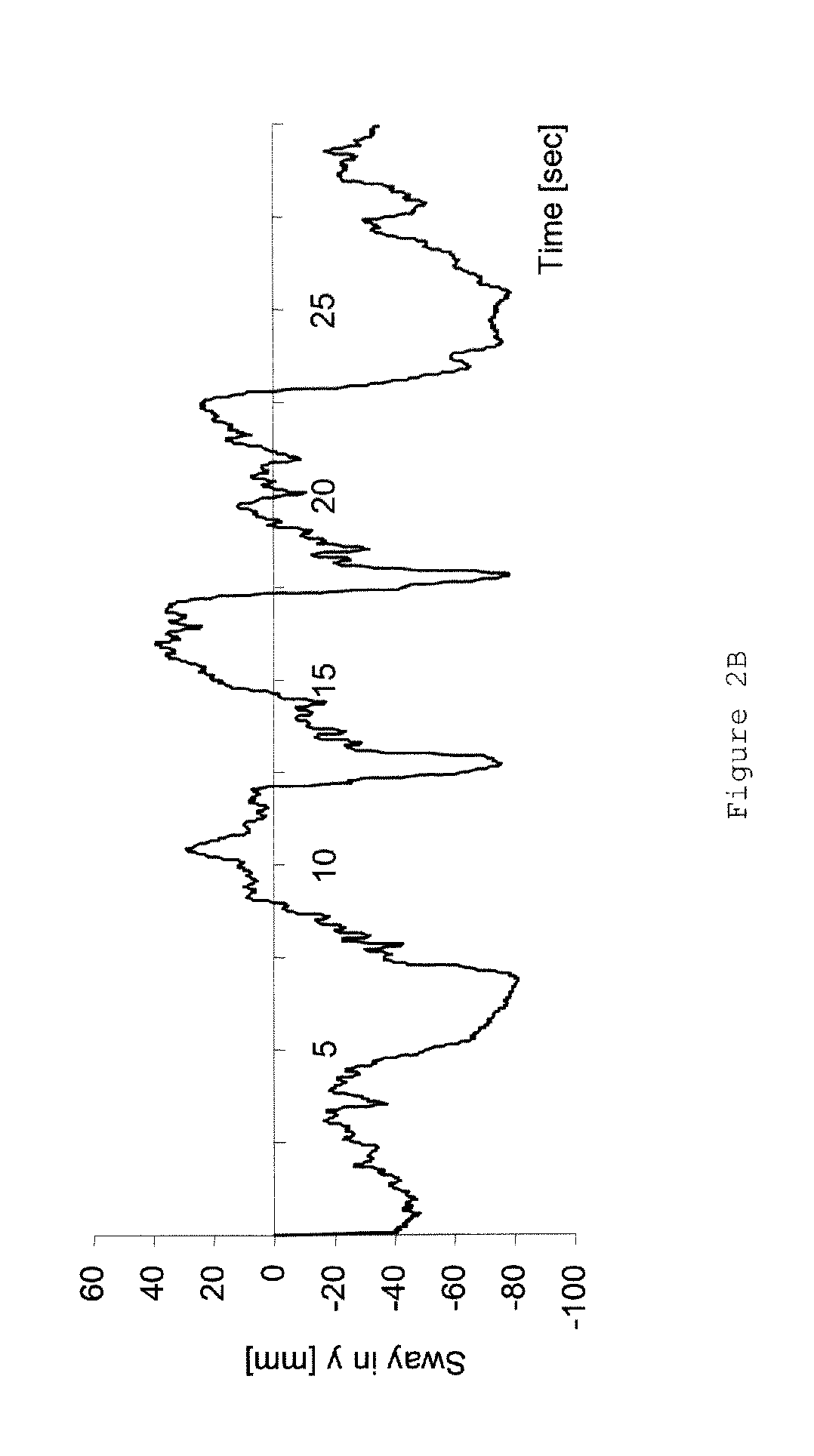
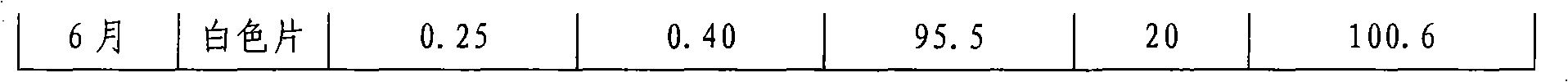Patents
Literature
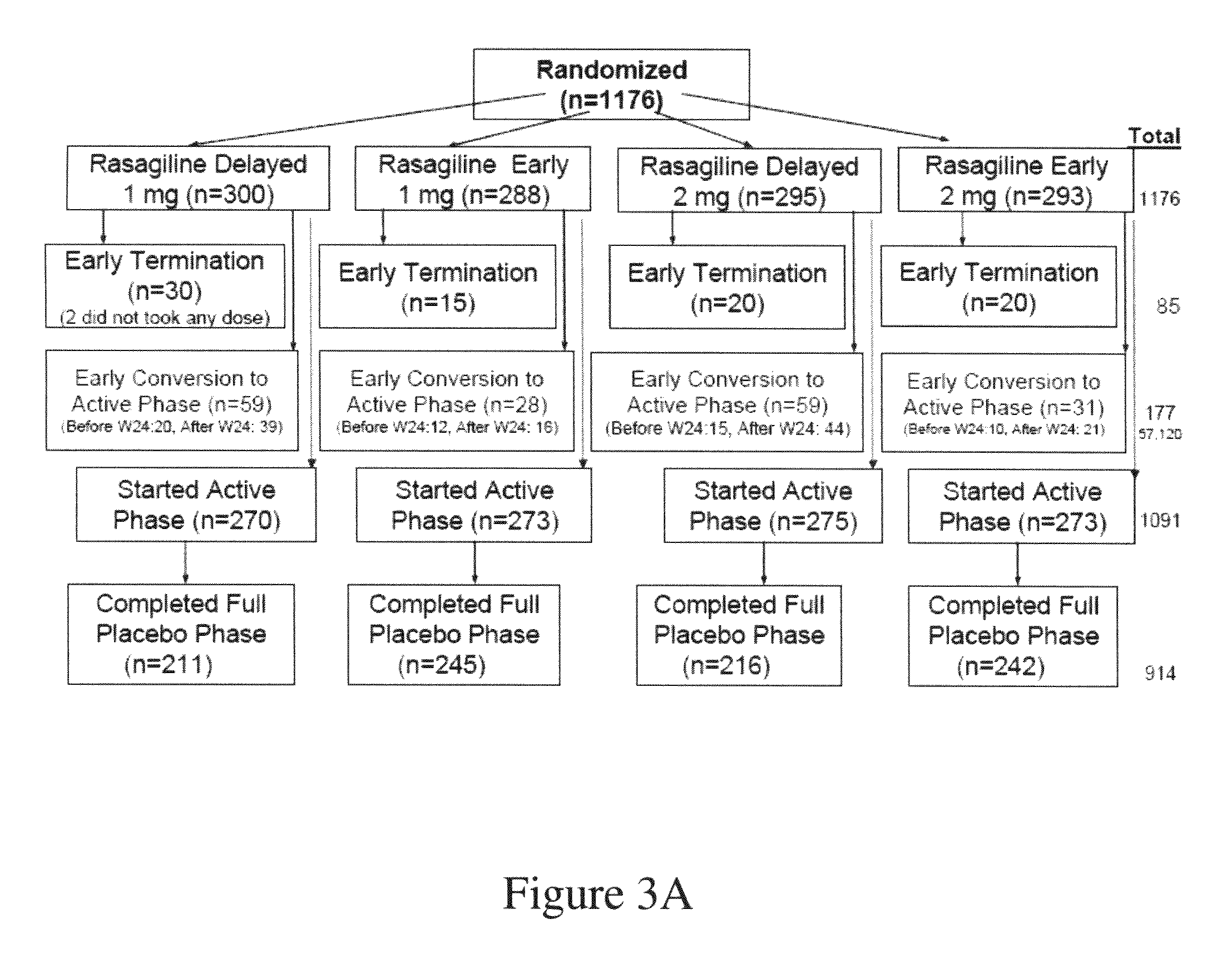
84 results about "Rasagiline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
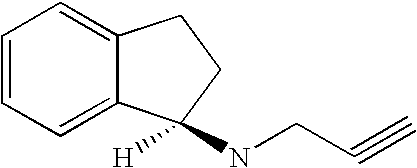
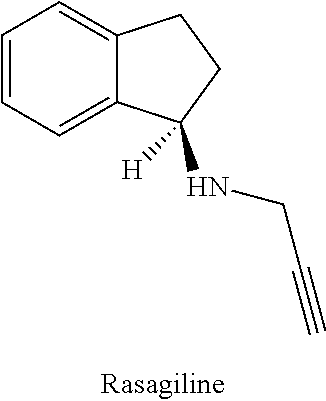
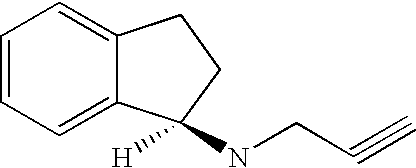
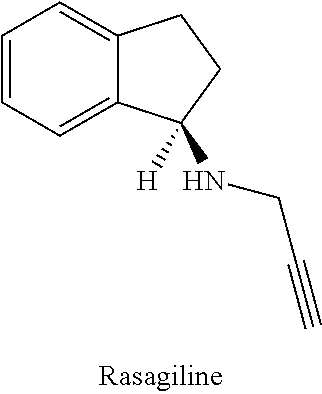
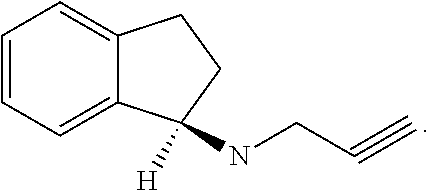
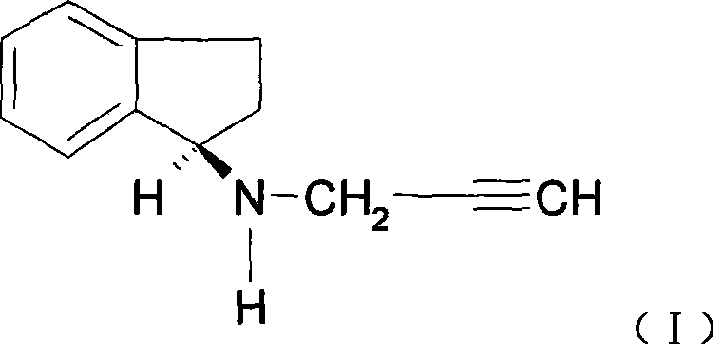
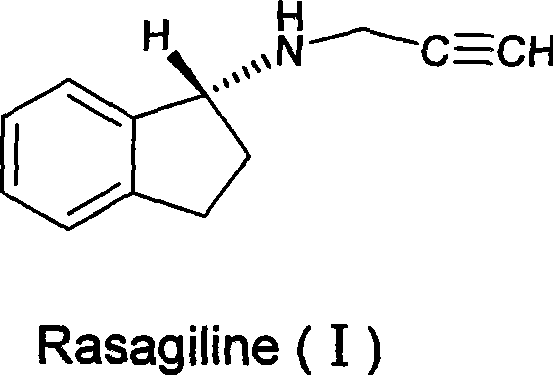
Rasagiline is used alone or with other medications (such as levodopa/carbidopa) to treat symptoms of Parkinson's disease.
