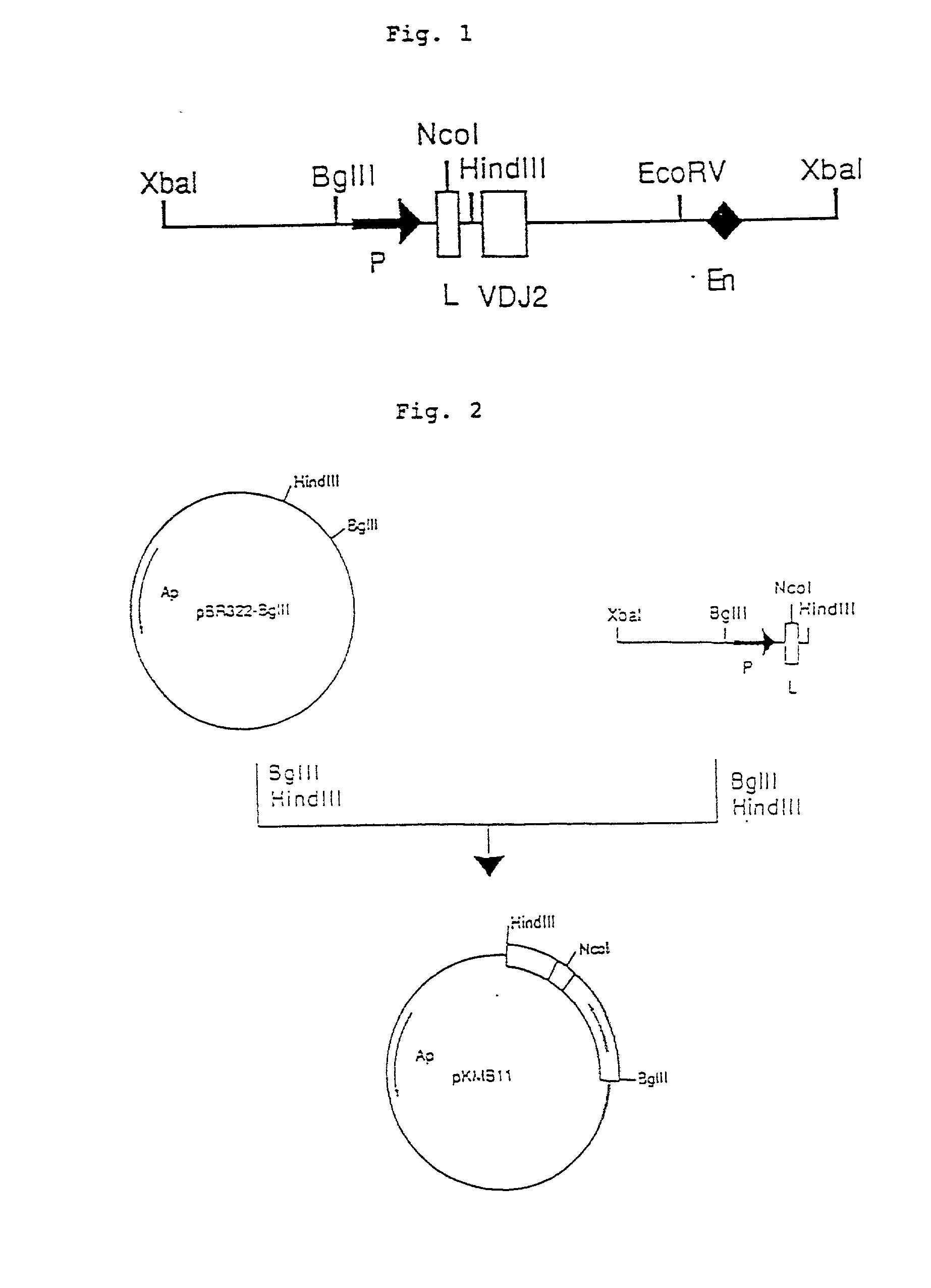
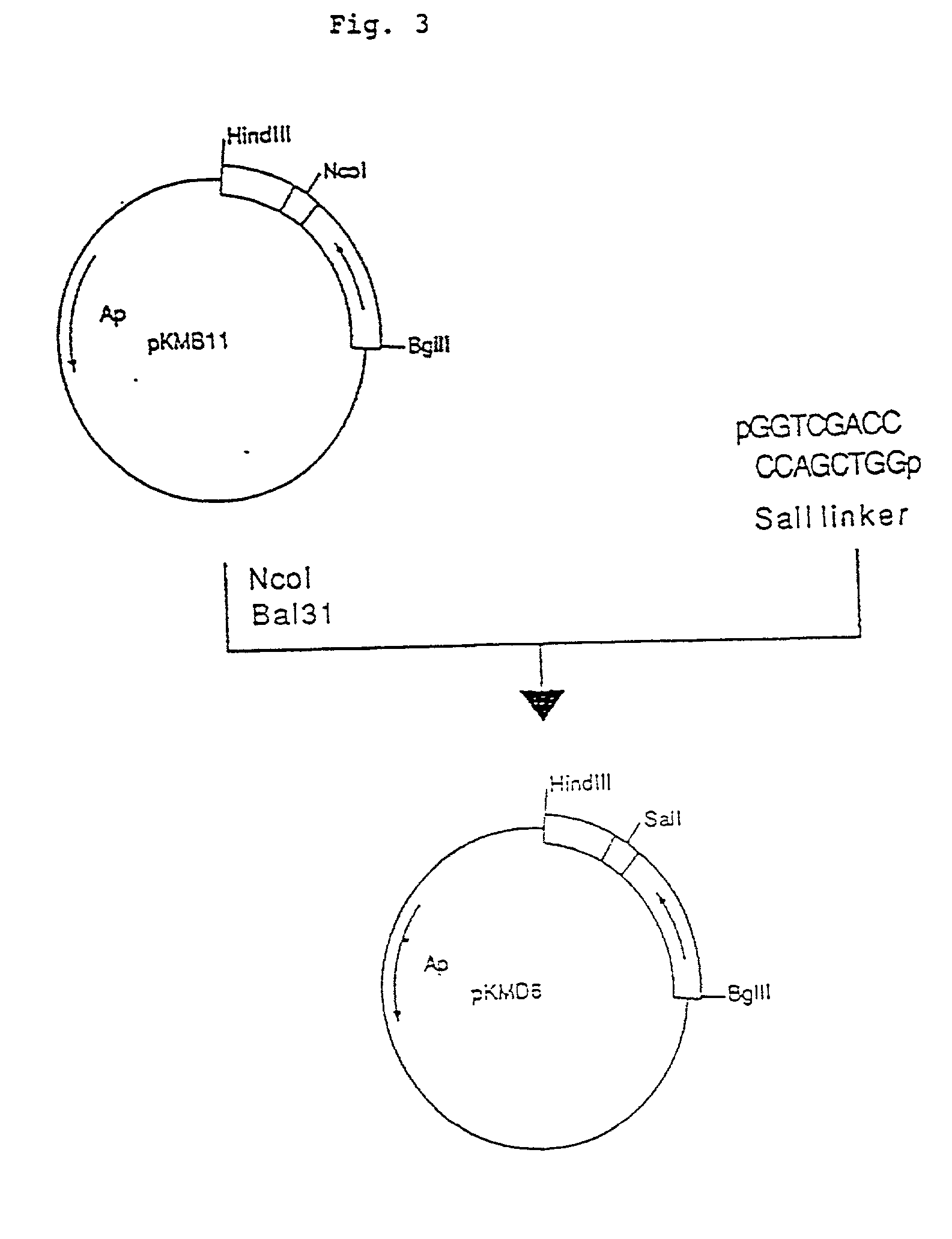
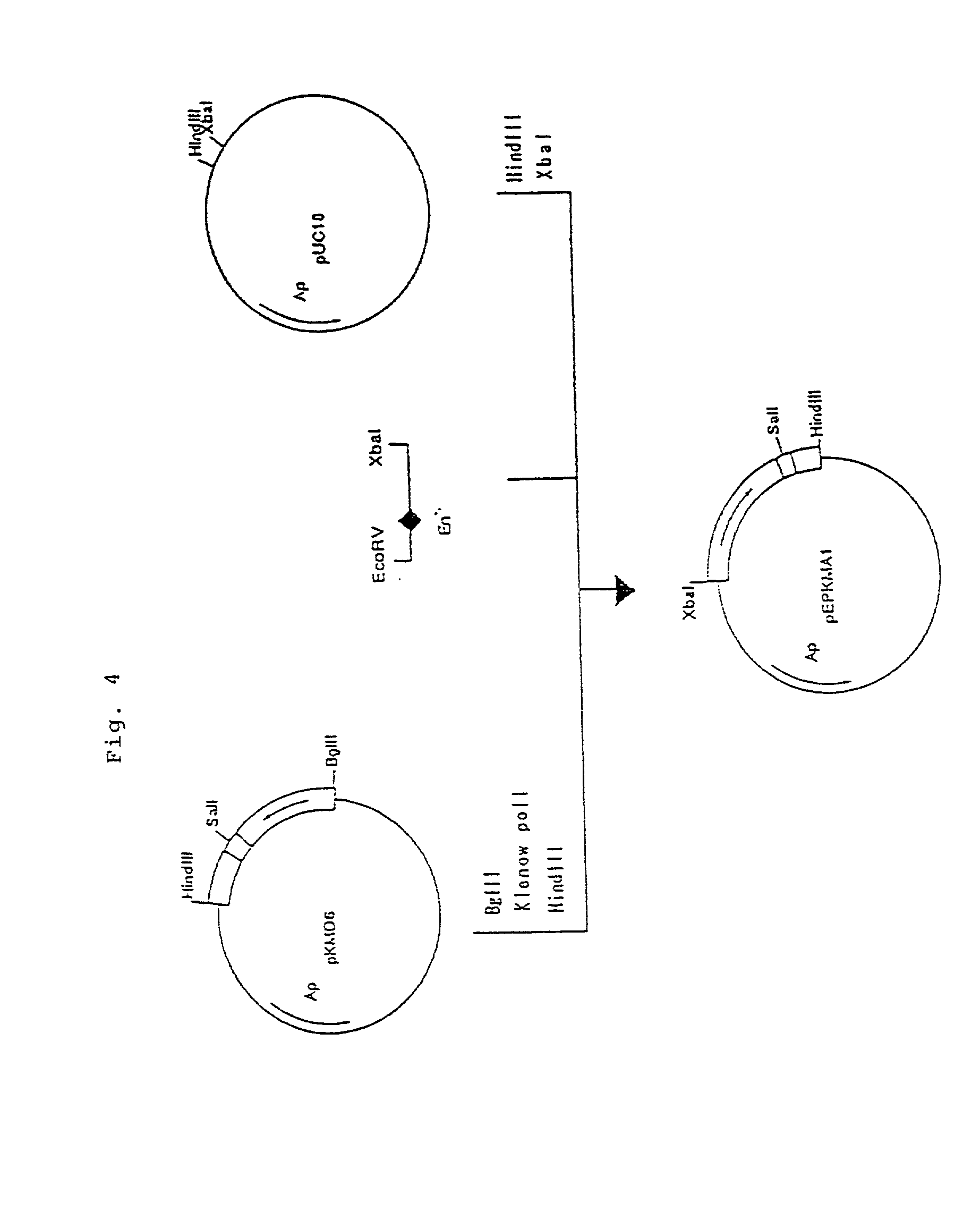
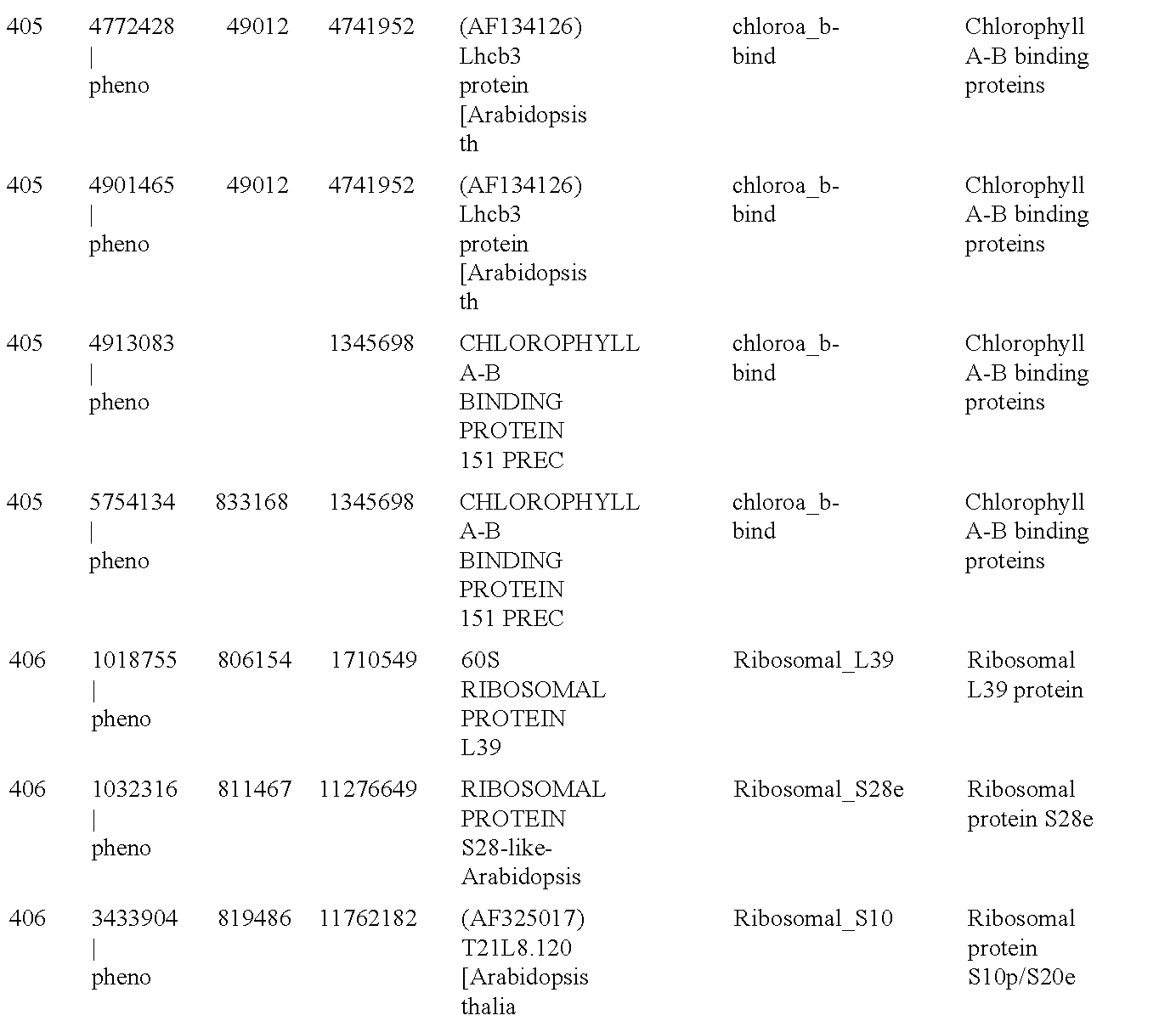
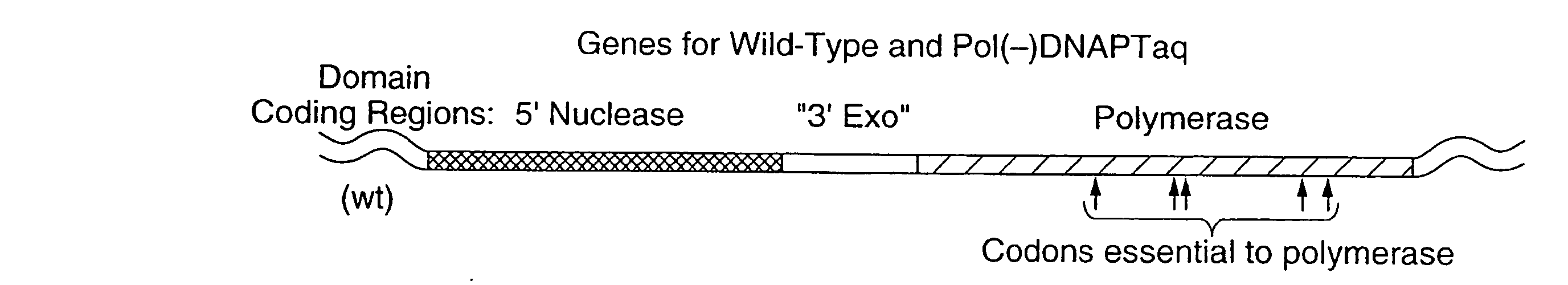
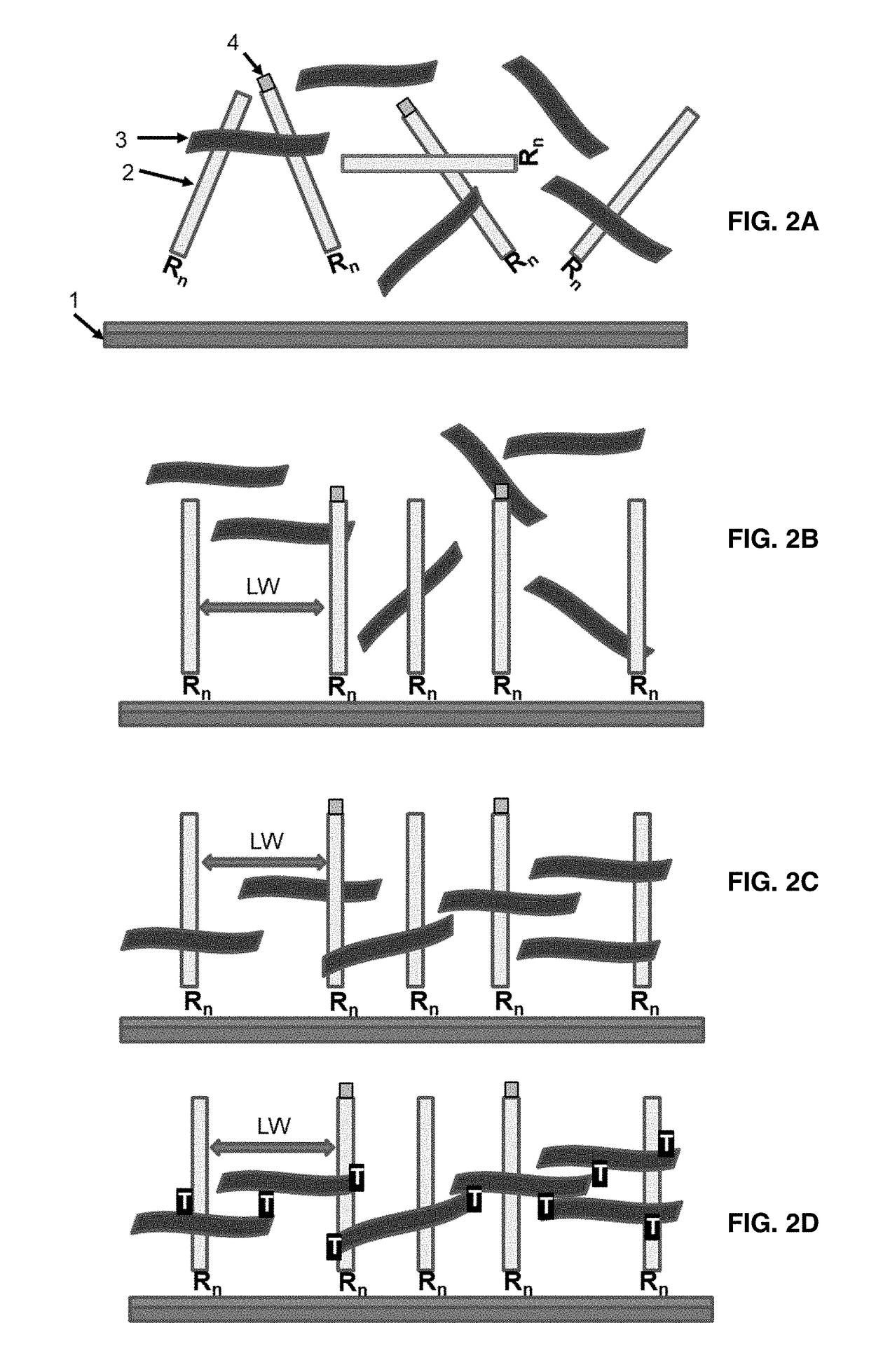
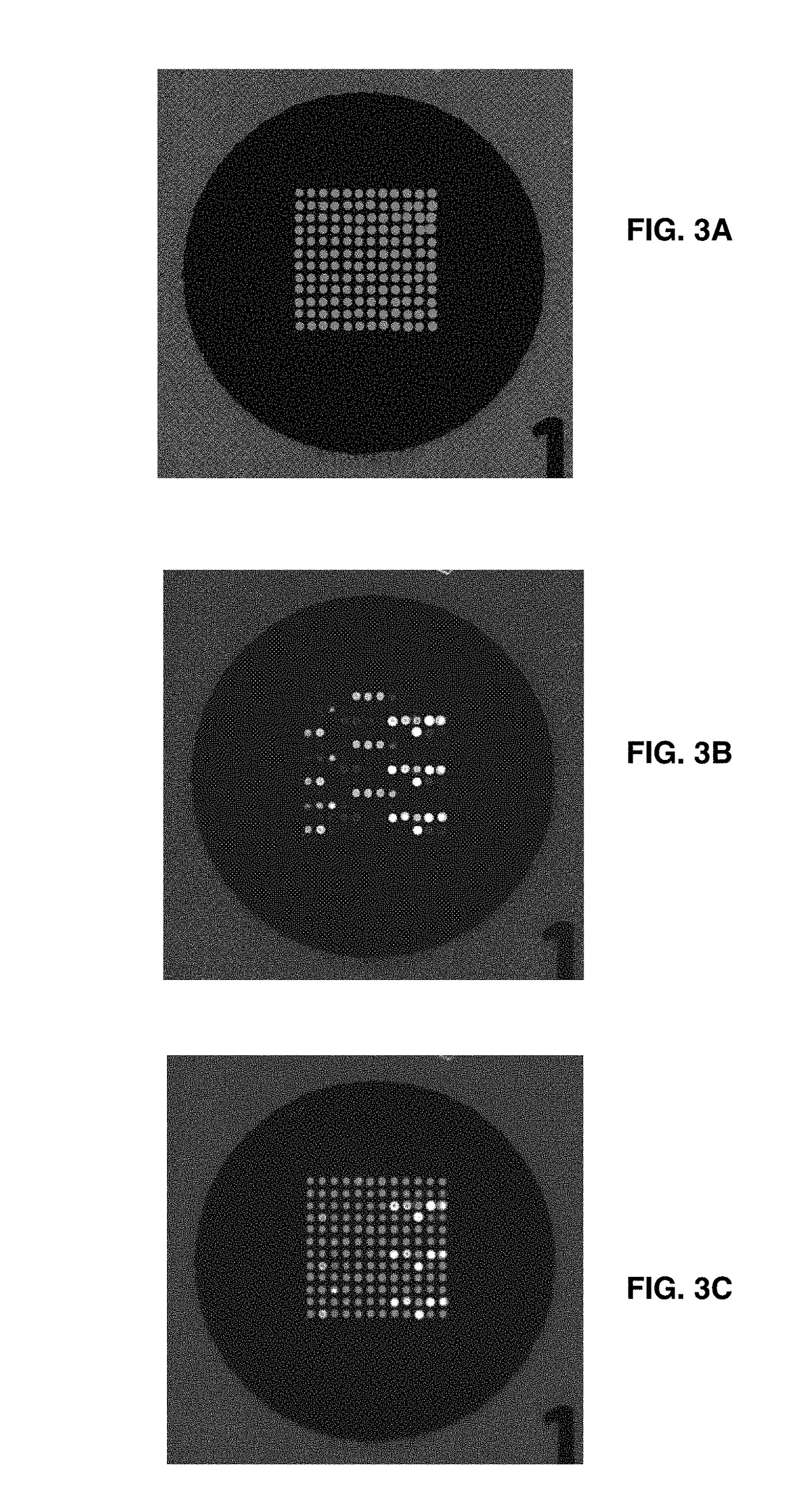
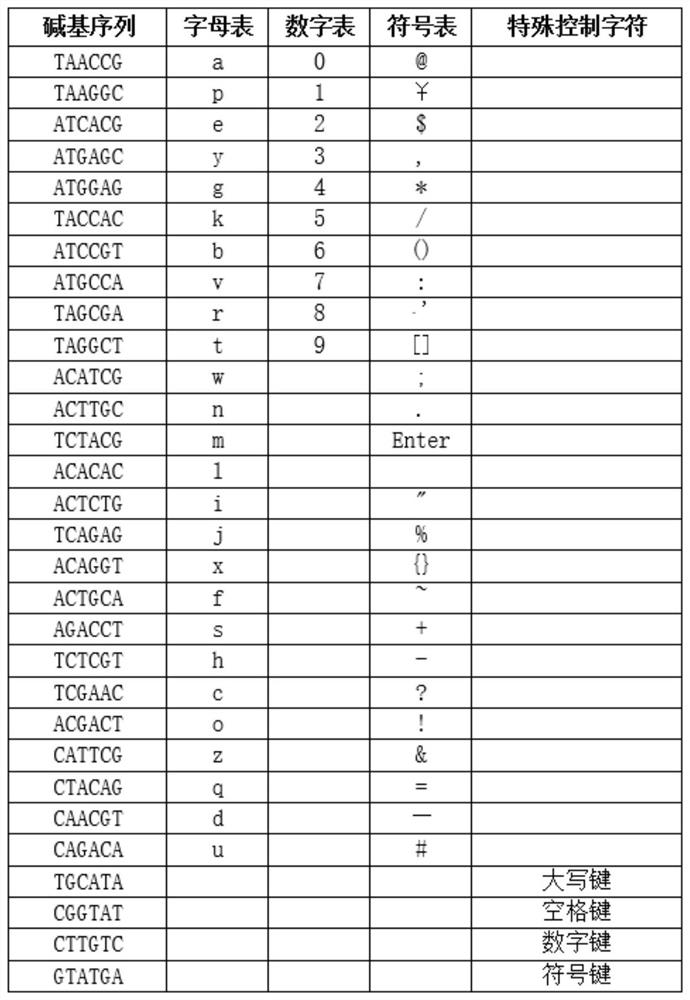
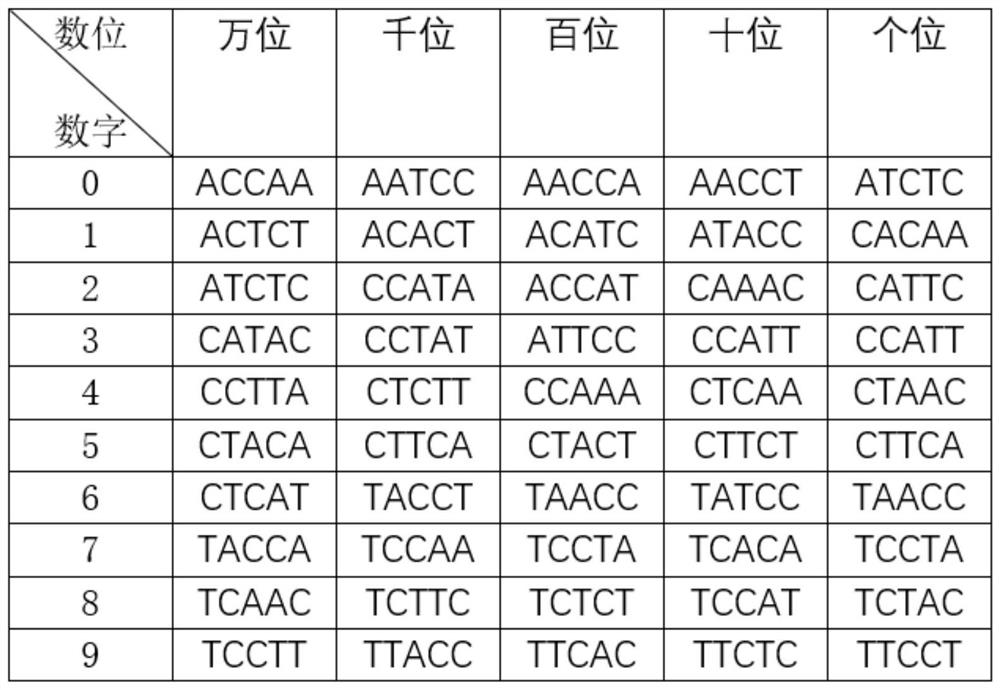
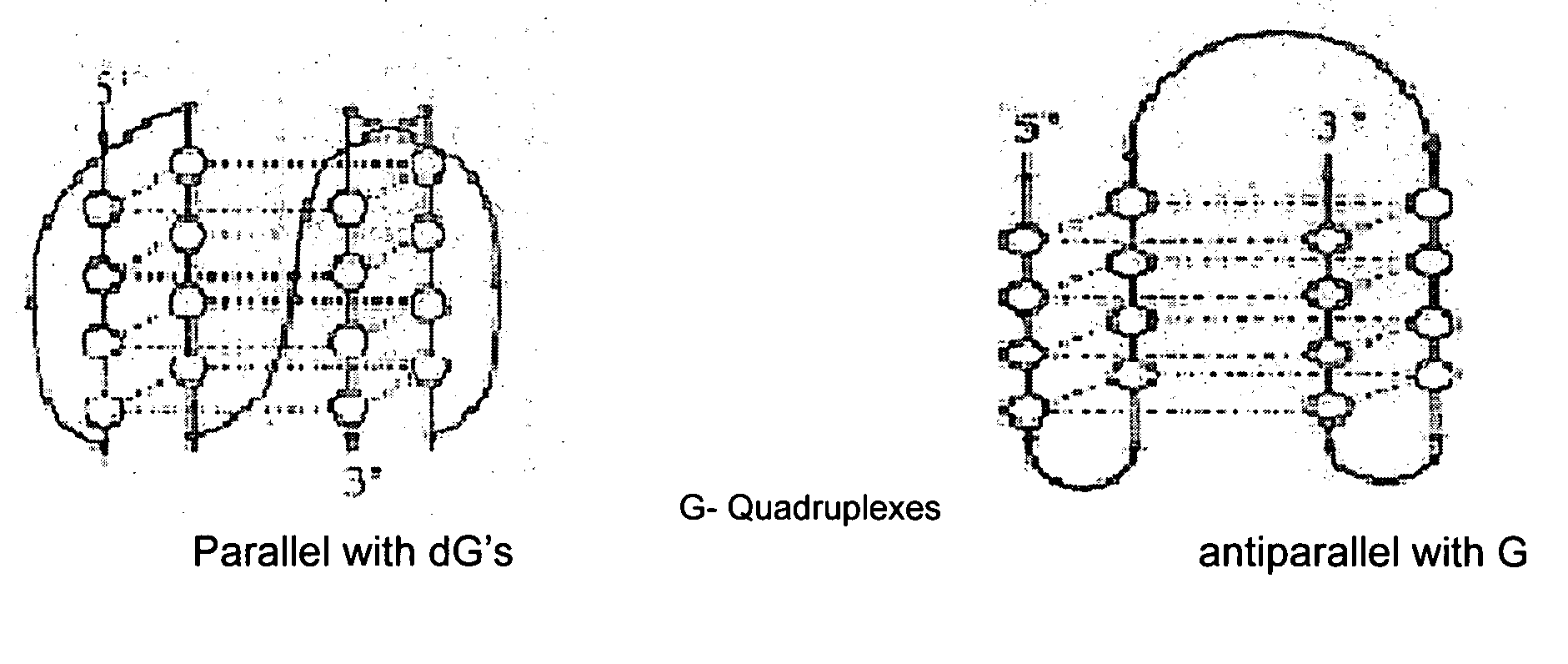Patents
Literature
96 results about "Synthetic DNA" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Protein/(poly)peptide libraries
InactiveUS6828422B1Reduce in quantityRemoving unwanted cleavages sitesPeptide/protein ingredientsAntibody mimetics/scaffoldsHuman DNA sequencingSynthetic DNA
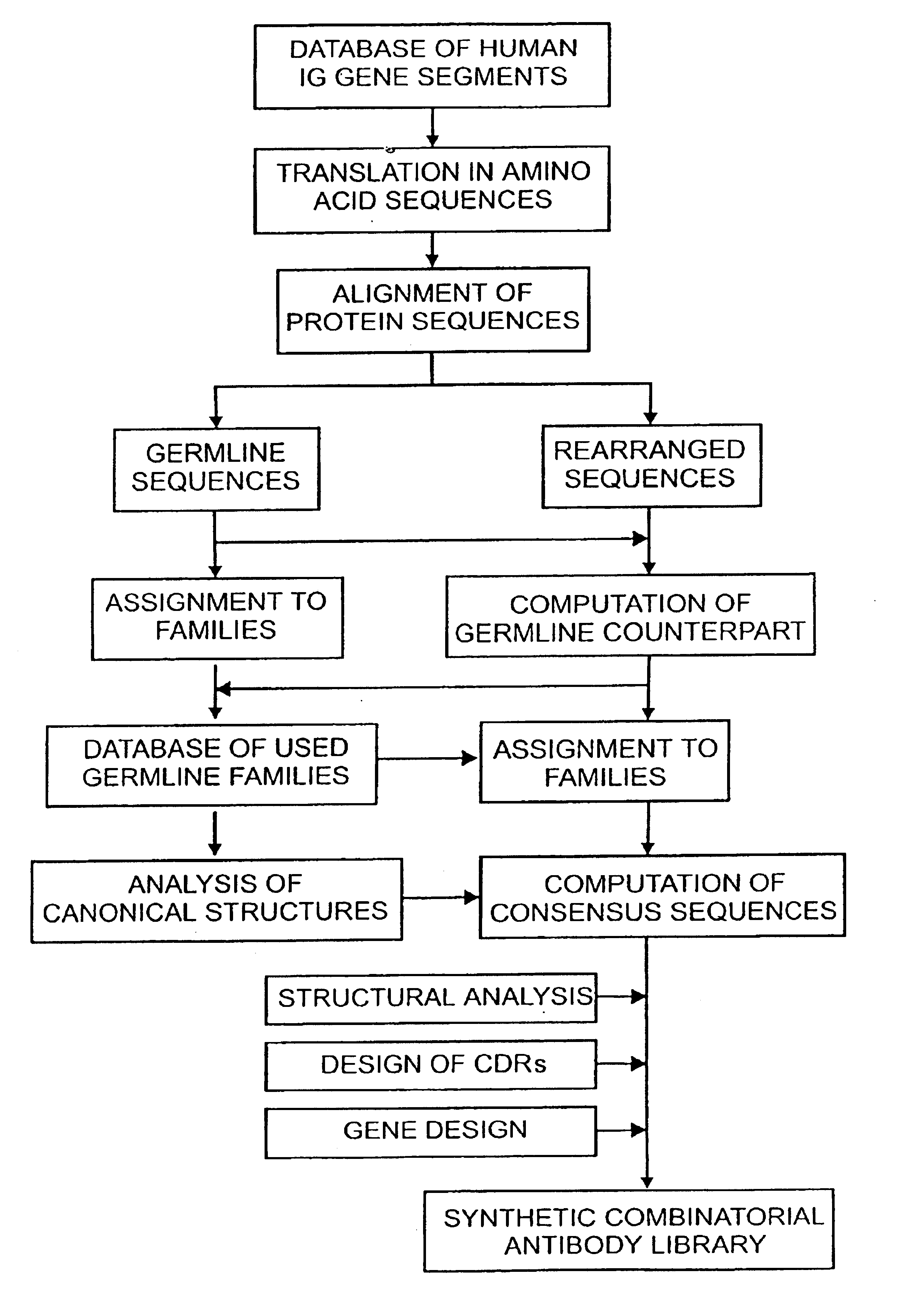
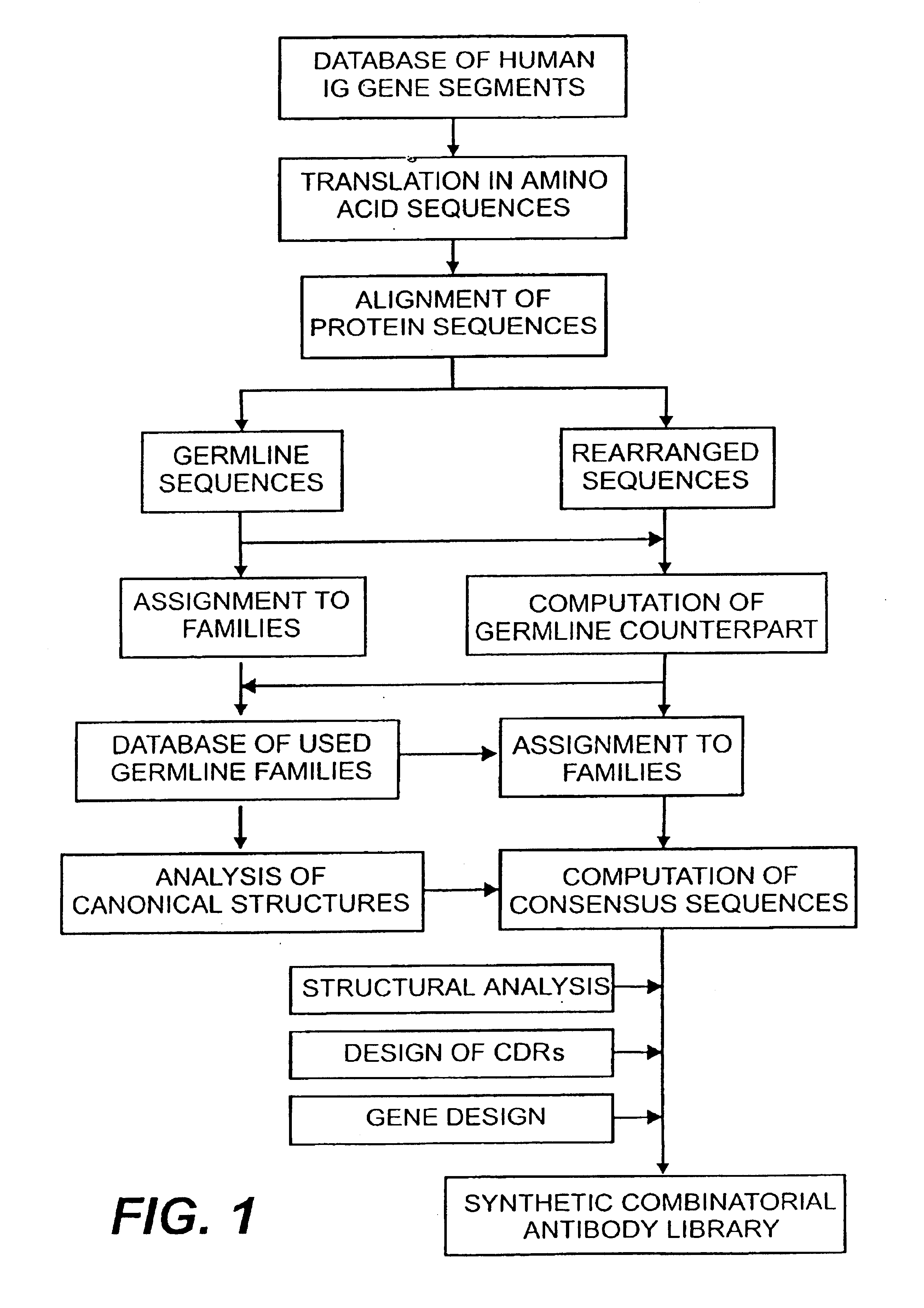
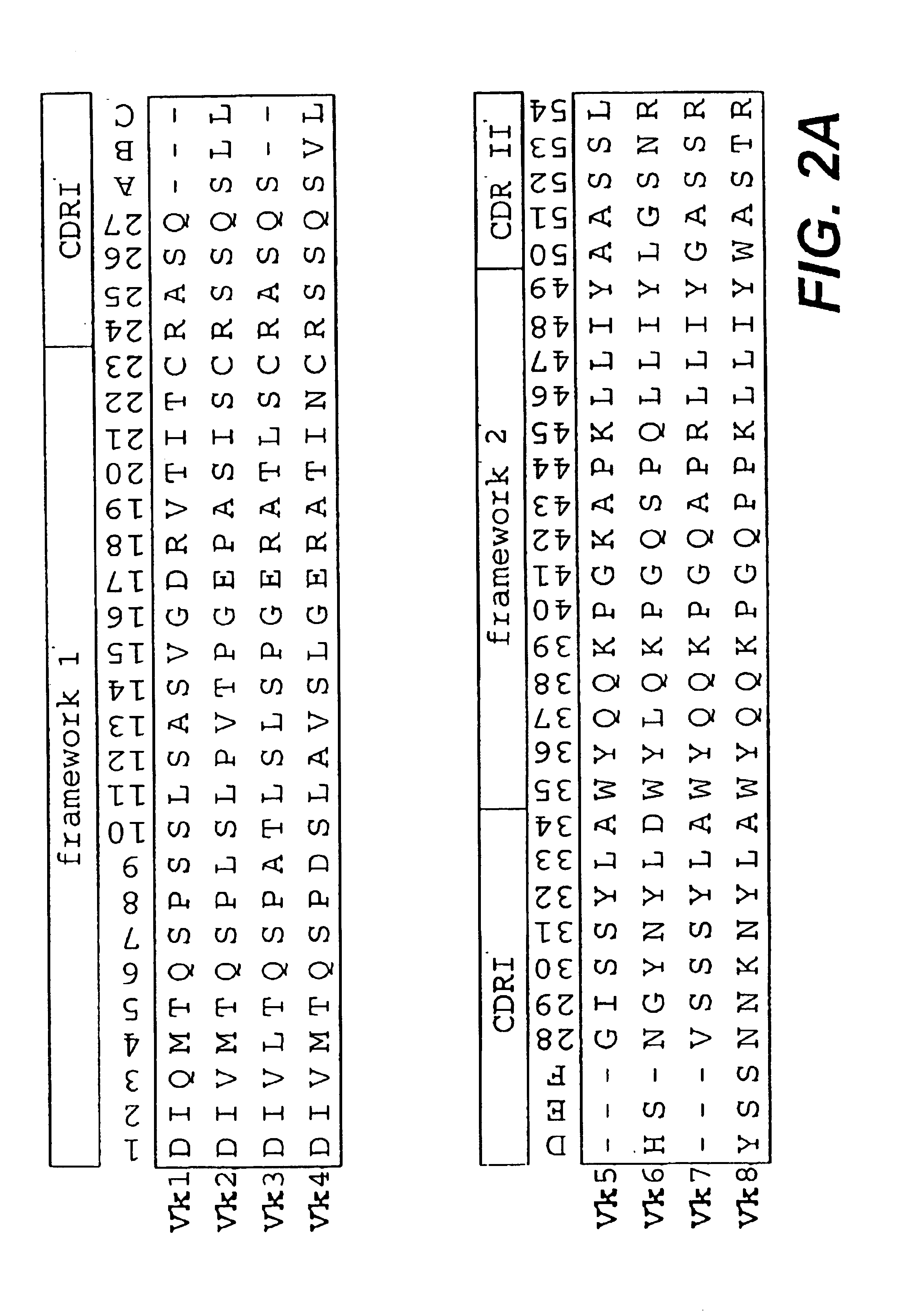
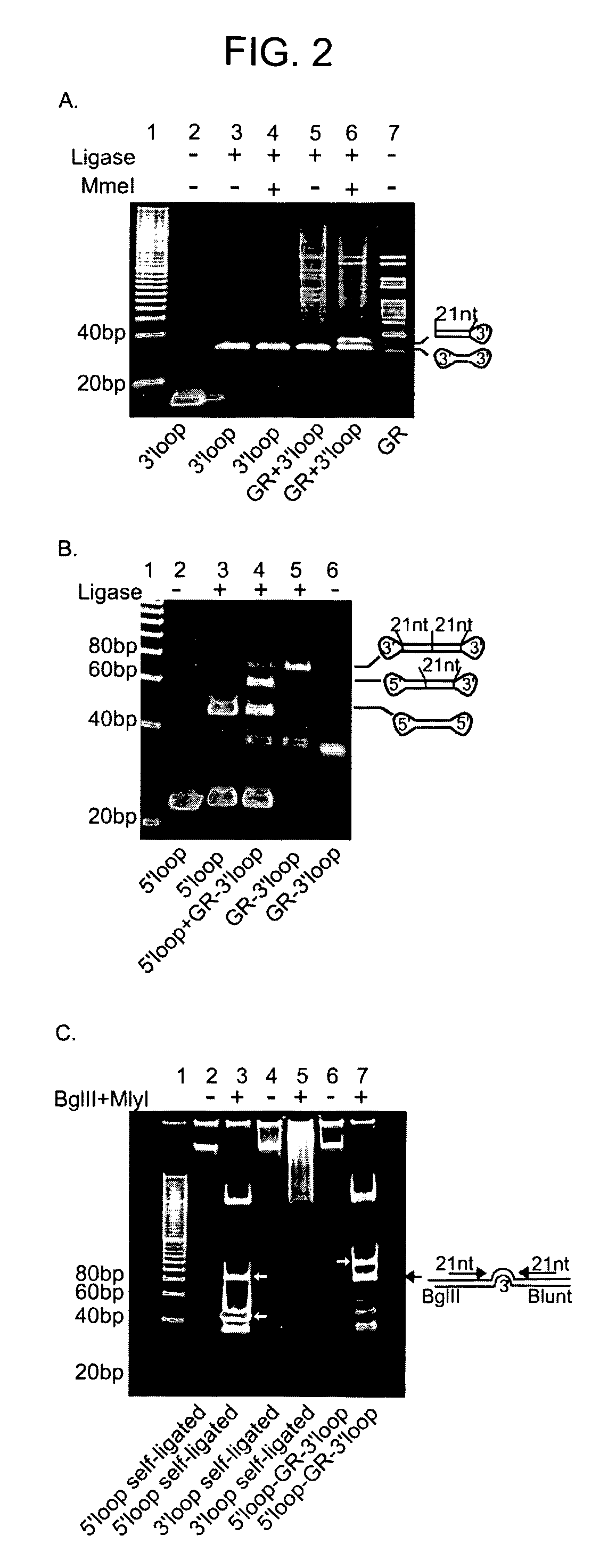
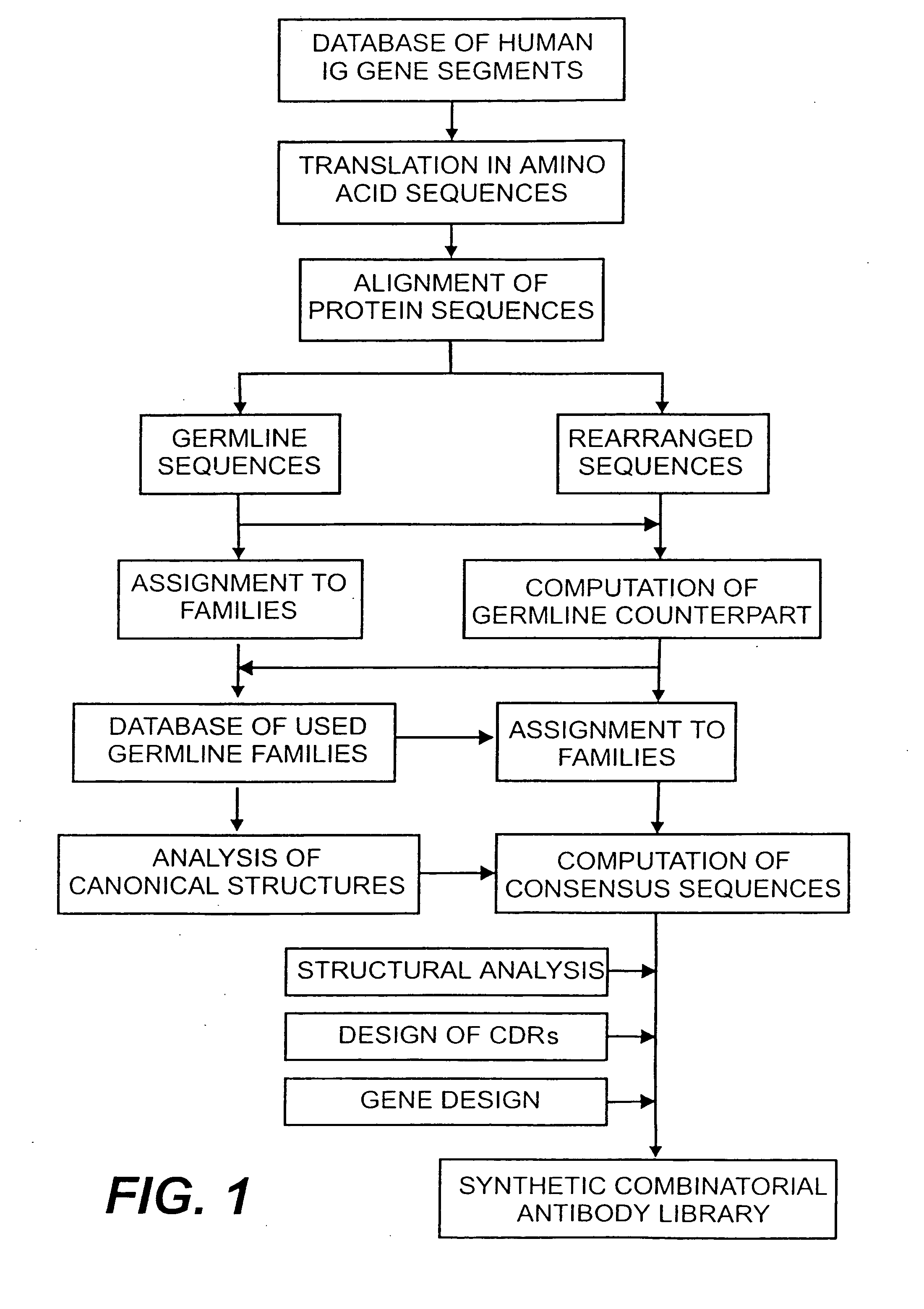
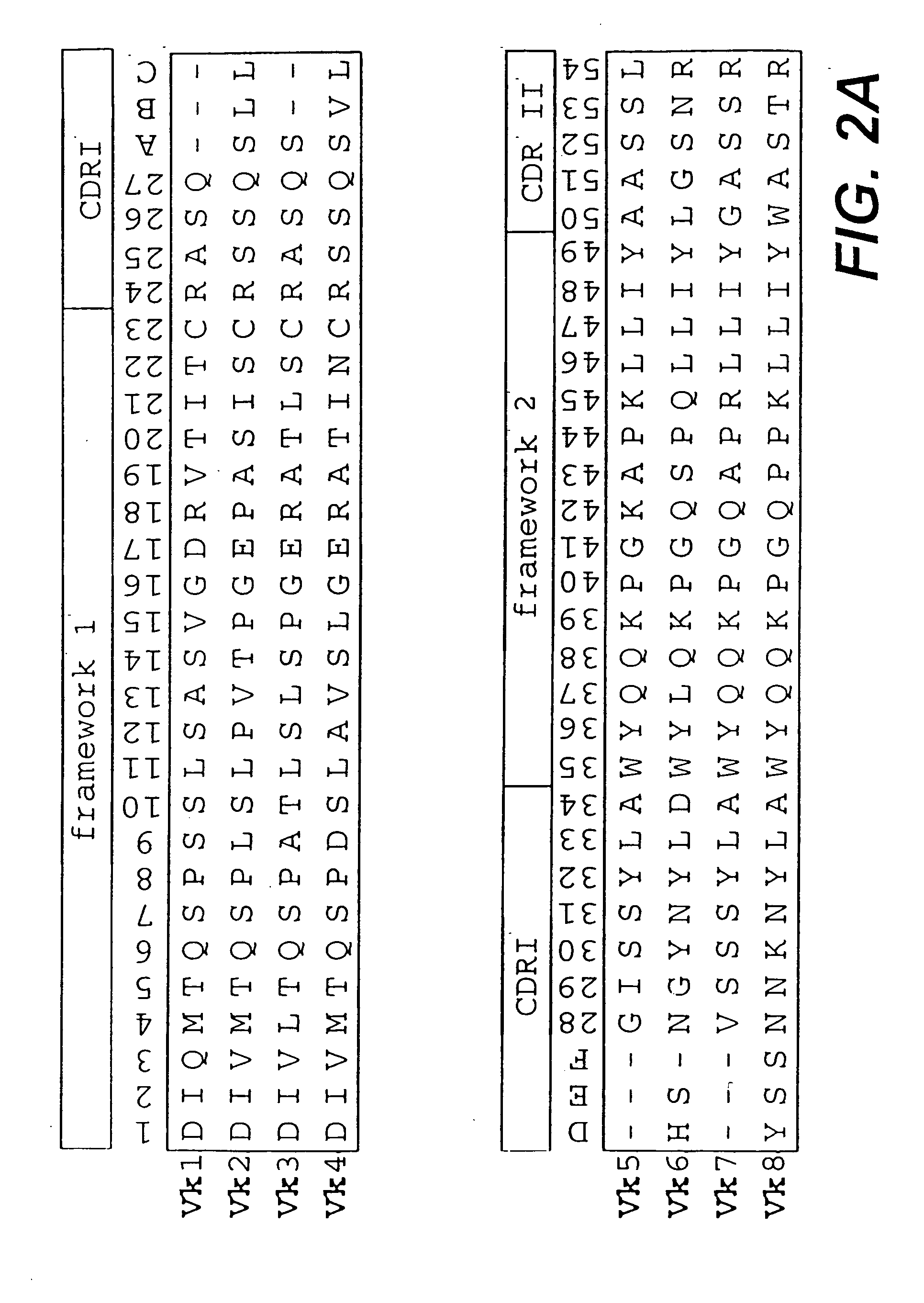
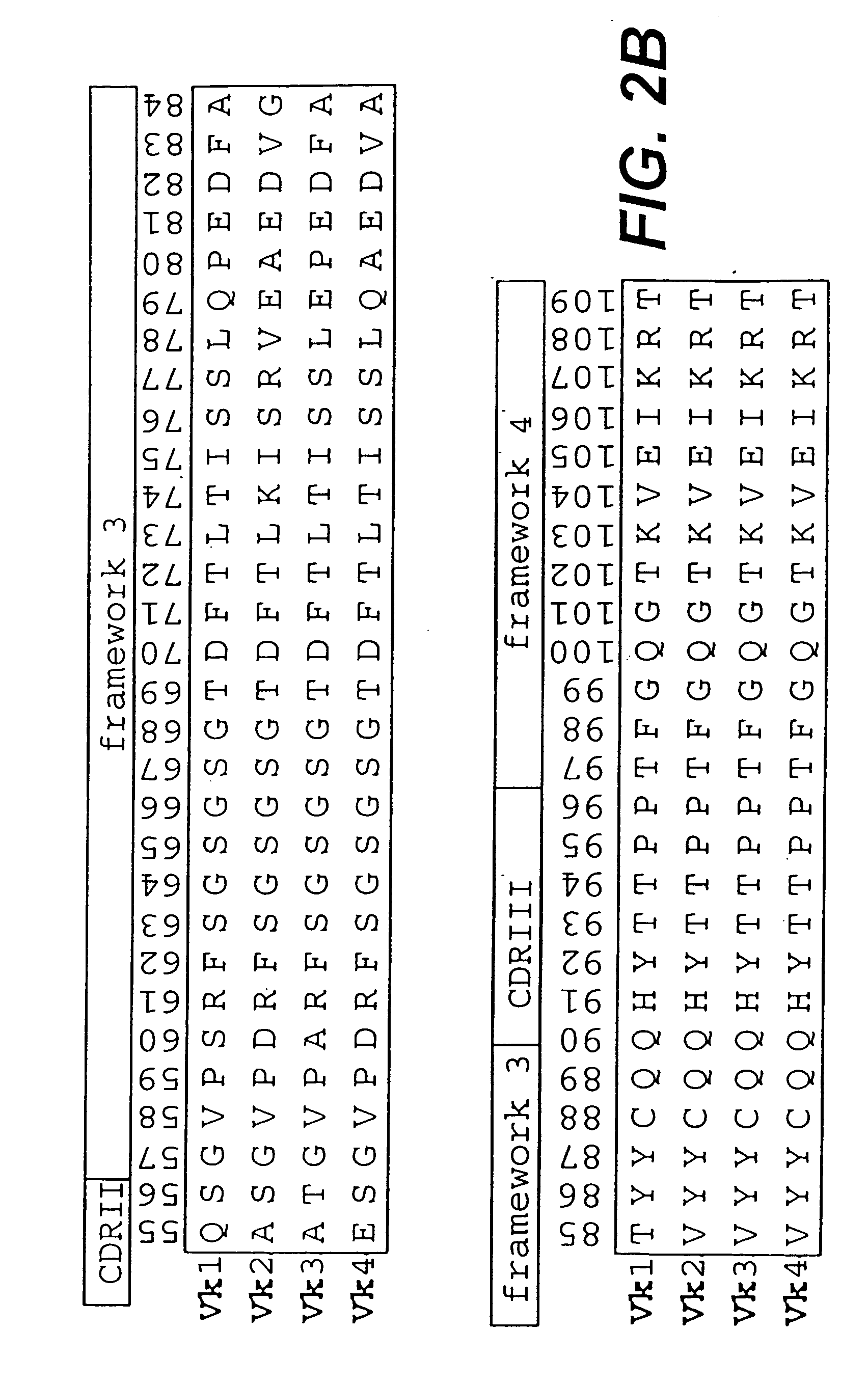
The present invention relates to synthetic DNA sequences which encode one or more collections of homologous proteins / (poly)peptides, and methods for generating and applying libraries of these DNA sequences. In particular, the invention relates to the preparation of a library of human-derived antibody genes by the use of synthetic consensus sequences which cover the structural repertoire of antibodies encoded in the human genome. Furthermore, the invention relates to the use of a single consensus antibody gene as a universal framework for highly diverse antibody libraries.
Owner:MORFOZIS AG
Promoter, promoter control elements, and combinations, and uses thereof
ActiveUS20120084885A1Enhancing and reducing regionImmunoglobulinsFermentationGene productSynthetic DNA
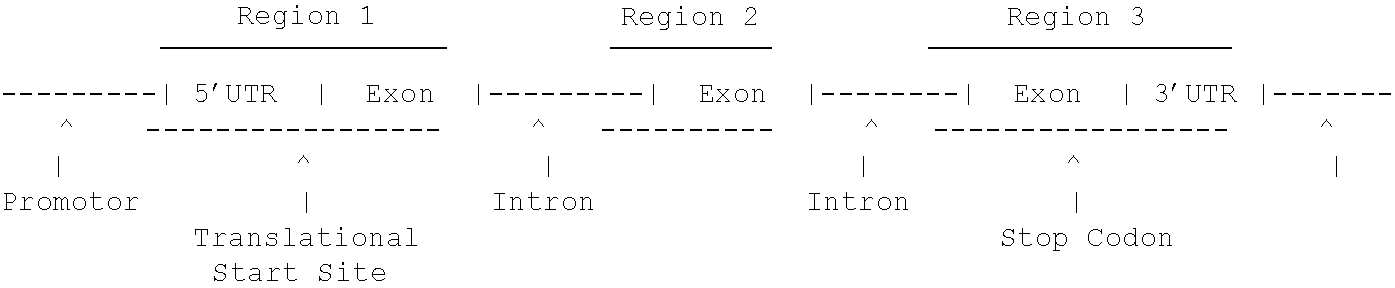
The present invention provides DNA molecules that constitute fragments of the genome of a plant, and polypeptides encoded thereby. The DNA molecules are useful for specifying a gene product in cells, either as a promoter or as a protein coding sequence or as an UTR or as a 3′ termination sequence, and are also useful in controlling the behavior of a gene in the chromosome, in controlling