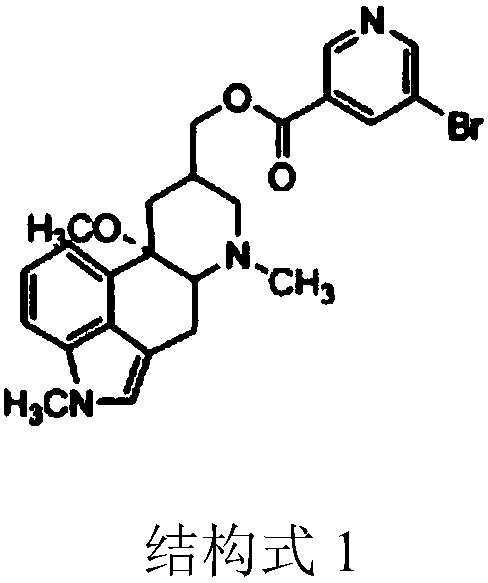
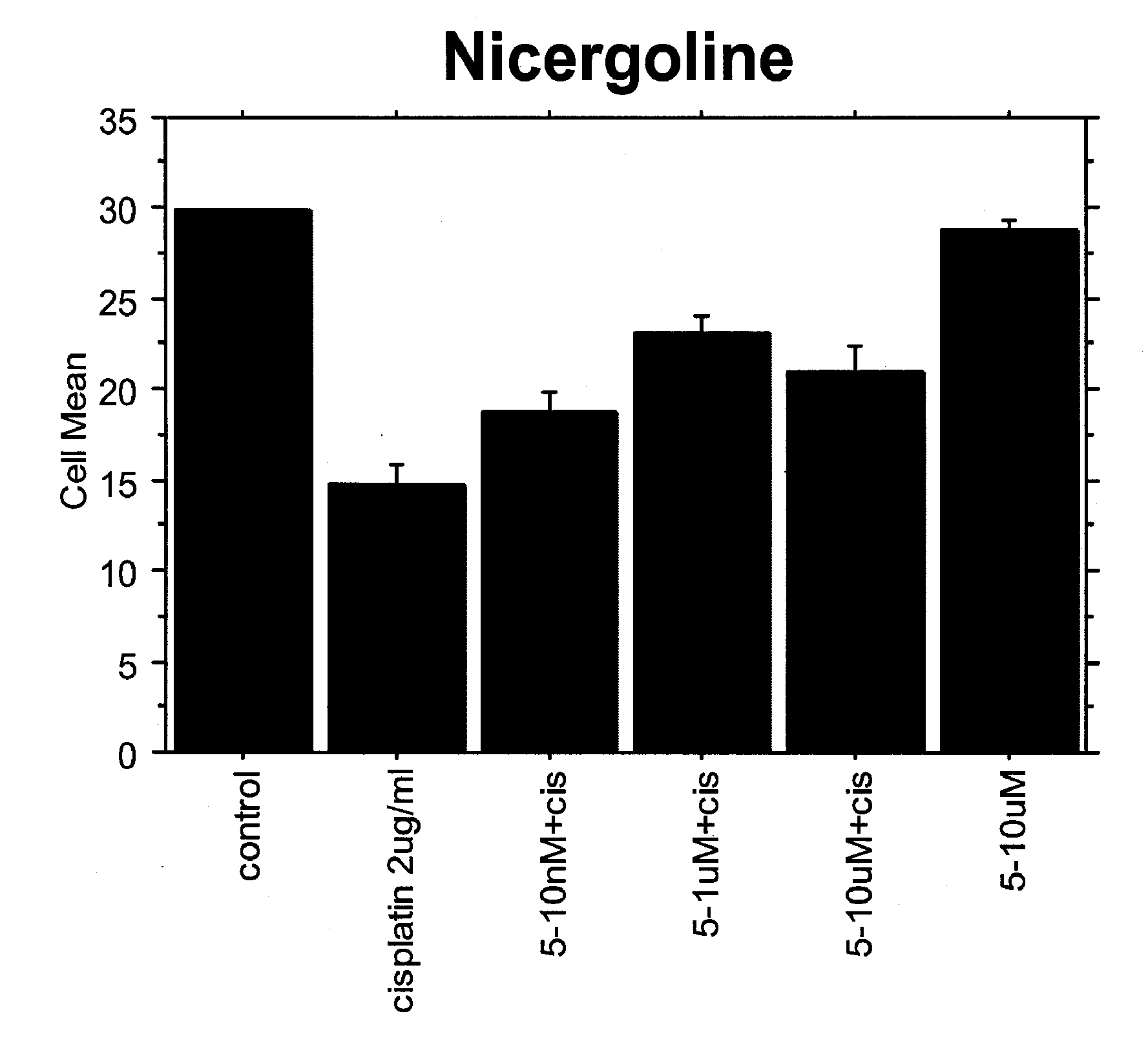
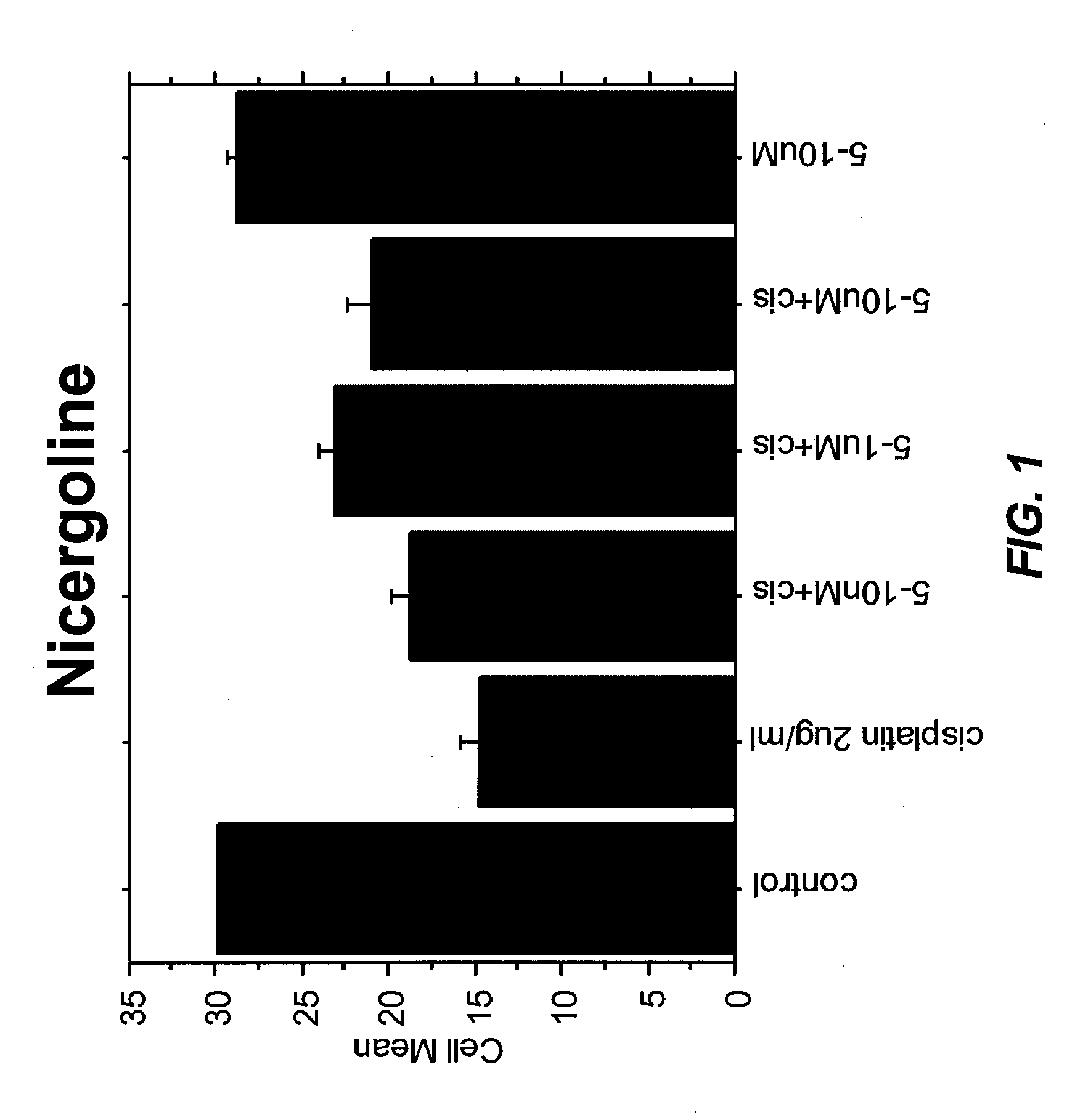
Patents
Literature
49 results about "Nicergoline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
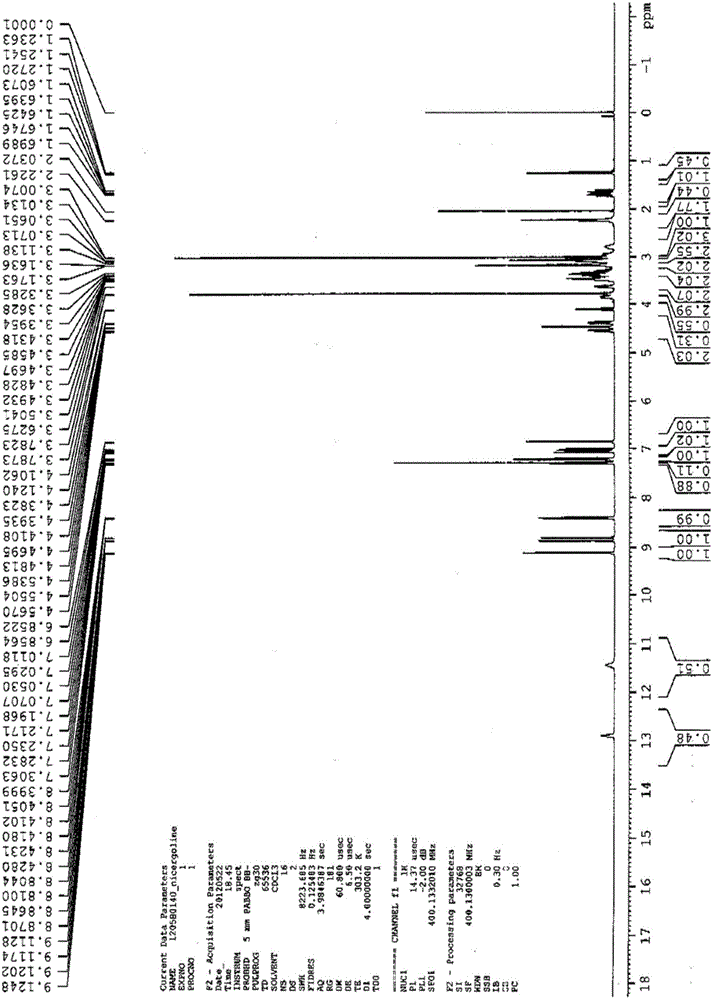
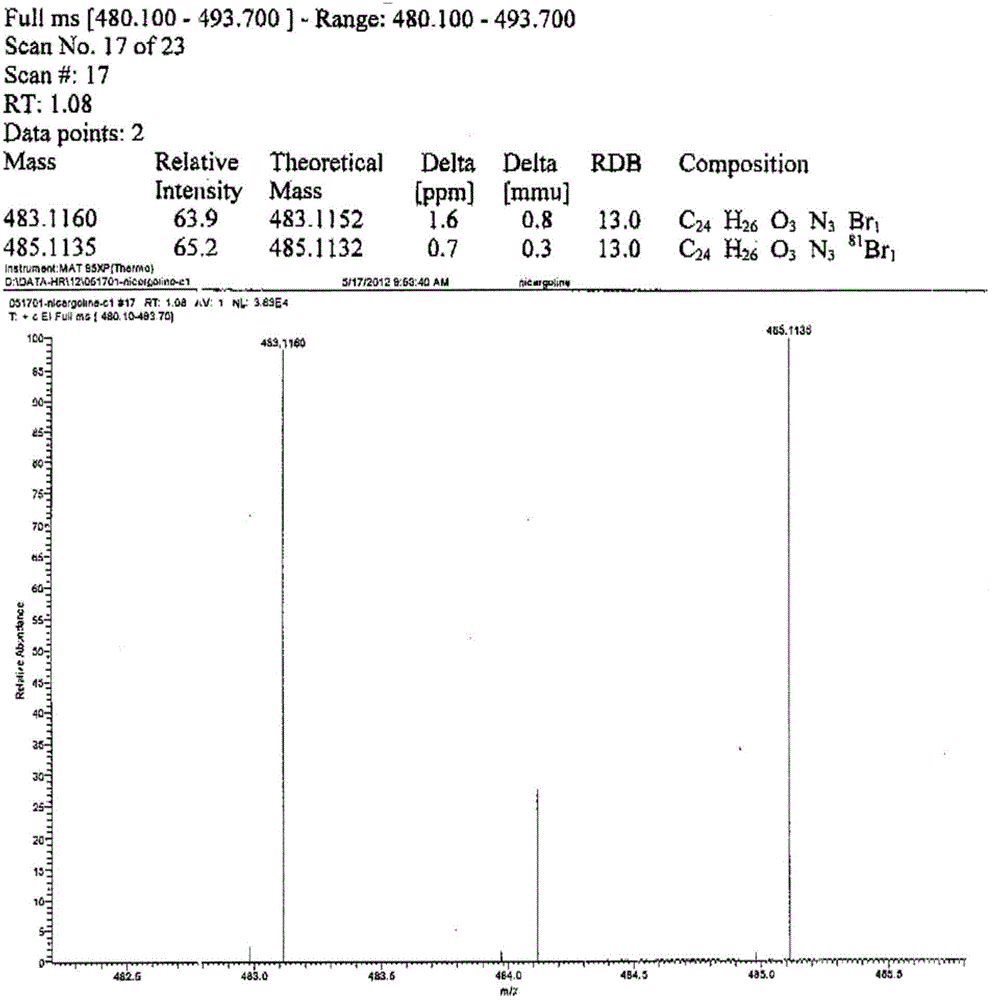
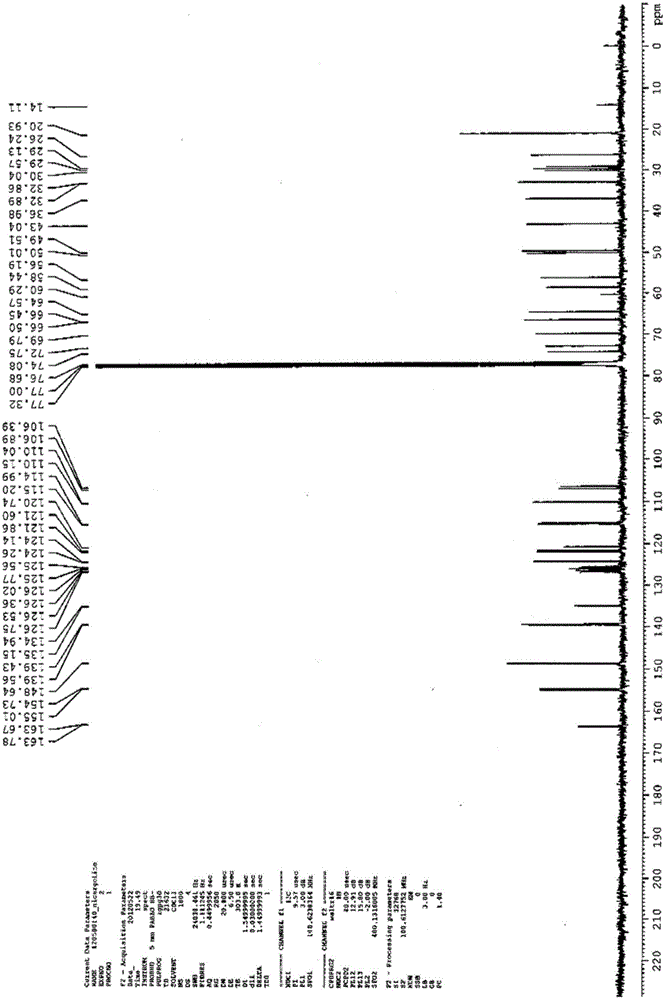
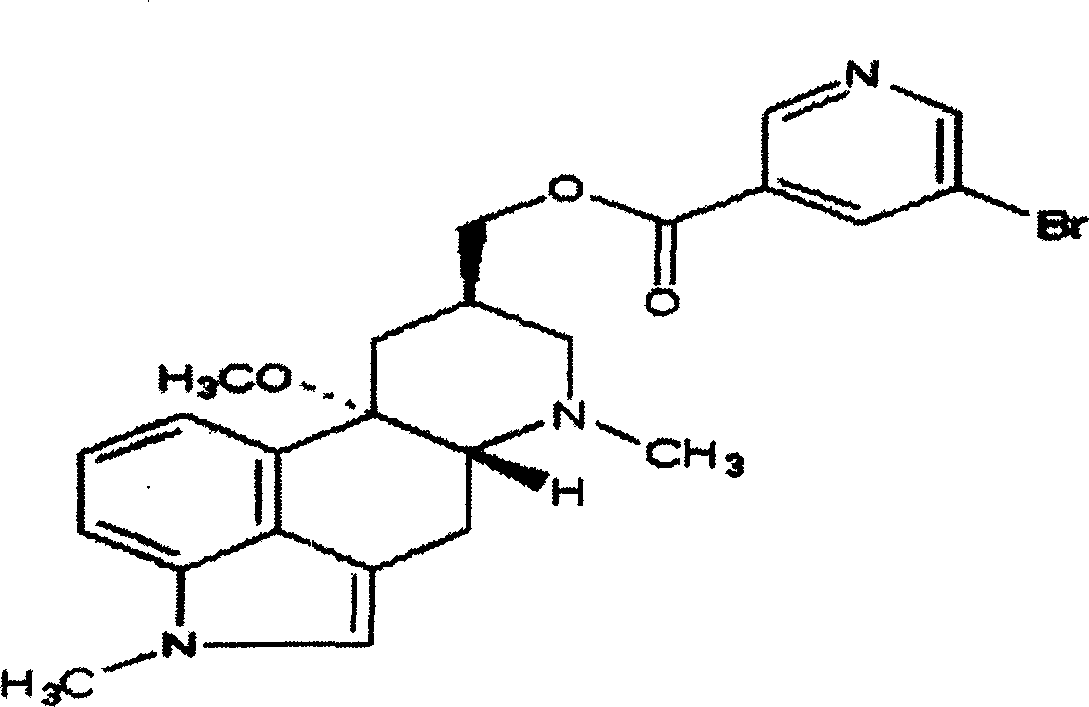
Nicergoline (INN, marketed under the trade name Sermion) is an ergot derivative used to treat senile dementia and other disorders with vascular origins. It decreases vascular resistance and increases arterial blood flow in the brain, improving the utilization of oxygen and glucose by brain cells. It has similar vasoactive properties in other areas of the body, particularly the lungs. Unlike many other ergolines, such as ergotamine, nicergoline is not associated with fibrosis...
