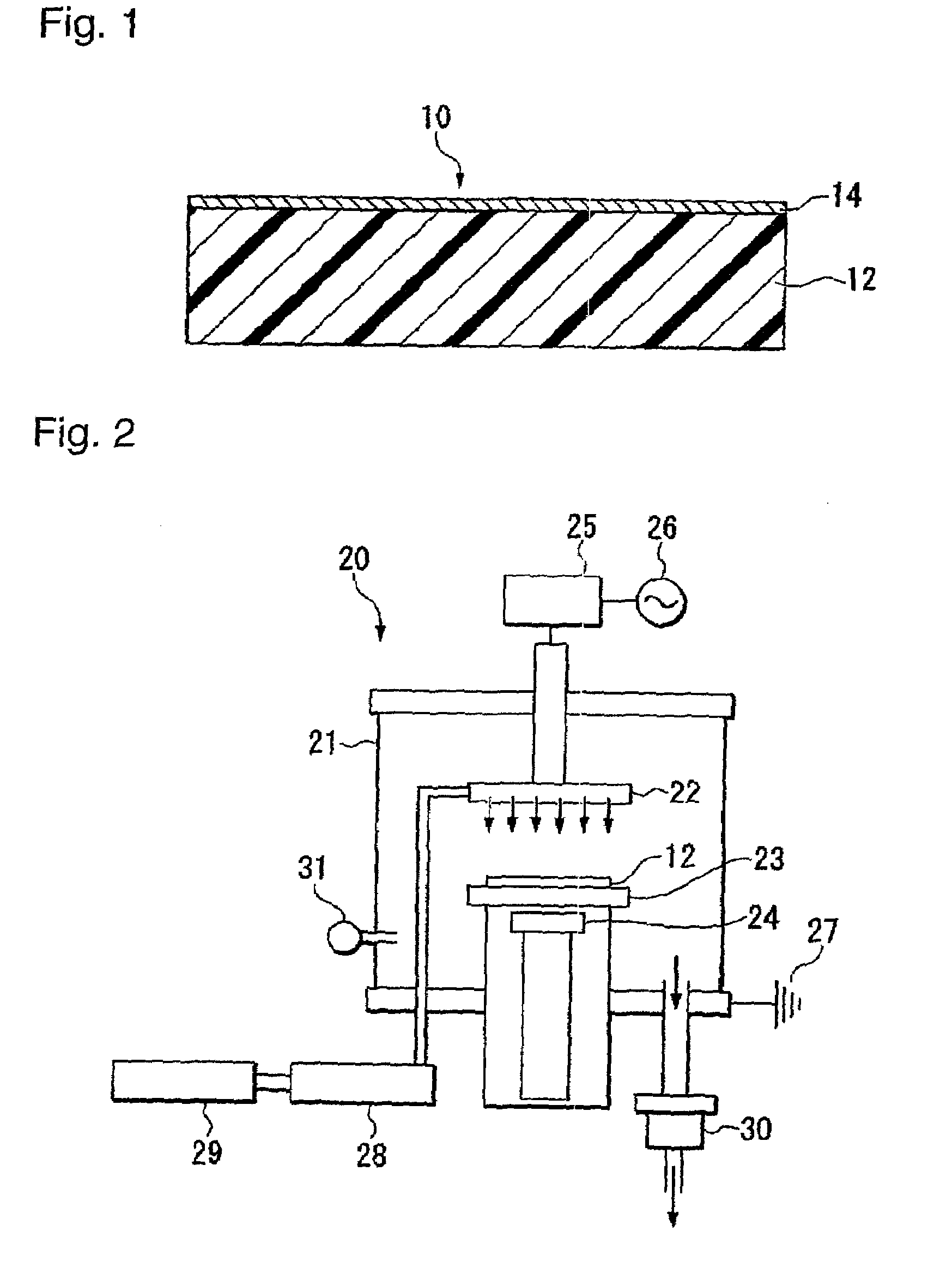
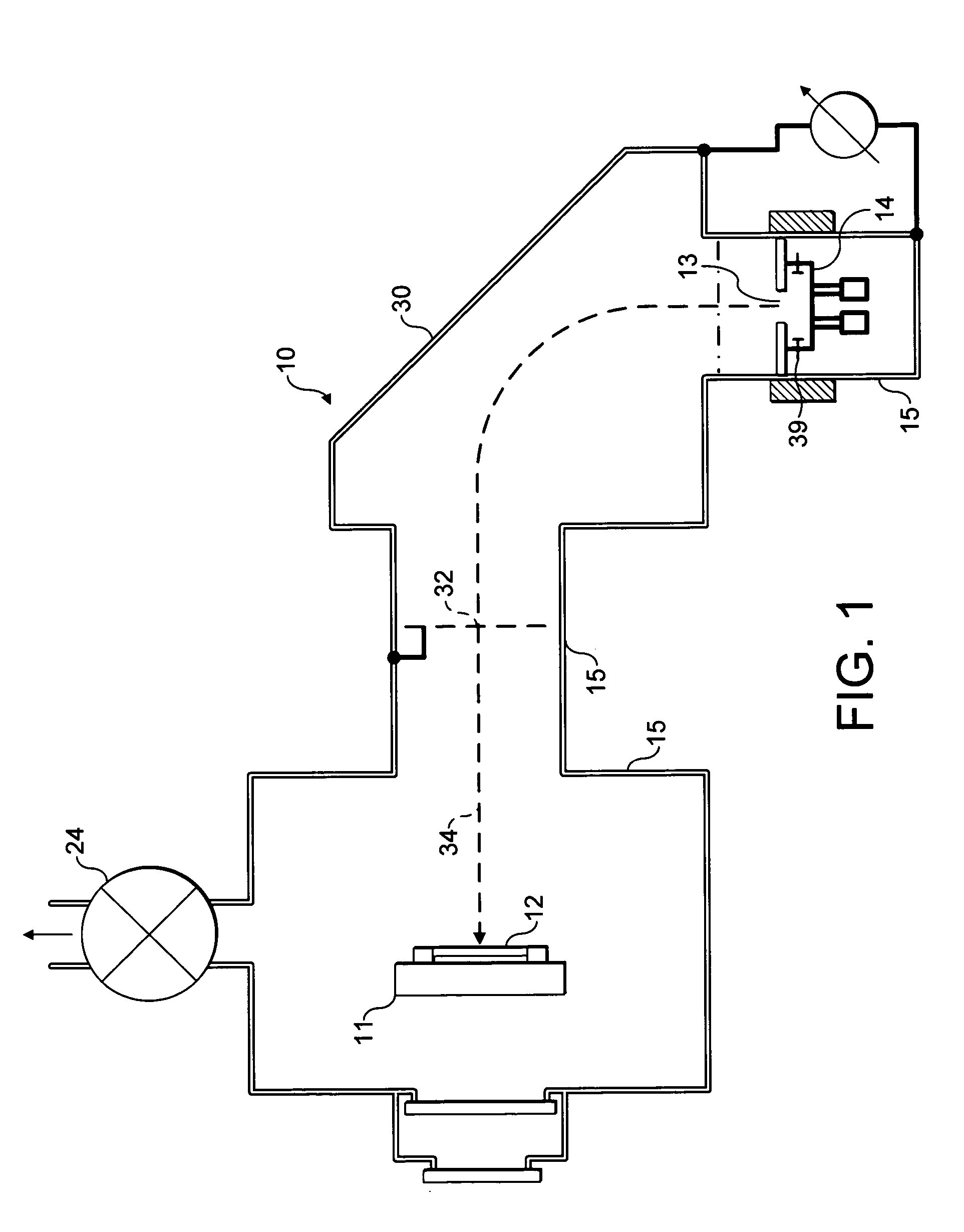
To provide an ethylene / tetrafluoroethylene (ETFE) copolymer molded product of which the surface has a high hydrophilicity and which is excellent in the light transmission property; and a method for producing the molded product.A method for producing an ETFE copolymer molded product comprising a substrate 12 made of a material containing at least 50 mass % of an ETFE copolymer, and a fluorine-doped silicon oxide film 14 formed on the surface of the substrate 12, characterized by supplying electric power between electrodes so that the electrical power density between the electrodes becomes from 0.5 to 1.1 W / cm3 to cause discharge and thus to plasmatize the following mixed gas so as to form the silicon oxide film 14 on the substrate 12: Mixed gas comprising silicon tetrafluoride, oxygen and a hydrocarbon, wherein the atomic ratio of oxygen atoms to carbon atoms (O / C) is from 1 to 10, and the atomic ratio of oxygen atoms to silicon atoms (O / Si) is from 1.7 to 25.
