Patents
Literature
595 results about "Transdermal patch" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
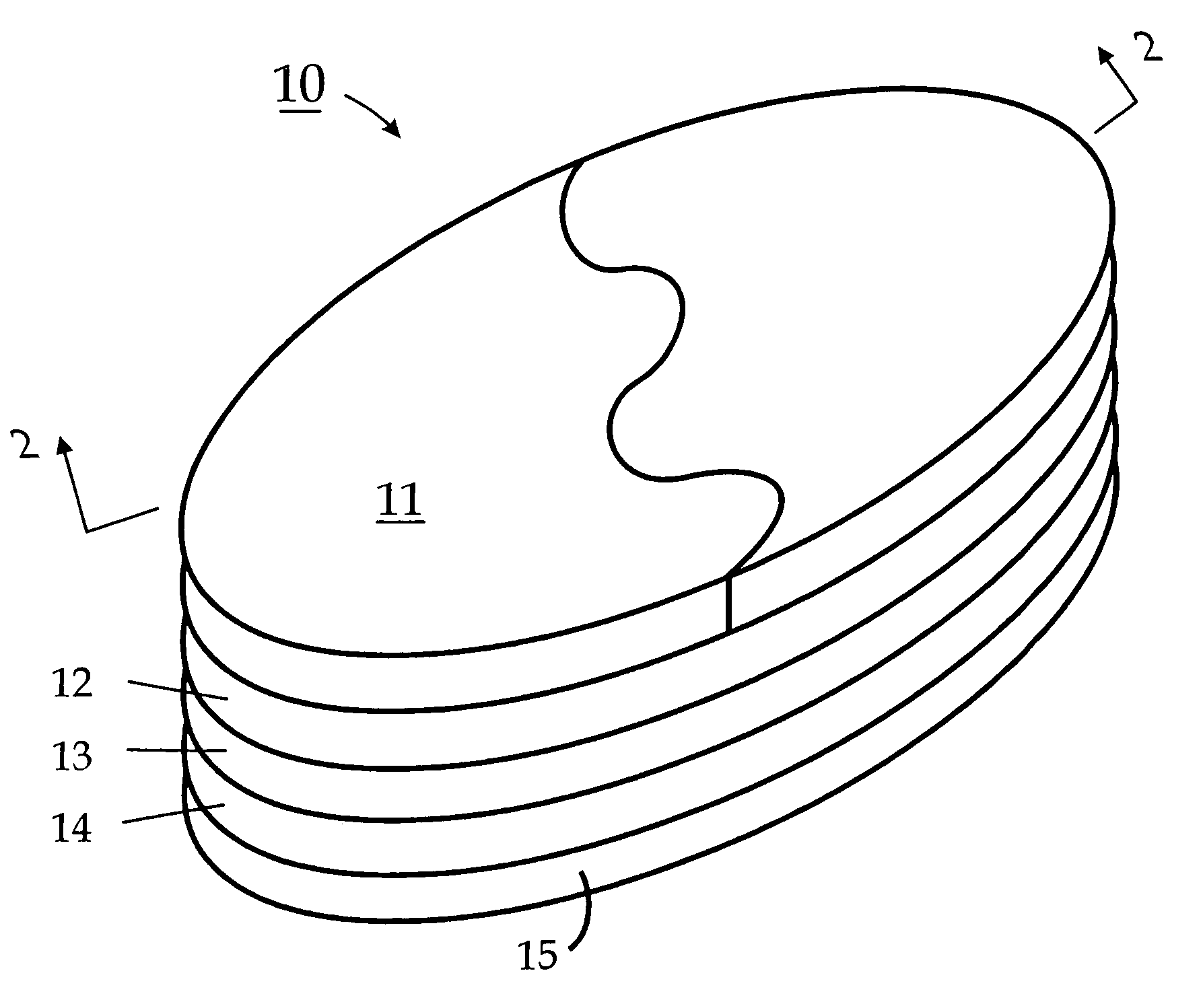
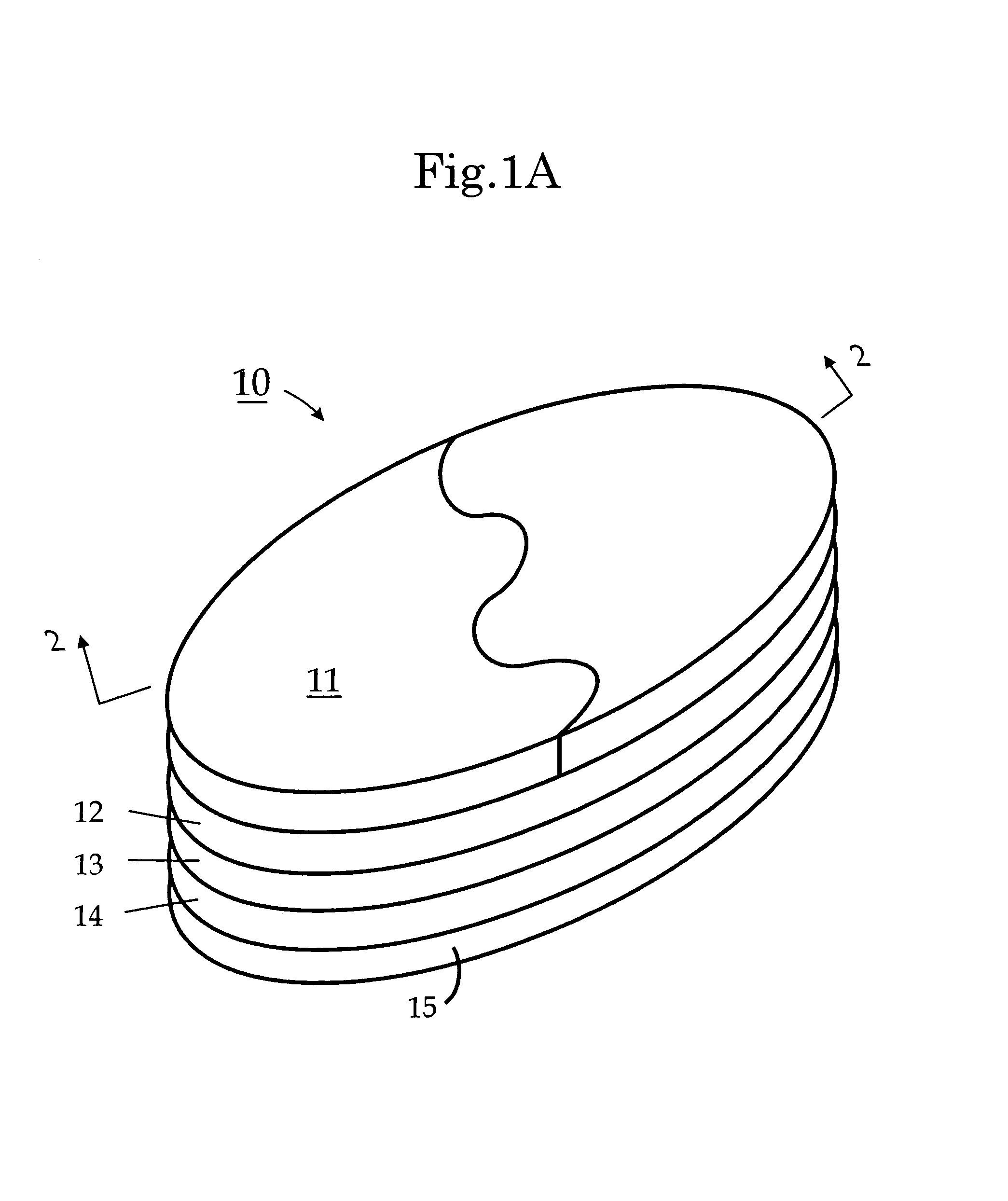
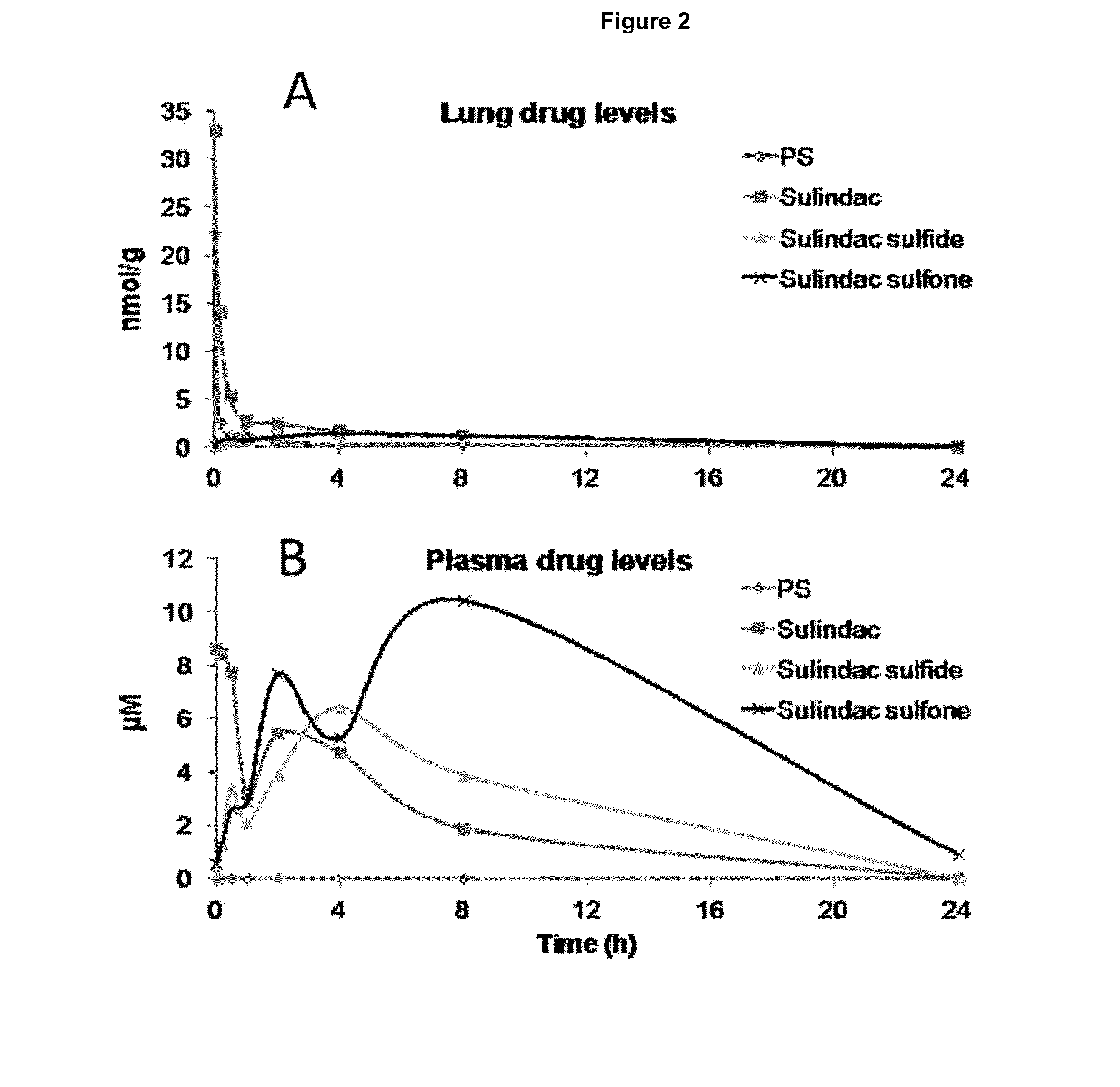
A transdermal patch is a medicated adhesive patch that is placed on the skin to deliver a specific dose of medication through the skin and into the bloodstream. Often, this promotes healing to an injured area of the body. An advantage of a transdermal drug delivery route over other types of medication delivery such as oral, topical, intravenous, intramuscular, etc. is that the patch provides a controlled release of the medication into the patient, usually through either a porous membrane covering a reservoir of medication or through body heat melting thin layers of medication embedded in the adhesive. The main disadvantage to transdermal delivery systems stems from the fact that the skin is a very effective barrier; as a result, only medications whose molecules are small enough to penetrate the skin can be delivered by this method. A wide variety of pharmaceuticals are now available in transdermal patch form.
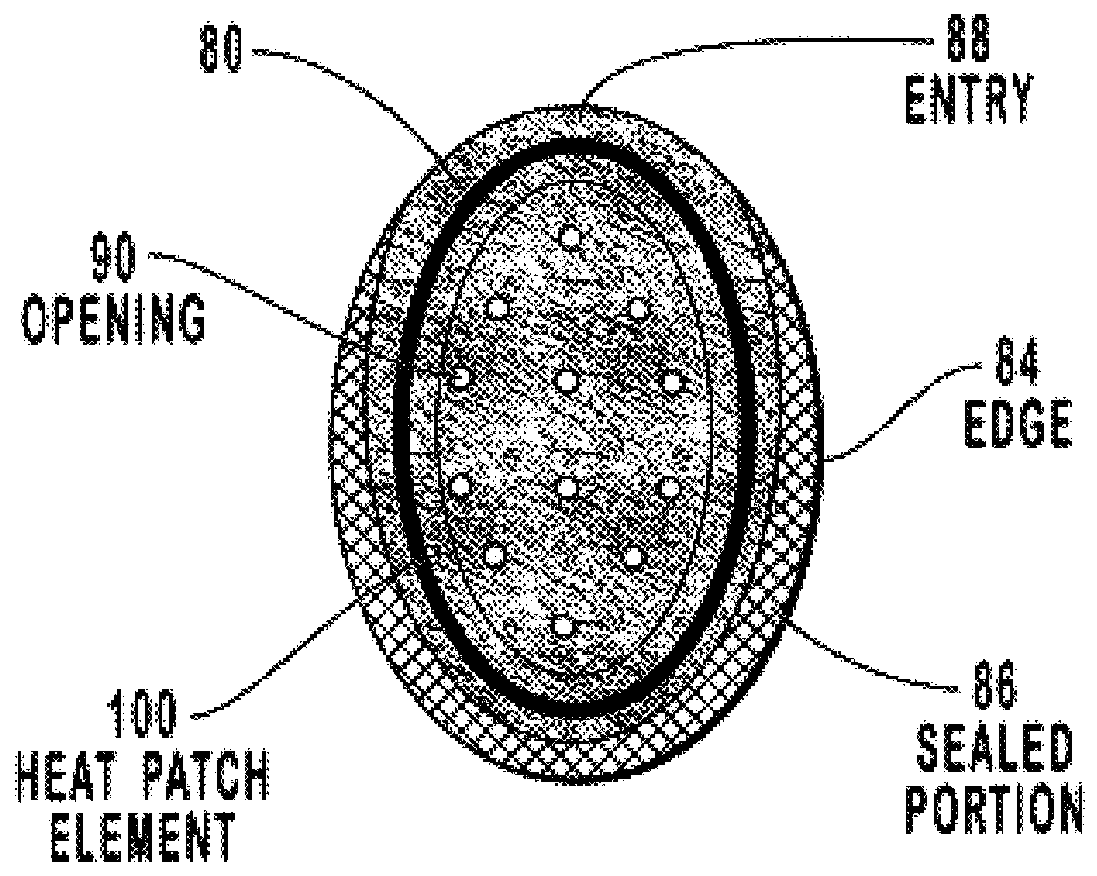
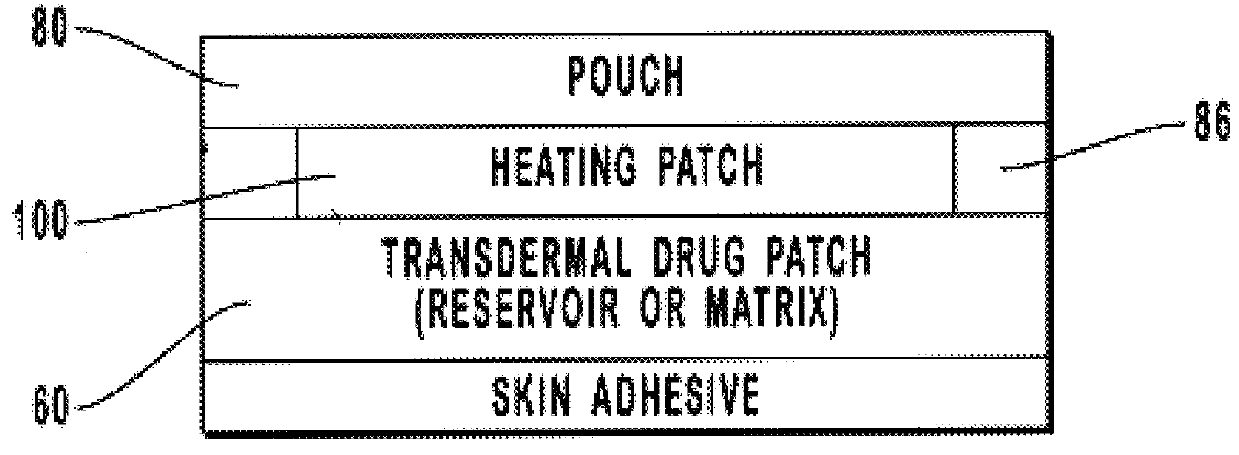
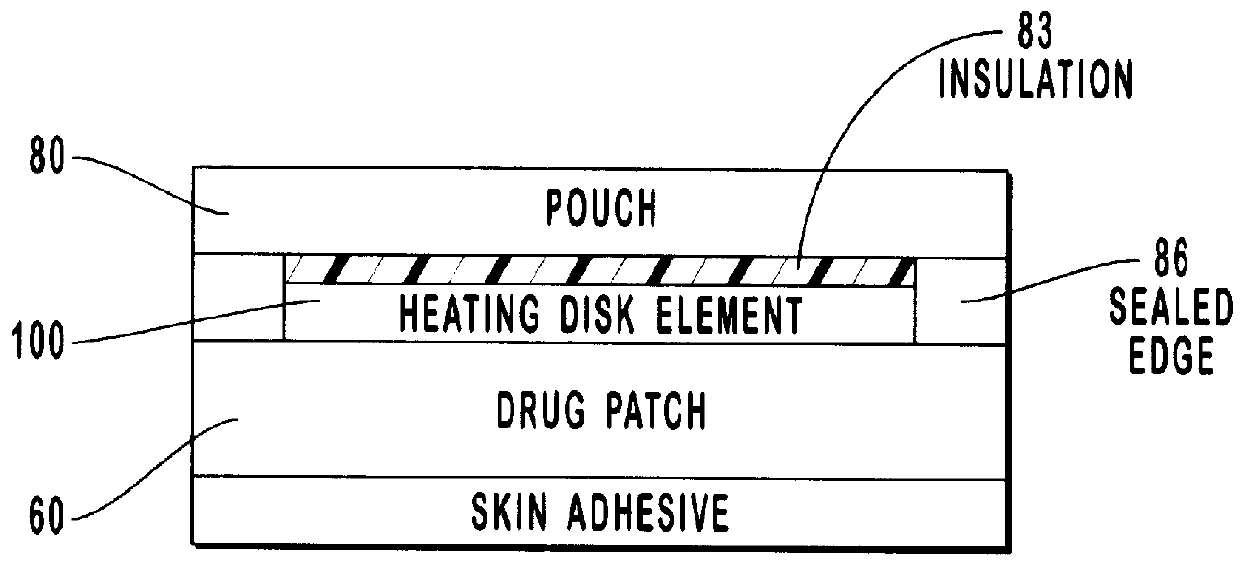
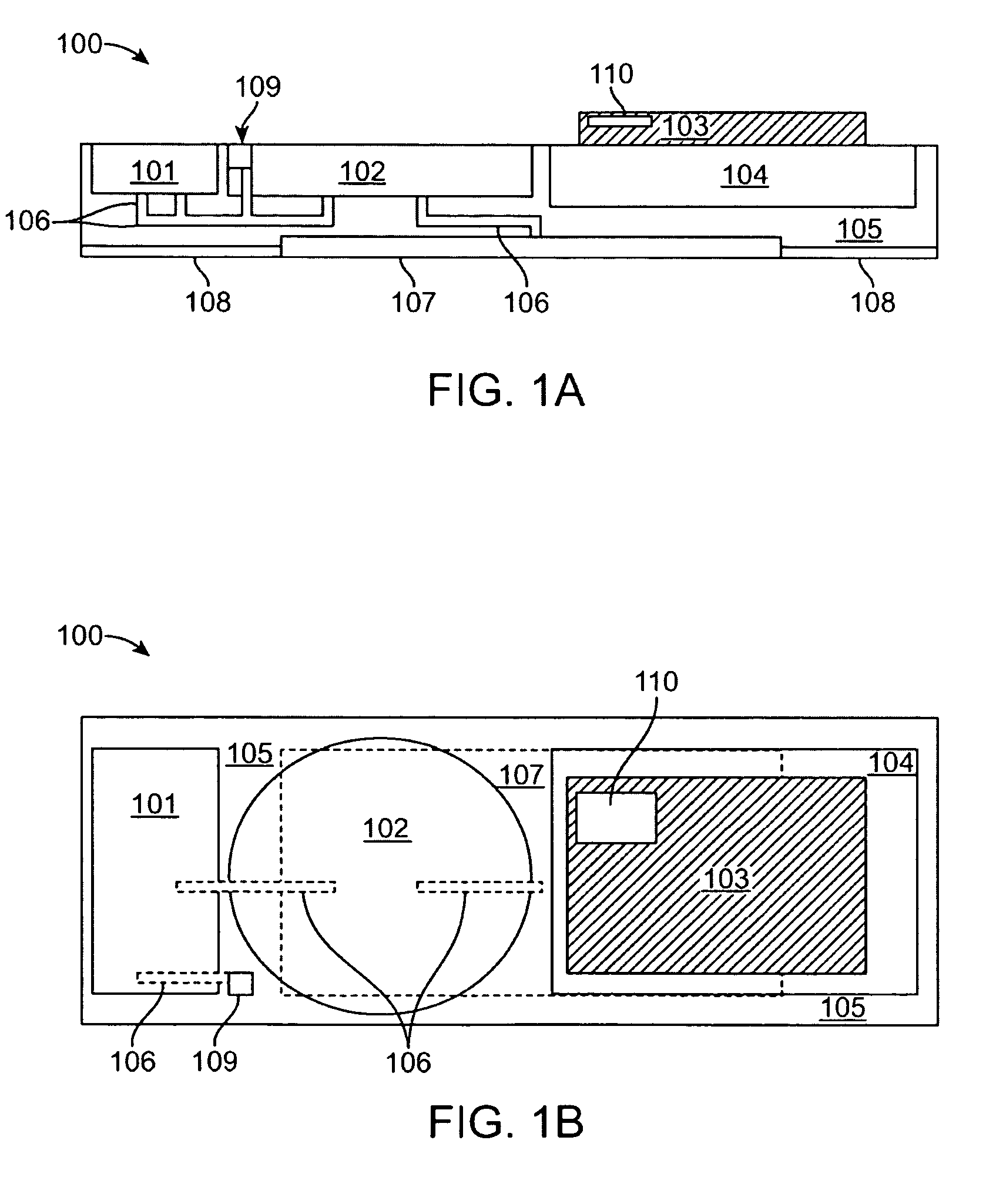
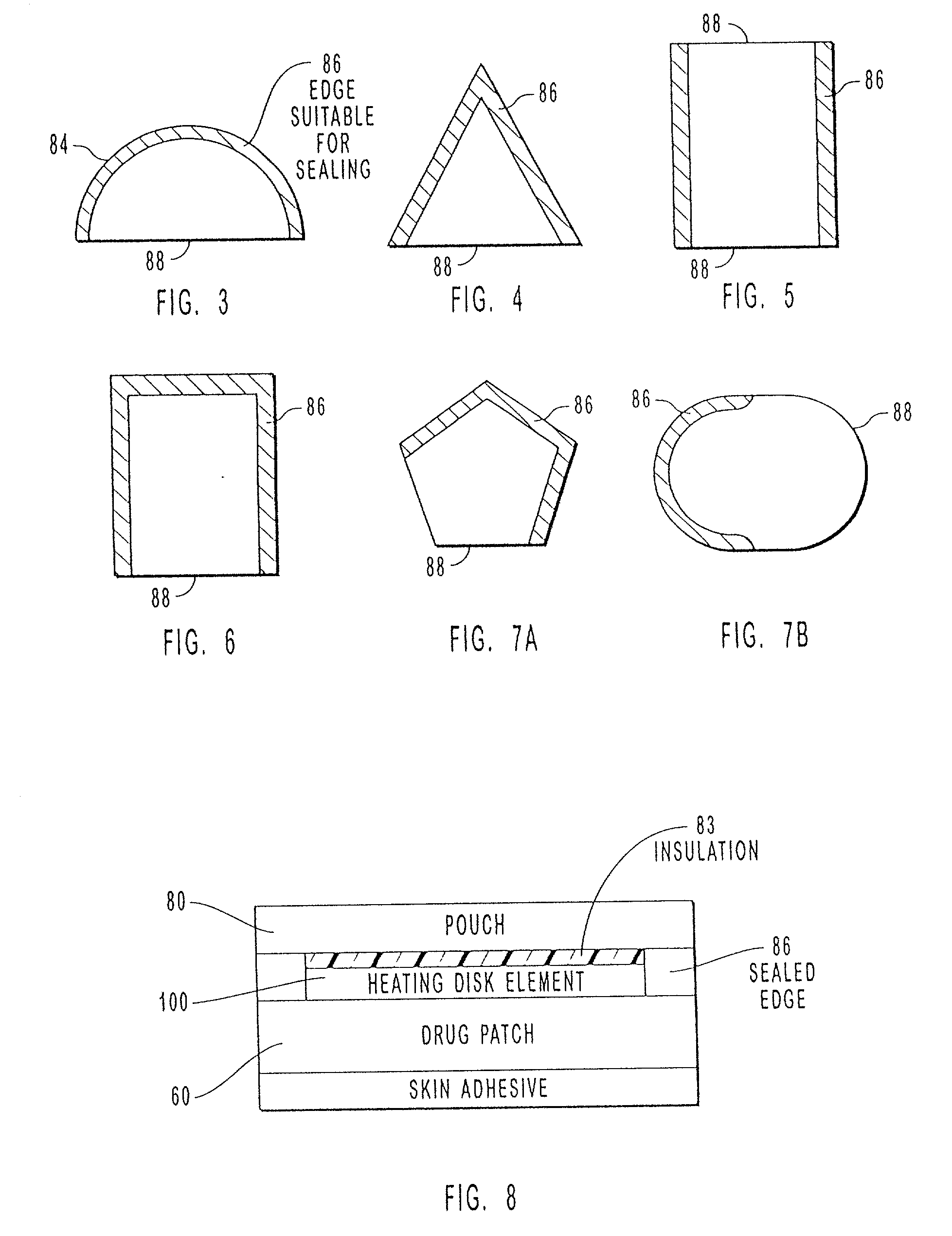
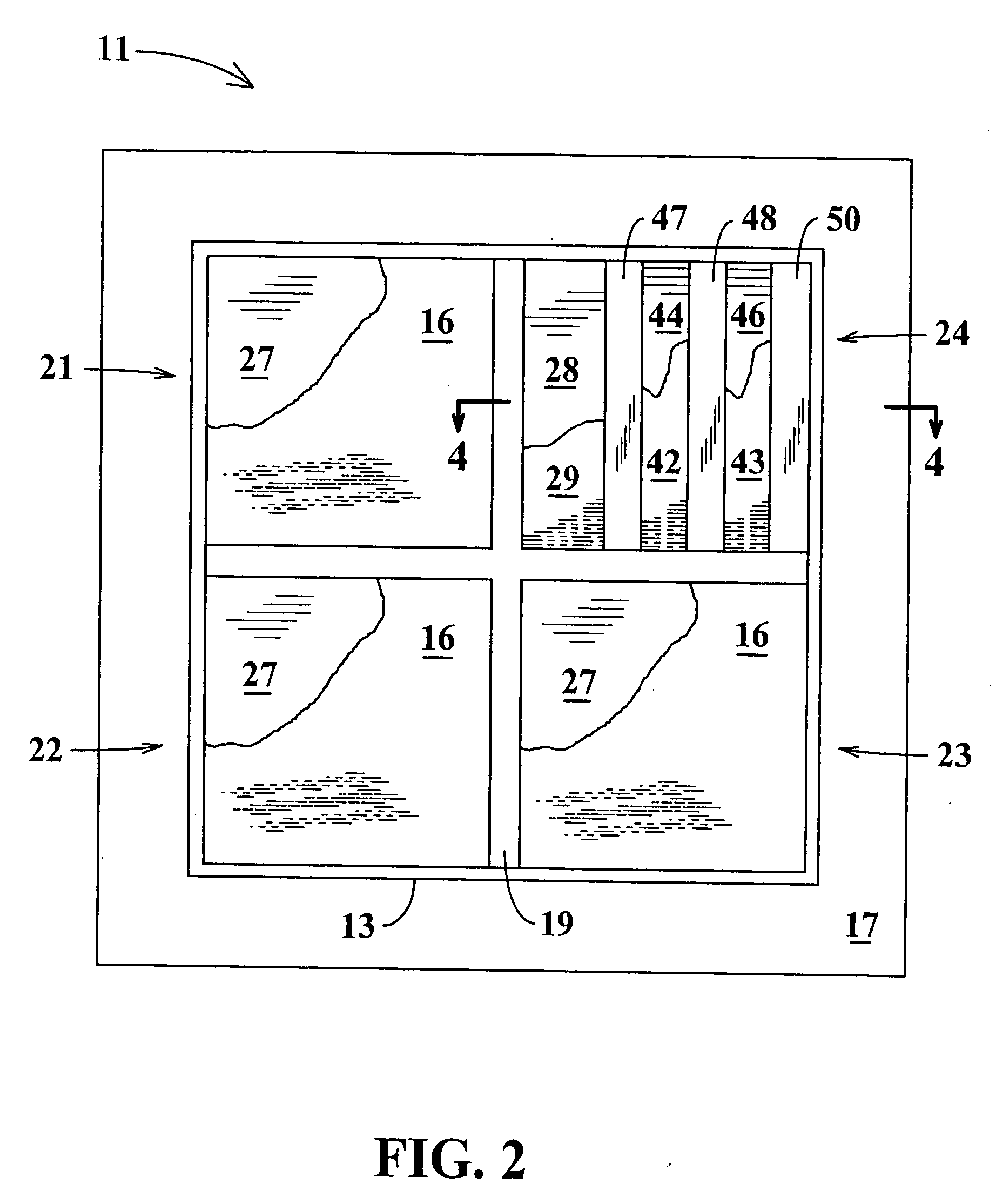
Transdermal drug patch with attached pocket for controlled heating device
InactiveUS6261595B1Shorten the timeEasy to replaceElectrotherapyMedical devicesTransdermal patchDrug administration
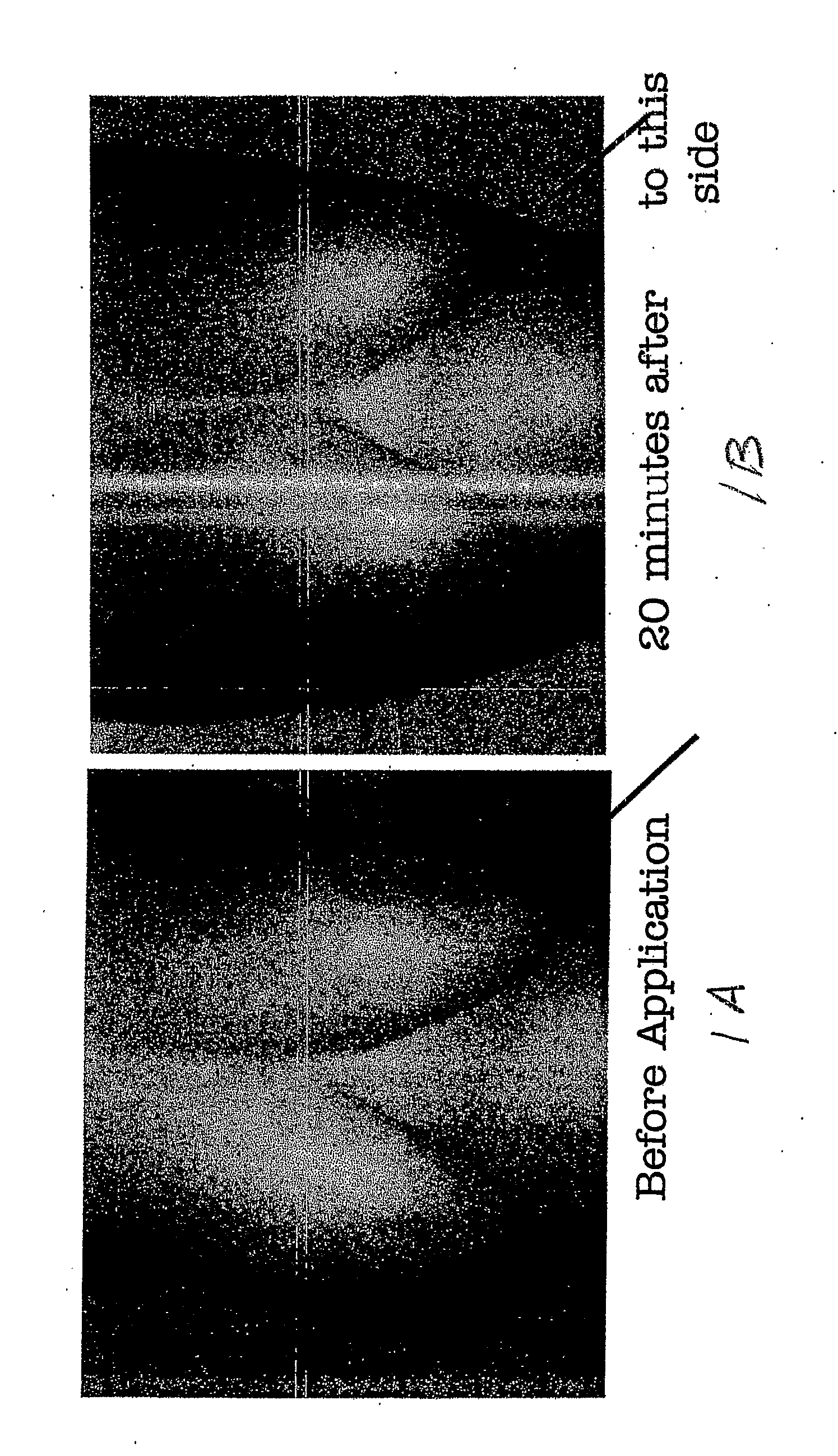
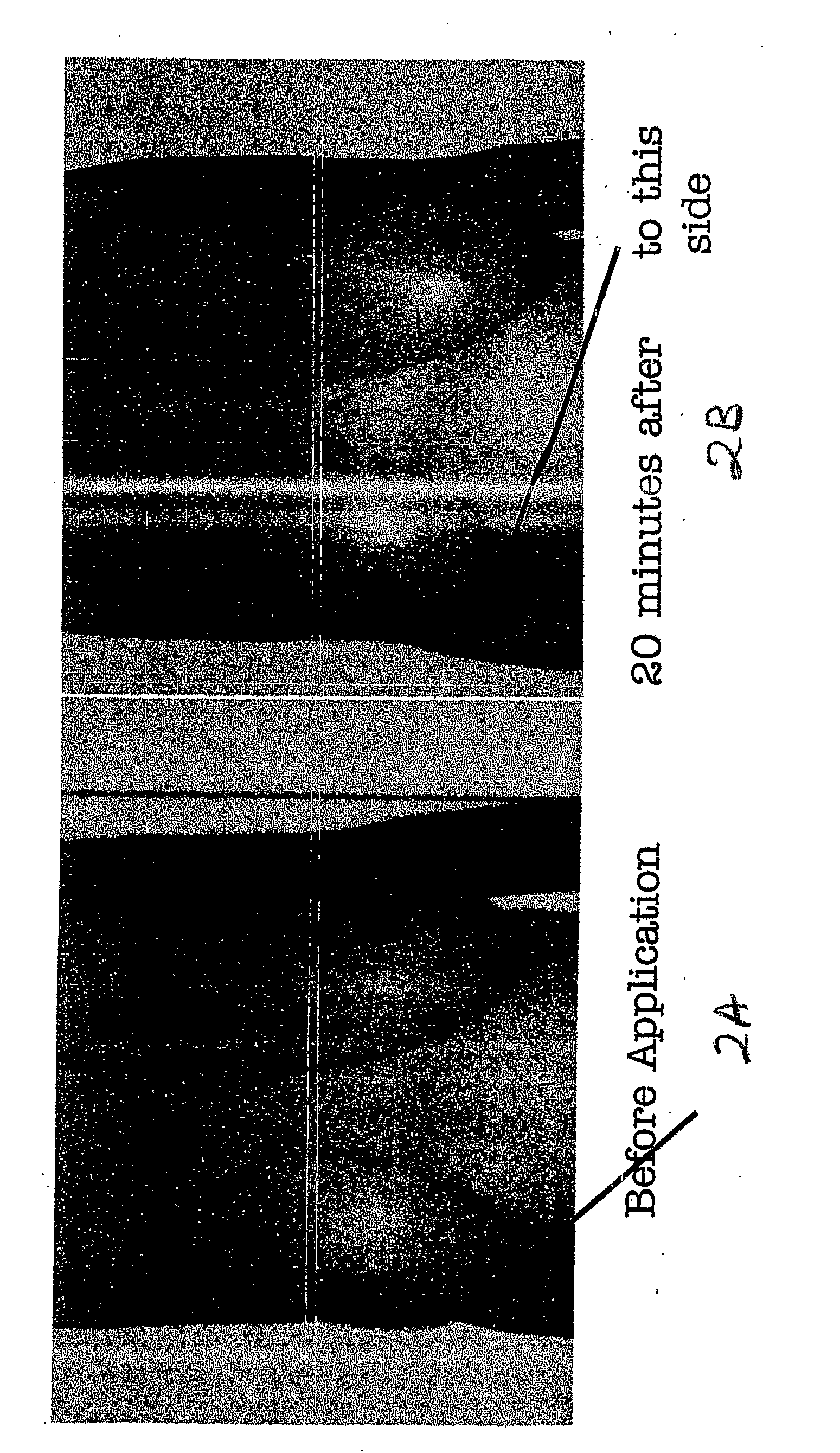