the expression of a gene or as tools for genetic mapping, recognizing or isolating identical or related DNA fragments, or identification of a particular individual organism, or for clustering of a group of organisms with a common trait. One of ordinary skill in the art, having this data, can obtain cloned DNA fragments, synthetic DNA fragments or polypeptides constituting desired sequences by recombinant methodology known in the art or described herein
Owner:CERES INC
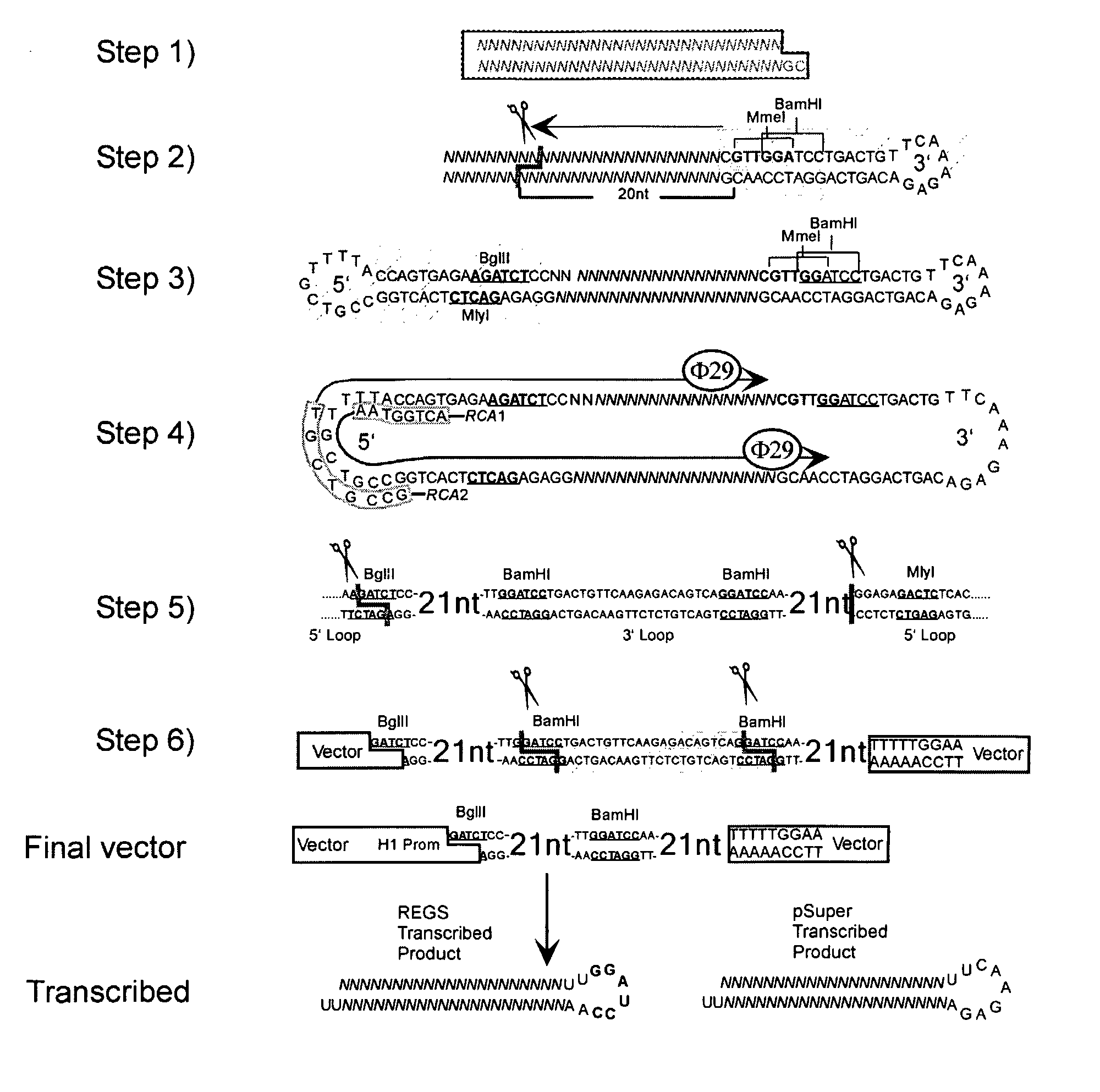
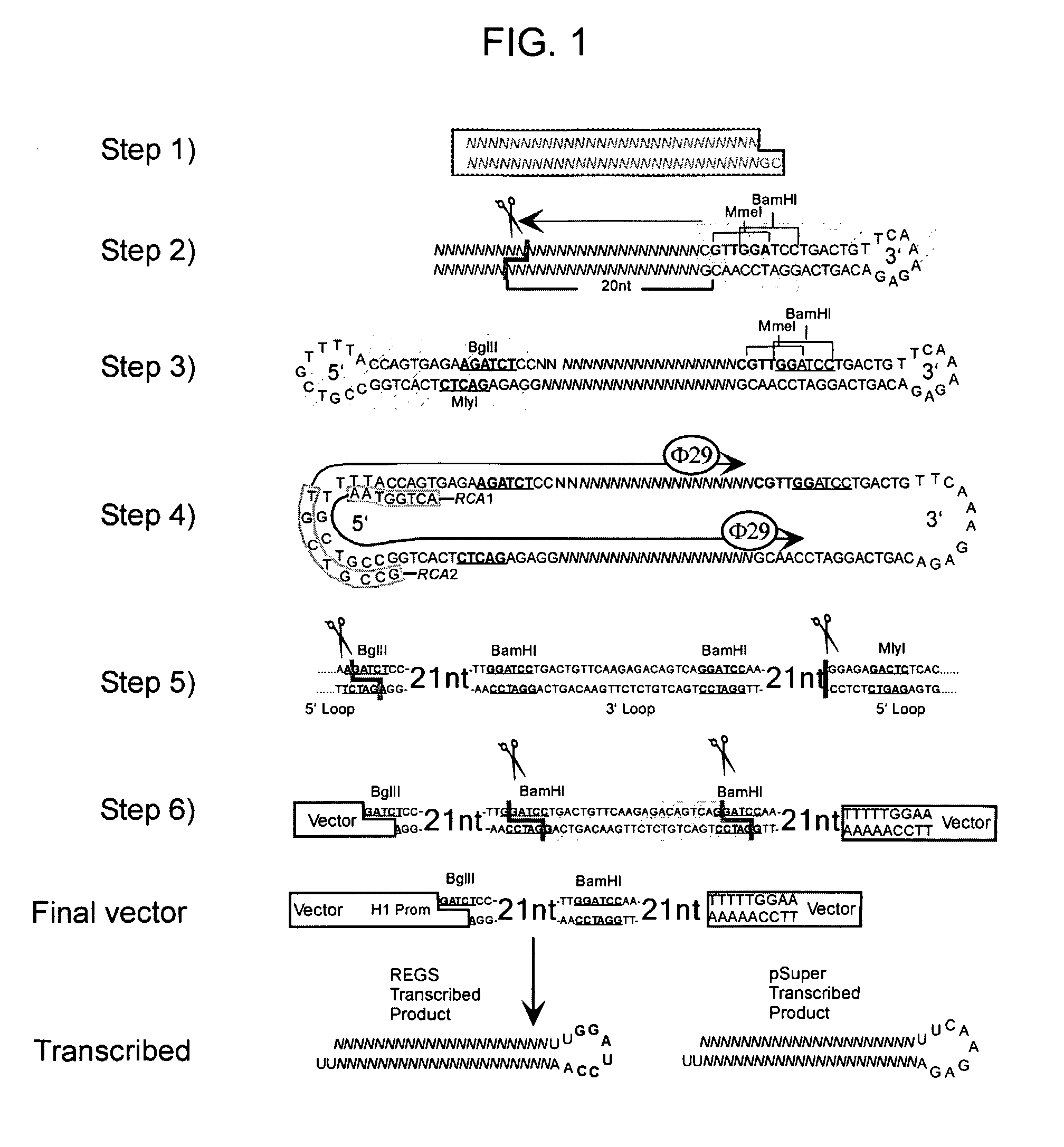
Methods and Compositions for Use in Preparing Hairpin Rnas
Methods and compositions for producing hRNA, e.g., shRNA, expression modules for specific target nucleic acids are provided. In the subject methods, an initial nucleic acid, e.g., dsDNA, synthetic DNA, etc., corresponding to the target nucleic acid of interest is converted to an intermediate nucleic acid. The resultant intermediate nucleic acid, following an optional size modification step, is then converted to a linear dsDNA that includes at least one copy of the hRNA expression module of interest, or a precursor (i.e., pro-shRNA expression module) thereof, where in certain embodiments conversion may include amplification. Also provided are reagents, systems and kits for use in practicing the subject methods. The subject methods and compositions find use in a variety of different applications.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Protein (poly)peptides libraries
InactiveUS20060003334A1High expressionMaximizes correspondenceFungiBacteriaHuman DNA sequencingSynthetic DNA
The present invention relates to synthetic DNA sequences which encode one or more collections of homologous proteins / (poly)peptides, and methods for generating and applying libraries of these DNA sequences. In particular, the invention relates to the preparation of a library of human-derived antibody genes by the use of synthetic consensus sequences which cover the structural repertoire of antibodies encoded in the human genome. Furthermore, the invention relates to the use of a single consensus antibody gene as a universal framework for highly diverse antibody libraries.
