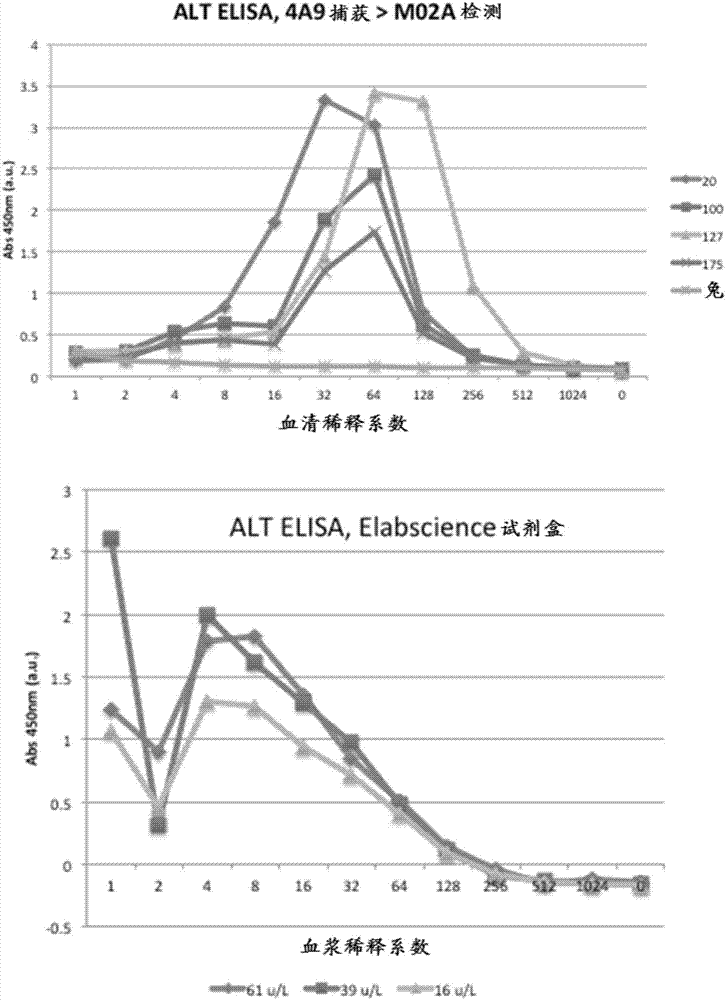
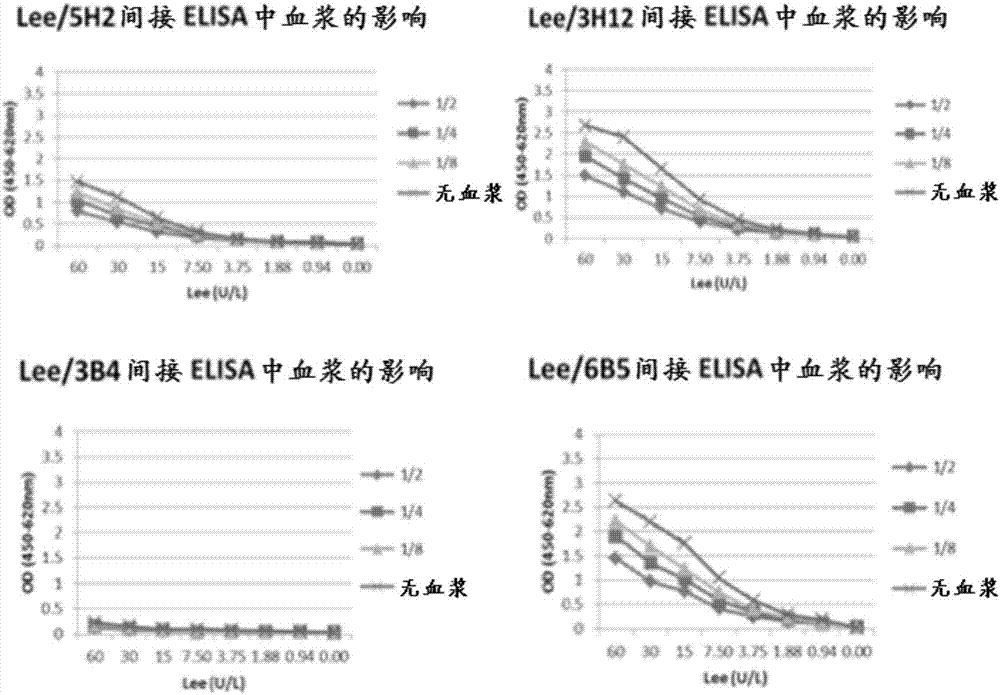
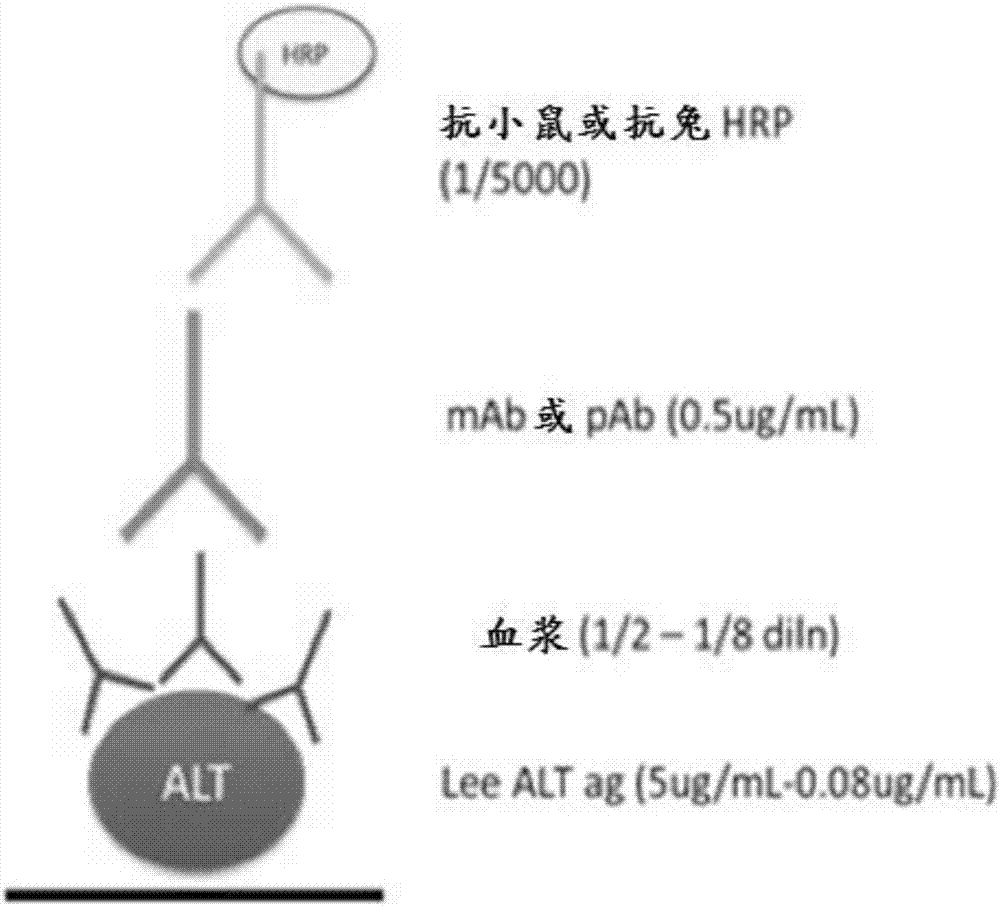
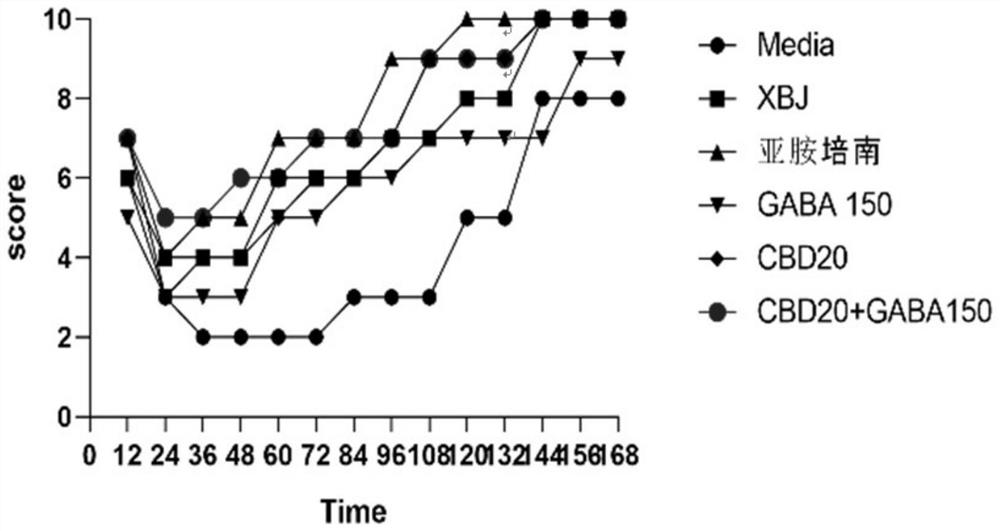
Patents
Literature
49 results about "Liver enzyme" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Liver enzymes are proteins located in the liver that speed up the rate of reactions to make them chemically feasible. The liver is the primary area of detoxification in the body and metabolizes many drugs and compounds that enter the body’s system. It is also the source of much of the stored glucose for energy.
Compositions and methods using nicotinic acid for treatment of hypercholesterolemia, hyperlipidemia nd cardiovascular disease
InactiveUS20070105793A1BiocideCarbohydrate active ingredientsCardiovascular problemsImmediate release
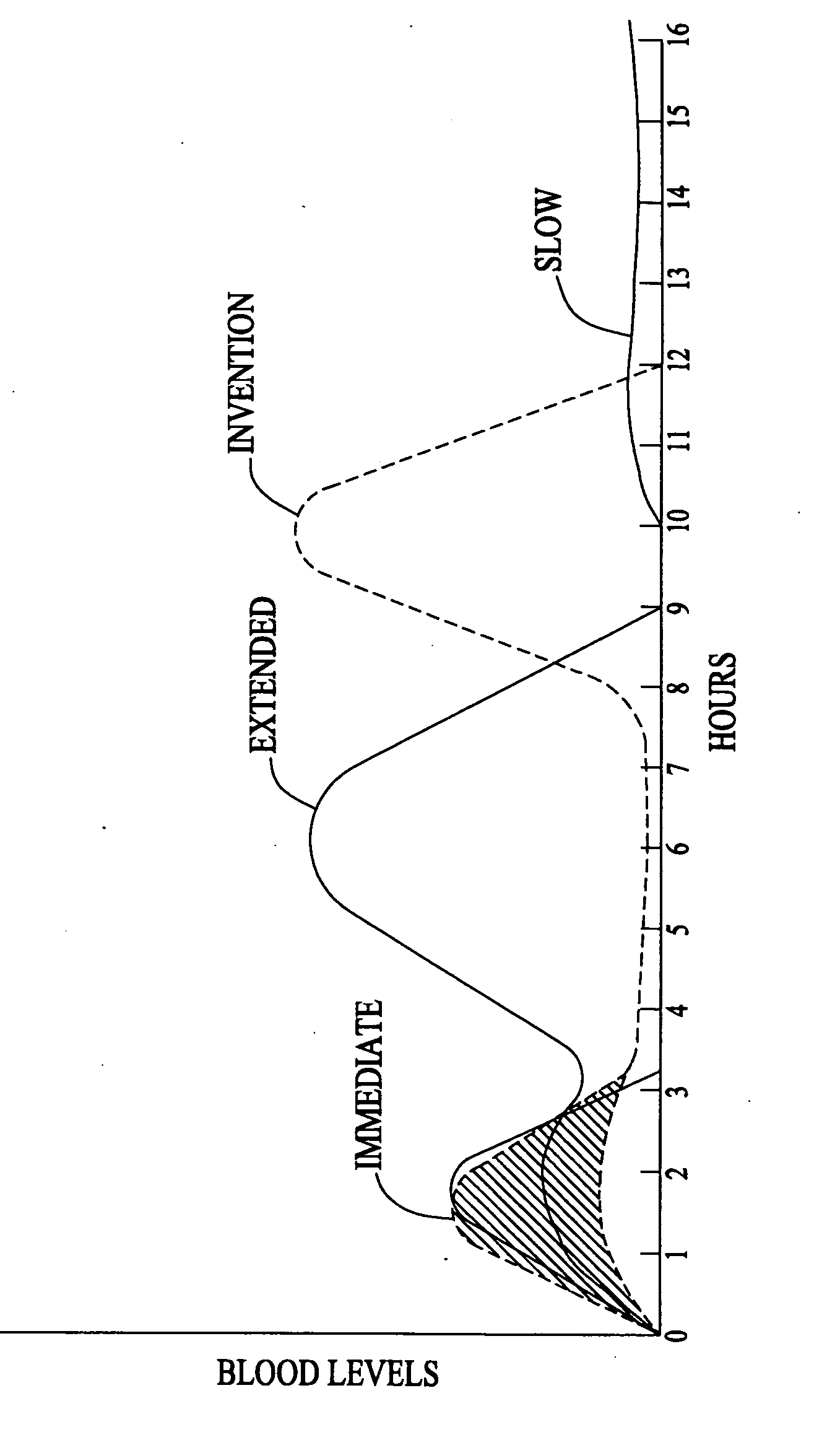
An improved method and composition for the treatment of hyperlipidemia, hypercholesterolemia, and hypertriglyceridemia as well as other disease states, particularly cardiovascular problems, uses a multiple release system that provides an immediate release of nicotinic acid followed by a subsequent release of nicotinic acid from the hydrolysis of a compound that generates nicotinic acid. In general, a composition according to the present invention comprises: (1) a quantity of nicotinic acid intended to saturate liver enzymes during a period from about 0.5 hours to about 2.5 hours after administration of the pharmaceutical composition but insufficient to trigger significant generation of nicotinuric acid; (2) a quantity of a derivative or analogue of nicotinic acid that is subject to hydrolysis after ingestion such that preferrably no more than about 10% of the derivative or analogue is hydrolyzed by about 8 hours after ingestion and such that substantially all of the derivative or analogue is hydrolyzed by about 12 hours after ingestion; and (3) at least one pharmaceutically acceptable carrier.