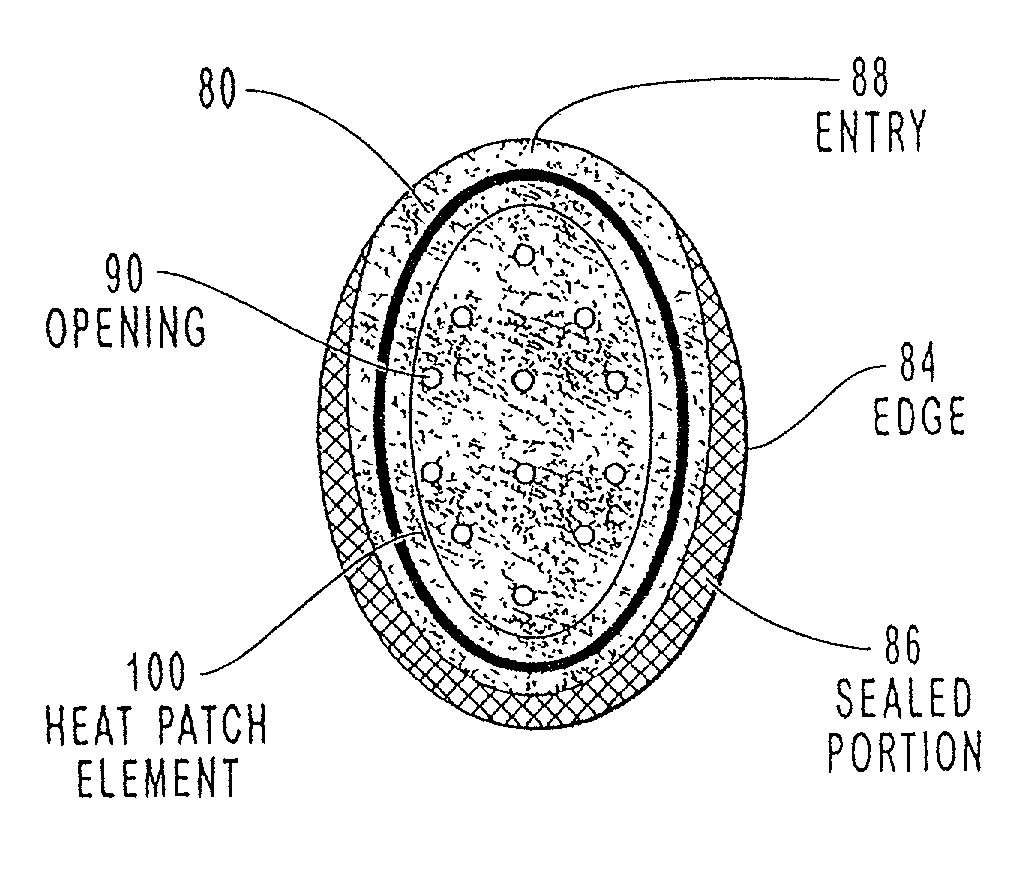
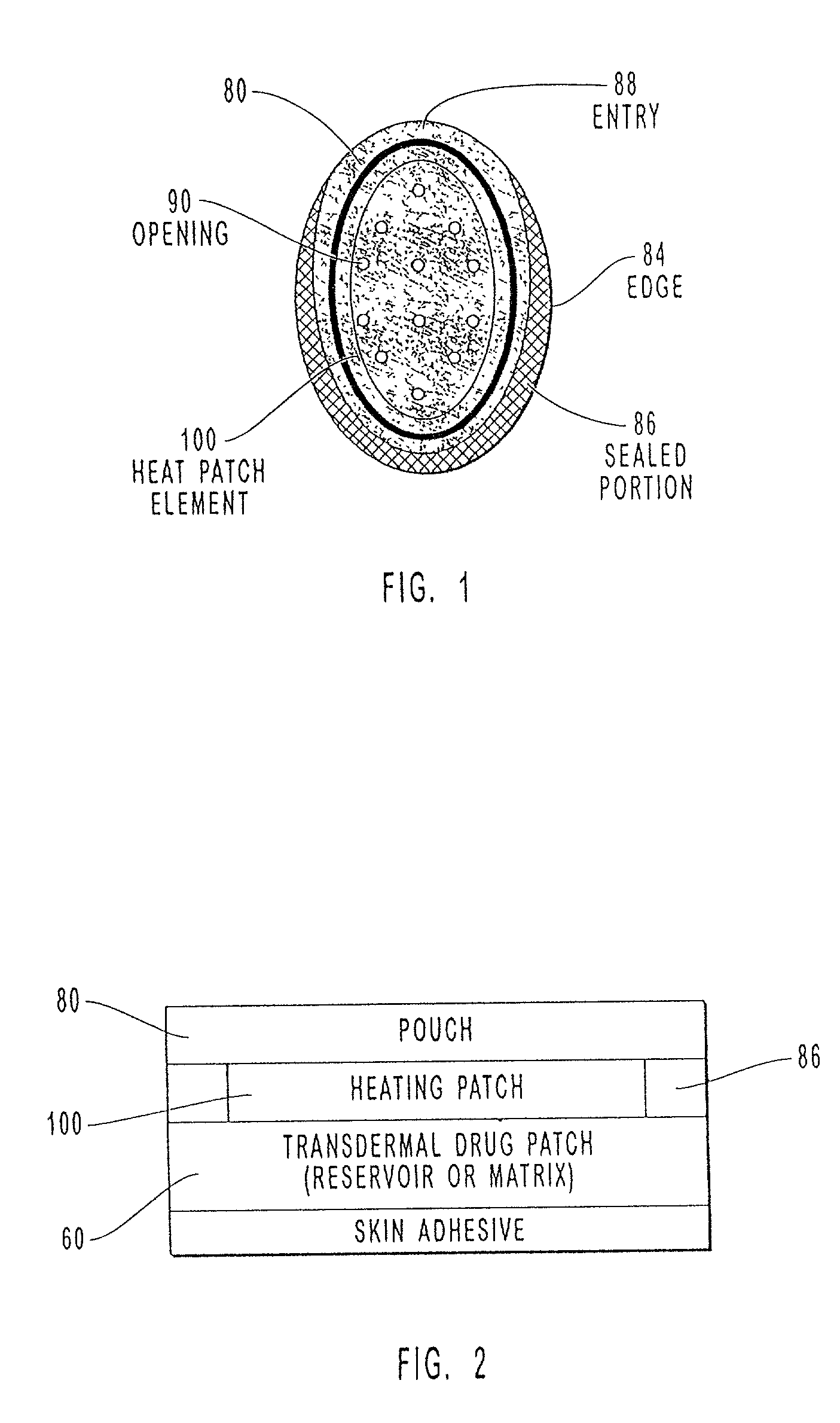
The present invention relates to a transdermal drug delivery system comprising a dermal drug delivery patch and a heating element compartment securable to the dermal drug delivery patch. A freely transferrable heating element is securable within the heating element compartment. A drug can be administered transdermally using the present invention by placing the dermal drug delivery patch upon a patient's skin at an administration site. A heating element compartment is secured to the dermal drug delivery patch and a freely transferrable heating element is placed within the heating element compartment. The heating element provides controlled heat to the dermal drug patch and the patient's skin aid thereby improves dermal drug administration.
Owner:ZARS INC
Transdermal patch system
InactiveUS20090259176A1Maintaining awarenessRemain alertElectrotherapyMicroneedlesTransdermal patchMedicine
A transdermal patch system configured as a patch or pump assembly may be placed into contact upon a skin surface to transport drugs or agents transdermally via any number of different mechanisms such as microporous membranes, microneedles, in-dwelling catheters, etc. The assembly may enclose or accommodate a reservoir configured as an elongate microchannel to contain the drug or agent suspended in a fluid vehicle. The reservoir may also be fluidly coupled via microchannels to transport the drugs into or against an underlying skin surface as driven or urged via a pump and controlled by an electronic control circuitry which may be programmed to affect any number of treatment regimens.