Owner:ASAHI GLASS CO LTD
Method for comprehensively recovering tungsten and fluorine from minerals
InactiveCN102586632AHigh recovery rateImprove solubilitySilicaProcess efficiency improvementDecompositionApatite
The invention provides a method for comprehensively recovering tungsten and fluorine from minerals, namely a mixed acid of phosphoric acid and sulfuric acid is adopted for decomposing complex calcium-containing minerals containing fluorite, scheelite, apatite, and calcite, wherein the fluorite is decomposed to fluorine hydride or silicon tetrafluoride to escape, and absorption treatment is performed for preparing hydrofluoric acid or a fluoride salt; and the scheelite is transformed to phosphotungstic acid to enter into a solution, and filtrate after filtration is supplemented into the consumed sulfuric acid and the phosphoric acid after extraction of the tungsten and returned to the new-round mineral leaching. The method disclosed by the invention has the advantages of comprehensively recovering the fluorine and the tungsten from the minerals, reducing the requirements on the fluorite or the tungsten ore raw material, reducing the pressure on a mineral dressing link, improving the comprehensive recovery rate and simultaneously ensuring the decomposition rate of the fluorite and the scheelite, wherein the decomposition rate of the fluorite is above 98%, and WO3 contained in decomposition slag is reduced to below 0.5%; furthermore, a leaching agent can be recycled, so that leaching cost and wastewater emission are greatly reduced; and the method also has the advantages of simple leaching equipment, convenience in operation and easiness in realization of industrialization.
Owner:CENT SOUTH UNIV
Method for preparing silicon tetrafluoride
ActiveCN101544374AImprove conversion rateTo avoidHalogenated silanesState of artSilicon tetrafluoride
The present invention discloses a method for preparing silicon tetrafluoride, including a) mixing a raw material powder containing NaAlF4 and Si source powder; b) heating the mixture of the step a) with sulfuric acid to 240 DEG C to 350 DEG C to react to obtain silicon tetrafluoride. The silicon tetrafluoride is prepared by a reaction of the raw material containing NaAlF4 powder and Si source and sulfuric acid under a heating condition, comparing with the present technology, it is not only capable of avoiding to use a great amount of fluorite to prepare the silicon tetrafluoride, which reduces cost, it is also capable of using the accessory substances NaAlF4 generated during a process for producing the silicone hydride effectively, and preparing the silicon tetrafluoride with a high percent conversion.
Owner:YINGLI ENERGY CHINA
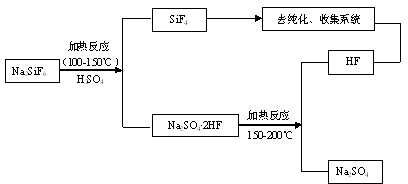
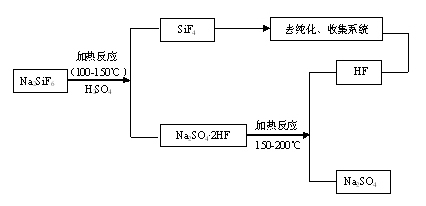
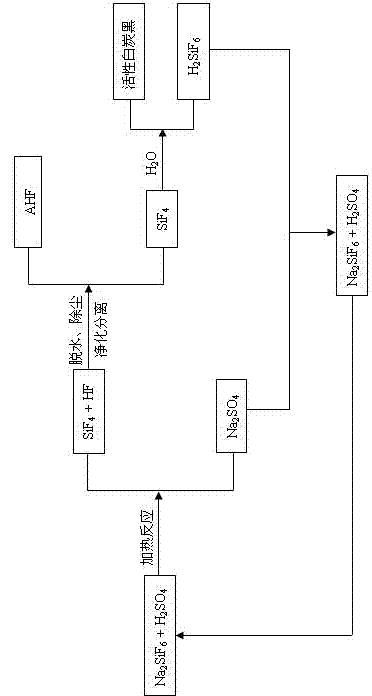
Method for preparing silicon tetrafluoride and anhydrous hydrogen fluoride from sodium fluosilicate acidified by sulfuric acid
InactiveCN101948114ATake advantage ofEfficient use ofFluorine/hydrogen-fluorideHalogenated silanesChemical industryHydrogen fluoride
The invention provides a method for preparing silicon tetrafluoride and anhydrous hydrogen fluoride from sodium fluosilicate acidified by sulfuric acid, which relates to the technical field of fluorine chemical industry, in particular to a method for preparing silicon tetrafluoride gas and anhydrous hydrogen fluoride gas by reaction of sodium fluosilicate and sulfuric acid. The method of the invention comprises the following steps: (a) stirring sodium fluosilicate and excessive sulfuric acid at the temperature lower than 150 DEG C for reacting to obtain silicon tetrafluoride gas, wherein hydrogen fluoride remains in solid residues; (b) dedusting, cooling, drying, refining and compressing the silicon tetrafluoride gas generated in the step (a) to obtain high-purity silicon tetrafluoride gas; (c) continuously heating to 200 DEG C to enable the hydrogen fluoride gas to escape; and (d) dedusting, cooling, drying, refining and compressing the hydrogen fluoride gas escaping in the step (c) to obtain the anhydrous hydrogen fluoride (AHF) gas. The invention enables the specific fluorine and silicon resources of phosphate fertilizer enterprises to be fully and efficiently utilized.
Owner:YUNNAN CHEM RES INST
Ion sources
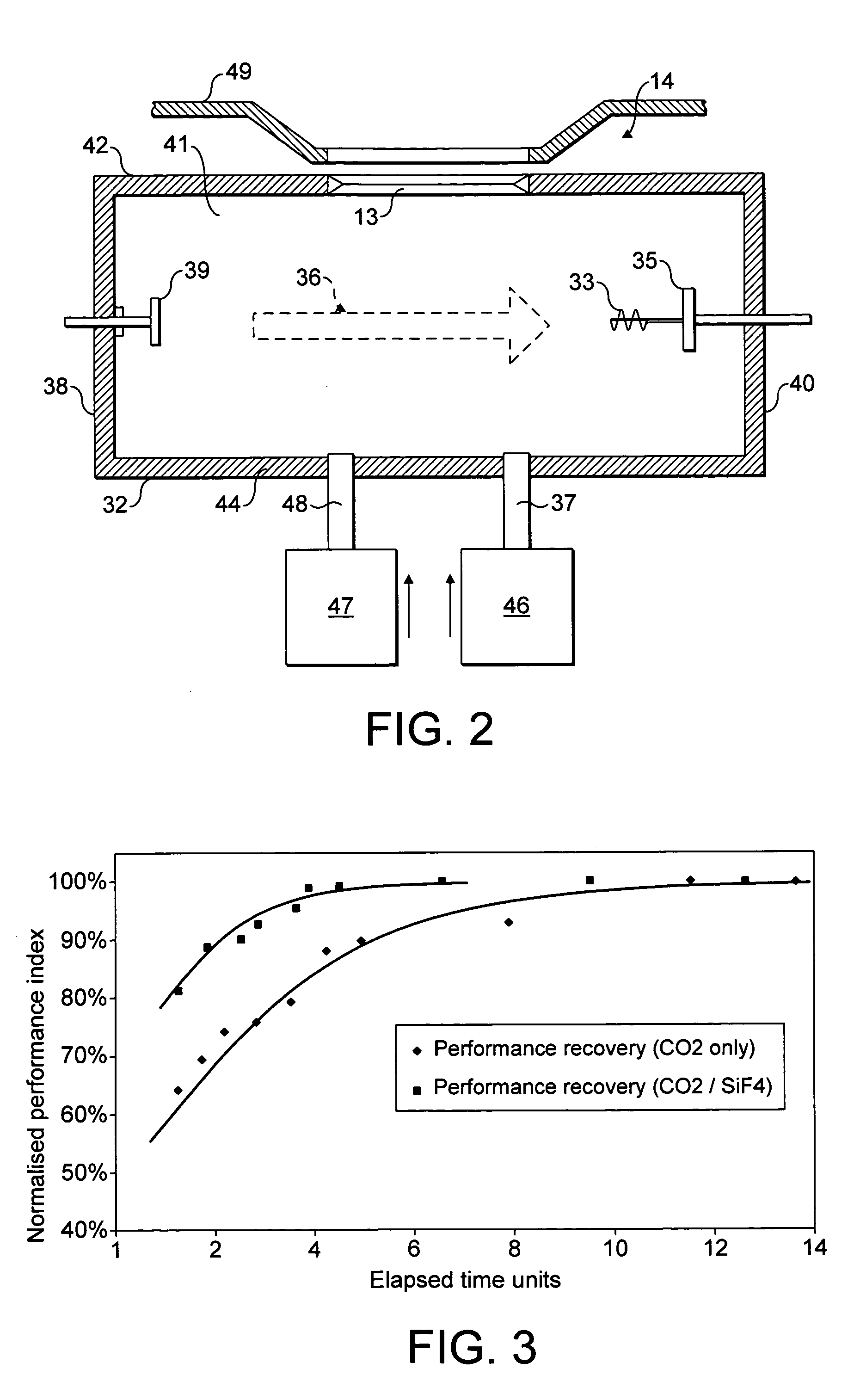
ActiveUS20050211923A1Reduce formationImprove efficiencySemiconductor/solid-state device manufacturingMaterial analysis by optical meansChemical reactionPhysical chemistry
The invention relates to methods of controlling the effect of ions of an ionisable source gas that can react with interior surfaces of an arc chamber, by introducing ions of a displacement gas into the arc chamber, where the displacement gas ions are more chemically reactive with the material of the interior surfaces than the ions of the source gas. The source gas ions may typically be oxygen ions and the displacement gas ions are then typically fluorine ions where the interior surfaces comprise tungsten. The fluorine ions may, by way of example, be sourced from fluorine, silicon tetrafluoride or nitrogen trifluoride.