Owner:MORFOZIS AG
Process for producing humanized chimera antibody
InactiveUS20020026036A1Easy to produceEasy constructionPeptide/protein ingredientsAntibody mimetics/scaffoldsRestriction enzyme digestionSynthetic DNA
A process for the production of humanized chimera antibody, wherein the chimera antibody is produced easily without changing any of the amino acids of its mouse antibody variable region, which comprises the steps of: (1) constructing a cassette vector by inserting a cDNA coding for a heavy chain constant region of human antibody into an expression vector for animal cell use and establishing a cloning site in the upstream region of the heavy chain constant region of said cassette vector for inserting a cDNA which encodes a heavy chain variable region of nonhuman animal antibody; (2) digesting a cDNA coding for the heavy chain variable region of nonhuman animal antibody with restriction enzymes; (3) inserting said cDNA coding for the heavy chain variable region of nonhuman animal antibody into the cassette vector, using a synthetic DNA which comprises a base sequence corresponding to the 5'-end side of said heavy chain constant region of human antibody and a base sequence corresponding to the 3'-end side of said heavy chain variable region of nonhuman animal antibody and is possessed of the restriction enzyme recognition sites on both of its ends, thereby constructing a humanized chimera antibody heavy chain expression vector in which said cDNA coding for the heavy chain constant region of human antibody and said cDNA coding for the heavy chain variable region of nonhuman animal antibody are linked together through said synthetic DNA; (4) constructing a cassette vector by inserting a cDNA coding for a light chain constant region of human antibody into an expression vector for animal cell use and establishing a cloning site in the upstream region of the light chain constant region of said cassette vector for inserting a cDNA which encodes a light chain variable region of nonhuman animal antibody; (5) digesting a cDNA coding for the light chain variable region of nonhuman animal antibody with restriction enzymes; (6) inserting said cDNA coding for a light chain variable region of nonhuman animal antibody into the cassette vector using a synthetic DNA which comprises a base sequence corresponding to the 5'-end side of said light chain constant region of human antibody and a base sequence corresponding to the 3'-end side of said light chain variable region of nonhuman animal antibody and is possessed of the restriction enzyme recognition sites on both of its ends, thereby constructing a humanized chimera antibody light chain expression vector in which said cDNA coding for the light chain constant region of human antibody and said cDNA coding for the light chain variable region of nonhuman animal antibody are linked together through said synthetic DNA; (7) introducing these expression vectors into host cells to obtain a transformant; and (8) culturing said transformant in an appropriate culture medium, thereby allowing the transformant to produce and accumulate a humanized chimera antibody, and collecting said humanized chimera antibody from the resulting culture broth.
Owner:KYOWA HAKKO KIRIN CO LTD
Promoter, promoter control elements, and combinations, and uses thereof
ActiveUS20110191912A1Enhancing and reducing regionPeptide/protein ingredientsImmunoglobulinsGene productSynthetic DNA
The present invention provides DNA molecules that constitute fragments of the genome of a plant, and polypeptides encoded thereby. The DNA molecules are useful for specifying a gene product in cells, either as a promoter or as a protein coding sequence or as an UTR or as a 3′ termination sequence, and are also useful in controlling the behavior of a gene in the chromosome, in controlling the expression of a gene or as tools for genetic mapping, recognizing or isolating identical or related DNA fragments, or identification of a particular individual organism, or for clustering of a group of organisms with a common trait. One of ordinary skill in the art, having this data, can obtain cloned DNA fragments, synthetic DNA fragments or polypeptides constituting desired sequences by recombinant methodology known in the art or described herein.
Owner:CERES INC
Targeted genomic rearrangements using site-specific nucleases
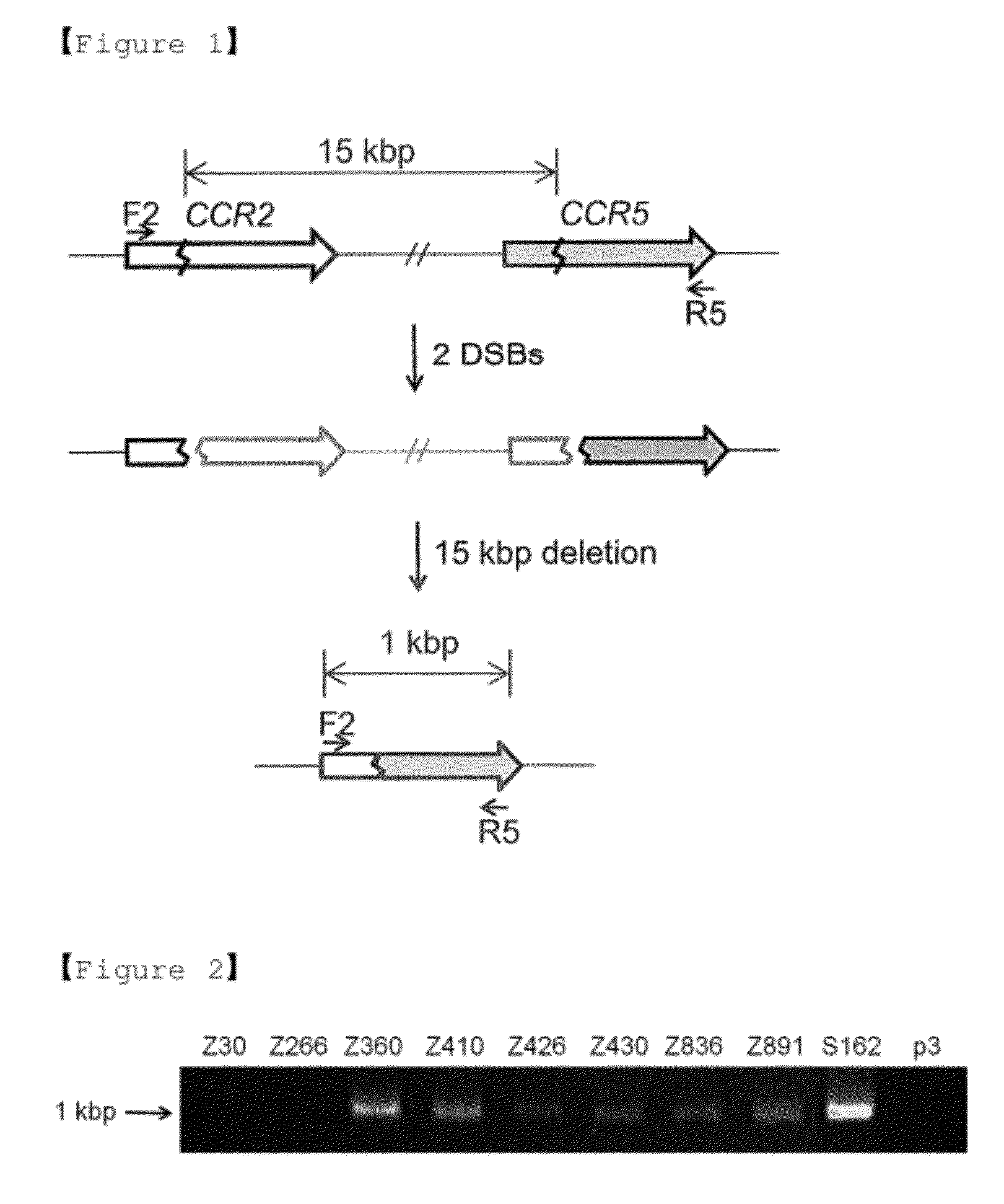
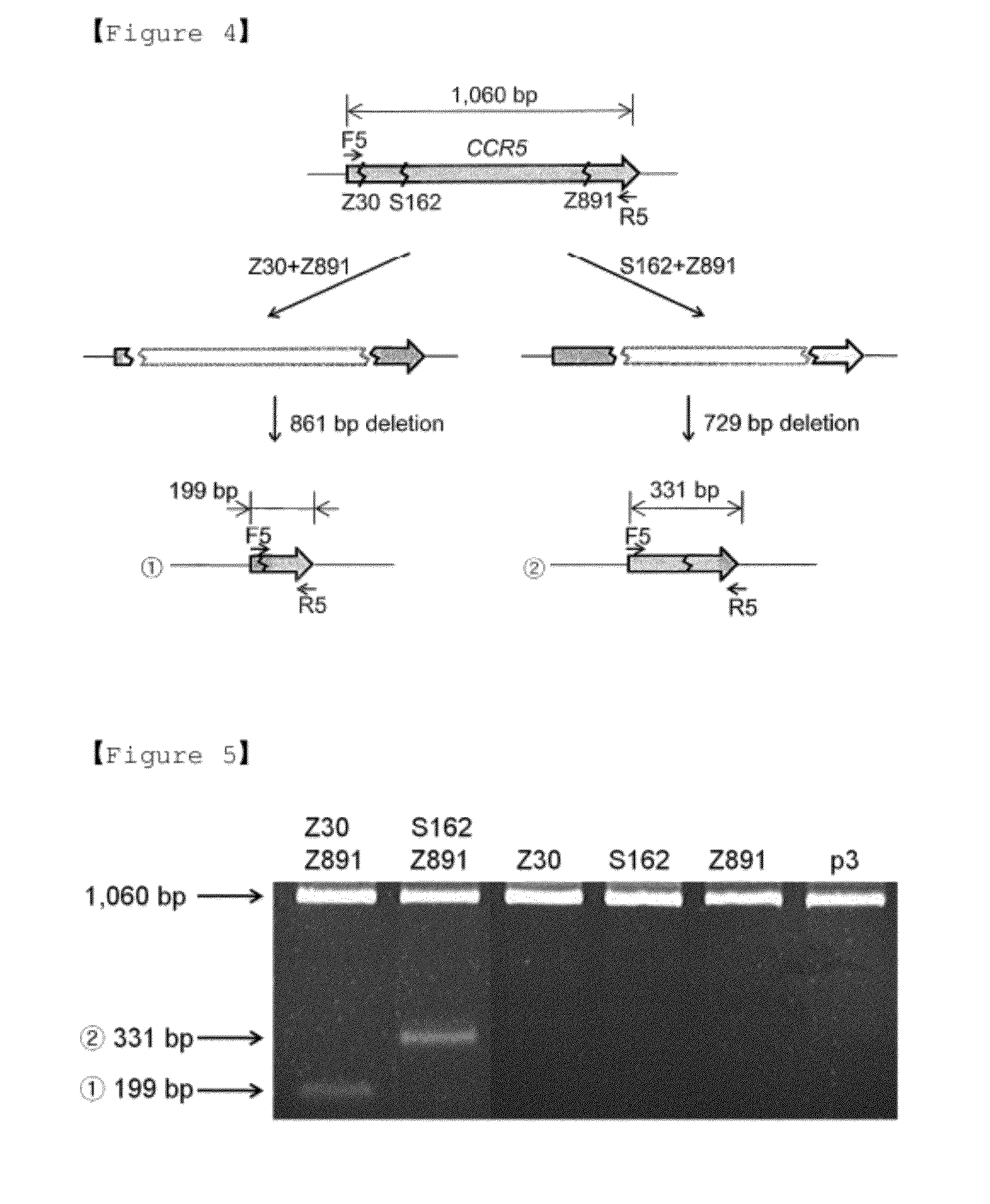
The present invention relates to a method for genomic DNA rearrangements, and more particularly, to a method for deletion, duplication, inversion, replacement, or rearrangement of genomic DNA using pairs of site-specific nucleases targeting two or more sites in the genome, a cell in which genomic DNA is deleted, duplicated, inverted, replaced, or rearranged by the same method, and a method for expressing the site-specific nucleases in cells. Further, the present invention relates to a method for inserting synthetic DNA molecules into the genome using site-specific nucleases targeting a pre-determined site in the genome, a cell in which DNA insertion occurs by the same method, and a method for expressing the site-specific nucleases in cells.
Owner:TOOLGEN INC
Synthetic human papillomavirus genes
InactiveUS7001995B1Easy to insertEasy to removeBiocideGenetic material ingredientsPolynucleotide VaccinesHuman papillomavirus
Synthetic DNA molecules encoding papillomavirus proteins are provided. The codons of the synthetic molecules are codons preferred by the projected host cell. The synthetic molecules may be used as a polynucleotide vaccine which provides effective immunoprophylaxis against papillomavirus infection through stimulation of neutralizing antibody and cell-mediated immunity.
Owner:MERCK SHARP & DOHME CORP
Methods and Compositions for Detecting Target Sequences
InactiveUS20080160524A1Polypeptide with localisation/targeting motifSugar derivativesSynthetic DNANucleic acid sequencing
The present invention relates to compositions and methods for the detection and characterization of nucleic acid sequences and variations in nucleic acid sequences. The present invention relates to methods for amplifying a synthetic DNA from a target nucleic acid, forming a nucleic acid cleavage structure on the synthetic DNA, and detecting cleavage of the nucleic acid cleavage structure as an indicator of the preset of the target nucleic acid.
Owner:THIRD WAVE TECH
Codon optimized cftr
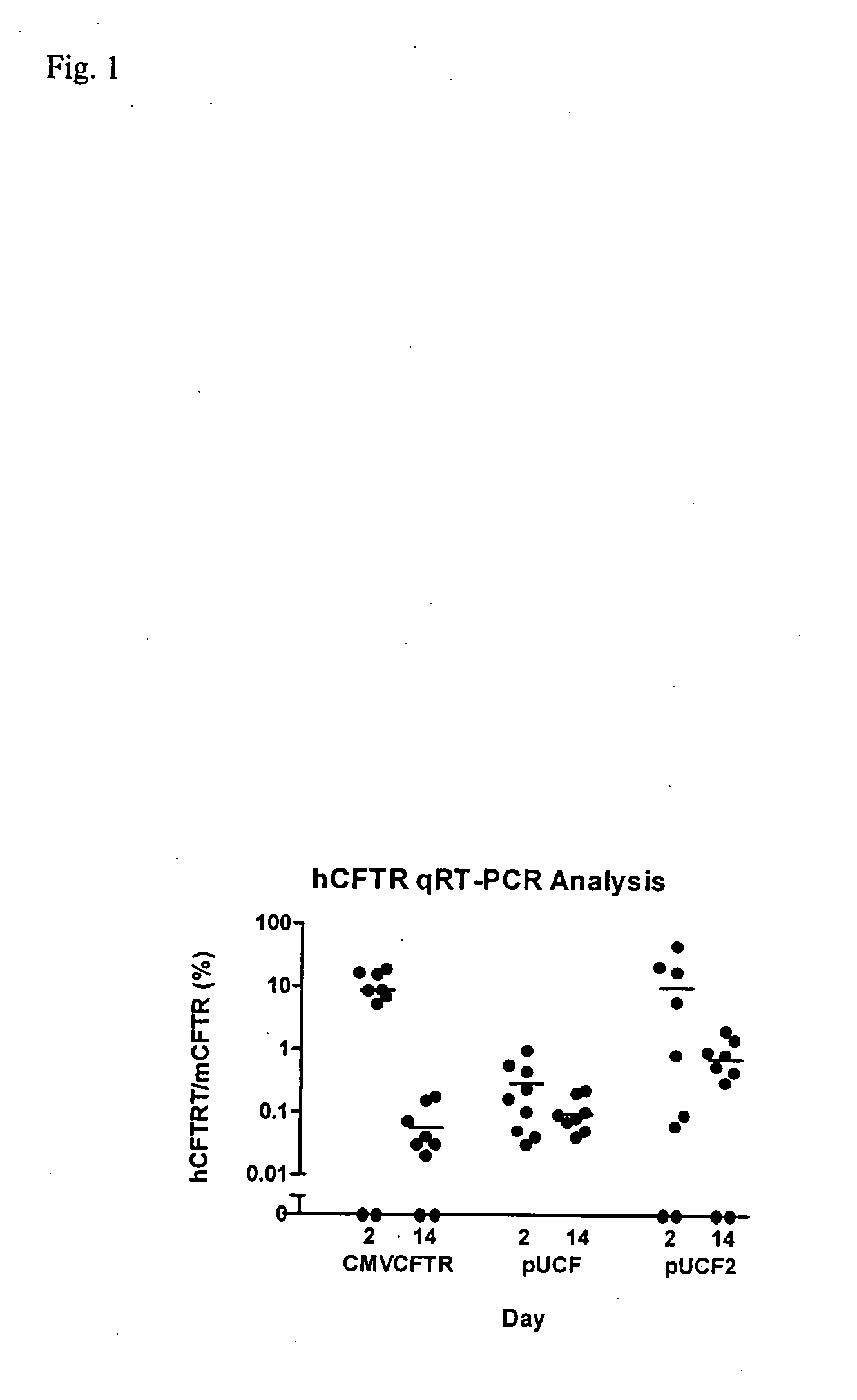
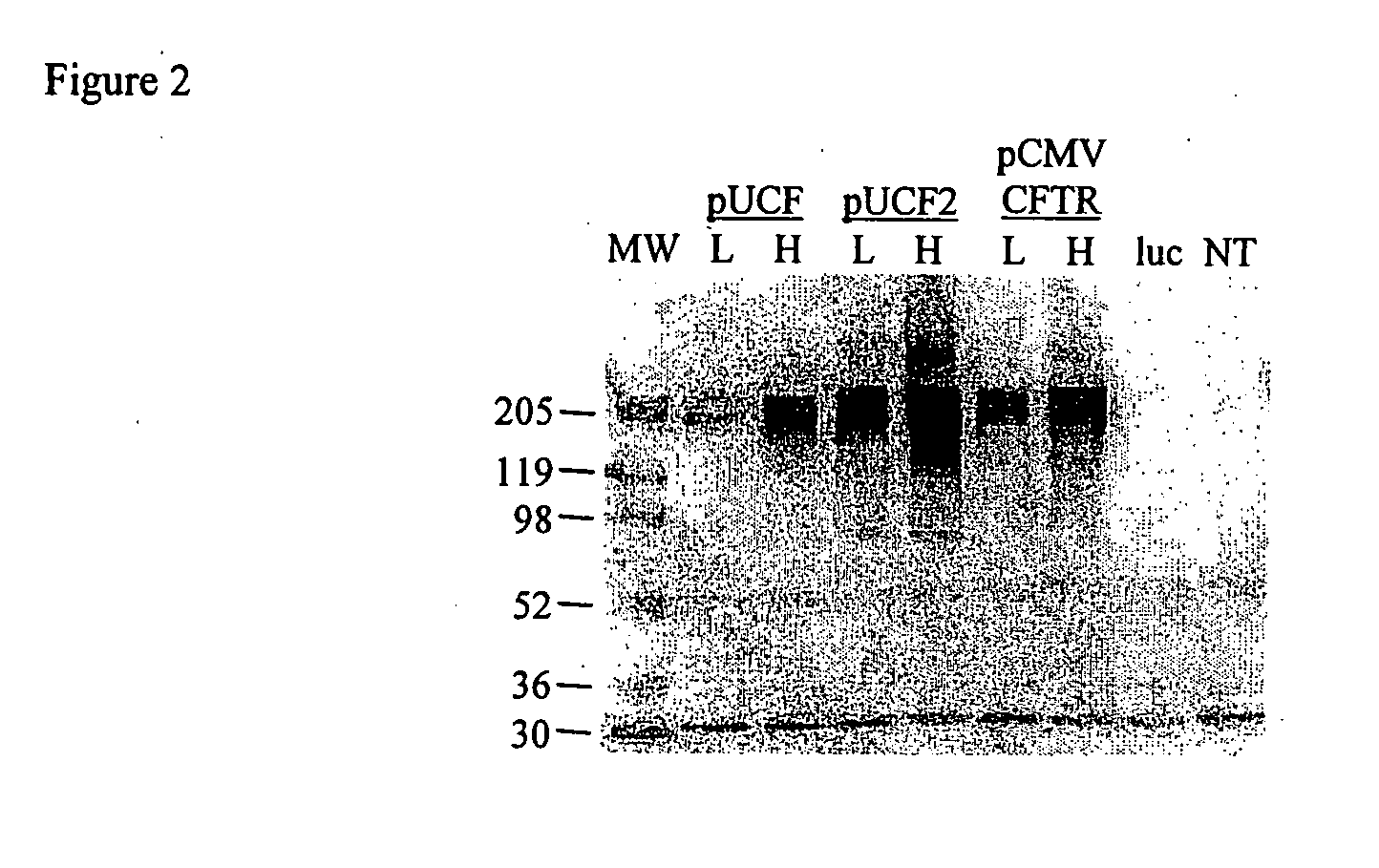
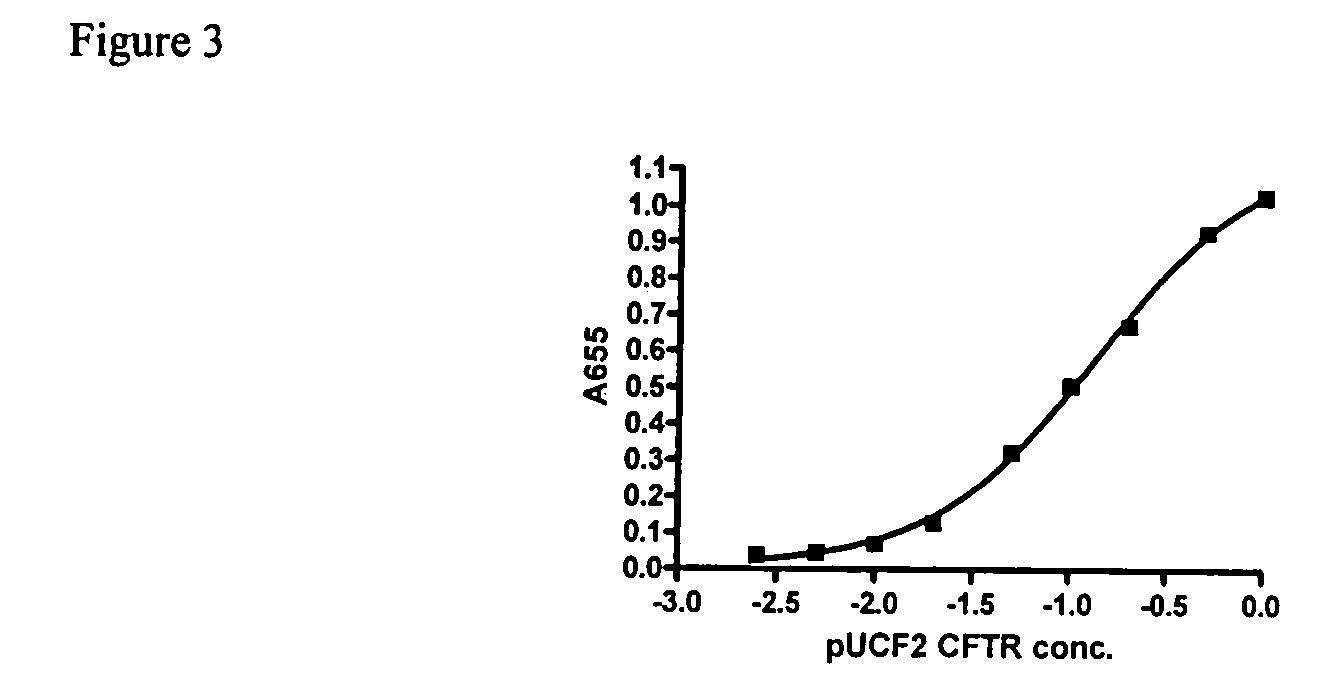
A synthetic hCFTR DNA sequence has been developed that produces remarkably high levels of hCFTR mRNA and protein in dosed murine lungs and human cells in culture compared to the natural hCFTR cDNA. This synthetic DNA addresses problems inherent in some natural cDNAs, such as premature transcriptional truncation sites introduced during cDNA synthesis. Introns are initially present in mRNA until the mRNA is processed. cDNA made from processed mRNA is devoid of introns. Thus DNA sequences (exon junctions) are present in a cDNA molecule which are not present in cells in nature. These exon junctions may affect transcription. Methods for improving expression of CFTR are based on sequence changes in cDNA molecules. The improvement methods may be applied to other cDNA molecules which are refractory to in vivo expression efforts. Compositions embodying the sequence changes increase the production of both transgenic mRNA and protein from cDNA molecules.
Owner:COPERNICUS THERAPEUTICS
Methods and Compositions for Detecting Target Sequences
InactiveUS20080220425A1Polypeptide with localisation/targeting motifPeptide/protein ingredientsSynthetic DNANucleic acid sequencing
The present invention relates to compositions and methods for the detection and characterization of nucleic acid sequences and variations in nucleic acid sequences. The present invention relates to methods for amplifying a synthetic DNA from a target nucleic acid, forming a nucleic acid cleavage structure on the synthetic DNA, and detecting cleavage of the nucleic acid cleavage structure as an indicator of the preset of the target nucleic acid.