Owner:LOS GATOS RES
Transdermal patch for delivering volatile liquid drugs
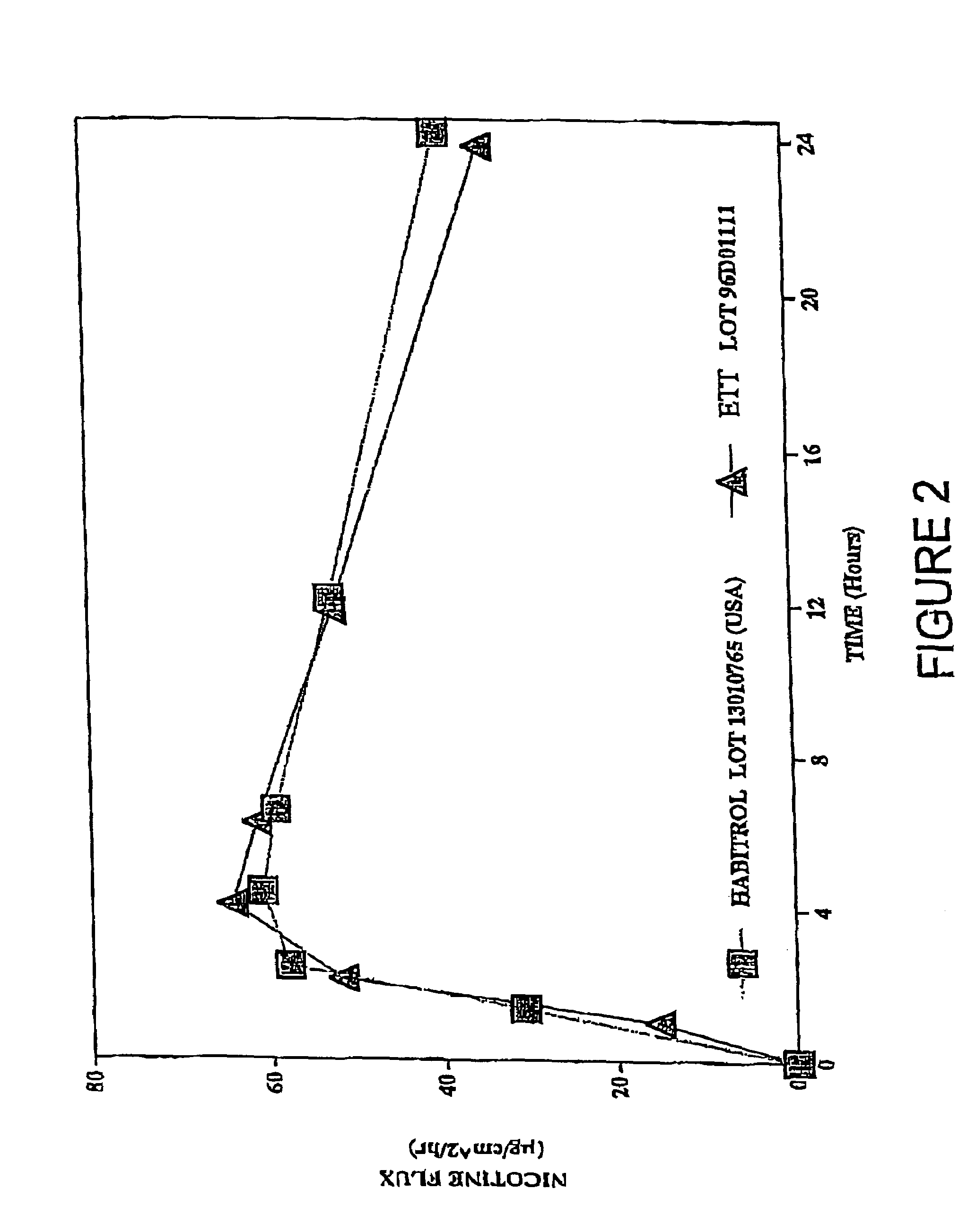
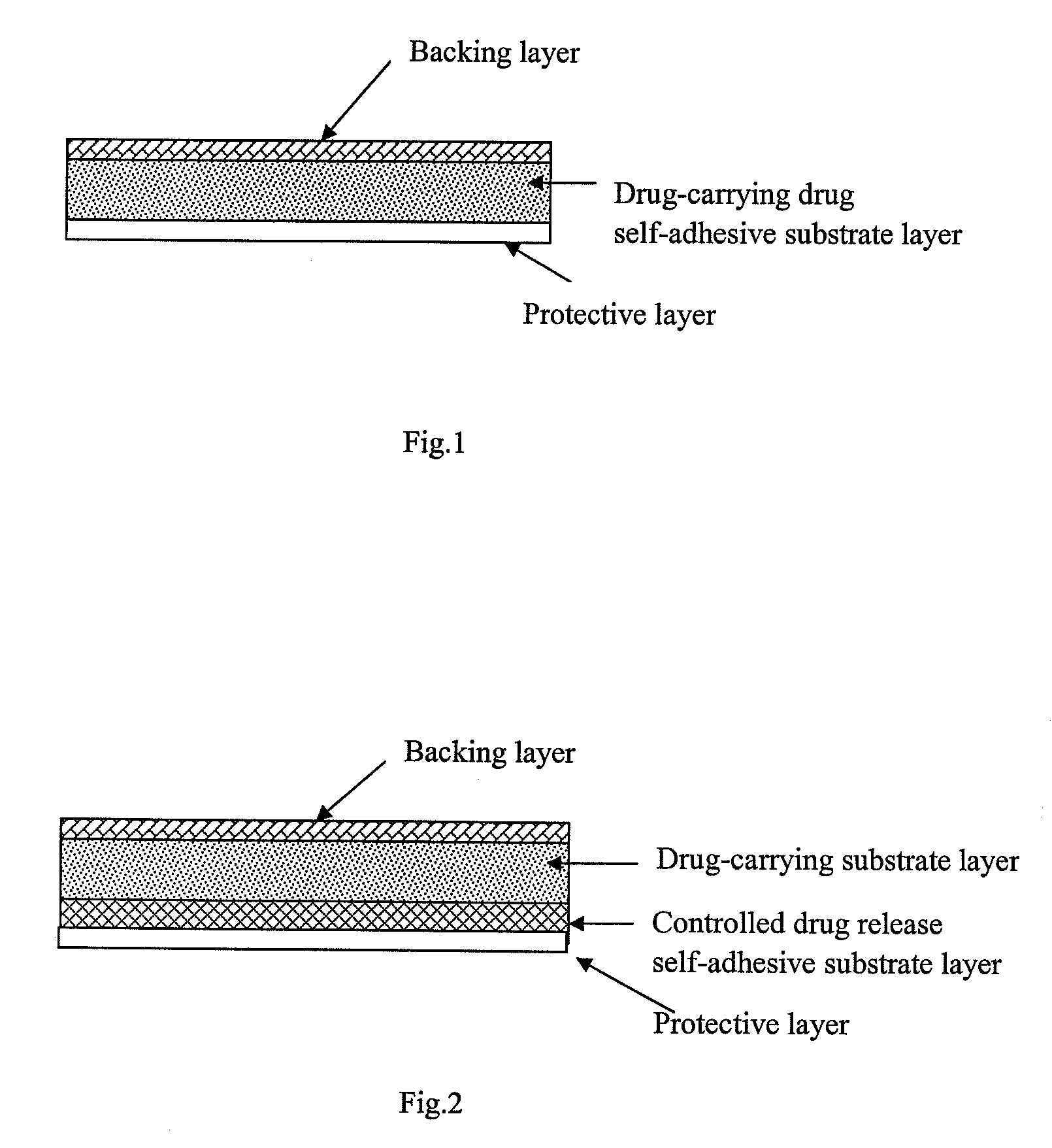
A transdermal patch for administering a volatile liquid drug, such as nicotine, transdermally to a patient comprising a four-layer laminated composite of: a top drug impermeable backing layer; a pressure sensitive silicone adhesive layer containing the drug; a pressure sensitive acrylic adhesive layer also containing the drug; and a removable siliconized release liner layer. Also disclosed is a method for treating a person for nicotine dependence and particularly for treating a woman for nicotine dependence.
Owner:ELAN PHRMA INT LTD +1
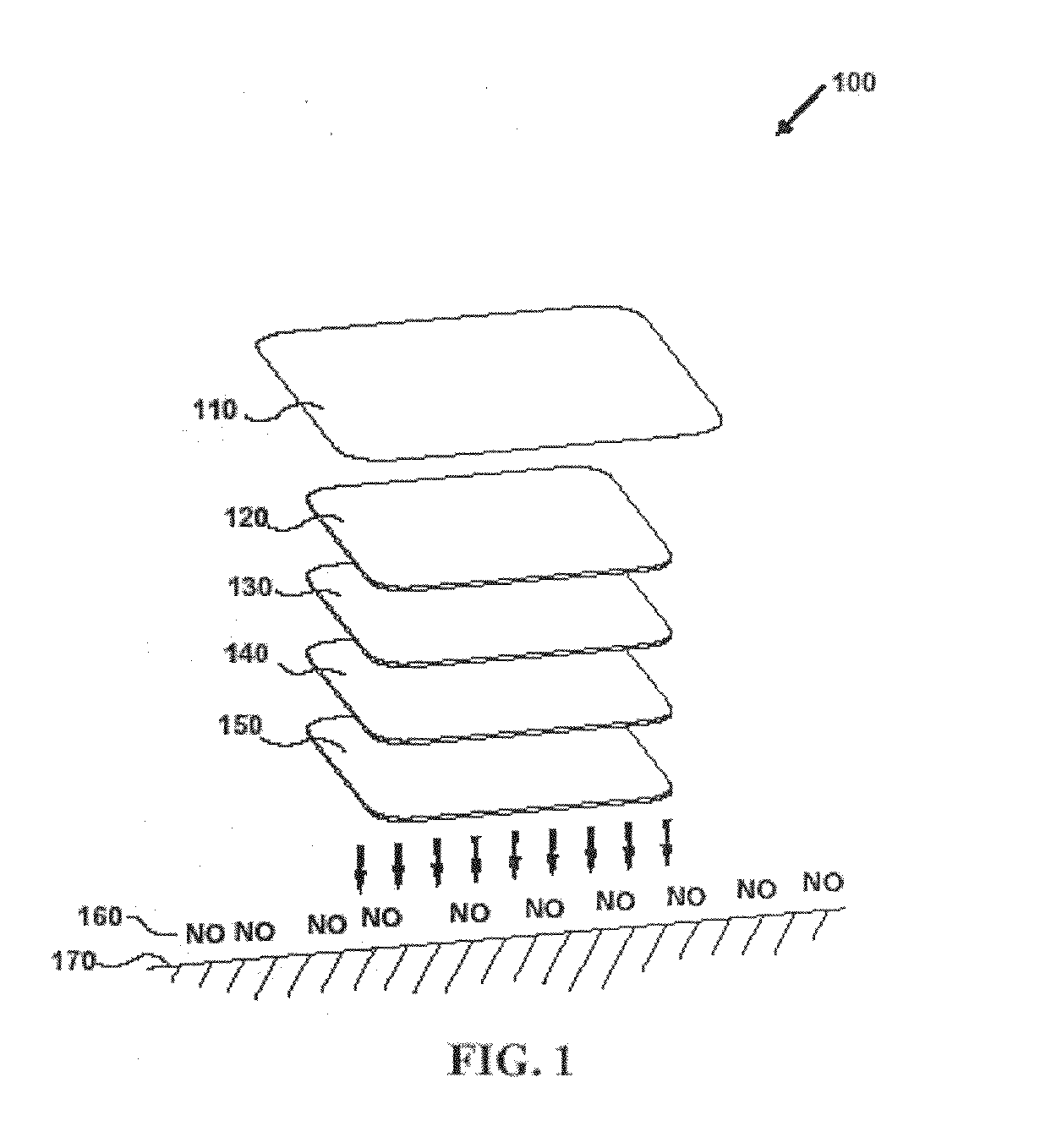
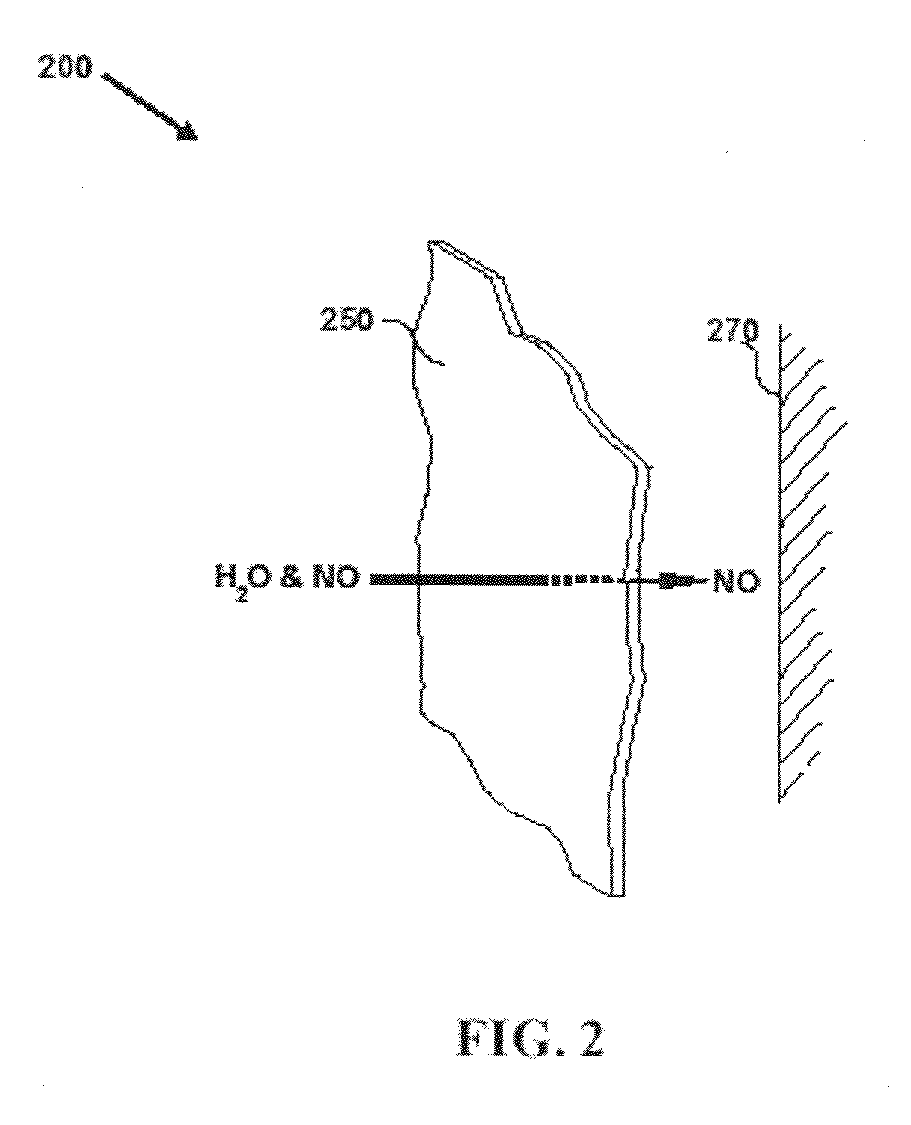
Systems and methods for topical treatment with nitric oxide
InactiveUS7048951B1Reduce skin irritationBiocideInorganic active ingredientsSandwich likeTransdermal patch
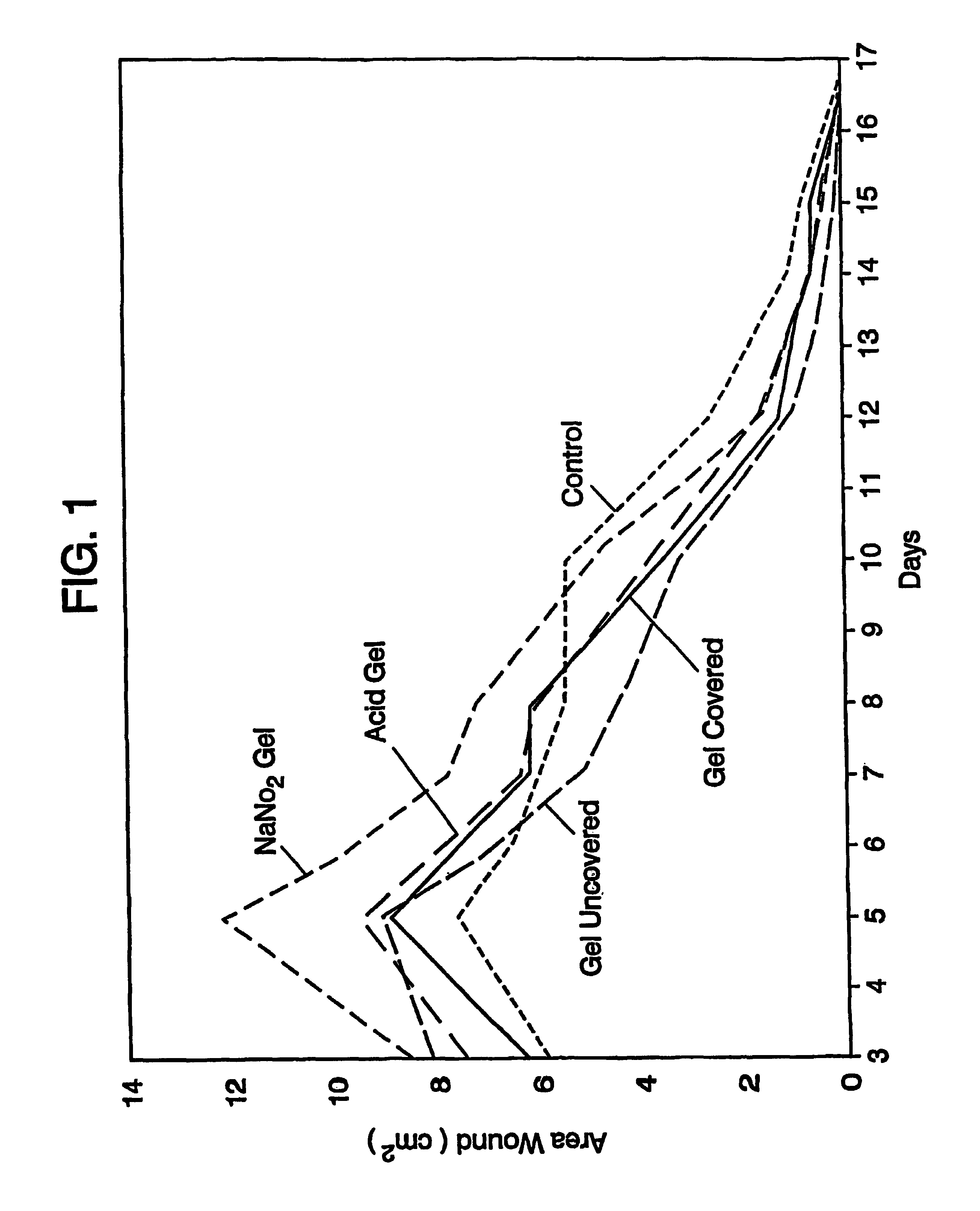
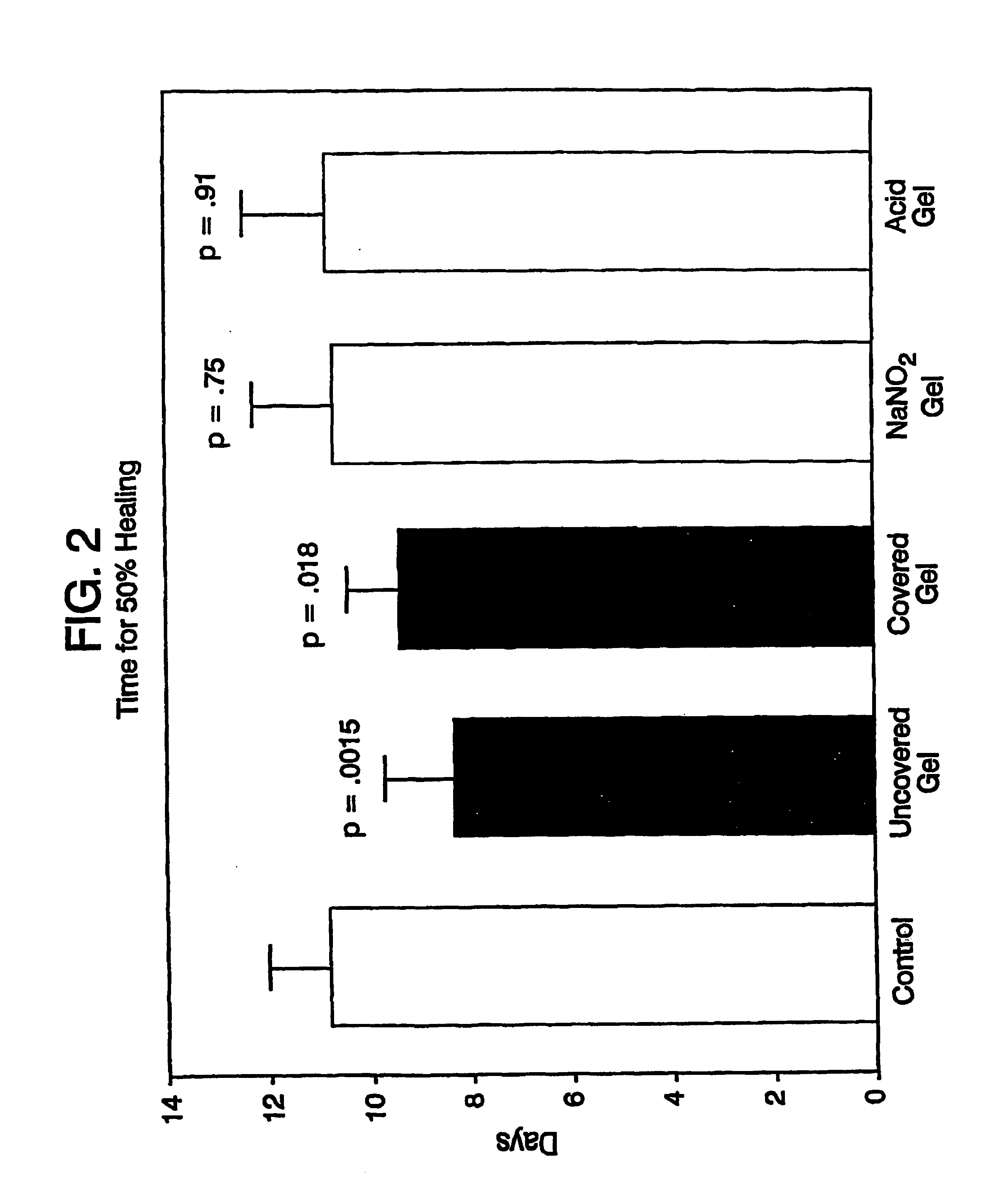
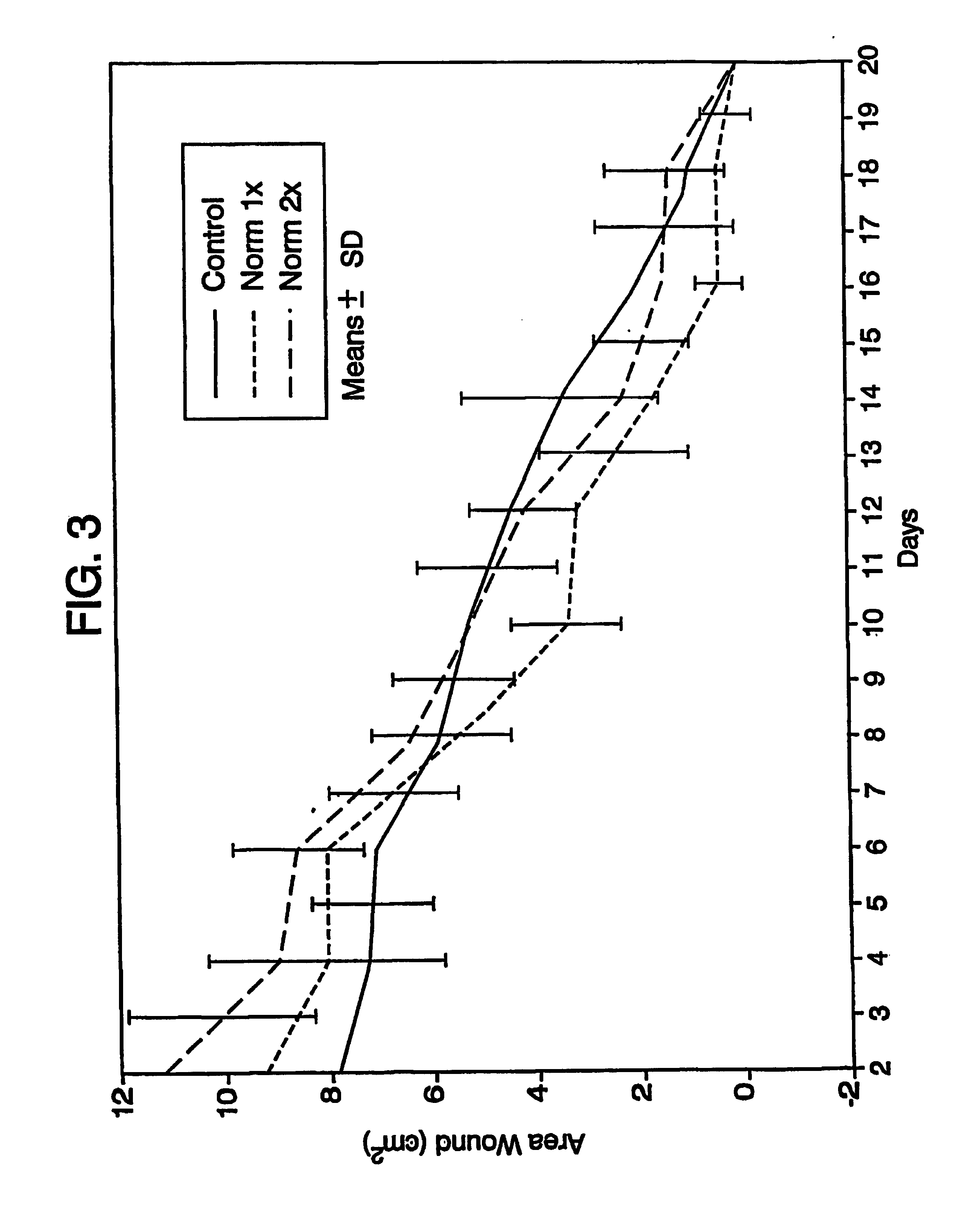
A simple, biocompatible system and procedure for generating nitric oxide (NO) is described. A mixture of powdered sodium nitrite, ascorbic acid, and maleic acid (or another organic acid of adequate strength) immediately generates nitric oxide (NO) on treatment with water. To slow down the NO generation, one may prepare an ointment from a nonaqueous medium (petrolatum, Vaseline™) and the three powdered ingredients, which on being applied topically on the skin will release NO as water permeates through this medium; alternatively, one may convert the aqueous sodium nitrite solution into a gel with hydroxyethylcellulose (or other gel-forming compound) and combine this gel with another gel obtained from aqueous ascorbic and maleic acids with hydroxyethylcellulose for topical application (on intact skin, burns intra-cavity, burns, intra-cavity, etc.). The two gels may be admixed immediately before use (possibly from a single container with separate chambers and dual nozzle, via pushing or squeezing the two gels through the nozzle), or may be applied in sandwich-like fashion (possibly as a transdermal patch) for further slowing down the delivery of NO.