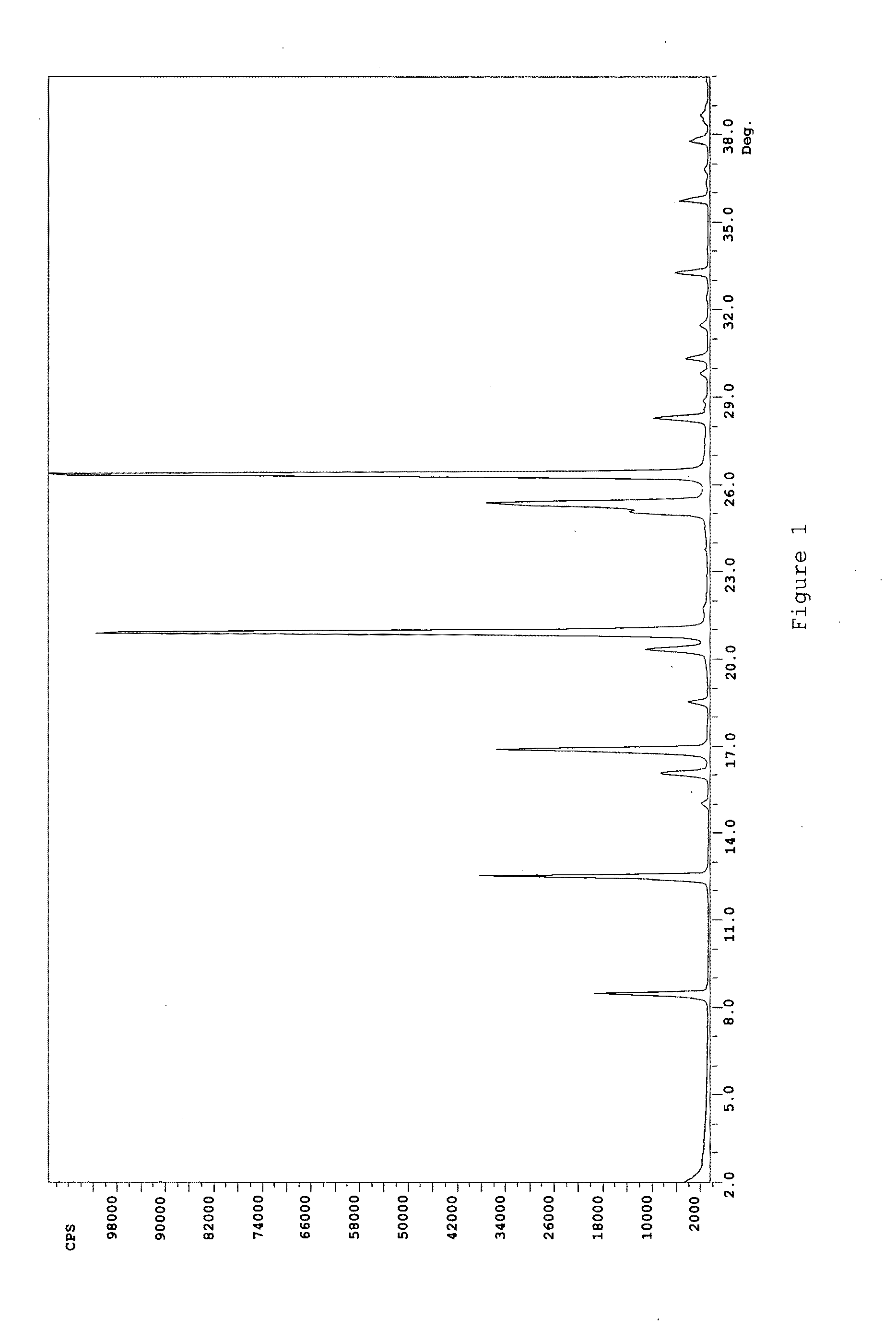
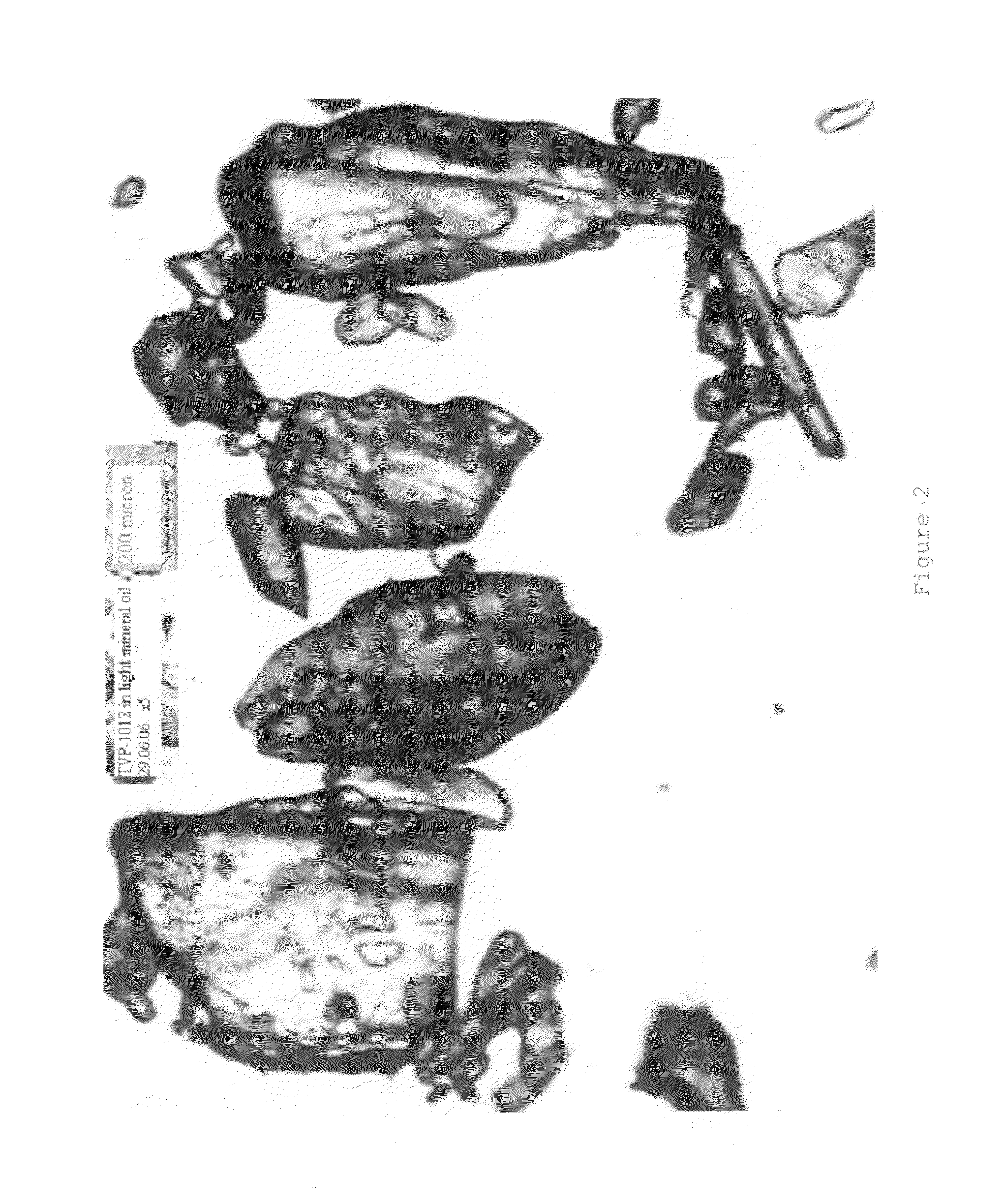
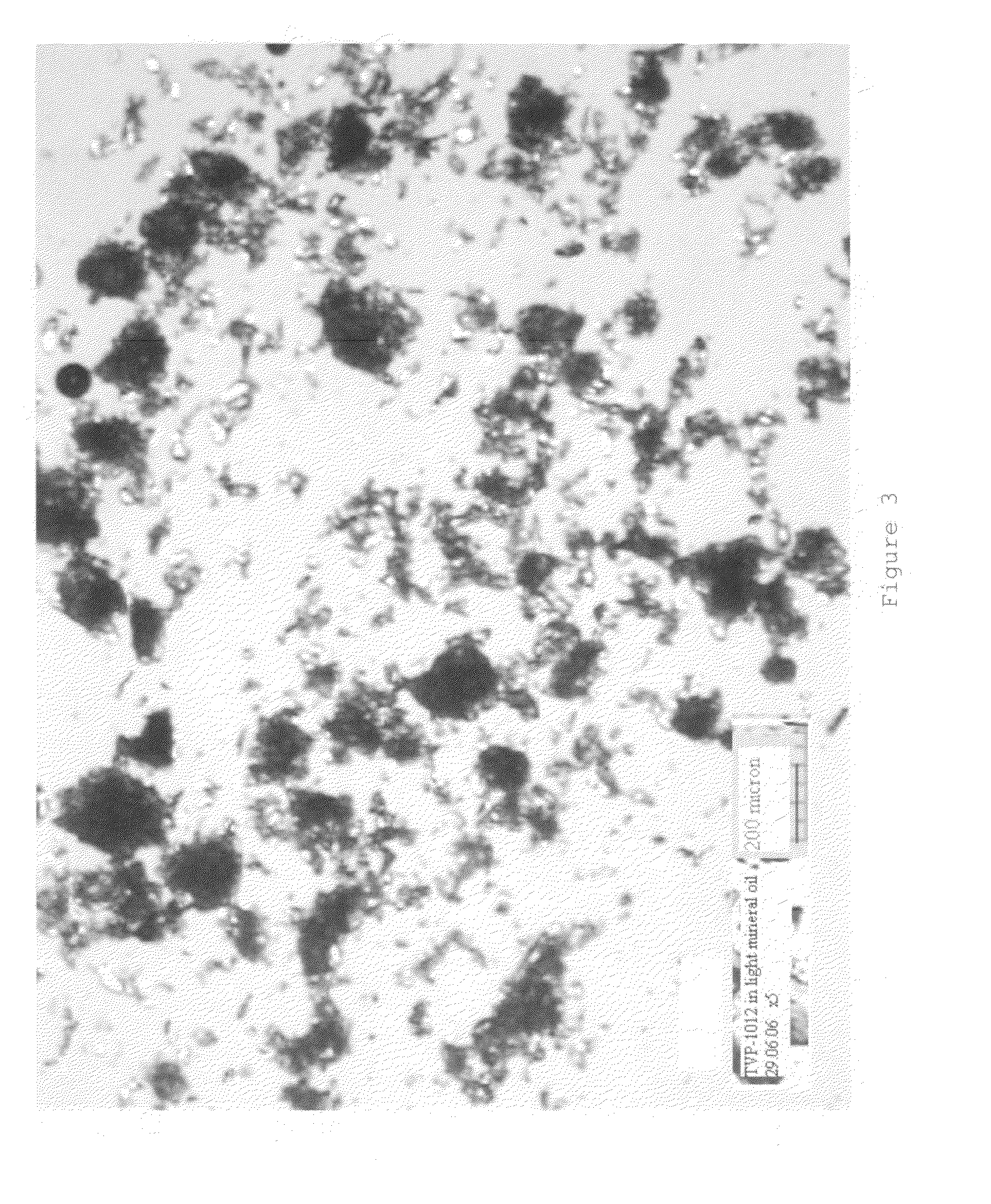
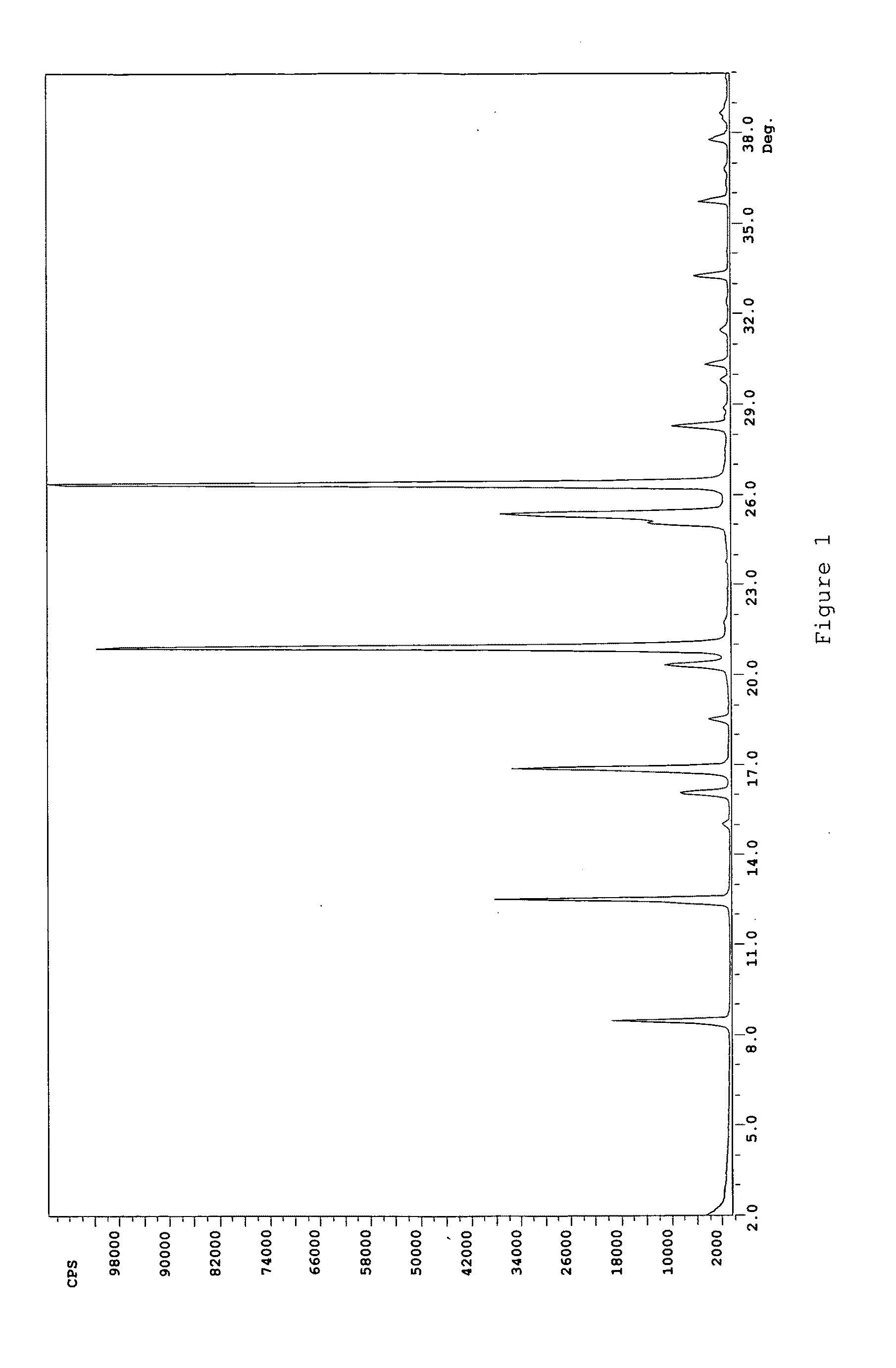
Rasagiline formulations of improved content uniformity
ActiveUS20060188581A1Good content uniformityImprove uniformityOrganic active ingredientsBiocideN-propargyl1-aminoindan
Owner:TEVA PHARMA IND LTD
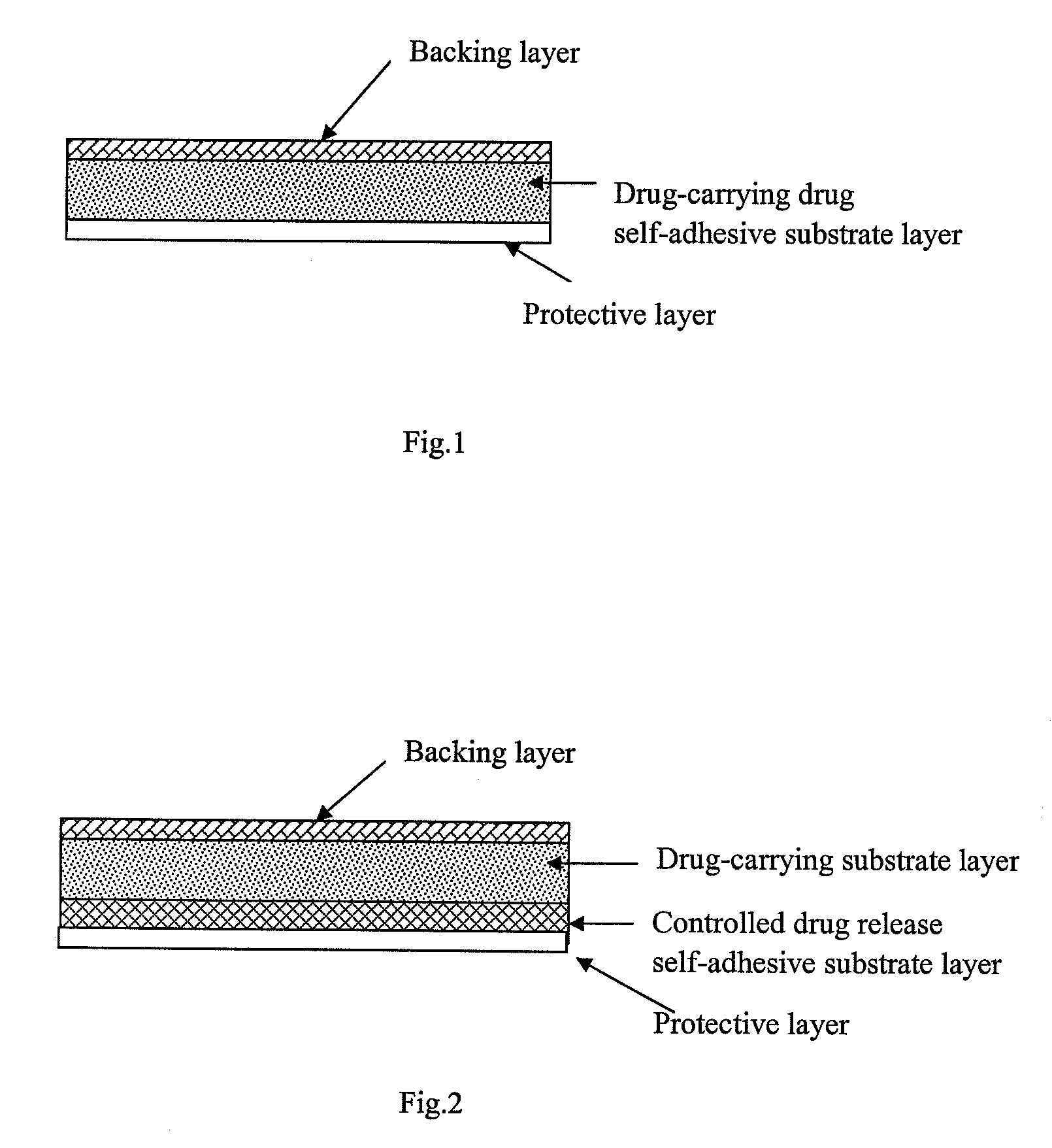
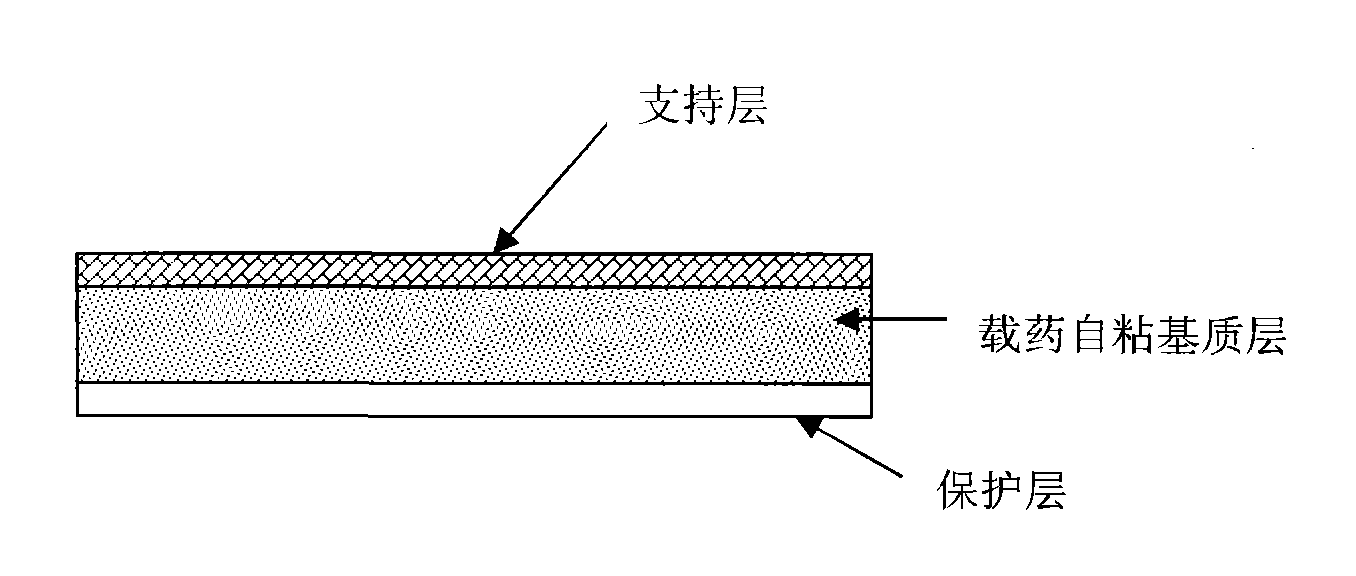
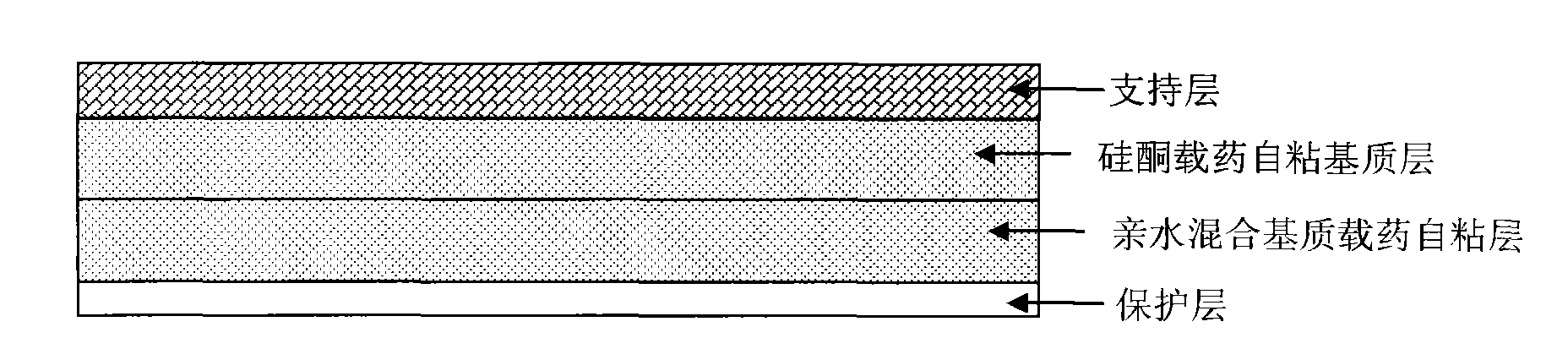
Transdermal patch containing rasagiline for treatment or prophylaxis of nervous system disease and its preparation process
InactiveUS20090136549A1Improve skin penetrationTreatment or prophylaxis of nervous system diseasesBiocideOrganic active ingredientsTransdermal patchPharmacy
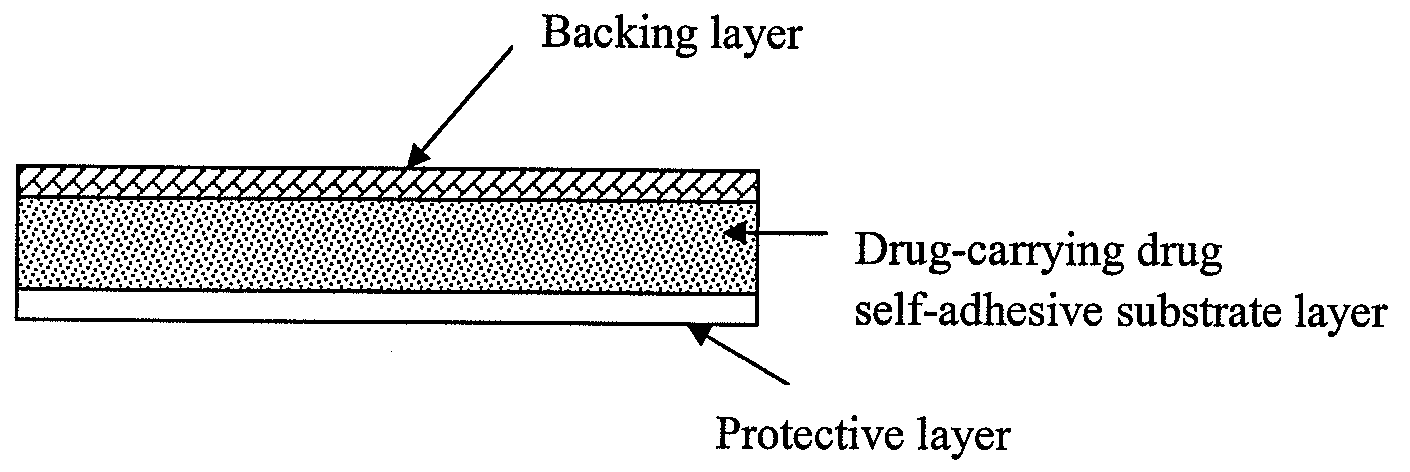
