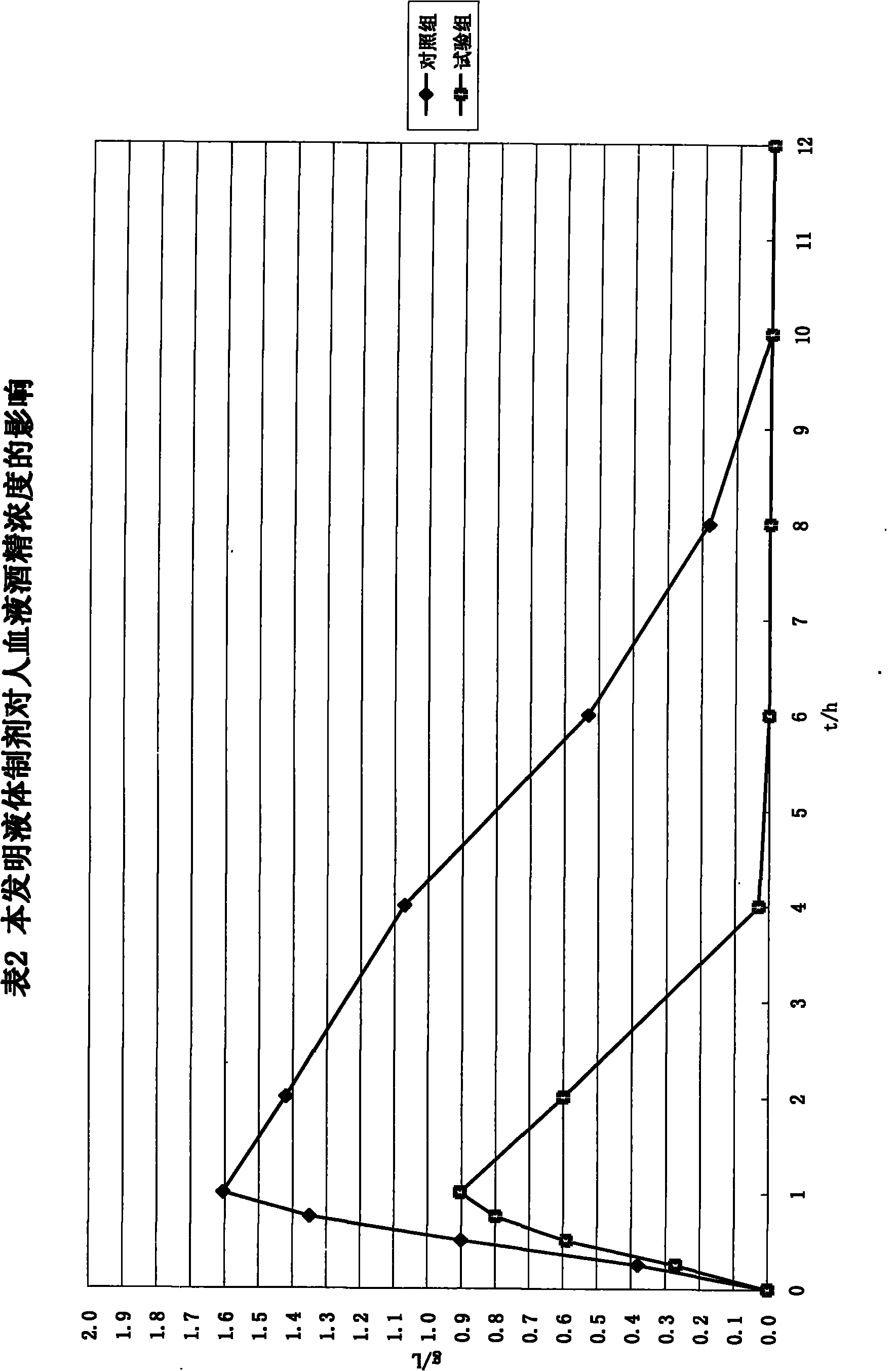
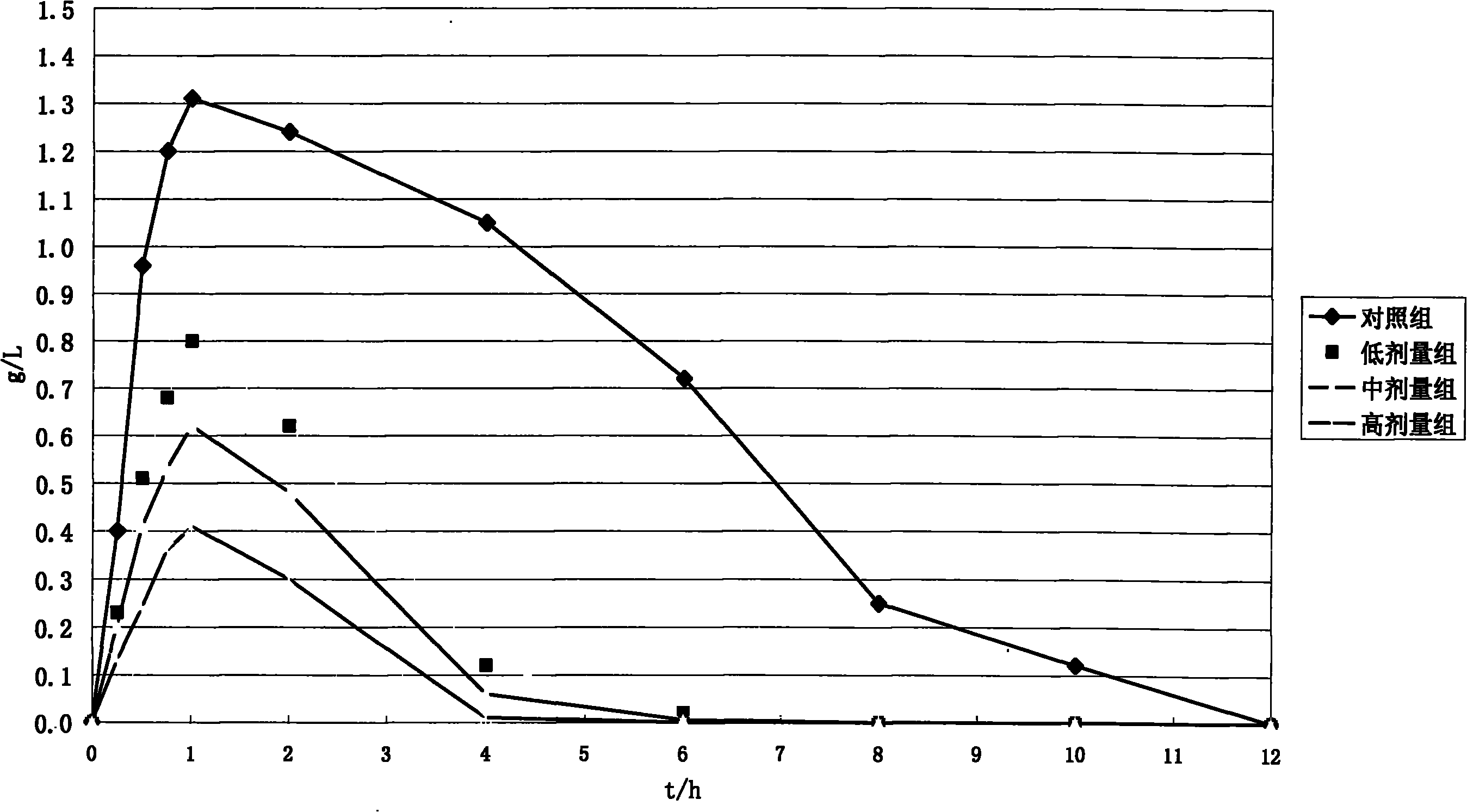
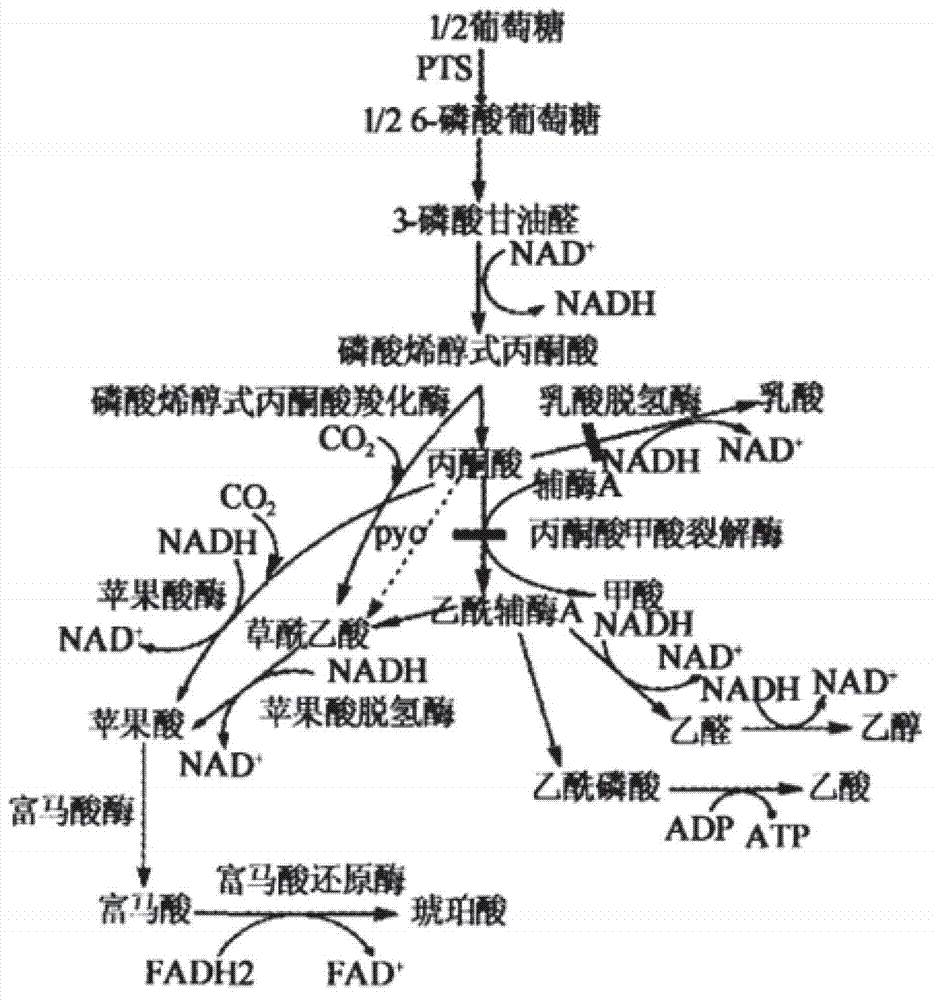
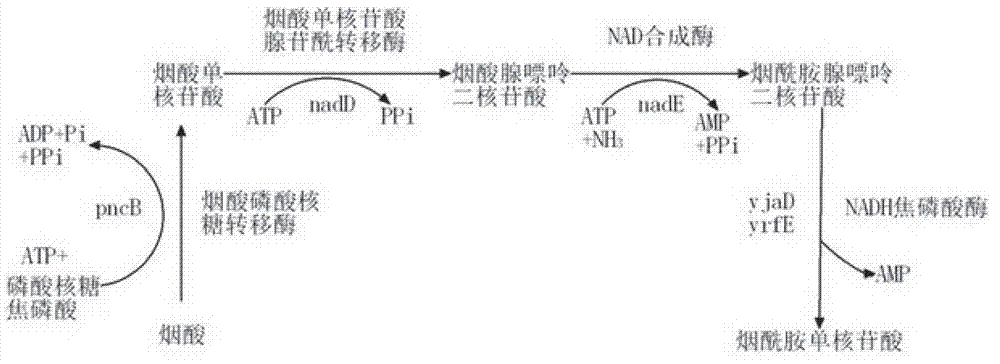
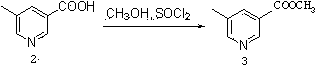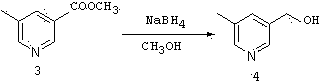Patents
Literature
162 results about "Nicotinuric acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
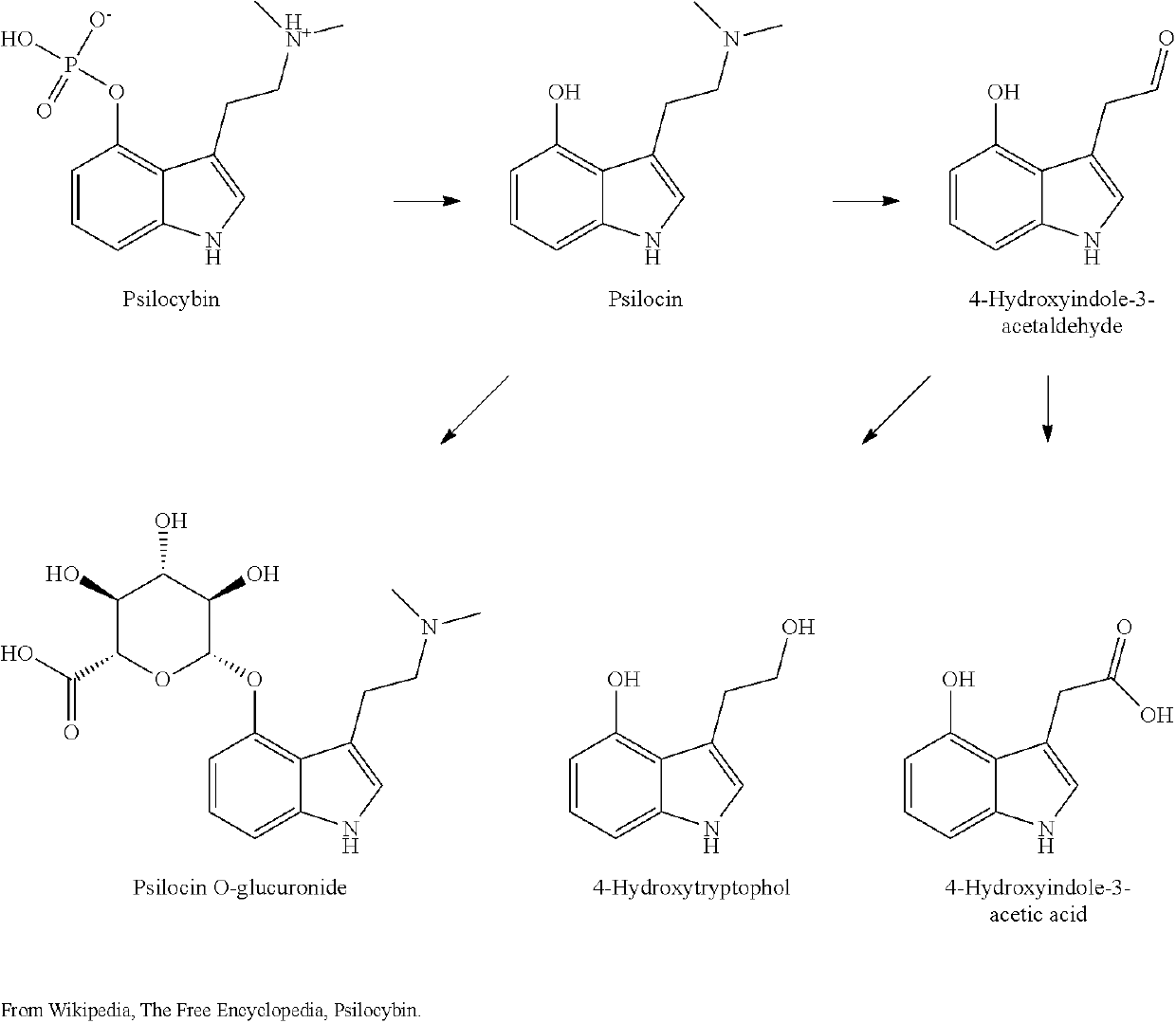
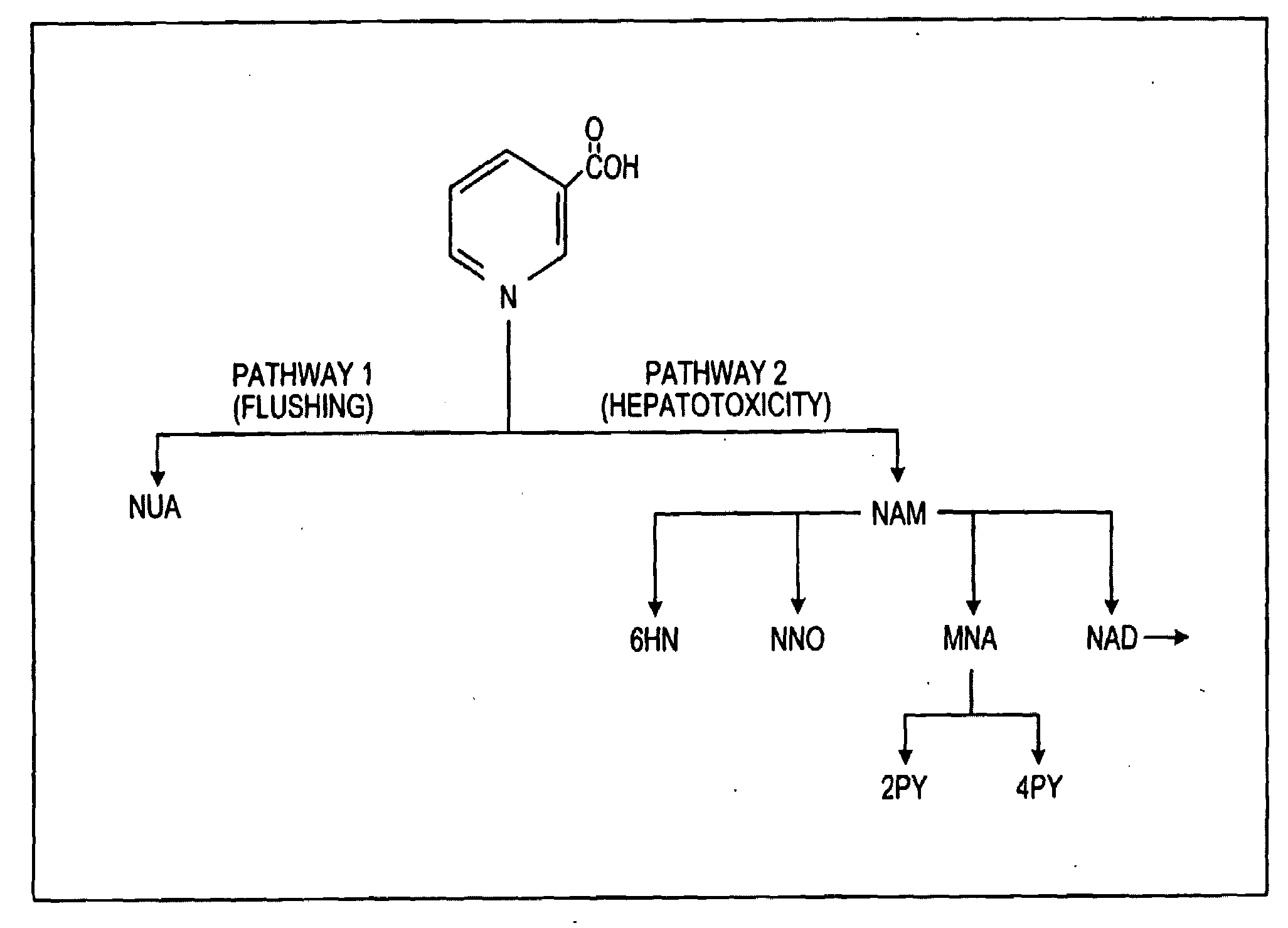
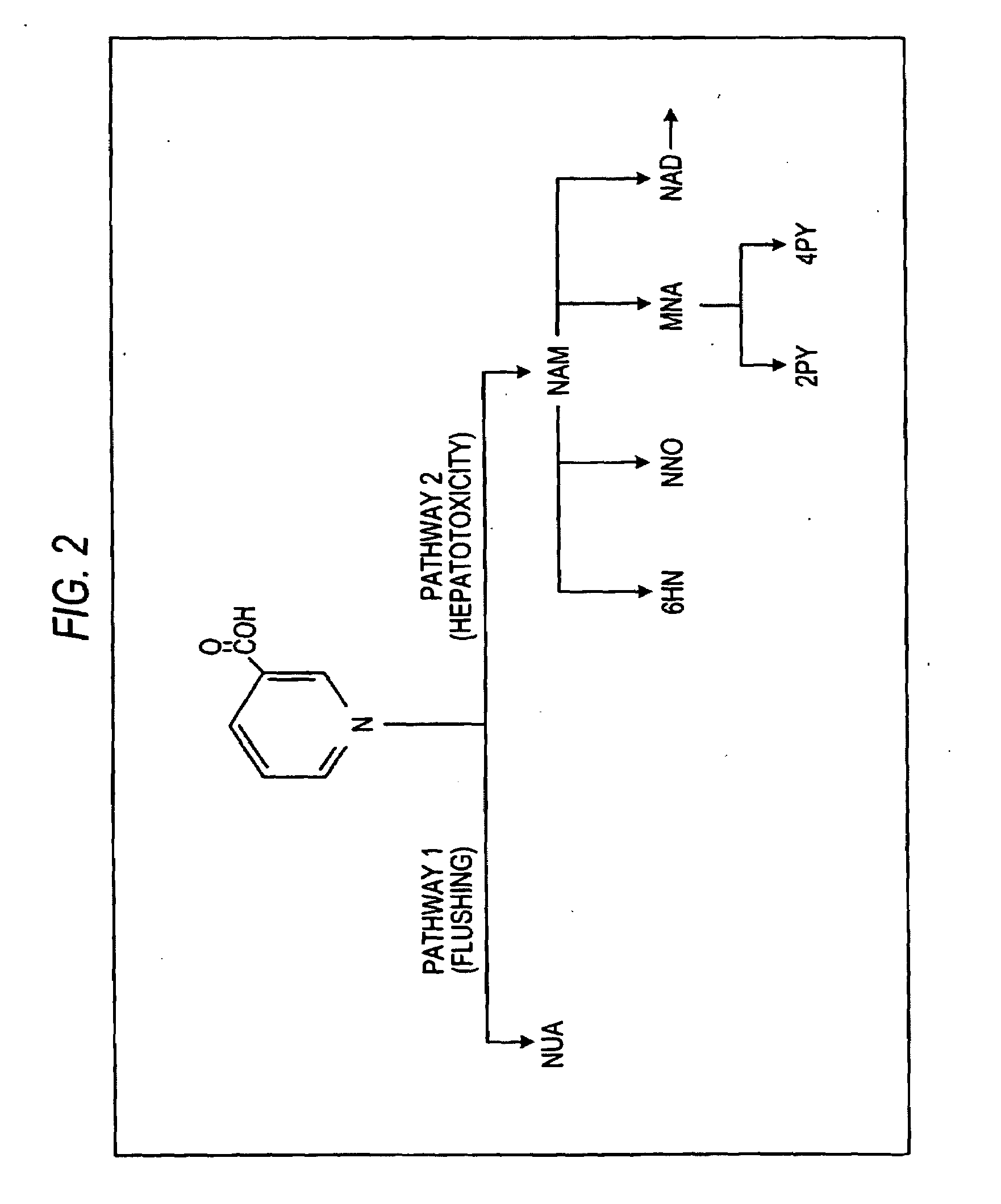
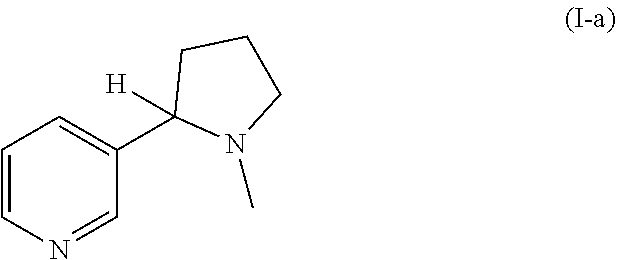
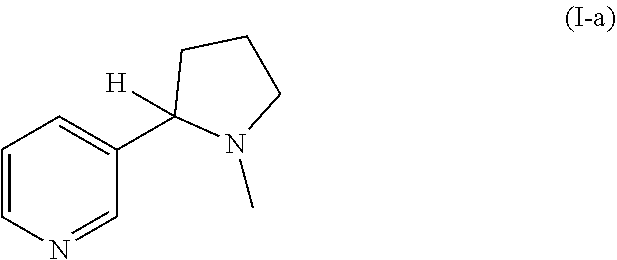
Nicotinuric acid is an acyl glycine. Acyl glycines are normally minor metabolites of fatty acids. However, the excretion of certain acyl glycines is increased in several inborn errors of metabolism.
Psilocybin compositions
ActiveUS20190105313A1Improve neurological functionReduce degradationOrganic active ingredientsFungi medical ingredientsPsilocinNeurogenesis
Methods and compositions are disclosed for enhancing neurogenesis, resolving neuropathy and improving neurological health and functioning using fungal extracts and their active ingredients, including species of mushrooms and mycelia containing psilocybin