Nicergoline pill and preparation method thereof
InactiveCN102949372AImprove liquidityPromote dissolutionNervous disorderPharmaceutical delivery mechanismOfficinalAdhesive
The invention discloses a nicergoline pill and a preparation method thereof. Preparation comprises nicergoline, diluent, disintegrant, adhesive, lubricant and film coating premixing agent. The preparation method includes uniformly mixing the diluent with the internally added disintegrant; preparing blank particles in proper quantity by the adhesive, drying, pelleting and reserving; independently pelleting main drug by the proper adhesive, quickly drying, pelleting and reserving at lower temperature; uniformly mixing the main drug particles with the blank particles, adding externally added auxiliaries, mixing totally, pressing, coating a thin film and packaging. Accordingly, flowability of the particles is guaranteed, the drug can be dissolved beneficially, and more importantly, the problems that content of the main drug and the auxiliaries is lowered and relevant substances are increased due to the high drying temperature and long time in pelleting are avoided. Compared with the prior art, the nicergoline pill has the advantages of reasonable components, simplicity in process, unique preparation method, high dissolving degree, good stability of the preparation, low cost and the like.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
Synthetic method for nicergoline
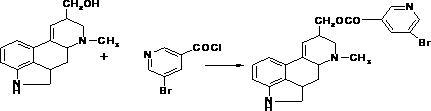
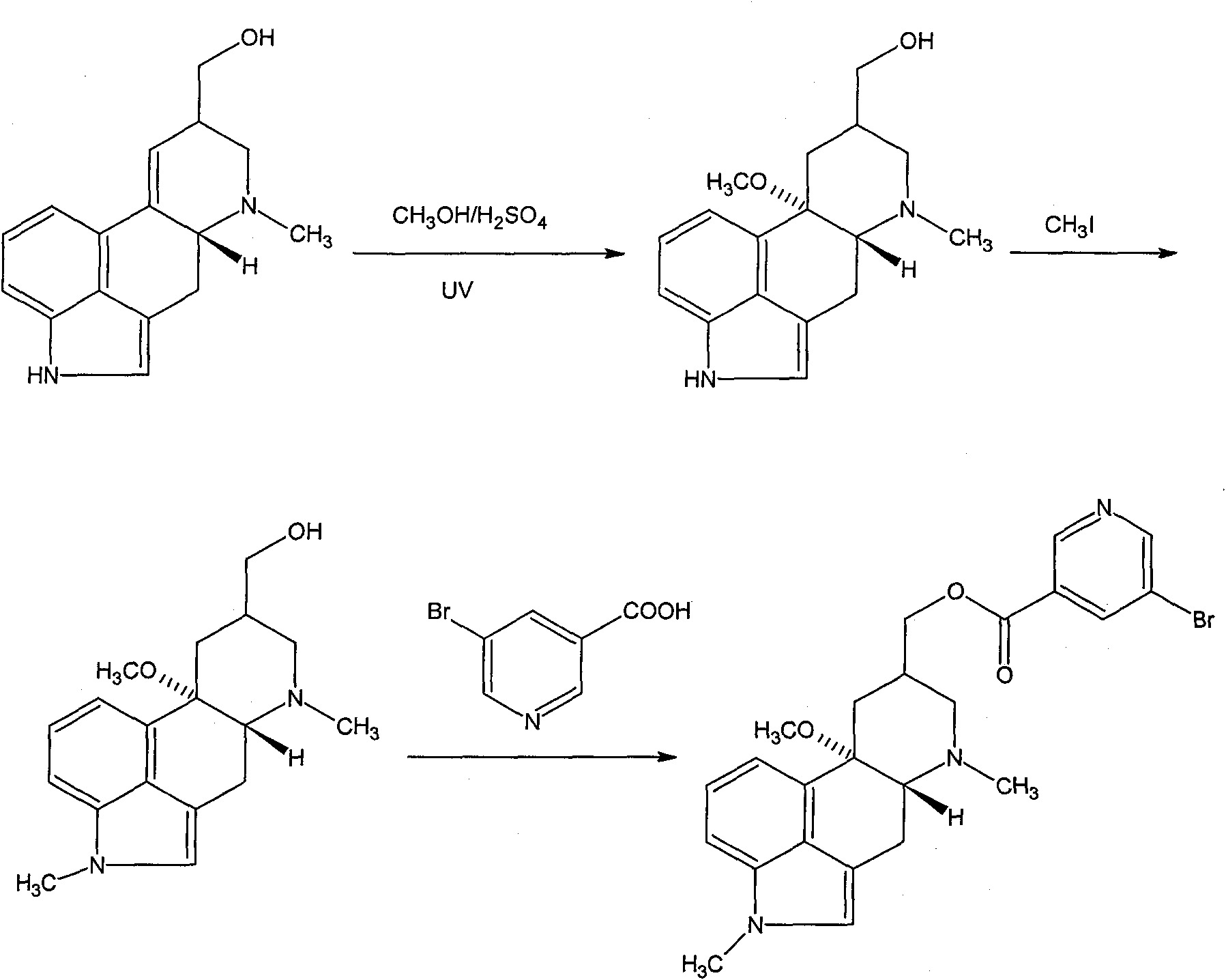
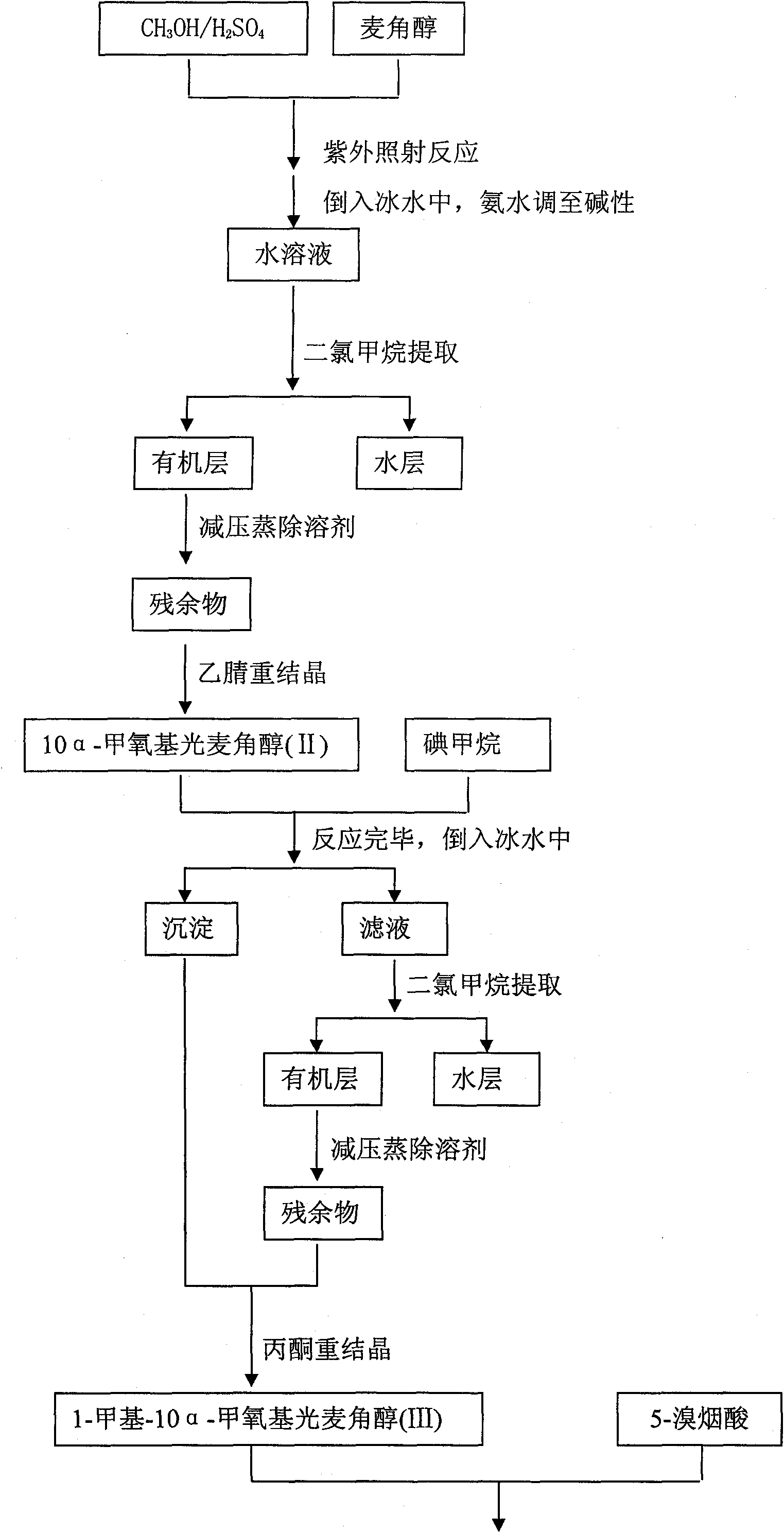
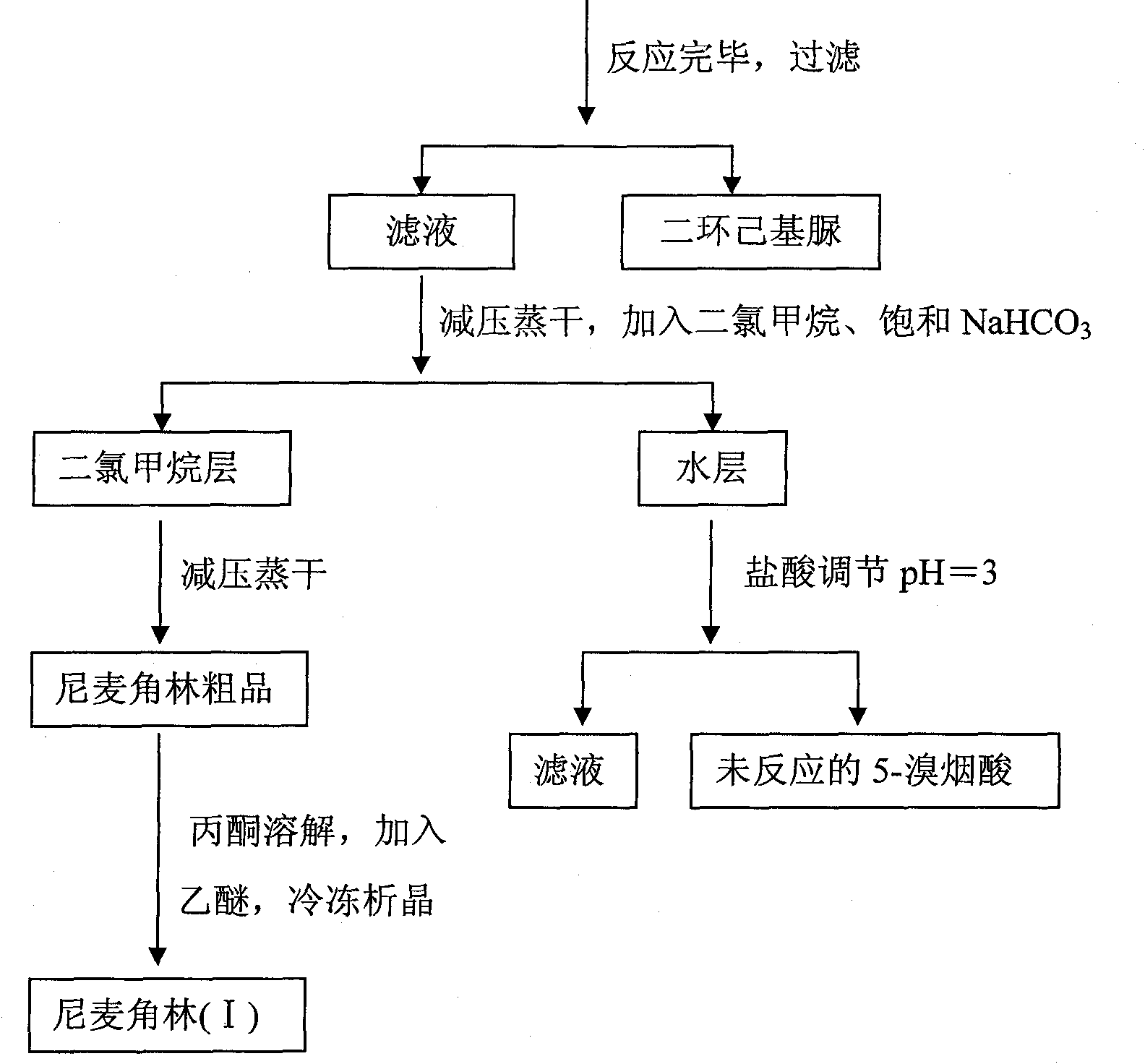
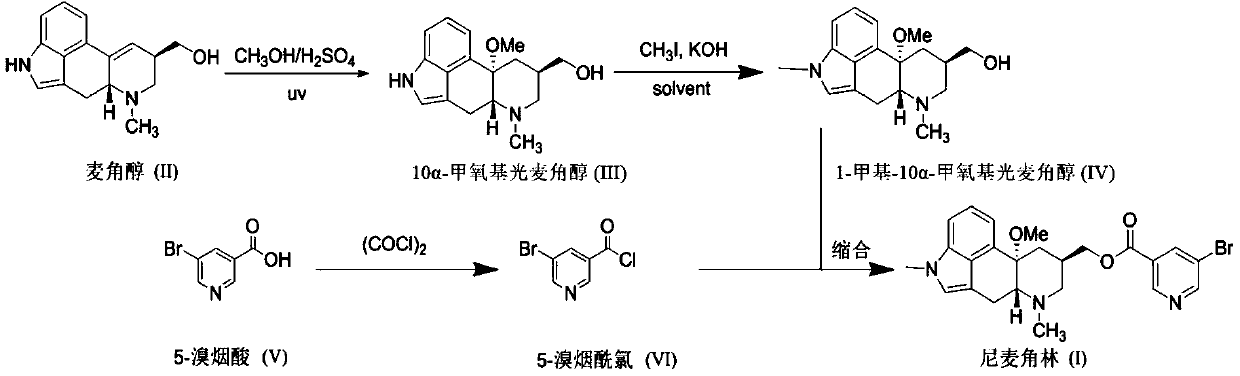
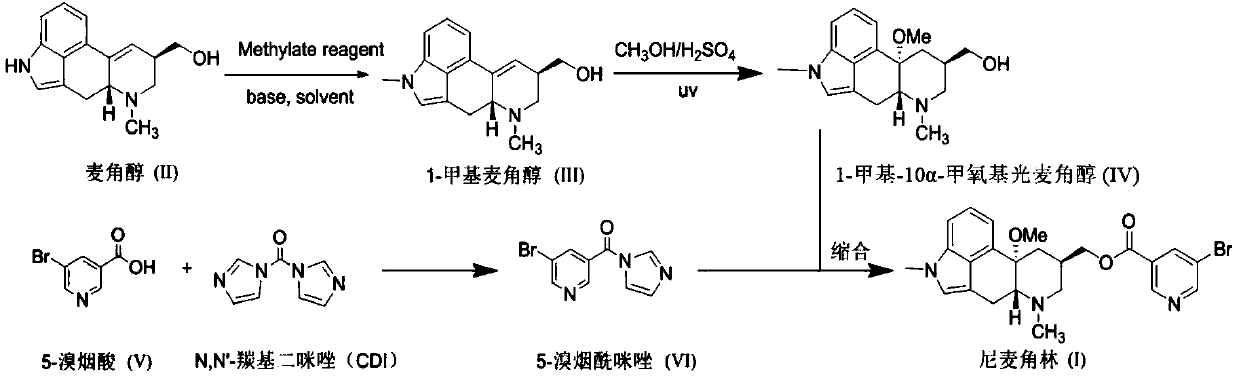
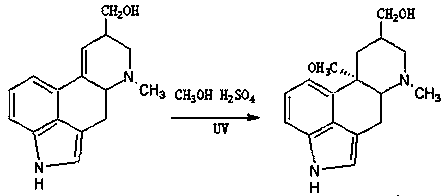
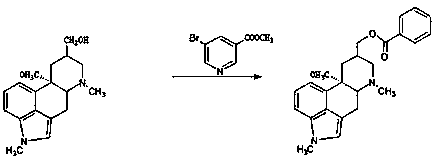
The invention discloses a synthetic method for nicergoline. The synthetic method comprises the following steps: allowing lysergol and methanol to generate a methoxylation reaction, and carrying out purifying so as to obtain 10alpha-methoxyl-dihydrolysergol; allowing the 10alpha-methoxyl-dihydrolysergol and trimethylsulphoxonium iodide to generate a methylation reaction, and carrying out purifyingso as to obtain 1-methyl-10alpha-methoxyl-dihydrolysergol; and allowing the 1-methyl-10alpha-methoxyl-dihydrolysergol and 5-bromonicotinoyl chloride to generate an acylation reaction, and carrying outrefining so as to obtain the nicergoline. The synthetic method provided by the invention has the advantages of simple process, mild reaction conditions, easy operation, low toxicity of raw materials,high quality of a finished product, and facilitation of industrial popularization and application.
Owner:广州普星药业有限公司
Novel preparation method for nicergoline
InactiveCN103159756AQuality improvementHigh selectivityOrganic chemistryPhotocatalytic reactionSynthesis methods
The invention discloses a novel synthesis method for the raw material medicine of nicergoline (shown in formula I). The novel synthesis method disclosed by the invention comprises the following steps of: reacting ergosterol with 5-bromonicotinate derivatives to generate carboxylic ester; then performing a photocatalytic reaction with methanol in an acidic condition; and then performing a methylation reaction with a methylation reagent to generate nicergoline. With the adoption of the novel synthesis method disclosed by the invention, the cumbersome operation of the methods disclosed by the existing patents is avoided, the used solvent is low in toxicity, and the yield is high. The novel synthesis method is beneficial to industrialized popularization and application.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
Prevention and treatment of hearing disorders
InactiveUS20070123555A1Low variabilityGood curative effectBiocideAnimal repellantsAntioxidantHearing perception
Compositions, and methods of use thereof, are provided for the prevention, treatment or alleviation of symptoms of hearing are provided. The methods employ nicergoline, MMDL or MDL as the sole active pharmaceutical agent or a combination of nicergoline, MMDL or MDL and another pharmaceutical agent, such as an antioxidant, a NMDA antagonist, an SSRI or a combined SSRI / NMDA antagonist agent. The method involves the use of nicergoline, MMDL or MDL alone or in combination with another API to prevent, treat or ameliorate one or more symptoms of hearing loss.
