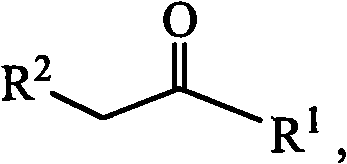
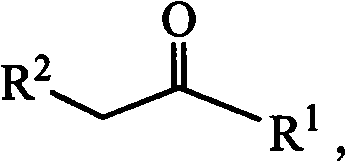
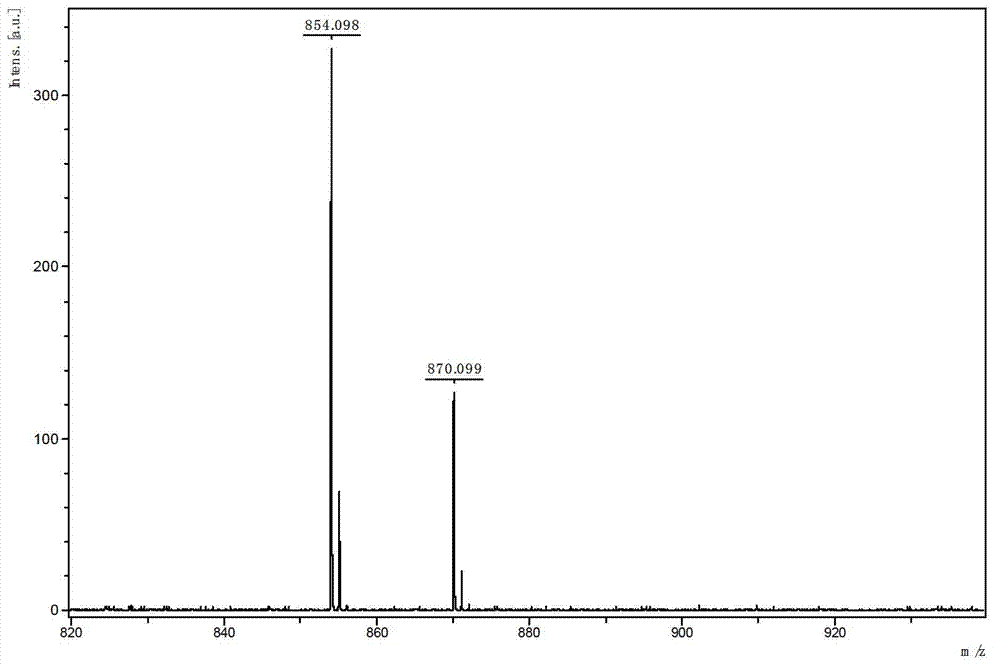
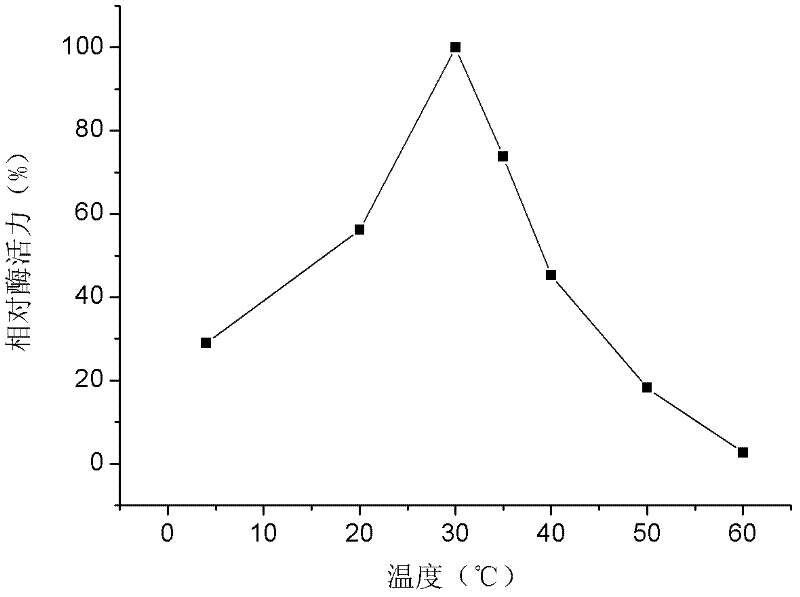
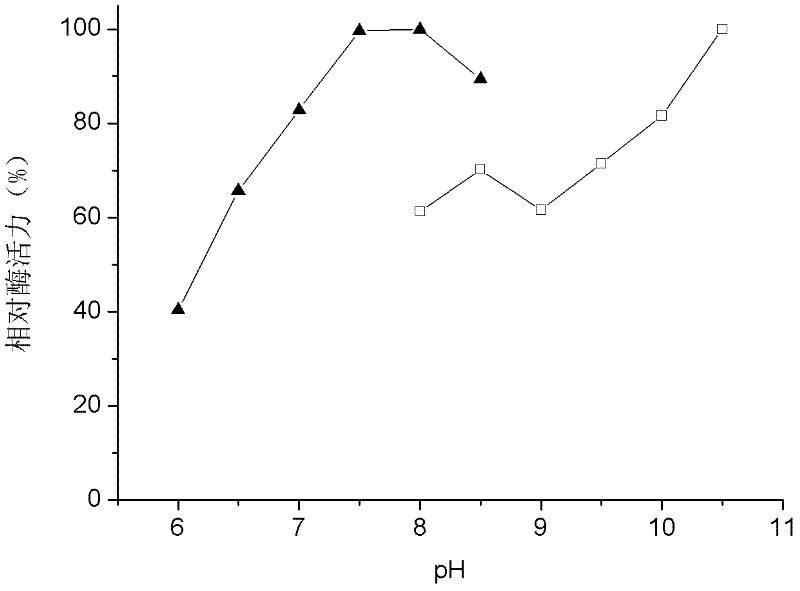
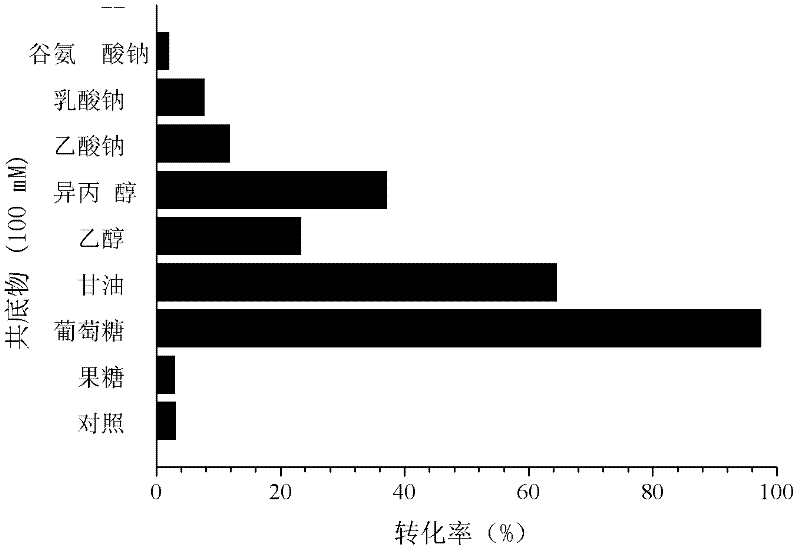
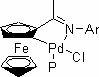Patents
Literature
129results about How to "Strong response specificity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
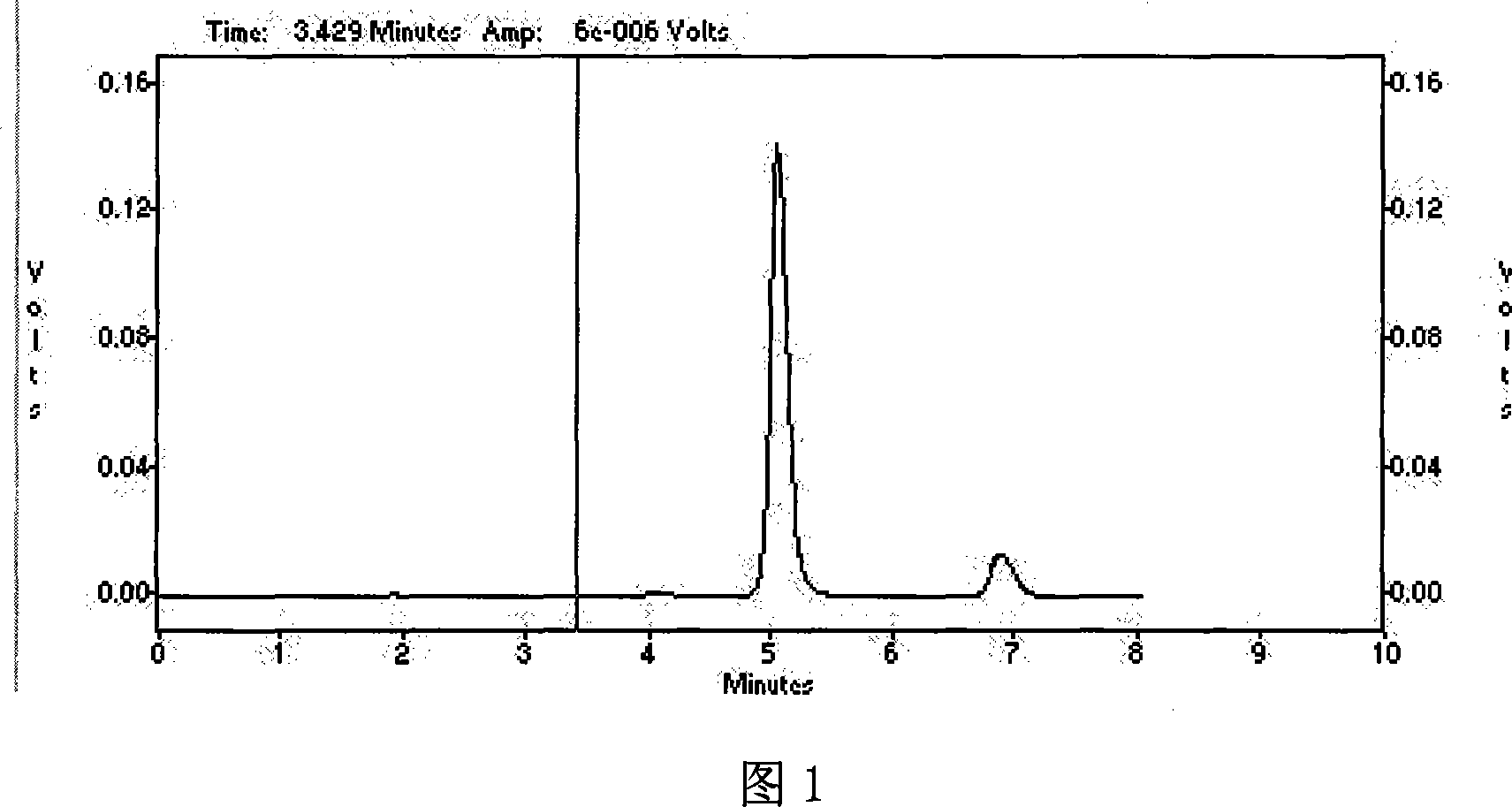
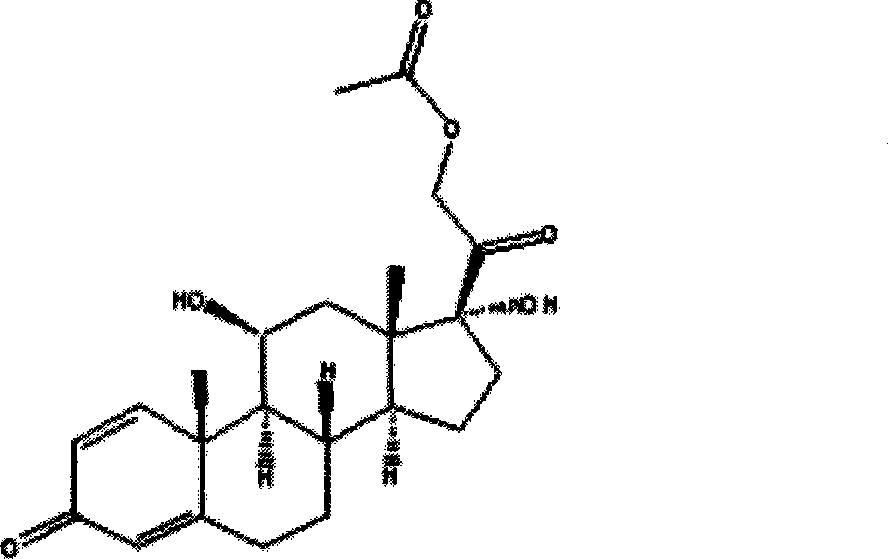
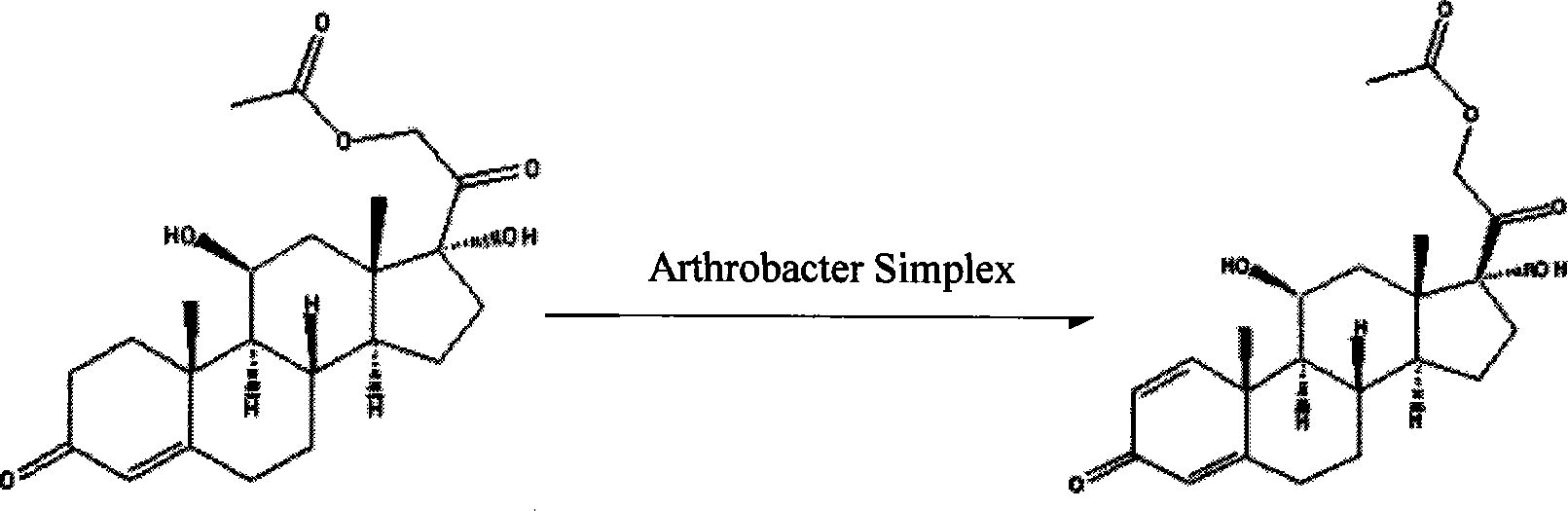
Method for producing prednisolone acetate
InactiveCN101210259AStrong response specificityEasy to operateMicroorganism based processesFermentationMicrobial transformationArthrobacter simplex
The invention belongs to the field of microbial pharmaceutics and pharmaceutical engineering, specifically relates to a production method of prednisone acetate by microbial transformation with Arthrobacter simplex as bacteria strain and hydrocortisone acetate as substrate. The method uses Arthrobacter simplex as bacteria strain and comprises the following steps of: performing primary seed culture, performing second fermentation culture, adding hydrocortisone acetate into the fermentation liquid of Arthrobacter simplex to transform hydrocortisone acetate into prednisone acetate, filtering, and collecting cake to obtain prednisone acetate. The bacteria can be prepared into double liquid phase, broken cells or protoplast, each of which has high transformation ratio. The inventive production method replaces cortisone acetate with hydrocortisone acetate as raw material, and has the advantages of high yield, simple process, good economical and practical performance, and less use of harmful reagents; and is important for steroids production with biotransformation method.
