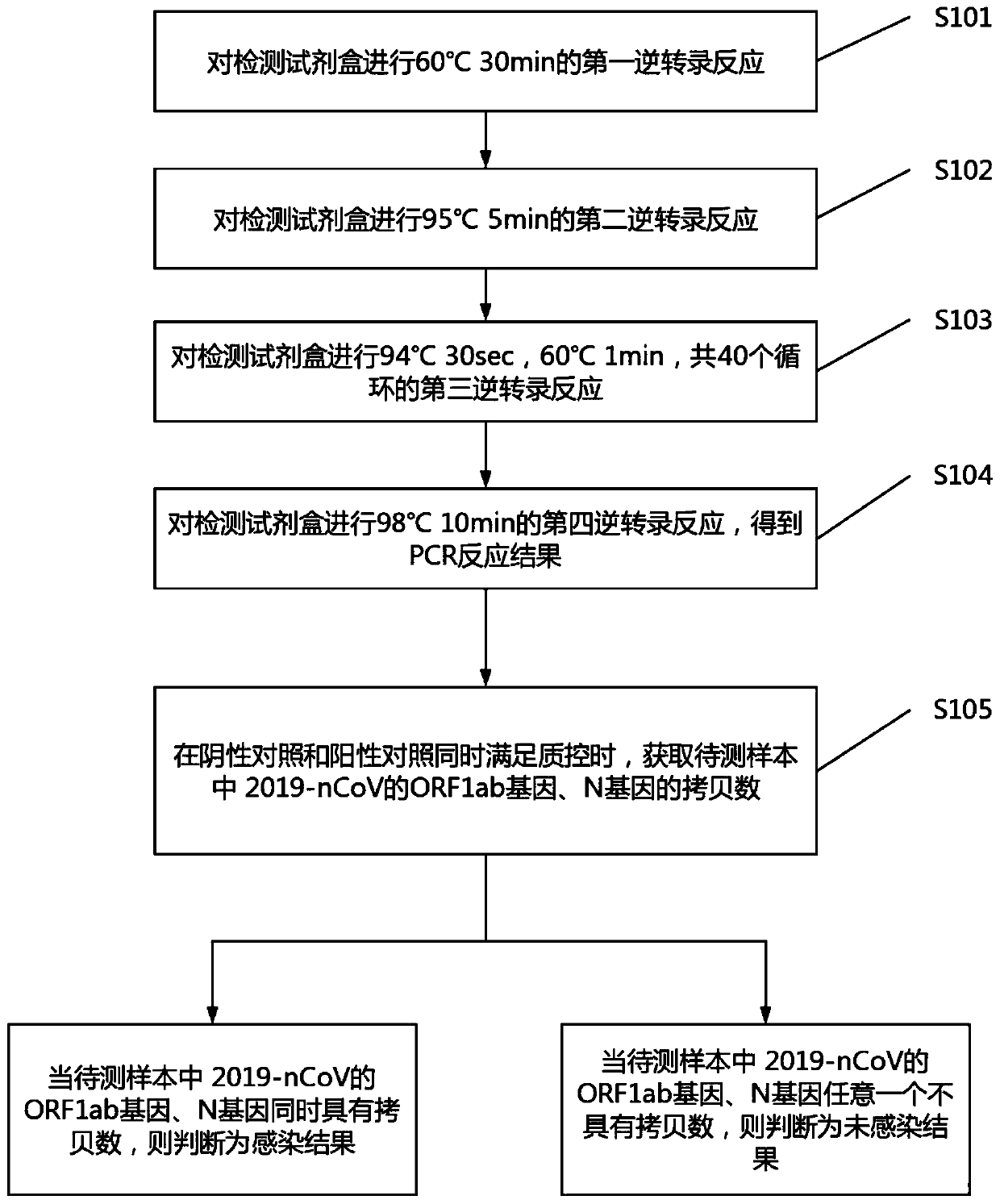
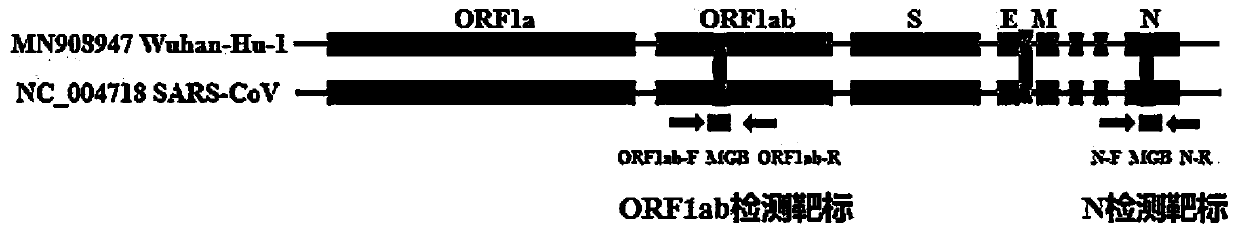
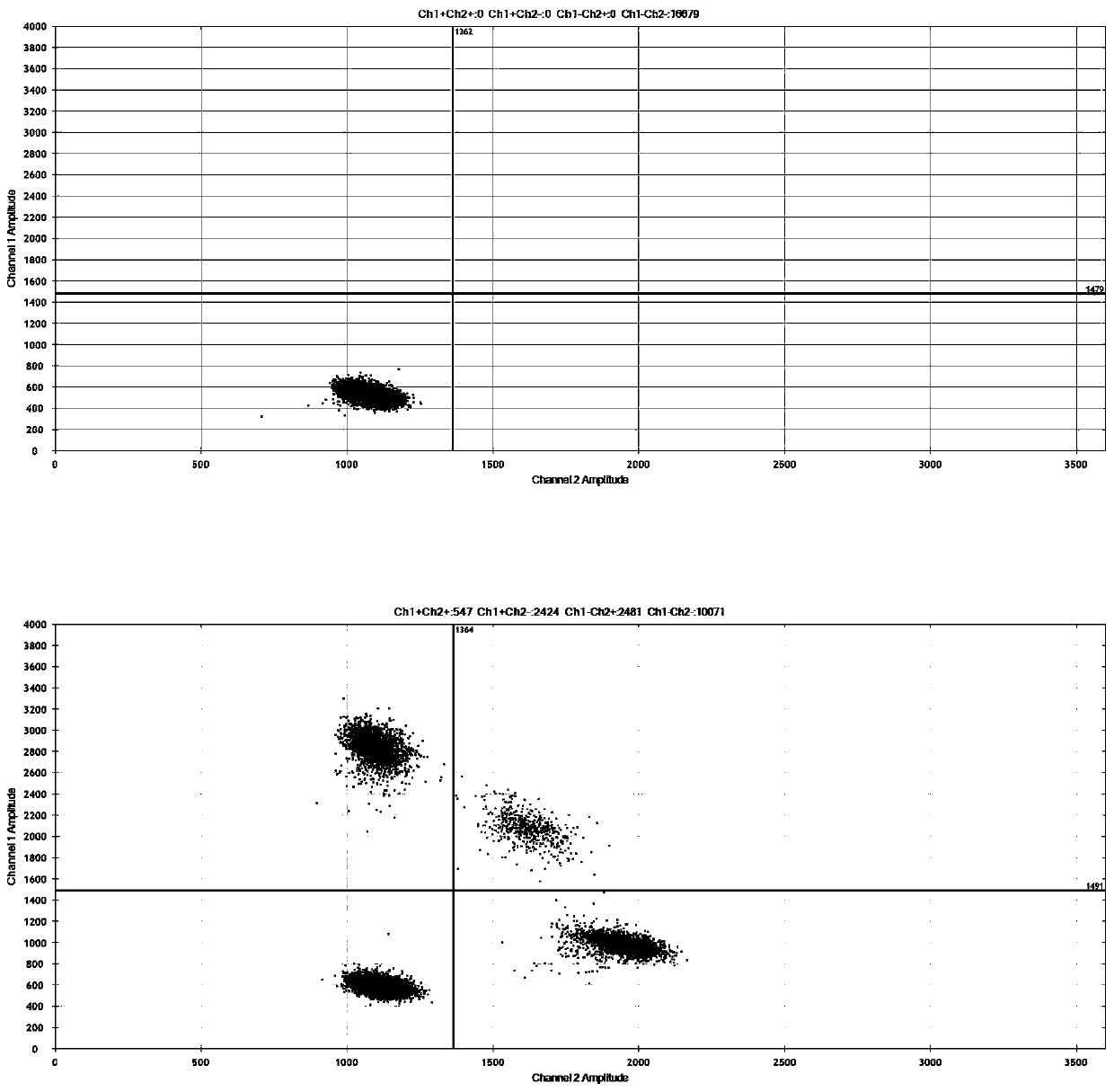
Digital PCR-based novel coronavirus nucleic acid quantitative detection kit and application
A detection kit, a technology for coronavirus, applied in the determination/inspection of microorganisms, resistance to vector-borne diseases, biochemical equipment and methods, etc., can solve the problems of false negatives, variations, and uninvolved diagnostic methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0087] Implementation case 1: Using the positive control of pseudovirus to detect the sensitivity of the droplet digital PCR system
[0088] In this implementation case, the positive control (pseudovirus) of 2019-nCoV was used for a series of gradient dilutions, and the corresponding viral RNA was extracted from the diluted series of pseudovirus solutions, and then the corresponding droplet digital PCR detection was performed. In the case of satisfying a minimum of 3 microdroplets in digital PCR, the minimum detectable sensitivity of the ORF1ab gene of the 2019-nCoV pseudovirus is 5copies / reaction, and the minimum detectable sensitivity of the N gene is 3copies / reaction, so the present invention will detect the sensitivity It is defined as 5copies / reaction, which is much higher than the fluorescent PCR product approved by the State Food and Drug Administration (the lowest sensitivity is 100copies / reaction).
Embodiment example 2
[0089] Implementation Case 2: Clinical Sample Testing
[0090] Due to the particularity of the novel coronavirus pneumonia, the kit described in the present invention only collects throat swabs from 10 employees after returning to work for fluorescent RT-PCR detection and droplet digital PCR detection of 2019-nCoV. RT-PCR detection uses products that have been approved by the State Food and Drug Administration, and 2019-nCoV droplet digital PCR detection uses the method described in the present invention.
[0091] According to the digital PCR detection process of the present invention and the commercial RT-PCR kit detection method process, the detection results of throat swabs of 10 employees are shown in Table 3.
[0092] Table 3 Summary of 2019-nCoV digital PCR and fluorescent RT-PCR detection results
[0093] sample number Droplet digital PCR test results Fluorescent RT-PCR detection results A01 Negative Negative A02 Negative Negative A03 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com