Method and equipment for preparing hydrogen fluoride from fluosilicic acid
A technology of hydrogen fluoride and fluosilicic acid, applied in the direction of fluorine/hydrogen fluoride, silicon oxide, silicon dioxide, etc., can solve problems such as excessive fluosilicic acid, achieve the effects of reducing emissions, increasing economic value, and saving energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
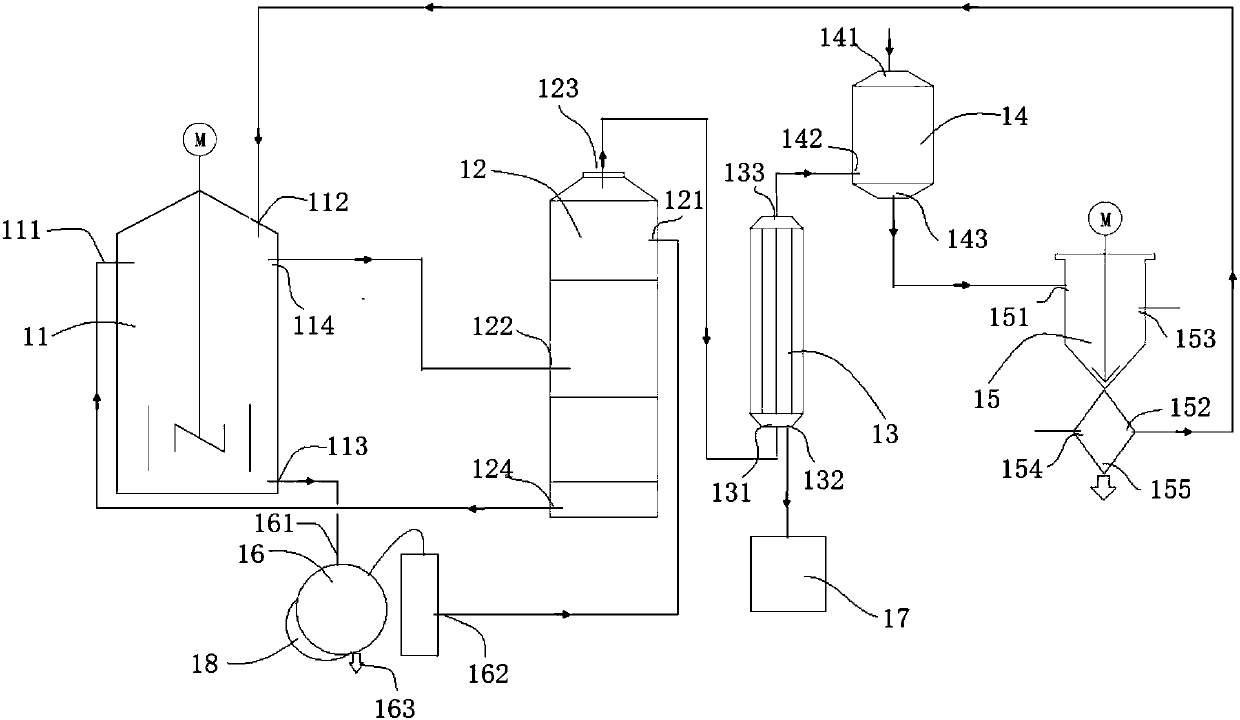
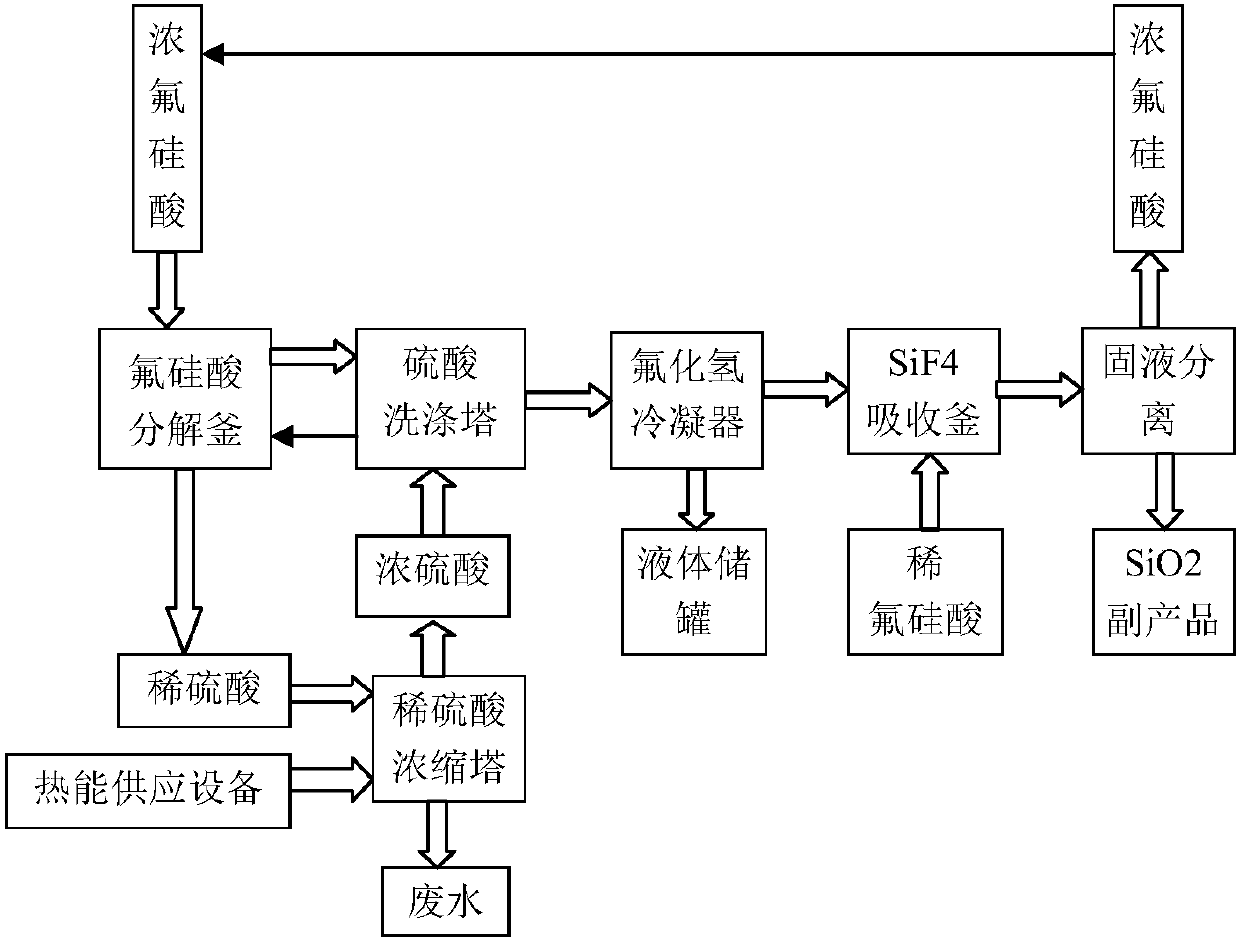
[0043] Such as figure 1Shown: a device for preparing hydrogen fluoride from fluorosilicic acid, including a decomposition kettle 11 , a washing tower 12 , a condenser 13 , an absorption kettle 14 and a solid-liquid separation device 15 . Wherein the decomposition kettle 11, the washing tower 12, the condenser 13, the absorption kettle 14, and the separation device 15 are connected in sequence through pipelines. The decomposition kettle 11 is used for decomposing fluosilicic acid, which includes a first inlet 111 for concentrated sulfuric acid, an inlet 112 for concentrated fluosilicic acid, an outlet 113 for dilute sulfuric acid, and a first outlet 114 for mixed gas of silicon tetrafluoride and hydrogen fluoride. The washing tower 12 is used to absorb the moisture entrained in the mixed gas of silicon tetrafluoride and hydrogen fluoride, which includes the second inlet 121 of concentrated sulfuric acid, the first inlet 122 of the mixed gas of silicon tetrafluoride and hydrogen...
Embodiment 2
[0046] Adopt the equipment preparation among the embodiment 1, take fluosilicic acid as the concrete steps of raw material preparation hydrogen fluoride as follows:
[0047] Pass 1,000kg of 30% fluosilicic acid and 1,750kg of 98% concentrated sulfuric acid into the decomposition kettle 11, mix and react at 90°C, and the gas generated is passed into the washing tower 12, and the moisture entrained in the mixed gas of silicon tetrafluoride and hydrogen fluoride is absorbed by concentrated sulfuric acid , then enter the condenser 13, and the condensed liquid hydrogen fluoride passes into the liquid storage tank 17. The concentrated sulfuric acid in the washing tower 12 continues to be used in the decomposition kettle 11.
[0048] The uncondensed gas is passed into the absorption tank 14 that has 1000kg30% fluosilicic acid, and then the reactant is passed into the separation device 15, and the silicon dioxide precipitate is cleaned to obtain 41.7kg of silicon dioxide and 1175kg of...
Embodiment 3
[0051] Adopt the equipment preparation among the embodiment 1, take fluosilicic acid as the concrete steps of raw material preparation hydrogen fluoride as follows:
[0052] Pass 1175kg of 42.5% fluosilicic acid and 1746.4kg of 98% concentrated sulfuric acid into the decomposition kettle 11, mix and react at 90°C, the gas generated is passed into the washing tower 12 and condenser 13 in turn, and the condensed liquid hydrogen fluoride is refined 138.7 kg of anhydrous hydrogen fluoride were obtained. The concentrated sulfuric acid in the washing tower 12 continues to be used in the decomposition kettle 11.
[0053] The uncondensed gas is passed into the absorption tank 14 that has 1000kg30% fluosilicic acid, and then the reactant is passed into the separation device 15, and the silica precipitate is cleaned to obtain 69.4kg of silica and 1291.4 kg of 49% fluosilicic acid. kg. The fluosilicic acid continues to be reused as a raw material. This silica is available as a takeawa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com