Owner:CYPRESS BIOSCI
Preparation method for nicergoline
The invention discloses a preparation method for nicergoline. The preparation method for the nicergoline comprises the following steps: 1) reacting ergot alcohol in a methanol-sulfuric acid system under photocatalysis to prepare 10alpha-methoxyl-light ergot alcohol; 2) performing methylation on the 10alpha-methoxyl-light ergot alcohol and a methylated reagent in an aprotic polar solvent under the catalytic action of strong alkali to prepare 1-methyl-10alpha-methyl-light ergot alcohol; and 3) performing acylation reaction on the1-methyl-10alpha-methyl-light ergot alcohol and 5-bromine nicotinoyl halide in an aprotic hydrophobic solvent under the catalytic action of weak alkali to prepare the nicergoline. The solvents and catalysts adopted by the method have the advantages of low toxicity, low price, simple aftertreament and the like, so that the reaction residues of the products can be reduced, the quality of the product can be improved, and the requirement of environment-friendly production can be met.
Owner:珠海润都制药股份有限公司
Freeze-dried composition contg. nicergoline and its prepn. method
InactiveCN1861076AGood stability of physical and chemical propertiesPowder deliveryNervous disorderFreeze-dryingExcipient
A freeze-dried powder injection is prepared from nicergoline, the acid as pH value regulator and the excipient for freeze-drying. Its preparing process is also disclosed.
Owner:范敏华
Method for producing nicergoline
InactiveCN101781296AFewer synthetic stepsMild reaction conditionsProductsOrganic chemistryLysergolNiacin
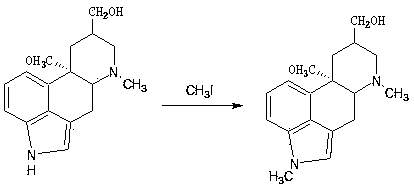
The invention discloses a method for producing nicergoline, which takes a multifunctional reaction kettle with a special kettle bottom valve as a reactor, a special filter as refining equipment and lysergol as an original raw material and comprises the following steps: directly carrying out photoreaction at room temperature in sulfuric acid-methanol to obtain 10alpha-methoxy optical lysergol; reacting the 10alpha-methoxy optical lysergol with methyl iodide under an alkaline condition to obtain 1-methyl-10alpha-methoxy optical lysergol; and directly forming ester with 5-bromine niacin to produce the nicergoline. Compared with the method for producing the nicergoline through current synthesis equipment, the disclosed production process of the nicergoline has the advantages of few reaction steps, mild reaction conditions, convenient refinement, simple operation, good effects of heat preservation, oxidation resistance, sealing property and environmental protection of the used special equipment, complete reaction, simple structure of reaction equipment, convenient operation, easy disassembling, assembling and cleaning, high filtering efficiency, high reaction quality and yield, and stable product quality.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Improved preparation method of nicergoline
ActiveCN111116580AEasy to recycleAvoid Risk of Residue Control ComplianceOrganic chemistryNicotinuric acidMethyl palmoxirate
The invention relates to an improved preparation method of nicergoline. The method comprises the following steps: (1) carrying out a photoreaction on ergosterol and methanol under the conditions of catalysis of a proper amount of concentrated sulfuric acid and ultraviolet irradiation to obtain 10alpha-methoxyergosterol; (2) adding an inorganic base into an amide aprotic solvent, and carrying out amethylation reaction on the 10alpha-methoxyergosterol and methyl iodide to generate 1-methyl-10alpha-methoxyergosterol; and (3) reacting 5-bromonicotinic acid with oxalyl chloride in a solvent with organic amine as an acid-binding agent to prepare a 5-bromonicotinyl chloride intermediate, and then carrying out a condensation reaction on the 5-bromonicotinyl chloride intermediate and 1-methyl-10alpha-methoxyergosterol to prepare the nicergoline. Compared with the prior art, the method of the invention has the advantages of no inert gas shielding in the reaction, small acid dosage, and easinessin recycling of reaction byproducts, makes the final yield of the product reach 50% or above and the purity of the product reach 99% or above, and is suitable for large-scale production.