Owner:NITRIC SOLUTIONS
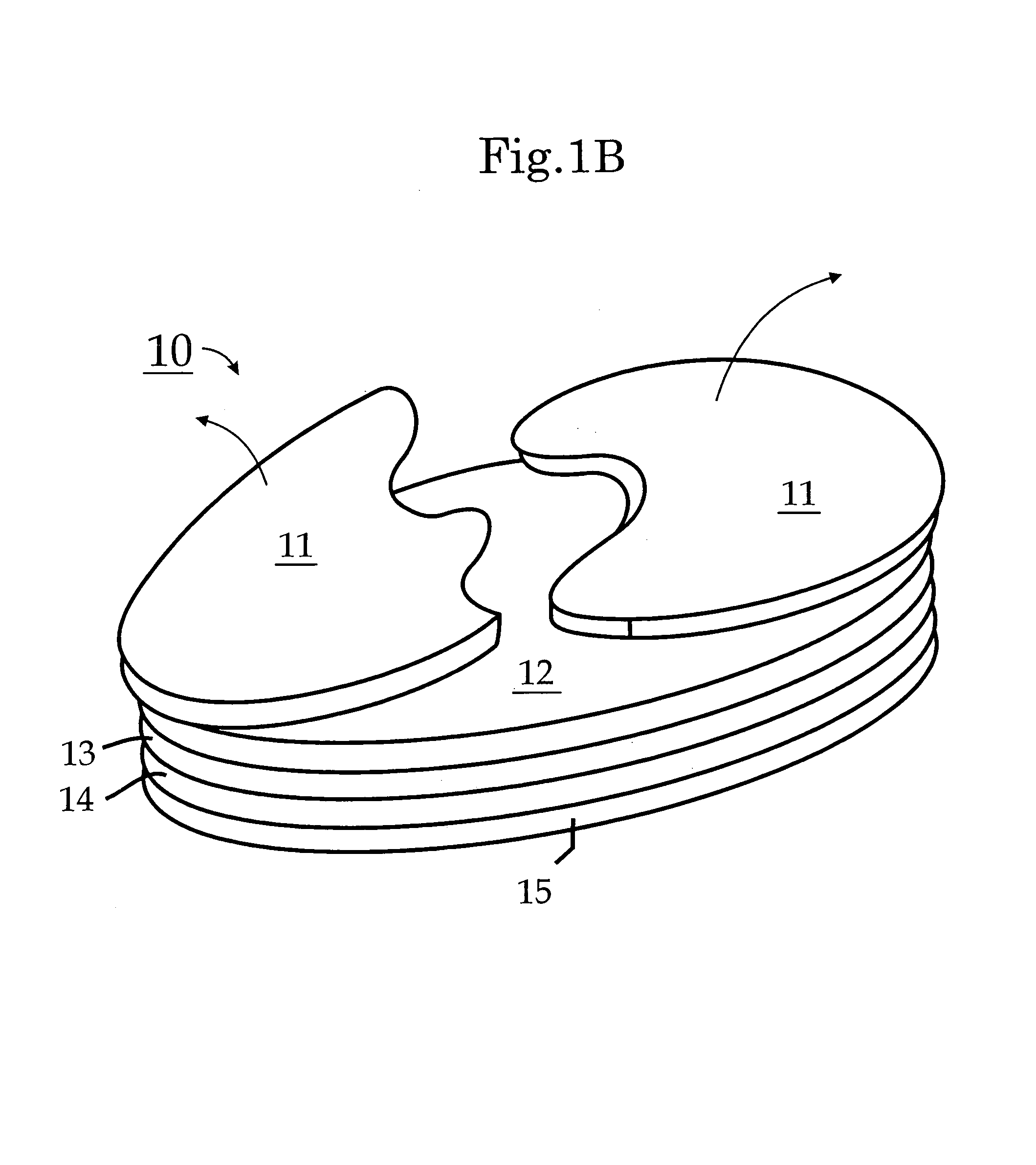
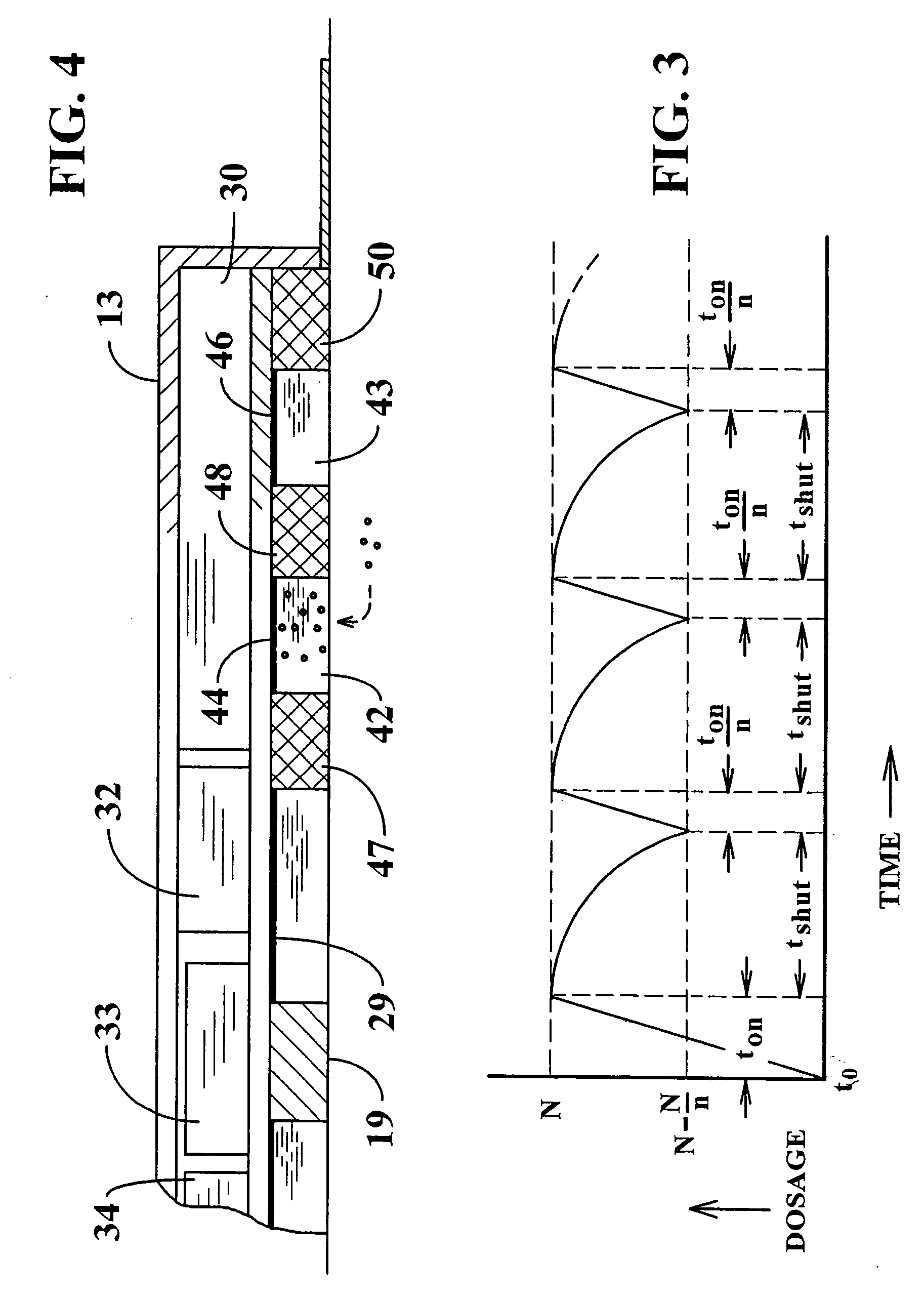
Transdermal patch containing microneedles
A transdermal patch that can easily deliver a controlled volume of a fluidic drug compound to skin is provided. More particularly, the patch contains a microneedle assembly that is configured to be placed in fluid communication with a drug delivery assembly. The microneedle assembly contains a support and a plurality of microneedles that extend outwardly from the support. The microneedles are formed with one or more channels of a certain dimension such that passive capillary flow drives a flow of the drug compound. The drug delivery assembly contains a reservoir for the drug compound that is in fluid communication with a rate control membrane that helps control a flow rate of the drug compound by modulating a pressure of the drug compound, downstream from the reservoir. A release member is also positioned adjacent to the microneedle and drug delivery assemblies. Prior to use, the release member acts as a barrier to the flow of the drug compound and thus inhibits premature leakage. In this manner, the patch can initially be provided in an “inactive” configuration in which the drug compound is securely retained. When it is desired to release the drug compound, the patch can simply be activated by at least partially separating the release member from the drug delivery and microneedle assemblies.
Owner:SORRENTO THERAPEUTICS INC
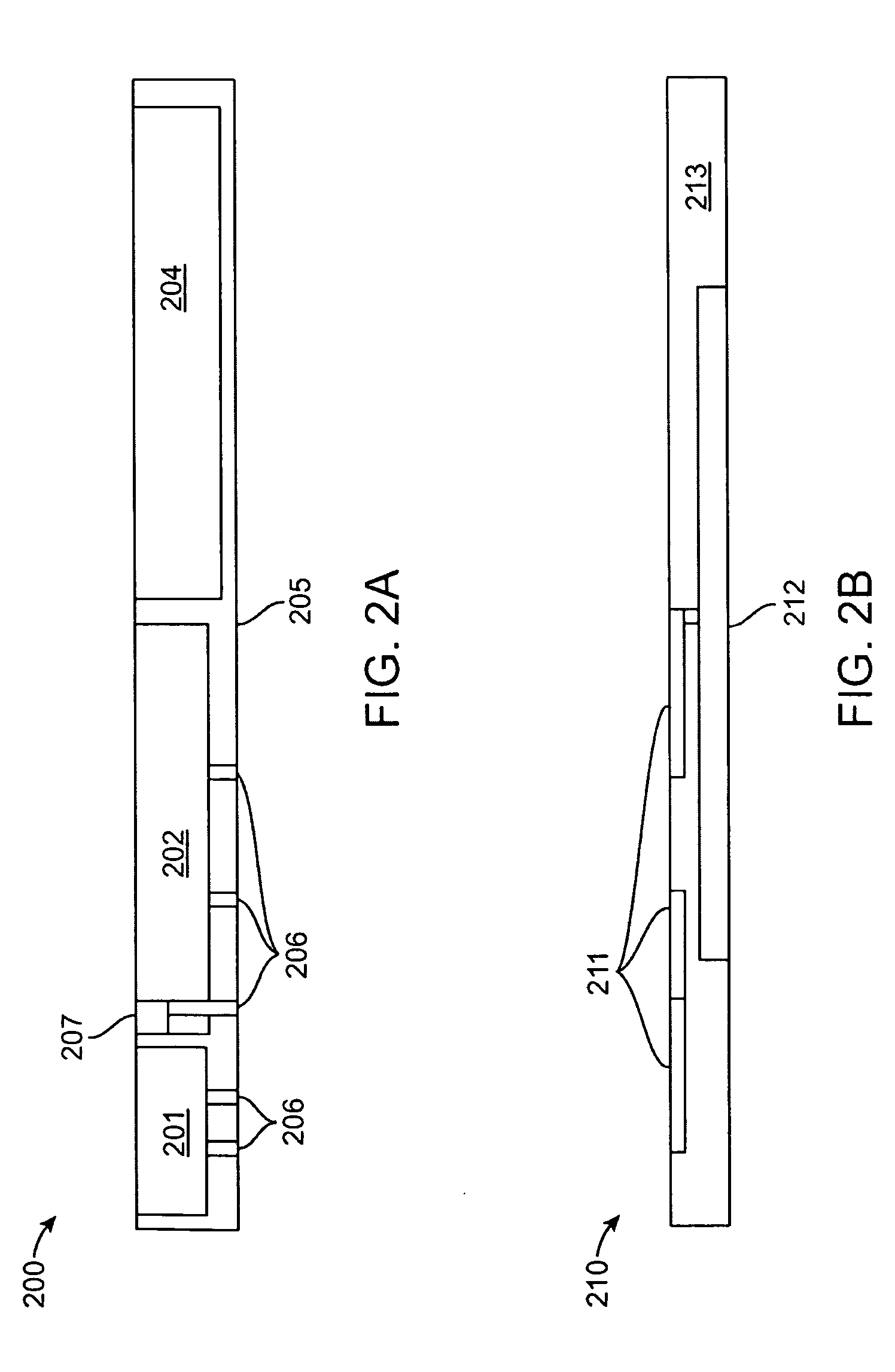
Multi-layer Transdermal Patch
This invention pertains to a construction consisting of in the order from the outside towards the inside: An occlusive or non occlusive external film layer; a non-curing pressure sensitive adhesive (PSA) that has been blended with a therapeutic concentration of at least one or a combination of cosmetic or pharmaceutical active ingredients; and a silicone gel adhesive that is used as the skin contact layer.
Owner:DOW CORNING CORP +1
Transdermal estradiol/progestogen agent patch and its production
PCT No. PCT / EP96 / 05759 Sec. 371 Date Sep. 14, 1998 Sec. 102(e) Date Sep. 14, 1998 PCT Filed Dec. 20, 1996 PCT Pub. No. WO97 / 23227 PCT Pub. Date Jul. 3, 1997The invention concerns a transdermal patch for the release through the skin of estradiol and a progestogen agent and a process for its production.
Owner:ROTTA RES
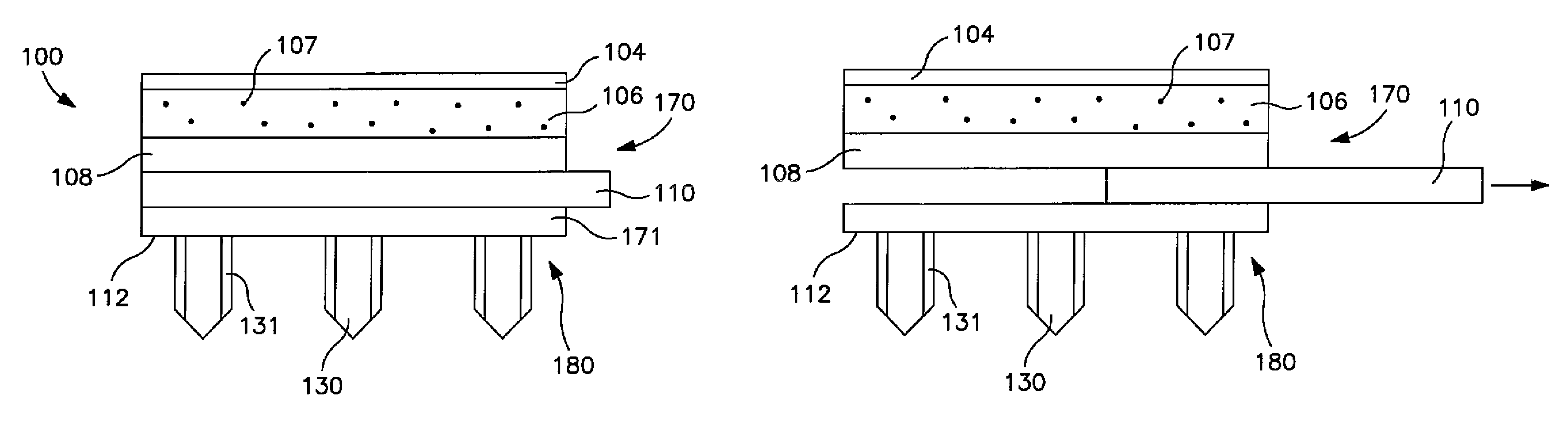
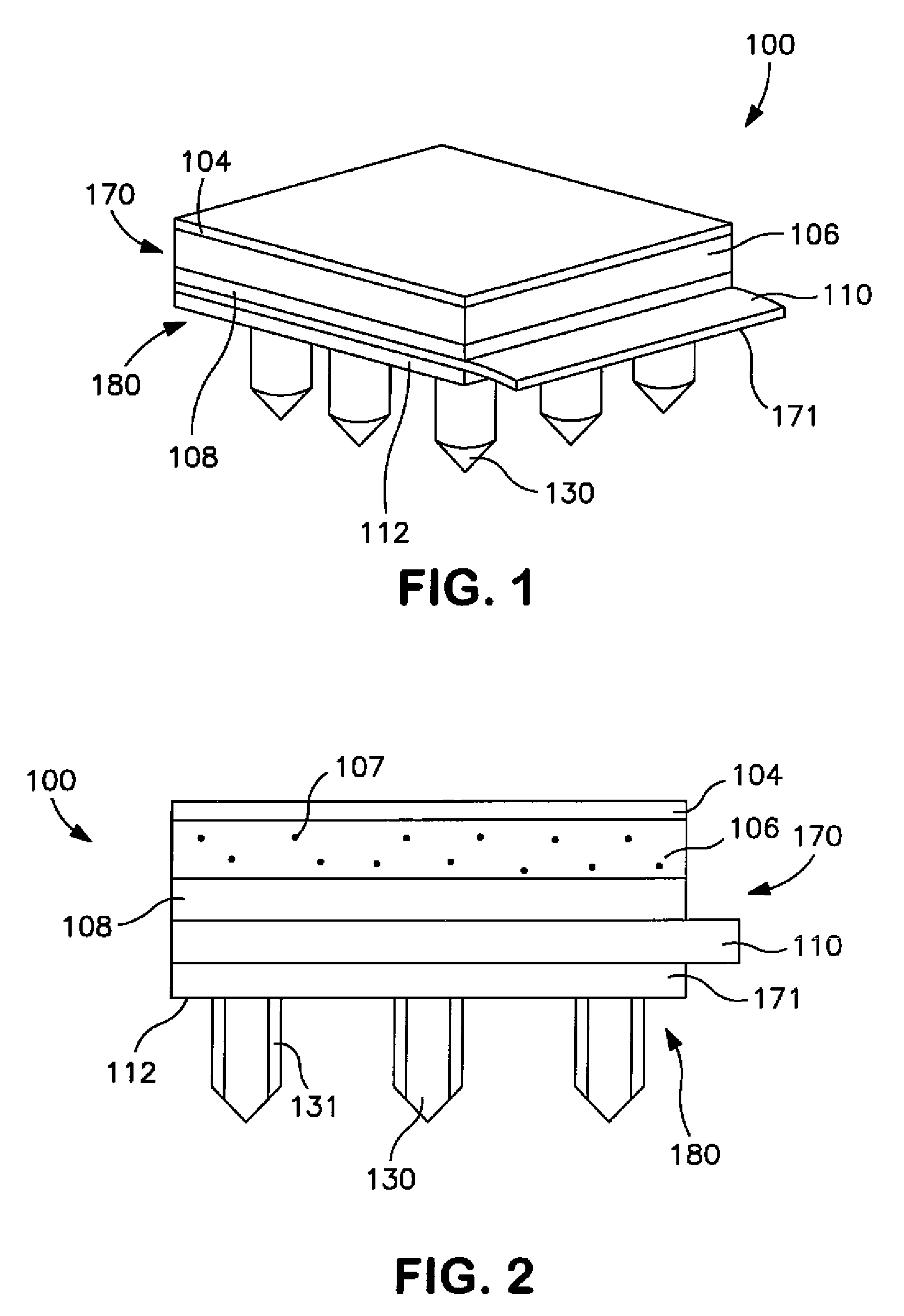
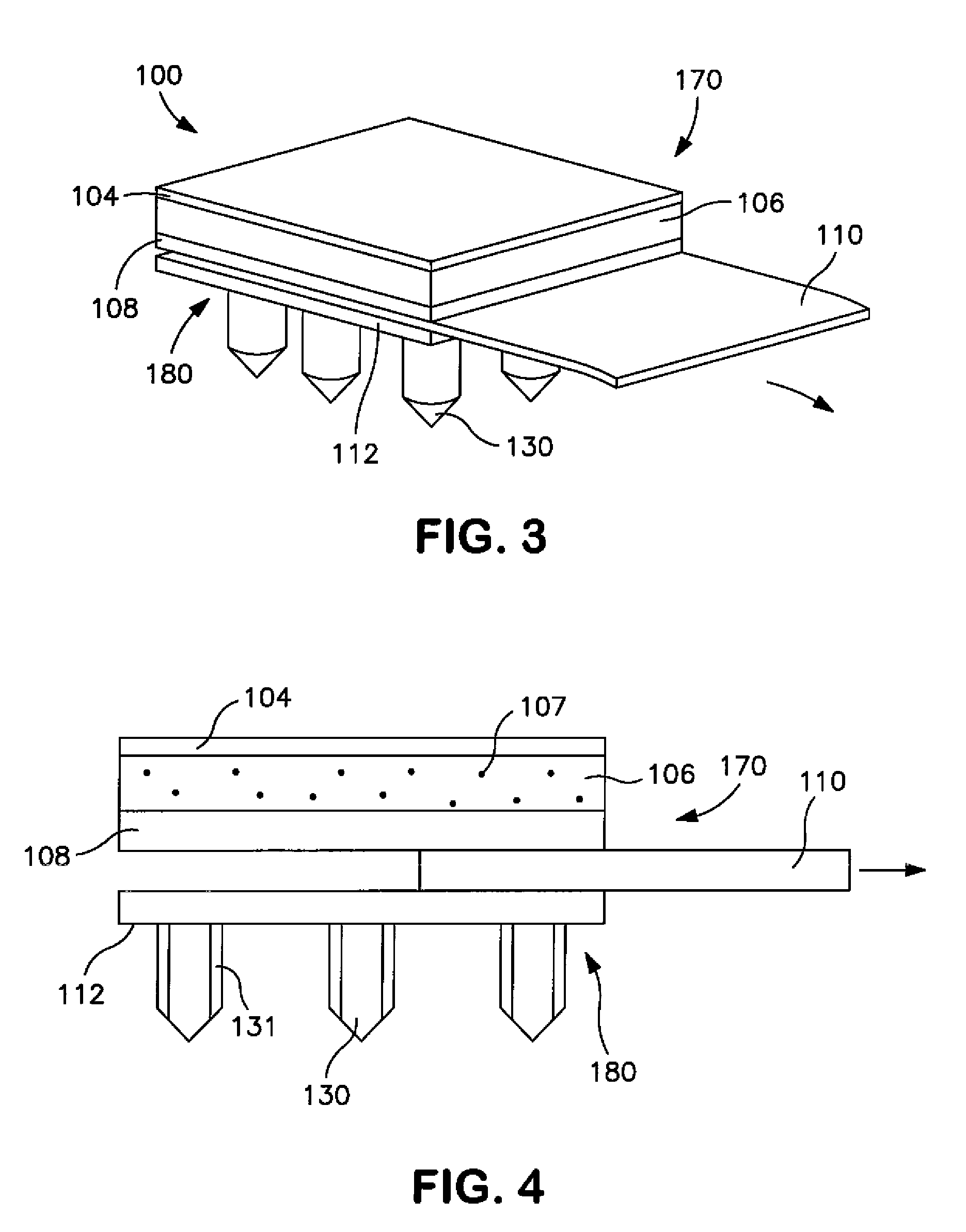
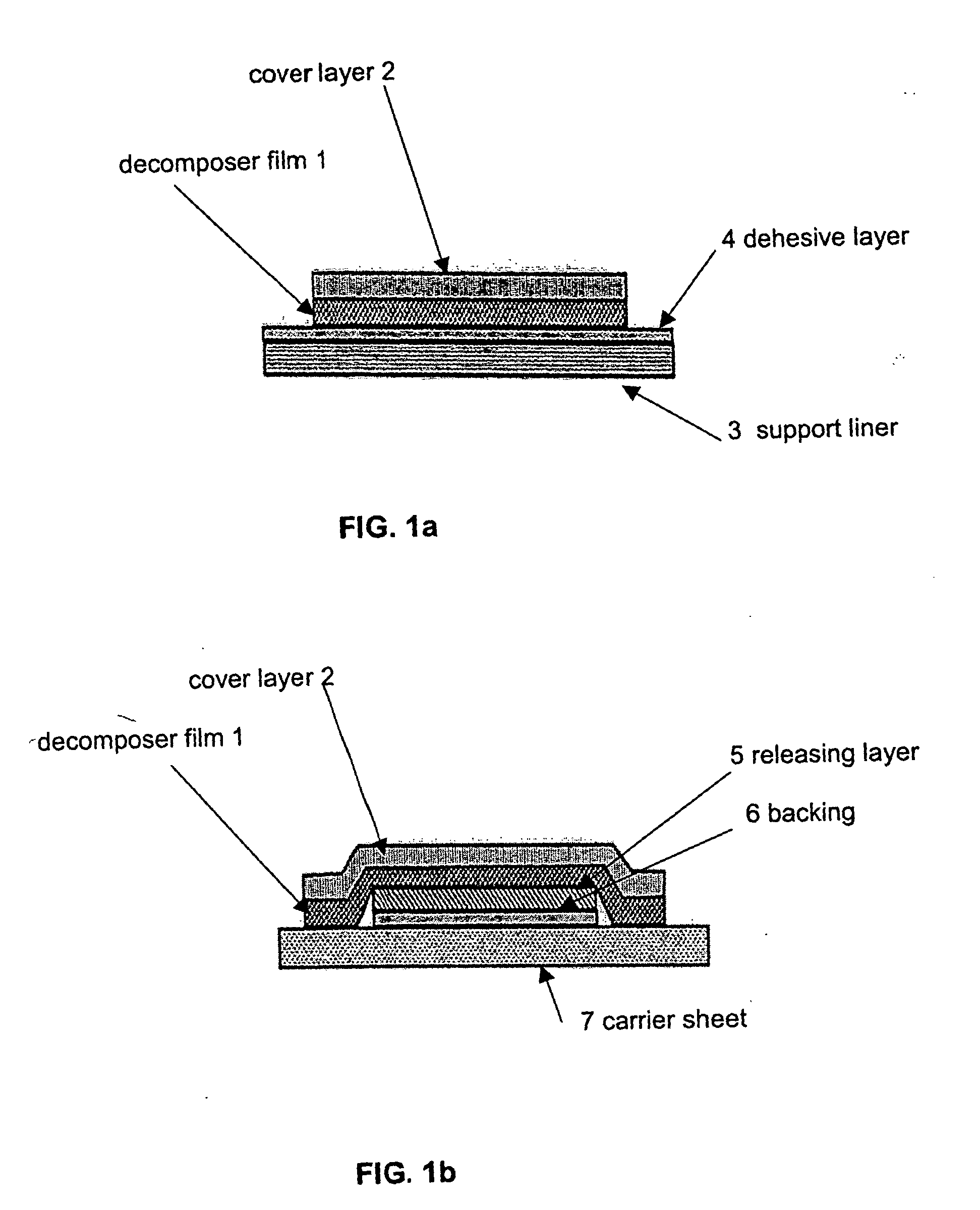
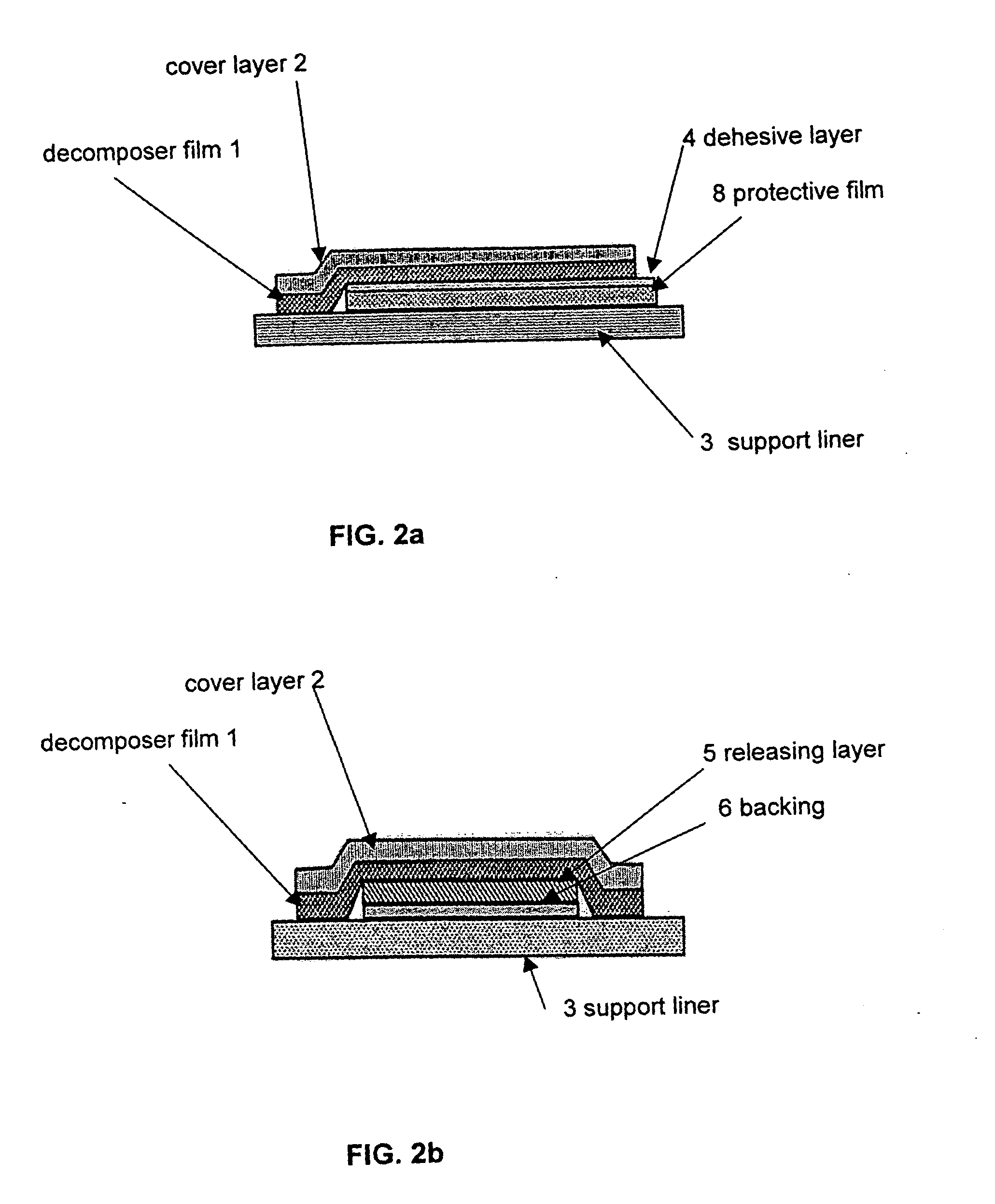
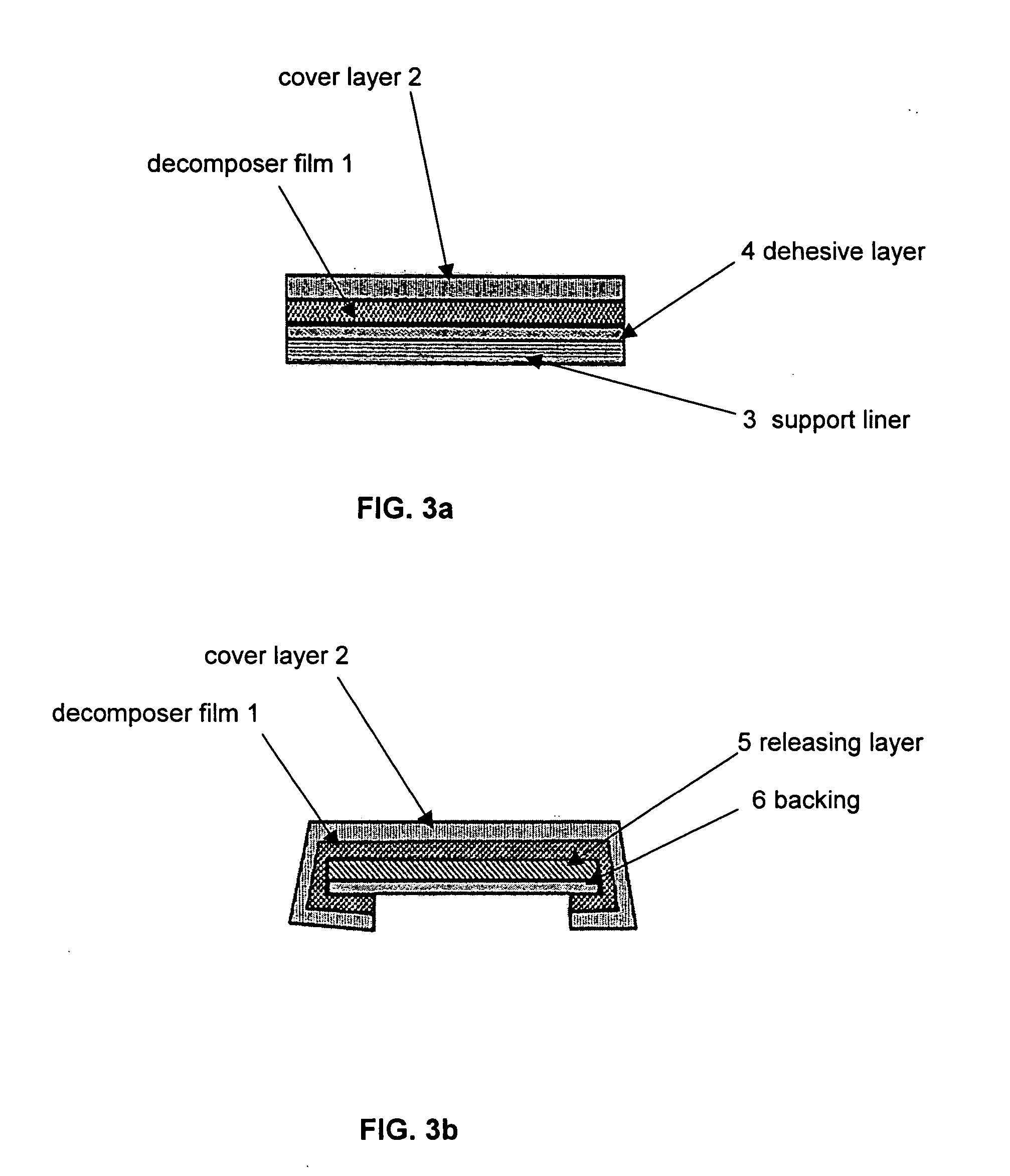
Decomposer film for transdermal patches
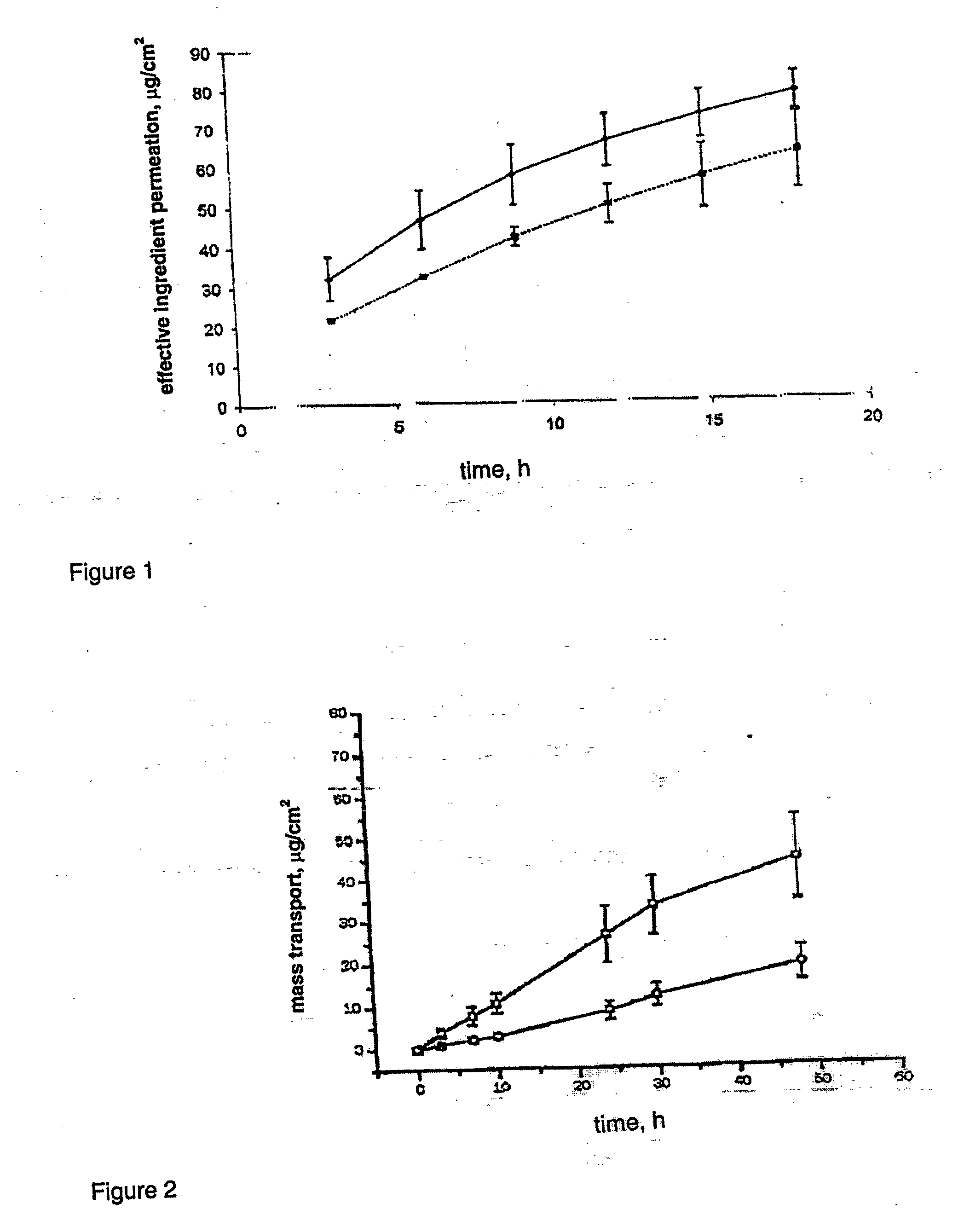
InactiveUS20070014839A1Increased internal surface areaAccelerate decomposition reactionOrganic active ingredientsAntipyreticTransdermal patchPolyvinyl alcohol
The decomposer film product has a polymeric decomposer layer, a cover layer for protecting the decomposer film from the surroundings and a releasable support liner, which is removed prior to use. The polymeric decomposer film contains a water-soluble or water-insoluble polymeric adhesive material and a decomposition accelerator, which acts to decompose an effective ingredient, such as a steroid hormone, of a worn or unused transderamal patch, when the effective ingredient releasing layer of the patch adheres to the polymer film, so that the pharmaceutical effective ingredient comes into contact with the decomposition accelerator by diffusion. The decomposition accelerator includes a chemically oxidizing substance, preferably urea peroxide, manganese (III) acetate or iron (III) citrate. The water-insoluble polymeric adhesive material is preferably an acrylates adhesive. The water-soluble polymeric adhesive material is preferably polyvinyl alcohol, polyvinyl pyrrolidone, a cellulose derivative or a polyacrylic acid.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