and psilocin, combined with erinacines and hericenones or fungal extracts containing those active ingredients, with the addition of nicotinic acid. The compositions may optionally be combined with nervine plants.
Owner:TURTLE BEAR HLDG LLC
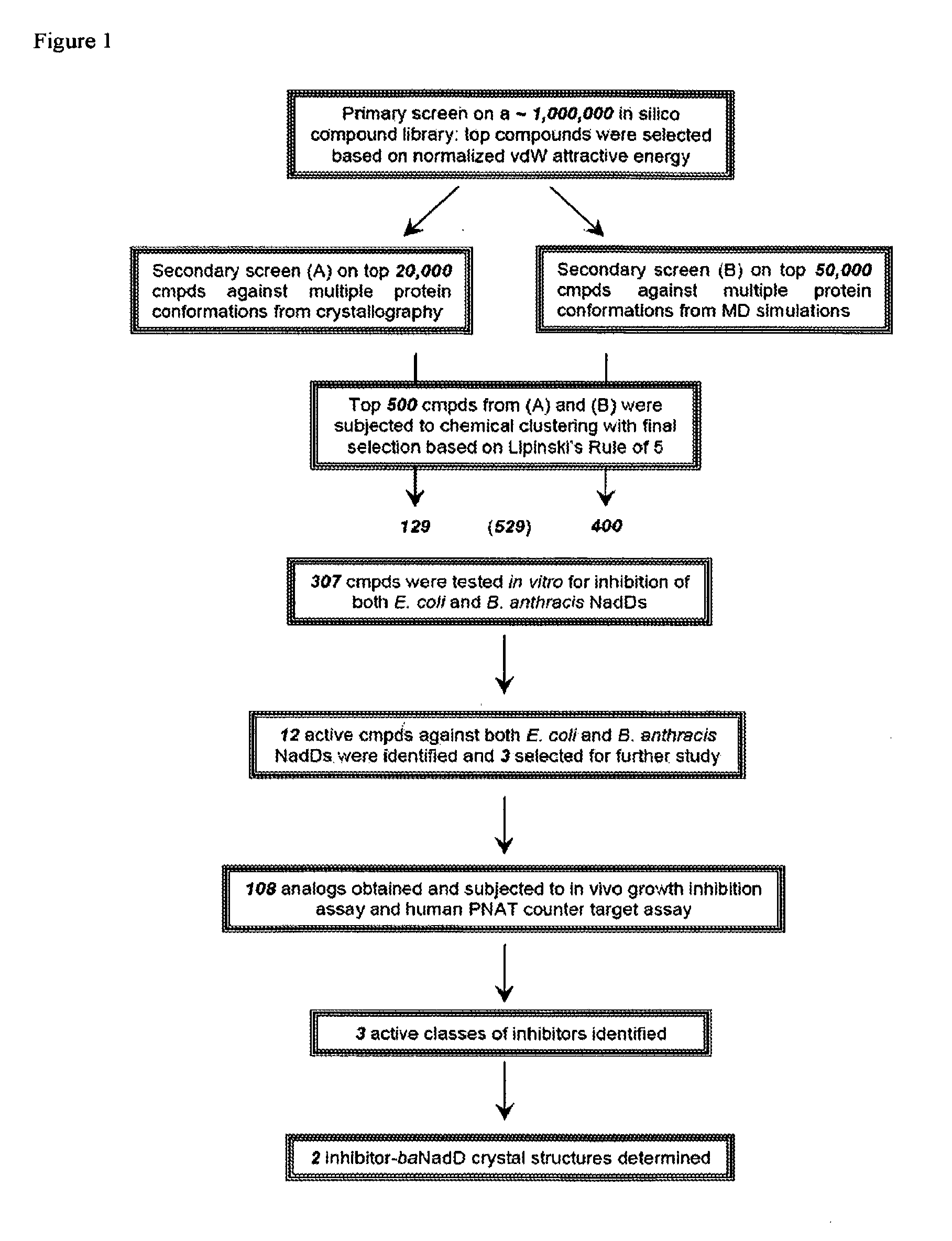
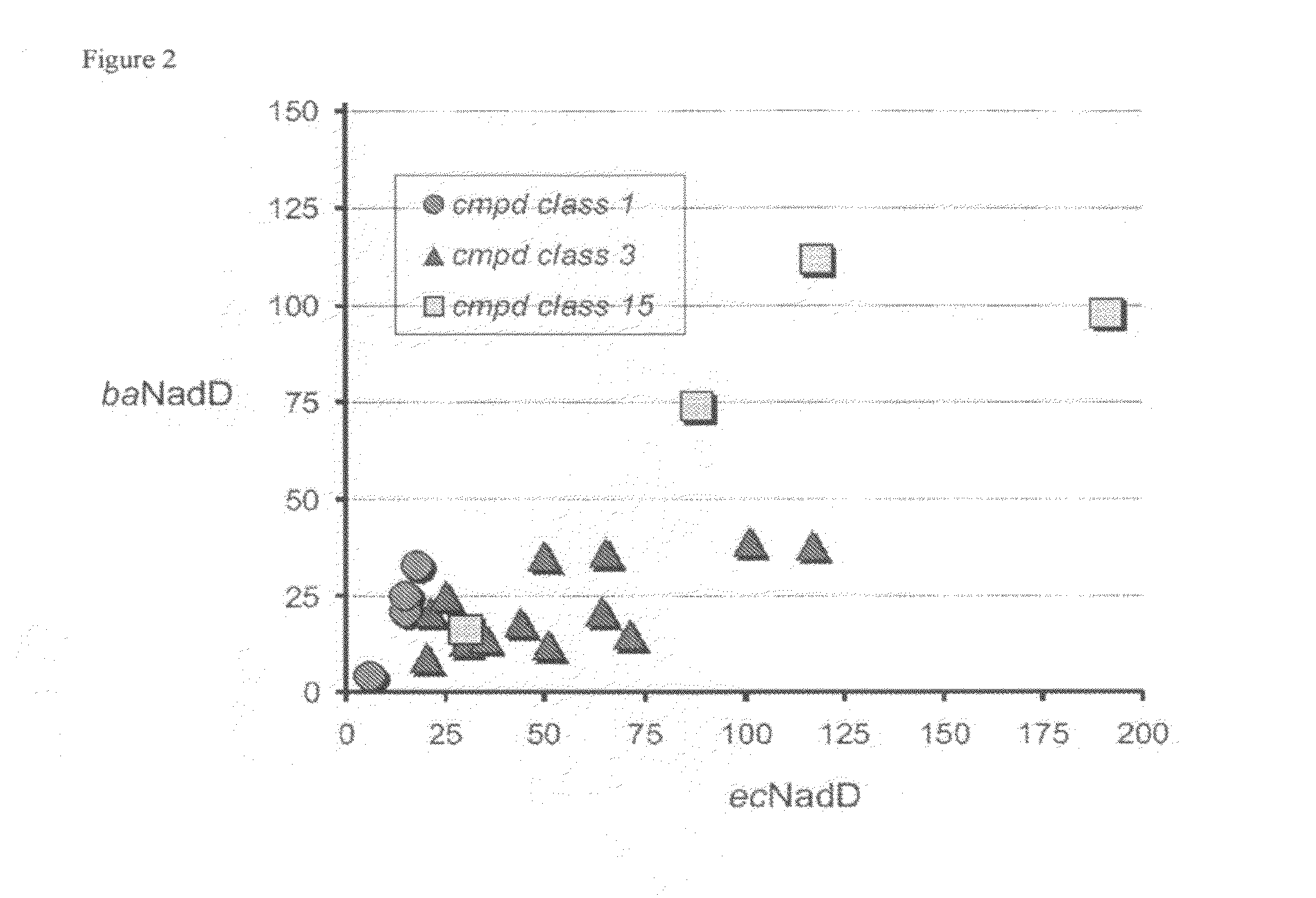
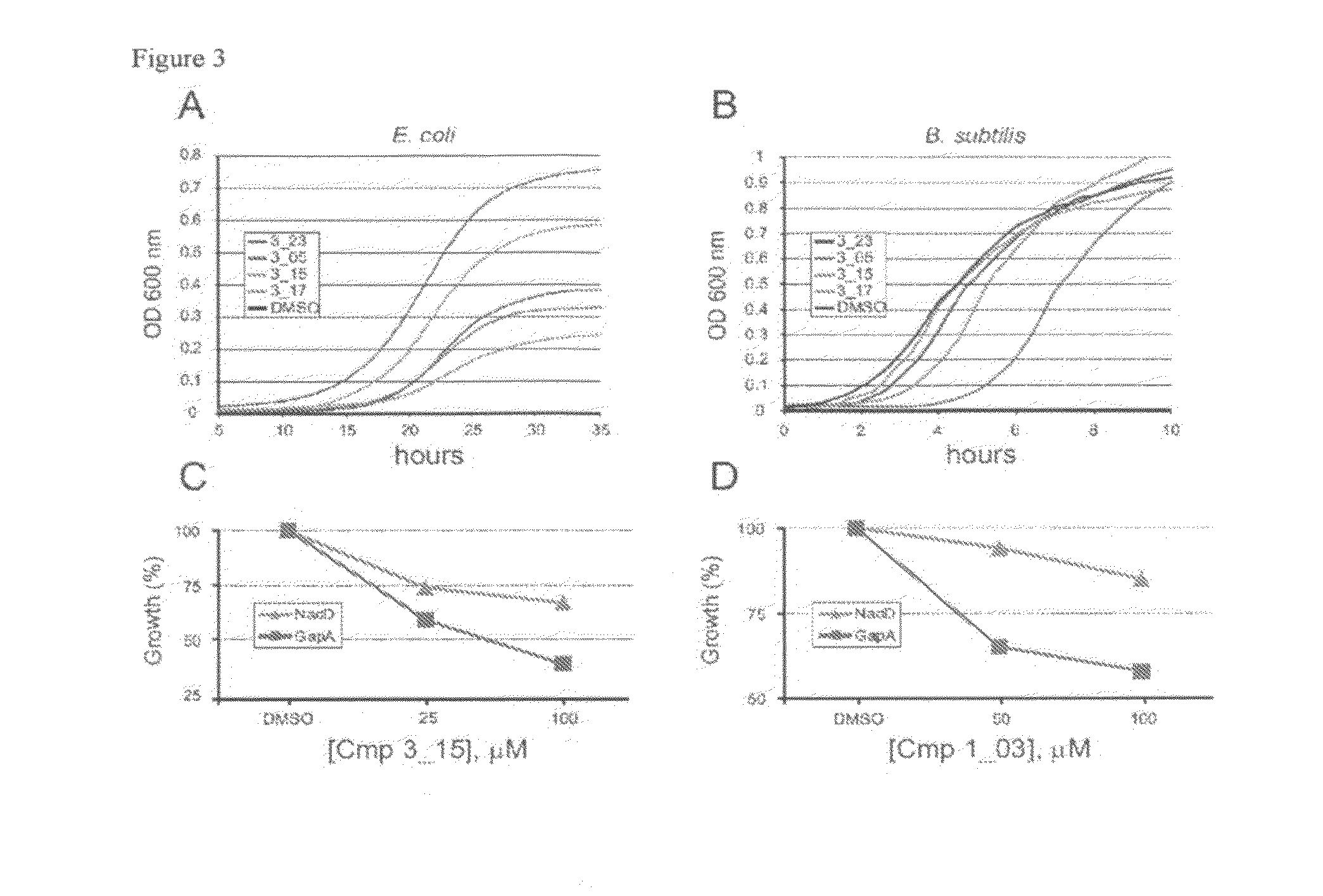
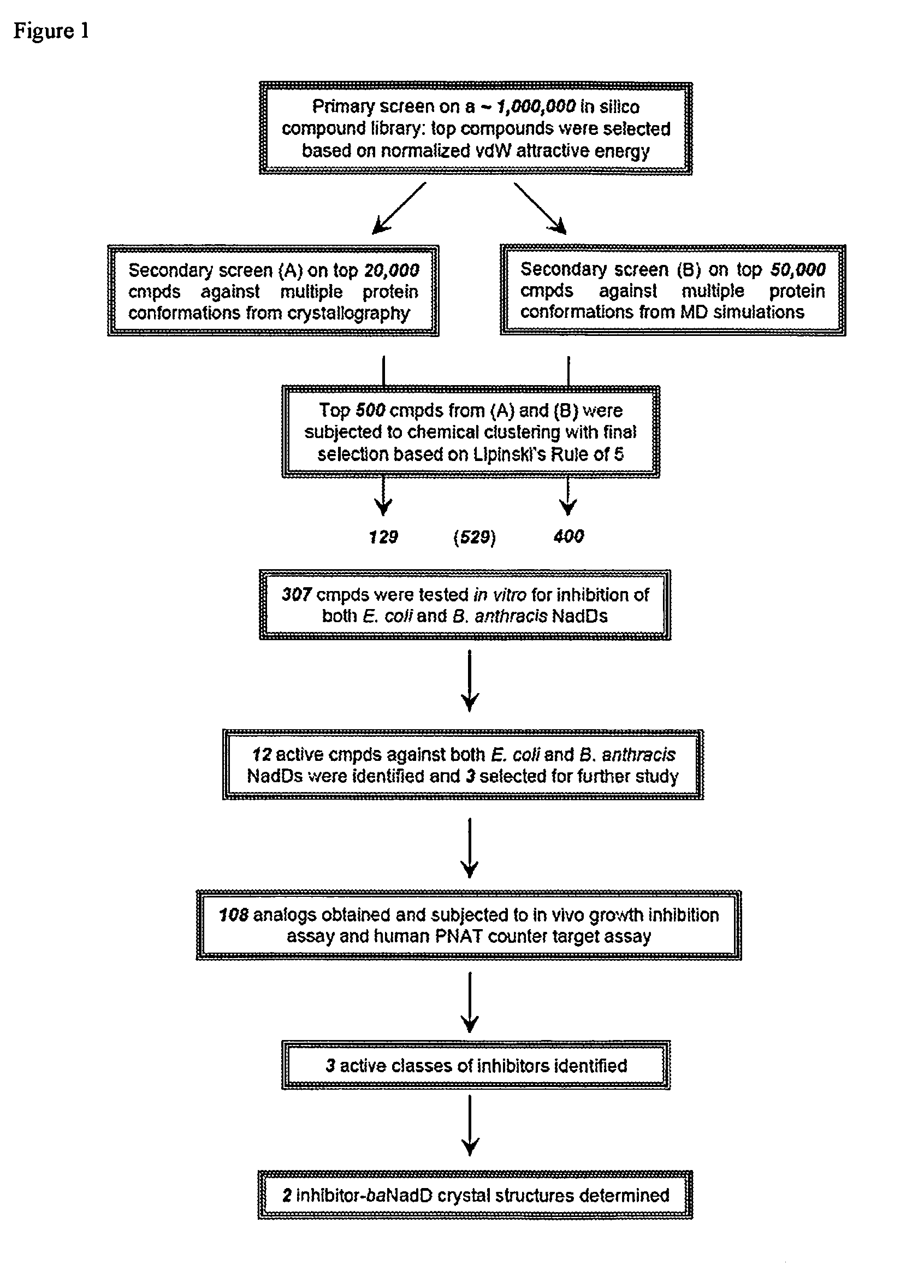
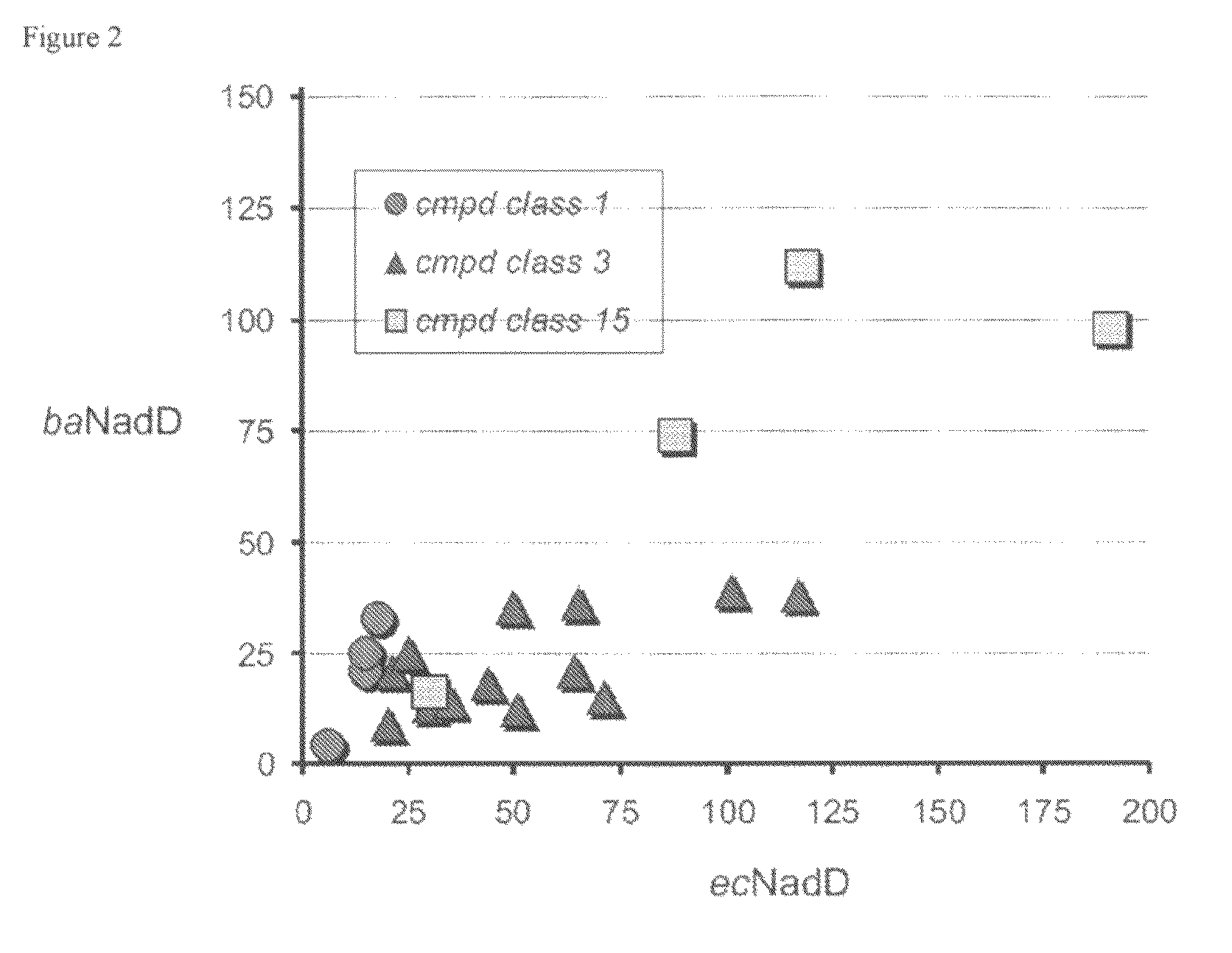
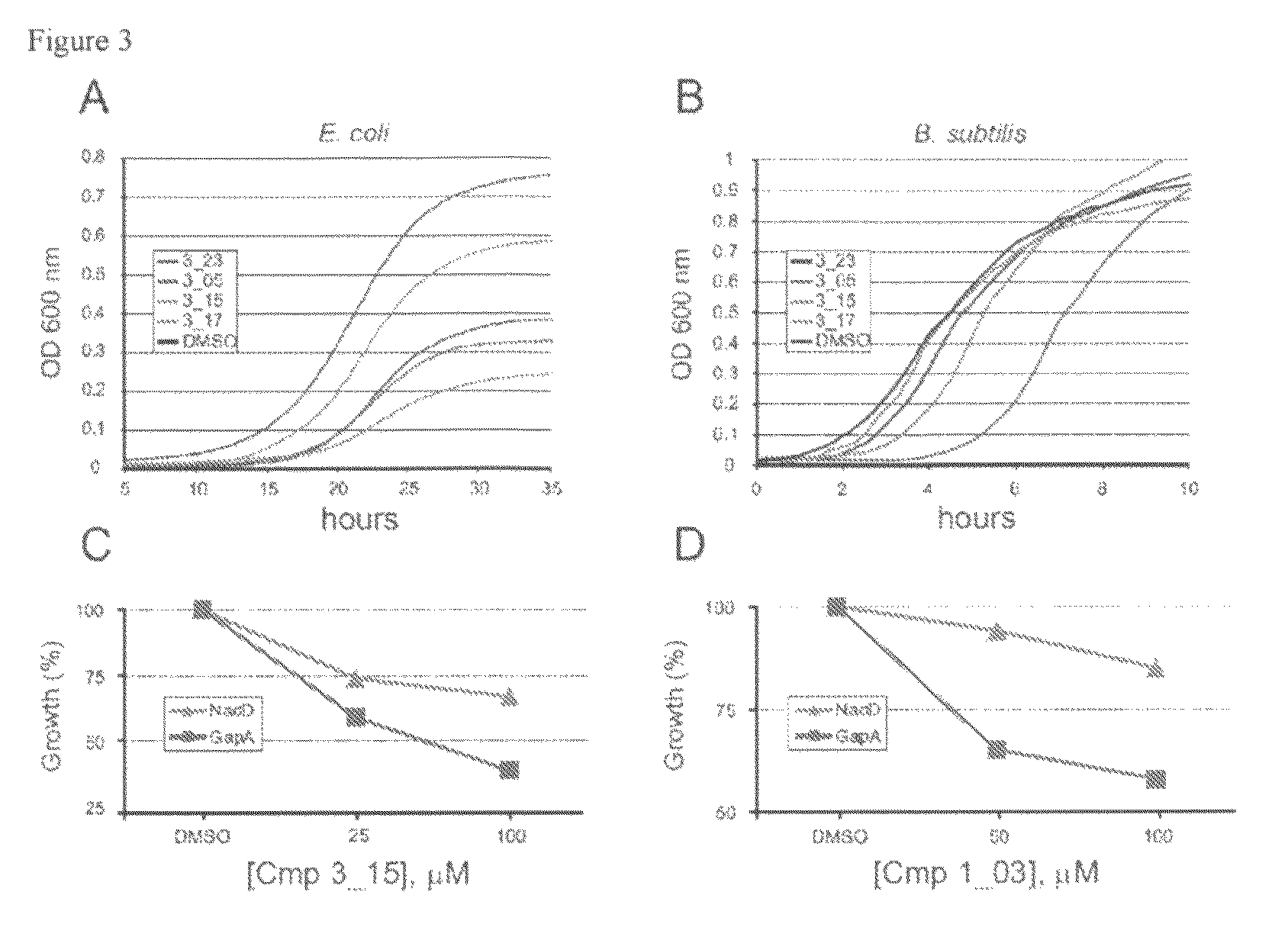
Targeting nad biosynthesis in bacterial pathogens
The emergence of multidrug-resistant pathogens necessitates the search for new antibiotics acting on previously unexplored targets. Nicotinate mononucleotide adenylyltransferase of the NadD family, an essential enzyme of NAD biosynthesis in most bacteria, was selected as a target for structure-based inhibitor development. To this end, the inventors have identified small molecule compounds that inhibit bacterial target enzymes by interacting with a novel inhibitory binding site on the enzyme while having no effect on functionally equivalent human enzymes.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +2
