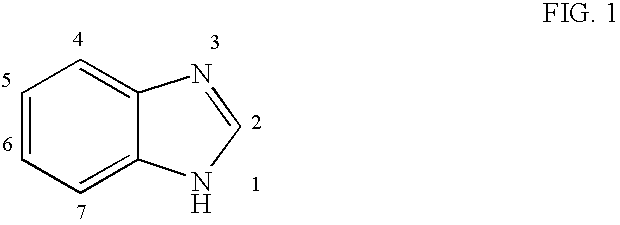Patents
Literature
248 results about "Viral load" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
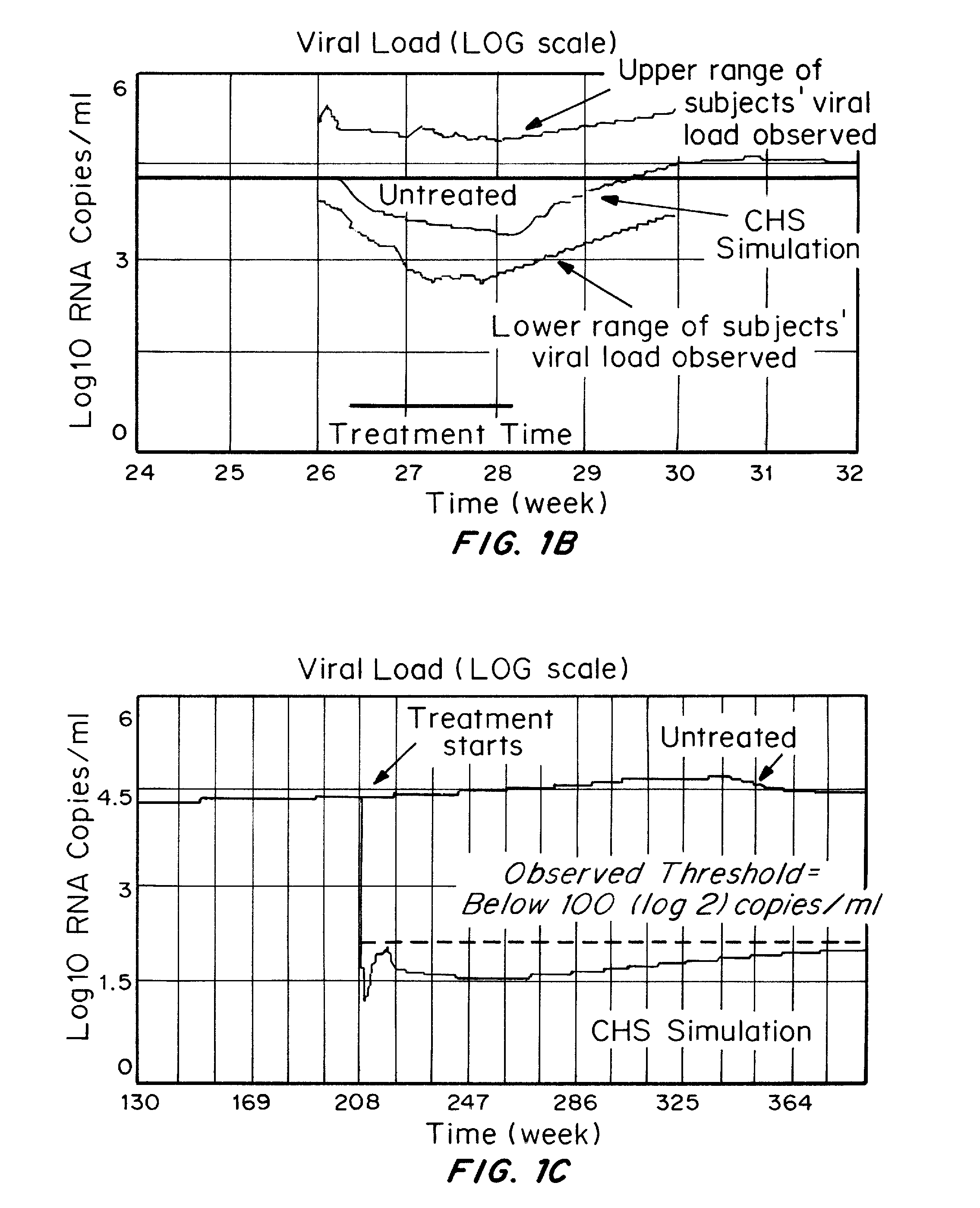
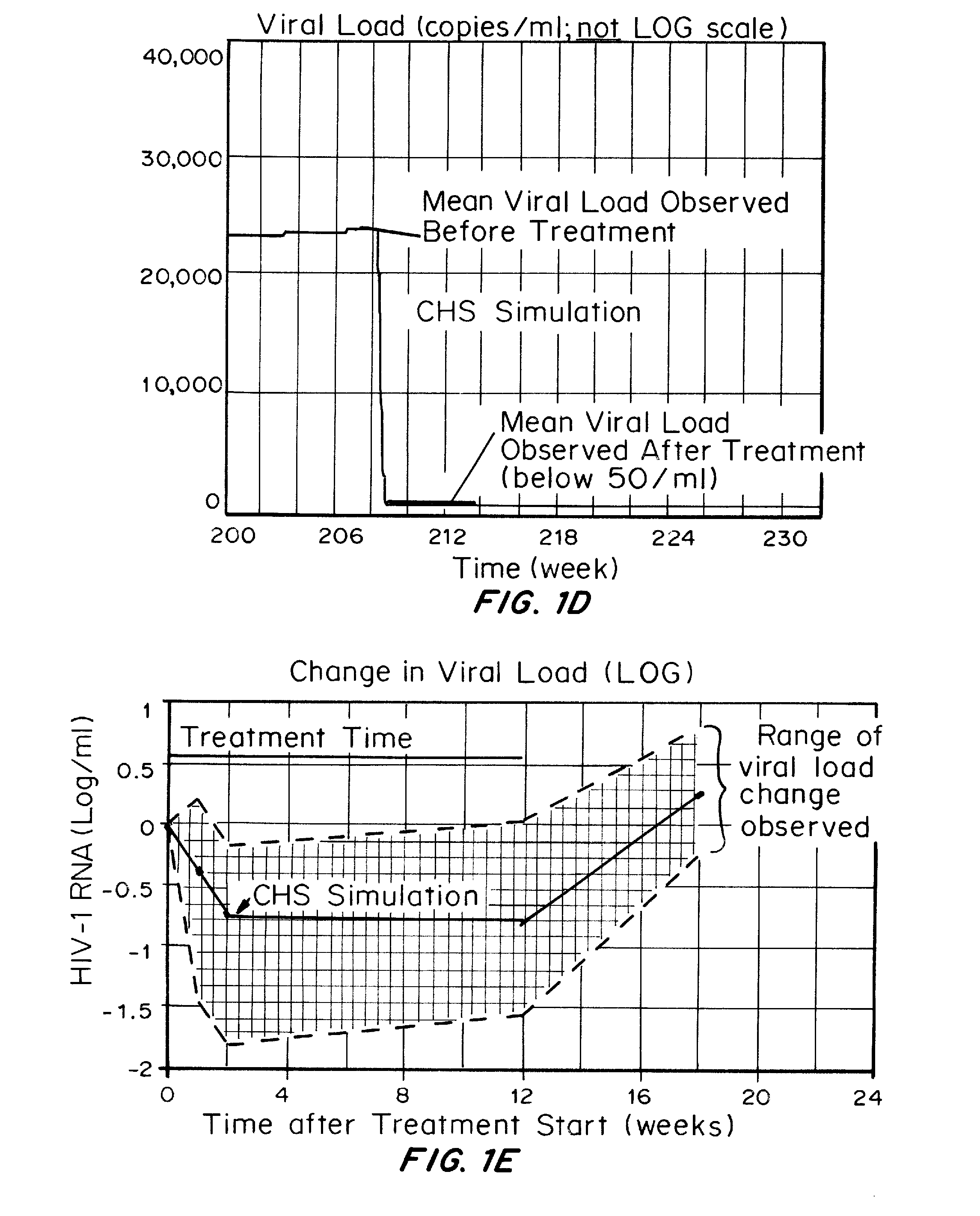
<ul><li>In patients with antiretroviral treatment,<200 copies/ml suggests that the treatment is effective enough to control the infection.</li><li>Higher than <200 copies/ml suggests ineffectiveness of the treatment.</li></ul>
Reduction of porcine circovirus-2 viral load with inactivated PCV-2
InactiveUS6517843B1Improving immunogenicityImprove performanceBiocideGenetic material ingredientsDiseaseStaining
Porcine circovirus-2 (PCV-2) is a recently identified agent wherein the potential spectrum of PCV-2-associated disease has been expanded by evidence of vertical and sexual transmission and associated reproductive failure in swine populations. PCV-2 was isolated from a litter of aborted piglets from a farm experiencing late term abortions and stillbirths. Severe, diffuse myocarditis was present in one piglet associated with extensive immunohistochemical staining for PCV-2 antigen. Variable amounts of PCV-2 antigen were also present in liver, lung and kidney of multiple fetuses. Inoculation of female pigs with a composition including an immunogen from PCV-2 or an epitope of interest from such an immunogen or with a vector expressing such an immunogen or epitope of interest prior to breeding, such as within the first five weeks of life, or prior to the perinatal period, or repeatedly over a lifetime, or during pregnancy, such as between the 6th and 8th and / or the 10th and 13th weeks of gestation, can prevent myocarditis, abortion and intrauterine infection associated with porcine circovirus-2. In addition, innoculation of male and / or female pigs with the aforementioned compositions can be carried out to prevent transmission of PCV-2 from male to female (or vice versa) during mating. Thus, the invention involves methods and compositions for preventing myocarditis, abortion and intrauterine infection associated with porcine circovirus-2.
Owner:QUEENS UNIV OF BELFAST +4
Composition comprising beta -hydroxy- beta -methylbutyric acid and at least one amino acid and methods of use
InactiveUS6031000ADecrease in fat massQuality improvementBiocidePeptide/protein ingredientsSerum igeDisease
The present invention provides a composition comprising HMB and at least one amino acid. The present invention also provides a method for the treatment of disease-associated wasting of an animal, a method for decreasing the serum-level of triglycerides of an animal, a method for decreasing the serum viral load of an animal, and a method for redistributing fat in an animal having a visceral region and a subcutaneous region. All methods comprise administering to the animal a composition comprising HMB and at least one amino acid.
Owner:IOWA STATE UNIV RES FOUND
Methods for removal, purification, and concentration of viruses, and methods of therapy based thereupon
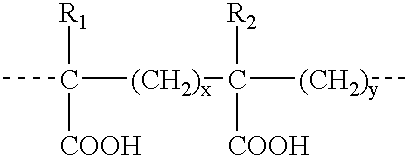
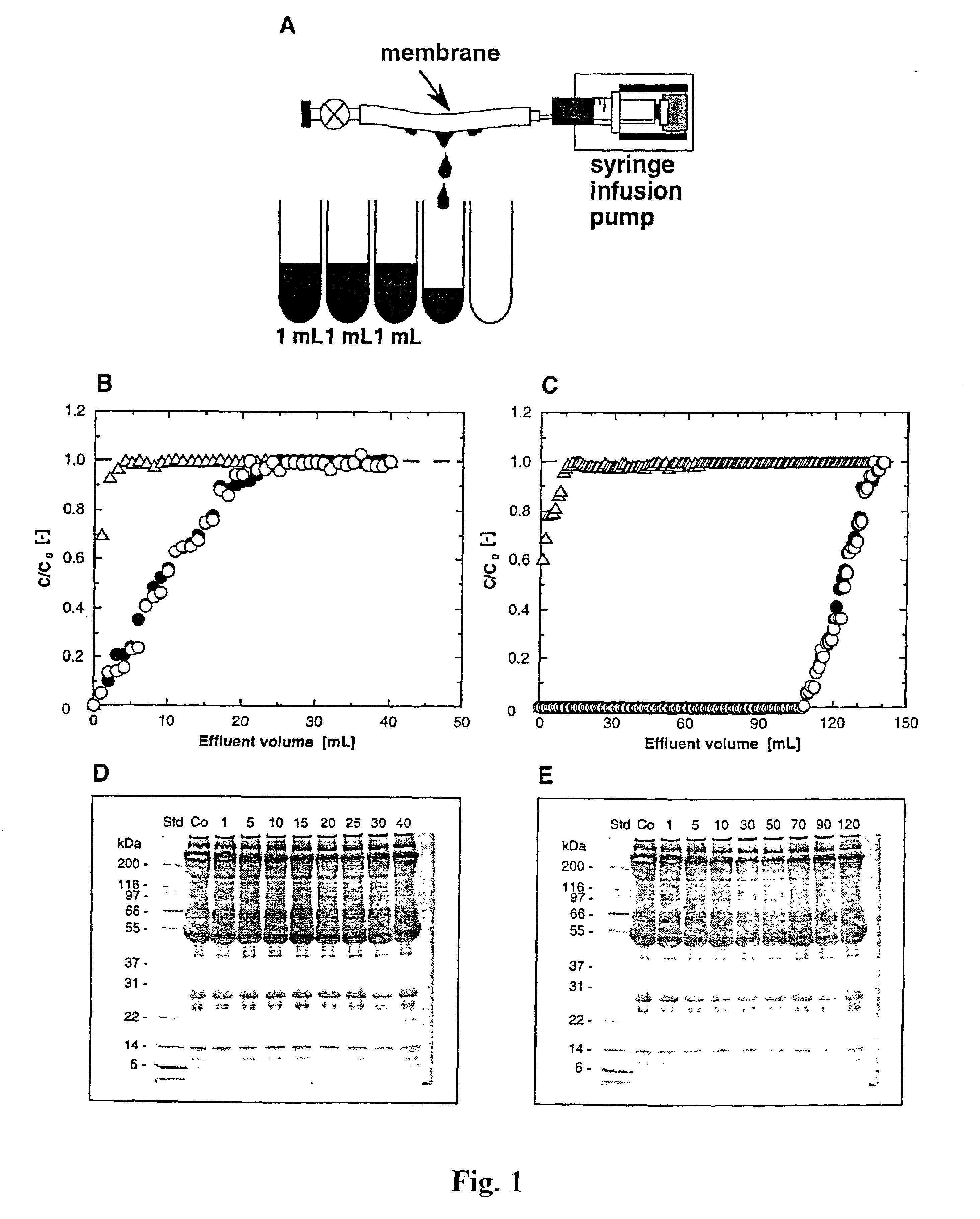
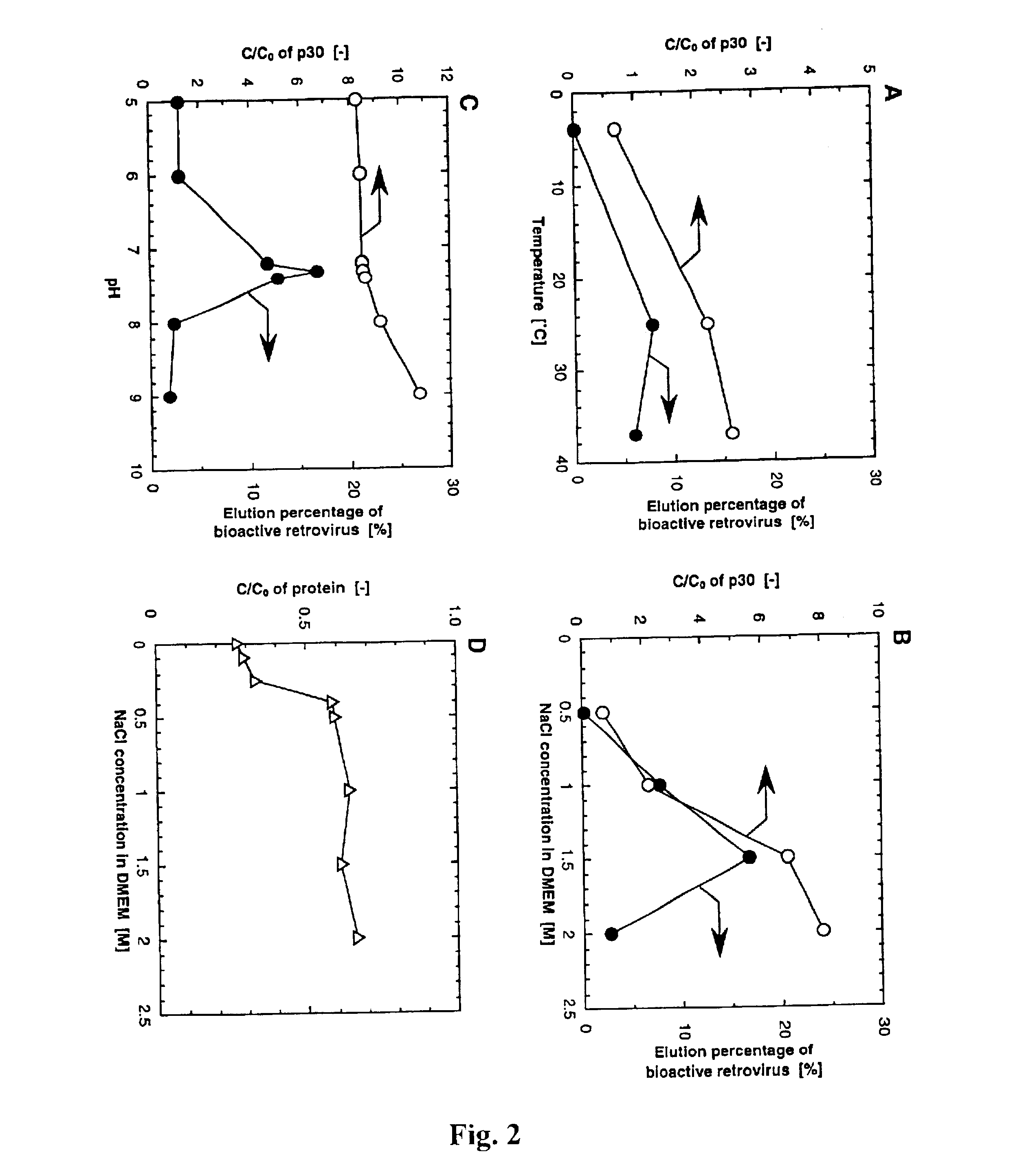
InactiveUS6861001B2Effect clotting timeEasy to zoom inBioreactor/fermenter combinationsBiological substance pretreatmentsSide chainBlood plasma
The invention is based on the discovery that certain membranes, which include side chains or molecular “brushes” having, for example, tertiary amino functional groups, can be used as highly effective filters to capture viruses / virus particles from liquids without removal of proteins. New methods based on this discovery include removing viruses from liquids such as blood or plasma, removing viruses from pharmaceuticals, concentrating and / or purifying viruses, e.g., for use in gene therapy, and producing recombinant viruses in new bioreactors. The invention also includes new methods of therapy or adjunct therapy for viral infections, in which a patient's blood or plasma is filtered through the membranes to remove viruses to reduce the viral load. The invention also includes new bioreactors and viral filters containing the membranes.