Owner:CONCOURSE HEALTH SCI
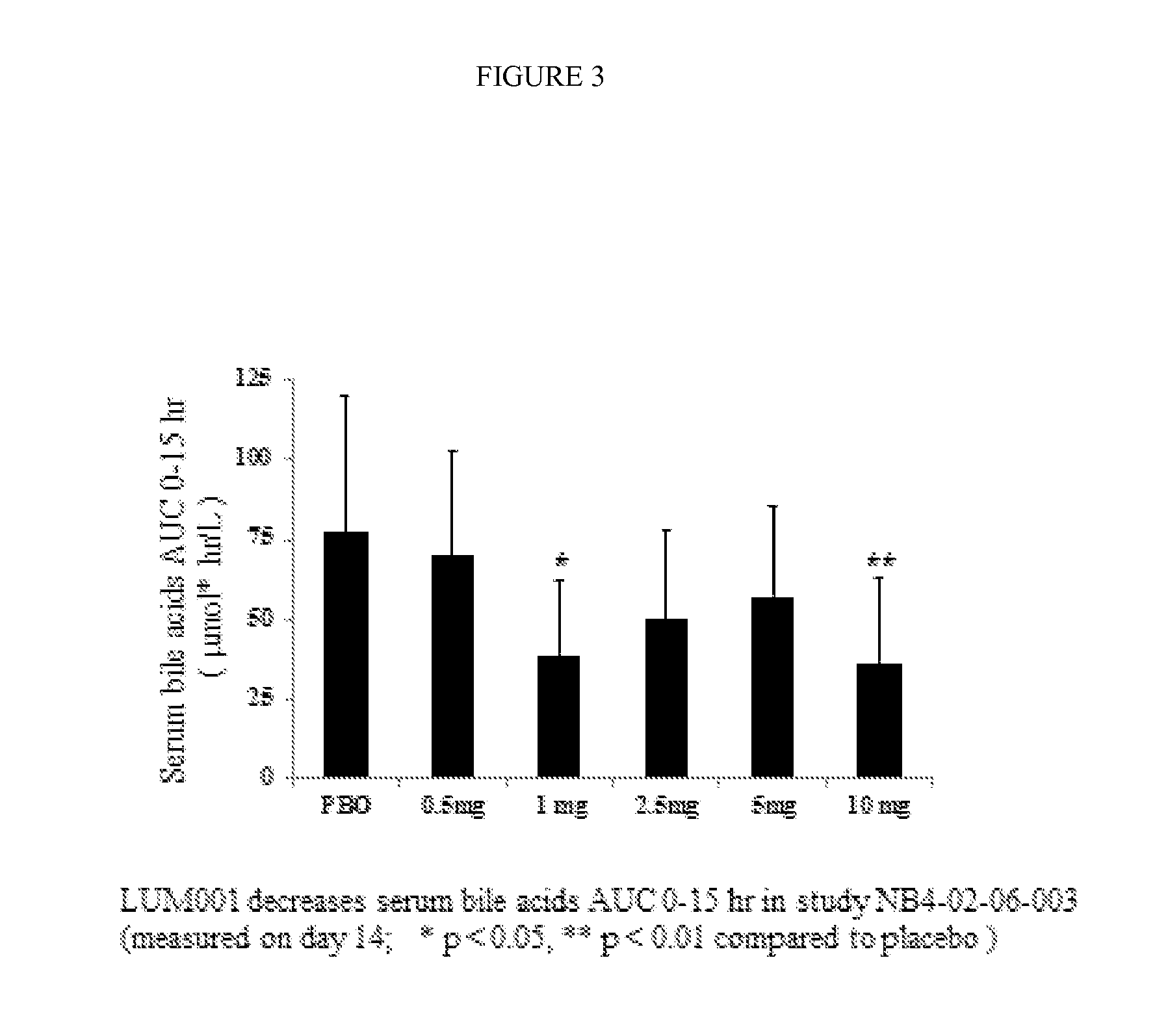
Bile Acid Recycling Inhibitors for Treatment of Hypercholemia and Cholestatic Liver Disease
InactiveUS20130108573A1Relieve symptomsReduce recurrenceBiocideCyclic peptide ingredientsDiseaseHepatic bile
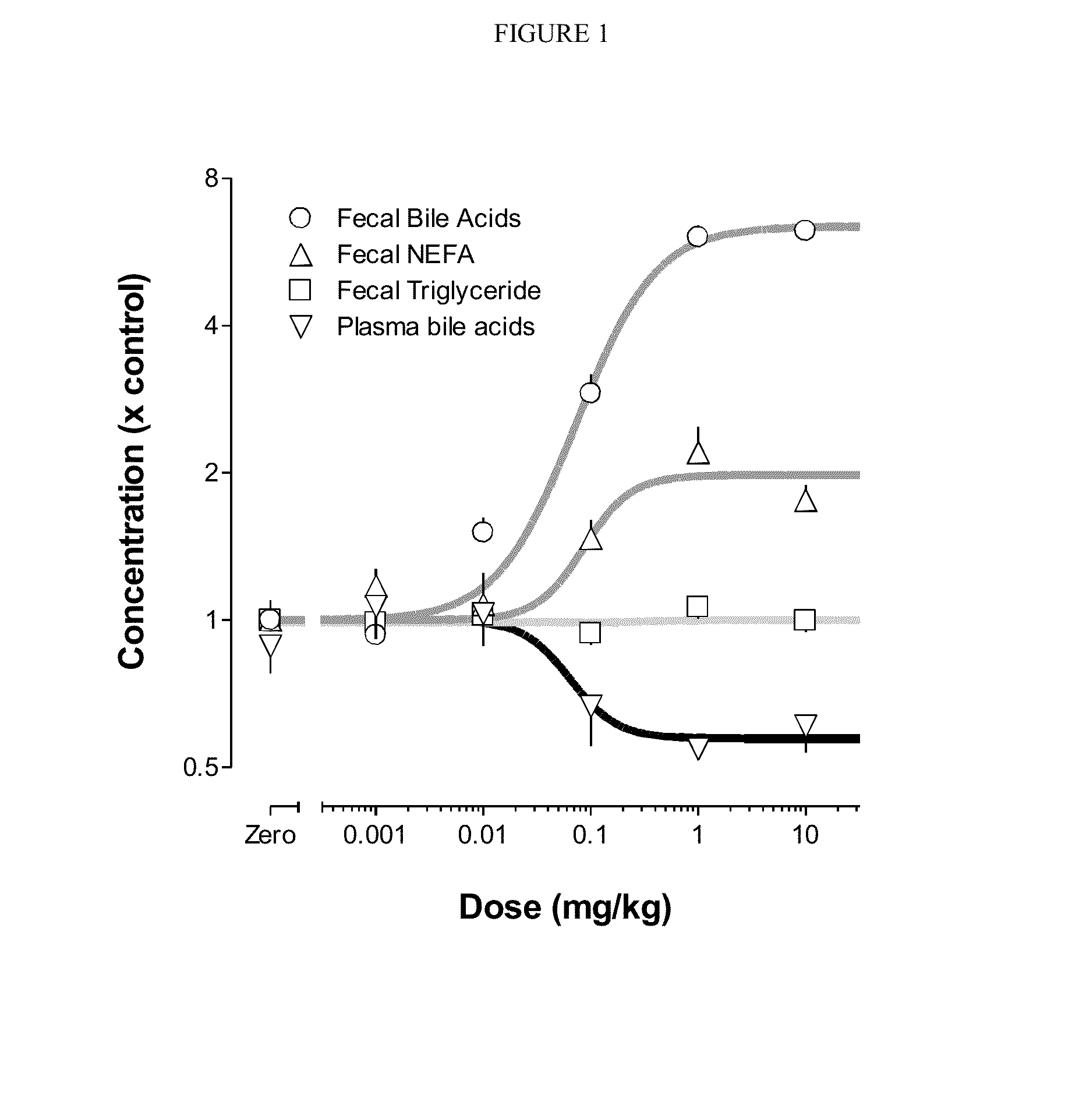
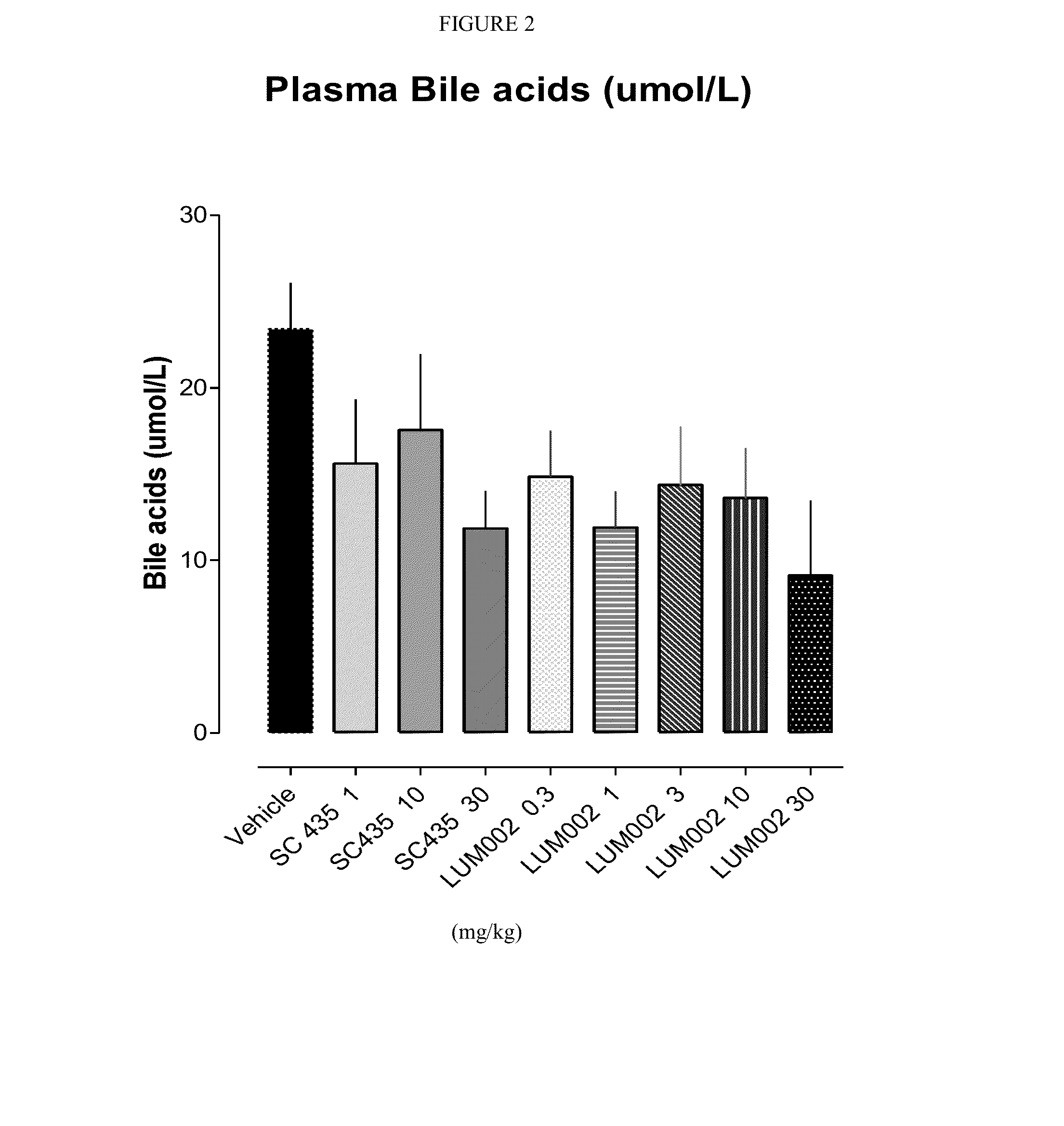
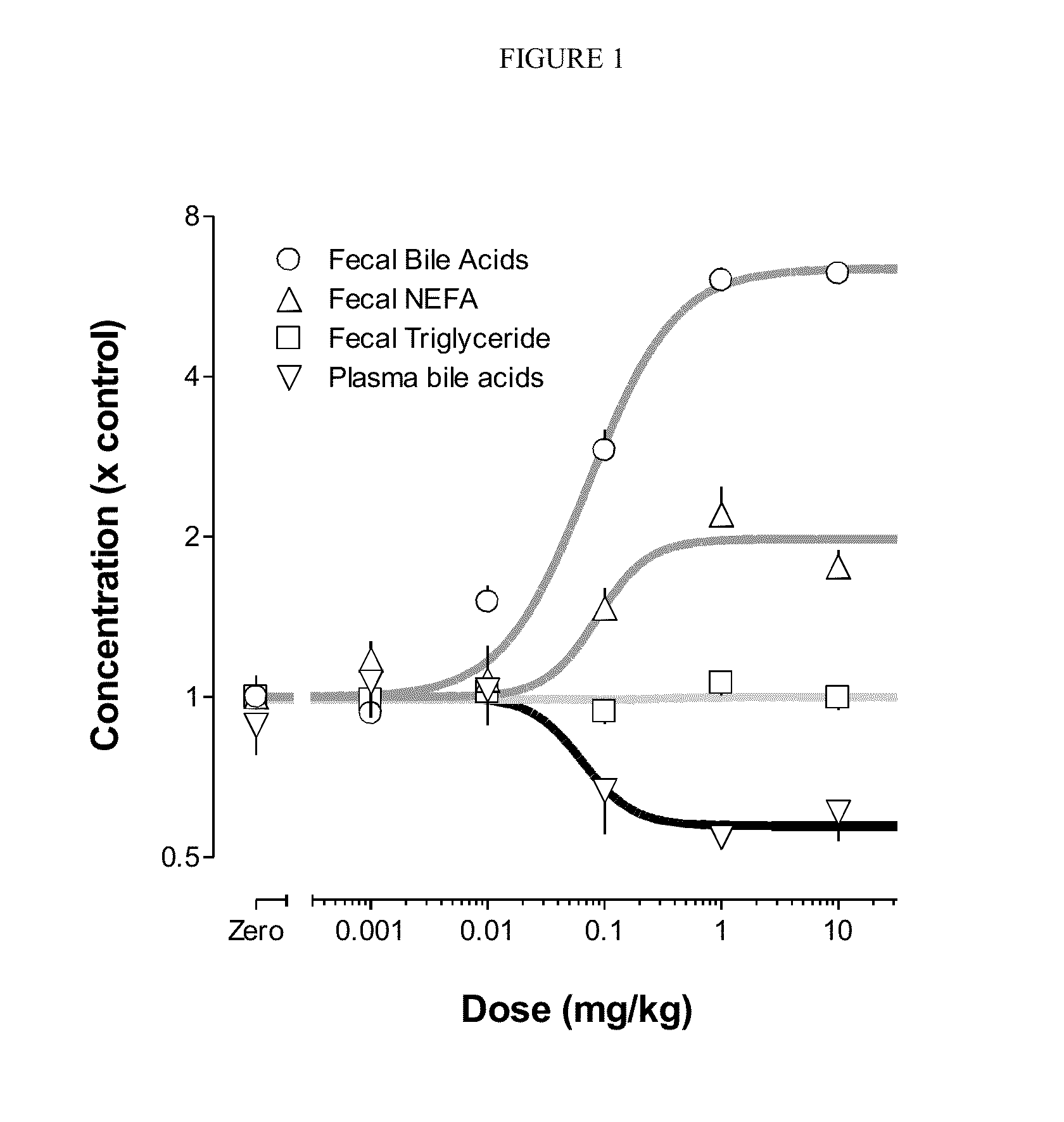
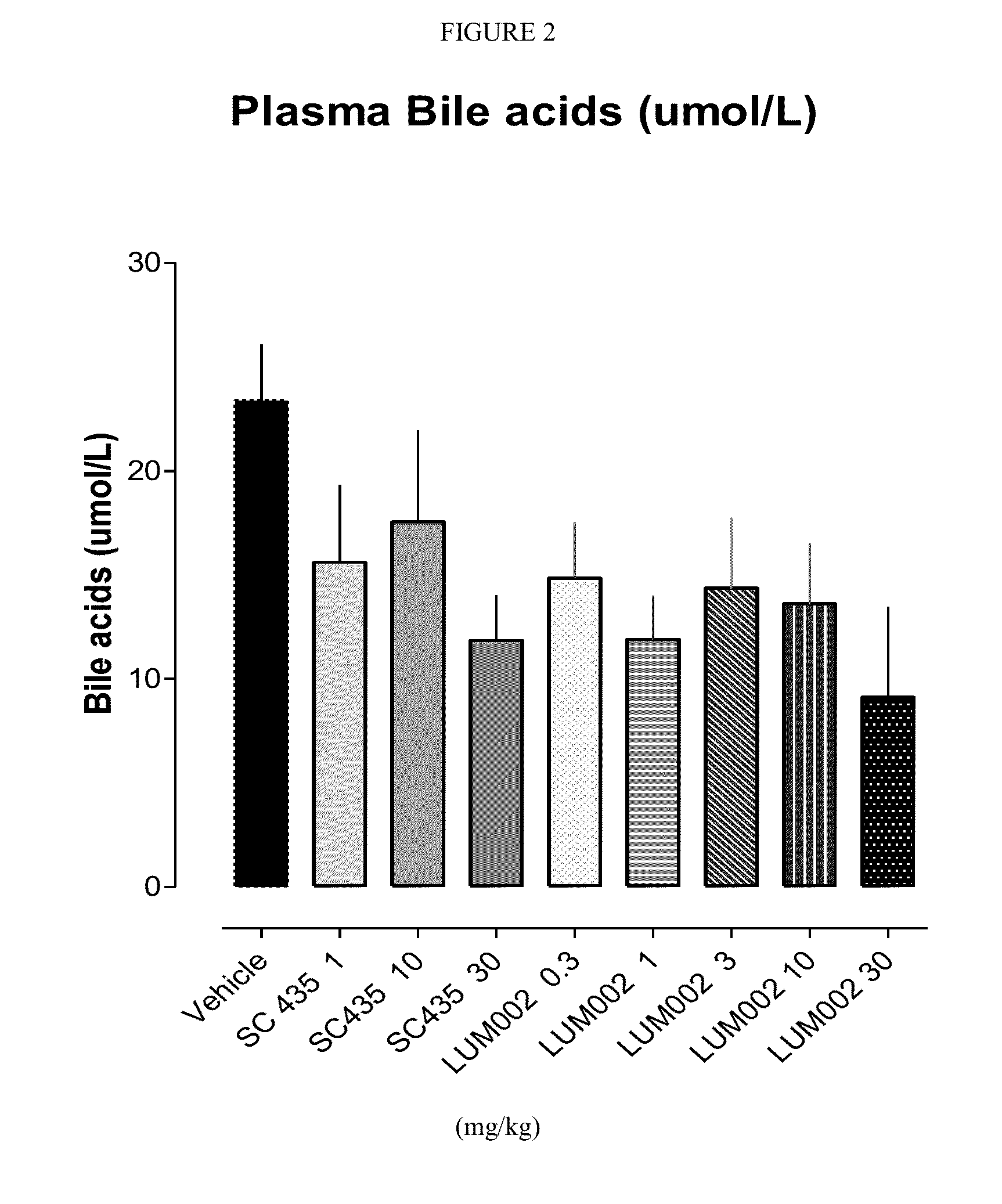
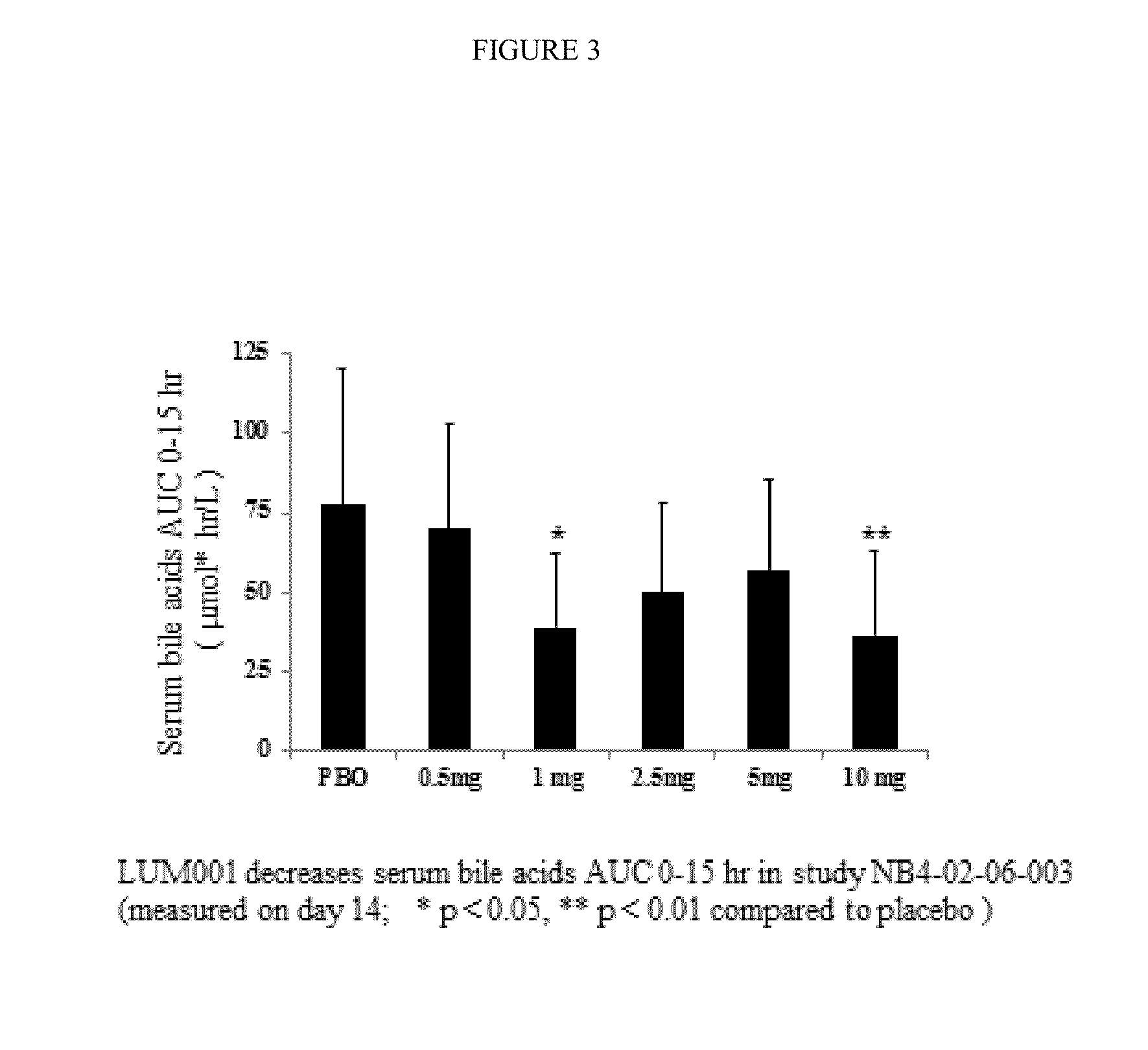
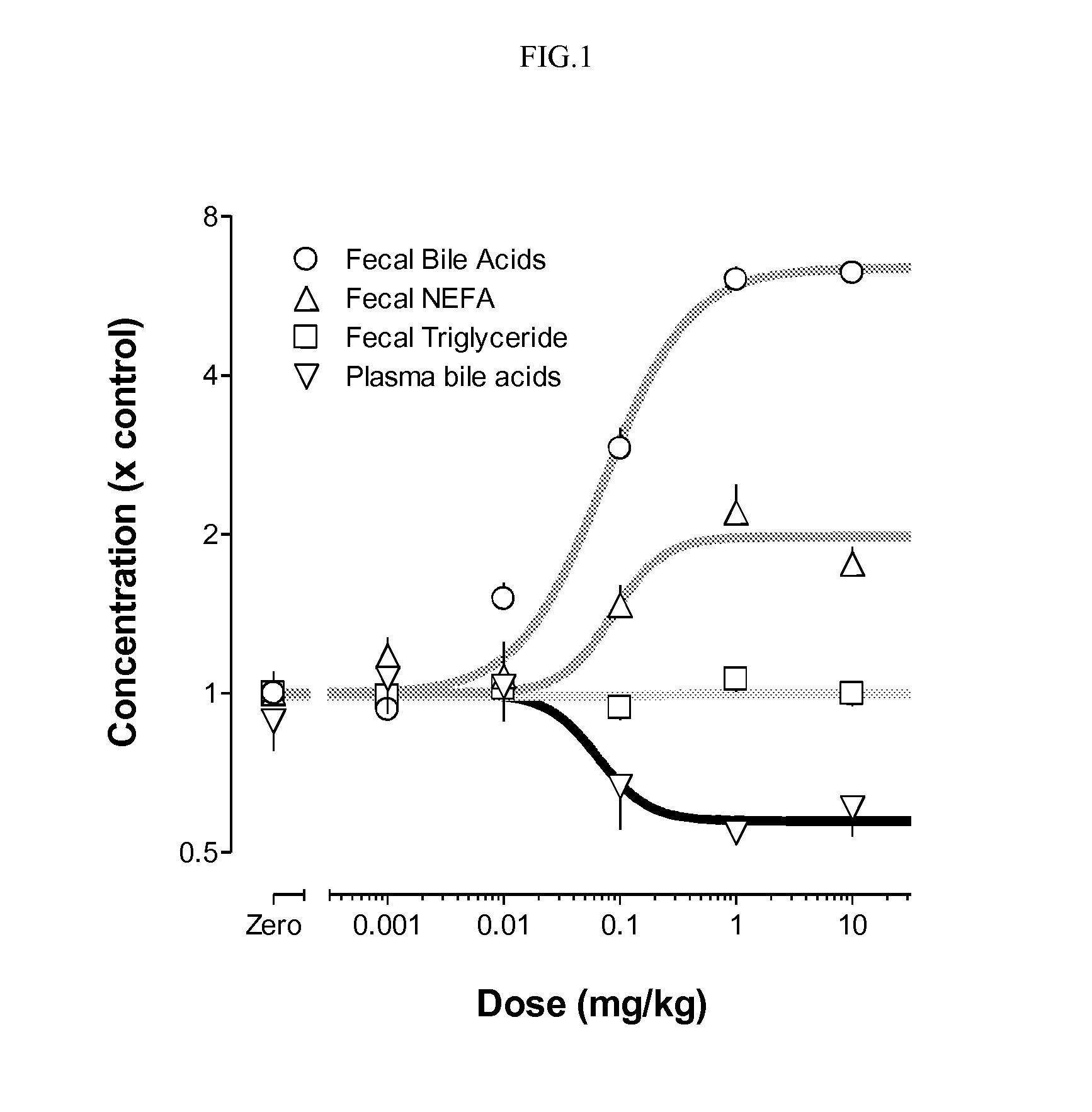
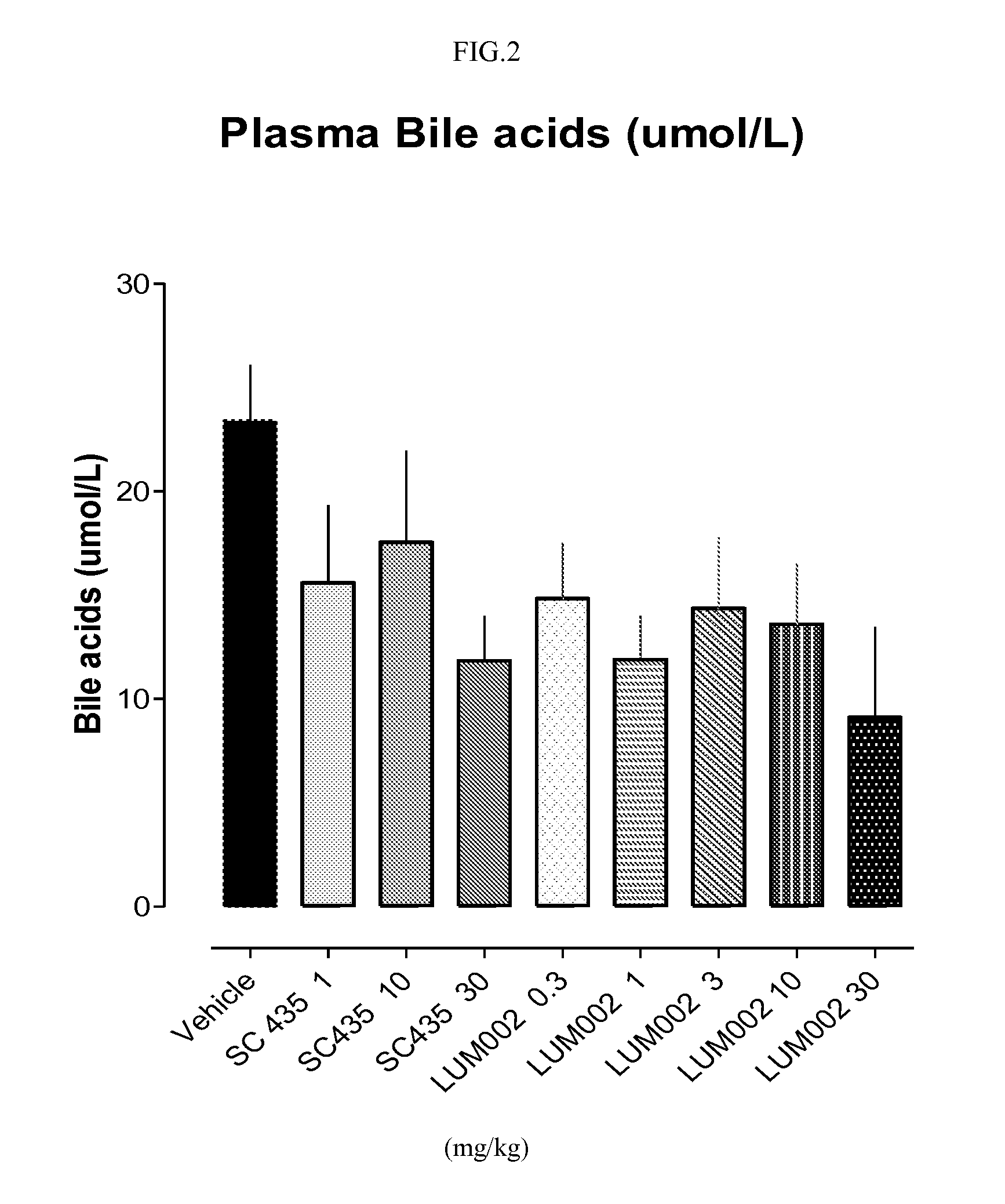
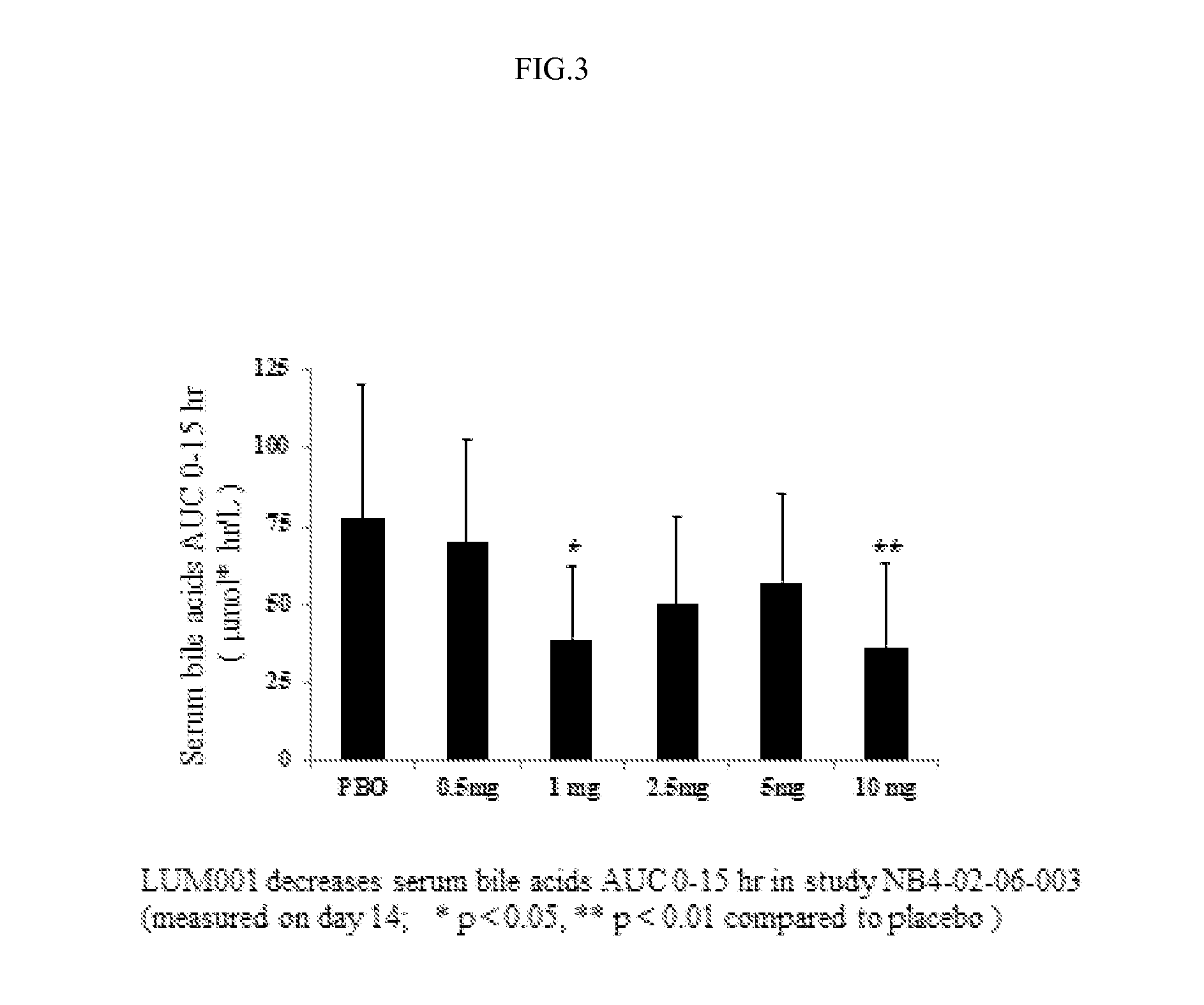
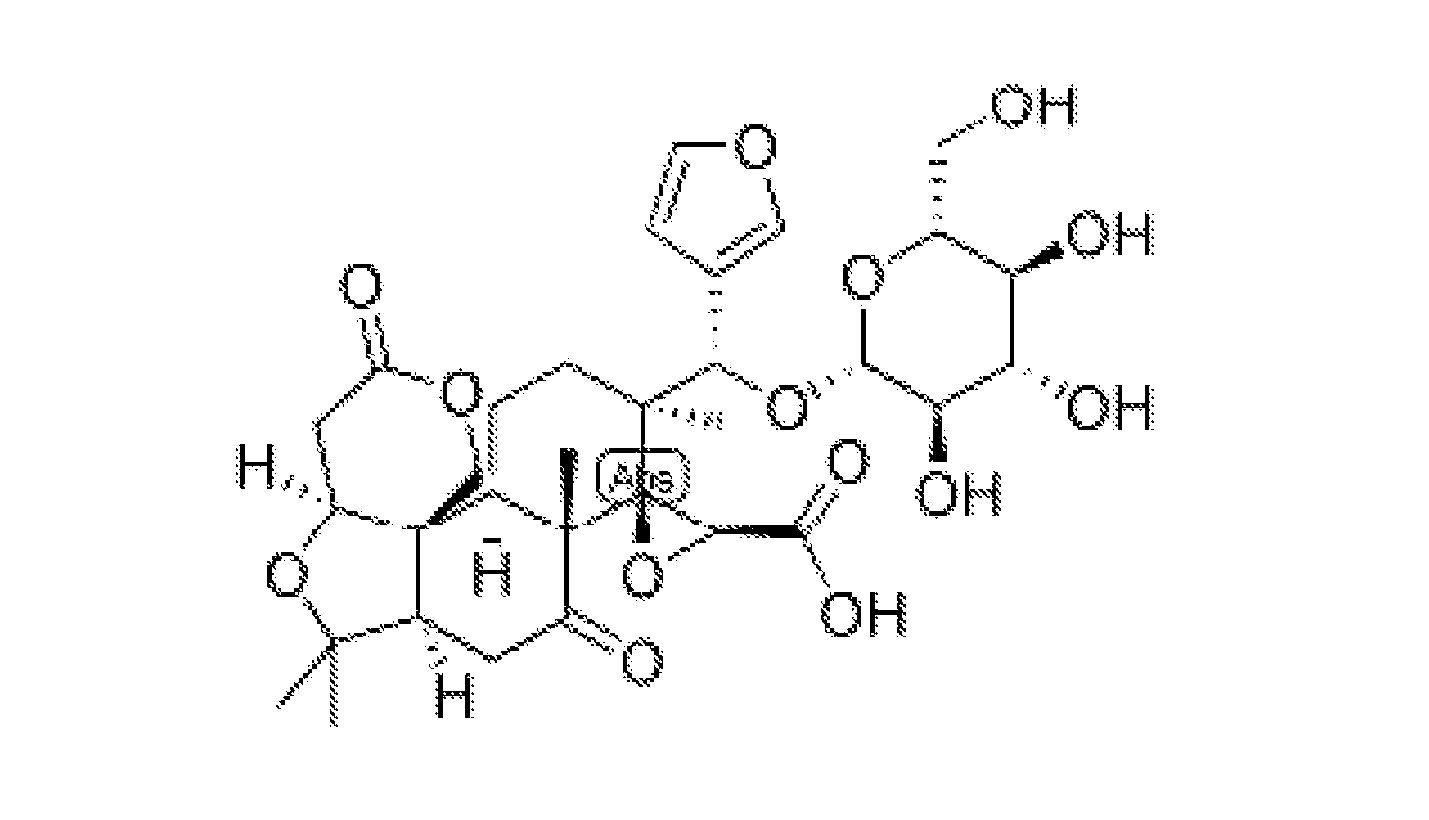
Provided herein are methods of treating or ameliorating hypercholemia or a cholestatic liver disease by administering to an individual in need thereof a therapeutically effective amount of an Apical Sodium-dependent Bile Acid Transporter Inhibitor (ASBTI) or a pharmaceutically acceptable salt thereof. Also provided are methods for treating or ameliorating a liver disease, decreasing the levels of serum bile acids or hepatic bile acids, treating or ameliorating pruritis, reducing liver enzymes, or reducing bilirubin comprising administering to an individual in need thereof a therapeutically effective amount of ASBTI or a pharmaceutically acceptable salt thereof.
Owner:LUMENA PHARMA INC
Bile Acid Recycling Inhibitors for Treatment of Pediatric Cholestatic Liver Diseases
InactiveUS20130109671A1Reduces and inhibits recyclingReduce harmBiocidePill deliveryHepatic DiseasesHepatic bile
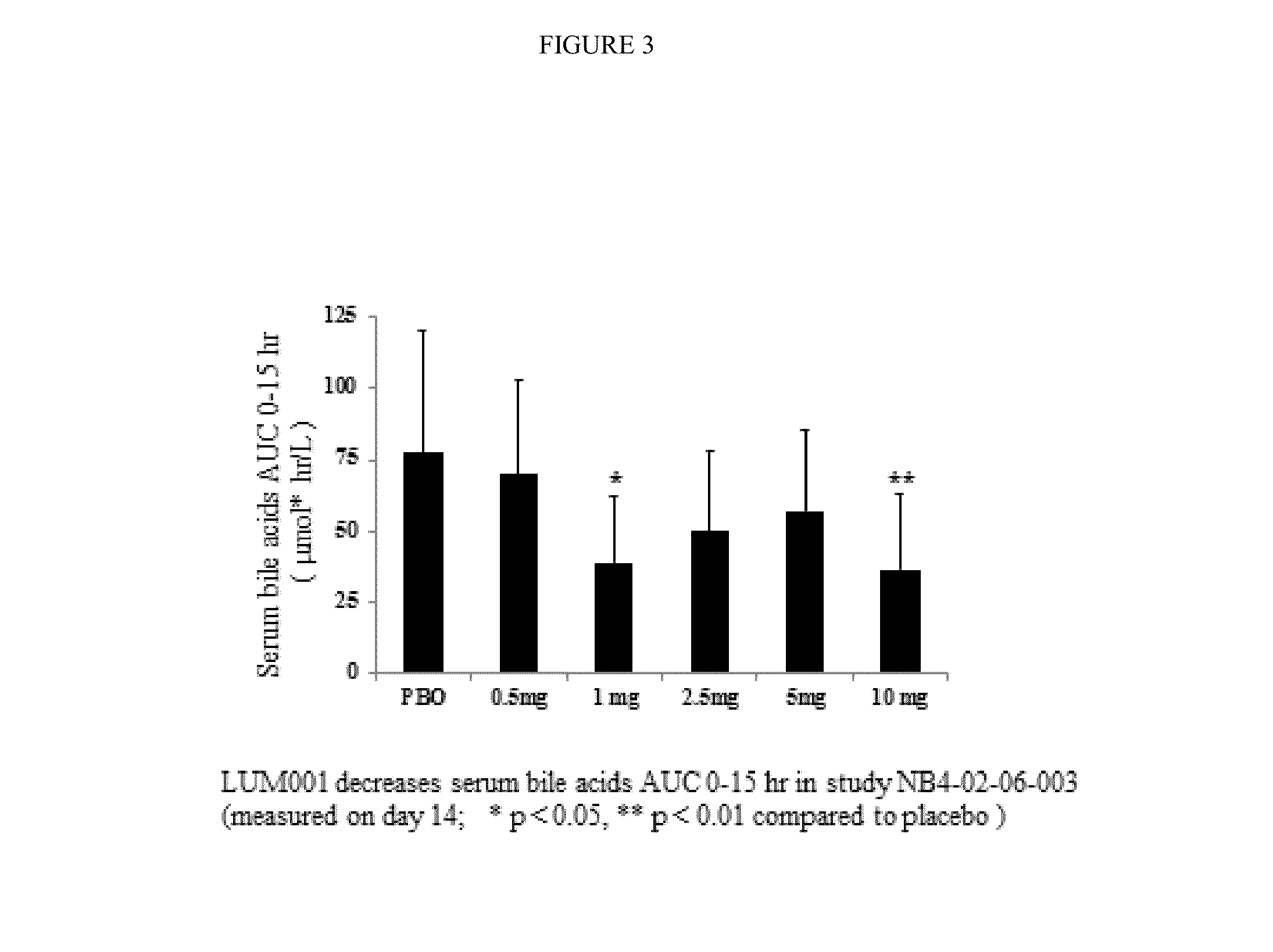