Composite premix feed for egg-laying quail and processing technique thereof
ActiveCN101485399ANutritionally balanced and richIncrease egg productionAnimal feeding stuffAccessory food factorsBiotechnologyPhytase
The invention relates to compound premix feed for laying quails, which comprises 3 to 7 kg of manganous sulfate, 2 to 6 kg of zinc sulfate, 1 to 5 kg of ferrous sulfate, 0.2 to 0.6 kg of copper sulphate, 15 to 21 kg of choline chloride, 3 to 7 kg of lysine, 26 to 34 kg of methionine, 1.6 to 2.4 kg of phytase, 180 to 261.2 kg of calcium carbonate, 240 to 310 kg of calcium hydrophosphate, 320 to 440 kg of fish meal, 35 to 65 kg of common salt, 0.26 to 0.85 kg of vitamin A, 0.08 to 0.25 kg of vitamin D3, 0.69 to 2.28 kg of vitamin E, 0.063 to 0.21 kg of vitamin K3, 0.035 to 0.11 kg of vitamin B1, 0.44 to 1.4 kg of vitamin B2, 0.19 to 0.63 kg of vitamin B6, 0.033 to 0.14 kg of vitamin B12, 0.04 to 0.13 kg of folic acid, 0.01 to 0.03 kg of biotin, 0.4 to 1.3 kg of nicotinic acid, 0.1 to 0.38 kg of calcium pantothenate and 1.059 to 2.29 kg of rice hull powder. The laying quails fed by the premix feed have high laying rate, and the ratio of the feed to the egg is 2.2-2.5:1.