Owner:THE GENERAL HOSPITAL CORP
Prevention of myocarditis, abortion and intrauterine infection associated with porcine circovirus-2
InactiveUS7211379B2Peptide/protein ingredientsGenetic material ingredientsLegally induced abortionViral carditis
Provided is a method for reducing viral load of porcine circovirus type 2 (PCV-2) in a pig by inducing an immune response against PCV-2 through the administration of an immunogenic composition comprising a PCV-2 antigen. A preferred antigen is a vector containing a PCV-2 nucleotide sequence. In a particularly preferred embodiment, the PCV-2 nucleotide sequence is ORF4, ORF13, or ORF4 and ORF13. In some embodiments, the immunogenic composition includes one or more additional pig pathogens.
Owner:MERIAL SAS +1
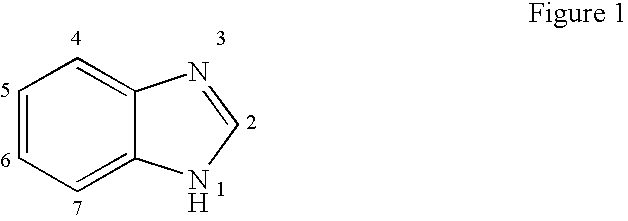
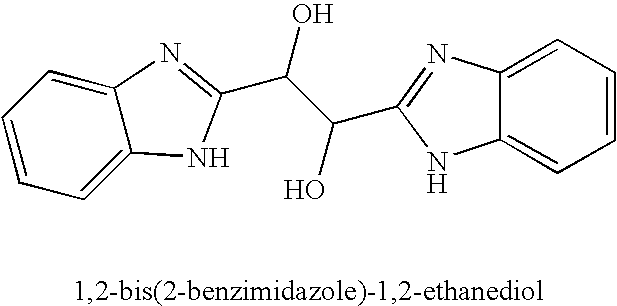
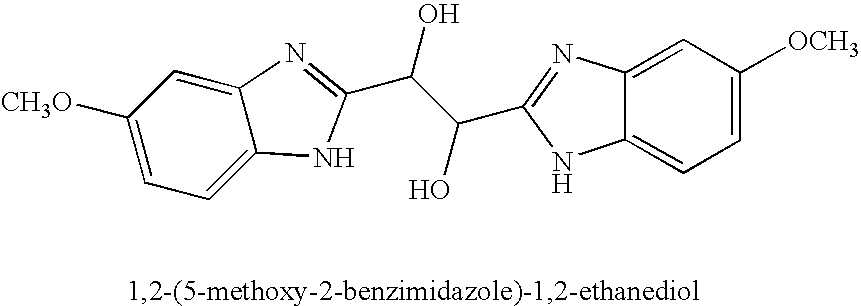
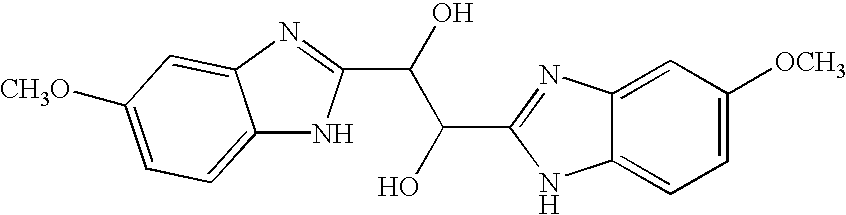
Hetero-substituted benzimidazole compounds and antiviral uses thereof
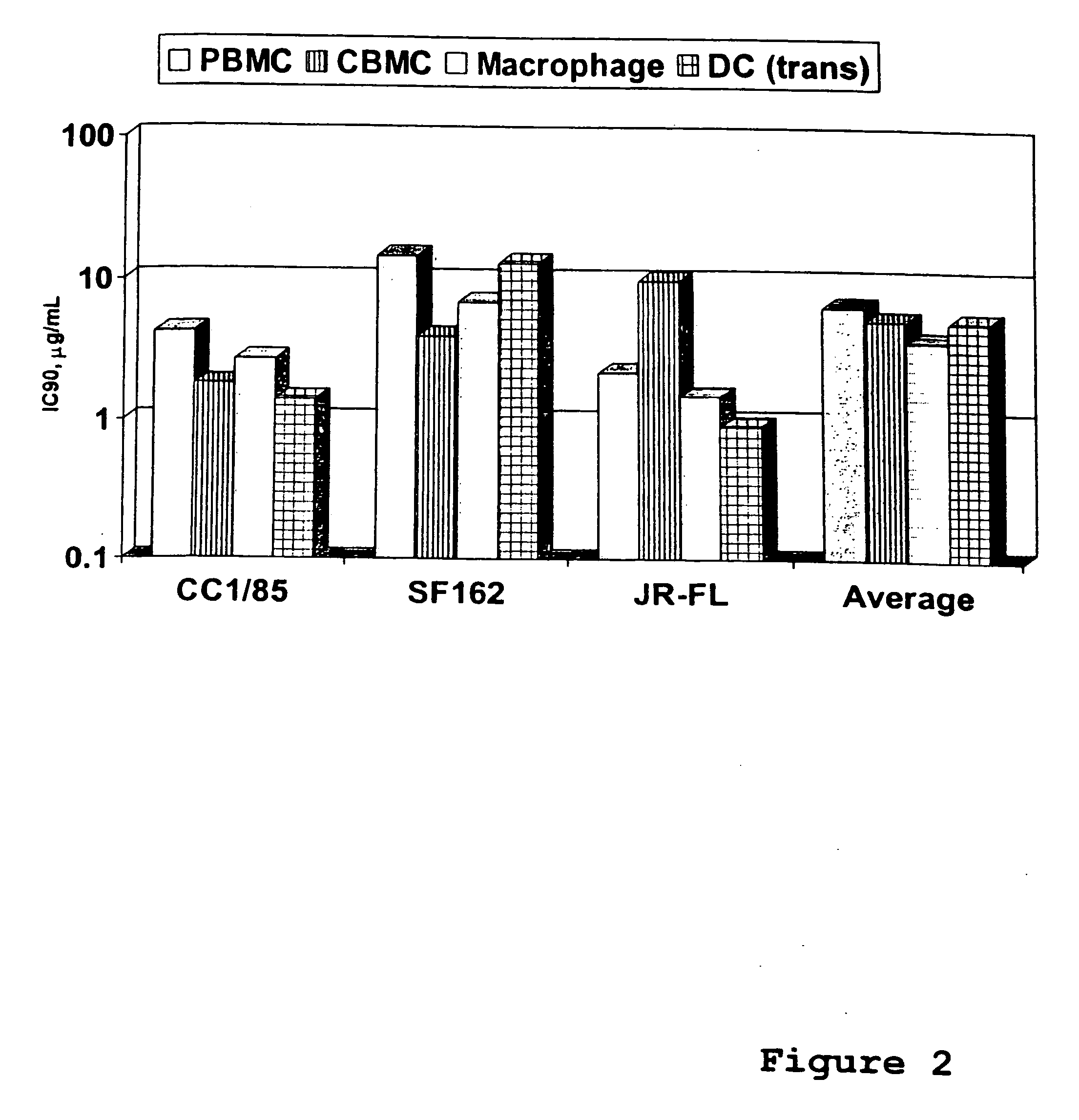
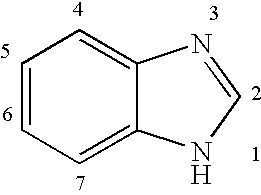
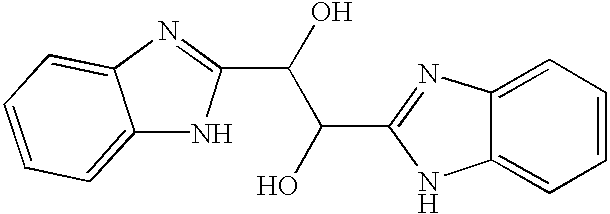
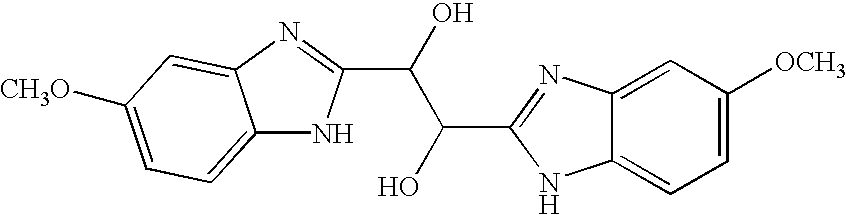
InactiveUS20040166137A1Many symptomReduce loadBiocideOrganic active ingredientsRespiratory infectionViral infection
The present invention relates to novel hetero-substituted benzimidazole compounds that have useful antiviral activity. More specifically, the invention encompasses hetero-substituted benzimidazole compounds that inhibit membrane fusion associated events such as viral transmission, reduce viral load or otherwise treat viral infections. The invention also encompasses the use of hetero-substituted benzimidazole compounds as inhibitors of membrane fusion associated events, such as viral transmission. In another embodiment, the invention encompasses processes for making hetero-substituted benzimidazole compounds, methods of using the hetero-substituted benzimidazole compounds and compositions comprising the hetero-substituted benzimidazole compounds. Finally, the invention provides methods for treating, preventing or ameliorating symptoms associated with respiratory infection, particularly that caused by Respiratory Syncytial Virus utilizing the novel benzimidazole compounds of the invention.
Owner:TRIMERIS
Dosing regimen for Flaviviridae therapy
An anti-hepatitis C agent which is an anti-metabolite to the host and cannot be administered on a daily or chronic basis as is usual in anti-viral therapy (referred to below as an “anti-HCV anti-metabolite”), can be administered using a traditional anti-cancer dosing regimen (for example via intravenous or parenteral injection), over a period of one, two, three, four, five, six, or seven days followed by cessation of therapy until rebound of the viral load is noted. This dosing regimen runs counter to conventional antiviral experience, wherein effective agents are usually administered over at least fourteen days of sustained therapy, and typically on an indefinite daily basis.
Owner:PHARMASSET
Recombinant SARS-CoV-2 vaccine using human replication-defective adenovirus as vector
ActiveCN111218459AReduce loadSimple manufacturing methodSsRNA viruses positive-senseViral antigen ingredientsProtective antigenCoronavirus vaccination
The invention provides a SARS-CoV-2 vaccine using human type-5 replication-defective adenovirus as a vector. The vaccine uses E1 and E3 to be combined with replication-defective human type-5 adenovirus as the vector and HEK293 cells integrating adenovirus E1 gene as a packaging cell line, and a protective antigen gene carried is the 2019 SARS-CoV-2 S protein gene (Ad5-nCoV) which is subjected to optimization design. After the S protein gene is optimized, the expression level in transfected cells is increased significantly. The vaccine has good immunogenicity in mouse and guinea pig models, andcan induce a body to produce a strong cellular and humoral immune response in a short time. Studies on the protective effect of hACE2 transgenic mice show that after 14 days of single immunization ofAd5-nCoV, the viral load in lung tissue can be significantly reduced, and it is indicated that the vaccine has a good immunoprotective effect on the 2019 SARS-CoV-2. In addition, the vaccine is quick, simple and convenient to prepare, and can be mass-produced in a short period of time to respond to sudden outbreaks.