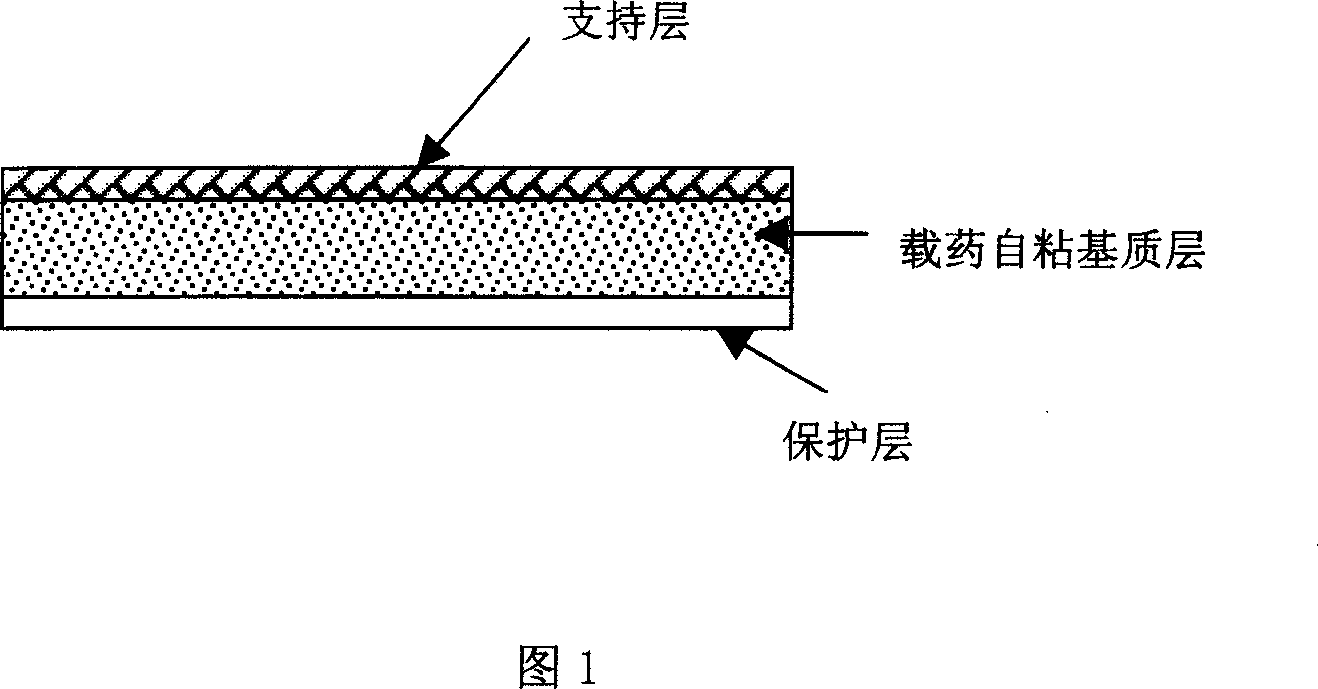
The present invention relates to a rasagiline transdermal patch for treatment or prophylaxis of nervous system diseases, in which the patch comprises an inert backing layer chemically inert to substrate ingredients, a substrate layer comprising rasagiline or a pharmaceutically acceptable salt thereof, and a protective layer to be peeled off before use. The substrate layer is an adhesive system comprising an organic polymer material as basis and an inorganic or organic material as filler, and a plurality of micro-reservoirs containing rasagiline. The substrate further comprises one or more substances for enhancing the transdermal absorption of rasagiline, in which the above organic polymer material in the substrate is used for the reservoir of rasagiline and as adhesive.