Owner:潍坊中基饲料有限公司
Refreshing nutrient milk tea
The invention discloses a refreshing nutrient milk tea and belongs to the field of beverage formula. Per 100 ml beverage contains 5-20g of soymilk powder, 0.5-3g of coffee powder, 5-20g of cane sugar, 6-8g of codonopsis lanceolata extract, 0.5-1g of American ginseng extract, 0.5-1.4mg of vitamin, 1-2mg of nicotinic acid, 10-50mg of taurine and the balance of water. The beverage provided by the invention has good mouthfeel, low cost, unique flavour, anti-fatigue effect and abundant nutrition.
Owner:CHENGDU SHENGERJIA SCI & TECH
Formula food with special medical application and preparation method thereof
ActiveCN105476012AHigh yieldReduce the likelihood of immune rejectionOrganic active ingredientsNervous disorderSide effectHigh absorption
The invention provides a formula food with special medical application and a preparation method thereof, and application of the formula food in preparation of a medical drug. The prepared lactalbumin small peptide contains folic acid accounting for one millionth to one ten thousandth of the lactalbumin peptide by mass and nicotinic acid accounting for one thousandth to five thousandths of the lactalbumin peptide by mass; the lactalbumin peptide with molecular weight smaller than 1000 Dalton accounts for more than or equal to 98%. The product has the characteristics of high activity, high absorption rate, high nutrition, high safety and no side effect. By adopting the method, the lactalbumin is subjected to sufficient enzymolysis, the lactalbumin peptide yield is high, the prepared lactalbumin pepetide is a polypeptide mixture consisting of 2-5 amino acids and is easy to absorb by the organism, the sensitization property is further reduced, and the product activity is high; and the preparation method is simple to operate and suitable for industrial production.
Owner:中健医用食品有限公司
Synthesis method of (R, S-) nicotine
ActiveCN112745294AHigh synthetic specificityHigh purityOrganic chemistryPhysical/chemical process catalystsPtru catalystPhenacyl
Owner:SHANDONG JINCHENG KERUI CHEMICAL CO LTD
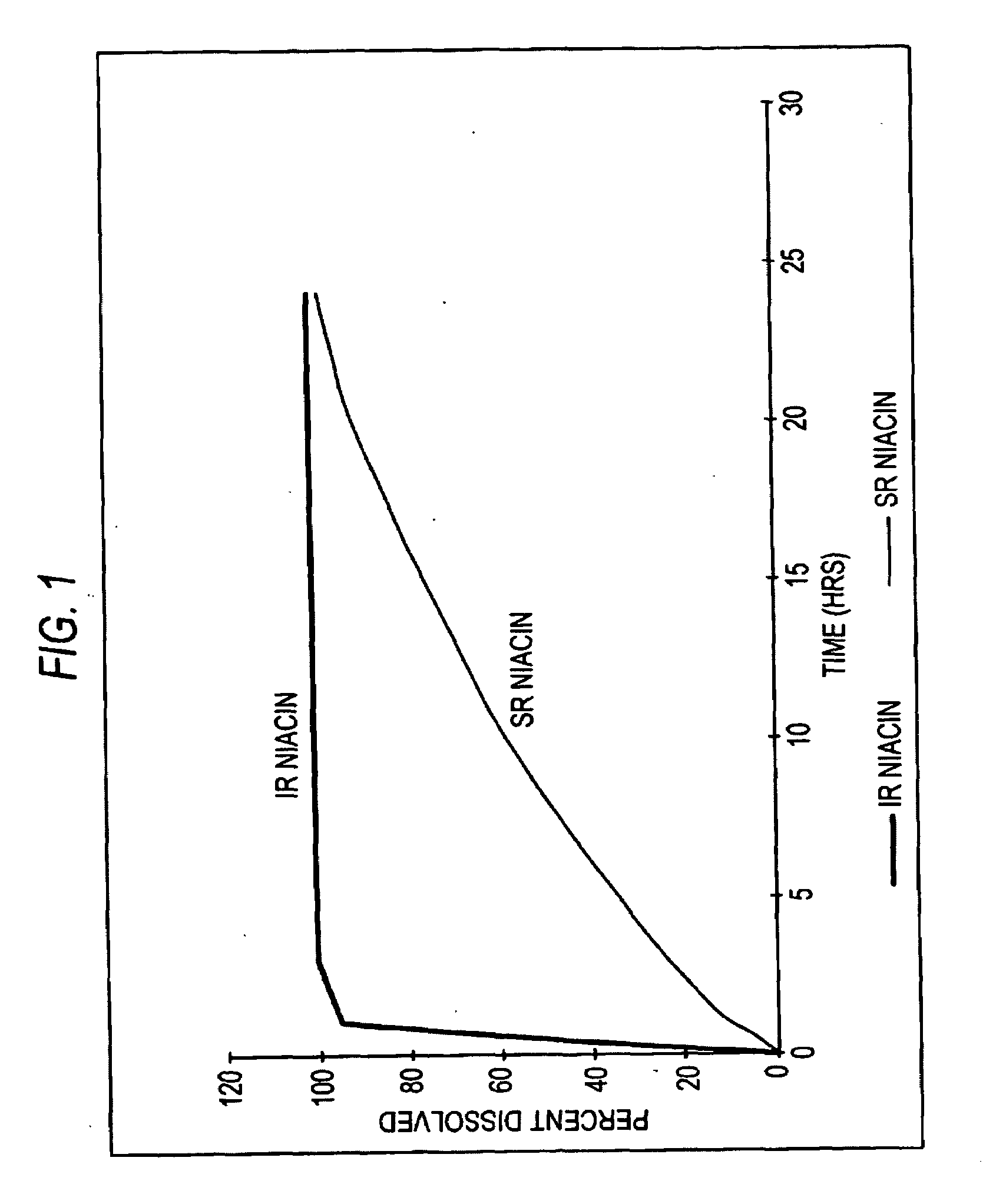
Intermediate Release Nicotinic Acid Compositions For Treating Hyperlipidemia Which Exhibit an In Vivo Stair-Stepped Absorption Curve
InactiveUS20090054499A1Lower Level RequirementsImprove the level ofBiocideAC motor controlDrug induced hepatotoxicityIn vivo
Owner:CEFALI EUGENIO A
Composite for disintoxicating and sobering
The invention discloses a composite (E) for disintoxicating and sobering, which is prepared by glucose and fructose, fruit glucose, honey, flower of kudzuvine, hoveniae semoveniae semen, L-alanine, L-ornithine, L-glutamine, L-carnitine according to a certain weight range. In order to enable the effect better, one or more of tea leaf, vitamin C, L-cysteine, taurine, L-asparaginic acid or L-aspartate, vitamin B1, vitamin B6, L-arginine, L-glutamic acid, L-proline, phaseomannite, vitamin B2, nicotinic acid, folic acid, vitamin B12 and pantothenic acid are also added. The composite can be prepared into liquid preparation, electuary and medicinal tea. The composite of the invention can be prepared into food and health food. The invention can quickly disintoxicate, sober, eliminate alcoholism symptom and continued effect of alcohol on a human body, and has no side effect.
Owner:克科
V emulsion nicotinate for injection and its preparing method
InactiveCN1562014AGood prevention effectInhibition of thrombusOrganic active ingredientsMetabolism disorderPoloxamerDosage form
A VE nicotinate injection in the form of emulsion for preventing cerebral infarction is prepared from VE nicotinate, oil for injection, glycerine, emulsifier, coemulsifier and pH regulator. Its preparing process is also disclosed.
Owner:SHENYANG PHARMA UNIVERSITY
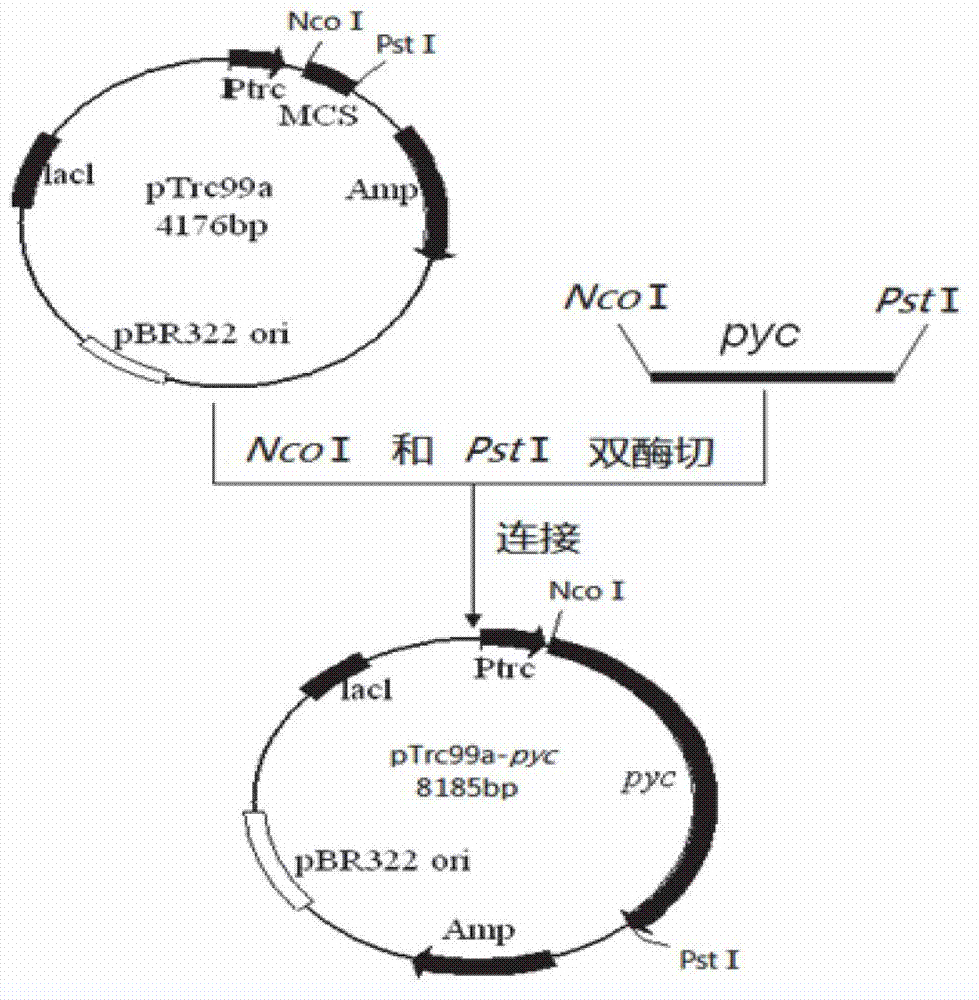
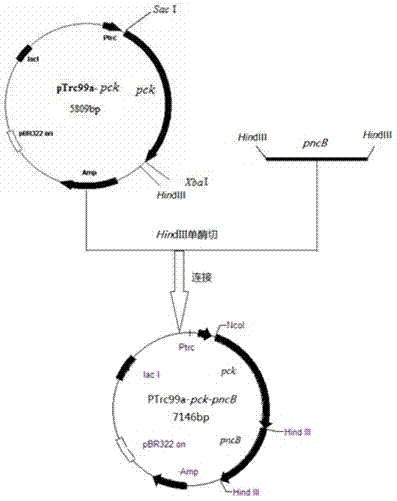
Genetic engineering bacterium for producing succinic acid, and construction and application thereof
ActiveCN102864116AEasy to buildThe fermentation method is simpleBacteriaMicroorganism based processesEscherichia coliButanedioic acid
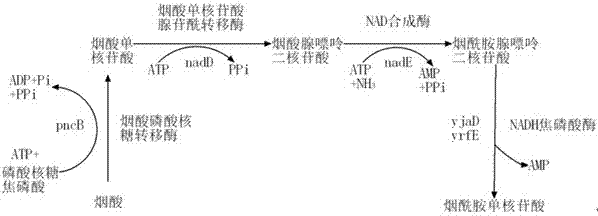
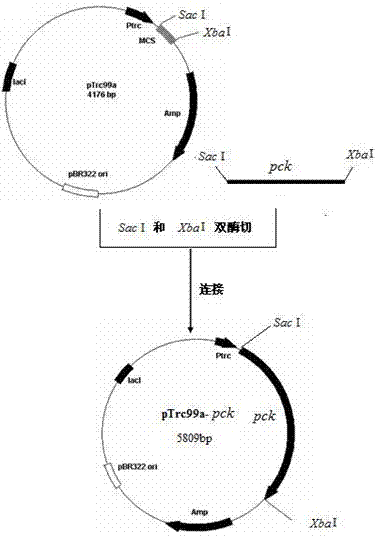
The invention provides a genetic engineering bacterium strain for producing succinic acid. The genetic engineering bacterium strain is named as Escherichia coli BA016, the preserving number registration number CCTCC NO is M 2012350. The invention further provides a construction method of the strain and a method for producing succinic acid by fermentation, recombinant escherichia coli can grow by glucose metabolism through joint excessive expression of exogenous pyruvic carboxylase and nicotinic acid ribose phosphate transferase, generation of by-product pyruvic acid is reduced, and accordingly the yield and production intensity of succinic acid are greatly improved.
Owner:NANJING UNIV OF TECH
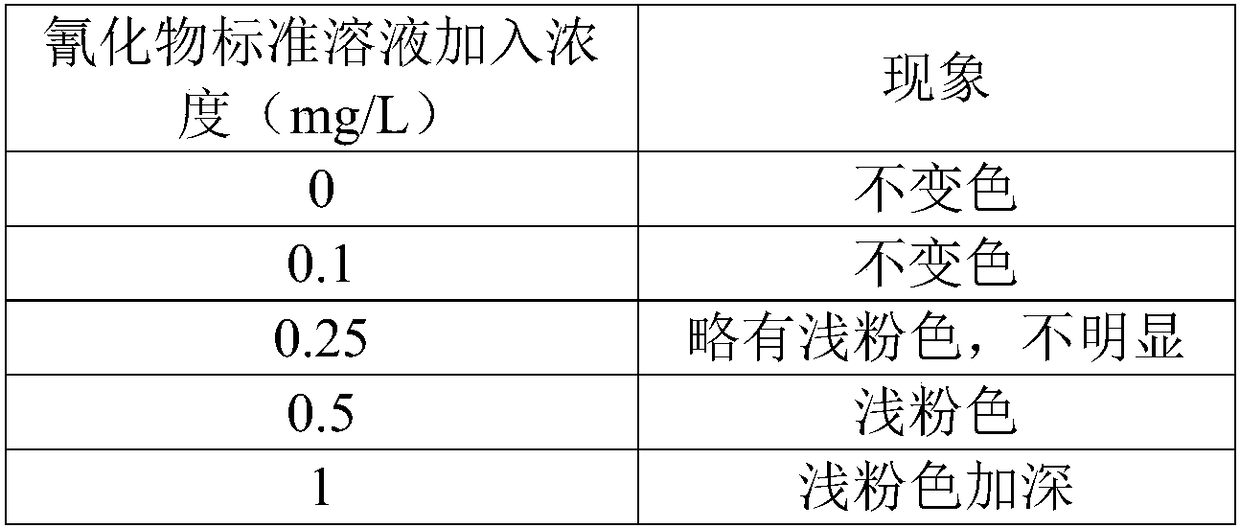
Rendering test paper for quick detection of cyanides and preparation and detection methods thereof
ActiveCN108535243AImprove detection efficiencyShort reaction timeMaterial analysis by observing effect on chemical indicatorBarbituric acidTriton X-100
The invention provides rendering test paper for quick detection of cyanides, comprising a cyanide detection layer. The cyanide detection layer is composed of, by weight, 3-12 parts of sodium hydroxide, 8-15 parts of isonicotinic acid, 1-5 parts of triton X-100, 3-15 parts of barbituric acid, and 30-90 parts of methyl vinyl ether-maleic anhydride copolymer. The rendering test paper herein uses no bitter acids and other flammable explosive dangerous reagents during the detection process, the reaction time is short, the operating process is simple and fast, the detection cost is low, the detection cost for single sample detection is about 0.1 Yuan, the detection sensitivity and accuracy are high, rendered color uniformity is good, and the shelf life is long.