Owner:ACADEMY OF MILITARY MEDICAL SCI +1
Method for removal of viruses from blood by lectin affinity hemodialysis
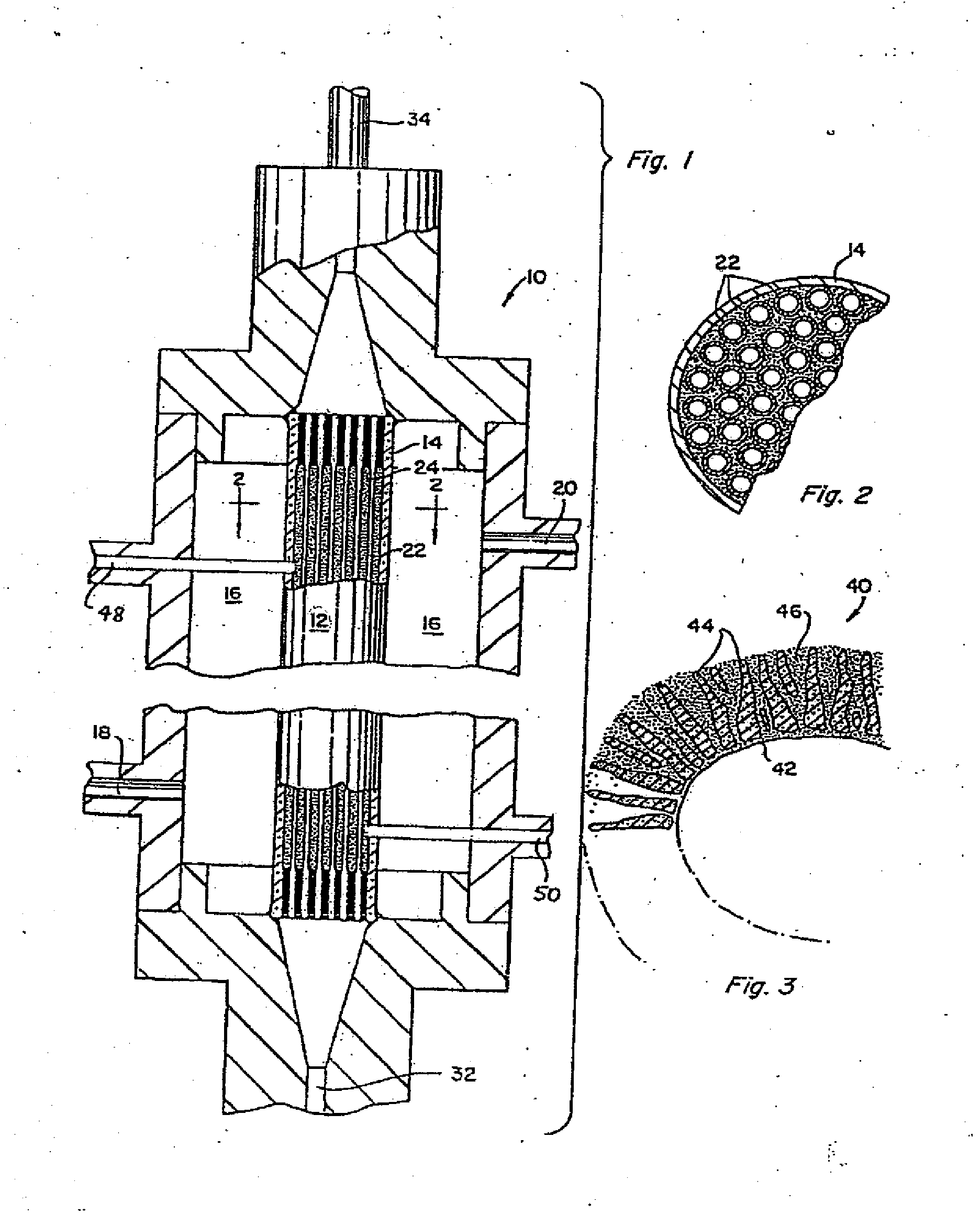
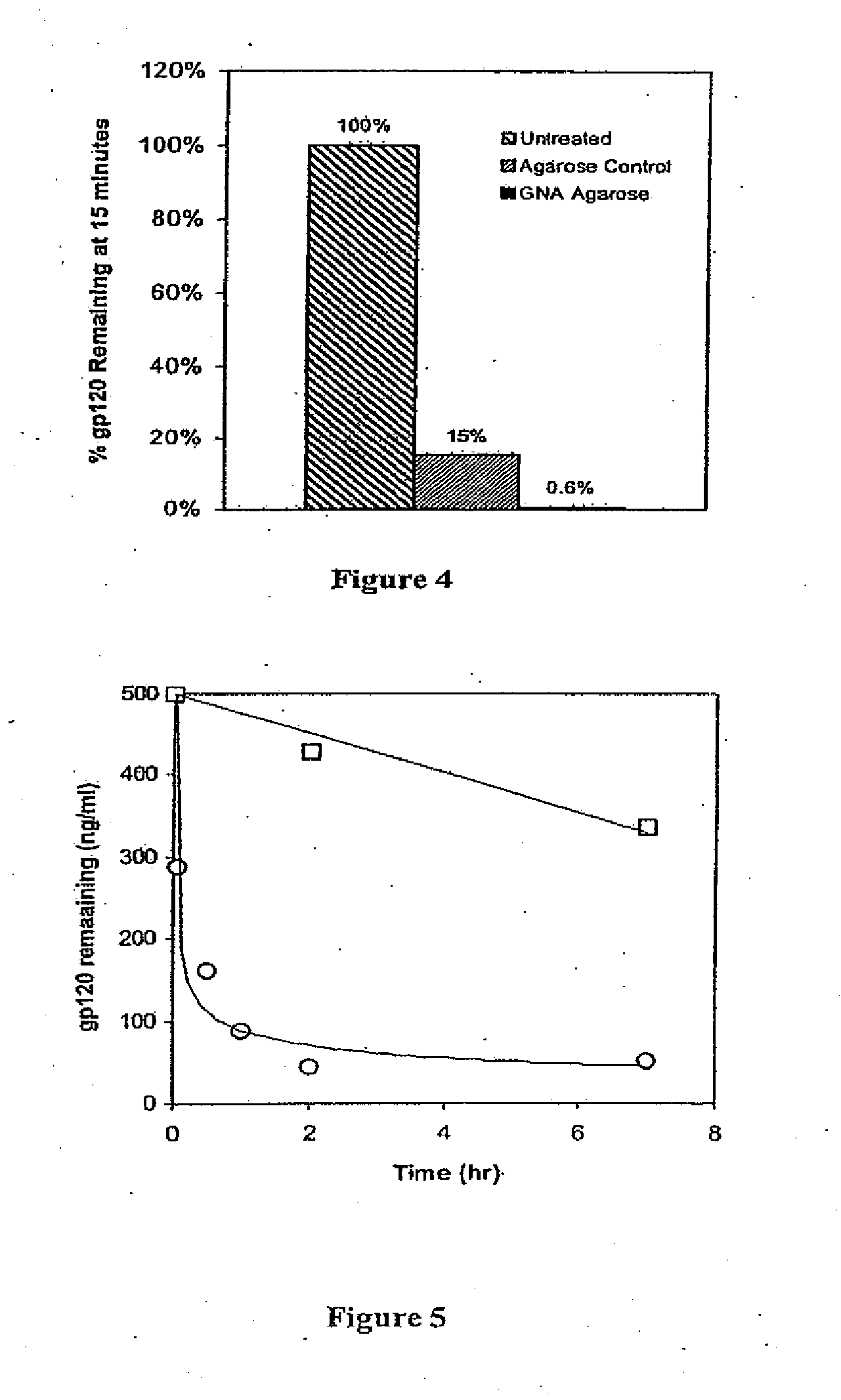
ActiveUS7226429B2Reduce loadReduce in quantityOther blood circulation devicesViral antigen ingredientsHemodialysisHigh mannose
The present invention relates to a method for using lectins that bind to pathogens having high mannose surface glycoproteins or fragments thereof which contain high mannose glycoproteins, to remove them from infected blood or plasma in an extracorporeal setting. Accordingly, the present invention provides a method for reducing viral load in an individual comprising the steps of obtaining blood or plasma from the individual, passing the blood or plasma through a porous hollow fiber membrane wherein lectin molecules are immobilized within the porous exterior portion of the membrane, collecting pass-through blood or plasma and reinfusing the pass-through blood or plasma into the individual.
Owner:AETHLON MEDICAL INC
Viricidal and microbicidal compositions and uses thereof
InactiveUS20120121679A1Reduced viabilityEfficient reductionAntibacterial agentsBiocideLevulinic acidActive agent
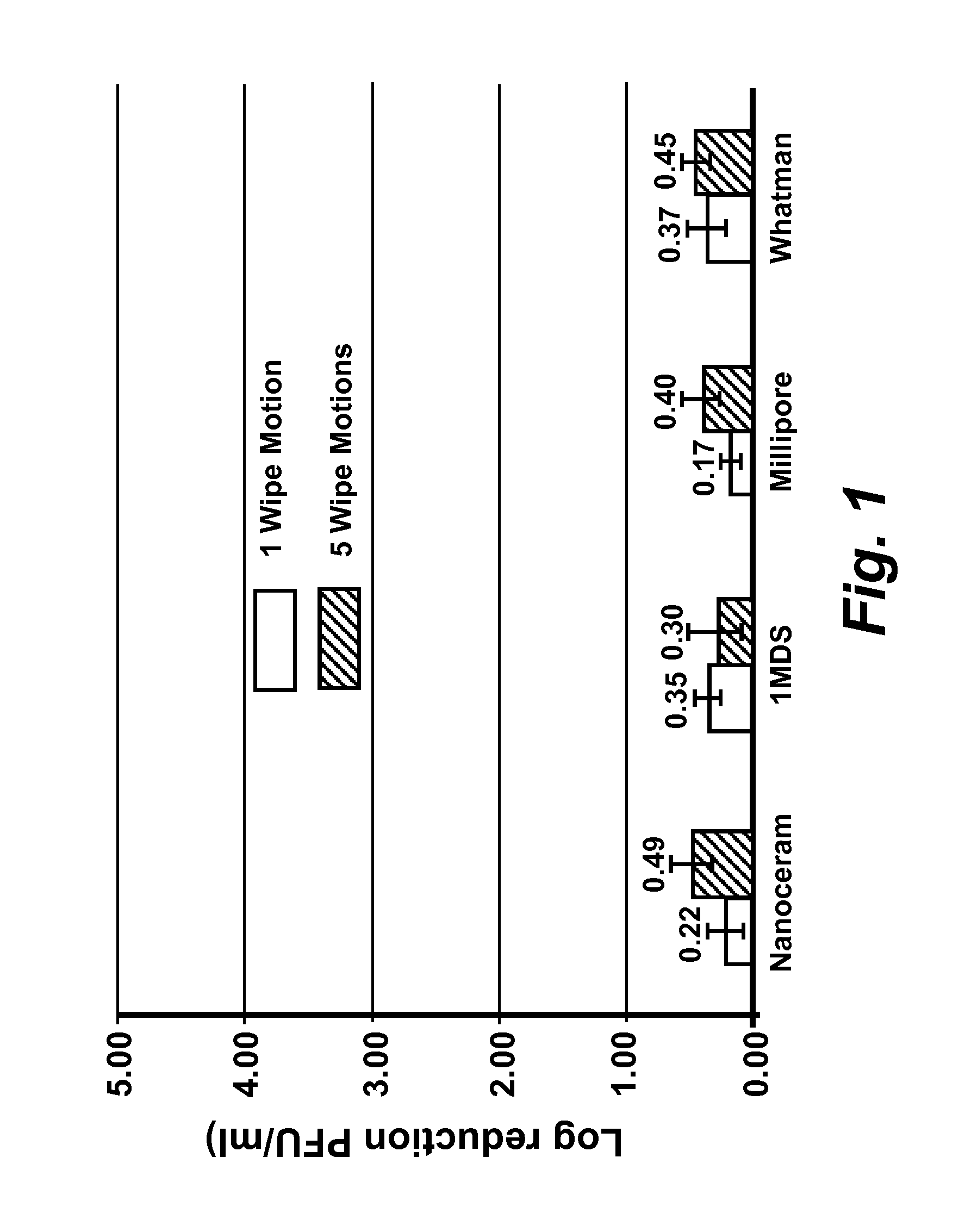
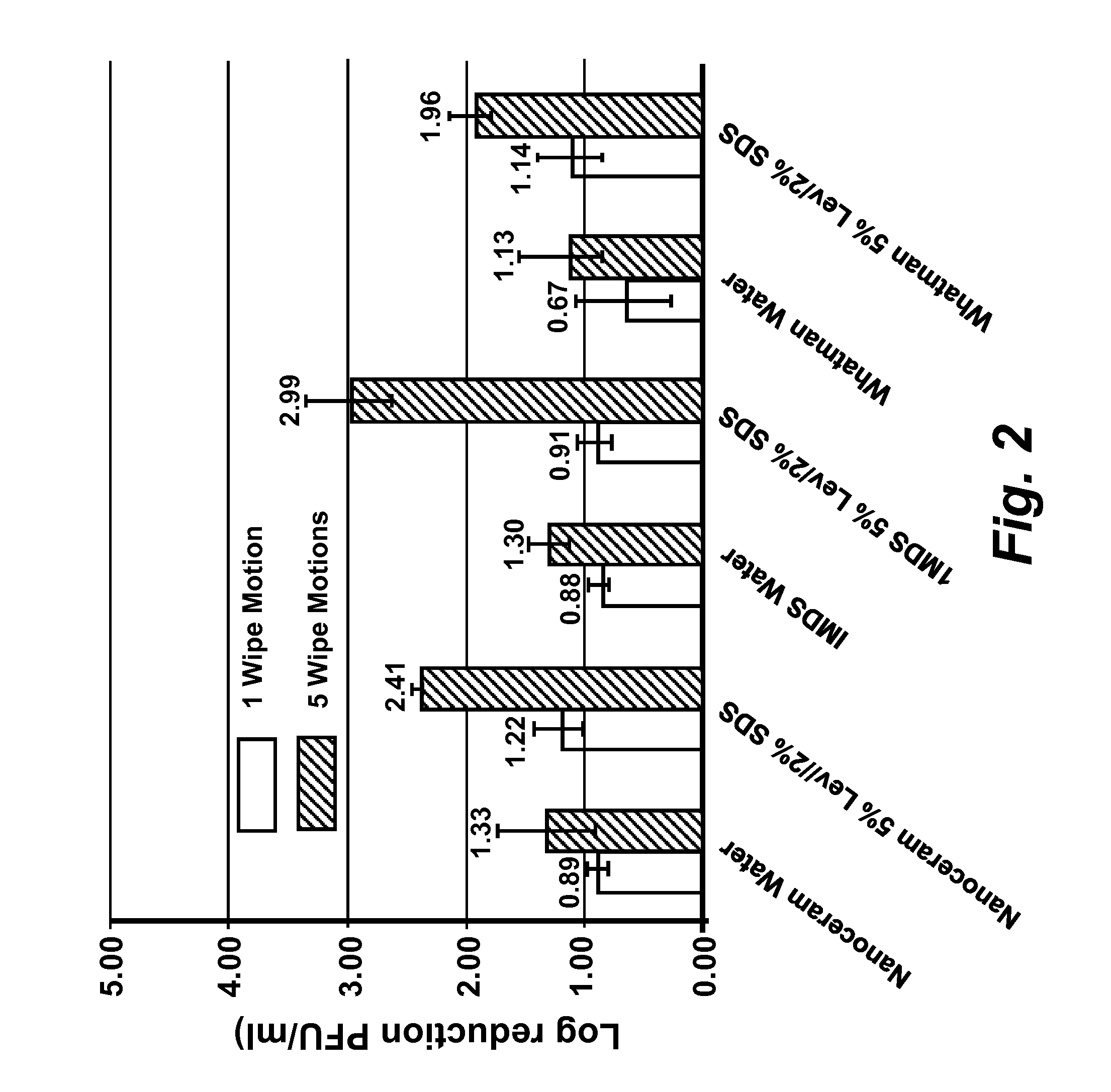
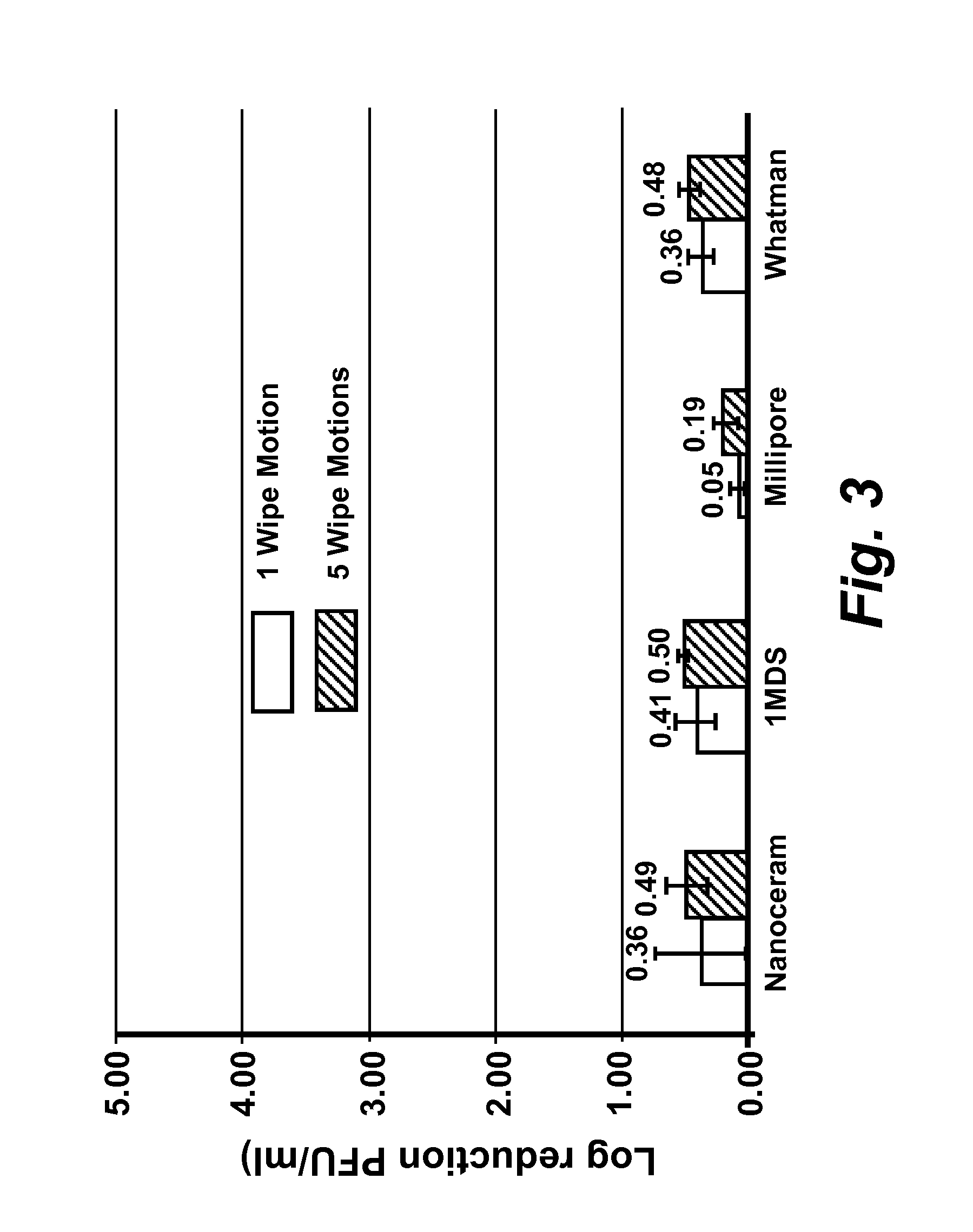
The present disclosure encompasses compositions comprising a surfactant, and an acid such as, but not limited to, levulinic acid, that together have a synergistic effect in reducing the viability of a virus population compared to the efficacy of the individual compounds. This synergy allows the formulation of compositions where the active agents (including an acid and a surfactant) are present at concentrations effective to inactivate viruses on surfaces, including human skin. The viricidal compositions disclosed herein are efficacious without damaging the surface to which they may be applied, or even altering the organoleptic properties of a treated food substance. The viricidal compositions and wipes containing such compositions are suitable for sanitizing any surface suspected of having a viral load thereon, or where it is desirable to ensure that a viral load is as low as possible.