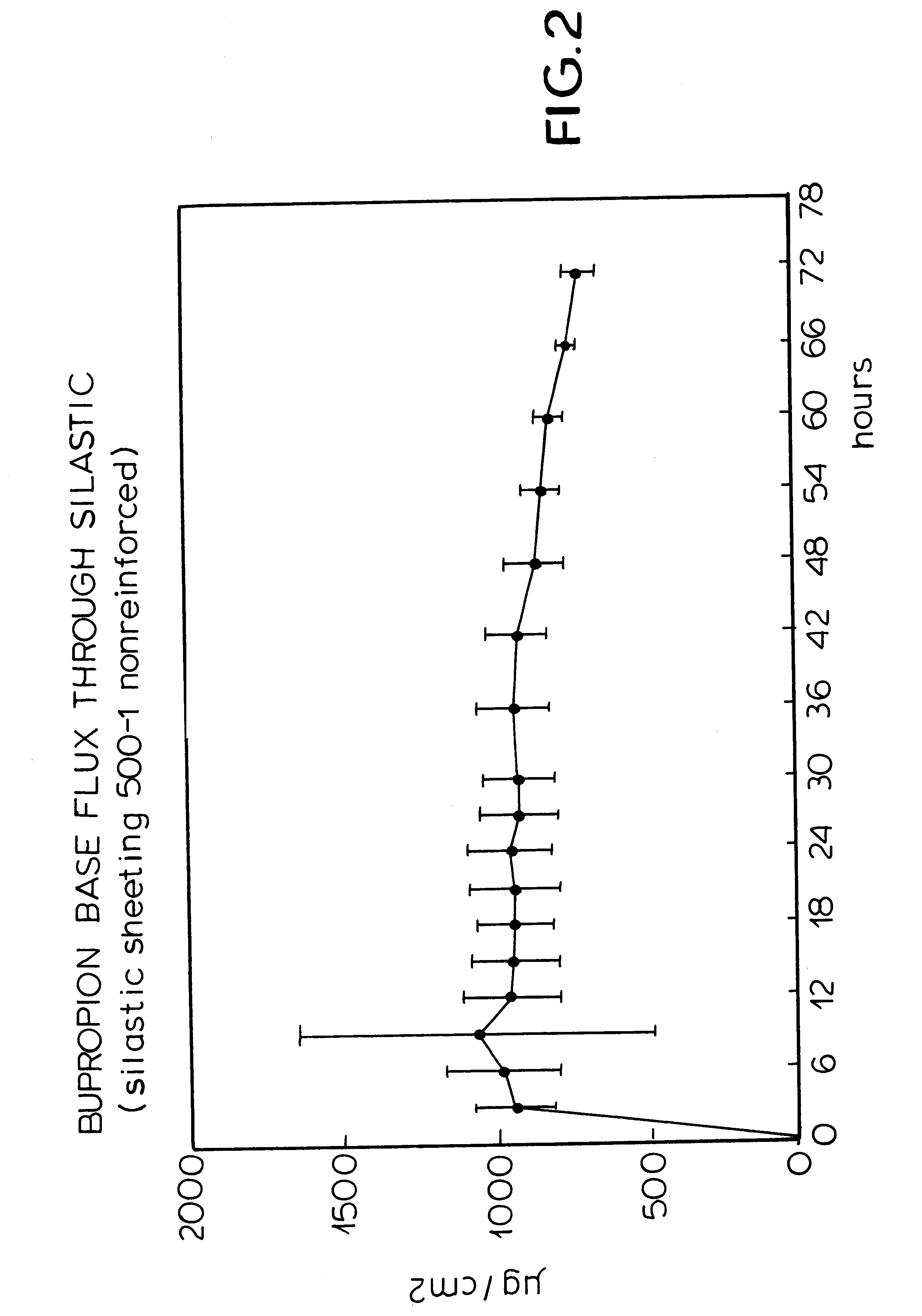
Patch and method for transdermal delivery of bupropion base
InactiveUS6312716B1Good for weight lossRelieve symptomsOrganic active ingredientsBiocideTransdermal patchDrug withdrawal symptoms
The invention includes a patch and method for transdermal delivery of bupropion base. In the method of this invention, a patient is administered a bupropion base in an amount effective to alleviate withdrawal symptoms and to prevent or reduce craving of nicotine in said patient. Alternatively, an effective amount of bupropion base is delivered to alleviate depression in a patient or to treat obesity. A transdermal patch includes a bupropion base. The bupropion base can be mixed with an acceptable pharmaceutical carrier.
Owner:COLLEGIUM PHARMA INC
Method of Delivery of Therapeutic Metal Ions, Alloys and Salts
A method for treating a bacterial, viral, fungal, or vector-induced disease state. A therapeutically effective dose of a metal substance is delivered to the body of a potentially infected organism using a delivery methodology selected from the group consisting of syringes, auto-injectors, pricking devices, buccal embedding, transdermal patches, needle transdermal patches, aerosol inhalers, ingestible dissolvable capsules, encapsulated boluses, needle encapsulated boluses, and electrode catheterization methodologies. The metal substance is selected from the group consisting of silver, gold, copper, zinc, selenium, platinum, and their ions, alloys, salts, and combinations thereof. Preferably, an electrical current is introduced substantially in the course of utilizing the delivery methodology. The electrical current is preferably substantially varied over time, and is still more preferably a reversing electrical current.
Owner:INT BIO THERAPEUTIC RES
Transdermal pharmaceutical preparation with a progesterone A-specific ligand (PRASL) as active ingredient
InactiveUS20060134188A1Avoid reactionReduce riskBiocideOrganic active ingredientsTransdermal patchAdditive ingredient
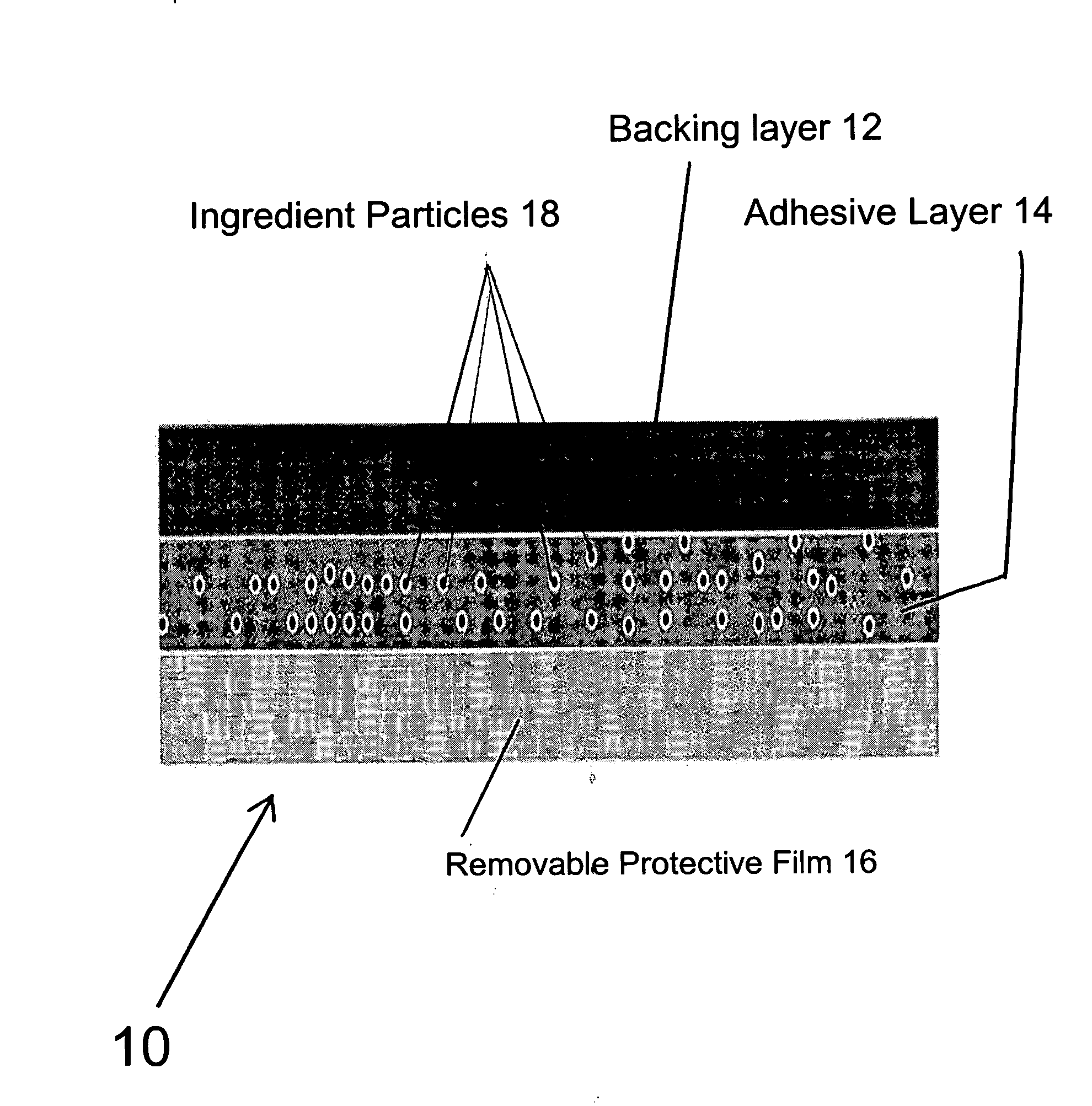
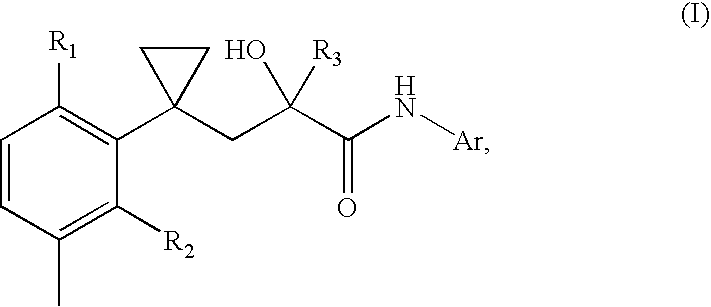
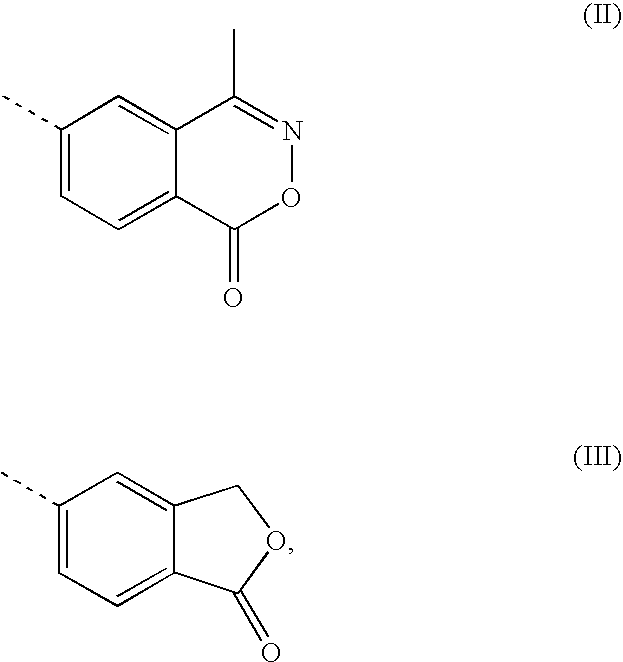
A transdermal patch for hormone therapy and fertility control has a backing layer, an effective-ingredient-containing adhesive layer adhering to the backing layer and a removable protective film. The adhesive layer includes a progestagenic effective ingredient and an estrogen in an adhesive matrix based on a silicone polymer, a polyisobutylene polymer (PIB), a polyacrylate polymer or a styrene block copolymer with butadiene or isoprene (SBS or SIS). The transdermal patch contains from 0.1 to 10%, based on a total weight of the adhesive matrix, of a progestagenic effective ingredient of formula I: wherein R1 and R2 each represent, independently of each other, H or F; R3 represents CH3 or CF3 and Ar is a group of formula II or III: or a pharmaceutically suitable derivative thereof.
