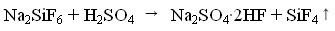Method for preparing silicon tetrafluoride and anhydrous hydrogen fluoride from sodium fluosilicate acidified by sulfuric acid
A technology of anhydrous hydrogen fluoride and silicon tetrafluoride, which is applied in the direction of fluorine/hydrogen fluoride, silicon halide compounds, halosilane, etc., can solve the problems of high cost, high energy consumption, complicated process, etc., and achieve low environmental load and high-efficiency utilization , high environmental benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
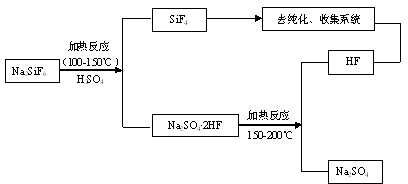
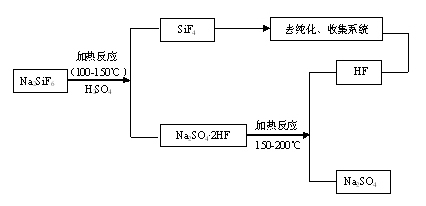
[0029] At a temperature of 100°C, add 100kg of sodium fluorosilicate and an excess of 20% sulfuric acid into the reactor and stir for 1 hour. The stirring rate is controlled at 200rpm and the concentration of sulfuric acid is 95%. Silicon tetrafluoride gas is obtained, and hydrogen fluoride remains in the solid residue. Among them, the decomposition rate is 49.7%, and the fluorine escaped into the gas phase accounts for 33.1% of the content of sodium fluorosilicate. Then the temperature was raised to 160°C, and hydrogen fluoride gas escaped. The two gases are strictly collected after dust removal, cooling, drying, refining and compression.
Embodiment 2
[0031] At 120°C, add 120kg of sodium fluorosilicate and an excess of 30% sulfuric acid into the reaction kettle for 1.2h, the stirring rate is controlled at 240rpm, and the concentration of sulfuric acid is 97%, to obtain silicon tetrafluoride gas, and hydrogen fluoride remains in the solid residue Among them, the decomposition rate is 56.9%, and the fluorine escaped into the gas phase accounts for 39.4% of the content of sodium fluorosilicate. Then the temperature was raised to 170°C, hydrogen fluoride gas escaped. The two gases are strictly collected after dust removal, cooling, drying, refining and compression.
Embodiment 3
[0033] At a temperature of 140°C, add 150kg of sodium fluorosilicate and excess 50% sulfuric acid into the reactor for 1.5h reaction, control the stirring rate at 300rpm, and sulfuric acid concentration of 98%, to obtain silicon tetrafluoride gas, and hydrogen fluoride remains in the solid residue Among them, the decomposition rate is 77.5%, and the fluorine escaped into the gas phase accounts for 51.2% of the content of sodium fluorosilicate. Then the temperature is raised to 180°C, and hydrogen fluoride gas escapes. The two gases are strictly collected after dust removal, cooling, drying, refining and compression.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com