Owner:APPLIED MATERIALS INC
Method for preparing silicon tetrafluoride through pyrolysis of sodium fluosilicate in rotary reaction furnace
ActiveCN101698482AAct as a diluentImprove liquidityHalogenated silanesWastewaterSilicon tetrafluoride
The invention provides a process for preparing silicon tetrafluoride through pyrolysis of sodium fluosilicate in a rotary reaction furnace, which comprises the steps of: performing drying and heat treatment on the sodium fluosilicate at a temperature of between 200 and 300 DEG C in a calciner to remove the moisture in the sodium fluosilicate, and maintaining a negative pressure in the calciner, wherein the negative pressure value is controlled to between 10 and 30mmH2O; and sending the dried sodium fluosilicate into the rotary reaction furnace for the pyrolysis at a temperature of between 500 and 900 DEG C (material temperature) for 1 to 2 hours, and obtaining the silicon tetrafluoride and sodium fluoride after the pyrolysis. The generated silicon tetrafluoride gas is dedusted, cooled, dried, compressed and collected to form high-purity silicon tetrafluoride gas. The process has the advantages of single reactant and no discharge of waste gas, waste water and waste residue, and belongs to an environmentally-friendly comprehensive utilization project.
Owner:西安三瑞实业有限公司 +1
Method for producing calcium fluoride
InactiveCN101134595AReduce total usageSave resourcesCalcium/strontium/barium fluoridesCalcium silicateSilicon tetrafluoride
The process of producing calciumfluoride with fluorosilicic acid and calcium oxide as material includes the following steps: 1. reacting fluorosilicic acid solution and calcium oxide for 10-60 min, filtering to obtain solid calcium fluorosilicate; 2. decomposing calcium fluorosilicate at 200-600 deg.c for 1-5 hr to produce solid calcium fluoride product and silicon tetrafluoride gas; and 3. absorbing silicon tetrafluoride gas with water and hydrolyzing, filtering to obtain fluorosilicic acid solution to be returned to the step 1 and solid silica, and washing and drying silica to obtain carbon white. The process utilizes fluorosilicic acid as one side product of phosphate fertilizer production as one main material, and has low cost and environment friendship.
Owner:DO FLUORIDE CHEM CO LTD
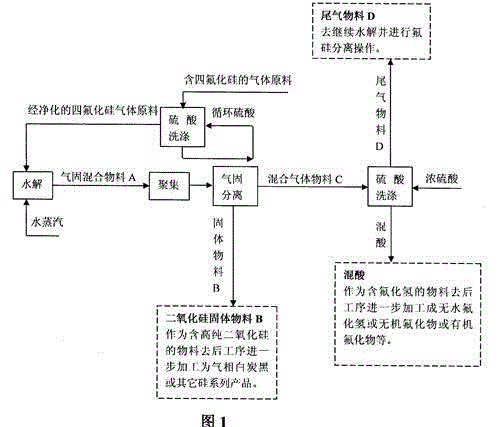
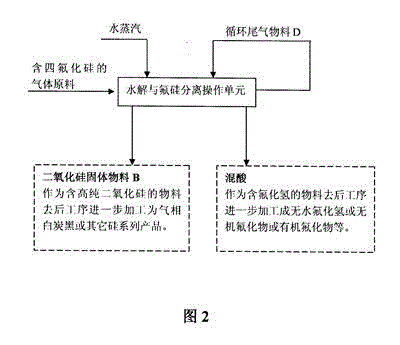
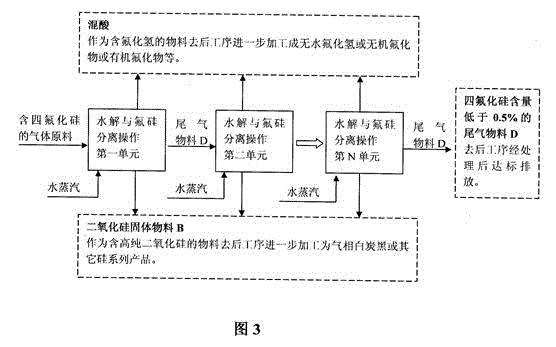
Gas phase hydrolysis and fluoride-silicon separation method of silicon tetrafluoride
InactiveCN103601195ASimple purification methodLow hydrolysis temperatureSilicaFluorine/hydrogen-fluorideChemical industrySilicon tetrafluoride
A two-step method is adopted to carry out low temperature gas phase hydrolysis and fluoride-silicon separation operations of silicon tetrafluoride. In a first step, a silicon tetrafluoride gas is hydrolyzed into a fluosilicic acid gas and silicon dioxide particles at a low temperature; in a second step, a hydrogen fluoride gas is dissolved by a washing operation of high concentrated sulfuric acid so as to promote complete decomposition of the fluosilicic acid into the hydrogen fluoride gas and a silicon tetrafluoride gas; decomposed and separated hydrogen fluoride gas is completely dissolved in high concentrated sulfuric acid; decomposed and separated silicon tetrafluoride can be continuously carried out hydrolysis and fluoride-silicon separation operations; and finally complete separation of the fluoride and silicon elements are realized. The method has the beneficial effects that a gas raw material containing relatively low silicon tetrafluoride content can be used; a method for purifying the raw material is simple; total conversion rate of silicon tetrafluoride introduced into a production apparatus system can reach over 98%; and hydrolysis temperature is relatively low, so that the method can realize industrial production relatively easily. The method is suitable for recovery and utilization of fluoride- and silicon-containing materials from industries such as phosphorus chemical industry, fluorine chemical industry, electron industry, glass processing industry and aluminium alloy processing industry.
Owner:班仁义
Method of utilizing biochar for adsorbing orange II dye waste water and catalytically degrading the orange II dye waste water with persulfate
InactiveCN106219724AImprove catalytic performanceGood removal effectPhysical/chemical process catalystsOther chemical processesCellulosePersulfate
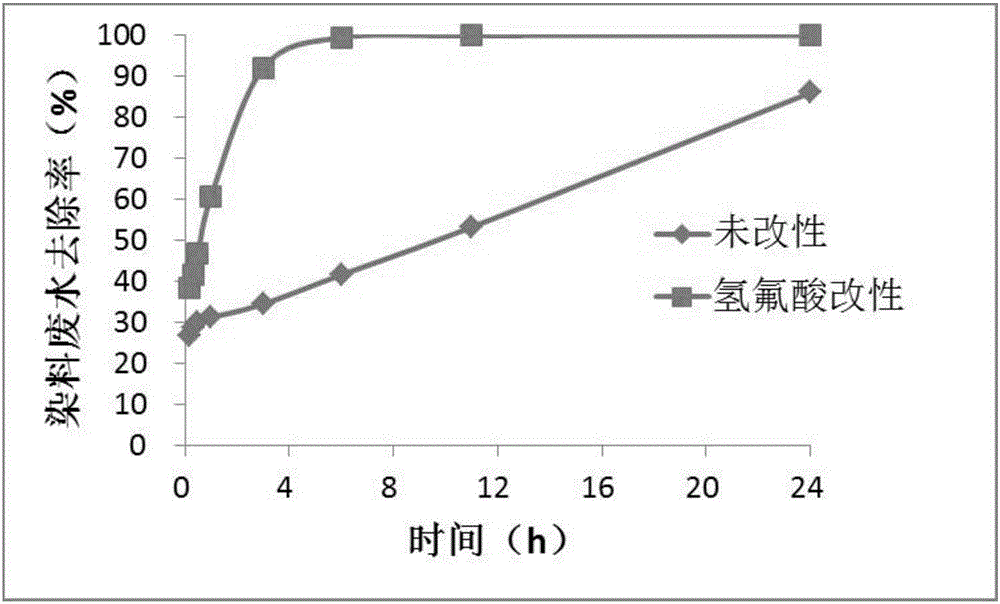
The invention provides a method of utilizing biochar for adsorbing orange II dye waste water and catalytically degrading the orange II dye waste water with persulfate. The method includes the steps of: 1) preparation of the biochar modified by hydrofluoric acid: adding rice hull to a hydrofluoric acid solution, mixing and stirring the solution, filtering the solution and washing the rice hull to neutralization, and drying the rice hull to prepare modified biomass, and pyrolyzing and carbonizing the modified rice hull biomass in a biochar producing apparatus to obtain the biochar; 2) dye waste water degradation: mixing persulfate with the orange II dye waste water and adding the biochar to the mixed solution until the pH value of the mixed solution is 3-10 to carry out a reaction for 4-12 h to complete the degradation of the orange II dye waste water. The rice hull, or rice straw, contains a large quantity of silicon dioxide existing in cellulose and lignin in a net-like status, so that through modification by the hydrofluoric acid, silicon is reacted with fluorine to obtain gas silicon tetrafluoride, thereby forming porous structures on the surface of the rice hull. The rich hull than is pyrolyzed into the biochar, wherein the porous structure are maintained and further improved, so that the modified bichar is significantly increased in specific surface area and has a more reasonable pore size distribution, which promote the process of catalyzing degradation of the dye waste water with persulfate.
Owner:ANHUI SCI & TECH UNIV
Industrial fluosilicic acid purification and white carbon black co-production method
ActiveCN104843712ALow costEasy to controlSilicaSilicon halogen compoundsSilicon tetrafluorideProduct gas
An industrial fluosilicic acid purification and white carbon black co-production method belongs to the technical field of inorganic acid chemical production and purification. The method comprises the following steps: contacting an industrial fluosilicic acid solution with silica powders and carrying out heating, and collecting silicon tetrafluoride gas obtained after heating; absorbing the obtained silicon tetrafluoride gas directly by absorption liquid, and carrying out hydrolysis reaction to obtain a mixture of a fluosilicic acid solution and hydrated silicon dioxide solid; and separating the mixture to obtain the fluosilicic acid solution and the hydrated silicon dioxide solid. The method does not waste fluorine atoms in the purification process, and thus application range and additional value of the industrial fluosilicic acid are improved.