Owner:UNIV OF GEORGIA RES FOUND INC
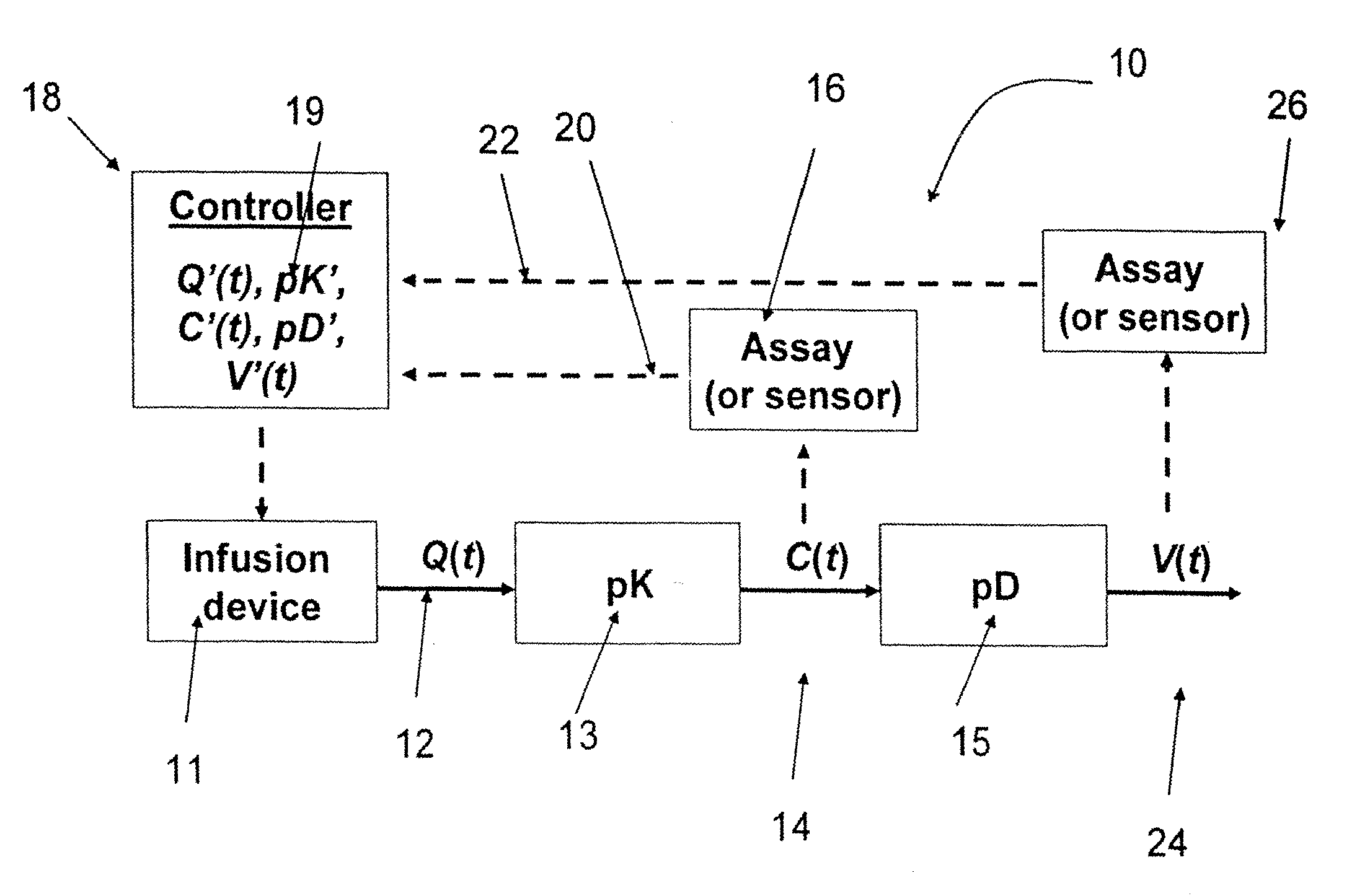
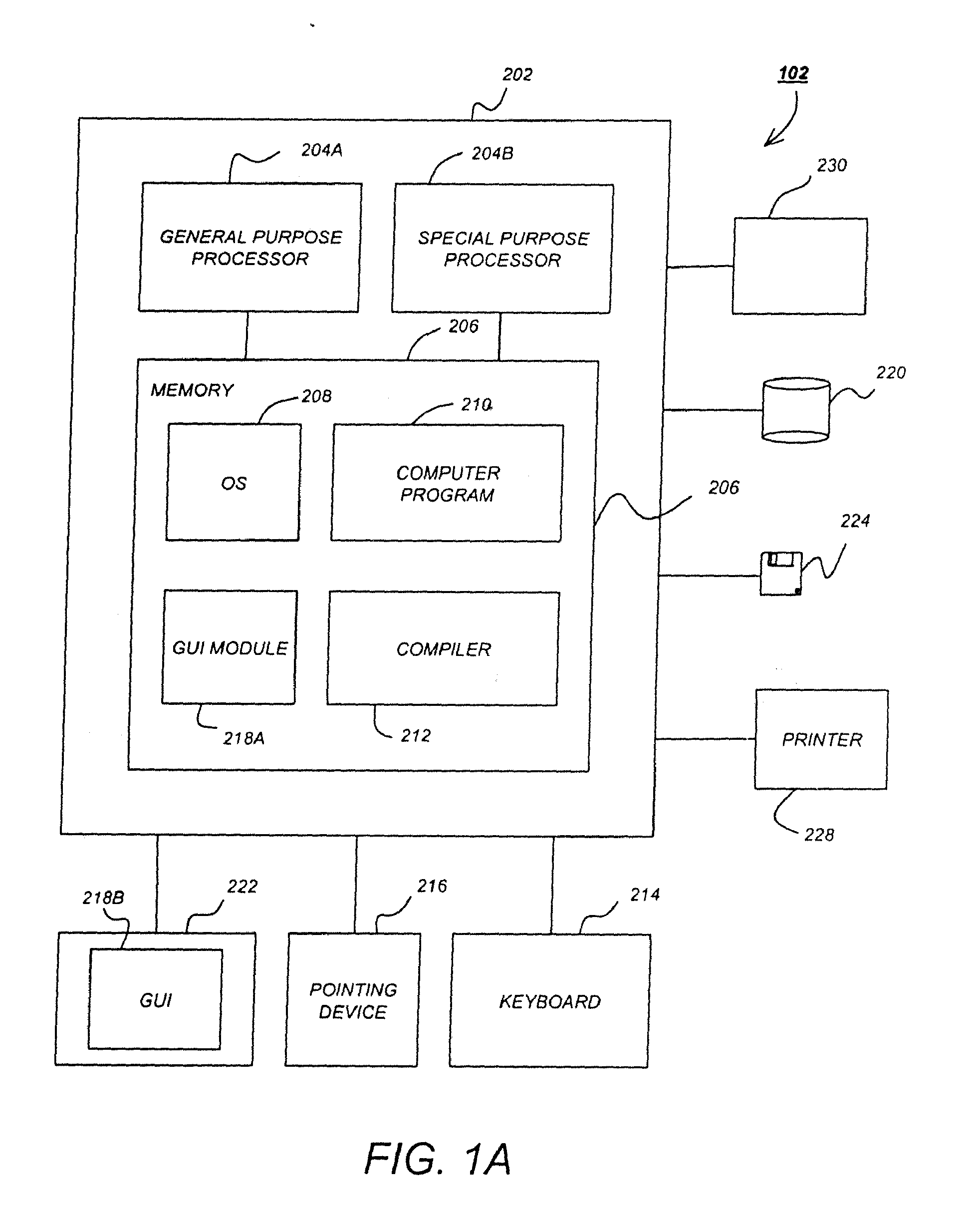
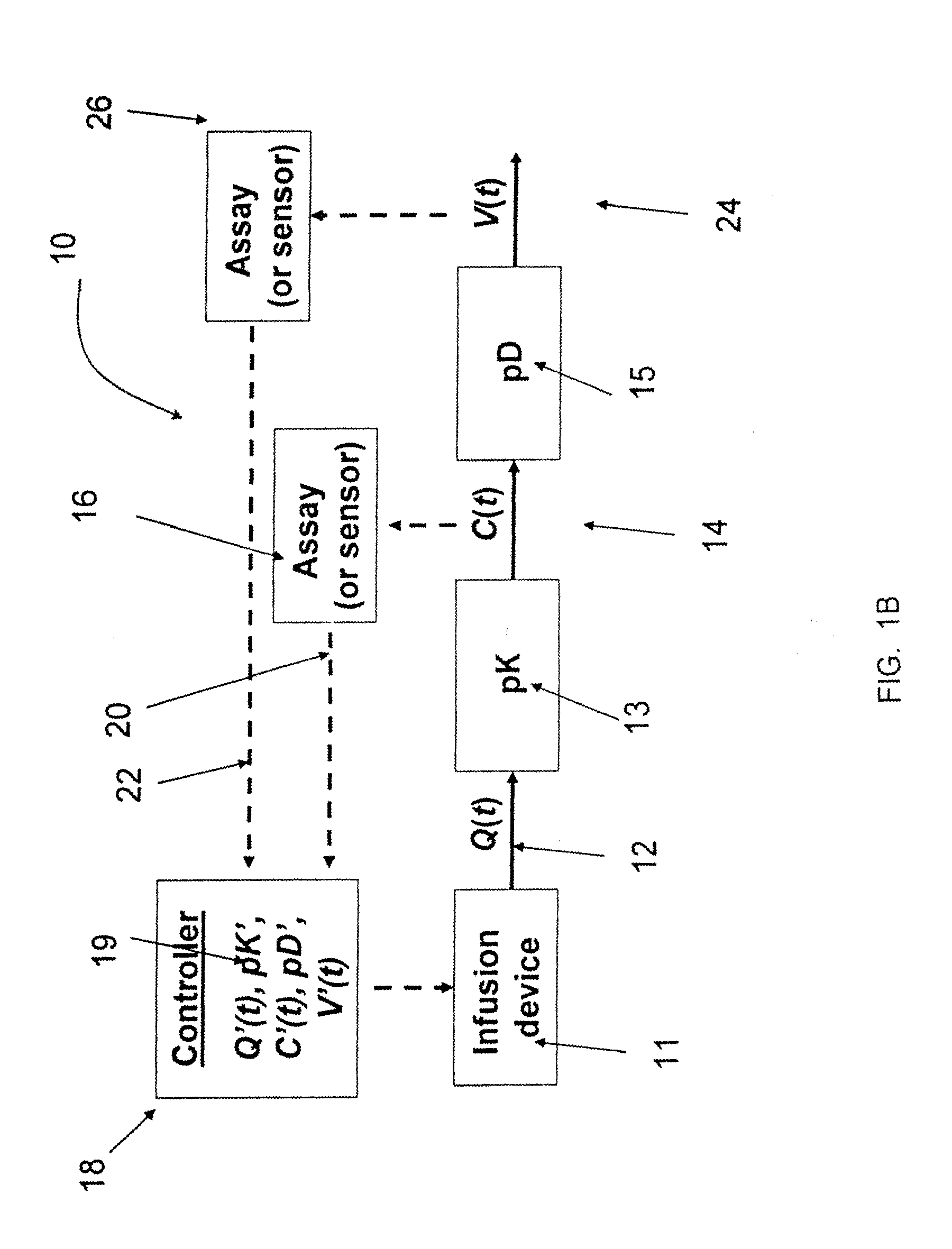
Method and system to define patient specific therapeutic regimens by means of pharmacokinetic and pharmacodynamic tools
Methods for treating Hepatitis infections are provided. In one embodiment, an initial dosage of interferon is administered to a patient, and interferon serum levels and viral load data is collected over time. This data can be used to determine patient-specific pharmacokinetic and pharmacodynamic parameters and then construct patient-specific interferon delivery profiles. Patient-specific delivery profiles can then be used to design patient-specific therapeutic regimens.
Owner:MEDTRONIC INC
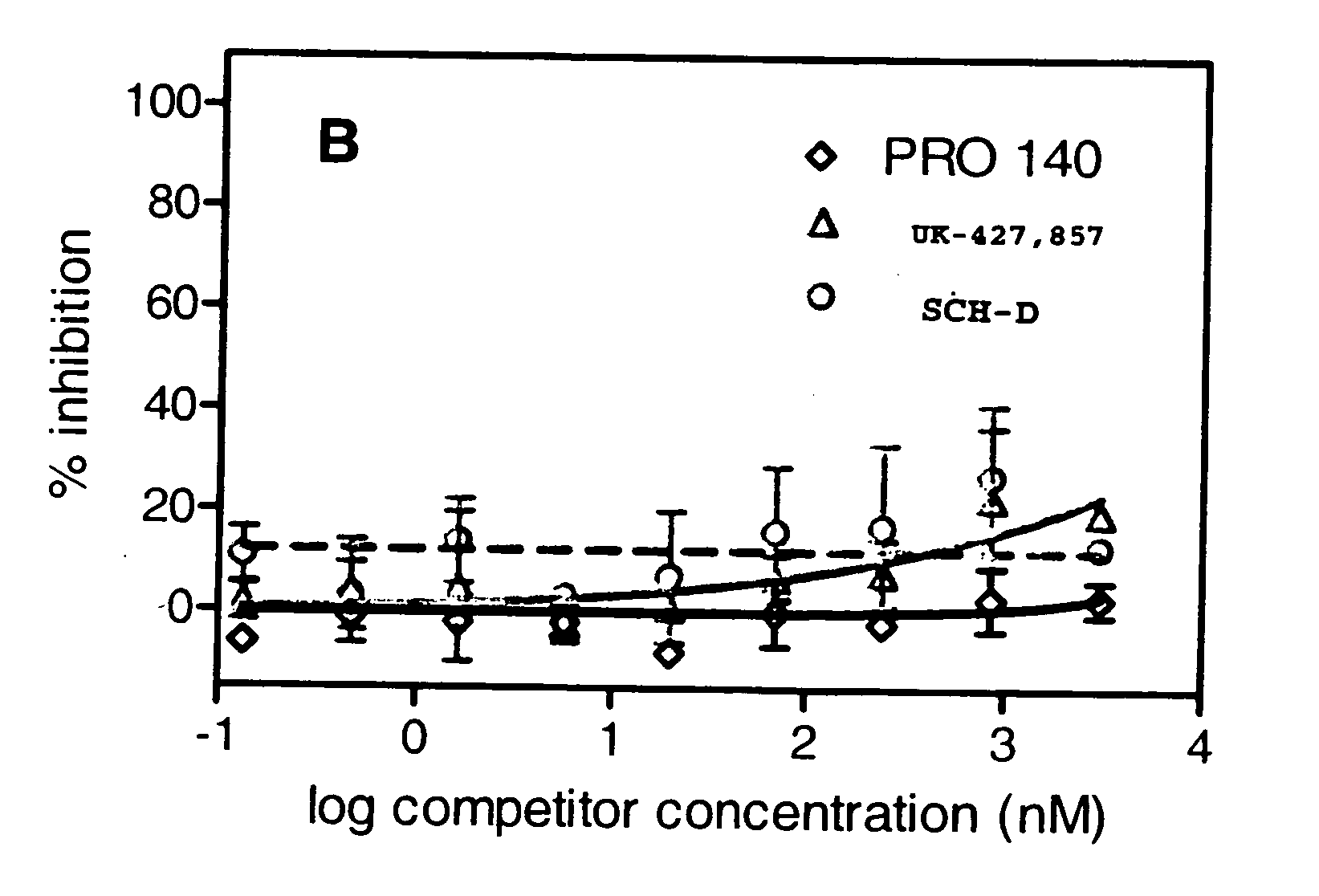
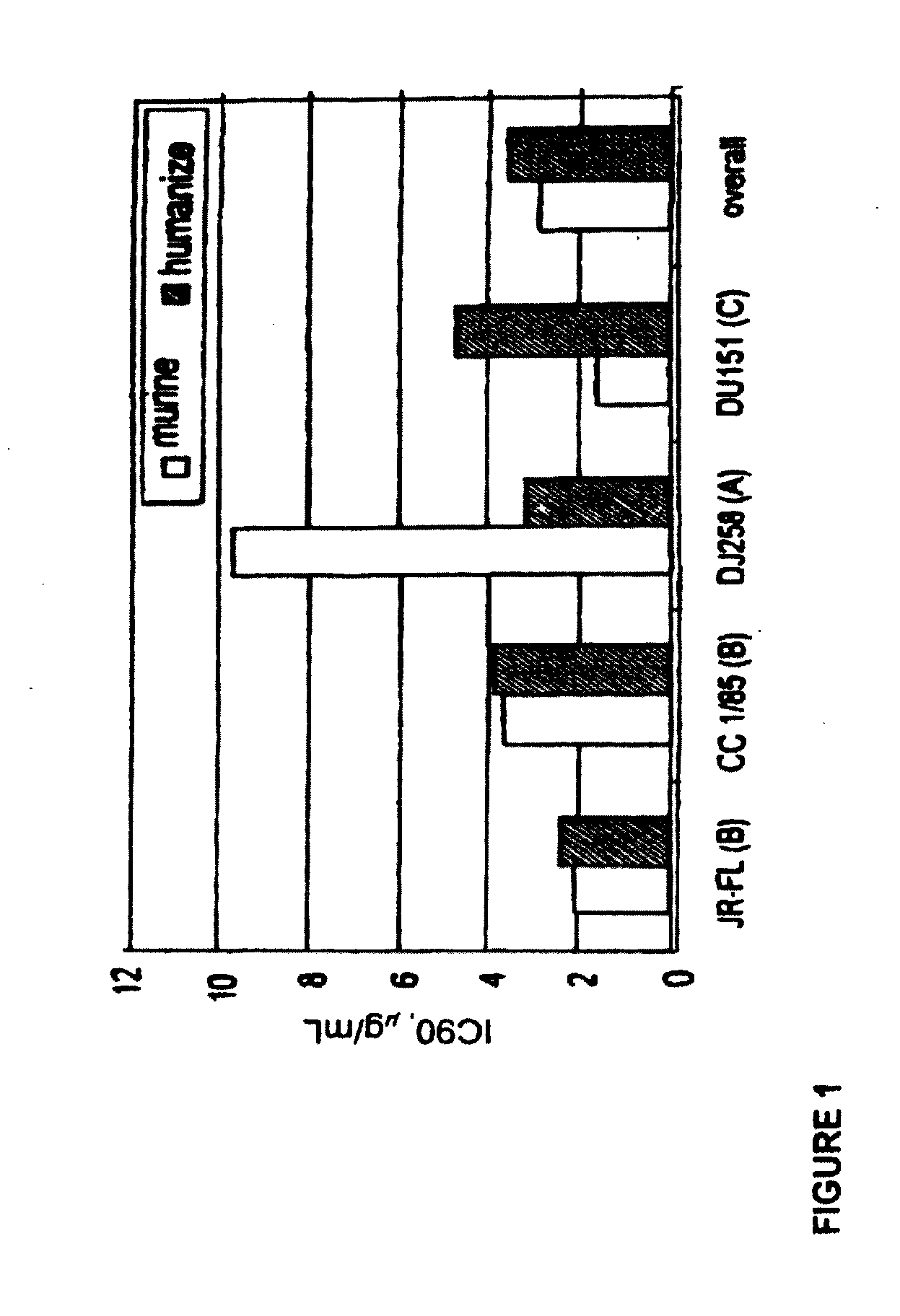
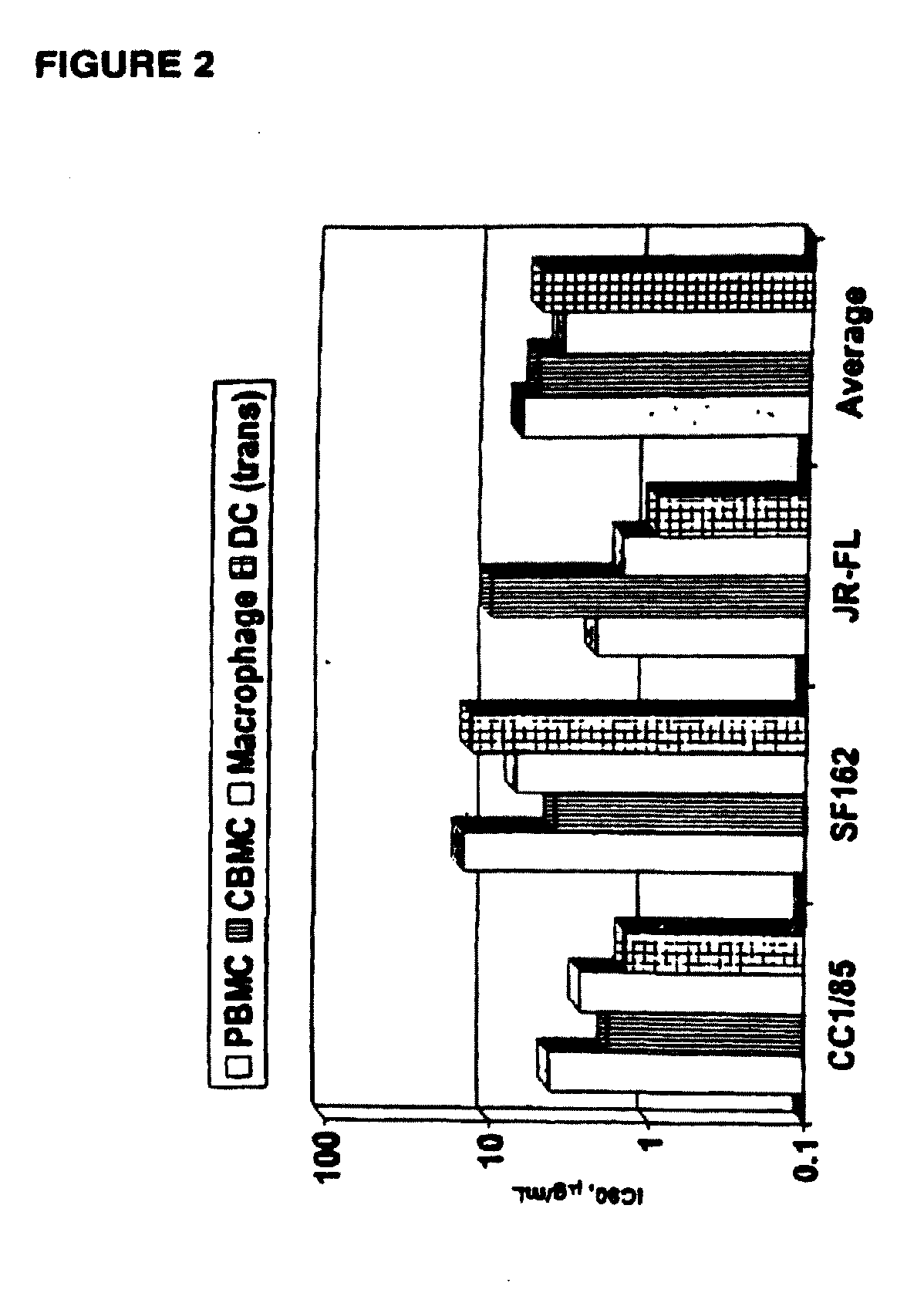
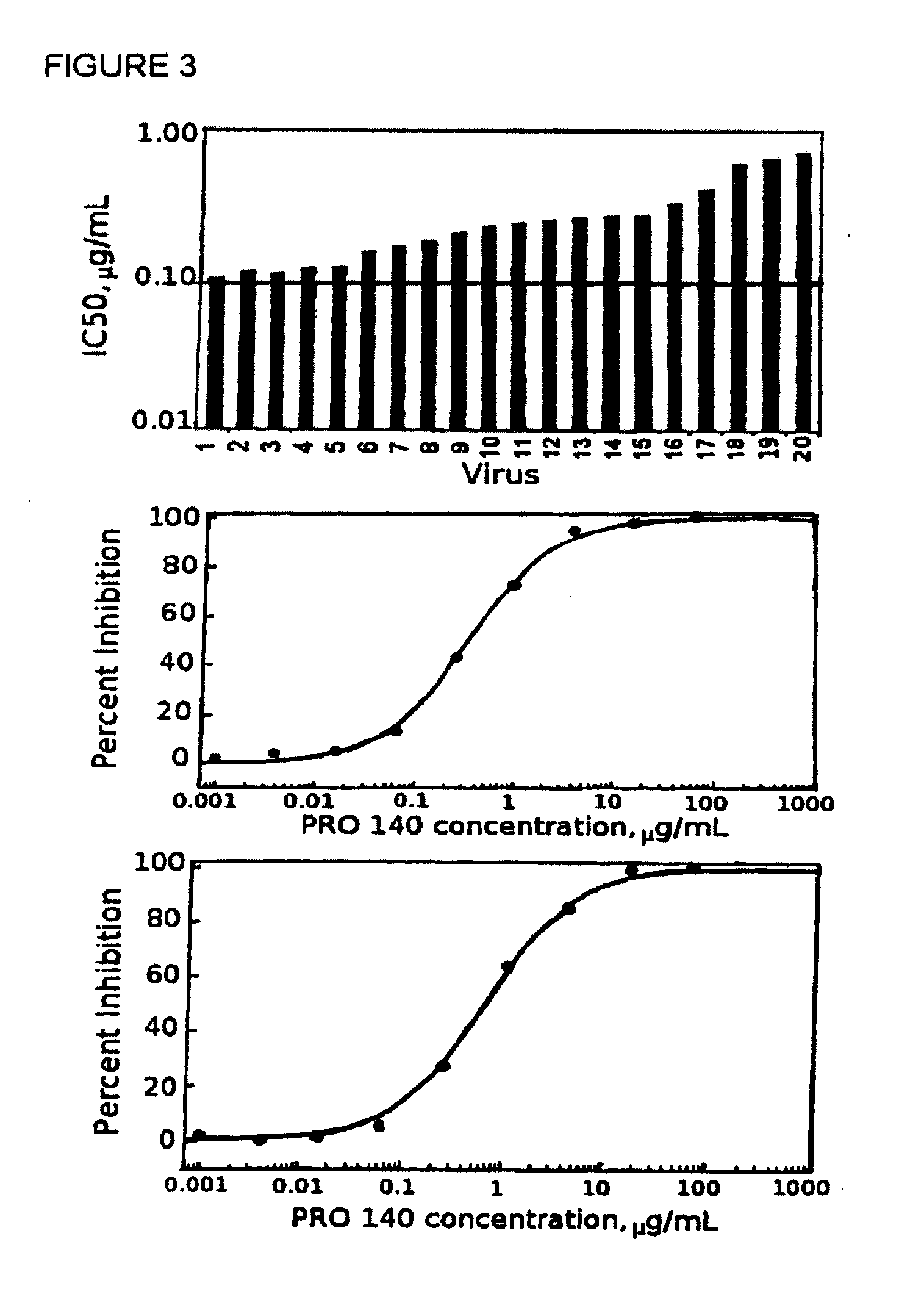
Methods for reducing viral load in HIV-1-infected patients
InactiveUS20070026441A1Reduce the possibilityStrong inhibitory activityPeptide/protein ingredientsMicrobiological testing/measurementReduced doseHIV receptor
This method provides a method for reducing HIV-1 viral load in an HIV-1-infected human subject which comprises administering to the subject at a predefined interval effective HIV-1 viral load-reducing doses of (a) a humanized antibody designated PRO 140, or of (b) an anti-CCR5 receptor monoclonal antibody. This invention also provides a method for inhibiting in a human subject the onset or progression of an HIV-1-associated disorder, the inhibition of which is effected by inhibiting fusion of HIV-1 to CCR5+CD4+ target cells in the subject. This invention also provides a method for treating a subject infected with HIV-1 comprising administering to the subject (a) a monoclonal antibody which (i) binds to a CCR5 receptor on the surface of the subject's CD4+ cells and (ii) inhibits fusion of HIV-1 to the subject's CCR5+CD4+ cells, and (b) a non-antibody CCR5 receptor antagonist, in amounts effective to treat the subject.
Owner:PROGENICS PHARMA INC
Compositions for the treatment of hepatitis C and methods for using compositions for the treatment of hepatitis C
InactiveUS20060229293A1Treating hepatitis CImprove the level ofBiocideCarbohydrate active ingredientsMetaboliteChronic hepatitis
The present invention pertains to a composition comprising Ibogaine, an indole alkaloid, its active salts and its principal metabolite noribogaine, a demethylated form of ibogaine, for the treatment of hepatitis C and hepatitis C related complications, administered in single or multiple dose regimens effective to reduce somatic complaints, liver enzyme values and viral load caused by chronic hepatitis C in patients, and methods of using the same.
Owner:ADDICTION RES INST INC
Benzimidazole compounds and antiviral uses thereof
InactiveUS20030119754A1Reducing viral titer viralReduce viral loadBiocideSugar derivativesRespiratory infectionViral infection
Owner:TRIMERIS