Owner:BEIJING PRIMEBIOTEK COMPANY
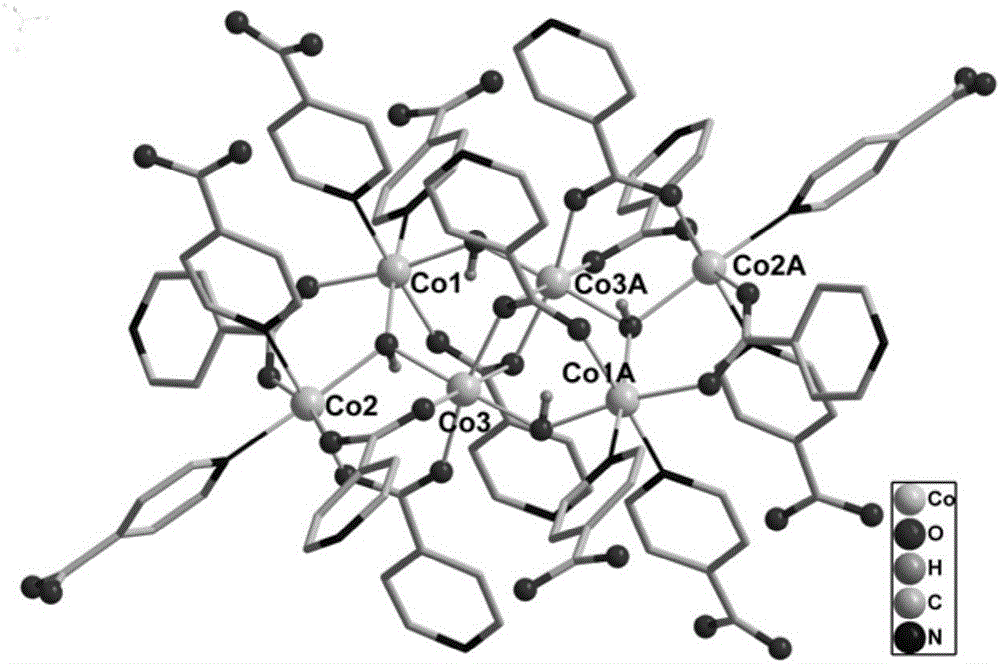
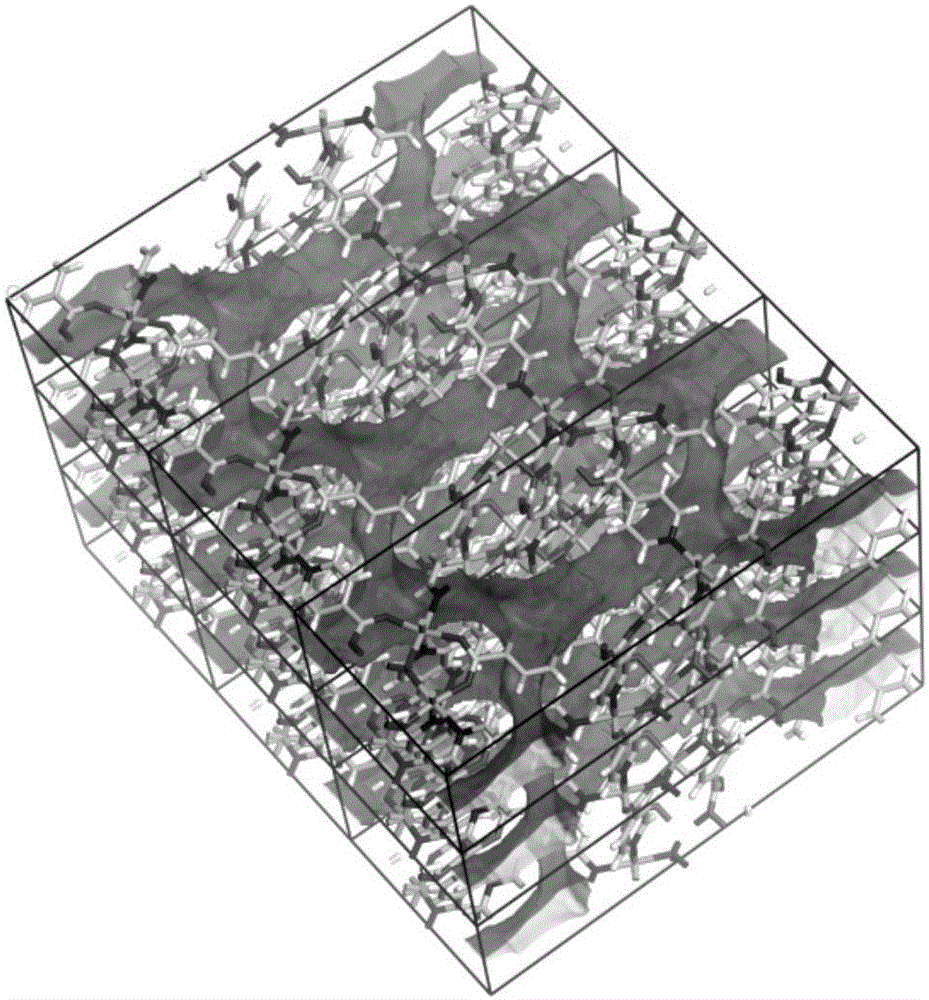
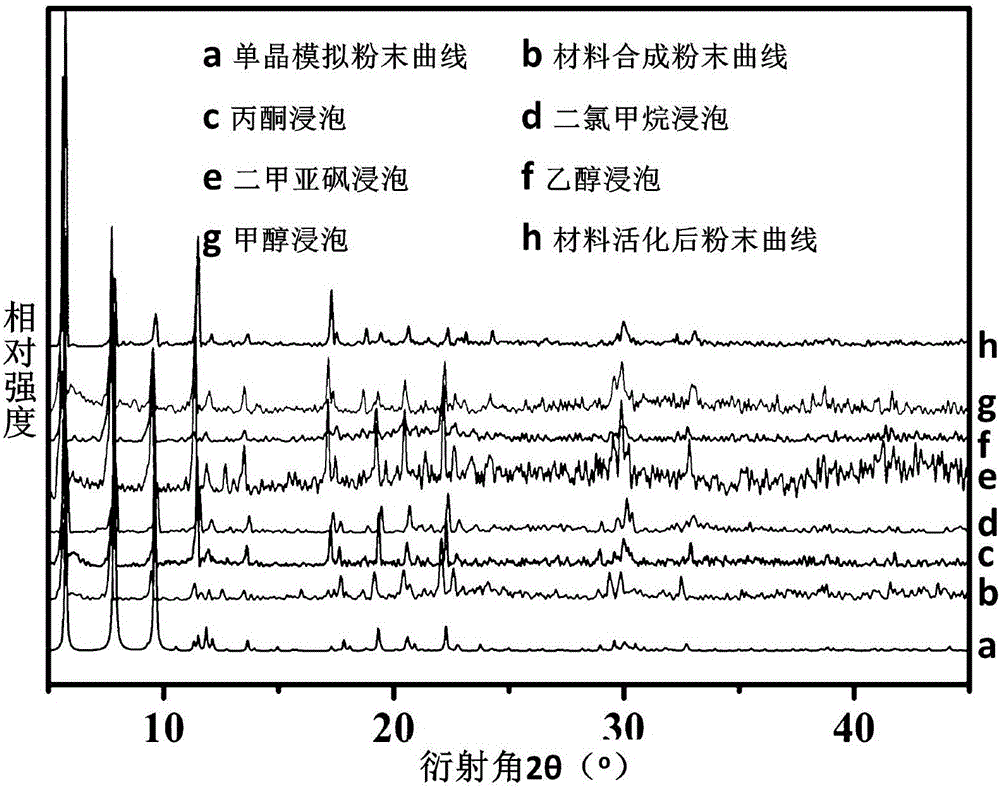
Cluster-base crystalline material, and preparation method and application thereof
InactiveCN105713209AMild conditionsEasy to prepare in large batchesOther chemical processesCrystalline materialsCluster based
The invention discloses a cluster-base crystalline material, and a preparation method and application thereof. The chemical formula of the cluster-base crystalline material is [Co6(mu3-OH)4(Ina)8](H2O)10(DMA)2, wherein Ina is an isonicotinic acid anion ligand, and DMA is N,N-dimethylacetamide. The cluster-base crystalline material is crystallized in the monoclinic system, the space group is P2[1] / c, a=9.2607(2)A, b=12.0677(3)A, c=31.0183(7)A, beta=97.604(3) degrees, and V=3435.98(15)A<3>. The cluster-base crystalline material has the advantages of favorable stability for organic solvents and higher heat stability; and the gas adsorption test result indicates that the material has favorable selective adsorptivity for C2H2 and CH4.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
Genetic engineering strain for producing succinic acid by utilizing glucose and acidogenic fermentation method thereof
InactiveCN102533626AOvercomes the inability to utilize glucoseBacteriaRecombinant DNA-technologyPhosphoenolpyruvate carboxylaseEscherichia coli
The invention belongs to the field of biology engineering technology, and relates to a genetic engineering strain for producing succinic acid by utilizing glucose and an acidogenic fermentation method of the genetic engineering strain. The genetic engineering strain for producing succinic acid by utilizing glucose is named as Escherichia coli BA205 and the preservation number is registered as CCTCC No.M2011447. In the construction process, Escherichia coli which is short of lactic dehydrogenase (LDH) gene and Pyruvate formate-lyase (PFL) gene activity is mainly used as an original strain; phosphoenolpyruvate carboxylase (PPC) gene is removed by utilizing a homologous recombination technology; and phosphoenolpyruvate carboxylase and nicotinic acid phosphoribosyl transferase are excessively co-expressed; therefore the synthesis efficiency of succinic acid is greatly increased. In the fermentation method, a two-stage fermentation manner is adopted, the biomass is improved in an aerobic stage and the acidogenic fermentation is carried out in an anaerobic stage.
Owner:NANJING UNIV OF TECH
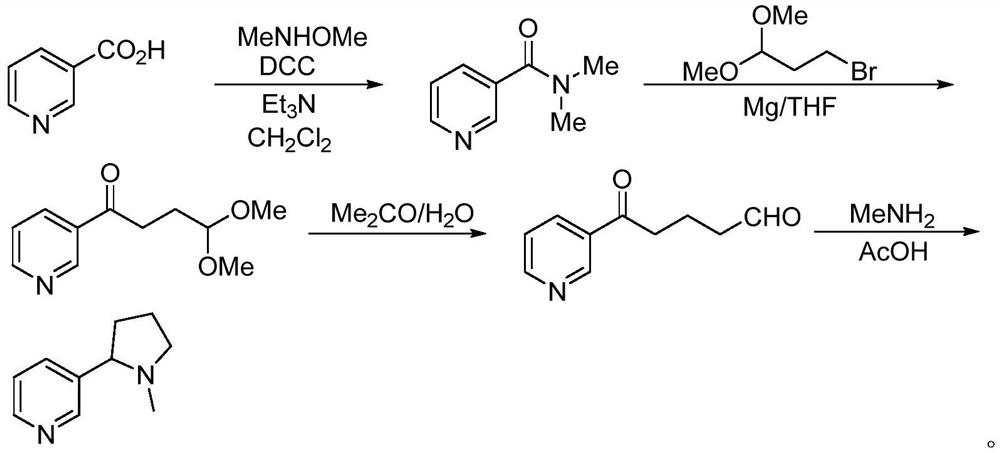
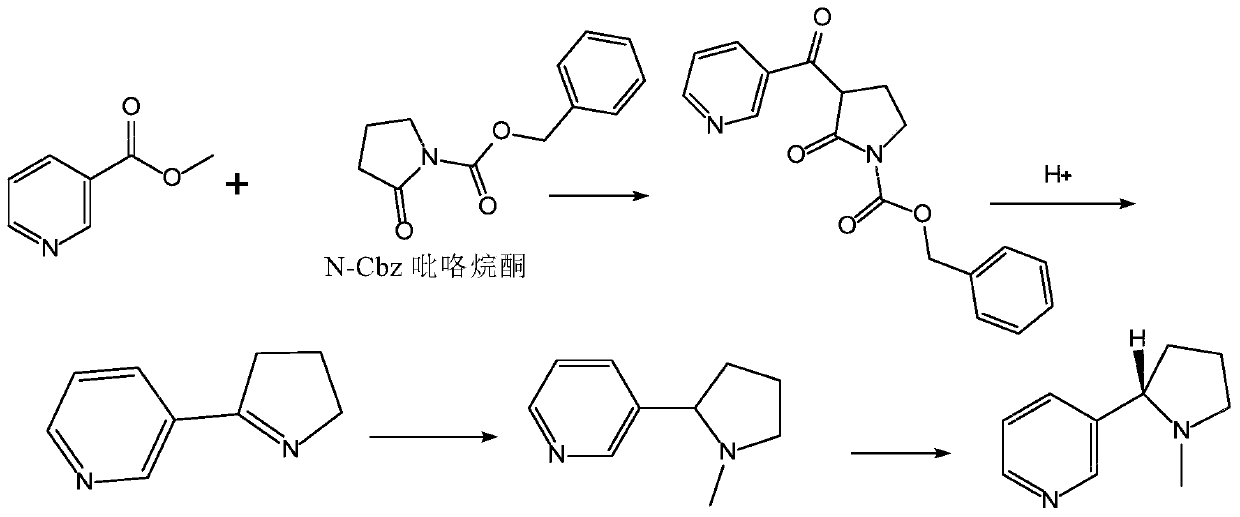
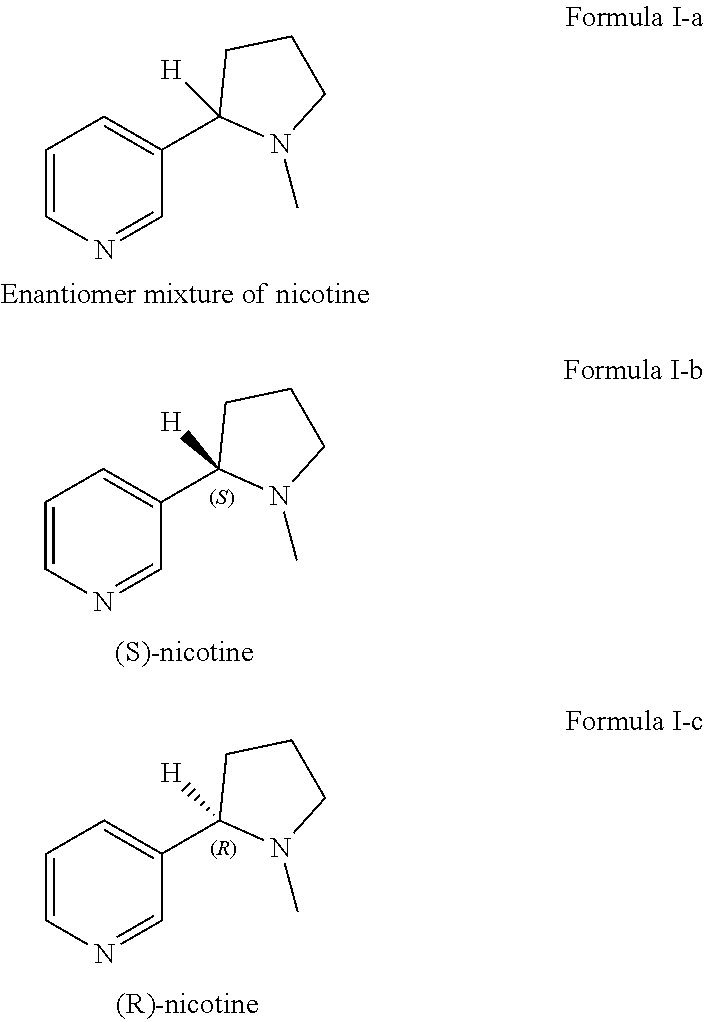
Method for preparing nicotine
The invention relates to a method for preparing nicotine. The method comprises the following steps: (1) adding N-Cbz pyrrolidone, nicotinate, an alkaline catalyst and a reaction solvent into a reaction container, carrying out a reaction, quenching until the system is neutral, and removing the reaction solvent to obtain a first solid mixture; (2) adding the first solid mixture into an acidic solution, and carrying out a reflux reaction to obtain a second reaction mixture; and (3) adding formic acid or formate solid and a formaldehyde solution into the second reaction mixture, reacting, and purifying the product to obtain racemic nicotine. The preparation method disclosed by the invention has high yield, and the prepared racemic nicotine and the S-nicotine have high purity.
Owner:SHENZHEN RELX TECH CO LTD
Method for preparing 5-methyl-3-bromomethylpyridine hydrobromide
The invention relates to a method for preparing 5-methyl-3-bromomethylpyridine hydrobromide. The method comprises a step of preparing methyl 5-methyl nicotinate; a step of preparing 5-methyl-3-pyridinemethanol; a step of preparing 5-methyl-3-pyridinemethanol hydrobromide; and a step of preparing 5-methyl-3-bromomethylpyridine hydrobromide. In the step of preparing methyl 5-methyl nicotinate, esterification of 5-methyl nicotinic acid and thionyl chloride is carried out under heating reflux to obtain methyl 5-methyl nicotinate; in the step of preparing 5-methyl-3-pyridinemethanol, a reduction reaction of methyl 5-methyl nicotinate and sodium borohydride is carried out in an organic solvent to obtain 5-methyl-3-pyridinemethanol; in the step of preparing 5-methyl-3-pyridinemethanol hydrobromide, 5-methyl-3-pyridinemethanol reacts with hydrobromic acid to obtain 5-methyl-3-pyridinemethanol hydrobromide; and in the step of preparing 5-methyl-3-bromomethylpyridine hydrobromide, 5-methyl-3-pyridinemethanol hydrobromide reacts with hydrobromic acid in an organic solvent and water is removed from the reaction system to obtain 5-methyl-3-bromomethylpyridine hydrobromide. According to the method, 5-methyl-3-bromomethylpyridine hydrobromide is obtained by four steps; the process is simple; and yield is high.