Owner:THIRD WAVE TECH
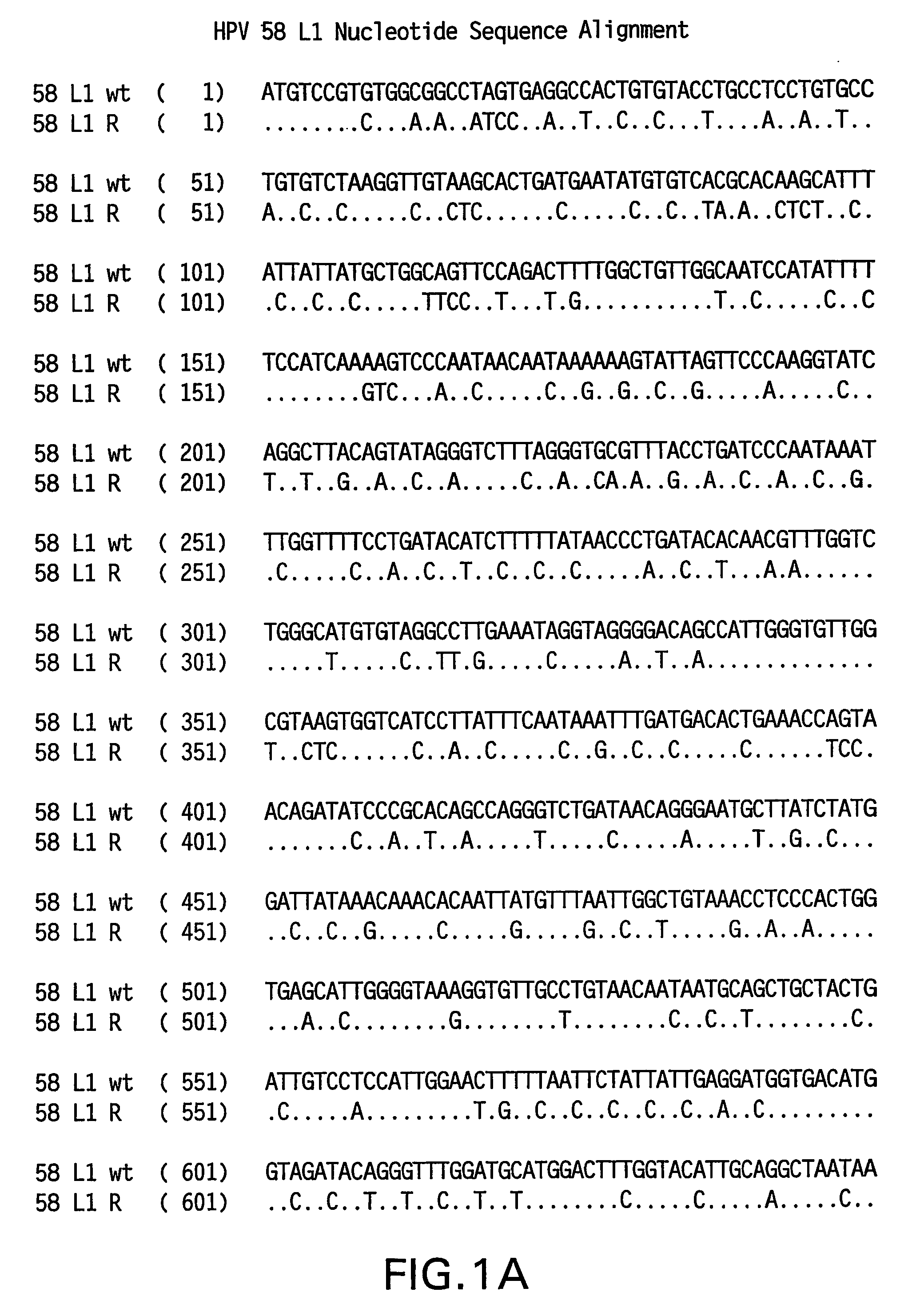
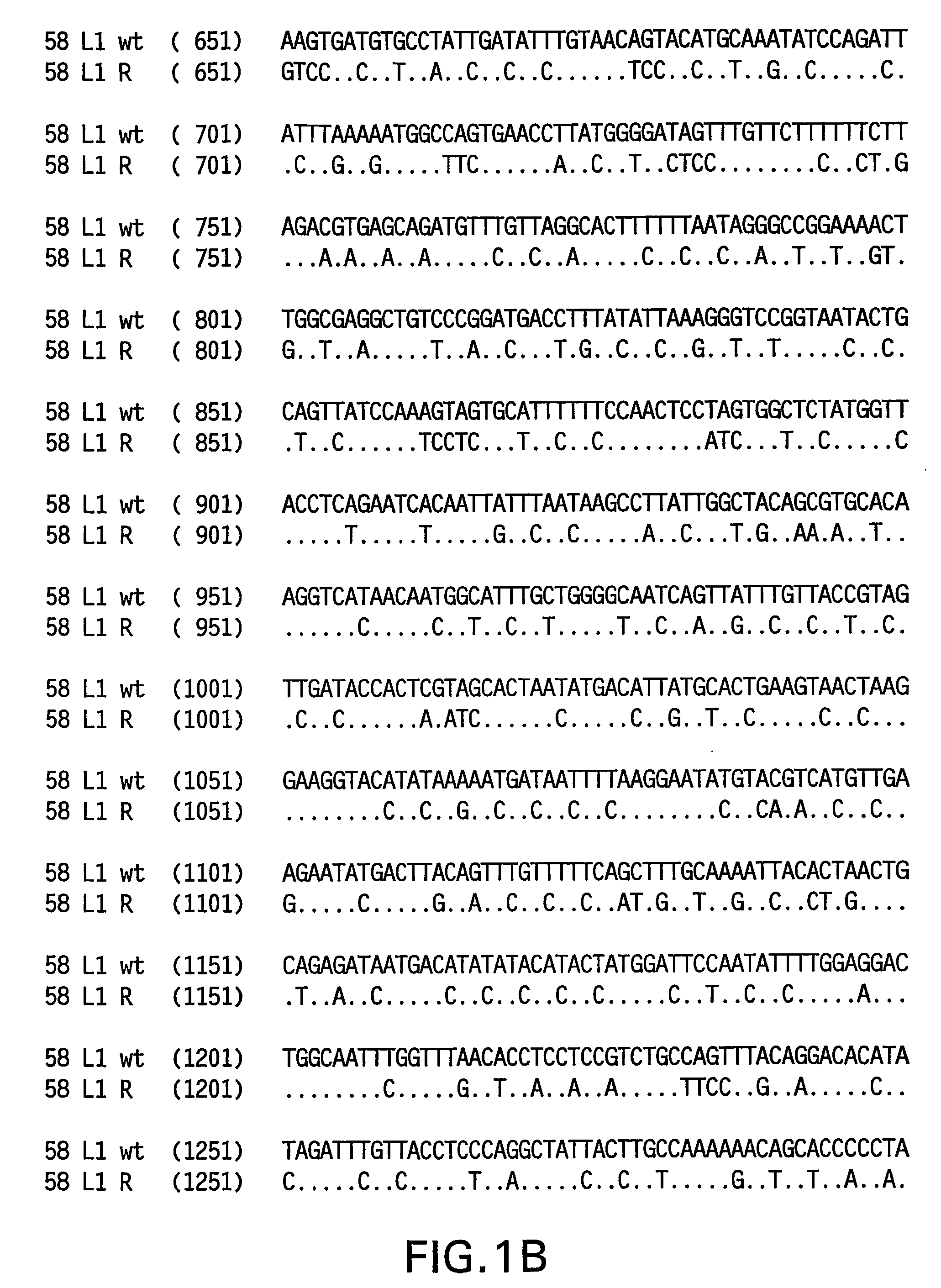
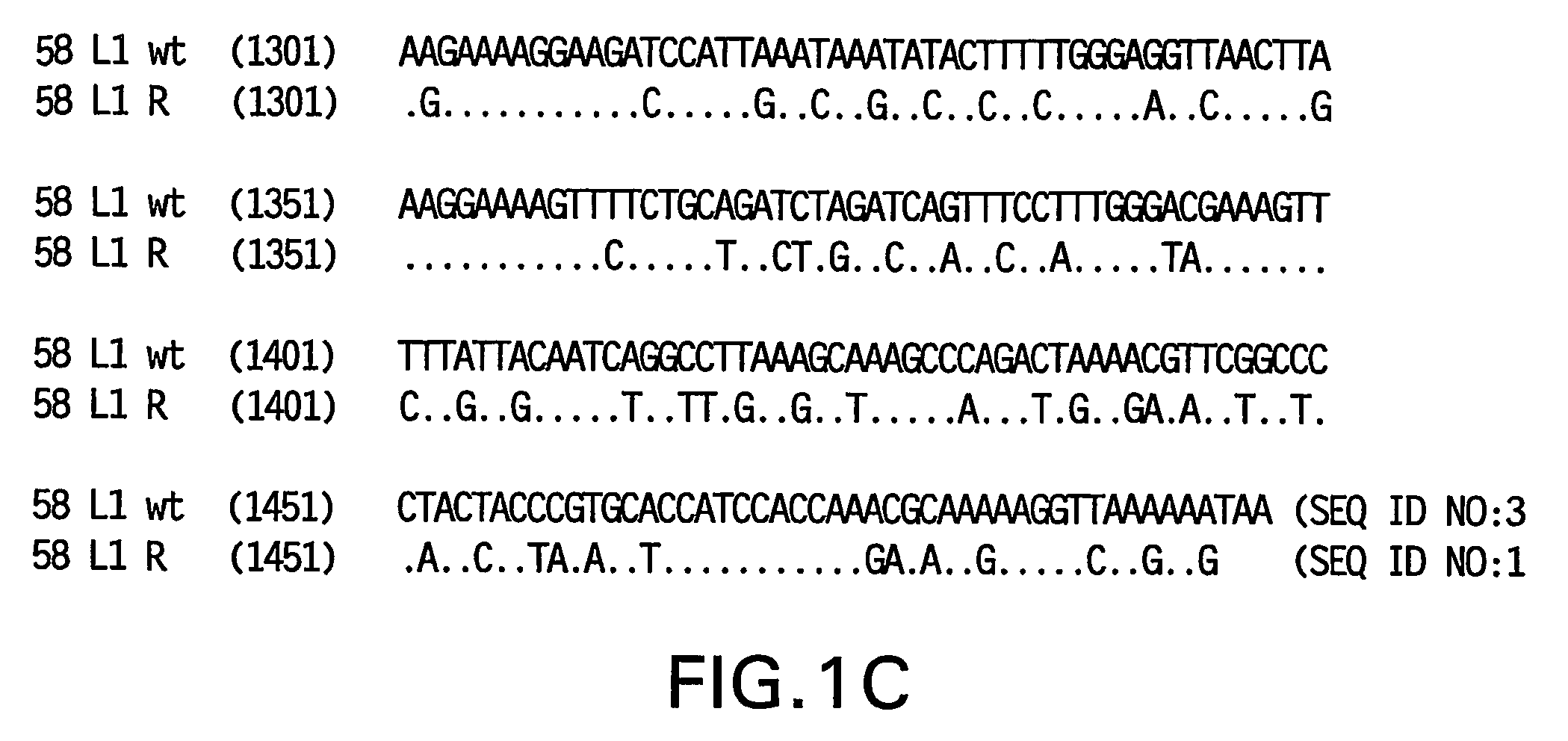
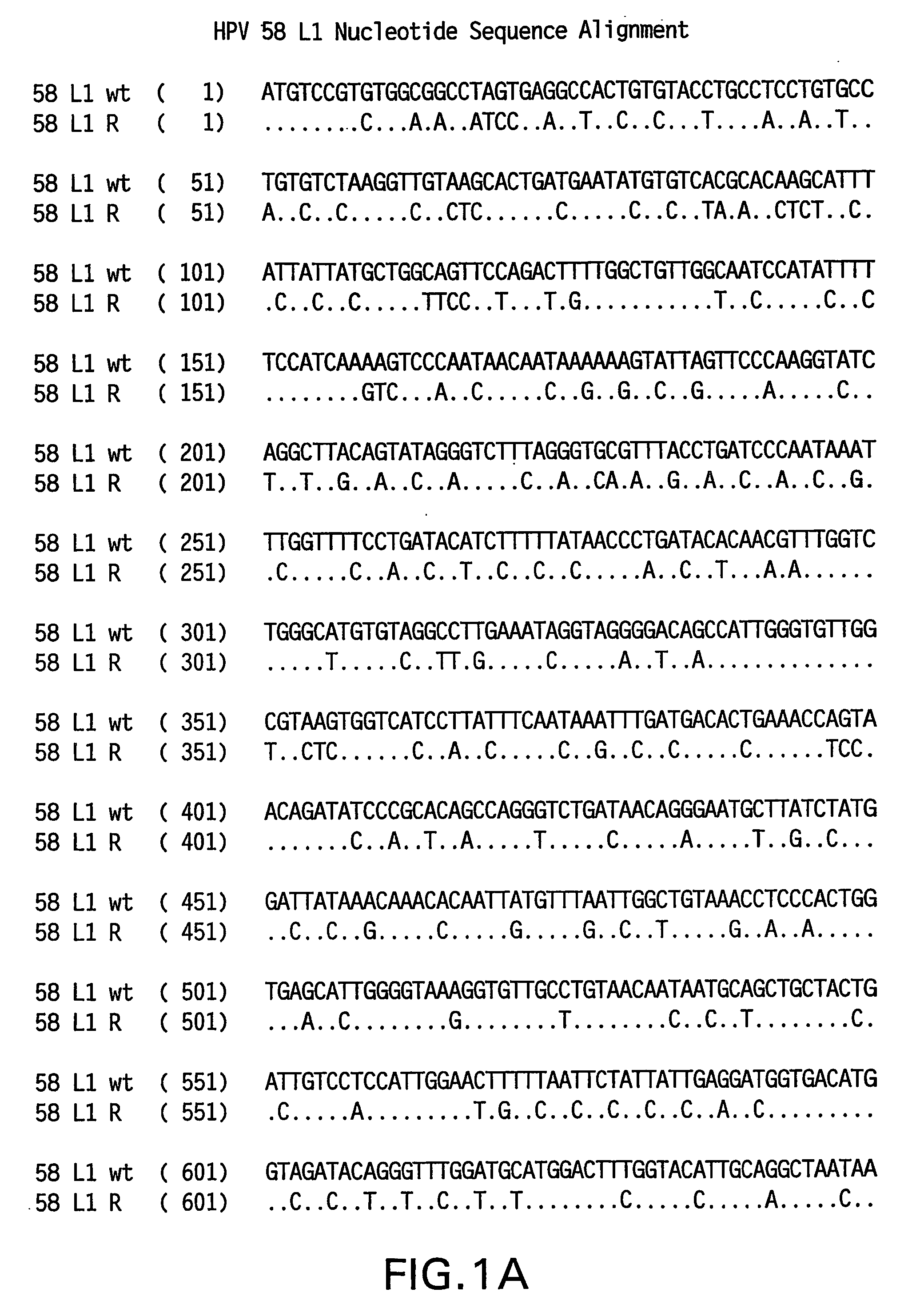
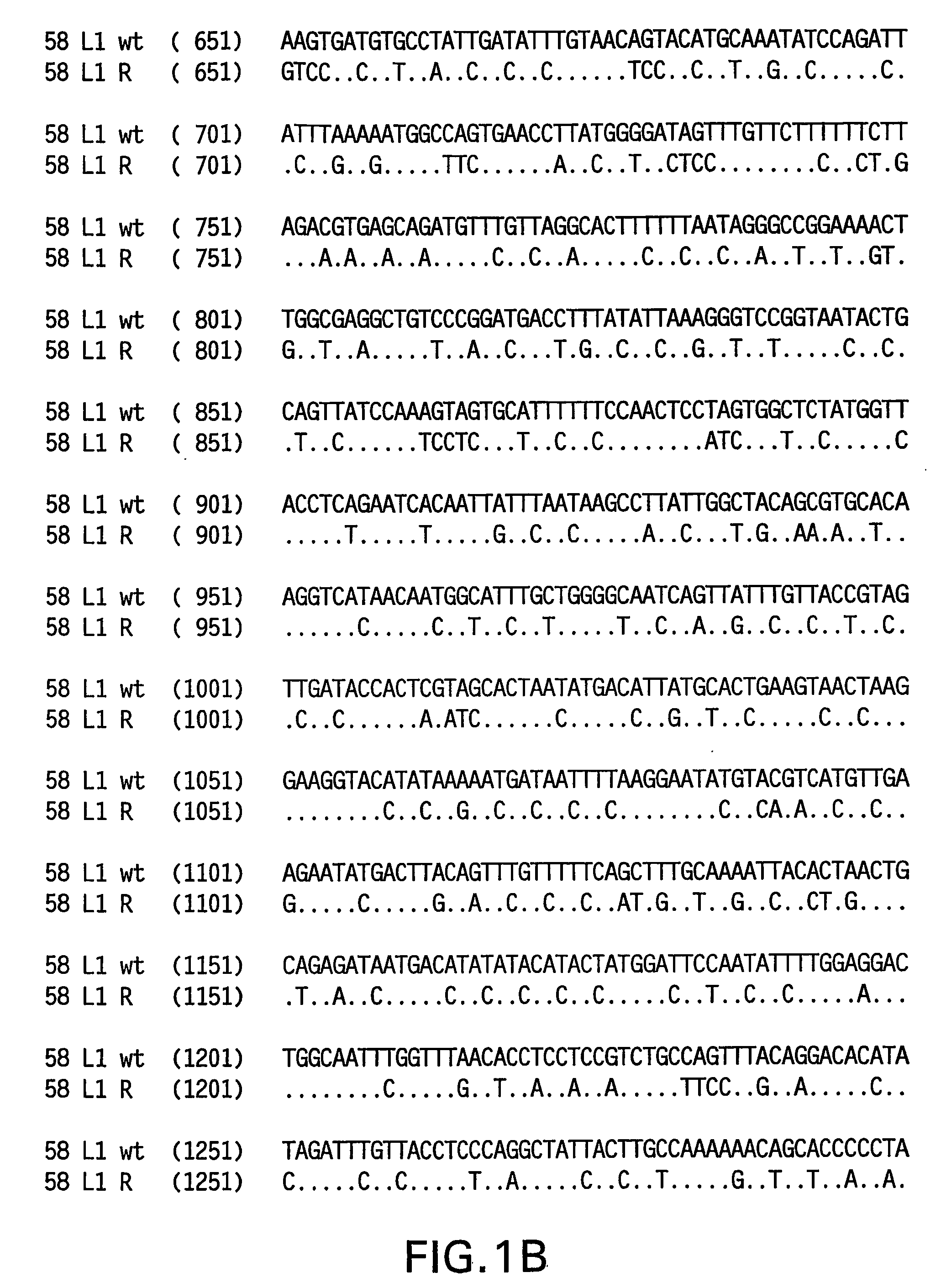
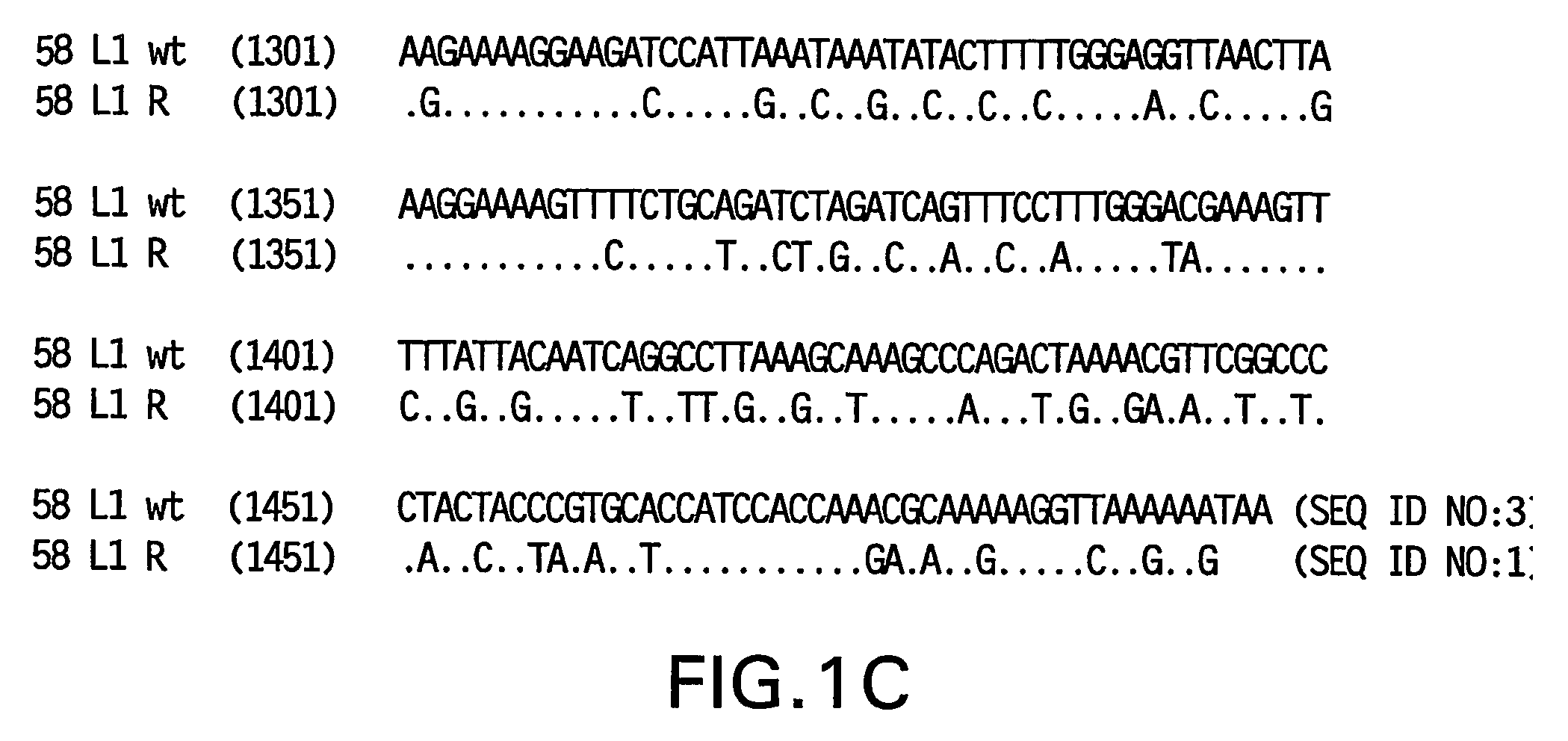
Optimized expression of HPV 58 L1 in yeast
Synthetic DNA molecules encoding the HPV58 L1 protein are provided. Specifically, the present invention provides polynucleotides encoding HPV58 L1 protein, wherein said polynucleotides are codon-optimized for high level expression in a yeast cell. The synthetic molecules may be used to produce HPV58 virus-like particles (VLPs), and to produce vaccines and pharmaceutical compositions comprising the HPV58 VLPs. The vaccines of the present invention provide effective imnunoprophylaxis against papillomavirus infection through neutralizing antibody and cell-mediated immunity and are also useful for treatment of existing HPV infections.
Owner:MERCK SHARP & DOHME LLC
Sequence-determined DNA fragments and corresponding polypeptides encoded thereby
InactiveUS20180223303A1Enhancing and reducing regionControls are responsiveMicrobiological testing/measurementImmunoglobulins against plantsBiological bodyGene product
The present invention provides DNA molecules that constitute fragments of the genome of a plant, and polypeptides encoded thereby. The DNA molecules are useful for specifying a gene product in cells, either as a promoter or as a protein coding sequence or as an UTR or as a 3′ termination sequence, and are also useful in controlling the behavior of a gene in the chromosome, in controlling the expression of a gene or as tools for genetic mapping, recognizing or isolating identical or related DNA fragments, or identification of a particular individual organism, or for clustering of a group of organisms with a common trait. One of ordinary skill in the art, having this data, can obtain cloned DNA fragments, synthetic DNA fragments or polypeptides constituting desired sequences by recombinant methodology known in the art or described herein.
Owner:CERES INC
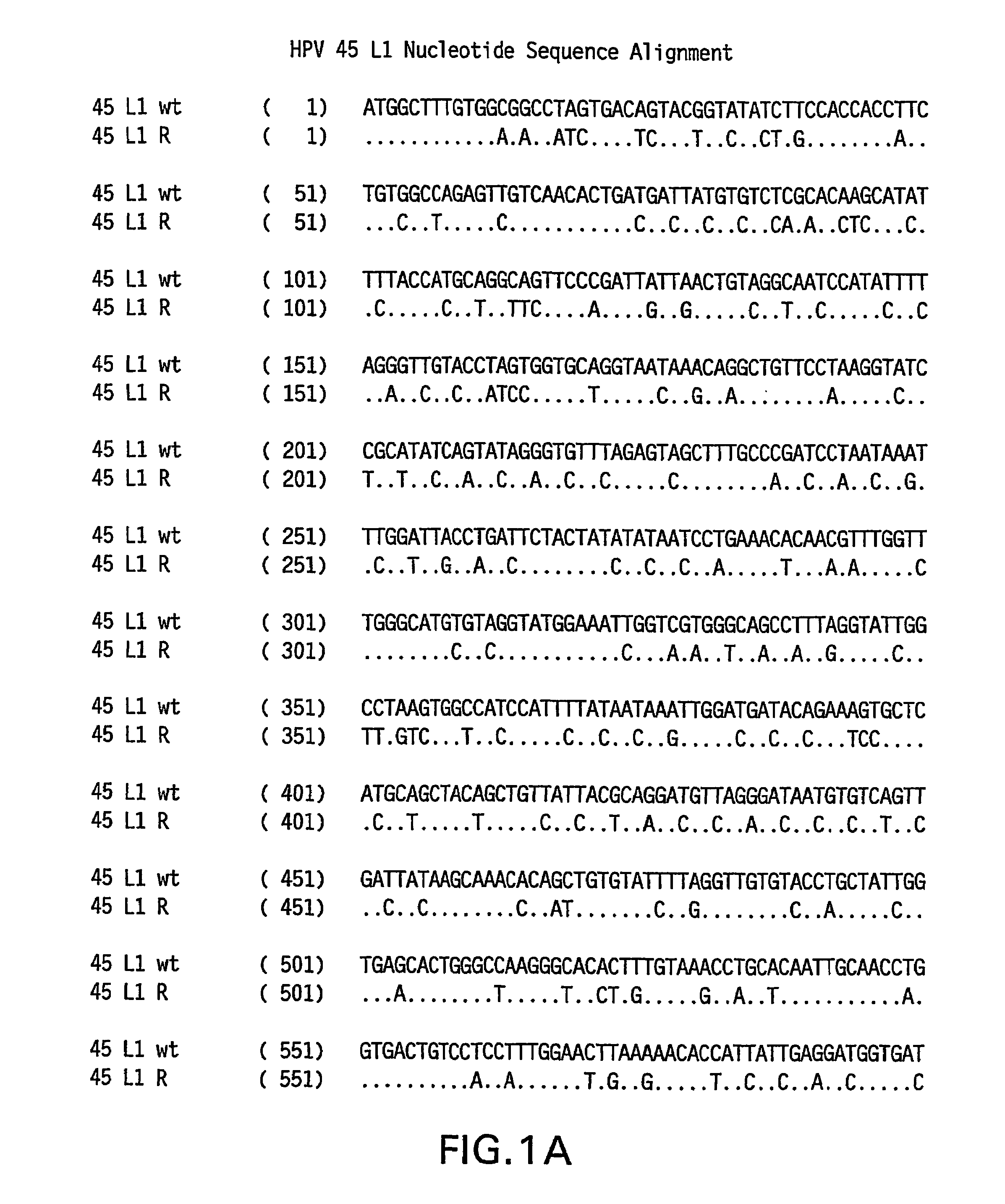
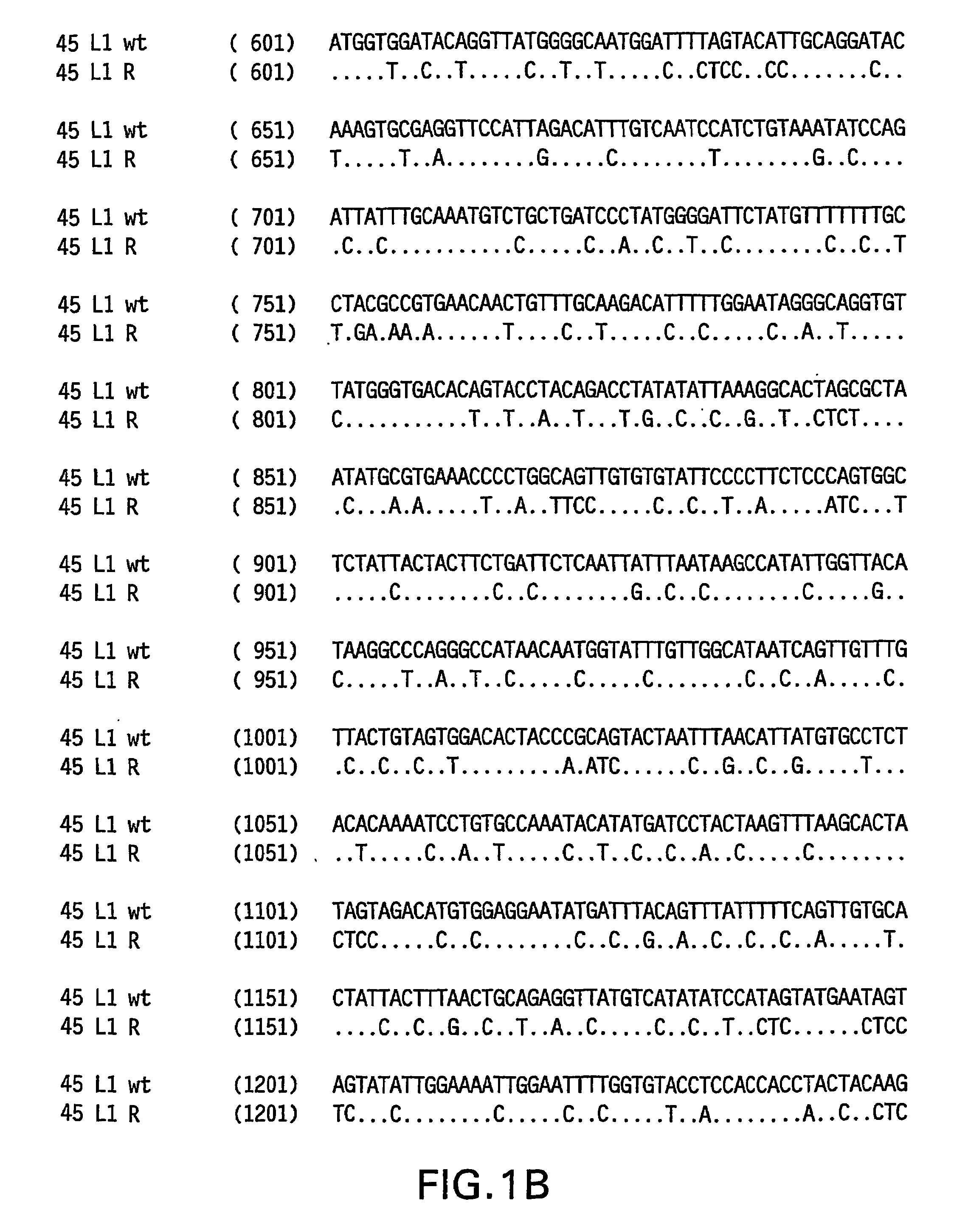
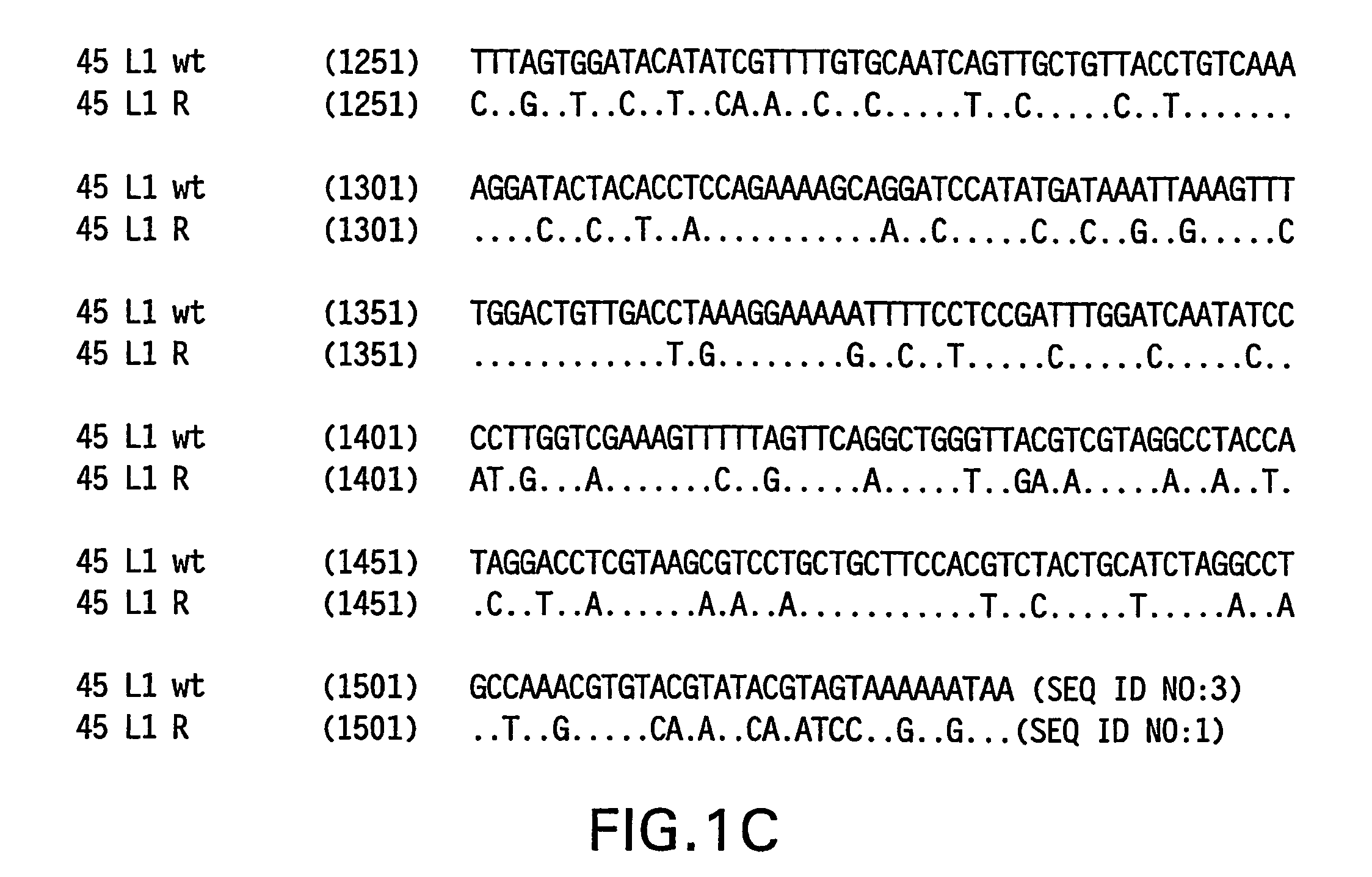
Optimized expression of HPV 45 L1 in yeast
Owner:MERCK SHARP & DOHME LLC
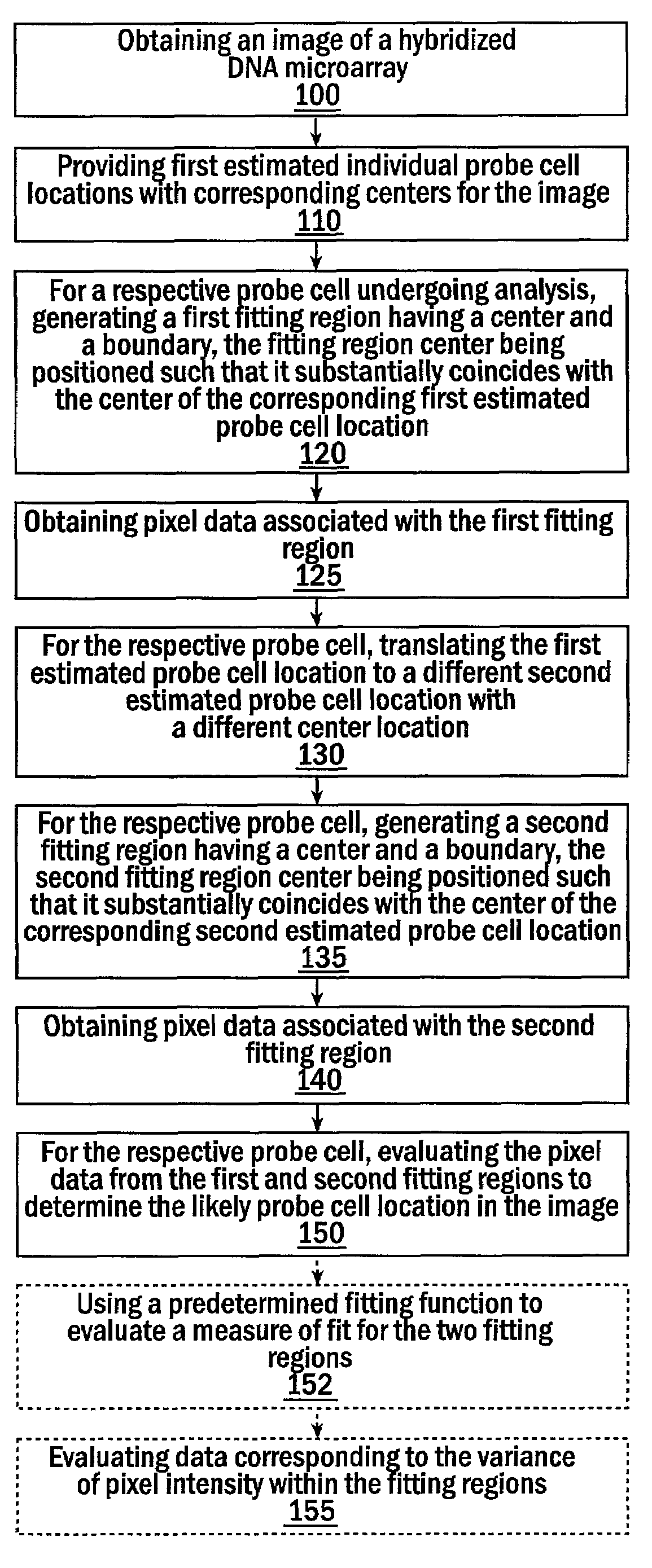
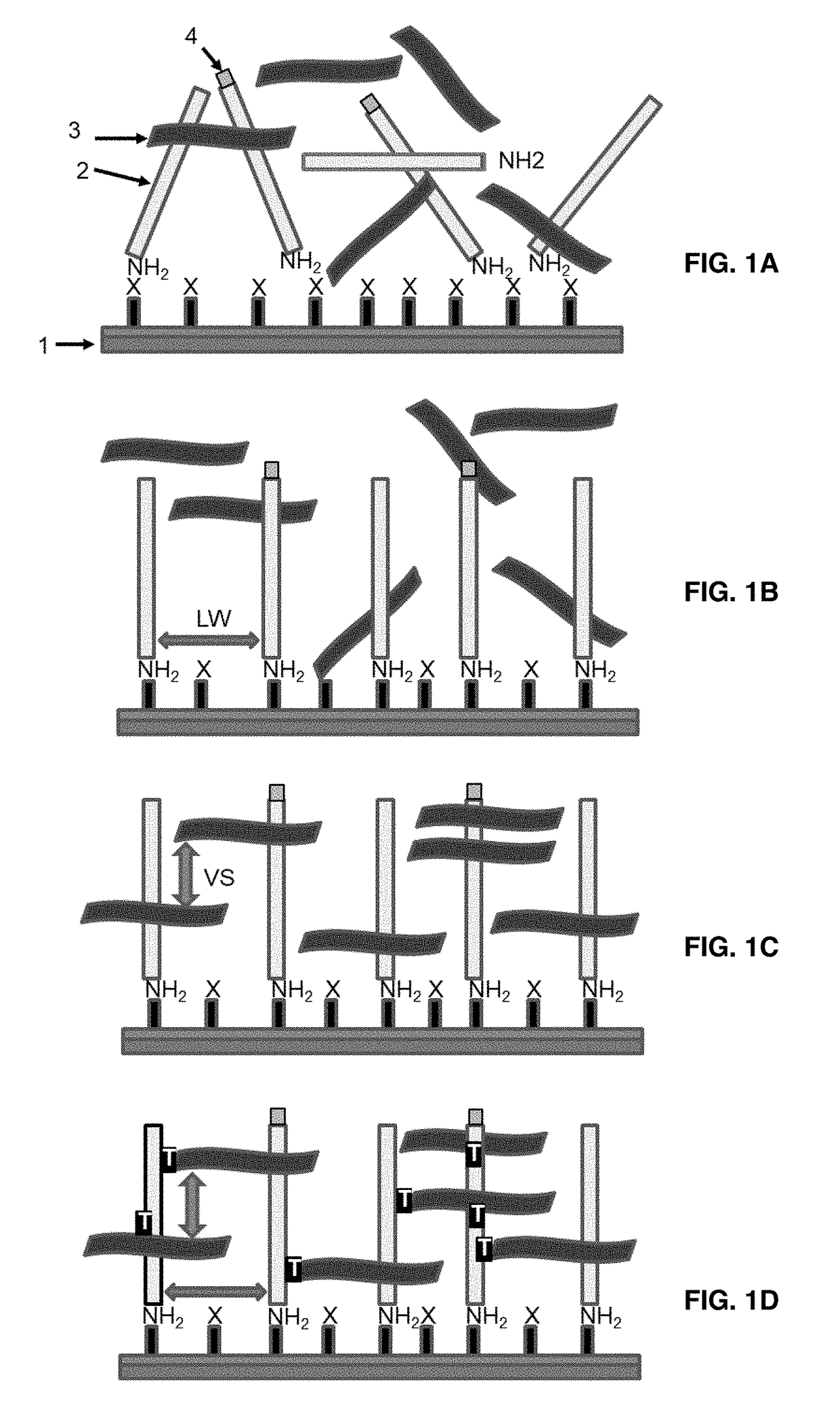
Methods for estimating probe cell locations in high-density synthetic DNA microarrays
InactiveUS6993173B2Easy to optimizeAccurate attributionImage enhancementImage analysisHigh-Density MicroarrayHigh density
Methods, systems, and computer program products for estimating the location of a probe cell in an image of a high-density microarray DNA chip interrogate a plurality of different closely spaced estimated locations to identify the most likely estimated location of the probe cell in the image.