Owner:SCHERING AG
Topical Delivery of a Nitric Oxide Donor to Improve and Skin Appearance
InactiveUS20080045909A1Promote absorptionGood lookingCosmetic preparationsToilet preparationsTransdermal patchSkin appearance
This invention generally relates to improvement of the body and skin appearance, for example enhancing the appearance of sagging, wrinkled, or cellulite-afflicted areas of the skin and body, through the local delivery of a nitric oxide donor, for example, using delivery vehicles such as lotions, creams, liquids, and / or transdermal patches. In some embodiments, a delivery vehicle containing a nitric oxide donor, for example, L-arginine (an important biological precursor) or its derivatives in a sufficient concentration to improve the appearance of a selected area of the body may be applied. In certain cases, one or more agents may also be included that aid in the transfer of the nitric oxide donor into the tissue, which may overcome the resistance to transfer into the skin. Non-limiting examples of suitable agents include agents able to create hostile biophysical environments, for instance, choline chloride, magnesium chloride, and / or sodium chloride.
Owner:STRATEGIC SCI & TECH
Transdermal drug patch with attached pocket for controlled heating device
InactiveUS20020004066A1Shorten the timeEasy to replaceElectrotherapyMedical devicesTransdermal patchDrug administration
The present invention relates to a transdermal drug delivery system comprising a dermal drug delivery patch and a heating element compartment securable to the dermal drug delivery patch. A freely transferrable heating element is securable within the heating element compartment. A drug can be administered transdermally using the present invention by placing the dermal drug delivery patch upon a patient's skin at an administration site. A heating element compartment is secured to the dermal drug delivery patch and a freely transferrable heating element is placed within the heating element compartment. The heating element provides controlled heat to the dermal drug patch and the patient's skin and thereby improves dermal drug administration.
Owner:ZARS INC
Chemical Combination and Method for Increasing Delivery of Coenzyme Q10
InactiveUS20110318405A1Reduce deliveryOvercome problemsBiocidePeptide/protein ingredientsTransdermal patchMedicine
The present invention relates to a chemical combination and method for increasing delivery of Coenzyme Q10. The chemical combination comprises Coenzyme Q10 mixed with at least one chemical. The at least one chemical includes cyclic terpene containing essential oil(s) that permit unprecedented levels of Coenzyme Q10 to be made available for delivery and absorption, increasing bioavailability, as well as overcoming the previous limits. A transdermal patch including a layer containing Coenzyme Q10 is also provided.
Owner:ERWIN CHARLES
Transdermal method and apparatus
Owner:ALDRED KATHERINE M
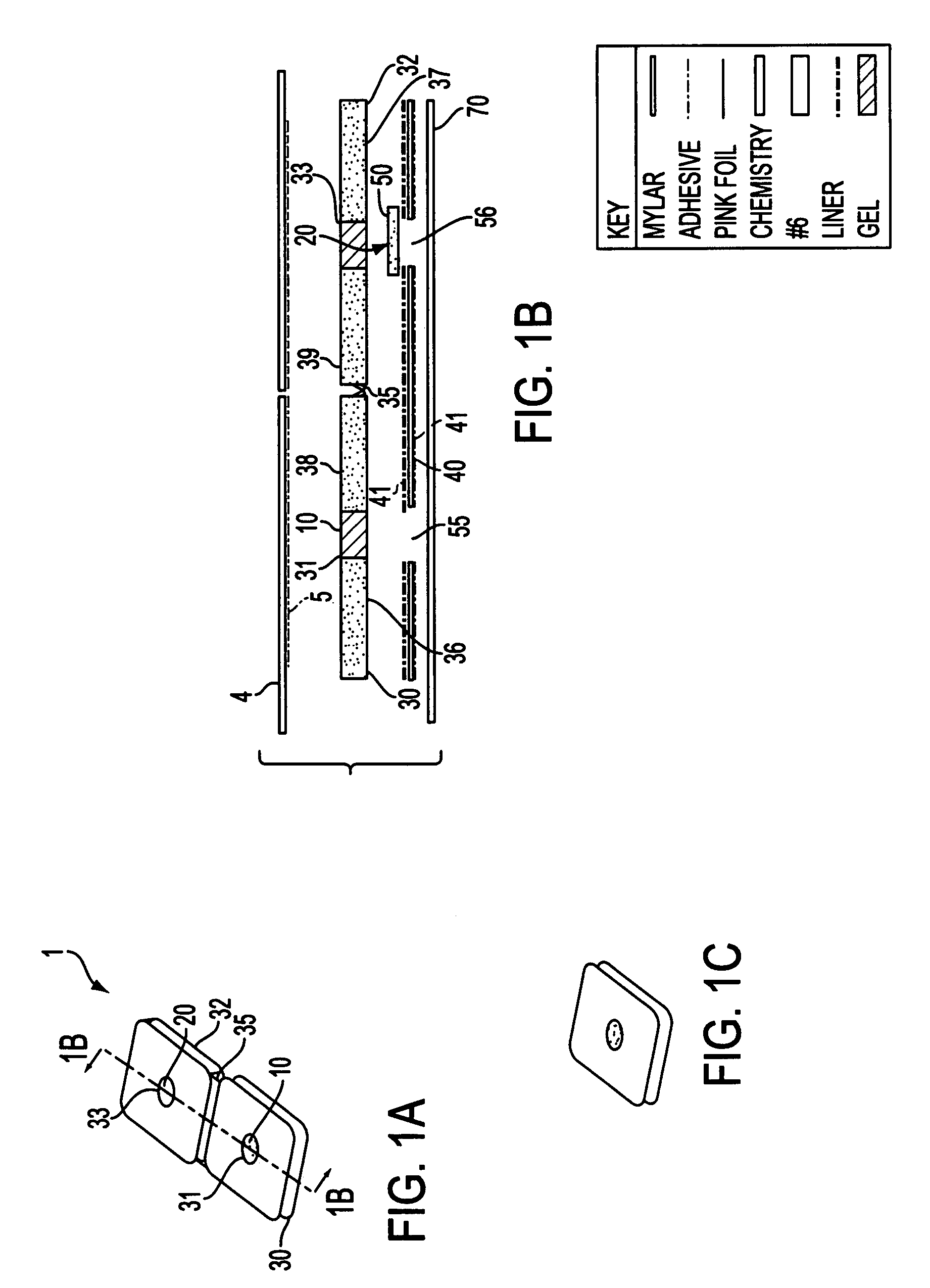
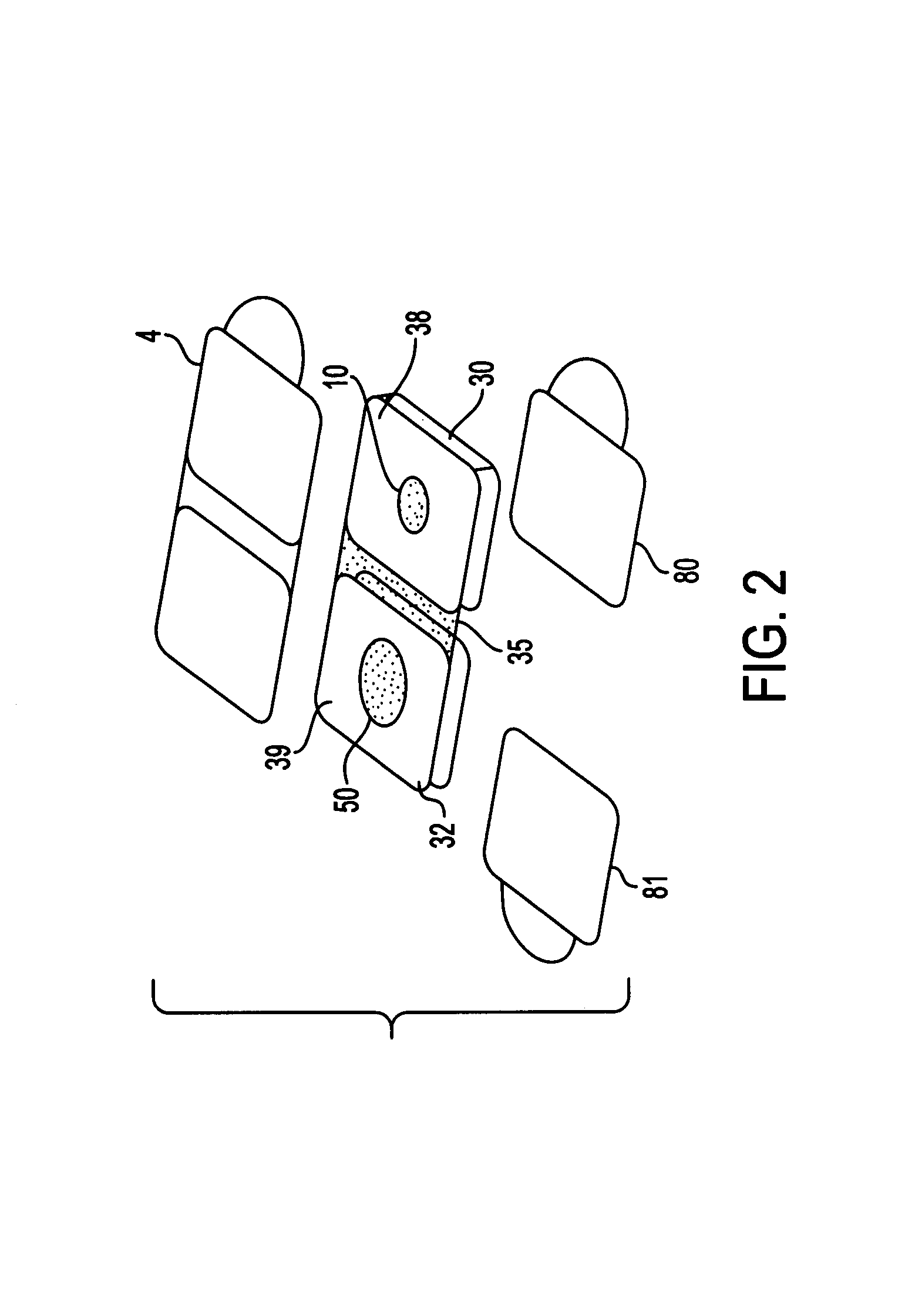
Noninvasive transdermal systems for detecting an analyte in a biological fluid and methods
InactiveUS7577469B1Easy to useImprove complianceInvestigating moving sheetsDiagnostic recording/measuringTransdermal patchColor changes
The present invention relates to noninvasive transdermal systems comprised of a noninvasive transdermal patch and a reflectometer. The noninvasive transdermal patches are comprised of a wet chemistry component and a dry chemistry component. The wet chemistry component is a liquid transfer medium in the form of a gel layer for the extraction and liquid bridge transfer of the analyte of interest from the biological fluid within or beneath the skin to the dry chemistry component. The dry chemistry component is a reagent system for interacting with the analyte of interest (glucose) to generate a color change. The reflectometers include a modulated light source for emitting light to illuminate a target surface which possesses a certain color and shade of color for detection by an optical detector. The output signal is processed for determining a corresponding quantity or quality measurement.
Owner:PLDHC ACQUISITIONS LLC
Transdermal matrix system
PCT No. PCT / FR96 / 01494 Sec. 371 Date Mar. 26, 1998 Sec. 102(e) Date Mar. 26, 1998 PCT Filed Sep. 25, 1996 PCT Pub. No. WO97 / 11687 PCT Pub. Date Apr. 3, 1997A novel transdermal matrix system for the percutaneous delivery of a hormone, including a carrier and an adhesive matrix, is disclosed. The matrix includes (a) 39-61 parts by weight of an ethylene / vinyl acetate copolymer, (b) 12-17 parts by weight of 2-octyldodecyl myristate, (c) 5-17 parts by weight of diethyl phthalate, (d) 10-16 parts by weight of a compound selected from N-alkyl-2-pyrrolidones, wherein the alkyl group is a C4-15 group, and (e) 1-12 parts by weight of at least one hormone selected from the group consisting of oestrogenic and progestogenic components. A method for preparing said transdermal matrix system and the therapeutical use of said system are also disclosed.
Owner:LABES DHYGIENE & DE DIETETIQUE L H D
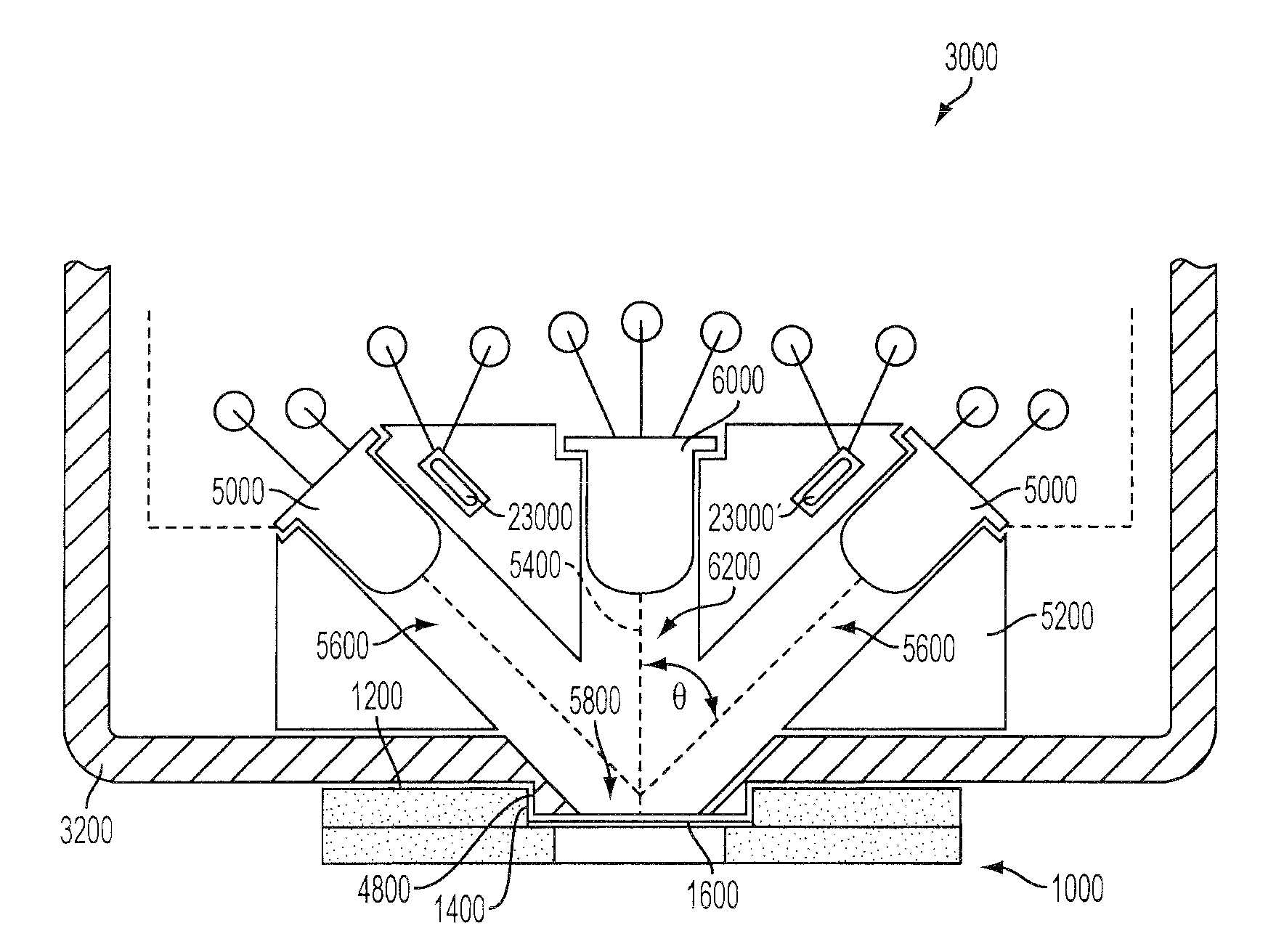
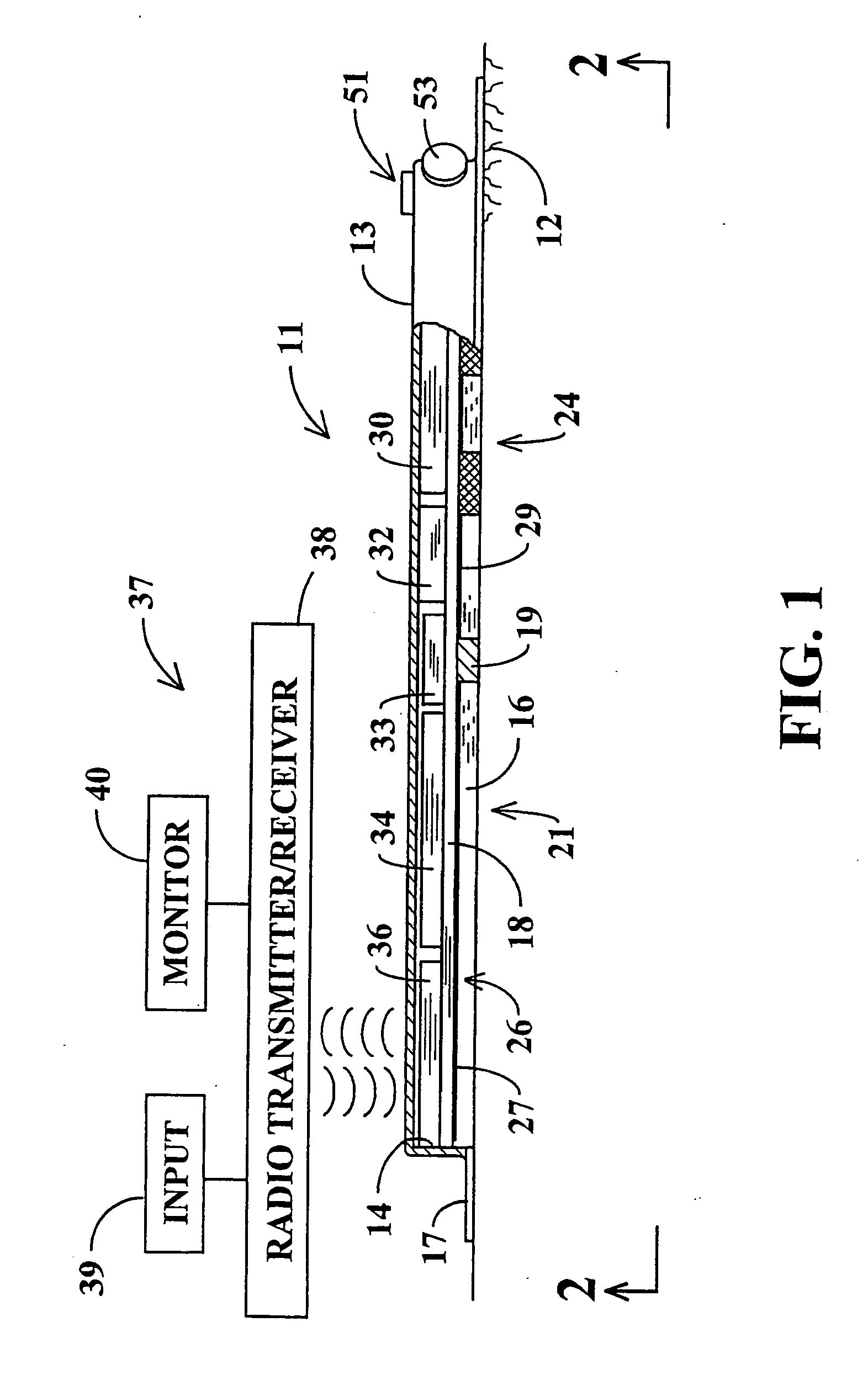
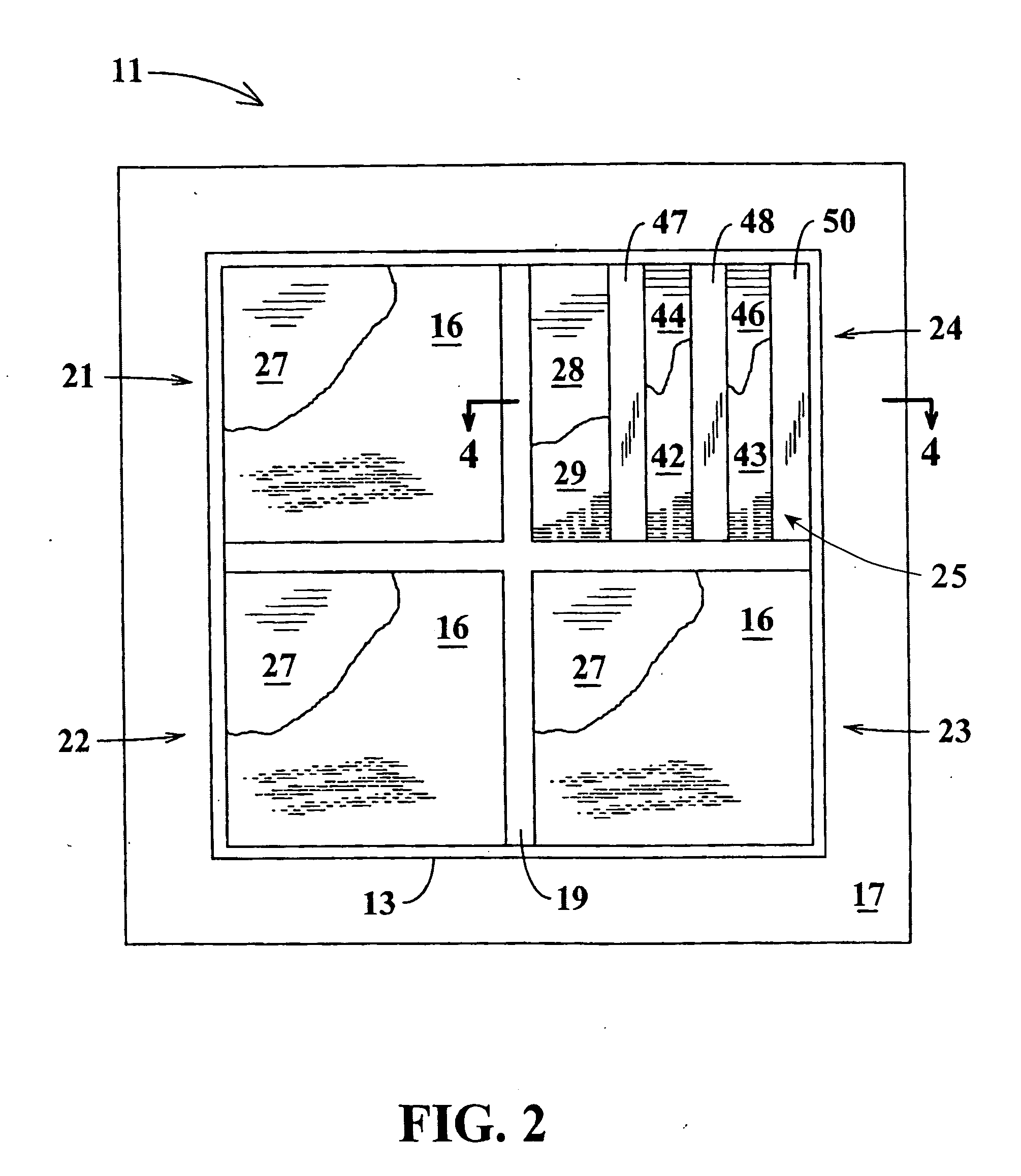
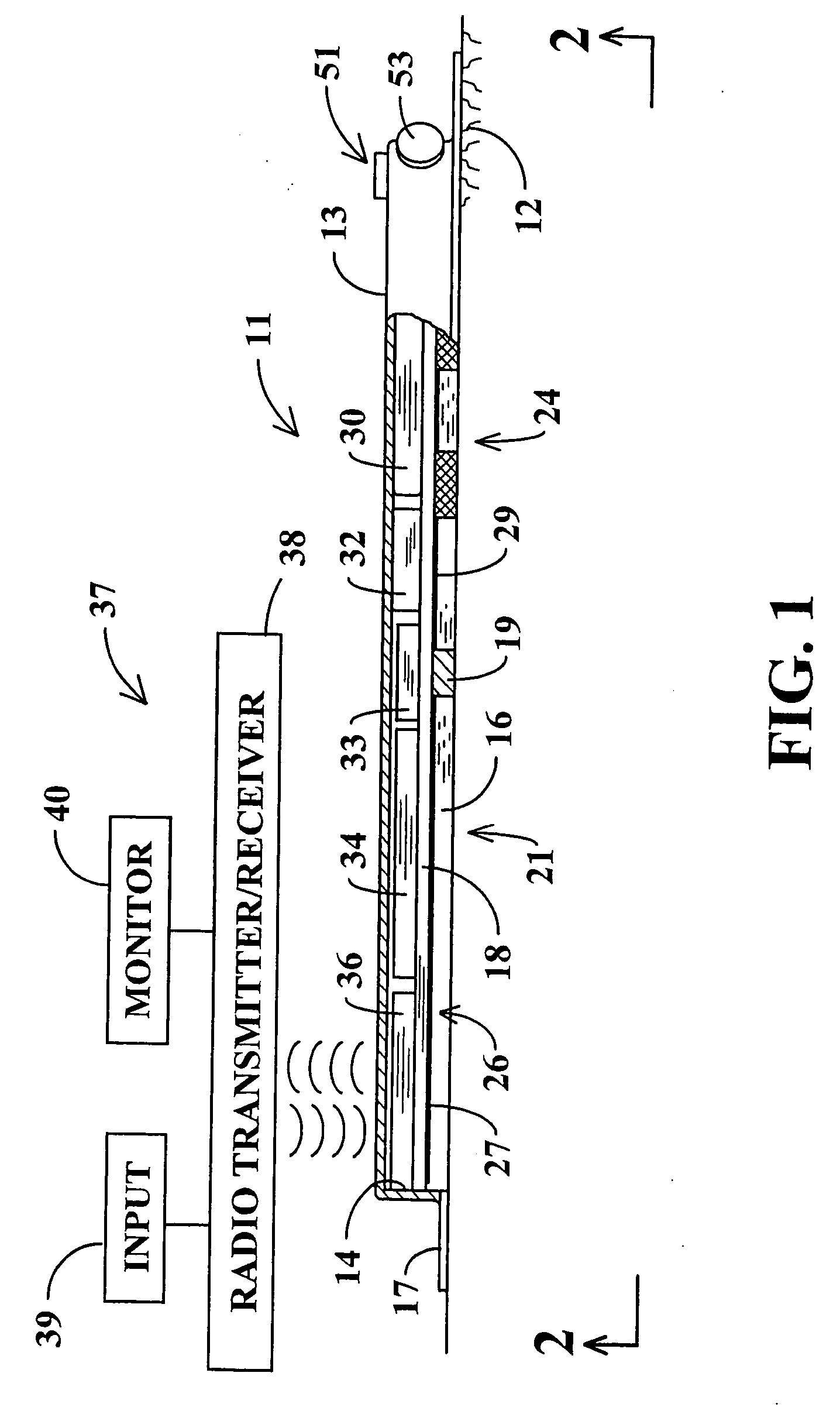
Non-invasive analysis and controlled dosage transdermal active patch
A programmable transdermal patch non-invasively delivers pharmaceuticals or other bio-active agents through the skin of a living body. The patch contains one or more agent storage pads and one or more active drivers that apply an electric current to the skin or produce ultrasound to drive the agent into the skin. A digital data processor controls the drivers to match administration of the agents to the needs of the body. The patch may contain a sensor, coupled to the data processor, for monitoring the concentration of a substance in the body in order to vary dosage of a therapeutic agent. A radio contained in the patch enables control by medical personnel from a remote location and / or transmission of sensor data to the remote location. The pads, drivers, sensor, data processor, radio and a battery are all contained within a unitary patch and need no physical connection to external devices.