Owner:SHANGHAI INST OF TECH +1
Application of bilobalide as synergist in preparation of drug for preventing cranial nerve injury diseases
InactiveCN106727501AActive ingredient clearQuality improvementNervous disorderHydroxy compound active ingredientsDiseaseSide effect
The present invention discloses application of bilobalide as a synergist in preparation of a drug or a heath product for preventing cranial nerve injury diseases. An inventor discovers that compounds of bilobalides, particularly bilobalide, bilobalide extracts, and hydroxyl derivatives of bilobalides, particularly glucoside derivatives, ester derivatives and ether derivatives can well promote drug molecules with a treating or health care function on cranial nerve injury diseases to enter brain tissues, wherein the drug molecules comprise ginsenoside, stibene glucoside, resveratrol, levodopa, edaravone, vinpocetine, nicergoline, citicoline, oxiracetam and the like; under the conditions of not increasing drug concentration in blood, the concentration of bilobalide can be increased to a large extent in the brain tissues, so that the efficacy of the drug can be improved, and side effects and adverse reactions of the drug can be reduced.
Owner:GUANGDONG PHARMA UNIV
Mini-pill type nicergoline capsule and preparation method thereof
InactiveCN104622850AImprove solubilityWidely distributedSenses disorderNervous disorderSide effectBiomedical engineering
The invention discloses a mini-pill type nicergoline capsule and a preparation method thereof. The mini-pill type nicergoline capsule is a sustained-release preparation and is prepared from a capsule shell and a nicergoline mini-pill accommodated in the capsule shell, wherein the nicergoline mini-pill sequentially comprises an empty pill core, a main drug layer and a pigmented layer from inside to outside; the main drug layer accounts for 15-60 percent of the empty pill core, and the pigmented layer accounts for 0.5-1 percent of the total weight of the empty pill core and the main drug layer. The mini-pill type nicergoline capsule has the advantages of small stimulation to intestines and stomach, high utilization rate, high stability, less toxic or side effects and capability of fully releasing effective components in time.
Owner:ZHEJIANG CHANGDIAN PHARMA +1
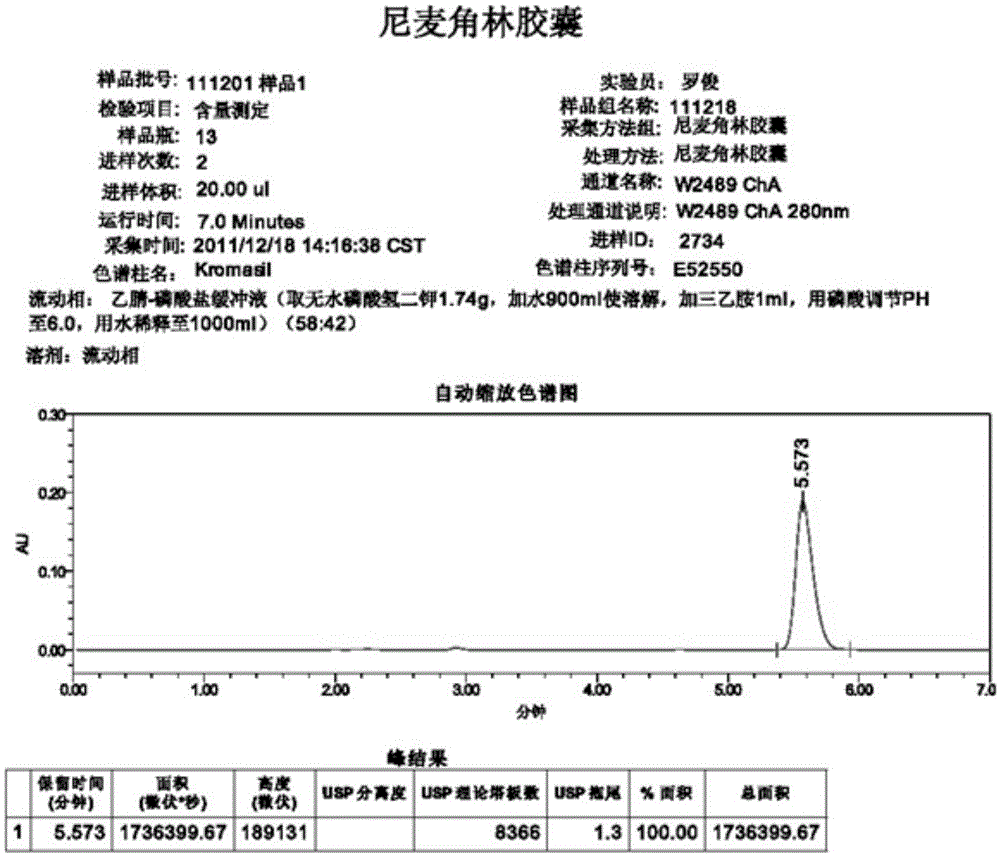
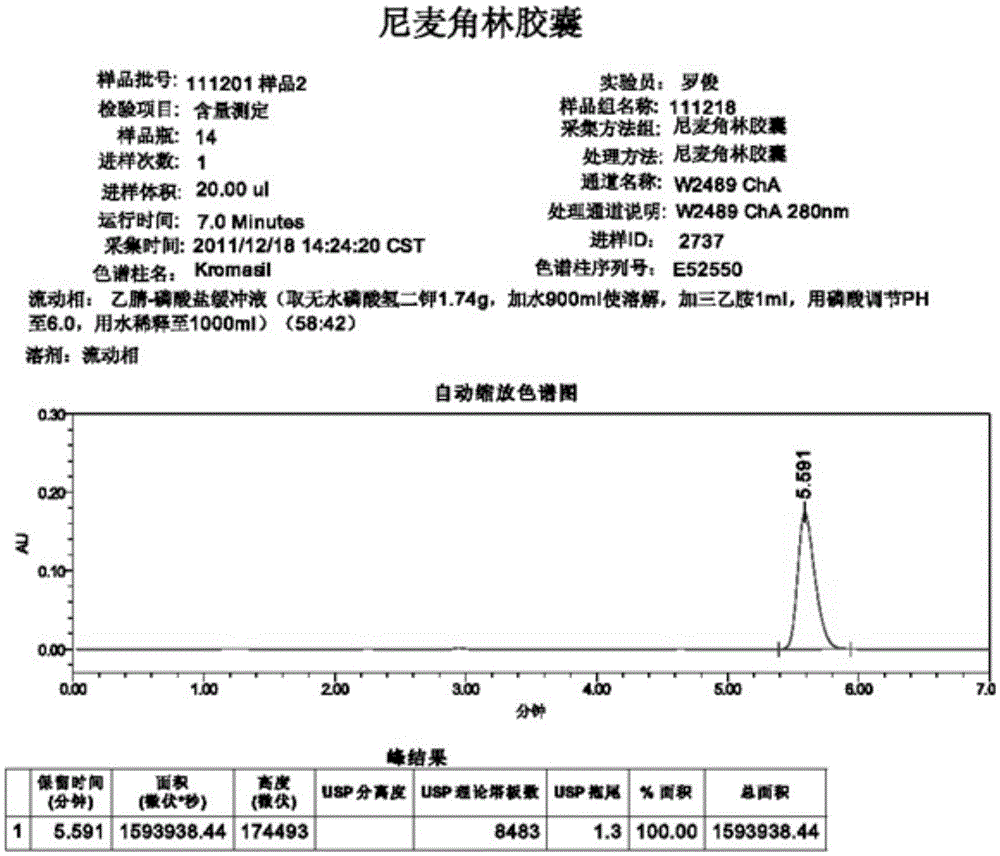
High performance liquid chromatography method for determining nicergoline related substances
ActiveCN108593818AReliable Quality Control MethodsStable quality control methodComponent separationIsocratic elutionColumn temperature
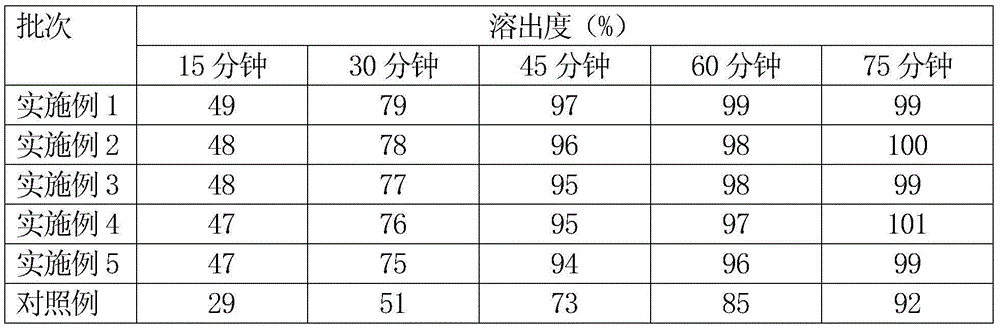
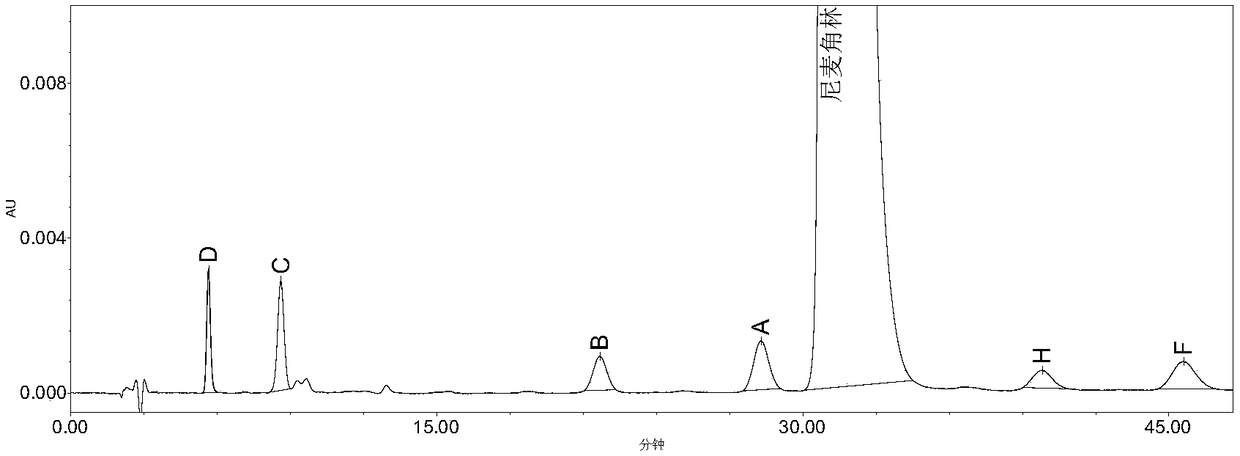
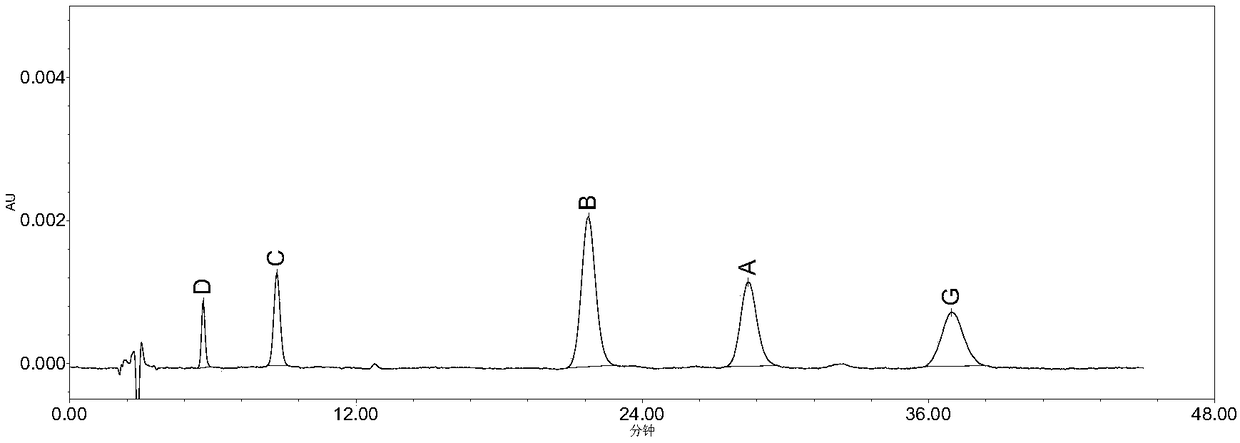
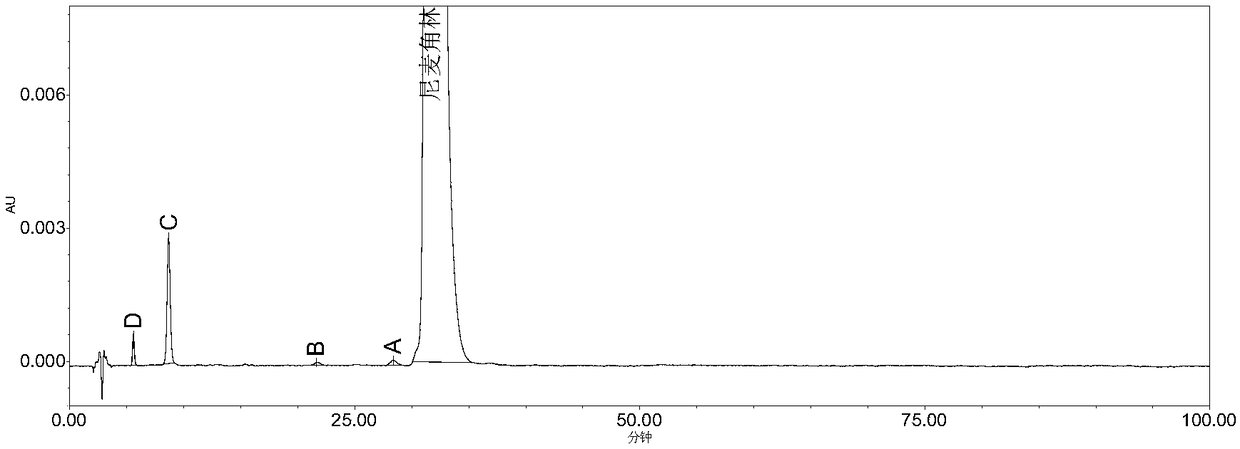
The invention belongs to the field of medicine analysis, and particularly relates to a high performance liquid chromatography method for determining nicergoline related substances. According to the high performance liquid chromatography method, a filler obtained by mixing strong cation exchange resin and a reversed phase C18 according to a volume ratio of 1 to 4 is used as a filling agent, acetonitrile is used as a mobile phase A, a phosphate buffer is used as a mobile phase B, the mobile phase A and the mobile phase B are mixed according to a ratio of 30 to 70 for isocratic elution, the detection wavelength is 288nm, and the column temperature is 30 DEG C. By the high performance liquid chromatography method, the content of an impurity D can be accurately determined and known impurities C, B, A, G, F and H and other unknown impurities in nicergoline can be simultaneously determined.
Owner:CHONGQING INST FOR FOOD & DRUG CONTROL
Novel preparation method of nicergoline
PendingCN111004233AAvoid it happening againFor follow-up productionOrganic chemistryLysergolOrganosolv
The invention relates to a new preparation method of nicergoline. The method comprises the following steps: (1) adding an inorganic alkali into a polar aprotic solvent, and carrying out a methylationreaction on lysergol (II) and a methylation reagent to generate 1-methyl lysergol (III); (2) carrying out a light reaction on the 1-methyl lysergol (III) and methanol under the catalysis of concentrated sulfuric acid to prepare 1-methyl-10 alpha-methoxy dihydrolysergol (IV); and (3) in a polar aprotic organic solvent, carrying out a reaction on 5-bromonicotinic acid (V) and N,N'-carbonyl diimidazole by taking the N,N'-carbonyl diimidazole as a condensing agent to prepare a 5-bromo nicotinyl imidazole (VI) intermediate, and then carrying out a condensation reaction on the 5-bromo nicotinyl imidazole (VI) intermediate and the 1-methyl-10 alpha-methoxy dihydrolysergol (IV) to prepare nicergoline (I). Compared with the method in the prior art, the method of the invention does not require inertgas protection, is small in acid consumption, good in product quality, high in yield, easy in reaction byproduct recovery and utilization and suitable for industrial production.
Owner:SHANGHAI INST OF TECH +1
Nicergoline oral cavity disintegration tablet and its preparation method
