Synthetic method for nicergoline
A kind of synthetic method, the technology of nicergoline, are applied in the synthetic field of nicergoline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
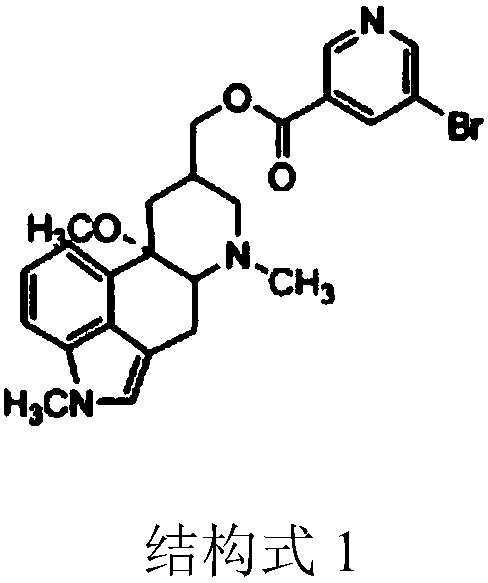
[0030] A kind of synthetic method of Nicergoline, comprising:
[0031] The preparation steps of intermediate product 1: methoxylation reaction occurs between ergot alcohol and methanol, and after purification, 10α-methoxyl-dihydroergot alcohol is obtained, namely intermediate product 1;
[0032] The preparation steps of intermediate product 2: 10α-methoxy-dihydroergool and trimethylsulfoxide iodide undergo methylation reaction, after purification, 1-methyl-10α-methoxy-dihydroergool is obtained , the intermediate product 2;
[0033] The preparation steps of Nicergoline: 1-methyl-10α-methoxy-dihydroergoline is subjected to acylation reaction with 5-bromonicotinoyl chloride, and after refining, Nicergoline is obtained.
[0034] As a preferred embodiment,
[0035] The preparation steps of intermediate product 1: under the protection of nitrogen, add ergot alcohol into the mixed solution of methanol and concentrated sulfuric acid, turn on the ultraviolet lamp to irradiate, contro...
Embodiment 1
[0048] A kind of synthetic method of Nicergoline, comprising:
[0049] The preparation steps of intermediate product 1: under the protection of nitrogen, add 20 g of ergot alcohol into the mixed solution of 500 ml of methanol and 100 ml of concentrated sulfuric acid, turn on the ultraviolet light, control the temperature at 20-40 ° C, react for 6 hours, and detect the reaction process by TLC (UV. 254, the volume ratio of methanol, chloroform, and ammonia water is 1:8:0.1), after the reaction is completed, add 400ml of chloroform, add dropwise ammonia water to adjust the pH value to 7, extract with chloroform, and then use chloroform Methane (400ml*2) was extracted twice, the chloroform layer was washed with purified water, the organic solvent was removed by distillation under reduced pressure, and the residue was recrystallized with 1000ml of acetonitrile to obtain 10α-methoxy-dihydroergool (intermediate product 1) 17.2 g, yield 82%.
[0050] The preparation steps of intermed...
Embodiment 2
[0053] A kind of synthetic method of Nicergoline, comprising:
[0054] Preparation steps of intermediate product 1: under nitrogen protection, add 100 g of ergot alcohol into a mixture of 2000 ml of methanol and 600 ml of concentrated sulfuric acid, turn on the ultraviolet light, control the temperature at 20-40 ° C, react for 6 hours, and detect the reaction progress by TLC (UV. 254, the volume ratio of methanol, chloroform, and ammonia water is 1:8:0.1), after the reaction is completed, add 2000ml of chloroform, drop ammonia to adjust the pH value to 7, extract with chloroform, and then use chloroform Methane (2000ml*2) was extracted twice, the chloroform layer was washed with purified water, the organic solvent was removed by distillation under reduced pressure, and the residue was recrystallized with 5000ml of acetonitrile to obtain 10α-methoxy-dihydroergool (intermediate product 1) 85.3 g, yield 85.3%.
[0055] The preparation steps of intermediate product 2: add powdery...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com