Owner:KORTZEBORN ROBERT N
Drospirenone-containing preparations for transdermal use
InactiveUS20050222106A1Increase supersaturationReduce dosageOrganic active ingredientsAerosol deliveryTransdermal patchDrospirenone
The pharmaceutical preparation for transdermal administration contains solvent ingredients, such as water and ethanol and / or propanol, and drospirenone. The drospirenone is contained in the preparation in an amount that is not above its saturation solubility in an initial state prior to application to skin. However after application to the skin the amount of drospirenone exceeds its saturation solubility due to escape or discharge of the solvent ingredients from the preparation. Preferably the saturation solubility is exceeded by at least a factor of five during application to the skin. The pharmaceutical preparation can also contain an estrogen, such as ethinyl estradiol. It can be in the form of a semi-solid or liquid preparation that is contained in a reservoir-type transdermal patch. A transdermal patch for contraception containing the pharmaceutical preparation including drospirenone and ethinyl estradiol is also disclosed.
Owner:SCHERING AG
Transdermal drug patch
InactiveUS20010033858A1Sufficient transdermal permeabilityConstant concentrationNervous disorderSheet deliverySolubilityTransdermal patch
The present invention is directed toward a formulation for supplying additional drug for delivery in a transdermal drug delivery device. The invention comprises a drug, such as fentanyl that is capable of transdermal delivery, and a solution having a pre-designed solubility for the drug. The solution dissolves only a portion of said drug and allows a significant portion of the drug to remain undissolved in solution, thus providing extra drug to be delivered at a consistent, controlled delivery rate. The invention may used in conjunction with controlled heat.
Owner:ZARS INC
Transdermal patch containing rasagiline for treatment or prophylaxis of nervous system disease and its preparation process
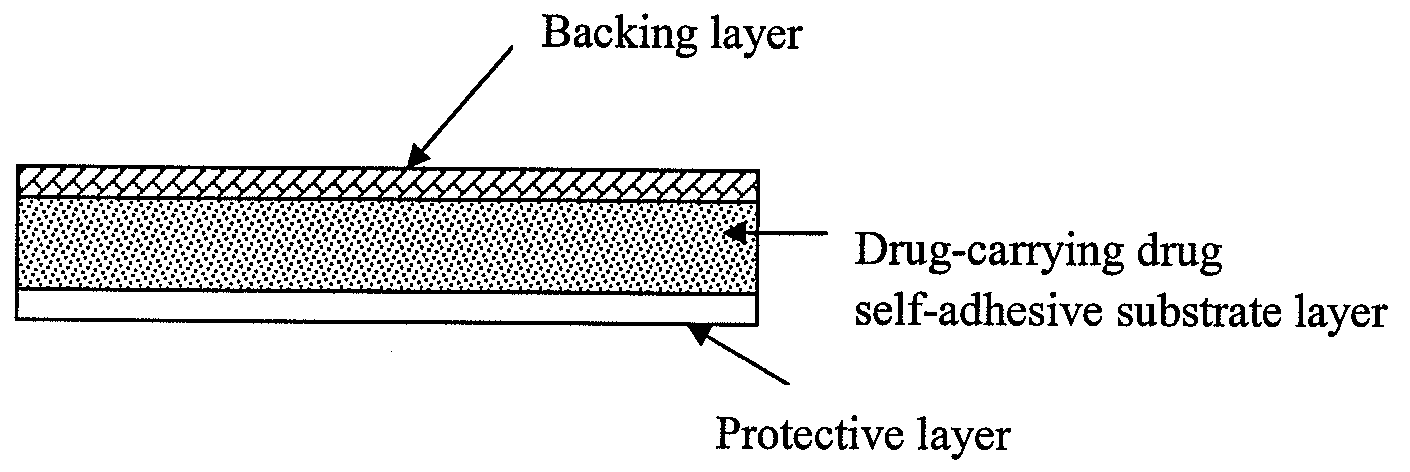
InactiveUS20090136549A1Improve skin penetrationTreatment or prophylaxis of nervous system diseasesBiocideOrganic active ingredientsTransdermal patchPharmacy
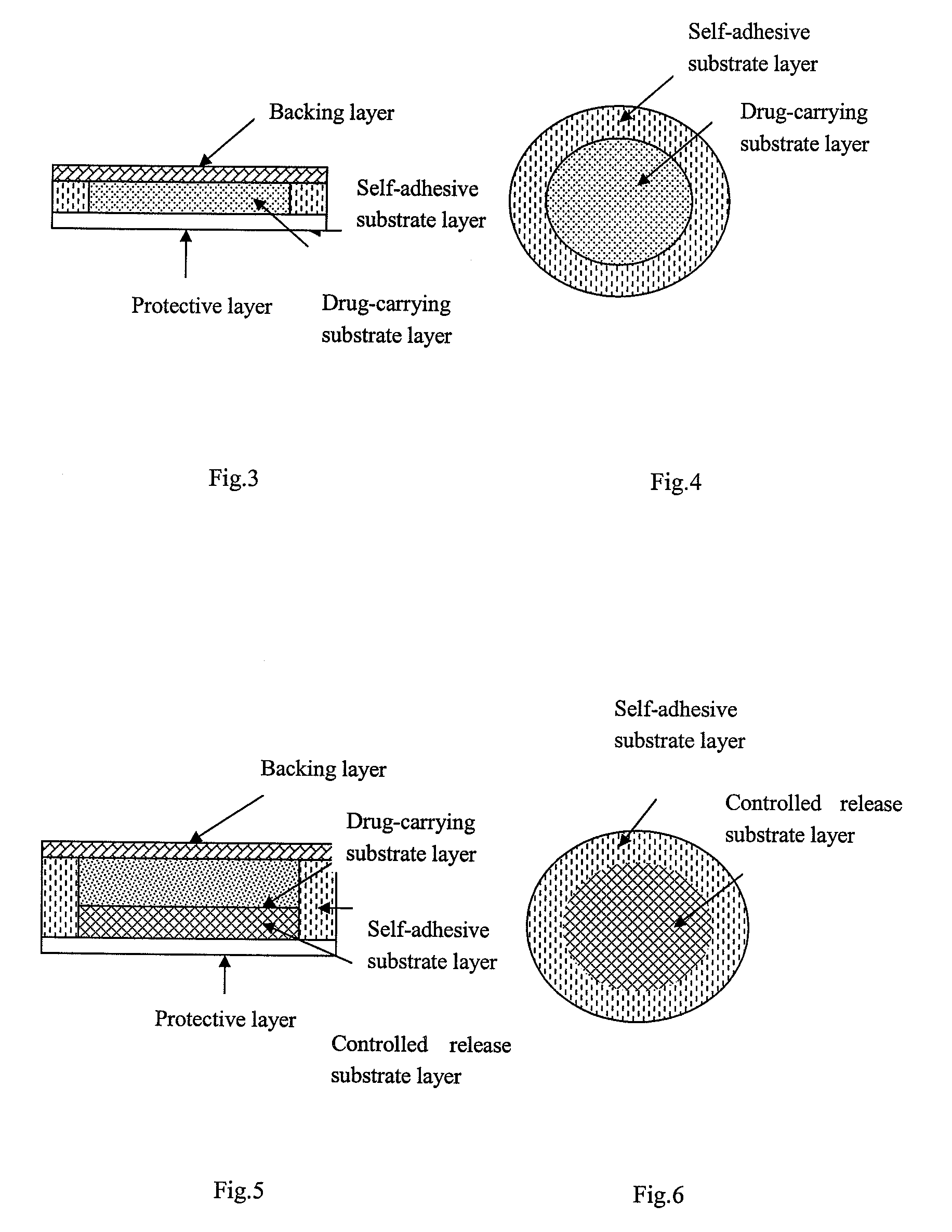
The present invention relates to a rasagiline transdermal patch for treatment or prophylaxis of nervous system diseases, in which the patch comprises an inert backing layer chemically inert to substrate ingredients, a substrate layer comprising rasagiline or a pharmaceutically acceptable salt thereof, and a protective layer to be peeled off before use. The substrate layer is an adhesive system comprising an organic polymer material as basis and an inorganic or organic material as filler, and a plurality of micro-reservoirs containing rasagiline. The substrate further comprises one or more substances for enhancing the transdermal absorption of rasagiline, in which the above organic polymer material in the substrate is used for the reservoir of rasagiline and as adhesive.
Owner:CHONGQING PHARMA RES INST +1
Topical delivery of a nitric oxide donor to improve body and skin appearance
InactiveUS20100196517A1Good lookingImprove the level ofBiocideCosmetic preparationsTransdermal patchSkin appearance
This invention generally relates to improvement of the body and skin appearance, for example enhancing the appearance of sagging, wrinkled, or cellulite-afflicted areas of the skin and body, through the local delivery of a nitric oxide donor, for example, using delivery vehicles such as lotions, creams, liquids, and / or transdermal patches. In some embodiments, a delivery vehicle containing a nitric oxide donor, for example, L-arginine (an important biological precursor) or its derivatives in a sufficient concentration to improve the appearance of a selected area of the body may be applied. In certain cases, one or more agents may also be included that aid in the transfer of the nitric oxide donor into the tissue, which may overcome the resistance to transfer into the skin. Non-limiting examples of suitable agents include agents able to create hostile biophysical environments, for instance, choline chloride, magnesium chloride, and / or sodium chloride.
Owner:STRATEGIC SCI & TECH
Transdermal methods and systems for the delivery of Anti-migraine compounds
Iontophoretic patches for the delivery of anti-migraine compounds and methods of using the patches are described.
Owner:TEVA PHARMACEUTICALS INTERNATIONAL GMBH
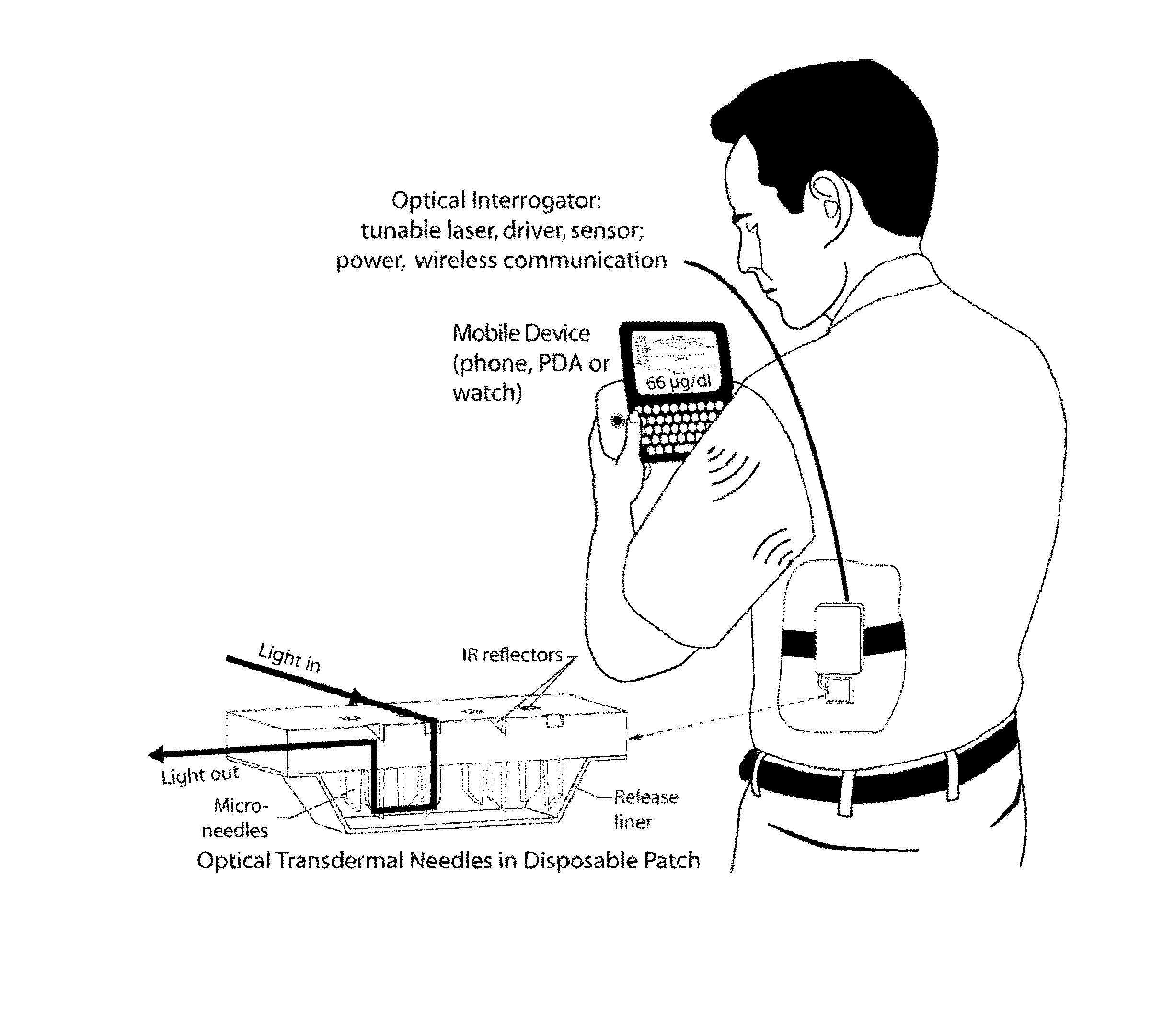
Optical microneedle-based spectrometer
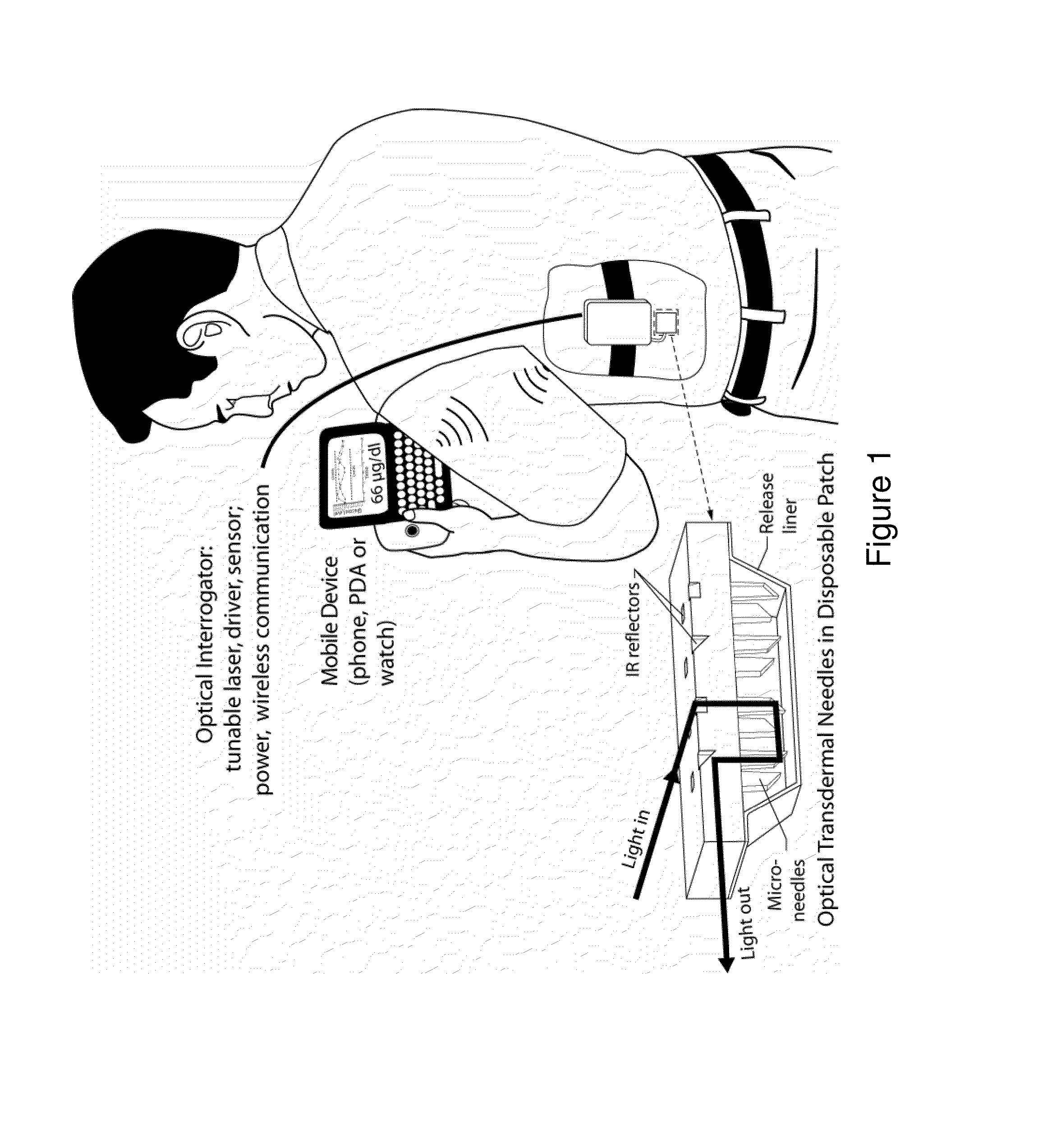
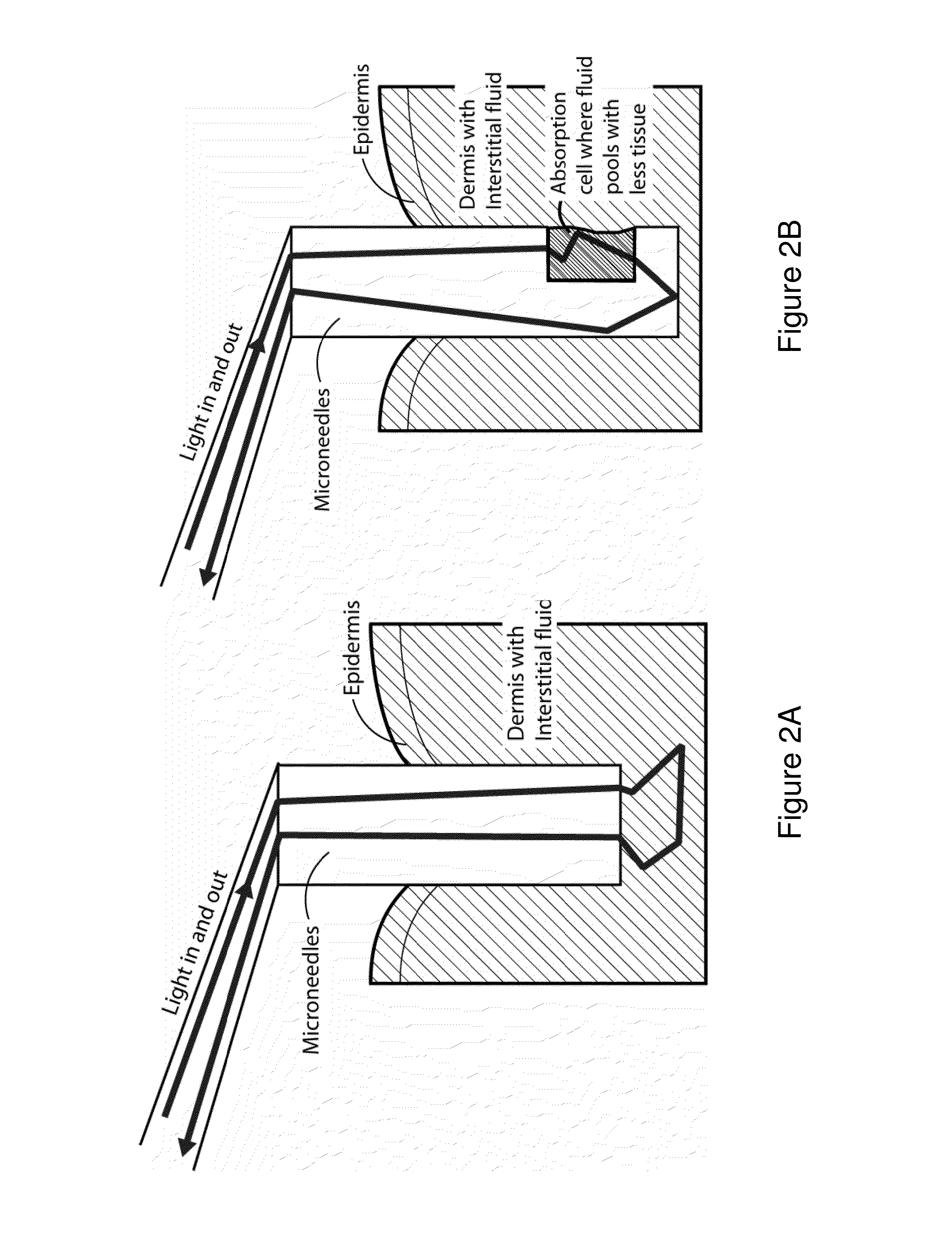
InactiveUS8452356B2Improved signal-to-noise ratio performanceImprove performanceCatheterDiagnostic recording/measuringTransdermal patchMid infrared
Optical microneedles are adapted for near-infrared or mid-infrared in vivo spectroscopic sensing; and provide a MEMS-based spectrometer for continuous lactate and glucose monitoring by means of a near-infrared or mid-infrared optical microneedle array in a transdermal patch.