Owner:CHONGQING PHARMA RES INST +1
Use of rasagilline for the treatment of restless legs syndrome
ActiveUS20070232700A1Effective treatmentRelieve symptomsBiocideNervous disorderN-propargylPediatrics
Disclosed are methods for the treatment of Restless Legs Syndrome comprising administering an amount of R(+)-N-propargyl-1-aminoindan or a pharmaceutically acceptable salt thereof.
Owner:TEVA PHARMA IND LTD
Citrate salt of Rasagiline
The subject invention provides rasagiline citrate, its compositions and processes for the manufacture thereof.
Owner:TEVA PHARMA IND LTD
Process for purifying rasagiline base
Disclosed is crystalline R(+)-N-propargyl-1-aminoindan and racemic N-propargyl-1-aminoindan characterized by colorless crystals a pharmaceutical composition comprising the same, and the process for the manufacture and the validation thereof. Also disclosed is pure liquid R(+)-N-propargyl-1-aminoindan and a pharmaceutical composition comprising the same, and the process for the manufacture thereof.
Owner:TEVA PHARMA IND LTD
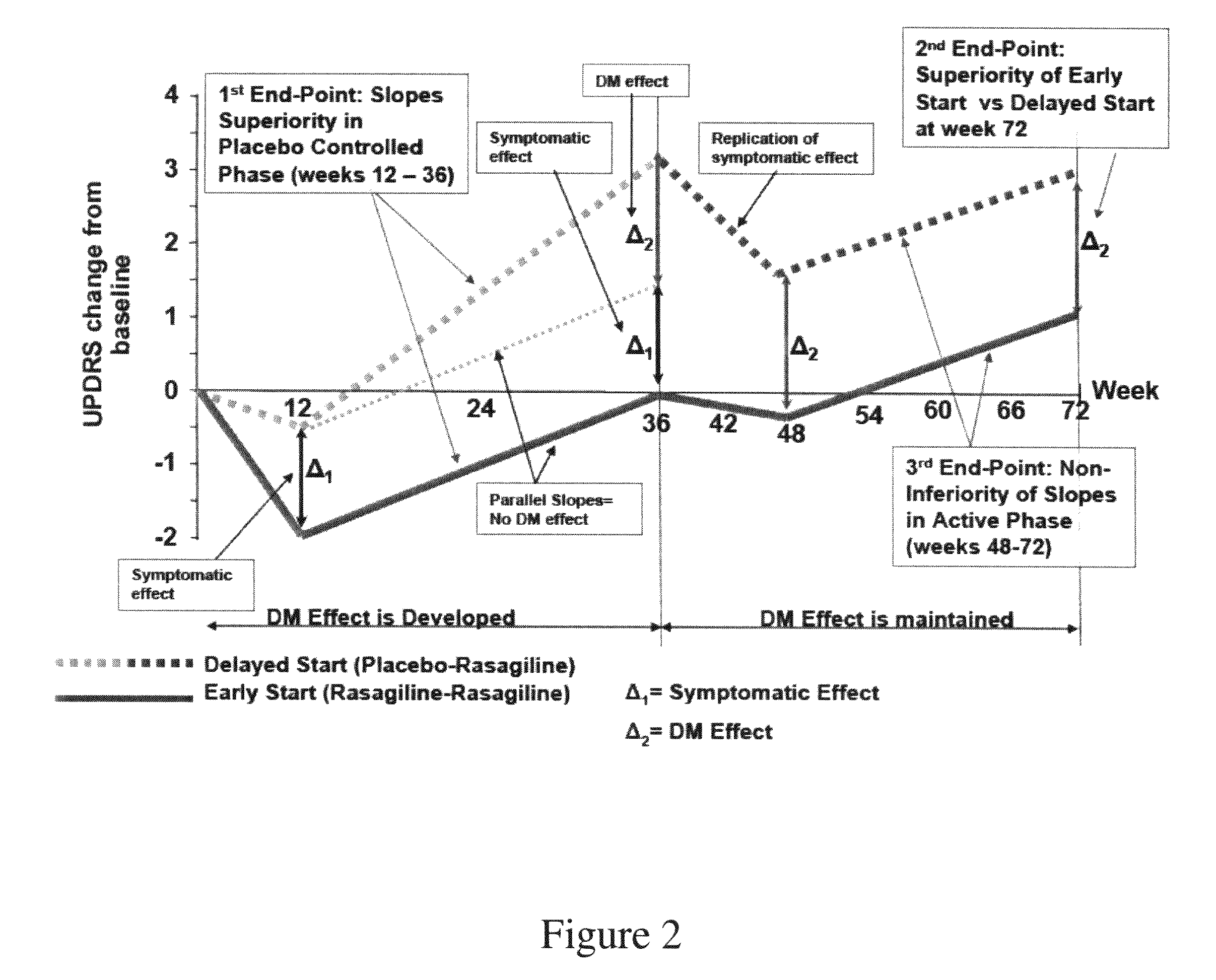
Rasagiline for parkinson's disease modification
InactiveUS20090312436A1Shorten the progressReduce rateBiocideOrganic active ingredientsFunctional declineDisease patient
A method for modifying Parkinson's disease by periodically administering a pharmaceutical composition comprising a therapeutically effective amount of rasagiline or a pharmaceutically acceptable salt of rasagiline to the patient, thereby modifying the disease. The method includes reducing the rate of progression; delaying the need for symptomatic anti-Parkinsonian therapy; reducing the risk of a Parkinson's disease patient requiring symptomatic anti-Parkinsonian therapy; and reducing the functional decline.
Owner:TEVA PHARMA IND LTD
Rasagiline Orally Disintegrating Compositions
InactiveUS20090111892A1Reduce brittlenessSolve the lack of hardnessBiocideOrganic active ingredientsAlcohol sugarsPharmacology
This invention provides a solid pharmaceutical composition comprising rasagiline or a pharmaceutically acceptable salt of rasagiline, and particles having a non-filamentous microstructure of at least two sugar alcohols. This invention also provides a solid pharmaceutical composition comprising rasagiline or a pharmaceutically acceptable salt of rasagiline, a mixture of a disintegrant, a flow agent and particles having a non-filamentous microstructure of at least two sugar alcohols, a supplemental sugar alcohol, a supplemental flow agent, and a supplemental disintegrant. This invention further provides a method of treating a subject afflicted with Parkinson's disease comprising administering to the subject a therapeutically effective amount of the solid pharmaceutical composition, thereby treating the subject. Finally, this invention provides a process of making such solid pharmaceutical compositions.
Owner:TEVA PHARMA IND LTD
Rasagiline formulations of improved content uniformity
Disclosed are pharmaceutical preparations of R(+)-N-propargyl-1-aminoindan salts having enhanced content uniformity, processes for preparation of the compositions, and their uses.
Owner:TEVA PHARMA IND LTD
Rasagiline transparent patch for curing and preventing neurological diseases and the preparing method thereof
The present invention relates to one kind of transdermal rasagiline medicine plaster for preventing and treating neurological diseases and its preparation process. The transdermal rasagiline medicine plaster includes one support layer without chemical reaction with the matrix components, one matrix layer containing rasagiline or its pharmaceutically acceptable salt, and operate protecting layer being torn off before the plaster is used. It features the matrix layer with polymer as the basic material, adhering system with inorganic or organic filler, stored rasagiline and matter(s) to promote the transdermal absorption of rasagiline.
Owner:CHONGQING PHARMA RES INST +1
Crystalline solid rasagiline base
Owner:TEVA PHARMA IND LTD
Crystalline solid rasagiline base
InactiveUS20100145101A1Organic active ingredientsAmino compound purification/separationN-propargyl1-aminoindan
The subject invention provides crystalline R(+)-N-propargyl-1-aminoindan, pharmaceutical compositions and methods of manufacture thereof.
Owner:TEVA PHARMA IND LTD
Use of rasagiline for the treatment of progressive supranuclear palsy
A method for the treatment of Progressive Supranuclear Palsy. Such method includes administering to a subject an amount of R(+)-N-propargyl-1-aminoindan or a pharmaceutically acceptable salt thereof.
Owner:TEVA PHARMA IND LTD
Solid oral prepn. of leishajilan
An orally taken solid of rasagiline with high disintegrating speed in oral cavity is proportionally prepared from rasagiline or its pharmacologic salt, filler and disintegrant.
Owner:CHONGQING PHARMA RES INST +1
Combination therapy with glatiramer acetate and rasagiline for the treatment of multiple sclerosis
InactiveUS20100167983A1Relieve symptomsNervous disorderPeptide/protein ingredientsMedicineCombination therapy
The subject invention provides a method of treating a subject afflicted with a form of multiple sclerosis comprising periodically administering to the subject an amount of glatiramer acetate and an amount of rasagiline or the pharmaceutically acceptable salt thereof, wherein the amounts when taken together are effective to alleviate a symptom of the form of multiple sclerosis in the subject so as to thereby treat the subject. The subject invention also provides a package comprising glatiramer acetate, rasagiline or the pharmaceutically acceptable salt thereof and instructions for use of the together to alleviate a symptom of a form of multiple sclerosis in a subject. The subject invention further provides a pharmaceutical combination comprising separate dosage forms of an amount of glatiramer acetate and an amount of rasagiline or the pharmaceutically acceptable salt thereof, which combination is useful to alleviate a symptom of a form of multiple sclerosis in a subject.