Owner:TIANJIN UNIV OF SCI & TECH
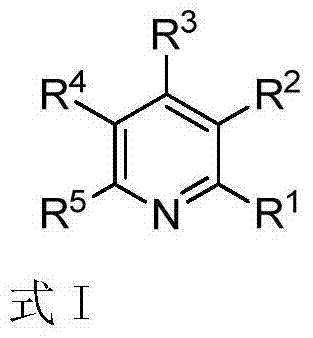
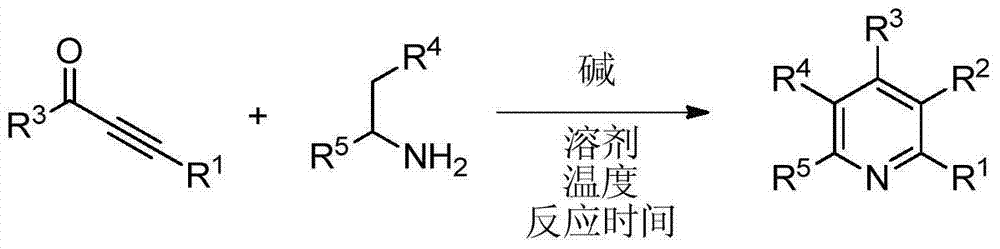
Polysubstituted pyridine derivative and preparation method thereof
The invention discloses a polysubstituted pyridine derivative and a preparation method thereof. The derivative has a structure as shown in specification, wherein R1, R2, R3, R4 and R5 all are any one selected from hydrogen atom, halogen atom, alkyl, aryl, substituted aryl, acyl, amino, nitryl and alkoxy; the invention also discloses a preparation method of the polysubstituted pyridine derivative; the preparation method comprises the following steps: by taking acetyenic ketone and 1-arylethylamine as raw materials, and under the action of appropriate alkali, heating to have a reaction in the solvent to obtain the polysubstituted pyridine derivative as shown in the formula at high yield. The preparation method is mild in reaction condition, short in reaction time, wide in substrate range, high in reaction specifity, high in yield and simple in after-treatment.
Owner:HUAQIAO UNIVERSITY
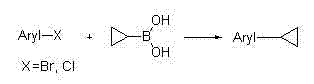
Method for synthesizing cyclopropyl alkyl aromatic compound
InactiveCN102391059AImprove stabilityEfficient catalytic activityCarboxylic acid nitrile preparationOrganic compound preparationArylOrganic synthesis
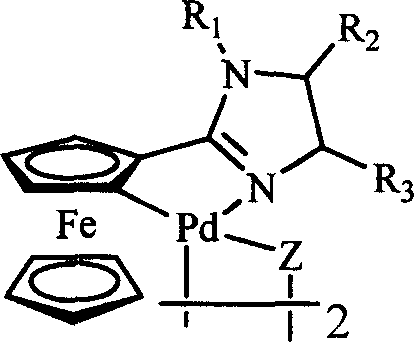
The invention belongs to the technical field of organic synthesis, and particularly relates to application of a cyclopalladated ferrocenylimines-phosphine adduct in the synthesis of a cyclopropyl alkyl aromatic compound. Halogenated aromatic hydrocarbon Aryl-X, alkali and cyclopropyl borate which serve as raw materials, and the cyclopalladated ferrocenylimines-phosphine adduct is used as a catalyst. The catalyst has high stability, efficient catalytic activity and wide application range, and a corresponding coupling product can be synthesized in a mode of high yield (which is up to 96 percent) on the premise of small catalytic quantity; and the method is mild in reaction condition, wide in range of substrates and high in specifity of reaction.
Owner:TETRANOV PHARMA CO LTD
Process for Producing Aliprazo
InactiveCN1576273AStrong response specificityHigh yieldOrganic chemistryPurification methodsAripiprazole
The present invention relates to the preparation process of Aripiprazole. Aripiprazole is prepared with two kinds of compound and through condensation. The preparation process of the present invention has mild reaction condition, less side products, simple operation and controllable quality of the intermediate, and the Aripiprazole product of the present invention may reach relevant medicine standard without needing several times of re-crystallization.
Owner:重庆凯林制药有限公司 +1