Owner:BEIJING UNIV OF CHEM TECH
Method for aminolysis of fluorosilicone compounds and separation of fluorine and silicon
InactiveCN1884077AOvercoming the disadvantages of separation methodsImprove conversion rateSilicon halogen compoundsAmmonium halidesPhosphoric acidSilicon tetrafluoride
The invention discloses a separating method of aminolysis and hydrofluosilicic element of silicofluoride, which comprises the following steps: 1. adding solid ammonium fluosilicate or silicon tetrafluoride gas in the saturated ammonium fluoride circulating reacting liquid; putting raw material amino to proceed aminolysis reaction; 2. reducing slurry temperature; screening the reacted slurry; 3. filtering the screened silica suspension and ammonium fluoride crystal slurry to obtain saturated ammonium fluoride solution, silica solid and ammonium fluoride crystal; returning to former procedures as the circulating reacting liquid; washing the ammonium fluoride crystal; drying through normal drying method to obtain high-purity product. The invention can improves technological and economical index, which is fit for wet-producing phosphoric acid, phosphate fertilizer and other phosphor businesses.
Owner:GUIYANG KAILIN FERTILIZER CO LTD
Method for preparing fumed silica and anhydrous hydrofluoric acid by utilizing phosphatic fertilizer by-product fluosilicate as raw material
InactiveCN103420383AIncrease profitHigh purityChemical industrySilicon oxidesDistillationSilicon tetrafluoride
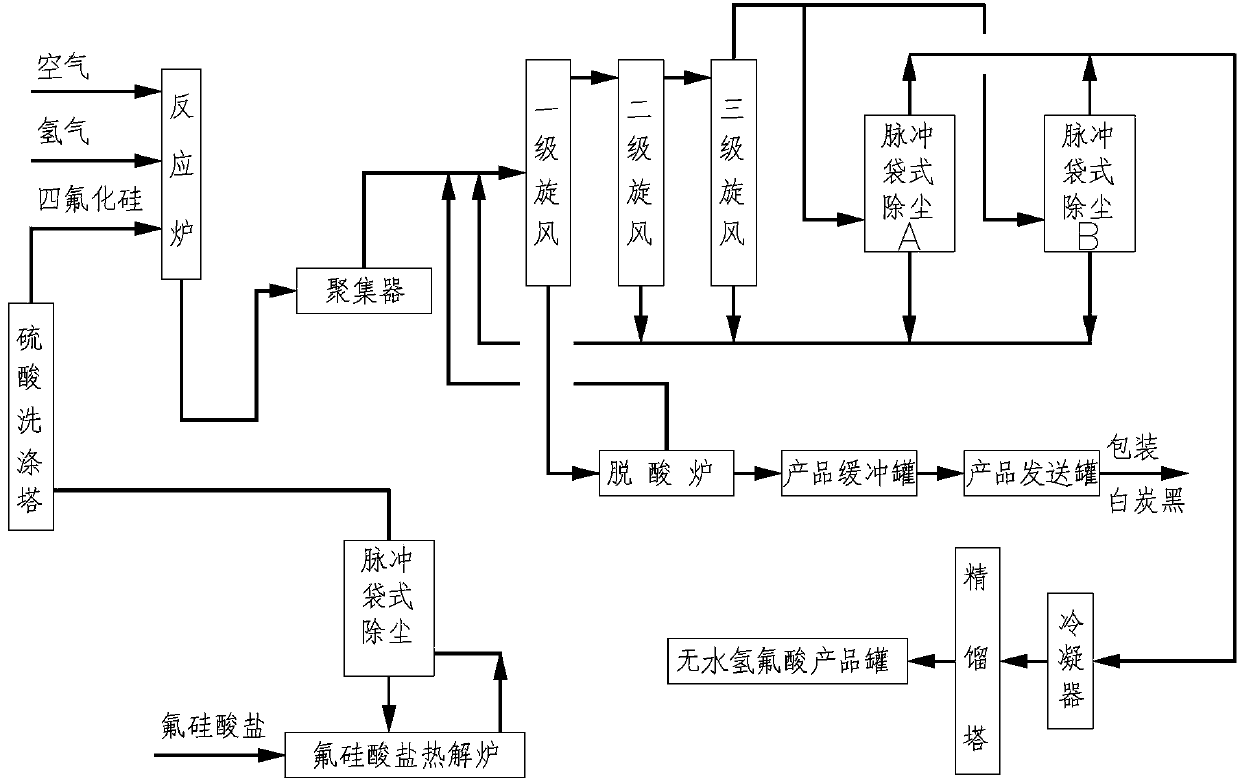
The invention discloses a method for preparing fumed silica and anhydrous hydrofluoric acid by utilizing phosphatic fertilizer by-product fluosilicate as raw material. The method comprises the steps as follows: performing pyrolysis on the phosphatic fertilizer by-product fluosilicate at the temperature of 200-400 DEG C to obtain silicon tetrafluoride, utilizing a filter to remove dust containing in the silicon tetrafluoride, washing the silicon tetrafluoride by 93-98 percent of concentrated sulfuric acid to remove the moisture and impurities, mixing the silicon tetrafluoride, air with hydrogen in a hydrolysis reactor via the volume ratio of 1:2:(0.05-0.3) and performing high-temperature hydrolysis reaction, and then performing aggregation, separation, dust removal, condensation and distillation to obtain the fumed silica and the anhydrous hydrofluoric acid respectively. The method has the advantages that the technical process is simple, the raw material is easy to get, the utilization rate of the raw material is high, energy conservation and environmental protection are realized, the corrosion to the equipment is less, the production cost is reduced, and the produced white carbon black is high in purity.
Owner:SEDIN NINGBO ENG
Method for producing aluminun fluoride
The present invention relates to process of producing aluminum fluoride with fluorosilicic acid and aluminum hydroxide as material. The process includes the following steps: 1. reacting fluorosilicic acid solution and sodium sulfate for 10-60 min, filtering to obtain solid sodium fluorosilicate and waste sulfuric acid solution to be treated and drained; 2. decomposing aluminum fluorosilicate at 300-800 deg.c for 1-5 hr to produce solid sodium fluoride and silicon tetrafluoride gas; 3. absorbing silicon tetrafluoride gas with water and hydrolyzing, filtering to obtain fluorosilicic acid solution to be returned to the step 1 and solid silica, and washing and drying silica to obtain carbon white; 4. reacting solid sodium fluoride and sulfuric acid, condensing, rectifying to obtain anhydrous hydrofluoric acid and solid sodium sulfate returned to the step 1; and 5. reacting anhydrous hydrofluoric acid and aluminum hydroxide to produce aluminum fluoride product. The process is environment friendly.