Provided herein are methods of treating or ameliorating a pediatric cholestatic liver disease by non-systemically administering to an individual in need thereof a therapeutically effective amount of a pediatric formulation comprising an Apical Sodium-dependent Bile Acid Transporter Inhibitor (ASBTI) or a pharmaceutically acceptable salt thereof. Also provided are methods for treating or ameliorating a pediatric liver disease, decreasing the levels of serum bile acids or hepatic bile acids, treating or ameliorating pruritis, reducing liver enzymes, or reducing bilirubin comprising non-systemically administering to an individual in need thereof a therapeutically effective amount of a pediatric formulation comprising an ASBTI or a pharmaceutically acceptable salt thereof.
Owner:LUMENA PHARMA INC
Light Therapy for Treating or Managing Diabetes and Metabolic Syndrome
Techniques for managing diabetes and pre-diabetes using light are disclosed herein. In one example, a light generating device is positioned to a body part of a diabetic or pre-diabetic patient, and a beam of light generated by the light generating device is directed to the body part of the patient to control blood sugar level of the patient. In various embodiments, the beam of light directed to the patient can also help to control the blood lipid level such as triglyceride level, blood liver enzyme of the patient, and various other symptoms of diabetes and pre-diabetes. In various embodiments, the body part includes body area rich in adipose tissue, such as the abdominal area, thigh, buttocks, and upper arms of the patient.
