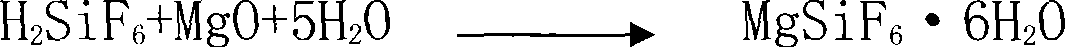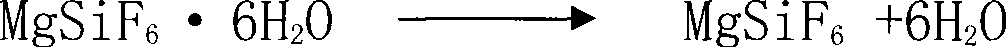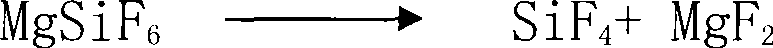Preparation of silicon tetrafluoride
A technology of silicon tetrafluoride and fluorosilicic acid, which is applied in the direction of silicon halides, halosilanes, etc., can solve the problems of complex process of by-product recovery method, difficulty in industrialization, and high cost of hydrofluoric acid method, so as to be easy to popularize and apply , good economic value and social value, the effect of excellent quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation method of silicon tetrafluoride of the present invention is to be raw material with fluosilicic acid and magnesium oxide, specifically comprises the following steps:
[0028] (1) react 15% fluosilicic acid solution with magnesium oxide solid for 40 minutes, filter to obtain magnesium fluosilicate solution, concentrate and crystallize to obtain magnesium fluosilicate hexahydrate crystal;
[0029] (2) Agitating the obtained magnesium fluorosilicate hexahydrate crystals in a fluidized bed with a 30°C air flow for 3 hours to remove attached water and crystal water;
[0030] (3) Calcining the magnesium fluorosilicate that has removed the attached water and crystal water for 2 hours at a temperature of 200° C. in a converter to obtain silicon tetrafluoride gas and magnesium fluoride;
[0031] (4) Refrigerating and purifying silicon tetrafluoride gas with liquid nitrogen to obtain high-purity silicon tetrafluoride gas.
[0032] The magnesium fluoride obtained ...
Embodiment 2
[0034] The preparation method of silicon tetrafluoride of the present invention, take fluosilicic acid and magnesium oxide as raw material, specifically comprise the following steps:
[0035] (1) react 25% fluosilicic acid solution with 20% magnesium oxide slurry for 30 minutes, filter to obtain magnesium fluosilicate solution, concentrate and crystallize to obtain magnesium fluosilicate hexahydrate crystals;
[0036] (2) Agitating the obtained magnesium fluorosilicate hexahydrate crystals in a fluidized bed with a 40°C air flow for 2 hours to remove attached water and crystal water;
[0037] (3) Calcining the magnesium fluorosilicate that has removed the attached water and crystal water for 2 hours at a temperature of 200° C. in a converter to obtain silicon tetrafluoride gas and magnesium fluoride;
[0038] (4) Refrigerating and purifying silicon tetrafluoride gas with liquid nitrogen to obtain high-purity silicon tetrafluoride gas.
Embodiment 3
[0040] The preparation method of silicon tetrafluoride of the present invention, using fluosilicic acid and magnesium oxide as raw materials, specifically comprises the following steps;
[0041] (1) Reacting a 35% fluorosilicate solution with a 30% magnesium oxide slurry for 20 minutes, filtering to obtain a magnesium fluorosilicate solution, concentrating and crystallizing to obtain magnesium fluorosilicate hexahydrate crystals;
[0042] (2) Agitate the obtained magnesium fluorosilicate hexahydrate crystals in a fluidized bed with a 55°C air flow for 1 hour to remove attached water and crystal water;
[0043] (3) Calcining the magnesium fluorosilicate that has removed the attached water and the crystal water at a temperature of 300° C. for 1 hour in a converter to obtain silicon tetrafluoride gas and magnesium fluoride;
[0044] (4) Refrigerating and purifying silicon tetrafluoride gas with liquid nitrogen to obtain high-purity silicon tetrafluoride gas.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com