Matrix and System for Preserving Biological Specimens for Qualitative and Quantitative Analysis
InactiveUS20140038172A1Accurate and reproducible quantificationSafe and convenient and simple deviceBioreactor/fermenter combinationsBiological substance pretreatmentsTarget analysisPolyolefin
The present invention provides a device, system, and methods of use comprising an absorbent hydrophobic polyolefin matrix, and methods of use thereof, for storage, preserving, and recovering liquid suspension of biological specimens containing analytes of interest in a dry state. The dried biological specimens containing analytes of interest absorbed on the polyolefin matrix are reconstituted such as with molecular-grade water and released by compressing the polyolefin matrix. The reconstituted biological analytes are qualified for subsequent analysis, such as for qualitative and quantitative analysis of viral nucleic acids, such a viral load testing, genotyping, and sequencing. Also provided are kits with instructions, and methods of use thereof, for storage, preserving, and recovering biological specimens containing analytes of interest using the compression device of the invention.
Owner:VIVEBIO
Detection, Capture and Quantification of Biological Moieties from Unprocessed Bodily Fluids Using Nanoplasmonic Platform
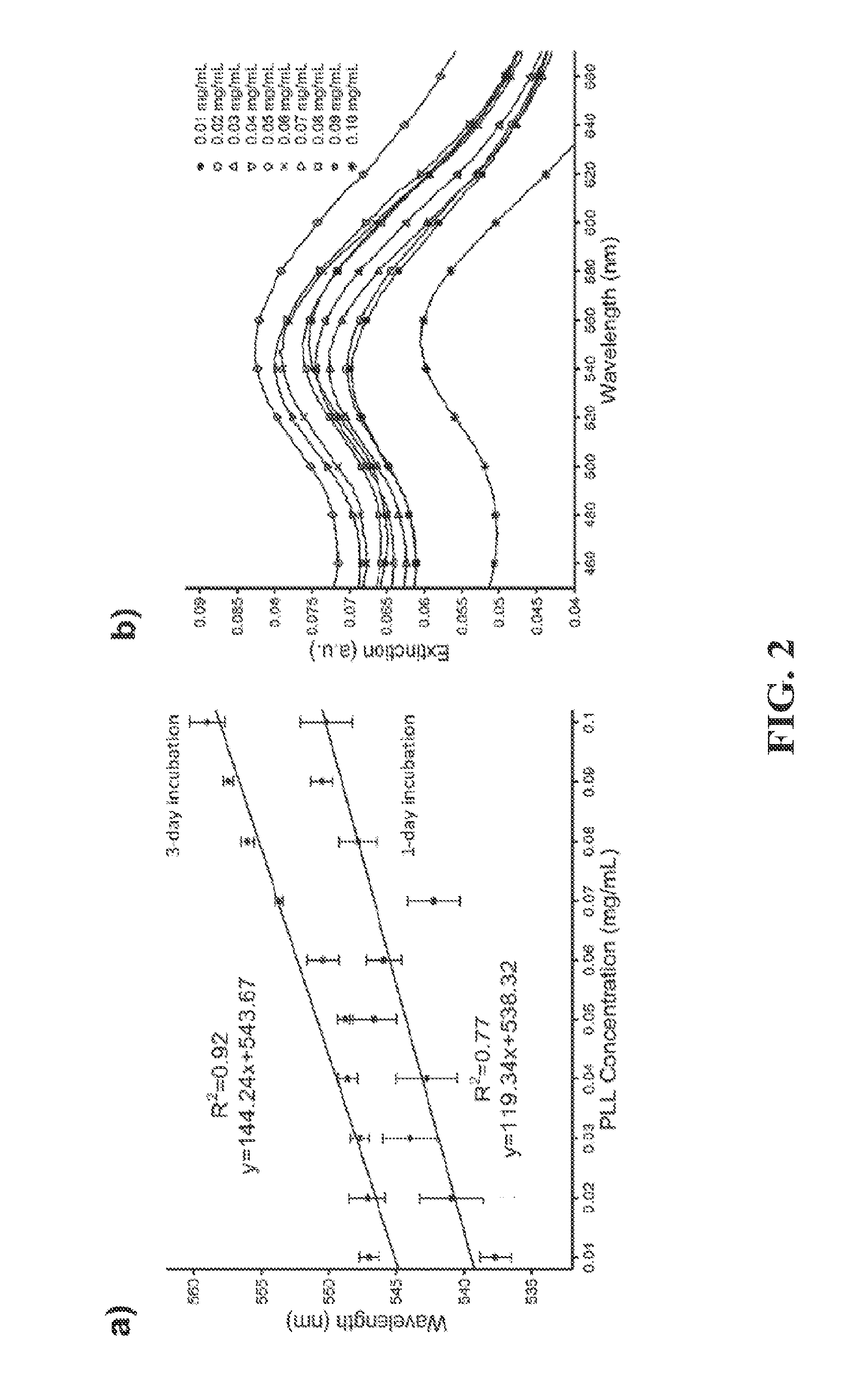
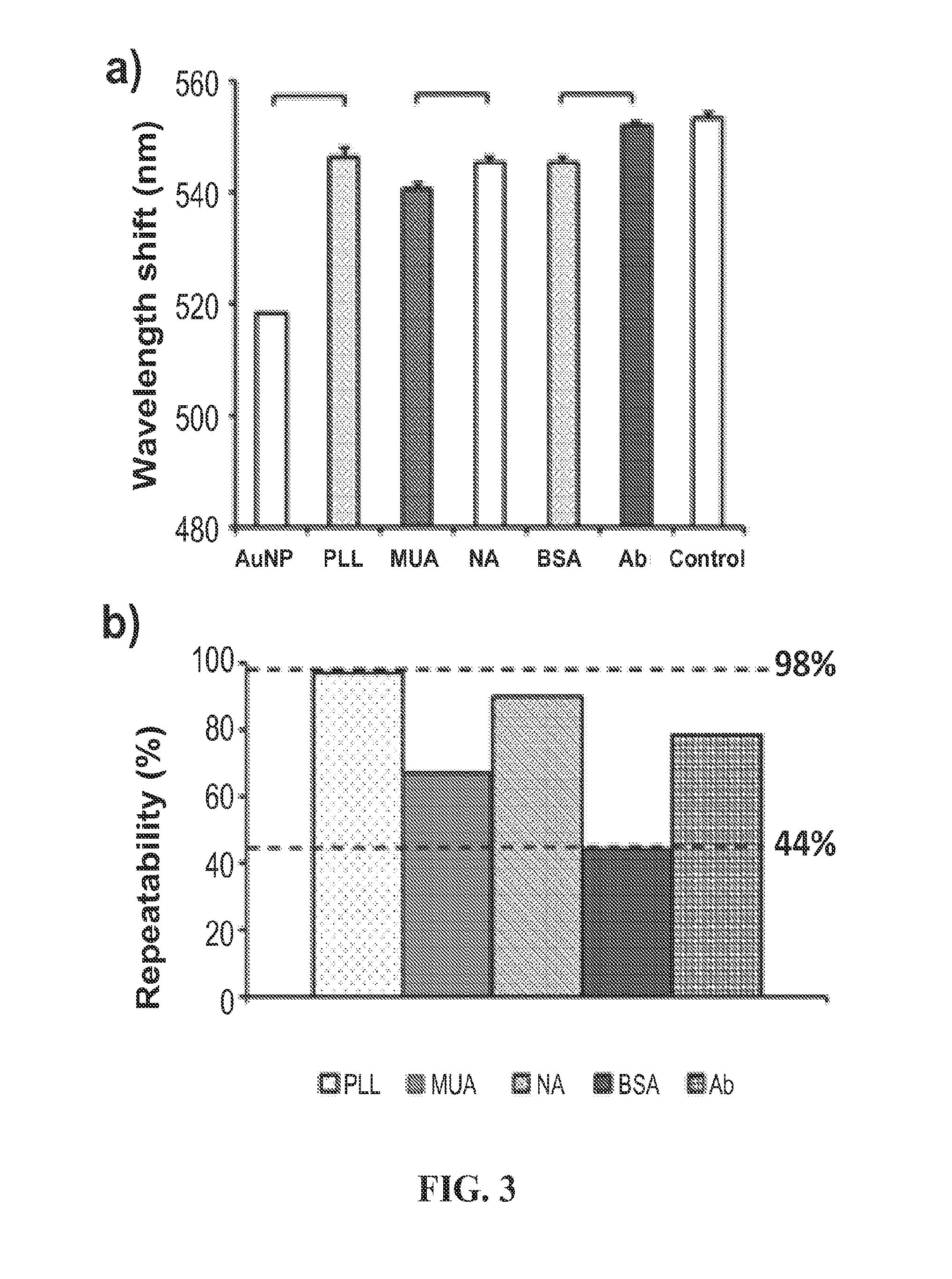
ActiveUS20150160218A1Good repeatabilityShorten the timeMicrobiological testing/measurementMaterial analysis by optical meansPoint of careBiology
A nanoplasmonic platform can be used for the detection and quantification of multiple HIV subtypes in whole blood with localized surface plasmon resonance. Among other things, this nanoplasmonic platform provides a viable way to detection and quantification of viral load at a point of care with significantly less cost, time, and laboratory resources than existing methods of detection. Although an example of HIV detection in whole blood is provided, the nanoplasmonic platform is adaptable to detect other pathogens and infectious agents or macromolecules.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Benzimidazole compounds and antiviral uses thereof
The present invention relates to novel benzimidazole compounds that have useful antiviral activity. More specifically, the invention encompasses benzimidazole compounds that inhibit membrane fusion associated events such as viral transmission, reduce viral load or otherwise treat viral infections. The invention also encompasses the use of benzimidazole compounds as inhibitors of membrane fusion associated events, such as viral transmission. In another embodiment, the invention encompasses processes for making benzimidazole compounds, methods of using the benzimidazole compounds and compositions comprising the benzimidazole compounds. Finally, the invention provides methods for treating, preventing or ameliorating symptoms associated with respiratory infection, particularly that caused by Respiratory Syncytial Virus utilizing the novel benzimidazole compounds of the invention.
