Application of bilobalide as synergist in preparation of drug for preventing cranial nerve injury diseases
A technology of bilobalide and synergist, which is applied in the directions of nervous system diseases, drug combinations, medical preparations containing active ingredients, etc., can solve problems such as no data display, etc. stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Ginkgo lactone and its extracts on the blood-brain barrier in vitro (in vitro BBB model) open research test:
[0044] 1. Experimental scheme:
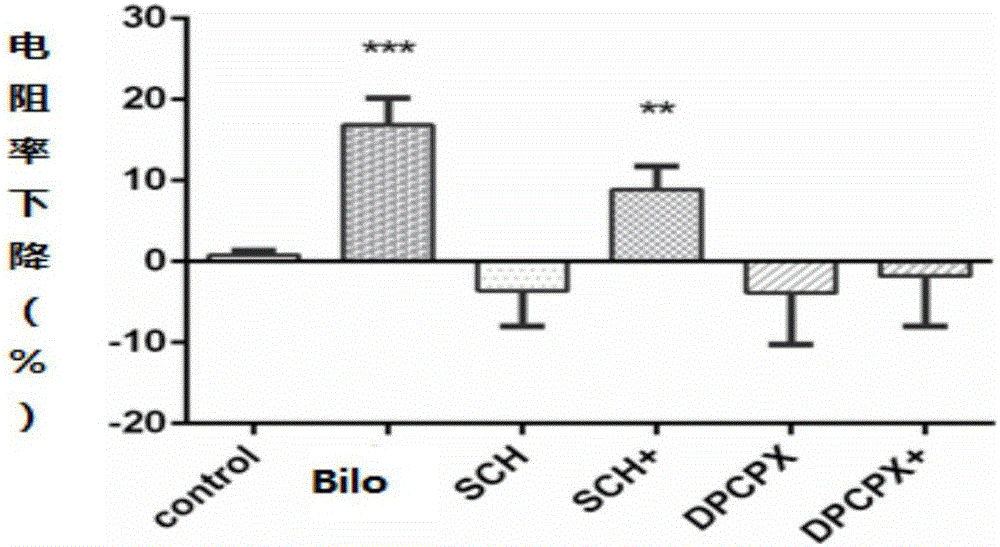
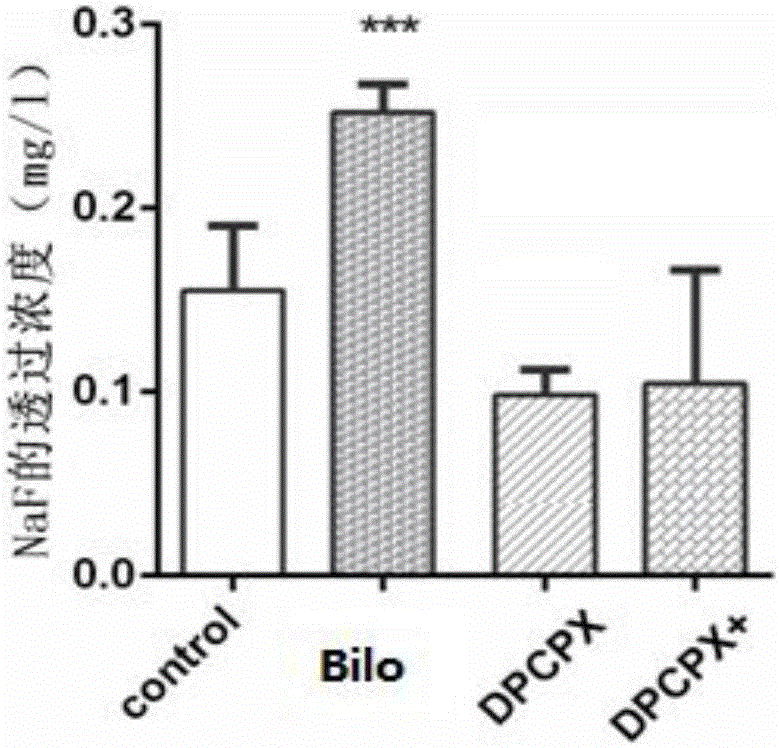
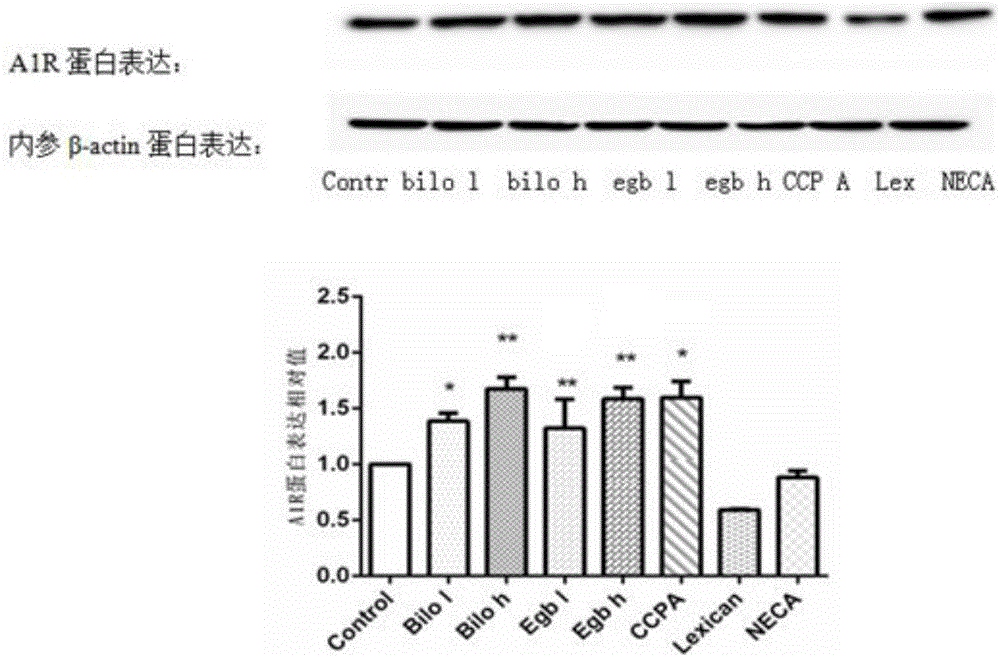
[0045] 1.1 Establishment of an in vitro blood-brain barrier model (BBB): culture and resuscitate human glial cells (HEB cells) and immortalized brain microvascular endothelial cells (hCMEC / D3 cells), after the cells are confluent, digest and centrifuge HEB cells for 3×10 5 Cells / ml were inoculated in the lower chamber of the transwell 12-well plate. After the cells adhered to the wall, low-glucose medium was added for culture. When the cells grew to 80%, the cells were placed in the upper layer of the transwell chamber with 7 × 10 cells. 5 hCMEC / D3 cells were seeded at a density of cells / ml. After the cells were confluent, the transendothelial cell electrical resistance (TEER) of the co-culture model was measured by Millicell ERS, which was the sum of endothelial cells (EC) and filter layer TEER. The culture pool was used as a...
Embodiment 2
[0054] Ginkgo lactone extract (ginkgo lactone content ≥ 99.0%) 5, 15g;
[0055] Active ingredients of traditional Chinese medicine: ginsenoside extract (ginsenoside Rg1 content ≥ 95%) 300g;