Owner:DO FLUORIDE CHEM CO LTD
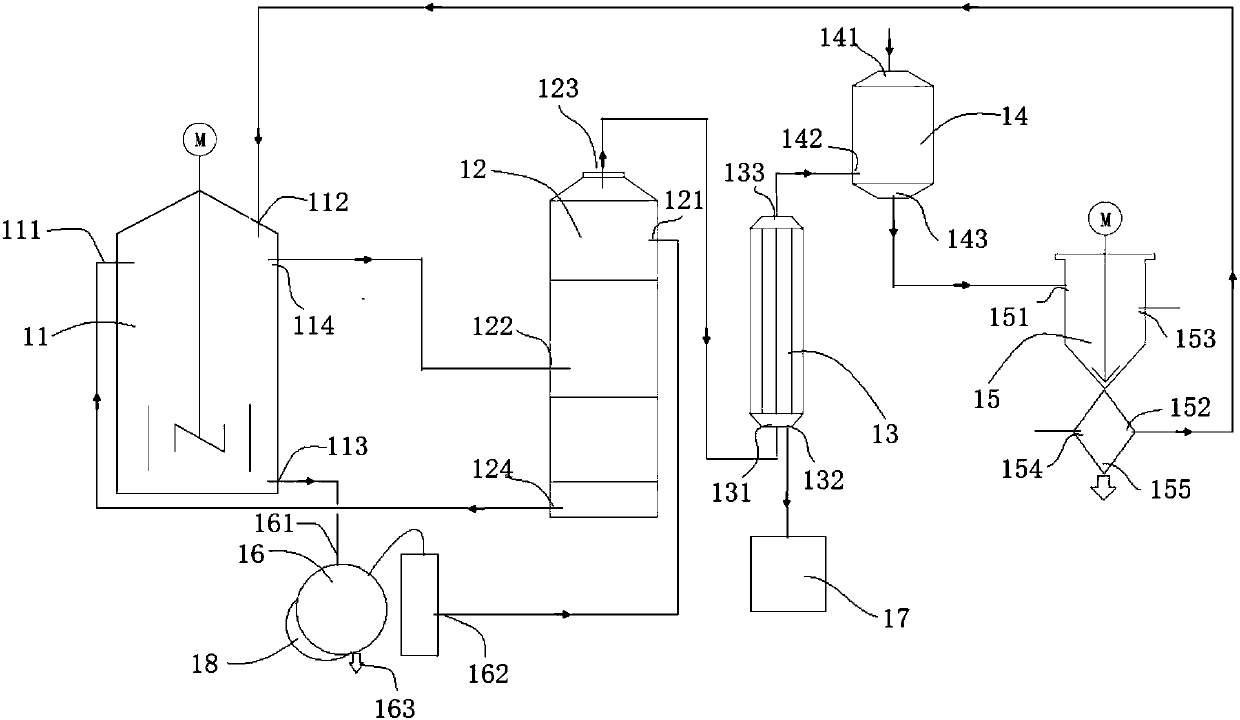
Method for preparing silicon tetrafluoride and anhydrous hydrogen fluoride by taking sodium fluorosilicate as raw material
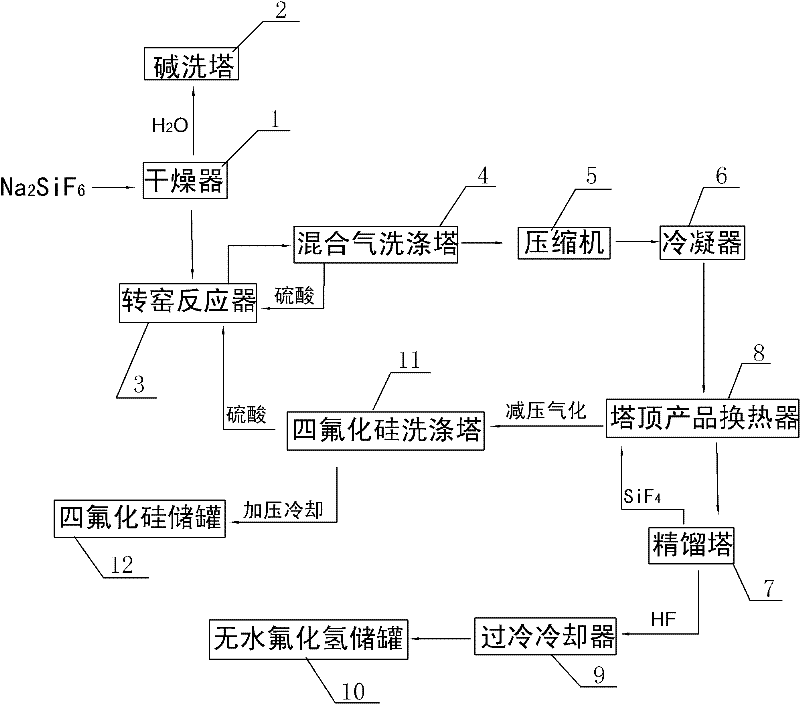
InactiveCN102557043AWell mixedAdequate responseFluorine/hydrogen-fluorideHalogenated silanesSilicon tetrafluorideSodium sulfate
The invention discloses a method for preparing silicon tetrafluoride and anhydrous hydrogen fluoride by taking sodium fluorosilicate as a raw material. The method is characterized by comprising the following steps of: (1) drying sodium fluorosilicate for removing water; (2) adding the dried sodium fluorosilicate and sulfuric acid into a rotary kiln reactor in the molar ratio of 1:1, and reacting at the temperature of 160-220 DEG C for 30-180 minutes to generate a sodium sulfate solid and a mixed gas of silicon tetrafluoride and hydrogen fluoride; (3) dedusting, pressurizing and precooling to between 20 DEG C below zero and 30 DEG C below zero; (4) feeding the precooled mixed gas into a rectifying tower for separating, controlling the pressure of the rectifying tower at 0.6-0.75 MPaG and the temperature of tower top condenser between 70 DEG C below zero and 80 DEG C below zero, and discharging anhydrous hydrogen fluoride from the tower bottom; and (5) discharging silicon tetrafluoride from the tower top in the form of a liquid phase, decompressing, gasifying, dedusting and feeding into a silicon tetrafluoride washing tower to obtain silicon tetrafluoride gas. The method has the advantages that: corrosion to equipment can be reduced greatly, the obtained silicon tetrafluoride and hydrogen fluoride products have high purity, and the production process has low energy consumption and is environmentally-friendly.
Owner:SEDIN NINGBO ENG
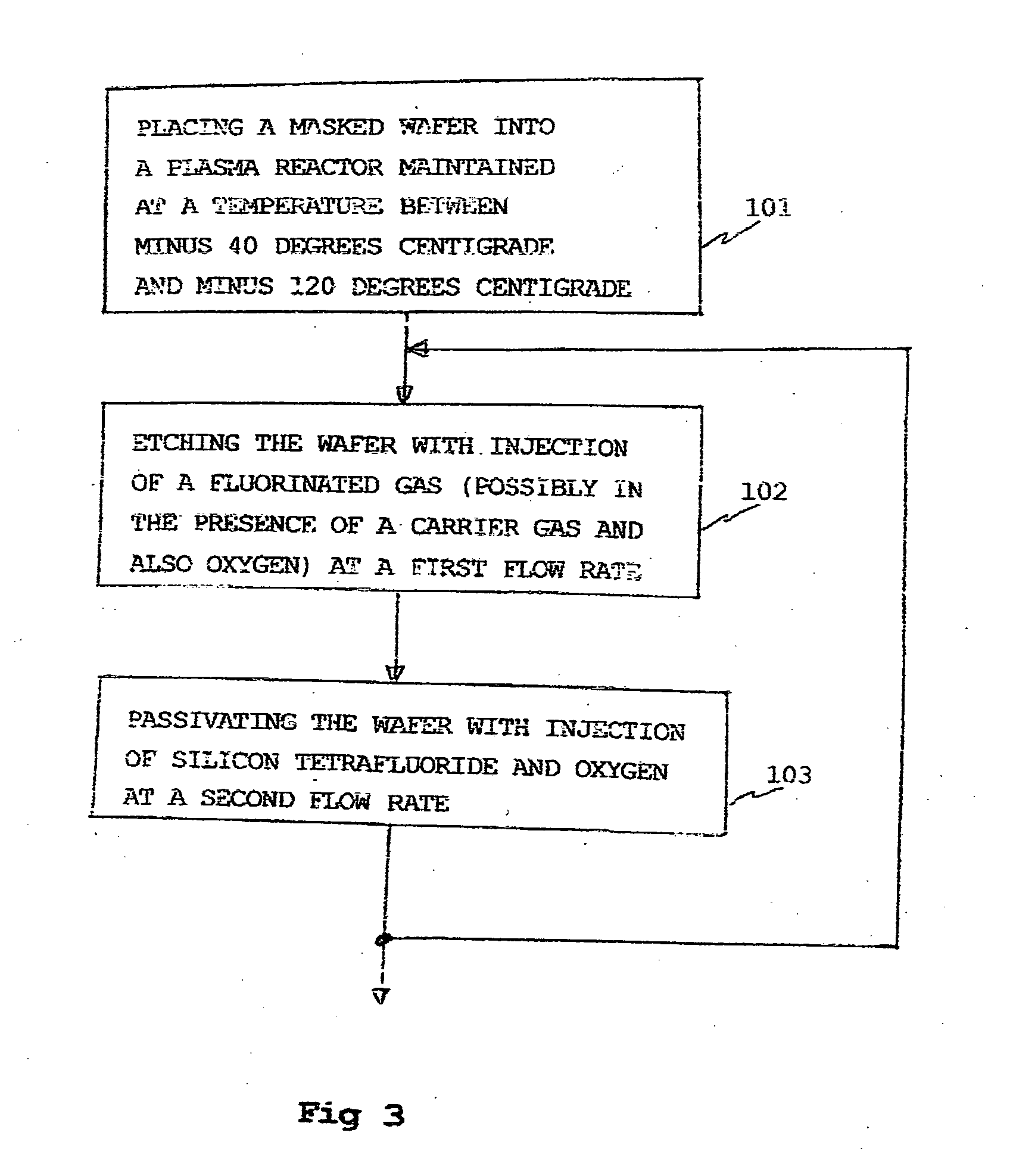
Deep anisotropic silicon etch method
ActiveUS20080293250A1Eliminate disadvantagesDecorative surface effectsSemiconductor/solid-state device manufacturingFluorinated gasesSilicon tetrafluoride
A method of anisotropic plasma etching of a silicon wafer, maintained at a temperature from −40° C. to −120° C., comprising alternated and repeated steps of:etching with injection of a fluorinated gas, into the plasma reactor, andpassivation with injection of silicon tetrafluoride, SiF4, and of oxygen into the plasma reactor, the flow rate of the gases in the plasma reactor being on the order of from 10% to 25% of the gas flow rate during the etch step.
Owner:STMICROELECTRONICS SRL +2
Method for preparing nano white carbon black from coal gangue
InactiveCN102701221ASolve the problem of stacking land occupationLow costSilicaSolid waste disposalDissolutionSilicon tetrafluoride
The invention relates to a method for preparing white carbon black from coal gangue, belongs to the technical field of coal chemical industry, and aims at overcoming the shortcomings of expensive raw materials and high production cost in the process for preparing nano white carbon black by using the traditional method. The method comprises the steps of: based on coal gangue as a raw material, carrying out high-temperature activation, nitric acid dissolution, burning and the like to extract silicon dioxide; corroding extracted silicon dioxide with hydrogen fluoride generated by reacting concentrated sulfuric acid and sodium fluoride so as to generate silicon tetrafluoride gas, reacting the silicon tetrafluoride gas with an aqueous solution with a surfactant dissolved to generate silicon gel, cleaning, drying and burning to remove water so as to produce the required nano white carbon black product. According to the method provided by the invention, the waste recycling of coal gangue is realized, and the cost for preparing nano white carbon black is lowered; and the particle size of the obtained product achieves a nano scale, and the indexes of the obtained product achieve the ministerial standard HG-1-125-64 or enterprise standard.