Owner:SRI INTERNATIONAL
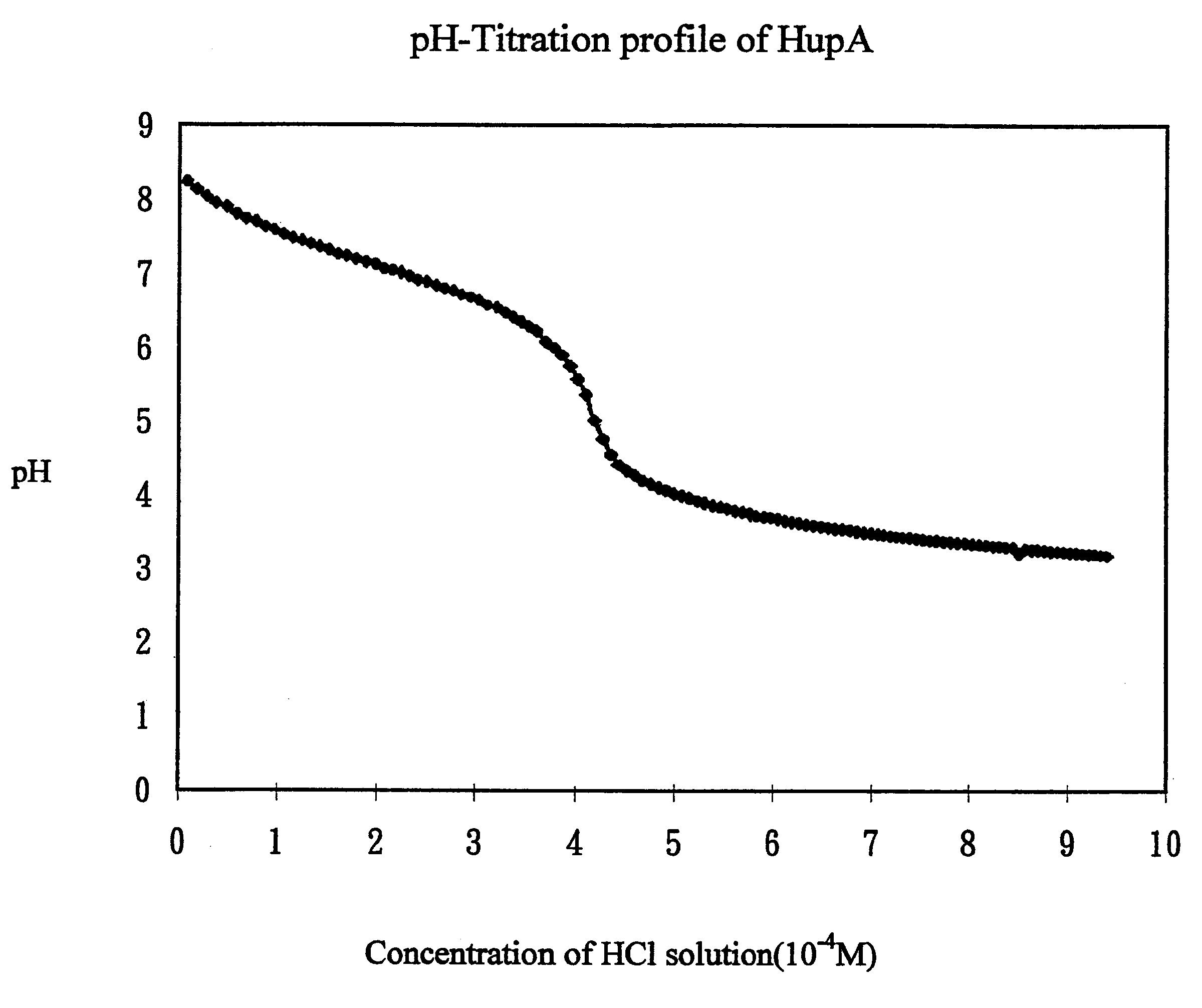
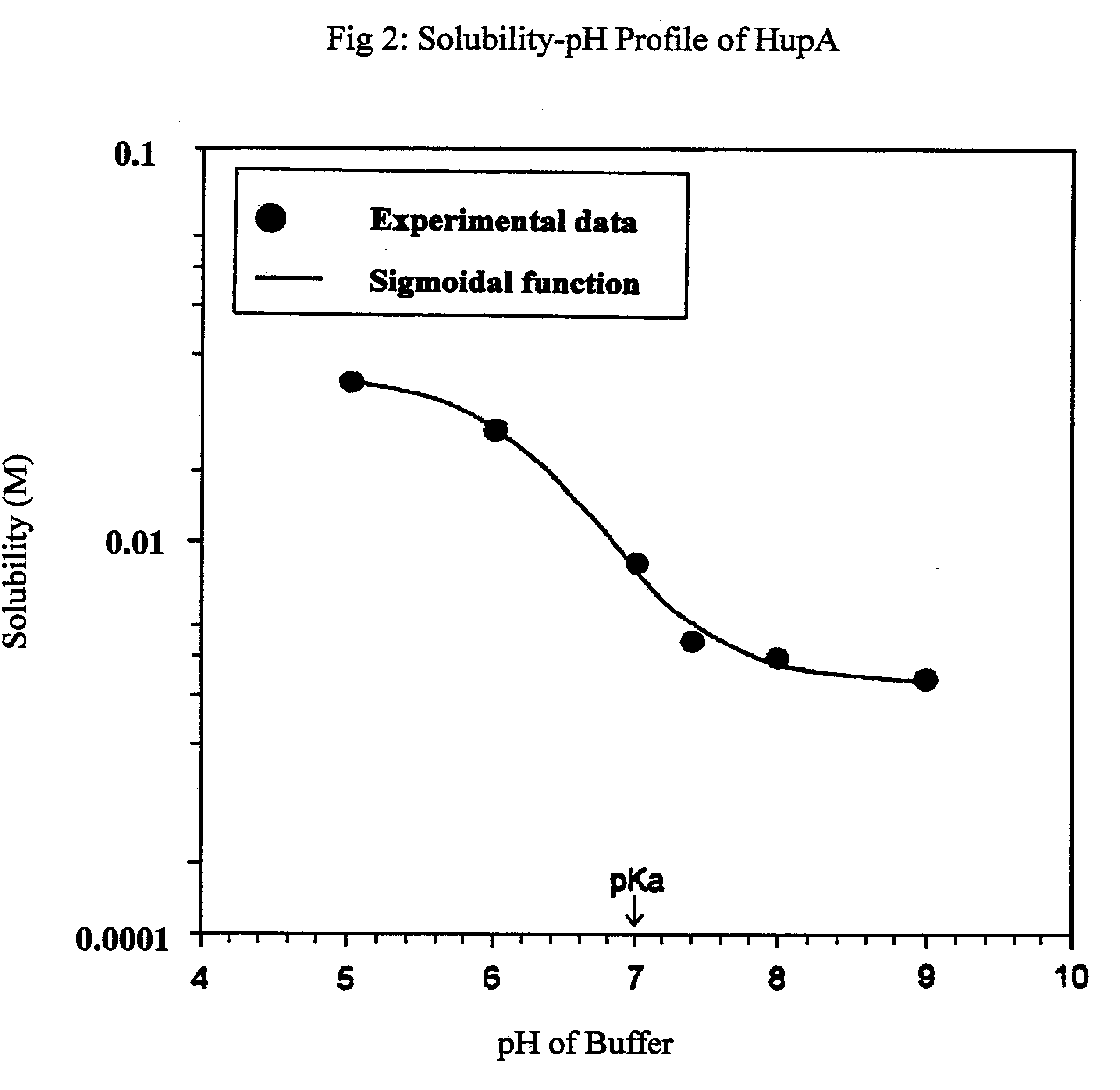
Transdermal rate-controlled delivery of Huperzine A for treatment of alzheimer's disease
This invention relates to a novel transdermal drug delivery system whereby Huperzine A ("Hup A"), a naturally occurred Acetylcholine esterase inhibitor traditionally used to alleviate memory problem, is formulated for transdermal administration suitable for the treatment of Alzheimer's Disease ("AD") to increase the efficacy and convenience for outpatient care of AD patients. A controlled-release skin patch designed for once-a-week application of Hup A is provided for easy AD medication according to the invention.
Owner:SAGITTARIUS LIFE SCI
Debridement Method Using Topical Nitric Oxide Donor Devices and Compositions
ActiveUS20110033437A1Easy to disassembleBiocidePeptide/protein ingredientsTransdermal patchNitric oxide
The present invention relates to methods for removal of dead tissue from wounds or skin using topical nitric oxide donor devices and / or compositions. In one embodiment, the present invention relates to a transdermal patch device that is designed to deliver nitric oxide. In another embodiment, the present invention relates to one or more devices and / or compositions that are designed to deliver nitric oxide and optionally one or more other compounds, where such devices / compositions include, without limitation, bandages, layered bandages, adhesive bandages, transdermal patches, creams, ointments, or a any combination of two or more thereof.
Owner:KCI USA +1
Non-invasive analysis and controlled dosage transdermal active patch
A programmable transdermal patch non-invasively delivers pharmaceuticals or other bio-active agents through the skin of a living body. The patch contains one or more agent storage pads and one or more active drivers that apply an electric current to the skin or produce ultrasound to drive the agent into the skin. A digital data processor controls the drivers to match administration of the agents to the needs of the body. The patch may contain a sensor, coupled to the data processor, for monitoring the concentration of a substance in the body in order to vary dosage of a therapeutic agent. A radio contained in the patch enables control by medical personnel from a remote location and / or transmission of sensor data to the remote location. The pads, drivers, sensor, data processor, radio and a battery are all contained within a unitary patch and need no physical connection to external devices.
Owner:KORTZEBOM ROBERT N
Product comprising a nicotine-containing material and an Anti-cancer agent
InactiveUS20140088045A1Prevention reductionReduce riskSmall article dispensingOrganic active ingredientsTransdermal patchAnticarcinogen
The present invention provides a composition comprising a nicotine-containing material and an anti-cancer agent usable in the treatment and / or prevention or reduction of the risk of cancer and precancerous conditions as well as for preventing or reducing the risk of cancer recurrence. Furthermore, a composition comprising a nicotine-containing material and an anti-inflammatory agent usable in the treatment and / or prevention or reduction of the risk of inflammation, is provided. The nicotine containing composition can also include both an anti-cancer agent and an anti-inflammatory agent A device for administering the composition of the present invention to subjects can be a cigarette, smoking pipe, smokeless tobacco, electronic cigarette, transdermal patch or the like.
Owner:RIGAS BASIL +1
Transdermal patch
InactiveUS20090258063A1Diffusibility is increasedSufficient amountOrganic active ingredientsUrinary disorderTransdermal patchCarbon number
The present invention provides a transdermal patch having excellent preservation stability and transdermal absorbency of DMAEs. The patch has a support and a plaster layer integrally laminated on one surface of the support, and the plaster layer includes: DMAEs; an acrylic adhesive prepared by copolymerizing monomers respectively containing 30 to 99% by weight of alkyl methacrylate having an alkyl group with a carbon number of 6 to 22 and 1 to 70% by weight of alkyl acrylate having an alkyl group with a carbon number of 2 to 20; and fatty acid ester prepared by dehydro-condensing saturated fatty acid having an alkyl group with a carbon number of 10 to 20 and saturated aliphatic monohydric alcohol having an alkyl group with a carbon number of 2 to 20, wherein solubility of the DMAEs is 0.05 to 5 g at 25° C. with respect to the fatty acid ester.
Owner:SEKISUI CHEM CO LTD
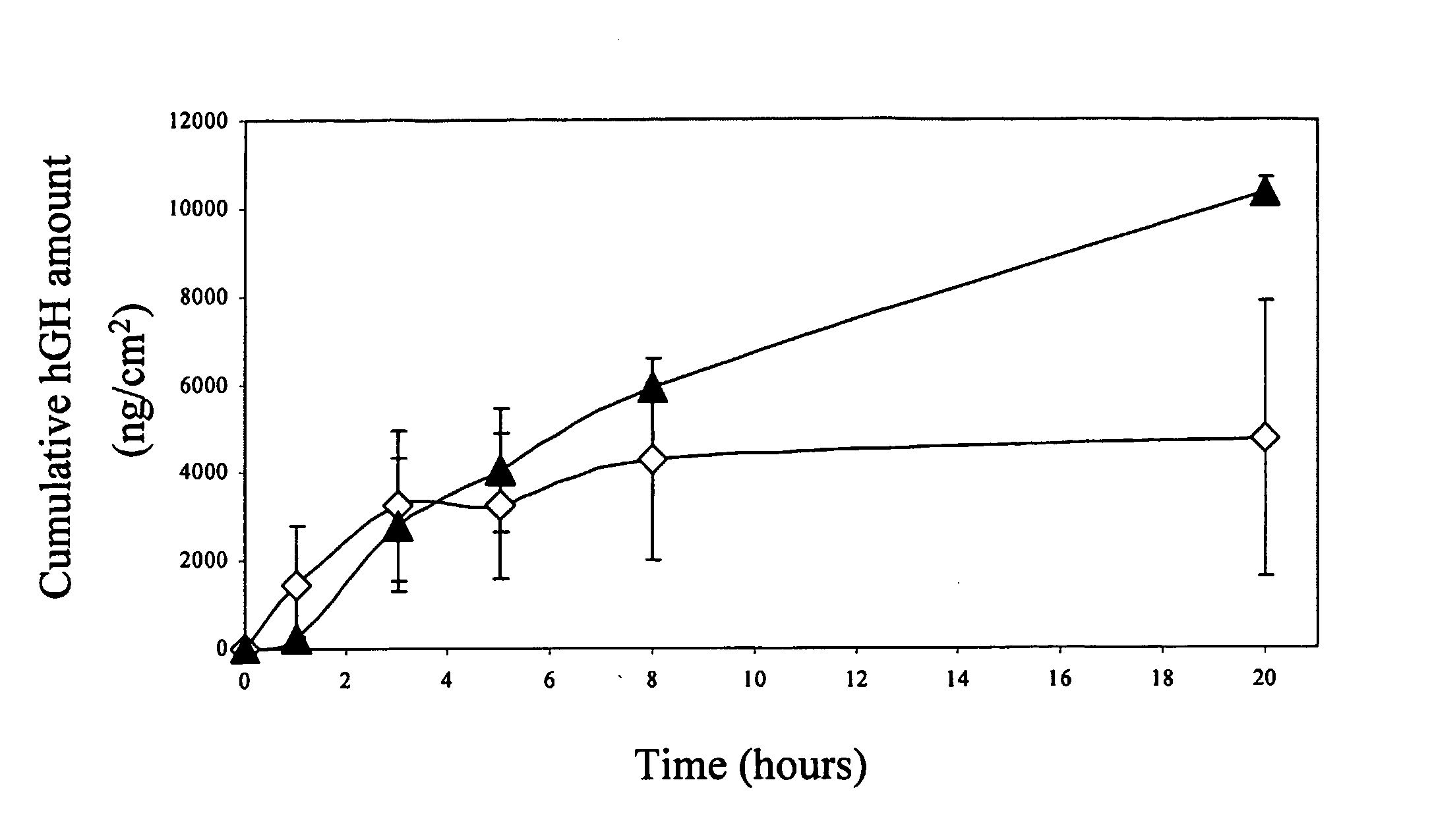
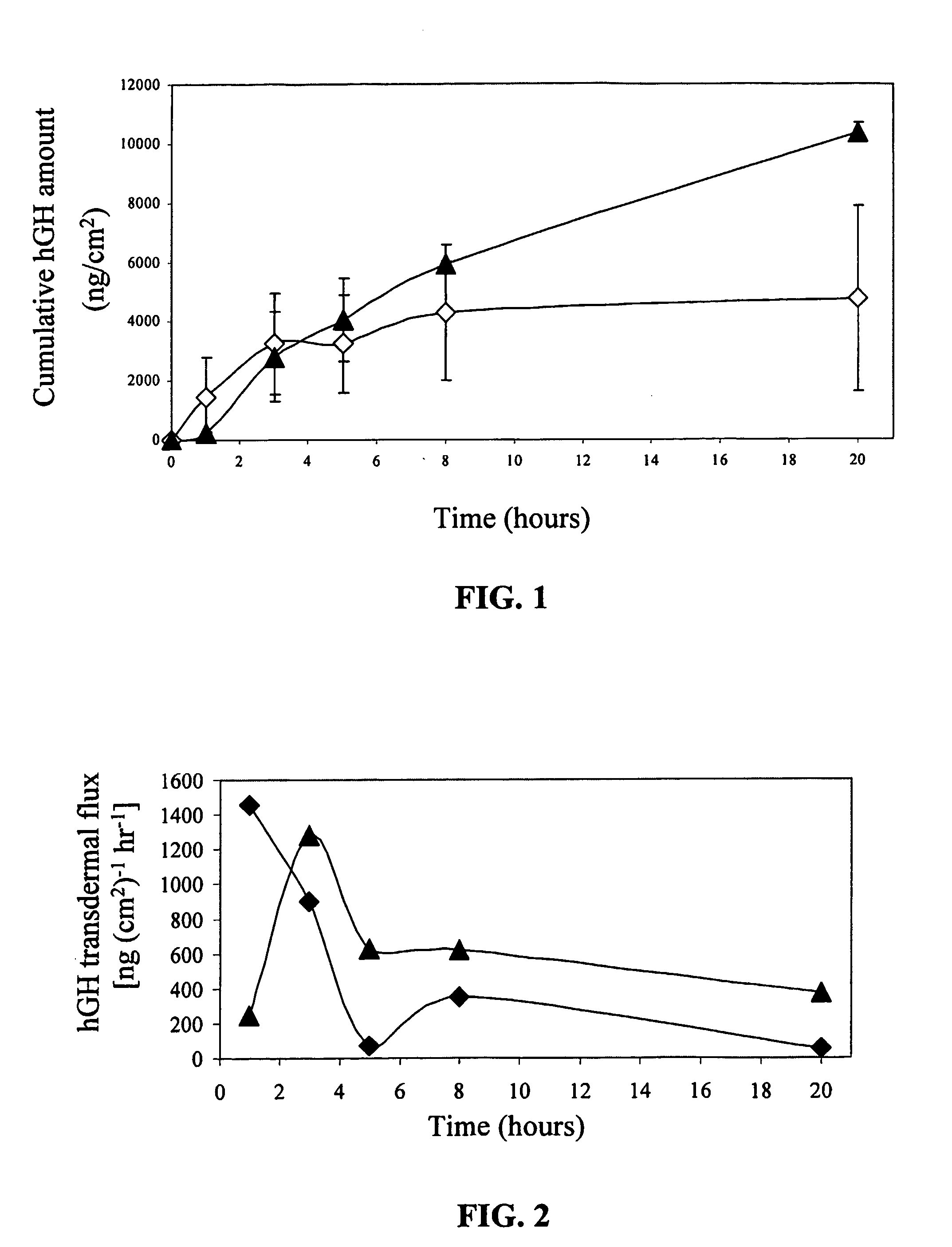
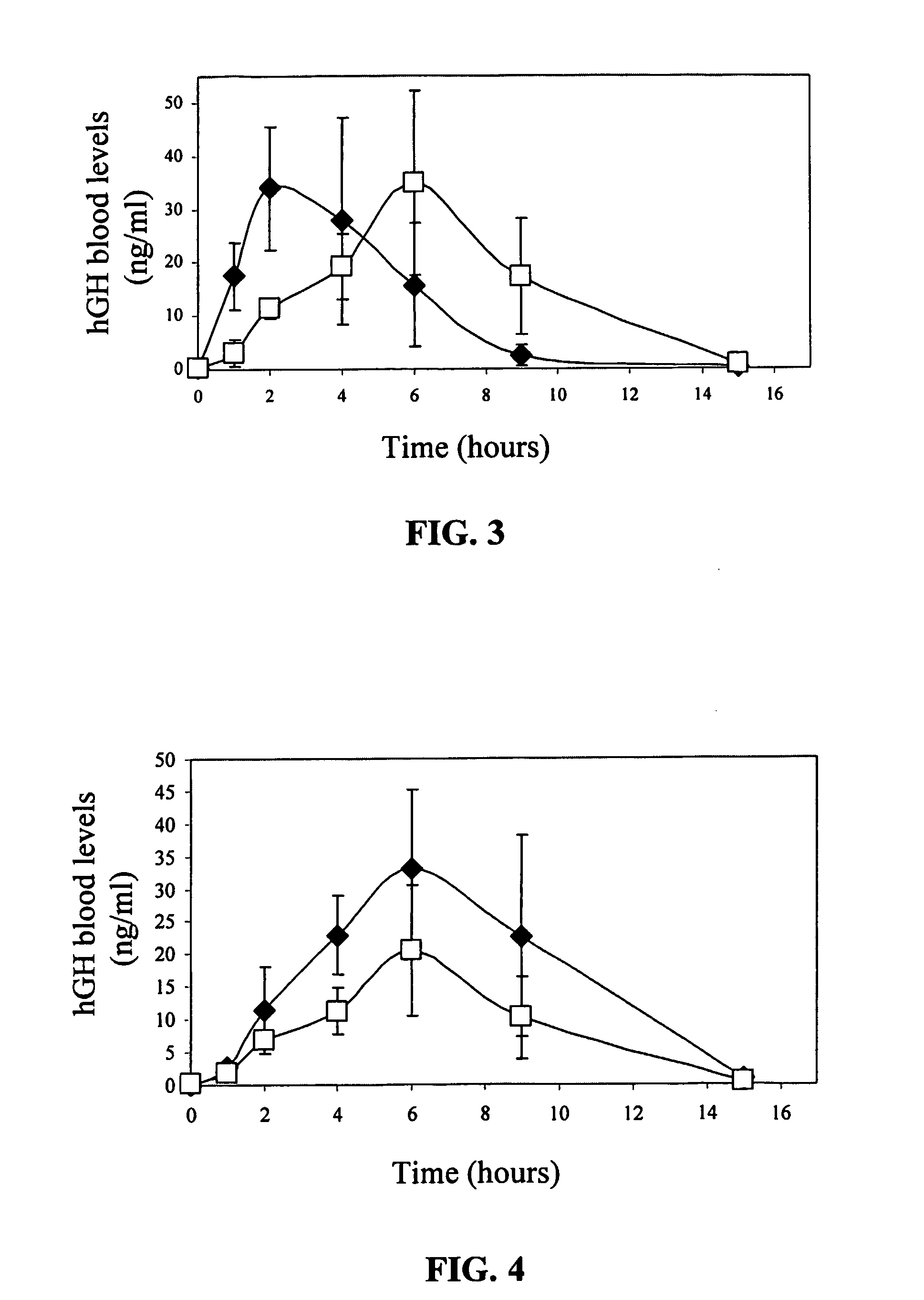
Transdermal System for Sustained Delivery of Polypeptides
InactiveUS20070287949A1Slow and sustained deliveryEffective levelingPowder deliveryElectrotherapyTransdermal patchDrug reservoir
A transdermal system for sustained delivery of high molecular weight hydrophilic drugs, especially peptide-, polypeptide- or protein-drugs, and methods of use thereof, are provided. The system includes an apparatus that generates micro-channels in the skin of a subject in combination with a transdermal patch comprising at least one drug reservoir layer comprising a polymeric matrix and a therapeutic or immunogenic peptide, polypeptide, or protein. The system provides sustained delivery of therapeutic or immunogenic agents, thereby achieving sustained therapeutic blood concentrations of these agents.
Owner:SYNERON MEDICAL LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com