Owner:PRESCOTT MARVIN A
Application of GLP-1R/GCGR double-target agonist polypeptide to treatment of fatty liver diseases, hyperlipidemia and arteriosclerosis
ActiveCN106046145AImprove biological activityExtended half-lifePeptide/protein ingredientsMetabolism disorderFibrosisDrug biological activity
The invention relates to application of a polypeptide compound having double agonist effects on a glucagon-like peptide-1 receptor and a glucagon receptor. The polypeptide compound has the characteristics of high enzymolysis stability, high bioactivity, no adverse reaction, etc., can alleviate abnormal rise in the levels of total cholesterol and triglyceride in blood induced by diabetes and high-meal diet, lowers the level of liver enzyme, improves liver damage and fibrosis stage and is applicable to prevention or treatment of diseases like non-alcoholic fatty liver diseases, hyperlipidemia and arteriosclerosis.
Owner:SHENZHEN TURIER BIOTECH CO LTD
Compositions for the treatment of hepatitis C and methods for using compositions for the treatment of hepatitis C
InactiveUS20060229293A1Treating hepatitis CImprove the level ofBiocideCarbohydrate active ingredientsMetaboliteChronic hepatitis
The present invention pertains to a composition comprising Ibogaine, an indole alkaloid, its active salts and its principal metabolite noribogaine, a demethylated form of ibogaine, for the treatment of hepatitis C and hepatitis C related complications, administered in single or multiple dose regimens effective to reduce somatic complaints, liver enzyme values and viral load caused by chronic hepatitis C in patients, and methods of using the same.
Owner:ADDICTION RES INST INC
Individual drug safety
InactiveUS20050037366A1Microbiological testing/measurementBiostatisticsProtein profilingActivity profiling
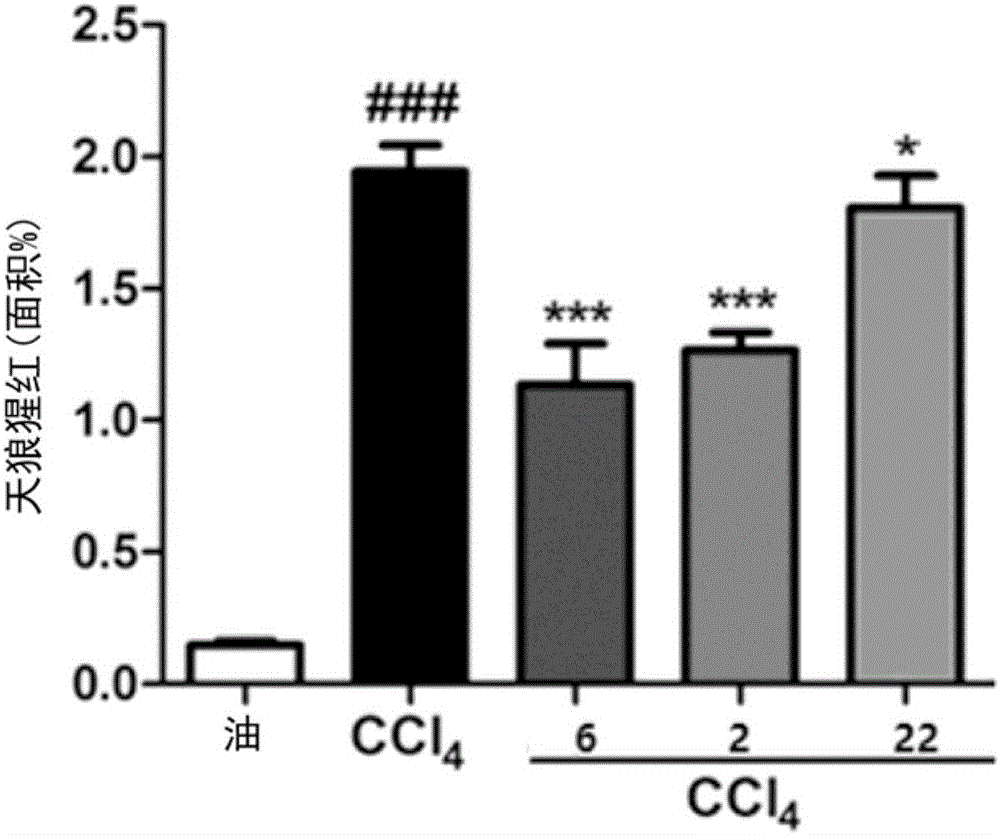
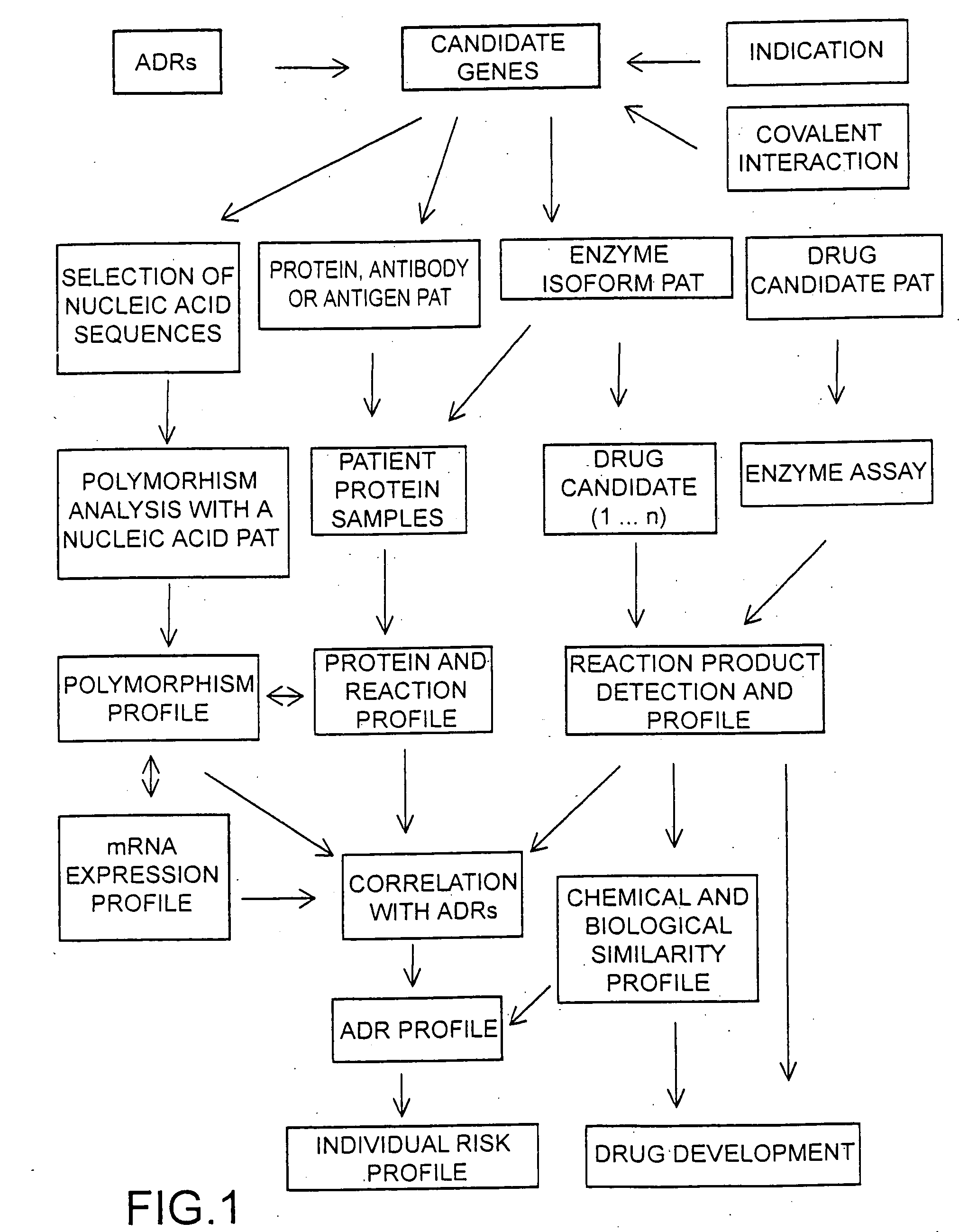
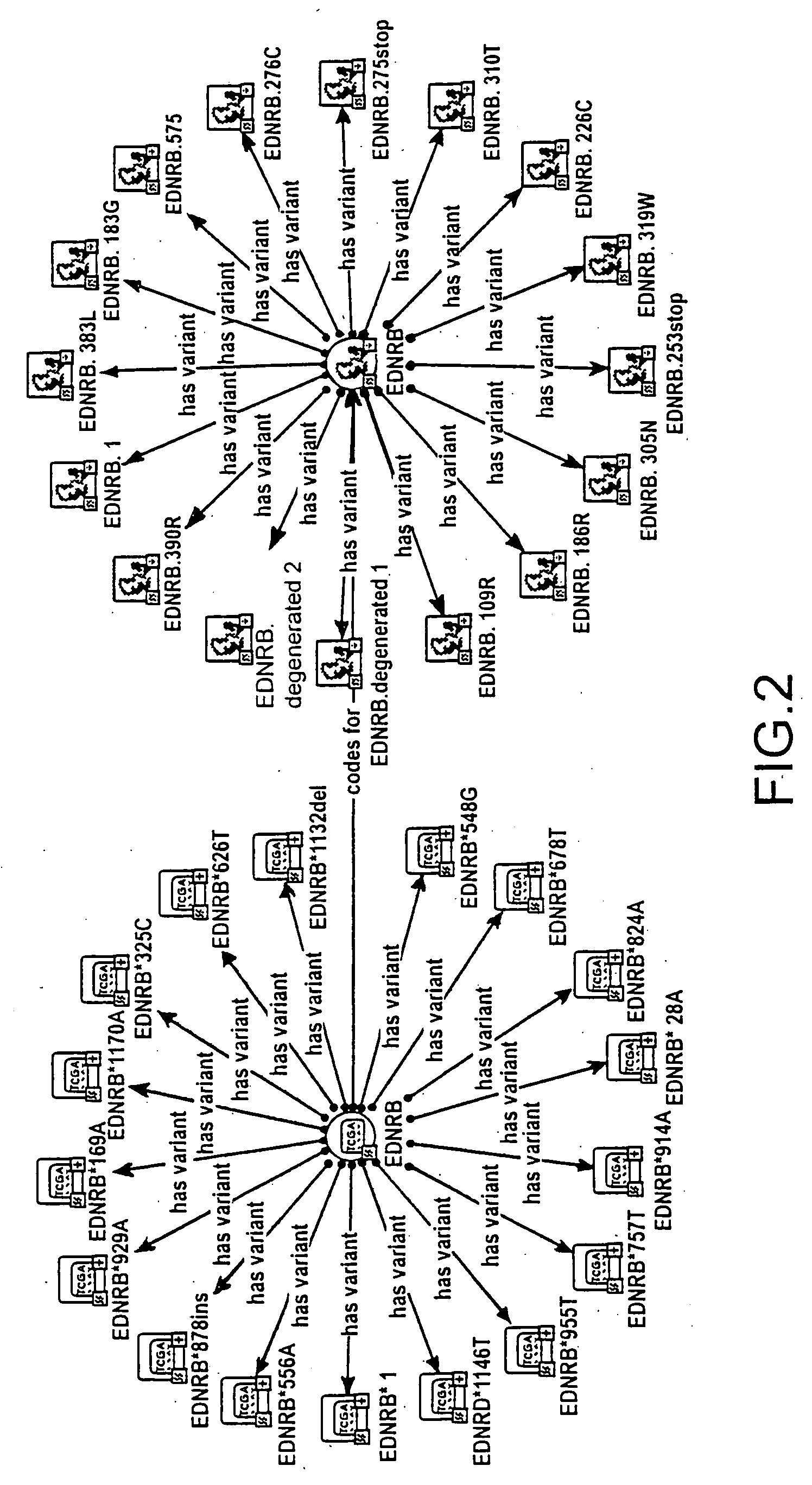
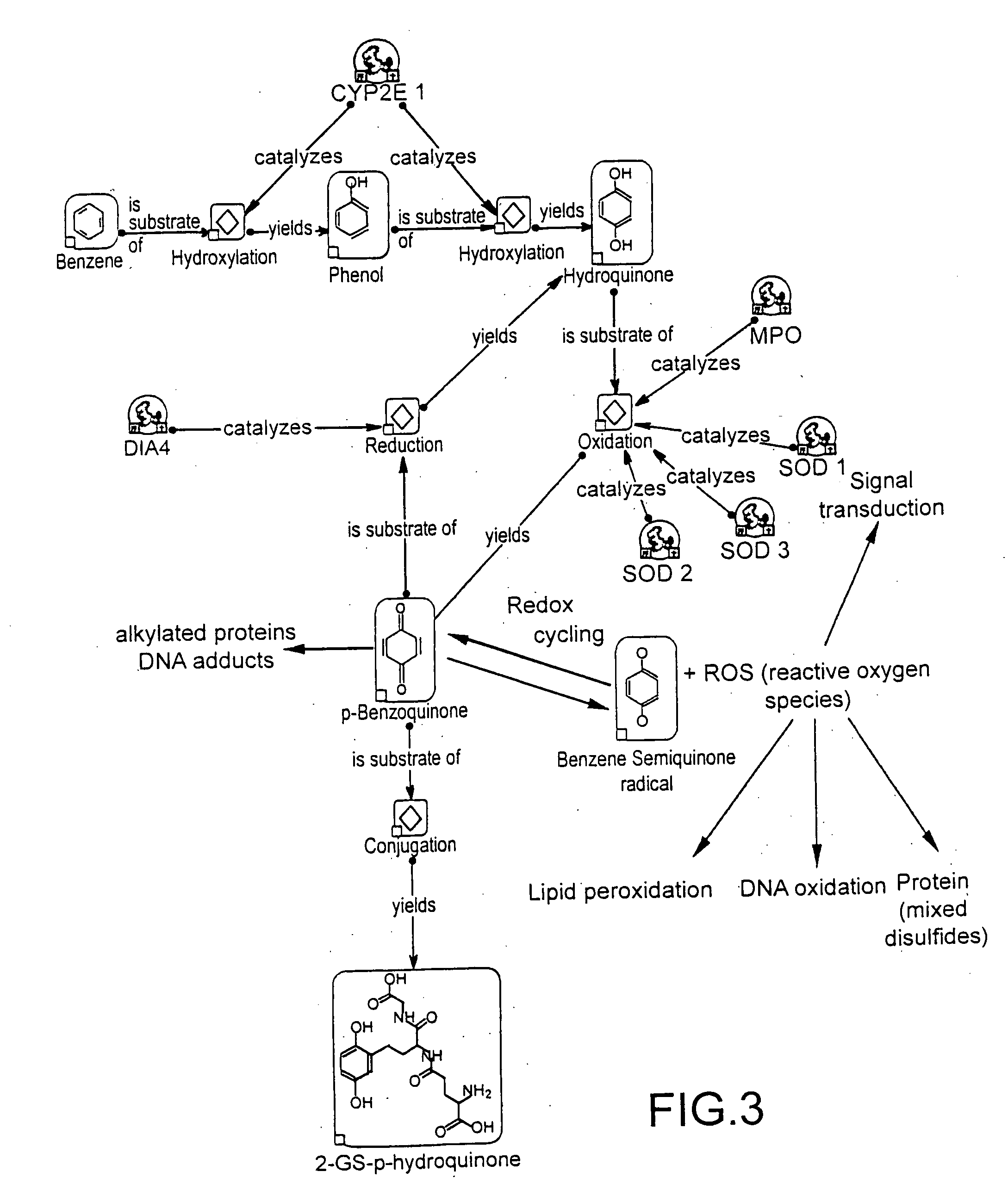
The invention provides means to determine the predisposition of individuals to adverse drug reactions (ADRs). The methods are based on genotyping or parallelized enzyme and protein profiling or both. Parallelized enzyme activity profiling can be used for drug screening and development. As examples of the invention we show the prediction of adverse drug reactions of pulmonary hypertension patients by identifying genes and alleles linked to known ADRs and liver enzyme reaction profiling with ADR correlation.
Owner:THERASTRAT
Microchip-based system for hiv diagnostics
InactiveUS20060234209A1Efficient captureEasy to detectMicrobiological testing/measurementPeptidesDiseaseCD4 Lymphocyte
The invention relates to microchip-based assays to measure HIV-associated analytes of interest (e.g., CD4 lymphocytes, HIV RNA and liver enzymes) in a sample from a subject infected with the HIV virus. Methods of the present invention are optimal for use in monitoring HIV disease in resource-poor settings.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Method for minim hepatic tissue in vitro incubation and detecting CYP450 enzymatic activity
InactiveCN101308119AHigh simulationSave costsComponent separationMicrobiological testing/measurementMetaboliteCytochrome p450 enzyme
Disclosed is a method for detecting CYP450 enzymatic activity by means of micro-liver tissue incubation in vitro, which is characterized in that: a liver trace puncturing method used in clinic takes micro-liver tissues of 0.1 to 0.2g, or kills a mouse to take out the liver in an animal experiment, and builds a micro-liver tissue in vitro incubation system, then uses a one-probe medicine to perform the liquid phase chromatography tandem mass spectrometry for detecting probe substrates and concentration of corresponding metabolites, and obtains the activity of P450 enzyme according to metabolite ratios thereof. The method has the advantages of: 1. micro scale: only micro-liver tissues of 0.1 to 0.2g are needed for detecting cytochrome P450enzyme activity, and can be used for animal experiment and checking clinic micro-liver tissue liver drug enzyme activity; 2. time saving and convenience: costly ultracentrifugation equipment used in the conventional method is saved, costs and time for detection are greatly saved, and liver enzyme metabolic condition simulation is better; and 3. establishing indexes for evaluating the enzyme metabolic activity: metabolite ratios.
Owner:CENT HOSPITAL XUHUI DISTRICT SHANGHAI CITY
Bile Acid Recycling Inhibitors for Treatment of Pediatric Cholestatic Liver Diseases
InactiveUS20130338093A1Reduces and inhibits recyclingReduce intraenterocyte bile acids/saltsBiocidePill deliverySerum igeHepatic bile
Owner:LUMENA PHARMA INC
Goose liver essence and preparation method thereof
The invention discloses a goose liver essence and a preparation method of the goose liver essence. The goose liver essence comprises the following components by weight ratio: 40-60 parts of goose liver enzyme hydrolysis solution, 1-10 parts of reducing sugar, 1-15 parts of amino acid, 0.1-1.5 parts of thiamine hydrochloride, 0.1-5 parts of disodium 5'-ribonucleotide, 0.1-5 parts of spice powder and 30-45 parts of hydrolyzed vegetable protein solution. The goose liver essence is prepared from the goose liver enzyme hydrolysis solution as well as the reducing sugar, the amino acid, the thiamine hydrochloride, the disodium 5'-ribonucleotide, the spice powder and the hydrolyzed vegetable protein solution through Maillard reaction, wherein the goose liver enzyme hydrolysis solution serves as the main raw material, and the reducing sugar, the amino acid, the thiamine hydrochloride, the disodium 5'-ribonucleotide, the spice powder and the hydrolyzed vegetable protein solution serve as the auxiliary raw materials. Therefore, the prepared product has strong real goose liver characteristic aroma, mellow taste and high nutritional value and can be widely used as the essence of instant noodle seasonings, food ingredients and meat products.