Owner:DUKE UNIV
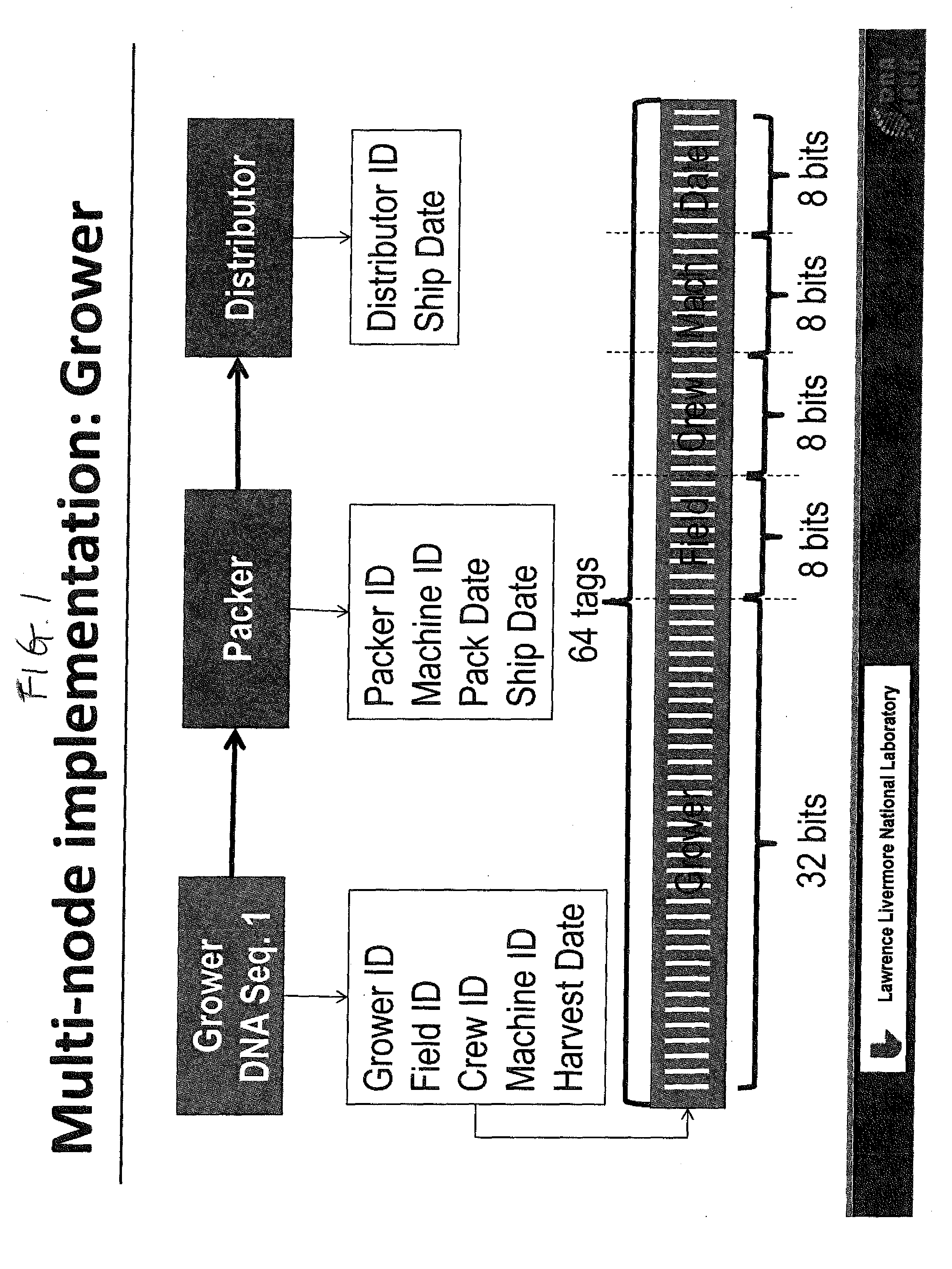
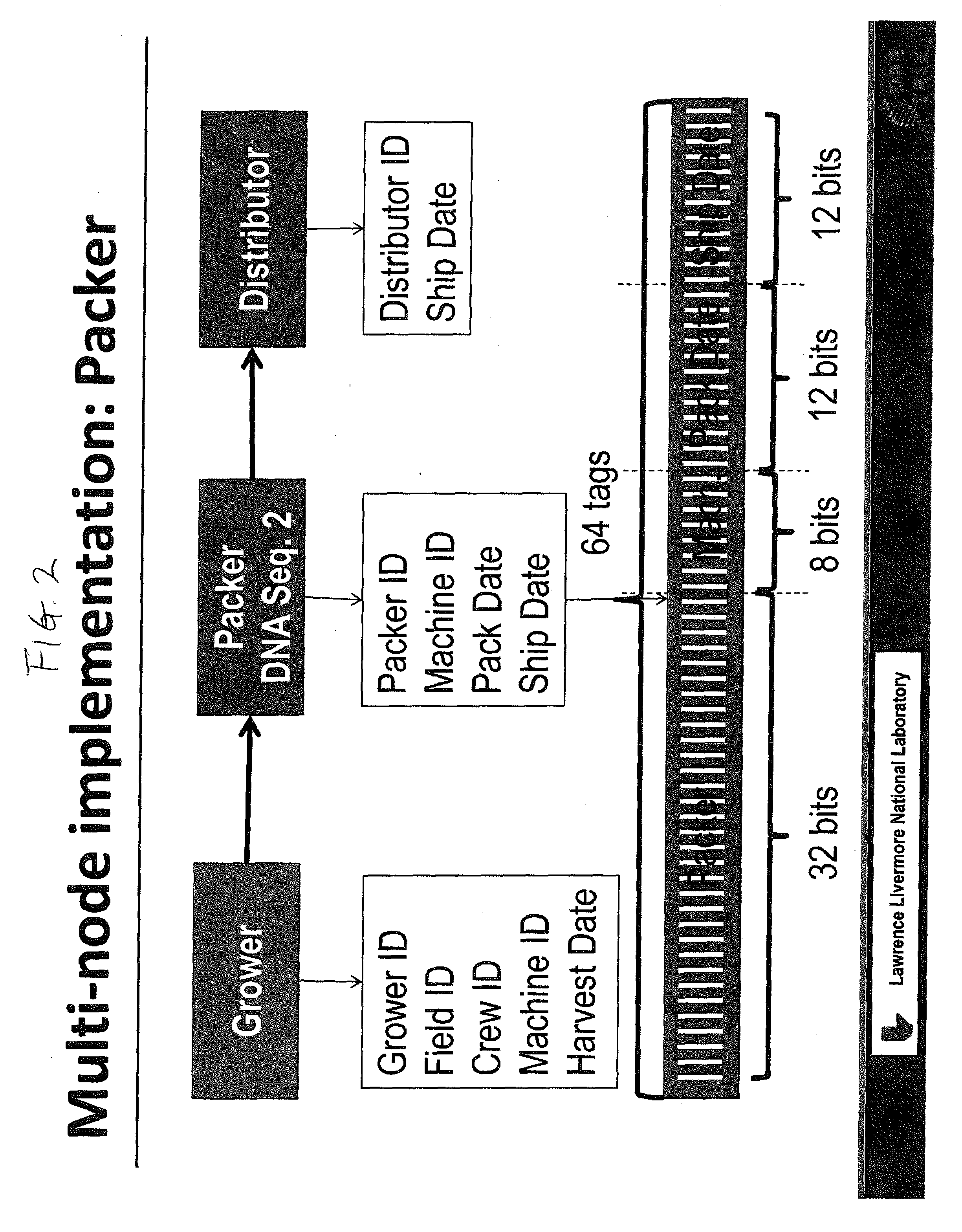
DNA Based Bar Code for Improved Food Traceability
Food distributed to consumers through a distribution chain may be traced by tagging the food with DNA tags that identify the origin of the food, such as the grower, packer and other points of distribution, and their attributes. This makes it much quicker and easier to trace the food in case of food contamination or adulteration. Preferably these attributes indicate the field, location, crew and machine used to grow and process the food, and the dates of the various steps of food harvesting, processing and distribution. Natural or synthetic DNA pieces may be used to tag items, including food items. Multidigit binary or other types of bar codes may be represented by multiple types of DNA. Each digit of the bar code may be represented by one, two or more unique DNA pieces.
Owner:LAWRENCE LIVERMORE NAT SECURITY LLC +1
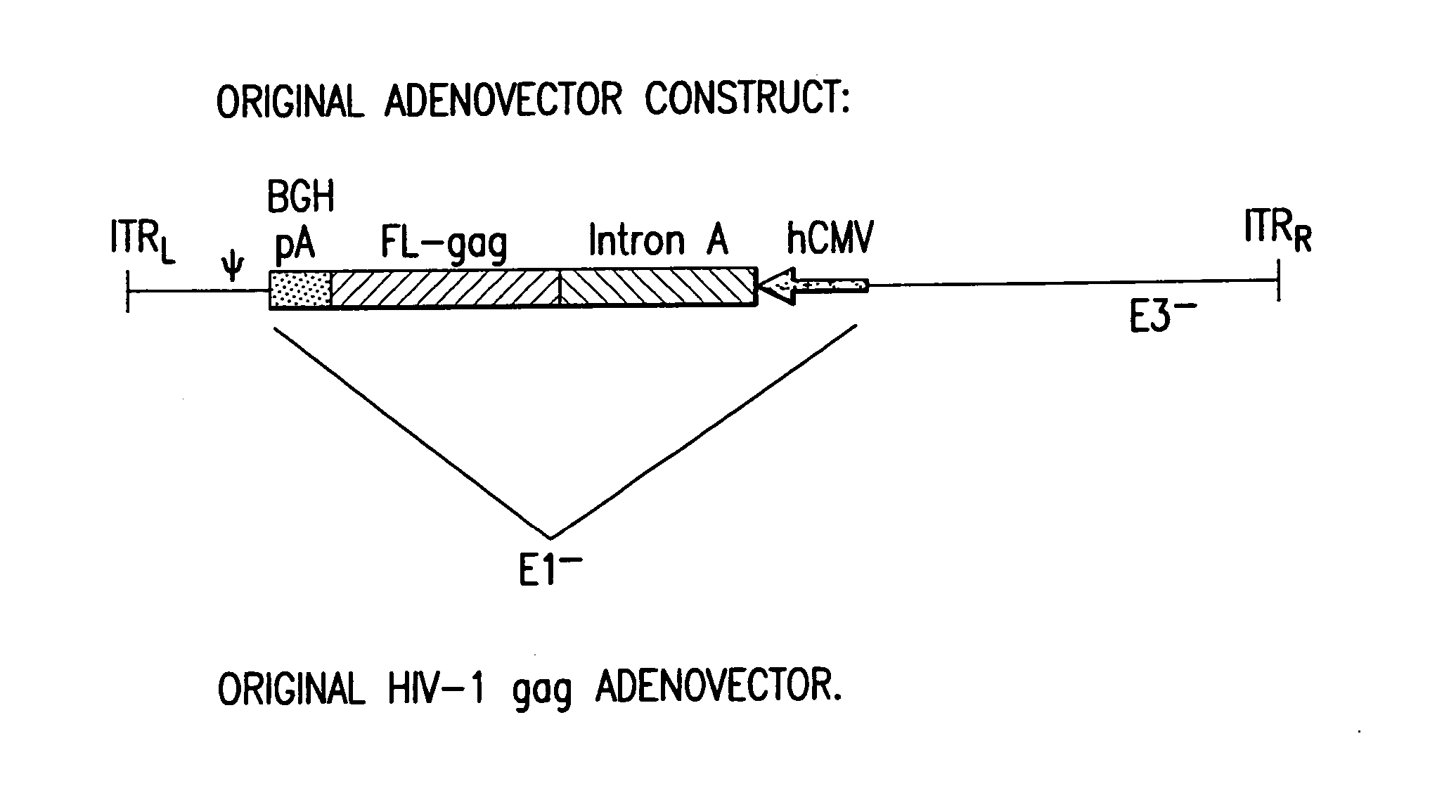
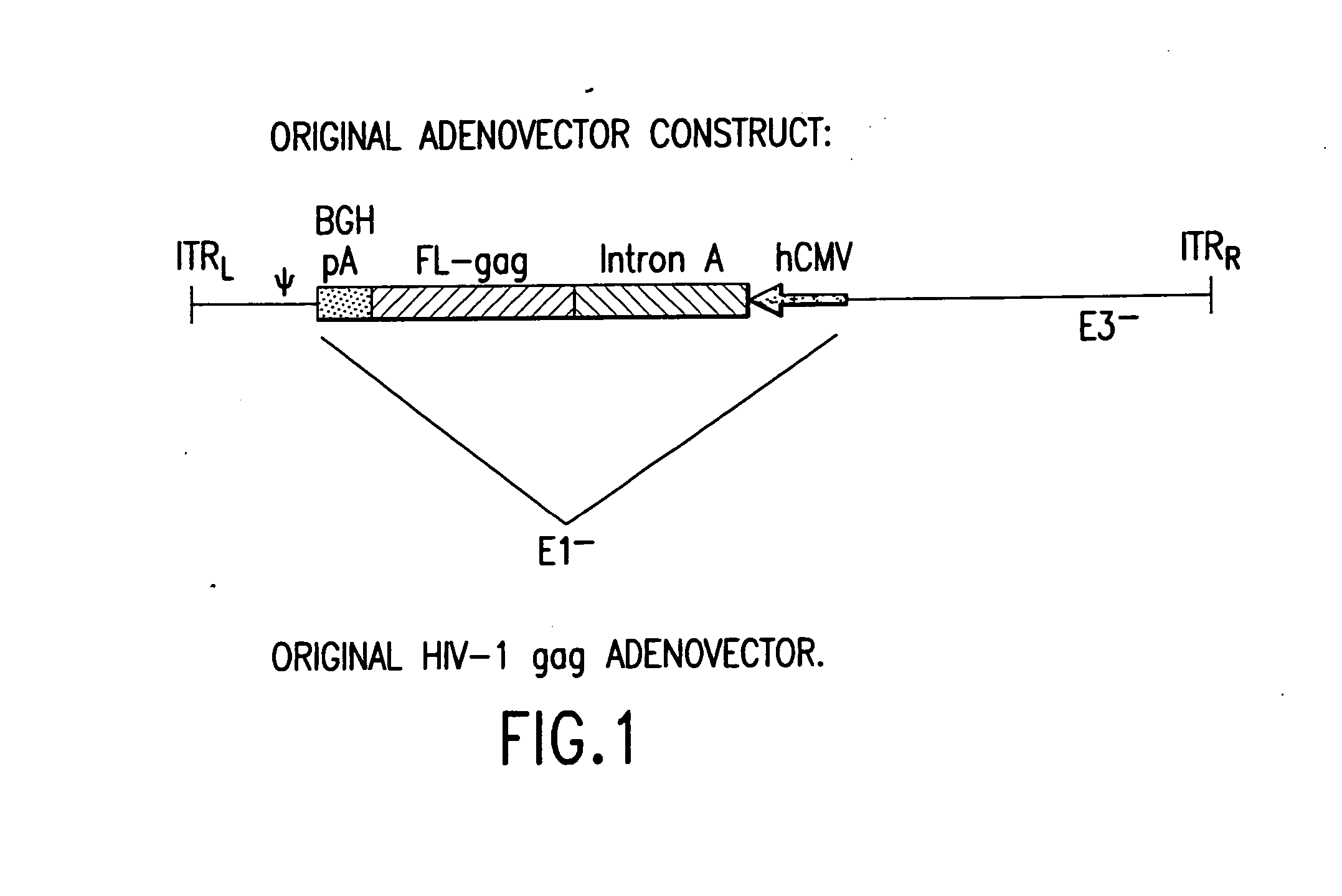
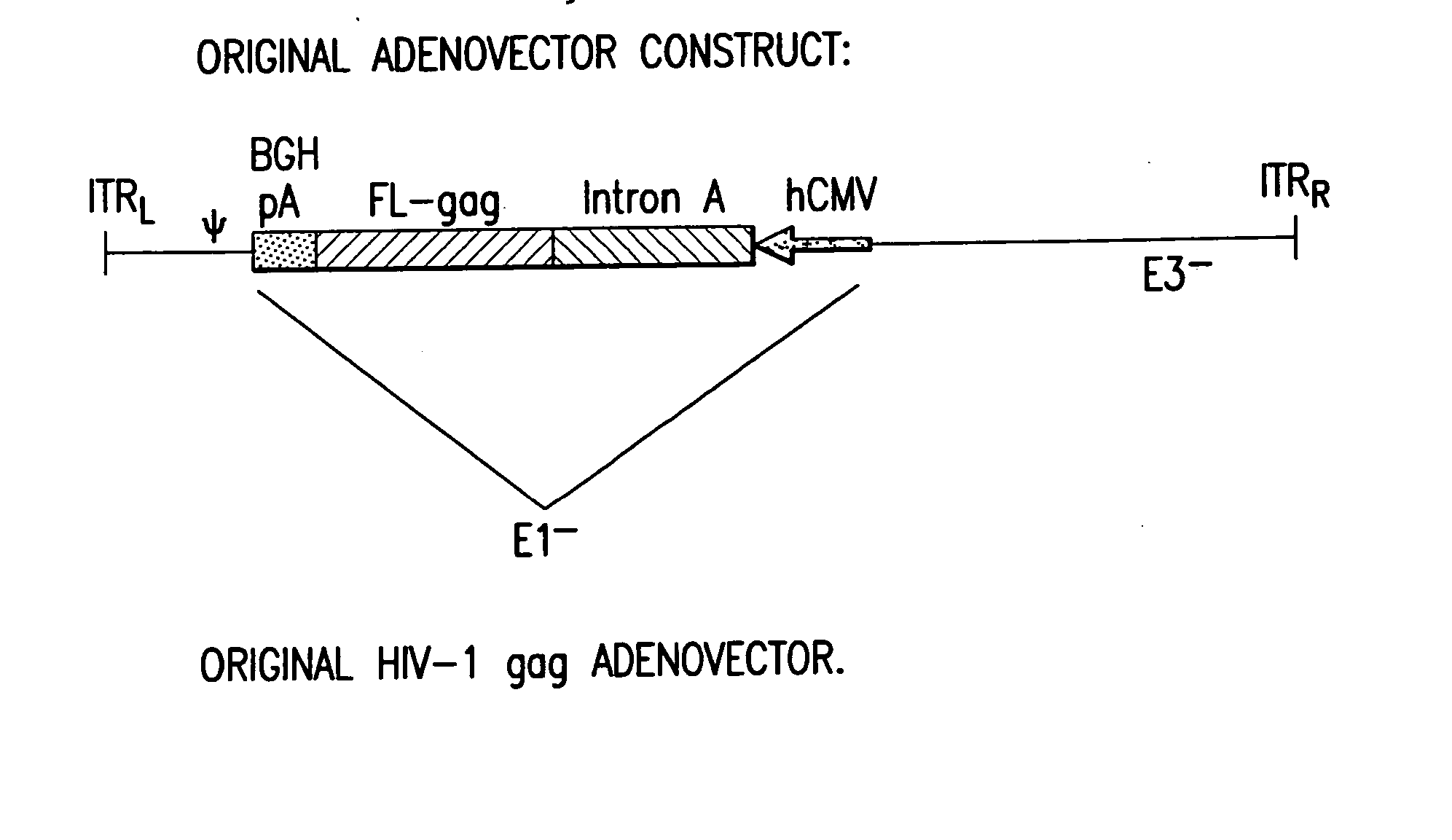
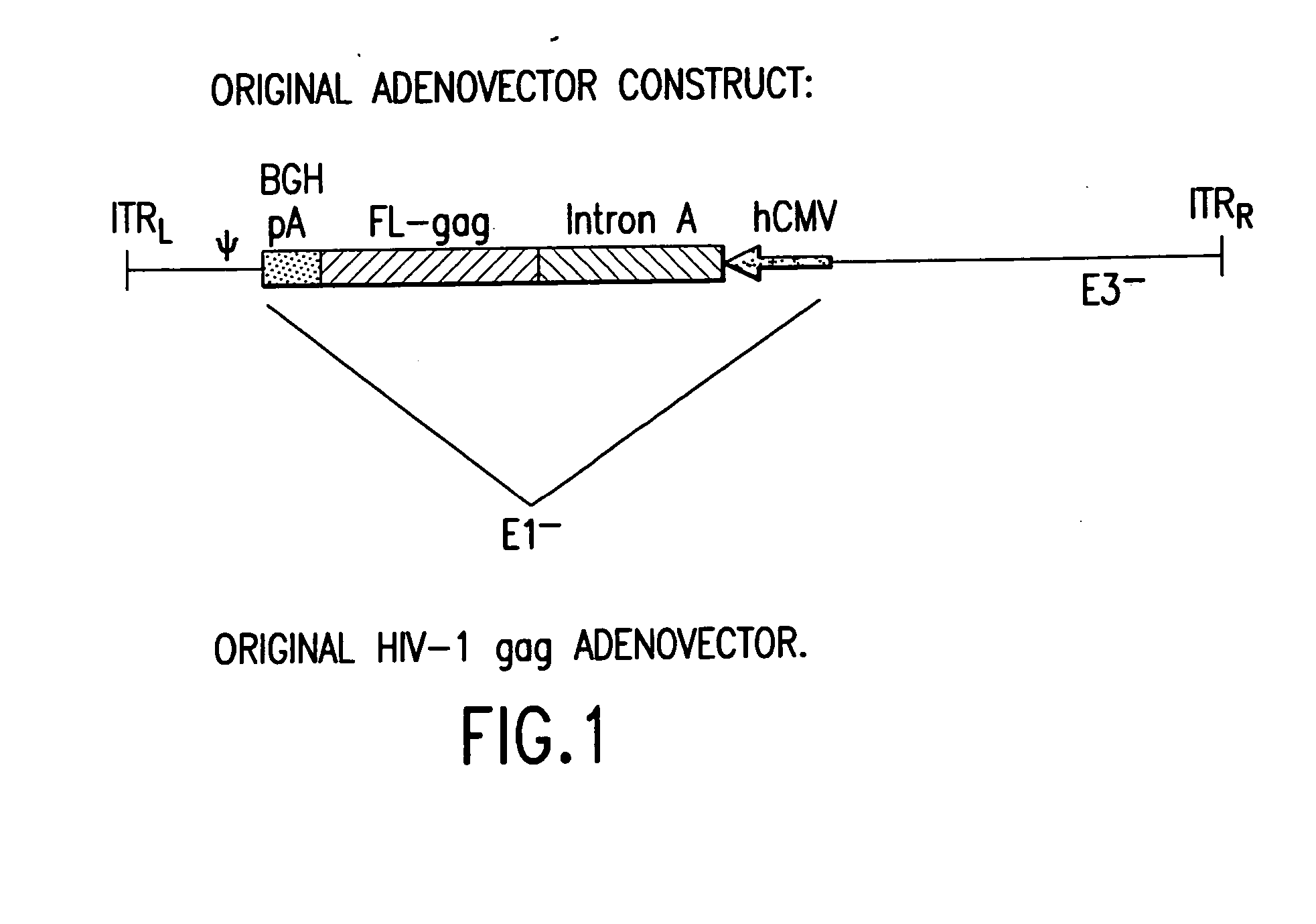
Enhanced first generation adenovirus vaccines expressing codon optimized HIV1-Gag, Pol, Nef and modifications
InactiveUS20070054395A1Genetically stableImprove featuresVectorsViral antigen ingredientsMammalReverse transcriptase
First generation adenoviral vectors and associated recombinant adenovirus-based HIV vaccines which show enhanced stability and growth properties and greater cellular-mediated immunity are described within this specification. These adenoviral vectors are utilized to generate and produce through cell culture various adenoviral-based HIV-1 vaccines which contain HIV-1 gag, HIV-1 pol and / or HIV-1 nef polynucleotide pharmaceutical products, and biologically relevant modifications thereof. These adenovirus vaccines, when directly introduced into living vertebrate tissue, preferably a mammalian host such as a human or a non-human mammal of commercial or domestic veterinary importance, express the HIV1-Gag, Pol and / or Nef protein or biologically modification thereof, inducing a cellular immune response which specifically recognizes HIV-1. The exemplified polynucleotides of the present invention are synthetic DNA molecules encoding HIV-1 Gag, encoding codon optimized HIV-1 Pol, derivatives of optimized HIV-1 Pol (including constructs wherein protease, reverse transcriptase, RNAse H and integrase activity of HIV-1 Pol is inactivated), HIV-1 Nef and derivatives of optimized HIV-1 Nef, including nef mutants which effect wild type characteristics of Nef, such as myristylation and down regulation of host CD4. The adenoviral vaccines of the present invention, when administered alone or in a combined modality regime, will offer a prophylactic advantage to previously uninfected individuals and / or provide a therapeutic effect by reducing viral load levels within an infected individual, thus prolonging the asymptomatic phase of HIV-1 infection.