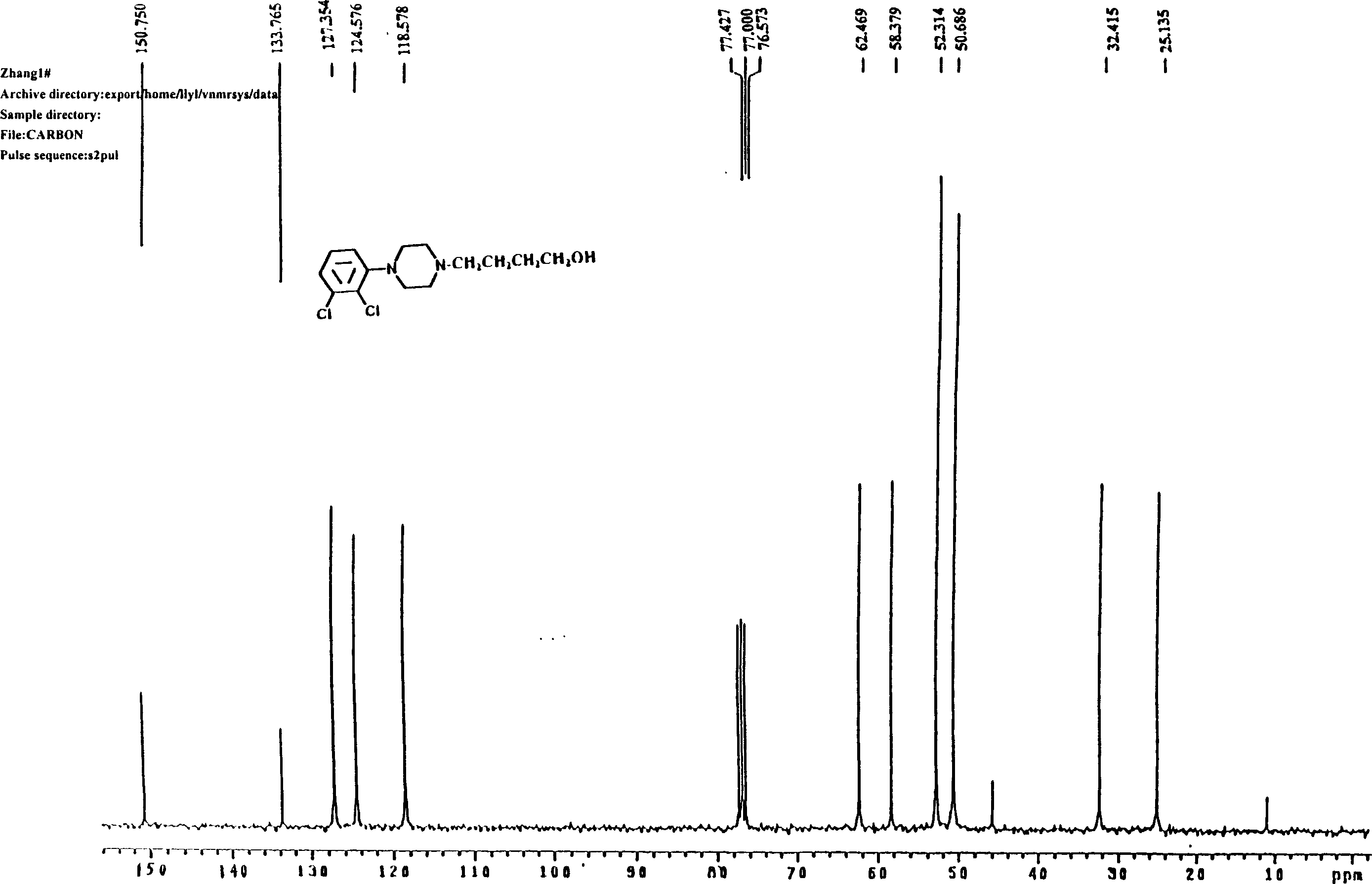
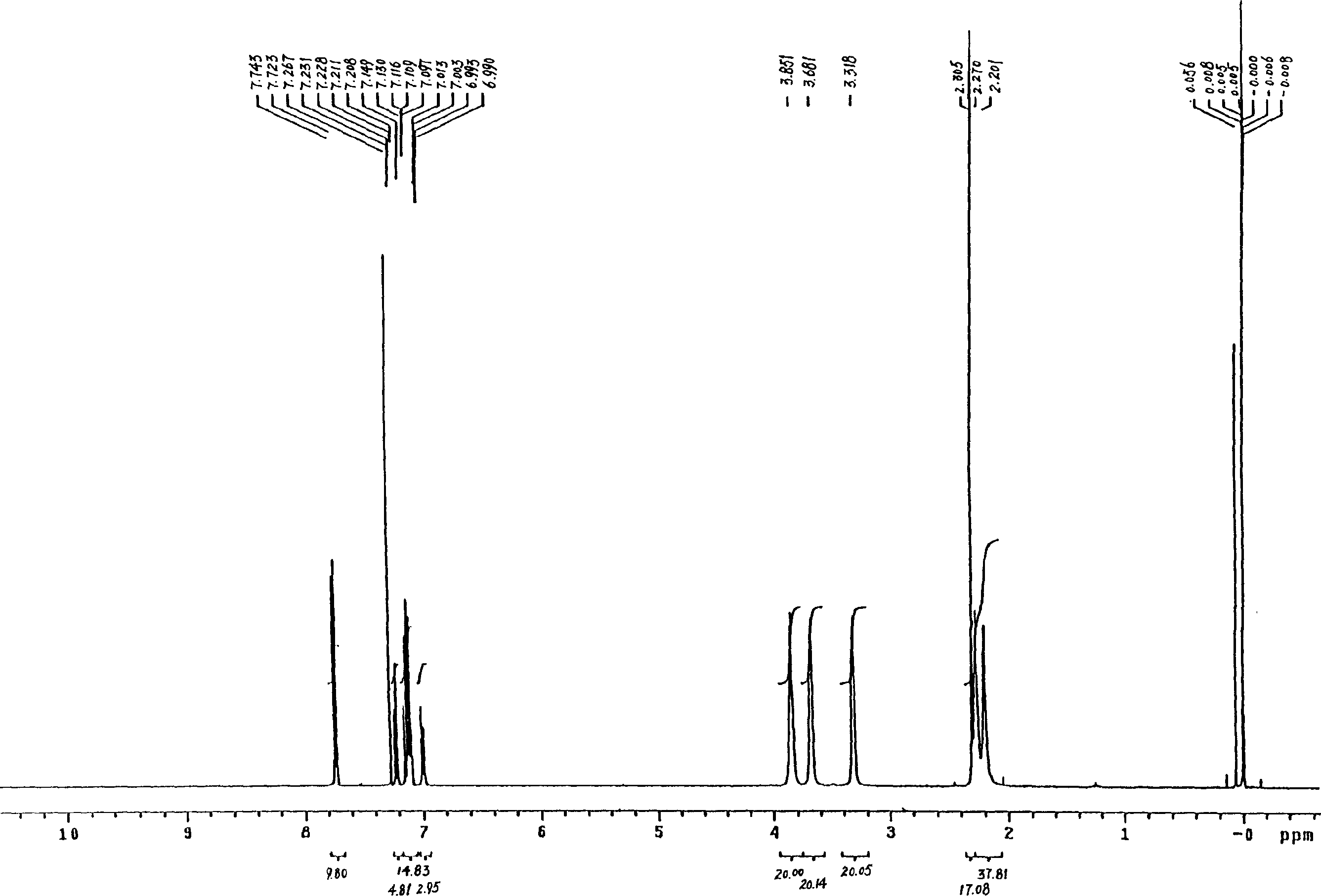
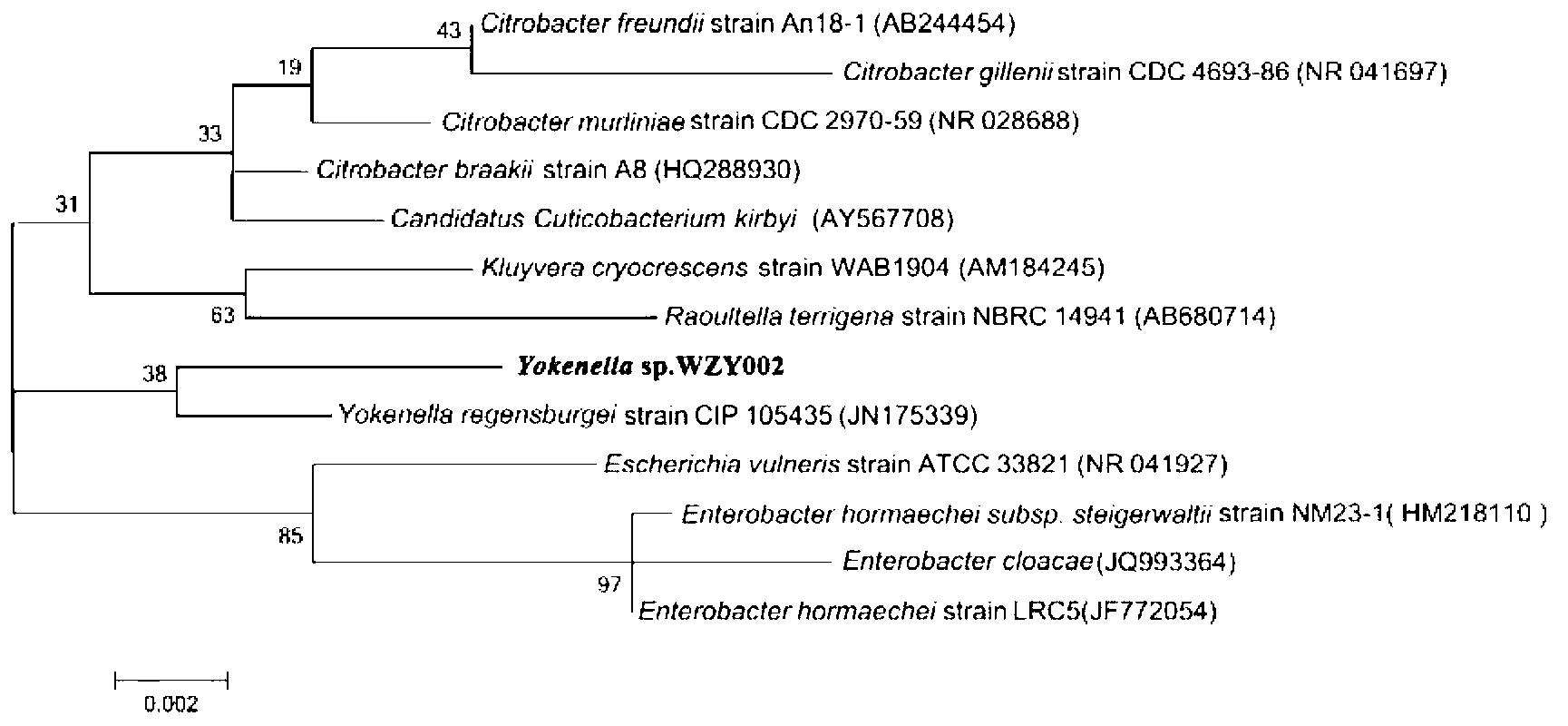
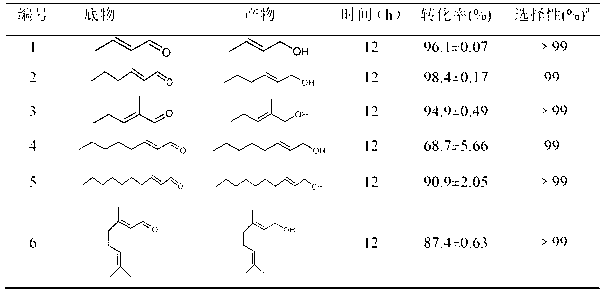
Yokenella sp. and application thereof in preparing alpha, beta-unsaturated enol and aromatic alcohol
ActiveCN103289922AHigh regional selectivityHigh stereoselectivityBacteriaMicroorganism based processesReaction temperatureKetone
The invention provides a novel bacterial strain-Yokenella sp. WZY002 and an application thereof in preparing alpha, beta-unsaturated olefine aldehyde (ketone) through regioselective reduction and aromatic alcohol through aromatic aldehyde (ketone) reduction. The bacterial strain is preserved in the China general microbiological culture collection center (CCTCC); the address is 430072, Wuhan University, Wuhan, China; the preservation number is CCTCC No: M2013099; the preservation date is March 22, 2013. The novel bacterial strain has the main beneficial effects of high regioselectivity, high stereoselectivity and high enzymatic activity, regioselective reduction of the alpha, beta-unsaturated enol is catalyzed so as to obtain various alpha, beta-unsaturated olefine aldehydes, and reduction of the aromatic aldehyde (ketone) can also be catalyze so as to obtain the aromatic alcohol. The bacterial strain is served as a biocatalyst and has high regioselectivity, high stereoselectivity and strong catalytic activity, the catalytic reaction does not need to add coenzyme, the reaction temperature is moderate, and the novel bacterial strain has a relatively high application value for industrial production.
Owner:ZHEJIANG UNIV OF TECH
Preparation of two kinds of high optical activity enantiomer of ethyl 4-cyano-3-hydroxybutyrate by biological catalysis method
InactiveCN101260415AHigh optical purityStrong response specificityMicroorganism based processesFermentationMicroorganismEnantiomer
The invention provides a method for preparing two high optical activity enantiomers of 4-cyano-3-hydroxy ethyl butyrate by biocatalysis. The microbial strain adopted in the method is Klebsiella pneumoniae Phe-E4 or Bacillus pumilus Phe-C3 which is used for catalysis. The product obtained by the biocatalysis of the strain Klebsiella pneumoniae Phe-E4 is (S)-4-cyano-3-hydroxy ethyl butyrate, and the product obtained by biocatalysis of the strain Bacillus pumilus Phe-C3 is (R)-4-cyano-3-hydroxy ethyl butyrate. The substrate percent conversion of the product obtained by the method can reach more than 98 percent. Chiral products with high optical purity can be obtained by the method, and the reaction is strong in specificity, byproducts are fewer and the post processing is simple. Therefore, the method has good prospect of industrial application and development.