Owner:广州百花香料股份有限公司
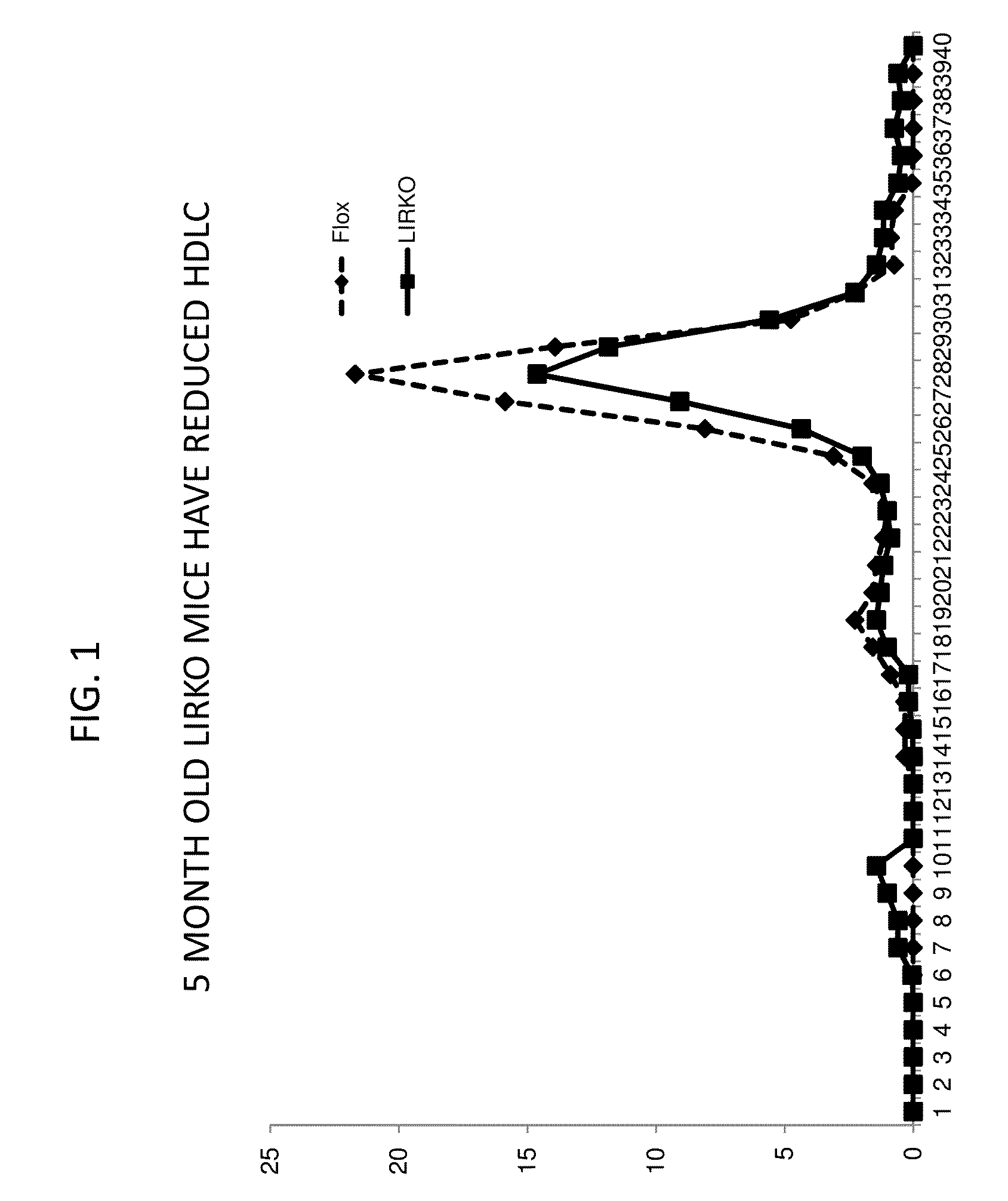
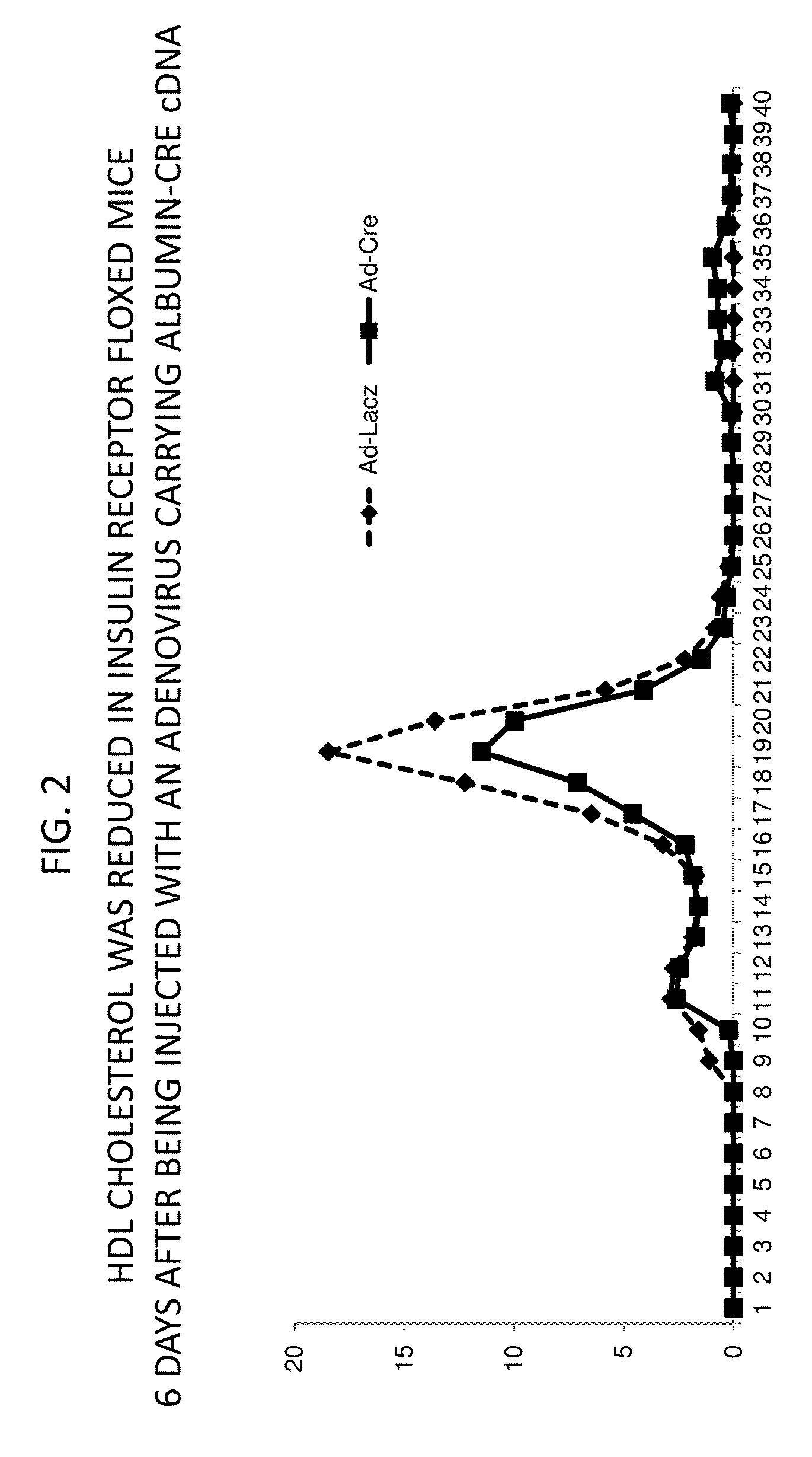
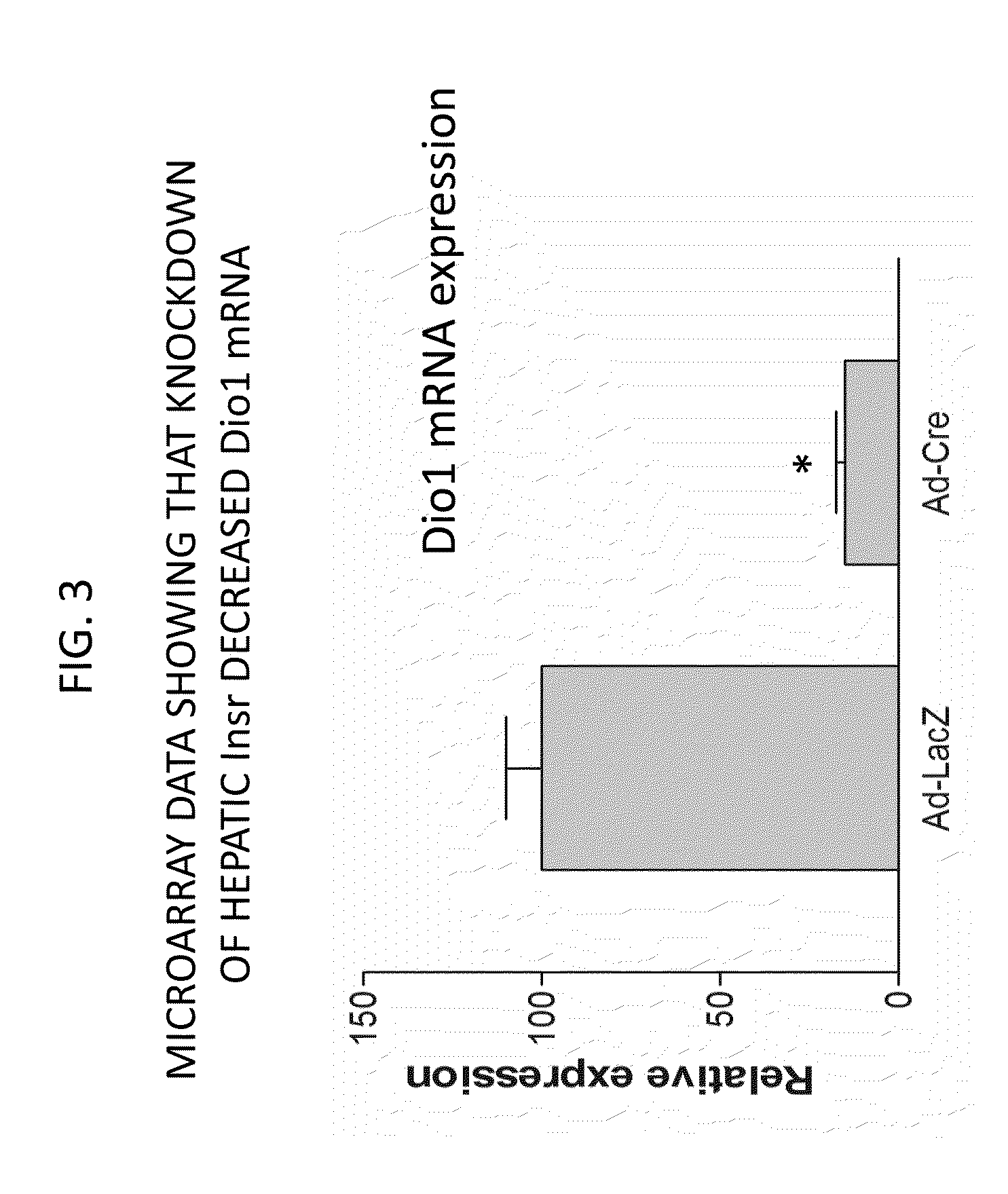
Methods For High Density Lipoprotein Cholesterol Regulation
InactiveUS20130017250A1Reduced insulin receptor expressionReduce biological activitySugar derivativesPeptide/protein ingredientsADAMTS ProteinsBlood plasma
It was discovered that insulin binding to insulin receptors signals the upregulation of expression of the liver enzyme deiodinase 1 (Dio1), which in turn activates the ApoA-1 promoter, thereby thereby increasing ApoA-1 expression (primarily in the liver), that in turn raises the levels of plasma ApoA-1, the major and necessary protein in HDLC. Certain embodiments of the invention are directed to methods for increasing circulating HDLC levels in an animal by administering therapeutically effective amounts of Dio1, or by increasing the level of Dio1 through gene therapy.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
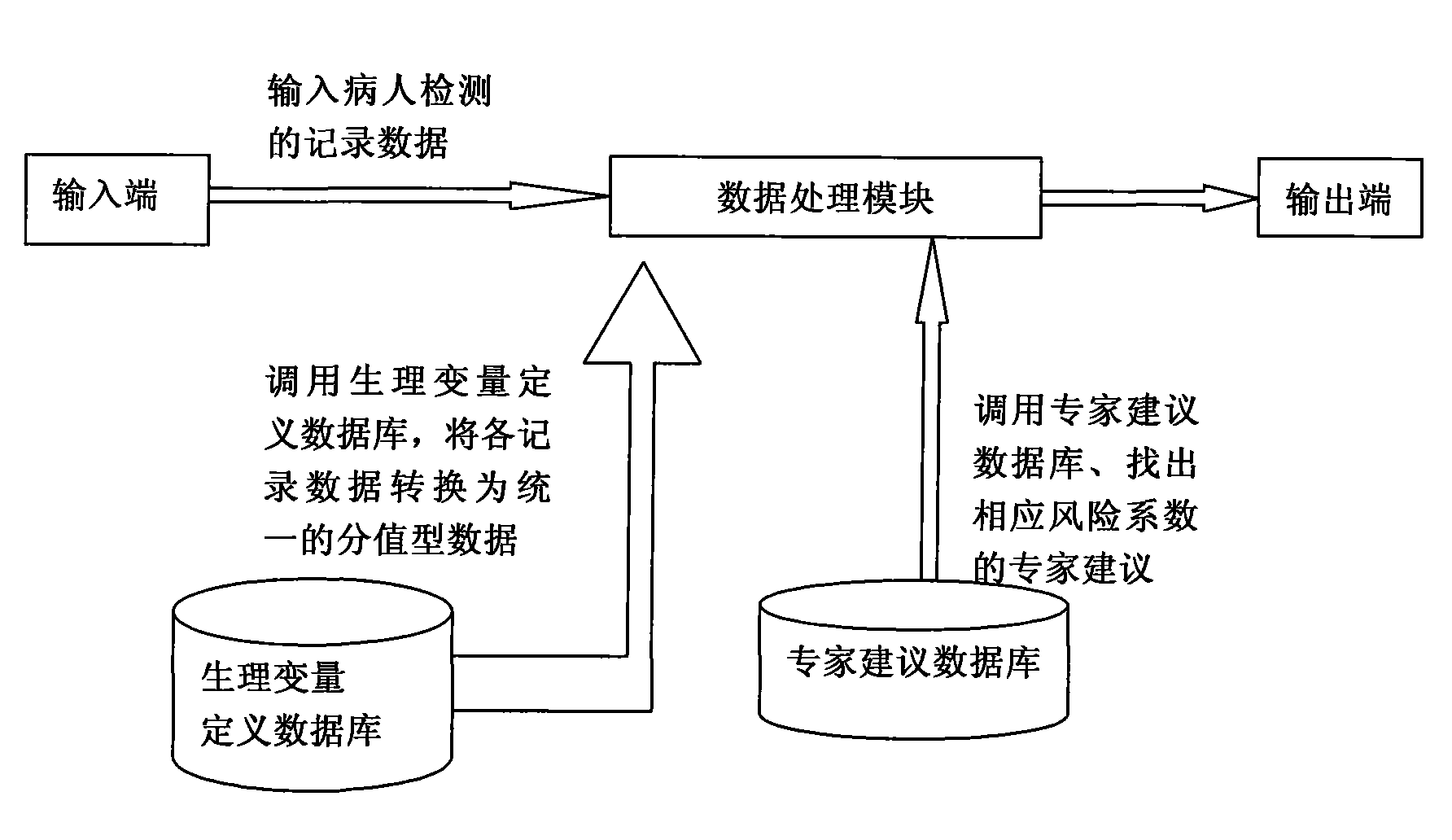
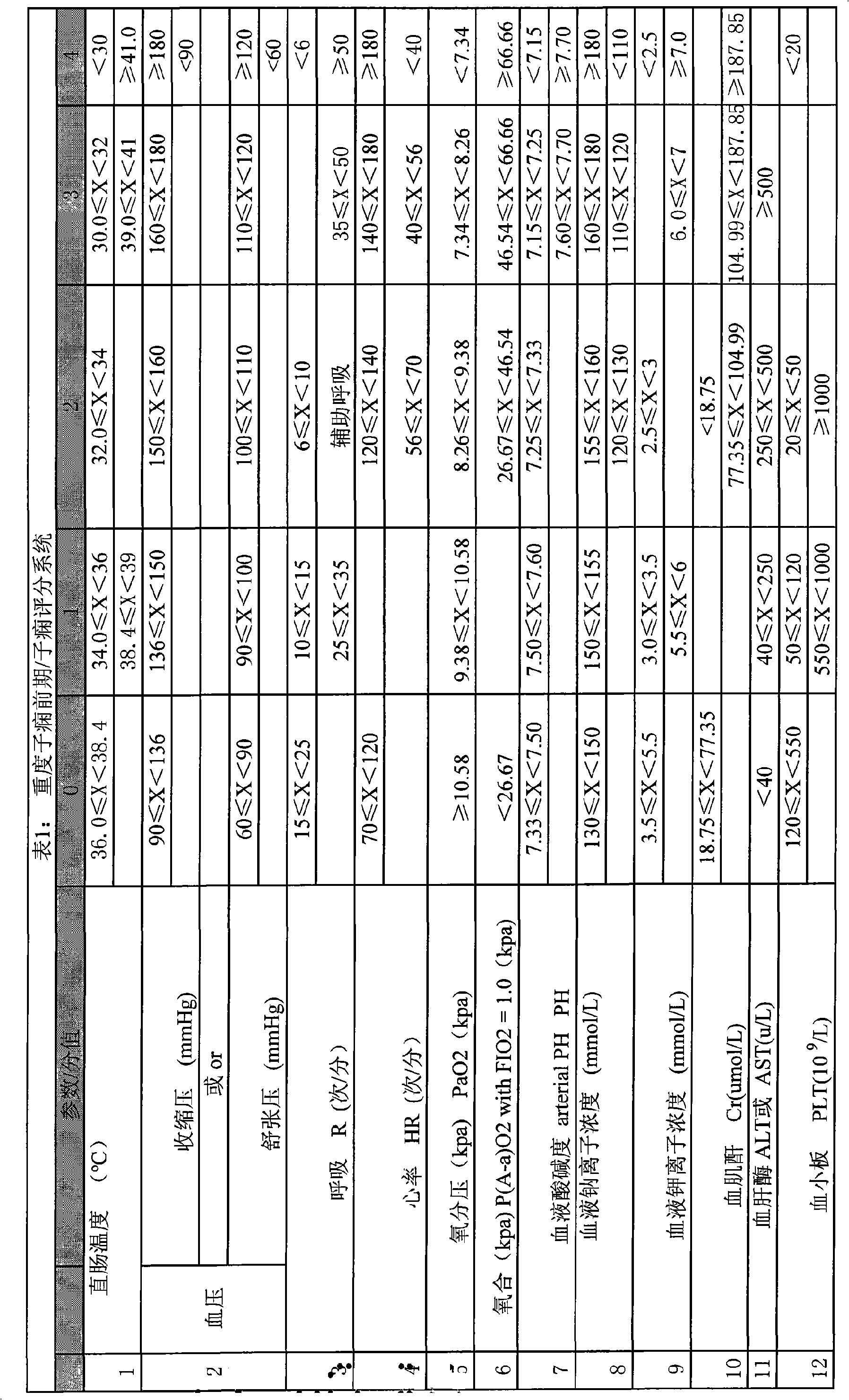
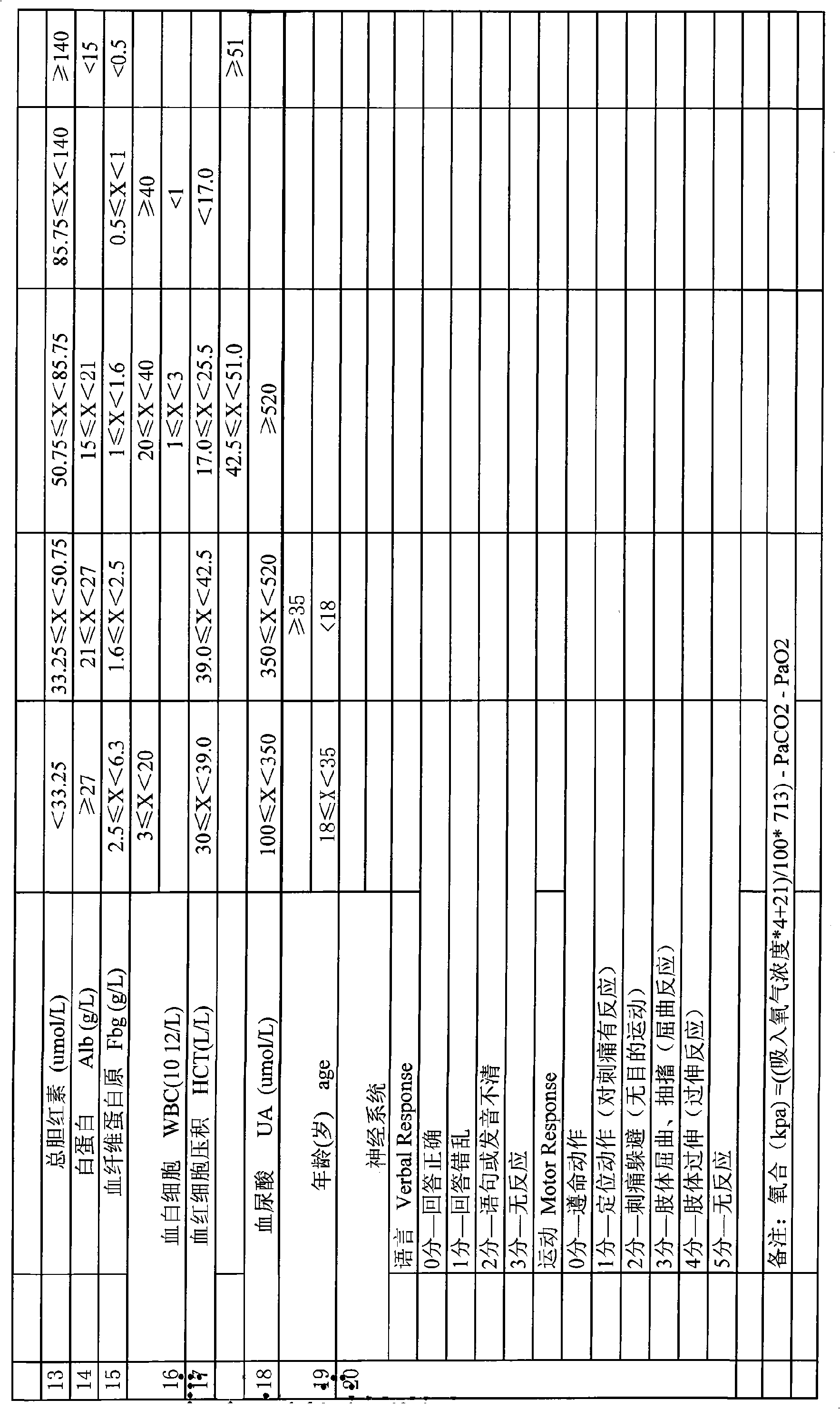
Serious preeclampsia/eclampsia illness state evaluation system
InactiveCN101548876AReduce mortalityReduce inappropriateness of criticality assessmentSurgeryDiagnostic recording/measuringNervous systemCreatinine rise
The invention discloses a serious preeclampsia / eclampsia illness state evaluation system which comprises an input end, an output end and a data processing module. Firstly, the measured heart rate, the blood pressure, the body temperature, the breathing rate, the pH, the oxygen partial pressure, the oxygenation, the sodium ion concentration, the hematokrit, the white cell count, the platelet count, the fibrinogen, the blood liver enzyme, the albumin, the bilirubin, the creatinine, the blood uric acid and an age scoring and nervous system scoring data input end of a patient are input to the serious preeclampsia / eclampsia illness state evaluation system; then the data processing module works out death risk factor; and finally, the output end directly reflects results of the patient and expert suggestions. The invention can quantificationally evaluate the illness state critical degree of a serious preeclampsia / eclampsia patient, dynamically evaluate the serious preeclampsia / eclampsia of the patient, predict death risks and provide clinical processing reference proposals and is beneficial to enhance the consistency and the comparability of a selected contrast and a clinical case, thereby lowering the mortality rate of newborn babies and pregnant women.