Owner:韩钊武
Method for producing potassium fluoride
The present invention relates to process of producing potassium fluoride with fluorosilicic acid and potassium chloride as material. The process includes the following steps: 1. reacting fluorosilicic acid solution and potassium chloride for 10-60 min, filtering to obtain solid potassium fluorosilicate and waste hydrochloric acid solution; 2. decomposing potassium fluorosilicate at 300-800 deg.c for 1-5 hr to produce solid potassium fluoride product and silicon tetrafluoride gas; and 3. absorbing silicon tetrafluoride gas with water and hydrolyzing, filtering to obtain fluorosilicic acid solution to be returned to the step 1 and solid silica, and washing and drying silica to obtain carbon white. The process utilizes fluorosilicic acid as one side product of phosphate fertilizer production as one main material, and has low cost and environment friendship.
Owner:DO FLUORIDE CHEM CO LTD
Preparation method of hydrofluoric acid
InactiveCN101134561ASilicon oxidesFluorine/hydrogen-fluorideSilicon tetrafluorideHexafluorosilicic acid
The process of producing hydrofluoric acid with fluorosilicic acid and magnesia as material includes the following steps: 1. reacting fluorosilicic acid solution and solid sodium sulfate for 10-60 min, filtering to obtain solid sodium fluosilicate, and treating waste sulfuric acid before draining; 2. decomposing sodium fluorosilicate at 300-800 deg.c for 1-5 hr to produce solid sodium fluoride and gaseous silicon tetrafluoride; 3. absorbing gaseous silicon tetrafluoride with water and hydrolyzing, and filtering to obtain fluorosilicic acid solution returned to the step 1 and silica, washing and drying to obtain carbon white; and 4. reacting the solid sodium fluoride and 98 % over sulfuric acid, condensing the produced gas condensing and rectifying to obtain hydrofluoric acid, and returning solid sodium sulfate to the step 1. The present invention utilizes fluorosilicic acid as side product from phosphate fertilizer production as material, and has low cost and environment friendship.
Owner:DO FLUORIDE CHEM CO LTD
Method for preparing silicon tetrafluoride by rotary kiln
InactiveCN102976337ASavingsAdequate responseHalogenated silanesAcid concentrationSilicon tetrafluoride
The invention discloses a method for preparing silicon tetrafluoride by a rotary kiln. The method is characterized in that sodium fluosilicate and sulfuric acid undergo a pre-reaction; reaction products are fed into a rotary kiln and then undergo a further reaction, wherein the pressure in the rotary kiln is in a range of -2 to -5 Kpa and temperatures of front, middle and rear sections in the rotary kiln are respectively controlled to 160-190 DEG C, 191-220 DEG C and 221-250 DEG C; and after the reaction, silicon tetrafluoride and HF mixed gas are separated. According to the invention, sodium fluosilicate and sulfuric acid undergo a reaction in the rotary kiln as a reactor; a by-product sodium fluosilicate produced by phosphate fertilizer plants can be used as a raw material; and a main solid waste sodium sulfate produced by the rotary kiln still can be recovered by the phosphate fertilizer plants and be used for producing sodium fluosilicate. By a three-section heating method, the raw materials react completely. In addition, intractable three wastes are not produced; a waste acid concentration device is avoided; and a device investment is greatly saved.
Owner:SP CHEM TAIXING
Preparation of silicon tetrafluoride
InactiveCN101481113ASimple purification processQuality improvementHalogenated silanesSocial benefitsSilicon tetrafluoride
The invention discloses a method for preparing silicon tetrafluoride, which takes hydrofluorosilicic acid and magnesia as raw materials, and comprises the following steps: (1) reacting hydrofluorosilicic acid solution with the magnesia for 10 and 60 minutes, filtering the mixture to obtain magnesium fluosilicate solution, and concentrating and crystallizing the mixture to obtain magnesium fluosilicate crystal hexahydrate; (2) pulsating the obtained magnesium fluosilicate crystal hexahydrate by air flow at a temperature of between 25 and 55 DEG C for 1 to 3 hours, and removing adhesive water and crystallization water; (3) sintering the magnesium fluosilicate removing the adhesive water and the crystallization water at a temperature of between 200 and 300 DEG C for 1 to 2 hours so as to obtain silicon tetrafluoride gas and magnesium flux; and (4) obtaining the high-purity silicon tetrafluoride gas after sublimating the silicon tetrafluoride gas. The invention has low production cost, the prepared silicon tetrafluoride has high quality, three wastes are not discharged; in addition, the co-produced magnesium flux can be taken as an additive in the aluminum electrolytic industry, the waste residue containing fluorine gas is not generated, and the invention has good social benefits and social benefits and is easily popularized and applied.
Owner:DO FLUORIDE CHEM CO LTD
Method for preparing nano silicon dioxide by vapor phase process
The invention provides a method for preparing nano silicon dioxide by a vapor phase process, which is characterized in that fly ash is hydrolyzed to produce silica gel, and the silica gel is dried to obtain the nano silicon dioxide. The method hydrolyzes silicon tetrafluoride in the fly ash to generate silica gel, and the silica gel is dried to obtain the nano silicon dioxide. The method has the advantages of low material cost, simple technique and easy production control. The obtained silicon dioxide is a white amorphous flocculent translucent solid, is innoxious, and has a large specific area (100-400 m<2> / g). The production has the advantages of high chemical purity, high dispersibility and higher surface activity.
Owner:SHANGHAI KEWANG NEW MATERIAL RES CENT
Method of preparing nanometer SiO2 from ophiolite
InactiveCN1911799ATo achieve the effect of resource utilizationSimple processSilicaNano sio2Silicon tetrafluoride
The present invention is superior process of preparing nanometer SiO2 with serpentine. Serpentine powder is first refluxed and leached to leach out soluble metal ions and solid matter with porous SiO2 as main component; and the solid matter is further prepared into nanometer SiO2 through a silicon tetrafluoride and sodium silicate process. The present invention has low cost and simple technological process.
Owner:SHANGHAI UNIV
Method and apparatus for preparing fumed silica from coal gangue
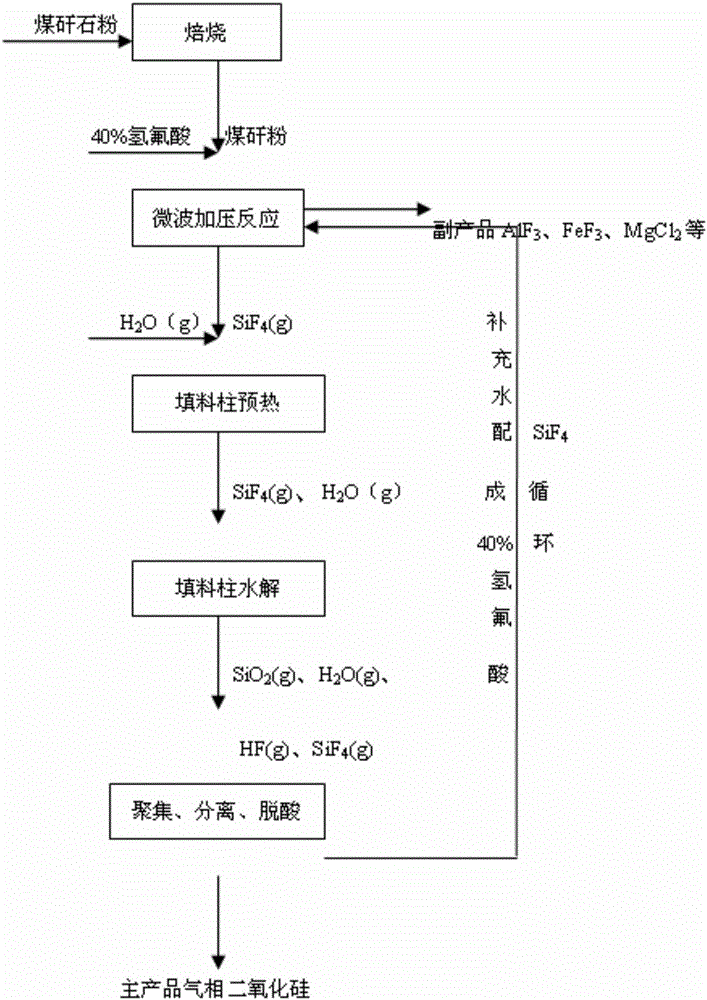
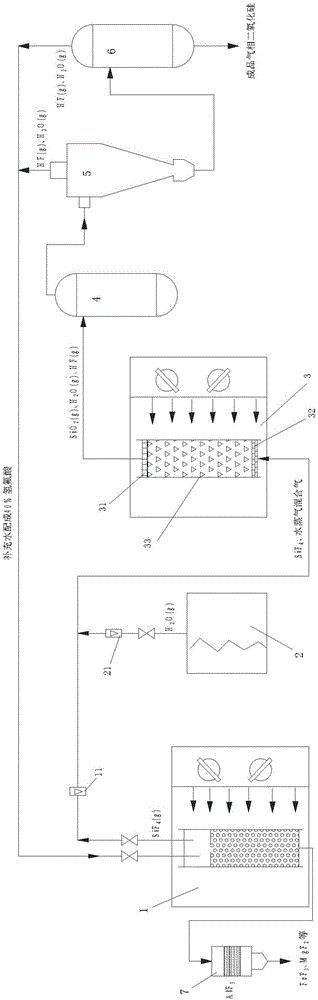
The invention discloses a method and an apparatus for preparing fumed silica from coal gangue. The method comprises: performing pressurized reaction, in microwave fields, on activated coal gangue powder and hydrofluoric acid, converging the generated silicon tetrafluoride gas with water vapor to obtain a mixture and introducing the mixture into a micorwave-absorbing agent packed column under microwave irradiation, and performing high-temperature pyrohydrolysis reaction on the mixture to generate gas silicon dioxide and hydrogen fluoride; performing shock chilling, gathering, separating, acid-removing and purging on the generated gas silicon dioxide and hydrogen fluoride as well as unreacted water vapor to prepare a fumed silica product; separating the hydrogen fluoride gas and the water vapor from a cyclone separator and the top of an acid removing furnace, and adding the water to prepare hydrofluoric acid for recycling, wherein the fumed silica product can be used as a thickening agent, a rubber reinforcing agent and a washing assistant agent, and the like; by-products are aluminum fluoride, iron fluoride and magnesium fluoride and the like; the microwave-absorbing agent in the present invention can significantly improve the direct hydrolysis efficiency of silicon tetrafluoride; the pressurized microwave reaction can significantly increase the reaction rate and conversion rate of silicon dioxide and hydrofluoric acid; and the microwave-absorbing agent is recycled and easy to regenerate, and has a high utilization rate, a simple process and low preparation cost.