Owner:中科院嘉兴中心应用化学分中心
Process for synthesizing ritonavir
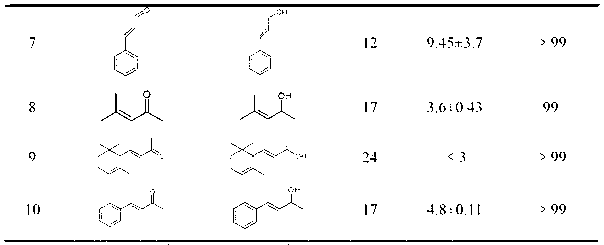
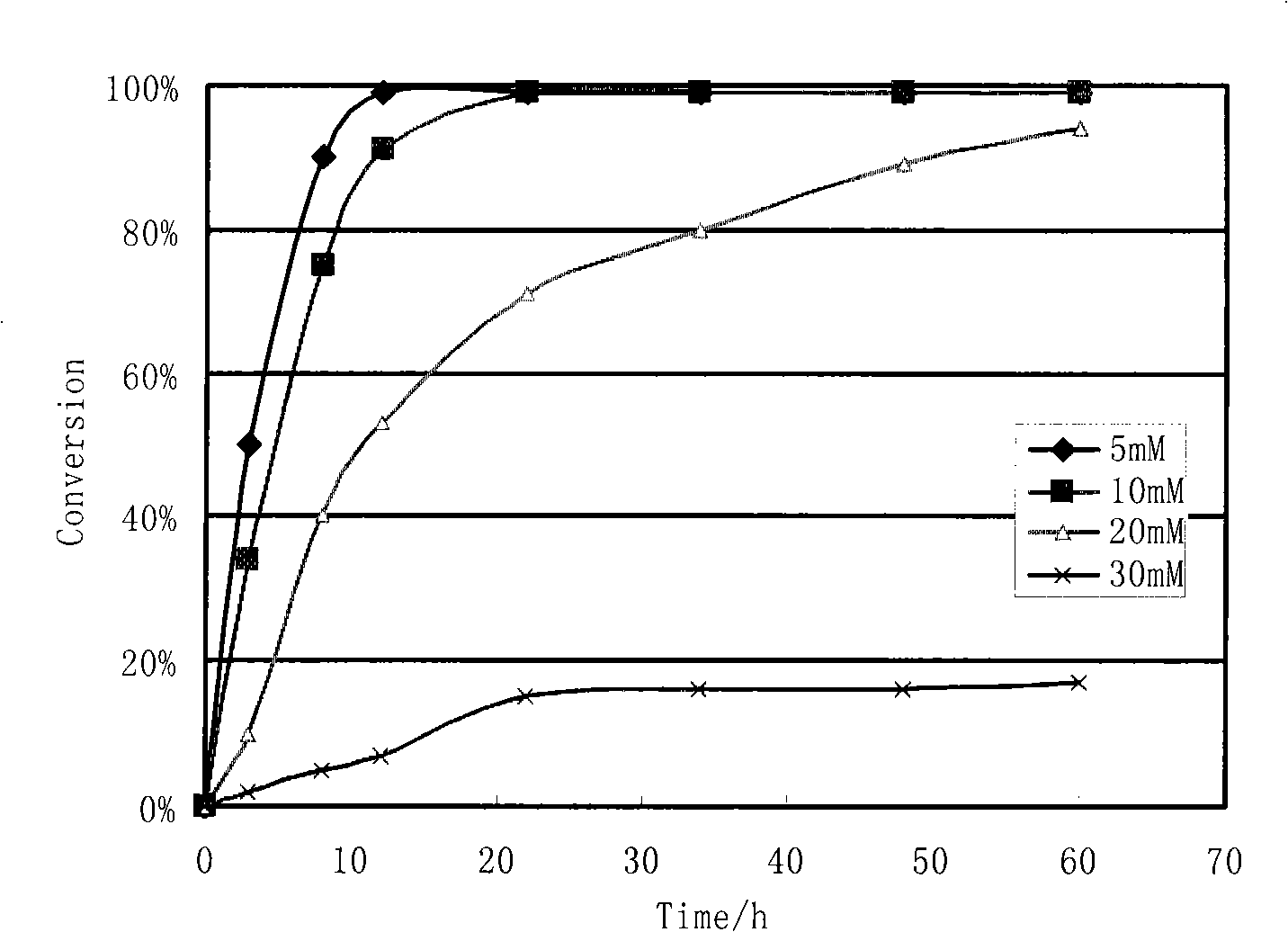
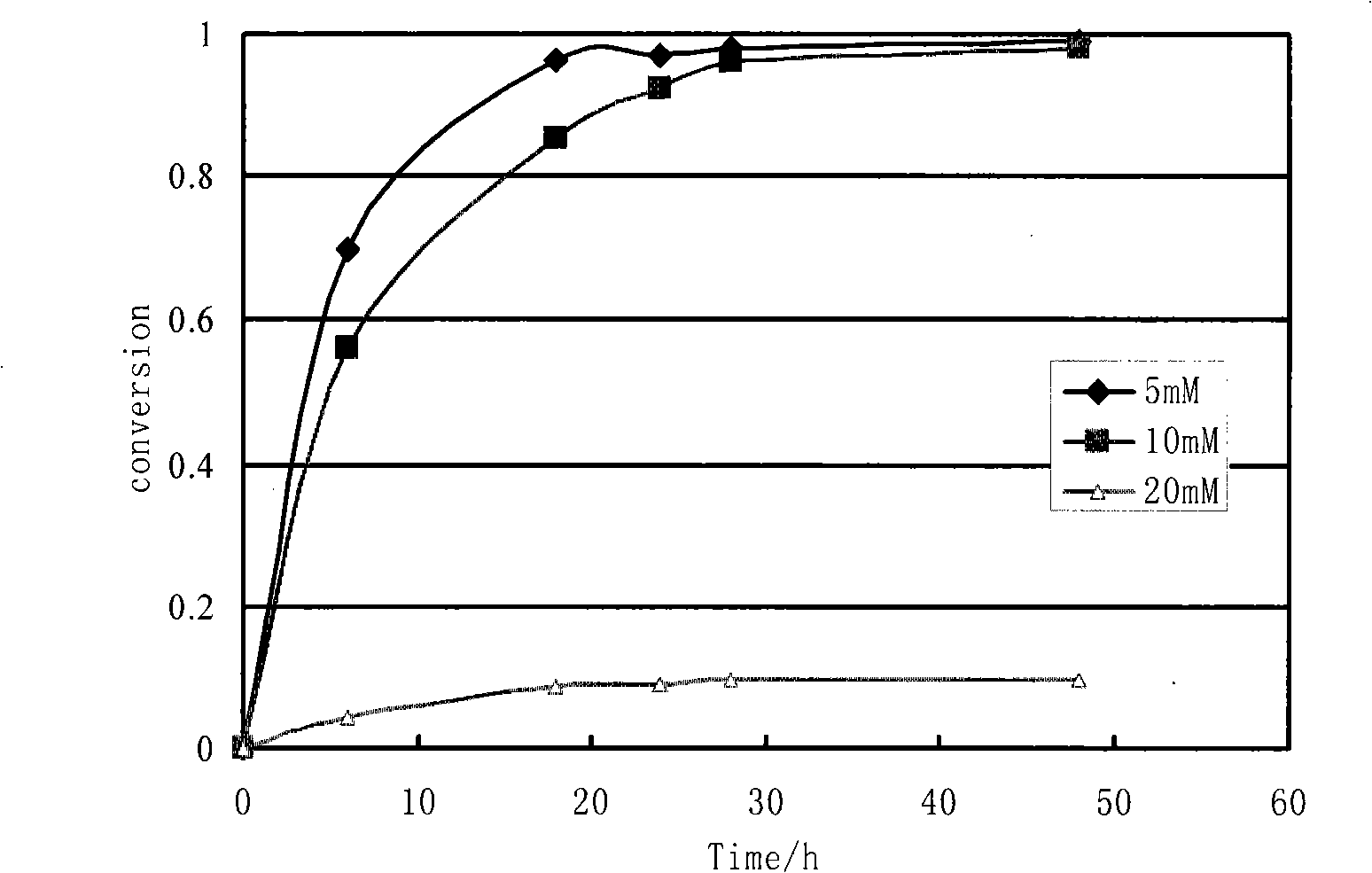
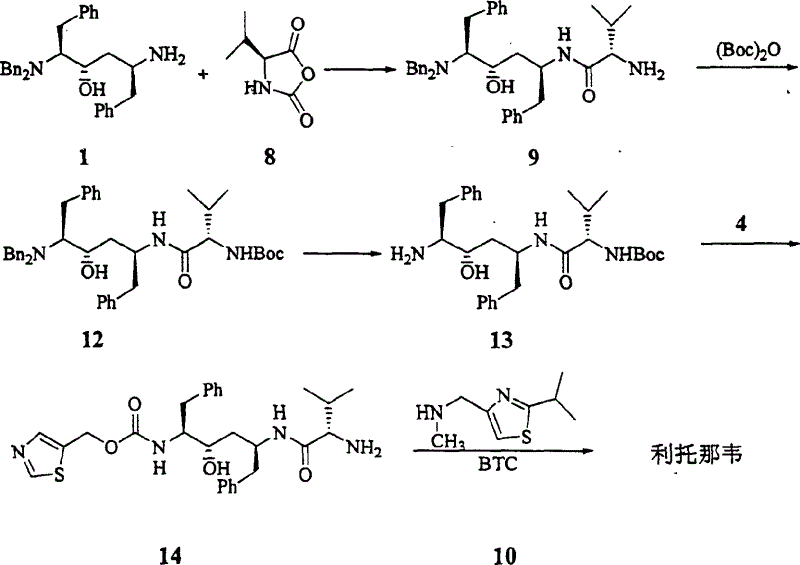
InactiveCN1554647AStrong response specificityLow costOrganic chemistryAntiviralsChloroformateHydrolysis
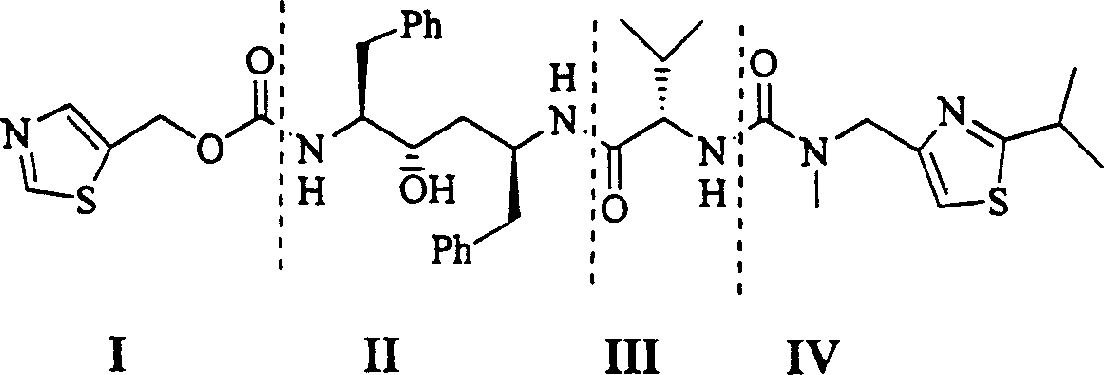
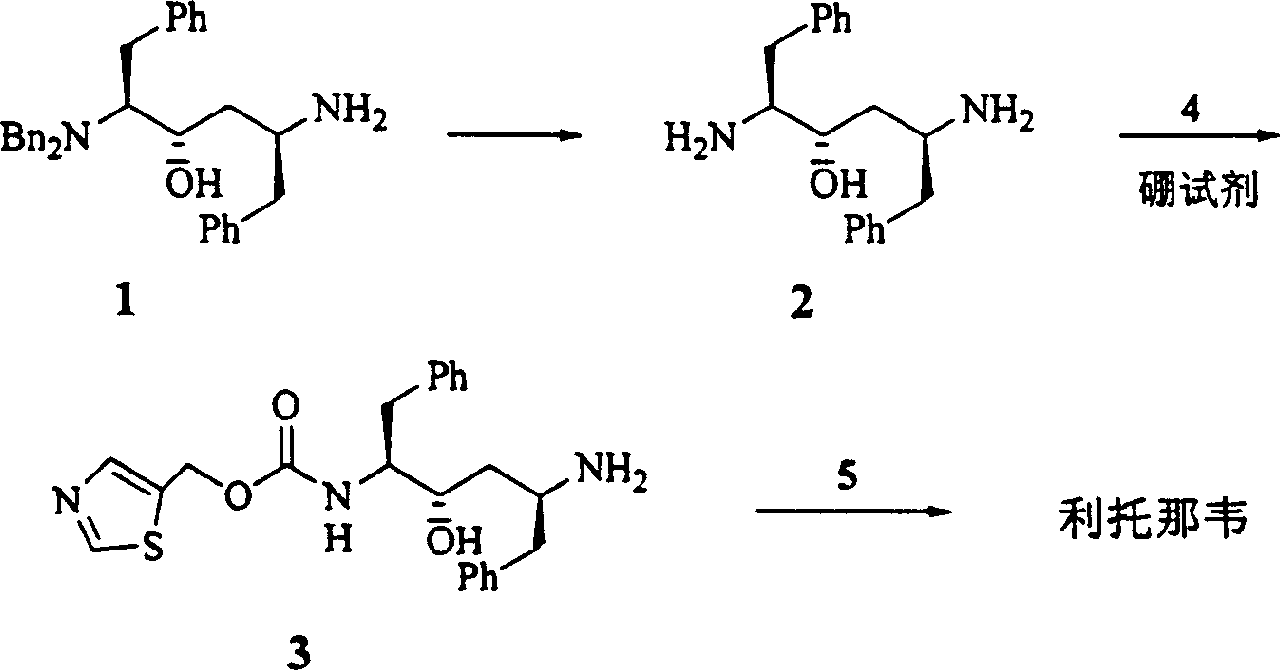
The present invention relates to the synthesis process of Ritonavir as one proteinase inhibitor for resisting AIDS. The synthesis process includes the condensation between benzylamino alcohol and valine NCA to obtain valyl benzylamino alcohol, the reaction between valyl benzylamino alcohol and ditert-butyl dicarbonate to obtain tert-butoxy acyl valyl benzylamino alcohol, the hydrogenolysis and debenzylation of tert-butoxy acyl valyl benzylamino alcohol in ammonium formate and Pd-C to obtain tert-butoxy acyl valyl amino alcohol, the active esterification reaction between tert-butoxy acyl valyl amino alcohol and 5-methylol thiazole, subsequent hydrolysis to obtain thiazolyl-5-methoxycarbonyl valyl amino alcohol, and the reaction of thiazolyl-5-methoxycarbonyl valyl amino alcohol and isopropyl thiazolyl methylamine under the action of BTC to obtain final product Ritonavir. The present invention has lowered cost and raised atomic utilization.
Owner:XIAMEN UNIV
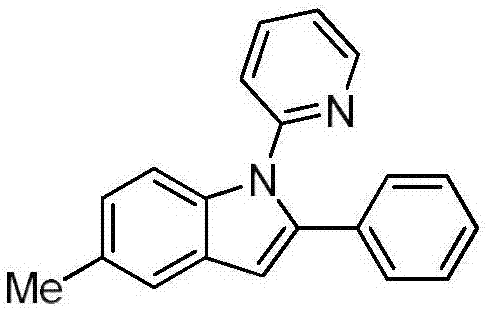
Preparation method of N-(2-pyridyl/pyrimidyl)indole derivative
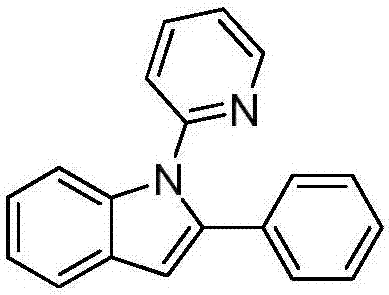
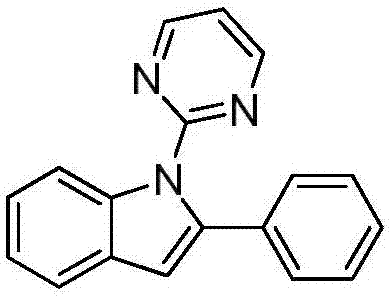
ActiveCN107973779ARaw materials are easy to getHigh yieldOrganic chemistryOrganic solventRoom temperature
The invention discloses a preparation method of an N-(2-pyridyl / pyrimidyl)indole derivative. The preparation method of the N-(2-pyridyl / pyrimidyl)indole derivative comprises the following steps: mixing a 2-substituted phenylaminopyridine / pyrimidine derivative, an alkenyl azide compound, a catalyst, an oxidizing agent, alkali and an organic solvent uniformly, heating to 60 to 80 DEG C under nitrogen or air, performing a cyclization reaction for 18 to 24 hours, cooling to room temperature after the reaction, and performing concentration and column chromatographic purification to obtain the N-(2-pyridyl / pyrimidyl)indole derivative. By the preparation method, the N-(2-pyridyl / pyrimidyl)indole derivative with various substituent groups which cannot be synthesized according to other methods canbe synthesized; furthermore, the used raw materials are easily available, the yield is high, the reaction condition is mild, the reaction time is short, the substrate range is wide, the reaction specificity is high and aftertreatment is simple, convenient and green.