Owner:SHANGHAI INST OF TECH
Therapeutics
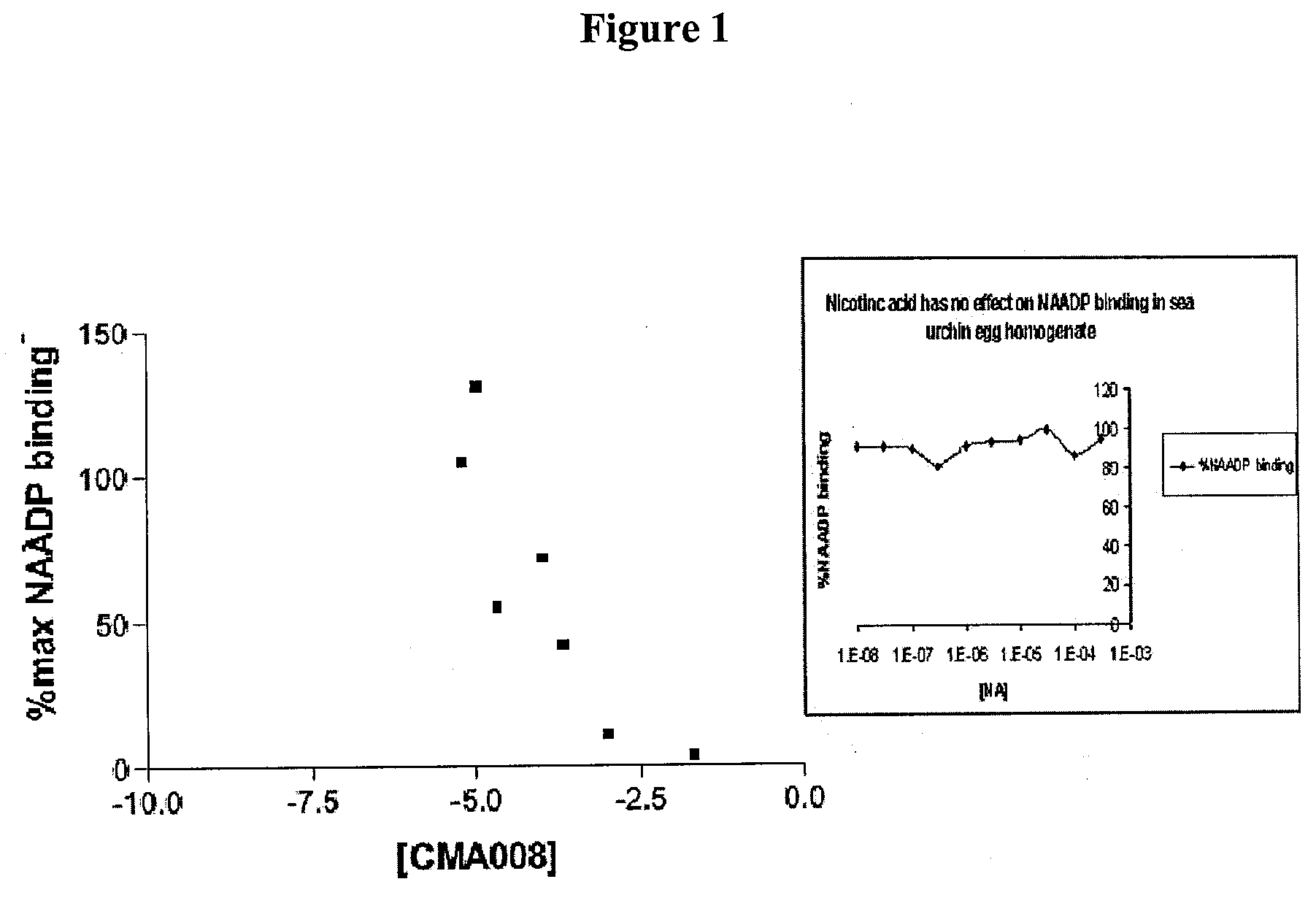
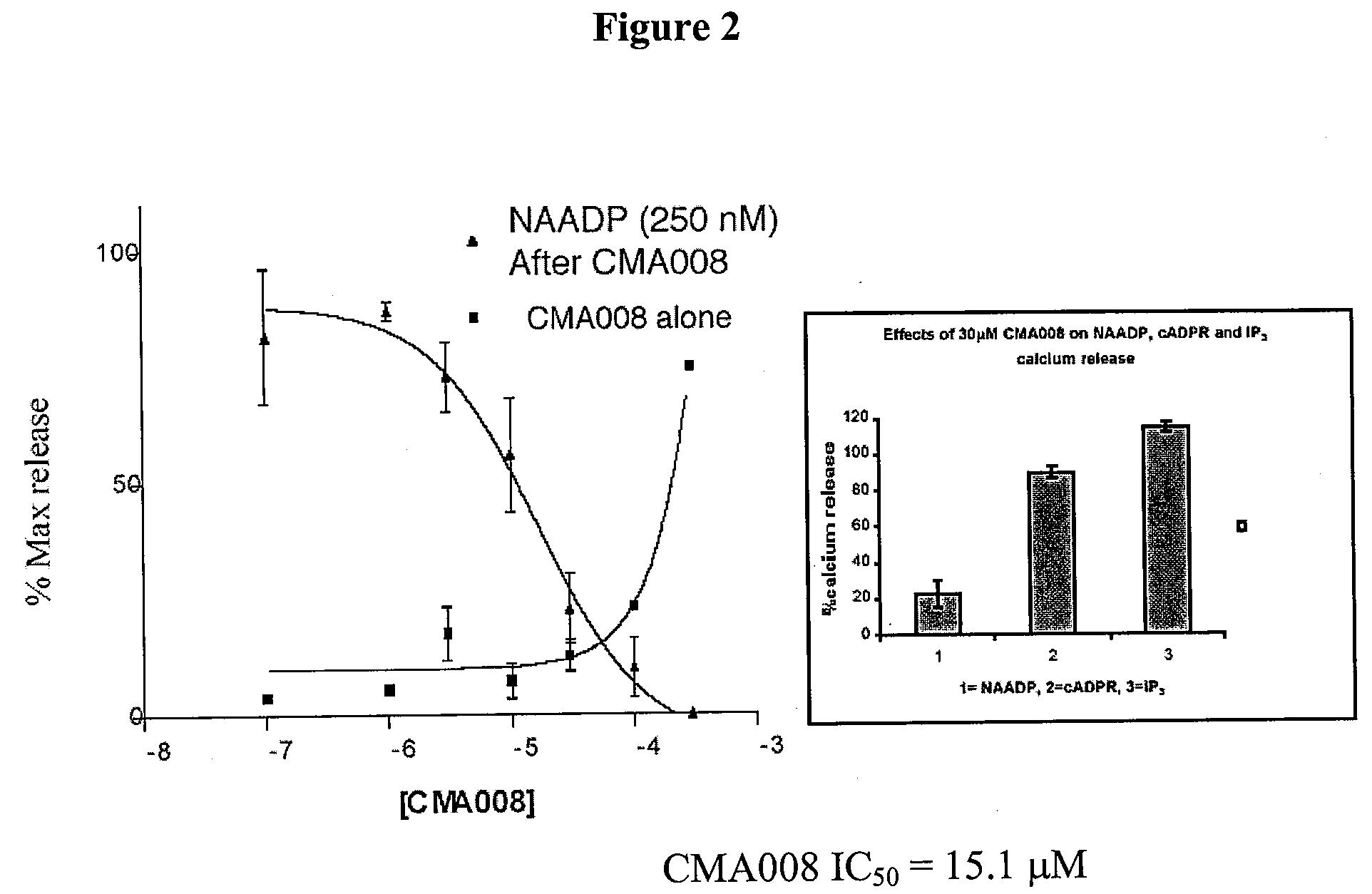
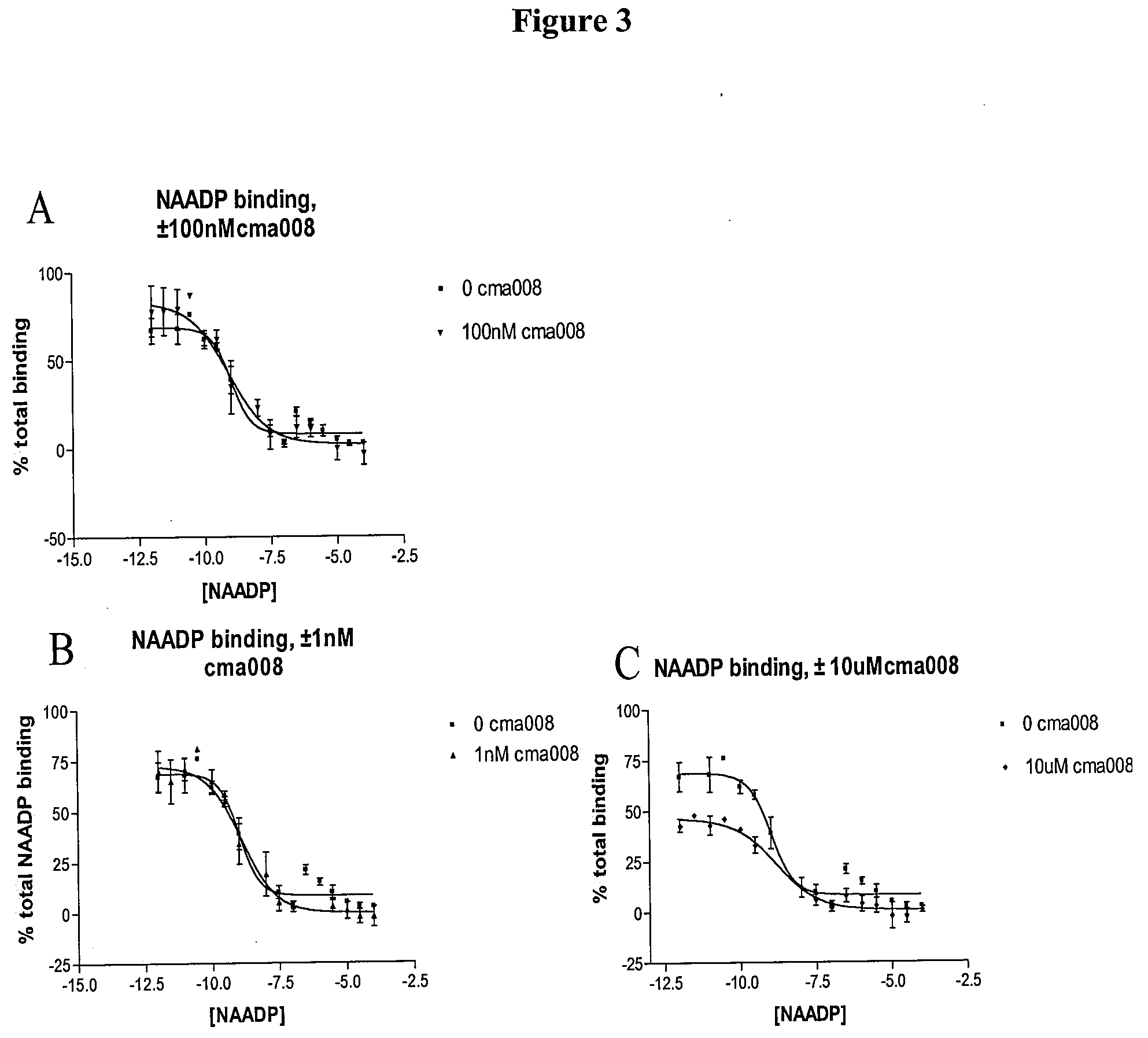
The present invention relates to the use of a compound of formula (1): wherein: R1 comprises a carbonyl group and R2 is a hydrocarbyl group; optionally wherein said ring is further substituted; or a pharmaceutically acceptable salt thereof; in the manufacture of a medicament for use in one or more of: modulating the release of intracellular calcium from a store controlled by nicotinic acid adenine dinucleotide phosphate; modulating calcium spikes in mammalian cells; treating diseases in one or more of brain, heart, pancreatic cells (e.g. pancreatic acinar and pancreatic beta cells), immune cells, T-cells, haemopoietic cells including phagocytes; treating diseases in one or more of brain, heart, pancreatic cells (e.g. pancreatic acinar and pancreatic beta cells), immune cells, T-cells, haemopoietic cells including phagocytes by modulating the release of intracellular calcium from a store controlled by nicotinic acid adenine dinucleotide phosphate; treating diseases in one or more of brain, heart, and T-cells by modulating calcium spikes in mammalian cells.
Owner:POTTER BARRY VICTOR LLOYD +4
Targeting NAD biosynthesis in bacterial pathogens
The emergence of multidrug-resistant pathogens necessitates the search for new antibiotics acting on previously unexplored targets. Nicotinate mononucleotide adenylyltransferase of the NadD family, an essential enzyme of NAD biosynthesis in most bacteria, was selected as a target for structure-based inhibitor development. To this end, the inventors have identified small molecule compounds that inhibit bacterial target enzymes by interacting with a novel inhibitory binding site on the enzyme while having no effect on functionally equivalent human enzymes.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +2
Improved preparation method of nicergoline
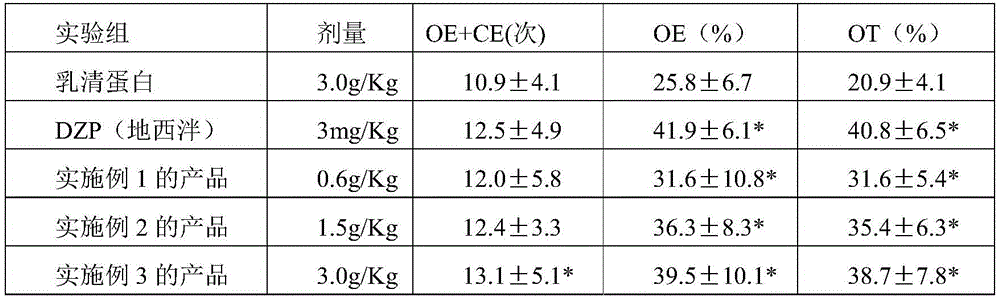
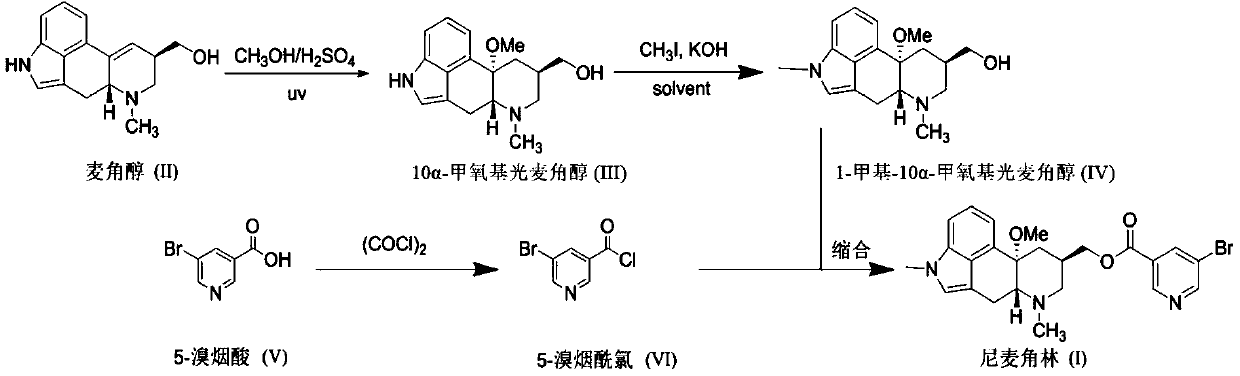
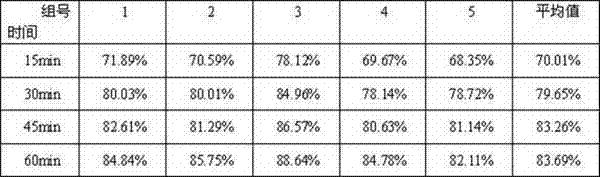
ActiveCN111116580AEasy to recycleAvoid Risk of Residue Control ComplianceOrganic chemistryNicotinuric acidMethyl palmoxirate
The invention relates to an improved preparation method of nicergoline. The method comprises the following steps: (1) carrying out a photoreaction on ergosterol and methanol under the conditions of catalysis of a proper amount of concentrated sulfuric acid and ultraviolet irradiation to obtain 10alpha-methoxyergosterol; (2) adding an inorganic base into an amide aprotic solvent, and carrying out amethylation reaction on the 10alpha-methoxyergosterol and methyl iodide to generate 1-methyl-10alpha-methoxyergosterol; and (3) reacting 5-bromonicotinic acid with oxalyl chloride in a solvent with organic amine as an acid-binding agent to prepare a 5-bromonicotinyl chloride intermediate, and then carrying out a condensation reaction on the 5-bromonicotinyl chloride intermediate and 1-methyl-10alpha-methoxyergosterol to prepare the nicergoline. Compared with the prior art, the method of the invention has the advantages of no inert gas shielding in the reaction, small acid dosage, and easinessin recycling of reaction byproducts, makes the final yield of the product reach 50% or above and the purity of the product reach 99% or above, and is suitable for large-scale production.