Owner:TEVA PHARMA IND LTD
Combination of rasagiline and pridopidine for treating neurodegenerative disorders, in particular huntington's disease
InactiveUS20140088145A1Effective treatmentBiocideNervous disorderHuntingtons choreaPharmaceutical drug
This invention provides a method of treating a patient afflicted with a neurodegenerative disorder, e.g., Huntington's disease, comprising administering to the patient rasagiline as an add-on therapy to or in combination with pridopidine. This invention also provides a package and a pharmaceutical composition comprising rasagiline and pridopidine for treating a patient afflicted with a neurodegenerative disorder. This invention also provides rasagiline for use as an add-on therapy or in combination with pridopidine in treating a patient afflicted with a neurodegenerative disorder. This invention further provides use of rasagiline and pridopidine in the preparation of a combination for treating a patient afflicted with a neurodegenerative disorder.
Owner:TEVA PHARMA IND LTD
Combination Therapy with Glatiramer Acetate and Rasagiline for the Treatment of Multiple Sclerosis
InactiveUS20080261894A1Relieve symptomsOrganic active ingredientsNervous disorderCombination therapyGlatiramer acetate
The subject invention provides a method of treating a subject afflicted with a form of multiple sclerosis comprising periodically administering to the subject an amount of glatiramer acetate and an amount of rasagiline or the pharmaceutically acceptable salt thereof, wherein the amounts when taken together are effective to alleviate a symptom of the form of multiple sclerosis in the subject so as to thereby treat the subject. The subject invention also provides a package comprising glatiramer acetate, rasagiline or the pharmaceutically acceptable salt thereof and instructions for use of the together to alleviate a symptom of a form of multiple sclerosis in a subject. The subject invention further provides a pharmaceutical combination comprising separate dosage forms of an amount of glatiramer acetate and an amount of rasagiline or the pharmaceutically acceptable salt thereof, which combination is useful to alleviate a symptom of a form of multiple sclerosis in a subject.
Owner:TEVA PHARMA IND LTD
Rasagiline orally disintegrating compositions
InactiveUS20120238636A1Reduce brittlenessSolve the lack of hardnessBiocideOrganic active ingredientsDiseaseAlcohol sugars