Owner:HUAQIAO UNIVERSITY
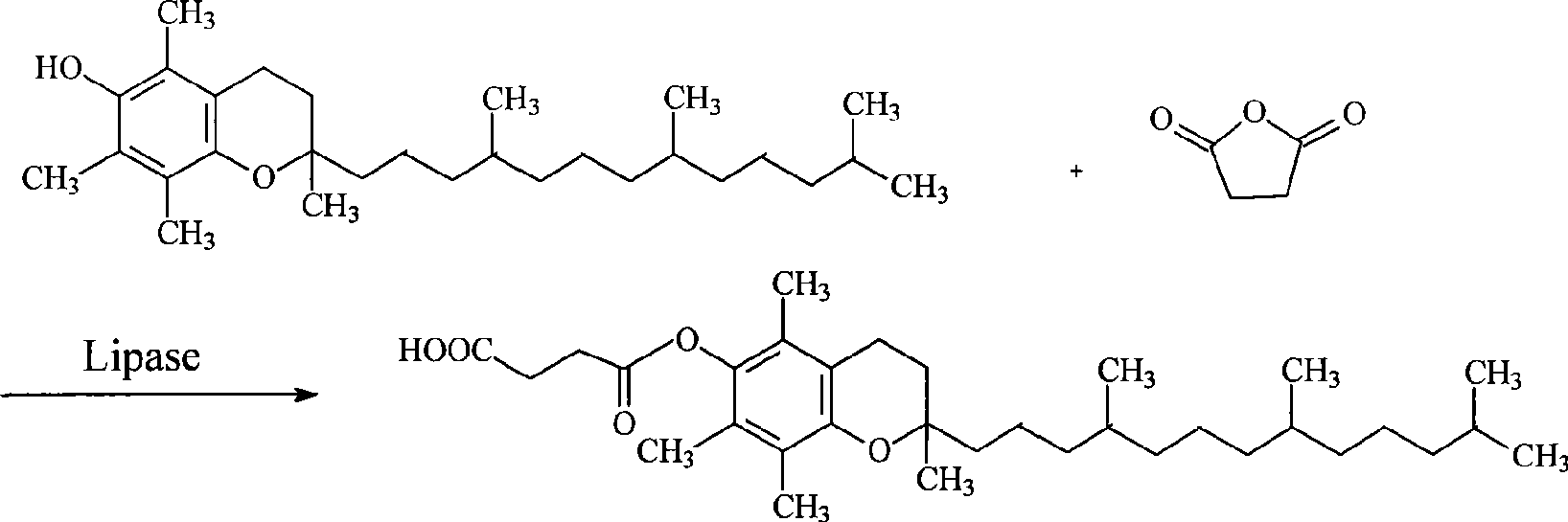
Method for synthesizing natural alpha-tocofecol tocopherol acid succinate by using lipase
A method for synthesizing natural alpha-tocopherol succinic acid monoester using lipase pertains to the field of biological chemicals, which is characterized in that the preparation method includes: adding the substrate of natural alpha-tocopherol and succinic anhydride at molar ratio of 1:1-1:8 in an organic solvent, adding lipase to start the reaction, oscillating or mixing reaction 20-72 hours under the conditions of 20-45 DEG C, wherein, the conversion rate of the alpha-tocopherol is 80-98.39%. Compared with the prior industry process in which esterification is performed at high-temperature by using high toxicity chemical catalyst under, the method has advantages of mild reaction conditions (room temperature, atmospheric pressure), low energy consumption, non-toxic catalyst, specificity, high reaction efficiency fewer accessory substances, and the like.
Owner:UNIV OF SCI & TECH BEIJING
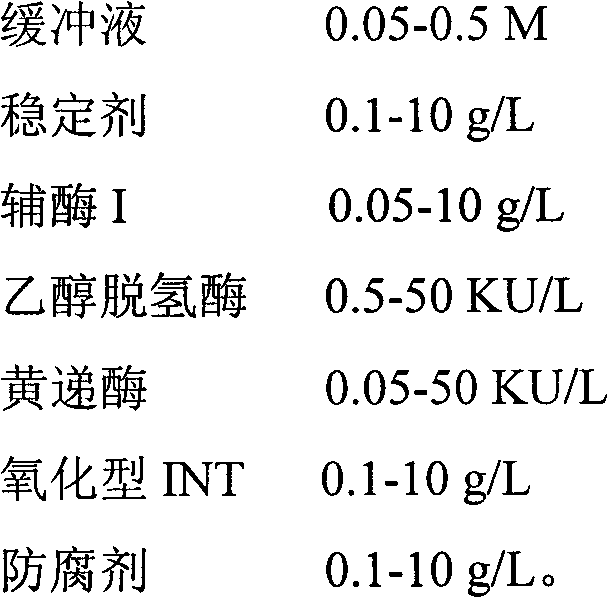
Method for determining alcohol concentration by using enzyme cycling method and alcohol determination kit
InactiveCN102564979AStrong response specificityHigh detection specificityColor/spectral properties measurementsEthanol dehydrogenasePreservative
The invention provides a method for determining alcohol concentration by using an enzyme cycling method and an alcohol determination kit. The method comprises the following steps of: generating reduced nicotinamide adenine dinucleotide I by using nicotinamide adenine dinucleotide I and alcohol under the action of alcohol dehydrogenase, reacting the reduced nicotinamide adenine dinucleotide I with oxidized iodonitrotetrazolium (INT) under the action of diaphorase to generate red reduced INT, detecting a change in the absorbance of a final reactant, and then calculating the alcohol concentration of a sample. The kit consists of the following components: 0.05 to 0.5M of a buffer solution, 0.1 to 10g / L of a stabilizer, 0.05 to 10g / L of nicotinamide adenine dinucleotide I, 0.5 to 50KU / L of alcohol dehydrogenase, 0.05 to 50KU / L of diaphorase, 0.1 to 10g / L of oxidized INT and 0.1 to 10g / L of a preservative. The method is high in reaction specificity, detection specificity and sensitivity, wide in linear range and low in cost; and the kit is high in stability and accuracy.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
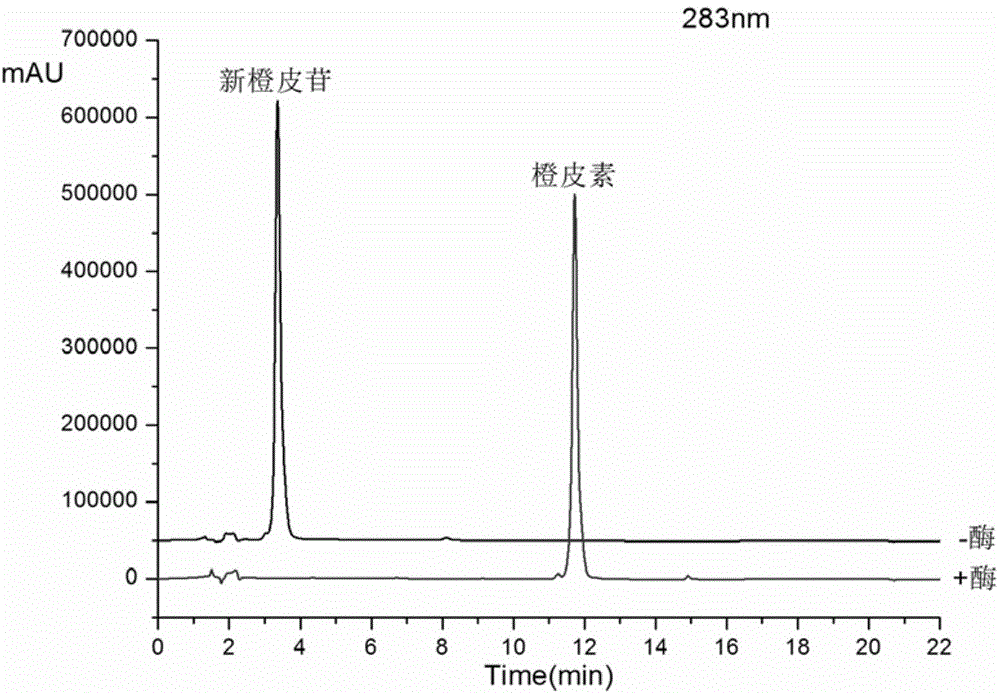
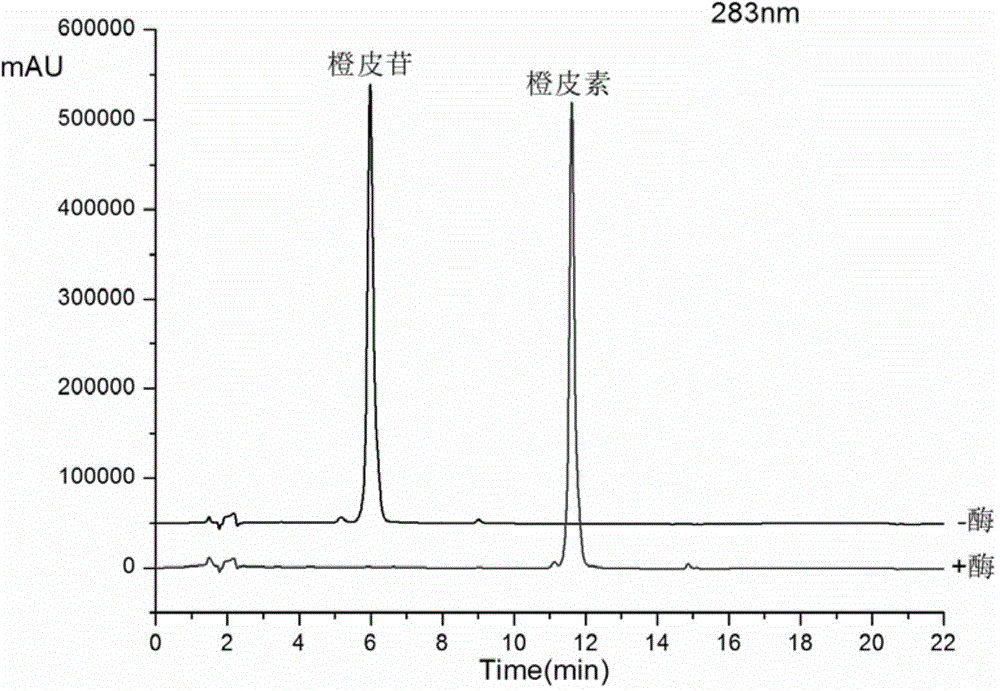
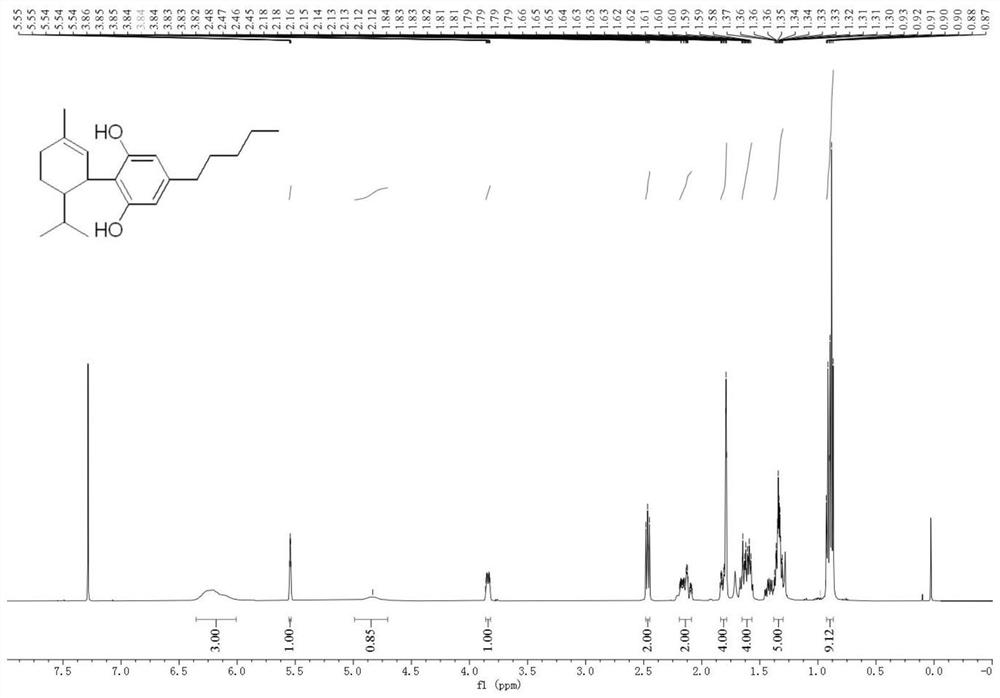
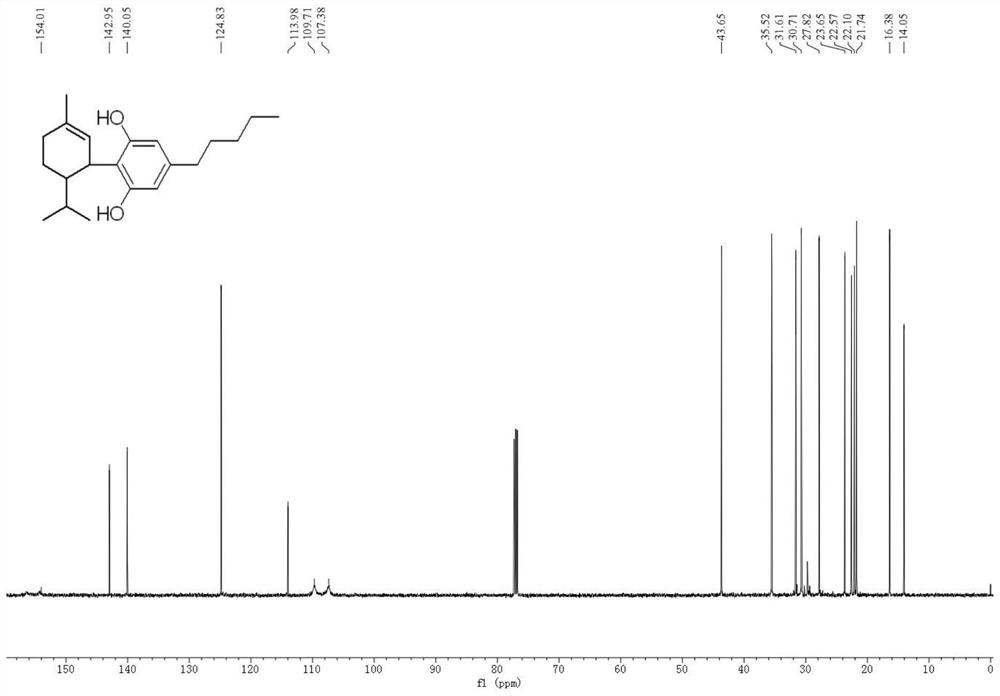
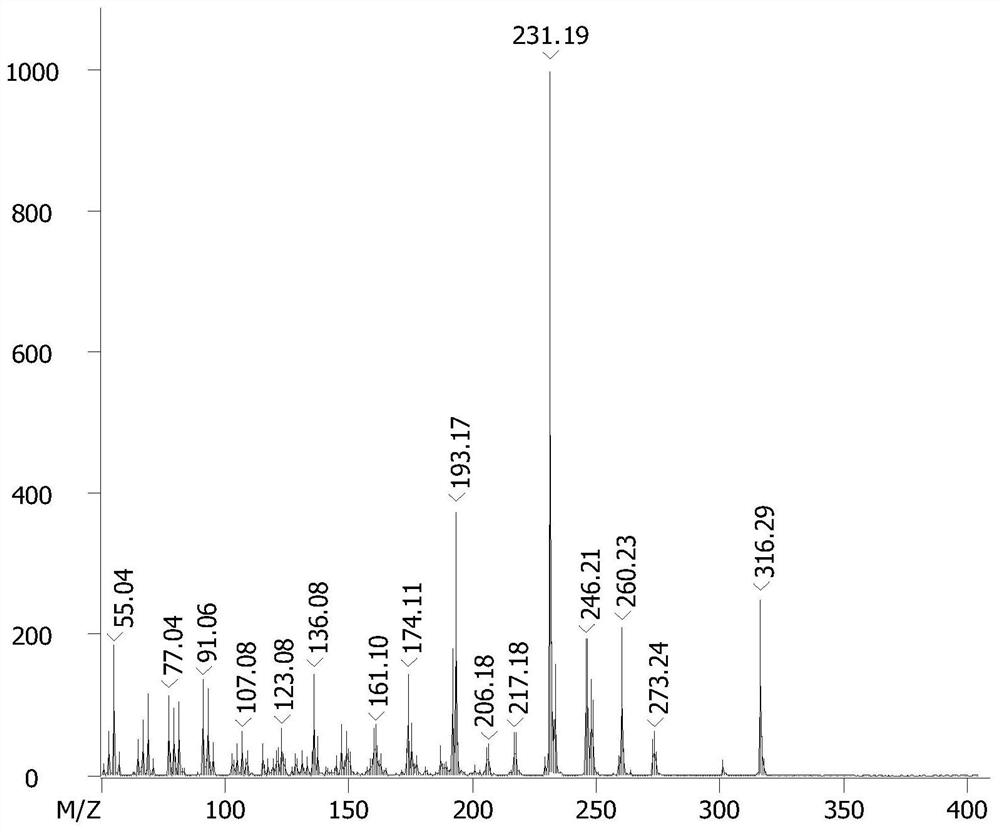
Method for preparing hesperetin from enzymatic hydrolysis neohesperidin or hesperidin
InactiveCN106148446AHigh purityHigh efficiency and high purityFermentationChromatographic separationOrganic solvent
The invention discloses a method for preparing hesperetin from enzymatic hydrolysis neohesperidin or hesperidin. Neohesperidin or hesperidin beta-D-glucoside bond can be hydrolyzed in one step through crude enzyme liquid to prepare corresponding aglycone hesperetin. The method includes the steps of preparing the crude enzyme liquid of Penicillium decumbens through fermentation, adjusting the content and reaction time of the crude enzyme liquid, directly converting neohesperidin or hesperidin into hesperetin, dissolving or extracting conversion liquid through organic solvent, and conducting chromatographic purification to obtain hesperetin. By means of the method, the defects that a traditional chemical method is strict in reaction condition, serious in pollution, low in conversion rate and the like when used for preparing hesperetin can be overcome, and the defects that when microbial fermentation is directly adopted, operation is complicated, products are not simplex, and products are difficult to purify can be overcome.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Process method for synthesizing ajoene from allicin
InactiveCN106957883AHigh purityStrong response specificityOn/in organic carrierFermentationSide reactionBioreactor
The invention belongs to the technical field of deep processing of garlic and particularly relates to a process method and a preparation method for synthesizing ajoene from allicin. The process method comprises the steps of extracting allinase, purifying allinase, immobilizing allinase, preparing allicin, synthesizing ajoene, and purifying ajoene. According to the process method, allinase is immobilized by virtue of an enzyme immobilization method and is converted into allicin through catalysis in a bioreactor, the reaction specificity is good, the catalytic efficiency is high, and a high-purity allicin product can be obtained; by aiming at characteristics of cleavage reaction of allicin, allicin is complexed by virtue of a hydrophilous complex in a complex catalysis reaction manner, so that the stability of allicin is improved; allicin is decomplexed in a reaction process and can timely react so as to generate ajoene, so that a large number of side reactions are avoided.
Owner:山东晨隆晟世生物科技有限公司
8, 9-dihydrocannabidiol as well as synthesis method and application thereof
ActiveCN113372196AStrong response specificityHigh yieldAntibacterial agentsOrganic chemistryPhellandrenePtru catalyst
The invention discloses 8, 9-dihydrocannabidiol as well as a synthesis method and an application thereof, and belongs to the field of organic synthesis. According to the invention, alpha-phellandrene and olive alcohol are taken as substrates, and under the action of a Lewis acid catalyst, 8, 9-dihydrocannabidiol is obtained through reaction. The raw materials are cheap, the used catalyst is cheap and easy to separate (filter removal), the usage amount is small (0.9% of the mass of reactants), the catalyst is mild (Lewis acid), the yield is high (90%), the industrialization cost is greatly reduced, and the operation is simple.
Owner:JIANGNAN UNIV
Application of cyclopalladated ferrocenylimine-phosphine adduct in synthesis of asymmetric biaryl compound
InactiveCN102125875AImplement responseAchieving BSC ResponsesCarboxylic acid nitrile preparationAmino preparation from aminesOrganic synthesisCoupling
The invention belongs to the technical field of organic synthesis, and in particular relates to an application of a cyclopalladated ferrocenylimine-phosphine adduct in synthesis of an asymmetric biaryl compound. In synthesis of the asymmetric biaryl compound, the cyclopalladated ferrocenylimine-phosphine adduct is utilized as a catalyst, and the catalyst has the advantages of good stability, efficient catalytic activity and wide applicability; and by utilizing the catalyst, a corresponding coupling product with high yield (up to 95%) can be obtained on the premise of smaller catalytic amount, and the synthesis process has the characteristics of mild reaction conditions, wide substrate range and strong reaction specificity.
Owner:ZHENGZHOU UNIV
Method for synthesizing grafted lysine viscose fibers
