Novel preparation method for nicergoline
A technology of nicergoline and a new method, applied in the field of preparation of nicergoline, can solve problems such as improvement, cumbersome operation steps, cumbersome operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
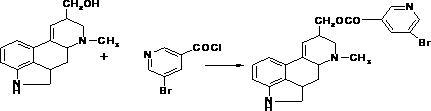
[0013] 1. Preparation of Ergool-5-bromo-3-pyridinecarboxylate (Intermediate 1)
[0014] Add 380 ml of dichloromethane, 50 grams of ergot alcohol and 25 grams of triethylamine into the reaction bottle, slowly add 60 grams of 5-bromonicotinoyl chloride after stirring, and control the temperature at about 30°C. The end point of the reaction was detected by thin-layer method, and the developing solvent was ethyl acetate:heptane=1:1. After the reaction was complete, water was added, the organic layer was separated, and the aqueous layer was extracted with dichloromethane. The organic layer was diluted with ammonia, washed with water, and anhydrous Na 2 SO 4 Dry and concentrate to dryness under reduced pressure to obtain crude Nicergoline. Acetone was added to the crude product, stirred and heated until completely dissolved, cooled to room temperature, crystallized by freezing, filtered, and dried under reduced pressure to obtain 102 g of the product. The yield is about 85%.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com