Owner:PATASHNIK SHULAMIT +2
Citrate Salt of Rasagiline
The subject invention provides rasagiline citrate, its compositions and processes for the manufacture thereof.
Owner:TEVA PHARMA IND LTD
Process for purifying rasagiline base
Disclosed is crystalline R(+)-N-propargyl-1-aminoindan and racemic N-propargyl-1-aminoindan characterized by colorless crystals a pharmaceutical composition comprising the same, and the process for the manufacture and the validation thereof. Also disclosed is pure liquid R(+)-N-propargyl-1-aminoindan and a pharmaceutical composition comprising the same, and the process for the manufacture thereof.
Owner:TEVA PHARMA IND LTD
Combination of rasagiline and pridopidine for treating neurodegenerative disorders, in particular huntington's disease
This invention provides a method of treating a patient afflicted with a neurodegenerative disorder, e.g., Huntington's disease, comprising administering to the patient rasagiline as an add-on therapy to or in combination with pridopidine. This invention also provides a package and a pharmaceutical composition comprising rasagiline and pridopidine for treating a patient afflicted with a neurodegenerative disorder. This invention also provides rasagiline for use as an add-on therapy or in combination with pridopidine in treating a patient afflicted with a neurodegenerative disorder. This invention further provides use of rasagiline and pridopidine in the preparation of a combination for treating a patient afflicted with a neurodegenerative disorder.
Owner:TEVA PHARM USA INC
Simple and novel process for preparing indenes derivatives
The invention provides a method for preparing indene derivative. Said compound can be used to prepare Rasagiline that can prevent Parkinson's disease.
Owner:北京德众万全医药科技有限公司
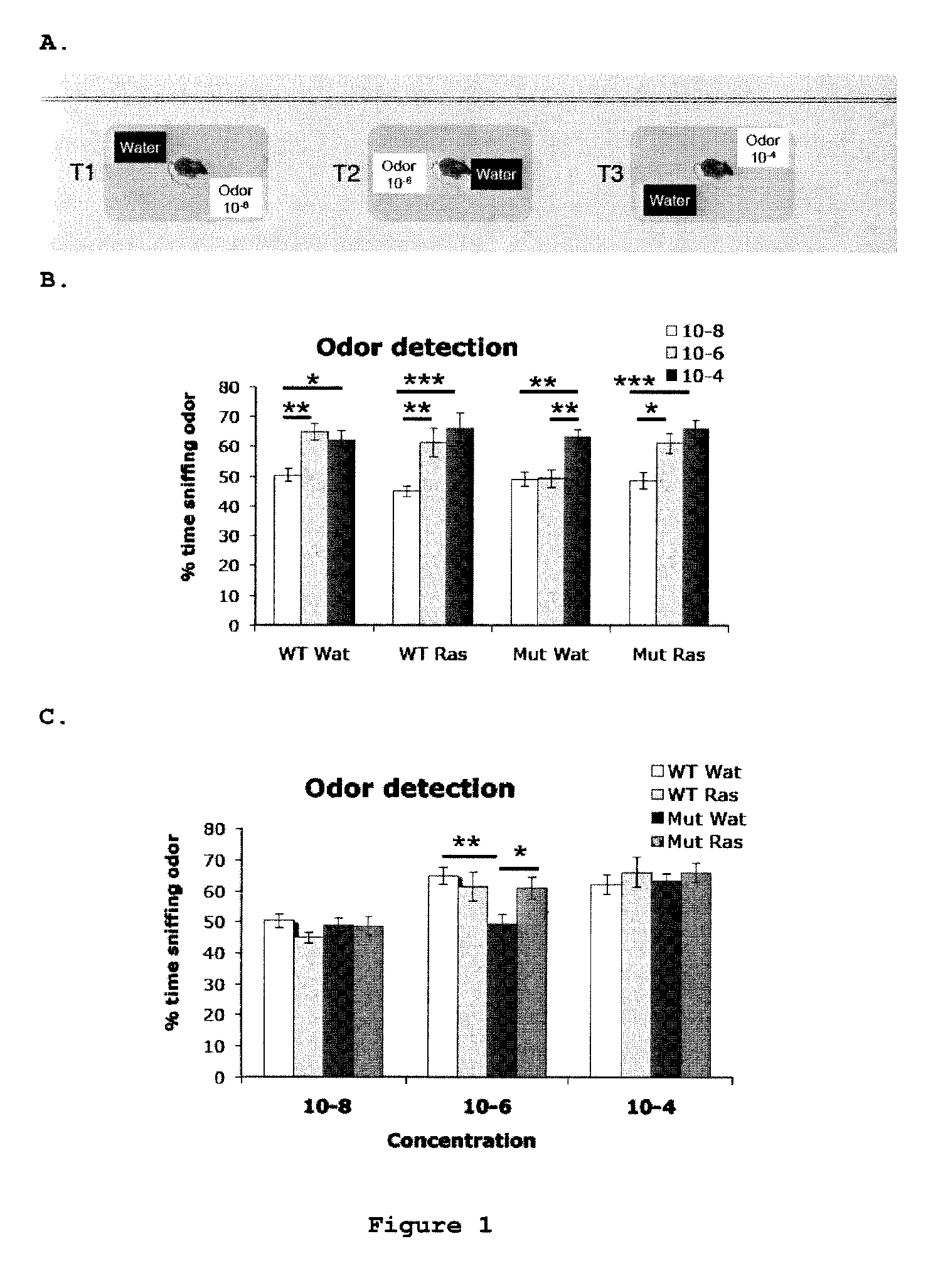
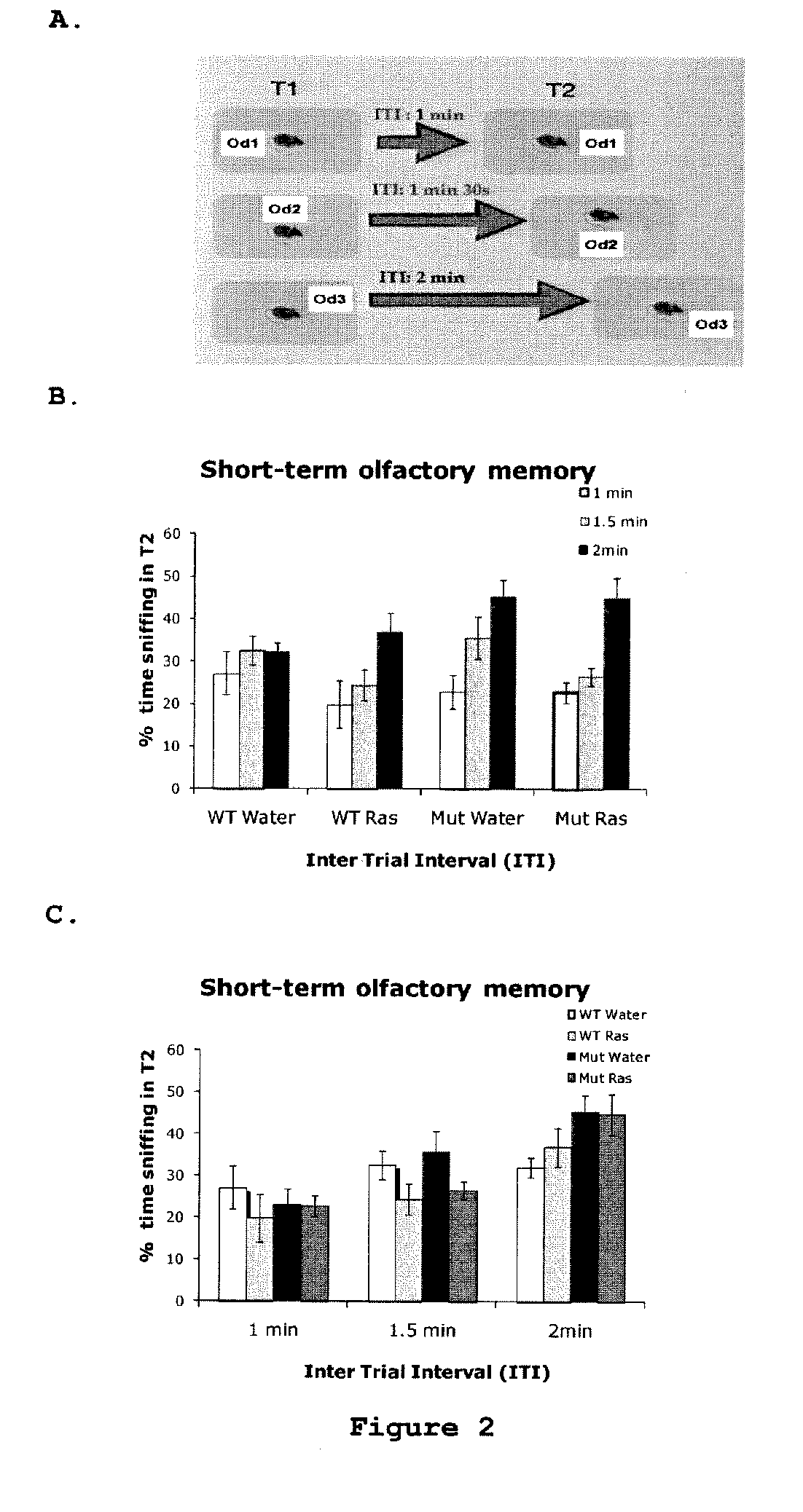
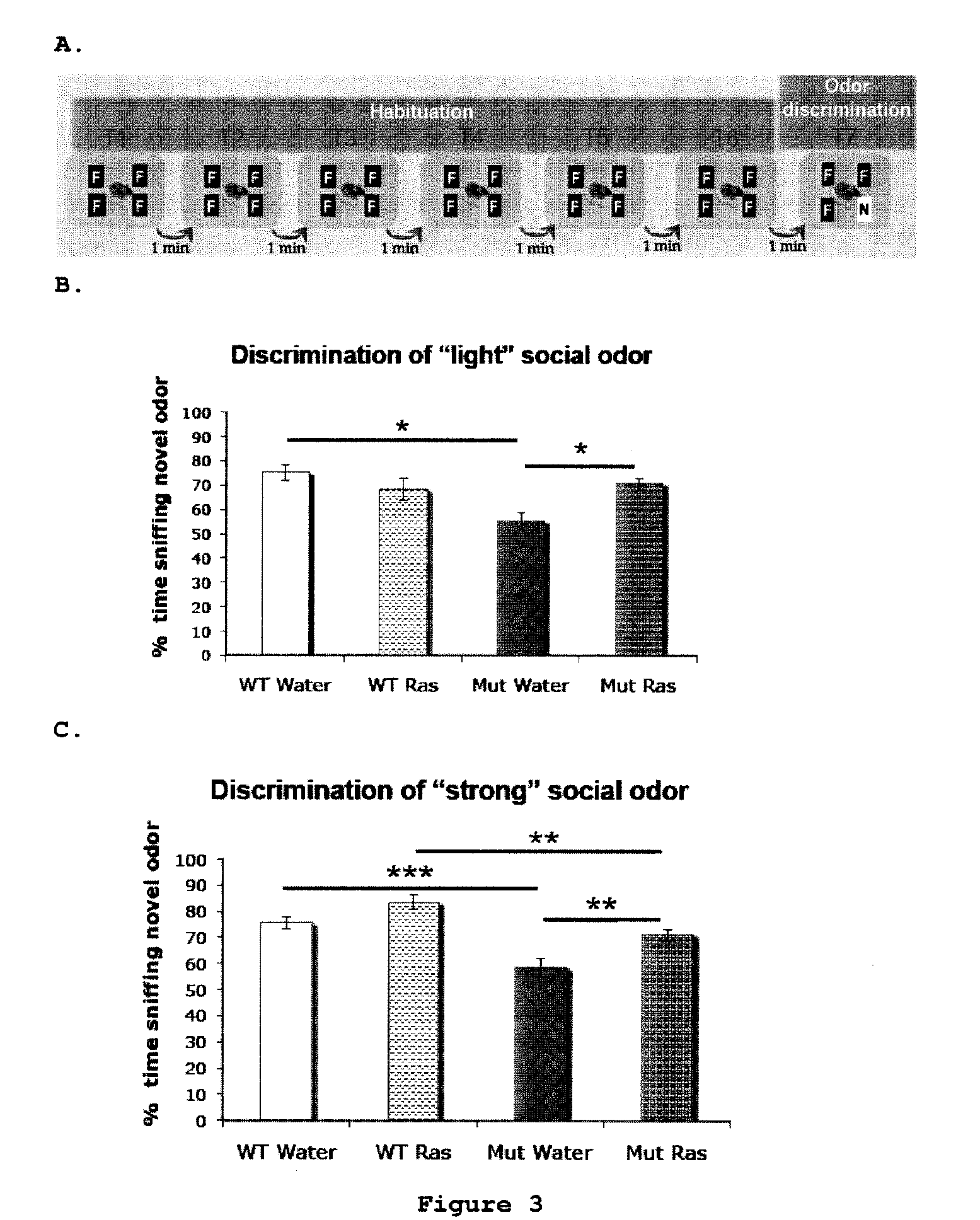
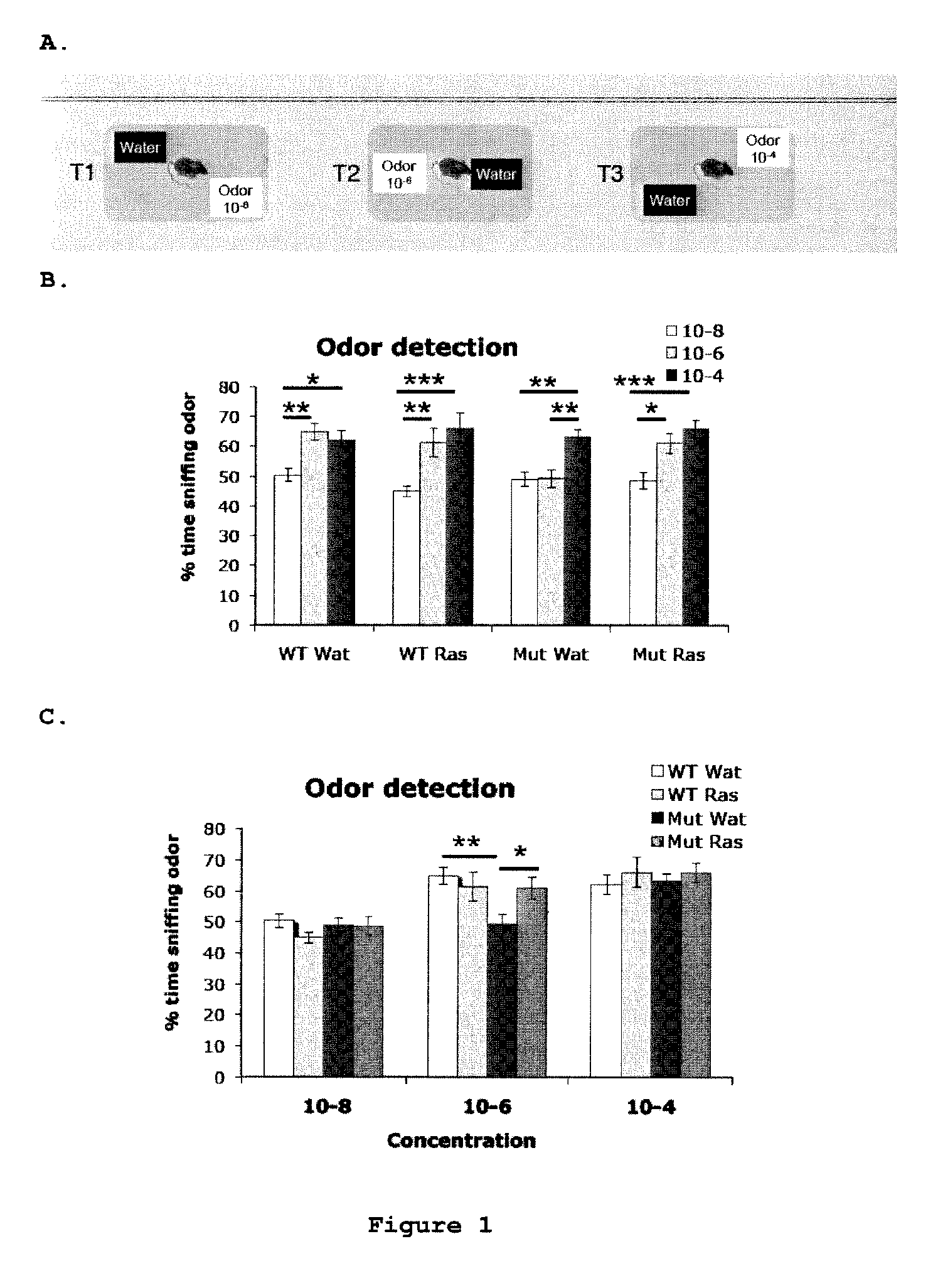
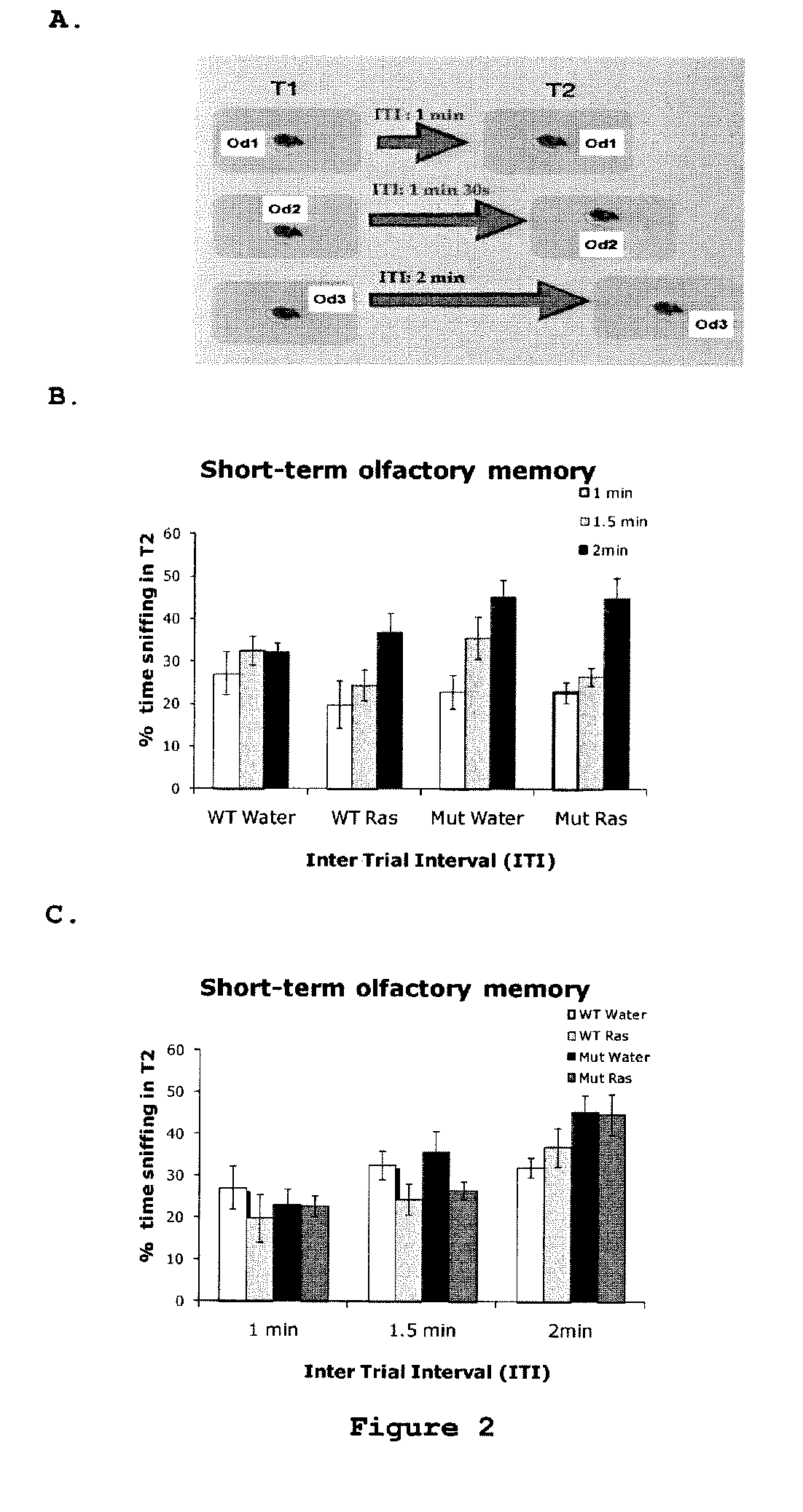
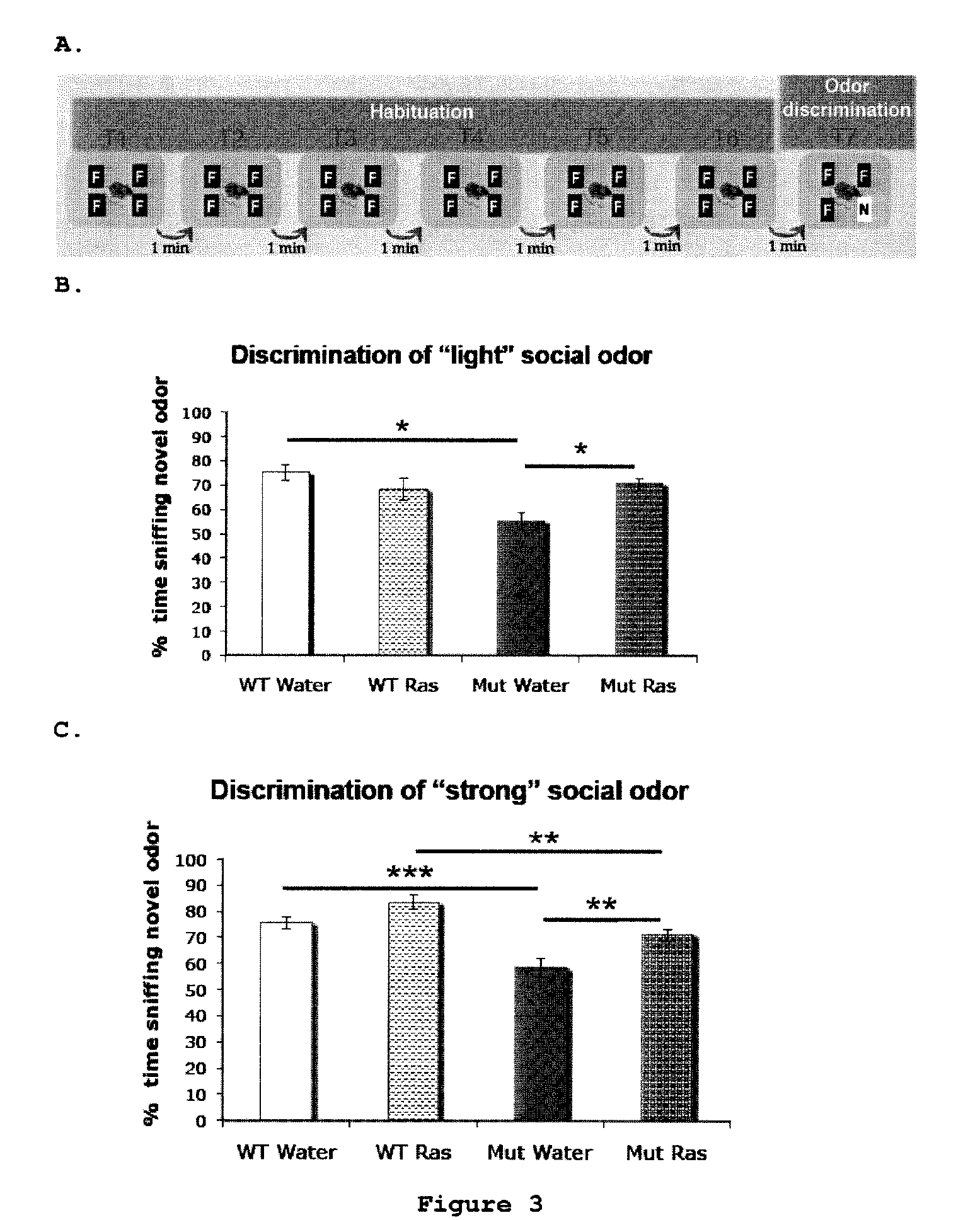
Use of rasagiline for the treatment of olfactory dysfunction
InactiveUS20120029087A1Reduction in rate of progressAvoid lostBiocideOrganic active ingredientsPharmacologyRasagiline
Disclosed are methods of treating olfactory dysfunction by periodically administering a therapeutically effective amount of rasagiline or a pharmaceutically acceptable salt of rasagiline to a subject.