InactiveCN107829300AMild reaction conditionsStrong response specificityBiochemical treatment with enzymes/microorganismsVegetal fibresChemistryDyeing
The invention provides a method for synthesizing grafted lysine viscose fibers and belongs to the technical field of modification of fiber materials. According to the method, commercially available viscose fibers and lysine are taken as raw materials, and the grafted lysine viscose fibers with good skin-friendly property, antibacterial property and dyeing property can be prepared stably through steps as follows: preparation of purified viscose fibers, preparation of carboxylated viscose fibers, preparation of ethylene glycol non-aqueous reaction media, preparation of the grafted lysine viscosefibers and preparation of purified ethylene glycol. The carboxylated viscose fibers and the grafted lysine viscose fibers are produced under catalysis of glucose oxidase and protease in sequence, thegrafted lysine viscose fibers are prepared in the ethylene glycol non-aqueous reaction media, reaction conditions are mild, reaction specificity and reaction efficiency are high, no by-products or three wastes are produced, and the method is a typical green production technology; the grafted lysine viscose fibers synthesized with the method are covered with amino acid, can be widely applied to the fields such as textiles, clothes, food and the like and have great social value and economic benefits.
Owner:CHONGQING UNIV
Chlorinating and shrink-proofing mercerization method for wool tops
ActiveCN105544200AThe process steps are simpleReduce yellowingLight resistant fibresBiochemical treatment with enzymes/microorganismsFiberEnzymatic hydrolysis
The invention discloses a chlorinating and shrink-proofing mercerization method for wool tops. The method comprises a wool top chlorinating step, a reduction step, a hydrolysis step, a softening and shrink-proofing step and the like. The wool tops exported from the step S1 are not padded, so that peptide chains, which are not subjected to enzymatic hydrolysis, of scales on the fiber surface are not completely stripped. A penetrant is brought into the reaction in the step S2 along the wool tops, so that improvement of the infiltration capacity of horse radish peroxidase is facilitated, and the rate of enzymatic reaction is accelerated. The wool treated by the method disclosed by the invention is full, and excellent in elasticity; the scales on the wool surface are thoroughly stripped after mercerization; and the wool has relatively high stability in a dye bath or a bleach bath after a shrink-proof agent for softening and shrink-proofing treatment forms a film.
Owner:TIANYU WOOL IND ZHANGJIAGANG FREE TRADE ZONE CO LTD
Phosphonium salt compound containing precursor of biphosphorus ylide cyclopentadienyl cyclocarbene as well as preparation method and application thereof
InactiveCN101717408AReduce dosageWide range of choicesOrganic compound preparationGroup 5/15 element organic compoundsPhosphonium saltOrganic synthesis
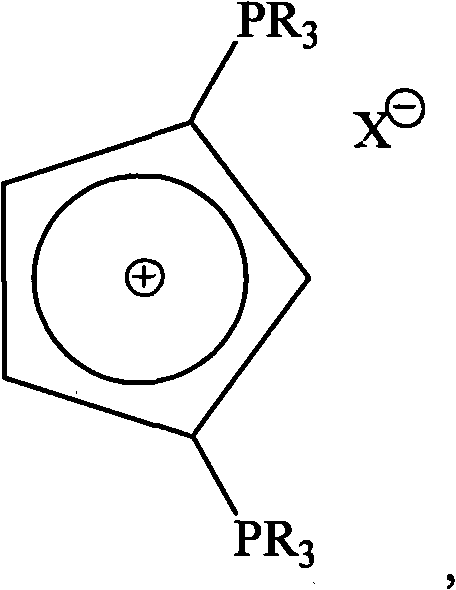
The invention relates to a phosphonium salt compound containing precursor of biphosphorus ylide cyclopentadienyl cyclocarbene as well as a preparation method and application thereof, which belong to the field of organic synthesis. The adopted technical scheme is as follows: the general formula of the phosphonium salt compound containing precursor of biphosphorus ylide cyclopentadienyl cyclocarbene: FORMULA, wherein R is alkyl or aryl, X is selected from F-, Cl-, Br-, I-, PF6-, BF4-, SbF6-, ClO4- or N3-. The preparation method of the phosphonium salt compound comprises the following steps of: adding 1, 1'-ferrocene bimercury compound, tertiary phosphine PR3 and palladium chloride to an organic solvent, refluxing under protection of N2, filtering, evaporating to dryness and recrystallizing to obtain the phosphonium salt compound. In addition, in the invention, the phosphonium salt compound is used as a catalyst ligand so that the phosphonium salt compound and the metal salt act togetherto catalyze and synthesize alpha-aryl derivatives of carbonyl compound by one step. The application test indicates that compared with traditional N-heterocyclocarbene ligand, the phosphonium salt compound has excellent catalytic activity.
Owner:LUOYANG NORMAL UNIV
Dendrimer with alkynyl core and outer amino acid shell, and Huisgen 1,3-dipolar cycloaddition synthetic method and application thereof
ActiveCN102731417AHigh reactivityEasy to purifyOrganic chemistryOther chemical processesDendrimer1,3-Dipolar cycloaddition
The invention discloses a dendrimer with an alkynyl core and an outer amino acid shell, and a Huisgen 1,3-dipolar cycloaddition synthetic method and application thereof. In the preparation process of the dendrimer, 0.5-G, 1.5-G, 2.5-G or 3.5-G dendrimer molecules with an outer azido shell are synthesized; the dendrimer molecules and ascorbic acid / copper sulfate composing a catalytic system are added into alkynylated tertbutyloxycarbonyl-amino acid dissolved in DMF, so that the dendrimer molecules with the outer azido shell and ethynylated amino acids are bonded together through Huisgen 1,3-dipolar cycloaddition; after a reaction at a temperature of 15 to 35 DEG C for 4 to 24 hours, dichloromethane is added to dilute a reaction solution, and then extraction with water is carried out. The invention has the following advantages: reaction specificity is good, reaction efficiency is high, and the outer shell of the dendrimer has a plurality of amino acid ligands, which enables a prepared adsorption material to have better protein separation or blood purification effects compared with common adsorption materials with a space arm as a single active site.
Owner:SOUTH CHINA UNIV OF TECH
Rhodococcus erythropolis and application thereof in microbe-catalyzed preparation of chiral aromatic alcohol
ActiveCN102417889AHigh stereoselectivityImprove conversion rateBacteriaMicroorganism based processesMicroorganismAromatic alcohol
The invention provides a novel bacterial strain - Rhodococcus erythropolis WZ010 and application thereof in selectively oxidizing racemic aromatic alcohol to prepare R-aromatic alcohol and asymmetrically reducing aromatic ketone to produce S-aromatic alcohol. The bacterial strain is preserved in China Center for Type Culture Collection, Wuhan University, Wuhan, China, the postcode is 430072, the preservation number is CCTCC No: M2011336, and the preservation date is 29 September, 2011. The invention mainly has the advantages that: the invention provides the novel bacterial strain with high stereoselectivity and high enzymatic activity, the R-aromatic alcohol and the S-aromatic alcohol can be obtained at the same time by selective oxidation and asymmetric reduction with the bacterial strain, the reaction specificity and catalytic activity of the bacterial strain are high, the production cost is greatly reduced, moreover, environment pollution is little, and the bacterial strain is suitable for industrialized production.