Owner:SHANGHAI INST OF TECH +1
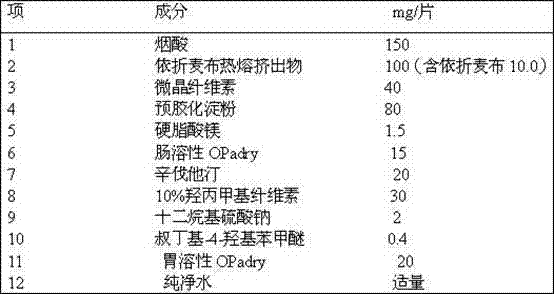
Ezetimibe, simvastatin and nicotinic acid compound preparation and preparation method of ezetimibe, simvastatin and nicotinic acid compound preparation
InactiveCN103239449AHigh dissolution rateGood effectOrganic active ingredientsMetabolism disorderProlonged-release tabletNicotinuric acid
The invention belongs to the field of pharmaceutical preparations and particularly relates to a compound preparation which takes ezetimibe, simvastatin and nicotinic acid as effective components and has the effects of reducing blood pressure and blood lipid and a preparation method of the compound preparation. The compound preparation is a sustained release tablet. Aiming at the problems of poor water solubility of the ezetimibe, unstability of the simvastatin to acid and oxygen, and the like, the preparation method of the compound preparation adopts a melt extrusion technology for improving the dissolution rate of the ezetimibe, and adopts a cladding membrane technology for enhancing the stability of the simvastatin in a body so as to give full play to the efficacy of all components of the compound preparation and have the best synergy effect. The specific process comprises the steps of preparing the nicotinic acid into a tablet core as a sustained release part, spraying an isolating layer on the tablet core, then spraying the simvastatin and the ezetimibe on an outer layer of the sustained release part as a quick release layer, and then carrying out film coating on the quick release layer. The compound preparation provided by the invention is mainly applied to the treatment or prevention of diseases and indications associated with heart and cerebral vessels.
Owner:LIAONING YILING KECHUANG BIOLOGICAL MEDICAL TECH +1
Method for preparing nevirapine
InactiveCN102167699AAvoid hyperbaric reactionsReduce security risksOrganic chemistryNucleoside Reverse Transcriptase InhibitorDiimide
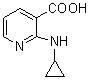
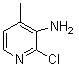
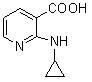
The invention discloses a method for preparing nevirapine, in particular a new method for preparing a non-nucleoside reverse transcriptase inhibitor of Nevirapine for treating human immunodeficiency virus (HIV). The method comprises the following steps of: reacting 2-chloronicotinic acid serving as an initial material with cyclopropanamine; performing amidation reaction with 2-chloro-3-amino-4-methyl pyridine in the presence of N-hydroxysuccinimide, dicyclohexylcarbodiimide and triethylamine; and closing a ring under the action of potassium tert-butoxide to obtain the Nevirapine. The method has the advantages of cheap and readily available raw materials, simple operation, light pollution and suitability for industrial production.
Owner:HUNAN BOAODE BIOPHARML TECH DEV
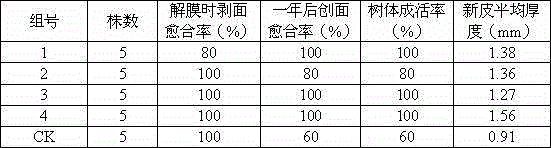
Bark regenerant and preparation method and application thereof
InactiveCN104396968AImprove survival rateShort regeneration timeBiocidePlant growth regulatorsIron sulfateThiamine hcl
The invention belongs to the field of bark regenerants, and particularly discloses a bark regenerant. The bark regenerant at least comprises one of a bark regenerant I, a bark regenerant II and a bark regenerant III, wherein the bark regenerant I is prepared from nicotinic acid, glycine, thiamine hydrochloride, pyridoxine hydrochloride, vitamin, naphthylacetic acid and indolebutyric acid; the bark regenerant II is prepared from vitamin C, amoxicillin sulbactam, a povidone iodine preparation, polyvinylpyrrolidone, naphthylacetic acid and indolebutyric acid; the bark regenerant III is prepared from inositol, nicotinic acid, glycine, thiamine hydrochloride, pyridoxine hydrochloride, vitamin C, potassium iodide, sodium sulfate, potassium nitrate, calcium nitrate tetrahydrate, magnesium sulfate heptahydrate, manganese sulfate tetrahydrate, zinc sulphate heptahydrate, sodium molybdate, copper sulfate pentahydrate, ferric sulfate, naphthylacetic acid and indolebutyric acid. The bark regenerant can effectively promote regeneration of new barks of peeled trees, is general and is extremely good in use effect in most tree species.
Owner:CHENGDU VOCATIONAL COLLEGE OF AGRI SCI & TECH
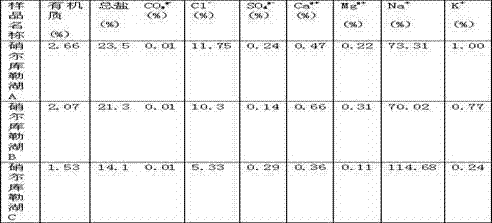
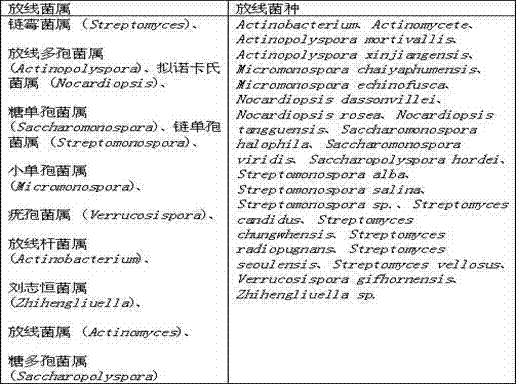
Culture medium for separating actinomycetes in high-salt environment and application thereof
InactiveCN102965308ASeparation fitMore separationBacteriaMicroorganism separationBiotechnologyVitamin b6
The invention discloses a culture medium for separating actinomycetes in a high-salt environment and application thereof. The culture medium is composed of 1% of mannitol, 0.1% of alanine, 0.1% of K2HPO4, 0.1% of MgSO4.7H2O, 0.02% of CaCO3, 0.001% of FeSO4.7H2O, 1.8% of Agar, 0.0003% of trace salt, 0.0004% of compound vitamin, 0.000025% of biotin and 0.005% of potassium dichromate, wherein the trace salt is composed of 0.0001% of FeSO4.7H2O, 0.0001% of MnCl2.4H2O and 0.0001% of ZnSO4.7H2O; and the compound vitamin is composed of 0.00005% of vitamin B1, 0.00005% of vitamin B2, 0.00005% of vitamin B6, 0.00005% of nicotinic acid, 0.00005% of inositol, 0.00005% of pantothenic acid, 0.00005% of paraaminobenzoic acid and 0.00005% of folic acid. The culture medium is suitable for separating actinomycetes in an oligotrophic high-salt environment which has a high salt content, and has wide application values.
Owner:TARIM UNIV
Preparation method of topiroxostat
The invention provides a preparation method of topiroxostat. The method comprises the following steps: subjecting isonicotinic acid as a starting material to oxidation with hydrogen peroxide to obtainisonicotinic acid-nitric oxide (an intermediate 1); then esterifying with methanol to obtain methyl isonicotinate-nitric oxide (intermediate 2); then performing hydrazinolysis with hydrazine hydrateto obtain isoniazide-nitric oxide (intermediate 3); then reacting with 4-cyanopyridine to obtain an intermediate 4; then cyaniding with trimethylsilyl cyanide to obtain an intermediate 5; and finallyperforming dehydration and cyclization to generate topiroxostat. According to the method, initial raw materials and reagents are cheap and easily available; experimental operation is simple and controllable, extreme reaction conditions are avoided, and the method is suitable for laboratory development and even industrial production; the total yield is high, and the production cost is reduced; purity of the finished product can be ensured.
Owner:孙哲
Sugar-free momordica grosvenori drink and production method thereof
The invention discloses a sugar-free momordica grosvenori drink and a production method thereof. The sugar-free momordica grosvenori drink is characterized by comprising the following raw materials in parts by weight: 70-80 parts of momordica grosvenori, 20-30 parts of Wuzhou parasitic loranthus tea, 10-15 parts of green plum, 5-10 parts of radices stellariae dichotomae, 2-4 parts of lotus seed, 2-4 parts of apple juice, 0.05-0.1 part of malic acid, 0.05-0.1 part of glacial acetic acid, 0.05-0.1 part of sodium citrate, 0.05-0.1 part of nicotinic acid and the balance of pure water. The sugar-free momordica grosvenori drink disclosed by the invention has the characteristics of removing heat to cool blood, regenerating body fluid and relieving cough, lubricating the intestines and expelling toxins, tendering the skin and beautifying, moistening lung for removing phlegm, increasing the appetite and generating unique taste.
Owner:邓小健
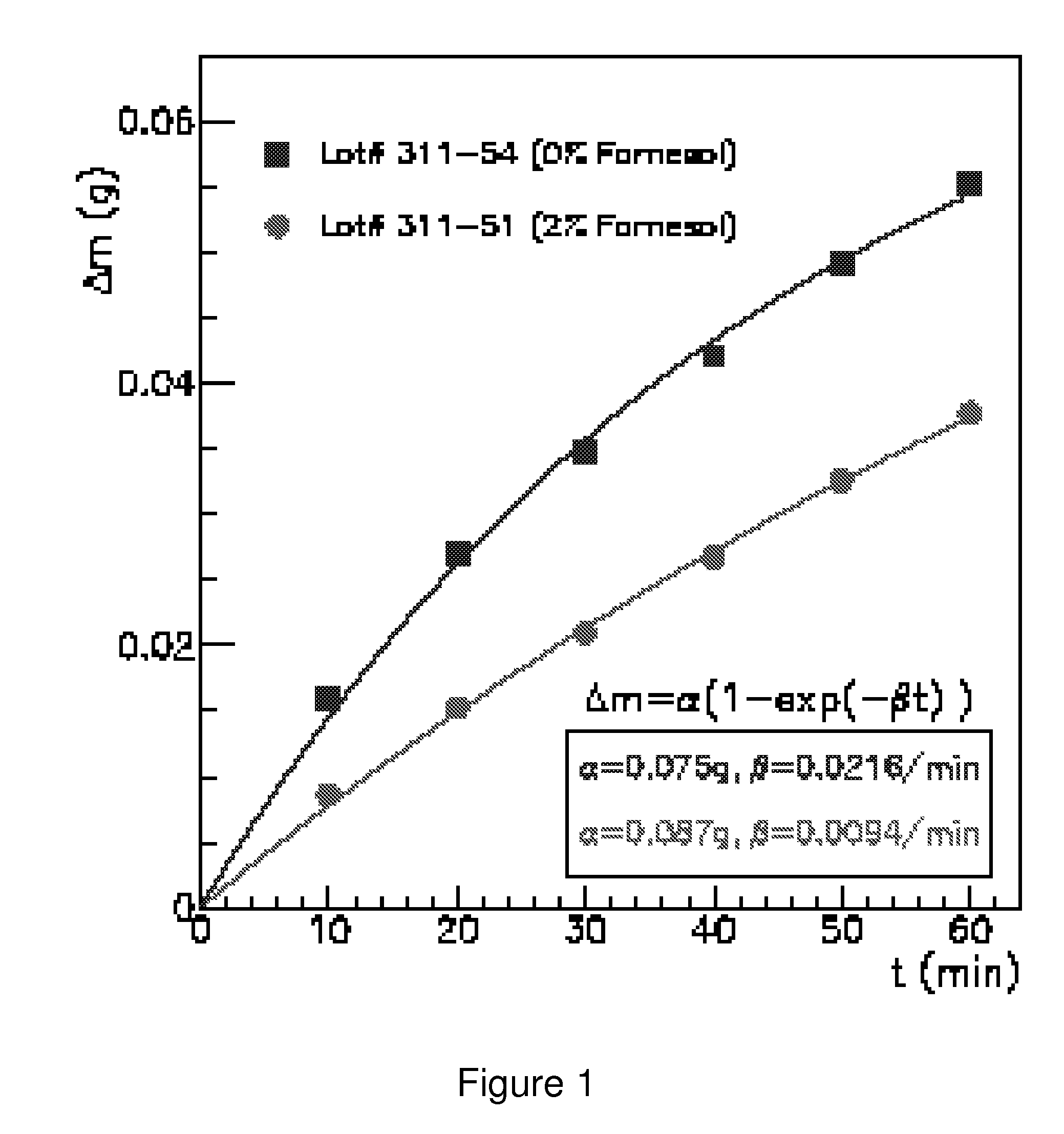
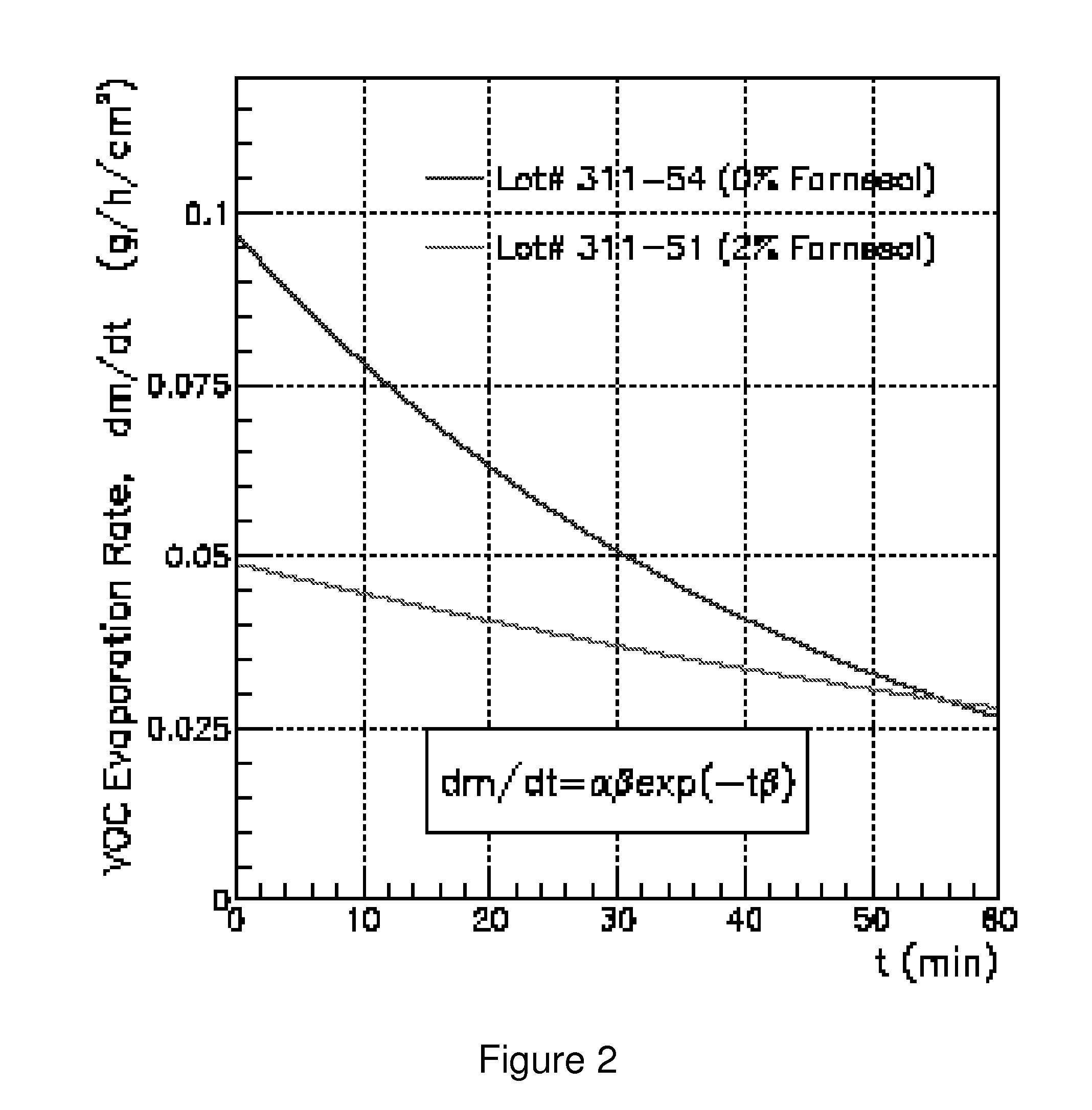
Odor suppression of volatile organic analgesic compounds and method of use
ActiveUS8697043B1Reduced tendencyNone is suitable for purposeBiocideOrganic chemistryWater in oilVolatile organic compound
Disclosed are odor suppressing analgesic compositions comprising:a) from about 0.1% to about 20%, by weight, of farnesol or other suitable deodorizing bacteriostatic agent; andb) from about 0.1% to about 60%, by weight, volatile organic compound wherein said volatile organic compound may be encapsulated and is chosen from the following (menthol, camphor, methyl salicylate, methyl nicotiniate, or combination of thereof); andc) a water-in-oil or oil-in-water emulsion, gel, patch, plaster, ointment, solution or spray carrier. An optimized odor suppressing analgesic composition can provide a combination of antimicrobial properties, odor suppression properties, skin cooling or heating properties, topical analgesic properties, low irritation and stability.