Owner:TRIMERIS
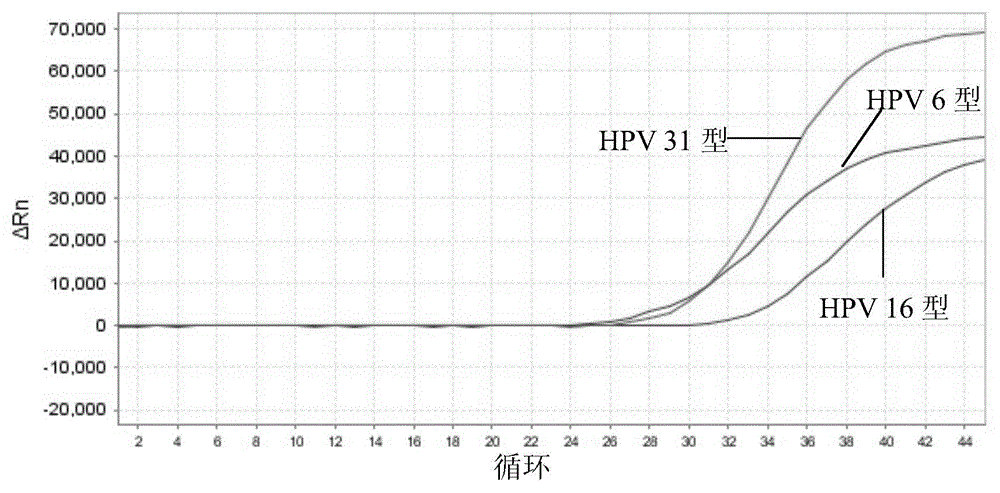
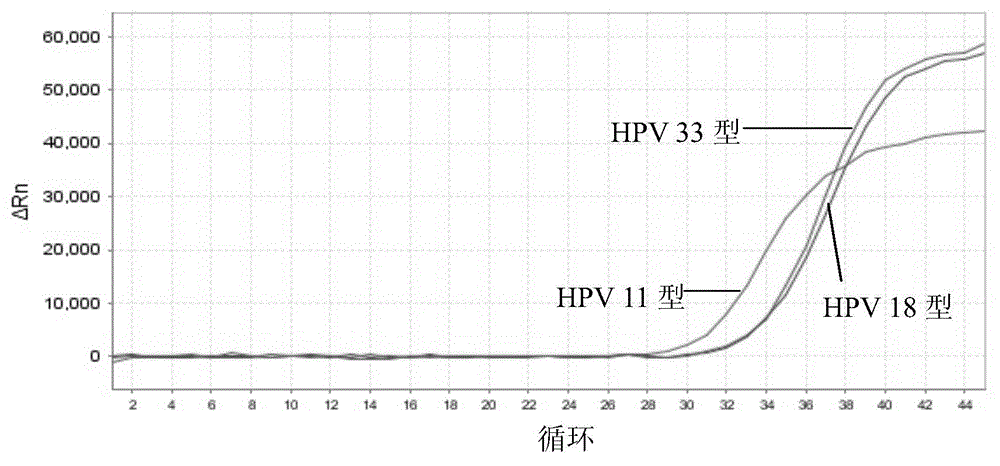
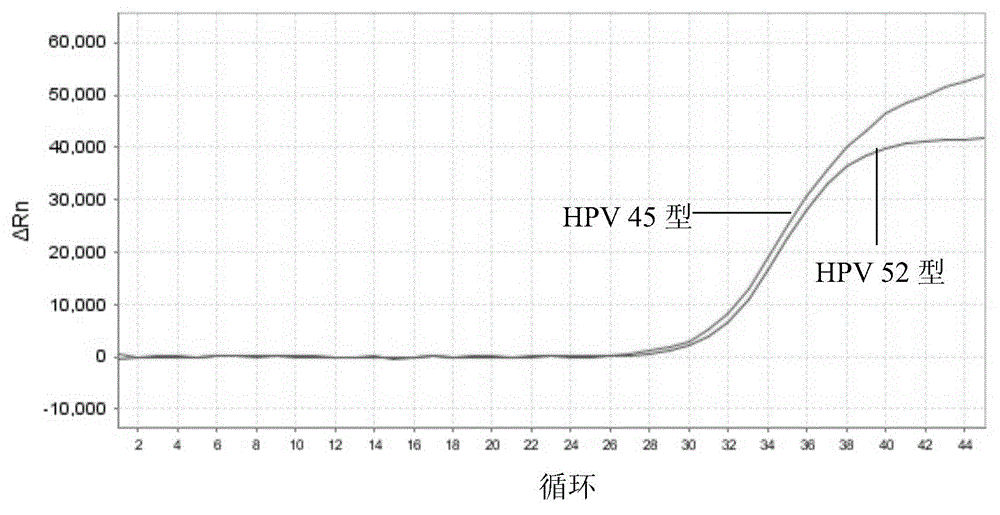
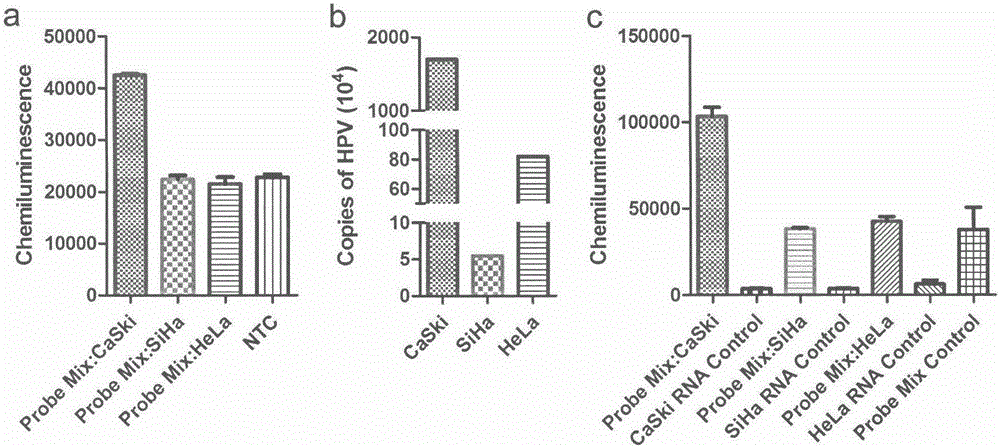
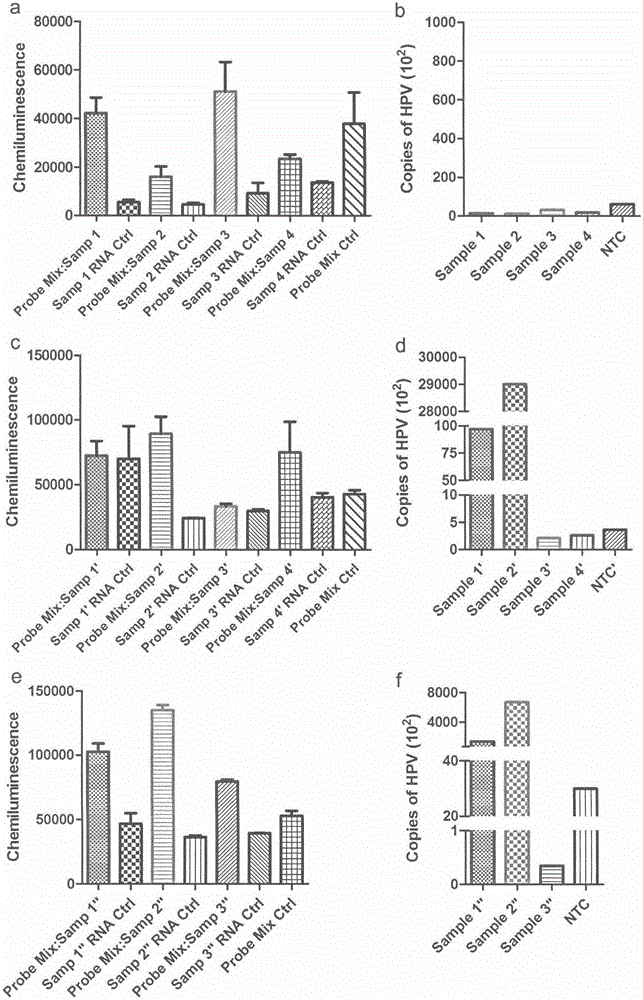
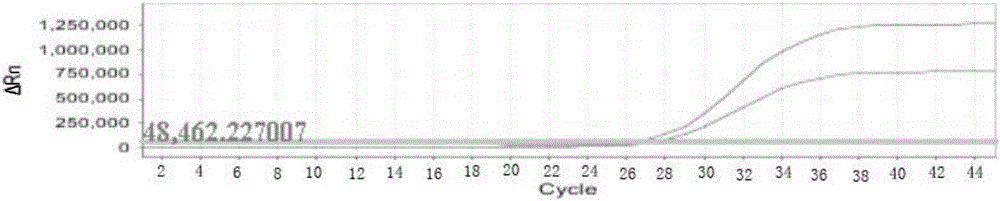
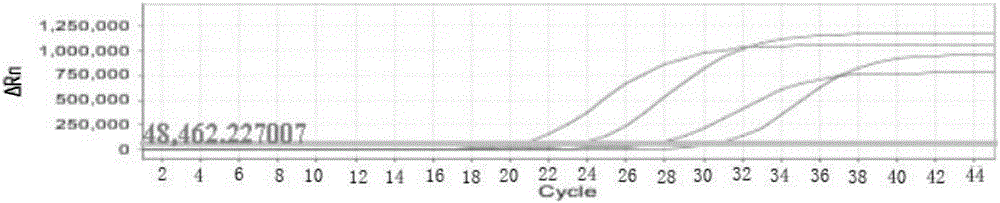
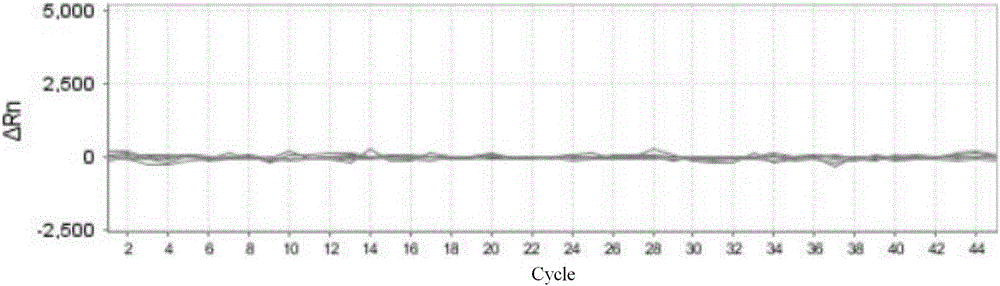
Nucleic acid detection kit for human papilloma virus, use method and application thereof
ActiveCN105506173AAvoid false negativesIncrease throughputMicrobiological testing/measurementMicroorganism based processesLower riskFluorescence
The invention discloses a nucleic acid detection kit for human papilloma virus, a use method and an application thereof. The kit includes a nucleic acid amplification reagent comprising a primer pair and a probe corresponding to the primer pair. The use method of the kit includes the following steps: 1) extracting nucleic acids from a sample; 2) preparing the reagent; 3) performing PCR amplification; and 4) performing fluorescent detection to a PCR amplification reaction product at 65-72 DEG C, and determining infection type of the HPV according to the changes on the Ct value of a target amplification curve and the Ct value of an internal standard amplification curve. The kit can be used for detecting low-risk and high-risk human papilloma virus and for typing the human papilloma virus. The kit can be used for calculating relative viral load of the HPV, can avoid pollution, can increase treatment throughput of samples, and can avoid missing detection.
