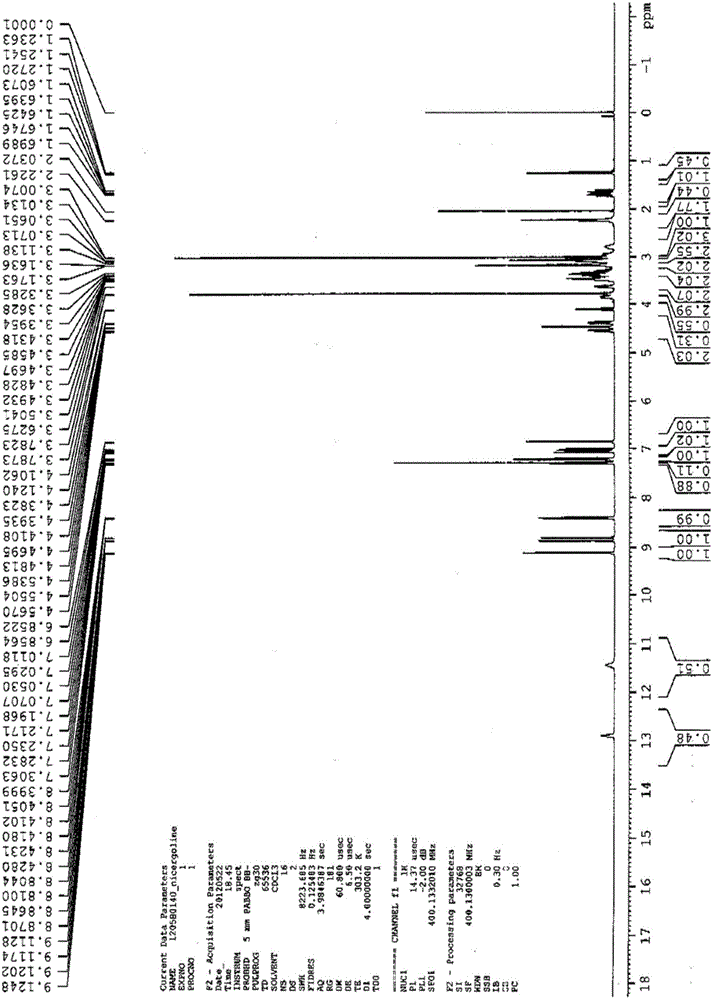
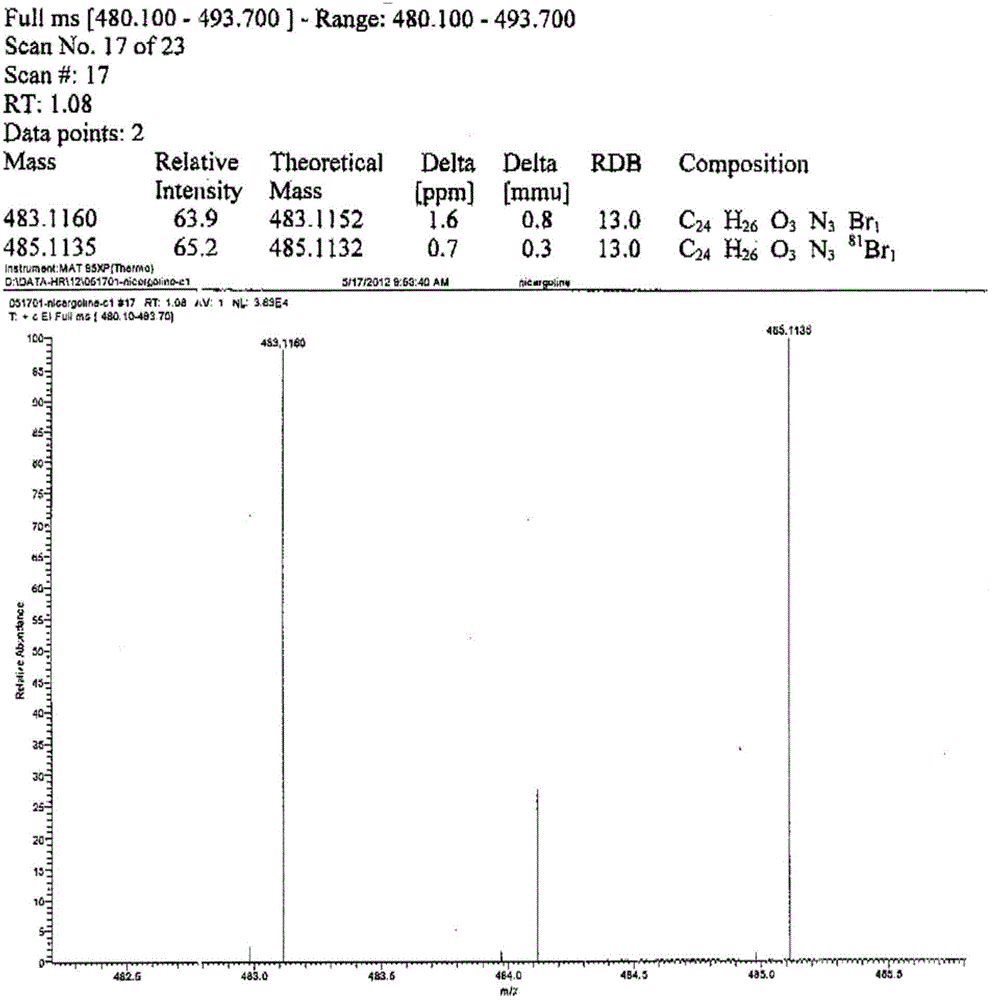
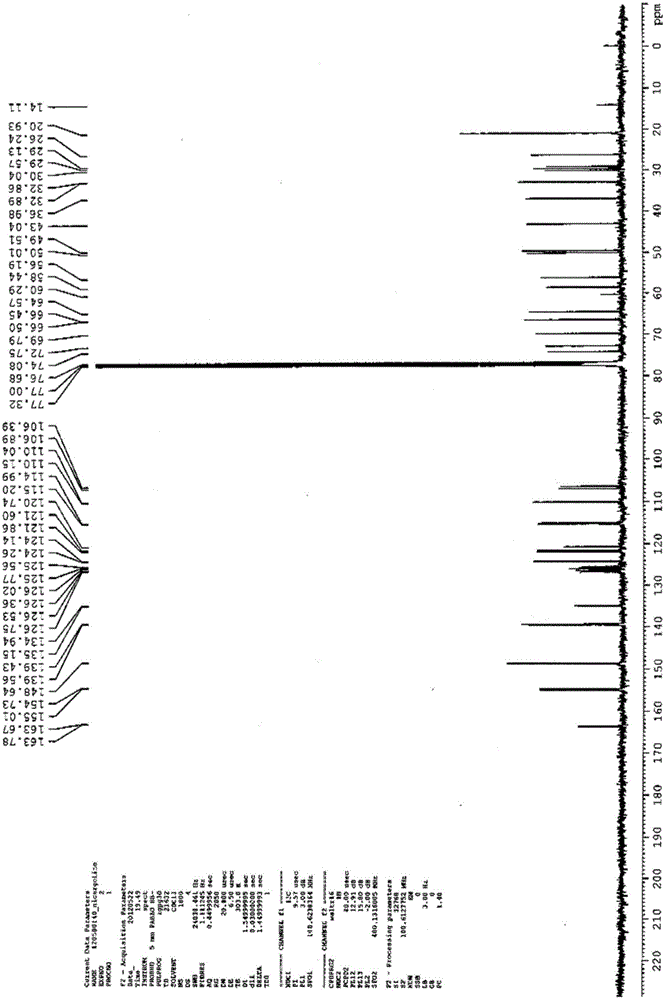
Preparation method for nicergoline
A kind of technology of nicergoline and ergot alcohol, which is applied in the field of preparation of nicergoline, can solve the problems of high toxicity and troublesome post-processing, and achieve the effects of low toxicity, simple equipment and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of 5-bromonicotinoyl chloride
[0035]
[0036] Dissolve 8.65kg of 5-bromonicotinic acid in 175kg of dichloromethane, add 0.5%~1% mass equivalent of N,N-dimethylformamide (DMF), add 7.5kg of thionyl chloride under stirring, and dissolve the mixture Stir and reflux for 5 hours. After the raw materials were completely reacted, methylene chloride was removed by distillation under reduced pressure, and 1.5 kg of anhydrous toluene was added to the resulting solid to remove residual thionyl chloride, and then the toluene was removed by distillation under reduced pressure to obtain 5-bromonicotinoyl chloride as a white powdery solid. The yield was 92.6%.
Embodiment 2
[0037] Example 2: Preparation of 10α-methoxy-photoergool (Compound IV)
[0038]Add 50kg of methanol and 5kg of concentrated sulfuric acid into the photocatalytic reactor to prepare methanol-concentrated sulfuric acid mixed solution, add 2.0kg of ergot alcohol (compound VI) under stirring, turn on the ultraviolet high-pressure mercury lamp with a wavelength range of 280nm~350nm and a power of 1500W , under the protection