Owner:刘慧姝 +1
Plant oil containing 4:1 ratio of linoleic acid and linolenic acid
InactiveCN1857088APrevention of cardiovascular and cerebrovascular diseasesEdible oils/fatsVegetable oilPerilla oil
The present invention relates to a kind of plant oil with linoleic acid and linolenic acid in the ratio of 4 to 1, and the plant oil is mixture of five kinds of plant oil, including perilla oil, linseed oil, sunflower seed oil, soybean oil and sesame oil in undetermined proportion and contains linoleic acid in 48 wt% and linolenic acid in 12 wt%. The plant oil has increased linolenic acid, and under the action of the liver enzyme inside human body, linolenic acid will have its carbon chain elongated to become eicosapentaenoic acid, so-called 'blood vessel cleaner', and docosahexenoic acid, so-called 'brain gold'. Therefore, the present invention is significant in preventing various kinds of cardiac and cerebral vascular diseases.
Owner:上海高寿康油脂有限公司
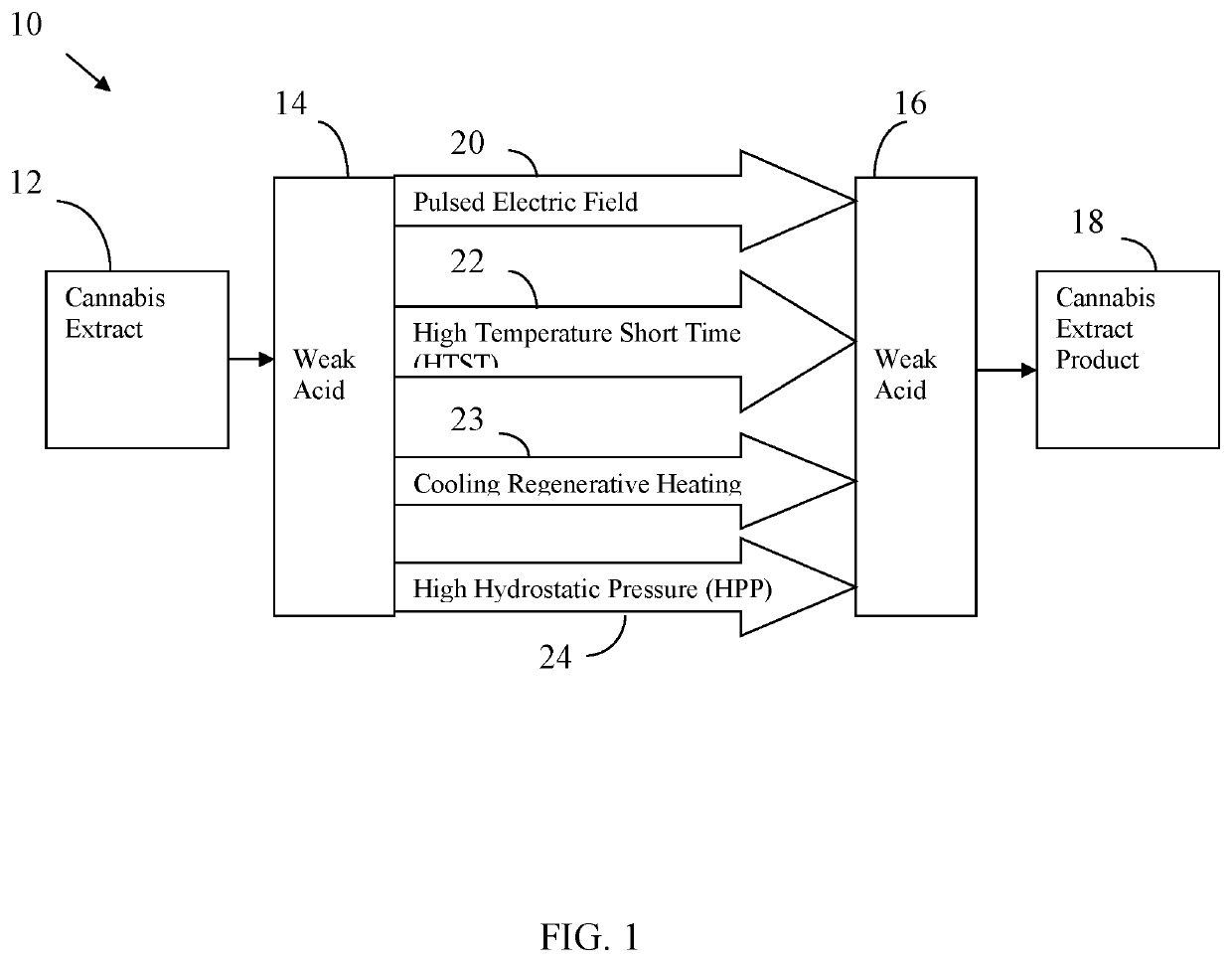
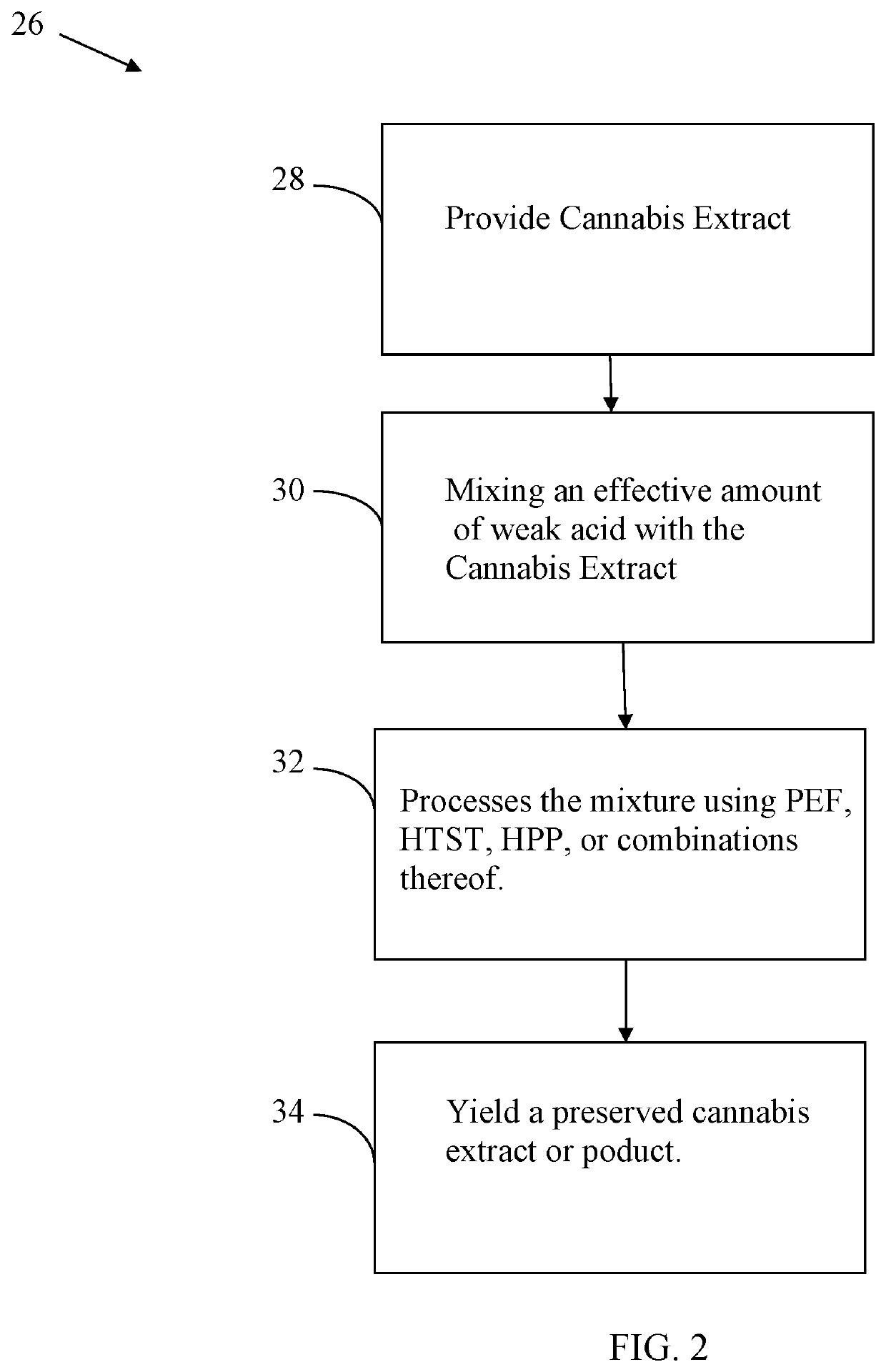
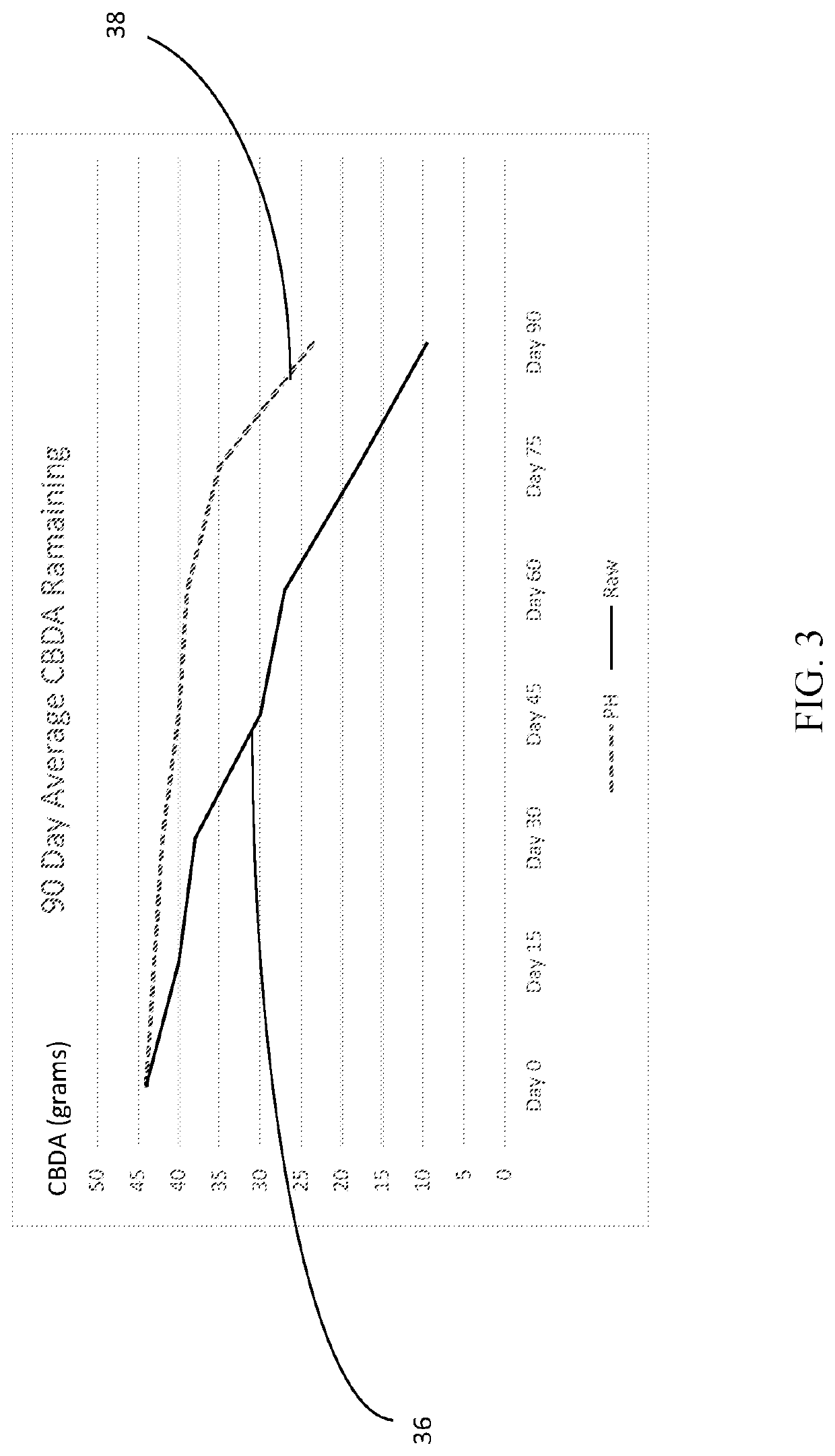
Cannabis extract and juice having improved shelf life and methods thereof
InactiveUS20200170283A1Reducing enzymatic browningDelay and maintain and inhibit and retardClimate change adaptationFood preservationBiotechnologyFruit juice
Cannabis extract subjected to a Pulsed Electric Fields (PEF), High Temperature Short Time (HTST), Heat, High Pressure Processing (HPP) and weak acid preserves the cannabis extract. This process delays, maintains, inhibits, retards and / or reduces the rate of plant derived and / or synthetically derived cannabinoid extract deterioration or degradation and is ideally used in beverages, foods and other consumer products, including a juice product. In addition to preserving the product, the weak acids are preferably citric acid and lemon juice to both inhibit action of oxidative enzymes in the packaged product, but to also inhibit hepatic enzymes in vivo to improve the duration of bio-activity, improve bio-availability, and optimize bio-effects of cannabinoids.
Owner:REGENERATIVE FOODS & JUICES CO
Purified limonin glucoside for prevention and treatment of chronic diseases
One exemplary embodiment of the disclosure provides methods and compositions for treating a subject suffering from Alcoholic Liver Disease, Non-Alcoholic Fatty Liver Disease, type 2 diabetes, metabolic syndrome, cardiovascular disease, chronic kidney disease, and certain cancers, or a combination thereof, by administering a therapeutically effective amount of purified liminoid glucoside or a pharmaceutically acceptable salt thereof to the subject. In another exemplary embodiment, the disclosure provides methods and compositions for treating a subject suffering from elevated circulating concentrations of liver enzymes wherein the liver enzymes are selected from gamma glutamyl transferase, alanine amino transferase, alkaline phosphatase and complement fraction 3 or a combination thereof, the method comprising administering a therapeutically effective amount of purified liminoid glucoside or a pharmaceutically acceptable salt thereof to the subject wherein the treating results in a decreased circulating concentration of the liver enzymes.