Owner:ZHEJIANG UNIV OF TECH
Preparation method and application of non-natural ginsenoside
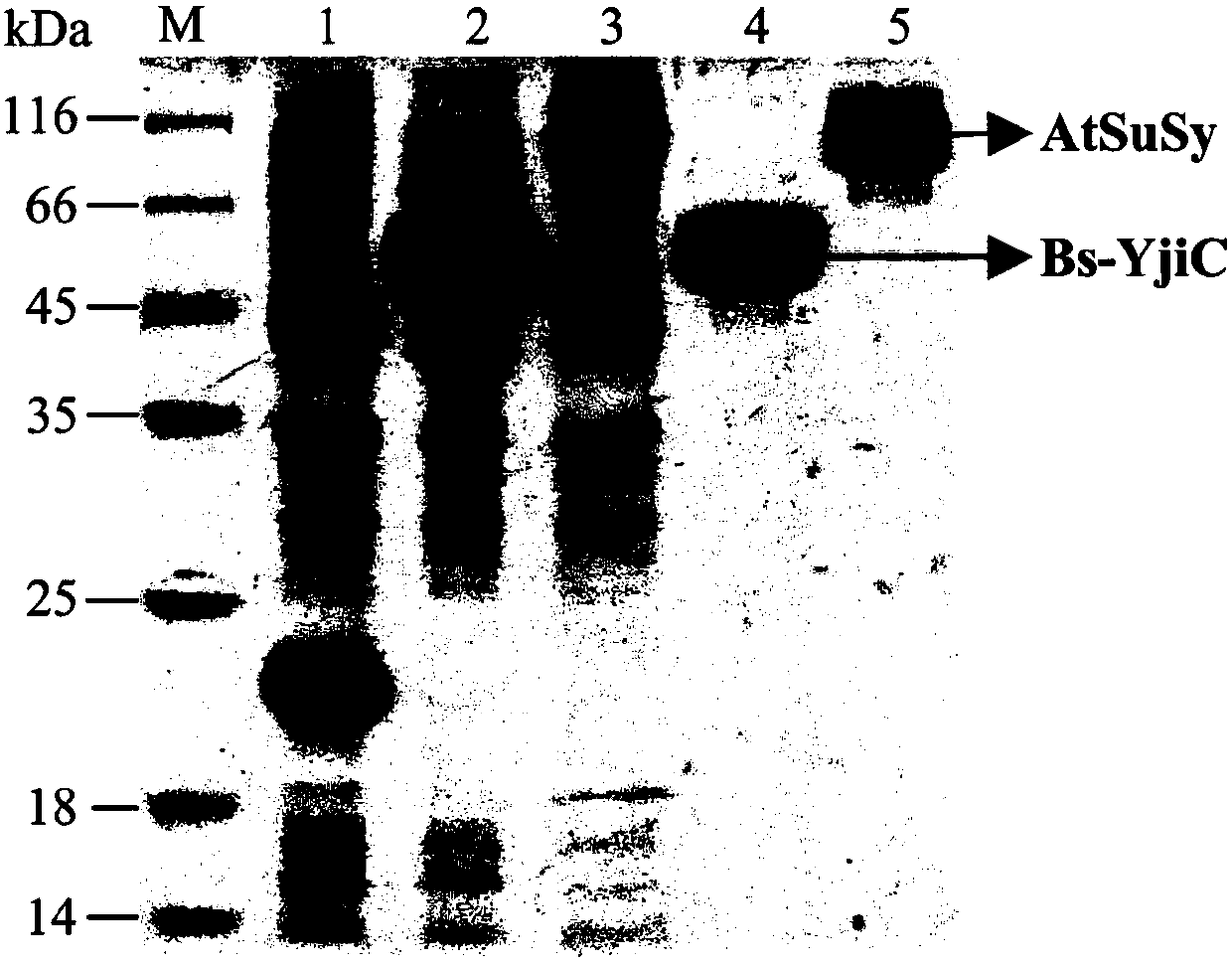
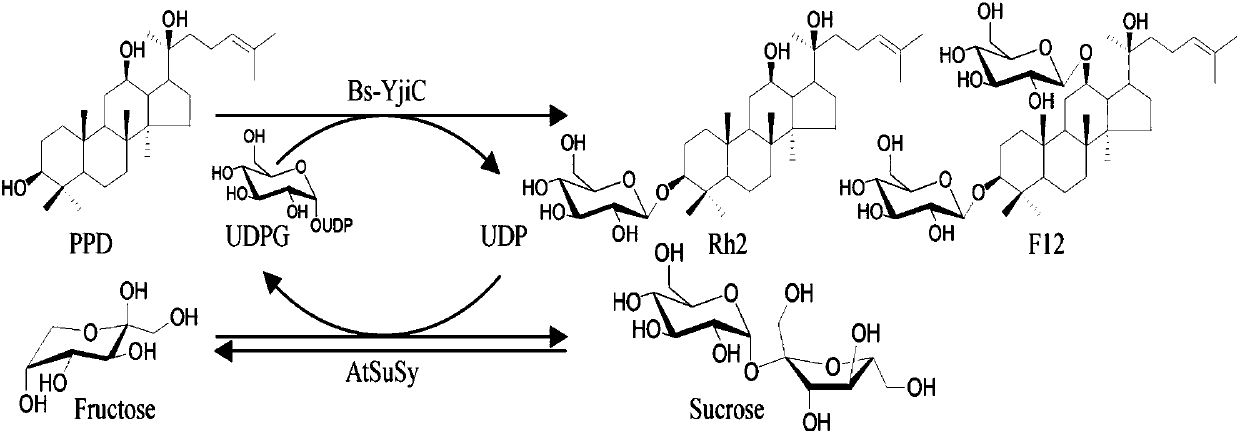
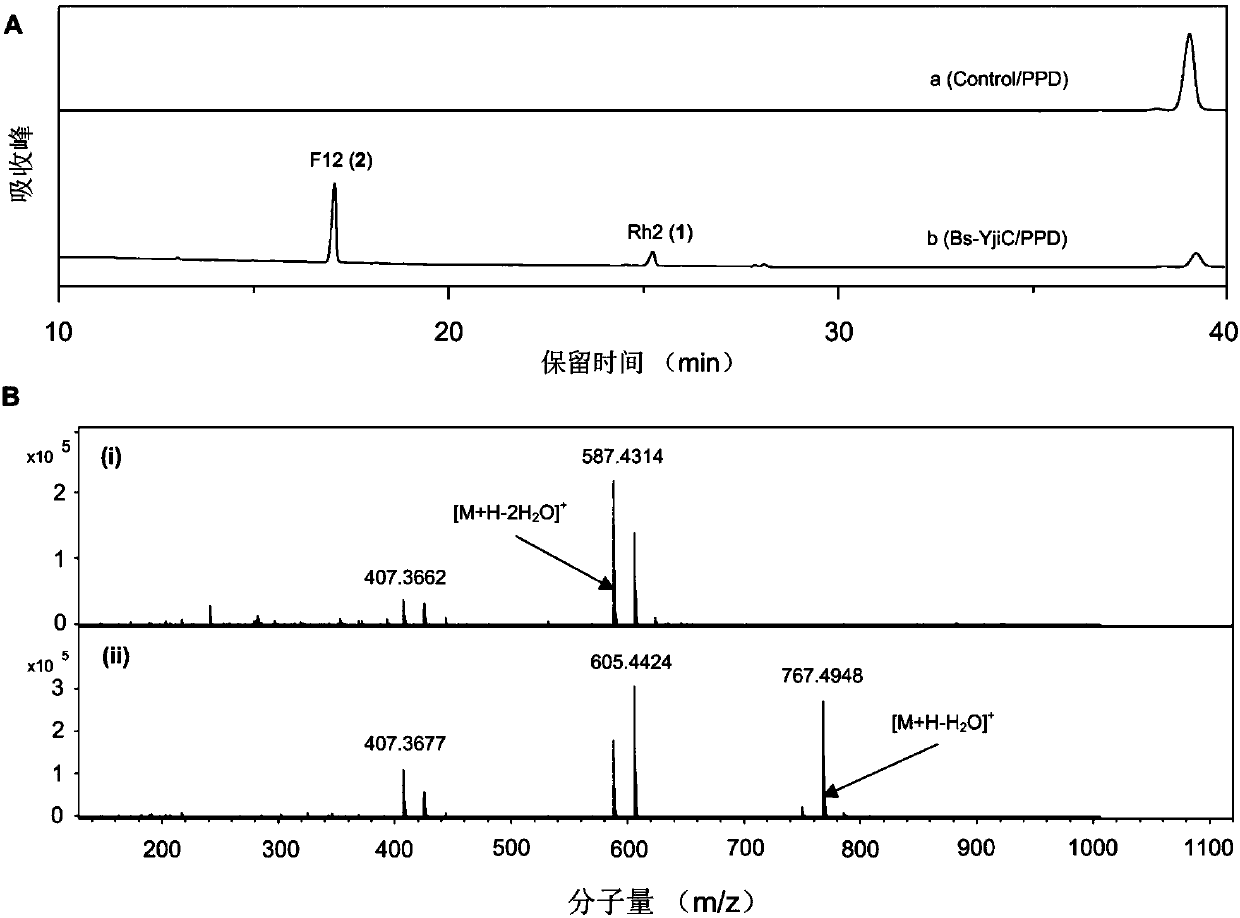
ActiveCN107929296AEnhanced inhibitory effectWide variety of sourcesOrganic active ingredientsGlycoside steroidsSucrose synthetaseProtopanaxadiol
The invention relates to a preparation method and application of non-natural ginsenoside, specifically to an antitumor drug containing the non-natural ginsenoside F12 (3-O-beta-D-glucopyranosyl-12-O-beta-D-glucopyranosyl-20(S)-protopanaxadiol). The antitumor drug of the invention has significant inhibitory effect on colon cancer cells, liver cancer cells, lung cancer cells and gastric cancer cells. According to the invention, cheap sucrose is used as a glucose glycosyl donor, glycosyltransferase and sucrose synthase are used as coupled catalysts, and protopanaxadiol (PPD) is used as a glycosylacceptor so as to realize high-efficiency catalysis of glycosylation of hydroxyl groups at the position C3 and the position C12 of protopanaxadiol for production of the non-natural ginsenoside F12; and the method has the characteristics of wide sources of a substrate, good specificity of reactions, high catalysis efficiency, simple purification of the non-natural ginsenoside, etc.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Method for preparing analogue of carnosine
This invention discloses a method for preparing carnosine analog. The method uses enzyme as the catalyst, and does not need group protection or condensation agent during the reaction, thus having such advantages as simple process, mild reaction conditions, easy operation, complete utilization of resources, no pollution and low cost. The prepared canosine analog has high purity (93.5-95.5%), good quality and strong antioxidative effect, and can be used as antioxidative active substances in medicine, food, health product, cosmetic and feed.