Owner:SUZHOU SYM BIO LIFESCI CO LTD
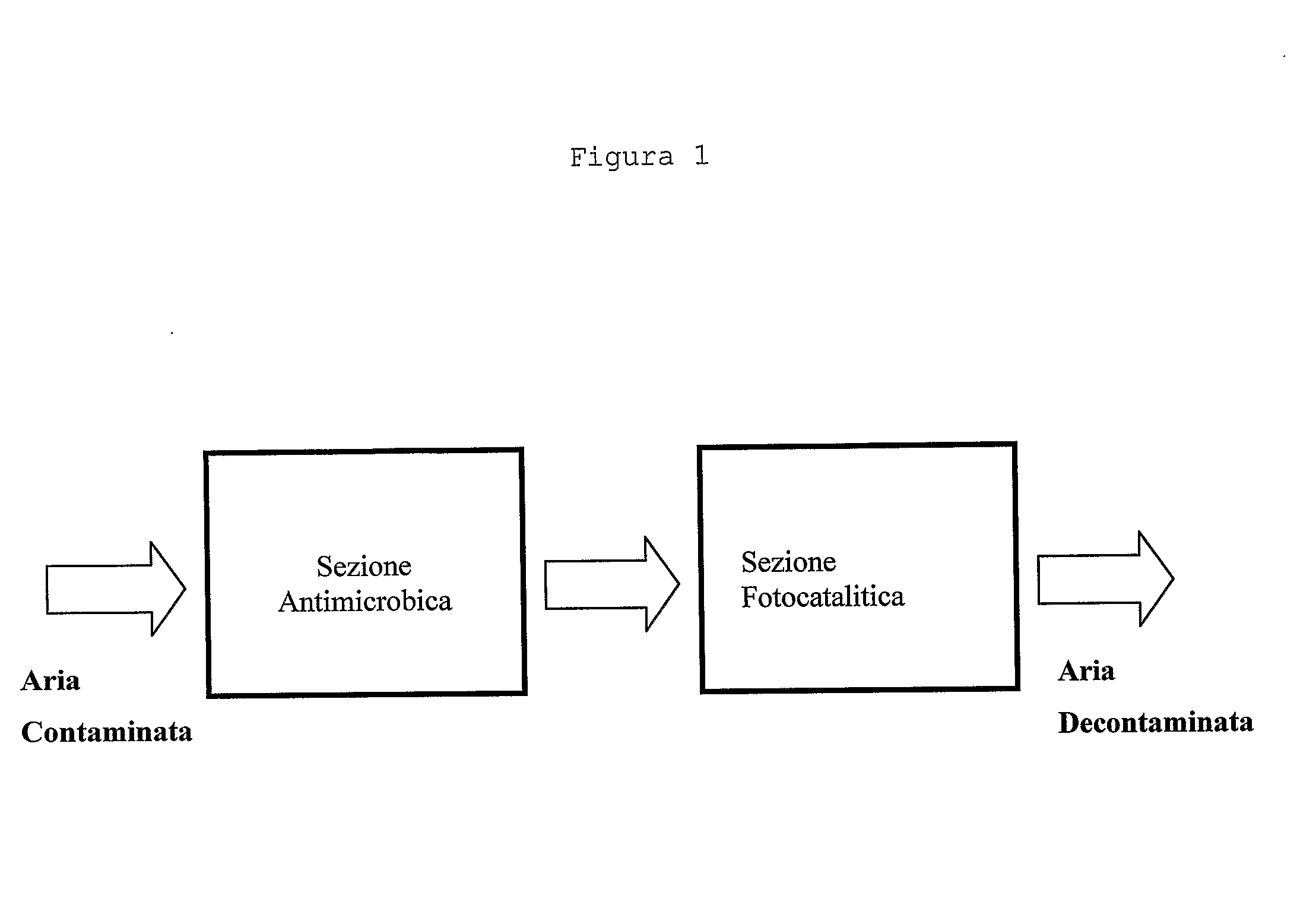
Air decontamination equipment
InactiveUS20110223057A1Most efficientReduce volatilityGas treatmentDispersed particle separationAir decontaminationVirus processing
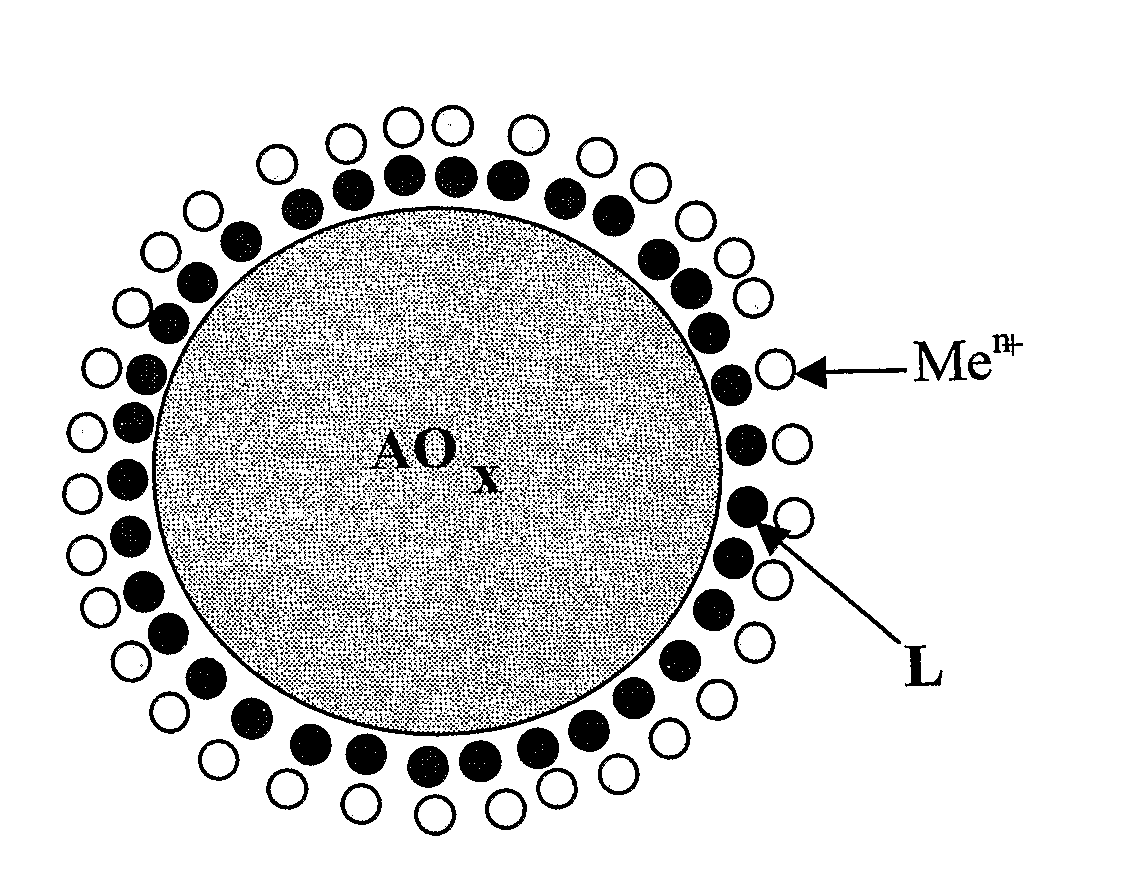
The present invention relates to an air decontamination equipment, from both odours or pollutants, and bacterial or viral loads. More particularly, the present invention relates to a decontaminating equipment (1) for the treatment of air, comprising a shell (2) which is divided in a first and a second compartments (3, 4), which are arranged in a contiguous position in any sequence order, in the second of said compartments (3, 4) suction means (6) being arranged, in which one of said first and second compartments (3, 4) is for the antibacterial / antiviral treatment of air, and one of said first and second compartments (3, 4) is for the photocatalytic treatment of air, and comprises UV illumination means (9), said first and second compartments (3, 4) comprising a material with antibacterial and antiviral activity and a material with photocatalytic activity, respectively.
Owner:NM TECH NANOMATERIALS & MICRODEVICES TECH
Methods and compositions for treatment of HIV infection
InactiveUS20160095850A1Reduce viral loadBiocidePeptide/protein ingredientsImmunodeficiency virusCD4 antigen
Methods and compositions for treatment of human immunodeficiency virus (HIV) infections have been developed which dampen immune activation with a bias more on the CD4 T cells relative to the CD8 T cell response, inhibit HIV replication, reactivate latent HIV, and inhibit infection of cells by HIV. Pushing latent HIV into active infections with hindrance of cell infection by the reactivated HIV can substantially reduce the number of cells infected with HIV and the viral load of HIV, which is not achieved using just the combination of ART and compounds which activate latent HIV. The methods involve administering to an HIV-infected subject three or more compounds which collectively dampen immune activation with a bias more on the CD4 T cells relative to the CD8 T cell response, inhibit HIV replication, reactivate latent HIV, and inhibiting infection of CD4 T cells by HIV.
Owner:COOPER HUMAN SYST
Polyherbal compositions and methods for treating viral infections
The present invention relates to pharmaceutical or veterinary or nutritional compositions of polyherbal extracts useful as anti-viral or immune-supporting agents. Particularly, the present invention of polyherbal composition comprises of extracts of Withania somnifera, Mangifera indica and purified Shilajit. This cost effective immune-supporting agent is ideal for use during the maintenance phase of the treatment, following an initial viral load reduction phase in which it is used as an adjuvant to conventional anti-viral drug therapy. The anti-viral and immune-supporting composition of this invention can perhaps be the sole basis of treatment where affordability is an issue. Additionally, this composition is used for the treatment, prevention or management of immune-supporting system in primates in need, especially humans.
Owner:INDIAN HERBS RES & SUPPLY +1
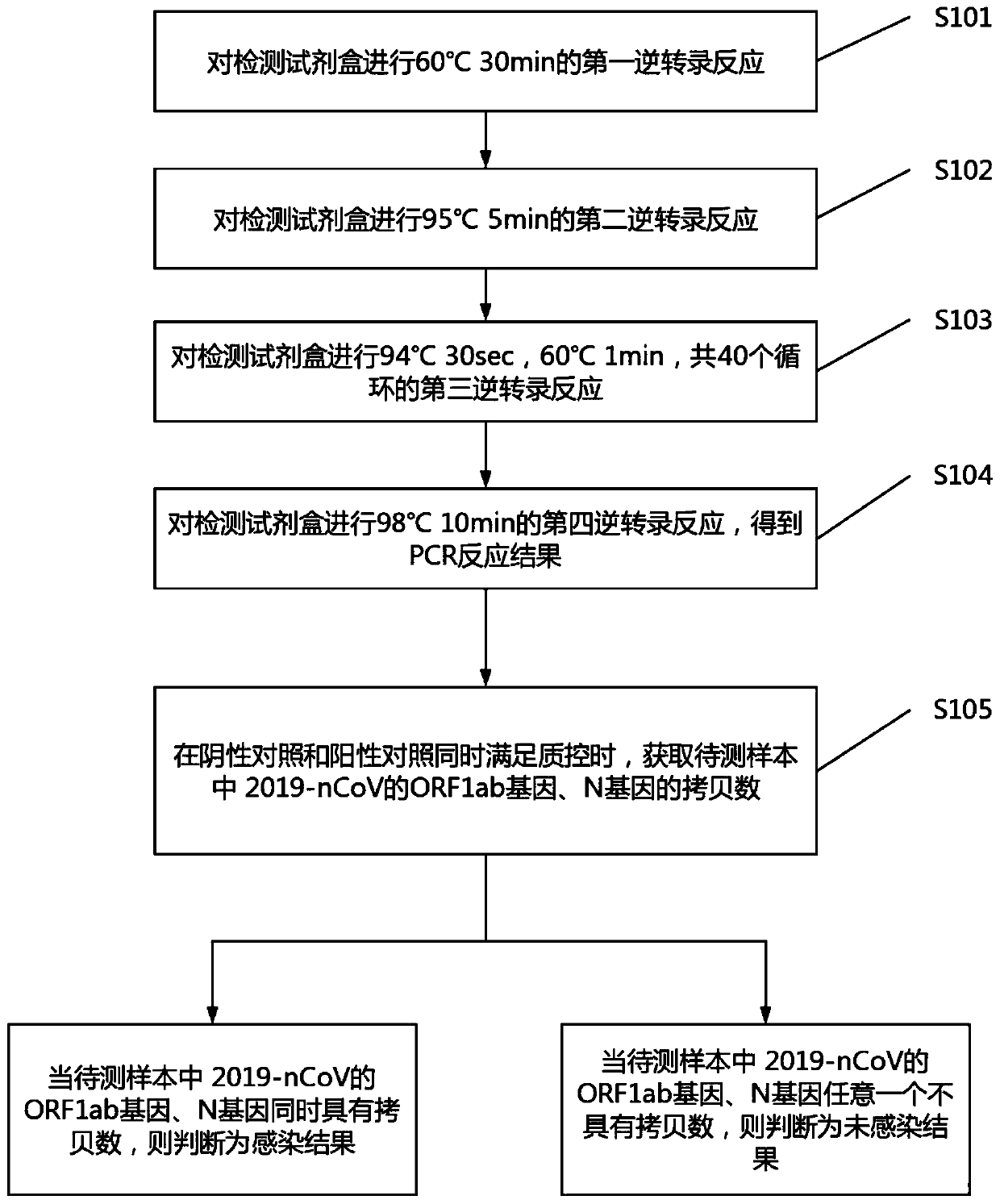
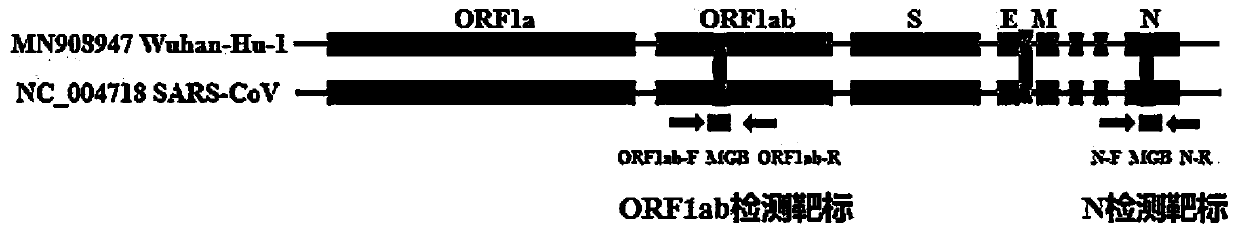
Digital PCR-based novel coronavirus nucleic acid quantitative detection kit and application
ActiveCN111394519AHigh sensitivityStrong specificityMicrobiological testing/measurementAgainst vector-borne diseasesRNA extractionAbsolute quantification
The invention discloses a digital PCR-based novel coronavirus nucleic acid quantitative detection kit and an application. The reaction total system of the digital PCR-based novel coronavirus nucleic acid quantitative detection kit has 20 ul, comprising 10ul of 2x One-Step RT-ddPCR Supermix, 0.8ul of 25mM manganese acetate solution, 5ul of to-be-detected sample RNA, 1ul of ORFlab gene primer probeworking solution, 1ul of N gene primer probe working solution, 1ul of RPP30 gene primer probe working solution and 1.2ul of Nuclease-Free Water, wherein 5ul of negative control RNA extraction solutionand 5ul of positive control RNA extraction solution are adopted to replace the to-be-detected sample RNA in a negative control reaction system and a positive control reaction system respectively. Based on the innovative RNA one-step reverse transcription microdroplet type digital PCR technology, nucleic acid absolute quantification is carried out specific to highly conservative ORFlab gene and Ngene in the novel coronavirus (2019-nCoV) genome, so that the detection accuracy is improved, and the kit can be used for clinical assisted diagnosis and viral load analysis of novel coronavirus (2019-nCoV) infection, and has a wide clinical application value.