Owner:TEVA PHARMA IND LTD
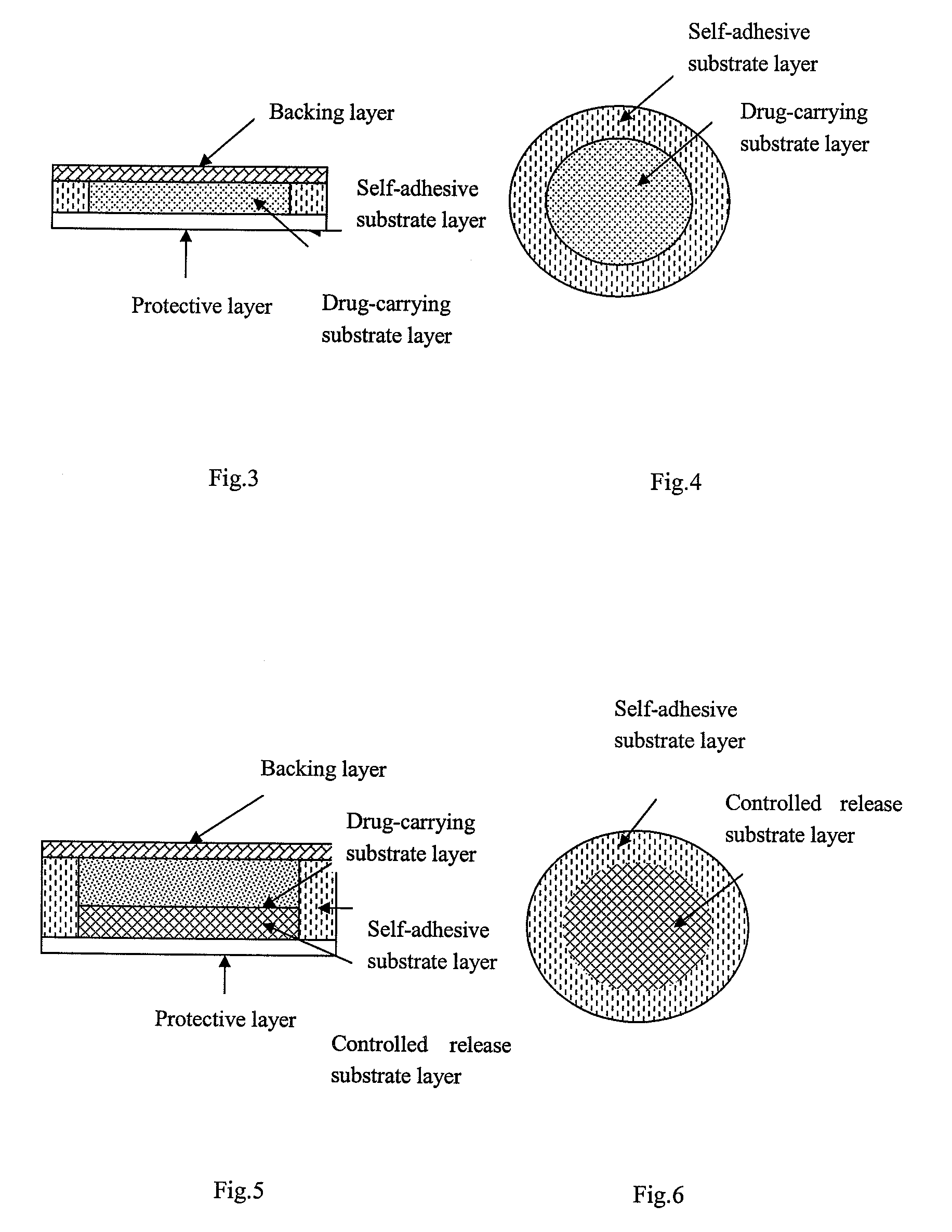
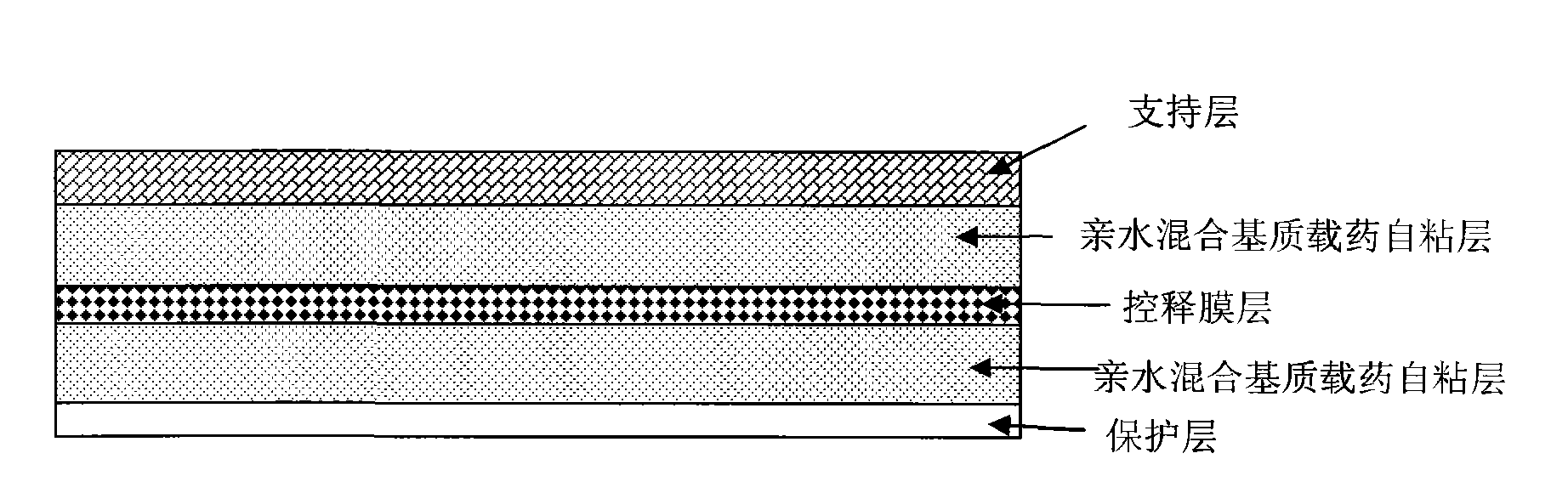
Stable controlled-release rasagiline transdermal patch and preparation method thereof
ActiveCN101606923ALong lastingEasy to manufactureOrganic active ingredientsNervous disorderTransdermal patchControlled release
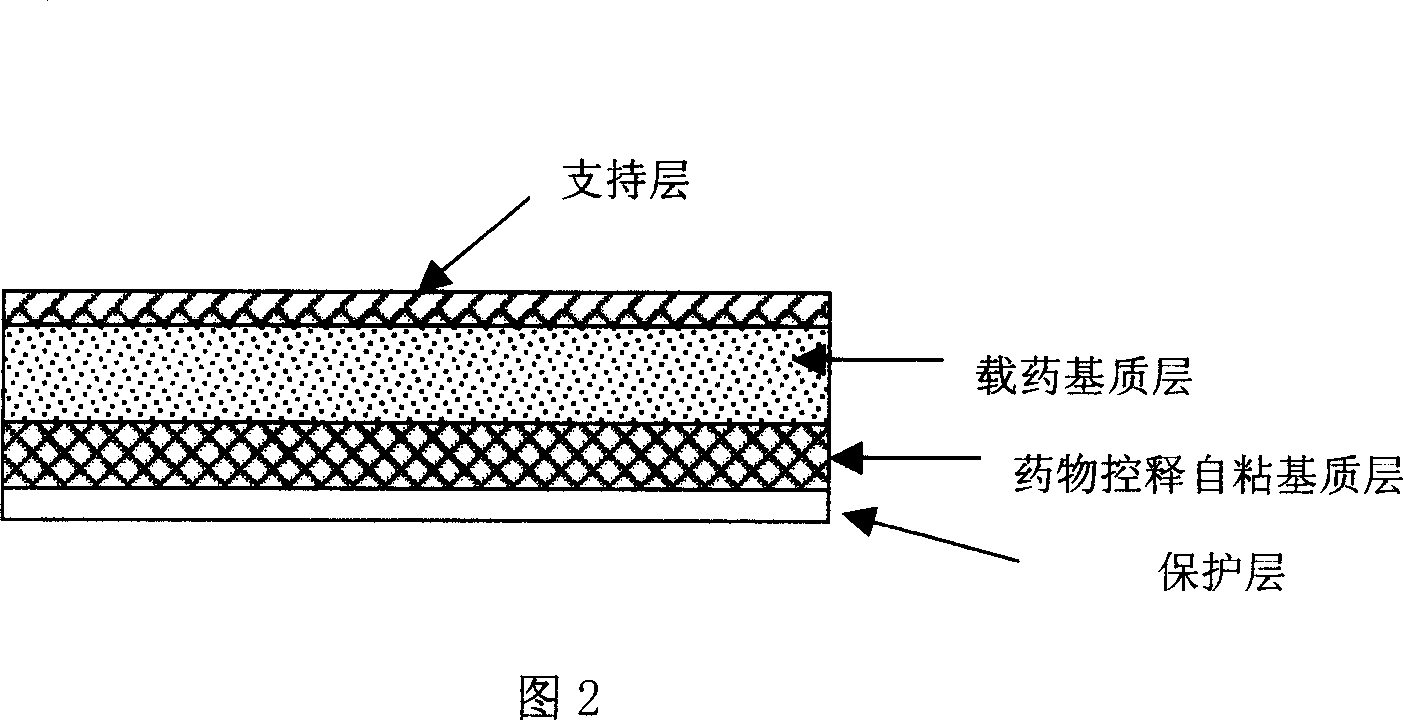
The invention relates to rasagiline for treating or preventing nervous system diseases and a transdermal patch of a pharmaceutically acceptable slat of rasagiline. The transdermal patch comprises the rasagiline or the pharmaceutically acceptable slat of rasagiline and at least one hydrophilic polymer substrate. And the pH value of the patch ranges from 3.5 to 6.5. The patch has the characteristics of excellent stability and good control over transdermal release of the rasagiline.