Owner:KELLEY DARSHAN SINGH
Double enzyme coupling preparation of ornithine hydrochloride
The invention relates to a method for preparing ornithine hydrochloride, in particular to a double-enzyme coupling method for preparing the ornithine hydrochloride. The invention provides an economic and environment-friendly double-enzyme coupling method for preparing the ornithine hydrochloride. The method fully utilizes biological enzymes to prepare the product of the ornithine hydrochloride and is distinguished from the most production methods which depend on chemical reagent too much in the prior art. The method adopts urease extracted from fresh soybeans and bovine liver enzyme solution extracted from fresh bovine livers to prepare the ornithine hydrochloride.
Owner:NINGBO ZHENHAI HAIDE BIOCHEM
Microorganism feed additive capable of protecting pig livers and preparation method of microorganism feed additive
InactiveCN106387317AGrowth inhibitionInhibition of reproductionFungiBacteriaErythrocyte membranePig liver
The invention relates to a microorganism feed additive capable of protecting pig livers and a preparation method of the microorganism feed additive. All strains adopted by the microorganism feed additive are screened out from intestinal tracts of animals, and a strain compound culturing method is adopted, so that an antagonism mechanism of the strains is avoided, and fermented products are optimized; and in the whole production technology, beneficial microorganisms are furthest maximized, and a main fermented product namely glutathione is wholly recovered from intracellular and extracellular positions. According to the microorganism feed additive and the preparation method thereof disclosed by the invention, oxygen free radicals can be effectively eliminated, so that the stability of liver cell membranes is improved, the activity of liver enzymes is promoted, the absorption of iron can also be promoted, the integrity of red cell membranes can be maintained, the biosynthesis of DNA is maintained, the normal growth of cells is maintained, and cell immunity is maintained; as a micro-ecologic preparation, the microorganism feed additive can effectively promote the digestion and the absorption of the feed by the animals, the lean meat percentage is increased, toxin disoperation can be alleviated, the feed conversion rate is increased, and the production capacity of bred animals is improved.
Owner:上海绿博生物科技发展有限公司
Bile acid recycling inhibitors for treatment of hypercholemia and cholestatic liver disease
InactiveUS20140323412A1Reduces and inhibits recyclingReduce intraenterocyte bile acids/saltsEsterified saccharide compoundsBiocideDiseaseHepatic bile
Provided herein are methods of treating or ameliorating hypercholemia or a cholestatic liver disease by administering to an individual in need thereof a therapeutically effective amount of an Apical Sodium-dependent Bile Acid Transporter Inhibitor (ASBTI) or a pharmaceutically acceptable salt thereof. Also provided are methods for treating or ameliorating a liver disease, decreasing the levels of serum bile acids or hepatic bile acids, treating or ameliorating pruritis, reducing liver enzymes, or reducing bilirubin comprising administering to an individual in need thereof a therapeutically effective amount of ASBTI or a pharmaceutically acceptable salt thereof.
Owner:LUMENA PHARMA LLC
Purified limonin glucoside for prevention and treatment of chronic diseases
ActiveUS9066965B1Decreased circulating concentrationReduce concentrationOrganic active ingredientsSugar derivativesGamma glutamyl transferaseGlucoside
One exemplary embodiment of the disclosure provides methods and compositions for treating a subject suffering from Alcoholic Liver Disease, Non-Alcoholic Fatty Liver Disease, type 2 diabetes, metabolic syndrome, cardiovascular disease, chronic kidney disease, and certain cancers, or a combination thereof, by administering a therapeutically effective amount of purified liminoid glucoside or a pharmaceutically acceptable salt thereof to the subject. In another exemplary embodiment, the disclosure provides methods and compositions for treating a subject suffering from elevated circulating concentrations of liver enzymes wherein the liver enzymes are selected from gamma glutamyl transferase, alanine amino transferase, alkaline phosphatase and complement fraction 3 or a combination thereof, the method comprising administering a therapeutically effective amount of purified liminoid glucoside or a pharmaceutically acceptable salt thereof to the subject wherein the treating results in a decreased circulating concentration of the liver enzymes.
Owner:KELLEY DARSHAN SINGH
Prognostic marker to determine the risk for early-onset preeclampsia
InactiveUS20160025738A1Increase opportunitiesGood blood pressureMicrobiological testing/measurementScattering properties measurementsObstetricsCvd risk
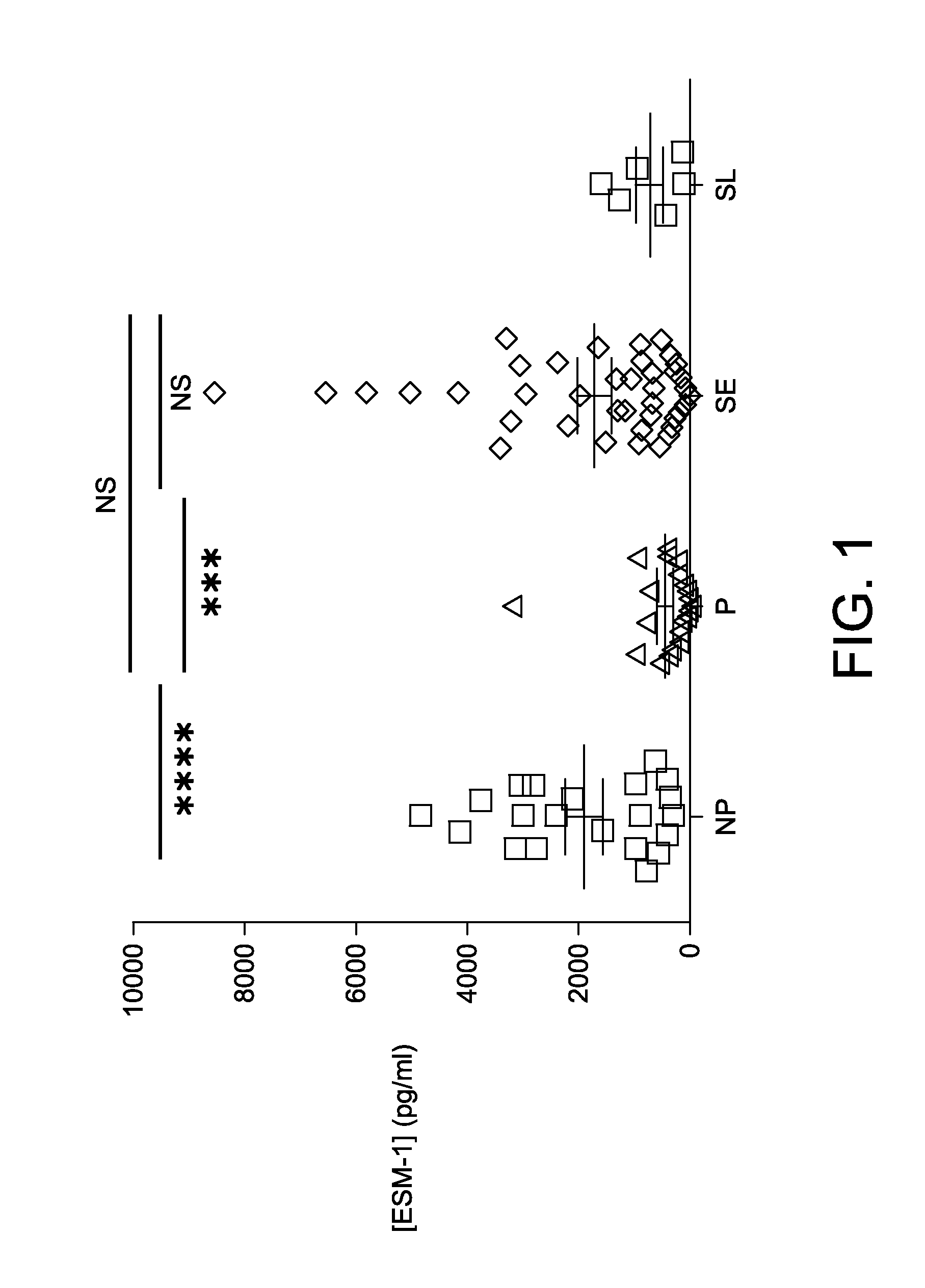
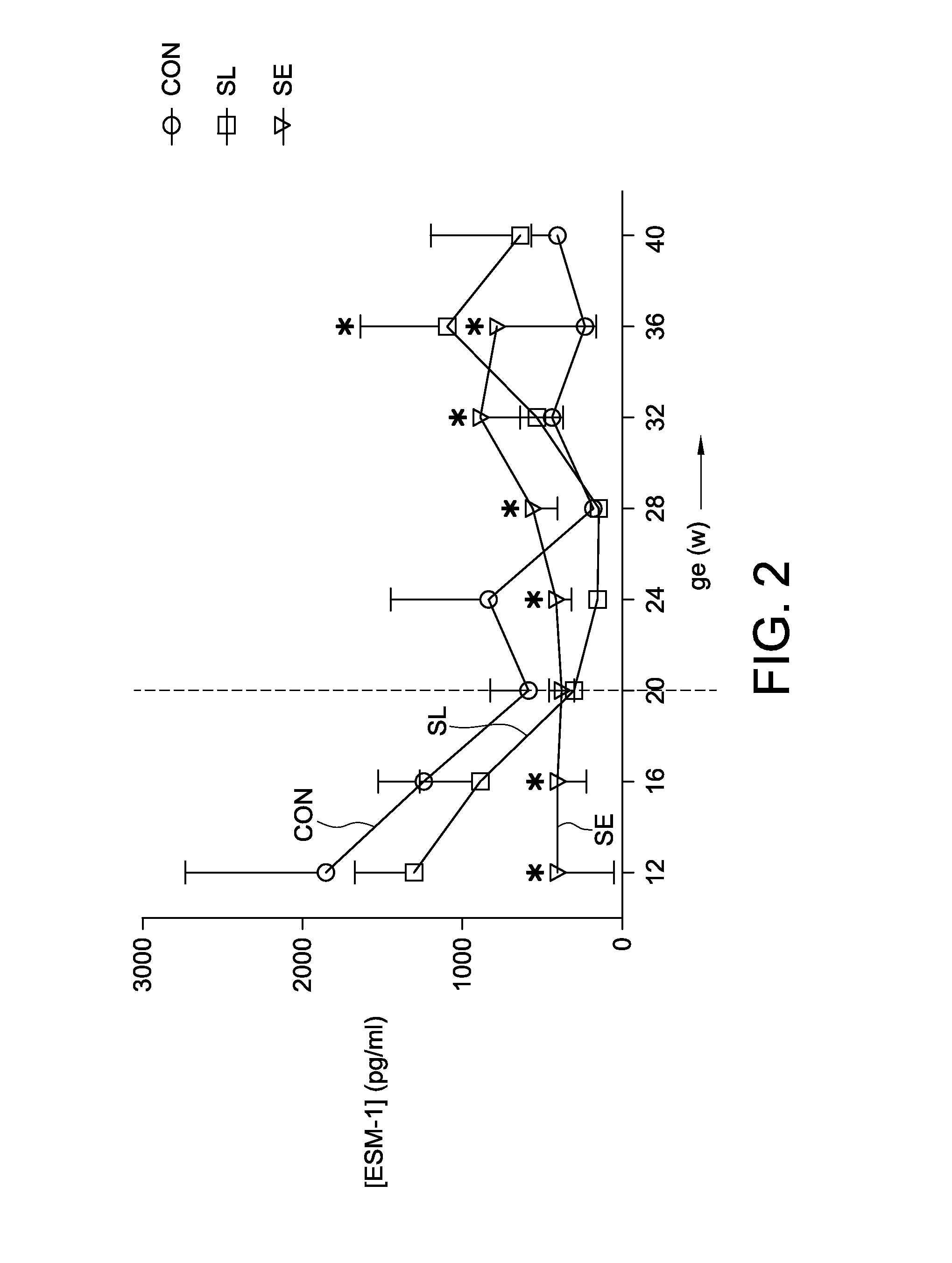
The present application relates to an in vitro method for identifying a pregnancy related syndrome selected from the group consisting of pre-eclampsia, eclampsia, Hemolysis Elevated Liver enzymes and Low Platelets (HELLP) and intra-uterine growth restriction (IUGR), the method including (i) measuring the amount of ESM-1 in a biological fluid sample from a pregnant subject, wherein the pregnant subject is between week 1 to 20 of gestation; (ii) comparing said amount of ESM-1 to a reference value, and (iii) identifying the subject as being likely to have or develop the pregnancy related syndrome based on a comparison of the amount of ESM-1 to the reference value. A device and a kit for identifying such a pregnancy related syndrome are also claimed.
Owner:IQ PROD
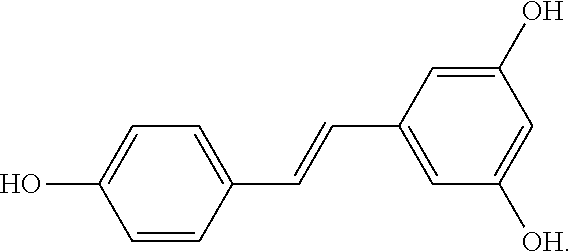
Use of reservatrol for the treatment of non-alcoholic fatty liver disease (NAFLD)
ActiveUS20190209488A1Reduce fatImprove antioxidant capacityHydroxy compound active ingredientsDigestive systemInsulin resistanceHepatic enzyme
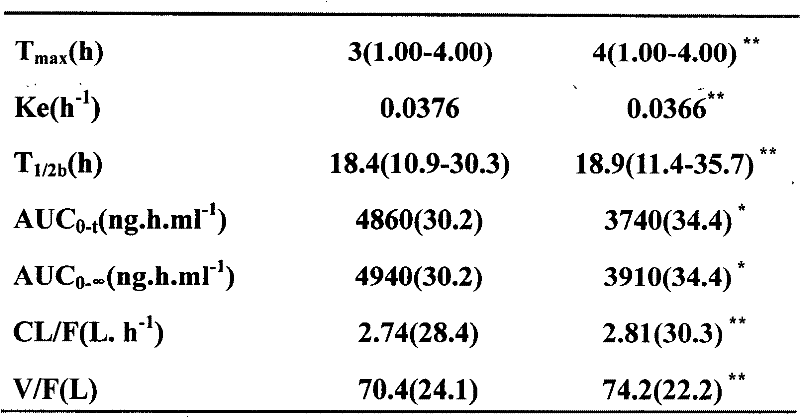
Micronized trans-resveratrol is provided in 50-200 mg unit dosage form for use as a single unit dose daily for administration to human patients the treatment or prevention of non-alcoholic fatty liver disease and / or for the treatment, prevention or reversal of non-alcoholic hepatic steatosis, e.g. for administration to patients exhibiting evidence of fatty liver on ultrasonography. A reported study shows the effects of resveratrol micronized formulation in reducing the liver fat, decreasing hepatic enzymes, serum glutamate pyruvic transaminase (SGPT) and gamma-glutamyl transpeptidase (g-GT) and decreasing insulin resistance. At the end of the study, statistical analysis showed a strongly statistically significant reduction in the liver fat, which in some patients continued for an extended period after treatment was discontinued. These results demonstrate that use of resveratrol in micronized formulation improves features of NAFLD, prevents liver damage and that resveratrol micronized formulation can be an effective treatment for NAFLD.
Owner:THEODOTOU MARIOS ANDREOU
Tadalafil chewing gum and manufacturing method and application
InactiveCN101143145BSymptoms improvedImprove erectile dysfunctionOrganic active ingredientsChewing gumTadalafilSexual dysfunction drugs
A tadalafil chewing gum and the manufacturing method and the application relate to a medical chewing gum for remedying male sexual dysfunction and the preparation method. Because of the indissolubility and the low biological utilization degree of the tadalafil, the noneffective dosage is large, and a plurality of adverse reactions occurs. The component of the tadalafil chewing gum includes a chewing gum matrix, which contains tadalafil or tadalafil slat, and the ratio of the weight portion is 100 portions of chewing gum matrix to 0.1 to 10 portions of tadalafil. The product is used for improving the administration method of the drug of tadalafil for the male sexual dysfunction and can promote the stable absorption of the drug. The drug can be directly absorbed through the sublingual vein by orally chewing, which reduces the damage towards the drug because of gastrointestinal enzyme and liver enzyme (first-pass effect) and improves the biological utilization degree and reduces the dosage of the drug; the invention can also reduce the stimulation of the drug toward the gastrointestinal tract, which reduces the occurrence of the adverse reaction and increases the safety.
Owner:北京康业元投资顾问有限公司
Compound alcoholism-relieving and liver-protecting kudzuvine-flower ginseng oral liquid and preparation method thereof
The invention discloses a kudzu flower and ginseng compound anti-alcohol and liver-protecting oral liquid and a preparation method thereof. The kudzu flower and ginseng are used as the monarch medicine and supplemented with six traditional Chinese medicines of ganoderma lucidum, chrysanthemum, skullcap, astragalus, mulberry and thyme. The effective essence of traditional Chinese medicine is extracted by CO2 supercritical, and the extract powder is dried and sieved, and then 9% of the extract powder, 15% of jujube honey, 26% of fresh lotus root juice, and 50% of purified water are mixed and stirred to make an oral liquid. Taking it in time before and after drinking can enhance the function of the liver enzyme system, accelerate the decomposition of alcohol, and improve alcohol tolerance to prevent drunkenness and sobering up. Effectively relieves headache, dizziness, polydipsia and vomiting caused by alcoholism, protects and repairs liver cell damage caused by alcoholism; daily use can also nourish and protect the liver, and remove alcohol poisoning accumulated in the body for many years; it is simple and convenient to take, without side effects; and The curative effect is remarkable, the effect is quick, the course of treatment is short, and the total effective rate reaches 100%.
Owner:闫战军
Nursing point determination method
The invention relates to an immunoassay for evaluating liver diseases or functions by virtue of a nursing point. The immunoassay comprises the following steps: (i) enabling a blood sample coming froma human subject to be contacted with a specific binding agent used for identifying an epitope of a liver enzyme or metabolite, so as to form an antigen-binding agent composite, and detecting the composite by using a second binding agent connected with a detectable reporter, wherein a rodent antibody can not identify the epitope in presence of human blood plasma; and (ii) detecting the liver diseases or functions in the subject according to mass concentration of the liver enzyme or metabolite in the sample.
Owner:NANJING BIOPOINT DIAGNOSTIC TECH CO LTD
Composition containing cannabidiol and use of composition in treatment of systemic inflammatory response syndrome
ActiveCN113521050AReduce joinLow THC contentAntibacterial agentsNervous disorderTherapeutic effectOrgan damage
The present invention relates to a composition containing cannabidiol and use of the composition in the treatment of systemic inflammatory response syndrome. The composition provided by the invention comprises cannabidiol and gamma-aminobutyric acid. Various preparations are prepared by compounding and emulsifying cannabidiol and gamma-aminobutyric acid, and can be used for treating or relieving systemic inflammatory response syndromes caused by various external causes or internal causes or reducing cytokine storm and the degree of tissue and organ injury caused by cytokine storm. In the application of the composition, in order to avoid the first pass effect of liver enzyme metabolism and improve the bioavailability of fat-soluble substances and water-soluble substances, oral administration, nasal spray, nasal smearing and transdermal administration (acupoint application, massage or injection) modes can be adopted. The composition disclosed by the invention has an obvious treatment effect on the systemic inflammatory response syndrome caused by acute body injuries such as bacterial infection, trauma and shock, and also has a potential application prospect in the systemic inflammatory response syndrome occurring in virus infection, advanced cancer or an immunotherapy process.