Owner:南京实践医学检验有限公司
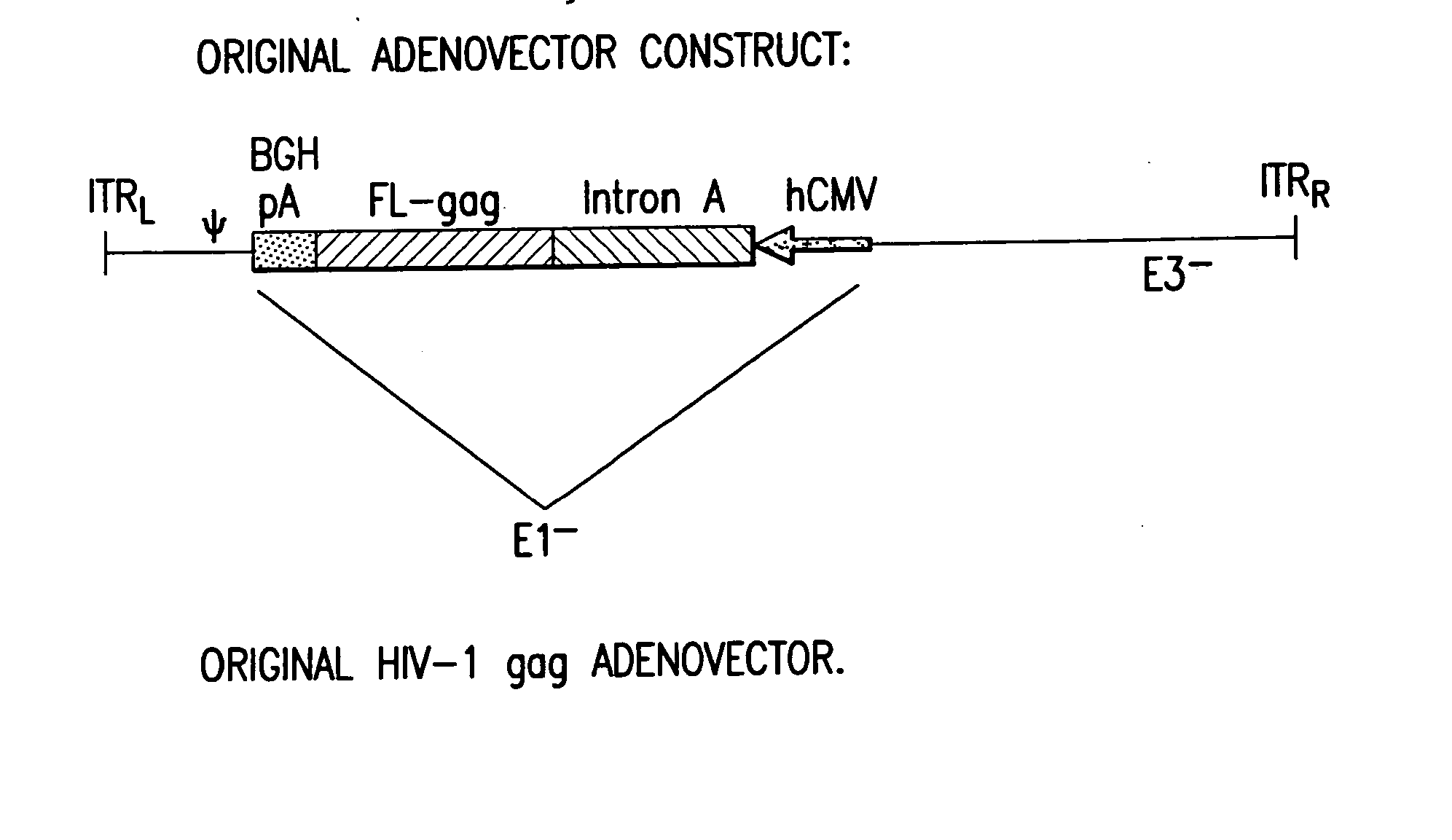
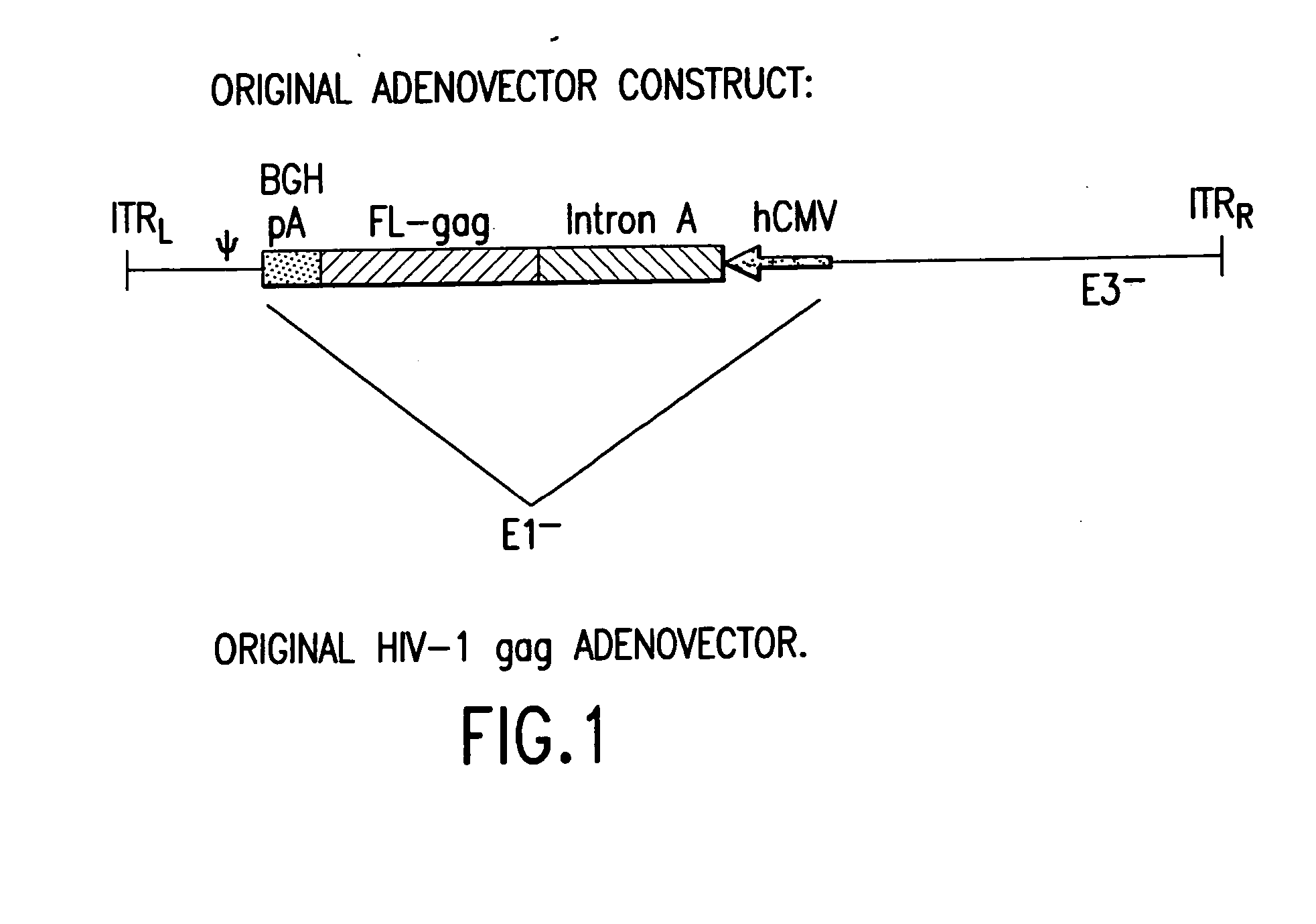
Enhanced first generation adenovirus vaccines expressing condon optimized HIV1-Gag, Pol, Nef and modifications
InactiveUS20070077257A1Improved cellular-mediated immune responseLow transmission rateViral antigen ingredientsVirus peptidesTreatment effectNucleotide
First generation adenoviral vectors and-recombinant adenovirus-based HIV vaccines which contain HIV-1 gag, HIV-1 pol and / or HIV-1 nef polynucleotide pharmaceutical products, and biologically relevant modifications thereof are described. The adenovirus vaccines, when directly introduced into living vertebrate tissue, express the relevant proteins, inducing a cellular immune response which specifically recognizes HIV-1. The exemplified polynucleotides of the present invention are synthetic DNA molecules encoding HIV-1 Gag, HIV-1 Pol, HIV-1 Nef, and derivatives thereof. The adenoviral vaccines of the present invention, alone or in combination, will offer a prophylactic advantage to previously uninfected individuals and / or provide a therapeutic effect by reducing viral load levels within an infected individual, thus prolonging the asymptomatic phase of HIV-1 infection.
Owner:EMINI EMILIO A +7
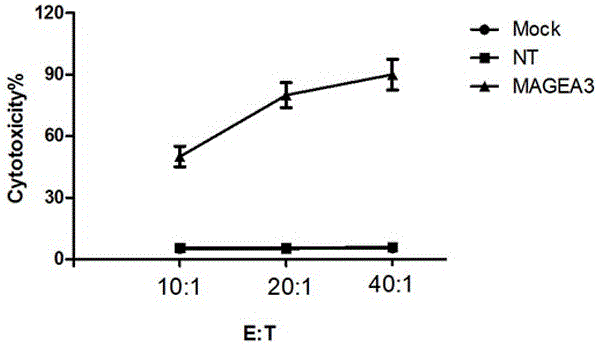
Novel viral vaccine for treating non-small cell lung cancer and preparation method thereof
InactiveCN105039269ALittle side effectsEnhanced ability to clear tumor cellsCarrier-bound antigen/hapten ingredientsViruses/bacteriophagesViral antibodyViral Vaccine
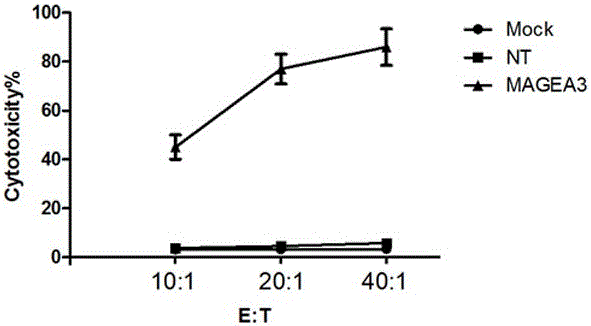
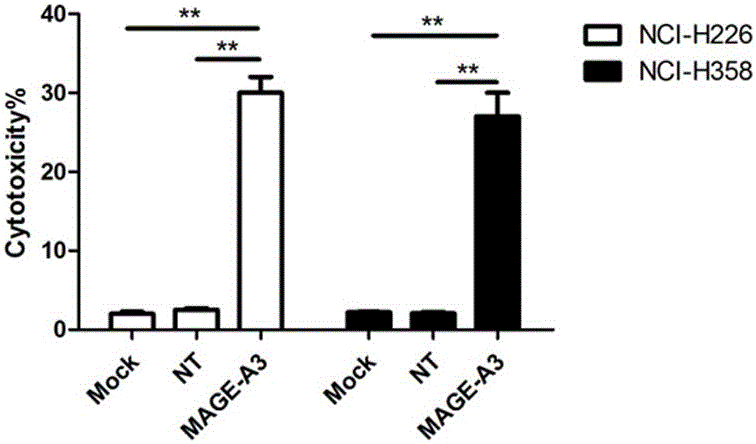
The invention provides a novel viral vaccine for treating a non-small cell lung cancer and a preparation method thereof. The preparation method of the viral vaccine comprises the first step of constructing a carrier of an MVA virus and the second step of obtaining specificity presenting MAGE-3 antigen DC cells. According to the viral vaccine prepared through the method, the biological stability is high, adverse effects on human bodies are not produced, and pathogenic dangers do not exist; a virus antibody can obtain a large number of viral particles with high purity easily; MAGE-A3 expressed by the viral vaccine has better immunogenicity, more epitopes are achieved, and drug resistance is not prone to being caused; the viral load needed in inoculation of the viral vaccine is smaller than other viruses.
Owner:BEIJING DCTY BIOTECH CO LTD
Non-amplification nucleic acid hybrid capture system and application thereof in nucleic acid test
InactiveCN107523611AFast detection methodLow costMicrobiological testing/measurementNucleic acid chemistryNucleic acid detection
The invention belongs to the technical field of molecular biology, immunology and nucleic acid chemistry and in particular relates to a non-amplification nucleic acid hybrid capture system and application thereof. The kit is an immunoassay method for capturing hybrid nucleic acids based on a non-amplification anti-nucleic antibody; inverse transcription, PCR (Polymerase Chain Reaction) and other amplification technologies are not needed in the detection process, while a probe complementary to a to-be-detected nucleic acid is subjected to annealing hybridization with the to-be-detected nucleic acid so as to form a structure which can be recognized by the anti-nucleic antibody, and the detection signal is amplified by utilizing enzyme linked immunosorbent assay. The detection method has the advantages of being rapid, low in cost, high in sensitivity, high in specificity, free in complicated amplification and detection instruments and capable of detecting initial viral load.
Owner:NANJING UNIV
Kit for flavivirus quick typing and virus load detection
InactiveCN106086242ANo cross reactionAchieve quantitative goalsMicrobiological testing/measurementAgainst vector-borne diseasesZika virusMicroorganism
The invention belongs to the field of microbial molecular detection, and particularly relates to a kit for flavivirus quick typing and virus load detection. The kit for flavivirus quick typing and virus load detection comprises specific primers and probes for flaviviruses, including specific primers and a probe for dengue virus, specific primers and a probe for Zika virus, specific primers and a probe for yellow fever virus, and specific primers and a probe for Chikungunya virus. The kit for flavivirus quick typing and virus load detection has the advantages of high detection speed and accurate detection and quantification results, can simultaneously detect 3 different flavivirus pathogens and 1 togavirus pathogen capable of causing similar clinical symptoms at one time, can detect and diagnose flavivirus-pathogen-infected suspicious cases in time, and enhances the detection accuracy of flavivirus pathogen infection.
Owner:GUANGZHOU EIGHTH PEOPLES HOSPITAL
Polyherbal compositions and methods for treating viral infections
The present invention relates to pharmaceutical or veterinary or nutritional compositions of polyherbal extracts useful as anti-viral or immune-supporting agents. Particularly, the present invention of polyherbal composition comprises of extracts of Withania somnifera, Mangifera indica and purified Shilajit. This cost effective immune-supporting agent is ideal for use during the maintenance phase of the treatment, following an initial viral load reduction phase in which it is used as an adjuvant to conventional anti-viral drug therapy. The anti-viral and immune-supporting composition of this invention can perhaps be the sole basis of treatment where affordability is an issue. Additionally, this composition is used for the treatment, prevention or management of immune-supporting system in primates in need, especially humans.
Owner:INDIAN HERBS RES & SUPPLY +1
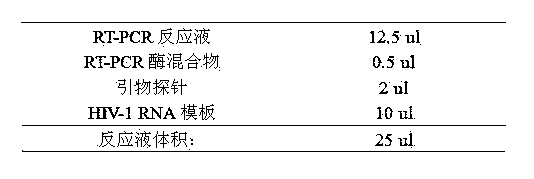
Human immunodeficiency virus type 1 one-step fluorescence quantitative RT-PCR detection kit
InactiveCN103966356AQuick analysisAccurate analysisMicrobiological testing/measurementFluorescence/phosphorescenceBioinformaticsHuman immuno deficiency virus
The present invention provides a human immunodeficiency virus type 1 one-step fluorescence quantitative RT-PCR detection kit, which comprises a RT-PCR reaction solution, a RT-PCR enzyme mixture, a primer probe, a negative control, a strong positive control, a weak positive control, and calibration substances No.1-5. According to the present invention, a one-step fluorescence quantitative reaction can be directly performed on the extracted HIV-1RNA, the virus loading of the HIV-1RNA in a sample can be detected, the reference gene is adopted as the internal control, and the UNG enzyme is adopted to prevent pollution; and the kit has characteristics of simple one-step amplification method, short procedure, easy operation, pollution prevention, strong detection result specificity, high sensitivity, clear result and high result reliability, and can be used for detection of the virus loading of the human immunodeficiency virus type 1 (HIV-1) RNA in blood serum.
Owner:SHANGHAI XINGYAO MED TECH DEV CO LTD +1
Methods for reducing viral load in hiv-1 infected patients
ActiveUS20130216526A1Decrease in HIV RNAReduce viral loadAntibody ingredientsImmunoglobulinsReduced doseHumanized antibody
This invention provides a method of reducing viral load in an HIV-1-infected human subject which comprises administering to the subject an effective HIV-1 viral load reducing dose of a CCR5 receptor antagonist, such as a humanized antibody designated PRO 140 or an anti-CCR5 receptor monoclonal antibody, wherein the viral load reducing dose achieves an average maximum decrease of viral load in the subject of at least 1.83 log10 to 2.5 log10 at about ten days following administration of the CCR5 receptor antagonist and wherein the viral load reducing dose further achieves a mean viral load reduction of 1.7 log10 at about nine days following administration of the CCR5 receptor antagonist.
Owner:CYTODYN
Kit for real-time fluorescence quantification RT-qPCR detection of hepatis c viral nucleic acid by means of one-step method
ActiveCN106119415AShorten the lengthReduce the chance of being degraded by enzymesMicrobiological testing/measurementFluorescenceReverse transcriptase
The invention discloses a kit for real-time fluorescence quantification RT-qPCR detection of hepatis c viral nucleic acid by means of a one-step method. The kit comprises a viral nucleic acid extraction reagent, a probe containing detection target polynucleotide and an internal control, RT-qPCR reaction buffer used for amplification of a target polynucleotide primer, an RT-PCR enzyme mixture containing warm start DNA polymerase, Revert AidTM M-MLV reverse transcriptase and RiboLockTM ribonuclease inhibitor, and a quantitative criterion reference containing virus-like particles. By the adoption of the novel hepatitis c virus target sequence probe and primer, a secondary structure and an incision enzyme site which cause degradation easily in a hepatis c virus 5'-UTR area are avoided innovatively, original viral load can still be reflected to the maximum degree even if viral nucleic acid is unstable, all six genotypes of hepatis c viral nucleic acid RNA in samples subjected to improper acquisition, transportation, storage and treatment can be tested, and accurate basis is provided for auxiliary diagnosis of hepatis c virus infection and monitoring of drug therapy of infectors.
Owner:山东探克生物科技股份有限公司
Method for removal of viruses from blood by lectin affinity hemodialysis
InactiveUS20070218458A1Reduce loadReduce in quantityOther blood circulation devicesHaemofiltrationHemodialysisHollow fibre membrane
The present invention relates to a method for using lectins that bind to pathogens having high mannose surface glycoproteins or fragments thereof which contain high mannose glycoproteins, to remove them from infected blood or plasma in an extracorporeal setting. Accordingly, the present invention provides a method for reducing viral load in an individual comprising the steps of obtaining blood or plasma from the individual, passing the blood or plasma through a porous hollow fiber membrane wherein lectin molecules are immobilized within the porous exterior portion of the membrane, collecting pass-through blood or plasma and reinfusing the pass-through blood or plasma into the individual.
Owner:AETHLON MEDICAL INC
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com