Owner:LIUPANSHUI NORMAL UNIV
Preparation method of fluoride
ActiveCN103043613ATake advantage ofEfficient use ofSilicaFluorine/hydrogen-fluorideSilicon tetrafluorideHydrolysis
The invention relates to a preparation method of fluoride, which comprises the following steps: reacting sodium fluosilicate and concentrated sulfuric acid at 80-300 DEG C to obtain the product gases of silicon tetrafluoride and hydrogen fluoride and the byproduct sodium sulfate, and carrying out dehydration, dust removal, purification and separation on the two gases to obtain an anhydrous hydrogen fluoride product; introducing the silicon tetrafluoride gas into water, and controlling the hydrolysis reaction conditions to obtain active white carbon black and a fluosilicic acid solution; and reacting the fluosilicic acid solution with the byproduct sodium sulfate to obtain sodium fluosilicate and sulfuric acid, thereby implementing cyclic utilization. The reaction between the sodium fluosilicate and concentrated sulfuric acid and the hydrolysis reaction of the silicon tetrafluoride are carried out to finally obtain the products anhydrous hydrogen fluoride and active white carbon black. The low-value byproduct sodium fluosilicate in phosphate fertilizer enterprises is utilized to prepare the high-added-value products anhydrous hydrogen fluoride and active white carbon black; the byproduct sodium fluosilicate in the phosphate fertilizer enterprises is subjected to high-efficiency high-benefit comprehensive utilization of fluorine and silicon resources; and no unmanageable waste can be generated in the preparation process.
Owner:KUNMING DAOERSEN TECH
Process for the preparation of pure silica
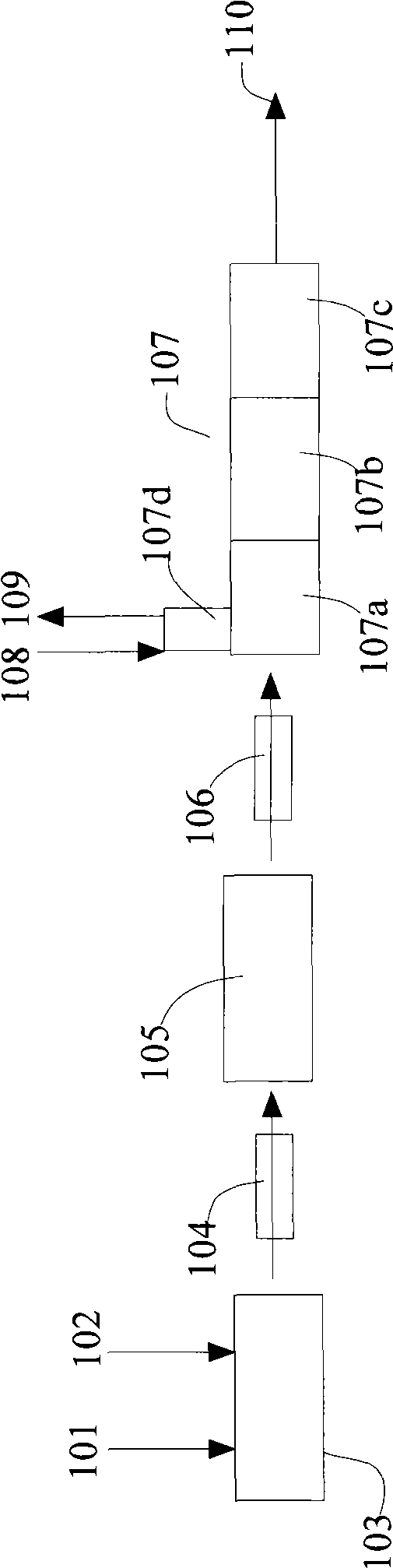
InactiveUS20050106091A1Simple and efficient and cost-effectiveSilicaFluosilicic acidSilicon tetrafluorideSilicon dioxide
A production process for producing high-purity silica from a crude silica source by means of fluosilic acid, including the steps of: (a) subjecting the crude silica souce and the fluosilicic acid to a reaction in a reaction stage, so as to produce silicon tetrafluoride and water; (b) selectively evaporating the silicon tetrafluoride with respect to at least a portion of at least one impurity derived from the crude silica source, and (c) reacting the silicon tetrafluoride with water to produce the high-purity silica, wherein the reaction stage (a) is performed at a temperature above 75° C.
Owner:ATI ALUMINUM TECH ISRAEL
Method for recycling waste gas containing fluorine
InactiveCN101708418AAchieve high temperature generationAchieve water balanceDispersed particle separationSilicon oxidesSolubilityAmmonium fluorosilicate
The invention relates to a method for recycling waste gas containing fluorine, belonging to the technical field of chemical industrial production. The method comprises the following steps: absorbing silicon tetrafluoride by an ammonium fluoride solution to produce ammonium fluosilicate, then ammoniating the ammonium fluosilicate to reduce the ammonium fluosilicate to ammonium fluoride, and simultaneously, generating precipitate silica (silica white); after separating the precipitate silica (silica white), by using the changing solubility of ammonium fluoride resulting from the change of temperature, separating the ammonium fluoride by cooling and crystallization; simultaneously, in order to ensure water balance of the overall system and diversification of products, enabling part of the ammonium fluosilicate and potassium chloride (or potassium sulfate) to carry out metathetical reaction to generate potassium fluosilicate precipitate and ammonium chloride (or ammonium sulfate); and after the precipitate silica is separated, concentrating and crystallizing a mother solution to obtain ammonium chloride (or ammonium sulfate) fertilizer. The invention is mainly applied to the fertilizer production industry.