An oral disintegrating tablet of nicergoline contains nicergoline and auxiliaries including sorbitol, methylacryl copolymer resin (Eudragit L30D), citric acid, sodium dicarbonate, magnesium stearate, SiO2 and lemon perfume.
Owner:HAINAN PULIN PHARMA +2
Rapidly disintegrated nicergoline tablet and preparation method thereof
ActiveCN110051639ADisintegrates quicklyImprove stabilityNervous disorderPharmaceutical non-active ingredientsCalcium biphosphatePrill
The invention relates to a preparation method of a rapidly disintegrated nicergoline tablet. The method comprises a necessary key step: that is, microcrystalline cellulose-sodium carboxymethylcellulose granules are first prepared by a wet granulation method and then mixed with other auxiliary materials, so that the prepared nicergoline tablet can be rapidly disintegrated by the granules. The tableting process provided by the invention is a powder direct compression process: the prepared microcrystalline cellulose-sodium carboxymethylcellulose granules, nicergoline, dibasic calcium phosphate and magnesium stearate are directly mixed; and then tabletting, coating and aluminum-plastic packaging are executed to obtain a finished product. Each inspection index of the nicergoline tablet preparedby the method is qualified, the disintegration time is less than or equal to 2 minutes, and the nicergoline tablet can rapidly and completely disintegrate in an inspected dissolution medium to realize rapid dissolution. A disintegrating agent easily absorbing moisture is not used in the prescription, and the prepared nicergoline tablet has excellent stability. The preparation method of the nicergoline tablets p is novel in method and simple in process, and well solves the problems of no disintegration and poor stability of the existing marketed nicergoline tablets.
Owner:湖北科莱维生物药业有限公司
Nicergoline sublingual tablet and preparation method thereof
InactiveCN101711749AImprove stabilityEasy to shapeNervous disorderPharmaceutical product form changeFiller ExcipientBioavailability
The invention discloses nicergoline sublingual tablet and preparation method thereof. The nicergoline sublingual tablet is a sublingual mucosal administration non-quick-acting preparation prepared by comprising nicergoline raw material medicine, a filling agent, a disintegrating agent and a lubricating agent. In the sublingual tablet, the weight parts of the nicergoline is 1-20, the weight parts of the filling agent is 20-150, and the weight parts of the lubricating agent is 0.3-3.5. The nicergoline sublingual tablet has convenient use, small adverse reaction, good compliance, stable quality, slower release, sufficient absorption on the sublingual mucosa and high bioavailability.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Nicergoline for injection
InactiveCN108743528AAvoid hydrolysisReasonable collocationNervous disorderPharmaceutical delivery mechanismFreeze-dryingMannitol
The invention provides nicergoline for injection. The nicergoline for injection comprises the following components in parts by weight: 2-5 parts of nicergoline, 35-70 parts of mannitol, 5-30 parts ofTrehalose, and a proper amount of citric acid. A pH value of an aqueous solution formed in 1000 parts of water with nicergoline is 3.2-3.8. The invention also provides a preparation method of nicergoline for injection. The method comprises the following steps: 1) weighing 2-5 parts of nicergoline, 35-70 parts of mannitol, and 5-30 parts of Trehalose by weight; 2) mixing the materials and placing amixture in 1000 parts of water, uniformly stirring the materials, performing assistant dissolving by using citric acid to adjust the pH value to 3.2-3.8, adding medicinal active carbon with 0.03 w% of a solution amount, and uniformly stirring the mateirials; 3) performing filtering by a ultrafilter membrane with a thickness being 0.45 [mu]m and removing active carbon; S4) performing filtering bythe ultrafilter membrane with the thickness being 0.22 [mu]m; and 6) performing loading and freeze-drying. The nicergoline has excellent dissolving performance and good stability, and the effective component content with long-time preservation cannot be reduced.
Owner:HAINAN GENERAL & KANGLI PHARMA
Synthetic method of nicergoline
ActiveCN111116579AReact SafeHigh yieldOrganic chemistryBiochemical engineeringEsterification reaction
The invention discloses a synthetic method of nicergoline. The synthetic method comprises the following steps: (1) photocatalytic addition reaction; (2) purification of 10-methoxy-dihydroergosterol; (3) methylation reaction; (4) purification of 10-methoxy-1, 6-dimethane-8-carbinol-ergoline; (5) esterification reaction; and (6) purifying of the nicergoline. According to the Synthetic method of thenicergoline, the mild photocatalytic addition, methylation and esterification reactions in the reaction process are adopted, and the raw materials and reagents with high safety are selected, so that the whole synthesis process is safe and controllable, the post-processing process is simplified, and the product obtained by the reaction is high in yield, high in purity, good in quality and suitablefor industrial production.