Owner:EMINI EMILIO A +7
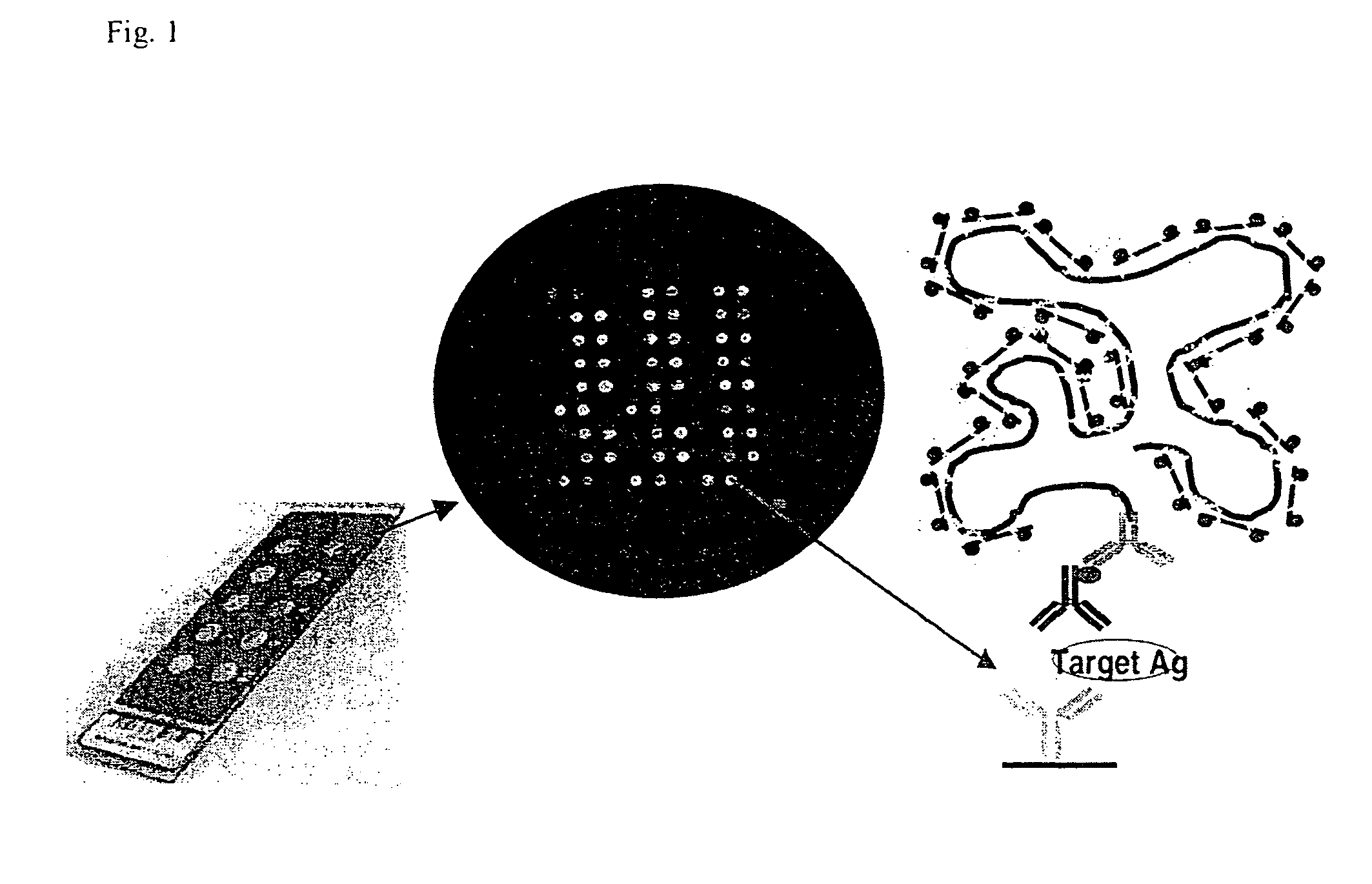
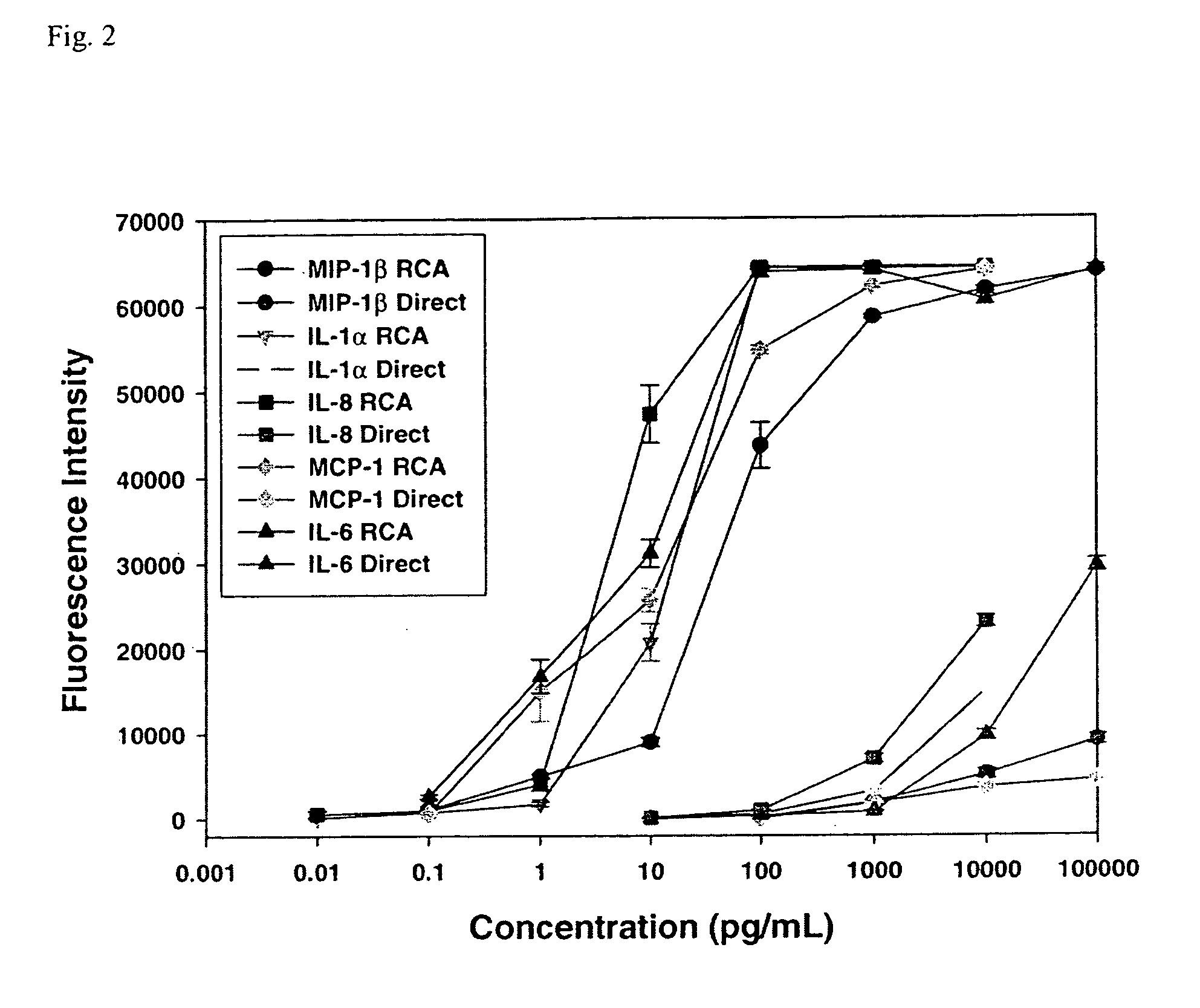
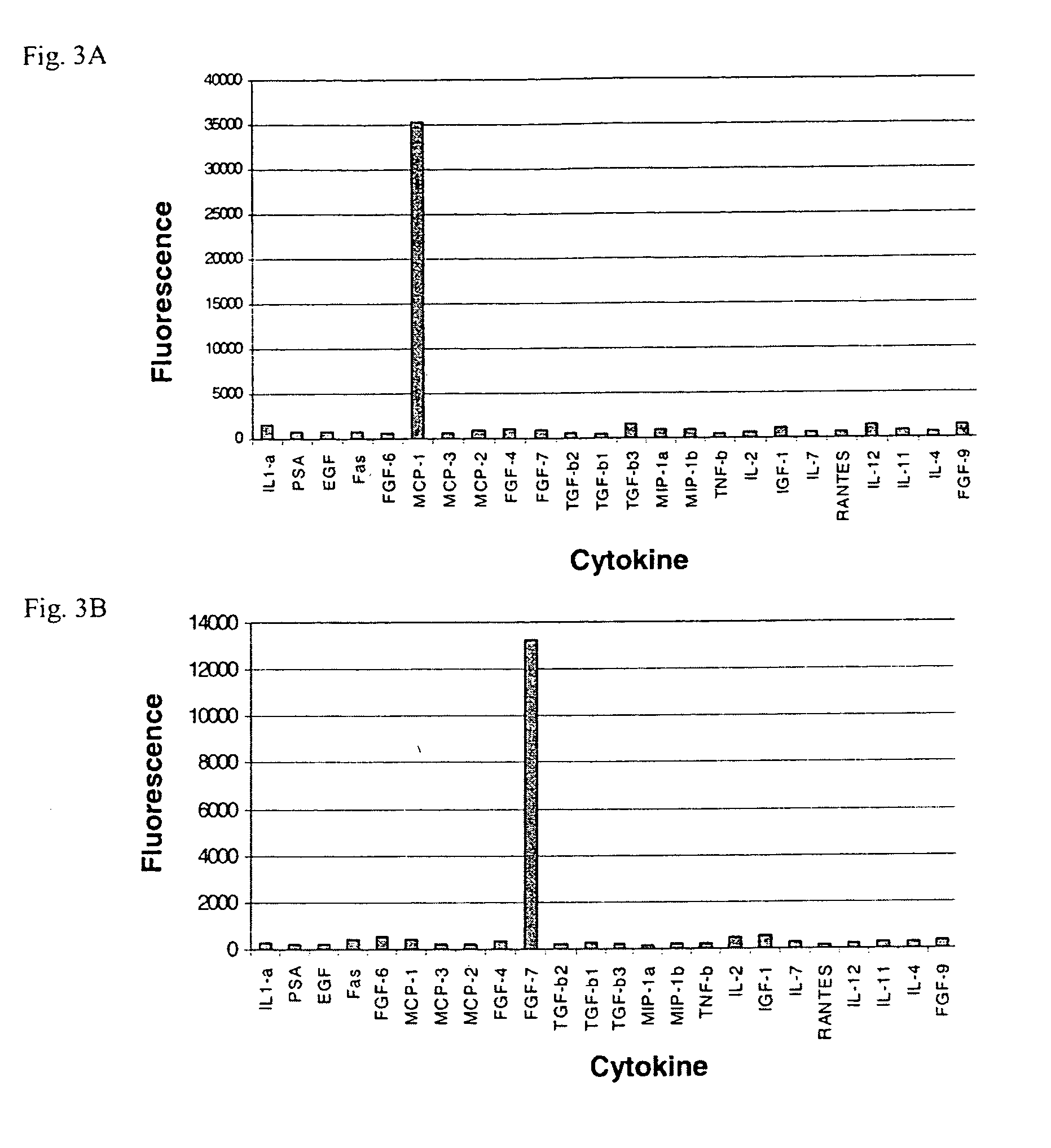
Use of cytokines secreted by dendritic cells
A prerequisite of proteomics is the ability to quantify many selected proteins simultaneously. Fluorescent sandwich immunoassays on microarrays hold appeal for such studies, since equipment and antibodies are readily available, and assays are simple, scalable and reproducible. To attain adequate sensitivity and specificity, however, a general method of immunoassay amplification is required. Coupling of isothermal rolling circle amplification (RCA) to universal antibodies can be used for this purpose: RCA on a synthetic DNA circle is initiated by a complementary oligonucleotide attached to an anti-biotin antibody; single-stranded RCA product remains attached to the antibody, and is detected by hybridization of complementary, fluorescent oligonucleotides. 51 cytokines were measured simultaneously on microarrays with signal amplification by RCA with high specificity, femtomolar sensitivity and 4 log quantitative range. This cytokine microarray was used to measure secretion from human Dendritic cells (DCs) induced by lipopolysaccharide (LPS) or tumor necrosis factor-alpha (TNF-(alpha). Rapid secretion of inflammatory cytokines such as MIP-1beta, IL-8, and IP-10 was induced by LPS. Eotaxin-2 and I-309 were found to be induced by LPS, and MDC, TARC, sIL-6R, and sTNF-RI were found to be induced by TNF-alpha. Since microarrays can accommodate ~1000 sandwich immunoassays of this type, a relatively small number of RCA microarrays appears to offer a tractable approach for proteomic surveys.
Owner:MOLECULAR STAGING
Optimized expression of hpv 58 l1 in yeast
ActiveUS20070036824A1High level expressionImprove immunityFungiVirus peptidesYeastHigh level expression
Synthetic DNA molecules encoding the HPV58 L1 protein are provided. Specifically, the present invention provides polynucleotides encoding HPV58 L1 protein, wherein said polynucleotides are codon-optimized for high level expression in a yeast cell. The synthetic molecules may be used to produce HPV58 virus-like particles (VLPs), and to produce vaccines and pharmaceutical compositions comprising the HPV58 VLPs. The vaccines of the present invention provide effective imnunoprophylaxis against papillomavirus infection through neutralizing antibody and cell-mediated immunity and are also useful for treatment of existing HPV infections.
Owner:MERCK SHARP & DOHME LLC
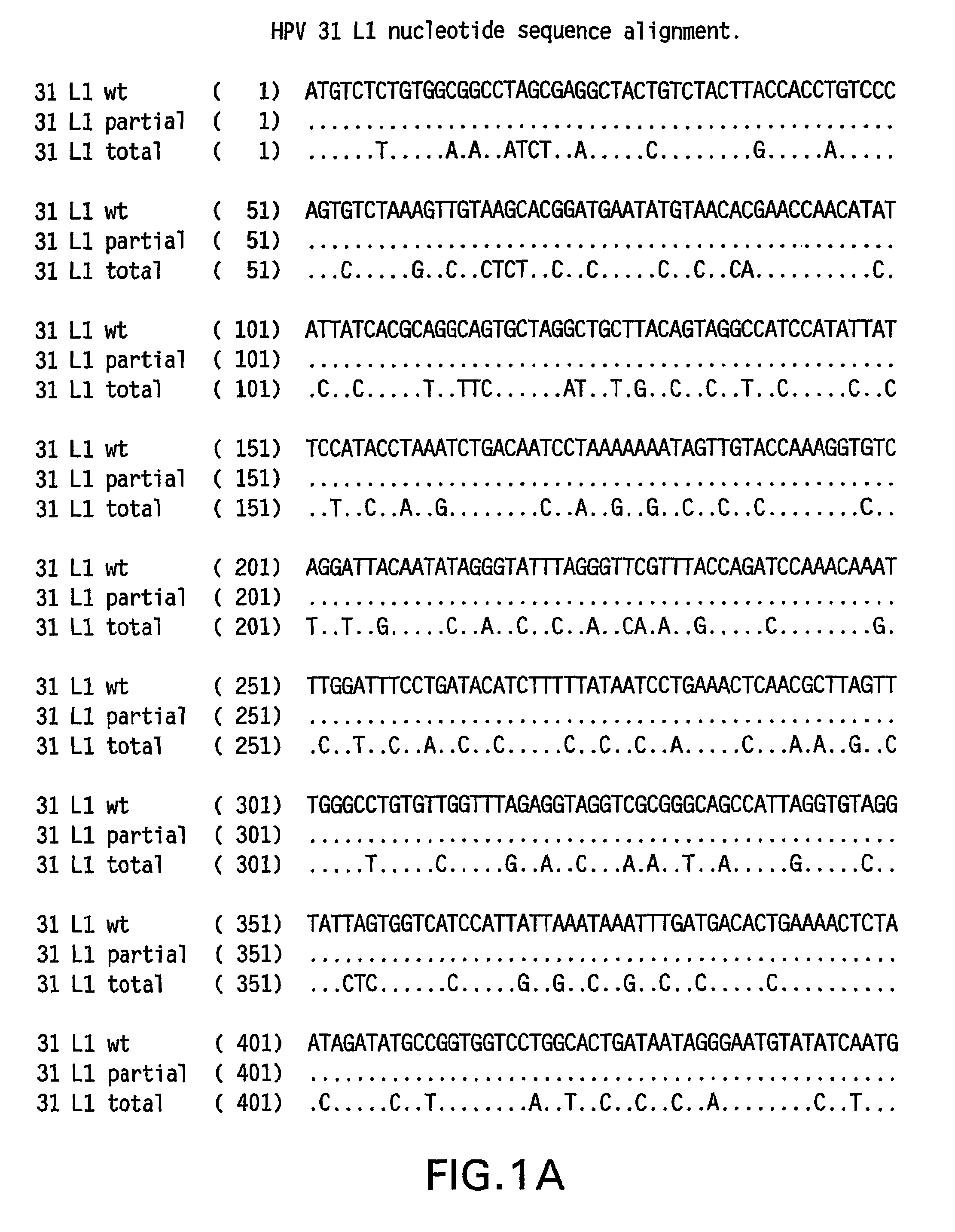
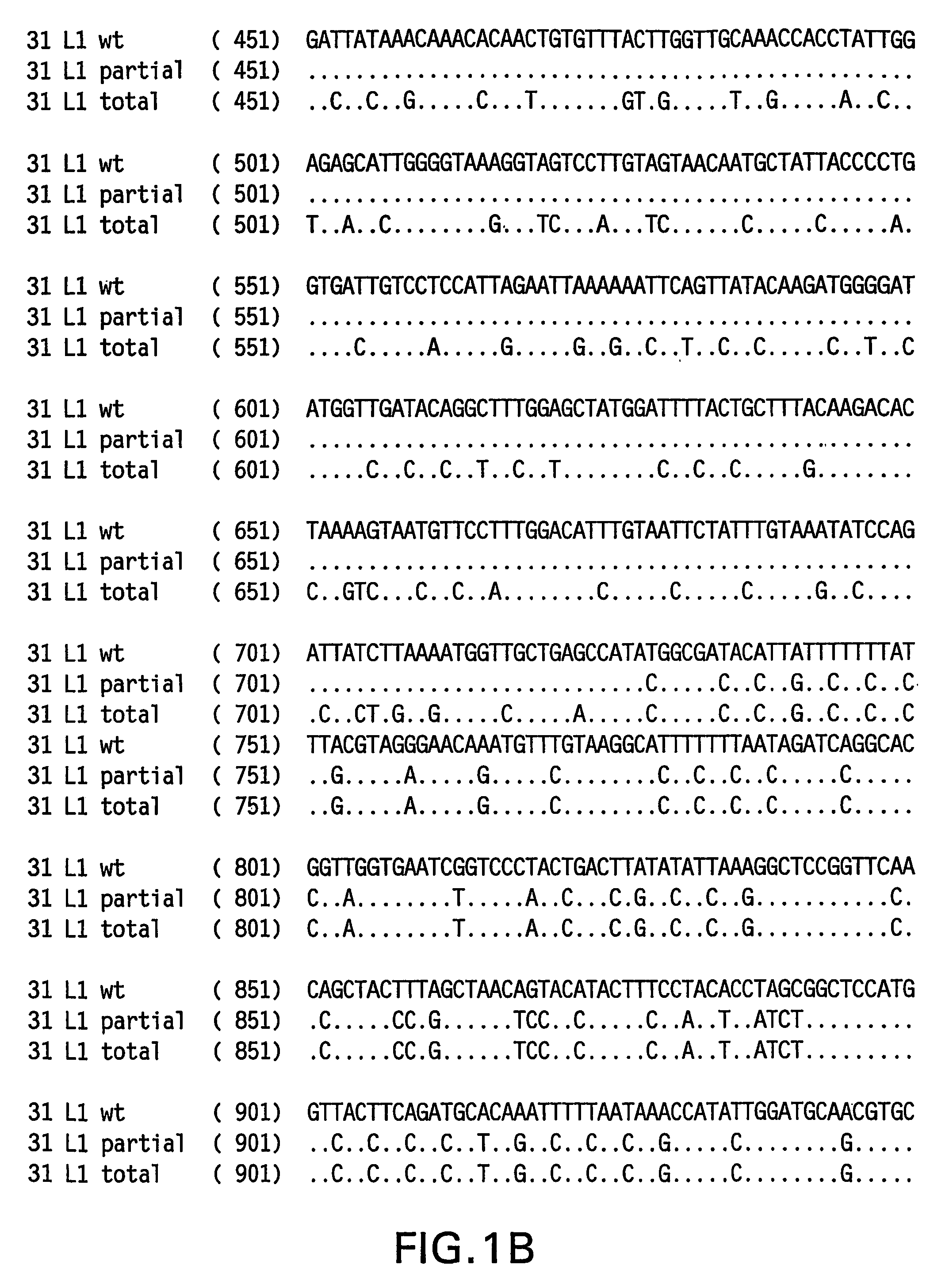
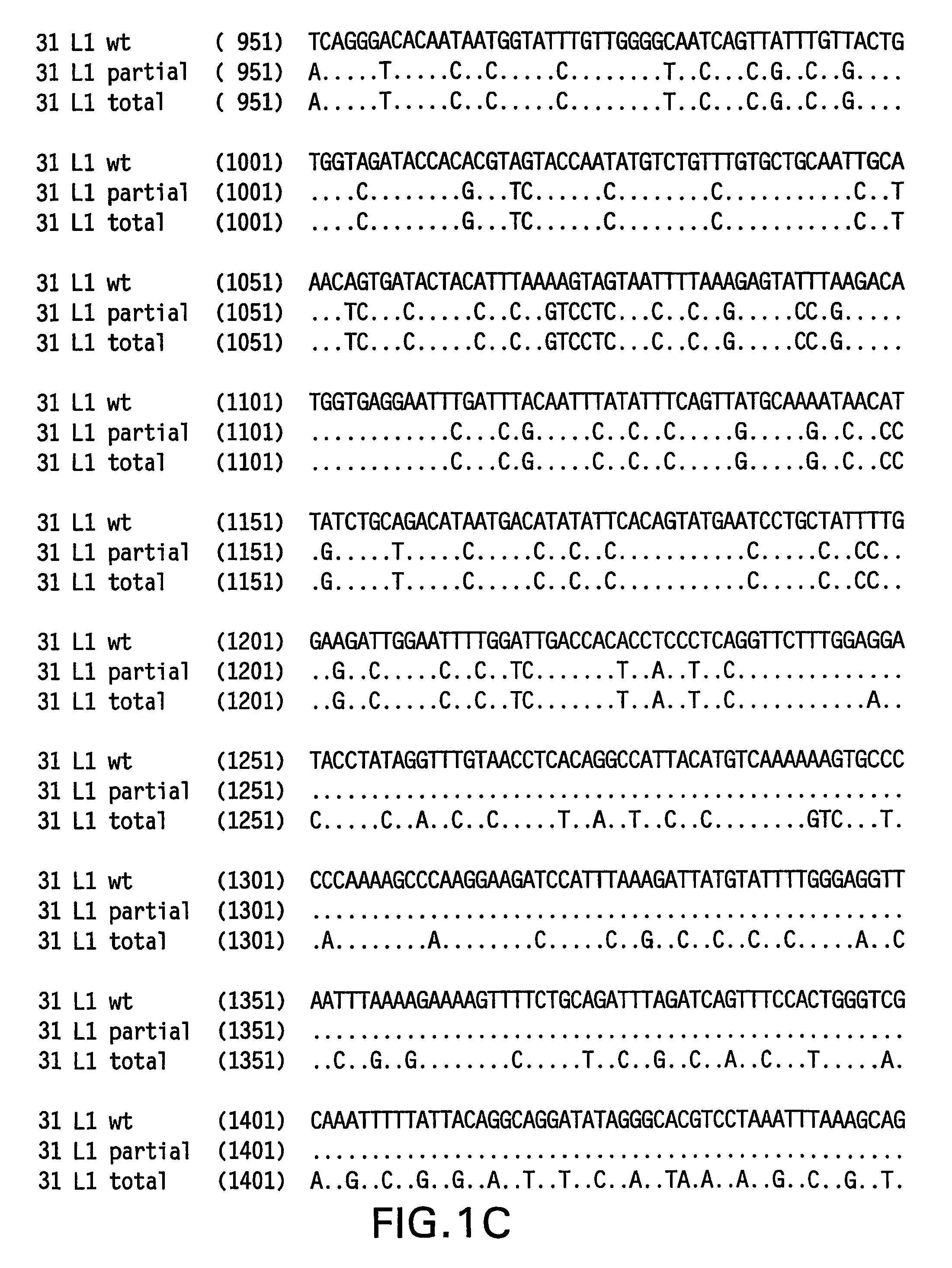
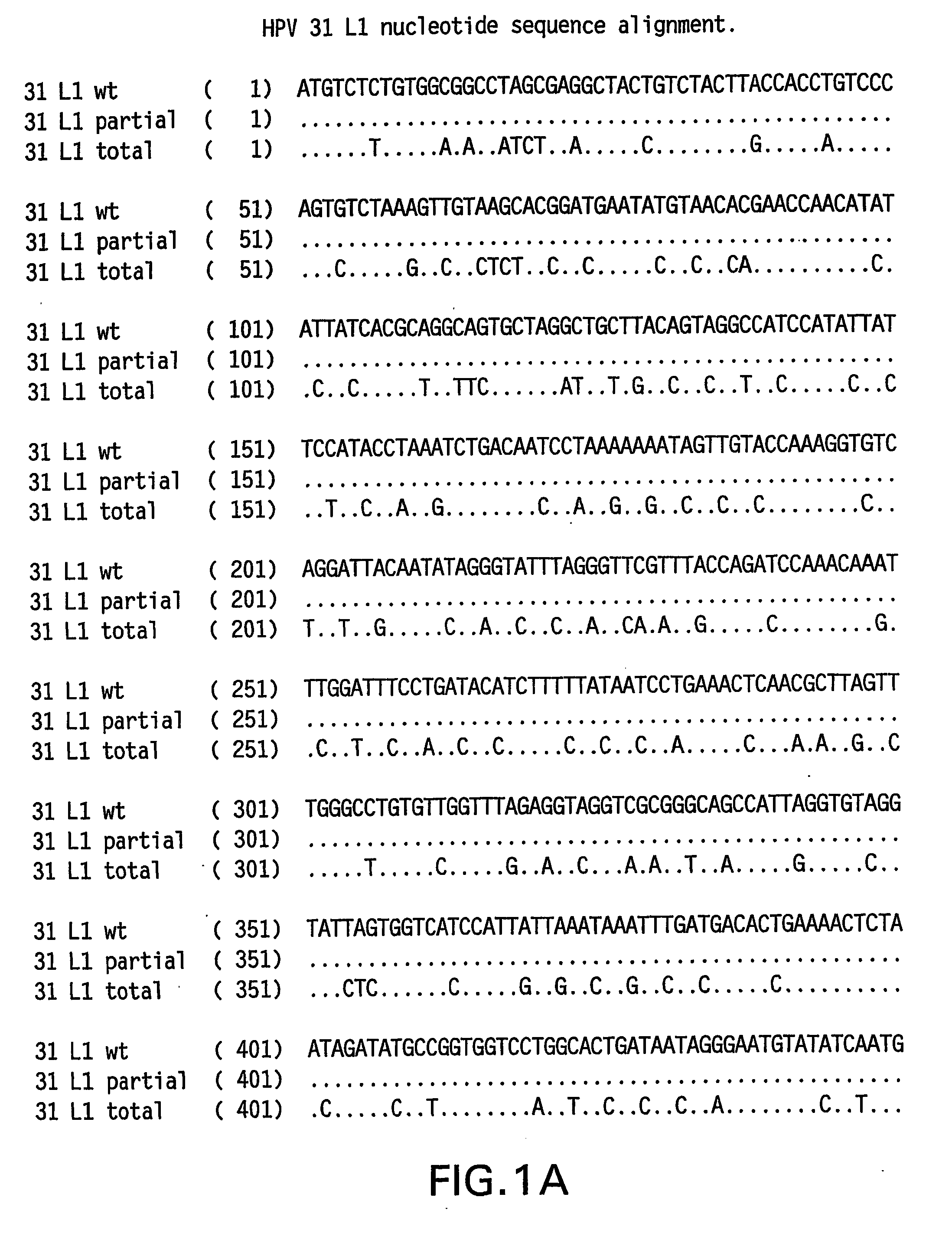
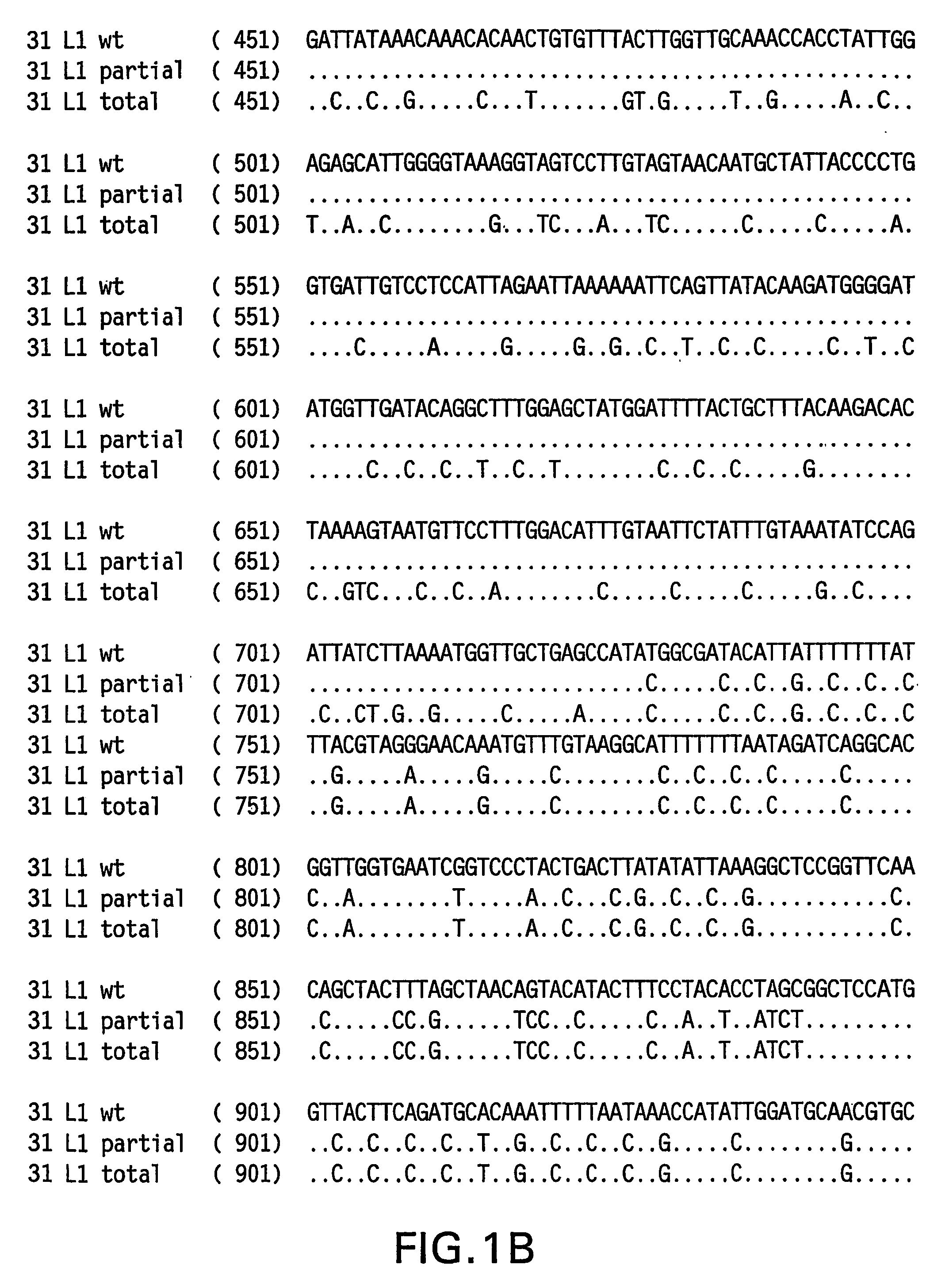
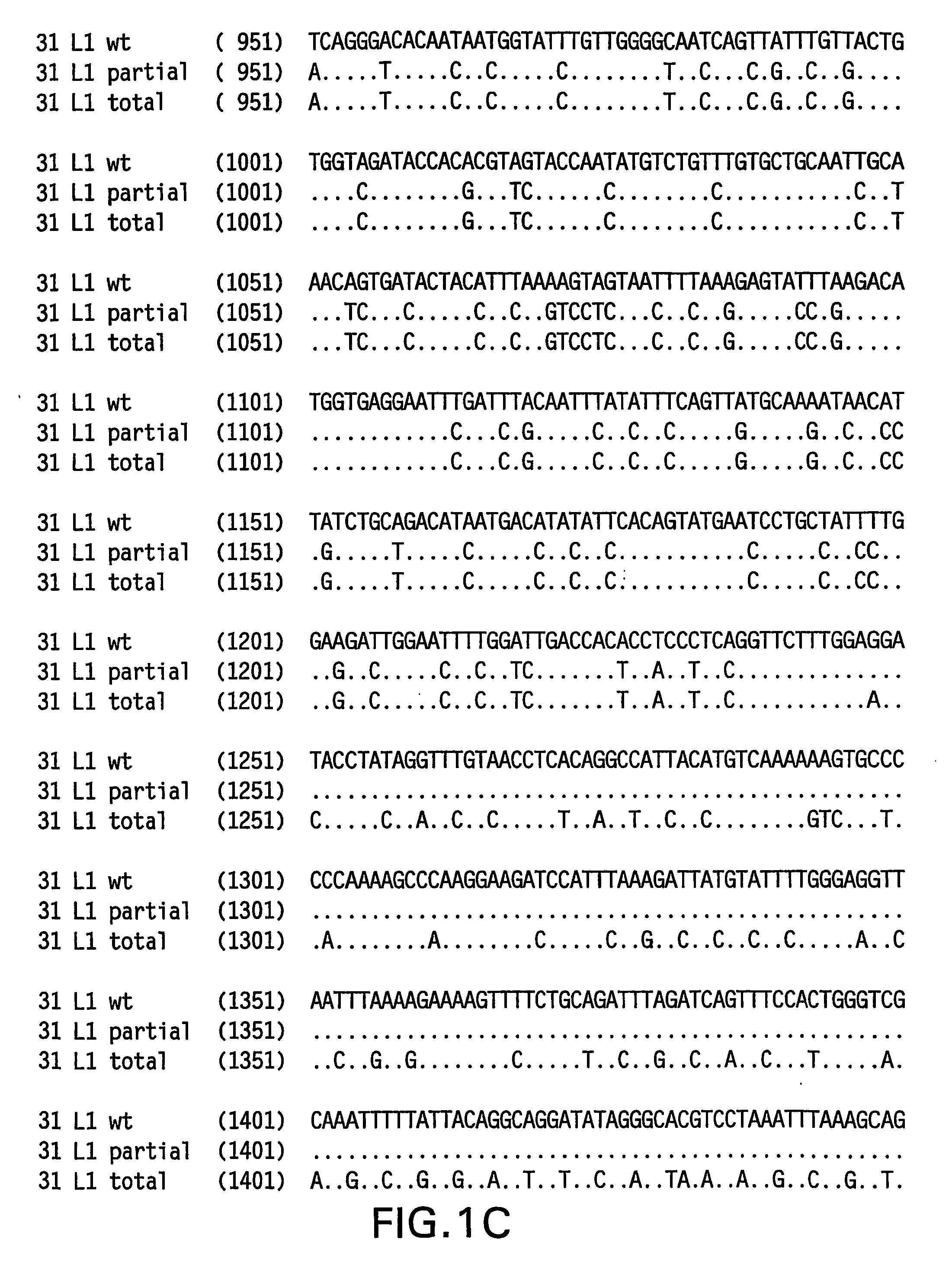
Optimized expression of HPV31 L1 in yeast
Synthetic DNA molecules encoding the HPV31 L1 protein are provided. Specifically, the present invention provides polynucleotides encoding HPV31 L1 protein, wherein said polynucleotides are free from internal transcription termination signals that are recognized by yeast. Also provided are synthetic polynucleotides encoding HPV31 L1 wherein the polynucleotides have been codon-optimized for high level expression in a yeast cell. The synthetic molecules may be used to produce HPV31 virus-like particles (VLPs), and to produce vaccines and pharmaceutical compositions comprising the HPV31 VLPs. The vaccines of the present invention provide effective immunoprophylaxis against papillomavirus infection through neutralizing antibody and cell-mediated immunityHPV31 L1 total rebuild nucleotide and aminoacid sequence: M S L W R P S 1 ATGTCTTTGT GGAGACCATC E A T V Y L P P V PTGAAGCTACC GTCTACTTGC CACCAGTCCC V S K V V S T 51 AGTCTCTAAG GTCGTCTCTA D E Y V T R T N I YCCGACGAATA CGTCACCAGA ACCAACATCT Y H A G S A 101 ACTACCACGC TGGTTCTGCTR L L T V G H P Y YAGATTGTTGA CCGTCGGTCA CCCATACTAC S I P K S D N 151 TCTATCCCAA AGTCTGACAA P K K I V V P K V SCCCAAAGAAG ATCGTCGTCC CAAAGGTCTC G L Q Y R V F 201 TGGTTTGCAA TACAGAGTCT R V R L P D P N K FTCAGAGTCAG ATTGCCAGAC CCAAACAAGT G F P D T S 251 TCGGTTTCCC AGACACCTCTF Y N P E T Q R L VTTCTACAACC CAGAAACCCA AAGATTGGTC W A C V G L E 301 TGGGCTTGTG TCGGTTTGGA V G R G Q P L G V GAGTCGGTAGA GGTCAACCAT TGGGTGTCGG I S G H P L L 351 TATCTCTGGT CACCCATTGT N K F D D T E N S NTGAACAAGTT CGACGACACC GAAAACTCTA R Y A G G P 401 ACAGATACGC TGGTGGTCCAG T D N R E C I S MGGTACCGACA ACAGAGAATG TATCTCTATG D Y K Q T Q L 451 GACTACAAGC AAACCCAATT C L L G C K P P I GGTGTTTGTTG GGTTGTAAGC CACCAATCGG E H W G K G S 501 TGAACACTGG GGTAAGGGTT P C S N N A I T P GCTCCATGTTC TAACAACGCT ATCACCCCAG D C P P L E 551 GTGACTGTCC ACCATTGGAAL K N S V I Q D G DTTGAAGAACT CTGTCATCCA AGACGGTGAC N V D T G F G 601 ATGGTCGACA CCGGTTTCGG A N D F T A L Q D TTGCTATGGAC TTCACCGCTT TGCAAGACAC K S W V P L D 651 CAAGTCTAAC GTCCCATTGG I C N S I C K Y P DACATCTGTAA CTCTATCTGT AAGTACCCAG Y L K M V A 701 ACTACTTGAA GATGGTCGCTE P Y G D T L F F YGAACCATACG GCGACACCTT GTTCTTCTAC L R R E Q M F 751 TTGCGTAGAG AACAGATGTT V R H F F N R S G TCGTAAGGCAC TTCTTCAACA GATCCGGCAC V G E S V P T 801 CGTAGGTGAA TCTGTCCCAA D L Y I K G S G S TCCGACCTGTA CATCAAGGGC TCCGGTTCCA A T L A N S 851 CCGCTACCCT GGCTAACTCCT Y F P T P S G S NACCTACTTCC CAACTCCATC TGGCTCCATG V T S D A Q I 901 GTCACCTCCG ACGCTCAGAT F N K P Y W M Q R ACTTCAACAAG CCATACTGGA TGCAGCGTGC Q G H N N G I 951 ACAGGGTCAC AACAACGGTA C W G N Q L F V T VTCTGTTGGGG TAACCAGCTG TTCGTGACTG V D T T R S1001 TGGTCGATAC CACGCGTTCTT N N S V C A A I AACCAACATGT CTGTCTGTGC TGCAATCGCT N S D T T F K1051 AACTCTGACA CTACCTTCAA S S N F K E Y L R HGTCCTCTAAC TTCAAGGAGT ACCTGAGACA G E E F D L Q1101 TGGTGAGGAA TTCGATCTGC F I F Q L C K I T LAATTCATCTT CCAGTTGTGC AAGATCACCC S A D I N T1151 TGTCTGCTGA CATCATGACCY I H S M N P A I LTACATCCACA GTATGAACCC TGCCATCCTG E D W N F G L1201 GAGGACTGGA ACTTCGGTCT T T P P S G S L E DGACCACTCCA CCTTCCGGTT CTTTGGAAGA.