Owner:CHONGQING PHARMA RES INST
Pharmaceutical composition containing rasagiline
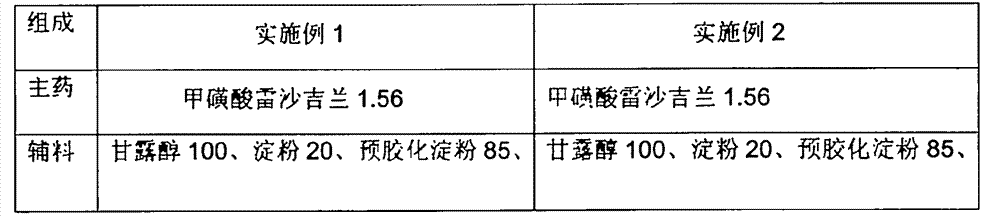
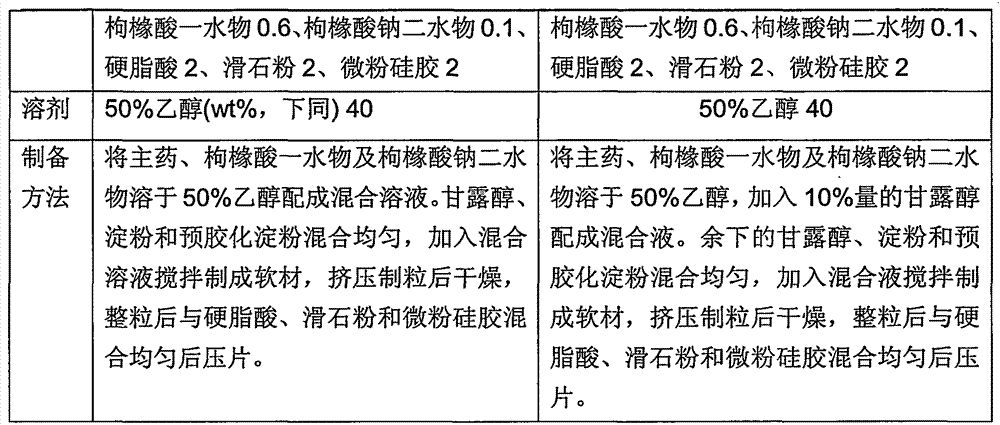
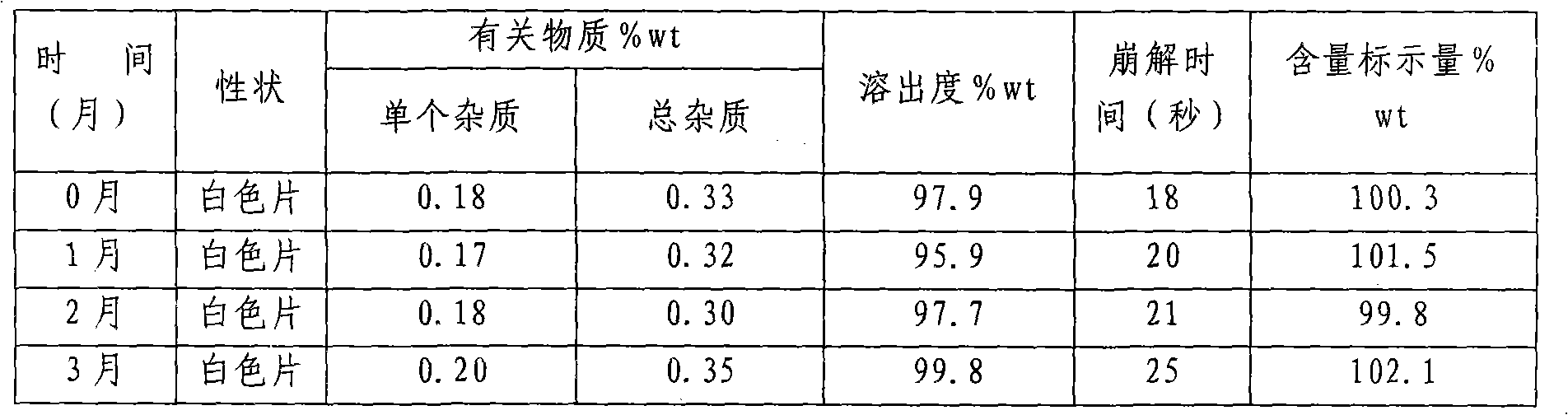
The present invention discloses an oral solid medicinal combination, comprises 0.5 weight percent to 3 weight percent of rasagiline and salt of rasagiline, less than 60 weight percent of pentahydric alcohol and / or hexahydric alcohol and 0.5 weight percent to 3 weight percent of organic acid. The present invention is used for curing the Parkinsonism.
Owner:BEIJING D VENTUREPHARM TECH DEV
Combination therapy with glatiramer acetate and rasagiline for the treatment of multiple sclerosis
InactiveUS20120027718A1Nervous disorderPeptide/protein ingredientsCombination therapyGlatiramer acetate
The subject invention provides a method of treating a subject afflicted with a form of multiple sclerosis comprising periodically administering to the subject an amount of glatiramer acetate and an amount of rasagiline or the pharmaceutically acceptable salt thereof, wherein the amounts when taken together are effective to alleviate a symptom of the form of multiple sclerosis in the subject so as to thereby treat the subject. The subject invention also provides a package comprising glatiramer acetate, rasagiline or the pharmaceutically acceptable salt thereof and instructions for use of the together to alleviate a symptom of a form of multiple sclerosis in a subject. The subject invention further provides a pharmaceutical combination comprising separate dosage forms of an amount of glatiramer acetate and an amount of rasagiline or the pharmaceutically acceptable salt thereof, which combination is useful to alleviate a symptom of a form of multiple sclerosis in a subject.
Owner:TEVA PHARMA IND LTD
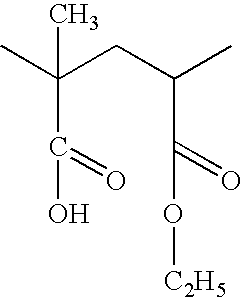
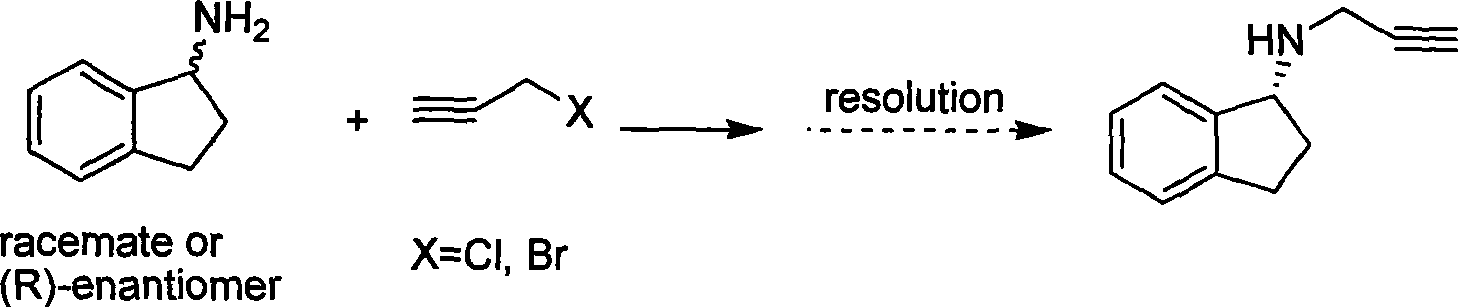
Preparation method of (R)-(+)-N-propargyl-1-indan amines
InactiveCN101381314AStrong response specificityHigh yieldAmino preparation by functional substitutionBulk chemical productionN-propargylSide effect
The invention discloses a method for preparing rasagiline which has simple and convenient operation and is suitable for industrialized production. In the method, primary amine group on 1-indan amine is protected by o-Nos, and the 1-indan amine removes the protecting group after the 1-indan amine is substituted by propargyl chloride (or propargyl bromide); compared with the prior method, two steps of reactions are added in the method, but after the 1-indan amine is protected by the o-Nos, the reaction specificity of the 1-indan amine and the propargyl chloride (or the propargyl bromide) is greatly improved; and the protecting group can be easily removed after reaction without other side effects, so a single product is generated. Besides, raw materials and reaction reagent used in the method is cheap and easily obtained. The method has the advantages of simple and easy operation, mild reaction condition, easy control, good reaction selectivity, total yield improvement and cost reduction, and has excellent industrialized prospect.
Owner:成都和康药业有限责任公司
Long-acting sustained-release preparation for resisting Parkinson's disease and preparation method thereof
ActiveCN107049985AReduce fluctuations in concentrationNo drug burstOrganic active ingredientsNervous disorderMicrosphereMicrometer
The invention discloses a long-acting sustained-release preparation for resisting Parkinson's disease and a preparation method thereof. The long-acting sustained-release preparation refers to microspheres, each microsphere comprises rasagiline or pharmaceutically acceptable salt thereof and biodegradable high-biocompatibility molecular polymer, and the microspheres includes the microspheres with the average particle size of 0.5-5 micrometers and the microspheres with the average particle size of 20-150 micrometers. The two kinds of microspheres different in particle size are properly matched and used in the pharmaceutical composition, the fluctuation of the concentration of rasagiline in plasma can be significantly reduced, and there is no obvious delayed release period. At the same time, the long-acting sustained-release preparation has no drug releasing phenomenon under the condition of high drug loading.
Owner:AC PHARMA CO LTD
Use of rasagiline for the treatment of olfactory dysfunction
InactiveUS8569379B2Shorten the progressReduce rateBiocideOrganic active ingredientsPharmacologyRasagiline
Disclosed are methods of treating olfactory dysfunction by periodically administering a therapeutically effective amount of rasagiline or a pharmaceutically acceptable salt of rasagiline to a subject.
Owner:TEVA PHARMA IND LTD
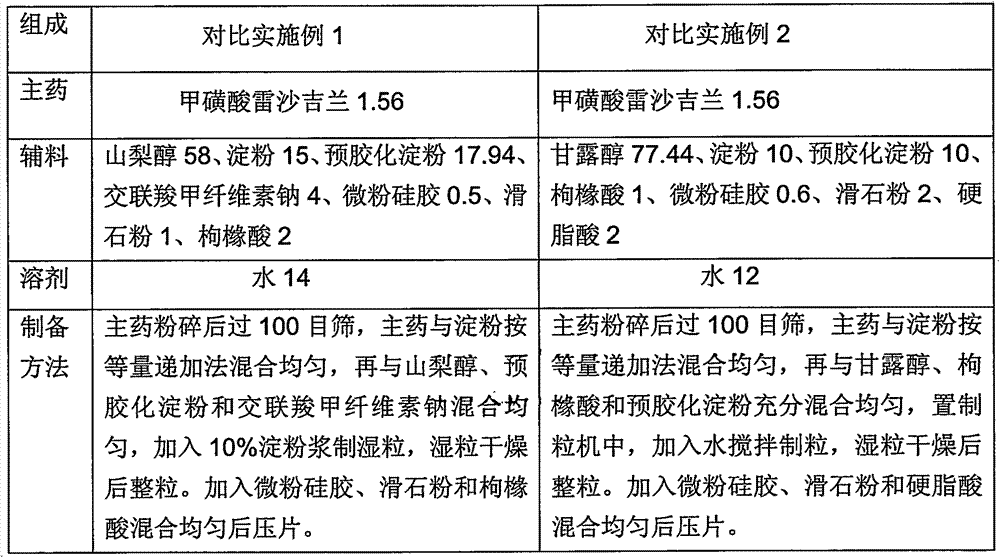
Rasagiline preparation and preparation method thereof
ActiveCN103315983AHigh dissolution rateImprove stabilityOrganic active ingredientsNervous disorderDissolutionPharmacology
The invention provides a rasagiline preparation and a preparation method thereof. The rasagiline preparation provided by the invention comprises rasagiline pharmaceutically acceptable salt and pharmaceutically acceptable auxiliary materials comprising a stabilizer and a filling agent. With the method provided by the invention, dissolution rate and stability of the rasagiline preparation are improved, preparation content uniformity is ensured, treatments of crushing and sieving are avoided, and loss and pollution are reduced. The method also has the advantages of simple operation, low cost, and no requirement on special equipment. The method is suitable to be applied on industrial productions.
Owner:SHANGHAI ZHONGXI PHARMA
Orally disintegrating tablet of Rasagiline or medicine salts thereof and preparation method thereof
ActiveCN101874790ADisintegrates quicklyQuality improvementOrganic active ingredientsNervous disorderDiseaseMANNITOL/SORBITOL
The invention belongs to the technical field of medicines and relates to an orally disintegrating tablet of Rasagiline or medicine salts thereof and a preparation method thereof. The orally disintegrating tablet comprises the following components in percentage by weight: 0.25-2 percent of Rasagiline or medicine salts thereof, 85-91 percent of spray drying mannitol, 3-10 percent of disintegrant, 0.5-2 percent of flow agent, 0.5-2 percent of lubricant, 0-2 percent of sweetener and 0-2 percent of flavoring agent. The raw materials and the auxiliary materials are uniformly mixed to obtain uniform fine powder which is directly squashed to form tablets. The orally disintegrating tablet of the Rasagiline or the medicine salts thereof has enough hardness, can satisfy the requirements of production, packaging, storage and transportation and also has good taste and shorter disintegration time.
Owner:QILU PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com