Owner:JIANGSU HI STONE PHARMA
Injection used nicergoline freeze-dried powder injection and preparation method thereof
InactiveCN107669643AImprove stabilityImprove solubilityPowder deliveryNervous disorderPorosityMANNITOL/SORBITOL
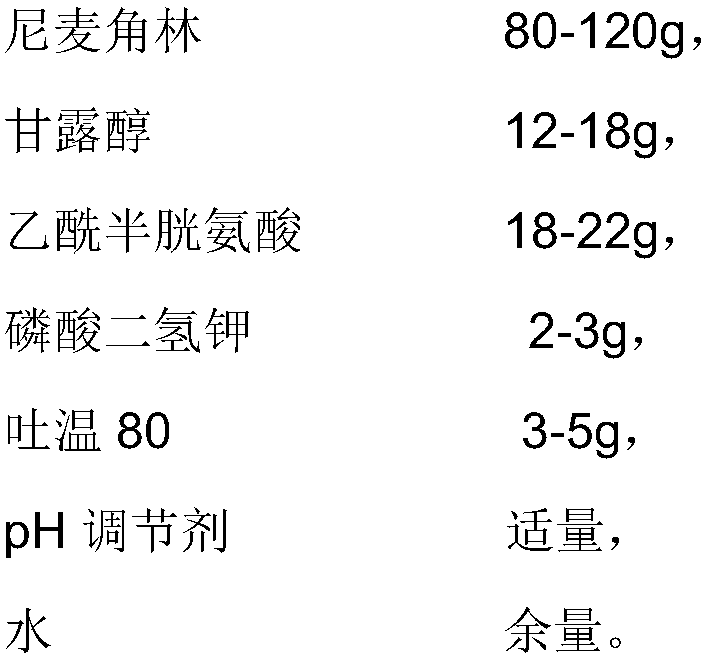
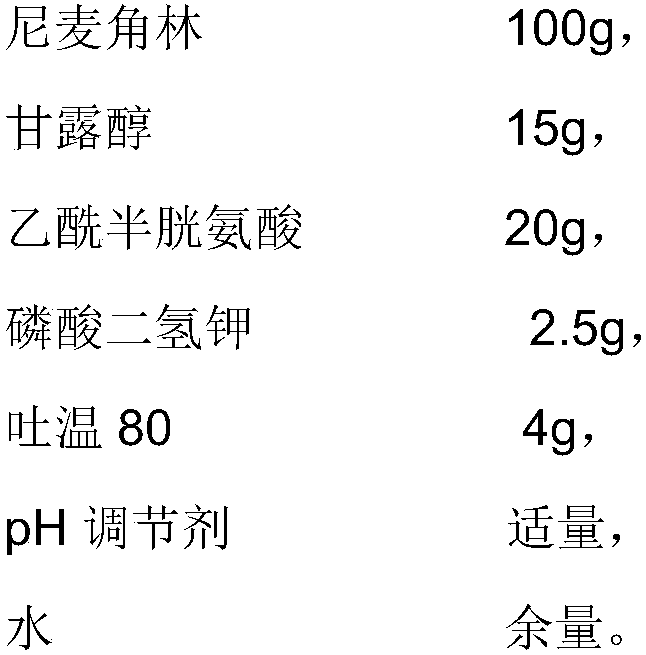
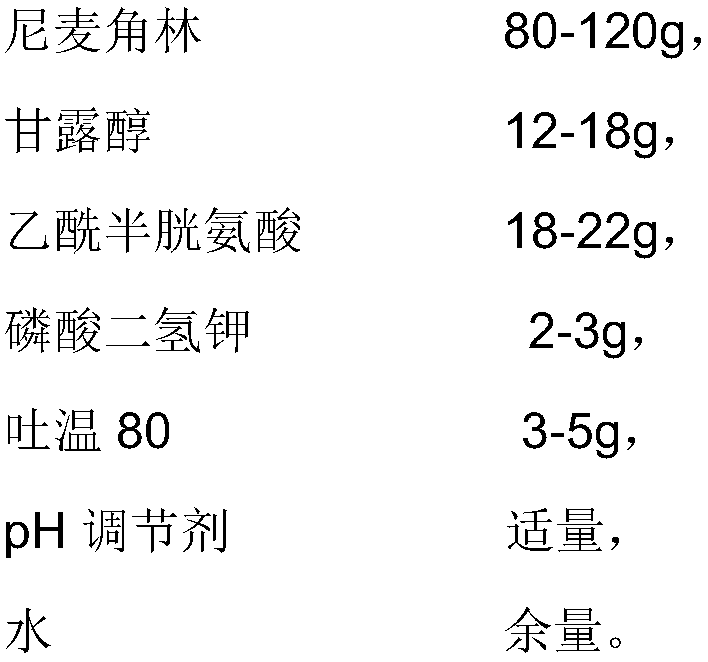
The invention provides an injection used nicergoline freeze-dried powder injection which is prepared by freeze-drying a nicergoline medicine solution with the pH value being 3.2-3.5. Every 2000 ml ofthe nicergoline medicine solution contains 80-120 g of nicergoline, 12-18 g of mannitol, 18-22 g of acetylcysteine, 2-3 g of monopotassium phosphate, 3-5 g of Tween 80, a proper amount of a pH regulating agent, and the balance of water. In the formula of the nicergoline freeze-dried powder injection, the proper amounts of ingredients, such as mannitol, acetylcysteine, Tween 80 and monopotassium phosphate, are added, all components play a synergistic effect, the appearance shape and porosity of the freeze-dried product can be improved, and the surface of the product is smoother; meanwhile, medicine properties are more stable, and storage and transportation of the medicine are facilitated.
Owner:HAINAN GENERAL & KANGLI PHARMA
Nicergoline capsule and preparation method thereof
ActiveCN105640919AReduce typesImprove liquidityNervous disorderPharmaceutical non-active ingredientsSide effectCurative effect
The invention relates to the technical field of medicine, in particular to a nicergoline capsule and a preparation method thereof. The preparation method is characterized in that first, nicergoline and starch are mixed to be uniform, then a binder is added to be prepared into a mother pill, and finally a pill core is prepared; in the whole preparation process, the adopted kinds of auxiliary materials are fewer (2 kinds of auxiliary materials), the preparation process is simple, operation is easy, the pill core is good in fluidity, filling is easy, meanwhile, the main drug dissolution rate is high, the curative effect in clinical application is good, the side effects are fewer, and the compliance of a patient taking the medicine is good; according to similar kinds of medicine sold on the market, a large number of kinds of auxiliary materials are adopted, the preparation process is complicated, operation is hard, the curative effect in clinical application is poor, a large number of side effects are produced, and the compliance of the patient taking the medicine is poor. It shows that according to the novel nicergoline capsule and the preparation method thereof, the quality performance of the product is good, and medication safety of the patient is ensured.
Owner:CHINA MEHECO SANYANG PHARMA CO LTD
Nicergoline sustained-release dropping pill and preparation method thereof
InactiveCN101269035AIncrease surface areaImprove solubilityNervous disorderPharmaceutical delivery mechanismAdditive ingredientHemangiectasis
The invention relates to a drug compound playing the role of retarding Alpha-receptors and expanding blood vessels, and is used for improving low desires and affective disorders due to cerebral arteriosclerosis and stroke sequelae and treating vascular dementia. The drug compound aims to supplement the deficiency of the prior oral pharmaceutical formulation used for hemangiectasis and provide a drug compound oral formulation, sustained-release nicergoline dropping pill which has high bioavailability, steady plasma concentration, low frequency of drug taking, low cost and absence of contamination during the production. The sustained-release nicergoline dropping pill adopts nicergoline as the ingredient and is prepared jointly with the medicinal carrier used as the stroma.
Owner:北京博智绿洲医药科技有限公司
Serum-free culture medium and culture method of CAR-T cells
PendingCN113817685AIncrease proliferative activityGood tumor killing activityCulture processBlood/immune system cellsT cellBlood plasma
The invention discloses a serum-free culture medium of CAR-T cells. The serum-free culture medium consists of a basic culture medium and the following components added into the culture medium: nicergoline, icariin, ferulate, IL-2, IL-15, vitamin E and L-glutamine. The nicergoline and the icariin are added into the serum-free culture medium of the CAR-T cells in the invention, so that the proliferation activity of the CAR-T cells can be effectively improved; and a large number of CAR-T cells can be harvested within a short time. The ferulate is also added, so that the number of living cells in the harvested cells is easily increased; and the cell survival rate is increased. The components, the L-2, the IL-15, the vitamin E and the L-glutamine jointly act; the proliferation activity of the CAR-T cells is improved; the used components are safe; blood components such as plasma are not added; the culture cost of the CAR-T cells is reduced; and the burden of a patient is relieved. The invention further provides a culture method of the CAR-T cells; the culture method is simple and convenient to operate and easy to implement; and popularization and application of the CAR-T cells in the field of tumour immunotherapy are facilitated.
Owner:曾献芳
Nicergoline nasal powder and preparation method thereof
InactiveCN101716164AImprove stabilityImprove securityNervous disorderCapsule deliveryNasal cavityBioavailability
The invention discloses nicergoline nasal powder and a preparation method thereof. In the nicergoline nasal powder, nicergoline dry powder and carrier dry powder are in a form of capsules or vesicles, the atomized nicergoline is sprayed to the nasal cavity by using a Helioeast dry powder administration device, and a preparation which is in brain targeting administration and treats vascular cretinism is absorbed by the mucous membrane of the nasal cavity. The nicergoline nasal powder contains 1-16 parts by weight of nicergoline dry powder with the particle diameter thereof being 80-250mum and 15-45 parts by weight of carrier dry powder with the particle diameter thereof being 80-250mum.The nicergoline nasal powder features convenient use, good conformability, quick acting of the brain targeting function and high bioavailability.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Preparation method of nicergoline tablet
InactiveCN111904938AShorten the dissolution timeProlong the action timeNervous disorderMacromolecular non-active ingredientsPhospholipidTraditional medicine
The invention relates to the technical field of medicine, in particular to a preparation method of a nicergoline tablet. The nicergoline tablet is sequentially provided with a pill, a second main medicine layer, a coating inner layer, a first main medicine layer and a coating outer layer from inside to outside; and the thickness ratio of the second main medicine layer to the coating inner layer tothe first main medicine layer to the coating outer layer is (1-2):(4-5):(1-2):(1-2). a tablet inner layer is arranged, so that the medicine can be disintegrated hierarchically, the action time of themedicine is prolonged, and absorption is facilitated; and the nicergoline is uniformly dispersed in phospholipid, so that the affinity between the nicergoline and grease is increased, the dissolutionrate of the nicergoline is further increased, and the utilization rate is increased. By arranging a hollow tablet shell, the dissolution time of the tablet shell is shortened, the medicine is exposedas soon as possible to play a role, and a patient is treated with the medicine early; in addition, the tablet shell contains gelatin and corn starch, so that the tablet shell is softened and decomposed when meeting water and can be absorbed by the human body, and the dissolution time of the tablet shell is also shortened;.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
Nicergoline lipid microspheres agent
InactiveCN104958266AProlong degradation timeGood membrane permeabilityPowder deliveryNervous disorderIrritationCurative effect
A nicergoline lipid microspheres agent is mainly prepared by nicergoline, oil for injection, an emulsifying agent and an isotonic agent. The nicergoline lipid microspheres agent has a perfect curative effect, the problems that the nicergoline cannot be dissolved in water and unwelcome reactions can be produced when the dosage of the nicergoline is large are solved, the medicine stability is improved, irritation and toxic reaction are reduced, the quality of the nicergoline lipid microspheres agent is improved, and the nicergoline lipid microspheres agent is suitable to be industrially produced widely.