Owner:MERCK SHARP & DOHME LLC
Enhanced first generation adenovirus vaccines expressing condon optimized HIV1-Gag, Pol, Nef and modifications
InactiveUS20070077257A1Improved cellular-mediated immune responseLow transmission rateViral antigen ingredientsVirus peptidesTreatment effectNucleotide
First generation adenoviral vectors and-recombinant adenovirus-based HIV vaccines which contain HIV-1 gag, HIV-1 pol and / or HIV-1 nef polynucleotide pharmaceutical products, and biologically relevant modifications thereof are described. The adenovirus vaccines, when directly introduced into living vertebrate tissue, express the relevant proteins, inducing a cellular immune response which specifically recognizes HIV-1. The exemplified polynucleotides of the present invention are synthetic DNA molecules encoding HIV-1 Gag, HIV-1 Pol, HIV-1 Nef, and derivatives thereof. The adenoviral vaccines of the present invention, alone or in combination, will offer a prophylactic advantage to previously uninfected individuals and / or provide a therapeutic effect by reducing viral load levels within an infected individual, thus prolonging the asymptomatic phase of HIV-1 infection.
Owner:EMINI EMILIO A +7
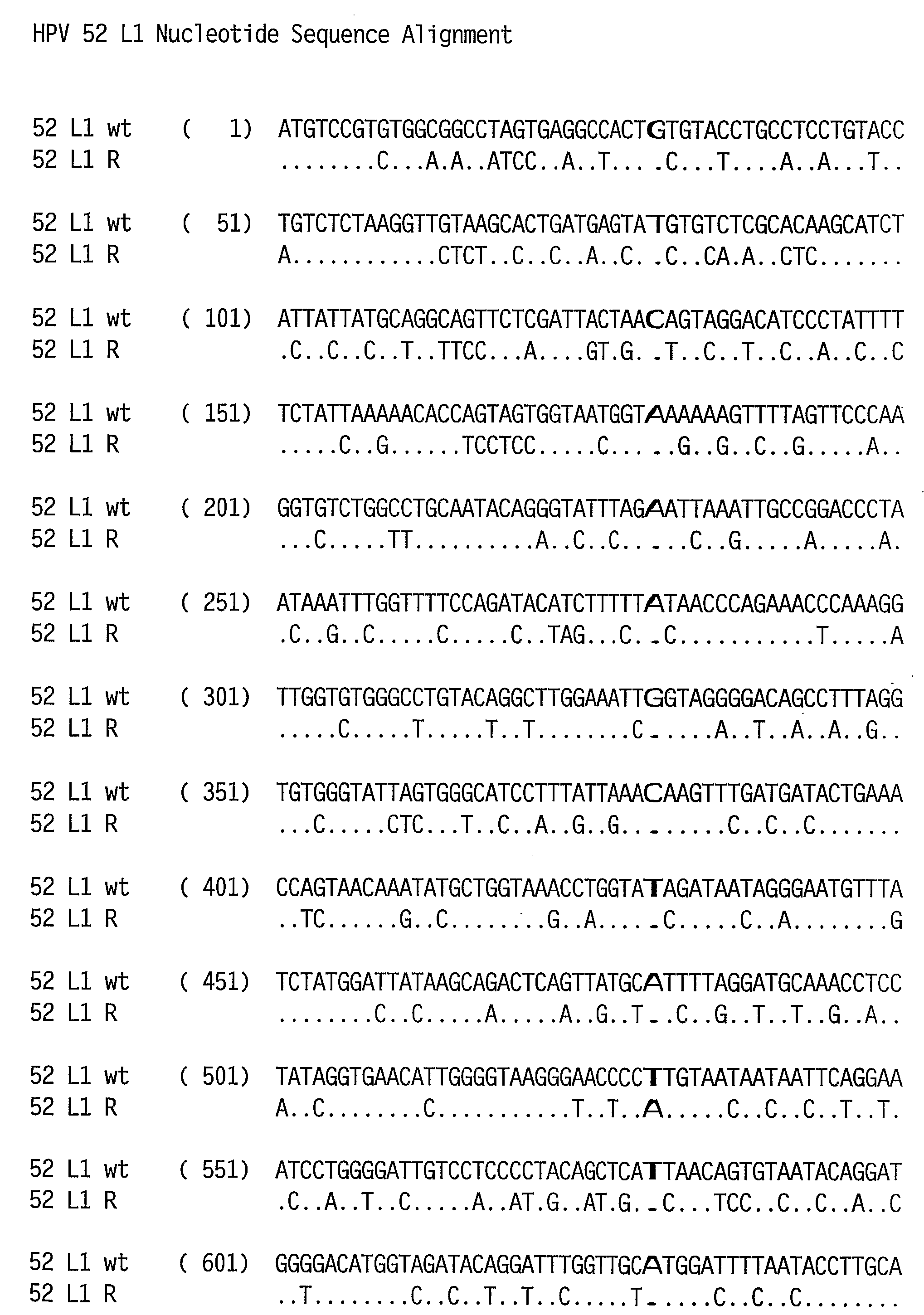
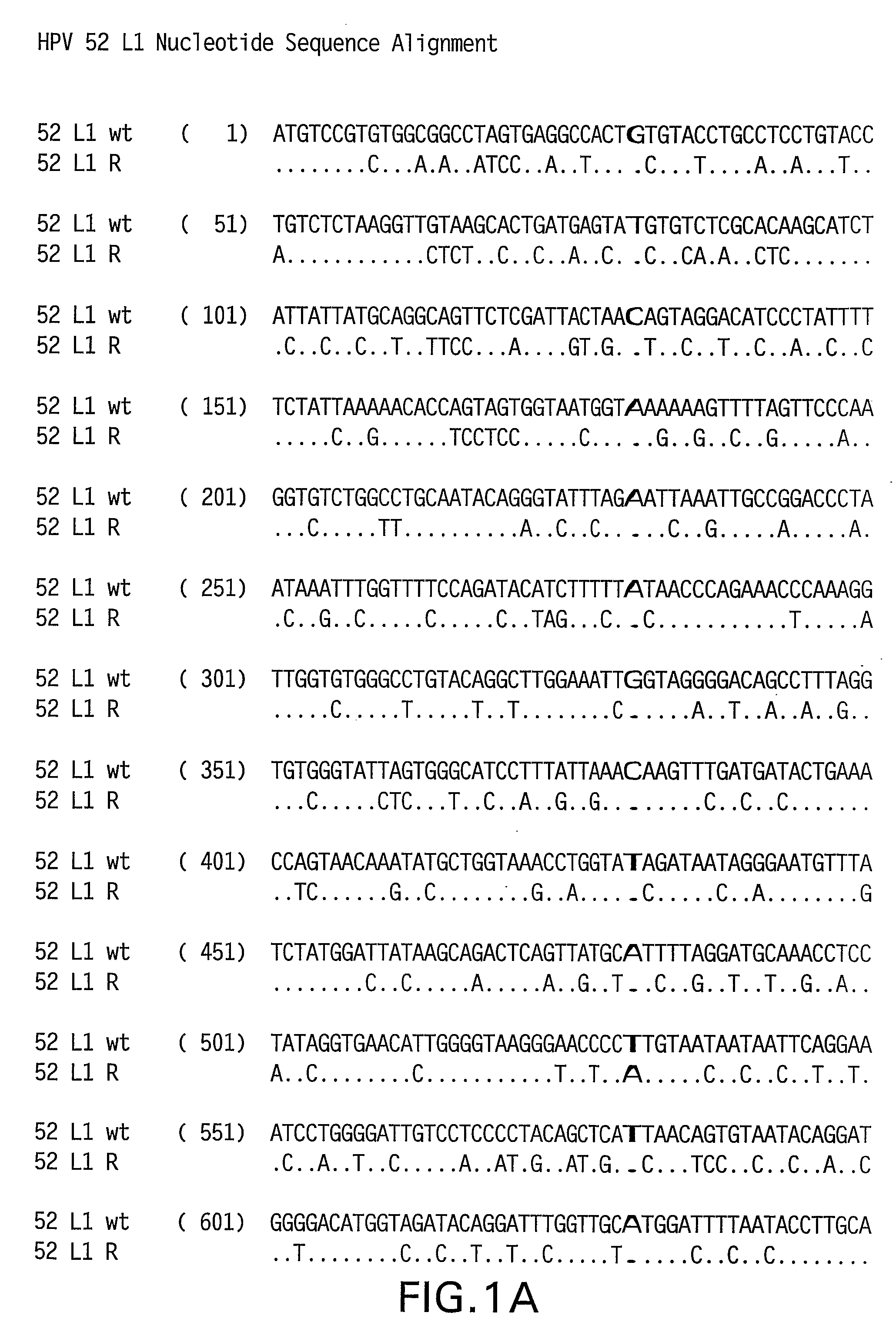
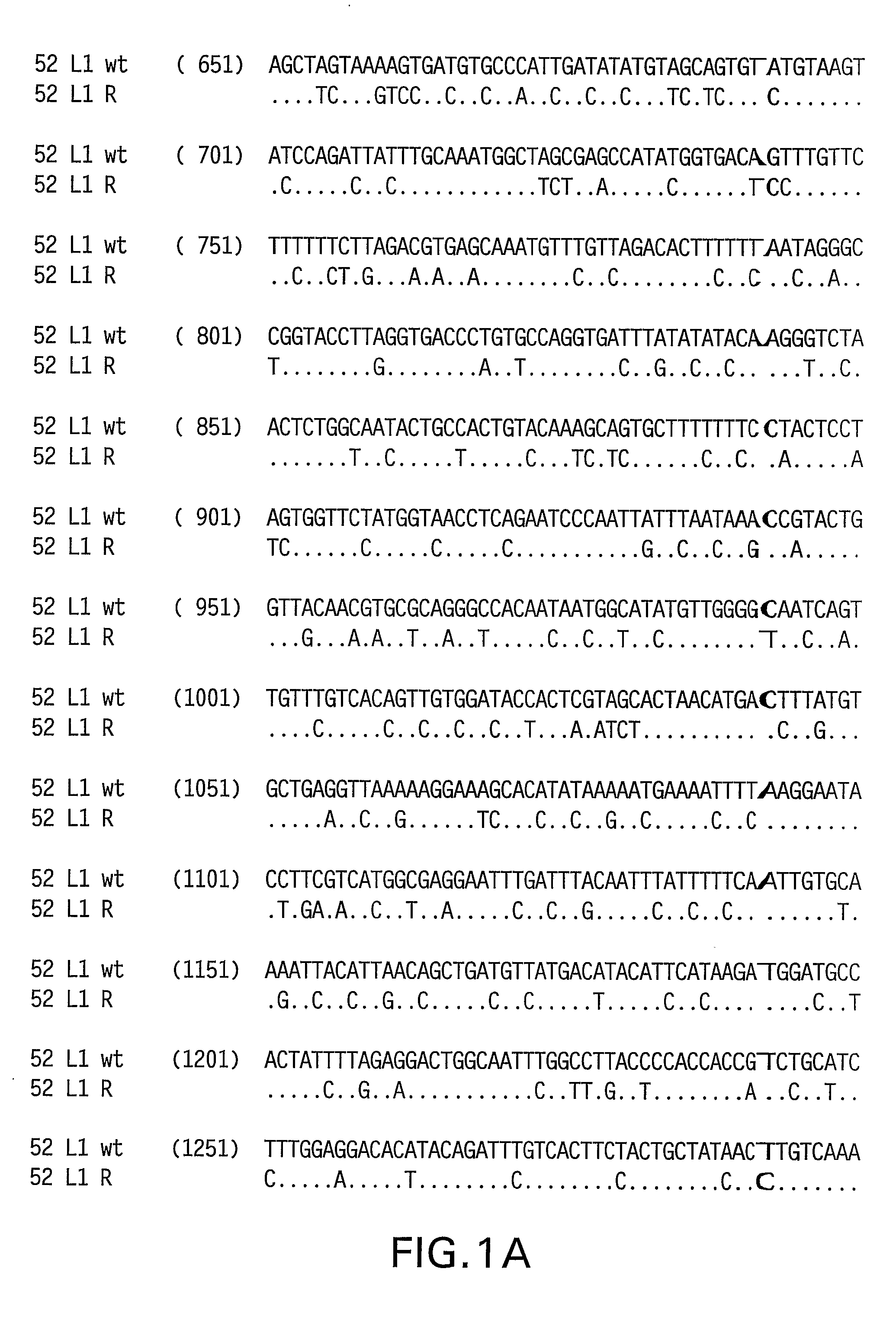
Optimized Expression of Hpv 58 L1 in Yeast
Synthetic DNA molecules encoding the HPV 52 L1 protein are provided. Specifically, the present invention provides polynucleotides encoding HPV 52 L1 protein, wherein said polynucleotides are codon-optimized for high level expression in a yeast cell. In alternative embodiments of the invention, the nucleotide sequence of the synthetic molecule is altered to eliminate transcription termination signals that are recognized by yeast. The synthetic molecules may be used to produce HPV 52 virus-like particles (VLPs), and to produce vaccines and pharmaceutical compositions comprising the HPV 52 VLPs. The vaccines of the present invention provide effective immunoprophylaxis against papillomavirus infection through neutralizing antibody and cell-mediated immunity and may also be useful for treatment of existing HPV infections.
Owner:MERCK SHARP & DOHME LLC
Synthetic human papilloma virus genes
Synthetic DNA molecules encoding papillomavirus proteins are provided. The codons of the synthetic molecules are codons preferred by the projected host cell. The synthetic molecules may be used as a polynucleotide vaccine which provides effective immunoprophylaxis against papillomavirus infection through stimulation of neutralizing antibody and cell-mediated immunity.
Owner:MERCK SHARP & DOHME CORP
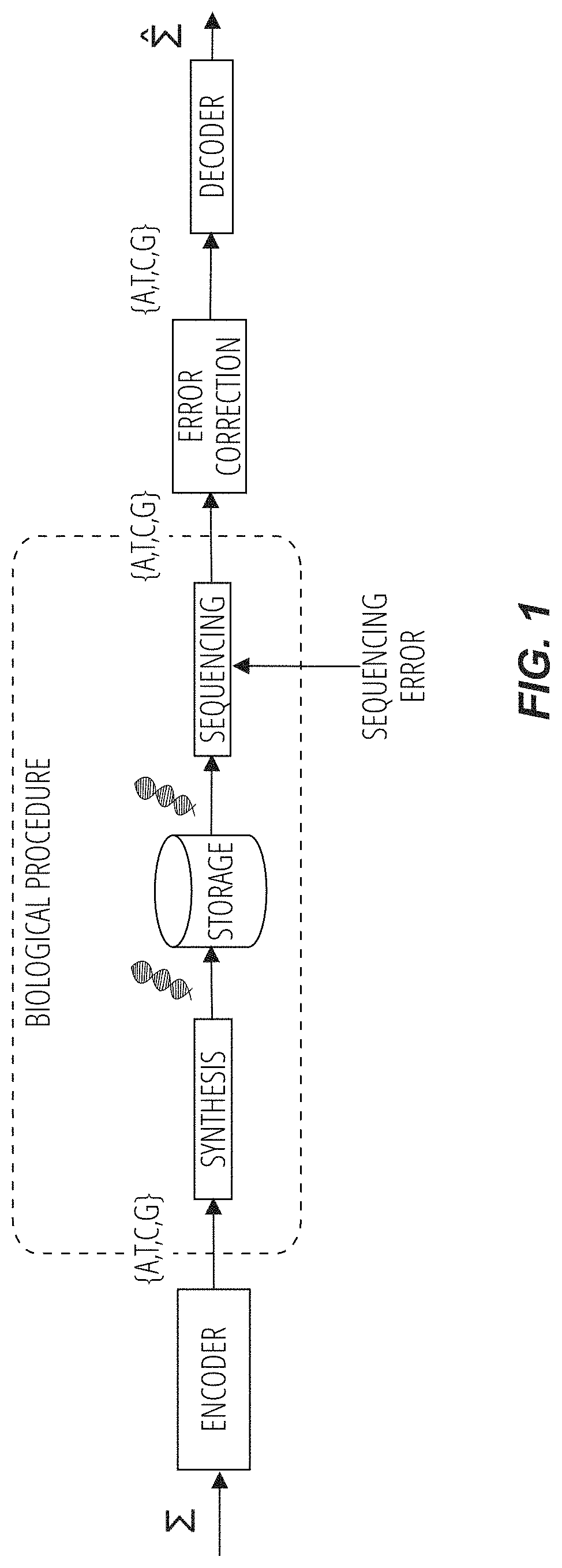
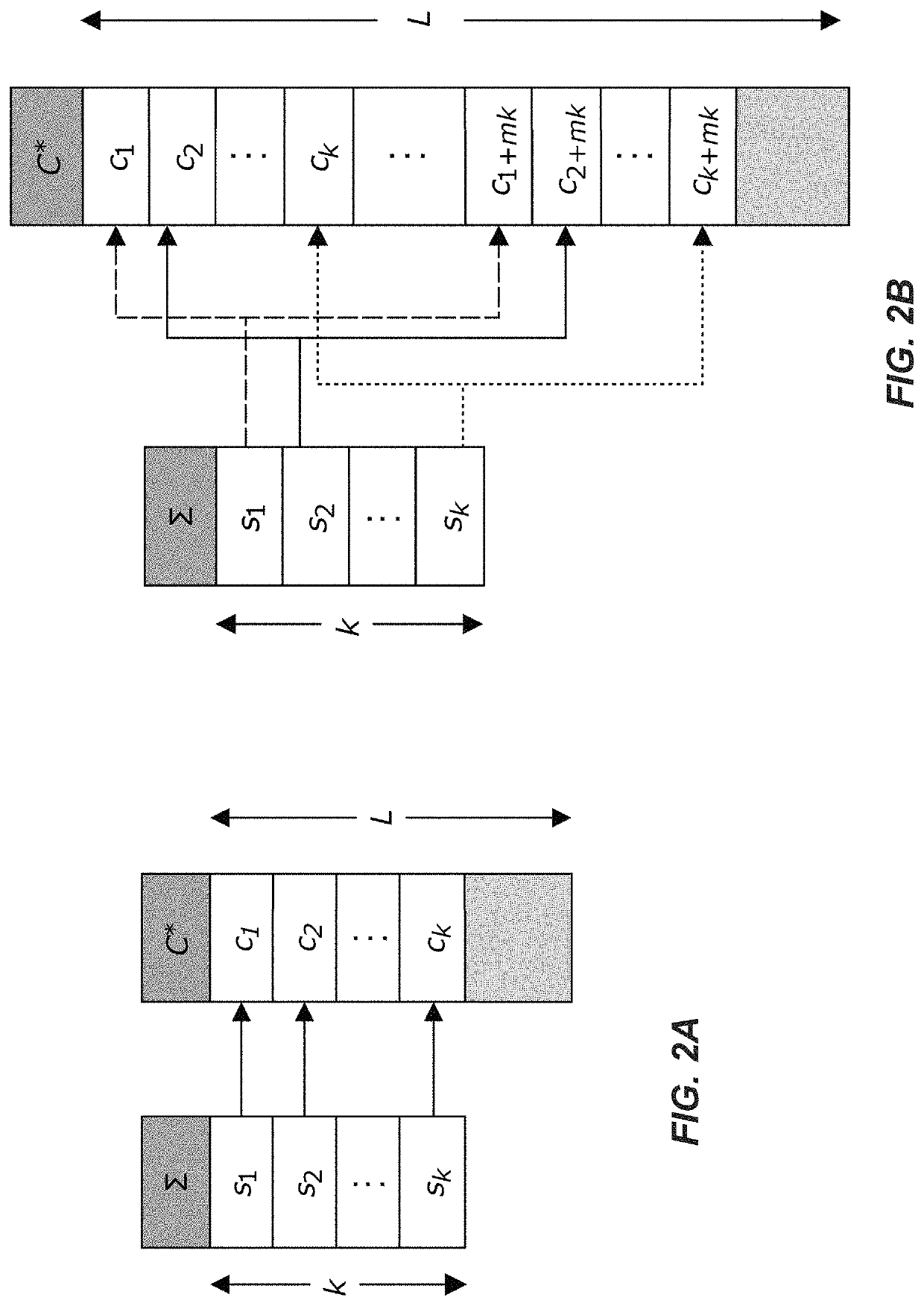
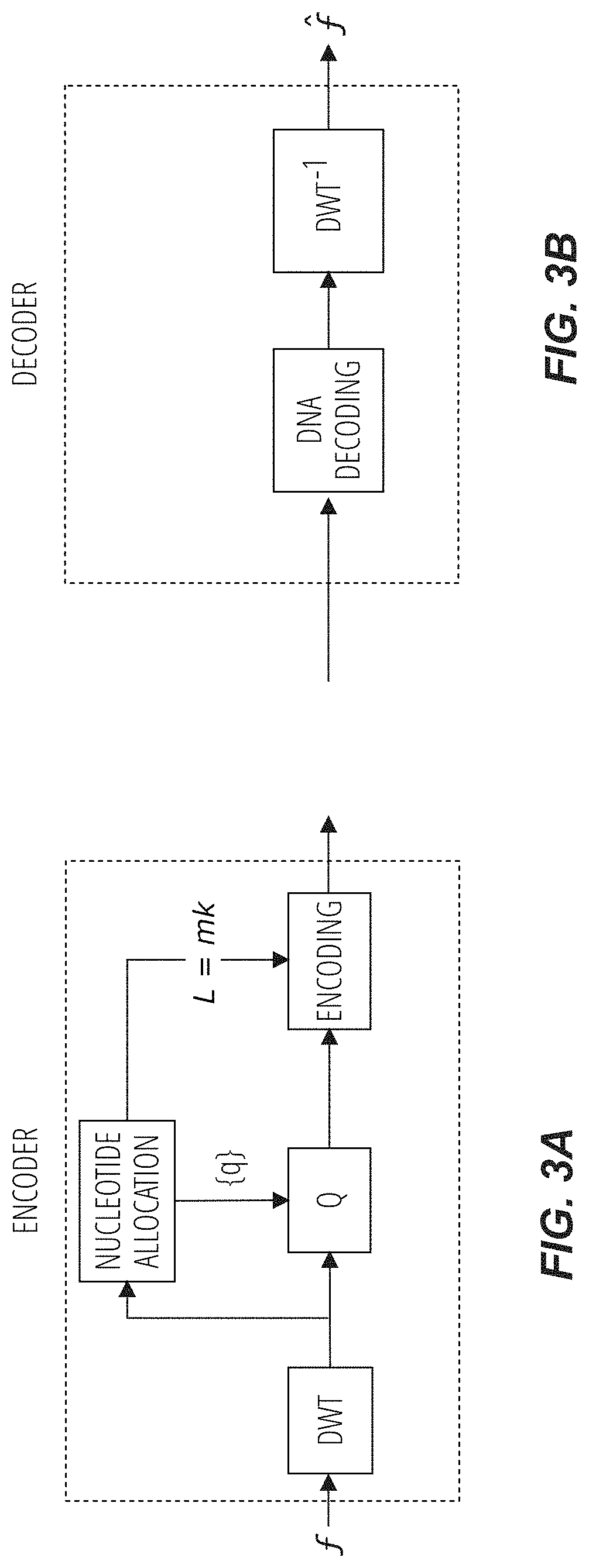
Methods for storing digital data as, and for transforming digital data into, synthetic DNA
Methods for encoding data in synthetic DNA include, for an input data sequence, constructing a codebook of a number of unique codewords, of a nucleotide length, which are constructed such that, for each codeword, the codeword is formed from a selection of nucleotide pairs from a first predefined dictionary of nucleotide pairs, and, if the nucleotide length of the codeword is odd, an additional selection of a nucleotide from a second predefined dictionary of individual nucleotides. For each symbol from the input data sequence, at least one codeword is designated as associable therewith, and each symbol is coded as one codeword selected from the designated at least one codeword associable with that symbol. A code is formed from the codewords, arranged in corresponding order to that of their respective symbols in the input data sequence. A DNA sequence is synthetized with nucleotides ordered to match the code.
Owner:CENT NAT DE LA RECHERCHE SCI +1
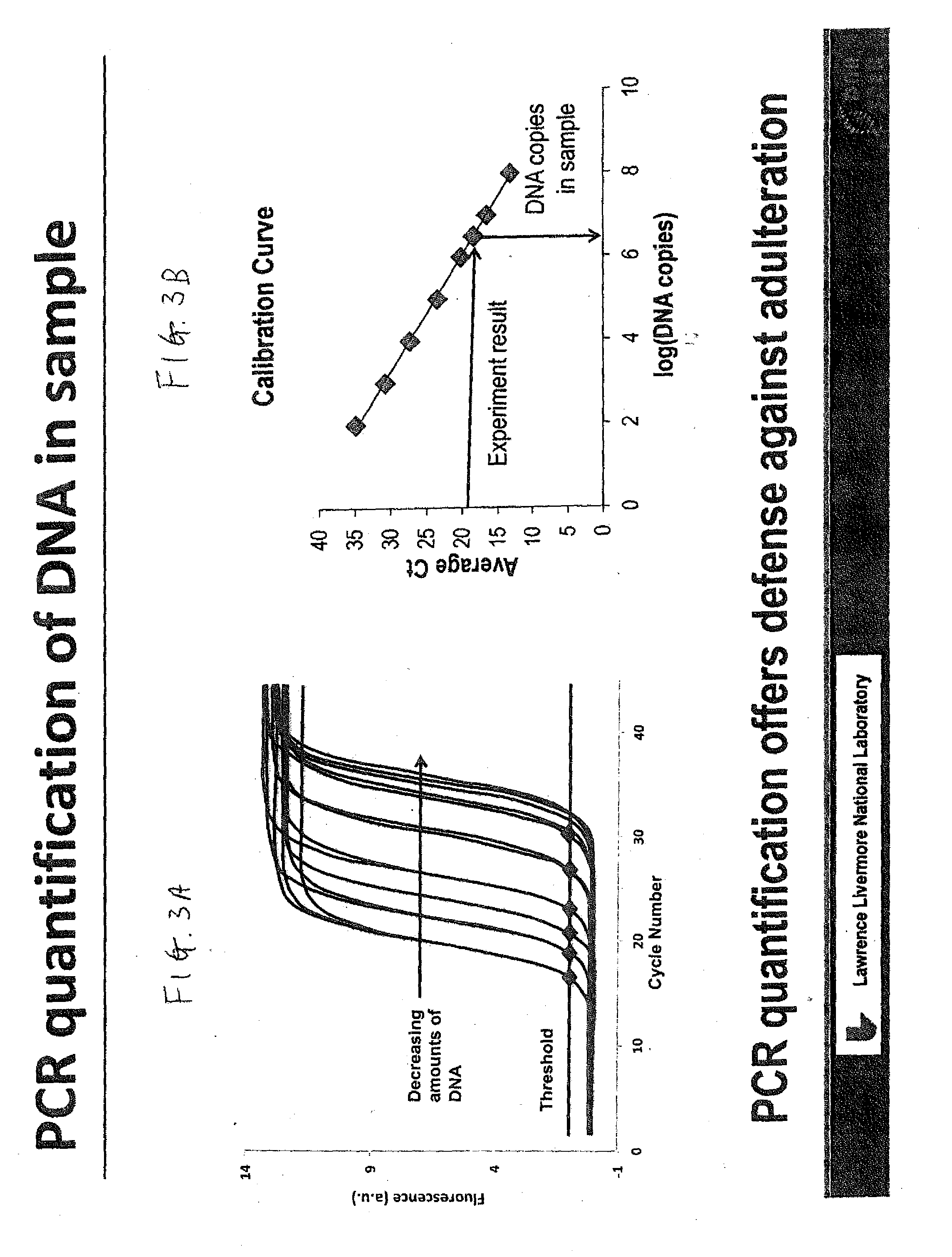
Microarray Based Multiplex Pathogen Analysis and Uses Thereof
Provided herein is an internal standard method for determining copy number of a pathogen DNA in an unpurified nucleic acid sample by using a known copy number of synthetic DNA that shares a consensus region sequence with the pathogen. The sample is subject to two amplification steps using locus-specific primers and fluorescent primers respectively to obtain fluorescent amplicons for the pathogen and synthetic DNA. These are hybridized with immobilized pathogen-specific and synthetic DNA-specific nucleic acid probes and imaged to obtain fluorescent signals for pathogen-specific and synthetic DNA-specific amplicons. Signal intensities are correlated with the known copy number of synthetic DNA to determine copy number of pathogen DNA in the plant. Also described herein is a method to simultaneously quantitate using the above method, copy numbers of both pathogen and plant DNA in a sample.
Owner:PATHOGENDX INC
Codon-optimized papilloma virus sequences
Synthetic DNA molecules encoding the HPV31 L1 protein are provided. Specifically, the present invention provides polynucleotides encoding HPV31 L1 protein, wherein said polynucleotides are free from internal transcription termination signals that are recognized by yeast. Also provided are synthetic polynucleotides encoding HPV31 L1 wherein the polynucleotides have been codon-optimized for high level expression in a yeast cell. The synthetic molecules may be used to produce HPV31 virus-like particles (VLPs), and to produce vaccines and pharmaceutical compositions comprising the HPV31 VLPs. The vaccines of the present invention provide effective immunoprophylaxis against papillomavirus infection through neutralizing antibody and cell-mediated immunity.