Owner:CHATTEM
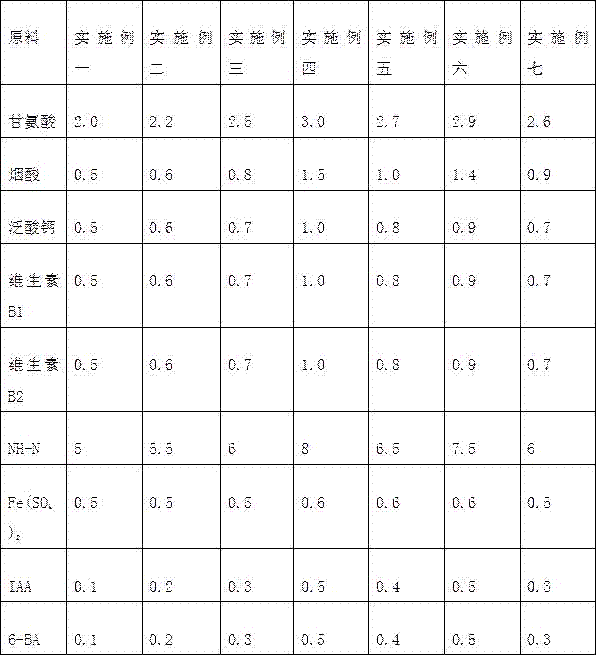
Medium used for growing paper mulberry seedlings
The invention discloses a medium used for growing paper mulberry seedlings. The medium contains glycine, nicotinic acid, calcium pantothenate, vitamin B1, vitamin B2, NH-N, Fe(SO4)2, IAA and 6-BA. The medium solves the problems of slow growth and too long cultivation period of paper mulberry germs in a medium.
Owner:中科天华生物科技有限公司
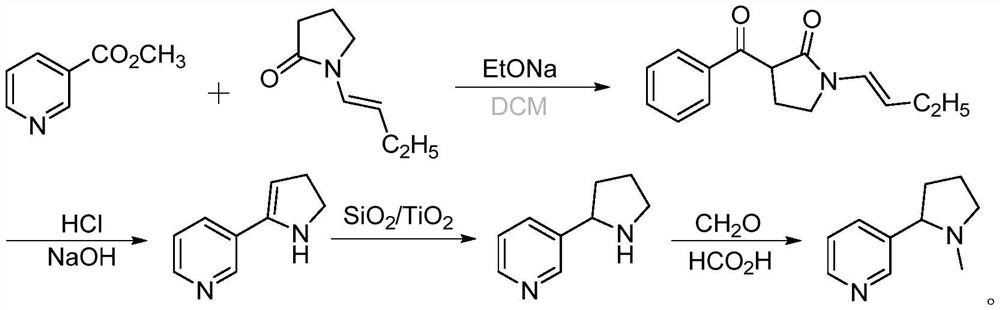
Preparation of racemic nicotine by reaction of ethyl nicotinate with n-vinylpyrrolidone in the presence of an alcoholate base and subsequent process steps
ActiveUS20200331884A1Organic baseOrganic active ingredientsNervous disorderPyrrolidinonesEthyl ester
The present invention relates to a method of preparing racemic nicotine comprising: (i) reacting ethyl nicotinate and N-vinylpyrrolidone in the presence of an alcoholate base to 3-nicotinoyl-1-vinylpyrrolidin-2-one; (ii) reacting the 3-nicotinoyl-1-vinylpyrrolidin-2-one with an acid to myosmine; (iii) reducing the myosmine to nornicotine using a reducing agent; and (iv) methylating the nornicotine to obtain the racemic nicotine.
Owner:CONTRAF NICOTEX TOBACCO GMBH +1
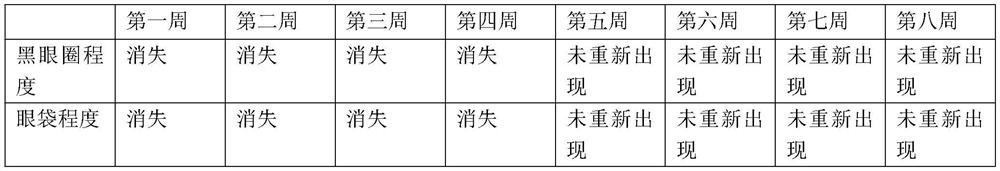
Eye cream for removing dark circles and eye bags and preparation method
ActiveCN111759786APromote circulationExcellent for removing dark circlesCosmetic preparationsToilet preparationsSalvia miltiorrhizaOphthalmology
The invention discloses eye cream for removing dark circles and eye bags and a preparation method. The eye cream is prepared from radix salviae miltiorrhizae extract, rhizoma gastrodiae extract, semenpersicae extract, radix curcumae extract, nicotinic acid, camellia oil, a humectant, a grease emollient, an emulsifier, a thickener, a preservative, a coloring agent and deionized water. Effective components are extracted from salvia miltiorrhiza, rhizoma gastrodiae, semen persicae and radix curcumae, nicotinic acid, camellia oil and the like are used as auxiliary effects, and the composition offunctional extracts is optimized, so that the composition can be used for solving the problems of dark circles and eye bags caused by unsmooth blood at the eye bags, continuous contraction of blood capillaries and the like; the dark circle and eye bag removing skin cream has more excellent dark circle and eye bag removing effects, and can fundamentally solve the dark circle and eye bag problems for a long time as the dark circle and eye bag removing skin cream does not act on melanin but can continuously improve the circulation of blood vessels around eyes for a long time.
Owner:山茶元素(北京)科技研发有限公司
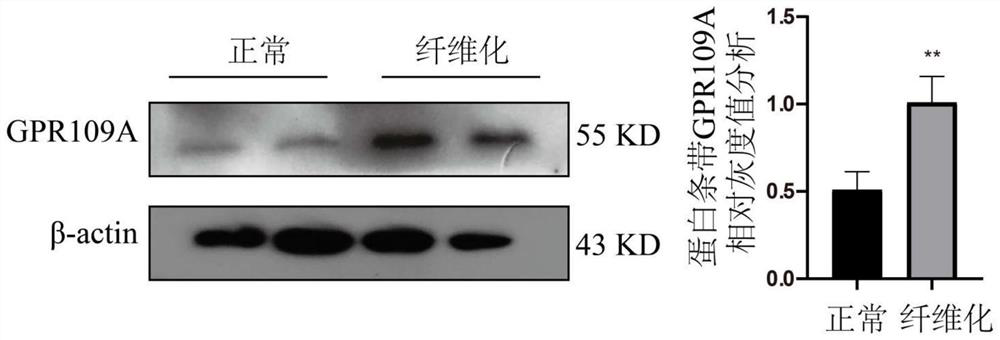
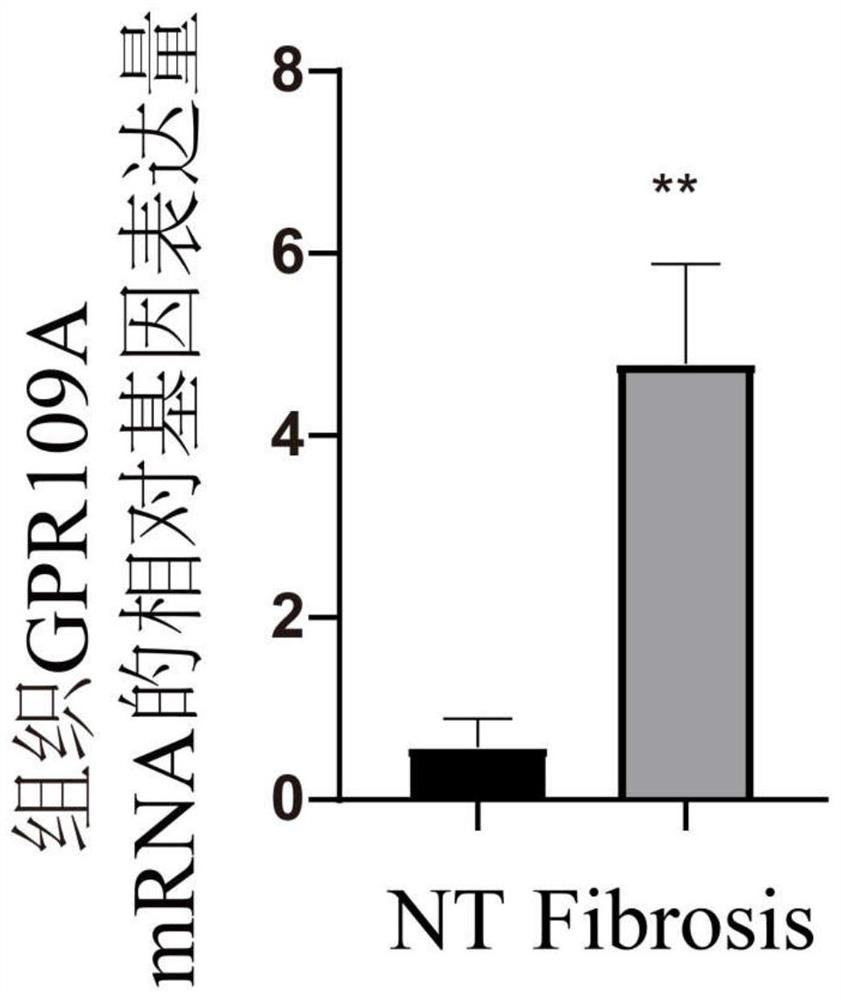
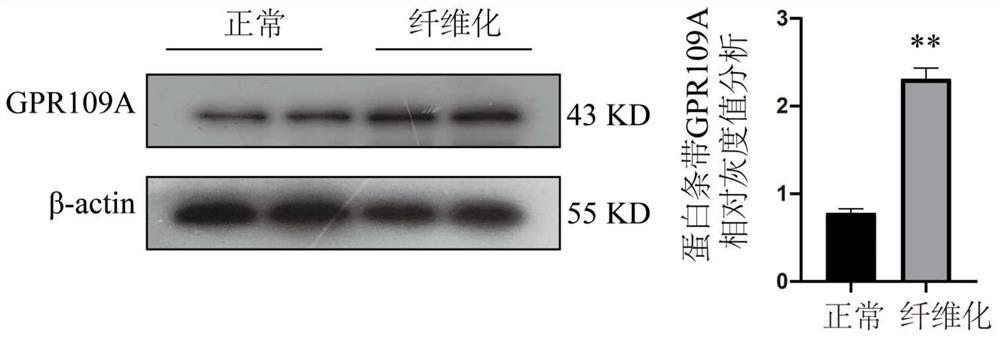
Application of nicotinic acid in preparation of drug for relieving mammary gland fibrosis of dairy cows through GPR109A receptors
ActiveCN113564221AAlleviate fibrosis tendencyMitigation protectionOrganic active ingredientsCompound screeningDiseasePhysiology
The invention discloses an application of nicotinic acid in preparation of a drug for relieving mammary gland fibrosis of dairy cows through GPR109A receptors. The application comprises the following steps that S1, GPR109A receptor expression difference conditions are analyzed in mammary glands of normal dairy cows and fibrosis-prone dairy cows through in-vivo and in-vitro experiments, and it is determined that the GPR109A receptors are highly expressed in the mammary glands of the dairy cows with mammary gland fibrosis; and S2, fibrosis phenotypes in mammary epithelial cells of the dairy cows are detected, specifically, the change conditions of TGF-beta 1, alpha-SMA, Collagen2 and E-cadherin in the mammary epithelial cells of the dairy cows are specifically and correspondingly detected after the nicotinic acid is relieved. According to the application of nicotinic acid in preparation of the drug for relieving mammary gland fibrosis of the dairy cows through the GPR109A receptors, a brand-new relieving strategy for the mammary gland fibrosis tendency of the dairy cows is formulated, and the relieving effect of nicotinic acid on the mammary gland fibrosis tendency of the dairy cows is comprehensively evaluated by collecting normal mammary gland tissues and mammary gland fibrosis mammary gland tissues, separating primary mammary gland epithelial cells of the dairy cows and utilizing a molecular biology method. Nicotinic acid can effectively reduce rise of fibrosis phenotypes in mammary gland epithelial cells, so that the purpose of preventing the occurrence of mammary gland fibrosis diseases is achieved.
Owner:JILIN UNIV
Novel preparation method of nicergoline
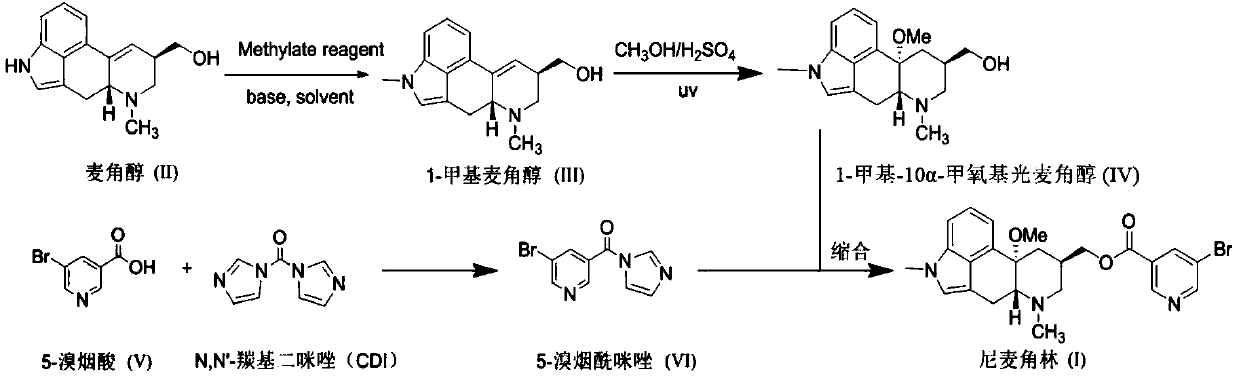
PendingCN111004233AAvoid it happening againFor follow-up productionOrganic chemistryLysergolOrganosolv
The invention relates to a new preparation method of nicergoline. The method comprises the following steps: (1) adding an inorganic alkali into a polar aprotic solvent, and carrying out a methylationreaction on lysergol (II) and a methylation reagent to generate 1-methyl lysergol (III); (2) carrying out a light reaction on the 1-methyl lysergol (III) and methanol under the catalysis of concentrated sulfuric acid to prepare 1-methyl-10 alpha-methoxy dihydrolysergol (IV); and (3) in a polar aprotic organic solvent, carrying out a reaction on 5-bromonicotinic acid (V) and N,N'-carbonyl diimidazole by taking the N,N'-carbonyl diimidazole as a condensing agent to prepare a 5-bromo nicotinyl imidazole (VI) intermediate, and then carrying out a condensation reaction on the 5-bromo nicotinyl imidazole (VI) intermediate and the 1-methyl-10 alpha-methoxy dihydrolysergol (IV) to prepare nicergoline (I). Compared with the method in the prior art, the method of the invention does not require inertgas protection, is small in acid consumption, good in product quality, high in yield, easy in reaction byproduct recovery and utilization and suitable for industrial production.
Owner:SHANGHAI INST OF TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com