Antibiotic peptide buforin II and porcine INF-alpha fusion expression pichia pastoris, and preparation method and applications thereof
A technology of -6his-bufforinii-zeocin and Pichia pastoris, applied in the field of biotechnology, can solve the problems of inability to directly apply, troublesome purification and the like, and achieve the effects of easy purification, high expression amount and simple purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
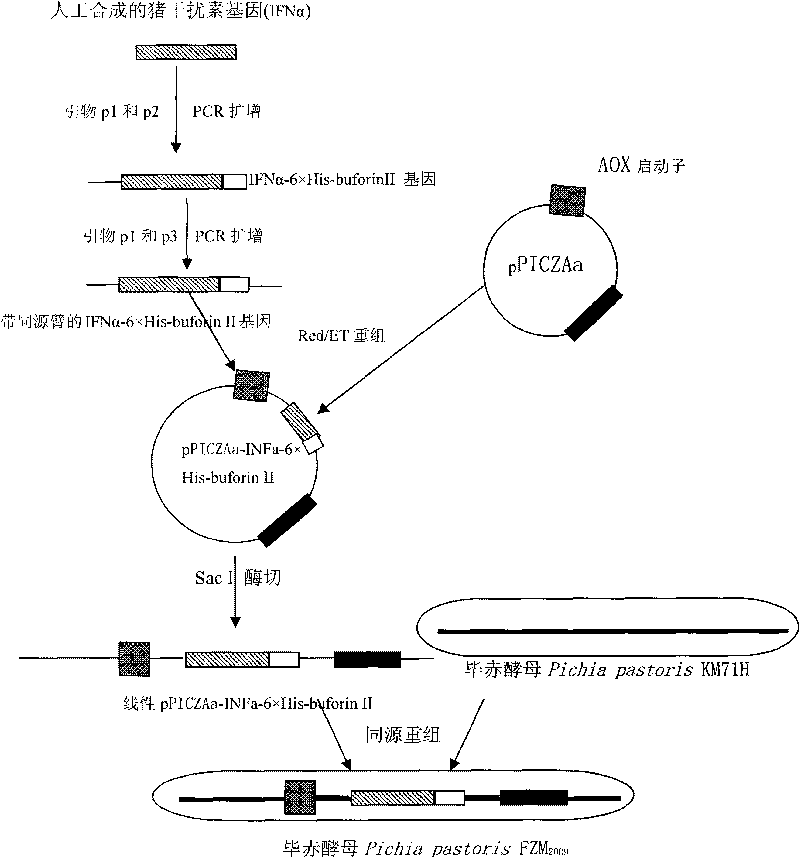
[0042] The construction of Pichia pastoris engineered bacteria expressing the fusion expression of antibacterial peptide buforin II and porcine INF-α and the preparation method of antibacterial peptide buforin II.
[0043] 1 material
[0044] 1.1 Strain and carrier
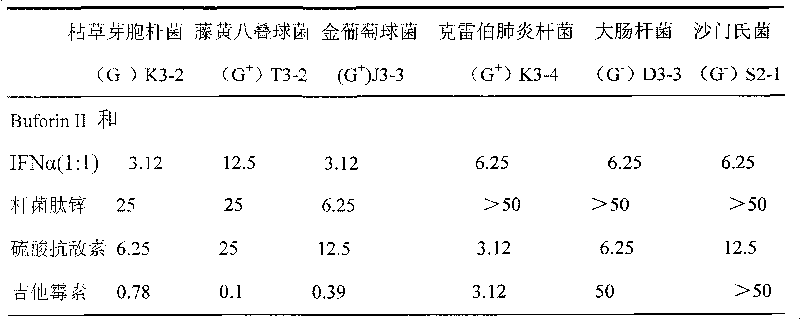
[0045] See Table 1 for strains and vector genotypes and sources.
[0046] Table 1 Strain and carrier genotype and source
[0047]
[0048] 1.2 Tool enzyme
[0049] RNaseA and Proteinase K were purchased from Shanghai Shenggong Company; restriction endonucleases were purchased from promega; 2 * PCR master Mix was purchased from Beijing Tiangen Biotechnology Company.
[0050] 1.3 Kit
[0051] Small, medium and large extraction plasmid kits, and yeast genome kits are products of QIAGEN.
[0052] 1.4 Medium
[0053] LB liquid medium: 5g of yeast extract; 10g of peptone; 5g of NaCl; add water to make up to 1L.
[0054] LB solid medium: add agar powder at a final concentration of 1.5% (m / v) to the LB liquid m...
Embodiment 2
[0063] An engineering bacterium Pichia pastoris FZM 2009 The method for preparing fusion protein buforin II and INF-α, the steps are as follows:
[0064] A. Material and solution preparation
[0065] The engineering bacterium used is Pichia pastoris FZM provided by the present invention 2009 .
[0066] The engineering bacteria medium is an optimized medium for experimental fermentation, and the mass fraction% of each component is: glucose 4%, ammonia water 150μL, KH 2 PO 4 0.7%, MgSO 4 .7H 2 O, 0.03% FeSO 4 .7H 2 O, 0.05% MnSO 4 .H 2 O0.05%, 0.1% Peptone, pH 5.5, the culture condition is 30°C, add ammonia every 8h, add methanol once every 24h, 0.4% PTM1 (v / v).
[0067] The formula of PTM1 is: copper sulfate (CuSO 4 ) 6g / L, potassium iodate (KI) 0.08g / L, magnesium sulfate monohydrate (MnSO 4 ·H 2 O) 3g / L, sodium molybdate dihydrate 0.2g / L, boric acid (H 3 BO 3 ) 0.02g / L, zinc chloride (ZnCl 2 ) 20g / L, cobalt chloride 0.5g / L, iron sulfate heptahydrate (FeSO 4 ....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com