Owner:铸鼎北京医学技术发展有限公司
Method of deriving mature hepatocytes from human embryonic stem cells
ActiveUS20160032287A1Reduce loadReduce inflammationHepatocytesPeptide/protein ingredientsDrug metabolismCell differentation
A method for producing mature hepatocytes having functional hepatic enzyme activity from human pluripotent cells is disclosed. The method includes the step of transferring an external vector comprising the DNA sequence coding for a microRNA having the seed sequence of the microRNA miR-122, the DNA sequence coding for a microRNA having the seed sequence of the microRNA miR-let-7c, a microRNA having the seed sequence of the microRNA miR-122, a microRNA having the seed sequence of the microRNA miR-let-7c, or a combination thereof into one or more fetal hepatocytes. The resulting cells differentiate into mature hepatocytes that exhibit functional hepatic enzyme activity, and can be used in drug metabolism and toxicity testing, in the study of viruses that target hepatic tissue, and as therapeutics.A related method of maintaining the functional hepatic enzyme activity of primary hepatocytes over time is also disclosed. The method includes the step of transferring an external vector comprising the DNA sequence coding for a microRNA having the seed sequence of the microRNA miR-122 into one or more cultured primary hepatocytes.
Owner:WISCONSIN ALUMNI RES FOUND
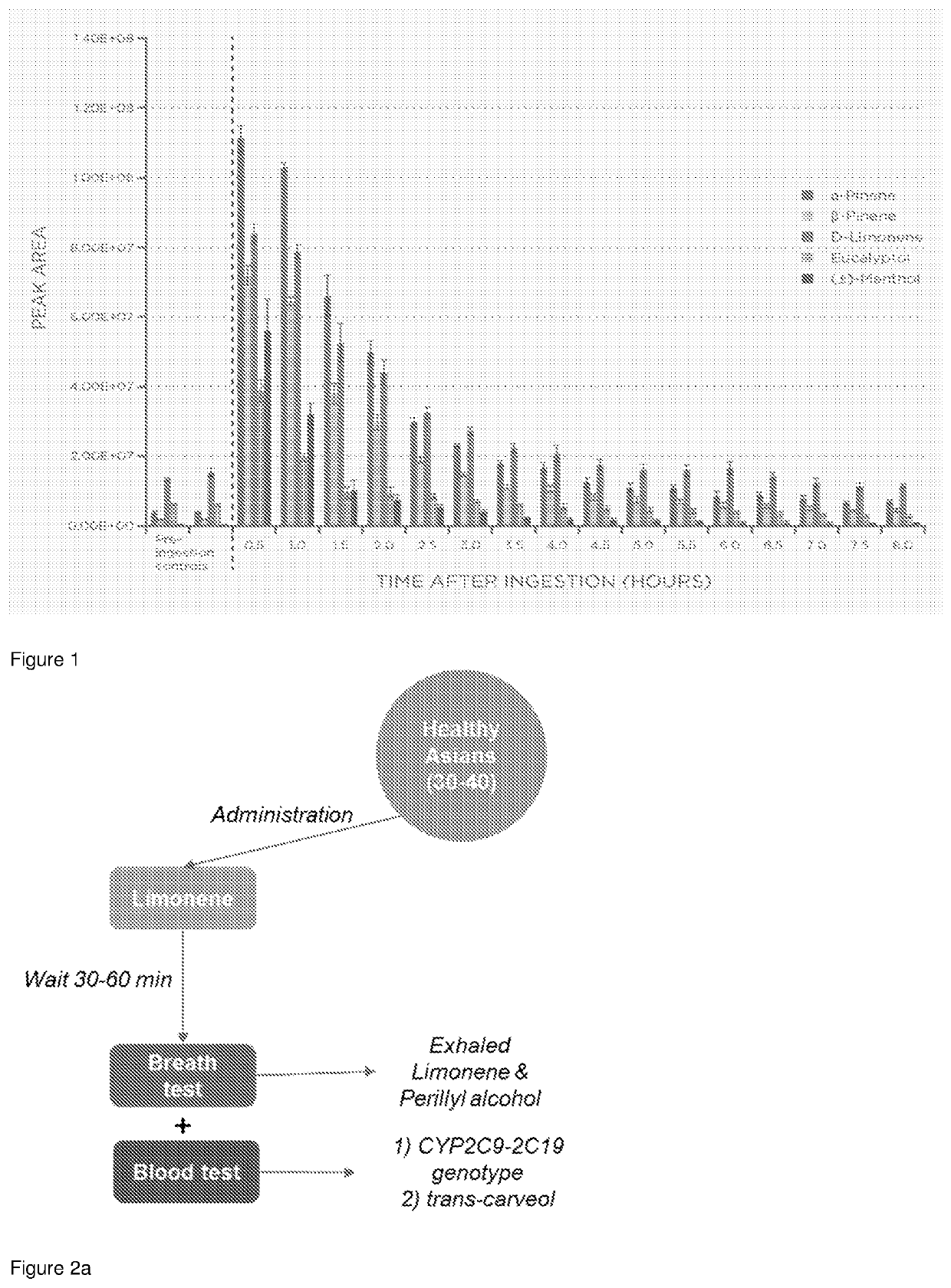
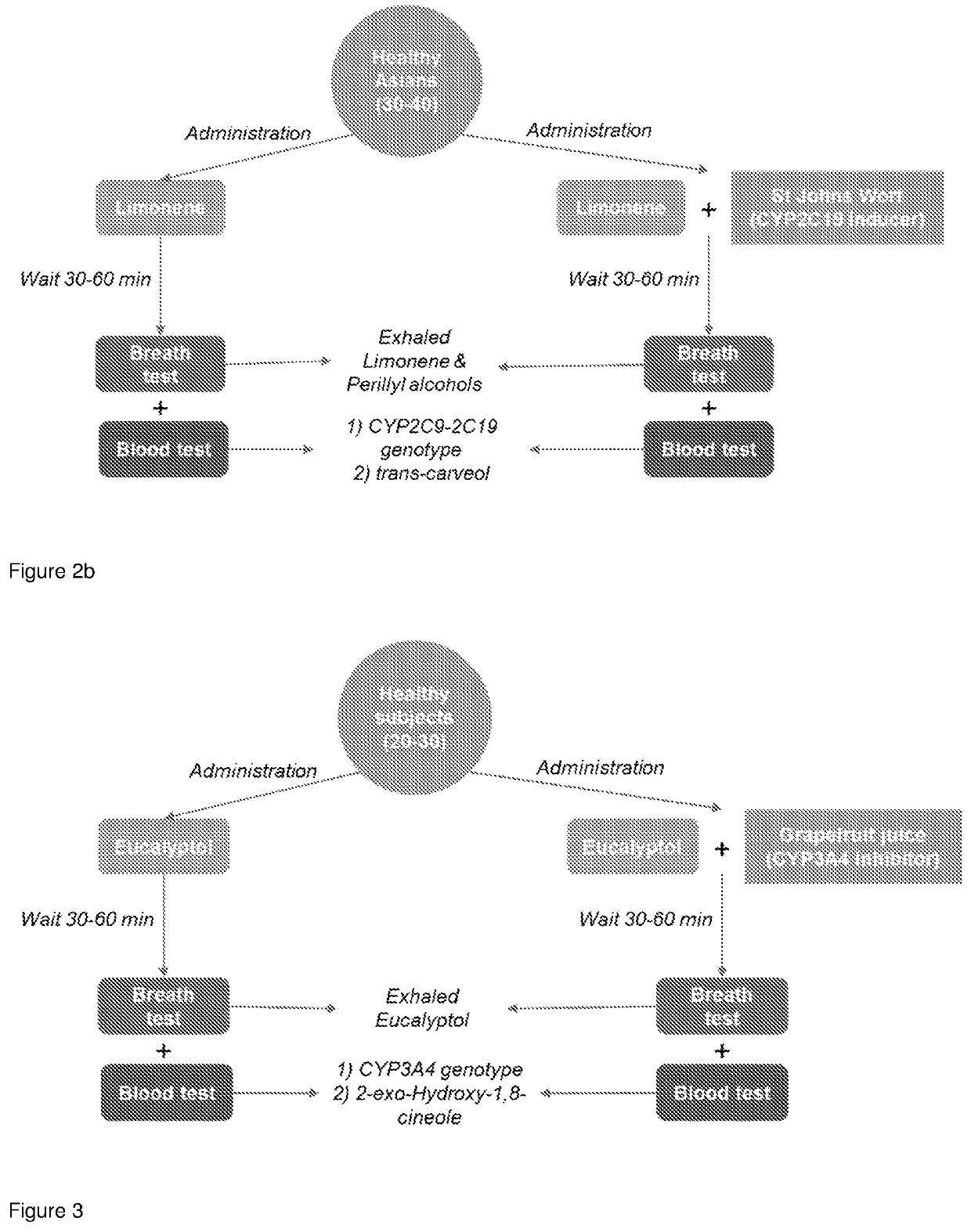
Method To Evaluate Metabolic Activity Of Liver Enzymes
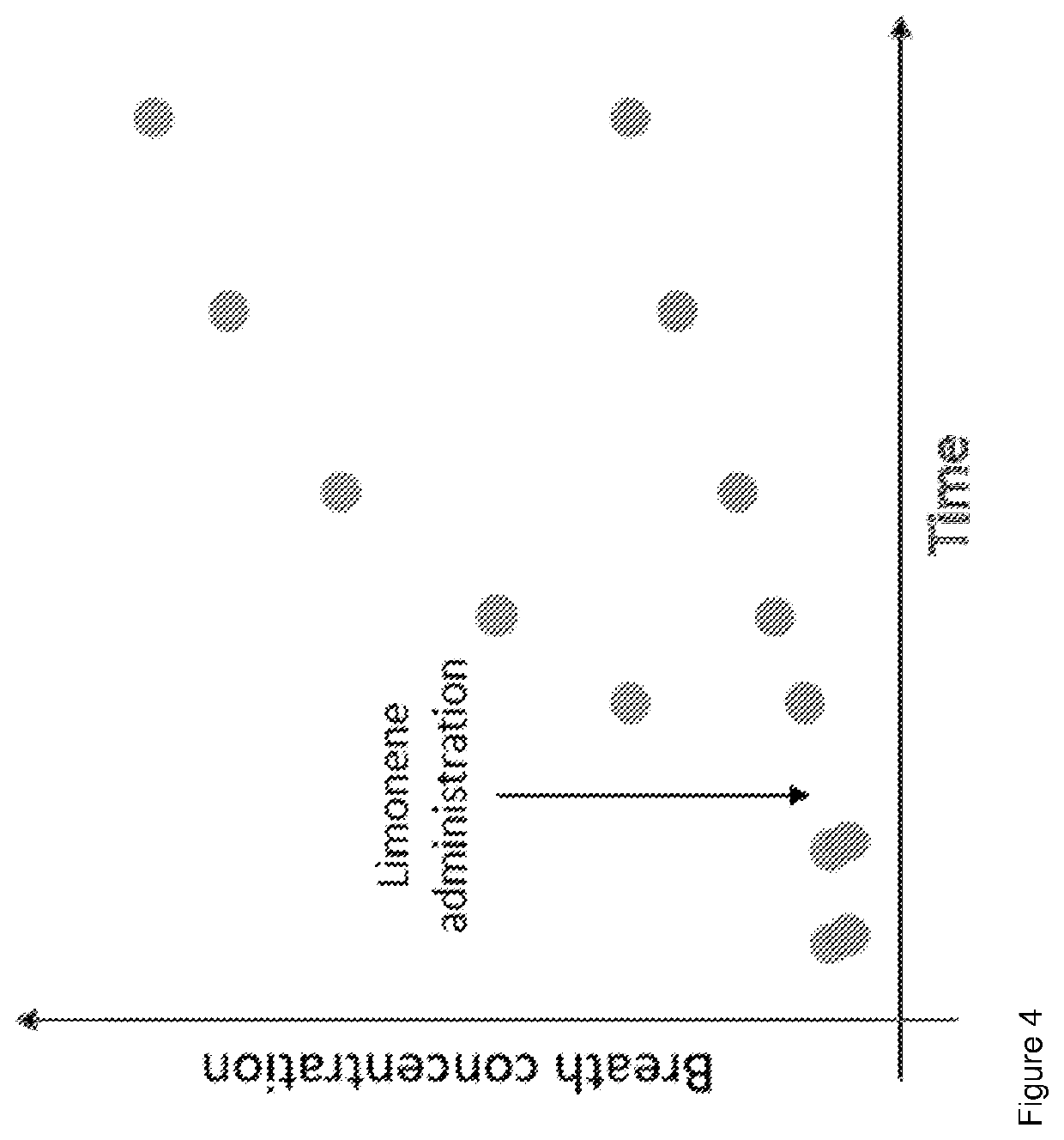
PendingUS20220228192A1Microbiological testing/measurementDisease diagnosisPhysiologyMetabolic phenotype
The invention provides a breath test for assessing a metabolic phenotypeand / or assessing a disease state.
Owner:OWLSTONE MEDICAL LTD
Duck fatty liver oil and preparation method thereof
InactiveCN107267277AEasy to separateImprove oil yieldFatty-oils/fats refiningFatty-oils/fats productionFatty liverRotary evaporator
The invention discloses a preparation method of a duck fatty liver oil. The method specifically includes the steps of: (1) preparation of duck fatty liver slurry: chopping duck fatty liver and performing tissue homogenization to obtain a slurry; (2) duck fatty liver enzymolysis: mixing the slurry and a buffer solution according to a ratio of 1:0-2.5, and adding protease for enzymolysis of the slurry; and (3) extraction of duck fatty liver oil: after enzymolysis, mixing the slurry with petroleum ether, then performing ultrasonic extraction, then carrying out high speed centrifugation, taking the supernatant and performing filtering, and utilizing a vacuum rotary evaporator to remove petroleum ether from the filtrate. The invention adopts an ultrasonic assisted enzymatic extraction method to prepare duck fatty liver oil, and the method has the advantages of full extraction of grease from duck fatty liver, reduction of temperature, time saving, simple process, and high maneuverability.
Owner:QINGDAO AGRI UNIV
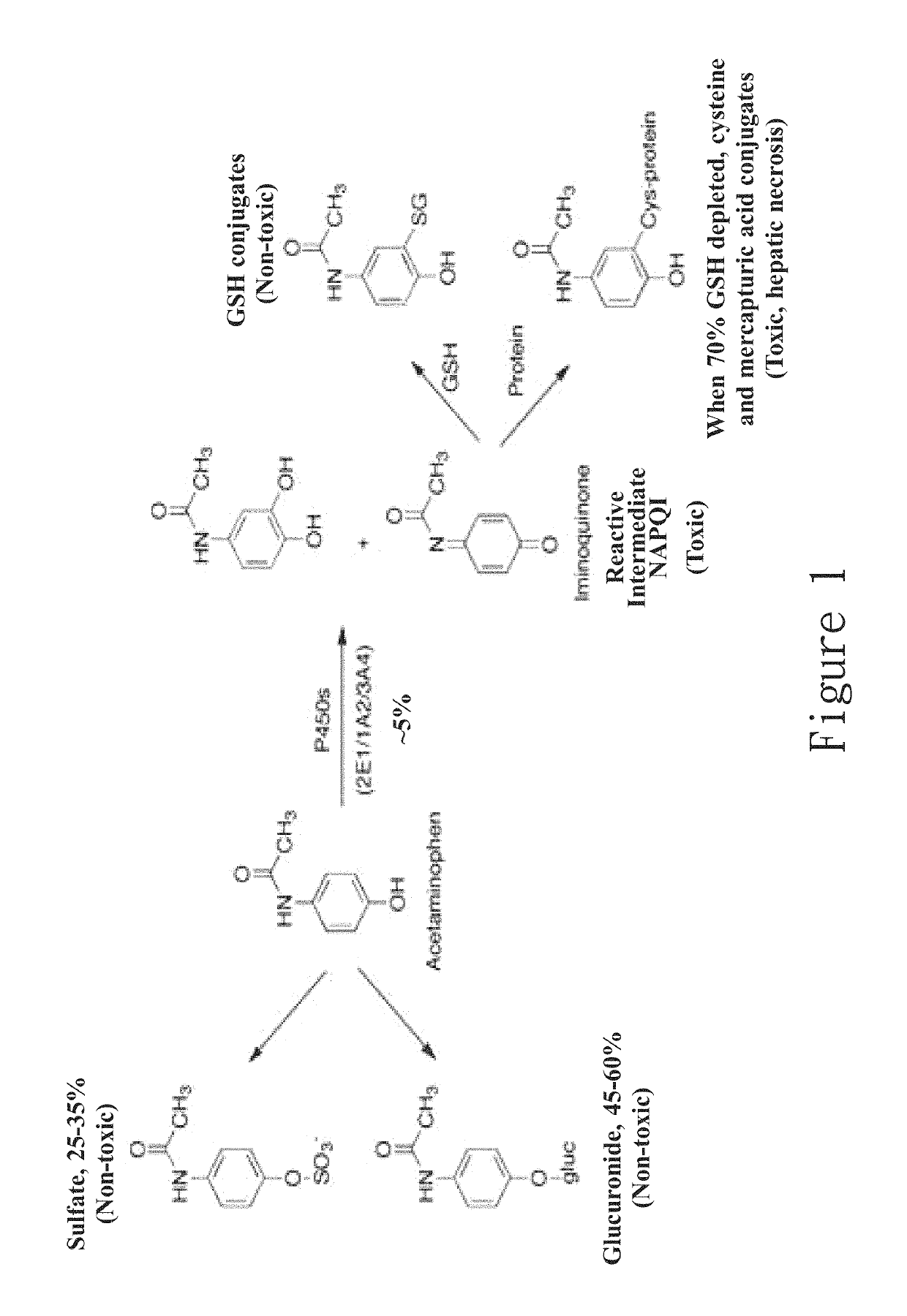
Hepatotoxicity-free pharmaceutical composition containing acetaminophen drugs
A new compound composition that is free of a side effect to a liver and used for alleviating the toxicity of an acetaminophen (APAP) medicament to the liver. The compound composition comprises (a) a pharmaceutically effective amount of acetaminophen and (b) a frequently-used safe and pharmaceutically acceptable excipient that can be combined with one or more than two medicaments that can reduce the toxicity of a drug via liver enzyme CYP2E1 metabolism to the liver. The compound is selected from the following group: Tween 20, microcrystalline cellulose, dicalcium phosphate, polyoxyethylene 23 lauryl ether, saccharin, mannitol, polyoxyethylene alkyl ether, sucralose, pyrrolidone, sodium starch glycolate, acrylic resin S100, carboxymethyl cellulose sodium, polyoxyethylene polyoxypropylene, menthol, low-substituted hydrocarbon propyl cellulose, pregelatinized starch, Dextrates NF hydrated, citric acid, polyoxyethylene castor oil, colloidal silica, polyethylene glycol monostearate aliphatic ester, sorbic acid, lemon oil, hydroxypropyl cellulose, sorbitol, acesulfame potassium, hypromellose phthalate, lactose monohydrate, maltodextrin, Brij 58, Brij 76, Tween 80, Tween 40, PEG 400, PEG 4000, PEG 2000, and the like, so as to reduce the side effect of the toxicity caused by acetaminophen to the liver.
Owner:INT EDUCATION FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com