Owner:CHONGQING UNIV
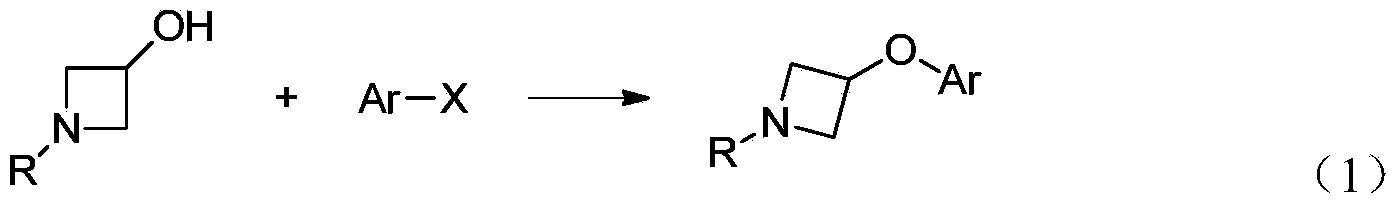
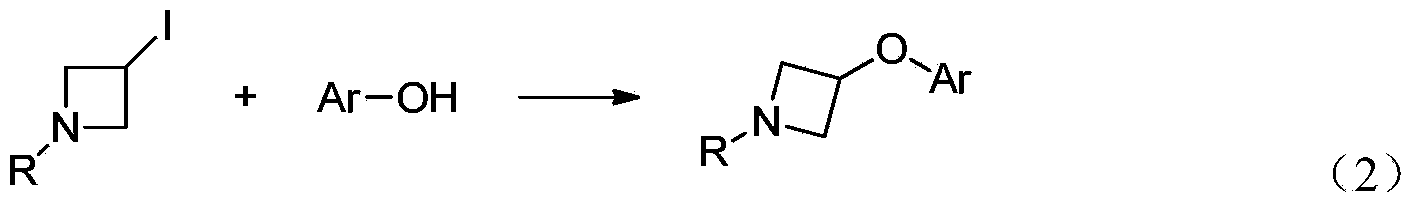
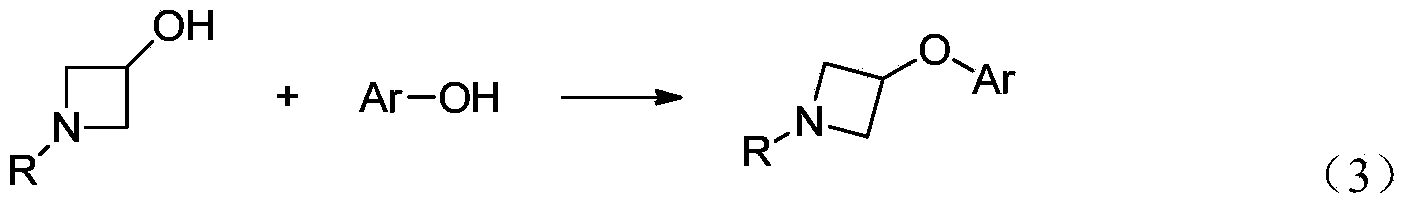
Preparation method for N-tert-butyloxycarboryl-azetidine aromatic ether/aromatic heterocyclic ether compounds
ActiveCN104140387AWide applicabilityImprove stabilityOrganic chemistryTert-Butyloxycarbonyl protecting groupOrganic synthesis
The invention discloses a preparation method for N-tert-butyloxycarbonyl-azetidine aromatic ether / aromatic heterocyclic ether compounds, and belongs to the technical field of organic synthesis. The method is as follows: by taking 1-tert-butyloxycarboryl-3-iodoazetidine and arylboronic acid or nitrogen-containing heterocyclic boric acid as materials, producing the N-tert-butyloxycarboryl-azetidine aromatic ether / aromatic heterocyclic ether compound by virtue of copper-catalyzed carbon-oxygen cross-coupling reaction. The reaction conditions are gentle, the substrate applicability is good, the reaction specificity is strong, and a medium to high yield can be obtained. A catalyst system for the reaction has good stability, efficient catalytic activity and extensive applicability, and can effectively avoid damages of strong-basicity reaction conditions on a certain functional groups; moreover, the materials are cheap and easy to obtain, so that the cost is low.
Owner:TYK MEDICINES ZHENGZHOU INC +1
Etimicin sulfate preparation method
ActiveCN105524129AEasy to operateEasy to industrializeSugar derivativesSugar derivatives preparationGentamicin C1aAcetylation
The invention provides an etimicin sulfate preparation method. The method comprises the following steps: taking gentamycin C1a base, dissolving the gentamycin C1a base in a certain proportion of a solvent, adding a certain proportion of a complexing agent to complex, adding a certain proportion of an amino group protection agent, keeping for a certain time, adding a certain proportion of a precipitating agent, stirring above materials, filtering the obtained mixture, adding a certain proportion of an acetylation reagent to the above obtained filtrate, reacting for a certain time, adding a certain proportion of a reducing agent, reacting for a certain time, removing an amino protection group, adding a certain proportion of water, carring out vacuum concentration under certain conditions to remove the solvent, adjusting the pH value with ammonia water, adsorbing with macro-porous resin, carrying out gradient separating purification with diluted ethanol, collecting an etimicin solution with a certain purity, carrying out vacuum concentration under certain conditions, adding sulfuric acid to adjust the pH value, adding a certain proportion of active carbon, decolorizing, filtering, and drying to obtain etimicin sulfate according with relevant standards.
Owner:WUXI JIMIN KEXIN SHANHE PHARMA +1
Synthetic method of doxylamine succinate intermediate
InactiveCN103058916AStrong response specificityRapid responseOrganic chemistryGrignard reagentPyridine
The invention discloses a synthetic method of a doxylamine succinate intermediate, which is characterized by comprising the following steps of: A, 2-pyridine-phenyl methyl methanol synthesis: dissolving acetophenone into methyl tertiary butyl ether, adding a catalyst, then agitating and dropwise adding a Grignard reagent, and obtaining a target product, i.e. 2- pyridine-phenyl methyl methanol after separation and desolvation; and B, 2-pyridine-phenyl methyl methanol purification: dissolving the 2-pyridine-phenyl methyl methanol in the step A into methanol, dropwise adding acetone slowly into solution till a large amount of 2-pyridine-phenyl methyl methanol is crystallized and separated out. The invention aims to overcome the defects in the prior art and provides the synthetic method of the doxylamine succinate intermediate, which has the advantages of simple, safe and reliable process, high efficiency and low production cost.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
Preparation method of trans-trisubstituted alkene derivative
ActiveCN109879899AHigh regional selectivityRaw materials are easy to getGroup 3/13 element organic compoundsPalladium catalystAlkyne
The invention discloses a preparation method of a trans-trisubstituted alkene derivative. The preparation method comprises following steps: 1, an alkali, an alkyne, a palladium catalyst, a ligand, a halogenated aromatic hydrocarbon, a precursor compound, and an organic solvent are introduced into a reaction container, nitrogen gas is adopted for repeat filling, and reaction is carried out for 12 to 36h at 60 to 140 DEG C; 2, a product obtained in step 1 is diluted with Ethyl Acetate, is washed with water, and is separated so as to obtain an organic phase; and 3, the organic phase obtained in step 2 is subjected to drying, filtering, concentrating, and column chromatography or thin-layer chromatography processing so as to obtain the trans-trisubstituted alkene derivative. According to the preparation method, construction of one carbon boron bond is realized in construction of the trans-tri aromatic hydrocarbon-substituted alkenes, excellent zone selectivity is achieved, and the trans-trisubstituted alkene derivative which is not easily synthesized using other methods is synthesized.
Owner:HUAQIAO UNIVERSITY
Preparation method for betulinic acid
The invention relates to a preparation method for a betulinic acid. According to the present invention, betulin is adopted as a raw material; the method comprises a process route, wherein the process route comprises the following steps: (1) preparation of a betulinic acid crude product by selective oxidation of the betulin; (2) purification of the betulinic acid crude product to obtain the betulinic acid product. With the present invention, the direct production method for the betulinic acid by selective oxidation of the betulin is provided, the process route is simple, and the produced product is easy to purify.
Owner:NORTHEAST FORESTRY UNIVERSITY
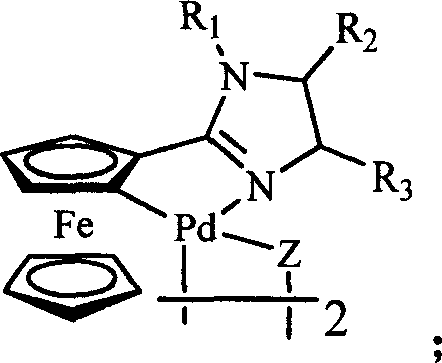
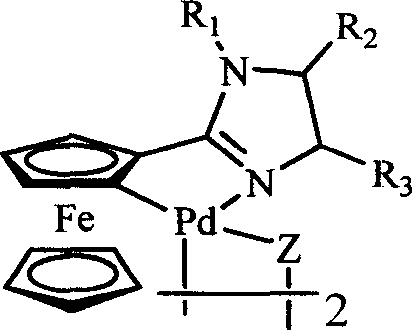
Ferrocenyl imidazoliny palladium compound, its preparation method and its uses in catalytic synthesis of coupling product
InactiveCN101007825AGood structural controlHigh catalytic activityOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbon by hydrocarbon condensationHalidePalladium compound
The invention discloses a ferrocenyl imidazoline palladacycle complexe, the preparation method and its application in catalizing coupling product. The general formula of compound is in the right. The method comprises: carrying out reaction with ferrocenyl imidic acid methyl ester hydrochlorate and vicinal diamine, getting ferrocenyl imidazoline compound through post treatment; carrying out reaction with mentioned compound above with palladium lithium halides and NaOAc in organic solvent while being stirred at room temperature, and getting final product. The catalytic synthesis of coupling product with siad compound comprises following steps: dissloving catalyst, aryl boracic acid, alkali and halogenated aromatic compound in organic solvent, heating for reaction, cooling for extraction, purifying and getting coupling product. The invention is characterized by high catalytic property, environmental- friendly, insenstive to aor and water, good thermal stability of non-phosphine ligand, easy processing route, temperate reaction condition, and high specificity.
Owner:ZHENGZHOU UNIV
Method for producing 6 alpha-methylprednisolone and its derivatives
InactiveCN1978457AStrong response specificityHigh yieldSteroids preparationKetone6Alpha-Methylprednisolone
This invention discloses a 6alpha-methylprednisolone and production method of its derivate. This invention uses cortisone acetate and its derivate as raw material to produce 6alpha-methylprednisolone and its derivate. Its production method includes steps such as: lead-in methenyl at sixth position, methenyl is transformed to alpha-methyl, 1,2 position is dehydrogenated to 6alpha-methyl prednisoni acetasby by method of arthrobacterium biotransformation, then selectively hydrogenise 11-ketone to 11 beta-hydroxy group. Using this invention can obtain higher 6alpha-methylprednisolone and yield and quality of its derivate. This invention has characteristics such as: position specificity is high, yield is high. It has important significance for preparing steroid medicine.
Owner:ZHEJIANG UNIV
Preparation method for substituted isocoumarin derivatives
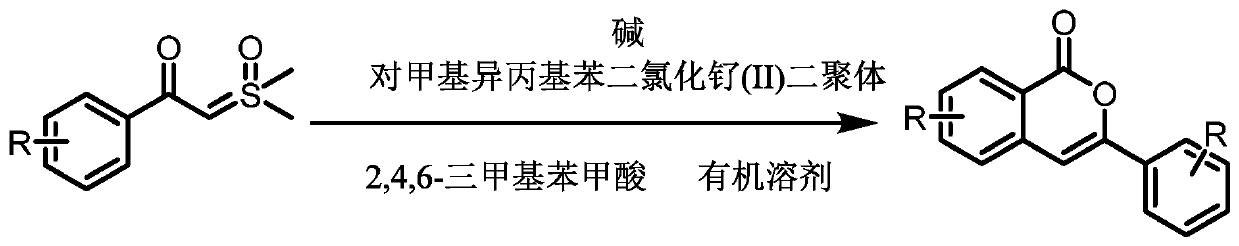
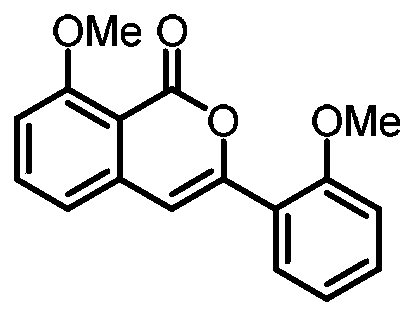
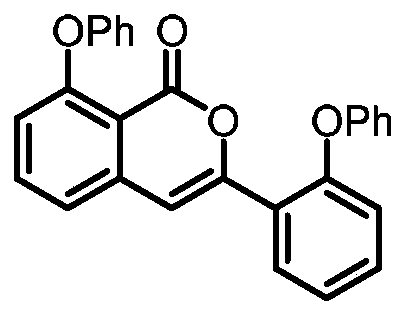
ActiveCN110128393ARaw materials are easy to getHigh yieldOrganic chemistryCarbon–oxygen bondOrganic solvent
The invention discloses a preparation method for substituted isocoumarin derivatives. The method comprises the following steps: a novel carbon-carbon bond and a carbon-oxygen bond are constructed while a carbon-carbon bond and a carbon-hydrogen bond are cut, a base, dichloro(p-cymene)ruthenium(II) dimer, 2,4,6-trimethylbenzoic acid and sulfur ylide are reacted in an organic solvent, and thereforethe substituted isocoumarin derivatives are prepared. According to the method provided by the invention, the raw materials used in the method are easy to obtain, the yield is high, the reaction conditions are mild, the reaction time is short, the substrate range is wide, the reaction specificity is strong, and the post-treatment is simple and green.
Owner:HUAQIAO UNIVERSITY
Method for preparing L-(+)-lactic acid
The invention discloses a method for preparing L-(+)-lactic acid, relates to an enzyme method for synthesizing the lactic acid, and is a technology for producing the L-(+)-lactic acid by catalyzing asymmetric hydrolytic ethyl lactate in the enzyme method in an organic phase. The method has the steps that the ethyl lactate and water are taken to be added into a reactor, and then organic solvent and lipase catalyst are added, wherein, the materials has the compounding ratio that the mass ratio of the ethyl lactate and the water is 3 to 15:1, the dosage of the lipase catalyst is 0.4 to 1 percent of the mass of the ethyl lactate, and the volume of the water is 1 to 10 percent of the volume of the organic solvent. After being sealed, the reactor is put into a thermostat water bath shaker, the number of revolution of the shaker is 220 to 50 revolution / minute, the reaction temperature is 30 to 70 DEG C, the reaction time is 3 to 28 hours, and after the reaction is finished, the product, namely, the L-(+)-lactic acid can be obtained by distilling the molecules of the resultant. The invention has simple method for preparing the L-(+)-lactic acid, the reaction condition is mild and the environmental pollution is small; the stereo selectivity of the obtained product is high, the ee value of the L-(+)-lactic acid is 50 to 94 percent, and the yield rate thereof is 10 to 40 percent.
Owner:HEBEI UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com