Owner:HAINAN LINGKANG PHARMA CO LTD
Application of nicergoline and gastrodin in synergistic prevention and treatment of Alzheimer's disease (AD)
InactiveCN111991396AEasy to change functionsImprove pathological changesNervous disorderHeterocyclic compound active ingredientsAmyloidCognitive impairment
The invention discloses application of nicergoline and gastrodin in synergistic prevention and treatment of Alzheimer's disease (AD), and relates to the field of clinical medical small molecule drugs.The nicergoline and the gastrodin are obtained by inhibiting beta-amyloid oligopeptide mediated [alpha]1-adrenergic receptor chronic activation-like cerebrovascular function change, AD-like pathological change and preclinical research of cognitive impairment for natural small molecular compounds; according to the application in preventing and treating AD, in the application of prevention and treatment of the AD, it is proved from the molecular, cellular and animal levels that the nicergoline and the gastrodin are independently used or jointly used for inhibiting beta-amyloid oligopeptide mediated [alpha]1-adrenergic receptor chronic activation-like cerebrovascular smooth muscle cell molecular signaling pathways and cerebrovascular tissue activity, and improving cerebrovascular function change and AD-like molecular pathological damage and behavioral disorder of AD model mice.
Owner:谭骏
Nicergoline dry emulsion and its preparation method and application
InactiveCN101057848AImprove stabilityGood curative effectNervous disorderEmulsion deliveryOil phaseAcute urine retention
The invention discloses a medicine preparation form, especially discloses a nicergoline dry emulsion and the preparing method and application. It takes nicergoline in the dry emulsion as effective component, and also comprises oil phase, emulsifying agent and other findings. The dry emulsion is characterized by mature preparation process, easy industrialization, good stability, easy transportation and storage, controllable medicine content and good effect. The invention is used for improving cerebral arteriosclerosis and affective disorder caused by cerebral apoplexy after-effect, acute and chronic circulatory disturbance and acute urine retention resulted from blood vessel dementia and prostate gland proliferation.
Owner:陈云生 +1
Nicergoline lyophilized preparation with excellent stability
The invention provides a nicergoline lyophilized preparation with excellent stability. The nicergoline lyophilized preparation is prepared by freeze-drying a nicergoline drug solution, and each 1000 gof the nicergoline drug solution comprises: 2-4 g of nicergoline, 0.5-1 g of tartaric acid, 0.2-9 g of sodium chloride, 20-40 g of an excipient, and an appropriate amount of water for injection, andthe pH value of the drug solution is 3.0-5.0. In the formula of the lyophilized preparation, mannitol is used to replacing lactose in an original development agent to be a lyophilized excipient, which can avoid the risks of allergies, pyrogens, and mad cow disease caused by lactose as an injection adjuvant, and overcomes the instability caused by the absence of lactose in the prior art. The mostnotable feature of the preparation is the combination of mannitol, sorbitol and the like with sodium chloride, and the nicergoline for injection still has excellent stability without using of nicergoline. The lyophilized product of the invention has rapid reconstitution and loose appearance, and is convenient for transportation and preservation and clinical use.
Owner:湖北科莱维生物药业有限公司
Traditional Chinese medicine preparation for treating senile dementia
InactiveCN109663086AImprove securityWide applicabilityNervous disorderGinkgophyta medical ingredientsLicorice rootsSenile dementia
The invention provides a traditional Chinese medicine preparation for treating senile dementia. The preparation is prepared from, by weight, 50-60 parts of bitter cardamon, 30-45 parts of acorus calamus, 9-15 parts of ginseng, 7-13 parts of ginkgo leaves, 8-14 parts of Tibetan hawksbeard roots, 10-16 parts of live charcoal brittle faslsepimpernel herb, 9-14 parts of vicia sativa, 7-15 parts of vitamin E, 9-16 parts of Beautiful Phyllodium Twig and Leaves, 15-20 parts of rhizoma atractylodis, 11-20 parts of nicergoline, 12-30 parts of licorice roots and 5-10 parts of eupatorium. The preparationis a pure traditional Chinese medicine preparation, and is high in safety, wide in application range and easily acceptable by a patient; the preparation is developed according to the traditional Chinese medicine principle, and has the effects of tonifying the kidney, reinforcing bones, soothing the nerves, restoring consciousness and the like; the preparation is good in curative effect, free of adverse reaction, low in cost and free of economic burdens after the patient takes the preparation for a long time.
Owner:姜为荣
Nicergoline oral cavity disintegration tablet and its preparation method
An oral disintegrating tablet of nicergoline contains nicergoline and auxiliaries including sorbitol, methylacryl copolymer resin (Eudragit L30D), citric acid, sodium dicarbonate, magnesium stearate, SiO2 and lemon perfume.
Owner:HAINAN PULIN PHARMA +2
Application of nicergoline and derivative thereof in preparation of medicine for treating tumors
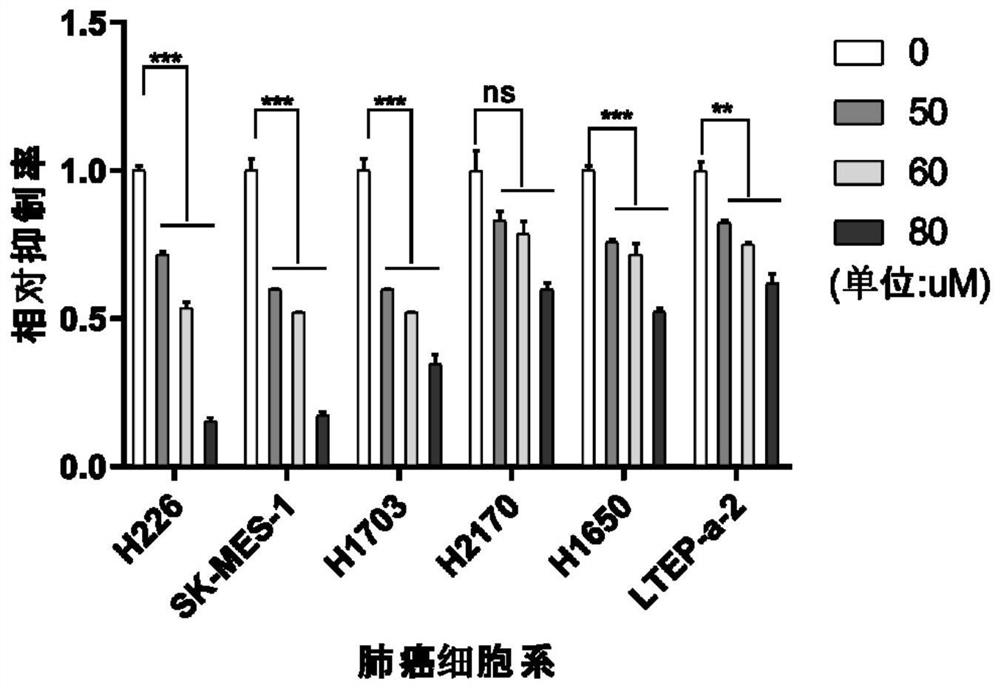
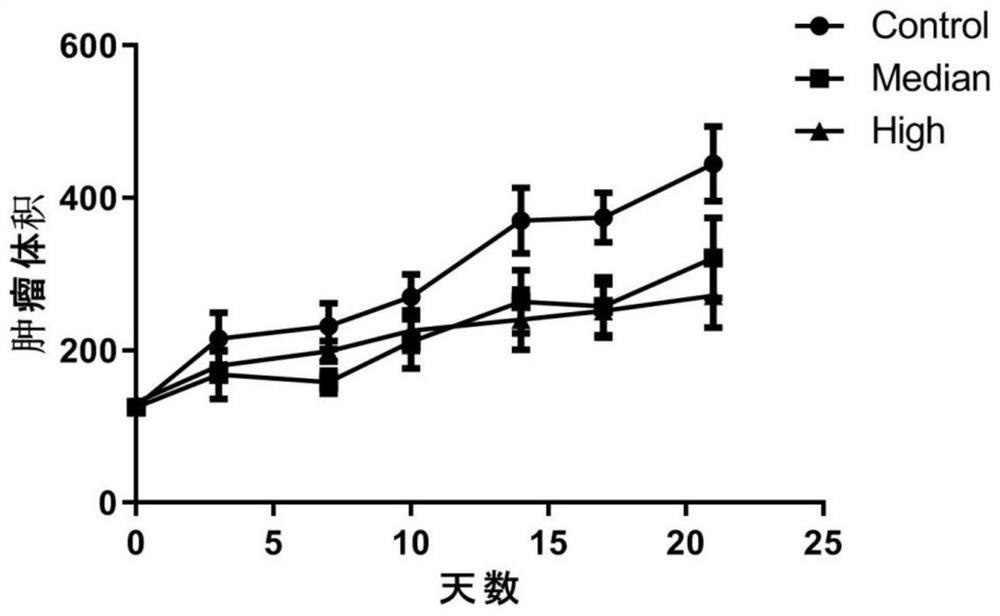
PendingCN114504576AAntineoplastic agentsHeterocyclic compound active ingredientsLung squamous cell carcinomaPharmaceutical drug
The invention relates to the technical field of preparation of medicines for treating tumors, in particular to application of nicergoline and derivatives thereof in preparation of medicines for treating tumors. The nicergoline is used as a medicine for treating tumors and can be used for effectively treating tumors, especially lung squamous cell carcinoma, so that the lifetime of tumor patients, especially lung squamous cell carcinoma patients, is prolonged.
Owner:SHANGHAI HI TECH BIOENG +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com