[0056] Weigh the above-mentioned various extracts in the recipe quantity, and mix them uniformly according to the equal increment method, and the obtained mixture is recorded as YXN1-1 (containing 5g ginkgo lactone extract and 300g ginsenoside extract) and YXN1-2 (containing 15g ginkgo internal ester extract and 300g ginsenoside extract).
[0057] Efficacy test:
[0058] Treatment group: YXN1-1 and YXN1-2 were made into a suspension, and the YXN1-1 treatment group was given a dose of 101.6 mg / kg (equivalent to 100 mg / kg of ginsenoside extract), 20 ml / kg volume, and 5 SD rats were given orally. Rats; YXN1-2 treatment group was given 5 SD rats by gavage at a dose of 105 mg / kg (equivalent to 100 mg / kg of ginsenoside extract) and a volume of 20 ml / kg;
[0059] Control group: another ginsen...
Embodiment 3
[0067] Bilobalide extract (bilobalide content ≥ 20.0%) 20, 40g;
[0068] Active ingredients of traditional Chinese medicine: ginsenoside extract (ginsenoside Rg1 content ≥ 30%) 200g;
[0069] Take the above-mentioned various extracts of the prescription amount, mix them uniformly by the method of equal increase, and the obtained mixture is recorded as YXN2-1 (containing 20g bilobalide extract and 200g ginsenoside extract) and YXN2-2 (containing 40g bilobalide extract). ester extract and 200g ginsenoside extract).
[0070] Drug efficacy experiment:
[0071] Treatment group: YXN2-1 and YXN2-2 were made into suspension, and YXN2-1 treatment group was dosed at 220mg / kg (equivalent to 200mg / kg of ginsenoside extract) and 20ml / kg capacity, and 5 SD large Rats; YXN2-2 treatment group was given a dose of 240mg / kg (equivalent to 200mg / kg of ginsenoside extract), 20ml / kg capacity, and 5 SD rats were administered orally;
[0072] Control group: another ginsenoside extract was taken to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com