Owner:MERCK SHARP & DOHME LLC
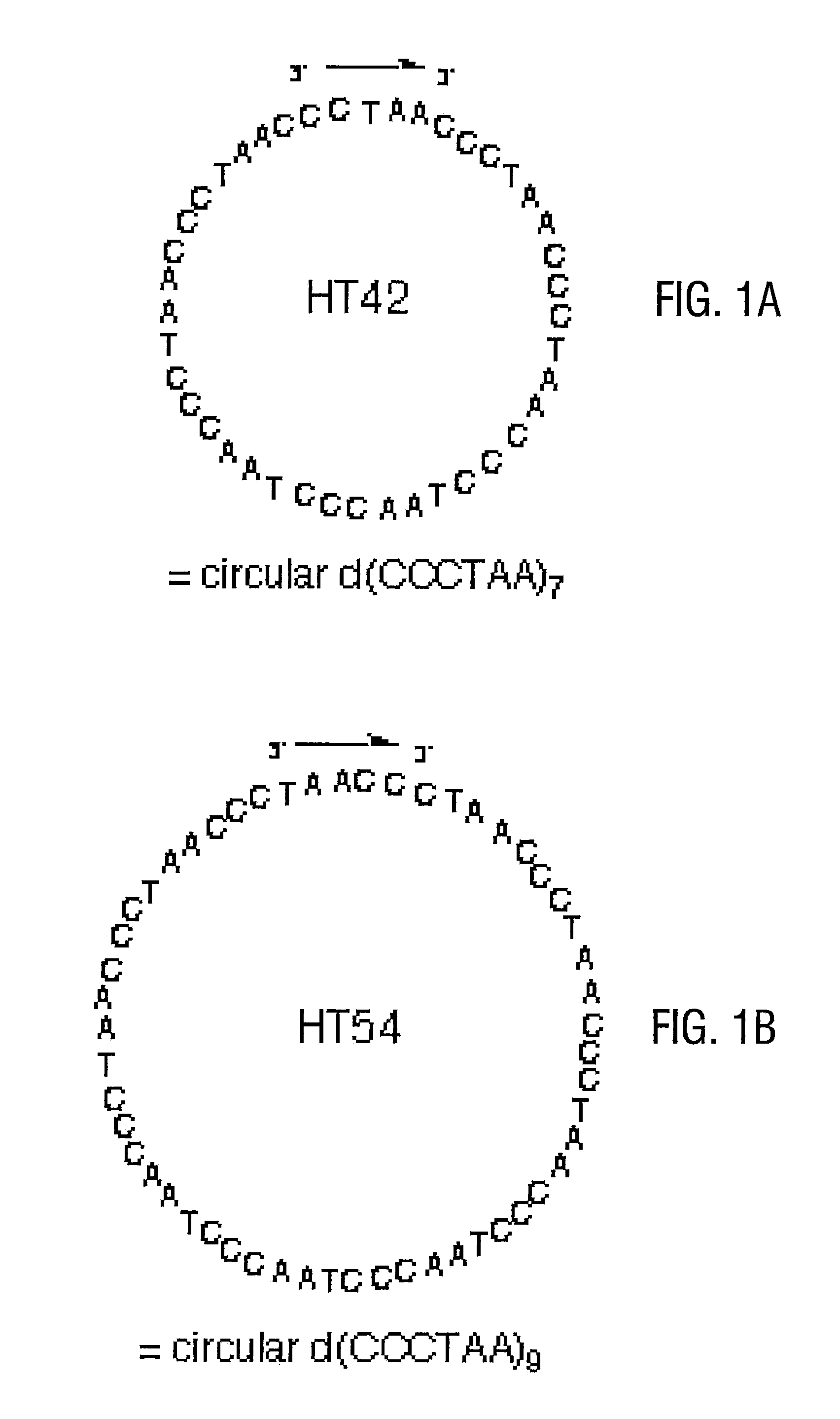
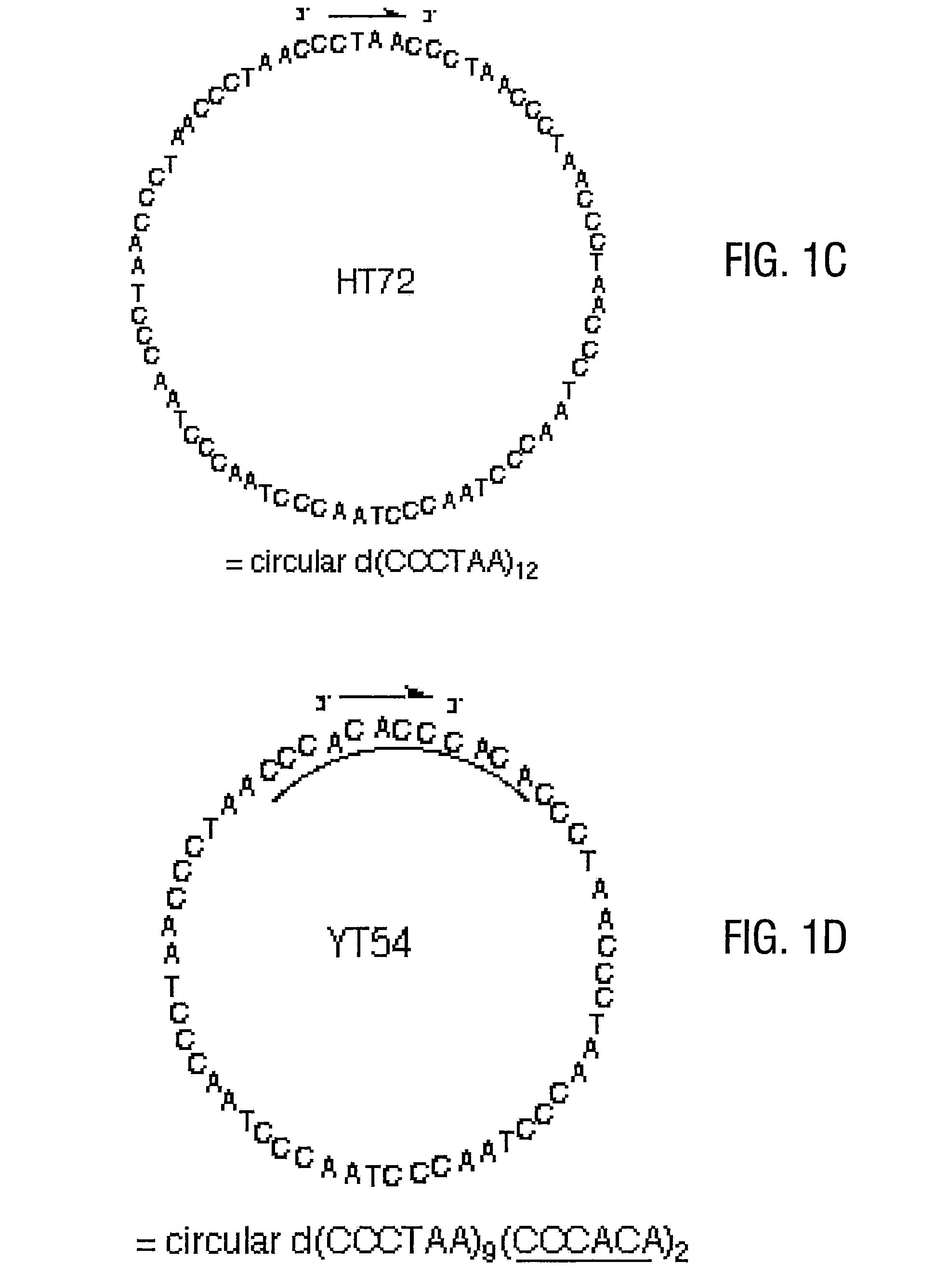
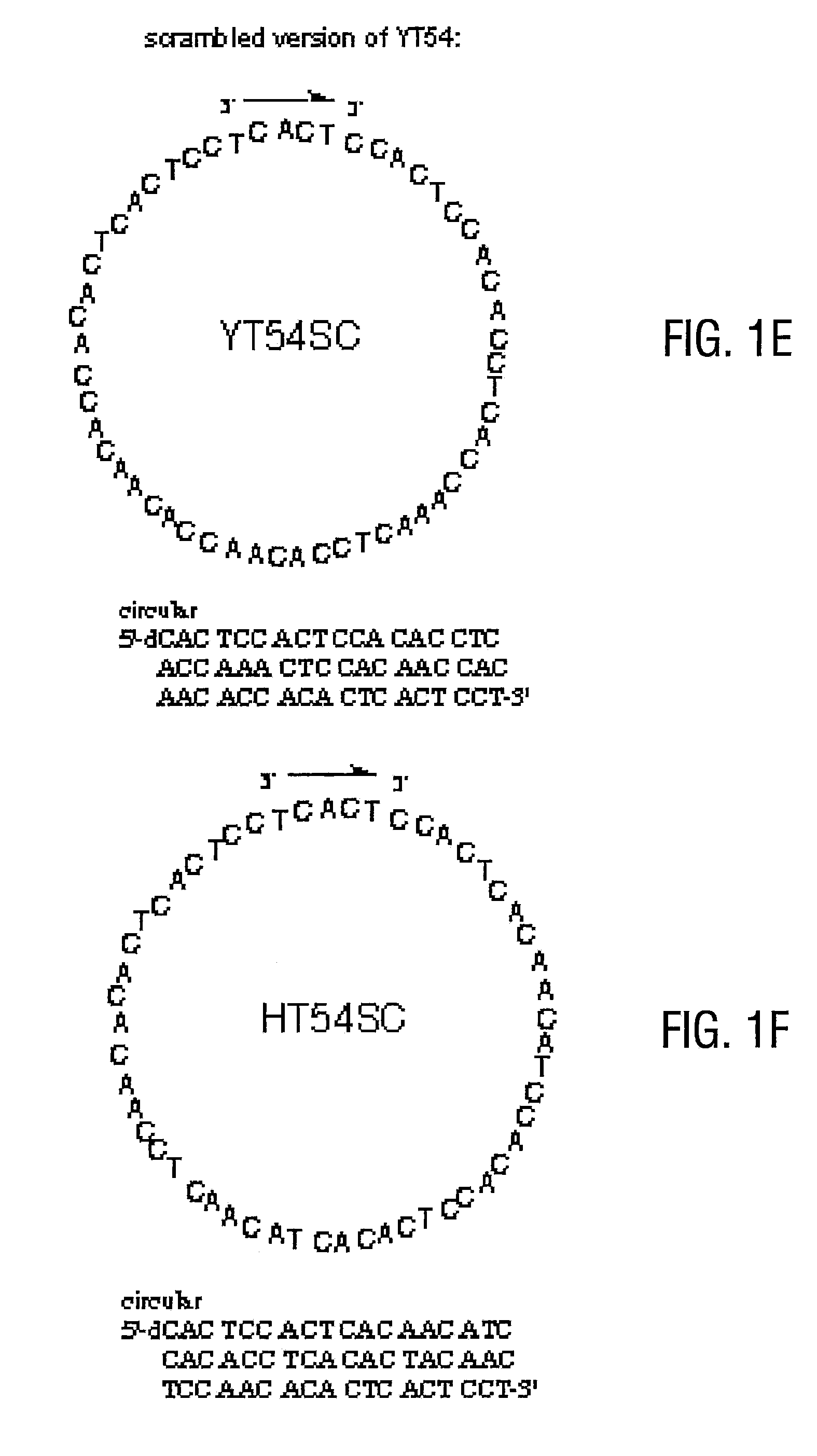
Telomere-encoding synthetic DNA nanocircles, and their use for the elongation of telomere repeats
InactiveUS8323975B2Easy to produceEasy to storeOrganic active ingredientsSugar derivativesCancer cellSynthetic DNA
Telomere-encoding nucleic acid nanocircles, methods for their preparation, and methods for their use are disclosed. The nanocircles can be constructed containing multiple repeats of the complement of telomere repeat sequences. The telomere-encoding nanocircles are useful for extending telomeres both in vitro and in vivo, for treating macular degeneration, the effects of skin aging, liver degeneration, and cancer. The nanocircles are further useful for treating cell cultures to produce long-lived non-cancerous cell populations. This use has wide applicability in scientific research, tissue engineering, and transplantation.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
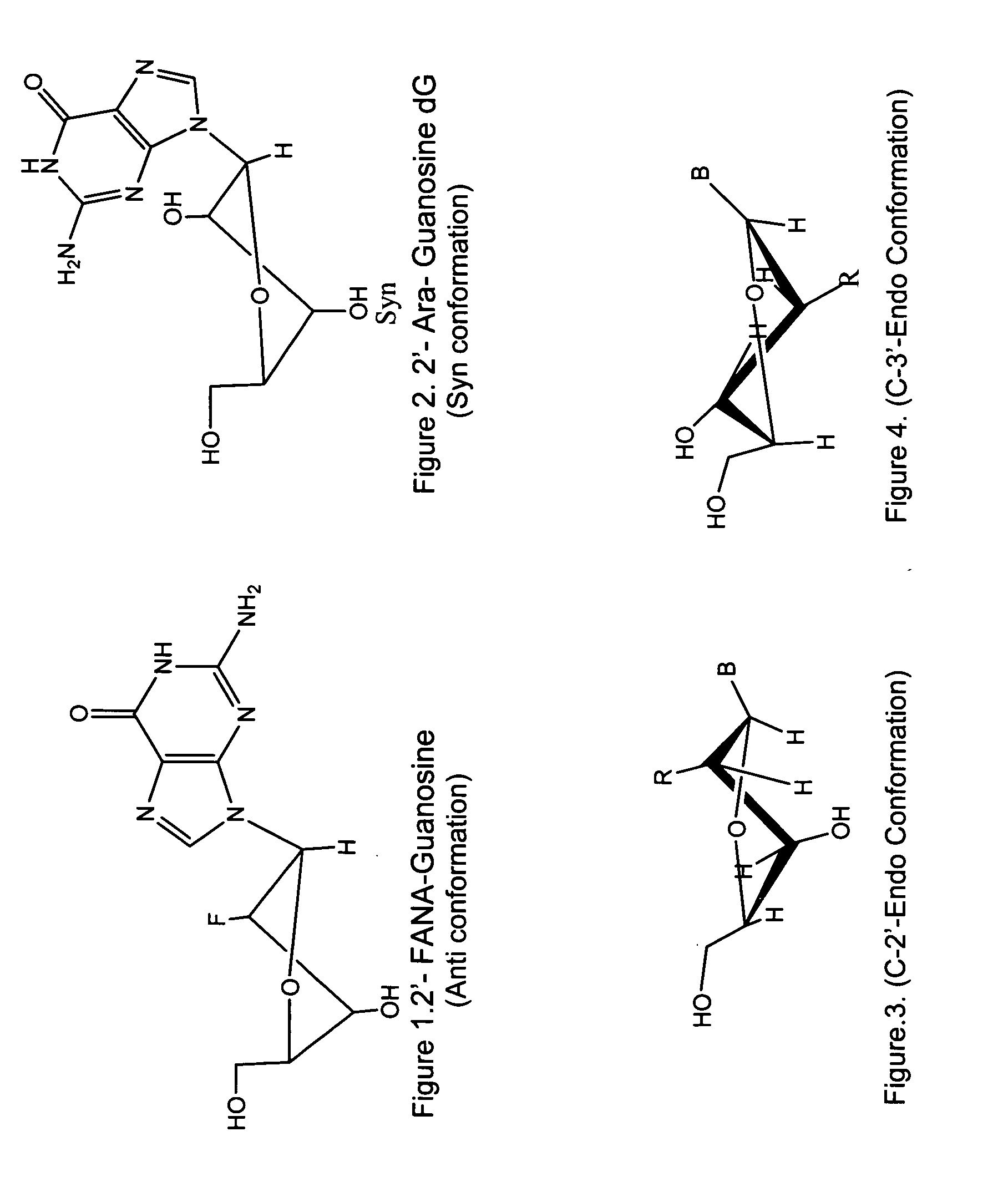
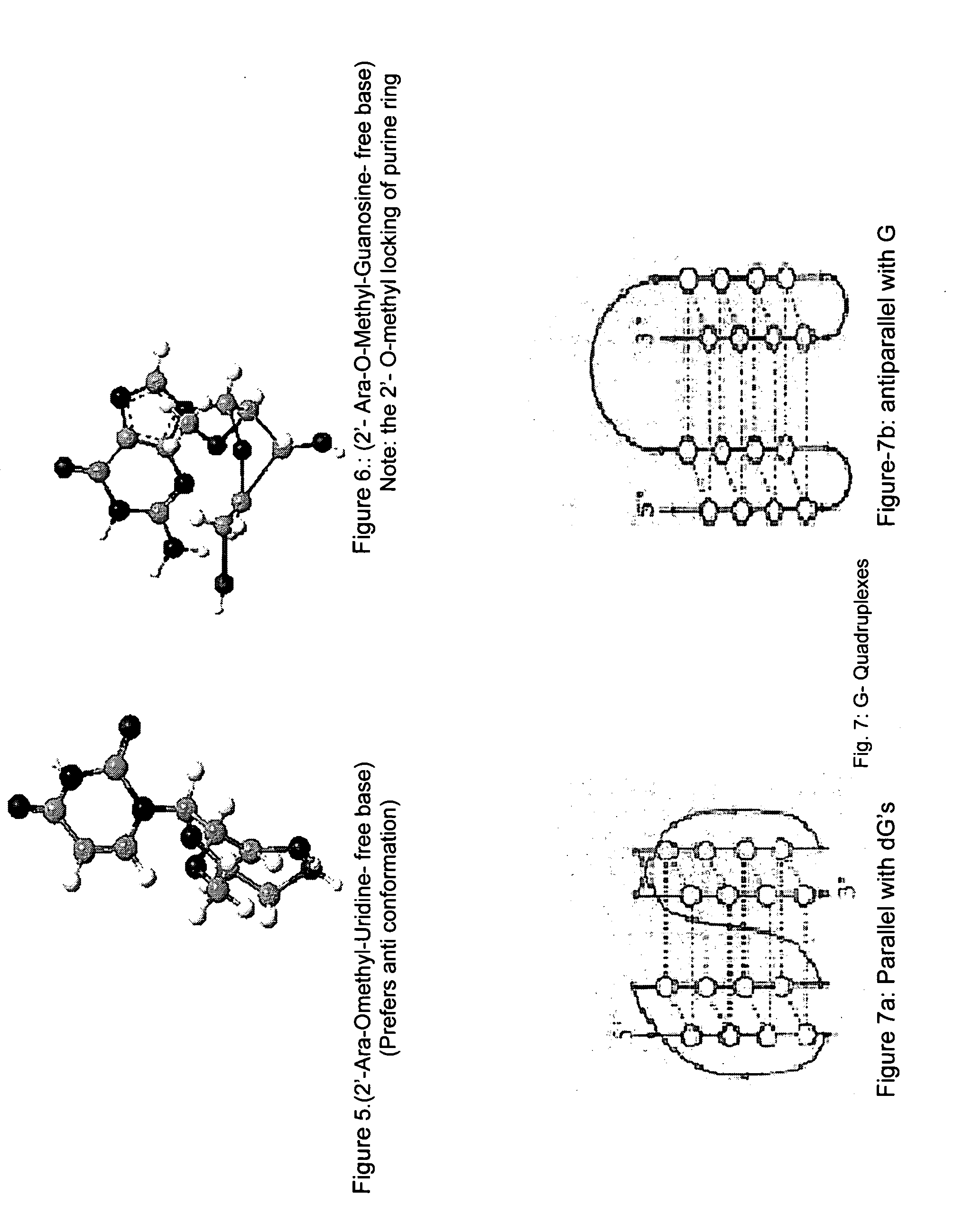
Synthesis of ara-2'-o-methyl-nucleosides, corresponding phosphoramidites and oligonucleotides incorporating novel modifications for biological application in therapeuctics, diagnostics, g- tetrad forming oligonucleotides and aptamers
InactiveUS20120149888A1Prolong lifeEffective interactionOrganic active ingredientsSugar derivativesPhosphateSynthetic DNA
The present invention relates to synthesis, purification and methods to obtain high purity novel 2′-arabino-O-methyl nucleosides and the corresponding phosphoramidites of various arabinonucleoside bases and introduction of such units into defined sequence synthetic DNA and RNA. Various synthetic oligonucleotides, such as HIV integrase inhibitor 14-mer and thrombin binding oligonucleotide, thrombin-1, bearing ara-2′-omethyl modification have been synthesized. It is anticipated the oligonucleotides incorporating these monomers will exhibit biological activities related to antisense approach approach, design of better SiRNA's, diagnostic agents. Similarly, it is anticipated that oligonucleotides incorporating such novel nucleosides will be useful to develop therapeutic candidates designing stable G-quadruplexes and Aptamers for oligonucleotide structure, folding topology, evaluation of biochemical properties and design and develop as therapeutic agents. It is further anticipated that the nucleosides, phosphates and triphosphates of this invention could develop as therapeutic agents.
Owner:CHEMGENES CORP
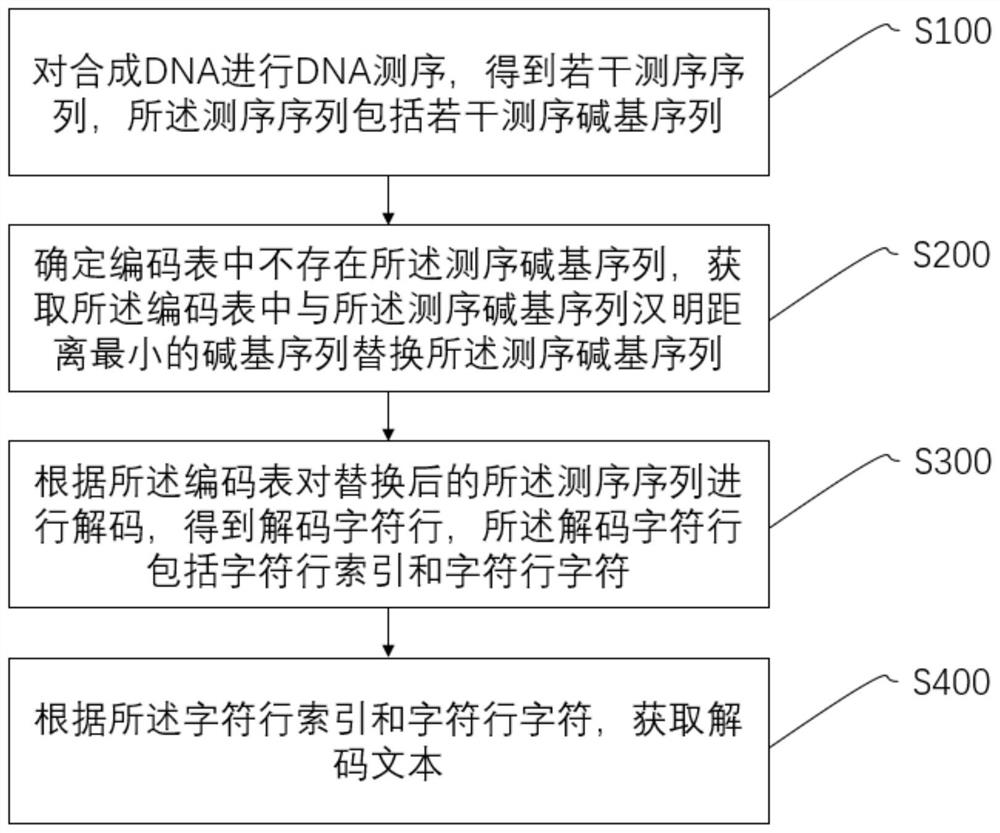
DNA storage method and system and storage medium
ActiveCN112100982AWith error correction abilityImprove storage efficiencyNatural language data processingSequence analysisSynthetic DNAA-DNA
The invention discloses a DNA storage method and system and a storage medium, and the method comprises the steps of carrying out the sequencing of synthetic DNA, and obtaining a plurality of sequencing sequences; determining that no corresponding sequencing base sequence exists in the coding table, and obtaining the base sequence with the smallest Hamming distance from the sequencing base sequencein the coding table to replace the sequencing base sequence; decoding the replaced sequencing sequence according to the coding table to obtain a decoded text, wherein the Hamming distance between anytwo base sequences in the character coding table is greater than a first threshold value. According to the embodiment of the invention, error correction is carried out on the sequencing base sequenceobtained by sequencing by utilizing the Hamming distance with the base sequence, and the sequencing base sequence after error correction is decoded to obtain a decoded text. Due to the fact that theHamming distance between any two base sequences in the coding table is larger than the first threshold value, the coding table has certain error correction capacity, and compared with existing redundant code error correction, the DNA storage efficiency is greatly improved. The invention can be widely applied to the field of molecular biology.
Owner:GUANGZHOU UNIVERSITY
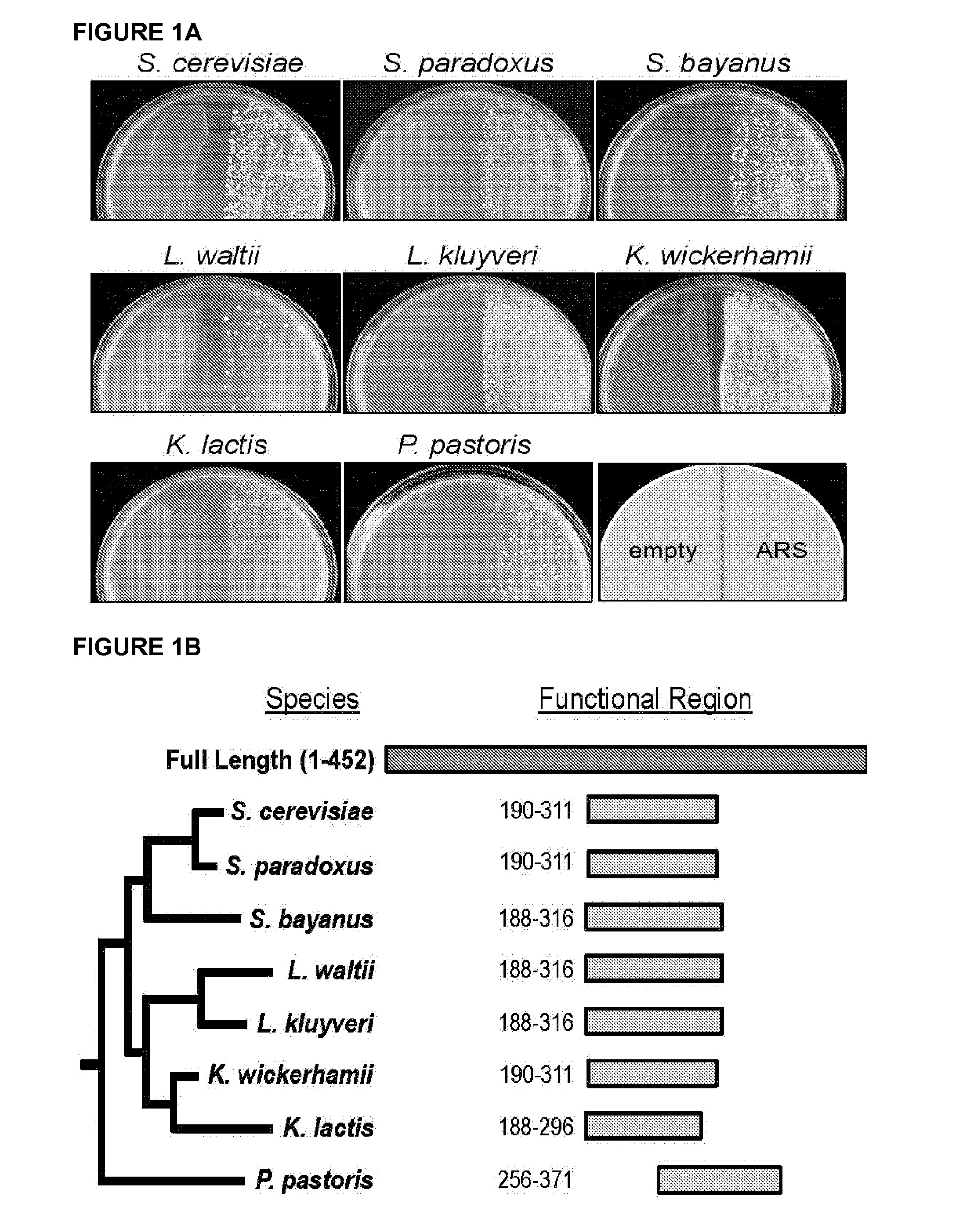
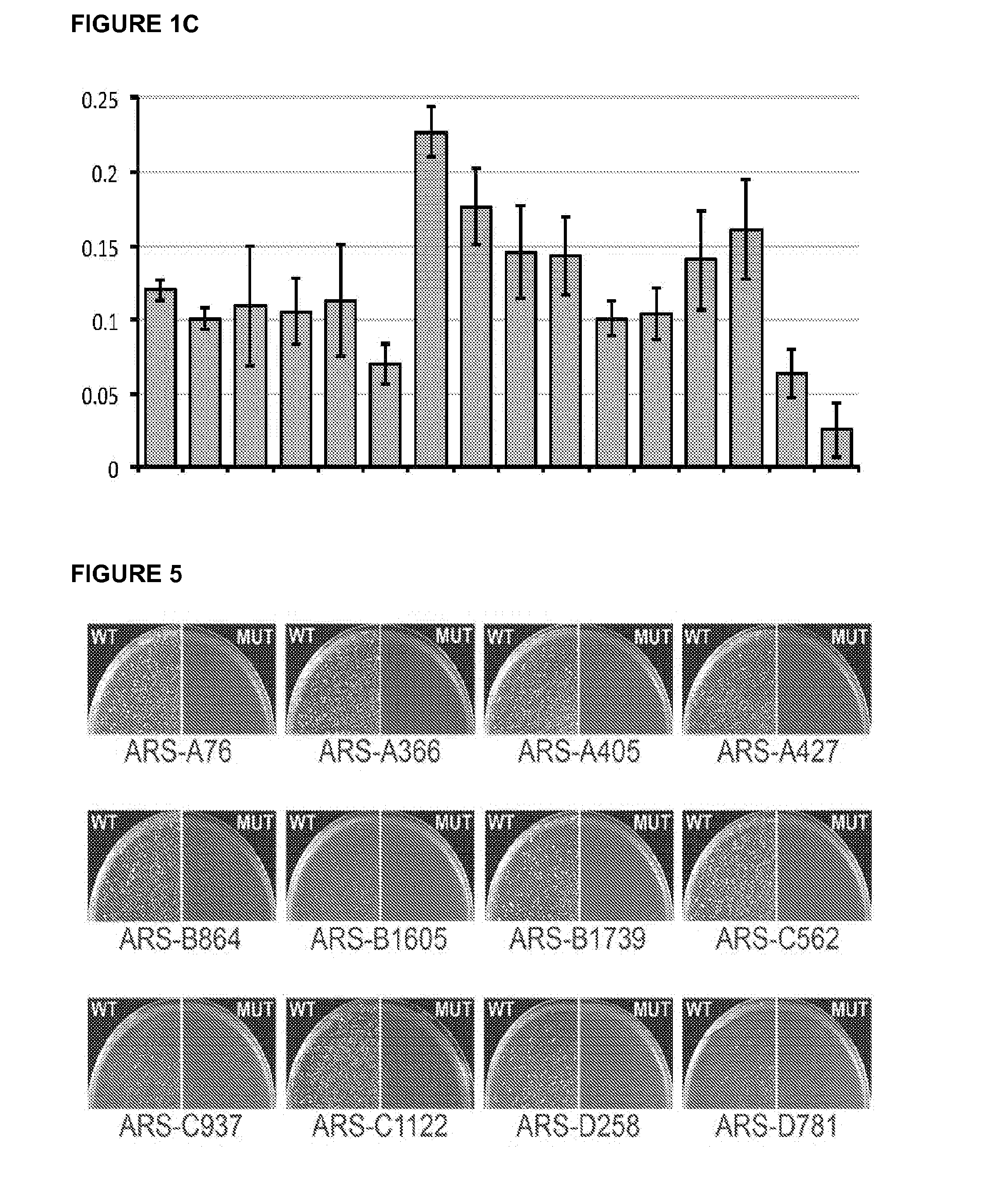
Pan-yeast autonomously replicating sequence
ActiveUS20160002647A1Faster ratePositive effect on ARS functionSugar derivativesMicroorganismsBiotechnologyLachancea kluyveri
A DNA sequence that functions as an origin in many different yeast species. From 1 to 17 mutations can be introduced into this sequence to improve its function across multiple yeasts. The resulting synthetic DNA sequence confers stable plasmid replication function in all yeast species tested, including but not limited to Saccharomyces cerevisiae, Lachancea kluyveri, Kluyveromyces lactis, Kluyveromyces wickerhammii, Hansenula polymorpha, and Pichia pastoris. Also provided are sequences that function as an optimal origin in the industrially useful Pichia pastoris.
Owner:UNIV OF WASHINGTON CENT FOR COMMERICIALIZATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com