Owner:SHANDONG HONGRI ACRON CHEM
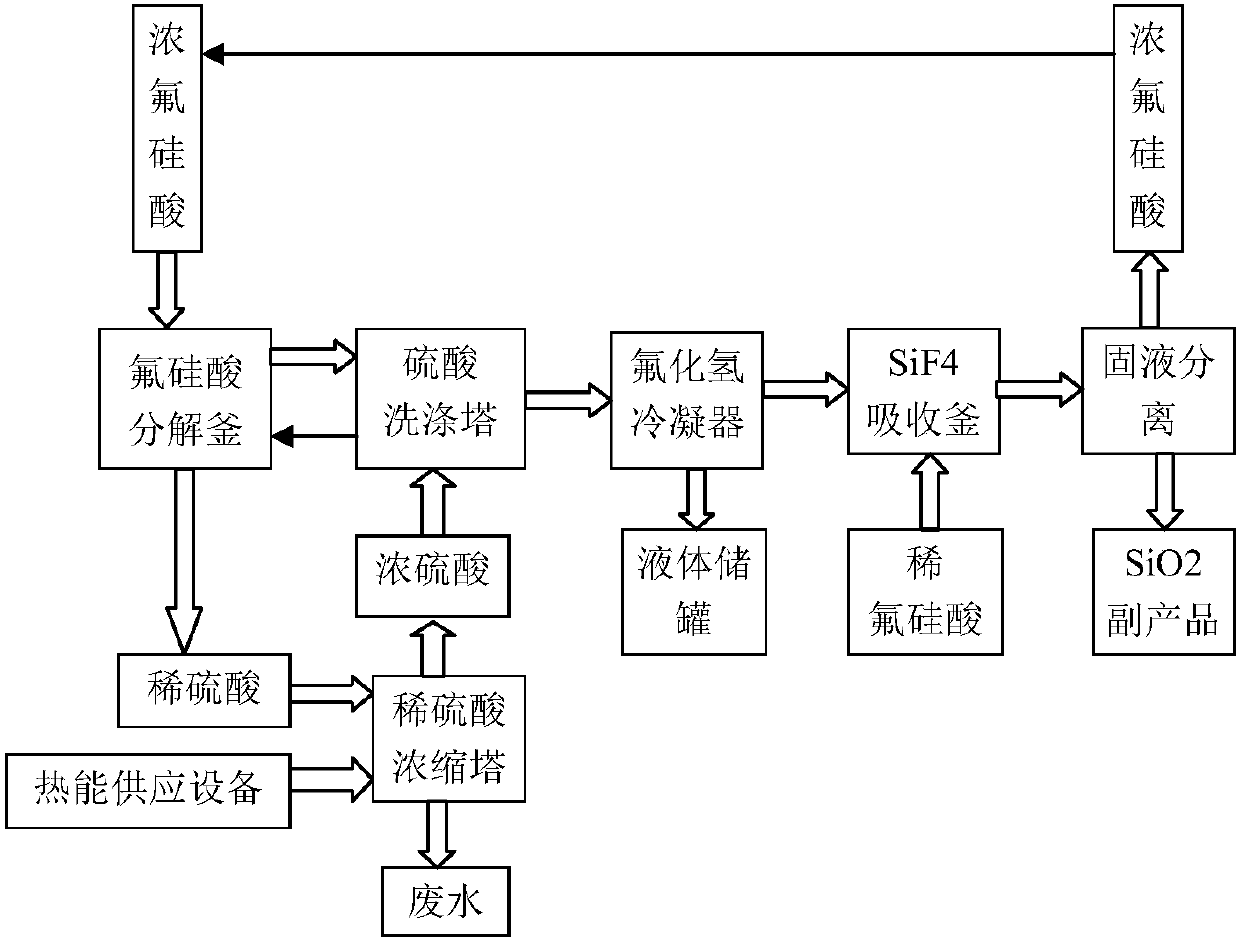
Method and equipment for preparing hydrogen fluoride from fluosilicic acid
PendingCN107601434AEfficient use ofEmission reductionSilicaFluorine/hydrogen-fluorideHydrogen fluorideSilicon tetrafluoride
The invention provides a method and equipment for preparing hydrogen fluoride from fluosilicic acid. The method comprises the following steps: concentrated fluosilicic acid and concentrated sulfuric acid are mixed, after reaction, mixed gas of silicon tetrafluoride and hydrogen fluoride and dilute sulfuric acid are obtained; washing and condensation are carried out; silicon tetrafluoride gas is added into dilute fluosilicic acid, and silica solid and concentrated fluosilicic acid clear liquid are obtained. The equipment comprises the following components: a decomposing pot, a washing tower, acondenser, an absorption kettle and a separator, which are communicated in order. Substances generated in the steps can be used as raw materials, cost is saved, and at the same time post-treatment process is reduced, production efficiency is improved, and large scale application of factory is facilitated. Absorption function of concentrated sulfuric acid is used, fluosilicic acid is decomposed into hydrogen fluoride and silicon tetrafluoride gas, so that effective utilization of fluosilicic acid is obtained. Dilute sulfuric acid is concentrated into concentrated sulfuric acid again, dischargeof dilute sulfuric acid is reduced, sulfuric acid becomes an intermediate, and fluosilicic acid is decomposed into hydrogen fluoride and silica.
Owner:衢州市鼎盛化工科技有限公司
Bright etched frosted glass and preparation method thereof
Owner:YIWU HUAHONG CULTURE CREATIVE CO LTD
Method for producing silicon tetrafluoride from uranium oxyfluoride
InactiveUS6033642AReduced thermodynamic stabilityAvoid vaporizationUranium dioxideHalogenated silanesSilicon tetrafluorideSilicon dioxide
A method for producing silicon tetrafluoride includes combining uranium oxyfluoride and silicon dioxide; heating the combination below the melting point of the uranium oxyfluoride to sufficiently react the uranium oxyfluoride and the silicon dioxide to produce non-radioactive silicon tetrafluoride and an oxide of uranium; and removing the silicon tetrafluoride.
Owner:INT ISOTOPES
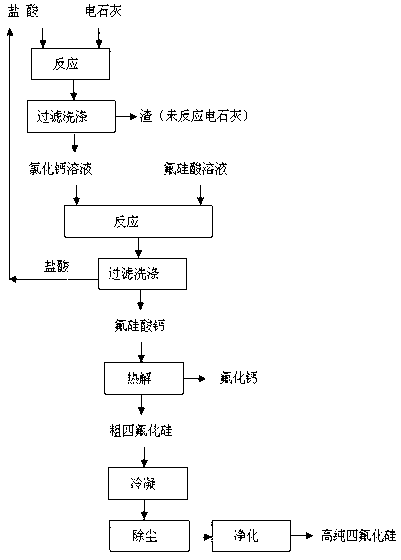
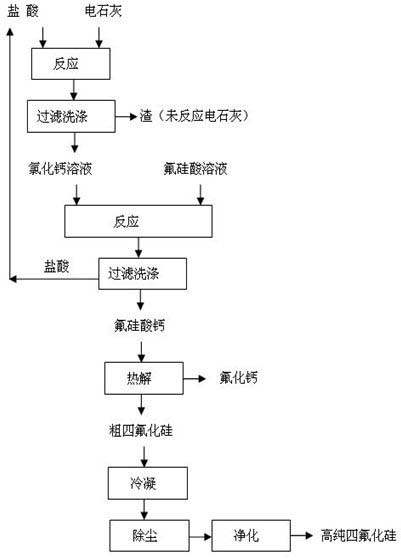
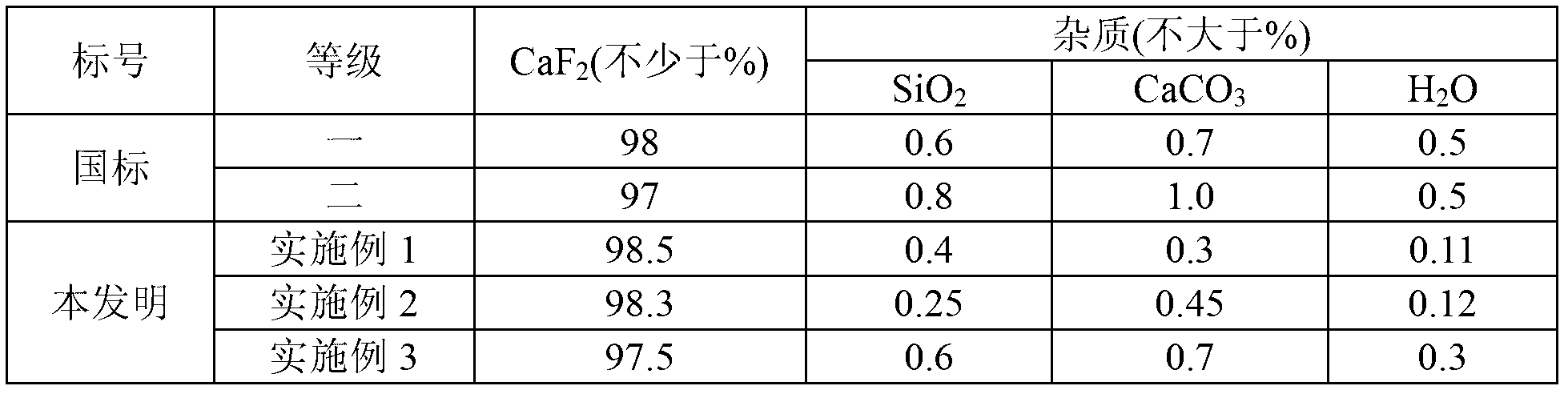
Method for preparing silicon tetrafluoride co-production with calcium fluoride by using fluosilicic acid and calcium carbide dust
InactiveCN102701215AImprove conversion rateIncrease added valueCalcium/strontium/barium fluoridesHalogenated silanesHexafluorosilicic acidSilicon tetrafluoride
The invention discloses a method for preparing silicon tetrafluoride co-production with calcium fluoride by using fluosilicic acid and calcium carbide dust. The method disclosed by the invention comprises the following steps of: reacting the calcium carbide dust with hydrochloric acid at 5-100 DEG C to obtain a calcium chloride solution, and concentrating to obtain the calcium chloride solution with the mass percentage of 40-60%; then reacting the calcium chloride solution with the mass percentage of 40-60% with the fluosilicic acid to obtain calcium fluosilicate, wherein the filtrate hydrochloric acid is recyclable; thermally decomposing the dried calcium fluosilicate to obtain crude silicon tetrafluoride and calcium fluoride; condensing the crude silicon tetrafluoride, removing the dust and purifying to obtain highly pure crude silicon tetrafluoride. The method disclosed by the invention makes use of the by-product fluosilicic acid of phosphorous fertilizer and the calcium carbide dust with low added value to prepare the silicon tetrafluoride with high added value; the prepared calcium fluoride meets the standard of preparing acid-grade fluorite and can completely replace the strategic resource fluorite; the conversion rate of the raw material is high, the application field of the product is wide, and the efficient utilization of fluorine and silicon resources is realized completely. The method disclosed by the invention is remarkable in economical, environmental and social benefits.
Owner:DO FLUORIDE CHEM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com