of nitrogen, the temperature was controlled below 25°C, and the reaction was continued with stirring for 48 hours. After the raw materials are completely reacted, pour the reaction solution into 1000kg of ice water, add concentrated ammonia water under stirring, and adjust the pH to 9~10 to neutralize the sulfuric acid in the reaction system. Extracted with dichloromethane (20kg×3 times), the extract was washed with 100kg of saturated brine, and then the solvent was distilled off under reduced pressure at 35°C to obtain 2.0kg of a crude red powder solid. The c...
Embodiment 3
[0039] Example 3: Preparation of 1-methyl-10α-methoxy-photoergool (Compound VII)
[0040] Add 4.8kg powdered potassium hydroxide to 40kg dimethyl sulfoxide, stir for 15 minutes, add 12.3kg 10α-methoxyl-photoergool (compound IV), continue stirring at room temperature for 30 minutes, and control the temperature at At 15°C to 20°C, slowly add 7.92 kg of iodomethane in dimethyl sulfoxide solution dropwise, and stir for 1 hour to react. After the raw materials were completely reacted, the reaction solution was poured into 400 kg of ice water and stirred for 10 minutes. Extract with dichloromethane (100kg x 3 times), wash the extract with 200kg of saturated brine, and distill off the solvent under reduced pressure at 35°C to obtain a crude red powder solid. The crude product was recrystallized with 530kg of acetone to obtain 8.2kg of light red crystal 1-methyl-10α-methoxyl-photoergool with a yield of 60%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com